ABSTRACT
Diabetes is the most prevalent metabolic disorder and it is estimated to affect more than 460 million people globally. In the United States, 34.2 million Americans, or 10.5% of the population, have diabetes. Patients with diabetes have a 3-fold greater chance of hospitalization compared to those without diabetes. In 2016 in the U.S., there were over 7.8 million hospital stays for patients with diabetes. Hyperglycemia, defined as a blood glucose greater than 140 mg/dl (7.8 mmol/l), is reported in 22-46% of non- critically ill hospitalized patients. Extensive data indicates that inpatient hyperglycemia, in patients with or without prior diagnosis of diabetes, is associated with an increased risk of complications and mortality. Recently the American Diabetes Association recommended a target glucose between 140 mg/dl (7.8 mmol/l) and 180 mg/dl (10.0 mmol/l) for critically ill patients in the ICU as well as for most patients admitted to general medicine and surgery in the non-ICU setting. Insulin remains the best way to control hyperglycemia in the inpatient setting especially in the critically ill patient. Intravenously administered insulin is the preferred method to achieve the recommended glycemic target in the ICU. The use of oral antidiabetic agents was not recommended in previous guidelines because the lack of safety and efficacy studies in the inpatient setting. However, increasing evidence indicates that treatment with oral agents such as DPP4 inhibitors, alone or in combination with basal insulin, is safe and effective in general medicine and surgery patients with mild to moderate hyperglycemia.
INTRODUCTION
Diabetes is a prevalent metabolic disorder that affects more than 460 million people globally, and is projected to rise to 700 million (10.9% of the adult population) by 2045 (1). In the United States, data from the National Diabetes Statistics Report in 2020 estimated that a total of 34.2 million Americans, or 10.5% of the population, had diabetes (2). The percentage of the population with diagnosed diabetes is expected to rise, with one study projecting that as many as one in three U.S. adults will have diabetes by 2050 (3). People with diabetes have a 35% greater chance of referral for elective operations and an up to 4-fold greater chance of hospitalization compared to those without diabetes (4-7). Data from the US and Scotland estimate that of those individuals with a discharge diagnosis of diabetes, 30% will require 2 or more hospitalizations in any given year (5,6,8). In 2016 in the U.S., there were over 7.8 million hospital stays for people with diabetes (i.e., diabetes as either a principal diagnosis for hospitalization or as a secondary diagnosis, coexisting condition) (2), and in the UK the annual National Diabetes Inpatient Audit suggested that the prevalence of diabetes amongst inpatients had risen from 15% in 2010 to almost 20% in 2019 (9). In addition, those hospitalized with a diagnosis of diabetes stay in the hospital for longer than those without a diagnosis of diabetes admitted for the same condition (10,11).
Hyperglycemia is defined as a blood glucose concentration of greater than 140 mg/dl (7.8 mmol/l) (15,16). It is reported in 22% to 46% of non-critically ill hospitalized patients (8,15). Extensive observational and trial data indicate that inpatient hyperglycemia, in patients with or without a prior diagnosis of diabetes, is associated with an increased risk of complications and mortality, a longer hospital stay, a higher admission rate to the intensive care unit (ICU), and a higher need for transitional or nursing home care after hospital discharge (8,17,18).
Several studies and meta-analyses have shown that attempting ‘tight’ glycemic control using intensive insulin therapy is associated with increased risk of hypoglycemia (19-23). This has been associated with increased morbidity and mortality in hospitalized patients (15,24-28). Thus, while insulin therapy is recommended for the management of hyperglycemia in hospitalized patients, the concern about hypoglycemia have led to revised glucose target recommendations from leading professional organizations around the world (16,22,29-32).
This chapter reviews the pathophysiology of hyperglycemia during illness, the mechanisms for increased complications and mortality due to hyperglycemia and hypoglycemia, and reviews the evidence supporting different therapies and approaches for the management of inpatient diabetes and hyperglycemia in the critical care and in the general medicine and surgical settings.
PREVALENCE OF DIABETES AND HYPERGLYCEMIA IN THE HOSPITALIZED PATIENT
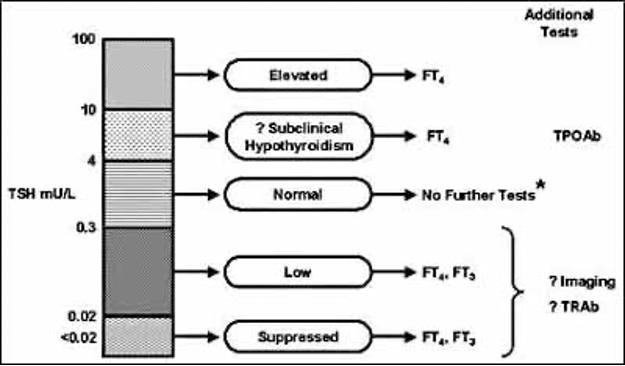
Observational studies have reported a prevalence of hyperglycemia and diabetes ranging from 38% to 40% in hospitalized patients (8), and in 70-80% of those with diabetes who have a critical illnesses or cardiac surgery (33-35). A 2017 report using point-of-care bedside glucose tests data in almost 3.5 million people (653,359 ICU and 2,831,436 non-ICU) from 575 hospitals in the United States reported a prevalence of hyperglycemia, (defined as a glucose level >180 mg/dl [10.0 mmol/l]) of 32.2% in ICU patients and in 32.0% of non-ICU patients (33). These numbers included those with newly identified or stress hyperglycemia as well as those with a prior diagnosis of diabetes. The American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) consensus on inpatient hyperglycemia defined stress hyperglycemia or hospital-related hyperglycemia as any blood glucose concentration >140 mg/dl (>7.8 mmol/l) in patients without a prior history of diabetes (15,16). Although stress hyperglycemia typically resolves as the acute illness or surgical stress abates, a significant proportion (up to 60% in some reports) had confirmed diabetes at 6-12 months after discharge (36,37). A guide from the UK on the management of ‘diabetes at the front door’, also recommends that any individual with diabetes who presents acutely unwell should have a capillary glucose measurement and blood/urine ketone measurement taken, but that if it is high on admission (i.e. >140mg/dl [7.8 mmol/l]) and subsequently goes down to normal, then a diagnosis of stress hyperglycemia should be made and documented to the primary care team (38).
Measurement of HbA1c is indicated in people with hyperglycemia without a history of diabetes to differentiate between stress induced hyperglycemia and previously undiagnosed diabetes (38-41). The Endocrine Society and the UK Joint British Diabetes Societies for Inpatient Care recommendations indicate that people hospitalized with both an elevated blood glucose >140 mg/dl (7.8 mmol/l) and an HbA1c of 6.5% (48 mmol/mol) or higher can be identified as having diabetes (15,38).
PATHOPHYSIOLOGY OF HYPERGLYCEMIA DURING ILLNESS
In subjects without diabetes during the fasted state, plasma glucose is maintained between 70 – 100 mg/dl (3.9 – 5.6 mmol/l) by a finely regulated balance between hepatic glucose production and glucose utilization in peripheral tissues. Maintenance of normal glucose concentration is essential for cardiovascular functioning as well as central nervous system function because the brain can neither synthesize nor store glucose (42,43).
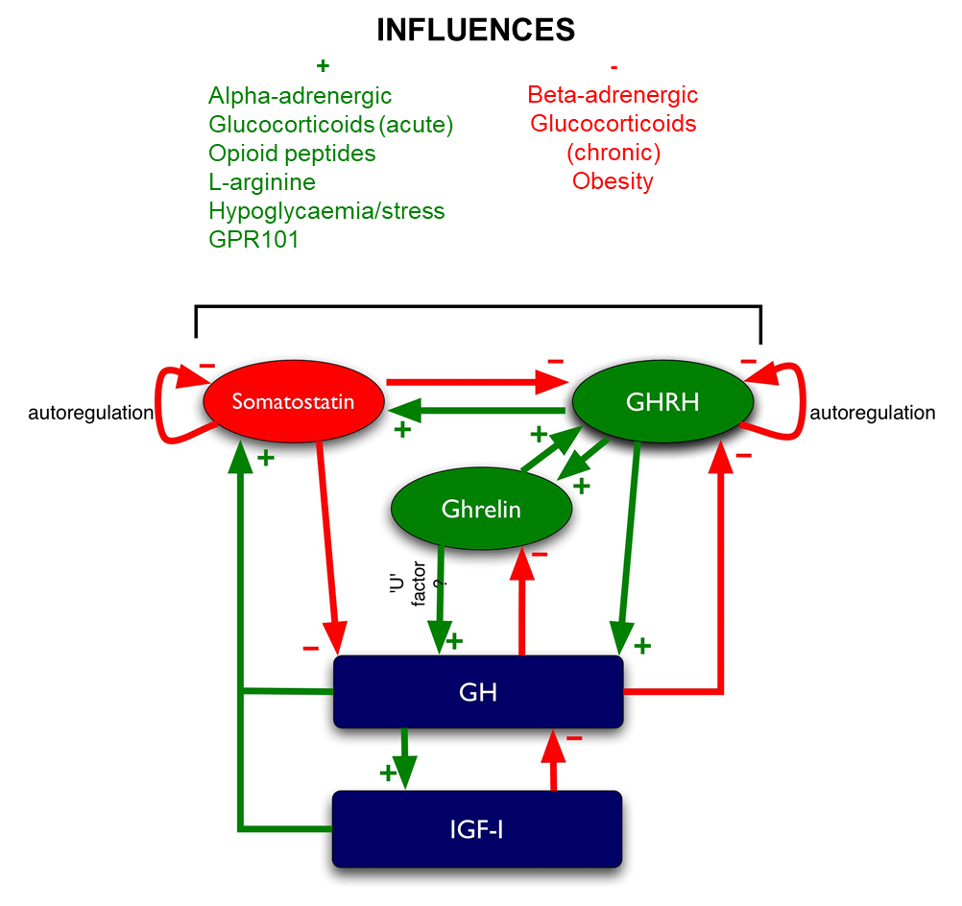
Systemic glucose balance is maintained by a dynamic, minute-to-minute regulation of endogenous glucose production and of glucose utilization by peripheral tissues (44). Glucose production is accomplished by gluconeogenesis or glycogenolysis primarily in the liver and to a lesser degree by the kidneys (45). Gluconeogenesis results from conversion of non-carbohydrate precursors such as lactate, alanine, and glycerol to glucose in the liver (46). Excess glucose is polymerized to glycogen, which is mainly stored in the liver and muscle. Hyperglycemia develops because of three processes: 1) increased gluconeogenesis, 2) accelerated glycogenolysis, and 3) impaired glucose utilization by peripheral tissues (Figure 1).
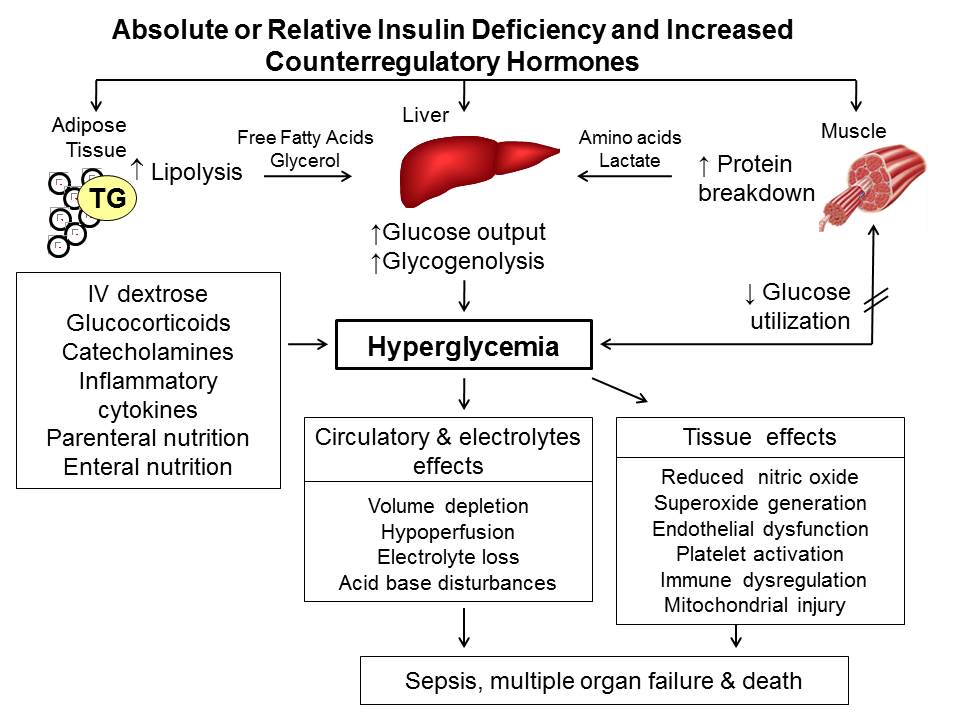
Figure 1. Pathogenesis of hyperglycemia. Hyperglycemia results from increased hepatic glucose production and impaired glucose utilization in peripheral tissues. Reduced insulin and excess counter-regulatory hormones (glucagon, cortisol, catecholamines and growth hormone) increase lipolysis and protein breakdown (proteolysis), and impair glucose utilization by peripheral tissues. Hyperglycemia causes osmotic diuresis that leads to hypovolemia, decreased glomerular filtration rate, and worsening hyperglycemia. At the cellular level, increased blood glucose levels result in mitochondrial injury by generating reactive oxygen species, and endothelial dysfunction by inhibiting nitric oxide production. Hyperglycemia increases levels of pro-inflammatory cytokines such as TNF-α and IL-6 leading to immune system dysfunction. These changes can eventually lead to increased risk of infection, impaired wound healing, multiple organ failure, prolonged hospital stay and death. Adapted from ref (20).
