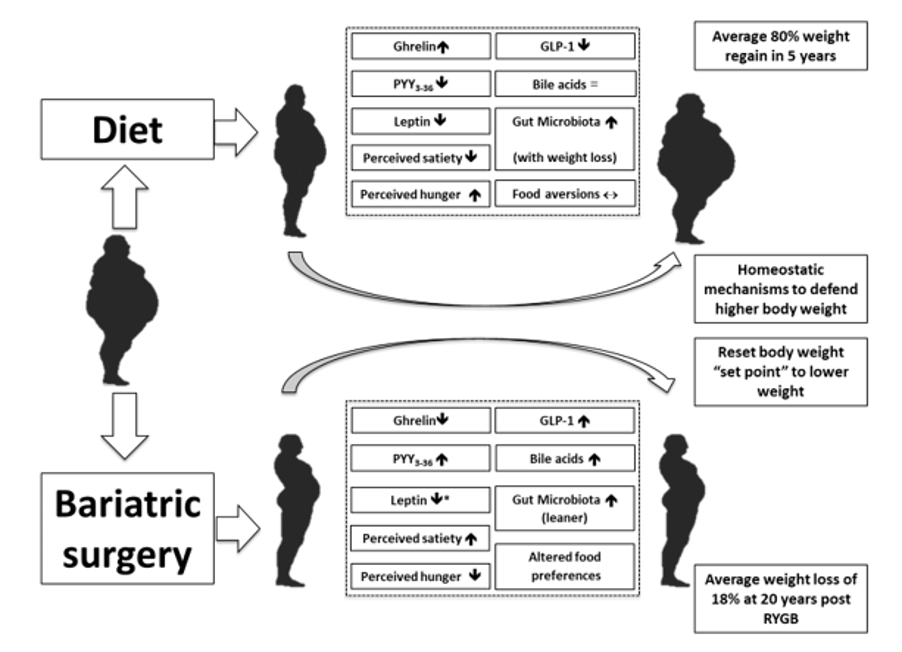
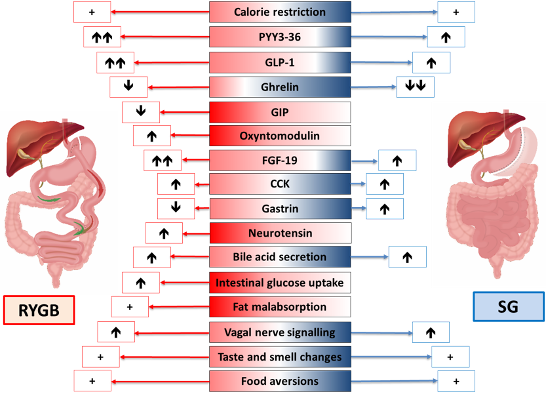
ABSTRACT
The chromosomal sex of the embryo is established at fertilization. However, 6 weeks elapse in humans before the first signs of sex differentiation are noticed. Sex differentiation involves a series of events whereby the sexually indifferent gonads and genitalia progressively acquire male or female characteristics. Believed initially to be governed entirely by the presence or absence of the SRY gene on the Y chromosome, gonadal determination has proven to rely on a complex network of genes, whose balanced expression levels either activate the testis pathway and simultaneously repress the ovarian pathway or vice versa. The presence or absence of primordial germ cells, of extragonadal origin, also has a sexually dimorphic relevance. Subsequently, internal and external genitalia will follow the male pathway in the presence of androgens and anti-Müllerian hormone (AMH), or the female pathway in their absence. Here we review the sexually undifferentiated stage of embryonic development, and the anatomic, histologic, physiologic and molecular aspects of the fetal sexual differentiation of the gonads, the internal reproductive tract and the external genitalia.
INTRODUCTION
Genital sex differentiation involves a series of events whereby the sexually indifferent embryo progressively acquires male or female characteristics in the gonads, genital tract and external genitalia. Sex development consists of several sequential stages. Genetic sex, as determined by the chromosome constitution, drives the primitive gonad to differentiate into a testis or an ovary. Subsequently, internal and external genitalia will follow the male pathway in the presence of specific testicular hormones, or the female pathway in their absence. Since the presence of the fetal testis plays a determining role in the differentiation of the reproductive tract, the term "sex determination" has been coined to designate the differentiation of the gonad during early fetal development.
THE BIPOTENTIAL GONAD
No sexual difference can be observed in the gonads until the 6th week of embryonic life in humans and 11.5 days post-coitum (dpc) in mice. Undifferentiated gonads of XX or XY individuals are apparently identical and can form either ovaries or testes. This period is therefore called indifferent or bipotential stage of gonadal development.
The Gonadal Ridge
The urogenital ridges are the common precursors of the urinary and genital systems and of the adrenal cortex (1). In the human, they develop during the 4th week post-fertilization at the ventral surface of the cranial mesonephroi, and are formed by intermediate mesoderm covered by coelomic epithelium. Each urogenital ridge divides into a urinary and an adreno-gonadal ridge in the 5th week (Table 1). The adreno-gonadal ridge is the common precursor of the gonads and adrenal cortex. The gonadal ridge is bipotential and can develop into an ovary or a testis. Gonads are subsequently colonized by the primordial germ cells, of extra-gonadal origin. The mesonephroi also give rise to components of the internal reproductive tract and of the urinary system.
The molecular mechanisms underlying the specific location of the gonads on the surface of the mesonephroi begin to be unveiled in chicken embryos, where Sonic hedgehog (SHH) signaling mediated by the bone morphogenetic protein 4 (BMP4) establishes the dorsoventral patterning of the mesoderm and induces coelomic epithelium cell ingression, thus probably initiating gonadal development (2). However, since there are significant differences in gonadal development between birds and mammals, these mechanisms need to be explored to establish whether they are conserved amongst vertebrates.
|
TABLE 1. Chronology of Human Sex Differentiation*
|
|
Age from conception
|
CR length (mm)
|
Event
|
|
22 days
|
2-3
|
Intermediate mesoderm becomes visible
Primordial germ cells in the yolk sac
|
|
24 days
|
2.5-4.5
|
Formation of solid Wolffian ducts
Primordial germ cells migrate to the hindgut
|
|
26 days
|
3-5
|
Wolffian ducts develop a lumen
Primordial germ cells in the hindgut
|
|
28 days
|
4-6
|
Primordial germ cells migrate to the urogenital ridges
|
|
32 days
|
5-7
|
Gonadal primordia develop
Growth of Wolffian ducts
|
|
33-37 days
|
7-11
|
Primordial germ cells reach gonadal ridge
Urogenital sinus is distinguishable
Differentiation of Müllerian ducts
Genital tubercle and urethral folds are visible
|
|
41-44 days
|
11-17
|
Seminiferous cord differentiation
Differentiation between pelvic and phallic parts of the urogenital sinus
|
|
44-50 days
|
15-20
|
Seminiferous cords with germ cells
|
|
50-60 days
|
30
|
Beginning of secretion of AMH
Leydig cell differentiation
Cranial part of Müllerian ducts begins to regress
|
|
9 weeks
|
40
|
Leydig cells produce testosterone
Beginning of masculinization of urogenital sinus and external genitalia
|
|
10 weeks
|
45-50
|
Meiotic entry of oocytes in the medulla
Beginning of degeneration of female Wolffian ducts
Male Müllerian ducts have disappeared
Prostatic buds appear
|
|
12 weeks
|
55-60
|
The vaginal cord is formed
Primordial follicles appear
Seminal vesicles develop
Testis at internal inguinal ring
|
|
14 weeks
|
70
|
Completion of male urethral organogenesis
|
|
16 weeks
|
100
|
Primary follicles appear
|
|
20 weeks
|
150
|
Testosterone serum level is low
Formation of prostatic utricle
|
|
22 weeks
|
180
|
Vagina reaches perineum
|
|
24 weeks
|
200
|
Graafian follicles appear
Beginning of penile growth
|
|
27-30 weeks
|
230-265
|
Inguino-scrotal descent of the testis
|
|
36 weeks
|
300
|
Secondary and tertiary follicles produce AMH
|
* According to O’Rahilly (3).
Several general transcription factors belonging to the large homeobox gene family play an important role in the stabilization of the intermediate mesoderm and the formation of the urogenital ridges (Table 2). Mice in which Lhx1 (4), Emx2 (5, 6) or Pax2 (7) have been inactivated fail to develop urogenital derivatives. Most of these ubiquitous factors are essential for the development of other vital embryonic structures. However, another LIM homeobox gene, Lhx9, seems to be essential only for the proliferation of somatic cells of the gonadal ridge (8) by interacting with Wt1 to regulate Sf1 (9). LHX9 expression increases in both XX and XY undifferentiated gonads, and then decreases as Sertoli and granulosa cells differentiate (10, 11). Several other factors are involved in cell proliferation in the gonadal primordium both in XX and XY embryos. For instance, impairment of the signaling pathway of the insulin/insulin-like growth factor family in mouse knockout models with disrupted Insr, Igf1r and Insrr leads to a significant reduction of the size of adreno-gonadal ridges in both XX and XY embryos (12). Also in mice with a knockout of Tcf21, gonads are severely hypoplastic in both XX and XY fetuses (13). GATA4 (14) and the homeoproteins SIX1 and SIX4 are also essential for early proliferation of gonadal precursor cells and for FOG2- and SF1-regulated SRY expression (15). The Notch signaling pathway is also involved in somatic cell lineage commitment during early gonadogenesis in mice. Conditional knockout of Numb and Numbl (antagonists of Notch signaling) in the undifferentiated gonad results in disruption of the coelomic epithelium and reduction of somatic cell numbers in the gonads (16). Finally, NRG1 is also required in a dose-dependent manner in order to induce somatic cell proliferation in the gonads (17). Since cell proliferation is more important in the male than in the female early developing gonad (18, 19), sex-reversal is often observed in XY embryos with an alteration of gonadal cell proliferation (12). It has been suggested that this is due to a reduction in the number of SRY-expressing pre-Sertoli cells, resulting in very low levels of SRY expression that are insufficient to trigger testicular differentiation (discussed in ref. (20).
|
TABLE 2. Factors Involved in Early Gonadal Ridge Development
|
|
Gene
|
Chromosomal localization
|
Expression
|
Function
|
|
ATRX (Alpha-thalassemia/mental retardation syndrome, Helicase 2, X-Linked)
|
Xq21.1
|
Widespread
|
Nucleotide excision repair and initiation of transcription
|
|
CITED2 (CBP/p300-interacting transactivator, with glu/asp-rich c-terminal domain, 2)
|
6q24.1
|
Widespread
|
WT1 cofactor, regulating SF1expression in the adrenogonadal primordium
|
|
EMX2 (homolog of empty spiracles homeobox gene 2)
|
10q26.11
|
Telencephalon and epithelial components of the urogenital system
|
Arealization of the neocortex and induction of the mesenchyme
|
|
GATA4 (GATA-binding protein 4)
|
8p23.1
|
Widespread
|
Regulation of coelomic epithelium thickening
|
|
INSR (Insulin receptor)
IGF1R (Insulin growth factor 1 receptor)
INSRR (Insulin receptor-related receptor)
|
19p13.2
15q26.3
1q23.1
|
Widespread
|
Metabolic, cell proliferation
|
|
JMJD1A, or KDM3A(Lysine-Specific Demethylase 3A)
|
2p11.2
|
Testis, ovary, kidney, lung, heart, brain, liver, skeletal muscle, pancreas, and spleen
|
Demethylases histone H3 (epigenetic regulation by modification of chromatin conformation)
|
|
LHX1 (LIM homeobox gene 1)
|
17q12
|
Primitive streak, prechordal and intermediate mesoderm, brain, thymus, tonsil
|
Differentiation and development of the head, neural and lymphoid tissues and urogenital structures
|
|
LHX9 (LIM homeobox gene 9)
|
1q31.3
|
Central nervous system, forelimb and hind limb mesenchyme and urogenital system
|
Activation of SF1 in gonadal primordia
|
|
NR5A1 (Nuclear receptor subfamily 5, group A, member 1, also SF1: Steroidogenic factor 1, or AD4BP: Adrenal 4 binding protein, or FTZF1: Fushi tarazu factor homolog 1)
|
9q33.3
|
Gonadal ridges, adrenal gland primordia, hypothalamus and pituitary
|
Stabilization of intermediate mesoderm, and transcriptional regulation of several genes (StAR, steroid hydroxylases, aromatase, AMH, DAX1 and many other)
|
|
NRG1 (Neuregulin 1)
|
8p12
|
Widespread, including progenitors of somatic gonadal cells
|
Progenitor cell proliferation in the gonads
|
|
NUMB
and
NUMBL
|
14q24.2-q24.3
and
19q13.2
|
Widespread, including coelomic epithelium
|
Antagonize NOTCH signaling, involved in mediating asymmetric division of cells in the coelomic epithelium
|
|
PAX2 (Paired box gene 2)
|
10q24.31
|
Mesonephros, metanephros, adrenals, spinal cord, hindbrain and optic and otic vesicles
|
Regulation of WT1 expression and of mesenchyme- to- epithelium transition
|
|
SIX1 / SIX 4 (Sine oculis homeobox 1 and 4)
|
14q23.1
|
Urogenital ridge derivatives
|
Regulation of gonadal precursor cell proliferation, and of Fog2 and Sf1
|
|
TCF21 (Transcription factor 21, also POD1: Podocyte-expressed 1)
|
6q23.2
|
Epithelium of the developing gastrointestinal, genitourinary, and respiratory systems
|
Basic helix-loop-helix transcription factor
|
|
WT1 (Wilms tumor associated gene 1)
|
11p13
|
Urogenital ridge derivatives
|
DNA- and RNA-binding protein with transcriptional and post-transcriptional regulating capacity
|
The differentiation of the gonadal ridge from the intermediate mesoderm requires the expression of sufficient levels of WT1 and SF1. WT1 was initially isolated from patients with Wilms' tumor, an embryonic kidney tumor arising from the metanephric blastema. By alternative splicing and alternative translation initiation, WT1 encodes more than 20 isoforms of a zinc-finger protein acting as transcriptional and/or post-transcriptional regulator (20). The -KTS splicing variant of WT1, lacking the three amino acids lysine (K), threonine (T) and serine (S) at the end of the third zinc finger, is required for cell survival and proliferation in the indifferent gonad, whereas the +KTS variant is involved in the regulation of SRY expression (21). The first indication of a role for WT1 in gonadal and renal development was its expression pattern in the urogenital ridges (22). During gonadal differentiation, WT1 is expressed in the coelomic epithelium and later in Sertoli and granulosa cells (23). In mice with a knockout of WT1, neither the kidneys nor the gonads develop (24). In humans, mutations in the WT1 gene do not completely prevent urogenital ridge development but may result in gonadal dysgenesis associated with nephroblastoma (Wilms' tumor) and/or nephrotic syndrome owing to glomerular diffuse mesangial sclerosis (25-27).
SF1, also known as Ad4BP or FTZF1 (HGNC approved gene symbol: NR5A1), initially described as a regulator of steroid hydroxylases, is an orphan nuclear receptor expressed in the hypothalamus, the pituitary, the gonads and the adrenal glands (reviewed in refs. (28-30). In mice with a knockout of the SF1 gene, the intermediate mesoderm is not stabilized and the gonadal and adrenal primordia soon degenerate (31). SF1 also plays an important role in spermatogenesis, Leydig cell function, ovarian follicle development and ovulation, as demonstrated by a gonad-specific disruption of SF1 (32). A recurrent heterozygous p.Arg92Trp variant of the gene is associated with testicular development in XX subjects (33, 34). WT1, through interaction with CITED2 (35, 36), and LHX9 (8) regulate the expression of SF1 upstream of the gonadal development cascade. GATA4 and SOX-family factors also regulate SF1 expression in the gonad (28). In humans, the phenotype resulting from SF1 mutations does not exactly match that of Sf1 knockout mice: the clinical spectrum includes severe and partial forms of testicular dysgenesis, anorchidism, and even male infertility in normally virilized individuals; adrenal insufficiency is not always present. In 46,XX females, SF1 mutations have been described in patients with primary ovarian insufficiency (29, 30). SF1 is one of the increasing number of examples of dosage-sensitive mechanisms in human sex differentiation, since mutations at the heterozygous state are sufficient to induce sex reversal in XY individuals (reviewed in refs. (29, 30).
