Archives
Pituitary and Other Tumors
Posterior Pituitary
Physiology
Anatomy
Adrenal Androgens
ABSTRACT
Adrenal androgens (AA) are 19 carbon (C19) steroids that are secreted by the adrenal cortex through complicated biosynthetic pathways, which are regulated by complex mechanisms not completely understoodas of yet. Adrenal steroidogenesis differs between the fetal and adult adrenal not only in regard tothe site of production, but also in their significance for the human organism. The production of the AA is coordinated bya large number of adrenal and non-adrenal regulators. These steroids exerta number of effects in normal physiology and their excess may cause a number of different kinds of disorders.
INTRODUCTION
Adrenal androgens (AAs), normally secreted by the fetal adrenal zone and the zona reticularis of the adrenal cortex, are steroid hormones with weak androgenic activity. They includedehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEAS), androstenedione (A4), androstenediol (Α5) and 11β-hydroxyandrostenedione (11βOHA4) (1). DHEA and DHEAS are secreted in greater quantities than the other adrenal androgens. Although these steroids have little androgenic activity, theyprovide a pool of circulating precursors for peripheral conversion to more potent androgens (e.g. testosterone, T) and estrogens, (e.g. estradiol) (2-6). The production of T by the adrenal glands is minimal (7). Although adrenal androgens do not appear to play a major role in the fully androgenized adult man, they seem to play a role in the adult woman and in both sexes before puberty. Girls, women, and prepubertal boys may be negatively affected by AA hypersecretion in contrast to adult men. This chapter reviews AA biosynthesis, regulation, physiology and biological action. New data suggest that the principal androgen made by the human adrenal is 11-ketotestosterone (11-KT), a rarely studied steroid.
ADRENAL GLAND ANATOMY
Fetal Adrenal Gland(Figure 1)

Figure 1: Ontogenesis of steroidogenic enzymes in the human fetal adrenal gland. This schematic representation is divided into portions showing the fetal adrenal gland (right) at the first, second and third trimesters of pregnancy, and the adult adrenal gland (left). During the first trimester, the fetal gland is composed of a definitive zone (DZ, light grey) and a fetal zone (FZ, darker grey). Fetal zone (FZ) - expressing the P450C17 cytochrome, is responsible for massive secretion of DHEA and DHEA/S, used by the placenta as estrogen precursors. Second trimester - chromaffin cells (CC, darkest grey) originating from the neural crests migrate through the fetal cortex to progressively colonize the center of the gland to form the future medulla (Med). Third trimester - the newly constituted transitional zone (TZ, medium grey) acquires the enzyme 3ß-HSD while the expression of P450C17 remains, thus allowing the production of fetal cortisol. Near birth, cells of the definitive zone which express only 3ß-HSD, acquire the P450aldo and begin to secrete mineralocorticoids such as aldosterone. Neonatal - the fetal adrenal regresses strongly (mainly due to the regression of the fetal zone) and recovers progressively during the first years of extra-uterine life. Adult - adult adrenal gland is composed of the zona glomerulosa (ZGlo, light grey), zona fasciculata (ZFasc, medium grey) and zona reticularis (ZRet, darker grey) responsible for the production of mineralocorticoids (aldosterone), glucocorticoids (cortisol) and androgens (DHEA-DHEA/S), respectively. P450scc - cytochrome P450 side chain cleavage; Pregn. – pregnenolone; P450C17 - cytochrome P450 17a-hydroxylase, 17-20 lyase; 17OHP5 - 17-hydroxy-pregnenolone; DHEA/S - dehydroepiandrosterone-sulfate; S-Tfase - DHEA sulfotransferase; 3ß-HSD - 3ß-hydroxysteroid dehydrogenase; Prog. – progesterone; 17OHP4 - 17-hydroxyprogesterone; P450C21 - cytochrome P450 21-hydroxylase; P450C11 - cytochrome P450 11ß-hydroxylase; P450aldo - cytochrome P450 aldosterone synthase.
Fetal adrenal cortex arises from mesodermal cells migrating from the celomic epithelium very early in the embryonic period. Thus, adrenocortical tissue can be found in the ovaries, spermatic cord and testes. By the second month of gestation, the developing human fetal adrenal acquires two rudimentary, but distinct, zones: the inner fetal zonewhich consists of large eosinophilic cells, and the outer definitive zone, which is comprised of small, densely packed basophilic cells (8-9). At about the ninth week of gestation, the developing human fetal adrenal is completely encapsulated. Ultrastructural studies also have revealed a third zone between the inner fetal zone and the definitive zone, the transitional zone(10). Cells in this zone show intermediate characteristics (11) and they demonstrate the capacity to synthesize cortisol, being histologically similar to cells of the zona fasciculataof the adult adrenal cortex. By the 30th week of gestation, the human fetal adrenal cortex manifests a rudimentary form of the adult adrenal cortex; the definitive zoneand the transitional zonebegin to resemble the zona glomerulosaand the zona fasciculata, respectively (12). Although the fetal zone is functionally similar to the adult zona reticularis(where DHEA-S is produced), it produces, unlike the adult zona reticularis, largeamounts of other sulfated D5 steroids, including pregnenolone sulfate and 17a-hydroxypregnenolone sulfate.
Soon after birth, human fetal adrenal undergoes rapid involution due to the rapid regression of the inner fetal zone followed by a decrease in androgen secretion (12-17). Thus, the total weight of the glands decreases by approximately 50%. (11,18). Dramatic remodeling of the postnatal adrenal gland involves a complex combination of inner fetal zone regression and development of the zone glomerulosaand fasciculate(12,19). Because morphological studies have identified rudimentary zone glomerulosaand fasciculataduring late gestation, the development of these zones may occur from their primordial structures, although there has been a general belief that the adult cortical zones develop from the persistent definitive zone (13).
Various genetic disorders of steroidogenesis, which constitute human “gene knockout experiments of nature”, indicate that fetal adrenal steroidogenesis, and the fetal adrenal zone itself, are not essential for fetal development, survival, or parturition (20). Aging results in tissue-rearrangements within the adrenal cortex while there is a relative increase of the outer cortical zones (21). As far it regards to the zona reticularis, after a continuous growth until young adulthood (20 to 25 years), it remains at a plateau for 5 to 10 years, and it regresses gradually after the reproductive period of life (22-23).
Adult Adrenal Gland
The adult adrenal glands, consisting of cortex and medulla, have a roughly pyramidal shape, lie above the upper poles of the kidneys in the retroperitoneum and weigh approximately 4g each. They are well supplied with arterial blood from branches of the phrenic arteries, the aorta, and the renal arteries, which give rise to the superior, middle, and inferior adrenal arteries, respectively. Arterial blood enters from the outer cortex, flows through fenestrated capillaries between the cords of cells, and drains into venules in the medulla. On the right side, the adrenal vein directly enters the inferior vena cava; on the left side, it usually drains into the left renal vein. The adrenal cortex is divided into three histologic and functional zones: the outer, aldosterone-secreting, called zona glomerulosa; the intermediate, predominantly cortisol and corticosterone secreting, called zona fasciculata; and the inner, predominantly androgens secreting called zona reticularis (Figure 2). Each one is characterized by the expression of specific steroidogenic enzymes, which result in the production of different steroid hormones. Zona glomerulosa constitutes about 15% of cortical volume. Zona fasciculata is the thickest part of the adrenal cortex, constructing about 75% of the cortex, produces cortisol as well as small amounts of androgens and estrogens. Zona reticularis surrounds the medulla and produces the adrenal androgens and small amounts of cortisol and estrogens (24). Zona glomerulosa is deficient in 17a – hydroxylase activity and thus cannot produce cortisol and androgens. Whereas zona glomerulosais primarily regulated by angiotensin II and corticotropin (ACTH), both zona fasciculataand zona reticularisare regulated by ACTH (25).Both of these zones become hypofunctional and atrophic when ACTH is deficient while they become hypertrophic and hyperplastic when ACTH is secreted in excess.
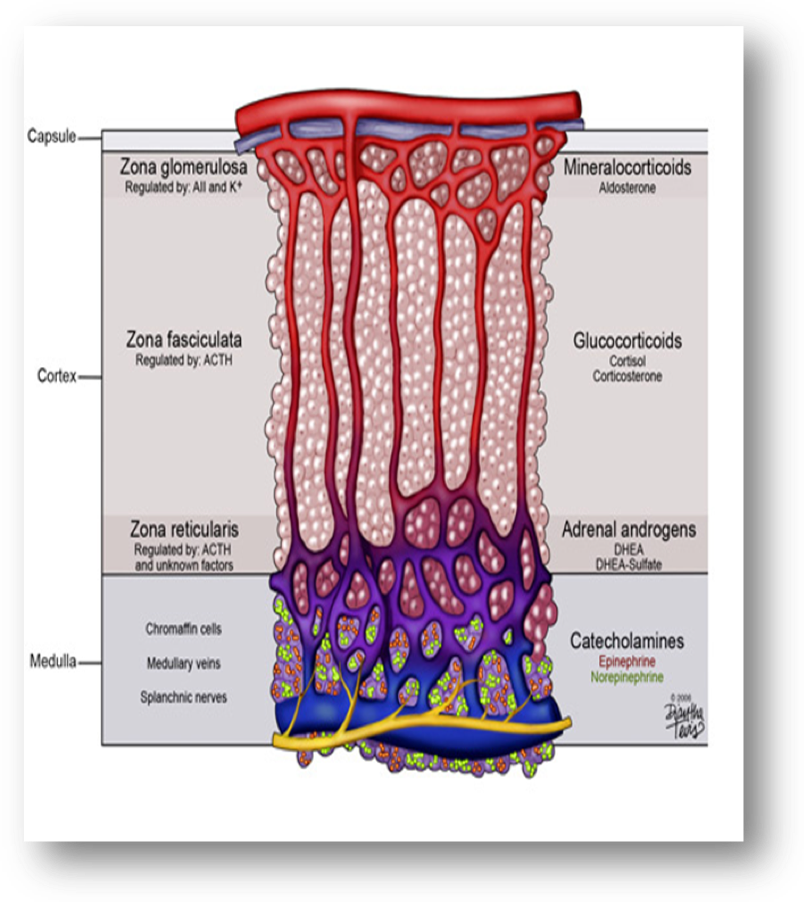
Figure 2: Schematic presentation of the adrenal zones and the main products of each zone. Downloaded from: georgiahealth.edu
The anatomical alterations of the adrenal cortex that occur during lifespan are followed by a marked decline in circulating adrenal C19 steroids and their resulting androgen metabolites. This decline takes place mainly between the age groups of 20-30 and 50-60 yr, with smaller changes observed after the age of 60 yr (26).
ADRENAL STEROIDS AND BIOSYNTHESIS OF ADRENAL ANDROGENS
Main Biosynthetic Pathway of Adrenal Steroids
All human steroid hormones derive from cholesterol. Plasma lipoproteins are the major source of adrenal cholesterol. Low–density lipoprotein (LDL) accounts for about 80% of cholesterol delivered to the adrenal gland. There are specific cell surface LDL receptors on the adrenal tissue. Synthesis within the gland from acetyl-coenzyme A also occurs. A small pool of free cholesterol within the adrenal is available for acute response when stimulation occurs. Acute stimulation leads to hydrolysis of stored cholesteryl esters to free cholesterol, increased uptake from plasma lipoproteins and increased cholesterol synthesis within the gland (27). In addition, there is evidence that the adrenal can utilize high density lipoprotein HDL cholesterol through HDL receptor, SR-B1 (28).
Cholesterol enters the steroidogenic pathwayby the action of the enzyme cholesterol esterase and transferred from the outer mitochondrial membrane of steroidogenic cells to the inner mitochondrial membrane by the steroid acute regulatory (STAR) protein. This transport is followed by the conversion of cholesterol to pregnenolone which is the first step of steroid synthesis and the major action of ACTH on adrenals (Figure 3). The conversion of cholesterol to pregnenolone requires the action of the cholesterol side-chain cleavage enzyme, commonly referred to as P450scc, encoded by the CYP11A gene located on chromosome 15 in mitochondria. This cleavage gives birth to 21-carbon (C21) molecules resulting from the C27 cholesterol molecule. These reactions require molecular oxygen and a pair of electrons. The electrons are donated by nicotinamide adenine dinucleotide phosphate (NADPH) to adrenodoxin reductase (flavoprotein) and then to adrenodoxin (an iron-sulfur protein) and finally to P450scc. Electron transport to microsomal cytochrome P450 involves the enzyme P450 reductase (Figure 4).The steroid hormones produced by the adrenal cortex are members of a large family of compounds derived from the cyclopentanoperhydrophenanthrene ring structure that comprises three cyclohexane rings and one cyclopentane ring. P450scc is needed for the production of all steroids in the human body, including those produced by the adrenal. It is expressed in all adrenal cortex zones, and although its expression is obligatory for the synthesis of C19 steroids (DHEA, DHEA-S, and A4) it is the presence of downstream enzymes that determines whether these cells produce C21 corticosteroids or C19 steroids (Figure 5).
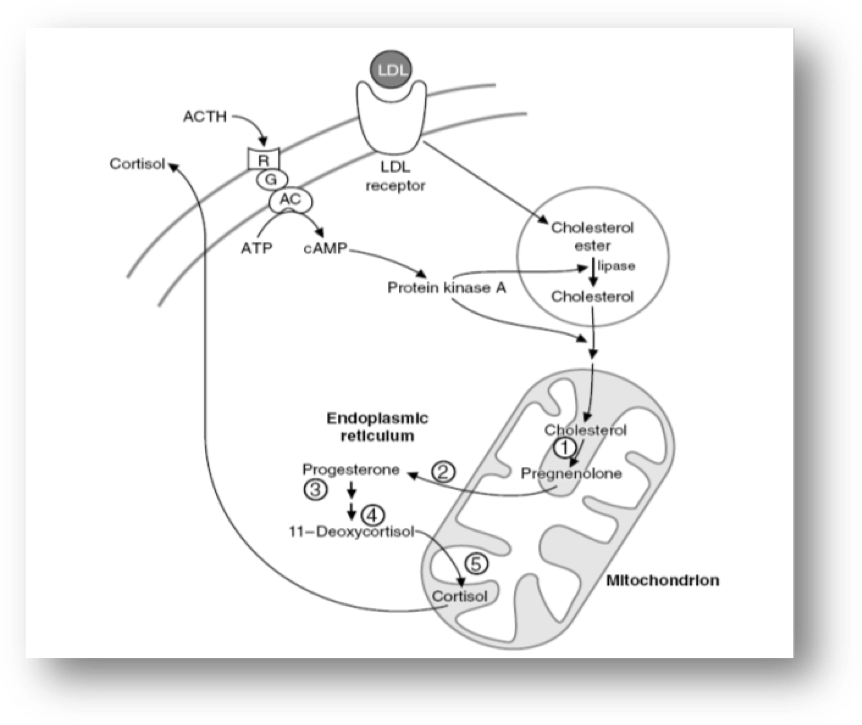
Figure 3: The role of the mitochondrion in the adrenal steroidogenesis.

Figure 4: Reaction mechanism of hydroxylations catalyzed by cytochrome P-450s of adrenal cortex mitochondria. Abbreviations: XH = substrate; XOH = product; Fp = flavoprotein; ISp = iron-sulfur protein.

Figure 5: Adrenal androgen biosynthetic pathway.
Biosynthesis of Adrenal Androgens.
The second step of adrenal steroidogenesis is mediated by cytochrome P450 17A1 enzyme which acts both as a hydroxylase hydroxylating pregnenolone, and as a lyase splitting the C17–C20 bond of 17-hydroxypregnenolone (17OHP5),resulting in the production of DHEA (29-31). Specifically, CYP17A1 gene encodes a protein that catalyzes two metabolic pathways, the 17a-hydroxylation (principal for the androgen and glucocorticoid pathway) and the 17,20 lyase reaction (specific for androgen pathway). Even though the affinity of the human Cytochrome P450 17A1 is similar either forΔ5 steroid substrate (pregnenolone) or Δ4 steroid substrate (progesterone), the predominant pathway for the 17,20 lyase reaction is viathe 17 OH pregnenolone (Δ5 substrate) (32). This 17,20-lyase activity is predominant in zona reticularis. There, the presence of cytochrome b5, form Aenzyme (encoded by the CYB5A gene), a cofactor/regulator of cytochrome P450 17A1 function, promotes 17,20-lyase activity (33).
DHEA is then converted to DHEAS sulfate by an adrenal sulfokinase (encoded by the SULT2A1 gene). This enzyme, present mostly in the cytoplasm of adrenocortical cells in zona reticularis, mediates the sulfo conjunctionof the Δ5 steroids (pregnenolone, 17α-hydroxypregnenolone, DHEA, and A5. Although all of these adrenal steroids, in fetal life, act as substrates for the corresponding sulfated products; in adult life the main substrate for the production of DHEAS is DHEA (34). During embryonic development DHEAS is supplied in maternal circulation from the fetal adrenals and acts as a substrate for estrogen synthesis from the placenta in such a way that the concentration of maternal estriol (produced in the placenta) reflects fetoplacental steroidogenesis (5). After birth however the sulfation of DHEA to DHEAS has a preventive role for androgen production by preventing excessive amounts of DHEA, a substrate for HSD3B2, to produce increased A4 and finally T (35). Of note, the expression of SULT2A1 in zona reticularisincreases during adrenarche (36).
DHEA is also converted to A4 by the enzyme 3-β-hydroxysteroid dehydrogenase (3βHSD) encoded by the HSD3B2 gene. This pathway represents the predominant pathway for the production of DHEA in humans.3-β-Hydroxysteroid dehydrogenasehas a major role in the synthesis of androgens but also of mineralocorticoids and glucocorticoids as it catalyzes the conversion of Δ5 (pregnenolone, 17α-hydroxypregnenolone, DHEA and A5) to Δ4 steroids (progesterone, 17α-hydroxyprogesterone, Α4 and T). In fetal adrenal the expression of 3βHSDpeaks at the 8thto 9thgestational week, resulting in the production of cortisol at 8thto 10thgestational week, decreasing thereafter and being undetectable at 14thgestational week.The decrease of HSD3B2 expression is followed by a decrease of cortisol synthesis. The transient cortisol synthesis by the 10thgestational week may exert a negative feedback on ACTH secretion suppressing adrenal synthesis of androgenic C19 steroids during the time of genital differentiation. Thus, in humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development (9,37). Given that fetal adrenal cell has a low expression of HSD3B2 gene until the end of 2ndtrimester (38-39) it becomes evident that the fetal adrenal gland is more likely to produce Δ5 steroids, especially DHEA, than Δ4 steroids with mineralocorticoid and glucocorticoid activities. This low HSD3B2 expression is apparent also in adrenarche where the characteristic expansion of zona reticularis, demonstrating lower concentration of HSD3B2 compared to the adjacent zona fasciculata, facilitates the increased amount of DHEAS which marks the prepubertal to adult life transition (40-42,36).
Finally, Δ4 can be converted to T, although adrenal secretion of this hormone is minimal (Figure 5) (43-45). Human type 5 17β-hydroxysteroid dehydrogenase (encoded by the 17β-HSD) catalyzes the conversion of A4 to T (46-47). The fetal as well as the postnatal adrenal also expresses AKR1C3 gene in zona reticularis, which appears to be responsible for the small amount of T produced directly by the adrenal glands (48) and is likely responsible for the larger amounts of androgens produced in congenital adrenal hyperplasia.
CIRCULATIONAND METABOLISM
Adrenal androgens are secreted from the adrenal cortex in an unbound state. Bound steroids are biologically inactive. Androstenedione, DHEA and DHEAS bind mainly to albumin. About 90% of adrenal androgens are bound to albumin and 3% approximately are bound to sex hormone-binding globulin (SHBG). The binding globulins have high affinity and low capacity, whereas, albumin has low affinity and high capacity for steroids. Adrenal androgens can follow two different pathways after entering the circulation. Their metabolism turns either towards degradation and inactivation or towards peripheral conversion to their more potent derivatives T and dihydrotestosterone (DHT).Adrenal androgens and their metabolites are inactivated or degradedin various tissues, including liver and kidney (49). Major biochemical routes for inactivation and excretion are conjugation of androgens to glucuronate or sulfate residues to produce hydrophilic glucuronides or sulfates, respectively, excreted in the urine (Figure 6A).
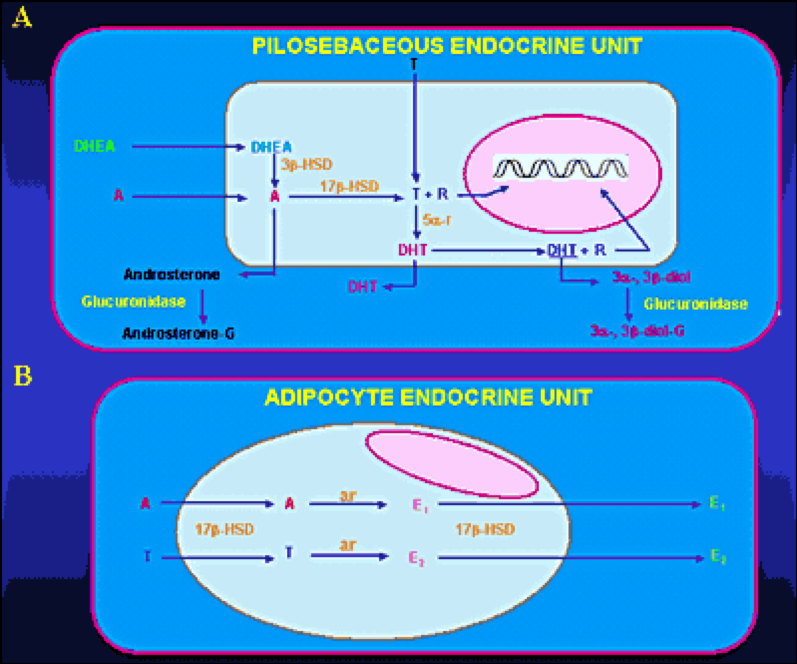
Figure 6: Metabolism of adrenal androgens in the pilosebaceous unit (A) and adipocyte tissue (B). Abbreviations: A, Δ4-androstenedione; T, testosterone; DHT, dihydrotestosterone; R, receptor; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; 5α-r, 5α-reductase; 3α-, 3β -diol, 3α-androstenediol, 3β-androstenediol; 3α-, 3β- diol-G, 3α-diol-glucuronide, 3β-diol-glucuronide; androsterone-G, androsterone glucuronide; ar, aromatase; E1, estrone; E2, 17β-estradiol
DHEA, DHEAS, and A4 are converted to the potent androgens T and DHT in peripheral tissues. Major conversions are those of A4 to T and T to DHT by the enzymes 17β-hydroxysteroid dehydrogenase (17β-HSD) and 5α-reductase, respectively. Major peripheral sites of androgen conversion are the hair follicles, the sebaceous glands (Figure 6A), the prostate, the external genitalia and the adipose tissue (50-51). DHEASis the sulfated version of DHEA. This conversion is catalyzed by sulfotransferase (SULT2A1) primarily in the adrenals, the liver, the kidney and small intestine. The concentrations of DHEAS in the circulation are about 300 times greater than those of free DHEA. The former show no diurnal variation, whereas the latter reach their peak in the early morning hours. DHEA secreted by the adrenal gland can be also converted to A4. Both DHEA and DHEAS are also metabolized to 7αand 16α– hydroxylated derivatives and by 17βreduction to Α5 and its sulfate. Androstenedione is converted either to T or by reduction of its 4,5-double bond to etiocholanolone or androsterone. Testosterone is converted to DHT in androgen-sensitive tissues by 5βreduction. The product is mainly metabolized by 3αreduction to 3α androstanediol. The metabolites of these androgens are conjugated either as glucuronides or sulfates and excreted in the urine.Active uptake of androgens and in situestrogen synthesis occur in peripheral adipose tissue (Figure 6B) through the enzymes 17β-HSD and aromatase, respectively (52-55). Peripheral conversion contributes significantly to circulating T concentration in women, but not in men, in whom T is largely produced by the testis. Three main enzyme complexes are involved in the synthesis of estrogens in peripheral tissues (56-58):
- Aromatase for the aromatization of androstenedione to estrone.
- Estrone sulfatase (E1-STS), which catalyses the formation of estrone from estrone sulfate.
- Estradiol-17-β-hydroxysteroid dexydrogenase (17β-HSD) Type 1 which is responsible for the reduction of estrone to the biologically active estrogen, estradiol.
Finally, according to recent studies, 11-KT has been found to be the principal androgen made by the human adrenal. Both A4 and T may undergo 11-hydroxylation catalyzed by P450c11b (CYP11B1 gene) to yield 11OHA4 and 11OH-testosterone (11OH-T), respectively. These 11-hydroxysteroids may be oxidized by 11β-hydroxysteroid dehydrogenase type 2 (HSD-11B2), which is more known for its role in the oxidation of cortisol to cortisone, to 11-ketoandrostenedione (11-KA4) and 11-KT, respectively (Figure 7). These 11-keto steroids may then be 5α-reduced by 5α-reductase type 2 (SRD5A2 gene) in peripheral tissues, and possibly also by 5α-reductase type 1 (SRD5A1 gene) in the adrenal itself, to 5α-androstanedione and 5α-dihydrotestosterone (5αDHT), respectively. Both 11-KT and 11-ketodihydrotestosterone (11-KDHT) are bona fideandrogens that bind to and transactivate the androgen receptor. Whereas most studies have addressed the synthesis of these steroids in castration resistant prostate cancer, other studies showed that they may have an important role also in other disease states (e.g. congenital adrenal hyperplasia).
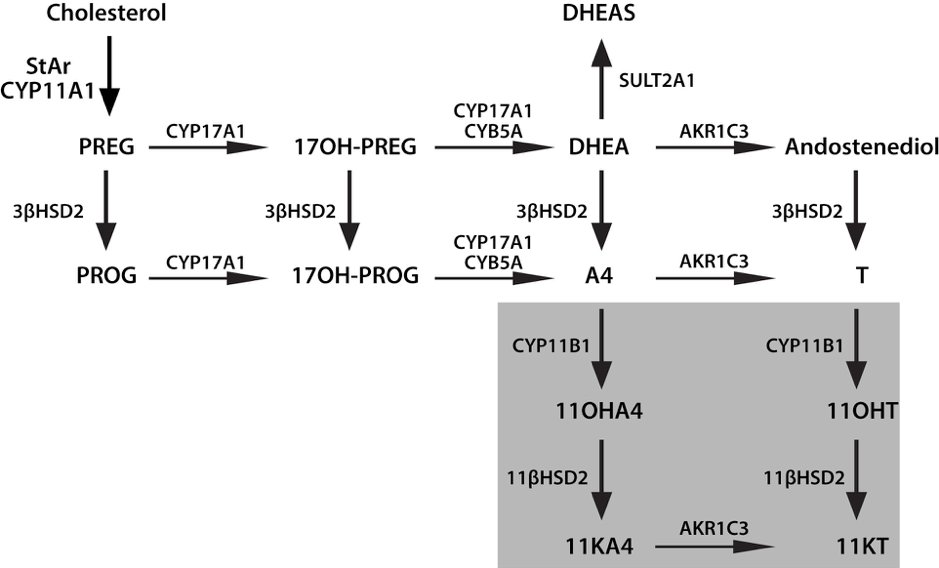
Figure 7: Novel Adrenal Androgens. 3bHSD2, 3b-hydroxysteroid dehydrogenase type 2; 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11b-hydroxyandrostenedione; 11OHT, 11b-hydroxytestosterone; 17OH-PREG, 17a-hydroxypregnenolone; 17OH-PROG, 17a-hydroxyprogesterone; A4, androstenedione; AKR1C3, aldo-keto reductase 1C3; CYB5A, cytochrome b5; CYP11A1, cytochrome P450 cholesterol side-chain cleavage; CYP11B1, cytochrome P450 11b-hydroxylase; CYP17A1, cytochrome P450 17a-hydroxylase/17,-20-lyase; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; PREG, pregnenolone; StAR, steroidogenic acute regulatory protein; T, testosterone, SULT2A1, Sulfotransferase Family 2A Member 1; PROG, progesterone; CYP17A2 cytochrome P450 family 17 polypeptide 2; 11βHSD2, 11-β-hydroxysteroid dehydrogenase type 2.
ANDROGEN RECEPTOR
The inactive androgen precursors secreted by the adrenal glands are converted to T and DHT and exert their effects in most peripheral tissues by interacting with high-affinity receptor proteins. The androgen receptor (AR), member of the steroid receptor superfamily, also known as NR3C4 (nuclear receptor subfamily 3, group C, member 4) is a type of nuclear receptor that is activated by binding to T or DHT in the cytoplasm and then trans-locates into the nucleus. The AR is most closely related to the progesterone receptor, while progestins in higher dosages can block AR. Androgen receptors are encoded by the AR gene located on the X-chromosome at Xq11-12 (59). This gene contains a polymorphic CAG microsatellite repeat within exon 1, encoding for a variable length of polyglutamine chain at the amino terminal, the transactivation domain of the AR protein. Triplet-repeat DNA sequences can be sites of genetic instability, and their expansion in a variety of genes has been associated with human genetic diseases, such as fragile X-syndrome (60-61) and myotonic dystrophy (62). In the case of the AR gene, an inverse correlation of the number of CAG repeats with the risk for prostate cancer was described (63-66) and its expansion was documented in Kennedy's disease (spinal and bulbar muscular atrophy), a disorder associated with primary hypogonadism due to androgen insensitivity(67). In vitrostudies showed that progressive expansion of the repeat length in the AR was associated with a linear decrease in its transactivation function (68). These observations support the idea that there is an optimal number of repeats, which varies in the population from 11 to 31 (average size: 21±2) (63). Methylation of deoxycytosine residues is another process involved in the modulation of gene expression. Belmont et al. (69) showed that the methylation of HpaII and HhaI sites near the polymorphic CAG repeats in the first exon of the human AR (HUMARA) locus correlated with X-inactivation.
Patients with idiopathic hirsutism were shown to have a normal number of CAG repeats but with a preponderance of the shortest and most active alleles (70). These patients had also a preferential methylation of the longer AR allele compared to normal subjects, leading to inactivation of the functionally weaker gene. This skewing could allow the shorter, more active AR allele (64,68) to be preferentially expressed explaining the peripheral hypersensitivity to androgens in hirsute patients.
Multiple "coactivators" were identified enhancing transcription of the AR gene (71) including AP-1 (72), Smad3 (73-74), nuclear factor kB (NF-kB) (75-76) sex-determining region Y (SRY) (77) and the Ets family of transcription factors (78). The relative importance of these molecules for any particular cell type remains unclear, since the ability of a putative coregulator to alter the transcriptional activity is typically examined in transient transfection experiments. Although AR is normally thought to function as a homodimer, it was also shown to heterodimerize with other nuclear receptors including the estrogen receptor (ER) (79) glucocorticoid receptor (GR) (80) and testicular orphan receptor 4 (TR4) (81). One of the major mechanisms through which coregulators might function is by forming a bridge between the DNA-bound nuclear receptor and the basal transcriptional machinery (type I regulators) (82). Coactivators may also facilitate ligand binding, promote receptor nuclear translocation, or mediate signal transduction (type II coregulators). The role of "corepressors" in AR function is poorly defined. Three corepressors of androgen-bound AR have been identified to date, cyclin D1, calreticulin, and HBO1. However, relatively little is known about the mechanism of their repressive effect.
ADRENAL ANDROGEN PHYSIOLOGY AND REGULATION
Regulation
Adrenal androgens are secreted by the adrenal glands in response to ACTH, a 39-amino acid peptide synthesized and secreted by the anterior pituitary (Figure 8).It is derived from proopiomelanocortin (POMC), a large precursor molecule from which β-lipotropin hormone and β-endorphin are also derived (83-84). ACTH is the predominant form of corticotropin in plasma and has a half-life of approximately 10 minutes (85). Its synthesis and secretion are primarily regulated by corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP), both of which are produced by parvocellular neurons of the paraventricular nucleus of the hypothalamus and act in synergy with each other (86-87). Under ACTH regulation, adrenal androgens are secreted synchronously with cortisol. There are three mechanisms of neuroendocrine control: [1] episodic secretion and the circadian rhythm of ACTH, [2] stress responsiveness of the hypothalamic-pituitary-adrenal axis (HPA), [3] feedback inhibition of ACTH secretion by cortisol. [1]
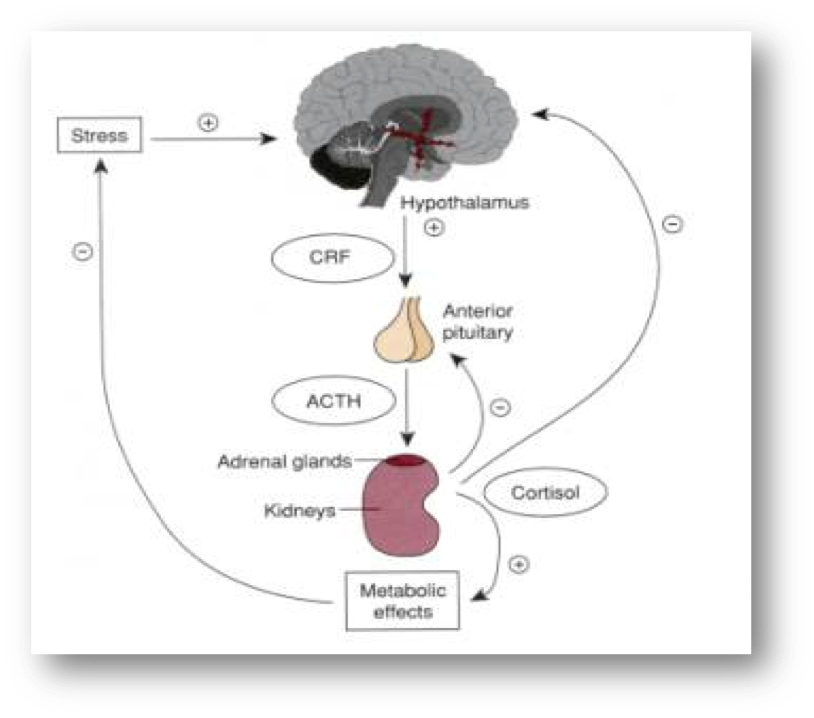
Figure 8: Schematic presentation of the adrenal androgen regulation. Downloaded from: wikis.lib.ncsu.edu
The circadian rhythm is the result of the central nervous system regulation of CRH and ACTH nyctohemeral secretory episodes. The major secretory episodes begin in the sixth to eighth hour of sleep and then begin to decline as wakefulness occurs. Cortisol secretion then gradually declines during the day with fewer secretory episodes (88). The circadian rhythm of adrenal androgens is typical in different physiologic and pathologic conditions. Patients with nonclassical 21-hydroxylase deficiency, for example, have a distinct pattern of adrenal steroid secretion characterized by a high-frequency 17-hydroxyprogesterone release accompanied by a relative nocturnal cortisol deficiency (89-90). [2] Plasma ACTH and cortisol secretion are secreted within minutes following the onset of physical stress. This response abolishes circadian periodicity if the stress is prolonged. Stress responses originate in the CNS and result to CRH and ACTH secretion. [3] Corticotropin-stimulated cortisol exerts major feedback inhibitory influences at the concentration of both the hypothalamus and the anterior pituitary by suppressing CRH and ACTH synthesis and secretion.
Plasma DHEA, A4, and T concentrations parallel closely the circadian rhythm of plasma cortisol. Plasma DHEA-S concentrations do not exhibit a circadian rhythm because of the much longer circulating half-life of this sulfated steroid (91-92). Numerous other endocrine signals (93) were proposed as coregulators of adrenal androgen secretion. Among these are prolactin (PRL) (94), estrogen (95-99), epidermal growth factor (100), prostaglandins (101), angiotensin (102), GH (103), gonadotropins (104-105), β-lipotropin, and β-endorphin. Glasow et al. reported the presence of PRL receptors in the human adrenal gland and suggested a direct effect of PRL on adrenal steroidogenesis that may be of particular relevance in clinical disorders characterized by hyperprolactinemia (106). Interestingly, adults with hyperprolactinemia have increased secretion of AAs by the zona reticularis, which is corrected by reduction of PRL secretion with bromocriptine (107). In women with PRL-secreting tumors there is a correlation between PRL concentration and DHEA-S (108). Pabon et al. (109) have detected the presence of LH- HCG receptors in zona reticularis and fasciculata. The receptor bearing cells were positive for steroidogenic enzymes, indicating that the receptors could be coupled to DHEAS secretion (110-112).
Cytokines interfere with steroidogenesis at the level of the adrenals, testes and ovaries. Within the adrenal adrenocortical and chromaffin cells cytokines such as interleukin (IL) 1, IL-6, tumor necrosis factor (TNF), leukemia inhibitory factor (LIF) and IL-18 are produced. They have a key role in the immune-adreno-cortical communication. Thus, in autoimmune and inflammatory diseases an adequate adrenal stress response is observed. In addition, cytokines such as IL-8 and monocyte chemotactic protein-1 (MCP-1) are involved in steroidogenesis (113). ΙL-6 also is known to activate the HPA axis by stimulating both the CRH and the AVP -secreting neurons of the paraventricular nucleus of the hypothalamus, and their terminals at the median eminence, the corticotrophs of the anterior pituitary, and the cortisol-secreting adrenal cells in rats. In the latter it acts through specific receptors expressed mainly in the zona fasciculata and reticularis, but also with lower density in the zona glomerulosa (114-115). The ability of IL-6 to stimulate glucocorticoids, mineralocorticoids, and androgens suggests that this cytokine might have a role in coordinating the response of all adrenocortical zones. Its secretion is regulated by different substances, such as CRH, ACTH, angiotensin II, or immune products such as IL-1 α/βindicating that IL-6 may play a major role in the interaction of the adrenal function with the immune/inflammatory reaction (116).
Interleukin 1 and TNF regulate the activity of HPA axis at several levels. Studies investigated their action on adrenal steroidogenesis and indicated that IL-1αand IL-1βincrease cortisol, A4, DHEA, DHEAS production and the accumulation of mRNAs for STAR, 17α-hydroxylase/17,20-lyase (CYP17A1) and HSD3B2 in these cells. TNF induced cortisol production (117).
Both ACTH and PRL stimulate AAs secretion by the fetal adrenal zone. In addition, placental CRH appears to play a major role in sustaining this zone and stimulating androgen secretion together with corticotropin and/or PRL (118).
Physiology
Adrenal androgens are secreted in small amounts during infancy and early childhood. DHEAS is maintained at minimum concentrations for 5 years in both male and females, after which a gradual increase is observed (115). Their secretion gradually increases with age, paralleling the growth of zona fasciculata and zona reticularis.Disturbances in both enzymatic activity in zona fasciculata and zona reticularis and its regulators (ACTH or peptides of hypothalamic – pituitary origin, such as PRL) may result in syndromes of hirsutism and virilization in females. Adrenal cortex normally secretes androgens in increasing amounts beginning at about 6-7 years of age in girls and 7-8 years of age in boys. This rise continues until late puberty. Adrenarche (secretion of adrenal androgens) occurs years before gonadarche (secretion of gonadal sex steroids). The appearance of pubic hair (pubarche) results from a rise in adrenal androgen concentration (adrenarche) (116-117). The mechanism(s) by which zona fasciculata and zona reticularis develop with age, as well as the regulation of adrenarche onset are not understood. The biochemical hallmark of adrenarche is accelerated DHEAS production from the adrenal gland. The axillary and pubic hair regions are the most sensitive androgen-dependent regions and they represent the clinical manifestation of adrenarche. Children with premature pubarche demonstrate hormonal responses to CRH stimulation test similar in magnitude to those of prepubertal children of comparable age, ruling out a prominent role of CRH in premature pubarche (118). Gell et al. suggested that as children mature, a decrease of HSD3B2 activity in the adrenal zona reticularis occurs, leading to an increased production of DHEA and DHEA-S, as seen during adrenarche, by shifting pregnenolone through the 17α-hydroxylase/17, 20 lyase pathway (Figure 5) (39).
Activation of the type 1 insulin-like growth factor (IGF1) receptor was shown to enhance steroidogenic responsiveness of the fetal zone cells to ACTH by modulating the ACTH signal transduction pathway at some point downstream from ACTH receptor binding (119). Also, locally produced IGF2 modulates fetal adrenocortical cells function by increasing responsiveness to ACTH viaactivation of the IGF1 receptor and increases the capacity of those cells for androgen synthesis by directly augmenting the expression of P450c17 (119). Thus, IGF2 may play a pivotal role in AA production, both physiologically in uteroand at adrenarche, as well as in conditions of hyperandrogenemia (119). All together, these data indicate that the IGF system is important in the regulation of the differential function of adult human adrenocortical cells (120). The rise in plasma concentrations of the AAs at adrenarche occurs in the presence of constant cortisol concentrations, suggesting that factors other than corticotropin are involved. The influences of sex and age are minor in the modulation of adrenal steroidogenesis supporting the conceptthat extra-adrenal factors prevail in the differential modulation of AAs and cortisol (121). These may include POMC-derived or other still uncovered peptides. An increased serine phosphorylation of human P450c17 might have a role in the development of both the excessive adrenarche and hyperandrogenism of patients with the polycystic ovary syndrome (PCOS) resulting in a substantial increase in 17-20-lyase activity (122-124) (Figure 5). P450c17 is the key enzyme that regulates androgen synthesis. (125). It is the only enzyme known to be able to convert C21-precursors to the androgen pro-hormones, the 17-ketosteroids. It is a single enzyme with two activities, 17 a-hydroxylase and 17,20-lyase (Figure 5) and serine phosphorylation appears to modulate the activity of P450c17. In particular, it promotes the 17,20-lyase activity, and at the same time it inhibits the activity of the insulin receptor (123-124,126-128). It was postulated that a single abnormal serine kinase might hyperphosphorylate both P450c17 and the insulin receptor, accounting for the hyperandrogenism and hyperinsulinism responsible for both premature pubarche and PCOSlater in life(129). In vitrostudies, however, failed to find evidence for increased autophosphorylation of the insulin receptor-βsubunit and P450c17 in PCOS (130). The reason for this might be related to the many different factors needed for P450c17 optimal activity and not normally expressed in the cell line used for that study (48).
BIOLOGIC EFFECTS
In adult men, the conversion of adrenal A4 to T accounts for less than 5% of the production rate of the latter, making its participation in the physiologic androgenization of the male negligible. Excessive AA secretion appears to have no major clinical consequences in the adult man, although this may be debated. Adrenal androgens hypersecretion in prepubertal boys, on the other hand has clearly been associated with isosexual precocious puberty.
In adult women, adrenal A4 and A4 generated from peripheral conversion of DHEA contribute substantially to total androgen production and effects. In the follicular phase of the menstrual cycle, adrenal precursors account for two thirds of Tproduction and half ofDHTproduction. At midcycle, the ovarian contribution increases, and the adrenal precursors account for 40% of T production. In women, increased AA production may be manifested as cystic acne, hirsutism, male type baldness, menstrual irregularities, oligoovulation or anovulation, infertility, and/or frank virilization. Excessive adrenal androgen secretion in prepubertal or pubertal girls can cause heterosexual precocious puberty.
Abnormalities in the timing and intensity of adrenarche are associated with PCOS, congenital adrenal hyperplasia (CAH) and insulin resistance conditions. Recently, we have shown that in postmenopausal PCOS women, androgen concentration at baseline are greater in PCOS than control women and remain increased after ACTH stimulation, while the results of the dexamethasone suppression test in postmenopausal PCOS women suggest that DHEAS and total T are partially of adrenal origin (131). Although the ovarian contribution was not fully assessed, increased A4 production suggests that the ovary also contributes to hyperandrogenism in postmenopausal PCOS women. In conclusion, this study indicates that postmenopausal PCOS women are exposed to higher adrenal and ovarian androgen concentrations than non-PCOS women (131).
Studies conducted over the past few years have investigated the use of DHEA to treat female infertility (132-133). Women with poor ovarian reserve, after DHEA supplementation 4 to 12 weeks prior an in vitrofertilization (IVF) cycle, had a 50-80% reduction in miscarriages (134). However, its efficacy in treating infertility remains controversial (135-137).
Reports demonstrate DHEA as a replacement therapy in the elderly (138-139). At 70-80 years of age, peak DHEA concentrations are about 10-20% of those in young adults. These reports suggest DHEA as replacement treatment in menopausal women; it has been reported to restore both the androgenic and estrogenic environment and reduce most of the symptoms of menopause (140-142). Other reports have suggested that oral DHEA in doses of 25-50 mg/d may restore plasma T concentrations to normal in some women with hypopituitarism who have diminished libido despite adequate estrogen therapy (143-145). In addition, DHEA replacement therapy has been investigated for the conditions of adrenopause and adrenal insufficiency (146-149). In spite of these few reports so far DHEA does not appear to be effective for perimenopausal symptoms (135) nor has it been shown to be effective as an “anti-aging” agent, as its effects in trials on cognitive function, body composition, insulin resistance, and well-being have been inconsistent (150-157,146). Based upon available data, the Endocrine Society guidelines, suggested against the routine use of DHEA for sexual function (or other indications) in postmenopausal women because of its limited efficacy and lack of long-term safety data (158). Clinical trial data on the efficacy of DHEA therapy in women with primary adrenal insufficiency are mixed.
In several studies of women with premature ovarian insufficiency (POI), serum ovarian androgen concentrations (A4and/or T) were lower than those of age-matched women without ovarian insufficiency, but similar to those seen in older postmenopausal women (159-161). In contrast, DHEAS concentrations were normal (although they would be expected to be low in those women with coexisting primary adrenal insufficiency).Potential side effects of androgen replacement include hirsutism and acne, and with oral preparations (e.g. DHEA), dyslipidemia. However, in women with autoimmune ovarian failure and coexisting adrenal insufficiency, adrenal androgen therapy with DHEA may be beneficial.
Other studies investigate the role of DHEA and DHEAS in the immune response and suggest that adrenal androgens have opposite biological effects to those of corticosteroids (162). DHEA is a cortisol antagonist (163). Research studies indicate DHEA supplementation has an anti-depressant effect (164-166). Exogenous DHEA has been proposed to have a number of potential benefits (on sexual function, depression, cognition, and inflammation), but available clinical trial data do not support these claims (115,167-168,137). It is widely available in some countries as a dietary supplement; however, quality control of these products has been shown to be quite poor (169,170).
Both gonadal and AAs contribute to the positive impact of androgenic steroids on bone cell metabolism in vitro(171). Interestingly, a study found that the potential anabolic effect of androgens on bone might not be mediated at the level of the mature osteoblast but at the level of fetal, less differentiated, osteoblastic cell lines (172).
Finally, although A4 seems to increase serum T and estrone concentrations when administered acutely to women, (173) the impact of regular use on sexual function or its potential androgenic side effects in women are unknown
CONCLUSIONS
The physiology of adrenal androgens follows the different periods of life starting from the fetal period. During this period, the secretion of these hormones from the fetal adrenal is important. It is not clarified as yet its role in the fetal development or survival, while it is of major importance for parturition. DHEA is the most prevalent steroid hormone in the body. After birth DHEA(S) concentrations fall rapidly with the involution of the fetal adrenal and rise slowly during childhood accelerating at adrenarche before the onset of puberty. The physiology of adrenarche is well described although its trigger has not been identified yet. DHEA concentrations drop dramatically with aging. There are pronounced differences in the average DHEA concentrations between men and women, with women on average having lower DHEA concentrations. The spectrum of women and men that would benefit from DHEA therapy is not clearly defined. Further studies are needed to investigate the side effects of the DHEA replacement therapy and to define the range of dosage that is more effective without complications. During menopause transition mean circulating DHEAS concentrations exhibit a positive inflection starting in the early perimenopause, continuing through the early post menopause and returning to early perimenopausal concentrations by late post menopause. This rise in mean DHEAS is accompanied by concomitant rises in T, DHEA, A4, and an equal rise in A5. Studies have shown that the mean A4 and T concentrations changed the least while mean DHEAS and A5 changed the most. The role of these changes in altering the estrogen/androgen balance in menopause is not known.
REFERENCES
- Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. The Journal of clinical endocrinology and metabolism. 2013; 98:1182–1188.
- Kaufman FR, Stanczyk FZ, Matteri RK, et al. Dehydroepiandrosterone and dehydroepiandrosterone sulfate metabolism in human genital skin. Fertility and sterility. 1990; 54:251–254.
- Luu-The V. Assessment of steroidogenesis and steroidogenic enzyme functions. J Steroid Biochem Mol Biol. 2013; 137:176–182.
- Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best practice & research Clinical endocrinology & metabolism. 2008; 22:223–228.
- Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Seminars in reproductive medicine. 2004; 22:327–336.
- Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. The journal of investigative dermatology Symposium proceedings/the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 2005; 10:205–208
- Longcope C. Adrenal and gonadal androgen secretion in normal females. In: Horton R, Lobo RA, eds. Clinics in Endocrinology and Metabolism. Philadelphia: W.B. Saunders, 1986:213-228.
- Hanley NA, Rainey WE, Wilson DI, et al. 2001. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol 15:57–68.
- Goto M, Piper Hanley K, Marcos J, et al. 2006. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 116:953–960.
- Mesiano S, Coulter CL, Jaffe RB. 1993. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17 α-hydroxylase/17, 20-lyase, and 3β-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab 77:1184–1189.
- McNutt NS, Jones AL. 1970. Observations on the ultrastructure of cytodifferentiation in the human fetal adrenal cortex. Lab Invest 22:513–527.
- Sucheston ME, Cannon MS. 1968. Development of zonular patterns in the human adrenal gland. J Morphol 126:477–491.
- Keene MF, Hewer EE. 1927. Observations on the development of the human suprarenal gland. J Anat 61:302–324.
- Grueters A, Korth-Schutz S. 1982. Longitudinal study of plasma dehydroepiandrosterone sulfate in preterm and fullterm infants. J Clin Endocrinol Metab 55:314–320.
- Honour JH, Wickramaratne K, Valman HB. 1992. Adrenal function in preterm infants. Biol Neonate 61:214–221.
- Kojima S, Yanaihara T, Nakayama T. 1981. Serum steroid levels in children at birth and in early neonatal period. Am J Obstet Gynecol 140:961–965.
- Wiener D, Smith J, Dahlem S, et al 1987. Serum adrenal steroid levels in healthy full-term 3-day-old infants. J Pediatr 110:122–124.
- Lanman JT. 1953. The fetal zone of the adrenal gland: its developmental course, comparative anatomy, and possible physiologic functions. Medicine (Baltimore) 32:389–430.
- Bocian-Sobkowska J. 2000. Morphometric study of the human suprarenal gland in the first postnatal year. Folia Morphol (Warsz) 58:275–284.
- Walter L. Miller. Steroidogenesis: Unanswered Questions Review. Trends Endocrinol Metab. 2017 Nov;28(11):771-793.
- Parker CRJ, Mixon RL, Brissie RM, et al. Aging alters zonation in the adrenal cortex of men. J.Clin.Endocrinol.Metab. 1997; 82:3898-3901.
- Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf). 2004 Mar;60(3):288-96. Review.
- Auchus RJ. The physiology and biochemistry of adrenarche. Endocr Dev. 2011; 20:20-7.
- Guyton Physiology 11th ed. Chapter 77. Adrenocortical Hormones.
- Davis JO. Regulation of aldosterone secretion. In: Einstein AB e, ed. The adrenal cortex. Boston: Little, Brown, 1967:203-247.
- Labrie F, Belanger A, Cusan L, et al. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin.Endocrinol. Metab. 1997; 82:2396-2402.
- Kronenberg. Williams Textbook of Endocrinology 11th ed. Chapter 14. The adrenal cortex.
- Hoekstra M., Van Berkel Theo JC., Van Eck M. A multi-purpose player in cholesterol and steroid metabolism. World J Gastroenterol. 2010 December 21;16(47):5916-5924.
- Bradshaw KD, Waterman MR, Couch RT, et al. Characterization of complementary deoxyribonucleic acid for human adrenocortical 17 alpha-hydroxylase: a probe for analysis of 17 alpha- hydroxylase deficiency. Mol Endocrinol. 1987;1(5):348–354.
- Kagimoto M, Winter JS, Kagimoto K, et al. Structural characterization of normal and mutant human steroid 17 alpha- hydroxylase genes: molecular basis of one example of combined 17 alpha- hydroxylase/17,20 lyase deficiency. Mol Endocrinol. 1998;2(6):564–570.
- Swart P, Estabrook RW, Mason JI, et al. Catalytic activity of human and bovine adrenal cytochromes P-450 17 alpha, lyase expressed in Cos 1 cells. Biochem Soc Trans. 1989;17(6):1025–1026.
- Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 1998; 273:3158–3165.
- Turcu et al. Adrenal androgens and androgen precursors: definition, synthesis, regulation and physiologic actions. Compr Physiol. 2014 Oct; 4(4): 1369–1381.
- Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. The Journal
of steroid biochemistry and molecular biology. 2008; 108:281-6.
- Noordam C, Dhir V, McNelis JC, et al. Inactivating PAPSS2 mutations in a patient with premature pubarche. The New England journal of medicine. 2009; 360:2310–2318.
- Suzuki T, Sasano H, Takeyama J, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clinical endocrinology. 2000; 53:739–747.
- Asby Daniel J, Arlt Wiebke, Hanley Neil A. The adrenal cortex and sexual differentiation during early human development. Reviews in Endocrine and Metabolic Disorders 2008.
- Goldman AS, Yakovac WC, Bongiovanni AM. Development of activity of 3 beta-hydroxysteroid dehydrogenase in human fetal tissues and in two anencephalic newborns. J Clin Endocrinol Metab. 1966 Jan;26(1):14-22.
- Simonian MH, Capp MW. Characterization of steroidogenesis in cell cultures of the human fetal adrenal cortex: comparison of definitive zone and fetal zone cells. J Clin Endocrinol Metab. 1984 Oct;59(4):643-51.
- Gell JS, Atkins B, Margraf L, et al. Adrenarche is associated with decreased 3 beta-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocrine research. 1996; 22:723–728.
- Gell JS, Carr BR, Sasano H, et al. Adrenarche results from development of a 3beta-hydroxysteroid dehydrogenase-deficient adrenal reticularis. The Journal of clinical endocrinology and metabolism. 1998; 83:3695-701.
- Hui XG, Akahira J, Suzuki T, et al. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. The Journal of endocrinology. 2009; 203:241–252.
- Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. 2005 Jun;34(2):293-313, viii. Review.
- Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001 Mar;15(1):79-94. Review.
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004 Dec;25(6):947-70.
- Deyashiki Y, Ogasawara A, Nakayama T, et al. Molecular cloning of two human liver 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase isoenzymes that are identical with chlordecone reductase and bile-acid binder. The Biochemical journal. 1994;299 (Pt 2):545–552.
- Dufort I, Rheault P, Huang XF, et al. Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999; 140:568–574.
- Nakamura Y, Hornsby PJ, Casson P, et al. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. The Journal of clinical endocrinology and metabolism. 2009; 94:2192–2198
- Norman AW LG. Androgens. In: Norman and Litusassk eds, ed. Hormones. San Diego: 1987:483-513
- Schweikert HU, Wilson JD. Regulation of human hair growth by steroid hormones. I. Testosterone metabolism in isolated hairs. J. Clin.Endocrinol. Metab. 1974; 38:811-819.
- Schweikert HU, Milewich L, Wilson JD. Aromatization of androstenedione by isolated human hairs. J. Clin.Endocrinol. Metab. 1975; 40:413-417.
- Deslypere JP, Verdonck L, Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J. Clin.Endocrinol. Metab. 1985; 61:564-570.
- Kirschner MA, Samojlik E, Drejka M, et al. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J. Clin.Endocrinol. Metab. 1990; 70:473-479.
- McNatty KP, Makris A, Reinhold VN, et al. Metabolism of androstenedione by human ovarian tissues in vitro with particular reference to reductase and aromatase activity. Steroids 1979; 34:429-443.
- Ackerman GE, Smith ME, Mendelson CR, et al: Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab 1981, 53:412-417.
- Simpson ER, Ackerman GE, Smith ME, et al: Estrogen formation in stromal cells of adipose tissue of women: induction by glucocorticoids. Proc Natl Acad Sci USA 1981, 78:5690-5694.
- Zhao Y, Nichols JE, Bulnn SE, et al: Aromatase P450 gene expression in human adipose tissue. J Biol Chem 1995, 270:16449-16457.
- Simpson ER, Mahendroo MS, Means GD, et al: Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev 1994.
- Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell 1995; 83:835-839.
- Kremer EJ, Pritchard M, Lynch M, et al. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science 1991;252:1711-1714.
- Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991; 65:905-914.
- Fu YH, Pizzuti A, Fenwick RGJ, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 1992; 255:1256-1258.
- Schoenberg MP, Hakimi JM, Wang S, et al. Microsatellite mutation (CAG24-->18) in the androgen receptor gene in human prostate cancer. Biochem. Biophys. Res. Commun. 1994; 198:74-80.
- Hardy DO, Scher HI, Bogenreider T, et al. Androgen receptor CAG repeat lengths in prostate cancer: correlation with age of onset. J. Clin.Endocrinol. Metab. 1996; 81:4400-4405.
- Coetzee GA, Ross RK. Re: Prostate cancer and the androgen receptor. J.Natl.Cancer Inst. 1994;86:872-873.
- Irvine RA, Yu MC, Ross RK, et al. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res. 1995; 55:1937-1940.
- La Spada AR, Wilson EM, Lubahn DB, et al. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991; 352:77-79.
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic.Acids.Res. 1994;22:3181-3186.
- Allen RC, Zoghbi HY, Moseley AB, et al. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am.J.Hum.Genet. 1992; 51:1229-1239.
- Vottero A, Stratakis CA, Ghizzoni L, et al. Androgen receptor-mediated hypersensitivity to androgens in women with nonhyperandrogenic hirsutism: skewing of X-chromosome inactivation. J. Clin.Endocrinol Metab 1999;84:1091-1095.
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr.Rev.2002. Apr.;23. (2.):175.-200. 23:175-200.
- Sato N, Sadar MD, Bruchovsky N, et al. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J.Biol.Chem. 1997;272:17485-17494.
- Hayes SA, Zarnegar M, Sharma M, et al. SMAD3 represses androgen receptor-mediated transcription. Cancer Res.2001. Mar.1.;61. (5.):2112.-8. 61:2112-2118.
- Kang HY, Lin HK, Hu YC, et al. From transforming growth factor-beta signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc.Natl.Acad.Sci.U.S.A.2001. Mar.13.;98. (6.):3018.-23. 98:3018-3023.
- Palvimo JJ, Reinikainen P, Ikonen T, et al. Mutual transcriptional interference between RelA and androgen receptor. J.Biol.Chem. 1996;271:24151-24156.
- Aarnisalo P, Palvimo JJ, Janne OA. CREB-binding protein in androgen receptor-mediated signaling. Proc.Natl.Acad.Sci.U.S.A. 1998;95:2122-2127.
- Yuan X, Lu ML, Li T, et al. SRY interacts with and negatively regulates androgen receptor transcriptional activity. J.Biol.Chem.2001. Dec.7.;276. (49.):46647.-54. 276:46647-46654.
- Schneikert J, Peterziel H, Defossez PA, et al. Androgen receptor-Ets protein interaction is a novel mechanism for steroid hormone-mediated down-modulation of matrix metalloproteinase expression. J.Biol.Chem. 1996;271:23907-23913.
- Panet-Raymond V, Gottlieb B, Beitel LK, et al. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol.Cell Endocrinol.2000. Sep.25.;167. (1.-2.):139.-50. 167:139-150
- Chen S, Wang J, Yu G, et al. Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J.Biol.Chem. 1997;272:14087-14092.
- Lee YF, Shyr CR, Thin TH, et al. Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: a unique signaling pathway in the steroid receptor superfamily. Proc.Natl.Acad.Sci.U.S.A. 1999;96:14724-14729
- Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev.2000. Oct.15.;14. (20.):2551.-69. 14:2551-2569. 94.
- Brown JD, Doe RP. Pituitary pigmentary hormones. Relationship of melanocyte-stimulating hormone to lipotropic hormone. JAMA 1978; 240:1273-1278.
- Krieger DT, Liotta AS, Suda T, et al. Human plasma immunoreactive lipotropin and adrenocorticotropin in normal subjects and in patients with pituitary-adrenal disease. J. Clin.Endocrinol. Metab. 1979; 48:566-571.
- Nicholson WE, Liddle RA, Puett D, et al. Adrenocorticotropic hormone biotransformation, clearance, and catabolism. Endocrinology 1978; 103:1344-1351.
- Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213:1394-1397.
- Lamberts SW, Verleun T, Oosterom R, et al. Corticotropin-releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J. Clin.Endocrinol. Metab. 1984; 58:298-303.
- Gardner D.G., Shoback D. Greenspan’s Basic & Clinical Endocrinology. 8th edition. Lange. Mc Graw Hill
- Ghizzoni L, Bernasconi S, Virdis R, et al. Dynamics of 24-hour pulsatile cortisol, 17-hydroxyprogesterone, and androstenedione release in prepubertal patients with nonclassic 21- hydroxylase deficiency and normal prepubertal children. Metabolism 1994; 43:372-377.
- Ghizzoni L, Mastorakos G, Vottero A, et al. Spontaneous cortisol and growth hormone secretion interactions in patients with nonclassic 21-hydroxylase deficiency (NCCAH) and control children. J. Clin.Endocrinol. Metab. 1996; 81:482-487.
- Rosenfeld RS, Rosenberg BJ, Fukushima DK, et al. 24-Hour secretory pattern of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J. Clin.Endocrinol. Metab. 1975; 40:850-855.
- Feuillan P, Pang S, Schurmeyer T, et al. The hypothalamic-pituitary-adrenal axis in partial (late-onset) 21-hydroxylase deficiency. J. Clin.Endocrinol. Metab. 1988; 67:154-160.
- Parker LN. Control of adrenal androgen secretion. Endocrinol Metab Clin.North Am. 1991;20:401-421.
- Thomas G, Frenoy N, Legrain S, et al. Serum dehydroepiandrosterone sulfate levels as an individual marker. J. Clin.Endocrinol. Metab. 1994; 79:1273-1276.
- Warne GL, Carter JN, Faiman C, et al. Hormonal changes in girls with precocious adrenarche: a possible role for estradiol or prolactin. J. Pediatr. 1978; 92:743-747.
- Wathen NC, Perry L, Hodgkinson S, et al. The relationship between prolactin, dehydroepiandrosterone sulphate and testosterone in normally menstruating females. Acta Endocrinol. (Copenh.) 1985;109:173-175.
- Sklar CA, Kaplan SL, Grumbach MM. Lack of effect of oestrogens on adrenal androgen secretion in children and adolescents with a comment on oestrogens and pubic hair growth. Clin.Endocrinol. (Oxf.) 1981;14:311-320.
- Zachmann M, Manella B, Eiholzer U, et al. Influence of oestrogen in high and low doses on plasma steroid concentrations in girls with tall stature and Turner syndrome. Acta Endocrinol. (Copenh.) 1984;106:368-373.
- Sobrinho LG, Kase NG, Grunt JA. Changes in adrenocortisol function of patients with gonadal dysgenesis after treatment with estrogen. J. Clin.Endocrinol. Metab. 1971; 33:110-114.
- Mattila AL, Perheentupa J, Pesonen K, et al. Epidermal growth factor in human urine from birth to puberty. J. Clin.Endocrinol. Metab. 1985; 61:997-1000
- Keymolen V, Dor P, Borkowski A. Output of oestrogens, testosterone and their precursors by isolated human adrenal cells as compared with that of glucocorticosteroids. J.Endocrinol. 1976; 71:219-229
- Parker LN, Lifrak ET, Kawahara CK, et al. Angiotensin II potentiates ACTH-stimulated adrenal androgen secretion. J. Steroid Biochem. 1983; 18:205-208.
- Zadik Z, Chalew SA, McCarter RJJ, et al. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J. Clin.Endocrinol. Metab. 1985; 60:513-516
- Lee PA, Kowarski A, Migeon CJ, et al. Lack of correlation between gonadotropin and adrenal androgen levels in agonadal children. J. Clin.Endocrinol. Metab. 1975; 40:664-669.
- Sizonenko PC, Paunier L. Hormonal changes in puberty III: Correlation of plasma dehydroepiandrosterone, testosterone, FSH, and LH with stages of puberty and bone age in normal boys and girls and in patients with Addison's disease or hypogonadism or with premature or late adrenarche. J. Clin.Endocrinol. Metab. 1975; 41:894-904.
- Glasow A, Breidert M, Haidan A, et al. Functional aspects of the effect of prolactin (PRL) on adrenal steroidogenesis and distribution of the PRL receptor in the human adrenal gland. J. Clin.Endocrinol. Metab. 1996; 81:3103-3111.
- Lobo RA, Kletzky OA, Kaptein EM, et al. Prolactin modulation of dehydroepiandrosterone sulfate secretion. Am.J.Obstet.Gynecol. 1980;138:632-636
- Yamaji T, Ishibashi M, Takaku F, et al. Role of prolactin in age-related change in serumdehydroepiandrosterone sulphate concentrations. Acta Endocrinol. (Copenh.) 1989;120:655-660.
- Pabon JE, Li X, Lei ZM, et al. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab. 1996 Jun;81(6):2397-400.
- Harold E. Carlson. Human adrenal cortex hyperfunction due to LH/hCG. Molecular and Cellular Endocrinology 269 (2007) 46–50. Review.
- Rao C.V. Human adrenal LH/hCG receptors and what they could mean for adrenal physiology and pathology. Molecular and Cellular Endocrinology 329 (2010)33-36. Review.
- Rao Ch., Zhou X.L, and Lei Z.M. Functional Luteinizing Hormone/Chorionic Gonadotropin Receptors in Human Adrenal Cortical H295R Cells. Biology of Reproduction 71, 579–587 (2004).
- Bornstein S.R., Rutkowski H., Vrezas I. Cytokines and steroidogenesis. Molecular and Cellular Endocrinology 215(2004)135-141.
- Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J. Clin.Endocrinol. Metab. 1993; 77:1690-1694.
- Mastorakos G, Weber JS, Magiakou MA, et al. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J. Clin.Endocrinol. Metab. 1994; 79:934-939
- Path G, Bornstein SR, Ehrhart-Bornstein M, et al. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J. Clin.Endocrinol. Metab. 1997; 82:2343-2349.
- Tkachenko IV, Jääskeläinen T, Jääskeläinen J, et al. Interleukins 1α and 1β as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids. 2011 Sep-Oct;76(10-11):1103-15.
- Smith R, Mesiano S, Chan EC, et al. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J. Clin.Endocrinol. Metab. 1998; 83:2916-2920
- Guran T1, Firat I, Yildiz F, et al. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin Endocrinol (Oxf). 2015 May;82(5):712-8.
- Smail PJ, Faiman C, Hobson WC, et al. Further studies on adrenarche in nonhuman primates. Endocrinology 1982; 111:844-848.
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004 Nov;22(4):337-47. Review.
- Ghizzoni L, Virdis R, Ziveri M, et al. Adrenal steroid, cortisol, adrenocorticotropin, and beta-endorphin responses to human corticotropin-releasing hormone stimulation test in normal children and children with premature pubarche. J. Clin.Endocrinol. Metab. 1989; 69:875-880
- Mesiano S, Katz SL, Lee JY, et al. Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J. Clin.Endocrinol. Metab. 1997; 82:1390-1396
- Fottner C, Engelhardt D, Weber MM. Regulation of steroidogenesis by insulin-like growth factors (IGFs) in adult human adrenocortical cells: IGF-I and, more potently, IGF-II preferentially enhance androgen biosynthesis through interaction with the IGF-I receptor and IGF-binding proteins. J. Endocrinol. 1998; 158:409-417
- Fearon U, Clarke D, McKenna TJ et al. Intra-adrenal factors are not involved in the differential control of cortisol and adrenal androgens in human adrenals. Eur.J.Endocrinol. 1998; 138:567-573.
- Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids 1997; 62:133-142.
- Zhang LH, Rodriguez H, Ohno S, et al. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc.Natl.Acad.Sci.U.S.A. 1995;92:10619-10623.
- Dunaif A, Xia J, Book CB, et al. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J. Clin. Invest. 1995; 96:801-810.
- Qin KN, Rosenfield RL. Role of cytochrome P450c17 in polycystic ovary syndrome. Mol.Cell Endocrinol. 1998; 145:111-121.
- Li M, Youngren JF, Dunaif A, et al. Decreased Insulin Receptor (IR) Autophosphorylation in Fibroblasts from Patients with PCOS: Effects of Serine Kinase Inhibitors and IR Activators. J. Clin.Endocrinol. Metab.2002. Sep.;87. (9.):4088.-93. 87:4088-4093.
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr. Rev. 1997;18:774-800.
- Takayama S, White MF, Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J.Biol.Chem. 1988;263:3440-3447.
- Chin JE, Dickens M, Tavare JM, Roth RA. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J.Biol.Chem. 1993;268:6338-6347.
- Martens JW, Geller DH, Arlt W, et al. Enzymatic activities of P450c17 stably expressed in fibroblasts from patients with the polycystic ovary syndrome. J. Clin.Endocrinol Metab 2000.Nov.;85. (11.):4338.-46. 85:4338-4346.
- Auchus RJ. The regulation of human P450c17 activity: relationship to premature adrenarche and the polycystic ovary syndrome. Trends Endocrinol Metab 1998; 9:47-50.
- Markopoulos MC, Rizos D, Valsamakis G, et al. Hyperandrogenism in women with polycystic ovary syndrome persists after menopause. J Clin Endocrinol Metab. 2011 Mar;96(3):623-31.
- Casson PR, et al. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod, 2000;15:2129-2132.
- Barad and Gleicher. Effect of dehydroepiandrostenone on oocyte and embryo yields, embryo grade and cell numbers in IVF. Human Reproduction 2006 Nov.Vol. 21, Issue 11, Pp. 2845-2849.
- Gleicher N., Ryan, E., Weghofer, A., et al (2009). "Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: a case control study". Reproductive Biology and Endocrinology 2009, 7:108.
- Barnhart KT, Freeman E, Grisso JA, et al. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999 Nov;84(11):3896-902.
- Genazzani AR, Pluchino N. DHEA therapy in postmenopausal women: the need to move forward beyond the lack of evidence. Climacteric. 2010 Aug;13(4):314-6. Review.
- Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011 Jun;96(6):1642-53.
- Genazzani AD, Lanzoni C, Genazzani AR. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging. 2007;24(3):173-85. Review.
- Labrie Fernand. DHEA, important source of sex steroids in men and even more in women. L. Martini (Eds.) Progress in Brain Research, Vol. 182 (2010).
- Panjari M, Davis SR. Vaginal DHEA to treat menopause related atrophy: A review of the evidence. Maturitas 2010 Clinical review: DHEA replacement for postmenopausal women.
- Saltzman E, Guay A. Dehydroepiandrosterone therapy as female androgen replacement. Semin Reprod Med. 2006 Apr;24(2):97-105. Review.
- Buvat J. Androgen therapy with dehydroepiandrosterone. World J Urol. 2003 Nov;21(5):346-55.
- Allolio B, Arlt W, Hahner S. DHEA: why, when, and how much--DHEA replacement in adrenal insufficiency. Ann Endocrinol (Paris). 2007 Sep;68(4):268-73.
- Neary N, Nieman L. Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes. 2010 Jun;17(3):217-23. Review.
- Arlt, W, Callies, F., Van Vlijmen, J.C., et al. (1999) Dehydroepiandrosterone replacement in women with adrenal insufficiency. New England Journal of Medicine, 341, 1013–1020.
- Arlt, W, Callies, F., Koehler, I., et al. (2001) Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. Journal of Clinical Endocrinology and Metabolism, 86, 4686–4692.
- Martina, V.; Benso, A.; Gigliardi, V. R. et al. (2006). "Short-term dehydroepiandrosterone treatment increases platelet cGMP production in elderly male subjects". Clin. Endocrinol. (Oxf.) 11 (March;64(3)): 260–4.
- Zang H, Davis SR. Androgen replacement therapy in androgen-deficient women with hypopituitarism. Drugs. 2008;68(15):2085-93. Review.
- Rice SP, Agarwal N, Bolusani H, et al. Effects of dehydroepiandrosterone replacement on vascular function in primary and secondary adrenal insufficiency: a randomized crossover trial. J Clin Endocrinol Metab. 2009 Jun;94(6):1966-72.
- Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97(8):4279
- Morales AJ, Nolan JJ, Nelson JC, et al. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78(6):1360.
- Labrie F, Diamond P, Cusan L, et al. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82(10):3498
- Morales AJ, Haubrich RH, Hwang JY, et al. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998;49(4):421.
- Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, et al. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999;84(5):1527.
- Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647.
- Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292(18):2243.
- Jankowski CM, Gozansky WS, Schwartz RS, et al. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. 2006;91(8):2986.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(10):3489.
- Bernardi F, Hartmann B, Casarosa E, et al. High levels of serum allopregnanolone in women with premature ovarian failure. Gynecol Endocrinol. 1998;12(5):339.
- Bachelot A, Meduri G, Massin N, et al. Ovarian steroidogenesis and serum androgen levels in patients with premature ovarian failure. J Clin Endocrinol Metab. 2005;90(4):2391.
- Elias AN, Pandian MR, Rojas FJ. Serum levels of androstenedione, testosterone and dehydroepiandrosterone in patients with premature ovarian failure to age-matched menstruating controls. Gynecol Obstet Invest. 1997;43(1):47.
- Chen CC, Parker CR Jr. Adrenal androgens and the immune system. Semin Reprod Med. 2004 Nov;22(4):369-77
- Hechter, A. Grossman and R.T. Chatterton Jr (1887). "Relationship of dehydroepiandrosterone and cortisol in disease". Medical Hypotheses 49 (1): 85–91.
- Wolkowitz, O. M.; Reus, V. I.; Keebler, A. et al. (2006). "Double-blind treatment of major depression with dehydroepiandrosterone". Psychopharmacology (bo-controlled study) 188 (4): 541–551.
- Young, E. A.; Haskett, R. F.; Grunhaus, L. et al (1994). "Increased evening activation of the hypothalamic–pituitary–adrenal axis in depressed patients". Archives of General Psychiatry 51 (9): 701–707.
- Gallagher Peter BSc (Hons) and Young Allan MB, ChB, MPhil, Ph.D., MRCPsych (2002). "Cortisol/DHEA Ratios in Depression". Neuropsychopharmacology 26
- Grimley Evans J, Malouf R, Huppert F et al. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006;
- Elraiyah T, Sonbol MB, Wang Z et al. Clinical review: The benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014 Oct;99(10):3536-42.
