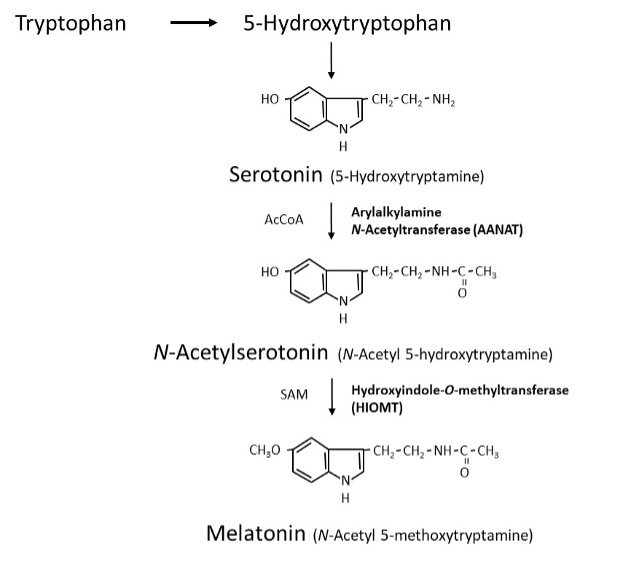
ABSTRACT
Low levels of physical activity combined with food intake in excess of daily energy expenditure over extended time periods precede weight gain and promote increases in body fat. Obesity and related insulin resistance are common sequelae of a chronically positive energy balance, potentially resulting in type 2 diabetes (T2D) and nonalcoholic/metabolic dysfunction associated fatty liver disease (NAFLD/MAFLD). The percentage of individuals considered as obese and morbidly obese is continuously rising and developing countries are catching up quickly as compared to industrialized nations. If the observed trend continues, global obesity prevalence will prospectively reach more than 21% in women and 18% in men by 2025. In addition to poor dietary habits, physical activity levels have decreased in recent decades in parallel with an increase in sedentary behavior. Given the technological advances in domestic, community, and working spaces in the last century it is not uncommon for people in industrialized countries to spend one half of their day sitting. As well, for a majority of people, voluntary physical exercise remains of minor importance. Non-Exercise Activity Thermogenesis (NEAT) refers to that portion of daily energy expenditure resulting from spontaneous physical activity that is not specially the result of voluntary exercise. Levels of NEAT ranges widely, with variance of up to 2000 kilocalories per day between two individuals of similar size. These differences are related to complex interactions of environmental and biological factors, including people’s differing occupations, leisure-time activities, individual molecular and genetic factors, and evidence that food intake has independent effects on spontaneous physical activity. Available data support the hypothesis that targeting NEAT could be an essential tool for body weight control. This comprehensive review systematically describes the definition, evaluation methods, and environmental and biological factors involved in the regulation of NEAT. It further emphasizes the association with obesity and related disorders and suggests practical relevant implications, but also potential limitations for the integration of NEAT in daily life.
INTRODUCTION
The prevalence of obesity and resultant adverse health implications continues to rise in both sexes in the United States and worldwide (1). In 2011 Finucane et al. estimated international trends of mean, age-standardized, body mass index (BMI) and suggested that an estimated 1.46 billion adults worldwide had BMI of 25 kg/m² or greater, of these 205 million men and 297 million women were considered obese (2). Indeed, between 1980 and 2008 the prevalence of adult obesity (BMI ≥ 30 kg/m2) has risen in every sub-region of the world except for Central Africa and South Asia (2). The mean BMI increase was reported to be 0.4 kg/m2 per decade for men and 0.5 kg/m2 for women (2). Comparable data have been presented by the NCD Risk Factor Collaboration Trial (3). With a focus on industrialized countries, the most prominent increases of mean BMI have been observed in the United States, followed by the United Kingdom and Australia. The United States had the highest BMI of all studied high-income countries, recent data from the U.S. National Health and Nutrition Examination Survey indicate that obesity affects approximately 35% of the male and 40% of the female population (1, 4, 5). Similarly, populations of European and Eastern Mediterranean countries show an overweight prevalence of up to 50% (6). Further data indicate that compared to 1975, childhood obesity is much more common in 2016, with one obesogenic key driver in this group representing reduced physical activity (3, 7). This continuous rise in the incidence and prevalence of overweight and obesity in children, adolescents, and adults in the past century has created major burdens for national health care systems worldwide.
Obesity-related diseases are typically of chronic nature and include type 2 diabetes mellitus (T2D), nonalcoholic/metabolic dysfunction associated fatty liver disease (NAFLD/MAFLD), peripheral artery and cardiovascular disease, sleep apnea, hypertension, as well as various cancers. In the United States, seven out of the leading ten causes of premature death and disability are represented by chronic diseases related to obesity (8). In fact, obesity is one of the most important avoidable risk factors for significant morbidity and premature mortality, leads to impaired quality of life, can elevate disability rates, and statistically reduces expected life span by seven years (9, 8, 10, 11).
The etiology of obesity is multifactorial, resulting from genetic and epigenetic, physiological, behavioral, sociocultural, and environmental factors leading to an imbalance between energy intake and expenditure (12). To this end excess body fat consistently results from a period of sustained net-positive energy balance, followed by homeostatically regulated weight maintenance. The roles of diet and caloric intake in these processes have been the principal focus of most mechanistic studies. In countries with high obesity prevalence rates, nutritional quality is mostly poor, but there is a controversy as to whether increased energy intake alone can explain the obesity epidemic. Trends for improved dietary quality and reduced energy intake have been observed in recent decades in the United Kingdom and among parts of the U.S.-population (13–15). For instance, since the 1980s obesity prevalence has substantially risen in Great Britain, while caloric intake appeared to decline on a population level (12). In order to explain such findings it was hypothesized that if physical activity decreases, body weight may increase if energy intake is not altered (16). Therefore, under conditions of unchanged caloric intake, the amount of daily physical activity emerged as major predictor (1, 17). Accordingly, the National Weight Control Registry of the United States has identified strategies for maintaining weight loss, which include engagement in higher levels of physical activity (1). Therefore, greater attention has been paid to addressing the energy expenditure side of the caloric balance equation, in particular with regards to prevention of body weight regain after successful weight loss.
NON-EXERCISE ACTIVITY THERMOGENESIS: A SIGNIFICANT COMPONENT OF DAILY ENERGY EXPENDITURE
Daily total energy expenditure (TEE) is the net amount of energy utilized by animals and humans to maintain core physiological functions and locomotion. Three main components of energy balance determine TEE: Basal metabolic rate (BMR), the thermic effect of food (TEF), also called diet-induced thermogenesis, and the energy expended for physical activity (18). Additional components may exist (e.g. energy costs of emotion), but apparently play a minor role with respect to energy balance (19–22). Multiple factors significantly affect our daily energy needs, including age, body composition, thyroid hormone status, catecholamine levels/sympathoadrenergic activity, ambient and body temperature, disease states, and certain medications (19, 18).
Basal Metabolic Rate
Basal metabolic rate (BMR) represents the minimal amount of energy expended to maintain all the body’s vital processes at rest (the basal state). BMR is essentially a function of lean body mass (LBM), which is estimated to account for 80% of BMR within and across species (23–29) . BMR is sometimes misinterpreted to imply the lowest level of energy expenditure during the day. This is, however, not true, since during sleep or undernutrition metabolic functions may be lower than that observed under basal conditions (27). In free-living individuals, BMR accounts for the main percentage of TEE (Figure 1), in healthy subjects with mainly sedentary occupations it predicts around 60% of the variance (22). Otherwise it is proportionally larger in individuals with minimal physical activity (e.g., in sedated and ventilated patients on an intensive care unit) (30).
Of note, it is not uncommon that the term resting energy expenditure (REE) is synonymously interchanged with BMR. However, BMR is measured in completely rested subjects, both before and during the measurements. Therefore, measurements are classically taken in the morning after 8 hours of sleep. Subjects should be lying supine, fully awake, and be fasted for at least 10-12 hours. The environment in which the measurements are taken should be thermo-neutral (22–26 °C) so that no thermoregulatory effect on heat production biases the results (19); and subjects should be free from emotional stress and familiar with the measurement apparatus (27). By contrast, REE is equivalent to postabsorptive energy expenditure at complete rest at any time of the day and can vary as much as 10% from BMR (21).
Some studies suggest BMR is reduced in those prone to become obese (31–33). It is, however, a matter of controversy as to whether a reduction of energy expenditure due to a decrease in BMR sufficiently explains the positive net energy balance that contributes to excess weight gain in the majority of subjects who become obese (34–36). On the other hand, LBM can be enlarged or even preserved by means of regular physical activity, which in turn can favorably modulate BMR (1, 37–39).
Thermic Effect of Food
Thermic effect of food (TEF) is the increment of energy expenditure above REE following meal ingestion that reflects the energy cost (burned) during food digestion, absorption, and storage. It is a relatively stable component of total energy expenditure (Figure 1). Thermic effect of food typically ranges between 8-15% of TEE and the variance of TEF has been associated with nutrient composition and energy content of consumed foods (40).
Physical Activity: EAT and NEAT
Physical activity is the second most significant contributor to TEE in most people (Figure 1). It is defined as the additional energy expenditure above REE and TEF that is required for performing daily activities and therefore also called physical activity-related or simply activity-related energy expenditure (PEE/AEE). PEE/AEE can be categorized into exercise-related activity thermogenesis (EAT) and non-exercise activity thermogenesis (NEAT). Both vary widely within and between individuals.
For the majority of subjects in industrialized countries, exercise is believed to be negligible (20). According to NHANES data, 36.1% of the studied US population was categorized as sedentary, while a further 47.6% were physically active at low levels (40, 41). Remarkably, only around 16% of subjects in NHANES met recommended guidelines for physical activity or were highly active. Even so, the latter subjects did not necessarily exercise (42, 43, 41). Thus, it is reasonable to conclude that on a population level the percentage of subjects engaging in regular, intense physical exercise is low. In those who habitually participate in purposeful physical training, EAT is believed to maximally account for 15-30% of TEE (18, 44). Other authors suggest that the majority of subjects undergoing regular physical training, defined as “bodily exertion for the sake of developing and maintaining physical fitness,” do not exercise more than two hours a week, accounting for an average energy expenditure of 100 kilocalories (kcal) per day(45). Such expenditure would contribute to only 1-2% to the variance of TEE. Taken together, for most human subjects EAT seems to not be a major contributor to TEE variance.
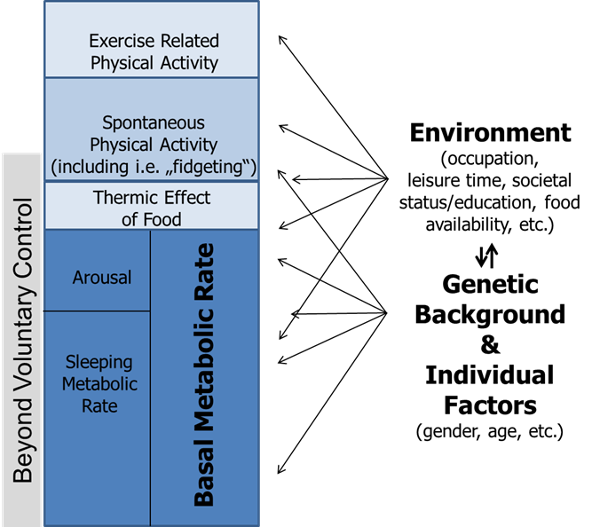
Figure 1. Model of human energy expenditure components (adapted from (46)). Exercise-related physical activity is comparable to exercise-related activity thermogenesis (EAT), while spontaneous physical activity is comparable to non-exercise activity thermogenesis (NEAT); for further explanations see text. Note that parts of spontaneous physical activity are beyond voluntary control, also called “fidgeting.” Arrows symbolize the multiple and varying impacts of individual factors, genetic background, and environment.
In contrast, NEAT comprises the largest share of daily activity-related thermogenesis, including for most subjects engaging in regular physical training. It is important to note that both NEAT and spontaneous physical activity are not interchangeable but represent complementary concepts (47): NEAT refers to energy expenditure, while spontaneous physical activity describes the types of bodily activity that are not defined as purposeful movements but still contribute to NEAT. Some authors categorize NEAT into three main subcomponents comprising body posture, ambulation, and all other spontaneous movements including “fidgeting” (48). Accordingly, a certain percentage of spontaneous physical activity (and therefore NEAT) is beyond voluntary control (e.g., “fidgeting”).
NEAT corresponds to all the energy expended with occupation, leisure time activity, sitting, standing, stair climbing, ambulation, toe-tapping, shoveling snow, playing the guitar, dancing, singing, cleaning, and more (20, 22). These activities do not characteristically involve moderate- to vigorous-intensity activities in comparable manner as voluntary exercise, but occur at a low energy workload for minutes and up to hours (12).
The importance of NEAT becomes apparent when considering the following points: The variability in BMR between individuals of similar age, BMI and of equal gender ranges around 7-9% (49), while the contribution of TEF is maximally 15%. Thus, BMR and TEF are relatively fixed in amount and account for approximately three quarters of daily TEE variance. As EAT is believed to be negligible on a population level, NEAT consequently represents the most variable component of TEE within and across subjects. It is responsible for 6-10% of TEE in individuals with a mainly sedentary lifestyle and for 50% or more in highly active subjects (47, 22, 29).
Taken together, with respect to body mass regulation and modification of energy balance, NEAT must be considered as a factor with potentially major impact. This is of particular interest in terms not only of developing therapeutic strategies for the patient with obesity, but also for designing obesity-prevention strategies for populations. The following sections will focus on these issues, including the environmental and biological modification of NEAT.
Measurement of NEAT
For a better understanding of the potential role of NEAT in the context of obesity it is essential to recognize the strengths and limitations of available techniques used for the quantification of NEAT.
NEAT can be principally measured by two approaches: 1) by assessing total NEAT, and 2) by using the factorial approach (for extensive reviews of available techniques see (50, 51)).
TOTAL NEAT
Assuming that EAT is negligible, total NEAT can be calculated by subtracting BMR and TEF from TEE (16):
Equation 1: NEAT = TEE – (BMR + TEF)
To complete equation 1, TEE, BMR and TEF need to be measured. Under free-living conditions TEE can be reliably measured by using the doubly-labeled water method (16). This approach requires the application of stable isotopes (deuterium/2H2 and O18) containing water (2H2O18). The difference in the clearance rate of the two isotopes equals carbon dioxide (CO2) production generated during energy production and thus represents quantification of TEE (52)(Figure 2).