Recent studies using single-cell RNA sequencing (scRNA-seq) has shed light on the initial steps of lineage trajectories and cell fate in the developing gonads (1, 37). A subset of cells of the coelomic epithelium expressing GATA4, SF1 and WT1 are likely to be the precursors of the somatic lineages of the undifferentiated gonads: both the supporting (Sertoli and granulosa) and the steroidogenic (Leydig and theca) cell populations of the differentiating gonads seem to derive from SF1 and WT1-expressing cells present in the genital ridge (1, 37, 38).
The Germ Cells
Initially formed exclusively by somatic cells, the gonads are subsequently colonized by the primordial germ cells (PGCs). PGCs derive from pluripotent cells of the posterior proximal epiblast, which move, at a very early stage of embryonic life, through the primitive streak into the extra-embryonic region at the base of the allantois (39). Not all of these cells are committed to a germ cell lineage since they also give rise to extra-embryonic mesoderm cells (40).
The mechanisms responsible for specification of epiblast cells to become PGCs vary between species (41-43). In mice, PCG specification involves several extraembryonic ectoderm-derived factors, including bone morphogenetic protein 2 (BMP2) (44), BMP4 (45-47), BMP8B (46) and WNT3 (48). Cells of the adjacent epiblast become determined to develop through the germline as they start expressing BLIMP1 (44), encoded by Prdm1. BLIMP1 represses somatic fate in the epiblast cells, and together with PRDM14 and AP2G (encoded by Tfap2c), constitute a tripartite genetic network necessary and sufficient for mouse PGC specification (49). PRDM14 regulates the restoration of pluripotency and epigenetic reprogramming in PGCs, reestablishing the expression of the pluripotency factors OCT3/4 (encoded by Pou5f1), SOX2 and NANOG (41).
Instead, embryos of other mammals do not form a structure equivalent to the extraembryonic ectoderm, and the origin of the signals that initiate PGC specification remain largely unknown. Notably, in the human embryo, PGC-like cells express very low or no PRDM14, maintain NANOG expression, and do not express SOX2. Furthermore, the expression of SOX17 is detected before that of BLIMP1 and could be involved in the regulation of PGC specification and maintenance of their pluripotency in humans (49, 50).
Widespread chromatin modifications are observed: PGCs undergo genome-wide demethylation including erasure of genomic imprints (44), thus reaching a ‘ground state’ in terms of epigenetic marks. Re-methylation of germ cell genome occurs later during fetal life: in XY germ cells when they have committed to the spermatogenic fate, and in XX germ cells just before ovulation (45).
In the 4thweek, PGCs have migrated and are present in the yolk sac near the base of the allantois. They can be identified by their expression of alkaline phosphatase, OCT3/4 and the tyrosine kinase receptor C-KIT (Fig. 1A) (40). Subsequently, PGCs become embedded in the wall of the hind gut, gain motility and migrate through the dorsal mesentery to reach the gonadal ridges in the 5thweek (Fig. 1B). Early migration of PGCs is dependent on the expression of interferon-induced transmembrane proteins 1 and 3 (IFITM1 and IFITM3) in the surrounding mesoderm (51). During migration, PGCs proliferate actively but do not differentiate (40). Germ cell migration through the dorsal mesentery to the gonadal ridges and survival/proliferation in both XX and XY embryos is driven by signaling between kit ligand (KITL, also known as Stem cell factor [SCF], Steel factor or mast cell growth factor [MGF]), which is expressed in somatic cells of the gonadal ridge and the hind gut along the pathway of PGC migration, and its receptor present in germ cells, C-KIT (Fig. 1) (52). PGC migration and genital ridge colonization is also dependent on stromal cell-derived factor 1 (SDF1, also known as CXCL12) and its receptor CXCR4 (53) and on interactions with extracellular matrix proteins, like fibronectin and laminin, while proliferation and/or survival involve many other factors (39, 40, 52, 54).
PGCs are in a bipotential state when they colonize the gonadal ridges, i.e. they still have the capacity to enter either spermatogenesis or oogenesis. Shortly afterwards, induced by the gonadal environment, PGCs begin to express DAZL, DDX4 (also known as MVH) and low levels of SYCP3 (43), probably owing to promoter demethylation (55). DAZL seems to induce PGCs capacity to respond to specific male or female gonadal signals (56, 57).
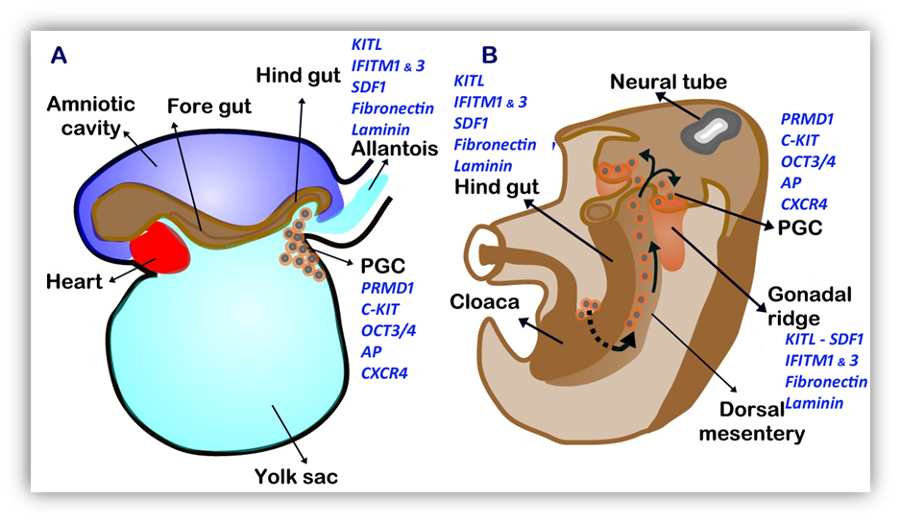
FIGURE 1. Regulation of Germ Cell Migration. A: 4-week embryo. Differentiation of primordial germ cells (PGC) occurs from epiblast-derived cells present in the yolk sac near the base of the allantois. PGCs express PMRD1, the receptors C-KIT and CXCR4, OCT3/4 and alkaline phosphatase. Fibronectin and laminin, together with KITL, SDF1 and IFITM 1 and 3 are expressed in the mesoderm along the PGC pathway. B: 5-week embryo. PGCs migrate along the dorsal mesentery of the hind gut to the gonadal ridges.
SEX DETERMINATION
The Determining Role of Testicular Differentiation
The pioneering experiments of fetal sexual differentiation carried out by Alfred Jost in the 1940’s clearly established that the existence of the testes determines the sexually dimorphic fate of the internal and external genitalia (Fig. 2)(58, 59). Irrespective of their chromosomal constitution, when the gonadal primordia differentiate into testes, all internal and external genitalia develop following the male pathway. When no testes are present, the genitalia develop along the female pathway. The existence of ovaries has no effect on fetal differentiation of the genitalia. The paramount importance of testicular differentiation for fetal sex development has prompted the use of the expression “sex determination” to refer to the differentiation of the bipotential or primitive gonads into testes.
In the next section, we describe the morphological aspects of fetal testicular and ovarian differentiation and the underlying molecular mechanisms, involving genes mapping to sex-chromosomes (Fig. 3) and autosomes (Table 3).
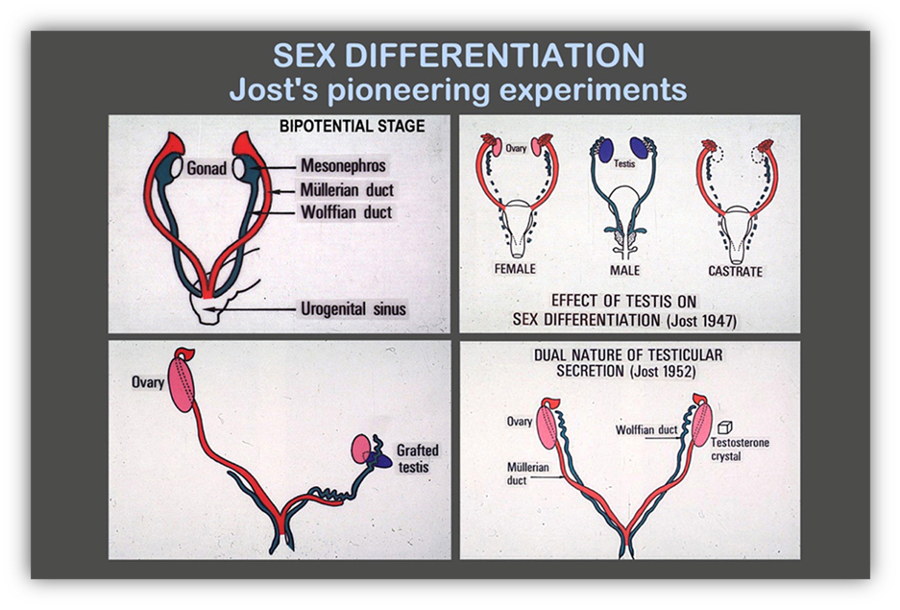
FIGURE 2. Determining role of the testes in fetal sex differentiation. In normal females, Müllerian ducts are maintained, Wolffian ducts regress. In males, the opposite occurs. In castrated fetuses, irrespective of genetic or gonadal sex, the reproductive tract differentiates according to the female pattern.
The Fate of the Undifferentiated Gonadal Ridge
As already mentioned, the gonadal ridges are bipotential until the 6th week after conception in humans, i.e. they have the capacity to follow the testicular and the ovarian pathways. The discovery of the testis-determining factor SRY in 1990 was followed by the progressive unveiling of robust networks of genes, whose balanced expression levels either activate the testis pathway and simultaneously repress the ovarian pathway or vice versa (Fig. 4). During the formation of the undifferentiated gonadal ridges, a common genetic program is established in the supporting-cell lineage deriving from the multipotent somatic progenitor cells in both XX and XY embryos, characterized by a balanced expression of pro-Sertoli (SOX9, FGF9, PGD2) and pregranulosa (WNT4, RSPO1, FST and CTNNB1) genes (37, 60). Under physiological conditions in the XY gonad, the upregulation of SRY induces a destabilization of that balance, initiating the testis cascade.
THE MALE DETERMINING PATHWAY
Sex-Determining Region on the Y Chromosome (SRY)
Compelling evidence for the importance of the Y chromosome for the development of the testes, irrespective of the number of X chromosomes present, has existed since 1959 (61, 62). However, the identification of the testis-determining factor (TDF) on the Y chromosome did not prove easy and several candidates (e.g. HY antigen, ZFY) were successively proposed and rejected until the SRY (Sex-determining region on the Y) gene was cloned in 1990 in man (63) and mouse (64). Experimental (65, 66) and clinical (67, 68) evidence clearly established that SRY was the testis-determining factor. Considerable progress has been made since SRY was identified, and it has become clear that sex determination is a far more complex process, regulated by competing molecular pathways in the supporting cell lineage of the bipotential gonad. SRY has lost much of its prestige because it has a very weak transactivation potential, is expressed very transiently in the mouse, weakly at best in other mammals and not at all in sub-mammalian species (reviewed in ref. (20). Instead, its target gene encoding the transcription factor SOX9 has emerged as the master regulator of testis determination, the main role of SRY consisting in upregulating the expression of SOX9 during a very narrow critical time window (69). Once time is up, either SOX9 is able to maintain its own expression with the help of feed-forward enhancing mechanisms succeeding in triggering Sertoli cell differentiation or it is silenced by an opposing set of genes which impose ovarian differentiation. Timing and expression level determine which team wins (20, 70, 71) but the battle is never over, even after birth, at least in mice (72).
SRY is a member of a family of DNA-binding proteins bearing a high mobility group (HMG) box; its gene maps to the short arm of the Y chromosome (Table 3), very close to the pseudoautosomal region 1 (PAR1) (Fig. 3). PAR1 on Yp and PAR2 on Yq are the only regions of the Y chromosome that undergo meiotic recombination with homologous sequences of the X chromosome during male spermatogenesis. The proximity of SRY to PAR1 makes it susceptible to translocation to the X chromosome following aberrant recombination and provides an explanation for 80% of XX males (73) and for a low proportion of XY females. Indeed, mutations and deletions of the SRY locus only account for 15% of XY females (74, 75).
While SRY gene exists in almost all mammals as a single copy gene, the rat carries 6 copies and the mouse Sry gene has a distinct structure from other mammalian SRY genes because of the presence of a long-inverted repeat. Also, SRY expression varies between species: in mice a functional transcript is present only in pre-Sertoli cells for a very short period during early gonadogenesis, in goats SRY is expressed in all somatic and germ cells of the gonad during fetal life and restricted to Sertoli cells and spermatogonia in the adult testis. Human SRY is expressed in both Sertoli cells and germ cells at fetal and adult stages (reviewed in ref. (20). Proteins that interact with SRY and could have a relevant function in gonadal differentiation include SIP-1/NHERF2 (76) and KRAB-O (77).
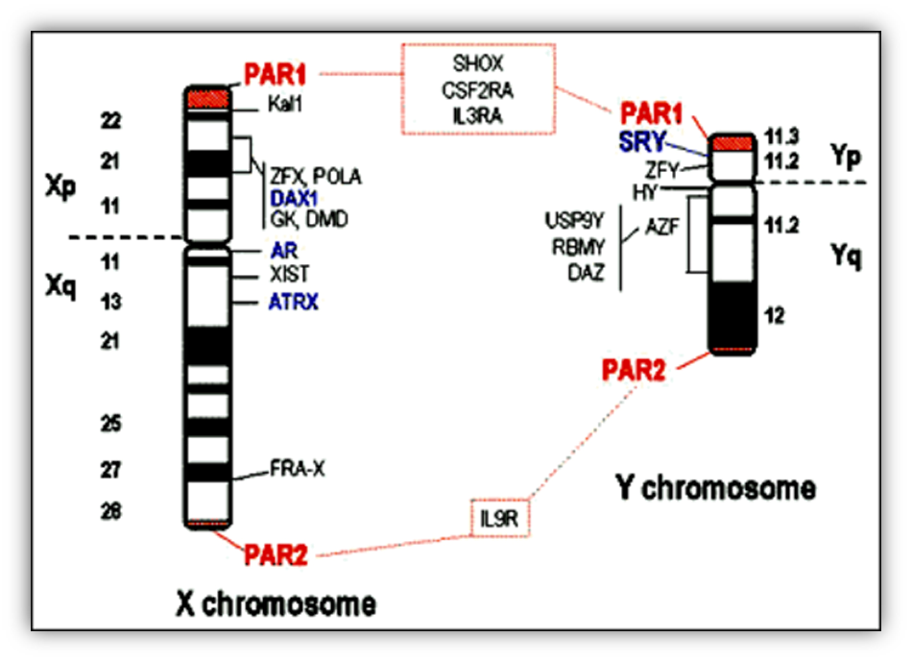
FIGURE 3. X and Y chromosome genes involved in sex determination and differentiation.
SRY: Sex-determining region Y chromosome; DAX1: DSS-AHC critical region X chromosome gene 1; AR: Androgen receptor; and ATRX: Alpha-thalassemia/mental retardation syndrome X-linked are involved in in sex determination and differentiation. Other genes present in the X and Y chromosomes are: AZF: azoospermia factor; CSF2RA: Colony-stimulating factor 2 receptor alpha; DAZ: Deleted in azoospermia; FRA-X: Fragile X syndrome; DMD: Duchenne muscular dystrophy; GK: Glycerol kinase; HY: Histocompatibility antigen Y; IL3RA: Interleukin 3 receptor alpha; IL9R: Interleukin 9 receptor; Kal1: Kallmann syndrome 1; PAR: Pseudo-autosomal regions; POLA: DNA polymerase alpha; RBMY: RNA-binding motif protein Y chromosome; SHOX: Short stature homeo box; USP9Y: Ubiquitin-specific protease 9 Y chromosome; XIST: X inactivation-specific transcript; ZFX: Zinc finger protein X-linked; ZFY: Zinc finger protein Y-linked.
