ABSTRACT
Amenorrhea not due to pregnancy, lactation, or menopause is a relatively common abnormality of the reproductive years and indicative of a defect somewhere in the hypothalamic-pituitary-ovarian-uterine axis. This chapter considers the various causes of amenorrhea and their treatment. It also considers the diagnosis and treatment of abnormal uterine bleeding at all stages of life.
AMENORRHEA
Amenorrhea may be defined as 1) the absence of menstruation for 3 or more months in women with past menses (i.e., secondary amenorrhea) or 2) the absence of menarche by the age of 15 years in girls who have never menstruated (i.e., primary amenorrhea). Recent data suggest that pubertal development, and hence menarche, continues to begin earlier in American girls (3). Consequently, some clinicians would consider initiating evaluation of a girl with primary amenorrhea by age 14, particularly if 5 or more years had passed since the first evidence of pubertal development. Women who menstruate fewer than 9 times in any 12-month period (defined as oligomenorrhea) should be evaluated identically to women with secondary amenorrhea. These women are typically oligo- or anovulatory. The separation of amenorrhea into the categories primary and secondary is artificial and should not be considered in the evaluation of the amenorrheic woman. Likewise, the term “postpill” amenorrhea, sometimes used to refer to women who do not menstruate within 3 months of discontinuing oral contraceptives, conveys nothing about the cause of the amenorrhea and should not alter the evaluation.
Amenorrhea is not a diagnosis in itself but rather a sign of a disorder. In general, menses general occur at intervals of 28 ± 3 days in two-thirds of women, with a normal range of 18-40 days.
It is useful to think about 3 broad categories of amenorrhea:
- Anatomic causes, including pregnancy, that almost always can be identified by physical examination alone.
- Ovarian failure.
- Chronic anovulation resulting from any of a number of endocrine disturbances.
These three categories are delineated in Table 1.
Table 1. Categories of Amenorrhea
| 1. Anatomic Causes |
| 1. Pregnancy
2. Müllerian agenesis or dysgenesis (uterine, cervical, or vaginal) 3. Cervical stenosis 4. Various disorders of sexual differentiation 5. Intrauterine adhesions (Asherman syndrome) |
| 2. Ovarian Failure |
| 1. Menopause
2. Genetic abnormalities 1. X chromosomal causes 1. Structural alterations, mutations in, or absence of an X chromosome 1. Gonadal dysgenesis with stigmata of Turner syndrome (most 45,X) 2. Gonadal dysgenesis without stigmata of Turner syndrome 1. Pure gonadal dysgenesis (46,XX) 2. Premature ovarian failure with mutations in the X chromosome 1. Mutations in POF1 (Xq26-q28) 2. Mutations in POF1 together with Fragile X (FMR1) premutations (Xq27.3) 3. Mutations in POF2A or 2B (Xq22 or Xq21) 4. Mutations in POF4 together with mutations in bone morphogenetic protein 15 (Xp11.2) 2. Trisomy X with or without mosaicism 3. Mutations with a 46, XY karyotype (Pure gonadal dysgenesis) 1. Mutations in Xp22. 11-21.2 (Swyer syndrome) 2. Mutations in 5 cen 4. Autosomal causes 1. In association with myotonia dystrophica or other abnormalities 2. Mutations involving enzymes with reproductive effects 1. 17α-Hydroxylase deficiency (CYP17A)(10q24.3 2. Galactosemia (Galactose- 1 – phosphate uridyltransferase deficiency)(9p13) 3. 20,22-Lyase (P450scc) and aromatase (P450arom) deficiency 3. Mutations involving reproductive hormones, their receptors, and actions 1. Mutations inactivating LH or FSH (theorectical) 2. Mutations in inhibin A (INHA) 3. Receptor mutations 1. FSH receptor (2p21-p16) 2. LH receptor (2p21) 4. Mutations in the hormone action pathways 4. Known genetic alterations of other specific genes 1. FOXL2 (a forkhead transcription factor associated with the blepharophimosis/ptosis/epicanthus inverse syndrome) 2. ELF2B (a family of genes associated with CNS leukodystrophy and ovarian failure) 3. BMP15 (bone morphogenetic factor 15, involved with folliculogenesis) 4. PMM2 (phosphomannomutase) 5. AIRE (autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome) 6. STAG3 (encoding a meiosis-specific subunit of cohesion)(7q21.3-22.2) 3. Immune Dysfunction 1. Association with other autoimmune disorders (15-20% of cases, 4% with steroidogenic cell autoimmunity) 2. Isolated 3. In association with congenital thymic aplasia 4. Physical Insults 1. Chemotherapeutic (especially alkylating) agents 2. Ionizing radiation 3. Viral agents 4. Surgical extirpation 5. Gonadotropin-Secreting Pituitary Tumors (Extremely Rare) 6. Idiopathic |
| 3. Chronic Anovulation |
| 1. Hypothalamic
1. Psychogenic, including pseudocyesis 2. Exercise-associated 3. Eating disorders, nutritional 4. 2□ to systemic illness 5. Hypothalamic neoplasms 6. Some forms of isolated (idiopathic) hypogonadotropic hypogonadism (including Kallmann syndrome) 2. Pituitary 1. Some forms of isolated (idiopathic) hypogonadotropic hypogonadism (including Kallmann Syndrome) 2. Hypopituitarism 3. Pituitary neoplasms, including mucroadenomas 3. With inappropriate steroid feedback 1. Functional androgen excess (PCOS) 2. Adrenal hyperplasia 3. Neoplasms producing androgens or estrogens 4. Neoplasms producing hCG (including trophoblastic disease) 5. Liver and renal disease 6. Obesity 4. Other endocrine disorders 1. Thyroid dysfunction 2. Adrenal hyperfunction |
It is generally impossible to distinguish between ovarian failure and chronic anovulation without laboratory testing.
The most important aspect of the clinical evaluation is the history and physical examination. During the physical examination, special attention should be directed toward evaluating:
- Body dimensions and habitus.
- Distribution and extent of terminal androgen-stimulated body hair.
- Extent of breast development by Tanner staging and the presence or absence of any breast secretions.
- External and internal genitalia, with emphasis on evidence of exposure to androgens and estrogens.
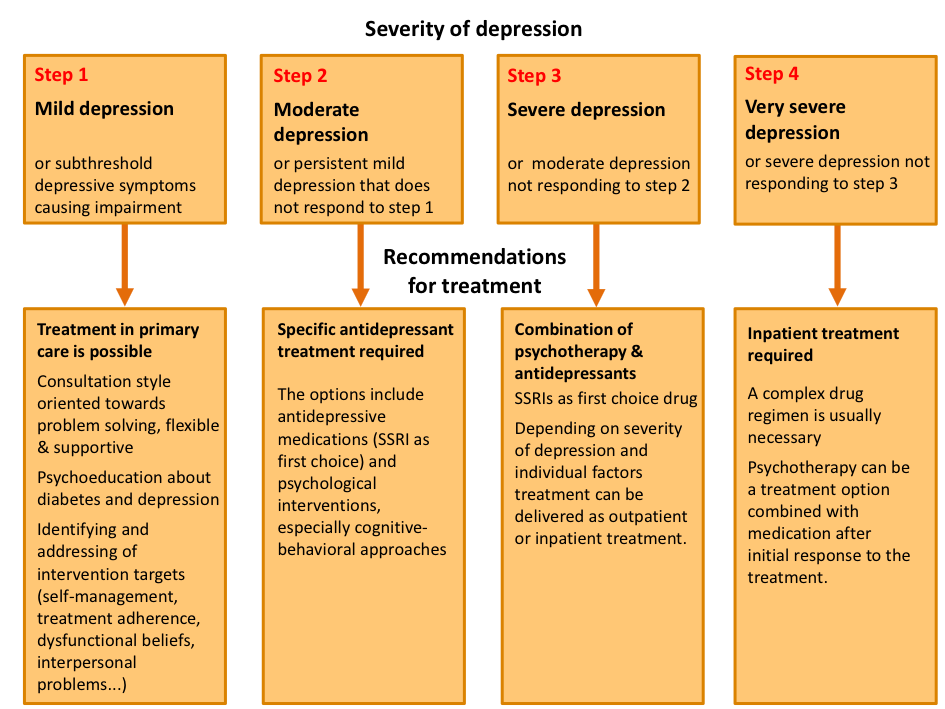
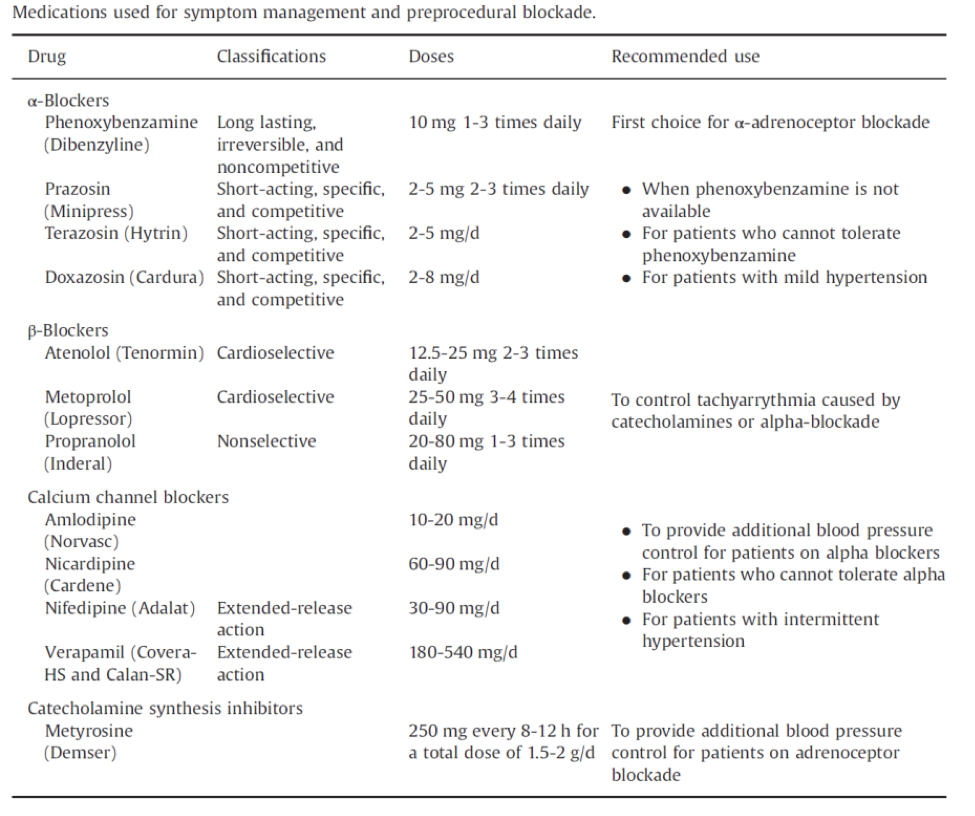
History, physical examination and determination of basal concentrations of follicle-stimulating hormone (FSH), thyroid-stimulating hormone (TSH), and prolactin will identify the most common causes of amenorrhea. Administration of exogenous progestin has been recommended in the past, both as a clinical aid to diagnosis and to evaluate the biological levels of estrogen. Either progesterone in oil (100 - 200 mg im) or medroxyprogesterone acetate (5 - 10 mg orally daily for 5 - 10 days) can be given. Any genital bleeding within 10 days of the completion of these regimens is regarded as a positive test. If the test is negative (suggesting low levels of endogenous estrogen), then an estrogen and a progestin together (e.g., oral conjugated estrogen, 2.5 mg daily for 25 days, together with oral medroxyprogesterone acetate, 5-10 mg for the last 10 days of estrogen therapy) should induce bleeding if the endometrium is normal. This test will determine with certainty if the outflow tract is intact. However, the results are not always definitive. In fact, in one survey almost half the women with so-called premature ovarian failure bled in response to progestin (4). Thus, progestin challenge should never be used as the sole diagnostic test by which amenorrheic women should be evaluated. In women with evidence of hirsutism, at least total testosterone and dehydroepiandrosterone sulfate levels should be determined to rule out any serious cause (Figure 1).
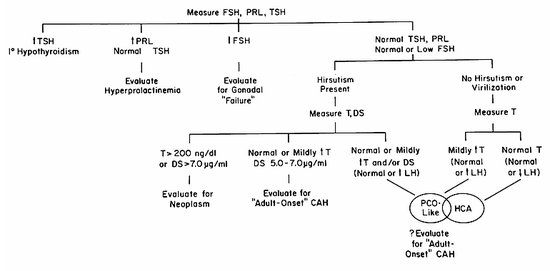
Figure 1. Flow diagram for the laboratory evaluation of amenorrhea. Such a scheme must be considered as an adjunct to the clinical evaluation of the patient. CAH -= congenital adrenal hyperplasia; DHEAS = dehydroepiandrosterone sulfate; FSH = follicle-stimulating hormone; HCA = hypothalamic chronic anovulation; PCO = polycystic ovary syndrome; PRL = prolactin; T = testosterone; TSH = thyroid-stimulating hormone. Originally from Rebar RW, The ovaries. In: Smith LH Jr, ed. Cecil textbook of medicine, ed. 18. Philadelphia. WB Saunders, 1992:1367)
The World Health Organization has divided women with amenorrhea into three groups originally based upon a suggestion of Insler (5):
Group I (hypothalamic-pituitary forms of amenorrhea) consists of women with no evidence of endogenous estrogen production (based on urinary measurements), normal prolactin levels, and normal or low FSH levels.
Group II (polycystic ovary syndrome) consists of women with evidence of endogenous estrogen production (on urinary measurement) and normal levels of prolactin and FSH.
Group III (gonadal failure) consists of women with elevated FSH levels.
HYPERGONADOTROPIC AMENORRHEA (Primary Hypogonadism; Gonadal Failure; Primary Ovarian Insufficiency)
It is frequently impossible to diagnose hypergonadotropic amenorrhea, also called presumptive ovarian failure and, more recently, primary ovarian insufficiency, without the measurement of basal serum FSH levels. This is especially true because ovarian failure may occur at any time from embryonic development onward. The ovaries normally fail at the time of menopause, when virtually no viable oocytes remain. Premature ovarian failure (POF) or premature menopause generally is defined as consisting of the triad of amenorrhea, hypergonadotropinism, and hypoestrogenism in women under the age of 40 years (5a). From what is known about follicular development and atresia, it appears that premature ovarian failure can arise from abnormalities in the recruitment and selection of oocytes. The follicles may undergo atresia at an accelerated rate or a smaller than normal pool may undergo atresia at the normal rate to yield early oocyte depletion. FSH must be involved because it is the principal hormonal regulator of folliculogenesis. Circulating gonadotropin levels rise whenever ovarian failure occurs because of decreased negative estrogen feedback to the hypothalamic-pituitary unit. Gonadotropin levels sometimes increase even in the presence of viable oocytes, but the explanation for such increases is unclear. Thus, use of the term POF is inappropriate. In 5-10% of patients, spontaneous pregnancy has occurred many years after the initial diagnosis (4,6). Thus, it is more appropriate to refer to this disorder as hypergonadotropic amenorrhea, primary hypogonadism, hypergonadotropic hypogonadism, or primary ovarian insufficiency, but the term premature ovarian failure is well established in the literature.
TYPES OF PREMATURE OVARIAN FAILURE
It is now clear that POF is a heterogeneous disorder. Premature loss of oocytes could result from a reduced germ cell endowment in utero, accelerated atresia, or failure of all germ cells to migrate to the genital ridges in early development. There may be marked differences in oocyte endowment and rates of follicular atresia among women (7,8). Only now are investigators learning about the molecular factors that regulate oocyte number and development. Because information in this field is changing rapidly, it is probably impossible to provide a definitive classification of the disorder, but it is possible to enumerate many of the apparent causes (Table 1).
It is becoming clear that genetic abnormalities are perhaps the most important cause of premature ovarian failure (Table 1). Although it is estimated that only 10-15% of women with POF have a recognized genetic cause for their disorder(8a), this will no doubt increase with time. New genetic causes are identified almost monthly, and it is impossible to list all genetic causes in any such table.