One necessary precondition of the doubly-labeled water method is that the O2 of expired CO2 is in equilibrium with the O2 in body water. Once ingested, O18 will be readily distributed in the systemic H2O, H2CO3 and CO2 pools. 2H2 will be dispensed in body water and the H2CO3 pool (equation 2).
Equation 2: CO2 + H2O ↔ H2CO3
The concentration of labeled O2 in body water will then decrease over time due to a loss of CO2 with expiration, perspiration, and in excreted body water (urine). 2H2 is mainly lost through excreted body fluids, yet a small percentage can be incorporated into body fat or protein. Since body water becomes tagged with known amounts of tracers at the same time at the beginning of the measurement period, the difference of elimination rates of both tracers equals CO2 excretion.
Doubly labeled water is usually administered at baseline after samples of blood, urine and saliva have been collected (21). Subsequent sample collections occur within 7-21 days after administration, when the isotopes are completely dispersed within body compartments. Isotope-ratio mass spectroscopy is used to measure 2H2 and O18 enrichments in collected samples, which are needed to finally calculate CO2 production. Thereby, TEE can be ascertained under free-living conditions with an error of about 6-8%. This error can be minimized by repeatedly gathering samples over the measurement period rather than relying on only two time points.
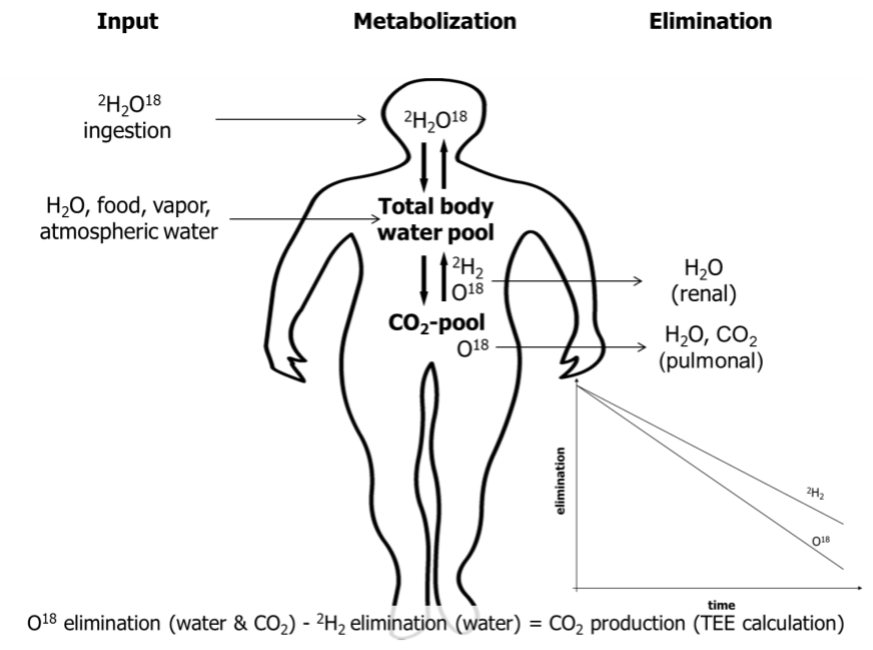
Figure 2. Schematic picture of the doubly labeled water method. CO2, carbon dioxide; 2H2, deuterium; O18, labeled oxygen.
It should be mentioned that the study of TEE classically relied on direct calorimetry (49, 18). Direct calorimetry, or direct measurement of heat loss from a subject, is achieved by using well-controlled environmental chambers. However, this requires participants to be confined to small rooms, which fails to capture the enormous variety of NEAT components in free-living individuals (21, 22).
By contrast, indirect calorimetry is an easily accessible and portable method commonly used to assess BMR, RMR and TEF. Indirect calorimetry is based on the principle that by measuring oxygen consumption (VO2), CO2 excretion (VCO2), or both over a defined time span, the metabolic conversion of fats, carbohydrates, and proteins into energy can be calculated using established formulae (18). The net energy released is typically expressed as kcal or Kilojoule (kJ). The small percentage of physiological protein oxidation can be estimated by nitrogen excretion in urine, or can be neglected without adding a substantial error in subjects who are in stable nitrogen balance (18).
Indirect calorimetry is accordingly one of the most commonly used approaches in clinical investigations, either in controlled metabolic-ward studies or in field settings. For instance, Levine et al. in their study on human NEAT applied indirect calorimetry to measure BMR and TEF (53). To measure TEF they provided participants with a meal containing a third of the subject’s daily weight maintenance energy needs. To estimate TEF over 24 hours, the measured energy expenditure area-under-the-curve obtained when they ate the standardize meal was multiplied by three (16). Alternatively, study protocols may ignore TEF as a non-substantial variable, or simply multiply TEE by 0.1 to yield a crude estimate.
When sensitive techniques are not available, BMR can be alternatively estimated by using validated age-, gender- and population-specific equations. For instance, the Harris-Benedict equation is widely used in clinical settings (18). However, this equation was derived from about 300 healthy, normal-weight Caucasian adults between 1907 and 1917 and its application, therefore, has limitations (18). Substantial error could be introduced by applying it to an inappropriate collective, including subjects who are with obesity, which is also true for other validated equations estimating BMR (18).
The physical activity level (PAL) is frequently used in studies to provide an index of activity when measurement of total NEAT is not possible. It is calculated by expressing TEE relative to BMR (15). Sedentary subjects in industrialized countries typically have a PAL of approximately 1.5 (see Table 1). The PAL rises to values of 2.0-2.4 with strenuous work and under certain conditions it can increase to values up to 3.5-4.5 (22). Using PAL, however, can also introduce significant bias in the assessment of NEAT, as under free-living conditions the cumulative error of PAL measurements can be as high as 7% (21).
|
Table 1. Physical Activity Levels (PAL) Predicted From Lifestyle (from 16). |
|
|
Chair or bed bound |
1.2 |
|
Seated work with no option of moving around and little or no strenuous leisure activity |
1.4 – 1.5 |
|
Seated work with discretion and requirement to move around but little or no strenuous leisure activity |
1.6 – 1.7 |
|
Standing work (e.g., homemaker, shop assistant) |
1.8 – 1.9 |
|
Strenuous work or highly active leisure |
2.0 – 2.4 |
THE FACTORIAL APPROACH OF NEAT MEASUREMENT
Measuring total NEAT or using PAL provides no information regarding the individual components contributing to PEE/AEE. Therefore, the factorial approach is widely used to estimate NEAT constituents. Accordingly, all physical activities of a subject of interest are recorded over a defined time span, typically seven days. The energy equivalent of each activity is determined, and these equivalents are then apportioned according to the time spent by the individual with the respective activities. Finally, the data is totaled to get an estimate of the energy expenditure attributable to NEAT (21, 22). The first step when using the factorial approach is the quantification of a subject’s physical activities. Several methods are available to obtain such information, including questionnaires, interviews, and activity diaries. These approaches might be useful for estimates of particular activities, such as those related to occupational duties. Otherwise, they have substantial limitations, including inadequate or incomplete data recording, alteration of habits during assessment periods, and others (21, 22).
Measuring NEAT is a complex task when intensities of transition movements and fidgeting-like activities are the matter of research interest. By applying a combination of direct calorimetry and motion detectors, Ravussins’s group have shown that there is a considerable inter-person variability of daily energy expenditure, even after adjustment for differences in LBM (29). A large percentage of this variability among individuals was due to variability in the degree of e.g., "fidgeting." Thus, estimation of fidgeting-like activities represents a substantial subcomponent under conditions of NEAT measurement. However, subcomponents such as “fidgeting” might not be captured by many methods. Levine et al. proposed an accelerometer method that could predict 86% of activity related energy expenditure for the posture and locomotion component of NEAT compared with a room calorimeter, but predicted only 50% of the variance in fidgeting and did not discriminate various types of fidgeting (50). Therefore, more technically advanced approaches have been developed. Among these are motion detectors, floor-pressure-pad displacement, cine photography, pedometers, accelerometers, and global positioning systems (GPS) (21, 22). Recently, portable intelligent devices for energy expenditure and activity assessment were used to study subtypes of NEAT by the method of mechanical modeling (54).
However, depending on the degree of sophistication, all these approaches have limitations. While data obtained from triaxial accelerometers typically found in wearable activity monitors is often used as an estimate of activity-related energy expenditure, they only measure actual movements (not energy expended), and a combination of methods probably yields the most appropriate estimates (21, 22). For example, energy costs of single NEAT components are typically measured by means of indirect calorimetry, for which highly sophisticated portable systems are now available. Alternatively, tables with listed energy costs of NEAT activities may be used. This latter method is convenient and inexpensive, but for similar reasons as pointed out for PAL, substantial systematic errors can be introduced (21). Similar problems will arise when NEAT is assessed by multiple linear regression approaches at a population level (55).
Furthermore, principal problems ascend with the NEAT measurement per se, as little validated information is available concerning the time necessary to representatively assess spontaneous physical activity. Many studies report extrapolation of measured results of an individual’s energy expenditure obtained during a limited timeframe to longer intervals. In this regard, experienced investigators believe that measurements of approximately seven days will likely provide a representative assessment regarding a 2-4 month time span (21).
Taken together, the difficulty of getting true estimates under free-living conditions is a major reason why available information on human NEAT physiology is limited. Representative studies are needed that are conducted over appropriate time spans (months) and utilize a combination of the factorial approach and total NEAT measurement to overcome major limitations in current estimates.
INDIVIDUAL AND ENVIRONMENTAL DETERMINANTS OF NEAT
There is a close interplay between environmental factors and individual characteristics with respect to NEAT as discussed below.
Effects of Occupation, Gender, and Age
Highly physically-active individuals expend up to three times more energy in 24-hours than subjects with negligible bodily activity (56). As previously discussed, NEAT accounts for a significant proportion of the observed differences at the population level TEE. Thereby, occupation clearly represents the key determinant of NEAT (45). Indeed, NEAT can vary by as much as 2000 Kcal per day when comparing two adults of similar body size, lean body mass, age and gender (Figure 3).
For an average worker spending most of her/his time in a seated position, occupational NEAT is relatively low and associated energy costs range at a maximum of 700 kcal per day (Figure 3). A comparable person working mainly in a standing position can increase their occupational NEAT to up to 1400 kcal per day, while an agricultural occupation would theoretically result in NEAT categories ranging around 2000 kcal per day or more (Figure 3) (45). Thus, occupations relying on intense physical activity can expend 1500 kcal per day more than a sedentary job.
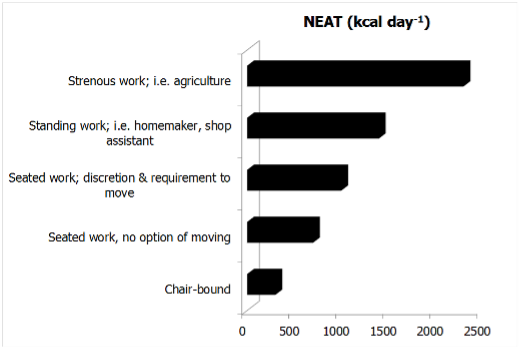
Figure 3. The effect of occupational intensity on NEAT (adapted from (45)). The data assume a BMR of 1600 kcal per day.
This variation in daily NEAT between individuals and populations becomes even more accentuated when considering research from non-industrialized countries (57, 58). For instance, a study by Levine and colleagues included more than 5000 inhabitants of agricultural regions of the Ivory Coast of Africa (Figure 4).
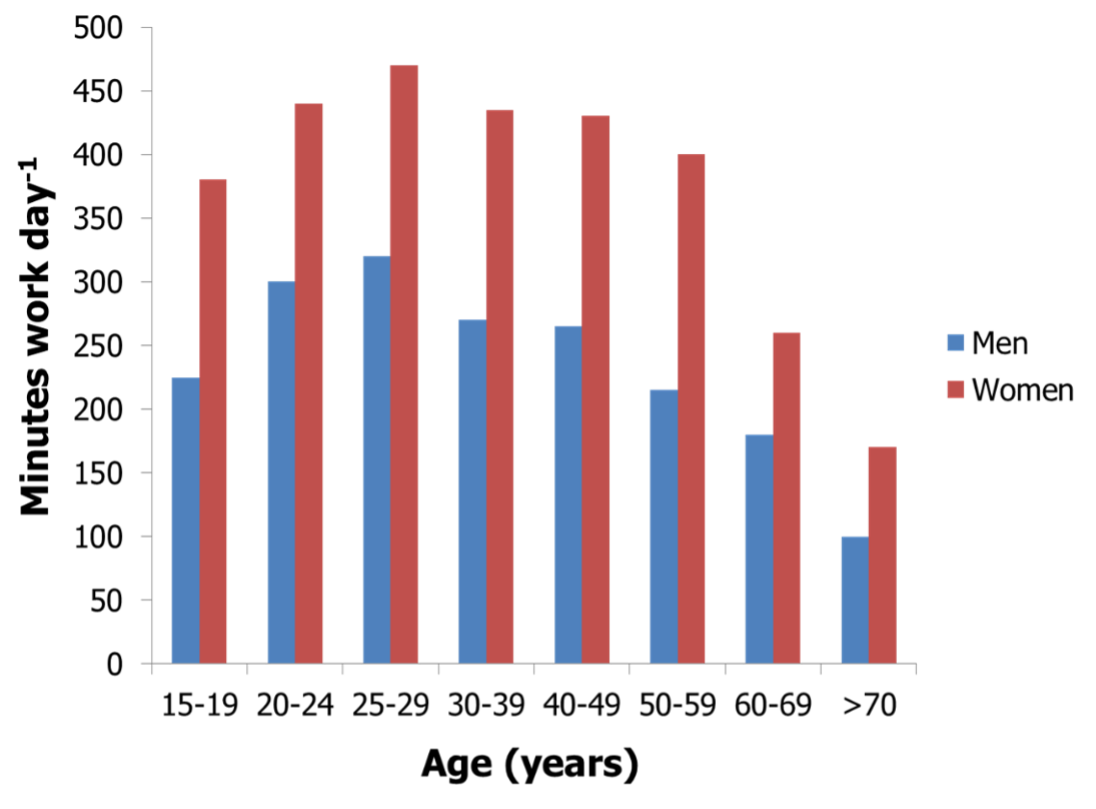
Figure 4. Gender- and age-dependent physical activity levels (time engaged in work activities) of people living in agricultural communities of the Ivory Coast in Africa (adapted from reference (58)).
