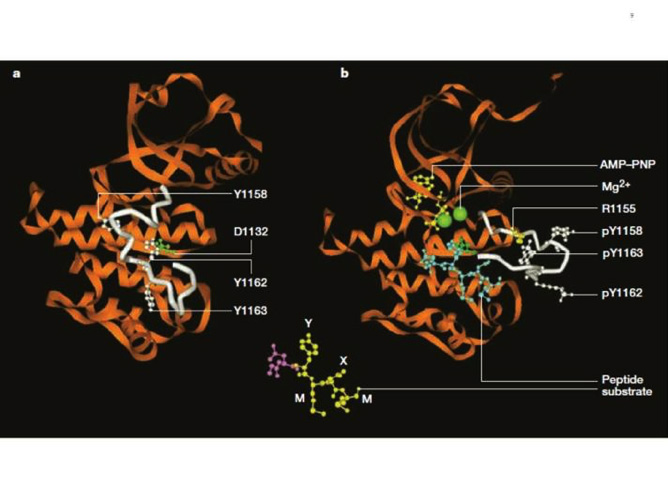
Figure 8. Structure of the inactive and activated insulin receptor tyrosine kinase (with bound ATP analogue AMP-PNP, peptide substrate and Mg2+). This figure illustrates the autoinhibition mechanism whereby Tyr 1162, one of the three tyrosines that are autophosphorylated in the activation loop (shown in white) in response to insulin (1158, 1162, 1163) is bound in the active site, hydrogen bonded to a conserved Asp 1132 in the catalytic loop (left). Tyr 1162 in effect competes with protein substrates before autophosphorylation. In the activated state (right), the activation loop is tris-phosphorylated and moves out of the active site. Tyr 1163 becomes hydrogen-bonded to a conserved Arg 1155 in the beginning of the activation loop, which stabilizes the repositioned loop. Also shown is the peptide substrate with the WMXM motif. From reference 25.