Individuals with the various forms of gonadal dysgenesis typically present with hypergonadotropic amenorrhea regardless of the extent of pubertal development and the presence or absence of associated anomalies or stigmata. It is well known that cytogenetic abnormalities of the X chromosome can impair ovarian development and function. Studies of 46,XX individuals and those with various X chromosomal depletions have confirmed that two intact X chromosomes are necessary for maintenance of oocytes (9) The gonads of 45,X fetuses contain the normal complement of oocytes at 20 to 24 weeks of fetal age, but these rapidly undergo atresia so that none are typically present by the time of birth (10). Primary or secondary amenorrhea typically occurs in women with deletions in either the short or the long arm of one X chromosome.9 Mutations at independent loci on the X chromosome at Xq26-28 (POF1), Xq13.3-22 (POF2), and Xp11.2 have been identified that also are linked to POF. One gene in the POF2 region has homology to the DIA allele in Drosophila, mutants of which result in male and female infertility. A breakpoint in the last intron of the DIAPH2 gene (the homologue of the Drosophila diaphanous gene) has been associated with familial POF in women (11).
Although individuals with Turner syndrome usually are apparent on physical examination, patients with pure and mixed gonadal dysgenesis typically have no obvious identifying features. Women with pure gonadal dysgenesis, who generally present with sexual infantilism and primary amenorrhea, are of normal height and have none of the somatic abnormalities associated with Turner syndrome (12,9) Affected individuals have either a 46,XX or 46,XY karyotype. In mixed gonadal dysgenesis, a germ cell tumor or testis accounts for one gonad, with a streak, rudimentary gonad, or no gonad accounting for the other (9,13). The 45,X/46,XY karyotype is most frequent, but affected individuals may have any of several other reported karyotypes. The vast majority of affected individuals are raised as females, with mild to moderate virilization occurring at puberty. Abnormal genitalia may be noted at birth. Because of the malignant potential of intraabdominal gonads in individuals with a Y chromosomal component (14-16) the gonads should be removed.
Additional X chromosomes also are present in some women with POF (17). These women typically develop normally and may bear children early in adulthood and commonly develop POF after age 30.
Mutations in the Familial Mental Retardation-1 (FMR1) gene, located at Xq27 and which can lead to fragile X syndrome, can also lead to POF (18). Although the gene changes involved in the abnormalities associated with this gene are quite complex, the basic principles can be summarized. Normal individuals have 5-50 repeats of the cytosine-guanine-guanine (CGG) trinucleotide in the gene. Expansion of this trinucleotide to greater than 200 repeats inactivates the gene and leads to the fragile X syndrome. In addition to mild to severe mental retardation, affected males typically present with long narrow faces, increased head circumference, dysmorphic ears, prominent jaws and foreheads, and large testes. Females are less severely affected, presumably because one of the two X chromosomes is inactivated independently in every cell in the body, and only one of the chromosomes carries the mutations. Some individuals have 50-200 repeats of the CGG sequences, and these individuals are considered premutation carriers. The women who are carriers of this unstable premutation can have further expansion of the trinucleotide in their germ cells and transmit the full syndrome to their offspring; in some families, the carrier state can be transmitted for several generations before expansion occurs. Men who are premutation carriers virtually never have further expansion in germ cells but can transmit the premutation to their female offspring. It is now recognized that POF develops in about 20% of female premutation carriers (19,20). In addition, about 2% of women with sporadic POF and 14% of women with familial POF have this unstable mutation (21,22). These observations make a convincing argument for testing women with POF for mutations of FMR1. Men with the premutation are known to sometimes develop tremors and ataxia as well as more subtle neurological and emotional difficulties (the so-called Fragile X-Associated Tremor-Ataxia Syndrome, FXTAS).
Several other specific gene mutations (not necessarily located on the X chromosome) also can result in POF. These include mutations involving the inhibin alpha gene (INHA), a gene at chromosome 3q23 involving a forkhead transcription factor associated with blepharophimosis-ptosis-epicanthus inversus (BPES) type I syndrome (23,24), a family of genes associated with central nervous system leukodystrophy and ovarian failure (EIF2B) (25), the gene involving bone morphogenetic factor 15 (BMP15) which is known to play a role in folliculogenesis (26), the phosphomannomutase (PMM) gene, and the gene associated with the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome (AIRE) (27). More recently, a mutation altering a meiosis-specific subunit of the cohesion ring, which ensures correct sister chromatid cohesion, has been identified as a cause of POF in one large family (8a). No doubt other mutations will be identified as causes of POF in some affected women. Several rare inherited enzymatic defects also may be associated with premature ovarian failure. These include partial deficiencies in four enzymes in the steroidogenic pathway, 17a-hydroxylase, 17,20-desmolase, 20,22-desmolase, and aromatase, as well as galactosemia.
Girls with 17α-hydroxylase deficiency (involving the CYP17A gene) who survive to their teens present with sexual infantilism; primary amenorrhea; increased circulating levels of LH, FSH, deoxycorticosterone, and progesterone; and hypertension with hypokalemic alkalosis (28-30). Ovarian biopsy has revealed no evidence of orderly follicular maturation but instead has demonstrated numerous, large cysts and primordial follicles. Presumably, the enzyme deficiency does not permit normal follicular development. The startling observation that normal follicular growth and development with successful fertilization in vitro can be achieved with exogenous gonadotropins in individuals with 17α-hydroxylase deficiency raises significant questions about why there is no follicular development in affected girls (31).
Several case reports have described individuals with mutations in the CYP19 (aromatase P450) gene (32-34). Aromatase deficiency appears to be inherited in an autosomal recessive manner and is manifested in 46,XX individuals by female pseudohermaphroditism with clitoromegaly and posterior labioscrotal fusion at birth; enlarged cystic ovaries associated with elevated FSH levels during childhood; lack of pubertal development in association with further enlargement of the clitoris, normal development of pubic and axillary hair, and continued existence of enlarged multicystic ovaries during the teenage years; and severe estrogen deficiency, virilization, and enlarged multicystic ovaries in association with markedly elevated gonadotropin levels in adulthood. Administration of exogenous estrogen results in prompt lowering of circulating gonadotropin levels. Ovarian biopsy showed many closely packed primordial follicles in an affected 17-month old (33), but biopsy in a 13-year old showed excessive atresia (34).
Girls with galactosemia, a disorder in which galactose-1-phosphate uridyltransferase activity is decreased and that is characterized by mental retardation, cataracts, hepatosplenomegaly, and renal tubular dysfunction, also may develop premature ovarian failure with hypergonadotropinism even when a galactose-restricted diet is introduced early in infancy (35).
Data from a variety of sources indicate that abnormalities in the structure, secretion, metabolism, or action of gonadotropins can cause POF. It is now known that at least one form of premature ovarian failure is caused by mutations in the FSH receptor (FSHR). Affected individuals present with primary or secondary amenorrhea and elevated levels of FSH and may have ovarian follicles present on transvaginal ultrasound. One specific mutation on chromosome 2p (C566T: alanine to valine) in exon 7 of the FSHR was identified in several Finnish families (36,37), but the mutation must be very rare outside of Finland because it has not been detected in some other populations (38,39).
The “resistant ovary” syndrome may be the result of a gonadotropin postreceptor defect. As originally described, the Savage syndrome (named after the first patient described) consisted of young amenorrheic women with elevated peripheral gonadotropin concentrations, normal but immature follicles in the ovaries on biopsy, 46,XX karyotype with no evidence of mosaicism, complete sexual development, and hyposensitivity (i.e., resistance) to exogenous gonadotropin stimulation (40).
Altered forms of immunoreactive LH and FSH in urinary extracts from women with POF compared to those from oophorectomized and postmenopausal women have been reported, suggesting that metabolism and/or excretion of gonadotropin is altered in some cases (41). Some individuals with POF and evidence of intermittent follicular activity may have low molecular weight receptor-binding activity that antagonizes normal FSH binding (42).
Destruction of oocytes by any of several environmental insults, including ionizing radiation, various chemotherapeutic (especially alkylating) agents, and certain viral infections may accelerate follicular atresia (43). Although there is no evidence that cigarette smoking will result in POF, cigarette smokers experience menopause several months before nonsmokers.
More and more girls and young women who are treated for a variety of malignancies are surviving free of disease and subsequently presenting with transient or permanent ovarian failure. Strategies for reducing the likelihood of ovarian failure in women cured of their malignancies are being investigated by several groups. Cryopreservation of oocytes and ovarian tissue before therapy remains experimental at this point in time. Still, both males and females of reproductive age should be appraised of the potential for preserving gametes before treatment of any malignancy is initiated.
Approximately half of all individuals receiving 400-500 rads to the ovaries over four to six weeks will develop permanent ovarian failure (44). For any given dose of radiation, the older the woman, the greater the likelihood of her developing ovarian failure. It appears that about 800 rads is sufficient to result in permanent ovarian failure in all women. The transient nature of the hypergonadotropic amenorrhea in some women suggests that some follicles may be damaged but not destroyed by lower doses of radiation. To minimize the dose of radiation received by the ovaries, transposition to the pelvic sidewalls, often by laparoscopy, is recommended. One series noted preservation of ovarian function in about 90% of women undergoing transposition (45). Similarly, the older the woman at the of chemotherapy, the more likely is the ovarian failure (46). In general, it appears that the greater the number of oocytes present in the ovaries at the time of therapy with radiation or chemotherapy, the more likely it is that normal ovarian function will continue. Although the data are limited, the frequency of congenital anomalies does not appear to be increased in the children of women previously treated with chemotherapeutic agents (47).
Premature ovarian failure may be associated with a number of autoimmune disorders (4). The most common association may be with thyroiditis. Ovarian failure occurs commonly in women with polyglandular failure, including hypoparathyroidism, hypoadrenalism, and mucocutaneous candidiasis4. The heterogeneous nature of this disorder is suggested by the many different endocrinopathies that may be associated with premature ovarian failure. Autoimmune ovarian failure may occur independently of any other autoimmune disorder.
Autoimmune lymphocytic oophoritis was originally reported in association with adrenal insufficiency (Addison disease). Women with steroidogenic cell autoimmunity have lymphocytic oophoritis resulting in the ovarian failure. When POF occurs in association with adrenal insufficiency, the ovarian failure presents first about 90% of the time. The presence of antibodies to the 21-hydroxylase enzyme measured by a commercially available immunoprecipitation assay will identify women who may have occult adrenal insufficiency at the time of initial presentation as well as those who should be followed closely for the subsequent development of adrenal insufficiency (48,49). At the present time there is no good test to document the presence of antibodies to any specific ovarian antigens. The best evidence of antibodies to ovarian tissue comes from a study documenting FSH receptor antibodies in two women with myasthenia gravis and hypergonadotropic amenorrhea (50). Immunoglobulins that block the trophic actions of FSH but not LH also have been reported (51).
The thymus gland influences reproductive function (52). Congenitally athymic girls have ovaries devoid of oocytes (53). Irradiation and chemotherapeutic (especially alkylating) agents used to treat various malignancies are increasingly causes of premature ovarian failure (54-56). Inexplicably, both of these modalities have been associated with “reversible” ovarian failure. Ovulation and cyclic menses return in some individuals after prolonged intervals of hypergonadotropic amenorrhea associated with signs and symptoms of profound hypoestrogenism. Preliminary studies suggest that gonadotropin-releasing hormone analogues (but not oral contraceptive agents) may provide some protection from ovarian damage (57). Rarely, the mumps virus can affect the ovaries and cause ovarian failure (58).
DIAGNOSIS AND THERAPY OF PREMATURE OVARIAN FAILURE
Individuals with premature ovarian failure warrant thorough evaluation to eliminate potentially treatable causes and to identify associated disorders that may require therapy (5a). In general, young women who experience loss of regular menses for three or more consecutive months should be evaluated. Failure to initiate pubertal development by age 13 or begin menstruating by age 15 also warrants evaluation.
Several laboratory tests are indicated in women with POF, beginning with measurement of basal levels of prolactin, FSH, and TSH (after pregnancy is ruled out). FSH levels are typically greater than 30 mIU/ml in women with ovarian failure. If the FSH level is greater than 15 mIU/ml on initial screening, then the measurement should be repeated and serum estradiol should be measured as well to document hypogonadism. In addition, the simultaneous measurement of basal LH levels may be helpful in discerning if any oocytes remain. In general, if the estradiol concentration is greater than 50 pg/mL or if the LH level is significantly greater than the FSH level (in terms of mIU/mL) in any sample, the probability of viable oocytes is considerable. Irregular uterine bleeding, as an indication of estrogen stimulation, also provides good evidence of remaining functional ovarian follicles. It is not uncommon to identify women with intermittent menstruation, hypoestrogenism, and hypergonadotropinism. Visualization of follicles on transvaginal ultrasound also provides evidence of functional oocytes. Because a number of pregnancies have occurred after biopsy of ovaries devoid of oocytes, ovarian biopsy cannot be recommended for affected women.
Other indicated laboratory tests include measurement of thyroid-stimulating immunoglobulins (because of the frequency of thyroiditis), adrenal antibodies, fasting glucose, electrolytes, and bone density by dual-energy X-ray absorptiometry. Also indicated are an analysis of karyotype and fragile X premutation screening, particularly in the presence of a family history of mental retardation. If adrenal antibodies are detected, then a corticotropin stimulation test is indicated to identify women with adrenal insufficiency. One series evaluated 119 women with karyotypically normal spontaneous POF and found that 32 patients had hypothyroidism (27%) and 3 had adrenal insufficiency (2.5%) (59).
Even women with X-chromosomal abnormalities have delivered normal children and subsequently developed POF prior to age 40. Thus, neither parity nor age rules out the possibility of a chromosomal abnormality. Unexpected karyotypic findings that may be inherited have important implications for other family members. Also, by finding an explanation for the POF, patients with normal karyotypes may be reassured, and the patients with abnormal karyotypes can be counselled. Surgical removal of the gonads is indicated in any individual in whom a Y chromosome is identified.
Women who experience spontaneous POF are unprepared for the diagnosis. Taking the time to present the findings with sensitivity and to counsel appropriately is most important. Patients may benefit from referral to a psychologist and/or to an organization such as the Premature Ovarian Failure Support Group or Rachel’s Well ( www.rachelswell.org). Patients should be reassessed at intervals of one to two years for the presence of other disorders associated with POF.
Even in women with intermittent ovarian failure, estrogen replacement is appropriate to prevent the accelerated bone loss that occurs in affected women (4). Although exogenous estrogen may be given either as part of combined estrogen-progestin therapy or in the form of combined oral contraceptives, sequential therapy with exogenous estrogen and progestin is most physiologic. The estrogen should always be given with a progestin to prevent endometrial hyperplasia. Because women with ovarian failure may conceive while on estrogen therapy (including combined oral contraceptive agents), affected women should be counseled appropriately and cautioned to have a pregnancy test if withdrawal bleeding does not occur or if signs and symptoms suggestive of pregnancy develop. Despite these considerations, probably no other contraceptive agent is required for those women who do not wish pregnancy but who are sexually active, because pregnancy occurs in less than 10% (4). Although rare pregnancies in women with premature ovarian failure have occurred after ovulation induction with human menopausal and chorionic gonadotropins, the low likelihood should lead the physician to discourage patients from selecting such therapy. There is no evidence that ovulation and pregnancy occur more commonly in response to ovulation induction than spontaneously in these patients. Hormone replacement treatment to mimic the normal menstrual cycle, with oocyte donation for embryo transfer, provides the greatest possibility for pregnancy in women desiring pregnancy (60,61).
There are no data documenting safety of estrogen-progestin in young women with POF, but there are no reports of excessive risks either. Findings documenting risks in postmenopausal women do not apply to women with POF for whom estrogen therapy really represents replacement. Similarly, there are no data documenting the optimal form of estrogen and progestin to use in women with POF. It is important to remember that these young patients typically require twice as much estrogen as postmenopausal women to relieve any signs and symptoms of estrogen deficiency. Thus, one reasonable regimen would be 100 mm of estradiol per day by a skin patch, combined with 5-10 mg of medroyprogesterone acetate for 12 calendar days each month. The skin patch deliver a constant infusion of estradiol, avoids the first pass effect on the liver, and will maintain regular menses and be well tolerated by most patients.
CHRONIC ANOVULATION
Chronic anovulation may be viewed as a steady state in which the monthly rhythms associated with ovulation are not functional. Although amenorrhea is common, irregular menses and oligomenorrhea may occur as well. Chronic anovulation further implies that viable oocytes remain in the ovary and that ovulation can be induced with appropriate therapy.
Chronic anovulation is the most common pathological cause of oligomenorrhea or amenorrhea in women of reproductive age (Table 2). Appropriate management requires determination of the cause of the anovulation. However, anovulation can be interrupted transiently by nonspecific induction of ovulation in most affected women.