Each subject was studied over seven days and all of their daily tasks were recorded by a trained evaluator (58). Several lines of available evidence regarding NEAT were established from this study. First, a substantial effect of gender was observed as women apparently worked more than men in these societal constructs. Women were responsible for more than 95% of domestic and for an additional 30% of agricultural tasks. Otherwise, men exclusively worked in agricultural occupations and had more leisure time, resulting in substantially lower NEAT than women (45, 58). In contrast, men in Canada, Australia or the United Kingdom are reported to be up to three times more physically active than females, while data from the United States indicate comparable activity levels of genders (59, 60). Even less data is available on NEAT in children, but in general boys seem to be reproducibly more active than girls (61, 62).
A second important observation from the study of Levine et al. is the fact that decreased occupational NEAT was observed with aging (Figure 4) (58). An age-related decline of NEAT had been previously reported across species (63). The underpinnings for this age-effect on NEAT in free-living humans has been reported by Harris et al. (64), who showed that the substantial decrease in elderly as compared to younger subjects was mainly attributable to the ambulation subcomponent of NEAT. Elderly subjects walked less distance despite having a comparable number of daily walking periods compared to their younger counterparts. Indeed, elderly subjects performed 29% less non-exercise activity, corresponding to three miles less ambulation per day (64).
A further factor that should be considered to potentially impact NEAT could be sleep restriction. It was shown that subjects with 5.5 compared to 8.5 hours nighttime sleep opportunity had 31% fewer daily activity counts, spent 24% less time engaged in moderate plus vigorous physical activity and became more sedentary (65). However, the significance of these findings is currently limited to time spans over few weeks and it remains therefore unclear as to whether the results are also valid in terms of common problems, such as chronic sleep insufficiency or shift working. Although, some short-term experiments support the latter findings, conflicting data have also been reported on that matter (66–69). Future research will need to validate these associations and, if true, better understand mechanisms underlying restricted sleep time and alterations in PEE/AEE. Sleep restriction should be nevertheless regarded as potential biasing factor in future research projects on NEAT.
Industrialization and Societal Status
Industrialization and societal status are distinctive factors affecting NEAT. Urban environment and mechanization are associated with a decrease in daily physical activity. For example, it was shown that sales of labor-saving domestic machines (e.g. washing machines) and obesity rates are closely correlated in the U.S. population, while this association was not present with respect to energy intake (20, 22). Furthermore, it is known that poverty and societal aspects comprising neighborhood and educational status are associated with increased consumption of energy-dense foods, which is thought to contribute to higher obesity rates reported in lower socioeconomic groups (70, 71). Highly educated individuals report more leisure-time physical activity and are three times more likely to be physically active as compared to less educated individuals (20, 22).
Seasonal Effects
Seasonal variations also play a role for NEAT. Time spent in activity is twice as likely during the summer as compared to winter months and contributions to activity from agricultural or construction work plays a greater role in the summer season (20).
Together, available data indicate close relationships between environmental conditions and biological aspects of NEAT. Evidence suggests that the contribution of occupational activity to NEAT can vary by 2000 kcal per day. Since the accumulation of excess body fat is a result of positive energy balance, subtle perturbations of energy balance over sustained time periods are theoretically capable of contributing to unwanted weight gain. But once weight stability is again established at a higher weight, energy expenditure and energy intake need to be precisely matched to achieve a long-term persistency of body mass, as an error of 1% would lead to a gain or loss of 1 kg per year or some 40 kg between the ages of 20-60 years. Therefore, small alterations of energy balance by means of NEAT could theoretically result in significant effects with respect to severity of obesity in an individual and the prevalence of obesity in a population. Consequently, when considering how to influence NEAT as a strategy for the treatment or prevention of obesity, a central question arises: In what fashion does NEAT interact with another environmentally-influenced factor involved in energy balance, that of energy intake?
Alteration of NEAT with Varying Energy Availability from Foods
Alteration of energy balance is followed by multiple adaptations, but relatively few studies have addressed modulation of spontaneous physical activity and NEAT by variations in nutritional energy intake. The latter is part of the concept of adaptive thermogenesis (72).
Adaptive thermogenesis refers to changes in REE, EAT and NEAT, which are independent of changes in LBM and are, instead, modulated by other factors, including increased or decreased caloric intake (72). With caloric restriction, both REE and non-resting energy expenditure substantially contribute to changes in adaptive thermogenesis (72). Interestingly, in most studies, alterations in adaptive thermogenesis in response to overfeeding are in large part explained by raised non-resting energy expenditure (e.g., NEAT). However, the individual ability to adapt NEAT to manipulations of energy balance is highly variable between subjects and situations, particularly with respect to overfeeding (39). But if an individual is able to generate a significant upregulation of NEAT in the face of positive energy balance brought about by an increase in food intake, they may be more able to prevent obesity (34).
NEAT UNDER CONDITIONS OF OVERFEEDING
As discussed above, by using a combination of direct calorimetry and motion detectors, Ravussin et al. have shown that the inter-person variability of TEE is considerable and remains significant even after adjustment for LBM (29). A substantial percentage of this variability is explained by differences in spontaneous physical activity including “fidgeting” (29). Fidgeting-like activities accounted for a range in energy expenditure of 100-800 kcal per day in the subjects. Interestingly, the researchers observed elevated spontaneous physical activity levels in subjects with obesity as compared to those who were lean, indicating a positive relationship with elevated body mass. However, the latter study was performed under isocaloric conditions and over a short observation period. Therefore, the importance of this report was to establish the significance of spontaneous physical activity for energy balance, but whether acute variability in energy intake affects NEAT was not addressed. In their “weight clamping” study, Leibel et al. (73) overfed adults who were lean and overweight/obese by 10%, or approximately with 5000-8000 kcal per day over 4-10 weeks, followed by a weight maintenance period. Body composition, fecal caloric loss, TEE, REE, TEF and spontaneous physical activity were measured using multiple technical approaches including indirect calorimetry, the doubly labeled water method, and activity trackers in a subgroup (73). They showed that maintaining a 10% elevated body mass induced a significant increase of TEE by 9±7 kcal per kg LBM per day in subjects who had not been previously obese and by 8±4 kcal per kg LBM per day in those who had formerly been obese (73). The majority of this rise in TEE was attributable to non-resting energy expenditure. The magnitude of this effect was comparable in those who had previously not been obese and those who had been overweight or obese, indicating similar adaptations to overfeeding conditions (73). However, a related review pointed out the large inter-individual variability regarding the capability to adjust energy expenditure with overfeeding in the “weight clamping” experiments (46) and emphasized that in other human over-feeding studies there is a wide range of individual weight gain per unit of excessively consumed energy. Therefore, it can be proposed that some individuals show a remarkable capacity to increase energy expenditure in response to overfeeding, while others do not (46). It was further suggested that spontaneous physical activity, and accordingly NEAT, likely play an important role regarding the impact on body weight under such conditions.
Levine’s group was the first to systematically investigate the effect of overfeeding on the individual ability to adapt NEAT in free-living subjects (53). Using sophisticated methods and measuring NEAT over a representative time span, the authors overfed 16 volunteers (12 males, 4 females; age ranging from 25-36 years) by 1000 kcal per day over their weight maintenance requirements (20% of the calories came from protein, 40% from fat, 40% from carbohydrates). The energy surplus was paralleled by a mean TEE increment of 554 kcal per day. In this study 14% of this TEE increase was attributable to a rise in REE, approximating by 79 kcal per day. A further 25% of the TEE increment (136 kcal per day) corresponded to an increase in TEF, probably due to the relatively high percentage of protein intake. The most prominent effect was, however, attributable to enhanced physical activity thermogenesis, corresponding to around 336 kcal per day. As volitional exercise of the study subjects remained at a constant low level and since the authors did not detect changes in exercise efficiency, they concluded that about 60% of the increase in TEE due to overfeeding was attributable to NEAT (53). Again, however, the change in NEAT varied remarkably between subjects, ranging from -98 to +692 kcal per day. The maximal individual increase of NEAT constituted 69% of the excessively consumed 1000 kcal per day in this study (74). Moreover, the change in NEAT was directly predictive of the individual vulnerability or resistance to body fat accumulation (53).
A recent randomized study enrolled young aged female and male participants with variable BMI (19-30 kg/m2) and used gold standard methods to investigate not just the effect of caloric overfeeding, but also of protein intake on PEE/AEE (75). 140% of caloric needs including 5, 15, or 25% of energy from protein was fed for 56 days. Caloric overfeeding resulted in increased physical activity and correspondingly PEE/AEE including NEAT, even after adjustment for changes in body composition. By contrast, changes in physical activity were not related to protein intake. The authors concluded that increased PEE/AEE in response to weight gain might be one mechanism of modulating adaptive thermogenesis (75). But the results indicate also that the observed increase in activity was not likely effective at attenuating weight gain.
Other recent reports have failed to detect compensatory increases in spontaneous physical activity during short-term overfeeding in humans (76–80). While in several cases, methodological differences make them less directly comparable to Levine’s study, (76, 56, 77, 79), a study by Siervo et al. used state-of-the-art methods and applied a highly standardized 17-week protocol with progressive overfeeding from 20-60% energy in excess of maintenance needs (81) in lean, healthy men. Three sequential intervals of stepwise overfeeding were each separated by one week of ad libitum energy intake. In agreement with previous findings, between-subject variability concerning weight change during ad libitum phases was high (63). However, in contrast to Levine’s study (53), Siervo et al. failed to detect a significant systematic change in spontaneous physical activity (81). The authors therefore concluded that systematic elevations in energy intake induce very limited counter-regulatory responses in energy expenditure (63).
In summary, these data underscore the highly individual compensatory response regarding adaptive thermogenesis to overfeeding. From Levine and Apolzan et al., supportive evidence is provided for the susceptibility to gain body mass under standardized overfeeding conditions and identifies an individual’s ability to adapt NEAT as a potential modulator (75, 53, 20, 34). In accordance with findings of other overfeeding studies (reviewed in (74)), adaptations in thermogenesis by changes in NEAT observed between individuals may explain why some individuals are particularly susceptible or resistant to weight gain. The potential impact of age on these adaptational mechanisms remains unclear and should be addressed by future research.
THE IMPACT OF CALORIC DEPRIVATION ON NEAT
While evidence with respect to systematic NEAT upregulation under overfeeding conditions remains somewhat controversial, TEE and spontaneous physical activity are consistently influenced with energy deprivation in the majority of available studies (72, 80). This is of importance as most subjects who are overweight or have obesity try to lose body mass through various calorie-restricted dietary strategies.
The first study to quantitatively investigate adaptive thermogenesis under conditions of food restriction was the Minnesota Starvation Experiment (reviewed in (46, 49, 72)). This evaluation showed a marked reduction in TEE that was later reproduced by numerous studies, independent of pre-starvation BMI or method used to induce weight loss (reviewed in (72)). For instance, Leibel et al. in a second arm of their “weight clamping” experiment evaluated the effect of a 10% body mass reduction, followed by weight maintenance at this level (73). While overfeeding was accompanied by a 16% increase of adjusted TEE, weight reduction from underfeeding induced a 15% decrement (57). As compared to overfeeding, it is well accepted that both REE and non-resting energy expenditure are affected by energy deprivation in humans and animal models (82, 72, 80).
These findings are supported by results from the so-called Biosphere 2 experiment, where the authors demonstrated a major reduction of TEE and spontaneous physical activity with body mass reduction over a two-year time period, which persisted after six months of body weight regain (46, 83). Demonstrated TEE reductions by adaptive thermogenesis in weight-regainers has been reproduced in other human studies (72). Of note, when subjects recover from starvation they spontaneously overeat and, moreover, if weight loss-induced adaptive thermogenesis persists (e.g. reduced NEAT), there is a significant risk of regaining weight and “overshooting” pre-starvation body mass (72). Thus, changes in non-resting energy expenditure with underfeeding (or overfeeding) appear to occur in a direction tending to return subjects to their initial body mass and to conserve body energy as recently described in humans as so-called “thrifty phenotype” compared to a “spendthrift phenotype” (84).
Regarding this, findings from a recent study in sedentary overweight females are of potential practical relevance (85). In this investigation, 140 pre-menopausal females reduced their body mass by an average of ~11 kg while undergoing a total of 800 kcal per day diet. Subjects were randomly assigned to one of three groups: no exercise, a structured aerobic training schedule, or an exercise program incorporating resistance training. Body composition, strength, TEE, REE and NEAT were measured using various approaches. One main finding was that TEE, REE and NEAT all declined with weight loss in the no-exercise group. Non-exercise activity thermogenesis was reduced by 150 kcal per day, which was equivalent to 27% compared to baseline levels. However, in subjects undergoing the exercise regimens only REE was reduced, while NEAT remained unaffected. Thus, since caloric deprivation appears to consistently decrease spontaneous physical activity, untrained subjects with obesity who plan to lose body mass by means of dietary intervention alone would be advised to engage in a concomitant exercise program so as to avoid negative effects on NEAT and mitigate weight cycling after reaching a lower body weight. Of note, the authors of this study emphasized that, consistent with the concept that “more is not always better,” exercising two days a week was capable of increasing NEAT but, paradoxically, an intense three days weekly schedule was followed by a substantial decrement in NEAT (85).
At this end, one further study of Levine’s group should be considered (78). NEAT as measured by gold standard methods and posture allocation was evaluated in mildly obese subjects and normal weight controls over a period of ten days. This baseline evaluation showed a 164 minute per day longer seated time in obese compared to lean subjects. A subset of eight obese subjects then underwent an intentional weight loss program over a time span of eight weeks and thereby lost significant body weight. Posture allocation was measured for another ten days after intervention. Interestingly, the weight losing obese subjects did not further reduce their NEAT in this experiment (78). The latter study has several limitations, e.g., the small number of enrolled subjects and the relatively short intervention period when compared to some of the above-mentioned trials. Levine and colleagues themselves emphasized that it was a pilot experiment (78). The data otherwise suggest that interindividual differences in posture allocation could at least in part be biologically determined and are therefore not necessarily influenced in every individual by e.g., reduced energy intake over short time periods. Over- and underfeeding studies in identical twins indeed suggest an important role of genetics regarding the variability underlying body mass regulation (86, 87). Specifically regarding NEAT, it is likely that due to the polygenetic nature of obesity, numerous pathways are involved in the regulation of spontaneous physical activity, as the latter has multiple environmental cues and affects a multitude of behaviors (8, 88). Zurlo et al. have shown that spontaneous physical activity levels cluster in families and could prospectively help to explain the propensity for weight gain (89). Moreover, according to a recent review up to 57% of the variability of spontaneous activity has been attributed to inheritance (90).
