Archives
PITUITARY
THYROID
Endometriosis
ABSTRACT
Endometriosis is a disease in which endometrial glands and stroma implant and grow in areas outside the uterus. The disease has a multi-factorial etiology including genetic and environmental factors. Exposure to ovarian hormones appears to be essential. Estrogens stimulate ectopic endometrium growth and aberrations in estrogen signaling have been associated with the disease. Caucasians appear to be more likely to suffer from endometriosis than African Americans or Asians. There is also an increased prevalence in taller women and those with a lower BMI. Genetic factors contribute to approximately 51% of endometriosis risk. Patients with an affected first-degree relative have an approximate 7 to 10-fold increased risk of developing endometriosis.
The prevalence of endometriosis ranges from 2-50% of reproductive aged women. The morbidity associated from endometriosis is great, as it can cause both chronic pelvic pain and infertility. In women with infertility, the prevalence ranges from 20-50%. Endometriosis is present in 71-87% of women with chronic pelvic pain.
In the treatment of infertility associated with endometriosis, the age, history, health status, physical exam, and wishes concerning treatment should be weighed. There are certain situations where surgery may be indicated for the treatment of infertility associated with endometriosis. Controlled ovarian stimulation with IUI or IVF is often indicated.
DEFINITION
Endometriosis is a disease in which endometrial glands and stroma implant and grow in areas outside the uterus (Fig. 1). The most common place to find implants is in the peritoneal cavity (involving the ovary, cul-de-sac, uterosacral, broad and round ligaments, fallopian tubes, colon, and appendix), but endometriosis lesions have occasionally been found in the pleural cavity, liver, kidney, gluteal muscles, bladder, abdominal scars, and even in men (1).
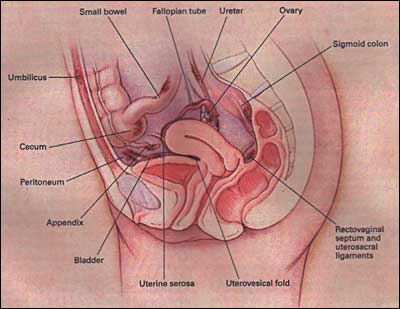
Figure 1.Common locations of endometriosis within the pelvis and abdomen. (Reprinted by permission from the New England Journal of Medicine 2001. Olive DL, Pritts EA. Treatment of Endometriosis. Vol. 345:267).
There are three typical types of endometriotic lesions: 1) superficial peritoneal and ovarian implants, 2) endometriomas (ovarian cysts that are lined with endometrioid mucosa), and 3) deep infiltrating endometriosis (complex nodules comprised of endometriotic tissue, adipose tissue, and fibromuscular tissue) (2,3). The anatomical location and inflammatory response to these lesions are believed to account for the symptoms and signs associated with endometriosis.
ETIOLOGY
Classically, three theories exist to explain the etiology of endometriosis; 1) Sampson’s theory, 2) Meyer’s theory, and 3) Halban’s theory. The most often quoted theory, and to date the one supported by the most evidence, is Sampson’s theory of transplantation and implantation. This theory stemmed from observations made during surgeries in the 1920’s that many women shed endometrial debris through their fallopian tubes into the peritoneum during menstruation (4). Not only has viable endometrial tissue been found in the fallopian tubes and peritoneal fluid of women, but in humans and other animals, endometrial tissue will grow if placed ectopically. Further supporting this theory, endometriosis seems to occur most commonly in the gravitationally dependent parts of the pelvis (5). Finally, the incidence of endometriosis is significantly increased in patients with mullerian anomalies or genital tract obstructions, both which increase the likelihood of retrograde flow (6). One problem with this theory is that retrograde menstruation has been shown in 76-90% of menstruating women, which is much higher than the prevalence of endometriosis (7,8). This discrepancy suggests that additional factors beyond the presence of ectopic tissue are needed to establish the disease, such as the amount of endometrial debris that reaches the peritoneal cavity, the immunocompetency of the woman to clear the debris, and the molecular abnormalities/properties inherent in the ectopic tissue. Meyer’s theory (9) suggests that metaplasia of the coelomic epithelium is the origin of endometriosis. This theory is logical, as cells from both the peritoneum and endometrium are derived from a common embryological precursor: the coelomic cell. However, this has been a difficult theory to support scientifically. If this postulate were correct, one would expect much higher rates of pleural endometriosis than are observed. Halban’s theory is one that suggests that distant lesions are established by the hematogenous or lymphogenous spread of viable endometrial cells. Although this metastatic theory explains rare endometriotic lesions in the brain or lung, it does not explain the gravitationally-dependent location of most foci of endometriosis. Sampson’s transplantation and implantation theory is the most widely accepted, but most researchers agree that it grossly simplifies the disease process. Whilst retrograde menstruation can transplant tissue fragments into the peritoneal cavity, the cells themselves must escape apoptosis (10), adhere to the underlying peritoneum (11,12), degrade the underlying extracellular matrix (13), generate a new vascular supply (14), and evade the immune surveillance system (15) in order to survive. Clear molecular differences exist between endometriotic lesions and eutopic endometrium. Secondary to the inflammation associated with endometriosis, there are increased levels of prostaglandins, chemokines, and cytokines, such as interleukin-1ß, intlerleukins-1, 6, 8 and tumor necrosis factor (TNF), which are thought to enhance the adhesion of endometriotic implants to the peritoneal surface. These are associated with overproduction of prostaglandins, cytokines, and chemokines. Proteolytic membrane metalloproteinases, also increased in endometriosis, promote implantation. Angiogenesis and apoptosis are also altered in endometriosis in favor of survival of the implant. Granulocytes, macrophages, and natural killer cells are attracted by the increased monocyte chemoattractant protein 1, interleukin-8, and RANTES (regulated upon activation normal T-cell expressed and secreted), present in endometriosis. These inflammatory mediators accumulate in endometriotic tissue by autoregulatory positive feedback loops (16). Erythrocyte breakdown from endometriotic lesions is internalized by macrophages and the non-protein-bound catalytic iron increases the production of reactive oxygen species, thereby perpetuating peritoneal damage and inflammation. Studies have found no association between the oxidative stress and antioxidants such as Vitamins A and E (17), but the association between Vitamin D and endometriosis is more complex. Several studies suggest a role for Vitamin D and its metabolites as local autocrine and paracrine agents involved in endometriosis etiology and pathology (18-20), but the exact mechanism has yet to be elucidated (21). While steroidogenic factor 1 (SF1) is present in ectopic endometrial tissue, it is not found in the eutopic endometrium. SF1 is involved in estradiol synthesis. The increased amount of estradiol seen in the peritoneal fluid of patients with endometriosis increases local cyclo-oxygenase -2 (COX-2) activity, resulting in stimulation of prostaglandin E2 formation, which upregulates aromatase activity, resulting in additional estradiol to perpetuate the symptoms and lesions present in endometriosis (16). There is elevated expression of both estrogen receptor α and estrogen receptor ß leading to a downregulation of progesterone receptors (2), ultimately causing the characteristic hormonal profile seen in endometriosis.
DEMOGRAPHY
Endometriosis is diagnosed in women aged 12-80 and the average age at diagnosis is approximately 28. The disease has a multi-factorial etiology including genetic and environmental factors. Exposure to ovarian hormones appears to be essential with the vast majority of the cases found in women aged 20-50. Estrogens stimulate ectopic endometrium growth and aberrations in estrogen signaling have been associated with the disease (2, 22). Caucasians appear to be more likely to suffer from endometriosis than African Americans or Asians (23). There is also an increased prevalence in taller women and those with a lower BMI (24). Genetic factors contribute to approximately 51% of endometriosis risk. Patients with an affected first-degree relative have an approximate 7 to 10-fold increased risk of developing endometriosis (25, 26). Several at risk loci have been identified: WNT4, CDKN2B-AS1, and GREB1 (27,28). WNT4 encodes for gene products that are important for the development of the female reproductive tract and for steroidogenesis. The CDKN2B-AS1 gene is located in an enhancer region and includes a gene cluster that encodes tumor suppressor proteins such as p15, p16-NK4a, and p14ARF. GREB1 encodes an early-response gene in the estrogen receptor regulated pathway (27,28). Risk factors for this disease include nulliparity, early menarche, and frequent or prolonged menses. Protective factors include pregnancy, menopausal status, multiparity, and periods of lactation (29).
PREVALENCE
The prevalence of endometriosis ranges from 2-50% of reproductive aged women. Unfortunately, this number is widely disparate depending on the study. In women with infertility, the prevalence ranges from 20-50% (30-33). This may be due to the fact that endometriosis plays a causative role in infertility, but it also may be due to a diagnostic selection bias, as women with infertility often undergo laparoscopy as part of their clinical evaluation. Endometriosis is present in 71-87% of women with chronic pelvic pain (34-36). Between the years 1965 and 1984, endometriosis increased from 10% to19% as the primary indication for hysterectomy in the USA. Interestingly, this happened during a time in which a trend towards more conservative therapies as treatment modalities for endometriosis occurred (37). This finding suggests a true increase in the incidence of the disease. Varying theories have been proposed to explain this increase, including a delay in childbearing, declining use of oral contraceptives, and exposure to environmental toxins such as dioxin as a causative agent (38). A recent study involving mice has demonstrated that bisphenol A (one of the highest volume chemicals produced worldwide) is able to elicit an endometriosis-like effect in mice who were prenatally exposed (39). Human studies, however, are equivocal (40-42). Other studies have found higher phthalate concentrations in women with endometriosis than those without the disease (40, 42-48).
SYMPTOMS
Pain
The most common symptom for women who have endometriosis is pelvic pain, although the pathophysiologic mechanisms are not well understood and many women with endometriosis may not have this complaint. The pain is most often cyclic, but may also be chronic in nature. The pain usually begins just before menses and continues throughout the duration of menstrual flow. Dysmenorrhea and deep dyspareunia are the most common pain complaints with 80% and 30% prevalence, respectively (49-51). Dysuria, dyschezia, and intermenstural pelvic pain are less common and are associated with bladder or bowel lesions. The pain may also be perceived in musculoskeletal regions, such as the flank, low back, or thighs. The heterogeneity of the disease process makes it difficult to ascertain the exact etiology of the pain. Actively bleeding lesions can certainly cause discomfort. Pain may also be produced by the production of inflammatory mediators and neurologic stimulation. It is likely that different types of lesions cause pain through differing pathways. Early papular lesions contain higher levels of prostaglandins than older lesions. These prostaglandins may activate afferent neuronal pathways. Lesions deeply penetrating the peritoneum also have an increased propensity to cause pain, probably by direct irritation and invasion of pelvic nerves. Endometriomas may cause a mass-effect, which can result in the perception of pain. Adhesions and fibrosis cause pain by compromising the blood supply to certain neuronal plexi, or by placing small nerves on tension (52). In addition to the perturbation of nerve fibers by the lesions, studies have demonstrated that neuronal fibers are present in endometriotic lesions found on the ovaries. A nerve growth factor (a substance important for the maintenance of sensory nerves), S 100 (a neurotrophic factor), and PGP9.5 (an immunoreactive nerve fiber) have all been demonstrated in these lesions, which may contribute to the generation of pain (53). Other studies have shown that there is an increased amount of nerve fibers present in the endometrium in patients with endometriosis (54-57). The significance of these observations remains to be determined, but it has been suggested that the presence of nerve fibers in the endometrium may be used to diagnose endometriosis. With a chronic inflammatory environment in endometriosis, it is theorized that there may be a feedback cycle that promotes nociceptor sensitization with activation and neogenesis of sensory nerve fibers resulting in hyperalgesia (58). Finally, endometriosis can be associated with increased pain perception secondary to abnormal modulation of nociceptive input and an increase in intensity of neuronal signaling to the brain (59-61). Macrophages in the peritoneal fluid in women suffering from endometriosis are increased in concentration and activity (62). As discussed previously, macrophages are part of an inflammatory cascade, which perpetuates the sensation of pain, as does the constant elevated production of prostaglandins.
Infertility
The next most common symptom is that of infertility. Women with moderate and severe endometriosis, particularly those in which the ovaries and oviducts are involved with adhesive disease, have decreased fertility rates. It is theorized that this stems from the mechanical obstruction between the ovary and oviduct, with subsequent failure of gamete transport into the tubal ampulla. Many women with moderate to severe endometriosis have undergone operative management for their disease ; as a result, they may have decreased amounts of functional ovarian tissue and thereby suffer from decreased fecundity. Interestingly, women with only minimal or mild disease may also have decreased fertility when compared to those without clinical evidence of endometriosis. It remains controversial whether endometriosis is the cause of this subfertility. Some studies report that even minimal stage disease is associated with decreased fecundity, while other studies report no effect on fertility and pregnancy outcome (63, 64). The monthly fecundity rate in normal couples is 15-20%, while the monthly fecundity rate in women with untreated endometriosis and infertility is 2-10% (65).
In addition to the anatomic causes of infertility mentioned above, there are two other major theories that may explain the decreased monthly fecundity rates seen in women with endometriosis: 1) inflammation and 2) a locally altered hormonal profile. The altered cellular immunity results in an increased amount of inflammatory mediators, such as activated macrophages, RANTES (regulated on activation, normal T-cell expressed and secreted), interleukins, and tumor necrosis factor in follicular fluid and in peritoneal fluid. This results in a locally hostile, cytotoxic environment, characterized by oxidative stress, that negatively impacts most aspects of reproduction by causing altered folliculogenesis, oocyte degradation, structural DNA damage, decreased spermatozoal integrity, decreased fertilization potential, embryo fragmentation, impaired tubal function, and decreased endometrial receptivity. The altered humoral immunity results in the presence of autoantibodies with the capability of binding to the endometrium and, possibly the oocyte, sperm, embryo, and tube. The presence of elevated levels of steroidogenic factor-1, estrogen, and estrogen receptors in endometriotic cells ultimately leads to a down regulation of local progesterone receptors, and thus, an altered local reproductive hormonal profile (2,16, 66). Altered folliculogenesis has been proposed as one of the contributing factors to infertility. Characteristics of in vitro fertilization cycles in women with endometriosis include a slower follicular growth rate and reduced follicle size. Changes in the cell-cycle kinetics of the granulosa cell that favor an increase in the amount of cells in the S phase and in cells undergoing apoptosis may play a role in this phenomenon. There is a decreased amount of vascular endothelial growth factor present in follicular fluid, which is believed to be responsible for the decreased follicular health and vascularization seen in endometriosis.
Endometriosis has a multifactorial impact on the health and function of spermatozoa. The increased generation of reactive oxygen species seen in women with endometriosis results in the loss of spermatozoal membrane integrity and enzyme inactivation, thereby decreasing the potential for fertilization. Decreased sperm motility, mediated by glycoprotein-130 binding in sperm, is also a result of the negative impact endometriosis has on sperm function. The endosalpinx in women with endometriosis tends to bind spermatozoa more tightly, resulting in a decreased number of available spermatozoa. A decreased ability of spermatozoa to bind to the zona pellucida is evident in patients with endometriosis, resulting in impaired fertilization. Taken together, it is clear that endometriosis has a negative impact on the spermatozoa (16).
There are a few proposed mechanisms by which endometriosis impairs implantation. Explanations include the delayed histologic maturation or the biochemical alterations of the endometrium, both seen in endometriosis. In another mechanism, αvβ 3 integrin, an adhesion factor which is normally increased during the optimum implantation window, is decreased or absent in patients with endometriosis (16). Evidence indicates that the eutopic endometrium of women who suffer from endometriosis is abnormal in its expression of implantation-associated proteins, e.g., complement protein (C3) (67), IL-6 (68), HOX A 10 and 11 (69), and b3 integrins (70). The zona pellucida of the embryo is compromised by the cytotoxic effects of the reactive oxidative species, which may also lead to impaired implantation (16).
Other Symptoms and Associations
Other symptoms of endometriosis can include abnormal menstrual bleeding, diarrhea, constipation, and chronic fatigue. There are elevated rates of autoimmune diseases, including hypothyroidism, rheumatoid arthritis, lupus erythematosus, Sjogren’s syndrome, and multiple sclerosis in patients with endometriosis. Reports of allergies, asthma, chronic fatigue syndrome, and fibromyalgia are also more common in women with endometriosis than in women in the general US population (71).
PHYSICAL EXAM FINDINGS
Unfortunately, due to the diffuse and often varying nature of endometriotic lesions, the physical examination is typically unrevealing. The findings are variable and of limited precision in either localization or diagnosis of endometriosis. However, tender nodules may be palpable along the uterosacral ligaments, rectovaginal septum, or within the cul-de-sac, especially if the examination is performed just before menses. The astute clinician can appreciate pain or induration in the vicinity of otherwise non-palpable lesions, most commonly in the cul-de-sac, along the uterosacral ligaments, or rectovaginal septum. The clinician may also appreciate uterine or adnexal fixation or a tender adnexal mass. Because much disease is found in the dependent areas of the pelvis, it is critical to perform a systematic rectovaginal examination. In that way, the practioner is assured of evaluating the cul-de-sac and uterosacral ligaments as well as the adnexa (72).
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of endometriosis includes pelvic inflammatory disease, tubo-ovarian abscess, ectopic pregnancy, irritable bowel syndrome, interstitial cystitis, adenomyosis, pelvic adhesions, uterine fibroids, chronic or acute endometritis, ovarian neoplasms, musculoskeletal disease, gastrointestinal neoplasms, appendicitis, and diverticular disease.
DIAGNOSTIC MODALITIES
Laparoscopy with visualization of lesions is considered the gold standard for the diagnosis of endometriosis. Dogma states that a biopsy of the lesion is the only way to truly confirm a disease diagnosis. For those surgeons trained in advanced laparoscopy, direct visual recognition of endometriosis also allows treatment to be instituted immediately. Laparoscopy is preferred over laparotomy as it provides visualization of the entire abdomen and pelvis at magnified views, has less morbidity than laparotomy, and carries a decreased risk of adhesion formation (73, 74). One of the major limitations of diagnostic laparoscopy for pelvic pain is the assumption that those lesions seen (or not seen) directly correlate with the symptoms of pain. Attempts to develop noninvasive radiological imaging techniques as diagnostic tools for endometriosis have been compromised by lack of specificity. Computed tomography was used initially to diagnose endometriomas in the setting of adnexal masses. Unfortunately, not only was it difficult to distinguish between benign versus malignant masses, it was often impossible to distinguish adnexal structures and loops of bowel (56, 57). Hence, this modality is of little value for this purpose. Transvaginal ultrasound is considered the first-line imaging modality. The typical ultrasound appearance of an endometrioma is a homogeneously hypoechoic unilocular cystic mass with low-level internal echoes and a ground glass appearance. Occasionally, endometriomas may be multilocular, though they typically have fewer than five locules. The vaginal probe can further be used in the evaluation of endometriosis by helping to determine the mobility of pelvic organs. For example, if the ovary appears to be affixed to the uterus, the probe can be used to apply pressure to the ovary. If the ovary does not move with the applied pressure, one can conclude that there are adhesions preventing such movement (75).
The addition of cystoscopy, colonoscopy/ sigmoidoscopy, renal ultrasound, intravenous pyelogram, or barium enema should be considered if there is cyclic bowel/bladder dysfunction or back pain to rule out ureteral, bladder, or rectal involvement of deep lesions or other malignancy. Because of the broad range of symptoms that are included in endometriosis, a multi-disciplinary approach is often required for diagnosis and management (76-82). At present, magnetic resonance imaging is the best imaging for identifying endometriosis, and it can identify implants as small as 3mm in size (83, 84). It also has been shown to differentiate benign from malignant lesions, with excellent sensitivity and specificity, and is the method of choice for preoperative evaluation of any adnexal mass (85, 86). Because of the large disparity in cost of an MRI versus a transvaginal ultrasound, physicians may resort to using an MRI in cases of ultrasonographically indeterminate pelvic masses (3). Much research has been devoted to discovering a serum marker that will allow diagnosis and monitoring of treatment, to no avail. Monoclonal antibodies raised against a high molecular weight ovarian cancer epithelial cell antigen, CA-125, have been used as a biochemical marker of endometriosis. Moderate and severe endometriosis is associated with elevated levels of CA-125 in the peripheral blood (87). Unfortunately, this marker is relatively non-specific, being increased in ovarian cancer and other gynecologic malignancies (fallopian tube carcinoma, germ cell tumors, adenocarcinoma of the cervix, sertoli-leydig cell tumors), benign gynecologic conditions (benign pelvic neoplasms, adenomyosis, pregnancy, pelvic inflammation), liver disease, colitis, colon cancer, diabetes, congestive heart failure, some autoimmune and rheumatologic disorders, ascites, breast cancer, lung cancer, and even during menstruation of women without any disease. Although it may help define progression of disease in individual patients, its diagnostic utility is limited. Studies have reported an increased density of nerve fibers exist in the eutopic endometrium of patients with endometriosis, but there is controversy as to whether aberrant innervation in the endometrium is a reflective of gynecologic pathology in general (53, 90, 91, 94, 95) or a specific feature of endometriosis (55, 92-97). It was once thought that endometrial biopsies may prove to be useful in the diagnosis of endometriosis, but recent studies demonstrated the presence of neuronal markers in endometrial pipelle biopsies from women both with and without endometriosis (98).
CLINICAL APPEARANCE
Grossly, peritoneal endometriosis can take on many visual appearances. Classically, it was taught that endometriosis implants were blue-black “powder-burns” or “mulberry lesions” of the peritoneum. More recently, several stages of implant development have been appreciated, each with a corresponding appearance. Early, active lesions can appear as papular excrescences or vesicles, and can range in color from clear to pink, or bright red (98). About a third of these lesions are in phase with the eutopic endometrium, and have a tendency to spontaneously grow and regress, suggesting a fluctuation of proliferation in association with the cyclic hormone production during the menstrual cycle (99). Advanced, active lesions are associated with inflammation, fibrosis and hemorrhage, and take on a more classic appearance identifiable at surgery. These lesions can express a myriad of colors, from black to brown, purple, red, or green. These are due to the presence of heme degradation products as the foci undergo hemorrhage and fibrosis. Dormant and healed lesions take on either a white or calcified appearance, and represent remnants of glands embedded in fibrous tissue (98). The surface of peritoneum may also be puckered or contain windows (Allen-Masters windows). A specific manifestation is the endometrioma or chocolate cyst. These ovarian cysts gained their moniker by the characteristic chocolate syrup appearance of their contents often seen at rupture. They arise after implantation of ectopic endometrial tissue and subsequent invasion into the normal ovarian cortex. The cell types present in these cysts include endometrial epithelium, both as glands and flattened cells, endometrial stroma, and hemosiderin-laden macrophages. In some cases, ciliated cells, similar to those of oviduct epithelium, have been observed (56 100). Under scanning electron microscopy, microscopic lesions have been found in the normal appearing peritoneum of women with and without endometriosis (101). The clinical significance of these findings is presently unknown, but the existence and potential tissue activity in these occult lesions may contribute to the recurrence/occurrence of endometriosis or persistence/recurrence of symptoms in women even after successful ablation or excision of visible lesions.
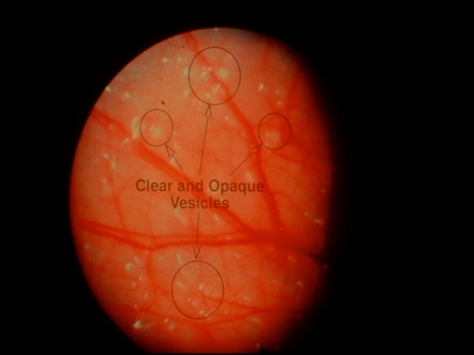
Fig 2a
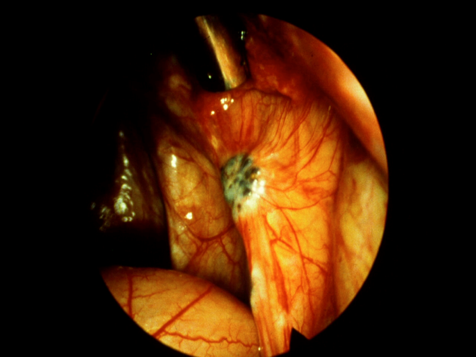
Fig 2b
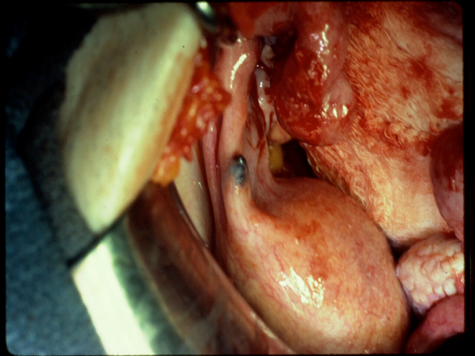
Fig 2c
Figure 2. Intraoperative appearance of endometriosis. (a) The vesicular appearance of early, active lesions. (b) Peritoneal windows. (c) The characteristic blue-black appearance of more advanced, active implants.
HISTOLOGY
Although the histopathologic finding of ectopic endometrial glands and stroma is the sine qua non for establishing the diagnosis of disease, only about 50-70% of presumed endometriosis specimens fulfill these criteria. Many specimens harbor fibrosis, chronic inflammation, and/or hemosiderin-laden macrophages. Most pathologists and clinicians accept these latter findings as highly suggestive of disease status. Eutopic endometrium and endometriotic implants are histologically similar, but as mentioned previously, they are functionally, biochemically, and hormonally different from each other.
CLASSIFICATION
The scheme most widely used to classify the extent of disease is the one derived from the American Society for Reproductive Medicine (ASRM), revised in 1996 (103)(Fig. 3 and 4). This scheme designates disease extent based upon the total 3-dimensional volume of endometriosis. Importance is placed upon the size, depth of invasion, bilaterality, ovarian involvement, extent of cul-de-sac involvement, as well as density of associated adhesions. From this system, point scores are assigned and tallied, with scores of 1-15 representing minimal or mild disease, 16-40 moderate, and >40 severe. It is important to note that this staging system was established to predict fertility outcomes and does not correlate with the more common symptom of pelvic pain. Unfortunately, the classification system is not good at predicting which women will suffer from infertility, as infertility may even occur in women with mild endometriosis. Commonly, practitioners classify endometriosis as minimal (isolated small implants), mild (superficial implants <5cm total, only located on the ovaries and peritoneum, no adhesions), moderate (multiple superficial and invasive implants with or without adhesions), or severe (endometriomas), and the classification system by the ASRM is sometimes reserved for use in studies, where standardization of the classification is important. Use of the ASRM classification system should be encouraged, however, because it best describes the full extent and location of endometriotic lesions. It provides clinicians caring for women with endometriosis with a detailed description of the location and extend of disease.
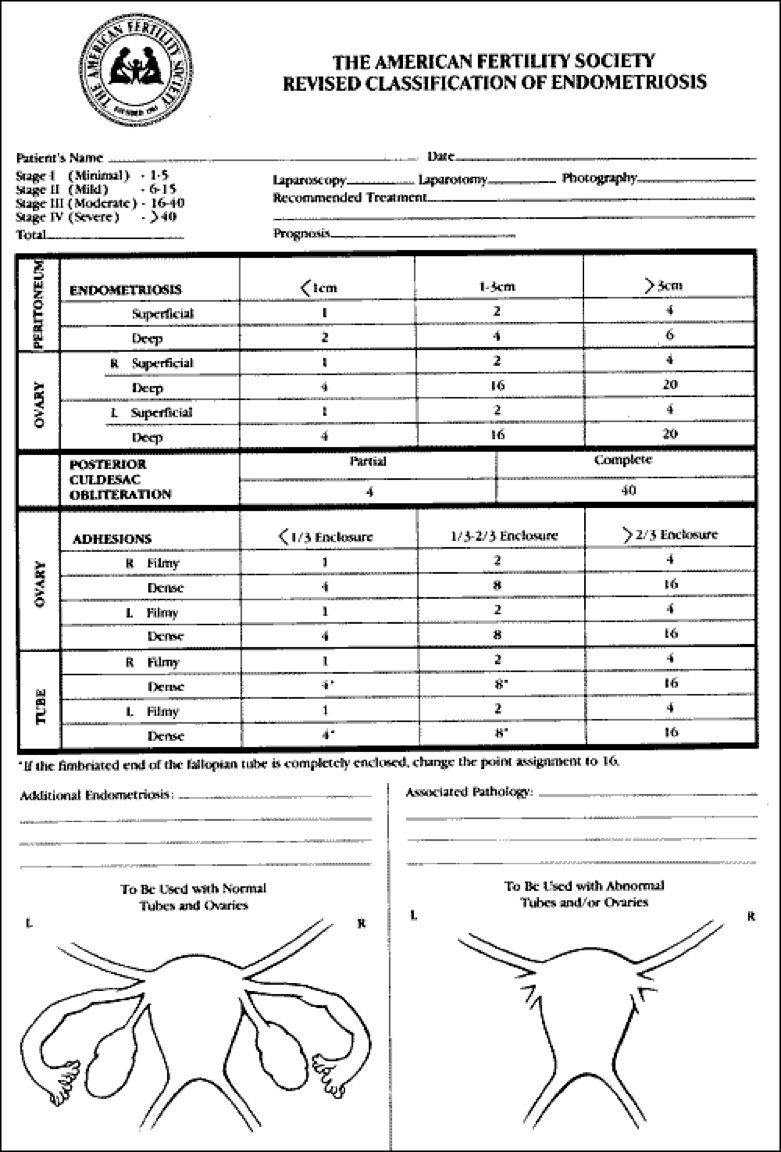
Figure 3. The American Fertility Society Revised Classification of Endometriosis. (Reprinted by permission from Fertil Steril 1985;43:351).
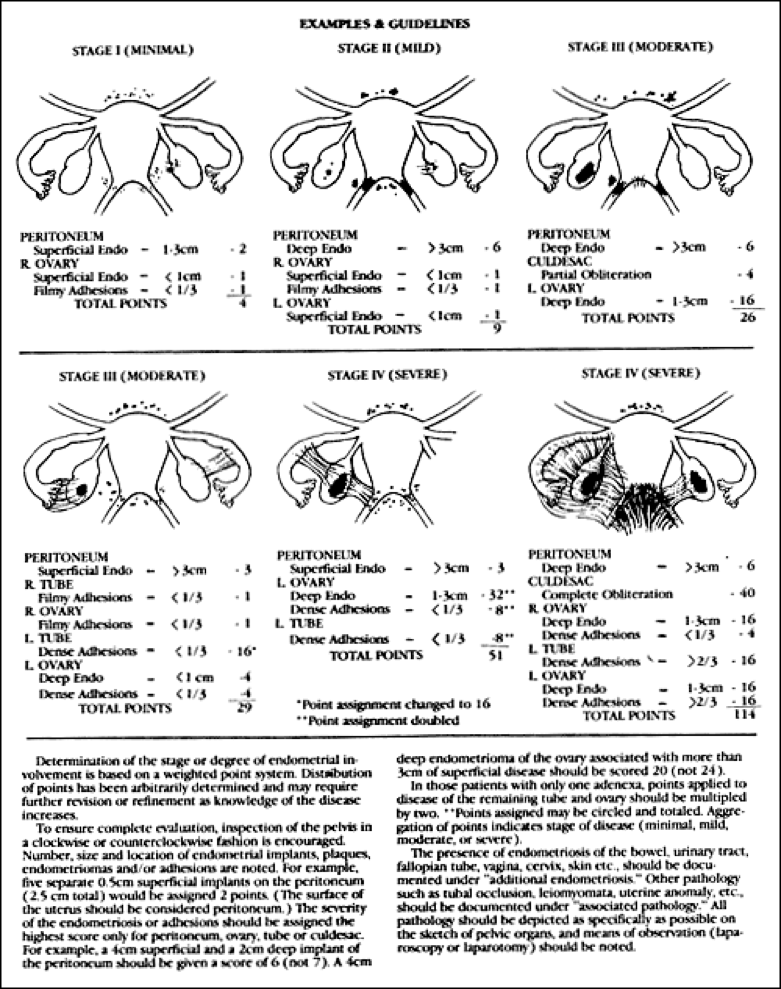
Figure 4. Examples and guidelines for use of the American Fertility Society Revised Classification of Endometriosis. (Reprinted by permission from Fertil Steril 1985;43:352).
TREATMENT
Treatment for endometriosis differs depending on the symptom being targeted and whether or not the patient is trying to become pregnant in the near future . Treatment is either aimed at pain reduction, fertility restoration, or evaluation and treatment of a pelvic mass. Unfortunately, there are few treatment options for those who desire both fertility in the near future and the treatment of pelvic pain. However, sometimes pregnancy will temporarily relieve the pain associated with endometriosis.
Pain
Pain is the most commonly reported symptom of endometriosis, an estrogen-dependent condition characterized by chronic peritoneal and pelvic inflammation. Since endometriosis is a chronic disease, it would be most beneficial to use agents that can be safely used long-term. Dysmenorrhea is one of the most common complaints in women with endometriosis, so many of the hormonal agents aim to cause amenorrhea. These treatments may also relieve deep dyspareunia, non-cyclic pelvic pain, and dyschezia. Hormonal agents may have an additional effect on reducing the nerve fiber density present in endometriotic lesions, which is believed to be one of the factors involved in the origin of the pain caused by endometriosis (104). Oral contraceptives, progestins, danazol, gestrinone, medroxyprogesterone acetate, and GnRH agonists are all supported by clinical trials showing approximately equal benefit over placebo (Fig. 5). These hormonal agents create a hypoestrogenic (GnRH agonist), hyperandrogenic (danazol, gestrinone) or hyperprogestogenic (oral contraceptives, medroxyprogesterone acetate) state that suppresses endometrial cell proliferation. Their side-effect profiles and costs lead one agent to be preferred over another. However, once the agent is discontinued, the symptoms tend to recur. A definitive diagnosis of endometriosis can only be made with surgery. If there is sufficient clinical suspicion of endometriosis, it is reasonable to try empiric therapy with a single agent or a combination of agents. Oftentimes, the pain will improve and surgery can be avoided.
The combined oral contraceptive pill (COCP) has been used for the treatment of endometriosis-associated pain for several years. Its efficacy in reducing symptoms of pain is well-established and works by decreasing retrograde menstruation, inducing a pseudo-pregnant state and causing decidualization and subsequent atrophy of eutopic and ectopic endometrium. Advantages include a mild side-effect profile, several combinations from which to choose, and the choice of cyclic or continuous administration. However, not all women are candidates for the use of COCPs. COCPs are FDA approved for contraception, but they are used off-label for the treatment of endometriosis-associated pain.
There are many progestins (synthetic derivatives of progesterone) that have been used to treat endometriosis-associated pain. Examples include norethindrone (norethisterone), medroxyprogesterone, and levonorgestrel. There are several routes of administration. They do not contain estrogen; thus, they are believed to be safer for use in women who have a contraindication to estrogen. Norethindrone acetate is a progestin that is taken orally and is initiated at the dose of 5 mg/day and can be increased by increments of 2.5 mg to achieve amenorrhea or to a total dose of 20 mg/day. Side effects include break-through bleeding and breast tenderness. Favorable effects on bone-mineral density (short term) and lipid metabolism have been reported (105). Norethindrone acetate is FDA approved for the treatment of endometriosis-associated pain. Medroxyprogesterone, a 17-hydroxy derivative of progesterone, taken orally, has moderate androgenic activity and minor effects on the lipid profile. The dose may range from 15-50 mg/day. Side effects may include breakthrough bleeding and depression/anxiety. The intramuscular dose or subcutaneous dose of depot medroxyprogesterone acetate is 150 mg given every 3 months. There is concern about the decrease in bone mineral density with long-term use of medroxyprogesterone. However, studies have shown that after discontinuation of this medication, the bone mineral density profile improves. It takes an average of 7 months for menses to return. The abnormal bleeding and lipid profiles are still concerns for long-term use (105). Medroxyprogesterone is FDA approved for the treatment of endometriosis-associated pain.
The levonorgestrel-containing intrauterine device releases 20 µg/day and induces amenorrhea by causing the endometrium to become atrophic and inactive. It has been shown to improve dysmenorrhea, relieve deep dyspareunia, and, as expected, reduce monthly blood loss. Approximately one third of users will develop amenorrhea. Reasons for discontinuation include irregular bleeding, pelvic pain, breast tenderness, and weight gain. There is a 5% expulsion rate and a 1.5% infection rate associated with use of the intrauterine device. Advantages of this system are that it doesn’t induce a systemic hypoestrogenic state and it is effective, without any further medical intervention, for five years. It is currently being evaluated for postoperative use in women who undergo laparoscopy for endometriosis (105). It is not FDA approved for the treatment of endometriosis-associated pain, but it is approved for the treatment of heavy menstrual bleeding.
For the relief of pain caused by the deep infiltrative type of endometriosis, a combination of different types of agents may be of benefit. A study with continuous oral ethinyl estradiol 0.01 mg/day with cyproterone acetate 3 mg/day or only norethisterone 2.5 mg/day demonstrated that both regimens substantially decreased dysmenorrhea, non-cyclic pelvic pain, deep dyspareunia, and dyschezia, but both caused slight weight gain and undesirable changes in lipid profiles. The group treated by norethisterone reported slightly better symptom improvement but also registered additional androgenic side effects (105).
GnRH agonists have been shown to decrease dysmenorrhea, dyspareunia, and non-cyclic pelvic pain by creating a hypoestrogenic environment. Some agonists are given subcutaneously either once a month at a dose of 3.75 mg or once every three months at a dose of 11.25 mg. The side effects of GnRH agonists include a reduction in bone mineral density and, therefore are not recommended for periods longer than 6 months. Other side effects include hot flushes, emotional lability, vaginal dryness, insomnia, and loss of libido. “Add-back therapy” with a low dose estrogen/progestin combination has been introduced to prevent the loss of bone mineral density and control the other side effects resulting from the hypoestrogenic environment, while continuing to control the symptoms of endometriosis (105). GnRH agonists are FDA approved for the treatment of endometriosis-associated pain.
GnRH antagonists are newer compounds and avoid the undesirable “flare” caused by the agonists. Unfortunately, they are not as well studied and must be administered subcutaneously at least once a week at a dose of 3 mg (105). More recent studies have looked at oral formations of GnRH antagonists (106). These show promising results in terms of reduced dysmenorrhea and non-menstrual pelvic pain. However, much is still unknown regarding reversibility of adverse outcomes such as decreased bone mineral density and altered lipid profiles. Thus far, it appears that oral GnRH antagonists have less complete suppression of the hypothalamic-pituitary-ovarian axis compared to GnRH agonists. It may be inferred that hypoestrogenic side effects may be less with GnRH antagonists compared to GnRH agonists; however, studies showed incomplete suppression of ovulation, and pregnancy may still occur with the use of GnRH antagonists (106). Clinical trials are on-going, but currently GnRH antagonists are not FDA approved for the treatment of endometriosis-associated pain.
Danazol, an oral agent, induces an amenorrheic state by suppressing the hypothalamic-pituitary-ovarian axis, and is characterized by hyperandrogenemia and hypoestrogenemia. The hormonal cyclicity of the menstrual cycle is interrupted, thereby disrupting steroidogenesis and estrogen production from the ovary that leads to the undesirable painful symptoms in endometriosis. Its use is losing favor because of its undesirable side effects. Weight gain, fluid retention, breast atrophy, acne, oily skin, hot flushes, hirsutism, and unfavorable changes in the lipid profile are among the side effects of danazol. Alternative routes of delivery may result in a beneficial relief of symptoms, while significantly reducing the side effect profile. Studies have looked at a danazol-releasing intrauterine device, vaginal ring, or vaginal capsules, but these preparations are not available at this time (105). Danazol is FDA approved for the treatment of endometriosis-associated pain.
Gestrinone, an oral agent, is a 19-norsteroid derivative that was originally designed as an oral contraceptive. It has the ability to block follicular development and estradiol production by causing both agonist and antagonist effects when bound to progesterone receptors. Its ability to bind to androgen receptors, however, is responsible for its undesirable side effects: an unfavorable lipid profile, weight gain, hirsutism, seborrhea, and acne. Like danazol, this medication is not frequently used (105). Gestrinone is available in many countries for the treatment of endometriosis-associated pain, but is not approved for use in the US.
Aromatase inhibitors have recently become part of the armamentarium against endometriosis-associated pain. Aromatase is the enzyme responsible for the conversion of androgens into estrogens, and is normally expressed in granulosa cells, skin fibroblasts, adipocytes, and syncytiotrophoblasts. Although, steroidogenic factor-1 is found in endometriotic tissue, it is not found in eutopic endometrium. Steroidogenic factor-1 activates aromatase gene transcription, thereby increasing the production of estrogen. Because endometriosis is estrogen-dependent, and aromatase is responsible for estrogen production, aromatase inhibitors have therefore been employed to alleviate the painful symptoms caused by endometriosis. Combination therapy of an aromatase inhibitor with a progestin, oral contraceptive agent, or GnRH agonist is recommended in the treatment of endometriosis in pre-menopausal women because of their ovarian stimulatory properties in this population and to prevent pregnancy. Aromatase inhibitors have a tolerable side-effect profile and don’t reduce bone mineral density (105). Aromatase inhibitors are not FDA approved for the treatment of endometriosis-associated pain, but clinical trials are ongoing.
Historically, there has been a well-established role for prostaglandin inhibitors, such as ibuprofen, in the treatment of endometriosis-associated pain. Recently, a Cochrane Review evaluated non-steroidal anti-inflammatory use for the treatment of endometriosis (107). It discovered two studies that met their inclusion criteria. They found inconclusive evidence to show that non-steroidal anti-inflammatory agents are effective for the treatment of pain associated with endometriosis. Despite this review, prostaglandin inhibitors are relatively safe, have a tolerable side-effect profile, and can generally be taken on a long-term basis by most patients, so they remain part of the first-line therapy for the treatment of endometriosis-associated pain. However, their use is associated with undesirable and potentially severe gastrointestinal side-effects that may cause them not to be candidates for use in every patient. Motrin (ibuprofen), a commonly used prostaglandin inhibitor is FDA approved for the treatment of endometriosis-associated pain.
| Figure 5: Options for the Treatment of Pain Associated with Endometriosis | |||||||||||||||||||||
| Agent | Route | Side-Effects | |||||||||||||||||||
| Oral Contraceptive Agents | Oral | Mild nausea, vomiting | |||||||||||||||||||
| Progestins | Oral, Injection, or Intrauterine | Breakthrough bleeding, breast tenderness | |||||||||||||||||||
| Some have unfavorable effects on bone mineral density and lipid profile | |||||||||||||||||||||
| Some have androgenic side-effects | |||||||||||||||||||||
| GnRH Agonists | Injection or Intranasal | Symptoms of a hypoestrogenic state | |||||||||||||||||||
| (hot flushes, mood irritability, vaginal dryness, sleep disturbances, and decreased bone mineral density) | |||||||||||||||||||||
| GnRH Antagonists* | Oral | Symptoms of hypoestrogenic state | |||||||||||||||||||
| (hot flushes, decreased bone mineral density, unfavorable changes in the lipid profile) | |||||||||||||||||||||
| Aromatase Inhibitors | Oral | Ovarian stimulation in pre-menopausal women | |||||||||||||||||||
| Danazol | Oral | Weight gain, fluid retention, breast atrophy, acne, oily skin, hot flushes, hirsutism | |||||||||||||||||||
| Unfavorable changes in the lipid profile | |||||||||||||||||||||
| Gestrinone | Oral | Unfavorable changes in the lipid profile | |||||||||||||||||||
| Weight gain, hirsuitism, seborrhea, and acne | |||||||||||||||||||||
| Prostaglandin Inhibitors | Oral | Unfavorable gastrointestinal side-effects | |||||||||||||||||||
Figure 5. Pharmacologic options in the treatment of pain associated with endometriosis.
*GnRH antagonists are not yet FDA approved, but may be a potential future treatment.
As previously mentioned, endometriosis is associated with a peritoneal and pelvic inflammatory cascade, and anti-inflammatory compounds may alleviate the pain associated with endometriosis. Since the cyclo-oxygenase-2 pathway is upregulated, COX-2 inhibitors may have a role in treatment, but their main role in treatment of this disease is not well established. Other emerging therapies have targeted the molecular steps involved in endometriosis pathogenesis. This includes agents that enhance cell-mediated immunity, agents that counteract tumor necrosis factor-α (TNF-α), anti-angiogenic agents, metalloproteinase inhibitors, hypocholesterolemic agents, and selective progesterone modulators. A Cochrane Review examined the use of TNF-α inhibitors for the treatment of endometriosis-associated pain and found no benefit. (108). Several antiangiogenic agents are in preclinical testing and show promise. Statin medications are also being evaluated as well as trichostatin A and valproic acid (109). These agents are not FDA approved for the treatment of endometriosis-associated pain.
From the available evidence, it is clear that medical treatment is effective for endometriosis-associated pelvic pain. In general, a non-steroidal anti-inflammatory agent alone or in combination with an oral contraceptive agent or a progestin derivative should be considered as first-line therapy. GnRH analogues with add-back therapy and possibly aromatase inhibitors (with oral contraceptive agents, progestins, or a GnRH analog in premenopausal patients), should be regarded as second-line agents. Danazol and gestrinone should be reserved for cases that have failed other medical treatments. If a patient continues to have symptoms of pain despite medical therapy, or needs pain relief but desires pregnancy and thus must avoid hormonal compounds that interfere with ovulation, then conservative surgery should be performed with resection or ablation of lesions and lysis of adhesions. In double-blind randomized control trials, laparoscopic laser treatment of pelvic pain associated with minimal-moderate endometriosis was found to decrease pain significantly(109, 111). Follow up re-operation rates after initial surgical removal of lesions was 21%, 47%, and 55% at 2, 5, and 7 years of follow-up, respectively (112). The highest predictor of re-operation was associated with younger age of the patient. The evidence shows that both ablation and resection of lesions are equally effective techniques. Excision of ovarian endometriomas, however, is associated with better pain relief, lower recurrence, and higher pregnancy rates than cyst vaporization or coagulation (113). There is no evidence supporting the performance of a uterosacral nerve ablation; however, if there is significant midline pain, presacral neurectomy may be of benefit (114). It should be noted that presacral neurectomy requires excellent surgical skills, as there is significant risk of damaging the neurovascular plexus or causing retroperitoneal bleeding severe enough to require transfusion or re-operation. Resection of rectovaginal lesions had an approximate 10% complication rate especially when a colorectal resection is performed (115, 116). Good pain relief is usually achieved during the first year after bowel resection for deep endometriosis, but in a systematic literature review, pain recurrence was observed in one out of four patients and re-intervention was required in one out of five of these recurrences (117). A debatable issue is the use of medical therapy, such as a GnRH agonist or medroxyprogesterone acetate, as a neoadjuvant or adjuvant to surgical management. Preoperative medical therapy may be helpful to decrease the pelvic vascularity and size of the endometriotic implants, reducing intraoperative blood loss and surgical resection required. However, by reducing the endometriotic load, the disease may be understaged, which may affect management. Postoperative medical therapy may eradicate the residual implants. A Cochrane Review, however, found insufficient evidence to show a benefit of hormonal suppression either before or after surgery when compared with surgery alone for long-term difference in pain relief from endometriosis (118). There were two trials that compared pre-surgical medical therapy with surgery alone, but American Fertility Society scores were significantly improved in the medical treatment group in one study and not in the other. Post-surgical hormonal suppression of endometriosis versus surgery alone (either no medical therapy or placebo) showed a modest reduction in pain after one year, but results were inconsistent and pain recurrence in both groups indicated no benefit beyond one year after treatment (119). Further, there was no evidence that medical therapy pre- or post-surgery improves pregnancy rates. There have been no trials comparing medical and surgical treatment to reduce endometriosis-associated pain. The studies employing each treatment modality, though, have similar success rates. With this in mind, it is reasonable to conclude that the treatments are equally effective. With the development of newer medical therapies with better tolerated side-effect profiles, surgery may be avoided or delayed. Ultimately, in women who no longer desire future childbearing, hysterectomy with bilateral salpingo-oophorectomy, often is considered as definitive therapy for the treatment. Narcotics should never be considered for treatment of the chronic pain associated with endometriosis. Referral to a multidisciplinary pain center may be of value. In the absence of other factors, if the pain continues to be present after surgery, a diagnosis of adenomyosis should be considered.
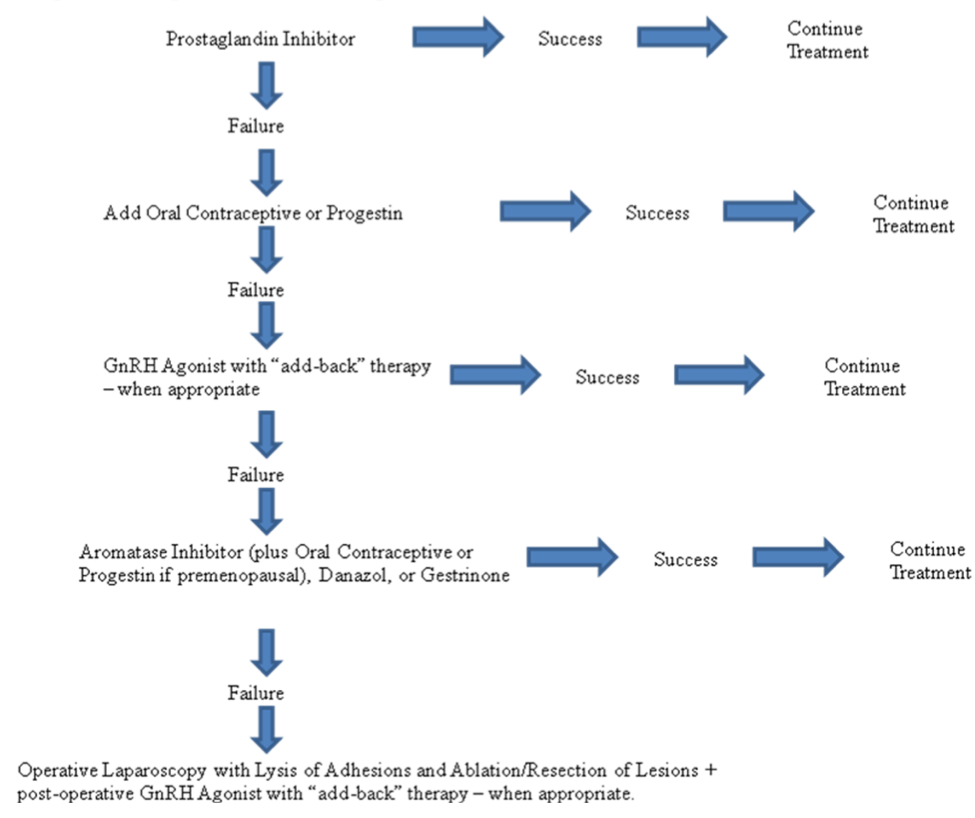
Figure 6. Treatment algorithm for pain associated with endometriosis
Infertility
The treatment of infertility, in the absence of pain, may involve expectant management, surgery, ovulation induction/stimulation with intrauterine insemination (IUI), or assisted reproductive techniques, such as in vitro fertilization (IVF). Infertility clinics differ in their use of diagnostic laparoscopy in the evaluation of the etiology of infertility, at which time asymptomatic endometriosis may be diagnosed and excised or ablated. Physicians that do not perform diagnostic laparoscopy as part of their evaluation for infertility would argue that there are significant anesthetic and surgical risks afforded by laparoscopy, ovarian reserve may be compromised by the incidental removal/destruction of normal ovarian tissue, surgery is a cause of adhesions, and that the practice of restoring normal tubal anatomy has fallen out of favor as the success of IVF continues to rise. In women with stage I/II endometriosis, laparoscopic ablation of lesions may offer a small, but significant improvement in live birth rates. A Cochrane Review evaluated two trials in which women were randomized to operative laparoscopy or diagnostic laparoscopy (120). The two randomized trials addressed the question of whether laparoscopic surgery improved outcomes in patients with otherwise unexplained infertility (121, 122). When combining live birth rates and clinical on-going pregnancy rates after 20 weeks, the meta-analysis demonstrated an advantage of laparoscopic surgery when compared to diagnostic laparoscopy alone, with an odds-ratio (OR) of 1.64 [95% Confidence Interval (CI) 1.05-2.57]. However, the two studies had incompatible results, which should be taken into account in this interpretation. One study (121) reported a large positive effect, while the other (122) reported a small negative effect. The number needed to treat, resulting from this analysis, was 12 laparoscopies for one additional pregnancy. The decision to perform surgery should, therefore, be balanced with the patient’s age, history, health status, and wishes, and should be entered into with informed consent about the risks and possible benefits. For those that do undergo surgery, the Endometriosis Fertility Index (EFI), an intraoperative staging system, can be used to help predict future pregnancy rates. Based on the EFI, a patient may be better counseled regarding her likelihood of spontaneous conception and, if her prognosis is poor, more readily seek appropriate treatment (123). Suppressive medical therapy (such as in the treatment for pain) should not accompany surgery or be involved in the treatment of infertility, as it does not improve fertility and may increase the time to pregnancy. Viable options for the medical treatment of endometriosis-related infertility are discussed. Ovulation induction in women with stage I/II endometriosis confers a fertility benefit. The three randomized trials in the literature addressing this issue, employing either GnRH agonists with follicle-stimulating hormone and luteinizing hormone, clomiphene citrate/IUI, or follicle-stimulating hormone/IUI, all showed increased pregnancy rates relative to the control arm, where the patient did not receive treatment (114). Thus, both ovulation induction plus IUI and assisted reproductive technology have a place in the treatment of women with endometriosis-associated infertility.
If the patient is under the age of 35, initial treatment options include expectant management or controlled ovarian stimulation with clomiphene citrate or gonadotropins combined with IUI. In a woman over the age of 35, because of the age-associated decrease in fecundity, controlled ovarian stimulation combined with intrauterine insemination or IVF are reasonable therapies. In women with stage III/IV endometriosis, surgery is likely to be of benefit and is recommended by the ASRM Practice Committee, but there are no randomized controlled trials to document efficacy and some studies show a negative effect of surgery on pregnancy rates. (65, 124). Advanced staged disease is often associated with a complex surgical history which, combined with an extensive amount of disease, causes the operation to be difficult, sometimes requiring advanced laparoscopic techniques or laparotomy. These higher acuity surgeries have increased risk of serious complications. In situations when the initial surgery fails to restore fertility, IVF, rather than another operation for infertility, is likely a better option. However, randomized controlled trials are lacking. If surgery is performed, then the subsequent treatment for infertility should be controlled ovarian stimulation (with gonadotropins) and IUI, or IVF, especially in women of advanced reproductive age (66). If the plan is to go directly to IVF, then surgery may not show a benefit, as the slight possible benefit afforded by the surgery is greatly overshadowed by the benefits of IVF and indirect evidence suggests that surgery may not be beneficial in women with deep infiltrating disease (125). There has been some debate about the management of endometriomas in stage IV disease in the absence of pain. Only case series have been published, but the overall likelihood of pregnancy following endometrioma excision is around 50% (125); though this may be an overestimate with women achieving pregnancy through IVF.
The question then remains, should the endometriomas be removed prior to IVF? A meta-analysis evaluated five studies that compared surgery versus no treatment for an endometrioma (121). In this analysis, there were no differences in the pregnancy rates or responses to controlled ovarian stimulation in the two groups, favoring against surgical resection. There are no randomized controlled trials that evaluate this question. Surgery for endometriomas should be performed to relieve pain and to confirm the diagnosis in situations when it is in question. Overall, endometriosis is a common indication for IVF. IVF allows the abnormal anatomy to be overcome by avoiding the tubes; the oocyte is retrieved directly from the ovaries, and the embryos are placed directly in the endometrial cavity, thereby bypassing the effects of the abnormal peritoneal environment. Despite this, endometriosis is associated with overall lower chances of success. This may be due to the negative impact of endometriosis on folliculogenesis and endometrial receptivity (126). A meta-analysis demonstrated significantly lower pregnancy rates for women with endometriosis compared to women with tubal factor infertility controls. Pregnancy rates were also significantly lower for women with severe endometriosis versus women with milder disease (127). However, the difference in pregnancy rates does not appear to be attributable to differing aneuploidy rates. A recent retrospective cohort study demonstrated no difference in aneuploidy rates between age-matched controls of women with endometriosis undergoing IVF compared to those without endometriosis (128).
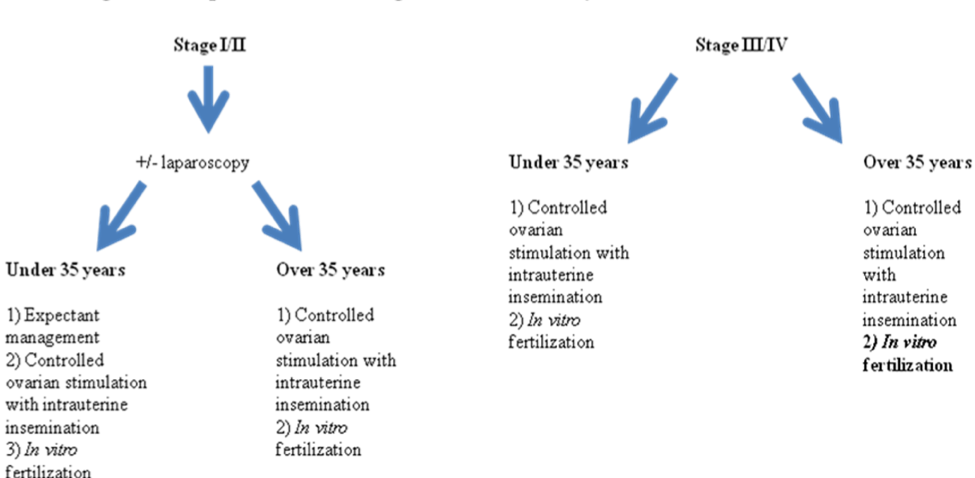
Figure 7. Treatment algorithm for infertility associated with endometriosis.
Other Therapeutic Modalities
There have been many studies exploring complementary alternative medicine (CAM) for the treatment of symptoms associated with endometriosis. Although these approaches are not FDA or USDA approved for the treatment of endometriosis, they may offer relief by suppressing cytokines and inflammatory pathways, inhibiting cyclooxygenase-2 (COX-2) pathways, acting as antioxidants, and alleviating pain by other mechanisms (129). High frequency transcutaneous electrical nerve stimulation, acupuncture, vitamin B1, magnesium, reflexology, traditional Chinese medicine, herbal treatments, and homeopathy are examples of CAM that have been explored for use in endometriosis-associated pain. Even though the risks associated with some of these modalities appear minimal, the standards of evidence-based medicine on aspects of safety and efficacy have not been applied to most studies involving CAM. A promising note for CAM is that there is now a National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (NIH). Hopefully, this will allow for proper evidence-based evaluation, so that their safety and efficacy can be compared with the more traditional approaches (129). Another therapeutic modality that may be considered is patient self-help and support groups. Information can be found at www.endometriosis.org (76).
ENDOMETRIOSIS-ASSOCIATED OVARIAN CANCER
Although it is not considered a pre-malignant condition, there are data to suggest that endometriosis has neoplastic potential (130). Endometriosis shares several similarities with malignant diseases such as reduced apoptosis, invasion of endometrial cells into adjacent organs (bowel, bladder), increased angiogenesis, ability to spread distantly, etc. (131-133). Women with endometriosis have twice the risk of developing epithelial ovarian cancer compared to controls, and a 4-fold increased risk if they also have subfertility (134). The association is limited to clear cell (OR 3.05, 95% CI 2.43-3.84), endometroid (OR 2.04, 95% CI 1.67-2.48), and low-grade serous (OR 2.11, 95% CI 1.39-3.2) tumors (135). The carcinogenic pathways, however, poorly understood. There is thought that oxidative stress, inflammation and the estrogen dependent environment may play a role (136). In women without malignant appearing features on ultrasound, 1-3% are found to have atypical endometriotic cells (125). Generally, endometriosis associated malignancies arise from endometriomas. Reactive oxygen species generated by catalytic iron from phagocytosed heme causes cell damage, DNA mutations, and genetic instability may be aided by the local ovarian environment of endometriomas (137-139). This oxidative stress is thought to activate proto-oncogenes or disrupt tumor suppressor genes, such as ARID1A (140-142). Clear cell and endometroid tumors also develop due to hormonally dependent and independent pathways. Endometroid cell types are often estrogen receptor (ER) and progesterone (PR) receptor positive. Clear cell tumors, however, generally have low receptor expression (143). Endometroid histology is also thought to be estrogen-dependent based on the fact that there is a higher than expected rate of synchronous primary, estrogen-dependent endometrial endometroid carcinoma (144) concurrently with endometriosis-associated ovarian endometroid carcinoma. (145-147). Clear cell carcinomas overexpress hepatocyte nuclear factor 1ß, which is a transcriptional factor that increases survival of endometrial cells during oxidative stress by inhibiting apoptosis, whereas endometroid cell types do not overexpress hepatocyte nuclear factor 1ß (137). Knowing the increased risk of carcinoma in endometriosis patients, the question remains as to how to prevent progression. There are limited data available regarding medical and surgical interventions for prevention. It is known that oral contraceptives are associated with an 80% reduction in risk among women with endometriosis when used for greater than 10 years. Since endometroid subtypes are ER and PR positive, whereas clear cell subtypes are not, those at risk for the endometroid subtype are more likely to benefit from hormonal therapy while those more likely to develop the clear cell subtype would benefit from surgery. In Western countries the endometroid subtype is more prevalent than clear cell, 10-20% versus 5-10%, respectively. However, the opposite is true in Asian countries. This could suggest a population based treatment approach (144-146).
CONCLUSION
Endometriosis is an enigmatic disease, the pathophysiology of which we are just beginning to understand. Symptomatic women with endometriosis, suffering from infertility or pain, are often difficult patients to treat because we have few treatment options to offer. The therapies themselves are imperfect, with none that permanently eradicate the disease. A multidisciplinary approach is often required for the diagnosis and management of this disease. In deciding how to treat women with pelvic pain or infertility, we must consider the best available evidence to form our decisions. In women with pelvic pain and suspected endometriosis, first line treatment would be non-steroidal anti-inflammatory medications with/without oral contraceptives or progestins. Continuous combined oral contraceptives have been shown to provide significant pain reduction from baseline over cyclic combined oral contraceptives(148). If these conservative approaches fail, three alternative treatments would be empiric: GnRH agonist therapy with estrogen and progestin add-back therapy, aromatase inhibitors, or operative laparoscopy. The laparoscopy should include lysis of adhesions and excision or ablation of endometriosis. Surgery for pain may be followed by medical treatment with GnRH agonist and add-back therapy (Fig. 6). It is important to note that at no time are narcotics advocated for the treatment of endometriosis-associated pelvic pain except immediately pre-operatively or post-operatively. If the above measures fail, patients should be enrolled into a multi-disciplinary chronic pelvic pain treatment group. This should include physicians from many subspecialties, including psychiatry, anesthesiology, gynecology, and often gastroenterology and urology.
REFERENCES
- Gustofson, RL, Kim, N, Liu, S, Stratton, P. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril 2006; 86:298.
- Bulun, Serdar E. Endometriosis. N Engl J Med 2009; 360:268-278.
- Hsu, Albert, L., Khachikyan, Izabella, and Stratton, Pamela. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin Obstet Gynecol 2010; 53:413-419.
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927; 14:422-69.
- Olive DL, Schwartz LB. Endometriosis. N Engl J Med 1993; 328:1759-69.
- Olive, DL, Henderson, DY. Endometriosis and Mullerian anomalies. Obstet Gynecol 1987; 69:412.
- Blumenkrantz JM, Gallagher N, Bashore RA, Tenckhoff, H. Retrograde menstruation in women undergoing chronic peritoneal dialysis. Obstet Gynecol 1981; 57:667-70.
- Kruitwagen RF, Poels LG, Willemsen WN, de Ronde IJ, Jap PH, Rolland R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil Steril 1991; 55:297-303.
- Meyer R. Uber den staude der frage der adenomyosites adenomyoma in allgemeinen und adenomyometritis sarcomastosa. Zentralb Gynakol 1919; 36:745.
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of tissue is impaired in women with endometriosis. Fertil Steril 1998; 69:1042-4.
- Evers JLH. The defense against endometriosis. Fertil Steril 1996; 66:351-3.
- Witz CA, Montoya-Rodriguez IA, Miller DM, Schneider BG, Schenken RS. Mesothelium expression of integrins in vivo and in vitro. J Soc Gynecol Invest 1998; 5:87-93.
- Osteen KG, Bruner KL, Sharpe-Timms KL. Steroid and growth factor regulation of matrix metalloproteinase expression and endometriosis. Semin Reprod Endocrinol 1996; 14:247-55.
- Taylor RN, Ryan IP, Moore ES, Hornung D, Shifren JL, Tseng JF. Angiogensis and macrophage activation in endometriosis. Ann NY Acad Sci 1997; 828:194-207.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril 2001; 75:1-10.
- Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis associated infertility. Fertil Steril 2008; 90:247-257.
- Jackson LW, Schisterman EF, Dey-Rao R, et al. Oxidative stress and endometriosis. Hum Reprod 2005; 20:2014–20.
- Somigliana E, Panina-Bordignon P, Murone S, et al. Vitamin D reserve is higher in women with endometriosis. Hum Reprod 2007; 22:2273–8.
- Vigano P, Lattuada D, Mangioni S, et al. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J Mol Endocrinol 2006; 36:415–24.
- McLaren J, Prentice A, Charnock-Jones DS, et al. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest 1996; 98:482–9.
- Sayegh L, Fuleihan GE, Nassar AH. Vitamin D in endometriosis: A causative or confounding factor? Metabolism Clinical and Experimental. 63 (2014) 32–41.
- Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012 Jan; 30(1):39-45.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004; 160:784.
- Hediger, ML, Hartnett, HJ, Louis, GM. Association of endometriosis with body size and figure. Fertil Steril 2005; 84:1366.
- Malinak LR, Buttram VC Jr, Elias S, Simpson JL. Heritage aspects of endometriosis. II. Clinical characteristics of familial endometriosis. Am J Obstet Gynecol 1980; 137:332–7.
- Matalliotakis IM, Arici A, Cakmak H, Goumenou AG, Koumantakis G, Mahutte NG. Familial aggregation of endometriosis in the Yale Series. Arch Gynecol Obstet 2008; 278:507–11.
- Nyholt DR, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012 Dec; 44(12):1355-9. doi: 10.1038/ng.2445. Epub 2012 Oct 28.
- Pagliardini L, Gentilini D, Vigano' P, Panina-Bordignon P, Busacca M, Candiani M, Di Blasio AM. An Italian association study and meta-analysis with previous GWAS confirm WNT4, CDKN2BAS and FN1 as the first identified susceptibility loci for endometriosis. J Med Genet. 2013 Jan; 50(1):43-6. doi: 10.1136/jmedgenet-2012-101257.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Malspeis S, Willett WC, Hunter DJ. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol 2004; 104:965.
- Balasch J, Creus M, Fabregues F, Carmona F, Ordi J, Martinez-Roman S, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11:387–91.
- Rawson JM. Prevalence of endometriosis in asymptomatic women. J Reprod Med 1991; 36:513–5.
- Strathy JH, Molgaard CA, Coulam CB, Melton LJ 3rd. Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril 1982; 38:667–72.
- Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc 1987; 74:671–5.
- Carter JE. Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J Am Assoc Gynecol Laparosc 1994; 2:43–7.
- Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometrio- sis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril 1991; 55:759–65.
- Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol 1999; 93:51–8.
- National Center for Health Statistics. Hysterectomies in the United States, 1965-84. Hyattsville, MD. National Center for Health Statistics, 1987(Vital and Health Statistics, Series 13, Data from the National Health Survey, No. 92, DHHS Publ No. (PHS. 88-1753).
- Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (macaca mulatta. following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fund Applied Toxicol 1993; 21:433-41.
- Signorile PG, Spugnini EP, Mita L, Mellone P, D'Avino A, Bianco M, Diano N, Caputo L, Rea F, Viceconte R, Portaccio M, Viggiano E, Citro G, Pierantoni R, Sica V, Vincenzi B, Mita DG, Baldi F, Baldi A. Prenatal exposure of mice to bisphenol A elicits an endometriosis- like phenotype in female offspring. Gen Comp Endocrinol 2010.
- Parazzini F, Cipriani S, Bravi F, Pelucchi C, Chiaffarino F, Ricci E, Viganò P. A meta analysis on alcohol consumption and risk of endometriosis. Am. J. Obstet. Gynecol. 2013 209; 106.e1–10.
- Noble, L. S., Simpson, E. R., Johns, A. & Bulun, S. E. Aromatase expression in endometriosis. J. Clin. Endocrinol. Metab. 1996; 81, 174–179.
- Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, Varner MW, Kennedy A, Giudice L, Fujimoto VY, Sun L, Wang L, Guo Y, Kannan K. Bisphenol A and phthalates and endometriosis: the Endometriosis: Natural History, Diagnosis and Outcomes Study. Fertil Steril 2013; 100:162–9.
- M., Mesrine. S., Clavel-Chapel, F. & Boutron-Ruault, M. C. Endometriosis risk in relation to naevi, freckles and skin sensitivity to sun exposure: the French E3N cohort. Int. J. Epidemiol. 2009; 38, 1143–1153.
- Somigliana E, Viganò P, Abbiati A, Gentilini D, Parazzini F, Benaglia L, Vercellini P, Fedele L. ‘Here comes the sun’: pigmentary traits and sun habits in women with endometriosis. Hum. Reprod. 2010; 25, 728–733.
- Vitonis, A. F., Hankinson, S. E., Hornstein, M. D. & Missmer, S. A. Adult physical activity and endometriosis risk. Epidemiology 2010; 21, 16–23.
- Viganò P, Somigliana E, Panina P, Rabellotti E, Vercellini P, Candiani M. Principles of phenomics in endometriosis. Hum. Reprod. Update. 2012; 18, 248–259.
- Lafay Pillet MC, Schneider A, Borghese B, Santulli P, Souza C, Streuli I, de Ziegler D, Chapron C. Deep infiltrating endometriosis is associated with markedly lower body mass index: a 476 case–control study. Hum. Reprod. 2012; 27, 265–272.
- Shah, D. K., Correia, K. F., Vitonis, A. F. & Missmer, S. A. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 2013; 28, 1783–1792.
- Vercellini, P. Endometriosis: What a pain it is. Semin. Reprod. Endocrinol. 1997; 15, 251–261.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis on 1000 patients. Hum. Reprod. 2007; 22, 266–271.
- Ballard, K. D., Seaman, H. E., de Vries, C. S. & Wright, J. T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case–control study—Part 1. BJOG 2008; 115, 1382–1391.
- Olive DL, Blackwell RE, Copperman AB. Endometriosis and pelvic pain. In: Blackwell RE, Olive DL, editors. Chronic Pelvic Pain: Evaluation and Management. Springer, New York, 1997; pgs 61-83.
- Bokor, Xinmei, Yao, Huijiao, Huang, Xiufeng, Lu, Bangchun, Xu, Hing, and Zhou, Caiyun. Nerve fibres in ovarian endometriotic lesions in women with ovarian endometriosis. Hum Reprod 2010; 25:392-397.
- Al-Jefout, M, Dezarnaulds, G, Cooper, M, Tokushige N, Luscombe GM, Markham R, Fraser IS. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod. 2009; 24:3019-3024.
- Bokor, A, Kyama, CM, Vercruysse, L, Fassbender A, Gevaert O, Vodolazkaia A, De Moor B, Fülöp V, D'Hooghe T. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod. 2009; 24:3025-3032.
- Anaf V, Simon P, El Nakadi I, Fayt I, Buxant F, Simonart T, Peny MO, Noel JC. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod 2000; 15:1744.
- Wang, G, Tokushige, N, Markham, R, Fraser, IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod 2009; 24:827.
- Howard, F. M. Endometriosis and mechanisms of pelvic pain. J. Minim. Invasive Gynecol. 2009; 16, 540–550.
- Bajaj,P., Bajaj,P., Madsen,H. & Arendt- Nielsen, L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J. Pain 2003; 4, 372–380.
- Berkley, K. J., Rapkin, A. J. & Papka, R. E. The pains of endometriosis. Science 2005; 308, 1587–1589.
- Stratton, P. & Berkley, K. J. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum. Reprod. Update 2011; 17, 327–346.
- Tran, Lu Vinh Phuc, Tokushige, Natusuko, Berbic, Marina, Markham, Robert, and Fraser, Ian. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod. 2009; 24:835-841.
- Guzick DS, Silliman NP, Adamson GD, Buttram VC Jr, Canis M, Malinak LR, Schenken RS. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine's revised classification of endometriosis. Fertil Steril 1997; 67:822-829.
- Inoue M, Kobayashi Y, Honda I, Awaji H, Fujii A. The impact of endometriosis on the reproductive outcome of infertile patients. Am J Obstet Gynecol 1992; 167:278-282.
- The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and Infertility. Fertil Steril 2006; 86:S156-S160.
- Cahill, DJ. What is the optimal medical management of infertility and minor endometriosis?: Analysis and future prospects. Hum Reprod 2002; 17:1135.
- Isaacson KB, Galman M, Coutifaris C, Lyttle CR. Endometrial synthesis and secretion of complement component-3 by patients with and without endometriosis. Fertil Steril 1990; 53:836-41.
- Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. In terleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab 1996; 81:1118-1122.
- Taylor HS, Bagot C, Kardana A, Olive DL, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 1999; 14:1328-31.
- Lessey BA, Young SL. Integrins and other cellular adhesion molecules in endometrium and endometriosis. Semin Reprod Endocrinol 1997; 15:291-9.
- Sinaii, N, Cleary, SD, Ballweg, ML, Nieman, LK, and Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome, and atopic diseases among women with endometriosis: a survey analysis. Hum Repro 2002; 17:2715-2724.
- Contemporary Concepts in Clinical Management. Schenken RS, editor. J.B.Lippincott Company, Philadelphia, 1989.
- Lundberg W, Wall J, Mathers J. Laparoscopy in evaluation of pelvic pain. Obstet Gynecol 1973; 42:872-76.
- Diamond M, Daniell J, Johns D, et al. Postoperative adhesion development after operative laparoscopy: evaluation at early second look procedures. Fertil Steril 1991; 55:700-4.
- Hoyos L, Johnson S, Puscheck E. Endometriosis and Imaging. Clin Obstet Gynecol 2017; 60:503-516.
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A, Saridogan E; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 2005; 20:2698-2704.
- Hudelist G, Tuttlies F, Rauter G, Pucher S, Keckstein J. Can transvaginal sonography predict infiltration depth in patients with deep infiltrating endometriosis of the rectum? Hum Reprod 2009; 24:1012.
- Bazot M, Malzy P, Cortez A, Roseau G, Amouyal P, Daraï E. Accuracy of transvaginal sonography and rectal endoscopic sonography in the diagnosis of deep infiltrating endometriosis. Ultrasound Obstet Gynecol 2007; 30:994.
- Faccioli N, Manfredi R, Mainardi P, Dalla Chiara E, Spoto E, Minelli L, Mucelli RP. Barium enema evaluation of colonic involvement in endometriosis. AJR Am J Roentgenol 2008; 190:1050.
- Piketty M, Chopin N, Dousset B, Millischer-Bellaische AE, Roseau G, Leconte M, Borghese B, Chapron C. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod 2009; 24:602.
- Hudelist G, Oberwinkler KH, Singer CF, Tuttlies F, Rauter G, Ritter O, Keckstein J. Combination of transvaginal sonography and clinical examination for preoperative diagnosis of pelvic endometriosis. Hum Reprod 2009; 24:1018.
- Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril 2009; 92:1825.
- Arrive L, Hricak H, Martin MC. Pelvic endometriosis: MR imaging. Radiology 1989; 171:687.
- 83 Togashi K, Nishimura K, Kimura I, Tsuda Y, Yamashita K, Shibata T, Nakano Y, Konishi J, Konishi I, Mori T. Endometrial cysts: diagnosis with MR imaging. Radiology 1991; 180:73.
- Mawhinney RR, Powell MC, Worthington BS, Symonds EM. Magnetic resonance imaging of benign ovarian masses. Br J Radiol 1988; 61:179.
- Woodward PJ, Gilfeather M. Magnetic resonance imaging of the female pelvis. Semin Ultrasound CT MR 1998; 19:90-103.
- Pittaway DE, Fayez JA. The use of CA-125 in the diagnosis and management of endometriosis. Fertil Steril 1986; 46:790.
- Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril 2009; 92:1799 – 1801.
- Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil Steril 2010; 94:730– 737.
- Barcena de Arellano ML, Arnold J, Sacher F, Blochle M, Staube M, Bartley J, Vercellino GF, Chiantera V, Schneider A, Mechsner S. Eutopic endometrium from women with endometriosis does not exhibit neurotrophic properties. J Neuroimmunol 2012a; 249:49 – 55.
- Barcena de Arellano ML, Wagner MF, Oldeweme J, Arnold J, Ebert A, Schneider A, Mechsner S. Neurotrophin expression is not affected in uteri of women with adenomyosis. J Mol Neurosci 2012b; 47:495 – 504.
- Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod 2006; 21:782– 787.
- Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril 2007; 88:795 – 803.
- Tokushige N, Markham R, Russell P, Fraser IS. Effects of hormonal treatment on nerve fibers in endometrium and myometrium in women with endometriosis. Fertil Steril 2008; 90:1589 – 1598.
- Aiamkitsumrit B, Zhang X, Block TM, Norton P, Fraser NW, Su YH. Herpes simplex virus type 1 ICP4 deletion mutant virus d120 infection failed to induce apoptosis in nerve growth factor-differentiated PC12 cells. J Neurovirol 2007; 13:305 – 314.
- Al-Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, Markham R, Fraser IS. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod 2009; 24:3019– 3024
- Aghaey Meibody F, Mehdizadeh Kashi A, Zare Mirzaie A, Ghajarie Bani Amam M, Shariati Behbahani A, Zolali B, Najafi L. Diagnosis of endometrial nerve fibers in women with endometriosis. Arch Gynecol Obstet 2011; 284:1157– 1162.
- Newman TA, Bailey JL, Stocker LJ, Woo YL, Macklon NS, Cheong YC. Expression of neuronal markers in the endometrium of women with and those without endometriosis. Human Reproduction, Vol.28, No.9 pp. 2502 –2510, 2013.
- Nisolle M, Casanas-Roux F, Donnez J. Histogenesis of peritoneal endometriosis. In: Nezhat CR, Berger GS, Nezhat FR, Buttram VC Jr, Nezhat CH, editors. Endometriosis: Advanced Management and Surgical Techniques. Springer-Verlag, New York. 1995; 19-25.
- Evers J. The second look laparoscopy for the evaluation of the results of medical treatment of endometriosis should not be performed during ovarian suppression. Fertil Steril 1987; 47:502-504
- Nisolle-Pochet M, Casanas-Roux F, Donnez J. Histologic study of ovarian endometriosis after hormonal therapy. Fertil Steril 1988; 49:423.
- Brosens IA, Vasquez G, Gordts S. Scanning electron microscopic study of the pelvic peritoneum in unexplained infertility and endometriosis. Fertil Steril 1984; 41(Suppl):S21.
- American Society for Reproductive Medicine. (1997) Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 67, 817-21.
- Tokushige N, Markham R, Russell P, Fraser IS. Effects of hormonal treatment on nerve fibers in endometrium and myometrium in women with endometriosis. Fertil Steril. 2008; 90: 1589-1598.
- Vercellini, Paolo, Somigliano, Edgardo, Vigano, Paola, Abbiati, Annalisa, Barbara, Giussy, and Crosignani, Pier Giorgio. Endometriosis: current therapies and new pharmacological developments. Drugs 2009; 69:649-675.
- Taylor, H.S. et al. Treatment of Endometriosis Associated Pain with Elagolix, an Oral GnRH Antagonist. N Engl J Med 2017, 377 (1), 28-40.
- Allan, C, Hopewell, S, Prentice, A, and Gregory, D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. The Cochrane Collaboration 2009; 2:1-19.
- Lv, D, Song, H, Shi G. Anti- TNF-α treatment for pelvic pain associated with endometriosis (review). The Cochrane Collaboration 2010; 3:1-31.
- Soares, S. R., Martinez-Varea, A., Hidalgo- Mora, J. J. & Pellicer, A. Pharmacologic therapies in endometriosis: a systematic review. Fertil. Steril. 2012; 98, 529–555.
- Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril 1994; 62:696–700.
- Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril 2004; 82:878–84.
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery [published erratum appears in Obstet Gynecol 2008; 112:710]. Obstet Gynecol 2008; 111:1285–92.
- Hart, R. J., Hickey, M., Maouris, P. & Buckett, W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database of Systematic Reviews, Issue 2. Art. No.: CD004992. http://dx.doi.org/10.1002/ 14651858.CD004992.pub3.
- Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med 2001; 345:266-275.
- Vercellini, P. et al. Surgery for deep endometriosis: a pathogenesis-oriented approach. Gynecol. Obstet. Invest. 2009; 68, 88–103.
- Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Viganò P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum. Reprod. Update 2009; 15, 177–188.
- De Cicco, C. et al. Bowel resection for deep endometriosis: a systematic review. BJOG 2011; 118, 285–291.
- Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2004;(3):CD003678.
- Regidor PA, Regidor M, Schmidt M, Ruwe B, Lubben G, Fortig P, Kienle E, Schindler AE. Prospective randomized study comparing the GnRH-agonist leuprorelin acetate and the gestagen lynestrenol in the treatment of severe endometriosis. Gynecol Endocrinol 2001; 15:202-9.
- Jacobson, TZ, Duffy, JMN, Barlow, D, Farquhar, C, Koninckx, PR, and Olive, D. Laparoscopic surgery for subfertility associated with endometriosis (Review). The Cochrane Collaboration. 2010; 1:1-20.
- Marcoux, S, Maheux, R, Berube, S, for The Canadian Collaborative Group on Endometriosis. Laparoscopic Surgery in Infertile Women with Minimal or Mild Endometriosis. N Engl J Med 1997; 337:217-222.
- Gruppo Italiano per lo Studio dell'Endometriosi. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Hum Reprod 1999; 14(5):1332-4.
- Adamson , G, Pasta, D. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril 2010; 94:1609-1615.
- Tsoumpou, I, Kyrgiou, M, Gelbaya, T, and Nardo, L. The effect of surgical treatment for endometrioma on in vitro fertilization outcomes: a systematic review and meta-analysis. Fertil Steril 2009; 92:75-87.
- Vercellini, P. et al. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: literature review. Reprod. Biomed. Online 2012; 24, 389–395.
- De Ziegler, D., Borghese, B. & Chapron, C. Endometriosis and infertility: pathophysiology and management. Lancet 2010; 376, 730–738.
- Barnhart, K., Dunsmoor-Su, R. & Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002; 277, 1148–1155.
- Juneau,C, et al. Patients with endometriosis have aneuploidy rates equivalent to their age- matched peers in the in vitro fertilization population. Fertil Steril 2017; 108: 284-288.
- Wieser, F, Cohen, M, Gaeddert, A, Yu, J, Burks-Wicks, C, Berga, S, and Taylor, RN. Evolution of medical treatment for endometriosis: Back to the roots? Hum Reprod 2007; 13:487-499.
- Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012 Jan; 124(1):164-9.
- Agic A, Xu H, Altgassen C, Noack F, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha- hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25- hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 2007; 14:486–97.
- Varma R, Rollason T, Gupta JK, et al. Endometriosis and the neoplastic process. Reproduction 2004; 127:293–304.
- Donnez J, Squifflet J, Pirard C, et al. The efficacy of medical and surgical treatment of endometriosis-associated infer- tility and pelvic pain. Gynecol Obstet Invest 2002; 54(1):2–7.
- Oral E, Ilvan S, Tustas E, et al. Prevalence of endometriosis in malignant epithelial ovary tumours. Eur J Obstet Gynecol Reprod Biol 2003; 109:97–101.
- Pearce CL, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012 Apr; 13(4):385-94.
- Worley MJ, Welch WR, Berkowitz RS, Ng S-W. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci 2013; 14:5367– 5379.
- Kobayashi, H. et al. The role of hepatocyte nuclear factor-1β in the pathogenesis of clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2009; 19, 471–479.
- Vercellini, P. et al. The “incessant menstruation” hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum. Reprod. 2011; 26, 2262–2273.
- Yamada, Y. et al. Redox-active iron-induced oxidative stress in the pathogenesis of clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2011; 21, 1200–1207.
- Wiegand, K. C. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010; 363, 1532–1543.
- Maeda, D. & Shih, I. M. Pathogenesis and the role of ARID1A mutation in endometriosis- related ovarian neoplasms. Adv. Anat. Pathol. 2013; 20, 45–52.
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Loss of arid1a protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with pik3ca mutations. Mod. Pathol. 2012, 25, 615–624.
- Soslow, R.A. Histologic subtypes of ovarian carcinoma: An overview. Int. J. Gynecol. Pathol. 2008, 27, 161–174.
- Llauradó M. et al. Molecular bases of endometrial cancer: New roles for new actors in the diagnosis and the therapy of the disease. Mol. Cell. Endocrinol. 2012; 358, 244–255.
- Herrinton, L. J., Voigt, L. F., Weiss, N. S., Beresford, S. A. A. & Wingo, PA. Risk factors for synchronous primary endometrial and ovarian cancers. Ann. Epidemiol. 2001; 11, 529–533.
- Mangili, G. et al. Unraveling the two entities of endometrioid ovarian cancer: a single center clinical experience. Gynecol. Oncol. 2012; 126, 403–407.
- Liu, Y., Li, J., Jin, H., Lu, Y. & Lu, X. Clinicopathological characteristics of patients with synchronous primary endometrial and ovarian cancers: A review of 43 cases. Oncol. Lett. 2013; 5, 267–270.
- Vercellini P, Frontino G, De Giorgi O, Pietropaolo G, Pasin R, Crosignani PG. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril 2003; 80:560–3.
Adrenal Insufficiency
ABSTRACT
Adrenal insufficiency is a serious pathologic condition characterized by decreased production or action of glucocorticoids and/or mineralocorticoids and adrenal androgens. This life-threatening disorder may be classified as primary, secondary or tertiary, resulting from diseases affecting the adrenal cortex, the anterior pituitary gland or the hypothalamus, respectively. The clinical manifestations of adrenal insufficiency include anorexia, abdominal pain, weakness, weight loss, fatigue, hypotension, salt craving and hyperpigmentation of the skin in case of primary adrenal insufficiency. The diagnosis of adrenal insufficiency can be confirmed by demonstrating inappropriately low cortisol secretion, determining whether the cortisol deficiency is secondary or primary, and defining the cause of the disorder. Treatment with glucocorticoid and/or mineralocorticoid replacement should be initiated when glucocorticoid and or mineralocorticoid deficiency is suspected. This chapter will provide an overview of the epidemiology, etiology, pathophysiology, clinical manifestations, diagnosis and treatment of adrenal insufficiency. Finally, special conditions of adrenal insufficiency, including critical illness, pregnancy, infancy and childhood will also be discussed. For complete coverage of this and related areas of Endocrinology, please visit our free online textbook, WWW.ENDOTEXT.ORG.
INTRODUCTION
Adrenal insufficiency is a disorder first described by Thomas Addison in 1855, which is characterized by deficient production or action of glucocorticoids and/or mineralocorticoids and adrenal androgens. This life-threatening disease may result from disorders affecting the adrenal cortex (primary), the anterior pituitary gland (secondary), or the hypothalamus (tertiary) (Figure 1) (1-3). The clinical symptoms of adrenal insufficiency include weakness, fatigue, anorexia, abdominal pain, weight loss, orthostatic hypotension, salt craving, and characteristic hyperpigmentation of the skin occurring with primary adrenocortical failure (4, 5). Regardless of etiology, adrenal insufficiency was an invariably fatal disorder, until the synthesis of cortisone by Kendall, Sarett, and Reichstein (6-9) in 1949, and the introduction of substitution therapy with life-saving synthetic glucocorticoids subsequently. However, despite this progress, there are still numerous challenges regarding the diagnosis and treatment of patients with adrenal insufficiency.

