ABSTRACT
It is recognized that traditional measures of glucose control (such as hemoglobin A1c [A1C]) provide little information regarding the need for day-to-day changes in therapies. While intermittent self-monitored blood glucose (SMBG) provides additional information with which to make treatment decisions, significant barriers to its use exist, such asinconvenience and lack of timely and regular feedback. Furthermore, important information regarding glucose trends may be missed. Continuous glucose monitoring (CGM) has become increasingly reliable and has demonstrated efficacy in terms of improving A1C, reducing hypoglycemia, and improving the time in target glucose range. Incremental progress continues to be made toward a fully functional artificial pancreas, of which CGM will play a vital role. As more and more data are presented to patients and providers, it has become increasingly paramount that the data are organized in a standardized way and that communication of data is streamlined using patients’ mobiledevices where available and within the existing clinic infrastructure. Systems that provide immediate feedback to patients and decision support tools for patients and providers have demonstrated superior outcomes compared to routine SMBG alone. Alternate markers of glucose control may provide complementary information about glucose control and long-term prognosis. This chapter will review the latest evidence for use of professional and personal CGM, mobile glucose monitoring approaches, and biomarkers of glycemic control.
INTRODUCTION
The current technology for monitoring of glucose levels has been well established since the 1980′s. This practice is beneficial to patients with diabetes from both a clinical and an economic standpoint when used optimally. Knowledgeof the glucose levels that are measured can allow a patient to select an appropriate dose of insulin or implement dietary or other lifestyle changes to regulate their glucose levels. Expert groups provide recommendations for glucose targets, including A1C, self-monitored blood glucose (SMBG), and interstitial glucose (1,2). Although targets vary, expert groups recommend individualization based upon risk of hypoglycemia, polypharmacy, comorbidities, and other characteristics that may affect long-term benefit and individual patient characteristics. The ADA has expanded recommendations for assessing overall glucose levels to include the A1C or CGM metrics such as % Time in Range(TIR, the % of time spent 70-180 mg/dl), or the Glucose Management Indicator (GMI), which is an estimate of A1C that is derived from a 14-day CGM report for routine assessment of glucose levels (1).
The landscape of glucose monitoring technologies is expanding and rapidly changing. For a full review of glucose monitoring technologies, the reader is referred to one of many excellent reviews referenced throughout this chapter. Several trends are emerging in glucose monitoring and will be reviewed in more detail in this chapter:
- CGM: This practice is becoming more widely established as evidence supporting its use has accumulated. The data available through CGM can permit significantly more fine-tuned adjustments in insulin dosing and other therapies than spot testing from self-monitoring of blood glucose (SMBG) can CGM technologies forautomatic collection of data have spurred interest in noninvasive glucose monitoring as an additional tool for obtaining information about glucose levels.
- Closed loop control (CLC): Also known as an “artificial” or “bionic” pancreas, this technology links CGM withautomatically controlled insulin The first steps toward CLC are now in use.
- Mobile Technology and Decision Support: In recent years, increasing connectivity between glucose monitoringtechnologies and mobile devices has facilitated ongoing improvements in self-care and communication of data.
- Alternate Markers of Glucose Control: Finally, the use of additional analytes besides glucose is still being established.
This chapter analyzes the technology, benefits, and problems with the use of intermittent SMBG and CGM, mobile technology and decision support, and alternate biomarkers of glycemic control.
CONTINUOUS GLUCOSE MONITORS
CGM measures glucose levels (typically interstitial glucose) continuously and updates the glucose level display every 5 minutes. Most CGMs consist of 1) a monitor to display the information (in some cases, this is the patient’s mobile device), 2) a sensor that is usually inserted into the subcutaneous tissue, and 3) a transmitter that transmits the sensor data to the monitor. Previously, all devices were approved for adjunctive use only due to limitations in accuracy; in this case patients must still perform fingerstick glucose monitoring in order to guide therapy and perform calibrations. However, in 2016, the FDA approved the use of the Dexcom G5 as the first CGM for stand-alone use.Newer technologies have eliminated the requirements for calibration of CGM with a fingerstick glucose. The accuracy of all commercially available CGMs is still the lowest in the hypoglycemic range, which is where the need for sensitivity and specificity is great in terms of serving as an alarm for hypoglycemia.
CGM can provide both retrospective as well as real-time information to detect: 1) hypoglycemic and hyperglycemic excursions; 2) predict impending hypoglycemia; and 3) wide fluctuations in glucose levels, also known as glycemicvariability. 24-hour telephone support is available for all FDA approved CGM devices. Use of CGM can help both thepatient and their medical provider make fine tune adjustments to medication therapy and provide insight to the patient on behavioral changes to achieve glycemic control. Additionally, current efforts to link CGM measurement with automatically controlled insulin delivery, has progressed incrementally toward a fully functional artificial pancreas.Systems can be divided according to their intended use as professional CGM (which is a clinic-owned device and provides either retrospective or real-time glucose data) and personal CGM (which is patient-owned and provides real-time glucose data).
PROFESSIONAL CGM
Professional CGM describes CGM data that are obtained via healthcare provider owned equipment. It does not necessarily provide the glucose results in real time, but downloads the readings after they have been collected, similar to a 24-hour cardiac Holter monitor that provides information about cardiac rhythms after they have occurred. This allows the health care provider to obtain relatively unbiased glucose patterns during typical everyday life. The Endocrine Society recommendations state that professional CGM may be of benefit in adults with diabetes to detectnocturnal hypoglycemia, dawn phenomenon, postprandial hyperglycemia and to assist in management of diabetes therapies (3). Professional CGM is more readily reimbursed than personal CGM, but interpretation of both personal and professional CGM reports by qualified healthcare professionals may be reimbursed on a monthly basis.
Some personal CGM systems can be operated in a blinded fashion in order to provide professional glucose data. These systems will be discussed in more detail later (see “Personal [Real-time] Continuous Glucose Monitoring”). The first device for reading blood glucose levels continuously was a professional CGM that was approved by the FDA inJune 1999. This device was the Continuous Glucose Monitor System (CGMS) manufactured by Medtronic MiniMed (Medtronic Diabetes, Northridge, CA) (4). Since then, newer models have shown improvements in accuracy and patient acceptance. In a meta-analysis of 22 articles, professional CGM resulted in a greater reduction in A1c(-0.28%, 95% CI -0.36% to -0.21%, P < 0.00001) as well as TIR (5.59%, 95% CI 0.12 to 11.06, P = 0.05) compared to usual practice (5).
FreeStyle Libre Pro
The FreeStyle Libre Pro utilizes the same sensor as the Libre personal CGM. The Libre is factory calibrated andtherefore does not require self-monitored blood glucose calibrations. This may be a potential advantage since capillary blood glucose testing is subject to various system and user errors, which in addition to the physiologic lag time between blood and interstitial glucose (which is magnified in the postprandial period) could contribute to CGM error. Itcollects up to 14 days of glucose readings, which are recorded every 15 minutes. The glucose sensor is fully disposable and a single reader is used to activate and scan multiple devices, allowing multiple patients in one office to undergo the procedure simultaneously. Reports are obtained through the LibreView website, which offers a secure cloud-based system, or the FreeStyle Libre desktop reporting software. Reports provide daily patterns, an assessmentof glucose variability and hypoglycemia risk, a daily glucose report, and an overall snapshot report.
The overall MARD (Mean Absolute Relative Difference which is calculated by averaging the absolute values of relative differences between CGM measurement results and corresponding comparison method results) for the FreeStyle Libre is 11.4%, 86.7% of readings were in Zone A of the Consensus Error Grid analysis, and 99.7% of results were in Zones A and B (6). It is important to note that sensor accuracy is lower on day 1 and in thehypoglycemia range (MARD 20.3% for values <72 mg/dl in one study) (7). Accuracy improves and remains steady over the 14-day wear period. The Libre utilizes glucose oxidase in a “direct signaling” approach that is not dependenton oxygen and minimizes interference by other substances, such as acetaminophen, which may falsely elevated readings on other devices.
Dexcom Professional
The Dexcom G6 Pro was approved by the FDA in March 2018 and is available in blinded or unblinded mode depending upon whether the goal is to observe glucose patterns without intervention, to provide immediate feedback to educate and inform patients about their medications and behaviors, or to facilitate decisions about pursuingpersonal CGM. The sensor, transmitter, and receiver are essentially identical to the personal Dexcom G6 system and features expedited startup time and no calibration. The device measures interstitial glucose levels every 5 minutes and is approved for 10 days of use. The device is downloaded using Dexcom CLARITY, a web-based software program that is also used to download and review personal data.
Analysis of Retrospective Data
Data from all CGM devices can be studied retrospectively after downloading (8). It is recommended that diet, activity, symptom, and insulin data are collected during professional CGM to assist with interpretation, either via patient diary,direct entry of events into the device, or use of an accompanying app, depending on the system. Three time periods should be analyzed. These are:
- Overnight: Out-of-target overnight glucose levels can be modified by adjusting the basal insulin dose.
- Pre-prandial Period: Out-of-target pre-prandial glucose levels can be modified by adjusting the previous meal bolus, meal, or exercise pattern.
- Post-prandial period: Out-of-target postprandial glucose levels can be modified by adjusting the immediate meal bolus, meal, or exercise pattern.
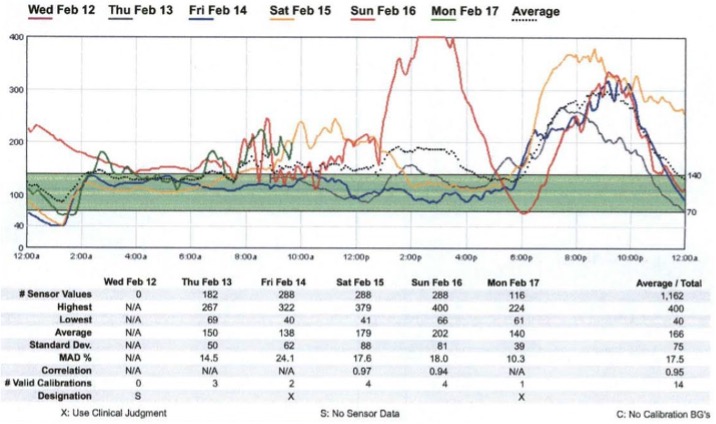
In certain special situations, targets may need to be adjusted. Other important elements of a professional CGM analysis are shown in Table 1. An example of a patient who used CGM is presented in Figure 1. The CGMdemonstrated high glucose levels from 6:00 PM to 11:00 PM post-supper and low glucose levels from 12:00 AM to 2AM. Recognition of these patterns allowed appropriately timed treatment interventions.
|
Table 1. Elements of Professional Continuous Glucose Monitoring Analysis |
|
Overall Control Glucose Variability (Standard Deviation, Coefficient of Variation) Daily Detail Diurnal Patterns: dawn phenomenon, overnight Meal effects Correction Exercise effects Other patterns (work days vs. weekend, menstrual cycles) Hypoglycemia Precipitating factors Corresponding meter glucose (recognition) |
Ambulatory Glucose Profile
The ambulatory glucose profile (AGP, Figure 2) is a standardized reporting format for glucose data that was developed by an expert panel of diabetes specialists and sponsored by the Helmsley Charitable Trust and is customized for insulin pumps or injection therapy (9). The universal report is intended to simplify and facilitate interpretation of otherwise complex and lengthy reports with varying terminology. It is anticipated that a standardized report would “help clinicians develop expertise in CGM use, enhance quality of care through enhanced pattern recognition, improve practice efficiencies with minimal disruption of workflow, and engage patients, thereby reinforcing consistent use of CGM technology.” A single page report that the medical team can view and file into a patient’s electronic medical record and that can be used as a shared decision-making tool with people with diabetes wasconsidered to be of great value in the report of the 12th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2019) (10). The AGP is currently employed by many reporting systems and consists of 3 components:
- Statistical Summary, which utilizes standard metrics and terminology to summarize the number of values,percentage of values, and time in target, above target, and below target, as well as an assessment of glucose variability.
- Modal day report which collapses data from days or weeks to a single day in order to identify patterns by time of day. Data are presented graphically as 5 distribution curves, representing the median, interquartile range, and10th to 90th percentiles, on the backdrop of target range.
- Daily View, which facilitates review of within day
Composite Metrics
As a measure of the quality of glycemia, the time in range (TIR), similar to the A1C is limited in its assessment of hypoglycemia. Multiple composite metrics have thus been reported (11). However, the use of multiple metricsincreases complexity and is subject to issues with collinearity. The Glycemia Risk Index (GRI) is a composite metricthat was developed using input from 330 clinical experts who analyzed 14-day tracings from 225 adults with diabetes (12). GRI more heavily weights very high or very low glucose values and correlates with clinician rankings more closely than TIR or time below range (%time < 70 mg/dl, TBR) alone.
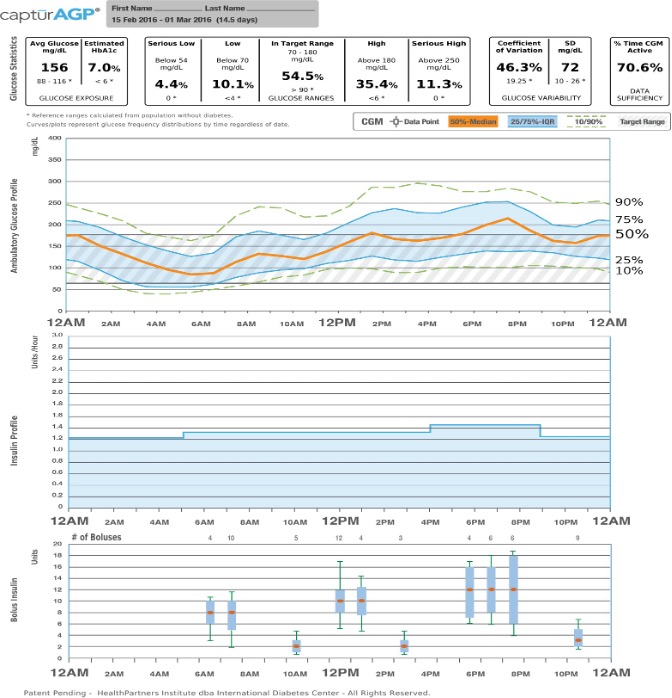
Figure 2. Ambulatory Glucose Profile for Insulin Pumps.
Glucose Statistics: Metrics include mean glucose, estimated A1C, glucose ranges, coefficient of variation and standard deviation.
Glucose Profile: Daily glucose profiles are combined to make a one-day (24-hour) picture. Ideally, lines would stay within grey shaded area (target range).
Orange: median (middle) glucose line.
Blue: area between blue lines shows 50% of the glucose values.
Green: 10% of values are above (90% top line) and 10% are below (10% bottom line). Insulin Profile Graph: Shows basal insulin pump settings over a 24-hour period.
Bolus Insulin Graph: Combines all bolus insulin doses into one graph to make a one-day (24-hour) picture. Each box on the graph covers 60 minutes of doses.
Orange: median (middle) dot.
Blue: shaded box shows 50% of the bolus dosages in the hour.
Green: lines above and below the shaded box (whiskers) show how many of the bolus dosages per hour were between 75 - 90% and between 10 - 25%.
PERSONAL REAL-TIME CGM (RT-CGM) OR INTERMITTENTLY SCANNED CGM (IS-CGM)
RT-CGM devices not only display the current glucose every few minutes, but may also alert the patient for impending (projected alert) or actual (threshold alert) hyperglycemia or hypoglycemia or rate of change in glucose. Bycomparison, is-CGM requires patient interaction with the device to obtain readings but may still provide alerts for hypoglycemia or hyperglycemia. While few head to head studies are available, some studies suggest greater reduction in hypoglycemia and improvement in TIR with RT-CGM compared to is-CGM in persons with type 1 diabetes (13,14), even up to 24 months (15).
Over time, accuracy with RT-CGM and is-CGM has improved substantially (16,17,18). In fact, some devices, including the Dexcom and Freestyle Libre are approved for stand-alone use, meaning that under specified conditions, the device may be used to make treatment decisions without confirmatory blood glucose measure. However, the user will still experience a tradeoff between a high alarm sensitivity and specificity for detecting hypoglycemic events, particularly where glucose levels are changing rapidly (Figure 3). Current and recent glucose levels, trend information,and a visual alarm are all presented so that a patient can predict future low or high glucose excursions. Using this information will allow the patient to take actions to spend more time in the euglycemic range and less time in the hypoglycemic or hyperglycemic ranges. This potential decrease in glycemic variability will not necessarily be reflected in an improved A1C value, which reflects mean glycemic levels.
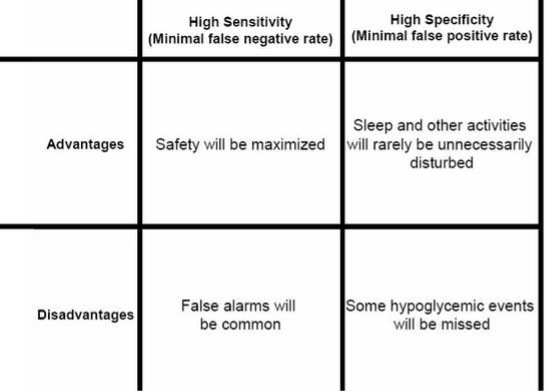
Figure 3. Tradeoffs between emphasis on high sensitivity compared to emphasis on high specificity in a hypoglycemic alarm that is part of a continuous glucose monitor.
Evidence- Type 1 Diabetes
Studies may be divided according to background therapies (insulin pump or injection therapy).
STUDIES UTILIZING EITHER INSULIN PUMP OR INJECTIONS AS BACKGROUND THERAPIES
- The seven-country GuardControl Study was the first randomized controlled trial to ever demonstrate a statisticallysignificant improvement in A1C levels with the use of RT-CGM (19). The Guardian RT was used either continuously or biweekly for three months and both regimens were compared to control treatment which did not include use of CGM. At one month and at three months the continuous users had significantly lower A1C levelsthan the controls. The biweekly users had intermediate improvement which did not reach statistical significance compared to the outcomes in the control group.
- In 2008, the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group evaluated 322 adults and children with type 1 diabetes (either injection or insulin pump therapy) and A1C 7-10% who wererandomized to either RT-CGM or usual care (20). RT-CGM was associated with a 53% reduction in A1Ccompared to usual care (p<0.001), but was only significant among subjects over age 24 due to lack of consistent use in younger patients. Hypoglycemia was infrequent and was not different between groups.
- In 2011, 120 children and adults with type 1 diabetes on insulin pump or injection therapy and A1C <7.5% wererandomly assigned to RT-CGM (Freestyle Navigator—not available in the US) or masked CGM every other week (21). The time spent in hypoglycemia was reduced over 50% at 26 weeks, and patients spent more time in 70-180 mg/dl range.