From the quantitative standpoint, inappropriately increased hepatic glucose production represents the major pathogenic disturbance. Increased hepatic glucose production results from the high availability of gluconeogenic precursors including the amino acids alanine and glutamine, as a result of accelerated proteolysis and decreased protein synthesis; lactate as a result of increased muscle glycogenolysis; and glycerol as a result of increased lipolysis; and from the increased activity of gluconeogenic enzymes (phosphoenol pyruvate carboxykinase, fructose-1,6-bisphosphatase, and pyruvate carboxylase) (45,46).
Glucose metabolism is maintained by an interaction of glucoregulatory hormones – insulin and counter-regulatory hormones (glucagon, cortisol, catecholamines and growth hormone). Insulin controls hepatic glucose production by suppressing hepatic gluconeogenesis and glycogenolysis. Depending on the concentration in the circulation, insulin promotes protein anabolism in insulin-sensitive tissues such as muscle, glucose uptake and glycogen synthesis, and inhibits glycogenolysis and protein breakdown (44,47,48). In addition, insulin is a powerful inhibitor of lipolysis, free fatty acid oxidation, and ketogenesis (47,48).
Counter-regulatory hormones (glucagon, cortisol, catecholamines and growth hormone) also play an important role in the regulation of glucose production and utilization. Glucagon is the most important glycogenolytic hormone, therefore, regulates hepatic glucose production during normal state and in every state of hyperglycemia (45). During stress, excess concentration of counter-regulatory hormones results in altered carbohydrate metabolism by inducing insulin resistance, increasing hepatic glucose production, and reducing peripheral glucose utilization. In addition, high epinephrine levels stimulate glucagon secretion and inhibits insulin release by pancreatic β-cells (49,50).
The development of hyperglycemia results in an inflammatory state characterized by an elevation of pro-inflammatory cytokines and increased oxidative stress markers (51-53). Circulating levels of tumor necrosis factor-α (TNF-α), interleukin [IL]-6, IL1-ß, IL-8, and C-reactive protein are significantly increased two- to fourfold on admission in people with severe hyperglycemia compared with control subjects, and levels returned to normal levels after insulin treatment and resolution of hyperglycemic crises (51). Raised concentrations of TNF-α lead to insulin resistance at the level of the insulin receptor and through altered regulation of the insulin-signaling pathway. Increasing evidence indicates that during acute stressful states, increased concentrations of these inflammatory cytokines can increase insulin resistance by interfering with insulin signaling (52,54). In addition, by preventing insulin-mediated activation of phosphatidylinositol 3- kinase TNF-α reduces insulin- stimulated glucose uptake in peripheral tissues (52,54,55).
CONSEQUENCES OF HYPERGLYCEMIA IN THE HOSPITALIZED PATIENTS
A large body of literature including observational and prospective randomized clinical trials, in people with and without diabetes, as well those who are critically or non-critically ill has shown a strong association between hyperglycemia and poor clinical outcomes, such as mortality, infections and hospital complications (5,56-64). This association correlates with severity of hyperglycemia on admission as well as during the hospital stay (62,65,66). Of interest, increasing evidence indicates an increased risk of complications and mortality in patients without a history of diabetes (stress induced) compared to patients with known diagnosis of diabetes (8,59,65,67,68). It is not clear if stress hyperglycemia is the direct cause of poor outcomes or it is a general marker of severity of illness.
The mechanisms implicated on the detrimental effects of hyperglycemia during acute illnesses are not completely understood. Current evidence indicates that severe hyperglycemia results in impaired neutrophil granulocyte function, high circulating free fatty acids, and overproduction of pro-inflammatory cytokines and reactive oxygen species (ROS) that can result in direct cellular damage, and vascular and immune dysfunction (69).
The majority of evidence linking hyperglycemia and poor outcomes comes from studies in the ICU. Falciglia et al in a retrospective study of over 250,000 veterans admitted to various ICUs reported that hyperglycemia is an independent risk factor for mortality and complications (65). In a nonrandomized, prospective study, Furnary followed 3,554 patients with diabetes that underwent coronary artery bypass graft. Patients treated with subcutaneous insulin (SCI) who had an average blood glucose of 214 mg/dl (11.9 mmol/l) and patients treated with continuous insulin infusion (CII) with an average blood glucose of 177 mg/dl (9.8 mmol/l) had significantly more deep sternal wound infections (63) and a 50% higher risk-adjusted mortality (70). In a different ICU study, patients with blood glucose levels >200 mg/dl (>11.1 mmol/l) were shown to have higher mortality compared to those with blood glucose levels <200 mg/dl (<11.1 mmol/) (5.0% vs. 1.8%, p < 0.001) (62). Importantly however, once again it has been shown that it was those people who were not previously known to have diabetes who developed hyperglycemia in the ICU who fared worst (71). This was confirmed by another ICU study with almost 350,000 people, looking at the outcomes in those with sepsis (72). These authors showed that having hyperglycemia was associated with increased stay in hospital and ICU, and greater 90-day mortality (72). However, there was no difference in outcomes for those with diabetes, unless they had experienced severe hypoglycemia, in which case mortality rose (OR 2.95 95%CI 1.19-7.32) (72). Thus, despite a large amount of work having been done, the optimal blood glucose concentration for people on ICU has yet to be determined (73).
The association of hyperglycemia and poor outcomes also applies to those not in the ICU, but admitted to general medicine and surgery services. In such individuals, hyperglycemia is associated with poor hospital outcomes including prolonged hospital stay, infections, disability after hospital discharge, and death (5,8,56,57,66,74). In a retrospective study of 1,886 patients admitted to a community hospital, mortality in the general floors was significantly higher in patients with newly (stress) diagnosed hyperglycemia and with known diabetes compared to subjects with normal glucose values (10% vs. 1.7% vs. 0.8%, respectively; p < 0.01) (8). In a prospective cohort multicenter study of 2,471 patients with community-acquired pneumonia, those with an admission glucose levels >198 mg/dl (>11.0 mmol/l) had a greater risk of mortality and complications than those with glucose levels <198 mg/dl (<11.0 mmol/l) (68). The risk of complications increased 3% for each 18 mg/dl (1.0 mmol/l) increase in admission glucose (68). In a retrospective study of 348 patients with chronic obstructive pulmonary disease and respiratory tract infection, the relative risk of death was 2.1 in those with a blood glucose of 126-160 mg/dl (7.0-8.9 mmol/l), and 3.4 for those with a blood glucose of >162 mg/dl (9.0 mmol/l) compared to patients with a blood glucose of 108 mg/dl (6.0 mmol/l) (74).
General surgery patients with hyperglycemia during the perioperative period are also at increased risk for adverse outcomes. A systematic review of diabetes and the risk of surgical site infection across a variety of surgical specialties showed that high peri-operative glucose levels were associated with an increased risk of infection (75). In a case-control study, elevated preoperative glucose levels increased the risk of postoperative mortality in patients undergoing elective non-cardiac non-vascular surgery (76). Patients with glucose levels of 110-200 mg/dl (5.6-11.1 mmol/l) and those with glucose levels of >200 mg/dl (>11.1 mmol/l) had, respectively, 1.7-fold and 2.1-fold increased mortality compared to those with glucose levels <5.6 mmol/l (<110 mg/dl) (76). In another study, patients with glucose levels >220 mg/dl (>12.2 mmol/l) on the first postoperative day had a rate of infection 2.7 times higher than those who had serum glucose levels <220 mg/dl (<12.2 mmol/l) (77). A more recent study showed an increase of postoperative infection rate by 30% for every 40mg/dl (2.2 mmol/l) rise in postoperative glucose level above 110 mg/dl (6.1 mmol/l) (78) [67]. Furthermore, a study looking at perioperative glycemic control and the effect on surgical site infections in diabetic patients undergoing foot and ankle surgery showed that 11.9% of those with a serum glucose ≥200 mg/dl (11.1 mmol/l) during the admission developed a surgical site infection versus only 5.2% of those with a serum glucose <200 mg/dl (11.1 mmol/l) (odds ratio = 2.45; 95% CI 1.09-5.52, P = 0.03) (79). Lastly, a prospective randomized study looking at the impact of glycemic control at 1-year post liver transplant showed that in those randomized to a glycemic control of blood glucose below 140 mg/dl (7.8 mmol/l) any infection within 1 year occurred in 35 of the 82 patients (42.7%) versus 54 of 82 (65.9%) in those randomized to a glycemic control of 180 mg/dl (10.0 mmol/l) (P = 0.0046) (80). There is now emerging evidence to suggest that early intervention and the use of technology allowing pro-active identification of people at risk, helps to reduce hospital acquired infection rates, episodes of hyper- and hypoglycemia, as well as, in some cases, reduced length of stay (81-84). A meta-analysis also shows that improving peri-operative glycemic control reduced postoperative infection rates (85).
GLYCEMIC TARGETS IN THE ICU AND NON-ICU SETTINGS
The American Diabetes Association (ADA) and American Association of Clinical Endocrinologist (AACE) task force on inpatient glycemic control and other groups recommended different glycemic targets in the ICU setting (16) (Table 1). These guidelines suggest targeting a glucose level between 140 and 180 mg/dl (7.8 and 10.0 mmol/l) for the majority of ICU patients and lower glucose targets between 110 and 140 mg/dl (6.1 and 7.8 mmol/l) in selected ICU patients (i.e., centers with extensive experience and appropriate nursing support, cardiac surgical patients, patients with stable glycemic control without hypoglycemia). Glucose targets >180 mg/dl (>10.0 mmol/l) or <110 mg/dl (<6.1 mmol/l) are not recommended in ICU patients. There is an argument to say that lowering glucose thresholds for those in hospital is likely to be associated with harm (27,86) and an equally persuasive argument to suggest that the implementation of the thresholds as advocated by national and organizational guidelines have led to safer care (87).
The most recent guidelines from the Society of Critical Care Medicine (SCCM) for the management of hyperglycemia in critically ill (ICU) patients recommended that a blood glucose ≥150 mg/dl (≥8.3 mmol/l) should trigger interventions to maintain blood glucose below that level and absolutely <180 mg/dl (<10.0 mmol/l) (30). They also suggest that the insulin regimen and monitoring system be designed to avoid and detect hypoglycemia (blood glucose <70 mg/dl [<3.9 mmol/l]) and to minimize glycemic variability (30). The technology to allow this to occur is being development and may enter routine clinical use relatively soon (88-92).
|
Table 1. Major Guidelines for Treatment of Hyperglycemia in a Hospital Setting |
||
|
|
ICU |
Non-ICU |
|
ADA/AACE (16) |
Initiate insulin therapy for persistent hyperglycemia (glucose >180 mg/dl [>10 mmol/l]). Treatment goal: For most people, target a glucose level between 140 – 180 mg/dl (7.8 – 10.0 mmol/l]. More stringent goals (110 – 140 mg/dl [6.1 – 7.8 mmol/l]) may be appropriate for selected individuals, if achievable without significant risk for hypoglycemia. |
No specific guidelines. If treated with insulin, pre-meal glucose targets should generally be <140 mg/dl (<7.8 mmol/l), with random glucose levels <180 mg/dl (<10.0 mmol/l). More stringent targets may be appropriate for those with previously tight glycemic control. Less stringent targets may be appropriate in people with severe comorbidities.
|
|
ACP (22) |
Recommends against intensive insulin therapy in those with or without diabetes in surgical / medical ICUs Treatment goal: target glucose between 140 – 200 mg/dl (7.8 – 11.0 mmol/l), in people with or without diabetes, in surgical / medical ICUs |
|
|
Critical Care Society (30) |
Glucose >150 mg/dl (>8.3 mmol/l) should trigger insulin therapy Treatment goal: maintain glucose <150 mg/dl (<8.3 mmol/l) for most adults in ICU. Maintain glucose levels <180 mg/dl (10.0 mmol/l) while avoiding hypoglycemia. |
|
|
Endocrine Society (15) |
|
Pre-meal glucose target <140 mg/dl (<7.8mmol/l) and random blood glucose <180 mg/dl (<10.0 mmol/l). A lower target range may be appropriate in people able to achieve and maintain glycemic control without hypoglycemia. A glucose of <180 – 200 mg/dl (<10.0 – 11.0 mmol/l) is appropriate in those with terminal illness and/or with limited life expectancy or at high risk for hypoglycemia. Adjust antidiabetic therapy when glucose falls <100 mg/dl (<5.6 mmol/l) to avoid hypoglycemia. |
|
Society of Thoracic Surgeons (93) (Guidelines specific to adult cardiac surgery) |
Continuous insulin infusion preferred over SC or intermittent intravenous boluses. Treatment goal: Recommend glucose <180 mg/dl (<10.0 mmol/l) during surgery (≤110 mg/dl [≤6.1 mmol/l] in fasting and pre-meal states) |
|
|
Joint British Diabetes Society for Inpatient Care (94) |
|
Target blood glucose levels in most people of between 108 – 180 mg/dl (6.0 – 10 mmol/l) with an acceptable range of between 72 – 216 mg/dl (4.0 – 12.0 mmol/l). |
AACE/ADA, American Association of Endocrinologists and American Diabetes Association joint guidelines; ACP, American College of Physicians; ADA, American Diabetes Association; ICU, intensive care unit;
In the non-ICU setting, the Endocrine Society and the ADA/AACE Practice Guidelines recommended a pre-meal glucose of <140 mg/dl (<7.8 mmol/l) and a random glucose of <180 mg/dl (<10.0 mmol/l) for the majority of non-critically ill patients treated with insulin (15,16,29). More recently the American Diabetes Association has recommended that target glucose for most general medicine and surgery patients in non-ICU settings should be between 140 – 180 mg/dl (7.8 – 10.0 mmol/l) (31). To avoid hypoglycemia <70 mg/dl (<3.9 mmol/l), the total basal and prandial insulin dose should be reduced if glucose levels fall between 70 – 100 mg/dl (3.9 – 5.6 mmol/l). In contrast, higher glucose ranges (>200 mg/dl [>11.1 mmol/l]) may be acceptable in terminally ill patients or in patients with severe comorbidities as a way of avoiding symptomatic hyperglycemia (15,95).