CHRONIC ANOVULATION OF CENTRAL ORIGIN
Functional Hypothalamic Chronic Anovulation (FHA)
Functional hypothalamic chronic anovulation may be defined as anovulation in which dysfunction of hypothalamic signals to the pituitary gland causes failure to ovulate. It remains unclear whether the primary abnormality is always present within the hypothalamus or sometimes occurs as a result of altered inputs to the hypothalamus. The term is used to refer to women who may be affected with suprahypothalamic or hypothalamic chronic anovulation. Although isolated gonadotropin deficiency frequently is caused by hypothalamic dysfunction, it is preferable to consider such individuals separately. However, partial forms of isolated gonadotropin deficiency may be virtually impossible to differentiate from hypothalamic chronic anovulation.
Numerous studies have documented an increased incidence of amenorrhea in women who exercise strenuously, diet excessively, or are exposed to severe emotional or physical stresses of any kind (1,62.63). Such amenorrheic persons fall into this group of women considered as having hypothalamic chronic anovulation, which is sometimes called functional amenorrhea. The diagnosis of FHA is suggested by the abrupt cessation of menses in women younger than 30 years of age who have no clinically evident anatomic abnormalities of the hypothalamic-pituitary-ovarian axis or any other endocrine abnormalities. The term hypothalamic amenorrhea was first proposed by Klinefelter and colleagues in 1943 for anovulation in which hypothalamic dysfunction is thought to interfere with the pituitary secretion of gonadotropin (64).
Although FHA is a common cause of oligomenorrhea and amenorrhea, relatively little is known about its pathophysiologic basis. The diversity of women presenting with FHA indicates that this is a heterogeneous group of disorders with similar manifestations. Compared with a matched control population, young women with secondary amenorrhea are more likely to be unmarried, to engage in intellectual occupations, to have had stressful life events, to use sedative and hypnotic drugs, to be underweight, and to have a history of previous menstrual irregularities (1). Although it has been suggested that the percentage of body fat controls the maintenance of normal menstrual cycles, it is more likely that diet, exercise, stress, body composition, and other unrecognized nutritional and environmental factors contribute in various proportions to amenorrhea.
Hormonally, basal circulating concentrations of pituitary (i.e., LH, FSH, TSH, prolactin, growth hormone), ovarian (i.e., estrogens, androgens), and adrenal hormones (i.e., dehydroepiandrosterone, DHEAS, cortisol) typically are within the normal range for women of reproductive age (65). However, mean serum gonadotropin, gonadal steroid, and DHEAS levels often are slightly decreased, and circulating and urinary cortisol levels are generally increased compared with those in normal women in the early follicular phase of the menstrual cycle (63,66). Despite low levels of circulating estrogen, affected women rarely have symptoms related to estrogen deficiency. Typically, the pulsatile secretion of gonadotropin is diminished, but these individuals respond normally to exogenous gonadotropin-releasing hormone.
In a comprehensive guideline issued in 2017, the Endocrine Society noted that one common feature of women with FHA is that all have a relative “energy deficit” (65a). The underlying cause of FHA may be a young woman’s overzealous approach to “healthy” behavior – and those habits can be difficult to change.
ANOREXIA NERVOSA, BULIMIA NERVOSA AND ATYPICAL EATING DISORDERS.
Eating disorders are common in adolescents and young women and may represent the most severe forms of functional hypothalamic chronic anovulation (67,68). Eating disorders are generally divided into three diagnostic categories: 1) anorexia nervosa, 2) bulimia nervosa, 3) binge eating disorders, and 4) other atypical eating disorders.
All eating disorders are characterized by altered eating habits or weight-control behavior. Poor nutrition can impact physical health. In addition, disturbed behavior in bulimia and anorexia is not due to any general medical disorder or any other psychiatric condition.
The constellation of amenorrhea often preceding weight loss, a distorted and bizarre attitude toward eating, food, or weight, extreme inanition, and a disordered body image makes the diagnosis of anorexia nervosa obvious in almost all cases (69-71). Demographically, 90% to 95% of anorectic women are white and come from middle- and upper-income families. In DSM-5 amenorrhea is no longer required to make the diagnosis of anorexia nervosa.
What distinguishes bulimia nervosa from anorexia nervosa is repeated binges at least once each week during which there is loss of self-control with unusually large amounts of food eaten. In most cases, binge eating is followed by compensatory self-induced vomiting or laxative abuse. Individuals with bulimia seldom have body weights that are significantly altered from ideal. Thus, body weight is the most obvious difference that distinguishes bulimia from anorexia nervosa. Many women with bulimia are ashamed or distress by their actions and are often more willing to accept treatment than individuals with anorexia. Symptoms of depression and anxiety disorders are common.
Anorexia nervosa most commonly arises in the mid-adolescent years. Self-induced dietary restrictions quickly get out of control. In some cases, the disorder is of short-standing and self-limited, whereas in others the disorder becomes well entrenched and long-standing.
Bulimia nervosa usually begins later in adolescence. Often bulimia begins similarly to anorexia. However, episodes of binge eating eventually interrupt the dietary restriction, and body weight increases to near normal levels. Women with bulimia commonly seek treatment more than five years after disease onset.
Peripheral gonadotropin and gonadal steroid levels generally are lower than in the early follicular phase of the menstrual cycle (72). As patients with anorexia undergo therapy, gain weight, and improve psychologically, sequential studies of the ultradian gonadotropin rhythms show progressive gonadotropin changes paralleling those normally seen during puberty. Initially, there is a nocturnal rise in gonadotropins, followed by an increase in mean basal gonadotropin levels throughout the 24-hour period (73-75). The responses of severely ill anorectics to GnRH are also similar to those observed in prepubertal children and become adult-like with recovery or with treatment with pulsatile GnRH (76). Because the metabolism of estradiol and testosterone is also abnormal, normalizing with weight gain, some of the gonadotropin changes may be secondary to peripheral alterations in steroids (77).
Several abnormalities indicate hypothalamic dysfunction, including mild diabetes insipidus and abnormal thermoregulatory responses to heat and cold (71). Affected individuals have altered body images as well (78).
Still other central and peripheral abnormalities exist. There is evidence of chemical hypothyroidism, with affected patients having decreased body temperature, bradycardia, low serum triiodothyronine (T3) levels, and increased reverse T3 concentrations (79,80). Circulating cortisol levels also are elevated, but the circadian cortisol rhythm is normal (81). The increased cortisol seems to be caused by the reduced metabolic clearance of cortisol as a result of the reduced affinity constant for corticosteroid binding globulin (CBG) present in such patients (82). Moreover, like women with endogenous depression, anorectics suppress significantly less after dexamethasone administration than do normal subjects (83). Anorectics also have reduced ACTH responses to exogenous corticotropin-releasing hormone (CRH), suggesting normal negative pituitary feedback by the increased circulating cortisol (84).
Although rigorous studies have not been performed in women with bulimia, presumably such individuals have endocrine disturbances similar to those of women with anorexia nervosa.
SIMPLE WEIGHT LOSS AND AMENORRHEA
Societal attitudes encourage dieting and pursuit of thinness, particularly in young women. Several reproductive problems, including hypothalamic chronic anovulation, have been associated with simple weight loss. Affected women are distinctly different from anorectics in that they do not fulfill the psychiatric criteria for anorexia. The cessation of menses does not occur before significant weight loss in such women, although this sequence is common in anorectics. The few studies that have been conducted in amenorrheic women with simple weight loss suggest that the abnormalities are similar to those observed in anorectics, but are more minor and more easily reversed with weight gain (85). Although it has been suggested that the amenorrhea in these women is secondary to metabolic defects resulting from undernutrition, the possibility of separate central defects has not been excluded (86). The importance of normal body weight to normal reproductive function is evident in studies of a tribe of desert-dwelling hunter-gatherers in Botswana (87). The weights of the women vary markedly with the season, being greatest in the summer, and the peak incidence of parturition follows exactly 9 months after the attainment of maximal weight.
EXERCISE-ASSOCIATED AMENORRHEA
Regular endurance training in women is associated with at least three distinct disorders of reproductive function: delayed menarche, luteal dysfunction, and amenorrhea (88,89). In 1992 the American College of Sports Medicine coined the term the “female athletic triad” to describe the three disorders recognized as sometimes occurring together in female athletes: disordered eating, amenorrhea, and osteoporosis (90). Activities associated with an increased frequency of reproductive dysfunction include those favoring a slimmer, lower-body-weight physique such as middle and long distance running, ballet dancing, and gymnastics. Swimmers and bicyclists appear to have lower rates of amenorrhea despite comparable training intensities. The cause of these disorders remains to be established and may involve many factors. Dietary changes, the hormonal effects of acute and chronic exercise, alterations in hormone metabolism because of the increased lean-to-fat ratio, and the psychological and physical “stress” of exercise itself may all contribute and may vary in importance in different individuals. Women engaged in endurance training frequently also have disordered attitudes toward eating, and a number of studies have documented low leptin levels and the absence of normal circadian leptin variation (91-94).
In untrained women who underwent a program of strenuous aerobic exercise (running 4-10 miles/day) combined with caloric restriction, menstrual dysfunction was induced (95). The spectrum of abnormalities in these women included luteal phase dysfunction, loss of the midcycle LH surge, prolonged menstrual cycles, altered patterns of gonadotropin secretion, and amenorrhea. Subsequent studies have indicated that luteal phase defects can occur soon after beginning endurance training in the majority of untrained women (96). However, in contrast to these findings, others observed that a progressive exercise program of moderate intensity did not affect the reproductive system of gynecologically mature (mean age, 31.4 years), untrained, eumenorrheic women (97). It was suggested that relatively young gynecologic age or an earlier age of training onset in particular adversely affects menstrual cyclicity.
Many amenorrheic athletes welcome the onset of amenorrhea. However, significant osteopenia, usually affecting trabecular bone, has been reported in these women (98-100). It appears that the loss in bone density secondary to hypoestrogenism nullifies the beneficial effects of weight-bearing exercise in strengthening and remodeling bone (99,101). Such women are at risk for stress fractures, particularly in the weight-bearing lower extremities, and bone density may remain below those of eumenorrheic athletes even after resumption of menses (102).
Stress is generally acknowledged to play a role in the cause of this form of amenorrhea, even though the term stress itself remains difficult to define. Amenorrheic runners subjectively associate greater stress with running than do runners with regular menses (103) (Fig.2).
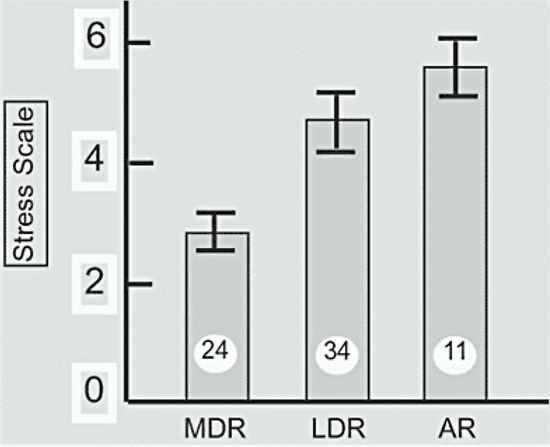
Figure 2. Subjective stress associated with running. Subjects were asked to evaluate the stress associated with running on a scale from 0 to 10, with 10 being maximal. The means + standard errors are shown. The number of subjects in each group is shown in the bar. MDR - middle distance runners (15-30 miles per wee) with regular menses; LDR - long distance runners (>30 miles per week) with regular menses; AR = amenorrheic runners. Stress was significantly greater (p<0.001) in both long distance and amenorrheic runners compared to middle distance runners. (Data from Schwartz et al., reference 103).
However, no increase in amenorrhea was observed in a competitive group of young classical musicians, who presumably were experiencing similar stress, compared with a group of young ballet dancers, in whom the incidence of amenorrhea was quite high (104). Basal levels of circulating cortisol and urinary free cortisol excretion, indicative of increased stress, are increased in both eumenorrheic and amenorrheic runners (105) (Fig.3). It is likely even the eumenorrheic runners in this particular study had subtle reproductive abnormalities.
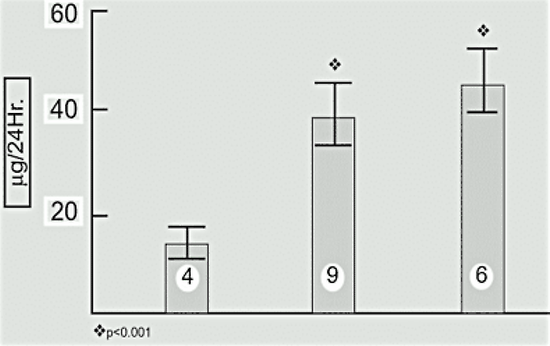
Figure 3. 24 Hour-urinary free cortisol excretion in normal control subjects (NC) eumenorrheic runners (R) and amenorrheic runners (AR). The number of subjects is shown in each bar. (Data from Villaneuva et al, reference 105).
Because levels of CBG, the disappearance rate of cortisol from the circulation, and the response of cortisol to adrenocorticotropin (ACTH) were not altered in the women runners compared with sedentary control subjects, secretion of ACTH and possibly of CRH must be increased in women who run. Abnormalities of the hypothalamic-pituitary-adrenal axis also are indicated by the observations that serum ACTH and cortisol responses to exogenous CRH are blunted as are the responses to meals (105,106).
The observation that amenorrheic runners also have subtle abnormalities in hypothalamic-pituitary-thyroidal function provides support for the concept that exercise-associated amenorrhea is similar to other forms of hypothalamic amenorrhea (107).
PSYCHOGENIC HYPOTHALAMIC AMENORRHEA
Amenorrhea may occur in women with a definite history of psychological and socioenvironmental trauma (86,108). The incidence of amenorrhea is quite high among depressed women, and the effects of lifestyle and nutritional status are difficult to differentiate from variables such as stress. Studies of individuals in whom a definite psychological traumatic event preceded the onset of amenorrhea have revealed low to normal basal levels of serum gonadotropins with normal responses to GnRH, prolonged suppression of gonadotropins in response to estradiol, and failure of a positive feedback response to estradiol (86,108-110). Increased basal levels of cortisol and decreased levels of DHEAS also have been noticed in women with psychogenic amenorrhea compared with eumenorrheic women (62). The mean levels of circulating cortisol are increased in such women largely because of an increase in the amplitude of the pulses of cortisol (111). Moreover, studies of depressed women have revealed abnormal circadian rhythms of cortisol and early “escape” from dexamethasone suppression (112,113).
The mechanism by which emotional states or stressful experiences causes psychogenic amenorrhea is not yet established. Evidence suggests, however, that a cascade of neuroendocrine events that may begin with limbic system responses to psychic stimuli impairs hypothalamic-pituitary activity (114). It has been suggested that increased hypothalamic b-endorphin is important in inhibiting gonadotropin secretion (114).
Psychological studies have found several social and psychological correlates of psychogenic amenorrhea: a history of previous pregnancy losses, including spontaneous abortion (115,116), stressful life events within the 6-month period preceding the amenorrhea (117,118), and poor social support or separation from significant family members during childhood and adolescence (113,118). Many women with psychogenic amenorrhea report stressful events associated with psychosexual problems and socioenvironmental stresses during the teenage years.108 Women with psychogenic amenorrhea also tend to have negative attitudes toward sexually related body parts, more partner-related sexual problems, and greater fear of or aversion to menstruation than do eumenorrheic women (117). Distortions of body image and confusion about basic bodily functions, especially sexuality and reproduction, are common (116).
DIMINISHED GONADOTROPIN-RELEASING HORMONE AND LUTEINIZING HORMONE SECRETION IN ALL FORMS
The various forms of hypothalamic chronic anovulation associated with altered lifestyles just discussed have several features in common. Altered GnRH and LH secretion seems to be the common result from altered hypothalamic input. It remains unclear if these disorders form a single disorder or several closely related disorders. Moreover, similar forms of amenorrhea are sometimes seen in women with severe systemic illnesses or with hypothalamic damage from tumors, infection, irradiation, trauma, or other causes.
TREATMENT
The treatment of patients with functional hypothalamic chronic anovulation is controversial and difficult. The Endocrine Society recommends a multidisciplinary approach including medical, dietary, and mental health support (65a). Psychological therapy and support or a change in lifestyle may cause cyclic ovulation and menses to resume. However, ovulation does not always resume, even after the lifestyle is altered. The treatment of affected women in whom menses do not resume and who do not desire pregnancy is difficult. Most physicians now advocate the use of exogenous sex steroids to prevent osteoporosis. Therapy consisting of oral conjugated estrogens (0.625-1.25 mg), ethinyl estradiol (20 mg), micronized estradiol-17β (1-2 mg), or estrone sulfate (0.625-2.5 mg) or of transdermal estradiol-17b (0.05-0.1 mg) continuously with oral medroxyprogesterone acetate (5 to 10 mg) or oral micronized progesterone (200 mg) added for 12-14 days each month is appropriate. Sexually active women can be treated with oral contraceptive agents. These women appear to be particularly sensitive to the undesired side effects of sex steroid therapy, and close contact with the physician may be required until the appropriate dosage is established. If sex steroid therapy is provided, patients must be informed that the amenorrhea may still be present after therapy is discontinued.