- Panjari M, Davis SR. DHEA for postmenopausal women: a review of the evidence Maturitas. 2010 Jun;66(2):172-9.
- Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA. 1998;280(18):1565.
- Thompson RD, Carlson M, Thompson RD et al. Liquid chromatographic determination of dehydroepiandrosterone (DHEA) in dietary supplement products. J AOAC Int. 2000;83(4):847.
- Kasperk CH, Wakley GK, Hierl T et al. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J. Bone Miner’s. 1997;12:464-471
- Hofbauer LC, Hicok KC, Khosla S. Effects of gonadal and adrenal androgens in a novel androgen-responsive human osteoblastic cell line. J. Cell Biochem. 1998; 71:96-108.
- Leder BZ, Leblanc KM, Longcope C et al. Effects of oral androstenedione administration on serum testosterone and estradiol levels in postmenopausal women J Clin Endocrinol Metab. 2002;87(12):5449.
The Role of Lipids and Lipoproteins in Atherosclerosis
ABSTRACT
Atherosclerosis is the underlying cause of heart attack and stroke. Early observations that cholesterol is a key component of arterial plaques gave rise to the cholesterol hypothesis for the pathogenesis of atherosclerosis. Population studies have demonstrated that elevated levels of LDL cholesterol and apolipoprotein B (apoB) 100, the main structural protein of LDL, are directly associated with risk for atherosclerotic cardiovascular events (ASCVE). Indeed, infiltration and retention of apoB containing lipoproteins in the artery wall is a critical initiating event that sparks an inflammatory response and promotes the development of atherosclerosis. Arterial injury causes endothelial dysfunction promoting modification of apoB containing lipoproteins and infiltration of monocytes into the subendothelial space. Internalization of the apoB containing lipoproteins by macrophages promotes foam cell formation, which is the hallmark of the fatty streak phase of atherosclerosis. Macrophage inflammation results in enhanced oxidative stress and cytokine/chemokine secretion, causing more LDL/remnant oxidation, endothelial cell activation, monocyte recruitment, and foam cell formation. HDL, apoA-I, and endogenous apoE prevent inflammation and oxidative stress and promote cholesterol efflux to reduce lesion formation. Macrophage inflammatory chemoattractants stimulate infiltration and proliferation of smooth muscle cells. Smooth muscle cells produce the extracellular matrix providing a stable fibrous barrier between plaque prothrombotic factors and platelets. Unresolved inflammation results in formation of vulnerable plaques characterized by enhanced macrophage apoptosis and defective efferocytosis of apoptotic cells resulting in necrotic cell death leading to increased smooth muscle cell death, decreased extracellular matrix production, and collagen degradation by macrophage proteases. Rupture of the thinning fibrous cap promotes thrombus formation resulting in clinical ischemic ASCVE. Surprisingly, native LDL is not taken up by macrophages in vitro but has to be modified to promote foam cell formation. Oxidative modification converts LDL into atherogenic particles that initiate inflammatory responses. Uptake and accumulation of oxidatively modified LDL (oxLDL) by macrophages initiates a wide range of bioactivities that may drive development of atherosclerotic lesions. Lowering LDL-cholesterol with statins reduces risk for cardiovascular events, providing ultimate proof of the cholesterol hypothesis. All of the apoB containing lipoproteins are atherogenic, and both triglyceride rich remnant lipoproteins and Lp(a) promote atherothrombosis. Non-HDL cholesterol levels capture all of the apoB containing lipoproteins in one number and are useful in assessing risk in the setting of hypertriglyceridemia. Measures of apoB and LDL-P are superior at predicting risk for ASCVE, when levels of LDL-C and LDL-P are discordant. Here, we also describe the current landscape of HDL metabolism. Epidemiological studies have consistently shown that HDL-C levels are inversely related to ASCVE. We highlight recent clinical trials aimed at raising HDL-C that failed to reduce CVE and the shifting clinical targets of HDL-C, HDL particle numbers, and HDL function (e.g. cholesterol efflux capacity). Furthermore, we describe many beneficial properties of HDL that antagonize atherosclerosis and how HDL dysfunction may promote cardiometabolic disease.
PATHOPHYSIOLOGY OF ATHEROSCLEROSIS
Atherosclerosis in Cardiovascular Disease
As the underlying cause of heart attack, stroke, and peripheral vascular disease, atherosclerosis is the major cause of death and morbidity in the United States and the industrial world (1). The discovery by Virchow more than 100 years ago that atheroma contained a yellow fatty substance, later identified as cholesterol by Windaus, suggested a role for lipids in the pathogenesis of atherosclerosis (2). Indeed, the goal of this chapter is to focus on the role of lipids and lipoproteins in the pathogenesis of atherosclerosis as well as their critical roles in risk assessment and as targets of therapy. The recognition that atherosclerosis is an inflammatory disease has led to tremendous progress in our understanding of the pathogenesis of atherosclerosis (3). First, we provide brief description of the cellular and molecular events in the key stages of atherosclerosis.
Initiation and Fatty Streak Phase of Atherosclerotic Lesions
The endothelial lining of arteries responds to mechanical and molecular stimuli to regulate tone, (4)hemostasis, (5)and inflammation (6)throughout the circulation. Endothelial cell dysfunction is an initial step in atherosclerotic lesion formation and is more likely to occur at arterial curves and branches that are subjected to low shear stress and disturbed blood flow (atherosclerosis prone areas) (7,8). These mechanical stimuli activate signaling pathways leading to a dysfunctional endothelium lining that is barrier compromised, prothrombotic, and proinflammatory (9). In atherosclerosis susceptible regions, the endothelial cells have cuboidal morphology, a thin glycocalyx layer, and a disordered alignment (8,10,11). In addition, these regions have increased endothelial cell senescence and apoptosis as evidenced by ER stress markers (12-14).In contrast, less atherosclerosis prone endothelium is exposed to laminar shear stress causing activation of signaling pathways that maintain endothelial cell coaxial alignment, proliferation, (13,14)glycocalyx layer, (15)and survival (12,16). In atherosclerosis resistant regions, the transcription factors, Kruppel-like factors (KLF) 2 and 4, are activated via MEK5/ERK5/MEF2 signalingwhich enhances expression of endothelial nitric oxide synthase (eNOS) (17-19). The increased nitric oxide (NO) production promotes endothelial cell migration and survival thereby maintaining an effective barrier (20). In addition, the expression of superoxide dismutase (SOD) is increased to reduce cellular oxidative stress (18). In atherosclerosis susceptible regions, reduced expression of eNOS and SOD leads to compromised endothelial barrier integrity (Figure 1), leading to increased accumulation and retention of subendothelial atherogenic apolipoprotein B (apoB)-containing lipoproteins (low-density lipoproteins (LDL)) and remnants of very low-density lipoproteins (VLDL) and chylomicrons) (21,22). KLF2, KLF4, and NO production inhibit activation of the nuclear factor kappa B (NF‐κB) pathway.Increased NF‐κBactivation in atherosclerosis susceptible areas leads to endothelial cell activation (Figure 1), as evidenced by increased expression of monocyte adherence proteins (VCAM-1, ICAM-1,and P-selectin) and proinflammatory receptors (toll-like receptor 2, TLR2) and cytokines (MCP-1 and IL-8) (19,23,24). In addition, endothelial cell activationleads to increased production of reactive oxygen species (25)that can cause oxidative modification of apoB-containing lipoproteins (26). Besides mechanical stimuli, endothelial cell activation is increased by various molecular stimuli, including oxidized LDL, cytokines,advanced glycosylation end products, and pathogen-associated molecules (27-30). In contrast, an atheroprotective function of HDL is to prevent endothelial activation and enhance NO production to maintain barrier integrity (see details below) (31).

Figure 1. Initiation of the atherosclerotic lesion. The fatty streak phase of atherosclerosis begins with dysfunctional endothelial cells and the retention of apoB-containing lipoproteins (LDL, VLDL, and apoE remnants) in the subendothelial space. Retained lipoproteins are modified (oxidation, glycation, enzymatic), which, along with other atherogenic factors, promotes activation of endothelial cells. Activated endothelial cells have increased expression of monocyte interaction/adhesion molecules (selectins, VCAM-1) and chemoattractants (MCP-1) leading to attachment and transmigration of monocytes into the intimal space. Activated endothelial cells also promote the recruitment of other immune cells including dendritic cells, mast cells, regulatory T (T-reg) cells, and T helper 1 (Th-1) cells. The monocytes differentiate into macrophages and express receptors that mediate the internalization of VLDL, apoE remnants, and modified LDL to become foam cells. In addition, inflammatory signaling pathways are activated in macrophage foam cells leading to more cell recruitment and LDL modification.
Immune Cell Recruitment and Foam Cell Formation
Activation of endothelial cells causes a monocyte recruitment cascade involving rolling, adhesion, activation and transendothelial migration (Figure 1). Selectins, especially P-selectin, mediate the initial rolling interaction of monocytes with the endothelium (32). Monocyte adherence is then promoted by endothelial cell immunoglobulin-G proteins including VCAM-1 and ICAM-1(32). Potent chemoattractant factors such as MCP-1 and IL-8 then induce migration of monocytes into the subendothelial space (33-35). Ly6himonocytes, versus Ly6lo, preferentially migrate into the subendothelial space to convert to proinflammatory macrophages in mice (36-38). The enhanced migration of Ly6hiversus Ly6lomonocytes likely results from increased expression of functional P-selectin glycoprotein ligand-1 (39). In addition, the number of blood monocytes originating from the bone marrow and spleen, especially Ly6hicells, increases in response to hypercholesterolemia (36). Furthermore, hypercholesterolemia and atherosclerosis increase monocytosis in humans (40,41). Importantly, increased numbers of inflammatory CD14++CD16+monocytes independently predicted cardiovascular death, myocardial infarction, and stroke in patients undergoing elective coronary angiography (42). Intimal macrophages also result from proliferation of monocyte/macrophages, especially in more advanced lesions (43). During the initial fatty streak phase of atherosclerosis (Figure 1), the monocyte-derived macrophages internalize the retained apoB-containing lipoproteins, which are degraded in lysosomes, where excess free cholesterol is trafficked to the endoplasmic reticulum (ER) to be esterified by acyl CoA:cholesterol acyltransferase (ACAT), and the resulting cholesteryl ester (CE) is packaged into cytoplasmic lipid droplets, which are characteristic of foam cells (42)(Figure 2) (44,45). Modification of apoB lipoproteins via oxidation and glycation enhances their uptake through a number of receptors not down-regulated by cholesterol including CD36, scavenger receptor A, and lectin-like receptor family (see details below) (Figure 2) (46,47). Enzyme-mediated aggregation of apoB lipoproteins enhances uptake via phagocytosis (Figure 2) (48,49). In addition, native remnant lipoproteins can induce foam cell formation via a number of apoE receptors (LRP1 and VLDLR) (Figure 2) (50,51). Uptake of native LDL by fluid phase pinocytosis may also contribute to foam cell formation (Figure 2) (52,53).
Figure 2. Macrophage Cholesterol Metabolism. Native LDL is recognized by the LDL receptor (LDLR). The LDL is endocytosed and trafficked to lysosomes, where the cholesteryl ester (CE) is hydrolyzed to free cholesterol (FC) by the acid lipase. The FC is transported to the endoplasmic reticulum (ER) to be esterified by acyl CoA:cholesterol acyltransferase (ACAT). Increased FC in an ER regulatory pool initiates a signaling cascade resulting in down-regulation of the LDL receptor. Cholesterol regulation of the LDLR prevents foam cell formation via this receptor in the setting of hypercholesterolemia. ApoB containing lipoproteins that also contain apoE (apoE remnants, VLDL) can cause cholesterol accumulation via interaction of apoE with apoE receptors including the LRP1 and the VLDL receptor, which are not regulated by cellular cholesterol. Uptake of native LDL by fluid phase pinocytosis may also contribute to foam cell formation. Modifications of apoB containing lipoproteins induce significant cholesterol accumulation via a number of mechanisms. Enzyme-mediated aggregation of apoB lipoproteins enhances uptake via phagocytosis. Oxidation and/or glycation enhances internalization via a number of receptors that are not regulated by cholesterol, including CD36, scavenger receptor A (SRA), lectin-like receptors (LOX), and toll-like receptors (TLR4). The CE generated by ACAT is stored in cytoplasmic lipid droplets, where there is a continual cycle of hydrolysis to FC by neutral cholesterol esterase and re-esterification by ACAT. Cytoplasmic CE is cleared by two main pathways. In one pathway, removal of FC from the plasma membrane stimulates transport of FC that has been generated by neutral cholesterol esterase away from ACAT to the plasma membrane. Alternatively, cytoplasmic CE is packaged into autophagosomes, which are transported to fuse with lysosomes, where the CE is hydrolyzed by acid lipase and the resulting FC is then transported to the plasma membrane. The efflux of FC to lipid-poor apolipoproteins or HDL occurs by a number of mechanisms to reduce foam cell formation. Exogenous lipid-free apoA-I or endogenous apoE that is produced by the macrophages interacts with ABCA1 to stimulate the efflux of phospholipid and FC to form nascent HDL particles (e.g. apoA-I or apoE containing phospholipid discs). ApoE produces the most buoyant, FC-enriched particles. ABCA1 plays a major role in the clearance of cytoplasmic CE via autophagy. The apoA-I/apoE discs as well as mature HDL containing apoA-I and/or ApoE stimulate FC efflux via three major mechanisms including ABCG1, SR-BI, and aqueous diffusion. ABCG1 may also play a role in the intracellular trafficking of cholesterol.
The triggering of macrophage inflammatory pathways is also a critical event in lesion development. Inflammatory M1 phenotype macrophages exhibit increased oxidative stress, impaired cholesterol efflux and enhanced cytokine/chemokine secretion, leading to more LDL/remnant oxidation, endothelial cell activation, monocyte recruitment, and foam cell formation (54-59). Oxidative stress, modified lipoproteins, and other lesion factors (bioactive lipids, pattern recognition molecules, cytokines) are capable of inducing inflammation via receptors (54,55,60). In addition, plasma membrane cholesterol in macrophage foam cells enhances signaling via inflammatory receptors (61,62). Recently, inflammasome activation of IL-1β and IL-18 has been implicated in atherogenesis (63,64). Indeed, a recent clinical trial showed that subjects treated with the IL-1β monoclonal antibody, canakinumab, had a significantly lower rate of recurrent cardiovascular events which were independent of cholesterol lowering (65). Macrophage foam cell formation and cholesterol dependent inflammatory receptor signaling can be reduced by the removal of cholesterol by atheroprotective HDL and apoA-I via a number of mechanisms including ABCA1, ABCG1, SR-BI, and aqueous diffusion (Figure 2) (61,66-68)(see details below). Lipid-poor apoA-I stimulates efflux via ABCA1, whereas lipidated apoA-I or mature HDL are the main drivers of efflux via ABCA1, ABCG1, SR-BI, and aqueous diffusion (Figure 2) (61,69-71). Cytoplasmic CE is cleared by two major pathways. One route involves the hydrolysis of cytoplasmic CE by neutral cholesterol esterase and the resulting free cholesterol is mobilized away from the ACAT pool (72,73)and made available for efflux via ABCA1, ABCG1, SR-BI, and aqueous diffusion (Figure 2). Alternatively, cytoplasmic CE is packaged into autophagosomes, which are trafficked to lysosomes, where the CE is hydrolyzed by acid lipase(73,74), generating free cholesterol that is made available for efflux mainly via ABCA1(Figure 2) (73,74). Furthermore, HDL and apoA-I protect against atherosclerosis by reducing inflammation via mechanisms independent of cholesterol efflux (31,75)(see details below). In addition, small non-coding RNAs have been found to impact atherosclerosis development by regulating inflammation and/or cholesterol homeostasis in different cell types in lesions (76,77). MiR-33a and MiR-33b promote atherosclerosis by impairing cholesterol efflux and promoting inflammatory M1 macrophage conversion (78-80).Other microRNAs including MiR-223 and MiR-93 exhibit atheroprotective effects by increasing cholesterol efflux and conversion to the anti-inflammatory M2 macrophage phenotype (76,81-83). HDL carry small non-coding RNAs (77), which can also reduce or promote atherosclerosis development depending upon composition of individual non-coding RNAs (see details below).
Although macrophages are the main infiltrating cells, other cells contribute to the development of lesions including dendritic cells (84,85), mast cells, T cells, and B cells (Figure 1) (86,87). Dendritic cells promote the priming of reactive T cell clones and secrete cytokines, functioning in a largely pro-inflammatory capacity(88). They also take up lipid, which leads to inflammasome activation and increased pro-inflammatory cytokine secretion (89). Mast cells produce interferon-g(IFNg) and IL-6 and appear to promote lesion development(90). Atherosclerotic plaques also contain a significant number of adaptive immune cells, including T and B lymphocytes. The role of T cells is subset-dependent and atherosclerotic plaques have been shown to contain CD4+and CD8+effector T cells as well as T helper 1 (Th-1), Th-2, Th-17, and regulatory T (T-reg) cells. Antigen-specific Th-1 cells produce IFNgthat converts macrophages to a proinflammatory M1 phenotype. Th-17 cells have also been identified in atherosclerotic plaques and have been shown to produce IFNg. However, their specific role in atherosclerosis has not yet been elucidated (91). Classical T-reg cells produce anti-inflammatory cytokines (TGF-β and IL-10) and inhibit activation of Th-1 cells, leading to more anti-inflammatory M2 macrophages. As atherosclerosis progresses, T effector cell numbers increase or remain constant, while T-reg numbers decline. This reduction in T-regs is due in part to their heightened susceptibility to cell death as well as their impaired trafficking into lesions (91). Further, T-regs may appear fewer in number because they undergo phenotypic switching into other T-reg subtypes. Several subclasses of these ‘former’ T-regs have been identified in the atherosclerotic lesions of mice, including Th1-Tregs (CD4+CCR5+IFN-g+FoxP3+T-bet+) and T follicular helper cells (CXCR5+PD1+Bcl6+CD62LloCD44hiCD4+Foxp3-), and these have been shown to have both impaired regulatory and enhanced inflammatory function, therefore contributing to atheroprogression (91,92). B cells preferentially reside in the adventitial layer of arteries neighboring sites of plaque, in regions known as tertiary lymphoid organs (TLOs). The function of B lymphocytes is also subset dependent, with B-1 cells being atheroprotective and B-2 cells being atherogenic. B-1 cells undergo limited or no affinity maturation and produce natural antibodies (NAbs) that have broad specificity and low binding affinity. Among these are NAbs, found within atherosclerotic plaques, that can bind to oxidation motifs in LDL and block the uptake of oxLDL by macrophages (93). Mice engineered to overexpress a single-chain variable fragment of E06, an IgM NAb directed against oxidized phospholipids (oxPL), were found to have reduced atherosclerosis and features consistent with greater overall plaque stability, confirming the atheroprotective nature of these B-1 cell-derived antibodies (94). B-2 cells produce high-affinity IgA, IgE and IgG antibodies. While the role of IgA in atherosclerosis remains controversial, IgG and IgE are atherogenic. IgG forms immune complexes with oxLDL and promotes an inflammatory macrophage phenotype while IgE also stimulates macrophages and mast cells to produce proatherogenic cytokines (95).
ApoE in Atherosclerosis
In addition to apoA-I and HDL, the endogenous production of apoE by macrophages is critical in preventing atherosclerotic lesion formation. The majority of apoE in plasma is produced by the liver, but macrophages are responsible for producing 5 -10% of apoE in plasma (96). ApoE serves as the ligand for clearance of all of the apoB containing lipoproteins from the blood by the liver except for LDL. Gene knockout of apoE in mice results in hypercholesterolemia and spontaneous atherosclerotic lesion development (97,98). Hence, ApoE deficient mice have been used widely to study mechanisms of atherosclerotic lesion development. Bone marrow transplantation studies were used to examine the role of macrophage apoE in lipoprotein metabolism[Linton, 1998 #5676]. Transplantation of Apoe-/-mice with wildtype bone marrow, resulted in normalization of plasma cholesterol levels and protection from atherosclerosis (99), demonstrating the ability of macrophage apoE to exchange between lipoproteins and to serve as a vehicle for cellular gene therapy of atherosclerosis. Furthermore, reconstitution of wildtype (100)or LDL receptor deficient mice(Ldlr-/-)(101)with Apoe-/-bone marrow accelerates atherosclerotic lesion development without affecting plasma cholesterol levels, demonstrating an atheroprotective role for macrophage apoE. Interestingly, ApoE protects against atherosclerosis via several mechanisms. Expression of apoE by hematopoietic stem cells reduces monocyte proliferation and infiltration into the intima (102). In addition, apoE on apoB lipoproteins reduces the lysosomal accumulation of cholesterol by enhancing the expression of acid lipase (103). Importantly, secretion of apoE by macrophages stimulates efflux in the absence and presence of exogenous acceptors, including HDL and lipid-free apoA-I (Figure 2) (104-107). Recent studies demonstrated that macrophage apoE facilitates reverse cholesterol transport in vivo(108). Macrophage apoE stimulates phospholipid and cholesterol efflux via ABCA1, and the apoE particles formed then promotecholesterol efflux through ABCG1, SR-BI, and aqueous diffusion (104,109-111). Endogenous apoE is required for efficient formation of the most buoyant, cholesterol-enriched particles by macrophages (Figure 2) (104,112-116). In addition to cholesterol efflux, macrophage apoE prevents inflammation (117-120)and oxidative stress (121-124). The local production of apoE is likely a critical atheroprotective mechanism considering that areas of atherosclerotic lesions have limitedaccessibilityto plasma apoA-1 and HDL (100,101,125). Humans express three common apoE polymorphisms that predict CAD rates independently from plasma cholesterol levels (126). ApoE3 (C112, R158) is the most common isoform and is functionally similar to mouse apoE. Compared to apoE3 and apoE2 (C112, C158), apoE4 (R112, R158) are impaired in stimulating cholesterol efflux (127-130)and in preventing inflammation and oxidation (117,124,131). Consistent with the compromised function of apoE4, human carriers exhibit increased risk of CAD compared to humans expressing apoE3 or apoE2 (heterozygous) (126,132,133).
Progression to Advanced Atherosclerotic Lesions
Fatty streaks do not result in clinical complications and can even undergo regression. However, once smooth muscle cells infiltrate, and the lesions become more advanced, regression is less likely to occur (134,135). Small populations of vascular smooth muscle cells (VSMCs) already present in the intima proliferate in response to growth factors produced by inflammatory macrophages (136). In addition, macrophage-derived chemoattractants cause tunica media smooth muscle cells to migrate into the intima and proliferate (Figure 3). Critical smooth muscle cell chemoattractants and growth factors include PDGF isoforms, (137)matrix metalloproteinases, (138)fibroblast growth factors, (139)and heparin-binding epidermal growth factor (Figure 3) (140). HDL prevents smooth muscle cell chemokine production and proliferation. The accumulating VSMCs produce a complex extracellular matrix composed of collagen, proteoglycans, and elastin to form a fibrous cap over a core comprised of foam cells (Figure 4) (141). A vital component of the fibrous cap is collagen, and macrophage-derived TGF-bstimulates its production (Figure 4) (142). In addition, HDL maintains plaque stability by inhibiting degradation of the fibrous cap extracellular matrix through its anti-elastase activity (143).A subset of VSMCs accumulates CE and resides in the lesion core (Figures 3 and 4). This smooth muscle cell phenotype produces less a-actin and expresses macrophage markers, including CD68, F4/80 and Mac2 (144-146). While studies have shown that VSMCs express the VLDL receptor and various scavenger receptors, (145,147,148)data showing that these cells robustly load with CE, (147)similar to macrophages via these mechanisms is lacking. As lesions advance, substantial extracellular lipid accumulates in the core, in part due to large CE-rich particles arising from dead macrophage foam cells (149,150). Earlier in vitrostudies showed that these CE-rich particles effectively cholesterol load VSMCs (151,152). Regardless of the mechanisms of cholesterol enrichment, VSMCs compared to macrophages are inefficient at lysosomal processing and trafficking of cholesterol (152,153)and express much less ABCA1(154), which all contribute to impaired cholesterol efflux (155). However, macrophages in more advanced plaques also have reduced lysosome function and trapping of free and esterified cholesterol within their lysosomes contributes to the overall sterol accumulation in the lesion (156-158). The reduced lysosome function appears multifactorial but includes direct and indirect inhibition of lysosomal acid lipase, the enzyme responsible for hydrolysis of cholesteryl esters in lysosomes, and a reduced capacity for transferring cholesterol from lysosomes (159-162). In cell culture models of human macrophage foam cells, the inability to clear cholesterol from macrophages with compromised lysosome function continues even in the presence of compounds that stimulate efflux (161,163). Proteomic analysis of foam cells shows that changes in a number of lysosome proteases are related to macrophage sterol accumulation (164). Thus, at least in the advanced stages of atherosclerosis, lysosome dysfunction contributes to the overall lesion severity. As the intimal volume enlarges due to accumulating cells, there is vascular remodeling to lessen protrusion of the lesion into the lumen (Figure 4), thereby decreasing occlusion and the appearance of clinical symptoms for much of the life of the lesion (165-167).

Figure 3. Progression of the atherosclerotic plaque. Macrophage foam cell and endothelial cell inflammatory signaling continues to promote the recruitment of more monocytes and immune cells into the subendothelial space. Transition from a fatty streak to a fibrous fatty lesion occurs with the infiltration and proliferation of tunica media smooth muscle cells. Macrophage foam cells and other inflammatory cells produce a number of chemoattractant and proliferation factors, including transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF) isoforms, matrix metalloproteinases, fibroblast growth factors (FGF), and heparin-binding epidermal growth factor (HB-EGF). Smooth muscle cells are recruited to the luminal side of the lesion to proliferate and generate an extracellular matrix network to form a barrier between lesional prothrombotic factors and blood platelets and procoagulant factors. A subset of smooth muscle cells express macrophage receptors and internalize lipoproteins to become foam cells. Fibrous fatty lesions are less likely to regress than fatty streaks.

Figure 4. Features of the stable fibrous plaque. As the cell volume of the intima increases, there is vascular remodeling so that the lumen is only partially occluded, substantially lessening clinical events resulting from occlusion. The stable plaque contains a generous fibrous cap composed of layers of smooth muscle cells ensconced in a substantial extracellular matrix network of collagen, proteoglycans, and elastin. The thick fibrous cap of the stable plaque provides an effective barrier preventing plaque rupture and exposure of lesion prothrombotic factors to blood, thereby limiting thrombus formation and clinical events. Maintenance of a thick fibrous cap is enabled by regulation of the inflammatory status of the foam cell core of the lesion. Regulatory T (T-reg) cells produce transforming growth factor-β (TGF-β) and IL-10. In addition, T-reg cells inhibit antigen-specific activation of T helper 1 (Th-1) cell to produce interferon gamma (IFNg). Increased TGF-β and IL-10 and decreased IFNg reduce the proinflammatory macrophage phenotype leading to reduced cell death, effective efferocytosis (phagocytosis of dead cells), and anti-inflammatory cytokine production (i.e. TGF-β, IL-10). Thus, stable plaques have small necrotic cores containing macrophage debris and extracellular lipid resulting from secondary necrosis of noninternalized apoptotic macrophage foam cells. The production of TGF-β by T-reg cells and macrophages maintains fibrous cap quality by being a potent stimulator of collagen production in smooth muscle cells.
Vulnerable Plaque Formation and Rupture
The advanced atherosclerotic lesion is essentially a nonresolving inflammatory condition leading to formation of the vulnerable plaque, increasing the risk of plaque rupture. The vulnerable plaque is characterized by two fundamental morphological changes: 1) Formation of a necrotic core and 2) Thinning of the fibrous cap. Sections of the atheroma with a deteriorated fibrous cap are subject to rupture (Figures 4 and 5) (168,169). A recent lipidomics study showed that stable versus unstable plaques have different lipid subspecies profiles (170). Compared to plasma and control arteries, stable plaques have increased CE containing polyunsaturated fatty acids (170), which have increased susceptibility to oxidation. The CE containing polyunsaturated fatty acids are decreased in unstable plaques compared to stable plaques of the same subjects (170). In addition, 18:0 containing lysophosphatidylcholine is increased in unstable plaques indicating enhanced oxidation (170). Plaque rupture leads to acute exposure of procoagulant and prothrombotic factors from the necrotic core of the lesion to platelets and procoagulant factors in the lumen, thereby causing thrombus formation (Figure 5) (168,169). Thrombus formation at sites of plaque rupture accounts for the majority of clinical events with acute occlusive luminal thrombosis causing myocardial infarction, unstable angina, sudden cardiac death, and stroke (168,169).
Figure 5. Formation of the vulnerable plaque. The vulnerable plaque results from a heightened, unresolved inflammatory status of the lesion foam cell core. Antigen-specific activation of T helper 1 (Th-1) cells produces interferon gamma (IFNg) resulting in a proinflammatory macrophage phenotype. The proinflammatory macrophage foam cells exhibit enhanced inflammatory cytokine secretion and apoptosis susceptibility. There is less secretion of the anti-inflammatory cytokines, TGF-β and IL-10. In addition, proinflammatory macrophages have impaired atheroprotective functions including cholesterol efflux and efferocytosis. The defective efferocytosis of inflammatory apoptotic macrophages results in secondary necrosis leading to an enlarged necrotic core composed of leaked oxidative and inflammatory components. This unresolved inflammation causes thinning of the fibrous cap resulting from increased smooth muscle cell death, enhanced extracellular matrix degradation and decreased extracellular matrix production. Areas of thin fibrous cap are prone to rupture exposing prothrombotic components to platelets and procoagulation factors leading to thrombus formation and clinical events.
Macrophage Cell Death and Efferocytosis Influence Plaque Stability
The necrotic core results from a combination of accelerated macrophage death and impaired efferocytosis (receptor-mediated phagocytosis of apoptotic cells) (Figure 5) (171,172). As apoptotic cells accumulate and fail to be internalized by phagocytes, they undergo secondary necrotic death leading to the leakage of intracellular oxidative and inflammatory components, which then propagate more inflammation, oxidative stress, and death in neighboring cells (Figure 5) (173). Multiple triggers likely occur in lesions to accelerate macrophage death, including oxidative stress, death receptor activation, and nutrient deprivation (174). Prolonged ER stress and activation of the unfolded protein response (UPR) contribute to macrophage apoptosis as substantiated by studies showing that apoptosis and the UPR effector, CHOP, increase with each stage of atherosclerosis in humans, but the largest increase is observed in the vulnerable plaque (175). In diabetes and obesity, accelerated formation of an enlarged necrotic core is likely instigated by defective macrophage insulin signaling (176)and saturated fatty acids (177,178), which are potent inducers of ER stress. In addition, other triggers act in tandem with ER stress to accelerate apoptosis. In particular, activation of toll-like receptors (TLR) (TLR2 and TLR4) and scavenger receptors (CD36 and SR-A) by oxidized phospholipids induces apoptotic signaling (178-181). Death is also accelerated by simultaneous suppression of survival pathways such as pAkt and NF‐κB via these same receptors. Accelerated apoptotic macrophage death is not sufficient to promote necrosis. Apoptotic cells undergo secondary necrotic death if they are not internalized by phagocyte efferocytosis receptors. Necrotic death leads to the leakage of intracellular oxidative and inflammatory components, which then propagate more inflammation, oxidative stress, and death in neighboring cells (Figure 5) (173). The presence of necrotic tissue together with apoptosis is consistent with defective efferocytosis in human plaques. Studies have shown that the majority of apoptotic cells are free in advanced human lesions, whereas in tonsils apoptotic cells are macrophage-associated (182). Efferocytosis also becomes defective in advanced atherosclerosis through several different mechanisms. First, accumulating evidence has shown that the expression and function of key efferocytosis receptors,MerTK (183), LRP1 (184), and SR-BI (185)are impaired in advanced atherosclerosis. These receptors recognize apoptotic cell ligands such as phosphatidylserine (185,186). and efferocytosis efficiency is enhanced by bridging molecules such as apoE and MFG-E8 that interact with efferocytosis receptors to enhance their efficiency and also have reduced expression in advanced lesions (186-190). Compared to apoE3, apoE4 is defective at facilitating efferocytosis of apoptotic cells (191). Efferocytosis via LRP1 (187)and SR-BI (185)also stimulates signaling pathways leading to pAkt production to promote phagocyte survival. In addition, anti-inflammatory signaling (185,192)is activated so that phagocytes secrete TGF-β and IL-10 (Figures 4 and 5). In addition, efferocytosis may be limited by competition for apoptotic cell binding. For example, oxPLs bind efferocytosis receptors and effectively compete for apoptotic cell recognition. In addition, lesional autoantibodies to oxPL and oxLDL are able to bind to ligands on the apoptotic cell themselves in order to prevent their binding and ingestion. Finally, apoptotic cells in advanced lesions appear to become poor substrates for efferocytosis. CD47, which typically acts as a “don’t eat me” signal expressed by live cells, is upregulated by apoptotic cells within human and murine atherosclerotic plaques, allowing them to evade uptake by phagocytes. When given to atheroprone Apoe-/-mice, a CD47-blocking antibody enhanced lesional efferocytosis and resulted in smaller necrotic cores(193). Similarly, mice that express low levels of the “eat me” signal, calreticulin, have increased necrotic cores compared to control mice and apoptotic cells from these mice demonstrate resistance to uptake by phagocytes (194).
Components of the necrotic core promote thinning of the fibrous cap. Loss of extracellular matrix is in part due to death of fibrous cap smooth muscle cells, resulting from macrophage-derived Fas receptor ligand (195), inflammatory cytokines (196), and oxidation products (Figure 5) (197,198). Smooth muscle cells are inefficient at efferocytosis (199)relying on macrophages to internalize apoptotic smooth muscle cells. As such, the impaired efferocytosis by lesional macrophages likely leads to uncontrolled VSMC death (Figure 5). In addition, impaired production of TGF-β by phagocytes (185,200)reduces collagen production by healthy smooth muscle cells (Figures 4 and 5). The extracellular matrix components are degraded by macrophage-derived matrix metalloproteinases, (201-203)elastases, and cathepsins (Figure 5) (204). HDL can reduce VSMC apoptosis and elastin degradation induced by elastases (143,205).
Importantly, HDL can prevent efferocyte apoptosis via ER stress by its cholesterol efflux and anti-oxidant functions (179,206,207). Furthermore, HDL drives conversion to the anti-inflammatory M2 macrophages which have enhanced efferocytosis ability compared to inflammatory M1 macrophages (56,208)leading to increased plaque stability. Once plaque rupture occurs, critical HDL functions may also include prevention of platelet activation and thrombus formation. In addition to the role of HDL in stabilizing plaques, recent studies have focused on the lesional loss of specialized proresolving mediators (SPM) versus proinflammatory factors (i.e. leukotriene B4)in promoting uncontrolled inflammation and formation of vulnerable plaques (209). Studies on human atherosclerotic lesions have shown that unstable versus stable plaques have decreased lipid-derived SPM including resolvin D1 and lipoxin A4 (210). In addition, resolving D1 treatment ofLdlr-/-mice with established atherosclerosis increased lesional efferocytosis and collagen content and reduced the necrotic area and reactive oxygen species content (210). Similar results were observed in Apoe-/-micetreated with the phospholipase D derived proresolving lipid, palmitoylethanolamide(211).Other lipid derived resolving mediators which impact atherosclerotic plaques include maresin 1 and resolvin D2 (212). Protein SPM have also been identified including annexin 1 and IL-10 (209). Administration of lesion targeting nanoparticles containing the bioactive annexin 1 peptide, Ac2-26, to Ldlr-/-mice with atherosclerosis reduced both lesional oxidative stress and necrosis while increasing collagen content and fibrous cap thickness (213). Enhancing the lesional IL-10 content also improved atherosclerotic lesion stability (214). In addition, Treg cells likely control atherosclerotic lesion inflammation resolution as recent studies demonstrated that Treg cells regulate efferocytosis in atherosclerotic lesions by secreting IL-13 to stimulate macrophage production of IL-10 to induce Vav-1 activation of Rac1 and increased efferocytosis (215).
Summary
Atherosclerotic lesions initiate with endothelial cell dysfunction causing modification of apoB containing lipoproteins (LDL, VLDL, remnants) and infiltration of immune cells, particularly monocytes, into the subendothelial space (Figure 1). The macrophages internalize the retained apoB containing lipoproteins to become foam cells forming the fatty streak (Figure 1). Macrophage inflammatory pathways are also activated leading to increased oxidative stress and enhanced cytokine/chemokine secretion, causing more LDL/remnant oxidation, endothelial cell activation, monocyte recruitment, and foam cell formation (Figure 1). HDL, apoA-I, and endogenous apoE reduce lesion formation by preventing endothelial cell activation, inflammation, and oxidative stress and also by promoting cholesterol efflux from foam cells. As the lesion progresses to fibrotic plaques as a result of continued inflammation, macrophage chemoattractants stimulate infiltration and proliferation of smooth muscle cells (Figure 3). Smooth muscle cells produce the extracellular matrix providing a stable fibrous barrier between plaque prothrombotic factors and platelets (Figure 4). Unresolved inflammation results in formation of vulnerable plaques, which have large necrotic cores and a thinning fibrous cap (Figure 5). Enhanced macrophage apoptosis and defective efferocytosis of apoptotic cells results in necrotic cell death causing heightened inflammation leading to increased smooth cell death, decreased extracellular matrix production, and collagen degradation by macrophage proteases. An imbalance between inflammatory factors and SPMs is prominent in facilitating formation of the vulnerable plaque. Rupture of the thinning fibrous cap promotes thrombus formation resulting in clinical ischemic cardiovascular events (Figure 5).
THE ROLE OF CHOLESTEROL AND LIPOPROTEINS IN ATHEROGENESIS
Metabolism of ApoB Containing Lipoproteins
Apolipoprotein B (apoB) occurs in two isoforms, apoB100 and apoB48. ApoB100 is the main structural apolipoprotein of low-density lipoproteins (LDL), and there is only one molecule of apoB100 per LDL particle (216). ApoB100 is produced mainly by the liver, where it is required for the synthesis and secretion of triglyceride-rich very low-density lipoprotein (VLDL) particles (Figure 6). In the circulation, VLDL is metabolized to the cholesteryl ester-enriched intermediate low-density lipoprotein (IDL) and LDL particles through the progressive hydrolysis of triglycerides by lipoprotein lipase (LPL) and hepatic lipase (Figure 6). In humans, apoB48 is produced exclusively in the intestine through an unique RNA editing mechanism by the apobec-1 enzyme complex (217). ApoB100 is the full-length protein, which contains 4536 amino acids, whereas apoB48 contains the first 48% of the amino terminal amino acids. ApoB48 is required for the synthesis and secretion of triglyceride-rich chylomicrons, which play a critical role in the intestinal absorption of dietary fats and fat-soluble vitamins. Similar to the metabolism of VLDL, chylomicrons are metabolized in the circulation through the hydrolysis of triglycerides by LPL and hepatic lipase to form cholesteryl ester-enriched chylomicron remnants, which release free fatty acids that can be used for energy by the tissues.
Figure 6. Metabolism of ApoB100 containing lipoproteins. ApoB100 is critical for the production and secretion of very low-density lipoprotein (VLDL) by the liver. Plasma VLDL is metabolized to cholesteryl ester-enriched intermediate low-density lipoprotein (IDL) and LDL particles via hydrolysis of triglycerides by lipoprotein lipase (LPL) and hepatic lipase (HL). In addition, cholesteryl ester transfer protein (CETP) transfers CE from HDL to VLDL in exchange for triglyceride (TG) to HDL. ApoCII and apoCIII are transferred from HDL to VLDL and act as an activator or inhibitor of LPL activity, respectively. ApoB100 is the ligand for hepatic LDL receptor-mediated clearance of LDL. VLDL acquires apoE from HDL, and apoE mediates the clearance of triglyceride-enriched remnants and IDL. In addition, HDL can directly transfer cholesterol to liver via interaction with SR-BI. VLDL and IDL remnants can induce foam cell formation by internalization via apoE receptors on macrophages. LDL, IDL, and VLDL can be modified (oxidation, glycation) and internalized by a number of macrophage receptors including scavenger receptors and lectin-like receptors. HDL and lipid-poor apoA-I reduce foam cell formation by stimulating cholesterol efflux.
Static measurements of cholesterol in the LDL pool (LDL-C) represent the steady state of production of VLDL, its metabolism to LDL, and the receptor-mediated clearance of LDL by the LDL receptor (LDLR). Mutations in the Ldlrgene are the most common cause of familial hypercholesterolemia (FH), an autosomal dominant disorder associated with elevated levels of LDL-C and increased risk for premature cardiovascular disease (218). ApoB100 serves as the ligand for receptor-mediated clearance of LDL by the liver (Figure 6). In contrast, apoE mediates the clearance of triglyceride-rich remnants (IDL and chylomicron remnants) either through the LDLR or the remnant receptor pathway (Figure 6). The existence of the remnant receptor pathway was suggested by the fact that patients with homozygous FH, who completely lack LDLR function, have severely elevated levels of LDL-C but normal blood levels of triglycerides. The clearance of these remnant lipoproteins involves binding to heparin sulfate proteoglycans and the LDLR like protein -1 (LRP1) in the hepatic space of Disse, in a process called secretion capture that requires local enrichment by hepatic expression of apoE
The Cholesterol Hypothesis
Studies by Anitschkow showing that feeding cholesterol in oil to rabbits caused the formation of atheroma, similar to those seen in humans, demonstrated a causal role of cholesterol in the pathogenesis of atherosclerosis in 1913 (220). In 1939, Muller described families with inherited high cholesterol and increased risk for cardiovascular disease (221). Yet it would take several decades before compelling evidence from epidemiological studies, such as Framingham (221)and MRFIT (222), demonstrated that elevated blood cholesterol levels were associated with increased risk of cardiovascular events (CVE). Subsequently, LDL-C levels were found to be directly associated with CVE (223); whereas HDL-C levels were shown to be inversely related to risk of CVE (224). The Seven Countries Studies by Ancel Keys showed that coronary heart disease (CHD) mortality rates were higher in countries with higher blood levels of cholesterol (e.g. Finland, Norway, and the USA) than in countries of southern Europe and Japan with lower blood levels of cholesterol (225). The high levels of cholesterol were proposed to be associated with the amount of saturated fat in the diet. As such, the cholesterol hypothesis was born, proposing that lowering LDL-C would reduce CVE(226).
Response to Retention Hypothesis for the Initiation of Atherosclerosis
The response to retention hypothesis holds that retention of atherogenic lipoproteins in the artery wall is a critical initiating event that sparks an inflammatory response and promotes the development of atherosclerosis (Figure 1). First articulated in 1995 by Williams and Tabas (227), the hypothesis was based on more than two decades of work demonstrating that apoB-containing lipoproteins are retained in the artery wall by interaction with proteoglycans (228,229). Proteoglycans consist of a protein core bound covalently to one or more glycosaminoglycans (GAGs). The most common proteoglycans in the artery wall are decorin, biglycan, perlecan, versican, and syndecan-4 (230). There is ionic binding between the positively charged GAGs and negatively charged amino acids of apoB100 (229). Boren et al.identified the principal proteoglycan-binding site in LDL and showed that a single point mutation in apoB100 impaired binding to proteoglycans (231). The major proteoglycan binding site consists of residues 3359-3369 in apoB100 (site B), which is in the C-terminal half of apoB100. Furthermore, mutation of “site B” in mice resulted in reduced retention of apoB100 in the artery wall and reduced atherosclerosis, providing in vivosupport for the response to retention hypothesis (232). Subsequently, proteoglycan binding sites were identified for apoB48 (233)and a second site (site A) on apoB100, which is exposed when LDL is modified by secreted phospholipase A2 (sPLA2), forming a small dense LDL particle (234).
Surprisingly, native LDL, despite the strong evidence for its critical role in promoting atherosclerosis, does not induce macrophage foam cell formation or much in the way of inflammation in vitro. These observations led to the hypothesis that LDL has to be modified to promote foam cell formation and induce inflammation. Binding of proteoglycans induces structural changes in LDL impacting both the configuration of apoB100 and the lipid composition (234). Hence, the binding of LDL to proteoglycans makes the LDL more susceptible to oxidation and aggregation, which promotes foam cell formation and a proinflammatory response, and the process is self-perpetuating. Oxidized LDL (oxLDL) can induce further production of proteoglycans by vascular smooth muscle cells, retaining more LDL in the arterial wall. Furthermore, macrophages express LPL, which can serve as bridging molecules, binding both lipoproteins and proteoglycans (235,236). Consistent with an important role for LPL in atherogenesis, the loss of macrophage LPL expression protects mice from atherosclerosis (237,238). In addition, macrophages secrete sphingomyelinase, which has been reported to act synergistically with LPL to promote binding of LDL and lipoprotein (a) (Lp(a)) to vascular smooth muscle cells (VSMC) and the extracellular matrix promoting their retention in the artery (239,240). Furthermore, sphingomyelinase induces aggregation and fusion of LDL particles, promoting increased binding to proteoglycans and induces foam cell formation (241). Thus, interfering with the retention of apoB-containing lipoproteins in the artery wall is a potential strategy for preventing atherosclerosis.
OXIDATION OF PHOSPHOLIPIDS AND PROTEINS IN LIPOPROTEINS AND THEIR ROLE IN ATHEROSCLEROSIS
Overview
The response to retention hypothesis for the initiation of atherosclerosis posits that retention of LDL in the artery wall leads to its modification into highly atherogenic particles that initiate inflammatory responses. A key overall point is that retention of LDL leads to oxidative modification of LDL, allowing this oxidized LDL (oxLDL) to be recognized by scavenger receptors on macrophages and other cells. Uptake of oxLDL by macrophages leads to marked accumulation of cholesterol, converting them to foam cells and initiating development of atherosclerotic lesions. In addition to serving as a substrate for cholesterol accumulation, oxLDL exerts a wide range of bioactivities that are consistent with it being critical for driving atherogenesis (Table 1). In mouse models, loss of enzymes that modulate LDL oxidation increases atherosclerosis, and dietary antioxidants that reduce levels of oxLDL also inhibit atherosclerosis. Although human trials with dietary antioxidants have failed to reduce disease outcomes, it is important to recognize that these interventions are less efficacious in reducing oxLDL levels in humans than in rodent models. Additional studies are needed to determine optimal interventions for lowering oxLDL levels and whether such interventions will be effective for preventing or treating atherosclerosis.
| Table 1-Potential Atherogenic Activities of Oxidized LDL (oxLDL) | |
| Macrophages | Smooth muscle cells |
| Serves as ligand for recognition by scavenger receptors 256, 257, 258 | Induces proliferation, migration, and transition to inflammatory phenotype 276, 277, 278, 279 |
| Serves as substrate for unregulated cholesterol uptake 262 | |
| Induces expression and secretion of inflammatory cytokines 280, 281, 282, 283 | Lymphocytes |
| Induces polarization to M1 (minimally oxidized LDL) or M2-phenotype (highly oxidized LDL) 284 | Serves as a neo-antigen 274 |
| Inhibits egress from atherosclerotic lesions 289 | Induces chemotaxis 275 |
| Induces macrophage apoptosis and rupture of atherosclerotic plaques 290, 291, 292 | Increases antibody production 275 |
| Other cell types | |
| Endothelial cells | Induces chemotaxis of monocytes, PMN, and eosinophils 285, 286, 287, 288 |
| Induces surface expression of adhesion molecules 266, 268, 269, 270 | Increases platelet aggregation 293, 294, 295, 296 |
| Induces inflammatory genes including cytokine release 271, 272 | Activates dendritic cells and induces their release of T cells stimulating cytokines 284 |
Peroxidation of Polyunsaturated Fatty Acids Generates Oxidatively Modified Lipoproteins
The outer shell of lipoproteins is composed of phospholipids with polyunsaturated fatty acid (PUFA) side chains. These PUFAs (and to a lesser extent the PUFAs of cholesterol esters and triglycerides in the lipoprotein core) are highly vulnerable to oxidation by free radical species, particularly hydroxyl radicals (●OH). This vulnerability results from the relatively low energy required for free radicals to abstract hydrogen atoms located between two adjacent double bonds (bis-allelic hydrogens). Hydrogen abstraction by free radicals creates a lipid radical that reacts nearly instantaneously with any molecular oxygen present in the environment.
The resulting lipid peroxide radical (LOO●) can then propagate the radical reaction by abstracting hydrogens from neighboring phospholipids or can react with itself to create a large number of secondary peroxidation products (Figure 7). Secondary products that may be relevant to atherogenesis can be thought of in two broad classes: oxidized lipids (primarily oxidized phospholipids but also oxidized cholesterol esters) and reactive lipid aldehydes that exert their effects by modifying proteins and other macromolecules. Oxidized phospholipids (oxPL) include chain shortened oxPL such as 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC)(242), 1-O-alkyl-2-azelaoyl-sn-glycero-3-phophorylcholine (azPAF) (243), and 1-(Palmitoyl)-2-(5-keto-6-octene-dioyl) phosphatidylcholine (KOdiA-PC) and cyclized oxPL such as 1-palmitoyl-2-(5,6)-epoxyisoprostane E2-sn-glycero-3-phosphocholine (PEIPC) (244)and 1-palmitoyl-2-F2-isoprostane-sn-glycero-3-phosphocholine (F2IsoP-PC) (245). Reactive lipid species include malondialdehyde (246), 4-hydroxynonenal (246), and isolevuglandins (247)that modify proteins associated with lipoprotein particles including ApoB100 (Figure 7).

Figure 7. Oxidation of Phospholipid Polyunsaturated Fatty Acids. Oxidation of phospholipids containing polyunsaturated fatty acids present in plasma lipoproteins results in formation of a variety of reactive lipid aldehydes and oxidized phospholipids that convert these lipoproteins to atherogenic particles. Reactive lipid species include malondialdehyde (MDA), isolevuglandins (IsoLG), methyglyoxal (MGO), 4-oxononenal (ONE), and 4-hydroxynonenal (HNE). Oxidized phospholipids include 1-palmitoyl-2-oxovaleroyl-sn-glycero-3-phosphorylcholine (POVPC), 1-O-alkyl-2-azelaoyl-sn-glycero-3-phophorylcholine (azPAF), 1-(Palmitoyl)-2-(5-keto-6-octene-dioyl) phosphatidylcholine (KOdiA-PC), 1-palmitoyl-2-F2-isoprostane-sn-glycero-3-phosphocholine (F2IsoP-PC), and 1-palmitoyl-2-(5,6)-epoxyisoprostane E2-sn-glycero-3-phosphocholine (PEIPC).
It is critical to keep in mind that oxidatively modified LDLs (oxLDLs) are in fact highly heterogeneous and complex particles, even though oxLDL is usually referred to as a discrete entity. Oxidation of LDL in vitro has been used extensively to study the biological activities of oxLDL, but, even here, the actual species present varies significantly based on the oxidation method (exposure to air, to copper, or to oxidases) and length of oxidation. Many of the methods commonly used to measure the concentration of oxLDL in vivo only measure general characteristics of oxLDL. For instance, because the reaction of reactive lipid species with lysine residues of ApoB100 converts LDL from a positively charged particle to a negatively charged particle, oxLDL is often detected by increased mobility during agarose gel electrophoresis. Alternatively, oxLDL in plasma and other tissues can be quantified by the immunoreactivity of the natural IgM autoantibody E06. However, while E06 recognizes a variety of oxidized phosphatidylcholines, it does not necessarily recognize all oxPL equally. Therefore, equivalent E06 immunoreactivity does not necessarily mean exposure to identical oxLDL particles. While modification of LDL with malondialdehyde (MDA-LDL) (248)is often used as a model of oxLDL for bioactivity assays, modification of LDL by other reactive lipid species can exert unique effects from MDA-LDL, and MDA-LDL does not include any of the various oxPL species. Therefore, it is important to keep in mind that in vivo oxLDL is a mixture of many different compounds and that the atherogenic activities of oxLDL represent the net cellular responses to the full range of compounds present.
While oxLDL has been studied in greatest detail, all lipoproteins are vulnerable to oxidation at least in vitro, and this oxidative modification alters their biological activities in ways that may be atherogenic. The species of plasma lipoprotein that has the highest content of oxidized phospholipids (oxPL) depends on the species of oxPL under consideration. This suggests that not all oxPL are formed in situ on the lipoprotein where they are found and might instead be transferred from other lipoproteins or tissues. Lp(a) is the major carrier in plasma of oxPLs that are detected by E06 immunoreactivity (249)and these oxPLs associate with Lp(a) in preference to native LDL particles in human plasma (250). E06 immunoreactive oxPL generated in chemically oxidized LDL can rapidly transfer to Lp(a) (249), so the high content of these lipids in Lp(a) isolated from human plasma may be due either to direct oxidation of Lp(a) or by transfer of the oxPL from oxLDL to Lp(a). In LDL and Lp(a) isolated from human plasma, levels of MDA-modified lysine (based on E014 immunoreactivity) are higher in LDL than Lp(a), while E06 immunoreactivity is much greater for Lp(a) than for LDL (249). Because MDA-modified proteins do not readily transfer between particles, these findings suggest that oxidation initially occurs in LDL with subsequent transfer of oxPL to Lp(a). Thus, a physiological role has been proposed for Lp(a) in binding and transporting oxPL in the plasma (251). Unlike oxPL detected by E06 immunoreactivity that are highest in Lp(a), the F2-IsoP-phospholipids forms of oxPL are highest in HDL (252). As with Lp(a), the high levels of these oxPLs in HDL may well be the result of transfer from other oxidized lipoproteins and tissues. Because oxidation is unlikely to occur in the circulation, the rate that oxPL are transferred from tissue to various plasma lipoproteins could potentially be an important determinant of the risk for atherosclerosis.
Significant correlations have been found between levels of oxLDL and extent of atherosclerosis in human patients. Measurement of oxLDL using E06 antibody showed that: 1) significant elevation of oxLDL in acute coronary syndromes (250), 2) treatment with a statin markedly reduced these levels (253), 3) oxLDL levels are higher in children with familial hypercholesterolemia compared to their siblings (254), and 4) oxLDL levels predict the presence and progression of atherosclerosis and symptomatic cardiovascular disease (255). Measurement of oxLDL using antibodies against MDA-LDL found that oxLDL were elevated in patients with coronary artery disease (CAD) (256), that elevated levels of oxLDL predicted future cardiac events in diabetic patients with CAD, that oxLDL were particularly elevated in patients with rheumatoid arthritis and CAD compared to either alone (257), and that treatment with fibrates decreased levels of oxLDL (258). Thus, there is a clear correlation between the presence of oxLDL and cardiovascular disease.
Mechanisms of Lipoprotein Oxidation In Vitro And In Vivo
The precise mechanisms that generate oxidized lipoproteins in vivo are still only partially understood. LDL circulating in the plasma appears to be protected from oxidation, both by dietary antioxidants such as vitamin E and C (259)and by protective enzymes including glutathione peroxidases (260,261), peroxiredoxins, PAF-acetylhydrolase (also known as lipoprotein-PLA2) (262,263), and paraoxonases (PON) (264,265). Penetration of LDL into the artery wall occurs at branch points in the aorta and other places with turbulent flow and shear stress. Retention of LDL in the intima, due to interactions with extracellular matrix such as chondroitin sulfate-rich proteoglycans, sequesters LDL away from the antioxidant environment of the plasma and exposes LDL to oxidation. A variety of oxidases and peroxidases generate strong oxidants that can readily oxidize LDL. These include myeloperoxidase (MPO) (266), xanthine oxidase (XO) (267), NADPH oxidases (NOXs) (268), and inducible nitric oxide synthase (iNOS) (269). Oxygenases such as lipoxygenases (LOX) have also been shown to oxidize LDL in vitro (270,271). The extent that each of these enzymes contributes to lipoprotein oxidation in vivo and thus to atherosclerosis remains to be fully elucidated, and there is much we do not understand about these individual processes. This is illustrated by studies on MPO and the 12/15-Lipoxygenase, the two enzymes most closely linked to lipoprotein oxidation.
MYELOPEROXIDASE (MPO)
MPO released from activated neutrophils (and to a lesser extent from monocytes/macrophages) can accumulate in the subintimal space of the artery wall(272), so neutrophil activation indirectly increases the chance for lipoprotein oxidation. Increased plasma levels of MPO correlate with increased levels of oxLDL in hypercholesterolemic children (273). Increased MPO blood levels also associate with increased risk for atherosclerosis (274-277)and polymorphisms in the MPO gene that lower MPO activity reduce the risk for atherosclerosis (278,279).
Incubation of lipoproteins with MPO generates oxidized phospholipids that serve as ligands for CD36(280). A putative binding site for MPO with the apoB of LDL has been identified (281), although further verification is needed. Of interest, MPO also associates with HDL via binding to ApoAI and PON1 in a ternary complex(282), so that the binding of MPO to HDL and subsequent generation of reactive oxygen species may account for the high levels of oxidized lipids carried by HDL. Association of MPO with HDL leads to modification of tyrosine 71 of paraoxonase, reducing PON1 activity (282). It also generates reactive lipid dicarbonyls such as isolevuglandins that modify ApoAI (283)and phosphatidylethanolamine (284). In the presence of small molecules that scavenge lipid dicarbonyls, the ability of MPO to crosslink ApoAI is markedly reduced (283). Modification of HDL by lipid dicarbonyls such as isolevuglandins and MDA reduce its ability to drive cholesterol efflux from macrophages and protect against inflammatory stimuli such as LPS (283,285).
Mouse models have been used to directly examine the contribution of MPO to atherosclerosis, although these studies carry the caveat that mouse MPO levels are only 10-20% that of humans (286,287). Transplantation of bone marrow from genetically altered mice into atherosclerosis susceptible strains (e.g. Ldlr-/- and Apoe-/- mice) after lethal irradiation to ablate host hematopoietic cells is commonly used to study the effect of specific genes expressed by macrophages and other hematopoietic cells on atherogenesis (99). Reconstitution of Ldlr-/- mice with macrophages overexpressing MPO markedly increased their susceptibility to atherosclerosis (288). However, transplantation of MPO-/- macrophages into Ldlr-/- mice, also markedly increased atherosclerosis, and this was confirmed in MPO-/-/Ldlr-/- double knockout mice compared to MPO+/+/Ldlr-/- controls (289). The reasons for these paradoxical findings with both MPO overexpression and deletion remain unclear. Perhaps the complete lack of MPO activity is harmful because it allows overgrowth of specific microbes that incite atherosclerosis via alternative mechanisms. In contrast to effects of complete ablation, a recently developed selective MPO inhibitor (e.g. INV315) that only partially reduces MPO activity markedly reduced atherosclerosis in Apoe-/- mice (290). Thus, clinical studies with selective MPO inhibitors are needed to determine if this will be a meaningful therapeutic approach to the treatment of atherosclerosis in humans.
12/15-LIPOXYGENASES
Although the primary substrates for lipoxygenases are non-esterified fatty acids, exposure of LDL to 15-LOX also leads to oxidation of phospholipids and cholesterol esters (270,271). In mice, the gene analogous to the human 15-LOX encodes a lipoxygenase that converts arachidonyl chains to both 12-HPETE and 15-HPETE and is thus a 12/15-LOX. 12/15-LOX-/- mice on Apoe-/- background have reduced atherosclerosis compared to Apoe-/- mice (291). Importantly, they also have lower levels of autoantibodies against oxLDL and MDA-LDL (291). These results support the notion that 12/15-LOX can directly contribute to atherosclerosis via LDL oxidation. Nevertheless, the role of 15-LOX in human atherogenesis is less clear-cut. While homozygotes of an Alox15 variant that almost completely ablates 15-LOX activity tended to have a reduced risk for coronary artery disease, heterozygotes paradoxically have increased risk of disease (292). Other polymorphisms in the Alox15 gene encoding 15-LOX increase risk for coronary artery calcification (293), yet others have no effect (294). Direct correlations between Alox15 polymorphisms and biochemical measurements of oxidized lipoproteins or oxPL and oxidized cholesterol esters have not been reported to date in humans, but are clearly needed.
Biological Activities of OxLDL And Receptors That Mediate These Activities
Perhaps the most important atherogenic effect of LDL oxidation is that this modification of LDL shifts recognition and internalization of the lipoprotein from the LDL receptor (LDLR) to scavenger receptors (295-297). While internalization of LDL by the LDLR in hepatocytes downregulates cholesterol synthesis to maintain cholesterol homeostasis, internalization of oxLDL by scavenger receptors fails to trigger this inhibition (298,299,300). Thus, cholesterol synthesis continues unabated despite the fact that peripheral cells are accumulating large amounts of cholesterol. In particular, macrophages express scavenger receptors and gluttonously take up large quantities oxLDL to form foam cells in the initial atherosclerotic lesion (301).
OxLDL also activates a number of cellular responses in macrophages, dendritic cells, endothelial cells, T cells, and smooth muscle cells that in aggregate promote inflammation, lesion formation, atherogenesis, and unstable atherosclerotic plaques (302-304). OxLDL induces surface expression of adhesion molecules and the release of chemokines from endothelial cells (305-311), all of which are important steps in recruitment of leukocytes to sites of lesions. Exposure to oxLDL activates dendritic cells so that they induce T-cell proliferation and production of IL-17 (312). OxLDL itself also serves as a neo-antigen (313). OxLDL also induces increased antibody generation by lymphocytes (314). OxLDL also promotes smooth muscle cell proliferation, migration, and transition to a proinflammatory phenotype (315-318). OxLDL induces secretion by macrophages of inflammatory cytokines (e.g. TNF, IL-1, MCP-1, and IL-8) that activate other inflammatory cell types (319-322). OxLDL polarizes macrophages towards the M1-like phenotype or M2-like phenotype depending on its extent of oxidation (323). OxLDL promotes the chemotaxis of monocytes, neutrophils, eosinophils, and T cells (314,324-327), bringing them into the arterial wall. In contrast, oxLDL inhibits macrophage emigration out of atherosclerotic lesions, because it induces netrin-1 (328). OxLDL induces apoptosis of macrophages and development of unstable plaques prone to rupture (329-331). Thrombotic arterial occlusion in the aftermath of plaque rupture is a critical cause of mortality, therefore the fact that oxLDL increases platelet aggregation (332-335)suggests an additional mechanism whereby elevated circulating oxLDL may increase risk of mortality during acute coronary events (336). As discussed in detail below, identification of cognate receptors for various components of oxLDL and other oxidized lipoproteins has provided important insight into the mechanisms by which these oxidized lipoproteins exert their pathophysiological effects.
MACROPHAGE SCAVENGER RECEPTOR (SR-AI)
In 1979, Brown and Goldstein demonstrated that macrophages had specific binding sites for acetylated LDL (AcLDL) that allowed uptake of this modified LDL even in the presence of high cellular cholesterol levels (298). This was in contrast to LDL uptake by the LDLR, which is markedly downregulated when cellular cholesterol levels rise (Figure 2). Cholesterol synthesis is also downregulated by LDL uptake by LDLR (337). The lack of feedback inhibition during uptake of modified LDL by this unidentified receptor suggested a plausible mechanism for the massive accumulation of cholesterol in macrophages that generates foam cells. The putative receptor mediating this binding was named the macrophage scavenger receptor (MSR). Later, oxLDL (338)and MDA-LDL (248)were shown to compete with AcLDL for binding and uptake by macrophages, suggesting they were native ligands for MSR. In 1990, Kodama et al. purified and sequenced this scavenger receptor, allowing identification of the MSRgene (339). Through alternative gene splicing, this gene gives rise to Scavenger Receptor A–I (SR-AI), SRA-II, and SRA-III. Deletion of the MSR gene in C57BL6 mice fed butterfat diet substantially reduced atherosclerotic lesions and deletion of MSR in Ldlr-/-mice also reduced lesion formation (340).
CD36 AND OTHER SCAVENGER RECEPTORS
Subsequent work has shown that in addition to SR-AI, macrophages express a wide range of scavenger receptors that recognize oxidized lipoproteins including MARCO, scavenger receptor-B1, -B2, -B3 (CD36), and Lectin-like oxLDL Receptor-1 (LOX-1) (341). These scavenger receptors belong to a larger family of pattern recognition receptors, all of which are individually capable of binding to a wide spectrum of ligands. Quantitatively, SR-A1 and CD36 account for the vast majority of all oxLDL uptake by macrophages (342). The specific ligands of the two receptors on oxLDL appear to diverge (342). SR-AI appears to preferentially recognize more rigorously oxidized LDL and seems to primarily recognize modified lysine residues like MDA-lysines. In contrast, the primary ligands of CD36 on oxLDL appear to be oxidized phospholipids, in particular fragmented phosphatidylcholine including azPAF (243), POVPC (343)and KOdiA-PC (280). Apoe-/- mice lacking CD36 are more vulnerable to some bacterial infections (344)but also have less atherosclerosis when fed a high cholesterol diet (345).
Recent findings suggest that SR-AI and other scavenger receptors have both pro- and anti-atherosclerotic effects, depending on the context. For instance, deletion of the MSR gene actually increased lesion size in male Apoe-/-mice (346); however, deletion of both MSR and CD36 greatly reduces lesion complexity and vulnerable plaques, the most critical aspect of lesion development (347). The complex results of scavenger receptor deletion should not be surprising given that scavenger receptors have multiple ligands and that an important role of scavenger receptors expressed by macrophages is to allow these macrophages to remove bacteria and damaged cells from surrounding tissues. Under normal physiological conditions, uptake of oxLDL by macrophages is probably generally protective, because subsequent efflux of the cholesterol from the macrophages to HDL via reverse cholesterol transport as well as emigration of these macrophages from the arterial wall to lymph nodes serves to minimize the accumulation of cholesterol-laden macrophages in the arterial wall. However, under conditions where reverse cholesterol transport capacity is reduced or where emigration of macrophages is inhibited, uptake of oxLDL by macrophages leads to its accumulation and initiation of pathophysiological processes.
TOLL-LIKE RECEPTORS AND OTHER TARGETS OF OXLDL
In addition to scavenger receptors, other pattern recognition receptors also recognize components of oxLDL. Perhaps most important among these are the Toll-like Receptors (TLR) including TLR-2 (348,349), TLR-4 (350), TLR-6 (351), TLR-7 (352), and TLR-9 (352). TLRs can interact with scavenger receptors, for instance, CD36 forms complexes with TLR4 and TLR6 that recognize oxLDL and activate NFkappaB (351).While bacterial components such as bacterial lipopolysaccharide (LPS) are full agonists for TLRs, oxLDL components like POVPC often appear to act functionally as partial agonists of TLRs, so that activation of macrophages and dendritic cells by full agonists like LPS is reduced in the presence of oxLDL (353,354).
In addition to TLRs, another important pattern recognition receptor for oxLDL is the receptor for advanced glycation end-products (RAGE) (355). Other factors of the innate immune response that bind oxidized phospholipids including C-reactive protein(CRP) (356,357)and natural IgM antibodies like E06 (358,359). While scavenger and pattern recognition receptors tend to recognize broad classes of compounds, a number of G-protein coupled receptors (GPCRs) recognize specific oxidized phospholipids. These include the receptor for platelet-activating factor (PAFR) (360-362), prostaglandin receptor EP2 (363,364), and sphingosine-1-phosphate receptor 1 (S1P1) (365). Intracellular receptors for oxidized phospholipids include nuclear hormone receptors PPAR alpha (366)and PPAR gamma (243). Non-receptor, intracellular targets for oxLDL include c-SRC (367)and NRF-2 (368,369).
Mechanisms Protecting Against LDL Oxidation In Vivo
Given the susceptibility of LDL to oxidation, it is perhaps not surprising that a number of mechanisms appear to exist in order to protect LDL from oxidation. These include small molecule antioxidants circulating in plasma and enzymes that catabolize oxidized lipids. How essential each of these mechanisms are to the control of oxLDL levels and preventing the development of atherosclerosis remains an area of active investigation. Obviously, a better understanding of the relationship between changes in protective mechanism and atherogenesis might allow identification of particularly vulnerable individuals and the development of novel therapeutic approaches.
SMALL MOLECUE ANTIOXIDANTS
Circulating small molecule antioxidants such as ascorbate (vitamin C), alpha-tocopherol (vitamin E), urate, and bilirubin serve as sacrificial targets reacting with free radicals and reactive oxygen species to prevent lipid and protein oxidation. Thus, even when strong oxidants are added to plasma ex vivo, there is relatively little generation of oxLDL until the oxidants have depleted these small molecule antioxidants, most specifically ascorbate (370). Depletion of vitamin C and vitamin E increase atherosclerosis in Apoe-/-mice(371). Importantly, plasma ascorbate levels inversely correlate with prevalence of cardiovascular disease in humans (372). Supplementation with vitamin C appears to play a role in preventing endothelial dysfunction in humans (373). However, it is not clear that supplementing dietary antioxidants beyond those typically obtained in a well-balanced diet endows any additional atheroprotective effects. Supplementation with dietary antioxidants inhibits development of atherosclerosis in susceptible mice (374-378). While a few human trials with dietary antioxidants have demonstrated reduced atherosclerosis and cardiovascular disease (379-382), most large-scale trials have failed to demonstrate any disease reduction (383-387). The reasons underlying these failures continue to be investigated and debated (388,389). Because it had not been fully appreciated that relatively high doses of these antioxidants were needed to markedly alter lipid peroxidation rates in humans (390), one possibility is that the doses used in most large scale prevention trials were simply insufficient (390,391) . However, the ability to use very high doses of small molecule antioxidants like vitamin E for extended periods of times may be limited by the toxicity of these high doses (392).
ANTIOXIDANT ENZYMES
Antioxidant enzymes appear to play a more critical role than dietary antioxidants in limiting lipoprotein oxidation. Two families of nonheme peroxidases, the glutathione peroxidases and the peroxiredoxins, appear to be the most critical. Glutathione peroxidases (Gpx) 1-4 are selenoproteins that convert glutathione to glutathione disulfide while reducing peroxides (including lipid peroxides) to water (393,394). Polymorphisms in glutathione peroxidase 1 (Gpx1) are associated with increased risk for atherosclerosis in various human populations (395-397). Furthermore, genetic deletion of Gpx1 markedly exacerbates atherosclerosis in Apoe-/- mice(398,399), while overexpression of Gpx4 in Apoe-/-mice inhibits atherogenesis (400). Peroxiredoxins (Prdx) are cysteine containing proteins where the cysteine is oxidized to sulfenic acid during reduction of peroxides (401). Deletion of either Prdx1 or Prdx2 increases atherosclerosis in Apoe-/- mice(402,403). Overexpression of Prdx4 inhibits atherosclerosis in Apoe-/- mice (404). In contrast, overexpression of Prdx6 failed to inhibit atherosclerosis in C57BL6 mice fed an atherogenic diet (405).
In general, studies looking for associations between risk for atherosclerosis and polymorphisms or deficiencies in other major antioxidant genes including catalase, SOD-1, -2, and -3, and glutathione S-transferase have been negative (406,407). In fact, SOD-1 overexpression may even increase fatty streak lesions in mice (408). However, SOD-1 does inhibit proliferation and migration of smooth muscle cells induced by oxLDL in vitro (315), and overexpression of both SOD-1 and catalase reduce atherosclerosis in Apoe-/-mice (409). Sod2+/-mice crossed with Apoe-/-mice have increased atherosclerosis compared to control Apoe-/-mice (410), but there is little effect on atherosclerosis of crossing Sod3-/-mice with Apoe-/-mice (411). Several studies have demonstrated an association between SOD2and hypertriglyceridemia (412,413).
ENZYMES THAT CATABOLIZE LIPIDS
In addition to anti-oxidant enzymes, several enzymes specifically catabolize oxidized phospholipids including secreted Platelet-Activating Factor Hydrolases (sPAF-AH) and Paraoxonases (PON). sPAF-AH, also known as lipoprotein associated PLA2 (LP-PLA2) is a calcium independent PLA2secreted by macrophages that primarily circulates on LDL and to a lesser extent on HDL (414,415). sPAF-AH does not hydrolyze phospholipids with the typical long-chain fatty acids, but efficiently cleaves phospholipids with oxidatively fragmented (e.g. azPAF and POVPC) (362,416,417)or oxidatively cyclized (e.g. F2-isoprostane-PC) sn-2 chains (418). Whether this effect results in a net gain of pro- or anti-inflammatory lipids is controversial, because only some of these oxPL are highly potent inflammatory mediators, while others are partial agonists that might therefore antagonize inflammatory responses to other mediators like LPS. Furthermore, this hydrolysis generates lysoPC and lysoPAF, which are proinflammatory at high concentrations. This ambivalent effect is also seen in vivo. While a large number of clinical studies have found that increased sPAF-AH predicts increased risk for atherosclerosis (419,420), whether increased sPAF-AH actually contributes to atherogenesis or simply reflects a compensatory increase in response to elevated oxLDL is unclear (421,422). Some gene polymorphisms in sPAF-AH that reduce its activity (i.e. Val279Phe) appear to increase the risk of myocardial infarction (423), yet another polymorphism (i.e. Ala379Val) appears to have little effect (424). The interpretation that increased sPAF-AH activity caused an increased risk of atherosclerotic cardiovascular disease (ASCVD) led to the development of selective sPAF-AH inhibitors and their clinical trials (425). However, two recently completed phase III trials with one such inhibitor, darapladib, found that while this drug significantly reduced circulating PAF-AH activity, it had no effect on ASCVD events (426,427).
Paraoxonases (PONs) were originally named for their ability to hydrolyze the neurotoxin paraoxon and this activity is still routinely used to assay paraoxonase activity in plasma. However, in terms of atherosclerosis, the most important physiological function of PONs appears to be their ability to protect against LDL oxidation (428). PON-1 and PON-3 circulate bound to HDL. HDL treated with specific inhibitors of PON fails to protect LDL from oxidation (429). Treatment of oxLDL with purified PON1 markedly decreases its ability to induce endothelial cell activation and monocyte binding (264). Genetic deletion of PON1 markedly increases atherosclerosis in C57BL6 mice(430), and this is further exacerbated in Apoe-/-mice (431). Conversely, overexpression of PON-1 reduces atherosclerotic lesions in both wild-type mice fed high cholesterol diets and Apoe-/- mice (432). Adenovirus expression of PON-2 and PON-3 also inhibits atherosclerosis in Apoe-/-mice (433,434), indicating that all three PON enzymes have protective effects. However, transgenic Apoe-/- mice overexpressing the entire gene cluster of PON genes (PON-1, -2, -3) were not further protected compared to Apoe-/-mice with transgenic expression of PON-1 or PON-3 alone (435), suggesting these effects are redundant rather than additive. These mouse studies appear relevant to human disease, as a large number of studies have shown that polymorphisms in PON1 are associated with increased risk for atherosclerosis (265). It should be noted that PON activity varies greatly even in persons with the same polymorphism, suggesting that environmental factors leading to PON inactivation may also be important in determining disease risk.