Owing to its Y-chromosome localization, SRY can only be expressed in the XY gonadal ridge, thus playing a paramount role in tilting the balance between testicular and ovarian promoting genes towards the male pathway.
A tight regulation of SRY expression is essential for fetal gonadogenesis: both timing and level of expression are determinant, as revealed by experiments in mouse showing that SRY levels must reach a certain threshold at a certain stage of fetal development to induce testis differentiation (69). SRY expression commences between days 41 and 44 post-fertilization in humans (78). The mechanisms underlying the initiation of SRY expression begin to be unraveled (Fig. 4). The +KTS splice variant of WT1 (21, 79, 80), SF1 (20) and SP1 (81, 82) are able to activate SRYtranscription. The transcriptional co-factor CITED2 acts in the gonad with WT1 and SF1 to increase SRY levels to attain a critical threshold to efficiently initiate testis development (35). The +KTS isoform of WT1 might also act as a posttranscriptional stabilizer of SRY mRNA (70).
The implication of GATA4 on SRY expression is less straightforward. The interaction between GATA4 and its cofactor FOG2 in the gonadal primordium is required for normal Sry expression and testicular differentiation in mice (83). However, whether the effect is specific on Sry transcription or more general on gonadal somatic cell development was not evaluated. Functional GATA-binding sites are present in the mouse and pig Sry promoter but not in the human SRY (84, 85). One possibility is that GATA4 interacts with WT1 (Fig. 4), mainly the +KTS isoform, which binds to the SRY promoter and increases its transcriptional activity (84). Alternatively, it has been proposed that GATA4 directly acts on the SRY promoter, based on the experimental observation that GADD45G binds and activates the mitogen-activated protein kinase kinase MAP3K4 (also known as MEKK4) to promote phosphorylation and activation of the p38 kinase (Table 3), which in turn phosphorylates GATA4 thus enhancing its binding to the Sry promoter (85, 86)(Fig. 4). These results are in line with those indicating that MAP3K4 is essential for testicular differentiation in mice (87).
SRY expression is also epigenetically regulated: the demethylase KDM3A, also known as JMJD1A, positively regulates the expression of Sry in mice, as shown by the absence of testicular development and consequent sex reversal in Jmjd1a-deficient XY mice (88). Histone methylation is an important mechanism of epigenetic regulation: methylation of lysine 9 of histone H3 (H3K9) is a hallmark of transcriptionally suppressed chromatin. JMJD1A demethylates H3K9, thus allowing transcriptional activation of Y chromosome genes, amongst which is SRY. ATRX,also known as XH2, is an X-encoded DNA-helicase whose mutation results in mental retardation, α-thalassemia and gonadal dysgenesis in XY individuals (89-91). ATRX has a more general effect on chromatin remodeling, which seems to play an important role in the epigenetic regulation of sex determination (92).
Several other experimental models impairing the expression of signaling molecules, which are expressed SRY in the early gonadal ridge in normal conditions, show reduced or absent SRY expression, develop gonadal agenesis and a female phenotype of the internal and external genitalia. LHX9 (8) is a potential regulator of SRY expression. A direct effect of LHX9 on the SRY gene has not been demonstrated but an indirect effect through SF1 upregulation has been postulated (20). Loss-of-function mutations of the mouse genes encoding the insulin receptor (Insr), the IGF1 receptor (Igf1r) and the insulin related receptor (Insrr) also result in decreased or absent Sry expression (12). However, these factors and signaling pathways affect cell proliferation, and decreased SRY expression might only reflect the reduced number of cells in the gonadal primordium. Indeed, many of these potential regulators have not yet been proven to affect SRY expression directly.
|
TABLE 3. Factors Involved in Gonadal Differentiation
|
|
Gene
|
Chromosomal localization
|
Expression
|
Function
|
|
ATRX (Alpha-thalassemia/mental retardation syndrome, Helicase 2, X-Linked)
|
Xq21.1
|
Widespread
|
Nucleotide excision repair and initiation of transcription
|
|
CBX2 (Chromobox homolog gene 2; or M33 mouse homolog of)
|
17q25.3
|
Widespread
|
Regulation of homeotic genes. Represses WNT4 signaling
|
|
CITED2 (CBP/p300-interacting transactivator, with glu/asp-rich c-terminal domain, 2)
|
6q24.1
|
Widespread
|
WT1 and SF1 cofactor, regulating SRYexpression in the gonad
|
|
COUP-TF2 (Chicken ovalbumin upstream promoter transcription factor 2), or NR2F2(Nuclear receptor subfamily 2, group F, member 2)
|
15q26.2
|
Widespread
|
Transcription factor (orphan nuclear receptor) likely involved in mesenchymal-epithelial interactions
|
|
CTNNB1 (β-catenin)
|
3p22.1
|
Widespread
|
Upregulates WNT4, FST and FOXL2
|
|
DAX1: DSS-AHC critical region on the X chromosome 1); orNR0B1 (Nuclear receptor subfamily 0, group B, member 1).
|
Xp21.2
|
Gonads, pituitary, adrenals
|
Antagonizes SRY, SOX9. Essential for normal testicular and ovarian development
|
|
DHH (Desert hedgehog)
|
12q13.12
|
Sertoli cells (testis), Schwann cells (peripheral nerves)
|
Morphogenesis
|
|
DKK1 (Dickkopf, xenopus, homolog of, 1)
|
10q21.1
|
Widespread
|
Represses WNT4 binding to the LRP5/6 co-receptor
|
|
DMRT1 (Doublesex- and mab3-related transcription factor 1)
|
9p24.3
|
Gonads and several other tissues
|
Antagonizes FOXL2
|
|
FGF9 (Fibroblast growth factor 9)
|
13q12.11
|
Gonads and several other tissues
|
Upregulation of SOX9 and downregulation of WNT4
|
|
FGFR2 (FGF receptor 2)
|
10q26.13
|
Gonads and several other tissues
|
Upregulation of SOX9 and downregulation of WNT4
|
|
FOG2 (Friend of GATA, gene 2, or ZFPM2: zinc finger protein multitype 2)
|
8q23.1
|
Widespread
|
Repression of DKK1
|
|
FOXL2 (Forkhead transcription factor 2)
|
3q22.3
|
Gonads and eyelids
|
Antagonizes SOX9. Survival of meiotic germ cells
|
|
FST (Follistatin)
|
5q11.2
|
Widespread
|
Antagonizes Activins. Survival of meiotic germ cells
|
|
GADD45G (Growth arrest- and DNA damage-inducible gene, gamma)
|
9q22.2
|
Widespread
|
Phosphorylation of GATA4
|
|
GATA4 (GATA-binding protein 4)
|
8p23.1
|
Widespread
|
Regulation of SRY expression
|
|
HHAT (Hedgehog acyltransferase)
|
1q32.2
|
Gonads
|
Two INHBB subunits form Activin B dimer, which induces vascular endothelial cell migration to the gonad
|
|
INHBB (Inhibin βB, Activin βB)
|
2q14.2
|
Gonads
|
Two INHBB subunits form Activin B dimer, which induces vascular endothelial cell migration to the gonad
|
|
JMJD1A; or KDM3A(Lysin-specific demethylase 3A)
|
2p11.2
|
Testis, ovary, kidney, lung, heart, brain, liver, skeletal muscle, pancreas, and spleen
|
Demethylases histone H3 (epigenetic regulation by modification of chromatin conformation)
|
|
MAP3K1 (MAP/ERK Kinase Kinase 1; MEKK1; MAPKKK1; MEK Kinase)
|
5q11.2
|
Widespread
|
Phosphorylation of GATA4
|
|
MAPK14 (Mitogen-activated protein kinase 14; or p38-MAPK)
|
6p21.31
|
Widespread
|
Phosphorylation of GATA4
|
|
NR5A1 (Nuclear receptor subfamily 5, group A, member 1, also SF1: Steroidogenic factor 1, or AD4BP: Adrenal 4 binding protein, or FTZF1: Fushi tarazu factor homolog 1)
|
9q33.3
|
Gonadal ridges, adrenal gland primordia, hypothalamus and pituitary
|
Transcriptional regulation of several genes (SRY, SOX9, STAR, steroid hydroxylases, aromatase, AMH, DAX1 and many other)
|
|
PDGFB (Platelet-derived growth factor, beta polypeptide)
|
22q13.1
|
Endothelial cells
|
Increase in cell proliferation in the gonadal interstitial tissue
|
|
PDGFRA (PDGF receptor α)
|
4q12
|
Gonadal interstitial cells and several other tissues
|
Increase in cell proliferation in the gonadal interstitial tissue
|
|
PTGDS (or PGDS2: Prostaglandin D2 synthase)
|
9q34.3
|
Gonads and several other tissues
|
Synthesis of prostaglandin D2 (PGD2), upregulation of SOX9 and its nuclear translocation
|
|
RSPO1 (R-spondin family, member 1)
|
1p34.3
|
Gonads and skin
|
Upregulates WNT4 by sequestering the transmembrane E3 ubiquitin ligases ZNRF3 and RNF43.
Cooperates with WNT4 signaling, by antagonizing DKK1, to stabilize β-catenin and FST
|
|
SOX8 (SRY box 8)
|
16p13.3
|
Gonads and several other tissues
|
Transcriptional regulation of SOX9, in cooperation with SF1
|
|
SOX9 (SRY box 9)
|
17q24.3
|
Testis, cartilage
|
Triggers testis differentiation, and regulates several testis-specific genes
|
|
SOX10 (SRY box 10)
|
22q13.1
|
Gonads and several other tissues
|
Transcriptional regulation of SOX9, in cooperation with SF1
|
|
SP1 (Specificity protein 1)
|
12q.13.13
|
Widespread
|
Regulation of SRY expression
|
|
SRY (Sex-determining region on the Y chromosome)
|
Yp11.31
|
Male gonadal ridge
|
Regulates SOX9 and triggers testis differentiation
|
|
VEGFA (Vascular endothelial growth factor A)
|
6p21.1
|
Mesenchymal cells of the gonadal ridge and other organs
|
Induces vascular endothelial cell migration to the gonad
|
|
WNT4 (Wingless-type MMTV integration site family, member 4)
|
1p36.12
|
Gonads and several other tissues
|
Induces β-catenin and silences FGF9 and SOX9 by binding to Frizzled receptor
|
|
WT1 (Wilms tumor associated gene 1)
|
11p13
|
Urogenital ridge derivatives
|
Transcriptional regulation and post-transcriptional stabilization of SRY
|
|
ZNRF3 (Zinc finger and ring finger protein 3)
|
22q12.1
|
Widespread
|
Inhibition of WNT signaling by targeting Frizzled receptor for degradation by ubiquitination and increased membrane turnover
|
SOX9: A Target of SRY
SOX9, an autosomal member of the HMG-box protein superfamily mapped to chromosome 17 q24 (93), is the master regulator of Sertoli cell differentiation (94). In the mouse, SOX9 is expressed at low levels in the bipotential gonads of both sexes under SF1 regulation (95), but persists only in testicular Sertoli cells after SRY expression has peaked (96-98). SRY and SF1 directly bind to several sites within a 3.2-kb testis-specific enhancer (TES) or 1.4-kb of its core element (TESCO), present approximately 14 kb upstream of the Sox9 promoter and responsible for this expression pattern (95, 99). Together with SF1, SOX9 also binds and activates TES, thus maintaining its own expression by autoregulation after transient SRY expression has ceased in the mouse.
SOX9 mimics SRY effects independently of SRY expression. In fact, overexpression of SOX9 during early embryogenesis induces testicular differentiation in two different models of transgenic XX mice (100, 101). Functional analysis of SOX9 during sex determination, by conditional gene targeting in mice, has shown that homozygous deletion of Sox9 in XY gonads interferes with sex cord development and with activation of testis specific markers (102). Further evidence for the role of SOX9 in testicular development comes from observations in humans, in whom a double dose of SOX9 expression is required. Heterozygous mutations result in haploinsufficiency resulting in campomelic dysplasia, a polymalformative syndrome that includes sex-reversal due to gonadal dysgenesis in XY individuals (93, 103), whereas gain-of-function of SOX9 in XX individuals leads to sex reversal (104).
In humans more distant regulatory regions of SOX9 have been identified (105), and confirmed by observations in patients with XY gonadal dysgenesis. No mutation has been found in the TES sequence (106), instead a 1.9 kb SRY-responsive subfragment of a 32.5 kb interval lying 607.1–639.6 kb upstream of SOX9 —termed XY SR for XY Sex Reversal— seems to be the core of the Sertoli-cell enhancer of human SOX9. Heterozygous deletions encompassing these sequences were identified in four families with SRY-positive 46,XY gonadal dysgenesis without campomelic dysplasia (107) and a deletion of a 557–base pair element named enhancer 13 (Enh13), reproduced in mice, led to XY sex reversal (108). This region is included in a 1.2-Mb deletion previously described in a case of 46,XY DSD with gonadal dysgenesis and no skeletal phenotype (109). Finally, in line with these observations, overexpression of SOX9 is supposed to underlie testicular development in familial 46,XX SRY-negative males with a 178-kb duplication or a 96-kb triplication in sequences lying 500–600 kb upstream of SOX9 (110, 111).
SOX9 also affects the differentiation of the reproductive tract by upregulating the expression of anti-Müllerian hormone (AMH) (112, 113), a Sertoli cell factor involved in male differentiation of the internal genitalia (see below).
SOX8 and SOX10 are two other members of the SOX family expressed in the gonads and in several other tissues. During mouse embryo development, the expression of SOX8 and SOX10 is triggered shortly after that of SOX9, but at lower level (114-117). SOX8 is regulated by SOX9 (102). Like SOX9 itself, SOX8 and SOX10 can synergize with SF1 and upregulate SOX9 expression (Fig. 4) upon binding to TESCO (20). SOX8 can bind the canonical target DNA sequences and activate AMH transcription acting synergistically with SF1, but with less efficiency than SOX9 (114, 118). Later during fetal development, an interaction between SOX9 and SOX8 is required for basal lamina integrity of testis cords and for suppression of FOXL2, two events essential to the normal development of testis cords (117).
An X-linked member of the SOX family, SOX3, although not involved in the normal pathway of fetal gonadal differentiation, is capable of inducing SOX9 expression and testis differentiation when ectopically expressed in the XX gonad (119). It is also possible that indirect mechanisms mediate Sox9 activation, in line with the hypothesis indicating that SRY might act as a repressor of a negative regulator of the male cascade (120). For instance, targeted disruption of Foxl2 leads to SOX9 upregulation in the XX gonad (121), and prostaglandin D2 (PGD2) has been shown to upregulate SOX9 in the absence of SRY (122).
SOX9 expression is maintained at high levels in the male gonad despite down-regulation of SRY soon after testicular determination, at least in the mouse (97, 98). As mentioned, SOX9 is capable of autoregulating its expression (95), and other members of the SOX family like SOX3, SOX8 and SOX10 are also able to interact with SF1 to maintain SOX9 expression in the male gonad (20, 117).
Observations made in XY intersex patients with normal SRY together with the discovery of proteins showing a sexually dimorphic pattern of expression in the gonads following SRY peak have helped to identify other loci, likely to be involved in testicular differentiation, which are discussed below.
FGF9 and PGD2: Maintaining SOX9 Expression Levels
SOX9 upregulates the expression of FGF9 and the synthesis of prostaglandin D2 (PGD2) catalyzed by PGD synthase. FGF9 interacts with its receptor FGFR2, initiating a feed-forward loop that maintains SOX9 expression and also results in downregulation of WNT4 expression (123-126) (Fig. 4). Independently of FGF9, PGD2 interacts with its receptor DP to induce SOX9 expression (122, 127) and its nuclear translocation (127, 128), thus increasing its availability to target genes (80).