- In the IMPACT trial, 241 adults with type 1 diabetes with an A1C less than or equal to 7.5% were randomly assigned to Freestyle flash glucose monitoring (described in more detail under “Overview of Stand-Alone Personal CGM systems”) vs. SMBG. In this group 68% of the patients were treated with multiple daily injections and 32% with CSII. The amount of time spent in hypoglycemia was decreased by nearly 90 minutes per day (P<0.0001) when patients had access to CGM data (22). It must be noted that this technology does not provide real-time alerts for impending hypoglycemia or hyperglycemia and data are accessed via a hand-held device on Ina small study of patients with hypoglycemia unawareness or recent severe hypoglycemia, RT-CGM more effectively reduced the time spent in hypoglycemia compared to flash glucose monitoring (23).
- The CITY study was a randomized study among 153 adolescents and young adults with type 1 diabetes. CGM resulted in a -0.37% greater reduction in A1C compared to usual care (p=0.01) (24). In this study, only 68% of participants used CGM at least 5 days per week in month 6, which is significantly lower than studies reported inadults (25). However, this is more than twice that reported in the pivotal JDRF study of 2008 (20). Moreover, this study utilized an earlier generation CGM which required twice daily calibration; thus, it is possible that newer technologies may support greater persistence with use.
- Among 203 older adults (median age 68) with type 1 diabetes randomized to CGM or usual care, CGM resulted in less hypoglycemia at 26 weeks (estimated treatment difference 27 minutes/day, p<0.001) as well as modest improvement in A1C (estimated treatment difference -0.3%, p<0.001) (26). The improvement in hypoglycemia was sustained over 52 weeks, at which point CGM use was still >90% (27).
STUDIES UTILIZING INSULIN PUMP THERAPY AS BACKGROUND
- In the largest study to date, the STAR3 study, 485 adults and children with A1C 4-9.5% were randomized to sensor-augmented pump therapy (Medtronic Paradigm Revel) or multiple daily injections per day (28). Sensor-augmented pump therapy resulted in better A1C reduction with between-group difference of 0.6%, p<0.001.Hypoglycemia did not differ between groups, but only short-term CGM data were available for comparison and patients with a history of severe hypoglycemia were excluded.
STUDIES UTILIZING INJECTION THERAPY AS BACKGROUND
- In 2016, a 6-month randomized controlled trial, the DIAMOND study, compared RT-CGM (using Dexcom G4system) versus SMBG in 158 patients with type 1 diabetes on multi-dose injection therapy and demonstrated a significantly lower A1C (between group difference 0.6%, p<0.0001), decrease in hypoglycemia (43 minutes vs. 80 minutes per day, p=0.0002) and less glucose variability with RT-CGM compared to SMBG. This study did not address hypoglycemia frequency in the two groups (25).
- The GOLD trial studied 161 patients with type 1 diabetes receiving multiple daily injections with either RT-CGM (Dexcom G4) or standard care in a random order cross-over trial. The mean difference in A1C was 0.43% (p<0.001), favoring RT-CGM. One subject in the CGM group compared to 5 subjects in the standard care group experienced a severe hypoglycemic event. The percentage of time spent in hypoglycemia numerically favored the CGM group but statistical analyses were not presented. There was a significant reduction in standard deviationand MAGE (measures of glucose variability). Overall well-being, diabetes treatment satisfaction, and fear of hypoglycemia improved (29).
- In the FLASH-UK study, 156 participants with type 1 diabetes were randomized to intermittently scanned glucosemonitoring or usual care (30). The intervention group had a significantly greater reduction in HbA1 (adjusted treatment difference -0.5%, p<0.001), higher % TIR, and lower % TBR.
- A randomized controlled trial among 104 adults with type 1 diabetes found that intermittently scanned glucose monitoring improved A1c (estimated treatment difference 0.3% [95% CI, 0.0%-0.6%; P = 0.04) and TIR but not TBR compared to blood glucose monitoring (31).
META-ANALYSES
A Cochrane review and another meta-analysis found modest A1c reductions, particularly among patients who were not using insulin pumps, patients under age 18, and among patients with lower adherence (32). The results were heavily influenced by the STAR3 trial, and the JDRF study did not report a difference between pump users and patients using multiple dose injection therapy. Severe hypoglycemia rates did not differ. However, the quality of most studies was limited due to small sample size, lack of blinding, and lack of sufficient data to compare hypoglycemia rates. Meta-analyses may be hampered by the inclusion of studies with obsolete technology or lack of consideration for the intended use of the device in the study (33,34). In another meta-analysis, studies that specifically enrolled patients at risk for hypoglycemia and used blinded CGM to assess it did show improvement in hypoglycemia (35).
More recently, a meta-analysis of 21 studies published between 2011-2020 encompassing 2149 individuals with type 1 diabetes revealed that CGM led to a significant reduction in A1C by 0.23% (p=0.0005), with larger treatment effect at higher baseline A1C (>8%), and no effect on severe hypoglycemia or DKA (36). However, the meta-analysis did not report CGM derived metrics such as TIR or TBR or clinically significant hypoglycemia. In a 2023 meta-analysis of 22 randomized controlled trials that included participants with type 1 diabetes, there was an overall improvement in A1c,TIR and TBR (37). Reduction in A1C was limited to nonadjunctive devices but all devices resulted in improvement in TIR.
PATIENTS WITH HYPOGLYCEMIC UNAWARENESS
Many older studies specifically excluded patients with a history of severe hypoglycemia or were underpowered todetect significant hypoglycemia. Recent studies have examined the use of RT- CGM in patients with hypoglycemiaunawareness, which is a risk factor for severe hypoglycemia (events requiring outside assistance to treat).
- In the HypoCOMPaSS trial, 96 patients with a history of hypoglycemia unawareness determined by the GOLD Score of at least 4 or more were randomly assigned in a 2x2 factorial design to insulin pump or injection therapy, both with access to a bolus insulin calculator, and either RT-CGM (Medtronic Continuous Glucose Monitoring System) or SMBG. All patients had diabetes education with a goal toward hypoglycemia avoidance (38). Theresults demonstrated a similar reduction in severe hypoglycemia and improvement in hypoglycemia unawareness and fear of hypoglycemia without a significant treatment interaction between insulin or glucose monitoring interventions. Treatment satisfaction was higher with insulin pump compared to injection therapy but similar between RT-CGM and
- The IN CONTROL trial evaluated patients with Type 1 diabetes and hypoglycemia unawareness receiving either injection or insulin pump therapy in a crossover study comparing RT-CTM (Medtronic Paradigm Veo system with a MiniLink transmitter and an Enlite glucose sensor) or SMBG (39). Hypoglycemia was significantly reduced with RT-CGM compared to SMBG (including a 9.8% reduction in events <70 mg/dl and 44% reduction in events <40 mg/dl). Severe hypoglycemic events were significantly reduced but hypoglycemia unawareness was unchanged.
- In a smaller study of 52 adults with type 1 diabetes and problematic hypoglycemia, immediate randomization to CGM was more effective for preventing severe hypoglycemia (39% fewer events, p<0.05) than a dedicated hypoglycemia avoidance education program alone (40). CGM also lead to greater reduction in A1c (treatment difference -0.47%, p<0.05), but impaired awareness was restored in 31% of both groups, supporting the conceptthat CGM assists in earlier recognition and treatment of impending hypoglycemia as opposed to effecting fundamental change in counterregulatory responses.
Differences between studies may be explained by differences in populations and the technologies utilized. In the InCONTROL study, contact with patients was less frequent, sensor use was greater (89 vs. 57% in HypoCOMPaSS) and there were no insulin adjustment protocols. Therefore, more studies are needed to understand the potential role of background therapy, other technologies, and clinical support.
PATIENT REPORTED OUTCOMES
Generic Quality of life scores generally do not improve with RT-CGM but treatment-specific measures, such asdiabetes distress, hypoglycemic confidence, fear of hypoglycemia and to a lesser extent, measures of convenience, efficacy and performance, may be improved (28,41,42).
Evidence- Type 2 Diabetes
In patients with type 2 diabetes, even in patients not on insulin, RT-CGM may act as a motivator and positive influence for patients to improve lifestyle. The change in behavior can potentially lead to better glycemic control and weight loss(43). Moreover, periodic (every 3 months) short- term (14 day) use of real-time CGM may be sufficient to achieve andmaintain clinically relevant improvements in A1c in this population (44).
- In 2012, Vigersky et al. randomized 100 patients with type 2 diabetes on basal insulin and anti-hyperglycemic agents into either a group that used real-time RT-CGM intermittently (2 weeks on, 1 week off) or a group that recorded SMBG four times per day for 12 weeks. At 12 weeks, they found a statistically significantly greater reduction in A1c by 1.0% in the CGM group compared to 5% reduction in the SMBG group. The effect persistedup to the 40-week follow-up, 0.8% and 0.5% reduction in A1c in the RT-CGM versus SMBG group respectively (45).
- In 2017, Beck et al conducted a randomized study to evaluate benefit of RT-CGM use in 158 patients with type 2 diabetes with mean A1C of 8.5% treated using multiple daily injections (46). Over a 24-week period the A1C decreased to 7.7% in the RT-CGM group compared to 8% in the group with usual care (mean difference -0.3%,p=0.022). RT-CGM derived hypoglycemia and quality of life did not differ.
- The Dexcom MOBILE study assessed patients with type 2 diabetes on basal insulin randomly assigned to the Dexcom G6 or usual care for 8 months and reported a significant reduction in A1C, improved TIR andhypoglycemia (47). This was accomplished without an appreciable change in insulin or other medication use, indicating that CGM improves glucose levels by facilitating behavioral changes. Moreover, subsequent discontinuation of CGM for 6 months resulted in loss of about half of the improvement in TIR (48). Moreover, the benefit was similar in older (≥65 years old) vs. younger adults (49).
- In a 10-week study of 101 patients with type 2 diabetes on multiple daily injections of insulin, patients randomized flash glucose monitoring (Freestyle Libre) had greater A1C reduction (-0.82 vs -0.33%, p=0.005), found their treatment to be significantly more flexible and were more likely to recommend it to others (50).
- Among 141 adults with type 2 diabetes treated with insulin or sulfonylurea and recent myocardial infarction, thoserandomized to intermittently scanned glucose monitoring had significantly less TBR (-80 minutes, 95% CI -118, -43 minutes) at 90 days, but marginal difference in A1c or TIR, and the intervention was reported to be cost-effective (51).
- In a randomized trial of 116 adults with type 2 diabetes using non-insulin therapies, intermittently scanned glucose monitoring in combination with diabetes self-management education demonstrated superior A1c reduction at 16 weeks (treatment difference 0.3%, 95% CI 0 to 7%, p=0.048), larger increase in TIR (9.9%, p<0.01), and greatersatisfaction compared to education alone (52).
Real World Outcomes
- In a study of over 29,000 pediatric patients with type 1 diabetes in the Type 1 diabetes Exchange Registry or the German/Austrian DPV Initiative, pediatric CGM use was associated with lower mean A1C regardless of insulindelivery modality (pump or injection) (53).
- In a study of 106 UK hospitals incorporating 16,427 participants, 1241 with repeated TIR data, improvements inTIR were associated with improvement in hypoglycemia unawareness and diabetes related Moreover, TIR>70% was associated with reduced resource utilization (hospital admissions for hypoglycemia or hyperglycemia, paramedic visits, and severe hypoglycemia (54).
- In the Swedish National Diabetes Registry that included 14,372 adults with type 1 diabetes, intermittently scanned glucose monitoring was associated with a small (0.11%, p<0.0001) reduction in A1C after 15-24 months and reduction in severe hypoglycemic episodes (OR 0.79, 95% CI 0.69-0.91) (55).
- Using the French national claims database, a total of 74,011 patients with type 1 or type 2 diabetes initiatedintermittently scanned glucose monitoring and over 98% persisted with the device at 12 months (56). Following initiation of the device, patients had a 39-49% reduction in hospitalizations for acute complications and a 32-40%reduction in diabetes-related Moreover, the reduction in hospitalizations persisted after 2 years (57).
- In Belgium, a study of 1913 adults with type 1 diabetes were studied before and after nationwide reimbursement of intermittently scanned continuous glucose monitoring (58). Following the policy change, treatment satisfaction improved, there was a significant reduction in admissions for acute complications (severe hypoglycemia orketoacidosis), and there were fewer absences from work.
- Among 41,753 patients with insulin requiring diabetes in an integrated health care delivery system, 3806 patientsinitiated CGM, which was associated with a greater reduction in A1C (adjusted treatment difference 0.40%, p<0.001), emergency department or hospitalization for hypoglycemia (adjusted difference -2.7%, p=0.001), areduction in number of outpatient visits and an increase in telephone visits (59). However, there was no difference in hospitalizations for hyperglycemia or ketoacidosis.
- In a Medicare supplemental and commercial claims database study of 2463 patients with type 2 diabetes on multiple injections of insulin/day, intermittently scanned CGM was associated with a reduction in acute diabetes events (HR 0.39, 95% CI 0.30-0.51) and all cause hospitalizations (HR 68, 95% CI 0.59-0.78) at 6 monthscompared to the 6 months prior to initiation (60).
Recommendations
Patients should be adequately informed of the benefits and limitations of this technology, particularly with respect to the role for SMBG. At a minimum, structured education programs encompassing concepts such as carbohydrate counting and active insulin time (insulin on board) should be completed prior to considering RT-CGM, and payers mayrequire that patients demonstrate that they can reliably and consistently perform SMBG (61). Several expert groups have issued guidance in the use of RT-CGM.
- In 2016, the Endocrine Society, co-sponsored by The American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology, published guidelines for use ofinsulin pumps and CGM. The guidelines recommended RT-CGM in adults with type 1 diabetes and any A1C who are willing and able to use the devices nearly daily. The panel suggested short-term intermittent use for patientswith type 2 diabetes (not requiring prandial insulin) who had an A1C ≥7% and are willing and able to use the device (3).
- The American Diabetes Association (ADA) Standards of Care recommend CGM in adults with type 1 diabetes andthose with hypoglycemia unawareness or frequent hypoglycemia (Table 2a). Among pediatric patients, the ADAnotes that CGM may reduce missed school days with regular usage (1).
|
Table 2a. ADA 2023 Recommendations for CGM |
||||
|
Group |
Recommendation (Level of Evidence) |
|||
|
|
Real-time CGM |
Intermittently Scanned CGM |
||
|
|
Adults |
Youth |
Adults |
Youth |
|
MDI or CSII insulin use |
Should be offered (A) |
Should be offered(B-T1D, E-T2D) |
Should be offered(B) |
Should be offered (E- T1D) |
|
|
Should be used as close to daily as possible (A) |
Should be scanned frequently, at least every 8 hours (A) |
||
|
Basal insulin use |
A |
NA |
C |
NA |
|
All |
· Devices are recommended for individuals or caregivers who can use the devices safely · The choice of device should be individualized based on patient centered factors. · People should have uninterrupted access to supplies to minimize gaps in monitoring (A) · Periodic RT-CGM, intermittently scanned CGM, or professional CGM can be helpful where continuous use is not possible (C) |
|||
|
Diabetes and pregnancy |
CGM can help to achieve A1C targets in pregnancy when used as an adjunct to pre- and postprandial SMBG (B) |
|||
A=Clear evidence from well-conducted, generalizable randomized controlled trials that are adequately powered;B=Supportive evidence from well-conducted cohort studies; C= Supportive evidence from poorly controlled or uncontrolled studies; E=expert consensus.
T1D=type 1 diabetes, T2D=type 2 diabetes, SMBG=self-monitored blood glucose
|
Table 2b. AACE Recommendations for CGM by Methodology |
||||
|
Method |
Background/Therapy |
Evidence* |
BEL* |
Grade^ |
|
RT-CGM |
• Problematic hypoglycemia • Lifestyle and other factors should also be considered |
Low- Intermediate |
1 |
B |
|
isCGM |
• Newly diagnosed T2D • Non-hypoglycemic therapies • Motivated to scan device several times/day • Low hypoglycemia risk, desire for more data |
Low/Expert Opinion |
4 |
D |
|
Diagnostic/ professional CGM |
• Newly diagnosed T2D • problematic hypoglycemia, but no access to personal CGM • non-insulin therapies as an educational tool • Trial use |
Intermediate |
1 |
B |
|
Intermittent CGM |
persons …who are reluctant or unable to commit to routine CGM use. |
Intermediate |
1 |
C |
* Level of Evidence:
- High (1) = randomized controlled trial (RCT) or meta-analysis of RCT
- Intermediate (2) = meta-analysis including nonrandomized studies, network meta-analysis, nonrandomizedcontrolled trial, prospective cohort, case control, cross-sectional, hypothesis driven epidemiologic, open label extension, post-hoc analysis
- Weak (3) = discovery/exploratory, economic, consecutive case series, case report, safety/feasibility, high impact basic research
- None (4) = consensus, position, policy, guideline, any highly flawed study, lower impact basic science
BEL=best evidence level
^Grade is based upon evidence level, recommendation qualifiers, subjective factors, and consensus
|
Table 2c. AACE Recommendations for CGM—Patient Characteristics |
|||
|
Background/Therapy |
Evidence rating |
BEL |
Grade* |
|
3+ injections/day or CSII |
High |
1 |
A |
|
Frequent/severe or nocturnal hypoglycemia or unawareness |
Intermediate-High |
1 |
A |
|
Children/adolescents with T1D |
Intermediate-High |
1 |
A |
|
Pregnant, 3+ injection/day |
Intermediate-High |
1 |
A |
|
Gestational DM on insulin |
Intermediate |
1 |
A |
|
Gestational DM no insulin |
Intermediate |
1 |
B |
|
T2D, on insulin |
Intermediate |
1 |
B |
* Level of Evidence:
- High (1) = randomized controlled trial (RCT) or meta-analysis of RCT
- Intermediate (2) = meta-analysis including nonrandomized studies, network meta-analysis, nonrandomizedcontrolled trial, prospective cohort, case control, cross-sectional, hypothesis driven epidemiologic, open label extension, post-hoc analysis
- Weak (3) = discovery/exploratory, economic, consecutive case series, case report, safety/feasibility, high impact basic research
- None (4) = consensus, position, policy, guideline, any highly flawed study, lower impact basic science
BEL=best evidence level
^Grade is based upon evidence level, recommendation qualifiers, subjective factors, and consensus
The 2021 American Association of Clinical Endocrinologists recommendations for use are summarized in Table 2band 2c. These include all adults and children with type 1 diabetes, especially those with severe hypoglycemia or hypoglycemia unawareness, and all patients with type 2 diabetes on multiple insulin injections, basal insulin, or sulfonylureas who are at risk for hypoglycemia (2).