Otherwise, biological and genetic determinants can only partially explain NEAT variance. Models of human energy budgets related to exercise can provide a conceptual framework to better understand NEAT regulation in humans (reviewed in (39)). For instance, the so-called independent model predicts that changes in basal TEE have no impact on the energy budgeted for behavior, e.g., spontaneous physical activity. This model is analogous to the factorial model of exercise in humans that assumes that exercise has an additive effect on TEE (39). In contrast, the allocation model suggests that the total energy budget is constrained, and therefore, an increase in the energy cost related to maintaining basal functions will reduce the amount of available energy to support other functions, finally altering an individual’s behavior (39). The independent model would for example predict that exercise additively increases TEE, while the allocation model predicts that exercise leads to a reduction in some components, e.g., NEAT. Indeed there is evidence that each of these models is evident in different human populations, although studies suggest that e.g. allocation may be affected by confounders such as age (older more likely to reallocate), sex (males more likely to reallocate), and exercise volume (allocation more likely with higher volumes) (39). Such aspects have to be considered and could help to integratively explain the contradictory findings of Levine et al. and from other researchers.
In summary, an integrative view of existing human overfeeding studies proposes that the magnitude of adaptations regarding energy expenditure varies largely between individuals. Subjects capable of responding with increased spontaneous physical activity to overfeeding can be categorized as “compensators” and are therefore less susceptible to obesity (“spendthrift phenotype”). Subjects unable to respond to a continued energy surplus with increased NEAT (e.g., “non-compensators” or “thrifty phenotype”) seemingly represent the majority of subjects on a population level, of which a major percentage will attempt dietary weight-loss strategies at some time in their lives. However, REE and non-resting energy expenditure including NEAT are reduced with underfeeding in the majority of subjects. They therefore represent a risk factor for weight regain after dietary intervention. Mild voluntary exercise during and maybe shortly after periods of dietary weight loss could be a promising strategy to avoid decreases in NEAT, although this alone will probably not fully compensate for the obligate decline in REE. Whether the majority of dieting subjects with obesity will continuously adhere to a structured exercise program during extended periods of weight maintenance as suggested by current recommendations is also uncertain (and unlikely) (42, 8, 43, 41).
Therefore, to advance anti-obesity strategies that systematically increase or even conserve NEAT it is essential to understand the mechanisms contributing to regulation of spontaneous physical activity.
PHYSIOLOGICAL AND MOLECULAR DETERMINANTS OF NEAT
Principles of NEAT Regulation
The ability to adapt thermogenesis, and specifically NEAT, presupposes existing mechanisms in the body that sense, accumulate, and integrate internal and external directionality signals with respect to energy balance (20). Figure 5shows a proposed model of the principal regulation of spontaneous physical activity and correspondingly NEAT. In this model, physiological data regarding caloric intake, energy depots and energy expenditure are compiled centrally by the brain. These signals converge at the data acquisition center, are rectified to a common signal, and directed to a NEAT accumulator, which is constantly summing the net amount of NEAT per unit of time. The latter continuously refers to the energy balance integrator, which, in turn, modulates spontaneous physical activity in response to changes in energy intake. Thereby, energy balance resulting from these three components is in constant flux.
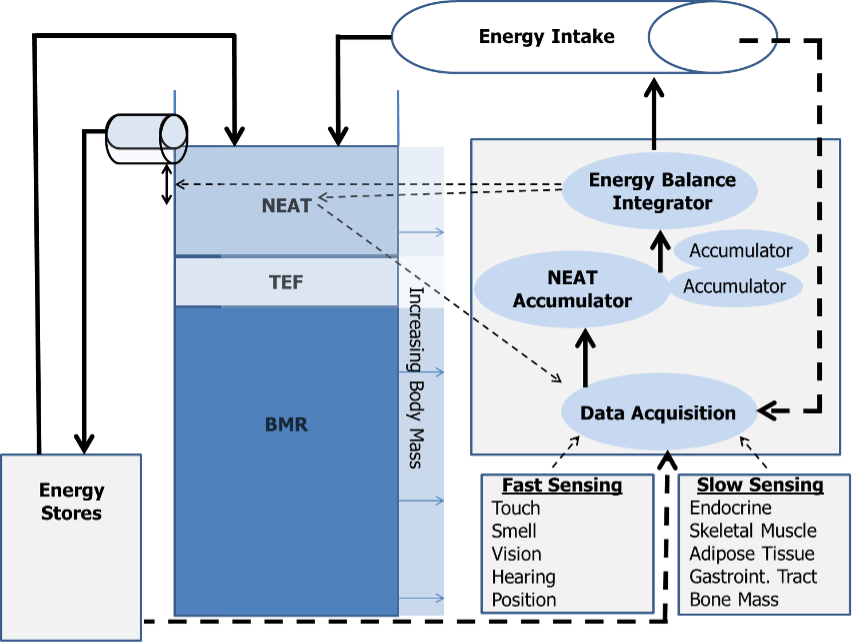
Figure 5. The NEAT hypothesis of energy balance (adapted from (20)).
Continuous arrows represent energy flow through the system, broken arrows stand for putative signaling pathways. BMR, basal metabolic rate; NEAT, non-exercise activity thermogenesis; TEF, thermic effect of food.
For example, under circumstances of positive energy balance during increased food intake, the energy balance integrator may mediate deposition of the caloric surplus in energy stores (adipose tissue) or alternatively dissipate the excess energy in the form of NEAT. More likely, since BMR is essentially fixed, some energy will be spent on food digestion and absorption (increased TEF), some will be deposited as body fat or in other depots, and the rest will be dissipated as NEAT (20). This model also supports the observation that although BMI and energy balance are continuously oscillating around a weight set point (or “set range”) under daily life conditions, body mass is remarkably stable in the long term. This is exemplified by data from the Framingham Study showing a mean body mass increase of only 10% over a 20 year time span in average adults (cited in (91, 92)).
Thus, it appears that the regulation of energy balance and body mass is realized by a complex network of precisely working auto-regulatory structures controlling effector systems, one of which being spontaneous physical activity. However, while ample available data detail the regulation of food intake, there is lack of information concerning the biological mechanisms driving NEAT (34). Most animal evidence supports the concept that NEAT can help to protect against obesity (93). Therefore, spontaneous physical activity has certainly potential for impacting body mass and energy balance. It is very likely that many of the biological systems involved in the regulation of energy intake are also involved in gathering and integrating information regarding NEAT and determining NEAT adaptation in humans (34).
Tissues, Organs, and Central Mechanisms Involved in NEAT Regulation
Several well-described central neuroendocrine systems are thought to regulate NEAT through interactions with peripheral tissues/organs known to affect energy balance (Figure 6).
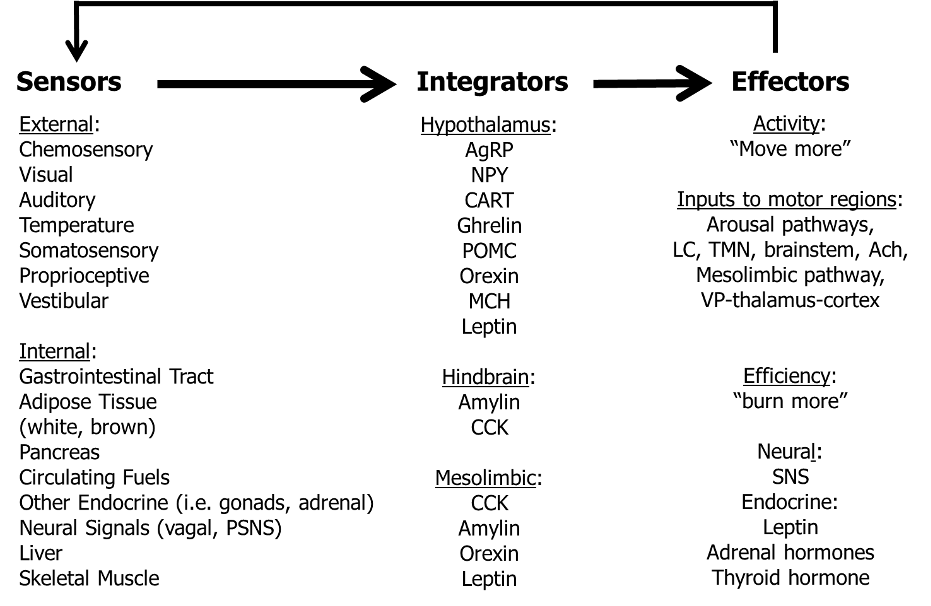
Figure 6. Model for the neuroendocrine regulation of NEAT in the service of energy balance (adapted from (34)). Multiple external and internal signals are sensed and integrated, whereby defined brain structures (e.g., arcuate nucleus of the hypothalamus, area postrema and nucleus of the solitary tract of the hindbrain, dopamine pathway of the mesolimbic system) interpret a multitude of sensory cues of energy availability. The involved brain systems have multiple ascending and descending projections affecting the amount of physical activity through arousal and limbic pathways, and descending neural projections and endocrine signals to modulate the energy efficiency of physical activity. Thereby, the central nervous system could adapt NEAT to adjust energy balance under conditions of caloric excess or starvation. Ach, acetylcholine; AgRP, agouti-related peptide; CART, cocaine- and amphetamine-regulated transcript; CCK, cholecystokinin; LC, locus ceruleus; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; (P)SNS, (para-)sympathetic nervous system; SNS, sympathetic nervous system; TMN, tuberomammilary nucleus; VP, ventral pallidum
These associated neural mechanisms of brain regions involve a multitude of neurotransmitters and neuropeptides (for extensive review see (34) and (94)). While all are important, this section will focus on the biological role of central orexin peptides as promising and potentially targeted regulators of spontaneous physical activity (for extensive review see (47)).
Experimental data indicate that injections of orexin A into defined brain regions reproducibly induce a dose dependent increase in spontaneous physical activity along with significant increments in NEAT. Moreover, current knowledge suggests a role for the orexin system in the control of arousal and sleep, and for reward and stress reactions. This has led to conduction of extensive research showing that the orexins A and B are produced by cleavage from a single pro-peptide (44). The majority of orexin A is synthesized in the lateral hypothalamus and perifornical area. Orexin A binds to two G protein-coupled receptors, namely orexin receptor type 1 (OXR1) and 2 (OXR2). OXR1 has high affinity for orexin A, while OXR2 has equal affinity for the A and B peptide. Receptor binding of orexin A is associated with increased intracellular calcium levels and followed by enhanced neuronal signaling (44).
Physiologically, orexin A is capable of modulating spontaneous physical activity, food intake, and sleep as evidenced by an obesity-resistant rat model with high orexin A activity (34). These animals show more ambulatory and vertical movement, independent of age or food availability (34). Intriguingly, this rat model exhibits lower body weight gain when fed a high-fat diet, despite consuming significantly more kcal per gram body mass. From this study, it appears that orexin A simultaneously enhances feeding behavior and induces physical activity, but the consumed calories are outweighed by those expended with spontaneous physical activity (47, 34). These data were confirmed by a mouse model with postnatal loss of orexin neurons (47). These mice exhibit hypophagia, decreased spontaneous physical activity, and develop spontaneous-onset obesity while consuming a regular chow-diet. Furthermore, in recent years so called DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) have been developed and were applied to manipulate orexin neuron activity in specific animal models. Compared with previous work of targeted infusions of orexin and/or orexin agonists into the brain, the use of DREADDs allows for the sustained activation of the neurons of interest without needing to inject directly into the brain (95).
A high-fat diet model was used to evaluate the effect of DREADDs on NEAT, since spontaneous physical activity can be decreased under such circumstances. It was shown that NEAT was decreased in animals on the high-fat diet, and was increased to the NEAT level of control animals following activation of orexin neurons with DREADDs (95). Thus, these preliminary results clearly support the hypothesis that the orexin signaling system could be a promising target of modulation by drugs.
However, from the perspective of humans, data on orexin are currently scarce. Otherwise it was recently shown in a pilot study that plasma levels of orexin A correlated with physical activity levels including many NEAT features (96). Therefore, plasma orexin A could be considered as a biomarker of NEAT in future studies. Furthermore, the orexin system has been shown to be defective in the sleep disorder narcolepsy. Since obesity is a recognized comorbidity of narcolepsy in both animal models and humans (47), regulation of this signaling system has the potential to impact clinical endpoints, probably including the development of overweight and obesity.
The Role of Peripheral Tissues for NEAT Regulation: Adipose Tissue and Adipokines
Peripheral tissues are also believed to contribute to NEAT regulation (Figure 6). That NEAT can be influenced by over- and underfeeding has been extensively discussed in earlier sections; but it is essential to also reconsider that longitudinal studies of fasting humans show significant decreases in adaptive thermogenesis with starvation, while refeeding may induce a variable elevation (72). These adaptations are likely the result of feedback mechanisms between thermogenesis regulation and energy depots (Figures 5 and 6). It can be further hypothesized that adaptations of thermogenesis in response to fasting or overfeeding result, at least in part, from an adipose tissue-specific impact on thermogenesis (reviewed in (46)). Indeed, studies of white adipose tissue (WAT) have led to the recognition that this energy store represents an important endocrine organ communicating with the brain through secretion of so-called “adipokines.” Accordingly, adipokines could play a role for NEAT regulation (Figure 7) (97).