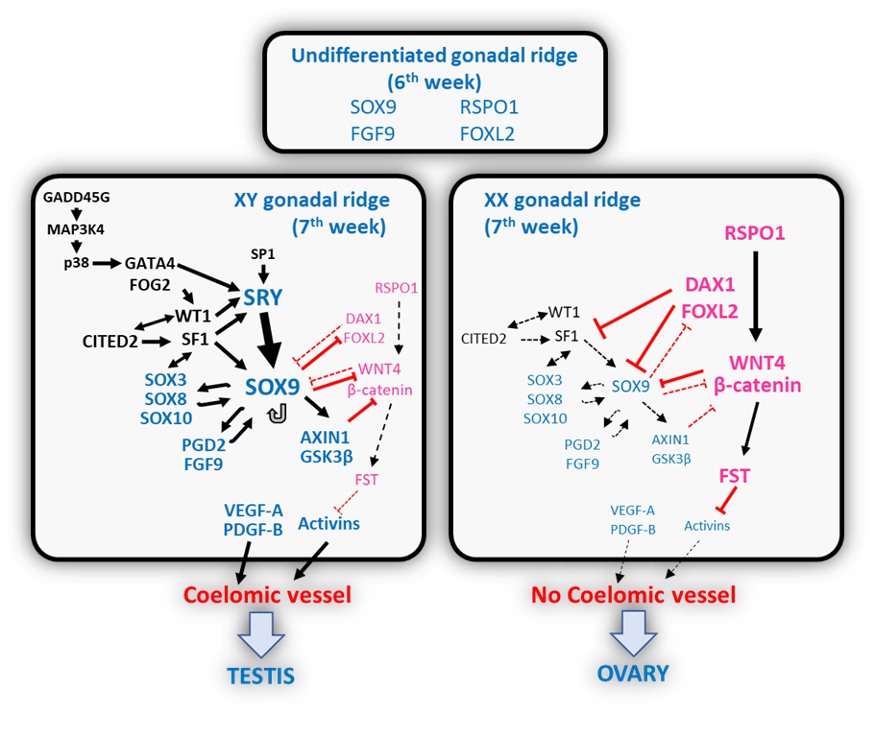
FIGURE 4. Schematic representation of molecular mechanisms involved in determining the fate of the undifferentiated gonadal ridge. Black arrows indicate a positive regulation; double arrows indicate a positive feedback loop; red lines indicate a negative regulation; double red lines indicate a mutual antagonism. In the 6th week of embryonic life, the gonadal ridge is sexually undifferentiated, and various factors are expressed at the same levels in the XX and the XY gonads. During the 7th week, in the XY gonad, SRY expression is triggered, and the male pathway prevails driving to the formation of the coelomic vessel. In the XX gonad, the female pathway prevails, and there is no formation of the coelomic vessel. Reprinted with permission from ref. (129) Freire AV, Ropelato MG, Rey R. Ovaries and Testes. In: Kovacs C, Deal C, Eds. Maternal-Fetal and Neonatal Endocrinology. 1st Edition. Boston: Academic Press-Elsevier, 2020, pp. 625-641. ISBN 9780128148235. Copyright © 2020 Elsevier Inc.
As already discussed, somatic cell proliferation is critical for early testicular differentiation (18). FGF9 and WNT4 act as antagonistic signals in the first steps of differentiation of the gonadal ridge (130). FGF9 controls cell proliferation in a sexually dimorphic fashion: the disruption of FGF9 expression by targeted deletion in transgenic mice does not affect XX gonads but prevents testicular differentiation and results in sex reversal in XY mice (131). In the mouse, FGF9 and WNT4 are expressed in the undifferentiated XX and XY gonads at the same levels: FGF9 near the coelomic surface and WNT4 near the mesonephric border (130). When SRY expression is initiated and upregulates SOX9 in the XY gonadal ridge, the balance between FGF9 and WNT4 is disrupted: SOX9 enhances FGF9 expression which in turn maintains high SOX9 levels thus resulting in a feed-forward loop that accelerates commitment to the male pathway. WNT4 expression is downregulated when a threshold level of FGF9 is reached (130). FGF9 controls the proliferation of a cell population that gives rise to Sertoli progenitors (19). In Fgf9 knockout mice, initial Sertoli cell differentiation is not hindered: SRY and SOX9 expression is observed but soon weakens resulting in an aborted differentiation of Sertoli cell precursors (130). Although in experimental conditions, FGF9 is capable of inducing proliferation of coelomic epithelium cells in XX gonadal ridges, this does not result in Sertoli cell differentiation, clearly indicating that increasing cell proliferation is not sufficient to induce testicular differentiation, and that other pro-testicular signals are also required (131). FGF9 and SOX9 also upregulate AXIN1 and GSK3β, which promote the destabilization of β-catenin and, thus, serve to block ovarian development (132).
DMRT1, DAX1 and Other Factors Modulating Testis Versus Ovary Antagonism
DMRT1 is a member of the DM domain transcription factor family which appears to play a conserved role in vertebrate male gonad development. In mice, DMRT1 –but not DMRT2 or DMRT3– is expressed and required in both germ cells and Sertoli cells of the testis (133). Overexpression of DMRT1 in XX mice inhibits WNT4 and FOXL2 expression and results in partial testicular differentiation and male genital development (134), while loss of DMRT1 expression activates FOXL2 and reprograms Sertoli cells into granulosa cells, even in postnatal life, suggesting that DMRT1 is essential to maintain mammalian testis differentiation life-long in mice (135, 136).
In humans, deletions of chromosome 9p involving DMRT1, DMRT2 and DMRT3 genes are associated with XY male-to-female sex reversal due to gonadal dysgenesis. Patients also present with mental retardation and typical craniofacial dysmorphia, including trigonocephaly, upward-slanting palpebral fissures, and less frequently hypertelorism, epicanthus, flat nasal bridge, low-set ears, microstomia, micrognathia, short neck, widely spaced nipples, square hyperconvex nails, dolichomesophalangy and hypotonia (137, 138).
DAX1 (HGNC approved gene symbol: NR0B1), encoding for an orphan nuclear receptor and mapping to the DSS(Dosage Sensitive Sex-reversal) region on Xp21, was the first putative testis repressor and/or ovarian determining gene. A duplication of DSS results in sex-reversal in 46,XY patients (139), and DAX1 overexpression in transgenic XY mice impairs testis differentiation by antagonizing the ability of SF1 to synergize with SRY action on SOX9 (140, 141)(Fig. 4). However, the disruption of Dax1 gene in XX mice does not prevent ovarian differentiation (142). Furthermore, DAX1 is essential for normal testicular cord formation (143, 144). These observations in rodent models, together with DAX1 expression pattern in the human fetus showing persistently low levels in both XX and XY gonads from 33 days post-fertilization (i.e. the bipotential stage) through 15 fetal weeks (78), strongly suggest that low DAX1 levels are necessary for gonadal development in both sexes. Abnormally low or high DAX1 expression result in abnormal gonadal differentiation (145).
CBX2, the human homolog of murine M33 (146), does not seem to activate SRY expression as initially proposed (147), but may act as a stabilizer of SRY action and the testis pathway by repressing WNT4 downstream target LEF1, involved in ovarian differentiation (148). Interestingly, biallelic mutations in CBX2 were found in a 46,XY girl with ovarian tissue (149), and XY mice with inactivated Cbx2 developed as female (146).
MAP3K1, unlike MAP3K4 (87), is not essential for testicular differentiation and development in mice (150), but it modulates the balance between testicular and ovarian male pathways by sequestration of AXIN1 (see “Genetic pathways of ovarian differentiation”). In humans, mutations in the MAP3K1 gene have been associated with testicular dysgenesis (151, 152).
Similarly, inactivating variants that disrupt ZNRF3 function result in 46,XY DSD in humans and to sex reversal in mice, likely due to gonadal dysgenesis (153).ZNRF3 is an E3 ubiquitin ligase that promotes the degradation by ubiquitination and the turnover of Frizzled, a WNT receptor (Fig. 5) (154, 155).
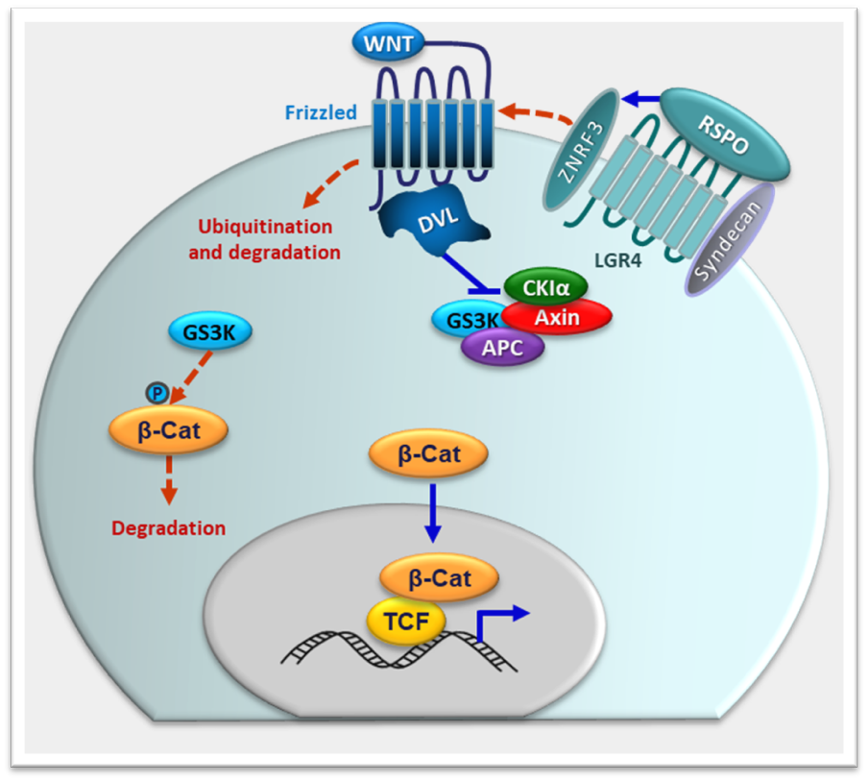
FIGURE 5. WNT and RSPO actions. Under steady state conditions (red dotted arrows), ZNRF3 provokes the ubiquitination and degradation of Frizzled, receptor of WNT family factors. GSK3 phosphorylates β-catenin, which is then degraded. R-spondin (RSPO) family members binding to their receptors LGR4/5/6 results in complex formation with ZNRF3. Consequently, more Frizzled molecules become available for WNT signaling. Under these conditions (blue full arrows), the complex formed by GSK3, Axin, CKIα and APC is recruited to the WNT–receptor complex and inactivated, allowing β‑catenin to translocate to the nucleus and regulate target genes.
COUP-TF2, encoded by NR2F2, is a transcription factor likely involved in mesenchymal-epithelial interactions required for organogenesis. In the fetal gonads, COUP-TF2 expression increases as the ovaries develop, and loss-of-function mutations in NR2F2 have been described in 46,XX ovotesticular SRY-negative DSD (156), indicating that COUP-TF2 may be involved in driving the balance towards ovarian differentiation.
MAMLD1 is expressed in fetal Sertoli and Leydig cells, under the control of SF1 (157, 158), and gene variants have been associated with a broad phenotypic spectrum of DSD (159). However, Mamld1 knockout mice depict a very mild reproductive phenotype (160). The precise role of MAMLD1 still needs to be established.
Stabilization of Testis Differentiation: Vascular, Cellular and Molecular Pathways
In the XY fetus, the initially amorphous cluster of gonadal cells becomes segregated in two compartments, testicular cords and interstitial tissue, during the 7th week of gestation (3). These architectural changes are heralded by gonadal ridge vascularization, a highly dynamic and sexually dimorphic process. At variance with the differentiating ovary that recruits vasculature by typical angiogenesis, the XY gonad recruits and patterns vasculature by a remodeling mechanism: pre-existing mesonephric vessels disassemble and generate a population of endothelial cells that migrate to the gonad, below the coelomic epithelium, where they reaggregate to form the coelomic vessel, an arterial vessel that runs the length of the testis at its antimesonephric margin (161, 162). The formation of this vessel is one of the earliest hallmarks of testis development that distinguishes it morphologically from the developing ovary (161, 163). Evidence now exists for a close spatial relationship between testis vascularization and cord formation (162, 164). Furthermore, all of the cells migrating from the mesonephros to the coelomic zone of the differentiating testis express endothelial markers such as VE-cadherin, an indication that incoming endothelial, rather than peritubular myoid cells, are required for testicular cord formation (164). Subsequently, Sertoli cells aggregate and enclose germ cells. The interaction between differentiating peritubular myoid cells and Sertoli cells results in the formation of basement membrane of the testicular cords. Mesenchymal cells and matrix and blood vessels fill the interstitial space, in which Leydig cells will soon appear. Beyond vascularization, which is necessary to allow efficient export of testosterone, cell migration from the mesonephros largely contributes to testicular organogenesis (165, 166) and is antagonized by the initiation of meiosis in germ cells (167).
The molecular mechanisms underlying sex-specific gonadal vascularization are being progressively unraveled. A vascular-mesenchymal cross-talk between VEGFs and PDGFs drives gonadal patterning during early fetal life (Fig. 4). VEGF-A, expressed in interstitial mesenchymal cells of the undifferentiated gonadal ridge, induces vascular endothelial cell migration to the gonad. In turn, PDGF-B expressed by the endothelial cells is responsible for an increase in cell proliferation in the gonadal interstitium, upon binding to its receptor PDGFRα. Disruption of vascular development blocks formation of testis cords (168, 169) while not affecting Sertoli and Leydig cell specification(169). In the XX gonadal ridge, WNT4 and its downstream target follistatin (FST) repress endothelial cell migration, probably by antagonizing Activin B (Fig. 4). In the XY gonad, the SRY/SOX9 pathway downregulates WNT4/FST thus allowing Activin B, VEGF and other potential as yet unidentified factors to induce male-specific gonadal vascularization (170). Genes involved in male sex determination are shown in Fig. 6.
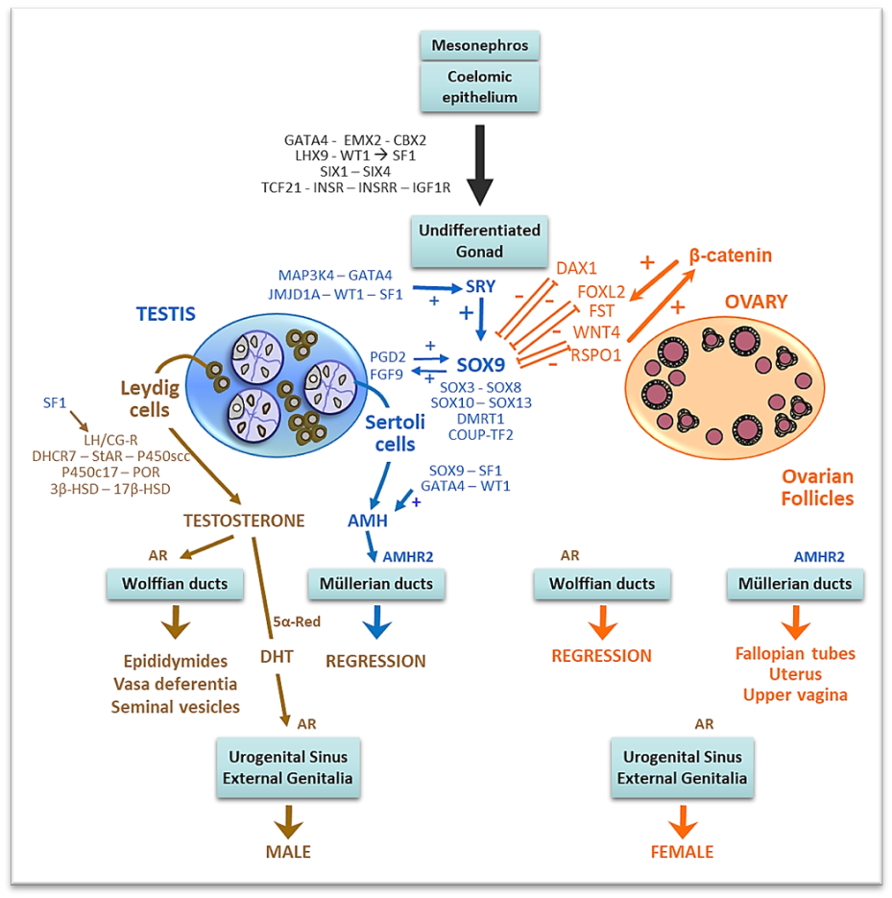
FIGURE 6. Sex determination and differentiation. Reprinted with permission from ref. (171): Grinspon RP, Rey RA Molecular Characterization of XX Maleness. International Journal of Molecular Sciences (2019) 20:6089, © 2019 by the authors. Licensee MDPI, Basel, Switzerland.