In 2017, the Advanced Technologies & Treatments for Diabetes (ATTD) Congress organized an international consensus panel, consisting of physicians, researchers, and individuals with diabetes to analyze the existing literature and to provide guidance for utilizing, interpreting, and reporting CGM data (62). This was updated in 2019 (Table 3 and 4). These recommendations are supported by recent data from the DCCT demonstrating that a 10% reduction in time in target glucose range derived from 7-point self-monitored glucose profiles is associated with a 40% reduction in risk of microalbuminuria and a 64% reduction in risk of incident or progressive retinopathy (63).
|
Table 3. CGM-Based Targets for Different Diabetes Populations |
||
|
Glucose Range |
%Time in Range |
|
|
|
Non-Pregnant Patients |
|
|
Type 1 and Type 2 Diabetes |
Older/High Risk Diabetes |
|
|
>250 mg/dl (13.9 mmol/L) |
<5% |
<10% |
|
>180 mg/dl (10 mmol/L)* |
<25% |
<50% |
|
70-180 mg/dl (3.9-10 mmol/L) |
>70% |
>50% |
|
<70 mg/dl (3.9 mmol/L)** |
<4% |
<1% |
|
<54 mg/dl (3.0 mmol/L) |
<1% |
|
|
|
Pregnant Patients |
|
|
Type 1 Diabetes |
Gestational and Type 2 Diabetes # |
|
|
>140 mg/dl (7.8 mmol/L) |
<25% |
- |
|
63-140 mg/dl (3.5-7.8 mmol/L) |
>70% |
- |
|
<65 mg/dl (3.5 mmol/L) |
<4% |
- |
|
<54 mg/dl (3.0 mmol/L) |
<1% |
- |
*Includes time >250, **Includes time <54 mg/dl, #Insufficient data
|
Table 4. Summary of ATTD Recommendations for CGM |
|
|
Limitations of A1C |
CGM should be utilized when there is a discrepancy in A1C and other measures of glucose control. CGM should be utilized to assess hypoglycemia and glucose variability. |
|
Guiding management and assessing outcomes |
CGM should be considered for patients with type 1 diabetes and insulintreated type 2 diabetes who are not achieving targets or those with hypoglycemia. All patients should receive training education regarding how to interpret andrespond to their data, utilizing standardized programs with follow-up. |
|
Performance |
No accepted standard exists for CGM system performance. However, a mean absolute relative difference ≤10% provides little additional benefit forinsulin dosing. |
|
Definition and assessment of hypoglycemia |
Clinical classification Level 1: 54-70 mg/dl with or without symptoms Level 2: <54 mg/dl with or without symptoms (clinically significant) Level 3: cognitive impairment requiring external assistance for recovery Quantification using CGM % of values or time below a given threshold (54 or 70 mg/dl) Number of events (defined as CGM readings persistently below threshold for at least15 min. with recovery defined as persistent readings over the threshold forat least 15 min.) over a given reporting period) |
|
Glycemic Variability |
Coefficient of Variation should be the primary measure |
|
Time in Range |
The % time in hyperglycemia, hypoglycemia, and target range should be reported. |
|
CGM Metrics |
Standardized reporting using the AGP and integration into electronic health records is recommended. Key metrics: · Number of days worn (14 days recommended) · % time CGM is active (70% of data from 14 days recommended) · Mean glucose · Glucose Management Indicator · Glycemic variability (%CV, target <36%) · % Time in Range (TIR): 70-180 mg/dl (3.9-10.0 mmol/L) %Time Above Range (TAR): 181-250 mg/dl (10.1-13.9 mmol/L), and >250 mg/dl (>13.9 mmol/L) · %Time Below Range (TBR): 54-69 mg/dl (3.0-3.8 mmol/L), and <54 mg/dl (3.0 mmol/L) |
Also, in 2017 the ADA and the European Association for the Study of Diabetes published a joint statement providing recommendations for systematic improvements in clinical use and regulatory handling of CGM devices (64).
Hospital Use
CGM is not currently approved for use in the hospital setting. However, during the COVID-19 pandemic, the FDA announced that it would not object to their use in an effort to support reduction in use of personal protective equipmentand risk of exposures to staff. Thus, there has been increasing interest in their use. Moreover, an increasing number of patients are using these devices in the ambulatory setting and want to continue their use in the hospital. Recent randomized trials support the use of CGM on the hospital wards, where it has been shown to be safe, and may reduce the frequency of hypoglycemia (65,66). In the ICU, there is concern that CGM may be less accurate due to factors such as edema, hypoperfusion, and acidosis but preliminary studies suggest use of CGM in conjunction with periodic point of care (POC) blood glucose (BG) within well-established protocols is safe and may reduce the need for POC BG (67).
In 2020, the Diabetes Technology Society sponsored a panel of experts in inpatient diabetes management to reviewthe evidence for us of CGM in the hospital (68). The panel agreed that CGM had the potential to improve clinicaloutcomes, particularly for patients who are unable to communicate signs or symptoms of hypoglycemia, but use is limited by lack of data demonstrating accuracy (particularly in the hypoglycemic range or in case of diabetic ketoacidosis, poor perfusion, or acetaminophen use) and clinical utility, and a lack of decision support systems, including infrastructure for communicating results to care teams and to the electronic medical record. The panelagreed that patients who are admitted with personal CGM devices should be allowed to continue use of such devices under the condition that they are able to self-manage the devices on their own and are followed by an endocrinologist or experienced practitioner who is specifically trained in their use. In particular, the panel advised implementing institutional policies that recommend continued capillary or blood glucose monitoring, ensuring that CGM data are not used for inpatient insulin dosing (since no CGM device is FDA approved in the inpatient setting), and requiring patients to sign safety waivers which illustrate the potential risks and benefits of continued use. Devices must beremoved for any MR or CT imaging. The panel made the specific recommendations for clinical care including:
- Consider use of CGM to reduce exposures (such as for point of care glucose) and need for personal protective equipment in persons with highly contagious diseases.
- Barring use in the setting of highly contagious disease, CGM values should be confirmed with point of care (POC) glucose prior to making treatment decisions.
- Hospitals should develop implementation plans which include a process map, protocol, provider/staff/patient education and order sets.
- Providers should recognize CGM pattern caused by compression of the device, which can cause a falsely low value.
- Providers should ensure patients are not taking medications or supplements that can interfere with CGM.
- Nurses should be adequately trained on use of CGM, inspect the insertion site every shift, and set expectations that POC values are still necessary to support ongoing use of CGM (typically every 6 hours).
- Hospitals need to develop security protocols, data storage, visualization tools, and integration within the electronic medical record to support the use of CGM.
- Hospitals need to identify CGM values in the electronic medical record to distinguish values from blood glucose values.
- Hospitals need to adopt the Unique Device Identifier (UDI) to track devices in the electronic medical record.
Limitations of Use
It should be emphasized that most prospective randomized controlled trials enroll highly motivated patients. In the real-world setting, there are concerns about limited resources for training, and less motivated patients may be overwhelmed with the additional data, particularly where complex algorithms are required. Nevertheless, in the Type 1 Diabetes Exchange Registry, CGM use increased from 7% in 2010-2012 to 30% in 2016-2018, and rose more than 10-fold in children (69). A1C levels were lower in CGM users compared to nonusers. While CGM use has improved substantially over time, more than half of respondents cited cost or insurance coverage as a significant barrier to use (70). Moreover, disparities in prescribing patterns and implicit bias have been described (71,72,73). Modifiablereasons for avoiding use include the hassle of devices (47%) and aversion to having a device attached to the body (35%). Skin reactions and/or difficulty with adhesion are well known and are an important cause of discontinuation (74). Methods of addressing this barrier such as use of barriers, overlay patches, or topical antihistamines and corticosteroids have been described but additional research is needed (75).
In a multi-national study of 263 patients, persistent sensor use for 12 months was only 30% (76). Improvement in A1C was associated with higher A1C at baseline, older age, and more frequent sensor use. Diabetes related hospital admissions were reduced following the initiation of sensor augmented pump therapy and fear of hypoglycemia improved. In the 6-month follow- up phase of the JDRF-CGM trial, RT-CGM was initiated in the control group in a manner that more closely approximates clinical practice (77). Investigators found a significant reduction in CGM use inall age groups over time. However, increasing sensor use was associated with A1C reduction. It is likely that adherence will improve as technologies improve.
Other limitations include possible interference with acetaminophen, ascorbate, and other active agents in glucose-oxidase based electrochemical sensors. They are also dependent on both the sensitivity and specificity on the enzyme availability on the electrode surface. There are well known delay artifacts due to the time lag between glucose concentration in the interstitial fluid and blood glucose. These time ranges, often between 5 and 10 minutes, are not crucial to analyzing retrospective data, but can be critical when CGM is indicated for real-time decision making (78).
Daily Use
Patients must be aware that sensor readings can deviate from actual blood glucose measurements, particularly during rapid glucose changes such as that which occurs post-meal or during exercise. Calibration, where necessary, should not be performed when trend arrows indicate rapid swings in glucose. While systems are becoming more reliable, patients may need to verify sensor readings before taking action such as meal boluses or treatment of hypoglycemia depending on the device, even if a device is approved for nonadjunctive use.
Alarm thresholds should be set in order to maximize patient compliance, keeping in mind that the sensitivity fordetecting hypoglycemia decreases as the threshold is reduced below 70 mg/dl. Conversely, specificity improves to amuch smaller degree at lower thresholds, and thus false alarms may not be reduced substantially.
Several algorithms have been published that provide specific guidance to patients for responding to trend arrows and alarms and are summarized below. All algorithms are complex and are not integrated within bolus calculators ofexisting insulin pumps. Therefore, they should only be implemented in patients who have demonstrated anunderstanding of CGM technology, including lag times between CGM and BGM, calibration procedures, alerts andtrend arrows, as well as understanding of insulin action time and the risks of insulin stacking. In one small study, trend arrows were accurate approximately 79% of the time outside of mealtime windows (30 minutes before and 120 min after carbohydrate intake) but this dropped to ~60% within mealtime windows (80). Thus, algorithms are not intended for use post-meal. The use of automated insulin delivery systems should increase safety and efficacy and reduce the complexity of the trend arrow approach.
- The algorithm by Jenkins et al. provides tiered recommendations that are based upon the meter glucose and sensor trend arrows (81). In addition, the algorithm advises patients how to review downloads of the data periodically (weekly) and make adjustments. Patients who were randomly assigned to sensor augmented pump with the algorithm had lower A1C and reported better quality of life at 16 weeks compared to patients who did not get the The effect on quality of life persisted at the 32-week follow-up, and was associated with A1Creduction. Importantly, patients who received the algorithm at 16 weeks after initiating sensor augmented pump did not benefit.
- The DirecNet study algorithm (for use with the Navigator system) recommended that patients increase or decreasethe meal + correction bolus by 10-20% based upon the rate of change and provided specific instructions for responding to alarms (82). Algorithm use was high in the first 3 weeks but dropped off by week 13, despite increasing insulin self- adjustments, possibly as patients became more independent over time.
- Subsequent methods recommended adjustment of only the correction insulin dose by the amount needed to cover a glucose level that is incrementally higher or lower than the current glucose, based upon the trend arrow (83,84).
- Klonoff and Kerr proposed a more straightforward correction dose (in 5-unit increments), based upon the trend arrow and the patient’s insulin sensitivity (85).
- A consensus statement facilitated by the Endocrine Society provides expert guidance on the use of trend arrows for making treatment decisions (86). The guidance recommends adjustment of boluses pre-meal and no sooner than 4 hours post-meal in 0.5-unit increments based upon the trend arrow and the patient’s sensitivity. The statement recommends no additional treatment within 2 hours of a previous meal bolus, and correction bolus using the bolus calculator or usual correction dose only in the 4 hours after a meal. Similar expert guidance has been developed for the Freestyle Libre system (87).
- A more recent adaptation of the Endocrine Society guidance incorporated pre-meal glucose levels in addition to theinsulin sensitivity (88) and a small randomized study demonstrated it was more effective than the incorporation of insulin sensitivity alone, particularly among insulin pump patients (89).
Overview of Stand-alone Personal RT-CGM and IS-CGM Systems
The first RT-CGM (Guardian, MedtronicR) was approved in 2004. Since then, additional models and other devices have entered the market, and accuracy and patient satisfaction have improved. Several personal continuous glucosemonitors have been approved by the US Food and Drug Administration (FDA) for use in the United States or carry CE marking for use in Europe and are currently on the market (Table 5). For a full review of regulatory requirements for glucose monitoring devices, the reader is referred to one of several excellent reviews (90).
|
Table 5. Comparison of Subcutaneous Continuous Glucose Monitoring Devices |
|||||||
|
|
Calibration required |
Confirmatory Fingersticks requiredprior to treatment |
Real-time alerts |
Sensor Life (days) |
Warm-up (hrs) |
Removefor MRI, CT diathermy |
Acetaminophen interference |
|
Dexcom G5 |
Y |
N |
Y |
7 |
2 |
Y |
Y |
|
Dexcom G6 |
N |
N |
Y |
10 |
2 |
Y |
N |
|
Dexcom G7 |
N |
N |
Y |
10 |
0.5 |
Y |
N |
|
Medtronic Guardian 3 |
Y |
Y |
Y |
7 |
2 |
Y |
Y |
|
FreeStyle Libre 14 day |
N |
N |
N |
14 |
1 |
Y |
N |
|
FreeStyle Libre 2 |
N |
N |
Y |
14 |
1 |
Y |
N |
|
FreeStyle Libre 3 |
N |
N |
Y |
14 |
1 |
Y |
N |
|
Eversense (surgical implant) |
Y |
N |
Y |
180 |
NA |
Y |
No |
GUARDIAN CONNECT
The Guardian Connect utilizes the Medtronic Guardian Sensor 3, the Guardian Connect transmitter, and the GuardianConnect app to transmit data via Bluetooth every 5 minutes to the user’s smart phone or device (initially only available on iOS devices) via the Guardian Connect App on smartphones and via CareLink personal and professional software. A separate receiver is not available with this system. Data can be shared with others remotely, and SMS messages can be sent in times of hypoglycemia. The system is only approved for adjunctive use and at least 2 daily fingerstick calibrations are required.
DEXCOM G6
The Dexcom CGM utilizes a glucose oxidase sensor at the tip of a wire that is implanted in the subcutaneous space. The data are transmitted wirelessly and are displayed on a separate receiver (personal smartphone or device specificreceiver). The Dexcom G6 has a sensor life of 10 days, no longer requires calibrations, and minimizes interference by acetaminophen. G6 is also associated with a smartphone app that allows the patient to log activity, set reminders or alarms, and physically see their glucose levels and trends throughout their time wearing the device. The Dexcom CLARITY Diabetes Management Software organizes and presents the patient’s blood glucose data. Dexcom SHARE app allows users to share data with up to 10 other individuals.
DEXCOM G7
The Dexcom G7 features a 60% smaller size (the size of 3 stacked quarters) vs. the G6, is fully disposable, and has a shorter 30-minute warm up time and a 12-hour grace period to replace completed sensors. The overall MARD was reported to be 8.2% with the abdomen and 9.1% on the arm (17). Consistent with previous studies, accuracy is lower at lower sensor glucose, higher glucose rate of change, and on day one of wear.
FREESTYLE LIBRE 14 Day
The sensor utilizes Wired Enzyme™ technology in which the enzyme and mediator are co- immobilized on the sensor. It offers factory calibration, and therefore nearly eliminates the need for fingerstick monitoring. However, patients are still advised to perform SMBG whenever an alert appears on the reader display (which occurs when the glucose isrising or falling rapidly) or whenever the glucose value does not fit the patient’s symptoms. The reader contains a built-in meter for this purpose. The sensor is FDA approved for 14 days of use. The system is not approved for use in children under age 18, or during pregnancy or in persons requiring hemodialysis. The Libre has minimized the interference by acetaminophen which is present in other devices but interference from other substances such as ascorbic acid or aspirin may be possible. The Libre 14 day differs from other CGM devices in that the system does not alert the user for glucose values surpassing a high or low threshold. In addition, glucose values are not automatically made available to the user but are easily and instantly accessed by scanning the sensor with a handheld reader or theassociated app FreeStyle LibreLink. However, this product may be attractive option for patients who are averse to the hassle imposed by other RT-CGM devices. Glucoses are measured every minute and recorded every 15 minutes. Data can be accessed using the reader or downloaded to LibreView cloud based online management system, or using the FreeStyle Libre desktop software. The MARD is reported by the manufacturer to be 9.7% overall, and as with other CGM devices, less accurate on day 1 of wear and in hypoglycemia range (91).
FREESTYLE LIBRE 2
The FreeStyle Libre 2 system offers real-time alerts for high or low glucose values and improved accuracy, approved for ages 4 years and older (92). However, users must continue to scan the device to obtain glucose readings.Moreover, similar to the Libre 14 day, the sensor memory is only 8 hours and glucose data are lost if the sensor is scanned less frequently.
FREESTYLE LIBRE 3
The FreeStyle Libre 3 is even smaller than other devices (the size of 2 stacked pennies), does not require scanningunlike older models, but does require the use of a compatible smartphone. The bluetooth range is improved from 20 to 33 feet. Accuracy is improved compared to the Libre 2, with an overall MARD of 9.2% in adults and 9.7% in children (16).
EVERSENSE
The Eversense system (Senseonics) is a 90-day implantable sensor that uses fluorescent technology to sendmeasures via a rechargeable transmitter which rests just above the skin to a smartphone app titled Eversense NOW (93). In a pivotal clinical study of 71 patients with type 1 and type 2 diabetes, there were no device-related serious adverse events, and the MARD was 11.1%, with over 99% of samples in clinically acceptable error zones A and B of Clarke Error Grid Analysis (94). One study reported interference with tetracycline and mannitol, but not with acetaminophen or ascorbic acid (95). The Eversense XL CGM system consists of a 180-day implantable sensor thathas been shown to have acceptable safety and accuracy with an overall MARD of 9.1% (96). This option may be particularly useful for patients with privacy concerns, physical disability, needle phobia, allergies or other difficulty with adhesion, or activities or professions that may be barriers to external wear (97).
Sensor Augmented Pumps
To date the largest A1C reductions have been observed when sensors are initiated with insulin pump technology. In the observational (nonrandomized) COMISAIR study, patients initiating CGM (with or without insulin pump) achieved significantly larger reductions in A1C (-1.2%) compared to subjects initiating insulin pump alone (-0.6%) or remaining on injections alone (- 0.3%) (98). There was no difference in outcomes between the DexCom G4 and Enlite sensor.
A reduction in time spent in hypoglycemia was observed only in patients using CGM (8% vs 6%, p<0.001).
STEPS TOWARDS AN ARTIFICIAL PANCREAS
Until recently, RT-CGM technology has operated completely independently of insulin delivery. By combining continuous basal insulin delivery during fasting periods with discrete bolus doses of insulin at mealtimes, insulindelivery can be crafted to mimic the natural pattern of pancreatic insulin release. An artificial pancreas consists of: 1) an automatic and continuous glucose monitor; 2) an implanted continuous insulin delivery system; 3) a control processor to link the insulin delivery rate to the glucose level; and 4) a signal to send the glucose level to the body surface for continuous display onto a monitor. Limitations to full implementation include sensor accuracy and lag time, inadequate onset and offset of currently available rapid acting insulin analogs, meal challenges, and changes in insulinsensitivity due to circadian rhythms, exercise, menstrual cycles, and intercurrent illness (99). However, even incremental advances improve glucose control without increasing the complexity of decision-making on the part of the patient. These include:
- Low glucose (threshold) suspend: the insulin pump suspends when the glucose decreases below a pre-set value.