Guidelines from the Joint British Diabetes Society's Inpatient Care Group in the UK published over the last few years, aim for target blood glucose levels in most people between 108 – 180 mg/dl (6.0 – 10.0 mmol/l) with an acceptable range of between 72 – 216 mg/dl (4.0 – 12.0 mmol/l) (94). Table 1 summarizes the currently available guidelines for the management of hyperglycemia in the hospital setting.
EVIDENCE FOR CONTROLLING HYPERGLYCEMIA IN ICU AND NON – ICU SETTINGS
The Leuven surgical ICU study set the stage for promoting intensive glycemic control in the critical care setting (96). This study randomized 1,548 people admitted to the surgical ICU (63% cardiac cases, 13% with diabetes, most patients received early parenteral nutrition). Individuals were randomized to either conventional therapy with a target glucose between 180 – 200 mg/dl (10.0 – 11.1 mmol/l) or intensive therapy to a target glucose between 80 – 110 mg/dl (4.4 – 6.1 mmol/l). Those in the conventional arm had a mean daily glucose average of 153 mg/dl (8.5 mmol/l) and those in the intensive arm had an average glucose of 103 mg/dl (5.7 mmol/l). Those in the intensive group had significantly less bacteremia, less antibiotic requirements, lower length of ventilator dependency, lower number of ICU days, and an overall 34% reduction in mortality (96). Following a similar study design, the same group of investigators randomized people in a medical ICU (18% with diabetes) and reported that intensive insulin therapy (mean daily glucose of 111 mg/dl [6.2 mmol/l]) resulted in less ICU and total hospital complications in those with 3 days of insulin treatment (97).
A large number of well-designed randomized controlled trials and meta-analyses however, have shown that such low glucose targets are difficult to achieve, even in environments with high staff to patient ratios without increasing the risk for severe hypoglycemia (19,98-100). In addition, these and other studies failed to show improvement in clinical outcomes and have even shown increased mortality risk with intensive glycemic control (Table 2) (26,98-102). The Glucontrol trial, a seven-country multicenter trial, randomized people in medical and surgical ICUs to tight glycemic control (80 – 110 mg/dl [4.4 – 6.1 mmol/l]) versus conventional glycemic control (140 – 180 mg/dl [7.8 – 10.0 mmol/l]). The study did not find a difference in mortality between the two groups (103). The Efficacy of Volume Substitution and Insulin Therapy in Sepsis (VISEP) study was another trial that attempted to reproduce the data from the Leuven trial (98). The study was a multicenter study in Germany that randomized people with sepsis to receive intensive insulin therapy to maintain glucose levels between 180 – 200 mg/dl (10.0 – 11.1 mmol/l) versus the intensive arm of 80 – 110 mg/dl (4.4 – 6.1 mmol/l) (98). The investigators evaluated differences between the groups in 28- and 90-day mortality, sepsis-related organ failure, ICU stay and frequency of hypoglycemia (glucose < 40mg/dl [<2.2 mmol/l]). The trial was stopped prematurely after reaching only ~2/3 of the projected enrollment due to an interim analysis that showed no difference in 28- or 90-day mortality between patients treated in the conventional arm versus those in the intensive arm (21.6% vs. 21.9%; 29.5 vs. 32.8%, respectively), but those in the intensive arm experienced a significantly greater amount of severe hypoglycemia (12.1 vs. 2.1%) (98).
The Normoglycemia in Intensive Care Evaluation and Surviving Using Glucose Algorithm Regulation (NICE-SUGAR) trial randomized over 6104 subjects to receive either conventional glycemic control to a target glucose <180 mg/dl [<10.0 mmol/l]) or intensive glycemic control (target 81 – 108 mg/dl [4.5 – 6.0 mmol/l]) reported no difference in hospital mortality, but found increased mortality at 90 days of follow-up (24.9% vs. 27.5%, p=0.02) (19). In a subsequent analysis of the trial, the NICE SUGAR investigators reported a higher frequency of hypoglycemia in the intensive arm (6.8% vs. 0.5%) and those with hypoglycemia had ~2-fold increase in mortality compared to patients without hypoglycemia (24).
|
Table 2. Clinical Trials of Intensive Glycemic Control in ICU Populations |
||||
| Study | Setting | Population | Percentage with diabetes | Clinical Outcome |
| Malmberg, 1994 (104) | CCU | People with diabetes with suspected or confirmed acute MI | 100 | 28% decrease mortality after 1 year |
| Furnary, 1999 (63)* | CCU | People with diabetes undergoing CABG | 100 | 65% decrease in deep sternal wound infection rate |
| Van den Berghe, 2001 (96) | Surgical ICU | Mixed, with CABG | 13 | 34% decrease in mortality |
| Furnary, 2003 (70)* | CCU | People with diabetes undergoing CABG | 100 | 50% decrease in adjusted mortality rate |
| Krinsley, 2003 (62)* | Medical and surgical ICU | Mixed | 22.4 | 27% decrease in mortality |
| Lazar, 2004 (105) | Operating room and ICU | People with diabetes undergoing CABG | 100 | 60% decrease of post - operative atrial fibrillation |
| Van den Berghe, 2006 (97) | Medical ICU | Mixed | 17 | 18% decrease mortality |
| Gandhi, 2007 (106) | Operating Room | Mixed, undergoing cardiac surgery | 19.6 | No difference in mortality; increase in stroke rate in the intensive treatment arm |
| VISEP, 2008 (98) | Medical ICU | Mixed, admitted with sepsis | 30 | No differences in 28-day or 90-day mortality, end-organ failure, length of stay |
| De La Rosa, 2008 (99) | Medical and surgical ICU | Mixed | 12 | No differences in 28-day mortality or infection rate |
| Glucontrol, 2009 (103) | Medical and surgical ICU | Mixed | 18 | No difference in 28-day mortality |
| NICE-SUGAR, 2009/2012 (19,24) | Medical and surgical ICU | Mixed | 20 | No difference in 90-day mortality |
| Boston Children’s (SPECS), 2012 (107,108) | Cardiac ICU | Cardiac surgery, people without diabetes | 0 | No differences in 30-day mortality, length of stay, in the cardiac ICU, length of hospital, duration of mechanical ventilation and vasoactive support, or measures of organ failure |
| ChiP, 2014 (109) [148] | Pediatric ICU | Critical illness/injury/major surgery, those without diabetes. | 0 | No difference in 30-day mortality. Increased hypoglycemia in the intensive treated group |
| CGAO–REA, 2014 (110,111) | Medical ICU | Mixed | 23 | No difference in 90-day mortality. Increased hypoglycemia in the intensive treated group |
| Okabayashi, 2014 (112) | Surgical ICU | Mixed | 25.3 | Decreased surgical site infection in the intensive treated group |
MI, myocardial infarction, ICU – Intensive Care Unit, CABG – Coronary artery bypass graft
*These are observational studies (reference 62, 63, 70) and all the other studies were randomized studies. Mixed – study enrolled those with and without diabetes
The GLUCO-CABG trial was a randomized open-label clinical study that included those with and without diabetes undergoing CABG who experienced perioperative hyperglycemia, defined as a glucose >140 mg/dl (>7.8 mmol/l) (60). A total of 302 people between 18 and 80 years of age were randomized to the intensive glycemic control group (target glucose 100 – 140 mg/dl [5.6 – 7.8 mmol/l]) or to conservative – or conventional – control (glucose 141 – 180 mg/dl [7.9 – 10.0 mmol/l]) in the ICU. After transition from ICU to the telemetry floor, patients were managed with a single treatment protocol aimed to maintain a glucose target <140 mg/dl (<7.8 mmol/l) before meals during the hospital stay. The primary outcome included differences between intensive and conservative glucose control on a composite of perioperative complications including sternal wound infection, bacteremia, respiratory failure, pneumonia, acute kidney injury, and major adverse cardiovascular events including acute coronary syndrome, stroke, heart failure and cardiac arrhythmias (60). The mean glucose during the ICU stay was 132±47 mg/dl (7.3±2.6 mmol/l) in the intensive and 152±17 mg/dl (8.4±1.0 mmol/l) in the conservative group. Intensive glucose treatment resulted in a 20% reduction in perioperative complications compared to the conservative group (42% vs. 52%; p=0.08). Of interest, there were no differences in the rate of complications among patients with diabetes treated with intensive or conservative regimens (49.3% vs. 45.8%, p=0.68); however, in patients without diabetes intensive treatment was associated with significantly lower rate of complications compared to the conservative group (35% vs. 58%, p=0.006) (60). Hospitalization costs were lower in the intensive group (median [IQR] $36,681 [28,488 – 46,074] vs. $40,913 [31,464 – 56,629], p=0.04), with an average total cost savings of $3,654 per case compared to conservative glucose control (113).
To date, no large studies have been conducted to determine if improved control in those not in an ICU may result in reduced morbidity and mortality in general medical and surgical patients. For most people in the hospital with diabetes while there are observational data to show that dysglycemia is harmful, there were little data to show that improving glycemic control helps (114). A randomized controlled trial and a meta-analysis reported that improved glucose control may reduce hospital complications in general surgery patients (61). Improving glucose control with a basal bolus regimen resulted in a significant reduction in the frequency of composite complications including postoperative wound infection, pneumonia, bacteremia, and acute renal and respiratory failure (61). In that study, treatment with basal bolus insulin reduced average total inpatient costs per day by 14% or $751 compared to treatment with sliding scale alone (115).
HYPOGLYCEMIA
Hypoglycemia is the commonest side effect of treatment of all types of diabetes and stress hyperglycemia in the hospital setting. It presents a major barrier to satisfactory long-term glycemic control. Hypoglycemia results from an imbalance between glucose supply, glucose utilization and current insulin levels. Hypoglycemia is defined as a lower-than-normal level of blood glucose. For the purposes of hospital inpatients, hypoglycemia is defined as any glucose level <70 mg/dl (<3.9 mmol/l) (31,116). Severe hypoglycemia has been defined by many as <40 mg/dl (<2.2 mmol/l) (117). The incidence of severe hypoglycemia among the different trials ranged between 5% and 28% depending on the intensity of glycemic control in the ICU (118). Rates from trials using subcutaneous insulin in non-critically ill patients range from less than 1% to 33% (61,119,120). In 2017, the UK National Diabetes Inpatient Audit (NaDIA) data showed 18% of people with diabetes in hospital experienced one or more hypoglycemic episodes with a blood glucose <72mg/dl (<4.0 mmol/l) – down from 26% in 2011, with 7% (1 in 14) experiencing episodes requiring third party assistance to administer rescue therapy (121). The NaDIA data also showed that those with type 1 diabetes had the highest prevalence, with 25% experiencing a severe hypoglycemic episode (121). Furthermore 1.3% (1 in 80) of those in hospital with diabetes required some form of injectable rescue treatment (i.e. IV glucose or IM glucagon), down from 2.1% in 2011 (121). The same data showed that the highest proportion of episodes took place overnight (28%) between 05:00 and 09.00am when snack availability was likely to have been lowest (121).
The key predictors of hypoglycemic events in those hospitalized include older age, greater illness severity, diabetes, and the use of oral glucose lowering medications and/or insulin (122-124). In-hospital processes of care that contribute to risk for hypoglycemia include unexpected changes in nutritional intake that are not accompanied by associated changes in the glycemic management regimen (e.g., cessation of nutrition for procedures, adjustment in the amount of nutritional support), interruption of the established routine for glucose monitoring, deviations from the established glucose control protocols, and failure to adjust therapy when glucose is trending down or steroid therapy is being tapered (124-126). A common cause of inpatient hypoglycemia is insulin prescription errors including misreading poorly written prescriptions – when ‘U’ is used for units (i.e. 4U becoming 40 units) or confusing the insulin name with the dose (e.g. Humalog Mix25 becoming Humalog 25 units) (127).