Differentiation of Sertoli and Leydig Cells
As already mentioned, both the supporting and the steroidogenic cell lineages derive from WT1-positive somatic progenitors present in the undifferentiated gonadal ridges. Wt1-positive cells can express HES1, a Notch effector, or not (38). In the subset of WT1-positive and HES1-negative cells, having delaminated from the coelomic epithelium in the central part of the indifferent gonad, SRY expression is induced giving rise to the supporting cell lineage (pre-Sertoli cells) (38, 172-174). SRY-expressing pre-Sertoli cells lying beneath the coelomic epithelium play a central role in the migration of cells from the mesonephric mesenchyme into the differentiating gonad (175). Experimental work using XX-XY chimeras has shown that not 100% of Sertoli cell precursors need to express SRY to differentiate along the male pathway: in fact, up to 10% of Sertoli cells were XX. However, a threshold number of SRY expressing –i.e. XY– cells seems to be essential in order for Sertoli cell differentiation, and thus testicular development, to be guaranteed (176).
Along with SRY, FGF9 might have a role in inducing mesonephric cell migration into the developing fetal testis and Sertoli cell differentiation. FGF9 is expressed in Sertoli cells of the fetal testis and Fgf9-null mice have dysgenetic gonads (131, 177) (see below).
Vanin-1, a cell-surface molecule involved in the regulation of cell migration, might also be responsible for differentiating Sertoli cell association with, and adhesion to, migrating peritubular cells (178). Nexin-1, expressed by early Sertoli cells, could act to maintain the integrity of the basal lamina (178).
Desert hedgehog (DHH) and its receptor PATCHED2 might also play a role in Sertoli-peritubular cell interaction and basal lamina deposition (179, 180). DHH is a protein secreted by fetal Sertoli cells, but not by somatic components of the fetal ovary, immediately after testicular determination (181). Patched2 is expressed in germ, peritubular and interstitial cells of the testis (182). Testes develop abnormally during fetal life in Dhh null mice, resulting in XY sex-reversal. Seminiferous cords are disorganized owing to defects in the basal lamina and peritubular cells, with germ cells occasionally lying in the interstitial tissue, and Leydig cells are hypoplastic (179, 180). Homozygous mutations of DHH in 46,XY patients are associated with gonadal dysgenesis (183, 184).
DHH, like other members of the hedgehog family, undergoes post-translational modifications including N-terminal palmitoylation by HHAT (hedgehog acyl-transferase), which is essential for efficient signaling. A mutation leading to defective HHAT function was found to cause complete gonadal dysgenesis and female phenotype in two 46,XY patients (185).
Testicular cord formation can be detected in human fetuses 13-20 mm crown-rump length (43-50 days) beginning in the central part of the gonad (186). Cord formation is heralded by the development of a new type of cell, the primitive Sertoli cell, characterized by a polarized, large and clear cytoplasm with abundant rough endoplasmic reticulum and complex membrane interdigitations (187), a downregulation of desmin and an upregulation of cytokeratins (188), and the expression of SOX9 (97), AMH (189, 190) and DHH (184, 191, 192). Differentiating Sertoli cells also express growth factors, like nerve growth factors (NGFs), which can induce cell migration from the mesonephros acting through their receptors TRKA (NTRK1) and TRKC (NTRK3) (193, 194). Sertoli cells aggregate around large, spherical germ cells, with a large nucleus and pale cytoplasm, called gonocytes at this stage, which can be observed in the center of testicular cord cross-sections (186). The structural basis of cord formation seems to be dependent on basal lamina deposition between Sertoli and peritubular cells with myofibroblastic characteristics (166). In the interstitial compartment, connective tissue, blood vessels and Leydig cells can be observed. As described above, one particular feature of testicular vasculature is the formation of the coelomic vessel, a large vessel that appears below the coelomic epithelium very early in testicular differentiation (161, 195). Surrounding the gonad, the basement membrane layer underlying the coelomic epithelium thickens to form the tunica albuginea.
Sertoli and germ cell numbers increase exponentially in the human fetal testis throughout the second trimester (196)in response to FSH acting through its receptor in Sertoli cells (197-199), and androgens acting indirectly through the peritubular myoid cells (200). This probably explains why newborns with congenital hypogonadotropic hypogonadism have small testes and low serum levels of Sertoli cell markers, such as AMH and inhibin B (201, 202). Sertoli cells do not reach a mature state, and meiosis is not initiated in the human testis until pubertal age, when all Sertoli cells reach a high expression level of the androgen receptor (203-206). In mice, NRG1 and its receptors ERBB2/3 are also essential for Sertoli cell proliferation, and Nrg1 gene invalidation leads to Sertoli cell hypoplasia and micro-orchidism (17).
Morphologically and functionally distinct from testicular cords, the interstitial compartment contains developing Leydig cells (Fig. 7), the main androgen producing cells in the male. The origin of Leydig cells has not been clearly established: the precursors of fetal Leydig cells have been proposed to be either migrating cells from the coelomic epithelium, the mesonephros or the neural crest or resident cells present in the adreno-gonadal primordium (reviewed in refs (20, 207, 208). According to the latter hypothesis, a subset of SF1-expressing cells gives rise to all steroidogenic lineages of the gonads and adrenal cortex. This is supported by the finding of adrenal markers (209)and adrenal-like cells in the fetal testis (210, 211) and of adrenal rests in the testes of male patients with congenital adrenal hyperplasia (212). Mesonephric cells expressing nestin, a cytoskeletal filament initially characterized in neural stem cells, are a multipotent progenitor population that gives rise to Leydig cells, pericytes and smooth muscle cells. However, the first cohort of Leydig cells derive from nestin-negative cells, confirming the multiple origins of fetal Leydig cells (213).
Another particular feature of the mouse testis is that Leydig cell populations can be divided into fetal and adult Leydig cells according to the time they arise. Fetal Leydig cells disappear after birth and are replaced by adult Leydig cells at puberty (214). Despite their similar functions, fetal and adult Leydig cells show morphologic and gene expression differences: some progenitor cells that lose Wt1 expression and are HES1-negative/GLI1-negative become located to the interstitial tissue, do not express SOX9 and differentiate into fetal Leydig cells, under the effect of the Notch signaling pathway. Another subset of cells that expresses HES1 and GLI1, under the Hedgehog signaling pathway, are not initially steroidogenic, but give rise to adult Leydig cells in postnatal life (38).
In the human fetus, Leydig cells can be identified in the interstitial tissue by the beginning of the 8th week (215) —after testicular cords have completely formed— and soon begin to produce testosterone, which plays an essential role in the stabilization of Wolffian ducts and the masculinization of external genitalia. Leydig cells also produce insulin-like growth factor 3 (INSL3), a growth factor responsible for the transabdominal phase of testicular descent (216-218). Although the initial differentiation of fetal Leydig cells depends, at least partially, on Sertoli cell-secreted PDGFs binding to PDGFRα (219) independently of gonadotropin action (220), further Leydig cell differentiation and proliferation depends on placental hCG in the first and second trimesters of fetal life and on fetal pituitary LH thereafter acting on the LH/CG receptor (221). At mid-gestation, interstitial tissue is literally packed with Leydig cells; afterwards their number decreases (196, 215).
SF1 action, is suppressed by WNT4-activated DAX1 expression (222). By counteracting WNT4, and thus downregulating DAX1 in interstitial cells of XY gonads, SRY might indirectly enhance SF1 action (223, 224). Finally, ARX is an X-chromosome gene identified in patients with X-linked lissencephaly and genital abnormalities probably associated with a block in Leydig cell differentiation (225). FGF9 (131, 177) and DHH (180) are Sertoli cell-secreted signals involved in Leydig cell differentiation.
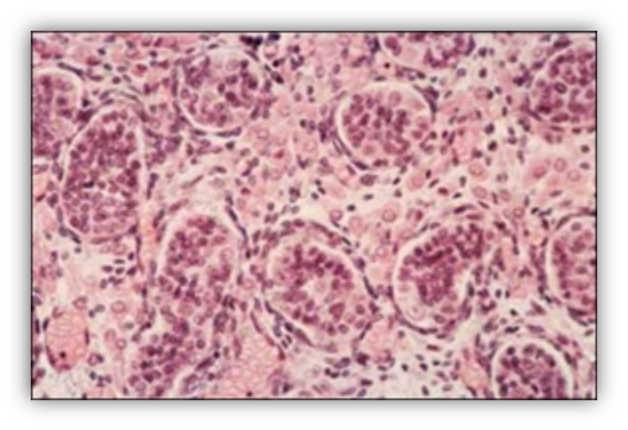
FIGURE 7. Leydig cells accumulate in the testicular interstitial tissue of a 90-mm male human fetus (11th week). Large eosinophilic Leydig cells with a prominent nucleus are interspersed with mesenchymal cells.
Timing of Testicular Differentiation
In order for the fetal testis to adequately differentiate and secrete masculinizing hormones, not only do all these factors need to be present at sufficient levels in the right cell lineage, but their expression must also be initiated within a narrow time window. In mice, the ability of SRY to induce testis development is limited to a time window of only 6 hours after the normal onset of expression in XY gonads. If SRY is expressed later, Sox9 gene activation is not maintained due to failure of FGF9/WNT4 signaling to switch to a male pattern (69).
Germ Cell Interaction with Somatic Cells in the Developing Testis: Repression of Meiosis
Upon arriving in the undifferentiated genital ridge, by the end of the 5th week, germ cells continue to proliferate by mitosis and maintain bipotentiality for approximately one week. Then germ cells in the male gonad become enclosed in the seminiferous cords and differentiate into the spermatogonial lineage, which does not enter meiosis until the onset of puberty. Gonocyte proliferation in the fetal testis is inhibited by androgens (226). Prevention of entry into meiosis was first thought to be a specific effect of male somatic cells since germ cells entering a prospective ovary or those which have failed to enter gonads of either sex enter meiosis at approximately the same time and develop into oocytes, irrespective of their chromosomal pattern (227). Subsequent studies shed light on the sexually dimorphic evolution of gametogenesis in the fetal gonads. The mesonephros from the indifferent gonad, as well as the lung and adrenal gland, synthesize retinoic acid that acts as a meiosis inducer (228, 229). Germ cells embedded in the seminiferous cords do not enter meiosis because they are protected from retinoic acid action: mouse Sertoli cells express two factors that prevent meiosis onset: FGF9 (230) and CYP26B1, an enzyme that catabolizes retinoic acid (231, 232). NANOS2, expressed in germ cells, is also a meiosis-preventing protein, since in the fetal testis it represses the expression of STRA8 (233) (for details on STRA8, see “Genetic control of oogenesis and folliculogenesis“. In human fetal testis, CYP26B1 does not seem to be expressed, and the mechanism underlying the inhibition of germ cell entry into meiosis needs to be elucidated (234, 235).
Chromosomal constitution does not influence sex differentiation of germ cells: XX germ cells surrounded by Sertoli cells differentiate into spermatogonia, whereas XY germ cells in an ovarian context differentiate into oogonia and then enter meiosis (236). However, germ cells whose karyotype is discordant with the somatic lineages fail to progress through gametogenesis and enter apoptosis later in life.
The influence of germ cells on the developing gonad is sexually dimorphic: Germ cell progression through meiosis is essential for the maintenance of the fetal ovary, otherwise prospective follicular cells degenerate and streak gonads result. In contrast, the development of the testes is not hindered by the lack of germ cells (195).
STABILIZATION OF OVARIAN DIFFERENTIATION: CELLULAR AND MOLECULAR PATHWAYS
Genetic Pathways of Ovarian Differentiation
The pathway leading to ovarian differentiation and stabilization is far more complex than what was originally hypothesized. In humans, the absence of an active SRY gene –e.g. SRY mutations or deletions of the Y chromosome involving the SRY locus– results in gonadal dysgenesis of variable degrees, but is not sufficient to allow ovarian differentiation: no oocyte meiotic progression or follicle development has been described, even during fetal life. Recent findings suggest that most probably the coordinated action of several factors is needed for the differentiation and stabilization of the ovaries (237-239) (Table 3, Figs. 4, 6 and 8).
WNT4 is a secreted protein that functions as a paracrine factor to regulate several developmental mechanisms. WNT proteins bind to the frizzled (FZ) family of membrane receptors and LRP5/6 co-receptors, leading to the activation of the phosphoprotein disheveled (DVL) and a subsequent increase in cytoplasmic β-catenin levels owing to an inhibition of its degradation rate (240). In turn, WNT4 is upregulated by the action of β-catenin, which establishes a positive feedback loop, and also indirectly by the GATA4/FOG2 complex, which represses DKK1 (241). DKK1 is capable of binding to the LRP5/6 co-receptor, thus preventing the formation of the WNT-FZ-LRP5/6 signaling complex. WNT4 is expressed at similar levels in the XY and XX bipotential gonads. When SRY upregulates SOX9 in XY gonads, and the feed-forward loops with FGF9 and PGD2 are established, WNT4 is silenced (130) (Fig. 4). In XX gonads, the absence of SRY releases WNT4 expression, which stabilizes β-catenin and silences FGF9 and SOX9 (130). WNT4 also up-regulates DAX1 (222), which antagonizes SF1 and thereby inhibits steroidogenic enzymes. WNT4-deficient XX mice express the steroidogenic enzymes 3b-hydroxysteroid dehydrogenase and 17a-hydroxylase, which are required for the production of testosterone and are normally suppressed in the developing female ovary (242). In humans, a duplication of chromosome 1 containing 1p36.12, where human WNT4 maps, causes ambiguous genitalia of XY patients, probably due to low testosterone production (222), whereas inactivation of both copies of WNT4 in XX human fetuses results in alterations in gonadal morphology, ranging from ovotestes to testes, associated with renal agenesis, adrenal hypoplasia, and pulmonary and cardiac abnormalities (SERKAL syndrome: Sex reversal with kidney, adrenal and lung abnormalities) (243). WNT4 is also involved in the development of the internal genital tract (see below).
Like WNT4, RSPO1 is expressed in the undifferentiated gonadal ridge of XY and XX embryos and increases in the XX gonads in the absence of SRY. RSPO1 binds to G protein–coupled receptors LGR4 and LGR5 (244), stimulates the expression of WNT4 and cooperates with it to increase cytoplasmic β-catenin (Fig. 5) and FST levels (245-248). RSPO1 is thought to facilitate WNT-FZ-LRP complex formation through fending off DKK1 and by sequestering ZNRF3, which promotes FZ degradation by ubiquitination and increased turnover (154, 155, 249). The increase in WNT4/β-catenin counteracts SOX9, thus leading to the ovarian pathway (170). Loss of function mutations in the human RSPO1 gene and Rspo1 gene ablation in mice result in the formation of ovotestes in the XX fetus probably owing to SOX9 upregulation (75, 170, 250).