- Suspend before low: insulin pump suspends when hypoglycemia is
- Hybrid closed loop: insulin delivery increases or decreases based upon the sensor glucose value but meal boluses are still required.
- Closed loop control: fully closed loop delivery without the need for meal boluses
- Dual hormone systems: these are hybrid closed loop or closed loop control systems that utilize glucagon or other peptides (such as amylin) in an effort to more closely mimic the physiology of the endocrine pancreas.
The long-term safety, efficacy, cost, and cost-effectiveness of an artificial pancreas are still largely unknown at this time. However, the urgency of this technology is demonstrated by the #WeAreNotWaiting movement, which has given rise to home-grown, crowd-sourced, patient driven systems that utilize existing devices which are linked by open-source software, such as Open Artificial Pancreas System, and Loop. Recently Tidepool Loop received FDA approval (100). A retrospective observational study of patients with Type 1 diabetes demonstrated lower mean glucose, higher time in target range, and less time in hypoglycemia using Open Artificial Pancreas Systems (OpenAPS) compared to sensor augmented pump use alone (101). In general, open-source systems carry safety concerns, particularly among less tech-savvy patients, in the absence of regulatory approval (102). However, the healthcare provider can providesafety recommendations as well as a back-up plan in case of system failure (103). The reader is referred to one of several reviews as a detailed review is beyond the scope of this chapter (104,105).
Threshold Suspend
Progress is expected toward a fully functional closed loop system in incremental steps. The first step toward a fully automated “artificial pancreas” is the low glucose suspend feature, which is now available. The Medtronic 530Gsystem, containing the Veo insulin pump and Enlite sensor, is the first sensor augmented pump with low threshold suspend and uses the same sensor as the more recent 630G system. The Enlite sensor accuracy is significantly improved over the previous Sof-sensorR, with a MARD of 13.6% when used with the 530G (106). The Enlite is also one-third of the size of Sof-sensor and the filament is 38% shorter. The Enlite sensor may be worn up to six days. The low threshold suspend SmartGardTM technology suspends the pump for up to two hours in the event of sensor detected hypoglycemia in which the user does not respond to the alarm. Prior to suspension, a “siren” sounds which is distinct from other high or low alerts, and the suspension can be overridden at any time. The MiniMed Connect mobile accessory sends sensor data to an app on a mobile device where data can be viewed (available only with the 530G system). A study that enrolled 247 patients with type 1 diabetes and documented nocturnal hypoglycemia to sensor-augmented pump with or without a low-glucose threshold-suspend feature demonstrated similar A1C between groups at 3 months but lower frequency of nocturnal hypoglycemia (107). Similar findings were demonstrated in an Australian study of 95 patients, in which the incidence rate ratio for hypoglycemia was 3.6 (95% CI 1.7-7.5, p<0.001) (108). There were no reports of DKA in either study.
Suspend Before Low
The next incremental step in closed loop systems is the suspend before low feature, currently available in theMedtronic 640G (approved only in Europe) and the 670G systems. This feature automatically suspends insulin delivery 30 minutes before a low glucose threshold is predicted and resumes delivery once the glucose recovers, without alerting the patient. In a 6-month randomized study of 154 children and adolescents with type 1 diabetes, the 640G system reduced the time spent in hypoglycemia from 2.6 to 1.5% without causing a change in A1C (109). The t:slim X2 Insulin Pump incorporates Basal IQ technology with predictive low glucose suspend using the Dexcom G5 or G6 sensor. In a randomized cross-over study of 103 participants with type 1 diabetes age 6-72 years of age, predictivelow glucose suspend resulted in a 31% reduction in time spent in hypoglycemia < 70 mg/dl without a change in meanglucose or time in hyperglycemia (110).
Hybrid Closed Loop (HCL)
This step refers to sensor glucose driven automatic adjustment of basal insulin with or without additional auto boluses,and still requires the patient to bolus for meals. In a recent consensus statement, an ideal candidate for automated insulin delivery systems (103):
- Is technically capable of managing a pump, has basic carbohydrate counting skills, and is able to implement a back-up plan (including the use of manual injections).
- Has realistic expectations of system In particular, several situations that are unique to HCL are worth emphasizing:
- Bolusing: pre-blousing approximately 15 minutes prior to meals is critical to maintain In many systems,delayed boluses not only cause early postmeal hyperglycemia but also precipitate delayed hypoglycemia as the system has already begun to augment insulin delivery in response to hyperglycemia.
- Exercise management: Similarly, carbohydrate loading prior to exercise while using HCL systems will only stimulate insulin delivery and thus is recommended that users implement other means for management such as setting a higher target, typically with a designated exercise mode, or exiting to manual mode with temporarybasal insulin reduction or temporary suspension of the pump.
- Hypoglycemia management: HCL users typically need fewer carbohydrates (about half) to manage hypoglycemia since the pump has generally already suspended insulin delivery based upon glucose trends.
- Has adequate support, including diabetes education, insurance coverage, and caregiver or other social support where relevant.
- Has the ability to transmit data to the healthcare
- Is mentally and psychologically able to implement AID
On the other hand, there does not appear to be an ideal threshold A1c for determining candidates for HCL therapy, asthose with lower A1c may benefit by reducing TBR and those with higher A1c benefit from reductions in hyperglycemia without the perceived risk of ketoacidosis traditionally attributed to initiation of insulin pump therapy (111). Thus, less ideal candidates may obtain the greatest benefit in terms of achieving glycemic targets.
HCL demonstrates improvements in a range of glycemic outcomes and may confer psychological benefits as well.Most studies have enrolled patients with type 1 diabetes. More data are needed for special populations including type 2 diabetes, especially those with very high insulin requirements, pregnancy, acute illness, steroid use, renaldysfunction, and persons in assisted living facilities (112).
As with CGM, it is important to evaluate these systems in the real-world setting, where user experience can differ from that of the highly controlled and supportive research environment. Cost and insurance hassles, as well as user wear issues are the most commonly reported barriers to use of any diabetes related device. These barriers contribute todiabetes distress and depressive symptoms which can impede self-management behaviors (70,112). HCL systems improve glucose control but may also introduce additional alarms or alerts which are needed for safety (such as threshold alerts for hyperglycemia or hypoglycemia or HCL mode exits due to insulin delivery exceeding the system’s guardrail) or ongoing functionality such as calibration of the CGM (113). Newer systems have attempted to address many of these barriers through improved algorithms or other features (Table 6). Devices differ considerably with respect to algorithms used for insulin adjustment and a number of other features (Table 6) (114,115,116). There are few head-to-head studies comparing the efficacy and safety of available HCL systems. Details of select systems are presented next.
MEDTRONIC 670G
The first system to gain FDA approval is the Medtronic 670G, which adjusts basal insulin delivery every 5 minuteswhen in auto mode. This system utilizes the Guardian sensor 3, which offers enhanced sensor accuracy, with an overall MARD of 9.64% (117). The system was associated with a reduction in A1C from 7.4 to 6.9% and there were trends in improvement of time in target range and hypoglycemia in a non-randomized study of 124 patients with type 1 diabetes (118). A subsequent randomized trial of 151 adults and children demonstrated a significant reduction in A1c and TBR compared to insulin pump without CGM (119). There are few studies addressing long-term use however. In a 1-year prospective observational study of 84 patients, 28% stopped using auto mode by 3 months, and 33% discontinued by 12 months (120). The most common reasons for discontinuation included sensor issues (62%) and difficulty obtaining supplies (12%), fear of hypoglycemia (12%), and preference for injections (8%) or sports (8%). In astudy of 92 youth, 30% discontinued HCL, typically between 3 and 6 months after initiation, due to issues such asdifficulty with calibrations, alarms, and extra time needed for operation (121).
MEDTRONIC ADVANCED HYBRID CLOSED LOOP (AHCL) SYSTEM (780G)
This HCL system is approved for use in Europe and features substantially reduce frequency of alerts, improved time in auto mode, remote software updating, an adjustable target setting as low as 100 mg/dl and enable users to view data via an app on mobile devices (122). In a single arm study of 157 adolescents and adults the 780G system resulted in nearly 95% time in automated mode with 1.2 exits per week, improved A1C, TIR, and TBR (123). In a randomized study of 82 persons with type 1 diabetes using multiple injections per day and isCGM, AHCL resulted in improvements in A1C (-1·42%, 95% CI -1·74 to -1·10; p<0·0001) and TIR, but no difference in hypoglycemia (124). There was an improvement in treatment satisfaction, fear of hypoglycemia, and similar diabetes quality of life. By comparison in a randomized study of 41 participants with type 1 diabetes who were naïve to both CGM or insulin pump technologies, AHCL also resulted in improvements in TBR (125). In a real-world study of 3211 youth (<age 15 years) and 8874 individuals >age 15 years, ACHL demonstrated >90% treatment persistence over 6 months (126). The ACHL system was reported to have better glucose monitoring treatment satisfaction but similar diabetes distress, technology attitudes, and fear of hypoglycemia compared to the 670G system (127).
TANDOM CONTROL-IQ
This system utilizes a t:slim X2 insulin pump with a calibration-free Dexcom G6 sensor (128). In a 6-month trial of 168 patients (age 14-71) randomized 2:1 to hybrid closed loop vs. sensor augmented pump alone, the % time in target 70-180 mg/dl was increased by 11% more in the hybrid closed loop group compared to sensor augmented pump(p<0.0001), with improvements in hypoglycemia, mean glucose and A1c. Moreover, real-world outcomes among 1435 persons with type 1 diabetes included reduced impact of diabetes on life, improved device related treatment satisfaction, and improved emotional well-being (129).
OMNIPOD 5
The Omnipod 5 is a HCL system that uses the Omnipod DASH platform (130). In a single arm study, the Omnipod 5 demonstrated a reduction in A1C of 0.38%, increase in TIR of 9.3% and decrease in TBR of 1.6% (131).
|
Table 6. Comparison of Hybrid Closed Loop Systems |
|||||
|
|
Medtronic 670G/770G |
Medtronic780G |
Tandem T:Slim with Control IQ |
Omnipod 5 |
iLet BionicPancreas |
|
Insulin delivery |
Tubing |
Tubing |
Tubing |
Tubeless (pod) |
Tubing |
|
CGM |
Guardian 3 |
Guardian 4 |
Dexcom G6 |
Dexcom G6 |
Dexcom G6 |
|
Reservoir capacity(unit) |
300 |
300 |
300 |
200 |
180 |
|
Calibration needed |
Yes |
No |
No |
No |
No |
|
Supplies |
DME company |
DME company |
DME company |
Pharmacy |
To be determined |
|
Control via smartphone |
Dataviewable from Smartphone |
Data viewablefrom Smartphone |
Smartphone bolus |
Compatible smartphone controller |
No |
|
Algorithm initiation |
48 hours in manualmode to estimate TDI |
48 hours in manual mode to estimate TDI |
Weight and TDI entry with maximumdelivery of 50% TDI over 2 hr |
TDI estimated from programmed basal rates |
Weight entry |
|
Bolus automation |
No |
Yes, every 5 minutes |
up to 1 auto-correction bolus//hr if glucose predicted >180 mg/dl |
No |
unknown |
|
Other inputs |
CIR, AIT Unable to overridebolus dose |
CIR, AIT |
CIR, ISF, AIT is fixedat 5 hours |
CIR, ISF, AIT Boluscalculator uses CGM rate of change |
“Usual for Me”, “More”, or“Less” customized by meal |
|
Extended bolus |
No |
No |
Yes, up to 2 hr |
No |
No |
|
Algorithm adjustment |
Every 6 days |
Every 6days |
TDI used to scalebasal changes |
Every 3 days with pod change |
Continuously or based on change in entered weight |
|
Target |
120 mg/dl |
100, 110, 120 mg/dl |
112.5-160 mg/dl |
110, 120, 130, 140, 150 mg/dl, customizableby time of day |
100, 110, 120, 130 mg/dl, customizable by time of day |
|
Exercise |
Temp target 150 mg/dl for 2-12 hour |
Temp target 150 mg/dlfor 2-12 hour |
Exercise target 140-160 mg/dl—cannotprogram duration Sleep mode: target 112.5-120 mg/dlwithout auto-correction bolus, programmable |
Target 150 mg/dl, “less aggressive”,for 1-24 hr |
None |
|
Safety mode* |
Yes, for 670/770G results in forced exits from HCL |
Yes, exits to Safe Basal up to 4hours (5.7 vs. 1.7 x/week with 670G) (130). |
Not applicable(defaults to basal rate settings) |
Yes, forced exits “rare” |
Yes, BG-run mode uses manuallyentered BG upto 72 hours, forces switch to alternate therapy. |
PID=proportional integral derivative (system with continual change in response to error between actual and targetvalues). MPC=model predictive control (dynamic reference model serves as a basis. TDI=total daily insulin dose,CIR=carbohydrate to insulin ratio, AIT=active insulin time, ISF=insulin sensitivity factor. *Safety mode provides amechanism to ensure insulin delivery in case of loss of sensor input or threshold for insulin delivery guardrail is reached.
Closed Loop Systems (CLC)
Additional steps toward closed loop control (CLC) insulin delivery require algorithmic insulin adjustments, whicharguably present additional safety concerns. Overnight CLC insulin delivery is relatively straightforward, whereas post-meal control and exercise effects remain the most challenging of events to manage. Until recently, most randomized studies have been small and reported only short-term outcomes, often in controlled settings.
ILET BIONIC PANCREAS
The iLet Bionic pancreas was approved by the FDA in 2023. This insulin pump is initiated using the patient’s body weight and requires meal announcements (designated as small, medium, or large) but not formal carb counting and thus represents an incremental step toward a fully closed loop insulin pump. In A 13-week multi-center randomized study of 219 participants with type 1 diabetes demonstrated a greater reduction in A1c with the iLet bionic pancreas (-0.5%, [95% CI -0.6 to -0.3; P<0.001) but no difference in hypoglycemia compared to standard care (132).
OTHER SYSTEMS
Systems have utilized single hormone (rapid acting insulin only) or dual hormone (both fast- acting insulin analog and glucagon to imitate normal physiology) as directed by a computer algorithm (Figure 4) (133). At this time, there areinsufficient data demonstrating the superiority of one system or algorithm compared to others. The three most common algorithms are:
- Model Predictive Control (MPC): predicts future glucose levels and adjusts insulin delivery in response.
- Proportional Integral Derivative (PID): calculates the deviation of glucose from target to determine insulin delivery.
- Fuzzy Logic (FL): mimics insulin dosing based upon clinical
A meta-analysis of 40 randomized studies (35 studies using insulin alone and 9 dual hormone studies) including 1027participants with type 1 diabetes demonstrated a significant increase in % time in target range (70-180 mg/dl, weighted mean difference 9.6%, 95% CI 7.5-12%), as well as less time in hypoglycemia or hyperglycemia, regardless of type of system (134). In another meta-analysis of 24 studies and 585 participants (7 studies using dual-hormone therapy and 20 studies of insulin only) reported greater improvement in time in target with artificial pancreas systems (12.6%, 95% CI 9.0-16.2, p<0.0001), and greater improvement with dual hormone compared to single hormone systems (135). Another meta-analysis of studies with at least 8 weeks duration confirmed these findings (136). A systematic review and meta-analysis of 25 studies in 504 children demonstrated superior %TIR with CLC and bi-hormonal systems vs. single hormone systems (137).
Figure 4. Dual hormone Closed Loop Control system.
MINIMALLY INVASIVE AND NON-INVASIVE GLUCOSE MONITORS
Continuous hypoglycemia detection systems using current sensing technology must be either implanted (fully or partially, either subcutaneously or into a blood vessel). Implantation is more secure, but may be associated with biocompatibility problems or local irritation. Less invasive methods may be categorized as minimally invasive or noninvasive. Minimally invasive techniques extract fluid (tears or interstitial fluid) while noninvasive technologies do not.
Minimally invasive methods include electrical, nanotechnology, and optical approaches while noninvasive techniquesrely on some form of radiation without the need to access bodily fluids. Noninvasive methods frequently incorporate electric, thermal, optical, or nanotechnology methods for detection. Many noninvasive devices under development are aimed for non- continuous monitoring as they often require controlled surroundings including factors such as light, motion and temperature.
- Optical approaches utilize reflective, absorptive, or refractive properties of infrared and optical bands of the light spectrum to detect glucose. Pure optical methods under development utilize Raman and Near infra-red spectroscopy.
- Thermal methods detect glucose via the far-infrared band of the spectrum and provide noninvasive approaches for glucose monitoring.
- Electric methods use electromagnetic radiation, currents, or ultrasound approaches to detect dielectric properties of Reverse iontophoresis has been employed with early minimally invasive approaches while bioimpedance spectroscopy has been used in recent noninvasive approaches.
- Nanotechnologies aim to miniaturize existing technologies, including fluorescence and surface plasmon resonance (SPR) approaches (138).
Few devices (other than interstitial CGMs discussed above) have demonstrated high levels of accuracy recommendedby expert groups, though several have been approved by CE or FDA (139).
MOBILE TECHNOLOGY AND DECISION SUPPORT
It has become increasingly clear that the isolated use of glucose monitoring technologies without a specific plan toaddress the data provides minimal benefit, particularly among patients with type 2 diabetes or who are not using insulin (140). In order for glucose monitoring to provide the most benefit, patients and providers must be able to easilyobtain and communicate the data. Data must be organized in such a way that patterns can be identified, and patients must receive feedback at the point of care. The widespread use of mobile devices provides opportunities for data collection, analysis, and communication of results with health care providers as well as facilitates digital or remote clinical models of care (141). Finally, as healthcare providers are inundated with more data and spend increasingamounts of time using electronic medical records, it has also become paramount that devices and or reports from the devices communicate or interface with these systems (142).
Hurdles to wider implementation of mobile technology include the lack of usability (both for patients, as well as providers who may be expected to review and act upon reports), safety, efficacy (including long-term adherence), and cost-effectiveness studies (143). The lack of data is in part due to the rapidly changing technology itself, which rendersthe technology obsolete by the time a vigorous clinical trial is conducted and published. The fee for service model is amajor barrier to adapting many glucose monitoring technologies, which often require frequent feedback and treatment adjustments, efforts that are not reimbursed without an actual office visit. Finally, cyber security is a big concern for all medical devices, especially for devices that are controlled by a smartphone (144).
Device Downloading, Connectivity, and Interoperability
Manual recording of glucose data is fraught with inaccuracies (145). Most monitors can be downloaded, via a tethering cable or wireless connection, either by the patient or healthcare provider. Each glucose monitoring devicegenerally works with its own proprietary management software. However, several programs (Tidepool,Glooko/Diasend, Carelink by Medtronic, Accu- chek) are capable of downloading and organizing data for multiple different devices (146).
Reports are standardized across all device downloads, facilitating efficient and actionable patient and healthcare provider review. These programs also facilitate population health and telehealth strategies (discussed below). The Nightscout Project is a crowd sourced application that provides a free mobile technology platform for patients whowant to access their devices in real time on any mobile device (147). Recent data suggest that retrospective weekly review of data is associated with improved TIR (148,149) as well as patient reported outcomes including confidence in avoiding hypoglycemia, overall well-being and diabetes distress (150).
