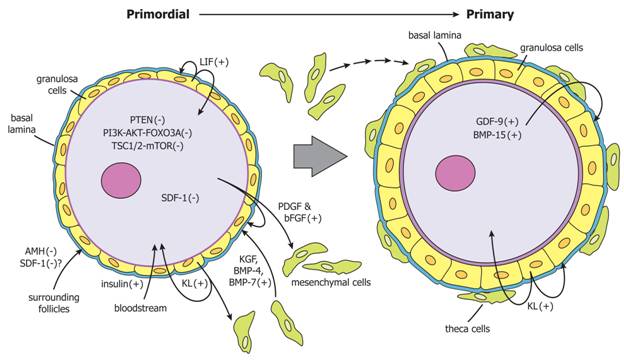
Figure 6. Regulation of primordial follicle activation. Primordial follicle activation is driven by the collective actions of primordial follicles themselves, surrounding mesenchymal cells, surrounding follicles, and endocrine factors. The model shown is based mainly on experimental evidence from rodent model systems. PTEN, Foxo3a, and SDF-1 generated by primordial oocytes restrain their own activation. Primordial oocytes secrete PDGF and bFGF that stimulate pre-granulosa cells to increase secretion of KL and promote recruitment of mesenchymal cells to the follicle. KL secreted by pregranulosa cells promotes oocyte growth and follicle activation, and promotes recruitment of mesenchymal cells. KGF, BMP-4, and BMP-7 secreted by surrounding mesenchymal cells stimulate follicle activation. AMH and possibly SDF-1 secreted from surrounding growing follicles negatively regulate primordial follicle activation. Insulin from the circulation may promote follicle activation. In primary follicles, granulosa cells continue to secrete KL, which further promotes follicle activation. Oocytes of primary follicles secrete GDF-9 and BMP-15, which promote granulosa cell proliferation, KL expression and theca formation. PTEN, phosphatase and tensin homolog; Foxo3a; forkhead box O3A; SDF-1, stromal derived factor-1; PDGF, platelet-derived growth factor; bFGF, basic fibroblast growth factor; KL, kit ligand; KGF, keratinocyte growth factor; BMP, bone morphogenetic protein; AMH, anti-Mullerian hormone; GDF-9, growth differentiation factor-9.
