ABSTRACT
Despite the health consequences of osteoporosis and the availability of effective treatments, it is under-diagnosed and under-treated. For example, although 90% of patients with hip fractures have osteoporosis, in 2007 only 20% of patients with fragility fractures were evaluated and treated. In a retrospective study of patients with hip fractures, less than 15% of subjects were diagnosed and less than 13% were treated with medications for osteoporosis, including calcium and vitamin D. Fracture patients require evaluation of secondary causes and treatment of osteoporosis to help prevent subsequent fractures. The preceding chapters summarize the pathogenesis and the clinical evaluation of osteoporosis. This chapter will review established therapeutic options and new approaches for the prevention and treatment of osteoporosis. Strategies include both lifestyle and medical approaches to enhance bone strength.
INTRODUCTION
Osteoporosis is a major growing global health problem, resulting in 200 million osteoporotic fractures worldwide each year (1,2). Characterized by reduced bone mass and architectural deterioration, it leads to an increased risk of fragility fractures often occurring with minimal trauma such as falling from a standing height. These fractures rise exponentially with advancing age and most commonly involve the spine, hip or distal forearm. An estimated 1 in 2 women and 1 in 4 men aged 50 years and older will suffer a fragility fracture in their remaining lifetime. Hip fractures are the most serious of these fractures, given the high rates of morbidity and mortality. Approximately 50% of patients who sustain a hip fracture lose the ability to walk independently and 12-24% of women suffering a hip fracture die within the 1st year, compared to 33% of men (3-5). Vertebral compression fractures are the most common osteoporotic fractures, but they are often asymptomatic and found incidentally on imaging done for other reasons. Vertebral fractures are, however, associated with high rates of morbidity involving height loss, kyphosis, restrictive lung disease, back pain, and functional impairment. Vertebral fractures are associated with a 5-fold increased risk of future vertebral fractures and a 2 to 3-fold risk of other fragility fractures. Although there are very effective treatments to reduce fracture risk, only 30% of patients with fragility fractures have a bone density test and/or are treated for their underlying osteoporosis. There are currently critically needed national and international efforts to improve fracture care and bone health in women and men. Identification of osteoporosis at the time of a hip, spine, or other fragility fracture is imperative so that patients with fragility fractures can be evaluated for secondary causes of osteoporosis and treated with osteoporosis medications for their underlying bone disease.
The preceding chapters summarize the pathogenesis and the clinical evaluation of osteoporosis. This chapter will focus on reviewing established therapeutic options and new approaches for the prevention and treatment of osteoporosis. Strategies include both lifestyle and medical approaches to enhance bone strength and reduce fractures.
PATHOPHYSIOLOGY
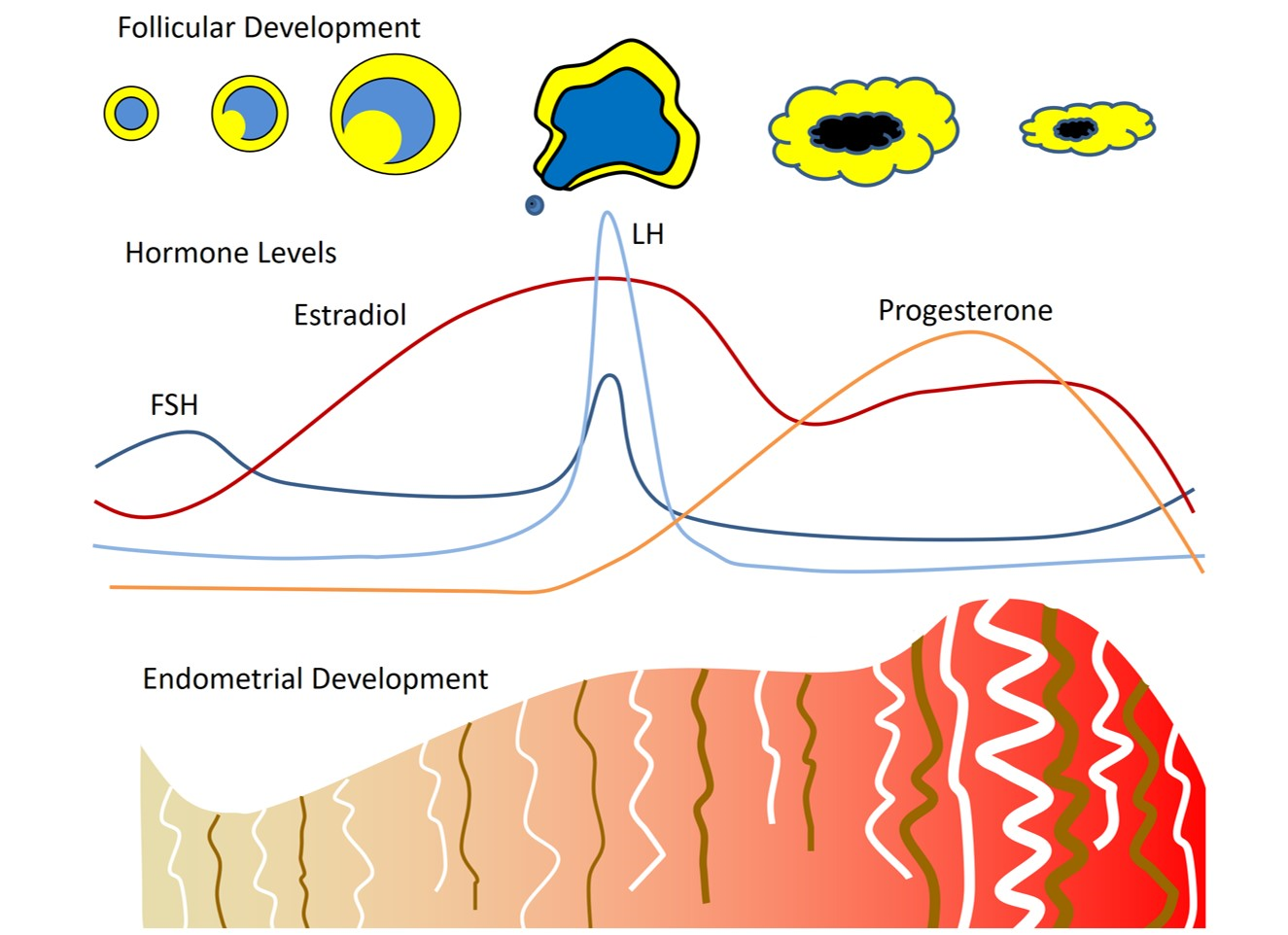
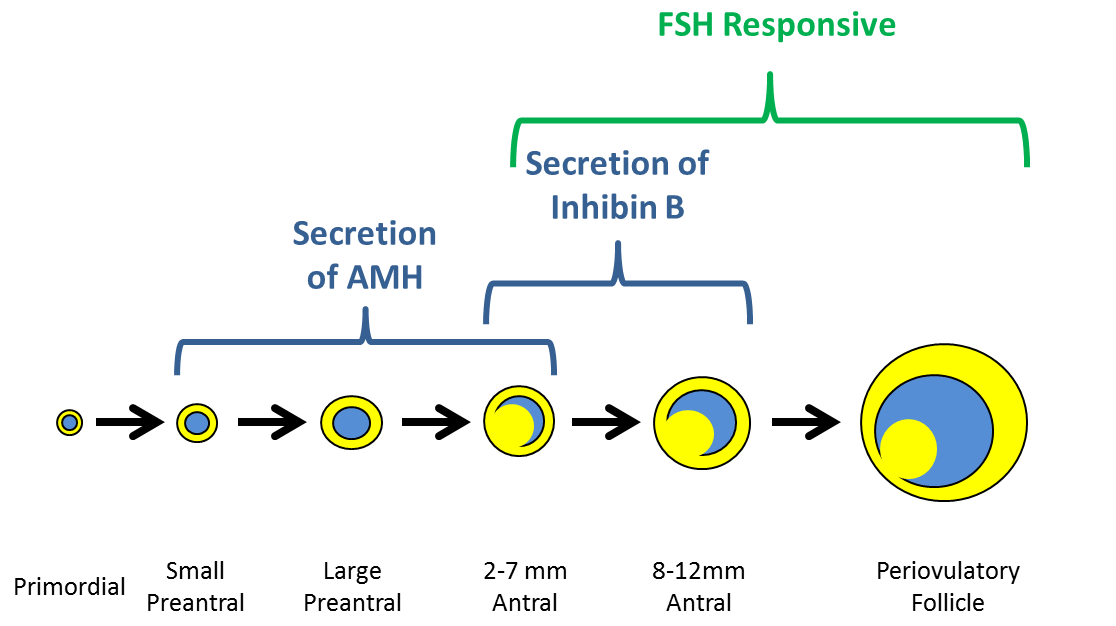
Bone is a dynamic organ with continuous remodeling occurring as osteoclasts resorb bone and osteoblasts form new bone. Among the key regulators of this process is the receptor activator of nuclear factor-kappa B (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) system. Interaction between RANKL, produced by the osteoblast lineage, and RANK receptor stimulates osteoclastic differentiation and activity; OPG, made by osteoblasts, is an endogenous decoy receptor that binds with RANKL, inhibiting bone resorption. In addition, the Wnt signaling pathway is involved in activation of transcription of genes that direct the differentiation and proliferation of osteoblasts. In the skeletal life cycle, there is acquisition of peak bone mass during adolescence and young adulthood. For women, bone loss is accelerated surrounding the time of menopause with decreases in bone mineral density (BMD) of approximately 2-3%/year. With advancing age, the decline in BMD occurs at a slower rate of approximately 0.1 to 0.5% per year in women and men.
DIAGNOSIS
BMD testing is typically measured in the proximal femur and lumbar spine, though the distal radius should be measured in patients with hyperparathyroidism or in those in whom the other major sites cannot be adequately assessed. Each SD below peak bone mass represents approximately 2-fold increase in fracture risk. Osteopenia is present when the BMD is between 1.0 and 2.5 SDs below bone density of young healthy individuals. More than 50% of fragility fractures occur in these patients (6). Osteoporosis is defined as a BMD≤-2.5 SDs of young normal, healthy individuals.
Vertebral imaging by DXA or X-ray is useful for identification of spinal fractures that frequently are not clinically evident. The Bone Health and Osteoporosis Foundation (BHOF, previously the National Osteoporosis Foundation) currently recommends DXA for women ≥65 years and men ≥70 years, or earlier if clinical risk factors are present. Physicians should routinely perform height measurements preferably with a stadiometer as there is an association between height loss and spinal fractures. The BHOF Clinical Guide recommends vertebral imaging for spinal fractures in the presence of height loss of 1.5 inches or more and longitudinal height loss of 0.8 inches or more for postmenopausal women and men age 50-69. Vertebral imaging is also recommended in women and men age 70 and 80 years and older, respectively (7). When the diagnosis of a low bone density compared with age-adjusted controls or osteoporosis is made, a work-up to look for secondary causes of osteoporosis should be considered. See Table 1.
|
Table 1. Secondary Causes of Osteoporosis
|
|
Endocrinological Abnormalities
|
Glucocorticoid excess, hyperthyroidism, hypogonadism (androgen insensitivity, Turner’s and Klinefelter’s Syndrome, hyperprolactinemia, premature menopause), anorexia, athlete triad, vitamin D deficiency, hyperparathyroidism, diabetes mellitus (Types 1 and 2)
|
|
Cardiovascular, Renal, Pulmonary and Miscellaneous Disorders
|
Chronic kidney disease, post-transplant bone disease, congestive heart failure, chronic obstructive lung disease, AIDS/HIV
|
|
Connective Tissue Disorders
|
Osteogenesis imperfecta, Ehlers-Danlos syndrome, Marfan Syndrome, ankylosing spondylitis,
|
|
Gastrointestinal Diseases
|
Celiac sprue, Inflammatory bowel disease, post-gastrectomy, primary biliary cirrhosis, bariatric surgery
|
|
Hematological Disorders
|
Multiple myeloma, mastocytosis, leukemia, hemophilia, sickle cell disease, leukemia, lymphoma, thalassemia
|
|
Other Genetic Disorders
|
Homocystinuria, cystic fibrosis, hemochromatosis, hypophosphatasia
|
|
Rheumatological Disorders
|
Ankylosing spondylitis, rheumatoid arthritis
|
|
Medications
|
Aromatase inhibitors, heparin (long term), anticonvulsants, methotrexate, cytoxan, gonadotropin-releasing hormone (GnRH) agonists and antagonists, tamoxifen (in premenopausal women), excess thyroid hormone, lithium, cyclosporine A, tacrolimus, glucocorticoids, thiazolidinediones, depo-medroxyprogesterone (premenopausal women) proton-pump inhibitors, selective serotonin reuptake inhibitors (SSRIs), tenofovir
|
Laboratory evaluation may include the following: Calcium, phosphorus, liver function tests (including alkaline phosphatase), complete blood count, 25-hydroxyvitamin D, 24-hour urine calcium +/- PTH, TSH (if clinical evidence of hyperthyroidism or those already on thyroid hormone replacement), and serum testosterone in men. For select cases, one may consider sending specialized tests for gastrointestinal disorders (tissue transglutaminase with an IgA level for celiac sprue), infiltrative diseases (serum tryptase for mastocytosis), neoplastic (serum and urine protein electrophoresis), or excess glucocorticoid (cortisol levels, dexamethasone suppression test for Cushing’s syndrome).