Figure 1: Types of adrenal insufficiency. CRH: corticotropin-releasing hormone, ACTH: adrenocorticotropic hormone.
EPIDEMIOLOGY
The prevalence of chronic primary adrenal insufficiency in Europe has been doubled from 40–70 cases per million population in the 1960s (10, 11) to 93–144 cases per million population by the end of the last century and in recent years (12-16). The currently estimated incidence of this disorder is 4.4–6 new cases per million population per year (15). Primary adrenal insufficiency affects more frequently women, and clinical manifestations can present at any age, although most often between 30 and 50 years (12).
Secondary adrenal insufficiency occurs more frequently than primary adrenal insufficiency (1). Its estimated prevalence is 150–280 per million and is more common in women than men (14, 17-20). Affected patients are mostly diagnosed in the sixth decade of life (18, 19).
The most common cause of tertiary adrenal insufficiency is chronic exogenous administration of synthetic glucocorticoids, which causes prolonged suppression of hypothalamic corticotropin-releasing hormone (CRH) secretion through negative feedback mechanisms (21).
CAUSES OF ADRENAL INSUFFICIENCY
Causes of Primary Adrenal Insufficiency
The etiology of primary adrenal insufficiency has changed over time. Prior to 1920, the most common cause of primary adrenal insufficiency was tuberculosis, while since 1950, the majority of cases (80-90%) have been ascribed to autoimmune adrenalitis, which can be isolated (40%) or in the context of an autoimmune polyendocrinopathy syndrome (60%) (1, 2, 22-24).
Autoimmune adrenalitis (Addison’s disease): This condition is characterized by destruction of the adrenal cortex by cell-mediated immune mechanisms. Antibodies that react against steroid 21-hydroxylase are detected in approximately 90% of patients with autoimmune Addison’s disease (16), but only rarely in patients with other causes of adrenal insufficiency or normal subjects (25). Considerable progress has been made in identifying genetic factors that predispose to the development of autoimmune adrenal insufficiency (2). In addition to the major histocompatibility complex (MHC) haplotypes DR3-DQ2 and DR4-DQ8, other genetic factors, such as protein tyrosine phosphatase non-receptor type 22 (PTPN22), cytotoxic T lymphocyte antigen 4 (CTLA-4), and the major histocompatibility complex class II transactivator (CIITA) have been associated with this condition (23-29).
Primary adrenal insufficiency may also present as part of autoimmune polyendocrinopathy syndromes. Patients with autoimmune polyendocrinopathy syndrome type 1 (APS1) or APECED (Autoimmune Polyendocrinopathy, Candidiasis, Ectodermal Dystrophy) syndrome may present with chronic mucocutaneous candidiasis, adrenal insufficiency, hypoparathyroidism, hypoplasia of the dental enamel and nail dystrophy, while type 1 Diabetes Mellitus (T1DM) or pernicious anemia, may develop later in life (30, 31). Clinical manifestations of autoimmune polyendocrinopathy syndrome type 2 (APS2) include autoimmune adrenal insufficiency, autoimmune thyroid disease and/or T1DM, whereas autoimmune polyendocrinopathy syndrome type 4 (APS4) is characterized by autoimmune adrenal insufficiency and one or more other autoimmune diseases, such as atrophic gastritis, hypogonadism, pernicious anemia, celiac disease, myasthenia gravis, vitiligo, alopecia and hypophysitis, but without any autoimmune disorders of APS1 or APS2 (23, 24, 26, 31-33).
Adrenoleukodystrophy: This is an X-linked recessive disorder affecting 1 in 20.000 males (2). The molecular basis of this condition has been ascribed to mutations in the ABCD1 gene, which result in defective beta oxidation of very long chain fatty acids (VLCFAs) within peroxisomes. The abnormally high concentrations of VLCFAs in affected organs, including the adrenal cortex, result in the clinical manifestations of this disorder, which include neurological impairment due to white-matter demyelination and primary adrenal insufficiency, with the latter presenting in infancy or childhood (1-3, 34).
Hemorrhagic infarction: Bilateral adrenal infarction caused by hemorrhage or adrenal vein thrombosis may also lead to adrenal insufficiency (35, 36). The diagnosis is usually made in critically ill patients in whom a computed tomography (CT) scan of the abdomen shows bilateral adrenal enlargement. Several coagulopathies and the heparin-induced thrombocytopenia syndrome have been associated with adrenal vein thrombosis and hemorrhage, while the primary antiphospholipid syndrome has been recognized as a major cause of adrenal hemorrhage (37). Adrenal hemorrhage has been mostly associated with meningococcemia (Waterhouse-Friderichsen syndrome) and Pseudomonas aeruginosa infection (38).
Infectious adrenalitis: Many infectious agents may attack the adrenal gland and result in adrenal insufficiency, including tuberculosis (tuberculous adrenalitis), disseminated fungal infections and HIV-associated infections, such as adrenalitis due to cytomegalovirus and mycobacterium avium complex (39-41).
Drug-induced adrenal insufficiency : Drugs that may cause adrenal insufficiency by inhibiting cortisol biosynthesis, particularly in individuals with limited pituitary and/or adrenal reserve, include aminoglutethimide (antiepileptic), etomidate (anesthetic-sedative) (42, 43), ketoconazole (antimycotic) (44) and metyrapone (45). Drugs that accelerate the metabolism of cortisol and most synthetic glucocorticoids by inducing hepatic mixed-function oxygenase enzymes, such as phenytoin, barbiturates, and rifampicin can also cause adrenal insufficiency in patients with limited pituitary or adrenal reserve, as well as those who are on replacement therapy with glucocorticoids (46). Furthermore, some of novel tyrosine kinase-targeting drugs (e.g. sunitinib) have been shown in animal studies to cause adrenal dysfunction and hemorrhage (47).
Other causes of primary adrenal insufficiency are listed in Table 1.
Table 1. Causes of Primary Adrenal Insufficiency
| Disease | Pathogenetic Mechanism |
| Autoimmune adrenalitis | |
| Isolated |
Associations with HLA-DR3-DQ2, HLADR4-DQ8, MICA, CTLA-4, PTPN22, CIITA, CLEC16A, Vitamin D receptor |
| APS type 1 (APECED) | AIRE gene mutations |
| APS type 2 |
Associations with HLA-DR3, HLA-DR4, CTLA-4 |
| APS type 4 | Associations with HLA-DR3, CTLA-4 |
| Infectious adrenalitis | |
| Tuberculous adrenalitis | Tuberculosis |
| AIDS | HIV-1, cytomegalovirus |
| Fungal adrenalitis |
Histoplasmosis, cryptococcosis, coccidiodomycosis |
| Syphilis | Treponema pallidum |
| African Trypanosomiasis | Trypanosoma brucei |
| Bilateral adrenal hemorrhage |
Meningococcal sepsis (Waterhouse- Friderichsen syndrome), primary antiphospholipid syndrome |
| Bilateral adrenal metastases |
Primarily lung, stomach, breast and colon cancer |
| Bilateral adrenal infiltration |
Primary adrenal lymphoma, amyloidosis, haemochromatosis |
| Bilateral adrenalectomy |
Unresolved Cushing’s syndrome, bilateral adrenal masses, bilateral pheochromocytoma |
| Drug-induced adrenal insufficiency | |
|
Anticoagulants (heparin, warfarin), tyrosine kinase inhibitors (sunitinib) |
Hemorrhage |
| Aminoglutethimide | Inhibition of P450 aromatase (CYP19A1) |
| Trilostane |
Inhibition of 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) |
| Ketoconazole, fluconazole, etomidate |
Inhibition of mitochondrial cytochrome P450-dependent enzymes (e.g. CYP11A1, CYP11B1) |
| Phenobarbital |
Induction of P450-cytochrome enzymes (CYP2B1, CYP2B2), which enhance cortisol metabolism |
| Phenytoin, rifampin, troglitazone |
Induction of P450-cytochrome enzymes (primarily CYP3A4), which enhance cortisol metabolism |
| Genetic disorders | |
|
Adrenoleukodystrophy or adrenomyeloneuropathy |
ABCD1 and ABCD2 gene mutations |
| Congenital adrenal hyperplasia | |
| 21-Hydroxylase deficiency | CYP21A2 gene mutations |
| 11β-Hydroxylase deficiency | CYP11B1 gene mutations |
|
3β-hydroxysteroid dehydrogenase type 2 deficiency |
HSD3B2 gene mutations |
| 17α-Hydroxylase deficiency | CYP17A1 gene mutations |
| P450 Oxidoreductase deficiency | POR gene mutations |
| P450 side-chain cleavage deficiency | CYP11A1 gene mutations |
| Congenital lipoid adrenal hyperplasia | StAR gene mutations |
| Smith-Lemli-Opitz syndrome | DHCR7 gene mutations |
| Adrenal hypoplasia congenita | |
| X-linked | NR0B1 gene mutations |
| Xp21 contiguous gene syndrome |
Deletion of the Duchenne muscular dystrophy, glycerol kinase and NR0B1 genes |
| SF-1 linked | NR5A1 gene mutations |
| IMAGe syndrome | CDKN1C gene mutations |
| Kearns-Sayre syndrome | Mitochondrial DNA deletions |
| Wolman’s disease | LIPA gene mutations |
|
Sitosterolaimia (also known as phytosterolemia) |
ABCG5 and ABCG8 gene mutations |
|
Familial glucocorticoid deficiency (FGD, or ACTH insensitivity syndromes) |
|
| Type 1 | MC2R gene mutations |
| Type 2 | MRAP gene mutations |
| Variant of FGD | MCM4 gene mutations |
| FGC - Deficiency of mitochondrial ROS detoxification | NNT, TXNRD2, GPX1, PRDX3 gene mutations |
|
Primary Generalized Glucocorticoid Resistance or Chrousos syndrome |
NR3C1 gene mutations |
| Sphingosine-1-phosphate lyase 1 deficiency | SPGL1 gene mutations |
| Infantile Refsum disease | PHYH, PEX7 gene mutations |
| Zellweger syndrome | PEX1 and other PEX gene mutations |
| Triple A syndrome (Allgrove’s syndrome) | AAAS gene mutations |
Modified from References (48, 49)
Causes of Secondary and Tertiary Adrenal Insufficiency
Secondary adrenal insufficiency may be caused by any disease process that affects the anterior pituitary and interferes with ACTH secretion. The ACTH deficiency may be isolated or occur in association with other pituitary hormone deficits.
Tertiary adrenal insufficiency can be caused by any process that involves the hypothalamus and interferes with CRH secretion. The most common cause of tertiary adrenal insufficiency is chronic administration of synthetic glucocorticoids that suppress the hypothalamic-pituitary-adrenal (HPA) axis (50).
Other causes of secondary and tertiary adrenal insufficiency are listed in Tables 2 and 3 respectively.
Table 2. Causes of Secondary Adrenal Insufficiency.
| Disease | Pathogenetic Mechanism |
| Space occupying lesions or trauma | |
|
Pituitary tumors (adenomas, cysts, craniopharyngiomas, ependymomas, meningiomas, rarely carcinomas) or trauma (pituitary stalk lesions) |
Decreased ACTH secretion |
|
Pituitary surgery or irradiation for pituitary tumors, tumors outside the HPA axis or leukemia |
Decreased ACTH secretion |
|
Infections or Infiltrative processes (lymphocytic hypophysitis, hemochromatosis, tuberculosis, meningitis, sarcoidosis, actinomycosis, histiocytosis X, Wegener’s granulomatosis) |
Decreased ACTH secretion |
| Pituitary apoplexy | Decreased ACTH secretion |
|
Sheehan’s syndrome (peripartum pituitary apoplexy and necrosis) |
Decreased ACTH secretion |
| Genetic disorders | |
|
Transcription factors involved in pituitary development |
|
| HESX homeobox 1 | HESX1 gene mutations |
| Orthodentical homeobox 2 | OTX2 gene mutations |
| LIM homeobox 4 | LHX4 gene mutations |
| PROP paired-like homeobox 1 | PROP1 gene mutations |
| SRY (sex-determining region Y) – box 3 | SOX3 gene mutations |
| T-box 19 | TBX19 gene mutations |
|
Congenital Proopiomelanocortin (POMC) deficiency |
POMC gene mutations |
| Prader-Willi Syndrome (PWS) |
Deletion or silencing of genes in the imprinting center for PWS |
Modified from Reference (48)
Table 3. Causes of Tertiary Adrenal Insufficiency.
| Disease | Pathogenetic Mechanism |
| Space occupying lesions or trauma | |
|
Hypothalamic tumors (craniopharyngiomas or metastasis from lung, breast cancer) |
Decreased CRH secretion |
|
Hypothalamic surgery or irradiation for central nervous system or nasopharyngeal tumors |
Decreased CRH secretion |
|
Infections or Infiltrative processes (lymphocytic hypophysitis, hemochromatosis, tuberculosis, meningitis, sarcoidosis, actinomycosis, histiocytosis X, Wegener’s granulomatosis) |
Decreased CRH secretion |
| Trauma, injury (fracture of skull base) | Decreased CRH secretion |
| Drug-induced adrenal insufficiency | |
|
Glucocorticoid therapy (systemic or topical) or endogenous glucocorticoid hypersecretion (Cushing’s syndrome) |
Decreased CRH and ACTH secretion |
| Mifepristone |
Tissue resistance to glucocorticoids through impairment of glucocorticoid signal transduction |
|
Antipsychotics (chlorpromazine), antidepressants (imipramine) |
Inhibition of glucocorticoid-induced gene transcription |
Modified from Reference (48)
PATHOPHYSIOLOGIC MECHANISMS OF ADRENAL INSUFFICIENCY
Pathophysiology of Primary Adrenal Insufficiency
In primary adrenal insufficiency, although the above mentioned causes lead to gradual destruction of the adrenal cortex, the symptoms and signs of the disease appear when the loss of adrenocortical tissue is higher than 90% (37). At the molecular and cellular level, a viral infection, even subclinical, or an excessive tissue response to inflammatory signals may potentially lead to apoptosis or necrosis of adrenocortical cells. Cellular components, such as 21OH-derived peptides, trigger the activation of local dendritic cells, which then transport and present these antigens to CD4+ Th1 cells. Upon activation, CD4+ Th1 cells help the committed clonal expansion of cytotoxic lymphocytes and autoreactive B cells releasing antibodies against 21-hydroxylase and possibly other antibodies. The gradual destruction of adrenocortical tissue seems to be mediated by four distinct and complementary molecular mechanisms: (a) direct cytotoxicity by lymphocytes that induce apoptosis; (b) direct cytotoxic actions by IFN-γ and lymphotoxin-α released by activated CD4+ Th1 cells; (c) cellular cytotoxicity by autoantibodies or by autoantibody-mediated activation of the complement system; and (d) cytotoxic effects of inflammatory cytokines (IL-1β, TNF-α) and free radicals (superoxide, NO) secreted by monocytes/macrophages or by the adrenal cells (51).
In the initial phase of chronic gradual destruction, the adrenal reserve is decreased and although the basal steroid secretion is normal, the secretion in response to stress is suboptimal. Consequently, any major or even minor stressor can precipitate an acute adrenal crisis. With further loss of adrenocortical tissue, even basal steroid secretion is decreased, leading to the clinical manifestations of the disease. Low plasma cortisol concentrations result in the increase of production and secretion of ACTH due to decreased negative feedback inhibition (37). The elevated plasma ACTH concentrations are responsible for the well-recognized hyperpigmentation observed in these patients.
Pathophysiology of Secondary and Tertiary Adrenal Insufficiency
In secondary or tertiary adrenal insufficiency, the resultant ACTH deficiency leads to decreased secretion of cortisol and adrenal androgens, while mineralocorticoid production remains normal. In the early stages, basal ACTH secretion is normal, while stress-induced ACTH secretion is impaired (37). With further loss of basal ACTH secretion, there is atrophy of zonae fasciculata and reticularis of the adrenal cortex. Therefore, basal cortisol secretion is decreased, but aldosterone secretion by the zona glomerulosa is preserved.
CLINICAL MANIFESTATIONS OF ADRENAL INSUFFICIENCY
The clinical manifestations of adrenal insufficiency depend upon the extent of loss of adrenal function and whether mineralocorticoid production is preserved. The onset of adrenal insufficiency is often gradual and may go undetected until an illness or other stress precipitates an adrenal crisis (50, 52).
Adrenal Crisis: Adrenal crisis or acute adrenal insufficiency may complicate the course of chronic primary adrenal insufficiency, and may be precipitated by a serious infection, acute stress, bilateral adrenal infarction or hemorrhage. It is rare in patients with secondary or tertiary adrenal insufficiency. The main clinical manifestation of adrenal crisis is shock, but patients may also have nonspecific symptoms, such as anorexia, nausea, vomiting, abdominal pain, weakness, fatigue, lethargy, confusion or coma. Hypoglycemia is rare in acute adrenal insufficiency, but more common in secondary adrenal insufficiency.
The major factor precipitating an adrenal crisis is mineralocorticoid deficiency and the main clinical problem is hypotension. Adrenal crisis can occur in patients receiving appropriate doses of glucocorticoid if their mineralocorticoid requirements are not met (53), whereas patients with secondary adrenal insufficiency and normal aldosterone secretion rarely present in adrenal crisis. However, glucocorticoid deficiency may also contribute to hypotension by decreasing vascular responsiveness to angiotensin II, norepinephrine and other vasoconstrictive hormones, reducing the synthesis of renin substrate, and increasing the production and effects of prostacyclin and other vasodilatory hormones (54, 55).
Chronic Primary Adrenal Insufficiency: The clinical manifestations of chronic primary adrenal insufficiency are owing to deficient concentrations of all adrenocortical hormones (mineralocorticoids, glucocorticoids, adrenal androgens) and include general malaise, fatigue, weakness, anorexia, weight loss, nausea, vomiting, abdominal pain or diarrhea, which may alternate with constipation, hypotension, electrolyte abnormalities (hyponatremia, hyperkalemia, metabolic acidosis), hyperpigmentation, autoimmune manifestations (vitiligo), decreased axillary and pubic hair, and loss of libido and amenorrhea in women (50, 52). The onset of chronic adrenal insufficiency is often insidious and the diagnosis may be difficult in the early stages of the disease.
Secondary or Tertiary Adrenal Insufficiency: The clinical features of secondary or tertiary adrenal insufficiency are similar to those of primary adrenal insufficiency. However, hyperpigmentation of the skin does not occur, because the secretion of ACTH is not increased. Also, since the production of mineralocorticoids by the zona glomerulosa is mostly preserved, dehydration and hyperkalemia are not present, and hypotension is less prominent. Hyponatremia and increased intravascular volume may be the result of “inappropriate” increase in vasopressin secretion. Hypoglycemia is more common in secondary adrenal insufficiency possibly due to concomitant growth hormone insufficiency and in isolated ACTH deficiency. Clinical manifestations of a pituitary or hypothalamic tumor, such as symptoms and signs of deficiency of other anterior pituitary hormones, headache or visual field defects, may also be present (50, 52).
DIAGNOSIS OF ADRENAL INSUFFICIENCY
The clinical diagnosis of adrenal insufficiency can be confirmed by demonstrating inappropriately low cortisol secretion, determining whether the cortisol deficiency is secondary or primary and, hence, dependent or independent of ACTH deficiency, and detecting the cause of the disorder (50, 52).
Basal morning serum cortisol concentrations: The diagnosis of adrenal insufficiency depends upon the demonstration of inappropriately low cortisol secretion. Serum cortisol concentrations are normally highest in the early morning hours (06:00h – 08:00h), ranging between 10 – 20 mcg/dL (275 – 555 nmol/L) than at other times of the day. Serum cortisol concentrations determined at 08:00h of less than 3 µg/dL (80 nmol/L) are strongly suggestive of adrenal insufficiency (56), while values below 10 µg/dL (275 nmol/L) make the diagnosis likely. Simultaneous measurements of cortisol and ACTH concentrations confirm in most cases the diagnosis of primary adrenal insufficiency.
Morning salivary cortisol concentrations: Adrenal insufficiency is excluded when salivary cortisol concentration at 08:00h is higher than 5.8 ng/mL (16 nmol/L), whereas the diagnosis is more possible for values lower than 1.8 ng/mL (5 nmol/L).
Urinary free Cortisol (UFC): Basal urinary cortisol and 17-hydroxycorticosteroid excretion is low in patients with severe adrenal insufficiency, but may be low-normal in patients with partial adrenal insufficiency. Generally, baseline urinary measurements are not recommended for the diagnosis of adrenal insufficiency.
Basal plasma ACTH, renin and aldosterone concentrations: Basal plasma ACTH concentration at 08:00h, when determined simultaneously with the measurement of basal serum cortisol concentration, may both confirm the diagnosis of adrenal insufficiency and establish the cause (57). The normal values of basal 08:00h plasma ACTH concentrations range between 20-52 pg/mL (4.5-12 pmol/L). In primary adrenal insufficiency, the 08:00h plasma ACTH concentration is elevated, and is coupled with increased concentration or activity of plasma renin, low aldosterone concentrations, hyperkalemia and hyponatremia. In the cases of secondary or tertiary adrenal insufficiency, plasma ACTH concentrations are low or low normal, associated with normal values of plasma concentrations of renin and aldosterone.
Standard dose ACTH stimulation test: Adrenal insufficiency is usually diagnosed by the standard-dose ACTH test, which determines the ability of the adrenal glands to respond to 250 mcg intravenous or intramuscular administration of ACTH(1-24) by measurement of serum cortisol concentrations at 0, 30 and 60 min following stimulation. The test is defined as normal if peak cortisol concentration is higher than 18–20 mcg/dL (500–550 nmol/L), thereby excluding the diagnosis of primary adrenal insufficiency and almost all cases of secondary adrenal insufficiency. However, if secondary adrenal insufficiency is of recent onset, the adrenal glands will have not yet atrophied, and will still be capable of responding to ACTH stimulation normally. In these cases, a low-dose ACTH stimulation test or an insulin-induced hypoglycemia test may be required to confirm the diagnosis (58-60).
Low-Dose ACTH stimulation test: This test theoretically provides a more sensitive index of adrenocortical responsiveness because it results in physiologic plasma ACTH concentrations. This test should be performed at 14:00h, when the endogenous secretion of ACTH is at its lowest. The results might not be valid if it is performed at another time. At 14:00h, a blood sample is collected for determination of basal cortisol concentrations. The low dose of ACTH(1-24) (500 nanograms ACTH(1-24)/1.73 m2 ) is then administered as an intravenous bolus. In normal subjects, this dose results in a peak plasma ACTH concentration about twice that of insulin-induced hypoglycemia (60). Subsequently, blood samples are collected at +10 min, +15 min, +20 min, +25 min, +30 min, +35 min, +40 min and +45 min after stimulation for determination of serum cortisol concentrations (51). A value of 18 µg/dL (500 nmol/L) or more at any time during the test is indicative of normal adrenal function. The advantage of this test is that it can detect partial adrenal insufficiency that may be missed by the standard-dose test (58-62). The low-dose test is also preferred in patients with secondary or tertiary adrenal insufficiency (63-66).
Prolonged ACTH Stimulation Tests: Prolonged stimulation with exogenous administration of ACTH helps differentiate between primary and secondary or tertiary adrenal insufficiency. In secondary or tertiary adrenal insufficiency, the adrenal glands display cortisol secretory capacity following prolonged stimulation with ACTH, whereas in primary adrenal insufficiency, the adrenal glands are partially or completely destroyed and do not respond to ACTH. The prolonged ACTH test consists of the intravenous administration of 250 μg of ACTH as an infusion over eight hours (8-hour test) or over 24 hours on two (or three) consecutive days (two-day test), and the measurement of serum cortisol, and 24-hour urinary cortisol and 17-hydroxycorticoid (17-OHCS) concentrations before and after the infusion (67).
Insulin-induced hypoglycemia test: This test provides an alternative choice for confirmation of the diagnosis when secondary adrenal insufficiency is suspected. The insulin tolerance test helps in the investigation of the integrity of the HPA axis and has the ability to assess growth hormone reserve. Insulin, at a dose of 0.1-0.15 U/kg, is administered to induce hypoglycemia, and measurements of cortisol concentrations are determined at 30 min intervals for at least 120 min (68, 69). This test is contraindicated in patients with cardiovascular disease or a history of seizures, and requires a high degree of supervision.
Corticotropin-releasing hormone (CRH) test: This test is used to differentiate between secondary and tertiary adrenal insufficiency. It consists of intravenous administration of CRH (1 mcg/kg up to a maximum of 100 mcg) and determination of serum cortisol and plasma ACTH concentrations at 0, 15, 30, 45, 60, 90 and 120 min following stimulation. Patients with secondary adrenal insufficiency demonstrate little or no ACTH response, whereas patients with tertiary adrenal insufficiency show an exaggerated and prolonged response of ACTH to CRH stimulation, which is not followed by an appropriate cortisol response (70, 71).
Autoantibody screen: Adrenocortical antibodies or antibodies against 21-hydroxylase can be detected in more than 90% of patients with recent onset autoimmune adrenalitis. Furthermore, antibodies that react against other enzymes involved in the steroidogenesis (P450scc, P450c17) and anti-steroid-producing cell antibodies are present in some patients (1, 3, 22-26, 33, 72-74).
Very long chain fatty acids: To exclude adrenoleukodystrophy, plasma very long chain fatty acids should be determined in male patients with isolated Addison’s disease and negative autoantibodies (34).
Imaging: Patients without any associated autoimmune disease and negative autoantibody screen should undergo a computed tomography (CT) scan of the adrenal glands. In cases of tuberculous adrenalitis, the CT scan shows initially hyperplasia of the adrenal glands and subsequently spotty calcifications during the late stages of the disease. Bilateral adrenal lymphoma, adrenal metastases or adrenal infiltration (sarcoidosis, amyloidosis, hemochromatosis) may also be detected by CT scan. If central adrenal insufficiency is suspected, a magnetic resonance imaging (MRI) scan of the hypothalamus and pituitary gland should be performed. This may detect any potential disease process, such as craniopharyngiomas, pituitary adenomas, meningiomas, metastases and infiltration by Langerhans cell histiocytosis, sarcoidosis or other granulomatous diseases (75, 76). It should be noted that imaging is not required when adrenal cortex autoantibodies are detected.
TREATMENT OF ADRENAL INSUFFICIENCY
Adrenal insufficiency is one of the most life-threatening disorders. Treatment should be administered to the patients as soon as the diagnosis is established, or even sooner if an adrenal crisis occurs (77, 78).
Treatment of Chronic Adrenal Insufficiency: One of the most important aspects of the management of chronic primary adrenal insufficiency is patient and family education. Patients should understand the reason for life-long replacement therapy, the need to increase the dose of glucocorticoid during minor or major stress and to inject hydrocortisone, methylprednisolone or dexamethasone in emergencies.
Emergency precautions: Patients should wear a medical alert (Medic Alert) bracelet or necklace and carry the Emergency Medical Information Card, which should provide information on the diagnosis, the medications and daily doses, and the physician involved in the patient’s management. Patients should also have supplies of dexamethasone sodium phosphate and should be educated about how and when to administer them.
Glucocorticoid replacement therapy: Patients with adrenal insufficiency should be treated with hydrocortisone, the natural glucocorticoid, or cortisone acetate if hydrocortisone is not available. The hydrocortisone daily dose is 10-12 mg per meter square body surface area and can be administered in two to three divided doses with one half to two thirds of the total daily dose being given in the morning (1-5, 77, 79-85). Small reductions of bone mineral density (BMD) probably due to higher than recommended doses (86), as well as impaired quality of life (87, 88) were observed in patients treated with hydrocortisone. A longer-acting synthetic glucocorticoid, such as prednisone, prednisolone or dexamethasone, should be avoided because their longer duration of action may produce manifestations of chronic glucocorticoid excess, such as loss of lean body mass and bone density, and gain of visceral fat (89). Recently, preparations of hydrocortisone that lead to both delayed and sustained release of this compound have been developed and are under clinical investigation (90, 91). These formulations maintain stable cortisol concentrations during 24 hours and physiologic circadian rhythmicity with the cortisol peak occurring during the early morning after oral intake of the preparation at bed-time. Furthermore, a novel once-daily (OD) dual-release hydrocortisone tablet has been developed to maintain more physiologic circadian-based serum cortisol concentrations. Compared to the conventional treatment, the OD dual-release hydrocortisone improved glucose metabolism, cardiovascular risk factors and quality of life (92). Regardless of the type of the formulation used, glucocorticoid replacement should be monitored clinically, evaluating weight gain/loss, arterial blood pressure, annualized growth velocity and presence of Cushing features (93).
Glucocorticoid replacement during minor illness or surgery: During minor illness or surgical procedures, glucocorticoids should be given at a dosage up to three times the usual maintenance dosage for up to three days. Depending on the nature and severity of the illness, additional treatment may be required.
Glucocorticoid replacement during major illness or surgery: During major illness or surgery, high doses of glucocorticoid analogues (10 times the daily production rate) are required to avoid an adrenal crisis. A continuous infusion of 10 mg of hydrocortisone per hour or the equivalent amount of dexamethasone or prednisolone eliminates the possibility of glucocorticoid deficiency. This dose can be halved the second postoperative day, and the maintenance dose can be resumed at the third postoperative day.
Mineralocorticoid replacement therapy: Mineralocorticoid replacement therapy is required to prevent intravascular volume depletion, hyponatremia and hyperkalemia. For these purposes, fludrocortisone (9-alpha-fluorohydrocortisone) in a dose of 0.05 - 0.2 mg daily should be taken in the morning. The dose of fludrocortisone is titrated individually based on the findings of clinical examination (mainly the body weight and arterial blood pressure) and the levels of plasma renin activity. Patients receiving prednisone or dexamethasone may require higher doses of fludrocortisone to lower their plasma renin activity to the upper normal range, while patients receiving hydrocortisone, which has some mineralocorticoid activity, may require lower doses. The mineralocorticoid dose may have to be increased in the summer, particularly if patients are exposed to temperatures above 29ºC (85ºF) (77, 79-85). If patients receiving mineralocorticoid replacement develop hypertension, the dose of fludrocortisone should be reduced accordingly (93). In case of uncontrolled blood pressure, patients should be encouraged to continue fludrocortisone and initiate antihypertensive therapy, such as angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, or dihydropyridine calcium blockers (93, 94).
Androgen replacement: In women, the adrenal cortex is the primary source of androgen in the form of dehydroepiandrosterone and dehydroepiandrosterone sulfate. Treatment with DHEA enhances mood and general well being both in adult patients and in children and adolescents with adrenal insufficiency (80-85, 87, 95-101). A single oral morning dose of DHEA of 25-50 mg may be sufficient to maintain normal serum androgen concentrations in premenopausal women with primary adrenal insufficiency, who present with decreased libido, anxiety, depression, and low energy levels (93). If symptoms are still present during a period of 6 months, patients are advised to discontinue DHEA replacement (93). Naturally, women should be encouraged to report any side effects of androgen therapy. Finally, DHEA replacement should be monitored by determining serum DHEA concentrations in the morning before patient receives her daily DHEA dose (93).
Treatment of adrenal crisis: The aim of initial management in adrenal crisis is to treat hypotension, hyponatremia and hyperkalemia, and to reverse glucocorticoid deficiency. Treatment should be started with immediate administration of 100 mg hydrocortisone i.v. and rapid rehydration with normal saline infusion under continuous cardiac monitoring, followed by 100–200 mg hydrocortisone in glucose 5% per 24-hour continuous iv infusion; alternatively, hydrocortisone could be administered iv or im at a dose of 50-100 mg every 6 hours depending on body surface area and age (80). With daily hydrocortisone doses of 50 mg or more, mineralocorticoids in patients with primary adrenal insufficiency can be discontinued or reduced because this dose is equivalent to 0.1 mg fludrocortisone (79). Once the patient’s condition is stable and the diagnosis has been confirmed, parenteral glucocorticoid therapy should be tapered over 3-4 days and converted to an oral maintenance dose (1-3, 77, 79-85). Patients with primary adrenal insufficiency require life-long glucocorticoid and mineralocorticoid replacement therapy.
Treatment of chronic secondary and tertiary adrenal insufficiency: In chronic secondary or tertiary adrenal insufficiency, glucocorticoid replacement is similar to that in primary adrenal insufficiency, however, measurement of plasma ACTH concentrations cannot be used to titrate the optimal glucocorticoid dose. Mineralocorticoid replacement is rarely required, while replacement of other anterior pituitary deficits might be necessary.
ADRENAL INSUFFICIENCY IN CRITICALLY ILL PATIENTS
Clinical manifestations of adrenal insufficiency are common in critically ill patients, specifically in patients with severe pneumonia, adult respiratory distress syndrome, sepsis, trauma, HIV infection or after treatment with etomidate (2, 102-106).
The molecular pathogenetic mechanisms underlying adrenal insufficiency in critical illness have not been fully elucidated. However, it seems that both inadequate cortisol secretion and impaired glucocorticoid receptor signaling are convincingly involved. Indeed, proinflammatory cytokines may compete with ACTH on its receptor (107) and/or induce tissue resistance to glucocorticoids (108-110). Moreover, the widely used medications during the treatment of sepsis may impair both glucocorticoid production and glucocorticoid signaling. Furthermore, other neuropeptides, signaling molecules, components of oxidative stress and the impaired adrenal blood flow contribute to adrenal insufficiency.
To provide recommendations on the diagnosis and management of adrenal insufficiency in critically ill patients, the American College of Critical Care Medicine suggested that the diagnosis is best made by a delta total serum cortisol of < 9 mcg/dL following ACTH (250 microg) administration or a random total cortisol of < 10 mcg/dL. Hydrocortisone at a dose of 200 mg/day in four divided doses or as a continuous infusion at a dose of 240 mg/day (10 mg/hr) for at least 7 days is recommended for patients with septic shock. Methylprednisolone at a dose of 1 mg/kg/day for at least 14 days is recommended in patients diagnosed with severe early acute respiratory distress syndrome. The role of glucocorticoid therapy in other critically ill patients remains to be further elucidated (111).
ADRENAL INSUFFICIENCY DURING PREGNANCY
Although adrenal insufficiency is relatively rare in pregnancy, it may be associated with significant maternal and/or fetal morbidity and mortality if it remains undiagnosed or untreated (112, 113). Symptoms are usually “nonspecific”, such as nausea, vomiting and fatigue, making the diagnosis of adrenal insufficiency challenging. The current diagnostic tests are serum cortisol concentrations and the cosyntropin stimulation test (93, 113). However, it should be emphasized that the peak cortisol response following ACTH stimulation is higher in pregnant than in non-pregnant women during the second and third trimesters, as a result of physiologic pregnancy-associated hypercortisolism and elevations of cortisol-binding globulin (114). Regarding glucocorticoid replacement during pregnancy, hydrocortisone, cortisone acetate, prednisolone or prednisone can be administered; in contrast,, fluorinated glucocorticoids such as dexamethasone should be avoided because they cross the placenta at higher rates (93). Mineralocorticoid replacement is usually more complicated to assess during pregnancy because of the “nonspecific” symptoms often observed in physiologic pregnancy (93). A hydrocortisone stress dose (bolus intravascular injection of 50-100 mg hydrocortisone followed by continuous infusion of 100-200 mg hydrocortisone/24h) should be administered at the beginning of active labor (93, 115, 116).
ADRENAL INSUFFICIENCY IN INFANCY AND CHILDHOOD
Children with primary adrenal insufficiency should be treated with hydrocortisone phosphate at a daily dose of 10-12 mg per meter square body surface area divided into two or three doses (93). Alternatively, cortisone acetate can be administered with safety also as two to three daily doses. Intermediate-acting or long-acting glucocorticoid analogues, such as prednisolone/prednisolone or dexamethasone respectively, are not recommended due to undesirable chronic side effects, such as glucose intolerance or osteopenia/osteoporosis. The hydrocortisone daily dose should be adjusted according to the increasing body surface area of the child. Caution should be paid to decreased growth velocity, excessive weight gain or other clinical manifestations suggestive of iatrogenic Cushing syndrome. Children with primary adrenal insufficiency also require fludrocortisone at a daily dose of 50-300 μg (93). During the first 6 months, infants require supplementation of sodium chloride at a dose of 1-2 g/day administered in multiple feedings, because the infant kidney is physiologically resistant to mineralocorticoids and the infant milk (breast milk or formula) has relatively low sodium content (93, 117).
REFERENCES
- Arlt W, Allolio B. Adrenal insufficiency. Lancet 2003; 361:1881-1893.
- Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med 2009; 360(22):2328-39.
- Neary N, Nieman L. Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes 2010; 17(3):217-23.
- Addison T. On the constitutional and local effects of disease of the supra-renal capsules. London: Samuel Highley, 1855.
- Løvås K, Husebye ES. Addison's disease. Lancet 2005; 365(9476):2058-61.
- Sarett LH. Partial synthesis of pregnene-4-triol-17(β), 20(β), 21-dione-3, 11 and pregnene-4-diol-17(β),21-trione-3,11,20 monoacetate. J Biol Chem 1946; 162;601-632.
- Kendall EC. Hormones of the adrenal cortex in health and disease. Proceedings of the American Philosophical Society. 1953; 97:8-11.
- Reichstein T. The most important hormones of adrenal cortex. Acta Endocrinol (Copenh) 1954; 17(1-4):375-84.
- Hillier SG. Diamonds are forever: the cortisone legacy. J Endocrinol 2007; 195(1):1- 6.
- Mason AS, Meade TW, Lee JA, Morris JN. Epidemiological and clinical picture of Addison’s disease. Lancet 1968; 2(7571):744-77.
- Nerup J. Addison’s disease – clinical studies. A report of 108 cases. Acta Endocrinol (Copenh) 1974; 76(1):127-41.
- Kong MF, Jeffocoate W. Eighty-six cases of Addison’s disease. Clin Endocrinol (Oxf ) 1994; 41(6):757-61.
- Willis AC, Vince FP. The prevalence of Addison’s disease in Coventry, UK. Postgrad Med J 1997; 73(859):286-88.
- Laureti S, Vecchi L, Santeusanio F, Falorni A. Is the prevalence of Addison’s disease underestimated? J Clin Endocrinol Metab 1999; 84(5):1762.
- Løvås K, Husebye ES. High prevalence and increasing incidence of Addison’s disease in western Norway. Clin Endocrinol (Oxf) 2002; 56(6):787-91.
- Erichsen MM, Lovas K, Skinningsrud B, Wolff AB, Undlien DE, Svartberg J, Fougner KJ, Berg TJ, Bollerslev J, Mella B, Carlson JA, Erlich H, Husebye ES. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observation from a Norwegian registry. J Clin Endocrinol Metab 2009; 94(12):4882-90.
- Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab 1996; 81:1169-72.
- Nilsson B, Gustavasson-Kadaka E, Bengtsson BA, Jonsson B. Pituitary adenomas in Sweden between 1958 and 1991: incidence, survival, and mortality. J Clin Endocrinol Metab 2000; 85:1420-5.
- Regal M, Páramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol 2001; 55:735-40.
- Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet 2001; 357(9254):425-31.
- Gomez MT, Magiakou MA, Mastorakos G, Chrousos GP. The pituitary corticotroph is not the rate-limiting step in the postoperative recovery of the hypothalamic-pituitary-adrenal axis in patients with Cushing syndrome. J Clin Endocrinol Metab 1993; 77(1):173-7.
- Betterle C, Morlin L. Autoimmune Addison's disease. Endocr Dev 2011; 20:161-72.
- Mitchell AL, Pearce SH. Autoimmune Addison disease: pathophysiology and genetic complexity. Nat Rev Endocrinol 2012; 8(5):306-16.
- Husebye E, Løvås K. Pathogenesis of primary adrenal insufficiency. Best Pract Res Clin Endocrinol Metab 2009; 23(2):147-57.
- Husebye ES, Løvås K. Immunology of Addison's disease and premature ovarian failure. Endocrinol Metab Clin North Am 2009; 38(2):389-405.
- Napier C, Pearce SH. Autoimmune Addison's disease. Presse Med 2012; 41(12 P2):e626-35.
- Baker PR, Baschal EE, Fain PR, Triolo TM, Nanduri P, Siebert JC, Armstrong TK, Babu SR, Rewers MJ, Gottlieb PA, Barker JM, Eisenbarth GS. Haplotype analysis discriminates genetic risk for DR3-associated endocrine autoimmunity and helps define extreme risk for Addison's disease. J Clin Endocrinol Metab 2010; 95(10):E263-70.
- Baker PR, Baschal EE, Fain PR, Nanduri P, Triolo TM, Siebert JC, Armstrong TK, Babu SR, Rewers MJ, Gottlieb PA, Barker JM, Eisenbarth GS. Dominant suppression of Addison's disease associated with HLA-B15. J Clin Endocrinol Metab 2011; 96(7):2154-62.
- Skinningsrud B, Lie BA, Lavant E, Carlson JA, Erlich H, Akselsen HE, Gervin K, Wolff AB, Erichsen MM, Løvås K, Husebye ES, Undlien DE. Multiple loci in the HLA complex are associated with Addison's disease. J Clin Endocrinol Metab 2011; 96(10):E1703-8.
- Akirav EM, Ruddle NH, Herold KC. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol 2011; 7(1):25-33.
- Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol 2010; 6(5):270-7.
- Betterle C, Lazzarotto F, Presotto F. Autoimmune polyglandular syndrome Type 2: the tip of an iceberg? Clin Exp Immunol 2004; 137(2):225-33.
- Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev 2002; 23(3):327- 64.
- Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta. 2012; 1822(9): 1465-74.
- Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med 1989; 110(3):227-35.
- Rao RH. Bilateral massive adrenal hemorrhage. Med Clin North Am 1995; 79(1):107-29.
- Aron DC, Findling JW, Tyrrell JB. Glucocorticoids and adrenal androgens. In: Greenspan’s Basic and Clinical Endocrinology. Gardner DG, Shoback D (eds). Mc Graw Hill 2007; pp 367-378.
- Caron P, Chabannier MH, Cambus JP, Fortenfant F, Otal P, Suc JM. Definitive adrenal insufficiency due to bilateral adrenal hemorrhage and primary antiphospholipid syndrome. J Clin Endocrinol Metab 1998; 83(5):1437-9.
- Bhatia E, Jain SK, Gupta RK, Pandey R. Tuberculous Addison's disease: lack of normalization of adrenocortical function after anti-tuberculous chemotherapy. Clin Endocrinol (Oxf) 1998; 48(3):355-9.
- Walker BF, Gunthel CJ, Bryan JA, Watts NB, Clark RV. Disseminated cryptococcosis in an apparently normal host presenting as primary adrenal insufficiency: diagnosis by fine needle aspiration. Am J Med 1989; 86(6 Pt 1):715-7.
- Norbiato G, Galli M, Righini V, Moroni M. The syndrome of acquired glucocorticoid resistance in HIV infection. Baillieres Clin Endocrinol Metab 1994; 8(4):777-87.
- Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med 1984; 310(22):1415-21.
- Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard-Hibon A, Vivien B, Bertrand L, Beltramini A, Gamand P, Albizzati S, Perdrizet D, Lebail G, Chollet-Xemard C, Maxime V, Brun-Buisson C, Lefrant JY, Bollaert PE, Megarbane B, Ricard JD, Anguel N, Vicaut E, Adnet F; KETASED Collaborative Study Group. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet 2009; 374(9686):293-300.
- Sonino N. The use of ketoconazole as an inhibitor of steroid production. N Engl J Med 1987; 317:812.
- Schoneshofer M, Claus M. Multiple-sites of inhibition by intravenous metyrapone of human adrenal steroidogenesis. Acta Endocrinol (Copenh) 1985; 109(3):378-85.
- Elias AN, Gwinup G. Effects of some clinically encountered drugs on steroid synthesis and degradation. Metabolism 1980; 29:582.
- Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, Pazdur R. Food and Drug Administration drug approval summary: Sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist 2007; 12(1):107-13.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014; 383(9935): 2152-2167.
- Flück CE. MECHANISMS IN ENDOCRINOLOGY: Update on pathogenesis of primary adrenal insufficiency: beyond steroid enzyme deficiency and autoimmune adrenal destruction. Eur J Endocrinol. 2017; 177(3):R99-R111.
- Stewart PM. The adrenal cortex. In: Williams Textbook of Endocrinology. Larsen PR, Kronenberg HM, Melmed S, Polonsky KS (eds). Saunders, Philadelphia, PA. 2003; pp 525-532.
- Bratland E, Husebye ES. Cellular immunity and immunopathology in autoimmune Addison's disease. Mol Cell Endocrinol 2011; 336(1-2):180-90.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335(16):1206-12.
- Cronin CC, Callaghan N, Kearney PJ, Murnaghan DJ, Shanahan F. Addison disease in patients treated with glucocorticoid therapy. Arch Intern Med 1997; 157:456.
- Ohtani R, Yayama K, Takano M, Itoh N, Okamoto H. Stimulation of angiotensinogen production in primary cultures of rat hepatocytes by glucocorticoid, cyclic adenosine 3',5'-monophosphate, and interleukin-6. Endocrinology 1992; 130:1331.
- Jeremy JY, Dandona P. Inhibition by hydrocortisone of prostacyclin synthesis by rat aorta and its reversal with RU486. Endocrinology 1986; 119:661.
- Hägg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol 1987; 26:221.
- Oelkers W, Diederich S, Bähr V. Diagnosis and therapy surveillance in Addison's disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab 1992; 75(1):259-64.
- Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol 1996; 44:151.
- Thaler LM, Blevins LS Jr. The low dose (1-µg) adrenocorticotropin stimulation test in the evaluation of patients with suspected central adrenal insufficiency. J Clin Endocrinol Metab 1998; 83:2726.
- Nye EJ, Grice JE, Hockings GI, Strakosch CR, Crosbie GV, Walters MM, Jackson RV. Comparison of adrenocorticotropin (ACTH) stimulation tests and insulin hypoglycemia in normal humans: low dose, standard high dose, and 8-hour ACTH-(1-24) infusion tests. J Clin Endocrinol Metab 1999; 84(10):3648-55.
- Abdu TAM, Elhadd TA, Neary E, Clayton RN. Comparison of the low dose short Synacthen test (1 µg), the conventional dose short synacthen test (250 µg), and the insulin tolerance test for assessment of the hypothalamic-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 1999; 84:838.
- Kazlauskaite R, Evans AT, Villabona CV, Abdu TA, Ambrosi B, Atkinson AB, Choi CH, Clayton RN, Courtney CH, Gonc EN, Maghnie M, Rose SR, Soule SG, Tordjman K; Consortium for Evaluation of Corticotropin Test in Hypothalamic-Pituitary Adrenal Insufficiency. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab 2008; 93(11):4245-53.
- Stewart PM, Clark PM. The low-dose corticotropin-stimulation test revisited: the less, the better? Nat Clin Pract Endocrinol Metab 2009; 5(2):68-9.
- Mushtaq T, Shakur F, Wales JK, Wright NP. Reliability of the low dose synacthen test in children undergoing pituitary function testing. J Pediatr Endocrinol Metab 2008; 21(12):1129-32.
- Park YJ, Park KS, Kim JH, Shin CS, Kim SY, Lee HK. Reproducibility of the cortisol response to stimulation with the low dose (1 microg) of ACTH. Clin Endocrinol (Oxf) 1999; 51(2):153-8.
- Wade M, Baid S, Calis K, Raff H, Sinaii N, Nieman L. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur J Endocrinol 2010; 162(1):109-13.
- Rose LI, Williams GH, Jagger PI, Lauler DP. The 48-hour adrenocorticotropin infusion test for adrenocortical insufficiency. Ann Intern Med 1970; 73:49.
- Chrousos GP, Kino T, Charmandari E. Evaluation of the hypothalamic-pituitary-adrenal axis function in childhood and adolescence. Neuroimmunomodulation 2009; 16(5):272-83.
- Grossman AB. Clinical Review#: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab 2010; 95(11):4855-63.
- Schulte HM; Chrousos GP; Avgerinos P; Oldfield EH; Gold PW; Cutler GB Jr; Loriaux DL. The corticotropin-releasing hormone stimulation test: A possible aid in the evaluation of patients with adrenal insufficiency. J Clin Endocrinol Metab 1984; 58:1064.
- Gold PW, Kling MA, Khan I, Calabrese JR, Kalogeras K, Post RM, Avgerinos PC, Loriaux DL, Chrousos GP. Corticotropin releasing hormone: relevance to normal physiology and to the pathophysiology and differential diagnosis of hypercortisolism and adrenal insufficiency. Adv Biochem Psychopharmacol 1987; 43:183-200.
- Reato G, Morlin L, Chen S, Furmaniak J, Smith BR, Masiero S, Albergoni MP, Cervato S, Zanchetta R, Betterle C. Premature ovarian failure in patients with autoimmune Addison's disease: clinical, genetic, and immunological evaluation. J Clin Endocrinol Metab 2011; 96(8): E1255-61.
- Laureti S, Aubourg P, Calcinaro F, Rocchiccioli F, Casucci G, Angeletti G, Brunetti P, Lernmark A, Santeusanio F, Falorni A. Etiological diagnosis of primary adrenal insufficiency using an original flowchart of immune and biochemical markers. J Clin Endocrinol Metab 1998; 83(9):3163-8.
- Falorni A, Laureti S, De Bellis A, Zanchetta R, Tiberti C, Arnaldi G, Bini V, Beck-Peccoz P, Bizzarro A, Dotta F, Mantero F, Bellastella A, Betterle C, Santeusanio F; SIE Addison Study Group. Italian addison network study: update of diagnostic criteria for the etiological classification of primary adrenal insufficiency. J Clin Endocrinol Metab 2004; 89(4):1598-604.
- Boland GW. Adrenal imaging: from Addison to algorithms. Radiol Clin North Am 2011; 49(3):511-28.
- Ouyang T, Rothfus WE, Ng JM, Challinor SM. Imaging of the pituitary. Radiol Clin North Am 2011; 49(3):549-71.
- Løvås K, Gjesdal CG, Christensen M, Wolff AB, Almås B, Svartberg J, Fougner KJ, Syversen U, Bollerslev J, Falch JA, Hunt PJ, Chatterjee VK, Husebye ES. Glucocorticoid replacement therapy and pharmacogenetics in Addison's disease: effects on bone. Eur J Endocrinol 2009; 160(6):993-1002.
- Bleicken B, Hahner S, Loeffler M, Ventz M, Decker O, Allolio B, Quinkler M. Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2010; 72(3):297-304.
- Charmandari E, Kino T, Chrousos GP. Glucocorticoids. In Neonatal and Pediatric Pharmacology, 4th Edition. Yaffe SJ and Aranda JV (eds), Lippincott Williams & Wilkins, Philadelphia, PA, USA. 2010; Chapter 51; pages: 760-772.
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab 2009; 94(4):1059-67.
- Løvås K, Husebye ES. Replacement therapy for Addison's disease: recent developments. Expert Opin Investig Drugs 2008; 17(4):497-509.
- Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab 2009; 23(2):167-79.
- Quinkler M, Hahner S. What is the best long-term management strategy for patients with primary adrenal insufficiency? Clin Endocrinol (Oxf) 2012; 76(1):21-5.
- Falorni A, Minarelli V, Morelli S. Therapy of adrenal insufficiency: an update. Endocrine 2013; 43(3):514-28.
- Reisch N, Arlt W. Fine tuning for quality of life: 21st century approach to treatment of Addison's disease. Endocrinol Metab Clin North Am 2009; 38(2):407-18.
- Bleicken B, Hahner S, Loeffler M, Ventz M, Allolio B, Quinkler M. Impaired subjective health status in chronic adrenal insufficiency: impact of different glucocorticoid replacement regimens. Eur J Endocrinol 2008; 159(6):811-7.
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med 1999; 341(14):1013-20.
- Callies F, Fassnacht M, van Vlijmen JC, Koehler I, Huebler D, Seibel MJ, Arlt W, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency: effects on body composition, serum leptin, bone turnover, and exercise capacity. J Clin Endocrinol Metab 2001; 86(5):1968-72.
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 2009; 11(9):1093-102.
- Newell-Price J, Whiteman M, Rostami-Hodjegan A, Darzy K, Shalet S, Tucker GT, Ross RJ. Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin Endocrinol (Oxf) 2008; 68(1):130-5.
- Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell- Price J, Darzy K, Merke DP, Arlt W, Ross RJ. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab 2009; 94(5):1548-54.
- Johannsson G, Nilsson AG, Bergthorsdottir R, Burman P, Dahlqvist P, Ekman B, Engström BE, Olsson T, Ragnarsson O, Ryberg M, Wahlberg J, Biller BM, Monson JP, Stewart PM, Lennernäs H, Skrtic S. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. J Clin Endocrinol Metab 2012; 97(2):473-81.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016; 101(2):364-89.
- Inder WJ, Meyer C, Hunt PJ. Management of hypertension and heart failure in patients with Addison's disease. Clin Endocrinol (Oxf) 2015; 82(6):789-92.
- Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterjee VK. Improvement in mood and fatigue after dehydroepiandro- sterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab 2000; 85:4650-4656.
- Brooke AM, Kalingag LA, Miraki-Moud F, Camacho-Hubner C, Maher KT, Walker DM, Hinson JP, Monson JP. Dehydroepiandrosterone improves psychological well-being in male and female hypopituitary patients on mainte- nance growth hormone replacement. J Clin Endocrinol Metab 2006; 91:3773-3779.
- Gurnell EM, Hunt PJ, Curran SE, Conway CL, Pullenayegum EM, Huppert FA, Compston JE, Herbert J, Chatterjee VK. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J Clin Endocrinol Metab 2008; 93:400-409.
- Bhagra S, Nippoldt TB, Nair KS. Dehydroepiandrosterone in adrenal insufficiency and ageing. Curr Opin Endocrinol Diabetes Obes 2008; 15(3):239-43.
- Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab 2009; 94(10):3676-81.
- Binder G, Weber S, Ehrismann M, Zaiser N, Meisner C, Ranke MB, Maier L, Wudy SA, Hartmann MF, Heinrich U, Bettendorf M, Doerr HG, Pfaeffle RW, Keller E; the South German Working Group for Pediatric Endocrinology. Effects of dehydroepiandrosterone therapy on pubic hair growth and psycho- logical well-being in adolescent girls and young women with central adrenal insufficiency: a double-blind, randomized, placebo-controlled phase III trial. J Clin Endocrinol Metab 2009; 94:1182-1190.
- Arlt W. Androgen therapy in women. Eur J Endocrinol 2006; 154(1):1-11.
- Fleseriu M, Loriaux DL. "Relative" adrenal insufficiency in critical illness. Endocr Pract 2009; 15(6):632-40.
- Annetta M, Maviglia R, Proietti R, Antonelli M. Use of corticosteroids in critically ill septic patients: a review of mechanisms of adrenal insufficiency in sepsis and treatment. Curr Drug Targets 2009; 10(9):887-94.
- Cohen J, Venkatesh B. Relative adrenal insufficiency in the intensive care population; background and critical appraisal of the evidence. Anaesth Intensive Care 2010; 38(3):425-36.
- Moraes RB, Czepielewski MA, Friedman G, Borba EL. Diagnosis of adrenal failure in critically ill patients. Arq Bras Endocrinol Metabol 2011; 55(5):295-302.
- Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Med 2011; 37(6):901-10.
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab 2008; 19(5):175-80.
- Charmandari E, Kino T, Ichijo T, Chrousos GP. Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder. J Clin Endocrinol Metab 2008; 93(5):1563-72.
- Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids 2010; 75(1):1-12.
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 2012; 1261:55-63.
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M; American College of Critical Care Medicine. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008; 36(6):1937-49.
- Yuen KC, Chong LE, Koch CA. Adrenal insufficiency in pregnancy: challenging issues in diagnosis and management. Endocrine 2013; 44(2):283-92.
- Langlois F, Lim DST, Fleseriu M. Update on adrenal insufficiency: diagnosis and management in pregnancy. Curr Opin Endocrinol Diabetes Obes. 2017; 24(3):184-192.
- Suri D, Moran J, Hibbard JU, Kasza K, Weiss RE. Assessment of adrenal reserve in pregnancy: defining the normal response to the adrenocorticotropin stimulation test. J Clin Endocrinol Metab 2006; 91(10):3866-72.
- Lebbe M, Arlt W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin Endocrinol (Oxf) 2013; 78(4):497-502.
- Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol 2015; 3(3):216-26.
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC; Endocrine Society. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95(9):4133-60.
Primary Generalized Glucocorticoid Resistance or Chrousos Syndrome
ABSTRACT
Primary Generalized Glucocorticoid Resistance or Chrousos Syndrome is a rare endocrinologic condition, which affects almost all tissues, and is characterized by resistance of tissues to glucocorticoids. The clinical spectrum of Chrousos syndrome is broad, ranging from asymptomatic cases to severe cases of mineralocorticoid and/or androgen excess. At the molecular level, Chrousos syndrome has been associated with defects in the NR3C1 gene that encodes the human glucocorticoid receptor (hGR). We and others have applied molecular and structural biology methods to investigate the molecular mechanisms of action of the mutant hGRs. We demonstrated that the defective hGRs impair several steps of glucocorticoid signaling cascade depending on the position of the NR3C1 gene defect. In clinical practice, when Chrousos syndrome is suspected, a detailed personal and family history should be obtained, while physical examination should include an assessment for signs of mineralocorticoid and/or androgen excess. Suspected patients should then undergo a detailed endocrinologic evaluation with particular emphasis on the measurement of serum cortisol concentrations and determination of the 24-hour urinary free cortisol (UFC) excretion on 2 or 3 consecutive days. Affected subjects demonstrate resistance of the HPA axis to dexamethasone suppression, which may vary depending on the severity of the condition. The diagnosis of Chrousos syndrome is confirmed by sequencing of the coding region of the NR3C1 gene, including the intron/exon junctions. Treatment of Chrousos syndrome involves administration of high doses of mineralocorticoid-sparing synthetic glucocorticoids, which activate the mutant and/or wild-type hGRα, and suppress the endogenous secretion of ACTH in affected subjects. For complete coverage of this and related areas in Endocrinology, visit our free web-books, www.endotext.org and www.thyroidmanager.org.
GLUCOCORTICOIDS
THE GLUCOCORTICOID RECEPTOR GENE (NR3C1) AND PROTEIN ISOFORMS
At the molecular level, glucocorticoids signal through an intracellular protein, the glucocorticoid receptor (GR) (3, 5, 6). The human (h) GR is a member of the steroid/thyroid/retinoic acid nuclear receptor superfamily of transcription factor proteins and functions as a ligand-dependent transcription factor that influences the transcription rate of numerous glucocorticoid target genes in a positive or negative fashion. The hGR gene (NR3C1) consists of 9 exons and is located on chromosome 5q31.3. Exons 2-9 constitute the protein-coding exons, whereas exon 1 consists of untranslated sequence with the transcription start site connected to multiple promoters. Alternative splicing of the NR3C1 gene in exon 9 generates two highly homologous receptor isoforms, the hGRα and hGRβ. These protein isoforms share 727 common amino acids, but then diverge, with hGRα having an additional 50 amino acids and hGRβ having an additional, nonhomologous 15 amino acids. The hGRα resides primarily in the cytoplasm of cells and represents the classic glucocorticoid receptor that binds natural and synthetic glucocorticoids and mediates the genomic and most of the nongenomic actions of these hormones. The hGRβ, on the other hand, does not bind glucocorticoid agonists, may or may not bind the synthetic glucocorticoid antagonist RU486, has intrinsic, hGRa-independent, gene-specific transcriptional activity, and exerts a dominant negative effect upon the transcriptional activity of hGRa (3, 5-8). Moreover, recent studies have shown that hGRβ is implicated in insulin signaling (9) and participates in the molecular pathogenetic mechanisms of glioma formation through regulation of β-catenin/T-cell factor/lymphoid enhancer factor (TCF/LEF) transcriptional activity (10, 11).
The hGRα mRNA further expresses multiple isoforms by using at least 8 alternative amino-terminal translation initiation sites (12). All these hGRα isoforms are differentially distributed in the cytoplasm and/or the nucleus in the absence of ligand, have different transcriptional activity following ligand-induced activation and display distinct transactivation or transrepression patterns on global gene expression examined by cDNA microarray analyses (12). Therefore, these hGRα isoforms may differentially transduce the glucocorticoid signal to target tissues depending on their selective relative expression and inherent activities. Since hGRβ shares a common amino-terminal domain that contains the same translation initiation sites with the hGRα, the hGRβ variant mRNA might also be translated through the same initiation sites to a similar host of hGRβ isoforms. It is likely that differential cell-specific production and functional differences might also be present between the putative hGRβ translational isoforms.
The NR3C1 gene has at least three different promoters, A, B and C. Promoter A can be used with three untranslated exons, 1A1, 1A2 and 1A3, that contain unique promoter fragments (13). Therefore, the NR3C1 gene can produce five different transcripts from different promoters that encode the same hGR proteins. Through differential use of these promoters, the expression levels of hGR proteins may vary considerably among tissues. The splice and translational hGR isoforms expressed from different promoters appear to form up to 256 different combinations of homo- and hetero-dimers with varying transcriptional activities. The marked complexity in the transcription/translation of the NR3C1 gene enables target tissues to differentially respond to circulating glucocorticoid concentrations and accounts for the highly stochastic nature of the glucocorticoid signaling pathway (14).
The hGRα protein consists of four functional domains (5). The N-terminal or immunogenic domain (NTD) is encoded by exon 2 and represents the largest domain of the receptor containing amino acids that undergo several post-translational modifications, as well as the activation function-1 (AF-1) domain that is used by the receptor as a molecular platform for the molecular interactions with coactivators (5). The DNA-binding domain (DBD) is expressed by exons 3 and 4, and lies between amino acids 420 and 480. This domain consists of the characteristic motif of two zinc fingers, which facilitates the interaction between the receptor and its target DNA sequences in the promoter regions of glucocorticoid-responsive genes (5). The ligand-binding domain (LBD) is located at the carboxyl-terminal fragment of the receptor and corresponds to amino acids 481 to 777. Encoded by exons 5-9, this region contains amino acids responsible for the binding of the receptor to natural and synthetic glucocorticoids, for the cytoplasmic-to-nuclear translocation following ligand-induced activation of the receptor, as well as for the transactivation and interaction of the receptor with coactivator molecules (AF-2 domain) in a ligand-dependent fashion. Finally, a hinge region lies between the DBD and LBD. This protein domain provides the appropriate structural flexibility to the receptor and allows the interaction of the latter with several different glucocorticoid-responsive genes (5).
GENOMIC AND NONGENOMIC hGR ACTIONS
At the target cell, the inactivated hGRα resides primarily in the cytoplasm as part of a hetero-oligomeric complex consisting of chaperone heat shock proteins (HSPs) 90, 70 and 50, immunophilins, as well as other proteins (15). HSP90 regulates ligand binding, as well as cytoplasmic retention of hGRα by exposing the ligand-binding site and masking the two nuclear localization sequences (NLS), NL1 and NL2, which are located adjacent to the DNA-binding domain (DBD) and in the ligand-binding domain (LBD) of the receptor, respectively. Upon ligand-induced activation, the receptor undergoes a conformational change that results in dissociation from this multiprotein complex and translocation into the nucleus (Figure 1) (15, 16). Within the nucleus, the receptor binds as a dimer to tandem glucocorticoid-response elements (GREs) in the promoter regions of target genes, and regulates their expression positively or negatively depending on GRE sequence and promoter context (17, 18). The GRE-bound hGRα stimulates the transcription of target genes by facilitating the formation of the transcription initiation complex, including the RNA polymerase II and its ancillary components (19). To initiate transcription, hGRα uses its transcriptional activation domains, AF-1 and AF-2, as surfaces to interact with nuclear receptor coactivators and chromatin-remodeling complexes. Several coactivators form a bridge between the DNA-bound hGRα and the transcription initiation complex, and facilitate the transmission of the glucocorticoid signal to the RNA polymerase II (20-22). These include: (1) The p300 and the homologous cAMP-responsive element-binding protein (CREB)-binding protein (CBP), which also serve as macromolecular docking “platforms” for transcription factors from several signal transduction cascades, including nuclear receptors, CREB, AP-1, NF-kB, p53, Ras-dependent growth factor, and STATs. In view of their central position in many signal transduction cascades, the p300/CBP coactivators are also called co-integrators; (2) The p300/CBP-associated factor (p/CAF), which interacts with p300/CBP, but is also a broad transcription coactivator; and (3) The p160 family of coactivators, which preferentially interact with the steroid hormone receptors, and include the steroid receptor coactivator-1 (SRC-1), SRC-2 and SRC-3 (20-22). The hGRα also interacts with several other distinct chromatin modulators through its transactivation domains, such as the mating-type switching/sucrose non-fermenting (SWI/SNF) complex and components of the vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein (DRIP/TRAP) complex (20-22) (Figure 1).

Figure 1: Genomic, nongenomic and mitochondrial glucocorticoid actions. Upon binding to the ligand, the activated hGRα dissociates from heat shock proteins (HSPs) and translocates into the nucleus, where it homodimerizes and binds to glucocorticoid response elements (GREs) in the promoter region of target genes or interacts with other transcription factors (TFs), such as activator protein-1 (AP-1), nuclear factor-κB (NF-κB) and signal transducer and activator of transcription-5 (STAT5), ultimately modulating the transcriptional activity of respectively GRE- or TFRE-containing genes. In addition to the well-known genomic actions, most of the nongenomic glucocorticoid actions are mediated by membrane-bound GRs, which trigger the activation of kinase signaling pathways. Furthermore, accumulating evidence suggests that the ligand-bound hGRα influences the expression of mitochondrial DNA in a direct or indirect fashion. GR: glucocorticoid receptor; HSP: heat shock proteins; FKBP: immunophillins; p160: nuclear receptor coactivators p160; SWI/SNF: switching/sucrose non-fermenting complex; DRIP/TRAP: vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein complex; MAPK: mitogen-activated protein kinases; cPLA2α: cytosolic phospholipase A2 alpha; PI3K: phosphatidylinositol 3-kinase; eNOS: endothelial nitric oxide synthetase; NO: nitric oxide.
The ligand-activated hGRα can also modulate gene expression independently of binding to GREs, by interacting possibly as a monomer with other transcription factors, such as activator protein-1 (AP-1), nuclear factor-κB (NF-κB), p53 and signal transducers and activators of transcription (STATs) (23, 24) (Figure 1). Therefore, hGRα may affect signal transduction cascades through protein-protein interactions with specific transcription factors by influencing their ability to stimulate or inhibit the transcription rates of respective target genes. This activity may be more important than the GRE-mediated one, given that mice harboring a mutant GR, which is active in terms of protein-protein interactions but inactive in terms of transactivation via DNA, survive and procreate, in contrast to mice with a deletion of the entire NR3C1 gene that die immediately after birth from severe respiratory distress syndrome (25). The protein-protein interactions of GR with other transcription factors may take place on the promoters that do not contain GREs, as well as on the promoters that have both GRE(s) and responsive element(s) of transcription factors that interact with GR (“composite promoters”). Suppression of transactivation of other transcription factors through protein-protein interactions may be particularly important in suppression of immune function and inflammation by glucocorticoids (25, 26). Most of the effects of glucocorticoids on the immune system may be mediated by the interaction between GR and NF-kB, AP-1 and STATs (27, 28).
Following transcriptional activation or inhibition of glucocorticoid-responsive genes, the hGRα dissociates from the ligand and has a lower affinity for binding to GREs. The unliganded hGRα remains within the nucleus for a considerable length of time and is then exported to the cytoplasm; both within the nucleus and within the cytoplasm the hGR may be recycled and/or degraded in the proteasome (3, 5) (Figure 1).
Although the transcriptional activity of GR is primarily governed by ligand binding, accumulating evidence suggests that post-translational modifications (PTMs) play an important additional role. These include phosphorylation, ubiquitination, acetylation and sumoylation of the receptor. These covalent changes may affect receptor stability, subcellular localization, as well as the interaction between GR and other proteins (5).
Further to the above-described genomic actions, mounting evidence suggests that glucocorticoids also signal within seconds or minutes. These effects are termed as “nongenomic”, since they do not require hGRα transcriptional activity. Although the underlying mechanisms are not fully understood, recent studies have demonstrated that most of the nongenomic glucocorticoid actions are triggered by membrane-bound GRs, which induce the activity of kinase signaling pathways, such as the mitogen-activated protein kinase (MAPK) or the phosphatidylinositol 3-kinase (PI3K) cascades (29-32) (Figure 1). Some representative examples of these actions are: (i) the immediate suppression of ACTH release from the anterior lobe of pituitary by glucocorticoids (33); (ii) the increased frequency of excitatory post-synaptic potentials in the hippocampus (34); (iii) the cardioprotective role of glucocorticoids through nitric oxide (NO)-mediated vasorelaxation in patients with myocardial infarction or stroke (35); and (iv) some immunomodulatory glucocorticoid effects via disruption of T-cell receptor signaling (36).
In addition to genomic and nongenomic actions, glucocorticoids exert some effects through mitochondrial hGRs, granted that many regulatory sites (D-loop) of the mitochondrial genome have functional GREs (37) (Figure 1). Several studies have shown that the ligand-activated hGRα translocates from the cytoplasm to mitochondrion and influences substantially mitochondrial gene expression (37-39). Moreover, many mitochondrial RNA-processing enzymes or transcription factors are expressed under the control of nuclear hGRα, suggesting a dynamic interrelation between glucocorticoids, mitochondria and the nucleus (40). Importantly, the mitochondrial hGRα has been early recognized as a potent therapeutic target, because of its involvement in the programmed cell death (apoptosis) of malignant cells. Indeed, nowadays, synthetic glucocorticoids are the cornerstone of several therapeutic protocols of hematologic malignancies (40).
CHROUSOS SYNDROME
The internal equilibrium of all living organisms, termed homeostasis, is adequately achieved by the optimal effect of all homeostatic systems that occurs in the middle range of homeostatic activity. Too much or too little activity ultimately leads to dysfunction of homeostasis, termed allostasis or cacostasis (41). Alterations in any step of glucocorticoid signal transduction may cause impaired tissue sensitivity to glucocorticoids, which may present with clinical manifestations of glucocorticoid resistance or glucocorticoid hypersensitivity, conditions with significant morbidity (42, 43). One such condition that we have extensively investigated both at clinical and molecular level over the years is Primary Generalized Glucocorticoid Resistance or Chrousos syndrome (44-56).
Clinical Manifestations
Primary Generalized Glucocorticoid Resistance is a condition first described and elucidated by Chrousos et al. as a rare, familial or sporadic, genetic disorder characterized by generalized, partial, end-organ insensitivity to glucocorticoids (47-58). Because of the generalized glucocorticoid resistance, the glucocorticoid negative feedback inhibition at the hypothalamic and anterior pituitary levels is decreased, leading to compensatory activation of the HPA axis, inferred hypersecretion of corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP) in the hypophysial portal system and increased secretion of adrenocorticotropic hormone (ACTH) in the systemic circulation (47-58) (Figure 2). The excess ACTH secretion results in adrenocortical hyperplasia and increased secretion of cortisol and adrenal steroids with mineralocorticoid [deoxycorticosterone (DOC) and corticosterone] and/or androgenic [androstenedione, dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS)] activity (47-58) (Figure 2). In recognition of Professor George P. Chrousos' extensive and ground-breaking research work in this field, it has been proposed that the term “Chrousos Syndrome” is used in place of “Primary Generalized Familial or Sporadic Glucocorticoid Resistance” (48).

Figure 2: Pathophysiologic mechanisms and clinical manifestations of Chrousos syndrome. CRH: corticotropin-releasing hormone; AVP: arginine-vasopressin; ACTH: adrenocorticotropic hormone. Modified from Reference (54).
The clinical presentation of Chrousos syndrome reflects the pathophysiologic alterations described above and is mainly associated with, respectively, hypertension and/or hypokalemic alkalosis and hyperandrogenism (47-58) (Table 1) (Figure 2). Clinical manifestations of glucocorticoid deficiency might occur, but are rare and were only reported in a young child with hypoglycemic generalized tonic-clonic seizures during the course of a febrile illness (59), in a newborn baby with severe hypoglycemia, excessive fatigability with feeding, increased susceptibility to infections and concurrent growth hormone deficiency (60), and in several adult patients with chronic fatigue. The latter might indicate inadequate glucocorticoid target tissue compensation at the central nervous system (CNS) and/or the skeletal muscles by the increased circulating cortisol concentrations (47-58). Clinical manifestations of androgen excess include ambiguous genitalia in a karyotypic female at birth and gonadotropin-independent precocious puberty in children of either gender; acne, hirsutism and decreased fertility in both sexes; male-pattern hair loss, menstrual irregularities and oligo-anovulation in females; and oligospermia in males (Table 1). The impaired fertility in both sexes has been attributed in part to the feedback inhibition of gonadotropin secretion by the elevated androgen concentrations, while the profound anxiety observed in some subjects is probably due to compensatory increases in hypothalamic CRH and AVP secretion. The latter might also predispose the patients to the development of an ACTH-secreting pituitary adenoma. Finally, the elevated circulating ACTH concentrations may be responsible for the observed growth of intra-testicular adrenal rests and oligospermia (47-58).
The clinical spectrum of Chrousos syndrome is broad, ranging from most severe to mild forms, and a number of patients may be asymptomatic, displaying biochemical alterations only (47-58) (Table 1). This variable clinical phenotype is due to variations in the tissue sensitivity of the glucocorticoid, mineralocorticoid and/or androgen receptor signaling pathways; variations in the activity of key hormone-inactivating or -activating enzymes, such as the 11β-hydroxysteroid dehydrogenase (61) and 5α-reductase (62); and other genetic or epigenetic factors, such as the presence of insulin resistance and visceral obesity (58).
Molecular Mechanisms
The molecular basis of Chrousos syndrome has been ascribed primarily to mutations in the NR3C1 gene, which impair the molecular mechanisms of hGR action and decrease tissue sensitivity to glucocorticoids. The pathologic NR3C1 gene mutations causing Chrousos syndrome that have been reported to date are shown in Table 2 (56, 57, 59, 60, 63-82) and Figure 3. Eight-teen out of 22 of these mutations are heterozygous (4 are homozygous), while all mutations partially inactivate hGR function. Although studies of GR knock-out mice suggested that complete loss-of-function of the GR is incompatible with extrauterine life (83), one out of 15 of the mutations completely inactivated hGR function (60). The first described hGR gene mutation was a homozygotic adenine to thymine substitution at nucleotide position 1922, which resulted in substitution of aspartic acid to valine at amino acid residue 641 (57). In vitro studies showed that the mutant receptor hGRαD641V demonstrated decreased ability to transactivate glucocorticoid-responsive genes, had lower affinity for the ligand, showed delayed nuclear translocation when exposed to ligand and interacted with the glucocorticoid receptor interacting protein 1 (GRIP1) less effectively (57).