Summary for Oxidized Lipoproteins
In summary, substantial evidence has accumulated over the past several decades for a causative role for oxidized lipoproteins in the initiation and progression of atherosclerosis and the need to reduce lipoprotein oxidation in order to reduce disease burden. Nevertheless, significant questions remain including which mechanisms are most important for driving lipoprotein oxidation, what treatment strategies can effectively reduce lipoprotein oxidation, and what are the key components of oxidized lipoproteins that drive atherogenesis?
ELEVATED LDL-C AND RISK FOR ASCVD
Genetic Causes of Elevated LDL-C
As described above, FH is an autosomal dominant inherited disorder associated with elevated levels of LDL-C and premature ASCVD, and provides some of the most compelling evidence for a causal role for LDL-C in atherosclerosis. Brown and Goldstein discovered the LDLR pathway and found that mutations in the Ldlrgene cause FH (300). Heterozygotes for loss-of-function mutations have cholesterol levels that are about twice normal, and these subjects are at increased risk of premature CVE. In contrast, individuals with homozygous FH have extremely high levels of LDL-C (> 500 mg/dL) and often develop severe coronary atherosclerosis and supravalvular aortic stenosis in early childhood. The prevalence of heterozygous FH is around 1/200-250 in the USA, whereas homozygous FH is extremely rare affecting only about 1/160,000 to 1/250,000 individuals (436). Nonetheless, about 20% of people having myocardial infarctions (MI) before 40 years of age have heterozygous FH. Thus, FH offers an important opportunity to target therapies to prevent atherosclerosis (437), but FH remains under recognized with recent evidence suggesting that only 1-10% of subjects with FH have been identified (438). Most individuals with significant hypercholesterolemia do not have classic monogenic autosomal dominant inherited dyslipidemias, but polygenic factors contributing to susceptibility to environmental factors underlie the observed increase in LDL-C levels. A recent study suggests that among individuals with LDL cholesterol ≥190 mg/dl, gene sequencing identified a monogenic FH mutation in only <2%of subjects (439). However, for any observed LDL cholesterol, FH mutation carriers are at substantially increased risk for CAD (439). Pathogenic variants in three genes (LDLR, APOB, and PCSK9) account for the majority of monogenic FH cases. Recent genome-wide association studies (GWAS) have identified more than 50 discrete genetic loci that are associated with an increased risk of CVE(440,441). Many of these genetic loci are associated with genes previously known to impact LDL-C levels and cardiovascular risk (e.g. Ldlr, APOB, PCSK9), but novel loci that impact both LDL-C levels and risk for MI have also been identified, e.g. sortilin-1 (SORT1)(442,443). Most importantly, inherited low levels of LDL-C due to loss-of-function mutations in the PCSK9gene have been shown to be associated with dramatic reductions in risk for ASCVD events in the Atherosclerosis Risk in Communities study (444). Hence, genetic disorders of lipoprotein metabolism provide strong evidence that the impact of LDL-C on the development of atherosclerosis is dose- and time-dependent (445), supporting a causal role for LDL-C in atherosclerosis.
Lowering LDL-C Reduces ASCVD
Large randomized outcomes trials of cholesterol lowering drugs have provided critical proof of the cholesterol hypothesis (446). The Coronary Drug Project, conducted between 1966 and 1975, found niacin treatment showed modest benefit in decreasing definite nonfatal recurrent myocardial infarction by 26% (10.2% for niacin group vs 13.8% for placebo group) (447). However, there was no benefit in primary endpoint, total mortality. Impressively, with a mean follow-up of 15 years, nearly 9 years after termination of the trial, all-cause mortality was 11% lower in niacin group than in the placebo group (448). The Lipid Clinics Research trial was another early major outcomes trial to show that lowering cholesterol reduced cardiovascular events. Treatment with cholestyramine, a bile acid binding inhibitor, resulted in a 12% reduction in LDL-C levels and a 19% reduction in CHD events (449). The early lipid lowering cardiovascular outcomes trials were limited by a lack of highly effective approaches for lowering LDL-C levels, and several trials raised concerns that cholesterol lowering did not reduce total mortality and might increase the risk of cancer, accidental death and suicide (446). The advent of the statin drug class (HMG-CoA reductase inhibitors) provided a much more effective approach to lowering LDL-C and laid to rest the concerns raised by the earlier trials. The 4S trial was a landmark clinical trial of cholesterol lowering with simvastatin in patients with coronary artery disease (CAD) and severely elevated levels of LDL-C that was designed to look at total mortality as the primary endpoint (450). The 4S showed for the first time that lowering LDL-C levels by 35% with simvastatin resulted in a 30% reduction in total mortality with a 42% reduction in CHD deaths and a 34% reduction in the risk of Major Coronary Events (450). A large number of subsequent trials extended these results to populations with CHD with low levels of LDL-C and to subjects without known CAD (primary prevention) with high or low levels of LDL-C (451). It is important to note that the relationship between on-treatment LDL-C lowering and reduction in cardiovascular events in secondary prevention trials was similar for both statin and non-statin approaches to lowering LDL-C levels. A large meta-analysis of 26 statin trials involving over 170,000 subjects demonstrated that statin treatment for 5–years reduced the combined incidence of major coronary events, coronary revascularization, and stroke by 20% per every 1 mmol/l (38.7 mg/dL) reduction in LDL-C (452). These results have been extended by a recent large meta-analysis of 49 trials involving 9 different interventions to lower LDL that included more than 300,000 patients and approximately 40,000 major vascular events, each 1mmol/l (38.7mg/dl) reduction in LDL-C was associated with 23% relative reduction in the risk of major vascular events (453). This raised the question of whether further lipid lowering would be of additional benefit. With the recent development of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, dramatic additional LDL lowering up to 50 -70% is now possible. In the FOURIER trial, patients with prior stable CAD who received the PCSK9 inhibitor, evolocumab, in combination with statin therapy achieved median LDL-C levels of 30 mg/dl. This was associated with a 15% reduction in the composite endpoint of cardiovascular death, MI, stroke, hospitalization for unstable angina or coronary revascularization (454). Similarly, ODYSSEY demonstrated that administration of alirocumab to acute coronary syndrome patients already on maximally tolerated statin therapy led to LDL-C values <50 mg/dl and was associated with a 15% reduction in the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke or unstable angina requiring hospitalization, and this benefit approached 24% in the subgroup of patients with initial LDL-C values >100 mg/dL (455). Together, the results of these PCSK9 trials reinforce the “lower is better” hypothesis.
Although statins are very effective in preventing CVE, many patients on statins do still have CVE, a phenomenon referred to as residual risk (456). This residual risk is likely attributable at least in part to inflammation. Indeed, definitive support for this hypothesis recently came from the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS), where administration of an anti-IL1bantibody to patients with prior MI and elevated serum hsCRP successfully reduced recurrent CVE independent of lipid lowering (>90% of patients were receiving concurrent statin therapy) (457). A secondary analysis of the FOURIER trial also demonstrated that, while relative risk for the primary cardiovascular endpoint was consistent across groups, the absolute risk reduction with evolocumab was greatest in patients with elevated hsCRP (458). These results suggest that targeting both LDL and inflammation will provide the most robust strategy for lowering ASCVD risk.
Levels of LDL-C, ApoB-100, Non-HDL Cholesterol and LDL-P as Markers for ASCVD Risk.
Based on the strength of the direct association of LDL-C levels and risk for ASCVD, the guidelines for treatment of hypercholesterolemia have focused on LDL-C levels for risk assessment, stratification and treatment recommendations. Indeed, the terms LDL-C and LDL are often, though incorrectly, used interchangeably in practice. It is important to understand that LDL is a collection of particles defined by density (d = 1.019 – 1.063 g/ml) that are heterogeneous, consisting of a large variety of lipids and proteins (459). In addition, LDL particles vary in size and cholesterol content. The relationship between LDL-C levels and risk for ASCVD is “J-shaped”, and the predictive value of LDL-C levels is better at higher levels of LDL-C. Surprisingly, the majority of subjects presenting to the hospital with acute coronary artery syndrome do not have elevated levels of LDL-C, but tend to have low levels of HDL-C and elevated triglycerides (460). There has been tremendous interest in whether other measures of LDL, including subpopulations, apoB100, or particle number, might serve as a better predictors of CVE than quantifying LDL cholesterol content.
Groundbreaking studies by Krauss and co-workers (461)described two major patterns for LDL subpopulations based on size and density of the LDL particles. Pattern A is characterized by large buoyant LDL (lbLDL) particles, whereas Pattern B is associated with small dense LDL (sdLDL). Importantly, sdLDL is associated with increased triglyceride levels and low HDL-C, which is referred to as the lipid triad, a phenotype common in insulin resistance. Hence, Pattern B is commonly seen in subjects with obesity, metabolic syndrome and type 2 diabetes mellitus. A number of studies have reported that Pattern B is associated with an increased risk of CVE (462). Several different approaches have been used to characterize LDL phenotypes, including gradient gel electrophoresis, ultracentrifugation (sequential and vertical), ion mobility and nuclear magnetic resonance (NMR) (462,463). A number of mechanisms have been proposed to underlie the proatherogenic properties of sdLDL, including increased susceptibility of oxidation (464)and glycation (465), promoting arterial retention and increased macrophage foam cell formation. Cholesteryl ester transfer protein (CETP), which transfers CE from HDL to VLDL/LDL and triglycerides in the opposite direction, and hepatic lipase, which hydrolyses triglycerides, impacts the lipid composition and size of sdLDL. As such, increased levels of sdLDL have the potential to provide additional information regarding risk of CVE in individuals with normal LDL-C levels but elevated triglycerides and low HDL. Alternatively, it has been proposed that the real impact of sdLDL is due to increased LDL particle number.
Each LDL particle contains one molecule of apoB100, and the majority of apoB100 in plasma is on LDL particles (466). Hence, levels of apoB100 correlate directly with LDL particle (LDL-P) number. A large number of studies have shown that levels of apoB100 are superior markers of ASCVD risk compared to LDL-C (467). Because the mass of cholesterol in LDL particles varies, LDL-C levels will result in overestimation apoB levels and the number of LDL particles, when LDL particles are cholesterol-enriched (Figure 8) and underestimate apoB and LDL particle number when the particles are cholesterol depleted (Figure 8) (468).
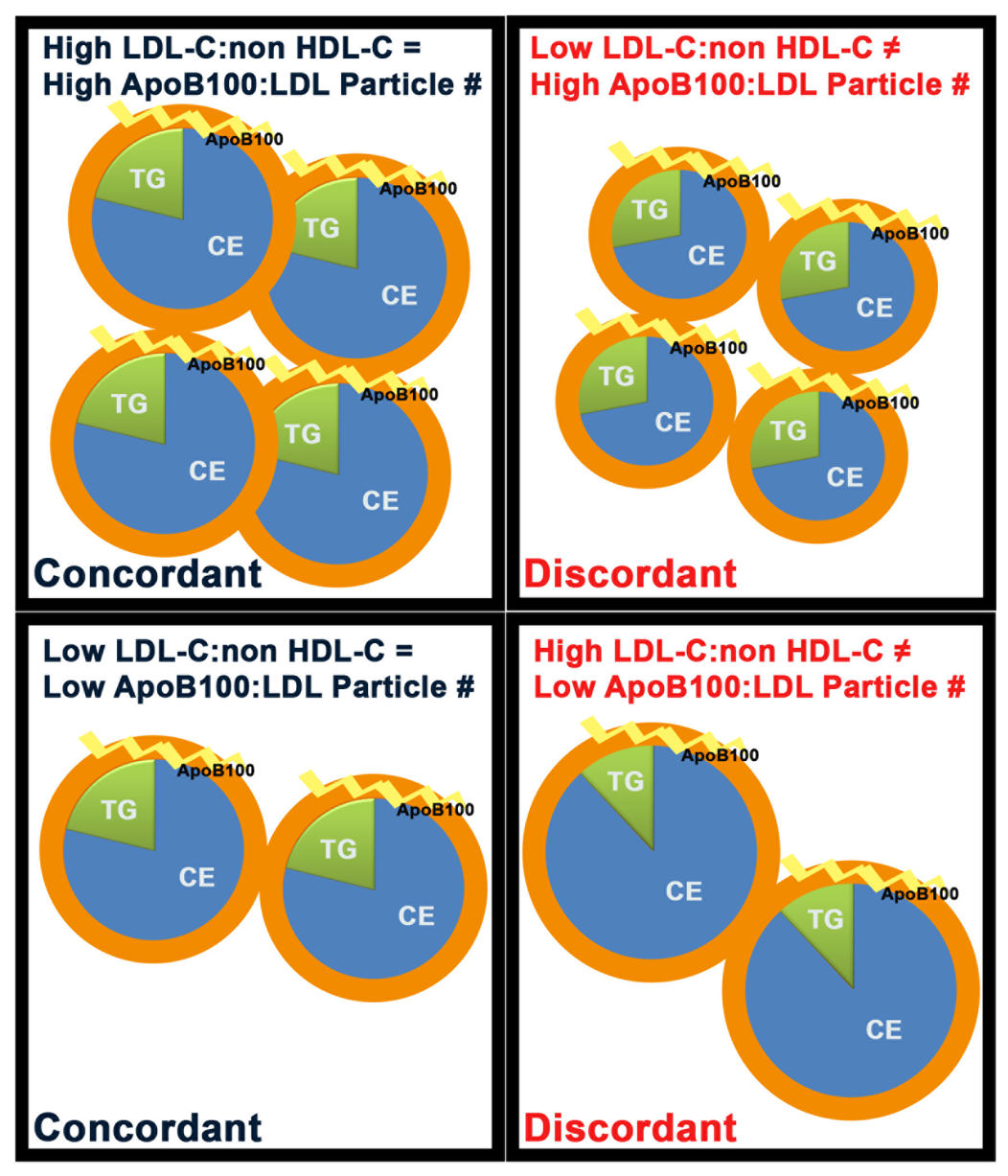
Figure 8. Schematic of the Relationship Between Measurements of LDL-C Versus ApoB100 Particle Number in Concordant and Discordant Human Populations. Subjects with hypertriglyceridemia have enhanced numbers of small dense LDL where each particle is enriched in triglyceride (TG) and relatively poor in cholesteryl ester (CE) content compared to normal subjects, and measurement of LDL-C underestimates particle numbers and apoB100 levels. Other subjects have enlarged CE-enriched LDL particles and measurement of LDL-C overestimates the number of LDL particles and apoB100 molecules. Thus, LDL particle number or apoB100 levels are a more accurate predictor of cardiovascular risk in the setting of discordance between the percentiles for measures of cholesterol carried by LDL (LDL-C or Non-HDL-C) and particle number (apoB100 or LDL-P). Adapted from Sniderman, A.D. et al. Curr Opin Lipidol 2014, 25:461–467.
Furthermore, all of the major atherogenic lipoproteins contain apoB (LDL, triglyceride rich remnants of VLDL, IDL, chylomicron remnants, and Lp(a)). LDL-C is routinely calculated using the Friedewald formula (LDL-C = TC – HDL-C – TG/5), but this formula is not accurate when serum TG levels are > 400 mg/dl. It has long been recognized that LDL-C underestimates risk of ASCVD in the setting of hypertriglyceridemia (467). Non-HDL cholesterol is the mass of cholesterol in all of the apoB-containing particles: Non-HDL-C = TC – HDL-C. The ATPIII guidelines recommended using Non-HDL-C to estimate risk of ASCVD, when TG > 200 mg/dL (469,470). A meta-analysis by Sniderman et al.found that Non-HDL-C was a slightly better marker of ASCVD risk than LDL-C, but apoB was far superior to Non-HDL-C (471). NMR spectroscopy is another way to measure LDL-P concentrations. Table 2 includes selected percentiles for mean levels for the various LDL-related markers from the Framingham Offspring Study (472). In an analysis of the Framingham Offspring Study, LDL-P determined by NMR was more strongly related to incident CVD events than LDL-C levels, and the ability of Non-HDL-C to predict risk was less than LDL-P, but better than LDL-C (473). In addition, they found that low LDL-P numbers were a better index of low CVD risk than low LDL-C (473). In contrast, an earlier meta-analysis from the Emerging Risk Factors Collaboration found LDL-C, Non-HDL and apoB to be equivalent markers of CVE (474). The lack of difference may relate to the population studied. When the LDL particles have normal cholesterol content, then LDL-C, Non-HDL and apoB are equivalent markers (Figure 8) of risk (471,473). Interestingly, data from the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that when LDL-C and LDL-P are discordant (Figure 8), then LDL-P proves to be a better predictor of risk for incident CVD events than LDL-C (475).
| Table 2. Equivalent Percentiles in the Framingham Offspring Study | ||||
| Percentile % | LDL-C mg/dL | Non-HDL-C mg/dL | ApoB mg/dL | LDL-P nmol/L |
| 2 | 70 | 83 | 54 | 720 |
| 20 | 100 | 119 | 78 | 1100 |
| 50 | 130 | 153 | 97 | 1440 |
| 80 | 160 | 187 | 118 | 1820 |
| 95 | 191 | 224 | 140 | 2210 |
| Adapted from Contois JH, et al. Clinical Chemistry 2009; 55:407-419 | ||||
For several decades the guidelines for treatment of hypercholesterolemia have focused on LDL-C levels both for risk stratification and as the principal target of therapy to prevent ASCVD. Indeed, therapeutic goals for LDL-C of < 100 mg/dL and < 70 mg/dL for subjects at high-risk and very high risk of CVE, respectively, were recommended by the 2004 update of the NCEP ATPIII, guidelines (469,470). The 2013 ACC/AHA guidelines for treatment of hypercholesterolemia abandoned these targets in favor of recommending the use of high-intensity statins in high risk individuals (476), there are numerous sets of guidelines that have maintained the recommendation for LDL-C targets, including those of the National Lipid Association (NLA) (477), the American Association for Clinical Endocrinologists (478), and the European Guidelines (479). The Canadian guidelines include targets for levels of apoB(480), and the NLA guidelines include targets for both LDL-C and Non-HDL-C(477). Table 2 includes the percentiles for mean levels for these various LDL markers from the Framingham Offspring Study. The levels of LDL-C shown in Table 2 closely coincide with levels that have been widely used in guidelines for lipid management for decision-making regarding levels at which to initiate therapy and goals of therapy. The recent NLA guidelines recommend using both LDL-C and Non-HDL-C as targets of therapy with only two sets of targets: LDL-C < 70 mg/dL and Non-HDL-C < 100 mg/dL for very high-risk subjects and LDL-C < 100 mg/dL and Non-HDL-C < 130 mg/dL for high, moderate or low risk subjects (who qualify for drug therapy). Most recently, AHA/ACC published a new version of guidelines for cholesterol management (481). These guidelines included evidence from recent 2 large randomized clinical end-point trials of PCSK9 inhibitors (454,455)and a long-awaited ezetimibe trial in patients with recent acute coronary syndromes (482). The new guidelines re-introduced LDL-C treatment goals in some high-risk patient groups, such as those with high risk of ASCVD and those with very high baseline LDL-C. The new AHA/ACC guidelines also stated that elevated apoB particle number, elevated Lp(a) and hypertriglyceridemia are all additional risk factors for ASCVD.
Lp(a)
Lp(a) has been shown to be an independent risk factor for atherothrombotic events, including heart attack, stroke and peripheral vascular disease, in multiple prospective studies (451,483). The new AHA/ACC guidelines list elevated Lp(a) a one of the risk-enhancing factors for developing ASCVD (481). Lp(a) consists of an LDL particle in which apoB100 is covalently linked via a disulfide bridge to apo(a), a glycoprotein with repeating Kringle units that share homology with plasminogen. Although apo(a) is synthesized by the liver, the Lp(a) particles are not formed in the liver but in the plasma. Despite being a modified LDL particle, Lp(a) levels are independent of LDL-C levels. The catabolism of Lp(a) is poorly understood, but Lp(a) is not cleared by the LDLR (484). The number of repeating Kringle units is highly variable but largely genetically determined, and this contributes to tremendous heterogeneity in size of Lp(a). The plasma levels of Lp(a) vary tremendously in humans, and plasma Lp(a) levels are generally inversely related to the size of the apo(a) isoform (485). Thus, smaller Lp(a) particles with fewer Kringle repeats are present at higher levels in the plasma. In American Caucasians, the increased levels of smaller Lp(a) particles is largely explained by the size of the LPAgene, based on the size of the repeated KIV2domain (486), which is believed to be due to difficulty of hepatic secretion of larger apo(a) isoforms. Nevertheless, this relationship varies in different ethnic populations. Early studies suggested that even though Lp(a) levels are higher in African Americans that Lp(a) levels did not appear to be an independent risk factor for cardiovascular events in this group(487). However, by determining allele specific Lp(a) concentrations, a larger more recent analysis demonstrated that elevated Lp(a) levels associated with small apo(a) isoform sizes serve as an independent risk factor for CHD in both African Americans and Caucasians (488). Similarly, a 20 year follow up study of the ARIC cohort found that elevated levels of Lp(a) are associated with a similar degree of risk in in both African Americans and Caucasians (489). A recent meta-analysis by the Emerging Risk Factors Collaboration evaluated 36 prospective studies with 126,634 subjects found that Lp(a) is an independent risk factor for CHD (490). In contrast to previous studies that suggested Lp(a) was only relevant as a risk factor when levels were extremely elevated, the meta-analysis demonstrated that risk and that Lp(a) levels are continuously associated with CHD risk (490). The Ile4399Met polymorphism (rs3798220) in the protease-like domain of apo(a) is particularly associated with increased risk for severe CAD (491). Subsequently, Clarke et al.found that the rs3798220 and rs10455872 variants were associated with small apo(a) isoform size, increased Lp(a) levels and substantially increased risk of CAD (492). Furthermore, a Mendelian randomization study by Kamstrup et al.demonstrated that a genetically determined doubling of Lp(a) plasma levels leads to a 22% increase in the risk of MI, strongly supporting a causal role for elevated levels of Lp(a) and risk for MI (493).
The proatherogenic mechanisms for Lp(a) remain incompletely understood, but recent studies suggest an important role for oxidative modification of Lp(a) by oxidized phospholipids (OxPL) (251). Mounting evidence supports an important role for OxPLs in the development of atherosclerosis (251). Interestingly, OxPLs associate with Lp(a) in preference to native LDL particles in human plasma (250). Hence a physiological role has been proposed for Lp(a) for binding and transporting OxPL in the plasma (251). Although, Lp(a) is found only in humans and Old-World monkeys, mice expressing human Lp(a) have been developed to examine the role of Lp(a) in atherogenesis and lipoprotein metabolism. The first transgenic mice expressing high levels of human apoB100 were created using a 79.5-kb human genomic DNA fragment containing the entire human APOBgene that was isolated from a P1 bacteriophage library, and crossing these mice with apo(a) transgenic mice produced high levels of human Lp(a) in plasma (494). In a study of transgenic mice expressing high and low concentrations of Lp(a), high levels of OxPLs were found in transgenic mice with very high levels of Lp(a), but not in LDL of apoB transgenic control mice (495). These studies support the concept of preferential transfer of OxPL to Lp(a). In the Dallas Heart Study, levels of OxPL on apoB were strongly correlated with Lp(a) levels, and inversely related to the size of the apo(a) isoforms (496). In the European Prospective Investigation of Cancer (EPIC)–Norfolk prospective study the impact of OxPL and Lp(a) levels on CHD risk was additive (497). Further studies are needed to define the extent to which the preferential binding of OxPL by Lp(a) is responsible for mediating the increased risk of atherothrombotic events attributable to Lp(a).
Lp(a) is considered an emerging risk factor, but the approach to managing patients with elevated levels of Lp(a) has not been well established. Elevated levels of Lp(a) do not respond well to changes in diet or statin therapy. Analysis of data from the Familial Atherosclerosis Treatment Study (FATS) showed that substantial lowering of LDL-C (with lovastatin plus colestipol or niacin plus colestipol) in subjects with CAD and high apoB100 eliminated the increased risk attributable to having very high Lp(a) (498). The JUPITER trial showed that treatment of subjects with low levels of LDL-C, but increased hsCRP, with rosuvastatin (20 mg) reduced CVE. In JUPITER, elevated Lp(a) was a significant determinant of residual risk, but the reduction in relative risk with rosuvastatin was similar among participants with high or low Lp(a) (499,500). Treatment with niacin reduces Lp(a) by 20-30%, and the European guidelines recommend treating patients with elevated Lp(a) who are at intermediate to high risk of CVD with extended release niacin to obtain levels of Lp(a) < 50 mg/dL (501). Nonetheless, the recent failure of the AIM-HIGH and HPS-2 THRIVE studies have cast doubt on the use of extended release niacin in subjects fitting the profile of those studies (CAD with LDL well treated on a statin). LDL apheresis is approved and effective for lowering Lp(a) in individuals with recurring CVE in the setting of very high levels of Lp(a). There are a number of new therapies that may prove useful in treating patients with elevated levels of Lp(a). The recently approved monoclonal antibodies to PCSK9 significantly lower Lp(a) by around 30% in addition to lowering LDL-C by 30-50%. Furthermore, a Phase 1 clinical trial of a second-generation antisense to apo(a) has recently reported potent, dose-dependent, selective reductions of plasma Lp(a) (502). This approach has the appeal of specifically targeting apo(a) to reduce Lp(a) levels. Hopefully, these new approaches will ultimately yield an effective approach to lower levels of Lp(a) that translates into reduced cardiovascular events.
INTESTINAL LIPID METABOLISM AND CHYLOMICRON ASSEMBLY
Intestinal Lipid Absorption
Through absorption of dietary lipids, the intestine is a key regulator of stored and circulating lipids. Primarily it is enterocytes in the small intestine that actively regulate the release of dietary lipids into circulation (503-505). The predominant lipids derived from diet are triglycerides, phospholipids and cholesteryl esters. In the intestinal lumen, ingested lipids are emulsified by bile salts to enhance their hydrolysis by lipases (Figure 9) (506-509). Triglycerides make up the largest percentage of the intestinal lipids. Lipolysis of triglycerides releases free fatty acids (non-esterified fatty acids) and monoacylglycerides (Figure 9). These are absorbed on the luminal surface of the enterocytes both by free diffusion and actively by protein-mediated transport into the enterocyte cytosol (Figure 9) (508-510). The principal transporters identified to date are CD36 (now known as SR-B2 (511)) and several fatty acid binding and transport proteins (512-514).
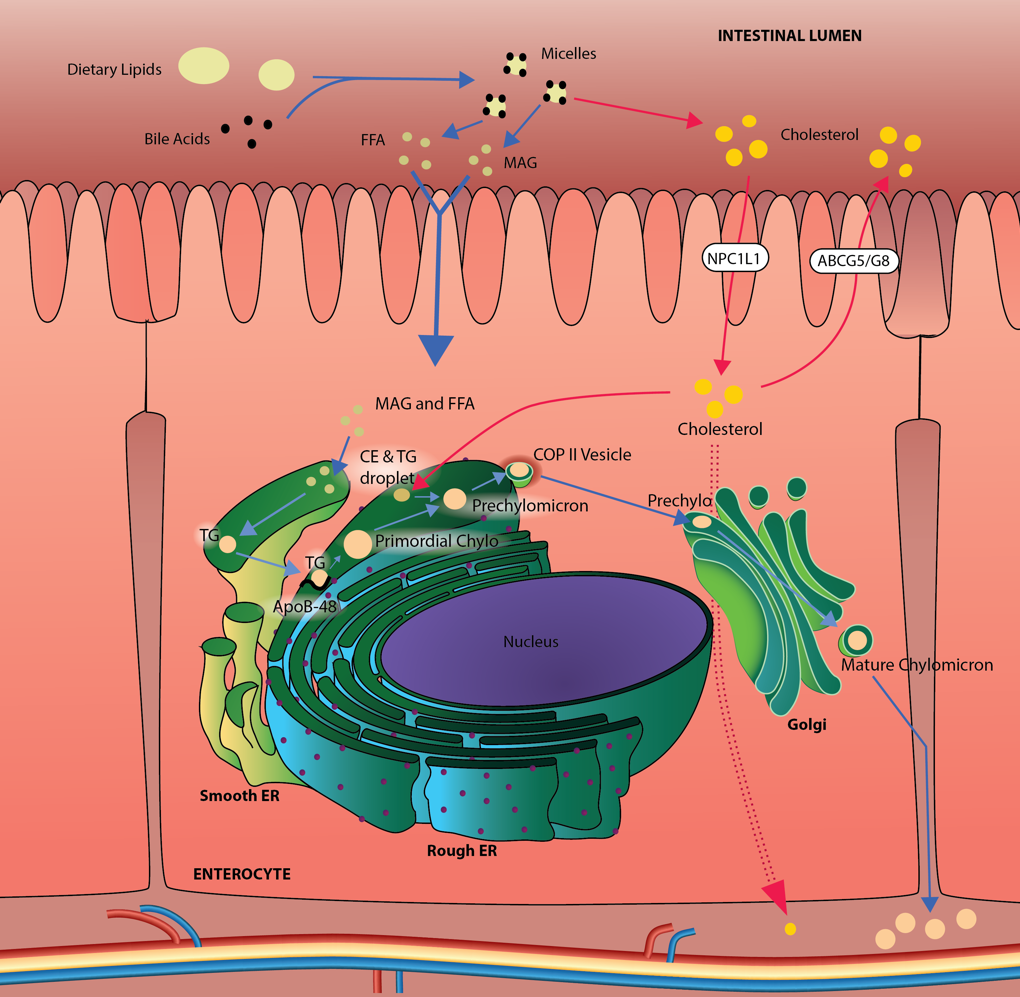
Figure 9. Intestinal Triglyceride and Cholesterol Metabolism. In the intestinal lumen, dietary triglyceride (TG) and cholesterol are emulsified by bile salts which enhance their uptake. Lipases in the intestinal lumen digest triglycerides to free fatty acids (FFA) and monoacylglycerides (MAG). These are absorbed into the enterocyte where they are used in the synthesis of TG, phospholipid and cholesteryl ester (CE). Much of the synthesized TG in enterocytes is packaged, along with phospholipids, cholesterol and proteins into chylomicrons, which are secreted at the basolateral surface of the enterocyte and enter the lymphatic system. The assembly of chylomicrons begins in the endoplasmic reticulum. During the synthesis of apolipoprotein B48 (apoB48), the protein acquires phospholipid from the endoplasmic reticulum membrane and also cholesterol and TG to form a primordial chylomicron. Continued acquisition of TG and CE and smaller, exchangeable proteins (e.g. apolipoprotein A-IV and apolipoprotein C-III) in the endoplasmic reticulum enlarges the particle to form a prechylomicron. Prechylomcirons are transported to the Golgi apparatus in specialized COPII vesicles. In the Golgi apparatus, the prechylomicron matures into a chylomicron. The maturation process includes the glycosylation of apoB48, the acquisition of additional proteins (e.g. apolipoprotein A-I) and lipid. Secretory vesicles formed from the Golgi carry the mature chylomicrons to the basolateral surface of the enterocyte. Fusion of the secretory vesicle membrane with the plasma membrane releases the chylomicron into the extracellular space where it is taken up into lacteals near the enterocyte and, thus, enters the lymphatic circulation. Dietary cholesterol in the intestinal lumen is taken into the enterocyte by a process involving Niemann-Pick C1-like protein 1 (NPC1L1). Enterocyte cholesterol and CE can be incorporated into chylomicrons and secreted with TG. In addition, enterocyte cholesterol can be directly excreted into the intestinal lumen using the heterodimer ATP-binding cassette transporter G5 and G8 (ABCG5/G8). Enterocyte cholesterol can also be transported to and incorporated into the basolateral membrane for efflux into the circulation.
Chylomicron Assembly and Secretion
In the enterocyte, the free fatty acids and monoacylglycerides are used to synthesize triglycerides, phospholipids, and cholesteryl esters (Figure 9) (508,509,513,515-517). The majority of the triglycerides formed in the enterocytes are repackaged into large, buoyant lipoproteins, called chylomicrons, and secreted from the basolateral surface of the cell (Figure 9). These particles play a central role in the transport of triglycerides and fat-soluble vitamins to the rest of the body (518).
The assembly of the chylomicron particle from precursors is a complex process. Each particle contains a single copy of apolipoprotein B48 and assembly begins with the synthesis of this protein in the rough endoplasmic reticulum. Apolipoprotein B48 is a truncated form of apolipoprotein B100 that is formed by posttranscriptional editing (519,520). As apolipoprotein B48 is synthesized and translocated across the endoplasmic reticulum membrane, it becomes lipidated to form a phospholipid-rich, dense primordial chylomicron in the lumen of the endoplasmic reticulum (Figure 9). The primordial chylomicron contains apolipoprotein B48, phospholipid, cholesterol and minor amounts of cholesteryl ester and triglyceride (513,521,522). The assembly process requires microsomal triglyceride transfer protein(523). In the absence of sufficient lipid, or if microsomal triglyceride transfer protein function is impaired, apolipoprotein B48 is ubiquitinated and targeted for proteasome degradation (524). The importance of this initiating assembly step is seen in patients with a defect in the MTP gene leading to the rare recessive disorder abetalipoproteinemia. Individuals with abetalipoproteinemia have almost undetectable levels of apoB or and very low total cholesterol levels in their plasma because of the inability to assemble apoB-containing lipoproteins in their enterocytes or hepatocytes. Among the sequelae experienced by these patients are accumulation of triglycerides in their intestines and livers and a deficiency of lipid-soluble vitamins in their plasma (525,526). If untreated, these patients develop severe neurological problems; mostly related to vitamin E and A deficiency.
After formation, the initial primordial particle expands by the acquisition of additional triglyceride and cholesteryl ester (Figure 9). The additional lipid is acquired by fusion with non-apolipoprotein B48 containing particles that are rich in triglyceride and cholesteryl ester. The exact origin of these lipid particles and their precise composition is currently actively debated (504,505,513,527,528), but the fusion of the primordial chylomicron with the apolipoprotein B48-free particles occurs in the endoplasmic reticulum (513). The resulting particle is a prechylomicron (Figure 9). In addition to apolipoprotein B48, the prechylomicron surface can contain multiple copies of other small, exchangeable apoproteins including apolipoprotein A-IV and apolipoprotein C-III. Exchangeable apoproteins are soluble proteins that are not as tightly adherent to the particle surface and so can be exchanged between lipid particles.
Prechylomicrons are transported out of the endoplasmic reticulum and delivered to the Golgi apparatus for further processing (Figure 9). Transport occurs in specialized vesicles that can accommodate their large size. The unique vesicles contain a number of specific proteins necessary for the transport and docking process. Vesicle-associated membrane protein-7, coatomer protein II and Sar1b, a small GTPase component of the coatomer protein II vesicle assembly machinery (Figure 9) are among the specialized proteins on the lipid transport vesicles (505,529-531). The maturation of the particle in the Golgi apparatus includes further glycosylation of apolipoprotein B48 and the addition of apolipoprotein A-I to the surface (505,532,533). After processing, the mature chylomicron is packaged into Golgi-derived secretory vesicles and transported to the basolateral surface and exocytosed into the lymph (Figure 9) (527,534,535).
The assembly of chylomicrons in enterocytes is a complex process requiring a number of coordinated steps and specific factors to work in unison. A failure in any of these can lead to lipid-related disease states. For instance, mutations in the SAR1B gene lead to retention of prechylomicrons within membrane-bound structures in the enterocytes (529). The condition is marked in childhood by decreased blood cholesterol levels, lipid accumulation in the enterocytes, chronic fat malabsorption with steatorrhea, and deficiencies in fat-soluble vitamin and essential fatty acids.
Chylomicron Cholesterol
Although chylomicrons are triglyceride-rich, they also carry substantial amounts of cholesterol (536,537). The cholesterol in chylomicrons comes from the general pool of enterocyte cholesterol. Enterocytes acquire cholesterol by uptake at the luminal surface, acquisition from lipoproteins at the basal lateral surface, and by de novo synthesis within the enterocyte. Niemann-Pick C1-Like 1 protein is a key component of the luminal acquisition machinery (Figure 9) (538), while the low density lipoprotein receptor appears to be a major mediator of cholesterol acquisition at the basolateral surface (539,540). The incorporation of cholesterol into chylomicrons contributes to the circulating levels of cholesterol, and increases in intestinal synthesis of chylomicrons due to increased dietary lipids contributes to cardiovascular risk and atherosclerosis, albeit by complex mechanisms (516,541,542).
Non-Chylomicron Intestinal Lipid Metabolism
Enterocytes can also regulate circulating lipids by means other than chylomicron secretion. In the presence of excess fatty acids or cholesterol, the enterocyte can store excess lipid in their esterified forms (triglycerides and cholesteryl esters, respectively) within cytoplasmic lipid droplets (543-545). The neutral lipids in the droplets can subsequently be mobilized by hydrolysis as needed by the cell. The free fatty acids liberated from storage droplets can be incorporated into the chylomicron production pathway to become part of secreted chylomicrons.
Finally, the intestine also regulates circulating cholesterol levels by taking up excess circulating cholesterol and excreting it into the intestinal lumen for clearance in the feces. This process is known as trans-intestinal cholesterol excretion. It acts as an adjunct to liver biliary secretion and can account for as much as 30% of neutral sterol excretion (546). Trans-intestinal cholesterol excretion occurs at the luminal surface of the enterocytes by a process that primarily utilizes the ATP-binding cassette transporter pair ABCG5/G8 (Figure 9) but can use other pathways as well (547).
Summary
It is clear that intestinal lipid processing is a key contributor to the circulating levels of both triglyceride and cholesterol. Dietary, genetic and metabolic factors that disrupt the process of enterocyte lipid metabolism potentially can alter lipid homeostasis and produce disease states.
TRIGLYCERIDES, CARDIOVASCULAR DISEASE AND ATHEROSCLEROSIS
Causes of Hypertriglyceridemia
The prevalence of high circulating triglyceride levels is increasing worldwide, particularly in developed countries. In the United States there has been a greater than 7 fold increase in average plasma triglyceride concentration over the last 30 years (548). This increase coincides, in part, with increased instances of obesity and type 2 diabetes (T2DM) although the relationship of these conditions to hypertriglyceridemia is complex (549-553). Most classifications of hypertriglyceridemia are based, at least in part, on the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (469). These guidelines classified circulating triglyceride levels <150 mg/dL as normal. Values between 150 mg/dL and 199 mg/dL are considered borderline high and anything above 200 mg/dL are classified as high, with those above 500 mg/dL deemed to be very high (469,470). Hypertriglyceridemia is generally the result of increases in one or more of the triglyceride-rich lipoproteins; chylomicrons, VLDL, or their remnants. The increase occurs because of increased synthesis, decreased catabolism or both, with the underlying cause generally being the result of alterations in metabolic factors such as apolipoprotein C-II, apolipoprotein C-III, CETP and lipoprotein lipase. However, hypertriglyceridemia can also be secondary to other disease states (e.g. diabetes mellitus, hypothyroidism, renal disease, and nephrotic syndrome) (548,554). Not surprisingly, environmental conditions, particularly a diet with high fat or high glycemic index content and in which energy intake is out of balance with energy utilization, are associated with hypertriglyceridemia as is excess alcohol consumption (548,554). In fact, dietary choices and lack of exercise are widely held to be a major contributor to the recent rise in circulating triglyceride levels in developed countries.
Hypertriglyceridemia as an Independent Risk Factor for Cardiovascular Disease
Individuals with elevated triglyceride levels are at increased risk for cardiovascular complications, particularly atherosclerosis (555,556). The Framingham Study was one of the first large studies to associate hypertriglyceridemia with cardiovascular disease, particularly in women (557). However, many other studies before and since have also shown a univariate association of high triglycerides and increased risk of cardiovascular disease. In many of these studies, however, the affect went away after accounting for other major risk factors (558-561),calling into question whether triglycerides represent an independent risk factor. For instance, a meta-analysis of the Emerging Risk Factors Collaboration data revealed triglycerides as a strong risk factor for cardiovascular disease and stroke, but, after adjusting for standard risk factors (primarily lipoprotein-associated cholesterol), the researchers concluded that triglyceride levels provided no additional predictive value (474). The authors did note, as have other studies (562-565), that patients with triglyceride levels above 500 mg/dL are at increased risk of pancreatitis; providing impetus for measuring triglyceride levels in patients and treating those with high levels irrespective of cardiovascular risk. The lack of strong association of triglyceride concentration with cardiovascular disease (after accounting for other risk factors such as elevated LDL-C and low HDL-C) has led some to question whether measuring triglyceride levels has any utility for cardiovascular patient management. In contrast, we argue below that there are a number of important reasons for evaluating triglyceride levels in patients, particularly those with cardiovascular disease, metabolic syndrome or diabetes.
First, we would point out that the difficulty in substantiating an independent association of triglycerides with cardiovascular disease may simply reflect the fact that a number of interrelated risk factors make it difficult to determine to what extent triglycerides independently contribute to cardiovascular events. One key issue is that, even in studies suggesting independence, the effect size has been small compared to traditional risk factors like LDL-C (548). Therefore, independence is very hard to detect in small studies. There are also issues regarding the way triglyceride levels are determined, the high variability of triglyceride concentrations in a single individual, and the association of triglyceride levels with other atherogenic conditions such as low HDL-C, obesity and T2DM (548,566-571). These confounding issues are not always considered by authors when drawing conclusions. Moreover, endpoints have differed widely among studies. Despite the confounding issues, an increasing number of case control studies do indicate triglycerides as an independent risk factor for cardiovascular disease even when adjusting for total cholesterol, LDL-C and HDL-C (572-578). The PROCAM study, for instance, found increases in risk for cardiovascular events as triglyceride levels increased and residual risk remained after accounting for other major risk factors (579), and the PROVE IT-TIMI 22 study revealed that triglyceride levels had a substantial impact on cardiovascular outcomes in patients with acute coronary syndrome that was independent of LDL-C (580). Moreover, Mendelian randomization studies strongly suggest a causal relationship between factors involved in regulating triglyceride rich lipoprotein levels and cardiovascular disease (581-583). For instance, analysis of data from the Copenhagen City Heart Study showed that genetic variants of lipoprotein lipase that resulted in reduced circulating triglyceride levels also reduced all-cause mortality (583).
Meta-analyses of randomized, prospective trials probably provide the strongest evidence for triglyceride levels as an independent risk factor. One such analysis assessing the effects of lowering circulating cholesterol levels with statins, indicated that in patients with preexisting coronary heart disease, there was a reduction in residual risk not associated with lowering LDL-C that could be related to other lipoproteins, such as triglyceride-rich lipoproteins (584). Most convincingly, a recent meta-analysis of 29 prospective studies showed that considering triglyceride concentrations yielded an adjusted odds ratio of 1.72 (95% Confidence interval=1.56-1.90) for those in the top tertile of triglyceride levels even after adjusting for other common risk factors (556). A similar odds ratio was reported in a meta-analysis that included data from 26 prospective studies in Asian and Pacific populations (585).
Given the increasing evidence that hypertriglyceridemia is indicative of increased cardiovascular disease risk, a key question is whether reducing triglyceride levels are protective. The results of several studies do suggest that reducing TG levels can reduce risk of cardiovascular events. An analysis of two secondary prevention trials of pravastatin suggests that high HDL-C and low triglycerides were significant predictors of reduced risk for CHD events (586). A recent meta-analysis of 18 trials evaluating the effects of fibrates on cardiovascular outcomes reported a 10% relative risk reduction for major cardiovascular events in individuals with hypertriglyceridemia alone or in combination with low HDL-C (587). Other meta-analyses have generally shown small but significant associations of low triglycerides and protection from cardiovascular events independent of other major risk factors (588).
Thus, the evidence is mounting for an independent role of circulating triglyceride levels in mediating cardiovascular risk and certainly has established the utility of determining triglyceride levels in at-risk patients. However, the studies also suggest that the association between high triglycerides and cardiovascular disease is complicated, multidimensional, and possibly indirect.
Is There a Direct Role for Triglycerides in Promoting Cardiovascular Disease?
If hypertriglyceridemia does directly affect cardiovascular disease, the mechanism(s) remain to be fully elucidated. Nonetheless, several hypotheses have been put forward. As the most prevalent form of cardiovascular disease, atherosclerosis has been the target for most explorations of a direct role for triglycerides in cardiovascular disease, and there is growing evidence, albeit circumstantial, that triglycerides can directly influence specific aspects of atherosclerotic lesion development. Many of the hypotheses are based on the fact that triglyceride rich lipoproteins (VLDL, chylomicron) also contain significant amounts of cholesterol (536)and could promote foam cell formation by contributing cholesterol to the lesion. Remnants of VLDL and chylomicrons are created by partial hydrolysis of their triglycerides through the action of lipoprotein lipase. These particles have an increased percentage of cholesterol(537,589)and can acquire additional cholesterol by transfer from HDL through the action of cholesterol ester transfer protein(CETP) (590). In hypertriglyceridemia, there is increased VLDL synthesis, delayed clearance and often increases in remnant particles (591,592). In fact, it has been argued that nonfasting triglyceride levels primarily reflect remnant lipoproteins, particularly in hypertriglyceridemia, and these particles may be the atherogenic moiety (593). Although chylomicrons and, to some extent, very low density lipoproteins are generally too large to cross the endothelial layer and invade the arterial intima, conversion to remnants allows these particles to accumulate within atherosclerotic lesions and to deposit their cholesterol (594-596). This would imply that levels of lipoprotein lipase, by increasing remnants, could influence atherosclerotic lesion development and there are animal studies showing just such a correlation (237,238,597). Evidence for the importance of remnants in atherogenesis also comes from individuals with type III hyperlipoproteinemia. Patients with type III hyperlipoproteinemia have decreased clearance of remnant lipoproteins and develop premature atherosclerosis (598). ApoE is crucial for the normal clearance of chylomicrons and VLDL remnants, but the ApoE-2 isoform has reduced ability to bind to lipoprotein receptors and mediate clearance (599). Type III hyperlipoproteinemia occurs most often in subjects who are homozygous for APOE2, but the majority of E2/E2 individuals do not have the Type III phenotype, suggesting that a second hit is required to express the phenotype (600). Interestingly, rare genetic variants of APOE have been described that cause an autosomal dominant form of Type III hyperlipoproteinemia (601,602)and ApoE deficiency in humans is extremely rare but is associated with the Type III phenotype (600,603).
One mitigating factor in evaluating how much delivery of cholesterol in triglyceride-rich particles contributes to atherosclerosis is the fact that, although triglyceride-rich particles and their remnants contain large amounts of cholesterol, they also contain significant amounts of triglyceride. At least with respect to cellular cholesterol accumulation in macrophage foam cells (a hallmark of atherosclerosis), the presence of triglyceride in cells actually promotes the hydrolysis of cholesteryl esters to cholesterol (604,605). Cholesterol stored in foam cells is primarily in the form of cholesteryl esters. In order to be removed from the cell and eventually from the plaque, esterified cholesterol must first be converted to unesterified cholesterol (606). The presence of triglyceride intermixed with cholesteryl esters in foam cells facilitates the hydrolysis and removal of cholesterol (604,605,607). The differing effects of circulating triglyceride levels on cardiovascular disease risk and their cellular effects on cholesterol metabolism have yet to be reconciled.
There are mechanisms other than cholesterol delivery by which triglycerides could influence atherosclerosis. Lipolysis of triglyceride rich particles not only concentrates cholesterol in the particles it also produces free fatty acids and monoglycerides. Cell culture studies have demonstrated that long-chain fatty acids, particularly saturated fatty acids like palmitate and stearate, are cytotoxic (608-610). Thus, the presence of triglyceride lipolysis within atherosclerotic lesions could raise toxic free fatty acid levels in cells of the arterial wall, which would promote cell death and resulting inflammation. Both increased cell death and increased inflammatory signaling are key attributes of atherogenesis (611-614). In support of triglyceride lipolysis as an atherogenic driver, macrophages make and secrete lipoprotein lipase (lipoprotein lipase) and it is estimated that macrophages are the primary source of lipoprotein lipase in atherosclerotic plaques (615). Localized lipolysis of triglyceride-rich lipoproteins and their remnants can also liberate other oxidized fatty acids, which can promote cytotoxicity and inflammation (616-619); key players in atherosclerotic lesion development. Increases in macrophage lipoprotein lipase do stimulate macrophage cytotoxicity (620), while diminution of macrophage lipoprotein lipase in mice reduces atherosclerotic plaque size (237,621,622). Thus, localized hydrolysis of triglyceride-rich particles by macrophages have the potential to produce cytotoxic and inflammatory effects.
It is also becoming clear that the dietary fatty acid composition of lipoproteins, including triglyceride-rich lipoproteins, affects their metabolism in complex and not completely understood ways. The fatty acid composition of lipoproteins (as well as phospholipids and cholesteryl esters) is strongly influenced by dietary intake of fatty acids. Although dietary intake of saturated fatty acids is popularly believed to be bad, whether consuming saturated fat, per se, increases cardiovascular risk is somewhat controversial based on available evidence (623,624). However, in subjects with FH, increased saturated fat in the diet clearly increases LDL-C levels. What also appears clear is that replacing saturated fatty acids in the diet with polyunsaturated fatty acids (PUFA) reduces cardiovascular events (623-627). Omega-6 PUFA are the primary PUFA found in western diets. There is evidence these lower triglyceride levels, in part, by increasing lipolysis of triglyceride-rich lipoproteins (628). Omega-3 PUFA are the other major source of dietary PUFA. Fish are a rich source of long-chain omega-3 PUFA, and there is compelling evidence that omega-3 PUFA (at least from marine sources) reduce both triglyceride levels and cardiovascular risk (629-631). A recent large scale randomized controlled trial (REDUCE-IT) using an EPA only fish oil product reduced major cardiovascular event by 25% in patients who have hypertriglyceridemia (632). Replacing saturated fat with monounsaturated fatty acids may provide some reduction in cardiovascular events, but PUFA appear to have a stronger correlation with improved cardiovascular risk compared to monounsaturated fatty acids (633-635). In contrast to cis fatty acids, trans unsaturated fatty acids, which are common in processed foods, have been convincingly associated with increased cardiovascular risk (623,636,637). Given this and other evidence, a recent report from the National Lipid Association’s Expert Panel recommends, for patients with low or moderate risk for cardiovascular disease, that intake of saturated fatty acids be reduced to <7% of total energy and trans fatty acids should be avoided (638). The reduction in saturated and trans fats should be replaced with PUFA, protein and carbohydrate (638). The guidelines also suggest eating fish twice weekly. For individuals with high triglyceride levels, the Expert Panel also recommends supplementation with omega-3 polyunsaturated fatty acids from marine sources (638). The 2018 AHA/ACC listed persistent hypertriglyceridemia as a risk enhancer for developing ASCVD and recommend using omega-3 fish oil for individuals with high triglyceride levels to prevent pancreatitis. However, the AHA/ACC guidelines did not include the evidence of the REDUCE-IT trial. Therefore, in these individuals with hypertriglyceridemia and other risk factors for ASCVD, one should consider initiating omega-3 fish oil or intensifying statin therapy(481).
Lipoprotein lipase-mediated hydrolysis of triglyceride is not the only mechanism in the artery wall for the metabolism of triglyceride rich particles to produce potentially atherogenic compounds. The foam cell macrophages are also capable of the endocytic uptake of VLDL and remnant particles, which can then be catabolized in the lysosome (Figure 2) (639-642). Interestingly, there is evidence that under atherogenic influences, including macrophage sterol engorgement, the route of triglyceride metabolism in macrophages can shift to favor endocytic delivery of triglyceride-rich lipoproteins rather than surface hydrolysis (641,643). Whereas surface hydrolysis of triglycerides by surface lipases primarily delivers only free fatty acids to cells, endocytic uptake of particles would include the delivery of the particle’s full content, including its sterol, which would exacerbate foam cell sterol accumulation.
Another potential way that triglyceride-rich lipoproteins could influence atherosclerosis focuses on the apolipoprotein CIII content of VLDL and remnants. ApoCIII inhibits lipoprotein lipase, inhibits remnant uptake by the liver, and its levels are associated with hypertriglyceridemia (644-648). Thus, high apolipoprotein CIII concentrations could promote arterial retention of VLDL and remnants making them more atherogenic, suggesting apolipoprotein CIII as a therapeutic target. In fact, individuals with certain mutations in APOC3 have low triglycerides and LDL-C (649,650). Two recent studies show that loss-of-function mutations in apoCIII lowered serum triglycerides by >39%, significantly reduced LDL-C and raised HDL-C, and lowered the incidence of cardiovascular events by >36% (651,652). An antisense oligonucleotide selective inhibitor of apoCIII has been developed that lowers serum apoCIII and triglycerides in mice, non-human primates, and humans and is currently in a phase 2 clinical trial (653). These studies indicate that reduction of apoCIII by antisense oligonucleotide inhibition significantly reduces circulating triglyceride levels (654,655). Besides their effects on circulating lipids, Apo CIII-containing lipoproteins also stimulate a range of processes including activation of monocytes, inflammation, endothelial cell NO production resulting in vascular dysfunction and increased lipid oxidation and binding of lipoproteins to PG which can stimulate macrophage foam cell formation (227,239,656-658).
A final way in which triglyceride levels could influence atherogenesis is related to the finding that patients with hypertriglyceridemia also tend to have increased circulating levels of thrombotic factors such as fibrinogen and plasminogen activator inhibitor and inflammatory mediators (TNF-alpha, IL-6, VCAM-1 and MCP-1) (659-661). Thrombosis and inflammation are key factors in atherosclerosis and its progression to heart attack and stroke.
Reducing Circulating Triglyceride Levels
It is clear, therefore, that there are a variety of ways in which the triglyceride-containing particles in hypertriglyceridemic plasma could contribute either directly or indirectly to multiple aspects of atherosclerotic lesion development. Regardless of whether triglycerides are directly causative of cardiovascular disease, the evidence is mounting that assessment of triglyceride levels has an important role in evaluating and managing cardiovascular risk, and treating elevated triglyceride levels may reduce risk for cardiovascular events (548,662). This is particularly true for patients with coronary heart disease or diabetes (548,662-664). Several agents have shown efficacy in reducing triglyceride levels and also in reducing cardiovascular disease risk. The reduced risk is thought to occur to a large extent by reducing atherosclerosis. Currently, therapeutic agents recommended for treating hypertriglyceridemia are fibrates, statins, niacin and omega-3 PUFA but others are being developed. Unfortunately, clinical trials of the impact of triglyceride lowering medications on cardiovascular events in subjects with severe hypertriglyceridemia have not been undertaken.
Fibrates are the most effective approach for directly lowering triglyceride levels. Fibrates have been shown to lower triglyceride levels by 30%-50% depending on the baseline levels (548). More importantly, fibrate therapy with gemfibrozil has been shown to reduce cardiovascular risk in patients with elevated triglycerides (665-667). Unfortunately, the trials of combination therapy of statins with fenofibrate have failed to meet their primary endpoints in terms of reducing cardiovascular events (668,669). However, posthoc analysis of all of the fibrate trials show significant benefits in terms of reducing CVD events, when looking at the subgroup of patients with elevated triglycerides and low HDL-C and features of the metabolic syndrome or diabetes (670,671).
Statins inhibit HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis, and produce dramatic reductions in LDL-C levels; typically from 20%-60% depending upon the particular statin used and dosage (672). However, they also reduce circulating levels of triglycerides (673)and Non-HDL-C (477). Levels of LDL-C are often low in the setting of hypertriglyceridemia, so Non-HDL-C levels are a more useful measure of the burden of atherogenic apoB-containing lipoproteins than LDL-C in patients with hypertriglyceridemia. Indeed, the National Lipid Association recommends using goals for Non-HDL-C levels of < 130 mg/dl or < 100 mg/dl, in subjects at high- and very-high risk of cardiovascular events, respectively (477). Niacin is a B vitamin that can lower overall circulating lipid levels when given in high doses. The mechanism of action is not entirely clear but niacin reduces VLDL production by the liver. Unfortunately, clinical trial data regarding the use of niacin on cardiovascular outcomes in severe hypertriglyceridemia is lacking. Although in a subgroup analysis of the AIM-HIGH trial, niacin showed a trend toward benefit in the tertile of subjects with the highest triglycerides (>198mg/dl) and lowest HDL-C (<33mg/dl), which is consistent with the post hoc analysis of fibrate trials (674). Finally, evidence indicates that daily intake of omega-3 PUFA from marine sources (which primarily containing eicosapentaenoic and docosahexaenoic acids) can significantly reduce circulating triglyceride levels (675-678). Treatment with 3 – 4 grams a day of omega-3 PUFA (EPA+DHA) is effective in lowering triglycerides. A large meta-analysis of omega-3 FA in 20 studies including 63,000 participants did not see an impact on a combined cardiovascular endpoint or coronary events, but there was a reduction in vascular death (679). However, most omega-3 outcome trials used less than one gram of omega-3 PUFA, which was probably too low of a dose to have meaningful triglyceride lowering effects that could yield clinical efficacy. The JELIS trial, compared the effect of 1.8 g EPA vs. placebo on top of statin in a hypercholesteremic but relatively normal triglycerides patient population (mean LDL-C 182 mg/dl and triglycerides 151 mg/dl) (95). JELIS found a 19% relative risk reduction in CV events but a more pronounced 53% reduction in the subgroup with mixed dyslipidemia, specifically the subgroup with triglycerides >150mg/dl and HDL<40 mg/dl(680). Most recently, a large randomized controlled trial (REDUCE-IT) of an EPA only fish oil product reduced major cardiovascular event by 25% in patients who have hypertriglyceridemia (632). Whether the clinical benefit was confined to EPA only or it can be generalized to all omega-3 PUFA is still yet to be determined. In contrast to marine-derived omega-3 PUFA, plant-derived omega-3 PUFA have generally not shown efficacy for lowering triglycerides(681,682). A number of new approaches for treating hypertriglyceridemia are in development including antisense oligonucleotides to ApoC3(654,655).
Summary
The association of elevated triglyceride levels and cardiovascular disease has been well established (555,556,579,588). What remains a subject of ongoing debate is the extent to which triglycerides directly promote atherogenesis or, alternatively, simply represent a biomarker for other processes that influence cardiovascular risk. (542,548,554,561,570,592,683,684). Nonetheless, the evidence supports measuring triglycerides and including triglyceride and Non-HDL-C reduction in treatment regimens is strengthening especially in patients with metabolic syndrome, diabetes, or cardiovascular disease.
HDL METABOLISM AND ATHEROSCLEROSIS
HDL and Reverse Cholesterol Transport
Apolipoprotein A-I (apoA-I) is the major protein on HDL and provides both structure and function. Lipid-poor apoA-I and mature HDL both contribute to removing cholesterol from macrophages and prevent foam cell formation (Figure 2). Although cholesterol flux from macrophages to HDL (or apoA-I) alleviates cholesterol-accumulation in lesions, the net flux of cholesterol from the lesion has little to no effect on systemic cholesterol levels. Nevertheless, macrophage cholesterol efflux to HDL reduces inflammation and the atherosclerotic burden, and is the first step in reverse cholesterol transport (RCT) (Figure 10) (685-687). This pathway was first described in 1966 (688). The rate at which cholesterol flows through the RCT pathway is of greater importance than steady state levels of HDL-cholesterol (HDL-C). Interestingly, cholesterol movement from macrophages to HDL occurs through at least 4 routes (70). First, lipid-poor apoA-I stimulates the efflux of phospholipid and free cholesterol through interaction with ATP-binding cassette transporter A1 (ABCA1) (Figure 2), which generates pre-beta HDL and nascent discoidal particles (689). The more lipidated the apoA-1 becomes, the discoidal HDL particles transition into a spherical structure and lose their ability to interact with ABCA1 and stimulate cholesterol efflux through ABCA1. Both discoidal HDL particles and mature spherical HDL particles can also promote free cholesterol efflux from another transporter, ATP-binding cassette transport G1 (ABCG1), which is thought to reside on sub-cellular organelles as opposed to the plasma membrane (Figure 2) (690,691). This transporter is a critical regulator of intracellular cholesterol trafficking cellular cholesterol availability, and cholesterol export (690,692). HDL’s primary receptor for cholesteryl ester (CE) uptake, scavenger receptor BI (SR-BI), is also a bidirectional free cholesterol transporter in that it facilitates the efflux and influx of free cholesterol between cells and mature HDL (693-695)(Figure 2). The net direction of cholesterol flux is determined by the cholesterol concentration gradient (plasma membrane and HDL ratio of free cholesterol to phospholipid) (696)as well as by the phospholipid subspecies (697,698). Finally, cholesterol can simply move from the plasma membrane to HDL through passive aqueous diffusion, which is a major route of cholesterol efflux from macrophages (Figure 2) (70,687,695). On HDL free cholesterol is solubilized in the phospholipid surface layer and is rapidly esterified by lecithin:cholesterol acyltransferase (LCAT) (Figure 6), and the hydrophobic CE is then mobilized to HDL’s core (699,700).

Figure 10. Beneficial Functions of HDL. HDL mediates a number of atheroprotective processes. HDL is critical in reverse cholesterol transport where it mediates the first step of removing cholesterol from the periphery and macrophage foam cells for clearance by the liver. HDL can directly mediate the last step in reverse cholesterol transport by delivering cholesterol to the liver via interaction with SR-BI. HDL reduces LDL oxidation and cell oxidative status by removing lipid hydroperoxides from LDL and cells. HDL also prevents LDL oxidation via its anti-oxidant enzymes (PON1, LCAT, and Lp-PLA2) and by the reduction of lipid hydroperoxides by apoA-I. HDL maintains the endothelial cell barrier by stimulating vasorelaxation resulting from enhanced nitric oxide production from HDL induced signaling via a number of endothelial cell receptors (SR-BI, S1P, ABCG1). HDL prevents thrombus formation by inhibiting coagulation factors and by stimulating efflux of cholesterol from platelets via SR-BI to reduce platelet aggregation. HDL prevents endothelial cell and macrophage apoptosis by signaling pathways which modulate expression of the pro-apoptotic protein, Bid, and the anti-apoptotic factor, Bcl-xl. HDL also reduces apoptosis susceptibility by alleviating endoplasmic reticulum stress by removing excess free cholesterol and lipid hydroperoxides from cells. HDL limits atherosclerotic lesion inflammation by inhibiting endothelial cell activation resulting in less monocyte recruitment. HDL also reduces lesion inflammation by promoting the macrophage anti-inflammatory M2 phenotype via ABCA1/ JAK2 signaling to enhance anti-inflammatory cytokine production (IL-10, TGF-β). HDL inhibits conversion to the macrophage inflammatory M1 phenotype by preventing antigen-specific activation of T helper 1 (Th-1) cell to produce interferon gamma. HDL contains an array of proteins and bioactive lipids that regulate HDL function. In addition, HDL controls a number of atheroprotective processes by modulating gene expression by transferring microRNAs to recipient cells.
Spherical mature HDL then transports CE to peripheral cells and tissues, and back to the liver as part of the RCT pathway (Figure 10). HDL delivers CE to the liver through 2 primary routes. HDL delivers CE to the liver through binding to SR-BI (Figure 6), which drives selective uptake of core lipids (694). Another major route of cholesterol delivery to the liver is mediated through LDL and the LDL receptor (LDLR) (Figure 6) (701). In the circulation, HDL exchanges CE for TG from VLDL and LDL through cholesteryl ester transfer protein (CETP) activity (Figure 6), and this action is responsible for directing CE through the LDL receptor pathway (702). Besides these major routes holoparticle uptake of HDL may also contribute to delivery of HDL-CE to the liver. Hepatocytes, and many other cell types in other tissues, likely participate in HDL retro-endocytosis where apoA-I or HDL particles are taken up by endocytosis and resecreted without degradation in late endosomes and lysosomes (703,704). SR-BI and CD36 may participate in this process as well as other potential HDL receptors (705-707). For example, the F0F1ATPase and P2Y13receptor have been reported to facilitate the uptake of the entire HDL particle(703,704,708,709). The liver then excretes both cholesterol and bile acids-derived from cholesterol into the bile which are removed from the body in feces, thus completing RCT from peripheral macrophages to bile through HDL and the liver (710). Recent evidence suggests there is also likely an HDL-independent pathway for systemic cholesterol removal through transintestinal cholesterol excretion (TICE) (711). Historically, HDL’s anti-atherogenic properties were largely attributed to HDL’s role in RCT and removing excess cholesterol from macrophages and peripheral tissues; however, continually emerging alternative HDL functions likely significantly contribute to HDL’s protection against CVD.
HDL Levels and Risk of CVD
Historically, HDL-C was synonymous with the term HDL; however, the amount of cholesterol in the HDL pool (HDL-C) and the number and quality of HDL particles (HDL-P) are independent concepts that are important to consider in the context of HDL function. Several decades of high-quality epidemiological studies have clearly shown that HDL-C levels are inversely correlated to CVD risk and events, independent of race, gender, and ethnicity (712). In well-controlled studies assessing CVD risk using multivariate approaches to adjust for covariates, both apoA-I and HDL-C are strong independent predictors of CVD risk (474). Nonetheless, HDL-C levels are also inversely correlated to insulin resistance, obesity, and triglycerides. As such, HDL-C’s causality in protection from CVD is difficult to define and is somewhat controversial, mainly due to epidemiological discrepancies between the dose-response of HDL-C levels to CVD outcomes. It is possible that HDL-C levels may simply be a biomarker for CVD and not play a causal role in atherosclerosis; however, an increasing number of functional studies clearly support HDL’s functional relevance in biochemical mechanisms of atherosclerosis. In any case, epidemiological studies over the past 50 years have provided many insights into HDL-C and CVD risk. The first evidence came from the Framingham Heart Study in 1966 demonstrating a link between HDL-C and ASCVD (713). In 1975, HDL-C levels were found to be inversely associated with CVD in a Norwegian trial (Tromso Heart Study) (714). In subsequent years, the Honolulu Heart Study (1976) (715)and Framingham Heart Study (1977) (559)both reported that many CVD patients had low HDL-C levels. Over the years, low HDL-C levels have consistently been reported to be associated with increased risk of ASCVD and events (716-718). By the late 1980s and early 1990s, the relationship between HDL-C and CVD was generally accepted, as studies during this period established that low HDL-C levels were associated with CVD risk independent of other risk factors even in patients with normal total cholesterol levels (719-721).
Clinical Outcomes Trials
Prior to the statin-era, results from randomized controlled clinical trials suggested that increasing HDL-C levels 1 mg/dL or 1% reduces mortality from CVD by 3-4% (722,723). In the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS), treatment of men and women withaverage TC and LDL-C levels and below-average HDL-C levels with lovastatin (20-40 mg) reduced LDL-C by 25% and raised HDL-C 6%, resulting in a 37% reduction in the risk for the first major acute coronary event (724). These results showed that statin therapy was effective in reducing risk for CVE in subjects with low-HDL-C. The extent to which the benefit came from HDL-C raising is unclear. Studies completed in subjects on statins have yielded inconsistent results with regard to the importance of raising HDL-C partly due to evidence suggesting that statin (fluvastatin) use in low HDL-C subjects decreased coronary artery disease (CAD) with little to no increase in HDL-C levels (725-727). In the fluvastatin regression study, low HDL-C subjects on placebo showed increased disease (angiographic) progression compared to subjects with high HDL-C levels (727). Collectively, evidence from these and a large number of epidemiological studies overwhelmingly support a clear inverse association between HDL-C levels and CVD risk. This is demonstrated clinically as raising HDL levels through injections of reconstituted HDL (rHDL) resulted in atherosclerotic plaque regression, as determined by intravascular ultrasound (728). A number of animal studies clearly support the HDL-C hypothesis. For example, raising HDL in mice and rabbits consistently blocks atherogenesis( 729-731). However, raising HDL-C levels by mono- or combined therapy to reduce risk and events has proven challenging. Two major clinical outcomes trials of raising HDL with niacin failed to show a benefit. In subjects with CAD with LDL-C levels well controlled with a statin, the addition of extended release niacin in AIM-HIGH (732)and extended release niacin plus laropiprant (prostaglandin D2 receptor blocker to inhibit flushing) in HPS-2THRIVE (733)failed to reduce cardiovascular outcomes. However, structural limitations of the two Niacin trials design complicated their interpretation (734). In addition, major cardiovascular outcomes trials of 3 CETP inhibitors torcetrapib (735), dalcetrapib (736), evacetrapib have now failed to show a benefit in reducing cardiovascular events. More recently, the CETP inhibitor (anacetrapib) was tested in the REVEAL trial, which was a positive outcomes trial (737). However, the benefit of anacetrapib in reducing CVE seems to be largely explained by lowering of non-HDL, rather than increases in HDL-C (738). More recently, two recombinant apoA-I products MDCO-216 and CER001 showed no benefit in imaging studies (739,740). Collectively, the failure of these clinical studies has raised doubts about the HDL hypothesis. Indeed, raising HDL-C is presently not a primary target for therapeutic intervention. Nevertheless, HDL infusion in humans has been reported to improve endothelial function, which should contribute to inhibiting atherogenesis (741). At this time, HDL particle infusion therapies have not been proven to be an effective approach to reduce cardiovascular events (742); however, clinical trials with reconstituted HDL are still ongoing. Furthermore, recent studies indicate that HDL particle number and cholesterol efflux capacity are better indicators of CHD risk than HDL-C levels (743,744). The therapeutic targeting of HDL non-cholesterol cargo, quality, and function are emerging and gaining support, as HDL have many other biological properties that likely contribute to prevention of atherosclerosis and CVD (745). In addition, quantifying HDL function, including cholesterol efflux capacity, will provide a better risk index than steady-state HDL-C levels (746).
Particle Number and Cholesterol Efflux
A major blow to HDL causality in atherosclerosis comes from genetic studies. Mendelian disorders resulting in very low HDL-C levels have yielded conflicting data, as mutations to critical lipoprotein genes (e.g. apoA-I) were found to be associated with protection from atherosclerosis in one study (747)and increased risk in another study (748). The ApoA-I Milano mutation is associated with low levels of HDL-C and reduced risk of CVD (747). Infusion of recombinant apoA-I Milano was reported to induce regression of atherosclerosis (749), but there has not been clear progress in developing it as an approach to therapy since the initial regression study was published. The evidence that some genetic causes of low HDL-C are associated with increased risk for premature atherosclerosis, whereas others are not, supports the notion that HDL function may be more important than HDL-C levels. Nonetheless, Mendelian disorders of low HDL-C levels are rare, and thus the sample sizes in these studies are limited and it is difficult to draw accurate conclusions. To address this issue, genome-wide association studies were completed to attempt to resolve if HDL-C is a risk index or causal factor. These studies are limited in that many variants that raise or lower HDL-C levels also affect other lipoproteins, namely LDL-C levels. For example, variants in CETPraise HDL-C levels and reduce LDL-C levels, which complicates risk prediction based on HDL-C levels (750). Nevertheless, studies have found that variance solely associated with HDL-C levels is not linked to cardiovascular events. For example, single nucleotide polymorphisms (SNPs) in endothelial lipase (LIPG), which raises HDL-C levels, are not associated with decreased CVD(751).
As failed clinical trials aimed at raising HDL-C levels and genetic studies do not uniformly support causality for HDL-C in CVD, HDL functional tests in future prospective studies will likely provide more resolution to HDL’s causal role in CVD. Cholesterol efflux capacity, a marker of HDL function, has been reported to be inversely associated with CVD risk independent of HDL-C levels (744,746). This was first demonstrated in a cross-sectional study using radio-tracing of cholesterol efflux (746). A subsequent study also found an inverse association between HDL efflux capacity and atherosclerosis, but reported a positive link to cardiovascular events (752). In a third study assessing HDL cholesterol efflux in a US cohort using a fluorescence method, efflux was again linked to decreased risk of CVD (743). Recently, HDL cholesterol efflux capacity was found to be inversely associated with CVD risk and events in a large nested case-control prospective study (n=3,494 subjects) from the EPIC-Norfolk Study (744,753). These associations were independent of many other co-founding factors, including HDL-C, T2DM, obesity, LDL-C, and age amongst others (744).
In addition to HDL cholesterol efflux and functional indices as risk predictors, HDL particle number (HDL-P) has also been reported to provide biomarker potential. HDL-P numbers can be quantified using nuclear magnetic resonance (754)or calibrated ion mobility assays (755). HDL-P was found to be inversely associated with carotid intima medial thickness (cIMT) and coronary heart disease (CHD) independent of LDL particle numbers and HDL-C levels in the large multi-ethnic study of atherosclerosis (MESA) (756). Importantly, HDL-P remains inversely associated to CHD after adjusting for triglycerides and apolipoprotein B (apoB), thus suggesting that HDL-P is far superior to HDL-C levels as a biomarker of ASCVD and events (757,758). Furthermore, neither HDL-C levels nor HDL-P levels correlate to cholesterol efflux from macrophages; therefore, the rate of cholesterol efflux is still critical to understanding RCT and HDL function. Likewise, HDL quality is more important than apoA-I levels, which also do not correlate with HDL function, e.g. RCT (759). Serum samples with identical apoA-I and HDL-C levels were found to have differing cholesterol acceptance capacities, mostly due to pre-beta HDL levels, which contributed to altered ABCA1-mediated cholesterol efflux (759). These studies strongly suggest that HDL function (cholesterol efflux capacity), as opposed to HDL-C, HDL-P, and apoA-I levels, provide a more important risk assessment and better predictor of future events as well as a more reasoned therapeutic target for reducing CVD risk and events. However, clinical assays for apoA-I and HDL-P are widely available and well-established, whereas assays for cholesterol efflux capacity have not been standardized and remain a research tool at present.
HDL Composition and Analysis
Historically, HDL have been isolated by density-gradient ultracentrifugation (DGUC) based on isopycnic equilibrium, and HDL have been defined by their density 1.063-1.21 g/mL since the 1950s (760,761). Based on mass, HDL can also be separated from other lipoproteins by size-exclusion chromatography (fast protein liquid chromatography, FPLC), and HDL’s molecular weight ranges from 175,000 - 360,000 Da (762). In addition to DGUC and FPLC, affinity chromatography can also be used to purify HDL from plasma using antibodies against apoA-I (763)or apoA-II, as HDL heterogeneity includes particles containing apoA-I:apoA-II (75%) or apoA-I only (25%) (763,764). Furthermore, asymmetric flow field-flow fractionation is now being used to isolate and characterize HDL (765). HDL can also be separated by non-denaturing gradient gel electrophoresis, e.g. polyacrylamide gel electrophoresis. Large HDL (HDL2, 8.8-12.9 nm in diameter) and small HDL (HDL3, 7.2-8.8 nm) are both α migrating particles (high negative charge), whereas pre-β HDL (5.4-7 nm) are β migrating particles for which they are defined. To quantify pre-β HDL particles, 2-D gel electrophoresis is often used to separate pre-β from mature HDL (766). HDL-P numbers can be quantified by either nuclear magnetic resonance spectroscopy or calibrated ion mobility assays. HDL can also be quantified and qualified by other methods, including vertical rotor ultracentrifugation, and transmission electron microscopy.
