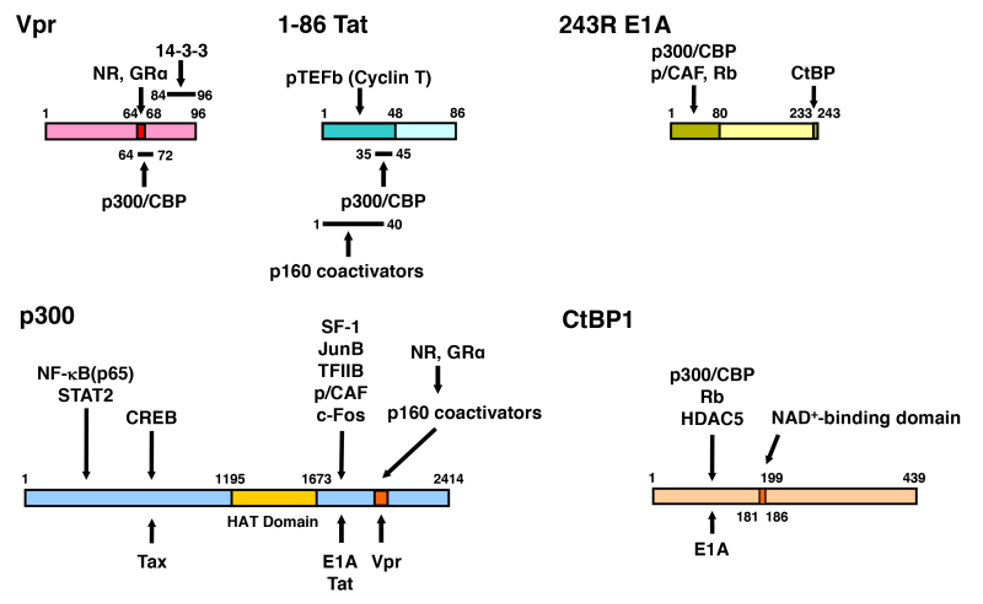
Figure 20. Linearized Vpr, Tat, E1A, p300 and CtBP1 molecules and their mutual interaction domains. Vpr interacts with GR and several other NRs through its LxxLL motif located at amino acids 64 to 69. Binding sites of Vpr and p160-type HAT coactivators overlap with each other on p300. Since Vpr has a LxxLL motif similar to p160 coactivators, Vpr mimics host p160 coactivators and enhances GR transcriptional activity. Tat also binds both p300 and p160 coactivators. p300 facilitates attraction of many transcription factors, cofactors and general transcription complexes, and loosens the histone/DNA interaction through acetylation of the histone tails by its histone acetyltransferase (HAT) domain. E1A binds p300 at the latter’s C-terminal portion, while it physically interacts with the N-terminal portion of CtBP1 through its C-terminal end. The N-terminal portion of CtBP1 physically interacts with HDAC5 and Retinoblastoma protein (Rb), which have repressive activity on transcription. CtBP1 regulates its interaction to binding partners by sensing cellular NAD+ levels through its NAD+-binding domain. The HAT domain of p300 and the NAD+-binding domain of CtBP1 are indicated in grey. Modified from (478). CREB: CRE-binding protein, HAT: histone acetyltransferase, HDAC5: histone deacetylases 5, NF-B: nuclear factor-B, NAD: nicotinamide adenine dinucleotide, NR: nuclear hormone receptor, p/CAF: p300/CBP-associating factor, pTEFb: positive-acting transcription elongation factor b, Rb: retinoblastoma protein, SF-1: steroidogenic factor-1, STAT2: signal transducer and activator of transcription 2, TFIIB: transcription factor IIB.
