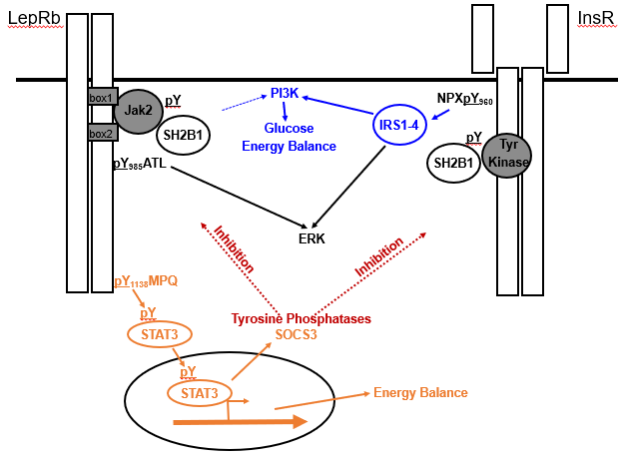
Figure 3. Signaling by and inhibition of LepRb and InsR. LepRb, which exists as a preformed homodimer in complex with the Jak2 tyrosine kinase, recruits and phosphorylates (pY) STAT3 via phosphorylated pY1138 to control many aspects of energy balance. InsR, which also exists as a preformed dimer, but has intrinsic tyrosine kinase activity, autophosphorylates the juxtamembrane Tyr960 to recruit the insulin receptor substrate (IRS) proteins IRS1-IRS4. IRS-proteins strongly activate the phosphatidylinositol 3-kinase (PI3K), which play roles in the brain control of energy balance and glucose homeostasis. Leptin also activates PI3K, albeit much more weakly than InsR, and by undefined mechansims. Both LepRb and InsR activate the ERK pathway. The adapter protein, SH2B1 also enhances signaling by both receptors. In addition to decreasing food intake and increasing energy expenditure, LepRb-mediated STAT3 signaling promotes the expression of SOCS3, which acts as a feedback inhibitor of LepRb and InsR signaling. A variety of tyrosine phosphatases also inhibit the activity of both receptors.