Some physicians believe that only periodic observation of affected women is indicated, with barrier methods of contraception recommended for fertility control. Contraception is necessary for sexually active women with hypothalamic chronic anovulation because spontaneous ovulation may resume at any time (before menstrual bleeding) in these mildly affected individuals. Women who refuse sex steroid therapy should be encouraged to have spinal bone density evaluated at intervals to document that bone loss is not accelerated. Adequate calcium ingestion should be encouraged in all affected women.
For women desiring pregnancy who do not ovulate spontaneously, clomiphene citrate (50-100 mg/d for 5 days beginning on the third to fifth day of withdrawal bleeding) can be used. However, clomiphene is frequently ineffective in these hypoestrogenic women. Treatment with human menopausal and chorionic gonadotropins (hMG-hCG) or with pulsatile GnRH may be effective in women who do not ovulate in response to clomiphene. Because the underlying defect in hypothalamic amenorrhea is decreased endogenous GnRH secretion, administration of pulsatile GnRH to induce ovulation can be viewed as physiologic; it offers the additional advantages of decreased need for ultrasonographic and serum estradiol monitoring, a decreased risk of multiple pregnancies, and a virtual absence of ovarian hyperstimulation. A starting intravenous dose of GnRH of 5 mg every 90 minutes is effective (119). After ovulation is detected by urinary LH testing or ultrasound, the corpus luteum can be supported by continuation of pulsatile GnRH or by hCG (1500 IU every 3 days for four doses). Ovulation rates of 90% and conception rates of 30% per ovulatory cycle can be expected (120). Unfortunately GnRH is no longer available in the United States because it was used so infrequently.
Cognitive behavioral therapy (CBT) has also been shown to be effective (120a).In a small trial involving 16 patients randomized to CBT or observation for 20 weeks, six women in the CBT group and only one in the observation group resumed ovulation. The CBT focused on changing attitudes towards eating habits, exercise, body image, problem-solving skills, and stress reduction.
One report noted improvements in reproductive function in a group of eight women with hypothalamic amenorrhea due to strenuous exercise or low weight who received recombinant human leptin for up to three months (121). As might be expected for a heterogeneous disorder, however, only three of the women ovulated in response to this therapy. Another study suggests that kisspeptin-54 may have utility in the future in treating hypogonadotropic hypogonadism by increasing LH pulsatility (121a).
In general, women with anorexia and bulimia nervosa should not have ovulation induced until their disease is in remission. It is clear that cognitve-behavioral therapy that focuses on modification of the specific behaviors and ways of thinking that support the patient’s eating disorder should be a part of any treatment plan (122). Addition of antidepressant drugs, especially selective serotonin reuptake inhibitors, may be of additional benefit in treating women with bulimia nervosa.
Isolated Hypogonadotropic Hypogonadism
Individuals with isolated (also termed idiopathic) hypogonadotropic hypogonadism fail to undergo pubertal maturation. Most have functional GnRH deficiency, but some appear to have abnormalities of gonadotropin deficiency localized to the pituitary gland (122a).
As originally described in 1944, Kallmann syndrome consisted of the triad of anosmia, hypogonadism, and color blindness in men (123). Women may be affected as well, and other midline defects may be associated (124-126). Because autopsy studies have shown partial or complete agenesis of the olfactory bulb, the term olfactogenital dysplasia also has been used to describe the syndrome (127). Because isolated gonadotropin deficiency may also occur in the absence of anosmia, the syndrome is considered to be quite heterogeneous.
Data indicate that in many patients the defect is a failure of GnRH neurons to form completely in the medial olfactory placode of the developing nose or the failure of GnRH neurons to migrate from the olfactory bulb to the medial basal hypothalamus during embryogenesis (128). In some patients, structural defects of the olfactory bulbs can be seen on magnetic resonance imaging (129). It appears likely that this disorder forms a structural continuum with other midline defects, with septo-optic dysplasia representing the most severe disorder.
Some individuals with isolated hypogonadotropic hypogonadism are normosmic. The molecular abnormalities identified thus far explain why some are anosmic and others are not (122a). The first mutations identified in Kallmann syndrome involve a cell surface adhesive gene, KAL1, which prevented normal development of the olfactory bulb and the neurologic tract responsible for transport of GnRH to the median eminence of the hypothalamus. Since that time, mutations in several other genes needed for development of the olfactory bulb and the neurologic tract needed for GnRH transport have been identified. Gene defects in individuals with normal smell include defects in the GnRH receptor gene, the gene responsible for GnRH production (GNRH1), the gene responsible for GnRH processing (PCSK1), and GnRH secretion (GPR54). Mutations of the KISS1-derived peptide receptor GPR54 have been particularly studied and indicate that the hypothalamic neuropeptide kisspeptin is a component of the GnRH pulse generator (122b).
Gene mutations localized to the pituitary and resulting in isolated hypogonadotropic hypogonadism include those associated with the GnRH receptor (GNRHR) and the production of the β subunit of gonadotropin. Mutations with the leptin, leptin receptor, and DAX1 genes appear to cause hypogonadotropic hypogonadism within both the hypothalamus and pituitary. These latter mutations appear to be associated with extreme obesity. There are also a number of mutations associated both with hypogonadotropic hypogonadism and other pituitary or endocrine deficiencies.
Clinically, affected individuals typically present with sexual infantilism and a eunuchoidal habitus, but moderate breast development may also occur. Primary amenorrhea is the rule. The ovaries usually are small and appear immature, with follicles rarely developed beyond the primordial stage (130). These immature follicles respond readily to exogenous gonadotropin with ovulation and pregnancy, and exogenous pulsatile GnRH can also be used to induce ovulation (131). Replacement therapy with estrogen and progestin should be given to affected women not desiring pregnancy.
Circulating LH and FSH levels generally are quite low. The response to exogenous GnRH is variable, sometimes being diminished and sometimes normal in magnitude, but rarely may be absent (132,133). Although the primary defect in most individuals appears to be hypothalamic, with reduced GnRH synthesis or secretion, a primary pituitary defect may occasionally be present. In addition, partial gonadotropin deficiency may be more frequent than has been appreciated.
Hyperprolactinemic Chronic Anovulation
About 15% of amenorrheic women have increased circulating concentrations of prolactin, but prolactin levels are increased in more than 75% of patients with galactorrhea and amenorrhea (134). Radiologic evidence of a pituitary tumor is present in about 50% of hyperprolactinemic women, and primary hypothyroidism always must be considered. Individuals with galactorrhea-amenorrhea (i.e., hyperprolactinemic chronic anovulation) frequently complain of symptoms of estrogen deficiency, including hot flushes and dyspareunia. However, estrogen secretion may be essentially normal (135). It is not clear if the hyperprolactinemia or the “hypoestrogenism” causes the accelerated bone loss seen in such individuals (136). Signs of androgen excess are observed in some women with hyperprolactinemia; androgen excess may rarely result in PCOS. In hyperprolactinemic women, serum gonadotropin and estradiol levels are low or normal.
Most hyperprolactinemic women have disordered reproductive function, and it appears that the effects on gonadotropin secretion are primarily hypothalamic. The mechanism by which hypothalamic GnRH secretion is disrupted is unknown but may involve an inhibitory effect of tuberoinfundibular dopaminergic neurons (135, 137). It has been proposed that increased hypothalamic dopamine is present in hyperprolactinemic women with pituitary tumors but is ineffective in reducing prolactin secretion by adenomatous lactotropes. The dopamine can, however, reduce pulsatile LH secretion and produce acyclic gonadotropin secretion through a direct effect on hypothalamic GnRH secretion.
It has been suggested that mild nocturnal hyperprolactinemia may be present in some women with regular menses and unexplained infertility (138). Galactorrhea in women with unexplained infertility may reflect increased bioavailable prolactin and may be treated appropriately with bromocriptine (139). Bromocriptine or cabergoline therapy may also be indicated in normoprolactinemic women with amenorrhea and increased prolactin responses to provocative stimuli (140).
Hypopituitarism
Hypopituitarism may be obvious on cursory inspection or it may be quite subtle. The clinical presentation depends on the age at onset, the cause, and the woman’s nutritional status. Loss of axillary and pubic hair and atrophy of the external genitalia should lead the physician to suspect hypopituitarism in a previously menstruating young woman who develops amenorrhea. In such cases, a history of past obstetric hemorrhage suggesting postpartum pituitary necrosis (i.e., Sheehan syndrome) should be sought (141). Failure to develop secondary sexual characteristics or to progress in development once puberty begins must always prompt a workup for hypopituitarism.
Individuals with pituitary insufficiency often complain of weakness, easy fatigability, lack of libido, and cold intolerance. Short stature may occur in individuals developing hypopituitarism during childhood. Symptoms of diabetes insipidus may be observed if the posterior pituitary gland is involved. On physical examination, the skin is generally thin, smooth, cool, and pale (i.e., “alabaster skin”) with fine wrinkling about the eyes; the pulse is slow and thready; and the blood pressure is low.
An evaluation of thyroid and adrenal function is of paramount importance in these individuals. Thyroid replacement therapy must be instituted and the patient must be euthyroid before adrenal testing is initiated. Serum gonadotropin and gonadal steroid levels typically are low in hypopituitarism. Responses to exogenously administered hypothalamic hormones often fail to localize the cause to the hypothalamus or the pituitary gland in affected patients.
Radiographic evaluation of the sella turcica is indicated in any individual with suspected hypopituitarism. The ovaries appear immature and unstimulated, but because oocytes still are present, ovulation can be induced with exogenous gonadotropins when pregnancy is desired. Exogenous pulsatile GnRH may also be used to induce ovulation if the disorder is hypothalamic. Moreover, oocytes may undergo some development, and even ovarian cysts may appear in the absence of significant gonadotropic stimulation. When pregnancy is not desired, maintenance therapy with cyclic estrogen and progestin is indicated to prevent signs and symptoms of estrogen deficiency.
CHRONIC ANOVULATION DUE TO INAPPROPRIATE FEEDBACK IN POLYCYSTIC OVARY SYNDROME (PCOS)
A Heterogeneous Disorder
In 1935, Stein and Leventhal focused attention on a common disorder in which amenorrhea, hirsutism, and obesity were frequently associated (142) (Fig.4). Since that time, this syndrome has been the topic of innumerable studies (142a,142b).
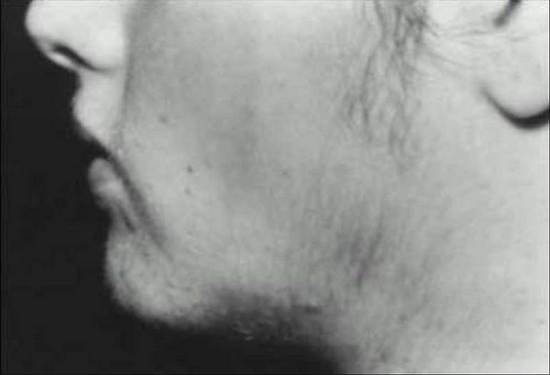
Figure 4. Facial hirsutism in a 17-year-old woman with polycystic ovary syndrome (PCOS).
With the development of radioimmunoassays for measuring reproductive hormones, it became clear that women with what is called PCOS shared several distinctive biochemical features. Compared with eumenorrheic women in the early follicular phase of the menstrual cycle, affected women typically have elevated serum LH levels and low to normal FSH levels (143). Virtually all serum androgens are moderately increased, and estrone levels are generally greater than estradiol levels (144). Ovarian inhibin physiology is normal (145). Also increased in women with PCOS are levels of anti-mullerian hormone (AMH); it appears that the more severe the disorder, the higher are the levels of AMH (145a). The increased AMH levels appear due to the increased number of small antral follicles as well as to intrinsic characteristics of the granulosa cells in PCOS.
Many women with the biochemical features of PCOS have small or even morphologically normal ovaries and are not overweight or hirsute. Not all women with PCOS present with the characteristic features. Moreover, excess androgen from any source or increased conversion of androgens to estrogens can lead to the constellation of findings observed in PCOS (146). Included are such disorders as Cushing syndrome, congenital adrenal hyperplasia, virilizing tumors of ovarian or adrenal origin, hyperthyroidism and hypothyroidism, and obesity. In all of these disorders, the ovaries may be morphologically polycystic. Although no clinical and biochemical criteria describe the syndrome strictly, a conference convened by the National Institutes of Health (147) developed diagnostic criteria for PCOS:
- Clinical evidence of hyperandrogenism (e.g., hirsutism, acne, androgenetic alopecia) and/or hyperandrogemia (e.g., elevated total or free testosterone).
- Oligoovulation (i.e., cycle duration >35 days or <8 cycles per year).
- Exclusion of related disorders (e.g., hyperprolactinemia, thyroid dysfunction, androgen-secreting tumors, 21-hydroxylase-deficient nonclassical congenital adrenal hyperplasia.)
A subsequent expert conference convened in Rotterdam, The Netherlands, in 2003 and sponsored in part by the American Society for Reproductive Medicine (ASRM) and the European Society for Human Reproduction and Embryology (ESHRE) recommended that PCOS be defined when at least two of the following three features are present: 1) oligo- and/or anovulation, 2) clinical and/or biochemical signs of hyperandrogenism, and 3) polycystic ovaries. This definition also states that other androgen excess or related disorders should be excluded prior to assigning the diagnosis of PCOS (148,149). By these criteria neither hyperandrogenism nor ovulatory dysfunction is required to make the definition of PCOS. This latter definition is now the most widely accepted; it is becoming increasingly common to spell out the phenotypes of patients reported in studies of PCOS as recommended by an NIH consensus conference in 2012 (149a).
A subsequent consensus conference on PCOS noted that there has been no overall agreement as to how to diagnose PCOS in adolescence (149b). Because both acne and irregular menstrual cycles are common in adolescents, while hirsutism develops slowly over time, it has been suggested that all three elements of the Rotterdam criteria should be present in teens in order to make the diagnosis of PCOS (149c). These investigators suggest that amenorrhea or oligomenorrhea should be present for at least two years after menarche (or until at least age 16), that the ovaries on ultrasound should be enlarged to greater than 10 cm3 (because numerous small cysts are commonly present during adolescence), and that hyperandrogenemia and not just signs of androgen excess should be present to diagnose PCOS in teenagers.
PCOS may be viewed as a state of chronic anovulation associated with LH-dependent ovarian overproduction of androgens. Clinically, the perimenarcheal onset of symptoms is a common feature. It has been estimated that PCOS affects approximately 5% of women of reproductive age, making it the most common form of chronic anovulation (150, 151). Some clinicians believe that PCOS may be the most common endocrinopathy. Although the cause of this disorder remains unknown, there is some evidence of autosomal dominant transmission in some affected individuals (152, 153). Disorders presenting similarly but with different underlying causes can be considered as having chronic anovulation with inappropriate feedback.
Polycystic Ovaries
Grossly, the ovaries of most women with PCOS are bilaterally enlarged and globular. (Fig.5)
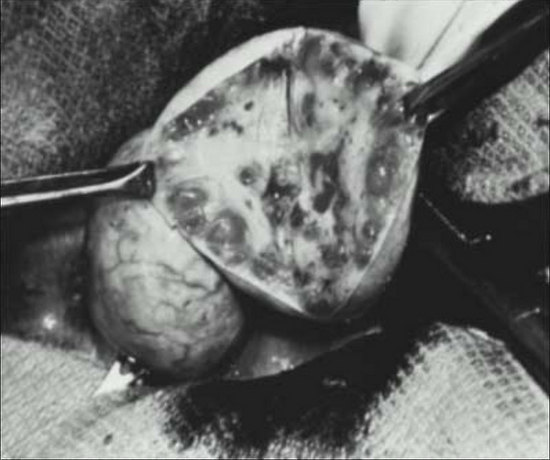
Figure 5. Gross and cut appearance of typical polycystic ovaries. Multiple small follicular cysts are apparent in the cut section.