To quantify an individual’s absolute fracture risk, the World Health Organization (WHO) developed the FRAX® calculator (http://www.shef.ac.uk/FRAX), an integrative measure of various risk factors and femoral neck bone mineral density. In addition to BMD, the following risk factors are included - ethnicity, age, BMI, prior fracture history (designated as a previous fracture in adult life that occurred spontaneously or a fracture arising from trauma, which in a healthy individual would not have resulted in a fracture), glucocorticoid use, excessive alcohol (≥3 units per day), smoking, rheumatoid arthritis, and certain secondary causes of osteoporosis. These secondary causes include Type 1 diabetes, osteogenesis imperfecta, long-standing hyperthyroidism, hypogonadism, premature menopause, malnutrition, malabsorption, or liver disease. If the 10-year absolute fracture risk is ≥3% for hip fractures or ≥20% for other major osteoporotic fractures, pharmacologic therapy should be considered (7). The FRAX® calculator should be utilized in postmenopausal woman ≥ 40 years and men ≥ 50 years with osteopenia. Although there are data analyzing the use of FRAX® in patients who have been treated with osteoporosis medications, its use is not currently validated for patients currently or formerly treated with pharmacotherapy for osteoporosis. Additionally, the FDA has approved the use of trabecular bone score (TBS), a structural measure derived from spinal bone density images that is associated with bone microarchitecture and fracture risk. Combining TBS and the FRAX score may increased the predictive value of the absolute fracture risk assessment (8).
Although the FRAX® calculator has greatly enhanced treatment of osteopenic women and men at risk for fractures, certain risk factors predictive of fracture risk are not accurately measured in this calculator. Patients on chronic glucocorticoids may warrant treatment earlier or at a lower threshold than determined by FRAX®; further, this tool does not include current or cumulative glucocorticoid doses or duration of treatment (9). Also, of note, spine BMD is not included in the algorithm. Once an initial bone density is measured, a follow-up BMD should be done 1-2 years after the initial screening depending on whether pharmacologic therapy was initiated. Biochemical bone turnover markers, collagen breakdown products, (e.g., N-telopeptide, C-telopeptide) may be helpful in select patients as an indicator of skeletal remodeling or to determine patient’s adherence to treatment.
EXERCISE
While pharmacological therapies are a major focus of this chapter, exercise and strategies to strengthen muscles and prevent falls are important components of osteoporosis care. Skeletal loading and mechanical loads from muscle forces have important effects on bone strength (10). Meta-analyses and clinical investigations have shown that exercise produces modest increases in BMD often ranging between 1% and 3% (11). Physical activity helps to maximize BMD during adolescence and young adulthood, diminish bone loss during aging, and improve stability and strength to minimize falls and fractures in the elderly (11-14). However, these benefits come from slow skeletal adaptations to training over time. Because it takes three to four months to complete the bone remodeling cycle of bone resorption, formation, and mineralization, a minimum of at least six to eight months of an exercise intervention is likely required to achieve a change in bone mass that is quantifiable (15,16). The benefits of exercise are lost when people stop exercising, therefore lifelong physical activity at all ages is strongly endorsed by the BHOF. Exercise recommendations generally should include weight-bearing, muscle-strengthening, and balance training exercises for 30 minutes 5 days per week or 75 minutes twice weekly, often consistent with other general health recommendations. Weight-bearing exercises are activities that make the body move against gravity such as walking, jogging, dancing, tennis, and Tai Chi. To protect the spine in patients with low spinal bone density, maintaining a straight spine and avoiding arching and twisting are generally recommended.
CALCIUM
Adequate calcium intake is essential to prevent calcium mobilization from the bone where 99% of calcium is stored. The effects of calcium supplementation on bone depend on age, menopausal status, calcium intake, and vitamin D sufficiency. Increased calcium intake is necessary during acquisition of peak bone mass and with advancing age. Calcium has modest effects on bone density (17). It is ineffective or minimally effective for prevention of bone loss in women within five years of menopause when there may be predominant effects of estrogen deficiency and other hormonal changes.
The Institute of Medicine's recommendations for daily calcium intake that meet the requirements of 97% of the population are shown in Table 2 (18). Unless a patient has an underlying disorder of calcium homeostasis, the upper limit of safety is considered 2,500 mg for adults aged 19 to 50 years and 2,000 mg for those >50 years (19). As maximum absorption of elemental calcium is about 500 to 600 mg at once, calcium intakes need to be divided into multiple doses throughout the day.
|
Table 2. Recommended Daily Elemental Calcium Intake (Adapted from 2011 IOM Report)
|
|
9-18 years
Lactating Women
|
1,300 mg
|
|
Women 19-50 years, Men 19-70 years
|
1,000 mg
|
|
Women > 50 years, Men > 70 years
|
1,200 mg
|
Obtaining calcium through the diet is preferred. While dairy products contain the largest amount of endogenous calcium, many foods including juices, cereals, and cereal bars, may contain added calcium. An 8-ounce glass of milk or calcium-supplemented orange juice contains ~300 mg of elemental calcium, calcium-supplemented soy and almond milk contains ~450 mg, one ounce (or 1 cubic inch) of cheese contains ~200 mg, and certain cereals contain as much as 1000 mg. It is important for physicians to calculate the dietary calcium intake. Resources helpful for patients to calculate their calcium intake include the U.S. dairy council of California website, http://www.healthyeating.org/Healthy-Eating/Healthy-Eating-Tools/Calcium-Quiz.aspx?action=quiz, the International Osteoporosis Foundation website, https://www.iofbonehealth.org/calcium-calculator, and the NOF Clinical Guide also available on the website https://link.springer.com/article/10.1007/s00198-021-05900-y (7). The former allows patients to check off the type and quantity of calcium-containing foods they usually consume and then calculates total daily calcium, with suggestions on how to increase calcium intake to recommended levels. The latter provides an easy tool to calculate calcium intakes from calcium-rich, dietary sources.
Supplemental calcium should be used if an individual’s dietary calcium intake does not meet the recommended daily calcium intake. Calcium carbonate contains 40% of elemental calcium and is a commonly used calcium supplement (e.g., Tums™, Oscal™, Caltrate™, and generic preparations). Calcium carbonate should be taken with food because patients with achlorhydria (or those on proton pump inhibitors chronically) cannot absorb this calcium salt well on an empty stomach (20). Adverse effects of calcium carbonate may include bloating and constipation. Calcium citrate (e.g., Citracal™), which contains 24% elemental calcium, is more bioavailable than calcium carbonate, can be taken while fasting and as a result is the formulation suggested when patients are on proton pump inhibitors chronically.
There have been a number of concerns related to the use of supplemental calcium and the risk of kidney stones and cardiovascular disease. Data from epidemiologic research and clinical trials suggest that vitamin D reduces the incidence of fractures and may also prevent falls and declining physical function, yet the available data are not consistent (21). Data from the Women’s Health Initiative (WHI) calcium and vitamin D clinical trial (CT) of supplemental calcium (1000 mg daily) plus vitamin D (400 IU daily) versus placebo in 36,282 women showed a 17% increased risk of developing renal stones in those assigned calcium plus vitamin D. However, among those compliant with the calcium plus vitamin D regimen versus placebo, there was a 29% reduced risk of hip fracture over seven years (22). Some evidence suggests that calcium supplements but generally not dietary calcium may be associated with vascular calcifications and an increased risk for myocardial infarction (23). In a prospective study in the National Institutes of Health AARP Diet and Health Study of 388,229 women and men in whom baseline calcium intakes were ascertained after an average of 12 years of follow-up, supplemental but not dietary calcium intakes were associated with excess cardiovascular death in men but not women; adverse cardiovascular effects were only observed among smokers (23) (24). An analysis of the WHI randomized placebo-controlled calcium and vitamin D trial (CT) and the WHI prospective observational study (OS) showed that in the CT, in postmenopausal women who did not take supplemental calcium and vitamin D at baseline, supplemental calcium (1000 mg/day) and vitamin D (400 IU/D) versus placebo for ≥ 5years was associated with a 38% reduction in the risk of hip fracture. In a combined analysis of data from the CT and OS, supplemental calcium and vitamin D reduced the risk of a hip fracture by 35%. In these subset analyses of the large WHI, it is important to note that there were no adverse effects of supplemental calcium plus vitamin D on risks of myocardial infarction, stroke, or other cardiovascular disease (25). Although additional analyses are ongoing, calcium intakes within the ranges recommended by the IOM appear not to increase cardiovascular risk.
Recently, however, the United States Preventive Services Task Force (USPSTF) recommended against supplemental calcium (≤1000 mg/day) and low-dose vitamin D (≤400 IU/D) in healthy postmenopausal women due to lack of evidence of benefit in fracture reduction and evidence for increased risk of kidney stones. Thus, the risk of renal stones with calcium supplementation needs to be balanced with fracture reduction. These recommendations did not apply to adults with osteoporosis or vitamin D deficiency (22,26).
VITAMIN D
Vitamin D insufficiency and deficiency is a common problem in many individuals. Individuals at increased risk for low vitamin D levels include the elderly and those with low vitamin D intake, malabsorption, inadequate sunlight exposure, use of sunblock, dark skin pigment, obesity, chronic kidney disease, and use of medications that increase the metabolism of vitamin D. Vitamin D deficiency and insufficiency are common in adults with hip fractures (30,31). Vitamin D deficiency can lead to reduced calcium absorption, secondary hyperparathyroidism, and increased risk of fractures (30,32-34). Mild vitamin D insufficiency may not cause symptoms, but contributes to low bone mass. Severe vitamin D deficiency causes osteomalacia. In addition, although more data are needed, vitamin D deficiency has been associated with proximal muscle weakness, impaired physical performance, increased risk of falls, and possibly increased risks of some cancers (including colorectal, breast among others) (19,35-41). Deficient levels of vitamin D are generally defined as a 25-(OH) vitamin D <20 ng/ml, relative insufficiency as 21 to 29 ng/ml, and sufficient levels of vitamin D to prevent the rise in parathyroid hormone levels as a 25-(OH) vitamin D ≥ 30 to 32 ng/ml (42). The National Health and Nutrition Examination Survey (NHANES) report showed that 32% of Americans have vitamin D deficiency (43).
Sources of dietary intake of vitamin D are limited and these include vitamin D-fortified milk and some soy milks (100 IU/glass), certain cereals, egg yolk, and oily fishes (e.g., salmon, mackerel, and sardines). Multivitamins typically contain 400 IU to 1,000 IU of vitamin D3, and many calcium preparations are supplemented with vitamin D. The NOF recommends 800 to 1000 IU vitamin D daily for adults aged 50 years and older, as do the International Osteoporosis Foundation and Endocrine Society (44,45). The IOM Committee report on the Dietary Reference Intakes for 97.5% of the population in North America was 600 IU/d of vitamin D for children and adults until age 70 and 800 IU/day for adults 71 years and older (46).
The USPTF recommended supplemental vitamin D for reduction in fall risk in women aged 65 and older. Although a meta-analysis of 31,022 individuals indicated that the highest quartile of vitamin D intakes (median 800 IU (and range 792 to 2000 IU/d) was associated with a 30% and 14% reduction in the risks of hip fractures and non-vertebral fractures, respectively, the USPSTF reported that recommendations concerning the safety and efficacy of higher doses of vitamin D on fracture reduction await additional research (26,27).
In the Vitamin D and Omega-3 Fatty Acid trial, a large randomized, placebo-controlled trial in 25,874 women and men across the United States of the effects of supplemental 2000 IU/d of cholecalciferol versus placebo determined the effect on the primary prevention of cardiovascular, fractures, cancer and other health outcomes. In addition, detailed in-person visits in a sub cohort provide extensive information on effects of supplemental vitamin D and/or omega-3 fatty acids on cardiovascular outcomes, bone health and many other clinical outcomes (28,29). This study found that in general, in a healthy population not preselected for low vitamin D levels or osteoporosis, supplemental vitamin D had no effect on bone density or bone structural measures or incident falls or fractures (194,195,196).
Patients with vitamin D deficiency need much higher doses. The upper limit of safety for vitamin D is 4000 IU/day. There are currently differing recommendations regarding the optimal 25-hydroxyvitamin D (25-OHD) level for bone health with the IOM committee recommending a 25-OH D level ≥20-29 ng/mL while several other societies recommend a 25-OHD level ≥30 ng/mL (44,45).
In the presence of vitamin D deficiency, it is safe to normalize vitamin D levels to a 25-(OH)D level of 30 ng/ml to prevent the compensatory rise in parathyroid hormone (PTH) level (33,47). This may be done in a variety of ways. High doses of vitamin D may be needed [e.g., 50,000 IU of D2 (ergocalciferol) or equivalent dose of D3(cholecalciferol) weekly for 8 weeks or according to the 25-hydroxyvitamin D level] (45). Individuals with malabsorption often require very high doses of supplemental vitamin D, and may benefit from evaluation by a bone specialist.
TREATMENT AND/OR PREVENTION OF OSTEOPOROSIS
There are effective therapies for osteoporosis and promising therapeutics under development. The antiresorptive therapies that reduce bone turnover include: bisphosphonates; estrogen or hormone therapy, estrogen agonists/antagonists [selective estrogen-receptor modulators (SERMs)]; calcitonin; and denosumab, a human monoclonal antibody to RANK-ligand. At present there are two FDA-approved anabolic or bone forming osteoporosis therapy, teriparatide [PTH (1-34)] and abaloparatide. Romosozumab is a monoclonal antibody to sclerostin and stimulates bone formation and inhibits bone resorption. In selection of the optimal therapy for a given individual, it is important to consider patient preference, cost, mode of administration, duration of treatment, and the effects of a treatment on reduction of spine, hip and other non-spine fractures. Tables 4 and 5 lists the currently available osteoporosis drugs approved by the FDA, their dosage, indication, and general efficacy for fracture reduction.