Figure 3: Location of the known mutations of the NR3C1 gene causing Chrousos syndrome. Mutations in the upper panel are located in the LBD of the receptor, while mutations in the lower panel are located in the DBD. Modified from Reference (56).
The molecular mechanisms through which these various natural hGR mutants affected glucocorticoid signal transduction were systematically investigated in all reported cases with the condition. These mechanisms included: i) the transcriptional activity of the mutant receptors; ii) the ability of the heterozygous mutant receptors to exert a dominant negative effect upon the wild-type receptor; iii) the concentrations and affinity of the mutant receptors for the ligand; iv) the subcellular localization of the mutant receptors and their nuclear translocation following exposure to the ligand; v) the ability of the mutant receptors to bind to GREs; vi) the interaction of the mutant receptors with the glucocorticoid receptor-interacting protein 1 (GRIP1) coactivator, which belongs to the p160 family of nuclear receptor coactivators and plays an important role in hGRa-mediated transactivation of glucocorticoid-responsive genes; vii) the motility of the mutant receptors inside the nucleus; viii) the ability of the mutant receptors to transrepress the NF-κB signaling pathway; and ix) structural biology studies (56, 57, 59, 60, 63-82).
The molecular defects that have been elucidated in cases with Chrousos syndrome are summarized in Table 2. Compared with the wild-type receptor, all mutant receptors demonstrated variable reduction in their ability to transactivate glucocorticoid-responsive genes following exposure to dexamethasone, with the most severe impairment observed in the cases of V423A, R477H, I559N, V571A, D641V, R477S and L672P mutations (56, 57, 59, 60, 63-82). Furthermore, the mutant receptors hGRαI559N, hGRαR714Q, hGRαF737L, hGRαI747M and hGRαL773P exerted a dominant negative effect upon the wild-type receptor, which might have contributed to manifestation of the disease at the heterozygote state (59, 63, 67, 70, 72, 74). All mutant receptors in which the mutations were located in the LBD of the receptor showed a variable reduction in their affinity for the ligand, with the most severe reduction observed in the cases of I559N, I747M and V571A mutations (56, 57, 59, 60, 63-82). The only mutant receptors that demonstrated normal affinity for the ligand were the hGRαV423A, the hGRαR477H, the hGRαR477S and the hGRαY478C in which the mutations were located at the DBD (73, 77, 82).
In subcellular localization and nuclear translocation studies, the pathologic mutant receptors were observed primarily in the cytoplasm of cells in the absence of ligand, except for the hGRaV729I and hGRaF737L receptors, which were localized both in the cytoplasm and the nucleus of cells. Exposure to dexamethasone induced a slow translocation of the mutant receptors into the nucleus, which ranged from 20 min (R477H) to 180 min (I559N and F737L) compared with the wild-type hGRa, which required only 12 min for complete translocation (56, 57, 59, 60, 63-82). These findings suggest that all hGR mutations affect the nucleocytoplasmic shuttling of the receptor, probably through impairment of the nuclear localization signal (NL)-1 and/or NL2 functions (84). Interestingly, the mutant receptors hGRαV423A, hGRαR477S and hGRαY478C also show delayed cytoplasmic-to-nuclear translocation upon exposure to dexamethasone, compared with the wild-type hGRa. Although these mutations are located in the DBD of the receptor, they indirectly affect the function of NL1 through R477, possibly leading to delayed cytoplasmic-to-nuclear translocation (77, 82).
All mutant receptors in which the mutations were located in the LBD preserved their ability to bind to DNA (56, 57, 59, 60, 63-82). The six mutant receptors that failed to bind to DNA were the hGRαR469X, the hGRαR477S, the hGRαR477C, the hGRαR477H and the hGRαY478C, in which the mutations were located at the C-terminal zinc finger of the DBD, and the hGRαV423A, in which the point substitution was found in the first zinc finger of the DBD of the receptor (73, 75, 77, 82). A major function of the C-terminal zinc finger of the DBD of hGRα is to contribute to receptor homodimerization, a prerequisite for potent receptor binding to GREs and efficient transactivation of glucocorticoid-responsive genes (85). The majority of the mutant receptors displayed an abnormal interaction with the GRIP1 (SRC-2) coactivator in vitro (56, 57, 59, 60, 63-82). Moreover, all mutant receptors had dynamic motility defects inside the nucleus of living cells, possibly caused by their inability to properly interact with key partner nuclear molecules of the transcription initiation complex necessary for full activation of glucocorticoid-responsive genes (86). Furthermore, using new-applied reporter assays, we investigated the ability of the mutant receptors hGRαV423A, hGRαV575G, hGRαH726R and hGRαT556I to transrepress the NF-κB signaling pathway. We showed that the hGRαV575G and the hGRαT556I significantly increased the transrepression of the NF-κB signaling pathway, whereas the hGRαH726R displayed decreased ability to transrepress the NF-κB signaling pathway (77-80).
Structural biology studies were conducted for the majority of the mutations in the NR3C1 gene in an attempt to explain how alterations in the structure of the mutant receptors may cause generalized glucocorticoid resistance (59, 77-80, 82, 87). Most of the mutant receptors, in which the mutations were located in the LBD, had a defective ligand-binding pocket and/or an impaired AF-2 domain that binds to the LXXLL motifs of coactivators (59, 78-80, 82, 87). These mutations resulted in loss or reduction of the electrostatic interaction between the mutant receptors and dexamethasone, ultimately leading to decreased affinity of the receptors for the ligand (87). The impaired interaction of the mutant receptors with coactivators was mostly due to disrupted electrostatic bonds with the non-core leucines of the LXXLL motif, as well as because of the decreased noncovalent interactions with the core leucine residues (87). Structural biology assays were also performed for the V423A, R477S and Y478C mutations located in the DBD (77, 82). In the case of V423A mutation, the substitution of valine (V) to alanine (A) destroyed the protective environment created by the hydrophobic valine for the four zinc-binding cysteines (C421, C424, C438 and C441) and permitted molecules of water to diffuse into the zinc-binding region of the receptor, therefore reducing the DNA-binding ability of the hGRαV423A (77). The replacement of arginine (R) by serine (S) at amino acid position 477 in the DBD of the receptor resulted in the loss of two hydrogen bonds with GREs, leading to decreased DNA binding of the mutant receptor (82). The substitution of tyrosine (Y) by cysteine (C) in the hGRαY478C caused a significant loss of van der Waals contacts formed between tyrosine and neighboring amino acid residues, therefore destabilizing the 3D structure of the mutated DBD (82).
CLINICAL EVALUATION OF THE PATIENTS
The first step in evaluating a patient with suspected Chrousos syndrome is to obtain a complete personal and family history, with particular attention to evidence suggesting hyperactivity of the HPA axis and ACTH hypersecretion-related pathology (47-52, 54). In addition, any evidence suggesting possible CNS dysfunction, such as headaches, visual impairment or seizures, should be noted. In female subjects, the regularity of menstrual cycles should be documented. In children and adolescents, growth and sexual maturation should be evaluated carefully, given that progressive hyperandrogenism is almost invariably associated with an increased growth velocity, an advanced bone age and changes in pubertal development (47-52, 54).
The physical examination should include an assessment for signs of hyperandrogenism and/or virilization, such as acne, hirsutism, pubic and axillary hair development, male-pattern hair loss and clitoromegaly. Hirsutism should be assessed using the Ferriman-Gallwey score (88), while pubic hair development should be classified according to Tanner (89, 90). Arterial blood pressure should be recorded and preferably monitored over a 24-hour period. All subjects should be screened for signs suggestive of Cushing syndrome and undergo a complete neurologic examination.
Endocrinologic Evaluation of the Patients
The concentrations of plasma ACTH, renin activity (recumbent and upright) and aldosterone, as well as those of serum cortisol, testosterone, androstenedione, DHEA and DHEAS should be recorded in the morning (47-52, 54). Determination of the 24-hour urinary free cortisol (UFC) excretion on 2 or 3 consecutive days is central to the diagnosis, given that patients with the condition demonstrate increased 24h UFC excretion in the absence of clinical manifestations suggestive of hypercortisolism (47-52, 54). The 24-hour UFC excretion may be up to 50-fold higher compared with the highest value of its normal range, while serum cortisol concentrations may be up to 7- fold higher compared with the upper normal range. Plasma ACTH concentrations may be normal or high. However, the circadian pattern of ACTH and cortisol secretion and their responsiveness to stressors are preserved, albeit at higher concentrations (47-52, 54).
The responsiveness of the HPA axis to exogenous glucocorticoids should also be tested with dexamethasone suppression testing (47-52, 54). Increasing doses of dexamethasone (0.3, 0.6, 1.0, 1.5, 2.0, 2.5, 3.0 mg) should be given orally at midnight every other day, and a serum sample should be drawn at 0800h the following morning for determination of serum cortisol and dexamethasone concentrations. Affected subjects demonstrate resistance of the HPA axis to dexamethasone suppression, which may vary depending on the severity of the condition. The concurrent measurement of serum dexamethasone concentrations is suggested in order to exclude the possibility of increased metabolic clearance or decreased absorption of this medication (54).
Cellular and Molecular Studies in the Patients
Thymidine incorporation assays and dexamethasone-binding assays on peripheral blood mononuclear cells in association with sequencing of the NR3C1 gene are necessary to confirm the diagnosis and to provide genetic counseling (47-52, 54) (Table 1). In affected subjects, the thymidine incorporation assays reveal resistance to dexamethasone-induced suppression of phytohemaglutinin-stimulated thymidine incorporation, while the dexamethasone-binding assays often show decreased affinity of the hGR receptor for the ligand compared to control subjects. Sequencing of the coding region of the NR3C1 gene, including the intron/exon junctions, will reveal insertions, deletions or mutations in most (56, 57, 59, 60, 63-82) but not all (91) cases with Chrousos syndrome. Finally, once the sequence defect is determined, its adverse effects on receptor function should be confirmed using in vitro mutagenesis and standardized assays that examine the ability of the mutant receptor to transactivate glucocorticoid-responsive genes.
Management of the Patients
The aim of treatment in Chrousos syndrome is to suppress the excess secretion of ACTH, thereby suppressing the increased production of adrenal steroids with mineralocorticoid and/or androgenic activity. Treatment involves administration of high doses of mineralocorticoid-sparing synthetic glucocorticoids, which activate the mutant and/or wild-type hGRα, and suppress the endogenous secretion of ACTH in affected subjects (47-52, 54). Adequate suppression of the HPA axis is of particular importance in cases of severe impairment of hGRα action, given that long-standing corticotroph hyperstimulation in association with decreased glucocorticoid negative feedback inhibition at the hypothalamic and pituitary levels may lead to the development of an ACTH-secreting adenoma (63). Long-term dexamethasone treatment should be carefully titrated according to the clinical manifestations and biochemical profile of the affected subjects (47-52, 54).
CONCLUSIONS AND RECOMMENDATIONS
The glucocorticoid receptor is a ubiquitously expressed intracellular, ligand-dependent transcription factor, which mediates the action of glucocorticoids and influences physiologic functions essential for life. Mutations, deletions or insertions in the NR3C1 gene may impair one or more of the molecular mechanisms of glucocorticoid action, thereby altering tissue sensitivity to glucocorticoids. A consequent increase in the activity of the HPA axis compensates for the reduced sensitivity of peripheral tissues to glucocorticoids at the expense of ACTH hypersecretion-related pathology. The variable clinical phenotype of Chrousos syndrome, including chronic fatigue, mild hypertension and hyperandrogenism, in association with the difficulties encountered in establishing the correct diagnosis may account for the low reported prevalence of the condition, given that many cases may be unrecognized and misclassified. We recommend screening with 24h UFC excretion and sequencing of the NR3C1 gene in patients with manifestations of mineralocorticoid and androgen excess (hypertension, hirsutism, menstrual irregularities, oligo-anovulation, impaired fertility), in whom detailed investigations fail to reveal an underlying etiology.
Although Chrousos syndrome has been associated with genetic defects in the NR3C1 gene, some patients with clinical manifestations of this condition did not have any mutations, deletions or insertions in the gene encoding the hGR. In the era of novel technologies, we speculate that the application of whole genome/exome sequencing will delineate yet unknown molecular pathogenetic mechanisms of Chrousos syndrome, by identifying new partners that regulate the expression of the NR3C1 gene and/or influence hGR activity.
REFERENCES
- Kino T, Chrousos GP. Glucocorticoid effects on gene expression. In Handbook of Stress and the Brain, T. Steckler, N. H. Kalin, J. M. H. M. Reul, Eds. (Elsevier, Amsterdam, 2005), pp. 295–311.
- Chrousos GP, Charmandari E, Kino T. Glucocorticoid action networks–an introduction to systems biology. J Clin Endocrinol Metab 2004; 89:563-564.
- Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med 2004; 117:204-207.
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 2002; 16(1):61-71.
- Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids 2010; 75(1):1-12.
- Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol 2006; 102(1-5):11-21.
- Kino T, Manoli I, Kelkar S, Wang Y, Su YA, Chrousos GP. Glucocorticoid receptor (GR) β has intrinsic, GRa-independent transcriptional activity. Biochem Biophys Res Commun 2009; 381(4):671-675.
- Oakley RH, Jewell CM, Yudt MR, Bofetiado DM, Cidlowski JA. The dominant negative activity of the human glucocorticoid receptor β isoform. Specificity and mechanisms of action. J Biol Chem 1999; 274(39):27857-66.
- Stechschulte LA, Wuescher L, Marino JS, Hill JW, Eng C, Hinds TD Jr. Glucocorticoid receptor β stimulates Akt1 growth pathway by attenuation of PTEN. J Biol Chem 2014; 289:17885-94.
- Yin Y, Zhang X, Li Z, Deng L, Jiao G, Zhang B, Xie P, Mu H, Qiao W, Zou J. Glucocorticoid receptor β regulates injury-mediated astrocyte activation and contributes to glioma pathogenesis via modulation of β-catenin/TCF transcriptional activity. Neurobiol Dis 2013; 59:165-176.
- Wang Q, Lu PH, Shi ZF, Xu YJ, Xiang J, Wang YX, Deng LX, Xie P, Yin Y, Zhang B, Mu HJ, Qiao WZ, Cui H, Zou J. Glucocorticoid Receptor β Acts as a Co-activator of T-Cell Factor 4 and Enhances Glioma Cell Proliferation. Mol Neurobiol 2015; 52(3):1106-1118.
- Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell 2005; 18:331-342.
- Breslin MB, Geng D, Vedeckis WV. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol Endocrinol 2001; 15:1381-1395.
- Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE 2005; 2005(304):pe48.
- Pratt WB. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem 1993; 268(29):21455-8.
- Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 2007; 318(5855):1412-6.
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 1996; 17(3):245-61.
- Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Steroid Biochem Mol Biol 2002; 83(1-5):37-48.
- Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev 1996; 17:587-609.
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 1999; 20(3):321-44.
- McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002; 108:465-474.
- Auboeuf D, Honig A, Berget SM, O'Malley BW. Coordinate regulation of transcription and splicing by steroid receptor coregulators. Science 2002; 298:416-419.
- Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 1990; 62(6):1189-204.
- Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS Jr. Characterization of mechanisms involved in transrepression of NF-kB by activated glucocorticoid receptors. Mol Cell Biol 1995; 15(2):943-53.
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 1998; 93:531-541.
- Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schutz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J 2001; 20:7168-7173.
- Barnes PJ, Karin M. Nuclear factor-kB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997; 336:1066-1071.
- Didonato JA, Saatcioglu F, Karin M. Molecular mechanisms of immunosuppression and anti-inflammatory activities by glucocorticoids. Am J Respir Crit Care Med 1996; 154:S11-15.
- Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB J 2012; 26:4805-4820.
- Song IH, Buttgereit Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol 2006; 246:142-146.
- Lee SR, Kim HK, Youm JB, Dizon LA, Song IS, Jeong SH, Seo DY, Ko KS, Rhee BD, Kim N, Han J. Non-genomic effect of glucocorticoids on cardiovascular system. Pflugers Arch 2012; 464:549-559.
- Bellavance MA, Rivest S. The neuroendocrine control of the innate immune system in health and brain diseases. Immunol Rev 2012; 248:36-55.
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res 2000; 17:1273-1277.
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 2005; 102:19204-19207.
- Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 2002; 8:473-479.
- Löwenberg M, Verhaar AP, Bilderbeek J, Marle J, Buttgereit F, Peppelenbosch MP, van Deventer SJ, Hommes DW. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep 2006; 7:1023-1029.
- Demonacos C, Djordjevic-Markovic R, Tsawdaroglou N, Sekeris CE. The mitochondrion as a primary site of action of glucocorticoids: the interaction of the glucocorticoid receptor with mitochondrial DNA sequences showing partial similarity to the nuclear glucocorticoid responsive elements. J Steroid Biochem Mol Biol 1995; 55(1):43-55.
- Demonacos C, Tsawdaroglou NC, Djordjevic-Markovic R, Papalopoulou M, Galanopoulos V, Papadogeorgaki S, Sekeris CE. Import of the glucocorticoid receptor into rat liver mitochondria in vivo and in vitro. J Steroid Biochem Mol Biol 1993; 46(3):401-413.
- Psarra AM, Sekeris CE. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: role of the mitochondrial glucocorticoid receptor. Biochim Biophys Acta 2011; 1813(10):1814-1821.
- Lee SR, Kim HK, Song IS, Youm J, Dizon LA, Jeong SH, Ko TH, Heo HJ, Ko KS, Rhee BD, Kim N, Han J. Glucocorticoids and their receptors: insights into specific roles in mitochondria. Prog Biophys Mol Biol 2013; 112(1-2):44-54.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 2009; 5:374-381.
- Chrousos GP. Hormone Resistance and Hypersensitivity States. In: Modern Endocrinology Series. Chrousos GP, Olefsky JM, Samols E. (eds). Lippincott, Williams & Wilkins; Philadelphia, PA, 2002; page 542.
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol 2003; 85(2-5):457-467.
- Kino T, Vottero A, Charmandari E, Chrousos GP. Familial/sporadic glucocorticoid resistance syndrome and hypertension. Ann N Y Acad Sci 2002; 970:101-11.
- Charmandari E, Kino T, Chrousos GP. Familial/sporadic glucocorticoid resistance: clinical phenotype and molecular mechanisms. Ann N Y Acad Sci 2004; 1024:168-81.
- Charmandari E, Kino T. Novel causes of generalized glucocorticoid resistance. Horm Metab Res 2007; 39(6):445-50.
- Charmandari E, Kino T, Ichijo T, Chrousos GP. Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder. J Clin Endocrinol Metab 2008; 93(5):1563-72.
- Charmandari E, Kino T. Chrousos syndrome: a seminal report, a phylogenetic enigma and the clinical implications of glucocorticoid signalling changes. Eur J Clin Invest 2010; 40:932-942.
- Charmandari E. Primary generalized glucocorticoid resistance and hypersensitivity. Horm Res Paediatr 2011; 76:145-155.
- Chrousos G. Q&A: primary generalized glucocorticoid resistance. BMC Med 2011; 9:27.
- Charmandari E. Primary generalized glucocorticoid resistance and hypersensitivity: the end-organ involvement in the stress response. Sci Signal 2012; 5:pt5.
- Charmandari E, Kino T, Chrousos GP. Primary generalized familial and sporadic glucocorticoid resistance (Chrousos syndrome) and hypersensitivity. Endocr Dev 2013; 24:67-85.
- Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Recent advances in the molecular mechanisms determining tissue sensitivity to glucocorticoids: novel mutations, circadian rhythm and ligand-induced repression of the human glucocorticoid receptor. BMC Endocr Disord 2014; 14:71.
- Nicolaides NC, Charmandari E. Chrousos syndrome: from molecular pathogenesis to therapeutic management. Eur J Clin Invest 2015; 45:504-514.
- Nicolaides N, Lamprokostopoulou A, Sertedaki A, Charmandari E. Recent advances in the molecular mechanisms causing primary generalized glucocorticoid resistance. Hormones (Athens) 2016; 15(1):23-34.
- Nicolaides NC, Charmandari E. Novel insights into the molecular mechanisms underlying generalized glucocorticoid resistance and hypersensitivity syndromes. Hormones (Athens) 2017; 16(2):124-138.
- Chrousos GP, Vingerhoeds A, Brandon D, Eil C, Pugeat M, DeVroede M, Loriaux DL, Lipsett MB. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest 1982; 69(6):1261-9.
- Chrousos GP, Detera-Wadleigh SD, Karl M. Syndromes of glucocorticoid resistance. Ann Intern Med 1993; 119(11):1113-24.
- Nader N, Bachrach BE, Hurt DE, Gajula S, Pittman A, Lescher R, Kino T. A novel point mutation in the helix 10 of the human glucocorticoid receptor causes Generalized Glucocorticoid Resistance by disrupting the structure of the ligand-binding domain. J Clin Endocrinol Metab 2010; 95(5):2281-5.
- McMahon SK, Pretorius CJ, Ungerer JP, Salmon NJ, Conwell LS, Pearen MA, Batch JA. Neonatal complete generalized glucocorticoid resistance and growth hormone deficiency caused by a novel homozygous mutation in Helix 12 of the ligand binding domain of the glucocorticoid receptor gene (NR3C1). J Clin Endocrinol Metab 2010; 95(1):297-302.
- Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 2004; 25(5):831-66.
- Wilson JD, Griffin JE, Russell DW. Steroid 5a-reductase 2 deficiency. Endocr Rev 1993; 14(5):577-93.
- Karl M, Lamberts SW, Koper JW, Katz DA, Huizenga NE, Kino T, Haddad BR, Hughes MR, Chrousos GP. Cushing's disease preceded by generalized glucocorticoid resistance: clinical consequences of a novel, dominant-negative glucocorticoid receptor mutation. Proc Assoc Am Physicians 1996; 108(4):296-307.
- Hurley DM, Accili D, Stratakis CA, Karl M, Vamvakopoulos N, Rorer E, Constantine K, Taylor SI, Chrousos GP. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest 1991; 87(2):680-686.
- Karl M, Lamberts SW, Detera-Wadleigh SD, Encio IJ, Stratakis CA, Hurley DM, Accili D, Chrousos GP. Familial glucocorticoid resistance caused by a splice site deletion in the human glucocorticoid receptor gene. J Clin Endocrinol Metab 1993; 76(3):683-689.
- Malchoff DM, Brufsky A, Reardon G, McDermott P, Javier EC, Bergh CH, Rowe D, Malchoff CD. A mutation of the glucocorticoid receptor in primary cortisol resistance. J Clin Invest 1993; 91(5):1918-1925.
- Kino T, Stauber RH, Resau JH, Pavlakis GN, Chrousos GP. Pathologic human GR mutant has a transdominant negative effect on the wild-type GR by inhibiting its translocation into the nucleus: importance of the ligand-binding domain for intracellular GR trafficking. J Clin Endocrinol Metab 2001; 86(11):5600-5608.
- Ruiz M, Lind U, Gafvels M, Eggertsen G, Carlstedt-Duke J, Nilsson L, Holtmann M, Stierna P, Wikstrom AC, Werner S. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf) 2001; 55(3):363-371.
- Mendonca BB, Leite MV, de Castro M, Kino T, Elias LL, Bachega TA, Arnhold IJ, Chrousos GP, Latronico AC. Female pseudohermaphroditism caused by a novel homozygous missense mutation of the GR gene. J Clin Endocrinol Metab 2002; 87(4):1805-1809.
- Vottero A, Kino T, Combe H, Lecomte P, Chrousos GP. A novel, C-terminal dominant negative mutation of the GR causes familial glucocorticoid resistance through abnormal interactions with p160 steroid receptor coactivators. J Clin Endocrinol Metab 2002; 87(6):2658-2667.
- Charmandari E, Kino T, Vottero A, Souvatzoglou E, Bhattacharyya N, Chrousos GP. Natural glucocorticoid receptor mutants causing generalized glucocorticoid resistance: Molecular genotype, genetic transmission and clinical phenotype. J Clin Endocrinol Metab 2004; 89(4):1939-1949.
- Charmandari E, Raji A, Kino T, Ichijo T, Tiulpakov A, Zachman K, Chrousos GP. A novel point mutation in the ligand-binding domain (LBD) of the human glucocorticoid receptor (hGR) causing generalized glucocorticoid resistance: the importance of the C terminus of hGR LBD in conferring transactivational activity. J Clin Endocrinol Metab 2005; 90(6):3696-705.
- Charmandari E, Kino T, Ichijo T, Zachman K, Alatsatianos A, Chrousos GP. Functional characterization of the natural human glucocorticoid receptor (hGR) mutants hGRaR477H and hGRaG679S associated with generalized glucocorticoid resistance. J Clin Endocrinol Metab 2006; 91(4):1535-43.
- Charmandari E, Kino T, Ichijo T, Jubiz W, Mejia L, Zachman K, Chrousos GP. A novel point mutation in helix 11 of the ligand-binding domain of the human glucocorticoid receptor gene causing generalized glucocorticoid resistance. J Clin Endocrinol Metab 2007; 92(10):3986-90.
- Bouligand J, Delemer B, Hecart AC, Meduri G, Viengchareun S, Amazit L, Trabado S, Fève B, Guiochon-Mantel A, Young J, Lombès M. Familial glucocorticoid receptor haploinsufficiency by non-sense mediated mRNA decay, adrenal hyperplasia and apparent mineralocorticoid excess. PLoS One 2010; 5:e13563.
- Zhu HJ, Dai YF, Wang O, Li M, Lu L, Zhao WG, Xing XP, Pan H, Li NS, Gong FY. Generalized glucocorticoid resistance accompanied with an adrenocortical adenoma and caused by a novel point mutation of human glucocorticoid receptor gene. Chin Med J (Engl) 2011; 124:551-555.
- Roberts ML, Kino T, Nicolaides NC, Hurt DE, Katsantoni E, Sertedaki A, Komianou F, Kassiou K, Chrousos GP, Charmandari E. A novel point mutation in the DNA-binding domain (DBD) of the human glucocorticoid receptor causes primary generalized glucocorticoid resistance by disrupting the hydrophobic structure of its DBD. J Clin Endocrinol Metab 2013; 98:E790-E795.
- Nicolaides NC, Roberts ML, Kino T, Braatvedt G, Hurt DE, Katsantoni E, Sertedaki A, Chrousos GP, Charmandari E. A novel point mutation of the human glucocorticoid receptor gene causes primary generalized glucocorticoid resistance through impaired interaction with the LXXLL motif of the p160 coactivators: dissociation of the transactivating and transreppressive activities. J Clin Endocrinol Metab 2014; 99:E902-E907.
- Nicolaides NC, Geer EB, Vlachakis D, Roberts ML, Psarra AM, Moutsatsou P, Sertedaki A, Kossida S, Charmandari E. A Novel Mutation of the hGR Gene Causing Chrousos Syndrome. Eur J Clin Invest 2015; 45:782-791.
- Nicolaides NC, Skyrla E, Vlachakis D, Psarra AM, Moutsatsou P, Sertedaki A, Kossida S, Charmandari E. Functional characterization of the hGRαT556I causing Chrousos syndrome. Eur J Clin Invest 2016; 46(1):42-49.
- Velayos T, Grau G, Rica I, Pérez-Nanclares G, Gaztambide S. Glucocorticoid resistance syndrome caused by two novel mutations in the NR3C1 gene. Endocrinol Nutr 2016; 63(7):369-371.
- Vitellius G, Fagart J, Delemer B, Amazit L, Ramos N, Bouligand J, Le Billan F, Castinetti F, Guiochon-Mantel A, Trabado S, Lombès M. Three novel heterozygous point mutations of NR3C1 causing glucocorticoid resistance. Hum Mutat 2016; 37(8):794-803.
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 1995; 9(13):1608-21.
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol 1999; 19(2):1025-37.
- Dahlman-Wright K, Wright A, Gustafsson JA, Carlstedt-Duke J. Interaction of the glucocorticoid receptor DNA-binding domain with DNA as a dimer is mediated by a short segment of five amino acids. J Biol Chem 1991; 266(5):3107-12.
- Kino T, Liou SH, Charmandari E, Chrousos GP. Glucocorticoid receptor mutants demonstrate increased motility inside the nucleus of living cells: time of fluorescence recovery after photobleaching (FRAP) is an integrated measure of receptor function. Mol Med 2004; 10(7-12):80-8.
- Hurt DE, Suzuki S, Mayama T, Charmandari E, Kino T. Structural Analysis on the Pathologic Mutant Glucocorticoid Receptor Ligand-Binding Domains. Mol Endocrinol 2016; 30(2):173-188.
- Ferriman D, Gallwey J. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab 1961; 83:2694-2698.
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44(235):291-303.
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45:13-24.
- Huizenga NA, de Lange P, Koper JW, de Herder WW, Abs R, Kasteren JH, de Jong FH, Lamberts SW. Five patients with biochemical and/or clinical generalized glucocorticoid resistance without alterations in the glucocorticoid receptor gene. J Clin Endocrinol Metab 2000; 85(5):2076-81.
TABLE 1: Clinical Manifestations and Diagnostic Evaluation of Chrousos Syndrome *
Clinical Presentation
Apparently normal glucocorticoid function in most cases
Asymptomatic
Hypoglycemia, chronic fatigue (glucocorticoid deficiency?)
Mineralocorticoid excess
Hypertension
Hypokalemic alkalosis
Androgen excess
Children: Ambiguous genitalia at birth**, clitoromegaly, premature adrenarche, gonadotropin-independent precocious puberty
Females: Acne, hirsutism, male-pattern hair loss, menstrual irregularities, oligo-anovulation, hypofertility
Males: Acne, hirsutism, oligospermia, adrenal rests in the testes, hypofertility
Increased HPA axis activity (CRH/AVP and ACTH hypersecretion)
Anxiety
Adrenal rests (oligospermia)
Pituitary corticotropinoma
Diagnostic Evaluation
Absence of clinical features of Cushing syndrome
Normal or elevated plasma ACTH concentrations
Elevated serum or plasma cortisol concentrations
Increased 24-hour urinary free cortisol excretion
Normal circadian and stress-induced pattern of cortisol and ACTH secretion
Resistance of the HPA axis to dexamethasone suppression
Thymidine incorporation assays: Increased resistance to dexamethasone-induced suppression of phytohemaglutinin-stimulated thymidine incorporation compared to control subjects
Dexamethasone-binding assays: Decreased concentration or affinity of the glucocorticoid receptor for the ligand compared to control subjects
Molecular studies: Mutations/deletions of the glucocorticoid receptor; functional studies of mutant receptors
* Modified from Reference (48).
** This is the only case of ambiguous genitalia documented in a child with 46,XX karyotype who also harbored a heterozygous mutation of the 21-hydroxylase gene.
TABLE 2: Mutations of the NR3C1 Gene Causing Chrousos Syndrome
| Author (Reference) | cDNA | Amino acid | Molecular Mechanisms | Genotype | Phenotype |
|
Chrousos et al. (57) Hurley et al. (64) Charmandari et al. (71) |
1922 (A®T) | 641 (D®V) |
Transactivation ¯ Affinity for ligand ¯ (x 3) Nuclear translocation: 22 min Abnormal interaction with GRIP1 |
Homozygous |
Hypertension Hypokalemic alkalosis |
| Karl et al. (65) | 4 bp deletion in exon-intron 6 |
hGRα number: 50% of control Inactivation of the affected allele |
Heterozygous |
Hirsutism Male-pattern hair-loss Menstrual irregularities |
|
|
Malchoff et al. (66) Charmandari et al. (71) |
2185 (G®A) | 729 (V®I) |
Transactivation ¯ Affinity for ligand ¯ (x 2) Nuclear translocation: 120 min Abnormal interaction with GRIP1 |
Homozygous |
Precocious puberty Hyperandrogenism |
|
Karl et al. (63) Kino et al. (67) Charmandari et al. (71) |
1676 (T®A) | 559 (I®N) |
Transactivation ¯ Decrease in hGR binding sites Transdominance (+) Nuclear translocation: 180 min Abnormal interaction with GRIP1 |
Heterozygous |
Hypertension Oligospermia Infertility
|
|
Ruiz et al. (68) Charmandari et al. (73) |
1430 (G®A) | 477 (R®H) |
Transactivation ¯ No DNA binding Nuclear translocation: 20 min |
Heterozygous |
Hirsutism Fatigue Hypertension |
|
Ruiz et al. (68) Charmandari et al. (73) |
2035 (G®A) | 679 (G®S) |
Transactivation ¯ Affinity for ligand ¯ (x 2) Nuclear translocation: 30 min Abnormal interaction with GRIP1 |
Heterozygous |
Hirsutism Fatigue Hypertension
|
|
Mendonca et al. (69) Charmandari et al. (71) |
1712 (T®C) | 571 (V®A) |
Transactivation ¯ Affinity for ligand ¯ (x 6) Nuclear translocation: 25 min Abnormal interaction with GRIP1 |
Homozygous |
Ambiguous genitalia Hypertension Hypokalemia Hyperandrogenism |
|
Vottero et al. (70) Charmandari et al. (71) |
2241 (T®G) | 747 (I®M) |
Transactivation ¯ Transdominance (+) Affinity for ligand ¯ (x 2) Nuclear translocation ¯ Abnormal interaction with GRIP1 |
Heterozygous |
Cystic acne Hirsutism Oligo-amenorrhea |
| Charmandari et al. (72) | 2318 (T®C) | 773 (L®P) |
Transactivation ¯ Transdominance (+) Affinity for ligand ¯ (x 2.6) Nuclear translocation: 30 min Abnormal interaction with GRIP1 |
Heterozygous |
Fatigue Anxiety Acne Hirsutism Hypertension |
| Charmandari et al. (74) | 2209 (T®C) | 737 (F®L) |
Transactivation ¯ Transdominance (+) Affinity for ligand ¯ (x 1.5) Nuclear translocation: 180 min |
Heterozygous |
Hypertension Hypokalemia |
| McMahon et al. (60) |
2 bp deletion at nt 2318-9 |
773 |
Transactivation ¯ Affinity for ligand: absent No suppression of IL-6 |
Homozygous |
Hypoglycemia Fatigability with feeding Hypertension |
| Nader et al. (59) | 2141 (G®A) | 714 (R®Q) |
Transactivation ¯ Transdominance (+) Affinity for ligand ¯ (x 2) Nuclear translocation ¯ Abnormal interaction with GRIP1 |
Heterozygous |
Hypoglycemia Hypokalemia Hypertension Mild clitoromegaly Advanced bone age Precocious pubarche |
| Bouligand et al. (75) | 1405 (C®T) | 469 (R®X) |
Transactivation ¯ Ligand-binding sites ¯ No DNA binding No nuclear translocation |
Heterozygous |
Adrenal hyperplasia Hypertension Hypokalemia
|
|
Zhu Hui-juan et al. (76) Nicolaides et al. (80) |
1667 (G®T) | 556 (T®I) |
Transactivation ¯ Transrepression Affinity for ligand ¯ Nuclear translocation ¯ Abnormal interaction with GRIP1 |
Heterozygous |
Adrenal incidentaloma
|
| Roberts et al. (77) | 1268 (T®C) | 423 (V®A) |
Transactivation ¯ Affinity for ligand: Normal No DNA binding Nuclear translocation: 35 min Interaction with GRIP1: Normal |
Heterozygous |
Fatigue Anxiety Hypertension |
| Nicolaides et al. (78) | 1724 (T®G) | 575 (V®G) |
Transactivation ¯ Transrepression Affinity for ligand ¯ (x 2) Nuclear translocation ¯ Abnormal interaction with GRIP1 |
Heterozygous |
Melanoma Asymptomatic daughters |
| Nicolaides et al. (79) | 2177 (A®G) | 726 (H®R) |
Transactivation ¯ Transrepression ¯ Affinity for ligand ¯ (x 2) Nuclear translocation ¯ Abnormal interaction with GRIP1 |
Heterozygous |
Hirsutism Acne Alopecia Anxiety Fatigue Irregular menstrual cycles |
| Velayos et al. (81) | 1429 (C®T) | 477 (R®C) | Not studied yet | Heterozygous |
Mild hirsutism Asymptomatic mother |
| Velayos et al. (81) | 1762_1763insTTAC | 588 (H®L*5) | Not studied yet | Heterozygous |
Hirsutism Anxiety Chronic fatigue |
| Vitellius et al. (82) | 1429 (C®A) | 477 (R®S) |
No Transactivation Affinity for ligand: Normal No DNA binding Nuclear translocation ¯ |
Heterozygous | Adrenal incidentaloma |
| Vitellius et al. (82) | 1433 (A®G) | 478 (Y®C) |
Transactivation ¯ Affinity for ligand: Normal DNA binding ¯ Nuclear translocation ¯ |
Heterozygous | Adrenal incidentaloma |
| Vitellius et al. (82) | 2015 (T®C) | 672 (L®P) |
No Transactivation No Affinity for ligand No DNA binding No Nuclear translocation |
Heterozygous | Adrenal incidentaloma |
.
DISCLOSURE STATEMENT:
The authors N.C.N., T.K., G.P.C. and E.C. have nothing to disclose.
TSH Receptor Mutations and Diseases
ABSTRACT
The thyroid-stimulating hormone (TSH) receptor (TSHR) is a member of the glycoprotein hormone receptors (GPHRs), a sub-group of class A G protein-coupled receptors (GPCRs). TSHR and its ligand thyrotropin are of essential importance for growth and function of the thyroid gland. The TSHR activates different G-protein subtypes and signaling pathways, of which Gs and Gq induced signaling are of highest importance in the thyroid gland. A proper interplay between TSH and TSHR is pivotal for thyroid growth and regulated production and release of thyroid hormones (TH). Autoimmune (antibody binding) or non-autoimmune (occurrence of mutants) TSHR dysfunctions are the underlying cause of several pathologies, including rare cancer-development. The sequential processes of TSHR binding, signal transduction across the cell-membrane and activation of intracellular effectors involve elaborate specific structural properties of the receptor and several interacting proteins. In consequence, different pathogenic mutations at TSHR or TSH may have diverse impact on particular molecular functions, but finally result in either hypo- or hyperthyroid states accompanied or not by various growth anomalies. We here summarize current knowledge regarding naturally occurring TSHR mutations, associated diseases and related molecular pathogenic mechanisms at the level of TSHR structure and function. For complete coverage of this and related areas in Endocrinology, visit our free web-books, www.endotext.org and www.thyroidmanager.org
GAIN-OF-FUNCTION MUTATIONS
On a theoretical basis, for a hormone receptor, “gain-of-function” may have several meanings: (i) activation in the absence of ligand (constitutively); (ii) increased sensitivity to its normal agonist; (iii) increased, or de novo sensitivity to an allosteric modulator; (iv) broadening of its specificity. When the receptor is part of a chemostat, as is the case for the TSHR, the first situation is expected to cause tissue autonomy, whereas the second would simply cause adjustment of TSH to a lower value. In the third and fourth cases, inappropriate stimulation of the target will occur because the illegitimate agonists or modulators are not expected to be subject to the normal negative feedback. If a gain-of-function mutation of the first category occurs in a single cell normally expressing the receptor (somatic mutation), it will become symptomatic only if the regulatory cascade controlled by the receptor is mitogenic in this particular cell type or, during development, if the mutation affects a progenitor contributing significantly to the final organ. Autonomous activity of the receptor will cause clonal expansion of the mutated cell. If the regulatory cascade also positively controls function, the resulting tumor may progressively take over the function of the normal tissue and ultimately result in autonomous hyperfunction. If the mutation is present in all cells of an organism (germline mutation) autonomy will be displayed by the whole organ.
From what we know of thyroid cell physiology it is easy to predict the phenotypes associated with gain-of-function of the cAMP-dependent regulatory cascade. Two observations provide pertinent models of this situation. Transgenic mice made to express ectopically the adenosine A2a receptor in their thyroid display severe hyperthyroidism associated with thyroid hyperplasia (1). As the A2a adenosine receptor is coupled to Gs and displays constitutive activity due to its continuous stimulation by ambient adenosine (2), this model mimics closely the situation expected for a gain-of-function germline mutation of the TSHR. Patients with the McCune-Albright syndrome are mosaïc for mutations in the Gs protein (Gsp mutations) leading to the constitutive stimulation of adenylyl-cyclase (3). Hyperfunctioning thyroid adenomas develop in these patients from cells harboring the mutation, making them a model for gain-of-function somatic mutations of the TSHR. A transgenic model in which Gsp mutations are targeted for expression in the mouse thyroid has been constructed. Though with a less dramatic phenotype this represents also a pertinent model for a gain-of-function of the cAMP regulatory cascade (4).
Since the TSHR is capable of activating both Gs and Gq (though with lower potency) the question arises whether mutations with a different effect on the two cascades would be associated with different phenotypes. Studies in mice (5) and rare patients (6) suggests that activation of Gq may be required to observe goitrogenesis in patients with non-autoimmune familial hyperthyroidism. However, when tested in transfected non-thyroid cells, all identified gain-of-function mutations of the TSHR stimulate constitutively Gs, with only a minority capable of stimulating both Gs and Gq (7,8). Also, thyroid adenomas or multinodular goitre are frequent in McCune Albright syndrome, which is characterized by pure Gs stimulation (9).
Familial non-autoimmune hyperthyroidism or hereditary toxic thyroid hyperplasia
The major cause of hyperthyroidism in adults is Graves' disease in which an autoimmune reaction is mounted against the thyroid gland and auto-antibodies are produced that recognize and stimulate the TSHR (10,11). This may explain why the initial description by the group of Leclère of a family showing segregation of thyrotoxicosis as an autosomal dominant trait in the absence of signs of autoimmunity was met with skepticism (12). Re-investigation of this family together with another family from Reims identified two mutations of the TSHR gene, which segregated in perfect linkage with the disease (13). A series of additional families have been studied since (14-28) [Figure 1 and Table 1, For a completed list of naturally occurring TSHR single amino acid substitutions with their functional characteristics, see the glycoprotein hormone receptor information resource “SSFA” (29,30) available under: http://www.ssfa-gphr.de]. The functional characteristics of these mutant receptors confirm that they are constitutively stimulated (see below). This nosological entity, hereditary toxic thyroid hyperplasia (HTTH), sometimes called Leclère’s disease, is characterized by the following clinical characteristics: autosomal dominant transmission; hyperthyroidism with a variable age of onset (from infancy to adulthood, even within a given family); hyperplastic goiter of variable size, but with a steady growth; absence of clinical or biological stigmata of auto-immunity. An observation common to most cases is the need for drastic ablative therapy (surgery or radioiodine) in order to control the disease, once the patient has become hyperthyroid (13,31). The autonomous nature of the thyroid tissue from these patients has been elegantly demonstrated by grafting in nude mice (32). Contrary to tissue from Graves' disease patients, HTTH cells continue to grow in the absence of stimulation by TSH or TSAb.
The prevalence of hereditary toxic thyroid hyperplasia is difficult to estimate. It is likely that many cases are still mistaken for Graves' disease. This may be explained by the high frequency of thyroid auto-antibodies (anti-thyroglobulin, anti-thyroperoxidase) in the general population. It is expected that wider knowledge of the existence of the disease will lead to better diagnosis. This is not a purely academic problem, since presymptomatic diagnosis in children of affected families may prevent the developmental or psychological complications associated with infantile or juvenile hyperthyroidism (for a review, see (33)). A country-wide screening for the condition has been performed in Denmark. It was found in one out of 121 patients with juvenile thyrotoxicosis (0.8%; 95% CI: 0.02-4.6%), which corresponds to one in 17 patients with presumed non-autoimmune juvenile thyrotoxicosis (6%; 95% CI:0.15-28.69) (34).
Sporadic toxic thyroid hyperplasia
Cases with toxic thyroid hyperplasia have been described in children born from unaffected parents (35-39). Conspicuously, congenital hyperthyroidism was present in most of the cases and required aggressive treatment. Mutations of one TSHR allele were identified in the children, but were absent in the parents. As paternity was confirmed by mini- or microsatellite testing, these cases qualify as true neo-mutations. When comparing the amino acid substitutions implicated in hereditary and sporadic cases, for the majority, they do not overlap (see Table 1). Whereas most of the sporadic cases harbor mutations that are also found in toxic adenomas, most of the hereditary cases have "private" mutations. Although there may be exceptions, the analysis of the functional characteristics of the individual mutant receptors in COS cells, and the clinical course of individual patients, suggest an explanation for this observation: "sporadic" and somatic mutations seem to have a stronger activating effect than "hereditary" mutations (40). From their severe phenotypes, it is likely that newborns with neo-mutations would not have survived, if not treated efficiently. On the contrary, from inspection of the available pedigrees, it seems that the milder phenotype of patients with “hereditary" mutations has only limited effect on reproductive fitness. The fact that "hereditary" mutations are rarely observed in toxic adenomas is compatible with the suggestion that they would cause extremely slow tissue growth and, accordingly, would rarely cause thyrotoxicosis if somatic. There is, however, no a priori reason for neo-mutations to cause stronger gain-of-function than hereditary mutations. Accordingly, an activating mutation of the TSHR gene has been found in a six month child with subclinical hyperthyroidism presenting with weight loss as the initial symptom (41).
Somatic mutations: autonomous toxic adenomas
Soon after mutations of Gαs had been found in adenomas of the pituitary somatotrophs (42), similar mutations (also called Gsp mutations) were found in some toxic thyroid adenomas and follicular carcinomas (43-46). The mutated residues (Arg201, Glu227) are homologous to those found mutated in the ras proto-oncogenes: i.e. the mutations decrease the endogenous GTPase activity of the G protein, resulting in a constitutively active molecule. Toxic adenomas were found to be a fruitful source of somatic mutations activating the TSHR, probably because the phenotype is very conspicuous and easy to diagnose (47). Whereas mutations are distributed all along the serpentine portion of the receptor and even in the extracellular amino-terminal domain (48-54), there is clearly a hotspot at the cytoplasmic end of the sixth transmembrane segment (see Figure 1). The clustering reflects the pivotal role of this portion in the activation mechanism observed in the TSHR and in class A GPCRs generally [e.g. (55-61)].
Despite some dispute about the prevalence of TSHR mutations in toxic adenomas (which may be due to different origin of patients (62,63) or different sensitivity of the methodology) the current consensus is that activating mutations of the TSHR are the major cause of solitary toxic adenomas and account for about 60 to 80% of cases (15,7,64-66). Contrary to initial suggestions (63), the same percentage of activating TSHR mutations is observed in Japan, an iodine-sufficient country with low prevalence of toxic adenomas (67). In some patients with a multinodular goiter and two zones of autonomy at scintigraphy, a different mutation of the TSHR was identified in each nodule (68,36,69,70). This indicates that the pathophysiological mechanism responsible for solitary toxic adenomas can be at work on a background of multinodular goiter.
Table 1
| CODONS | Substitution |
Somatic mutation |
Germline neo- mutation |
Germline familial |
Cancer |
Stimulation of basal cAMP |
Stimulation of IP/Ca | |
| Ser | 281 | Asn | + | + | - | |||
| Thr | + | + | - | |||||
| Ile | + | + | - | |||||
| Asp | 403 | deletion | + | + | nd | |||
| Asn | 406 | Ser | + | + | nd | |||
| Ser | 425 | Ile | + | + | - | |||
| Ala | 428 | Val | + | nd | nd | |||
| Gly | 431 | Ser | + | + | + | |||
| Met | 453 | Thr | + | + | + | + | - | |
| Met | 463 | Val | + | + | - | |||
| Ala | 485 | Val | + | + | - | |||
| Ile | 486 | Phe | + | + | + | |||
| Met | + | + | ± | |||||
| Asn | + | + | - | |||||
| Ser | 505 | Arg | + | + | - | |||
| Asn | + | + | - | |||||
| Val | 509 | Ala | + | + | - | |||
| Leu | 512 | Arg | + | + | nd | |||
| Gln | + | + | nd | |||||
| Ile | 568 | Thr | + | + | + | ± | ||
| Phe | + | + | ± | |||||
| Glu | 575 | Lys | + | + | nd | |||
| Ala | 593 | Asn | + | + | nd | |||
| Val | 597 | Leu | + | + | nd | |||
| Phe | + | nd | ||||||
| Y613-F631 | deletion | - | ||||||
| Tyr | 601 | Asn | + | + | - | |||
| Asp | 617 | Tyr | + | + | ± | |||
| Asp | 619 | Gly | + | + | - | |||
| Thr | 620 | Ile | + | + | + | nd | ||
| Ala | 623 | Ile | + | + | ± | |||
| Val | + | + | + | - | ||||
| Ser | + | + | + | - | ||||
| Phe | + | + | nd | |||||
| Met | 626 | Ile | + | + | nd | |||
| Ala | 627 | Val | + | + | nd | |||
| Leu | 629 | Phe | + | + | + | - | ||
| Ile | 630 | Leu | + | + | - | |||
| Phe | 631 | Leu | + | + | - | |||
| Cys | + | + | + | - | ||||
| Ile | + | + | - | |||||
| Thr | 632 | Ile | + | + | + | - | ||
| Thr | 632 | Ala | + | + | + | nd | ||
| Asp | 633 | Tyr | + | + | + | - | ||
| Glu | + | + | - | |||||
| His | + | + | + | - | ||||
| Ala | + | + | - | |||||
| Ile | 635 | Val | + | + | nd | |||
| Cys | 636 | Trp | + | + | - | |||
| Arg | + | + | ± | |||||
| Pro | 639 | Ser | + | + | + | + | ||
| Ile | 640 | Lys | + | + | nd | |||
| Asn | 650 | Tyr | + | + | - | |||
| Val | 656 | Phe | + | + | - | |||
| Del 658-661 | + | - | ||||||
| Asn | 670 | Ser | + | + | - | |||
| Cys | 672 | Thr | + | + | - | |||
| Leu | 677 | Val | + | + | nd | |||
Table 1: Gain-of-function TSHR mutations. The nature of the mutations is indicated with their origins (somatic, germline sporadic, germline familial, cancer) and effects on intracellular regulatory cascades. nd - not determined; “-“ decreased functional property; “+” enhanced; “+/-“ similar to wild type.
In agreement with this notion, activating mutations of the TSHR have been identified in hyperfunctioning areas of multinodular goiter (70,19,65,23). The independent occurrence of two activating mutations in a patient may seem highly improbable at first. However, the multiplicity of the possible targets for activating mutations within the TSHR makes this less unlikely. It is also possible that a mutagenic environment is created in glands exposed to a chronic stimulation by TSH, resulting in H2O2 generation (71,72). Finally the involvement of TSHR mutations in thyroid cancers has been implicated in a limited number of follicular thyroid carcinoma (73-81).
Figure 1

Legend to figure 1: Structural model of the TSHR with interacting proteins and highlighted positions for gain-of-function mutations. Left: The model shows different parts of the receptor for which homologous structural information is available. The leucine-rich repeat domain (LRRD) and the hinge region are both harboring determinants for hormone (TSH model (surface) based on the FSH structure (82)) and antibody binding. The hinge region (colored pink) structurally links the LRRD with the serpentine domain made of transmembrane helices (H) 1-7 connected by intracellular (I1-3) and extracellular (E1-3) loops. Three cysteine bridges (yellow spheres) between the C-terminal LRRD and the C-terminus of the hinge region are indicated that are required for correct receptor arrangement and function. Wild type positions of constitutively activating mutations are indicated by side-chain representation (red sticks). Right: The known activating mutations (Table 1) are distributed over the entire serpentine portion of the receptor structure with clustering in the central core and specifically in helix 6. In contrast to other glycoprotein-hormone receptors (GPHRs), naturally occurring activating mutations were also identified in the extracellular loops and in the hinge region (Ser281).
Structure-function relationships of the TSHR
An important observation has been that the wild-type TSHR itself displays significant constitutive activity [(83,47) and review (84)]. This characteristic is not exceptional amongst GPCRs (e.g. (85)), but interestingly, it is not shared, at least to the same level, by its close relatives, the human luteinizing hormone/chorionic gonadotropin (LH/CG) receptor (LHCGR) and the human follitropin (FSH) receptor (FSHR). Compared to the TSHR, the LHCGR displays minimal basal activity and the human FSH receptor is totally silent (86). Together with the observation that mutations in residues distributed over most of the serpentine portion of the TSHR are equally effective in activating it (which does not seem to be a general characteristic in all GPCRs) this suggests that the unliganded TSHR might be less constrained than other GPCRs. As a consequence, being already “noisy” it would be more prone to further destabilization by a wide variety of mutations affecting multiple structural elements (Figure 1).
The effect of activating mutations must accordingly be interpreted in terms of “increase in constitutive activity”. Most constitutively active mutant receptors (also referred to as “CAMs”) found in toxic adenomas and/or toxic thyroid hyperplasia share common characteristics: i) they increase the constitutive activity of the receptor toward stimulation of adenylyl cyclase; ii) with a few notable exceptions (see Table 1 and below) (48), they do not display constitutive activity toward the inositol phosphate/diacylglycerol pathway; iii) their expression at the cell surface is decreased (from slightly to severely); iv) most, but not all of them keep responding to TSH for stimulation of cAMP and inositol phosphate generation, with a tendency to do so at decreased median effective concentrations; and v) they bind 125I-labeled bovine TSH with an apparent affinity higher than that of the wild-type receptor. Of note, CAMs with mutations at Ser281 (to Ile) (37) in the extracellular N-terminal part, at Ile486 (to Phe or Met) (48) and Ile568 (to Thr) (48) in the first and second extracellular loops, respectively, and at both Asp633 (to His) (7) and Pro639 (to Ser) (69) in transmembrane helix 6 are exceptional in that in addition to stimulating adenylyl cyclase, they cause constitutive activation of the inositol phosphate pathway. The constitutive activity of these mutants is interesting as it points to positions and structural fragments of the wild type receptor which may be of high relevance for its physiological coupling to both Gs and Gq (Figure 1 and Table 1).
No direct relationship is found between the level of cAMP achieved by different mutants in transfected COS cells and their level of expression at the cell membrane (87), which means that individual mutants have widely different “specific constitutive activity” (measured as the stimulation of cAMP accumulation/receptor number at the cell surface). Although this specific activity may tell us something about the mechanisms of receptor activation, it is not a measure of the actual phenotypic effect of the mutation in vivo. Indeed, one of the relatively mild mutations, observed up to now only in a family with HTTH (Cys672Tyr) (13), is among the strongest according to this criterion.
Differences between the effects of the mutants in transfected COS or HEK293 cells and thyrocytes in vivo render these correlations a difficult exercise. Indeed, most of the activating mutations of the TSHR have been studied by transient expression in COS or HEK293T cells and there is no guarantee that the mutants will function in an identical way in these artificial systems as they do in thyrocytes (88). In thyrocytes, a better relation has been observed between adenylylcyclase stimulation and differentiation than with growth (88). However, the built-in amplification associated with transfection of constructs in COS or HEK 293T cells has the advantage of allowing detection of even slight increases in constitutive activity of certain TSHR mutants.
According to a current model of GPCR activation, the receptor would exist under at least two interconverting conformations: R (silent conformations) and R* (active forms) (89,55,90,91) (Figure 2). The unliganded wild type receptor would shuttle between both forms, the equilibrium being in favor of R (90). Binding of the agonistic ligand is believed to stabilize the R* conformation.
Figure 2

Legend to figure 2: Left. Schematic representation of the equilibria between inactive (R) and active (R*) conformations of TSHR. The triangles indicate the equilibrium point of the wild type receptor (pink) and hypothetical mutants with increasing constitutive activity (brown, red). The situation of a receptor which would be devoid of basal activity is also indicated (blue triangle). Note that the wild type receptor (pink) has basal activity. Right. The concentration action curves corresponding to the hypothetical mutants and wild type receptors are indicated with the same color code.
The resulting R-to-R* transition was supposed to involve a conformational change that modifies the relative position of transmembrane helices to each other, which in turn would translate into conformational changes in the cytoplasmic crevice between the intracellular loops and transmembrane helices interacting with the hetero-trimeric G-protein. This model is strongly supported by solved crystal structures of active GPCR conformations (59,61) reviewed in (92)]. They reveal that receptor activation and signal transduction is characterized by specific movements of transmembrane helices (TMHs) 5, 6 and 7 leading to modification of their distances relative to each other. Helix 6 is a major player in this process, its cytoplasmic end moves away from that of TMH3 by turning around a pivotal helix-kink. The result is an “opening” of the cytoplasmic crevice of the receptor allowing interaction with the G protein (59). (A more detailed description of the activation mechanics, adapted to a model of the TSHR, is given in the legend to Figure 3.) This essential function of TMH6 for determination of an active state (59,61) might explain, why most activating TSHR mutations were found in this particular helix (Figure 1). This conclusion is in accordance with the early concept that the silent form of GPCRs would be submitted to structural constraints requiring the wild-type primary structure of the helix 6 and the connected third intracellular loop (93,90,91), and explains why these constraints could be released by a wide spectrum of amino acid substitutions in this segment as observed also for the TSHR (60).
Figure 3

Legend to figure 3: Model of the TSHR structure in complex with TSH and G-protein and illustration of the putative activation mechanism. The receptor is displayed as a backbone cartoon in complex with the hetero-dimeric hormone and a hetero-trimeric G-protein molecule (surface representations). For the serpentine portion of the receptor, the model is based on the solved structure of the β2-adrenergic/Gs crystal (61). The ectodomain (in complex with TSH) and the hinge region were modeled based on a determined and homologous FSHR-FSH structure (82). A selection of residues affected by known activating mutations are shown as red sticks and identified by their position in the primary structure of the protein (see Figure 1). Their positions tentatively illustrate the “path” followed by the activation signal, from outside the membrane (in the ectodomain) to the cytoplasmic surface of the receptor, via the transmembrane helices. Briefly, the hormone binds to both the Leucine rich repeat domain (LRRD) and the hinge region (e.g. sulfated tyrosine 385 (sTyr)). This initial signal is transduced into a conformational change of a module (intramolecular agonist) constituted by the “hinge” region of the ectodomain and the exoloops (E1-3) of the serpentine domain. In favor of this model, several residues belonging to this module (Ser281 in the ectodomain; Asp403 and Asn406 at the ectodomain-serpentine domain border; Ile486, Ile568, Val656 in the exoloops) can activate the receptor constitutively when mutated. Together with the observation that a truncated receptor devoid of ectodomain displays significant increase in constitutive activity, this suggests that activation of the TSHR involves switching of specific extracellular portions from a tethered inverse agonist (maintenance of the basal state) to an intramolecular agonist (94). The resulting structural changes affecting the exoloops are expected to be directly conveyed to the transmembrane helices with the resulting breakage of silencing locks (arrows). From comparison of the inactive and active rhodopsin (59) or beta-2 adrenoreceptor structures (61), the largest spatial movement affects TMH6, involving a combination of horizontal and rotational (wound arrow) movements around a pivotal helix-kink at a proline (TSHR Pro639). These global changes result in the partial “opening” of the intra-helical crevice on the cytoplasmic side of the receptor (horizontal double-head arrows), allowing complete binding and activation of G-proteins.
In addition to the release of structural locks stabilizing the inactive conformation of GPCRs, activation of the GPHRs has been shown to involve a triggering mechanism exerted by an “intramolecular agonist” constituted by segments of the exoloops and the C-terminal portion of their ectodomain (94-96). According to this model, it is this module, activated by the binding of TSH, thyroid stimulating auto-antibodies, thyrostimulin (97,98) or mutations (see below) which would be the immediate agonist of the serpentine portion of the receptor (94,95,99,96,100,101). In all cases, however, mutations are expected to affect the local three dimensional structure of the receptor with a resulting global effect on its activation state. Amongst these are modification of “knob and hole” interactions (e.g. by repulsion) in tightly packed local microdomains and breakage, or creation of intramolecular interactions by changing the biophysical characteristics of side chains (e.g. (6)). As exclusive examples of these, mutations at Asp633 (57,102) or Asp619 (47) are expected to break interhelical locks between transmembrane helices 6 and 7 or 3, respectively. Interestingly, even mutations affecting an important residue of the trigger in the ectodomain (Ser281) seem also to be responsible for a “loss-of-local structure”. Indeed, substitution of the wild type residue (serine) by almost any amino acid results in constitutive activation (103,104). This implicates that predictions of phenotype-genotype relationships must always be considered with much caution if they are not backed by detailed structural and functional knowledge.
Familial gestational hyperthyroidism
Some degree of stimulation of the thyroid gland by human chorionic gonadotrophin (hCG) is commonly observed during early pregnancy. It is usually responsible for decrease in serum thyrotropin with an increase in free thyroxine concentrations that remains within the normal range (for references see (105)). When the concentrations of hCG are abnormally high, like in molar pregnancy, true hyperthyroidism may ensue. The pathophysiological mechanism is believed to be the promiscuous stimulation of the TSHR by excess hCG, as suggested by the rough direct or inverse relation between serum hCG and free T4 or TSH concentrations, respectively (106,105). A convincing rationale is provided by the close structural relationships of the glycoprotein hormones and their receptors, respectively (107).
A new syndrome has been described in 1998 in a family with dominant transmission of hyperthyroidism limited to pregnancy (Figure 4) (108). The proposita and her mother had severe thyrotoxicosis together with hyperemesis gravidarum during the course of each of their pregnancies. When non pregnant they were clinically and biologically euthyroid. Both patients were heterozygous for a K183R mutation in the extracellular amino-terminal domain (Figure 3) of the TSHR gene. When tested by transient transfection in COS cells, the mutant receptor displayed normal characteristics towards TSH. However, providing a convincing explanation to the phenotype, it showed higher sensitivity to stimulation by hCG, when compared with wild type TSHR (108).
The amino acid substitution responsible for the promiscuous stimulation of the TSHR by hCG is surprisingly conservative. Also surprising is the observation that residue 183 is a lysine in both the TSH and LH/CG receptors. When placed on the available three dimensional model of the hormone-binding domain of the TSHR (109), residue 183 belongs to one of the beta-sheets which constitute the putative surface of interaction with the hormones (Figure 3).
Figure 4

Legend to figure 4: Familial gestational hyperthyroidism secondary to mutation of the TSHR gene. Upper panel displays the pedigree, with the two ladies affected, together with a “snake plot “of the TSHR, with the mutation indicated. Lower right panel illustrates the increased sensitivity of the K183R mutant TSHR vis-à-vis hCG. Binding of TSH, or hCG to the ectodomain of the TSHR, according to models based on crystallographic data from (110,82) is visualized in figure 3.
Detailed analysis of the effect of the K183R mutation by site-directed mutagenesis indicated that any amino acid substitution at this position confers a slight increase in stability to the illegitimate hCG/TSHR complex (111). This increase in stability would be enough to cause signal transduction by the hCG concentrations achieved in pregnancy, but not by the LH concentrations observed after menopause. Indeed, the mother of the proposita remained euthyroid after menopause. This finding is compatible with a relatively modest gain-of-function of the K183R mutant upon stimulation by hCG. A second family with the same phenotype has recently been identified. Interestingly, the mutation affects the same residue (K183N) (112).
Contrary to other mammals, human and primates rely on chorionic gonadotropin for maintenance of corpus luteum in early pregnancy (113). The frequent partial suppression of TSH observed at peak hCG levels during normal pregnancy indicates that evolution has selected physiological mechanisms operating very close to the border of thyrotoxicosis. This may provide a rationale to the observation that, in comparison to other species, the glycoprotein hormones of primates display a lower biological activity due to positive selection by evolution of specific amino acid substitutions in their alpha-subunits (114). Up to now no spontaneous mutation has been identified which would increase the bioactivity of hCG. An interesting parallel may be drawn between familial gestational hyperthyroidism and cases of spontaneous ovarian hyperstimulation syndrome (sOHSS) (86,115). In sOHSS, mutations of the FSH receptor gene render the receptor abnormally sensitive to hCG. The result is recurrent hyperstimulation of the ovary, on the occasion of each pregnancy.
LOSS OF FUNCTION MUTATIONS
Loss-of-function mutations in the TSHR gene are expected to cause a syndrome of “resistance to TSH.” The expected phenotype is likely to resemble that of patients with mutations in TSH itself. These mutations have been described early because of the prior availability of information on TSH alpha and beta genes (114). Mouse models of resistance to TSH are available as natural (hyt/hyt mouse) (116) or experimental TSHR mutant lines (117,118). Interestingly, and contrary to the situation in human (see below), the thyroid of newborn TSHR knockout mice is of normal size. As expected, the homozygote animals displayed profound hypothyroidism. Their thyroids do not express the sodium-iodide symporter, but showed significant (non-iodinated) thyroglobulin production. From this information one would expect patients with two TSHR mutated alleles to exhibit a degree of hypothyroidism in accordance with the extent of the loss-of-function, going from mild, compensated, hypothyroidism, to profound neonatal hypothyroidism with absent iodide trapping. Heterozygous carriers are expected to be normal or display minimal increase in plasma TSH.
Clinical cases with the mutations identified
A few patients with convincing resistance to TSH had been described before molecular genetics permitted identification of the mutations (119,120). The first cases described in molecular terms were euthyroid siblings with elevated TSH (121). Sequencing of the TSHR gene identified a different mutation in each allele of the affected individuals, which made them compound heterozygotes. The substitutions were in the extracellular amino-terminal portion of the receptor (maternal allele, P162A; paternal allele, I167N). The functional characteristics of the mutant receptors showed that the paternal allele was virtually completely non-functional, whereas the maternal allele displayed an increase in the median effective TSH concentration for stimulation of cAMP production. Current knowledge of the structure of part of the ectodomain of the receptor allows to establish structure-function relationships for mutations affecting this portion of the receptor (122,109,123-126,101).
A large number of familial cases with loss-of-function mutations of the TSHR have been identified in the course of screening programs for congenital hypothyroidism (127-140) [(Figure 5, For a complete list of naturally occurring and side-directed TSHR single amino acid substitutions with their functional characteristics see the GPHR information resource “SSFA” available under: http://www.ssfa-gphr.de (29,30)]. Some of the patients displayed the usual criteria for congenital hypothyroidism, including high TSH, low free T4, and undetectable trapping of 99Tc. In some cases, plasma thyroglobulin levels were normal or high. The patients can be compound heterozygotes for complete loss of function mutations (129), or homozygotes, born to consanguineous (128) or apparently unrelated parents (134).
Figure 5

Legend to figure 5: Structural model of the TSHR with indication of loss-of-function mutations. The location and substitutions responsible for known loss-of-function mutations (side-chains as blue sticks) are indicated on a three-dimensional receptor model. Single letter abbreviations of amino-acids are used. In contrast to activating mutations, many inactivating mutations are located also in the LRRD. As observable in this complex model inactivating mutations can have different molecular effects on TSHR functions dependent on their localization, like diminishing hormone binding (location in the LRRD, e.g. R109Q), G-protein binding (intracellular localization, e.g. M527T, R531W), leading to a decreased receptor cell surface expression by modification of the three-dimensional structure (e.g. mutations at extracellular cysteines which interrupts stabilizing disulfide bridges, e.g. C390W), or interrupting the signal transport in the serpentine domain (located at the helices, e.g. A593V).
In agreement with the phenotype of knock-out mice with homozygous invalidation of the TSHR, patients with complete loss-of-function of the receptor display an in-place, thyroid with completely absent iodide or 99Tc trapping. However, in contrast with the situation in mice, the gland is hypoplastic. Activation of the cAMP pathway, while dispensable for the anatomical development of the gland and thyroglobulin production, is thus absolutely required for expression of the NIS gene and, at least in human, for normal growth of the tissue during fetal life. This explains that in the absence of thyroglobulin measurements or expert echography, loss-of-function mutations of the TSHR may easily be misdiagnosed as thyroid agenesis. In the heterozygous state, complete loss of function mutations of the TSHR is a cause of moderate hyperthyrotropinemia (subclinical hypothyroidism), segregating as an autosomal dominant trait (141).
Resistance to thyrotropin not linked to the TSHR gene
Finally, it must be stressed that an autosomal dominant form of partial resistance to TSH has been demonstrated in families in which linkage to the TSHR gene has been excluded (142). A locus has been identified on chromosome 15q25.3-26.1 but the gene responsible for the phenotype has not been identified yet (143).
Polymorphisms
A series of single nucleotide polymorphisms affecting the coding sequence have been identified in the TSHR gene. After the initial suggestion that some of these (D36H, P52T, D727E) would be associated with susceptibility to autoimmune thyroid diseases (144-146) the current consensus is that they represent neutral alleles with no pathophysiological significance (147-150). However, a genome-wide study involving a large cohort of patients has recently demonstrated association between non-coding SNPs at the TSHR gene locus and Graves’disease (151,152). The genetic substratum responsible for this association is still under study (152). However, a large meta-analysis of genome wide association studies failed to identify the TSHR as a locus affecting plasma TSH values (153).
One polymorphic residue deserves special mention: position 601 was found to be a tyrosine or a histidine in the two initial reports of TSHR cloning (154,155). Characterization of the two alleles by transfection in COS cells indicated interesting functional differences: the Tyr601 allele displayed readily detectable constitutive activity, whereas the His601 was completely silent; the Tyr601 allele responded to stimulation by TSH by activating both the adenylylcyclase and phospholipase C dependent regulatory cascades, when the His601 allele was only active on the cAMP pathway (156,157). The Tyr601 allele is by far the most frequent in all populations tested. A Tyr601Asn mutation was found in a toxic adenoma. Characterization of the mutant demonstrated increase in constitutive activation of the cAMP regulatory cascade (157), making the 601 residue an interesting target for structure-function studies.
Acknowledgments
Research in the laboratory of the author was supported by the Interuniversity Attraction Poles Programme-Belgian State-Belgian Science Policy (6/14), the Fonds de la Recherche Scientifique Médicale of Belgium, the Walloon Region (program “Cibles”) and the non-for-profit Association Recherche Biomédicale et Diagnostic. This work was supported by the Deutsche Forschungsgemeinschaft (DFG), projects KL2334/2-2 and Cluster of Excellence ‘Unifying Concepts in Catalysis’ (Research Field D3/E3-1) to G.Kl..
REFERENCES
1. Ledent C, Dumont JE, Vassart G, et al. Thyroid expression of an A2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. The EMBO journal 1992; 11:537-542
2. Maenhaut C, Van Sande J, Libert F, et al. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochemical and biophysical research communications 1990; 173:1169-1178
3. Weinstein LS, Shenker A, Gejman PV, et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. The New England journal of medicine 1991; 325:1688-1695
4. Michiels FM, Caillou B, Talbot M, et al. Oncogenic potential of guanine nucleotide stimulatory factor alpha subunit in thyroid glands of transgenic mice. Proceedings of the National Academy of Sciences of the United States of America 1994; 91:10488-10492
5. Kero J, Ahmed K, Wettschureck N, et al. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. The Journal of clinical investigation 2007; 117:2399-2407
6. Winkler F, Kleinau G, Tarnow P, et al. A new phenotype of nongoitrous and nonautoimmune hyperthyroidism caused by a heterozygous thyrotropin receptor mutation in transmembrane helix 6. The Journal of clinical endocrinology and metabolism 2010; 95:3605-3610
7. Parma J, Duprez L, Van Sande J, et al. Diversity and prevalence of somatic mutations in the thyrotropin receptor and Gs alpha genes as a cause of toxic thyroid adenomas. The Journal of clinical endocrinology and metabolism 1997; 82:2695-2701
8. Corvilain B, Van Sande J, Dumont JE, et al. Somatic and germline mutations of the TSH receptor and thyroid diseases. Clinical endocrinology 2001; 55:143-158
9. Chanson P, Salenave S, Orcel P. McCune-Albright syndrome in adulthood. Pediatric endocrinology reviews : PER 2007; 4 Suppl 4:453-462
10. Rapoport B, Chazenbalk GD, Jaume JC, et al. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev 1998; 19:673-716
11. Davies TF, Ando T, Lin RY, et al. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. The Journal of clinical investigation 2005; 115:1972-1983
12. Thomas JS, Leclere J, Hartemann P, et al. Familial hyperthyroidism without evidence of autoimmunity. Acta endocrinologica 1982; 100:512-518
13. Duprez L, Parma J, Van Sande J, et al. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nature genetics 1994; 7:396-401
14. Tonacchera M, Van Sande J, Cetani F, et al. Functional characteristics of three new germline mutations of the thyrotropin receptor gene causing autosomal dominant toxic thyroid hyperplasia. The Journal of clinical endocrinology and metabolism 1996; 81:547-554
15. Fuhrer D, Holzapfel HP, Wonerow P, et al. Somatic mutations in the thyrotropin receptor gene and not in the Gs alpha protein gene in 31 toxic thyroid nodules. The Journal of clinical endocrinology and metabolism 1997; 82:3885-3891
16. Esapa CT, Duprez L, Ludgate M, et al. A novel thyrotropin receptor mutation in an infant with severe thyrotoxicosis. Thyroid 1999; 9:1005-1010
17. Khoo DH, Parma J, Rajasoorya C, et al. A germline mutation of the thyrotropin receptor gene associated with thyrotoxicosis and mitral valve prolapse in a Chinese family. JClinEndocrinolMetab 1999; 84:1459-1462
18. Fuhrer D, Warner J, Sequeira M, et al. Novel TSHR germline mutation (Met463Val) masquerading as Graves' disease in a large Welsh kindred with hyperthyroidism. Thyroid 2000; 10:1035-1041
19. Tonacchera M, Agretti P, Rosellini V, et al. Sporadic nonautoimmune congenital hyperthyroidism due to a strong activating mutation of the thyrotropin receptor gene. Thyroid 2000; 10:859-863
20. Alberti L, Proverbio MC, Costagliola S, et al. A novel germline mutation in the TSH receptor gene causes non-autoimmune autosomal dominant hyperthyroidism. EurJEndocrinol 2001; 145:249-254
21. Biebermann H, Schoneberg T, Hess C, et al. The first activating TSH receptor mutation in transmembrane domain 1 identified in a family with nonautoimmune hyperthyroidism. The Journal of clinical endocrinology and metabolism 2001; 86:4429-4433
22. Claus M, Maier J, Paschke R, et al. Novel thyrotropin receptor germline mutation (Ile568Val) in a Saxonian family with hereditary nonautoimmune hyperthyroidism. Thyroid 2005; 15:1089-1094
23. Gozu H, Avsar M, Bircan R, et al. Two novel mutations in the sixth transmembrane segment of the thyrotropin receptor gene causing hyperfunctioning thyroid nodules. Thyroid 2005; 15:389-397
24. Nwosu BU, Gourgiotis L, Gershengorn MC, et al. A novel activating mutation in transmembrane helix 6 of the thyrotropin receptor as cause of 25.Nishihara E, Nagayama Y, Amino N, et al. A novel thyrotropin receptor germline mutation (Asp617Tyr) causing hereditary hyperthyroidism. EndocrJ 2007; 54:927-934
26. Akcurin S, Turkkahraman D, Tysoe C, et al. A family with a novel TSH receptor activating germline mutation (p.Ala485Val). Eur J Pediatr 2008; 167:1231-1237
27. Liu Z, Sun Y, Dong Q, et al. A novel TSHR gene mutation (Ile691Phe) in a Chinese family causing autosomal dominant non-autoimmune hyperthyroidism. JHumGenet 2008; 53:475-478
28. Nishihara E, Chen CR, Higashiyama T, et al. Subclinical nonautoimmune hyperthyroidism in a family segregates with a thyrotropin receptor mutation with weakly increased constitutive activity. Thyroid 2010; 20:1307-1314
29. Kleinau G, Brehm M, Wiedemann U, et al. Implications for molecular mechanisms of glycoprotein hormone receptors using a new sequence-structure-function analysis resource. Mol Endocrinol 2007; 21:574-580
30. Kreuchwig A, Kleinau G, Kreuchwig F, et al. Research resource: Update and extension of a glycoprotein hormone receptors web application. Mol Endocrinol 2011; 25:707-712
31. Bircan R, Miehle K, Mladenova G, et al. Multiple relapses of hyperthyroidism after thyroid surgeries in a patient with long term follow-up of sporadic non-autoimmune hyperthyroidism. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association 2008; 116:341-346
32. Leclere J, Bene MC, Duprez A, et al. Behaviour of thyroid tissue from patients with Graves' disease in nude mice. The Journal of clinical endocrinology and metabolism 1984; 59:175-177
33. Hebrant A, van Staveren WC, Maenhaut C, et al. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. European journal of endocrinology 2011; 164:1-9
34. Lavard L, Jacobsen BB, Perrild H, et al. Prevalence of germline mutations in the TSH receptor gene as a cause of juvenile thyrotoxicosis. Acta paediatrica 2004; 93:1192-1194
35. Kopp P, van Sande J, Parma J, et al. Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. The New England journal of medicine 1995; 332:150-154
36. Holzapfel HP, Wonerow P, von Petrykowski W, et al. Sporadic congenital hyperthyroidism due to a spontaneous germline mutation in the thyrotropin receptor gene. JClinEndocrinolMetab 1997; 82:3879-3884
37. Kopp P, Muirhead S, Jourdain N, et al. Congenital hyperthyroidism caused by a solitary toxic adenoma harboring a novel somatic mutation (serine281-->isoleucine) in the extracellular domain of the thyrotropin receptor. The Journal of clinical investigation 1997; 100:1634-1639
38. Gruters A, Schoneberg T, Biebermann H, et al. Severe congenital hyperthyroidism caused by a germ-line neo mutation in the extracellular portion of the thyrotropin receptor. The Journal of clinical endocrinology and metabolism 1998; 83:1431-1436
39. Karges B, Krause G, Homoki J, et al. TSH receptor mutation V509A causes familial hyperthyroidism by release of interhelical constraints between transmembrane helices TMH3 and TMH5. JEndocrinol 2005; 186:377-385
40. Gozu HI, Lublinghoff J, Bircan R, et al. Genetics and phenomics of inherited and sporadic non-autoimmune hyperthyroidism. Molecular and cellular endocrinology 2010; 322:125-134
41. Pohlenz J, Pfarr N, Kruger S, et al. Subclinical hyperthyroidism due to a thyrotropin receptor (TSHR) gene mutation (S505R). Acta paediatrica 2006; 95:1685-1687
42. Landis CA, Masters SB, Spada A, et al. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 1989; 340:692-696
43. Lyons J, Landis CA, Harsh G, et al. Two G protein oncogenes in human endocrine tumors. Science 1990; 249:655-659
44. O’Sullivan C, Barton CM, Staddon SL, et al. Activating point mutations of the gsp oncogene in human thyroid adenomas. Molecular carcinogenesis 1991; 4:345-349
45. Suarez HG, du Villard JA, Caillou B, et al. gsp mutations in human thyroid tumours. Oncogene 1991; 6:677-679
46. Goretzki PE, Lyons J, Stacy-Phipps S, et al. Mutational activation of RAS and GSP oncogenes in differentiated thyroid cancer and their biological implications. World journal of surgery 1992; 16:576-581; discussion 581-572
47. Parma J, Duprez L, Van Sande J, et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature 1993; 365:649-651
48. Parma J, Van Sande J, Swillens S, et al. Somatic mutations causing constitutive activity of the thyrotropin receptor are the major cause of hyperfunctioning thyroid adenomas: identification of additional mutations activating both the cyclic adenosine 3',5'-monophosphate and inositol phosphate-Ca2+ cascades. Mol Endocrinol 1995; 9:725-733
49. Duprez L, Parma J, Costagliola S, et al. Constitutive activation of the TSH receptor by spontaneous mutations affecting the N-terminal extracellular domain. FEBS letters 1997; 409:469-474
50. Fuhrer D, Wonerow P, Willgerodt H, et al. Identification of a new thyrotropin receptor germline mutation (Leu629Phe) in a family with neonatal onset of autosomal dominant nonautoimmune hyperthyroidism. JClinEndocrinolMetab 1997; 82:4234-4238
51. Holzapfel HP, Fuhrer D, Wonerow P, et al. Identification of constitutively activating somatic thyrotropin receptor mutations in a subset of toxic multinodular goiters. The Journal of clinical endocrinology and metabolism 1997; 82:4229-4233
52. Parma J, Duprez L, Van Sande J, et al. Diversity and prevalence of somatic mutations in the thyrotropin receptor and Gs alpha genes as a cause of toxic thyroid adenomas. JClinEndocrinolMetab 1997; 82:2695-2701
53. Wonerow P, Chey S, Fuhrer D, et al. Functional characterization of five constitutively activating thyrotrophin receptor mutations. Clinical endocrinology 2000; 53:461-468
54. Castro I, Lima L, Seoane R, et al. Identification and functional characterization of two novel activating thyrotropin receptor mutants in toxic thyroid follicular adenomas. Thyroid 2009; 19:645-649
55. Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 2000; 21:90-113
56. Govaerts C, Lefort A, Costagliola S, et al. A conserved Asn in transmembrane helix 7 is an on/off switch in the activation of the thyrotropin receptor. J Biol Chem 2001; 276:22991-22999
57. Neumann S, Krause G, Chey S, et al. A free carboxylate oxygen in the side chain of position 674 in transmembrane domain 7 is necessary for TSH receptor activation. Mol Endocrinol 2001; 15:1294-1305
58. Kleinau G, Claus M, Jaeschke H, et al. Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem 2007; 282:518-525
59. Scheerer P, Park JH, Hildebrand PW, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature 2008; 455:497-502
60. Kleinau G, Hoyer I, Kreuchwig A, et al. From Molecular Details of the Interplay between Transmembrane Helices of the Thyrotropin Receptor to General Aspects of Signal Transduction in Family A G-protein-coupled Receptors (GPCRs). J Biol Chem 2011; 286:25859-25871
61. Rasmussen SG, DeVree BT, Zou Y, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 2011; 477:549-555
62. Russo D, Arturi F, Wicker R, et al. Genetic alterations in thyroid hyperfunctioning adenomas. The Journal of clinical endocrinology and metabolism 1995; 80:1347-1351
63. Takeshita A, Nagayama Y, Yokoyama N, et al. Rarity of oncogenic mutations in the thyrotropin receptor of autonomously functioning thyroid nodules in Japan. The Journal of clinical endocrinology and metabolism 1995; 80:2607-2611
64. Trulzsch B, Krohn K, Wonerow P, et al. Detection of thyroid-stimulating hormone receptor and Gsalpha mutations: in 75 toxic thyroid nodules by denaturing gradient gel electrophoresis. Journal of molecular medicine 2001; 78:684-691
65. Georgopoulos NA, Sykiotis GP, Sgourou A, et al. Autonomously functioning thyroid nodules in a former iodine-deficient area commonly harbor gain-of-function mutations in the thyrotropin signaling pathway. European journal of endocrinology 2003; 149:287-292
66. Palos-Paz F, Perez-Guerra O, Cameselle-Teijeiro J, et al. Prevalence of mutations in TSHR, GNAS, PRKAR1A and RAS genes in a large series of toxic thyroid adenomas from Galicia, an iodine-deficient area in NW Spain. European journal of endocrinology 2008; 159:623-631
67. Vanvooren V, Uchino S, Duprez L, et al. Oncogenic mutations in the thyrotropin receptor of autonomously functioning thyroid nodules in the Japanese population. European journal of endocrinology 2002; 147:287-291
68. Duprez L, Hermans J, Van Sande J, et al. Two autonomous nodules of a patient with multinodular goiter harbor different activating mutations of the thyrotropin receptor gene. JClinEndocrinolMetab 1997; 82:306-308
69. Tonacchera M, Chiovato L, Pinchera A, et al. Hyperfunctioning thyroid nodules in toxic multinodular goiter share activating thyrotropin receptor mutations with solitary toxic adenoma. The Journal of clinical endocrinology and metabolism 1998; 83:492-498
70. Tonacchera M, Vitti P, Agretti P, et al. Activating thyrotropin receptor mutations in histologically heterogeneous hyperfunctioning nodules of multinodular goiter. Thyroid 1998; 8:559-564
71. Krohn K, Fuhrer D, Bayer Y, et al. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev 2005; 26:504-524
72. Maier J, van Steeg H, van Oostrom C, et al. Deoxyribonucleic acid damage and spontaneous mutagenesis in the thyroid gland of rats and mice. Endocrinology 2006; 147:3391-3397
73. Russo D, Arturi F, Schlumberger M, et al. Activating mutations of the TSH receptor in differentiated thyroid carcinomas. Oncogene 1995; 11:1907-1911
74. Spambalg D, Sharifi N, Elisei R, et al. Structural studies of the thyrotropin receptor and Gs alpha in human thyroid cancers: low prevalence of mutations predicts infrequent involvement in malignant transformation. The Journal of clinical endocrinology and metabolism 1996; 81:3898-3901
75. Camacho P, Gordon D, Chiefari E, et al. A Phe 486 thyrotropin receptor mutation in an autonomously functioning follicular carcinoma that was causing hyperthyroidism. Thyroid 2000; 10:1009-1012
76. Mircescu H, Parma J, Huot C, et al. Hyperfunctioning malignant thyroid nodule in an 11-year-old girl: pathologic and molecular studies. The Journal of pediatrics 2000; 137:585-587
77. Fuhrer D, Tannapfel A, Sabri O, et al. Two somatic TSH receptor mutations in a patient with toxic metastasising follicular thyroid carcinoma and non-functional lung metastases. Endocrine-related cancer 2003; 10:591-600
78. Niepomniszcze H, Suarez H, Pitoia F, et al. Follicular carcinoma presenting as autonomous functioning thyroid nodule and containing an activating mutation of the TSH receptor (T620I) and a mutation of the Ki-RAS (G12C) genes. Thyroid 2006; 16:497-503
79. Garcia-Jimenez C, Santisteban P. TSH signalling and cancer. Arquivos brasileiros de endocrinologia e metabologia 2007; 51:654-671
80. Nikiforova MN, Wald AI, Roy S, et al. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. The Journal of clinical endocrinology and metabolism 2013; 98:E1852-1860
81. O'Hayre M, Vazquez-Prado J, Kufareva I, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nature reviews Cancer 2013; 13:412-424
82. Jiang X, Liu H, Chen X, et al. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proceedings of the National Academy of Sciences of the United States of America 2012; 109:12491-12496
83. Kosugi S, Okajima F, Ban T, et al. Mutation of alanine 623 in the third cytoplasmic loop of the rat thyrotropin (TSH) receptor results in a loss in the phosphoinositide but not cAMP signal induced by TSH and receptor autoantibodies. J Biol Chem 1992; 267:24153-24156
84. Kleinau G, Biebermann H. Constitutive activities in the thyrotropin receptor: regulation and significance. Advances in pharmacology 2014; 70:81-119
85. Berg KA, Harvey JA, Spampinato U, et al. Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors. Trends in pharmacological sciences 2005; 26:625-630
86. Smits G, Olatunbosun O, Delbaere A, et al. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. The New England journal of medicine 2003; 349:760-766
87. Van Sande J, Parma J, Tonacchera M, et al. Somatic and germline mutations of the TSH receptor gene in thyroid diseases. The Journal of clinical endocrinology and metabolism 1995; 80:2577-2585
88. Fuhrer D, Lewis MD, Alkhafaji F, et al. Biological activity of activating thyroid-stimulating hormone receptor mutants depends on the cellular context. Endocrinology 2003; 144:4018-4030
89. De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 1980; 255:7108-7117
90. Kenakin T. Principles: receptor theory in pharmacology. Trends in pharmacological sciences 2004; 25:186-192
91. Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 2009; 459:356-363
92. Lebon G, Warne T, Tate CG. Agonist-bound structures of G protein-coupled receptors. Current opinion in structural biology 2012; 22:482-490
93. Lefkowitz RJ, Cotecchia S, Samama P, et al. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends in pharmacological sciences 1993; 14:303-307
94. Vlaeminck-Guillem V, Ho SC, Rodien P, et al. Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol 2002; 16:736-746
95. Kleinau G, Jaschke H, Neumann S, et al. Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem 2004; 279:51590-51600
96. Bruser A, Schulz A, Rothemund S, et al. The Activation Mechanism of Glycoprotein Hormone Receptors with Implications in the Cause and Therapy of Endocrine Diseases. J Biol Chem 2016; 291:508-520
97. Nakabayashi K, Matsumi H, Bhalla A, et al. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. The Journal of clinical investigation 2002; 109:1445-1452
98. Karponis D, Ananth S. The role of thyrostimulin and its potential clinical significance. Endocrine regulations 2017; 51:117-128
99. Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends in biochemical sciences 2004; 29:119-126
100. Schoneberg T, Kleinau G, Bruser A. What are they waiting for?-Tethered agonism in G protein-coupled receptors. Pharmacological research 2016; 108:9-15
101. Kleinau G, Worth CL, Kreuchwig A, et al. Structural-Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work. Frontiers in endocrinology 2017; 8:86
102. Claeysen S, Govaerts C, Lefort A, et al. A conserved Asn in TM7 of the thyrotropin receptor is a common requirement for activation by both mutations and its natural agonist. FEBS letters 2002; 517:195-200
103. Ho SC, Van Sande J, Lefort A, et al. Effects of mutations involving the highly conserved S281HCC motif in the extracellular domain of the thyrotropin (TSH) receptor on TSH binding and constitutive activity. Endocrinology 2001; 142:2760-2767
104. Jaeschke H, Neumann S, Kleinau G, et al. An aromatic environment in the vicinity of serine 281 is a structural requirement for thyrotropin receptor function. Endocrinology 2006; 147:1753-1760
105. Glinoer D, Spencer CA. Serum TSH determinations in pregnancy: how, when and why? Nature reviews Endocrinology 2010; 6:526-529
106. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 1997; 18:404-433
107. Grossmann M, Weintraub BD, Szkudlinski MW. Novel insights into the molecular mechanisms of human thyrotropin action: structural, physiological, and therapeutic implications for the glycoprotein hormone family. Endocr Rev 1997; 18:476-501
108. Rodien P, Bremont C, Sanson ML, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. The New England journal of medicine 1998; 339:1823-1826
109. Sanders J, Chirgadze DY, Sanders P, et al. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 2007; 17:395-410
110. Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature 2005; 433:269-277
111. Smits G, Govaerts C, Nubourgh I, et al. Lysine 183 and glutamic acid 157 of the TSH receptor: two interacting residues with a key role in determining specificity toward TSH and human CG. Mol Endocrinol 2002; 16:722-735
112. Coulon AL, Savagner F, Briet C, et al. Prolonged and Severe Gestational Thyrotoxicosis Due to Enhanced hCG Sensitivity of a Mutant Thyrotropin Receptor. The Journal of clinical endocrinology and metabolism 2016; 101:10-11
113. Stewart HJ, Jones DS, Pascall JC, et al. The contribution of recombinant DNA techniques to reproductive biology. Journal of reproduction and fertility 1988; 83:1-57
114. Szkudlinski MW, Fremont V, Ronin C, et al. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure-function relationships. Physiological reviews 2002; 82:473-502
115. Vasseur C, Rodien P, Beau I, et al. A chorionic gonadotropin-sensitive mutation in the follicle-stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. The New England journal of medicine 2003; 349:753-759
116. Stein SA, Oates EL, Hall CR, et al. Identification of a point mutation in the thyrotropin receptor of the hyt/hyt hypothyroid mouse. Mol Endocrinol 1994; 8:129-138
117. Marians RC, Ng L, Blair HC, et al. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:15776-15781
118. Postiglione MP, Parlato R, Rodriguez-Mallon A, et al. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:15462-15467
119. Stanbury JB, Rocmans P, Buhler UK, et al. Congenital hypothyroidism with impaired thyroid response to thyrotropin. The New England journal of medicine 1968; 279:1132-1136
120. Codaccioni JL, Carayon P, Michel-Bechet M, et al. Congenital hypothyroidism associated with thyrotropin unresponsiveness and thyroid cell membrane alterations. The Journal of clinical endocrinology and metabolism 1980; 50:932-937
121. Sunthornthepvarakui T, Gottschalk ME, Hayashi Y, et al. Brief report: resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. The New England journal of medicine 1995; 332:155-160
122. Smits G, Campillo M, Govaerts C, et al. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. The EMBO journal 2003; 22:2692-2703
123. Kleinau G, Krause G. Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev 2009; 30:133-151
124. Sanders P, Young S, Sanders J, et al. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol 2011; 46:81-99
125. Krause G, Kreuchwig A, Kleinau G. Extended and structurally supported insights into extracellular hormone binding, signal transduction and organization of the thyrotropin receptor. PloS one 2012; 7:e52920
126. Kleinau G, Neumann S, Gruters A, et al. Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr Rev 2013; 34:691-724
127. de Roux N, Misrahi M, Brauner R, et al. Four families with loss of function mutations of the thyrotropin receptor. The Journal of clinical endocrinology and metabolism 1996; 81:4229-4235
128. Abramowicz MJ, Duprez L, Parma J, et al. Familial congenital hypothyroidism due to inactivating mutation of the thyrotropin receptor causing profound hypoplasia of the thyroid gland. The Journal of clinical investigation 1997; 99:3018-3024
129. Biebermann H, Schoneberg T, Krude H, et al. Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. The Journal of clinical endocrinology and metabolism 1997; 82:3471-3480
130. Clifton-Bligh RJ, Gregory JW, Ludgate M, et al. Two novel mutations in the thyrotropin (TSH) receptor gene in a child with resistance to TSH. The Journal of clinical endocrinology and metabolism 1997; 82:1094-1100
131. Gagne N, Parma J, Deal C, et al. Apparent congenital athyreosis contrasting with normal plasma thyroglobulin levels and associated with inactivating mutations in the thyrotropin receptor gene: are athyreosis and ectopic thyroid distinct entities? The Journal of clinical endocrinology and metabolism 1998; 83:1771-1775
132. Tonacchera M, Agretti P, Pinchera A, et al. Congenital hypothyroidism with impaired thyroid response to thyrotropin (TSH) and absent circulating thyroglobulin: evidence for a new inactivating mutation of the TSH receptor gene. The Journal of clinical endocrinology and metabolism 2000; 85:1001-1008
133. Bretones P, Duprez L, Parma J, et al. A familial case of congenital hypothyroidism caused by a homozygous mutation of the thyrotropin receptor gene. Thyroid 2001; 11:977-980
134. Jordan N, Williams N, Gregory JW, et al. The W546X mutation of the thyrotropin receptor gene: potential major contributor to thyroid dysfunction in a Caucasian population. The Journal of clinical endocrinology and metabolism 2003; 88:1002-1005
135. Park SM, Clifton-Bligh RJ, Betts P, et al. Congenital hypothyroidism and apparent athyreosis with compound heterozygosity or compensated hypothyroidism with probable hemizygosity for inactivating mutations of the TSH receptor. Clinical endocrinology 2004; 60:220-227
136. Kanda K, Mizuno H, Sugiyama Y, et al. Clinical significance of heterozygous carriers associated with compensated hypothyroidism in R450H, a common inactivating mutation of the thyrotropin receptor gene in Japanese. Endocrine 2006; 30:383-388
137. Tsunekawa K, Onigata K, Morimura T, et al. Identification and functional analysis of novel inactivating thyrotropin receptor mutations in patients with thyrotropin resistance. Thyroid 2006; 16:471-479
138. Yuan ZF, Mao HQ, Luo YF, et al. Thyrotropin receptor and thyroid transcription factor-1 genes variant in Chinese children with congenital hypothyroidism. Endocrine journal 2008; 55:415-423
139. Sura-Trueba S, Aumas C, Carre A, et al. An inactivating mutation within the first extracellular loop of the thyrotropin receptor impedes normal posttranslational maturation of the extracellular domain. Endocrinology 2009; 150:1043-1050
140. Tenenbaum-Rakover Y, Grasberger H, Mamanasiri S, et al. Loss-of-function mutations in the thyrotropin receptor gene as a major determinant of hyperthyrotropinemia in a consanguineous community. The Journal of clinical endocrinology and metabolism 2009; 94:1706-1712
141. Alberti L, Proverbio MC, Costagliola S, et al. Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. The Journal of clinical endocrinology and metabolism 2002; 87:2549-2555
142. Xie J, Pannain S, Pohlenz J, et al. Resistance to thyrotropin (TSH) in three families is not associated with mutations in the TSH receptor or TSH. The Journal of clinical endocrinology and metabolism 1997; 82:3933-3940
143. Grasberger H, Vaxillaire M, Pannain S, et al. Identification of a locus for nongoitrous congenital hypothyroidism on chromosome 15q25.3-26.1. Human genetics 2005; 118:348-355
144. Bohr UR, Behr M, Loos U. A heritable point mutation in an extracellular domain of the TSH receptor involved in the interaction with Graves' immunoglobulins. Biochimica et biophysica acta 1993; 1216:504-508
145. Bahn RS, Dutton CM, Heufelder AE, et al. A genomic point mutation in the extracellular domain of the thyrotropin receptor in patients with Graves' ophthalmopathy. The Journal of clinical endocrinology and metabolism 1994; 78:256-260
146. Gabriel EM, Bergert ER, Grant CS, et al. Germline polymorphism of codon 727 of human thyroid-stimulating hormone receptor is associated with toxic multinodular goiter. The Journal of clinical endocrinology and metabolism 1999; 84:3328-3335
147. Simanainen J, Kinch A, Westermark K, et al. Analysis of mutations in exon 1 of the human thyrotropin receptor gene: high frequency of the D36H and P52T polymorphic variants. Thyroid 1999; 9:7-11
148. Muhlberg T, Herrmann K, Joba W, et al. Lack of association of nonautoimmune hyperfunctioning thyroid disorders and a germline polymorphism of codon 727 of the human thyrotropin receptor in a European Caucasian population. The Journal of clinical endocrinology and metabolism 2000; 85:2640-2643
149. Ban Y, Greenberg DA, Concepcion ES, et al. A germline single nucleotide polymorphism at the intracellular domain of the human thyrotropin receptor does not have a major effect on the development of Graves' disease. Thyroid 2002; 12:1079-1083
150. Ho SC, Goh SS, Khoo DH. Association of Graves' disease with intragenic polymorphism of the thyrotropin receptor gene in a cohort of Singapore patients of multi-ethnic origins. Thyroid 2003; 13:523-528
151. Wellcome Trust Case Control C, Australo-Anglo-American Spondylitis C, Burton PR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature genetics 2007; 39:1329-1337
152. Brand OJ, Barrett JC, Simmonds MJ, et al. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves' disease. Human molecular genetics 2009; 18:1704-1713
153. Porcu E, Medici M, Pistis G, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS genetics 2013; 9:e1003266
154. Libert F, Lefort A, Gerard C, et al. Cloning, sequencing and expression of the human thyrotropin (TSH) receptor: evidence for binding of autoantibodies. Biochemical and biophysical research communications 1989; 165:1250-1255
155. Nagayama Y, Kaufman KD, Seto P, et al. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochemical and biophysical research communications 1989; 165:1184-1190
156. Biebermann H, Schoneberg T, Schulz A, et al. A conserved tyrosine residue (Y601) in transmembrane domain 5 of the human thyrotropin receptor serves as a molecular switch to determine G-protein coupling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 1998; 12:1461-1471
157. Arseven OK, Wilkes WP, Jameson JL, et al. Substitutions of tyrosine 601 in the human thyrotropin receptor result in increase or loss of basal activation of the cyclic adenosine monophosphate pathway and disrupt coupling to Gq/11. Thyroid 2000; 10:3-10
Hashimoto’s Thyroiditis
ABSTRACT
Hashimoto's thyroiditis is characterized clinically as a commonly occurring, painless, diffuse enlargement of the thyroid gland occurring predominantly in middle-aged women. The patients are often euthyroid, but hypothyroidism may develop. The thyroid parenchyma is diffusely replaced by a lymphocytic infiltrate and fibrotic reaction; frequently, lymphoid germinal follicles are visible. Persons with Hashimoto's thyroiditis have serum antibodies reacting with TG, TPO, and against an unidentified protein present in colloid. In addition, many patients have cell mediated immunity directed against thyroid antigens, demonstrable by several techniques. The incidence is on the order of three to six cases per 10,000 population per year, and prevalence among women is at least 2%. The gland involved by thyroiditis tends to lose its ability to store iodine, produces and secretes iodoproteins that circulate in plasma, and is inefficient in making hormone. Thus, the thyroid gland is under increased TSH stimulation, fails to respond to exogenous TSH, and has a rapid turnover of thyroidal iodine.
Diagnosis is made by the finding of a diffuse, smooth, firm goiter in a young woman, with strongly positive titers of TG Ab and/or TPO Ab and a euthyroid or hypothyroid metabolic status. A patient with a small goiter and euthyroidism does not require therapy unless the TSH level is elevated. The presence of a large gland, progressive growth of the goiter, or hypothyroidism indicates the need for replacement thyroid hormone. Surgery is rarely indicated. Development of lymphoma, though very unusual, must be considered if there is growth or pain in the involved gland.
HISTORICAL REVIEW
In 1912 (Fig. 8-1) Hashimoto described four patients with a chronic disorder of the thyroid, which he termed struma lymphomatosa. The thyroid glands of these patients were characterized by diffuse lymphocytic infiltration, fibrosis, parenchymal atrophy, and an eosinophilic change in some of the acinar cells.(1) Clinical and pathologic studies of this disease have appeared frequently since Hashimoto's original description. The disease has been called Hashimoto's thyroiditis, chronic thyroiditis, lymphocytic thyroiditis, lymphadenoid goiter, and recently autoimmune thyroiditis. Classically, the disease occurs as a painless, diffuse enlargement of the thyroid gland in a young or middle-aged woman. It is often associated with hypothyroidism. The disease was thought to be uncommon for many years, and the diagnosis was usually made by the surgeon at the time of operation or by the pathologist after thyroidectomy. The increasing use of the needle biopsy and serologic tests for antibodies have led to much more frequent recognition, and there is reason to believe that it may be increasing in frequency.(2) It is now one of the most common thyroid disorders.