HDL are very dynamic and should be acknowledged as a heterogeneous pool of sub-classes with differing sizes, shapes, densities, protein compositions, and lipid diversity. Lipid-free apoA-I is secreted from the liver and small intestine as an amphipathic helix, and it quickly becomes lipidated by ABCA1 to form pre-β HDL, which then becomes discoidal after accepting phospholipid and free cholesterol from hepatocytes and peripheral cells. Upon further lipidation and cholesterol accumulation and esterification, nascent spherical HDL forms that range 7-12 nm in diameter. Mature HDL contains 3-4 apoA-I molecules of which 1 remains on the particle and the other apoA-I are free to (dis)associate (exchange) on and off the particle with other HDL. This is predominantly associated with rearrangement of HDL’s aqueous phase and surface area (767). As such, HDL are in a constant state of remodeling and interconversion. Each spherical HDL particle has approximately 50-130 phospholipids, 10-50 free cholesterol molecules, 30-90 CE molecules, and 10-20 triglyceride (TG) molecules (536). Phosphatidylcholine makes up the largest amount of lipid on HDL (approximately 90%); however, over 200 species of lipids have been reported, including sphingolipids, acylglycerols, isoprenoids, glycerophospholipids, and vitamins (768,769). The HDL proteome has been extensively studied and there is a general consensus of approximately 80 proteins (770,771). In addition to apoA-I and apoA-II, HDL transports over a dozen other apolipoproteins, as well as many enzymes and other factors. HDL have also been found to transport small RNAs, namely microRNAs (miRNA), which were found to be altered in hypercholesterolemia and atherosclerosis (772,773). Most interestingly, HDL have been demonstrated to transport a wide-variety of exogenous non-host small RNAs, including rRNA and tRNA fragments derived from bacterial and fungal species present in the microbiome and environment (774).The size of HDL is determined by the amount of CE and triglyceride (TG) in the hydrophobic core, and HDL is generally separated into 5 sub-classes based on size. Distinct HDL sub-species have been associated with CVD risk, and the sub-species have differential biological functions, e.g. large HDL are less anti-inflammatory (775-777). Many of the cargo or components of HDL are enriched in the small HDL sub-class which provides many of the alternative functions to the total HDL pool (778,779). The concentration of all HDL particles in plasma is approximately 20 umol/L; however, small HDL particles are the most abundant sub-class at approximately 10 umol/L. HDL are heterogeneous particles that transport a wide-variety of proteins, lipids, and nucleic acids, which confer many of HDL’s biological properties and beneficial functions in health and dysfunction in specific diseases.
HDL Cell Signaling
Many of HDL’s cellular functions – cell survival, proliferation, vasodilation -- are mediated by HDL-induced cell signaling cascades (780). As such, HDL can be characterized as hormone-like agonists. Although substantial work still remains in identifying HDL binding proteins and receptors on the cell surface, HDL have been found to activate many signaling cascades through various receptors. The most studied example of this is HDL’s ability to bind to the plasma membrane and through cell signaling mobilize cholesterol from intracellular stores in organelles to the plasma membrane for efflux. This has been attributed to HDL-induced activation of protein kinase C (PKC) (781). Specifically, apoA-I binds to ABCA1 and activates phosphatidylcholine lipases, which activate PKC leading to the movement of cellular cholesterol from intracellular stores to the plasma membrane for efflux, as well as PKC-mediated phosphorylation of ABCA1, which increases the transporter’s stability and efflux activity (782-784). This is a prime example of HDL-induced cell signaling that contributes to HDL cholesterol efflux capacity, which reduces the cholesterol burden for macrophages in the lesion, prevents foam cell formation, and antagonizes atherogenesis. Other HDL-induced signaling pathways that result in increased cholesterol and lipid efflux include protein kinase A (PKA) (785,786), cell division control protein 42 (Cdc42) (787), and Janus kinases-2 (JAK2) (788,789)cascades. HDL (i.e. apoA-I)-induced cell signaling through ABCA1 also suppresses macrophage M1 phenotype activation and pro-inflammatory cytokine production (Figure 10), and promotes M2 phenotype anti-inflammatory cytokine secretion (e.g. interleukin 10 (IL-10)) through JAK2 signaling and activation of signal transducer and activator of transcription 3 (STAT3) (75). In addition, the apoA-I:ABCA1:JAK2 axis was reported to suppress inflammation in endothelial cells through cyclooxygenase-2 (COX-2) activation leading to increased prostaglandins (PGI2), which also suppresses atherogenesis (790). HDL have also been reported to induce cell signaling through SR-BI. HDL binding to SR-BI’s extracellular loop was reported to trigger activation of SR-BI’s cytoplasmic C terminal domain leading to the phosphorylation of protein kinase Src and activation of both liver kinase B1 (LKB1) and calmodulin-dependent protein kinase (CAMK) (791,792). This results in cell signaling through downstream kinases – AMP-activated protein kinases (AMPK) (792), protein kinase Akt (791), and mitogen-activated protein kinase (MAPK)(791)– which ultimately regulates angiogenesis (ubiquitin ligase Siah (Siah1/2) and hypoxia-inducible factor 1α (HIF1α) (793)), insulin sensitivity (glucose transporter 4 (Glut4)(794)), re-vascularization (Rac1(795)), and vasodilation (COX(796), endothelial nitric oxide synthase (eNOS)(797,798)). Interestingly, macrophage SR-BI has recently been shown to mediate efferocytosis (phagocytosis of dead cells) in the setting of atherosclerosis via a Src/Akt/Rac1 signaling pathway, reducing necrosis in lesions (185). All of these downstream effects contribute to HDL function, and to a lesser degree atherogenesis.
The most robust HDL signaling activation is mediated by bioactive lipids on HDL, namely the lysosphingolipid sphingosine-1-phosphate (S1P). A majority of S1P in circulation is associated with HDL, and HDL-S1P activates the G-coupled S1P receptors (S1P1-5) on the surface of many vascular cell types, including macrophages, endothelial cells, and smooth muscle cells. Activation of S1P1and S1P2 receptors turns on a host of signaling cascades and factors that directly contribute to the many anti-atherogenic properties of HDL, including increasing endothelial barrier function (799)and angiogenesis (800,801)while decreasing inflammation (802)and apoptosis (803). HDL were also found to inhibit smooth muscle migration through S1P signaling, a key factor in restenosis and plaque development (804). All of these are critical processes to atherogenesis. In support of these studies, subjects with CAD were found to have decreased HDL-S1P levels (805). The key terminal effector factors in these G-protein receptor signaling cascades are focal adhesion kinase (FAK), nuclear factor κ beta (NF-κB), nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, eNOS, STAT3, and B-cell lymphoma-extra-large (Bcl-xl) (780). This HDL-S1P signaling pathway has also been linked to vasorelaxation (806)and cytoprotection (e.g. cardiomyocytes) (807). In addition to these direct pathways, HDL also likely activates cell signaling indirectly through ATP (β-ATPase/P2Y12/13)(808)or toll-like receptors (809). Collectively, HDL induced cell signaling in vascular and inflammatory cells underlies HDL’s anti-atherogenic properties in health, and deficits in HDL signaling likely link HDL dysfunction in metabolic diseases to increased risk of atherosclerosis.
Anti-Inflammatory HDL
Outside reverse cholesterol transport, HDL’s anti-inflammatory properties have been the most extensively studied HDL function and likely play a large role in HDL’s anti-atherogenicity (Figure 10). HDL’s anti-inflammatory properties are conferred by numerous mechanisms in many types of cells. In addition to providing the vascular barrier, endothelial cells control vascular inflammation through expressing adhesion molecules that aid in monocyte adhesion and ultimate migration into the atherosclerotic lesion. Moreover, activated endothelial cells secrete cytokines and recruit monocytes through chemokine release. The induction of adhesion molecules, cytokines, and chemokines in activated endothelial cells is largely due to NF-B transcriptional activation. In humans, injection of apoA-I resulted in decreased adhesion molecule expression in atherosclerotic plaques (810). One mechanism by which HDL suppresses endothelial cell and monocyte activation is through inhibiting NF-kB activity by attenuating IkB kinase activity (811). Nonetheless, HDL decreases adhesion molecule expression through multiple mechanisms. Cells pre-treated with HDL or apoA-I are protected from TNFα or oxidized LDL (oxLDL)-induced adhesion molecule expression. In addition, HDL binding to SR-BI may also contribute to inhibition of adhesion molecule expression, as SR-BI-mediated Akt activation promoted heme oxygenase-1 expression. In addition, up-regulation of 3-beta-hydroxysteroid-delta 24 (DHCR24) by HDL binding to SR-BI was reported to underlie HDL’s ability to suppress adhesion molecules (812). Furthermore, HDL suppression of intracellular adhesion molecule-1 (ICAM-1) in endothelial cells was found to be mediated, in part, through the transfer of miR-223 to recipient cells (773). Recent studies also suggest that TGFβ and AMPK also contribute to HDL’s suppression of adhesion molecule expression (813).
In addition to HDL’s profound effects on vascular endothelium, HDL suppresses myelopoiesis, monocyte recruitment, macrophage activation, proliferation, and emigration from atherosclerotic lesions. Similar to its impact on endothelial cells, HDL also suppresses adhesion molecule expression in monocytes, which inhibits monocyte adhesion and migration to atherosclerotic lesions (814). HDL and apoA-I were demonstrated to suppress CD11b expression on human monocytes through both ABCA1-dependent and independent mechanisms (814). HDL inhibition of monocyte activation, which includes suppression of cytokines and adhesion molecules, is mediated through both peroxisome proliferator-activated receptor gamma (PPARγ) and NF-kB transcription factors (815). Suppression of chemokine and cytokines in myeloid cells inhibits infiltration and migration of circulating monocytes, and thus antagonizes atherosclerosis. HDL have also been reported to mediate macrophage reprogramming through the transcription factor ATF3 that reduces Toll-like receptor signaling (816). Importantly, much of HDL’s (and apoA-I’s) inhibition of macrophage activation is mediated through altering cholesterol levels in plasma membrane lipid rafts through cholesterol efflux mediated by ABCG1/SR-BI and ABCA1; however, apoA-I induced signaling through ABCA1 and the JAK/STAT pathway independent of cholesterol efflux may also contribute to HDL’s effect, as described above (75,817)(814,818). HDL have also been demonstrated to promote macrophage emigration through removing excess cholesterol and induction of signaling pathways (208). In addition to HDL’s impact on monocytes and macrophages, HDL also strongly suppresses neutrophil activation and vascular smooth muscle cell secretion of monocyte chemoattractant protein-1 (MCP1) (819).
In addition to HDL’s roles in innate immunity, recent evidence suggests that HDL play multiple roles in adaptive immunity (820). Mice lacking apoA-I develop autoimmunity when challenged with a high cholesterol (diet and background, Ldlr-/-), which includes T cell activation and production of autoantibodies (821,822). This phenotype was rescued by apoA-I injections. HDL have also been reported to repress both antigen-presenting cell (APC) activation of T cells and T cell activation of monocytes, thus preventing the secretion of proinflammatory cytokines and chemokines (Figure 10) (823,824). ApoA-I also prevents the phenotypic switching of T-regs into pro-inflammatory follicular helper T cells during atheroprogression (92). Moreover, cholesterol efflux to HDL and apoA-I have been reported to suppress myelopoiesis and proliferation of myelopoietic stem and progenitor cells, as loss of function for both Abca1and Abcg1in mice resulted in increased myelopoiesis (820). Injection of apoA-I was also found to rescue this phenotype (825). In addition, HDL and cholesterol efflux were reported to suppress megakaryocyte progenitor proliferation, platelet levels, and thrombocytosis (826). Collectively, HDL and apoA-I inhibit circulating levels of hematopoietic progenitor cells, monocytes, neutrophils, and platelets all of which contribute to HDL’s capacity to limit inflammation and atherosclerosis.
Antithrombotic HDL
Another anti-atherogenic function of HDL is the capacity to directly and indirectly inhibit platelet activation, aggregation, and thrombus formation (Figure 10). HDL-C levels were found to be inversely associated with thrombus formation in humans (827). HDL is required to remove excess cholesterol from the plasma membrane of platelets for proper function, and platelets isolated from mice lacking SR-BI to mediate cholesterol efflux to HDL were found to be more susceptible to activation (828,829). Both HDL and cyclodextrin-mediated cholesterol efflux were found to inhibit platelet aggregation (828). However, HDL-induced cell signaling through binding to glycoprotein IIb/IIIa on the surface of platelets was reported to activate phospholipase C (PLC) and PKC, thus leading to flux through the Na+/H+ antiport system (717). This pathway can result in alkalization of the cytoplasm and calcium release, which can reduce platelet activation (830). Furthermore, HDL dose-dependently inhibits stimulated platelet activation, which leads to reduced platelet aggregation, granule secretion and fibrinogen binding. In rats, apoA-I injections inhibited thrombus formation and reduced thrombus mass (831). HDL’s anti-thrombotic effects are also mediated, in part, through HDL’s ability to inhibit tissue factor and factors X, Va, and VIIIa (Figure 10)(832). HDL also prevents thrombus formation through cell signaling and nitric oxide (NO) production in endothelial cells (828), and suppression of tissue factor and platelet-activating factor expression in endothelial cells (833,834). HDL also reduces erythrocyte influence on thrombus formation (835). Collectively, HDL has multiple biological mechanisms that inhibit thrombus formation, and thus, contribute to HDL’s anti-atherogenic properties.
Pro-Vasodilatory HDL
The endothelium significantly contributes to vascular tone, and HDL confer protection against endothelial cell activation, apoptosis, and loss of barrier function, which is critical to atherogenesis. HDL have been reported to induce endothelium-dependent vasodilation in aortic rings (806), and individuals with low HDL have reduced endothelium-dependent vasorelaxation (Figure 10) (741). HDL’s benefit to endothelial cells is largely mediated by cell signaling through phosphatidylinositol 3-kinase (PI3K) and Akt and is induced by bioactive lipids and associated proteins on HDL, including lysosulfatide, S1P, and sphingosylphosphorylcholine (SPC) (791,798,806). A key outcome of HDL-induced cell signaling is the production of NO (Figure 10) through both signaling induced phosphorylation of eNOS and increased eNOS expression (791,836). HDL can trigger eNOS-phosphorylation through SR-BI, S1P receptor (S1P1-5), and ABCG1-mediated cholesterol efflux (806,837). HDL-induced NO underlies many of HDL’s beneficial properties to endothelial cells, including HDL-induced vasodilation, tightening of cell-to-cell junctions and increased barrier function, differentiation of endothelial progenitor cells, cell survival and proliferation, cell migration, inhibition of apoptosis, and suppression of adhesion molecule expression. In addition, HDL also has NO-independent properties on endothelial cells, including induced proliferation, increased barrier function, suppressed inflammation and decreased apoptosis (838). These studies clearly define a beneficial role for HDL in vascular integrity, which underlies HDL protection against atherosclerosis.
Anti-Apoptotic HDL
HDL have multiple anti-apoptotic properties that enhance cell survival (Figure 10). By various metrics, HDL support mitochondrial function and prevent the release of apoptotic signals, including cytochrome C (205,839). Moreover, HDL drives the expression of Bcl-xl, which is a strong anti-apoptotic factor and suppresses Bid, which is a pro-apoptic protein (839,840). HDL mediates these gene expression changes through cell signaling and NO production through activation of surface receptors by HDL-associated proteins and bioactive lipids, including apolipoprotein J (apoJ) and S1P (803,840). In addition, there are likely alternative anti-apoptotic mechanisms resulting from HDL-induced signaling. Nonetheless, HDL has been demonstrated to suppress apoptosis in endothelial cells (Figure 10) activated with tumor necrosis factor (TNFα) and oxLDL (839,841,842). HDL proteins (apolipoprotein M, apoM) and apoM-binding lipids (S1P) contribute to HDL’s ability to increase tight junctions and endothelial cell survival (843). Mice deficient in apoM have reduced S1P levels and loss of endothelium barrier function (843). HDL’s ability to support the endothelium barrier function is a key feature of its anti-atherosclerosis properties and represents a classic example of HDL’s control of cellular gene expression and phenotype that are beneficial to vascular health. However, HDL also have many capacities in the extracellular space (e.g. plasma) that protect against atherosclerosis.
Anti-Oxidative HDL
A key factor in monocyte activation and chemotaxis in the vascular wall is the accumulation of oxLDL, which is more pro-inflammatory and pro-atherogenic than unmodified LDL. LDL can become oxidized by a variety of endogenous mechanisms (844). In the vascular wall, LDL can be modified (oxidized) by many cell types, including vascular smooth muscle cells, endothelial cells, and macrophages (776). Remarkably, HDL prevents the oxidation of LDL (Figure 10) and recent evidence suggests that this may occur through 4 distinct proteins circulating on HDL – apoA-I (845,846), LCAT (847), lipoprotein-associated phospholipase A2 (Lp-PLA2)(848,849), and paraoxonase 1 (PON1) (430,846). First, HDL can simply soak up oxidized lipids or oxidizing factors from cells preventing their association with LDL and their modification of LDL lipids and proteins. In addition, HDL removes lipid hydroperoxides from LDL particles (846). Specifically, small apoAI containing HDL particles are the most efficient at accepting lipid hydroperoxides, which are reduced to their inactive lipid hydroxides via oxidation of the methionine residues in apoA-I (850). Compared to apoA-II the methionine residues in apoA-I are more conformationally conducive to reducing lipid hydroperoxides (851,852). In addition, HDL with low surface free cholesterol and sphingomyelin are more efficient at accepting lipid hydroperoxides (745,853). The capacity of HDL to prevent oxidation via this mechanism is also maintained by the selective removal of HDL lipid hydroperoxides and hydroxides by hepatocyte SR-BI (854). In addition, ApoA-I methionine sulfoxide is reduced to methionine by methionine sulfoxide reductases.(850). LCAT circulates on HDL and has also been reported to block LDL oxidation, as LCAT over-expression in mice reduced LDL oxidation as determined by reduced LDL autoantibodies (855). Lp-PLA2appears to be pro-atherogenic on LDL and anti-atherogenic on HDL (856). Its activity on HDL likely contributes to HDL’s anti-oxidative capacity, as inhibition of HDL-associated Lp-PLA2attenuated HDL’s ability to block LDL oxidation (848). The strongest anti-oxidative HDL protein is likely PON1. Over-expression of PON1 in mice confers enhanced HDL anti-oxidative capacity, and PON1 itself prevents LDL oxidation in vitro (432). Most importantly, HDL isolated from mice lacking PON1 have reduced ability to prevent LDL oxidation. HDL’s anti-oxidative capacity likely plays a large role in preventing inflammation and atherogenesis, and like many of the other alternative functions, confer HDL’s beneficial role in health.
HDL Intercellular Communication
HDL also likely participate in intercellular communication through the transfer of nucleic acids between tissues. Recently, HDL have been reported to transport miRNA (Figure 10), which are small non-coding RNAs that suppress gene expression through binding to complimentary target sites in the 3’ untranslated region of mRNAs, and thus inhibit translation and induce mRNA degradation (772). Most interestingly, the HDL-miRNA profile is significantly altered in hypercholesterolemia and atherosclerosis (772). miRNAs have been reported to be exported from macrophages to HDL, and HDL has been demonstrated to transfer specific miRNAs to recipient hepatoma cells (Huh7) and endothelial cells, likely through HDL’s receptor SR-BI (773). In endothelial cells, HDL was found to deliver miR-223 to recipient cells, where it directly targeted intracellular adhesion molecule-1 (ICAM-1) expression (Figure 10), and thus inhibited neutrophil adhesion to the cells (773). miR-223 is not transcribed or processed in endothelial cells and HDL delivery of mature miR-223 to endothelium likely confers, in part, HDL’s anti-inflammatory capacity associated with adhesion molecule suppression. Future studies are needed to determine the physiological relevance and functional impact of HDL-miRNAs in humans and animal models in the context of atherosclerosis and other inflammatory diseases.
Anti-Infectious HDL
HDL also contributes to innate immunity by modulating immune cell function. However, this hypothesis has not been extensively studied in the context of atherosclerosis. HDL are anti-infectious, anti-parasitic, and anti-viral. HDL have the unique capacity to prevent endotoxic shock and readily binds to lipopolysaccharides (LPS) and contributes to removing LPS through biliary excretion thus aiding innate immunity (857-859). Amongst the many proteins that circulate on HDL, apolipoprotein L1 (apo-L1) (also known as trypanosome lytic factor) is present in specific sub-classes of HDL (860,861). This factor kills Trypanosome bruceiand Trypanosome brucei rhdesiense, parasites that cause sleeping sickness, through creating ionic pores in endosomes (860-862). Although promising, future studies are required to define how HDL regulation of innate immunity contributes to the inhibition of atherogenesis.
HDL Dysfunction
HDL confer many anti-atherogenic properties that are lost in atherosclerosis and other inflammatory and metabolic diseases. These include 9 key processes –
- Loss of cholesterol efflux capacity from macrophages
- Reduced ability to inhibit LDL oxidation
- Decreased vasodilation through reduced NO production in endothelial cells
- Reduced ability to inhibit monocyte chemotactic activity
- Loss of the ability to metabolize hydroperoxides on erythrocyte membranes
- Reduced ability to suppress TNFα-induced NF-κB activation and adhesion molecule expression
- Loss of anti-apoptotic capacity in endothelial cells
- Decreased capacity to block TNFα-induced NADPH oxidase activity and superoxide production
- Suppression of cytokine inhibition in activated inflammatory cells.
Many of these defects are due to changes in HDL cargo, e.g. decreased PON1 levels or increased serum amyloid A (SAA) levels. Moreover, changes in the content of bioactive lipids or increased oxidative modifications to HDL’s lipids and protein cargo likely confer dysfunction. HDL-miRNAs have been shown to be significantly altered in hypercholesterolemia and atherosclerosis (772). It is unknown how these changes contribute to HDL’s loss of anti-atherogenic properties, but they hold great potential for future studies. In CHD, acute coronary syndrome (ACS), and ischemic cardiomyopathy, HDL have reduced ability to inhibit oxidation of LDL, likely through reduced PON1 levels as reported in CHD (845,863,864). Loss of PON1 also reduces HDL’s ability to prevent oxidation of its own lipids and proteins, which has been reported in metabolic syndrome as oxidation of apoA-I impairs HDL’s RCT and anti-inflammatory functions (865). Reduced HDL-PON1 levels are also found in other cardiometabolic diseases, including type 2 diabetic mellitus (T2DM) (866,867), type 1 diabetes mellitus (T1DM) (868), rheumatoid arthritis (RA) (869,870), dyslipidemia (e.g. hyperalphalipoproteinemia (HALP) (871)), and patients after cardiac surgery (872). In subjects with ACS and CAD, HDL have been reported to have decreased ability to prevent endothelial cell apoptosis likely through decreased activation (phosphorylation) of Bcl-xl and increased activation of Bcl-2, which are anti-apoptotic and pro-apoptotic proteins, respectively (840). Loss of HDL’s anti-apoptotic capacity has been proposed to be due to increased apoCIII and possibly decreased clusterin levels on HDL (840). HDL from subjects with CHD also have decreased ability to prevent monocyte adhesion to endothelial cells and recruitment in arterial wall co-cultures, which could be associated with reduced PON1 levels amongst other cargo (845,873,874). HDL from ischemic cardiomyopathy and CAD subjects also have reduced cholesterol efflux acceptance capacity, which likely leads to increased foam cell formation in the atherosclerotic lesion and increased atherogenesis (746,863). Although the molecular basis for all of HDL’s loss of anti-atherogenicity in CHD is not known, other functions of HDL are compromised in these subjects, including the ability to reduce hydroperoxides on erythrocyte membranes (875). This loss of HDL’s anti-oxidant capacity is also found in T2DM (875)and T1DM (868,876). HDL in metabolic syndrome have been reported to have decreased capacity to prevent oxidation of LDL and inhibit endothelial cell apoptosis (877,878). This loss of anti-atherogenic properties is also found in hypertension(879), T2DM (867,880,881), end-stage renal disease (ESRD) (882,883), RA (869,870,884,885), systemic erythematosus lupus (SLE) (884), obstructive sleep apnea (886), and dyslipidemia (HALP) (871). Reduced ability of HDL to stimulate NO production from endothelial cells and decreased vasorelaxation properties are reported for T2DM (887,888), T1DM (889), mild chronic kidney disease (CKD) (890), and rare forms of autoimmunity (ALPS) (891). Loss of HDL-mediated cholesterol efflux capacity has been found in patients with hyperhomocysteinemia (892), sepsis (893), psoriasis (894), SLE (895), RA (885,896), ESRD (897,898), and T2DM (899,900). Not only does HDL dysfunction result from loss of key proteins and cargo, HDL can gain pro-atherogenic cargo and properties in cardiometabolic diseases. Due to loss of PON1, HDL accumulate malonaldehydes, which inhibits NO production through increased phosphorylation of eNOS through LOX-1 receptor signaling (901).
HDL Summary
Years of sound epidemiological studies have clearly established an inverse relationship between HDL-C levels and risk of CVD. Nevertheless, recent GWAS studies suggest that individuals with high HDL-C levels are not protected from CVD. Furthermore, clinical studies aimed at raising HDL-C levels through niacin and CETP inhibitors have failed to reduce risk of cardiovascular events and have been stopped prematurely due to lack of efficacy or increased number of events. Although they’re often lumped together, HDL-C levels do not represent HDL particle numbers or HDL function (e.g. cholesterol efflux capacity); both of which have been reported to be better indicators of CVD risk than HDL-C. In addition to HDL’s transport of cholesterol and lipids in the RCT pathway, HDL transports a wide-variety of cargo, including a diverse group of proteins, small RNAs, bioactive lipids, and many other small molecules. These alternative cargos may confer many of HDL’s alternative functions outside of RCT. In fact, HDL have many beneficial properties, including anti-inflammatory, anti-oxidative, anti-thrombotic, anti-infectious, anti-apoptotic, intercellular communication, and pro-vasodilatory capacities. Recently, HDL dysfunction has been reported in many cardiometabolic diseases, including CAD, T2D, and CKD. Current and future challenges include the need to better define HDL anti-atherogenic properties in health and pro-atherogenic influences in disease to better control HDL function to potentially prevent and treat CVD.
ACKNOWLEGEMENTS
:
This work was supported in part by National Institutes of Health grants HL116263 and HL127173.
REFERENCES
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015; 131:e29-322
- Mayerl C, Lukasser M, Sedivy R, Niederegger H, Seiler R, Wick G. Atherosclerosis research from past to present--on the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Archiv : an international journal of pathology 2006; 449:96-103
- Ross R. Atherosclerosis--an inflammatory disease. New England Journal of Medicine 1999; 340:115-126
- Luscher TF, Dohi Y, Tanner FC, Boulanger C. Endothelium-dependent control of vascular tone: effects of age, hypertension and lipids. Basic research in cardiology 1991; 86 Suppl 2:143-158
- Dahlback B. Blood coagulation. Lancet 2000; 355:1627-1632
- Luscinskas FW, Gimbrone MA, Jr. Endothelial-dependent mechanisms in chronic inflammatory leukocyte recruitment. Annual review of medicine 1996; 47:413-421
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiological reviews 1995; 75:519-560
- Gimbrone MA, Jr., Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Annals of the New York Academy of Sciences 2000; 902:230-239; discussion 239-240
- Gimbrone MA, Jr., Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology 2013; 22:9-15
- Nerem RM, Levesque MJ, Cornhill JF. Vascular endothelial morphology as an indicator of the pattern of blood flow. Journal of biomechanical engineering 1981; 103:172-176
- Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. Journal of internal medicine 2006; 259:393-400
- Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr., Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circulation research 2009; 105:453-461
- Gerrity RG, Richardson M, Somer JB, Bell FP, Schwartz CJ. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. The American journal of pathology 1977; 89:313-334
- Hansson GK, Chao S, Schwartz SM, Reidy MA. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. The American journal of pathology 1985; 121:123-127
- Koo A, Dewey CF, Jr., Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. American journal of physiology Cell physiology 2013; 304:C137-146
- Zeng L, Zampetaki A, Margariti A, Pepe AE, Alam S, Martin D, Xiao Q, Wang W, Jin ZG, Cockerill G, Mori K, Li YS, Hu Y, Chien S, Xu Q. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proceedings of the National Academy of Sciences of the United States of America 2009; 106:8326-8331
- Chiplunkar AR, Curtis BC, Eades GL, Kane MS, Fox SJ, Haar JL, Lloyd JA. The Kruppel-like factor 2 and Kruppel-like factor 4 genes interact to maintain endothelial integrity in mouse embryonic vasculogenesis. BMC developmental biology 2013; 13:40
- Topper JN, Cai J, Falb D, Gimbrone MA, Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proceedings of the National Academy of Sciences of the United States of America 1996; 93:10417-10422
- SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr., Garcia-Cardena G, Jain MK. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. The Journal of experimental medicine 2004; 199:1305-1315
- Lei J, Vodovotz Y, Tzeng E, Billiar TR. Nitric oxide, a protective molecule in the cardiovascular system. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society 2013; 35:175-185
- Gerrity RG, Naito HK, Richardson M, Schwartz CJ. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. The American journal of pathology 1979; 95:775-792
- Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis 1989; 9:908-918
- Yurdagul A, Jr., Chen J, Funk SD, Albert P, Kevil CG, Orr AW. Altered nitric oxide production mediates matrix-specific PAK2 and NF-kappaB activation by flow. Molecular biology of the cell 2013; 24:398-408
- Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. The Journal of biological chemistry 2007; 282:13769-13779
- Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxidants & redox signaling 2006; 8:1121-1129
- Steinbrecher UP. Role of superoxide in endothelial-cell modification of low-density lipoproteins. Biochim Biophys Acta 1988; 959:20-30
- Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. The Journal of biological chemistry 2000; 275:12633-12638
- Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1995; 9:899-909
- Park S, Yoon SJ, Tae HJ, Shim CY. RAGE and cardiovascular disease. Frontiers in bioscience 2011; 16:486-497
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Laboratory investigation; a journal of technical methods and pathology 2006; 86:9-22
- Kratzer A, Giral H, Landmesser U. High-density lipoproteins as modulators of endothelial cell functions: alterations in patients with coronary artery disease. Cardiovascular research 2014; 103:350-361
- Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2007; 27:2292-2301
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Molecular Cell 1998; 2:275-281
- Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr., Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999; 398:718-723
- Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. Journal of Clinical Investigation 1998; 101:353-363
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007; 117:195-205
- Libby P, Nahrendorf M, Swirski FK. Monocyte heterogeneity in cardiovascular disease. Seminars in immunopathology 2013; 35:553-562
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325:612-616
- Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arteriosclerosis, thrombosis, and vascular biology 2011; 31:1506-1516
- Christensen JJ, Osnes LT, Halvorsen B, Retterstol K, Bogsrud MP, Wium C, Svilaas A, Narverud I, Ulven SM, Aukrust P, Holven KB. Altered leukocyte distribution under hypercholesterolemia: A cross-sectional study in children with familial hypercholesterolemia. Atherosclerosis 2017; 256:67-74
- van der Valk FM, Kuijk C, Verweij SL, Stiekema LCA, Kaiser Y, Zeerleder S, Nahrendorf M, Voermans C, Stroes ESG. Increased haematopoietic activity in patients with atherosclerosis. European heart journal 2017; 38:425-432
- Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. Journal of the American College of Cardiology 2012; 60:1512-1520
- Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine 2013; 19:1166-1172
- Brown M, Goldstein J, Krieger M, Ho Y, Anderson R. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol 1979; 82:597-613
- Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 1983; 52:223-261
- Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arteriosclerosis, thrombosis, and vascular biology 2006; 26:1702-1711
- Younis N, Sharma R, Soran H, Charlton-Menys V, Elseweidy M, Durrington PN. Glycation as an atherogenic modification of LDL. Current opinion in lipidology 2008; 19:378-384
- Torzewski M, Lackner KJ. Initiation and progression of atherosclerosis--enzymatic or oxidative modification of low-density lipoprotein? Clinical chemistry and laboratory medicine : CCLM / FESCC 2006; 44:1389-1394
- Torzewski M, Suriyaphol P, Paprotka K, Spath L, Ochsenhirt V, Schmitt A, Han SR, Husmann M, Gerl VB, Bhakdi S, Lackner KJ. Enzymatic modification of low-density lipoprotein in the arterial wall: a new role for plasmin and matrix metalloproteinases in atherogenesis. Arteriosclerosis, thrombosis, and vascular biology 2004; 24:2130-2136
- Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochimica et biophysica acta 2012; 1821:858-866
- Fujioka Y, Cooper A, Fong L. Multiple processes are involved in the uptake of chylomicron remnants by mouse peritoneal macrophages. J Lipid Res 1998; 39:2339-2349
- Anzinger JJ, Chang J, Xu Q, Barthwal MK, Bohnacker T, Wymann MP, Kruth HS. Murine bone marrow-derived macrophages differentiated with GM-CSF become foam cells by PI3Kgamma-dependent fluid-phase pinocytosis of native LDL. J Lipid Res 2012; 53:34-42
- Kruth HS. Fluid-phase pinocytosis of LDL by macrophages: a novel target to reduce macrophage cholesterol accumulation in atherosclerotic lesions. Current pharmaceutical design 2013; 19:5865-5872
- Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunological reviews 2014; 262:153-166
- Adamson S, Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Current opinion in lipidology 2011; 22:335-342
- Bolick DT, Skaflen MD, Johnson LE, Kwon SC, Howatt D, Daugherty A, Ravichandran KS, Hedrick CC. G2A deficiency in mice promotes macrophage activation and atherosclerosis. Circulation research 2009; 104:318-327
- Wang XQ, Panousis CG, Alfaro ML, Evans GF, Zuckerman SH. Interferon-gamma-mediated downregulation of cholesterol efflux and ABC1 expression is by the Stat1 pathway. Arteriosclerosis, thrombosis, and vascular biology 2002; 22:e5-9
- Brand K, Mackman N, Curtiss LK. Interferon-gamma inhibits macrophage apolipoprotein E production by posttranslational mechanisms. J Clin Invest 1993; 91:2031-2039
- Peled M, Fisher EA. Dynamic Aspects of Macrophage Polarization during Atherosclerosis Progression and Regression. Frontiers in immunology 2014; 5:579
- Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Current opinion in lipidology 2011; 22:365-372
- Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Current drug targets 2011; 12:647-660
- Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nature reviews Immunology 2015; 15:104-116
- Lu X, Kakkar V. Inflammasome and atherogenesis. Current pharmaceutical design 2014; 20:108-124
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464:1357-1361
- Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Group CT. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018; 391:319-328
- Yancey PG, Jerome WG, Yu H, Griffin EE, Cox BE, Babaev VR, Fazio S, Linton MF. Severely altered cholesterol homeostasis in macrophages lacking apoE and SR-BI. J Lipid Res 2007; 48:1140-1149
- Fioravanti J, Medina-Echeverz J, Berraondo P. Scavenger receptor class B, type I: a promising immunotherapy target. Immunotherapy 2011; 3:395-406
- Kellner-Weibel G, de la Llera-Moya M. Update on HDL receptors and cellular cholesterol transport. Current atherosclerosis reports 2011; 13:233-241
- Phillips MC. Molecular mechanisms of cellular cholesterol efflux. The Journal of biological chemistry 2014; 289:24020-24029
- Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arteriosclerosis, thrombosis, and vascular biology 2003; 23:712-719
- Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. The Journal of biological chemistry 2003; 278:52379-52385
- Ghosh S, Zhao B, Bie J, Song J. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascul Pharmacol 2010; 52:1-10
- Ouimet M, Marcel YL. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arteriosclerosis, thrombosis, and vascular biology 2012; 32:575-581
- Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 2011; 13:655-667
- Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. The Journal of biological chemistry 2009; 284:32336-32343
- Donaldson CJ, Lao KH, Zeng L. The salient role of microRNAs in atherogenesis. Journal of molecular and cellular cardiology 2018; 122:98-113
- Michell DL, Vickers KC. Lipoprotein carriers of microRNAs. Biochimica et biophysica acta 2016; 1861:2069-2074
- Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S, Kinoshita M, Horiguchi M, Nakamura T, Chonabayashi K, Hishizawa M, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE-/- mice. Journal of the American Heart Association 2012; 1:e003376
- Nishino T, Horie T, Baba O, Sowa N, Hanada R, Kuwabara Y, Nakao T, Nishiga M, Nishi H, Nakashima Y, Nakazeki F, Ide Y, Koyama S, Kimura M, Nagata M, Yoshida K, Takagi Y, Nakamura T, Hasegawa K, Miyamoto S, Kimura T, Ono K. SREBF1/MicroRNA-33b Axis Exhibits Potent Effect on Unstable Atherosclerotic Plaque Formation In Vivo. Arteriosclerosis, thrombosis, and vascular biology 2018; 38:2460-2473
- Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. The Journal of clinical investigation 2011; 121:2921-2931
- Ganta VC, Choi MH, Kutateladze A, Fox TE, Farber CR, Annex BH. A MicroRNA93-Interferon Regulatory Factor-9-Immunoresponsive Gene-1-Itaconic Acid Pathway Modulates M2-Like Macrophage Polarization to Revascularize Ischemic Muscle. Circulation 2017; 135:2403-2425
- Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, Palmisano BT, Tabet F, Cui HL, Rye KA, Sethupathy P, Remaley AT. MicroRNA-223 coordinates cholesterol homeostasis. Proceedings of the National Academy of Sciences of the United States of America 2014; 111:14518-14523
- Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 2012; 125:2892-2903
- Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. The Journal of experimental medicine 2009; 206:497-505
- Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circulation research 2010; 106:383-390
- Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circulation research 2004; 94:253-261
- Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nature reviews Cardiology 2009; 6:399-409
- Gil-Pulido J, Zernecke A. Antigen-presenting dendritic cells in atherosclerosis. Eur J Pharmacol 2017; 816:25-31
- Pirillo A, Bonacina F, Norata GD, Catapano AL. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr Atheroscler Rep 2018; 20:12
- Abdolmaleki F, Gheibi Hayat SM, Bianconi V, Johnston TP, Sahebkar A. Atherosclerosis and immunity: A perspective. Trends Cardiovasc Med 2018;
- Tabas I, Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017; 47:621-634
- Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, Sorci-Thomas MG, Hedrick CC. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun 2018; 9:1095
- Srikakulapu P, McNamara CA. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol 2017; 312:H1060-H1067
- Que X, Hung MY, Yeang C, Gonen A, Prohaska TA, Sun X, Diehl C, Maatta A, Gaddis DE, Bowden K, Pattison J, MacDonald JG, Yla-Herttuala S, Mellon PL, Hedrick CC, Ley K, Miller YI, Glass CK, Peterson KL, Binder CJ, Tsimikas S, Witztum JL. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018; 558:301-306
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPAlisI. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007; 369:1090-1098
- Linton MF, Hasty AH, Babaev VR, Fazio S. Hepatic ApoE expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J Clin Invest 1998; 101:1726-1736
- Zhang S, Reddick R, Piedrahita J, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992; 258:468-471
- Plump A, Smith J, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J, Rubin E, Breslow J. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992; 71:343-353
- Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science 1995; 267:1034-1037
- Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in C57BL/6 mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci USA 1997; 94:4647-4652
- Fazio S, Babaev VR, Burleigh ME, Major AS, Hasty AH, Linton MF. Physiological expression of macrophage apoE in the artery wall reduces atherosclerosis in severely hyperlipidemic mice. J Lipid Res 2002; 43:1602-1609
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest 2011;
- Wu D, Sharan C, Yang H, Goodwin JS, Zhou L, Grabowski GA, Du H, Guo Z. Apolipoprotein E-deficient lipoproteins induce foam cell formation by downregulation of lysosomal hydrolases in macrophages. J Lipid Res 2007; 48:2571-2578
- Yancey PG, Yu H, Linton MF, Fazio S. A Pathway-Dependent on ApoE, ApoAI, and ABCA1 Determines Formation of Buoyant High-Density Lipoprotein by Macrophage Foam Cells. Arteriosclerosis, thrombosis, and vascular biology 2007; 27:1123-1131
- Mazzone T, Reardon C. Expression of heterolgous human apolipoprotein E by J774 macrophages enhances cholesterol effux to HDL3. J Lipid Res 1994; 35:1345-1353
- Langer C, Yadong H, Cullen P, Wiesenhutter B, Mahley RW, Assmann G, von Eckardstein A. Endogenous apolipoprotein E modulates cholesterol efflux and cholesteryl ester hydrolysis mediated by high-density lipoprotein-3 and lipid-free apoproteins in mouse peritoneal macrophages. J Mol Med 2000; 78:217-222
- Huang Z, Mazzone T. ApoE-dependent sterol efflux from macrophages is modulated by scavenger receptor class B type I expression. J Lipid Res 2002; 43:375-382
- Zanotti I, Pedrelli M, Poti F, Stomeo G, Gomaraschi M, Calabresi L, Bernini F. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arteriosclerosis, thrombosis, and vascular biology 2011; 31:74-80
- Kim WS, Rahmanto AS, Kamili A, Rye KA, Guillemin GJ, Gelissen IC, Jessup W, Hill AF, Garner B. Role of ABCG1 and ABCA1 in regulation of neuronal cholesterol efflux to apolipoprotein E discs and suppression of amyloid-beta peptide generation. The Journal of biological chemistry 2007; 282:2851-2861
- Lammers B, Out R, Hildebrand RB, Quinn CM, Williamson D, Hoekstra M, Meurs I, Van Berkel TJ, Jessup W, Van Eck M. Independent protective roles for macrophage Abcg1 and Apoe in the atherosclerotic lesion development. Atherosclerosis 2009; 205:420-426
- Chroni A, Nieland T, Kypreos K, Krieger M, Zannis V. SR-BI mediates cholesterol efflux via its interactions with lipid-bound apoE. Structural mutations in SR-BI diminish cholesterol efflux. Biochemistry 2005; 44:13132-13143
- Ito J, Zhang L-Y, Asai M, Yokoyama S. Differential generation of high-density lipoprotein by endogenous and exogenous apolipoproteins in cultured fetal rat astrocytes. J of Neurochemistry 1999; 72:2362-2369
- Lin C, Duan H, Mazzone T. Apolipoprotein E-dependent cholesterol efflux from macrophages:kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein. J Lipid Res 1999; 40:1618-1626
- Bielicki J, McCall M, Forte T. Apolipoprotein A-I promotes cholesterol release and apolipoprotein E recruitment from THP-1 macrophage-like foam cells. J Lipid Res 1999; 40:85-92
- Kruth H, Skarlatos S, Gaynor P, Gamble W. Production of cholesterol-enriched nascent high density lipoproteins by human monocyte-derived macrophages is a mechanism that contributes to macrophage cholesterol efflux. The Journal of biological chemistry 1994; 269:24511-24518
- Zhang W, Gaynor P, Kruth H. Apolipoprotein E produced by human monocyte-derived macrophages mediates cholesterol efflux that occurs in the absence of added cholesterol acceptors. The Journal of biological chemistry 1996; 271:28641-28646
- Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun 2007; 357:319-324
- Ali K, Middleton M, Pure E, Rader DJ. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circulation research 2005; 97:922-927
- Yu S, Duan RS, Chen Z, Quezada HC, Bao L, Nennesmo I, Zhu SW, Winblad B, Ljunggren HG, Zhu J. Increased susceptibility to experimental autoimmune neuritis after upregulation of the autoreactive T cell response to peripheral myelin antigen in apolipoprotein E-deficient mice. J Neuropathol Exp Neurol 2004; 63:120-128
- Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-kappaB-driven inflammation and atherosclerosis. Circulation research 2015; 117:e1-e11
- Maor I, Kaplan M, Hayek T, Vaya J, Hoffman A, Aviram M. Oxidized monocyte-derived macrophages in aortic atherosclerotic lesion from apolipoprotein E-deficient mice and from human carotid artery contain lipid peroxides and oxysterols. Biochem Biophys Res Commun 2000; 269:775-780
- Rosenblat M, Coelman R, Aviram M. Increased macrophage glutathione content reduces cell-mediated oxidation of LDL and atherosclerosis in apolipoprotein E deficient mice. Atheroscler 2002; 163:17-28
- Rosenblat M, Aviram M. Oxysterols-induced activation of macrophage NADPH oxidase enhances cell-mediated oxidation of LDL in the atherosclerotic apolipoprotein E deficient mouse: inhibitory role for vitamin E. Atherosclerosis 2002; 100:69-80
- Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and b-amyloid peptides. Nature Genetics 1996; 14:55-61
- Ishiguro H, Yoshida H, Major AS, Zhu T, Babaev VR, Linton MF, Fazio S. Retrovirus-mediated expression of apolipoprotein A-I in the macrophage protects against atherosclerosis in vivo. The Journal of biological chemistry 2001; 276:36742-36748
- Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 1988; 8:1–21
- Okoro EU, Zhao Y, Guo Z, Zhou L, Lin X, Yang H. Apolipoprotein E4 is deficient in inducing macrophage ABCA1 expression and stimulating the Sp1 signaling pathway. PloS one 2012; 7:e44430
- Cullen P, Cignarella A, Brennhausen B, Mohr S, Assmann G, von Eckardstein A. Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J Clin Invest 1998; 101:1670-1677
- Gong JS, Kobayashi M, Hayashi H, Zou K, Sawamura N, Fujita SC, Yanagisawa K, Michikawa M. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. The Journal of biological chemistry 2002; 277:29919-29926
- Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem 2000; 74:1008-1016
- Huebbe P, Lodge JK, Rimbach G. Implications of apolipoprotein E genotype on inflammation and vitamin E status. Molecular nutrition & food research 2010; 54:623-630
- Utermann G, Hardewig A, Zimmer F. Apolipoprotein E phenotypes in patients with myocardial infarction. Hum Genet 1984; 65:237–241
- Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Annals of internal medicine 2004; 141:137-147
- Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arteriosclerosis, thrombosis, and vascular biology 2012; 32:2813-2820
- Puri R, Nissen SE, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Raichlen JS, St John J, Wolski K, Uno K, Kataoka Y, Nicholls SJ. Factors underlying regression of coronary atheroma with potent statin therapy. European heart journal 2013; 34:1818-1825
- Schwartz SM. The intima : A new soil. Circulation research 1999; 85:877-879
- Raines EW. PDGF and cardiovascular disease. Cytokine & growth factor reviews 2004; 15:237-254
- Johnson JL. Matrix metalloproteinases: influence on smooth muscle cells and atherosclerotic plaque stability. Expert review of cardiovascular therapy 2007; 5:265-282
- Yang X, Liaw L, Prudovsky I, Brooks PC, Vary C, Oxburgh L, Friesel R. Fibroblast growth factor signaling in the vasculature. Current atherosclerosis reports 2015; 17:509
- Molloy CJ, Taylor DS, Pawlowski JE. Novel cardiovascular actions of the activins. The Journal of endocrinology 1999; 161:179-185
- Libby P. Changing concepts of atherogenesis. Journal of internal medicine 2000; 247:349-358
- Bobik A. Transforming growth factor-betas and vascular disorders. Arteriosclerosis, thrombosis, and vascular biology 2006; 26:1712-1720
- Ortiz-Munoz G, Houard X, Martin-Ventura JL, Ishida BY, Loyau S, Rossignol P, Moreno JA, Kane JP, Chalkley RJ, Burlingame AL, Michel JB, Meilhac O. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2009; 23:3129-3139
- Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circulation research 2014; 115:662-667
- Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proceedings of the National Academy of Sciences of the United States of America 2003; 100:13531-13536
- Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nature medicine 2015; 21:628-637
- Argmann CA, Sawyez CG, Li S, Nong Z, Hegele RA, Pickering JG, Huff MW. Human smooth muscle cell subpopulations differentially accumulate cholesteryl ester when exposed to native and oxidized lipoproteins. Arteriosclerosis, thrombosis, and vascular biology 2004; 24:1290-1296
- Zingg JM, Ricciarelli R, Andorno E, Azzi A. Novel 5' exon of scavenger receptor CD36 is expressed in cultured human vascular smooth muscle cells and atherosclerotic plaques. Arteriosclerosis, thrombosis, and vascular biology 2002; 22:412-417
- Chao F, Blanchette-Mackie E, Chen Y, Dickens B, Berlin E, Amende L, Skarlatos S, Gamble W, Resau J, Mergner W, Kruth H. Characterization of two unique cholesterol-rich lipid particles isolated from human atherosclerotic lesions. The American journal of pathology 1990; 136:169-179
- Kruth H. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. The American journal of pathology 1984; 114:201-208
- Minor LK, Rothblat GH, Glick JM. Triglyceride and cholesteryl ester hydrolysis in a cell culture model of smooth muscle foam cells. J Lipid Res 1989; 30:189-197
- Minor LK, Mahlberg FH, Jerome WG, Lewis JC, Rothblat GH, Glick JM. Lysosomal hydrolysis of lipids in a cell culture model of smooth muscle foam cells. Exp Molec Pathol 1991; 54:159-171
- Jerome WG, Minor LK, Glick JM, Rothblat GH, Lewis JC. Lysosomal lipid accumulation in vascular smooth muscle cells. Exp Molec Pathol 1991; 54:144-158
- Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014; 129:1551-1559
- Li Q, Komaba A, Yokoyama S. Cholesterol is poorly available for free apolipoprotein-mediated cellular lipid efflux from smooth muscle cells. Biochemistry 1993; 32:4597-4603
- Goldfischer S, Schiller B, Wolinsky H. Lipid accumulation in smooth muscle cell lysosomes in primate atherosclerosis. The American journal of pathology 1975; 78:497-504
- Haley N, Fowler SD, deDuve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res 1980; 21:961-969
- Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation research 2006; 9:245-255
- Hoppe G, O'Neil J, Hoff HF. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J Clin Invest 1994; 94:1506-1512
- Cox BE, Griffin EE, Ullery JC, Jerome WG. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidification. J Lipid Res 2007; 48:1012-1021
- Yancey PG, Jerome WG. Lysosomal cholesterol derived from mildly oxidized low density lipoprotein is resistant to efflux. J Lipid Res 2001; 42:317-327
- Griffin EE, Ullery JC, Cox BE, Jerome WG. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J Lipid Res 2005; 46:2052-2060
- Yancey PG, Jerome WG. Lysosomal sequestration of free and esterified cholesterol from oxidized low density lipoprotein in macrophages of different species. J Lipid Res 1998; 39:1349-1361
- Suzuki M, Becker L, Pritchard DK, Gharib SA, Wijsman EM, Bammler TK, Beyer RP, Vaisar T, Oram JF, Heinecke JW. Cholesterol accumulation regulates expression of macrophage proteins implicated in proteolysis and complement activation. Arteriosclerosis, thrombosis, and vascular biology 2012; 32:2910-2918
- Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014; 383:1933-1943
- Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest 2012; 122:70-79
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annual review of physiology 2012; 74:13-40
- Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. Journal of interventional cardiology 2002; 15:439-446
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. The New England journal of medicine 2013; 368:2004-2013
- Stegemann C, Drozdov I, Shalhoub J, Humphries J, Ladroue C, Didangelos A, Baumert M, Allen M, Davies AH, Monaco C, Smith A, Xu Q, Mayr M. Comparative lipidomics profiling of human atherosclerotic plaques. Circulation Cardiovascular genetics 2011; 4:232-242
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature reviews Immunology 2010; 10:36-46
- Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ J 2016; 80:2259-2268
- Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. Journal of leukocyte biology 2009; 86:1089-1095
- Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circulation research 2010; 107:839-850
- Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 2007; 116:1226-1233
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab 2006; 3:257-266
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nature medicine 2009; 15:1383-1391
- Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 2010; 12:467-482
- Yvan-Charvet L, Pagler TA, Seimon TA, Thorp E, Welch CL, Witztum JL, Tabas I, Tall AR. ABCA1 and ABCG1 protect against oxidative stress-induced macrophage apoptosis during efferocytosis. Circulation research 2010; 106:1861-1869
- Ben J, Zhang Y, Zhou R, Zhang H, Zhu X, Li X, Zhang H, Li N, Zhou X, Bai H, Yang Q, Li D, Xu Y, Chen Q. Major vault protein regulates class A scavenger receptor-mediated tumor necrosis factor-alpha synthesis and apoptosis in macrophages. The Journal of biological chemistry 2013; 288:20076-20084
- Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol 2005; 171:61-73
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2005; 25:1256-1261
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arteriosclerosis, thrombosis, and vascular biology 2008; 28:1421-1428
- Yancey PG, Ding Y, Fan D, Blakemore JL, Zhang Y, Ding L, Zhang J, Linton MF, Fazio S. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation 2011; 124:454-464
- Tao H, Yancey PG, Babaev VR, Blakemore JL, Zhang Y, Ding L, Fazio S, Linton MF. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J Lipid Res 2015; 56:1449-1460
- Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends in cell biology 2006; 16:189-197
- Yancey PG, Blakemore J, Ding L, Fan D, Overton CD, Zhang Y, Linton MF, Fazio S. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arteriosclerosis, thrombosis, and vascular biology 2010; 30:787-795
- Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol 2004; 173:6366-6375
- Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. European journal of immunology 2011; 41:2515-2518
- Yurdagul A, Jr., Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med 2017; 4:86
- Cash JG, Kuhel DG, Basford JE, Jaeschke A, Chatterjee TK, Weintraub NL, Hui DY. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. The Journal of biological chemistry 2012; 287:27876-27884
- Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. The Journal of biological chemistry 2006; 281:6707-6717
- Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016; 536:86-90
- Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest 2014; 124:1083-1097
- Geng YJ, Henderson LE, Levesque EB, Muszynski M, Libby P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology 1997; 17:2200-2208
- Boyle JJ, Weissberg PL, Bennett MR. Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arteriosclerosis, thrombosis, and vascular biology 2003; 23:1553-1558
- Lee JY, Jung GY, Heo HJ, Yun MR, Park JY, Bae SS, Hong KW, Lee WS, Kim CD. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicology letters 2006; 166:212-221
- Fruhwirth GO, Moumtzi A, Loidl A, Ingolic E, Hermetter A. The oxidized phospholipids POVPC and PGPC inhibit growth and induce apoptosis in vascular smooth muscle cells. Biochimica et biophysica acta 2006; 1761:1060-1069
- Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arteriosclerosis, thrombosis, and vascular biology 2015; 35:535-546
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998; 101:890-898
- Quillard T, Araujo HA, Franck G, Tesmenitsky Y, Libby P. Matrix metalloproteinase-13 predominates over matrix metalloproteinase-8 as the functional interstitial collagenase in mouse atheromata. Arteriosclerosis, thrombosis, and vascular biology 2014; 34:1179-1186
- Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proceedings of the National Academy of Sciences of the United States of America 1995; 92:402-406
- Schneider F, Sukhova GK, Aikawa M, Canner J, Gerdes N, Tang SM, Shi GP, Apte SS, Libby P. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation 2008; 117:931-939
- Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2004; 24:1359-1366
- Nofer JR, Junker R, Pulawski E, Fobker M, Levkau B, von Eckardstein A, Seedorf U, Assmann G, Walter M. High density lipoproteins induce cell cycle entry in vascular smooth muscle cells via mitogen activated protein kinase-dependent pathway. Thrombosis and haemostasis 2001; 85:730-735
- Muller C, Salvayre R, Negre-Salvayre A, Vindis C. Oxidized LDLs trigger endoplasmic reticulum stress and autophagy: prevention by HDLs. Autophagy 2011; 7:541-543
- Niculescu LS, Sanda GM, Sima AV. HDL inhibits endoplasmic reticulum stress by stimulating apoE and CETP secretion from lipid-loaded macrophages. Biochem Biophys Res Commun 2013; 434:173-178
- Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:7166-7171
- Fredman G, Tabas I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. The American journal of pathology 2017; 187:1211-1221
- Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature communications 2016; 7:12859
- Rinne P, Guillamat-Prats R, Rami M, Bindila L, Ring L, Lyytikainen LP, Raitoharju E, Oksala N, Lehtimaki T, Weber C, van der Vorst EPC, Steffens S. Palmitoylethanolamide Promotes a Proresolving Macrophage Phenotype and Attenuates Atherosclerotic Plaque Formation. Arteriosclerosis, thrombosis, and vascular biology 2018; 38:2562-2575
- Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ Res 2016; 119:1030-1038
- Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokzhad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Science translational medicine 2015; 7:275ra220
- Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, Milton J, Carvalho Leitao R, Rosa Fernandes L, Hasan M, Gao H, Nguyen V, Harris J, Tabas I, Farokhzad OC. Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS nano 2016; 10:5280-5292
- Proto JD, Doran AC, Gusarova G, Yurdagul A, Jr., Sozen E, Subramanian M, Islam MN, Rymond CC, Du J, Hook J, Kuriakose G, Bhattacharya J, Tabas I. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018; 49:666-677 e666
- Fazio S, Linton MF. Regulation and Clearance of Apoliprotein B-Containing Lipoproteins. In: Ballantyne CM, ed. Clinical Lipidology, a companion to Braunwald’s Heart Disease. Vol Second Edition: Elsevier Saunders; 2015:11-24.
- Anant S, Davidson NO. Identification and regulation of protein components of the apolipoprotein B mRNA editing enzyme. A complex event. Trends in cardiovascular medicine 2002; 12:311-317
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. Vol II. 7th ed. New York: McGraw-Hill; 1995:1981-2030.