HORMONE REPLACEMENT THERAPY
In postmenopausal women, it is well known that estrogen therapy (ET) and hormone therapy [estrogen plus progesterone (HT)] prevent bone loss and increase BMD through interaction with estrogen receptors on bone cells, activation of tissue-specific genes and proteins, and/or a reduction in cytokines that stimulate osteoclast function (51-54). In addition to the bone density benefit, the Women’s Health Initiative (WHI) did show that HT resulted in a 34% reduction in the risk of hip fractures and clinical spine fractures (55). However, the risks – increases in breast cancer, coronary heart disease (CHD), pulmonary embolism (PE), and stroke, outweighed the benefits. In addition, after cessation of ET or HT, the benefit of fracture reduction is not sustained (56,57). Although data from the WHI show that ET and HT reduce fractures, ET and HT are FDA-approved for the prevention of fractures but not for the treatment of osteoporosis (55,58).
Data has shown potential cardiovascular safety with use of ET in early menopause, though this remains controversial (the “critical window” hypothesis) (59-61).
Unlike oral estrogens, in postmenopausal women transdermal estrogens do not adversely affect clotting factors, and are therefore preferred. Transdermal estrogens prevent bone loss and are available in low doses (e.g., 0.014 to 0.0375 mg daily patch applied 2x/week). In women with premature or early menopause, hormone replacement can be considered until the natural age of menopause (51.3 years) (62). Before estrogen is prescribed, the benefits versus the risks of cardiovascular disease, stroke, and breast cancer should be reviewed. When prescribing estrogen, the FDA recommends the following: consider all non-estrogen preparations first for osteoporosis prevention; use the lowest dose of HT/ET for the shortest time interval to achieve therapeutic goals; and prescribe HT/ET when benefits outweigh risks in a given woman.
Estrogen Agonist/Antagonists
Estrogen Selective agonists/antagonists previously classified as selective estrogen receptor modulators (SERMs) are a class of drugs that bind to estrogen receptors and can selectively function as agonists or antagonists in different tissues. Raloxifene (Evista™) is Food and Drug Administration (FDA) approved for the prevention and treatment of osteoporosis. Raloxifene was also approved by the FDA in 2007 for reduction in the risk of invasive breast cancer in post-menopausal women with osteoporosis and postmenopausal women at high risk for invasive breast cancer. The Multiple Outcomes of Raloxifene Evaluation (MORE) study was a randomized clinical trial of the effects of raloxifene versus placebo on bone density and fractures in 7,705 postmenopausal women (mean age of 67 years) with osteoporosis. Compared with placebo, raloxifene treatment for three years increased BMD of the spine by 2.6% and of the femoral neck by 2.1%. Over three years, raloxifene reduced spine fractures by 55% in women without prevalent vertebral fractures and by 30% in women with more than one prevalent vertebral fracture (63). Raloxifene therapy did not lead to a reduction in hip or wrist fractures, which was further confirmed in the Continuing Outcomes Relevant to Evista (CORE) trial (64). Additional benefits of raloxifene include the reduction in invasive breast cancer risk and mild decreases in LDL-cholesterol, with no effect on the risk of cardiovascular disease.
The side effects of raloxifene include an increase in deep venous thrombosis similar to use of estrogen, along with a small increase in hot flashes and leg cramps, and a small increased risk of fatal stroke in the Raloxifene Use for the Heart (RUTH trial).
Tamoxifen, a SERM used for the prevention and treatment of estrogen receptor-positive breast cancer, has estrogen-like effects in bone. It also stimulates the endometrium and can result in uterine hyperplasia or malignancy (65). Bazedoxifene, lasofoxifene, and arzoxifene are third-generation SERMs, none of which appear to cause endometrial hyperplasia (66,67). In a study of 7492 postmenopausal women with osteoporosis, women who received bazedoxifene (20 mg or 40 mg daily) compared with placebo had a lower incidence of new vertebral fractures, but not non-spine fractures (68). In a 7-year phase III, placebo-controlled study of 7492 women with osteoporosis , bazodoxifene versus placebo resulted in a 36.5% (40 mg daily dose) and 30.4% (20 mg daily dose) reduction in morphogenic spine fractures and no effect of overall incidence of nonvertebral fractures (69). In October, 2013, a combination of conjugated estrogens plus bazedoxifene (DuaveeTM) was FDA-approved for the treatment of moderate-severe vasomotor symptoms related to menopause and to prevent osteoporosis after menopause.
At present raloxifene and bazodoxifene, are the only estrogen agonist/antagonist that are FDA-approved for prevention (raloxifene and bazodoxifene) and treatment (raloxifene only) of osteoporosis.
|
Table 4. Effects of FDA-Approved Hormonal Osteoporosis Therapies on Fractures
|
|
Drug
|
Most Common Dosage
|
Fracture Risk Reduction
|
FDA Indications*
|
|
Estrogen Therapy (ET) Hormone Therapy (HT)
|
Many oral and transdermal preparations
|
Spine, total hip
|
PMO-Prevention
|
|
Selective Estrogen Receptor Modulators
Raloxifene
|
60 mg PO once daily
|
Spine
|
PMO - Prevention & Treatment; Reduce risk of invasive breast cancer in patients with osteoporosis and increased risk of breast cancer.
|
|
Basodoxifene + conjugated estrogens
|
20 mg/0.45 mg PO once daily
|
Spine
|
PMO- Prevention
|
PMO: postmenopausal osteoporosis; GIO: Glucocorticoid-induced osteoporosis
CALCITONIN
Calcitonin is a 32-amino acid peptide produced by the parafollicular cells of the thyroid that inhibits bone resorption through direct effects on the osteoclasts. Calcitonin is a highly conserved protein, with human and salmon calcitonin differing by only one amino acid. Injectable salmon calcitonin was approved by the FDA in 1984 for the treatment of osteoporosis, although current use is limited because of the availability of other more effective medications for the treatment of osteoporosis. Calcitonin nasal spray (Miacalcin™ and Fortical™ 200 IU daily) is a form of calcitonin (70) approved by the FDA for the treatment of osteoporosis in women more than five years past menopause. Although studies have shown calcitonin nasal spray to decrease spine fractures, there is no effect on the prevention of hip and other non-spine fractures. Current and future use of calcitonin for osteoporosis has been limited, however, because of data analyses showing a potential increased risk of cancers, particularly liver cancer with calcitonin use, though this remains controversial (71). An FDA review found no causal relationship between calcitonin use and cancer but cautioned that physicians should evaluate the potential benefit to relative risk of calcitonin use in patients.
BISPHOSPHONATES
Bisphosphonates are analogs of pyrophosphate that inhibit bone turnover and because of their phosphorous-carbon-phosphorous structure are resistant to hydrolysis. They have a strong affinity for calcium crystals and bind avidly to the surface of bone. Bisphosphonates suppress bone resorption and interrupt osteoclast activity directly through several mechanisms including inhibition of acid production, lysosomal enzymes, and the mevalonate pathway (72-74) and indirectly through their effects on osteoblasts and macrophages. They also inhibit osteoclast recruitment and induce osteoclast apoptosis. Thus, through various mechanisms, bisphosphonates reduce the depth of resorption pits (thereby producing positive bone balance at individual bone remodeling units) and decrease the formation of new bone remodeling units.
Pharmacodynamics
Oral bisphosphonates are poorly absorbed. Less than 3% is absorbed in the fasting state, and absorption is significantly reduced if these drugs are taken with food, calcium, or beverages other than water. The skeleton rapidly takes up approximately half of the absorbed bisphosphonate, and the remainder is excreted unchanged by the kidney within hours. The drug remains at the bone surface for several weeks before becoming embedded in bone, where it is biologically inert. The embedded drug then remains in bone for many years and is slowly released, although the skeletal retention varies among bisphosphonates. Potency and side effects of the bisphosphonates vary according to the side chains (75,76).
Effective Therapies for Osteoporosis
Alendronate (Fosamax™), risedronate (Actonel™, Atelvia™), ibandronate (Boniva™), and zoledronic acid (Reclast™) are all FDA approved for osteoporosis prevention and/or treatment. Their indication and specific fracture benefits on fracture reduction are shown in Table 5. It is important to select an osteoporosis medication that reduces spine, hip and non-spine fractures, especially in high-risk individuals. Since around 50% of patients discontinue bisphosphonates within 1 year of treatment, it is essential to review compliance and adherence with patients. Of the approved bisphosphonates, Alendronate, Risedronate, and Zoledronic acid are now generic, making them affordable options for patients.
ALENDRONATE
Several longitudinal studies have shown that oral alendronate increases BMD and decreases the risk of osteoporotic fractures, and can be used for primary and secondary prevention
In a meta-analysis of randomized controlled trials published between 1966 and 2007, the efficacy of alendronate in the primary and secondary prevention of osteoporotic fractures in postmenopausal women was evaluated (77). Eleven studies were selected, including three primary prevention studies (78-80) and eight secondary prevention studies involving women with low BMD on DXA and/or high prevalence of vertebral fracture (81-88). A total of 12,068 women received at least one year of oral alendronate (6543 women) or placebo (5525 women). Three trials, including the largest secondary prevention trial, Fracture Intervention Trial (FIT), used an initial daily dose of 5 mg and then switched to 10 mg for the remaining study duration. Other studies used 5 mg, 10 mg, or 20 mg of alendronate daily. The length of follow-up ranged from one to four years, and the mean ages were 53 to 78 years. With alendronate 10 mg daily for secondary prevention, there was a significant 45% relative risk reduction (RRR) in vertebral fractures, 23% RRR in non-vertebral fractures, and 53% RRR in hip fractures. For primary prevention, the RRR was only significant for vertebral fractures (45%). No statistically significant differences in adverse events were found in any included study.
The prevalence of osteoporosis is lower in men than in women. It is estimated that one out of two women and one out of four men over age 50 will develop an osteoporotic fracture (89). Several longitudinal studies have evaluated the efficacy of treatment interventions on bone in osteoporotic men. Orwoll et al. enrolled 241 men with a femoral neck T score of ≤ -2 with a lumbar spine T score ≤ -1 or a history of osteoporotic fracture and a femoral neck T score ≤ -1. Compared with placebo, alendronate significantly increased BMD at each site and decreased markers of bone turnover over two years. From baseline, alendronate increased BMD by 3.1% in the total hip and by 7.1% in the lumbar spine and decreased urinary N-telopeptides by 59% and bone-specific alkaline phosphatase by 38%. The incidence of vertebral fractures was 7.1% in the placebo group versus 0.8% in the alendronate group; there was insufficient power to assess the effects of alendronate on non-vertebral fractures (90). Similar results were seen in a smaller study of hypogonadism-induced osteoporosis, indicating no difference in the skeletal response to alendronate in the presence of hypogonadism.
Alendronate is also effective in the treatment of glucocorticoid-induced osteoporosis. In glucocorticoid-treated men and women, alendronate resulted in increases in BMD (91,92) and decreases in incidence of radiographic vertebral fractures at two years (6.8% vs. 0.7%) (92).
Data show that weekly alendronate (70 mg) is effective and well tolerated, and this dosage has become the standard of care for use of this oral bisphosphonate. Alendronate is suitable for weekly dosing because of its long skeletal retention. It is often the first line treatment that is cost-effective as a generic preparation.
Long-term treatment with alendronate has beneficial effects on BMD. Bone et al. showed that spine BMD continued to rise in small increments during 10 years of treatment. Femoral neck and trochanter BMD increased during the first three years and then remained stable (93,94).
In an extension of FIT, the FIT Long-term Extension (FLEX) trial, 1099 women who had received alendronate (5 mg daily for two years and 10 mg daily thereafter) were again randomized to receive either 5 or 10 mg alendronate daily or placebo for five more years. With a pooled analysis of the alendronate doses, after five years, the alendronate-treated subjects had significantly better BMD changes at the total hip, femoral neck, lumbar spine, total body, and forearm. These changes included less loss of BMD at the total hip (placebo 3.38% decrease, pooled alendronate 1.02% decrease) and more gain in BMD at the lumbar spine (placebo 1.52% increase, pooled alendronate 5.26% increase). Subjects on placebo had increases in bone turnover markers compared with alendronate users. Alendronate users had lower risk of clinically recognized vertebral fractures, but the cumulative risk of nonvertebral fractures was not significantly different between the alendronate-treated women and those who received placebo. The authors concluded that for many women the discontinuation of alendronate for up to five years did not appear to significantly increase fracture risk, but women at high risk of vertebral fractures with a history of spinal fracture and a BMD T-score of -2 or less as well as those with osteoporosis according to BMD testing (T-score less than -2.5) after 5 years of treatment may benefit from continued alendronate use (95,96). This trial has limitations because patients with severe osteoporosis were excluded from enrollment, while those with osteopenia were included. There was an uncontrolled phase between FIT completion and FLEX enrollment. There was also a high dropout rate, limiting statistical power (97). As summarized below in the section on a bisphosphonate holiday, with these limitations, risk of fracture versus benefit of continuing treatment should be individualized.