Table 3 describes the most common risk factors for developing hypoglycemia in the hospital (124,128). However, other factors may also be involved, such as concurrent use of drugs with hypoglycemic agents e.g., warfarin, quinine, salicylates, fibrates, sulfonamides (including co-trimoxazole), monoamine oxidase inhibitors, NSAIDs, probenecid, somatostatin analogues, or selective serotonin reuptake inhibitors. Secondary causes of inpatient hypoglycemia include loss of counter-regulatory hormone function, e.g., Addison’s disease, growth hormone deficiency, hypothyroidism, or hypopituitarism.
|
Table 3. Common Risk Factors for Developing Hypoglycemia in the Hospital |
|
Prior episode of hypoglycemia |
|
Older age |
|
Chronic kidney disease |
|
Congestive heart failure |
|
Liver Failure |
|
Sepsis |
|
Malnutrition |
|
Erratic eating patterns / Nutritional interruptions / Lack of access to carbohydrates |
|
Malignancies |
|
Insulin regimen |
|
Type 1 diabetes |
|
Mental status changes |
|
Certain concomitant use of medications |
|
Duration of diabetes |
The development of hypoglycemia is associated with poor hospital outcomes (25,97,100,102,103,129-136). Turchin et al examined data from 4,368 admission episodes for people with diabetes of which one third were on regular insulin therapy (25). Patients experiencing inpatient hypoglycemia experienced a 66% increased risk of death within one year and spent 2.8 days longer in hospital compared to those not experiencing hypoglycemia. The odds ratio (95% confidence interval) for mortality associated with one or more episodes was 2.28 (1.41-3.70, p=0.0008) among a cohort of 5,365 patients admitted to a mixed medical-surgical ICU (122). In a larger cohort of over 6,000 patients, hypoglycemia was associated with longer ICU stay and greater hospital mortality especially for patients with more than one episode of hypoglycemia (24). A 2019 systematic review and meta-analysis of hospital acquired hypoglycemia in non-ICU patients suggested that adults exposed to glucose levels <72mg/dl (<4.0 mmol/l) experienced a mean increased length of hospital stay of 4.1 days (95% CI 2.36 – 5.79) compared to those who did not experience hypoglycemia (129). The same dataset suggested an increased relative risk of in-hospital mortality for non-ICU patients of 2.09 (95% CI 1.64 – 2.67) (129). There was a non-significant reduction in mortality for those in the ICU of 0.75 (95% CI 0.49 – 1.16) (129). These data strengthen the argument to have potentially less strict glycemic targets for those not in the ICU (27). For example, if an individual has a glucose of 75 mg/dl (4.2 mmol/l), and is on an intravenous insulin infusion, by the time their bedside capillary glucose is next measured, they may have a glucose well below 72 mg/dl (4.0 mmol/l); thus, they may have potential harm. Indeed, data published from previous NaDIA surveys using data from over 100 hospitals across the UK showed several serious adverse events including seizures; permanent cerebral damage; cardiac arrests; and deaths. Insulin therapy was implicated in several of these events (28,137). The counter argument is that there are initiatives to reduce the risk of developing inpatient hypoglycemia and having national guidance has led to improved patient care overall (87,138).
Bedside point-of-care (POC) capillary glucose monitoring is recommended to assess glycemic control in hospitalized patients. Clinical guidelines recommend bedside capillary POC testing before meals and at bedtime to assess glycemic control and to adjust insulin therapy in the hospital (15). This approach; however, has been shown to fail to detect hypoglycemia, in particular nocturnal hypoglycemia and asymptomatic hypoglycemia, a common scenario in the hospital setting (139,140). Hence, the reported rates of inpatient hypoglycemia are significant and improved methods to monitor glycemic control in the hospital setting may reduce the risk of hypoglycemia. As mentioned, technological advances are currently being evaluated to allow the use of continuous glucose monitoring to help manage patients in the ICU and general wards (88-92).
Hypoglycemia has been associated with adverse cardiovascular outcomes, such as increased myocardial contractility, prolonged QT interval (possibly due to the rapid drop in potassium concentrations due to the increased circulating epinephrine and norepinephrine), ischemic electrocardiogram changes and repolarization abnormalities, angina, arrhythmias, increased inflammation, and sudden death, (43,141-143). The mechanisms for the poor outcome are not completely understood, but hypoglycemia has been associated with increases in pro-inflammatory cytokines (TNFα, IL-1β, IL-6, and IL-8), markers of lipid peroxidation, acute changes in endothelial dysfunction with associated vasoconstriction, increased blood coagulability, cellular adhesion, and oxidative stress (144-147).
Despite these observations, the direct causal effect of iatrogenic hypoglycemia on outcomes is still debatable. Kosiborod et al reported that spontaneous hypoglycemia, but not insulin – induced hypoglycemia was associated with higher in hospital mortality (133). Similarly, another study of 31,970 patients also reported that hypoglycemia is associated with increased in-hospital mortality, but the risk was limited to patients with spontaneous hypoglycemia and not to patients with drug-associated hypoglycemia (148). These studies raised the possibility that hypoglycemia, like hyperglycemia, and despite the biochemical and other changes described, is a marker of disease burden rather than a direct cause of death.
RECOMMENDATIONS FOR MANAGING HYPERGLYCEMIA IN THE HOSPITAL ENVIRONMENT
Knowledge of Diabetes Management Amongst Medical Staff
The burden on inpatient diabetes falls most frequently to junior medical staff who often have little or no specialist diabetes training. As such, it is perhaps not surprising that errors occur. In the UK, a survey of junior doctors showed that unlike other commonly encountered medical conditions, such as acute asthma or angina, their knowledge about and confidence in managing diabetes was significantly lower (149). In 2019, this was also shown in a multicenter study from the US – with the major difference being that the while most staff felt confident and comfortable managing diabetes, when challenged on how to manage certain situations, and in particular identifying glucose targets for those who were critically ill or the threshold for defining hypoglycemia, their confidence was far higher than their knowledge – a potentially devastating combination (150). Given the high prevalence of diabetes amongst hospital inpatients, basic diabetes management should be part of mandatory training.
Management of Inpatient Hyperglycemia in the ICU
Insulin is the best way to control hyperglycemia in the inpatient setting especially in the critically ill patient. A variable rate, intravenous insulin infusion is the preferred method to achieve the recommended glycemic target. The short half-life of intravenous insulin makes it ideal in this setting because of flexibility in the event of unpredicted changes in an individual’s health, medications, and nutrition.
When someone is identified as having hyperglycemia (blood glucose ≥180 mg/dl [≥10.0 mmol/l]), a variable rate intravenous insulin infusion should be started to maintain blood glucose levels <180 mg/dl (<10.0 mmol/l). A variety of intravenous infusion protocols have been shown to be effective in achieving glycemic control with a low-rate of hypoglycemic events, and in improving hospital outcomes (63,70,96,104,151-156). A proper protocol should allow flexible blood glucose targets modified based on the individuals’ clinical situation. Further, it should have clear instructions about the blood glucose threshold for initiating insulin infusion and the initial rate. The appropriate fluids should also be prescribed. It should be validated in order to avoid hyperglycemia if adjusted too slowly and hypoglycemia if adjusted too fast. Accurate insulin administration requires a reliable infusion pump that can deliver the insulin dose in increments of 0.1 unit per hour (118,154).
There is no ideal protocol for the management of hyperglycemia in the critically ill patient. In addition, there is no clear evidence demonstrating the benefit of one protocol/algorithm versus any other. The implementation of any of these algorithms requires close follow up by the nursing staff and is prone to human errors. Some institutions have developed computerized protocols that can be implemented in order to avoid errors in dosing (157-161). Essential elements that increase protocol success of continuous insulin infusion are: 1) rate adjustment that considers the current and previous glucose value and the current rate of insulin infusion, 2) rate adjustment that considers the rate of change (or lack of change) from the previous reading, and 3) frequent glucose monitoring (hourly until stable glycemia is established, and then every 2 – 3 hours) (118,152,162-164).
Several computer-based algorithms aiming to direct the nursing staff adjusting the insulin infusion rate have become commercially available (158-160,165). Retrospective cohorts have been reported, as well as controlled trials have reported a more rapid and tighter glycemic control with computer-guided algorithms than standard paper form protocols in ICU patients (157,159), as well as lower glycemic variability than patients treated with the standard insulin infusion regimens. Despite differences in glycemic control between insulin algorithms, another study showed no difference between computerized protocols versus conventional glucose control (110). Thus, most insulin algorithms appear to be appropriate alternatives for the management of hyperglycemia in critically ill patients, and the choice depends upon the physician’s preferences and cost considerations.
Managing Hyperglycemia in the Non-ICU Setting
Subcutaneous insulin is the preferred therapeutic agent for glucose control in those admitted to non-ICU settings (general medicine and surgery). Several studies have shown that the commonly used subcutaneous sliding scale insulin (SSI) is not acceptable as the single regimen in people with diabetes, because it results in undesirable levels of hypoglycemia and hyperglycemia (166-168). It has become evident in recent years that the use of scheduled subcutaneous insulin therapy with basal (e.g., glargine, detemir or degludec) once daily or with intermediate acting insulin (NPH) given twice daily alone or in combination with short (regular) or rapid acting insulin (lispro, aspart, glulisine) prior to meals is effective and safe for the management of most patients with hyperglycemia and diabetes (16,169).
The basal-bolus (prandial) insulin regimen is considered the physiologic approach as it addresses the three components of insulin requirement: basal (what is required in the fasting state), nutritional (what is required for peripheral glucose disposal following a meal), and supplemental (what is required for unexpected glucose elevations, or to dispose of glucose in hyperglycemia (169).
A prospective, randomized multi-center trial compared the efficacy and safety of a basal/bolus insulin regimen with sliding scale insulin in people with type 2 diabetes admitted to a general medicine service (119). The use of basal-bolus insulin regimen had greater improvement in blood glucose control than subcutaneous sliding scale alone. A blood glucose target <140 mg/dl (<7.8 mmol/l) was achieved in 66% of those in the glargine plus glulisine group and 38% in the sliding scale group (119). The incidence of hypoglycemia, defined as a glucose <60 mg/dl (<3.3 mmol/l), was less than 5% in those treated with basal bolus or SSI. A different study in general surgery inpatients also compared efficacy and safety of a basal bolus regimen to SSI in those with type 2 diabetes (61). The basal bolus regimen resulted in a significant improvement in glucose control and in a reduction in the frequency of the composite of postoperative complications including wound infection, pneumonia, respiratory failure, acute renal failure, and bacteremia.
The use of multi-dose human NPH and regular insulin has been compared to basal bolus treatment with insulin analogs in an open-label, controlled, multicenter trial in 130 medical admissions with type 2 diabetes (170). This study found that both treatment regimens resulted in significant improvements in inpatient glycemic control with a glucose target of <140 mg/dl (<7.8 mmol/l) before meals, as well as no difference in the rate of hypoglycemic events. Thus, it appears that similar improvement in glycemic control can be achieved with either basal bolus therapy with insulin analogs or with NPH/regular human insulin in people with type 2 diabetes.
Most people in the hospital have reduced caloric intake due to lack of appetite, medical procedures or surgical intervention. In the Basal Plus trial people with type 2 diabetes who were treated with diet, oral antidiabetic agents, or low-dose insulin (≤ 0.4 unit/kg/day) prior to admission were randomized to receive a standard basal bolus regimen with glargine once daily and glulisine before meals or a single daily dose of glargine. In addition, supplemental doses of glulisine were administered for correction of hyperglycemia (>140 mg/dl [>7.8 mmol/l]) per sliding scale (171). This study reported that the basal approach resulted in similar improvement in glycemic control and in the frequency of hypoglycemia compared to a standard basal bolus regimen (171). Thus, in insulin naive individuals or in those receiving low-dose insulin on admission, as well as those with reduced oral intake, the use of a basal plus regimen is an effective alternative to basal bolus.
The recommended total daily insulin dose for most people should start between 0.3 to 0.5 units per Kg (119,126,172,173). Starting doses greater than 0.6 – 0.8 unit/kg/day have been associated with 3-fold higher odds of hypoglycemia than doses lower than 0.2 U/kg/day. In elderly individuals or those with impaired renal function, lower initial daily doses (≤ 0.3 units/kg) may lower the risk of hypoglycemia (174).
Hospital Use of Non-Insulin Therapy in Non-Critical Care Settings
There are several other classes of non-insulin glucose lowering agents that have been tried in the hospital setting. However, most are not suitable for use. Metformin, while first line for type 2 diabetes in the outpatient setting may not be appropriate where there is any evidence of dehydration or renal impairment. Thiazolidinediones are excellent at lowering glucose, but they take several weeks to reach their maximum effect, may precipitate heart failure, and may cause peripheral oedema due to the fluid retention (175). Thus they are not suitable for the inpatient setting (176). Sulfonylureas work rapidly, and are often the drugs of choice for worsening of diabetes in an outpatient setting (177). However, they increase the risk of hypoglycemia. There are data to show that they remain one of the most frequent causes of inpatient hypoglycemia, thus extending length of hospital stay and increased risk of inpatient mortality (121,178).
Oral glucose lowering medication use is limited by the delay and unpredictability of onset of action and there is also concern regarding the cardiovascular effects with sulfonylureas and the inability to use metformin in patients with renal or liver dysfunction (15,179). Recent work using the sodium-glucose co-transporter 2 inhibitor dapagliflozin for corticosteroid-induced hyperglycemia in acute exacerbation of COPD failed to demonstrate an improvement in hyperglycemia (180).