- Raffai RL, Hasty AH, Wang Y, Mettler SE, Sanan DA, Linton MF, Fazio S, Weisgraber KH. Hepatocyte-derived ApoE is more effective than non-hepatocyte-derived ApoE in remnant lipoprotein clearance. Journal of Biological Chemistry 2003; 278:11670-11675
- AnitschkowNNC, S. Ueber experimentelle Cholesterinsteatose und ihre Bedeutung fur die Entstehung einiger pathologischer Prozesse. Zentralbl Allg Pathol 1913; 24:1-9
- Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J, 3rd. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Annals of internal medicine 1961; 55:33-50
- Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356 222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). J Am Med Assoc 1986; 256:2823-2828
- Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. Jama 1986; 256:2835-2838
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber RT. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med 1977; 62:707-714
- Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, Kromhout D, Nedeljkovic S, Punsar S, Seccareccia F, Toshima H. The diet and 15-year death rate in the Seven Countries Study. Am J Epidemiol 1986; 124:903-915
- Steinberg D. In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J Lipid Res 2013; 54:2946-2949
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arteriosclerosis, thrombosis, and vascular biology 1995; 15:551-561
- Camejo G, Lalaguna F, Lopez F, Starosta R. Characterization and properties of a lipoprotein-complexing proteoglycan from human aorta. Atherosclerosis 1980; 35:307-320
- Iverius PH. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. The Journal of biological chemistry 1972; 247:2607-2613
- Fogelstrand P, Boren J. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutrition, metabolism, and cardiovascular diseases : NMCD 2012; 22:1-7
- Boren J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest 1998; 101:2658-2664
- Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002; 417:750-754
- Flood C, Gustafsson M, Richardson PE, Harvey SC, Segrest JP, Boren J. Identification of the proteoglycan binding site in apolipoprotein B48. The Journal of biological chemistry 2002; 277:32228-32233
- Flood C, Gustafsson M, Pitas RE, Arnaboldi L, Walzem RL, Boren J. Molecular mechanism for changes in proteoglycan binding on compositional changes of the core and the surface of low-density lipoprotein-containing human apolipoprotein B100. Arteriosclerosis, thrombosis, and vascular biology 2004; 24:564-570
- Olivecrona G, Olivecrona T. Triglyceride lipases and atherosclerosis. Curr Opin Lipidol 1995; 6:291-305
- Merkel M, Kako Y, Radner H, Cho IS, Ramasamy R, Brunzell JD, Goldberg IJ, Breslow JL. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proceedings of the National Academy of Sciences of the United States of America 1998; 95:13841-13846
- Babaev V, Fazio S, Gleaves L, Carter K, Semenkovich C, Linton M. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest 1999; 103:1697-1705
- Babaev V, Patel M, Semenkovich C, Fazio S, Linton M. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. The Journal of biological chemistry 2000; 275:26293-26299
- Tabas I, Li Y, Brocia RW, Xu SW, Swenson TL, Williams KJ. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. The Journal of biological chemistry 1993; 268:20419-20432
- Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arteriosclerosis, thrombosis, and vascular biology 2008; 28:1723-1730
- Oorni K, Hakala JK, Annila A, Ala-Korpela M, Kovanen PT. Sphingomyelinase induces aggregation and fusion, but phospholipase A2 only aggregation, of low density lipoprotein (LDL) particles. Two distinct mechanisms leading to increased binding strength of LDL to human aortic proteoglycans. The Journal of biological chemistry 1998; 273:29127-29134
- Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. The Journal of biological chemistry 1997; 272:13597-13607
- Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, Hilaire AS, Prescott SM, Zimmerman GA, McIntyre TM. Oxidized Alkyl Phospholipids Are Specific, High Affinity Peroxisome Proliferator-activated Receptor γ Ligands and Agonists. The Journal of biological chemistry 2001; 276:16015-16023
- Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. The Journal of biological chemistry 2002; 277:7271-7281
- Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ, 2nd. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proceedings of the National Academy of Sciences of the United States of America 1992; 89:10721-10725
- Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proceedings of the National Academy of Sciences of the United States of America 1989; 86:1372-1376
- Brame CJ, Salomon RG, Morrow JD, Roberts LJ, 2nd. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. The Journal of biological chemistry 1999; 274:13139-13146
- Fogelman AM, Shechter I, Seager J, Hokom M, Child JS, Edwards PA. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci USA 1980; 77:2214-2218
- Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res 2008; 49:2230-2239
- Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol 2003; 41:360-370
- Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers in medicine 2011; 5:673-694
- Proudfoot JM, Barden AE, Loke WM, Croft KD, Puddey IB, Mori TA. HDL is the major lipoprotein carrier of plasma F2-isoprostanes. J Lipid Res 2009; 50:716-722
- Tsimikas S, Witztum JL, Miller ER, Sasiela WJ, Szarek M, Olsson AG, Schwartz GG, Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study I. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 2004; 110:1406-1412
- Rodenburg J, Vissers MN, Wiegman A, Miller ER, Ridker PM, Witztum JL, Kastelein JJ, Tsimikas S. Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J Am Coll Cardiol 2006; 47:1803-1810
- Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, Xu Q, Bergmark C, Weger S, Oberhollenzer F, Witztum JL. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol 2006; 47:2219-2228
- Tanaga K, Bujo H, Inoue M, Mikami K, Kotani K, Takahashi K, Kanno T, Saito Y. Increased circulating malondialdehyde-modified LDL levels in patients with coronary artery diseases and their association with peak sizes of LDL particles. Arteriosclerosis, thrombosis, and vascular biology 2002; 22:662-666
- Wang J, Hu B, Meng Y, Zhang C, Li K, Hui C. The level of malondialdehyde-modified LDL and LDL immune complexes in patients with rheumatoid arthritis. Clin Biochem 2009; 42:1352-1357
- Kondo A, Morita H, Nakamura H, Kotani K, Kobori K, Ito S, Manabe M, Saito K, Kanno T, Maekawa M. Influence of fibrate treatment on malondialdehyde-modified LDL concentration. Clin Chim Acta 2004; 339:97-103
- Esterbauer H, Rotheneder M, Striegel G, Waeg G, Ashy A, Sattler W, Jurgens G. Vitamin E and other lipophilic antioxidants protect LDL against oxidation. Fat Sci Technol 1989; 8:316-324
- Hussein O, Rosenblat M, Refael G, Aviram M. Dietary selenium increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low-density lipoprotein in kidney transplant recipients. Transplantation 1997; 63:679-685
- Guo Z, Van Remmen H, Yang H, Chen X, Mele J, Vijg J, Epstein CJ, Ho YS, Richardson A. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arteriosclerosis, thrombosis, and vascular biology 2001; 21:1131-1138
- Steinbrecher UP, Pritchard PH. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res 1989; 30:305-315
- Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, Hooper S, Le Trong H, Cousens LS, Zimmerman GA, Yamada Y, McIntyre TM, Prescott SM, Gray PW. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature 1995; 374:549-553
- Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995; 96:2882-2891
- Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2001; 21:473-480
- Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 1997; 99:2075-2081
- George J, Struthers AD. Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vascular health and risk management 2009; 5:265-272
- Aviram M, Rosenblat M, Etzioni A, Levy R. Activation of NADPH oxidase required for macrophage-mediated oxidation of low-density lipoprotein. Metabolism: clinical and experimental 1996; 45:1069-1079
- Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life sciences 2006; 79:525-531
- Upston JM, Neuzil J, Stocker R. Oxidation of LDL by recombinant human 15-lipoxygenase: evidence for alpha-tocopherol-dependent oxidation of esterified core and surface lipids. J Lipid Res 1996; 37:2650-2661
- Ezaki M, Witztum JL, Steinberg D. Lipoperoxides in LDL incubated with fibroblasts that overexpress 15-lipoxygenase. J Lipid Res 1995; 36:1996-2004
- Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest 2001; 108:1759-1770
- Pignatelli P, Loffredo L, Martino F, Catasca E, Carnevale R, Zanoni C, Del Ben M, Antonini R, Basili S, Violi F. Myeloperoxidase overexpression in children with hypercholesterolemia. Atherosclerosis 2009; 205:239-243
- Puntoni M, Sbrana F, Bigazzi F, Minichilli F, Ferdeghini E, Sampietro T. Myeloperoxidase modulation by LDL apheresis in familial hypercholesterolemia. Lipids in health and disease 2011; 10:185
- Karakas M, Koenig W, Zierer A, Herder C, Rottbauer W, Baumert J, Meisinger C, Thorand B. Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg study. Journal of internal medicine 2012; 271:43-50
- Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation 2010; 122:2505-2513
- Michowitz Y, Kisil S, Guzner-Gur H, Rubinstein A, Wexler D, Sheps D, Keren G, George J. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart failure. The Israel Medical Association journal : IMAJ 2008; 10:884-888
- Dolley G, Lamarche B, Despres JP, Bouchard C, Perusse L, Vohl MC. Myeloperoxidase gene sequence variations are associated with low-density-lipoprotein characteristics. Journal of human genetics 2008; 53:439-446
- Nikpoor B, Turecki G, Fournier C, Theroux P, Rouleau GA. A functional myeloperoxidase polymorphic variant is associated with coronary artery disease in French-Canadians. American heart journal 2001; 142:336-339
- Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. The Journal of biological chemistry 2002; 277:38503-38516
- Sokolov AV, Chekanov AV, Kostevich VA, Aksenov DV, Vasilyev VB, Panasenko OM. Revealing binding sites for myeloperoxidase on the surface of human low density lipoproteins. Chem Phys Lipids 2011; 164:49-53
- Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X, Fu X, Wagner MA, Besler C, Gerstenecker G, Zhang R, Li XM, DiDonato AJ, Gogonea V, Tang WH, Smith JD, Plow EF, Fox PL, Shih DM, Lusis AJ, Fisher EA, DiDonato JA, Landmesser U, Hazen SL. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 2013; 123:3815-3828
- May-Zhang LS, Yermalitsky V, Huang J, Pleasent T, Borja MS, Oda MN, Jerome WG, Yancey PG, Linton MF, Davies SS. Modification by isolevuglandins, highly reactive gamma-ketoaldehydes, deleteriously alters high-density lipoprotein structure and function. J Biol Chem 2018; 293:9176-9187
- Guo L, Chen Z, Amarnath V, Davies SS. Identification of novel bioactive aldehyde-modified phosphatidylethanolamines formed by lipid peroxidation. Free Radic Biol Med 2012; 53:1226-1238
- Shao B, Pennathur S, Pagani I, Oda MN, Witztum JL, Oram JF, Heinecke JW. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem 2010; 285:18473-18484
- Noguchi N, Nakano K, Aratani Y, Koyama H, Kodama T, Niki E. Role of myeloperoxidase in the neutrophil-induced oxidation of low density lipoprotein as studied by myeloperoxidase-knockout mouse. Journal of biochemistry 2000; 127:971-976
- Rausch PG, Moore TG. Granule enzymes of polymorphonuclear neutrophils: A phylogenetic comparison. Blood 1975; 46:913-919
- McMillen TS, Heinecke JW, LeBoeuf RC. Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 2005; 111:2798-2804
- Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest 2001; 107:419-430
- Liu C, Desikan R, Ying Z, Gushchina L, Kampfrath T, Deiuliis J, Wang A, Xu X, Zhong J, Rao X, Sun Q, Maiseyeu A, Parthasarathy S, Rajagopalan S. Effects of a novel pharmacologic inhibitor of myeloperoxidase in a mouse atherosclerosis model. PloS one 2012; 7:e50767
- Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice [see comments]. Journal of Clinical Investigation 1999; 103:1597-1604
- Assimes TL, Knowles JW, Priest JR, Basu A, Borchert A, Volcik KA, Grove ML, Tabor HK, Southwick A, Tabibiazar R, Sidney S, Boerwinkle E, Go AS, Iribarren C, Hlatky MA, Fortmann SP, Myers RM, Kuhn H, Risch N, Quertermous T. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis 2008; 198:136-144
- McGeachie M, Ramoni RL, Mychaleckyj JC, Furie KL, Dreyfuss JM, Liu Y, Herrington D, Guo X, Lima JA, Post W, Rotter JI, Rich S, Sale M, Ramoni MF. Integrative predictive model of coronary artery calcification in atherosclerosis. Circulation 2009; 120:2448-2454
- Burdon KP, Rudock ME, Lehtinen AB, Langefeld CD, Bowden DW, Register TC, Liu Y, Freedman BI, Carr JJ, Hedrick CC, Rich SS. Human lipoxygenase pathway gene variation and association with markers of subclinical atherosclerosis in the diabetes heart study. Mediators of inflammation 2010; 2010:170153
- Jurgens G, Hoff HF, Chisolm GM, 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation--characterization and pathophysiological implications. Chemistry and physics of lipids 1987; 45:315-336
- Greaves DR, Gough PJ, Gordon S. Recent progress in defining the role of scavenger receptors in lipid transport, atherosclerosis and host defence. Current opinion in lipidology 1998; 9:425-432
- van Berkel TJ, Fluiter K, van Velzen AG, Vogelezang CJ, Ziere GJ. LDL receptor-independent and -dependent uptake of lipoproteins. Atherosclerosis 1995; 118 Suppl:S43-50
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA 1979; 76:333-337
- Brown MS, Goldstein JL. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci USA 1979; 76:3330–3337
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986; 232:34-47
- Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2003; 27:S35-40
- Greig FH, Kennedy S, Spickett CM. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation. Free Radic Biol Med 2012; 52:266-280
- Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calo LA. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators of inflammation 2013; 2013:714653
- Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Current opinion in lipidology 2003; 14:437-445
- Takei A, Huang Y, Lopes-Virella MF. Expression of adhesion molecules by human endothelial cells exposed to oxidized low density lipoprotein. Influences of degree of oxidation and location of oxidized LDL. Atherosclerosis 2001; 154:79-86
- Vielma SA, Mironova M, Ku JR, Lopes-Virella MF. Oxidized LDL further enhances expression of adhesion molecules in Chlamydophila pneumoniae-infected endothelial cells. J Lipid Res 2004; 45:873-880
- Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest 1995; 95:1262-1270
- Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proceedings of the National Academy of Sciences of the United States of America 1999; 96:12010-12015
- Vora DK, Fang ZT, Liva SM, Tyner TR, Parhami F, Watson AD, Drake TA, Territo MC, Berliner JA. Induction of P-selectin by oxidized lipoproteins. Separate effects on synthesis and surface expression. Circulation research 1997; 80:810-818
- Berliner JA, Schwartz DS, Territo MC, Andalibi A, Almada L, Lusis AJ, Quismorio D, Fang ZP, Fogelman AM. Induction of chemotactic cytokines by minimally oxidized LDL. Adv Exp Med Biol 1993; 351:13-18
- Parhami F, Fang ZT, Fogelman AM, Andalibi A, Territo MC, Berliner JA. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest 1993; 92:471-478
- Liu A, Ming JY, Fiskesund R, Ninio E, Karabina SA, Bergmark C, Frostegard AG, Frostegard J. Induction of dendritic cell-mediated T-cell activation by modified but not native low-density lipoprotein in humans and inhibition by annexin a5: involvement of heat shock proteins. Arteriosclerosis, thrombosis, and vascular biology 2015; 35:197-205
- Horkko S, Binder CJ, Shaw PX, Chang MK, Silverman G, Palinski W, Witztum JL. Immunological responses to oxidized LDL. Free Radic Biol Med 2000; 28:1771-1779
- Huang YH, Ronnelid J, Frostegard J. Oxidized LDL induces enhanced antibody formation and MHC class II-dependent IFN-gamma production in lymphocytes from healthy individuals. Arteriosclerosis, thrombosis, and vascular biology 1995; 15:1577-1583
- Oinuma T, Yamada T, Sakurai I. Effects of copper-zinc type superoxide dismutase on the proliferation and migration of cultured vascular smooth muscle cells induced by oxidized low density lipoprotein. Journal of atherosclerosis and thrombosis 1997; 4:79-84
- Liu J, Ren Y, Kang L, Zhang L. Oxidized low-density lipoprotein increases the proliferation and migration of human coronary artery smooth muscle cells through the upregulation of osteopontin. International journal of molecular medicine 2014; 33:1341-1347
- Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N, Owens GK. Oxidized phospholipids induce type VIII collagen expression and vascular smooth muscle cell migration. Circulation research 2009; 104:609-618
- Kiyan Y, Tkachuk S, Hilfiker-Kleiner D, Haller H, Fuhrman B, Dumler I. oxLDL induces inflammatory responses in vascular smooth muscle cells via urokinase receptor association with CD36 and TLR4. J Mol Cell Cardiol 2014; 66:72-82
- Jovinge S, Ares MP, Kallin B, Nilsson J. Human monocytes/macrophages release TNF-alpha in response to Ox-LDL. Arteriosclerosis, thrombosis, and vascular biology 1996; 16:1573-1579
- Frostegard J, Huang YH, Ronnelid J, Schafer-Elinder L. Platelet-activating factor and oxidized LDL induce immune activation by a common mechanism. Arteriosclerosis, thrombosis, and vascular biology 1997; 17:963-968
- Terkeltaub R, Banka CL, Solan J, Santoro D, Brand K, Curtiss LK. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler Thromb 1994; 14:47-53
- Wang GP, Deng ZD, Ni J, Qu ZL. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophages. Atherosclerosis 1997; 133:31-36
- Seo JW, Yang EJ, Yoo KH, Choi IH. Macrophage Differentiation from Monocytes Is Influenced by the Lipid Oxidation Degree of Low Density Lipoprotein. Mediators of inflammation 2015; 2015:235797
- Sedgwick JB, Hwang YS, Gerbyshak HA, Kita H, Busse WW. Oxidized low-density lipoprotein activates migration and degranulation of human granulocytes. Am J Respir Cell Mol Biol 2003; 29:702-709
- Hashimoto K, Kataoka N, Nakamura E, Tsujioka K, Kajiya F. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis 2007; 194:e9-17
- Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest 1997; 100:1209-1216
- Han KH, Hong KH, Ko J, Rhee KS, Hong MK, Kim JJ, Kim YH, Park SJ. Lysophosphatidylcholine up-regulates CXCR4 chemokine receptor expression in human CD4 T cells. Journal of leukocyte biology 2004; 76:195-202
- Ramkhelawon B, Yang Y, van Gils JM, Hewing B, Rayner KJ, Parathath S, Guo L, Oldebeken S, Feig JL, Fisher EA, Moore KJ. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arteriosclerosis, thrombosis, and vascular biology 2013; 33:1180-1188
- Baird SK, Hampton MB, Gieseg SP. Oxidized LDL triggers phosphatidylserine exposure in human monocyte cell lines by both caspase-dependent and -independent mechanisms. FEBS letters 2004; 578:169-174
- Reid VC, Hardwick SJ, Mitchinson MJ. Fragmentation of DNA in P388D1 macrophages exposed to oxidised low-density lipoprotein. FEBS letters 1993; 332:218-220
- Hardwick SJ, Hegyi L, Clare K, Law NS, Carpenter KL, Mitchinson MJ, Skepper JN. Apoptosis in human monocyte-macrophages exposed to oxidized low density lipoprotein. The Journal of pathology 1996; 179:294-302
- Selley ML, Bartlett MR, Czeti AL, Ardlie NG. The role of (E)-4-hydroxy-2-nonenal in platelet activation by low density lipoprotein and iron. Atherosclerosis 1998; 140:105-112
- Chen R, Chen X, Salomon RG, McIntyre TM. Platelet activation by low concentrations of intact oxidized LDL particles involves the PAF receptor. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:363-371
- Magwenzi S, Woodward C, Wraith KS, Aburima A, Raslan Z, Jones H, McNeil C, Wheatcroft S, Yuldasheva N, Febbriao M, Kearney M, Naseem KM. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015; 125:2693-2703
- Wraith KS, Magwenzi S, Aburima A, Wen Y, Leake D, Naseem KM. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood 2013; 122:580-589
- Zhang YC, Tang Y, Chen Y, Huang XH, Zhang M, Chen J, Sun YG, Li YG. Oxidized low-density lipoprotein and C-reactive protein have combined utility for better predicting prognosis after acute coronary syndrome. Cell biochemistry and biophysics 2014; 68:379-385
- Ho YK, Faust JR, Bilheimer DW, Brown MS, Goldstein JL. Regulation of cholesterol synthesis by low density lipoprotein in isolated human lymphocytes. Comparison of cells from normal subjects and patients with homozygous familial hypercholesterolemia and abetalipoproteinemia. The Journal of experimental medicine 1977; 145:1531-1549
- Parthasarathy S, Fong L, Otero D, Steinberg D. Recognition of solubilized apoproteins from delipidated, oxidized low density lipoprotein (LDL) by the acetyl-LDL receptor. Proc Natl Acad Sci USA 1987; 84:537-540
- Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains a-helical and collagen-like coiled coils. Nature 1990; 343:531-535
- Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arteriosclerosis, Thrombosis & Vascular Biology (Online) 2000; 20:2593-2599
- Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cellular and molecular life sciences : CMLS 1998; 54:628-640
- Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. The Journal of biological chemistry 2002; 277:49982-49988
- Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res 2005; 46:969-976
- Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol 2005; 170:477-485
- Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovascular research 2008; 78:185-196
- Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest 2005; 115:2192-2201
- Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:19-26
- Chavez-Sanchez L, Madrid-Miller A, Chavez-Rueda K, Legorreta-Haquet MV, Tesoro-Cruz E, Blanco-Favela F. Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Human immunology 2010; 71:737-744
- Kadl A, Sharma PR, Chen W, Agrawal R, Meher AK, Rudraiah S, Grubbs N, Sharma R, Leitinger N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic Biol Med 2011; 51:1903-1909
- Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circulation research 2009; 104:1355-1363
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 2010; 11:155-161
- Karper JC, Ewing MM, Habets KL, de Vries MR, Peters EA, van Oeveren-Rietdijk AM, de Boer HC, Hamming JF, Kuiper J, Kandimalla ER, La Monica N, Jukema JW, Quax PH. Blocking toll-like receptors 7 and 9 reduces postinterventional remodeling via reduced macrophage activation, foam cell formation, and migration. Arteriosclerosis, thrombosis, and vascular biology 2012; 32:e72-80
- von Schlieffen E, Oskolkova OV, Schabbauer G, Gruber F, Bluml S, Genest M, Kadl A, Marsik C, Knapp S, Chow J, Leitinger N, Binder BR, Bochkov VN. Multi-hit inhibition of circulating and cell-associated components of the toll-like receptor 4 pathway by oxidized phospholipids. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:356-362
- Bluml S, Kirchberger S, Bochkov VN, Kronke G, Stuhlmeier K, Majdic O, Zlabinger GJ, Knapp W, Binder BR, Stockl J, Leitinger N. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol 2005; 175:501-508
- Sun L, Ishida T, Yasuda T, Kojima Y, Honjo T, Yamamoto Y, Yamamoto H, Ishibashi S, Hirata K, Hayashi Y. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovascular research 2009; 82:371-381
- Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:13043-13048
- van Tits L, de Graaf J, Toenhake H, van Heerde W, Stalenhoef A. C-reactive protein and annexin A5 bind to distinct sites of negatively charged phospholipids present in oxidized low-density lipoprotein. Arteriosclerosis, thrombosis, and vascular biology 2005; 25:717-722
- Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996; 98:800-814
- Horkko S, Miller E, Dudl E, Reaven P, Curtiss LK, Zvaifler NJ, Terkeltaub R, Pierangeli SS, Branch DW, Palinski W, Witztum JL. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids. Recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J Clin Invest 1996; 98:815-825
- Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, Prescott SM. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest 1995; 96:2322-2330
- Smiley PL, Stremler KE, Prescott SM, Zimmerman GA, McIntyre TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. The Journal of biological chemistry 1991; 266:11104-11110
- Marathe GK, Davies SS, Harrison KA, Silva AR, Murphy RC, Castro-Faria-Neto H, Prescott SM, Zimmerman GA, McIntyre TM. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. The Journal of biological chemistry 1999; 274:28395-28404
- Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circulation research 2006; 98:642-650
- Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem 2002; 83:463-470
- Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circulation research 2009; 104:978-986
- Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, Nagy L. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circulation research 2000; 87:516-521
- Yeh M, Gharavi NM, Choi J, Hsieh X, Reed E, Mouillesseaux KP, Cole AL, Reddy ST, Berliner JA. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. The Journal of biological chemistry 2004; 279:30175-30181
- Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arteriosclerosis, thrombosis, and vascular biology 2010; 30:1007-1013
- Jyrkkanen HK, Kansanen E, Inkala M, Kivela AM, Hurttila H, Heinonen SE, Goldsteins G, Jauhiainen S, Tiainen S, Makkonen H, Oskolkova O, Afonyushkin T, Koistinaho J, Yamamoto M, Bochkov VN, Yla-Herttuala S, Levonen AL. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circulation research 2008; 103:e1-9
- Frei B. Ascorbic acid protects lipids in human plasma and low-density lipoprotein against oxidative damage. Am J Clin Nutr 1991; 54:1113S-1118S
- Babaev VR, Li L, Shah S, Fazio S, Linton MF, May JM. Combined vitamin C and vitamin E deficiency worsens early atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 2010; 30:1751-1757
- Frikke-Schmidt H, Lykkesfeldt J. Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin Pharmacol Toxicol 2009; 104:419-433
- May JM, Harrison FE. Role of vitamin C in the function of the vascular endothelium. Antioxidants & redox signaling 2013; 19:2068-2083
- Crawford RS, Kirk EA, Rosenfeld ME, LeBoeuf RC, Chait A. Dietary antioxidants inhibit development of fatty streak lesions in the LDL receptor-deficient mouse. Arteriosclerosis, thrombosis, and vascular biology 1998; 18:1506-1513
- Black TM, Wang P, Maeda N, Coleman RA. Palm tocotrienols protect ApoE +/- mice from diet-induced atheroma formation. J Nutr 2000; 130:2420-2426
- Witting PK, Pettersson K, Letters J, Stocker R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic Biol Med 2000; 29:295-305
- Peluzio MC, Homem AP, Cesar GC, Azevedo GS, Amorim R, Cara DC, Saliba H, Vieira EC, Arantes RE, Alvarez-Leite J. Influences of alpha-tocopherol on cholesterol metabolism and fatty streak development in apolipoprotein E-deficient mice fed an atherogenic diet. Braz J Med Biol Res 2001; 34:1539-1545
- Suarna C, Wu BJ, Choy K, Mori T, Croft K, Cynshi O, Stocker R. Protective effect of vitamin E supplements on experimental atherosclerosis is modest and depends on preexisting vitamin E deficiency. Free Radic Biol Med 2006; 41:722-730
- Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Lakka TA, Rissanen T, Leskinen L, Tuomainen TP, Valkonen VP, Ristonmaa U, Poulsen HE. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. Journal of internal medicine 2000; 248:377-386
- Azen SP, Qian D, Mack WJ, Sevanian A, Selzer RH, Liu CR, Liu CH, Hodis HN. Effect of supplementary antioxidant vitamin intake on carotid arterial wall intima-media thickness in a controlled clinical trial of cholesterol lowering. Circulation 1996; 94:2369-2372
- Levy AP, Friedenberg P, Lotan R, Ouyang P, Tripputi M, Higginson L, Cobb FR, Tardif JC, Bittner V, Howard BV. The effect of vitamin therapy on the progression of coronary artery atherosclerosis varies by haptoglobin type in postmenopausal women. Diabetes Care 2004; 27:925-930
- Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens 2007; 20:392-397
- Pruthi S, Allison TG, Hensrud DD. Vitamin E supplementation in the prevention of coronary heart disease. Mayo Clin Proc 2001; 76:1131-1136
- Tornwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK. Alpha-tocopherol (vitamin E) and beta-carotene supplementation does not affect the risk for large abdominal aortic aneurysm in a controlled trial. Atherosclerosis 2001; 157:167-173
- Kinlay S, Behrendt D, Fang JC, Delagrange D, Morrow J, Witztum JL, Rifai N, Selwyn AP, Creager MA, Ganz P. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. J Am Coll Cardiol 2004; 43:629-634
- Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, de Lemos JA, Jialal I. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr 2007; 86:1392-1398
- Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetiere P, Hercberg S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arteriosclerosis, thrombosis, and vascular biology 2004; 24:1485-1491
- Traber MG, Frei B, Beckman JS. Vitamin E revisited: do new data validate benefits for chronic disease prevention? Current opinion in lipidology 2008; 19:30-38
- Roberts LJ, 2nd, Traber MG, Frei B. Vitamins E and C in the prevention of cardiovascular disease and cancer in men. Free Radic Biol Med 2009; 46:1558
- Roberts LJ, 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med 2007; 43:1388-1393
- Michels AJ, Frei B. Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients 2013; 5:5161-5192
- Khanna S, Heigel M, Weist J, Gnyawali S, Teplitsky S, Roy S, Sen CK, Rink C. Excessive alpha-tocopherol exacerbates microglial activation and brain injury caused by acute ischemic stroke. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2015; 29:828-836
- Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling 2011; 15:1957-1997
- Park JG, Yoo JY, Jeong SJ, Choi JH, Lee MR, Lee MN, Hwa Lee J, Kim HC, Jo H, Yu DY, Kang SW, Rhee SG, Lee MH, Oh GT. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circulation research 2011; 109:739-749
- Winter JP, Gong Y, Grant PJ, Wild CP. Glutathione peroxidase 1 genotype is associated with an increased risk of coronary artery disease. Coron Artery Dis 2003; 14:149-153
- Nemoto M, Nishimura R, Sasaki T, Hiki Y, Miyashita Y, Nishioka M, Fujimoto K, Sakuma T, Ohashi T, Fukuda K, Eto Y, Tajima N. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol 2007; 6:23
- Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, Nanjo K. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes 2004; 53:2455-2460
- Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 2007; 27:850-857
- Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation 2007; 115:2178-2187
- Guo Z, Ran Q, Roberts LJ, 2nd, Zhou L, Richardson A, Sharan C, Wu D, Yang H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic Biol Med 2008; 44:343-352
- Butterfield LH, Merino A, Golub SH, Shau H. From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxidants & redox signaling 1999; 1:385-402
- Kisucka J, Chauhan AK, Patten IS, Yesilaltay A, Neumann C, Van Etten RA, Krieger M, Wagner DD. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circulation research 2008; 103:598-605
- Park JG, Oh GT. The role of peroxidases in the pathogenesis of atherosclerosis. BMB Rep 2011; 44:497-505
- Guo X, Yamada S, Tanimoto A, Ding Y, Wang KY, Shimajiri S, Murata Y, Kimura S, Tasaki T, Nabeshima A, Watanabe T, Kohno K, Sasaguri Y. Overexpression of peroxiredoxin 4 attenuates atherosclerosis in apolipoprotein E knockout mice. Antioxidants & redox signaling 2012; 17:1362-1375
- Phelan SA, Wang X, Wallbrandt P, Forsman-Semb K, Paigen B. Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med 2003; 35:1110-1120
- Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxidants & redox signaling 2011; 15:1583-1606
- Shuvalova YA, Kaminnyi AI, Meshkov AN, Shirokov RO, Samko AN. Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Mol Cell Biochem 2012; 370:241-249
- Tribble DL, Gong EL, Leeuwenburgh C, Heinecke JW, Carlson EL, Verstuyft JG, Epstein CJ. Fatty streak formation in fat-fed mice expressing human copper-zinc superoxide dismutase. Arteriosclerosis, thrombosis, and vascular biology 1997; 17:1734-1740
- Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circulation research 2004; 95:1075-1081
- Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation 2002; 106:544-549
- Sentman ML, Brannstrom T, Westerlund S, Laukkanen MO, Yla-Herttuala S, Basu S, Marklund SL. Extracellular superoxide dismutase deficiency and atherosclerosis in mice. Arteriosclerosis, thrombosis, and vascular biology 2001; 21:1477-1482
- Chen H, Yu M, Li M, Zhao R, Zhu Q, Zhou W, Lu M, Lu Y, Zheng T, Jiang J, Zhao W, Xiang K, Jia W, Liu L. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol Cell Biochem 2012; 363:85-91
- Santl Letonja M, Letonja M, Ikolajevic-Starcevic JN, Petrovic D. Association of manganese superoxide dismutase and glutathione S-transferases genotypes with carotid atherosclerosis in patients with diabetes mellitus type 2. Int Angiol 2012; 31:33-41
- Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy 2009; 23:73-83
- Karabina SA, Liapikos TA, Grekas G, Goudevenos J, Tselepis AD. Distribution of PAF-acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochimica et biophysica acta 1994; 1213:34-38
- Stremler KE, Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. The Journal of biological chemistry 1989; 264:5331-5334
- Kriska T, Marathe GK, Schmidt JC, McIntyre TM, Girotti AW. Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides. The Journal of biological chemistry 2007; 282:100-108
- Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ, 2nd. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. The Journal of biological chemistry 2006; 281:4616-4623
- Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arteriosclerosis, thrombosis, and vascular biology 2005; 25:923-931
- Cai A, Zheng D, Qiu R, Mai W, Zhou Y. Lipoprotein-associated phospholipase A2 (Lp-PLA(2)): a novel and promising biomarker for cardiovascular risks assessment. Dis Markers 2013; 34:323-331
- Stafforini DM, Zimmerman GA. Unraveling the PAF-AH/Lp-PLA2 controversy. J Lipid Res 2014; 55:1811-1814
- Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res 2012; 53:1767-1782
- Shimokata K, Yamada Y, Kondo T, Ichihara S, Izawa H, Nagata K, Murohara T, Ohno M, Yokota M. Association of gene polymorphisms with coronary artery disease in individuals with or without nonfamilial hypercholesterolemia. Atherosclerosis 2004; 172:167-173
- Campo S, Sardo MA, Bitto A, Bonaiuto A, Trimarchi G, Bonaiuto M, Castaldo M, Saitta C, Cristadoro S, Saitta A. Platelet-activating factor acetylhydrolase is not associated with carotid intima-media thickness in hypercholesterolemic Sicilian individuals. Clin Chem 2004; 50:2077-2082
- Riley RF, Corson MA. Darapladib, a reversible lipoprotein-associated phospholipase A2 inhibitor, for the oral treatment of atherosclerosis and coronary artery disease. IDrugs 2009; 12:648-655
- O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP, Investigators S-T, Steen DL. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014; 312:1006-1015
- Stability Investigators, White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez-Sendon J, Manolis AJ, Mohler ER, 3rd, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L. Darapladib for preventing ischemic events in stable coronary heart disease. The New England journal of medicine 2014; 370:1702-1711
- Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998; 101:1581-1590
- Aviram M, Billecke S, Sorenson R, Bisgaier C, Newton R, Rosenblat M, Erogul J, Hsu C, Dunlop C, La Du B. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arteriosclerosis, thrombosis, and vascular biology 1998; 18:1617-1624
- Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998; 394:284-287
- Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. The Journal of biological chemistry 2000; 275:17527-17535
- Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002; 106:484-490
- Ng CJ, Hama SY, Bourquard N, Navab M, Reddy ST. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Molecular genetics and metabolism 2006; 89:368-373
- Ng CJ, Bourquard N, Hama SY, Shih D, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 2007; 27:1368-1374
- She ZG, Zheng W, Wei YS, Chen HZ, Wang AB, Li HL, Liu G, Zhang R, Liu JJ, Stallcup WB, Zhou Z, Liu DP, Liang CC. Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice. Circulation research 2009; 104:1160-1168
- Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, Kuivenhoven JA, Nordestgaard BG, Descamps OS, Steinhagen-Thiessen E, Tybjaerg-Hansen A, Watts GF, Averna M, Boileau C, Boren J, Catapano AL, Defesche JC, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Ray KK, Stalenhoef AF, Stroes E, Taskinen MR, Wiegman A, Wiklund O, Chapman MJ, European Atherosclerosis Society Consensus Panel on Familial H. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. European heart journal 2014; 35:2146-2157
- Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. Journal of clinical lipidology 2011; 5:S9-17
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. European heart journal 2013; 34:3478-3490a
- Khera AV, Won HH, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, Morrison AC, Brody JA, Gupta N, Nomura A, Kessler T, Duga S, Bis JC, van Duijn CM, Cupples LA, Psaty B, Rader DJ, Danesh J, Schunkert H, McPherson R, Farrall M, Watkins H, Lander E, Wilson JG, Correa A, Boerwinkle E, Merlini PA, Ardissino D, Saleheen D, Gabriel S, Kathiresan S. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol 2016; 67:2578-2589
- Kathiresan S, Consortium MIG. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet 2009; 41:334-341
- Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013; 45:25-33
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 2008; 40:189-197
- Rader DJ. Human genetics of atherothrombotic disease and its risk factors. Arteriosclerosis, thrombosis, and vascular biology 2015; 35:741-747
- Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. The New England journal of medicine 2006; 354:1264-1272
- Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009; 50 Suppl:S172-177
- Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation 2000; 101:207-213
- Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. J Am Med Assoc 1975; 231:360–381
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. Journal of the American College of Cardiology 1986; 8:1245-1255
- The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984; 251:365-374
- Scandinavian, Simvastatin, Survival, Study, Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the scandinavian simvastatin survival study (4S). Lancet 1994; 344:1383-1389
- McKenney JR, EM. Statins. In: Ballantyne CM, ed. Clinical Lipidology, a companion to Braunwald’s Heart Disease. Vol Second Edition: Elsevier Saunders; 2015:227-256.
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376:1670-1681
- Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016; 316:1289-1297
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, Committee FS, Investigators. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017; 376:1713-1722
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM, Committees OO, Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med 2018; 379:2097-2107
- Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Current atherosclerosis reports 2012; 14:1-10
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine 2017; 377:1119-1131
- Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, Lira Pineda A, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS. Inflammatory and Cholesterol Risk in the FOURIER Trial. Circulation 2018; 138:131-140
- Diffenderfer MR, Schaefer EJ. The composition and metabolism of large and small LDL. Current opinion in lipidology 2014; 25:221-226
- Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Jr., Dai D, Hernandez A, Fonarow GC. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. American heart journal 2009; 157:111-117 e112
- Krauss RM. Low-density lipoprotein subclasses and risk of coronary artery disease. Curr Opin Lipidol 1991; 2:248-252
- Hirayama S, Miida T. Small dense LDL: An emerging risk factor for cardiovascular disease. Clin Chim Acta 2012; 414:215-224
- Sninsky JJ, Rowland CM, Baca AM, Caulfield MP, Superko HR. Classification of LDL phenotypes by 4 methods of determining lipoprotein particle size. Journal of investigative medicine : the official publication of the American Federation for Clinical Research 2013; 61:942-949
- Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. The American journal of medicine 2001; 110:103-110
- Hurt-Camejo E, Camejo G, Rosengren B, Lopez F, Wiklund O, Bondjers G. Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. J Lipid Res 1990; 31:1387-1398
- Young SG. Recent progress in understanding apolipoprotein B. Circulation 1990; 82:1574-1594
- Sniderman AD, De Graaf J, Couture P. Low-density lipoprotein-lowering strategies: target versus maximalist versus population percentile. Current opinion in cardiology 2012; 27:405-411
- Sniderman AD, Lamarche B, Contois JH, de Graaf J. Discordance analysis and the Gordian Knot of LDL and non-HDL cholesterol versus apoB. Current opinion in lipidology 2014; 25:461-467
- National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report.[see comment]. Circulation 2002; 106:3143-3421
- Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ, National Heart L, Blood I, American College of Cardiology F, American Heart A. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines.[erratum appears in Circulation. 2004 Aug 10;110(6):763]. Circulation 2004; 110:227-239
- Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circulation Cardiovascular quality and outcomes 2011; 4:337-345
- Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, Warnick GR. Apolipoprotein B and cardiovascular disease risk: position statement from the AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Clin Chem 2009; 55:407-419
- Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PW, D'Agostino RB. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study - Implications for LDL Management. Journal of clinical lipidology 2007; 1:583-592
- Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009; 302:1993-2000
- Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC, Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. Journal of clinical lipidology 2011; 5:105-113
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr., Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1-45
- Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. Journal of clinical lipidology 2015; 9:129-169
- Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah-Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M, Zangeneh F, Bush MA. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY GUIDELINES FOR MANAGEMENT OF DYSLIPIDEMIA AND PREVENTION OF CARDIOVASCULAR DISEASE - EXECUTIVE SUMMARYComplete Appendix to Guidelines available at http://journals.aace.com/. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2017; 23:479-497
- Authors/Task Force M, Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016; 253:281-344
- Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, Couture P, Dufour R, Fodor G, Francis GA, Grover S, Gupta M, Hegele RA, Lau DC, Leiter L, Lewis GF, Lonn E, Mancini GB, Ng D, Pearson GJ, Sniderman A, Stone JA, Ur E. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. The Canadian journal of cardiology 2009; 25:567-579
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, Investigators I-I. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. The New England journal of medicine 2015; 372:2387-2397
- Koschinsky MLB, MB, Marcovina, SM. Lipoprotein(a). In: Ballantyne CM, ed. Clinical Lipidology, a companion to Braunwald’s Heart Disease. Vol Second Edition: Elsevier Saunders; 2015:109-127.
- Rader DJ, Mann WA, Cain W, Kraft HG, Usher D, Zech LA, Hoeg JM, Davignon J, Lupien P, Grossman M, et al. The low density lipoprotein receptor is not required for normal catabolism of Lp(a) in humans. J Clin Invest 1995; 95:1403-1408
- Rader DJ, Cain W, Ikewaki K, Talley G, Zech LA, Usher D, Brewer HB, Jr. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J Clin Invest 1994; 93:2758-2763
- Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest 1992; 90:52-60
- Moliterno DJ, Jokinen EV, Miserez AR, Lange RA, Willard JE, Boerwinkle E, Hillis LD, Hobbs HH. No association between plasma lipoprotein(a) concentrations and the presence or absence of coronary atherosclerosis in African-Americans. Arteriosclerosis, thrombosis, and vascular biology 1995; 15:850-855
- Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, Berglund L. High levels of Lp(a) with a small apo(a) isoform are associated with coronary artery disease in African American and white men. Arteriosclerosis, thrombosis, and vascular biology 2000; 20:2619-2624
- Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, Coresh J, Mosley TH, Morrisett JD, Catellier DJ, Folsom AR, Boerwinkle E, Ballantyne CM. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2012; 125:241-249
- Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009; 302:412-423
- Luke MM, Kane JP, Liu DM, Rowland CM, Shiffman D, Cassano J, Catanese JJ, Pullinger CR, Leong DU, Arellano AR, Tong CH, Movsesyan I, Naya-Vigne J, Noordhof C, Feric NT, Malloy MJ, Topol EJ, Koschinsky ML, Devlin JJ, Ellis SG. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology 2007; 27:2030-2036
- Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. The New England journal of medicine 2009; 361:2518-2528
- Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009; 301:2331-2339
- Linton MF, Farese RV, Jr., Chiesa G, Grass DS, Chin P, Hammer RE, Hobbs HH, Young SG. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a). J Clin Invest 1993; 92:3029-3037
- Schneider M, Witztum JL, Young SG, Ludwig EH, Miller ER, Tsimikas S, Curtiss LK, Marcovina SM, Taylor JM, Lawn RM, Innerarity TL, Pitas RE. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J Lipid Res 2005; 46:769-778
- Tsimikas S, Clopton P, Brilakis ES, Marcovina SM, Khera A, Miller ER, de Lemos JA, Witztum JL. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation 2009; 119:1711-1719
- Tsimikas S, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Sandhu MS, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Boekholdt SM. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol 2010; 56:946-955
- Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). Jama 1995; 274:1771-1774
- Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation 2014; 129:635-642
- Kronenberg F. Lipoprotein(a): there's life in the old dog yet. Circulation 2014; 129:619-621
- Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman M, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011; 217:3-46
- Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, Crooke RM, Witztum JL. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015;
- Abumrad N, el-Maghrabi M, Amri E, Lopez E, Grimaldi P. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. The Journal of biological chemistry 1993; 268:17665-17668
- Cartwright I, Plonne D, Higgins J. Intracellular events in the assembly of chylomicrons in rabbit enterocytes. J Lipid Res 2000; 41:1728-1739
- Mansbach C, 2nd, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol 2007; 293:G645-650
- Mu H, Hoy CE. The digestion of dietary triacylglycerols. Prog Lipid Res 2004; 43:105-133
- Phan C, Tso P. Intestinal lipid absorption and transport. Front Biosci 2001; 6:D299-319
- Iqbal J, Hussain M. Intestinal lipid absorption. Am J Physiol Endocrinol Metab 2009; 296:E1183-1194
- Pan X, Hussain M. Gut triglyceride production. Biochimica et biophysica acta 2012; 1821:727-735
- Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiological reviews 2012; 92:1061-1085
- Prabhudas M, Bowdish D, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, Means T, Moestrup S, Post S, Sawamura T, Silverstein S, Wang X, El Khoury J. Standardizing scavenger receptor nomenclature. J Immunol 2014; 192:1997-2006
- Storch J, Thumser A. Tissue-specific functions in the fatty acid-binding protein family. The Journal of biological chemistry 2010; 285:32679-32683
- Mansbach C, 2nd, Siddiqi S. The biogenesis of chylomicrons. Annual review of physiology 2010; 72:315-333
- Glatz J, Luiken J. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res 2018; 59:1084-1093
- Adeli K, Lewis G. Intestinal lipoprotein overproduction in insulin-resistant states. Current opinion in lipidology 2008; 19:221-228
- Hussain M, Leung T, Zhou L, Abu-Merhi S. Regulating intestinal function to reduce atherogenic lipoproteins. Clin Lipidol 2013; 8
- Shakir K, Sundaram S, Margolis S. Lipid synthesis in isolated intestinal cells. J Lipid Res 1978; 19:433-442
- Hamilton R. Synthesis and secretion of plasma lipoproteins. Adv Exp Med Biol 1972; 26:7-24
- Davidson N, Shelness G. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu Rev Nutr 2000; 20:169-193
- Anant S, Davidson N. Molecular mechanisms of apolipoprotein B mRNA editing. Current opinion in lipidology 2001; 12:159-165
- Kumar N, Mansbach C, 2nd. Prechylomicron transport vesicle: isolation and partial characterization. Am J Physiol 1999; 276:G378-386
- Levy E, Stan S, Delvin E, Menard D, Shoulders C, Garofalo C, Slight I, Seidman E, Mayer G, Bendayan M. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. The Journal of biological chemistry 2002; 277:16470-16477
- Chen Z, Davidson N. IRE1a-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab 2012; 16:473-486
- Liang S, Wu X, Fisher E, Ginsberg H. The amino-terminal domain of apolipoprotein B does not undergo retrograde translocation from the endoplasmic reticulum to the cytosol. Proteasomal degradation of nascent apolipoprotein B begins at the carboxyl terminus of the protein, while apolipoprotein B is still in its original translocon. The Journal of biological chemistry 2000; 275:32003-32010
- Kane J, Havel R. Disorders of the biogenesis and secretion of lipoproteins containing the B apolipoproteins. In: Scriver C, Beaudet A, Sly W, Valle D, eds. The Metabolic Basis of Inherited Disease, 6th Edition. New York, NY: McGraw-Hill; 1989:1139-1164.
- Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta 1997; 1345:136-150
- Hussain M. A proposed model for the assembly of chylomicrons. Atherosclerosis 2000; 148:1-15
- Yamaguchi J, Conlon D, Liang J, Fisher E, Ginsberg H. Translocation efficiency of apolipoprotein B is determined by the presence of b-sheet domains, not pause transfer sequences. The Journal of biological chemistry 2006; 281:27063-27071
- Shoulders C, Stephens D, Jones B. The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Current opinion in lipidology 2004; 15:191-197
- Siddiqi S, Mahan J, Siddiqi S, Gorelick F, Mansbach C, 2nd. Vesicle-associated membrane protein 7 is expressed in intestinal ER. J Cell Sci 2006; 119:943-950
- Siddiqi S, Saleem U, Abumrad N, Davidson N, Storch J, Siddiqi S, Mansbach C, 2nd. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res 2010; 51:1918-1928
- Berriot-Varoqueaux N, Dannoura A, Moreau A, Verthier N, Sassolas A, Cadiot G, Lachaux A, Munck A, Schmitz J, Aggerbeck L, Samson-Bouma M. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson's disease. Gastroenterology 2001; 121:1101-1108
- Siddiqi S, Siddiqi S, Mahan J, Peggs K, Gorelick F, Mansbach C, 2nd. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. The Journal of biological chemistry 2006; 281:20974-20982
- Jones B, Jones E, Bonney S, Patel H, Mensenkamp A, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam S, Naoumova R, Angelin B, Infante R, Levy E, Roy C, Freemont P, Scott J, Shoulders C. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet 2003; 34:29-31
- Sabesin S, Clark S, Holt P. Ultrastructural features of regional differences in chylomicron secretion by rat intestine. Experimental and molecular pathology 1977; 26:277-289
- Shen B, Scanu A, Kezdy F. Structure of human serum lipoproteins inferred from compositional analysis. Proceedings of the National Academy of Sciences of the United States of America 1977; 74:837-841
- Mead J. Lipid Metabolism. Annual review of biochemistry 1963; 32:241-268
- Sane A, Sinnett D, Delvin E, Bendayan M, Marcil V, Menard D, Beaulieu J, Levy E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J Lipid Res 2006; 47:2112-2120
- Stange E, Dietschy J. Cholesterol synthesis and low density lipoprotein uptake are regulated independently in rat small intestinal epithelium. Proc Nat Acad Sci, USA 1983; 80:5739-5743
- Le May C, Berger J, Lespine A, Pillot B, Prieur X, Letessier E, Hussain M, Collet X, Cariou B, Costet P. Transintestinal Cholesterol Excretion Is an Active Metabolic Process Modulated by PCSK9 and Statin Involving ABCB1. Arteriosclerosis, thrombosis, and vascular biology 2013; 33:1484-1493
- Tomkin G, Owens D. The chylomicron: relationship to atherosclerosis. International journal of vascular medicine 2012; 2012:784536
- Brinton E. Management of Hypertriglyceridemia for Prevention of Atherosclerotic Cardiovascular Disease. Cardiology clinics 2015; 33:309-323
- Thiam A, Farese R, Jr., Walther T. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol 2013; 14:775-786
- Demignot S, Beilstein F, Morel E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie 2014; 96:48-55
- Lehner R, Lian J, Quiroga A. Lumenal lipid metabolism: implications for lipoprotein assembly. Arteriosclerosis, thrombosis, and vascular biology 2012; 32:1087-1093
- van der Veen J, van Dijk T, Vrins C, van Meer H, Havinga R, Bijsterveld K, Tietge U, Groen A, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. The Journal of biological chemistry 2009; 284:19211-19219
- Temel R, Brown J. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends in pharmacological sciences 2015; 36:440-451
- Miller M, Stone N, Ballantyne C, Bittner V, Criqui M, Ginsberg H, Goldberg A, Howard W, Jacobson M, Kris-Etherton P, Lennie T, Levi M, Mazzone T, Pennathur S, American Heart Association Clinical Lipidology T, Prevention Committee of the Council on Nutrition PA, Metabolism, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular N, Council on the Kidney in Cardiovascular D. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011; 123:2292-2333
- Christian J, Bourgeois N, Snipes R, Lowe K. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States adults. The American journal of cardiology 2011; 107:891-897
- Ford E, Li C, Zhao G, Pearson W, Mokdad A. Hypertriglyceridemia and its pharmacologic treatment among US adults. Archives of internal medicine 2009; 169:572-578
- Maki K, Bays H, Dicklin M. Treatment options for the management of hypertriglyceridemia: strategies based on the best-available evidence. Journal of clinical lipidology 2012; 6:413-426
- Carroll M, Lacher D, Sorlie P, Cleeman J, Gordon D, Wolz M, Grundy S, Johnson C. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA 2005; 294:1773-1781
- Flegal K, Carroll M, Ogden C, Johnson C. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002; 288:1723-1727
- Yuan G, Al-Shali K, Hegele R. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2007; 176:1113-1120
- Austin M, Hokanson J, Edwards K. Hypertriglyceridemia as a cardiovascular risk factor. The American journal of cardiology 1998; 81:7B-12B
- Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt S, Khaw K, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007; 115:450-458
- Castelli W. The triglyceride issue: a view from Framingham. American heart journal 1986; 112:432-437
- Rhoads G, Blackwelder W, Stemmermann G, Hayashi T, Kagan A. Coronary risk factors and autopsy findings in Japanese-American men. Laboratory investigation; a journal of technical methods and pathology 1978; 38:304-311
- Robertson T, Kato H, Gordon T, Kagan A, Rhoads G, Land C, Worth R, Belsky J, Dock D, Miyanishi M, Kawamoto S. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California. Coronary heart disease risk factors in Japan and Hawaii. The American journal of cardiology 1977; 39:244-249
- Yano K, Rhoads G, Kagan A, Tillotson J. Dietary intake and the risk of coronary heart disease in Japanese men living in Hawaii. Am J Clin Nutr 1978; 31:1270-1279
- Hulley S, Rosenman R, Bawol R, Brand R. Epidemiology as a guide to clinical decisions. The association between triglyceride and coronary heart disease. The New England journal of medicine 1980; 302:1383-1389
- Lindkvist B, Appelros S, Regner S, Manjer J. A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. Pancreatology : official journal of the International Association of Pancreatology 2012; 12:317-324
- Murphy M, Sheng X, MacDonald T, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA internal medicine 2013; 173:162-164
- Preiss D, Tikkanen M, Welsh P, Ford I, Lovato L, Elam M, LaRosa J, DeMicco DA, Colhoun H, Goldenberg I, Murphy M, MacDonald T, Pedersen T, Keech A, Ridker P, Kjekshus J, Sattar N, McMurray J. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA 2012; 308:804-811
- Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis G. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids in health and disease 2011; 10:157
- Jacobs D, Jr., Barrett-Connor E. Retest reliability of plasma cholesterol and triglyceride. The Lipid Research Clinics Prevalence Study. American journal of epidemiology 1982; 116:878-885
- NIH Consensus conference. Triglyceride, high-density lipoprotein, and coronary heart disease. NIH Consensus Development Panel on Triglyceride, High-Density Lipoprotein, and Coronary Heart Disease. JAMA 1993; 269:505-510
- Miller M. Chapter 9. High-density lipoprotein cholesterol and triglycerides in coronary heart disease risk assessment. In: Ballantyne C, ed. Clinical lipidology: A companion to Braunwald's Heart Disease, Second Edition. Philadelphia: Elsevier Saunders; 2015:100-108.
- Pejic R, Lee D. Hypertriglyceridemia. Journal of the American Board of Family Medicine : JABFM 2006; 19:310-316
- Talayero B, Sacks F. The role of triglycerides in atherosclerosis. Current cardiology reports 2011; 13:544-552
- Katcher H, Hill A, Lanford J, Yoo J, Kris-Etherton P. Lifestyle approaches and dietary strategies to lower LDL-cholesterol and triglycerides and raise HDL-cholesterol. Endocrinology and metabolism clinics of North America 2009; 38:45-78
- Brunner D, Altman S, Loebl K, Schwartz S, Levin S. Serum cholesterol and triglycerides in patients suffering from ischemic heart disease and in healthy subjects. Atherosclerosis 1977; 28:197-204
- Castelli W, Doyle J, Gordon T, Hames C, Hjortland M, Hulley S, Kagan A, Zukel W. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 1977; 55:767-772
- Fager G, Wiklund O, Olofsson S, Wilhelmsen L, Bondjers G. Multivariate analyses of serum apolipoproteins and risk factors in relation to acute myocardial infarction. Arteriosclerosis 1981; 1:273-279
- Gotto A, Gorry G, Thompson J, Cole J, Trost R, Yeshurun D, DeBakey M. Relationship between plasma lipid concentrations and coronary artery disease in 496 patients. Circulation 1977; 56:875-883
- Hamsten A, Walldius G, Dahlen G, Johansson B, De Faire U. Serum lipoproteins and apolipoproteins in young male survivors of myocardial infarction. Atherosclerosis 1986; 59:223-235
- Kukita H, Imamura Y, Hamada M, Joh T, Kokubu T. Plasma lipids and lipoproteins in Japanese male patients with coronary artery disease and in their relatives. Atherosclerosis 1982; 42:21-29
- Scott D, Gotto A, Cole J, Gorry G. Plasma lipids as collateral risk factors in coronary artery disease--a study of 371 males with chest pain. Journal of chronic diseases 1978; 31:337-345
- Assmann G, Cullen P, Schulte H. The Munster Heart Study (PROCAM). Results of follow-up at 8 years. European heart journal 1998; 19 Suppl A:A2-A11
- Miller M, Cannon C, Murphy S, Qin J, Ray K, Braunwald E, Investigators PI-T. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2008; 51:724-730
- Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T, Lin SY, Lindstrom J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Muller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancakova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrieres J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Jarvelin MR, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, Marz W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nature genetics 2013; 45:1345-1352
- Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, Tragante V, van Iperen EP, Sivapalaratnam S, Shah S, Elbers CC, Shah T, Engmann J, Giambartolomei C, White J, Zabaneh D, Sofat R, McLachlan S, Doevendans PA, Balmforth AJ, Hall AS, North KE, Almoguera B, Hoogeveen RC, Cushman M, Fornage M, Patel SR, Redline S, Siscovick DS, Tsai MY, Karczewski KJ, Hofker MH, Verschuren WM, Bots ML, van der Schouw YT, Melander O, Dominiczak AF, Morris R, Ben-Shlomo Y, Price J, Kumari M, Baumert J, Peters A, Thorand B, Koenig W, Gaunt TR, Humphries SE, Clarke R, Watkins H, Farrall M, Wilson JG, Rich SS, de Bakker PI, Lange LA, Davey Smith G, Reiner AP, Talmud PJ, Kivimaki M, Lawlor DA, Dudbridge F, Samani NJ, Keating BJ, Hingorani AD, Casas JP. Mendelian randomization of blood lipids for coronary heart disease. European heart journal 2015; 36:539-550
- Thomsen M, Varbo A, Tybjaerg-Hansen A, Nordestgaard BG. Low nonfasting triglycerides and reduced all-cause mortality: a mendelian randomization study. Clinical chemistry 2014; 60:737-746
- Baigent C, Keech A, Kearney P, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists C. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267-1278
- Patel A, Barzi F, Jamrozik K, Lam T, Ueshima H, Whitlock G, Woodward M, Asia Pacific Cohort Studies C. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation 2004; 110:2678-2686
- Sacks F, Tonkin A, Craven T, Pfeffer M, Shepherd J, Keech A, Furberg C, Braunwald E. Coronary heart disease in patients with low LDL-cholesterol: benefit of pravastatin in diabetics and enhanced role for HDL-cholesterol and triglycerides as risk factors. Circulation 2002; 105:1424-1428
- Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls S, Grobbee D, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875-1884
- Hokanson J, Austin M. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996; 3:213-219
- Desnuelle P, Savary P. Specificities of Lipases. J Lipid Res 1963; 4:369-384
- Brewer H, Jr. Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. The American journal of cardiology 1999; 83:3F-12F
- Zheng C, Khoo C, Furtado J, Sacks F. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 2010; 121:1722-1734
- Ooi T, Ooi D. The atherogenic significance of an elevated plasma triglyceride level. Critical Rev Clin Lab Sci 1998; 35:489-516
- Nordestgaards B. The vascular endothelial barrier-selective retention of lipoproteins. Current opinion in lipidology 1996; 7:269–273
- Zilversmit D. A proposal linking atherogenesis to the interaction of endothelial lipoprotein lipase with triglyceride-rich lipoproteins. Circulation research 1973; 33:633-638
- Mamo J, Proctor S, Smith D. Retention of chylomicron remnants by arterial tissue; importance of an efficient clearance mechanism from plasma. Atherosclerosis 1998; 141 Suppl 1:S63-S69
- Rutledge J, Mullick A, Gardner G, Goldberg IJ. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery : roles of VLDL and HDL. Circulation research 2000; 86:768-773
- Van Eck M, Zimmermann R, Groot P, Zechner R, Van Berkel T. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2000; 20:E53-62
- Mahley RW, Huang Y, Rall SC, Jr. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res 1999; 40:1933-1949
- Mahley R, Ji Z. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res 1999; 40:1-16
- Mahley R, Weisgraber K, Farese R. Disorders of lipid metabolism. Williams textbook of endocrinology. Vol 91998:1099-1119.
- Abou Ziki MD, Strulovici-Barel Y, Hackett NR, Rodriguez-Flores JL, Mezey JG, Salit J, Radisch S, Hollmann C, Chouchane L, Malek J, Zirie MA, Jayyuosi A, Gotto AM, Jr., Crystal RG. Prevalence of the apolipoprotein E Arg145Cys dyslipidemia at-risk polymorphism in African-derived populations. The American journal of cardiology 2014; 113:302-308
- de Villiers WJ, van der Westhuyzen DR, Coetzee GA, Henderson HE, Marais AD. The apolipoprotein E2 (Arg145Cys) mutation causes autosomal dominant type III hyperlipoproteinemia with incomplete penetrance. Arteriosclerosis, thrombosis, and vascular biology 1997; 17:865-872
- Schaefer E, Gregg R, Ghiselli G, Forte T, Ordovas J, Zech L, Brewer H, Jr. Familial apolipoprotein E deficiency. J Clin Invest 1986; 78:1206-1219
- Lada A, Rudel L, St Clair R. Effects of LDL enriched with different dietary fatty acids on cholesteryl ester accumulation and turnover in THP-1 macrophages. J Lipid Res 2003; 44:770-779
- Lada AT, Willingham MC, St. Clair R. Triglyceride depletion in THP-1 cells alters cholesteryl ester physical state and cholesterol efflux. J Lipid Res 2002; 43:618-628
- Rothblat G, Bamberger M, Phillips M. Reverse cholesterol transport. Methods in Enzymology 1986; 129:628-644
- Ullery-Ricewick J, Cox B, Griffin E, Jerome W. Triglyceride alters lysosomal cholesteryl ester metabolism in cholesteryl-ester laden macrophage foam cells. J Lipid Res 2009; 50:2014-2026
- Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer research 2000; 60:6353-6358
- Maedler K, Spinas G, Dyntar D, Moritz W, Kaiser N, Donath M. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 2001; 50:69-76
- Paumen M, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. The Journal of biological chemistry 1997; 272:3324-3329
- Chang W, Lin J, Dong J, Li D. Pyroptosis: an inflammatory cell death implicates in atherosclerosis. Medical hypotheses 2013; 81:484-486
- Tabas I. Apoptosis and plaque destabilization in atherosclerosis: the role of macrophage apoptosis induced by cholesterol. Cell Death and Differentiation 2004; 11:S12-S16
- Leitinger N, Schulman I. Phenotypic polarization of macrophages in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 2013; 33:1120-1126
- Hansson G. Immune mechanisms in plaque formation. Atherosclerosis rev 1991; 23:169-176
- Yla-Herttuala S, Lipton B, Rosenfeld M, Goldberg I, Steinberg D, Witztum J. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proceedings of the National Academy of Sciences of the United States of America 1991; 88:10143-10147
- Zhang W, Schwartz E, Wang Y, Attrep J, Li Z, Reaven P. Elevated concentrations of nonesterified fatty acids increase monocyte expression of CD11b and adhesion to endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 2006; 26:514-519
- Chung B, Segrest J, Smith K, Griffin F, Brouillette C. Lipolytic surface remnants of triglyceride-rich lipoproteins are cytotoxic to macrophages but not in the presence of high density lipoprotein. J Clin Invest 1989; 83:1363-1374
- Kume N, Cybulsky M, Gimbrone M, Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest 1992; 90:1138-1144
- Wang MD, Kiss RS, Franklin V, McBride HM, Whitman SC, Marcel YL. Different cellular traffic of LDL-cholesterol and acetylated LDL-cholesterol leads to distinct reverse cholesterol transport pathways. J Lipid Res 2007; 48:633-645
- Wehinger A, Tancevski I, Schgoer W, Eller P, Hochegger K, Morak M, Hermetter A, Ritsch A, Patsch J, Foeger B. Phospholipid transfer protein augments apoptosis in THP-1-derived macrophages induced by lipolyzed hypertriglyceridemic plasma. Arteriosclerosis, thrombosis, and vascular biology 2007; 27:908-815
- Benlian P, De Gennes JL, Foubert L, Zhang H, Gagne SE, Hayden M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. The New England journal of medicine 1996; 335:848-854
- Clee S, Bissada N, Miao F, Miao L, Marais A, Henderson H, Steures P, McManus J, McManus B, LeBoeuf R, Kastelein J, Hayden M. Plasma and vessel wall lipoprotein lipase have different roles in atherosclerosis. J Lipid Res 2000; 41:521-531
- de Souza R, Mente A, Maroleanu A, Cozma A, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J, Anand S. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Bmj 2015; 351:h3978
- Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS medicine 2010; 7:e1000252
- Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. The Cochrane database of systematic reviews 2015; 6:CD011737
- Jakobsen M, O'Reilly E, Heitmann B, Pereira M, Balter K, Fraser G, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett W, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009; 89:1425-1432
- Li Y, Hruby A, Bernstein A, Ley S, Wang D, Chiuve S, Sampson L, Rexrode K, Rimm E, Willett W, Hu F. Saturated Fats Compared With Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J Am Coll Cardiol 2015; 66:1538-1548
- Yamagishi K, Nettleton JA, Folsom AR, Investigators AS. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. American heart journal 2008; 156:965-974
- Stampfer M, Hu F, Manson J, Rimm E, Willett W. Primary prevention of coronary heart disease in women through diet and lifestyle. The New England journal of medicine 2000; 343:16-22
- Hu F, Willett W. Diet and coronary heart disease: findings from the Nurses' Health Study and Health Professionals' Follow-up Study. The journal of nutrition, health & aging 2001; 5:132-138
- Mozaffarian D, Wu J. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011; 58:2047-2067
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, Investigators R-I. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. The New England journal of medicine 2018;
- Maki K, Lawless A, Kelley K, Kaden V, Geiger C, Dicklin M. Corn oil improves the plasma lipoprotein lipid profile compared with extra-virgin olive oil consumption in men and women with elevated cholesterol: results from a randomized controlled feeding trial. Journal of clinical lipidology 2015; 9:49-57
- Wagner K, Tomasch R, Elmadfa I. Impact of diets containing corn oil or olive/sunflower oil mixture on the human plasma and lipoprotein lipid metabolism. European journal of nutrition 2001; 40:161-167
- Lichtenstein A, Ausman L, Carrasco W, Jenner J, Gualtieri L, Goldin B, Ordovas J, Schaefer E. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arterioscler Thromb 1993; 13:1533-1542
- Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nature reviews Endocrinology 2009; 5:335-344
- Wang Q, Imamura F, Lemaitre R, Rimm E, Wang M, King I, Song X, Siscovick D, Mozaffarian D. Plasma phospholipid trans-fatty acids levels, cardiovascular diseases, and total mortality: the cardiovascular health study. Journal of the American Heart Association 2014; 3
- Jacobson T, Maki K, Orringer C, Jones P, Kris-Etherton P, Sikand G, Forge R, Daniels S, Wilson D, Morris P, Wild R, Grundy S, Daviglus M, Ferdinand K, Vijay K, Deedwania P, Aberg J, Liao K, McKenney J, Ross J, Braun L, Ito M, Bolick J, Dicklin M, Kirkpatrick C, Rhodes K, Smith N, Blackett P, DeFerranti S, Gidding S, Davey R-E, McCrindle B, McNeal C, Urbina E, Dayspring T, Underberg J, Lopez J, Pirzada A, Roderguez C, Fichtenbaum C, Gallant J, Horberg M, Longenecker C, Myerson M, Overton E, Coblyn J, Curtis J, Plutzky J, Solomon D, Bays H, Brown W. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. Journal of clinical lipidology 2015; In Press
- Bates S, Murphy P, Feng Z, Kanazawa T, Getz G. Very low density lipoproteins promote triglyceride accumulation in macrophages. Arteriosclerosis 1984; 4:103-114
- Lindqvist P, Ostlund-Lindqvist AM, Witztum JL, Steinberg D, Little JA. The role of lipoprotein lipase in the metabolism of triglyceride-rich lipoproteins by macrophages. J Biol Chem 1983; 258:9086-9092
- Gianturco S, Ramprasad M, Lin A, Song R, Bradley W. Cellular binding site and membrane binding proteins for triglyceride-rich lipoproteins in human monocyte-macrophages and THP-1 monocytic cells. J Lipid Res 1994; 35:1674-1687
- Evans A, Sawyez C, Wolfe B, Connelly P, Maquire G, Huff M. Evidence that cholesteryl ester and triglyceride accumulation in J774 macrophages induced by very low density lipoprotein subfractions occurs by different mechanisms. J Lipid Res 1993; 34:703-717
- Tabas I, Myers J, Innerarity T, Xu X, ArnoldJ, Maxfield F. The influence of particle size and multiple apoprotein E-receptor interactions on the endocytic targeting of b-VLDL in mouse. J Cell Biol 1991; 115:1547-1560
- Brown W, Levy R, Fredrickson D. Studies of the proteins in human plasma very low density lipoproteins. The Journal of biological chemistry 1969; 244:5687-5694
- Brown W, Baginsky M. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun 1972; 46:375-382
- Windler E, Havel R. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res 1985; 26:556-565
- Ginsberg H, Le N, Goldberg I, Gibson J, Rubinstein A, Wang-Iverson P, Norum R, Brown W. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest 1986; 78:1287-1295
- Ito Y, Azrolan N, O'Connell A, Walsh A, Breslow J. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science 1990; 249:790-793
- Pollin T, Damcott C, Shen H, Ott SH, Shelton J, Horenstein R, Post W, McLenithan J, Bielak L, Peyser P, Mitchell B, Miller M, O'Connell J, Shuldiner A. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008; 322:1702-1705
- Bochem A, van Capelleveen J, Dallinga-Thie G, Schimmel A, Motazacker M, Tietjen I, Singaraja R, Hayden M, Kastelein J, Stroes E, Hovingh G. Two novel mutations in apolipoprotein C3 underlie atheroprotective lipid profiles in families. Clinical genetics 2014; 85:433-440
- Crosby J, Peloso G, Auer P, Crosslin D, Stitziel N, Lange L, Lu Y, Tang Z, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang H, Xue C, Goel A, Farrall M, Duga S, Merlini P, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox C, Hveem K, Holmen O, Nikpay M, Farlow D, Assimes T, Franceschini N, Robinson J, North K, Martin L, Gupta N, Escher S, Jansson J, Van Zuydam N, Palmer C, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig I, Kruppa J, Degenhardt F, Gottesman O, Bottinger E, O'Donnell C, Psaty B, Ballantyne C, Abecasis G, Ordovas J, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos R, McPherson R, Willer C, Erdmann J, Hall A, Samani N, Deloukas P, Schunkert H, Wilson J, Kooperberg C, Rich S, Tracy R, Lin D, Gabriel S, Nickerson D, Jarvik G, Cupples L, Reiner A, Boerwinkle E, Kathiresan S, Institute THWGotESPNHLB. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. The New England journal of medicine 2014; 371:22-31
- Jorgensen A, Frikke-Schmidt R, Nordestgaard B, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. The New England journal of medicine 2014; 371:32-41
- Graham M, Lee R, Bell T, 3rd, Fu W, Mullick A, Alexander V, Singleton W, Viney N, Geary R, Su J, Baker B, Burkey J, Crooke S, Crooke R. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circulation research 2013; 112:1479-1490
- Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, Graham MJ, Hughes SG, Yu R, Singleton W, Baker BF, Bhanot S, Crooke RM. Antisense-Mediated Lowering of Plasma Apolipoprotein C-III by Volanesorsen Improves Dyslipidemia and Insulin Sensitivity in Type 2 Diabetes. Diabetes care 2016; 39:1408-1415
- Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M. Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. Journal of the American College of Cardiology 2017; 69:789-800
- Kawakami A, Aikawa M, Alcaide P, Luscinskas F, Libby P, Sacks F. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 2006; 114:681-687
- Kawakami A, Osaka M, Tani M, Azuma H, Sacks F, Shimokado K, Yoshida M. Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation 2008; 118:731-742
- Hiukka A, Stahlman M, Pettersson C, Levin M, Adiels M, Teneberg S, Leinonen E, Hulten L, Wiklund O, Oresic M, Olofsson S, Taskinen M, Ekroos K, Boren J. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes 2009; 58:2018-2026
- Georgieva A, Cate H, Keulen E, van Oerle R, Govers-Riemslag J, Hamulyak K, van der Kallen C, Van Greevenbroek M, De Bruin T. Prothrombotic markers in familial combined hyperlipidemia: evidence of endothelial cell activation and relation to metabolic syndrome. Atherosclerosis 2004; 175:345-351
- Sutherland J, McKinley B, Eckel R. The metabolic syndrome and inflammation. Metabolic syndrome and related disorders 2004; 2:82-104
- Norata G, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, Eriksson P, Catapano A. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis 2007; 193:321-327
- Berglund L, Brunzell J, Goldberg A, Goldberg I, Sacks F, Murad M, Stalenhoef A, Endocrine s. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism 2012; 97:2969-2989
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Circulation 2002; 106:3143-3421
- Brunzell J, Davidson M, Furberg C, Goldberg R, Howard B, Stein J, Witztum J, American Diabetes A, American College of Cardiology F. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008; 31:811-822
- Manninen V, Tenkanen L, Koskinen P, Huttunen J, Manttari M, Heinonen O, Frick M. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation 1992; 85:37-45
- Rubins H, Robins S, Collins D, Fye C, Anderson J, Elam M, Faas F, Linares E, Schaefer E, Schectman G, Wilt T, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. The New England journal of medicine 1999; 341:410-418
- Robins S, Collins D, Wittes J, Papademetriou V, Deedwania P, Schaefer E, McNamara J, Kashyap M, Hershman J, Wexler L, Rubins H, Trial V-HSGVAH-DLI. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA 2001; 285:1585-1591
- Ginsberg H, Elam M, Lovato L, Crouse J, 3rd, Leiter L, Linz P, Friedewald W, Buse J, Gerstein H, Probstfield J, Grimm R, Ismail-Beigi F, Bigger J, Goff D, Jr., Cushman W, Simons-Morton D, Byington R. Effects of combination lipid therapy in type 2 diabetes mellitus. The New England journal of medicine 2010; 362:1563-1574
- Keech A, Simes R, Barter P, Best J, Scott R, Taskinen M, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi Y, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, investigators Fs. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849-1861
- Barter P, Rye K. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arteriosclerosis, thrombosis, and vascular biology 2008; 28:39-46
- Elam M, Lovato L, Ginsberg H. Role of fibrates in cardiovascular disease prevention, the ACCORD-Lipid perspective. Current opinion in lipidology 2011; 22:55-61
- Freeman M, Walford G. Chapter 41. Lipoprotein metabolism and the treatment of lipid disorders. In: Jameson J, De Groot L, eds. Endocrinology, adult and pediatric, Seventh Edition2015.
