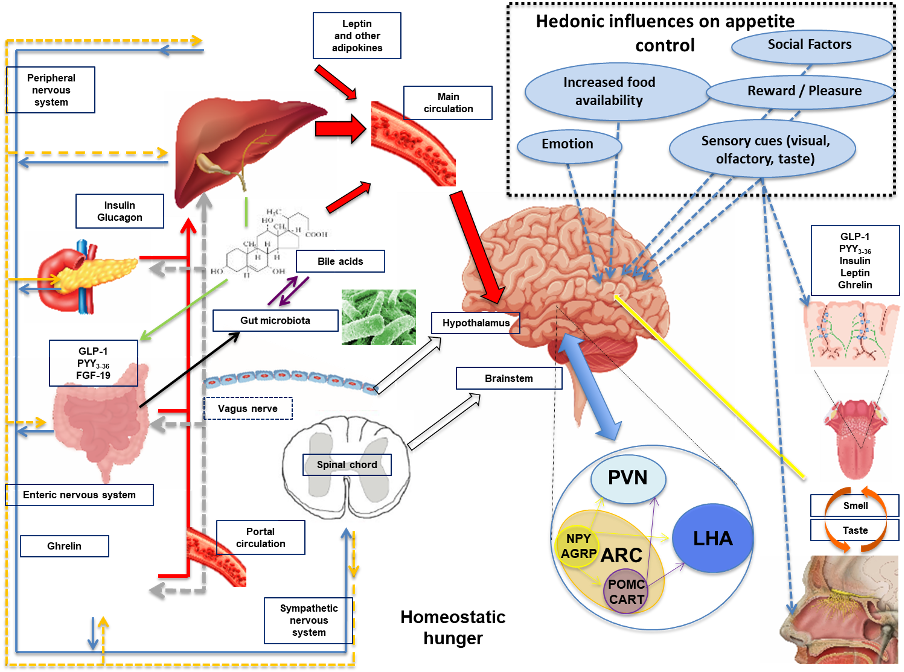
Figure 4. Schematic diagram illustrating the mechanisms involved in regulating feeding behavior. Nutrient entry into the GI tract causes gastric and intestinal distension, secretion of pancreatic enzymes and BA, altered enteric and vagal nerve signaling and exposure of EECs to nutrients with altered circulating gut hormone levels (e.g. decrease in orexigenic hormone acyl-ghrelin and increase in anorectic hormones PYY3-36 and GLP-1). Gut-derived signals (nutrients, hormones, and neural) and adipokines (e.g. leptin and others) act directly and indirectly upon the brainstem and hypothalamic areas (see Figure 3 for a detailed description of hypothalamic nuclei controlling energy homeostasis). All of these factors are involved in the regulation of homeostatic hunger. Social factors, emotion, reward, pleasure, increased food availability and sensory cues can influence brain reward and higher cognitive brain regions leading to altered feeding behavior (hedonic influences on hunger and appetite control). Taste and olfactory signals can also influence energy intake acting on both homeostatic and brain reward systems. Insulin leptin, GLP-1, PYY and ghrelin are present in saliva with cognate receptors on taste buds and olfactory neurons. Abbreviations: AgRP, agouti-related peptide; ARC, arcuate nucleus; CART, cocaine and amphetamine-regulated transcript; EEC’s, enteroendocrine cells; FGF-19, fibroblast growth factor-19; GLP-1, glucagon-like peptide 1; LHA, lateral hypothalamic area; NPY, neuropeptide Y, peripheral nervous system, PNS; PVN, paraventricular nucleus; PYY, peptide tyrosine-tyrosine 3-36; POMC, pro-opiomelanocortin, sympathetic nervous system, SNS.