The use of oral antidiabetic agents was not recommended in previous guidelines because of the lack of safety and efficacy studies in the inpatient setting (16). However, increasing evidence indicates that treatment with dipeptidyl peptidase-4 (DPP4) inhibitors, alone or in combination with basal insulin, is safe and effective in general medicine and surgery patients with mild to moderate hyperglycemia. In a pilot study, in general medicine and surgery patients with a blood glucose between 140 and 400 mg/dl (7.8 – 22.2 mmol/l) treated with diet, oral antidiabetic drugs, or low- dose insulin (≤0.4 U/kg/day) who were randomized to sitagliptin once daily, sitagliptin and basal insulin, or basal bolus insulin (181). All groups received correction doses of lispro before meals and bedtime for blood glucose >140 mg/dl (>7.8 mmol/l). In those with mild-moderate hyperglycemia (<180 mg/dl [<10 mmol/l]), the use of sitagliptin plus supplemental (correction doses) or in combination with basal insulin resulted in no significant differences in mean daily blood glucose, frequency of hypoglycemia, or in the number of treatment failures compared to a basal bolus regimen (181). The SITA-HOSPITAL trial, a multicenter, randomized controlled study in 279 general medicine and surgery patients with type 2 diabetes previously treated with oral anti-diabetic agents or low-dose insulin (<0.6 U/kg/d) also reported similar glycemic control, hypoglycemia rate, hospital length-of-stay, treatment failures, or hospital complications (including acute kidney injury or pancreatitis) between the combination of oral sitagliptin plus basal insulin to the more labor-intensive basal-bolus insulin regimen (182).
In 2020 a pooled analysis from three prospective studies using DPP4-i in general medicine and surgery patients with type 2 diabetes reported that treatment with DPP4-i alone or with basal insulin plus correctional doses with rapid-acting insulin for glucose >140mg/dl. reported no differences in mean hospital daily blood glucose, percentage of glucose readings within target of 70-180 mg/dl, length of stay, or complications. Importantly there was less hypoglycemia with DPP4-i compared to basal bolus insulin regimen (183).
Glucose Monitoring in the Hospital
All patients admitted to the hospital with a diagnosis of diabetes and those with newly discovered hyperglycemia should be monitored closely (38). The frequency of monitoring and the schedule of the blood glucose checks will depend on the nutritional intake, patient treatment, and schedule of insulin. There is some controversy regarding the best method to monitor blood glucose. However, considering the convenience and wide availability of the capillary point of care (POC) testing we suggest this as the best approach as long as it is done with a monitoring device that has demonstrated accuracy (184,185). It is important that when using POC blood glucose meters, that several things be kept in mind, in particular overall clinical conditions that might affect the POC value such as hemoglobin level, perfusion, and medications. Table 4 summarizes potential schedules for blood glucose monitoring based on the patient’s nutritional intake and medical regimen.
Several studies have reported on the efficacy of continuous glucose monitoring (CGM) in insulin treated patients in the hospital (88-92). The CGM devices provide estimated glucose values every 5-15 minutes resulting in a better assessment of glycemic control than capillary POC testing (186-188). Recent observational studies have shown increased detection of hypoglycemic events using CGM in the hospital in insulin treated patients (139,189). Additionally, a recent randomized trial by Singh et al. showed the ability of CGM to prevent and reduce hypoglycemia in high-risk hospitalized patients with diabetes through the use of remote CGM alarms (190). There are some concerns including the accuracy of CGM data when acute physiologic disturbances are present (i.e., hypoxemia, vasoconstriction, and rapidly changing glucose levels in diabetic ketoacidosis) or interference with glucose readings (such as salicylic acid, acetaminophen). They should also be removed for certain procedures – with each company having their own list –such as MRIs, CT scans, and diathermy. The use of CGM in the hospital has not been approved by regulatory agencies and remains investigational. Ongoing studies (NCT03832907) with factory-calibrated CGM are testing its accuracy in diverse inpatient populations and the use of a Glucose Telemetry System (GTS) with which glucose values can be wirelessly transmitted from the patient's bedside (CGMS) to a monitor device at the nursing station (NCT03508934, NCT03877068).
|
Table 4. Glucose Monitoring Schedule Based on Nutritional Intake, Insulin Regimen, and Special Patient Situations |
|||
|
Diet |
Regimen |
Glucose monitoring |
Special caveats |
|
NPO |
Intravenous insulin infusion |
Every 1-2 hrs |
|
|
NPO |
SC regular insulin every 6 hrs (6 am, noon, 6pm, midnight) |
Every 6 hrs (6 am, noon, 6pm, midnight) prior to SC insulin dose |
|
|
NPO |
Basal insulin alone (e.g., glargine or detemir) |
Every 6 hrs (6 am, noon, 6pm, midnight) |
|
|
Eating 3 meals per day |
Basal/bolus regimen with long acting (e.g., glargine, detemir) and rapid- acting insulin with meals (aspart, lispro, glulisine) |
4 times per day: before breakfast, before lunch, before evening meal, and bedtime. |
Consider a 3 am blood glucose check in those at risk of hypoglycemia |
|
Nocturnal tube feeds and daytime oral intake |
Regimen varies depending on clinical status. Basal insulin plus corrections or basal bolus with long and rapid-acting insulin. Basal in AM and low- dose NPH insulin at the start of the nocturnal tube feeds |
5 times per day: before breakfast, before lunch, before evening meal, bedtime and 3 am. |
|
|
Continuous tube feeds |
Basal insulin plus correction with regular insulin every 4-6 hours. NPH 2 or 3 times daily or regular insulin every 6 hrs |
Every 6 hrs (6 am, noon, 6pm, midnight) |
|
|
Patients eating small multiple meals per day. (e.g., cystic fibrosis) |
Basal/bolus with long-acting insulin and rapid – acting insulin with meals – e.g. (carbohydrate counting) |
At least 4 times per day: before breakfast, before lunch, before evening meal, and bedtime |
More frequent checks might be warranted in order to include post prandial blood glucose |
|
Those on high dose corticosteroids |
Basal/bolus with long-acting insulin and rapid-acting insulin with meals. May add small dose of NPH to basal bolus regimen in those on morning dose of steroids. AM dose of NPH may be used without basal insulin in persons on intermediate acting steroids (prednisone, methylprednisone) administered once daily |
4 times per day: before breakfast, before lunch, before evening meal, and bedtime |
|
|
NPO or eating 3 meals per day |
Those on insulin pumps |
4-8 times per day: before breakfast, before lunch, before evening meal, and bedtime. Consider post-prandial checks |
|
NPO – nothing by mouth. NPH – Neutral Protamine Hagedorn, SC - subcutaneous
Medical Nutrition Therapy (MNT) in Hospitalized Patients with Diabetes
Medical nutrition therapy is a key component of the comprehensive management of diabetes and hyperglycemia in the inpatient setting. Maintaining adequate nutrition is important for glycemic control and to meet adequate caloric demands. Caloric demand in acute illness will differ from that in the outpatient setting. Achieving the proper nutritional balance in the inpatient setting is challenging. Anyone admitted to the hospital with diabetes or hyperglycemia should be assessed to determine the need for a modified diet in order to meet caloric demand.
The general approach to address MNT in the inpatient setting is usually based on expert opinions and patient need. There is limited data regarding what is the best approach or method to achieve the ideal caloric supply. To determine the best approach, method, and caloric needs of their patients, providers should work closely with a nutrition professional.
All patients with diabetes or hyperglycemia should receive an individualized assessment. In general, most patients will receive adequate caloric needs with 3 discrete meals per day. Further, the metabolic need for patients with diabetes is usually provided by 25 to 35 calories/kg where some critically ill patients might require less than 15 to 25 calories/kg per day (191). A consistent carbohydrate meal-planning system might help to facilitate glycemic control and insulin dosing in the inpatient setting. Most patients will require 1,500-2000 calories per day with 12-15 grams of carbohydrates per meal (15). Ideally, the carbohydrates should come from low glycemic index foods such as whole grains and vegetables.
Those individuals not able to achieve these goals should be evaluated in order to determine the need for enteral or parenteral nutrition. Enteral nutrition is the second-best option after oral nutrition and should be preferred over parenteral nutrition in hospitalized individuals (192,193). There are several advantages of enteral feeding versus parenteral feeding including: low cost, low risk of complications, physiologic route, less risk for gastric mucosa atrophy, and lower risk of infectious and thrombotic complications compared with the latter form of therapy (192,194). The benefit of parental nutrition has been documented in the critically ill patient. However, some research has shown a detrimental effect on patients with diabetes and hyperglycemia. Parental nutrition should be considered only in patients that are not able to receive enteral nutrition and should be coordinated with the institution parenteral nutrition team.
Enteral and parenteral nutrition can prevent the effects of starvation and malnutrition (192). The preference for use of EN over PN whenever possible is due to a lower risk of infectious and thrombotic complications (194,195). Standard enteral formulas reflect the reference values for macro- and micronutrients for a healthy population and contain 1-2 cal/ml. Most standard formulas contain whole protein, lipid in the form of long-chain triglycerides, and carbohydrates. Standard diabetes-specific formulas provide low amounts of lipids (30% of total calories) combined with a high carbohydrates (HCH) content (55–60% of total calories); however, newer diabetic formulas have replaced part of the carbohydrates with monounsaturated fatty acids (up to 35% of total calories) and also include 10-15 g/l dietary fiber and up to 30% fructose (196,197).
Diabetic enteral formulas containing low-carbohydrate high–monounsaturated fatty acids (LCHM) are preferable to standard high-carbohydrate formulas in hospitalized patients with type 1 and type 2 diabetes (196,198). A meta-analysis of studies comparing relatively newer enteral low-carbohydrate high-monounsaturated fatty acid (LCHM) formulas with older formulations, the postprandial rise in blood glucose was reduced by 18- 29 mg/dl with the newer formulations (198). Table 5 depicts the composition of standard and diabetic specific enteral formulas commonly used in hospitalized patients.
|
Table 5. Composition of Standard and Diabetic Specific Enteral Formulas Commonly Used in Hospitalized Patients in the USA |
|||||||
|
|
Calories (kcal/mL) |
Carbohydrate (g/l) |
Fat (g/l) |
Protein (g/l) |
Manufacture |
||
|
Standard formula |
|
||||||
|
Jevity® 1.0 Cal |
1.0 |
155 |
35 |
44 |
Abbott Nutrition |
||
|
Nutren® 1.0 |
1.0 |
127 |
38 |
40 |
Nestle Nutrition |
||
|
Osmolite® 1.2 Cal |
1.2 |
158 |
39 |
55 |
Abbott Nutrition |
||
|
Jevity® 1.2 |
1.2 |
169 |
39 |
56 |
Nestle Nutrition |
||
|
Fibersource® HN |
1.2 |
160 |
39 |
53 |
Nestle Nutrition |
||
|
Isosource® 1.5 Cal |
1.5 |
170 |
65 |
68 |
Nestle Nutrition |
||
|
Jevity® 1.5 |
1.5 |
216 |
50 |
64 |
Nestle Nutrition |
||
|
Diabetes specific formula |
|||||||
|
Glucerna® 1.0 Cal |
1.0 |
96 |
54 |
42 |
Abbott Nutrition |
||
|
Nutren® Glytrol® |
1.0 |
100 |
48 |
45 |
Nestle Nutrition |
||
|
Glucerna® 1.2 Cal |
1.2 |
115 |
60 |
60 |
Abbott Nutrition |
||
|
Diabetisource® AC |
1.2 |
100 |
59 |
60 |
Nestle Nutrition |
||
|
Glucerna® 1.5 Cal |
1.5 |
133 |
75 |
82 |
Abbott Nutrition |
||
The UK Joint British Diabetes Societies has produced specific guidelines for the management of diabetes in those who are parenterally fed (193).
Corticosteroid Therapy – Impact on Blood Glucose
Steroid use in hospitalized patients is common. A single center cross sectional study showed that 12.8% of all the people in the hospital were on glucocorticoids (199). Steroids may be administered by various regimes and at variable doses. A single daily dose of steroid (e.g., prednisolone/prednisone) in the morning may be the commonest mode of administration (177,199,200). In susceptible individuals, this will often result in a rise in blood glucose by late morning that continues through to the evening. Overnight the blood glucose generally often falls back to baseline levels by the next morning. Thus, treatment should be tailored to treating the hyperglycemia, while avoiding nocturnal and early morning hypoglycemia. Multiple daily doses of steroid, be it intravenous hydrocortisone or oral dexamethasone, can cause a hyperglycemic effect throughout the 24-hour period. It may be, however that a twice daily premixed or basal bolus regimen may need to be started if oral medication, or once daily insulin proves insufficient to control hyperglycemia. Close attention will therefore need to be paid to blood glucose monitoring and early intervention may be necessary.
Glucose levels in most individuals can be predicted to rise approximately 4 to 8 hours following the administration of oral steroids, and sooner following the administration of intravenous steroids. Again, capillary blood glucose monitoring is paramount to guide appropriate therapeutic interventions. Conversely, glucose levels may improve to pre-steroid levels 24 hours after intravenous steroids are discontinued. If oral steroids are weaned down over several weeks, the glucose levels may decline in a dose dependent fashion, but this may not occur, particularly in those with pre-existing undiagnosed diabetes.
At the commencement of steroid therapy, or for those already on a supraphysiological dose of corticosteroid, capillary blood glucose testing should occur before meals and at bedtime, in particular before lunch or evening meal, when the hyperglycemic effects of a morning dose of steroid is likely to be greatest.