- Branchi A, Fiorenza A, Rovellini A, Torri A, Muzio F, Macor S, Sommariva D. Lowering effects of four different statins on serum triglyceride level. European journal of clinical pharmacology 1999; 55:499-502
- Guyton JR, Slee AE, Anderson T, Fleg JL, Goldberg RB, Kashyap ML, Marcovina SM, Nash SD, O'Brien KD, Weintraub WS, Xu P, Zhao XQ, Boden WE. Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes). J Am Coll Cardiol 2013; 62:1580-1584
- Kris-Etherton P, Harris W, Appel L, American Heart Association. Nutrition C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002; 106:2747-2757
- Harris W. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997; 65:1645S-1654S
- Lungershausen Y, Abbey M, Nestel P, Howe P. Reduction of blood pressure and plasma triglycerides by omega-3 fatty acids in treated hypertensives. Journal of hypertension 1994; 12:1041-1045
- Jacobson T. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr 2008; 87:1981S-1990S
- Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 Fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circulation Cardiovascular quality and outcomes 2012; 5:808-818
- Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT, Jr., Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM, Investigators R-I. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol 2017; 40:138-148
- Prasad K. Flaxseed and cardiovascular health. Journal of cardiovascular pharmacology 2009; 54:369-377
- Brenna J, Salem N, Jr., Sinclair A, Cunnane S, International Society for the Study of Fatty A, Lipids I. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, leukotrienes, and essential fatty acids 2009; 80:85-91
- Goldberg I, Eckel R, McPherson R. Triglycerides and heart disease: still a hypothesis? Arteriosclerosis, thrombosis, and vascular biology 2011; 31:1716-1725
- Freedman D, Gruchow H, Anderson A, Rimm A, Barboriak JJ. Relation of triglyceride levels to coronary artery disease: the Milwaukee Cardiovascular Data Registry. American journal of epidemiology 1988; 127:1118-1130
- Rosenson RS, Brewer HB, Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 2012; 125:1905-1919
- Barter PJ, Rye KA. Molecular mechanisms of reverse cholesterol transport. Current opinion in lipidology 1996; 7:82-87
- von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arteriosclerosis, thrombosis, and vascular biology 2001; 21:13-27
- Glomset JA, Janssen ET, Kennedy R, Dobbins J. Role of plasma lecithin:cholesterol acyltransferase in the metabolism of high density lipoproteins. J Lipid Res 1966; 7:638-648
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arteriosclerosis, thrombosis, and vascular biology 2003; 23:1178-1184
- Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proceedings of the National Academy of Sciences of the United States of America 2004; 101:9774-9779
- Tarling EJ, Edwards PA. Intracellular Localization of Endogenous Mouse ABCG1 Is Mimicked by Both ABCG1-L550 and ABCG1-P550-Brief Report. Arterioscler Thromb Vasc Biol 2016; 36:1323-1327
- Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:19719-19724
- Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. The Journal of biological chemistry 1994; 269:21003-21009
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518-520
- Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 2007; 48:2453-2462
- de La Llera-Moya M, Connelly MA, Drazul D, Klein SM, Favari E, Yancey PG, Williams DL, Rothblat GH. Scavenger receptor class B type I affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL. J Lipid Res 2001; 42:1969-1978
- Yancey PG, de la Llera-Moya M, Swarnakar S, Monza P, Klein S, Connelly M, Johnson W, Williams D, Rothblat GH. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. The Journal of biological chemistry 2000; 275:36596-35604
- Kellner-Weibel G, de La Llera-Moya M, Connelly MA, Stoudt G, Christian AE, Haynes MP, Williams DL, Rothblat GH. Expression of scavenger receptor BI in COS-7 cells alters cholesterol content and distribution. Biochemistry 2000; 39:221-229
- Jahani M, Lacko AG. A study of the interaction of lecithin: cholesterol acyltransferase with subfractions of high density lipoproteins. J Lipid Res 1981; 22:1102-1110
- Calabresi L, Franceschini G. Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends in cardiovascular medicine 2010; 20:50-53
- Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circulation research 2005; 96:1221-1232
- Barter P, Rye KA. Cholesteryl ester transfer protein: its role in plasma lipid transport. Clinical and experimental pharmacology & physiology 1994; 21:663-672
- Rohrl C, Stangl H. HDL endocytosis and resecretion. Biochim Biophys Acta 2013; 1831:1626-1633
- Zanoni P, Velagapudi S, Yalcinkaya M, Rohrer L, von Eckardstein A. Endocytosis of lipoproteins. Atherosclerosis 2018; 275:273-295
- Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer C, Volf I, Pavelka M, Eckhardt ER, van der Westhuyzen DR, Schutz GJ, Stangl H. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J Biol Chem 2006; 281:11193-11204
- Sun B, Eckhardt ER, Shetty S, van der Westhuyzen DR, Webb NR. Quantitative analysis of SR-BI-dependent HDL retroendocytosis in hepatocytes and fibroblasts. J Lipid Res 2006; 47:1700-1713
- Brundert M, Heeren J, Merkel M, Carambia A, Herkel J, Groitl P, Dobner T, Ramakrishnan R, Moore KJ, Rinninger F. Scavenger receptor CD36 mediates uptake of high density lipoproteins in mice and by cultured cells. J Lipid Res 2011; 52:745-758
- Malaval C, Laffargue M, Barbaras R, Rolland C, Peres C, Champagne E, Perret B, Terce F, Collet X, Martinez LO. RhoA/ROCK I signalling downstream of the P2Y13 ADP-receptor controls HDL endocytosis in human hepatocytes. Cellular signalling 2009; 21:120-127
- Cardouat G, Duparc T, Fried S, Perret B, Najib S, Martinez LO. Ectopic adenine nucleotide translocase activity controls extracellular ADP levels and regulates the F1-ATPase-mediated HDL endocytosis pathway on hepatocytes. Biochim Biophys Acta Mol Cell Biol Lipids 2017; 1862:832-841
- Nijstad N, Gautier T, Briand F, Rader DJ, Tietge UJ. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology 2011; 140:1043-1051
- van der Velde AE, Brufau G, Groen AK. Transintestinal cholesterol efflux. Current opinion in lipidology 2010; 21:167-171
- Castelli WP. Epidemiology of coronary heart disease: the Framingham study. The American journal of medicine 1984; 76:4-12
- Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation 1966; 34:679-697
- Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975; 1:16-19
- Rhoads GG, Gulbrandsen CL, Kagan A. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. The New England journal of medicine 1976; 294:293-298
- Pearson TA, Bulkley BH, Achuff SC, Kwiterovich PO, Gordis L. The association of low levels of HDL cholesterol and arteriographically defined coronary artery disease. American journal of epidemiology 1979; 109:285-295
- Nofer JR, Tepel M, Kehrel B, Walter M, Seedorf U, Assmann G, Zidek W. High density lipoproteins enhance the Na+/H+ antiport in human platelets. Thrombosis and haemostasis 1996; 75:635-641
- Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arteriosclerosis, thrombosis, and vascular biology 1997; 17:107-113
- Abbott RD, Wilson PW, Kannel WB, Castelli WP. High density lipoprotein cholesterol, total cholesterol screening, and myocardial infarction. The Framingham Study. Arteriosclerosis 1988; 8:207-211
- Miller M, Mead LA, Kwiterovich PO, Jr., Pearson TA. Dyslipidemias with desirable plasma total cholesterol levels and angiographically demonstrated coronary artery disease. The American journal of cardiology 1990; 65:1-5
- Ginsburg GS, Safran C, Pasternak RC. Frequency of low serum high-density lipoprotein cholesterol levels in hospitalized patients with "desirable" total cholesterol levels. The American journal of cardiology 1991; 68:187-192
- Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr., Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989; 79:8-15
- Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. The New England journal of medicine 1987; 317:1237-1245
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM, Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615-1622
- Olsson AG, Schwartz GG, Szarek M, Sasiela WJ, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A. High-density lipoprotein, but not low-density lipoprotein cholesterol levels influence short-term prognosis after acute coronary syndrome: results from the MIRACL trial. European heart journal 2005; 26:890-896
- Ray KK, Cannon CP, Cairns R, Morrow DA, Ridker PM, Braunwald E. Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: results from PROVE IT-TIMI 22. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:424-430
- Ballantyne CM, Herd JA, Ferlic LL, Dunn JK, Farmer JA, Jones PH, Schein JR, Gotto AM, Jr. Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation 1999; 99:736-743
- Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J, Effect of r HDLoA-S, Efficacy I. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007; 297:1675-1682
- Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proceedings of the National Academy of Sciences of the United States of America 1994; 91:9607-9611
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265-267
- Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990; 85:1234-1241
- Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. The New England journal of medicine 2011; 365:2255-2267
- Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. The New England journal of medicine 2014; 371:203-212
- Song WL, FitzGerald GA. Niacin, an old drug with a new twist. J Lipid Res 2013; 54:2586-2594
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, Investigators I. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine 2007; 357:2109-2122
- Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal OI. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine 2012; 367:2089-2099
- HPS3/TIMI55–REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. The New England journal of medicine 2017; 377:1217-1227
- Tall AR, Rader DJ. Trials and Tribulations of CETP Inhibitors. Circulation research 2018; 122:106-112
- Nicholls SJ, Andrews J, Kastelein JJP, Merkely B, Nissen SE, Ray KK, Schwartz GG, Worthley SG, Keyserling C, Dasseux JL, Griffith L, Kim SW, Janssan A, Di Giovanni G, Pisaniello AD, Scherer DJ, Psaltis PJ, Butters J. Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial. JAMA Cardiol 2018; 3:815-822
- Nicholls SJ, Puri R, Ballantyne CM, Jukema JW, Kastelein JJP, Koenig W, Wright RS, Kallend D, Wijngaard P, Borgman M, Wolski K, Nissen SE. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol 2018; 3:806-814
- Bisoendial RJ, Hovingh GK, Levels JH, Lerch PG, Andresen I, Hayden MR, Kastelein JJ, Stroes ES. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation 2003; 107:2944-2948
- Karalis I, Jukema JW. HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option? Curr Cardiol Rep 2018; 20:66
- Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. The New England journal of medicine 2014; 371:2383-2393
- Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. The lancet Diabetes & endocrinology 2015; 3:507-513
- Zerrad-Saadi A, Therond P, Chantepie S, Couturier M, Rye KA, Chapman MJ, Kontush A. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:2169-2175
- Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine 2011; 364:127-135
- Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, Salvetti M, Monteduro C, Zulli R, Muiesan ML, Agabiti-Rosei E. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation 2001; 103:1949-1954
- Hovingh GK, Brownlie A, Bisoendial RJ, Dube MP, Levels JH, Petersen W, Dullaart RP, Stroes ES, Zwinderman AH, de Groot E, Hayden MR, Kuivenhoven JA, Kastelein JJ. A novel apoA-I mutation (L178P) leads to endothelial dysfunction, increased arterial wall thickness, and premature coronary artery disease. J Am Coll Cardiol 2004; 44:1429-1435
- Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003; 290:2292-2300
- Johannsen TH, Frikke-Schmidt R, Schou J, Nordestgaard BG, Tybjaerg-Hansen A. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J Am Coll Cardiol 2012; 60:2041-2048
- Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 2012; 380:572-580
- Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arteriosclerosis, thrombosis, and vascular biology 2013; 33:1696-1705
- Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, Wareham N. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. British journal of cancer 1999; 80 Suppl 1:95-103
- Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in laboratory medicine 2006; 26:847-870
- Hutchins PM, Ronsein GE, Monette JS, Pamir N, Wimberger J, He Y, Anantharamaiah GM, Kim DS, Ranchalis JE, Jarvik GP, Vaisar T, Heinecke JW. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem 2014; 60:1393-1401
- Mackey RH, Greenland P, Goff DC, Jr., Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2012; 60:508-516
- El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Annals of internal medicine 2009; 150:84-93
- Virani SS, Catellier DJ, Pompeii LA, Nambi V, Hoogeveen RC, Wasserman BA, Coresh J, Mosley TH, Otvos JD, Sharrett AR, Boerwinkle E, Ballantyne CM. Relation of cholesterol and lipoprotein parameters with carotid artery plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) carotid MRI study. Atherosclerosis 2011; 219:596-602
- de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arteriosclerosis, thrombosis, and vascular biology 2010; 30:796-801
- Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 1955; 34:1345-1353
- Gofman JW, Lindgren FT, Elliott H. Ultracentrifugal studies of lipoproteins of human serum. The Journal of biological chemistry 1949; 179:973-979
- Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Current opinion in lipidology 2010; 21:229-238
- Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. The Journal of biological chemistry 1984; 259:12201-12209
- James RW, Hochstrasser D, Tissot JD, Funk M, Appel R, Barja F, Pellegrini C, Muller AF, Pometta D. Protein heterogeneity of lipoprotein particles containing apolipoprotein A-I without apolipoprotein A-II and apolipoprotein A-I with apolipoprotein A-II isolated from human plasma. J Lipid Res 1988; 29:1557-1571
- Kuklenyik Z, Jones JI, Gardner MS, Schieltz DM, Parks BA, Toth CA, Rees JC, Andrews ML, Carter K, Lehtikoski AK, McWilliams LG, Williamson YM, Bierbaum KP, Pirkle JL, Barr JR. Core lipid, surface lipid and apolipoprotein composition analysis of lipoprotein particles as a function of particle size in one workflow integrating asymmetric flow field-flow fractionation and liquid chromatography-tandem mass spectrometry. PLoS One 2018; 13:e0194797
- Castro GR, Fielding CJ. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry 1988; 27:25-29
- Pownall HJ, Ehnholm C. The unique role of apolipoprotein A-I in HDL remodeling and metabolism. Current opinion in lipidology 2006; 17:209-213
- Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res 2009; 50:574-585
- Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res 2013; 54:2950-2963
- Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 2013; 54:2575-2585
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 2007; 117:746-756
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature cell biology 2011; 13:423-433
- Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L, Levin MG, Thacker S, Sethupathy P, Barter PJ, Remaley AT, Rye KA. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nature communications 2014; 5:3292
- Allen RM, Zhao S, Ramirez Solano MA, Zhu W, Michell DL, Wang Y, Shyr Y, Sethupathy P, Linton MF, Graf GA, Sheng Q, Vickers KC. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J Extracell Vesicles 2018; 7:1506198
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL--an evolving field. Nature clinical practice Endocrinology & metabolism 2006; 2:504-511
- Van Lenten BJ, Navab M, Shih D, Fogelman AM, Lusis AJ. The role of high-density lipoproteins in oxidation and inflammation. Trends in cardiovascular medicine 2001; 11:155-161
- Eren E, Yilmaz N, Aydin O. High Density Lipoprotein and it's Dysfunction. The open biochemistry journal 2012; 6:78-93
- Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RB, Sr., Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: a consensus statement from the National Lipid Association. Journal of clinical lipidology 2013; 7:484-525
- Rached FH, Chapman MJ, Kontush A. HDL particle subpopulations: Focus on biological function. BioFactors 2015; 41:67-77
- Nofer JR. Signal transduction by HDL: agonists, receptors, and signaling cascades. Handbook of experimental pharmacology 2015; 224:229-256
- Mendez AJ, Oram JF, Bierman EL. Protein kinase C as a mediator of high density lipoprotein receptor-dependent efflux of intracellular cholesterol. The Journal of biological chemistry 1991; 266:10104-10111
- Walter M, Reinecke H, Nofer JR, Seedorf U, Assmann G. HDL3 stimulates multiple signaling pathways in human skin fibroblasts. Arteriosclerosis, thrombosis, and vascular biology 1995; 15:1975-1986
- Yamauchi Y, Hayashi M, Abe-Dohmae S, Yokoyama S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. The Journal of biological chemistry 2003; 278:47890-47897
- Yamauchi Y, Chang CC, Hayashi M, Abe-Dohmae S, Reid PC, Chang TY, Yokoyama S. Intracellular cholesterol mobilization involved in the ABCA1/apolipoprotein-mediated assembly of high density lipoprotein in fibroblasts. J Lipid Res 2004; 45:1943-1951
- Ma L, Dong F, Denis M, Feng Y, Wang MD, Zha X. Ht31, a protein kinase A anchoring inhibitor, induces robust cholesterol efflux and reverses macrophage foam cell formation through ATP-binding cassette transporter A1. The Journal of biological chemistry 2011; 286:3370-3378
- See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. The Journal of biological chemistry 2002; 277:41835-41842
- Nofer JR, Remaley AT, Feuerborn R, Wolinnska I, Engel T, von Eckardstein A, Assmann G. Apolipoprotein A-I activates Cdc42 signaling through the ABCA1 transporter. J Lipid Res 2006; 47:794-803
- Tang C, Vaughan AM, Oram JF. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. The Journal of biological chemistry 2004; 279:7622-7628
- Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J Lipid Res 2006; 47:107-114
- Liu D, Ji L, Tong X, Pan B, Han JY, Huang Y, Chen YE, Pennathur S, Zhang Y, Zheng L. Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1. American journal of physiology Cell physiology 2011; 301:C739-748
- Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. The Journal of biological chemistry 2003; 278:9142-9149
- Kimura T, Tomura H, Sato K, Ito M, Matsuoka I, Im DS, Kuwabara A, Mogi C, Itoh H, Kurose H, Murakami M, Okajima F. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. The Journal of biological chemistry 2010; 285:4387-4397
- Tan JT, Prosser HC, Vanags LZ, Monger SA, Ng MK, Bursill CA. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1alpha. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2014; 28:206-217
- Zhang Q, Zhang Y, Feng H, Guo R, Jin L, Wan R, Wang L, Chen C, Li S. High density lipoprotein (HDL) promotes glucose uptake in adipocytes and glycogen synthesis in muscle cells. PloS one 2011; 6:e23556
- Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circulation research 2006; 98:63-72
- Zhang QH, Zu XY, Cao RX, Liu JH, Mo ZC, Zeng Y, Li YB, Xiong SL, Liu X, Liao DF, Yi GH. An involvement of SR-B1 mediated PI3K-Akt-eNOS signaling in HDL-induced cyclooxygenase 2 expression and prostacyclin production in endothelial cells. Biochem Biophys Res Commun 2012; 420:17-23
- Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest 2005; 115:969-977
- Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nature medicine 2001; 7:853-857
- Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. The Journal of biological chemistry 2008; 283:25074-25081
- Matsuo Y, Miura S, Kawamura A, Uehara Y, Rye KA, Saku K. Newly developed reconstituted high-density lipoprotein containing sphingosine-1-phosphate induces endothelial tube formation. Atherosclerosis 2007; 194:159-168
- Miura S, Fujino M, Matsuo Y, Kawamura A, Tanigawa H, Nishikawa H, Saku K. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 2003; 23:802-808
- Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, Murakami M, Okajima F. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. The Journal of biological chemistry 2006; 281:37457-37467
- Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, Seedorf U, Assmann G. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. The Journal of biological chemistry 2001; 276:34480-34485
- Tamama K, Tomura H, Sato K, Malchinkhuu E, Damirin A, Kimura T, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein inhibits migration of vascular smooth muscle cells through its sphingosine 1-phosphate component. Atherosclerosis 2005; 178:19-23
- Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic research in cardiology 2010; 105:821-832
- Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 2004; 113:569-581
- Morel S, Frias MA, Rosker C, James RW, Rohr S, Kwak BR. The natural cardioprotective particle HDL modulates connexin43 gap junction channels. Cardiovascular research 2012; 93:41-49
- Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezon E, Champagne E, Pineau T, Georgeaud V, Walker JE, Terce F, Collet X, Perret B, Barbaras R. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 2003; 421:75-79
- Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circulation research 2009; 104:455-465
- Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circulation research 2008; 103:1084-1091
- Park SH, Park JH, Kang JS, Kang YH. Involvement of transcription factors in plasma HDL protection against TNF-alpha-induced vascular cell adhesion molecule-1 expression. The international journal of biochemistry & cell biology 2003; 35:168-182
- McGrath KC, Li XH, Puranik R, Liong EC, Tan JT, Dy VM, DiBartolo BA, Barter PJ, Rye KA, Heather AK. Role of 3beta-hydroxysteroid-delta 24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:877-882
- Norata GD, Callegari E, Marchesi M, Chiesa G, Eriksson P, Catapano AL. High-density lipoproteins induce transforming growth factor-beta2 expression in endothelial cells. Circulation 2005; 111:2805-2811
- Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arteriosclerosis, thrombosis, and vascular biology 2008; 28:2071-2077
- Bursill CA, Castro ML, Beattie DT, Nakhla S, van der Vorst E, Heather AK, Barter PJ, Rye KA. High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arteriosclerosis, thrombosis, and vascular biology 2010; 30:1773-1778
- De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, Xu D, Zimmer S, Lahrmann C, Schildberg FA, Vogelhuber J, Kraut M, Ulas T, Kerksiek A, Krebs W, Bode N, Grebe A, Fitzgerald ML, Hernandez NJ, Williams BR, Knolle P, Kneilling M, Rocken M, Lutjohann D, Wright SD, Schultze JL, Latz E. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014; 15:152-160
- Vaughan AM, Tang C, Oram JF. ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation. J Lipid Res 2009; 50:285-292
- Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008; 118:1837-1847
- Tolle M, Pawlak A, Schuchardt M, Kawamura A, Tietge UJ, Lorkowski S, Keul P, Assmann G, Chun J, Levkau B, van der Giet M, Nofer JR. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arteriosclerosis, thrombosis, and vascular biology 2008; 28:1542-1548
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010; 328:1689-1693
- Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arteriosclerosis, thrombosis, and vascular biology 2009; 29:843-849
- Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr., Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr-/-, ApoA-I-/- mice. The Journal of biological chemistry 2010; 285:36158-36169
- Yu BL, Wang SH, Peng DQ, Zhao SP. HDL and immunomodulation: an emerging role of HDL against atherosclerosis. Immunology and cell biology 2010; 88:285-290
- Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, 3rd, Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood 2001; 97:2381-2389
- Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell stem cell 2012; 11:195-206
- Murphy AJ, Bijl N, Yvan-Charvet L, Welch CB, Bhagwat N, Reheman A, Wang Y, Shaw JA, Levine RL, Ni H, Tall AR, Wang N. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nature medicine 2013; 19:586-594
- Naqvi TZ, Shah PK, Ivey PA, Molloy MD, Thomas AM, Panicker S, Ahmed A, Cercek B, Kaul S. Evidence that high-density lipoprotein cholesterol is an independent predictor of acute platelet-dependent thrombus formation. The American journal of cardiology 1999; 84:1011-1017
- Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, Sviridov D, Cooper ME, Kingwell BA, Jackson SP. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation 2009; 120:2095-2104
- Brodde MF, Korporaal SJ, Herminghaus G, Fobker M, Van Berkel TJ, Tietge UJ, Robenek H, Van Eck M, Kehrel BE, Nofer JR. Native high-density lipoproteins inhibit platelet activation via scavenger receptor BI: role of negatively charged phospholipids. Atherosclerosis 2011; 215:374-382
- Nofer JR, Brodde MF, Kehrel BE. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clinical and experimental pharmacology & physiology 2010; 37:726-735
- Li D, Weng S, Yang B, Zander DS, Saldeen T, Nichols WW, Khan S, Mehta JL. Inhibition of arterial thrombus formation by ApoA1 Milano. Arteriosclerosis, thrombosis, and vascular biology 1999; 19:378-383
- Carson SD. Plasma high density lipoproteins inhibit the activation of coagulation factor X by factor VIIa and tissue factor. FEBS letters 1981; 132:37-40
- Sugatani J, Miwa M, Komiyama Y, Ito S. High-density lipoprotein inhibits the synthesis of platelet-activating factor in human vascular endothelial cells. Journal of lipid mediators and cell signalling 1996; 13:73-88
- Viswambharan H, Ming XF, Zhu S, Hubsch A, Lerch P, Vergeres G, Rusconi S, Yang Z. Reconstituted high-density lipoprotein inhibits thrombin-induced endothelial tissue factor expression through inhibition of RhoA and stimulation of phosphatidylinositol 3-kinase but not Akt/endothelial nitric oxide synthase. Circulation research 2004; 94:918-925
- Epand RM, Stafford A, Leon B, Lock PE, Tytler EM, Segrest JP, Anantharamaiah GM. HDL and apolipoprotein A-I protect erythrocytes against the generation of procoagulant activity. Arterioscler Thromb 1994; 14:1775-1783
- Ramet ME, Ramet M, Lu Q, Nickerson M, Savolainen MJ, Malzone A, Karas RH. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J Am Coll Cardiol 2003; 41:2288-2297
- Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest 2008; 118:3701-3713
- Annema W, von Eckardstein A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circulation journal : official journal of the Japanese Circulation Society 2013; 77:2432-2448
- de Souza JA, Vindis C, Negre-Salvayre A, Rye KA, Couturier M, Therond P, Chantepie S, Salvayre R, Chapman MJ, Kontush A. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. Journal of cellular and molecular medicine 2010; 14:608-620
- Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Luscher TF, Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 2013; 127:891-904
- Suc I, Escargueil-Blanc I, Troly M, Salvayre R, Negre-Salvayre A. HDL and ApoA prevent cell death of endothelial cells induced by oxidized LDL. Arteriosclerosis, thrombosis, and vascular biology 1997; 17:2158-2166
- Sugano M, Tsuchida K, Makino N. High-density lipoproteins protect endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Biochem Biophys Res Commun 2000; 272:872-876
- Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proceedings of the National Academy of Sciences of the United States of America 2011; 108:9613-9618
- Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res 2004; 45:993-1007
- Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, Subbanagounder G, Faull KF, Reddy ST, Miller NE, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res 2000; 41:1481-1494
- Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res 2000; 41:1495-1508
- Vohl MC, Neville TA, Kumarathasan R, Braschi S, Sparks DL. A novel lecithin-cholesterol acyltransferase antioxidant activity prevents the formation of oxidized lipids during lipoprotein oxidation. Biochemistry 1999; 38:5976-5981
- Watson AD, Navab M, Hama SY, Sevanian A, Prescott SM, Stafforini DM, McIntyre TM, Du BN, Fogelman AM, Berliner JA. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest 1995; 95:774-782
- Noto H, Hara M, Karasawa K, Iso ON, Satoh H, Togo M, Hashimoto Y, Yamada Y, Kosaka T, Kawamura M, Kimura S, Tsukamoto K. Human plasma platelet-activating factor acetylhydrolase binds to all the murine lipoproteins, conferring protection against oxidative stress. Arteriosclerosis, thrombosis, and vascular biology 2003; 23:829-835
- Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. The Journal of biological chemistry 1998; 273:6088-6095
- Bashtovyy D, Jones MK, Anantharamaiah GM, Segrest JP. Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function. J Lipid Res 2011; 52:435-450
- Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arteriosclerosis, thrombosis, and vascular biology 2003; 23:1881-1888
- Kumpula LS, Kumpula JM, Taskinen MR, Jauhiainen M, Kaski K, Ala-Korpela M. Reconsideration of hydrophobic lipid distributions in lipoprotein particles. Chemistry and physics of lipids 2008; 155:57-62
- Christison J, Karjalainen A, Brauman J, Bygrave F, Stocker R. Rapid reduction and removal of HDL- but not LDL-associated cholesteryl ester hydroperoxides by rat liver perfused in situ. The Biochemical journal 1996; 314 ( Pt 3):739-742
- Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, Stengel D, Ninio E, Navab M, Mackness B, Mackness M, Holvoet P. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation 2003; 107:1640-1646
- Rallidis LS, Tellis CC, Lekakis J, Rizos I, Varounis C, Charalampopoulos A, Zolindaki M, Dagres N, Anastasiou-Nana M, Tselepis AD. Lipoprotein-associated phospholipase A(2) bound on high-density lipoprotein is associated with lower risk for cardiac death in stable coronary artery disease patients: a 3-year follow-up. J Am Coll Cardiol 2012; 60:2053-2060
- Wu A, Hinds CJ, Thiemermann C. High-density lipoproteins in sepsis and septic shock: metabolism, actions, and therapeutic applications. Shock 2004; 21:210-221
- Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. The Journal of experimental medicine 1994; 180:1025-1035
- Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America 1993; 90:12040-12044
- Hajduk SL, Moore DR, Vasudevacharya J, Siqueira H, Torri AF, Tytler EM, Esko JD. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. The Journal of biological chemistry 1989; 264:5210-5217
- Perez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homble F, Vanhamme L, Tebabi P, Pays A, Poelvoorde P, Jacquet A, Brasseur R, Pays E. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005; 309:469-472
- Lecordier L, Vanhollebeke B, Poelvoorde P, Tebabi P, Paturiaux-Hanocq F, Andris F, Lins L, Pays E. C-terminal mutants of apolipoprotein L-I efficiently kill both Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. PLoS pathogens 2009; 5:e1000685
- Patel PJ, Khera AV, Wilensky RL, Rader DJ. Anti-oxidative and cholesterol efflux capacities of high-density lipoprotein are reduced in ischaemic cardiomyopathy. European journal of heart failure 2013; 15:1215-1219
- Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti-oxidative capacity of high-density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol 2011; 58:2068-2075
- Smith JD. Dysfunctional HDL as a diagnostic and therapeutic target. Arteriosclerosis, thrombosis, and vascular biology 2010; 30:151-155
- Li C, Gu Q. Protective effect of paraoxonase 1 of high-density lipoprotein in type 2 diabetic patients with nephropathy. Nephrology 2009; 14:514-520
- Nobecourt E, Jacqueminet S, Hansel B, Chantepie S, Grimaldi A, Chapman MJ, Kontush A. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia 2005; 48:529-538
- Ferretti G, Bacchetti T, Busni D, Rabini RA, Curatola G. Protective effect of paraoxonase activity in high-density lipoproteins against erythrocyte membranes peroxidation: a comparison between healthy subjects and type 1 diabetic patients. The Journal of clinical endocrinology and metabolism 2004; 89:2957-2962
- Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, Lee TD, Reddy ST. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis and rheumatism 2012; 64:1828-1837
- Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, Park G, McMahon M, Paulus HE, Fogelman AM, Reddy ST. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis and rheumatism 2009; 60:2870-2879
- Kontush A, de Faria EC, Chantepie S, Chapman MJ. Antioxidative activity of HDL particle subspecies is impaired in hyperalphalipoproteinemia: relevance of enzymatic and physicochemical properties. Arteriosclerosis, thrombosis, and vascular biology 2004; 24:526-533
- Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 1995; 96:2758-2767
- Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 2003; 108:2751-2756
- Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 2001; 42:1308-1317
- Mastorikou M, Mackness B, Liu Y, Mackness M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabetic medicine : a journal of the British Diabetic Association 2008; 25:1049-1055
- Kalogerakis G, Baker AM, Christov S, Rowley KG, Dwyer K, Winterbourn C, Best JD, Jenkins AJ. Oxidative stress and high-density lipoprotein function in Type I diabetes and end-stage renal disease. Clinical science 2005; 108:497-506
- Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. The Journal of clinical endocrinology and metabolism 2004; 89:4963-4971
- de Souza JA, Vindis C, Hansel B, Negre-Salvayre A, Therond P, Serrano CV, Jr., Chantepie S, Salvayre R, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis 2008; 197:84-94
- Xu Y, Jin X, Ping Q, Cheng J, Sun M, Cao F, You W, Yuan D. A novel lipoprotein-mimic nanocarrier composed of the modified protein and lipid for tumor cell targeting delivery. Journal of controlled release : official journal of the Controlled Release Society 2010; 146:299-308
- Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E, Reddy ST. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 2011; 60:2617-2623
- Gowri MS, Van der Westhuyzen DR, Bridges SR, Anderson JW. Decreased protection by HDL from poorly controlled type 2 diabetic subjects against LDL oxidation may Be due to the abnormal composition of HDL. Arteriosclerosis, thrombosis, and vascular biology 1999; 19:2226-2233
- Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney international 2009; 76:437-444
- Moradi H, Pahl MV, Elahimehr R, Vaziri ND. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Translational research : the journal of laboratory and clinical medicine 2009; 153:77-85
- McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, Badsha H, Kalunian K, Charles C, Navab M, Fogelman AM, Hahn BH. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis and rheumatism 2006; 54:2541-2549
- Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Annals of the rheumatic diseases 2012; 71:1157-1162
- Tan KC, Chow WS, Lam JC, Lam B, Wong WK, Tam S, Ip MS. HDL dysfunction in obstructive sleep apnea. Atherosclerosis 2006; 184:377-382
- Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horvath T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von Eckardstein A, Drexler H, Landmesser U. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010; 121:110-122
- Persegol L, Verges B, Foissac M, Gambert P, Duvillard L. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2006; 49:1380-1386
- Persegol L, Foissac M, Lagrost L, Athias A, Gambert P, Verges B, Duvillard L. HDL particles from type 1 diabetic patients are unable to reverse the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2007; 50:2384-2387
- Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Luscher TF, Fliser D, Bahlmann FH, Landmesser U. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013; 38:754-768
- Charakida M, Besler C, Batuca JR, Sangle S, Marques S, Sousa M, Wang G, Tousoulis D, Delgado Alves J, Loukogeorgakis SP, Mackworth-Young C, D'Cruz D, Luscher T, Landmesser U, Deanfield JE. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA 2009; 302:1210-1217
- Holven KB, Aukrust P, Retterstol K, Otterdal K, Bjerkeli V, Ose L, Nenseter MS, Halvorsen B. The antiatherogenic function of HDL is impaired in hyperhomocysteinemic subjects. J Nutr 2008; 138:2070-2075
- Annema W, Nijstad N, Tolle M, de Boer JF, Buijs RV, Heeringa P, van der Giet M, Tietge UJ. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A(2). J Lipid Res 2010; 51:743-754
- Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, El-Gamal D, Wadsack C, Heinemann A, Marsche G. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res 2012; 53:1618-1624
- Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Annals of the rheumatic diseases 2014; 73:609-615
- Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, Heinecke JW, Saran R, Kaplan MJ, Pennathur S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Annals of the rheumatic diseases 2013; 72:1725-1731
- Yamamoto S, Yancey PG, Ikizler A, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V. Dysfunctional High-Density Lipoprotein in Patients on Chronic Hemodialysis. Journal of the American College of Cardiology 2012; 60:2372-2379
- Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G. Uremia Alters HDL Composition and Function. J Am Soc Nephrol 2011; 22:1631-1641
- Cavallero E, Brites F, Delfly B, Nicolaiew N, Decossin C, Degeitere C, Fruchart JC, Wikinski R, Jacotot B, Castro G. Abnormal Reverse Cholesterol Transport in Controlled Type-Ii Diabetic-Patients - Studies on Fasting and Postprandial Lpa-I Particles. Arterioscl Throm Vas 1995; 15:2130-2135
- Zhou HL, Tan KCB, Shiu SWM, Wong Y. Increased serum advanced glycation end products are associated with impairment in HDL antioxidative capacity in diabetic nephropathy. Nephrol Dial Transpl 2008; 23:927-933
- Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Luscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest 2011; 121:2693-2708
Sight-Threatening Graves’ Ophthalmopathy
CLINICAL RECOGNITION
Ophthalmopathy may develop any time in the course of Graves’ disease, or infrequently in association with primary thyroid failure or apparent Hashimoto’s thyroiditis, and is infrequently accompanied by thyroid dermopathy. Graves’ ophthalmopathy (GO) is usually mild to moderately severe, and about 75% of Graves’ patients apparently have no ocular involvement. However, GO may be sight threatening in 1-2% of cases. The latter represent an emergency requiring immediate treatment. GO-related sight loss may be due to corneal breakdown or, more frequently to dysthyroid optic neuropathy (DON). Corneal involvement and/or DON require an urgent referral to specialists. As shown in Table 1, these risky conditions should be suspected in patients with unexplained reduction in visual acuity (blurred vision which does not clear with blinking or closing one eye), changes in the intensity or quality of colors, history of popping out of the eyeballs (globe subluxation), presence of corneal opacity, incomplete closure of the eyelids (lagophthalmos), if associated with poor Bell’s phenomenon, spontaneous or gaze-evoked orbital pain, if associated with up-gaze restriction. DON may develop acutely (hours) or insidiously (weeks to months).
Table 1. Symptoms and Signs of Sight-Threatening Graves’ Ophthalmopathy |
| Symptoms |
| Severe eye pain and scratchy sensation |
| Acute or subacute blurred vision not clearing with blinking (abnormalities of tear film) or closing one eye (abnormality in eye movements) |
| Deterioration in the quality or intensity of color vision |
| Episode(s) of globe subluxation (popping out eyes) |
| Signs |
| Corneal opacity |
| Lagophthalmos (incomplete eye closure) particularly if associated with visible cornea on attempted eye closure |
| Pale or swollen disc, choroidal folds at fundoscopy |
PATHOPHYSIOLOGY
Graves’ ophthalmopathy is an autoimmune disorder triggered by autoreactive T-lymphocytes recognizing antigen(s) shared by the thyroid and the orbit. Culprit antigens may be the TSH receptor and the IGF-1 receptor. After antigen recognition, a cascade of events is triggered leading to orbital fibroblast proliferation, preadipocyte fibroblast differentiation into adipocytes, secretion of a number of cytokines in turn stimulating fibroblast growth, infiltration of extraocular muscles, and increased secretion of the hydrophilic glycosaminoglycans. These reactions eventually cause an expansion of the fibroadipose tissue, swelling and dysfunction of extraocular muscles, edema of orbital and periorbital tissues. These changes mechanically explain the most relevant clinical manifestations of the disease, such as exophthalmos, diplopia (double vision), and sight loss due to compression of the optic nerve.
DIAGNOSIS AND DIFFERENTIAL
The diagnosis of GO is usually easy in patients with Graves’ disease and bilateral ocular involvement. It may be more difficult when it is not associated with thyroid dysfunction (euthyroid Graves’ disease) or ocular involvement is asymmetrical or apparently unilateral. In these cases, other causes of exophthalmos and dysmotility must be ruled out. The latter include orbital tumors, vascular causes (e.g., arteriovenous fistulas), idiopathic myositis, and other inflammatory disorders.
Diagnostic Tests
Minor corneal abnormalities can be detected by slit lamp examination of the cornea which reveals punctate fluorescein staining. Severe corneal damage, usually occurring in patients with marked exophthalmos, is evident simply using a strong light. This shows a marked redness of the lower conjunctiva, a grey corneal opacity, or even a corneal abscess. The eyelids do not close over the cornea and the cornea is visible on attempted eye closure.
DON is due to optic nerve compression, most frequently occurring at the orbital apex (apical crowding), by the enlarged extraocular muscles, or to optic nerve stretching in the event of extreme exophthalmos. Although no single test is sufficient to establish or rule out DON, optic nerve involvement should be investigated by assessing best corrected visual acuity, color vision (e.g., using Ishihara charts), pupil responses by the swinging flashlight test for a relative afferent pupil defect, fundoscopy (optic disc pallor or swelling, choroidal folds), perimetry, or visual evoked potentials. Measurement of intraocular pressure (IOP), particularly in upward gaze, is useful to detect increases due to tightness of the inferior rectus muscle (Table 2). Orbital imaging (CT or MRI) are fundamental to show apical crowding and other features, such as intraorbital fat prolapsed and bony orbital angles, correlated with DON.
Table 2. Testing for Corneal Damage or Optic Neuropathy |
| Cornea |
| Direct visual examination |
| Slit lamp examination with corneal fluorescein staining |
| Optic Nerve |
| Best-corrected visual acuity |
| Color vision (Ishihara charts or others) |
| Pupil responses to swinging flashlight test (relative afferent papillary defect, RAPD) |
| Fundoscopy |
| Perimetry |
| Visual evoked potentials |
| Measurement of intraocular pressure (particularly in up-gaze) |
| Orbital imaging (CT or MRI) |
THERAPY
Corneal Breakdown
Frequent (hourly) use of topical lubricants and antibiotics is warranted. If these and other measures, such as moisture chambers, are not sufficient to prevent corneal ulceration and perforation, temporary measures to improve eyelid closure are necessary. These include blepharroraphy, tarsorraphy, emergency gluing, amnion membranes, and botulinum toxin. After controlling the acute situation, permanent improvement of eyelid closure is mandatory (Table 3). Corneal grafting may be then necessary.
Table 3. Managing Corneal Breakdown or Optic Neuropathy |
| Corneal Breakdown |
| Intensive (hourly) topical lubricants and antibiotics |
| Moisture chambers |
| Temporary measures to improve eye closure: blepharroraphy, tarsorraphy, amnion membranes, botulinum toxin, emergency gluing |
| Optic neuropathy |
| First-line treatment: intravenous methylprednisolone (0.5-1 gram in slow 2-3-hour infusion) for 3 consecutive days to be repeated on the next week |
| Second-line treatment: orbital decompression, if response is absent or poor after two weeks |
Optic neuropathy
DON must be treated aggressively. Intravenous glucocorticoids are the first-line treatment. Evidence of the best therapeutic regimen is missing. A commonly used protocol is based on the slow (2-3 hour) infusion of 0.5-1-gram methylprednisolone for three consecutive days. Gastric protection is required. Control of blood glucose and electrolytes is needed, as well as frequent measurement of blood pressure during and for a few hours after infusion. This treatment can be repeated during the next week. If, however, the response to treatment is poor or absent within two weeks or glucocorticoid treatment causes severe side effects, the patient should be promptly submitted for orbital decompression to prevent irreversible damage and sight loss (Table 3).
Treatment of ON (as well as of corneal breakdown) should be performed in specialized centers. New therapies using immune-suppression with agents such as rituximab or teprotumumab (antibody to IGF-1 receptor) are under investigation, but their role in the setting of sight-threatening Graves’ ophthalmopathy is unsettled. In particular, rituximab cannot prevent the occurrence of DON and, therefore, should not be used in patients with impending or overt DON.
FOLLOW-UP
After the emergency treatment (medical and/or surgical), residual manifestations of Graves’ ophthalmopathy should be treated, as appropriate. If the disease is still active, glucocorticoid treatment can be continued using either oral or intravenous glucocorticoids. It is recommended not to exceed a cumulative dose of 8 grams of intravenous methylprednisolone per cycle because of potential severe hepatotoxicity. If the ophthalmopathy is inactive, rehabilitative surgery (orbital decompression and/or squint surgery and/or eyelid surgery) is often necessary for cosmetic and/or functional reasons.
All patients should be urged to refrain from smoking, because the latter is associated with more severe forms of GO and a decreased effectiveness of glucocorticoids (and orbital radiotherapy). The dilemma of the optimal long-term treatment for hyperthyroidism in patients with GO remains unsolved in the absence of sound evidence based on randomized clinical trials. Comparative benefits of anti-thyroid drugs, RAI, and surgery are described in the first reference below.
GUIDELINES
Bartalena L, Baldeschi L, Dickinson A, et al., Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J. Endocrinol 2008; 158: 273-285).
Bartalena L, Baldeschi L, Boboridis K et al., The 2016 European Thyroid Association/European Group in Graves’ Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 2016; 5: 9-26.
REFERENCES
Bartalena L., Graves’ Disease: Complications. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-2018 Feb 20
Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease. J Endocrinol Invest 2014; 37: 691-700.
Familial Or Sporadic Adrenal Hypoplasia Syndrome
ABSTRACT
Congenital adrenal hypoplasia is a rare cause of primary adrenocortical failure, which was first described in 1948. During the last two decades, the genetic basis for several forms of familial adrenal insufficiency syndromes has been elucidated. The molecular mechanisms for these disorders involve a broad spectrum of cellular and physiologic processes, including metabolism, nuclear protein import, oxidative stress defense-mechanisms, and regulation of cell cycle. Adrenal hypoplasia can occur: 1) secondary to defects in transcription factors involved in pituitary development or (2) defects in ACTH synthesis and secretion; 3) as a primary defect in the development of the adrenal gland; 4) as part of rare syndromes associated with adrenal hypoplasia/aplasia, which are inherited in an autosomal recessive or autosomal dominant manner; and 5) in the context of chromosomal abnormalities. Early diagnosis and management are crucial because of the life-threatening nature of the condition. Depending on the etiology, adrenal crisis may occur in early infancy or could insidiously develop over the course of childhood or adolescence. Moreover, some of these conditions previously thought to occur only in childhood, may also be diagnosed later in adulthood and present with variable phenotypes, including isolated infertility or disorders of sex differentiation. The clinical manifestations of primary adrenal insufficiency (PAI) result from deficiency of all adrenocortical hormones (aldosterone, cortisol, androgens). The acute presentation can be precipitated by physiologic stress, such as surgery, trauma, or an intercurrent infection. Patients may present with signs and symptoms of complete adrenal insufficiency, usually early in life, including hypoglycemic convulsions, hyponatremia, hyperkalemia, metabolic acidosis or later with hyperpigmentation, vomiting and poor weight gain. It should be remembered, that the most common cause of PAI in children is congenital adrenal hyperplasia due to 21-hydroxylase deficiency and can be excluded by measuring baseline or ACTH-stimulated 17-hydroxyprogesterone levels in serum. Screening for autoimmune Addison disease includes detection of 21-hydroxylase antibodies. Males with negative 21-hydroxylase antibodies should be tested for adrenoleukodystrophy measuring very–long-chain fatty acids concentrations in plasma. The presence of alacrima in patients with PAI should raise suspicion for Triple A syndrome, whereas the combination of PAI and hypogonadotropic hypogonadism in a male patient point towards X-linked adrenal hypoplasia congenita. To date, molecular genetic testing is commercially available for the identification of several genes involved in adrenal hypoplasia syndromes. The early identification of these diseases can have important prognostic and therapeutic implications for patients with respect to surveillance for associated conditions, initiation of early treatment or screening of family members who are at risk. Adrenal insufficiency is potentially life threatening, thus treatment should be initiated as soon as the diagnosis is confirmed, or sooner if the patient presents in adrenal crisis. Therapy consists of life-long replacement therapy with glucocorticoids and mineralocorticoids. Hypogonadism or other associated disorders should be treated appropriately. Screening of family members for the disease or carrier status may also be indicated and can be critical for family planning. When a monogenic cause of adrenal failure is identified, genetic counseling is indicated. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
The adrenal glands consist of two anatomically and functionally distinct subunits, the cortex and the medulla. The adrenal cortex secretes glucocorticoids, mineralocorticoids and androgens. The glucocorticoid, cortisol, is secreted by the cells of the intermediate zona fasciculata. Its secretion is tightly regulated by the hypothalamic corticotropin-releasing hormone (CRH) and vasopressin (AVP) and by the pituitary adrenocorticotropic hormone (ACTH) (1). Glucocorticoids regulate a broad spectrum of physiologic functions essential for life and play an important role in the maintenance of basal and stress-related homeostasis. The mineralocorticoid, aldosterone, is produced by the outer adrenal zona glomerulosa. This steroid regulates water and electrolyte homeostasis and its secretion is primarily under the control of the renin-angiotensin system, although it may be weakly influenced by ACTH. The adrenal androgens, dehydroepiandrosterone (DHEA), its sulfate (DHEA-S) and androstenedione, are secreted by the inner zona reticularis under the control of ACTH.
CONGENITAL ADRENAL HYPOPLASIA
Congenital adrenal hypoplasia is a rare cause of primary adrenocortical failure, which was first described in 1948. It has an estimated frequency of 1:12,500 live births (2). During the last decade there have been significant advances in our understanding of the genetic etiology of several forms of adrenal insufficiency with a presentation in infancy or childhood. Several of these conditions affect adrenal development and are commonly known as adrenal hypoplasia. Adrenal hypoplasia may be due to (3-9):
- Secondary to defects in transcription factors involved in pituitary development (e.g. HESX1, LHX4, SOX3) or defects in ACTH synthesis (TPIT), processing and release (e.g. POMC or PC1);
- Part of an ACTH resistance syndrome [MC2R/ACTH receptor, MRAP, AAAS (triple A syndrome), StAR, CYP11A1, MCM4, NNT, TXNRD2, GPX1, PRDX3 mutations];
- A primary defect in the development of the adrenal gland itself (primary/congenital adrenal hypoplasia; X-linked form/DAX1 gene mutations or deletions, autosomal recessive form/SF-1 gene mutations or deletions, autosomal recessive form of uncertain etiology, IMAGe syndrome, MIRAGE syndrome, Familial steroid-resistant nephrotic syndrome with adrenal insufficiency due to SGPL1 deficiency);
- Part of rare syndromes associated with adrenal hypoplasia/aplasia, which are inherited in an autosomal recessive (Meckel-Gruber syndrome, Pena-Shokeir syndrome, Pseudotrisomy 13, Hydrolethalus syndrome, Galloway-Mowat syndrome) or autosomal dominant (Pallister-Hall syndrome) manner; and
- In the context of chromosomal abnormalities (tetraploidy, triploidy, trisomy 18, trisomy 21, 5p duplication, monosomy 7 and the 11q syndrome), which are often associated with central nervous system (CNS) abnormalities.
There are two distinct histological patterns of the adrenal cortices in this rare syndrome, the miniature adult and cytomegalic forms. In the miniature adult form of adrenal hypoplasia congenital (AHC), the small amount of residual adrenal cortex is composed primarily of permanent adult cortex with normal structural organization. The miniature adult form is either sporadic or inherited in an autosomal recessive manner, and is frequently associated with abnormal CNS development, including anencephaly or pituitary gland abnormalities.
In the cytomegalic form of AHC, the residual adrenal cortex is structurally disorganized with scattered irregular nodular formations of eosinophilic cells, with the adult permanent zone absent or nearly absent. Enlarged cells are present, some with abundant vacuolated cytoplasm. The cytomegalic form is generally considered to be X-linked, but there may be one or more autosomal genes associated with this phenotype (6, 10, 11).
Genetic causes of adrenal hypoplasia and aplasia syndromes are summarized in Table 1. However, this review focuses on ACTH resistance syndromes and disorders of adrenal gland development.
TABLE 1: Genetic Causes of Adrenal Hypoplasia and Aplasia |
||
| Genetics | Associated Clinical Manifestations | |
|
Adrenal dysgenesis Primary/congenital adrenal hypoplasia |
||
| Pallister-Hall syndrome |
GLI3 -autosomal dominant, 25% de novo mutation -transcription factor, mediator of Shh signaling |
Hypothalamic hamartomas, mesoaxial and postaxial polydactyly, bifid epiglottis, imperforate anus, genitourinary anomalies, laryngotracheal cleft, pituitary insufficiency |
| Meckel-Gruber syndrome |
MKS1 -autosomal recessive -protein localized to the basal body, required for formation of the primary cilium in ciliated epithelial cells |
Cystic renal disease, CNS malformation – occipital encephalocele, polydactyly, hepatic abnormalities |
| Pena-Shokeir syndrome |
-DOK7 (homozygous truncating mutation) non-catalytic cytoplasmic adaptor protein that is expressed specifically in muscle and is essential for the formation of neuromuscular synapses -RAPSN (homozygosity for a frameshift mutation) postsynaptic protein that connects and stabilizes acetylcholine receptors at the neuromuscular junction -autosomal recessive |
Arthrogryposis, facial anomalies, IUGR, camptodactyly, fetal akinesia, polyhydramnion, pulmonary hypoplasia, cardiac defects, intestinal malrotation |
| Pseudotrisomy 13 | Genetic cause unclear; thought to be autosomal recessive | Holoprosencephaly, polydactyly, craniofacial anomalies |
| Hydrolethalus syndrome |
HYLS1 -protein incorporated into centrioles as they are formed, required for the formation of cilia -autosomal recessive |
Hydrocephaly, micrognathia, polydactyly, abnormal genitalia, congenital heart defects, respiratory organ defects |
| Galloway-Mowat syndrome |
WDR73 - protein found in the cytoplasm during interphase, but accumulates at the spindle poles and astral microtubules during mitosis - reduced expression results in abnormalities in the size and morphology of the nucleus -autosomal recessive |
Nephrotic syndrome, microcephaly, encephalopathy, diaphragmatic hernia |
| X-linked | NR0B1 (DAX1) |
Males: hypogonadotropic hypogonadism. In some cases, normal puberty, central or gonadotropin-independent precocious puberty Infertility, attention deficit disorder, short stature, growth hormone deficiency, inappropriate tall stature, renal ectopy, macrophalia in infancy
Females carrying homozygous or heterozygous mutations: isolated hypogonadotropic hypogonadism or extreme pubertal delay, respectively |
| Xp21 contiguous gene syndrome | Deletion of genes for Duchenne muscular dystrophy, glycerol kinase, and NR0B1 | Duchenne muscular dystrophy, glycerol kinase deficiency, psychomotor retardation, hepatic iron deposition |
| SF-1 linked |
NR5A1 (SF-1) -autosomal recessive or dominant |
XY sex reversal, gonadal insufficiency, 46,XX ovotesticular/testicular DSD, gonadoblastoma, germ cell neoplasia in situ (GCNIS), splenic anomalies, ovarian insufficiency Microdeletions of chromosome 9q33.3, involving NR5A1: genitopatellar syndrome, developmental delay, ovotestes, XY sex reversal |
| IMAGe syndrome |
CDKN1C -imprinted mode of inheritance/maternal transmission |
Intrauterine growth retardation, metaphyseal dysplasia, genital abnormalities, hypercalcemia, dysmorphic facial features, soft tissue calcifications, growth hormone deficiency, skeletal abnormalities, hydronephrosis, hypercalciuria-associated nephrocalcinosis, oligohydramnios |
| MIRAGE syndrome |
SAMD9 -autosomal dominant |
Myelodysplasia, infection, restriction of growth, genital phenotypes, enteropathy, dysmorphic features, bronchopulmonary dysplasia, neurologic abnormalities, skeletal abnormalities, renal defects, apneas, reduced body fat |
| Metabolic Disorders | ||
| Familial steroid-resistant nephrotic syndrome with adrenal insufficiency |
SGPL1 -autosomal recessive |
Adrenal calcifications, ichthyosis, immunodeficiencies, dermatologic, ophthalmologic, neurologic, skeletal and genital abnormalities, hypothyroidism, muscular hypotonia, fetal demise, fetal hydrops, facial dysmorphism, hypocalcemia, dilated cardiomyopathy, intestinal malrotation, capillary leak syndrome. |
| ACTH Resistance Syndromes | ||
|
Familial glucocorticoid deficiency (FGD) Type 1
|
MC2R gene mutations -autosomal recessive
|
Hyperpigmentation, tall stature, characteristic facial features, such as hypertelorism and frontal bossing, lethargy and muscle weakness but normal blood pressure (mostly normal production of MC) |
|
FGD Type 2
|
MRAP gene mutations --autosomal recessive |
Hyperpigmentation, normal height, hypoglycemia, lethargy, and muscle weakness, but normal blood pressure (mostly normal production of MC), obesity |
|
Nonclassic CLAH (FGD variant) |
partial loss-of-function mutations of - StAR* - CYP11A1 - - autosomal recessive |
Milder phenotype of FGD with no gonadal derangement potentially hypogonadism and compromised fertility in adulthood |
| Variant of FGD (DNA repair defect) |
MCM4 gene mutations -autosomal recessive |
Growth failure, microcephaly, increased chromosomal breakage, natural killer cell deficiency, recurrent viral infections |
| Variant of FDG (Deficiency of mitochondrial radicals detoxification) |
NNT -autosomal recessive
TXNRD2 -autosomal recessive
GPX1 PRDX3 -autosomal recessive |
Precocious puberty associated with testicular nodules**, hypothyroidism, hypertrophic cardiomyopathy, azoospermia associated with testicular adrenal rests and elevated FSH levels, plagiocephaly Left ventricular noncompaction¶ Only glucocorticoid deficiency Dilated cardiomyopathy‡
Only glucocorticoid deficiency Only glucocorticoid deficiency |
| Triple A syndrome (Allgrove’s syndrome) |
AAAS gene mutations -autosomal recessive |
Achalasia, alacrima, deafness, mental retardation, hyperkeratosis, neurodegeneration, short stature, osteoporosis, xerostomia, nasal speech, angular cheilitis, glossitis and fissured tongue, enamel defect, poor wound healing, hypolipoproteinemia type IIb, scoliosis, pes cavus, long QT syndrome, microcephaly, dysmorphic features, premature loss of permanent teeth
|
AAAS=achalasia, adrenocortical insufficiency, alacrima syndrome. CDKN1C= Cyclin-dependent kinase inhibitor 1C (p57, Kip2). CLAH=Congenital Lipoid Adrenal Hyperplasia. CYP11A1= Cytochrome P450, family 11, subfamily A, polypeptide 1. DAX1= Dosage sensitive sex reversal, Adrenal hypoplasia congenita, critical region on X chromosome, gene-1. DOX7=Docking protein 7. FGD: familial glucocorticoid deficiency. FSH: Follicle stimulating hormone. GLI3=gene responsible for Greig cephalopolysyndactyly syndrome (GCPS), Pallister-Hall syndrome (PHS), Preaxial polydactyly type IV and Postaxial polydactyly type-A1 and B. GPX1= Glutathione Peroxidase 1. HYLS1= Hydrolethalus syndrome protein 1. IMAGe=Intrauterine growth restriction (IUGR), Metaphyseal dysplasia, Adrenal hypoplasia congenita, and Genitourinary abnormalities. MC=Mineralocorticoids. MC2R=Melanocortin 2 receptor. MCM4= Minichromosome maintenance complex component 4. MIRAGE=Myelodysplasia, Infection, Restriction of growth, Adrenal hypoplasia, Genital phenotypes and Enteropathy. MKS1=gene responsible for Meckel syndrome, type 1 and Bardet-Biedl syndrome type 13. MRAP=Melanocortin 2 receptor accessory protein. NNT= Nicotinamide nucleotide transhydrogenase. NR0B1= Nuclear Receptor subfamily 0, group B, member 1. NR5A1= Nuclear receptor subfamily 5 group A member 1. PRDX3=Peroxiredoxin 3. RAPSN=Receptor-associated protein of the synapse. SAMD9=Sterile Alpha Motif Domain-Containing 9. SF-1=Steroidogenic factor 1. SGPL1= Sphingosine-1-Phosphate Lyase 1. Shh= Sonic hedgehog. StAR= Steroidogenic acute regulatory protein. TXNRD2= Thioredoxin reductase 2. WDR73= WD repeat domain 73.
* To date, nine StAR mutations have been reported in patients with NCLAH (30).
**Leydig cell adenoma identified in one case (40).
¶ Heterozygous loss of function mutations in NNT gene (42).
‡ TXNRD2 mutations have been detected in 3 out of 227 patients with a diagnosis of dilated cardiomyopathy, however, no data are available on their adrenal function (15).
ADRENAL HYPOPLASIA AS PART OF AN ACTH RESISTANCE SYNDROME
ACTH resistance syndromes include two distinct genetic disorders, both of which are inherited in an autosomal recessive manner and are characterized by ACTH insensitivity:
- Familial Glucocorticoid Deficiency (FGD)
- Allgrove syndrome or Triple A syndrome
Familial Glucocorticoid Deficiency (FGD)
Familial (isolated) glucocorticoid deficiency (FGD), which is also known as hereditary unresponsiveness to ACTH, is a rare autosomal recessive disorder characterized by glucocorticoid deficiency (12, 13). The underlying genetic defect is known in approximately 70% of patients with FGD.
CLINICAL AND LABORATORY FEATURES OF FGD
Patients with FGD are usually diagnosed during the neonatal period or in early childhood. However, the oldest affected member of the kindred, carrying MCM4 and TXNRD2 mutations (see Genetics below), presented at the age of 8.5 years and 10.8 years, respectively (14, 15). Patients with FDG may present with hypoglycemic seizures, hyperpigmentation, recurrent infections, transient neonatal hepatitis, failure to thrive, collapse and coma. The long-term neurological sequelae of FGD can vary from learning difficulties to spastic quadriplegia, which may reflect the severity and number of hypoglycemic episodes in childhood. There may be a family history of unexplained neonatal death, history of other family member(s) affected with FGD and/or parental consanguinity (12, 16).
The clinical manifestations of FGD reflect resistance to ACTH. The typical hormonal profile in FGD is a combination of low cortisol but high plasma ACTH concentrations, in the presence of normal plasma renin activity and aldosterone concentrations. Most patients with FGD have markedly elevated ACTH concentrations, which correlate with the degree of ACTH resistance. Hyperpigmentation is often observed during the first months of life owing to the effect of ACTH on the melanocortin-1 receptors in melanocytes (12).
ADRENAL IMAGING
In the MRI or CT scans, the adrenal glands appear small in size.
HISTOPATHOLOGY
Absence of fasciculata or reticularis cells and disorganization of glomerulosa cells have been observed (17).
GENETICS
FGD was first described by Shepard et al. (18) in 1959, when he reported two siblings with “familial Addison’s disease”. It took 30 years for the first inactivating ACTH receptor mutations to be detected (19, 20). To date, FGD has been associated with mutations in seven genes: MC2R (ACTH receptor/melanocortin 2 receptor) (OMIM 202200), MRAP (MC2R accessory protein) (OMIM 607398), StAR (steroidogenic acute regulatory protein) (OMIM 201710), CYP11A1 (cytochrome P450, family 11, subfamily A, polypeptide 1) (OMIM 613743), NNT (nicotinamide nucleotide transhydrogenase) (OMIM 614736), MCM4 (the mini chromosome maintenance-deficient 4 homolog gene) (OMIM 609981), TXNRD2 (thioredoxin reductase 2) (OMIM 617825), GPX1 (Glutathione Peroxidase 1) and PRDX3 (peroxiredoxin 3) (9, 21,). Mutations in the MC2R and MC2R accessory protein (MRAP) account for approximately 50% of all cases.
The ACTH receptor MC2R is a 7-membrane G-protein coupled receptor located almost exclusively in the adrenocortical cells. To date, more than 50 mutations have been described in the MC2R gene (Human Gene Mutations Database, www.hgmd.cf.ac.uk) and represent the most common cause of FGD (25% of cases, FGD type 1) (8, 16). Some of them are shown in Table 2. FGD type 1 patients usually present in early childhood. Tall stature has been observed in some cases (22).
TABLE 2: Mutations of the MC2R in FGD Patients |
||
| Mutation | Probable Effect of Mutation | Reference |
| p.D107G | Failure to bind ACTH | Aza-Carmona et al,13. |
| p.R145C | Trafficking defect | Aza-Carmona et al,13. |
| c.459_460insC | Translation frame shift after codon 154 and a premature termination codon at 248 of the MC2R mRNA (p.I154fsX248) | Al Kandari et al,43. |
| p.Leu225Arg | Unknown | Akin et al,44. |
| K289fs | Impaired cell surface expression (Loss of C terminus of MC2R) | Hirsch et al,45. |
| G116V | Impaired cell surface expression | Collares et al,46. |
| T159K | Impaired cell surface expression | Elias et al,47. |
| D20N | Possible loss of ligand affinity | Chung et al,48. |
| H170L | Loss of signal transduction | Chung et al,48 |
| D103N | Loss of signal transduction and loss of ligand affinity | Berberoglu et al,49, Chung et al,48. |
| R137W | Loss of signal transduction | Ishii et al,50. |
| P273H | Possible structural disruption | Wu et al,51. |
| S120R | Possible structural disruption | Tsigos et al,20,52. |
| R201X | Truncated receptor | Tsigos et al,20. |
| S74I | Possible loss of ligand affinity | Clark et al,19. |
| I44M | Possible loss of ligand affinity | Weber et al,53. |
| Y254C | Possible structural disruption | Tsigos et al,52,54. |
| R146H | Loss of signal transduction | Weber et al,53. |
| R128C | Loss of signal transduction | Weber et al,53 . |
| L192fs | Truncated receptor | Weber et al,53. |
| D107N | Loss of ligand affinity and loss of signal transduction | Naville et al,55, Chung et al,48. |
| C251F | Possible structural disruption | Naville et al,55. |
| G217fs | Truncated receptor | Naville et al,55. |
| p.Pro281GlnfsX9 | Frameshift mutation | Delmas et al,56. |
In 2005, a second gene was identified, located at 21q22.1 and encoding MC2R accessory protein (MRAP), a 19-kDa single-transmembrane domain protein. In humans, MRAP is expressed in the adrenal cortex, pituitary, brain, testis, ovary, breast, thyroid, lymph node, skin, and fat. This protein serves as an essential cofactor of MC2R to promote its trafficking from the endoplasmic reticulum to the cell surface and subsequent signaling in response to ACTH (16, 23-25). Mutations in MRAP are responsible for a further 15-20% of FGD cases (FGD type 2). Most patients with FGD type 2 present in the neonatal period or in very early infancy. However, missense MRAP mutations are associated with a milder phenotype and late onset adrenal insufficiency (AI) (26). Interestingly, obesity has been reported in a patient harboring homozygous MRAP mutations and his heterozygous family members, whereas the only unaffected member of the family had normal weight (25). Studies on Mrap-/- mice demonstrated the important role of MRAP plays in both steroidogenesis and the regulation of adrenal cortex zonation. Mrap-/- mice were shown to have isolated GC deficiency with normal aldosterone and catecholamine production and small adrenal glands with gross impairment of the adrenal capsular morphology and cortex zonation. Furthermore, progenitor cell differentiation was significantly impaired, with dysregulation of WNT4/b-catenin and sonic hedgehog pathways (27). MRAP mutations are summarized in Table 3.
TABLE 3: Mutations of the MRAP in FGD Patients |
||
| Mutation | Probable Effect of Mutation | References |
| c.106+2_3dupTA | Skipping of exon 3 (No protein or lack transmembrane domain) | Jain et al,16. |
| c.3G>A | Unknown | Chung et al,57, Collares et al,46, McEachern et al,58. |
| c.175T>G | Full-length protein with amino acid change-impaired cAMP generation | Hughes et al,59. |
| c.76T>C | Full-length protein with amino acid change-impaired cAMP generation | Hughes et al,59. |
| c.106+2insT | Skipping of exon 3 (No protein or lack transmembrane domain) | Chung et al,57, Metherell et al,23. |
| c.106+1G>T | Skipping of exon 3 (No protein or lack transmembrane domain) | Chung et al,57, Metherell et al,23 |
| c.106+1G>A | Skipping of exon 3 (No protein or lack transmembrane domain) | Chung et al,57, Metherell et al,23. |
| c.106+1G>C | Skipping of exon 3 (No protein or lack transmembrane domain) | Chung et al,57, Metherell et al,23. |
| c.106+1delG | Skipping of exon 3 (No protein or lack transmembrane domain) | Chung et al,57., Metherell et al,23., Akin et al60. |
| c.33C>A | Shortened protein if translated | Chan et al,12. |
| c.17-23delACGCCTC | Shortened protein if translated | Modan-Moses et al,61. |
| c.128delG (p.V44X) | Frameshift mutation causing a premature termination (V44X) in exon 4 | Metherell et al,23, Rumie et al,25 |
Interestingly, mutations in steroidogenic acute regulatory protein (StAR) and more rarely cytochrome P450 family 11 subfamily A member 1 (CYP11A1) have also been detected in patients with FGD (StAR: approximately 5% of FGD patients). Mutations in these two enzymes usually result in Congenital Lipoid Adrenal Hyperplasia (CLAH), a severe disorder with both adrenal and gonadal steroid insufficiencies. However, certain, partial loss-of-function mutations may be associated with a milder phenotype with no gonadal derangement, termed non-classic CLAH (NCLAH). To date, nine StAR mutations have been reported in patients with NCLAH. Of note, affected individuals require life-long monitoring of both adrenal and gonadal function because their disorder may evolve. Hypogonadism and infertility may occur in adulthood. (28-31).
Recently, mutations in the mini chromosome maintenance-deficient 4 (MCM4) homolog gene have been identified in an Irish travelling community presenting with a variant of FGD. These patients had short stature, chromosomal breakage, natural killer cell deficiency and progressive primary adrenal insufficiency (PAI) characterized by ACTH resistance with glucocorticoid deficiency and normal mineralocorticoids (MC) levels. Typically, patients started with normal adrenal function and developed PAI over time. The MCM4 gene, mapped on 8q11.2 chromosome, is part of a heterohexameric helicase complex, which is important for DNA replication and genome integrity. MCM4 deficiency leads to genomic instability and is associated with increased incidence of cancer and developmental defects. Therefore, it is recommended that patients carrying this mutation are followed-up closely. The c.71-1insG splice site mutation found in the Irish travelling community was predicted to lead to a frameshift with a prematurely terminated translation product (p.Pro24ArgfsX4) (32, 33).
ΝΝΤ (nicotinamide nucleotide transhydrogenase), a highly conserved gene, encodes a redox-driven proton pump of the inner mitochondrial membrane. This enzyme uses energy from the mitochondrial proton gradient to produce high concentrations of NADPH. Detoxification of reactive oxygen species (ROS) in mitochondria by glutathione peroxidases (GPX) depends on this NADPH for regeneration of reduced glutathione (GSH) from oxidized glutathione (GSSG) to maintain a high GSH/GSSG ratio (Figure 1). The adrenal cortex contains high amounts of P450 steroid enzymes, which use NADPH for their catalytic activity. Its function is therefore very sensitive to ROS (34). ROS may suppress StAR protein synthesis and thus inhibit steroidogenesis (9). In addition, the peroxiredoxin system (PRDX), another antioxidant defense mechanism which removes H2O2 and lipid peroxides also requires NADPH (9, 34). PRDX3 is a mitochondrial protein highly expressed in human adrenals. Inactivation of PRDX3 results in accumulation of H2O2, activation of p38 MAPK signaling pathways, suppression of StAR protein synthesis and inhibition of steroidogenesis (9, 35). Mutations in GPX1 and PRDX3 have been rarely identified in patients with FGD (9, 36).
Figure 1. Detoxification of reactive oxygen species in the mitochondria. ΝΝΤ (nicotinamide nucleotide transhydrogenase) is a key enzyme, located in the inner mitochondrial membrane, that plays an important role in maintaining the mitochondrial redox balance. It utilizes the electrochemical proton gradient to generate NADPH from NADH and NADP. NNT provides high concentrations of NADPH for detoxification of H2O2 by the glutathione and thioredoxin pathways. Manganese superoxide dismutase catalyzes the conversion of the superoxide radical Ο2.- to H2O2. The peroxiredoxin system (PRDX), another antioxidant defense mechanism, which removes H2O2 and lipid peroxides also requires NADPH. NNT loss would result in compromised NADPH production, thereby rendering the mitochondria more susceptible to oxidative stress. Modified by Prasad et al (34) and Flück (9).
StAR: Steroidogenic acute regulatory protein; CYP11A1= Cytochrome P450, family 11, subfamily A, polypeptide 1; GSR: glutathione reductase; GSH: reduced glutathione; TXNRD2: thioredoxin reductase 2; TXN2: thioredoxin 2; GLRX2: glutaredoxin 2; NNT: nicotinamide nucleotide transhydrogenase; PRDX3: peroxiredoxin 3; GPX: glutathione peroxidase; MnSOD: manganese superoxide dismutase.
NNT mutations account for 5–10% of FGD patients. The first mutations in the NNT gene were identified six years ago in 20 patients with FGD (candidate region localized on chromosome 5p13–q12), in whom mutations of MC2R, MRAP and StAR had not been detected. A novel homozygous missense mutation at exon 5 of the NNT gene was subsequently reported in a Japanese patient and was predicted to have a loss-of-function effect (c.644T>C, p.Phe215Ser) (37, 38). In mice with Nnt loss, higher levels of adrenocortical cell apoptosis and impaired glucocorticoid production were observed. NNT knockdown in a human adrenocortical cell line resulted in impaired redox potential and increased ROS levels.
It is of great interest, that two patients from non-consanguineous parents of East Asian and South African origin were diagnosed with FGD at the ages of 21 and 8 months respectively, caused by compound heterozygous mutations in NNT, i.e. a heterozygous intron 20 mutation (pseudoexon activation) in combination with a heterozygous stop-gain mutation in exon 3 of NNT gene (p.Arg71) (21).
Recent studies provide new insights into the effects of NNT deletion. Altered mitochondrial morphology, lower ATP content and increased ROS levels have been observed in fibroblasts derived from a patient harboring biallelic NNT mutations (35). Most recently, it was shown that both NNT loss and overexpression can negatively affect steroidogenesis and cause redox imbalance, resulting in reduced protein levels of two mitochondrial antioxidant enzymes (Prdx3 and thioredoxin reductase 2/Txnrd2) and CYP11A1. Transcriptomic analysis of Nnt−/− mice demonstrated upregulation of heat shock proteins, alpha- and beta-hemoglobins, possibly reflecting activations of compensatory mechanisms to cope with oxidative stress (39).
To date, more than 40 pathogenic variants of NNT gene have been identified. They are scattered throughout the gene, including abolishment of the initiating methionine, and splice, missense and nonsense mutations (35, 40, www.hgmd.cf.ac.uk). Phenotypic heterogeneity has been observed among patients carrying the same mutation or within the same family. Unlike “classic FGD”, adrenal dysfunction is not restricted to glucocorticoid deficiency, but may include mineralocorticoid deficiency as well (35, 40). AI is usually diagnosed around the first year of life, may be severe and present with hypoglycemic seizures
Although, NNT mutations have been known to affect preferentially the adrenal glands, all tissues rich in mitochondria may be affected. Extra-adrenal features have been first demonstrated in Nnt-mutant mice, which had reduced insulin secretion and high-fat diet-induced diabetes mellitus, in addition to adrenal dysfunction (27). More recently, extra-adrenal manifestations were also noted in patients harboring homozygous or compound heterozygous NNT mutations, including: precocious puberty associated with testicular nodules (Leydig cell adenoma identified in one case), hypothyroidism, hypertrophic cardiomyopathy, azoospermia associated with testicular adrenal rests and elevated FSH levels and mild plagiocephaly (40, 41).
Of note, heterozygous loss of function mutations in NNT have been recently identified in two patients presenting with left cardiac ventricular noncompaction, an autosomal-dominant cardiomyopathy, which is frequently associated with mitochondrial disorders and cardiac hypertrophy (42).
In 2014, Prasad et al described the first homozygous mutation in the thioredoxin reductase 2 (TXNRD2) gene in an extended consanguineous Kashmiri kindred presenting with FGD (stop gain mutation, c.1341T>G; p.Y447X within exon 15). The selenoprotein TXNRD2, one of three thioredoxin reductases, is mitochondria specific and contributes to the maintenance of redox homeostasis. Particularly high TXNRD2 mRNA levels have been noted in the adrenal cortex compared with the other human tissues investigated, suggesting a susceptibility of the adrenal cortex and especially zona fasciculata to oxidative stress. Given that the final step of cortisol production, which is catalyzed by CYP11B1 in the mitochondria, accounts for approximately 40% of the total electron flow from NAPDH directed at reactive oxygen species production during steroidogenesis, individuals with TXNRD2 and NNT mutations primarily develop glucocorticoid deficiency. Extra-adrenal manifestations, associated with TXNRD2 mutations have also been reported. Txnrd2 deletion in mice is embryonically lethal, resulting in fatal cardiac and hematopoietic defects. In humans, two novel heterozygous mutations in TXNRD2 were identified in 3 of 227 patients with a diagnosis of dilated cardiomyopathy, however, no data are available on their adrenal function (15, 34).
Oxidative stress has been implicated in other causes of adrenal insufficiency, including triple A syndrome and X-linked adrenoleukodystrophy (ALD). In ALD, mutations in ABCD1 (encoding the peroxisomal ABCD transporter) result in the accumulation of very long-chain fatty acids in the tissues and plasma, the toxic effects of which are thought to result from an increase in steady-state ROS production, depletion of glutathione and dysregulation of the cell redox homeostasis. The adrenal and CNS are most susceptible to the disease process (34).
Triple A Syndrome
Triple A syndrome (OMIM 231550) is an autosomal recessive disorder characterized by ACTH-resistant adrenal insufficiency, achalasia of the esophagus, alacrima (absence of tears) and a variety of progressive central, peripheral and autonomic neurological defects (62). It was first described by Jeremy Allgrove in 1978 (63). It has been estimated that Triple A accounts for approximately 1% of all cases of primary adrenal insufficiency (PAI) with a prevalence of 1 per 1,000,000 individuals (64, 65).
CLINICAL FEATURES OF TRIPLE A SYNDROME
The spectrum of clinical manifestations is unique and encompasses a range of phenotypic abnormalities that vary even within families. Alacrima is the most consistent sign, and is attributed to both autonomic dysregulation and structural abnormalities of the lacrimal glands. Achalasia usually presents within the first two decades of life and may precede the adrenal failure by several years (62, 66). Older children/adults usually complain of dysphagia especially for liquids (67). The pathogenesis of achalasia includes a decrease in non-adrenergic and non-cholinergic neurons, as well as a lack of neuronal nitric oxide synthase in autonomic plexus (68). Adrenal failure does not occur in the immediate postnatal period. It usually presents during the first, or more rarely, the second decade of life, suggesting progressive adrenal destruction or degeneration. However, in some cases it may be the presenting symptom leading to the diagnosis of the condition. AI in Triple A syndrome typically manifests as isolated glucocorticoid deficiency, with less than 15% of patients having evidence of mineralocorticoid deficiency (69, 70).
Neurodegenerative disease may include progressive central, peripheral, autonomic neuropathy (pupillomotor, lacrimotor, erectile dysfunction), sensory and motor defects, hyperreflexia, cerebellar dysfunction, bulbospinal syndrome, distal amyotrophy, amyotrophic lateral sclerosis, spastic paraparesis, syringomyelia, atrophy and myofasciculations of the tongue, epilepsy, pyramidal syndrome, dystonia, dysarthria, ataxia, optic atrophy chorea, deafness, mental retardation, Parkinsonism and dementia (64, 65, 67-69).
Based on data of 133 index cases, alacrima was present in all but one patient (99.2%), achalasia in 93.2%, AI in 90.1% and ND in 79.4%. The most common presenting features were AI and achalasia, followed by neurological dysfunction and alacrima. Eight percent of patients developed clinical features of the syndrome in the 3rd to 5th decade of life, however, none presented with AI (70). The above data support previous recommendations, that in cases of presence of alacrima and at least one more symptom of triple A syndrome, adrenal function testing and molecular analysis should be performed (71).
Moreover, a number of associated features have been described in association with Triple A syndrome, including palmo‐plantar and punctate hyperkeratosis, short stature, osteoporosis, xerostomia, nasal speech, angular cheilitis, glossitis and fissured tongue, enamel defect, poor wound healing, hypolipoproteinemia type IIb, scoliosis, pes cavus, long QT syndrome, microcephaly and dysmorphic features, such as long narrow face, long philtrum, down-turned mouth, thin upper lip, and lack of eyelashes. Premature loss of permanent teeth has also been reported (62, 64-70, 72-74).
DIAGNOSIS
The diagnosis should be confirmed by the Schirmer test, basal and dynamic endocrine testing, genetic analysis and detailed gastroenterological and neurological evaluation (75). The diagnosis may be extremely challenging, given that the clinical manifestations may evolve at a variable time. Therefore, patients who undergo surgery for achalasia may be at risk of life-threatening adrenal crisis during anesthesia.
GENETICS
The first step towards in identifying the genetic etiology of triple A syndrome was the chromosomal localization by linkage analysis of the gene responsible for this condition to an 6cM area in chromosome 12
(76). Subsequently, homozygote or compound heterozygote mutations were found in the AAAS gene on 12q13 in families with triple A syndrome (77). This gene encodes a 60-kDa nuclear pore protein, termed ALADIN (alacrima-achalasia-adrenal insufficiency, neurologic disorder) (62). AAAS belongs to WD-repeat regulatory protein family, which exhibits wide functional diversity, in that they are involved in signal transduction, RNA processing, vesicular trafficking, cytoskeleton assembly and cell division control. WD-repeat proteins are characterized by the presence of four or more repeating units containing a conserved core of approximately 40 amino acids that usually end with tryptophan-aspartic acid (WD). AAAS mRNA and the ALADIN protein are ubiquitously expressed with predominance in the adrenal and CNS structures in humans and rats (34, 77). ALADIN is the only nucleoporin to be associated with hereditary adrenal disease and the first to be associated with hereditary neurodegenerative disease.
Screening of patients with triple A syndrome worldwide revealed that the IVS14+1G A splice donor mutation is the most common AAAS mutation. In the Puerto Rican and Middle Eastern/southern European populations, the frequent presence of this mutation is the result of a founder effect. A variety of disease-associated missense, nonsense, splice-site and frameshift mutations have been shown to result in either ALADIN deficiency or mis-localization of the abnormal protein, found predominantly into the cytoplasm, suggesting that correct targeting of ALADIN to the nuclear pore complex is required. Splice-site, indel, intronic region, regulatory element and 5′ UTR mutations have been also detected in affected individuals (70). Over 75 different mutations have been described in the literature (www.hgmd.cf.ac.uk), some of which are shown in Table 4 (62, 64, 77-85). However, there is little phenotype/genotype correlation, even between affected siblings, suggesting that other factors may be involved in disease progression (86). A recent review of the literature, showed that AI was more prevalent and diagnosed at a younger age in patients harboring truncating mutations. On the other hand, neurological dysfunction was more prevalent, with an older age at onset, in patients carrying non-truncating mutations (70). In addition, patients with truncating mutations were more likely to present with symptomatic AI, while those with non-truncating mutations with neurological dysfunction.
Table 4. Mutations of the AAAS Gene |
||
| Mutation | Probable Effect of Mutation | Reference |
| 125CàA | Deduced peptide sequence Q15K | Handschug et al,77. |
| 869TàC | Deduced peptide sequence S263P | Handschug et al,77. |
| 333GàA | Deduced peptide sequence W84X | Handschug et al,77. |
| 561AàG | Deduced peptide sequence H160R | Handschug et al,77. |
| 552-553delTT | Deduced peptide sequence F157fs | Handschug et al,77. |
| 869TàC | Deduced peptide sequence S263P | Handschug et al,77. |
| 1471delC | Deduced peptide sequence S463fs | Handschug et al,77. |
| 869TàC | Deduced peptide sequence S263P | Handschug et al,77. |
| 938CàT | Deduced peptide sequence R286X | Handschug et al,77. |
| 1106CàT | Deduced peptide sequence R342X | Handschug et al,77. |
| IVS14+1GàC | Defective nuclear transportation of Ferritin Heavy Chain protein (FTH1) | Storr et al,62. |
| p.Q387X | Defective nuclear transportation of Ferritin Heavy Chain protein (FTH1) | Storr et al,62. |
| H71fs | Defective nuclear transportation of Ferritin Heavy Chain protein (FTH1) | Storr et al,62. |
| R230X | Defective nuclear transportation of Ferritin Heavy Chain protein (FTH1) | Storr et al,62. |
| IVS11+1GàA | May interfere with the formation of WD repeats | Sandrini et al,78. |
| 43CàA | Defective preservation of stability of ALADIN β-strands | Sandrini et al,78. |
| c.130delA | Frameshift after phenylalanine at amino acid position 435 | Thummler et al,79. |
| c.1292-1294delTTCinsA | Change of phenylalanine at amino acid position 431 into a stop codon | Thummler et al,79. |
| R194X | Deduced peptide sequence | Marin et al,80. |
| p.Ala167Val | Change of alanine at position 167 into valine | Moschos et al,81. |
| p.Ser207fs | Frameshift mutation | Krull et al,82. |
| c.577C>T | p.Gln193X in exon 7 | Yang et al,83. |
| c.1062_1063insAC |
p.Ser355fsX416 in exon 11 Frameshift mutation |
Yang et al,83. |
| c.887C>A | p.Ser296Tyr in exon 9 | Dumić et al,84. |
| c.123+2T>C | Splice defect | Milenkovic et al,71. |
| c.1261_1262insG | Truncated protein (p.V421fs), most probably not functional | Milenkovic et al,71. |
| c.56A > G | p.Tyr 19 Cys | Capataz Ledesma et al,85. |
| 10-bp deletion c.1264_1273del |
Frameshift introducing an aberrant stop codon after 126 amino acids p.Q422NfsX126 |
Kurnaz E et al,64. |
| c.1144_1147delTCTG | Frameshift with a premature stop codon (p.Ser382ArgfsX33) | de Freitas MRG et al,65. |
| c.755G>C | p. (Trp252Ser) missense | Roucher-Boulez F et al,69. |
| c.1331+1G>A | Splice-site mutation | Patt H et al,70. |
Oxidative stress may play a role in the pathogenesis of this complex disorder. Data derived from experimental in vitro models of the disease, have shown that dermal fibroblasts of patients with triple A syndrome have higher basal intracellular ROS and are more sensitive to oxidative stress than wild-type fibroblasts. It has been suggested, that the failure of the nuclear accumulation of DNA repair proteins, aprataxin, and DNA ligase I together with the antioxidant protein ferritin heavy chain in skin fibroblasts of patients with triple A syndrome may render these cells more susceptible to oxidative stress. A disruption in redox homeostasis is suggested in the ALADIN-deficient adrenal cells with a depletion of reduced GSH, a major endogenous antioxidant and a cofactor of the antioxidant enzyme glutathione peroxidase. Moreover, AAAS knockdown results in cell cycle arrest and an increase in cell death by apoptosis. Increased chromosomal fragility has also been reported (34, 87). ALADIN protein has been shown to localize around the mitotic spindle and at spindle poles in Drosophila and human cells. It interacts with the microsomal protein progesterone receptor membrane component 2 (PGRMC2), regulator of cell cycle and activity regulator of CYP P450 enzymes, as well as with the inactive form of Aurora A, a serine/threonine kinase involved in various mitotic events. Recent studies suggest that ALADIN protein has functions in cell division. Interestingly, mitotic spindle assembly errors have been observed in cultured fibroblasts of patients with Triple A syndrome (88, 89). Finally, AAAS gene deficiency affects steroidogenesis and results in a reduction in StAR and P450c11β protein expression, and consequently in a significant reduction of cortisol production, an effect that is partially reversed with antioxidant N-acetylcysteine treatment (87). In addition, AAAS knock-down induces downregulation of genes coding for 17α-hydroxylase/17,20-lyase (CYP17A1), 21-hydroxylase (CYP21A2) and their electron donor cytochrome P450 oxidoreductase (POR), resulting in decreased production of glucocorticoid and androgen precursors (90).
Mutations in the AAAS gene have been identified in 90-95% of patients with a clinical diagnosis of Triple A syndrome (69, 70). The remaining cases may result from unidentified large deletions, mutations in uncharted intronic or regulatory regions, or mutations in two novel genes that may produce a “triple-A-like” phenotype without AI. GMPPA (guanosine diphosphate (GDP)-mannose pyrophosphorylase A) mutations were reported to cause an autosomal-recessive disorder characterized by achalasia, alacrima, and neurological deficits. Very recently, a homozygous splice mutation in TRAPPC11 gene, encoding for trafficking protein particle complex subunit 11, has been detected in patients presenting with achalasia, alacrima, myopathy and neurological symptoms (91, 92).
PRIMARY/CONGENITAL ADRENAL HYPOPLASIA
Five forms of AHC have been identified: 1) The X-linked form (OMIM 300200) caused by a mutation or deletion of the DAX1 gene (Dosage-sensitive sex reversal Adrenal hypoplasia congenita critical region of the X chromosome gene-1; NR0B1) on the X chromosome; 2) The autosomal recessive form owing to a mutation or deletion of the gene that encodes for the steroidogenic factor 1 (SF-1)/NR5A1 on chromosome 9q33 (OMIM 184757); 3) An autosomal recessive form of uncertain etiology (OMIM 240200); and 4) The IMAGe syndrome (Intrauterine growth restriction, Metaphyseal dysplasia, Adrenal hypoplasia congenita, and Genital abnormalities) (OMIM 614732) 5) The MIRAGE syndrome (Myelodysplasia, Infection, Restriction of growth, Adrenal hypoplasia, Genital phenotypes and Enteropathy) (OMIM 617053).
Most recently, mutations in the gene encoding sphingosine-1-phosphate (S1P) lyase 1 (SGPL1), located on chromosome 10q22.1 have been associated with a syndrome comprising primary adrenal insufficiency and steroid-resistant nephrotic syndrome 9, 10) (OMIM: 617575).
X-linked Adrenal Hypoplasia Congenita (AHC)
The incidence of X-linked AHC is unknown. The latest reports estimate it to be less than 1:70,000 live male births (5, 93). X-linked AHC is characterized by infantile-onset acute adrenal insufficiency at an average age of 3 weeks in approximately 60% of affected individuals. Onset in childhood accounts for 40% of the cases, whilst only a few individuals are diagnosed in adulthood due to infertility.
CLINICAL FEATURES OF X-LINKED AHC
Adrenal insufficiency typically presents acutely with vomiting, feeding difficulties, dehydration and shock owing to salt-wasting. Hypoglycemia, frequently presenting with seizures, may be the first symptom. If untreated, adrenal insufficiency may lead to hyperkalemia, metabolic acidosis, hypoglycemia, hypovolemic shock and death. Cryptorchidism may be present. Affected males typically present with delayed puberty due to hypogonadotropic hypogonadism and are infertile. Carrier females may occasionally have symptoms of adrenal insufficiency or hypogonadotropic hypogonadism (5, 94). Imaging studies may reveal small, ectopic, or normal in size adrenal glands (5).
DIAGNOSIS
Primary adrenal insufficiency, as evidenced by hyponatremia, hyperkalemia, metabolic acidosis, low aldosterone and elevated ACTH concentrations in the presence of normal or low 17-hydroxyprogesterone concentrations, in a male infant strongly suggests X-linked AHC (5). Serum cortisol concentrations in the first weeks of life vary from very low to high (95). An ACTH test would detect cortisol deficiency, whilst a GnRH test would most possibly reveal impaired gonadotropin secretion (94, 96, 97).
Elevated 11-deoxycortisol concentrations have been documented in kindreds with DAX1 mutations, but only when determined very early in life. A mouse model that displays elevated 11-deoxycorticosterone concentrations and evidence of hyperplasia of the zona glomerulosa has recently been described. DAX1 testing may be considered in patients with evidence of 11β-hydroxylase deficiency, especially in those with severe salt-wasting (98).
GENETICS
Males with the above manifestations should undergo genetic analysis for the DAX1 gene. The DAX1 gene also known as NR0B1, (Nuclear Receptor subfamily 0, group B, member 1) is located on chromosome Xp21.2 and is responsible for the X-linked AHC (93, 97, 99). The NR0B1 gene (MIM#300473) encodes an orphan member of the nuclear receptor superfamily that is expressed in the hypothalamus, the anterior pituitary, the adrenal glands and the gonads. Nuclear receptors are thought to play a functional role in the establishment and maintenance of steroidogenic tissues. They are transcription factors that regulate gene networks important for reproduction, development and homeostasis in response to various extracellular and intracellular signals. The DAX1 carboxy-terminal domain (CTD) shares high similarity to the ligand-binding domain (LBD) of other nuclear receptors. The amino-terminal region is an atypical DNA binding domain, consisting of 3.5 repeats of 66–67 amino acid repeat motifs (100). At this time, DAX1 lacks a known ligand and is therefore named an orphan nuclear receptor.
The molecular mechanism of DAX1 action during development remains unclear. However, many studies have shown that DAX1 functions as a transcriptional repressor of steroid biosynthesis pathways regulated by other nuclear receptors, such as the SF1-mediated transactivation of genes StAR, 3β-hydroxysteroid dehydrogenase and cholesterol side-chain cleavage enzyme (P450scc). In addition to SF1, it acts as a repressor to other nuclear receptors, such as the estrogen receptor (ER) (101), progesterone receptor (PR), glucocorticoid receptor (GR) (102), androgen receptor (AR) (103) and the liver receptor homologue-1 (LRH-1) (104). DAX1 has also been proposed to act as a shuttling RNA binding protein associated with ribonucleoprotein structures in the nucleus and polyribosomes in the cytoplasm, raising the possibility that it plays an additional regulatory role in post-transcriptional processes (105). Other studies have demonstrated that DAX-1 may activate gene transcription (5, 100). It has been suggested that DAX-1 represses adrenal stem cell differentiation during organ development so that a pool of progenitor stem cells can be expanded before these cells differentiate into mature steroidogenic cells. Loss of DAX-1 function, would lead to premature differentiation of progenitor cells into mature cells before expansion of cell number takes place, resulting in a transient overactivity of the gland followed by adrenal hypoplasia.