Figure 1. Dr. Hakaru Hashimoto
The first indication of an immunologic abnormality in this disease was an elevation of the plasma gamma globulin fraction detected by Fromm et al.(3) This finding, together with abnormalities in serum flocculation test results(4) indicated that the disease might be related to a long-continued autoimmune reaction. Rose and Witebsky(5) showed that immunization of rabbits with extracts of rabbit thyroids produced histologic changes in the thyroid glands resembling those seen in Hashimoto's thyroiditis. They also found antithyroglobulin antibodies in the blood of the animals. Subsequently, Roitt et al.(6) observed that a precipitate formed when an extract of human thyroid gland was added to serum from a patient with Hashimoto's thyroiditis. Thus, it appeared that the serum contained antibodies to a constituent of the human thyroid and that these antibodies might be responsible for the disease process. These original observations led directly to entirely new concepts of the causation of disease by autoimmunization.
PATHOLOGY
The goiter is generally symmetrical, often with a conspicuous pyramidal lobe. Grossly, the tissue involved by Hashimoto's thyroiditis is pinkish-tan to frankly yellowish and tends to have a rubbery firmness. The capsular surface is gently lobulated and non-adherent to peri-thyroid structures. Microscopically, there is a diffuse process consisting of a combination of epithelial cell destruction, lymphoid cellular infiltration, and fibrosis. The thyroid cells tend to be slightly larger and assume an acidophilic staining character; they are then called Hurthle or Askanazy cells and are packed with mitochondria. The follicular spaces shrink, and colloid is absent or sparse. Fibrosis may be completely absent or present in degrees ranging from slight to moderate; it may be severe, as observed in subacute or Riedel's thyroiditis. Foreign body giant cells and granulomas are not features of Hashimoto's thyroiditis, in contrast to subacute thyroiditis. In children, oxyphilia and fibrosis are less prominent, and hyperplasia of epithelial cells may be marked. Deposits of dense material representing IgG are found along the basement membrane on electron microscopy (Fig. 8-2).

Figure 2. Electron microscopy image of thyroid tissue from a patient with Hashimoto's thyroiditis, showing electron dense deposits of IgG and TG along the basement membrane of follicular cells.
Within the follicles may be seen clusters of macrophage-like cells. The lymphoid infiltration in the interstitial tissue is accompanied by actual follicles and germinal centers (Fig. 8-3, below). Plasma cells are prominent. Totterman has studied the characteristics of the lymphocytes in the thyroid and reports that they are made up of equal proportions of T and B cells.(7) Most infiltrating T cells have alpha/beta T cell receptors. Gamma/delta T cells are rare(8), although their proportion in intrathyroidal lymphocytes is higher than that in peripheral lymphocytes(9). CD4+CD8+ cells and CD3lo-TCRalpha/beta-lo/CD4-CD8- cells also are present in the infiltrate in the thyroid(9). Infiltrating T cells are considered to be a highly restricted population, based on the study of T cell receptor V alpha(10) and beta(11) gene expression. Heuer et al. studied cytokine mRNA expression in intrathyroidal T cells and found increased expression of IFN-gamma, IL-2 and CD25, which are Th1-related cytokines(12) in Hashimoto's thyroiditis. Thyroglobulin-binding lymphocytes were increased in percentage relative to their occurrence in blood.

Figure 3. Pathology of Hashimoto's thyroiditis. In this typical view of severe Hashimoto's thyroiditis, the normal thyroid follicles are small and greatly reduced in number, and with the hematoxylin and eosin stain are seen to be eosinophilic. There is marked fibrosis. The dominant feature is a profuse mononuclear lymphocytic infiltrate and lymphoid germinal center formation.
The quantity of parenchymal tissue left in the thyroid is variable. In some instances it is actually increased, perhaps as a compensatory hyperplastic response to inefficient iodide metabolism. Typically, the pathologic process involves the entire lobe or gland. Focal thyroiditis, which is microscopically similar, may be found in thyroid glands with diffuse hyperplasia of Graves' disease, in association with thyroid tumors, or in multinodular thyroid glands. The thymus, which is frequently enlarged in thyroiditis as it is in Graves' disease, does not present the picture of enhanced immunologic activity(13),(14). Histologic feature in painless (or silent) thyroiditis is almost similar to that of Hashimoto's thyroiditis. All specimens show chronic thyroiditis, focal or diffuse type: and lymphoid follicles were present in about half of the specimen(15). The follicular distruptions are characteristic and common histologic feature at the time of destructive thyrotoxicois but disappear during the late recovery phase of disease. Thus painless thyroiditis may be induced by the activation of autoimmune reaction within the thyroid gland in patients with Hashimoto's thyroiditis.
PATHOGENESIS
The putative causes of autoimmune thyroid disease (AITD) are reviewed in Chapter 7, and the basic concepts reviewed there apply of course to Hashimoto's thyroiditis. In Hashimoto's thyroiditis, the immunologic attack appears to be typically aggressive and destructive, rather than stimulatory, as in Graves' disease, and the difference is most likely due to the characteristics of the immune response. Hashimoto's thyroiditis is reported to occur in two varieties, an atrophic variety, perhaps associated with HLA-DR3 gene inheritance, and a goitrous form associated with HLA-DR5. The large UK Caucasian HT case control cohort study demonstrated clear differences in association within the HLA class II region between Hashimoto's thyroiditis and Graves' disease, differences in HLA class II genotype may, in part, contribute to the different immunopathological processes and clinical presentation of these related diseases (15a). In studies of autoimmune hypothyroidism in monozygotic twins, the concordance rate is below 1 and thus environmental factors are also etiologically important.(16) Concerning susceptibility genes for Hashimoto's thyroiditis, non-MHC class II genes have been recently investigated. A number of data accumulated, demonstrating an association between cytotoxic T cell antigen-4 (CTLA-4), which is a major negative regulator of T-cell mediated immune functions, and autoimmune diseases including Hashimoto's thyroiditis. New studies have appeared on the zinc-finger gene in AITD susceptibility region gene (ZFAT), the thyroglobulin gene, and the protein tyrosine phosphatase-22 (PTPN22) gene. Genome-wide association studies (GWAS) detected other genes including FCRL3, FOXE1 and IL2RA. (16a) Many of the genes associated with AITD are also associated with other autoimmune diseases, which highlights a key role for disrupted T cell central tolerance, antigen monitoring and peripheral immune tolerance in autoimmune onset. Association of polymorphisms in miroRNA genes (miR499A and miR125A) with autoimmune thyroid diseases were reported (16b).
Regarding environmental factors, high iodine intake, selenium deficiency, pollutants such as tobacco smoke, infectious diseases such as chronic hepatitis C, and certain drugs are implicated in the development of autoimmune thyroiditis (16.1: Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab. 2008 Jul 8. [Epub ahead of print]). Long-term iodine exposure leads to increased iodination of thyroglobulin, which increases its antigenicity and initiates the autoimmune process in genetically susceptible individuals. Selenium deficiency decreases the activity of selenoproteins, including glutathione peroxidases, which can lead to raised concentrations of hydrogen peroxide and thus promote inflammation and disease. Such environmental pollutants as smoke, polychlorinated biphenyls, solvents and metals have been implicated in the autoimmune process and inflammation. Environmental factors have not yet, however, been sufficiently investigated to clarify their roles in pathogenesis, and there is a need to assess their effects on development of the autoimmune process and the mechanisms of their interactions with susceptibility genes.
High titers of antibody against thyroglobulin (TG) and thyroid peroxidase (TPO) are present in most patients with Hashimoto's thyroiditis(17), and TPO antibodies are complement fixing and may be cytotoxic. However, the evidence for cytotoxicity is scant, especially since normal transplacental antibody passage of anti-TPO Ab to the human fetus does not usually induce thyroid damage.
Thus it is speculated that cytotoxic T cells, or killer (K) or natural killer (NK) cells, or regulatory T (Treg) or suppressor T cells, may play an important role. A few reports do show T cell line or clone cytotoxicity toward isologous thyroid epithelial cells, and experimental thyroiditis can be transferred by lymphocytes. T cells from patients with Hashimoto's disease proliferate when exposed to TG and TPO. These responses are known to be directed to specific sequences in the TPO molecule, including epitopes at aa 110-129, 210-230, 420-439, and 842-861(18). T cells from mice immunized to TPO react strongly to TPO sequence 540-559, and when immunized with this peptide, develop hypothyroidism and thyroiditis. This peptide may be a central factor in immunity to TPO(18.1). Muixí et al. identified natural HLA-DR-associated peptides in autoimmune organs that will allow finding peptide-specific T cells in situ (18.2). This study reports a first analysis of HLA-DR natural ligands from ex vivo Graves' disease-affected thyroid tissue. Using mass spectrometry, they identified 162 autologous peptides from HLA-DR-expressing cells, including thyroid follicular cells, with some corresponding to predominant molecules of the thyroid colloid. Most interestingly, eight of the peptides were derived from a major autoantigen, thyroglobulin. In vitro binding identified HLA-DR3 as the allele to which one of these peptides likely associates in vivo. Computer modeling and bioinformatics analysis suggested other HLA-DR alleles for binding of other thyroglobulin peptides. Increased K and NK cell function has been reported in Hashimoto's thyroiditis (19). Dysfunction of regulatory (or suppressor) CD4+ T cell populations may lead to the development of various organ-specific autoimmune diseases including Hashimoto’s thyroiditis (19.1). Despite the lack of understanding of the primary cause(s), it is certain that thyroid autoimmunity drives the lymphocyte collection in the thyroid and is responsible for thyroid epithelial cell damage. Progressive thyroid cell damage can change the apparent clinical picture from goitrous hypothyroidism to that of primary hypothyroidism, or "atrophic" thyroiditis. Primary hypothyroidism is considered to be the end stage of Hashimoto's thyroiditis. In the TSHR-immunized murine model of Graves’ disease, Treg depletion (particularly CD25) induced thyroid lymphocytic infiltrates with transient or permanent hypothyroidism (19.2). Lymphocytic infiltration was associated with intermolecular spreading of the TSHR antibody response to other self thyroid antigens, murine thyroid peroxidase and thyroglobulin. These data suggest a role for Treg in the natural progression of hyperthyroid Graves' disease to Hashimoto's thyroiditis and hypothyroidism in humans.
An alternative cause of "atrophic" hypothyroidism is the development of thyroid stimulation blocking antibodies (TSBAb), which, as the name implies, prevent TSH binding to TSH-R, but do not stimulate thyroid cells and produce hypothyroidism. It has been proposed that TSBAb bind to epitopes near the carboxyl end of the TSH-R extracellular domain, in contrast to thyroid stimulating antibodies (TSAb), which bind to epitopes near aa 40 at the amino terminus(20). This syndrome occurs in neonates, children and adults. The prevalence of TSBAb in adult hypothyroid patients has been reported to be 10%(21). However, in contrast to the usual progressive and irreversible thyroid damage occurring in the usual setting, these blocking antibodies tend to follow the course of TSAb--that is, they decrease or disappear over time, and the patient may become euthyroid again(22). A change from a predominant TSAb response to a predominant TSBAb response can cause patients to have sequential episodes of hyper- and hypothyroid function(23). HLA antigens of hypothyroid patients with TSBAb were found to be different from patients with idiopathic myxedema or Hashimoto's thyroiditis, and rather similar to patients with Graves' disease(24).
In patients with autoimmune hypothyroidism, thyroid dysfunction might be induced by cytokine-mediated apoptosis of thyroid epithelial cells and infiltrating T lymphocytes may not directly be involved in thyrocyte cell death during Hashimoto' s thyroiditis. Fragmented DNA, a characteristic feature of apoptosis, was frequently found in the thyroid follicular cells in Hashimoto's thyroiditis(25). The ligand for Fas(Fas L)was shown to be constitutively expressed on thyrocytes and lL-1alpha, abundantly produced in the thyroid gland of Hashimoto's thyroiditis, induced Fas expression on thyrocytes. Thus Fas-FasL interaction on thyrocytes may induce apoptosis and thyroid cell destruction(26). In the thyroid follicle cells of Hashimoto's thyroiditis, Fas and FasL are strongly stained and immunostaining of Bcl-2 is weak, suggesting that cytokines cause up-regulation of apoptosis(27). Increased serum TSH may inhibit Fas-mediated apoptosis of thyrocytes(28). In contrast TSBAb block the inhibitory action of TSH toward Fas-mediated apoptosis and thus induce thyroid atrophy. On the other hand, transgenic expression of Fas L on thyroid follicular cells actually prevents autoimmune thyroiditis, possibly through inhibition of lymphocyte infiltation(29). Other death-receptor ligands might participate in and TNF-related apoptosis-includingathyrocyte killing, including TNF- ligand(TRAIL)(30) . In relation to the Fas-Fas L system, Dong et al. reported that mutations of Fas, which induce loss of function, were found in thyroid lymphocytes in 38.1% of patients with Hashimoto's thyroiditis(31). These mutations are found in 65.4% of patients with malignant lymphoma(32), which usually develops from Hashimoto's thyroiditis. These changes are possibly important for progression of Hashimoto's thyroiditis.
Apparent de-novo development of antibodies, augmentation of pre-existing thyroid autoimmunity, goiter, and hypothyroidism, are induced in some cancer patients, when given courses of IL2, IL2a plus lymphokine activated K cells and/or IFN-gamma. It is thought that the phenomenon may reflect activation of lymphocytes by the lymphokine and lymphokine and cell-mediated attack on thyroid tissue(33). Activated lymphocytes release TNFalpha and IFNgamma, which can injure or suppress TEC function. IFNgamma may also augment thyrocyte HLA-DR expression, which could make the thyrocyte able to present self-antigens. Interferon alpha therapy for chronic active type C hepatitis also augments pre-existing thyroid autoimmunity and can induce autoimmune hypothyroidism. A humanised anti-CD52 monoclonal antibody, Campath-1H may permit the generation of antibody-mediated thyroid autoimmunity (33a,b). Campath-1H depletes lymphocytes and monocytes, and may cause the immune response to change from the Th1 phenotype.
T helper type 17 (Th17) lymphocytes, which produce a proinflammatory cytokine IL-17, have recently been shown to play a major role in numerous autoimmune diseases that had previously been thought to be Th1-dominant diseases, such as Hashimoto’s thyroiditis. It is reported that there is an increased differentiation of Th17 lymphocytes and an enhanced synthesis of Th17 cytokines in Hashimoto's disease (33c). In a mouse model of Hashimoto's thyroiditis, iodine-induced autoimmune thyroiditis in nonobese diabetic-H2(h4) mice, both Th1 and Th17 cells are found to be critical T(eff) subsets for the pathogenesis of spontaneous autoimmune thyroiditis (33d). Imbalance of Th17/Treg is reported in different subtypes of autoimmune thyroid diseases. Increased Th17 lymphocytes may play a more important role in the pathogenesis of HT and GO while decreased Treg may be involved in Graves’ disease (33d.1). In contrast, a significant decrease in the ratios of CD4 + IL17+/CD4 + CD25 + CD127 - (p < 0.0001) and CD4 + IL17+/CD4 + CD25 + CD127 - FoxP3 + (p < 0.0001) T cells was obsereved in Hashimoto’s thyroiditis in comparison to healthy children (33d.2).
The IgG4-related disease (IgG4-RD) is a new disease entity first proposed in relation to autoimmune pancreatitis (AIP) by Hamano et al. in 2001 (33e). A high prevalence of hypothyroidism has been reported in patients with AIP (33f). In 2009, it was reported that on the basis of the immunohistochemistry of IgG4, HT can be divided into two groups, which were proposed as IgG4 thyroiditis (IgG4-positive plasma cell-rich group) and non-IgG4 thyroiditis (IgG4-positive plasma cellpoor group) (33g). The IgG4 thyroiditis group shows indistinguishable histological features and may have a close relationship with IgG4-RD in other organs. In 2010, it was demonstrated that IgG4 thyroiditis is clinically associated with a lower female-to-male ratio, more rapid progress, subclinical hypothyroidism, diffuse low echogenicity, and a higher level of circulating thyroid autoantibodies than non-IgG4 thyroiditis (33h). Riedel thyroiditis (RT) is another candidate for IgG4-RD. It is a rare form of chronic thyroiditis, characterized by inflammatory proliferative fibrosis which involves the thyroid parenchyma and surrounding tissue structures. In 2010, Dahlgren et al. reported that IgG4-RD was the underlying condition in a part of the cases of RT (33i). When IgG4-RD occurs in a systemic pattern, the thyroid involvement may present as RT rather than HT (33j).
Iodine consumption influences the incidence of Hashimoto's thyroiditis and hypothyroidism (see below: “Iodide Metabolism and Effects” in this chapter). Smoking has also been identified as a risk factor for hypothyroidism, but the reason for the association is unknown (34).
An increase in the prevalence of thyroid autoantibodies (ATAs) was reported 6-8 yr after the Chernobyl accident in radiation-exposed children and adolescents (34a). TPOAb prevalence in adolescents exposed to radioactive fallout was still increased in Belarus 13-15 yr after the Chernobyl accident (34b). This increase was less evident than previously reported and was not accompanied by thyroid dysfunction. These data suggest that radioactive fallout elicited a transient autoimmune reaction, without triggering full-blown thyroid autoimmune disease. Longer observation periods are needed to exclude later effects.
Celiac disease was positively associated with hypothyroidism (Hazard Ratio = 4.4; 95% Confidence Interval = 3.4-5.6; p < 0.001), thyroiditis (3.6; 1.9-6.7; p < 0.001) and hyperthyroidism (2.9; 2.0-4.2; p < 0.001) (34c). The highest risk estimates were found in children (hypothyroidism 6.0; 3.4-10.6, thyroiditis 4.7; 2.1-10.5 and hyperthyroidism 4.8; 2.5-9.4). In post-hoc analyses, where the reference population was restricted to inpatients, the adjusted HR for hypothyroidism was 3.4 (2.7-4.4; p < 0.001), thyroiditis 3.3 (1.5-7.7; p < 0.001) and hyperthyroidism 3.1 (2.0-4.8; p < 0.001).This indicates shared etiology and that these individuals are more susceptible to autoimmune disease.
Hashimoto thyroiditis is often associated with type 1 diabetes and other autoimmune disorders such as coeliac disease, type 2 and type 3 polyglandular autoimmune disorders (APS). Type 2 APS is defined by the occurrence of Addison's disease with thyroid autoimmune disease and/or Type 1 diabetes mellitus. Type 3 APS is thyroid autoimmune diseases associated with other autoimmune diseases (excluding Addison's disease and/or hypoparathyroidism). Clinically overt disorders are considered only the tip of the autoimmune iceberg, since latent forms are much more frequent (34d). Hashimoto thyroiditis is also often associated in lymphocytic hypophysitis (34e).
There is a report that microRNAs (miRNAs) miR-146a1, miR-155_2, and miR-200a1 are altered in AITD. In the thyroid tissue of the GD group, miR-146a1 was significantly decreased in comparison to the control group (mean relative expression 5.17 vs. 8.37, respectively, p = 0.019). In the HT group, miR-155_2 was significantly decreased in comparison to the control group (8.30 vs. 11.20, respectively, p = 0.001), and miR-200a1 was significantly increased (12.02 vs. 8.01, p = 0.016) (34f). The expression levels of miRNAs in plasma and peripheral blood mononuclear cells showed wide individual variation, and the these levels may be associated with the pathogenesis of autoimmune thyroid diseases (34g). Accumulating data suggest that miRNAs crucially control innate and adaptive immune responses, and implicate some miRNAs as having an important role in the pathophysiology of immunity and autoimmunity. (34h) For example, miR-155_2 was previously shown to possess important functions in the mammalian immune system. (34i) MicroRNA-142-5p may contribute to Hashimoto's thyroiditis by targeting CLDN1.(34j)
INCIDENCE AND DISTRIBUTION
The incidence of Hashimoto's thyroiditis seen in practice is unknown but is roughly equal to that of Graves' disease (on the order of 0.3 - 1.5 cases per 1,000 population per year.)(35-37) The disease is 15 - 20 times as frequent in women as in men. It occurs especially during the decades from 30 to 50, but may be seen in any age group, including children. It is certain that it exists with a much higher frequency than is diagnosed clinically, and its frequency seems to be increasing. Family studies always bring to light a number of relatives with moderate enlargement of the thyroid gland suggestive of Hashimoto's thyroiditis. Many of these persons have TG and TPO antibodies, and most are entirely asymptomatic. Inoue et al. found 3% of Japanese children aged 6 - 18 to have thyroiditis(38). In most instances, biopsy revealed focal rather than diffuse thyroiditis.
In addition to overt thyroiditis, roughly 10% of most populations have positive TG and TPO antibody test results(35-37) in the apparent absence of thyroid disease by physical examination. In a classic study of an entire community, Tunbridge et al.(37) found that 1.9 - 2.7% of women had present or past thyrotoxicosis, 1.9% had overt hypothyroidism, 7.5% had elevated TSH levels, 10.3% had test results positive for TPO (microsomal antigen) Ab measured by hemagglutination assay (MCHA), and about 15.0% had goiter. Men had 10 to 4-fold lower incidence of thyroid abnormalities. In a study of children whose parents had history of thyroid disease, Carey et al.(39) found a 24% prevalence of thyroid "abnormalities", including a prevalence of 6.9% abnormal thyroids, and 9.3% with positive TG Ab measured by hemagglutination assay (TGHA) and 7.8% positive MCHA assays. Gordin et al.(35) found that 8% of adult Finns had positive TGHA results, and 26% had positive MCHA results. TSH levels were elevated in 30% of these persons. On the basis of positive antibody titers and elevated TSH levels, 2 - 5% were believed to have asymptomatic thyroiditis. These test results correlate with focal collection of lymphocytes on histologic examination of the thyroid glands(40), are frequently associated with elevated levels of TSH(41), and probably represent one end of a spectrum of thyroid damage. Women with both positive antibody test results and raised TSH levels become hypothyroid at the rate of 5%/year(42). A reasonable approximation of the prevalence of positive antibody tests in women is greater than 10%, and of clinical disease is at least 2%. Men have one-tenth this prevalence. A number of small datasets have suggested a potential role for skewed X chromosome activation (XCI), away from the expected 50:50 parent of origin ratio, as an explanation for the strong female preponderance seen in the common autoimmune thyroid diseases (AITD), Graves’ disease (GD) and Hashimoto’s thyroiditis (HT). (42a) A possible role for fetal cell microchimerism in triggering an autoimmune process has been repeatedly proposed, based on the evidence that autoimmune diseases have a higher prevalence in females, with peak incidence in women of childbearing age. Fetal microchimeric cells have been found to be significantly more represented within the thyroid gland of women with Hashimoto's thyroiditis and Graves' disease compared to those without thyroid autoimmunity, suggesting a pathogenic role. (42b, c, d)
COURSE OF THE DISEASE (Table 8-1)
Hashimoto's thyroiditis begins as a gradual enlargement of the thyroid gland and gradual development of hypothyroidism. It is often discovered by the patient, who finds a fullness of the neck or a new lump while self-examining because of a vague discomfort in the neck. Perhaps most often, it is found by the physician during the course of an examination for some other complaint.
Table 1. Presentations of Hashimoto's Thyroiditis
|
1. Euthyroidism and goiter 2. Subclinical hypothyroidism and goiter 3. Primary thyroid failure 4. Hypothyroidism 5. Adolescent goiter 6. Painless thyroiditis or silent thyroiditis 7. Postpartum painless thyrotoxicosis 8. Alternating hypo- and hyperthyroidism |
In some instances the thyroid gland may enlarge rapidly; rarely, it is associated with dyspnea or dysphagia from pressure on structures in the neck, or with mild pain and tenderness. Rarely, pain is persistent and unresponsive to medical treatment and requires medical therapy or surgery. The goiter of Hashimoto's thyroiditis may remain unchanged for decades(37), but usually it gradually increases in size. Occasionally the course is marked by symptoms of mild thyrotoxicosis, especially during the early phase of the disease. Symptoms and signs of mild hypothyroidism may be present in 20% of patients when first seen(41), or commonly develop over a period of several years. Progression from subclinical hypothyroidism (normal FT4 but elevated TSH) to overt hypo-thyroidism occurs in a certain fraction (perhaps 3-5%) each year. Eventually thyroid atrophy and myxedema may occur(43). This assertion is based on the clinical observation that patients with Hashimoto's thyroiditis often develop myxedema, and the knowledge that patients with myxedema due to atrophy of the thyroid have a high incidence of TG Ab in their serum. The disease frequently produces goitrous myxedema in young women, and we have occasionally observed a goitrous and hypothyroid patient who went on to develop thyroid atrophy.Occasionally, patients with Hashimoto痴 thyroiditis have persistent pain which is unresponsive to nonsteroidal anti-inflammatory drugs, replacement with thyroid hormone, and recurs after therapy with steroids. Kon and DeGroot recently reported seven patients who finally came to subtotal or near-total thyroidectomy, some of whom received subsequent radioactive iodide thyroid ablation, with final relief of symptoms (Kon, YC; DeGroot, LJ. Painful Hashimoto痴 thyroiditis as an indication for thyroidectomy: clinical characteristics and outcome in seven patients. J Clin Endocrinol Metab 88 2667-2672 2003).
Generally the progression from euthyroidism to hypothyroidism has been considered an irreversible process due to thyroid cell damage and loss of thyroidal iodine stores (Fig. 8-4). However, it is now clear that up to one-fourth of patients who are hypothyroid may spontaneously return to normal function over the course of several years. This sequence may reflect the initial effect of high titers of thyroid stimulation blocking antibodies which fall with time and allow thyroid function to return(23).

Figure 4. Fluorescent thyroid scan in thyroiditis. The normal thyroid scan (left) allows identification of a thyroid with normal stable (127I) stores throughout both lobes. A marked reduction in 127I content is apparent throughout the entire gland involved with Hashimoto's thyroiditis (right).
Within the past few years, several unusual syndromes believed to be associated with or part of the clinical spectrum of Hashimoto's thyroiditis have been described. Occasional patients develop amyloid deposits in the thyroid (44). Shaw et al.(45) described five patients with a relapsing steroid-responsive encephalopathy including episodes like stroke and seizures, high CSF protein, abnormal EEG, and normal CAT scans (see Hashimoto's encephalopathy below). Khardon et al.(46) described a steroid responsive lymphocytic interstitial pneumonitis in four patients. It remains uncertain how these illnesses relate to lymphocytic thyroiditis, which has until now been largely identified as an organ specific disease.
At 5 years of follow-up of the natural course of euthyroid Hashimoto's thyroiditis in Italian children, more than 50% of the patients remained or became euthyroid (46-1). The presence of goiter and elevated TGab at presentation, together with progressive increase in both TPOab and TSH, may be predictive factors for the future development of hypothyroidism.
Hashimoto's thyroiditis and hypothyroidism are associated with Addison's disease, diabetes mellitus, hypogonadism, hypopara-thyroidism, and pernicious anemia. Such combinations are described as the polyglandular failure syndrome. Two forms of polyglandular autoimmunity have been recognized(47). In the Type I syndrome patients have hypoparathyroidism, muco-cutaneous candidiasis, Addison's disease, and occasionally hypothyroidism. Type II, more frequent, often includes familial associations of diabetes mellitus, hypothyroidism, hypoadrenalism, and occasionally gonadal or pituitary failure. In these syndromes, antibodies reacting with the affected end organs are characteristically present. Vitiligo, hives, and alopecia are associated with thyroiditis. There is also a clear association with primary and secondary Sjogren's syndrome(48). Some patients appear to start with Hashimoto's thyroiditis, and progress with time to the picture of Riedel's thyroiditis including the frequently-associated retroperitoneal fibrosis(49).
Musculoskeletal symptoms, including chest pain, fibrositis, and rheumatoid arthritis, occur in one-quarter of patients(50), and of course, any of the musculoskeletal symptoms of hypothyroidism may likewise occur.
It has been suggested that thyroiditis predisposes to vascular disease and coronary occlusion. Abnormally elevated titers of thyroid autoantibodies and the morphologic changes of thyroiditis are said to occur with an increased frequency among patients with coronary artery disease. Mild hypothyroidism(51) associated with asymptomatic atrophic thyroiditis could predispose patients to heart disease. Others have failed to find increased TG Ab in-patients with coronary artery disease(52) or increased coronary disease in association with thyroiditis.
Although chronic inflammation, leading to neoplastic transformation, is a well-established clinical phenomenon, the link between Hashimoto’s thyroiditis and thyroid cancer remains controversial (52a, b). Larson et al. reported that patients with Hashimoto’s thyroiditis were three times more likely to have thyroid cancer, suggesting a strong link between chronic inflammation and cancer development (52-1). PI3K/Akt expression was increased in both Hashimoto’s thyroiditis and well-differentiated thyroid cancer, suggesting a possible molecular mechanism for thyroid carcinogenesis. Thyroid cancer may be associated with less aggressive disease and better outcome in patients with coexisting Hashimoto’s thyroiditis. (52b, c, d)
In children, retarded growth, retarded bone age, decreased hydroxyproline excretion, and elevated cholesterol levels may be seen (Fig. 8-5).

Figure 5. Identical male twins with Hashimoto's thyroiditis were photographed at age 12. At age 8, they had the same height and appearance. During the intervening 4 years, small goiters developed and the growth of the twin on the right almost stopped. Biopsy indicated Hashimoto's thyroiditis in each twin's thyroid.
Hashimoto's Thyroiditis in Identical Twin Boys*
D.L. was seen at age 12 for failure to grow over the past 4 years. The patient had an identical twin, whose development up to age 8 had been entirely normal. Pubertal changes had developed at age 11. No goiter had been noted.
On physical examination, he was a short, cooperative, pubertal boy of normal intelligence, 129 cm in height and 35 kg in weight. The thyroid gland was smooth and firm, and of normal size. The skin was dry, cool, and mottled. Reflex relaxation was delayed. Estimated T4 levels were < 4 ug/dl, and the 24-hour RAIU was 4%. Thyroid scan showed a normal thyroid gland. Bone age was 8 years. The potassium thiocyanate discharge test result was negative. Thyroid biopsy showed a moderately diffuse lymphocytic infiltrate with lymphoid germinal centers and a diffuse, dense fibrous reaction.
R.L. was seen simultaneously with D.L. and was an active, healthy-appearing boy with early pubertal changes. His height was 149 cm, and his weight was 39.7 kg. The pulse was 104. The skin was normal. The thyroid gland was enlarged to about three times the normal size and was not nodular. PBI levels were 6.4 and 7.2 ug/dl, and the 24- hour RAIU was 21%. Bone age was 11 years. A potassium thiocyanate discharge test caused no decrease in neck radioactivity. Biopsy showed diffuse lymphocytic infiltration, lymphoid follicles and germinal centers, atrophy of thyroid follicles, oxyphilic cytoplasm, and dense fibrosis.
Similar fingerprints, similar lip and ear shapes, and identity of 15 blood factors indicated that they were identical twins. There was no family history of thyroid disease.
Iodide kinetic studies showed rapid turnover of thyroid iodide and production of excess quantities of plasma butanol-insoluble iodine. Hemagglutination test results for TG Ab were negative, but an immunofluorescence assay showed a strongly positive reaction against a cytoplasmic antigen. Bioassay of the serum for thyroid-stimulating activity gave a TSH-type response.* These patients were studied in cooperation with Dr. William H. Milburn, to whom we are greatly indebted.
When goiter is induced by iodine administration, lymphocytic thyroiditis is frequently found and thyroid autoantibodies are often present(53).
Remission of Hashimoto's thyroiditis, with loss of goiter, hypothyroidism, and serum thyroid autoantibodies, has been reported during pregnancy, with relapse after delivery(54). Antibody levels usually fall during pregnancy(55). These phenomena may reflect the immunosuppressive effects of pregnancy. After delivery thyroid autoantibody levels rise, and after 2-6 months there may be sudden development (? return) of goiter and hypothyroidism (56). Concerning management of thyroid dysfunction during pregnancy and postpartum, an Endocrine Society Clinical Practice Guideline was developed (56a, Chapter 14). Management of thyroid diseases during pregnancy requires special considerations because pregnancy induces major changes in thyroid function, and maternal thyroid disease can have adverse effects on the pregnancy and the fetus. Care requires coordination among several healthcare professionals. Avoiding maternal (and fetal) hypothyroidism is of major importance because of potential damage to fetal neural development, an increased incidence of miscarriage, and preterm delivery. Maternal hyperthyroidism and its treatment may be accompanied by coincident problems in fetal thyroid function. Autoimmune thyroid disease is associated with both increased rates of miscarriage, for which the appropriate medical response is uncertain at this time, and postpartum thyroiditis. Fine-needle aspiration cytology should be performed for dominant thyroid nodules discovered in pregnancy. Radioactive isotopes must be avoided during pregnancy and lactation. Universal screening of pregnant women for thyroid disease is not yet supported by adequate studies, but case finding targeted to specific groups of patients who are at increased risk is strongly supported. One report recommended screening all pregnant women for autoimmune thyroid disease in the first trimester in terms of cost-effectiveness (56b).
Of course maternal antibodies cross the placenta, and as in Graves' disease, may affect the fetus and neonate. TPO and TG Ab typically appear to have no adverse effect. Some evidence suggests cytotoxic antibodies, which are thought to be different from TPO Ab or TG Ab, could cause fetal hypothyroidism(57). However, TSBAb can rarely produce neonatal hypothyroidism, which is self-limiting over 4-6 weeks as the maternal IgG is metabolized. Women with positive TPO antibody before assisted reproduction have a significantly increased risk for miscarriage, with an odds ratio of 3.77 (Poppe, K; Glinoer, D; Tournaye, H; Devroey, P; van Steirteghem, A; Kaufman, L; Velkeniers, B. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab 88 4149-4152 2003).
Y.L.C., 24-Year-Old Woman, Postpartum, Not-So-Transient Hypothyroidism
The patient had menarche at age 16 and had regular periods. She married at age 24 and was not able to conceive. After receiving danazol therapy for 7 months for treatment of extensive endometriosis, she became pregnant and delivered after 36 weeks' gestation. During the course of this pregnancy, her thyroid gland was noted to be normal; no thyroid function tests were done. After delivery, she nursed the infant for 1 week. She then stopped nursing, but galactorrhea and amenorrhea continued for the next 5 months. After the fourth month, she was noted to have an enlarged thyroid gland; the FT4I was found to be 3.4 (normal, 6.0 - 10.5) and TSH level 27 uU/ml. There were symptoms of mild hypothyroidism, with some lowering of the voice and increase in fatigue. A sister had an overactive thyroid and mild exophthalmos.
Her thyroid was estimated to weigh about 40 g, with a smooth surface and an enlarged lobe. Skin was dry, and there was some delay in the reflex relaxation. TGAb were present at a titer of 1/160 and TPOAb at 1/20480. Serum T3 level was 123 ng/dl, and the RAIU was 16% at 4 hours and 32% at 24 hours. The thyroid scan was within normal limits. Prolactin (PRL) level was elevated at 43 ng/ml. Sella turcica X-ray films and a CT scan of the head were normal.
It was hypothesized that the patient had postpartum hypothyroidism due to transient exacerbation of thyroiditis and that this condition might resolve spontaneously. Whether the hyperprolactinemia, amenorrhea, and galactorrhea were secondary to the hypothyroidism or were independent problems was at first unclear. The patient was treated expectantly, since she appeared to be in no distress and there was no evidence of pituitary tumor. One month after the initial observations, the TSH level had fallen to 13.5 uU/ml and the T3 level remained at 126 ng/dl. Eight weeks later, the FT4I had risen to 5.8, the T3 level was 113 ng/dl, TSH 9.1 uU/ml, and the PRL remained at 66 ng/ml. Later, all test results became normal.
Painless (silent) and Postpartum Thyroiditis
In the last decade several syndromes involving clinically significant, but self-limited, exacerbations of AITD have been delineated(54)-(59). Silent (painless) thyroiditis is a syndrome that has a clinical course of thyroid dysfunction similar to subacute thyroiditis but with no anterior neck pain and no tenderness of the thyroid. Initially, patients have a thyrotoxic phase, later passing through euthyroidism to hypothyroidism and, finally, return to euthyroidism. Postpartum thyroiditis occurs within 6 months after delivery and runs an identical clinical course(57). Postpartum thyroiditis is now considered to be identical to silent thyroiditis, and this term is used for patients who developed silent thyroiditis in the postpartum period(57). After delivery, other forms of autoimmune thyroid dysfunction also occur, including Graves' disease, transient hypothyroidism without preceding destructive thyrotoxicosis, and persistent hypothyroidism (Fig. 8-6). In recent years, the term painless thyroiditis also has been used frequently, and the same disorder has been described using different names, such as thyrotoxicosis with painless thyroiditis(60), occult subacute thyroiditis(61), hyperthyroiditis(64), lymphocytic thyroiditis with spontaneously resolving hyperthyroidism(62), painless thyroiditis and transient hyperthyroidism without goiter(63), and transient hyperthyroidism with lymphocytic thyroiditis(65). The thyrotoxicosis is induced by leakage of intrathyroidal hormones into the circulation caused by damage to thyroid epithelial cells from inflammation. Thus the thyroid radioactive iodine uptake (RAIU) is low(59). Therefore, the early phase of thyrotoxicosis in silent thyroiditis, postpartum thyroiditis, and subacute thyroiditis can be grouped together as destruction-induced thyrotoxicosis or simply as destructive thyrotoxicosis(66). When the measurement of radioactive iodine uptake is difficult, the measurement of anti-TSH receptor antibody and/or thyroid blood flow by ultrasonography may be useful to differentiate between destruction-induced thyrotoxicosis and Graves' thyrotoxicosis. The quantitative measurement by power Doppler ultrasonography was more effective than that of anti-TSH receptor antibody for differential diagnosis of these two types of thyrotoxicosis and may omit the radioactive iodine uptake test (66-1).

Figure 6.
Much evidence, including histopathological and immunological studies, indicates that this disorder is an autoimmune thyroid disease(68). It is believed to be due to autoimmune induced damage to the thyroid causing excess hormone release, and for this reason is not responsive to antithyroid drugs, KI or KCLO4, but does, if treatment is necessary, respond to prednisone(67). During the clinical course of subclinical or very mild autoimmune thyroiditis, aggravating factors cause exacerbation of the destructive process. All women with subclinical autoimmune thyroiditis(40) and antithyroid microsomal antibodies of more than 1:5120 before pregnancy develop postpartum thyroiditis(57). A significant percentage of patients with silent thyroiditis have personal or family histories of autoimmune thyroid disease. Most patients have a complete remission, but some develop persistent hypothyroidism(70). Some patients have had alternating episodes of typical "high-uptake" thyrotoxicosis and episodes of "transient" low-uptake thyrotoxicosis(69). Recurrence of disease is common in silent thyroiditis but very rare in subacute thyroiditis. Considering all these data, it is assumed that silent thyroiditis is caused by an exacerbation of autoimmune thyroiditis induced by aggravating factors. Thyroiditis frequently recurs, and seasonal allergic rhinitis is reported to be an initiation factor(71). Physically vigorous massage on the neck also was reported to be a contributing factor for silent thyroiditis(72). The prevalence of silent thyroiditis, including postpartum disease, is around 5 per cent of all types of thyrotoxicosis. Spontaneous silent thyroiditis is three times more frequent than postpartum thyroiditis.
An immune rebound mechanism has been established for the induction of postpartum thyroiditis(57). Postpartum thyroid destruction is associated with an increase in NK cell counts and activity(57). Cessation of steroid therapy has initiated silent thyroiditis in a patient with autoimmune thyroiditis and rheumatoid arthritis(73), presumably because this also allows immune rebound. In patients with Cushing's syndrome who have associated subclinical autoimmune thyroiditis, silent thyroiditis has occurred after unilateral adrenalectomy(74). Typically, painless thyroiditis or destructive thyrotoxicosis occurs at 2 to 4 months postpartum. The prevalence of postpartum thyroiditis ranges from 3 to 8 per cent of all pregnancies(57).POSSIBLE PREVENTION OF PPT-In a randomized prospective controlled study, 77 TPO+ pregnant women received 200 ug selenomethionine daily starting at the 12th week of pregnancy, and 74 TPO+ women received a placebo. The treated group had significantly lower TPO antibody levels at the end of pregnancy and during the post-partum while on treatment. The incidence of PPT was reduced from 48.6 to 28.6% in the treated group, and the incidence of permanent hypothyroidism was equivalently reduced. Thyroid hormone levels did not differ.( Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007 Apr;92(4):1263-8) .
Hashimoto's encephalopathy
Hashimoto's encephalopathy or encephalitis is a very rare complication of Hashimoto's thyroiditis. Neurological complications are sometimes associated with thyroid dysfunction but patients with this encephalopathy are usually euthyroid. It is treatable, steroid-responsive, progressive or relapsing encephalopathy associated with elevation of thyroid specific autoantibodies (75). This condition was first described in 1966 (76) and may present as a subacute or acute encephalopathy with seizures and stroke-like episodes, often in association with myoclonus and tremor (77). It is associated with abnormal EEG and high CSF proteins without pleocytosis. Some patients suffer from a significant residual disability(78). Antibody to α-enolase has been identified in some patients (79) but this antibody is also frequently found in other autoimmune diseases. Sawka et al. reported that this condition is not caused by thyroid dysfunction or antithyroid antibodies but represents an association of an uncommon autoimmune encephalopathy with a common autoimmune thyroid disease (80). Identification of antibodies to brain specific antigens may disclose the real pathogenesis of this condition. Recently, autoantibodies against the amino (NH2)-terminal of α-enolase (referred to as NAE) were reported to be highly specific in sera from a limited number of HE patients (68-83% with HE; 11%, 2 of 17 with HT without any neuropsychiatric features; none of controls [50 individuals] including those with other neurological or immunological conditions involving encephalopathy [25 individuals]) (80.1, 80.2). Steroid reversible cerebral hypometabolism was recently documented by PET scanning in this condition. (80.3) There is a report that Hashimoto’s encephalopathy associated with elevated intrathecal and serum IgG4 levels. (80.3a) Additional case studies, including histological investigations as well as measurements of IgG4, are needed to elucidate the pathological role of IgG4 in Hashimoto’s encephalopathy.
Hashimoto's ophthalopathy
Thyroid-associated orbitopathy (TAO) usually occurs in Graves’s disease with hyperthyroidism, and sometimes in euthyroid and hypothyroid patients. Since most euthyroid and hypothyroid patients with orbitopathy are thyrotropin receptor antibody (TRAb)-positive, they are diagnosed as having euthyroid Graves’ disease or hypothyroid Graves’ disease. When euthyroid and hypothyroid patients with orbitopathy are TRAb-negative but associated with Hashimoto’s thyroiditis, “Hashimoto's ophthalopathy” may be considered (80.4, 80.5). Because patients with Hashimoto’s thyroiditis test negative for TRAb, other autoantibodies against an eye muscle antigen, such as calsequestrin, flavoprotein, or G2s are postulated (80.6).
IODIDE METABOLISM AND EFFECTS
Many patients with Hashimoto's thyroiditis do not respond to injected TSH with the expected increase in RAIU or release of hormone from the gland(81). These findings probably mean that the gland is partially destroyed by the autoimmune attack and is unable to augment iodine metabolism further. Further, the thyroid gland of the patient with Hashimoto's disease does not organify normally(82) (Fig. 8-4). Administration of 400 mg potassium perchlorate 1 hour after giving a tracer iodide releases 20 - 60% of the glandular radioactivity. Also, a fraction of the iodinated compounds in the serum of patients with Hashimoto's thyroiditis is not soluble in butanol, as are the thyroid hormones, but is an abnormal peptide-linked iodinated component. This low-weight iodoprotein is probably serum albumin that has been iodinated in the thyroid gland. A similar iodoprotein is also found in several other kinds of thyroid disease, including carcinoma, Graves' disease, and one form of goitrous cretinism. It may be formed as part of the hyperplastic response. TG is also detectable in their serum.
Iodide is actively transported from blood to thyrocytes and recently the sodium / iodide symporter (NIS) has been cloned. Antibodies against NIS were found in autoimmune thyroid disease(83). This antibody has an inhibitory activity on iodide transport and may modulate the thyroid function in Hashimoto's thyroiditis. More recent studies reported rather low prevalence (less than 10%) of anti-NIS antibodies in Hashimoto's disease and clinical relevance is still unknown(84),(85).
In animal experiment iodine depletion prevents the development of autoimmune thyroiditis(86). It is suggested that mild iodine deficiency partly protect against autoimmune thyroid disease(87), although it is controversial(88). In a region where iodine-containing food (such as seaweed) is common, as in Japan, excessive dietary iodine intake (1000 micro g/day or more) may cause transient hypothyoidism in patients with subclinical autoimmune thyroiditis. This condition is easily reversible with a reduction in iodine intake(89). Iodine is important not only for thyroid hormone synthesis but also for induction and modulation of thyroid autoimmunity. In general, iodine deficiency attenuates, which iodine excess accelerates autoimmune thyroiditis in autoimmune prone individuals(90). In animal experiment, it is revealed that enhanced iodination of thyroglobulin facilitates the selective processing and presentation of a cryptic phatogenic peptide in vivo or in vitro. Moreover, it is suggested that iodine excess stimulates thymus development and effects function of various immune cells(91).
DIAGNOSIS
Diagnosis involves two considerations -- the differential diagnosis of the thyroid lesion and the assessment determination of the metabolic status of the patient.
A diffuse, firm goiter with pyramidal lobe enlargement, and without signs of thyrotoxicosis, should suggest the diagnosis of Hashimoto's thyroiditis. Most often the gland is bosselated or "nubbey." It is usually symmetrical, although much variation in symmetry (as well as consistency) can occur. The trachea is rarely deviated or compressed. The association of goiter with hypothyroidism is almost diagnostic of this condition, but is also seen in certain syndromes due to defective hormone synthesis or hormone response, as described in Chapter 9. Pain and tenderness are unusual but may be present. A rapid onset is also unusual, but the goiter may rarely grow from normal to several times the normal size in a few weeks. Most commonly the gland is two to four times the normal size. Satellite lymph nodes may be present, especially the Delphian node above the isthmus. Multinodular goiter occurs in significant incidence in adult women; thus the co-occurrence of multinodular goiter and Hashimoto's thyroiditis is not rare, and may provide the finding of a grossly nodular gland in a patient who is mildly hypothyroid and has positive antibody tests.
The T4 concentration and the FT4 range from low to high but are most typically in the normal or low range(92). The RAIU (rarely required) is variable and ranges from below normal to elevated values, depending on such factors as TSH levels, the efficiency of use of iodide by the thyroid, and the nature of the components being released into the circulation. Gammaglobulin levels may be elevated, although usually they are normal(93). This alteration evidently reflects the presence of high concentrations of circulating antibodies to TG, for an antibody concentration as high as 5.2 mg/ml has been reported.
T4 and FTI are normal or low(92). Serum TSH reflects the patient's metabolic status. However, some patients are clinically euthyroid, with normal FTI and T3 levels, but have mildly elevated TSH. Whether this "subclinical hypothyroidism" represents partial or complete compensation is a matter of debate. TPOAb, and less frequently TGAb are present in serum. High levels are diagnostic of autoimmune thyroid disease. TGAb are positive in about 80% of patients, and if both TGAb and TPOAb are measured, 97% are positive. Young patients tend to have lower and occasionally negative levels. In this age group, even low titers signify the presence of thyroid autoimmunity.
FNA can be a useful diagnostic procedure but is infrequently required, except in patients that seem to have- or have- a discreet nodule in the gland. FNA typically reveals lymphocytes, macrophages, scant colloid, and a few epithelial cells which may show Hurthle cell change. In this context Hurthle cells do not represent a discrete adenoma. However if only abundant Hurthle cells dominate the specimen, and there are few or no lymphocytes or macrophages, the biopsy must be interpreted as a possible Hurthle cell tumor. Biopsy results are less frequently diagnostic in children(95).
Thyroid isotope scan is not usually necessary, but can be helpful. The image is characteristically that of a diffuse or mottled uptake in an enlarged gland, in striking contrast to the focal "cold" and "hot" areas of multinodular goiter. Focal loss of isotope accumulation may occur in severely diseased portions of the thyroid.
Table 2. Guideline for the diagnosis of Hashimoto's thyroiditis (Chronic thyroiditis)
| * Some clinicians don't use the term Hashimoto's thyroiditis if patients have no goiter, although association of positive antibodies and lymphocytic infiltration in the thyroid gland was proved by histological examination. |
|
1. Clinical findings Diffuse swelling of the thyroid gland without any other cause (such as Graves' disease) 2. Laboratory findings a. Positive for anti-thyroid microsomal antibody or anti-thyroid peroxidase(TPO) antibody b. Positive for anti-thyroglobulin antibody c. Lymphocytic infiltration in the thyroid gland confirmed with cytological examination |
|
1. A patient shall be said to have Hashimoto's thyroiditis if he/she has satisfied clinical criterion and any one laboratory criterion.Notes a. A patients shall be suspected to have Hashimoto's thyroiditis, if he/she has primary hypothyroidism without any other cause to induce hypothyroidism. b. A patient shall be suspected to have Hashimoto's thyroiditis, if he/she has anti-thyroid microsomal antibody and/or anti-thyroglobulin antibody without thyroid dysfunction nor goiter formation.* c. If a patient with thyroid neoplasm has anti-thyroid antibody by chance, he or she should be considered to have Hashimoto's thyroiditis. d. A patient is possible to have Hashimoto's thyroiditis if hypoechoic and/or inhomogeneous pattern is observed in thyroid ultrasonography. |
Ultrasound may display an enlarged gland with normal texture, a characteristic picture with very low echogenicity, or a suggestion of multiple ill-defined nodules. Diagnostic guidelines made by The Japan Thyroid Association are shown in Table 8-2. The flow chart of diagnosis is shown in Figure 8-7.The incidental finding of diffusely increased (18)F-FDG uptake in the thyroid gland is mostly associated with chronic lymphocytic (Hashimoto's) thyroiditis and does not seem to be affected by thyroid hormone therapy (95.1).
DIFFERENTIAL DIAGNOSIS
Hashimoto's thyroiditis is to be distinguished from nontoxic nodular goiter or Graves' disease. The presence of gross nodularity is strong evidence against Hashimoto's thyroiditis, but differentiation on this basis is not infallible. In multinodular goiter, thyroid function test results are usually normal, and the patient is only rarely clinically hypothyroid. Thyroid autoantibodies tend to be absent or titers are low, and the scan result is typical. FNA can resolve the question but is usually unnecessary. In fact, the two conditions quite commonly occur together in adult women. Whether this is by chance, or due to the effect of thyroid growth stimulating antibodies (or other causes) is unknown.
Moderately and diffusely enlarged thyroid glands in teenagers are usually the result of thyroiditis, but some may be true adolescent goiters; that is, the enlargement may result from moderate hyperplasia of the thyroid gland in response to a temporarily increased demand for hormone. This condition is more often diagnosed than proved. Thyroid function test results should be normal. Antibody assays may resolve the issue. The diagnosis can be settled with certainty only by a biopsy disclosing normal or hyperplastic thyroid tissue and absence of findings of thyroiditis. The possibility of colloid goiter may be entertained in the differential diagnosis. Colloid goiter is a definite pathologic entity, as described in Chapter 17. Presumably it is the resting phase after a period of thyroid hyperplasia.
Tumor must also be considered in the differential diagnosis, especially if there is rapid growth of the gland or persistent pain. The diffuse nature of autoimmune thyroiditis, the characteristic hypothyroidism and involvement of the pyramidal lobe are usually sufficient for differentiation. FNA is indicated if there is uncertainty. However, it must be remembered that lymphoma or a small-cell carcinoma of the thyroid can be and has been mistaken for Hashimoto's thyroiditis. Clusters of nodes at the upper poles strongly suggesting papillary cancer may disappear when thyroid hormone replacement therapy is given. However, we have seen a sufficient number of patients with both thyroiditis and tumor to know that one diagnosis in no way excludes the other. Thyroid lymphoma must always be considered if there is continued (especially asymmetric) enlargement of a Hashimoto's gland, or if pain, tenderness, hoarseness, or nodes develop. Thyroiditis is a risk factor for thyroid lymphoma, although the incidence is very low. Thyroid lymphoma develops in most cases in glands which harbor thyroiditis. Distinguishing thyroid lymphoma from Hashimoto's thyroiditis is sometimes quite difficult Reverse transcription-polymerase chain reaction (RT-PCR) detecting the monoclonality of immunoglobulin heavy chain mRNA is useful for differentiation between the two(99). This condition and its management are discussed in Chapter 18.
Occasionally the picture of Hashimoto's thyroiditis blends rather imperceptibly into that of thyrotoxicosis, and some patients have symptoms of mild thyrotoxicosis, but then develop typical Hashimoto's thyroiditis. In fact, it is best to think of Graves' disease and Hashimoto's thyroiditis as two very closely related syndromes produced by thyroid autoimmunity. Categorization depends on associated eye findings and the metabolic level, but the pathogenesis, histologic picture, and function may overlap.
Likewise, we have seen patients who appear to have a mixture of Hashimoto's thyroiditis and subacute thyroiditis, with goiter, positive thyroid autoantibodies, normal or low FT4, and biopsies which have suggested Hashimoto's on one occasion and included giant cells on another. A form of painful chronic thyroiditis with amyloid infiltration has also been described, and is probably etiologically distinct from Hashimoto's thyroiditis(100).
THERAPY
Many patients need no treatment, for frequently the disease is asymptomatic and the goiter is small. This approach is justified by the study of Vickery and Hamlin(101), who found, on both clinical and pathologic grounds, that the disease may remain static and the clinical condition unchanged over many years.
If the goiter is a problem because of local pressure symptoms, or is unsightly, thyroid hormone therapy is indicated. Thyroid hormone often causes a gratifying reduction in the size of the goiter after several months of treatment(100). We have been especially impressed with this result in young people. It seems likely that in older patients there may be more fibrosis and therefore less tendency for the thyroid to shrink. In young patients the response often occurs within 2 - 4 weeks, but in older ones the thyroid decreases in size more gradually. Aksoy et al (100a) report that "prophylactic" thyroid hormone treatment is associated after 15 months with a decrease in thyroid size and in thyroid antibody levels. Thyroid hormone in a full replacement dose is, of course, indicated if hypothyroidism is present. Therapy is probably indicated if the TSH level is elevated and the FT4 is low normal, since the onset of hypothyroidism is predictable in such patients. There is no evidence that thyroid replacement actually halts the ongoing process of thyroiditis, but in some patients receiving treatment, antibody levels gradually fall over many years(102).