β-catenin also activates FOXL2 winged helix/forkhead transcription factor, expressed in germ and somatic cells, more strongly in the female than the male fetal gonad from the 8th fetal week (251) and involved in granulosa cell differentiation (252, 253). The high levels of WNT4/β-catenin and FOXL2 counteract FGF9 and SOX9, thus leading to the stabilization of the ovarian differentiation pathway (238, 239). FOXL2 also represses SF1 expression by antagonizing WT1 in the XX mouse fetus (254). FOXL2 and FST are needed for the survival of meiotic germ cells (72, 255, 256). In the XY fetus, SOX9 represses FOXL2 expression in the gonad (257). Conversely, inducible deletion of Foxl2 in adult mouse ovarian follicles leads to upregulation of Sox9 and reprogramming of adult ovaries to testes (72). In goats, XX males develop in the event of a deletion in the autosomal PIS locus (258), where FOXL2 has been identified. In humans, FOXL2 mutations result in a variety of phenotypes, from streak gonads to adult ovarian failure associated with eyelid abnormalities characterized by blepharophimosis, ptosis and epicantus inversus (BPES) (259).
Germ cell entry into meiosis is a specific feature of initial ovarian differentiation (Table 3, Figs. 4 and 9). Once stabilized by the cooperative action of WNT4 and RSPO1, cytoplasmic β-catenin migrates to the nucleus and induces the expression of FST. The latter antagonizes Activin B, thus repressing endothelial cell migration and the coelomic vessel formation, one of the earliest testis-specific events (170). Wnt4 has a similar effect (256).
MAP3K1 modulates the balance between female and male pathways. As explained above (see “FGF9 and PGD2: maintaining SOX9 expression levels”), SOX9 and FGF9 upregulate AXIN1 and GSK3β, which promote the destabilization of β-catenin, thus blocking ovarian development. MAP3K1 sequestrates AXIN1; consequently, there is a stabilization of β-catenin, which favors the ovarian pathway (132). In XY patients with mutations of MAP3K1 that result in increased binding to AXIN1, there is an increase of β-catenin leading to defective testicular differentiation and finally resulting in gonadal dysgenesis (151).
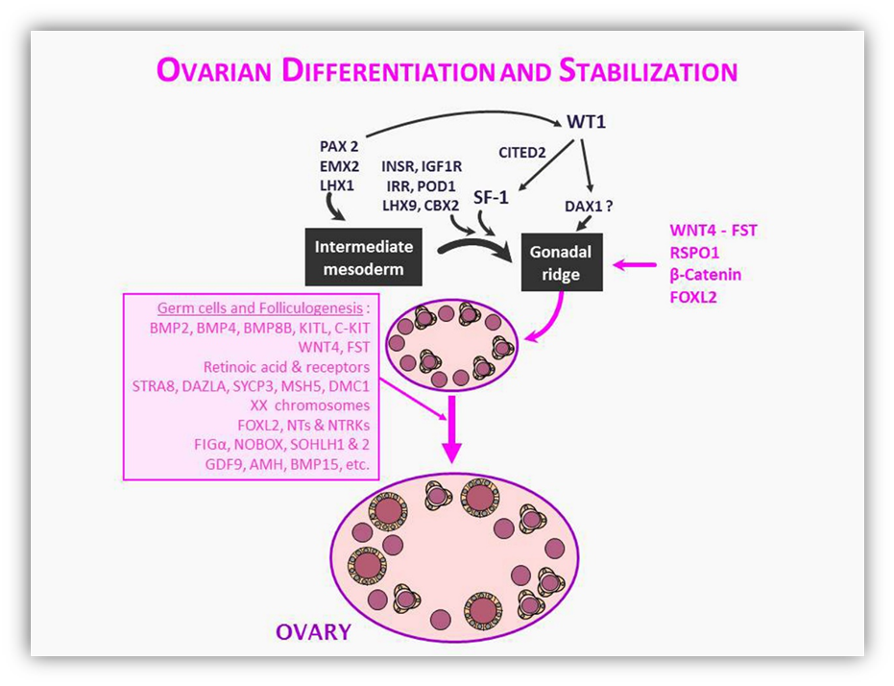
FIGURE 8. Female sex determination. As in the male, general transcription factors, as LHX1, EMX2 and PAX2, are necessary for intermediate mesoderm development. The gonadal ridge differentiates from the intermediate mesoderm following the action of SF1, LHX9 and WT1. WNT4, FST, RSPO1 and β-Catenin should be expressed to antagonize testis differentiation and promote early ovarian differentiation. Germ cell development (dependent on BMP family members, KIT ligand and its receptor C-KIT, WNT4, FST, retinoic acid and its receptors, the existence of two X chromosomes as well as several factors like DAZLA, MSH5, STRA8 and DMC1) are essential for fetal ovary stabilization. A number of other factors are involved in early folliculogenesis (FOXL2, neurotrophins and neurotrophin tyrosine kinase receptors, FIGα, NOBOX, SOHLH and members of the TGFβ family like GDF9, AMH and BMP15).
Ovarian Morphogenesis
In the XX fetus, the gonad remains histologically undifferentiated after the 7th week from a histological standpoint, but a functional differentiation is already detectable: XX gonads become capable of estradiol production at the same time as XY gonads begin to synthesize testosterone (260). PGCs proliferate by mitosis and differentiate to oogonia. Ovarian maturation proceeds from the center to the periphery. At week 10, oogonia in the deepest layers of the ovary enter meiotic prophase, the first unequivocal sign of morphological ovarian differentiation. Subsequently, oogonia become surrounded by a single layer of follicular (granulosa) cells, they enter meiosis, become oocytes and form primordial follicles (Fig. 9). Initiation of meiosis in the fetal ovary is heralded by the increase in retinoic acid levels synthesized by retinaldehyde dehydrogenase isoform 1 (encoded by ALDH1A1), expressed in the developing female gonad (261).
The earliest primary follicles appear at 15-16 weeks and the first Graafian follicles at 23-24 weeks (262, 263). By the end of the 7th month of gestation, mitotic activity has ceased and almost all germ cells have entered meiotic prophase. Oocytes proceed to the diplotene stage, where they remain until meiosis is completed at the time of ovulation in adult life. However, not all oocytes undergo meiosis: from 6-7 million ovarian follicles at 25 weeks, only 2 million persist at term (264). Most oocytes undergo apoptosis and follicles become atretic. AMH is produced, albeit in low amounts, after the 23th week of development (265) by granulosa cells from primary to antral follicles, but not by primordial follicles (266-268). The dynamics of follicle development and entry of germ cells into meiosis is notably different in rodents, in whom meiosis and folliculogenesis only progress after birth (170).
The involvement of germ cells in the stabilization of the gonadal structure is one major difference between the ovary and the testis, with germ cells being critical only in the ovaries in terms of maintenance of the somatic component of the gonad. In fact, while fetal testis development progresses normally in the absence of germ cells (269), ovarian follicles do not develop when germ cells are absent (263, 270). Furthermore, if germ cells are lost after formation of follicles, these rapidly degenerate (263, 271, 272).
In XX gonads, very few endothelial cells migrate from the mesonephros to the gonad, which suggests that cortical and medullary domains of the ovary are already established in early gonadogenesis, although no morphological boundaries are evident, consistently with molecular evidence of discrete gene expression domains specified by 12.5 dpc in the mouse ovary (255). The coelomic vessel formation, characteristic of the differentiating testis, does not occur in the normal XX gonadal ridge.
Granulosa cells, the equivalent of the Sertoli cells of the testes, originate from 3 possible sources: the ovarian surface epithelium, mesonephric cells from the adjacent rete ovarii, and the existing mesenchymal cells of the genital ridge (170, 273). Recent evidence in mice shows that many coelomic epithelial cells ingress to ovarian cortex and give rise to FOXL2-positive granulosa cells (274), confirming that other potential granulosa cell precursors are present in the gonadal ridge prior to the start of coelomic cell migration (173, 274). Theca cells, the counterpart of testicular Leydig cells, are thought to derive from fibroblast-like precursors in the ovarian stroma under the control of granulosa cells (275).
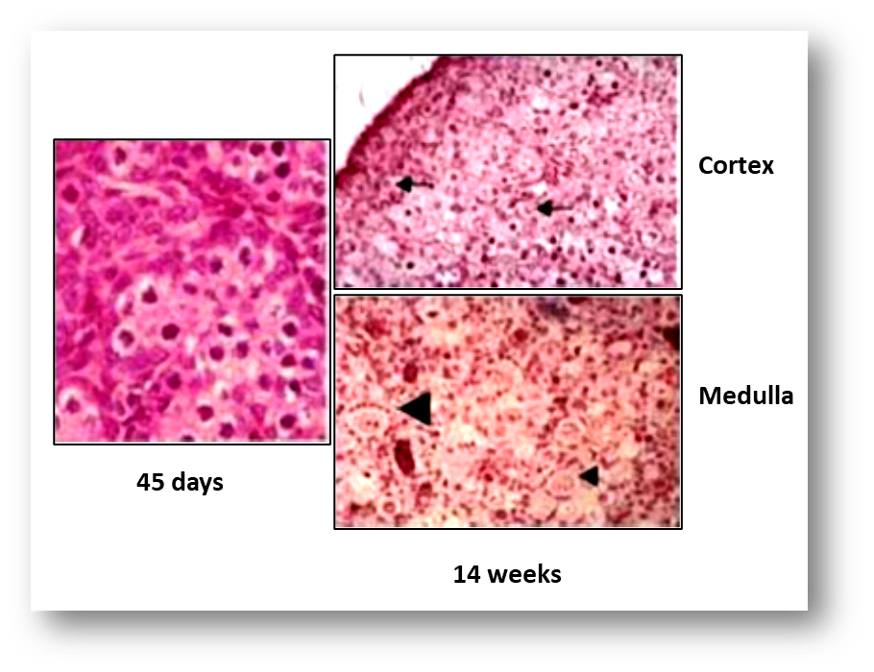
FIGURE 9. Developing human fetal ovaries. At 45 days, the ovary is recognizable only because it has not yet undergone testicular differentiation. In the cortex of the 14-week-old gonad, germ cells are aligned in rows, some of them have entered the meiotic prophase (arrows). In the medulla, primordial (small arrow head) and primary (large arrowhead) follicles are visible.
Genetic Control of Oogenesis and Folliculogenesis
Two major steps mark ovarian development: germ cell migration, proliferation and meiosis onset, followed by folliculogenesis. For a long time, it has been known that two intact X chromosomes are required in the human for ovarian differentiation and development –in contrast to the mouse, in which XY oocytes can occur in experimental conditions (65)– for ovarian differentiation and development. The lack of two X chromosomes, e.g. in Turner syndrome, results in germ cell loss and, subsequently, gonadal dysgenesis (263, 271). Therefore, all the factors involved in the proliferation and migration of PGCs in early embryogenesis (see “The Germ Cells” section) are essential for ovarian formation.
In the female gonad, germ cells continue to proliferate by mitosis. Meiotic entry is delayed until the 10th week in the human fetus and the 13th day in the mouse fetus (Table 1), due to the suppressive effect of the Polycomb repressive complex 1 (PRC1), which represses STRA8 and other factors involved in the differentiation of primordial germ cells and in early meiosis programs until retinoic acid reaches a threshold (276). Retinoic acid, synthesized by retinaldehyde dehydrogenases present in the mesonephros and the developing ovary (261, 277, 278), binds to the retinoic acid receptor (RAR) present in the germ cells and induces the expression of STRA8 (229, 234), a transcription factor that upregulates DAZL and SYCP3, two proteins involved in the formation of the synaptonemal complex essential for the onset of meiosis (39). Stabilization of oocytes requires the expression of MSH5, a protein involved in DNA mismatch repair (279). In Msh5 null mice, oocytes are lost before the diplotene stage resulting in ovarian dysgenesis. The expression of STRA8 takes place in an anterior-to-posterior wave and is followed by the upregulation of another meiotic gene Dmc1 (280). For a detailed description of other factors involved in oocyte development, see refs. (281) and (282).
A number of genes are upregulated in the human ovary before and during primordial follicle formation; their functional implications still need to be elucidated (283). In mice, neurotrophins (NTs) and their NTRK tyrosine kinase receptors facilitate follicle assembly and early follicular development (284). Factors involved in germ cell meiosis are also important. Although not essential to ovarian differentiation, several factors are involved in the development of ovarian follicles. FIGα is crucial for the formation of primordial follicles (285). AMH regulates the recruitment of primordial follicles into subsequent steps of folliculogenesis (286, 287), NOBOX, SOHLH1 and SOHLH2 are critical transcription factors during the transition from primordial to primary follicles (reviewed in ref. (39). GDF9 (288, 289) and BMP15 (290, 291) are important for follicle growth beyond the primary stage. An increasing number of factors are involved in later steps of folliculogenesis (for review, see ref. (39).
THE INTERNAL REPRODUCTIVE TRACT
The Indifferent Stage
Up to 8 weeks in the human embryo, the internal reproductive tract is similar in both sexes and consists of a set of two unipotential ducts, the Wolffian and Müllerian ducts (Fig. 10).
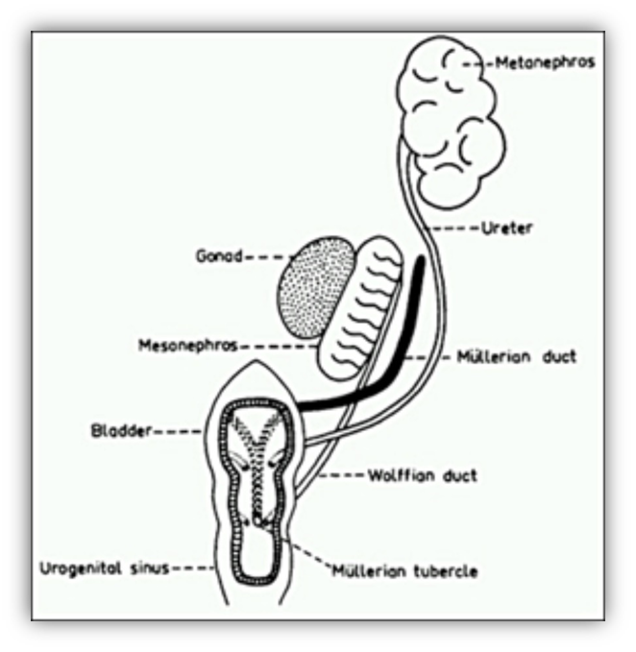
FIGURE 10. Undifferentiated reproductive tract. Both Wolffian and Müllerian ducts are present. Müllerian ducts open in the urogenital sinus at the level of the Müllerian tubercle between the orifices of the Wolffian duct.
Wolffian Ducts
In both the XX and the XY human embryo, Wolffian (mesonephric) ducts originate in the intermediate mesoderm, laterally to somites 8-13 in embryos 24 to 32 days old (Table 1) (3). Wolffian ducts elongate caudally and induce the formation of nephric tubules through a mesenchymal‑epithelial transition process. These tubules give rise, in a cephalic-to- caudal direction, to the three kidney primordia: pronephros, mesonephros and metanephros. While the pronephros and mesonephros are transient structures that soon degenerate, the metanephros is one of the main sources of the definitive kidney. Because Wolffian ducts are crucial for kidney development, abnormal formation of the Wolffian ducts is usually associated with other malformations in the urinary or genital systems.
Several factors have been identified in the induction and development of the Wolffian ducts (292, 293): PAX2 and PAX8, acting through GATA3, induce the initial formation, and LIM1 is required for the extension of the Wolffian ducts (293). EMX2 is necessary for their maintenance, whereas FGF8 and its receptors FGFR1 and FGFR2 seem to be important in the development and maintenance of different segments (cranial or caudal) of the Wolffian ducts (293).
A single ureteric bud evaginates from the Wolffian duct and grows dorsally, in response to inductive signals from metanephric mesenchyme involving GREMLIN1, BMP4 and BMP7 (294). RET signaling is involved in multiple aspects of early Wolffian duct development (295). Growing caudally, Wolffian ducts undergo extensive elongation and coiling while progressively acquiring a lumen. Factors involved in Wolffian duct stabilization, elongation and coiling include the SFRP1 and SFRP2, VANGL2, WNT5A and PKD1 (293).