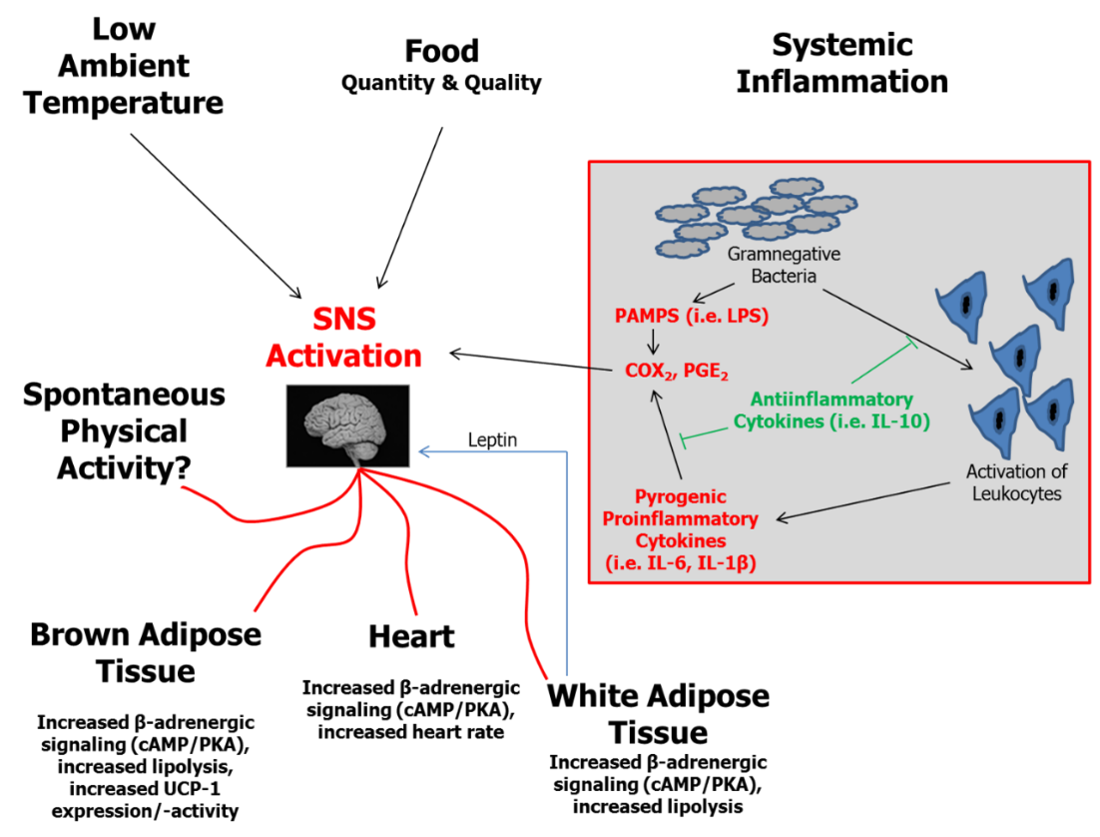
Figure 7. Essential elements in the regulation of thermogenesis including effects of WAT (adapted from (34, 98)). BAT, brown adipose tissue; cAMP, cyclic 5” adenosine monophosphate; COX, cyclooxygenase; IL, interleukin; LPS, lipopolysaccharide; PAMPs, pathogen associated molecular pattern; PGE, prostaglandin E; PKA, protein kinase A; SNS, sympathetic nervous system; UCP, uncoupling protein; WAT, white adipose tissue.
Leptin is such an adipokine signal that is the product of the rodent obese (ob) gene and human homolog (LEP) first characterized in 1995 (99). Due to its role regarding the sensing of energy stores and regulation of food intake, it was also suggested as an important signal with respect to adaptive thermogenesis (reviewed in (34, 98)). For instance, Dauncey and Brown studied ob⁄ob mice lacking leptin production and their lean littermates at comparable body weights (100). Leptin-deficient mice expended less energy and showed less motor activity as compared to control animals. The energy expended relative to metabolic size was greater in lean littermates, who were also more active than the ob⁄ob mice. It was calculated that activity-related energy expenditure accounted for at least part of the body mass difference observed between ob⁄ob and wild-type mice (100, 101). A central mediator of leptin action on NEAT partly includes the orexin signaling system, since the activity of orexin neurons has been shown to be modulated by leptin among other metabolic indicators (reviewed in (93)).
With respect to humans, Franks et al. have shown that fasting leptin levels were significantly associated with physical activity-related energy expenditure (101). Moreover, it was pointed out in a recent review that replacement of leptin in weight-reduced subjects was capable of reversing neuroendocrine adaptations that accompany caloric reduction as evidenced by an increase in SNS activity, suggesting that this component of the non-resting energy expenditure and adaptive thermogenesis is at least in part under endocrine control (72). In response to caloric restriction, the authors therefore suggest an adaptation model where in an initial fast “first set,” reduced insulin secretion and glycogen stores are accompanied by a reduction of both REE and non-resting energy expenditure within days, while in the long term, with maintenance of reduced body weight, a “second set” mediated by a drop in leptin levels becomes active that keeps energy expenditure low so as to preserve fat depots and maintain reproductive function (72). This endocrine action resulting from the drop in leptin levels with related activation of multiple pathways then enhances the risk of weight regain (72).
Overall, available data suggest that leptin’s central actions include not only reducing food intake but potentially also increasing energy expenditure mediated, in part, through increasing physical activity levels (reviewed in (34)). The potential role of leptin on NEAT in human obesity is of particular interest given the phenomenon of central leptin insensitivity under conditions of overfeeding and related obesity (102, 103). However, it must be also kept in mind that the leptin system represents only one component of peripheral tissue signaling that impacts NEAT. Other endocrine systems, such as thyroid hormone status, also have major influences on energy expenditure. For example, hyperthyroid rats have been shown to have significantly increased NEAT (104).
Energy availability from food and internal energy stores, ambient and body temperature, and systemic inflammation are further determinants of thermogenesis regulation. In WAT, two relevant factors converge: WAT represents the principal energy storage depot and, under conditions of significant obesity, WAT is a site of low-grade inflammatory activity that contributes to systemic inflammation. Recent epidemiological data from humans support this hypothesis (105). It was shown that levels of systemic low-grade inflammation (e.g., circulating interleukin-6 and interleukin-1β) were related to non-exercise physical activity. Although no causal relationship can be drawn from these findings, inflammation is theoretically one signaling system involved in NEAT regulation.
Signals from WAT can induce enhanced sympathetic nervous system (SNS) activity and affect downstream regulators of adaptive thermogenesis (Figure 7). Indeed, experimental data indicate that NEAT is one component affected by WAT signaling (93). Related to the hypothesis of “adaptive thermogenesis”, Dulloo et al. in their review regarding human starvation and overfeeding suggest SNS as key regulatory effector mechanism (46). Of note, activation of brown adipose tissue, which has recently gained a lot of interest with respect to human obesity (106–108), is suggested to be an additional site of adaptive thermogenesis.
Skeletal Muscle as Peripheral NEAT Modulator
Skeletal muscle is a major contributor not only to REE, but also to spontaneous physical activity and has been newly recognized as important NEAT effector. Human subjects maintaining a 10% weight loss in response to caloric restriction have increased skeletal muscle work efficiency (92, 109). This mechanism is believed to be responsible for the phenomenon that, even after adjustment for the reduced body mass, weight-loss maintaining subjects need proportionally fewer calories for performing comparable physical-activity tasks compared to before weight loss (92, 109). Therefore, adaptations of skeletal muscle work efficiency can contribute to reduced NEAT after low-calorie induced weight loss. Several recent animal models are in line with this hypothesis (82, 110). For example, a recent study in rats investigated the contribution of REE and non-resting energy expenditure to reduced TEE observed after three weeks of 50% energy deprivation (82). They reported a 42% reduction in TEE, even after correction for the loss in body weight and LBM (82). Moreover, 48% of the detected TEE reduction was explained by a significant decline in non-resting energy expenditure, e.g. spontaneous physical activity (82). Reduced baseline and activity-related muscle thermogenesis was also found in this study. Reduced skeletal muscle norepinephrine turnover as a surrogate of SNS drive and increased expression of the more energy “economic” myosin heavy chain (MHC) 1 isoform were identified potential mechanisms for the observed changes (82, 92, 109). Thus, according to these findings, restriction of caloric intake apparently not only suppresses spontaneous physical activity, but in addition reduces related caloric demand, which is mediated by reduced SNS activity to skeletal muscle and altered expression of MHC isoforms. These findings are supported by data from another recent animal study investigating rodents with “high intrinsic physical activity” and counterparts with “low intrinsic running capacity” under eucaloric conditions (87). It was found that animals with high intrinsic physical activity were lean, had lower skeletal muscle economy along with increased skeletal muscle heat dissipation during activity. This resulted in higher TEE and NEAT and was related to increased activation of skeletal muscle by SNS (110).
Taken together, skeletal muscle is an intrinsic site for NEAT and not only of great importance quantitatively, but also represents an important effector system for NEAT mediated through modifications on the level of SNS activity and gene expression, particularly under conditions of altered caloric intake. Therefore, evidence indicates that NEAT is modified by a complex network regulation of central and peripheral systems. The latter involve a variety of redundantly organized immediate and delayed mechanisms, including central neuropeptides and signaling systems to and from peripheral tissues. Involvement of such biochemical signaling systems identifies spontaneous physical activity and NEAT as a potential site for pharmaceutical targeting. Pharmaceutical manipulation of such a system could be envisioned to either increase the amounts of activities people undertake, or elevate activity-related energy expenditure.
TREATMENT OPTIONS FOR OBESITY AND RELATED DISORDERS: IMPLICATIONS FOR NEAT-ENHANCED LIVING
As stated in the introduction, obesity is associated with a many adverse health outcomes (1, 111). Energy expenditure in occupational activities has declined by a mean of 140 kcal/d since 1960 in the United States and this reduction is thought to account for a significant portion of the observed increase in mean BMI (reviewed in (1)).The latter hypothesis is supported by observational data showing that reduced energy expenditure of 100 kcal/d below expected values corresponds to a 0.2 kg/year weight gain, of which 0.1 kg/year are fat mass (112). In addition there is ample evidence from epidemiological, cross-sectional, and longitudinal studies showing that individuals with higher physical activity levels have lower mean body weight, gain less weight and fat mass over time, and show reduced weight regain after intended loss in body mass (113–117). Moreover, regular physical activity can moderate or even eliminate weight gain among those carrying a risk at the FTO (fat mass and obesity associated) gene variants ((118), reviewed in (93)). It has further been evidenced that levels of habitual or spontaneous physical activity are positively related to reduced risk of major cardiovascular disease and premature mortality, while an increase of sitting time correlates with a raise in complications (119–123).
Associations of NEAT with Type 2 Diabetes Mellitus and NAFLD/MAFLD as Common Sequelae of Obesity
In industrialized countries the daily time spent sitting is estimated to be 6-7 hours per day, which is closely correlated not only with obesity rates but also with the incidence of T2D (120, 124). A plethora of data suggest, on the other hand, beneficial effects of lifestyle interventions on insulin resistance, metabolic control, pain and clinical endpoints in these patients (e.g. (125, 126), reviewed in (127)). In subjects with impaired glucose tolerance or prediabetes, lifestyle interventions have proven to decrease the incidence of T2D, of which prominent examples comprise the DaQuing Study, the Finish Diabetes Prevention Study, the Diabetes Prevention Program and others (128–131). This was newly confirmed by the lifestyle intervention and impaired glucose tolerance Maastricht (SLIM) trial, which has shown to impact clinical endpoints even four years after stopping the intervention (132, 133).
The prevalence of NAFLD/MAFLD parallels the pandemic rise in T2D. A recent meta-analysis indicates that on a global perspective more than 55% of T2D patients suffer from bland liver steatosis, while a further 37% show signs of non-alcoholic steatohepatitis (NASH) (reviewed in (127)). “NAFLD” was defined in the 1980s to describe excess hepatocellular lipid accumulation in absence of significant alcohol intake, autoimmune, or viral liver disease, and the course of this pathology was long believed to follow the so-called “two-hit hypothesis” (134). Manifestation of bland steatosis was defined as first hit, while histological signs of liver inflammation, fibrosis and hepatocyte injury were proposed as succeeding second hit (NASH). Insulin resistance has now been recognized as the most valid predictive parameter of NAFLD/MAFLD progression to NASH. Moreover, presence of insulin resistance puts these patients to an elevated risk of morbidity and mortality, while sedentary behavior constitutes a complementary risk factor and correlates with clinical outcomes (reviewed in (135, 127)).
Since physical exercise is capable of improving and maybe reversing insulin resistance, while short term decreases in physical activity reduce multiorgan insulin-sensitivity and in parallel increase liver fat (reviewed in (127)), it is reasonable to assume that physical activity represents a potent treatment modality for NAFLD/MAFLD. There is indeed some evidence from prospective controlled randomized intervention studies that liver fat can be reduced independently from changes in body weight by structured exercise regimens in a limited, yet significant manner, while effects on liver inflammation and fibrosis remain to be investigated (extensively reviewed in (127)). Although no direct research of NEAT effects on NAFLD/MAFLD is currently available, one well-designed study using brisk walking as an intervention has shown reduced liver fat after 6 and 12 months (136). This suggests that low to moderate intensity activities potentially impact hepatic steatosis, which should be further evaluated.
For weight maintenance purposes, current recommendations encourage subjects with obesity to engage in 200-300 minutes per week in activities as e.g. walking (8). Yet, patients with T2D show markedly reduced TEE (< 300 kcal per day), number of steps taken (<1500 per day), physical activity duration (<130 min per day), and activity related energy expenditure (<300 kcal per day) as compared to subjects without diabetes (137). Accordingly, only 28% of patients with T2D in the U.S. achieve the recommended physical activity levels and less than 40% engage in regular voluntary exercise (120, 138, 139). In line with this, recent observational studies have shown that reduced NEAT is related to measures of insulin resistance, glycated hemoglobin A1c, and other features of the metabolic syndrome, consistent with the hypothesis that increasing spontaneous physical activity could be beneficial for those with T2D and NAFLD/MAFLD (140–142). This is supported by data coming from the Nurses’ Health Study showing that regular walking at normal pace (e.g. 3.2-4.8 km h-1) was associated with a 20-30% relative risk reduction of T2D, while in another study, frequent walking was correlated with an equally remarkable risk reduction of mortality in T2D (120, 143, 144).
On the other hand, it must be acknowledged that data on NEAT, T2D and NAFLD/MAFLD are limited. First, evidence suggests interindividual differences in the response to a standardized exercise schedule at a given dose and there is, moreover, strong indication of genetic components to the variation in human trainability (reviwed in (145)). Second, available evidence almost exclusively comes from observational and cross-sectional studies. Furthermore, the data on lifestyle interventions on sequalae of insulin resistance and manifest T2D also have limitations (146). For instance the 10-year prospective randomized LookAHEAD study in T2D patients was stopped early based on a futility analysis, since the trial was unable to detect substantial effects on cardiovascular events, although the intervention group experienced significant weight loss (147). Otherwise, secondary analyses of this hallmark study were able to show that the magnitude of weight loss may be predictive of outcome measures (148). In more detail, obese and overweight subjects suffering from T2D were examined regarding the association of the magnitude of fitness change (n=4406 and weight loss (n=4834) over a median of 10 years of follow-up for the primary outcome. The primary outcome was a composite of death, cardiovascular disease, myocardial infarction, hospitalization for angina, and stroke. Subjects losing more than 10% of body mass in the first year of the intervention had a 21% lower risk of the primary outcome relative to participants with stable body mass or weight gain. Interestingly, achieving a > 2 metabolic equivalents (MET) fitness change was not significantly associated with the primary, but with the secondary endpoint (composite of coronary–artery bypass grafting, carotid endartectomy, percutaneous coronary intervention, hospitalization for congestive heart failure, peripheral vascular disease, or total mortality (148). Therefore, it appears that life style modifications are principally capable of beneficially modulating clinical endpoints in, while the optimal exercise (and lifestyle intervention) therapy for individuals with T2D, particularly with co-existing complications, remains unknown (120). These findings suggest that priority should be made for subjects who are obese to engage in any type of regular physical activity before T2D and NAFLD/MAFLD become manifest ((144), reviewed in (120, 8)). Accordingly, the “Step It Up! (The Surgeon General’s Call to Action to Promote Walking and Walkable Communities)” program was recently released, focusing on promoting optimal health before disease occurs (149).