Figure 7. Diagnosis of Hashimoto’s thyroiditis (chronic thyroiditis)
The dosage of thyroxine should normally be that required to bring the serum TSH level to the low normal range, such as .3 - 1 uU/ml. This is typically achieved with 1 ug L-T4/lb body weight/day, ranges from 75 - 125 ug/day in women, and 125 - 200 ug/day in men. It is sensible to initiate therapy with a partial dose, since in some instances the thyroid gland may be nonsuppressible even though functioning at a level below normal. Once thyroxine treatment is initiated, it is required indefinitely in most patients. However, it has been found that up to 20% of initially hypothyroid individuals will later recover and have normal thyroid function if challenged by replacement hormone withdrawal. This may represent subsidence of cytotoxic antibodies, modulation of TSBAb, or some other mechanism(22). These individuals can be identified by administration of TRH, which will induce an increase in serum T4 and T3 if the thyroid has recovered(103). Replacement T4 therapy should be taken several hours before or after medications such as cholesterol binding resins, carafate, and FSO4, which can reduce absorption(104). (See Chapter 9) Autoimmune disease is usually takes an ongoing process and Hashimoto's thyroiditis develops into hypothyroidism. Recent trial of proplylactic treatment with T4 (1.0 ~ 2.0µg/Kg/day) for one year in euthyroid patients with Hashimoto's thyroiditis showed decrease of anti-TPO antibodies and thyroid B-lymphocytes(105), suggesting prophylactic T4 therapy might be useful to stop progression of disease. The long-term clinical benefit should be established in the future.Whether or not subclinical hypothyroidism should be treated is still under debate (see Chapter 9.10 SUBCLINICAL HYPOTHYROIDISM). Cardiac dysfunction may be associated with subclinical hypothyroidism, even when serum TSH is still in the normal range. These abnormalities are reversible with l-T4 replacement therapy (22-1).
In some instances the acute onset of the disease, in association with pain, has prompted therapy with glucocorticoids. This treatment alleviates the symptoms and improves the associated biochemical abnormalities, and in some studies has been shown to increase plasma T3 and T4 levels by suppression of the autoimmune process(106). Blizzard and co-workers(107) have given steroids over several months to children in an attempt to suppress antibody production and possibly to achieve a permanent remission. The adrenocortical hormones dramatically depress clinical activity of the disease and antibody titers, but all return to pre-therapy levels when treatment is withdrawn. We cannot recommend steroid therapy for this condition because of the undesirable side effects of the drug. Chloroquine has been reported in one study to reduce antibody titers(108). Because of toxicity, its use is not advised. X-ray therapy also results in a decrease in goiter size, and frequently in myxedema, but should not be used because of the possible induction of thyroid carcinoma.
SELENIUM- In a randomized prospective controlled study, 77 TPO+ pregnant women received 200 ug selenomethionine daily starting at the 12th week of pregnancy, and 74 TPO+ women received a placebo. The treated group had significantly lower TPO antibody levels at the end of pregnancy and during the post-partum while on treatment. The incidence of PPT was reduced from 48.6 to 28.6% in the treated group, and the incidence of permanent hypothyroidism was equivalently reduced. Thyroid hormone levels did not differ. This one report is certainly most interesting, but needs confirmation before this treatment can be suggested for general application (108.1). Confirming earlier studies, in Hashimoto’s patients, 200 mug Se in the form of l-selenomethionine orally for 6 months caused a significant decrease of 21% in serum anti-TPO levels. Cessation caused an increase in the anti-TPO concentrations.(108.2). A slightly opposing study, however, was reported no immunological benefit of selenium in patients with moderate disease activity (in terms of TPOAb and cytokine production patterns) may not (equally) benefit as patients with high disease activity (108.3). Selenium responsiveness may be different among patients with Hashimoto’s thyroiditis. A systematic review and meta-Analysis revealed that selenium supplementation reduced serum TPOAb levels after 3, 6, and 12 months in an LT4-treated Hashimoto’s population, and after three months in an untreated population (108.4). However, no effect of selenium supplementation on thyroid stimulating hormone, health-related quality of life or thyroid ultrasound was found in levothyroxine substitution-untreated individuals, and sporadic evaluation of clinically relevant outcomes in levothyroxine substitution-treated patients (108.5). Future well-powered RCTs, evaluating e.g. disease progression or health-related quality of life, are warranted before determining the relevance of selenium supplementation in autoimmune thyroiditis. Further, combined treatment with Myo-inositol and selenium was reported that the beneficial effects obtained by selenomethionine treatment on patients affected by subclinical hypothyroidism were further improved by cotreatment with Myo-Inositol (108.6). Myo-Inositol s an isomer of a C6 sugar alcohol an plays an important role in several cellular processes. In particular, it has been demonstrated that Myo-Inositol is the precursor for the synthesis of phosphoinositides, which are part of the phosphatidylinositol (PtdIns) signal transduction pathway. In one study. the administration of myo- inositol plus selenium has been reported to be effective in decreasing TSH, TPOAb, and TgAb levels, as well as enhancing thyroid hormones and personal wellbeing, therefore restoring euthyroidism in patients diagnosed with Hashimoto’s thyroiditis (108.7).
Anatabine- Anatabine, an alkaloid found in Solanaceae plants including tobacco, has been reported to ameliorate a mouse model of Hashimoto's thyroiditis. (108.8). In a double-blind, randomized, placebo-controlled multi-site study for three months, anatabine treated patients had a significant reduction in absolute serum TgAb levels from baseline by study end relative to those on placebo (p=0.027) (108.9). Further studies are warranted to dissect longer-term effects and possible actions of anatabine on the course of Hashimoto's thyroiditis.
Surgery has been used as a method of therapy. This treatment, of course, removes the goiter but usually results in hypothyroidism. We believe that it is not indicated unless significant pain, cosmetic, or pressure symptoms remain after a fair trial of thyroid therapy, and probably steroid therapy, but is appropriate in some cases. Among patients with postpartum thyroid dysfunction, the most common type is destructive thyrotoxicosis and simple symptomatic treatment, using beta-adrenergic--antagonists, is usually sufficient(109). In the case of postpartum hypothyroidism, replacement with a submaximal dose of T3 is useful to relieve symptoms more quickly and to predict spontaneous recovery which is detected by an increase of T4.
Some patients do not fit easily into the usual diagnostic categories; accordingly, choosing an appropriate course of therapy is more difficult. Frequently, it is impossible to differentiate Hashimoto's thyroiditis from multinodular goiter short of performing an open biopsy. In these cases, if there is no suggestion of carcinoma, it is logical to treat the patient with hormone replacement and to observe closely. A reduction in the goiter justifies continuation of the therapy, even in the absence of a diagnosis.
In some patients, especially teenagers, the examination discloses peri-thyroidal lymph nodes or an apparent discrete nodule, in addition to the diffusely enlarged thyroid of Hashimoto's thyroiditis. Such nodules should be evaluated by FNA, ultrasound and possibly scintiscan. Thyroid hormone treatment may cause regression of the nodes or nodule. If after full evaluation uncertainty persists, if nodes remain present, or if a nodule grows, surgical exploration is indicated.
Treatment of children and adolescents with 1.3ug/kg/day thyroxine for 24 months was shown in a recent study to cause significant reduction in thyroid size in patients with Autoimmune thyroiditis, but not affect antibody levels, or significantly alter TSH or freeT4. (110)
Occasionally, symptoms of serositis or arthritis suggest the coincident occurrence of another autoimmune disorder. We have given thyroid hormone to decrease thyroid activity and possibly reduce a tendency to antibody formation, and have treated the generalized disorder independently as indicated.
SUMMARY
Hashimoto's thyroiditis is characterized clinically as a commonly occurring, painless, diffuse enlargement of the thyroid gland occurring predominantly in middle-aged women. The patients are often euthyroid, but hypothyroidism may develop. The thyroid parenchyma is diffusely replaced by a lymphocytic infiltrate and fibrotic reaction; frequently, lymphoid germinal follicles are visible. Attention has been focused on this process because of the demonstration of autoimmune phenomena in most patients. Persons with Hashimoto's thyroiditis have serum antibodies reacting with TG, TPO, and against an unidentified protein present in colloid. In addition, many patients have cell mediated immunity directed against thyroid antigens, demonstrable by several techniques. Cell mediated immunity is also a feature of experimental thyroiditis induced in animals by injection of thyroid antigen with adjuvants.
All theories also emphasize a basic abnormality in the immune surveillance system, which in some way allows autoimmunity to develop against thyroid antigens, and as well against other tissues, including stomach, adrenal, and ovaries, in many patients with thyroiditis.
We suggest that Hashimoto's thyroiditis, primary myxedema, and Graves' disease are different expressions of a basically similar autoimmune process, and that the clinical appearance reflects the spectrum of the immune response in the particular patient. This response may include cytotoxic antibodies, stimulatory antibodies, blocking antibodies, or cell mediated immunity. Thyrotoxicosis is viewed as an expression of the effect of circulating thyroid stimulatory antibodies. Hashimoto's thyroiditis is predominantly the clinical expression of cell mediated immunity leading to destruction of thyroid cells, which in its severest form produces thyroid failure and idiopathic myxedema.
The clinical disease is more frequent than Graves' Disease when mild cases are included. The incidence is on the order of three to six cases per 10,000 population per year, and prevalence among women is at least 2%.
The gland involved by thyroiditis tends to lose its ability to store iodine, produces and secretes iodoproteins that circulate in plasma, and is inefficient in making hormone. Thus, the thyroid gland is under increased TSH stimulation, fails to respond to exogenous TSH, and has a rapid turnover of thyroidal iodine.
Diagnosis is made by the finding of a diffuse, smooth, firm goiter in a young woman, with strongly positive titers of TG Ab and/or TPO Ab and a euthyroid or hypothyroid metabolic status. A patient with a small goiter and euthyroidism does not require therapy unless the TSH level is elevated. The presence of a large gland, progressive growth of the goiter, or hypothyroidism indicates the need for replacement thyroid hormone. Surgery is rarely indicated. Development of lymphoma, though very unusual, must be considered if there is growth or pain in the involved gland.
References
- Hashimoto H. Zur Kenntniss der lymphomatosen Veranderung der Schilddruse (struma lymphomatosa), Arch Klin Chir 97:219, 1912.
- McConahey WM, Keating FR Jr, Beahrs OH, Woolner LB. On the increasing occurrence of Hashimoto's thyroiditis. J Clin Endocrinol Metab 22:542, 1962.
- Fromm GA, Lascano EF, Bur GE, Escalenta D. Tiroiditis cronica inespecifica. Rev Assoc Med Arg 67:162, 1953.
- Luxton RW, Cooke RT. Hashimoto's struma lymphomatosa: Diagnostic value and significance of serum-flocculation reactions. Lancet 2:105, 1956.
- Rose NR, Witebsky E. Studies on organ specificity. V. Changes in thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol 76:417, 1956.
- Roitt IM, Doniach D, Campbell PN, Hudson RV. Auto- antibodies in Hashimoto's thyroiditis (lymphadenoid goiter). Lancet 2:820, 1956.
- Totterman TH, Maenpaa J, Gordin A, Makinen T, Andersson AC. Blood and thyroid-infiltrating lymphocyte subclasses in juvenile autoimmune thyroiditis. Clin exp Immunol 30:193, 1977.
- Paolieri F, Pronzato C, Battifora M, Fiorino N, Canonica GW, Bagnasco M. Infiltrating gamma/delta T-cell receptor-positive lymphocytes in Hashimoto's thyroiditis, Graves' disease and papillary thyroid cancer. J Endocrinol Invest. 18 : 295-8, 1995.
- Iwatani Y, Hidaka Y, Matsuzuka F, Kuma K, Amino N. Intrathyroidal lymphocyte subsets, including unusual CD4+ CD8+ cells and CD3loTCR alpha beta lo/-CD4-CD8- cells, in autoimmune thyroid disease. Clin Exp Immunol. 93: 430-6, 1993.
- Davies TF, Martin A, Concepcion ES, Graves P, Cohen L, Ben Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 325: 238-44, 1991.
- Davies TF, Concepcion ES, Ben Nun A, Graves PN, Tarjan,G. T-cell receptor V gene use in autoimmune thyroid disease: direct assessment by thyroid aspiration. J Clin Endocrinol Metab. 76: 660-6, 1993.
- Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. Different cytokine mRNA profiles in Graves' disease, Hashimoto's thyroiditis, and nonautoimmune thyroid disorders determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Thyroid. 6: 97-106, 1996.
- Gunn A, Michie W, Irvine WJ. The thymus in thyroid disease. Lancet 2:776, 1964.
- Michie W, Beck JS, Mahaffy RG, Honein EF, Fowler GB. Quantitative radiological and histological studies of the thymus in thyroid disease. Lancet 1:691, 1967.
- Mizukami Y, Michigishi T, Hashimoto T, Tonami N, Hisada K, Matsubara F, Takazakura E. Silent thyroiditis: a histologic and immunohistochemical study. Hum Pathol 19:423-431, 1988.
15a. Zeitlin AA, Heward JM, Newby PR, Carr-Smith JD, Franklyn JA, Gough SC, Simmonds MJ. Analysis of HLA class II genes in Hashimoto's thyroiditis reveals differences compared to Graves' disease. Genes Immun. 2008 Jun;9(4):358-63
- Brix TH, Kyvik KO, Hegedus L: A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocr Metab 85:536-539, 2000
16a. Simmonds MJ. GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol. 2013 May;9(5):277-87. doi: 10.1038/nrendo.2013.56. Epub 2013 Mar 26.
16b. Cai T, Li J, An X, Yan N, Li D, Jiang Y, Wang W, Shi L, Qin Q, Song R, Wang G, Jiang W, Zhang JA. Polymorphisms in MIR499A and MIR125A gene are associated with autoimmune thyroid diseases. Mol Cell Endocrinol. 2017 Jan 15;440:106-115
16.1 Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab. 2008 Jul 8. [Epub ahead of print]
- Mori T, Kriss JP. Measurements by competitive binding radioassay of serum antimicrosomal and anti-thyroglobulin antibodies in Graves' disease and other thyroid disorders. J Clin Endocrinol Metab 33:688, 1971.
- Kawakami Y, Fisfalen M-E, DeGroot LJ. Proliferative responses of peripheral blood mononuclear cells from patients with autoimmune thyroid disease to synthetic peptide epitopes of human thyroid peroxidase. Autoimmunity, 13:17-26, 1992.
18.1 Ng HP, Kung AW Induction of autoimmune thyroiditis and hypothyroidism by immunization of immunoactive T cell epitope of thyroid peroxidase. Endocrinology. 2006 Jun;147(6):3085-92
18.2 Muixí L, Carrascal M, Alvarez I, Daura X, Martí M, Armengol MP, Pinilla C, Abian J, Pujol-Borrell R, Jaraquemada D. Thyroglobulin peptides associate in vivo to HLA-DR in autoimmune thyroid glands. J Immunol. 2008 Jul 1;181(1):795-807
- Hidaka Y, Amino N, Iwatani Y, Kaneda T, Nasu M, Mitsuda N, Tanizawa O, Miyai K Increase in peripheral natural killer cell activity in patients with autoimmune thyroid disease. Autoimmunity 11:239, 1992.
19.1 Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S 1999 Thymus and Autoimmunity: Production of CD25+CD4+ naturally anergic and suppressive T Cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 162: 5317-5326
19.2. McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B. The link between Graves' disease and Hashimoto's thyroiditis: a role for regulatory T cells. Endocrinology. 2007 Dec;148(12):5724-33
- Kosugi S, Ban T, Akamizu T, Kohn LD. Identification of separate determinants on the thyrotropin receptor reactive with Graves' thyroid-stimulating antibodies and with thyroid-stimulating blocking antibodies in idiopathic myxedema: These determinants have no homologous sequence on gonadotropin receptors. Molecul Endocrinol 6:168-180, 1992.
- Tamaki H, Amino N, Kimura M, Hidaka Y, Takeoka K, Miyai K. Low prevalence of thyrotropin receptor antibody in primary hypothyroidism in Japan. J Clin Endocrinol Metab 71:1382, 1990.
- Okamura K, Sato K, Yoshinari M, Ikenoue H, Kuroda T, Nakagawa M, Tsuji H, Washio M, Fujishima M. Recovery of the thyroid function in patients with atrophic hypothyroidism and blocking type TSH binding inhibitor immunoglobulin. Acta Endocrinol (Copenh) 122:107-114, 1990.
22-1. Mariotti S, Zoncu S, Pigliaru F, Putzu C, Cambuli VM, Vargiu S, Deidda M, Mercuro G. Cardiac effects of l-thyroxine administration in borderline hypothyroidism. Int J Cardiol. 2007 May 9; [Epub ahead of print]
- Takasu N, Yamada T, Takasu M, Komiya I, Nagasawa Y, Asawa T, Shinoda T, Aizawa T, Koizumi Y. Disappearance of thyrotropin-blocking antibodies and spontaneous recovery from hypothyroidism in autoimmune thyroiditis. N Engl J Med 326:513-518, 1992.
- Mori T, Akamizu T, Kosugi S, Sugawa H, Inoue D, Okuda J, Ueda Y. Recent progress in TSH receptor studies with a new concept of "Autoimmune TSH receptor disease". Endocr J 41:1-11, 1994.
- Kotani T, Aratake Y, Hirai K, Fukazawa Y, Sato H, Ohtaki S. Apoptosis in thyroid tissue from patients with Hashimoto's thyroiditis. Autoimmunity 20:231, 1995.
- Giordano C, Stassi G, Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroidiitis. Science 275:960, 1997.
- Mitsiiades N, Poulaki V, Kotoula V, Mastorakos G, Balafouta S, Koutras DA, Tsokos M.Fas/Fas ligand up-regulation and BCL-2 down-regulation may be significant in thepathogenesis of Hashimoto' thyroiditis. J Clin Endo Metab 83:2199,1998.
- Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Kawabe Y, Ishikawa N, Ito K, Nagataki S.Thyroid-stimulating hormone inhiibits Fas antigen-mediated apoptosis ofhuman thyrocytes in vitro. Endocrinology 137:3163, 1996.
- Batteux F, Lores P, Bucchini D, Chiocchia G. Transgenic expression of Fas ligand on thyroid follicular cells prevents autoimmune thyroiditis. J Immunol 164:1681-1688, 2000.
- Stassi G, DeMaria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nat Rev Immunol 2:195-204, 2002.
- Dong Z, Takakuwa T, Takayama H, Luo WJ, Takano T, Amino N, Matsuzuka F, Aozasa K. Fas and Fas ligand gene mutations in Hashimoto's thyroiditis. Lab Invest 82: 1611-1616, 2002.
- Takakuwa T, Dong Z, Takayama H, Matsuzuka F, Nagata S, Aozasa K. Frequent mutations of Fas gene
- Atkins MB, Mier JW, Parkinson DR, Gould JA, Berkman EM, Kaplan MM. Hypothyroidism after treatment with interleukin- 2 and lymphokine-activated killer cells. N Engl J Med 318:1557-1563, 1988.
33a Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, Weetman A, Hale G, Chatterjee VK, Waldmann H, Compston A (1999) Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 354:1691–1695
33b Hirst CL, Pace A, Pickersgill TP, Jones R, McLean BN, Zajicek JP, Scolding NJ, Robertson NP. Campath 1-H treatment in patients with aggressive relapsing remitting multiple sclerosis. J Neurol. 2008 Feb;255(2):231-8
33c Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2010 Feb;95(2):953-62
33d Horie I, Abiru N, Nagayama Y, Kuriya G, Saitoh O, Ichikawa T, Iwakura Y, Eguchi K. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology. 2009 Nov;150(11):5135-42
33d.1. 1. Li C, Yuan J, Zhu YF, Yang XJ, Wang Q, Xu J, He ST, Zhang JA. Imbalance of Th17/Treg in Different Subtypes of Autoimmune Thyroid Diseases. Cell Physiol Biochem. 2016;40(1-2):245-252.
33d.1.2. 2. Bossowski A, Moniuszko M, Idźkowska E, Grubczak K, Singh P, Bossowska A, Diana T, Kahaly GJ. Decreased proportions of CD4 + IL17+/CD4 + CD25 + CD127- and CD4 + IL17+/CD4 + CD25 + CD127 - FoxP3+ T cells in children with autoimmune thyroid diseases (.). Autoimmunity. 2016 Aug;49(5):320-8.
33e. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001 Mar 8;344(10):732-8.
33f. Komatsu K, Hamano H, Ochi Y, Takayama M, Muraki T, Yoshizawa K, Sakurai A, Ota M, Kawa S. High prevalence of hypothyroidism in patients with autoimmune pancreatitis. Dig Dis Sci. 2005 Jun;50(6):1052-7
33g. Li Y, Bai Y, Liu Z, Ozaki T, Taniguchi E, Mori I, Nagayama K, Nakamura H, Kakudo K. Immunohistochemistry of IgG4 can help subclassify Hashimoto's autoimmune thyroiditis. Pathol Int. 2009 Sep;59(9):636-41.
33h. Li Y, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of hashimoto's thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010 Mar;95(3):1309-17
33i. Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken) 2010 62: 1312-1318.
33j. Kakudo K, Li Y, Taniguchi E, Mori I, Ozaki T, Nishihara E, Matsuzuka F, Miyauchi A. IgG4-related disease of the thyroid glands [Review]. Endocr J. 2011 Dec 2. [Epub ahead of print]
- Nystrom E, Bengtsson C, Lapidus L, Petersen K, Lindstedt G. Smoking--A risk factor for hypothyroidism. J Endocrinol Invest 16:129-131, 1993.
34a. Pacini F, Vorontsova T, Molinaro E, Kuchinskaya E, Agate L, Shavrova E, Astachova L, Chiovato L, Pinchera A. Prevalence of thyroid autoantibodies in children and adolescents from Belarus exposed to the Chernobyl radioactive fallout. Lancet. 1998 Sep 5;352(9130):763-6.
34b. Agate L, Mariotti S, Elisei R, Mossa P, Pacini F, Molinaro E, Grasso L, Masserini L, Mokhort T, Vorontsova T, Arynchyn A, Tronko MD, Tsyb A, Feldt-Rasmussen U, Juul A, Pinchera A. Thyroid Autoantibodies and Thyroid Function in Subjects Exposed to Chernobyl Fallout during Childhood: Evidence for a Transient Radiation-Induced Elevation of Serum Thyroid Antibodies without an Increase in Thyroid Autoimmune Disease. J Clin Endocrinol Metab. 2008 Jul;93(7):2729-36
34c Elfström P, Montgomery SM, Kämpe O, Ekbom A, Ludvigsson JF. Risk of Thyroid disease in individuals with Celiac disease. J Clin Endocrinol Metab. 2008 Jul 8. [Epub ahead of print]
34d Betterle C, Lazzarotto F, Presotto F. Autoimmune polyglandular syndrome Type 2: the tip of an iceberg? Clin Exp Immunol. 2004 Aug;137(2):225-33
34e Molitch ME, Gillam MP. Lymphocytic hypophysitis. Horm Res. 2007;68 Suppl 5:145-50
34f. Christian Bernecker, Luisa Lenz, Martin S. Ostapczuk, Sven Schinner, Holger Willenberg, Margret Ehlers, Stefan Vordenbäumen, Joachim Feldkamp, Matthias Schott. MicroRNAs miR-146a1, miR-155_2, and miR-200a1 Are Regulated in Autoimmune Thyroid Diseases. Thyroid. December 2012, 22(12): 1294-1295.
34g. Otsu H, Watanabe M, Inoue N, Masutani R, Iwatani Y. Intraindividual variation of microRNA expression levels in plasma and peripheral blood mononuclear cells and the associations of these levels with the pathogenesis of autoimmune thyroid diseases. Clin Chem Lab Med. 2017 May 1;55(5):626-635.
34h. Xiao C, Rajewsky K 2009 MicroRNA control in the immune system: basic principles. Cell 136: 26–36.
34i. Thai T-H, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K 2007 Regulation of the germinal center response by microRNA-155. Science 316: 604–606
34j. Zhu J, Zhang Y, Zhang W, Zhang W, Fan L, Wang L, Liu Y, Liu S, Guo Y, Wang Y, Yi J, Yan Q, Wang Z, Huang G. MicroRNA-142-5p contributes to Hashimoto's thyroiditis by targeting CLDN1. J Transl Med. 2016 Jun 8;14(1):166.
- Gordin A, Maatela J, Miettinen A, Helenius T, Lamberg B-A. Serum thyrotrophin and circulating thyroglobulin and thyroid microsomal antibodies in a Finnish population. Acta Endocrinol 90:33, 1979.
- Ling SM, Kaplan SA, Weitzman JJ, Reed GB, Costin G, Landing BH. Euthyroid goiters in children. Correlation of needle biopsy with other clinical and laboratory findings in chronic lymphocytic thyroiditis and simple goiter. Pediatrics 44:695, 1969.
- Tunbridge WMG, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community. The Whickham Survey. Clin Endocrinol 7:481, 1977.
- Inoue M, Taketani N, Sato T, Nakajima H. High incidence of chronic lymphocytic thyroiditis in apparently healthy school children: Epidemiological and clinical study. Endocrinol Jpn 22:483, 1975.
- Carey C, Skosey C, Pinnamaneni KM, Barsano CP, DeGroot LJ. Thyroid abnormalities in children of parents who have Graves' disease. Possible pre-Graves' disease. Metabolism 29:369, 1980.
- Yoshida H, Amino N, Yagawa K, Uemura K, Satoh M, Miyai K, Kumahara Y. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland : studies of seventy autopsied cases. J Clin Endocrinol Metab 46:859, 1978.
- Gordin A, Saarinen P, Pelkonen A, Lamberg B-A. Serum thyroglobulin and the response to thyrotropin releasing hormone in symptomless autoimmune thyroiditis and in borderline and overt hypothyroidism. Acta Endocrinol 75:274, 1974.
- Tunbridge WMG, Brewis M, French JM, Appleton D, Bird T, Clark F, Evered DC, Evans JG, Hall R, Smith P, Stephenson J, Young E. Natural history of autoimmune thyroiditis. Br Med J 282:258, 1981.
42a. Simmonds MJ, Kavvoura FK, Brand OJ, Newby PR, Jackson LE, Hargreaves CE, Franklyn JA, Gough SC. Skewed X chromosome inactivation and female preponderance in autoimmune thyroid disease: an association study and meta-analysis. J Clin Endocrinol Metab. 2014 Jan;99(1):E127-31
42b. Klintschar, M, , Schwaiger, P, , Mannweiler, S, , Regauer, S, , Kleiber, M, 2001 Evidence of fetal microchimerism in Hashimoto's thyroiditis. J Clin Endocrinol Metab 86:2494–2498
42c. Klintschar, M, , Immel, UD, , Kehlen, A, , Schwaiger, P, , Mustafa, T, , Mannweiler, S, , Regauer, S, , Kleiber, M, , Hoang-Vu, C, 2006 Fetal microchimerism in Hashimoto's thyroiditis: a quantitative approach. Eur J Endocrinol 154:237–241
42d. Renné, C, , Ramos Lopez, E, , Steimle-Grauer, SA, , Ziolkowski, P, , Pani, MA, , Luther, C, , Holzer, K, , Encke, A, , Wahl, RA, , Bechstein, WO, , Usadel, KH, , Hansmann, ML, , Badenhoop, K, 2004 Thyroid fetal male microchimerism in mothers with thyroid disorders: presence of Y-chromosomal immunofluorescence in thyroid infiltrating lymphocytes is more prevalent in Hashimoto's thyroiditis and Graves' disease than in follicular adenomas. J Clin Endocrinol Metab 89:5810–5814
- Buchanan WW, Harden RM. Primary hypothyroidism and Hashimoto's thyroiditis. Arch Intern Med 115:411, 1965.
- Moriuchi A, Yokoyama S, Kashima K, Andoh T, Nakayama I, Noguchi S. Localized primary anyloid tumor of the thyroid developing in the course of Hashimoto's thyroiditis. Acta Pathologica Japonica 42:210-216, 1992.
- Shaw PJ, Walls TJ, Newman PK, Cleland PG, Cartlidge NE. Hashimoto's encephalopathy: A steroid-responsive disorder associated with high antithyroid antibody titers -- report of five cases. Neurology 41:228-233, 1991.
- Khardori R, Eagleton LE, Soler NG, McConnachie PR. Lymphocytic interstitial pneumonitis in autoimmune thyroid disease. Amer J Med 90:649-652, 1991.
46-1. Radetti G, Gottardi E, Bona G, Corrias A, Salardi S, Loche S; Study Group for Thyroid Diseases of the Italian Society for Pediatric Endocrinology and Diabetes (SIEDP/ISPED).The natural history of euthyroid Hashimoto's thyroiditis in children. J Pediatr. 149:827-32, 2006.
- Eisenbart GS, Wilson PW, Ward F, Buckley C, Lebovitz H. The polyglandular failure syndrome. Disease inheritance, HLA type, and immune function. Ann Intern Med 91:528, 1979.
- Loviselli A, Mathieu A, Pala R, Mariotti S, Cau S, Marongiu C, Mazzoleni AP, Maggio P, Martino E. Development of thyroid disease in patients with primary and secondary Sjogren's syndrome. J Endocrinol Invest 11:653, 1988.
- Best TB, Munro RE, Burwell S, Volpe R. Riedel's thyroiditis associated with Hashimoto's thyroiditis, hypoparathyroidism, and retroperitoneal fibrosis. J Endocrinol Invest 14:767- 772, 1991.
- Becker KL, Ferguson RH, McConahey WM. The connective-tissue diseases and symptoms associated with Hashimoto's thyroiditis. N Engl J Med 268:277, 1963.
- Bastenie PA, Vanhaelst L, Golstein J, Smets P, Keys MJ, Karvonen MJ, Punsar S. Asymptomatic autoimmune thyroiditis and coronary heart-disease. Lancet 1:155, 1977.
- Heinonen OP, Aho K, Pyorala K, Gordin A, Punsar S, Puro K. Symptomless autoimmune thyroiditis in coronary heart disease. Lancet 1:785, 1972.
52a. Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013 Feb;98(2):474-82.
52b. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013 Feb 15;168(3):343-9.
52c. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. 2013 Jun;98(6):2409-14
52d. Marotta V, Guerra A, Zatelli MC, Uberti ED, Di Stasi V, Faggiano A, Colao A, Vitale M. BRAF mutation positive papillary thyroid carcinoma is less advanced when Hashimoto's thyroiditis lymphocytic infiltration is present. Clin Endocrinol (Oxf). 2013 Nov;79(5):733-8
52-1. Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007 May; 204 (5):764-73
- Hall R, Turner-Warwick M, Doniach D. Autoantibodies in iodide goiter and asthma. Clin Exp Immunol 1:285, 1966.
- Amino N, Miyai K, Onishi T, Hashimoto T, Arai K, Ishibashi K, Kumahara Y. Transient hypothyroidism after delivery in autoimmune thyroiditis. J Clin Endocrinol Metab 42:296, 1976.
- Amino N, Kuro R, Tanizawa O, Tanaka F, Hayashi C, Kotani K, Kawashima M, Miyai K, Kumahara Y. Changes of serum antithyroid antibodies during and after pregnancy in autoimmune thyroid diseases. Clin Exp Immunol 31:30, 1978.
- Amino N, Miyai K, Kuro R, Tanizawa O, Azukizawa M, Takai S, Tanaka F, Nishi K, Kawashima M, Kumahara Y. Transient postpartum hypothyroidism. Fourteen cases with autoimmune thyroiditis. Ann Intern Med 87:155, 1977.
56a. Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007 92; Aug (8 Suppl):S1-47.
56b. Dosiou C, Sanders GD, Araki SS, Crapo LM. Screening pregnant women for autoimmune thyroid disease: a cost-effectiveness analysis. Eur J Endocrinol. 2008 Jun;158(6):841-51.
- Amino N, Tada H, Hidaka Y. Postpartum autoimmune thyroid syndrome: a model of aggravation of autoimmune disease. Thyroid 9: 705-713, 1999.
- Roti E, Emerson CH. Clinical Review 29. Postpartum thyroiditis. J Clin Endocrinol Metab 74:3-5, 1992.
- Gluck FB, Nusynowitz ML, Plymate S. Chronic lymphocytic thyroiditis, thyrotoxicosis, and low radioactive iodine uptake: Reports of four cases. N Engl J Med 292:624, 1975.
- Woolf PD, Daly R: Thyrotoxicosis with painless thyroiditis. Am J Med 60: 73-79, 1976.
- Hamburger JI: Occult subacute thyroiditis: Diagnostic challenge. Mich Med 70: 1125- , 1976.
- Nikolai TF, Brosseau J, Kettrick MA, Roberts R, Beltaos E. Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (silent thyroiditis). Arch Intern Med 140:478, 1980.
- Dorfman SG, Copperman MT, Nelson RL, Depuy H, Peake RL, Young RL. Painless thyroiditis and transient hyperthyroidism without goiter. Ann Intern Med 86:24, 1977.
- Woolf PD. Transient painless thyroiditis with hyperthyroidism. A variant of lymphocytic thyroiditis. Endocrine Reviews 1:411, 1980.
- Gorman CA, Duick DS, Woolner LB, Wahner HW: Transient hyperthyroidism in patients with lymphocytic thyroiditis. Mayo Clin Proc 53: 359-365, 1978.
- Amino N, Yabu Y, Miyai K, Fujie T, Azukizawa M, Onishi T, Kumahara Y: Differentiation of thyrotoxicosis induced by thyroid destruction from Graves' disease. Lancet 2: 344-346, 1978.
66-1. Ota H, Amino N, Morita S, Kobayashi K, Kubota S, Fukata S, Kamiyama N, Miyauchi A.Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves' disease. Clin Endocrinol (Oxf). 67:41-5, 2007
- Nikolai TF, Coombs GJ, McKenzie AK, Miller RW, Weir Jr, GJ. Treatment of lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (silent thyroiditis). Arch Intern Med 142:2281-2283, 1982.
- Inada M, Nishikawa M, Naito K, Ishii H, Tanaka K, Imura H. Reversible changes of the histological abnormalities of the thyroid in patients with painless thyroiditis. J Clin Endocrinol Metab 52:431, 1981.
- Taylor HC, Sheeler LR. Recurrence and heterogeneity in painless thyrotoxic lymphocytic thyroiditis. Report of five cases. J Amer Med Assn 248:1085-1088, 1982.
- Amino N, Tada H, Hidaka Y. Autoimmune thyroid disease and pregnancy. J Endocrinol Invest 19:59,1996.
- Yamamoto M, Shibuya N, Chen LC, Ogata E: Seasonal recurrence of transient hypothyroidism in a patient with autoimmune thyroiditis. Endocr J 35: 135-142, 1988.
- Tachi J, Amino N, Miyai K: Massage therapy on neck: a contributing factor for destructive thyrotoxicosis? .Thyroidology 2: 25-27, 1990.
- Maruyama H, Kato M, Mizuno O, Kataoka K, Matusi S: Transient thyrotoxicosis occurred after cessation of steroid therapy in a patient with autoimmune thyroiditis and rheumatoid arthritis. Endocr J 29: 583-588, 1982.
- Takasu N, Komiya I, Nagasawa Y, et al.: Exacerbation of autoimmune thyroid dysfunction after unilateral adrenalectomy in patients with Cushing's syndrome due to an adrenocortical adenoma. N Engl J Med 322: 1708-1712, 1990.
- Peschen-rosin R, Schabet m, Dichgans J. Manifestation of Hashimoto's encephalopathy years before onset of thyroid disease. Eur Neurol 41: 79-84, 1999.
- Brain L, Jellinek EH, Ball K. Hashimoto's disease and encephalopathy. Lancet 2:512-514, 1966.
- Pozo-Rosich P, Villoslada P, Canton A, Simo R, Rovira A, Montalban X. Reversible white matter alterations in encephalopathy associated with autoimmune thyroid disease. J Neurol 249:1063-1065,2002.
- Canton A, de Fabregas O, Tintore M, Mesa J, Codina A, Simo R. Encephalopathy associated to autoimmune thyroid disease: a more appropriate term for an underestimated condition? J Neurol Sci 176:65-69,2000.
- Ochi H, Horiuchi I, Araki n, Toda T, Araki T, Sato K, Murai H, Osoegawa M, Yamada T, Okamura K, Ogino T, Mizumoto K, Yamashita H, Saya H, Kira J. Proteomic analysis of human brain identifies ソ-enolase as a nobel autoantigen in Hashimoto's encephalopathy. FEBS Lett 528:197-202, 2002.
- Sawka AM, Fatourechi V, Boeve BF, Mokri B. Rarity of encephalopathy associated with autoimmune thyroiditis: A case series from Mayo Clinic from 1950 to 1996. Thyroid 12:393-398, 2002.
80.1 Fujii A, Yoneda M, Ito T, Yamamura O, Satomi S, Higa H, Kimura A, Suzuki M, Yamashita M, Yuasa T, Suzuki H, Kuriyama M. Autoantibodies against the amino terminal of a-enolase are a useful diagnostic marker of Hashimoto's encephalopathy. J Neuroimmunol. 162:130-6, 2005.
80.2 Yoneda M, Fujii A, Ito A, Yokoyama H, Nakagawa H, Kuriyama M. High prevalence of serum autoantibodies against the amino terminal of a-enolase in Hashimoto's encephalopathy. J Neuroimmunol. 185:195-200, 2007
80.3 Seo SW, Lee BI, Lee JD, Park SA, Kim KS, Kim SH, Yun MJ. Thyrotoxic autoimmune encephalopathy: a repeat positron emission tomography study.J Neurol Neurosurg Psychiatry. 2003 Apr;74(4):504-6
80.3a. Hosoi Y, Kono S, Terada T, Konishi T, Miyajima H. J Neurol. Hashimoto's encephalopathy associated with an elevated intrathecal IgG4 level. 2013 Apr;260(4):1174-6. doi: 10.1007/s00415-013-6878-2. Epub 2013 Mar 8.
80.4 Tateno F, Sakakibara R, Kishi M, Ogawa E. Hashimoto's ophthalmopathy. Am J Med Sci. 2011 Jul;342(1):83-5
80.5 Yoshihara A, Yoshimura Noh J, Nakachi A, Ohye H, Sato S, Sekiya K, Kosuga Y, Suzuki M, Matsumoto M, Kunii Y, Watanabe N, Mukasa K, Inoue Y, Ito K, Ito K. Severe thyroid-associated orbitopathy in Hashimoto's thyroiditis. Report of 2 cases. Endocr J. 2011;58(5):343-8.
- Skillern PG, Crile G Jr, McCullaugh EP, Hazard JB, Lewis LA, Brown H. Struma lymphomatosa: Primary thyroid failure with compensatory thyroid enlargement. J Clin Endocrinol Metab 16:35, 1956.
- Paris J, McConahey WM, Tausie WN, Woolner LB, Bahn RC. The effect of iodides on Hashimoto's thyroiditis. J Clin Endocrinol Metab 21:1037, 1961.
- Endo T, Kaneshige M, Nakazato M, Kohgai T, Saito T, Onaya T. Autoantibody against thyroid iodide transporter in the sera from patients with Hashimoto's thyroiditis possesses iodide transport inhiibitory activity. Bioch Bioph Res Com 228:199, 1996
- Chin HS, Chin DK, Morgenthaler NG, Vassart G, Costagliola S. Rarity of anti- Na+/I-symporter (NIS) antibody with iodide uptake inhibiting activity in autoimmune thyroid diseases (AITD). J Clin Endocrinol Metab 85: 3937-3940, 2000.
- Seissler J, Wagner S, Schott M, Feldkamp J, Scherbaum WA, Morgenthaler NG. Low frequency of autoantibodies to the human Na+/I -symporter (NIS) in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 85: 4630-4634, 2000.
- Brown TR, Zhao G, Palmer KC, Sundick RS: Thyroid injury, autoantigen availability, and the initiation of autoimmune thyroiditis. Autoimmunity 27:1-12, 1998.
- Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR: Iodine intake and the pattern of thyroid disorders: A comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocr Metab 83:765-769, 1998.
- Nagata K, Takasu N, Akamine H, Ohshiro C, Komiya I, Murakami K, Suzawa A, Nomura T: Urinary iodine and thyroid antibodies in Okinawa, Yamgata, Hyogo, and Nagano, Japan: The differences in iodine intake do not affect thyroid antibody positivity. Endoc J 45:797-803, 1998.
- Tajiri J, Higashi K, Morita M, Umeda T, Sato T: Studies of hypothyroidism in patients with high iodine intake. J Clin Endocr Metab 63:412-417, 1986.
- Ruwhof C, Drexhage HA. Iodine and thyroid autoimmune disease in animal models. Thyroid 11:427-436, 2001.
- Dai YD, Rao VP, Carayanniois G. Enhanced iodination of thyroglobulin facilitates processing and presentation of a cryptic pathogenic peptide. J Immunol 168:5907-5911, 2002.
- McConahey WM, Keating FR, Butt HR, Owen CA. Comparison of certain laboratory tests in the diagnosis of Hashimoto's thyroiditis. J Clin Endocrinol Metab 21:879, 1961.
- Glynne A, Thomson JA. Serum immunoglobulin levels in thyroid disease. Clin Exp Immunol 12:71, 1972.
- Wilkin TJ, Beck JS, Hayes PC, Potts RC, Young RJ. A passive hemagglutination (TRC) inhibitor in thyrotoxic serum. Clin Endocrinol 10:507, 1979.
- Monteleone JA, Davis RK, Tung KSK, Ramos CV, Peden VH. Differentiation of chronic lymphocytic thyroiditis and simple goiter in pediatrics. J Pediatr 83:381, 1973.
95-1. Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 48:896-901, 2007
- Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H: The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid 10:251-259, 2000.
- Hoffer PB, Gottschalk A, Refetoff S. Thyroid scanning techniques. The old and the new. Curr Probl in Radiol 2:5, 1972.
- Jonckheer MH, VanHaelst L, DeConinck F, Michotte Y. Atrophic autoimmune thyroiditis. Relationship between the clinical state and intrathyroidal iodine as measured in vivo in man. J Clin Endocrinol Metab 53:476, 1981.
- Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, Amino N: Diagnosis of thyroid malignant lymphoma by reverse transcription-polymerase chain reaction detecting the monoclonality of immunoglobulin heavy chain messenger ribonucleic acid. J Clin Endocr Metab 85:671-675, 2000.
- McConahey WM, Woolner LB, Black BM, Keating FR, Jr. Effect of desiccated thyroid in lymphocytic (Hashimoto's) thyroiditis. J Clin Endocrinol Metab 19:45, 1959.
100a Aksoy DY, Kerimoglu U, Okur H, Canpinar H, Karaagaoglu E, Yetgin S, Kansu E,Gedik O. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto's thyroiditis. Endocr J. 2005 Jun;52(3):337-43.
- Vickery AL, Hamlin E Jr: Struma lymphomatosa (Hasimoto's thyroiditis): Observations on repeated biopsies in 16 patients. N Engl J Med 264:226, 1961.
- Papapetrou PD, MacSween RNM, Lazarus JH, Harden R McG. Long-term treatment of Hashimoto's thyroiditis with thyroxine. Lancet 2:7786, 1972.
- Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. Test for recovery from hypothyroidism during thyroxine therapy in Hashimoto's thyroiditis. Lancet 336:1084-1086, 1990.
- Campbell NRC et al. Effect of ferrous sulfate on thyroid hormone replacement in hypothyroidism. Ann Int Med 117:1010-1013, 1992.
- Padberg S, Heller K, Usadel KH, Schumm-Draeger PM. One-year prophylactic treatment of euthyroid hashimoto's thyroiditis patients with levothyroxine: Is there a benefit? Thyroid 11: 249-255, 2001.
- Yamada T, Ikejiri K, Kotani M, Kusakabe T. An increase of plasma triiodothyronine and thyroxine after administration of dexamethasone to hypothyroid patients with Hashimoto's thyroiditis. J Clin Endocrinol Metab 46:784, 1981.
- Blizzard RM, Hung M, Chandler RW, Aceto T Jr, Kyle M, Winship T. Hashimoto's thyroiditis. Clinical and laboratory response to prolonged cortisone therapy. N Engl J Med 267:1015, 1962.
- Ito S, Tamura T, Nishikawa M. Effects of desiccated thyroid, prednisolone and chloroquine on goiter and antibody titer in chronic thyroiditis. Metabolism 17:317, 1968.
108.1. Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H.The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 92:1263-8, 2007.
108.2: Mazokopakis EE, Papadakis JA, Papadomanolaki MG, Batistakis AG, Giannakopoulos TG, Protopapadakis EE, Ganotakis ES. Effects of 12 Months Treatment with l-Selenomethionine on Serum Anti-TPO Levels in Patients with Hashimoto's Thyroiditis.Thyroid. 2007 Aug;17(7):609-12
108.3 Karanikas G, Schuetz M, Kontur S, Duan H, Kommata S, Schoen R, Antoni A, Kletter K, Dudczak R, Willheim M. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid. 2008 Jan;18(1):7-12
108.4 Wichman J, Winther KH, Bonnema SJ, Hegedüs L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid. 2016 Dec;26(12):1681-1692
108.5 Winther KH, Wichman JE, Bonnema SJ, Hegedüs L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017 Feb;55(2):376-385
108.6 Nordio M, Pajalich R. Combined treatment with Myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. J Thyroid Res. 2013;2013:424163. doi: 10.1155/2013/424163. Epub 2013 Oct
108.7 Nordio M, Basciani S. Treatment with Myo-Inositol and Selenium Ensures Euthyroidism in Patients with Autoimmune Thyroiditis. Int J Endocrinol. 2017;2017:2549491. doi: 10.1155/2017/2549491. Epub 2017 Feb 15.
108.8 Caturegli P, De Remigis A, Ferlito M, Landek-Salgado MA, Iwama S, Tzou SC, Ladenson PW. Anatabine ameliorates experimental autoimmune thyroiditis. Endocrinology. 2012 Sep;153(9):4580-7
108.9 Schmeltz LR, Blevins TC, Aronoff SL, Ozer K, Leffert JD, Goldberg MA, Horowitz BS, Bertenshaw RH, Troya P, Cohen AE, Lanier RK, Wright C 4th. Anatabine supplementation decreases thyroglobulin antibodies in patients with chronic lymphocytic autoimmune (Hashimoto's) thyroiditis: A randomized controlled clinical trial. J Clin Endocrinol Metab. 2014 Jan;99(1):E137-42
- Amino N, Tada H, Hidaka Y. The spectrum of postpartum thyroid dysfunction: diagnosis, management, and long-term prognosis. Endoc Prac 2:406, 1996.
- Karges B, Muche R, Knerr I, Ertelt W, Wiesel T, Hub R, Neu A, Klinghammer A, Aufschild J, Rapp A, Schirbel A, Boehm BO, Debatin KM, Heinze E, Karges W.Levothyroxine in euthyroid autoimmune thyroiditis and type 1 diabetes: a randomized, controlled trial.J Clin Endocrinol Metab. 2007 May;92(5):1647-52.
Disorders of the Thyroid Gland in Infancy, Childhood and Adolescence
This chapter is, in part, based on the previous version written by Prof. Rosalind Brown.
ABSTRACT
Thyroid disorders in infancy, childhood and adolescence represent common and usually treatable endocrine disorders. Thyroid hormones are essential for normal development and growth of many target tissues, including the brain and the skeleton. Thyroid hormone action on critical genes for neurodevelopment is limited to specific time window, and even a short period of deficiency of TH can cause irreversible brain damage. During the first trimester of pregnancy fetal brain development is totally dependent on maternal thyroid function. Congenital hypothyroidism is one of the most preventable causes of mental retardation, but early diagnosis is needed in order to prevent irreversible SNC damage. Today more than 70% of the babies worldwide are born in areas without an organized screening program. New insights about genetic causes, screening strategies and treatment of congenital hypothyroidism are reported. Hyperthyroidism in newborns is usually a transient consequence of transplacental passage of TSH receptor stimulating antibodies. Hypothyroidism can be detected in infants born to hyperthyroid mothers, due to transplacental passage of TSH receptor antibodies or hypothalamic-pituitary suppression. In childhood and adolescence autoimmune thyroid disease (AITD) as chronic lymphocytic thyroiditis and Graves’ disease account for the main cause of hypothyroidism and hyperthyroidism, respectively. Incidence of AITD increase from infancy to adolescence. Other autoimmune disorders are frequently associated. An increased risk of thyroid nodules and cancer is suggested. Differentiated thyroid cancer and medullary thyroid carcinoma in childhood and adolescence require specific expertise. Follow up programs are advised for high risk patients as long term survivors of childhood cancer. For complete coverage of this and related areas of Endocrinology, please visit our free online textbook, WWW.ENDOTEXT.ORG.
INTRODUCTION
In the last several decades, there have been exciting advances in our understanding of fetal and neonatal thyroid physiology, and screening for congenital hypothyroidism has enabled the virtual eradication of the devastating effects of mental retardation due to sporadic congenital hypothyroidism in most developed countries of the world. In addition, advances in molecular biology have led to new insights regarding the early events in thyroid gland embryogenesis and mechanisms of thyroid action in the brain. At the same time, the molecular basis for many of the inborn errors of thyroid hormonogenesis and thyroid hormone action is being unraveled. However, new questions and new challenges arise. In particular, the survival of increasingly small and premature fetuses has resulted in a growing number of neonates with abnormalities in thyroid function and a continuing controversy as to which of these infants require therapy. This chapter will focus on current concepts regarding the ontogenesis of thyroid function in the fetus and will review the major disorders of thyroid gland function in infants and children.
ONTOGENESIS OF THYROID FUNCTION IN THE FETUS AND INFANT
The ontogeny of mature thyroid function involves the organogenesis and maturation of the hypothalamus, pituitary, and thyroid glands as well as the maturation of thyroid hormone metabolism and thyroid hormone action. The placenta also plays a key role in the transfer of hormones and factors other than T4 that impact on thyroid function. In the first half of pregnancy, maternal T4 provides an important source of hormone for the developing fetus. Much of our knowledge derives from work in animal models, particularly sheep and rat. In interpreting these data, it is important to remember potential limitations in these models because of differences both in the structure of the placenta and timing of maturation. For example, the rat thyroid gland is much less mature at birth than its human counterpart and significant maturation of the thyroid gland and of the hypothalamic-pituitary-thyroid axis in this species occurs in the first 2 or 3 weeks after birth in the absence of placental or maternal influence, as compared with the third trimester in human infants.
Thyroid Gland Embryogenesis
Thyroid gland development is extensively reviewed in an earlier chapter and is shown diagrammatically in Figure 1. In brief, the thyroid gland is derived from the fusion of a medial outpouching from the floor of the primitive pharynx, the precursor of the thyroxine (T4)-producing follicular cells, and bilateral evaginations of the fourth pharyngeal pouch, which gives rise to the parafollicular, or calcitonin (C) secreting cells. Commitment towards a thyroid-specific phenotype as well as the growth and descent of the thyroid anlage into the neck results from the coordinate action of a number of transcription factors, including thyroid transcription factor 1 (TTF1, now called NKX2 (1), TTF2 (now called FOXE1) and PAX8 (1,2). Because these transcription factors are also expressed in a limited number of other cell types, it appears to be the specific combination of transcription factors and possibly non-DNA binding cofactors acting coordinately that determine the phenotype of the cell.
Other transcription factors and growth factors that play a role in early thyroid gland organogenesis include HHEX1, HOXA3 (3) and members of the fibroblast growth factor family, i.e., FGF10, but the initial inductive signal is unknown. A role of the neighboring heart primordium in the specification of the thyroid anlage has been postulated. Studies of cadherin expression suggest that the caudal translocation of the thyroid anlage may also arise indirectly, as a result of the growth and expansion of adjacent tissues, including the major blood vessels (4). In late organogenesis, the sonic hedgehog (SHH) gene and its downstream target TBX1 appear to play an important role in the symmetric bilobation of the thyroid (5); SHH also suppresses the ectopic expression of thyroid follicular cells (6).
During caudal migration the pharyngeal region of the thyroid anlage contracts to form a narrow stalk, known as the thyroglossal duct, which subsequently atrophies. Usually no lumen is left in the tract of its descent but, occasionally, an ectopic thyroid and/or persistent thyroglossal duct or cyst form if thyroid descent is abnormal.
In the human, embryogenesis is largely complete by 10 to 12 weeks gestation. At this stage, tiny follicle precursors can be seen, iodine binding can be identified and thyroglobulin (Tg) detected in follicular spaces (7,8) . Thyroid hormones are detectable in fetal serum by gestational age 11 to 12 weeks with both thyroxine (T4) and triiodothyronine (T3) being measurable. However, as discussed in further detail below, it is likely that a fraction of the hormones detectable at this early stage is contributed by the mother through transplacental transfer. Thyroid hormones continue to increase gradually over the entire period of gestation as does serum thyroxine-binding globulin (TBG) (9,10) . TBG is present at levels of 100 nmol/L (5 mg/L) at gestational age 12 weeks and progressively increases up to the time of birth, reaching concentrations of 500 nmol/L (25 mg/L). The serum TBG concentrations are higher in the infant then in adult humans as a consequence of placental estrogen effects on the fetal liver. In addition to the increase in total T4 there is also a progressive increase of the free T4 concentration indicating a maturation of the hypothalamic- pituitary- thyroid axis. The increased total T4 / thyrotropin (TSH) and free T4 /TSH ratios also indicate an increased ability of the thyroid gland to respond to TSH due to upregulation of the TSH receptor (11). Whereas the TBG and total T4 levels rise throughout gestation, the concentrations of free T4, and TSH rise until 31 to 34 weeks, declining thereafter to term (12).
Tg can be identified in the fetal thyroid as early as the 5th week, and is certainly present in follicular spaces by 10 to11 weeks, but maturation of Tg secretion takes much longer and it is not known when circulating Tg first appears in the fetal serum (not shown). By the time of gestational age 27 to 28 weeks, however, Tg levels average approximately 100 mg/L, much higher than in the adult and they remain approximately stable until the time of birth (13,14) . Iodide concentrating capacity can be detected in the thyroid of the 10 to 11 week fetus, but maturation of the Wolff-Chaikoff effect (reduction of iodide trapping in response to excess iodide) does not appear until 36 to 40 weeks gestation. Thus the premature fetus is more sensitive than the full term neonate to the thyroid-suppressive effects of iodine exposure.
The Hypothalamic-pituitary Axis
TSH is detectable at levels of 3 to 4 mU/L at gestational age 12 weeks and increases moderately over the last two trimesters to levels of 6 to 8 mU/L (8,9).The maturation of the negative feedback control of thyroid hormone synthesis is observed by approximately mid-gestation (Figure 1), with elevated serum TSH concentrations being observed in hypothyroid infants as early as 28 weeks (8). When TSH-Releasing Hormone (TRH) is given to mothers, a rise in TSH in the fetal circulation has been noted as early as 25 weeks gestation (15). It is of interest that the fetal TSH increment after TRH is greater than is the paired-maternal response, a consequence either of enhanced TSH release or impaired TSH degradation, perhaps due to immaturity of the hepatic glycoprotein metabolic clearance system. Similarly TSH is reduced in the cord serum of infants with neonatal thyrotoxicosis due to the transplacental passage of thyroid-stimulating antibodies from mothers with Graves’ disease as early as the end of the 2nd trimester.
Serum levels of TRH are higher in the fetal circulation than in maternal blood, the result both of extrahypothalamic TRH production (placenta and pancreas) and the decreased TRH degrading-activity in fetal serum. The physiological significance of these increased levels of TRH in the fetal circulation is not known.
Maturation Of Peripheral Thyroid Hormone Metabolism
As discussed in an earlier chapter, there are three iodothyronine deiodinases involved in the activation and inactivation of thyroid hormone. All three are coordinately regulated during gestation and function to closely regulate the supply of T3 to developing tissues while at the same time protecting the fetus against the effects of excess thyroid hormone. The physiological rationale for the maintenance of reduced circulating T3 concentrations throughout fetal life is still unknown, but it has been suggested that its function may be to avoid tissue thermogenesis and potentiate the anabolic state of the rapidly growing fetus while at the same time permitting highly regulated, tissue- specific maturation in an orderly, temporal sequence.
The seleno-enzyme type 1 iodothyronine deiodinase (D1), an important activating enzyme in adult life, is low throughout gestation. In addition to catalyzing T4 to T3 conversion, D1 catalyzes the inactivation of the sulfated conjugates of T4. As a consequence, circulating T3 concentrations in the fetus are quite low whereas the serum levels of the biologically inactive isomer reverse T3 and of T3 sulfate are increased (10). Unlike D1, both the Type 2 deiodinase (D2), an activating enzyme and D3, an inactivating enzyme are present in fetal brain as early as 7 weeks ’ gestation (16) . D2 converts T4 to T3 while D3 converts T4 to reverse T3. D2 and D3 are the major isoforms present in the fetus and are especially important in defining the level of T3 in the brain and pituitary. The highest concentration of D2 is in brain, pituitary, placenta and brown adipose tissue. D3 is present in many fetal tissues, most prominently the brain, uteroplacental unit, skin, and gastrointestinal tract (17). This is consistent with the key role of D3 in protecting fetal tissues against high maternal T4 concentrations present either in the placenta or in amniotic fluid.
In the presence of hypothyroidism, D2 activity increases while D3 decreases These coordinate activities have been found to be critically important in defending the rat fetus against the effects of fetal hypothyroidism as long as maternal T4 levels are maintained at normal concentrations (18, 19). Despite the low levels of circulating T3, brain T3 levels are 60-80% those of the adult by fetal age 20-26 weeks (20). Thus, whereas the physiological interrelationships between the various deiodinases in the fetus and placenta seem designed to maintain circulating T3 concentrations at a reduced level, specific mechanisms have evolved for maintaining brain T3 concentrations so that normal development can proceed.
Role of the Placenta
Contributions of the maternal thyroid to fetal thyroid economy.
In the human infant under normal circumstances, the placenta has only limited permeability to thyroid hormone and the fetal hypothalamic-pituitary-thyroid system develops relatively independent of maternal influence. Placental thyroid hormone transporters, thyroid hormone binding proteins, iodothyronine deiodinases, sulfotransferases and sulfatases regulate the transport of maternal thyroid hormones to the fetus (20a,20b). The transport of iodine through the placenta is also important as the organ has shown to actively concentrate the anion (20c).
The human placenta expresses iodothyronine Type 2 deiodinase I (D2) (which activates T4 to T3) and Type 3 (D3) (which inactivates T4 and T3). Maternal T4 is metabolized by D3 having 200 times the activity of D2 (20b). Both D2 and D3 activity decrease with advancing gestation (20b). Thus, the relative impermeability of the human placenta to thyroid hormone is due to the presence of D3 which serves to inactivate most of the thyroid hormone presented from the maternal or fetal circulation. The iodide released in this way can then be used for fetal thyroid hormone synthesis. Iodine is actively transported from the maternal circulation to the fetus through the placenta that express placental sodium iodide transporter (NIS) (20c,20d). NIS actively concentrates Iodine. NIS protein levels are significantly correlated with gestational age during early pregnancy and increase with increased placental vascularization (20e).
Interest in the potential role of maternal T4 in the fetal thyroid economy was reawakened with the recognition that in infants with the congenital absence of thyroid peroxidase, the cord serum concentration of T4 is nonetheless between 25 and 50% of normal (21). Since these infants are completely unable to synthesize T4, the measured hormone must be maternal in origin. Similar results are obtained in retrospective studies of cord serum in infants with sporadic congenital athyreosis. This maternal T4 disappears rapidly from the newborn circulation with a half-life of approximately 3 to 4 days.
There is also evidence that maternal-fetal T4 transfer occurs in the first half of pregnancy, when fetal thyroid hormone levels are low (19,22). Low concentrations of T4, presumably of maternal origin, have been detected in human embryonic coelomic fluid as early as 6 weeks gestation and in fetal brain as early 10 weeks gestation prior to the onset of fetal thyroid function indicating its maternal origin (22a-22f). Furthermore, both D2 and D3 activity as well as thyroid hormone receptor (TR) isoforms are present in low concentrations in human fetal brain from the mid first trimester, indicating that the machinery to convert T4 to T3 and to respond to T3 is present. The mechanisms of actions of thyroid hormones in the developing brain are mainly mediated through two ligand activated thyroid hormone receptor isoforms (22b,22c). There is also an important role for the thyroid hormone transporters in one or more of these processes (22g).
Between 6-12 weeks gestation, if maternal total T4 concentration is 100%, the total T4 concentration in the coelomic fluid would be 0.07% and T4 in the amniotic cavity as little as 0.0003-0.0013% of maternal total T4 concentrations. Fetal circulating concentrations of T3 are at least 10 fold lower than T4, whereas by fetal age of 20-26 weeks T3 levels in the fetal brain are 68-80% of the adult brain (20). Unlike adults, the proportion of free unbound T4 is also higher than bound T4 in early gestation. Free T4 levels are determined by the fetal concentrations of the thyroid hormone binding proteins in the circulation and the amount of maternal T4 crossing the placenta (7-9). It seems likely that when fetal thyroid function is normal, the net flux of T4 from mother to fetus is relatively limited. However, when the fetus is hypothyroxinemic, there is significant bulk transfer of T4 to the fetal circulation. This can occur both at the level of the placental maternal capillary interface and via uptake of thyroid hormone from the amniotic fluid through the immature epidermis. T4 uptake by the fetus can also occur via fetal ingestion of amniotic fluid. While the T4 concentrations in amniotic fluid appear modest, the fraction of T4 free in amniotic fluid is approximately ten-fold higher than that of serum and thus the free T4 concentration in amniotic fluid is approximately equal to that in fetal serum at 20 weeks gestation. It has been shown on numerous occasions in both animals and humans that amniotic fluid iodothyronine concentrations reflect those in the maternal circulation (23).
Placenta is permeable to TRH (15) and to immunoglobulins G (IgG) from midgestation. At the time of delivery, cord blood TPOAb correlate with maternal TPOAb concentrations (23a). Maternal passage of TPOAb and TgAb are not associated with thyroid fetal dysfunction. On the contrary, maternal TSH receptor antibodies (both stimulating and blocking) can be dangerous for the fetus and the newborn.
Fetal and neonatal hyperthyroidism can be caused by transplacental passage of TSH receptor antibodies (TRAb), whereas hypothyroidism can be due to transpancental passage of blocking TSH receptor antibodies, from mothers with severe Graves’ disease or severe hypothyroidism due to chronic lymphocytic thyroiditis.( The placenta is also permeable to certain drugs (15). Thus, the administration to the mother of excess iodide, drugs (especially propylthiouracil or methimazole), can affect thyroid function in the fetus and the newborn.
Role of Maternal Thyroid Hormone for fetal brain development and neurocognitive development in the offspring
The essential role that thyroid hormones (TH) play on the fetal brain development starts long before the onset of fetal thyroid function (22-22a). Thus, during the first trimester of pregnancy, fetal brain development is totally dependent on maternal thyroid function. Because the action of TH on critical genes for fundamental neurobiological processes is limited to specific time window, even a short period deficiency of TH may cause permanent brain damage. TH deficiency may affect neuronal cell differentiation and migration, axonal and dendritic outgrowth, myelin formation and synaptogenesis (22b-22f). It is well known that severe Iodine deficiency during pregnancy causes inadequate thyroid hormone production and irreversible brain damage known as cretinism, still endemic in many areas of the world (23b). None of the neurological features of severe endemic cretinism (24) due to iodine deficiency are found in infants with sporadic congenital hypothyroidism whose mothers have normal thyroid function and who receive early and adequate postnatal treatment. Similarly, impaired hearing, when found is much milder and less frequent (25). This would appear to provide unequivocal evidence that the neurological damage sustained by infants with endemic cretinism can be largely prevented by maternal T4. In addition to endemic cretinism, significant developmental delay despite early and adequate postnatal therapy has also been reported in other models of combined maternal-fetal hypothyroidism, such as materno-fetal POU1F1 deficiency (26) and TSH receptor blocking antibody-induced congenital hypothyroidism (27).
In iodine sufficient areas the main cause of maternal thyroid dysfunction (hypothyroidism, subclinical hypothyroidism or hypothyroxinemia) is thyroid autoimmunity, detectable in up to 17% of women (27a). Several studies reported on the consequences of maternal thyroid dysfunction in the progeny. Studies in children born to women with non-iodine deficient hypothyroidism during pregnancy (28,29,29a,29b) as well as in children from hypothyroxinemic mothers (30,30a-30e) have been published. Different parameters and different periods of pregnancy (i.e., increased TSH, low T4, presence or absence of autoimmunitity, prevalent obstetrical or developmental outcome) were analyzed, reporting conflicting results and conclusions.
Impairment in psychomotor development in the offspring of pregnant women with thyroid dysfunction was first reported by Man et al (28). They examined 131 hypothyroxinemic untreated pregnant women and found 36% of their 7-year-old children scored in the dull normal range or below compared to 16% of children of euthyroid mothers (28). Haddow detected a seven point IQ deficit in 7 to 9 year old children whose mothers were retrospectively found to have been hypothyroid at 17 weeks gestation (29). Accordingly, Pop demonstrated that even babies born to women whose free T4 levels were in the lowest 10% of normal at 12 weeks gestation had a measurable impairment in psychomotor development at 2 years of age as compared with the rest of the population, but this effect was not observed if maternal thyroid function was normal at 32 weeks (30). At variance with the aforementioned studies, Liu and more recently, Momotami failed to demonstrate any IQ deficit in babies born to hypothyroid mothers as long as the hypothyroidism was corrected by the end of the second trimester (31a, 31b). Similar results were obtained by Downing et al in 3 children born after severe feto-maternal hypothyroidism due to TSH receptor blocking antibodies (31c). Attention deficit disorder (30f,30g) autistic symptoms in offspring (30h) and schizophrenia in later life (30K) have also been associated with maternal hypothyroxinemia. Attention deficit disorder was previously noted in offspring from mothers with thyroid autoimmunity (30i). Children from mothers with anti-thyroid peroxidase antibodies have been found to have intellectual impairment in early infancy (30j) and a reduced childhood cognitive performance at age 4 and 7 and sensineural hearing loss at both ages (30l). An interesting association study, derived from the Rotterdam cohort, (the population based prospective study from Rotterdam (Generation R) for the first time analyzed the effects of maternal thyroid function on brain morphology of the offspring. In this study 3839 mother-child pairs were included. Maternal serum samples were taken before 18 weeks of gestation (9-18w). MRI were performed in 646 children (mean age 8 years) and IQ determined at mean age 6 years. They found that both high maternal and low FT4 showed an inverted U shaped association with child IQ (-1.4-3.8 points), child grey matter volume and cortex volume (32c). Recently, in the prospective double blind randomized controlled antenatal thyroid screening study (CATS), levothyroxine treatment was started from the 13th week of gestation if serum TSH was >97.5th percentile and/or FT4 was <2.5th percentile. The outcome was the IQ in the offspring at 3 years. No significant differences in IQ values were found between 390 children of treated mothers compared to 404 children of untreated mothers (32).
The incidence of maternal hypothyroidism during pregnancy (3 per 1000 in iodine-sufficient populations (33) is almost ten times that of congenital hypothyroidism for which routine population screening is widespread. Because maternal hypothyroidism has been associated not only with potential adverse effects on fetal brain development but an increased risk of preterm delivery and of miscarriage as well (33a ), some have argued that all pregnant women should be screened for hypothyroidism, a position that has been endorsed by some but not other professional societies.
Updated guidelines for the management of thyroid disease during pregnancy have been recently released from ATA (33b).
THYROID FUNCTION IN THE NEONATE, THE INFANT, AND DURING CHILDHOOD
The Full-term Neonate
Marked changes occur in thyroid physiology at the time of birth in the full term newborn. One of the most dramatic changes is an abrupt rise in the serum TSH which occurs within 30 minutes of delivery, reaching concentrations as high as 60 to 70 mU/L (8). This causes a marked stimulation of the thyroid and an increase in the concentrations of both serum T4 and T3 (34). These consist of an approximate 50% increase in the serum T4 and an increase of three- to four-fold in the concentration of serum T3 to adult levels at 1 to 4 days of life. Serum levels of T4, free T4 and TBG remain elevated over cord levels at 7 days of postnatal life (Figure 2), decreasing thereafter. The T3 concentration rises strikingly at Day 7, and continues to rise for the first 28 days. Opposite effects are noted in the reverse T3 levels and T3 sulfate.
Studies in experimental animals suggest that the increase in TSH is a consequence of the relative hypothermia of the ambient extrauterine environment. However, while a significant portion of the marked increase in T3 from its low basal levels in cord serum can be explained by the abrupt increase in TSH, the simultaneous fall in reverse T3 and T3 sulfate are consistent with an increase in D1 activity occurring at the same time. D2 has been identified in human brown adipose tissue as well as brain and the acute increase in T3 in adipose tissue at birth is required for optimal uncoupling protein synthesis and thermogenesis (35,36).
Premature Infants
Thyroid function in the premature infant reflects, in part, the relative immaturity of the hypothalamic-pituitary-thyroid axis that is found in comparable gestational age infants in utero. Following delivery, there is a surge in T4 and TSH analogous to that observed in term infants, but the magnitude of the increase is less in premature neonates (8). In infants <31 weeks, the circulating T4 concentration may not increase and may even fall in the first 1 to 2 weeks of life (37) (Figure 2). This decrease in the T4 concentration is particularly significant in very premature infants, in whom the serum T4 may occasionally be undetectable. In most cases, the total T4 is more affected than the free T4 (38), a consequence of abnormal protein binding and/or the decreased TBG in these babies with immature liver function.

Figure 15-2. Postnatal changes in of T4, free T4, TBG, T3, rT3 and TSH according to gestational age. Values determined in babies born at gestational age of 23-27, 28-30, 31-34 and 37 weeks or more are reported. Note the increase in T4, free T4 and TBG in the full term infant in the first week of life. T3 also rises strikingly, while rT3 and TSH decline. The increase in T4 and free T4 is blunted in infants
The causes of the decrease in T4 observed postnatally in premature infants are complex. In addition to the clearance of maternal T4 from the neonatal circulation, preterm babies have decreased thyroidal iodide stores (39) (a problem of particular significance in borderline iodine-deficient areas of the world), they are frequently sicker than their more mature counterparts, are less able to regulate iodide balance, and they may be treated by drugs that affect neonatal thyroid function (particularly dopamine and steroids). In addition, since the capacity of the immature thyroid to adapt to exogenous iodide is reduced, there is an increase in sensitivity to the thyroid-suppressive effects of excess iodide found in certain skin antiseptics and drugs to which these babies are frequently exposed (see below).
Despite the reduced total T4 observed in some preterm babies, the TSH concentration is not significantly elevated in most of these infants. In some babies, transient elevations in TSH are seen, the finding of a TSH concentration >40 mU/L being more frequent the greater the degree of prematurity. Frank et al found, for example, that the prevalence of a TSH concentration >40 mU/L in very low birth weight, (<1.5 kg), i.e., very premature, infants was 8-fold higher and in low birth weight, (1.5 kg-2.5 kg) neonates 2-fold higher than the prevalence in term babies (40). Whereas in some cases, an elevated TSH concentration may reflect true primary hypothyroidism, in other instances this increase in TSH may reflect the elevated TSH observed in adults who are recovering from severe illness. Such individuals may develop transient TSH elevations that are associated with still reduced serum T4 and T3 concentrations. These have been interpreted as reflecting a “ re-awakening ” of the illness-induced suppression of the hypothalamic pituitary axis. As the infant recovers from prematurity associated illnesses such as respiratory distress syndrome (RDS), a recovery of the illness-induced suppression of the hypothalamic- pituitary- thyroid axis would also occur.

Figure 15-3. Cord blood levels of T4, free T4, TBG, T3, reverse T3 and TSH in the human infant. Note the low T3 and high reverse T3 concentrations as well as the discrepancy between the total T4 and free T4 levels in very premature babies. (Redrawn from Williams et al (10). See text for details).
Somewhat surprisingly, given the relative immaturity of the thyroid gland, serum Tg concentrations are higher in the premature than in the full term infant (41), particularly in those who are sick with respiratory distress syndrome. In view of the attenuated postnatal TSH rise in the latter babies, it is likely that impaired clearance and/or degradation of this glycoprotein from the circulation rather than increased secretion plays an important role.
Small-for-gestational-age (SGA) Infants
SGA infants have significantly higher TSH and lower total and free T4 values than do infants of normal weight (42). This can be related to the severity of the malnutrition in these infants, as well as to fetal hypoxemia and acidemia. Impaired placental perfusion and chronic starvation may also play a role. This pattern of reduced T4 and elevated TSH differs from the response to starvation in older individuals and healthy adults in whom TSH is reduced. The explanation for the relatively higher TSH in duch infants is not known.
Infants and Children
Following the acute perturbations of the neonatal period there is a slow and progressive decrease in the concentrations of T4, free T4, T3 and TSH during infancy and childhood (43). Younger children tend to have slightly higher serum concentrations of T3 and TSH, so age-specific normative values should always be consulted. The serum concentration of reverse T3 remains unchanged or increases slightly. Serum Tg levels also fall over the first year of life reaching concentrations typical of adults by about 6 months of age. Another important aspect of thyroid physiology in the infant and child is the markedly higher T4 turnover in this age group relative to that in the adult. In infants, T4 production rates are estimated to be on the order of 5 to 6 mcg/kg per day decreasing slowly over the first few years of life to about 2 to 3 mcg/kg/day at ages 3 to 9 years. This is to be contrasted with the production rate of T4 in the adult which is about 1.5 mcg/kg/day. The size of the infant thyroid gland increases quite slowly. The thyroid gland of the newborn weighs approximately 1 gram and increases about 1 gram per year until age 15 when it has achieved its adult size of about 15 to 20 g. In general, the size of the thyroid lobe is comparable to that of the terminal phalanx of the infant or child’s thumb.
THYROID DISEASE IN INFANCY
Congenital hypothyroidism
Non endemic congenital hypothyroidism is one of the commonest treatable causes of mental retardation. The association between goitrous hypothyroidism and mental retardation was first noted more than 400 years ago by Paracelsus in 1527, and Thomas Curling first described sporadic nongoitrous hypothyroidism in 1850. However, despite the demonstration by Murray in 1891 that thyroid extract could ameliorate many of the features of untreated cretinism, it was not until the 1970’ that the importance of early treatment in diminishing the neuro-psychological abnormalities of congenital hypothyroidism was demonstrated convincingly (45). The development by Dussault et al of a sensitive and specific radioimmunoassay for the measurement of T4 in dried whole blood eluated from filter paper (and later tests for T4 and TSH using 1/8 ″ discs) provided the technical means to screen all newborns for congenital hypothyroidism prior to the development of clinical manifestations (46). Thus, as summarized by Delange, congenital hypothyroidism includes all the characteristics of a disease for which screening is justified: 1) it is common (4-5 times more common than phenylketonuria for which screening programs were initially developed); 2) to prevent mental retardation, the diagnosis must be made early, preferably within the first few days of life; 3) at that age, clinical recognition is difficult if not impossible; 4) sensitive, specific screening tests and 5) simple, cheap effective treatment are available; and 6) the benefit-cost ratio is highly favorable (approximately 10/1, a ratio that does not include the loss of tax income that would result from impaired intellectual capacity in the untreated, but non-institutionalized, person) (47). Since the development of the first pilot screening program for the detection of congenital hypothyroidism in Quebec in 1972, newborn screening programs have been introduced throughout the industrialized nations and are under development in many other parts of the world. It has been estimated that as of 1999, some 150 million infants had been screened for congenital hypothyroidism worldwide with 42,000 cases detected (46). Although there continues to be some disagreement as to whether minor neuro-intellectual sequelae remain in the most severely affected infants, accumulating evidence suggests that a normal outcome is possible even in the latter group of babies as long as treatment is started sufficiently early and is adequate (48-50). Certainly, the main objective of screening, the eradication of mental retardation, has been achieved.
National screening programs are well organized in many developed countries. However, it must be emphasized that approximately 71% of babies worldwide are not born in an area with an established national screening program for CH. The economic burden of disability owing to congenital hypothyroidism is still a significant public health challenge (50a).
The prevalence of CH was approximately 1:7000 to 1:10000 in the prescreening era and decreased to1;3000 to 1.4000 in the 1970s and 1980s when the screening programs were applied. Rates ranging from 1:1400 to 1:2800 have been recently reported by screening programs in USA, Canada, Italy, Greece, and New Zealand (50b).
Lower TSH cut off values used in the screening programs and changes in birth population partially explained the higher incidence reported. Lower cutoff values for TSH have been adopted in many countries over the years, leading to the identification of milder forms of CH essentially with eutopic thyroid gland (thyroid in situ). Ford and LaFranchi in 2014 (50a) found that lowering the TSH cutoff value from greater than 20-25 uU/mL to greater than 6-10 approximately doubled the incidence of CH. A study from Italy reported that 21.6% of babies with permanent CH had TSH value at screening less than 15 uU/mL (applied between 2000 and 2006, cutoff TSH value ranged from 15 to 7uU/mL in different regions). The frequency of thyroid dysgenesis in this group was 19.6% and TSH levels at confirmation ranged from 9.9 to 708 uU/mL .It is important to remember that in this study TSH value at screening does not discriminate between transient and permanent forms of CH (50c).
Harris and Pass reported that CH incidence increased from 1:3373 in 1978 to 1:1415 in 2005 (50d). Changes in the demographics of the birth population in New York partially explained the increased incidence of CH. They found a 23% increase with a birth weight < 1500 gr., 50% increase of twin/multiple births, 41% increase in mothers >30 years of age (50d). Also changes in percentage of races or ethnicity of newborns play a role, as shown in the State of California. In this study, the incidence of CH in Asian Indian is reported to be 1:1200 and in Hispanic 1:1600, versus 1:11000 in Non Hispanic Black (50e). A further study from the Italian Study Group, based on data from the Italian National Registry from 1987 to 2008 showed an increased incidence of both permanent and transient CH, in more recent years (50f). The authors investigated trends in the incidence of CH between the period 1987-1998, and 1999-2008. They found an increasing of 38% (from 1:3200 to 1:2320) of the incidence of permanent CH and of 54% (from1:3000 to 1:1940) including the transient forms in the period 1999-2008. The most important factor was the lowering of cutoff TSH values (from greater than 20 to 7/15 uU/ml since 1999. Moreover an increment of 58% of preterm babies with permanent CH was also reported in the second period. Permanent CH due to thyroid dysgenesis had a slight increase, being the great majority of cases presented with normal/hyperplastic thyroid.
A national study from France, including 6622 cases of CH identified from 1982 to 2012 showed that the incidence rate CH due to eutopic glands increased by 4.4 fold in this period, regardless of the screening method adopted. Interestingly, also severe eutopic forms of CH increased by 2.1%. The incidence of dysgenesis did not change (50g).
Screening Strategies
Screening for primary CH worldwide should be performed on the basis of national resources. The aim of neonatal screening is the earliest identification of any form of congenital hypothyroidism, but particularly those patients with severe hypothyroidism in whom disability is greatest if not treated. The identification of Central Congenital Hypothyroidism (CCH) by screening programs is under debate. Two screening strategies for the detection of congenital hypothyroidism have evolved. In the primary T4/backup TSH method, still favored in much of North America and the Netherlands, T4 is measured initially while TSH is checked on the same blood spot in those specimens in which the T4 concentration is low. In the primary TSH approach, favored in most parts of Europe and Japan, blood TSH is measured initially.
A primary T4/backup TSH program will detect overt primary hypothyroidism, secondary or tertiary hypothyroidism, babies with a low serum T4 level but delayed rise in the TSH concentration, TBG deficiency and hypothyroxinemia; this approach may, however, miss subclinical hypothyroidism. A primary TSH strategy, on the other hand, will detect both overt and subclinical hypothyroidism, but will miss secondary or tertiary hypothyroidism, a delayed TSH rise, TBG deficiency and hypothyroxinemia. There are fewer false positives with a primary TSH strategy. Both programs will miss the rare infant whose T4 level on initial screening is normal but who later develops low T4 and elevated TSH concentrations. This pattern has been termed “atypical” congenital hypothyroidism or “delayed TSH” and is observed most commonly in premature babies with transient hypothyroidism or infants with less severe forms of permanent disease.
In a few regions, a second routine specimen is collected from all births at 2-4 weeks of age (51). Results from the Northwest Regional Screening program, coordinated in Oregon, (USA), that applied this method, have recently been published (51a). In 2014 the European Society for Pediatric Endocrinology, (ESPE) on behalf of all the scientific societies of pediatric endocrinologists worldwide (ESPE,PES, SLEP, JSPE, APEG, APPES, ISPAE) published updated guidelines about screening, diagnosis, and management of congenital hypothyroidism (51b, 51c).
According to the ESPE guidelines, the most sensitive test for detecting primary CH is the determination of TSH concentration that detects primary CH more effectively than primary T4 screening (51b,51c). Primary T4 screening with confirmatory TSH testing can detect some cases of CCH, but some cases of mild CH can be missed, depending on the cutoff T4 value used.
When available, screening strategies for the identification of CCH are: a) a combination of primary T4 and primary TSH screening, b) a combination of primary T4 screening with secondary TSH testing followed by T4 binding protein determination (TBG). The last one is employed by the Netherlands where, in addition to a primary T4/backup TSH approach, TBG is assessed in those filter paper specimens with the lowest 5% of T4 values (52). The T4/TBG ratio is used as an indirect reflection of the free T4, which is difficult to be measured directly in dried blood spots. This approach has been reported to result in improved sensitivity and specificity in detecting milder cases of primary congenital hypothyroidism that might otherwise be missed. An additional reported advantage was the identification of >90% of infants with central hypothyroidism compared with only 22% with primary T4 screening and none with a primary TSH approach. Since on subsequent testing > 80% of the babies with central hypothyroidism had multiple pituitary hormone deficiencies, a disorder associated with high morbidity and mortality for which effective treatment exists (53,53a), and in view of an apparent frequency (1 in 16,000) similar to that of phenylketonuria (1 in 18,000), the authors have argued that the goals of newborn thyroid screening should be extended to include the detection of babies with central hypothyroidism.
Recently a primary FT4 and TSH strategy was applied in Kanagawa Prefecture in Japan. A different method to determine FT4, based on enzyme-immunometric assays in filter paper blood eluates was used. They found a CCH prevalence of 1:31000 infants (53b,53c).
Measurement of T4 and/or TSH is performed on an eluate of dried whole blood (DBS) collected on filter paper by skin puncture on day 1-4 of life. Primary CH screening has been shown to be effective for the testing of cord blood or the blood collected on filter paper after the age of 24 hours. Blood is applied directly to the filter paper and after drying the card is sent to the laboratory. The best time to collect blood for TSH screening is 48 to 72 hours of age. The practice of early discharge from the hospital of otherwise healthy full term infants has resulted in a greater proportion of babies being tested before this time. For example, it has been estimated that in North America 25% or more of newborns are now discharged within 24 hours of delivery and 40% in the second 24 hours of life (54). Because of the neonatal TSH surge and the dynamic changes in serum T4 and T3 concentrations that occur within the first few days of life, early discharge increases the number of false positive results. It is important that in the screening laboratory the results of TSH are interpreted in relation to time of sampling. Ethnicity seems to play a role in determining mean TSH values at birth (54a).
Physicians caring for infants need to appreciate that there is always the possibility for human error in failing to identify affected infants, whichever screening program is utilized. This can occur due to poor communication, lack of receipt of requested specimens, or the failure to test an infant who is transferred between hospitals during the neonatal period (55). Therefore if the diagnosis of hypothyroidism is suspected clinically, the infant should always be tested (Figure 5).
Similarly, as is obvious from the discussion earlier in the chapter, adult normative values, provided by many general hospital laboratories, differ from those in the newborn period and should never be employed. Normal values according to both gestational and postnatal age for cord blood T4, free T4, TBG, T3, reverse T3, and TSH up to 28 days of life (10) are shown in Figure 2. Normal serum levels of Tg in premature and full-term infants (13,14) and normal serum levels of free T4 and TSH in the first week of life (56) have also been published, though it should be noted that precise values may vary somewhat, depending on the specific assays used.