RISEDRONATE
Risedronate increases BMD and decreases fracture risk among postmenopausal women with osteoporosis. Harris et al. reported data on 2,458 postmenopausal women with established osteoporosis (subjects had either two or more vertebral fractures or one vertebral fracture and lumbar spine T score of -2 or less) and who were randomized to risedronate (5 mg daily) or placebo. Over three years, risedronate increased lumbar spine BMD by 5.4% and femoral neck BMD by 1.6%. Risedronate decreased the risk of new vertebral fractures by 41% and decreased the risk of non-vertebral fractures by 39% at three years (98). Reginster et al. showed in osteoporotic women that risedronate reduced spine fractures within the first year of treatment (99).
Risedronate therapy also reduces fracture risk in men (100), and is effective in the prevention and treatment of glucocorticoid-induced osteoporosis in men and women (101).
Weekly risedronate (35 mg) preparation used clinically is effective and well tolerated (102-104). Brown et al. randomized 1,468 women to daily or weekly risedronate. The increase in lumbar spine BMD at one year was similar between groups. Weekly risedronate was well tolerated, and the occurrence of adverse events was similar in daily and weekly treatment groups (102). A weekly preparation of risedronate that can be taken after breakfast is also available for clinical use. Monthly dosing of risedronate is available (150 mg once a month). Both monthly dosing regimens were shown to be non-inferior in efficacy and safety to the 5 mg daily regimen at one year (105,106). Thus, monthly risedronate provides alternative regimen for the prevention and treatment of osteoporosis. A formulation that can be taken with food is also available.
ZOLEDRONIC ACID
Zoledronic acid, an intravenous bisphosphonate, has been FDA approved for years for the treatment of hypercalcemia of malignancy, multiple myeloma, and bone metastases from solid tumors. In August 2007, zoledronic acid (Reclast®) became the second intravenous bisphosphonate after ibandronate (Boniva®) to be FDA approved for treatment of postmenopausal osteoporosis. It is considerably more potent than other available bisphosphonates. Thus, small doses and longer dosing intervals may be used (107). Reid et al. showed that zoledronic acid (4 mg annually) increases BMD and decreases markers of bone turnover in postmenopausal women.
In the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) study, a double-blind, placebo-controlled trial of 7765 post-menopausal women with osteoporosis were randomly assigned to receive a single 15-minute infusion of 5 mg of zoledronic acid or placebo at baseline, at 12 months, and at 24 months. The patients were followed over 36 months. In addition to positive effects on BMD and reduction in bone turnover biomarkers, treatment with zoledronic acid was associated with 70% RRR in morphogenic vertebral fractures and 41% RRR in hip fractures compared with placebo (108). Nonvertebral fractures, clinical fractures, and clinical vertebral fractures were reduced by 25%, 33%, and 77%, respectively. While adverse events, including change in renal function, were similar in both study groups, serious atrial fibrillation (AF) occurred slightly more frequently in the zoledronic acid group in the 3-year but not the 6-year data (108). Further analysis of the trial data and possible risk factors for rare AF are presented below under Adverse Effects (109).
In a study in 9355 women randomized to zoledronic acid versus placebo, zoledronic acid resulted in an early reduction in clinical fractures at one year that persisted for 3 years (110). Zoledronic acid is also effective in decreasing fracture risk in men (111).
In Horizon Recurrent Fracture trial, a double-blind, placebo-controlled study in adults with hip fractures, zoledronic acid versus placebo administered two weeks to 90 days post-surgical repair resulted in a 35% reduction in new clinical fractures and a 28% reduction in mortality (112). In a sub-sample analysis of this multi-national study, vitamin D deficiency was common and the median 25(OH)D level was only 14.7 ng/ml in these hip fracture study participants (113). Most study participants received 50,000 to 125,000 IU vitamin D at least two weeks prior to the zoledronic acid infusion. Once yearly infusion of zoledronic acid administered 2 weeks to 3 months after a hip fracture and after vitamin D supplementation, therefore, produced a decrease in clinical fractures and evidence of improved survival. Zoledronic acid is only FDA-approved therapy to reduce clinical fracture risk in adults with new hip fractures and provides skeletal protection for hip fracture patients as a once a year dosing. Zoledronic acid administered every 18 months for 6 years also decreased fracture incidence in women with low bone mass (197)
OTHER BISPHOSPHONATES
Ibandronate (oral and IV) is FDA-approved for the prevention and treatment of postmenopausal osteoporosis. In the larger clinical trial, it increased bone density and decreased vertebral fractures with both an oral daily regimen (2.5 mg daily) and an intermittent regimen (20 mg every other day for 12 doses every three months, 150 mg monthly) without reduction in hip fractures (114-116). Thus, unlike other bisphosphonates, ibandronate was not effective in decreasing non-spine fractures.
Pamidronate is not FDA approved for use in osteoporosis; however, it is occasionally used “off-label” for patients in patients with esophageal abnormalities (i.e., stricture or achalasia), organ transplants, or osteogenesis imperfecta. In adults, usually 30 to 60 mg is infused over two to four hours every three months. Pamidronate has been shown to increase BMD, but no fracture data are available (117-121).
Adverse Effects
GI EFFECTS
In general, the bisphosphonates are safe medications. Studies showing the long-term safety of alendronate, risedronate, and zoledronic acid are available for up to 10, 7, and 6 years respectively. Oral bisphosphonates are associated with some GI symptoms, and rare cases of severe esophagitis have been reported with alendronate, although reports are not consistent. However, Lanza et al. carried out a placebo-controlled endoscopic study in 277 subjects and found that the incidence of upper GI symptoms and endoscopic lesions was similar in the placebo and weekly alendronate groups (122). While in controlled trials the incidence of GI adverse effects did not differ in alendronate versus placebo groups, in clinical practice some patients discontinue bisphosphonates because of adverse GI experiences.
Because of the risk of esophagitis, alendronate is contraindicated for patients with esophageal abnormalities that delay esophageal emptying such as stricture or achalasia, and both alendronate and risedronate should not be used in patients who are unable to stand or sit upright for at least 30 minutes after drug administration because of increased risk of adverse esophageal effects.
ATYPICAL FEMUR FRACTURES
There has been concern over long-term bisphosphonate use and the reported risk of atypical femur fractures (AFF). AFF are thought to be stress or insufficiency fractures, caused by anti-resorptive-mediated suppression of intracortical remodeling, though the definite pathogenesis remains unclear. The absolute risk of AFF for patients taking bisphosphonates ranges from 3.2 to 50 per 100,000 person-years, but the risk with long-term bisphosphonate use is higher, ~100 per 100,000 person-years.
The Second Task Force of the American Society for Bone and Mineral Research (ASBMR) has defined AFF for case recognition. AFF must be located along the femoral diaphysis distal to the lesser trochanter and proximal to the supracondylar flare, and satisfy 4/5 major features: 1) the fracture is associated with minimal or no trauma, 2) the fracture line originates at the lateral cortex and is substantially transverse in its orientation (but can also be oblique as it progresses medially), 3) a complex fracture extends through both cortices and may have medial spike; or an incomplete fracture involves the lateral cortex, 4) the fracture is noncomminuted or minimally comminuted, and/or 5) localized periosteal or endosteal thickening of the lateral cortex is present at the fracture site (“beaking” or “flaring”). Other common features (minor features) include generalized increase in thickness of the femoral diaphyses, prodromal symptom of dull or aching pain in the groin or thigh, bilateral incomplete or complete femoral diaphysis fractures, and delayed fracture healing, though these are not required for case definition. Risk factors include use of bisphosphonates for >3-5 years, low vitamin D levels, and use of glucocorticoids (123).
The consensus has been that the number of fractures prevented far exceeds the number of AFF occurring as a result of bisphosphonate therapy, though further data is needed to guide decision-making around AFF risk.
Management of AFF recommended by the ASBMR task force includes surgical management with intramedullary fixation nailing or plating if the fracture is complete or incomplete accompanied by pain, with discontinuation of anti-resorptives, and adequate calcium and vitamin D intake. If the fracture is incomplete and pain is minimal, a trial of conservative management may be considered with use of crutches for 2-3 months, though there is a risk of progression to complete fracture with this method. In addition, obtaining X-ray imaging of the contralateral femur is recommended by the FDA, as ~28% of AFF also affect the contralateral leg. AFF noted on X-ray imaging should be followed by higher-order imaging, such as MRI or CT (123). Lastly, teriparatide may be considered in those who do not heal with other therapy (124).
OSTEONECROSIS OF THE JAW
Bisphosphonate-associated osteonecrosis of the jaws (ONJ) has also drawn attention even though this is a rare occurrence in patients treated with antiresorptive therapies. The International Task Force on Osteonecrosis of the Jaw defines ONJ as exposed bone in the maxillofacial region that does not heal within 8 weeks after identification by a health care provider, with prior exposure to an antiresorptive agent, and no history of radiation to the craniofacial region (125). It has been hypothesized that ONJ is the result of bone remodeling suppression combined with additional factors such as dental intervention or infection (126). Although very rare, it is more common after dental procedures such as tooth extraction. In 2005, the FDA requested that all oral and IV bisphosphonates include a class “precaution” labeling for ONJ. There have been no cases reported in randomized, placebo-controlled trials of alendronate, risedronate, or ibandronate. However, in a 2006 Medline review, 368 published cases were found, 94% of which involved patients receiving intravenous bisphosphonates, 85% of which involved patients with multiple myeloma or metastatic cancer. Only 4% of patients had osteoporosis and data suggests a time- and dose-dependent effect. 60% of reported cases of ONJ occurred after dentoalveolar surgery for infections (tooth extractions), and the remaining 40% were likely related to infection, denture trauma, or other oral trauma (127). Based on both published and unpublished data, the risk of ONJ associated with oral bisphosphonate treatment for osteoporosis is low, estimated between one in 10,000 and less than one in 100,000 patient-treatment years (128). Some experts have suggested stopping bisphosphonates during a time before and after-invasive dental procedures. The American Dental Association 2011 Recommendations indicate that for patients receiving bisphosphonate therapy, the risk of developing osteonecrosis of the jaw is low and that for dental care they do not currently recommend stopping bisphosphonates (129,130). The American Dental Association does recommend maintenance of good dental hygiene and routine dental care.
The International Task Force on Osteonecrosis of the Jaw in 2015 reported an incidence of ONJ of 0.001% to 0.01% in osteoporosis patients, which is slightly higher than the incidence in the general population (<0.001%). Risk factors for ONJ included glucocorticoid use, maxillary or mandibular bone surgery, poor oral hygiene, chronic inflammation, diabetes mellitus, ill-fitting dentures, as well as other drugs such as antiangiogenic agents. Incidence is greater in the oncology population (1-15%), who are receiving significantly more frequent and higher doses of anti-resorptives than the osteoporotic population. The task force recommended prevention of ONJ by eliminating or stabilizing oral disease prior to initiation of antiresorptive therapy, and considering the withholding of antiresorptive therapy in those at high risk for ONJ, such as cancer patients receiving bisphosphonates or denosumab and following extensive oral surgery until the surgical site heals with mature mucosal coverage (125). In a 2022 update by the American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws (MRONJ), the risk of MRONJ in osteoporotic patients treated with bisphosphonates was 0.02 to 0.05 percent, compared to 0 to 0.02 percent with placebo (198).
ATRIAL FIBRILLATION
In the HORIZON trial, serious atrial fibrillation (AF) was seen more frequently in patients who received IV zoledronic acid (50 subjects, 1.5%) than in those who received placebo (20 subjects 0.5%) (108). Significant risk factors were active tachyarrhythmia, congestive heart failure, previous bisphosphonate use, and advanced age (109). In a review of the results from FIT, there were more serious AF cases in the alendronate group (N=47 subjects, 1.5%) than in the placebo group (N=31 subjects, 1.0%), but these differences were not significant (131). These findings raised concern about a risk of AF with bisphosphonate use. In a case-control study published in 2008, researchers found more AF subjects than controls had ever used alendronate (n=47, 6.5% versus n=40, 4.1%) (132). A review of data from multiple trials did not find an association between risedronate use and AF (133). It is unclear how bisphosphonates may increase the risk of AF. Hypotheses include the release of inflammatory cytokines when IV bisphosphonates are administered, calcium shifts that can occur with IV and potent oral bisphosphonates, and relative binding affinity of the various bisphosphonates to bone. Both cytokines and calcium shifts may increase the risk of AF. The FDA released a review of spontaneous post-marketing reports of AF associated with oral and IV bisphosphonates and did not identify a risk of AF (134,135). The FDA continues to monitor such reports.
Post-Hip Fracture Care
Given the high rates of morbidity and mortality, particularly within the first-year post-fracture, hip fractures are the most serious of the osteoporotic fractures. There is a high prevalence of low vitamin D levels among hip fracture patients that warrants correction at the time of fracture (30,31). Nationally and internationally there is a large gap in fracture care and only 20% of fracture patients are evaluated and treated for their underlying osteoporosis. A fracture liaison service that identifies patients with fractures and initiates bone density testing and treatment has been very effective in reducing costs and improving post-fracture care (136-139). At Brigham and Women’s Hospital (BWH) Endocrinologists and members of the Department of Orthopedic Surgery have worked together since 2004 to implement a hospital-based approach to advance fracture care and reverse the high prevalence of vitamin D deficiency among hip fracture patient using the electronic health record (140). This inter-disciplinary fracture pathway for hip fracture patients called the Brigham Fracture Intervention Team Initiative or “B-Fit®” includes testing of 25(OH)D, calcium, and creatinine levels on admission to the hospital, administration of one dose of 50,000 units of vitamin D, daily supplemental calcium and vitamin D, and an Endocrinology evaluation. Outpatient care coordination between endocrinologists and Orthopedic Surgeons include assessment for secondary causes of osteoporosis, bone density testing, and pharmacological intervention to reduce subsequent fractures (7, 140-142). Many national organizations are seeking to bring together stakeholders and improve patient care so patients with fragility fractures are evaluated and treated for their underlying osteoporosis (7).
