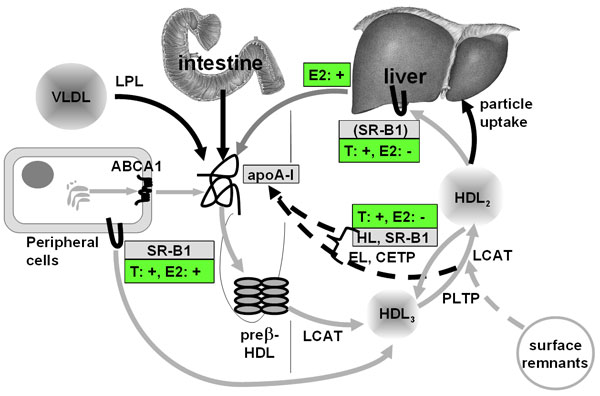
Figure 1 Pathways of HDL metabolism and regulation by testosterone and oestradiol Mature HDL 3 and HDL 2 are generated from lipid-free apoA-I or lipid-poor pre β-HDL as the precursors. These precursors are produced as nascent HDL by the liver or intestine or are released from lipolyzed VLDL and chlyomicrons, or by interconversion of HDL 3 and HDL 2 . ABCA1-mediated lipid efflux from cells is important for initial lipidation; LCAT-mediated esterification of cholesterol generates spherical particles which continue to grow upon ongoing cholesterol esterification, and PLTP-mediated particle fusion and surface remnant transfer. These mature HDL particles also continue to accept cellular cholesterol by processes which are facilitated by the scavenger receptor BI (SR-BI) and LCAT. Larger HDL 2 are converted into smaller HDL 3 upon CETP-mediated export of cholesteryl esters from HDL onto apoB-containing lipoproteins, SR-B1-mediated selective uptake of cholesteryl esters into liver and steroidogenic organs, and HL- and EL-mediated hydrolysis of phospholipids. HDL lipids are catabolized either separately from HDL proteins, i.e. by selective uptake or via CETP-transfer, or together with HDL proteins, ie. via uptake through as yet unknown HDL receptors or apoE receptors. Both the conversion of HDL 2 into HDL 3 and the PLTP-mediated conversion of HDL 3 into HDL 2 liberate lipid-free or poorly lipidated apoA-I, which is either re-used for the formation of mature HDL or is filtrated into the kidney. Grey arrows represent lipid transfer processes, black arrows represent protein transfer processes. The hepatic expression and activity of both HL and SR-B1 was shown to be up-regulated by testosterone and down-regulated by oestradiol. In addition oestradiol up-regulates the hepatic expression and secretion of apoA-I. These actions of testosterone and oestradiol are in good agreement with their lowering and increasing effect on HDL cholesterol, respectively. In addition both testosterone and oestradiol stimulate SR-BI expression in macrophages and thereby cholesterol efflux from these cells onto lipidated HDL.