Figure 15-4. Three month old male infant who was diagnosed clinically when he presented with a history of poor feeding at 3 months of age. The child was born in Puerto Rico prior to the development of newborn screening. Note the dull face, periorbital edema and enlarged tongue.
Screening in special categories of neonates at risk of CH
Special categories of neonates with CH can be missed at screening performed at usual time, particularly preterm babies and neonates with serious illnesses and multiple births. Drugs used in neonatal intensive care (i.e., dopamine, glucocorticoids that suppresses TSH), immaturity of hypothalamic-pituitary thyroid axis, decreased hepatic production of thyroid binding globulin, reduced transfer of maternal T4, reduced intake of iodine or excess iodine exposure, fetal blood mixing in multiple births can affect the first sample, and in many center a second specimen is required to rule out CH. (See section thyroid function in infants for more details).
Preterm babies have a higher incidence of a unique form of hypothyroidism, characterized by a delayed elevation of TSH. These babies can later develop low T4 and elevated TSH concentrations. This pattern has been termed “atypical” congenital hypothyroidism or “delayed TSH”. Preterm babies with a birth weight of less than 1500 gr. have an incidence of congenital hypothyroidism of 1:300. Survival of even extremely premature babies (<28 weeks of gestation) is around 90% in developed countries, and the incidence of prematurity is around 11.5 % in US and 11.8 % worldwide. So, an increasing subpopulation of preterm babies and high risk newborns deserves a special sight about screening and follow up of CH.
In these categories a second specimen 2-6 weeks from the first (ESPE guidelines suggested at about 15 days, or after 15 days from the first) may be indicated: preterm neonates with a gestational age of less than 37 weeks, Low Birth Weight and Very Low Birth Weight neonates and ill and preterm neonates admitted to neonatal intensive care unit, specimen collection within the first 24 hours of life, and multiple births, particularly in the case of same sex twins. The interpretation of the screening results should consider the results of a multiple sampling strategy, the age of sampling and the maturity (GA/birth weight) of the neonate.
Two recent papers (56a,56b) showed that a second screen (using a lower TSH cutoff) is able to detect the delayed elevation of TSH that occurs in these babies. Vigone et al (56a) revaluated the children with a diagnosis of CH detected at second screen and treated with L-thyroxine after 2 years of age and found 24% of cases with permanent congenital hypothyroidism, 52% with transient hypothyroidism and 24% with persistent hypertropinemia. Neither screening nor confirmatory TSH levels were able to predict the thyroid function after 2 years of age in these children.
Timing of normalization of thyroid hormones is critical for brain development (56c) and treatment should be started immediately if DBS TSH concentration is 40 mUI/l or more, after baseline TSH and FT4 serum determination, because this value strongly suggests decompensated hypothyroidism (56d). If TSH is < 40 mUI/l the clinician may postpone treatment, pending the serum results, for 1-2 days. ESPE guidelines (51b,51c) suggest treatment should be started if venous TSH concentration is persistently >20 mUI/l, even if serum FT4 is normal. Overtreatment can be dangerous for neurocognitive outcome and should be avoided, individualizing the dosage.
It is still a matter of debate if treatment can be beneficial in otherwise healthy babies with venous TSH concentration between 6-20 mUI/l and FT4 concentration within the normal limits for age. In these cases, diagnostic imaging is recommended to try to establish a definitive diagnosis. If TSH concentration remains high for more than 3 or 4 weeks, it is possible (in discussion with the family) either starting LT4 supplementation immediately and retesting, off treatment, at a later stage, or retesting two weeks later without treatment. Waiting for larger studies that are able to answer to this question, and given the irreversibility of a possible harm to the child, treating during early childhood and revaluating the thyroid function after myelination of the central nervous system is completed (by 36 to 40 months of age) can be a prudent behavior (56e). LT4 treatment must be started immediately if FT4 or TT4 levels are low, given the known adverse effect of untreated decompensated CH on neurodevelopment and somatic growth.
CH is defined on the basis of serum FT4 levels as severe when FT4 is <5 pmol/l, moderate when FT4 is 5 to 10 pmol/l and mild when FT4 is 10 to 15 pmol/l respectively. Determination of serum thyroglobulin (Tg) is useful, if below the detection threshold, to suggest athyreosis or a complete thyroglobulin synthesis defect. Measurement of Tg is most helpful when a defect in Tg synthesis or secretion is being considered. In the latter condition the serum Tg concentration is low or undetectable despite the presence of a normal or enlarged, eutopic thyroid gland. Serum Tg concentration also reflects the amount of thyroid tissue present and the degree of stimulation. For example, Tg is undetectable in most patients with thyroid agenesis, intermediate in babies with an ectopic thyroid gland and may be elevated in patients with abnormalities of thyroid hormonogenesis not involving Tg synthesis and secretion. Considerable overlap exists, and so, the Tg value needs to be considered in association with the findings on imaging. In patients with inactivating mutations of the TSH receptor discordance between findings on thyroid imaging and the serum Tg concentration has been described in some but not all studies (56f).
Clinical findings are usually difficult to appreciate in the newborn period except in the unusual situation of combined maternal-fetal hypothyroidism. Many of the classic features (large tongue, hoarse cry, facial puffiness, umbilical hernia, hypotonia, mottling, cold hands and feet and lethargy), when present, are subtle and develop only with the passage of time. In addition to the aforementioned findings, nonspecific signs that should suggest the diagnosis of neonatal hypothyroidism include: prolonged, unconjugated hyperbilirubinemia, gestation longer than 42 weeks, feeding difficulties, delayed passage of stools, hypothermia or respiratory distress in an infant weighing over 2.5 kg ( 57). A large anterior fontanelle and/or a posterior fontanelle > 0.5 cm is frequently present in affected infants but may not be appreciated. In general, the extent of the clinical findings depends on the cause, severity and duration of the hypothyroidism. Babies in whom severe feto-maternal hypothyroidism was present in utero tend to be the most symptomatic at birth. Similarly, babies with athyreosis or a complete block in thyroid hormonogenesis tend to have more signs and symptoms at birth than infants with an ectopic thyroid, the most common cause of congenital hypothyroidism. Unlike acquired hypothyroidism, babies with congenital hypothyroidism are of normal size. However, if diagnosis is delayed, subsequent linear growth is impaired. The finding of palpable thyroid tissue suggests that the hypothyroidism is due to an abnormality in thyroid hormonogenesis or in thyroid hormone action.
Bone maturation reflects the duration and the severity of hypothyroidism. Signs of delayed epiphyseal maturation on knee x-rays, persistence of the posterior fontanelle, a large anterior fontanelle, and a wide sagittal suture all reflect delayed bone maturation. The absence of one or both knee epiphyses has been shown to be related to T4 concentration at diagnosis and to IQ outcome, and is thus a reliable index of intrauterine hypothyroidism.
Imaging Techiniques in CH
Imaging studies are helpful to determine the specific etiology of CH. Both scintigraphy and ultrasound (US) should be considered in neonates with high TSH concentrations. Ideally, the association of US and scintigraphy gives the best information in a child with primary hypothyroidism. Scintigraphy shows the presence/absence (athyreosis), position (ectopic gland, in any point from the foramen caecum at the base of the tongue to the anterior mediastinum) and rough anatomic structure of the thyroid gland.
US, in experienced hands, is a valid tool in defining size and morphology of a eutopic thyroid gland, however, US alone is less effective in detecting ectopic glands. Color Doppler US improves the effectiveness of US (57a).
It is important to remember that an attempt to obtain an imaging of the thyroid in a newborn should never delay the initiation of treatment. Scintigraphy should be carried out within 7 days of starting LT4 treatment. Scintigraphy may be carried out with either 10-20 MBq of technetium-99m (99mTc) or 1-2 MBq of iodine-123 (I123). Tc is more widely available, less expensive, and quicker to use than I 123. Scintigraphy with I123, if available, is usually preferred because of the greater sensitivity and because, I123, unlike of technetium-99 is organified. Therefore, imaging with this isotope allows quantitative uptake measurements and tests for both iodine transport defects and abnormalities in thyroid oxidation. An enrichment of the tracer within the salivary gland can lead to misinterpretation, especially on lateral views, but this can be avoided by allowing the infant to feed before scintigraphy, thus empting the salivary glands and keeping the child calm under the camera. The perchlorate discharge test is considered indicative for a organification defect when a discharge of > 10% of I123 administred dose occurs in a thyroid in normal position (when perchlorate is given at 2 hours).
Excess iodine intake through exposure (i.e from antiseptic preparation), maternal TSH receptor blocking antibodies, inactivating mutation in the TSH receptor and in the sodium/iodide symporter (NIS), and TSH suppression from LT4 treatment can give interfere with the I123 uptake, showing no uptake in the presence of a thyroid in situ (apparent athyreosis).
Thyroid ultrasonography is performed with a high frequency linear array transducer (10-15 MHz) and allows a resolution of 0.7 to 1mm. Thyroid tissue is more echogenic than muscle and less echogenic than fat. In the case of absence of the thyroid fat tissue can be misdiagnosed as dysplastic thyroid gland in situ. Distinguish between thyroid hypoplasia and dysplastic non thyroidal tissue in a newborn requires an enormous experience, and reevaluation at later age can result in a different diagnosis (57a).
Combining scintigraphy and thyroid ultrasound improve diagnostic accuracy, and helps to address further investigations, including molecular genetic studies. Infants found to have a normal sized gland in situ in the absence of a clear diagnosis should undergo further reassessment of the thyroid axis and imaging at a later age.
Therapy
Replacement therapy with L-thyroxine (L-T4) should be begun as soon as the diagnosis of congenital hypothyroidism is confirmed. In babies whose initial results on newborn screening are suggestive of severe hypothyroidism therapy should be begun immediately without waiting for the results of the confirmatory serum. Severe hypothyroidism is defined by T4 <5 mcg/dL (64 nmol/L) and/or TSH >40 mU, or. accordingly with ESPE guidelines(51g,51k), CH is defined on the basis of serum FT4 levels as severe when FT4 is <5 pmol/l, moderate when FT4 is 5 to 10 pmol/l and mild when FT4 is 10 to 15 pmol/l. As noted above, treatment need not be delayed in anticipation of performing thyroid imaging studies as long as the latter are done within 5-7 days of initiating treatment (before suppression of the serum TSH). Parents should be counseled regarding the causes of congenital hypothyroidism, the importance of compliance and the excellent prognosis in most babies if therapy is initiated sufficiently early and is adequate and educational materials should be provided (58). An initial dosage of 10-15 mcg/kg/day of L-T4 is generally recommended to normalize the T4 as soon as possible. The highest dose is indicated in infants with severe disease, and the lower in those with a mild to moderate form. L-T4 Tablets can be crushed and given via a small spoon, with suspension, if necessary in a few milliliters of water or breast milk or formula or juice, but care should be taken that all of the medicine has been swallowed. Thyroid hormone should not be given with substances that interfere with its absorption, such as iron, calcium, soy, or fiber. Drugs such as antacids (aluminium hydroxide) or infantile colic drops (simethicone) can interfere with L-thyroxine absorption. Many babies will swallow the pills whole or will chew the tablets with their gums even before they have teeth. Reliable liquid preparations are not available commercially in the US, although they have been used successfully in Europe. L-T4 can also be administred in liquid form, but only if pharmaceutically produced and licensed L-T4 solutions are available. A brand name rather a generic formulation of L-T4 is recommended because they are not bioequivalent (58a).
The aims of therapy are to normalize the T4 as soon as possible, to avoid hyperthyroidism where possible, and to promote normal growth and development. When an initial dosage of 10-15 mcg/kg is used, the T4 will normalize in most infants within 1 week and the TSH will normalize within 1 month, Subsequent adjustments in the dosage of medication are made according to the results of thyroid function tests and the clinical picture. Often small increments or decrements of L-thyroxine (12.5 mcg) are needed. This can be accomplished by 1/2 tablet changes, by giving an alternating dosage on subsequent days, or by giving an extra tablet once a week.
As stated in ESPE guidelines: “ L-T4 alone is recommended as the medication of choice and should be started as soon as possible, no later than two weeks of life or immediately after confirmatory test results in infants identified in a second routine screening test. L-T4 should be given orally. If intravenous administration is necessary, the dose should be no more than 80% of the oral dose”. Serum or plasma FT4 (or TT4) and TSH concentration should be determined at least 4 hours after the last L-T4 administration. TSH should be maintained in the age-specific reference range and FT4 in the upper half of the age- specific reference range. “The first follow up examination is indicated after 1-2 weeks after the start of LT4 treatment and then every 2 weeks until TSH levels are completely normalized and then every 1- 3 months until 12 months of age. Between the age of one and three years, children should undergo frequent clinical and laboratory evaluations (every 2 to 4 months).” Thereafter, evaluations should be carried out every 3 to 12 months until growth is completed. “More frequent evaluations should be carried out if compliance is questioned or abnormal values are obtained. Any reduction of L-T4 dose should not be based on a single increase of FT4 concentration during treatment. “Measurements should be performed after 4-6 weeks any change in the dosage or in the L-T4 formulation”.
Re-evaluation and Trial Off Therapy
In hypothyroid babies in whom an organic basis was not established at birth and in whom transient disease is suspected, a trial off replacement therapy can be initiated after the age of 3 years when most thyroxine-dependent brain maturation has occurred, as shown by magnetic risonance imaging studies (56e). Re-evaluation is recommended if the treatment was started in a sick child (i.e. preterm), if thyroid antibodies were detectable, if no diagnostic assessment was completed, and in children who have required no increase in L-T4 dosage since infancy. Re-evaluation is recommended also in the case of a eutopic gland with or without goiter, if not enzyme defects have been detected, if any other cause of transient hypothyroidism is suspected.
Re-evaluation is not necessary if venous TSH concentration has risen during the first year of life, due to either LT4 underdosage or poor compliance. To perform a precise diagnosis LT4 treatment is suspended for 4-6 weeks, and biochemical testing and thyroid imaging are carried out. To establish the presence of primary hypothyroidism, without defining the cause, L-T4 dose may be decreased by 20-30% for 2 to 3 weeks. If TSH serum levels rise to > 10 mU/L during this period, the hypothyroidism can be confirmed.
Prognosis
Although all are agreed that the mental retardation associated with untreated congenital hypothyroidism has been largely eradicated by newborn screening, controversy persists as to whether subtle cognitive and behavioral deficits remain, particularly in the most severely affected infants (59-64). Both the initial treatment dose and early onset of treatment (before 2 weeks) are important. Time to normalization of circulating thyroid hormone levels, the initial free T4 concentration, maternal IQ, socioeconomic and ethnic status have also been related to outcome (59,62,63,64). The long term problems for these babies appear to be in the areas of memory, language, fine motor, attention and visual spatial. Inattentiveness can occur both in patients who are overtreated and those in whom treatment was initiated late or was inadequate. In addition to adequate dosage, assurance of compliance and careful long-term monitoring are essential for an optimal developmental outcome. More details about long term follow up are reported in ESPE guidelines (51g,51K). Progressive hearing loss in CH should be recognized and corrected, because strongly influenced the outcome). Recently, extensive reports on long term outcome of congenital hypothyroidism in young adults have been published (64a,64b). In the French cohort of 1202 CH young adults, hearing impairment was found at a mean age of 23.4 years in 9.5% versus 2.5% of general population, and the risk of developing hearing impairment was three times higher in these patients than in general population (64c). Also interesting data about pregnancy outcomes in young women with CH came out from the French cohort (64d).
CAUSES OF PERMANENT CONGENITAL HYPOTHYROIDISM
Permanent congenital thyroidal (primary) hypothyroidism can be the consequence of a disorder in thyroid development and/or migration (thyroid dysgenesis), or due to defects at every step in thyroid hormone synthesis (thyroid dyshormonogenesis). Although congenital hypothyroidism (CH) is in the great majority of cases a sporadic disease, the recent guidelines (51g,51k) for CH recommend genetic counseling in targeted cases. Positive family history for CH, association with cardiac or kidney malformation, midline malformation deafness, neurological sigs (i.e., choreoathetosis, hypotonia, any sign of Albright hereditary osteodystrophy, lung disorders, suggest genetic counseling, in order to assess the risk of recurrence and to provide further information about a possible genetic etiology of CH. Recently a targeted next-generation (NGS) panel, covering all exons of the major CH genes, has been proposed as a useful tool to identify the genetic etiology of CH (64e). Lowering TSH cut off value at screening increases the diagnosis of CH with eutopic thyroid. A targeted next-generation (NGS) panel has been applied to patients with CH and thyroid in situ (64f).
Thyroid Dysgenesis
Unlike in iodine-deficient areas of the world where endemic cretinism continues to be a major health hazard, the majority (85 to 90%) of cases of permanent congenital hypothyroidism in North America, Western Europe and Japan are due to an abnormality of thyroid gland development (thyroid dysgenesis). Thyroid dysgenesis may result in the complete absence of thyroid tissue (agenesis, 20-30%) owing to a defect in survival of the thyroid follicular cells precursors) or it may be partial (hypoplasia); the latter often is accompanied by a failure to descend into the neck (ectopy) mostly located in a sublingual position as a result of a premature arrest of its migratory process. Lowering of cut off TSH values for newborn screening increases the percentage of CH with thyroid in situ. Females are affected twice as often as males. In the United States, thyroid dysgenesis, is less frequent among African Americans and more common among Hispanics and Asians. Babies with congenital hypothyroidism have an increased incidence of cardiac anomalies, particularly atrial and ventricular septal defects (65). An increased prevalence of renal and urinary tract anomalies has also been reported recently (66). Most cases of thyroid dysgenesis are sporadic. Familial cases represent 2%. Discordance between monozigotic twins is inexplained (67). Although both genetic and environmental factors have been implicated in its etiology, in most cases the cause is unknown (67a).
The occasional familial occurrence, the higher prevalence of thyroid dysgenesis in babies of certain ethnic groups and in female versus male infants as well as the increased incidence in babies with Down syndrome (68) all suggest that genetic factors might play a role in some patients. Thyroid transcription factors would appear to be obvious candidate genes in view of their important role in thyroid organogenesis and in thyroid-specific gene expression. To date, however, abnormalities in these genes have been found in only a small proportion of affected patients, usually in association with other developmental abnormalities (68a).
Thyroid transcription factors (TTF) such as NKX2-1 (or formerly TTF1/TITF1), FOXE1 (Forkhread Box E1, formerly TTF2/TITF2), PAX8 (Paired box gene 8), and NKX2-5, are expressed during early phases of thyroid organogenesis (budding and migration), instead thyroid stimulating hormone receptor gene (TSHR) is expressed during the later phases of thyroid development. All these genes are involved in normal thyroid development and in thyroid dysgenesis. Alternately, epigenetic modifications, early somatic mutations or stochastic developmental events may play a role. Five monogenic forms due to mutations in TSHR, NXK2-1, PAX8, FOXE-1. NXK2-5 have been reported. Monogenic forms represent less than 10% in TD (68a).
TABLE 1. GENETIC CAUSES OF CONGENITAL HYPOTHYROIDISM
| 1.1 PRIMARY HYPOTHYROIDISM | Gene locus | Inheritance |
| Monogenic forms of thyroid dysgenesis | ||
| · Thyroid stimulating hormone receptor (TSHR) | AR | |
| · NK2 1 (NK2-1, TTF1) brain-lung thyroid syndrome | 14q13 | AD |
| · Paired box gene 8 (PAX8) | 2q11.2 | AD |
| · Forkhead boxE1 (FOXE1, TTF2) (Bambforth-Lazarus syndrome) | 9q22 | AR |
| · NK2 homeobox 5 (NKX2-5) | ||
| New candidates gene | ||
| · Nertrin 1 (NTN-1) | ||
| · JAG1 | 20p.12.2 | |
| Inborn errors of thyroid hormonogenesis | ||
| · Sodium/Iodide symporter (SLC5A5,NIS | 19p13.2 | AR |
| · Thyroid peroxidase (TPO) | 2p25 | AR |
| · Pendred syndrome (SLC26A4,PDS) | 7q31 | AR |
| · Thyroglobulin (TG) | 8q24 | AR |
| · Iodothyrosine deiodinase (IYD,DEHAL1) | 6q24-25 | AR |
| · Dual oxidase 2 (DUOX2) | 15q15.3 | AR/AD |
| · Dual oxidase maturation factor 2 (DUOXA2) | AR/AD | |
| B1.2 CENTRAL HYPOTHYROIDISM | ||
| Isolated TSH deficiency | ||
| · TRHR | 14q31 | AR |
| · TSHB | 1p13 | AR |
| Isolated TSH deficiency or combined pituitary hormone deficiency | ||
| Immunoglobulin superfamily member1 (IGSF1) gene defects | Xq26.1 | X-Linked |
| Combined pituitary hormone deficiency | ||
| · POU1F1 | 3p11 | AR,AD |
| · PROP1 | 5q | AR |
| · HESX1 | 3p21.2-21.2 | AR/AD |
| · LHX3 | 9q.34 | AR |
| · LHX4 | 1q25 | AD |
| · SOX3 | X-linked | |
| · OTX2 | AD |
Monogenic Forms of Thyroid Dysgenesis
Thyroid stimulating hormone receptor resistance (TSHR gene #OMIM 603372)
Described in 1968, is mostly caused by biallelic inactivating mutations in the TSH receptor gene (TSHR). TSH affects follicular thyroid cell proliferation and many cellular processes, including thyroidal iodine uptake, thyroglobulin iodination, and reuptake of iodinated thyroglobulin. Phenotype varies from mild hyperthyrotropinemia with normal thyroid gland to severe CH with thyroid hypoplasia and absence of tracer uptake at scintigraphy (apparent athyreosis).
Inactivating TSHR mutations are the most frequent cause of monogenic TD and non syndromic CH, with prevalence in CH cohorts around 4 % (68b). Clinically a classic and a non-classic TSH resistance form are described, based on different TSHR mutations (68c). Both Gs and Gq proteins are involved Heterozygous non polymorphic TSHR mutations were found in a high frequency (11.8-29%) in children and adolescents with isolated non-autoimmune hyperthyrotropinemia (68d).
NKX2-1 (OMIM 600635)
NKX2-1 (previously TITF-1, TTF-1) gene encodes for a transcription factor of the NK family. It is involved in early development of brain, thyroid and lung. In thyrocytes, NKX2-1 activates the transcription of TG, TPO, TSHR and PDS genes. In the lung is important for the branching of the lobar bronchi and regulates the expression of surfactant proteins in pneumocytes. In the brain, NKX2 is expressed in basal ganglia and forebrain and it is involved in the specification and migration of neurons. Haploinsufficiency of NKX2-1 is responsible for the brain-lung-thyroid (BLT) syndrome (OMIM 610978) characterized by CH, infant respiratory distress syndrome and benign hereditary chorea. NKX2-1 defects occur either as a sporadic cases or as familial cases inherited in an autosomal-dominant manner. The clinical presentation ranges from the complete BLT syndrome (50%) to incomplete forms with brain and thyroid disease (30%) or only benign hereditary chorea (13%), the mildest expression of the syndrome. TD ranges from hypoplasia (about 35%) to normal morphology (>50% of patients) (68e). Recently, a case of BLT syndrome has been reported with thyroid ectopy (68f).
The severity of symptoms varies widely, even in families with the same disease causing mutation. In a detailed study (68g) lung disease, if present at birth, manifests as a surfactant deficiency syndrome and can be fatal. Asthma, recurrent pneumonia in childhood, spontaneous pneumothorax, and interstitial lung disease has also been reported. Neurologic forms present with muscular hypotonia in early infancy and psychomotor delay, which progresses to benign hereditary chorea between 1 and 5 years. Additional non classical features including hypodontia o oligodontia, microcephaly, growth retardation, genitourinary abnormalities, skeletal disorders, and congenital heart defects have been reported in patients with large deletions on chromosome 14, including the NKX2-1 gene and surrounding genes. Interestingly, a more extended phenotype associating hypothalamic symptoms, frequent recurrence of fever without infection, dysrhytmic sleep, and abnormal height in patients with point NKX2-1 mutations was described (68g). So far, 116 NKK2-1 genetic anomalies have been reported worldwide (68h).
PAX8 (OMIM218700)
Paired box gene 8 (PAX8) codes for a TTF of the paired homeodomain transcription factors family. PAX8 is expressed during thyroid organogenesis in the median anlage and in the kidney development. In synergy with NKX2-1, PAX 8 influences the expression of TPO, TG and NIS in thyroid follicular cells. The prevalence of PAX8 mutations in CH patients is about 1%, ranging from 0.3 to 3.4% (68b,68i).Thyroid hypoplasia is the more common phenotype, but athyreosis to normal morphology have also been reported. Thyroid function varies from severe hypothyroidism to mild hypertropinemia, and different phenotypes can be found in the same family. The association with kidney malformations is possible, but remains a facultative sign in CH patients with PAX8 mutations. So far, 29 mutations have been reported (68h).
FOXE1 (OMIM#602617)
The Forkhead Box 1 E1 (FOXE1) gene encodes for a transcription factor of the forkhead/winged-helix transcription factor family. Foxe1 is expressed in the thyroid primordium, in the pharyngeal endoderm derivates such as the palate and the esophagus and in the hair follicoles (68j). Foxe1 interacts with TG and TPO promoters and with regulatory regions of DUOX2 and NIS genes (68k).
The Bamforth-Lazarus syndrome is caused by FOXE1 mutations. It is characterized by CH (usually athyreosis), cleft palate and spinky hair. Bifid epiglottis and choanal atresia can be present. So far, six mutation with loss of function (68h) and 1 mutation with gain of function have been reported in patients with Bamforh-Lazarus syndrome, showing the effect of FOXE1 gene dosage in this disorder (68m).
NKX2-5 (OMIM #600584)
Because an increased prevalence of heart congenital malformations have been reported in CH, genes involved in heart organogenesis as NKX2-5 have been suggested as a cause of CH. NKX2-5, that encodes for a transcription factor with a major role in heart development has been investigated in a cohort of 241 patients with thyroid dysgenesis. Heterozygous missense mutations had been reported in this study in 4 patients with ectopy and athyreosis, and all mutations were transmitted from one of the parents but only 1 patient had minor cardiac phenotype (68n).
A major pathogenetic role of NKX2-5 mutations in thyroid dysgenesis has been questioned: given the absence of TD in carriers of NKX2-5 mutations, and the high number of TD patients without mutations. Better defining the role of NKX2-5 in thyroid organogenesis need further studies (68o).
New Candidates Genes
NTN-1
A new gene Netrin-1 (NTN-1), has been recently identified in a patient with thyroid ectopy and ventricular sept defect, and considered as a possible link between thyroid and heart defects (68p).
JAG1 (20p12.2 OMIM 6019220)
A role for the Notch pathway in thyroid morphogenesis has recently been demonstrated in zebrafish (68q). JAG1 is a gene encoding one single pass transmembrane ligand of the notch receptors. Heterozygous variations of JAG1 are the cause of Alagille syndrome type 1, an autosomal dominant disorder characterized by paucity of intrahepatic bile ducts, cardiac malformations as pulmonary artery stenosis, coarctaction of aorta, atrio-ventricular septal defects and Fallot tetralogy. Many other organs as eye, skeleton, kidney, nervous system can be involved, with a characteristic facial phenotype. A study investigating the role of JAG1 loss of function variations in the pathogenesis of congenital thyroid defects in Alagille syndrome and in patients with congenital hypothyroidism supported the role of this gene as a predisposing factor in congenital hypothyroidism (68r). The authors reported, in a series of 21 patients affected with Alagille syndrome non autommune hypothyroidism in 6 patients (28%), two of them with thyroid hypoplasia. Analyzing 100 patients with congenital hypothyroidism for JAG1 variants they found JAG1 variants in 4. Interestingly, 2 of them had cardiac malformations.
Inborn Errors of Thyroid Hormonogenesis
Inborn errors of thyroid hormonogenesis (thyroid dyshormonogenesis) are responsible for most of the remaining cases (15%) of neonatal thyroidal hypothyroidism. Unlike thyroid dysgenesis, mostly a sporadic condition, these inborn errors of thyroid hormonogenesis are commonly associated with an autosomal recessive form of inheritance, consistent with a single gene abnormality. DUOX2 mutations can be transmitted in autosomal dominant way. Thyroid dysormonogenesis is caused by genetic defects in proteins involved in all steps of thyroid hormone synthesis (68s) often associated with goiter formation. Goiter can be present in utero or at birth.
.A number of different defects have been characterized based on radioiodine uptake and perchlorate test and include:
1) Iodide transport defect (ITD)
(SLC5A5, Sodium/Iodide Symporter NIS), that shows failure to concentrate iodide, with low or absent radioiodine uptake, also in salivary glands and gastric mucosa;
2) Iodide organification defect (IOD)
with normal radioiodine uptake and altered perchlorate discharge test. In these patients, less than 90% of the iodide is organified and remains stored in the follicles. Total IOD is defined as >90% of the given dose back to the blood. Partial IOD is defined as 10-90% of radioiodine washout after perchlorate application. Total IOD is due to Thyroid peroxidase mutations (TPO) and Dual Oxidase 2 (DUOX2), partial IOD is due to DUOX2, Dual Oxidase Maturation Factor 2 mutations (DUOX2A), SLC26A4, pendrin and TPO defects.
3) Forms with normal radioiodine uptake and a normal perchlorate test:
Thyroglobulin TG mutations, iodide recycling defects IYD, Iodothyrosine Deiodinase mutations (DEHAL1).
4) Iodide Transport Defect (OMIM 274400)
ITD is rather a rare form and is due a mutation of the Sodium/Iodide Symporter (NIS). The NIS is expressed at the basolateral membrane of the thyrocite and it is responsible for the active iodide uptake through the membrane into the thyrocite (69). This form of hypothyroidism is characterized by goiter and absence of radioiodine uptake. In contrast with athyreosis, uptake is lacking also in salivary glands and in the stomach (white scintigraphy).
The severity of hypothyroidism depends on the residual function of the mutated NIS protein, ranging with severe to mild forms, often detected in infancy or childhood.
Pendred Syndrome (OMIM274600)
Pendred syndrome is defined by the association of familial profound deafness with multinodular goiter. It is caused by biallelic mutation in the pendrin gene (70-71). Pendred syndrome is the only form of thyroid dyshormonogenesis associated with a malformation. The inner ear presents a characteristic malformation of the cochlea.
Congenital hypothyroidism is present in only 30% of cases, goiter occurs often in childhood. Thyroid phenotype is variable. Perchlorate test shows a partial organification defect. Pendred syndrome is the most frequent etiology of familial deafness. SLC264A mutations (mostly in the heterozygous state) have been also described in isolated enlargement of the vestibular aqueduct, with no thyroid disease (71a). More than 150 mutations have been described. Specific mutation cluster in Asia (H723R), and Europe (L236P, T416P, IVS8, 1-GA) (71b).
Thyroid peroxidase mutations (OMIM #274500)
Thyroid peroxidase (TPO) is a heme peroxidase that regulates two rate-limiting step of thyroid hormones synthesis, first the organification of iodide to iodinated thyrosyl residuates such as MIT and DIT, and then the coupling of MIT and DIT to T3 and T4. TPO action needs hydrogen peroxide as the final electron acceptor. Mutations are mostly in the heme-binding domain of the protein, encoded by exons 7-9 (71c). TPO mutations are a common form of thyroid dyshormonogenesis. Severe congenital hypothyroidism with goiter is present in the great part of patients, with a total IOD. Recently, a few patients with partial IOD have been reported (72,72a).
Dual Oxidase 2 and Dual Oxidase Maturation Factor 2 mutations (OMIM#607200 and 274900)
DUOX2 (formerly THOX2) and DUOXA2 are components of a nicotinamide adenine dinucleotide phosphate oxidase complex that produces hydrogen peroxide indispensable for TPO action.
The first mutation in DUOX2 has been reported in 2002. Heterozygous mutations have been found in a part of the patients, suggesting autosomal dominant and autosomal recessive inheritance both possible in this form (72b). Monoallelic mutations usually cause mild hypothyroidism; biallelic mutations are present in mild to severe hypothyroidism. In some cases, DUOX2 mutations lead to transient congenital hypothyroidism, with normalization of thyroid function at follow up. DUOX2 mutations usually cause partial IOD, but total IOD is also reported (72c). Mutations in DUOXA2 were described in patients detected by neonatal screening with mild CH. Partial IOD was found in these cases (72d).
Thyroglobulin Mutations (OMIM#274700)
Thyroglobulin (Tg) is a glycoprotein synthetized by the thyrocytes that serves as a matrix for thyroid hormones synthesis and storage in the follicles (68t). Tg is also in part released in the blood and it is a useful marker of thyroid tissue.
In CH, Tg serum determination can differentiate between a true and apparent athyreosis, the last with same residual dysgenetic tissue and Tg detectable.
In dyshomonogenesis, Tg levels are low in patients with Tg mutations, but are normal or high in the other defects of hormonogenesis (68t). CH due to Tg mutations is usually severe, with goiter in utero or at birth. Different mechanisms cause hypothyroidism in Tg mutations: a )Tg synthesis defects alter protein synthesis; b) Tg transport defects limit Tg excretion in the follicle; c) a abnormal structure of T impairs coupling of MIT and DIT; d) a large imperfect DNA inversion in Tg gene is a novel cause for CH (72e-72g).
Iodothyrosine Deiodinase Mutations cause Iodide Recycling defects (OMIM#274800)
DEAHAL 1(IYD) is the enzyme that regulates the recycling of iodide from MIT and DIT to the follicle, thus allowing the synthesis of thyroid hormones. Dietary Iodine is scarce in nature and it is the limitating factor for thyroid hormones synthesis. Failure of DEAHL1 cause iodotyrosine deiodinase deficiency, characterized by hypothyroidism, goiter and mental retardation. It is important to stress that these patients are not detected by neonatal screening for CH, probably because the maternal iodine protect for a period the newborn. Diagnosis is reported between 18 month and 16 years with hypothyroidism and mental retardation (72h). The first mutations in DEAHAL1 has been reported in 2008 (72i).
The use of MIT and DIT -as early markers to identify iodotyrosine deiodinase deficiency before mental retardation-is under investigation.
Central Congenital Hypothyroidism (CCH)
Central hypothyroidism (CCH) is caused by an insufficient thyroid hormone biosynthesis due to a defective stimulation by TSH, in the presence of an otherwise normal thyroid. This condition includes all causes of congenital hypothyroidism due to a pituitary or hypothalamic pathology (secondary or tertiary hypothyroidism). CCH was previously considered a very rare disease with a prevalence initially estimated to be 1:100000 in newborns (73). In more recent data, CCH had an incidence that could reach 1:16.000, as shown from results from screening for congenital hypothyroidism applied in the Neetherlands, based on T4/TSH/TBG determination (73a).
Also with this sophisticated method of screening, CCH is sometime not identified at birth, because the limiting step is “how low is a low T4”, low enough to be considered an effective cutoff value and allow the determination of TSH and TBG. Many cases are diagnosed in infancy or childhood, if not later in adulthood (73b). The majority of screening programs are based on TSH determination and a high index of suspicion is needed to identify CCH in the preclinical phase. Delayed diagnosis may result in neurodevelopment delay. More than 50% of children with CCH have moderate or severe hypothyroidism, so, if not treated, the risk of neurodevelopmental delay should not be underestimated (73c).
In the majority of cases identified early, TSH deficiency is a part of combined pituitary hormone deficiency. A timely correction of ACTH and cortisol deficiency, and/or GH deficiency may avoid life threatening emergencies.
CCH can be transient (mostly due to drugs or maternal hyperthyroidism), or permanent.
Genetic Central Hypothyroidism (Table 1)
Isolated Thyroid Stimulating Hormone deficiency
Two forms of non-efficient TSH are known, the first one is very rare and is due to defects in the receptor that regulates the action of TRH on thyrotropes (TRHR), the second form is due to several mutations in the β-subunit of TSH.
a)Thyrotropin-releasing hormone receptor (TRHR ) gene defects. TRHR mediates the correct action of TRH on thyrotropes toward the synthesis, glycosylation and secretion of TSH.
This is a very rare cause of central hypothyroidism. Mutations in TRHR gene have been described so far in 3 patients from 2 families, the first from Canada, the second from Italy, with autosomal recessive inheritance (73d,73e). Index cases were detected at 9 and 11 years for short stature and symptoms related to hypothyroidism. Neonatal hypothyroidism could not be proven because neonatal screening was based on TSH level. No psychomotor delay or intellectual deficit was reported in these children. TSH was in the low normal range with a suspected low bioactivity; T4 or FT4 were low, TRH test showed no response of TSH and PRL.
A compound heterozygosis with 2 different mutations in TRHR gene was found in the Canadian patient. The first mutation in the paternal allele was a premature stop codon R17X that completely inactivated protein function. The second one, on the maternal allele was a complex combination of mutations: 9-nucleotide deletion followed by a point mutation resulting in an in-frame deletion of three aminoacids (Ser115-Thir117) plus a missense change located at the cytoplasmatic end of the transmembrane domain of the receptor (73d).The Italian patient had a homozygous nonsense mutation (pR17X).
A novel homozygous missense mutation (P81R) in TRHR has been published in a female infant presented at age 19 days with prolonged jaundice due to isolated hyperbilirubinemia. Thyroid function showed CCH (TSH 2.2 mU/L (RR 0.4-3.5). FT4 7.9 pmol/L (RR10.7-21.8). She was treated with L-thyroxine and at 4 years of age growth and neurological development are in the normal range. The location of the mutated aminoacid (proline 81) in the second transmembrane helix underlines the functional role of this helix in hormone binding and receptor activation (73f).
b)TSH β Gene defects
TSH is a glycoprotein hormone with an α subunit common with FSH, LH and hCG, and a β-subunit, specific for TSH.
Mutations of the β-subunit of TSH are the cause of the most severe forms of central congenital hypothyroidism. All mutations described so far caused central hypothyroidism, either because truncated protein or alterations in key structural features required for heterodimeric integrity occur (74, 74a, 74b).
Another consequence of mutations of the β-subunit of TSH is the modification of bioactivity and immunoreacivity of the TSH heterodimer. Diagnosis of central hypothyroidism can be complicated because of impaired TSH immunoreactivity and/or bioactivity. For instance, TSH is not detectable when the heterodimer formation between TSHα and TSH β subunit is completely not allowed from mutations (i.e. p.G49, p.32), in other cases some mutant heterodimeric TSH is present and measurable in an immunoassay dependent manner (i. e. p.Q69, c.373 delT). TSH can be measurable but not shows normal bioactivity (74a). Interestingly, a variant (c223A>G, pR75) causing normal bioactive TSH, but with impaired immunoreactivity has been described (74c, 74d). These individuals are euthyroid, but erroneous diagnosis and inappropriate treatment have been reported.
In children affected with CCH due to mutations of the β-subunit of TSH, psychomotor and mental retardation can occur, depending on the time of diagnosis and treatment. Most are clinically diagnosed after 3 months of age because they are not identified by neonatal screening based on increased TSH levels. Hyperplastic pituitary, high levels of serum glycoprotein alfa-subunit and hypoplasic thyroid gland have been reported (74a). Several mutations have been reported, including missense, nonsense and frameshift mutations (74,74a), as well as slice mutations (74b). Recently a homozygous TSHβ mutation was found (74e). A novel missense mutation (c.2T>C) in which a methionin codon, is replaced by a threonine, has been very recently reported in a child with very low levels of TSH (0.45mU/l, (NR 0.4-3.5) and FT4.(<5.1 pmol/l (NR 13.8-22.5). This child was diagnosed at 3.5 months of age because feeding difficulties, somnolence, constipation and severe growth retardation. She was treated with L-thyroxine with a good response in growth, but she has severe neurodevelopmental deficits, with bilateral sensorineural deafness, nistagmus, motor and language development delay at age of 10. She was on autistic/Asperger spectrum and needed special education at school (74f).
Immunoglobulin superfamily member1 (IGSF1) gene defects
IGSF1 (immunoglobulin (Ig) superfamily member1) gene mutations were described in 2012 as a cause of central hypothyroidism, with an incidence of about 1:100.000 (75,75a). IGSF1 gene is located on X chromosome (Xq26.1) and encodes for a plasma membrane glycoprotein that is mainly expressed in the pituitary, brain and testes.
Several pathogenetic mutations in IGSF1 gene have been reported so far (75,75a,75b). An extensive case series, expanding the clinical phenotype has been published very recently (75c,75d). The first patient was diagnosed by neonatal screening in the Neetherlands where a screening program for congenital hypothyroidism that includes T4 determination (T4/TBG/TSH) is applied. Many other cases of central hypothyroidism were identified in this family and in others with an age at diagnosis ranging from 3 weeks to 69.9 years (75). Typical phenotype in adult males includes central hypothyroidism and macroorchidism (>30 ml by Prader orchidometer). Hypoprolactinemia and GH deficiency can be present. GH deficiency is usually transient and detectable in childhood. Body mass index tends to be elevated. Testicular volume is normal in childhood and increases at a normal age in puberty, but the testosterone rise is delayed, as well as the pubertal growth spurt and the appearance of secondary sexual characteristics. Thyroid volume is small, TSH is usually detectable, TSH response to TRH is diminished. No clear correlation genotype-phenotype has been established.
IGSF1 gene is located on X chromosome. Male are affected but 1/3 of females heterozygous carriers shows a milder phenotype, with central hypothyroidism, delayed menarche, mild prolactin deficiency and benign ovarian cysts sometime requiring surgical resection. Recently a familial form of isolated central hypothyroidism with neurological phenotypes due to a novel IGSF1 gene mutation has been reported from Israel (75e).
TSH deficiency in combined pituitary hormone deficiency
Central congenital hypothyroidism can be a component of combined pituitary hormone deficiency. This form represents the majority of cases detected by the neonatal screening when T4 determination is used (73b). Early diagnosis in these cases helps to prevent dangerous hypoglycemic and adrenal crisis due to associated GH and ACTH deficiency.
TSH deficiency can be present at diagnosis or occurs later, as a component of an evolving phenotype. In a minority of patients, mutations of known transcriptor factors (i.e POU1F1 ,PROP1,HESX1,LHX3,LHX4,SOX3 and OTX2) that are involved in pituitary development can be identified (76) (See Table 1).
Mutations in early transcriptor factors cause developmental abnormalities, i.e., septo-optic dysplasia, midline defects, holoprosencephaly, ocular or skeletal defects, intellectual impairment, associated with variable hypopituitarism. Mutations in HESX1, OTX2 and SOX3 have been found in patients with septo-optic dysplasia and TSH deficiency (76).
TSH deficiency in association with other pituitary hormone deficiencies may be associated with abnormal midline facial and brain structures (particularly cleft lip and palate, and absent septum pellucidum and/or corpus callosum) and should be suspected in any male infant with microphallus and persistent hypoglycemia (76a). One of the more common of these syndromes, septo-optic dysplasia, has been related in some cases to a mutation in the HESX 1 homeobox gene in some cases (76b). Other genetic causes of congenital hypopituitarism include molecular defects in the genes for the transcription factors LHX3 (76c), LHX4, POU1F 1 (76d) or PROP 1 (76d). POU1F 1 (Pit-1 in mice) is essential for the differentiation of thyrotrophs, lactotrophs and somatotrophs while PROP 1, a homeodomain protein that is expressed briefly in the embryonic pituitary, is necessary for POU1F 1 expression.
Defects of Thyroid Hormone Transport in Serum
For complete coverage of this and related areas visit the chapter entitled: “Defects of thyroid hormone transport in serum” by Samuel Refetoff, MD in this book.
Inherited abnormalities of the iodothyronine-binding serum proteins include TBG deficiency (partial or complete), TBG excess, transrethyretin (TTR) (prealbumin) variants and familial dysalbuminemic hyperthyroxinemia (FDH). In these conditions the concentration of free hormones is unaltered, but the abnormal total thyroxine concentrations can be misleading during neonatal screening and in the evaluation of thyroid function.
Impaired Sensitivity to Thyroid Hormone
For complete coverage of this and related areas visit the chapter entitled: “Impaired sensitivity to thyroid hormone: defects of transport, metabolism and action” by.Alexandra M. Dumitrescu, MD and Samuel Refetoff, MD, in this book.
Impaired sensitivity to thyroid hormone, previously known as “reduced sensitivity to thyroid hormone”, include defects in thyroid hormone action, transport and metabolism. They are classified in a)Thyroid hormone cell membrane transport defect (THCMTD),b) thyroid hormone metabolism defect (THMD) and c) thyroid hormone action defect that include Resistance to thyroid hormone (RTH) (77).The first defect, recognized almost 50 years ago, produces reduced sensitivity to TH and was given the acronym RTH, for resistance to thyroid hormone (77a, 77b). Its major cause, found in more than 3,000 individuals, is mutations in the TH receptor ß (THRB) gene. More recently mutations in the THRA gene were found to produce a different phenotype owing to the distinct tissue distribution of this TH receptor (77c, 77d). Two other gene mutations, affecting TH action, but acting at different sites have been identified in the last 10 years. One of them, caused by mutations in the TH cell-membrane transporter MCT8, with decreased T4 uptake into brain cells produces severe psychomotor defects (77e,77f). In this syndrome, first described as Allan Herdon Dudley syndrome, (77g) mutations in the monocarboxylate transporter 8 (MCT 8 gene, located on the X-chromosome), have been associated with male- limited hypothyroidism and severe neurological abnormalities, including global developmental delay, dystonia, central hypotonia, spastic quadriplegia, rotary nystagmus and impaired gaze and hearing (77e, 77f). Heterozygous females had a milder thyroid phenotype and no neurological defects. A defect of the intracellular metabolism of TH, identified in 11 members from 9 families, is caused by mutations in the SECISBP2 gene required for the synthesis of selenoproteins, including TH deiodinases (77h). Knowledge of the molecular mechanisms involved in mediation of TH action allows the recognition of the phenotypes caused by genetic defects in the involved pathways. While these defects have opened the avenue for novel insights into thyroid physiology, they continue to pose therapeutic challenges.
CAUSES OF TRANSIENT NEONATAL HYPOTHYROIDISM
Transient neonatal hypothyroidism should be distinguished from a ‘false positive’ result in which the screening result is abnormal but the confirmatory serum sample is normal. Causes of transient abnormalities of thyroid function in the newborn period are listed in Table 2. While iodine deficiency, iodine excess, drugs and maternal TSH receptor blocking antibodies are the most common causes of transient hypothyroidism, in some cases the cause is unknown.
TABLE 2. CAUSES OF TRANSIENT HYPOTHYROIDISM IN THE NEWBORN
| · | 2.1 PRIMARY HYPOTHYROIDISM |
| · | · Prenatal or postnatal iodine deficiency or excess |
| · | · Maternal antithyroid medication |
| · | · Maternal TSH receptor blocking antibodies |
| · | · Mild gene mutations (i.e. DUOX2, TSH-R ) |
| · | · Maternal hypothyroidism |
| · | · Prematurity, VLBW |
| · | · Drugs, (i.e. Dopamine, steroids) |
| · Hypothyroxinemia (low T4, normal TSH) | |
| · | 2.2 CENTRAL HYPOTHYROIDISM |
| · | · Prenatal exposure to maternal hyperthyroidism |
| · | · Prematurity (particularly <27 weeks gestation) |
| · | · Drugs |
Iodine Deficiency or Excess
In addition to iodine deficiency, both the fetus and newborn infant are sensitive to the thyroid-suppressive effects of excess iodine, whether administered to the mother during pregnancy, lactation or directly to the baby (78). This occurs, in part because, as noted earlier, recovery from the thyroid-suppressive effect of iodine does not mature before 36 weeks gestation; however, other factors, including increased skin absorption are also likely to play a role. Reported sources of iodine have included drugs (e.g., potassium iodide, amiodarone), radiocontrast agents and antiseptic solutions (e.g., povidone-iodine) used for skin cleansing or vaginal douches. In contrast to Europe, iodine-induced transient hypothyroidism has not been documented frequently in North America (79). For other information see the chapter “Iodine deficiency disorders” in this book.
Maternal Antithyroid Medication
Transient neonatal hypothyroidism may develop in babies whose mothers are being treated with antithyroid medication (either propylthiouracil, PTU or methimazole, MMI) for the treatment of Graves ’ disease. Even maternal PTU doses of 200 mg or less have been associated with an effect on neonatal thyroid function, illustrating the increased fetal sensitivity to these drugs (80). Babies with PTU- or MMI-induced hypothyroidism characteristically develop an enlarged thyroid gland and if the dose is sufficiently large, respiratory embarrassment may occur. Both the hypothyroidism and goiter resolve spontaneously with clearance of the drug from the baby’s circulation. Usually replacement therapy is not required.
Maternal TSH Receptor Antibodies
Maternal TSH receptor blocking antibodies, a population of antibodies closely related to the TSH receptor stimulating antibodies in Graves’ disease, (81) may be transmitted to the fetus in sufficient titer to cause transient neonatal hypothyroidism. The incidence of this disorder has been estimated to be 1 in 180,000 (81a,81b). TSH receptor blocking antibodies most often are found in mothers who have been treated previously for Graves’ disease or who have the non goitrous form of chronic lymphocytic thyroiditis (primary myxedema). Occasionally these mothers are not aware that they are hypothyroid and the diagnosis is made in them only after congenital hypothyroidism has been recognized in their infants (81b). Unlike TSH receptor stimulating antibodies that mimic the action of TSH, TSH receptor blocking antibodies inhibit both the binding and action of TSH (see below). Because TSH-induced growth is blocked, these babies do not have a goiter. Similarly, inhibition of TSH-induced radioactive iodine uptake may result in a misdiagnosis of thyroid agenesis (81c). Usually the hypothyroidism resolves in 3 or 4 months. Babies with TSH receptor blocking-antibody induced hypothyroidism are difficult to distinguish at birth from the more common thyroid dysgenesis but they differ from the latter in a number of important ways (Table 3). They do not require lifelong therapy, and there is a high recurrence rate in subsequent offspring due to the tendency of these antibodies to persist for many years in the maternal circulation. Unlike babies with thyroid dysgenesis in whom a normal cognitive outcome is found if postnatal therapy is early and adequate, babies with maternal blocking-antibody induced hypothyroidism may have a permanent deficit in intellectual development if feto-maternal hypothyroidism was present in utero (27).
TABLE 3. CLINICAL FEATURES OF THYROID DYSGENESIS VERSUS TSH RECEPTOR
- BLOCKING ANTIBODY INDUCED CONGENITAL HYPOTHYROIDISM
| Dysgenesis | Blocking Ab | |
| Severity of CH | + to ++++ | + to ++++ |
| Palpable thyroid | No | No |
| 123I uptake | None to low | None to normal |
| Clinical Course | Permanent | Transient |
| Familial risk | No | Yes |
| TPO Abs |
Variable
eele |
Variable |
| TSH Receptor Abs | Absent | Potent |
Transient Central Hypothyroidism Due to Maternal Hyperthyroidism
Occasionally, babies born to mothers who were hyperthyroid during pregnancy develop transient hypothalamic-pituitary suppression (81,81a,81b,81c). This hypothyroxinemia is usually self-limited, but in some cases it may last for years and require replacement therapy (82). In general the titer of TSH receptor stimulating antibodies in this population of infants is lower than in those who develop transient neonatal hyperthyroidism (see below).
Prematurity
Hypothyroxinemia in the presence of a ‘ normal ’ TSH occurs most commonly in premature infants in whom it is found in 50% of babies of less than 30 weeks gestation. Often the free T4 when measured by equilibrium dialysis is less affected than the total T4 (83). The reasons for the hypothyroxinemia of prematurity are complex. As well as hypothalamic-pituitary immaturity mentioned earlier, premature infants frequently have TBG deficiency due to both immature liver function and undernutrition, and they may have “sick euthyroid syndrome”. They may also be treated with drugs that suppress the hypothalamic-pituitary-thyroid axis. Hypothyroxinemia of prematurity may be associated with adverse neurodevelopmental outcomes. L-T4 treatment overall has no proven benefit and can be harmful (83a). Long term outcome evaluation in young adults did not find association between transient hypothyroxinemia of prematurity and neurodevelopmental outcome (83b). Whether or not premature infants with hypothyroxinemia should be treated remains controversial at the present time (83c,83d,83e). Although several retrospective, cohort studies have documented a relationship between severe hypothyroxinemia and both developmental delay and disabling cerebral palsy in preterm infants <32 weeks gestation a causal relationship could not be determined since the serum T4 in premature infants, as in adults, has been shown to reflect the severity of illness and risk of death (83c).
Drugs
Drugs that suppress the hypothalamic-pituitary axis include known agents such as steroids and dopamine, but also aminophylline, caffeine and diamorphine, other commonly used in sick premature infants (84).
Other causes of hypothyroidism in infancy
Chronic lymphocytic thyroiditis
Chronic lymphocytic thyroiditis (CLT) is a rare disease in infancy, but if not recognized and treated, can cause severe hypothyroidism in a short time with permanent brain damage (85). CLT can be associated with other autoimmune disease as type 1 diabetes or a manifestation of IPEX syndrome (85a). In the cases described by Foley, no goitrous was found. Clinical manifestations and biochemical hypothyroidism (TSH ranged from >42 to 928 mU/L) were severe and very high levels of antibodies were detectable.
IPEX related disorders
Lymphocytic thyroiditis has also been described in newborns with severe defects in tolerance and autoimmunity with immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome, a polyglandular disorder characterized by early-onset diabetes and colitis (85a,85b). IPEX disorders are an expanding spectrum of disease with mutations in FOXP3, CD25 deficiency, STAT5 deficiency and other.
Hepatic hemangiomas: consumptive hypothyroidism
Hepatic emangioendothelioma is a rare tumor typically presenting in infancy. Hypothyroidism is caused by a production of type 3 deiodinase by the vascular tumor (85c). D3 deoidinase increases inactivation of T4 and T3 to reverse T3 andT2 and large amount of LT4 (up to 94/ µg/kg/day) are needed to compensate this inactivation (85d). Frequent monitoring is required, adapting the LT4 treatment to the growing proliferative phase of the tumor. Today hemangioendotheliomas in infancy may successfully being treated with steroids and propranolol and may undergo spontaneous regression. Some babies underwent liver transplantation.
HYPERTHYROIDISM
Transient Neonatal Hyperthyroidism
Unlike congenital hypothyroidism which usually is permanent, neonatal hyperthyroidism almost always is transient and results from the transplacental passage of maternal TSH receptor antibodies. Hyperthyroidism develops only in babies born to mothers with the most potent stimulatory activity in serum (86,87,87a). This corresponds to 1-2% of mothers with Graves’ disease, or 1 in 50,000 newborns, an incidence that is approximately four times higher than is that for transient neonatal hypothyroidism due to maternal TSH receptor blocking antibodies (81a). Some mothers have mixtures of stimulating and blocking antibodies in their circulation, the relative proportion of which may change over time. Not surprisingly, the clinical picture in the fetus and neonate of these mothers is more complex and depends not only on the relative proportion of each activity in the maternal circulation at any one time but on the rate of their clearance from the neonatal circulation postpartum. Thus, one affected mother gave birth, in turn, to a normal infant, a baby with transient hyperthyroidism, and one with transient hypothyroidism (87b). In another neonate, the onset of hyperthyroidism did not become apparent until 1-2 months postpartum when the higher affinity blocking antibodies had been cleared from the neonatal circulation (87c). In the latter case, multiple TSH receptor stimulating and blocking antibodies were isolated from the maternal peripheral lymphocytes. Each monoclonal antibody recognized different antigenic determinants (“epitopes”) on the receptor and had different functional properties (87d).
Occasionally, neonatal hyperthyroidism may even occur in infants born to hypothyroid mothers. A prospective study showed that 40% of patients treated for Graves’ disease with radioactive iodine had TRAb detectable after 5 years (87e). In these situations, the maternal thyroid has been destroyed either by prior radioablation, surgery or by coincident destructive autoimmune processes so that potent thyroid stimulating antibodies, present in the maternal circulation, are silent in contrast to the neonate whose thyroid gland is normal (87d). Fetal/neonatal thyrotoxicosis can occur also in newborn from hypothyroid mothers with chronic lymphocytic thyroiditis (87f).
Clinical manifestations
Maternal TSH receptor antibody-mediated hyperthyroidism may present in utero. Fetal hyperthyroidism is suspected in the presence of fetal tachycardia (pulse greater than 160/min) especially if there is evidence of failure to thrive. Obstetric complications are common. Fetal goiter can by monitored by ultrasound. In the neonate infant most often the onset is during the first one-two weeks of life but can occur by 45 days. This is due both to the clearance of maternally-administered antithyroid drug (propylthiouracil, PTU, methimazole or carbimazole) from the infant ’s circulation and to the increased conversion of T4 to the more metabolically active T3 after birth. Rarely, as noted earlier, the onset of neonatal hyperthyroidism may be delayed until later if higher affinity blocking antibodies are also present. In the newborn infant, characteristic signs and symptoms include tachycardia, irritability, poor weight gain, and prominent eyes (Figure 5). Goiter, when present, may be related to maternal antithyroid drug treatment as well as to the neonatal Graves’ disease itself.

Figure 15.5. A baby with neonatal hyperthyroidism secondary to maternal Graves ‘disease. Note the prominent eyes in the baby and mother in whom Graves’ disease developed after radioiodine therapy for Hodgkin’s disease. In contrast, the father was unaffected.
Rarely, infants with neonatal Graves’ disease present with thrombocytopenia, jaundice, hepatosplenomegaly, and hypoprothrombinemia, a picture that may be confused with congenital infections such as toxoplasmosis, rubella, or cytomegalovirus (87g). In addition, arrhythmias and cardiac failure may develop and may cause death, particularly if treatment is delayed or inadequate. In addition to a significant mortality rate that approximates 20% in some older series, untreated fetal and neonatal hyperthyroidism is associated with deleterious long-term consequences, including premature closure of the cranial sutures (cranial synostosis), failure to thrive, and developmental delay (87h). The half-life of TSH receptor antibodies is 1 to 2 weeks. The duration of neonatal hyperthyroidism, a function of antibody potency and the rate of their metabolic clearance, is usually 2 to 3 months but may be longer.
Laboratory Evaluation
TSH receptor antibodies (TRAb) are Immunoglobulin of G class and freely cross the placenta. Different type of TRAb can be found: TRAb that bind to the TSH receptor and stimulates the production of thyroid hormones, (TSH receptor stimulating antibodies, TSI), TRAb that bind to the TSH receptor, do not stimulate the production of thyroid hormones and can block the binding of TSH (TSH receptor blocking antibodies TBI) .TSH receptor neutral antibodies have also been identified which do not block TSH binding and are unable to stimulate cAMP production (88)..
The receptor binding assays usually used to measure TRAb are not able to distinguish between TSH-receptor stimulating and blocking or neutral antibodies. Bioassays that measure TSI activity based on cAMP on cultured cells can be useful if TRAb are not detectable (88a,88b). The recent guidelines for management of hyperthyroidism (88c) and the updated guidelines for the management of thyroid disease during pregnancy released from the American Thyroid Association ATA (33b) both suggest to anticipate the determination of TRAb in pregnant women with Graves’ disease at 18-22 weeks instead of 20-24 weeks of gestation because a severe case of fetal Graves’ disease has occurred at 18 weeks of pregnancy (88d).
Because of the importance of early diagnosis and treatment, infants at risk for neonatal hyperthyroidism should undergo both clinical and biochemical assessment as soon as possible.
All neonates born from a woman with TRAb positivity in pregnancy should undergo determination of TRAb from cord blood at delivery. If TRAb are negative, the risk to neonatal hyperthyroidism is negligible (Sensitivity is around 100%). FT3, FT4 and TSH determination from cord blood did not predict neonatal hyperthyroidism. Determination of FT4 increase on day 3 to 5 seems to better indicate the onset of hyperthyroidism (88e) Situations that should prompt consideration of neonatal hyperthyroidism are listed in Table 4. A high index of suspicion is necessary in babies of women who have had thyroid ablation because in them a high titer of TSH receptor antibodies would not be evident clinically. Similarly, women with persistently elevated TSH receptor antibodies and with a high requirement for antithyroid medication are at an increased risk of having an affected child. The diagnosis of hyperthyroidism is confirmed by the demonstration of an increased concentration of circulating T4 (and free T4, and T3, if possible) accompanied by a suppressed TSH level in neonatal or fetal blood. The latter can be obtained by cordocentesis if someone experienced in this technique is available. Results should be compared with normal values during gestation. Fetal ultrasonography may be helpful in detecting the presence of a fetal goiter and in monitoring fetal growth. Demonstration in the baby or mother of a high titer of TSH receptor antibodies will confirm the etiology of the hyperthyroidism and, in babies whose thyroid function testing is normal initially, indicate the degree to which the baby is at risk.
TABLE 4. SITUATIONS THAT SHOULD PROMPT CONSIDERATION OF NEONATAL HYPERTHYROIDISM
| · Unexplained tachycardia, goiter or stare |
| · Unexplained petechiae, hyperbilirubinemia, or hepatosplenomegaly |
| · History of persistently high TSH receptor antibody titer in mother during pregnancy |
| · History of persistently high requirement for antithyroid medication in mother during pregnancy |
| · History of thyroid ablation for hyperthyroidism in mother |
| · History of previously affected sibling |
As noted in the case of TSH receptor blocking antibody-induced congenital hypothyroidism, the receptor binding assays are a cost-effective, rapid and technically feasible approach. In general, babies likely to become hyperthyroid have the highest TSH receptor antibody titer whereas if TSH receptor antibodies are not detectable, the baby is most unlikely to become hyperthyroid (87g, 89,89a). In the latter case, it can be anticipated that the baby will be euthyroid, have transient hypothalamic-pituitary suppression or have a transiently elevated TSH, depending on the relative contribution of maternal hyperthyroidism versus the effects of maternal antithyroid medication, respectively (89). Close follow up of all babies with abnormal thyroid function tests or detectable TSH receptor antibodies is mandatory.
Therapy
In the fetus, treatment is accomplished by maternal administration of antithyroid medication. Until recently PTU was the preferred drug for pregnant women in North America, but current recommendations suggest the use of MMI rather than PTU after the first trimester because of concerns about potential PTU-induced hepatotoxicity (123) (discussed under Graves’ disease, below). The goals of therapy are to utilize the minimal dosage necessary to normalize the fetal heart rate and render the mother euthyroid or slightly hyperthyroid.
In the neonate MMI (0.5 to 1.0 mg/kg/day) has been used initially in 3 divided doses. If the hyperthyroidism is severe, a strong iodine solution (Lugol’ s solution or SSKI, 1 drop every 8 hours) is added to block the release of thyroid hormone immediately. Often the effect of MMI is not as delayed in infants as it is in older children or adults, a consequence of decreased intrathyroidal thyroid hormone storage. Therapy with both antithyroid drug and iodine is adjusted subsequently, depending on the response. Propranolol (2 mg/kg/day in 2 or 3 divided doses) is added if sympathetic overstimulation is severe, particularly in the presence of pronounced tachycardia. If cardiac failure develops, treatment with digoxin should be initiated, and propranolol should be discontinued. Rarely, prednisone (2 mg/kg/day) is added for immediate inhibition of thyroid hormone secretion. Measurement of TSH receptor antibodies in treated babies may be helpful in predicting when antithyroid medication can be safely discontinued (87). Lactating mothers on antithyroid medication can continue nursing as long as the dosage of PTU or MMI does not exceed 400 mg or 40 mg, respectively. The milk/serum ratio of PTU is 1/10 that of MMI, a consequence of pH differences and increased protein binding, so one might anticipate less transmission to the infant, but concerns about potential PTU toxicity need to be considered. At higher dosages of antithyroid medication, close supervision of the infant is advisable.
A review about management of neonates born to mothers with Graves’ disease has been recently published (89b).
Permanent neonatal hyperthyroidism
Rarely, neonatal hyperthyroidism is permanent and is due to a germline mutation in the TSH receptor (TSH-R) resulting in its constitutive activation (90,90a,90b,90c). A gain of function mutation of the TSH-R should be suspected if persistent neonatal hyperthyroidism occurs in the absence of detectable TSH-R antibodies in the maternal circulation. Prematurity, low birth weight and advanced bone age are common. Most cases result from a mutation in exon 10 which encodes the transmembrane domain and intracytoplasmic tail of the TSH-R, a member of the G protein coupled receptor superfamily (90,90a,90b,90c). Less frequently, a mutation encoding the extracellular domain has been described (90d). An autosomal dominant inheritance has been noted in many of these infants; other cases have been sporadic, arising from a de novo mutation.
Early recognition is important because the thyroid function of affected infants is frequently difficult to manage medically (90a-90c), and, when diagnosis and therapy is delayed, irreversible sequelae, such as cranial synostosis and developmental delay may result (90c). For this reason early, aggressive therapy with either thyroidectomy or even radioablation has been recommended (90c).
Two clinical forms were described: the first one is the “familial non-autoimmune autosomal dominant hyperthyroidism” (FNAH). High variable age of manifestation from neonatal period to 60 years, with. variability also within the same family is reported. Goiter is present in children, with nodules in older age.
The second one is “Persistent sporadic congenital non autoimmune hyperthyroidism” (PSNAH) includes forms with sporadic (de novo) germline mutations in the TSH-R.
PSNAH is characterized by fetal-neonatal onset or within 11 months and more severe hyperthyroidism requiring early aggressive therapy. Guidelines about this rare condition have recently been published (90e).
McCune Albright syndrome
McCune Albright is a syndrome due to somatic activating mutations in Gsα gene, can rarely presents with neonatal hyperthyroidism (90f).
THYROID DISEASE IN CHILDHOOD AND ADOLESCENCE
Hypothyroidism and Thyroiditis
Chronic lymphocytic thyroiditis is the more common cause of acquired hypothyroidism in children and adolescents. Occasionally, patients with disorders classified as congenital hypothyroidism, i.e thyroid dysgenesis, inborn error of thyroid hormonogenesis, central hypothyroidism may be recognized later in childhood and adolescence.
Causes of hypothyroidism in children and adolescents are listed in table 5.
TABLE 5. CAUSES OF HYPOTHYROIDISM IN CHILDHOOD AND ADOLESCENCE
| PRIMARY HYPOTHYROIDISM |
| A) Congenital |
| Thyroid dysgenesis |
| Inborn error of thyroid hormonogenesis |
| Thyroidal Gsα protein abnormalities (pseudohypoparathyroidism 1B) |
| B) Acquired |
| Autoimmune |
| Chronic Lymphocytic Thyroiditis |
| Reversible autoimmune hypothyroidism (silent and postpartum thyroiditis, cytokine-induced thyroiditis |
| Infiltrative: Cystinosis, Hemocromathosis, Thalassemia,-Langerhans Cell Histiocytosis |
| Infective: acute, subacute thyroiditis |
|
Post ablative Surgery |
| Thyroiditis due to I 131, external irradiation of non-thyroidal tumors (i.e. lymphomas, brain tumors, TBI |
| Iodine deficiency and iodine excess |
| Drugs: antithyroid agents, lithium, natural and synthetic goitrogenic chemicals, tyrosine kinase inhibitors, lithium, thionamides, aminosalicylic acid, aminoglutethimide |
| Goitrogens (cassava, water pollutants, cabbage, sweet potatoes, cauliflower, broccoli, soya beans) |
| CENTRAL HYPOTHYROIDISM |
| A)Congenital |
| Pituitary hypoplasia, septo-optic dysplasia, basal encephalocele |
| Functional defects in TSH biosynthesis and release |
| Mutations in genes encoding for TRH receptor, TSHß, pituitary transcription factors (Pit-1, PROP1, LHX3, LHX4, HESX1), or LEPr, IGSF1 |
| B)Acquired |
| Tumors (pituitary adenoma, craniopharyngioma, meningioma, dysgerminoma, glioma, metastases) |
| Trauma surgery, irradiation, head injury |
| Infections |
| Vascular damage ischemic necrosis, hemorrhage, stalk interrruption, |
| Hypotalamic disorders |
| Drugs: dopamine; glucocorticoids; bexarotene; L-T4 withdrawal |
| “Peripheral” (extrathyroidal) hypothyroidism |
| Consumptive hypothyroidism (massive infantile hemangioma) |
| Mutations in genes encoding for MCT8, SECISBP2, TRα or TR β (impaired sensitivity to thyroid hormones) |
Chronic Lymphocytic Thyroiditis
Autoimmune thyroid diseases (AITD) are defined by the lymphocytic infiltration of the thyroid (91). Usually antibodies against thyroid antigens as thyroperoxidase (TPOAb), thyroglobulin (TgAb), and anti-TSH receptor (TRAb) are detectable in serum. Thyroid antibodies in serum correlate with the presence of lymphocytic infiltrate in the thyroid gland. The clinical spectrum of AITD ranges from hypothyroidism to hyperthyroidism and include chronic lymphocytic thyroiditis (CLT) and Graves’ disease. CLT is the most common cause of hypothyroidism in children and adolescents (91,91a).
Graves’ disease and CLT are closely associated and in fact overlapping syndromes .Patients can move from one to the other category, depending upon the stage of their illness. For example, an individual might first be observed with thyroid enlargement and positive antibody tests for anti-thyroglobulin or anti-TPO antibodies, and thus qualify as having CLT. At a later stage, this individual might become hyperthyroid (Hashitoxicosis) and fit in the category of Graves’ disease. Or, the patient with hyperthyroidism might have progressive destruction of the thyroid, or develops blocking antibodies, and become hypothyroid or ultimately develop myxedema.
Incidence
The prevalence of CLT in children and adolescents was reported to be 1.2% by Rallison in 1975 (91b). In this 6 year-survey study 5179 school children were examined in Arizona. Goiter was evaluated by palpation (91b). More recently, a study from Sardinia in 8040 children and adolescents aged 6-15 years reported TPOAb detectable in 2.9% (91c). Similar results were found in Berlin with a prevalence of TPOAb of 3.4% (mean age 11 years) (91d) and in Greece after correction of iodine deficiency. In this study examining 440 children and adolescents aged 5-18 years a prevalence of TPOAb and TgAb was reported to be 4.6% and 4.3% respectively. The prevalence of CLT, confirmed by ultrasound was 2.5% (91e).
In childhood the most common age at presentation is adolescence, but the disease may occur at any age, even infancy. CLT in infancy is rare, but can cause in a short time severe hypothyroidism and permanent damage to CNS if not recognized and treated (85). The female/male ratio in AITD is up to 6:1, but In prepubertal age the female/male ratio is lower than reported in adolescents and adults.
Etiology and Pathogenesis
CLT is thought to be caused by a combination of genetic susceptibility and environmental factors. Both thyroid-specific genes and genes involved in immune recognition and/or response have been identified (91f, 91g). (See chapter Autoimmunity, by A Weetman for an exhaustive information). Some genes are common to both disorders and some tend to predominate only in Graves’ disease. AITD has a striking predilection for females, but in prepubertal age the female/male ratio is lower. A family history of autoimmune thyroid disease (both chronic lymphocytic thyroiditis and Graves’ disease) is found in 30% to 40% of patients. A study about familial clustering of juvenile AITD found thyroid antibodies detectable in 56% of mothers and 25% of fathers. Interestingly, HLA DQ alleles and antibody status in fathers influenced the susceptibility to AITD in children (91h). Siblings recurrence in childhood is 20-30% (91i). AITD are often associated with other autoimmune disorders. The plethora of associations and their familial occurrence indicates that a defect in the immune system may be more likely than primary defects in each organ, as these diseases often share similar genetic associations, including HLA, CTLA-4, PTPN22 and CD25 gene polymorphisms. It is also clear however that there is a difference in the kind of clustering of other autoimmune disease in CLT and Graves’ disease, presumably related to differences between these two types of thyroid disease in genetic predisposition (91j,91k). There is also an increased incidence of CLT in patients with certain chromosomal abnormalities as Down syndrome (91l) Turner syndrome (91m), Klinefelter syndrome (91n) as well as in patients with Noonan syndrome (91o).
Environmental factors as infection, environmental toxins, substances as iodine, selenium, stress, smoking, estrogens, drugs (amiodarone, interferon alfa, lithium) have been suggested as precipitating factors for CLT (91p). The precise environmental trigger has not been yet established. An epigenetic mechanism may be implicated (91q).
Clinical Manifestations
Both goitrous (Hashimoto’s thyroiditis) and nongoitrous (atrophic thyroiditis, also called primary myxedema) as variants of chronic lymphocytic thyroiditis have been distinguished. The term “Hashimoto’s thyroiditis” is often used as a synonymous of CLT, not necessary linked to the presence of goiter (91, 91a). Goiter, present in approximately two-thirds of children with CLT is caused by lymphocytic infiltration that may be extensive, with lymphoid germinal centers, TSH stimulation, or production of antibodies that stimulate thyroid growth (92). Progressive thyroid cell damage, with cell mediated cytotoxicity and follicular cell apoptosis, can change the apparent clinical picture from goitrous hypothyroidism to that of “atrophic” thyroiditis. Atrophic thyroiditis, or primary hypothyroidism/mixedema, is considered to be the end stage of CLT (91).
Children with chronic lymphocytic thyroiditis may be euthyroid, or may have subclinical or overt hypothyroidism. Occasionally, children may experience an initial thyrotoxic phase due to the discharge of preformed T4 and T3 from the damaged gland. Alternatively, as indicated above, thyrotoxicosis may be due to concomitant thyroid stimulation by TSH receptor stimulatory antibodies (Hashitoxicosis).
The onset of hypothyroidism in childhood is insidious. Affected children often are recognized either because of the detection of a goiter on routine examination or because of a poor interval growth rate present for several years prior to diagnosis (92a). Because the deceleration in linear growth tends to be more affected than weight gain, these children can be relatively overweight for their height, although they rarely are significantly obese (Figure 6). If the hypothyroidism is severe and longstanding, immature facies with an underdeveloped nasal bridge and immature body proportions (increased upper-lower body ratio) may be noted. Dental and skeletal maturation are delayed, the latter often significantly. Patients with central hypothyroidism tend to be even less symptomatic than are those with primary hypothyroidism.