It is likely that subcutaneous insulin using a basal, or multiple daily injection regimen will be the most appropriate choice to achieve glycemic control in the event of hyperglycemia for the majority of patients. Morning administration of basal human insulin may closely fit the glucose excursion induced by a single morning dose of oral steroid. Basal analogue insulin may be appropriate if hyperglycemia is present for more prolonged periods. However, care should be taken to identify and protect against hypoglycemia overnight and in the early morning if long-acting insulin analogues are used in this context. Subsequent titration of the insulin dose may be required to allow maintenance of glucose control in the face of increasing or decreasing steroid dose.
When a patient is discharged from the hospital on steroid therapy, a clear strategy for the management of hyperglycemia or potential hyperglycemia, and the titration of therapy to address the hyperglycemia, should be communicated to the community diabetes team and primary care team. Patients commenced on steroids as an inpatient and discharged after a short stay with the intention of continuing high dose steroids, should receive standard education in regard to diabetes, encompassing the risks associated with hyperglycemia and hypoglycemia.
Closed-Loop Technology
Recent studies have reported that closed-loop systems, also referred to as artificial pancreas or automated insulin delivery systems have reported good efficacy with improved time in target and lower mean daily blood glucose without an increased rate of hypoglycemia in the ICU (201,202) and in non-ICU settings (203,204). In one non-ICU study, the time in target range between 100-180 mg/dl (5.6-10.0 mmol/l) was reported to be 59.8% in patients using the closed-loop technology compared to 38.1% with standard subcutaneous insulin regimen (204). Similarly, a closed-loop study in patients receiving nutritional support also reported higher time in target glucose range (68% vs 36.4%) and lower mean glucose values (153 vs 205 mg/dl [8.5-11.4 mmol/l]) compared to a standard insulin regimen (205). Same as the use of CGM in the hospital, treatment with artificial pancreas is experimental and larger studies are needed to prove safety and efficacy in ICU and non-ICU settings.
REFERENCES
- Federation ID. IDF atlas 9th edition. 2019; https://www.diabetesatlas.org/en/.
- Prevention CfDCa. National diabetes statistics report 2020. Estimates of diabetes and its burden in the United States. 2020; https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics 2010; 8:29
- Pournaras DJ, Photi ES, Barnett N, Challand CP, Chatzizacharias NA, Dlamini NP, Doulias T, Foley A, Hernon J, Kumar B, Martin J, Nunney I, Panagiotopoulou I, Sengupta N, Shivakumar O, Sinclair P, Stather P, Than MM, Wells AC, Xanthis A, Dhatariya K. Assessing the quality of primary care referrals to surgery of patients with diabetes in the East of England: A multi-centre cross-sectional cohort study. International Journal of Clinical Practice 2017; 71:e12971
- Jiang HJ, Stryer D, Friedman B, Andrews R. Multiple hospitalizations for patients with diabetes. Diabetes Care 2003; 26:1421-1426
- Donnan PT, Leese GP, Morris AD. Hospitalizations for people with type 1 and type 2 diabetes compared with the nondiabetic population of Tayside, Scotland: a retrospective cohort study of resource use. Diabetes Care 2000; 23:1774-1779
- Harding JL, Benoit SR, Gregg EW, Pavkov ME, Perreault L. Trends in rates of infections requiring hospitalization among adults with versus without diabetes in the U.S., 2000 - 2015. Diabetes Care 2020; 43:106-116
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. Journal of Clinical Endocrinology Metabolism 2002; 87:978-982
- Digital N. National Diabetes Inpatient Audit (NaDIA) - 2018. 2019; https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/2018.
- Sampson MJ, Dozio N, Ferguson B, Dhatariya K. Total and excess bed occupancy by age, speciality and insulin use for nearly one million diabetes patients discharged from all English Acute Hospitals. Diabetes Research & Clinical Practice 2007; 77:92-98
- Association AD. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018; 41:917-928
- Prevention CfDCa. National vital statistics report. Deaths: Final data for 2017. 2019; 9:https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf, 68.
- Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Barnighausen T, Vollmer S. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes and Endocrinology 2017; 5:423-430
- Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Barnighausen T, Davies J, Vollmer S. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care 2018; 41:963-970
- Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, Van den Berghe G. Management of hyperglycemia in hospitalized patients in non-critical care setting: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology Metabolism 2012; 97:16-38
- Moghissi ES, Korytkowski MT, Dinardo MM, Hellman R, Hirsch IB, Inzucchi S, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32:1119-1131
- Dhatariya K, Mustafa OG, Rayman G. Safe care for people with diabetes in hospital. Clinical Medicine 2020; 20:21-27
- Sampson M. A good inpatient diabetes service. https://abcd.care/sites/abcd.care/files/resources/A_good_Inpatient%20Service_FINAL_Aug_19.pdf 2019; https://abcd.care/sites/abcd.care/files/resources/A_good_Inpatient%20Service_FINAL_Aug_19.pdf.
- investigators NSs. Intensive versus conventional glucose control in critically ill patients. New England Journal of Medicine 2009; 360:1283-1297
- Hulkower RD, Pollack RM, Zonszein J. Understanding hypoglycemia in hospitalized patients. Diabetes Management (Lond) 2014; 4:165-176
- McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinology & Metabolism Clinics of North America 2012; 41:175-201
- Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P, Physicians ftCGCotACo. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine 2011; 154:260-267
- Cook CB, Kongable GL, Potter DJ, Abad VJ, Leija DE, Anderson M. Inpatient glucose control: a glycemic survey of 126 U.S. hospitals. Journal of Hospital Medicine 2009; 4:E7-E14
- investigators NSs. Hypoglycemia and risk of death in critically ill patients. New England Journal of Medicine 2012; 367:1108-1118
- Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009; 32:1153-1157
- Yamada T, Shojima N, Noma H, Yamauchi T, Kadowaki T. Glycemic control, mortality, and hypoglycemia in critically ill patients: a systematic review and network meta-analysis of randomized controlled trials. Intensive Care Medicine 2017; 43:1-15
- Levy N, Hall GM. National guidance contributes to the high incidence of inpatient hypoglycaemia. Diabetic Medicine 2019; 36:120-121
- Digital N. National Diabetes Inpatient Audit - Harms, 2019. 2019; https://digital.nhs.uk/data-and-information/clinical-audits-and-registries/national-diabetes-in-patient-audit-nadia-harms.
- Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, Blonde L, Bray GA, Cohen AJ, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda AP, Garber AJ, Garvey WT, Henry RR, Hirsch IB, Horton ES, Hurley DL, Jellinger PS, Jovanovic L, Lebovitz HE, LeRoith D, Levy P, McGill JB, Mechanick JI, Mestman JH, Moghissi ES, Orzeck EA, Pessah-Pollack R, Rosenblit PD, Vinik AI, Wyne K, Zangeneh F. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocrine Practice 2015; 21:1-87
- Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Duutschman C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Critical Care Medicine 2012; 40:3251-3276
- Association AD. Diabetes care in the hospital: Standards of medical care in diabetes - 2020. Diabetes Care 2020; 43:S193-S202
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009; 32:1335-1343
- Swanson C, Potter D, Kongable G, Cook C. Update on inpatient glycemic control in hospitals in the United States. Endocrine Practice 2017; 17:853-861
- Schmeltz LR, DeSantis AJ, Thiyagarajan V, Schmidt K, Shea-Mahler E, Johnson D, Henske J, McCarthy PM, Gleason TG, McGee EC, Molitch ME. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care 2007; 30:823-828
- Carpenter DL, Gregg SR, Xu K, Buchman TG, Coopersmith CM. Prevalence and impact of unknown diabetes in the ICU. Critical Care Medicine 2015; 43:e541-e550
- Van Ackerbroeck S, Schepens T, Janssens K, Jorens PG, Verbrugge W, Collet S, Van Hoof V, Van Gaal L, De Block C. Incidence and predisposing factors for the development of disturbed glucose metabolism and DIabetes mellitus AFter Intensive Care admission: the DIAFIC study. Critical Care 2015; 19:355
- Kar P, Plummer MP, Ali Abdelhamid Y, Giersch EJ, Summers MJ, Weinel LM, Finnis ME, Phillips LK, Jones KL, Horowitz M, Deane AM. Incident diabetes in survivors of critical illness and mechanisms underlying persistent glucose intolerance: A prospective cohort study. Critical Care Medicine 2019; 47:e103-e111
- Dhatariya K, James J, Kong M-F, Berrington R. Diabetes at the front door. A guideline for dealing with glucose related emergencies at the time of acute hospital admission from the Joint British Diabetes Society (JBDS) for Inpatient Care Group. Diabetic Medicine 2020; 37:1578-1589
- Pasquel FJ, Gomez-Huelgas R, Anzola I, Oyedokun F, Haw JS, Vellanki P, Peng L, Umpierrez GE. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care 2015; 38:e202-e203
- Greci LS, Kailasam M, Malkani S, Katz DL, Hulinsky I, Ahmadi R, Nawaz H. Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care 2003; 26:1064-1068
- Mazurek JA, Hailpern SM, Goring T, Nordin C. Prevalence of hemoglobin A1c greater than 6.5% and 7.0% among hospitalized patients without known diagnosis of diabetes at an urban inner city hospital. Journal of Clinical Endocrinology & Metabolism 2010; 95:1344-1348
- Cryer PE. Hypoglycemia, functional brain failure, and brain death. Journal of Clinical Investigation 2014; 117:868-870
- Amiel SA, Aschner P, Childs B, Cryer PE, de Galan BE, Frier BM, Gonder-Frederick L, Heller SR, Jones T, Khunti K, Leiter LA, Luo Y, McCrimmon RJ, Pedersen-bjergaard U, Seaquist ER, Zoungas S. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes & Endocrinology 2019; 7:385-396
- Corssmit EP, Romijn JA, Sauerwein HP. Regulation of glucose production with special attention to nonclassical regulatory mechanisms: A review. Metabolism 2014; 50:742-755
- Boden G. Gluconeogenesis and glycogenolysis in health and diabetes. Journal of Investigative Medicine 2004; 52:375-378
- van de Werve G, Jeanrenaud B. Liver glycogen metabolism: An overview. Diabetes/Metabolism Reviews 1987; 3:47-78
- Gerich JE, Lorenzi M, Bier DM, Tsalikian E, Schneider V, Karam JH, Forsham PH. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. Journal of Clinical Investigation 1976; 57:875-884
- Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. American Journal of Physiology - Endocrinology & Metabolism 1981; 240:E630-E639
- Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Lancet 2009; 373:1798-1807
- McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Critical Care Clinics 2001; 17:107-124
- Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004; 53:2079-2086
- Chaudhuri A, Umpierrez GE. Oxidative stress and inflammation in hyperglycemic crises and resolution with insulin: implications for the acute and chronic complications of hyperglycemia. Journal of Diabetes and its Complications 2012; 26:257-258
- Reyes-Umpierrez D, Davis G, Cardona S, Pasquel FJ, Peng L, Jacobs S, Vellanki P, Fayfman M, Haw S, Halkos M, Guyton RA, Thourani VH, Umpierrez GE. Inflammation and oxidative stress in cardiac surgery patients treated to intensive versus conservative glucose targets. Journal of Clinical Endocrinology & Metabolism 2016; 102:309-315
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002; 106:2067-2072
- Fan J, Li YH, Wojnar MM, Lang CH. Endotoxin-induced alterations in insulin-stimulated phosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock 1996; 6:164-170
- Evans NR, Dhatariya KK. Assessing the relationship between admission glucose levels, subsequent length of hospital stay, readmission and mortality. Clinical Medicine, Journal of the Royal College of Physicians 2012; 12:137-139
- Haddadin F, Clark A, Evans N, Dhatariya K. Admission blood glucose helps predict 1 year, but not 2 years, mortality in an unselected cohort of acute general medical admissions. International Journal of Clinical Practice 2014; 69:643-648
- Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33:1783-1788
- Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, Flum DR. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Annals of Surgery 2016; 261:97-103
- Umpierrez G, Cardona S, Pasquel F, Jacobs S, Peng L, Unigwe M, Newton CA, Smiley-Byrd D, Vellanki P, Halkos M, Puskas JD, Guyton RA, Thourani VH. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015; 38:1665-1672
- Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 Surgery). Diabetes Care 2011; 34:256-261
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proceedings 2003; 78:1471-1478
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Annals of Thoracic Surgery 1999; 67:352-362
- Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA 2002; 288:2167-2169
- Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Critical Care Medicine 2009; 37:3001-3009
- Bruno A, Gregori D, Caropreso A, Lazzarato F, Petrinco M, Pagano E. Normal glucose values are associated with a lower risk of mortality in hospitalized patients. Diabetes Care 2008; 31:2209-2210