Direct connectivity of blood glucose or CGM levels to cell phones or other devices not only improves data integrity but may also simplify the assimilation of glucose levels with other data such as insulin use, carbohydrate intake, andactivity levels for the purpose of facilitating insulin dose adjustments in real time or retrospectively. Cell phone connectivity may also improve communication with providers. A few meters with direct cellular capability are available.
Devices with direct cellular or Bluetooth connections may be paired with apps that facilitate collection, communication, and analysis of a variety of data and provide tools for education (such as nutrition information) at the point of care.
Currently, both the Tandem t:slim X2 and Insulet’s Omnipod 5 System are FDA approved for remote blousing via a cell phone app (151,152). A regulatory pathway has been developed for alternate controller enabled (ACE) infusion pumps which can be operated in conjunction with interchangeable components, particularly CGMs (153). In 2019, theFDA approved the first such devices (Tandem t:Slim X2 and Omnipod DASH system).
Diabetes Apps
A variety of stand-alone smart phone applications that support glucose monitoring are also available. Most provide information and track data (usually manually entered), some allow insulin or carbohydrate documentation, facilitate carbohydrate or calorie counting, promote weight loss, track or promote physical activity, enhance medication adherence, and use motivational or self-efficacy approaches, and a few provide an insulin dosing calculator. Simple apps provide information or tracking functions while more sophisticated approaches incorporate gaming theory and machine learning approaches that learn from the user’s previous experiences to optimize interactions. Apps have shown limited magnitude and sustainability of effect due to a variety of factors, including user fatigue, require continuous data entry (e.g., most apps do not connect directly with a glucose meter), and lack of integration with the health care team. Moreover, most apps have not been evaluated by the FDA or other regulatory agencies. Data privacy is also a concern, as no federal regulations currently prevent app developers from disclosing data to third parties. Most apps (81% in one survey of Android apps) do not have privacy policies, and of those that do, 49% share user data with third parties (154). Expert groups have developed policy or guidance statements to improvestandardization and functionality (155,156,157).
Efficacy
While the data are still evolving with respect to mobile diabetes applications, several systematic reviews and meta-analyses demonstrate modest (~0.5%) reductions in A1C in persons with type 2 diabetes, especially among younger patients, apps that provide healthcare provider feedback, or had other features including wireless entry of data (158,159,160,161). The Agency for Healthcare Research and Quality published a systematic review of comparative effectiveness studies assessing apps or programs available through a mobile device for the purpose of diabetes self-management (162). For type 1 diabetes, 6 apps were identified, 3 of which were associated with improvement in A1c, 2 of which were associated with improvement in hypoglycemia. Five apps for patients with type 2 diabetes were identified, 3 of which were associated with improvement in A1c. Efficacy is variable, in part because app features vary but also because apps are often studied as part of a multi-component intervention, making it difficult to assess individual elements, particularly the effect of additional health care provider support. Other researchers have focusedon identifying standard evidence-based features that should be included in diabetes apps, such as education, glucose monitoring, and reminders (163,164).
Usability
In a systematic review of 20 studies, only one third of the 20 apps met the authors’ health literacy standards (165).Usability was measured in 7 studies through satisfaction surveys from patients and experts, and ranged from 38-80%. The most common usability problems were multi-step tasks, limited functionality, and poor system navigation. While many apps are rated high quality for performing a single task, most do not address diabetes self-management tasks comprehensively (166) or otherwise do not function properly (165,167).
Decision Support
The use of pattern management software improves health care provider efficiency and accuracy in identifying needed therapeutic adjustments (168,169). Software programs provide graphs or charts and may in some cases provide dosing advice, either for the healthcare provider or directly to the patient.
Insulin Dosing Calculators
Insulin dosing calculators have been used for years as a means of incorporating glucose measures into routine practice, largely in concert with continuous insulin infusion pumps. While numerous apps have become available forbolus insulin calculation and basal insulin titration, it is important to note that only a few have been formally evaluated and approved by regulatory agencies. In addition, many still require manual data entry, few integrate within existing electronic medical records, and published evidence for efficacy is limited (170). All approved insulin calculators or dose titration apps require a prescription or need to be set up by a healthcare provider. Many such apps operate in conjunction with connected meters and insulin pens, which are subject to regulatory oversight and long-term support (171). Such support ensures safety and that software is updated to address any problems with operation and device compatibility. The functionality of connected pens ranges from insulin tracking functions, including insulin on board calculations and reminders to smart insulin pens which feature bolus dose calculators and more advanced decisionsupport such as dose titration and coaching features (172). A full review of insulin dosing apps is beyond the scope of this chapter.
Bolus calculators are known to substantially improve dosing accuracy and glycemic control in outpatients with type 1 diabetes (173,174,175). Bolus calculators might be particularly helpful for patients with poor numeracy. A number of stand-alone smart-phone apps for bolus insulin calculation have been developed but safety and efficacy remain a concern (176,177). Though algorithms typically incorporate the current glucose level, active insulin time, and carbohydrate intake, some do not account for activity or illness. Applications that improve the accuracy of carbohydrate counting, which is a major source of error (regardless of educational level), are desirable (178). Reports from connected pens provide insight into missed or altered insulin doses and when integrated with CGM data can alsofacilitate the evaluation of timing of boluses.
Likewise, basal insulin calculators have been developed to recommend ongoing adjustments in therapy, either fortitration or for mealtime insulin calculations. Unfortunately, efficacy and safety studies are not currently available for most apps. Most basal insulin titration apps account only for fasting glucose measures and not overnight trends.
Although there are a plethora of apps available, the ultimate choice should be individualized to the needs of thepatient. Those patients only needing a resource that assists with carbohydrate counting can be referred to common apps like MyFitnessPal or Calorie King. For glucose monitoring, apps that require manual entry of data should beminimized as they are not likely to be utilized long-term. Universal platforms that can download multiple devices can increase clinic efficiency. Where possible, patients should be invited to directly link with their clinic. This is particularly useful for telehealth visits. Smart insulin pens provide assistance with insulin dosing and can also be downloaded using some universal platforms.
Integration within the Electronic Health Record (EHR)
The major limitation of patient generated data is that it does not integrate within the EHR in a meaningful way. Someopportunities exist with the integration of Apple Health Kit and Samsung S-Health which can transmit data from a variety of apps but this process requires multiple steps and can be cumbersome (179,180). Recently, a consensus report from the Integration of Continuous Glucose Monitoring Data into the Electronic Health Record (iCoDE) project was published, setting standards for integration of CGM data within the EHR (181). Under these standards, data would be accessed by placing an order in the EHR. This would generate a notice to the patient via email or electronic message to obtain consent for sharing data. Once approved, standardized report is uploaded to the EHR. Importantly,none of these mobile health tools replace frequent patient contact and feedback (182).
BIOMARKERS OF GLYCEMIC CONTROL
Hemoglobin A1c (A1C)
A1C is the best biomarker indicator of glycemic control over the past 2-3 months due to strong data predicting complications (1,2). In addition, the American Diabetes Association has recommended its use for the diagnosis of diabetes (1).
Hemoglobin A1c refers to the nonenzymatic addition of glucose to the N-terminal valine of the hemoglobin beta chain. Assays are based upon charge and structural differences between hemoglobin molecules (183,184). Therefore, variants in hemoglobin molecules may lead to analytic interferences. It should be noted that some homozygous hemoglobin variants (HbC or HbD, or sickle cell disease) also alter erythrocyte life span and therefore, even if theassay does not show analytic interference, other methods of monitoring glycemia should be utilized, as A1C will be falsely low. Individual assay interferences are available at the National Glycohemohemoglobin Standardization Program website: www.ngsp.org (185). Several commercial home monitoring kits are also available (186). The two reference methods used to standardize A1c levels are 1) HPLC and electrospray ionization mass spectrometry or 2) a two- dimensional approach using HPLC and capillary electrophoresis with UV-detection (187). A brief summary of assay methods is described below.
- HPLC methods utilize the fact that glycated hemoglobin has a lower isoelectric point and migrates faster than otherhemoglobin As such it has variable interference with hemoglobinopathies that alter the charge of the molecule (such as HbF and carbamylated Hb), but these may be revealed through individual inspection of the chromatograms.
- Boronate affinity methods are based upon glucose binding to m-aminophenylboronic acid and measures glycation on the N-terminal valine on the beta chain but also glycation at other sites. There is minimal interference from hemoglobinopathies but this assay is not widely available.
- Immunoassays make use of antibody binding to glucose and N-terminal amino acids on the beta chain and therefore may be affected by hemoglobinopathies with structural changes at these sites, including HbF but notHbE, HbD, or carbamylated Some newer assays have attempted to correct for these interferences.
- Enzymatic methods lyse whole blood, releasing glycated N-terminal valines which are detected using achromogenic reaction and are not affected by hemoglobin
An Organization with links to governmental regulatory agencies, the National Glycohemoglobin Standardization Program (NGSP) (<http://www.ngsp.org/news.asp >), evaluates every laboratory and home test for A1C, sets accuracy standards, and certifies which methods meet their standards (188). The trend in industry is for monitors to become increasingly more accurate and the trend in regulatory organizations is to require increasing accuracy for ongoing certification.
A1C is an analyte found within red blood cells, comprised of glycated Hemoglobin. The glycation gap (formerly known as the glycosylation gap) (GG), based on fructosamine measurement, and the Hemoglobin Glycation Index (HGI), based on mean blood glucose, are two indices of between-individual differences in glycated hemoglobin adjusted forglycemia. GG is the difference between the measured A1C test and the A1C test result predicted from serum fructosamine testing based on a population regression equation of A1C on fructosamine (189). and HGI is the difference between the measured A1C test and A1C results predicted from the mean blood glucose level (calculated from self-monitored blood glucose tests) based on a population regression equation of A1C tests on mean blood glucose levels (190). These two indices are consistent within an individual over time and reflect an inherent tendency for an individual’s proteins to glycate (191,192). Patients with high GG and HGI indices might have falsely high A1C test results and might also be at increased risk of basement membrane glycosylation and development of microvascular complications. Whether between-individual biological variation in Hemoglobin A1c is an independent risk factor, distinct from that attributable to mean blood glucose or fructosamine levels, for diabetic microvascular complications is controversial (193).
Because the A1C test is supposed to reflect the mean level of glycemia, attempts have been made to correlate thiswidely-accepted measure with empirically measured mean blood glucose levels. In 2008, the A1c-Derived Average Glucose (ADAG) study compared A1C and continuous glucose monitoring derived mean glucose and 7-point glucose profiles among 507 patients with type 1 and type 2 diabetes and without diabetes from 10 international centers to derive an estimated average glucose (eAG) from A1C levels using the following equation: eAG(mg/dl) = (28.7* A1C)-46.7 (Table 6).
|
Table 6. A1C and Estimated Average Glucose |
||
|
A1C (%) |
eAG (mg/dl) |
eAG (mmol/l) |
|
5 |
97 |
5.4 |
|
6 |
126 |
7.0 |
|
7 |
154 |
8.6 |
|
8 |
183 |
10.2 |
|
9 |
212 |
11.8 |
|
10 |
240 |
13.4 |
|
11 |
269 |
14.9 |
|
12 |
298 |
16.5 |
Several lines of evidence support this disconnect from a tight correlation between mean glycemia and A1C levels. First, improvements in mean glycemia may not necessarily be reflected by improvements in A1C in intensively treated patients (194). A1C does not reflect short-term changes in glucose control, and therefore can be misleading wherethere have been recent changes in the clinical condition. In addition, glucose fluctuations, compared to chronic sustained hyperglycemia, have been shown to exhibit a more specific triggering effect on oxidative stress and endothelial function (195,196). Glycemic variability cannot be assessed by a global measure of mean glycemia, such as A1C, but requires multiple individual glucose values, such as what can be obtained from continuous glucose monitoring or from seven-point- per-day (or greater) self-glucose testing. Third, A1C does not permit specific adjustments in therapy, particularly among patients requiring insulin titration. Finally, A1C reliability may be affected by several conditions that alter red blood cell lifespan and its use in these circumstances can be misleading. Acomparison of the features and limitations in glucose markers is presented in Table 7 (197,198,199).
|
Table 7. Comparison of Markers of Glycemic Control |
|||
|
|
Biomarker mechanism |
Interval of time reflecting glucose control |
Cautions/Interferences |
|
A1C |
Hemoglobin glycation |
3 months |
Hemoglobinopathy (↑/↓*) Decrease in RBC survival (hemolysis, splenomegaly, pregnancy, drugs) (↓) Increase in RBC survival (Erythropoietin, iron replacement) (↑) Transfusion (↓) |
|
Fructosamine |
Protein glycation |
2 weeks |
Conditions resulting in hypoproteinemia (severe cirrhosis, nephrotic syndrome, enteropathy) (↓) High dose Vitamin C, severe hyperbilirubinemia/uremia/ hypertriglyceridemia (↑) |
|
1,5-AG |
Renal clearance |
1 week |
Chronic kidney disease (stage 4, 5) (↓) Glucosuria (pregnancy, renal tubular disorders SGLT2 inhibitors) (↓) Advanced cirrhosis (↓) High soy diet (↑) |
*Assay-dependent
Ethnic differences in A1C have also been reported (200). For example, recent data from the Type 1 Diabetes Exchange demonstrates a 0.4% higher A1C at a given mean glucose among black patients compared to whitepatients with type 1 diabetes, but no effect of race on glycated albumin or fructosamine (201). However, NHANES data do not demonstrate an effect of ethnicity on the association between A1C and retinopathy (202). Data from the ARIC study demonstrated that A1C, fructosamine, glycated albumin, and 1,5-AG were consistent with residualhyperglycemia among blacks compared to whites, and the prognostic value for incident cardiovascular disease, end stage renal disease and retinopathy were similar by race (203). It should be noted that the range of available A1C was relatively narrow in NHANES and ARIC, and further data across an expansive range is needed. In relation to CGMs, utility of A1C is further enhanced when used as a complement to glycemic data measured by CGM (10). Other biomarkers are becoming more widely used, however, A1C remains the most common biomarker. Other measures of average glycemia such as fructosamine and 1,5-anhydroglucitol are available, but their translation into average glucose levels and prognostic significance are not as clear as for A1C (1).
Fructosamine
A short to medium-term marker (reflecting the average glucose control over the past few weeks) may be useful for determining control over a period of days to weeks since A1C does not reflect recent changes in glucose control. Alternate markers may also be useful in patients with discrepant A1C and self-monitored blood glucose readings as well as patients with other hematologic conditions known to affect A1C. Fructosamine is a term that refers to a family of glycated serum proteins and this family is comprised primarily of albumin and to a lesser extent, globulins, and to an even lesser extent, other circulating serum proteins. No product exists for home use that measures serum fructosamine. A home blood fructosamine monitor, Duet Glucose Control System, was marketed in the early 2000′sand then withdrawn from the market. No subsequent home fructosamine test has been available since then. Randomized controlled trials have reported inconsistent effects of frequent monitoring on A1C lowering, possibly due to differences in execution of therapeutic interventions (204,205). Serial monitoring of short-term markers may also facilitate timely elective surgery in patients whose procedure is delayed due to an elevated A1C. In a recent study, fructosamine was a better predictor of post-operative complications in patients undergoing primary total joint arthroplasty (206).
GLYCATED ALBUMIN
The largest constituent of fructosamine is glycated albumin. Several investigators and companies are developing portable assays for glycated albumin to assess overall control during periods of rapidly changing glucose levels. In these situations, an A1C test may change too slowly to capture a sudden increase or decrease in mean glycemia. The components of the necessary technology appear to be in place to build a commercial instrument for home testing of glycated albumin. However, there is no randomized controlled trial showing that the measurement of glycated albumin improves outcomes. In the ARIC study, fructosamine, glycated albumin, and 1,5-AG were associated with incident diabetes, even after adjustment for baseline A1C and fasting glucose. In the ARIC study, both fructosamine and glycated albumin predicted incident retinopathy and nephropathy, even after adjusting for A1C (207). However, in adults with severe chronic kidney disease, none of the markers, including A1C, fructosamine, or glycated albumin were very highly correlated with fasting glucose, and there did not appear to be an advantage of one marker over another (208). In addition, baseline glycated albumin and fructosamine were associated with cardiovascular outcomes over a 20-year follow-up period after adjusting for other risk factors, but the overall magnitude of associations was similar to A1C (209). In the Diabetes Control and Complications Trial (DCCT), glycated albumin had a similar association with retinopathy and nephropathy as A1C, but the combination of both markers provided even better prediction (210). Short-term markers are also of interest for use in pregnancy, where glucose levels are changing more quickly than can be reflected by A1C. Unfortunately, glycated albumin does not predict gestational diabetes more effectively than A1C or fasting glucose (211). However, other preliminary data suggests that glycated albumin may be a better predictor of pregnancy complications than A1C (212).
1,5-Anhyroglucitol
The aforementioned biomarkers for measuring glycemic control, (A1C, fructosamine, and glycated albumin) only reflect mean levels of glycemia. These measures can fail to portray hyperglycemic excursions if they are balanced by hypoglycemic excursions. Plasma 1,5- anhydroglucitol (1,5-AG) is a naturally occurring dietary monosaccharide, witha structure similar to that of glucose (Figure 5). This analyte has been proposed as a marker for postprandial hyperglycemia (213). An automated laboratory grade assay named Glycomark is approved in the U.S. for measuring 1,5-AG as a short-term marker for glycemic control. A similar laboratory assay has been used in Japan. During normoglycemia, 1,5-AG is maintained at constant steady-state levels because of a large body pool compared with the amount of intake and because this substance is metabolically inert. Normally, 1,5-AG is filtered and completely reabsorbed by the renal tubules. During acute hyperglycemia when the blood glucose levels exceed 180 mg/dl, whichis the renal threshold for spilling glucose into the urine, serum 1,5-AG falls. This fall occurs due to competitive inhibition of renal tubular reabsorption by filtered glucose. The greater the amount of glucose in renal filtrate (due to hyperglycemia), the less 1, 5-AG is reabsorbed by the kidneys. The 1,5-AG levels respond sensitively and rapidly torises in serum glucose and a fall in the serum level of this analyte can indicate transient elevations of serum glucose occurring over as short a period as a few days. Measurement of 1,5-AG can be useful in assessing the prior 1-2 weeks for: 1) the degree of postprandial hyperglycemia; and 2) the mean short-term level of glycemia. This assay might prove useful in assessing the extent of glycemic variability that is present in an individual with a close-to-normal A1C level, but who is suspected to be alternating between frequent periods of hyperglycemia and hypoglycemia. In such a patient, the 1,5-AG level would be low, which would indicate frequent periods of hyperglycemia, whereas in a patient with little glycemic variability, the 1,5-AG levels would not be particularly depressed because of a lack of frequent hyperglycemic periods. In the ARIC study, 1,5-AG was associated with severe hypoglycemia after adjustmentfor other variables, an observation which is consistent with the role of 1,5-AG in reflecting glycemic variability, a known risk factor for hypoglycemia (214).
