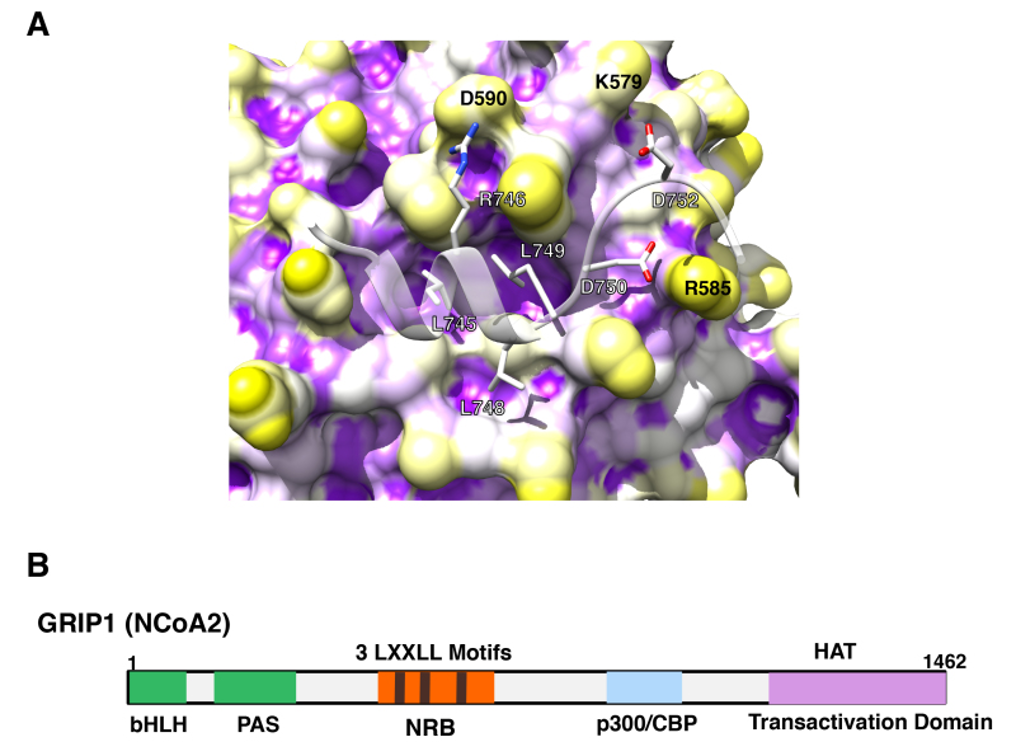
Figure 10. p160 coactivators physically interact with its multiple LxxLL motifs to the AF-2 surface of GR. A: 3-dimensional interaction image of GR AF-2 and the LXXLL peptide. The GR AF-2 surface has three large pockets into which core leucines (L745, L748 and L749) of the LXXLL peptide deeply bury themselves. There are additional intermolecular contacts that are important for peptide binding, including the electrostatic bonds created between (i) R746 (LXXLL peptide) and D590 (receptor), (ii) D750 (LXXLL peptide) and R585 (receptor) and (iii) D752 (LXXLL peptide) and K579 (receptor). From (89). B: p160-type coactivators (NCoAs) have 3 LxxLL motifs in their NR-binding box (NRB). Linearlized GRIP1 (NCoA2) molecule with NRB located in the middle portion is shown as a representative of the p160-type coactivators (NCoAs). In addition to NRB, GRIP1 has the basic helix-loop-helix (bLHL) and the PAS domains in its N-terminal portion, and p300/CBP-binding domain and one transactivation domain containing the HAT domain in the C-terminus.