They are often described as having an “oyster shell” appearance because they have smooth, glistening capsules and are the appropriate color. The tunica albuginea is often thickened diffusely, and many cysts 3 to 7 mm in diameter are present on cut section. Because ovulation rarely occurs, corpora lutea may be present. Histologically, the follicular cysts are usually lined by granulosa cells and surrounded by a thickened and luteinized theca interna and are in various stages of maturation and atresia. When islands of luteinized thecal cells are found scattered throughout the ovarian stroma, not just around the follicles, the term hyperthecosis is sometimes used. The clinical syndrome accompanying this pathologic finding is typically characterized by massive obesity, severe hirsutism reflecting particularly excessive ovarian overproduction of androgens, acanthosis nigricans, glucose intolerance with insulin resistance, and hyperuricemia. (Fig.6)
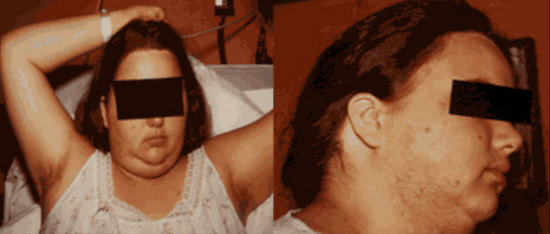
Figure 6. Appearance of a woman with hyperthecosis, sometimes referred to as the HAIR-AN syndrome (hyperandrogenism, insulin resistance, acanthosis nigricans). The obesity, hirsutism, acne, and acanthosis nigricans are obvious.
Insulin action at the target cell appears defective in these patients, with some individuals having antibodies to insulin receptors and others apparently having a postreceptor defect (154, 155). PCOS and hyperthecosis appear to represent facets of the same disease process rather than two distinct entities. Some authorities, however, maintain that the two represent different disorders.
The follicles in the ovaries of women with PCOS do not mature completely. However, in vitro studies have failed to detect any primary defect in the steroidogenic capacity of polycystic ovaries (156). Although there seems to be a relative deficiency in aromatase activity in the granulosa cells of polycystic ovaries, this deficiency can be corrected by FSH in vitro and in vivo.
Other Clinical and Biochemical Features
Although all women with PCOS produce androgens at increased rates compared with eumenorrheic women, only some present with hirsutism, largely because of varying sensitivity at the level of the hair follicle. The hyperandrogenism is rarely sufficient to produce overt virilization. Signs of markedly elevated androgen levels, including clitoromegaly, temporal balding, and deepening of the voice, may suggest an androgen-producing tumor, especially if these features developed rapidly. Women with PCOS invariably are well estrogenized, with normal breast development and abundant cervical mucus on examination. Because obesity is found in only about 50% of women with PCOS, it is doubtful that obesity is central to its cause.
About 50% of women with PCOS have amenorrhea, about 30% have irregular bleeding, and about 12% have “cyclic menses” (146). No particular pattern of menstrual bleeding is characteristic of women with PCOS, although a history of oligomenorrhea is probably most common. Because only about 75% of women with PCOS are infertile, women with PCOS do ovulate occasionally.
Two other biochemical features warrant discussion. First, obese and normal-weight women with PCOS generally release increased quantities of insulin in response to a standard glucose challenge compared with weight-matched eumenorrheic individuals (157, 158). Many investigators now regard the insulin resistance as the central abnormality in the disorder, but this has not been established with certainty. Thus, regardless of body weight, 30 to 80 percent of women with PCOS experience insulin resistance and compensatory hyperinsulinemia (159). Based on studies in a very well characterized subset of obese women with the disorder, the insulin resistance present in PCOS appears to represent a postreceptor signalling aberration and differs from the insulin resistance observed in non-insulin dependent diabetes mellitus and simple obesity (118). The compensatory hyperinsulinemia that results causes exaggerated effects in other tissues as well. These effects include increased ovarian androgen secretion; excessive growth of the basal cells of the skin leading to acanthosis nigricans in some women; increased vascular and endothelial reactivity, which may lead to hypertension and vascular disease; and abnormal hepatic and peripheral lipid metabolism, which may cause dyslipidemia. Thus, it is now recognized that women with PCOS are at increased risk of cardiovascular disease and non-insulin-dependent diabetes mellitus in addition to endometrial carcinoma because of anovulation. Because treatment with a GnRH analogue reduces ovarian androgen secretion but does not correct the insulin resistance in women with PCOS, the defect in insulin action presumably is not due to abnormal sex steroid levels (160). The possibility that a defect in the secretion or action of insulin or some related growth factor is central to the cause of PCOS cannot be entirely excluded and is gaining increasing support as the cause of hyperandrogenemia in women with PCOS (161). The pivotal role of insulin resistance in PCOS is strongly suggested by the beneficial effects of insulin-sensitizing agents such as metformin, troglitazone, and D-chiro-inositol on metabolic and reproductive function, regardless of the patient’s weight (162-165).
In addition, perhaps 10% to 15% of women with PCOS have mild hyperprolactinemia in the absence of radiographic evidence of a pituitary tumor, possibly because of chronic acyclic estrogen secretion (166). Although hyperprolactinemia is associated with increased adrenal production of DHEAS, the increased adrenal androgen production seen in women with PCOS usually does not correlate with the hyperprolactinemia.
Pathophysiology of the Chronic Anovulation
A growing body of evidence indicates that disordered insulin action precedes the increase in androgens in PCOS. The administration of insulin to women with PCOS increases circulating androgen levels (161, 167). The administration of glucose to hyperandrogenic women increases circulating levels of insulin and androgen (168). Weight loss decreases levels of insulin and androgens (169). The suppression of circulating insulin levels experimentally by diazoxide reduces androgen levels (170). The suppression of androgen secretion to normal levels with GnRH agonists does not lead to normal insulin responses to glucose tolerance testing in obese women with PCOS (160, 171, 172).
The hyperinsulinemia may cause hyperandrogenemia by binding to IGF-I receptors in the ovary (173). Activation of ovarian IGF-I receptors by insulin can lead to increased androgen production by thecal cells (174). Moreover, independent of any effect on ovarian steroid production, increased insulin inhibits the hepatic synthesis of SHBG (175). Insulin directly inhibits insulin-like growth factor binding protein-1 in the liver, permitting greater local activity of IGF-I in the ovary (176).
Regardless of the cause of PCOS, it is possible to construct a rational pathophysiologic mechanism to explain the disorder (Fig.7).
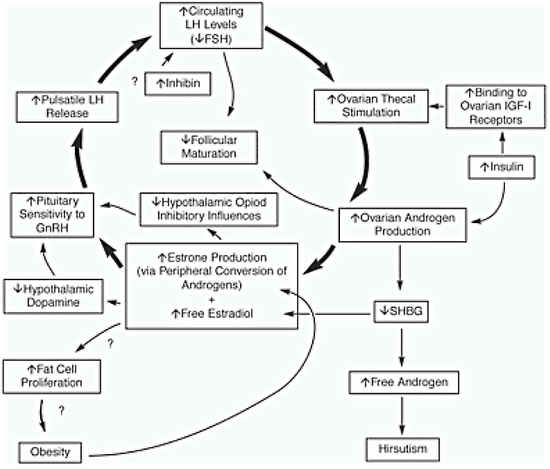
Figure 7. Pathophysiologic mechanisms associated with PCOS that may help explain the chronic anovulation.
Regardless of the source or cause of androgen excess, a vicious cycle of events causing persistent anovulation commences. The androgen is converted to estrogen, primarily estrone, in the periphery. The estrogen feeds back on the central nervous system-hypothalamic-pituitary unit to induce inappropriate gonadotropin secretion with an increased LH to FSH ratio. The estrogen stimulates GnRH synthesis and secretion in the hypothalamus, causing preferential LH release by the pituitary gland. The estrogen may also increase GnRH by decreasing hypothalamic dopamine. Selective inhibition of FSH secretion by increased ovarian inhibin may also occur in PCOS. Possible inhibition of FSH secretion by increased androgen secretion has not been considered. The increased LH secretion stimulates thecal cells in the ovary to produce excess androgen. The androgen also inhibits production of SHBG, resulting in increased free androgen and predisposing affected women to hirsutism. The morphologic ovarian changes undoubtedly are secondary to hormonal changes. The absence of follicular maturation and the reduced estradiol production by the ovaries apparently result from a combination of inadequate FSH stimulation and inhibition by the increased concentrations of intraovarian androgen. The low levels of SHBG probably facilitate tissue uptake of free androgen, leading to increased peripheral formation of estrogen and perpetuating the acyclic chronic anovulation. The androgenic basis for the inappropriate estrogen feedback is partly shifted from the site of origin to the ovaries. The increased estrogens (and perhaps androgens) may also stimulate fat cell proliferation, leading to obesity. The current data suggest that there is no defect in the hypothalamic-pituitary axis in PCOS but rather that peripheral alterations result in abnormal gonadotropin secretion.
Therapy
Appropriate therapy demands that potential causes such as neoplasms be eliminated. Besides facilitating fertility, the aims of treatment in women with PCOS are three-fold: to control hirsutism, to prevent endometrial hyperplasia from unopposed acyclic estrogen secretion, and to prevent the long-term consequences of insulin resistance. The treatment must be individualized according to the needs and desires of each patient.
For the anovulatory woman with PCOS who is not hirsute and who does not desire pregnancy, therapy with an intermittent progestin (e.g., medroxyprogesterone acetate, 5 to 10 mg orally, or micronized progesterone, 200 mg orally, for 10 to 14 days each month) or oral contraceptives if she is younger than 35 years of age, does not smoke, and has no other significant risk factor should be provided to reduce the increased risk of endometrial hyperplasia and carcinoma present in such a woman because of the unopposed estrogen secretion. The woman taking progestins intermittently should be informed of the need for effective barrier contraception if she is sexually active, because these agents as administered do not inhibit ovulation, and ovulation occasionally occurs in PCOS. There is no evidence that the use of low-dose combined oral contraceptive agents increases the risks associated with insulin resistance in women with PCOS, and the benefits in preventing endometrial hyperplasia are clearly established. The progestin-containing IUD can be used both for contraception and to prevent endometrial hyperplasia, especially for women who cannot or will not take oral contraceptives.
Therapy for the woman with PCOS who is hirsute is somewhat different in some circumstances. In general, oral contraceptives provide initial therapy for affected women with mild hirsutism and provide protection from endometrial hyperplasia.
For women with PCOS who are overweight, it is reasonable to encourage lifestyle changes (149a, 176a). It is also recommended that women with PCOS be screened for diabetes, typically by an oral glucose tolerance test, although measurement of hemoglobin A1C may suffice (176a). Weight loss alone (of even less than 10%) may result in decreased insulin resistance and resumption of ovulation (177-179). However, lifestyle changes are difficult for patients to adopt. The use of insulin-sensitizing agents such as metformin is increasing but is not approved by the FDA. Whether the use of such agents will decrease the likelihood of the consequences of the metabolic alterations associated with insulin resistance is unclear. At present no data regarding the long-term safety and efficacy of these agents exist. What is clear is that only short-term trials of perhaps 3 months’ duration are needed to determine if insulin-sensitizing agents will be useful: responsive individuals will resume cyclic menstruation and ovulation in this short time frame and insulin levels will fall substantially (162,163,180,181).
Predicting which individuals with PCOS will respond is not possible at the present time. However, many clinicians believe that the therapy is low in risk, and the agents are relatively inexpensive. The use of these agents should probably be contemplated only in women with well documented insulin resistance and PCOS. Metformin should be administered only if the patient’s creatinine is normal and should be discontinued during illnesses to prevent the occurrence of lactic acidosis. Individuals should be cautioned that they may anticipate nausea or diarrhea on beginning metformin. Consequently the drug should be increased slowly to the maximal dose of 2.5 g per day orally.
For the woman with PCOS who wants to conceive, clomiphene citrate is used initially because of its high success rate and relative simplicity and inexpensiveness. A randomized trial has documented that letrozole is more effective than clomiphene in women with PCOS and a BMI >30 (181a). In fact, data are accumulating to indicate that letrozole is as effective as clomiphene in all women with PCOS, whether obese or not (181b). Both clomiphene citrate and letrozole can now be considered as first-line agents for inducing ovulation in PCOS even though letrozole is not approved for this indication by the U.S. FDA. 181b Other possible therapeutic approaches to ovulation induction include the use of gonadotropins (perhaps preceded by a GnRH analogue), FSH alone, pulsatile GnRH, and wedge resection of the ovaries at laparotomy (181c). Wedge resection or any other surgical manipulation of the ovaries should be performed only after all other methods of ovulation induction fail, an ovarian tumor is possible because of ovarian size or circulating androgen levels, or fertility is unimportant, because pelvic adhesions frequently result from surgery and may contribute to infertility. Laparoscopic ovarian follicular cautery or laser vaporization can also be used successfully to induce ovulation (182,183). However, these procedures also cause adhesion formation in a significant percentage of women. In addition, the success of medical therapy does not justify routine use of these procedures.
There are some largely uncontrolled studies suggesting that insulin-sensitizing agents, alone or in combination with clomiphene citrate, may improve both ovulatory function and fertility in some women with PCOS (162, 163,180). A trial may be warranted in women who do not respond to clomiphene before considering the use of more expensive agents to induce ovulation. However, a large randomized clinical trial documented that clomiphene citrate is more effective than metformin in inducing ovulation and resulting pregnancy; moreover, there was no further improvement when the two agents were used concurrently (183a). Metformin should not be considered as first-line therapy for women with PCOS desiring pregnancy.
CHRONIC ANOVULATION DUE TO OTHER ENDOCRINE AND METABOLIC DISORDERS
Cushing Syndrome
Along with the well-known physical manifestations in Cushing syndrome-central obesity, moon facies, and pigmented striae-are the less visible endocrinologic changes of amenorrhea, hirsutism, and infertility. The mechanisms responsible for the chronic anovulation are unclear, but several possibilities exist. The various degrees of adrenal androgen excess in Cushing syndrome of all causes together with obesity may cause excessive extraglandular conversion of androgens to estrogens in fat cells and inappropriate acyclic feedback to the hypothalamic-pituitary unit (184). The increased levels of CRH and ACTH in Cushing disease may affect the hypothalamic-pituitary secretion of GnRH and LH, as suggested for hypothalamic chronic anovulation.
Thyroid Dysfunction
As a result of significant changes in the metabolism and interconversion of androgens and estrogens, hyperthyroidism and hypothyroidism are associated with menstrual disorders ranging from excessive and prolonged uterine bleeding to amenorrhea. The altered sex steroid metabolism leads to inappropriate feedback and chronic anovulation. The changes are corrected by appropriate treatment of the underlying thyroid disease.
ABNORMAL UTERINE BLEEDING IN WOMEN OF REPRODUCTIVE AGE
Etiology
Abnormal uterine bleeding (AUB) is the most common indication for gynecologic consultation. AUB is also believed to be the indication for 80-90% of D&C procedures performed in nonpregnant women in the United States, accounting for about 350,000 procedures annually (185). By some measures AUB is the second most common indication for hysterectomy in the U.S. after uterine leiomyomas, accounting for approximately 20% or 120,000 procedures annually (186,187).
AUB may be defined as uterine bleeding occurring at unexpected times or of abnormal duration and may take any of several forms, with the bleeding altered in frequency, duration, and/or amount. Because the terminology and definitions for disturbances of menstrual bleeding are so confused, it is best to use the term AUB and then to describe the bleeding pattern as precisely as possible (187a). AUB always must be differentiated from bleeding originating in the urinary or gastrointestinal tracts. Broadly speaking, AUB can be divided into “organic causes”, which are found in perhaps 25% of cases, and so-called “dysfunctional” (or anovulatory) uterine bleeding (Table 2)
Table 2. Etiology of Abnormal Uterine Bleeding
| 1. Organic Causes |
| a. Systemic disease
i. Coagulation disorders (Primary or secondary) ii. Thyroid dysfunction iii. Liver disease a. Pregnancy-related disorders b. Malignancies c. Benign uterine abnormalities (i.e., fibroids, polyps) d. Iatrogenic causes (i.e., IUDs, estrogens) e. Lower genital tract disease f. Functional ovarian cysts and other benign ovarian neoplasms |
| 2. Reproductive tract disease |
| a. Pregnancy-related disorders
b. Malignancies c. Benign uterine abnormalities (i.e., fibroids, polyps) d. Iatrogenic causes (i.e., IUDs, estrogens) e. Lower genital tract disease f. Functional ovarian cysts and other benign ovarian neoplasms |
| 3. “Dysfunctional” (anovulatory) uterine bleeding (DUB) |
Organic causes can be divided further into those associated with any of a number of systemic diseases and those associated with disorders of the reproductive tract. DUB may be defined as resulting from a functional abnormality of the hypothalamic-pituitary-ovarian axis and is present in the majority of women with AUB. It is perhaps best to refer to anovulatory bleeding rather than to DUB because many studies include in reports of DUB women who clearly have organic abnormalities.