As the Wolffian ducts elongate towards the cloaca, they induce the formation of the mesonephric tubules, most of which finally undergo regression, except close to the testes. There is a number of factors involved in mesonephric tubule development, including PAX2, PAX3, PAX8, GATA3, OSR1, WNT9B, WT1, SIX1, FGFR1, FGFR2, FGFR8 and SHH (292, 293, 296, 297). The mesonephric tubules give rise to the efferent ducts connecting the rete testis with the epididymis. WNT9B knockout male mice fail to develop the efferent ducts and the epididymis (298). Epididymal disjunction from the rete testis reflects a defect in these processes and can be found in approximately 40 % of patients with cryptorchidism (299).
The Wolffian ducts finally reach the caudal part of the hindgut, the cloaca. A spatiotemporally process of regulated apoptosis in both the Wolffian ducts and the cloaca is necessary for Wolffian duct insertion into the cloaca (300). The Wolffian ducts become incorporated into the male genital system when renal function is taken over by the definitive kidney, the metanephros (301).
Müllerian Ducts
Müllerian (paramesonephric) ducts, which give rise to most of the female reproductive tract, develop after Wolffian ducts in the urogenital ridges of both XX and XY embryos. They arise in 10-mm human embryo (5–6 weeks of gestation) as a cleft lined by the coelomic epithelium, between the gonadal and mesonephric parts of the urogenital ridge (3). This coelomic opening will later constitute the abdominal ostium of the Fallopian tube. The cleft is closed caudally by a solid bud of epithelial cells, which burrows in the mesenchyme lateral to the Wolffian ducts and then travels caudally inside their basal lamina. Initially, these cells are mesoepithelial, i.e. they exhibit characteristics of both the epithelium and the mesenchyme; they will become completely epithelial only in the female, at the time male ducts begin to regress (302, 303). At 8 weeks of development, the growing solid tip of the Müllerian duct, now in the pelvis, lies medial to the Wolffian duct, having crossed it ventrally in its downward course. For a while, the two Müllerian ducts are in intimate contact, then they fuse, giving rise to the uterovaginal canal (Fig. 11), which makes contact with the posterior wall of the urogenital sinus, causing an elevation, the Müllerian tubercle, flanked on both sides by the opening of the Wolffian ducts (Fig. 10).
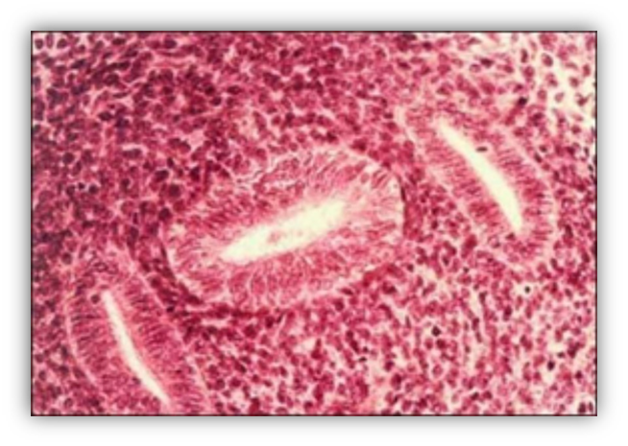
FIGURE 11. Fused Müllerian ducts flanked by Wolffian ducts in the lower reproductive tract of a 50-mm female human fetus (10th week).
Development of the Müllerian duct occurs in three phases (Fig. 12) (302, 303). First, cells of the coelomic epithelium are specified to a Müllerian duct fate. These can be identified by a placode-like thickening of the coelomic epithelium and by the expression of LHX1 (302, 304) and anti-Müllerian hormone receptor type II (AMHR2) (305, 306). Transcriptional co-factors DACH1 and DACH2 are required for the formation of Müllerian ducts, possibly by regulating the expression of LHX1 and WNT7A or other factors important for Müllerian duct formation (307, 308).
During the second phase, these primordial Müllerian cells invaginate from the coelomic epithelium to reach the Wolffian duct. WNT4 expression in the mesonephric mesenchyme is essential for the Müllerian duct progenitor cells to begin invagination (304, 309).
The third or elongation phase begins when the invaginating tip of the Müllerian duct contacts the Wolffian duct. This phase consists in the proliferation and caudal migration of a group of cells at the most caudal tip. Müllerian duct elongation continues in close proximity to the Wolffian duct, then Müllerian ducts cross Wolffian ducts ventrally and fuse centrally close to the urogenital sinus.
As could be expected, integrity of protein kinase pathways is required for cell proliferation (310). Close contact with the Wolffian duct is also necessary to Müllerian growth; indeed, the lack of transcription factors required for Wolffian development, such as LIM1 or PAX2, leads to Müllerian truncation (see Table 4). Wolffian ducts do not contribute cells to the elongating Müllerian tip (302, 311), but act by supplying WNT9B, secreted by Wolffian epithelium (298).
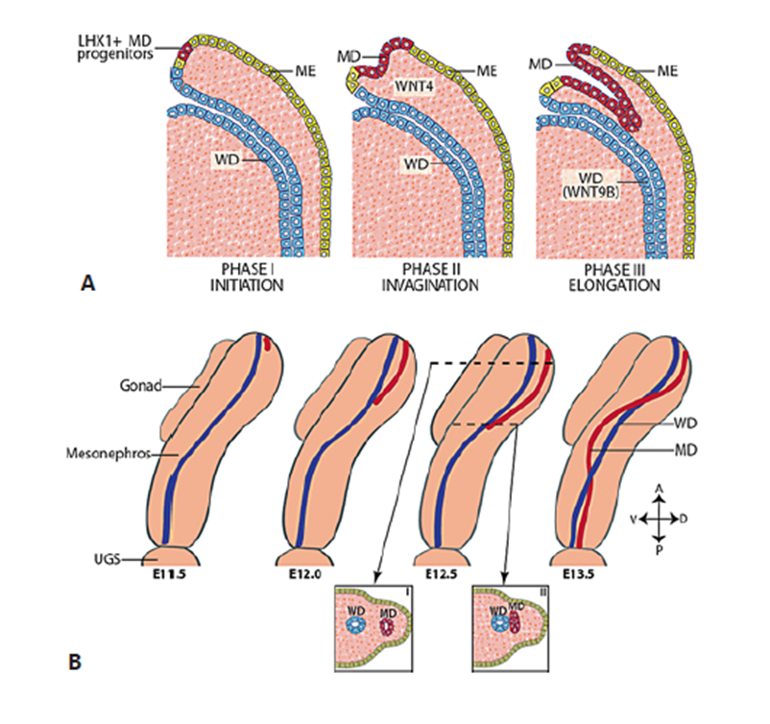
FIGURE 12. Müllerian duct (MD) development can be subdivided into three phases. A. Phase I (initiation): MD progenitor cells in the mesonephric epithelium (ME) (yellow) are specified and begin to express LHX1. Phase II (invagination): in response to WNT4 signaling from the mesenchyme, LHX1+ MD progenitor cells invaginate caudally into the mesonephros towards the WD (blue). Phase III (elongation): the tip of the MD contacts the WD and elongates caudally in close proximity to the WD requiring structure and WNT9B signaling from the WD. B. Beginning at ∼ E11.5 in mice, the MD invaginates and extends posteriorly guided by the WD. During elongation, mesenchymal cells separate the WD and MD anterior to the growing tip (inset I). However, at the MD tip, the MD and WD are in contact (inset II). At ∼ E12.5, the MD crosses over the WD to be located medially. Elongation is complete by ∼ E13.5 with the MD reaching the urogenital sinus (UGS). A = anterior (rostral); D = dorsal; P = posterior (caudal); V = ventral. Reprinted with permission from ref. (303): Mullen RD, Behringer RR. Molecular Genetics of Müllerian Duct Formation, Regression and Differentiation. Sexual Development 8:281-296 (2014), Copyright 2014, Karger.
Caudally each Müllerian duct contacts the urogenital sinus at the Müllerian tubercle. This is a critical step and its failure can lead to lower vaginal agenesis, as it has been observed in Lhfpl2 mutant mice (312). In weeks 7 and 8, the caudal portions of the Müllerian ducts lie between the two Wolffian ducts near the urogenital sinus. Then during the 8thweek, Müllerian ducts fuse in the midline, leaving temporarily an epithelial septum that disappears one week later giving rise to the midline uterovaginal canal. The degree of midline fusion of Müllerian ducts is extensive in humans, but it is almost inexistent in mice, exhibiting paired oviducts and large bilateral uterine horns. Defects in Müllerian duct fusion and retention of the midline septum can lead to various congenital malformations in humans, including separate hemiuteri, uterus didelphys or unicornis, double vagina or cervix, vagina with septum etc. (313).
MALE DIFFERENTIATION OF INTERNAL GENITALIA
Male differentiation of the internal genital tract is characterized by regression of Müllerian ducts and differentiation of the Wolffian duct into male accessory organs.
Müllerian Duct Regression
Müllerian regression, the first sign of male differentiation of the genital tract, occurs in 55 to 60 day-old human embryos (Fig. 13), triggered by anti-Müllerian hormone (AMH) at the center of a complex gene regulatory network (reviewed in ref (314)). Once initiated, the regression of the Müllerian duct extends caudally as well as cranially, sparing the cranial tip which becomes the Morgagni hydatid, and the caudal end, which participates in the organogenesis of the prostatic utricle. Müllerian regression of the cranial part of the Müllerian duct begins while the duct is still growing caudally towards the urogenital sinus (315) and is characterized by a wave of apoptosis spreading along the Müllerian duct (316, 317). Extra-cellular matrix is deposited in the peri-Müllerian mesenchyme (318), which progressively strangles the Müllerian duct epithelium and finally remains the only witness of its former existence. Mesenchymal changes are preceded by the dissolution of the basement membrane, which precipitates apoptosis and allows extrusion of epithelial cells and their transformation into mesenchymal cells (317, 319). Epithelial-mesenchymal transformation is an important factor of epithelial cell loss during Müllerian regression.
Integrity of the WNT/β-catenin pathway is required for complete Müllerian duct regression in the male, perhaps through amplification of the AMH signal (320). β-catenin accumulates in the nucleus (317) upregulating Osterix (Osx), also called Sp7, an AMH-induced gene that regulates the expression of matrix metallopeptidase 2 (MMP2) (321). Osxis expressed in male, but not female, Müllerian ducts before and during regression. Overexpression of human AMH in female fetuses induces Osx, and Amhr2 knockout males lose Osx expression. Additionally, conditionally invalidation of β-catenin in the Müllerian ducts leads to a reduction in Osx expression, indicating that OSX is downstream of β-catenin in the regression pathway.
Wif1 (WNT inhibitory factor 1) encodes a secreted frizzled-related protein that inhibits WNT signaling. WIF1 shows many similarities to OSX: it is expressed in the male, but not the female, Müllerian duct and is not detected in Amhr2knockout mice. However, Müllerian ducts are absent in Wif1 knockout male mice, which implies that WIF1 is not indispensable for Müllerian duct regression (322).
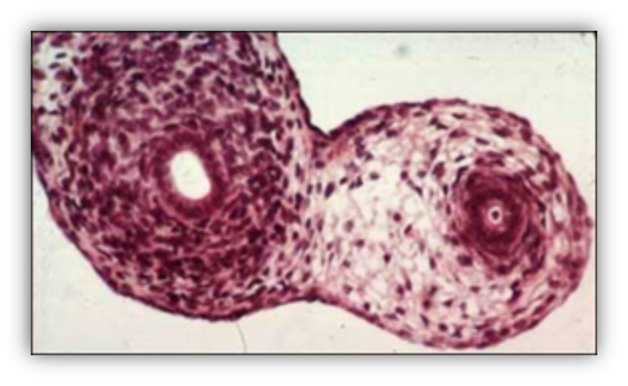
FIGURE 13. Regressing Müllerian duct in a 35-mm male human fetus (9th week). Note the fibroblastic ring surrounding the epithelium of the Müllerian duct (right), the Wolffian duct is visible on the left.
Stabilization and Differentiation of Wolffian Ducts
The second aspect of male differentiation of the internal genital tract is the stabilization and differentiation of the Wolffian ducts (323). After the loss of mesonephric functional activity, the mesonephric nephrons and caudal tubules degenerate but the cranial tubules persist to form the male efferent ducts. The connections between the mesonephric tubules and the gonadal primordium are permanently established in the sixth week; in the male, they give rise to the rete testis, while in the female, they form the rete ovarii. Between weeks 9 and 13 in the human embryo, the upper part of the Wolffian duct differentiates into the epididymis. Below, it is surrounded by a layer of smooth muscle and becomes the vas deferens, which opens into the urogenital sinus at the level of Müllerian tubercle. In sexually ambiguous individuals, in whom Wolffian and Müllerian ducts coexist, the vas deferens is embedded in the uterine and vaginal walls (reviewed in ref. (324). The seminal vesicle originates from a dilatation of the terminal portion of the vas deferens in 12-week-old fetuses.
Testicular Descent
During human fetal development, the testis migrates from its initial pararenal position to its terminal location in the scrotum (Fig. 14). Testicular descent has been subdivided into several phases (325). Initially, the upper pole of the testis is connected to the posterior abdominal wall by the cranial suspensory ligament while a primitive gubernaculum extends from the caudal pole to the inner inguinal ring. At 12 weeks, the cranial suspensory ligament dissolves and the gubernaculum testis swells and pulls the testis down to the inguinal ring. After 25 weeks, the gubernaculum bulges beyond the external inguinal ring and is hollowed out by a peritoneal diverticulum called the processus vaginalis. The second –inguinoscrotal– phase of testicular descent occurs between 27 and 35 weeks after conception. « Physiological » cryptorchidism is frequent in premature infants. In the female, the cranial ligament holds the ovary in a high position and the gubernaculum, now the round ligament, remains long and thin.
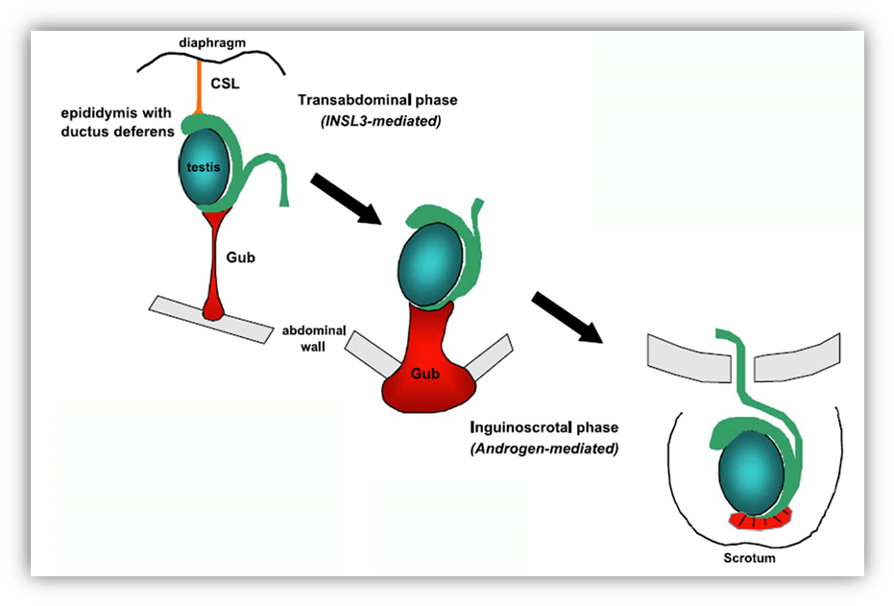
FIGURE 14. Testicular descent. Left, Initial phase: the primitive gonad is located near the kidney, held by the cranial suspensory ligament (CSL) and the gubernaculum testis. Center, Transabdominal descent: androgen-mediated dissolution of the CSL and insulin like factor 3 (INSL3) mediated swelling of the gubernaculum bring the testis to the internal orifice of the inguinal canal. Right, Inguino-scrotal migration: the testis passes through the inguinal canal into the scrotum, this phase is androgen-dependent. Reprinted from ref. (325): Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Developmental Biology, 270:1-18 (2004), Copyright 2004, with permission from Elsevier. http://www.sciencedirect.com/science/article/pii/S001216060400137X
FEMALE DIFFERENTIATION OF INTERNAL GENITALIA
Female differentiation of the internal genital tract is characterized by the disappearance of the Wolffian ducts, which is complete at 90 days of human fetal development, except for vestiges such as the Rosenmüller organs or Gartner canals. Traditionally, the regression of Wolffian ducts in the female fetus has been ascribed to a passive process deriving from the lack of androgen action. Recent work using a Nr2f2 (encoding COUP-TF2) deletion, conditionally targeted to the Wolffian mesenchyme, has shown that the regression of Wolffian ducts in female embryos is an active process induced by COUP-TF2 through inhibition of the expression of FGFs, which otherwise activate the p-ERK pathway in the Wolffian duct epithelium for its maintenance (326). How androgens interact with this mechanism in males needs to be elucidated.