Other Precautions
Bisphosphonates are excreted by the kidneys and should not be used for patients with severe renal insufficiency (creatinine clearance < 35ml/min, Creatinine clearance <30 ml/min for Ibandronate). Studies in cancer patients, in whom cumulative doses are several-fold higher than in osteoporosis patients, show that age, concomitant non-steroidal anti-inflammatory drug use, prior pamidronate use, history of hypercalcemia, renal disease, hypertension, and smoking are risk factors for renal failure (143,144).
Approximately 20% to 30% of subjects treated initially with intravenous administration of pamidronate or zoledronic acid (108,145) may develop an acute-phase reactions (e.g. fever, malaise, myalgia), which is typically less severe with subsequent infusions. Patients should be hydrated and often are premedicated with acetaminophen; symptoms are usually mild and transient.
Hypocalcemia may occur, but this is usually mild and asymptomatic. To avert marked hypocalcemia, it is important to ensure that the patient is vitamin D sufficient, which according to the authors’ practices, can best be achieved by checking a 25-hydroxy vitamin D level prior to each infusion. In addition, calcium and creatinine levels should be tested before each intravenous bisphosphonate treatment.
Bisphosphonate Holiday
Bisphosphonates have robust effects on fracture reduction when used for 3-5 years. There are concerns about the long-term use. According to the 2011 FDA review as summarized in the New England Journal of Medicine (146) there is no global regulatory restriction on duration of use. Post-hoc analyses of data from the FIT and FLEX studies for alendronate (up to 10 years of alendronate therapy) and the randomized extension to the HORIZON-Pivotal Fracture Trial (up to 6 years of zoledronic acid therapy) provide some guidance in these important clinical decisions (96,147).
According to the available data, alendronate and zoledronic acid may be discontinued in patients at low risk of fracture after 5 or 3 years of therapy, respectively. In the FLEX trial, continuation of alendronate to 10 years duration of therapy did reduce non-vertebral fractures in those with FN T-scores <-2.5 assessed at year 5, but not in those with T-scores >-2.0 at year 5 (96). In the HORIZON extension trial, stopping Zoledronic acid after 3 years duration of therapy did not significantly increase the risk of subsequent fracture in those with T-score >-2.5, no recent fractures, and no greater than 1 risk factor(148). The subgroups of patients who might benefit from continued therapy without holiday at 5 (oral) or 3 (IV) years of therapy include those with T-score <-2.5 at the hip, recent fracture on therapy, and prevalent spine fractures. Otherwise, annual evaluation while on holiday to assess each individuals fracture risk is recommended, in order to decide when to resume therapy (149). High risk individuals may benefit from use of an alternative treatment such as teriparatide or in some instances, raloxifene, during the time of bisphosphonate holiday. Ongoing evaluation of patients on a bisphosphonate holiday is important to reduce the risk of subsequent fractures (95,96,146,147,150).
The ASBMR Task Force for managing osteoporosis in patients on long-term bisphosphonate therapy included consideration of continuing therapy in any patients with history of hip, spine, or multiple other osteoporosis fractures before or during therapy, those with hip BMD T-score<=2.5 after treatment, or high fracture risk (151). However, these approaches do not replace clinical judgment.
Drug Administration
Oral bisphosphonates should be taken in the morning with water on an empty stomach. Because oral bisphosphonates are poorly absorbed, patients should wait at least 30 minutes before ingesting other beverages, food, or medications. To help patients avoid esophageal irritation, they are instructed to swallow oral bisphosphonates with six to eight ounces of water and to remain upright for at least 30 minutes and until they have had their first meal of the day (152). Intravenous preparations must be infused slowly to avoid renal toxicity.
When choosing an oral bisphosphonate and in the absence of contraindications, alendronate is often selected as initial therapy because of its efficacy in reduction of spine and non-spine fractures and its availability as a low cost, generic preparation. In addition to alendronate, risedronate has been on the market for more than 10 years and has favorable safety profiles when used in the indicated populations. While oral ibandronate is popular for its monthly dosing schedule, ibandronate reduces the incidence of spine but not non-spine fractures. In addition, ibandronate’s IV dosing is more expensive and requires more frequent dosing than the once-yearly, zoledronic acid. Thus, it has a limited role in osteoporosis treatment. In patients who are unable to comply with the administration requirements of the oral agents, and in those who experience intolerable GI effects, intravenous zoledronic acid is an effective therapy to reduce spine and non-spine fractures. Like alendronate and risedronate, it reduces the incidence of vertebral and nonvertebral fractures. Zoledronic acid (5 mg infusion once a year) should also be considered in patients with a recent hip fracture after two weeks to 90 days. A post-hoc analysis suggested a superior bone density response when zoledronic acid was administered 4-6 weeks after a hip fracture than at the earlier time points (153). Vitamin D deficiency should be optimally corrected prior to use of zoledronic acid.
DENOSUMAB
Denosumab is the first FDA-approved human monoclonal antibody that binds to the receptor activator of nuclear factor kappa B ligand (RANKL), an important regulator of bone remodeling. RANKL is secreted by osteoblast precursors and binds to its receptor, RANK, located on osteoclasts. Osteoprotegrin is an endogenous cytokine and decoy receptor that binds RANKL and inhibits osteoclast activation (154). The binding of RANKL to RANK promotes osteoclast proliferation, differentiation, activation, and survival. Denosumab inhibits RANKL and osteoclastogenesis and markedly reduces bone resorption.
Fracture Data
Denosumab is administered for osteoporosis treatment as a subcutaneous injection of 60 mg every 6 months. In its pivotal phase III randomized placebo-controlled study of 7868 osteoporotic women ages 60-90 years (FREEDOM), denosumab compared with placebo given twice yearly for 3 years was associated with a relative decrease in the risk of vertebral, hip, and nonvertebral fractures by 68%, 40%, and 20% respectively (155). In the extension of this trial, denosumab use for up to 10 years was associated with cumulative BMD gains of 21.7% at the lumbar spine and 9.2% at the total hip. Persistent reductions of bone turnover markers and fracture incidence was also noted, with a positive safety profile with up to 10 years of continued use (199).
Drug Administration
Denosumab may have advantages over current osteoporosis therapies: infrequent dosing (every six months), and rapid, effective, but reversible antiresorptive activity; drug adherence is, however, important to prevent the increase in bone turnover markers after 6 months of therapy.
Adverse Effects
Adverse effects of densoumab include hypocalcemia, nausea, musculoskeletal pain, serious skin infections (small risk), infections, dermatologic reactions, and cystitis. Infection risk has been a concern based on RANKL inhibition of non-skeletal immune cells causing theoretical immune suppression. The initial FREEDOM trial showed slightly higher infection rates (3 cases in densoumab arm vs. 0 cases in placebo arm of endocarditis, 0.4% risk in densoumab arm vs. <0.1% in placebo arm of severe skin events) while the extension trial showed no increased risk of infection compared to placebo. Furthermore, a meta-analysis failed to show an increased risk of serious infections with denosumab use (157). Given the unclear infection risk, its use in immunocompromised patients should be cautious. In addition, very rare osteonecrosis of the jaw and atypical femur fractures have occurred with denosumab use (similar to bisphosphonates). Stopping denosumab therapy has been shown to result in bone loss and, in some instances, spine fractures (200). Therefore, unlike bisphosphonates, a treatment holiday is not recommended. The FDA recommends initiation of antiresorptive therapy and a number of treatment regimens are undergoing evaluation in an effort to prevent this bone loss.
PARATHYROID HORMONE
Anabolic Action on Bone
Animal studies show that PTH is capable of both anabolic and catabolic actions on bone. PTH stimulates both bone formation and bone resorption; the net effect on BMD depends on the balance between these two processes (160). A continuous infusion of PTH increases both formation and resorption and leads to bone breakdown (160,161). However, intermittent exposure preferentially increases formation, thereby producing an anabolic effect on bone (160,162,163). Therefore, PTH can increase or decrease BMD depending on the pattern of exposure. Dosing PTH in a manner leading to stimulation of bone formation before causing bone resorption has become known as maximizing the “anabolic window” of PTH (164).
Cellular Mechanisms
PTH acts directly on osteoblasts and cells of the osteoblast lineage. PTH promotes differentiation of pre-osteoblasts to osteoblasts (161) and inhibits osteoblast apoptosis, thereby increasing the number of active osteoblasts (165). Furthermore, PTH triggers the production of several growth factors in bone cells, including insulin-like growth factor I (IGF-I) (161,166).
Teriparatide
In 2002, the FDA approved teriparatide (Forteo™), injectable recombinant human PTH (1-34), for the treatment of men and postmenopausal women with osteoporosis who are at high risk for fracture (see Table 5). The biologically active fragment PTH (1-34) has properties similar to the full-length molecule PTH (1-84), which is approved for use in Europe. Antiresorptive agents, such as bisphosphonates, increase BMD up to ~ 8%. However, many patients with osteoporosis have lost as much as 30% of their peak bone mass. Thus, agents that have an anabolic effect on bone are desirable (158). PTH directly stimulates bone formation before bone resorption, has robust effects on spinal BMD, improves bone structure, and reduces spine and non-spine fractures. The sequence of changes in bone formation and resorption leads to what is described as the anabolic window (159).
FRACTURE DATA
In a large multicenter, randomized placebo-controlled trial, Neer et al. reported the effects of PTH (1-34) on bone density and fractures in 1,637 postmenopausal women with baseline vertebral fractures randomized to 20 µg PTH daily, 40 µg PTH daily, or placebo. At a mean of 18 months’ follow-up, 20 µg PTH daily increased lumbar spine BMD by 9.7%, femoral neck BMD by 2.8%, and total hip BMD by 2.6%. There was a decrease of 0.1% at the distal radius, but this was not significantly different from the change seen in the placebo group. PTH (20 µg daily) reduced the risk of vertebral fractures by 65% and non-vertebral fragility fractures by 53% (and is the FDA-approved dose for treatment of osteoporosis). The two PTH (1-34) doses reduced fractures to a similar degree, but headache and nausea were more common in the group receiving the higher dose of 40 µg daily (167).
Abaloparatide
In 2017, an additional PTH analog was FDA approved for the treatment of post-menopausal osteoporosis. Abaloparatide (Tymlos™) is a parathyroid (1-34) hormone-related protein (PTHrp) analog drug that shares similar anabolic effects as teriparatide.
FRACTURE DATA
In the ACTIVE trial, a double-blind, placebo-controlled trial, Miller et al (202) studied the effect of abaloparatide 80 mcg daily versus placebo in 1901 women with osteoporosis and baseline vertebral fractures over 18 months. At a mean of 18 months’ follow-up, abaloparatide increased lumbar spine BMD by 11.2%, femoral neck BMD by 3.6%, and total hip BMD by 4.18%. New vertebral fracture incidence was 0.6% with abaloparatide versus 4.2% with placebo (86% relative risk reduction, p<0.001). There was a 43% relative risk reduction of non-vertebral fracture with abaloparatide, which just met statistical significance, P=0.049.
Combination Therapy of Teriparatide and Bisphosphonates or Denosumab
The effects of concurrent or sequential therapy with PTH and antiresorptive agents have been studied. Black et al. compared the effects of PTH (1-84), alendronate, or both in combination in postmenopausal women (168). At one year, spine DXA had increased in all three groups. There was no difference in spine DXA between the PTH group and the combination group. However, the PTH group had a significantly greater increase in volumetric BMD of the spine on quantitative CT than the alendronate and combination groups. Finkelstein et al. also carried out a study in men (169). PTH (1-34) was started at 6 months, and all three groups were followed for 30 months. Spine BMD as measured by both DXA and quantitative CT increased to a greater degree in the PTH group than in the alendronate and combination groups. Thus, these studies show no evidence of synergy between PTH and alendronate. Furthermore, alendronate administered prior to teriparatide may impair the anabolic activity of PTH. It is hypothesized that PTH is less effective when bone turnover is suppressed.
While concurrent treatment with PTH and alendronate does not appear to be additive, bisphosphonate therapy initiated immediately upon completion of PTH course is beneficial. Rittmaster et al. demonstrated that PTH followed by alendronate produces progressive increases in BMD. In this study, 66 postmenopausal women were randomized to either 50 µg of recombinant human PTH (1-84) daily or placebo for the first year, and then all subjects were treated with alendronate on an open label extension for the second year. During the first year, the PTH group gained 4.3% BMD at the lumbar spine while the placebo group gained 1.3%. During the second year, the PTH group gained 6.3% BMD at the lumbar spine while the placebo group gained 5.7%. Thus, subjects previously treated with PTH continued to gain BMD with subsequent alendronate therapy (158). Black et al. extended their trial mentioned above (168). Post-menopausal women who had received PTH (1-84) in year one were randomly assigned to an additional year of placebo (n = 60) or alendronate (n = 59). Over two years, alendronate after PTH (1-84) led to significant increases in BMD compared to placebo after PTH (1-84), most notable at trabecular bone areas of the spine as assessed by quantitative CT [31% increase in alendronate after PTH (1-84) group versus14% increase in placebo after alendronate group]. Significant BMD loss was seen in year two in the placebo after PTH (1-84)group (170). Kurland et al. reported similar findings in men (171). Twenty-one men were followed for up to two years after discontinuing PTH (1-34). Those who were treated with a bisphosphonate immediately upon completion of the PTH gained an additional 8.9% BMD at the lumbar spine at two years, while the men who did not go on bisphosphonate therapy lost 3.7% BMD at the lumbar spine at one year. These studies support the immediate use of bisphosphonates upon completion of the recommended 24-month course of PTH therapy to consolidate the increases in bone density.