The frequency of the various causes of AUB varies with the age of the patient. DUB is more common early and late in the reproductive years. Organic causes, especially neoplasms, increase with advancing age. In any woman of reproductive age, pregnancy must be ruled out, because bleeding related to complications of pregnancy is probably the most common cause of abnormal bleeding.
Different abnormalities cause AUB during the prepubertal years. Newborn girls sometimes spot for a few days after birth because of placental estrogenic stimulation of the endometrium in utero. Withdrawal of the estrogen at birth leads to sloughing of the endometrium. Accidental trauma to the vulva or vagina is the most common cause of bleeding during childhood. Vaginitis with spotting, most often because of irritation from a foreign body, also may occur. Prolapse of the urethral meatus and tumors of the genital tract also must be considered in the differential diagnosis. When the bleeding is due to the ingestion of estrogen-containing drugs (typically oral contraceptives) by children, there is rarely significant pubertal development. Of course, sexual abuse always must be considered in the young girl presenting with abnormal bleeding. Thus, it is clear that most of the prepubertal causes of bleeding are really not uterine in origin.
Although perhaps as many as half of all menstrual cycles are anovulatory when menses begin, the actual incidence of DUB in adolescents is low. Typically, anovulatory bleeding occurs at intervals longer than normal menstrual cycles, while bleeding due to organic causes tends to occur more frequently than regular menses. In most cases of anovulatory bleeding beginning in adolescence, there is spontaneous resolution. However, it is important to remember that up to 20% of patients with AUB during the teenage years have a primary coagulation disorder (188). It is also important to rule out pregnancy-related bleeding during the reproductive years.
Any woman over the age of 35 with AUB must be evaluated for a malignancy, despite the fact that most causes of such bleeding are benign. Endometrial hyperplasia clearly is a possibility in women who do not ovulate on a regular basis, even at a much earlier age than 40. The finding of endometrial hyperplasia after the menopause always should result in a search for a source of estrogen, either from exogenous therapy or from an endogenous (commonly ovarian) neoplasm.
Evaluation and Treatment
In the evaluation of the woman with AUB, obtaining a thorough history is of paramount importance. Emphasis should be placed on learning the pattern and quantity of bleeding. Because most women are poor at estimating blood loss and recalling exactly when they bled, all patients should be asked to keep a prospective menstrual calendar in which they record days and severity of bleeding. Menses lasting for more than 8 days or in which more than 80 ml of blood is lost are probably abnormal (i.e., menorrhagia). It has been estimated that up to 20% of women have excessive menstrual blood loss and that the incidence is similar for African-American and white U.S. women (189).
Obviously the physical examination also is important. The hemodynamic stability of any patient with abnormal bleeding should be assessed. The pelvic examination will rule out obvious organic causes. Warranted laboratory tests include measurement of hCG at the point of service, a complete blood count to assess hematological status, a platelet count and other coagulation studies to rule out a coagulation defect, and thyroid function studies to rule out a thyroid abnormality.
Just which patients should undergo further assessment of the endometrium is problematic, as is the type of evaluation to be undertaken. An endometrial biopsy is indicated in any woman over age 35 with AUB, in any woman with a prolonged history of irregular bleeding, and in most, if not all, women with severe bleeding. Measurement of endometrial thickness by transvaginal ultrasound appears to be of value in postmenopausal women who are not taking exogenous estrogen. Several studies have indicated that there is almost never any significant pathology when the endometrial thickness is less than 5 mm (190). Sonohysterography (SHG), sometimes termed saline infusion sonography (SIS), has become increasingly popular because it can be done in the office at the time of the initial evaluation and appears almost as accurate as hysteroscopy in diagnosing abnormalities within the uterine cavity (191,192). Some clinicians prefer hysteroscopy because it is generally superior to blind biopsy in identifying abnormalities and allows for treatment of many abnormalities at the time of diagnosis. Unfortunately it is also the most expensive of the various procedures; moreover, it is not clear that this procedure is needed to make the diagnosis in most cases. Until definitive data indicate when each of these procedures is warranted, physicians will need to exercise their individual judgment in evaluating women with AUB.
The management of AUB also requires judgment, but a few principles serve the clinician well. First, rule out an organic cause for the bleeding. Then remember that hormonal therapy can almost always stop anovulatory bleeding, but both the patient and the physician must recognize that bleeding will recur at a later (hopefully controlled and planned) time. In general, medical management is always preferred for the treatment of DUB, especially if the patient is interested in future childbearing or if menopause will occur shortly. The actual management of DUB depends on the severity of the problem, the age of the patient, and her desires regarding future fertility.
In young women, typically teenagers, with DUB, only reassurance and prospective charting may be necessary in those with mild irregular bleeding, especially because most adolescents will begin or resume regular ovulatory cycles within several months (188). In teens in whom the bleeding has been more prolonged and erratic such that there is some anemia (but the patient is hemodynamically stable), therapy must be individualized. If the young woman is sexually active (but not pregnant), a progestin-dominant oral contraceptive should control the bleeding and simultaneously provide contraception. Alternatively, a progestin such as medroxyprogesterone acetate (5-10 mg daily for 10-14 days) may be given every 30 to 60 days to induce intermittent “chemical curettage” and prevent chronic unopposed stimulation of the endometrium. However, it often takes several months before intermittent progestins can control irregular uterine bleeding. Oral iron therapy should always be provided as well. In general, the hormonal therapy can be discontinued, if desired, in 6 to 12 months. Most women will have regular menses when therapy is stopped, but thorough evaluation is warranted if irregular bleeding recurs.
In acute severe menorrhagia (with signs of acute blood loss such that the patient is hemodynamically unstable), blood transfusion may be required to restore hemodynamic stability. Once more hormonal therapy is almost always effective in controlling the bleeding. Any of several regimens may be utilized, but in general large doses of estrogen must be given initially and progestin must be added to stabilize the endometrium. For example, an oral contraceptive agent containing 35 micrograms of ethinyl estradiol may be given every 6 hours until the bleeding stops (generally within 48 hours). The dosage then may be tapered by reducing by one pill every other day (4,4,3,3,2,2,1,1 pills per day). Withdrawal bleeding may be permitted after the dosage has been reduced to one tablet each day or may be deferred for several days by continuing to administer one tablet daily. The patient then should be maintained on oral contraceptives given in the usual cyclic fashion for 6 to 12 months. If hormonal therapy cannot control the bleeding, the diagnosis of DUB should be questioned, and evaluation and biopsy of the endometrium are warranted.
Treatment of the woman over age 35 with AUB is more problematic. Organic causes of uterine bleeding are more common and mandate at least visualization if not sampling of the endometrium. Hormonal therapy with a progestin alone or with estrogen and a progestin can be used to control bleeding; combination therapy may be more effective. It is clear that low-dose combination oral contraceptive agents are effective in the majority of women with DUB (193). Hysterectomy is more commonly employed in this age group, particularly if the patient no longer desires childbearing.
A number of medications have proven effective in the treatment of menorrhagia associated with ovulatory menstrual cycles. Non-steroidal anti-inflammatory agents (NSAIDs) are clearly of benefit in some, but not all, women with increased menstrual blood loss. Five of seven randomized trials concluded that mean menstrual blood loss was less with NSAIDs than placebo, while two showed no significant difference (194). This therapy can be used for long-term treatment because side effects, mainly gastrointestinal, are mild with intermittent therapy administered only when the patient is bleeding. They can be given in combination with oral contraceptives or progestins to achieve more effective reduction in menstrual blood loss. Although not approved by the FDA for this purpose, studies from Europe indicate that progestin-containing IUDs may be the most effective therapy for menorrhagia, effecting a reduction in blood loss of as much as 90% in some women (195). The androgenic steroid danazol is also effective in reducing blood loss, even at relatively low doses of 200-400 mg per day, but side effects are common and more severe than with other medical therapies. Epsilon-aminocaproic acid (EACA), tranexamic acid (AMCA), and para-aminomethylbenzoic acid (PAMBA) are potent inhibitors of fibrinolysis and have been used effectively, particularly in Europe, to reduce menstrual blood loss, but side effects limit their utility (196,197). Tranexamic acid is now approved for use in the United States. Although extensive data are lacking, it is likely that GnRH analogs, perhaps with “add back” therapy to prevent bone loss, are very effective in reducing blood loss (198), but their expense mitigates using them except in those women who fail to respond to other methods of medical management and who wish to retain their childbearing capacity.
A few other comments are warranted. For women of reproductive age who desire childbearing, induction of ovulation is an effective means of controlling anovulatory bleeding. More than half of functional ovarian cysts, most commonly follicular and corpora luteal cysts, induce some form of menstrual irregularity, ranging from amenorrhea to menorrhagia, and most resolve spontaneously. Clearly abnormal bleeding is also a common complaint of women using hormonal and other forms of contraception. It is also important to remember that thyroid dysfunction may cause any disorder of bleeding ranging from amenorrhea to menorrhagia.
Lastly there is little if any role for the use of depot medroxyprogesterone acetate in the management of AUB. This is particularly true for the treatment of acute bleeding and for individuals in whom the cause of the bleeding has not been established with certainty. Although DMPA may be effective in some women, the drug is also known to cause irregular bleeding and may merely compound the problem. Other, more easily reversible forms of contraception are equally or more effective and should be used.
There are several approaches to the surgical treatment of abnormal uterine bleeding. The appropriate procedure depends on the individual circumstances.
Dilatation and curettage (D&C) is indicated for diagnostic purposes in those women in whom endometrial sampling is warranted but in whom endometrial biopsy in the office is not feasible or has been nondiagnostic. Although D&C has been found empirically to be effective in the management of acute uterine bleeding unresponsive to medical therapy, the therapeutic effect of the procedure is usually limited to the current bleeding episode. When D&C is performed for acute bleeding, it should be followed immediately by administration of cyclic exogenous estrogen and progestin in order to optimize long-term cycle control.
It has been estimated that the blind technique of D&C misses the diagnosis of intrauterine lesions in 10 to 25% of patients. Several studies have indicated that hysteroscopy with directed biopsy is at least as accurate as D&C in detecting endometrial abnormalities. Difficulties with hysteroscopy include its cost, the skill required to perform the procedure and evaluate what is seen, and the fact that it is not useful as a simple screening procedure. Hysteroscopy is probably most useful in individuals with AUB in whom no lesion is detected by other methods but in whom the abnormal bleeding persists.
Surgery that attempts to destroy the endometrium selectively, called endometrial ablation, has been reported for decades. Early approaches utilized thermocoagulation and irradiation. Hysteroscopic endometrial ablation can be conducted in several ways: using laser or electrical or thermal energy to coagulate or vaporize the tissue or resecting the endometrium with a loop electrode deployed via a modified urological resectoscope. Non-hysteroscopic endometrial ablation, involving blind destruction of the endometrium using computer-assisted energy delivery systems, is becoming increasingly popular because newly available approaches and those under development are less expensive than surgical approaches, require less training, and some can be performed in an office setting. Thermal balloon ablation systems are now available in the United States. Although trials comparing the various approaches are relatively uncommon, it appears that all the approved methods of endometrial ablation are equally effective (199-201). The reported incidence of complications with endometrial ablation is relatively low (200). A comprehensive survey of 87 Dutch hospitals indicates half of all complications are related to entry into the endometrial cavity (i.e., uterine perforation and cervical trauma) (202). Other complications include those related to anesthesia, failed access, hemorrhage, and the systemic absorption of distension media. Complications are more commonly encountered early in the experience of a given surgeon.
Data from several reported series suggest that endometrial ablation will result in initial amenorrhea in 50-75% of patients, acceptable reduction in blood loss in another 20-30%, and no significant reduction in blood loss in approximately 10% (199,201). Repeating the procedure a second time appears to be successful in over half the patients initially experiencing a treatment failure.
There is class I evidence from a Cochrane review that use of GnRH agonists prior to endometrial ablation results in shorter procedures, greater ease of surgery, a lower rate of post-operative dysmenorrhea, and a higher rate of post-surgical amenorrhea (203). Several randomized trials allowed a meta-analysis which documented that women undergoing hysteroscopic endometrial ablation had shorter hospital stays, fewer post-operative complications, and resumed activities earlier than those undergoing hysterectomy for increased menstrual bleeding (204). However, there was a significant advantage in favor of hysterectomy in the improvement in heavy menstrual bleeding and satisfaction rates up to 4 years after surgery compared with endometrial ablation. Moreover, rates of re-operation in women undergoing endometrial ablation increase steadily over time after the initial surgery, up to about 40% at 4 years (205). The direct costs of endometrial ablation may well be greater than hysterectomy if patients are followed long enough after their initial procedure (205). Thus, currently endometrial ablation may be an appropriate alternative to hysterectomy for the rare case of DUB or menorrhagia that is unresponsive to conservative management in a woman who is not desirous of future childbearing. Ablation may be very useful in women who are sufficiently ill such that they are poor candidates for hysterectomy.
The most common indication for the removal of uterine fibroids by myomectomy is menorrhagia, followed by pelvic pain or pressure and infertility. The reported effectiveness of myomectomy for menorrhagia is about 80%, but it is not clear what percentage of these patients have failed medical therapy. Although recurrence of myomas following myomectomy is observed in up to 50% of cases, reportedly only 10-15% of women undergoing myomectomy require subsequent surgery such as hysterectomy. MRI –guided focused ultrasound is being used increasingly to treat myomas without surgery.
The effectiveness of hysterectomy for AUB (virtually 100%) has contributed to its popularity as a primary treatment modality for this disorder. Unfortunately, the inefficiency of hysterectomy, due to its greater morbidity, mortality, and cost, makes it an inappropriate choice for management of the great majority of patients presenting with AUB. Current data would suggest that only 1-2% of women presenting with abnormal bleeding will ultimately require hysterectomy when given an appropriate trial of nonsurgical management. Hysterectomy usually should be reserved for the patient with other indications, such as leiomyomas or uterine prolapse. Hysterectomy should be used to treat persistent AUB after all other medical therapy has failed and the amount of menstrual blood loss has been documented to be excessive by some direct measurement (such as a fall in hematocrit).
ABNORMAL UTERINE BLEEDING IN POSTMENOPAUSAL WOMEN
Any bleeding in postmenopausal women not taking exogenous estrogen must be investigated. Vaginal, cervical, and rectal bleeding must be distinguished from uterine bleeding. Endometrial sampling is warranted in all postmenopausal women not on estrogen with uterine bleeding. The role of ultrasound in management continues to evolve: Many clinicians find it acceptable to defer biopsy if the endometrial thickness is less than 5 mm in diameter. In women not taking estrogen, the most common cause of uterine bleeding is endometrial atrophy.
Bleeding in postmenopausal women taking estrogen is more problematic. In women on sequential estrogen and progestogen, bleeding should occur only near the end or following the course of progestogen. If such is the case, endometrial sampling never may be indicated. An endometrial biopsy is warranted for bleeding at any other time. Despite the fact that a very few cases of endometrial cancer are associated with endometrial thickness of less than 5 mm in diameter on ultrasound, some clinicians are choosing to evaluate women with unscheduled bleeding by ultrasound alone.
When to sample women on continuous estrogen and progestogen is less clear. Sampling for bleeding occurring during the first 6 months these steroids are administered is rarely necessary. After the initial 6 months, any bleeding warrants biopsy. Biopsy would seem to be indicated at yearly intervals for women who continue to have some bleeding on continuous estrogen and progestogen.
A recent systematic review concluded that irregular bleeding was more than twice as common with a continuous as opposed to a sequential regimen, but with longer duration of treatment, continuous combined therapy was more protective than sequential therapy in preventing endometrial hyperplasia (206). There was also evidence of a higher incidence of hyperplasia under long cycle sequential therapy (progestogen every 3 months) compared to monthly sequential therapy.
REFERENCES
1.Fries HS, Nillus SJ, Petterson F. Epidemiology of secondary amenorrhea: II. A retrospective evaluation of etiology with specia regard to psychogenic factors and weight loss. Am J Obstet Gynecol 1974, 118:473.
2.Bachmann GA, Kemmann E. Prevalence of oligomenorrhea and amenorrhea in a college population. Am J Obstet Gynecol 1982; 144:98-102.
3.Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999;104:936-41.
4.Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril 1990;53:804810.
