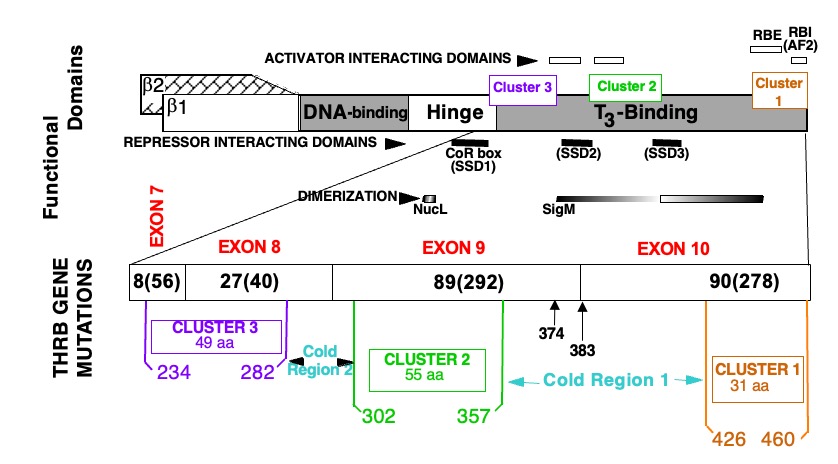
Figure 2. Location of mutations in the TRß protein in subjects with RTHß.
TOP PORTION: Schematic representation of TRß and its functional domains for interaction with TREs (DNA-binding), with hormone (T3-binding), with activating (298), repressing (299-301) cofactors and with nuclear receptor partners (dimerization) (74, 302, 303). Note their relationship to the three clusters of natural mutations.
BOTTOM PORTION: The T3-binding domain and distal end of the hinge region, which contain the three mutation clusters, are expanded. The four terminal exons containing all so far identified mutation are shown with the number different mutation and number of families in parenthesis (published and our unpublished data). Amino acids are numbered consecutively starting at the amino terminus of the TRß1 molecule according to the consensus statement of the First International Workshop on RTH (304). TRß2 has 15 additional residues at the N-terminus. Mutations occur in three clusters as indicated. A silent region between cluster 1 and 2, located in the dimerization domain contains two mutations (Q374K and R383H), indicated with arrows.
AF2, Hormone-dependent activation function (12th amphipathic helix) (305, 306); RBE, corepressor-binding enhancer; RBI, corepressor-binding inhibitor (306); SSD, silencing subdomain (301); NucL, nuclear localization (307); SigM, signature motif (308). aa, amino acid.