Longitudinal data from the ARIC study showed that 1,5-AG was associated with ESRD over a 19-year follow-up period, but the relationship was no longer significant after adjusting for glucose control with other markers (215). Among participants with diabetes and A1C <7%, each 5 mcg/mL decrease in 1,5-AG was associated with an increase in dementia risk by 16%, and at A1C >7%, there was also a significant association over a median 21-year follow-up period (216). There was also an association of 1,5-AG and cardiovascular outcomes in ARIC, which persisted, thoughwere attenuated after adjusting for A1C (217). Therefore, it is not yet clear whether 1,5-AG, as a measure of glucoseexcursions, provides incremental value beyond A1C for predicting long-term complications.
Figure 5. Structure of glucose (left) and 1,5-anhydroglucitol (right).
CONCLUSIONS
Many new types of technology are increasingly being developed and applied to fight diabetes and its complications. New technologies will improve the lives of people with diabetes by measuring glucose and other biomarkers of glycemic control and linking glucose levels with insulin delivery to improve the lives of people with diabetes.
REFERENCES
- ElSayed NA, Aleppo G, Aroda VR, et , American Diabetes Association. 7. Diabetes technology: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1): S111–S127.
- Grunberger G, Sherr J, Allende M, Blevins T, Bode B, Handelsman Y, Hellman R, Lajara R, Roberts VL, Rodbard D, Stec C, Unger J. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus. Endocr Pract. 2021Jun;27(6):505-537. doi: 10.1016/j.eprac.2021.04.008.
- Peters AL, Ahmann AJ, Battelino T, Evert A, Hirsch IB, Murad MH, Winter WE, Wolpert DiabetesTechnology-Continuous Subcutaneous Insulin Infusion Therapy and Continuous Glucose Monitoring in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(11):3922-3937.
- Gross TM, Bode BW, Einhorn D, Kayne DM, Reed JH, White NH, Mastrototaro JJ: Performance evaluation ofthe MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. 2000;2:49-56.
- Di Molfetta S, Caruso I, Cignarelli A, Natalicchio A, Perrini S, Laviola L, Giorgino F. Professional continuous glucose monitoring in patients with diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes 2023;25(5):1301-1310.
- Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther. 2015;17(11):787-94.
- Ólafsdóttir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, Theodorsson E, Lind A Clinical Trialof the Accuracy and Treatment Experience of the Flash Glucose Monitor FreeStyle Libre in Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19(3):164-172.
- Miele A, Weiland K, Dungan Clinical outcomes associated with referral-based continuous glucosemonitoring using a central standardized interpretation strategy. Diabetes Technol Ther. 2012;14(9):765-71.
- Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, Deeb L, Dolin RH, Garg SK, Goland R, Hirsch IB, Klonoff DC, Kruger DF, Matfin G, Mazze RS, Olson BA, Parkin C, Peters A, Powers MA,Rodriguez H, Southerland P, Strock ES, Tamborlane W, Wesley DM. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013;15(3):198-211.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring DataInterpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603.
- Rodbard D. Quality of Glycemic Control: Assessment Using Relationships Between Metrics for Safety and Diabetes Technol Ther. 2021 Oct;23(10):692-704. doi: 10.1089/dia.2021.0115.
- Klonoff DC, Wang J, Rodbard D, Kohn MA, Li C, Liepmann D, Kerr D, Ahn D, Peters AL, Umpierrez GE, Seley JJ, Xu NY, Nguyen KT, Simonson G, Agus MSD, Al-Sofiani ME, Armaiz-Pena G, Bailey TS, Basu A, BattelinoT, Bekele SY, Benhamou PY, Bequette BW, Blevins T, Breton MD, Castle JR, Chase JG, Chen KY, ChoudharyP, Clements MA, Close KL, Cook CB, Danne T, Doyle FJ 3rd, Drincic A, Dungan KM, Edelman SV, Ejskjaer N, Espinoza JC, Fleming GA, Forlenza GP, Freckmann G, Galindo RJ, Gomez AM, Gutow HA, Heinemann L, Hirsch IB, Hoang TD, Hovorka R, Jendle JH, Ji L, Joshi SR, Joubert M, Koliwad SK, Lal RA, Lansang MC, Lee WA, Leelarathna L, Leiter LA, Lind M, Litchman ML, Mader JK, Mahoney KM, Mankovsky B, Masharani U, Mathioudakis NN, Mayorov A, Messler J, Miller JD, Mohan V, Nichols JH, Nørgaard K, O'Neal DN, Pasquel FJ, Philis- Tsimikas A, Pieber T, Phillip M, Polonsky WH, Pop-Busui R, Rayman G, Rhee EJ, Russell SJ, Shah VN, Sherr JL, Sode K, Spanakis EK, Wake DJ, Waki K, Wallia A, Weinberg ME, Wolpert H, Wright EE, Zilbermint M, Kovatchev B. A Glycemia Risk Index (GRI) of Hypoglycemia and Hyperglycemia for Continuous Glucose Monitoring Validated by Clinician Ratings. J Diabetes Sci Technol. 2022 Mar 29:19322968221085273. doi: 1177/19322968221085273.
- Hásková A, Radovnická L, Petruželková L, Parkin CG, Grunberger G, Horová E, Navrátilová V, Kádě O,Matoulek M, Prázný M, Šoupal Real-time CGM Is Superior to Flash Glucose Monitoring for Glucose Control in Type 1 Diabetes: The CORRIDA Randomized Controlled Trial. Diabetes Care. 2020 Nov;43(11):2744-2750. doi: 10.2337/dc20-0112.
- Visser MM, Charleer S, Fieuws S, De Block C, Hilbrands R, Van Huffel L, Maes T, Vanhaverbeke G, Dirinck E, Myngheer N, Vercammen C, Nobels F, Keymeulen B, Mathieu C, Gillard P. Comparing real-time andintermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month,prospective, multicentre, randomised controlled trial. Lancet. 2021 Jun 12;397(10291):2275-2283. doi: 1016/S0140-6736(21)00789-3.
- Visser MM, Charleer S, Fieuws S, De Block C, Hilbrands R, Van Huffel L, Maes T, Vanhaverbeke G, Dirinck E, Myngheer N, Vercammen C, Nobels F, Keymeulen B, Mathieu C, Gillard P. Effect of switching from intermittently scanned to real-time continuous glucose monitoring in adults with type 1 diabetes: 24-monthresults from the randomised ALERTT1 trial. Lancet Diabetes Endocrinol. 2023;11(2):96-108.
- Alva S, Bailey T, Brazg R, Budiman ES, Castorino K, Christiansen MP, Forlenza G, Kipnes M, Liljenquist DR, Liu H. Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System With AdvancedAlgorithm in Pediatric and Adult Population With Diabetes. J Diabetes Sci Technol. 2022 Jan;16(1):70-77. doi: 1177/1932296820958754.
- Garg SK, Kipnes M, Castorino K, Bailey TS, Akturk HK, Welsh JB, Christiansen MP, Balo AK, Brown SA, Reid JL, Beck SE. Accuracy and Safety of Dexcom G7 Continuous Glucose Monitoring in Adults with Diabetes. Diabetes Technol 2022 Jun;24(6):373-380. doi: 10.1089/dia.2022.0011.
- Luijf YM, Mader JK, Doll W, Pieber T, Farret A, Place J, Renard E, Bruttomesso D, Filippi A, Avogaro A,Arnolds S, Benesch C, Heinemann L, DeVries AP@home consortium. Accuracy and reliability of continuous glucose monitoring systems: a head-to-head comparison. Diabetes Technol Ther. 2013;15(8):722-7
- Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip Improved glycemiccontrol in poorly controlled patients with type 1 diabetes using real- time continuous glucose monitoring. Diabetes Care. 2006; 29:2730-2732.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo- Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M,Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-76.
- Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring onhypoglycemia in type 1 Diabetes Care. 2011;34(4):795- 800.
- Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose- sensing technology andhypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263.
- Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med. 2017;35(4): 483-90.
- Laffel LM, Kanapka LG, Beck RW, Bergamo K, Clements MA, Criego A, DeSalvo DJ, Goland R, Hood K, Liljenquist D, Messer LH, Monzavi R, Mouse TJ, Prahalad P, Sherr J, Simmons JH, Wadwa RP, Weinstock RS,Willi SM, Miller KM; CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group; CDE10. Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults With Type 1Diabetes: A Randomized Clinical Trial. JAMA. 2020 Jun 16;323(23):2388-2396. doi: 1001/jama.2020.6940.
- Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, Kollman C, Kruger D, McGill JB, Polonsky W, Toschi E, Wolpert H, Price D; DIAMOND Study Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371-378.
- Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, Bhargava A, Bode BW, Carlson A, Chaytor NS, Fox DS, Goland R, Hirsch IB, Kruger D, Kudva YC, Levy C, McGill JB, Peters A, Philipson L, Philis-Tsimikas A, Pop-Busui R, Shah VN, Thompson M, Vendrame F, Verdejo A, Weinstock RS, Young L, Miller KM; Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group. Effect of Continuous Glucose Monitoring on Hypoglycemia in Older Adults With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2020 Jun 16;323(23):2397-2406. doi: 10.1001/jama.2020.6928.
- Miller KM, Kanapka LG, Rickels MR, Ahmann AJ, Aleppo G, Ang L, Bhargava A, Bode BW, Carlson A,Chaytor NS, Gannon G, Goland R, Hirsch IB, Kiblinger L, Kruger D, Kudva YC, Levy CJ, McGill JB, O'Malley G, Peters AL, Philipson LH, Philis-Tsimikas A, Pop- Busui R, Salam M, Shah VN, Thompson MJ, Vendrame F, Verdejo A, Weinstock RS, Young L, Pratley R. Benefit of Continuous Glucose Monitoring in Reducing Hypoglycemia Is Sustained Through 12 Months of Use Among Older Adults with Type 1 Diabetes. Diabetes Technol Ther. 2022 Jun;24(6):424-434. doi: 10.1089/dia.2021.0503.
- Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, Joyce C, Peoples T, Perkins BA, Welsh JB, Willi SM, Wood MA; STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapyin type 1 N Engl J Med. 2010;363(4):311-20.
- Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, Schwarz E, Ólafsdóttir AF, Frid A, Wedel H, Ahlén E, Nyström T, Hellman J. Continuous Glucose Monitoring vs Conventional Therapy for Glycemic Controlin Adults With Type 1 Diabetes Treated With Multiple Daily Insulin Injections: The GOLD Randomized Clinical Trial. JAMA. 2017;317(4):379-387.
- Leelarathna L, Evans ML, Neupane S, Rayman G, Lumley S, Cranston I, Narendran P, Barnard-Kelly K,Sutton CJ, Elliott RA, Taxiarchi VP, Gkountouras G, Burns M, Mubita W, Kanumilli N, Camm M, Thabit H,Wilmot EG; FLASH-UK Trial Study Group. Intermittently Scanned Continuous Glucose Monitoring for Type 1 Diabetes. N Engl J Med. 2022 Oct 20;387(16):1477-1487. doi: 10.1056/NEJMoa2205650.
- Yan J, Zhou Y, Zheng X, Zheng M, Lu J, Luo S, Yang D, Deng H, Xu W, Bi Y, Bao W, Weng J. Effects of intermittently scanned continuous glucose monitoring in adult type 1 diabetes patients with suboptimalglycaemic control: A multi-centre randomized controlled trial. Diabetes Metab Res Rev. 2023;39(4):e3614.
- Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoringsystems for type 1 diabetes Cochrane Database Syst Rev. 2012;1:CD008101.
- Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, Wilson LM, Haberl EB, Brick J, Bass EB, Golden SH. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta- analysis. Ann Intern Med. 2012;157(5):336-47.
- Pickup The evidence base for diabetes technology: appropriate and inappropriate meta-analysis. J Diabetes Sci Technol. 2013;7(6):1567-74.
- Pickup JC, Freeman SC, Sutton Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta- analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805.
- Teo E, Hassan N, Tam W, Koh S. Effectiveness of continuous glucose monitoring in maintaining glycaemiccontrol among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis. Diabetologia. 2022 Apr;65(4):604-619. doi: 10.1007/s00125-021-05648-4.
- Elbalshy M, Haszard J, Smith H, Kuroko S, Galland B, Oliver N, Shah V, de Bock MI, Wheeler Effect ofdivergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabet Med. 2022;39(8):e14854.
- Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, Chadwick TJ, Barendse S, Stocken DD, Brennand C, Marshall SM, Wood R, Speight J, Kerr D, Flanagan D, Heller SR, Evans ML, Shaw JA. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes 2014;37(8):2114- 22.
- van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed-Duijvestijn PH, Kramer MH, Diamant M, Snoek FJ, Serné EH. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open- label, crossover trial. Lancet Diabetes Endocrinol.2016;4(11):893-902.
- Serné EH, van den Berg IK, Racca C, van Raalte DH, Kramer MHH, de Wit M, Snoek Improved Effectiveness of Immediate Continuous Glucose Monitoring in Hypoglycemia- Prone People with Type 1 Diabetes Compared with Hypoglycemia-Focused Psychoeducation Following a Previous Structured Education: A Randomized Controlled Trial. Diabetes Technol Ther. 2023;25(1):50-61.
- Rubin RR, Peyrot M; STAR 3 Study Group. Health-related quality of life and treatment satisfaction in theSensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) Diabetes Technol Ther. 2012;14(2):143-51.
- Polonsky WH, Hessler D, Ruedy KJ, Beck RW; DIAMOND Study Group. The Impact of Continuous Glucose Monitoring on Markers of Quality of Life in Adults With Type 1 Diabetes: Further Findings From the DIAMONDRandomized Clinical Diabetes Care. 2017;40(6):736-741.
- Choe HJ, Rhee EJ, Won JC, Park KS, Lee WY, Cho YM. Effects of Patient-Driven Lifestyle Modification Using Intermittently Scanned Continuous Glucose Monitoring in Patients With Type 2 Diabetes: Results From theRandomized Open-label PDF Diabetes Care. 2022 Oct 1;45(10):2224-2230.
- Moon SJ, Kim KS, Lee WJ, Lee MY, Vigersky R, Park Efficacy of intermittent short- term use of a real-time continuous glucose monitoring system in non-insulin-treated patients with type 2 diabetes: A randomizedcontrolled trial. Diabetes Obes Metab. 2023 Jan;25(1):110-120.
- Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012 Jan;35(1):32-8.
- Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, McGill JB, Polonsky W, Price D, Aronoff S, Aronson R, Toschi E, Kollman C, Bergenstal R; DIAMOND Study Group. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann Intern 2017 Sep 19;167(6):365-374.
- Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, Pop-Busui R, Philis- Tsimikas A, Bao S,Umpierrez G, Davis G, Kruger D, Bhargava A, Young L, McGill JB, Aleppo G, Nguyen QT, Orozco I, Biggs W, Lucas KJ, Polonsky WH, Buse JB, Price D, Bergenstal RM; MOBILE Study Group. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. JAMA. 2021 Jun 8;325(22):2262-2272. doi: 1001/jama.2021.7444.
- Aleppo G, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, Pop-Busui R, Philis- Tsimikas A, Bao S, Umpierrez G, Davis G, Kruger D, Bhargava A, Young L, Buse JB, McGill JB, Martens T, Nguyen QT, Orozco I, Biggs W, Lucas KJ, Polonsky WH, Price D, Bergenstal RM; MOBILE Study Group; Type 2 Diabetes BasalInsulin Users: The Mobile Study (MOBILE) Study Group:. The Effect of Discontinuing Continuous Glucose Monitoring in Adults With Type 2 Diabetes Treated With Basal Insulin. Diabetes Care. 2021 Dec;44(12):2729-2737. doi: 10.2337/dc21-1304.
- Bao S, Bailey R, Calhoun P, Beck Effectiveness of Continuous Glucose Monitoring in Older Adults with Type 2 Diabetes Treated with Basal Insulin. Diabetes Technol Ther. 2022 May;24(5):299-306. doi: 10.1089/dia.2021.0494.
- Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, Rozenberg A, Karp M, Ish-Shalom M, Singer J, Wainstein J, Raz I. Effect of Flash Glucose Monitoring Technology on Glycemic Control and Treatment Satisfaction in Patients With Type 2 Diabetes. Diabetes Care. 2019 Jul;42(7):1178-1184. doi: 10.2337/dc18-0166.
- Ajjan RA, Heller SR, Everett CC, Vargas-Palacios A, Higham R, Sharples L, Gorog DA, Rogers A, Reynolds C,Fernandez C, Rodrigues P, Sathyapalan T, Storey RF, Stocken Multicenter Randomized Trial of Intermittently Scanned Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose in Individuals With Type 2 Diabetes and Recent-Onset Acute Myocardial Infarction: Results of the LIBERATES Trial.Diabetes Care. 2023 Feb 1;46(2):441-449.
- Aronson R, Brown RE, Chu L, Bajaj HS, Khandwala H, Abitbol A, Malakieh N, Goldenberg IMpact of flash glucose Monitoring in pEople with type 2 Diabetes Inadequately controlled with non-insulin AntihyperglycaemicThErapy (IMMEDIATE): A randomized controlled trial. Diabetes Obes Metab. 2023;25(4):1024-1031.
- DeSalvo DJ, Miller KM, Hermann JM, Maahs DM, Hofer SE, Clements MA, Lilienthal E, Sherr JL, Tauschmann M, Holl RW; T1D Exchange and DPV Registries. Continuous glucose monitoring and glycemic control amongyouth with type 1 diabetes: International comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes. 2018;19(7):1271-1275.
- Deshmukh H, Wilmot E, Pieri B, Choudhary P, Shah N, Gregory R, Kilvert A, Lumb A, Christian P, Barnes D, Patmore J, Walton C, Ryder REJ, Sathyapalan T. Time in range following flash glucose monitoring:Relationship with glycaemic control, diabetes-related distress and resource utilisation in the Association of British Clinical Diabetologists national audit. Diabet Med. 2022 Nov;39(11):e14942. doi: 10.1111/dme.14942.
- Nathanson D, Svensson AM, Miftaraj M, Franzén S, Bolinder J, Eeg-Olofsson Effect of flash glucose monitoring in adults with type 1 diabetes: a nationwide, longitudinal observational study of 14,372 flash users compared with 7691 glucose sensor naive controls. Diabetologia. 2021 Jul;64(7):1595-1603. doi: 10.1007/s00125-021-05437-z.
- Roussel R, Riveline JP, Vicaut E, de Pouvourville G, Detournay B, Emery C, Levrat- Guillen F, Guerci Important Drop Rate of Acute Diabetes Complications in People With Type 1 or Type 2 Diabetes After Initiation of Flash Glucose Monitoring in France: The RELIEF Study. Diabetes Care. 2021 Apr 20:dc201690. doi: 10.2337/dc20-1690.
- Riveline JP, Roussel R, Vicaut E, de Pouvourville G, Detournay B, Emery C, Levrat- Guillen F, Guerci B. Reduced Rate of Acute Diabetes Events with Flash Glucose Monitoring Is Sustained for 2 Years After Initiation: Extended Outcomes from the RELIEF Study. Diabetes Technol Ther. 2022 Sep;24(9):611-618. doi: 10.1089/dia.2022.0085.