5.Insler V, Melmed H, Mashiah S, Monselise M, Lunenfeld B, Rabau E. Functional classification of patients selected for gonadotropic therapy. Obstet Gynecol. 1968;32(5):620-6.
5a. Rebar RW. Premature ovarian failure. Obstet Gynecol 2009;113:1355.
6.Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab 1994;79:1470-1475.
7.Block E. Quantitative morphological investigations of the follicular system in women: variations at different ages. Acta Anat 1952; 14:108.
8.Block E. A quantitative morphological investigation of the follicular system in newborn female infants. Acta Anat 1953; 17:201.
8a. Caburet S, Arboleda VA, Llano E, Overbeek PA, Barbero JL, Oka K, et al. Mutant cohesion in premature ovarian failure. N Engl J Med, 2014. 370(10): p. 943-9.
9.Simpson JL and Rajkovic A. Ovarian differentiation and gonadal failure. Am J Med Genet, 1999. 89(4): p. 186-200.
10.Singh RP, Carr DH. The anatomy and histology of XO human embryos and fetuses. Anat Rec 1966;155:369-83.
11.Bione S, Sala C, Manzini C, Arrigo G, Zuffardi O, Banfi S, et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet 1998;62(3):533541.
12.Espiner EA, Veale AM, Sands VE and Fitzgerald PH. Familial syndrome of streak gonads and normal male karyotype in five phenotypic females. N Engl J Med, 1970. 283(1): p. 6-11.
13.Davidoff F and Federman DD. Mixed gonadal dysgenesis. Pediatrics, 1973. 52(5): p. 725-42.
14.Manuel M, Katayama PK and Jones HW, Jr. The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol, 1976. 124(3): p. 293-300.
15.Schellhas HF. Malignant potential of the dysgenetic gonad. Part 1. Obstet Gynecol, 1974. 44(2): p. 289-309.
16.Schellhas HF. Malignant potential of the dysgenetic gonad. II. Obstet Gynecol, 1974. 44(3): p. 455-62.
17.Villanueva AL and Rebar RW. Triple-X syndrome and premature ovarian failure. Obstet Gynecol, 1983. 62(3 Suppl): p. 70s-73s.
18.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: The International Collaborative POF in Fragile X Study preliminary data. Am J Med Genetics 1999;83:322-5.
19.Hagerman RJ, Hagerman PJ. The fragile X premutation: Into the phenotypic fold. Curr Opin Genet Dev 2002;12:278-83.
20.Hagerman RJ, Leavitt BR, Farzin F et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet 2004;74:1051-6.
21.Conway GS, Hettiarachchi S, Murray A, Jacobs PA. Fragile X premutations in familial premature ovarian failure. Lancet 1995;346:309-310.
22.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Hum Genet 2000;97:189-94.
23.Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 2001;27:159-166.
24.De Baere E, Dixon MJ, Small KW, et al. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype-phenotype correlation. Hum Molec Gen 2001;10:1591-1600.
25.Fogli A, Rodriguez D, Eymard-Pierre E et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am J Hum Genet 2003;72:1544-50.
26.Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 2004;75:106-11.
27.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 1990;322:1829-36.
28.Biglieri EG, Herron MA, Brust N. 17-Hydroxylation deficiency in man. J Clin Invest 1966; 45:1946.
29.Goldsmith O, Solomon DH, Horton R. Hypogonadism and mineralocorticoid excess: the 17-hydroxylase deficiency syndrome. N Engl J Med 1967;277:673.
30.Mallin SR. Congenital adrenal hyperplasia secondary to 17-hydroxylase deficiency: two sisters with amenorrhea, hypokalemia, hypertension, and cystic ovaries. Ann Intern Med 1969; 70:69.
31.Rabinovici J, Blankstein J, Goldman B, et al. IN vitro fertilization and primary embryonic cleavage are possible in 17α-hydroxylase deficiency despite extremely low intrafollicular 17β-estradiol. J Clin Endocrinol Metab 1989;68:693.
32.Shozu M, Akasofu K, Harada T, Kubota Y. A new cause of female pseudohermaphroditism: placental aromatase deficiency. J Clin Endocrinol Metab 1991;72:560-6.
33.Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. A syndrome of female hermaphroditism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom). J Clin Endocrinol Metab 1994;78:1287-92.
34.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 1995;80:3689.
35.Kaufman FR, Kogut MD, Donnel GN, et al. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med 1981; 304:994.
36.Aittomaki K, Dieguez Luccena JL, Pakarinen P, et al. Mutation in the follicle- stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 1995;82:959.
37.Aittomaki K, Herva R, Stenman UH, et al. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endocrinol Metab 1996;81:3722.
38.Layman LC, Amede S, Cohen DP, et al. The Finnish follicle-stimulating hormone receptor gene mutation is rare in North American women with 46,XX ovarian failure. Fertil Steril 1998;69:300.
39.Liu JY, Gromoll J, Cedars MI. Identification of allelic variants in the follicle-stimulating hormone receptor genes of females with or without hypergonadotropic amenorrhea. Fertil Steril 1998;70:326.
40.Jones GS, de Moraes-Ruehsen M. A new syndrome of amenorrhea in association with hypergonadotropism and apparently normal ovarian follicular apparatus. Am J Obstet Gynecol 1969; 104:597.
41.Silva de Sa MF, Matthews MJ and Rebar RW. Altered forms of immunoreactive urinary FSH and LH in premature ovarian failure. Infertility, 1988. 11: p. 1-11.
42.Sluss PM and Schneyer AL. Low molecular weight follicle-stimulating hormone receptor binding inhibitor in sera from premature ovarian failure patients. J Clin Endocrinol Metab, 1992. 74(6): p. 1242-6.
43.Verp M. Environmental causes of ovarian failure. Semin Reprod Endocrinol, 1983. 1: p. 101-111.
44.Ash P. The influence of radiation on fertility in man. Br J Radiol, 1980. 53(628): p. 271-8.
45.Bisharah M and Tulandi T. Laparoscopic preservation of ovarian function: an underused procedure. Am J Obstet Gynecol, 2003. 188(2): p. 367-70.
46.Damewood MD, Grochow LB. Prospects for fertility after chemotherapy or radiation for neoplastic disease. Fertil Steril 1986;45:443-59.
47.Green DM, Zevon MA, Lowrie G, Seigelstein N, Hall B. Congenital anomalies in children of patients who received chemotherapy for cancer in childhood and adolescence. N Engl J Med 1991;325:141-6.
48.Betterle C, Volpato M, Pedini B et al. Adrenal cortex autoantibodies and steroid-producing cells autoantibodies in patients with Addison’s desease: comparison of immunofluorescence and immunoprecipitation assays. J Clin Endocrinol Metab 1999;84:618-22.
49.Bakalov VK, Vanderhoof VH, Bondy CA, Nelson LM. Adrenal antibodies detect asymptomatic auto-immune adrenal insufficiency in young women with spontaneous premature ovarian failure. Hum Reprod 2002;17:2096-2100.
50.Chiauzzi V, Cigorraga S, Escobar ME, et al. Inhibition of follicle-stimulating hormone receptor binding by circulating immunoglobulins. J Clin Endocrinol Metab 1982; 54:1221.
51.van Weissenbruch MM, Hoek A, van Vliet-Bleeker I, et al. Evidence for existence of immunoglobulins that block ovarian granulosa cell growth in vitro. A putative role in resistant ovary syndrome. J Clin Endocrinol Metab 1991; 73:360.
52.Rebar RW. The thymus gland and reproduction: do thymic peptides influence reproductive lifespan in females? J Am Geriatr Soc 1982; 30:603.
53.Miller ME, Chatten J. Ovarian changes in ataxia telangiectasia. Acta Paediatr Scand 1967; 56:559.
54.Jacox HW. Recovery following human ovarian irradiation. Radiology 1939; 32:538.
55.Siris ES, Leventhal BG, Vaitukaitis JL. Effects of childhood leukemia and chemotherapy on puberty and reproductive function in girls. N Engl J Med 1976; 294:1143.
56.Koyama H, Wada J, Nishizawa Y, et al. Cyclophosphamide-induced failure and its therapeutic significance in patients with breast cancer. Cancer 1977; 39:1403.
57.Ataya KM, McKenna JA, Weintraub AM, et al. Treatment with LHRH agonist prevents chemotherapy induced follicular loss in rats. In: Abstracts of the 32nd annual meeting of the Society for Gynecologic Investigation, Arizona, 1985; 260.
58.Morrison JC, Givens JR, Wiser WL, Fish SA. Mumps oophoritis: a cause of premature menopause. Fertil Steril 1975; 26:655.
59.Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol 1997;89:777-9.
60.Rebar RW, Cedars MI. Hypergonadotropic amenorrhea. In: Filicori M, Flamigni eds. Ovulation induction. Basic science and clinical advances. Amsterdam: Elsevier Science, 1994:115.
61.Lydic ML, Liu JL, Rebar RW, et al. Success of donor oocyte in in vitro fertilization-embryo transfer in recipients with and without premature ovarian failure. Fertil Steril 1996;65:98.
62.Warren MP. The effects of altered nutritional states, stress, and systemic illness on reproduction in women. In: Vaitukaitis J, ed. Clinical reproductive neuroendocrinology. New York: Elsevier Biomedical, 1981:177.
63.Rebar RW. The reproductive age: chronic anovulation. In: Serra GB, ed. The ovary. New York: Raven Press, 1983:217.
64.Klinefelter HF, Albright F Jr, Griswold GC. Experience with a quantitative test for normal or decreased amounts of follicle-stimulating hormone in the urine in endocrinological diagnosis. J Clin Endocrinol Metab 1943; 3:529.
65.Berga S, Mortola J, Gierton L, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab 1989; 68:301.
65a. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017;102:1413-39.
66.Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91:1561-5.
67.Fairburn CG, Harrison PJ. Eating disorders. Lancet 2003;361:407-16.
68.Mehler PS. Clinical practice. Bulimia disorders. N Engl J Med 2003;349:875-81.
69.Bruch H. Perceptual and conceptual disturbances of anorexia nervosa. Psychosom Med 1962; 24:187.
70.Spitzer R, ed. Diagnostic and statistical manual of mental disorders, ed 4 :American Psychiatric Association, 1994:53.
71.Vigersky RA, Loriaux DL, Andersen MB. Anorexia nervosa: behavioral and hypothalamic aspects. Clin Endocrinol (Oxf ) 1976; 5:517.
72.Vigersky RA, Loriaux DL, Andersen AE, et al. Delayed pituitary hormone response to LRF and TRF in patients with anorexia nervosa and with secondary amenorrhea associated with simple weight loss. J Clin Endocrinol Metab 1976; 43:893.
73.Boyar R, Rosenfeld R, Kapen S, et al. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest 1974; 54:609.
74.Sherman BM, Halmi KA, Zamudio R. LH and FSH response to gonadotropin-releasing hormone in anorexia nervosa: effect of nutritional rehabilitation. J Clin Endocrinol Metab 1975; 41:135.
75.Katz JL, Boyar R, Roffwarg H, et al. Weight and circadian luteinizing hormonesecretory pattern in anorexia nervosa. Psychosom Med 1978; 40:549.
76.Warren MP, Jewelewicz R, Dyrenfurth I, et al. The significance of weight loss in the evaluation of pituitary response to hypothalamic releasing hormones in patients with anorexia nervosa. J Clin Endocrinol Metab 1975; 40:601.
77.Fishman J, Boyar RM, Hellman L. Influence of body weight on estradiol metabolism in young women. J Clin Endocrinol Metab 1975; 41:989.
78.Fries H. Studies on secondary amenorrhea, anorectic behavior, and body-image perception: importance for the early recognition of anorexia nervosa. In: Vigersky RA, ed. Anorexia nervosa. New York: Raven Press, 1977:163.
79.Mecklenberg RS, Loriaux DL, Thompson RH, et al. Hypothalamic dysfunction in patients with anorexia nervosa. Medicine (Baltimore) 1974; 53:147.
80.Warren MP, VandeWiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 1973; 117:435.
81.Boyar RM, Hellman LD, Roffwarg H, et al. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med 1977; 296:190.
82.Casper RC, Chatterton RT Jr, Davis JM. Alterations in serum cortisol and its binding characteristics in anorexia nervosa. J Clin Endocrinol Metab 1979; 49:406.
83.Takahara J, Hosogi H, Yunoki S, et al. Hypothalamic pituitary adrenal function in patients with anorexia nervosa. Endocrinol Jpn 1976; 23:451.
84.Gold PW, Gwirtsman H, Avgerinos PC, et al. Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa: pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med 1986; 314:1335.
85.Vigersky RA, Andersen AE, Thompson RH, Loriaux DL. Hypothalamic dysfunction in secondary amenorrhea associated with simple weight loss. N Engl J Med 1977; 297:1141.
86.Yen SSC, Rebar R, VandenBerg G, Judd H. Hypothalamic amenorrhea and hypogonadotropinism: responses to synthetic LRF. J Clin Endocrinol Metab 1973; 36:811.
87.Van der Walt LA, Wilmsen EN, Jenkins T. Unusual sex hormone patterns among desert-dwelling hunter-gatherers. J Clin Endocrinol Metab 1978; 46:658.
88.Rebar RW, Cumming DC. Reproductive function in women athletes. JAMA 1981; 246:1590.
89.Cumming DC, Rebar RW. Exercise and reproductive function in women: a review. Am J Ind Med 1983; 4:113.
90.American College of Sports Medicine. The female athlete triad: disordered eating, amenorrhea, and osteoporosis. Call to action. Sports Med Bulletin 1992;27:4.
91.Miller KK, Parulekar MS, Schoenfeld E, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab 1998;83:2309-12.
92.Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothatlamic amenorrhea: hypoleptinemia and disordered eating. J Clin Endocrinol Metab 1999;84:873-7.
93.Thong FS, McLean C, Graham TE. Plasma leptin in female athletes: relationship with body fat, reproductive, nutritional, and endocrine factors. J Appl Physiol 2000;88:2037-44.
94.Laughlin GA, Yen SSC. Hypoleptinemia in women athletes: absence of a diurnal rhythm with amenorrhea. J Clin Endocrinol Metab 1997;82:318-21.
95.Bullen BA, Skrinar GS, Beitins IZ, et al. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med 1985; 312:1349.
96.Beitins IZ, McArthur JW, Turnbull BA, et al. Exercise induces two types of human luteal dysfunction: confirmation by urinary free progesterone. J Clin Endocrinol Metab 1991; 72:1350.
97.Rogol AD, Weltman A, Weltman JY, et al. Durability of the reproductive axis in eumenorrheic women during 1 year of endurance training. J Appl Physiol 1992; 72:1571.
98.Drinkwater BL, Nilson K, Chestnut CH III, et al. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med 1984; 311:277.
99.Marcus R, Cann C, Madvig P, et al. Menstrual function and bone mass in elite women distance runners. Ann Intern Med 1985; 102:158.
100.Snead DB, Weltman A, Weltman JY, et al. Reproductive hormones and bone mineral density in women runners. J Appl Physiol 1992; 72:2149.
101.Myerson M, Gutin B, Warren MP, et al. Total body bone density in amenorrheic runners. Obstet Gynecol 1992; 79:973.
102.Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone density is compromised in amenorrheic women despite return of menses. A 2-year study. Obstet Gynecol 1993; 81:669.
103.Schwartz B, Cumming DC, Riordan E, et al. Exercise-associated amenorrhea: a distinct entity? Am J Obstet Gynecol 1981; 141:662.
104.Warren MP. The effects of exercise on pubertal progression and reproductive function in girls. J Clin Endocrinol Metab 1980; 51:1150.
105.Villanueva AL, Schlosser C, Hopper B, et al. Increased cortisol production in women runners. J Clin Endocrinol Metab 1986; 63:126.
106.Loucks AB, Mortola JF, Girton L, Yen SSC. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab 1989; 68:402.
107.Loucks AB, Laughlin GA, Mortola JF, et al. Hypothalamic-pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J Clin Endocrinol Metab 1992; 75:514.
108.Lachelin GCL, Yen SSC. Hypothalamic chronic anovulation. Am J Obstet Gynecol 1978; 130:825.
109.Vaitukaitis J, Becker R, Hansen J, Mecklenburg R. Altered LRF responsiveness in amenorrheic women. J Clin Endocrinol Metab 1974; 39:1005.
110.Rakoff JS, Rigg LA, Yen SSC. The impairment of progesterone-induced pituitary release of prolactin and gonadotropin in patients with hypothalamic chronic anovulation. Am J Obstet Gynecol 1978; 130:807.