The Denosumab and Teriparatide Administration (DATA) trial investigated the combination of denosumab and teriparatide vs. monotherapy for 2 years. Combination therapy of daily teriparatide and denosumab every 6 months showed increases in spine and hip bone density greater than either drug alone (172).In the absence of fracture outcomes, the role of combination teriparatide and denosumab therapy in osteoporosis remains to be determined, but this regimen may be a therapeutic option in patients with severe osteoporosis or in those who have failed conventional therapy. In the DATA-Switch study, an extension of the DATA trial, subjects who were on denosumab only were switched to teriparatide, and those on teriparatide only were switch to denosumab; the former group were found to have bone loss, whereas the latter group have continued BMD increase (173). This may indicate that the choice of initial and subsequent osteoporosis treatment is an important consideration.
In an overlap study of teriparatide with alendronate added to teriparatide after 9 months, found a greater increase in BMD with overlap compared to teriparatide alone (174). These findings may be due to a “reopening” of the anabolic window described with teriparatide use. Of note, fracture data is not available.
Adverse Effects
In general, teriparatide and abaloparatide, injections are well tolerated and have been safely used for a decade (175). PTH is cleared from the circulation within four hours of subcutaneous administration. A daily injection is necessary and transient redness at the injection site has been noted. Headache and nausea occur in less than 10% of subjects receiving a daily dose of teriparatide 20 µg. Mild, early, transient hypercalcemia can occur, but severe hypercalcemia is rare. Prior to starting a PTH or PTH-rp analog, it is suggested to obtain serum calcium, alkaline phosphatase, parathyroid hormone, 25-hydroxyvitamin D, and creatinine levels. Routine monitoring of serum calcium levels while on PTH or PTH-rp is not recommended by the manufacturer, though may be considered. Increases in urinary calcium (by 30 mg per day) and serum uric acid concentrations (by 13%) are seen but do not appear to have clinical consequences.
Fisher 344 rats treated with nearly life-long daily teriparatide or abaloparatide have an increased risk of osteosarcoma. Upon approval of teriparatide in 2002, the FDA placed a black box warning about osteosarcoma in rodents treated with teriparatide and the manufacturer has warned against using teriparatide in the following settings: Paget's disease or unexplained elevations of alkaline phosphatase, open epiphyses in children or young adults, bone metastases, prior radiation therapy involving the skeleton, metabolic bone disease other than osteoporosis, and hypercalcemia. As summarized by Cipriani et al in 2013, there have been 3 reported cases of osteosarcoma in adults treated with PTH (1-34), which does not appear to be greater than the prevalence of osteosarcoma in the population (175). In the Osteosarcoma Surveillance Study, a 15-year surveillance study with 7 years of follow-up, there has not been evidence of a causal relationship between use of teriparatide and risk of osteosarcoma in humans. Among the 1448 cases of osteosarcoma, no patient in this study had been previously treated with teriparatide (176).
In 2021, the FDA removed the black box warning for teriparatide based on 18 years of post-marketing surveillance using case-finding studies, which ruled out any but a small potential increase in risk of osteosarcoma in humans with the drug. The FDA no longer limits the lifetime use to a total of 2 years and longer use can be considered in patients at high fracture risk. The black box warning was also removed for abaloparatide, however, use is limited to 2 years in patient’s lifetime until more data is available. Use of teriparatide and abaloparatide, however, should be avoided in patients at risk for osteosarcoma (e.g., younger patients with open epiphyses or those with a history of skeletal malignancies, unexplained alkaline phosphatase, Paget’s disease of bone or radiation therapy to bone).
Off Label Uses
Teriparatide has been used off-label for numerous reasons, including improvement of bone healing with atypical femur fractures, and for treatment of vertebral fracture pain and fracture healing. More clinical data is needed in these areas. A systemic review of teriparatide use for healing of bisphosphonate-related AFF found anecdotal evidence of beneficial effects on fracture healing, noting the need for prospective data (124). In a small study of 34 patients with acute vertebral fractures given teriparatide vs. risedronate, those who received Teriparatide had lower rates of vertebral collapse, though had no significant difference in back pain scores (177).
Drug Administration
Teriparatide is supplied in a disposable pen device for subcutaneous injection into the thigh or abdomen. The pen requires refrigeration between uses. The recommended dosage is 20 µg once a day for two years, though its lifetime use may be extended beyond this in certain clinical situations (such as if a patient remains at or returns to a high risk of fracture). Abaloparatide is also supplied in a disposable pen device for subcutaneous injection into the thigh or abdomen, and can be stored at room temperature after first use for up to 30 days. The recommendation dosage is 80 µg once daily for no more than two years.
ROMOSOZUMAB
Romosozumab is a monoclonal antibody to sclerostin, a potent inhibitor of osteoblast differentiation and bone formation by way of Wnt signaling inhibition. Animal studies show that blocking the effect of sclerosin was associated with large increases in bone mass. In phase II trials, romosozumab administration shows increased BMD at the spine of 11.3%, as well as increased bone formation and decreased bone resorption (193). A dual effect of transiently increasing markers of bone formation (P1NP) while simultaneously lowering marker of bone resorption (CTX) was also demonstrated in the phase II trial.
Fracture Data
In its pivotal phase III trial (203) of 7180 women with osteoporosis, romosozumab reduced incidence of vertebral fractures compared to placebo by 73% at 12 months, and 75% at 24-months after transition to denosumab at 12 months. Non-vertebral fracture reduction was not demonstrated. In the ARCH trial (204), 4093 women with severe osteoporosis were randomized to Romosozumab or alendronate for 12 months. Incidence of new vertebral fractures was 4% with Romosozumab vs. 6.3% with alendronate (risk ratio 0.63, p=0.003). Changes in bone density were greater with Romosozumab compared to alendronate, 13.7% vs. 5% increase in lumbar BMD, and 6.2% vs. 2.8% increase in total hip BMD was demonstrated, respectively. In extension data, preservation of BMD accrual was achieved with transition to alendronate for up to 36 months based on trial duration.
Adverse Effects
Romosozumab has been associated with hypersensitivity reactions such as angioedema and urticaria. The most common side effects were arthralgia and headache (>5%). Cases of ONJ and AFF have been reported. Upon approval by the FDA in 2019, a black box warning was applied regarding a potential risk of heart attack, stroke, and cardiovascular death. In the ARCH trial, there was a higher rate of major adverse cardiac events (MACE), a composite endpoint of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. However, in post-hoc pooled analysis by the TIMI Group of both FRAME and ARCH data, a significantly high rate of cardiovascular event was not demonstrated. The Endocrine Society advises that women at high risk for cardiovascular disease or stroke should not be considered romosozumab pending further studies on its cardiovascular risk (201).
GLUCOCORTICOID-INDUCED OSTEOPOROSIS
Glucocorticoid induced osteoporosis (GIO) affects the spine greater than other sites. The 2010 American College of Rheumatology (ACR) guidelines can be used to help clinicians determine appropriate therapeutic options in those on glucocorticoid therapy (181). Epidemiological data has consistently shown that those taking glucocorticoids have fractures at higher T-scores. Glucocorticoids not only increase bone resorption, but also reduce bone formation. Thus, there are two important steps for targeted intervention—bisphosphonates and teriparatide, respectively. Rapid bone loss is prevalent in the first 6-12 months of glucocorticoid therapy; however, the increased fracture risk is already present within 3 months of initiating glucocorticoids. Thus, bone protection therapy should be started, at the onset, if the duration of glucocorticoids is anticipated to be 3 months or longer. For postmenopausal women and men over age 50, treatment for GIO is determined based on whether the patient’s risk for fracture—using FRAX® and clinical judgment—is low (<10%), moderate (10-20%), or high (>20%). For those taking prednisone dose >7.5 mg/day, the FDA has approved the following bisphosphonates—Risedronate, Alendronate, Zoledronate—and the anabolic agent, Teriparatide, for the treatment of GIO. In a 3-year randomized trial evaluating the prevention and treatment of GIO, teriparatide was statistically superior to alendronate in preventing BMD declines at the spine and hip (182).
|
Table 5. Effects of FDA-Approved Osteoporosis Therapies on Fractures
|
| |
Most Common Dosage
|
Fracture Risk Reduction
|
FDA Indications*
|
|
Alendronate
|
70 mg PO weekly
|
Spine, non-spine, hip
|
PMO Treatment and Prevention in women, Treatment of osteoporosis in men, GIO treatment.
|
|
Ibandronate
|
150 mg PO monthly;
3 mg IV every 3 months
|
Spine
|
PMO Treatment and Prevention in women.
|
|
Risedronate
|
35 mg PO weekly;
150 mg PO monthly
|
Spine, non-spine, hip
|
PMO Treatment and Prevention in women, Treatment of osteoporosis in men, GIO treatment.
|
|
Zoledronic Acid (ZA)
|
5 mg IV / year (Treatment)
5 mg every other year (Prevention)
|
Spine, non-spine, hip
|
PMO Treatment and Prevention in women, Treatment of osteoporosis in men, GIO treatment
|
|
RANKL inhibitor
Denosumab
|
60 mg SC every 6 months
|
Spine, non-Spine, hip
|
PMO-Treatment in women and men at high fracture risk;
|
|
PTH - Teriparatide (PTH 1-34)
|
20 mcg SC daily (for maximum of 2 years)
|
Spine, non-Spine
|
PMO and GIO Treatment in women and men at high risk of fracture
|
|
PTH- Abaloparatide (PTH-rp 1-34)
|
80 mcg SC daily (for maximum of 2 years)
|
Spine, non-spine
|
PMO treatment in women at high risk of fracture
|
|
Anti-Sclerostin Antibody- Romosozumab
|
210 mcg SC monthly (for maximum of 12 months)
|
Spine, non-spine
|
PMO treatment in women at high risk of fracture
|
PMO: postmenopausal osteoporosis; GIO: Glucocorticoid-induced osteoporosis
CONSIDERATIONS REGARDING SELECTION OF ANTI-FRACTURE TREATMENT
When approaching a patient at high risk for fracture, several considerations may help guide the initial treatment selection. Anabolic agents (i.e., romosozumab, abaloparatide, or teriparatide) should be considered as first-line agents In patients deemed “very high risk” for fracture. This may include patients with very low T-scores <-3.0 at the lumbar spine or hip, recent fragility fracture, multiple risk factors for fractures or fractures while on approved osteoporosis therapy or intolerance to osteoporosis therapies. If anabolic treatment is contraindicated or not available for a patient, a parental anti-resorptive agent should be considered.
Denosumab can also increase bone density and reduce fracture risk in women and men at high risk for fracture. Denosumab is FDA approved to treat glucocorticoid-induced osteoporosis in men and women at high risk for fracture, in women at high fracture risk on adjuvant aromatase inhibitor therapy for breast cancer, and in men treated with androgen deprivation for prostate cancer.
In patients with advanced chronic kidney disease, treatment options can be limited. Bisphosphonates are generally contraindicated in those with eGFR <30-35. Denosumab (Prolia) is the preferred agent for those with more advanced kidney disease, given lack of direct renal toxicity and renal metabolism compared to bisphosphonates. However, it is important to note that though denosumab has been shown to improve bone mineral density in those with advanced renal disease, there is little evidence of fracture reduction in this population. Since patients with CKD may have several different types of metabolic bone diseases including osteoporosis, use of denosumab should be approached with caution given the increased complexity of bone disease in these patients.
It is important to note that when selecting an anabolic agent or denosumab, a plan for the next agent in their treatment sequence should be considered at the onset. Anabolic agents are approved for 1-2 years of use, thereafter their effects wane. At present use of teriparatide can be used for more than a total of 2 years in patients at high risk of fracture. Anti-resorptive agents should ideally be given after completion of a course of anabolic in order to prevent the bone loss that occurs with discontinuation of these therapies. Regarding denosumab, this is approved for 5-10 years of continuous use, but at the point when denosumab is discontinued, it must be followed at the time of first missed dose or just after with an alternative anti-resorptive to prevent rapid rebound bone loss and spine fractures. Ongoing research is assessing different approaches to prevent the bone loss associated with the discontinuation of denosumab. In patients with intolerance or a renal contraindication to using bisphosphonates, the options for the treatment sequence must be taken into account and discussed with the patient as part of shared-decision making.
Zoledronic acid is an appropriate first-line option for several different patient scenarios. As mentioned in the Zoledronic acid (ZA) section above, it is the optimal choice in patients post-hip fracture given the benefit in morbidity and mortality in this setting. ZA should also be considered in patients at high fracture risk who have upper gastrointestinal/esophageal disease, or significant malabsorption (i.e. post-gastric bypass surgery), as oral bisphosphonates may be associated with increased risk of GI intolerance or poor absorption and efficacy, respectively. In patients with compliance difficulties or major transportation concerns, zoledronic acid may also be optimal given its infrequent and flexible dosing (once yearly, though less frequently may also be appropriate in select patients). This is in contrast to denosumab, which requires strict adherence to an every 6 month schedule of injections in order to avoid the consequence of rebound bone loss if doses are missed or significantly delayed.
