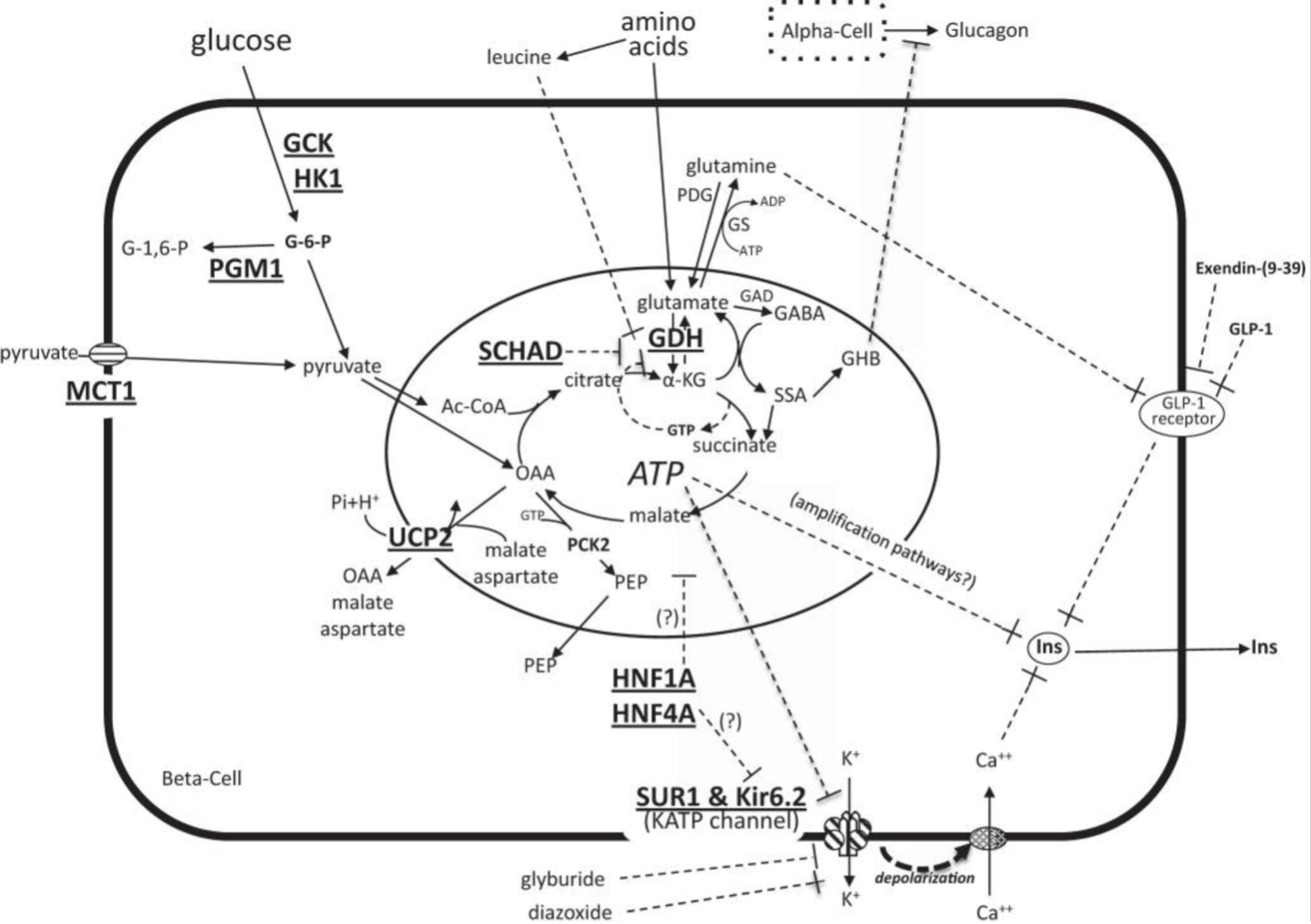
Figure 5. Diagram of the pathways stimulating beta-cell insulin secretion. Glucose enters the beta-cell via glucose transporters and is phosphorylated by GCK to G-6-phos. Oxidation of G-6-phos in mitochondria increases the ATP-to-ADP ratio, leading to closure of plasma membrane ATP-sensitive KATP channels (comprised of SUR1 and Kir6.2 subunits), inhibition of K+ efflux, membrane depolarization, opening of voltage-dependent Ca++ channels, Ca++ influx, and release of insulin from storage granules. Amino acids stimulate insulin secretion through glutamine-mediated amplification of GLP-1 receptor signaling. Leucine stimulates insulin secretion by increasing the oxidation of glutamate via activation of GDH, thereby increasing the ATP-to-ADP ratio and triggering the insulin secretion cascade. HK1 and MCT1 are not normally present in the beta-cell. Diazoxide suppresses insulin secretion by activating the KATP channel to remain open. Somatostatin suppresses insulin secretion downstream of Ca++ signaling. Known sites of defects associated with congenital hyperinsulinism are indicated by bold and underlined font and include GCK (glucokinase), HK1 (hexokinase 1), PGM1 (phosphoglucomutase 1), MCT1 (monocarboxylate transporter 1), UCP2 (uncoupling protein 2), SCHAD (short-chain 3-hydroxyacyl-coenzyme A dehydrogenase), GDH (glutamate dehydrogenase), HNF1A (hepatocyte nuclear factor 1A), HNFA (hepatocyte nuclear factor 4A), SUR1 (sulfonylurea receptor 1), Kir6.2 (inwardly rectifying potassium channel 6.2). Other abbreviations: OAA, oxaloacetate; PEP, phosphoenolpyruvate; α-KG, α-ketoglutarate; GAD, glutamic acid decarboxylase; GABA, γ-aminobutyrate, GHB, γ-hydroxybutyrate; SSA, succinic semialdehyde; Ins, insulin. Reprinted from Stanley CA, Perspective on the Genetics and Diagnosis of Congenital Hyperinsulinism Disorders, J Clin Endocrinol Metab, 2016, 101(3):815-826 by permission of Oxford University Press and Endocrine Society (31).