To date, more than 200 mutations of the DAX1 gene have been reported (www.hgmd.cf.ac.uk). These include large and small deletions, insertions, missense, nonsense, frameshift and splice site mutations (93, 106-111). Most missense mutations tend to cluster within the C-terminal region of the DAX-1 gene, indicating the essential role of the ligand-binding domain for the biological function of DAX1 protein (112). Gross deletions usually occur as a continuous gene deletion including the genes of glycerol kinase (GK) and Duchene muscular dystrophy (DMD). Of note, some of the patients with the contiguous gene syndrome also present with mental retardation.
DAX1 mutations have been detected in 58% of males with primary adrenal insufficiency of unknown etiology, in which common causes of adrenal failure, such as 21-hydroxylase deficiency, ALD or autoimmune disease had been excluded (93). A family history of AI (or unexplained death) or hypogonadism in male relatives is highly suggestive of X-linked AHC. Of note, positive adrenal (21-hydroxylase) antibodies and normal adrenal imaging have been recently reported in a male patient presenting with adrenal insufficiency who had a DAX-1 mutation (113). Two thirds of the patients have point mutations. Small deletions and insertions causing frameshift mutations, as well as nonsense mutations are mutations scattered throughout exons 1 and 2, whereas missense mutations are detected in exon 2 (encoding the putative ligand binding domain in the carboxyl-end of the protein).
It has been estimated that isolated and contiguous NR0B1 gene deletions account for 22 and 5% of all NR0B1 mutations, respectively. Mental retardation (MR) associated with AHC cannot be explained with GK deficiency or DMD in every case. Deletions extending to the IL1RAPL1 gene have been shown to be responsible for MR in several cases. Moreover, female carriers of NR0B1, as well as of GK or DMD mutations are at risk of developing symptoms, due to non-random X inactivation. Furthermore, in case of a contiguous gene deletion, the manifestation of the symptoms depends on the pattern of X inactivation in different tissues. Multiplex ligation-dependent probe amplification (MLPA) analysis is a valuable tool to detect NR0B1 and contiguous gene deletions in patients with AHC, showing a good genotype-phenotype correlation. It is especially helpful for the detection of IL1RAPL1 deletions causing MR, as no clinical markers for MR are available. Furthermore, MLPA has the advantage of identifying female carriers manifesting milder symptoms (114).
Patients with AHC harboring DAX1 mutations present with variable phenotypes. Typically, they develop primary adrenal failure during infancy but also later in childhood, adolescence or early adulthood. Of note, a milder form of AHC, presenting with isolated mineralocorticoid deficiency was described in an 11-yr-old boy carrying a W105C missense mutation in the amino-terminal region of DAX1 (115).
The hypogonadotropic hypogonadism may manifest as delayed puberty or pubertal arrest at about Tanner stage 3. Hypogonadotropic hypogonadism seems to involve combined hypothalamic and pituitary defects, as reflected by an impaired gonadotropin response to gonadotropin-releasing hormone (GnRH) stimulation. However, normal mini-puberty of infancy has been observed in affected boys, implying that hypothalamic-pituitary-gonadal axis defects may develop after early infancy. In addition, patients with normal puberty, gonadotropin-independent precocious puberty, central precocious puberty (5, 95, 116, 117), and impaired spermatogenesis with low inhibin B levels (5, 107, 118) have also been reported. Gonadotropin-independent precocious puberty in affected individuals may be due to a) enhanced stimulation of human melanocortin 1 receptors (MC1R) on Leydig cells by ACTH and b) an increased expression of testicular steroidogenesis activators secondary to a reduction of DAX1 repression activity. The above mechanisms may result in an increased testicular testosterone production, despite prepubertal gonadotropin levels.
Isolated infertility with normal pubertal development and normal integrity of the hypothalamic–pituitary–gonadal axis has been recently reported in a patient with adrenal insufficiency owing to a DAX1 mutation. The severely impaired spermatogenesis in this patient suggests that DAX1 mutations may lead to progressive deterioration of testicular function, independently of gonadotropin and testosterone production. The DAX1 represses aromatase production and therefore the production of estrogen in Leydig cells. It has been recently suggested that the deletion of the second exon of DAX1 may abolish the aforementioned repressor effect, resulting in aromatase overexpression and increased estrogen production. Consequently, this DAX1 dysfunction, through an indirect effect, may be able to disrupt spermatogenesis even in the presence of normal testosterone concentrations (119). Hence, semen preservation should be offered to young men with DAX1 mutations (120). Patients with oligo- or azoospermia usually fail to respond to gonadotropin treatment. Frapsauce et al reported a unique case of an infertile azoospermic patient harboring a nonsense mutation in DAX1, who was treated with FSH/hCG for 20 months and fathered a healthy boy following testicular sperm extraction-intracytoplasmic sperm injection (TESE-ICSI) (100, 121).There is no clear phenotype – genotype correlation, and the phenotypes are heterogeneous even within families, with respect to the age of onset of adrenal insufficiency, the severity of the disease and the occurrence (or not) of hypogonadotropic hypogonadism (95, 122-126). It is noteworthy, however, that adult-onset adrenal insufficiency and hypogonadotrophic hypogonadism have been linked to eight DAX1 mutations (127, 128). Interestingly, a novel non-sense p.Gln208X mutation in the amino terminal domain of the DAX-1 gene has been associated with both precocious puberty and hypogonadotropic hypogonadism in different members of a large pedigree, who had all presented with adrenal manifestations at different ages (129). This heterogeneity within families may be explained by the unique structure of the DAX-1 gene. It is also indicative of the presence of modifier genes or environmental effects on the expression of clinical manifestations (94, 130, 131). Although this is an X-linked condition, females carrying homozygous or heterozygous mutations may present with isolated hypogonadotropic hypogonadism or extreme pubertal delay, respectively. Moreover, adrenal insufficiency, moderate developmental delay and mild muscular dystrophy was reported in a girl with deletion at Xp21.2 on the maternal chromosome and skewed X inactivation (5, 108, 132-134).
Other phenotypic features such as attention deficit disorder, short stature and growth hormone deficiency have been noted in a few patients (135, 136). Inappropriate tall stature and renal ectopy associated with a DAX-1 missense mutation was reported in a single case (137). Macrophalia in infancy may be a rare feature of X-linked AHC (31). Hepatic iron deposition was documented in a male infant presenting with adrenal insufficiency as part of Xp21 deletion (138).
It is worth noting that DAX1 has anti-testis properties and antagonizes SRY (sex-determining gene region of the Y chromosome) action, required for male sex determination. NR0B1 locus duplications have been associated with 46,XY DSD/testicular dysgenesis (100).
Congenital Adrenal Hypoplasia Due to SF1 Mutations
The steroidogenic factor 1 (SF1) protein, encoded by the nuclear receptor subfamily 5 group A member 1 (NR5A1) gene, is also an orphan member of the nuclear receptor family. It was first recognized in 1992 as an element that regulates the proximal promoter region of the cytochrome p450 21-hydroxylase enzyme (139). The NR5A1 gene is located on chromosome 9q33 and encodes a protein of 461 amino acids, which is expressed in the adrenal gland, gonads, hypothalamus, anterior pituitary and spleen during development and postnatal life (140, 141). SF1 is considered the main regulator of enzymes involved in adrenal and gonadal steroidogenesis (142, 143). It is essential not only for adrenal and gonadal development and sex differentiation, but also for CNS function and metabolic homeostasis (144, 145). Among others, SF1 regulates the expression of luteinizing hormone/choriogonadotropin receptors (LHCGR), StAR, CYP11A1, and CYP17A1 in Leydig cells, SRY and SOX9 (testis-determining genes), anti-Müllerian hormone (AMH) and its receptor AMHR2 in Sertoli cells, insulin-like peptide 3 (INSL3), which is involved in testicular descent, and T-cell leukemia homeobox-11 (HOX11-TLX1), a transcription factor essential for spleen development (146, 147). SF1 expression in the hypothalamus and pituitary gland contributes to the differentiation of pituitary primordial cells into gonadotrophs (140).
CLINICAL CASES AND MUTATIONAL ANALYSIS
Targeted deletion of NR5A1 gene in mice resulted in adrenal and testicular agenesis, retained Mullerian structures and partial hypogonadotropic hypogonadism in males, as well as hyposplenism and late onset obesity (141, 144, 148-150). In the adrenals, SF1 represses the CYP11B2 (aldosterone synthase) gene (151) and facilitates CYP17 (cytochrome P450 family 17) transcription under the control of ACTH (152).
To date, more than 100 pathogenic SF1 mutations have been reported (153). A genotype-phenotype correlation cannot be observed and diverse clinical presentations even among family members carrying the same mutation may be attributed to incomplete penetrance, pathogenic variants in other testis/ovarian-determining genes, polymorphisms, environmental and epigenetic factors. The first mutation was detected in a patient with adrenal failure and complete 46,XY sex reversal, who presented during the first weeks of life with low circulating cortisol, low aldosterone and high ACTH concentrations. Although the karyotype of the patient was 46,XY, normal Müllerian structures and streak-like gonads containing poorly differentiated seminiferous tubules and connective tissue were detected (154). The patient had a de novo, heterozygous loss-of-function missense mutation (p.G35E) causing substitution of glycine at amino acid 35 by glutamate in the DNA-binding domain of the protein, abolishing its DNA-binding activity. Pituitary gonadotropins responded to GnRH stimulation, but testosterone did not respond to exogenous hCG administration, suggesting defective gonadal function. After introduction of estrogen and progesterone, the uterus grew and regular menstruation ensued. This case was the first to indicate that SF1 is essential for sex determination, steroidogenesis and reproduction.
The second patient was a phenotypically female infant, who presented with hypoglycemic convulsions, progressive hypotonia, weight loss, hyponatremia and hypokalemia. Genetic testing revealed homozygosity for the p.R92Q mutation, whilst her consanguineous parents and her sister were heterozygous for the mutation. Although DHEA concentrations were detectable, 17-hydroxyprogesterone concentrations were low. The abdominal CT scan demonstrated left adrenal hypoplasia and right adrenal agenesis. The patient’s karyotype was 46,XY and a uterus was seen on pelvic ultrasound and confirmed by magnetic resonance imaging (155).
A phenotypically and genotypically normal girl (46,XX), with adrenal failure and no apparent defect in ovarian maturation was described in 2000 (156). The patient had a heterozygous G to T transversion in exon 4 of the NR5A1 gene, resulting in the missense p.R255L mutation. The inability of the mutant NR5A1/SF1 to bind canonical DNA sequences offered a possible explanation for the failure of the mutant protein to transactivate target genes. This was the first report of a mutation in the NR5A1 gene in a genotypically female patient, suggesting that SF1 is not necessary for female gonadal development, although it plays a crucial role in adrenal gland formation in both sexes.
Since then, only two cases of isolated adrenal insufficiency (AI) have been reported (31, 157). One of them, a 46 XX female, with early-onset primary AI, was homozygous for the p.R92Q mutation, previously associated with 46XY DSD (31).
In contrast, there have been several reports of various types of NR5A1 mutations (including missense, nonsense, and frameshift), affecting the DNA binding domain of the protein in individuals with different forms of 46,XY disorders of sex differentiation (DSD) and associated adrenal insufficiency (93, 158, 159) or without an adrenal phenotype (160-165). Pathogenic NR5A1 variants have been identified in 10-20% of all 46 XY DSD cases. They usually arise de novo, but can be maternally inherited in a sex-limited dominant manner in 30% of cases (100). Phenotypic features include: female or ambiguous genitalia with inguinal or labial testes and remnant or no Müllerian structures (present in 24% of patients) (147), clitoral hypertrophy, labioscrotal folds, labioscrotal testes, bilateral anorchia (166), micropenis and hypospadias (164, 167-169). Biochemical evidence of hypogonadotrophic hypogonadism along with testicular dysfunction and borderline adrenal dysfunction was observed in a case of 46XY DSD dizygotic twins, harbouring a heterozygous frameshift mutation in the C-terminal region of NR5A1 (170). Of note, there are several reports of affected individuals, presenting with female external genitalia in the neonatal period followed by spontaneous and progressive virilization in adolescence. However, FSH levels remained persistently elevated in all cases, suggesting that Leydig cell function may be preserved while Sertoli cells are more severely affected (171).
Splenic anomalies may be an additional feature of patients with 46 XY DSD harboring SF1 mutations. A homozygous SF1 mutation, R103Q was found in a 46 XY patient presenting with complete sex reversal, asplenia and mildly elevated ACTH levels but no evidence of an AI. The SF1 R103Q mutant was shown to decrease the transcriptional activity of the spleen development gene TLX1, and impair the transcriptional activation of steroidogenic enzymes, without disrupting the synergistic effect of SF-1 with either SRY or SOX9 (146). Moreover, the de novo heterozygous deletion of 143 bp (c.616_758del) was identified in 6-week-old 46,XY female with complete sex reversal, AI and splenic hypoplasia. Finally, polysplenia was reported in a phenotypically female 46,XY-DSD patient carrying a heterozygous SF1 mutation, p.Tyr409* in the ligand-binding domain. The same mutation was found in her father, who had asplenia and hypospadias (172).
The phenotypic spectrum of SF1 mutations has been further expanded to include 46,XX ovotesticular/testicular DSD associated with the p.Arg92Trp and p.Arg92Gln variants. Affected patients may present with ambiguous genitalia with a uterus/hemi-uterus or as phenotypic males with testes (173-175). It has been suggested that p.Arg92Trp mutation results in downregulation of the pro-ovarian Wnt4/β-catenin pathways, thus leading to increased expression of SOX9 and other pro-testis genes at the gonadal level, switching organ fate from ovary to testis.
In addition, missense changes, in-frame deletions, frameshift, and nonsense mutations in NR5A1 have been found in 46,XX females with isolated ovarian insufficiency and account for about 1.4–1.6% of women presenting with sporadic primary ovarian insufficiency (POI) of unknown origin (100, 165, 176). Mothers or sisters who are heterozygous carriers may experience menstrual irregularities, decreased ovarian reserve, early menopause and rarely absence of puberty (100, 175).
Furthermore, NR5A1 mutations mostly located in the hinge region (100) may be found in 1.6-4% of men with otherwise unexplained severe impairment in spermatogenesis (177, 178). Gonadoblastoma and Germ Cell Neoplasia In Situ (GCNIS) have also been reported (179). Recent data indicate, that patients carrying NR5A1 mutations show distinct testicular histological features, i.e. reduced number of thin seminiferous tubules and focal aggregations of Leydig cells, containing cytoplasmic lipid droplets. Hence, testicular histology may be useful in identifying NR5A1 mutations in 46,XY patients with DSD before puberty. More recently, studies in mice indicate that lipid accumulation in the Leydig cells in 46 XY DSD is associated with decreased expression of StAR and CYP11A1, resulting in an increase in unmetabolized cholesterol (180, 181).
The above data indicate that SF1 mutations may lead to a wide range of endocrine phenotypes, which are only rarely related to adrenal insufficiency.
To date, microdeletions of chromosome 9q33.3, involving the NR5A1 gene have been reported in three patients with DSD. The first is a 3 Mb deletion in a 46,XY female, presenting with clinical features of Genitopatellar syndrome, developmental delay and ovotestes (182). The second is a unique 970kb microdeletion encompassing NR5A1, and resulting in XY sex reversal with clitoromegaly, neonatal male testosterone and AMH levels and a normal urine steroid profile (183). The third is a de novo 1.54 Mb microdeletion in a patient with 46,XY DSD and mild developmental delay (184).
Recently, a novel heterozygous p.Cys65Tyr mutation in NR5A1 gene has been identified in three 46,XY siblings of a Brazilian family, who presented with ambiguous genitalia without Müllerian derivatives and apparently normal Leydig function after birth and at puberty, respectively. Their mother, who reported symptoms suggestive of primary ovarian insufficiency was also heterozygous for this mutation. Basal ACTH and cortisol concentrations were slightly elevated and normal, respectively, in all three patients. After 1 mcg ACTH stimulation test, only the older sibling showed subnormal cortisol response. The above data indicate that NR5A1 analysis should be performed in 46,XY DSD patients with normal testosterone concentrations without AR mutations. Furthermore, a long-term follow-up for adrenal function is important for those patients (185).
IMAGE SYNDROME
CLINICAL FEATURES AND LABORATORY FINDINGS
The acronym IMAGe indicates the presence of Intrauterine growth restriction, Metaphyseal dysplasia, Adrenal hypoplasia congenita, and Genital anomalies (10, 186).
The life-threatening components of the adrenal insufficiency in this syndrome generally develop in the neonatal period. It usually manifests in the first few days of life with adrenal crises and may be the first sign of the disease. In some patients it may present later in childhood with failure to thrive and recurrent vomiting or in early adulthood. Hypoaldosteronism without evidence of glucocorticoid deficiency was also reported in one case (187). On imaging studies, the adrenal glands may appear small or normal in size. Radiologic identification of metaphyseal dysplasia is often crucial for the diagnosis, but this could be very mild and identifiable only in late infancy or in childhood and then progress with age. Additional radiographic features may include: epiphyseal dysplasia, mesomelia, osteopenia, gracile long bones, and delayed bone age (188).
A more precocious sign, i.e. delayed endochondral ossification associated with osteopenia, hypercalcemia, and/or hypercalciuria of unclear aetiology and of variable degree can be encountered in patients with this syndrome. Abnormalities in serum calcium concentrations may be present at birth and resolve later in infancy. Soft tissue calcifications have been occasionally reported (188).
Another endocrine involvement in these patients is GH deficiency and early substitution therapy could improve linear growth.
Specific dysmorphic craniofacial features in IMAGe syndrome include nonspecific signs, such as prominent forehead, macrocephaly, low-set ears, ear dysplasia, flat nasal bridge, and short nose, short arms and legs. micrognathia or retrognathia, cleft palate or cleft uvula, craniosynostosis, short palpebral fissures, smooth philtrum, microglossia, arachnodactyly, and bilateral 2–3 toe syndactyly (187-189).
Genital abnormalities seem to be confined to males and include micropenis, undescended testes, chordee and hypospadias of variable severity. Two female patients were reported to give birth to children. Labor may be complicated by cephalopelvic disproportion.
Additional features associated with the syndrome include:
- Skeletal abnormalities: progressive and severe scoliosis with onset before age five years, ovoid-shaped vertebral bodies, short first metatarsals, hallux valgus, hip dysplasia, fractures of the humerus and tibia present at birth
- Renal abnormalities: hydronephrosis, hypercalciuria-associated nephrocalcinosis
- Other: oligohydramnios (187-188).
GENETICS
IMAGe syndrome (OMIM 614732) is exclusively related to mutations of CDKN1C gene [cyclin-dependent kinase inhibitor 1C (p57, Kip2)] (190). Notably, familial analysis demonstrated de novo mutations or an imprinted mode of inheritance, exclusively with maternal transmission of the mutation. The responsible gene lies on 11p15, contains three exons and encodes p57 (KIP2), a potent tight-binding inhibitor of several G1 cyclin/Cdk complexes (cyclin E-CDK2, cyclin D2-CDK4, and cyclin A-CDK2). It is a negative regulator of cell proliferation, playing a role in the maintenance of the non-proliferative state throughout life, probably acting as a tumour suppressor gene. CDKN1C is expressed in the placenta, heart, brain, lung, skeletal muscle, kidney, pancreas, testis, eye, and in the subcapsular or developing definitive zone of the adrenal gland. To date, clinical manifestations suggestive of IMAGe syndrome have been described in 28 individuals. Six missense mutations have been documented in 17 out of 28 patients, all of which occur in the PCNA-binding domain in the carboxy-terminal region of CDKN1C (186, 188). Recently, Hamajima et al (191) demonstrated that the IMAGe-associated mutations cause a dramatically increased stability of the CDKN1C proteins, which probably results in a functional gain of growth inhibition properties. Further studies have shown that mutations in the PCNA-binding site of CDKN1C lead to a block in the G1 phase and impaired S-phase entry resulting in decreased cell proliferation (192). In contrast, loss-of-function CDKN1C mutations are associated with the Beckwith-Wiedemann syndrome (BWS), which represents an additional imprinting disorder with a mirror phenotype of IMAGe syndrome. BWS mutations are not clustered within a single domain and promote cell proliferation (186).
A novel CDKN1C mutation (c.842G>T, p. R281I) that did not entirely abrogate proliferating cell nuclear antigen binding has been recently associated with features of IMAGe syndrome, however, without adrenal insufficiency or metaphyseal dysplasia, but with early-adulthood-onset diabetes (189). A novel missense variant of CDKN1C (c.836G>[G;T], p.Arg279Leu) was also identified in a familial case of Russell Silver syndrome (193). Of note, both mutations were located within the PCNA-binding site of CDKN1C gene and were maternally inherited, thus producing phenotypic overlaps of IMAGe syndrome.
MIRAGE Syndrome
MIRAGE syndrome (OMIM 617053) is a rare form of syndromic adrenal hypoplasia, associated with high mortality rates during the first years of life. First described in 2016, MIRAGE stands for Myelodysplasia, Infection, Restriction of growth, Adrenal hypoplasia, Genital phenotypes and Enteropathy. The genetic basis of the syndrome has been linked to germline, mostly de novo, gain-of-function, heterozygous mutations in SAMD9 (sterile alpha motif domain-containing protein 9) gene. Homozygous loss-of-function SAMD9 mutations have been shown to result in normophosphatemic familial tumoral calcinosis (194).
GENETICS
SAMD9 gene resides on the long arm of chromosome 7 (7q21.2) and encodes a 1,589-amino acid protein that regulates cell proliferation and exhibits wide tissue expression, including in adrenal glands, colon, bone marrow, liver, immune system, lung, and testis (195, 196). SAMD9 facilitates endosome fusion and is likely to function as a growth repressor. It has been shown that expression of the wild-type SAMD9 resulted in decreased cell proliferation, whereas expression of mutants resulted in profound growth inhibition. At the cellular level, patient-derived fibroblasts displayed increased size of early endosomes, intracellular accumulation of giant vesicles and decreased plasma membrane epidermal growth factor receptor (EGFR) expression, likely due to defects in receptor recycling (194).
CLINICAL FEATURES AND LABORATORY FINDINGS
To date, heterozygous SAMD9 mutations associated with two or more components of MIRAGE syndrome have been reported in 24 patients (194-198).
Genital abnormalities may range from micropenis, cryptorchidism and hypospadias to ambiguous genitalia and completely feminized external genitalia in 46XY affected individuals. Of note, only 25% of reported cases were females, indicating that the syndrome may be underdiagnosed in girls. Histologically, the ovaries were markedly hypoplastic and dysgenetic in two patients, containing few primordial follicles (194, 195, 199).
Neonatal severe adrenal insufficiency is a common manifestation. Adrenal imaging may reveal hypoplasia or even absence of adrenal glands. Histologic studies have shown very small, highly disorganized, dysgenetic adrenal glands (194,195).
Thrombocytopenia and/or anemia, requiring transfusions may manifest within the first week of life, however spontaneous resolution has been reported in many cases (196, 197).
Myelodysplastic syndrome (MDS) associated with monosomy 7 or monosomy 7q was reported in 6 out of 24 MIRAGE-affected individuals. The researchers demonstrated that the preferential loss of the allele harboring the gain-of-function SAMD9 mutation, through the development of monosomy 7 (–7), deletions of 7q (7q–) or secondary somatic loss-of-function provide a survival advantage in affected hematopoietic cells. This is an example of an “adaptation by aneuploidy” mechanism, relieving the growth-restricting effect of the mutated gene, however at the expense of an increased risk for MDS (194, 195, 197, 199). Interestingly, two patients harboring two de novo SAMD9 mutations on the same allele, one activating SAMD9 mutation, and one second-site reversion nonsense mutation in the haematopoietic cells, exhibited no haematologic manifestations (198).
Additional features of the disorder include (194-196, 198-199):
- Moderate-to-severe growth restriction during both the prenatal and postnatal periods, premature delivery, fetal death
- Severe bacterial and viral infections, including sepsis, meningitis, and fungal infections thymus hypoplasia
- Chronic diarrhea with colonic dilation, feeding difficulties frequently requiring surgical feeding tube placement
- Dysmorphic features: frontal bossing, low-set ears, ptosis, down-turned corners of the mouth, round face, sparse hair, small feet and hands, tapered fingers, short phalanges, abnormal nails
- Bronchopulmonary dysplasia
- Neurologic abnormalities: dysautonomia hypolacrima, hyperhidrosis and blood pressure
dysregulation, syringomyelia, hypoplastic pons and cerebellum, hydrocephalus, bilateral auditory neuropathy, developmental delay
- Skeletal abnormalities: scoliosis, joint contracture in wrists and ankles
- Renal defects: renal tubular acidosis, glucosuria, defects in phosphate reabsorption and urinary concentration
- Apneas
- Reduced body fat
The majority of patients reported to date died within the first two years of life.
Familial Steroid-Resistant Nephrotic Syndrome with Adrenal Insufficiency
Most recently, in 2017, three study groups unraveled concurrently the genetic basis of a syndrome encompassing steroid-resistant nephrotic syndrome (SRNS) and primary adrenal insufficiency (PAI). Using whole exome sequencing analysis on patient cohorts with PAI or SRNS the researchers identified novel genetic mutations in the gene encoding sphingosine-1-phosphate (S1P) lyase 1 (SGPL1), located on chromosome 10q22.1 (200-202).
GENETICS
SGPL1 is an important endoplasmic reticulum (ER) enzyme that catalyzes the irreversible cleavage of the lipid molecule S1P to trans-2-hexadecenal and ethanolamine phosphate. S1P exhibits extracellular actions by activating a family of five differentially expressed extracellular G-protein-coupled receptors (G protein-coupled receptors (S1PRs) and intracellular functions via S1PR-independent mechanisms as well. S1P regulates multiple biological processes including cell migration, differentiation, angiogenesis, vascular maturation, cardiac development and immunity (200-202).
A total of 13 SGPL1 variants in 14 families have been reported so far (203). These were recessive loss-of-function mutations (homozygous or compound heterozygous) resulting in decreased or absent SGPL1 expression and/or enzyme activity, subcellular mis-localization of SGPL1 and altered levels of sphingolipid metabolism intermediates (200-202).
The pathogenesis of the syndrome may involve an excess of intracellular S1P, an imbalance of other sphingoid bases, S1P signaling through the S1P receptors or a lack of phosphoethanolamine production (201, 202).
SGPL1 is expressed in several mammalian tissues, among which in the adrenals and testes. Sgpl1–/– mice were shown to have impaired testicular and ovarian steroidogenesis and infertility. Recent studies have documented several histologic abnormalities in the adrenal glands of Sgpl1–/– mice, including compromised cortical zonation with less definition between zona glomerulosa (ZG) and zona fasciculata (ZF) and between ZF and X-zone as well as loss of vacuolization in the ZF. Furthermore, Sgpl1–/– adrenals displayed decreased cytochrome P450 side-chain cleavage (CYP11A1), reflecting impaired steroidogenesis. These data may indicate the potential role of SGPL1 on adrenal development (200).
CLINICAL FEATURES AND LABORATORY FINDINGS
Human SGPL1 mutations cause a multisystemic disorder, with the main components being PAI and SRNS (200-204).
PAI is manifested in almost all cases, usually during infancy and less frequently during childhood or later. Most patients exhibit an FDG phenotype, necessitating treatment with hydrocortisone only. However, in some cases additional mineralocorticoid treatment may be required. Of note, markedly low adrenal androgen levels were reported in one affected postpubertal patient. Adrenal imaging (U/S or MRI) performed in some cases revealed i) normal findings ii) calcifications in the adrenals and iii) bilateral enlarged adrenal glands in one case (200-202).
Most affected patients suffer from nephrotic syndrome (NS), which is typically manifested as congenital NS (clinical symptoms occurring during the 3 months after birth) or within the first year of life and is steroid-resistant, leading rapidly to end-stage renal disease requiring renal transplantation. Histologic examinations have shown mainly focal segmental glomerulosclerosis, but diffuse mesangial sclerosis and foci of calcification have also been reported (200-203).
The phenotypic spectrum of this syndrome is broad and associated features other than SRNS and PAI may include (200-204):
- Adrenal calcifications
- Dermatologic abnormalities: ichthyosis, acanthosis, hyperpigmentation, scaly lesions, calcinosis cutis
- Neurologic abnormalities: developmental delay, ptosis, strabismus, abnormal gait, ataxia, sensorineural deafness, seizures, microcephaly, cortical, cerebellar or corpus callosum hypoplasia, peripheral neuropathy, contrast enhancement of cerebellar structures and bilateral globus pallidus, medial thalamic nucleus and central pons, FLAIR-hyperintensity in hippocampus and brainstem.
- Ophthalmologic abnormalities: “salt and pepper” retinopathy, amblyopia
- Immunodeficiencies: lymphopenia, deficiency of cellular immunity, multiple bacterial infections, hypogammaglobulinemia, thrombocytopenia and anemia
- Genital abnormalities: micropenis, cryptorchidism, hypergonadotropic hypogonadism, microorchidism associated with low serum anti-Müllerian hormone
- Skeletal abnormalities: craniotabes, rachitic rosary, asymmetric skull, scoliosis, short stature
- Hypothyroidism
- Muscular hypotonia
- Fetal demise, fetal hydrops
- Other: facial dysmorphism (microstomia, hypertelorism, down-slanting palpebral fissures, epicanthus, dysplastic ears), hypocalcemia, mild dilated cardiomyopathy, intestinal malrotation, capillary leak syndrome.
Lovric et al have proposed the term Nephrotic syndrome, type 14 (NPHS14) to describe this syndromic form of SRNS associated with SGPL1 gene mutations (OMIM: 617575) (201).
REFERENCES
- Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 1994;23(3):451-466.
- Peter M, Viemann M, Partsch CJ, Sippell WG. Congenital adrenal hypoplasia: clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J Clin Endocrinol Metab. 1998;83(8):2666-2674.
- Ferraz-de-Souza B, Achermann JC. Disorders of adrenal development. Endocr Dev. 2008;13:19-32.
- Lin L, Ferraz-de-Souza B, Achermann JC. Genetic disorders involving adrenal development. Endocr Dev. 2007;11:36-46.
- Achermann JC, Vilain EJ. NR0B1-Related Adrenal Hypoplasia Congenita. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. Source GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. 2001 Nov 20 [updated 2018 Jan 25].
- McCabe ERB. Adrenal hypoplasias and aplasias. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, Gibson KM, Mitchell G, eds. The Online Metabolic and Molecular Bases of Inherited Disease. Chapter 167. New York, NY: McGraw-Hill; DOI: 10.1036/ommbid.199.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152-2167.
- Malikova J, Flück CE. Novel insight into etiology, diagnosis and management of primary adrenal insufficiency. Horm Res Paediatr. 2014;82(3):145-157.
- Flück CE. MECHANISMS IN ENDOCRINOLOGY: Update on pathogenesis of primary adrenal insufficiency: beyond steroid enzyme deficiency and autoimmune adrenal destruction. Eur J Endocrinol. 2017;177(3):R99-R111.
- Tan TY, Jameson JL, Campbell PE, Ekert PG, Zacharin M, Savarirayan R. Two sisters with IMAGe syndrome: cytomegalic adrenal histopathology, support for autosomal recessive inheritance and literature review. Am J Med Genet A. 2006;140(16):1778-1784.
- Phillips K, Arroyo MR, Duckworth LV. IMAGe association: report of two cases in siblings with adrenal hypoplasia and review of the literature. Pediatr Dev Pathol. 2014;17(3):204-208.
- Chan LF, Clark AJ, Metherell LA. Familial glucocorticoid deficiency: advances in the molecular understanding of ACTH action. Horm Res. 2008;69(2):75-82.
- Aza-Carmona M, Barreda-Bonis AC, Guerrero-Fernández J, González-Casado I, Gracia R, Heath KE. Familial glucocorticoid deficiency due to compound heterozygosity of two novel MC2R mutations. J Pediatr Endocrinol Metab. 2011;24(5-6):395-397.
- O'Riordan SM, Lynch SA, Hindmarsh PC, Chan LF, Clark AJ, Costigan C. A novel variant of familial glucocorticoid deficiency prevalent among the Irish Traveler population. J Clin Endocrinol Metab. 2008;93(7):2896-2899.
- Prasad R, Chan LF, Hughes CR, Kaski JP, Kowalczyk JC, Savage MO, Peters CJ, Nathwani N, Clark AJ, Storr HL, Metherell LA. Thioredoxin Reductase 2 (TXNRD2) mutation associated with familial glucocorticoid deficiency (FGD). J Clin Endocrinol Metab. 2014;99(8):E1556-1563.
- Jain V, Metherell LA, David A, Sharma R, Sharma PK, Clark AJ, Chan LF. Neonatal presentation of familial glucocorticoid deficiency resulting from a novel splice mutation in the melanocortin 2 receptor accessory protein. Eur J Endocrinol. 2011;165(6):987-991.
- Kelch RP, Kaplan SL, Biglieri EG, Daniels GH, Epstein CJ, Grumbach MM. Hereditary adrenocortical unresponsiveness to adrenocorticotropic hormone. J Pediatr. 1972;81(4):726-736.
- Shepard TH, Landing BH, Mason DG. Familial Addison's disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA J Dis Child. 1959;97(2):154-162.
- Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341(8843):461-462.
- Tsigos C, Arai K, Hung W, Chrousos GP. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest. 1993;92(5):2458-2461.
- Novoselova TV, Rath SR, Carpenter K, Pachter N, Dickinson JE, Price G, Chan LF, Choong CS, Metherell LA.NNT pseudoexon activation as a novel mechanism for disease in two siblings with familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2015;100(2):E350-354.
- Elias LL, Huebner A, Metherell LA, Canas A, Warne GL, Bitti ML, Cianfarani S, Clayton PE, Savage MO, Clark AJ. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol (Oxf). 2000;53(4):423-430.
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005 Feb;37(2):166-170.
- Cooray SN, Chan L, Metherell L, Storr H, Clark AJ. Adrenocorticotropin resistance syndromes. Endocr Dev. 2008;13:99-116.
- Rumié H, Metherell LA, Clark AJ, Beauloye V, Maes M. Clinical and biological phenotype of a patient with familial glucocorticoid deficiency type 2 caused by a mutation of melanocortin 2 receptor accessory protein. Eur J Endocrinol. 2007;157(4):539-542.
- Hughes CR, Chung TT, Habeb AM, Kelestimur F, Clark AJ, Metherell LA. Missense mutations in the melanocortin 2 receptor accessory protein that lead to late onset familial glucocorticoid deficiency type 2. J Clin Endocrinol Metab. 2010;95(7):3497-3501.
- Novoselova TV, Hussain M, King PJ, Guasti L, Metherell LA, Charalambous M, Clark AJL, Chan LF. MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation. FASEB J. 2018 J:fj201701274RR.
- Metherell LA, Naville D, Halaby G, Begeot M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone NP, Lin L, Racine M, Berney DM, Achermann JC, Arlt W, Clark AJ. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009;94(10):3865-3871.
- Flück CE, Pandey AV, Dick B, Camats N, Fernández-Cancio M, Clemente M, Gussinyé M, Carrascosa A, Mullis PE, Audi L. Characterization of novel StAR (steroidogenic acute regulatory protein) mutations causing non-classic lipoid adrenal hyperplasia. PLoS One. 2011;6(5):e20178.
- Burget L, Parera LA, Fernandez-Cancio M, Gräni R, Henzen C, Flück CE. A rare cause of primary adrenal insufficiency due to a homozygous Arg188Cys mutation in the STAR gene. Endocrinol Diabetes Metab Case Rep. 2018;2018 pii: 18-0003.
- Guran T, Buonocore F, Saka N, Ozbek MN, Aycan Z, Bereket A, Bas F, Darcan S, Bideci A, Guven A, Demir K, Akinci A, Buyukinan M, Aydin BK, Turan S, Agladioglu SY, Atay Z, Abali ZY, Tarim O, Catli G, Yuksel B, Akcay T, Yildiz M, Ozen S, Doger E, Demirbilek H, Ucar A, Isik E, Ozhan B, Bolu S, Ozgen IT, Suntharalingham JP, Achermann JC. Rare Causes of Primary Adrenal Insufficiency: Genetic and Clinical Characterization of a Large Nationwide Cohort. J Clin Endocrinol Metab. 2016;101(1):284-292.
- Hughes CR, Guasti L, Meimaridou E, Chuang C, Schimenti JC, King PJ, Costigan C, Clark AJL, Metherell LA. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012; 122(3): 814–820.
- Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, Picard C, Trouillet C, Eidenschenk C, Aoufouchi S, Alcaïs A, Smith O, Geissmann F, Feighery C, Abel L, Smogorzewska A, Stillman B, Vivier E, Casanova JL, Jouanguy E. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821-832.
- Prasad R, Kowalczyk JC, Meimaridou E, Storr HL, Metherell LA. Oxidative stress and adrenocortical insufficiency. J Endocrinol. 2014;221(3):R63-73.
- Weinberg-Shukron A, Abu-Libdeh A, Zhadeh F, Carmel L, Kogot-Levin A, Kamal L, Kanaan M, Zeligson S, Renbaum P, Levy-Lahad E, Zangen D. Combined mineralocorticoid and glucocorticoid deficiency is caused by a novel founder nicotinamide nucleotide transhydrogenase mutation that alters mitochondrial morphology and increases oxidative stress. J Med Genet. 2015;52(9):636-641.
- Chan LF, Campbell DC, Novoselova TV, Clark AJ, Metherell LA. Whole-Exome Sequencing in the Differential Diagnosis of Primary Adrenal Insufficiency in Children. Front Endocrinol (Lausanne). 2015;6:113.
- Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nürnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJ, Metherell LA. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44(7):740-742.
- Yamaguchi R, Kato F, Hasegawa T, Katsumata N, Fukami M, Matsui T, Nagasaki K, Ogata T. A novel homozygous mutation of the nicotinamide nucleotide transhydrogenase gene in a Japanese patient with familial glucocorticoid deficiency. Endocr J. 2013;60(7):855-859.
- Meimaridou E, Goldsworthy M, Chortis V, Fragouli E, Foster PA, Arlt W, Cox R, Metherell LA. NNT is a key regulator of adrenal redox homeostasis and steroidogenesis in male mice. J Endocrinol. 2018;236(1):13-28.
- Roucher-Boulez F, Mallet-Motak D, Samara-Boustani D, Jilani H, Ladjouze A, Souchon PF, Simon D, Nivot S, Heinrichs C, Ronze M, Bertagna X, Groisne L, Leheup B, Naud-Saudreau C, Blondin G, Lefevre C, Lemarchand L, Morel Y. NNT mutations: a cause of primary adrenal insufficiency, oxidative stress and extra-adrenal defects. Eur J Endocrinol. 2016;175(1):73-84.
- Jazayeri O, Liu X, van Diemen CC, Bakker-van Waarde WM, Sikkema-Raddatz B, Sinke RJ, Zhang J, van Ravenswaaij-Arts CM. A novel homozygous insertion and review of published mutations in the NNT gene causing familial glucocorticoid deficiency (FGD). Eur J Med Genet. 2015;58(12):642-649.
- Bainbridge MN, Davis EE, Choi WY, Dickson A, Martinez HR, Wang M, Dinh H, Muzny DM, Pignatelli R, Katsanis N, Boerwinkle E, Gibbs RA, Jefferies JL. Loss of Function Mutations in NNT Are Associated With Left Ventricular Noncompaction. Circ Cardiovasc Genet. 2015;8(4):544-552.
- al Kandari HM, Katsumata N, al Alwan I, al Balwi M, Rasoul MS. Familial glucocorticoid deficiency in five Arab kindreds with homozygous point mutations of the ACTH receptor (MC2R): genotype and phenotype correlations. Horm Res Paediatr. 2011;76(3):165-171.
- Akin MA, Akin L, Coban D, Ozturk MA, Bircan R, Kurtoglu S. A novel mutation in the MC2R gene causing familial glucocorticoid deficiency type 1. Neonatology. 2011;100(3):277-281.
- Ηirsch A, Meimaridou E, Fernandez-Cancio M, Pandey AV, Clemente M, Audi L, Clark AJ, Flück CE. Loss of the C terminus of melanocortin receptor 2 (MC2R) results in impaired cell surface expression and ACTH insensitivity. J Clin Endocrinol Metab. 2011;96(1):E65-72.
- Collares CV, Antunes-Rodrigues J, Moreira AC, Franca SN, Pereira LA, Soares MM, Elias Junior J, Clark AJ, de Castro M, Elias LL. Heterogeneity in the molecular basis of ACTH resistance syndrome. Eur J Endocrinol. 2008;159(1):61-68.
- Elias LL, Huebner A, Pullinger GD, Mirtella A, Clark AJ. Functional characterization of naturally occurring mutations of the human adrenocorticotropin receptor: poor correlation of phenotype and genotype. J Clin Endocrinol Metab. 1999;84(8):2766-27
- Chung TT, Webb TR, Chan LF, Cooray SN, Metherell LA, King PJ, Chapple JP, Clark AJ. The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. J Clin Endocrinol Metab. 2008;93(12):4948-49
- Berberoğlu M, Aycan Z, Ocal G, Begeot M, Naville D, Akar N, Adiyaman P, Evliyaoglu O, Penhoat A. Syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six patients. J Pediatr Endocrinol Metab. 2001;14(8):1113-1118.
- Ishii T, Ogata T, Sasaki G, Sato S, Kinoshita EI, Matsuo N. Novel mutations of the ACTH receptor gene in a female adult patient with adrenal unresponsiveness to ACTH. Clin Endocrinol (Oxf). 2000;53(3):389-392.
- Wu SM, Stratakis CA, Chan CH, Hallermeier KM, Bourdony CJ, Rennert OM, Chan WY. Genetic heterogeneity of adrenocorticotropin (ACTH) resistance syndromes: identification of a novel mutation of the ACTH receptor gene in hereditary glucocorticoid deficiency. Mol Genet Metab. 1998;64(4):256-265.
- Tsigos C, Tsiotra P, Garibaldi LR, Stavridis JC, Chrousos GP, Raptis SA. Mutations of the ACTH receptor gene in a new family with isolated glucocorticoid deficiency. Mol Genet Metab. 2000;71(4):646-6
- Weber A, Toppari J, Harvey RD, Klann RC, Shaw NJ, Ricker AT, Näntö-Salonen K, Bevan JS, Clark AJ. Adrenocorticotropin receptor gene mutations in familial glucocorticoid deficiency: relationships with clinical features in four families. J Clin Endocrinol Metab. 1995 Jan;80(1):65-71.
- Tsigos C, Arai K, Latronico AC, DiGeorge AM, Rapaport R, Chrousos GP. A novel mutation of the adrenocorticotropin receptor (ACTH-R) gene in a family with the syndrome of isolated glucocorticoid deficiency, but no ACTH-R abnormalities in two families with the triple A syndrome. J Clin Endocrinol Metab. 1995;80(7):2186-218
- Naville D, Barjhoux L, Jaillard C, Faury D, Despert F, Esteva B, Durand P, Saez JM, Begeot M. Demonstration by transfection studies that mutations in the adrenocorticotropin receptor gene are one cause of the hereditary syndrome of glucocorticoid deficiency. J Clin Endocrinol Metab. 1996;81(4):1442-144
- Delmas O, Marrec C, Caietta E, Simonin G, Morel Y, Girard N, Roucher F, Sarles J, Chabrol B, Reynaud R. Uncommon neonatal case of hypoglycemia: ACTH resistance syndrome. Arch Pediatr. 2014;21(12):1353-1358.
- Chung TT, Chan LF, Metherell LA, Clark AJ. Phenotypic characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clin Endocrinol (Oxf). 2010;72(5):589-5
- McEachern R, Drouin J, Metherell L, Huot C, Van Vliet G, Deal C. Severe cortisol deficiency associated with reversible growth hormone deficiency in two infants: what is the link? J Clin Endocrinol Metab. 2011;96(9):2670-2674.
- Hughes CR, Chung TT, Habeb AM, Kelestimur F, Clark AJ, Metherell LA.Missense mutations in the melanocortin 2 receptor accessory protein that lead to late onset familial glucocorticoid deficiency type 2. J Clin Endocrinol Metab. 2010;95(7):3497-3
- Akın L, Kurtoğlu S, Kendirici M, Akın MA. Familial glucocorticoid deficiency type 2: a case report. J Clin Res Pediatr Endocrinol. 2010;2(3):122-12
- Modan-Moses D, Ben-Zeev B, Hoffmann C, Falik-Zaccai TC, Bental YA, Pinhas-Hamiel O, Anikster Y. Unusual presentation of familial glucocorticoid deficiency with a novel MRAP mutation. J Clin Endocrinol Metab. 2006;91(10):3713-3717.
- Storr HL, Kind B, Parfitt DA, Chapple JP, Lorenz M, Koehler K, Huebner A, Clark AJ. Deficiency of ferritin heavy-chain nuclear import in triple a syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol Endocrinol. 2009;23(12):2086-2094.
- Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet. 1978;1(8077):1284-128
- Kurnaz E, Duminuco P, Aycan Z, Savaş-Erdeve Ş, Muratoğlu Şahin N, Keskin M, Bayramoğlu E Bonomi M, Çetinkaya S. Clinical and genetic characterisation of a series of patients with triple A syndrome. Eur J Pediatr. 2018;177(3):363-369.
- de Freitas MRG, Orsini M, Araújo APQC, Jr LJA, Barbosa GM, França MC, Correia L, Bastos VH, Trajano E, Jr MDS. Allgrove syndrome and motor neuron disease. Neurol Int. 2018;10(2):7436.
- Huebner A, Elias LL, Clark AJ. ACTH resistance syndromes. J Pediatr Endocrinol Metab. 1999;12 Suppl 1:277-2
- Misgar RA, Pala NA, Ramzan M, Wani AI, Bashir MI, Laway BA. Allgrove (Triple A) Syndrome: A Case Report from the Kashmir Valley. Endocrinol Metab (Seoul). 2015;30(4):604-606.
- Aftab S, Manzoor J, Talat N, Khan HS, Subhanie M, Khalid NA. Allgrove Syndrome: Adrenal Insufficiency with Hypertensive Encephalopathy. J Coll Physicians Surg Pak. 2016;26(9):790-792.
- Roucher-Boulez F, Brac de la Perriere A, Jacquez A, Chau D, Guignat L, Vial C, Morel Y, Nicolino M, Raverot G, Pugeat M. Triple-A syndrome: a wide spectrum of adrenal dysfunction. Eur J Endocrinol. 2018;178(3):199-207.
- Patt H, Koehler K, Lodha S, Jadhav S, Yerawar C, Huebner A, Thakkar K, Arya S, Nair S, Goroshi M, Ganesh H, Sarathi V, Lila A, Bandgar T, Shah N. Phenotype-genotype spectrum of AAA syndrome from Western India and systematic review of literature. Endocr Connect. 2017;6(8):901-913.
- Milenkovic T, Zdravkovic D, Savic N, Todorovic S, Mitrovic K, Koehler K, Huebner A. Triple A syndrome: 32 years experience of a single centre (1977-2008). Eur J Pediatr. 2010;169(11):1323-1328.
- Kimber J, McLean BN, Prevett M, Hammans SR. Allgrove or 4 "A" syndrome: an autosomal recessive syndrome causing multisystem neurological disease. J Neurol Neurosurg Psychiatry. 2003;74(5):654-657.
- Houlden H, Smith S, De Carvalho M, Blake J, Mathias C, Wood NW, Reilly MM. Clinical and genetic characterization of families with triple A (Allgrove) syndrome. Brain. 2002;125(Pt 12):2681-2690.
- Razavi Z, Taghdiri MM, Eghbalian F, Bazzazi N. Premature Loss of Permanent Teeth in Allgrove (4A) Syndrome in Two Related Families. Iran J Pediatr. 2010;20(1):101-10
- Salmaggi A, Zirilli L, Pantaleoni C, De Joanna G, Del Sorbo F, Koehler K, Krumbholz M, Huebner A, Rochira V. Late-onset triple A syndrome: a risk of overlooked or delayed diagnosis and management. Horm Res. 2008;70(6):364-372.
- Weber A, Wienker TF, Jung M, Easton D, Dean HJ, Heinrichs C, Reis A, Clark AJ. Linkage of the gene for the triple A syndrome to chromosome 12q13 near the type II keratin gene cluster. Hum Mol Genet. 1996;5(12):2061-2066.
- Handschug K, Sperling S, Yoon SJ, Hennig S, Clark AJ, Huebner A. Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet. 2001;10(3):283-290.
- Sandrini F, Farmakidis C, Kirschner LS, Wu SM, Tullio-Pelet A, Lyonnet S, Metzger DL, Bourdony CJ, Tiosano D, Chan WY, Stratakis CA. Spectrum of mutations of the AAAS gene in Allgrove syndrome: lack of mutations in six kindreds with isolated resistance to corticotropin. J Clin Endocrinol Metab. 2001;86(11):5433-5437.
- Thümmler S, Huebner A, Baechler-Sadoul E. Triple A syndrome: two novel mutations in the AAAS gene. BMJ Case Rep. 2009;2009. pii: bcr09.2008.0984.
- Marín S, Casano-Sancho P, Villarreal-Peña N, Sebastiani G, Pinillos S, Pérez-Dueñas B, Hwa V, Rosenfeld RG, Ibáñez L. Triple A syndrome in a patient with genetic growth hormone insensitivity: phenotypic effects of two genetic disorders. Horm Res Paediatr. 2012;77(1):63-68.
- Moschos MM, Margetis I, Koehler K, Gatzioufas Z, Huebner A. New ophthalmic features in a family with triple A syndrome. Int Ophthalmol. 2011;31(3):239-243.
- Krull I, M-Woelfle M, Bärlocher K, Koehler K, Huebner A, Brändle M. Two patients with an identical novel mutation in the AAAS gene and similar phenotype of triple A (Allgrove) syndrome. Exp Clin Endocrinol Diabetes. 2010;118(8):530-536.
- Yang H, Zhang H, Lu L, Wang O, Xing X, Zhang M, Lu Z. Clinical and genetic characterization of a Chinese patient with triple A syndrome and novel compound heterozygous mutations in the AAAS gene. J Pediatr Endocrinol Metab. 2013;26(3-4):389-391.
- Dumić M, Barišić N, Rojnić-Putarek N, Kušec V, Stanimirović A, Koehler K, Huebner A. Two siblings with triple A syndrome and novel mutation presenting as hereditary polyneuropathy. Eur J Pediatr. 2011;170(3):393-396.
- Capataz Ledesma M, Méndez Pérez P, Rodríguez López R, Galán Gómez E. Allgrove syndrome (triple A). Finding of a mutation not described in the AAAS gene. An Pediatr (Barc). 2013;78(2):109-112.
- Prpic I, Huebner A, Persic M, Handschug K, Pavletic M. Triple A syndrome: genotype-phenotype assessment. Clin Genet. 2003;63(5):415-417.
- Prasad R, Metherell LA, Clark AJ, Storr HL. Deficiency of ALADIN impairs redox homeostasis in human adrenal cells and inhibits steroidogenesis. 2013;154(9):3209-3218.
- Jühlen R, Landgraf D, Huebner A, Koehler K. Identification of a novel putative interaction partner of the nucleoporin ALADIN. Biol Open. 2016;5(11):1697-1705.
- Carvalhal S, Ribeiro SA, Arocena M, Kasciukovic T, Temme A, Koehler K, Huebner A, Griffis ER. The nucleoporin ALADIN regulates Aurora A localization to ensure robust mitotic spindle formation. Mol Biol Cell. 2015;26(19):3424-3438.
- Jühlen R, Idkowiak J, Taylor AE, Kind B, Arlt W, Huebner A, Koehler K. Role of ALADIN in human adrenocortical cells for oxidative stress response and steroidogenesis. PLoS One. 2015;10(4):e0124582.
- Koehler K, Malik M, Mahmood S, Gießelmann S, Beetz C, Hennings JC, Huebner AK, Grahn A, Reunert J, Nürnberg G, Thiele H, Altmüller J, Nürnberg P, Mumtaz R, Babovic-Vuksanovic D, Basel-Vanagaite L, Borck G, Brämswig J, Mühlenberg R, Sarda P, Sikiric A, Anyane-Yeboa K, Zeharia A, Ahmad A, Coubes C, Wada Y, Marquardt T, Vanderschaeghe D, Van Schaftingen E, Kurth I, Huebner A, Hübner CA. Mutations in GMPPA cause a glycosylation disorder characterized by intellectual disability and autonomic dysfunction. Am J Hum Genet. 2013;93(4):727-734.
- Koehler K, Milev MP, Prematilake K, Reschke F, Kutzner S, Jühlen R, Landgraf D, Utine E, Hazan F, Diniz G, Schuelke M, Huebner A, Sacher M. A novel TRAPPC11 mutation in two Turkish families associated with cerebral atrophy, global retardation, scoliosis, achalasia and alacrima. J Med Genet. 2017;54(3):176-185.
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years' experience. J Clin Endocrinol Metab. 2006;91(8):3048-3054.
- Jadhav U, Harris RM, Jameson JL. Hypogonadotropic hypogonadism in subjects with DAX1 mutations. Mol Cell Endocrinol. 2011;346(1-2):65-73.
- Landau Z, Hanukoglu A, Sack J, Goldstein N, Weintrob N, Eliakim A, Gillis D, Sagi M, Shomrat R, Kosinovsky EB, Anikster Y. Clinical and genetic heterogeneity of congenital adrenal hypoplasia due to NR0B1 gene mutations. Clin Endocrinol (Oxf). 2010;72(4):448-454.
- Tsai WY, Tung YC. Novel deletion mutations of the DAX1 (NR0B1) gene in two Taiwanese families with X-linked adrenal hypoplasia congenita. J Pediatr Endocrinol Metab. 2005;18(10):991-997.
- Peter M, Viemann M, Partsch CJ, Sippell WG.Congenital adrenal hypoplasia: clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J Clin Endocrinol Metab. 1998;83(8):2666-2674.
- Flint JL, Jacobson JD. Adrenal hypoplasia congenita presenting as congenital adrenal hyperplasia. Case Rep Endocrinol. 2013;2013:393584.
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372(6507):635-641.
- Suntharalingham JP, Buonocore F, Duncan AJ, Achermann JC. DAX-1 (NR0B1) and steroidogenic factor-1 (SF-1, NR5A1) in human disease. Best Pract Res Clin Endocrinol Metab. 2015;29(4):607-619.
- Zhang H, Thomsen JS, Johansson L, Gustafsson JA, Treuter E. DAX-1 functions as an LXXLL-containing corepressor for activated estrogen receptors. J Biol Chem. 2000;275(51):39855-39859.
- Agoulnik IU, Krause WC, Bingman WE 3rd, Rahman HT, Amrikachi M, Ayala GE, Weigel NL. Repressors of androgen and progesterone receptor action. J Biol Chem. 2003;278(33):31136-31148.
- Holter E, Kotaja N, Mäkela S, Strauss L, Kietz S, Jänne OA, Gustafsson JA, Palvimo JJ, Treuter E. Inhibition of androgen receptor (AR) function by the reproductive orphan nuclear receptor DAX-1. Mol Endocrinol. 2002;16(3):515-528.
- Suzuki T, Kasahara M, Yoshioka H, Morohashi K, Umesono K. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol. 2003;23(1):238-249.
- Lalli E, Ohe K, Hindelang C, Sassone-Corsi P. Orphan receptor DAX-1 is a shuttling RNA binding protein associated with polyribosomes via mRNA. Mol Cell Biol. 2000;20(13):4910-4921.
- Phelan JK, McCabe ER. Mutations in NR0B1 (DAX1) and NR5A1 (SF1) responsible for adrenal hypoplasia congenita. Hum Mutat. 2001;18(6):472-487.
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. 1994;372(6507):672-676.
- Seminara SB, Achermann JC, Genel M, Jameson JL, Crowley WF Jr. X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metab. 1999;84(12):4501-4509.
- Krone N, Riepe FG, Dörr HG, Morlot M, Rudorff KH, Drop SL, Weigel J, Pura M, Kreze A, Boronat M, de Luca F, Tiulpakov A, Partsch CJ, Peter M, Sippell WG. Thirteen novel mutations in the NR0B1 (DAX1) gene as cause of adrenal hypoplasia congenita. Hum Mutat. 2005;25(5):502-503.
- Achermann JC, Gu WX, Kotlar TJ, Meeks JJ, Sabacan LP, Seminara SB, Habiby RL, Hindmarsh PC, Bick DP, Sherins RJ, Crowley WF Jr, Layman LC, Jameson JL. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J Clin Endocrinol Metab. 1999;84(12):4497-4500.
- Li N, Liu R, Zhang H, Yang J, Sun S, Zhang M, Liu Y, Lu Y, Wang W, Mu Y, Ning G, Li X. Seven novel DAX1 mutations with loss of function identified in Chinese patients with congenital adrenal hypoplasia. J Clin Endocrinol Metab. 2010;95(9):E104-111.
- Ali JM, Jalaludin MY, Harun F. Late onset X-linked adrenal hypoplasia congenita with hypogonadotropic hypgonadism due to a novel 4-bp deletion in exon 2 of NR0B1. J Pediatr Endocrinol Metab. 2014;27(11-12):1189-1192.
- Bansal S, Castells S, Umpaichitra V, Perez-Colon S. Presence of 21-Hydroxylase Antibodies in a Boy with X-Linked Adrenal Hypoplasia Congenita. Horm Res Paediatr. 2015;84(6):408-413.
- Barbaro M, Bens S, Haake A, Peter M, Brämswig J, Holterhus PM, Lopez-Siguero JP, Menken U, Mix M, Sippell WG, Wedell A, Riepe FG. Multiplex ligation-dependent probe amplification analysis of the NR0B1(DAX1) locus enables explanation of phenotypic differences in patients with X-linked congenital adrenal hypoplasia. Horm Res Paediatr. 2012;77(2):100-107.
- Verrijn Stuart AA, Ozisik G, de Vroede MA, Giltay JC, Sinke RJ, Peterson TJ, Harris RM, Weiss J, Jameson JL. An amino-terminal DAX1 (NROB1) missense mutation associated with isolated mineralocorticoid deficiency. J Clin Endocrinol Metab. 2007;92(3):755-761.
- Takahashi I, Takahashi T, Shoji Y, Takada G. Prolonged activation of the hypothalamus-pituitary-gonadal axis in a child with X-linked adrenal hypoplasia congenita. Clin Endocrinol (Oxf). 2000;53(1):127-129.
- Guzzetti C, Bizzarri C, Pisaneschi E, Mucciolo M, Bellacchio E, Ibba A, Casula L, Novelli A, Loche S, Cappa M. Next-Generation Sequencing Identifies Different Genetic Defects in 2 Patients with Primary Adrenal Insufficiency and Gonadotropin-Independent Precocious Puberty. Horm Res Paediatr. 2018:1-9.
- Reutens AT, Achermann JC, Ito M, Ito M, Gu WX, Habiby RL, Donohoue PA, Pang S, Hindmarsh PC, Jameson JL. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999;84(2):504-511.
- Ponikwicka-Tyszko D, Kotula-Balak M, Jarzabek K, Bilinska B, Wolczynski S. The DAX1 mutation in a patient with hypogonadotropic hypogonadism and adrenal hypoplasia congenita causes functional disruption of induction of spermatogenesis. J Assist Reprod Genet. 2012;29(8):811-816.
- Raffin-Sanson ML, Oudet B, Salenave S, Brailly-Tabard S, Pehuet M, Christin-Maitre S, Morel Y, Young J. A man with a DAX1/NR0B1 mutation, normal puberty, and an intact hypothalamic-pituitary-gonadal axis but deteriorating oligospermia during long-term follow-up. Eur J Endocrinol. 2013;168(4):K45-50.
- Frapsauce C, Ravel C, Legendre M, Sibony M, Mandelbaum J, Donadille B, Achermann JC, Siffroi JP, Christin-Maitre S. Birth after TESE-ICSI in a man with hypogonadotropic hypogonadism and congenital adrenal hypoplasia linked to a DAX-1 (NR0B1) mutation. Hum Reprod. 2011;26(3):724-728.
- Calliari LE, Longui CA, Rocha MN, Faria CD, Kochi C, Melo MR, Melo MB, Monte O. A novel mutation in DAX1 gene causing different phenotypes in three siblings with adrenal hypoplasia congenita. Genet Mol Res. 2007;6(2):277-283.
- Wang CL, Fen ZW, Liang L. A de novo mutation of DAX1 in a boy with congenital adrenal hypoplasia without hypogonadotropic hypogonadism. J Pediatr Endocrinol Metab. 2014;27(3-4):343-347.
- Minari R, Vottero A, Tassi F, Viani I, Neri TM, Street ME, Ghizzoni L, Bernasconi S, Martorana D. A novel mutation in the NR0B1 gene in a family with monozygotic twin sisters and congenital adrenal hypoplasia affected children. Hormones (Athens). 2015;14(1):160-166.
- Xu XQ, Feng YY, Yuan WX, Huang K, Liang L, Fu JF. Novel mutations in DAX1 of X-linked adrenal hypoplasia congenita over several generations in one family. Endocr Pract. 2013;19(4):e105-111.
- Rojek A, Flader M, Malecka E, Niedziela M. A novel mutation in the NR0B1 (DAX1) gene in a large family with two boys affected by congenital adrenal hypoplasia. Hormones (Athens). 2014;13(3):413-419.
- Gerards J, Ritter MM, Kaminsky E, Gal A, Hoeppner W, Quinkler M. A novel stop mutation (p.(Gln22*)) of DAX1 (NR0B1) results in late-onset X-linked adrenal hypoplasia congenita. Endocrinol Diabetes Metab Case Rep. 2017;2017. pii: 17-0054.
- Kyriakakis N, Shonibare T, Kyaw-Tun J, Lynch J, Lagos CF, Achermann JC, Murray RD. Late-onset X-linked adrenal hypoplasia (DAX-1, NR0B1): two new adult-onset cases from a single center. Pituitary. 2017;20(5):585-593.
- Durmaz E, Turkkahraman D, Berdeli A, Atan M, Karaguzel G, Akcurin S, Bircan I. A novel DAX-1 mutation presented with precocious puberty and hypogonadotropic hypogonadism in different members of a large pedigree. J Pediatr Endocrinol Metab. 2013;26(5-6):551-555.
- Dipple KM, McCabe ER. Modifier genes convert "simple" Mendelian disorders to complex traits. Mol Genet Metab. 2000;71(1-2):43-50.
- Sekiguchi Y, Hara Y, Matsuoka H, Hayashi Y, Katsumata N, Hirata Y. Sibling cases of Addison's disease caused by DAX-1 gene mutations. Intern Med. 2007;46(1):35-39.
- Shaikh MG, Boyes L, Kingston H, Collins R, Besley GT, Padmakumar B, Ismayl O, Hughes I, Hall CM, Hellerud C, Achermann JC, Clayton PE. Skewed X inactivation is associated with phenotype in a female with adrenal hypoplasia congenita. J Med Genet. 2008;45(9):e1.
- Merke DP, Tajima T, Baron J, Cutler GB Jr. Hypogonadotropic hypogonadism in a female caused by an X-linked recessive mutation in the DAX1 gene. N Engl J Med. 1999;340(16):1248-1252.
- Bernard P, Ludbrook L, Queipo G, Dinulos MB, Kletter GB, Zhang YH, Phelan JK, McCabe ER, Harley VR, Vilain E. A familial missense mutation in the hinge region of DAX1 associated with late-onset AHC in a prepubertal female. Mol Genet Metab. 2006;88(3):272-279.
- Calliari LE, Rocha MN, Monte O, Longui CA. Mild adrenal insufficiency due to a NROB1 (DAX1) gene mutation in a boy presenting an association of hypogonadotropic hypogonadism, reduced final height and attention deficit disorder. Arq Bras Endocrinol Metabol. 2013;57(7):562-565.
- Chung ST, Chi CH, Haymond MW, Jeha GS. Infantile Growth Hormone Deficiency and X- Linked Adrenal Hypoplasia Congenita. Jacobs J Pediatr. 2015;1(1). pii: 003.
- Franzese A, Brunetti-Pierri N, Spagnuolo MI, Spadaro R, Giugliano M, Mukai T, Valerio G. Inappropriate tall stature and renal ectopy in a male patient with X-linked congenital adrenal hypoplasia due to a novel missense mutation in the DAX-1 gene. Am J Med Genet A. 2005;135(1):72-74.
- Montoya-Williams D, Mowitz M. Cholestasis and Hepatic Iron Deposition in an Infant With Complex Glycerol Kinase Deficiency. Pediatrics. 2017;140(1). pii: e20161479.
- Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249-1258.
- Ramayya MS, Zhou J, Kino T, Segars JH, Bondy CA, Chrousos GP. Steroidogenic factor 1 messenger ribonucleic acid expression in steroidogenic and nonsteroidogenic human tissues: Northern blot and in situ hybridization studies. J Clin Endocrinol Metab. 1997;82(6):1799-1806.
- Wong M, Ramayya MS, Chrousos GP, Driggers PH, Parker KL. Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J Mol Endocrinol. 1996;17(2):139-147.
- Achermann JC. The role of SF1/DAX1 in adrenal and reproductive function. Ann Endocrinol (Paris). 2005;66(3):233-239.
- Mello MP, França ES, Fabbri HC, Maciel-Guerra AT, Guerra-Júnior G. Multifunctional role of steroidogenic factor 1 and disorders of sex development. Arq Bras Endocrinol Metabol. 2011;55(8):607-612.
- Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michán S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14(3):301-312.
- Büdefeld T, Tobet SA, Majdic G. Steroidogenic factor 1 and the central nervous system. J Neuroendocrinol. 2012;24(1):225-235.
- Zangen D, Kaufman Y, Banne E, Weinberg-Shukron A, Abulibdeh A, Garfinkel BP, Dweik D, Kanaan M, Camats N, Flück C, Renbaum P, Levy-Lahad E. Testicular differentiation factor SF-1 is required for human spleen development. J Clin Invest. 2014;124(5):2071-2075.
- Domenice S, Machado AZ, Ferreira FM, Ferraz-de-Souza B, Lerario AM, Lin L, Nishi MY, Gomes NL, da Silva TE, Silva RB, Correa RV, Montenegro LR, Narciso A, Costa EM, Achermann JC, Mendonca BB. Wide spectrum of NR5A1-related phenotypes in 46,XY and 46,XX individuals. Birth Defects Res C Embryo Today. 2016;108(4):309-320.
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481-490.
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. 2002;143(2):607-614.
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18(3):361-377.
- Demura M, Wang F, Yoneda T, Karashima S, Mori S, Oe M, Kometani M, Sawamura T, Cheng Y, Maeda Y, Namiki M, Ino H, Fujino N, Uchiyama K, Tsubokawa T, Yamagishi M, Nakamura Y, Ono K, Sasano H, Demura Y, Takeda Y. Multiple noncoding exons 1 of nuclear receptors NR4A family (nerve growth factor-induced clone B, Nur-related factor 1 and neuron-derived orphan receptor 1) and NR5A1 (steroidogenic factor 1) in human cardiovascular and adrenal tissues. J Hypertens. 2011;29(6):1185-1195.
- Li D, Urs AN, Allegood J, Leon A, Merrill AH Jr, Sewer MB. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase theta facilitates induction of CYP17. Mol Cell Biol. 2007;27(19):6669-6685.
- Fabbri-Scallet H, de Mello MP, Guerra-Júnior G, Maciel-Guerra AT, de Andrade JGR, de Queiroz CMC, Monlleó IL, Struve D, Hiort O, Werner R. Functional characterization of five NR5A1 gene mutations found in patients with 46,XY disorders of sex development. Hum Mutat. 2018;39(1):114-123.
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125-126.
- Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, Jameson JL. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87(4):1829-1833.
- Biason-Lauber A, Schoenle EJ. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet. 2000;67(6):1563-1568.
- Guoying C, Zhiya D, Wei W, Na L, Xiaoying L, Yuan X, Defen W. The analysis of clinical manifestations and genetic mutations in Chinese boys with primary adrenal insufficiency. J Pediatr Endocrinol Metab. 2012;25(3-4):295-300.
- Malikova J, Camats N, Fernández-Cancio M, Heath K, González I, Caimarí M, del Campo M, Albisu M, Kolouskova S, Audí L, Flück CE. Human NR5A1/SF-1 mutations show decreased activity on BDNF (brain-derived neurotrophic factor), an important regulator of energy balance: testing impact of novel SF-1 mutations beyond steroidogenesis. PLoS One. 2014;9(8):e104838.
- Orekhova AS, Kalinchenko N, Morozov IA, Vasilyev EV, Rubtsov PM, Dedov II, Tiulpakov A. A Novel Mutation in the Critical P-Box Residue of Steroidogenic Factor-1 Presenting with XY Sex Reversal and Transient Adrenal Failure. Horm Res Paediatr. 2018;89(6):450-454.
- Correa RV, Domenice S, Bingham NC, Billerbeck AE, Rainey WE, Parker KL, Mendonca BB. A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab. 2004;89(4):1767-1772.
- Hasegawa T, Fukami M, Sato N, Katsumata N, Sasaki G, Fukutani K, Morohashi K, Ogata T. Testicular dysgenesis without adrenal insufficiency in a 46,XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab. 2004;89(12):5930-5935.
- Mallet D, Bretones P, Michel-Calemard L, Dijoud F, David M, Morel Y. Gonadal dysgenesis without adrenal insufficiency in a 46, XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab. 2004;89(10):4829-4832.
- Reuter AL, Goji K, Bingham NC, Matsuo M, Parker KL. A novel mutation in the accessory DNA-binding domain of human steroidogenic factor 1 causes XY gonadal dysgenesis without adrenal insufficiency. Eur J Endocrinol. 2007;157(2):233-238.
- Coutant R, Mallet D, Lahlou N, Bouhours-Nouet N, Guichet A, Coupris L, Croué A, Morel Y. Heterozygous mutation of steroidogenic factor-1 in 46,XY subjects may mimic partial androgen insensitivity syndrome. J Clin Endocrinol Metab. 2007;92(8):2868-2873.
- Lourenço D, Brauner R, Lin L, De Perdigo A, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, Maciel-Guerra AT, Achermann JC, McElreavey K, Bashamboo A. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360(12):1200-1210.
- Philibert P, Leprieur E, Zenaty D, Thibaud E, Polak M, Frances AM, Lespinasse J, Raingeard I, Servant N, Audran F, Paris F, Sultan C. Steroidogenic factor-1 (SF-1) gene mutation as a frequent cause of primary amenorrhea in 46,XY female adolescents with low testosterone concentration. Reprod Biol Endocrinol. 2010;8:28.
- Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA, Jameson JL, Sultan C, Dattani MT, Achermann JC. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92(3):991-999.
- Tantawy S, Mazen I, Soliman H, Anwar G, Atef A, El-Gammal M, El-Kotoury A, Mekkawy M, Torky A, Rudolf A, Schrumpf P, Grüters A, Krude H, Dumargne MC, Astudillo R, Bashamboo A, Biebermann H, Köhler B. Analysis of the gene coding for steroidogenic factor 1 (SF1, NR5A1) in a cohort of 50 Egyptian patients with 46,XY disorders of sex development. Eur J Endocrinol. 2014;170(5):759-767.
- Köhler B, Lin L, Ferraz-de-Souza B, Wieacker P, Heidemann P, Schröder V, Biebermann H, Schnabel D, Grüters A, Achermann JC. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat. 2008;29(1):59-64.
- Hattori A, Zukeran H, Igarashi M, Toguchi S, Toubaru Y, Inoue T, Katoh-Fukui Y, Fukami M. A novel C-terminal truncating NR5A1 mutation in dizygotic twins. Hum Genome Var. 2017;4:17008.
- Adachi M, Hasegawa T, Tanaka Y, Asakura Y, Hanakawa J, Muroya K. Spontaneous virilization around puberty in NR5A1-related 46,XY sex reversal: additional case and a literature review. Endocr J. 2018. EJ18-0218.
- Colson C, Aubry E, Cartigny M, Rémy AA, Franquet H, Leroy X, Kéchid G, Lefèvre C, Besson R, Cools M, Spinoit AF, Sultan C, Manouvrier S, Philibert P, Ghoumid J. SF1 and spleen development: new heterozygous mutation, literature review and consequences for NR5A1-mutated patient's management. Clin Genet. 2017;92(1):99-103.
- Swartz JM, Ciarlo R, Guo MH, Abrha A, Weaver B, Diamond DA, Chan YM, Hirschhorn JN. A 46,XX Ovotesticular Disorder of Sex Development Likely Caused by a Steroidogenic Factor-1 (NR5A1) Variant. Horm Res Paediatr. 2017;87(3):191-195.
- Baetens D, Stoop H, Peelman F, Todeschini AL, Rosseel T, Coppieters F, Veitia RA, Looijenga LH, De Baere E, Cools M. NR5A1 is a novel disease gene for 46,XX testicular and ovotesticular disorders of sex development. Genet Med. 2017;19(4):367-376.
- Bashamboo A, Donohoue PA, Vilain E, Rojo S, Calvel P, Seneviratne SN, Buonocore F, Barseghyan H, Bingham N, Rosenfeld JA, Mulukutla SN, Jain M, Burrage L, Dhar S, Balasubramanyam A, Lee B; Members of UDN, Dumargne MC, Eozenou C, Suntharalingham JP, de Silva K, Lin L, Bignon-Topalovic J, Poulat F, Lagos CF, McElreavey K, Achermann JC. A recurrent p.Arg92Trp variant in steroidogenic factor-1 (NR5A1) can act as a molecular switch in human sex development. Hum Mol Genet. 2016;25(23):5286.
- Warman DM, Costanzo M, Marino R, Berensztein E, Galeano J, Ramirez PC, Saraco N, Baquedano MS, Ciaccio M, Guercio G, Chaler E, Maceiras M, Lazzatti JM, Bailez M, Rivarola MA, Belgorosky A. Three new SF-1 (NR5A1) gene mutations in two unrelated families with multiple affected members: within-family variability in 46,XY subjects and low ovarian reserve in fertile 46,XX subjects. Horm Res Paediatr. 2011;75(1):70-77.
- Bashamboo A, Ferraz-de-Souza B, Lourenço D, Lin L, Sebire NJ, Montjean D, Bignon-Topalovic J, Mandelbaum J, Siffroi JP, Christin-Maitre S, Radhakrishna U, Rouba H, Ravel C, Seeler J, Achermann JC, McElreavey K. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010;87(4):505-512.
- Röpke A, Tewes AC, Gromoll J, Kliesch S, Wieacker P, Tüttelmann F. Comprehensive sequence analysis of the NR5A1 gene encoding steroidogenic factor 1 in a large group of infertile males. Eur J Hum Genet. 2013;21(9):1012-1015.
