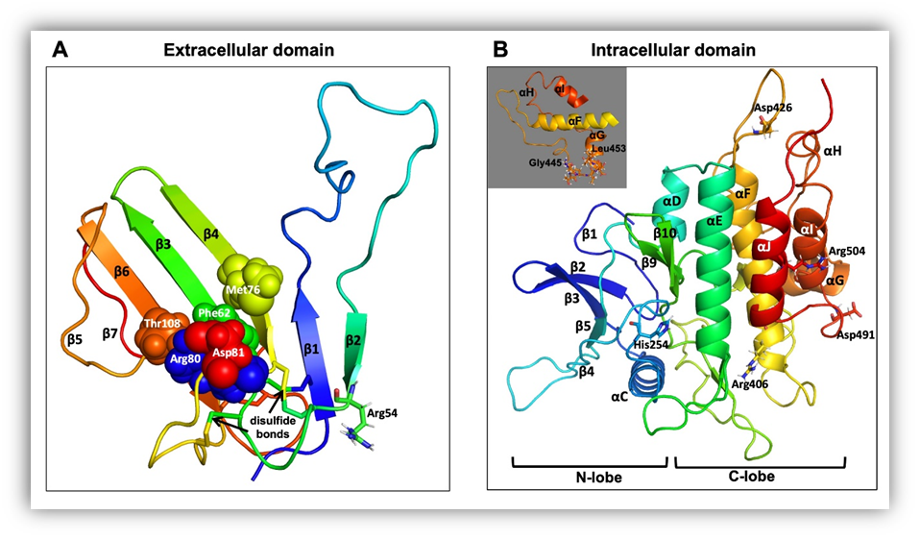
FIGURE 23. Molecular models of AMHR2 extracellular and intracellular domains. (A) The extracellular domain exhibits the general three-finger toxin fold of type II receptors and displays five disulfide bridges, four of which are conserved. Five amino acids (Phe62, Met76, Arg80, Asp81, and Thr108), implicated in binding AMH, are shown as spheres. (B) The intracellular domain exhibits the general fold of a two-domain kinase, with an N-lobe consisting mainly of a five-stranded β-sheet and a C-lobe, which is mainly α-helical. Some of the residues affected by PMDS mutations (Arg54, His254, Arg406, Asp426, Asp491, and Arg504) are shown as sticks. The inset shows residues affected by the p.((Gly445_Leu453del) mutation. Reprinted with permission from Elsevier, from ref. 364 (490): Josso N, Picard JY, Cate RL (2013). The Persistent Müllerian Duct Syndrome. In: New MI, Parsa A, Yuen TT, O’Malley BW, Hammer GD, eds. Genetic Steroid Disorders. New York, NY (USA): Elsevier
