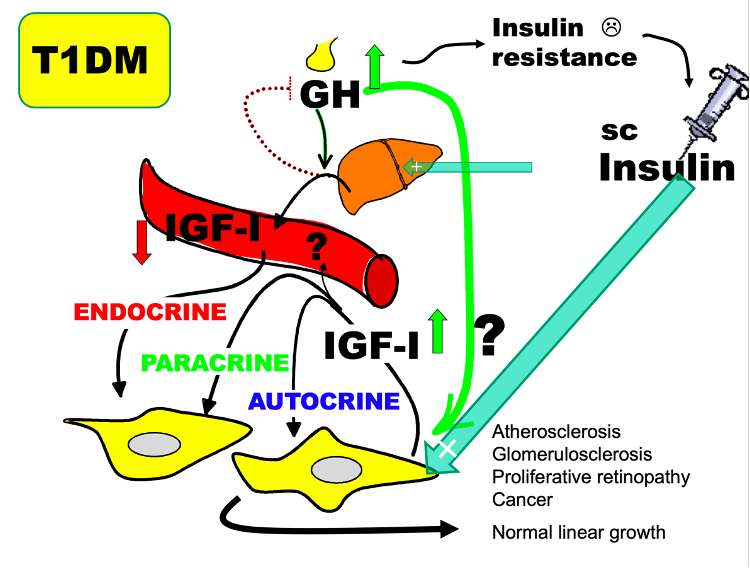
Figure 5. Changes in liver derived endocrine IGF-I measured in the circulation and paracrine/autocrine IGF-I are in most cases concordant. In the absence of practical and validated methods to measure IGF-I at the tissue site of action, paracrine/autocrine IGF-I activity is assessed by determining known physiological actions of IGF-I such as growth or glucose metabolism. Type 1 diabetes is a condition with discordant changes in endocrine vs. paracrine/autocrine changes in IGF-I that in many ways resembles those reported in a mouse model of conditional knock-out of IGF-1 expression in the liver. In type 1 diabetes, insulin deficiency in the liver, caused by a systemic rather than a portal insulin replacement therapy, results in a functional GHR signaling defect to IGF-I transcription (uncoupling). Low endocrine IGF-I production decreases circulating IGF-I and results in decreased negative pituitary feedback and GH hypersecretion. The lack of direct IGF-I effects on glucose uptake in muscle and the diabetogenic effects of GH (including maintained signaling to lipolysis) decreases insulin actions on glucose metabolism (known as insulin resistance). The portal insulin deficiency also fails to suppress hepatic glucose production. In other to maintain glycemic control, the increased insulin requirement can only be met by more subcutaneous insulin leading to systemic hyperinsulinemia. There is no direct information about local paracrine/autocrine IGF-I activity, but there are several indications that tissue hyperinsulinemia and GH hypersecretion results in a compensatory increase of tissue IGF-I activity. Firstly, linear growth is not impaired in children and adolescents with Type 1 Diabetes despite of their low endocrine IGF-I (comparable to levels in short stature children), indicating a compensatory upregulation of local IGF-I activity (IGF-I being the most important stimulator of longitudinal growth). Secondly, it is plausible that increased local IGF-I activity contributes to diabetes complications known to be tightly associated with increased rather than decreased IGF-I activity. While type 1 diabetes is not generally associated with increased risk of cancer, the increase in local production of IGF-I in obesity and type 2 diabetes may contribute.