- Robevska G, van den Bergen JA, Ohnesorg T, Eggers S, Hanna C, Hersmus R, Thompson EM, Baxendale A, Verge CF, Lafferty AR, Marzuki NS, Santosa A, Listyasari NA, Riedl S, Warne G, Looijenga L, Faradz S, Ayers KL, Sinclair AH. Functional characterization of novel NR5A1 variants reveals multiple complex roles in disorders of sex development. Hum Mutat. 2018;39(1):124-139.
- Nishina-Uchida N, Fukuzawa R, Numakura C, Suwanai AS, Hasegawa T, Hasegawa Y. Characteristic testicular histology is useful for the identification of NR5A1 gene mutations in prepubertal 46,XY patients. Horm Res Paediatr. 2013;80(2):119-128.
- Hatano M, Migita T, Ohishi T, Shima Y, Ogawa Y, Morohashi KI, Hasegawa Y, Shibasaki F. SF-1 deficiency causes lipid accumulation in Leydig cells via suppression of STAR and CYP11A1. 2016;54(2):484-496.
- Schlaubitz S, Yatsenko SA, Smith LD, Keller KL, Vissers LE, Scott DA, Cai WW, Reardon W, Abdul-Rahman OA, Lammer EJ, Lifchez CA, Magenis E, Veltman JA, Stankiewicz P, Zabel BU, Lee B. Ovotestes and XY sex reversal in a female with an interstitial 9q33.3-q34.1 deletion encompassing NR5A1 and LMX1B causing features of Genitopatellar syndrome. Am J Med Genet A. 2007;143A(10):1071-1081.
- van Silfhout A, Boot AM, Dijkhuizen T, Hoek A, Nijman R, Sikkema-Raddatz B, van Ravenswaaij-Arts CM. A unique 970kb microdeletion in 9q33.3, including the NR5A1 gene in a 46,XY female. Eur J Med Genet. 2009;52(2-3):157-160.
- Brandt T, Blanchard L, Desai K, Nimkarn S, Cohen N, Edelmann L, Mehta L. 46,XY disorder of sex development and developmental delay associated with a novel 9q33.3 microdeletion encompassing NR5A1. Eur J Med Genet. 2013;56(11):619-623.
- Fabbri HC, de Andrade JG, Soardi FC, de Calais FL, Petroli RJ, Maciel-Guerra AT, Guerra-Júnior G, de Mello MP. The novel p.Cys65Tyr mutation in NR5A1 gene in three 46,XY siblings with normal testosterone levels and their mother with primary ovarian insufficiency. BMC Med Genet. 2014;15:7.
- Milani D, Pezzani L, Tabano S, Miozzo M. Beckwith-Wiedemann and IMAGe syndromes: two very different diseases caused by mutations on the same gene. Appl Clin Genet. 2014;7:169-175.
- Bodian DL, Solomon BD, Khromykh A, Thach DC, Iyer RK, Link K, Baker RL, Baveja R, Vockley JG, Niederhuber JE. Diagnosis of an imprinted-gene syndrome by a novel bioinformatics analysis of whole-genome sequences from a family trio. Mol Genet Genomic Med. 2014;2(6):530-538.
- Bennett J, Schrier Vergano SA, Deardorff MA. IMAGe Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. SourceGeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. 2014 Mar 13 [updated 2016 Sep 8].
- Kerns SL, Guevara-Aguirre J, Andrew S, Geng J, Guevara C, Guevara-Aguirre M, Guo M, Oddoux C, Shen Y, Zurita A, Rosenfeld RG, Ostrer H, Hwa V, Dauber A. A novel variant in CDKN1C is associated with intrauterine growth restriction, short stature, and early-adulthood-onset diabetes. J Clin Endocrinol Metab. 2014;99(10):E2117-2122.
- Arboleda VA, Lee H, Parnaik R, Fleming A, Banerjee A, Ferraz-de-Souza B, Délot EC, Rodriguez-Fernandez IA, Braslavsky D, Bergadá I, Dell'Angelica EC, Nelson SF, Martinez-Agosto JA, Achermann JC, Vilain E. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat Genet. 2012;44(7):788-792.
- Hamajima N, Johmura Y, Suzuki S, Nakanishi M, Saitoh S. Increased protein stability of CDKN1C causes a gain-of-function phenotype in patients with IMAGe syndrome. PLoS One. 2013;8(9):e75137.
- Borges KS, Arboleda VA, Vilain E. Mutations in the PCNA-binding site of CDKN1C inhibit cell proliferation by impairing the entry into S phase. Cell Div. 2015;10:2.
- Brioude F, Oliver-Petit I, Blaise A, Praz F, Rossignol S, Le Jule M, Thibaud N, Faussat AM, Tauber M, Le Bouc Y, Netchine I. CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome. J Med Genet. 2013;50(12):823-830.
- Narumi S, Amano N, Ishii T, Katsumata N, Muroya K, Adachi M, Toyoshima K, Tanaka Y, Fukuzawa R, Miyako K, Kinjo S, Ohga S, Ihara K, Inoue H, Kinjo T, Hara T, Kohno M, Yamada S, Urano H, Kitagawa Y, Tsugawa K, Higa A, Miyawaki M, Okutani T, Kizaki Z, Hamada H, Kihara M, Shiga K, Yamaguchi T, Kenmochi M, Kitajima H, Fukami M, Shimizu A, Kudoh J, Shibata S, Okano H, Miyake N, Matsumoto N, Hasegawa T. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48(7):792-797.
- Buonocore F, Kühnen P, Suntharalingham JP, Del Valle I, Digweed M, Stachelscheid H, Khajavi N, Didi M, Brady AF, Blankenstein O, Procter AM, Dimitri P, Wales JKH, Ghirri P, Knöbl D, Strahm B, Erlacher M, Wlodarski MW, Chen W, Kokai GK, Anderson G, Morrogh D, Moulding DA, McKee SA, Niemeyer CM, Grüters A, Achermann JC. Somatic mutations and progressive monosomy modify SAMD9-related phenotypes in humans. J Clin Invest. 2017;127(5):1700-1713.
- Kim YM, Seo GH, Kim GH, Ko JM, Choi JH, Yoo HW. A case of an infant suspected as IMAGE syndrome who were finally diagnosed with MIRAGE syndrome by targeted Mendelian exome sequencing. BMC Med Genet. 2018;19(1):35.
- Schwartz JR, Wang S, Ma J, Lamprecht T, Walsh M, Song G, Raimondi SC, Wu G, Walsh MF, McGee RB, Kesserwan C, Nichols KE, Cauff BE, Ribeiro RC, Wlodarski M, Klco JM. Germline SAMD9 mutation in siblings with monosomy 7 and myelodysplastic syndrome. 2017;31(8):1827-1830.
- Shima H, Koehler K, Nomura Y, Sugimoto K, Satoh A, Ogata T, Fukami M, Jühlen R, Schuelke M, Mohnike K, Huebner A, Narumi S. Two patients with MIRAGE syndrome lacking haematological features: role of somatic second-site reversion SAMD9 mutations. J Med Genet. 2018;55(2):81-85.
- Jeffries L, Shima H, Ji W, Panisello-Manterola D, McGrath J, Bird LM, Konstantino M, Narumi S, Lakhani S. A novel SAMD9 mutation causing MIRAGE syndrome: An expansion and review of phenotype, dysmorphology, and natural history. Am J Med Genet A. 2018;176(2):415-420.
- Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, Hurcombe J, Bierzynska A, Barbagelata E, Bergadá I, Cassinelli H, Das U, Krone R, Hacihamdioglu B, Sari E, Yesilkaya E, Storr HL, Clemente M, Fernandez-Cancio M, Camats N, Ram N, Achermann JC, Van Veldhoven PP, Guasti L, Braslavsky D, Guran T, Metherell LA. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. J Clin Invest. 2017;127(3):942-953.
- Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, Shril S, Ashraf S, Tan W, Rao J, Airik M, Schapiro D, Braun DA, Sadowski CE, Widmeier E, Jobst-Schwan T, Schmidt JM, Girik V, Capitani G, Suh JH, Lachaussée N, Arrondel C, Patat J, Gribouval O, Furlano M, Boyer O, Schmitt A, Vuiblet V, Hashmi S, Wilcken R, Bernier FP, Innes AM, Parboosingh JS, Lamont RE, Midgley JP, Wright N, Majewski J, Zenker M, Schaefer F, Kuss N, Greil J, Giese T, Schwarz K, Catheline V, Schanze D, Franke I, Sznajer Y, Truant AS, Adams B, Désir J, Biemann R, Pei Y, Ars E, Lloberas N, Madrid A, Dharnidharka VR, Connolly AM, Willing MC, Cooper MA, Lifton RP, Simons M, Riezman H, Antignac C, Saba JD, Hildebrandt F. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest. 2017;127(3):912-928.
- Janecke AR, Xu R, Steichen-Gersdorf E, Waldegger S, Entenmann A, Giner T, Krainer I, Huber LA, Hess MW, Frishberg Y, Barash H, Tzur S, Schreyer-Shafir N, Sukenik-Halevy R, Zehavi T, Raas-Rothschild A, Mao C, Müller T. Deficiency of the sphingosine-1-phosphate lyase SGPL1 is associated with congenital nephrotic syndrome and congenital adrenal calcifications. Hum Mutat. 2017;38(4):365-372.
- Linhares ND, Arantes RR, Araujo SA, Pena SDJ. Nephrotic syndrome and adrenal insufficiency caused by a variant in SGPL1. Clin Kidney J. 2018;11(4):462-467.
- Bamborschke D, Pergande M, Becker K, Koerber F, Dötsch J, Vierzig A, Weber LT3, Cirak S. A novel mutation in sphingosine-1-phosphate lyase causing congenital brain malformation. Brain Dev. 2018;40(6):480-483.
Adrenal Insuffciency Due To X-Linked Adrenoleukodystrophy
ABSTRACT
X-linked adrenoleukodystrophy (X-ALD) is an inherited neurodegenerative disorder, involving mainly the white matter and axons of the central nervous system, the adrenal cortex, and the testis and a frequent but under-recognized cause of primary adrenocortical insufficiency. X-ALD is caused by a defect in the gene ABCD1 that maps to Xq 28 locus. The primary biochemical disorder is the accumulation of saturated very long chain fatty acids (VLCFA) secondary to peroxisomal dysfunction. The incidence in males is estimated to be 1:21,000 and in females 1:14,000, without any difference in the prevalence among different ethnicities. At least six distinct phenotypes have been described that differ in the age and severity of clinical presentation; however, there is no correlation between X-ALD phenotype and mutations in the ABCD1 gene. When suspected, the diagnosis is established biochemically and prenatal testing is possible in affected families. Currently, there is no satisfying treatment to prevent the onset or modify the progression of the chronic myelopathy of X-ALD. The administration of a mixture of glyceryl-trioleate and glyceryl- trierucate, also referred as Lorenzo's Oil, has been shown to prevent disease progression in asymptomatic patients with cerebral involvement of X-ALD. Allogeneic hematopoietic stem cell (HSC) transplantation is the treatment of choice for individuals with early stages of the cerebral form of the disease. An alternative option for patients without HLA-matched donors is autologous HSC-gene therapy with lentivirally corrected cells. Once adrenal insufficiency is present, hormonal replacement therapy is identical to that of autoimmune Addison’s disease. For complete coverage of this area and all of Endocrinology, visit www.endotext.org.
INTRODUCTION
Leukodystrophies are inherited neurodegenerative disorders, primary affecting the brain myelin. X-linked adrenoleukodystrophy (X-ALD; OMIM:300100)) is the most common leukodystrophy usually presenting as chronic myelopathy and peripheral neuropathy, a clinical entity called adrenomyeloneuropathy (AMN), frequently accompanied by adrenocortical insufficiency (1). The pattern of inheritance is X-linked and the disease is clinically evident in almost all male patients and in more than 80 % of female carriers older than 60 years, though with milder manifestations. Occasionally, male patients and very rarely female carriers may develop a rapidly progressive, devastating cerebral form of the disease known as Cerebral Adrenoleukodystrophy (CALD). The pathophysiological basis of the disease is peroxisome dysfunction and accumulation of very long chain fatty acids (VLCFA, >C22:0) due to impaired VLCFA degradation (2).
In the early 20th century, patients with signs and symptoms belonging in the Leukodystrophies spectrum were grouped under the name “Addison–Schilder disease”. It was not until the 1960s that Blaw introduced the term “adrenoleukodystrophy” as a distinct disease entity with X-linked inheritance (3). In 1976 it was shown that the principal biochemical disorder in X-ALD was the accumulation of VLCFA (4). In 1993, the gene responsible for the disease was identified at Xq28 locus and it was subsequently shown to be the ABCD1 gene, which encodes the Adrenoleukodystrophy Protein (ALDP) (5).
This chapter summarizes the latest data in the literature regarding the progress made in elucidating the pathogenesis of the disease, the strategies for early diagnosis, and the results of established as well as of newer experimental therapies.
GENETICS & PATHOPHYSIOLOGY
X-ALD is associated with the accumulation of saturated VLCFA, particularly hexacosanoic (C26:0) and lignoceric (C24:0) acids, due to impaired degradation by the peroxisomes (6,7).
The gene that is defective is referred to as ABCD1 (GenBank accession number: NM_000033). It is located on Xq28, covers 19.9 kb and contains 10 exons (5). It encodes a peroxisomal trans-membrane protein of 745 amino acids, ALDP, a member of the ATP binding cassette (ABC) transport protein family, which helps to form the channel through which VLCFAs move into the peroxisome as VLCFA-CoA (8).
The mode of inheritance of X-ALD is X-linked recessive, thus the possibility of a son of a female carrier developing X-ALD is 50%, whilst 50% of female off-springs will also be heterozygous carriers. All female off-springs of an affected male will be carriers but none of his male off-springs will be affected. Significant intra-familiar phenotype variability has been observed as different clinical phenotypes can occur even among monozygotic twins (9). Fifty percent of ABCD1 mutations lead to a truncated ALDP, whereas many missense mutations result in the formation of an unstable protein (10). The complete absence of a functional ALDP does not necessarily lead to the severe form of X-ALD, implicating the existence of additional factors that could modify disease’s clinical expression. Environmental factors, such as moderate head trauma, have been shown to trigger the progression of the disease to the severe central nervous system (CNS) form (11). In contrast, mutations with residual transporter activity or over-expression of ALDP-related protein (ALDRP, ABCD2), the closest homolog of ALDP, might prevent this progression (12). Variations in methionine metabolism have also been associated with the wide phenotypic spectrum of X-ALD (13).
The incidence of the disease is estimated to be 1:17,000 (1:21,000 in males and 1:14,000 in females), a disproportion that may reflect the morbidity related to adrenal insufficiency in males preceding the diagnosis of X-ALD. No difference has been observed in the prevalence among different ethnicities (2,14). More than 1000 different mutations have been identified in X-ALD patients and are updated in the website http://www.x-ald.nl. Of these mutations, 51% are missense mutations, 28% frame-shift mutations, 12% nonsense mutations, 6% point mutations and 3% larger deletions of one or more exons (10). Nine hotspot mutations have been identified, which together account for 20% of all cases; the most common among them being a micro-deletion in exon 5 (p.Gln472Argfs*83) (15). Near 4% of patients are affected by a de novo mutation; however in a recent study from Norway, this figure is reported to be as high as 19% (16).
Accumulation of abnormal VLCFA in affected organs is regarded to represent the underlying pathologic process in X-ALD, leading to cell death due to a combination of disruption of cell membranes as well as an induction of oxidative stress and apoptosis (17). Singh and co-workers have demonstrated that the β-oxidation of C24:0 and C26:0 is reduced in fibroblasts from X-ALD patients to approximately 25% of control levels (6), leading to the accumulation of VLCFA-CoA esters in the cytosol. These esters are prone to further elongation carried out by an elongase specific for VLCFA (ELOVL1), further increasing the intracellular levels of VLCFA (18). Transfection of X-ALD cell lines with normal ABCD1 gene restores their capacity to degrade VLCFA (19). Interestingly, injection of C24:0 complexed to phospholipids (C24:0–lysophosphatidylcholine) into the cortex of wild type mice caused widespread microglial activation and apoptosis (20). Such effects were not produced by injections of long-chain lipids (C16:0–lysophosphatidylcholine), implying a fatty-acyl chain length dependent cytotoxicity. In fact, VLCFA are extremely hydrophobic compounds and experimental data suggest that inclusion of C26:0 in a model membrane can disrupt its structure (21). This effect has been shown to be toxic mainly to the myelin-producing oligodendrocytes and Schwann cells, causing the breakdown or loss of the myelin sheath surrounding the nerve cells in the brain and the peripheral nerves respectively. The pathogenesis of X-ALD is summarized in Figure 1.
Figure 1. The pathogenesis of X- ALD: The mutated ABCD1 gene encodes a defective Adrenoleukodystrophy Protein (ALDP) that impedes Very Long Chain Fatty Acids (VLCFAs) from entering the peroxisome to undergo degradation. This leads to accumulation of VLCFAs in the cytosol, which is further aggravated by the elongation of LCFAs carried out by a specific VLCFA elongase (ELOVL1). VLCFA accumulation of has been shown to be toxic by causing the breakdown of cell membranes and by evoking mitochondrial dysfunction as a result of oxidative stress
Moreover, it is suggested that VLCFA can induce cell death by disturbing calcium homeostasis and/or evoking oxidative stress-related mitochondrial dysfunction (22,23). The theory of elevated oxidative stress has been supported by experiments showing that lymphocytes of X-ALD patients contain low amounts of total and reduced glutathione, whereas the proportion of oxidized glutathione forms is elevated (24). Oxidative stress may impair mitochondrial function by inducing the formation of the Mitochondrial Permeability Transition Pore (MPTP), which represents an increased permeability of the mitochondrial membranes to molecules of less than 1500 Daltons. Induction of the MPTP is associated with mitochondrial swelling and cell death. Cyclophilin D, the most studied component of MPTP has been found particularly expressed in the affected zones of the brain in patients with X-ALD and in the spinal cord of a mouse model of X-ALD. Notably, these changes can be experimentally reversed by treatment with anti-oxidants (25). In addition, the oxidation of cholesterol and linoleic acid, leads to the formation of cholesterol oxide derivatives oxidized at C7 (7-ketocholesterol (7KC), 7β-hydroxycholesterol (7β-OHC), which further aggravate peroxisomal dysfunction (26).
The pathogenic mechanism that triggers the progression to CALD has not been elucidated as yet. Inflammatory demyelination seems to play a key role and plasmatic VLCFA concentration has been positively correlated to the levels of pro-inflammatory cytokines (27). Moreover, a higher VLCFA content has been reported in the non-affected white matter of patients with CALD compared to patients with non-cerebral ALD, possibly representing a precursor lesion (28). Breakdown of the blood-brain-barrier is also implicated by recent studies that have demonstrated an elevation of the matrix metalloproteinases in the cerebrospinal fluid of CALD compared to AMN patients (29). Progression of the cerebral lesions has also been associated with elevated oxidative stress and impaired plasma antioxidant capacity as expressed by superoxide dismutase (SOD) levels. Plasma SOD levels from patients with CALD demonstrated an inverse correlation to brain magnetic resonance imaging (MRI) severity score, while longitudinal samples from the same patients showed a decrease in plasma SOD activity prior to and at the time of diagnosis (30).
Regarding the adrenal gland, abnormal VLCFA accumulation is believed to cause apoptosis and ultimately atrophy of the adrenal cortex. Increased esterification of cholesterol with VLCFAs may further impair cortisol secretion due to a relative shortage of substrate for steroidogenesis (31). Impaired cortisol response to ACTH stimulation usually precedes frank hypocortisolism indicating that loss of adrenal function is a gradual, progressive phenomenon (Figure 2). A possible explanation for this early adrenal dysfunction may be the incorporation of VLCFAs into the adrenocortical cell membrane, which may impair the stimulatory effects of adrenocorticotropin (ACTH) on the adrenocortical cells (32).
Similarly, male patients may present with testicular insufficiency due to the toxicity of VLCFAs on Sertoli and Leydig cells. Testosterone levels are usually in the lower‑normal range with elevated luteinizing hormone, while the response to human chorionic gonadotropin is blunted, indicating primary hypogonadism (33). However, in a recent case report, hypogonadism was attributed to tissue specific androgen resistance rather than to primary testicular failure, probably mediated through VLCFA accumulation at the androgen receptor and/or post-receptor levels (34).

Figure 2. Impaired Cortisol response to ACTH stimulation from adrenocortical cells cultivated in VLCFA (- - -) and (-----), compared to ethanol (…….) and linoleic acid (_._._.).
PATHOLOGY
In the CNS, ALDP is mostly expressed in oligodendrocytes, microglia, astrocytes and endothelial cells, but not in most neurons (35). Lipid inclusions containing cholesterol, phospholipids and gangliosides esterified with saturated VLCFA have been found in all affected tissues, even in morphologically normal regions, indicating that the biochemical abnormality precedes histopathological changes (17). Lesions of the spinal cord and peripheral neural system observed in AMN have been traditionally characterized as a non-inflammatory distal axonopathy with minimal myelin changes (36). Nevertheless, recent studies have shown that affected spinal cord microglia is also vulnerable to phagocytosis, allowing the injury of neurons that reside within an altered metabolic milieu (37). The regions that are mostly affected in AMN are the dorsal columns in the cervical cord segments and the cortico-spinal tracts in the lower thoracic and lumbar segments of the spinal cord (38). AMN can also insult peripheral nerves and this is evident in the epidermis where low nerve fiber densities can be found, indicating a loss of the thin unmyelinated nerve fibers (39). Such alterations may also appear in the optic nerve and can be detected by optical coherence tomography as thinning of the retinal nerve fiber layer (stratum opticum) and of the macula (40).
On the other hand, brain lesions in CALD are evident as large areas of demyelination of the white matter, while the cortex is typically spared. The parieto-occipital regions are affected in 85% of cases, with asymmetric progression of the lesions towards the frontal or temporal lobes, whereas the frontal lobes are involved in only 5% of cases (41). In general, arcuate fibers are spared, except in chronic cases, where axonal loss may be considerable, but myelin loss is usually greater. Lesions may sometimes involve the brainstem, especially the pons, whereas the spinal cord is usually spared, except in cases of bilateral cortico-spinal tract degeneration (42). Occasionally, demyelinated areas may be seen in the cerebral white matter of asymptomatic patients, however, these are scattered in a patchy manner and without signs of inflammation.
The severe and rapidly progressive cerebral form of X-ALD is associated with the evolution of an inflammatory process besides demyelination. Upon microscopic examination, these inflammatory lesions of CALD consist of three distinct concentric zones. The most outward zone contains many lipid-laden macrophages and destruction of myelin albeit with axonal sparing. The second zone also contains many macrophages and a mixture of myelinated and demyelinated axons. A hallmark finding in this zone is perivascular infiltration with lymphocytes (43,44). The pathological process involving this zone is responsible for the gadolinium enhancement observed on MRI scans. The third zone is the innermost and largest one, consisting of a dense grid of glial fibrils and scattered astrocytes. This distinct zonal pattern of CALD lesions can be also detected on MRI scans (45).
VLCFA accumulation is also evident in the adrenal cortex of patients with X-ALD, particularly in the zona reticularis and the zona fasciculate, with a relative sparing of the zona glomerulosa. Microscopically adrenocortical cells become ballooned and striated due to the accumulation of lamellae and lamellar-lipid profiles, which consist of cholesterol molecules esterified with saturated VLCFA (31). These distinct pathological features can also be demonstrable in the fetal adrenal gland, indicating that accumulation of VLCFA is present already in utero. Similar lesions can be found in the testes of X-Ald men, primary affecting Leydig cells that are responsible for steroidogenesis (46).
CLINICAL MANIFESTATIONS OF X-ALD
The range of clinical expression of X-ALD varies widely. Tables 1a and 1b summarize the principal clinical phenotypes. A hallmark that distinguishes X-ALD from other inherited neurodegenerative diseases is that patients are asymptomatic at birth (1); however, in male patients adrenocortical insufficiency may develop even during the first year of life (47). In contrast, AMN the most frequent form of the disease is rarely present before adulthood and reaches 100% penetration over the age of 55 years. The presence of asymptomatic males beyond this age is exceptional. In fact, ALD is more accurately considered as a progressive disorder and this phenotypic classification is due to systematic reasons: approximately 60% of male patients, including 20% of those initially diagnosed as AMN, will eventually present CALD during their lifespan with the childhood form being the most severe (48). The clinical course of the disease and particularly the presence of CALD is thought to be the result of an interplay between genetic and environmental factors (Figure 3).

Figure 3. The clinical course of X-ALD in men: patients are born asymptomatic, whereas Addison’s disease is usually the first manifestation of the disease, affecting up to 80% of men. Adrenomyeloneuropathy appears later in life (3rd decade) with an incidence increasing with increasing age and affecting almost 100% of men. Cerebral form of ALD may be apparent in early childhood, however it can emerge at any age and is thought to be the result of an interplay between genetic and environmental factors (modified from Kemp et al. 2016)
Adrenomyeloneuropathy
AMN is a disorder that affects mainly the long tracts of the spinal cord characterized by an absent or mild inflammatory response (36,38) and such patients may survive to the eighth decade of life. The disease onset is usually between the third and fourth decade and in two-thirds of patients, the neurologic disability progresses slowly over a span of 10-15 years. In the remaining, a more rapid progression is observed within 3–5 years. The primary manifestation is gait disorder due to spastic paraparesis and sensory ataxia with impaired vibration sense, which mainly affects the lower limbs; loss of dexterity or strength in the arms is exceptional (49). Sphincter dysfunction initially presenting as urge complaints, progressing to full incontinence as well as impotence are accompanying features; whereas in some cases a characteristic diffuse hair loss is observed (50). Signs of peripheral neuropathy may also be present, however they are usually masked by the most prominent clinical features of myelopathy (51). The course of AMN is gradually progressive, with most patients losing ambulation by the 6th decade of life (52).
Up to 63% of AMN patients are reported to have additional cerebral demyelination (53) and subtle cerebral manifestations are often present. The rate of depressive illness appears to be elevated at least two-fold (54) and mood change fluctuations according to the hormonal replacement of adrenal insufficiency frequently occur. Approximately 20-30% of the AMN patients may develop at a later stage progressive cerebral involvement in which the inflammatory response is present (55). In such cases, the survival is reported to be as poor as in childhood cerebral adrenoleukodystrophy. However, this risk decreases markedly after the age of 45 years.
Cerebral ALD
The risk of a newborn male carrier of the ABCD1 mutation developing CALD is 35 – 40% between the ages of 5 and 12 years. Disease onset prior to 3 years of age is rare and this risk is substantially lower among boys whose brain MRI remains normal until 7 years of age (56). The earlier the onset of disease, the more rapid the progression is, whereas patients may remain asymptomatic as long as demyelinating lesions are not visible on brain MRI. Generally, the onset of CALD is insidious, and can be confused with the Attention Deficit Hyperactivity Disorder which is characterized by hyperactivity, impulsiveness and an abrupt decline in school performance. Cognitive deficits can be accompanied by neurologic deficits such as hemiplegia or quadriparesis, cerebellar ataxia, impaired central auditory discrimination, visual field defects, cortical blindness, and often seizures (1).
The presentation in adults is similar and initially may appear as a psychiatric disturbance resembling the manifestations of obsessive-compulsive personality disorder (57). These psychiatric symptoms may precede frank motor or cognitive changes by some years. Infections or head trauma may trigger the onset of CALD, but usually no extrinsic factor is identified (58). Nevertheless, once the disease becomes inflammatory, as evidenced by the post-contrast enhancement of the borders of the brain lesions as shown in MRI, it usually progresses rapidly to a devastating form, leading to a vegetative state within two to five years (59). Interestingly, 10% of males with imaging evidence of CALD may never enter into the active inflammatory stage, a phenotype referred to as “chronic or arrested cerebral X-ALD ” (60); however it is possible that these lesions may be reactivated many years later.
Female Heterozygotes
Contrary to previous beliefs, that considered female heterozygotes as being asymptomatic, it is now accepted that approximately 65% of such individuals will develop an AMN-like syndrome by the age of 60. In general, the onset of neurologic symptoms occurs at a later age than in males, and there is a strong association between the onset of symptoms and age. Typically symptoms appear in the fourth to fifth decade of life and disease manifestations are less severe with a notable occurrence of early fecal incontinence (61). Scanty scalp hair can also be found in females (62). Only a few females have been reported to develop CALD and this has been attributed to skewed inactivation of the X-chromosome carrying the mutated ABCD1 gene (63).
Incidence of Primary Adrenal Deficiency in X-ALD
The incidence of primary adrenal insufficiency (PAI) in males with X-ALD has been reported to be 50-86% and the corresponding figures in the various phenotypes are shown in Tables 1 and 2. The incidence of PAI in the patients with the childhood cerebral forms of ALD appears to be higher than in the AMN patients. While many patients have both neurologic involvement and adrenal insufficiency, a considerable number has only one or the other. The patients with the "Addison only" phenotype by definition are free of demonstrable neurologic involvement; however, due to the progressive nature of the disease, many individuals in this category will later develop neurological involvement. ALD is the cause for up to 20 percent of male cases of idiopathic Addison’s disease. Biochemical evidence of adrenal insufficiency can be present for up to two years before the development of relevant clinical signs and the youngest boy detected with subclinical PAI was 5 months of age (47). Elevated ACTH levels and impaired cortisol response to ACTH administration are the most frequent findings. Frank hypoaldosteronism with salt wasting is not frequent, but impaired aldosterone response to ACTH may be observed in approximately one third of men with X-ALD (64).
Addison’s disease is rare in women heterozygous for X-ALD (1% or less), and considerably less frequent than the AMN-like syndrome, which develops in approximately 50% of women in middle age or later. Even though it is rare for heterozygous women to show clinically evident adrenal insufficiency, post-mortem studies have revealed adrenal abnormalities resembling those in affected males (65). When more subtle tests of adrenal function, such as the response to ovine corticotropin-releasing-hormone, were performed, subnormal responses were demonstrated in five of eight women with previously normal ACTH stimulation tests (66).
Table 1. X-ALD Phenotypes in Males |
|||
| Phenotype | Description | Estimated Relative Frequency | Adreno- cortical Insufficiency |
| Childhood cerebral | Onset 3-10 years. | 31-35% | 79% |
| Progressive behavioral, cognitive, neurologic deficits. | |||
| Total disability often within 3 years. | |||
| Adolescent cerebral | Like childhood cerebral; somewhat slower progression | 4-7% | 62% |
| Adult cerebral | Dementia, behavioral disturbances focal neurologic deficits without preceding adrenomyeloneuropathy | 2-3% | >50% |
| Adrenomyeloneuropathy | Onset 28 ± 9 years. | 40-46% | 50-70% |
| Slowly progressive paraparesis, sphincter disturbances | |||
| Addison only | Primary adrenal insufficiency without neurologic involvement. | Varies with age. Up to 50% in childhood | 100% |
| Most common onset 5-7 years. Most eventually develop AMN or cerebral forms | |||
| Asymptomatic | No demonstrable neurologic or adrenal involvement | Common before 4 years. Diminishes with age. | 50% plus with testing |
Table 2. Phenotypes in Female X-ALD Carriers |
||
| Phenotype | Description | Estimated relative frequency |
| Asymptomatic | No neurologic or adrenal involvement | Diminishes with age |
| Mild myeloneuropathy | Increased deep tendon reflexes and sensory changes in lower extremities |
Increases with age. ~ 50% at age >40 years. |
| Moderate to severe myeloneuropathy | Resembles AMN, but milder and later onset |
Increases with age >15% at age >40. |
| Clinically evident Addison’s disease | Rare at any age | <1% |
X-ALD appears to be a more frequent cause of Addison's disease in males than is generally recognized. Lauretti et al. found that 5 of 14 male patients between 12 and 45 years of age, previously diagnosed as having PAI, had abnormally high plasma VLCFA levels in the setting of X-ALD (67). Jorge et al. diagnosed X-ALD in ten of 37 patients with idiopathic Addison’s Disease (27%), and found that the incidence was 100% in patients in whom adrenal insufficiency became evident before 7.5 years of age (68). These findings are of great clinical importance, as prompt diagnosis of X-ALD has profound implications for prognosis, therapy and genetic counseling. It is therefore important that screening for X- ALD is carried out in all male patients with idiopathic Addison’s Disease. The need to do so is particularly relevant in patients in whom PAI was manifested before 7.5 years of age.
Other X-ALD Phenotypes
More than 200 X-ALD males have been reported to remain completely asymptomatic, even without signs of PAI, up to the age of 40 –50 years. This is referred to as the “asymptomatic-normal MRI” phenotype (14). Men with X-ALD may also present with gonadal dysfunction, however, neurological involvement in such patients is usually already evident at the time they develop testicular insufficiency. Therefore, the presence of erectile dysfunction might be related to myelopathy, while decreased libido may be associated with depression and/or the presence of a chronic disease rather than hypogonadism (33). Interestingly, a negative impact of X-ALD on male fertility has not been demonstrated so far (69).
DIAGNOSIS OF X-ALD
The plasma assay for VLCFA is the hallmark diagnostic procedure as it is very reliable for the identification of affected males (70). VLCFA levels are already increased on the day of birth and in untreated patients remain approximately the same throughout life. Testing, typically includes three VLCFA parameters: the level of hexacosanoic acid (C26:0) and tetracosanoic acid (C24:0), and the ratio of these two compounds to docosanoic acid (normal values of C24:0/C22:0 ratio <1.0 and C26:0/C22:0 ratio <0.02). Hexacosanoic acid is the one most consistently elevated, and is therefore considered to be diagnostic of the disease. It should be noted though, that VLCFA levels are also elevated in some other peroxisomal disorders, whereas they can be falsely elevated in patients on ketogenic diets (71). On the other hand, grape-seed and mustard-seed oils may cause false negative results. So far, no correlation has been established between the degree of VLCFA elevation and the severity of the disease or the onset of certain manifestations (72). The assay can also be used to identify asymptomatic patients by screening members of the extended family (49). Notably, false negative results may occur in approximately 15 to 20% of obligate female heterozygotes. In such patients, mutation analysis by molecular genetic testing of the ABCD1 gene locus is the most accurate method for a definitive diagnosis (73). Nevertheless, in some cases mutation analysis may reveal a sequence variant of the ABCD1 gene with unknown clinical significance, presenting a diagnostic conundrum for the clinician.
The diagnosis of X-ALD should be sought in:
- Boys with progressive behavioral, cognitive or neurologic disturbances beginning at 3 years of age or later.
- Males with Addison's disease in whom the etiology has not been defined (e.g. absent auto-antibodies against adrenal antigens). Since the plasma VLCFA assay is non-invasive, and the practical and genetic implications of the diagnosis of X-ALD are significant, the VLCFA assay could be part of the routine initial evaluation of male patients with Addison’s disease.
- Men and women with progressive myelopathy. AMN is often misdiagnosed as multiple sclerosis. Nevertheless, a relapsing and remitting evolution is never seen in AMN. The diagnosis of X-ALD should be considered even when there is no clinical or biochemical evidence of PAI. In a large series from Germany adrenal function was normal in 20 of 41 men with AMN, and PAI occurred in less than one percent of women with and AMN-like syndrome (74).
- Patients in whom PAI occurs in combination with neurologic disability (Table 3).
- Patients who are at genetic risk of having X-ALD on the basis of pedigree. Because X-ALD is X-linked recessive, a large number of relatives in the nuclear and extended family are at genetic risk. Detection of asymptomatic patients is particularly important, since therapeutic interventions have the greatest chance of success when clinical manifestations are still mild.
Table 3. Conditions in Which Adrenocortical Insufficiency is Associated with Neurologic Dysfunction. |
||
| Disorder | Nature of Neurologic Disturbance | |
| X-linked adrenoleukodystrophy | See text | |
| Neonatal adrenoleukodystrophy | Autosomal recessive; early onset; dysmorphic features, multiple organ involvement | |
| Triple A syndrome (OMIM 231550) | Achalasia, alacrima, adrenal insufficiency | |
| Peripheral neuropathy, cerebellar ataxia. | ||
| Mild dementia, autosomal recessive, gene defined | ||
| Glycerol kinase deficiency | Autosomal recessive. Psychomotor retardation | |
Newborn Screening
Newborn screening (NBS) is justified for a disorder, provided that a therapy is available and that early diagnosis allows timely implementation. This is particularly relevant for X-ALD after the promising results of hematopoietic stem cell transplantation (HSCT): early diagnosis at birth would allow the early detection of PAI in order to initiate timely adrenal steroid replacement therapy, whereas early detection of CALD would permit HSCT before severe neurologic impairment is established. Important improvements towards this target was the development of mass spectrometry methods to assess the presence of VLCFA in dried-blood spots as well as a combined liquid chromatography/tandem mass spectrometry (LC-MS/MS) high-throughput assay that could measure VLCFA enriched lysophosphatidylcholine (lysoPC , thus providing the technical background for NBS (75). Eventually, New York State (NYS) in 2013 was the first authority to include screening for X-ALD in the NBS program, while more states are expected to add screening for X-ALD to their own NBS program since it has been added to the Recommended Universal Screening Panel (RUSP) (76).
NYS NBS for X-ALD is based on a 3-tier algorithm. The first tier, refers to all newborns and includes C26:0 VLCFA assessment in dried blood spots. In case of a pathological result, the second more specific tier, measuring C26:0- lyso-PC is employed. If the C26:0 lyso-PC is also elevated, then sequencing of the 10 exons of the ABCD1 gene is performed as part of the third tier of screening. If sequencing reveals a relevant mutation, a confirmatory VLCFA analysis should be ordered in an independent laboratory. If ABCD1 mutation analysis is negative, then a rare peroxismal disorder should be sought (76). The whole procedure is reported to be both highly sensitive and specific and might be used as a template to diagnose X-ALD in symptomatic patients. It is however; still premature to draw conclusions about the health and social impact that NBS has on the diagnosed individuals and their families.
Genetic Counseling
As soon as an index case is detected either as a consequence of symptoms or as a result of NBS, genetic counseling should be offered to the family. If the index case is male, testing should be offered to his mother and female offspring. If the mother is confirmed to be a carrier for an ABCD1 mutation, testing should also include all the male siblings of the index case. If the index case is female, initial testing should include both parents. Regarding mutation testing of minor females of an affected family, there is no consensus whether it should be performed on a routine base. (76).
Prenatal diagnosis is an option for women who are heterozygous carriers of the ABCD1 gene (77). Recently, a non-invasive prenatal determination of fetal sex being able to detect Y chromosome sequences in maternal blood by molecular techniques (78). However, since a significant number of heterozygous women will develop AMN in adulthood, prenatal diagnosis may also be considered for a female fetus. Sex determination along with ABCD1 mutational analysis can be performed on a fresh chorionic villus sample (CVS) at 11–13 weeks of pregnancy. Amnioparacentesis can still be performed at 15–18 weeks of gestation; however, this option might delay the decision-making process since amniotic cell culture requires an additional 2 – 3 weeks. If the ABCD1 gene mutation has not been recognized but the maternal phenotype is highly suspicious, prenatal diagnosis of a male fetus can be done by the measurement of VLCFA levels in cultured CVS cells or amniotic cells (79). Preimplantation genetic diagnosis is an additional option, particularly useful for heterozygous female carriers who have already had pregnancy interruption due to prenatal diagnoses of an X-ALD male fetus.
Imaging
All individuals with confirmed ALD/AMN complex should undergo neuroimaging to determine if cerebral involvement is present. Brain MRI is the procedure of choice and should be performed every 6 months in pre-symptomatic male patients between 3 and 12 years of age and yearly after that up to 45 years (80). Brain MRI abnormalities precede symptoms in patients with the cerebral forms of X-ALD (56). Findings are always abnormal in symptomatic patients, demonstrating cerebral white matter demyelination (Figure 4). The lesions typically begin in the splenium of the corpus callosum before gradually expanding to the occipito-parietal region and they are usually bilateral, but occasionally can be limited to only one side, particularly if a previous head trauma has triggered CALD (11). The presence of contrast enhancement just behind the outermost edge of the lesions as seen in T1-weighted images (WI), heralds the progression to inflammatory devastating form of CALD (59). Loes et al. has introduced a grading system to assess the degree of MRI abnormalities in X-ALD (81). This is a 32-point scale score (0: normal, 32: most severe) that assesses the degree and extent of hyperintense lesions on FLAIR or T2W images as well as the degree of regional atrophy, and has proven to have predictive value for the response to HSCT (82). Regarding AMN, MRI of the spinal cord is unremarkable on standard sequences, it can however show atrophy in advanced cases (83). Contrast enhancement is not observed in AMN, since inflammation is not a feature of extra-cerebral lesions.
Functional imaging such as Proton MR Spectroscopy may detect white matter abnormalities that are not apparent on conventional MR imaging and may predict disease progression (84). A decrease of N-acetyl-aspartate (NAA)/creatinine ratio is observed, reflecting axonal loss and an increase of choline / creatinine and myo-inositol/creatine ratios associated lipid turnover changes (85). Brain F18 fludeoxy-glucose positron emission tomography (PET) may reveal hypometabolic regions particularly in cerebellum and temporal lobe areas, before lesions emerge in MRI (86). In contrast, hypermetabolism may be evident in the frontal lobes, related to the clinical severity of the disease (57).

Figure 4. MRI of a patient with CALD, showing reduced volume and increased signal intensity of the white matter localised mainly at the parieto-occipital regions. The anterior white matter is spared.
( http://en.wikipedia.org/w/index.php?title=Adrenoleukodystrophy&oldid=506277486).
Testing of Adrenal Function
Adrenal function should be evaluated as soon as the diagnosis of X-ALD is set by measurement of basal (8:00 AM) plasma ACTH and cortisol concentrations. A combined ACTH value more than twice the upper limit of normal (>100 pg/mL) with a cortisol value of less than 10 mcg/dL (270 nmol/L) make the diagnosis of PAI high likely and should prompt the initiation of proper cortisol replacement therapy (87). If results are equivocal (e.g. normal cortisol levels but elevated ACTH), a formal stimulation test following ACTH / cosyntropin administration should be offered. A response of cortisol less than 18 mg/dL, 60 minutes after the administration of cosyntropin is also indicative of PAI, which requires replacement therapy at least in situations of physical stress (surgery, acute febrile illness, vomiting etc.). In case the diagnosis of X-ALD is made in infancy, cosyntropin stimulation test is also indicated to diagnose PAI, since ACTH and cortisol production is not predictable until 6 to 12 months of age (88). Regarding asymptomatic patients with X-ALD, according to the NYS NBS guidelines, screening for PAI should be repeated every 6 months (76). Evaluation for mineralcorticoid deficiency is not currently recommended, due to the relative sparing of the zona glomerulosa, however it should be considered in the presence of symptoms, such as salt-craving and polyuria (89).
THERAPY
Allogeneic Hematopoietic Stem Cell Transplantation
Therapy of the neurologic aspects of X-ALD is a major challenge. Currently, there is no satisfying treatment to prevent the onset or modify the progression of the chronic myelopathy of X-ALD. Allogeneic HSCT is the treatment of choice for individuals with early stages of cerebral involvement of X-ALD, which may increase disease specific survival and can lead to long-term stabilization and occasionally improvement (90–92). Stem cells can be harvested from peripheral blood, bone marrow, and umbilical cord blood of immune-compatible donors. Although the mechanism of this effect is still unclear, bone marrow cells do express the ABCD1 gene and plasma VLCFA levels are reduced after bone marrow transplantation, offering a useful biomarker for the assessment of engraftment, graft failure, or rejection (93). It has been shown that bone marrow-derived cells do enter the brain-blood barrier and that a portion of perivascular microglia is gradually replaced by donor derived cells (94). HSCT may also diminish the brain inflammatory response as well as lipid peroxidation and protein damage. Stabilization of the disease is usually evident about 6 months after the transplantation. The outcomes of allogeneic HSCT for CALD have been mainly studied among adolescents: the 5-year survival among boys of Loes score < 10 is as high as 89%, whereas in those with a score ≥10 is only 40%. On the other hand, the cumulative incidence of transplantation-related mortality is 8% (92). A recent study has also evaluated the potential long-term neurological benefits and the complications of allogeneic HSCT in adult CALD, with less compelling results (95).
Current strategy is to monitor asymptomatic patients by MRI at 6-month to yearly intervals depending on their age and consider HSCT when the MRI abnormality is advancing and clinical disability is still mild (96). Because HSCT carries a substantial mortality risk (5%), it is not recommended for patients who already have advanced cerebral involvement (e.g. IQ<80 and a Loes score ≥10), because there is evidence that such an approach may not reverse severe deficits and in some instances may accelerate disease progression (97). HSCT has not been tested systematically in AMN because of concern that the risk-benefit ratio may not be favorable: up to 50% of AMN patients will never develop cerebral involvement, whereas it is high unlikely that HSCT will affect the non-inflammatory distal axonopathy which is the main pathological feature in AMN (36). Moreover, in retrospective series of patients who successfully underwent HSCT for CALD in childhood, it was shown that it could not prevent the onset of AMN in adulthood (98). It is still a question whether the progression of myelopathy in X-ALD might be slowed down by HSCT.
In case of patients without HLA-matched donors or adult patients with CALD (given the higher mortality risk of allogeneic HSCT compared to children), an alternative option is autologous HSC-gene therapy with lentivirally corrected cells (19). In this procedure, CD34+ cells from X-ALD patients are transfected ex vivo using a lentiviral vector encoding the wild-type ABCD1 cDNA. As a result of this therapy, 7-14% of granulocytes, monocytes, T and B lymphocytes express the lentivirally encoded ALDP. In a recent phase 2-3 study including 17 boys, short-term clinical outcomes were reported to be comparable to that of allogeneic HSCT (99). Nevertheless, concerns regarding long-term efficacy, biosafety of lentiviral vectors, as well as the high cost of this therapy need to be taken into account (100,101). An alternative approach is performing allogeneic HSCT from healthy siblings conceived after preimplantation HLA matching, which offers the possibility of selecting unaffected embryos that are HLA compatible with the sick child (102). Regarding adrenal function, there is no evidence for the reversal of adrenal failure after either HSCT or autologous HSC gene therapy (103).
Dietary Treatment
Other therapeutic options include dietary therapies with restriction of fat intake and particularly of VLCFAs and saturated fats to avoid their accumulation. In order to achieve this, total fat intake is restricted to 15% of the total calorie supply and a maximum of 5-10 mg of C26:0 are allowed on a daily basis (Table 4). However, since the majority of VLCFA are of endogenous origin (104), this approach is not sufficient. A mixture of glyceryl trioleate and glyceryl trierucate, also referred as Lorenzo's Oil (LO), which is shown to halt the elongation of VLCFA by inhibiting ELOVL1, has also been applied (105).
This therapy normalizes plasma VLCFA levels within four weeks and in a recent study involving 89 asymptomatic X-ALD patients with normal brain MRI, dietary treatment with LO resulted in a twofold or greater reduction in the risk of developing the childhood cerebral form of X-ALD (106). However, its therapeutic effects in patients who are already symptomatic has been disappointing. Besides, it is widely admitted that LO therapy does not improve adrenal function (52).The daily dosage of LO is 2-3 mL/kg/day and is usually well tolerated. Its most severe side effects are thrombocytopenia and lymphopenia, which usually revert to normal after treatment discontinuation. Treatment with LO may be continued for an indefinite time until disease progression and/or severe side effects occur. It is not recommended in children under one year of age, as it causes a decrease in the levels of other fatty acids, particularly of docosahexaenoic acid, which is essential for neurocognitive development.
Table 4. Dietary restrictions in X-ALD. Adopted form ref. 2. |
|
| Foods rich in VLCFAs | Foods rich in saturated fat |
|
Vegetable oils Fatty fish and meat Plant cover and cuticle Fruit peel and seeds Grains and nuts |
Vegetable oils Fatty fish and meat Milk and milk products Egg yolk Industrial pastry |
Experimental Therapies
Current research on novel treatment options for X-ALD is focused on a) agents that bypass the defective ALDP by inducing alternative pathways for VLCFA degradation, b) combinations of antioxidants that diminish oxidative stress, c) agents that halt VLCFA elongation and d) the use of neurotrophic factors
Apart from ALDP, three additional closely related ABC half-transporters exist: ALDRP, PMP70 and PMP69, which are located on the membrane of peroxysomes. ALDP must dimerize with one of these half-transporters to form a functional full-transporter (107). Over-expression of ABCD2, the gene producing ALDRP has been shown to compensate for ABCD1 deficiency and ameliorate VLCFA production from X-ALD cell series (12). Valproic acid (VPA), a widely used anti-epileptic drug, 4-phenylbutyrate, and other histone deacetylase inhibitors, are known inducers of the expression of ALDRP. In a 6-month pilot trial of VPA in X-ALD patients marked correction of the protein oxidative damage was observed (108). Other agents known to evoke induction of the ABCD2 gene are ligands to several nuclear receptors: fibrates for PPAR alpha, thyroid hormones and thyromimetics, retinoids, and lately LXR antagonists and are being tested in vitro and in vivo for the treatment of X-ALD (109–111). Lately, it has been shown that AMP-activated protein kinase (AMPKα1) is reduced in X-ALD, raising the question if metformin, a well-known AMPKα1 inducer, may have a therapeutic role for X-ALD (112).
Regarding the use of antioxidative treatments, experimental data show that treatment of ABCD1 null mice with a combination of antioxidants containing α-tocopherol, N-acetyl-cysteine and α-lipoic acid reversed oxidative damage, axonal degeneration, and locomotor impairment (22). Similar results have been observed with the oral administration of pioglitazone, an agonist of the PPAR gamma receptor, which restored oxidative damage to mitochondrial proteins and DNA, and reversed bioenergetic failure . Lately, bezafibrate, a PPAR pan agonist has been demonstrated to reduce VLCFA levels in X-ALD fibroblasts (113). The mechanism for this action is by decreasing the synthesis of C26:0 through a direct inhibition of ELOVL-1 and subsequent fatty acid elongation activity. Unfortunately, these actions could not be confirmed in vivo as in a recent clinical trial, bezafibrate was unable to lower VLCFA levels in plasma or lymphocytes of X-ALD patients (114).
The options for treatment of the advanced progressive form of CALD remain limited. Even though the presence of inflammatory lesions is well recognized, trials of immunosuppressive therapies have yielded poor results. Cyclophosphamide, interferon, IVIG, and other immunomodulators have been used without success (80,115). Promising results have been extracted by the use of the antioxidant N-acetyl-L-cysteine as adjunctive therapy to HSCT in patients with advanced CALD (116).
Treatment of Adrenal Insufficiency and Hypogonadism
For those patients with X-ALD who have impaired adrenal function glucocorticoid replacement therapy is mandatory. Glucocorticoid replacement requirements are generally the same as in other forms of PAI whereas most patients may not require mineralocorticoid replacement. While there is one report of substantial improvement of neurologic function when replacement therapy was administered to a patient with AMN (117), the general impression is that adrenal replacement therapy does not alter neurologic progression.
Male patients who present clinical manifestations of hypogonadism and confirmed low serum testosterone levels, should be treated with testosterone. Nevertheless, careful evaluation should be warranted, since impotence, in most instances may imply spinal cord involvement or neuropathy, rather than testosterone deficiency.
REFERENCES
- Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain. 1997;120 ( Pt 8:1485–508.
- Engelen M, Kemp S, Poll-The BT. X-linked adrenoleukodystrophy: pathogenesis and treatment. Curr Neurol Neurosci Rep. 2014;14(10):486.
- Blaw ME, Osterberg K, Kozak P, Nelson E. Sudanophilic Leukodystrophy and Adrenal Cortical Atrophy. Arch Neurol. 1964;11:626–31.
- Igarashi M, Schaumburg HH, Powers J, Kishmoto Y, Kolodny E, Suzuki K. Fatty acid abnormality in adrenoleukodystrophy. J Neurochem. 1976;26(4):851–60.
- Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361(6414):726–30.
- Singh I, Moser AE, Moser HW, Kishimoto Y. Adrenoleukodystrophy: impaired oxidation of very long chain fatty acids in white blood cells, cultured skin fibroblasts, and amniocytes. Pediatr Res. 1984;18(3):286–90.
- Singh I, Moser AE, Goldfischer S, Moser HW. Lignoceric acid is oxidized in the peroxisome: implications for the Zellweger cerebro-hepato-renal syndrome and adrenoleukodystrophy. Proc Natl Acad Sci U S A. 1984;81(13):4203–7.
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113.
- Pereira Fdos S, Matte U, Habekost CT, de Castilhos RM, El Husny AS, Lourenco CM, et al. Mutations, clinical findings and survival estimates in South American patients with X-linked adrenoleukodystrophy. PLoS One. 2012;7(3):e34195.
- Kemp S, Pujol A, Waterham HR, van Geel BM, Boehm CD, Raymond G V, et al. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum Mutat. 2001;18(6):499–515.
- Raymond G V, Seidman R, Monteith TS, Kolodny E, Sathe S, Mahmood A, et al. Head trauma can initiate the onset of adreno-leukodystrophy. J Neurol Sci. 2010;290(1–2):70–4.
- Netik A, Forss-Petter S, Holzinger A, Molzer B, Unterrainer G, Berger J. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum Mol Genet. 1999;8(5):907–13.
- Hudspeth MP, Raymond G V. Immunopathogenesis of adrenoleukodystrophy: current understanding. J Neuroimmunol. 2007;182(1–2):5–12.
- Bezman L, Moser AB, Raymond G V, Rinaldo P, Watkins PA, Smith KD, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49(4):512–7.
- Kemp S, Ligtenberg MJ, van Geel BM, Barth PG, Wolterman RA, Schoute F, et al. Identification of a two base pair deletion in five unrelated families with adrenoleukodystrophy: a possible hot spot for mutations. Biochem Biophys Res Commun. 1994 Jul 29;202(2):647–53.
- Horn MA, Retterstol L, Abdelnoor M, Skjeldal OH, Tallaksen CM. Adrenoleukodystrophy in Norway: high rate of de novo mutations and age-dependent penetrance. Pediatr Neurol. 2013;48(3):212–9.
- Reinecke CJ, Knoll DP, Pretorius PJ, Steyn HS, Simpson RH. The correlation between biochemical and histopathological findings in adrenoleukodystrophy. J Neurol Sci. 1985;70(1):21–38.
- Ofman R, Dijkstra IME, van Roermund CWT, Burger N, Turkenburg M, van Cruchten A, et al. The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Mol Med. 2010 Mar;2(3):90–7.
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Bougneres P, Schmidt M, Kalle C V, et al. Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy. Methods Enzym. 2012;507:187–98.
- Eichler FS, Ren JQ, Cossoy M, Rietsch AM, Nagpal S, Moser AB, et al. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol. 2008;63(6):729–42.
- Ho JK, Moser H, Kishimoto Y, Hamilton JA. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J Clin Invest. 1995;96(3):1455–63.
- Galea E, Launay N, Portero-Otin M, Ruiz M, Pamplona R, Aubourg P, et al. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: A paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta. 2012;1822(9):1475–88.
- Hein S, Schonfeld P, Kahlert S, Reiser G. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum Mol Genet. 2008;17(12):1750–61.
- Petrillo S, Piemonte F, Pastore A, Tozzi G, Aiello C, Pujol A, et al. Glutathione imbalance in patients with X-linked adrenoleukodystrophy. Mol Genet Metab. 2013;109(4):366–70.
- Lopez-Erauskin J, Galino J, Bianchi P, Fourcade S, Andreu AL, Ferrer I, et al. Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain. 2012;135(Pt 12):3584–98.
- Nury T, Zarrouk A, Ragot K, Debbabi M, Riedinger J-M, Vejux A, et al. 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: Potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J Steroid Biochem Mol Biol. 2017;169:123–36.
- Marchetti F, Rowan-Carroll A, Williams A, Polyzos A, Berndt-Weis ML, Yauk CL. Sidestream tobacco smoke is a male germ cell mutagen. Proc Natl Acad Sci. 2011;
- Asheuer M, Bieche I, Laurendeau I, Moser A, Hainque B, Vidaud M, et al. Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X-linked adrenoleukodystrophy. Hum Mol Genet. 2005;14(10):1293–303.
- Thibert KA, Raymond G V, Nascene DR, Miller WP, Tolar J, Orchard PJ, et al. Cerebrospinal fluid matrix metalloproteinases are elevated in cerebral adrenoleukodystrophy and correlate with MRI severity and neurologic dysfunction. PLoS One. 2012;7(11):e50430.
- Turk BR, Theisen BE, Nemeth CL, Marx JS, Shi X, Rosen M, et al. Antioxidant Capacity and Superoxide Dismutase Activity in Adrenoleukodystrophy. JAMA Neurol. 2017 May 1;74(5):519–24.
- Powers JM, Schaumburg HH. Adreno-leukodystrophy (sex-linked Schilder’s disease). A pathogenetic hypothesis based on ultrastructural lesions in adrenal cortex, peripheral nerve and testis. Am J Pathol. 1974;76(3):481–91.
- Whitcomb RW, Linehan WM, Knazek RA. Effects of long-chain, saturated fatty acids on membrane microviscosity and adrenocorticotropin responsiveness of human adrenocortical cells in vitro. J Clin Invest. 1988;81(1):185–8.
- Assies J, Gooren LJ, Van Geel B, Barth PG. Signs of testicular insufficiency in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy: a retrospective study. Int J Androl. 1997 Oct;20(5):315–21.
- Karapanou O, Vlassopoulou B, Tzanela M, Papadopoulos D, Angelidakis P, Michelakakis H, et al. X-linked adrenoleukodystrophy: are signs of hypogonadism always due to testicular failure? Horm. 2014;13(1):146–52.
- Fouquet F, Zhou JM, Ralston E, Murray K, Troalen F, Magal E, et al. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol Dis. 1997;3(4):271–85.
- Powers JM, DeCiero DP, Ito M, Moser AB, Moser HW. Adrenomyeloneuropathy: a neuropathologic review featuring its noninflammatory myelopathy. J Neuropathol Exp Neurol. 2000;59(2):89–102.
- Gong Y, Sasidharan N, Laheji F, Frosch M, Musolino P, Tanzi R, et al. Microglial dysfunction as a key pathological change in adrenomyeloneuropathy. Ann Neurol. 2017 Nov;82(5):813–27.
- Powers JM, DeCiero DP, Cox C, Richfield EK, Ito M, Moser AB, et al. The dorsal root ganglia in adrenomyeloneuropathy: neuronal atrophy and abnormal mitochondria. J Neuropathol Exp Neurol. 2001;60(5):493–501.
- Horn MA, Nilsen KB, Jorum E, Mellgren SI, Tallaksen CM. Small nerve fiber involvement is frequent in X-linked adrenoleukodystrophy. Neurology. 2014;82(19):1678–83.
- Aquino JJ, Sotirchos ES, Saidha S, Raymond G V, Calabresi PA. Optical coherence tomography in x-linked adrenoleukodystrophy. Pediatr Neurol. 2013 Sep;49(3):182–4.
- Poll-The BT, Gartner J. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochim Biophys Acta. 2012;1822(9):1421–9.
- van der Knaap MS, Valk J. The MR spectrum of peroxisomal disorders. Neuroradiology. 1991;33(1):30–7.
- Powers JM, Liu Y, Moser AB, Moser HW. The inflammatory myelinopathy of adreno-leukodystrophy: cells, effector molecules, and pathogenetic implications. J Neuropathol Exp Neurol. 1992;51(6):630–43.
- Ito M, Blumberg BM, Mock DJ, Goodman AD, Moser AB, Moser HW, et al. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. J Neuropathol Exp Neurol. 2001;60(10):1004–19.
- Musolino PL, Rapalino O, Caruso P, Caviness VS, Eichler FS. Hypoperfusion predicts lesion progression in cerebral X-linked adrenoleukodystrophy. Brain. 2012;135(Pt 9):2676–83.
- Powers JM, Moser HW, Moser AB, Schaumburg HH. Fetal adrenoleukodystrophy: the significance of pathologic lesions in adrenal gland and testis. Hum Pathol. 1982;13(11):1013–9.
- Dubey P, Raymond G V, Moser AB, Kharkar S, Bezman L, Moser HW. Adrenal insufficiency in asymptomatic adrenoleukodystrophy patients identified by very long-chain fatty acid screening. J Pediatr. 2005;146(4):528–32.
- Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M. Adrenoleukodystrophy - Neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol. 2016;12(10):606–15.
- Moser HW, Moser AB, Smith KD, Bergin A, Borel J, Shankroff J, et al. Adrenoleukodystrophy: phenotypic variability and implications for therapy. J Inherit Metab Dis. 1992;15(4):645–64.
- Lecumberri B, Giros ML, Coll MJ, Marco A, Casado M, Pallardo LF, et al. Diffuse hair loss in Addison disease: a reason for X-linked adrenoleukodystrophy screening. J Am Acad Dermatol. 2012;66(5):860–1.
- Chaudhry V, Moser HW, Cornblath DR. Nerve conduction studies in adrenomyeloneuropathy. J Neurol Neurosurg Psychiatry. 1996 Aug;61(2):181–5.
- van Geel BM, Assies J, Haverkort EB, Koelman JH, Verbeeten B. J, Wanders RJ, et al. Progression of abnormalities in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy despite treatment with “Lorenzo’s oil.” J Neurol Neurosurg Psychiatry. 1999;67(3):290–9.
- de Beer M, Engelen M, van Geel BM. Frequent occurrence of cerebral demyelination in adrenomyeloneuropathy. Neurology. 2014;83(24):2227–31.
- Walterfang MA, O’Donovan J, Fahey MC, Velakoulis D. The neuropsychiatry of adrenomyeloneuropathy. CNS Spectr. 2007 Sep;12(9):696–701.
- van Geel BM, Bezman L, Loes DJ, Moser HW, Raymond G V. Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann Neurol. 2001;49(2):186–94.
- Moser HW, Loes DJ, Melhem ER, Raymond G V, Bezman L, Cox CS, et al. X-Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31(5):227–39.
- Salsano E, Marotta G, Manfredi V, Giovagnoli AR, Farina L, Savoiardo M, et al. Brain fluorodeoxyglucose PET in adrenoleukodystrophy. Neurology. 2014;83(11):981–9.
- Raymond G V, Seidman R, Monteith TS, Kolodny E, Sathe S, Mahmood A, et al. Head trauma can initiate the onset of adreno-leukodystrophy. J Neurol Sci. 2009;290(1–2):70–4.
- Melhem ER, Loes DJ, Georgiades CS, Raymond G V, Moser HW. X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol. 2000;21(5):839–44.
- Cox CS, Dubey P, Raymond G V, Mahmood A, Moser AB, Moser HW. Cognitive evaluation of neurologically asymptomatic boys with X-linked adrenoleukodystrophy. Arch Neurol. 2006;63(1):69–73.
- Engelen M, Barbier M, Dijkstra IM, Schur R, de Bie RM, Verhamme C, et al. X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain. 2014;137(Pt 3):693–706.
- Restuccia D, Di Lazzaro V, Valeriani M, Oliviero A, Le Pera D, Colosimo C, et al. Neurophysiological abnormalities in adrenoleukodystrophy carriers. Evidence of different degrees of central nervous system involvement. Brain. 1997;120 ( Pt 7:1139–48.
- Maier EM, Kammerer S, Muntau AC, Wichers M, Braun A, Roscher AA. Symptoms in carriers of adrenoleukodystrophy relate to skewed X inactivation. Ann Neurol. 2002;52(5):683–8.
- Blevins LS, Shankroff J, Moser HW, Ladenson PW. Elevated plasma adrenocorticotropin concentration as evidence of limited adrenocortical reserve in patients with adrenomyeloneuropathy. J Clin Endocrinol Metab. 1994 Feb;78(2):261–5.
- Powers JM, Moser HW, Moser AB, Ma CK, Elias SB, Norum RA. Pathologic findings in adrenoleukodystrophy heterozygotes. Arch Pathol Lab Med. 1987;111(2):151–3.
- el-Deiry SS, Naidu S, Blevins LS, Ladenson PW. Assessment of adrenal function in women heterozygous for adrenoleukodystrophy. J Clin Endocrinol Metab. 1997;82(3):856–60.
- Laureti S, Casucci G, Santeusanio F, Angeletti G, Aubourg P, Brunetti P. X-linked adrenoleukodystrophy is a frequent cause of idiopathic Addison’s disease in young adult male patients. J Clin Endocrinol Metab. 1996;81(2):470–4.
- Jorge P, Quelhas D, Oliveira P, Pinto R, Nogueira A. X-linked adrenoleukodystrophy in patients with idiopathic Addison disease. Eur J Pediatr. 1994;153(8):594–7.
- Stradomska TJ, Kubalska J, Janas R, Tylki-Szymanska A. Reproductive function in men affected by X-linked adrenoleukodystrophy/adrenomyeloneuropathy. Eur J Endocrinol. 2012 Feb;166(2):291–4.
- Moser AB, Kreiter N, Bezman L, Lu S, Raymond G V, Naidu S, et al. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol. 1999;45(1):100–10.
- Theda C, Woody RC, Naidu S, Moser AB, Moser HW. Increased very long chain fatty acids in patients on a ketogenic diet: a cause of diagnostic confusion. J Pediatr. 1993 May;122(5 Pt 1):724–6.
- Korenke GC, Roth C, Krasemann E, Hüfner M, Hunneman DH, Hanefeld F. Variability of endocrinological dysfunction in 55 patients with X-linked adrenoleucodystrophy: clinical, laboratory and genetic findings. Eur J Endocrinol. 1997 Jul;137(1):40–7.
- Boehm CD, Cutting GR, Lachtermacher MB, Moser HW, Chong SS. Accurate DNA-based diagnostic and carrier testing for X-linked adrenoleukodystrophy. Mol Genet Metab. 1999;66(2):128–36.
- Brennemann W, Kohler W, Zierz S, Klingmuller D. Occurrence of adrenocortical insufficiency in adrenomyeloneuropathy. Neurology. 1996;47(2):605.
- Hubbard WC, Moser AB, Tortorelli S, Liu A, Jones D, Moser H. Combined liquid chromatography-tandem mass spectrometry as an analytical method for high throughput screening for X-linked adrenoleukodystrophy and other peroxisomal disorders: preliminary findings. Mol Genet Metab. 2006;89(1–2):185–7.
- Vogel BH, Bradley SE, Adams DJ, Aco KD, Erbe RW, Fong C, et al. Newborn screening for X-linked adrenoleukodystrophy in New York State : Diagnostic protocol , surveillance protocol and treatment guidelines. Mol Genet Metab. 2015;114(4):599–603.
- Moser AB, Moser HW. The prenatal diagnosis of X-linked adrenoleukodystrophy. Prenat Diagn. 1999;19(1):46–8.
- Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012 Dec 6;367(23):2175–84.
- Lan F, Wang Z, Ke L, Xie H, Huang L, Huang H, et al. A rapid and sensitive protocol for prenatal molecular diagnosis of X-linked adrenoleukodystrophy. Clin Chim Acta. 2010;411(23–24):1992–7.
- Berger J, Pujol A, Aubourg P, Forss-Petter S. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):845–56.
- Loes DJ, Hite S, Moser H, Stillman AE, Shapiro E, Lockman L, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol. 1994;15(9):1761–6.
- McKinney AM, Nascene D, Miller WP, Eisengart J, Loes D, Benson M, et al. Childhood cerebral X-linked adrenoleukodystrophy: diffusion tensor imaging measurements for prediction of clinical outcome after hematopoietic stem cell transplantation. AJNR Am J Neuroradiol. 2013;34(3):641–9.
- Dubey P, Fatemi A, Huang H, Nagae-Poetscher L, Wakana S, Barker PB, et al. Diffusion tensor-based imaging reveals occult abnormalities in adrenomyeloneuropathy. Ann Neurol. 2005;58(5):758–66.
- Eichler FS, Barker PB, Cox C, Edwin D, Ulug AM, Moser HW, et al. Proton MR spectroscopic imaging predicts lesion progression on MRI in X-linked adrenoleukodystrophy. Neurology. 2002;58(6):901–7.
- Ratai E, Kok T, Wiggins C, Wiggins G, Grant E, Gagoski B, et al. Seven-Tesla proton magnetic resonance spectroscopic imaging in adult X-linked adrenoleukodystrophy. Arch Neurol. 2008;65(11):1488–94.
- Renard D, Castelnovo G, Collombier L, Kotzki PO, Labauge P. Brain fludeoxyglucose F 18 positron emission tomography hypometabolism in magnetic resonance imaging-negative x-linked adrenoleukodystrophy. Arch Neurol. 2011;68(10):1338–9.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364–89.
- Tollenaar MS, Jansen J, Beijers R, Riksen-Walraven JM, de Weerth C. Cortisol in the first year of life: normative values and intra-individual variability. Early Hum Dev. 2010 Jan;86(1):13–6.
- Burtman E, Regelmann MO. Endocrine Dysfunction in X-Linked Adrenoleukodystrophy. Endocrinol Metab Clin North Am. 2016;45(2):295–309.
- Aubourg P, Blanche S, Jambaque I, Rocchiccioli F, Kalifa G, Naud-Saudreau C, et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322(26):1860–6.
- Shapiro E, Krivit W, Lockman L, Jambaque I, Peters C, Cowan M, et al. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356(9231):713–8.
- Miller WP, Rothman SM, Nascene D, Kivisto T, DeFor TE, Ziegler RS, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single-institution cohort report. Blood. 2011 Aug 18;118(7):1971–8.
- Stradomska TJ, Drabko K, Moszczynska E, Tylki-Szymanska A. Monitoring of very long-chain fatty acids levels in X-linked adrenoleukodystrophy, treated with haematopoietic stem cell transplantation and Lorenzo’s Oil. Folia Neuropathol. 2014;52(2):159–63.
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science (80- ). 1988;239(4837):290–2.
- Kühl J-S, Suarez F, Gillett GT, Hemmati PG, Snowden JA, Stadler M, et al. Long-term outcomes of allogeneic haematopoietic stem cell transplantation for adult cerebral X-linked adrenoleukodystrophy. Brain. 2017 Apr 1;140(4):953–66.
- Moser HW. Therapy of X-linked adrenoleukodystrophy. NeuroRx. 2006;3(2):246–53.
- Pierpont EI, Eisengart JB, Shanley R, Nascene D, Raymond G V, Shapiro EG, et al. Neurocognitive Trajectory of Boys Who Received a Hematopoietic Stem Cell Transplant at an Early Stage of Childhood Cerebral Adrenoleukodystrophy. JAMA Neurol. 2017 Jun 1;74(6):710–7.
- van Geel BM, Poll-The BT, Verrips A, Boelens JJ, Kemp S, Engelen M. Hematopoietic cell transplantation does not prevent myelopathy in X-linked adrenoleukodystrophy: a retrospective study. J Inherit Metab Dis. 2014;
- Eichler F, Duncan C, Musolino PL, Orchard PJ, De Oliveira S, Thrasher AJ, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630–8.
- Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr Gene Ther. 2013;13(6):453–68.
- Engelen M. Optimizing Treatment for Cerebral Adrenoleukodystrophy in the Era of Gene Therapy. N Engl J Med. 2017;NEJMe1709253.
- Kahraman S, Beyazyurek C, Yesilipek MA, Ozturk G, Ertem M, Anak S, et al. Successful haematopoietic stem cell transplantation in 44 children from healthy siblings conceived after preimplantation HLA matching. Reprod Biomed Online. 2014;29(3):340–51.
- Petryk A, Polgreen LE, Chahla S, Miller W, Orchard PJ. No evidence for the reversal of adrenal failure after hematopoietic cell transplantation in X-linked adrenoleukodystrophy. Bone Marrow Transpl. 2012;
- Tsuji S, Sano T, Ariga T, Miyatake T. Increased synthesis of hexacosanoic acid (C23:0) by cultured skin fibroblasts from patients with adrenoleukodystrophy (ALD) and adrenomyeloneuropathy (AMN). J Biochem. 1981;90(4):1233–6.
- Rizzo WB, Leshner RT, Odone A, Dammann AL, Craft DA, Jensen ME, et al. Dietary erucic acid therapy for X-linked adrenoleukodystrophy. Neurology. 1989;39(11):1415–22.
- Moser HW, Raymond G V, Lu SE, Muenz LR, Moser AB, Xu J, et al. Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo’s oil. Arch Neurol. 2005;62(7):1073–80.
- Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta. 2012;1822(9):1465–74.
- Fourcade S, Ruiz M, Guilera C, Hahnen E, Brichta L, Naudi A, et al. Valproic acid induces antioxidant effects in X-linked adrenoleukodystrophy. Hum Mol Genet. 2010;19(10):2005–14.
- Jang J, Kim HS, Kang JW, Kang HC. The genetically modified polysialylated form of neural cell adhesion molecule-positive cells for potential treatment of X-linked adrenoleukodystrophy. Yonsei Med J. 2013;54(1):246–52.
- Gondcaille C, Genin EC, Lopez TE, Dias AM, Geillon F, Andreoletti P, et al. LXR antagonists induce ABCD2 expression. Biochim Biophys Acta. 2014;1841(2):259–66.
- Park CY, Kim HS, Jang J, Lee H, Lee JS, Yoo JE, et al. ABCD2 is a direct target of beta-catenin and TCF-4: implications for X-linked adrenoleukodystrophy therapy. PLoS One. 2013;8(2):e56242.
- Singh J, Olle B, Suhail H, Felicella MM, Giri S. Metformin-induced mitochondrial function and ABCD2 up-regulation in X-linked adrenoleukodystrophy involves AMP-activated protein kinase. J Neurochem. 2016;138(1):86–100.
- Morato L, Galino J, Ruiz M, Calingasan NY, Starkov AA, Dumont M, et al. Pioglitazone halts axonal degeneration in a mouse model of X-linked adrenoleukodystrophy. Brain. 2013;136(Pt 8):2432–43.
- Engelen M, Tran L, Ofman R, Brennecke J, Moser AB, Dijkstra IME, et al. Bezafibrate for X-Linked Adrenoleukodystrophy. Baud O, editor. PLoS One. 2012 Jul 20;7(7):e41013.
- Horvath GA, Eichler F, Poskitt K, Stockler-Ipsiroglu S. Failure of repeated cyclophosphamide pulse therapy in childhood cerebral X-linked adrenoleukodystrophy. Neuropediatrics. 2012;43(1):48–52.
- Tolar J, Orchard PJ, Bjoraker KJ, Ziegler RS, Shapiro EG, Charnas L. N-acetyl-L-cysteine improves outcome of advanced cerebral adrenoleukodystrophy. Bone Marrow Transpl. 2007;39(4):211–5.
- Peckham RS, Marshall M. C. J, Rosman PM, Farag A, Kabadi U, Wallace EZ. A variant of adrenomyeloneuropathy with hypothalamic-pituitary dysfunction and neurologic remission after glucocorticoid replacement therapy. Am J Med. 1982;72(1):173–6.