In summary, since EAT is negligible in the vast majority of subjects and NEAT represents the main contributor to daily PEE/AEE and therefore TEE in Western populations NEAT can be recognized as a major potential target for lifestyle modification in subjects suffering from T2D and probably also under conditions of NAFLD/MAFLD.
Personalized Approaches versus Environmental Re-engineering for Promoting NEAT
In free-living individuals, counterbalancing effects of an obesity-promoting environment involves addressing several central questions:
- How can the amount of NEAT be increased or preserved under conditions of caloric restriction?
- What are realistic goals for daily spontaneous physical activity and what are appropriate activities for increasing NEAT?
When aiming to prevent or treat obesity, it can be argued that in an obesogenic environment each person needs to increase physical activity levels (45). Alternatively, it could be argued that the epidemic of obesity needs to be addressed at the population level, since obesity has emerged as a result of environmental pressures that have led to decreased population-wide activity levels (e.g., more sedentary jobs, more time at work and less in leisure) (45). In reality, these contributors are not mutually exclusive. However, in order to substantially increase NEAT it is useful to categorize these perspectives as the “individualized approach” versus the “environmental re-engineering approach”(45). Accordingly, Levine and Kotz have developed the “egocentric” and the “geocentric” models, which provide a theoretical framework to understand important environmental determinants of NEAT from these two perspectives (20).The egocentric model focuses on a single person. Accordingly, environmental factors that impact a particular person’s spontaneous physical activity levels are considered, such as “my occupation”, “my transportation to work”, or “my leisure time activities” (Figure 8) (20). By contrast, the geocentric model is focused on how the environment impacts NEAT of multiple subjects, such as city planning to ensure walk-friendly or bike-accessible environments (Figure 8) (20). These models may help to elucidate how NEAT can be effectively modulated.
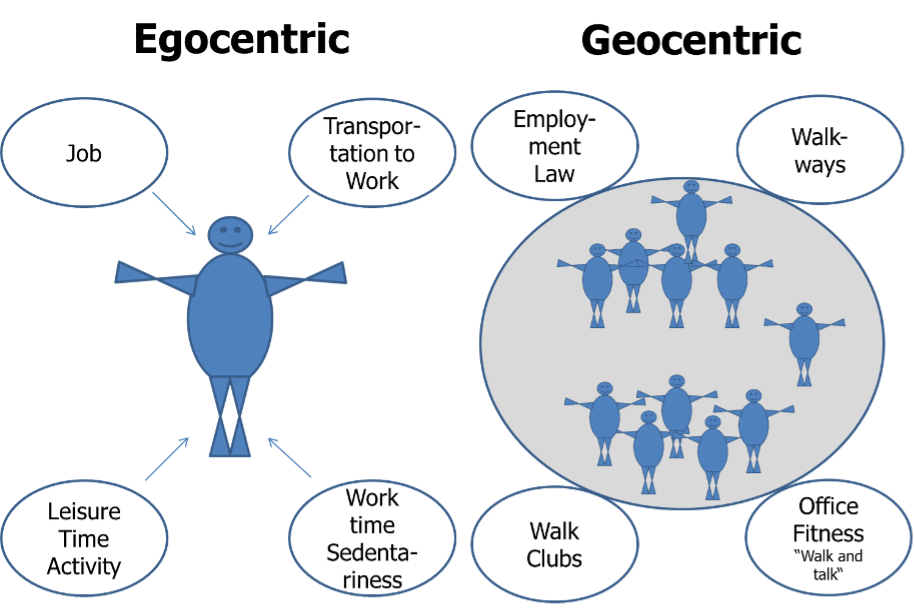
Figure 8. Environmental determinants of NEAT – the egocentric versus the geocentric model (adapted from (20)).
In the geocentric model a variety of environmental factors impact spontaneous physical activity. The most prominent negative influences on NEAT are represented by urbanization and mechanization, which are largely a phenomenon of high- and middle-income countries. Examples include televisions, drive-through restaurants, clothes washing machines, motorized walkways, and others (20).
Accordingly, when comparing daily energetic costs of mechanized tasks with the same tasks performed manually a century ago, the difference in daily energy costs approximates 111 kcal, or more than 40,000 kcal per year (150). Evidence such as this has been used in support of the proposal that urbanization and mechanization have likely had a dramatic impact on energy balance (20). Hence, proposals to increase NEAT on a population level have included environmental “re-engineering,” including provisions for adequate walkways, to build schools within walking distance, and to adapt employment laws for promoting office fitness. The problem with this argument is that it includes imposing high economic costs on business and localities without agreed upon standards for success (45).
It is self-evident that to intentionally increase NEAT over a protracted time period presupposes a significant amount of self-discipline. To facilitate behavior modification Levine has published approaches for promoting NEAT that focus on behavioral economic theory (for extensive review see (45)). Behavioral economic theory is a framework for conceptualizing how people make behavioral choices based upon their perceived relative value. When applied to NEAT, behavioral economic theory is concerned with how people choose between various activity/inactivity options. Consequently, Levine proposes four key elements (45): 1) It is critical to provide individuals with free-choice. By contrast, forcing a subject to choose a specific NEAT-promoting activity is likely to have the opposite to the intended effect. Otherwise, if an activity is self-selected, it is likely to be more reinforcing and consequently self-selected more often; 2) The delay between performing a NEAT-promoting behavior and the outcome needs to be minimized. While sedentary behaviors that people enjoy have immediately reinforcing consequences, health benefits of standing or ambulation may take longer to manifest. Therefore, it is important to choose NEAT-promoting physical activities; that are pleasing. For example, walking while listening to music or walk-and-talk with a friend. 3) Behavioral “costs” determine which sorts of activity/inactivity become selected. If a person has to work strenuously to participate in a given activity, they will be less likely to do it. For example, it is unlikely that many people will drive 40 minutes to the gym long-term just to engage in fitness training. People are more likely to choose physical activities that are easily accessible, such as home- or even office-based activities that do not require changing location or clothes; and 4) For an individual to choose a NEAT-promoting activity, it has to be more attractive than available alternatives. For example, behavior will change when providing a competing behavior that is more valued: a given person may prefer to surf the internet while seated rather than visiting the gym for a fitness workout. If, however, walk-and-talk with a friend was an option, the individual could choose that instead of internet-surfing. These behavioral components have been synthesized into a simplified approach termed, STRIPE (45): STRIPE is an acronym that represents S = Select a NEAT-activity that is enjoyed and start it; T = targeted, specific individual goals must be defined; R = rewards need to be identified for reaching the defined goals; I = identify barriers and remove them; P = plan NEAT-activity sessions; E = evaluate adherence and efficacy.
Overall, to increase NEAT in the prevention and treatment of obesity, egocentric and geocentric approaches should be considered. Occupation and leisure time are the two principal time frames that have to be targeted for promoting individual NEAT. To increase NEAT by means of changing behavior, the STRIPE approach is considered as safe and well-grounded in conceptual evidence. Unfortunately, there is no evidence as to whether an individualized or the population-based approach is more effective in terms of increasing physical activity levels and/or to affect body mass. Despite this, however, it is our opinion that both approaches should be still considered when counseling patients regarding ways they can improve their daily life conditions (45).
Increasing NEAT: Realistic Goals, Pitfalls and Appropriate Activities
Obesity is not a problem in only one component of energy balance and food restriction alone is surely not the complete answer on a long-term perspective. Hill et al. hypothesized that a person who is physically highly active could maintain energy balance and a healthy body weight by eating and expending at, say, an estimated 3000 kcal per day. This person, if adopting a sedentary lifestyle, could alternatively maintain energy balance and consequently body weight by eating and expending 2000 kcal per day. If, however, this sedentary person fails to strictly keep energy intake at 2000 kcal per day to match reduced energy expenditure over time, experiencing weight gain would be the unavoidable consequence (16). In other words, for a majority of subjects in a given population, maintaining energy balance at substantial higher levels by increasing NEAT could be in the long term much more realistic under free-living conditions (e.g. in an environment with high access to energy dense foods), than maintaining caloric intake at lower levels (16). Therefore, when attempting to increase NEAT, it is important to establish what goals have to be addressed for prevention or treatment of obesity. According to a review, the amount of physical activity necessary for weight loss approximates 2000-2500 kcal per week or about 2 ½ hours of additional daily ambulation, which is considered realistic for a majority of subjects with obesity (45). This is in line with current recommendations, suggesting ≥ 150 minutes per week of aerobic exercise in combination with a hypocaloric diet (8).
However, evidence is lacking from randomized, controlled studies as to whether strategies to promote NEAT in absence of dietary manipulation are effective for obesity prevention or treatment. Moreover, some lines of evidence suggest a compensatory upregulation of energy intake with rising physical activity energy expenditure, especially at higher workloads and energy expenditure levels (reviewed in (90, 112, 151, 80). Therefore, it remains unclear whether substantially increasing NEAT for body mass control under free-living conditions will be capable of inducing weight loss without caloric restriction. Probably this shows large inter-individual variability, since it was recently shown that subjects losing weight over a two year time span increased their daily activity by about 35 minutes, while subjects gaining weight showed a reciprocal decrease (113). Energy intake was comparable in both of these groups. Contrasting with that, subjects either losing body weight or weight maintainers did not show alterations in daily activity as compared to weight gainers in another study (152). Thus, even under real life conditions there might be “responders” and “non-responders” in terms of daily activity/NEAT and body weight control. These groups remain, however, to be better defined.
Recent work could help to better categorize “responders” and “non-responders” by applying the above mentioned constrained energy expenditure model (153). The authors hypothesized that total energy expenditure would increase with physical activity at low levels, while plateauing at higher activity levels. It was found that after adjusting for body size and composition, TEE is indeed positively associated with PEE/AEE, but the relationship was markedly stronger over the lower range of physical activity. For subjects in the upper range TEE plateaued (Figure 9). According to the author’s hypothesis, body fat and activity intensity appeared to modulate the metabolic response to physical activity (153). In other words, subjects who maintain high levels of physical activity in their daily lives could show a smaller response to attempts of further increasing NEAT as it contributes to TEE increment, while subjects with habitually low activity levels could increase NEAT contribution and can therefore possibly be categorized as “responders”.
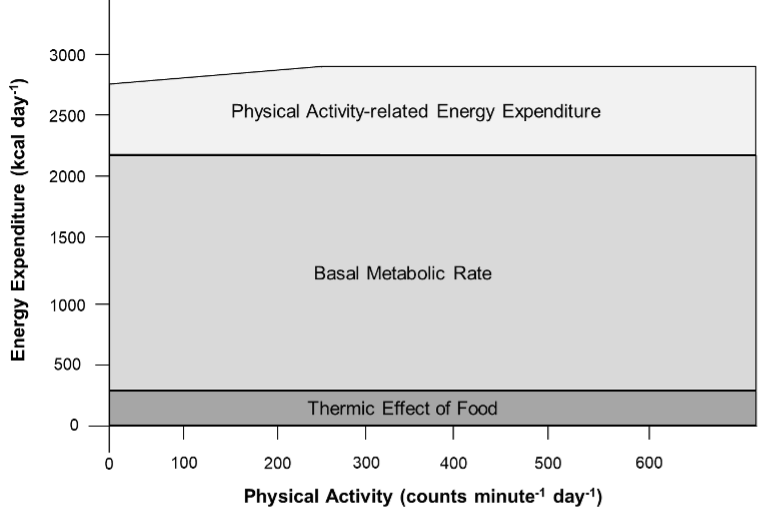
Figure 9. Effects of physical activity on PEE/AEE and its components (adapted from (153)).
Since hypocaloric diets can significantly reduce NEAT (see previous sections), a major goal has to include prevention of this reduction in NEAT. There is strong evidence that physical activity is essential for the prevention of weight regain after weight loss, and for weight maintenance (16, 80). For instance, it was shown that low physical activity levels are related to significant weight regain at follow up after hypocaloric diets (154). Furthermore, when subjects gaining weight under free-living conditions are compared to weight stable counterparts, the “weight gainers” demonstrated markedly lower physical activity related energy expenditure and less muscle strength (155). Of note, after a one year period, lower physical activity energy expenditure explained approximately 77% of body mass increase in the “weight gainers” as compared to “weight maintainers” (155). A recent meta-analysis on weight loss maintenance shows that physical activity has a significant treatment effect of approximately 1.6 kg in the short term (156). However, long term this effect was lost, probably due to reduced compliance since effects of voluntary physical exercise were examined (156).
As discussed above, for prevention of a decrease in NEAT resulting from hypocaloric dieting, an effective strategy could therefore be to recommend moderate voluntary exercising during weight reduction, and then to engage in NEAT-enhancing activities at the initiation of weight maintenance. This strategy could mitigate poor long-term compliance and substantially contribute to lower body mass preservation. However, when purposeful training is initiated in combination with a hypocaloric diet, the exercise schedule must be moderate as too intense of an exercise regimen during caloric restriction can disproportionally reduce NEAT rather than stabilize it (157, 85). Otherwise, even purposeful exercise training cannot completely overcome hypocaloric diet-induced reductions of TEE, as the REE-component remains largely unaffected by this kind of intervention even when LBM is preserved (85, 72, 36, 80).