- Charleer S, De Block C, Van Huffel L, Broos B, Fieuws S, Nobels F, Mathieu C, Gillard Quality of Life and Glucose Control After 1 Year of Nationwide Reimbursement of Intermittently Scanned Continuous Glucose Monitoring in Adults Living With Type 1 Diabetes (FUTURE): A Prospective Observational Real-World Cohort Study. Diabetes Care. 2020 Feb;43(2):389-397. doi: 10.2337/dc19-1610.
- Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott Association of Real-time Continuous Glucose Monitoring With Glycemic Control and Acute Metabolic Events Among Patients With Insulin-Treated Diabetes. JAMA. 2021 Jun 8;325(22):2273-2284. doi: 10.1001/jama.2021.6530.
- Bergenstal RM, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Hirsch IB. Flash CGM Is Associated WithReduced Diabetes Events and Hospitalizations in Insulin-Treated Type 2 Diabetes. J Endocr Soc. 2021 Feb 2;5(4):bvab013. doi: 10.1210/jendso/bvab013. PMID: 33644623; PMCID: PMC7901259.
- Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA. Interventions That Restore Awareness of Hypoglycemiain Adults With Type 1 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2015 Aug;38(8):1592-609.
- Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ 3rd, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017 Dec;40(12):1631-1640.
- Beck RW, Bergenstal RM, Riddlesworth TD, et Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400-405.
- Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann Improving the Clinical Value and Utility of CGM Systems: Issues and Recommendations: A Joint Statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2017;40(12):1614-1621.
- Spanakis EK, Urrutia A, Galindo RJ, Vellanki P, Migdal AL, Davis G, Fayfman M, Idrees T, Pasquel FJ, Coronado WZ, Albury B, Moreno E, Singh LG, Marcano I, Lizama S, Gothong C, Munir K, Chesney C, Maguire R, Scott WH, Perez-Guzman MC, Cardona S, Peng L, Umpierrez GE. Continuous Glucose Monitoring-Guided Insulin Administration in Hospitalized Patients With Diabetes: A Randomized Clinical Trial. Diabetes Care. 2022 Oct 1;45(10):2369-2375. doi: 10.2337/dc22-0716.
- Fortmann AL, Spierling Bagsic SR, Talavera L, Garcia IM, Sandoval H, Hottinger A, Philis- Tsimikas A. Glucose as the Fifth Vital Sign: A Randomized Controlled Trial of Continuous Glucose Monitoring in a Non-ICU Hospital Setting. Diabetes Care. 2020 Nov;43(11):2873- 2877. doi: 10.2337/dc20-1016.
- Buschur EO, Faulds E, Dungan CGM in the Hospital: Is It Ready for Prime Time? Curr Diab Rep. 2022 Sep;22(9):451-460. doi: 10.1007/s11892-022-01484-x.
- Galindo RJ, Umpierrez GE, Rushakoff RJ, Basu A, Lohnes S, Nichols JH, Spanakis EK, Espinoza J, Palermo NE, Awadjie DG, Bak L, Buckingham B, Cook CB, Freckmann G, Heinemann L, Hovorka R, Mathioudakis N,Newman T, O'Neal DN, Rickert M, Sacks DB, Seley JJ, Wallia A, Shang T, Zhang JY, Han J, Klonoff Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline. J Diabetes Sci Technol. 2020 Nov;14(6):1035-1064. doi: 10.1177/1932296820954163.
- Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV,Bergenstal R, Smith E, Olson BA, Garg State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72.
- Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes Device Use in Adults WithType 1 Diabetes: Barriers to Uptake and Potential Intervention Diabetes Care. 2017;40(2):181-187.
- Odugbesan O, Addala A, Nelson G, Hopkins R, Cossen K, Schmitt J, Indyk J, Jones NY, Agarwal S, Rompicherla S, Ebekozien O. Implicit Racial-Ethnic and Insurance-Mediated Bias to Recommending Diabetes Technology: Insights from T1D Exchange Multicenter Pediatric and Adult Diabetes Provider Cohort. Diabetes Technol Ther. 2022;24(9):619-
- Majidi S, Ebekozien O, Noor N, et al.: Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin Diabetes 2021;39:278–283.
- Agarwal S, Kanapka LG, Raymond JK, et al.: Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab 2020;105:e2960–e2969.
- Rigo RS, Levin LE, Belsito DV, Garzon MC, Gandica R, Williams KM. Cutaneous Reactions to Continuous Glucose Monitoring and Continuous Subcutaneous Insulin Infusion Devices in Type 1 Diabetes JDiabetes Sci Technol. 2021 Jul;15(4):786- 791. doi: 10.1177/1932296820918894.
- Messer LH, Berget C, Beatson C, Polsky S, Forlenza Preserving Skin Integrity with Chronic Device Use in Diabetes. Diabetes Technol Ther. 2018 Jun;20(S2):S254-S264. doi: 10.1089/dia.2018.0080.
- Nørgaard K, Scaramuzza A, Bratina N, Lalić NM, Jarosz-Chobot P, Kocsis G, Jasinskiene E, De Block C, Carrette O, Castañeda J, Cohen O; Interpret Study Group. Routine sensor-augmented pump therapy in type 1diabetes: the INTERPRET Diabetes Technol Ther. 2013;15(4):273-80.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes ResearchFoundation continuous glucose monitoring (JDRF- CGM) trial. Diabetes Care. 2010;33(1):17-22.
- Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous Glucose Monitoring Sensors for DiabetesManagement: A Review of Technologies and Diabetes and Metabolism Journal. 2019 Aug; 43(4): 383-397. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6712232/
- Bay C, Kristensen PL, Pedersen-Bjergaard U, Tarnow L, Thorsteinsson B. Nocturnal continuous glucose monitoring: accuracy and reliability of hypoglycemia detection in patients with type 1 diabetes at high risk ofsevere Diabetes Technol Ther. 2013;15(5):371-7.
- Freckmann G, Link M, Westhoff A, Kamecke U, Pleus S, Haug C. Prediction Quality of Glucose Trend Indicators in Two Continuous Tissue Glucose Monitoring Systems. Diabetes Technol Ther. 2018 Aug;20(8):550-556.
- Jenkins AJ, Krishnamurthy B, Best JD, Cameron FJ, Colman PG, Hamblin PS, O'Connell MA, Rodda C, Teede H, O'Neal DN. An algorithm guiding patient responses to real-time- continuous glucose monitoring improves quality of life. Diabetes Technol Ther. 2011 Feb;13(2):105-9.
- Diabetes Research In Children Network (DirecNet) Study Group, Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, Tsalikian E, Tamborlane W, Wysocki T, Ruedy K, Beck R. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator). Pediatr Diabetes. 2008;9(2):142-7.
- Scheiner Practical CGM: Improving Patient Outcomes Through Continuous Glucose Monitoring. 4th ed. Alexandria, VA: American Diabetes Association; 2015.
- Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data forinsulin adjustments in type 1 J Diabetes Sci Technol. 2017;11(1):138–147.
- Klonoff DC, Kerr D. A simplified approach using rate of change arrows to adjust insulin with real-timecontinuous glucose J Diabetes Sci Technol. 2017;11(6):1063– 1069.
- Aleppo G, Laffel LM, Ahmann AJ, Hirsch IB, Kruger DF, Peters A, Weinstock RS, Harris DR. A PracticalApproach to Using Trend Arrows on the Dexcom G5 CGM System for the Management of Adults with Diabetes. Journal of the Endocrine Society 2017;1(12):1445-1460.
- Ajjan RA, Cummings MH, Jennigs P, Leelarathna L, Rayman G, Wilmot E. Optimising use of rate-of-changetrend arrows for insulin dosing decisions using the FreeStyle Libre Flash Glucose Monitoring System. Diabetes and Vascular Disease Research. 2018;16(1):3-12.
- Ziegler, R.; von Sengbusch, S.; Kröger, J.; Schubert, O.; Werkmeister, P.; Deiss, D.; Siegmund, TherapyAdjustments Based on Trend Arrows Using Continuous Glucose Monitoring Systems. J. Diabetes Sci. Technol. 2019, 13, 763–773.
- Parise M, Di Molfetta S, Graziano RT, Fiorentino R, Cutruzzolà A, Gnasso A, Irace C. A Head-to-HeadComparison of Two Algorithms for Adjusting Mealtime Insulin Doses Based on CGM Trend Arrows in AdultPatients with Type 1 Diabetes: Results from an Exploratory Study. Int J Environ Res Public Health. 2023;20(5):3945.
- Gonzales WV, Mobashsher AT, Abbosh A. The Progress of Glucose Monitoring—A Review of Invasive toMinimally and Non-Invasive Techniques, Devices, and Sensors. 2019;19(4):800.
- FreeStyle Libre User Manual. https://freestyleserver.com/Payloads/IFU/2017_sep/ART38553-001_rev-C-Web.pdf. Accessed June 29, 2023.
- https://www.freestylelibre.us/system-overview/freestyle-libre-html?msclkid=4cedd02409fe16f5d98e8f09162393b1. Site accessed June 29, 2023.
- Aronson R, Abitbol A, Tweden First assessment of the performance of an implantable continuous glucose monitoring system through 180 days in a primarily adolescent population with type 1 diabetes. Diabetes Obes Metab. 2019;21(7):1689-1694.
- Kropff J, Choudhary P, Neupane S, Barnard K, Bain SC, Kapitza C, Forst T, Link M, Dehennis A, DeVries Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial. Diabetes Care. 2017 Jan;40(1):63-68.
- Lorenz C, Sandoval W, Mortellaro M. Interference Assessment of Various Endogenous and ExogenousSubstances on the Performance of the Eversense Long-Term Implantable Continuous Glucose Monitoring System. Diabetes Technol Ther. 2018;20(5):344-352.
- Garg SK, Liljenquist D, Bode B, Christiansen MP, Bailey TS, Brazg RL, Denham DS, Chang AR, Akturk HK, Dehennis A, Tweden KS, Kaufman FR. Evaluation of Accuracy and Safety of the Next-Generation Up to 180-Day Long-Term Implantable Eversense Continuous Glucose Monitoring System: The PROMISE Diabetes Technol Ther. 2022 Feb;24(2):84-92. doi: 10.1089/dia.2021.0182.
- Deiss D, Szadkowska A, Gordon D, Mallipedhi A, Schütz-Fuhrmann I, Aguilera E, Ringsell C, De Block C, Irace C. Clinical Practice Recommendations on the Routine Use of Eversense, the First Long-Term Implantable Continuous Glucose Monitoring System. Diabetes Technol Ther. 2019;21(5):254-264.
- Šoupal J, Petruželková L, Flekač M, Pelcl T, Matoulek M, Daňková M, Škrha J, Svačina Š, Prázný M. Comparison of Different Treatment Modalities for Type 1 Diabetes, Including Sensor-Augmented Insulin Regimens, in 52 Weeks of Follow-Up: A COMISAIR Study. Diabetes Technol Ther. 2016 Sep;18(9):532-8.
- Boughton CK, Hovorka Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36(3):279-286.
- Braune K, Hussain S, Lal The First Regulatory Clearance of an Open-Source Automated Insulin Delivery Algorithm. J Diabetes Sci Technol. 023:19322968231164166.
- Melmer A, Züger T, Lewis DM, Leibrand S, Stettler C, Laimer Glycaemic Control in Individuals with Type 1 Diabetes using an artificial open source pancreas system (OpenAPS). Diabetes Obesity and Metabolism. 2019; 21(10):2333-2337.
- https://www.fda.gov/news-events/press-announcements/fda-warns-against-use-unauthorized-devices-diabetes-management. Accessed June 29,
- Jennifer Sherr, Lutz Heinemann, G. Alexander Fleming, Richard M. Bergenstal, Daniela Bruttomesso, Hélène Hanaire, Reinhard W. Holl, John R. Petrie, Anne L. Peters, Mark Evans; Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetes Care 1 December 2022; 45 (12): 3058–3074. https://doi.org/10.2337/dci22-0018
- Lewis History and Perspective on DIY Closed Looping. J Diabetes Sci Technol. 2019;13(4):790-793.
- Hernando ME, Garcia-Sáez G, Gómez EJ, Pérez-Gandia C, Rodriguez-Herrero A. Automated InsulinDelivery: The Artificial Pancreas Technical American Journal of Therapeutics. 2019; 1075-2765
- Bailey TS, Ahmann A, Brazg R, Christiansen M, Garg S, Watkins E, Welsh JB, Lee SW. Accuracy andacceptability of the 6-day Enlite continuous subcutaneous glucose Diabetes Technol Ther. 2014;16(5):277-83.
- Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Ahmann AJ, Welsh JB, Lee SW, Kaufman FR; ASPIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of N Engl J Med. 2013;369(3):224- 32.
- Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor- augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patientswith type 1 diabetes: a randomized clinical JAMA. 2013;310(12):1240-7.
- Abraham MB, Nicholas JA, Smith GJ, Fairchild JM, King BR, Ambler GR, Cameron FJ, Davis EA, JonesTW; PLGM Study Reduction in Hypoglycemia With the Predictive Low-Glucose Management System: A Long-term Randomized Controlled Trial in Adolescents With Type 1 Diabetes. Diabetes Care. 2018;41(2):303-310.
- Predictive Low-Glucose Suspend Reduces Hypoglycemia in Adults, Adolescents, and Children WithType 1 Diabetes in an At-Home Randomized Crossover Study: Results of the PROLOG Trial. Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, Ekhlaspour L, Church MM, Weinzimer SA, Jost E, Marcal T, Andre C, Carria L, Swanson V, Lum JW, Kollman C, Woodall W, Beck RW. Diabetes Care. 2018;41(10):2155-2161.
- Ekhlaspour L, Town M, Raghinaru D, Lum JW, Brown SA, Buckingham BA. Glycemic Outcomes inBaseline Hemoglobin A1C Subgroups in the International Diabetes Closed- Loop Trial. Diabetes Technol Ther. 2022;24(8):588-591.
- Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetesdevice use in adolescents and potential intervention Diabetes Technol Ther 2020;22:760–767.
- Shivers JP, Mackowiak L, Anhalt H, Zisser “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol 2013;7:789–794.
- Zhou K, Isaacs Closed-Loop Artificial Pancreas Therapy for Type 1 Diabetes. Curr Cardiol Rep. 2022 Sep;24(9):1159-1167. doi: 10.1007/s11886-022-01733-1.
- Cobry EC, Berget C, Messer LH, Forlenza GP. Review of the Omnipod® 5 Automated Glucose ControlSystem Powered by Horizon™ for the treatment of Type 1 Ther Deliv. 2020 Aug;11(8):507-519. doi: 10.4155/tde-2020-0055.
- Nallicheri A, Mahoney KM, Gutow HA, Bellini N, Isaacs Review of Automated Insulin Delivery Systems for Type 1 Diabetes and Associated Time in Range Outcomes. touchREV Endocrinol. 2022 Jun;18(1):27-34. doi: 10.17925/EE.2022.18.1.27.
- Christiansen MP, Garg SK, Brazg R, Bode BW, Bailey TS, Slover RH, Sullivan A, Huang S, Shin J, LeeSW, Kaufman Accuracy of a Fourth-Generation Subcutaneous Continuous Glucose Sensor. Diabetes Technol Ther. 2017;19(8):446-456.
- Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA. 2016;316(13):1407-1408.
- Garg SK, Grunberger G, Weinstock R, Lawson ML, Hirsch IB, DiMeglio LA, Pop-Busui R, Philis-TsimikasA, Kipnes M, Liljenquist DR, Brazg RL, Kudva YC, Buckingham BA, McGill JB, Carlson AL, Criego AB, Christiansen MP, Kaiserman KB, Griffin KJ, Forlenza GP, Bode BW, Slover RH, Keiter A, Ling C, Marinos B,Cordero TL, Shin J, Lee SW, Rhinehart AS, Vigersky RA; Adult and Pediatric MiniMed™ HCL Outcomes 6-month RCT: HCL versus CSII Control Study Group. Improved Glycemia with Hybrid Closed-Loop Versus Continuous Subcutaneous Insulin Infusion Therapy: Results from a Randomized Controlled Trial. Diabetes Technol Ther. 2023;25(1):1-12.
- Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson One Year Clinical Experience of the First Commercial Hybrid Closed-Loop. Diabetes Care. 2019;42(12):2190-2196.
- Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors andperceptions of youth discontinuing the 670G system in the first 6 Pediatr Diabetes 2020;21:319–327.
- https://www.medtronicdiabetes.com/products/minimed-780g- system?utm_source=google&utm_campaign=Pumps+-+BRAND+-+Core+-+Exact++-+LP_Test_fy24_pumps_asbly&utm_medium=cpc&gclid=Cj0KCQjw1_SkBhDwARIsANbGpFvAwdARyEPkREpyy8EWXp5ysEutJ5OXqYmzQbyqn3raueXzLYRYSecaAssxEALw_wc B&gclsrc=aw.ds. Site accessed June 29,2023.
- Carlson AL, Sherr JL, Shulman DI, Garg SK, Pop-Busui R, Bode BW, et al. Safety and GlycemicOutcomes During the MiniMed™ Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2022;24(3):178–189. https:// doi. org/ 10. 1089/ dia. 2021. 0319.
- Choudhary P, Kolassa R, Keuthage W, Kroeger J, Thivolet C, Evans M, Ré R, de Portu S, Vorrink L, Shin J, Habteab A, Castañeda J, da Silva J, Cohen O; ADAPT study Group. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol. 2022 Oct;10(10):720-731. doi: 10.1016/S2213-8587(22)00212-1.
- Matejko B, Juza A, Kieć-Wilk B, Cyranka K, Krzyżowska S, Chen X, Cohen O, Da Silva J, Malecki MT, Klupa T. Transitioning of People With Type 1 Diabetes From Multiple Daily Injections and Self-Monitoring of Blood Glucose Directly to MiniMed 780G Advanced Hybrid Closed-Loop System: A Two-Center, Randomized, Controlled Study. Diabetes Care. 2022;45(11):2628-2635.
- Arrieta A, Battelino T, Scaramuzza AE, Da Silva J, Castañeda J, Cordero TL, Shin J, Cohen Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: Evidencefrom 12 870 real-world users. Diabetes Obes Metab. 2022 Jul;24(7):1370-1379. doi: 10.1111/dom.14714.
- Hood KK, Laffel LM, Danne T, Nimri R, Weinzimer SA, Sibayan J, Bailey RJ, Schatz D, Bratina N, Bello R, Punel A, Calhoun P, Beck RW, Bergenstal RM, Phillip M. Lived Experience of Advanced Hybrid Closed-Loop Versus Hybrid Closed-Loop: Patient- Reported Outcomes and Diabetes Technol Ther. 2021Dec;23(12):857-861. doi: 10.1089/dia.2021.0153.
- Brown SA, Kovatchev BP, Raghinaru D, et Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707-1717.
- Pinsker JE, Müller L, Constantin A, Leas S, Manning M, McElwee Malloy M, Singh H, Habif S. Real-World Patient-Reported Outcomes and Glycemic Results with Initiation of Control-IQ DiabetesTechnol Ther. 2021 Feb;23(2):120-127. doi: 10.1089/dia.2020.0388.