Figure 15-6 Sequential changes in physical appearance in a young girl who presented at 15 years of age with amenorrhea and hyperprolactinemia secondary to severe hypothyroidism. Note her poor linear growth since at least 11 years of age.
The classical clinical manifestations of hypothyroidism can be elicited on careful evaluation, though they often are not the presenting complaints. These include sluggishness, lethargy, cold intolerance, constipation, dry skin or hair texture, and periorbital edema. Bradycardia and delayed deep tendon reflexes can be present. In severe, long-standing hypothyroid children pericardial and pleural effusions may occur. School performance is not usually affected, in contrast to the severe irreversible neuro-intellectual sequelae that occur frequently in inadequately treated babies with congenital hypothyroidism. Causes of hypothyroidism associated with a goiter (CLT, inborn errors of thyroid hormonogenesis, thyroid hormone resistance) should be distinguished from non goitrous causes (primary myxedema, thyroid dysgenesis, central hypothyroidism). The typical thyroid gland in a longstanding chronic lymphocytic thyroiditis is diffusely enlarged and has a rubbery consistency. Although the surface is classically described as ’pebbly’ or bosselated, occasionally asymmetric enlargement occurs and must be distinguished from thyroid neoplasia. Alternatively, the thyroid may be normal in size and consistency or not palpable at all. A palpable lymph node superior to the isthmus (“Delphian node”) is often found and may be confused with a thyroid nodule. The thyroid gland, in thyroid hormone synthetic defects, on the other hand, tends to be softer and diffusely enlarged.
In patients with severe hypothyroidism of longstanding duration, the sella turcica may be enlarged due to thyrotrope hyperplasia. There is an increased incidence of slipped femoral capital epiphyses in hypothyroid children. The combination of severe hypothyroidism and muscular hypertrophy which gives the child a “Herculean” appearance is known as the Kocher-Debre-Semelaigne syndrome (92b).
Puberty tends to be delayed in hypothyroid children in proportion to the retardation in the bone age, although in longstanding severe hypothyroidism, sexual precocity has been described. Females with sexual precocity have menstruation, and breast development but relatively little sexual hair. Multicystic ovaries, the etiology of which is unknown, may be demonstrated on ultrasonography. In other cases, galactorrhea or severe menses have been the presenting features. In boys, testicular enlargement may be found (92c). An elevated serum prolactin, the latter possibly due to raised TRH which is known to stimulate prolactin as well as TSH, has been described in some cases, but gonadotropin levels are not consistently elevated. It has been hypothesized that this syndrome of pseudopuberty in hypothyroid patients is due to cross- interaction of the extremely elevated serum TSH with the FSH receptor (92d). Consistent with the latter hypothesis, there is little increase in serum testosterone as might be expected if the FSH, but not luteinizing hormone (LH) receptor is involved and serum gonadotropins are frequently not increased.
Long term follow up studies of children with chronic lymphocytic thyroiditis have suggested that while most children who are hypothyroid initially remain hypothyroid, spontaneous recovery of thyroid function may occur, particularly in those with initial compensated hypothyroidism (93,93a, 93b). A recent five-years prospective study in children and adolescents affected with CLT showed that thyroid dysfunction increased from 27.3% to 47.4% (93c). Therefore, close follow up is necessary.
Although chronic inflammation, leading to neoplastic transformation, is a well-established clinical phenomenon, if CLT can increase the risk for thyroid nodules and thyroid cancer remains controversial. In the past autoimmune thyroiditis has been thought to be protective from thyroid cancer, but several studies both in adults and in children suggested the opposite. Thyroid nodules in healthy children in iodine replete regions are detected in up to 1.6% (94). High prevalence of thyroid nodules, ranging from 13% to 31%, has been reported in children and adolescents with CLT. In a multicentric pediatric retrospective study from Italy, nodules were found in 115/365 patients with CLT (31.5%), and papillary thyroid carcinoma in 11/115 (9.5%) (94a). In a recent study from Turkey, thyroid nodules were detected in 39/300 (13%) of cases of pediatric CLT and papillary thyroid carcinoma was diagnosed in 2 of the 12 cases that underwent FNAB (94b). Recently, in. a retrospective study from United States examining ultrasound characteristic of the thyroid in 154 children and adolescents with goiter, nodules were reported in 20/154 (13%) and PTC in 4/154 (2.5% ) of children. In this study, the same prevalence of nodules (17%) was found in TPOAb positive and TPOAb negative children. Interestingly, one case of PTC was first classified at ultrasound as pseudonodule. Only 15 % of nodules and none of the papillary thyroid carcinoma in these series (PTC) were palpable, although PTC has a diameter ranging from 1.2 to 2.6 cm (94c). A rare variant of PTC, the diffuse sclerosing variant, has also been reported in children with CLT(94d).
Associated Disease
AITD are frequently associated with other common autoimmune disorders as type 1 diabetes (94e,94f) and celiac disease. AITD can be also the first manifestation of autoimmune polyendocrine syndromes (APS1 and 2) (95,95a,95b). A pletora of other autoimmune conditions, organ or non-organ specific disease, can be associated with AITD in childhood and adolescence. CLT is more frequently associated with adrenal and β cell autoimmunity than Graves’ disease (91j). Early identification and treatment of these disorders may be critical and even preserve children from life-threatening events. Long term surveillance is required, because a second autoimmune disorder may occur any time.
Type 1 diabetes. CLT is the most common associated autoimmune disease in type 1 diabetes. In a ten-years observational study of children and adolescents with type 1 diabetes (mean age at diagnosis 10 years), the prevalence of TPOAb and TgAb at diagnosis was 15.4% and 14.4% respectively. The cumulative incidence increased especially in females in mid puberty. In this study about 14% of patients required treatment with L-thyroxine (95c). Children with AITD had islet cell antibodies in 2.3% (95d). Screening for AITD is suggested at diagnosis and every 2-3 years if negative (ADA and ISPAD recommendation). Thyroid function should be checked every year or more frequently if needed, because thyroid dysfunction (both hypothyroidism and hyperthyroidism) affects metabolic control. In overt hypothyroidism hypoglycemia can occur because glucose absorption may be slow and the sensitivity and rate of degradation of insulin is increased. Hepatic gluconeogenesis and peripheral glucose utilization are also reduced. Long term dyslipidemia may affect cardiovascular risk in these patients.
Hyperthyroidism in type 1 diabetic children can precipitate acute complications. In a study on 60456 children and adolescents with type 1 diabetes, hyperthyroidism was diagnosed in 276 (0.46%). Hyperthyroid state was associated with diabetic ketoacidosis, hypoglycemia and hypertension (95e). Life-long surveillance is required.
Celiac disease. Another strong association is with celiac disease, which is found 3 times more commonly in patients with AITD. Intriguingly the autoantibodies which are the hallmark of celiac disease, directed against transglutaminase, can bind to thyroid cells and thus could be implicated directly in thyroid dysfunction (95f). A recent meta-analysis showed that the prevalence of celiac disease in AITD patients in higher in children (6.2%) than in adults (1.2%) (95g). Prevalence of AITD in celiac patients is about 20%. The presence of celiac disease in type 1 diabetes seems to increase the risk for AITD (95h). Undiagnosed celiac disease causes malabsorption with or without gastrointestinal symptoms. Delayed linear growth may be the first manifestation as unexplained change in L-T4 requirement (95i).
Addison’s disease. Addison’s disease is also associated with AITD. In an old report, the prevalence of adrenal antibodies in children with AITD, was found to be 2.3%, while the great majority of children affected with Addison’s disease presented with CLT (95d). Addison’ disease is more often a component of autoimmune polyendocrine syndromes (APS1 and 2) (95,95a,95b). Addison's disease and/or type 1 diabetes mellitus and AITD occasionally co-exist and form the classical autoimmune polyendocrine syndrome type2 (Schmidt syndrome). Undiagnosed adrenal insufficiency, is a life-threatening condition and can be exacerbated by L-thyroxine therapy, because L-thyroxine increases cortisol clearance. Moreover, symptoms of overt hypothyroidism can overlap with adrenal insufficiency manifestations. Adrenal insufficiency is a rare but non-obvious diagnosis in childhood and should be considered in when autoimmune disorders are diagnosed.
Autoimmune gastritis. Autoimmune gastritis was first described in association with AITD as thyrogastric syndrome. Common clinical manifestations in adults are iron deficient or pernicious anemia (95j). Perhaps 45% of patients with autoimmune thyroiditis have circulating gastric parietal cell antibodies. Also in children with AITD, early manifestations of gastric autoimmunity has been reported, with a prevalence of gastric parietal cell antibodies of 30%. In this series, 45% of PCA positive children presented with increased gastrin plasma levels (a marker of atrophic body gastritis) (95l).
Autoimmune polyendocrine syndromes (APS1 and 2)
CLT can be the first manifestation of autoimmune polyendocrine syndromes (APS1 and 2) (95,95a,95b). APS1 tends to present in early childhood and is characterized primarily by mucocutaneous candidiasis, hypoparathyroidism and adrenal deficiency. APS1 is also defined as autoimmune polyendocrinopaty-candidiasis-ectodermal dystrophy (APECED). APS1 results from mutations in the AIRE (autoimmune regulator) gene. It is a rare autosomal dominant disorder with incomplete penetrance (96). In APS1, chronic lymphocytic thyroiditis is found in approximately 10% of patients. APS1 was originally described in Europe. Recently, a report from USA described different clinical features and diagnostic criteria in APECED patients from Western Hemisphere i.e., as initial signs urticarial eruptions, intestinal dysfunction, enamel dysplasia. Classical triad presentation (mucocutaneous candidiasis, hypoparathyroidism and adrenal deficiency) was delayed. Life threatening endocrine complications can be prevented by an early diagnosis (96a). APS2, tends to occur later in childhood or in the adult with a polygenic predisposition. APS2 can be clustering in families with heterogeneous clinical phenotypes. Other disorders, including vitiligo, celiac disease, myasthenia gravis, premature ovarian failure and chronic active hepatitis may be present (95,95a). An extensive review of these associations has been published (96b) and large population data bases have clarified the strength of the various associations in adults (95k,96c).
Many other autoimmune conditions, organ or non-organ specific disease, can be associated with AITD in childhood and adolescence. Increased prevalence of CLT has been found in juvenile idiopathic arthritis (96d), non-segmental vitiligo (96e) and alopecia areata (96f). CLT may be associated with chronic uriticaria (96g) and rarely with immune-complex glomerulonephritis (96h). High prevalence of antinuclear antibodies (ANA) has recently been reported in a series of 93 children (86 with CLT and 8 with GD) without overt rheumatic disorders. In this series ANA positivity was found in 71% of children, ENA positivity in 4% and anti-DNA antibodies in 1% (96i). Growth hormone deficiency on an autoimmune basis has been suggested in a small number of children, of whom 43% have CLT (92j-96k). Nevertheless this is a rare association, hypothetically a growth disorder in a child with CLT can be due to other causes than hypothyroidism as celiac disease or GH deficiency. It is important that clinicians are cognizant of these associations in order to maintain a high index of vigilance.
Laboratory Evaluation
Measurement of TSH is the best initial screening test for the presence of primary hypothyroidism. If the TSH is elevated, then evaluation of the free T4 will distinguish whether the child has subclinical (normal free T4) or overt (low free T4) hypothyroidism. Measurement of TSH, on the other hand, is not helpful in central hypothyroidism. In these cases hypothyroidism is demonstrated by the presence of a low free T4 accompanied by an “inappropriate ‘’ TSH. In the past TRH testing (TRH 7 mcg/kg) was sometimes utilized to distinguish a hypothalamic versus pituitary origin of the hypothyroidism; in hypothalamic hypothyroidism there tends to be a delayed peak in TSH secretion (60-90 minutes versus the normal maximal response at 15-30 minutes) whereas in hypopituitarism there usually is little or no TSH response. However TRH is no longer available in the USA. Furthermore, the reliability of this test in the pediatric range has been questioned (97). Occasionally mild TSH elevation is seen in individuals with hypothalamic hypothyroidism, a consequence of the secretion of a TSH molecule with impaired bioactivity but normal immunoreactivity. Thyroid hormone resistance is characterized by elevated levels of T4 and T3 and an inappropriately normal or elevated TSH concentration. Antibodies to Tg and TPO, the thyroid antibodies measured in routine clinical practice, are detectable in over 90% of patients with chronic lymphocytic thyroiditis. Therefore, they are useful as markers of underlying autoimmune thyroid damage, TPO antibodies being more sensitive. TSH receptor antibodies also are found in a small proportion of patients with chronic lymphocytic thyroiditis. When stimulatory TSH receptor antibodies are present, they may give rise to a clinical picture of hyperthyroidism, the coexistence of chronic lymphocytic thyroiditis and Graves’ disease is known as Hashitoxicosis. In one study, TSH receptor blocking antibodies were found in <10% of children and adolescents with chronic lymphocytic thyroiditis, patients overall, but in 17.8% of those with severe hypothyroidism (defined as a serum TSH concentration >20 mU/L). Unlike in adults, they were found in goitrous as well as nongoitrous patients, and, when present at a high concentration, appeared to persist indefinitely, suggesting that the presence of potent TSH receptor blocking antibodies in adolescent females might identify patients at risk of having babies with transient congenital hypothyroidism in the future (97a,97b).
Imaging studies (thyroid ultrasonography and/or thyroid uptake and scan) may be performed if thyroid antibody tests are negative or if a nodule is palpable. If no goiter is present, imaging studies are helpful in identifying the presence and location of thyroid tissue, and therefore, of distinguishing primary myxedema from thyroid dysgenesis. Inborn errors of thyroid hormonogenesis beyond a trapping defect are usually suspected by an increased radioiodine uptake, and a large gland on scan. Other etiologies of hypothyroidism usually are evident on history. Ultrasound (US) is useful to define size, anatomy, echogenity of the thyroid. Occasionally the finding of heterogeneous echogenicity on ultrasound examination has been described prior to the appearance of antibodies. Diffuse reduction in echogenity, (hypoechoic), and pseudonodules are common findings (98). Moreover, US can be useful in detecting unsuspected thyroid nodules and cancer. In a study examinating US characteristic of the thyroid in 154 children and adolescents with goiter, nodules were reported in 20/154 (13%) and PTC in 4/154 (2.5%) of children. None of the papillary thyroid carcinoma in these series (PTC) was palpable, although a PTC diameter ranging from 1.2 to 2.6 cm was found. Interestingly, one case of PTC was first classified as pseudonodule (94c). The diffuse sclerosing variant of PTC, with a typical US appearance, has also been reported in children with CLT (94d). If there is a cost-effective benefit in performing US in all cases of children with CLT and/or goiter deserves sufficiently powered prospective studies.
Therapy
The typical replacement dose of L-thyroxine (derived from congenital hypothyroidism) in overt severe hypothyroidism is about 4 to 6 mcg/kg/day for children 1 to 5 years of age, 3 to 4 mcg/kg/day for these ages 6 to10 years and 2-3mcg/kg/day for these 11 ages and older. Lower dose as 1 to 3 mcg/kg/day may be sufficient in less severe cases. The dose should be individually titrated as the lowest useful to keep TSH in the normal range and FT4 or T4 in the upper half of the reference range. L-thyroxine should be given once daily preferably half to 1 hour before meal. Somministration of preparates (i.e., calcium, soya), or drugs that can interfere with absorption should be avoided. T4 and TSH should be measured after the child has received the recommended dosage for at least 6-8 weeks. Once a euthyroid state has been achieved, patients should be monitored every 6 to 12 months. In patients with a goiter a somewhat higher L-thyroxine dosage is used so as to keep the TSH in the low normal range, and thereby minimize its goitrogenic effect.
Close attention is paid to interval growth and bone age as well as to the maintenance of a euthyroid state. Thyroid hormone replacement is not associated with significant weight loss in overweight children, unless the hypothyroidism is severe (99b). Some children with severe, long standing hypothyroidism at diagnosis may not achieve their adult height potential even with optimal therapy (99c), emphasizing the importance of early diagnosis and treatment. Treatment is usually continued indefinitely.
Treatment of children and adolescents with subclinical hypothyroidism (normal free T4, elevated TSH) is controversial (100). Because normalization of TSH is also possible if the patient is not symptomatic, a reasonable option is to reassess thyroid function in 3- 6 months prior to initiating therapy because of the possibility that the thyroid abnormality will be transient.
In adults in whom the risk of progression to overt hypothyroidism is significant, treatment has been recommended whenever the serum TSH concentration is >10 mU/L ; if the TSH is 6-10 mU/L treatment on a case by case basis is suggested (100a). In children and adolescents, a recent five-years prospective study showed that in patients with CLT thyroid dysfunction increased from 27.3% to 47.4% (93c). Some considerations about “to treat or not to treat” “subclinical hypothyroidism” in children with a known cause of thyroid failure as CLT may be useful.
Well designed and adequately powered trials needed to establish the advantages of treating “subclinical hypothyroidism” are not available in adults and seem to be very difficult in children, also because some of the clinical consequences- i.e cardiovascular events- of untreated mild hypothyroidism may hypothetically occur later in adult life.
In adults, variations in thyroid function within the reference range may be associated with adverse health outcome (100b), and some data suggesting clinical consequences of subclinical hypothyroidism are also available in children and adolescents. A positive relationship was found for TSH levels and systolic and diastolic blood pressure (100c), atherogenic lipid profiles, (100d) and other risk factors for cardiovascular diseases (100e).
Moreover, adult patients with a thyroid nodule and TSH in the upper tertiles of the reference range may be at increased risk of malignancy (100f). Given an individual set point for TSH, more than an absolute TSH value (i.e more or less 6 7-, 8 UI/mL) the decision about treatment may consider the temporal trend of TSH in a patient. An increase of TSH value over the time suggests the progression of the grade of hypothyroidism. Both the decisions to “wait and see” or “to treat” require monitoring the thyroid function and clinical follow up. A careful discussion about “the state of the art” must be taken with the child and the family.
Guidelines about the management of subclinical hypothyroidism in pregnancy and in children have been published by the European Thyroid Association in 2014 (100g), but it as an evolving and open field (100).
Thyroid Dysgenesis and Inborn Errors of Thyroid Hormonogenesis
Occasionally, patients with thyroid dysgenesis will escape detection by newborn screening and present later in childhood with non goitrous hypothyroidism or with an enlarging mass at the base of the tongue or along the course of the thyroglossal duct. Similarly, children with inborn errors of thyroid hormonogesis may only be recognized later in childhood because of the detection of a goiter.
Drugs or Goitrogens
In addition to antithyroid medication, a number of drugs used in childhood may affect thyroid function, including certain anticonvulsants, lithium, amiodarone, aminosalicylic acid, aminoglutethimide and sertraline (101-101a). Similarly, a large number of naturally occurring goitrogens (broccoli, cabbage, sweet potatoes, cauliflower, soya beans, cassava and water pollutants) have been identified. Both radioiodine therapy and thyroidectomy, occasionally used in childhood for the definitive treatment of Graves’ disease, frequently cause permanent hypothyroidism.
Worldwide, iodine deficiency continues to be an important cause of hypothyroidism, affecting at least 800 million people living largely in developing countries. In addition, even in certain parts of Europe, an estimated 100-120 million individuals are thought to have borderline iodine deficiency (101b). Although one rarely sees iodine deficiency in North America, an iodine sufficient area, a 6 year old boy with goitrous hypothyroidism has been described in whom iodine deficiency was due to multiple food allergies and severe dietary restriction (101c). In addition, the child consumed a large intake of thiocyanate-containing foods that blocked organification of iodine.
Miscellaneous Causes of Acquired Hypothyroidism
Rarely, the thyroid gland may be involved in generalized infiltrative or infectious disease processes that are of sufficient severity to result in a disturbance in thyroid function (i.e., (Langerhans cell histiocytosis) (101 d). Alternatively, hypothyroidism may be a long term complication of mantle irradiation for Hodgkin’s disease or lymphoma. External irradiation of brain tumors in the posterior fossa of the brain may be associated with both primary and secondary hypothyroidism because of the inclusion of the neck in the radiation field. Rarely, hypothyroidism has been reported in infants with large hemangiomas (85b,85c). In these cases, the hypothyroidism was shown to be due to increased inactivation of T4 by the D3 activity of these tumors.
Central Hypothyroidism
Secondary or tertiary hypothyroidism in less severely affected children with the congenital abnormalities noted earlier in this chapter, may be recognized only later in childhood. Alternatively, secondary or tertiary hypothyroidism may develop as a result of acquired damage to the pituitary or hypothalamus, i.e., by tumors (particularly craniopharyngioma), granulomatous disease, head irradiation, infection (meningitis), surgery or trauma. Usually other trophic hormones are affected, particularly growth hormone.
Impaired Sensitivity to Thyroid Hormone (Thyroid Hormone Resistance)
In contrast to the neonatal period, children with thyroid hormone resistance usually come to attention when thyroid function tests are performed because of poor growth, hyperactivity, a learning disability or other nonspecific signs or symptoms. A small goiter may be appreciated. Affected patients have a high incidence of attention deficit hyperactivity disorder (102). Thyroid hormone resistance has also been described in patients with cystinosis (102a).
Other causes of goiter: Colloid or Simple (Nontoxic) Goiter
Colloid goiter is the second most common cause of euthyroid thyroid enlargement in childhood after CLT. The etiology of colloid goiter is unknown. Not infrequently there is a family history both of goiter, chronic lymphocytic thyroiditis and Graves’ disease, leading to the suggestion that colloid goiter, too, might be an autoimmune disease. Immunoglobulins that stimulated thyroid growth in vitro have been identified in a proportion of patients with simple goiter, but their etiological role is controversial (103). It is important to distinguish patients with colloid goiter from chronic lymphocytic thyroiditis because of the risk of developing hypothyroidism in patients with chronic lymphocytic thyroiditis, but not colloid goiter. Whereas many colloid goiters regress spontaneously, others appear to undergo periods of growth and regression, resulting ultimately in the large nodular thyroid glands later in life.
Clinical Manifestations and Laboratory Investigation
Evaluation of thyroid function by measurement of the serum TSH concentration is the initial approach to diagnosis. In euthyroid patients, the most common situation, chronic lymphocytic thyroiditis should be distinguished from colloid goiter. Clinical examination in both instances reveals a diffusely enlarged thyroid gland. Therefore, the distinction is dependent upon the presence of elevated titers of TPO and Tg antibodies in chronic lymphocytic thyroiditis but not colloid goiter. All patients with negative thyroid antibodies initially should have repeat examinations because some children with chronic lymphocytic thyroiditis will develop positive titers with time.
Therapy
Thyroid suppression in children with a euthyroid goiter is controversial (103a). A significant decrease in goiter size in patients with chronic lymphocytic thyroiditis as assessed by standard deviation score on ultrasonography has been demonstrated recently in patients treated for 3 years (103b). However, the absolute difference quantitatively was not reported and so, whether or not this difference was significant clinically remains unclear. Given the variability in response in different patients, it would be reasonable to attempt a therapeutic trial in patients whose goiter is large.
Painful thyroid: Acute suppurative thyroiditis, subacute thyroiditis
Painful thyroid enlargement is rare in pediatrics and suggests the probability of either acute (suppurative) (106) or subacute thyroiditis (106a). Rarely chronic lymphocytic thyroiditis may be associated with intermittent pain and be confused with the latter disorders. In acute suppurative thyroiditis, progression to abscess formation with the potential of rupture may occur rapidly so prompt recognition and antibiotic therapy are essential (106b). Acute suppurative thyroiditis is a potentially life-threatening endocrine emergency. It is often preceded by an upper respiratory infection, and can be initially misdiagnosed in a young child presenting with high fever, sore throat, and severe dysphagia. A tender very painful swelling in the region of the thyroid gland is present and the abscess can progress to the surrounding tissues and to the skin. Recurrent attacks and involvement of the left lobe suggest a pyriform sinus fistula between the oropharynx and the thyroid as the route of infection (106c). In the latter case, surgical extirpation of the pyriform sinus will frequently prevent further attacks. The management of this condition has recently been reviewed (106,106b). Subacute viral thyroiditis (or de Quervain or granulomatous thyroiditis) it is rarely reported in childhood and adolescence, but cases at 2-3 years of age are known (106a). Usually subacute thyroiditis presents with sore throat, fever and firm, painful tender enlargement of the thyroid. Mild signs of hyperthyroidism can be overlooked. Subacute thyroiditis may occur as a acute, subacute or rarely chronic disorder. A painless variant has been described also in children. (106d). Therapy is usually symptomatic, because the disease is self-limiting. Sometimes treatment with prednisone (0.5-1mg/kg/die) for a short period (i.e. one week) can be useful.
THYROTOXICOSIS AND HYPERTHYROIDISM
Thyrotoxicosis is defined as the clinical syndrome of hypermetabolism resulting from increased free thyroxine (T4) and/or free triiodothyronine (T3) serum levels (107)). The term thyrotoxicosis is not synonymous with hyperthyroidism, the elevation in thyroid hormone levels caused by an increase in their biosynthesis and secretion by the thyroid gland (Table 6). For example, thyrotoxicosis can result from the destruction of thyroid follicles and thyrocytes in the various forms of thyroiditis, or it can be caused by an excessive intake of exogenous thyroid hormone. It should also be noted that the elevation of free thyroid hormone levels does not always result in thyrotoxicosis in all tissues. In the syndrome of Resistance to Thyroid Hormone (RTH), dominant negative mutations in the thyroid hormone receptor β ( TR β) result in decreased thyroid hormone action in tissues where TRβ is the predominant receptor, for example in the liver and the pituitary, whereas other tissues such as the heart, which express mainly TR α, show signs of increased thyroid hormone action. The determination of the etiology of thyrotoxicosis is of importance in order to establish a rational therapy.
TABLE 6. CAUSES OF THYROTOXICOSIS IN CHILDHOOD AND ADOLESCENCE
| THYROTOXICOSIS DUE TO HYPERTHYROIDISM (increased production of T3, T4) |
| Autoimmune hyperthyroidism |
| · Graves’ disease |
| · Hashitoxicosis |
| Congenital non autoimmune hyperthyroidism |
| · Sporadic (de novo Persistent sporadic congenital non autoimmune hyperthyroidism (PSNAH) |
| · Hereditary familial non-autoimmune autosomal dominant hyperthyroidism (FNAH) |
| Autonomous functioning nodules |
| · Toxic adenoma |
| · Hyperfunctioning papillary or follicular carcinoma |
| · Toxic multinodular goiter |
| · McCune Albright disease |
| TSH-induced hyperthyroidism |
| · TSH-producing pituitary adenoma |
|
· Thyroid Hormone resistance Tumors |
| · Hydatiform mole, choriocarcinoma |
|
· Struma ovari, teratoma (autonomous function of thyroid tissue in ovarian)
|
| THYROTOXICOSIS WITHOUT HYPERTHYROIDISM- |
| Transient thyrotoxicosis (Release of stored hormones) |
| · Chronic lymphocytic thyroiditis |
| · Subacute thyroiditis |
| · Silent thyroiditis |
| · Drug-induced thyroiditis |
| · Exogenous causes |
| · Thyroid hormone ingestion |
| · Iodine -induced hyperthyroidism (iodine, radiocontrast agents, amiodarone) |
Graves’ Disease
Autoimmune thyroid disease (AITD), including Chronic lymphocytic thyroiditis and Graves’ disease share immunological abnormalities, histological changes in the thyroid, and genetic predisposition and associated diseases. (See chronic lymphocytic thyroiditis section). The clinical spectrum of AITD ranges from hypothyroidism to hyperthyroidism.
More than 95% of cases of hyperthyroidism in children and adolescents are due to Graves’ disease, (107a) an autoimmune disorder characterized by hyperthyroidism, goiter and a particular opthalmopathy. TSH receptor antibodies that mimic the action of TSH (TRAb), causing increased thyroid hormonogenesis and growth are specific of Graves’ disease, but other autoantibodies, as AbTPO and AbTg, are detectable.
Incidence
Graves’ disease is rare in children and adolescents. However, incidence rates of thyrotoxicosis below 15 years of age are increased in the last years. A study from Denmark reported an incidence of 1.58/100.000 person-years in the period 1998-2012 versus 0.79/100.000 person-years in 1982-1988 (107b). There is a strong female predisposition, the female:male ratio being 6 to 8:1. Although it can occur at any age, it is most common in adolescence. Prepubertal children tend to have more severe disease, to require longer medical therapy and to achieve a lower rate of remission as compared with pubertal children (107c). This appears to be particularly true in children who present at <5 years of age (107d). Graves’ disease has been described in children with other autoimmune diseases, both endocrine and non endocrine. These include diabetes mellitus, Addison’s disease, vitiligo, systemic lupus erythematosis, rheumatoid arthritis, myasthenia gravis, periodic paralysis, idiopathic thrombocytopenia purpura and pernicious anemia. (See also associated diseases in CLT). There is an increased risk of Graves’ disease in children with Down syndrome (trisomy 21) (107e).
Pathogenesis
The cause of Graves’ disease is unclear. For unknown reasons the immune system produces TSH receptor antibodies that mimic the action of TSH. Binding of ligand results in stimulation of adenyl cyclase and thyroid hormonogenesis and growth (107f, 107g). Presumably a complex interaction between genetic susceptibility (i.e., HLA, CTLA4, PTN22 genes) and environmental factors contribute. A familial history of AITD is often present, as well as for other autoimmune diseases.
Unlike chronic lymphocytic thyroiditis in which thyrocyte damage is predominant, the major clinical manifestations of Graves’ disease are hyperthyroidism and goiter. As noted earlier, TSH receptor blocking antibodies, in contrast, inhibit TSH-induced stimulation of adenyl cyclase. Both stimulatory and blocking TSH receptor antibodies bind to the extracellular domain of the receptor and appear to recognize apparently discrete linear epitopes in the context of a three-dimensional structure (107g). A number of different monoclonal stimulating Abs including one derived from a patient with Graves’ disease have now been generated (107h) and the crystal structure of the human monoclonal stimulating TSH receptor Ab complexed with a portion of the TSH receptor ectodomain has been accomplished (107i). Taken together, a picture has emerged of distinct but overlapping binding sites of both stimulating and blocking TSH receptor Abs and of TSH to the leucine rich TSH receptor ectodomain (107j). Current evidence suggests that it is the shed A subunit rather than the intact, holoreceptor that induces TSH receptor Abs leading to hyperthyroidism (107j). Studies employing monoclonal TSH receptor antibodies cloned from patients and recombinant mutant TSH receptor have demonstrated that there exist multiple TSH receptor antibodies each with different specificities and functional activities. There is evidence that stimulatory antibodies are mostly lambda and of the IgG1 subclass, strongly suggesting that they are monoclonal or pauciclonal (107k). Blocking antibodies, on the other hand, are not similarly restricted.
Clinical Manifestations
The major clinical manifestations of Graves’ disease are hyperthyroidism and goiter. Opthalmopathy is usually mild, pretibial myxedema and acropachy are not described in children and adolescents.
The onset of Graves’ disease in often insidious and a delay in diagnosis of several months is common, especially in prepubertal children (107c). In children below 4 years of age, the prolonged hyperthyroidism can be dangerous to the CNS (107d). Shortened attention span, and emotional lability may lead to behavioral and school difficulties. Sleep disturbances, and nightmares can occur. Some patients complain of polyuria and of nocturia, the result of an increased glomerular filtration rate. All but a few children with Graves’ disease present with some degree of thyroid enlargement, and most have symptoms and signs of excessive thyroid activity, such as tremors, inability to fall asleep, weight loss despite an increased appetite, diarrhea, proximal muscle weakness, heat intolerance and tachycardia. Acceleration in linear growth may occur, often accompanied by advancement in skeletal maturation (bone age). Adult height is not affected. In the adolescent child, puberty may be delayed. If menarche has occurred, secondary amenorrhea is a common concomitant. If sleep is disturbed, the patient may complain of fatigue. Clinical findings are usually related to hyperthyroid state and disappear with restoration to the euthyroid state (108).
Graves’ opthalmopathy in children and adolescents is reported in up to half of the children and is usually less severe than in adults. Eyelid retraction and “stare” are common and linked to hyperthyroid state (108a). Proptosis is subtle and often overlooked. Normal references for children should be used (108b). Some cases of prominent progressive proptosis requiring treatment have been reported (108c). Surgical therapy is infrequently necessary. In a series of 35 children with Graves’ opthalmopathy from Mayo Clinic 3 patients (8.6%) underwent transantral orbital decomprenssion for proptosis that caused discomfort and exposure keratitis and 1 patient (2.9%) required eyelid surgery. No compressive optic neuropathy was found (108d).
Laboratory Evaluation
The clinical diagnosis of hyperthyroidism is confirmed by the finding of increased concentrations of circulating thyroid hormones (T4 or, preferably, free T4 and T3 or FT3) and low- undetectable TSH. In hyperthyroidism, the circulating T3 concentration frequently is elevated out of proportion to the T4 because, like TSH, TSH receptor antibodies stimulate increased T4 to T3 conversion. Some patients may have at diagnosis high FT3 and normal FT4, a condition known as T3 thyrotoxicosis (109). Children with T3 thyrotoxicosis seem to be younger, with higher levels of TRAb and larger goiter than classical GD. The timing of T3 thyrotoxicosis onset is variable and can require higher doses of ATD to control hyperthyroidism (109a). Demonstration of a suppressed TSH excludes much rarer causes of thyrotoxicosis, such as TSH-induced hyperthyroidism and thyroid hormone resistance in which the TSH is inappropriately “normal” or slightly elevated. If the latter diseases are suspected, free α-subunit should be measured. Alternatively, an elevated T4 level in association with an inappropriately “normal” TSH may be due to an excess of thyroxine-binding globulins (either familial or acquired, for example a result of oral contraceptive use) or rarer binding protein abnormalities (for example, familial dysalbuminemic hyperthyroxinemia) (109b). In the latter cases, serum TBG concentration or electrophoresis of T4 binding proteins, respectively, should be measured. If pregnancy or an hCG-secreting tumor are suspected, serum or urinary hCG concentration can be measured. A low serum Tg can be demonstrated if thyrotoxicosis factitia is suspected (109c).
The diagnosis of Graves’ disease is confirmed by the demonstration of TSH receptor antibodies (TRAb) in serum. TRAb are disease specific antibodies and have a pathogenetic role in Graves’ disease. TRAb are usually determined by binding assays. Bioassays that measure Thyroid Stimulating Immunoglobulins activity based on cAMP on cultured cells can be useful if TRAb are not detectable by binding assays (88a,88b,88c). About 95% of children with GD have TRAb detectable. There is a positive correlation between severity of Graves’ disease and TR antibodies level. Higher levels of TRAb and thyroid hormones at presentation are associated with a need of prolonged ATD treatment (109d). Measurement of TSH receptor antibodies may be useful in distinguishing the toxic phase of chronic lymphocytic thyroiditis (TSH receptor antibody negative) from Graves’ disease. Tg and TPO antibodies are positive in 70% of children and adolescents with Graves’ disease but their measurement is not as sensitive or specific as measurement of TSH receptor antibodies. In contrast to adults, radioactive iodine uptake and scan are used to confirm the diagnosis of Graves’ disease only in atypical cases: for example, if measurement of TSH receptor antibodies is negative, or if a functioning thyroid nodule is suspected.
Therapy
The care of children with Graves’ disease can be complicated and requires physicians with expertise in this area. Treatment guidelines developed for adults guidelines cannot be simply applied to children. For instance, TRAb may be detectable in serum for several years, making the terms “remission” and “recidive” inapplicable in 1-2 years periods for the majority of children and adolescents.
The choice of which of the three therapeutic options (medical therapy, surgery radioactive iodine, or radioactive iodine) to use, should be individualized and discussed with the patient and his/her family. Each approach has its advantages and disadvantages with respect to efficacy, both short and long term complications, the time required to control the hyperthyroidism, and the requirement for compliance. In general, medical therapy with methimazole (MMI) is the initial choice of most pediatricians although radioiodine is gaining increasing acceptance, particularly in noncompliant adolescents, in children who are developmentally delayed, and in those about to leave home (for example, to go to college). Concern about the potential long term induction of cancer by RAI given to children is the discussed later. Alternately, surgery, the oldest form of therapy, may be the initial choice in specific cases if an experienced thyroid surgeon is available. ATA guidelines for hyperthyroidism including a pediatric section have recently been released (88c).
Medical therapy
The thiouracil compounds PTU, MMI and carbimazole (converted to MMI) exert their antithyroid effect by inhibiting the organification of iodine and the coupling of iodotyrosine residues on the Tg molecule to T3 and T4.
The aim of therapy with antithyroid drugs is to control hyperthyroidism for a period sufficient to go to spontaneous remission or until the child is old enough to afford definitive therapy as surgery or RAI. Remission is defined as a state of biochemically euthyroidism or hypothyroidism for one year or more after discontinuation of ATD and occurs in a minority of cases (see later).
Some important considerations arose in the last years: MMI is the drug that should be used, unless special conditions, because of the inacceptable risk of liver failure and transplantation (FDA Propilthyuracil warning) in patients using PTU. PTU can cause fulminant hepatic necrosis and death. The risk was estimated to be 1:2000 in children (110,110a,110b). Propylthiouracil and methimazole have for years been considered effectively interchangeable, and liver damage was considered a very rare event. Recently a commission appointed by the FDA reevaluated this problem, and concluded that the rare but severe complications of liver failure needing transplantation, and death, were sufficient to contraindicate the use of PTU as the normal first-line drug (110c). PTU can be used only in pediatric patients who are allergic to MMI, for a short term, and in whom permanent forms of therapy are not possible. MMI should be used alone, titrating the dosage at the lowest useful to maintain euthyroidism. The “block.and replace therapy”, adding L-thyroxine to MMI should be avoided, because it requires a higher dose of MMI, and the majority of side effect of MMI are dose dependent.
The initial dosage of MMI is 0.5 mg/kg/day (up to 1mg/kg/die, maximal dose 30 mg/die) given every 12 hours. The plasma half-life of methimazole in children is only 3-6 hours, but the drug is concentrated in the thyroid and maintains higher levels there for up to 24 hours after a dose (110d). The initial dosage of PTU is 5 mg/kg/day given every 8 hours. In severe cases, a beta-adrenergic blocker (atenolol, 25 to 50 mg daily or twice daily) can be added to control the cardiovascular overactivity until a euthyroid state is obtained.
Before FDA warning for PTU MMI was generally preferred over PTU because for an equivalent dose it requires taking fewer tablets, it has a longer half-life (and so, requires less frequent medication) and because it has a more favorable safety profile. PTU use has also been advocated in the first trimester of pregnancy. PTU but not MMI inhibits the conversion of T4 to the more active isomer T3.
Patients treated with MMI should be followed every 4 to 6 weeks until the serum concentration of T4 (or free T4 and total T3) normalizes. It should be noted that the TSH concentration may not return to normal until several months later. Therefore, measurement of TSH is useful as a guide to therapy only after it has normalized but not initially. Once the T4 and T3 have normalized, one can decrease the dosage of thioamide drug by 30% to 50%. Maintenance doses of MMI may be administered once daily. PTU may be given twice daily. Usually patients can be followed every 1-4 months once thyroid function has normalized.
As suggested by the ATA guidelines (88c), before starting therapy with ATD, a baseline complete blood count, including WBC with differential, and a liver including bilirubin, transaminases and alkaline phosphatase can be useful. This is because hyperthyroidism itself can determine low WBC count, and premorbid liver disease (i.e autoimmune hepatitis reported in 1% of GD) can exist (110e). Baseline information may help in a correct interpretation of side effects of MMI.
In most children and adolescents, circulating thyroid hormone levels can be normalized readily with antithyroid medication as long as compliance is not a problem. The optimal duration of therapy is controversial. There is no doubt that most children and adolescents, particularly prepubertal ones, require a longer course of therapy than adults. Therefore treatment guidelines developed for older individuals should not be applied to the young. In one retrospective study, TSH receptor Abs disappeared from the circulation in <20% of patients after 13-24 months of medical therapy (110f) in contrast to adults in most of whom TSH receptor Abs normalize by 6 to 12 months (110g,110h, 110i). In another study, approximately 25% of children remitted with every 2 years of therapy up to 6 years of treatment (110j). Equivalent results have been obtained by others (107c). In a recent prospective trial of 154 children with newly diagnosed Graves’ disease treated with carbimazole, 20% of children remitted after 4 years of therapy, 37% after 6 years and 45% after 8 years (110k). The median duration of therapy in most studies is 3 to 4 years, but therapy should be individualized. In patients treated with antithyroid drugs alone, a low drug requirement, small goiter, and lack of orbitopathy are favorable indicators that drug therapy can be tapered gradually and withdrawn. Lower initial degree of hyperthyroxinemia (T4<20 mcg/dL (257.4 nmol/L); T3:T4 ratio <20), lower initial TSH receptor Ab concentration (>4X upper limit of normal (111e) and postpubertal age are favorable prognostic indicators. Persistence of TSH receptor antibodies, on the other hand, indicates a high likelihood of relapse. Initial studies suggesting that combined therapy (i.e., antithyroid drug plus L-thyroxine) might be associated with an improved rate of remission (110l) have not been confirmed (110m).
Side effects
Side effects of drugs were reported in 20-30% of children treated both with PTU and MMI and major side effect are thought to be due to PTU. Cumulative data from more than 500 children (111) with Graves’ disease reported mild increase of liver enzymes in 28%, mild leucopenia in 26% skin reactions in 9%, arthritis in 2.4%, nausea in 1.1%, agranulocytosis and hepatitis in 0.4%. Rare complications can be loss of taste, hypothrombinemia, thrombocytopenia, aplastic anemia, nephrotic syndrome and death (111). Side effects of MMI occur in up to 19% of children. Urticaria, arthralgias, gastrointestinal problems and neutropenia (<1500 granulocytes/mm3) are the most common, myalgias (3%), and cholestatic liver injury (1%) were also reported in a series of 100 children with Graves’ disease exclusively treated with MMI. Side effects usually occur in the first 6 months of therapy (111a) but can occur any time.
Major side effects as Stevens-Johnson syndrome and vasculitis occur rarely (111). Vasculitis can be related with the development of anti-neutrophil-cytoplasmic antibodies (ANCA). ANCA positivity has been reported with MMI and PTU therapy and may develop after many years of therapy (111b). Manifestations of vasculitis typically are polyarthritis and purpuric skin lesions. Pulmonary and renal involvement are also described. In severe cases, glucocorticoids or other immunosuppressive therapy may be needed. Guma et al reported ANCA positivity in 67% of patients with Graves’ disease before medical treatment, suggesting an association with Graves’ disease, rather than a complication of antithyroid drugs (111c).
Rarely, more severe sequelae such as hepatitis, a lupus like syndrome, thrombocytopenia, and agranulocytosis may occur. Most reactions are mild and do not contraindicate continued use. The risk of agranulocytosis (<500 granulocytes/mm3) appears to be greatest within the first 3 months of therapy but it can occur at any time. There is some evidence that close monitoring of the white blood cell count during this initial time period may be useful in identifying agranulocytosis prior to the development of a fever and infection (111d), but most authors do not consider the low risk to be worth the cost of close monitoring. It is important to caution all patients to stop their medication immediately and consult their physician should they develop unexplained fever, sore throat, or gingival sores or jaundice. Unlike PTU, MMI is rarely associated with hepatocellular injury.
Children treated with PTU and MMI tends to excessive weight gain during the first 6 months of therapy and nutrition consultation should be considered if needed (111e). Approximately 10% of children treated medically will develop long term hypothyroidism, a consequence of coincident cell and cytokine-mediated destruction.
Patients with Graves’ disease showed a higher risk of thyroid cancer (111). The Collaborative Thyrotoxicosis Study Group found the incidence of thyroid carcinomas over 10-20 years of follow up 5 fold higher in adults with Graves’ disease treated with thionamides than in patients treated with definitive therapy (111g). Long term stimulation of TSAb can play a role. Patients treated for years with thionamides should be carefully monitored for the detection of thyroid nodules.
Surgery
Surgery, the second therapeutic modality, is performed less frequently now than in the past. The main argument favoring surgery is that it may correct the thyrotoxicosis with surety and speed, and result in less disruption of normal life and development that is associated with long-term administration of antithyroid drugs and the attendant constant medical supervision.
The most important limiting factor is the availably of a high-volume thyroid surgeon to reduce potential complications (112,112a,112b). Near-total thyroidectomy is the procedure of choice in order to minimize the risk of recurrence. Surgery usually is reserved for patients who have failed medical management, who have a markedly enlarged thyroid, who refuse radioactive iodine therapy, and for the rare patient with significant ophthalmopathy in whom radioactive iodine therapy is contraindicated. Often adolescents are unable to maintain the careful dosage schedule needed for control of the disease .and can choice a definitive treatment. Surgical complication rates are higher in younger children (112c). The most common potential complication is transient hypocalcemia which occurs in approximately 10% of patients. Starting therapy with calcitriol 3 days before surgery (0.25 to 0.5 µg twice a day), can reduce the need for calcium infusion and the length of stay (112c). Other, less common potential complications are keloid formation (2.8%), recurrent laryngeal nerve paralysis (2%), hypoparathyroidism (2%) and, rarely (0.08%) death (111). There are fewer complications with an experienced surgeon and when modern methods of anesthesia and pain control are used (112). Prior to surgery, it is important to treat with antithyroid medication in order to render the child euthyroid and prevent thyroid storm. Iodides (Lugols solution, 5 to 10 drops tid or potassium iodide, 2 to 10 drops daily or Na ipodate, 0.5-1 gm every 3 days) are added for 7 to 14 days prior to surgery in order to decrease the vascularity of the gland. L-thyroxine replacement therapy should be given within days of surgery. Following surgical thyroid ablation most patients become hypothyroid and require lifelong thyroid replacement therapy. On the other hand, if therapy is inadequate, hyperthyroidism may recur. Therefore long-term follow-up is mandatory.
131-I Therapy
Definitive therapy with either radioactive iodine or surgical thyroid ablation is usually reserved for patients who have failed drug therapy, developed a toxic drug reaction, or are noncompliant. In recent years, however, radioactive iodine is being favored increasingly, even as the initial approach to therapy (111). The advantages are the relative ease of administration, the reduced need for medical follow up and the lack of demonstrable long term adverse effects (111). The aim of RAI is to ablate completely the thyroid gland and thereby reduce the risk of future neoplasia. RAI should be administrated in a single dose.
Although a dose of 50 to 200 ïCi of 131I/estimated gram of thyroid tissue has been used, the higher dosage is recommended, particularly in younger children, in order to completely ablate the thyroid gland and thereby reduce the risk of future neoplasia. The size of the thyroid gland is estimated, based on the assumption that the normal gland is 0.5-1.0 gms/year of age, maximum 15-20 gms. The formula used is: Estimated thyroid weight in grams X 50-200 mcCi 131 -I/fractional 131I 24 hour uptake Thyroid size can be assessed by ultrasound because underestimation and consequent insufficient RAI treatment is frequent. Surgery may be indicated for goiters larger than 80 gr. Radioactive iodine therapy should be used with caution in children <10 years of age and particularly in those <5 years of age because of the increased susceptibility of the thyroid gland in the young to the proliferative effects of ionizing radiation (113). Pretreatment with antithyroid drugs prior to RAI therapy is advisable if the hyperthyroidism is severe. Thyroid hormone concentrations may rise transiently 4 to 10 days after RAI administration due to the release of preformed hormone from the damaged gland. Beta blockers may be useful during this time period. Similarly, analgesics may be employed if there is mild discomfort due to radiation thyroiditis. Other acute complications of RAI therapy (nausea, significant neck swelling) are rare. One usually sees a therapeutic effect within 6 weeks to 3 months. Worsening of ophthalmopathy, described in adults after RAI, does not appear to be common in childhood. However, if significant ophthalmopathy is present RAI therapy should be used with caution and pretreatment with steroids may be effective. Alternately, another permanent treatment modality (surgery) should be considered.
The question of an age limit below which RAI should not be used frequently arises. With lengthening experience these limits have been lowered. Several studies with average follow-up periods of 12 – 15 years attest to the safety of 131-I therapy in adults (111g,113a,113b). In two studies treated persons showed no tendency to develop thyroid cancer, leukemia, or reproductive abnormalities, and their children had no increase in congenital defects or evidence of thyroid damage (113c,113d). Franklyn and co workers (113e) reported on a population based study of 7417 patients treated with 131-I for thyrotoxicosis in England. They found an overall decrease in incidence of cancer mortality, but a specific increase in mortality from cancer of the small bowel (7 fold) and of the thyroid (3.25) fold 9 (113e). The absolute risk remains very low, and it is not possible to determine whether the association is related to the basic disease, or to radioiodine treatment.
There are less data about long term effects of RAI therapy in pediatric Graves’ disease. In an early report, 73 children and adolescents were so treated. Hypothyroidism developed in 43. Subsequent growth and development were normal (113f). In another group of 23 children treated with 131-I, there were 4 recurrences, at least 5 became hypothyroid, and one was found to have a papillary thyroid cancer 20 months after the second dose (113g). Safa et al. (113a) reviewed 87 children treated over 24 years and found no adverse effects except the well-known occurrence of hypothyroidism. Hamburger (115c) has examined therapy in 262 children ages 3 – 18 and concluded 131-I therapy to be the best initial treatment. Read et al (113h) reviewed experience with 131-I over a 36 year period, including six children under age 6, and 11 between 6 and 11 years. No adverse effects on the patients or their offsprings were found, and they advocate 131-I as a safe and effective treatment. In a review including approximately 1000 children with Graves’ disease treated with RAI and followed for <5 to >20 years to date, (111) there does not appear to be any increased rate of congenital anomalies in offspring nor in thyroid cancer. However, long term follow up data in a larger cohort are still lacking. The epidemic of thyroid cancer apparently induced by radioactive iodine isotopes in infants and children living around Chernobyl suggests caution in use of 131-I in younger children.
Since the possibility of a general induction of cancer by 131-I is of central concern, it is interesting to calculate the risk in children using the data presented by Rivkees et al (113i) who are proponents of use of RAI for therapy in young children. The risk of death from any cancer due specifically to radiation exposure is noted by these authors to be 0.16%/rem for children, and the whole body radiation exposure from RAI treatment at age 10 to be 1.45 rem/mCi administered. Rivkees et al advise treatment with doses of RAI greater than 160 uCi/gram thyroid, to achieve a thyroidal radiation dose of at least 150Gy (about 15000 rads). Assuming a reasonable RAIU of 50% and gland size of 40 gm, the administered dose would thus be 40(gm) x 160uCi/gm x 2 (to account for 50% uptake) =12.8 mCi. Thus the long term cancer death risk would be 12.8 (mCi) x 1.45 rem (per mCi) x 0.16% (per rem) = 3%. For a dose of 15mCi the theoretical incremental risk of a later radiation-induced cancer mortality would be 4% at age 5, 2% at age 10, and 1% at age 15.
Whether or not accepting a specific 2-4% risk of death from any cancer because of this treatment is of course a matter of judgment by the physician and family. However, this would seem to many persons to constitute a significant risk that might be avoided. We note that this is a theoretical risk, based on known effects of ionizing radiation to induce malignancies, but not so far proven in this setting.
Long term studies focused to establish an increased risk of non-thyroid malignancies in children treated with RAI for Graves’ diseases would require about 10.000 children treated below 10 years of age, thus today the decision should be taken on an individual base with the patient and the family. The choice between surgery and RAI therapy in Graves’ disease in children is one of the major long standing controversies in pediatric endocrinology. Most physicians remain concerned about the risks of carcinogenesis, and the experience of Chernobyl has accentuated this concern. Others believe that the risks of surgery and problems with antithyroid drug administration outweigh the potential risk of 131-I therapy. This problem was critically reviewed by Rivkees et al (113j). They point out the significant risks of reaction to antithyroid drugs, and of surgery. Surgery may have a mortality rate in hospital in children of about one per thousand operations, although this may have decreased in recent years. Among problems with radioactive iodide therapy, they note the whole body radiation, possibly worsening of eye disease, and the apparent lack of significant thyroid cancer risk so far reported among children treated with I-131 for Graves’ disease. They assumed that risk would be lower in children after age five, and especially after age ten, and if all thyroid cells were destroyed. They advise using higher doses of radioiodine to minimize residual thyroid tissue, and avoiding treatment of children under age five, but they believe that RAI is a convenient, effective, and useful therapy in children with Graves’ disease. However, as noted above in the section on risks related to use od 131-I, Rivkees own data indicate that treatment of children with conventional doses of RAI may induce a lifetime risk of any fatal cancer of over 2%, a very serious consideration (113i). Many physicians remain reluctant to use 131-I in children under age 15-18 as a first line therapy. Following thyroid ablation most patients become hypothyroid and require lifelong thyroid replacement therapy. On the other hand, if therapy is inadequate, hyperthyroidism may recur. Therefore longterm follow-up is mandatory.
Other Causes of Hyperthyroidism
Non autoimmune hyperthyroidism
Non autoimmune hyperthyroidism is caused by constitutive activation of the TSH receptor (TSHR) (Table 6). Two clinical forms including “familial non-autoimmune autosomal dominant hyperthyroidism (FNAH)” and “persistent sporadic congenital non autoimmune hyperthyroidism (PSNAH)” are described. FNAH is characterized by autosomal dominant inheritance and high variable age of manifestation from neonatal period to 60 years. Variability is present also within the same family. Goiter is present in children, with nodules in older age. PSNAH includes forms with sporadic (de novo) germline mutations in the TSHR. Guidelines about this rare condition have recently been published (90e).
Hyperfunctioning nodules
Hyperthyroidism may be caused by a functioning thyroid adenoma, or functioning thyroid carcinoma. Hyperfunctioning nodules are a rare cause of overt or subclinical hyperthyroidism. Somatic activating mutations within the genes encoding the TSH receptor or the Gs-alpha subunit can be detected (90f). Scintigraphy with Tc 99 or I 123 show hypercaptating nodule and absence of uptake of the surrounding thyroid parenchima. Hyperthyroidism can be controlled with methimazole. Autonomous nodules can be single or a part of multinodular goiter. A recent retrospective study on 31 pediatric cases from US indicated that 45% were overt hyperthyroid at diagnosis and 42% presented with multinodular goiter. Mean age at diagnosis was 15 years, with a range 3-18 yrs. Mean size of the autonomous nodule was 39 mm. In this series of 31 patients, only one patient developed a follicular carcinoma in the controlateral lobe seven years after lobectomy for a benign adenomatoid nodule (114). However, the risk of cancer has been reported up to one third of patients in a series of 31 patients from an iodine- deficient area (114a).
ATA Guidelines for pediatric thyroid nodules and cancer indicate surgery as treatment of children with overt hyperthyroidism due to hyperfunctioning nodules, and surgery is indicated in any nodule >4 cm, because of the decreased sensitivity of FNA to detect malignancy (114b).
Hyperthyroidism may be seen as part of the McCune Albright syndrome (90f) (Table 6). McCune Albright syndrome is due to somatic mutations in Gsα gene that can occur in different tissues as, skin, bones thyroid, adrenal glands.
TSH induced hyperthyroidism
Hyperthyroidism may be due to the inappropriate secretion of TSH by a pituitary adenoma, but thyroid hormone resistance should be excluded.
The syndrome of “inappropriate secretion of TSH” was described in 1975 to indicate two forms of central hyperthyroidism, characterized by high levels of FT3 and FT4 and non suppressed TSH levels(114c). TSH secreting pituitary adenomas are extremely rare in pediatric patients. Guidelines from the ETA has been recently released for these tumors (114d). It is important to consider that a pituitary tumor can be a manifestation of Multiple endocrine neoplasia type 1 and rarely of familial forms of isolated pituitary adenomas with AIP mutations (114e).
In thyroid hormone resistance (RTH) due to mutations of the β isoform of the thyroid hormone receptor hyperthyrodism TSH driven can occur. (See chapter entitled Impaired sensitivity to thyroid hormone. Defects of transport, metabolism and action. .Alexandra M. Dumitrescu, MD and Samuel Refetoff, MD, in this book for a detailed description of this condition).
Tumors secreting chorionic gonadotropin
Recently an adolescent female was described in whom hyperthyroidism resulted from an hCG-secreting hydatidiform mole (114f). Chorioncarcinoma, metastatic embryonal carcinoma of the testis can cause hyperthyroidism (114g).
Transient thyrotoxicosis
Thyrotoxicosis is caused by damage of thyroid cells and release of thyroid hormones stored in the gland. The duration of toxic phase (usually one to three months) depends on the amounts of the thyroid hormones released and the rate of metabolic clearance. Thyroid cell breakdown causes abrupt onset and short duration of symptoms.
Principal causes of transient thyrotoxicosis include:
- Autoimmune thyroiditis (silent thyroiditis): no local symptoms of local inflammation are present.
- Subacute Viral thyroiditis (or de Quervain or granulomatous thyroiditis) it is rarely reported in children and adolescents (106b). Usually presents with sore throat, fever and firm, painful tender enlargement of the thyroid. Mild signs of hyperthyroidism can be overlooked.
- Acute bacterial thyroiditis is rarely a cause of transient thyrotoxicosis.
- Drug-induced thyroiditis (amiodarone and thyrosine kinase inhibitors)
THYROID NODULES AND CANCER
For exhaustive information see also chapters “Thyroid nodules” and “Thyroid cancer “ By F. Pacini and Leslie de Groot in this book.
Recently, the first guidelines specifically elaborated for children with thyroid nodules and differentiated thyroid cancer have been published (114b). Hereditary syndromes (i.e. PTEN related sydromes, DICER1 syndrome, Carney complex, Familial adenomatous polyposis) associated with thyroid cancer in childhood are also been detailed (114b). Medullary thyroid carcinoma guidelines have also been revised, including genetic counseling and modified risk class for children with hereditary MTC (115a).
Thyroid nodules are rare in the first 2 decades of life, but when found, they are more likely to be carcinomatous than are similar masses in adults (115b). Follicular adenomas and colloid cysts account for the majority of benign nodules. Other causes of nodular enlargement include chronic lymphocytic thyroiditis and embryological defects, such as intrathyroidal thyroglossal duct cysts or unilateral thyroid agenesis. Like in adults, the most common form of thyroid cancer in childhood and adolescence is papillary thyroid carcinoma, but other histological types found in the adult may also occur (115c).
A high index of suspicion is necessary if the nodule is painless, of firm or hard consistency, if it is fixed to surrounding tissues or if there is a family history of thyroid cancer. Other worrisome findings include a history of rapid increase in size, associated cervical adenopathy, hoarseness or dysphagia. Even the findings of a cystic component or a functioning nodule, commonly used as favorable signs in adult patients, do not exclude the possibility of neoplasia (115c). Occasionally, thyroid cancer presents in childhood as unexplained cervical adenopathy, or neoplasia is found in patients who also have chronic lymphocytic thyroiditis (115c). The possibility of a rare medullary thyroid carcinoma should be considered if there is a family history of thyroid cancer or pheochromocytoma or if the child has multiple mucosal neuromas and a marfanoid habitus, findings suggestive of multiple endocrine neoplasia (MEN) types 2A and/or 2B (115d).
Children exposed previously to thyroid irradiation comprise a high-risk group. The increased risk of thyroid cancer in adults exposed during childhood to low levels of thyroid irradiation for benign conditions of the head and neck is well known (115e). The increased incidence of both benign and carcinomatous nodules in patients with Hodgkin disease who had received radiotherapy to the neck during childhood is also being documented increasingly (115f, 115g). Thyroid cancer is now known to be the most common second malignancy in childhood survivors of Hodgkin’s and is also seen with increased frequency in leukemia survivors (115h). Similarly, children exposed to high levels of radioactive iodine in the first decade of life or in utero, a consequence of the Chernobyl disaster, are at a markedly increased risk of developing papillary thyroid cancer (113). The risk of thyroid cancer is related to the dose of external irradiation and, unlike the 19 year average latency after low dose irradiation, the average latent period in survivors of Hodgkin disease appears to be only 9 years (115g). In Chernobyl victims, the latency was only 4 years (113). As compared with adults, there appears to be a higher prevalence of gene rearrangements in children with differentiated thyroid cancer, the clinical significance of which is unclear (115h).
Initial investigation of a thyroid nodule includes evaluation of thyroid function and TPO and Tg antibodies. A suppressed serum TSH concentration accompanied by an elevation in the circulating T4 and/or T3 suggests the possibility of a functioning nodule, which can be confirmed with a radionuclide scan. The finding of positive antibodies, on the other hand, usually indicates the presence of underlying chronic lymphocytic thyroiditis, but in some cases, positive antibodies may simply constitute evidence of an immune response to the presence of neoplastic cells. Ultrasonography provides information about whether the nodule is solid or cystic, and whether it is single or multifocal. Fine-needle aspiration biopsy, popular in the investigation of thyroid carcinoma in adults, is gaining increasing acceptance and is now considered to be the procedure of choice in the evaluation of nodules >0.5 cm (115k).
There is an increased incidence of both cervical node involvement and of pulmonary metastases at the time of diagnosis in children with thyroid carcinoma (115c). Nonetheless, the long term cancer specific mortality rate is no greater in children than in adults <40 years of age (115i). Guidelines specifically elaborated for management of children with thyroid nodules differentiated thyroid cancer have been published (114b). Excision of the tumor or lobe is the appropriate treatment for benign tumors and cysts, whereas total thyroidectomy with preservation of the parathyroid glands and recurrent laryngeal nerves is the initial therapy for malignant thyroid tumors. The latter procedure is followed by radioablation if there is evidence of residual gland or tumor after surgery. The issue of prophylactic lymph node dissection is controversial (115h). After radioiodine therapy, the dose of thyroxine is adjusted to keep the serum TSH concentration suppressed (between 0.05 mU/L and 0.1 mU/L in a sensitive assay). Measurement of serum Tg, a thyroid follicular cell-specific protein, is used to detect evidence of metastatic disease in differentiated forms of thyroid cancer, such as papillary or follicular carcinoma. This is best performed after a period (usually 6 weeks) of thyroxine withdrawal or after the exogenous administration of recombinant TSH (115). Measurement of circulating calcitonin is used as a tumor marker for medullary thyroid cancer (MTC), a C-cell derived malignancy (115m). Mutations of the RET protooncogene, detectable in nearly all familial forms of MTC, is of value in screening family members (115e, 115m). In families affected with multiple endocrine neoplasia type 2, screening of children as young as 5 years followed by total thyroidectomy has been successful in curing patients with microscopic MTC, an otherwise highly malignant neoplasm with a poor prognosis (115e). See Medullary Thyroid Carcinoma guidelines for updated genetic counseling and modified risk class for children with hereditary MTC (115a).
Optimal monitoring of patients with a history of thyroid irradiation during childhood remains controversial. Because of the insensitivity of clinical palpation, regular assessment of thyroid function (TSH and, as necessary free T4) as well as ultrasound examinations should be performed. There is evidence that thyroid suppression is associated with a reduction in the development of new nodules after partial surgical resection of an irradiated thyroid gland (115q) but whether it plays any role if the TSH is not elevated or in preventing neoplasia is unknown. Recently, a study that followed a cohort of 4338 5- years survivors of pediatric solid cancer suggested that chemotherapy (nitrosureas), splenectomy, and radiation dose to pituitary gland also play a role in predicting thyroid cancer risk (115r).
A retrospective study on the effects of total body irradiation (TBI) preceding hemopoietic cell transplation in childhood suggested short term and life-long monitoring for thyroid nodules and thyroid cancer in these patients (115s). Although it was a small size, retrospective study they found the time from TBI to thyroid carcinoma detection ranged from 2.2 years to 15.3 years. Follow up programs are advised for long term survivors of childhood cancer.
REFERENCES
- Missero C, Cobellis G, De Felice M, Di Lauro R. Molecular events involved in differentiation of thyroid follicular cells. Mol Cell Endocrinol. 1998;140:37-43.
- De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25:722-46.
- Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121;1989-2003.
- Fagman H, Grande M, Edsbagge J, Semb H, Nilsson M. Expression of classical cadherins in thyroid development: maintenance of an epithelial phenotype throughout organogenesis. Endocrinology. 2003;144:3618-24.
- Fagman H, Liao J, Westerlund J, Andersson L, Morrow BE, Nilsson M. The 22q11 deletion syndrome candidate gene Tbx1 determines thyroid size and positioning. Hum Mol Genet. 2007;16:276-85.
- Fagman H, Grande M, Gritli-Linde A, Nilsson M. Genetic deletion of sonic hedgehog causes hemiagenesis and ectopic development of the thyroid in mouse. Am J Pathol. 2004;164:1865-72.
- Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072-8.
- Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med. 1981;304:702-12.
- Thorpe-Beeston JG, Nicolaides KH, McGregor AM. Fetal thyroid function. Thyroid.1992;2:207-17.
- Williams FL, Simpson J, Delahunty C, et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab. 2004;89:5314-20.
- Brown RS, Shalhoub V, Coulter S, et al. Developmental regulation of thyrotropin receptor gene expression in the fetal and neonatal rat thyroid: relation to thyroid morphology and to thyroid-specific gene expression. Endocrinology. 2000;141:340-5.
- Hume R, Simpson J, Delahunty C, et al. Human fetal and cord serum thyroid hormones: developmental trends and interrelationships. J Clin Endocrinol Metab. 2004;89:4097-103.
- De Nayer P, Cornette C, Vanderschueren M, et al. Serum thyroglobulin levels in preterm neonates. Clin Endocrinol. 1984;21:149-53.
- Sobrero G, Munoz L, Bazzara L, et al. Thyroglobulin reference values in a pediatric infant population. Thyroid. 2007;17:1049-54.
- Roti E, Gnudi A, Braverman LE. The placental transport, synthesis and metabolism of hormones and drugs which affect thyroid function. Endocr Rev.1983;4:131-49.
- Kester MH, Kaptein E, Van Dijk CH, et al. Characterization of iodothyronine sulfatase activities in human and rat liver and placenta. Endocrinology. 2002;143:814-9.
- Van der Geyten S, Segers I, Gereben B, et al. Transcriptional regulation of iodothyronine deiodinases during embryonic development. Mol Cell Endocrinol. 2001;183:1-9.
- Ruiz de Ona C, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Developmental changes in rat brain 5′-deiodinase and thyroid hormones during the fetal period: the effects of fetal hypothyroidism and maternal thyroid hormones. Pediatr Res. 1988;24:588-94.
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975-87.
- Ferreiro B, Bernal J, Goodyer CG, Branchard CL. Estimation of nuclear thyroid hormone receptor saturation in human fetal brain and lung during early gestation. J Clin Endocrinol Metab. 1988;67:853-6.
20a. Chan SY, Vasilopoulou E, Kilby MD. The role of the placenta in thyroid hormone delivery to the fetus. Nat Clin Pract Endocrinol Metab. 2009; 5:45-54.
20b. Patel J.Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164-70.
20c. Burns R, O’Herlihy C, Smyth PP. The Placenta as a Compensatory Iodine Storage Organ. Thyroid. 2011; 21: 541-46.
20d. Burns R, O’Herlihy C, Smyth PP. Regulation of iodide uptake in placental primary cultures. Eur Thyroid J. 2013;2:243-51.
20e. Li H, Patel J, Mortimer RH, Richard K. Ontogenic changes in human placental sodium iodide symporter expression. Placenta. 2012;33:946-8.
- Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321:13-6.
- Calvo RM, Jauniaux E, Gulbis B, et al. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab. 2002;87:1768-77.
22a. Contempre B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab.1993; 77:1719-22.
22b. Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Practice. 2007; 3:249-259.
22c. Rovet JF. The role of thyroid hormones for brain development and cognitive function. Endocr Dev. 2014;26:26-43.
22d. Morreale de Escobar G, Obregon MJ, Escobar del Rey F: Maternal thyroid hormones early in pregnancy and fetal brain development. In: Best Practice & Research in Clinical Endocrinology and Metabolism: The Thyroid and Pregnancy. Editor: Glinoer D 2004; 18:225.
22e. Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. Neuroendocrinol. 2008;20:784-94.
22f. Schroeder AC, Privalsky ML. Thyroid hormones, T3 and T4, in the brain. Front Endocrinol. 2014;5:40. doi: 10.3389/fendo.2014.00040. eCollection 2014.
22g. Bernal J, Guadaño-Ferraz A, Morte B. Thyroid hormone transporters-functions and clinical implications. Nat Rev Endocrinol. 2015;11:406-17.
- Meinhold H, Dudenhausen JW, Wenzel KW, Saling E. Amniotic fluid concentrations of 3,3′,5′-tri-iodothyronine (reverse T3), 3,3′-di-iodothyronine, 3,5,3′-tri-iodothyronine (T3) and thyroxine (T4) in normal and complicated pregnancy. Clin Endocrinol.1979;10:355-65.
23a. Seror R, Amand G, Guibourdenche J, Ceccaldi PF, Luton D, Anti-TPO antibodies diffusion through the placental barrier during pregnancy. PloS One. 2014, 9:e84647.
23b. Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol. 2012; 26 (Supp1):108-117.
- Boyages SC. Clinical review 49: Iodine deficiency disorders. J Clin Endocrinol Metab. 1993;77:587-91.
- Rovet J, Walker W, Bliss B, Buchanan L, Ehrlich R. Long-term sequelae of hearing impairment in congenital hypothyroidism. J Pediatr. 1996;128:776-83.
- de Zegher F, Pernasetti F, Vanhole C, Devlieger H, Van den Berghe G, Martial JA. The prenatal role of thyroid hormone evidenced by fetomaternal Pit-1 deficiency. J Clin Endocrinol Metab. 1995;80:3127-30.
- Matsuura N, Konishi J. Transient hypothyroidism in infants born to mothers with chronic thyroiditis–a nationwide study of twenty-three cases. The Transient Hypothyroidism Study Group. Endocrinol Jpn. 1990;37:369-79.
27a. Hallowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braveman LE. Serum T4 and thyroid antibodies in the United States population (1988-1994): National Health and Nutrition Examination Survay (NHANES III). J Clin Endocrinol Metab. 2002; 489-499.
- Man EB, Jones WS, Holden RH, Mellits ED. Thyroid function in human pregnancy. 8. Retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am J Obstet Gynecol. 1971;111:905-16.
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med.1999;341:549-55.
29a. Smit BJ, Kok JH, Vulsma T, Briët JM, Boer K, Wiersinga WM. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr. 2000;89:291-5.
29b. Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, Hua T. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol. 2010;72:825-9.
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149-55.
30a. Pop VJ, Brouwers EP, Vader H, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol. 2003;59:282-8.
30b. Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM, Hofman A, Jaddoe VV, Visser W, Steegers EA, Verhulst FC, de Rijke YB, Tiemeier H. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95:4227-34.
30c. Suárez-Rodríguez M, Azcona-San Julián C, Alzina de Aguilar V. Hypothyroxinemia during pregnancy: the effect on neurodevelopment in the child. Int J Dev Neurosci. 2012;30:435-8.
30d. Finken MJ, van Eijsden M, Loomans EM, Vrijkotte TG, Rotteveel J. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab. 2013;98:1417-26.
30e. Julvez J, Alvarez-Pedrerol M, Rebagliato M, Murcia M, Forns J, Garcia-Esteban R, Lertxundi N, Espada M, Tardón A, Riaño Galán I, Sunyer J. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013;24:150-7.
30f. Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella G,Scaffidi G, Castagna MG, Mattina F, Violi MA, Crisà A, Artemisia A, Trimarchi F. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency:a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-60.
30g. Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, HofmanA, Verhulst FC, Ghassabian A. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr. 2015;169:838-45.
30h. Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW,Hofman A, de Rijke YB, Verhulst FC, Tiemeier H. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol. 2013;74:733-42.
30k. Gyllenberg D, Sourander A, Surcel HM, Hinkka-Yli-SalomäkiS, McKeague IW, Brown AS. Hypothyroxinemia During Gestation and Offspring Schizophrenia in a National Birth Cohort. Biol Psychiatry. 2015 19. pii: S0006-3223(15)00520-X.
30i. Ghassabian A, Bongers-Schokking JJ, de Rijke YB, van Mil N, Jaddoe VW, de Muinck Keizer-Schrama SM, Hooijkaas H, Hofman A, Visser W, Roman GC, Visser TJ, Verhulst FC, Tiemeier H. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study.Thyroid. 2012; 22:178-86.
30j. Pop VJ, de Vries E, van Baar AL, Waelkens JJ, de Rooy HA, Horsten M, Donkers MM, Komproe IH, van Son MM,Vader HL. Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired child development? J Clin Endocrinol Metab. 1995;80:3561-6.
30l. Wasserman EE, Pillion JP, Duggan A, Nelson K, Rohde C, Seaberg EC, Talor MV, Yolken RH, Rose NR. Childhood IQ, hearing loss, and maternal thyroid autoimmunity in the Baltimore Collaborative Perinatal Project. Pediatr Res. 2012;72:525-30.
30m. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-7.
30n. Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, Rovet J. Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies. Pediatr Res. 2005;57:902-7.
30o. Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, de Muinck Keizer-Schrama SM, Hooijkaas H, Steegers EA, Hofman A, Verhulst FC, van der Ende J, de Rijke YB, Tiemeier H. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. 2011;69:454-9.
- Momotani N, Iwama S, Momotani K. Neurodevelopment in children born to hypothyroid mothers restored to normal thyroxine (T₄) concentration by late pregnancy in Japan: no apparent influence of maternal T₄ deficiency. J Clin Endocrinol Metab. 2012;97:1104-8.
31a. Liu H, Momotani N, Noh JY, Ishikawa N, Takebe K, Ito K. Maternal hypothyroidism during early pregnancy and intellectual development of the progeny. Arch Intern Med. 1994;154:785-7.
31b. Downing S, Halpern L, Carswell J, Brown RS Severe maternal hypothyroidism corrected prior to the third trimester is associated with normal cognitive outcome in the offspring. Thyroid. 2012; 22:625-30.
- Lazarus JH, Bestwick JP, Channon S et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012; 366:493-501.
32a. Casey B. Effect of treatment of maternal subclinical hypothyroidism or hypothyroxinemia on IQ in offspring. American Journal of Obstetrics & Gynecology. Supplement to JANUARY 2016 Abstr 2 S2.
32b. Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96:3234-41.
32c. Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H, Peeters RP. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016; 4:35-43.
32d. Samadi A, Skocic J, Rovet JF. Children born to women treated for hypothyroidism during pregnancy show abnormal corpus callosum development.Thyroid. 2015; 25:494-502.
32e. Lischinsky JE, Skocic J, Clairman H, Rovet J. Preliminary Findings Show Maternal Hypothyroidism May Contribute to Abnormal Cortical Morphology in Offspring. Front Endocrino . 2016;25:7-16.
32f. Williams FL, Watson J, Ogston SA, Visser TJ, Hume R, Willatts P. Maternal and umbilical cord levels of T4, FT4, TSH, TPOAb, and TgAb in term infants and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. 2013;98: 829-38.
- Klein RZ, Haddow JE, Faix JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1991;35:41-6.
33a. Thangaratinam S, Tan A, Knox E, Kiliby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011; 342:d2616.
33b. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, grobman W, Laurberg P, Lazarus JK, Mandel SJ, Peeters R, Sullivan S. 2016 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2017, Jan 6. doi: 10.1089/thy.2016.0457. [Epub ahead of print]
- Abuid J, Stinson DA, Larsen PR. Serum triiodothyronine and thyroxine in the neonate and the acute increases in these hormones following delivery. J Clin Invest..1973;52:1195-9.
- Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest. 1987;79:295-300.
- Houstek J, Vizek K, Pavelka S, et al. Type II iodothyronine 5 ′ -deiodinase and uncoupling protein in brown adipose tissue of human newborns. J Clin Endocrinol Metab. 1993;77:382-7.
- Mercado M, Yu VY, Francis I, Szymonowicz W, Gold H. Thyroid function in very preterm infants. Early Hum Dev. 1988;16:131-41.
- Nelson JC, Weiss RM, Wilcox RB. Underestimates of serum free thyroxine (T4) concentrations by free T4 immunoassays. J Clin Endocrinol Metab. 1994;79:76-9.
- Ares S, Escobar-Morreale HF, Quero J, et al. Neonatal hypothyroxinemia: effects of iodine intake and premature birth. J Clin Endocrinol Metab. 1997;82:1704-12.
- Frank JE, Faix JE, Hermos RJ, et al. Thyroid function in very low birth weight infants: effects on neonatal hypothyroidism screening. J Pediatr. 1996;128:548-54.
- Kok JH, Tegelaers WH, de Vijlder JJ. Serum thyroglobulin levels in preterm infants with and without the respiratory distress syndrome. I. Cord blood study. Pediatr Res. 1986;20:996-1000.
- Thorpe-Beeston JG, Nicolaides KH, Snijders RJ, Felton CV, McGregor AM. Thyroid function in small for gestational age fetuses. Obstet Gynecol. 1991;77:701-6.
- Zurakowski D, Di Canzio J, Majzoub JA. Pediatric reference intervals for serum thyroxine, triiodothyronine, thyrotropin, and free thyroxine. Clin Chem. 1999;45:1087-91.
- Fisher D. Next generation newborn screening for congenital hypothyroidism? J Clin Endocrinol Metab. 2005;90:3797-9.
44b. Marvin L. Mitchell, Ho-Wen Hsu, Inderneel Sahai and the Massachusetts Pediatric Endocrine Work Group. The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol. 2011;75:806-10.
- Klein AH, Meltzer S, Kenny FM. Improved prognosis in congenital hypothyroidism treated before age three months. J Pediatr. 1972;81:912-5.
- Dussault JH. The anedoctal history of screening for congenital hypothyroidism. J Clin Endocrinol Metab. 1999;84:4332-4.
- Delange F. Neonatal screening for congenital hypothyroidism: results and perspectives. Horm Res.1997;48:51-61.
- Rovet J, Daneman D. Congenital hypothyroidism: a review of current diagnostic and treatment practices in relation to neuropsychologic outcome. Paediatr Drugs. 2003;5:141-9.
50a. Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Practice & Research Clinical Endocrinology & Metabolism. 2014;28:175-187.
50b. Wassner AJ, Brown RS. Congenital Hypothyroidism: recent advances. Curr Opin Endocrinol Diabetes Obes. 2015, 22:407-412.
50c. Olivieri A, Corbetta C, Weber G, Vigone MC, Fazzini C,Medda E, and The Italian Study Group for Congenital Hypothyroidism. Congenital hypothyroidism due to defects of thyroid development and mild increase of TSH at screening: data from the Italian registry of infants with congenital hypothyroidism. J Clin Endocrinol Metab. 2013; 98:140:3-1408.
50d. Harris KB and Pass KA. Increase of congenital hypothyroidism in New York State and in The United States. Molecular Genetics and Metabolism. 2007; 91:268-277.
50e. Hinton CF, Harris KB, Borgfeld L et al. trends in incidence of congenital hypothyroidism related to select demographic factors: data from the United States , California, Massachussets, New York and Texas. Pediatrics. 2010;125:S37-S47.
50f. Olivieri A, Fazzini C, Medda E. Multiple factors influencing the incidence of congenital hypothyroidism detected by neonatal screening. Horm Res Pediatr. 2015 83:86-93.
50g. Barry Y, Bonaldi C, Goulet V, Coutant R, Leger J, Paty AC, Delmas D, Cheillan D, Roussey M. Increased incidence of congenital hypotiroidism in France from 1982 to 2012: a nationwide multicenter analysis. Ann Epidemiol. 2016;26:100-105.
- LaFranchi SH, Hanna CE, Krainz PL, Skeels MR, Miyahira RS, Sesser DE. Screening for congenital hypothyroidism with specimen collection at two time periods: results of the Northwest Regional Screening Program. Pediatrics. 1985;76:734-40.
51a. Ford GA, Denniston S, Sessere D, Skeels MR, LaFranchi SH. Transient versus permanent congenital hypothyroidism after the age of 3 years in infants, detected on the first versus second newborn screening test in Oregon, USA. Horm Res Paediatr. 201616;86(3):169-177
51b. Leger J, Olivieri A, Donaldson M, Torresani T,Krude H, van Vliet G, Polak M, Butler G on behalf of ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE, and the Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology Consensus Guidelines on Screening, Diagnosis, and management of congenital hypothyroidism. Horm Res Pediatr. 2014;81:80-103.
51c. Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, Polak M, Butler G on behalf of ESPE-PES-SLEP-JSPE-APEG-APPE-ISPAE, and the Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology Consensus Guidelines on Screening, Diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014; 99:363-384.
- Lanting CI, van Tijn DA, Loeber JG, Vulsma T, de Vijlder JJ, Verkerk PH. Clinical effectiveness and cost-effectiveness of the use of the thyroxine/thyroxine-binding globulin ratio to detect congenital hypothyroidism of thyroidal and central origin in a neonatal screening program. Pediatrics. 2005;116:168-73.
- van Tijn DA, de Vijlder JJ, Verbeeten B, Jr., Verkerk PH, Vulsma T. Neonatal detection of congenital hypothyroidism of central origin. J Clin Endocrinol Metab. 2005; 90:3350-9.
53a. Nesbesio TD, McKenna MP, Nabhan ZM et al. Newborn screening results in children with central hypothyroidism. J Pediatr. 2010;156:990-3.
53b. Adachi M, Soneda A, Asakura Y, Muroya K, Yamagami Y, Hirahara F. Mass screening of newborns for congenital hypothyroidism of central origin by free thyroxine measurement of blood samples on filter paper. Eur J Endocrinol. 2012; 166:829-838.
53c. Soneda, A, Adachi M, Muroya K, Asakura Y, Yamagami Y, Hirahara F. Overall usefulness of newborn screening for congenital hypothyroidism by using free thyroxine measurements. Endocr J 2014; 61:025-1-30.
- American Academy of Pediatrics AAP Section on Endocrinology and Committee on Genetics, and American Thyroid Association Committee on Public Health: Newborn screening for congenital hypothyroidism: recommended guidelines. Pediatrics. 1993;91:1203-9.
54a. Peters C, Brooke I, Ifederu A, Langham S, Hindmarsh P, Cole TJ. Defining the newborn blood spot screening reference interval for TSH: impact of ethnicity. J Clin Endocrinol Metab. 2016; 101:3445-3449.
- 55. Fisher DA. Effectiveness of newborn screening programs for congenital hypothyroidism: prevalence of missed cases. Pediatr Clin North Am. 1987;34:881-90.
- Adams LM, Emery JR, Clark SJ, Carlton EI, Nelson JC. Reference ranges for newer thyroid function tests in premature infants. J Pediatr. 1995;126:122-7.
56a. Vigone MC, Caiulo S, Di Frenna M et al. Evolution of thyroid function in preterm infants detected by screening for congenital hypothyroidism. J Pediatrics. 2014;164: 1296-1302.
56b. LaFranchi SH. Screening preterm infants for congenital hypothyroidism: better the second time around. J Pediatr. 2014; 164:1259-1261.
56c. Zoller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observation and experimental findings. J Neuroendocrinol. 2004:16:809-818.
56d. Spencer CA, LoPresti JS, Patel A et al. Application of a new chemiluminometric thyrotropin assay to subnormal measurement. J Clin Endocrinol Metab. 1990;70:453-460.
56e. Parazzini C, Baldoli C, Scotti G, Triulzi F. Terminal zones of myelination: MR evaluation of children aged 20-40 months. Am J Neuroradiol. 2002:23:1669-1673.
56f. Gagne N, Parma J, Deal C, Vassart G, Van Vliet G. Apparent congenital athyreosis contrasting with normal plasma thyroglobulin levels and associated with inactivating mutations in the thyrotropin receptor gene: are athyreosis and ectopic thyroid distinct entities? J Clin Endocrinol Metab. 1998;83:1771-5.
- Smith DW, Klein AM, Henderson JR, Myrianthopoulos NC. Congenital hypothyroidism–signs and symptoms in the newborn period. J Pediatr. 1975;87(6 Pt 1):958-62.
57a. Karakoc-Aydiner E, Turan S, Akpinar I, Dede F, Isguven P, Adal E, Guran T, Akcay T, Bereket A. Pitfalls in the diagnosis of thyroid disgenesis by thyroid untrasonography and scintigraphy. Eur J Endocrinol. 2012; 166:43 -48.
- Brown RS, LaFranchi S, Rose SR. Patient information page from the hormone foundation. Congenital hypothyroidism. J Clin Endocrinol Metab. 2009;94:1835-6.
58a. Carswell JM, Gordon JH, Popovsky E, Hale A, Brown RS. Generic and brand-name L-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J Clin Endocrinol Metab. 2013;98: 610-617.
- Bongers-Schokking JJ, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on mental, psychomotor, and behavioral development in children with congenital hypothyroidism. J Pediatr. 2005;147:768-74.
- Heyerdahl S, Oerbeck B. Congenital hypothyroidism: developmental outcome in relation to levothyroxine treatment variables. Thyroid. 2003;13:1029-38.
- Simoneau-Roy J, Marti S, Deal C, Huot C, Robaey P, Van Vliet G. Cognition and behavior at school entry in children with congenital hypothyroidism treated early with high-dose levothyroxine. J Pediatr. 2004;144:747-52.
- Rovet JF. In search of the optimal therapy for congenital hypothyroidism. J Pediatr. 2004;144:698-700.
- Dimitropoulos A, Molinari L, Etter K, et al. Children with congenital hypothyroidism: long-term intellectual outcome after early high-dose treatment. Pediatr Res. 2009;65:242-8.
- Fisher DA. The importance of early management in optimizing IQ in infants with congenital hypothyroidism. J Pediatr. 2000;136:273-4.
64a. Leger J. Congenital hypothyroidism: a clinical update of long-term outcome in young adults. Eur J Endocrinol. 2015;172, R67-R77.
64b. Leger J, Ecosse E, Roussey M, Lanoe JL, Larroque B. French Congenital hypothyroidism study group. Subtle health impairment and socioeducational attainment in young adult patients with congenital hypothyroidism diagnosed by neonatal screening: a longitudinal population-based cohort study. J Clin Endocrinol Metab. 2011;96:1771-82.
64c. Lichtenberer-Geslin L, Dos Santos S, Hassani Y, Ecosse E, Van Den Abbele T, Leger J. Factors associated with hearing impairment in patient with congenital hypothyroidism treated since the neonatal period: a national population-based study. J Clin Endocrinol Metab. 2013; 98:3644-3652.
64d. Leger J, dos Santos S, larroque B, Ecosse E. Pregnancy outcomes and relationship to treatment adequacy in women treated early for congenital hypothyroidism: a longitudinal population-based study. J Clin Endocrinol Metab. 2015; 100: 800-809.
64e. Lof C, Patyra K, Kuulasmaa T, Vangipurapu J, Undeutsch H, Jaeschke H, Pajunen T, Kero A, Krude H, Biebermann, Kleinau G, Kuhnen P, Rantakari K, Miettinen P, Kirjavainen T, Pursiheimo JP, Mustila T, Jaaskelainen J, Ojaniemi M, Toppari J, Ignatius J, Laakso M, Kero J. Detection of novel gene variants associated with congenital hypothyroidism in a finnish patient cohort. Thyroid. 2016; 26:1215-1224.
64f. Nicholas AK, Serra EG, Cangui H, Alyaarubi S, Ullah I, Schoenmakes E, Deeb A, Habeb AM, AI Maghamsi M, Peters C, Nathwani N, Aycan Z, Saglam H, Bober E, Dattani M, Shenoy S, Murray PG, Babiker A, Willensen R, Thankarmony A, Lyons G, Irwin R, Padidela R, Tharian K, Davies JH, Puthi V, Park SM, Massoud AF, Gregory JW, Albanese A, Pease-Gevers E, Martin H, Brugger K, Maher ER, Chatterje K, Anderson CA, Schoenmakers N. Comprehensive screening of eight known causative genes in congenital hypothyroidism with gland-in-situ. J Clin Endocrinol Metab. 2016; 12:4521-31.
- Siebner R, Merlob P, Kaiserman I, Sack J. Congenital anomalies concomitant with persistent primary congenital hypothyroidism. Am J Med Genet. 1992;44:57-60.
- Kumar J, Gordillo R, Kaskel FJ, Druschel CM, Woroniecki RP. Increased prevalence of renal and urinary tract anomalies in children with congenital hypothyroidism. J Pediatr. 2009;154:263-6.
- Perry R, Heinrichs C, Bourdoux P, et al. Discordance of monozygotic twins for thyroid dysgenesis: implications for screening and for molecular pathophysiology. J Clin Endocrinol Metab. 2002;87:4072-7.
67a. Brown RS, Demmer LA. The etiology of thyroid dysgenesis-still an enigma after all these years. J Clin Endocrinol Metab. 2002;87:4069-71.
- Fort P, Lifshitz F, Bellisario R, et al. Abnormalities of thyroid function in infants with Down syndrome. J Pediatr. 1984;104:545-9.
68a. Stoupa A, Kariyawasam D, Carre’ A, Polak M. Update of thyroid development genes. Endocrinol Metab Clin N Am. 45:243-246, 2016.
68b. Szinnai G. genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab. 2014; 28:133-50.
68c. Satoshi Narumi and Tobonobu Hasegawa. TSH resistance revised. Endocrine Journal. 2015, 62:393-398.
68d. Calebiro D, Gelmini G, Cordella D et al. Frequent TSH receptor genetic alterations with variable signaling impairment in a large series of children with nonautoimmune isolated hyperthyrotropinemia. J Clin Endocrinol Metab. 2012; 97: E156-160.
68e. Carre A, Szinnai G, Castanet M et al. Five new TTF1/NKX2-1 mutations in brain–lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum Mol Genet. 2009;18:2266-76.
68f. de Filippis T, Marelli F, Vigone MC, Di Frenna M, Weber G, Persani L. Novel NKX2-1 frameshift mutations in patients with athypical phenotypes of the brain-lung-thyroid syndrome. Eu Thyroid J. 2014; 3: 227-233.
68g. Thowarth A, Schittert-Hubener S , Schrumpf P et al. Comprehensive genotyping and clinical characterization reveal 27 novel NKX2-1 mutations and expand the phenotypic spectrum. J Med Genet. 2014;51:375-387.
68h. Stenson PD, Mort M, Ball EV et al. The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133: 1-9.
68i. Narumi S, Muroya K, Asakura Y et al. Transcription factor mutations and congenital hypothyroidism: systematic genetic screening of a population-based cohort of Japanese patients. J Clin Endocrinol Metab. 2010: 95: 1981-5.
68j. Fernandez LP, Lopez- Marquez A, Santisteban P. Thyroid transcription factors in development, differentiation and disease. Nat Rev Endocrinol. 2015; 11:29-42.
68k. Fernandez LP, Lopez-Marquez A, Martinez AM, Gomez-Lopez G, Santisteban P l. New insights into FOXE1 functions: identification of direct FOXE1 targets in thyroid cells. PLoS One. 2013, 8:e62849.
68m. Carre A Hamza RT, Kariyawasam D, et al. A novel FOXE1 mutation (R73S) in Bamforth-Lazarus syndrome causing increased thyroid gene expression. Thyroid. 2014:24:649-65.
68n. Dentice M, Cordeddu V, Rosica A et al. Missense mutation in the transcription factor NKX2-5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab. 2006;91:1428-1433.
68o. Opitz R, Hitz MP, Vandernoot I et al. Functional zebrafish studies based on human genotyping point to netrin-1 as a link between aberrant cardiovascular development and thyroid dysgenesis. Endocrinology. 2015;156:377-388.
68q. Porazzi P, Marelli F, Benato F,de Filippis T,Calebiro D, Argenton F, Tiso N, Persani L . Disruptors of global and JAGGED1 mediated notch signaling affect thyroid morfogenesis in the zebrafish. Endocrinology. 2012; 153:5645-5658.
68r. De Filippis T, Marelli F. Nebbia G, Porazzi P, Corbetta S, Fugazzola L, Gastaldi R, Vigone MC, Biffanti R, Frizziero D, Mandara’ L, Prontera P, Salerno M, Maghnie M, Tiso N, Radetti G, Weber G, Persani L. JAG1 loss- of- function variations as a novel predisposing event in the pathogenesis of congenital thyroid defects. J Clin Endocrinol Metab. 2016;101:861-870.
68s. Knobel M, Madeires-Neto G. An outline of inherited disorders of the thyroid hormone generating system. Thyroid. 2003; 13:771-801.
- Portulano C, Paroder-Belenitsky M, Carrasco N. The Na+/I symporter (NIS): mechanism and medical impact. Endocr Rev. 2014;35 :106-149.
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani F, Nassir E, Baxevanis AD, Sheffied VC, Green ED. Pendred syndrome is caused by mutations in putative sulphate transporter gene (PDS). Nat Genet. 1997;17:411-422.
- 71. Kopp P. Pendred’s syndrome: identification of the genetic defect a century after its recognition. Thyroid. 1999;9:65-9.
71a. Ladsous M, Vlaeminck-Guillem V, Dumur V, Vincent C, Dubrulle F, Dhanens CM, Wemeau JL. Analysis of the thyroid phenotype in 42 patients with Pendred syndrome and non syndromic enlargement of the vestibular aqueduct. Thyroid. 2014; 24:639-648.
71b. Bizhanova A, Kopp P. Genetics and phenomics of Pendred syndrome. Mol Cell Endocrinol. 2010;322:83-90.
- Nascimiento AC, Guendes DR, Santos CS, Knobel M, Rubio IG, Medeiros-Neto G. Thyroperoxidase gene mutations in congenital goitrous hypothyroidism with total and partial iodide organification defect. Thyroid. 2003;13:1145-51.
72a. Delodoey J, Pfarr N, Vuissoz JM, Parma J, Vassart G, Biesterfeld S, Phlenz J, Van Vliet G. Pseudodominant inherence of goitrous congenital hypothyroidism caused byTPO mutations; molecular and in silico studies. J Clin Endocrinol Metab. 2008;93:627-633.
72b. Moreno JC,Bikker H, Kempers MJE, vanTrotsenburg AS, Baas F, deVijlder JJM, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002; 347:95-102.
72c. Muzza M, Rabbiosi S, Vigone MC, Zamproni I, Cirelio V, Maffini M, Maruca K, Schoenmarkers N, Beccaria L, Gallo F, Park SM, Beck-Peccoz P, Persani L, Weber G, Fugazzola L. The clinical and molecular characterization of patients with dyshormonogenetic congenital hypothyroidism reveals specific diagnostic clues for DUOX2 defects. J Clin Endocrinol Metab. 2014;99:E544-E553.
72d. Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, Onigata K, Fugazzola L, Refetoff S, Persani L, Weber G. Biallelic inactivation of the dual oxidation maturation factor 2 (DUAXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008; 93:605-610.
72e. Ieri T, Cochaux P, Targovinik HM, Suzuki M, Shimoda S, Perret J, Vassart G. A 3’splice site mutation in the thyroglobulin gene responsible for congenital goiter with hypothyroidism. J Clin Invest..1991:88:1901-1905.
72f. Medeiros-Neto G, Kim PS, Yoo SE, Vono J, Targovnik H, Camargo R, Hossain SA, Arvan P. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J Clin Invest. 1996; 98:2838-2844.
72g. Citterio CE, Rossetti LC, Souchon PF, Morales C, Thouvard-Viprey M, Salmon-Musial AS, Mauran PL, Doco-Fenzy M, Gonzales-Sarmiento R, Rivolta CM, De Brasi CD, Targovink HM. Novel mutational mechanism in the thyroglobulin gene: imperfect DNA inversion as a cause for hereditary hypothyroidism. Mol Cell Endocrinol. 2013; 381:220-229.
72h. Iglesias A, Garcia-Nimo L, Cocho de Juan JA, Moreno JC. Towards the pre-clinical diagnosis of hypothyroidism caused by iodotyrosine deiodinase (DEHAL1) deficts. Best Prac Res Clin Endocrinol Metab. 2014; 28:151-159.
72i. Moreno JC, Klootwijk W, van Toor H, Pinto G, D’Allessandro M, Leger A, Goundie D, Polak M, Gruters A, Visser TJ. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N Eng J Med. 2008;358:1856-1859.
- Fisher DA, Dussault JH, Foley TP, Klein AH, LaFranchi S, Larsen PR, Mitchell ML, Murphey WH, Walfish PG. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr. 1979;94: 700-705.
73a. Lanting CI, van Tijn DA, Loeber JG, Vulsma T, de Vijder JJ, Verkerk PH. Clinical effectiveness and cost-effectiveness of the use of the thyroxine-binding globulin ratio to detect congenital hypothyroidism of thyroidal and central origin in a neonatal screening program. Pediatrics. 2005;116:168-173.
73b. Kempers MJ, Lanting CI, van Heijst AF, van Trotsenburg AS, Wiedijk BM. De Vijder JJ,Vulsma T. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin and thyroxine-binding globulin measurement potentials and pitfalls. J Clin Endocrinol Metab.2006; 91:3370-3376.
73c. Zwaveling-Soonawala N, van Trotsenburg AS, Verkerk PH. The severity of congenital hypothyroidism of central origin should not be underestimated. J Clin Endocrinol Metab. 2015:100:E297-E300.
73d. Collu R, Tang J, Castagne’ J, Legace’ G, Masson N, Huot C, Deal C, Delvin E, Faccenda F, Edne KA, Van Vliet G. A novel mechanism for isolated central hypothyroidism: Inactivating mutations in the thyrotropin releasing-hormone receptor gene. J Clin Endocrinol Metab. 1997:82:1561-1565.
73e. Bonomi M, Busnelli M, Beck-Peccoz P, Costanzo D, Antonica F, Dolci C, Pilotta A, Buzi F, Persani L. A family with complete resistance to thyrotropin-releasing hormone receptor gene. N Engl J Med. 2009;360:731-734.
73f. Koulouri O, Nicholas AK, Shoenmakers E, Mokrosinki J, Lane F, Cole T, Kirk J, Farooqi IS, Chatterjee VK, Gurnell M, Shoenmakers N. A novel thyrotropin-releasing hormone receptor missense mutation (P81R) in central congenital hypothyroidism. J Clin Endocrinol Metab. 2016; 101:847-851.
- Dacou-Voutekatis C, Feltquate DM, Drakopoulou M, Kourides IA, Dracopoli NC. Familial hypothyroidism caused by a nonsense mutation in the thyroid stimulating hormone beta-subunit gene. Am J Hum Genet. 1990;46:988-993.
74a. Bonomi M, Proverbio MC, Weber G, Chiumello G, Beck-Peccoz P, Persani L. Hyperplastic pituitary gland, high serum glycoprotein hormone alfa-subunit, and variable circulating thyrotropin (TSH) levels as a hallmark of central hypothyroidism due to mutations of the TSH beta gene. J Clin Endocrinol Metab. 2001;86:1600-1604.
74b. Bequadano MS, Ciaccio M, Dujovne N et al. Two novel mutations of the TSH- β-subunit gene underlying congenital central hypothyroidism undetectable in neonatal TSH screening. J Clin Endocrinol Metab. 2010;95:E98-E103.
74c. Drees JC, Stone JA, Reamer CR, Arboleda VF, Huang K, Hrynkow J, Greene DN, Petrie MS, Hoke C, Lorey TS et al. Falsely undetectable TSH in a cohort of Sauth Asian euthyroid patients. J Clin Endocrinol Metab. 2014;99:1171-1179.
74d. PappaT, Johannesen J, Schemberg N, Torrent M, Dumitriescu A, Refetoff S. A TSHB variant with impaired immunoreactivity but intact biological activity and its clinical implications. Thyroid. 2015; 25:869-876.
74e. Hermanns P, Couch R, Leonard N, Klotz C, Pohlenz J. A novel deletion in the thyrotropin β-subunit gene identified by array comparative genomyc hybridization analysis causes central congenital hypothyroidism in a boy originating from Turkey. Horm ResPediatr. 2014;82:201-205.
74f. Nicholas AK, Jaleel S, Kyons E, Shoenmakers E, Dattani MT, Crowne E, Bernhard B, Kirk J, Roche EF, Chatterjee VK, Shoenmakers N. Molecular spectrum of TSHβ subunit gene defects in central hypothyroidism in the UK and Ireland. Clin Endocrinol. 2016; Jun 30 doi:10111/cen. 13149.
- Sun Y, Bak B, Schoenmaker N, van Trotsenburg AS, Oostdijk W, Voshol P et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet. 2012; 44:1375-1381.
75a. Tajima T, Nakamura A, Ishizu K. A novel mutation of IGSF1 in a Japanese patient of congenital central hypothyroidism without macroorchidism. Endocr J. 2013; 60:245-249.
75b. Nakamura A, Bak B, Silander TL, Lam J, Hotsubo T, Yorifuji T, Ishizu K, Bernard DJ, Tajima T. Three novel IGSF1 mutations in four Japanese patients with X-linked congenital central hypothyroidism. J Clin Endocrinol Metab. 2013;98:E1682-E1691.
75c. Joustra SD, Schoenmakers N, Persani L, Campi I, Bonomi M, Radetti G, Beck-Peccoz P, Zhu H, Davis TM, Sun Y, Corssmit EP, Appelman-Dijkstra NM, Heinen CA, Pereira AM, Varewick AJ, Janssen JA, Endert E, Hennekam RC, Lombardi MP, Mannens MM, Bak B, Bernard DI, Breuning MH, Chatterijee K et al. The IGSF1 deficiency syndrome:characteristics of male and females patients. J Clin Endocrinol Metab. 2013; 98:4942-4952.
75d. Joustra SD, Heinen CA, Schoenmakers N, Bonomi M, Ballieux MO, Turgeon D, Bernard E, Fliers E, van Trotsenburg ASP, Losekoot M, Persani L, Wit JM, Biermasz NR, Pereira AM, Oostdijk W, on behalf of the IGSF1 Clinical Care Group. IGSF1 Deficiency: lessons from an extensive case series and recommendations for clinical management. J Clin Endocrinol Metab. 2016; 101:1627-1636.
75e. Tenenbaum-Rakover Y, Turgeon MO, London S,Hermanns P,Pohlenz J, Bernard DJ, Bercovich D. Familial central hypothyroidism caused by a novel IGSF1 gene mutation. Thyroid. 2016 ;26:1693-1700..
- Alatzoglou KS, Dattani MT. Genetic forms of hypopituitarism and their manifestations in neonatal period. Early Hum Dev. 2009;85:705-712.
76a. Brown RS, Bhatia V, Hayes E. An apparent cluster of congenital hypopituitarism in central Massachusetts: magnetic resonance imaging and hormonal studies. J Clin Endocrinol Metab. 1991;72:12-8.
76b. Dattani ML, Martinez-Barbera J, Thomas PQ, et al. Molecular genetics of septo-optic dysplasia. Horm Res. 2000; 53 Suppl 1:26-33.
76c. Machinis K, Pantel J, Netchine I, et al. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet. 2001;69:961-8.
76d. Parks JS, Brown MR. Transcription factors regulating pituitary development. Growth Horm IGF Res. 1999;9 Suppl B:2-8; discussion -11.
- Refetoff S, Bassett JH, Beck-Peccoz P et al. Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport and metabolism. Thyroid. 2014; 24:407.
77a. Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993; 14:348-399.
77b. Weiss RE, Balzano S, Scherberg NH, Refetoff S. Neonatal detection of generalized resistance to thyroid hormone. Jama. 1990;264:2245-50.
77c. Bochukova E, Schoenmakers N, Agostini E, et al. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366:243-9.
77d. Moran C, Chatterje K, Resistance to thyroid hormone due to defective thyroid hormone receptor alpha. Best Pract Res Clin Endocrinol Metab. 2015; 29: 647.
77e. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168-75.
77f. Friesema EC, Gruters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 2004; 364:1435-1437.
77g. Allan W, Erdon CN, Dudley FC. Some exemples of the inheritance of mental deficiency apparently sex-linked idiocy and microcefaly. Am J Ment Defic. 1944, 48:325.
77h. Dumitrescu AM, Liao XH, Abdullah MS, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247-52.
- l’Allemand D, Gruters A, Beyer P, Weber B. Iodine in contrast agents and skin disinfectants is the major cause for hypothyroidism in premature infants during intensive care. Horm Res. 1987;28:42-9.
- Brown RS, Bloomfield S, Bednarek FJ, Mitchell ML, Braverman LE. Routine skin cleansing with povidone-iodine is not a common cause of transient neonatal hypothyroidism in North America: a prospective controlled study. Thyroid.1997;7:395-400.
- Cheron RG, Kaplan MM, Larsen PR, Selenkow HA, Crigler JF, Jr. Neonatal thyroid function after propylthiouracil therapy for maternal Graves’ disease. N Engl J Med. 1981;304:525-8.
- Mitsuda N, Tamaki H, Amino N, Hosono T, Miyai K, Tanizawa O. Risk factors for developmental disorders in infants born to women with Graves disease. Obstet Gynecol. 1992;80(3 Pt 1):359-64.
81a. Brown RS, Bellisario RL, Botero D, et al. Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor-blocking antibodies in over one million babies. J Clin Endocrinol Metab. 1996;81:1147-51.
81b. Brown RS, Keating P, Mitchell E. Maternal thyroid-blocking immunoglobulins in congenital hypothyroidism. J Clin Endocrinol Metab. 1990;70:1341-6.
81c. Connors MH, Styne DM. Transient neonatal ‘athyreosis’ resulting from thyrotropin-binding inhibitory immunoglobulins. Pediatrics. 1986;78:287-90.
- Mandel SH, Hanna CE, LaFranchi SH. Diminished thyroid-stimulating hormone secretion associated with neonatal thyrotoxicosis. J Pediatr. 1986;109:662-5.
- Deming DD, Rabin CW, Hopper AO, Peverini RL, Vyhmeister NR, Nelson JC. Direct equilibrium dialysis compared with two non-dialysis free T4 methods in premature infants. J Pediatr. 2007;151:404-8.
83a van Wassener-Leemhuis A, Ares S, Golombek S, Kok J, Paneth N, Kase J, LaGamma E. Thyroid hormone supplementation in preterm infants born before 28 weeks of gestational age and neurodevelopmental outcome at age 36 months. Thyroid. 2014; 24:1162-1169.
83b. Hollanders JJ, Israels J, van del Pal SM, Verkerk PH, Rotteveel J, Finken MJ, Duch POPS-19 collaborative study group. No Association Between Transient Hypothyroxinemia of Prematurity and Neurodevelopmental Outcome in Young Adulthood. J Clin Endocrinol Metab. 2015; 100:4648-53.
83c. Williams FL, Visser TJ, Hume R. Transient hypothyroxinaemia in preterm infants. Early Hum Dev. 2006;82:797-802.
83d. van Wassenaer AG, Kok JH, de Vijlder JJ, et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks’ gestation. N Engl J Med. 1997;336:21-6.
83e. van Wassenaer AG, Westera J, Houtzager BA, Kok JH. Ten-year follow-up of children born at <30 weeks’ gestational age supplemented with thyroxine in the neonatal period in a randomized, controlled trial. Pediatrics. 2005;116:e613-8.
- Williams FL, Ogston SA, van Toor H, Visser TJ, Hume R. Serum thyroid hormones in preterm infants: associations with postnatal illnesses and drug usage. J Clin Endocrinol Metab. 2005;90:5954-63.
- Foley TP, Jr., Abbassi V, Copeland KC, Draznin MB. Brief report: hypothyroidism caused by chronic autoimmune thyroiditis in very young infants. N Engl J Med. 1994;330:466-8.
85a. Verbansky JW, Chatila TA. Immune dysregulation, Poliendocrinopathy, Enteropathy, X linked (IPEX) and IPEX related disorders: an evolving web of heritable autoimmune disease. Curr Opin Pediatr. 2013; 25:708-714.
85b. Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. J Med Genet. 2002;39:537-45.
85c. Huang SA, Tu HM, Harney JW et al. Severe hypothyroidism caused by type 3 iodothyronine dieiodinase in infantile hemangiomas. N Eng J Med. 2000;343: 185-9.
85d. Balatz AE, Athanassaki I, Gunn SK, Tatevian N, Huang SA, Haymond MW, Karaviti L. Rapid resolution of consumptive hypothyroidism in a child with hepatic hemangioendothelioma following liver transplantation. Ann Cli Lab Sc. 2007;37:280-284.
- Zakarija M, McKenzie JM. Pregnancy-associated changes in the thyroid-stimulating antibody of Graves’ disease and the relationship to neonatal hyperthyroidism. J Clin Endocrinol Metab. 1983;57:1036-40.
- Skuza KA, Sills IN, Stene M, Rapaport R. Prediction of neonatal hyperthyroidism in infants born to mothers with Graves disease. J Pediatr. 1996;128:264-8.
87a. Abeillon-du Payret, Chikh K, Bossart N, Bretones P, Gaucherand P, Claris O, Charrie’ A, Raverot V, Orgiazzi J, Borzon- Chazot F, Bournaud C. Predictive value of maternal second-generation thyroid-binding inhibitory immunoglobulin assay for neonatal autoimmune hyperthyroidism. Eur J Endocrinol. 2014, 171:451-460.
87b. Fort P, Lifshitz F, Pugliese M, Klein I. Neonatal thyroid disease: differential expression in three successive offspring. J Clin Endocrinol Metab. 1988;66:645-7.
87c. Zakarija M, McKenzie JM, Munro DS. Immunoglobulin G inhibitor of thyroid-stimulating antibody is a cause of delay in the onset of neonatal Graves’ disease. J Clin Invest. 1983;72:1352-6.
87d. Kohn LD, Suzuki K, Hoffman WH, et al. Characterization of monoclonal thyroid-stimulating and thyrotropin binding-inhibiting autoantibodies from a Hashimoto’s patient whose children had intrauterine and neonatal thyroid disease. J Clin Endocrinol Metab. 1997;82:3998-4009.
87e. Launberg P, Wallin G, Tallstedt L, Abraham-Nording M, Lundell G, Terring O. TSH-receptor autoimmunity in Graves’ disease after therapy with antithyroid –drugs, surgery or radioiodine: a 5 year prospective randomized study. Eur J Endocrinol. 2008; 158:69-75.
87f. Kiefier FW, Kierbemass-Screhof K, Steiner M, Worda C, Kaisprian G, Tanja D, Kahaly G, Gess A. Fetal/neonatal thyrotoxicosis in a newborn from a hypothyroid woman with Hashimoto thyroiditis, J Clin Endocrinol Metab. 2017;102:6-9.
87g. Neal PR, Jansen RD, Lemons JA, Mirkin LD, Schreiner RL. Unusual manifestations of neonatal hyperthyroidism. Am J Perinatol. 1985;2:231-5.
87h. Daneman D, Howard NJ. Neonatal thyrotoxicosis: intellectual impairment and craniosynostosis in later years. J Pediatr. 1980;97:257-9.
- Morshed SA, Ando T, Latif R, Davies TF. Neutral antibodies to the TSH receptor are present in Graves' disease and regulate selective signaling cascades. Endocrinology. 151:5537-49, 2010.
88a. Barbesino G, Tomer Y. Clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab. 2013;98:2247-2255.
88b. Diana T, Brown RS, Bossowski A, Segni M, Niedziela M, Konig J, Bossowka A, Ziora K, Smith J, Pitz S, Kanitz M, Kahaly GJ. Clinical relevance of thyroid-stimulating autoantibodies in pediatric Graves’ disease. A multicenter study. J Clin Endocrinol Metab. 2014;99:1648-1655.
88c. Ross DS, Burch HB, Cooper DS, Greenlee MC, Launberg P, Maia AL, Rivkees S, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis.Thyroid.. 2016;2:1343-1421.
88d. Donnelly MA, Wood C, Casey B, Hobbins J, Barbour LA. Early severe fetal Graves disease in a mother after after thyroid ablation and thyroidectomy. Obstetric Gynecol. 2015; 125:141-152.
88e. Besancon A, Beltrand J, Le Gac I, Luton D, Polak M. Management of neonates born to women with Graves’ disease: a cohort study. Eur J Endocrinol. 2014;170:855-862.
- Matsuura N, Konishi J, Fujieda K, et al. TSH-receptor antibodies in mothers with Graves’ disease and outcome in their offspring. Lancet. 1988;1:14-7.
89a. Tamaki H, Amino N, Aozasa M, et al. Universal predictive criteria for neonatal overt thyrotoxicosis requiring treatment. Am J Perinatol. 1988;5:152-8.
89b. Van der Kaay D, Wasserman JD, Palmert MR. Management of neonates born to mothers with Graves’ disease. Pediatrics. 2016;137:e20151878.
- de Roux N, Polak M, Couet J, et al. A new mutation of the thyroid-stimulating hormone receptor in a severe neonatal hyperthyroidism. J Clin Endocrinol Metab. 1996;81:2023-6.
90a. Holzapfel HP, Wonerow P, von Petrykowski W, Henschen M, Scherbaum WA, Paschke R. Sporadic congenital hyperthyroidism due to a spontaneous germline mutation in the thyrotropin receptor gene. J Clin Endocrinol Metab. 1997;82:3879-84.
90b. Schwab KO, Gerlich M, Broecker M, Sohlemann P, Derwahl M, Lohse MJ. Constitutively active germline mutation of the thyrotropin receptor gene as a cause of congenital hyperthyroidism. J Pediatr. 1997;131:899-904.
90c. Kopp P, Muirhead S, Jourdain N, Gu WX, Jameson JL, Rodd C. Congenital hyperthyroidism caused by a solitary toxic adenoma harboring a novel somatic mutation (serine281–>isoleucine) in the extracellular domain of the thyrotropin receptor. J Clin Invest .1997;100:1634-9.
90d. Gruters A, Schoneberg T, Biebermann H, et al. Severe congenital hyperthyroidism caused by a germ-line neo mutation in the extracellular portion of the thyrotropin receptor. J Clin Endocrinol Metab. 1998;83:1431.
90e. Paschke R, Niedzela M, Vaidya B, Persani L, Rapaport B, Leclere J. The management of familial and persistent sporadic non-autoimmune hyperthyroidism caused by thyroid stimulating hormone receptor germline mutations. Eur Thyroid J. 2012;1:142-147.
90f. Yoshimoto M. Nakayama M, Baba T, Uehara y, Nikawa N, Ito M, Tsuji Y. A case of neonatal McCune Albright syndrome with Cushing syndrome and hyperthyroidism. Acta Paediatr Scand. 1991;80:984-987.
- Davies TF, Amino N. A new classification for human autoimmune thyroid disease. Thyroid. 1993; 3: 331-333,
91a. Fisher DA, Pandian MR, Carlton E. Autoimmune disease: an expanding spectrum. Pediatr Clin North Am. 1987; 34: 907-18.
91b. Rallison ML, Dobyns FR, Keating FR, Rall JE, Tyler FH, Occurrence and natural history of chronic lymphocytic thyroiditis in childhood. J Pediatr. 1975; 86: 675-82.
91c. Loviselli A, Veluzzi F, Mossa P, Cambosu MA, Secci G, Atzeni F, Taberlet A, Balestrieri A, Martino E, Grasso L, Songini M, Bottazzo GF, Mariotti S. Sardinian Schoolchildren Study Group. The Sardinian Autoimmunity Study: 3. Studies on circulating antithyroid antibodies in Sardinian schoolchildren: relationship to goiter prevalence and thyroid function.Thyroid. 2001;1: 849-57.
91d. Kabelitz M, Liesenkotter KP, Stach B, Willigerodt H, Stablen W, Singendok W, Jager-Roman E, Litzenborger H, EhnertB, Gruters A. The prevalence of anti-thyroid peroxidase antibodies and autoimmune thyroiditis in children and adolescents in an iodine replete area. Eur J Endocrinol. 2003;48:301-307.
91e. Kaloumenou I, Mastorakos G, Alevizaki M, Duntas LH, Mantzou E, Ladopoulos C, Antoniou A, Chiotis D, Popassotiriou I, Chousos GP, Dacou-Voutetakis-C. Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: correlation with gender, pubertal stage, and maternal thyroid autoimmunity. Thyroid. 2008;18:747-58.
91f. Davies TF. Really significant genes for autoimmune thyroid disease do not exist–so how can we predict disease? Thyroid. 2007;17:1027-9.
91g. Brown RS. Autoimmune thyroid disease: unlocking a complex puzzle. Curr Opin Pediatr. .2009;21:523-8.
91h .Segni M, Pani MA, Pasquino AM, Badenhoop K. Familial clustering of juvenile thyroid autoimmunity: higher risk is conferred by human leukocyte antigen DR3-DQ2 and thyroid peroxidase antibody status in fathers. J Clin Endocrinol Metab 2002; 87: 3779-82.
91i.Segni M, Wood J, Pucarelli I, Toscano V, Toscano R, Pasquino AM. Clustering of autoimmune thyroid diseases in children and adolescents: a study of 66 families. J Pediatr Endocrinol Metab. 2001, 14 Supp 5:1271-5.
91j. Wiebolt J, Achterbergh R, den Boer A, van der Leij S, Marsch E, Suelmann B, de Vries R, van Haeften TW. Clustering of additional autoimmunity behaves differently in Hashimoto's patients compared with Graves' patients. Eur J Endocrinol. 2011;164:789-94.
91k. Jenkins RC, Weetman AP. Disease associations with autoimmune thyroid disease. Thyroid. 2002;12:975-986.
91l. Karlsson B, Gustafsson G, Ivarson SA, Anneren G, Thyroid dysfunction in Down’s syndrome: relation to age and thyroid autoimmunity. Arch Dis Child. 1998;79:243-245.
91m. Eisheikh M, Wass JA, Conway GS. Autoimmune thyroid disease in women with Turner ‘s syndrome-the association with karyotype. Clin Endocrinol. 2001; 55:223-226,
91n. Seminog OO, Seminog AB, Yeates D, Goldacre MJ. Associations between Klinefelter’s syndrome and autoimmune diseases: English national recortd linkage studies. Autoimmunity. 2015;48: 125-280.
91o. Quaio CR, Carvalho JF, da Silva CA, Bueno C, Brasil AS, Pereira AC, Jorge AA, Malaquias AC, Kim CA, Bertola DR. Autoimmune disease and multiple autoantibodies in 42 patients with RASopathies. Am J Med Genet. 2012; 158A: 1077-82.
91p. Brent GA. Enviromental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761.
91q. Eshler DC, Hasam A, Tomer Y. Cutting edge: the etiology of autoimmune thyroid disease. Clin Rev Allergy Immunol. 2011; 41:190-197.
- Brown RS. Immunoglobulins affecting thyroid growth: a continuing controversy. J Clin Endocrinol Metab. 1995;80:1506-8.
92a. deVries L, Bulvik S, Philllip M. Chronic autoimmune thyroiditis in children and adolescents at presentation and during long-term follow up. Arch Dis Child. 2009; 94:33-7.
92b. Najjar SS. Muscular hypertrophy in hypothyroid children: the Kocher-Debre-Semelaigne syndrome; a review of 23 cases. J Pediatr. 1974;85:236-9.
92c. Hopwood NJ, Lockhart LH, Bryan GT. Acquired hypothyroidism with muscular hypertrophy and precocious testicular enlargement. J Pediatr. 1974;85:233-6.
92d. Anasti JN, Flack MR, Froehlich J, Nelson LM, Nisula BC. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab. 1995;80:276-9.
- Maenpaa J, Raatikka M, Rasanen J, Taskinen E, Wager O. Natural course of juvenile autoimmune thyroiditis. J Pediatr. 1985;107:898-904.
93a. Moore DC. Natural course of ‘subclinical’ hypothyroidism in childhood and adolescence. Arch Pediatr Adolesc Med. 1996;150:293-7.
93b. Lazar L, Frumkin RB, Battat E, Lebenthal Y, Phillip M, Meyerovitch J. Natural history of thyroid function tests over 5 years in a large pediatric cohort. J Clin Endocrinol Metab. 2009;94:1678-82.
93c. Aversa T, Corrias A, Salerno MC, Tessaris D, Di Mase R, Valenzise M, Corica D, De Luca F, Wasniewka M. Five-years prospective evaluation of thyroid function test evolution in children with Hashimoto’s thyroiditis presenting with either euthyroidism or subclinical hypothyroidism. Thyroid. 2016, 26:1450-1456.
- Hayashida N,Imaizumi M, Shimura H, Okubo N, Asari Y, Nigarawa T, Midorikava Sm Kotani K, Nakaji S, Otssuru A, Akamizu T, Kitaoka M, Suzuki S, Taniguchi N, Yamashita S, Takamura N. Thyroid ultrasound findings in children from three Japanese prefectures: Aomori, Yamanashi and Nagasaki. Plos One. 2013,8:e83220.
94a. Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M, Rapa A, Gastaldi R, Einaudi S, Baronio F, Vigone MC, Messina MF, Bal M, Bona G, De Santis C; for the study for thyroid diseases of the Italian Society for Pediatric Endocrinology and Diabetology. Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. 2008; 162:526-531.
94b. Keskin M, Savas-Erdeve S, Aycan Z. Co-existence of thyroid nodule and thyroid cancer in children and adolescents with Hashimoto thyroiditis: a single-center study. Horm Res Pediatr. 2016; 85:181-187.
94c. Kambalpalli M, Gupta A, Prasad UR, Francis GL. Ultrasound characteristics of the thyroid in children and adolescents with goiter: a single center experience. Thyroid. 2015; 25:176-182.
94d. Jeong SH, Hong HS, Lee FH, Kwak JJ. Papillary thyroid carcinoma arising in children and adolescent Hashimoto’s thyroiditis: ultrasonographic and pathologic findings. Int J Endocrinolol. 2016, 2397690.
94e. Gilani BB, MacGillivray MH, Voorhess ML, Mills BJ, Riley WJ, MacLaren NK. Thyroid hormone abnormalities at diagnosis of insulin-dependent diabetes mellitus in children. J Pediatr. 1984;105:218-22.
94f. Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes. Autoimmunity Reviews. 2015; 14:781-797.
- Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9:154-62.
95a. Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350:2068-2079.
95b. Betterle C, Greggio NA, Volpato M. Clinical review 93: Autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83:1049-55.
95c. Kordonouri O, Harmann R, Deiss D, Wilms M, Gruters-Kieslich A. Natural course of autoimmune thyroiditis in type 1 diabetes: association with gender, age, diabetes duration and and puberty. Arch Dis Child. 2005; 90:411-414.
95d. Bright GM, Blizzard RM, Kaiser DM, Clarke WL. Organ-specific autoantibodies in children with common endocrine disease. J Pediatr. 1982; 100:8-14.
95e. Dost A, Roher TR, Frolich-Reitherer E, Bollow E, Karges B, Bockmann A, Harmann J, Holl RW: DPV initiative and the German Competence Network Diabetes Mellitus. Horm Res Paediatr. 2015; 84: 190-8.
95f. Naiyer AJ, Shah J, Hernandez L, Kim SY, Ciaccio EJ, Cheng J, Manavalan S, Bhagat G, Green PH. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid. .2008;18:1171-8.
95g. Roy A, Laszowska M, Sundstrom J, Lebwohl B, green PH, Kampe O, Ludvigsoon JF. Prevalence of celiac disease in patients with autoimmune thyroid disease: a meta-analysis. Thyroid. 2016; 26:880-90.
95h. Kurien M, Mollazadegan K, Sanders DS, Ludvigssson JF. Celiac disease increased risk of thyroid disease in patients with type 1 diabetes: a nationwide cohort study. Diab Care. 2016 39:371-375.
95i. Virili C, Bassotti G, Santaguida MG, Iourio R, Del Duca SC, Mercuri V, Pocarelli A, Gargiulo P. Gargano L, Centanni M. Atypical celiac disease as a cause of increased need for thyroxine: a systematic study. J Clin Endocrinol Metab. 2012; 97:E419-22.
95j. Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, Manji N, Allahabadia A, Armitage M, Chatterjee KV, Lazarus JH, Pearce SH, Vaidya B, Gough SC, Franklyn JA. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-9.
95k Irvine WJ, Davies SH, Teitelbaum S, Delamore IW, Williams AW. The clinical and pathological significance of gastric parietal cell antibody. Ann NY Acad Sci.1965; 124:657-91.
95l. Segni M, Borrelli O, Pucarelli I, Delle Fave G, Pasquino AM, Annibale B. Early manifestations of gastric autoimmunity in patients with juvenile autoimmune thyroid diseases. J Clin Endocrinol Metab. 2004; 4944-8.
- Scott HS, Heino M, Peterson P, et al. Common mutations in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients of different origins. Mol Endocrinol.1998;12:1112-9.
96a. Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, Rosen LB, Break TJ, Gu W, Hunsberger S, Browne SK, Hsu AP, Rampertaap S, Swamydas M, Collar AL, Kong HH, Lee CR, Chascsa D, Simcox T, Pham A, Bondici A, Natarajan M, Monsale J, Kleiner DE, Quezado M, Alevizos I, Moutsopoulos NM, Yockey L, Frein C, Soldatos A, Calvo KR, Adjemian J, Similuk MN, Lang DM, Stone KD, Uzel G, Kopp JB, Bishop RJ, Holland SM, Olivier KN, Fleisher TA, Heller T, Winer KK, Lionakis MS. Redifined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI insight. 2016;1(13):e88782.
96b. Jenkins RC, Weetman AP. Disease associations with autoimmune thyroid disease. Thyroid. 2002; 12:975-986.
96c. Fallahi P, Ferrari SM, Ruffilli I, Elia G, Biricotti M, Vita R, Benvenga S, Antonelli A. The association of other autoimmune diseases in patients with autoimmune thyroiditis: Review of the literature and report of a large series of patients. Autoimm Rev. 2016;15:1125-1128.
96d. Stagi S, Giani T, Simonini G, Falcini F. Thyroid function, autoimmune thyroiditis and coeliac disease in juvenile idiopathic arthritis. Reumatology. 2005; 44:517-20.
96e. Kroon MW, Vrijman C, Chandeck C, Wind BS, Wolkerstofer A, Luiten RM, Bos JD, Geskus RB, van Trosenburg P, van der Veen JP. High prevalence of autoimmune thyroiditis in children and adolents with vitiligo. Horm Res Paediatr. 2013;79:137-44.
96f. Noso S, Park C, Babaya N, Hiromine Y, Harada T, Ito H, Taketomo Y, Kanto K, Oiso N, Kawada A, Suzuki T, Kawabata Y, Ikegami H. Organ specificity in autoimmune diseases: thyroid and islet autoimmunity in alopecia areata. J Clin Endocrinol Metab. 2015; 100:1976-1983.
96g. Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119:636-40.
96h. O’Regan S, Fong JS, Kaplan BS, Chadarevian JP, Lapointe N, Drummond KN. Thyroid antigen-antibody nephritis. Clin Immunol Immunopathol. 1976;6:341-6.
96i. Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res 2014, 150239.
96j. Manetti L, Lupi I, Morselli L, Albertini S, Cosottini M, Grasso L, Genovesi M, Pinna G, Mariotti S, Bogazzi F, Bartalena L, Martino E. Prevalence and functional significance of antipituitary antibodies in patients with autoimmune and non-autoimmune thyroid diseases. J Clin Endocrinol Metab. 2007.92:2176-81.
96k. De Bellis A, Bellastella G, Maiorino MI, Aitella E, Lucci E, Cozzolino D, Bellastella A, Bizzarro A, Giugliano D, Esposito K, Italian Autoimmune Hypophysitis network group. Longitudinal behavior of autoimmune GH deficiency: from childhood to transition age. Eur J Endocrinol. 2016;174:381-7.
- Mehta A, Hindmarsh PC, Stanhope RG, Brain CE, Preece MA, Dattani MT. Is the thyrotropin-releasing hormone test necessary in the diagnosis of central hypothyroidism in children?. J Clin Endocrinol Metab. 2003;88:5696-703.
97a. Feingold SB, Smith J, Houtz J, et al. Prevalence and functional significance of thyrotropin (TSH) receptor blocking antibodies in children and adolescents with chronic lymphocytic thyroiditis. J Clin Endocrinol Metab. 2009;94: 4742-8.
97b. Brown RS, Alter CA, Sadeghi-Nejad A. Severe unsuspected maternal hypothyroidism discovered after the diagnosis of thyrotropin receptor-blocking antibody-induced congenital hypothyroidism in the neonate: failure to recognize and implication to the fetus Horm Res Pediatr. 2015; 83:132-8.
- Pedersen OM, Aadal NP, Larssen TB, Varhuag JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000, 10:251-259.
- Rovet JF, Daneman D, Bailey JD. Psychologic and psychoeducational consequences of thyroxine therapy for juvenile acquired hypothyroidism. J Pediatr. 1993;122:543-9.
99a. Van Dop C, Conte FA, Koch TK, Clark SJ, Wilson-Davis SL, Grumbach MM. Pseudotumor cerebri associated with initiation of levothyroxine therapy for juvenile hypothyroidism. N Engl J Med.1983;308:1076-80.
99b. Lomenick JP, El-Sayyid M, Smith WJ. Effect of levo-thyroxine treatment on weight and body mass index in children with acquired hypothyroidism. J Pediatr. 2008. 152:96-100.
99c. Rivkees SA, Bode HH, Crawford JD. Long-term growth in juvenile acquired hypothyroidism: the failure to achieve normal adult stature. N Engl J Med. 1988;318:599-602.
- Salerno M, Capalbo D, Cerbone M, De Luca F. Subclinical hypothyroidism in childhood- current knowledge and open issues. Nat Rev Endocrinol. 2016; 734-746.
100a. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Jama. 2004;291:228-38.
100b. Taylor P, Razvi S, Pearce Sh, Colin D. A Review of the clinical consequences of variation in thyroid function whitin the reference range. J Clin Endocrinol Metab. 2013; 98: 3562-3571,
100c. Ittermann T, Thamm M, Wallschofski H, Rettig R, Volzke H. Serum thyroid-stimulating hormone levels are associated with blood pressure in children and adolescents. J Clin Endocrinol Metab. 2012; 97:828-834.
100d. Witte T, Ittermann T, Thamm M, Ribiet NB, Votzke H. Association between serum thyroid stimulating hormone levels and serum lipids in children and adolescents: a population-based study on german youth. J Clin Endocrinol Metab. 2015;100:2090-7.
100e. Nader NS, Bahn RS, Johnson ,MD, Weaver AL, Singh R, Kumar S. Relationships between thyroid function and lipid status or insulin resistance in a pediatric population. Thyroid. 2010;20:1333-1339.
100f. McLeod DS, Watters KF, Carpenter AD, Landenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2012; 97:2682-2692.
100g. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaydya B. European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014; 3: 76-94.
- Surks MI, Sievert R. Drugs and thyroid function. N Engl J Med. 1995;333:1688-94.
101a. McCowen KC, Garber JR, Spark R. Elevated serum thyrotropin in thyroxine-treated patients with hypothyroidism given sertraline. N Engl J Med. 1997;337:1010-1.
101b. Delange FM. Iodine Deficiency. In: Braverman LE, Utiger,R.D., ed. Werner & Ingbar’s The Thyroid. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
101c. Pacaud D, Van Vliet G, Delvin E, et al. A Third World endocrine disease in a 6-year-old North American boy. J Clin Endocrinol Metab. 1995;80:2574-6.
101d. Donadieu J, Rolon MA, Thomas C, et al. Endocrine involvement in pediatric-onset Langerhans ’ cell histiocytosis: a population-based study. J Pediatr. 2004;144:344-50.
- Hauser P, Zametkin AJ, Martinez P, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328:997-1001.
102a. Bercu BB, Orloff S, Schulman JD. Pituitary resistance to thyroid hormone in cystinosis. J Clin Endocrinol Metab. 1980;51:1262-8.
- van der Gaag RD, Drexhage HA, Wiersinga WM, et al. Further studies on thyroid growth-stimulating immunoglobulins in euthyroid nonendemic goiter. J Clin Endocrinol Metab. 1985;60:972-9.
- Rother KI, Zimmerman D, Schwenk WF. Effect of thyroid hormone treatment on thyromegaly in children and adolescents with Hashimoto disease. J Pediatr. 1994;124:599-601.
- Svensson J, Ericsson UB, Nilsson P, Olsson C, Jonsson B, Lindberg B, Ivarsson SA. Levothyroxine treatment reduces thyroid size in children and adolescents with chronic autoimmune thyroiditis. J Clin Endocrinol Metab. 2006. 91:1729-34.
- Smith SL, Pereira KD Suppurative thyroiditis in children. A management algorithm. Pediatr Emerg Care. 2008; 24:764-767.
106a. Greene JN. Subacute thyroiditis. Am J Med. 1971:51:97.
106b. Paes JE, Burman KD,.Cohen J, Franklyn J, McHemry CR, Shoham S, Kloos RT. Acute bacterial suppurative thyroiditis: a clinical review and expert opinion. Thyroid. 2010; 20:247-255.
106c. Mali VP, Prabhakaran K. Recurrent acute thyroid swellings because of pyriform sinus fistula. J Pediatr Surg. 2008;43:e27-30.
106d. Radfar N,Kenny FM, Larsen PR. Subacute thyroiditis in a lateral thyroid gland:evaluation of the pituitary-thyroid axis during the acute distructive and the recovery phases. J Pediatr. 1975; 87:34.
- Braverman L, Utiger R: Introduction to thyrotoxicosis. In: Braverman L, Utiger R eds. The Thyroid. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 453-455, 2005.
107a. Graves RJ: Clinical lectures. London Med Surg J (pt 2):516, 1835.
107b. Kjaer H, Andersen SM, Hansen D. Increasing incidence of juvenile thyrotoxicosis in Denmark: a nationwide study, 1998-2012. Horm Res Paediatr. 2015;84: 102-107.
107c. Shulman DI, Muhar I, Jorgensen EV, Diamond FB, Bercu BB, Root AW. Autoimmune hyperthyroidism in prepubertal children and adolescents: comparison of clinical and biochemical features at diagnosis and responses to medical therapy. Thyroid. 1997;7:755-60.
107d. Segni M, Leonardi E, Mazzoncini B, Pucarelli I, Pasquino AM. Special features of Graves ’ disease in early childhood. Thyroid. 1999;9:871-7.
107e. Goday-Arno A, Cerda-Esteva M, Flores-Le-Roux JA, Chillaron-Jordan JJ, Corretger JM, Cano-Perez JF. Hyperthyroidism in a population with Down syndrome (DS). Clin Endocrinol. 2009;71:110-4.
107f. Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673-716.
107g. Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007;17:923-38.
107h. Sanders J, Evans M, Premawardhana LD, et al. Human monoclonal thyroid stimulating autoantibody. Lancet. 2003;362:126-8.
107i. Sanders J, Miguel RN, Bolton J, et al. Molecular interactions between the TSH receptor and a thyroid-stimulating monoclonal autoantibody. Thyroid. 2007;17:699-706.
107j. Rapoport B, McLachlan SM. The thyrotropin receptor in Graves’ disease. Thyroid .2007;17:911-22.
107k. Williams RC, Jr., Marshall NJ, Kilpatrick K, et al. Kappa/lambda immunoglobulin distribution in Graves’ thyroid-stimulating antibodies. Simultaneous analysis of C lambda gene polymorphisms. J Clin Invest. 1988;82:1306-12.
- Segni M, Gorman CA. The aftermath of childhood hyperthyroidism. J Pediatr Endocrinol Metab. 2001; Suppl 5 1277-82.
108a. Goldstein SM, Katowitz WR, Modhang T, Katowitz JA. Pediatric Thyroid-Associated Orbitopathy: The children’s hospital of Philadelphia experience and literature review. Thyroid. 2008;18:997-999.
108b. Nucci P, Brancato R, Bandello F, Alfarano R, Bianchi S. Normal exophtalmometric values in children. Am J Opthal 1989;108:582-584.
108c. Liu GT, Helher KL, Katowitz JA, Kazim M, Moazami G, Moshang T, Teener JW, Sladeky J, Volpe NJ, Galetta SL. Prominent proptosis in childhood thyroid eye disease. Opthalmology. 1996;103:779-784.
108d. Durairaj VD, Bartley GB, Garrity JA. Clinical features and treatment of Graves’ opthalmopathy in pediatric patients. Opthal Plast Reconstr Surg 2006; 22:7-12.
- Sterling K, Refetoff S, Selenkow HA. T3 thyrotoxicosis due to elevated serum triiodothyronine levels. JAMA. 1970;213:571-5.
109a. Harvengt J, Boizeau P, Chevenne D, Zenaty D, Paulsen A, Simon D, Crepon SG, Alberti C, Carel JC, Leger J. Triiodothyronine- predominant Graves’ disease in childhood:detection and therapeutic inplications. Eur J Endocrinol. 2015; 172: 715-23.
109b. Ruiz M, Rajatanavin R, Young RA, Taylor C, Brown R, Braverman LE, Ingbar SH. Familial dysalbuminemic hyperthyroxinemia: a syndrome that can be confused with thyrotoxicosis. N Engl J Med. 1982;306:635-9.
109c. Mariotti S, Martino E, Cupini C, et al. Low serum thyroglobulin as a clue to the diagnosis of thyrotoxicosis factitia. N Engl J Med. 1982;307:410-2.
109d. Kaguelidou F, Alberti C, Castanet M, Guitteny MA, Czernichow P, Leger J. Predictors of autoimmune hyperthyroidism relapse in children after discontinuation of anthythyroid drug treatment. J Clin Endocrinol Metab. 2008; 93:3817-3826.
- Ichiki Y, Akahoshi M, Yamashita N, et al. Propylthiouracil-induced severe hepatitis: a case report and review of the literature. J Gastroenterol. 1998;33:747-50.
110a. Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018-23.
110b. Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med. 2009;360:1574-5.
110c. Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN. The Role of Propylthiouracil in the Management of Graves’ Disease in Adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid. 2009;19:673-4.
110d. Okuno A, Yano K, Inyaku F, Suzuki Y, Sanae N, Kumai M, Naitoh Y. Pharmacokinetics of methimazole in children and adolescents with Graves’ disease. Acta Endocrinol.1987. 115:112-118.
110e. Rivkees SA. Pediatric Graves’ disease: management in the post-propilthyuracil era .Inter J Pediatr Endocrinolol. 2014; 10:1-10.
110f. Smith J, Brown RS. Persistence of thyrotropin (TSH) receptor antibodies in children and adolescents with Graves’ disease treated using antithyroid medication. Thyroid. 2007;17:1103-7.
110g. Fenzi G, Hashizume K, Roudebush CP, DeGroot LJ. Changes in thyroid-stimulating immunoglobulins during antithyroid therapy. J Clin Endocrinol Metab. 1979;48:572-6.
110h. Teng CS, Yeung RT. Changes in thyroid-stimulating antibody activity in Graves’ disease treated with antithyroid drug and its relationship to relapse: a prospective study. J Clin Endocrinol Metab. 1980;50:144-7.
110i. Bliddal H, Kirkegaard C, Siersbaek-Nielsen K, Friis T. Prognostic value of thyrotrophin binding inhibiting immunoglobulins (TBII) in longterm antithyroid treatment, 131I therapy given in combination with carbimazole and in euthyroid ophthalmopathy. Acta Endocrinol. 1981;98:364-9.
110j. Collen RJ, Landaw EM, Kaplan SA, Lippe BM. Remission rates of children and adolescents with thyrotoxicosis treated with antithyroid drugs. Pediatrics. 1980;65(3):550-6.
110k. Leger J, Gelwane G, Kaguelidou F et al. Positive impact of long-term antithyroid drug treatment on the outcome of children with Graves disease: natural long-term cohort study. J Clin Endocrinol Metab. 2012; 97:110-9.
110l. Hashizume K, Ichikawa K, Sakurai A, et al. Administration of thyroxine in treated Graves’ disease. Effects on the level of antibodies to thyroid-stimulating hormone receptors and on the risk of recurrence of hyperthyroidism. N Engl J Med. 1991;324:947-53.
110m. McIver B, Rae P, Beckett G, Wilkinson E, Gold A, Toft A. Lack of effect of thyroxine in patients with Graves’ hyperthyroidism who are treated with an antithyroid drug. N Engl J Med 1996;334:220-4.
- Rivkees SA, Sklar C, Freemark M. Clinical review 99: The management of Graves’ disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab. 1998;83:3767-76.
111a. Rivkees SA, Stephenson K, Dinauer C. Adverse events associated with methimazole therapy of Graves’ disease in children. Int J Pediatr Endocrinol. 2010; 176970.
111b. Wada N, Mukai M, Kohono M, Notoya A, Ito T, Yoshioka N. Prevalence of serum anti-myeloperoxidase antineutrophil cytoplasmatic antibodies (MPO-ANCA) in patients with Graves’ disease trated with prophilthyuracil and thiamazole. Endocr J. 2002; 49: 329-334.
111c. Guma M, Salinas I, Reverter JL, Roca J, Valls-Roc M, Juan M, Olive’ A. Frequency of antineutrophil cytoplasmic antibody in Graves’ disease patients treated with methimazole. J Clin Endocrinol Metab. 2003, 88:2141-6.
111d. Tajiri J, Noguchi S, Murakami T, Murakami N. Antithyroid drug-induced agranulocytosis. The usefulness of routine white blood cell count monitoring. Arch Intern Med. 1990;150:621-4.
111e. van Veenendaal NR, Rivkees SA. Treatment of pediatric Graves’ disease is associated with excessive weight gain. J Clin Endocrinol Metab. 2011; 96:3257-63.
111f. Mazzaferri EL. Thyroid cancer and Graves’ disease. J Clin Endocrinol Metab. 1990; 70:825-829.
111g. Dobyns BM, Sheline GE, Workman JB, Tompkins EA, McConahey WM, Becker DV. Malignant and benign neoplasm of the thyroid in patients treated for hyperthyroidism: a report of the cooperative thyrotoxicosis therapy follow-up study. J Clin Endocrinol Metab. 1974; 38:976-988.
- Sherman J, Thompson GB, Lteif A, et al. Surgical management of Graves disease in childhood and adolescence: an institutional experience. Surgery. 2006;140:1056-61. Discussion 61-2.
112a.Tuggle CT, Roman SA, Wang TS, Thomas DC, Uldelsman R, Ann Sosa J. Pediatric endocrine surgery: who is operating on our children? Surgery. 2008; 144:869-877, Discussion 877.
112b. Kundel A, Thompson GB, Richards ML, Qiu LX, Cai Y, Schwenk FW, Lief AN, Pittock ST, Kumar S, Tebben PJ Hay ID, Grant CS. Pediatric endocrine surgery: a 20 year experience at the Mayo Clinic. J Clin Endocrinol Metab. 2014. 99: 399-406.
112c. Beuer CK, Solomon D, Donovan P, Rivkees SA, Udelsman R. Effect of patient age on surgical outcome for Graves’ disease: a case control study of 100 consecutive patients at a high volume thyroid surgical center. Int J Pediatr Endocrionol. 2013;1: 9856.
- Nikiforov Y, Gnepp DR, Fagin JA. Thyroid lesions in children and adolescents after the Chernobyl disaster: implications for the study of radiation tumorigenesis. J Clin Endocrinol Metab. 1996;81:9-14.
113a. Safa AM, Schumacher P, Rodriguez-Antunez A. Long-term follow-up results in children and adolescents treated with radioactive iodine (131-Iodine). N Engl J Med. 1975; 292:167-171.
113b. Holm LE, Dahlqvist I, Israelsson A, Lundell GM. Malignant thyroid tumors after 131-iodine therapy. N Engl J Med. 1980; 303:188-191.
113c. Hamburger JI. Management of hyperthyroidism in children and adolescents. J Clin Endocrinol Metab. 1985; 60:1019.
113d. Hayek A, Chapman E, Crawford JD. Long-term results of treatment of thyrotoxicosis in children and adolescents with radioactive iodine. N Engl J Med .1970; 283:949, 953.
113e. Franklyn JA, Maisonneuve P, Sheppard M, Betteridge PB.Bovie P. Cancer incidence and mortality after radioiodine treatment for hyperthyroidism: a population-based cohort study. Lancet. 1999;353:2111-5
113f. Starr P, Jaffe HL, Oettinger L Jr. Late results of 131-I treatment of hyperthyroidism in seventy-three children and adolescents. J Nucl Med.1964; 5:81-9.
113g. Kogut MD, Kaplan SA, Collipp PJ, Tiamsic T, Boyle D. Treatment of hyperthyroidism in children. N Engl J Med.1965; 272:217-22.
113h. Read CH Jr, Tansey MJ, Menda Y. A 36-year retrospective analysis of the efficacy and safety of radioactive iodine in treating young Graves’ patients. J Clin Endocrinol Metab. 2004;89:4229-33.
113i. Rivkees SA, Dinauer C. An optimal treatment for pediatric Graves’ disease is radioiodine. J Clin Endocrinol Metab. 2007;92:797-800.
113j. Rivkees SA. Controversies in the management of Graves’ disease in children. J Endocrinol Invest. 2016;39:1247-1257.
- Ly S, Frates MC, Benson CB, Peters HE, Grant FD, Drubach LA, Voss SD, Feldman HA, Smith JR, Barletta J, Hollowell M, Cibas ES, Moore FD, Modi BM, Shamberger RC, Huang SA. Features and outcome of autonomous thyroid nodules in children: 31 consecutive patients seen at a single center. J Clin Endocrinol Metab. 2016;101:3856-3862.
114a. Niedzela M, Breborowicz D, Trejster E, Koeman E. Hot nodules in children and adolents in western Poland from 1996 to 2000: clinical analysis of 31 patients. J Pediatr Endocrinol Metab. 2002;15:823-830.
114b. Francis GL, Waguenspack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015:716-759.
114c. Gershergorn MC, Weintraub BD. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone. A new syndrome of “inappropriate secretion of TSH”. J Clin Invest. 1975;56:633-642.
114d. Beck-Peccoz P, Lania A, Beckers A, Chatterrjee K, Wernau JL. European Thyroid association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Thyroid J. 2013;2-76-82.
114e. Daly AF, Tichomirova MA, Petronians P, Heliovaara E, Jaffrain-Rea ML, Barlus R et al. Clinical characteristics and therapeutic responses.in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010; 95:E373-E383.
114f. Misra M, Levitsky LL, Lee MM. Transient hyperthyroidism in an adolescent with hydatidiform mole. J Pediatr. 2002;140:362-6.
114g. Hershman JM. Human chorionic gonadotropin and the thyroid: hyperemesis gravidarum and throphoblastic tumors. Thyroid. 1999; 9:653-657.
115a. Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robison B, Rosental MS, Santoro M, Schulberger M, Shah M, Waguespack SG, American Thyroid Association Guidelines task force on medullary thyroid carcinoma. Thyroid. 2015; 25:567-610.
115b. Hung W, Anderson KD, Chandra RS, et al. Solitary thyroid nodules in 71 children and adolescents. J Pediatr Surg. 1992;27:1407-9.
115c . Schlumberger M, De Vathaire F, Travagli JP, et al. Differentiated thyroid carcinoma in childhood: long term follow-up of 72 patients. J Clin Endocrinol Metab. 1987;65:1088-94.
115d . Flannery TK, Kirkland JL, Copeland KC, Bertuch AA, Karaviti LP, Brandt ML. Papillary thyroid cancer: a pediatric perspective. Pediatrics 1996;98(3 Pt 1):464-6.
115e. Lairmore TC, Frisella MM, Wells SA, Jr. Genetic testing and early thyroidectomy for inherited medullary thyroid carcinoma. Ann Med. 1996;28:401-6.
115f. Sarne D, Schneider AB. External radiation and thyroid neoplasia. Endocrinol Metab Clin North Am. 1996;25:181-95.
115g. Healy JC, Shafford EA, Reznek RH, et al. Sonographic abnormalities of the thyroid gland following radiotherapy in survivors of childhood Hodgkin’s disease. Br J Radiol. 1996;69:617-23.
115h. Soberman N, Leonidas JC, Cherrick I, Schiff R, Karayalcin G. Sonographic abnormalities of the thyroid gland in longterm survivors of Hodgkin disease. Pediatr Radiol. 1991;21:250-3.
115k. Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol. 2008;20:59-65.
115i. Waguespack SG, Francis G. Initial management and follow up of differentiated thyroid cancer in children. J Natl Compr Canc Netw. 2010; 8:1289-300.
115l. Gharib H, Goellner JR. Fine-needle aspiration biopsy of thyroid nodules. Endocr Pract .1995;1:410-7.
115m. Zimmerman D, Hay ID, Gough IR, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988;104:1157-66.
115o. Ladenson PW, Braverman LE, Mazzaferri EL, et al. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N Engl J Med. 1997;337:888-96.
115p. Wohllk N, Cote GJ, Evans DB, Goepfert H, Ordonez NG, Gagel RF. Application of genetic screening information to the management of medullary thyroid carcinoma and multiple endocrine neoplasia type 2. Endocrinol Metab Clin North Am. 1996;25:1-25.
115q. Fogelfeld L, Wiviott MB, Shore-Freedman E, et al. Recurrence of thyroid nodules after surgical removal in patients irradiated in childhood for benign conditions. N Engl J Med. 1989;320:835-40.
115r. de Vathaire F1, Haddy N1, Allodji RS1, Hawkins M1, Guibout C1, El-Fayech C1, Teinturier C1, Oberlin O1, Pacquement H1, Diop F1, Kalhouche A1, Benadjaoud M1, Winter D1, Jackson A1, Bezin Mai-Quynh G1, Benabdennebi A1, Llanas D1, Veres C1, Munzer M1, Nguyen TD1, Bondiau PY1, Berchery D1, Laprie A1, Deutsch E1, Lefkopoulos D1, Schlumberger M1, Diallo I1, Rubino C. Thyroid Radiation Dose and Other Risk Factors of Thyroid Carcinoma Following Childhood Cancer. J Clin Endocrinol Metab. 2015 ;100:4282-90.
115s. Vivanco M1, Dalle JH, Alberti C, Lescoeur B, Yakouben K, Carel JC, Baruchel A, Léger J. Malignant and benign thyroid nodules after total body irradiation preceding hematopoietic cell transplantation during childhood. Eur J Endocrinol. 2012 ;167):225-233.
Endocrine Hypertension in Childhood
ABSTRACT
Hypertension in children is an important health issue and deserves a greater awareness among health care providers and the general population. When evaluating a suspected hypertensive child, it is essential that clinicians utilize proper tools to measure and interpret the blood pressure (BP) readings. The preferred method is auscultation using a mercury sphygmomanometer connected to the appropriate size cuff. Systolic blood pressure (BP) is determined by the onset of the "tapping" Korotkoff sounds (K1) while diastolic DBP is defined as the fifth Korotkoff sound (K5), or the disappearance of Korotkoff sounds. Automated devices can be used for BP measurement in newborns and young infants, in whom auscultation is difficult. An elevated BP reading obtained with an oscillometric device should be repeated with auscultation. To determine percentile of BP, the values are compared to normal BP in children and adults adjusted for age, sex and height. For complete coverage of this and related areas in Endocrinology, visit our free web-books, www.endotext.org and www.thyroidmanager.org.
INTRODUCTION
Hypertension is defined as average systolic BP and/or diastolic BP that is ≥95th percentile for gender, age, and height on ≥3 occasions (1, 2). Regulation of systemic BP is a function of three components: intravascular volume, cardiac output and peripheral resistance. The effect(s) of steroids on one or more of these components contribute to BP control. The binding of glucocorticoids (GCs) to its receptor enhances the vascular smooth muscle response to vasopressive agents. Activation of the mineralocorticoid (MC) receptor by the ligands leads to an increase in sodium resorption which results in water retention and intravascular volume expansion. These hemodynamic changes affect peripheral resistance and cardiac output, which in turn regulates systemic BP.