- Blaha J, Mraz M, Kopecky P, Stritesky M, Lips M, Matias M, Kunstyr J, Porizka M, Kotulak T, Kolnikova I, Simanovska B, Zakharchenko M, Rulisek J, Sachl R, Anyz J, Novak D, Linder J, Hovorka R, Svacina S, Haluzik M. Perioperative tight glucose control reduces postoperative adverse events in nondiabetic cardiac surgery patients. Journal of Clinical Endocrinology & Metabolism 2015; 100:3081-3089
- McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care 2005; 28:810-815
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radical Biology and Medicine 2011; 50:567-575
- Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. Journal of Thoracic & Cardiovascular Surgery 2003; 125:1007-1021
- Krinsley JS, Maurer P, Holewinski S, Hayes R, McComsey D, Umpierrez GE, Nasraway SA. Glucose control, diabetes status, and mortality in critically ill patients. Mayo Clinic Proceedings 2017; 92:1019-1029
- van Vught LA, Holman R, de Jong E, de Keizer NF, van der Poll T. Diabetes is not associated with increased 90-day mortality risk in critically ill patients with sepsis. Critical Care Medicine 2017; 45:e1026-e1035
- Vanhorebeek I, Gunst J, Van den Berghe G. Critical care management of stress-induced hyperglycemia. Current Diabetes Reports 2018; 18:17
- Baker EH, Janaway CH, Philips BJ, Brennan AL, Baines DL, Wood DM, Jones PW. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax 2006; 61:284-289
- Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, Bertran E, Jaber L. Diabetes and risk of surgical site infection: A systematic review and meta-analysis. Infection Control & Hospital Epidemiology 2016; 37:88-99
- Noordzij PG, Boersma E, Schreiner F, Kertai MD, Feringa HH, Dunkelgrun M, Bax JJ, Klein J, Poldermans D. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. European Journal of Endocrinology 2007; 156:137-142
- Pomposelli JJ, Baxter JK, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. Journal of Parenteral and Enteral Nutrition 1998; 22:77-81
- Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M, Rogers SO. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Annals of Surgery 2015; 248:585-591
- Sadoskas D, Suder NC, Wukich DK. Perioperative glycemic control and the effect on surgical site infections in diabetic patients undergoing foot and ankle surgery. Foot and Ankle Specialist 2016; 9:24-30
- Wallia A, Schmidt K, Oakes DJ, Pollack T, Welsh N, Kling-Colson S, Gupta S, Fulkerson C, Aleppo G, Parikh N, Levitsky J, Norvell JP, Rademaker A, Molitch ME. Glycemic control reduces infections in post-liver transplant patients: Results of a prospective, randomized study. Journal of Clinical Endocrinology & Metabolism 2017; 102:451-459
- Kyi M, Colman PG, Wraight PR, Reid J, Gorelik A, Galligan A, Kumar S, Rowan LM, Marley KA, Nankervis AJ, Russell DM, Fourlanos S. Early intervention for diabetes in medical and surgical inpatients decreases hyperglycemia and hospital-acquired infections: A cluster randomized trial. Diabetes Care 2019; 42:832-840
- Sheen YJ, Huang CC, Huang SC, Hung MD, Lin CH, Lee IT, Lin SY, Sheu WH. Implimentation of an electronic dashboard with a remote management system to improve glycemic management among hospitalized adults. Endocrine Practice 2020; 26:179-191
- Garg R, Schuman B, Bader A, Hurwitz S, Turchin A, Underwood P, Metzger C, Rein R, Lortie M. Effect of preoperative diabetes management on glycemic control and clinical outcomes after elective surgery. Annals of Surgery 2018; 267:858-862
- Gregory NS, Seley JJ, Ukena J, Shah S, Fred MR, Dargar SK, Mauer E, Kim RJ. Decreased rates of inpatient hypoglycemia following implementation of an automated tool in the electronic medical record for identifying root causes. Journal of Diabetes Science and Technology 2018; 12:63-68
- Wang YY, Hu SF, Ying HM, Chen L, Li HL, Tian F, Zhou ZF. Postoperative tight glycemic control significantly reduces postoperative infection rates in patients undergoing surgery: A meta-analysis. BMC Endocrine Disorders 2018; 18:42
- Yamamoto JM, Murphy HR. Inpatient hypoglycaemia; should we should we focus on the guidelines, the targets or our tools? Diabetic Medicine 2019; 36:122-123
- Dashora U, George S, Sampson M, Walden E. National guidelines have contributed to safer care for inpatients with diabetes. Diabetic Medicine 2019; 36:124-126
- Wernerman J, Desaive T, Finfer S, Foubert L, Furnary A, Holzinger U, Hovorka R, Joseph J, Kosiborod M, Krinsley J, Mesotten D, Nasraway S, Rooyackers O, Schultz M, Van Herpe T, Vigersky R, Preiser JC. Continuous glucose control in the ICU: report of a 2013 round table meeting. Critical Care 2014; 18:226
- Amrein K, Ellmerer M, Hovorka R, Kachel N, Fries H, von Lewinski D, Smolle K, Pieber TR, Plank J. Efficacy and safety of glucose control with space GlucoseControl in the medical intensive care unit - An open clinical investigation. Diabetes Technology & Therapeutics 2012; 14:690-695
- Leelarathna L, English SW, Thabit H, Caldwell K, Allen JM, Kumareswaran K, Wilinska ME, Nodale M, Mangat J, Evans ML, Burnstein R, Hovorka R. Feasibility of fully automated closed-loop glucose control using continuous subcutaneous glucose measurements in critical illness: a randomized controlled trial. Critical Care 2013; 17:R159
- Evans K, Green E, Hudson B, Stewart M, Evans M, Gregory R, Wilmot E, Soloman A, Karamat M, Narendran P. Clinical Guideline: Guidelines for managing continuous subcutaneous insulin infusion (CSII, or 'insulin pump') therapy in hospitalised patients. 2019; https://abcd.care/sites/abcd.care/files/CSII_DTN_FINAL%20210218.pdf.
- Wang M, Singh LG, Spanakis EK. Advancing the use of CGM devices in a non-ICU setting. Journal of Diabetes Science and Technology 2019; 13:674-681
- Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadhu AR, Bridges CR, Haan CK, Svedjeholm R, Taegtmeyer H, Shemin RJ. The Society of Thoracic Surgeons Practice Guideline Series: Blood glucose management during adult cardiac surgery. Annals of Thoracic Surgery 2009; 87:663-669
- Group JBDSIC. Guidelines for the management of inpatient diabetes. 2019; https://abcd.care/joint-british-diabetes-societies-jbds-inpatient-care-group.
- UK D. End of life diabetes care. 2018; https://www.diabetes.org.uk/resources-s3/2018-03/EoL_Guidance_2018_Final.pdf.
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Bouillon R, Lauwers P. Intensive insulin therapy in critically ill patients. New England Journal of Medicine 2001; 345:1359-1367
- Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. New England Journal of Medicine 2006; 354:449-461
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. New England Journal of Medicine 2008; 358:125-139
- De La Rosa G, Donado JH, Restrepo AH, Quintero AM, Gonzalez LG, Saldarriaga NE, Bedoya M, Toro JM, Velasquez JB, Valencia JC, Arango CM, Aleman PH, VAsquez EM, Chavarriaga JC, Yepes A, Pulido W, Cadavid CA. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Critical Care 2008; 12:R120
- Preiser J-C, Brunkhorst F. Tight glucose control and hypoglycemia. Critical Care Medicine 2008; 36:1391-1392
- Hall L, Fisher M. Aspirin for primary prevention in diabetes? Practical Diabetes International 2009; 26:6-8
- Marik PE. Tight glycemic control in acutely ill patients: low evidence of benefit, high evidence of harm! Intensive Care Medicine 2016; 42:1475-1477
- Preiser J-C, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve XM, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chiolero R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Medicine 2009; 35:1738-1748
- Malmberg KA, Efendic S, Ryden LE. Feasibility of insulin-glucose infusion in diabetic patients with acute myocardial infarction: A report from the multicenter trial: DIGAMI. Diabetes Care 1994; 17:1007-1014
- Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 2004; 109:1497-1502
- Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: A randomized trial. Annals of Internal Medicine 2007; 146:233-243
- Agus MS, Steil GM, Wypij D, Costello JM, Laussen PC, Langer M, Alexander JL, Scoppettuolo LA, Pigula FA, Charpie JR, Ohye RG, Gaies MG. Tight glycemic control versus standard care after pediatric cardiac surgery. New England Journal of Medicine 2012; 367:1208-1219
- Agus MS, Wypij D, Hirshberg EL, Srinivasan V, Faustino EV, Luckett PM, Alexander JL, Asaro LA, Curley MA, Steil GM, Nadkarni VM. Tight glycemic control in critically ill children. New England Journal of Medicine 2017; 376:729-741
- Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J, Parslow R, Tasker RC, Elbourne D. A randomized trial of hyperglycemic control in pediatric intensive care. New England Journal of Medicine 2014; 370:107-118
- Kalfon P, Giraudeau B, Ichai C, Guerrini A, Brechot N, Cinotti R, Dequin P-F, Riu-Poulenc B, Montravers P, Annane D, Dupont H, Sorine M, Riou B. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Medicine 2014; 40:171-181
- Cinotti R, Ichai C, Orban JC, Kalfon P, Feuillet F, Roquilly A, Riou B, Blanloeil Y, Asehnoune K, Rozec B. Effects of tight computerized glucose control on neurological outcome in severely brain injured patients: A multicenter sub-group analysis of the randomized-controlled open-label CGAO-REA study. Critical Care 2014; 18:498
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, Iiyama T, Sugimoto T, Kobayashi M, Yokoyama M, Hanazaki K. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 2014; 37:1516
- Cardona S, Pasquel FJ, Fayfman M, Peng L, Jacobs S, Vellanki P, Weaver J, Halkos M, Guyton RA, Thourani VH, Umpierrez GE. Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. Journal of Diabetes and its Complications 2017; 31:742-747
- Dhatariya K. Should inpatient hyperglycaemia be treated? British Medical Journal 2013; 346:f134
- Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. PharmacoEconomics - Open 2017; 1:109-115
- Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. Evaluation and management of adult hypoglycemic disorders: An Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism 2009; 94:709-728
- Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: A report of a workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care 2013; 36:1384-1395
- Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Current Opinion in Clinical Nutrition & Metabolic Care 2010; 13:198-204
- Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181-2186
- Rajendran R, Kerry C, Rayman G, group obotMs. Temporal patterns of hypoglycaemia and burden of sulfonylurea-related hypoglycaemia in UK hospitals: a retrospective multicentre audit of hospitalised patients with diabetes. BMJ Open 2014; 4
- Digital N. National Diabetes Inpatient Audit (NaDIA) - 2017. 2018; https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017.
- Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: Risk factors and outcomes. Critical Care Medicine 2007; 35:2262-2267
- Kagansky N, Levy S, Rimon E. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Archives of Internal Medicine 2003; 163:1825-1829
- Stanisstreet D, Walden E, Jones C, Graveling A, Amiel SA, Crowley C, Dhatariya K, Frier B, Malik R. The hospital management of hypoglycaemia in adults with diabetes mellitus. 2020. https://abcd.care/resource/hospital-management-hypoglycaemia-adults-diabetes.
- Smith WD, Winterstein AG, Johns T, Rosenberg E, Sauer BC. Causes of hyperglycemia and hypoglycemia in adult inpatients. American Journal of Health-System Pharmacy 2005; 62:714-719
- Maynard G, Lee J, Phillips G, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: Effect of structured subcutaneous insulin orders and an insulin management algorithm. Journal of Hospital Medicine 2009; 4:3-15
- Agency NPS. Insulin safety. Reducing harm associated with the unsafe use of insulin products. 2010; http://www.patientsafetyfirst.nhs.uk/Content.aspx?path=/interventions/relatedprogrammes/medicationsafety/insulin/
- Dendy J, Chockalingam V, Tirumalasetty N, Dornelles A, Blonde L, Bolton P, Meadows R, Andrews S. Identifying risk factors for severe hypoglycemia in hospitalized patients with diabetes. Endocrine Practice 2014; 20:1051-1056
- Lake A, Arthur A, Byrne C, Davenport K, Yamamoto JM, Murphy HR. The effect of hypoglycaemia during hospital admission on health-related outcomes for people with diabetes: a systematic review and meta-analysis. Diabetic Medicine 2019; 36:1349-1359
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. Canadian Medical Association Journal 2009; 180:821-827
- Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S. Severe hypoglycemia and risks of vascular events and death. New England Journal of Medicine 2010; 363:1410-1418
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA 2008; 300:933-944
- Kosiborod M, Inzucchi S, Goyal A, Krumholz HM, Masoudi FA, Xiao L, Spertus JA. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009; 301:1556-1564
- Arabi YM, Tamin HM, Rishu AH. Hypoglycemia with intensive insulin therapy in critically ill patients: Predisposing factors and association with mortality. Critical Care Medicine 2009; 37:2536-2544
- Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jong E, Roos YB, Schultz MJ, Rosendaal FR, Hoekstra JB. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Critical Care Medicine 2006; 34:2714-2718
- Krinsley JS, Schultz MJ, Spronk PE, Harmsen RE, van Braam Houckgeest F, van der Sluijs JP, Melot C, Preiser JC. Mild hypoglycemia is independently associated with increased mortality in the critically ill. Critical Care 2011; 15:R173
- Digital N. National Diabetes Inpatient Audit (NaDIA) - 2016. 2017; http://www.content.digital.nhs.uk/catalogue/PUB23539.