From a practical point of view this is a central issue, as after weight loss reduced TEE will persist over years. This was recently shown by a follow up study on participants of the “The Biggest Loser” televised weight loss competition in the U.S.(158). Fourteen subjects who were severely obese lost approximately 59 kg during a 30 week period of strenous physical exercise in combination with dieting. Fat mass was primarily lost, while LBM was largely preserved (158). Compared to baseline, by the end of the 30-week competition TEE decreased significantly by 800 kcal per day, which was mainly explained by the decline in RMR, approximating 600 kcal per day (158). Unfortunately, NEAT was not directly measured. However, keeping in mind that subjects underwent an intense physical exercise schedule it can be hypothesized that the observed difference of TEE and RMR (e.g. 200 kcal per day) was contributed to by a decline in spontaneous physical activity, which is comparable to earlier studies (157, 39, 72). After six years, the participants had regained 41 kg, TEE was reduced by roughly 400 kcal day-, and in spite of the significant weight regain, RMR remained reduced by 600 kcal per day (158). Therefore, after six years, “The Biggest Loser” televised weight loss competition resulted in a total weight loss of approximately 12 kg in subjects who were previously severely obese. The data indicate that these individuals will have to live with remarkably reduced dietary energy needs, even after adjustment for body weight and age (158) that contributes to their ongoing weight regain. Remarkably, the fact that after six years the reductions in RMR were lower than TEE indicates that some form of compensation is occuring, but it is unclear which component of daily energy needs is accounting for it (Figure 1). From the previously reported findings it can surmised that decreased NEAT was one of the factors contributing to the large weight regain (158, 39). Indeed, it was shown that participants of the “The Biggest Loser” television show with high levels of physical activity over years had significantly lower body mass regain compared to subjects with low physical activity levels. Weight loss maintainers had physical activity thermogenesis of roughly 12 kcal/kg/d as compared to weight regainers with approximately 8 kcal/kg/d, which was reflectetd by a significant negative correlation of body mass regain and physical activity levels (114).
Overall, moderate voluntary exercise results in significantly preserved weight loss during hypocaloric dieting (159, 160), which can be a helpful tool for preventing significant decreases in NEAT that will later help to stabilize body weight loss. With initiation of weight maintenance, subjects should be encouraged to engange in actvities increasing NEAT, to compensate at least in part for the obligatory weight loss-induced reduction in RMR. As stated previously, additional voluntary exercise is a useful tool for weight maintenance, but engagement in NEAT-related activities can be more easiliy integrated in daily life and will therefore likely result in higher adherence and compliance rates.
In terms of appropriate activities, it can be relatively easy to increase NEAT (Figure 10). Standing instead of sitting burns three times more kcal per hour, gum chewing increases energy expenditure four times, and stair climbing more than 40 times above resting levels (45). Ambulation (e.g., walking) in particular can raise NEAT and is easily performed at almost any place and at any time. But how can a goal of 2.5 hours of additional daily ambulation/standing time be realistically be integrated into daily routine? A key problem is that people’s occupations and personal lives regularly contribute to prohibit this degree of adaptation (45). As one’s occupation is the principal determinant of NEAT in adulthood and can represent an efficient means of promoting physical activity (45), how NEAT can be primarily integrated at the site of occupation needs examining. One important issue in this context addresses transportation to work. Similar to the NHANES data for the United States (42, 43, 41), recent results from the English and Welsh 2011 Census show that among the 23.7 million adult commuters, approximately 67% used private motorized transport as their usual main commute mode, while about 18% used public transport (161). In contrast, only 10.9% walked and 3.1% cycled (161). Thus, the majority of subjects rely on motorized transport. Promoting cycling or walking as a daily routine, not only with regard to transportation to work but also with respect to other daily needs (such as going to the grocery store), could represent a promising and realistic way for a majority of subjects to increase individual NEAT levels.
Figure 10. Effects of physical activity on PEE/AEE and its components (adapted from (153)).
Together, 2.5 hours of additional ambulation and standing time per day are proposed as goals for those considering weight loss. However, potential beneficial effects on weight loss could be at least partially compensated for by increasing energy intake from food. Increasing NEAT as an adjunct for weight maintenance could overcome potential compensatory effects to the low-calorie state and hypothetically represent an alternative strategy for body mass control.
Limitations to Increasing NEAT
A number of factors potentially affect time spent in NEAT, of which some (sociological and environmental factors) can be influenced, while others cannot (genetic and endocrine factors) (Figure 11). Two points are important to make. First, as was shown previously in this chapter, skeletal muscle can increase in fuel economy and work efficiency not only in response to the loss of body mass, but also so that benefits of NEAT may be diminished. Moreover, it has also been reported that under weight-loss conditions, subjects with obesity oxidize proportionally more carbohydrates and less fat as compared to lean counterparts, and that differences in skeletal muscle metabolism and SNS activity underlie some of the observed differences between subjects who are and are not obese (reviewed in (21)). And second, even though regular physical activity can increase the capacity of skeletal muscle to oxidize lipids and to store glycogen (85), regular physical activity can also increase work efficiency and thereby possibly reduce the energetic costs of NEAT (85). Even so, given the known contribution of physical activity to body weight maintenance, the recommendation to patients who are overweight and with obesity to increase NEAT-related activities such as ambulation or bicycling is logical, even before definitive evidence is reported.
Figure 11. Factors interfering with spontaneous physical activity and NEAT (adapted from (120)).
CONCLUSION
The epidemic of worldwide obesity in past decades is contributing to serious health concerns. Apart from poor diet, reduced physical activity and increased sedentary behavior contribute to the pathogenesis of obesity. Non-exercise activity thermogenesis is a highly variable component of daily total energy expenditure and essentially a function of environmental and individual factors. Whether caloric overfeeding systematically affects non-exercise activity thermogenesis is a matter of debate, while a negative energy balance due to voluntary caloric restriction and/or exercise can decrease it. As physical activity contributes to weight maintenance and prevention of body mass regain after hypocaloric dietary interventions, overcoming current obesogenic environmental pressures and increasing non-exercise activity thermogenesis could holds tremendous promise as a tool for body weight control.
REFERENCES
- Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, Hu FB, Jakicic JM, Ryan DH, Wolfe BM, Inge TH. The Science of Obesity Management: An Endocrine Society Scientific Statement, Endocr Rev. 2018; 39:79–132
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants, Lancet. 2011; 377:557–567
- Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults,Lancet. 2017; 390:2627–2642
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010, JAMA. 2012; 307:491–497
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014, JAMA. 2016; 315:2284–2291
- Pérez Rodrigo C. Current mapping of obesity, Nutr Hosp. 2013; 28 Suppl 5:21–31
- Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, Laxmaiah A, Kengne A-P, Bentham J. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action, BMC Med. 2019; 17:212
- Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity, N Engl J Med. 2017; 376:254–266
- Heo M, Allison DB, Faith MS, Zhu S, Fontaine KR. Obesity and quality of life: mediating effects of pain and comorbidities, Obes Res. 2003; 11:209–216
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century, N Engl J Med. 2005; 352:1138–1145
- Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability, N Engl J Med. 1998; 338:1035–1041
- Chung N, Park M-Y, Kim J, Park H-Y, Hwang H, Lee C-H, Han J-S, So J, Park J, Lim K. Non-exercise activity thermogenesis (NEAT): a component of total daily energy expenditure, J Exerc Nutrition Biochem. 2018; 22:23–30
- Johnston R, Poti JM, Popkin BM. Eating and aging: trends in dietary intake among older Americans from 1977-2010, J Nutr Health Aging. 2014; 18:234–242
- Popkin BM, Siega-Riz AM, Haines PS. A comparison of dietary trends among racial and socioeconomic groups in the United States, N Engl J Med. 1996; 335:716–720
- Slining MM, Mathias KC, Popkin BM. Trends in food and beverage sources among US children and adolescents: 1989-2010, J Acad Nutr Diet. 2013; 113:1683–1694
- Hill JO, Wyatt HR, Peters JC. Energy balance and obesity, Circulation. 2012; 126:126–132
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes, Diabetes Care. 2006; 29:2102–2107
- Psota T, Chen KY. Measuring energy expenditure in clinical populations: rewards and challenges, Eur J Clin Nutr. 2013; 67:436–442
- Chen KY, Brychta RJ, Abdul Sater Z, Cassimatis TM, Cero C, Fletcher LA, Israni NS, Johnson JW, Lea HJ, Linderman JD, O'Mara AE, Zhu KY, Cypess AM. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity, J Biol Chem. 2020; 295:1926–1942
- Levine JA, Kotz CM. NEAT--non-exercise activity thermogenesis--egocentric & geocentric environmental factors vs. biological regulation, Acta Physiol Scand. 2005; 184:309–318
- Levine JA. Non-exercise activity thermogenesis (NEAT), Best Pract Res Clin Endocrinol Metab. 2002; 16:679–702
- Levine JA. Non-exercise activity thermogenesis (NEAT), Nutr Rev. 2004; 62:S82-97
- Daan S, Masman D, Strijkstra A, Verhulst S. Intraspecific allometry of basal metabolic rate: relations with body size, temperature, composition, and circadian phase in the kestrel, Falco tinnunculus, J Biol Rhythms. 1989; 4:267–283
- Dériaz O, Fournier G, Tremblay A, Després JP, Bouchard C. Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding, Am J Clin Nutr. 1992; 56:840–847
- Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM. Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females, Am J Clin Nutr. 1991; 54:56–61
- Ford LE. Some consequences of body size, Am J Physiol. 1984; 247:H495-507
- Henry CJK. Basal metabolic rate studies in humans: measurement and development of new equations, Public Health Nutr. 2005; 8:1133–1152
- Ravussin E, Burnand B, Schutz Y, Jéquier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects, Am J Clin Nutr. 1982; 35:566–573
- Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber, J Clin Invest. 1986; 78:1568–1578
- Kreymann G, Adolph M, Mueller MJ. Energy expenditure and energy intake - Guidelines on Parenteral Nutrition, Chapter 3, Ger Med Sci. 2009; 7:Doc25
- Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain, N Engl J Med. 1988; 318:467–472
- Roberts SB, Savage J, Coward WA, Chew B, Lucas A. Energy expenditure and intake in infants born to lean and overweight mothers, N Engl J Med. 1988; 318:461–466
- Saltzman E, Roberts SB. The role of energy expenditure in energy regulation: findings from a decade of research, Nutr Rev. 1995; 53:209–220
- Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity, J Neuroendocrinol. 2007; 19:923–940
- Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women, J Clin Invest. 1995; 95:980–985
- Weinsier RL, Hunter GR, Zuckerman PA, Darnell BE. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women, Obes Res. 2003; 11:937–944
- Dolezal BA, Potteiger JA. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals, J Appl Physiol (1985). 1998; 85:695–700
- Glowacki SP, Martin SE, Maurer A, Baek W, Green JS, Crouse SF. Effects of resistance, endurance, and concurrent exercise on training outcomes in men, Med Sci Sports Exerc. 2004; 36:2119–2127
- Melanson EL. The effect of exercise on non-exercise physical activity and sedentary behavior in adults, Obes Rev. 2017; 18 Suppl 1:40–49
- Kinabo JL, Durnin JV. Thermic effect of food in man: effect of meal composition, and energy content, Br J Nutr. 1990; 64:37–44
- Sisson SB, Camhi SM, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Characteristics of step-defined physical activity categories in U.S. adults, Am J Health Promot. 2012; 26:152–159
- Barreira TV, Harrington DM, Katzmarzyk PT. Cardiovascular health metrics and accelerometer-measured physical activity levels: National Health and Nutrition Examination Survey, 2003-2006, Mayo Clin Proc. 2014; 89:81–86
- Loprinzi PD, Smit E, Mahoney S. Physical activity and dietary behavior in US adults and their combined influence on health, Mayo Clin Proc. 2014; 89:190–198
- Segal KR, Pi-Sunyer FX. Exercise and obesity, Med Clin North Am. 1989; 73:217–236
- Levine JA. Nonexercise activity thermogenesis--liberating the life-force, J Intern Med. 2007; 262:273–287
- Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance, Physiol Behav. 2004; 83:587–602
- Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance,Ann N Y Acad Sci. 2012; 1264:72–86
- McManus AM. Physical activity - a neat solution to an impending crisis, J Sports Sci Med. 2007; 6:368–373
- Elia M, Stratton R, Stubbs J. Techniques for the study of energy balance in man, Proc Nutr Soc. 2003; 62:529–537
- Levine J, Melanson EL, Westerterp KR, Hill JO. Measurement of the components of nonexercise activity thermogenesis, Am J Physiol Endocrinol Metab. 2001; 281:E670-5
- Levine JA. Measurement of energy expenditure, Public Health Nutr. 2005; 8:1123–1132
- Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers, Am J Clin Nutr. 1992; 56:641–655
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans, Science. 1999; 283:212–214
- Sun S, Tang Q, Quan H, Lu Q, Sun M, Zhang K. Energetic Assessment of the Nonexercise Activities under Free-Living Conditions, Biomed Res Int. 2016; 2016:8465976
- Jung W-S, Park H-Y, Kim S-W, Kim J, Hwang H, Lim K. Prediction of non-exercise activity thermogenesis (NEAT) using multiple linear regression in healthy Korean adults: a preliminary study, Phys Act Nutr. 2021; 25:23–29
- Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements, Eur J Clin Nutr. 1996; 50:72–92
- Coward WA. Contributions of the doubly labeled water method to studies of energy balance in the Third World,Am J Clin Nutr. 1998; 68:962S-969S
- Levine JA, Weisell R, Chevassus S, Martinez CD, Burlingame B, Coward WA. The work burden of women,Science. 2001; 294:812
- Caspersen CJ, Merritt RK. Physical activity trends among 26 states, 1986-1990, Med Sci Sports Exerc. 1995; 27:713–720
- United States. Public Health Service. Office of the Surgeon General., National Center for Chronic Disease Prevention and Health Promotion (U.S.), President's Council on Physical Fitness and Sports (U.S.). Physical activity and health : a report of the Surgeon General. Atlanta, Ga., Washington, D.C., Pittsburgh, PA: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, President's Council on Physical Fitness and Sports; 1996
