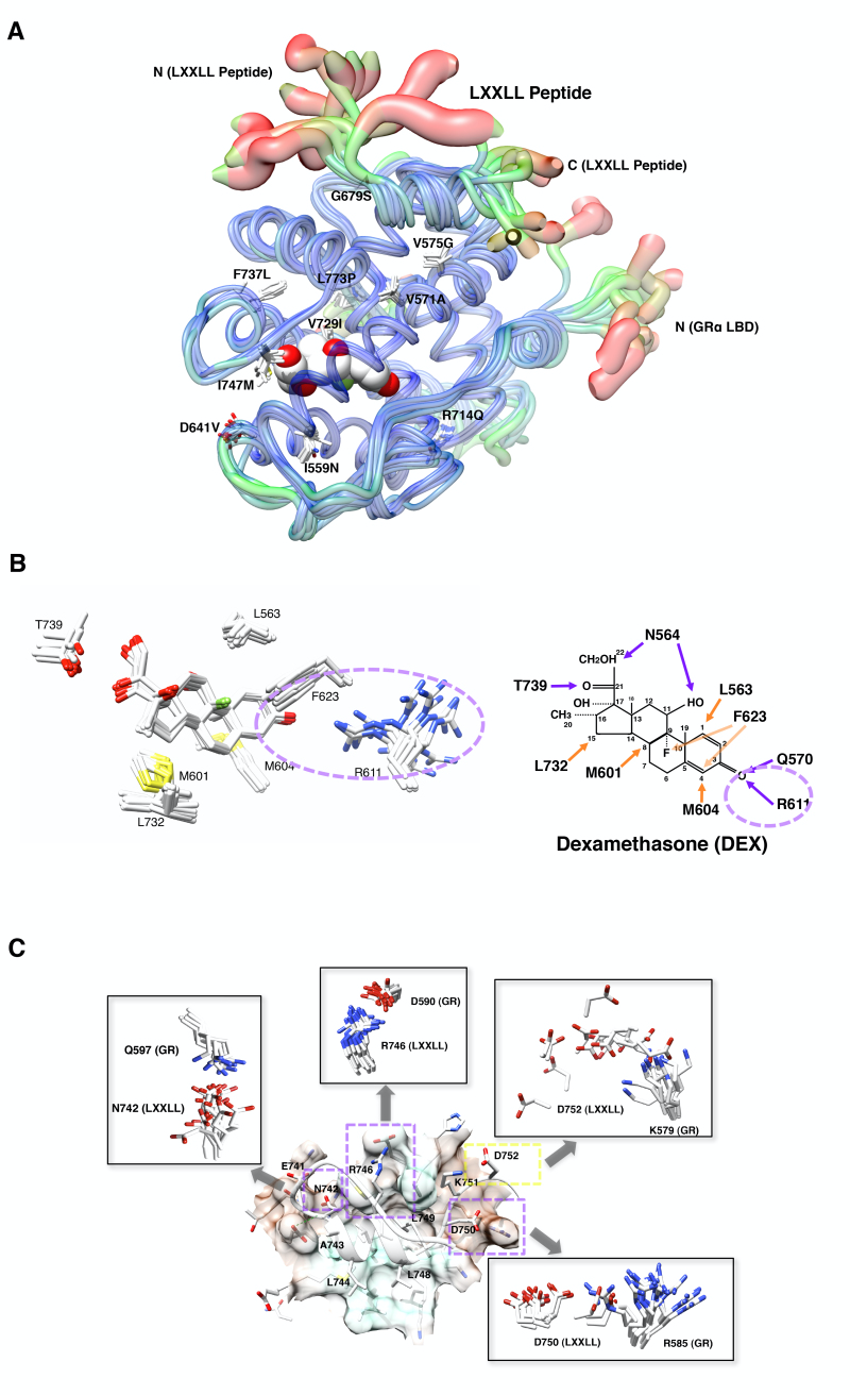
Figure 19. Impact of pathologic GR point mutations to the molecular structure of GR LBD. A: Distribution of the pathologic GR point mutations in its LBD and their overall impact on the 3-dimensional LBD peptide backbone. Thickness and color of the overlaid C-traces of the GR mutant receptor LBDs and the wild type GR LBD indicate the areas of least (thin and blue) to most (thick and red) motion over the course of simulation. Locations and side chains of the mutated amino acids are indicated, whereas dexamethasone (shown with the white and red spheres of space-filling model) is located inside LBP. B: Alteration of the electrostatic bond formed by arginine (R) 611 and threonine (T) 739 of pathologic GR mutants to dexamethasone may largely explain the reduced affinity of many pathologic GR mutants to this steroid. The left panel demonstrates superimposed 3-dimensional interaction images of dexamethasone and the key residues of all pathologic GRa mutants. Among the key amino acids of pathologic mutants participating in interaction with dexamethasone, R611 is largely deviated in these mutant receptors, which underlies reduced/disappeared electrostatic interaction between this residue and the carbonyl oxygen at carbon-3 of dexamethasone. Q570 and N564 are omitted from these panels. Major changes observed in the electrostatic bond formed by R611 and T739 are indicated with a purple dotted circle. The right panel shows schematic molecular interaction between wild type GRa and dexamethasone. Purple and orange arrows indicate electrostatic and non-covalent bonds, respectively. DEX: dexamethasone. C: Defective non-covalent bonds formed between Q597, D590, K579 and R585 of the pathologic GRa mutants and N742, R746, D750 and D752 of the LxxLL peptide mainly explain reduced interaction of the mutant receptor AF-2s to this peptide. The panel demonstrates 3-dimensional image of the molecular interaction between the LXXLL peptide and key residues of the wild type GRa. The LxxLL peptide forms important electrostatic bonds with its non-core leucine residues (N742, R746, D750 and D752) against the receptor residues (Q597, D590, R585 and K579, respectively) as marked with purple dotted boxes. Pathologic GRa mutants demonstrate significant shift of the side chains of some of these receptor residues among which the side chain of R585 shows the most significant deviation (shown in square inserts). Modified from (89).