- Dashora U, Sampson M, Castro E, Stanisstreet D, Hillson R. The best hypoglycaemia avoidance initiative in the UK. British Journal of Diabetes 2017; 17:74-77
- Gomez AM, Umpierrez GE. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. Journal of Diabetes Science and Technology 2014; 8:930-936
- Cardona S, Gomez PC, Vellanki P, Anzola I, Ramos C, Urrutia MA, Haw JS, Fayfman M, Wang H, Galindo RJ, Pasquel FJ, Umpierrez GE. Clinical characteristics and outcomes of symptomatic and asymptomatic hypoglycemia in hospitalized patients with diabetes. BMJ Open Diabetes Research & Care 2018; 6:e000607
- Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes—the 'dead in bed' syndrome revisited. Diabetologia 2009; 52:42-45
- Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia. Diabetes Care 2003; 26:1485-1489
- Petersen KG, Schluter KJ, Kerp L. Regulation of serum potassium during insulin-induced hypoglycemia. Diabetes 1982; 31:615-617
- Razavi Nematollahi L, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, Gozashti MH, Omidfar K, Taheri E. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism - Clinical and Experimental 2009; 58:443-448
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010; 33:1389-1394
- Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation. Diabetes Care 2010; 33:1591-1597
- Rana OA, Byrne CD, Greaves K. Intensive glucose control and hypoglycaemia: a new cardiovascular risk factor? Heart 2014; 100:21-27
- Boucai L, Southern WN, Zonszein J. Hypoglycemia-associated mortality is not drug-associated but linked to comorbidities. American Journal of Medicine 2011; 124:1028-1035
- George JT, Warriner D, McGrane DJ, Rozario KS, Price HC, Wilmot EG, Kar P, Stratton IM, Jude EB, McKay GA, Team obotTDS. Lack of confidence among trainee doctors in the management of diabetes: the Trainees Own Perception of Delivery of Care (TOPDOC) Diabetes Study. QJM 2011; 104:761-766
- Horton WB, Law S, Darji M, Conaway MR, Akbashev MY, Kubiak NT, Kirby JL, Thigpen SC. A multicenter study evaluating perceptions and knowledge of inpatient glycemic control among resident physicians: analyzing themes to inform and improve care. Endocrine Practice 2019; 25:1295-1303
- Brown G, Dodek P. Intravenous insulin nomogram improves blood glucose control in the critically ill. Critical Care Medicine 2001; 29:1714-1719
- Rea RS, Donihi AC, Bobeck M, Herout P, McKaveney TP, Kane-Gill SL, Korytkowski MT. Implementing an intravenous insulin infusion protocol in the intensive care unit. American Journal of Health-System Pharmacy 2007; 64:385-395
- Umpierrez GE, Kelly JP, Navarrete JE, Casals MM, Kitabchi AE. Hyperglycemic crises in urban blacks. Archives of Internal Medicine 1997; 157:669-675
- George S, Dale J, Stanisstreet D. A guideline for the use of variable rate intravenous insulin infusion in medical inpatients. Diabetic Medicine 2015; 32:706-713
- Miller EE, Lalla M, Zaidi A, Elgash M, Zhao H, Rubin DJ. Eating and glycemic control among critically ill patients receiving continuous intravenous insulin. Endocrine Practice 2020; 26:43-50
- Salinas PD, Mendez CE. Glucose management technologies for the critically ill. Journal of Diabetes Science and Technology 2019; 13:682-690
- Newton CA, Smiley D, Bode BW, Kitabchi AE, Davidson PC, Jacobs S, Steed RD, Stentz F, Peng L, Mulligan P, Freire AX, Temponi A, Umpierrez GE. A comparison study of continuous insulin infusion protocols in the medical intensive care unit: Computer-guided vs. standard column-based algorithms. Journal of Hospital Medicine 2010; 5:432-437
- Ullal J, Aloi JA. Subcutaneous insulin dosing calculators for inpatient glucose control. Current Diabetes Reports 2019; 19:120
- Bouw JW, Campbell N, Hull MA, Juneja R, Guzman O, Overholser BR. A retrospective cohort study of a nurse-driven computerized insulin infusion program versus a paper-based protocol in critically ill patients. Diabetes Technology & Therapeutics 2012; 14:125-130
- Juneja R, Roudebush C, Kumar N, Macy A, Golas A, Wall D, Wolverton C, Nelson D, Carroll J, Flanders SJ. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technology & Therapeutics 2007; 9:232-240
- Marvin MR, Inzucchi SE, Besterman BJ. Computerization of the Yale insulin infusion protocol and potential insights into causes of hypoglycemia with intravenous insulin. Diabetes Technology & Therapeutics 2013; 15:246-252
- Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004; 27:461-467
- Boutin JM, Gauthier L. Insulin infusion therapy in critically ill patients. Canadian Journal of Diabetes 2014; 38:144-150
- DeSantis AJ, Schmeltz LR, Schmidt K, O'Shea-Mahler E, Rhee C, Wells A, Brandt S, Peterson S, Molitch ME. Inpatient management of hyperglycemia: The Northwestern experience. Endocrine Practice 2006; 12:491-505
- Davidson PC, Steed RD, Bode BW. Glucommander. A computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care 2005; 28:2418-2423
- Hirsch IB. Sliding scale insulin--time to stop sliding. JAMA 2009; 301:213-214
- Umpierrez GE, Maynard G. Glycemic chaos (not glycemic control) still the rule for inpatient care. How do we stop the insanity? Journal of Hospital Medicine 2006; 1:141-144
- Swan E, Dhatariya K. Differential effects of intravenous and subcutaneous sliding scale insulin regimes used to improve blood glucose levels in a tertiary care setting. Practical Diabetes International 2009; 26:2i
- King AB, Armstrong DU. Basal bolus dosing: A clinical experience. Current Diabetes Reviews 2005; 1:215-220
- Umpierrez GE, Hor T, Smiley D, Temponi A, Umpierrez D, Ceron M, Munoz C, Newton C, Peng L, Baldwin D. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. Journal of Clinical Endocrinology Metabolism 2009; 94:564-569
- Umpierrez GE, Smiley D, Hermayer K, Khan A, Olson DE, Newton C, Jacobs S, Rizzo M, Peng L, Reyes D, Pinzon I, Fereira ME, Hunt V, Gore A, Toyoshima MT, Fonseca VA. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: Basal plus trial. Diabetes Care 2013; 36:2169-2174
- Pietras S, Hanrahan P, Arnold L, Sternthal E, McDonnell M. State-of-the-art inpatient diabetes care: The evolution of an academic hospital. Endocrine Practice 2010; 16:512-521
- Clement S, Bowen-Wright H. Twenty-four hour action of insulin glargine (Lantus) may be too short for once-daily dosing: A case report. Diabetes Care 2002; 25:1479-1147a
- Rubin DJ, Rybin D, Doros G, McDonnell ME. Weight-based, insulin dose-related hypoglycemia in hospitalized patients with diabetes. Diabetes Care 2011; 34:1723-1728
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure. Diabetes Care 2004; 27:256-263
- Willi SM, Kennedy A, Brant BP, Wallace P, Rogers NL, Garvey WT. Effective use of thiazolidinediones for the treatment of glucocorticoid-induced diabetes. Diabetes Research & Clinical Practice 2002; 58:87-96
- Roberts A, James J, Dhatariya K, Care oBotJBDSJfI. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabetic Medicine 2018; 35:1011-1017
- Nirantharakumar K, Marshall T, Kennedy A, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabetic Medicine 2012; 29:e445-e448
- Roumie CL, Greevy RA, Grijalva CG, Hung AM, Liu X, Murff HJ, Elasy TA, Griffin MR. Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA 2014; 311:2288-2296
- Gerards MC, Venema GE, Patberg KW, Kross M, Potter van Loon BJ, Hageman IM, Snijders D, Brandjes DP, Hoekstra JB, Vriesendorp TM, Gerdes VE. Dapagliflozin for prednisone-induced hyperglycemia in acute exacerbation of chronic obstructive pulmonary disease. Diabetes, Obesity and Metabolism 2018; 20:1306-1310
- Umpierrez GE, Gianchandani R, Smiley D, Wesorick DH, Newton C, Farrokhi F, Peng L, Lathkar-Pradhan S, Pasquel F. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: A pilot, randomized, controlled study. Diabetes Care 2013; 36:3430-3435
- Pasquel FJ, Gianchandani R, Rubin DJ, Dungan KM, Anzola I, Gomez PC, Peng L, Hodish I, Bodnar T, Wesorick D, Balakrishnan V, Osei K, Umpierrez GE. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes and Endocrinology 2017; 5:125-133
- Lorenzo-Gonzalez C, Atienza-Sanchez E, Reyes-Umpierrez D, Vellanki P, Davis GM, Pasquel FJ, Cardona S, Fayman M, Peng L, Umpierrez GE. Safety and efficacy of DDP-4 inhibitors for management of hospitalized general medicine and surgery patients with type 2 diabetes. Endocrine Practice 2020; 26:722-728
- Rice MJ, Smith JL, Coursin DB. Glucose measurement in the ICU: Regulatory intersects reality. Critical Care Medicine 2017; 45:741-743
- Pilackas K, El-Oshar S, Carter C. Clinical reliability of point-of-care glucose testing in critically ill patients. Journal of Diabetes Science and Technology 2020; 14:65-69
- Gomez AM, Umpierrez GE, Munoz OM, Herrera F, Rubio C, Aschner P, Buendia R. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. Journal of Diabetes Science and Technology 2016; 10:325-329
- Schaupp L, Donsa K, Neubauer KM, Mader JK, Aberer F, Holl B, Spat S, Augustin T, Beck P, Pieber TR, Plank J. Taking a closer look: Continuous glucose monitoring in non-critically ill hospitalized patients with type 2 diabetes mellitus under basal-bolus insulin therapy. Diabetes Technology & Therapeutics 2015; 17:611-618
- Davis GM, Galindo RJ, Migdal AL, Umpierrez GE. Diabetes technology in the inpatient setting for management of hyperglycemia. Endocrinology and Metabolism Clinics 2020; 49:79-93
- Galindo RJ, Migdal AL, Davis GM, Urrutia MA, Albury B, Zambrano C, Vellanki P, Pasquel FJ, Fayfman M, Peng L, Umpierrez GE. Comparison of the FreeStyle Libre Pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing (POC) in hospitalized patients with type 2 diabetes (T2D) treated with basal-bolus insulin regimen. Diabetes Care 2020; 43:2730-2735
- Singh LG, Satyarengga M, Marcano I, Scott WH, Pinault LF, Feng Z, Sorkin JD, Umpierrez GE, Spanakis EK. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: The glucose telemetry system, a randomized clinical trial. Diabetes Care 2020; 43:2736-2743
- Gosmanov AR, Umpierrez GE. Medical nutrition therapy in hospitalized patients with diabetes. Current Diabetes Reports 2012; 12:93-100
- McMahon MM, Nystrom E, Braunschweig C, Miles J, Compher C, Nutrition ASfPaE. A.S.P.E.N. clinical guidelines: Nutrition support of adult patients with hyperglycemia. Journal of Parenteral and Enteral Nutrition 2013; 37:23-36
- Roberts AW, Penfold S, Care obotJBDSJfI. Glycaemic management during the inpatient enteral feeding of people with stroke and diabetes. Diabetic Medicine 2018; 35:1027-1036
- Schafer RG, Bohannon B, Franz M, Freeman J, Holmes A, McLaughlin S, Haas LB, Kruger DF, Lorenz RA, McMahon MM. Translation of the diabetes nutrition recommendations for health care institutions. Diabetes Care 1997; 20:96-105
- McMahon MM, Rizza RA. Nutrition support in hospitalized patients with diabetes mellitus. Mayo Clinic Proceedings 1996; 71:587-594
- Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser J-C, van Zanten AR, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clinical Nutrition 2019; 38:48-79
- Via MA, Mechanick JI. Inpatient enteral and parental nutrition for patients with diabetes. Current Diabetes Reports 2011; 11:99-105
- Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: A systematic review and meta-analysis. Diabetes Care 2005; 28:2267-2279
- Narwani V, Swafe L, Stavraka C, Dhatariya K. How frequently are bedside glucose levels measured in hospital inpatients on glucocorticoids? Clinical Medicine 2014; 14:327-328
- Sudlow A, O'Connor HM, Narwani V, Swafe L, Dhatariya K. Assessing the prevalence of dexamethasone use in patients undergoing surgery, and subsequent glucose measurements: a retrospective cohort study. Practical Diabetes 2017; 34:117-121
- Yatabe T, Yamazaki R, Kitagawa H, Okabayashi T, Yamashita K, Hanazaki K, Yokoama M. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Critical Care Medicine 2011; 39:575-578
- Levitt DL, Spanakis EK, Ryan KA, Silver KD. Insulin pump and continuous glucose monitor initiation in hospitalized patients with type 2 diabetes mellitus. Diabetes Technology & Therapeutics 2018; 20:32-38
- Bally L, Thabit H, Hartnell S, Andereggen E, Ruan Y, Wilinska ME, Evans ML, Wertli MM, Coll AP, Stettler C, Hovorka R. Closed-loop insulin delivery for glycemic control in noncritical care. New England Journal of Medicine 2018; 379:547-556
- Thabit H, Hartnell S, Allen JM, Lake A, Wilinska ME, Ruan Y, Evans ML, Coll AP, Hovorka R. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. Lancet Diabetes & Endocrinology 2020; 5:117-124
- Boughton CK, Bally L, Martignoni F, Hartnell S, Herzig D, Vogt A, Wertli MM, Wilinska ME, Evans ML, Coll AP, Stettler C, Hovorka R. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial. Lancet Diabetes & Endocrinology 2019; 7:368-377