- Livingstone B. Epidemiology of childhood obesity in Europe, Eur J Pediatr. 2000; 159 Suppl 1:S14-34
- Pratt M, Macera CA, Blanton C. Levels of physical activity and inactivity in children and adults in the United States: current evidence and research issues, Med Sci Sports Exerc. 1999; 31:S526-33
- Westerterp KR. Daily physical activity and ageing, Curr Opin Clin Nutr Metab Care. 2000; 3:485–488
- Harris AM, Lanningham-Foster LM, McCrady SK, Levine JA. Nonexercise movement in elderly compared with young people, Am J Physiol Endocrinol Metab. 2007; 292:E1207-12
- Bromley LE, Booth JN, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes, Sleep. 2012; 35:977–984
- Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women, Obes Facts. 2008; 1:266–273
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men, Am J Clin Nutr. 2010; 91:1550–1559
- Roehrs T, Turner L, Roth T. Effects of sleep loss on waking actigraphy, Sleep. 2000; 23:793–797
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men, Am J Clin Nutr. 2009; 90:1476–1482
- Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs, Am J Clin Nutr. 2004; 79:6–16
- Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, Kessler RC, Kling JR, Lindau ST, Whitaker RC, McDade TW. Neighborhoods, obesity, and diabetes--a randomized social experiment, N Engl J Med. 2011; 365:1509–1519
- Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans, Curr Obes Rep. 2016; 5:413–423
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight, N Engl J Med. 1995; 332:621–628
- Vanltallie TB. Resistance to weight gain during overfeeding: a NEAT explanation, Nutr Rev. 2001; 59:48–51
- Apolzan JW, Bray GA, Smith SR, Jonge L de, Rood J, Han H, Redman LM, Martin CK. Effects of weight gain induced by controlled overfeeding on physical activity, Am J Physiol Endocrinol Metab. 2014; 307:E1030-7
- Apolzan JW, Bray GA, Hamilton MT, Zderic TW, Han H, Champagne CM, Shepard D, Martin CK. Short-term overeating results in incomplete energy intake compensation regardless of energy density or macronutrient composition, Obesity (Silver Spring). 2014; 22:119–130
- He J, Votruba S, Pomeroy J, Bonfiglio S, Krakoff J. Measurement of ad libitum food intake, physical activity, and sedentary time in response to overfeeding, PLoS One. 2012; 7:e36225
- Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity, Science. 2005; 307:584–586
- Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans, Obesity (Silver Spring). 2012; 20:2186–2193
- Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies, Nutr Rev. 2010; 68:148–154
- Siervo M, Frühbeck G, Dixon A, Goldberg GR, Coward WA, Murgatroyd PR, Prentice AM, Jebb SA. Efficiency of autoregulatory homeostatic responses to imposed caloric excess in lean men, Am J Physiol Endocrinol Metab. 2008; 294:E416-24
- Almundarij TI, Gavini CK, Novak CM. Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction, Physiol Rep. 2017; 5
- Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA, Ravussin E. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment, Am J Clin Nutr. 2000; 72:946–953
- Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, Votruba SB. A Human Thrifty Phenotype Associated With Less Weight Loss During Caloric Restriction, Diabetes. 2015; 64:2859–2867
- Hunter GR, Fisher G, Neumeier WH, Carter SJ, Plaisance EP. Exercise Training and Energy Expenditure following Weight Loss, Med Sci Sports Exerc. 2015; 47:1950–1957
- Bouchard C, Tremblay A, Després JP, Nadeau A, Lupien PJ, Thériault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins, N Engl J Med. 1990; 322:1477–1482
- Hainer V, Stunkard A, Kunesová M, Parízková J, Stich V, Allison DB. A twin study of weight loss and metabolic efficiency, Int J Obes Relat Metab Disord. 2001; 25:533–537
- McCarthy MI. Genomics, type 2 diabetes, and obesity, N Engl J Med. 2010; 363:2339–2350
- Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians, Am J Physiol. 1992; 263:E296-300
- Drenowatz C. Reciprocal Compensation to Changes in Dietary Intake and Energy Expenditure within the Concept of Energy Balance, Adv Nutr. 2015; 6:592–599
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight, N Engl J Med. 1995; 332:621–628
- Rosenbaum M, Leibel RL. Models of energy homeostasis in response to maintenance of reduced body weight,Obesity (Silver Spring). 2016; 24:1620–1629
- Kotz CM, Perez-Leighton CE, Teske JA, Billington CJ. Spontaneous Physical Activity Defends Against Obesity,Curr Obes Rep. 2017; 6:362–370
- Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis, Neuroendocrinology. 2008; 87:71–90
- Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet, Physiol Behav. 2017; 176:139–148
- Hao Y-Y, Yuan H-W, Fang P-H, Zhang Y, Liao Y-X, Shen C, Wang D, Zhang T-T, Bo P. Plasma orexin-A level associated with physical activity in obese people, Eat Weight Disord. 2017; 22:69–77
- Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines--energy regulation from the human perspective, J Nutr. 2006; 136:1935S-1939S
- Solinas G. Molecular pathways linking metabolic inflammation and thermogenesis, Obes Rev. 2012; 13 Suppl 2:69–82
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice, Science. 1995; 269:540–543
- Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice, Q J Exp Physiol. 1987; 72:549–559
- Franks PW, Farooqi IS, Luan J, Wong M-Y, Halsall I, O'Rahilly S, Wareham NJ. Does physical activity energy expenditure explain the between-individual variation in plasma leptin concentrations after adjusting for differences in body composition?, J Clin Endocrinol Metab. 2003; 88:3258–3263
- Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity, Am J Physiol Regul Integr Comp Physiol. 2002; 283:R941-8
- Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance, Diabetes. 2001; 50:2786–2791
- Levine JA, Nygren J, Short KR, Nair KS. Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats, J Appl Physiol (1985). 2003; 94:165–170
- Wu SH, Shu XO, Chow W-H, Xiang Y-B, Zhang X, Li H-L, Cai Q, Milne G, Ji B-T, Cai H, Rothman N, Gao Y-T, Zheng W, Yang G. Nonexercise physical activity and inflammatory and oxidative stress markers in women, J Womens Health (Larchmt). 2014; 23:159–167
- Herzig S, Wolfrum C. Brown and white fat: from signaling to disease, Biochim Biophys Acta. 2013; 1831:895
- Klingenspor M, Herzig S, Pfeifer A. Brown fat develops a brite future, Obes Facts. 2012; 5:890–896
- Virtanen KA, van Marken Lichtenbelt WD, Nuutila P. Brown adipose tissue functions in humans, Biochim Biophys Acta. 2013; 1831:1004–1008
- Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J-A, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects, Am J Physiol Regul Integr Comp Physiol. 2003; 285:R183-92
- Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis, Am J Physiol Endocrinol Metab. 2014; 306:E635-47
- Loeffelholz C von, Coldewey SM, Birkenfeld AL. A Narrative Review on the Role of AMPK on De Novo Lipogenesis in Non-Alcoholic Fatty Liver Disease: Evidence from Human Studies, Cells. 2021; 10
- Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass, J Clin Endocrinol Metab. 2013; 98:E703-7
- Drenowatz C, Hill JO, Peters JC, Soriano-Maldonado A, Blair SN. The association of change in physical activity and body weight in the regulation of total energy expenditure, Eur J Clin Nutr. 2017; 71:377–382
- Kerns JC, Guo J, Fothergill E, Howard L, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter PJ, Hall KD. Increased Physical Activity Associated with Less Weight Regain Six Years After "The Biggest Loser" Competition, Obesity (Silver Spring). 2017; 25:1838–1843
- Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults, Obesity (Silver Spring). 2014; 22:1406–1412
- Shook RP, Hand GA, Drenowatz C, Hebert JR, Paluch AE, Blundell JE, Hill JO, Katzmarzyk PT, Church TS, Blair SN. Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year, Am J Clin Nutr. 2015; 102:1332–1338
- Zhu W, Cheng Z, Howard VJ, Judd SE, Blair SN, Sun Y, Hooker SP. Is adiposity associated with objectively measured physical activity and sedentary behaviors in older adults?, BMC Geriatr. 2020; 20:257
- Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, O'Connell JR, Ducharme JL, Hines S, Sack P, Naglieri R, Shuldiner AR, Snitker S. Physical activity and the association of common FTO gene variants with body mass index and obesity, Arch Intern Med. 2008; 168:1791–1797
- Chipperfield JG. Everyday physical activity as a predictor of late-life mortality, Gerontologist. 2008; 48:349–357
- Hamasaki H. Daily physical activity and type 2 diabetes: A review, World J Diabetes. 2016; 7:243–251
- Lear SA, Hu W, Rangarajan S, Gasevic D, Leong D, Iqbal R, Casanova A, Swaminathan S, Anjana RM, Kumar R, Rosengren A, Wei L, Yang W, Chuangshi W, Huaxing L, Nair S, Diaz R, Swidon H, Gupta R, Mohammadifard N, Lopez-Jaramillo P, Oguz A, Zatonska K, Seron P, Avezum A, Poirier P, Teo K, Yusuf S. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study, The Lancet. 2017; 390:2643–2654
- Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D. Sitting Time, Physical Activity, and Risk of Mortality in Adults, J Am Coll Cardiol. 2019; 73:2062–2072
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida J-M, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice, Eur Heart J. 2021; 42:3227–3337
- Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, Groop P-H, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JPH, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis, Diabetes. 2017; 66:241–255
- Boulé NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study,Diabetes Care. 2005; 28:108–114
- Eitner A, Pester J, Vogel F, Marintschev I, Lehmann T, Hofmann GO, Schaible H-G. Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus, Pain. 2017; 158:1743–1753
- Loeffelholz C von, Roth J, Coldewey SM, Birkenfeld AL. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease, Biomedicines. 2021; 9
- Eriksson KF, Lindgärde F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmö Preventive Trial with diet and exercise, Diabetologia. 1998; 41:1010–1016
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, N Engl J Med. 2002; 346:393–403
- Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity,Diabetes Care. 2003; 26:3230–3236
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study, Diabetes Care. 1997; 20:537–544
- Boer AT den, Herraets IJT, Stegen J, Roumen C, Corpeleijn E, Schaper NC, Feskens E, Blaak EE. Prevention of the metabolic syndrome in IGT subjects in a lifestyle intervention: results from the SLIM study, Nutr Metab Cardiovasc Dis. 2013; 23:1147–1153
- Roumen C, Corpeleijn E, Feskens EJM, Mensink M, Saris WHM, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study, Diabet Med. 2008; 25:597–605
- Day CP, James OF. Steatohepatitis: A tale of two “hits”?, Gastroenterology. 1998; 114:842–845
- Cormier T. Exercise Programming for Nonalcoholic Fatty Liver Disease, Strength & Conditioning Journal. 2019; 41:89–93
- Zhang H-J, He J, Pan L-L, Ma Z-M, Han C-K, Chen C-S, Chen Z, Han H-W, Chen S, Sun Q, Zhang J-F, Li Z-B, Yang S-Y, Li X-J, Li X-Y. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial, JAMA Intern Med. 2016; 176:1074–1082
- Fagour C, Gonzalez C, Pezzino S, Florenty S, Rosette-Narece M, Gin H, Rigalleau V. Low physical activity in patients with type 2 diabetes: the role of obesity, Diabetes Metab. 2013; 39:85–87
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study, Diabet Med. 2005; 22:1379–1385
- Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey, Diabetes Care. 2006; 29:531–537
- Hamasaki H, Ezaki O, Yanai H. Nonexercise Activity Thermogenesis is Significantly Lower in Type 2 Diabetic Patients With Mental Disorders Than in Those Without Mental Disorders: A Cross-sectional Study, Medicine (Baltimore). 2016; 95:e2517
- Hamasaki H, Noda M, Moriyama S, Yoshikawa R, Katsuyama H, Sako A, Mishima S, Kakei M, Ezaki O, Yanai H. Daily Physical Activity Assessed by a Triaxial Accelerometer Is Beneficially Associated with Waist Circumference, Serum Triglycerides, and Insulin Resistance in Japanese Patients with Prediabetes or Untreated Early Type 2 Diabetes, J Diabetes Res. 2015; 2015:526201
- Uemura H, Katsuura-Kamano S, Yamaguchi M, Nakamoto M, Hiyoshi M, Arisawa K. Abundant daily non-sedentary activity is associated with reduced prevalence of metabolic syndrome and insulin resistance, J Endocrinol Invest. 2013; 36:1069–1075
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study, JAMA. 1999; 282:1433–1439
- Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes, Circulation. 2003; 107:2435–2439
- Sarzynski MA, Rice TK, Després J-P, Pérusse L, Tremblay A, Stanforth PR, Tchernof A, Barber JL, Falciani F, Clish C, Robbins JM, Ghosh S, Gerszten RE, Leon AS, Skinner JS, Rao DC, Bouchard C. The HERITAGE Family Study: A Review of the Effects of Exercise Training on Cardiometabolic Health, with Insights into Molecular Transducers, Med Sci Sports Exerc. 2022; 54:S1-S43
- Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I Figuls M, Metzendorf M-I, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus, Cochrane Database Syst Rev. 2017; 12:CD003054
- Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, van Hubbard S, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes, N Engl J Med. 2013; 369:145–154
- Gregg E, Jakicic J, Blackburn G, Bloomquist P, Bray G, Clark J, Coday M, Curtis J, Egan C, Evans M, Foreyt J, Foster G, Hazuda H, Hill J, Horton E, van Hubbard, Jeffery R, Johnson K, Kitabchi A, Knowler W, Kriska A, Lang W, Lewis C, Montez M, Nathan D, Neiberg R, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl P, Safford M, Stewart K, Trence D, Wadden T, Wing R, Yanovski S. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial, Lancet Diabetes Endocrinol. 2016; 4:913–921
- US Department of Health and Human Services. Step It Up! The Surgeon General’s Call to Action to Promote Walking and Walkable Communities. Washington (DC); 2015
- Lanningham-Foster L, Nysse LJ, Levine JA. Labor saved, calories lost: the energetic impact of domestic labor-saving devices, Obes Res. 2003; 11:1178–1181
- Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating, J Clin Endocrinol Metab. 2015; 100:3011–3020
- Elbelt U, Schuetz T, Knoll N, Burkert S. Self-Directed Weight Loss Strategies: Energy Expenditure Due to Physical Activity Is Not Increased to Achieve Intended Weight Loss, Nutrients. 2015; 7:5868–5888
- Pontzer H, Durazo-Arvizu R, Dugas LR, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Cooper RS, Schoeller DA, Luke A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Huumans, Curr Biol. 2016; 26:410–417
- Schoeller DA, Shay K, Kushner RF. How mch physical activity is needed to minimize weight gain in previously obese women?, Am J Clin Nutr. 1997; 66:551–556
- Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight, Am J Clin Nutr. 2002; 75:499–504
- Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials, Am J Clin Nutr. 2014; 99:14–23
- Hunter GR, Bickel CS, Fisher G, Neumeier WH, McCarthy JP. Combined aerobic and strength training and energy expenditure in older women, Med Sci Sports Exerc. 2013; 45:1386–1393
- Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition, Obesity (Silver Spring). 2016; 24:1612–1619
- Kempen KP, Saris WH, Westerterp KR. Energy balance during an 8-wk energy-restricted diet with and without exercise in obese women, Am J Clin Nutr. 1995; 62:722–729
- Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis, Obes Rev. 2009; 10:313–323
- Goodman A. Walking, cycling and driving to work in the English and Welsh 2011 census: trends, socio-economic patterning and relevance to travel behaviour in general, PLoS One. 2013; 8:e71790