111.Suh BY, Liu JH, Berga SL, et al. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J Clin Endocrinol Metab 1988; 66:733.
112.Sachar EJ, Hellman L, Roffwarg H, et al. Disrupted 24 hour patterns of cortisol secretion in psychotic depression. Arch Gen Psychiatry 1973; 28:19.
113.Amsterdam JD, Winokur A, Abelman E, et al. Cosyntropin (ACTH α1-24) stimulation test in depressed patients and healthy subjects. Am J Psychiatry 1983; 140:907.
114.Quigley ME, Sheenah KL, Casper RF, Yen SSC. Evidence for increased dopaminergic and opioid activity in patients with hypothalamic hypogonadotropic amenorrhea. J Clin Endocrinol Metab 1908; 50:949.
115.Ballenger JC, Post RM, Jimerson DC, et al. Biochemical correlates of personality traits in normals: an exploratory study. Personality Individ Differ 1983; 4:615.
116.Brown E, Bain J, Lerner P, Shaul D. Psychological, hormonal, and weight disturbances in functional amenorrhea. Am J Psychiatry 1983; 28:624.
117.Schreiber C, Florin I, Rost W. Psychological correlates of functional secondary amenorrhea. Psychother Psychosom 1983; 39:106.
118.Fava GA, Trombini G, Grandi S, et al. Depression and anxiety associated with secondary amenorrhea. Psychosomatics 1984; 25:905.
119.Liu JH, Yen SSC. The use of gonadotropin-releasing hormone for the induction of ovulation. Clin Obstet Gynecol 1984; 27:975.
120.Martin K, Santoro N, Hall J, et al. Management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab 1990; 71:1081A.
120a.Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavioral therapy. Fertil Steril 2003;80:976.
121.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004;351:987-97
121a. Jayasena CN, Abbara A, Veldhuis JD, Comninos AN, Ratnasabapathy R, De Silva A, et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of kisspeptin-54. J Clin Endocrinol Metab 2014;99:E953-61.
122.Hay PJ, Bacaltchuk J. Psychotherapy for bulimia anorexia and binging (Cochrane Review). In: The Cochrane Library, Issue 2, 2004, Chichester, UK: John Wiley & Sons, Ltd.
122a.Bhangoo A, Jacobson-Dickman E. The genetics of idiopathic hypogonadotropic hypogonadism: unraveling the biology of human sexual development. Pediatr Endocrinol Rev 2009;6:395.
122b. Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 2012(7);366:629-35.
123.Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidism. Am J Ment Defic 1944; 48:203.
124.Odell WD. Isolated deficiencies of anterior pituitary hormones: symptoms and diagnosis. JAMA 1966; 197:1006.
125.Spitz IM, Diamant Y, Rosen E, et al. Isolated gonadotropin deficiency: a heterogeneous syndrome. N Engl J Med 1974; 290:10.
126.Tagatz G, Fialkow PJ, Smith D, Spadoni L. Hypogonadotropic hypogonadism associated with anosmia in the female. N Engl J Med 1970; 283:1326.
127.De Morsier G. Studies in cranio-encephalic dysraphia. I. Agnesia of the olfactory lobe (lateral telencephaloschisis) and of the callous and anterior commissures (median telencephaloschisis); olfacto-genital dysplasia. Schweiz Arch Neurol Psychiatr. 1954; 74(1-2):309-61.
128.Schwanzel-Fukuda M, Pfaff DU. Origin of luteinizing hormone-releasing hormone neurons. Nature 1989; 338:161.
129.Klingmuller D, Dewes W, Krahe T, et al. Magnetic resonance imaging of the brain in patients with anosmia and hypothalamic hypogonadism (Kallmann’s syndrome). J Clin Endocrinol Metab 1987; 65:581.
130.Goldenberg RL, Powell RD, Rosen SW, et al. Ovarian morphology in women with anosmia and hypogonadotropic hypogonadism. Am J Obstet Gynecol 1976; 126:91.
131.Crowley WF Jr, McArthur JW. Simulation of the normal menstrual cycle in Kallman’s syndrome by pulsatile administration of luteinizing hormone-releasing hormone (LHRH). J Clin Endocrinol Metab 1980; 51:173.
132.Yen SSC, Rebar RW, VandenBerg G, et al. Pituitary gonadotropin responsiveness to synthetic LRF in subjects with normal and abnormal hypothalamic-pituitary-gonadal axis. J Reprod Fertil 1973; 20:137.
133.Yeh J, Rebar RW, Liu JH, Yen SSC. Pituitary function in isolated gonadotropin deficiency. Clin Endocrinol 1989; 31:375.
134.Molitch ME, Reichlin S. Hyperprolactinemic disorders. DM 1982; 28:1.
135.Lachelin GCL, Abu-Fadil S, Yen SSC. Functional delineation of hyperprolactinemic-amenorrhea. J Clin Endocrinol Metab 1977; 44:1163.
136.Klibanski A, Neer RM, Beitins IZ, et al. Decreased bone density in hyperprolactinemic women. N Engl J Med 1980; 303:1511.
137.Leblanc H, Lachelin GC, Abu-Fadil S, Yen SSC. Effects of dopamine infusion on pituitary hormone secretion in humans. J Clin Endocrinol Metab 1976; 43:668.
138.Board JA, Storlazzi E, Schneider V. Nocturnal prolactin levels in infertility. Fertil Steril 1983; 36:720.
139.DeVane GW, Gusick DS. Bromocriptine therapy in normoprolactinemic women with unexplained infertility and galactorrhea. Fertil Steril 1986; 46:1026.
140.Suginami H, Hamada K, Yano K, et al. Ovulation induction with bromocriptine in normoprolactinemic anovulatory women. J Clin Endocrinol Metab 1986; 62:899.
141.Sheehan HL, Davis JC. Pituitary necrosis. Br Med Bull 1968; 24:59.
142.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935; 29:181.
142a.Norman RJ, Dewailly D, Legros RS, et al. Polycystic ovary syndrome. Lancet 2007;370:685.
142b.Setji T, Brown A. Polycystic ovary syndrome: diagnosis and treatment. Am J Med 2007;120:128.
143.Rebar RW, Judd HL, Yen SSC, et al. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 1976; 57:1320.
144.DeVane GW, Czekala NM, Judd HL, Yen SSC. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol 1975; 121:496.
145.Buckler HM, McLachlan RI, MacLachlan VB, et al. Serum inhibin levels in polycystic ovary syndrome: basal levels and response to luteinizing hormone-releasing hormone agonist and exogenous gonadotropin administration. J Clin Endocrinol Metab 1988; 66:798.
145a.Dewailly D, Anderson CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014;20:370.
146.Yen SSC. The polycystic ovary. Clin Endocrinol (Oxf ) 1980; 12:177.
147.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR, eds. Polycystic Ovary Syndrome. Boston: Blackwell Scientific, 1992, p. 377.
148.ESHRE/ASRM. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19-25.
149.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41-7.
149a. Final Report. Evidence-based methodology workshop on polycystic ovary syndrome. December 3-5, 2012. https://prevention.nih.gov/docs/programs/pcos/FinalReport.pdf
149b. Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS). Hum Reprod 2012;27:14-24.
149c. Carmina E, Oberfield BE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 2010;203:201-5.
150.Hull MGR. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol 1987;1:235.
151.Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovarian syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078.
152.Franks S, Gharani N, Waterworth D. The genetic basis of polycystic ovary syndrome. Hum Reprod 1997;12:2641.
153.Legro RS, Driscoll D, Strauss JF III, et al. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 1998;95:14956.
154.Taylor SI, Dons RF, Hernandez E, et al. Insulin resistance associated with androgen excess in women with autoantibodies to the insulin receptor. Ann Intern Med 1982; 97:851.
155.Bar RS, Muggeo M, Roth J, et al. Insulin resistance, acanthosis nigricans, and normal insulin receptors in a young woman: evidence for a postreceptor defect. J Clin Endocrinol Metab 1978; 47:620.
156.Erickson GF, Hsueh AJW, Quigley ME, et al. Functional studies of aromatase activity in human granulose cells from normal and polycystic ovaries. J Clin Endocrinol Metab 1979;49:514.
157.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980; 50:113.
158.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in non-obese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983; 57:356.
159.Dunaif A. Insulin resistance ad the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774.
160.Geffner ME, Kaplan SA, Bersch N, et al. Persistence of insulin resistance in polycystic ovarian disease after inhibition of ovarian sex steroid secretion. Fertil Steril 1986; 45:327.
161.Dunaif A, Graf M. Insulin administration alters gonadal steroid metabolism independent of changes in gonadotropin secretion in insulin-resistant women with polycystic ovary syndrome. J Clin Invest 1989; 83:23.
162.Velazquez EM, Acosta A, Mendoza SG. Menstrual cyclicity after metformin therapy in polycystic ovary syndrome. Obstet Gynecol 1997;90:392.
163.Dunaif A, Scott D, Finegood D, et al. The insulin-sensitizing agent troglitazone improves metabolic reproductive abnormalities in the polycystic ovary syndromw. J Clin Endocrinol Metab 1996;81:3299.
164.Ehrmann DA, Schneider DJ, Sobel BE, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic overy syndrome. J Clin Endocrinol Metab. 1997;82:2108.
165.Nestler JE, Jakubowicz DJ, Reamer P, et al. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Eng L Med 1999;340:1314.
166.Luciano AA, Chapler FK, Sherman BM. Hyperprolactinemia in polycystic ovary syndrome. Fertil Steril 1984; 41:719.
167.Elkind-Hirsch KE, Valdes CT, McConnell TG, Malinak LR. Androgen responses to acutely increased endogenous insulin levels in hyperandrogenic and normal cycling women. Fertil Steril 1991; 55:486.
168.Smith S, Ravnikar V, Barbieri RL. Androgen and insulin response to an oral glucose challenge in hyperandrogenic women. Fertil Steril 1987; 48:72.
169.Kiddy DS, Hamilton-Fairley D, Seppala M, et al. Diet-induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-I. Clin Endocrinol 1989; 31:757.
170.Nestler JC, Barlascini CO, Matt DW, et al. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 1989; 68:1027.
171.Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyperandrogenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 1990; 70:699.
172.Dale PO, Tanbo T, Djoseland O, et al. Persistence of hyperinsulinemia in polycystic ovary syndrome after ovarian suppression by gonadotropin-releasing hormone agonist. Acta Endocrinol 1992; 126:132.
173.Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev 1987; 8:132.
174.Bergh C, Carlsson B, Olsson J-H, et al. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril 1993; 59:323.
175.Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 1991; 72:83.
176.Conover CA, Lee PDK, Kanaley JA, et al. Insulin regulation of insulin-like growth factor binding protein-1 in obese and nonobese humans. J Clin Endocrinol Metab 1992; 74:1355.
176a. Legros RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad HM, Pasquali R, Welt CK. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol 2013;98:4565-92.
177.Guzick DS, Wing R, Smith D. Endocrine consequences of weight loss in obese, hyperandrogenic anovulatory women. Fertil Steril 1994;61:598.
178.Anderson P, Selifeflot I, Abdelnoor M, et al. Increased insulin sensitivity and fibinolytic capacity after dietary intervention in obese women with polycystic ovary syndrome. Metabolism 1995;44;611.
179.Jakubowicz DJ, Nestler JE. 17α-Hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome after dietary weight loss. J Clin Endocrinol Metab 1997;82:556.
180.Diamanti-Kandarakis E, Kouli C, Tsianateli T, et al. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. Eur J Endocrinol 1998;138:269.
181.Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type 2 diabetes mellitus. N Engl J Med 1998;338:867.
181a. Legros RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014;371:119-29.
181b. Roque M, Tostes AC, Valle M, et al. Letrozole versus clomiphene citrate in polycystic ovary syndrome:systematic review and meta-analysis. Gyn Endocrinol 2015;31:917.
181c.The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 2008;89:505.
182.Greenblatt E, Casper RF. Endocrine changes after laparoscopic ovarian cautery in polycystic ovarian syndrome. Am J Obstet Gynecol 1987; 156:279.
183.Daniell JF, Miller W. Polycystic ovaries treated by laparoscopic laser vaporization. Fertil Steril 1989; 51:232.
183a. Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007;356:551.
184.Smals AGH, Kloppenborg PWC, Benraad TJ. Plasma testosterone profiles in Cushings syndrome. J Clin Endocrinol Metab 1977; 45:240.
185.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. National Center for Health Statistics. Vital Health Stat 13(139). 1998 pp 1-127.
186.Gambone JC, Reiter RD, Lench JB, Moore JB. The impact of a quality assurance process on the frequency and confirmation rate of hysterectomy. Am J Obstet Gynecol 1990;163:545-50.
187.Lepine LA, Hillis SD, Marchbanks, PA, Kovnim LM, Morrow B, Kieke BA, Wilcox LS. Hysterectomy Surveillance United States, 1980-1993. In: CDC Surveillance Summaries, Aug 8, 1997 MMWR 1997;46(No. SS-4); 1-15.
187a.Woolcock JG, Critchley HO, Munro MG, et al. Review of the confusion in current and historical terminology and definitions for disturbances of menstrual bleeding. Fertil Steril 2008;90:2269.
188.Falcone T, Desjardins C, Bourque J, Granger L, Hemmings R, Quiros E. Dysfunctional uterine bleeding in adolescents. J Reprod Med 1994;39:761-4.
189.Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from National Health Interview Survey, 1984-1992. Am J Public Health 1996;86:195-9.
190.deVries LD, Dijkhuizen FB, Mol BW, Brolmann HA, Moret E, Heintz AP. Comparison of transvaginal sonography, saline infusion sonography, and hysteroscopy in premenopausal women with abnormal uterine bleeding. J Clin Ultrasound 2000; 28:217-23.
191.Widrich T, Bradley LD, Mitchinson AR, Collinc RL. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol 1996;174:1327-34.
192.Saidi, MH, Sadler RK, Theis VD, Akright BD, Farhart SA, Villanueva GR. Comparison of sonography, sonohysterography, and hysteroscopy for evaluation of abnormal uterine bleeding. J Ultrasound Med 1997;16:587-91.
193.Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynceol. 2000;96:913-20.
194.Lethaby A, Augood C, Duckitt K. Nonsteroidal anti-inflammatory drugs for heavy menstrual bleeding (Cochran Review). Cochrane Database Syst Rev. 2002, (1):CD000400.
195.Crossignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, DeGiorgi O. Levonorgestrel-releasing intrauterine devide versus hysteroscopic endometrial resection in the treatment of hysteroscopic dysfunctional uterine bleeding. Obstet Gynecol 1997; 90:257-63.
196.Bonner J, Sheppard BL. Treatment of menorrhagia during menstration: randomized, controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ 1996;313:579-82.
197.Lethaby A, Farquhar C, Cooke I. Antifilrinolytics for heavy menstrual bleeding (Cochran Review). In: Cocharn Database Syst Rev 2000;4:000249.
198.Thomas EJ, Okuda KJ, and Thomas NM. The combination of a depot gonadotrophin releasing hormone agonist and cyclical hormone replacement therapy for dysfunctional uterine bleeding. Br J Obstet Gynaecol 1996;103:18-21.
199.Vercellini P, Oldani S, Yaylayan L, Zaina B, De Giorgi O, Crossignani PG. Randomized comparison of vaporizing electrode and cutting loop for endometrial ablation. Obstet Gynecol 1999;94:521-7.
200.Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders the MISTLETOE study. Minimally invasive surgical techniques - laser, endothermal, or endoresection. Br J ObstetGynecol 1997;102;1351-9.
201.Meyer WR, Walsh BW, Grainger DA, Peacock LM, Loffler FD, Steege JF. Thermal balloon and rollerball ablation to treat menorrhagia: a multicenter comparison. Obstet Gynecol 1998;92:98-103.
202.Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Obstet Gynecol, 2000 Aug; 96(2):266-70.
203.Sowter MC, Singla AA, Lethaby A. Pre-operative thinning agents before hysteroscopic surgery for heavy menstrual bleeding. Cochran Database Syst Rev 2000; (2): CD00124.
204.Lethaby A, Sheppard S, Cooke I, Farquher C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochran Database Syst Rev 2000;(2):CD00329.
205.Aberdeen Endometrial Ablation Trials Group. A randomized trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding outcome at four years. Br J Obstet Gynaecol 1999;106:360-6.
206.Lethaby A, Farquhar C, Sarkis A, Roberts H, Jepson R, Barlow D. Hormone replacement therapy in postmenopausal women: endometrial hyperplasia and irregular bleeding. Cochran Database Syst Rev 2000;(2):CD000402.