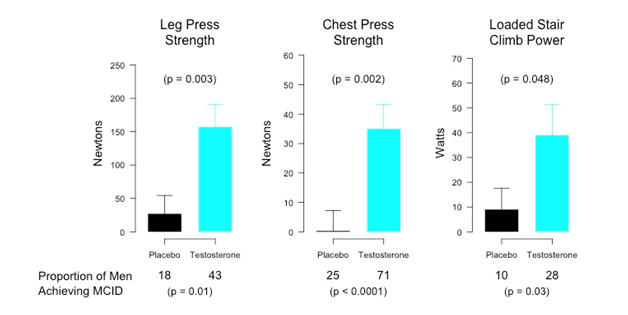
Figure 6. Effects of testosterone administration on maximal voluntary strength in the leg press and chest press exercises and on loaded stair climbing power in a randomized testosterone trial in older men with mobility limitation (The TOM Trial) The TOM Trial was a randomized, placebo-controlled trial in which men, 65 years of age or older with mobility limitation, were randomized to receive either placebo or testosterone gel daily for 6-months (Basaria et al, N Engl J Med 2010, and Travison et al, J Gerontol 2011). The dose of testosterone was adjusted to achieve target testosterone levels between 500 and 1000 ng/dL. The mean (SD) change from baseline to either the end of the intervention period or to the last measurement performed in subjects who dropped out before study completion. The minimal clinically important difference (MCID) for each outcome was determined using an anchor-based method within the trial. The proportion of men (percent) whose change from baseline either equaled or exceeded the MCID is shown below the figure along with the P-value for the comparison of placebo and testosterone groups. Figure reproduced with permission from Spitzer et al, Nature Reviews in Endocrinology 2013.
