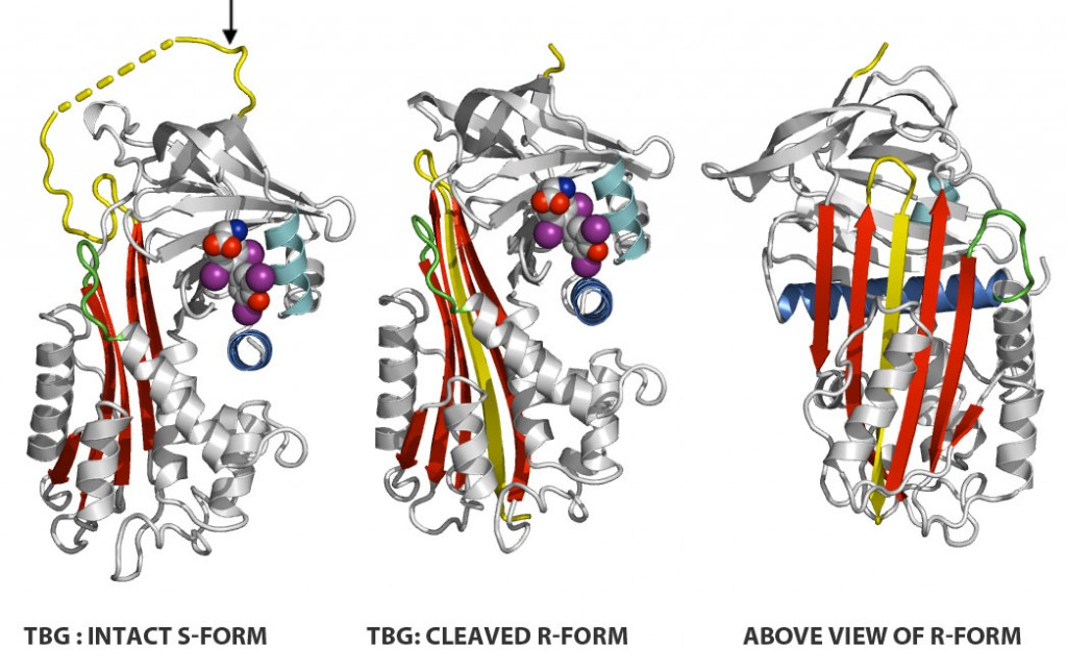
Figure 1. Structure of the TBG molecule: Reactive loop (in yellow). Insertion occurs following its cleavage by proteases to give an extra strand in the main sheet of the molecule but the T4-binding site can still retain its active conformation. This is in keeping with other findings showing that the binding and release of T4 is not due to a switch from an on to an off conformation but rather results from an equilibrated change in plasticity of the binding site. So, the S-to-R change in TBG results in a 6 -fold decrease but not a total loss of affinity. The important corollary is that that the release of thyroxine is a modulated process as notably seen in response to changes in temperature (19). (Courtesy of Dr, R.W. Carrell),
