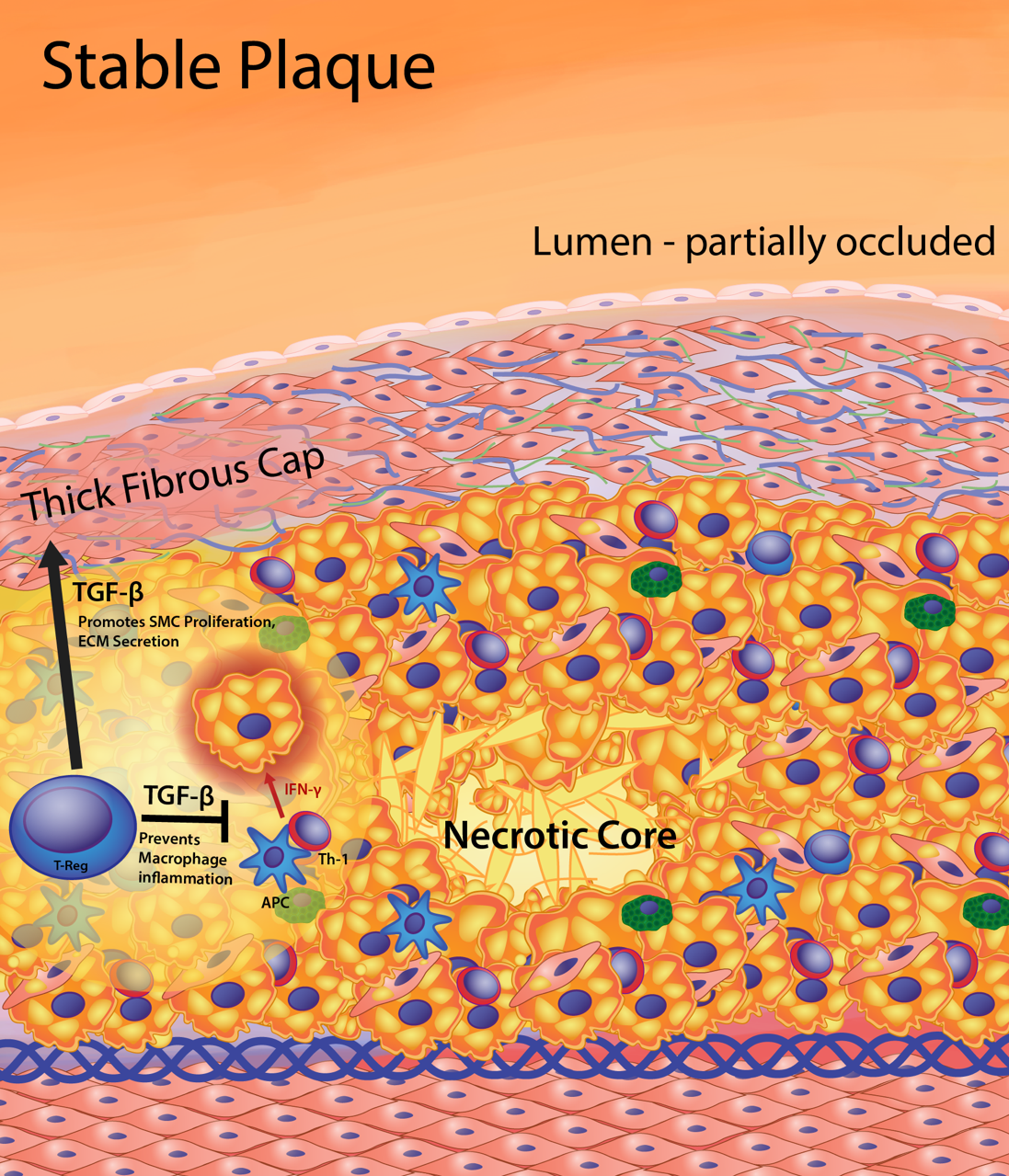
Figure 4. Features of the stable fibrous plaque. As the cell volume of the intima increases, there is vascular remodeling so that the lumen is only partially occluded, substantially lessening clinical events resulting from occlusion. The stable plaque contains a generous fibrous cap composed of layers of smooth muscle cells ensconced in a substantial extracellular matrix network of collagen, proteoglycans, and elastin. The thick fibrous cap of the stable plaque provides an effective barrier preventing plaque rupture and exposure of lesion prothrombotic factors to blood, thereby limiting thrombus formation and clinical events. Maintenance of a thick fibrous cap is enabled by regulation of the inflammatory status of the foam cell core of the lesion. Regulatory T (T-reg) cells produce transforming growth factor-β (TGF-β) and IL-10. In addition, T-reg cells inhibit antigen-specific activation of T helper 1 (Th-1) cell to produce interferon gamma (IFNg). Increased TGF-β and IL-10 and decreased IFNg reduce the proinflammatory macrophage phenotype leading to reduced cell death, effective efferocytosis (phagocytosis of dead cells), and anti-inflammatory cytokine production (i.e. TGF-β, IL-10). Thus, stable plaques have small necrotic cores containing macrophage debris and extracellular lipid resulting from secondary necrosis of noninternalized apoptotic macrophage foam cells. The production of TGF-β by T-reg cells and macrophages maintains fibrous cap quality by being a potent stimulator of collagen production in smooth muscle cells.
