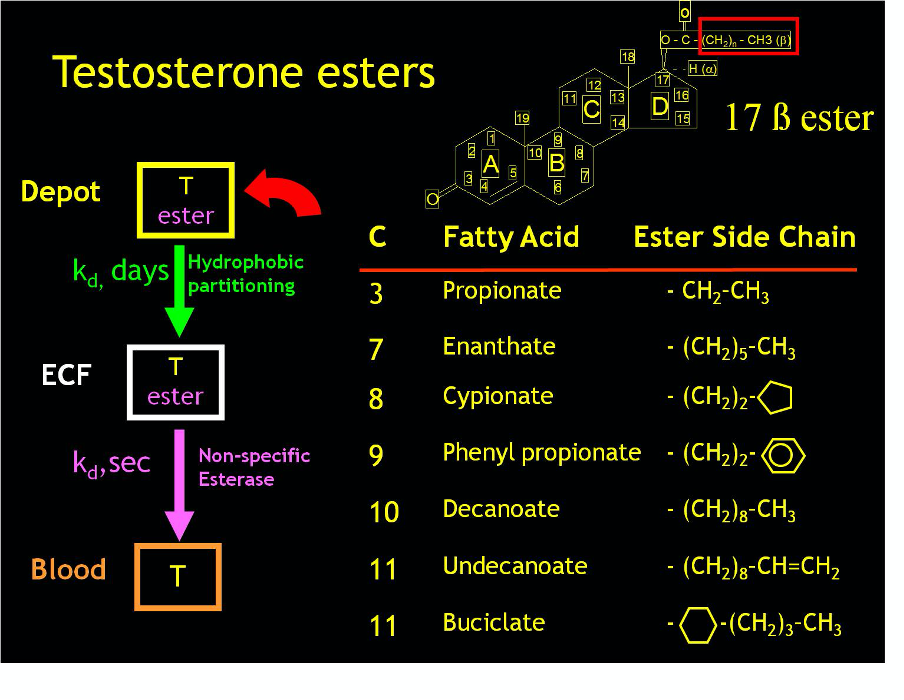
FIGURE 5. Schematic overview of the pharmacology of testosterone esters. Testosterone is esterified through the 17 β hydroxyl group with fatty acid esters of different aliphatic or other chain length which is a biologically inactive pro-drug. The esterified testosterone in an oil vehicle is injected deeply into a muscle forming a local drug depot from which the testosterone ester is released at a slow rate determined by its physico-chemical partitioning according to the testosterone ester’s hydrophobicity. Once the testosterone ester exits the depot and enters the extracellular fluids, it is rapidly hydrolyzed by ubiquitous non-specific esterases thereby releasing the testosterone into the general circulation.