- https://www.myomnipod.com/healthcareproviders/about-omnipod/innovation. Site accessed June29,2023.
- Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulindelivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44:1630–40.
- Bionic Pancreas Research Group; Russell SJ, Beck RW, Damiano ER, El-Khatib FH, Ruedy KJ, BalliroCA, Li Z, Calhoun P, Wadwa RP, Buckingham B, Zhou K, Daniels M, Raskin P, White PC, Lynch J, Pettus J, Hirsch IB, Goland R, Buse JB,
- Kruger D, Mauras N, Muir A, McGill JB, Cogen F, Weissberg-Benchell J, Sherwood JS, Castellanos LE, Hillard MA, Tuffaha M, Putman MS, Sands MY, Forlenza G, Slover R, Messer LH, Cobry E, Shah VN, PolskyS, Lal R, Ekhlaspour L, Hughes MS, Basina M, Hatipoglu B, Olansky L, Bhangoo A, Forghani N, Kashmiri H, Sutton F, Choudhary A, Penn J, Jafri R, Rayas M, Escaname E, Kerr C, Favela-Prezas R, Boeder S, Trikudanathan S, Williams KM, Leibel N, Kirkman MS, Bergamo K, Klein KR, Dostou JM, Machineni S, YoungLA, Diner JC, Bhan A, Jones JK, Benson M, Bird K, Englert K, Permuy J, Cossen K, Felner E, Salam M, Silverstein JM, Adamson S, Cedeno A, Meighan S, Dauber A. Multicenter, Randomized Trial of a Bionic Pancreas in Type 1 N Engl J Med. 2022 Sep 29;387(13):1161-1172.
- DeSalvo D, Buckingham Continuous glucose monitoring: current use and future directions.Curr Diab Rep. 2013;13(5):657-62.
- Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich AB, Hovorka R, Tsapas A. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310.
- Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems onglycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501-
- Jiao X, Shen Y, Chen Better TIR, HbA1c, and less hypoglycemia in closed-loop insulin system in patients with type 1 diabetes: a meta-analysis. BMJ Open Diabetes Res Care. 2022 Apr;10(2):e002633. doi: 10.1136/bmjdrc-2021-002633.
- Alexandraki K, Tentolouris N, Stefanadis C, Chrousos GP, Tousoulis Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta- analysis. Metabolism. 2019 Jan;90:20-30. doi: 10.1016/j.metabol.2018.10.002.
- Bailey TS, Grunberger G, Bode BW, Handelsman Y, Hirsch IB, Jovanovič L, Roberts VL, Rodbard D,Tamborlane WV, Walsh J; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE). American Association of Clinical Endocrinologists and American College of Endocrinology 2016 Outpatient Glucose Monitoring Consensus Statement. Endocr Pract. 2016;22(2):231-61.
- Ma R, Shao R, An X, Zhang Q, Sun S. Recent advancements in noninvasive glucose monitoring andclosed-loop management systems for J Mater Chem B. 2022 Jul 27;10(29):5537-5555. doi: 10.1039/d2tb00749e.
- Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;1:CD005060.
- Cahn A, Akirov A, Raz Digital health technology and diabetes management. J Diabetes. 2018;10(1):10-17.
- Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable Health Technology and Electronic Health RecordIntegration: Scoping Review and Future JMIR Mhealth Uhealth. 2019;7(9):e12861.
- Klonoff DC, Kerr Overcoming Barriers to Adoption of Digital Health Tools for Diabetes. J Diabetes Sci Technol. 2018;12(1):3-6.
- Klonoff DC, Kerr D, Kleidermacher Now Is the Time for a Security and Safety Standard for Consumer Smartphones Controlling Diabetes Devices. J Diabetes Sci Technol. 2017;11(5):870-873.
- Blackwell M, Wheeler Clinical review: the misreporting of logbook, download, and verbal self-measured blood glucose in adults and children with type I diabetes. Acta Diabetol. 2017;54(1):1-8.
- Beck Downloading Diabetes Device Data: Empowering Patients to Download at Home to Achieve Better Outcomes. Diabetes Technol Ther. 2015;17(8):536-7.
- Lee JM, Hirschfeld E, Wedding A Patient-Designed Do-It-Yourself Mobile Technology System for Diabetes: Promise and Challenges for a New Era in Medicine. JAMA. 2016 315(14):1447-8.
- van der Linden J, Welsh JB, Walker Sustainable Use of a Real-Time Continuous Glucose Monitoring System from 2018 to 2020. Diabetes Technol Ther. 2021 Jul;23(7):508-511. doi: 10.1089/dia.2021.0014.Akturk HK, Dowd R, Shankar K, Derdzinski M. Real-World Evidence and Glycemic Improvement Using Dexcom G6Features. Diabetes Technol Ther. 2021 Mar;23(S1):S21- S26. doi: 10.1089/dia.2020.0654.
- Polonsky WH, Soriano EC, Fortmann AL. The Role of Retrospective Data Review in the Personal Use ofReal-Time Continuous Glucose Monitoring: Perceived Impact on Quality of Life and Health Outcomes. Diabetes Technol Ther. 2022 Jul;24(7):492-501. doi: 1089/dia.2021.0526.
- https://investors.insulet.com/news/news-details/2019/Insulet-Omnipod-DASH-System-
- Mobile-Apps-Now-Available-for-iOS-Devices/default.aspx. Site accessed June 29,
- Tandem Diabetes Care announces FDA clearance for the t:slim X2 insulin pump to bolus using the t:connect Mobile App [press release]. San Diego, CA: Tandem Diabetes Care, Inc; February 16, 2022. Site accessed June 29, 2023. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable- insulin-pump-intended-allow-patients-customize-treatment-through. Site accessed June 29, 2023.
- Blenner SR, Köllmer M, Rouse AJ, Daneshvar N, Williams C, Andrews Privacy Policies of Android Diabetes Apps and Sharing of Health Information. JAMA 2016;315:1051-2.
- Jacques Rose K, Petrut C, L'Heveder R, de Sabata IDF Europe position on mobile applications in diabetes. Diabetes Res Clin Pract. 2019;149:139-46.
- Klonoff DC, Kerr D, Wong JC, Pavlovic Y, Koliwad S, Hu J, Salber P, Aguilera A, Long W, Hamilton G, Chen KY, Adi S. Digital Diabetes Congress 2017. J Diabetes Sci Technol. 2017;11(5):1045-1052.
- Rose KJ, Petrut C, L'Heveder R, de Sabata IDF Europe's position on mobile applications in diabetes. Diabetes Res Clin Pract. 2019;149:39-46.
- Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do Mobile Phone Applications Improve Glycemic Control (HbA1c) in the Self-management of Diabetes? A Systematic Review, Meta-analysis, and GRADE of 14Randomized Diabetes Care. 2016;39(11):2089- 2095.
- Cui M, Wu X, Mao J, Wang X, Nie M. T2DM Self-Management via Smartphone Applications: ASystematic Review and Meta-Analysis. PLoS One 2016;11(11):e0166718.
- Bonoto BC, de Araújo VE, Godói IP, de Lemos LL, Godman B, Bennie M, Diniz LM, Junior Efficacy of Mobile Apps to Support the Care of Patients With Diabetes Mellitus: A Systematic Review and Meta-Analysisof Randomized Controlled Trials. JMIR Mhealth Uhealth. 2017;5(3):e4.
- Wu Y, Yao X, Vespasiani G, Nicolucci A, Dong Y, Kwong J, Li L, Sun X, Tian H, Li S. Mobile App-Based Interventions to Support Diabetes Self-Management: A Systematic Review of Randomized Controlled Trials toIdentify Functions Associated with Glycemic Efficacy. JMIR Mhealth Uhealth. 2017;5(3):e35.
- Veazie S, Winchell K, Gilbert J, Paynter R, Ivlev I, Eden K, Nussbaum K, Weiskopf N, Guise JM, Helfand Mobile Applications for Self-Management of Diabetes [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 May.
- Adu MD, Malabu UH, Callander EJ, Malau-Aduli AE, Malau-Aduli Considerations for the Development of Mobile Phone Apps to Support Diabetes Self-Management: Systematic Review. JMIR Mhealth Uhealth. 2018 ;6(6):e10115.
- Martinez-Millana A, Jarones E, Fernandez-Llatas C, Hartvigsen G, Traver V. App Features for Type 1Diabetes Support and Patient Empowerment: Systematic Literature Review and Benchmark Comparison. JMIR Mhealth Uhealth. 2018;6(11):e12237.
- Fu H, McMahon SK, Gross CR, Adam TJ, Wyman Usability and clinical efficacy of diabetes mobile applications for adults with type 2 diabetes: A systematic review. Diabetes Res Clin Pract. 2017;131:70-81.
- Chavez S, Fedele D, Guo Y, Bernier A, Smith M, Warnick J, Modave Mobile Apps for the Management of Diabetes. Diabetes Care. 2017;40(10):e145-e146.
- Brzan PP, Rotman E, Pajnkihar M, Klanjsek Mobile Applications for Control and Self Management of Diabetes: A Systematic Review. J Med Syst. 2016;40(9):210.
- Katz LB, Dirani RG, Li G, Randoll RA, Mahoney Automated glycemic pattern analysis can improve health care professional efficiency and accuracy. J Diabetes Sci Technol. 2013;7(1):163-6.
- Parkin CG, Davidson Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol. 2009;3(3):500-8.
- Eiland L, McLarney M, Thangavelu T, Drincic App-Based Insulin Calculators: Current and Future State. Curr Diab Rep. 2018;18(11):123.
- Ahn Automated Bolus Calculators and Connected Insulin Pens: A Smart Combination for Multiple Daily Injection Insulin Therapy. J Diabetes Sci Technol. 2022 May;16(3):605- 609. doi: 10.1177/19322968211062624.
- MacLeod J, Vigersky A Review of Precision Insulin Management With Smart Insulin Pens: Opening Up the Digital Door to People on Insulin Injection Therapy. J Diabetes Sci Technol. 2023 Mar;17(2):283-289. doi: 10.1177/19322968221134546.
- Shashaj B, Busetto E, Sulli Benefits of a bolus calculator in pre- and postprandial glycaemic control and meal flexibility of paediatric patients using continuous subcutaneous insulin infusion (CSII). Diabet Med. 2008;25(9):1036–42.
- Sussman A, Taylor EJ, Patel M, Ward J, Alva S, Lawrence A, Ng R. Performance of a glucose meterwith a built-in automated bolus calculator versus manual bolus calculation in insulin-using subjects. J Diabetes Sci Technol. 2012;6(2):339-44.
- Maurizi AR, Lauria A, Maggi D, Palermo A, Fioriti E, Manfrini S, Pozzilli A novel insulin unit calculator for the management of type 1 diabetes. Diabetes Technol Ther. 2011;13(4):425–8.
- Huckvale, K, Adomaviciute, S, Prieto, JT, Leow, MK, Car, Smartphone apps for calculating insulin dose: a systematic assessment. BMC Med. 2015;13(1):106.
- Ahn Automated Bolus Calculators and Connected Insulin Pens: A Smart Combination for Multiple Daily Injection Insulin Therapy. J Diabetes Sci Technol. 2022 May;16(3):605- 609. doi: 10.1177/19322968211062624.
- Meade LT, Rushton Accuracy of carbohydrate counting in adults. Clin Diabetes. 2016;34:142–7.
- Weatherly J, Kishnani S, Aye T. Challenges with Patient Adoption of Automated Integration of BloodGlucose Meter Data in the Electronic Health Diabetes Technol Ther. 2019;21:671-674.
- Jung SY, Kim JW, Hwang H, et al. Development of Comprehensive Personal Health Records IntegratingPatient-Generated Health Data Directly From Samsung S-Health and Apple Health Apps: Retrospective Cross-Sectional Observational Study. JMIR Mhealth Uhealth. 2019;7:e12691.
- Espinoza J, Klonoff D, Vidmar A, et al. 2022 iCoDE Report: CGM-EHR Integration Standards and Diabetes Technol Soc. Published online November 7, 2022. Accessed March 14, 2023. https://www.diabetestechnology.org/icode/
- Lee EY, Cha SA, Yun JS, Lim SY, Lee JH, Ahn YB, Yoon KH, Hyun MK, Ko SH. Efficacy of PersonalizedDiabetes Self-care Using an Electronic Medical Record-Integrated Mobile App in Patients With Type 2 Diabetes: 6-Month Randomized Controlled Trial. J Med Internet Res. 2022 Jul 28;24(7):e37430. doi: 10.2196/37430.
- Gallagher EJ, Le Roith D, Bloomgarden Review of hemoglobin A(1c) in the management of diabetes. Journal of diabetes 2009;1:9-17.
- Little RR, Roberts A review of variant hemoglobins interfering with hemoglobin A1c measurement. Journal of diabetes science and technology 2009;3:446-51.
- http://www.ngsp.org. Site accessed June 29,
- Knaebel J, Irvin BR, Xie Accuracy and clinical utility of a point-of-care HbA1c testing device.Postgrad Med. 2013;125(3):91-8.
- Jeppsson JO, Kobold U, Barr J et Approved IFCC reference method for the measurement of HbA1cin human blood. Clin Chem Lab Med. 2002;40:78–89.
- Miedema Standardization of HbA1c and Optimal Range of Monitoring. Scand J Clin Lab Invest Suppl. 2005;240:61-72.
- Cohen RM, Snieder H, Lindsell CJ, Beyan H, Hawa MI, Blinko S, Edwards R, Spector TD, Leslie RD. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care. 2006; 29:1739-1743
- Chalew SA, McCarter RJ, Thomas J, Thomson JL, Hempe JM. A comparison of the Glycosylation Gapand Hemoglobin Glycation Index in patients with J Diabetes Complications. 2005;19:218-222.
- Rodríguez-Segade S, Rodríguez J, García Lopez JM, Casanueva FF, Camina F. Estimation of theglycation gap in diabetic patients with stable glycemic Diabetes Care 2012;35:2447 –2450.
- Nayak AU, Holland MR, Macdonald DR, Nevill A, Singh Evidence for consistency of the glycation gap in diabetes. Diabetes Care. 2011;34(8):1712-6.
- Sacks DB, Nathan DM, Lachin Gaps in the glycation gap hypothesis. Clin Chem 2011;57:150–152.
- Kilpatrick ES, Rigby AS, Atkin Variability in the Relationship between Mean Plasma Glucose and HbA1c: Implications for the Assessment of Glycemic Control. Clin Chem. 2007;53(5):897-901
- Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C andNAD(P)H-oxidase Diabetes 2003;52(11):2795-2804.
- Inhat M, Green D, Ross K, et al. Reduced antioxidant response of retinal and endothelial cells inresponse to chronic oscillating glucose Diabetes 2005;54(Suppl. 1):2314A.
- Radin Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014 Feb;29(2):388-94.
- Parrinello CM, Selvin Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep. 2014;14(11):548.
- Danese E, Montagnana M, Nouvenne A, Lippi Advantages and pitfalls of fructosamine and glycatedalbumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9(2):169-76.
- Herman WH, Cohen Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97(4):1067-72.
- Bergenstal RM, Gal RL, Connor CG, Gubitosi-Klug R, Kruger D, Olson BA, Willi SM, Aleppo G, Weinstock RS, Wood J, Rickels M, DiMeglio LA, Bethin KE, Marcovina S, Tassopoulos A, Lee S, Massaro E, Bzdick S, Ichihara B, Markmann E, McGuigan P, Woerner S, Ecker M, Beck RW; T1D Exchange RacialDifferences Study Racial Differences in the Relationship of Glucose Concentrations and HemoglobinA1c Levels. Ann Intern Med. 2017;167(2):95-102.
- Bower JK, Brancati FL, Selvin E. No ethnic differences in the association of glycated hemoglobin withretinopathy: the national health and nutrition examination survey 2005- 2008. Diabetes Care. 2013;36(3):569-73.
- Parrinello CM, Sharrett AR, Maruthur NM, Bergenstal RM, Grams ME, Coresh J, Selvin Racial Differences in and Prognostic Value of Biomarkers of Hyperglycemia. Diabetes Care. 2016;39(4):589-95.
- Lindsey CC, Carter AW, Mangum S, Greene D, Richardson A, Brown SJ, Essary JL, McCandless Aprospective, randomized, multicentered controlled trial to compare the annual glycemic and quality outcomes of patients with diabetes mellitus monitored with weekly fructosamine testing versus usual care. Diabetes TechnolTher. 2004;6(3):370-7.
- Edelman SV, Bell JM, Serrano RB, Kelemen Home testing of fructosamine improves glycemic control in patients with diabetes. Endocr Pract. 2001;7(6):454-8.
- Shohat N, Tarabichi M, Tischler EH, Jabbour S, Parvizi Serum Fructosamine: A Simple and Inexpensive Test for Assessing Preoperative Glycemic Control. J Bone Joint Surg Am. 2017;99(22):1900-1907.
- Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J. Fructosamine and glycatedalbumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2(4):279-88.
- Jung M, Warren B, Grams M, Kwong YD, Shafi T, Coresh J, Rebholz CM, Selvin E. Performance of non-traditional hyperglycemia biomarkers by chronic kidney disease status in older adults with diabetes: Resultsfrom the Atherosclerosis Risk in Communities Study. J Diabetes. 2018;10(4):276-285.
- Selvin E, Rawlings AM, Lutsey PL, Maruthur N, Pankow JS, Steffes M, Coresh J. Fructosamine andGlycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation. 2015;132(4):269-77.
- Nathan DM, McGee P, Steffes MW, Lachin JM; DCCT/EDIC Research Group. Relationship of glycatedalbumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63(1):282-90.
- Zhu J, Chen Y, Li C, Tao M, Teng The diagnostic value of glycated albumin in gestational diabetes mellitus. J Endocrinol Invest. 2018;41(1):121-128.
- Shimizu I, Hiramatsu Y, Omori Y, Nakabayashi M. Comparison of HbA1c and glycated albumin as acontrol marker for newborn complications in diabetic women in a multicentre study in Japan (Japan glycated albumin study group: study 2). Ann Clin Biochem. 2018;55(6):639-646.
- Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5- anhydroglucitol andpostprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29:1214-1219.
- Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin Risk Factors for Severe Hypoglycemia in Black and White Adults With Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2017;40(12):1661-1667.
- Rebholz CM, Grams ME, Chen Y, Gross AL, Sang Y, Coresh J, Selvin Serum Levels of 1,5-Anhydroglucitol and Risk of Incident End-Stage Renal Disease. Am J Epidemiol. 2017;186(8):952-960.
- Rawlings AM, Sharrett AR, Mosley TH, Ballew SH, Deal JA, Selvin Glucose Peaks and the Risk ofDementia and 20-Year Cognitive Decline. Diabetes Care. 2017;40(7):879-886.
- Selvin E, Rawlings A, Lutsey P, Maruthur N, Pankow JS, Steffes M, Coresh Association of 1,5-Anhydroglucitol With Cardiovascular Disease and Mortality. Diabetes. 2016;65(1):201-8.