In the middle adrenal zone, zona fasiculata, GC are produced. The principal GC in humans is cortisol, which serves many physiologic roles including glucose homeostasis and vascular integrity. The hypothalamic pituitary-adrenal or HPA axis determines the threshold for circulating GC concentration.
The inner zone, or zona reticuralis, is where adrenal androgens are produced (see chapter 3 in the Adrenal Physiology and Disease section). The clinical significance of its overproduction is evident in 11β-hydroxylase deficiency (11β-OHD). In this deficiency, steroid precursors proximal to the block shunted to androgen pathways which leads to virilization of the affected individual (see below).
Endocrine hypertension in children is usually mediated by the MC activities of cortisol, aldosterone and adrenal steroidogenic precursors with MC activity. Frequently in these cases, elevated BP is associated with suppressed renin activity, indicating a form of hypertension related with volume-overload and salt-sensitivity.
In the past few decades, considerable progress has been made toward unraveling the molecular genetics of some rare, or extremely rare, monogenic forms of hypertension (1).
CONGENITAL ADRENAL HYPERPLASIA (CAH); 11Β-OHD AND 17-OHD,
These include the following well-characterized disorders: two forms of
-hydroxylase deficiencies, glucocorticoid-remediable hyperaldosteronism (familial hyperaldosteronism type I), apparent mineralocorticoid excess, and Liddle's Syndrome. This chapter describes the important causes of endocrine hypertension in children as
well as some conditions with a similar presentation (Fig. 3).

Figure 3 Monogenetic mineralocorticoid hypertension syndromes. PRA = Plasma renin activity; FH = family hy- peraldosteronism; CAH = congenital adrenal hyperplasia; DOC = deoxycortisol; F/E = cortisol/cortisone ratio.
STEROID 11β- HYDROXYLASE DEFICIENCY CONGENTIAL ADRENAL HYPERPLASIA
CAH is a family of disorders characterized by enzymatic defects in one of the cortisol production steps. Steroid 11β-OHD is the second most common cause of CAH, accounting for 5-8% of all CAH cases (3). It occurs 1 in 100,00 live births (4) in the general population, but is more common in populations of North African origin (5).
Deficiency of 11β-hydroxylation causes a decrease in the conversion of 11-deoxycortisol (S) and 11-deoxycorticosterone (DOC) to cortisol and corticosterone, respectively (figure 1). Reduced cortisol feedback gives rise to an increase in ACTH secretion. Excessive ACTH secretion in turns leads to overproduction of precursors proximal to the enzyme block. These precursors serve as substrates for the unimpeded androgen pathways; therefore adrenal androgen secretion is increased. Virilization and hypertension are the salient clinical features of 11β-OHD.
The severity of in utero virilization of the external genitalia can vary from mild to severe, such that it is not uncommon to misassign an 11β-OHD affected female as a male (6,7). Males and females may manifest signs of androgen excess at any phase of postnatal development, including precocious pubic hair, advanced somatic and epiphyseal development, and central precocious puberty later in childhood. Without treatment, early epiphyseal maturation results in short stature.
Hypertension is a less consistent feature than virilization in 11β-OHD CAH. Despite failure of aldosterone production, upstream accumulation of deoxycorticosterone (DOC), a weak MC, causes salt retention and hypertension. Hypertension is usually not identified until later in childhood or in adolescence, although its appearance in an infant 3 months of age has been documented (8). In addition, hypertension correlates variably with biochemical values, or with the degree of virilization. Some of the severely virilized females were normotensive, whereas mildly virilized patients experienced severe hypertension, leading to fatal vascular accidents (9). An unusual presentation of neonatal salt wasting has also been reported (10). The complications of long standing uncontrolled hypertension, such as cardiomyopathy, retinal vein occlusion, and blindness have been reported in 11β-OHD patients (11,12). Potassium depletion develops concomitantly with sodium retention, but hypokalemia is variable.
Hormonal characteristics include elevation of compound S, DOC and androgens. Elevation of 17α-hydroxyprogesterone occurs, but not as greatly as in 21-hydroxylase deficiency (21OHD) CAH. Tetrahydro-11-deoxycortisol and tetrahydrodeoxycorticosterone, the principal metabolites of compound S and DOC, are significantly increased in the urine. Urinary 17-ketosteroids are elevated, reflecting the raised serum levels of adrenal androgens. Renin production is suppressed secondary to MC -induced sodium retention and volume expansion. Aldosterone production is low due to low serum potassium and low plasma renin.
Steroid 11β-OHD CAH is the result of autosomal recessive mutations in CYP11B1 gene. More than 50 mutations, including missense/nonsense, splicing, small/ gross deletions, insertions and complex rearrangement, which are responsible for 11β-OHD CAH have been described in CYP11B1 gene (14). A homozygous deletion of hybrid CYP11B2/CYP11B1, a reciprocal product of the recombination event as found in glucocorticoid remediable aldosteronism (GRA), leads to clinical phenotypes of neonatal salt wasting (due to diminished aldosterone synthase acitivity). This patient (10) also has 11β-OHD deficiency.
Treatment
Cortisol administration provides cortisol replacement and normalizes ACTH. This in turn removes the drive for oversecretion of DOC and in most cases brings about remission of hypertension, if diagnosed early in life. The goal is to replace deficient steroids while minimizing adrenal sex hormone and GC excess. Serum DOC and androgens are thus the indices of the adequate hormonal control. Plasma renin activity is also useful as a therapeutic index. In poor control cases with 11β-OHD, plasma renin is suppressed.
Similar to 21OHD CAH, oral hydrocortisone is preferred, because it is identical to physiologic GC. Typical dosing is 10–15 mg/m2·d in divided doses. Long-acting GCs may be an option at or near the completion of linear growth. Titration of the dose should be aimed at maintaining androgen levels at age and sex-appropriate levels and normalization of renin. Concurrently, over-treatment should be avoided because it can lead to Cushing syndrome. Depending on the degree of stress, stress dose coverage may require doses of up to 50-100 mg/m2/day. Each family must be given injectable hydrocortisone for emergency use (at the dose of 25 mg for infants, 50 mg for young children and 100 mg for adolescents and adults, intramuscularly). In the event of surgical procedure, a total of 5-10 times the daily maintainance dose (depending on the nature of the surgical procedure) may be required over the first 24 hours. Hydrocortisone dosage can be tapered down to maintenance dose during the first few days postoperatively, provided that there is no complication. Stress dose should not be given in the form of dexamethasone because of the delayed onset of action.
In children with advanced bone age, initiation of therapy may precipitate central precocious puberty, requiring treatment with a GnRH agonist. Growth hormone therapy improves height deficit in patients with poor height prediction (13). In patients with long duration of hypertension before diagnosis, additional spironolactone, calcium channel blockers or amiloride may be necessary. Reconstructive surgery of external genitalia should be performed by experienced surgeons.
Prenatal diagnosis and treatment can be accomplished using extracted fetal DNA for CYP11B1 analysis (4,15,16). An established protocol of prenatal treatment in 21OHD CAH can be applied to 11β-OHD CAH (also see Chapter 8 – Congenital Adrenal Hyperplasia)
STEROID -17 HYDROXYLASE DEFICIENCY CONGENTIAL ADRENAL HYPERPLASIA
17-OHD results from mutations in the cytochrome P450C17 enzyme which functions both as steroid 17α-hydroxylase and as 17, 20-lyase (17). The structural gene for cytochrome P450C17 (CYP17A1) has been mapped to chromosome 10q24.3 (18). Over 50 mutations in this gene have been described. Nucleotide substitution, causing missense or nonsense alterations, accounts for the majority of the patients reported (14). It is a rare disease identified in approximately 120 patients worldwide. The enzyme deficiency causes diminished production of cortisol and sex steroids, whose production requires the 17, 20-lyase function of the same 17α- hydroxylase enzyme (Figure 1). Because both adrenals and gonads share the enzyme defect, there is decreased biosynthesis of (i) androgens, results in an undervirilized phenotype in males (46,XY) at birth, and a failure of male pubertal development. (ii) estrogen, results in females at pubertal age presenting with primary amenorrhea and lack of development of secondary sex characteristics.
Reciprocal elevation of ACTH, due to low cortisol, increases synthesis of DOC and corticosterone via the unaffected 17-deoxy pathway. Therefore hypertension and hypokalemia may comprise the primary presentation at any age or can be associated with the abnormal sexual phenotype. As in 11β- OHD, the formation of aldosterone is reduced secondary to suppressed renin as a result of excess DOC.
Treatment
Treatment strategy in this condition is similar to other forms of CAH in term of GC replacement therapy and stress dose (see chapter 8 Congenital Adrenal Hyperplasia). In addition to GC, sex hormone replacement that is appropriate to sex of rearing is indicated at a developmentally appropriate time to allow patients to resemble their peers. (See also treatment section in Chapter 11 – 46,XY Disorders of Sexual Development)
GLUCOCORTICOID REMEDIABLE ALDOSTERONISM
GRA, also known as familial hyperaldosteronism type I (FH I), was first described by Sutherland et al. in 1966 (19). It is an autosomal dominant form of low renin hypertension characterized by hyperaldosteronism. Aldosterone secretion is controlled by ACTH rather than angiotensin II, and for this reason, the unique distinguishing feature of GRA is the complete and rapid suppression of aldosterone by exogenous GC (dexamethasone) administration.
GRA produces a volume expansion, salt-sensitive form of low renin hypertension. Variable presentation is not uncommon; hypertension is invariably present, but hypokalemia and metabolic alkalosis may be absent. The disease is characterized by early onset of moderate to severe hypertension with hyperaldosteronism and low renin values and by high incidence of premature cerebrovascular events. Additionally, children demonstrate normal growth and development, which distinguishes this disorder from 11β-OHD and apparent mineralocorticoid excess (AME) The serum aldosterone is elevated and plasma renin activity is suppressed, but the aldosterone-renin ratio is typically not as high as with primary aldosteronism (PA) caused by an aldosterone-producing adenoma.
Circadian measurement of plasma steroids in GRA patients has not only revealed excessive production of aldosterone following ACTH stimulation, but excessive secretion of two normally rare steroids: 18-hydroxycortisol and 18-oxocortisol (20). This can be explained by the molecular genetic finding of a chimeric gene between CYP11B1 and CYP11B2--two genes that reside within a 30-kilobase stretch on chromosome 8 that results from an unequal crossing over during meiotic reduction. CYP11B1 encodes 11β-hydroxylase, the enzyme that catalyzes the last step in cortisol synthesis in the zona fasiculata; CYP11B2 encodes aldosterone synthase, the enzyme that catalyzes the last step in aldosterone synthesis in the zona glomerulosa. The product of this chimera thus carries aldosterone synthase enzymatic activity but is regulated by ACTH. Indeed, direct genetic screening for the presence of the chimeric gene can be performed by the long template PCR method with oligonucleotides specific for CYP11B1 and CYP11B2. This test is 100% sensitive and specific, has a relatively low cost, and is more rapid and reliable, compared to conventional dexamethasone suppression test (21). However, both dexamethasone administration and genetic testing are of importance in making the diagnosis.
Treatment
Children with GRA who are treated with GCs usually experience resolution of their hypertension within 2 weeks after initiation of therapy. The recommended doses are similar to CAH during childhood and adulthood (also see Chapter 8 – Congenital Adrenal Hyperplasia), because the aim is to suppress ACTH secretion. Hydrocortisone is preferred during childhood period when dexamethasone is used in adults. A low sodium diet is recommended to lower BP because of the salt-sensitive volume expansion; this will also minimize potassium wasting. Typically, potassium supplement is not required. Normalization of urinary hybrid steroid levels and abolition of ACTH-regulated aldosterone production is not a requisite for hypertension control and, if used as a treatment goal, may unnecessarily increase the risk of Cushingoid side effects (22). The response to GCs is variable in adults, often requiring additional use of antihypertensive medications, such as spironolactone, amiloride and triamterene. It has been shown that even in the absence of hypertension, aldosterone excess is associated with increased left ventricular wall thicknesses and reduced diastolic function, initial changes that lead to cardiovascular morbidities. This leads to the recommendation to treat normotensive subjects diagnosed with FH I (23).
APPARENT MINERALOCORTICOID EXCESS
AME is a rare inherited form of hypertension caused by 11 β-hydroxysteroid dehydrogenase type 2 (11 β-HSD) deficiency. The disorder was first described biochemically and hormonally in 1977 by New et al in a Native American girl with severe hypertension (24). The syndrome is caused by non functional mutations in HSD11B2 gene on chromosome16q22. More than 40 causative mutations have been described. (14) In the past 4 decades since the original description of the disease, published data only included less than 100 patients worldwide.
AME defined an important “pre-receptor” pathway in steroid hormone action and their specificities to the receptor. The exploration and elucidation of this disease opened a new area in receptor biology as a result of the demonstration that the specificity of the MR function depends on a metabolic enzyme (11ßHSD2) rather than the receptor itself (25,26). This enzyme functions to protect the MR by inactivating cortisol to its inactive metabolite cortisone, thereby enabling the mineralocorticoid aldosterone to occupy the MR in vivo (27,28). Aldosterone is not metabolized by 11ßHSD2 because it forms a C11–C18 hemi-ketal group in aqueous solution. The MR is non-selective in vitro and cannot distinguish between the glucocorticoid cortisol and its natural ligand, aldosterone (29,30). Therefore, lack of protection of the receptor owing to the enzyme defect allows cortisol, which has higher circulating levels than aldosterone, to bind to the MR and to act as a mineralocorticoid. Clinical manifestations of AME mimic those of excessive mineralocorticoid activity, but no elevation of known mineralocorticoids is present in the AME patients. Three metabolite ratios are calculated, each reflecting a different aspect of enzyme function: (1) tetrahydrocortisol (THF) + allo-THF/ tetrahydrocortisone (THE) (global function of HSD) (31) ; (2) allo-THF/THF ratio (defect in 5ß-reductase activity) (32,33) ; (3) urinary free cortisol (UFF)/urinary free cortisone (UFE) (kidney HSD function)(34). Originally AME was described through the plasma half-life of [11-3H] cortisol (which when metabolized by 11ß-HSD yields tritiated water and cortisone), which may more accurately reflect renal 11ß-HSD2 activity (35).
AME usually presents in early life with low birth weight and postnatal failure to thrive, hypertension, and persistent polyuria and polydipsia. The disorder is characterized by hypokalemic alkalosis, hyporeninemia and undetectable serum concentrations of aldosterone. End-organ damage secondary to hypertension is common, even at a young age. Thirteen out of
fourteen AME patients demonstrated damage of one or more organs (kidney, heart, retina or central nervous system) at the time of diagnosis. In addition, most had hypercalcuria with nephrocalcinosis (36).
Treatment
The treatment of AME is primarily directed at the correction of hypokalemia and hypertension. Cortisol acts as the offending MC in AME, hence blockage of its binding to the MR reverses excess mineralocortocoidism. Spironolactone, an MR receptor antagonist, is the medication of choice: it binds competitively and protects the receptors against any MC in excess. The required dose of spironolactone in AME patients may go up to 3-5 mg/kg/day (or more than 400 mg per day in adults), to control blood pressure and to normalize renin. A reduction in dietary sodium and supplemental potassium are beneficial. Potassium supplement varies among patient to patient, range from 3-8 mEq/Kg/day. Patients with nephrocalcinosis require additional thiazide diuretic. In order to reduce urinary calcium and control blood pressure in these patients, either chlorothiazide at the dose of 20 mg/Kg/day or hydrochlorothiazide at the dose of 2 mg/Kg/day is recommended. Follow-up studies of AME patients treated with spironolactone revealed significant improvement in clinical symptoms. These outcomes demonstrate the importance of early diagnosis and adequate treatment (26,36). Another approach utilizing dexamethasone at the dose of 1.5-2.0 mg/day to suppress cortisol secretion demonstrated variable results. Normalization of BP occurred in approximately 60% of cases (37). Dexamethasone does not correct the hypokalemia and hypertension in all patients, and long-term therapy has excessive GC adverse effects. The low effectiveness of this treatment is not surprising based on theoretical grounds: in vitro data suggests that putative physiologic ligands to non-selective MR in the kidney include dexamethasone, as well as cortisol and other MCs (29). Therefore administering dexamethasone to suppress cortisol secretion, which is already lowered in AME, may supply an additional MR ligand to aggravate MC excess.
Additional antihypertensive medications, such as thiazides or amiloride, may be required during disease progression. Cure of AME was reported in one patient after kidney transplantation due to the normal 11β-HSD2 activity of the transplanted kidney (38,39). Advances in enhancing or inhibiting11βHSD2 activity by some medications may provide novel treatments for AME (40).
Although AME is very rare, mild or intermediate phenotypes of AME patients may be linked to common human disorders via alteration in cortisol-cortisone shuttle. These include several forms of hypertension, kidney failure, inflammatory processes (cirrhosis and cardiac fibrosis), low birth weight/ fetal programming of adult diseases and lately, carcinogenesis.
PRIMARY ALDOSTERONISM
Primary aldosteronism (PA) is a group of disorders, originally described by J.W.Conn in 1954 (41), in which there is a non-suppressible secretion of aldosterone. The major presentations are hypertension and hypokalemia. However, hypokalemia does not occur in the majority of patients with primary aldosteronism, with the prevalence ranging from 9 to 37% in adults (42). Various symptoms associated with hypokalemia can be found, including muscle weakness with various types of paresthesias, tiredness, thirst, polyuria and nocturia.
PA occurs in greater than 10% of hypertensive adult patients (43). Although it is considered rare in children, the high prevalence in the general adult population suggests that the disease
may develop in the pediatric population prior to its presentation of hypertension and vascular damage in adulthood [4]. Moderate to severe hypertension that does not respond to medication(s), spontaneous or diuretic induced hypokalemia and the presence of adrenal mass provide clues to diagnosis (43).
The major causes of PA are aldosterone-producing adenomas (often small tumors of less than 2 centimeters in diameter), bilateral or unilateral adrenal hyperplasia and rarely adrenal carcinoma. Plasma aldosterone-renin ratio (ARR) may be used as an initial screening test and should be repeated if the results are not conclusive or are difficult to interpret. Established ARR cut-offs in adults range between 20 to 40 (43). Further testing through suppressing aldosterone by oral sodium loading, saline infusion, and/or a challenge with either fludrocortisone or captopril can be used for diagnosis confirmation; however cut-off values and interpretation have only been established in adults. Adrenal computed tomography scan or an MRI image are used as the imaging study to identify the mass. The treatment options include unilateral adrenalectomy for unilateral diseases found on adrenal vein sampling and a MR antagonist such as spironolactone or eplerenone. (see details in Chapter 23 – Aldosterone Excess in ADRENAL PHYSIOLOGY AND DISEASES section)
PHEOCHROMOCYTOMA
Pheochromocytomas are reported to account for hypertension in 1 to 2% of children (44). They are catecholamine-producing tumors that arise from the chromaffin cells of the adrenal medulla and the sympathetic ganglia and they present with signs and symptoms that are related to the action of catecholamines. (See Chapter 34 in Adrenal Physiology and Disease section). Although the peak incidence is in the third to fourth decades, 10% to 20% occur in children, with increased frequency in boys, and a median age at presentation between 9.5 and 12.5 years (45). Certain symptoms are reported as occurring more commonly in children than adults. These include sweating, visual disturbances, nausea, vomiting, loss of weight, polyuria and polydipsia (46). In comparison with adults in whom the hypertension is often paroxysmal, it is sustained in 70 to 90% of children (47). However, hypertension is not invariable and can be absent in up to 20% of children (48). Furthermore, many pheochromocytomas, especially associated with MEN 2 and VHL disease, can be clinically silent.
OTHER CAUSES OF CHILDHOOD HYPERTENSION
Liddle’s syndrome is a rare autosomal dominant disease described by Liddle et al. in 1963 (49) causing arterial hypertension. Mutations in SCNN1B and SCNN1G, the genes that mapped to chromosome 16p12, have been described in Liddle’s syndrome patients (14). The clinical and biochemical findings other than elevated blood pressure are: chronic hypokalemia, increased urinary potassium excretion in conjunction with sodium retention, suppressed renin activity, aldosterone and angiotensin II. These presentations are similar to AME, but in contrast, Liddle’s syndrome is an autosomal dominant disorder that does not show a favorable response to spironolactone. (21)
Another rare cause is familial hyperaldosteronism type II (FHII), the first cases described by Gordon et al. in 1991 in three families with a familial variety of PA (50). It is distinguished from type I (GRA) by the failure of dexamethasone’s suppression of aldosterone and no hybrid gene mutation. FH-II is more common than FH-I, but their clinical presentations are indistinguishable from other forms of PA. Patients with FH II are older than those with FH I, perhaps owing to diagnosis of FH I at a younger age, made possible by genetic testing. No significance in age, sex, biochemical parameters, or aldosterone and renin levels was seen between patients with FH II and those with apparently sporadic PA. (21) It has been described both in families and in sporadic cases worldwide, with a range in age starting at 14 years and equal gender distribution (51). Although the inheritance in many families appears to be autosomal dominant, in sporadic cases it is still uncertain. Surgical treatment in the case of unilateral adrenal mass and medical treatment with MR antagonists can be effective (21).
Acknowledgement:
The author would like to express a special gratitude to C. Joan Riesland, M.Ed., BSN, RN for her editorial work on this article.
REFERENCES
- Corvol P, Persu A, Gimenez-Roqueplo AP, Jeunemaitre X Seven lessons from two candidate genes in human essential hypertension: Angiotensinogen and Epithelial Sodium Channel. Hypertension 1999; 33:1324-31
- The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114(2 Suppl 4th Report):555-76.
- Zachmann M, Tassinari D, Prader A 1983 Clinical and biochemical variability of congenital adrenal hyperplasia due to 11beta-hydroxylase deficiency. J Endocrinol Metab 56:222-229
- Curnow KM, Slutsker L, Vitek J, et al. 1993 Mutations in the CYP11B1 gene causing congenital adrenal hyperplasia and hypertension cluster in exons 6, 7, and 8. Proc Natl Acad Sci (USA) 90:4552-6
- Khemiri M, Ridane H, Bou YO, Matoussi N, Khaldi F 2006 [11 beta hydroxylase deficiency: a clinical study of seven cases]. Tunis Med 84:106-13
- al-Jurayyan NA 1995 Congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency in Saudi Arabia: clinical and biochemical characteristics. Acta Paediatr 84:651-4
- Rosler A, Leiberman E, Sack J 1982 Clinical variability of congenital adrenal hyperplaisa due to 11B-hydroxylase deficiency. Hormone Research 16:133
- Mimouni M, Kaufman H, Roitman A, Morag C, Sadan N 1985 Hypertension in a neonate with 11 beta-hydroxylase deficiency. Eur J Pediatr 143:231-3
- Rosler A, Leiberman E, Sack J 1982 Clinical variability of congenital adrenal hyperplaisa due to 11B-hydroxylase deficiency. Hormone Research 16:133
- Ezquieta B, Luzuriaga C 2004 Neonatal salt-wasting and 11 beta-hydroxylase deficiency in a child carrying a homozygous deletion hybrid CYP11B2 (aldosterone synthase)- CYP11B1 (11 beta-hydroxylase). Clin Genet 66:229-35
- Hague WM, Honour JW 1983 Malignant hypertension in congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. Clin Endocrinol (Oxf) 18:505-10
- Chabre O, Portrat-Doyen S, Chaffanjon P, et al. 2000 Bilateral laparoscopic adrenalectomy for congenital adrenal hyperplasia with severe hypertension, resulting from two novel mutations in splice donor sites of CYP11B1. J Clin Endocrinol Metab 85:4060-8
- Quintos JB, Vogiatzi MG, Harbison MD, New MI 2001 Growth hormone therapy alone or in combination with gonadotropin-releasing hormone analog therapy to improve the height deficit in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:1511-7
- Stenson et al (2009), The Human Gene Mutation Database
(HGMD®): 2008 Update. Genome Med 1(1):13
- Geley S, Kapelari K, Johrer K, et al. 1996 CYP11B1 mutations causing congenital adrenal hyperplasia due to 11 beta-hydroxylase deficiency. J Clin Endocrinol Metab 81:2896-901
- Cerame BI, Newfield RS, Pascoe L, et al. 1999 Prenatal diagnosis and treatment of 11beta-hydroxylase deficiency congenital adrenal hyperplasia resulting in normal female genitalia. J Clin Endocrinol Metab 84:3129-34
- Nakajin S, Shinoda M, Haniu M, Shively JE, Hall PF. C21 steroid side chain cleavage enzyme from porcine adrenal microsomes. Purification and characterization of the 17 alpha-hydroxylase/C17,20-lyase cytochrome P-450. J Biol Chem 1984;259(6):3971-6.
- Fan YS, Sasi R, Lee C, Winter JS, Waterman MR, Lin CC. Localization of the human CYP17 gene (cytochrome P450(17 alpha)) to 10q24.3 by fluorescence in situ hybridization and simultaneous chromosome banding. Genomics 1992;14(4):1110-1.
- Sutherland D, Ruse J, Laidlaw J. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J 1966;95(22): p1109-19.
- Dluhy R, Lifton R. Glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 1999;84(12): p4341-4.
- New MI, Geller DS, Fallo F, Wilson RC. Monogenic low renin hypertension. Trends Endocrinol Metab 2005;16(3):92-7.
- Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD. Treatment of familial hyperaldosteronism type I: only partial suppression of adrenocorticotropin required to correct hypertension. J Clin Endocrinol Metab 2000;85(9):3313-8.
- Stowasser M, Sharman J, Leano R, Gordon RD, Ward G, Cowley D, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab 2005;90(9):5070-6.
- New MI, Levine LS, Biglieri EG, Pareira J, Ulick S (1977) Evidence for an unidentified steroid in a child with apparent mineralocorticoid hypertension. J Clin Endocrinol Metab 44: 924-933
- New MI (1994) The prismatic case of apparent mineralocorticoid excess. J Clin Endocrinol Metab 79: 1-3
- Wilson RC, Nimkarn S, New MI (2001) Apparent mineralocorticoid excess. Trends Endocrinol Metab 12: 104-111
- Edwards C, Stewart P, Burt D, et al. (1988) Localisation of 11 beta-hydroxysteroid dehydrogenase--tissue specific protector of the mineralocorticoid receptor. Lancet 2: p986-989
- Funder J, Pearce P, Smith R, Smith A (1988) Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science 242: p583-585
- Krozowski ZS, Funder JW (1983) Renal mineralocorticoid receptors and hippocampal corticosterone-binding species have identical intrinsic steroid specificity. Proc Natl Acad Sci (USA) 80: 6056-6060
- Arriza JL, Weinberger C, Cerelli G, et al. (1987) Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237: 268-275
- Palermo M, Quinkler M, Stewart PM (2004) Apparent mineralocorticoid excess syndrome: an overview. Arq Bras Endocrinol Metabol 48: 687-696
- Monder C, Shackleton CH, Bradlow HL, et al. (1986) The syndrome of apparent mineralocorticoid excess: its association with 11 beta-dehydrogenase and 5 beta- reductase deficiency and some consequences for corticosteroid metabolism. J Clin Endocrinol Metab 63: 550-557
- Shackleton CH, Rodriguez J, Arteaga E, Lopez JM, Winter JS (1985) Congenital 11 beta-hydroxysteroid dehydrogenase deficiency associated with juvenile hypertension: corticosteroid metabolite profiles of four patients and their families. Clin Endocrinol (Oxf) 22: 701-712
- Palermo M, Shackleton CH, Mantero F, Stewart PM (1996) Urinary free cortisone and the assessment of 11 beta-hydroxysteroid dehydrogenase activity in man. Clin Endocrinol (Oxf) 45: 605-611
- Ulick S, Levine LS, Gunczler P, et al. (1979) A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. J Clin Endocrinol Metab 49: 757-764
- Dave-Sharma S, Wilson RC, Harbison MD, Newfield R, Razzaghy-Azar M, Krozowski Z, et al. Extensive Personal Experience: Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endo Metab 1998;83:2244-2254.
- Cushing's disease of the kidney. Lancet 1988;2(8618):1002.
- Palermo M, Cossu M, Shackleton CH. Cure of apparent mineralocorticoid excess by kidney transplantation [letter]. N Engl J Med 1998;339(24):1787-8.
- Palermo M, Delitala G, Sorba G, Cossu M, Satta R, Tedde R, et al. Does kidney transplantation normalise cortisol metabolism in apparent mineralocorticoid excess syndrome? J Endocrinol Invest 2000;23(7):457-62
- Riddle MC, McDaniel PA. Acute reduction of renal 11 beta-hydroxysteroid dehydrogenase activity by several antinatriuretic stimuli. Metabolism 1993;42(10):1370-
- Conn JW, Louis LH (1956) Primary aldosteronism, a new clinical entity. Ann Intern Med 44: 1-15
- Mulatero P, Stowasser M, Loh KC, et al. (2004) Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 89: 1045-1050
- Funder JW, Carey RM, Fardella C, et al. (2008) Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93: 3266-3281
- Dubois R, Chappuis J. [Pheochromocytoma: pediatric features]. Arch Pediatr 1997;4(12): p1217-25.
- Mircescu H, Wilkin F, Paquette J, Oligny LL, Decaluwe H, Gaboury L, et al. Molecular characterization of a pediatric pheochromocytoma with suspected bilateral disease. J Pediatr 2001;138(2):269-73.
- Fonseca V, Bouloux P. Phaeochromocytoma and paraganglioma. Baillieres Clin Endocrinol Metab 1993;7(2): p509-44.
- Ross J. Pheochromocytoma. Special considerations in children. Urol Clin North Am 2000;27(3): p393-402.
- Khafagi FA, Shapiro B, Fischer M, Sisson JC, Hutchinson R, Beierwaltes WH. Phaeochromocytoma and functioning paraganglioma in childhood and adolescence: role of iodine 131 metaiodobenzylguanidine. Eur J Nucl Med 1991;18(3):191-8.
- Liddle GW, Bledsoe T, Coppage WS, Jr. A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans. Assoc. Am. Phys. 1963;76:199-213.
- Gordon R, Stowasser M, Tunny T, Klemm S, Finn W, Krek A. Clinical and pathological diversity of primary aldosteronism, including a new familial variety. Clin Exp Pharmacol Physiol 1991;18(5): p283-6.
- Stowasser M, Gunasekera TG, Gordon RD. Familial varieties of primary aldosteronism. Clin Exp Pharmacol Physiol 2001;28(12):1087-90.