Lastly, raloxifene may be considered in patients within 10 years of menopause, who are at high fracture risk at the spine, and high risk for breast cancer based on familial history. Otherwise, an oral bisphosphonate (e.g., alendronate or risedronate) or intravenous bisphosphonate or denosumab is preferred over raloxifene as these therapies have been shown to reduce spine and non-spine fractures.
Clinical guides from the Bone Health and Osteoporosis Foundation (7), American Association of Clinical Endocrinologists/American College of Endocrinology (205), and the Endocrine Society (201) provide more detailed information on the management of osteoporosis in high- risk patients.
TREATMENT GAP IN OSTEOPOROSIS THERAPY
Despite having highly effective and well-tolerated available therapeutics for the treatment and prevention of osteoporosis, the rate of treatment of at-risk patients is much lower than desired. Based on prescription databases, bisphosphonate use declined by greater than 50% between 2008 and 2012 (183). In addition, the use of bisphosphonates among those with hip fractures declined from 15% in 2004 to only 3% in 2013, which is concerning given the high risk for future fracture in the setting of hip fracture (139). This decline in use temporally coincides with FDA warnings regarding potential risks related to anti-resorptive use, such as rare atypical femur fracture and osteonecrosis of the jaw, though the FDA has not restricted their use based on these risks (184). It is clear that many patients who would benefit from osteoporosis treatment are not receiving it, and this is a major concern for those who treat osteoporosis. Providers must be able to hold thorough and honest discussions with patients regarding the benefits and risks of osteoporosis treatment options in order for patients to accept and comply with needed treatment.
CONCLUSION
Osteoporosis is a major public health problem that affects approximately 50% of women and 25% of men aged 50 years and older and fractures increase exponentially with advancing age. At present, a number of safe and very effective osteoporosis therapies are available. Antiresorptive agents, such as the bisphosphonates, raloxifene, estrogen (not approved for treatment) and denosumab increase bone density and reduce fractures. Teriparatide, abaloparatide, and romosozumab are anabolic therapies and their treatment effects are best consolidated with an inhibitor of bone resorption such as a bisphosphonate or denosumab. A comprehensive review of the prevention and treatment of osteoporosis is summarized in the 2022 Bone Health and Osteoporosis Foundation Clinician’s Guide (7). A multifaceted approach including calcium and vitamin D, exercise, pharmacologic therapy, and fall prevention strategies can reduce the risk of fractures and promote independent healthy lives in older men and women.
ACKOWLEDGEMENTS
We wish to acknowledge Anjali Grover, MD, Kara Mikulec, MD and Kathryn E. Ackerman, MD, MPH, and thank them for their past contributions to the Endotext chapter and Jill MacLeod for her assistance in preparation of this review.
REFERENCES
- Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–289.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2007; 22:465-475.
- Chrischilles EA, Butler CD, Davis CS, Wallace RB. A model of lifetime osteoporosis impact. Arch Intern Med. 1991;151:2026–2032.
- Magaziner J, Lydick E, Hawkes W, Fox KM, Zimmerman SI, Epstein RS, Hebel JR. Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health. 1997;87:1630–1636.
- Melton LJ 3rd, Thamer M, Ray NF, Chan JK, Chesnut CH 3rd, Einhorn TA, Johnston CC, Raisz LG, Silverman SL, Siris ES. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:16–23.
- Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–1112.
- LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022; 33(10):2049-2102.
- McCloskey EV, Oden NC, Harvey WD, Leslie D, Hans H, Johansson, et al. A Meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016; 31; 940-948.
- Watts NB, Ettinger B, LeBoff MS. Perspective: FRAX Facts. Journal of Bone and Mineral Research. 2009;24:975–979.
- Kohrt WM, Barry DW, Schwartz RS. Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc. 2009;41:2050–2055.
- Shea B, Bonaiuti D, Iovine R, Negrini S, Robinson V, Kemper HC, Wells G, Tugwell P, Cranney A. Cochrane Review on exercise for preventing and treating osteoporosis in postmenopausal women. Eura Medicophys. 2004;40:199–209.
- Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2013;2013:741639.
- Khosla S, Bellido TM, Drezner MK, Gordon CM, Harris TB, Kiel DP, Kream BE, LeBoff MS, Lian JB, Peterson CA, Rosen CJ, Williams JP, Winer KK, Sherman SS. Forum on aging and skeletal health: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2011;26:2565–2578.
- Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position Stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–1996.
- Mundy G. Bone remodeling. In: Primer on the metabolic bone diseases and disorders of mineral metabolism, MJ Favus (ed.), Philadelphia: Lippincott Williams & Wilkins, 1999, 30–38.
- Watson SL, Weeks BK, Weis LJ, Horan SA, Beck BR. Heavy resistance training is safe and improves bone, function, and stature in postmenopausal women with low to very low bone mass: novel early findings from the LIFTMOR trial. Osteoporos Int. 2015;26(12):2889-2894.
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med. 1990;323:878–883.
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Flouride. Washington, DC, National Academy Press.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J Clin Endocrinol Metab. 2011;96:53–58.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med. 1985;313:70–73.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111:524–527.
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683.
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. Bmj. 2010;341:c3691.
- Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and Supplemental Calcium Intake and Cardiovascular Disease Mortality: The National Institutes of Health-AARP Diet and Health Study. JAMA internal medicine. 2013:1–8.
- Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, Datta M, Leblanc ES, Cauley JA, Rossouw JE. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–580.
- Moyer VA., USPSTF. Vitamin D and Calcium Supplementation to Prevent Fractures in Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013
- Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stahelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367:40–49.
- Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171.
- LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2015;41:259–268.
- Leboff MS, Hawkes WG, Glowacki J, Yu-Yahiro J, Hurwitz S, Magaziner J. Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos Int. 2008
- LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. Jama. 1999;281:1505–1511.
- Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146.
- Haden ST, Fuleihan GE, Angell JE, Cotran NM, LeBoff MS. Calcidiol and PTH levels in women attending an osteoporosis program. Calcif Tissue Int. 1999;64:275–279.
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642.
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758.
- Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351.
- Moyer VA. Prevention of Falls in Community-Dwelling Older Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2012;
- Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, Erwin PJ, Hensrud DD, Montori VM. The Effect of Vitamin D on Falls: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2011
- John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406.
- Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33:456–492.
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459.
- Dawson-Hughes B, Heaney R. P., Holick M. F., Lips P., Meunier P. J. R. V. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716.
- Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011:1–8.
- Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2010; 21:1151-1154.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96:1911–1930.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58.
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443.
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Jama. 2005;293:2257–2264.
- Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003;88:185–191.
- Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89-90:575–579.
- Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–86.
- Manolagas SC, Jilka RL. Cytokines, hematopoiesis, osteoclastogenesis, and estrogens. Calcif Tissue Int. 1992;50:199–202.
- Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989;86:2398–2402.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int. 1992;50:295–299.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333.
- Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, Chlebowski RT, Gass M, LaCroix A, Manson JE, Prentice RL, Rossouw J, Stefanick ML, Investigators WHI. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045.
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J, Investigators WHI. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314.
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Women's Health Initiative Memory S. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968.
- Manson JE. The Kronos Early Estrogen Prevention Study by Charlotte Barker. Womens Health (Lond Engl). 2013;9:9–11.
- Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, Miller VM, Neal-Perry G, Santoro N, Taylor HS, Vittinghoff E, Yan M, Hodis HN. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–260.
- Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP, Group ER. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med. 2016;374:1221–1231.
- North American Menopause S. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–271.
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama. 1999;282:637–645.
- Siris ES, Harris ST, Eastell R, Zanchetta JR, Goemaere S, Diez-Perez A, Stock JL, Song J, Qu Y, Kulkarni PM, Siddhanti SR, Wong M, Cummings SR. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res. 2005;20:1514–1524.
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856.
- Albertazzi P, Sharma S. Urogenital effects of selective estrogen receptor modulators: a systematic review. Climacteric. 2005;8:214–220.
- Burke TW, Walker CL. Arzoxifene as therapy for endometrial cancer. Gynecol Oncol. 2003;90:S40–46.
- Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923–1934.
- Palacios S, Silverman SL, de Villiers TJ, Levine AB, Goemaere S, Brown JP, De Cicco Nardone F, Williams R, Hines TL, Mirkin S, Chines AA. Bazedoxifene Study G. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause. 2015;22:806–813.
- Overgaard K, Riis BJ, Christiansen C, Podenphant J, Johansen JS. Nasal calcitonin for treatment of established osteoporosis. Clin Endocrinol (Oxf). 1989;30:435–442.
- Sun LM, Lin MC, Muo CH, Liang JA, Kao CH. Calcitonin nasal spray and increased cancer risk: a population-based nested case-control study. J Clin Endocrinol Metab. 2014;99:4259–4264.
- Zimolo Z, Wesolowski G, Rodan GA. Acid extrusion is induced by osteoclast attachment to bone. Inhibition by alendronate and calcitonin. J Clin Invest. 1995;96:2277–2283.
- Felix R, Graham R, Russell G, Fleisch H. The effect of several diphosphonates on acid phosphohydrolases and other lysosomal enzymes. Biochim Biophys Acta. 1976;429:429–438. ]
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 1998; 13:581-589.
- Fleisch H. Bisphosphonates in Bone Disease from the Laboratory to the Patient. Fourth ed: Academic Press.
- Watts NB. Treatment of osteoporosis with bisphosphonates. Endocrinol Metab Clin North Am. 1998;27:419–439.
- Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008:CD001155.
- Ascott-Evans BH, Guanabens N, Kivinen S, Stuckey BG, Magaril CH, Vandormael K, Stych B, Melton ME. Alendronate prevents loss of bone density associated with discontinuation of hormone replacement therapy: a randomized controlled trial. Arch Intern Med. 2003;163:789–794.
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082.
- Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338:485–492.
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Adams AL, Shi J, Takayanagi M, Dell RM, Funahashi TT, Jacobsen SJ. Ten-year hip fracture incidence rate trends in a large California population, 1997-2006. Osteoporos Int. 2013;24:373–376.
- Chesnut CH 3rd, McClung MR, Ensrud KE, Bell NH, Genant HK, Harris ST, Singer FR, Stock JL, Yood RA, Delmas PD, et al. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med. 1995;99:144–152.
- Dursun N, Dursun E, Yalcin S. Comparison of alendronate, calcitonin and calcium treatments in postmenopausal osteoporosis. Int J Clin Pract. 2001;55:505–509.
- Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, Yates J, de Papp AE, Palmisano J. Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc. 2002;77:1044–1052.
- Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 1998; 13:1431-1438.
- Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443.
- Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–468.
- Office of the Surgeon General. Bone health and osteoporosis: a report of the Surgeon General. Rockville, Md : U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General.
- Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610.
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292–299.
- Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, Lane NE, Kaufman JM, Poubelle PE, Hawkins F, Correa-Rotter R, Menkes CJ, Rodriguez-Portales JA, Schnitzer TJ, Block JA, Wing J, McIlwain HH, Westhovens R, Brown J, Melo-Gomes JA, Gruber BL, Yanover MJ, Leite MO, Siminoski KG, Nevitt MC, Sharp JT, Malice MP, Dumortier T, Czachur M, Carofano W, Daifotis A. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211.
- Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab. 2000;85:3109–3115.
- Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199.
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, Group FR. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
- Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, Wallace RB, Hochberg MC, Feldstein AC, Lombardi A, Black DM. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2010; 25:976-982.
- Briot K, Tremollieres F, Thomas T, Roux C. How long should patients take medications for postmenopausal osteoporosis? Joint Bone Spine. 2007;74:24–31.
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Jama. 1999;282:1344–1352.
- Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91.
- Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427–431. ]
- Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–2318.
- Brown JP, Kendler DL, McClung MR, Emkey RD, Adachi JD, Bolognese MA, Li Z, Balske A, Lindsay R. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int. 2002;71:103–111.
- Gordon MS, Gordon MB. Response of bone mineral density to once-weekly administration of risedronate. Endocr Pract. 2002;8:202–207.
- Delaney MF, Hurwitz S, Shaw J, LeBoff MS. Bone density changes with once weekly risedronate in postmenopausal women. J Clin Densitom. 2003;6:45–50.
- Delmas PD, Benhamou CL, Man Z, Tlustochowicz W, Matzkin E, Eusebio R, Zanchetta J, Olszynski WP, Recker RR, McClung MR. Monthly dosing of 75 mg risedronate on 2 consecutive days a month: efficacy and safety results. Osteoporos Int. 2008;19:1039–1045.
- Delmas PD, McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou CL, Man Z, Eusebio RA, Beary JF, Burgio DE, Matzkin E, Boonen S. Efficacy and safety of risedronate 150 mg once a month in the treatment of postmenopausal osteoporosis. Bone. 2008;42:36–42.
- Cheer SM, Noble S. Zoledronic acid. Drugs. 2001;61:799–805.
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, Trial HPF. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
- Cummings SR MP, Eriksen EF, Eastell R, Black DM. Risk factors for serious adverse events of atrial fibrillation in the HORIZON-PFT Trial of zoledronic acid. Am Soc Mineral Bone Res 29th Annual Meeting 2007, Abstract 1056.
- Boonen S, Eastell R, Su G, Mesenbrink P, Cosman F, Cauley JA, Reid IR, Claessens F, Vanderschueren D, Lyles KW, Black DM. Time to onset of antifracture efficacy and year-by-year persistence of effect of zoledronic acid in women with osteoporosis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 2012; 27:1487-1493.
- Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai HP, Witvrouw R, Eriksen E, Brixen K, Russo L, Claessens F, Papanastasiou P, Antunez O, Su G, Bucci-Rechtweg C, Hruska J, Incera E, Vanderschueren D, Orwoll E. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367:1714–1723.
- Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S. Zoledronic Acid in Reducing Clinical Fracture and Mortality after Hip Fracture. N Engl J Med 2007; 357:nihpa40967.
- Pieper CF, Colon-Emeric C, Caminis J, Betchyk K, Zhang J, Janning C, Shostak J, LeBoff MS, Heaney RR, Lyles KW. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5:335–340.
- Chesnut IC, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65:654–661.
- Cranney A, Wells GA, Yetisir E, Adami S, Cooper C, Delmas PD, Miller PD, Papapoulos S, Reginster JY, Sambrook PN, Silverman S, Siris E, Adachi JD. Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient data. Osteoporos Int. 2008
- Miller RG, Chretien KC, Meoni LA, Liu YP, Klag MJ, Levine MA. Comparison of intravenous pamidronate to standard therapy for osteoporosis: use in patients unable to take oral bisphosphonates. J Clin Rheumatol. 2005;11:2–7.
- Heijckmann AC, Juttmann JR, Wolffenbuttel BH. Intravenous pamidronate compared with oral alendronate for the treatment of postmenopausal osteoporosis. Neth J Med. 2002;60:315–319.
- Vis M, Bultink IE, Dijkmans BA, Lems WF. The effect of intravenous pamidronate versus oral alendronate on bone mineral density in patients with osteoporosis. Osteoporos Int. 2005;16:1432–1435.
- Cauza E, Etemad M, Winkler F, Hanusch-Enserer U, Partsch G, Noske H, Dunky A. Pamidronate increases bone mineral density in women with postmenopausal or steroid-induced osteoporosis. J Clin Pharm Ther. 2004;29:431–436.
- Chan SS, Nery LM, McElduff A, Wilmshurst EG, Fulcher GR, Robinson BG, Stiel JN, Gunton JE, Clifton-Bligh PB. Intravenous pamidronate in the treatment and prevention of osteoporosis. Intern Med J. 2004;34:162–166.
- Lanza F, Sahba B, Schwartz H, Winograd S, Torosis J, Quan H, Reyes R, Musliner T, Daifotis A, Leung A. The upper GI safety and tolerability of oral alendronate at a dose of 70 milligrams once weekly: a placebo-controlled endoscopy study. Am J Gastroenterol. 2002;97:58–64.
- Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O'Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23.
- Im GI, Lee SH. Effect of Teriparatide on Healing of Atypical Femoral Fractures: A Systemic Review. Journal of bone metabolism. 2015;22:183–189.
- Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J. International Task Force on Osteonecrosis of the J. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30:3–23.
- Allen MR. Bisphosphonates and Osteonecrosis of the Jaw: Moving from the Bedside to the Bench. Cells Tissues Organs. 2008
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761.
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491.
- Edwards BJ, Gounder M, McKoy JM, Boyd I, Farrugia M, Migliorati C, Marx R, Ruggiero S, Dimopoulos M, Raisch DW, Singhal S, Carson K, Obadina E, Trifilio S, West D, Mehta J, Bennett CL. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1166–1172.
- Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, Migliorati CA, Ristic H. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142:1243–1251. ]
- Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356:1895–1896.
- Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
- Karam R, Camm J, McClung M. Yearly zoledronic acid in postmenopausal osteoporosis. N Engl J Med. 2007;357:712–713.
- Cauley JA, Ensrud KE. Considering competing risks . . . Not all black and white. Arch Intern Med. 2008;168:793–795.
- Administration. UFaD. Early communication of an ongoing safety review (updated January 7, 2008). http://www.fda.gov/cder/drug/early_comm/bisphosphonates.htm.
- Adler RA, Bates DW, Dell RM, LeBoff MS, Majumdar SR, Saag KG, Solomon DH, Suarez-Almazor ME. Systems-based approaches to osteoporosis and fracture care: policy and research recommendations from the workgroups. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2011; 22 Suppl 3:495-500.
- Dell R, Greene D. Is osteoporosis disease management cost effective? Current osteoporosis reports. 2010;8:49–55.
- Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int. 2011;22 Suppl 3:457–460.
- Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE Jr, McLellan A, Mitchell PJ, Silverman S, Singleton R, Siris E. Prevention ATFoSF. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27:2039–2046.
- Glowacki J, Harris MB, Simon J, Wright J, Kolatkar NS, Thornhill TS, Leboff MS. Brigham fracture intervention team initiatives for hospital patients with hip fractures: a paradigm shift. Int J Endocrinol. 2010;2010:590751.
- Glowacki J, LeBoff MS, Kolatkar NS, Thornhill TS, Harris MB. Importance of vitamin D in hospital-based fracture care pathways. J Nutr Health Aging. 2008;12:291–293.
- Harris MB, LeBoff MS, Thornhill TS, Glowacki J. Evolution of comprehensive hospital care pathways to advance the treatment of fragility fractures. The Orthopaedic Journal at Harvard Medical School 2007:95-97.
- McDermott RS, Kloth DD, Wang H, Hudes GR, Langer CJ. Impact of zoledronic acid on renal function in patients with cancer: Clinical significance and development of a predictive model. J Support Oncol. 2006;4:524–529.
- Oh WK, Proctor K, Nakabayashi M, Evan C, Tormey LK, Daskivich T, Antras L, Smith M, Neary MP, Duh MS. The risk of renal impairment in hormone-refractory prostate cancer patients with bone metastases treated with zoledronic acid. Cancer. 2007;109:1090–1096.
- Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer JP, Kaufman JM, Jaeger P, Body JJ, Brandi ML, Broell J, Di Micco R, Genazzani AR, Felsenberg D, Happ J, Hooper MJ, Ittner J, Leb G, Mallmin H, Murray T, Ortolani S, Rubinacci A, Saaf M, Samsioe G, Verbruggen L, Meunier PJ. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661.
- Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for Osteoporosis - Where Do We Go from Here? N Engl J Med. 2012
- Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, Cummings SR, Hue TF, Lippuner K, Lakatos P, Leung PC, Man Z, Martinez RL, Tan M, Ruzycky ME, Su G, Eastell R. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243–254.
- Cosman F, Cauley JA, Eastell R, Boonen S, Palermo L, Reid IR, Cummings SR, Black DM. Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment? J Clin Endocrinol Metab. 2014;99:4546–4554.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565.
- Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing Bisphosphonate Treatment for Osteoporosis - For Whom and for How Long? N Engl J Med. 2012
- Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, McKinney R Jr, Pignolo RJ, Sellmeyer DE. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16–35.
- de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016–1021.
- Eriksen EF, Lyles KW, Colon-Emeric CS, Pieper CF, Magaziner JS, Adachi JD, Hyldstrup L, Recknor C, Nordsletten L, Lavecchia C, Hu H, Boonen S, Mesenbrink P. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24:1308–1313.
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319.
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
- von Keyserlingk C, Hopkins R, Anastasilakis A, Toulis K, Goeree R, Tarride JE, Xie F. Clinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysis. Seminars in arthritis and rheumatism. 2011;41:178–186.
- Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85:2129–2134.
- Rubin MR, Bilezikian JP. The anabolic effects of parathyroid hormone therapy. Clinics in geriatric medicine. 2003;19:415–432.
- Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110:506–512.
- Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709.
- Podbesek R, Edouard C, Meunier PJ, Parsons JA, Reeve J, Stevenson RW, Zanelli JM. Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology. 1983;112:1000–1006.
- Dobnig H, Turner RT. The effects of programmed administration of human parathyroid hormone fragment (1-34) on bone histomorphometry and serum chemistry in rats. Endocrinology. 1997;138:4607–4612.
- Bilezikian JP. Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Current osteoporosis reports. 2008;6:24–30.
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446.
- Rosen CJ, Bilezikian JP. Clinical review 123: Anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 2001;86:957–964.
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441.
- Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215.
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349:1216–1226.
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ, Pa THSI. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–565.
- Kurland ES, Heller SL, Diamond B, McMahon DJ, Cosman F, Bilezikian JP. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide. Osteoporos Int. 2004;15:992–997. [human parathyroid hormone(1-34)]
- Tsai J, Uihlein A, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013 6;382(9886):50-6.
- Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, Burnett-Bowie SA. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147–1155.
- Muschitz C, Kocijan R, Fahrleitner-Pammer A, Lung S, Resch H. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res. 2013;28:196–205.
- Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res. 2012;27:2419–2428.
- Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, Masica D. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 2012;27:2429–2437.
- Tsuchie H, Miyakoshi N, Kasukawa Y, Nishi T, Abe H, Segawa T, Shimada Y. The effect of teriparatide to alleviate pain and to prevent vertebral collapse after fresh osteoporotic vertebral fracture. Journal of bone and mineral metabolism. 2016;34:86–91.
- Michalska D, Luchavova M, Zikan V, Raska I Jr, Kubena AA, Stepan JJ. Effects of morning vs. evening teriparatide injection on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Osteoporos Int. 2012;23:2885–2891.
- Matsumoto T, Shiraki M, Hagino H, Iinuma H, Nakamura T. Daily nasal spray of hPTH(1-34) for 3 months increases bone mass in osteoporotic subjects: a pilot study. Osteoporos Int. 2006;17:1532–1538.
- Levin G MC. Transdermaly-delivered PTH(1-34), a new treatment for osteoporotic patients: results of phase I studies. Journal of Bone and Mineral Research. 2007;22 Suppl 1:S324.
- Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM, Volkmann E, Saag KG. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;62:1515–1526.
- Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, Dalsky GP, Marcus R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–2039.
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in Media Reports, Oral Bisphosphonate Prescriptions, and Hip Fractures 1996-2012: An Ecological Analysis. J Bone Miner Res. 2015;30:2179–2187.
- Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. J Bone Miner Res. 2016
- Leder BZ, O'Dea LS, Zanchetta JR, Kumar P, Banks K, McKay K, Lyttle CR, Hattersley G. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100:697–706.
- Miller P, Leder BZ, Hattersley G, Lau E, Alexandersen P, Hala T, Mustatea S, Storgaard Nedergaard B, Krogsaa A, Slesinger J, Zerbini CA, Valter I, Visockiene Z, Jendrych B, Kulak CA, Marquez F, Harris AG, Williams GC, Hu M, Riis BJ, Russo L, Christiansen C. Effects of Abaloparatide on Vertebral and Non-Vertebral Fracture Incidence in Postmenopausal Women with Osteoporosis- Results of the Phase 3 Active Trial. Endocr Rev. 2015;(Apr):36.
- Jee WSS, Li X, Tian XY, Paszty C, Ke HZ. Anti-Sclerostin Antibody Increases Bone Mass by Stimulating Bone Formation and Inhibiting Bone Resorption in a Hindlimb-Immobilization Rat Model. Abstracts of the 30th Annual Meeting of the American Society for Bone and Mineral Research 2008:S 1138.
- Li X, Warmington K, Niu QT, Grisanti M, Tan H, Dwyer D, Stolina M, Simonet WS, Kostenuik PJ, Paszty C, Ke HZ. Increases in BMD Observed with Anti-Sclerostin Antibody Treatment Are Reversible: A Longitudinal Ovariectomized Rat Study. Abstracts of the 30th Annual Meeting of the American Society for Bone and Mineral Research 2008:S 1211.
- Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30:216–224.
- McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420.
- LeBoff MS, Chou SH, Ratliff KA, Cook NR, Khurana B, Kim E, Cawthon PM, Bauer DC, Black D, Gallagher JC, Lee I, Buring JE, Manson JE. Supplemental vitamin D and incident fractures in midlife and older adults. NEJM. 2022;387(4):299-309.
- LeBoff MS, Murata EM, Cook NR, Cawthon P, Chou SH, Kotler G, Bubes V, Buring JE, Manson JE. VITamin D and OmegA-3 TriaL (VITAL): Effects of Vitamin D Supplements on Risk of Falls in the US Population. J Clin Endocrinol Metab. 2020 Jun 3;. doi: 10.1210/clinem/dgaa311. [Epub ahead of print] PubMed PMID: 32492153.
- LeBoff MS, Chou SH, Murata EM, Donlon CM, Cook N, Mora S, Lee I, Kotler G, Bubes V, Buring JE, Manson JE. Effects of Supplemental Vitamin D on Bone Health Outcomes in Women and Men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res. 2020. PMID: 31923341.
- Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, Wiessing KR, Bolland MJ, Bastin S, Gamble GD. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N Engl J Med. 2018;20:379(25):2407-2416. PMID: 30575489.
- Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. American Association of Oral and Maxillofacial Surgeons' Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J Oral Maxillofac Surg. 2022;80(5):920-943.
- Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017 Jul;5(7):513-523. doi: 10.1016/S2213-8587(17)30138-9. Epub 2017 May 22. PMID: 28546097.
- Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP. Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res. 2018;33(2):190-198.
- Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J Clin Endocrinol Metab. 2020;105(3)
202 Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C; ACTIVE Study Investigators. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016 Aug 16;316(7):722-33. doi: 10.1001/jama.2016.11136. Erratum in: JAMA. 2017 Jan 24;317(4):442. PMID: 27533157.
- Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med. 2016 Oct 20;375(16):1532-1543. doi: 10.1056/NEJMoa1607948. Epub 2016 Sep 18. PMID: 27641143.
- Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017 Oct 12;377(15):1417-1427. doi: 10.1056/NEJMoa1708322. Epub 2017 Sep 11. PMID: 28892457.
- Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB.. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract. 2020;26(suppl 1):1-46