Müllerian ducts persist, establish apico-basal characteristics and develop into an epithelial tube that will give rise to the endometrium (302), while the surrounding mesenchyme differentiates into the myometrium of the uterus and Fallopian tubes (306). The acquisition of true epithelial characteristics signals the end of the AMH-sensitive window of Müllerian ducts (302). Tubal differentiation involves formation of fimbriae and folds in the ampullary region (Fig. 15) and acquisition of cilia and secretory activity by the high columnar epithelium. The uterotubal junction is demarcated by an abrupt increase in the diameter of the uterine segment and by the development of epithelial crypts. The early endometrium is lined by a closely packed columnar epithelium in which gland formation and vacuolated cells can be recognized as gestation advances. The cervix occupies the distal two-thirds of the fetal uterus.
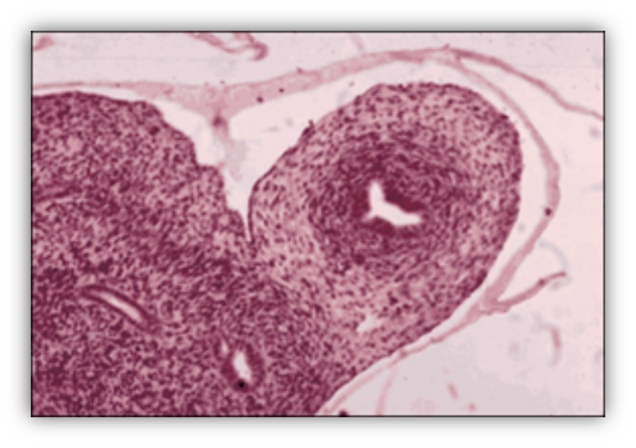
FIGURE 15. Müllerian ducts develop into the uterus and fallopian tubes
THE UROGENITAL SINUS AND EXTERNAL GENITALIA
The Indifferent Stage
Up to approximately 9 weeks, the urogenital sinus and external genitalia remain undifferentiated (Fig. 16). The urogenital sinus is individualized in 7-9 mm (~5 week) human embryos, when a transverse urorectal septum divides the cloaca into the rectum dorsally and the primitive urogenital sinus ventrally. The Müllerian tubercle demarcates the cranial vesicourethral canal from the caudal urogenital sinus.
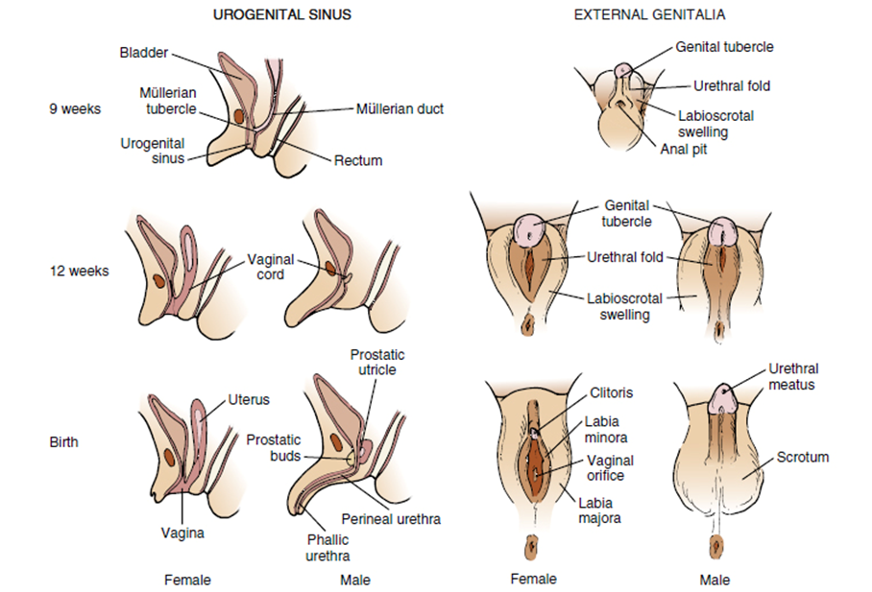
FIGURE 16. Sex differentiation of urogenital sinus (left) and external genitalia (right).
The cloaca is closed by the cloacal membrane, formed by ectoderm and endoderm, with no mesoderm in between. In the 5th week, mesodermal cells spread along the cloacal membrane and give rise to pair of swellings –the cloacal folds–, which form urogenital folds flanking the urogenital sinus and anal folds posteriorly. The urogenital folds fuse anteriorly to the cloacal membrane in the midline to form the genital tubercle. The cloacal membrane is divided by the urorectal septum into the genital membrane anteriorly and the anal membrane posteriorly. The genital membrane disappears in 20-22 mm (~8 week) embryos (327).
In embryos 8-15 mm long (~6 weeks), the opening of the urogenital sinus, the ostium, is surrounded by the labioscrotal swellings, which develop on each side of the urogenital folds. These are connected to the caudal poles of the genital ridges by fibrous bands which later develop into the gubernaculum testis in males and the round ligament in females.
The genital tubercle, consisting of lateral plate mesoderm and surface ectoderm, emerges as a ventral medial outgrowth just cranial to the opening of the ostium (328). Endodermal epithelial cells from the urogenital sinus are thought to invade the genital tubercle to form the midline epithelial urethral plate, which lies in the roof of the primary urethral groove and extends to the tip of the phallus (329, 330). After the corpora cavernosa and glans have differentiated, the ventral surface of the genital tubercle is depressed by a deep furrow, the urethral groove. The external genitalia remain undifferentiated up to approximately 9 weeks (327) (Fig. 16).
At 12 weeks in males and females alike, the vaginal primordium is formed by the caudal tips of the Müllerian ducts, and medial and lateral outgrowths of the urogenital sinus, the sinovaginal bulbs, which fuse to form the vaginal cord or plate. When the cells of the vaginal plate desquamate, the vaginal lumen is formed.
MALE DIFFERENTIATION
Urogenital Sinus and Prostate
Male orientation of the urogenital sinus is characterized by prostatic development and by the repression of vaginal development. Prostatic buds appear at approximately 10 weeks at the site of the Müllerian tubercle and grow into solid branching cords. Maturation of the prostatic gland is accompanied by development of the prostatic utricle. Two buds of epithelial cells, called the sino-utricular bulbs in the male, develop from the urogenital sinus close to the opening of the Wolffian ducts and grow inwards, fusing with the medial Müllerian tubercle, to form the sino-utricular cord, enclosed within the prostate gland, which canalizes at 18 weeks to form the prostatic utricle, the male equivalent of the vagina (331).
External Genitalia
Masculinization of the external genitalia begins in human male fetuses 35-40 mm long (~9 weeks) by lengthening of the anogenital distance (327) (Fig. 16). Fusion of the labioscrotal folds, in a dorsal to ventral fashion, forms the epithelial seam (332), which closes the primary urethral groove. The literature concerning penile development is controversial. Most textbooks describe it as a two-step process, with the proximal urethra forming by fusion of the urethral folds around the urethral plate and the distal urethra arising from an invagination of the apical ectoderm. However, according to Cunha and colleagues (333), the entire human male urethra is of endodermal origin, formed by the urethral plate dorsally and the fused urethral folds ventrally. The seam is remodeled into the tubularized urethra without connection to the epidermis. The ventrally discarded excess epithelial cells migrate into the ventral skin of the penis. Abnormalities of seam formation or remodeling could explain the vast majority of cases of hypospadias in which defects of androgen synthesis or metabolism cannot be demonstrated (334).
Urethral organogenesis is complete at 14 weeks, apart from a physiological ventral curvature, which can persist up to 6 months of gestation. However, surprisingly, no size difference exists between penile or clitoral size until 14 weeks (335) despite the fact that serum testosterone levels peak between 11 to 14 weeks in males (336). The insensitivity of the male genital tubercle to high levels of androgens during the second trimester does not correspond to a low expression of the androgen receptor or of 5α-reductase type 2 in the corpora cavernosa (337). Maximal phallic growth occurs during the third trimester of fetal life, at a time when male testosterone levels are declining. The action of the growth hormone-insulin-like growth factor system (GH-IGFs) is partly responsible for penile growth, independently of androgens (338-340).
FEMALE DIFFERENTIATION
Female orientation of the urogenital sinus is characterized by lack of prostatic differentiation and the acquisition of a separate vaginal opening on the surface of the perineum (Fig. 16). At the end of the ambisexual stage, the vaginal anlage is located just underneath the bladder neck. In females, the lower end of the vagina slides down along the urethra until the vaginal rudiment opens directly on the surface of the perineum at 22 weeks. The hymen marks the separation between the vagina and the diminutive urogenital sinus, which becomes the vestibule.
The embryological origin of the vagina is still hotly debated. In the generally accepted view, the upper part of the vagina derives from the Müllerian ducts and the lower part from the sinovaginal bulbs, which by fusion form the vaginal plate, derived from the urogenital sinus (341). It is now thought that the Wolffian ducts do not contribute cells to the sinovaginal bulbs but they may have a helper function during downward movement of the vaginal bud in the female (342). Atresia of the vagina in the Mayer-Rokitansky-Küster-Hauser syndrome could be explained by the failure of Wolffian and Müllerian ducts to descend caudally.
Development of female external genitalia is essentially static. The anogenital distance does not increase, the rims of the urethral groove do not fuse, the urethral plate persists as an epithelial cord, and the labioscrotal swellings give rise to the labia majora. The dorsal commissure forms at their junction. The genital folds remain separate and become the labia minora. When the vagina acquires a separate perineal opening, the diminutive pars pelvina and the pars phallica of the urogenital sinus become the vestibule.
CONTROL OF SEX DIFFERENTIATION
Growth Factors
Molecular genetic studies in the mouse have contributed to the identification of growth factors essential for the formation of the sexual ducts (Table 4) [see refs. (323) and (343) for review]. Since Wolffian ducts are required for the elongation of Müllerian ducts, absence of growth factors necessary to Wolffian development will per se induce Müllerian truncation. Many growth factors, such as LIM1, EMX2, HOXA13, PAX2 and 8 and VANGL2 are essential also for the development of other organs. In contrast the role of WNT4A and WNT7A, a subset of the Wnt family homologous to the Drosophila wingless gene, is restricted to reproductive organs. WNT4 is required in both sexes for the initial formation of Müllerian ducts (309), mutations of WNT4 have been reported in three cases of Müllerian aplasia associated with hyperandrogenism in girls (reviewed in refs. (344-347), but have not been detected in classical forms of the Rokitansky-Küster-Mayer syndrome (240, 348). WNT7 is required for the expression of AMHR2; in its absence the Müllerian ducts do not regress in male fetuses (349). Members of the dachsung gene family, DACH 1 and 2 also play a role by regulating the expression of LIM1 and WNT7 (307).
Congenital bilateral absence of the vas deferens affects 97-98%% of patients suffering from cystic fibrosis, a bronchial and pancreatic disease due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) (350). Whether efferent duct maldevelopment is a primary defect of cystic fibrosis or a secondary degenerative change resulting from obstruction by mucus is not known at the present time.
|
TABLE 4. Consequences of Null Mutations of Growth Factors on Morphogenesis of Genital Ducts
|
|
Growthfactors
|
Wolffian ducts
|
Müllerian ducts
|
Gonads
|
References
|
|
β-catenin
|
Normal
|
Lack of oviduct coiling.
Lack of regression
|
Loss of germ cells in the ovary.
Testes normal
|
(320, 351, 352)
|
|
DACH1/DACH2
|
Normal
|
Hypoplasia of female reproductive tract
|
Normal
|
(307)
|
|
DICER1
|
Normal
|
Hypoplasia of female reproductive tract
|
Reduced ovulation rate
|
(353)
|
|
EMX2
|
Early degeneration
|
Do not form
|
Absent
|
(5)
|
|
HOXA13
|
Rostral ureteral junction
|
Agenesis of caudal portion
|
Normal
|
(354)
|
|
IGF1
|
Agenesis of caudal portion
|
Infantile uterus
|
No ovulation.
Abnormal Leydig cells.
|
(355)
|
|
LIM1 (LHX1)
|
Do not form
|
Do not form
|
Normal
|
(304)
|
|
PI3K/AKT
|
Increased apoptosis
|
Increased apoptosis
|
|
(310)
|
|
PAX2
|
Early degeneration
|
Early degeneration
|
Normal
|
(356)
|
|
PAX8
|
Normal
|
Endometrium does not form
|
Normal
|
(356, 357)
|
|
Retinoic acid receptors
|
Agenesis of vas deferens and seminal vesicles
|
Agenesis of uterus and cranial vagina
|
Normal
|
(358)
|
|
WNT4
|
Persist in females
No regression in males
|
Do not form
|
Ovary produces testosterone
|
(320)
|
|
WNT7A
|
Normal
|
Persist in males
|
|
(349, 359, 360)
|
VAGINA, PROSTATE, URETHRA, AND EXTERNAL GENITALIA
Correct vaginal development requires Wnt, Pax and Vangl2 genes (Table 5). Vaginal abnormalities similar to those elicited by diethylstilbestrol (DES) administration, i.e. vaginal clear-cell adenocarcinoma, vaginal adenosis, transverse vaginal ridges and structural malformations of the cervix and uterus, occur in transgenic mice deficient in WNT7A, a signaling molecule expressed by the Müllerian epithelium, suggesting that DES exposure acts by deregulating WNT7A during uterine morphogenesis (361). WNT7A deficiency could act by interfering with normal mesenchymal-epithelial signaling, which is required for correct morphogenesis of the reproductive tract. Vaginal opening is regulated by PAX8 (357) and VANGL2 as shown in the mutated the loop-tail mouse (362).
SOX9 (363) and FGF10 (364) both play a role in early prostate bud differentiation.
The secreted frizzled-related proteins (SFRP1 and 2) are required for correct gubernaculum development and testicular descent (365).
Early patterning of external genitalia is regulated by a cascade of signaling molecules which orchestrate interaction between tissue layers and mesenchymal/epithelial tissues (Table 5). External genitalia are appendages emerging from the caudal body trunk, hence many genes which pattern distal limb development also play a predominant role during genital tubercle formation, for example BMPs (328, 366), Fgf-8 and 10, Hox gene families (for reviews, see refs. (325, 367). β-catenin activates Fgf8 expression in the urethra, required for normal genital tubercle outgrowth (368). Sonic hedgehog (SHH) signaling regulates many of the mesenchymal genes involved (325, 328, 369-371) (Fig. 17). The homeotic genes Hoxa13 and Hoxd13 act in a partially redundant manner since double null mutants show more severe urogenital abnormalities than those with at least one functional allele (372).
Ephrin family factor EFNB2 and receptors EPHB2 and EPHB3 mediate cell adhesion and patterning events occurring at the midline, including urethral closure and scrotal fusion, as well as palate fusion (328, 373). Diacylglycerol kinase K (DGKK), an enzyme that phosphorylates diacylglycerol, is expressed in the epithelial cells of the urethral plate (374). In humans, DGKK is strongly associated with hypospadias risk (375, 376). Regulation of urethral tube closure during the androgen-dependent phase of penile development is mediated by FGF10, signaling through the IIIb isoform of fibroblast growth receptor 2 (FGFR2-3b), suggesting that these genes are downstream targets of the androgen receptor (377).
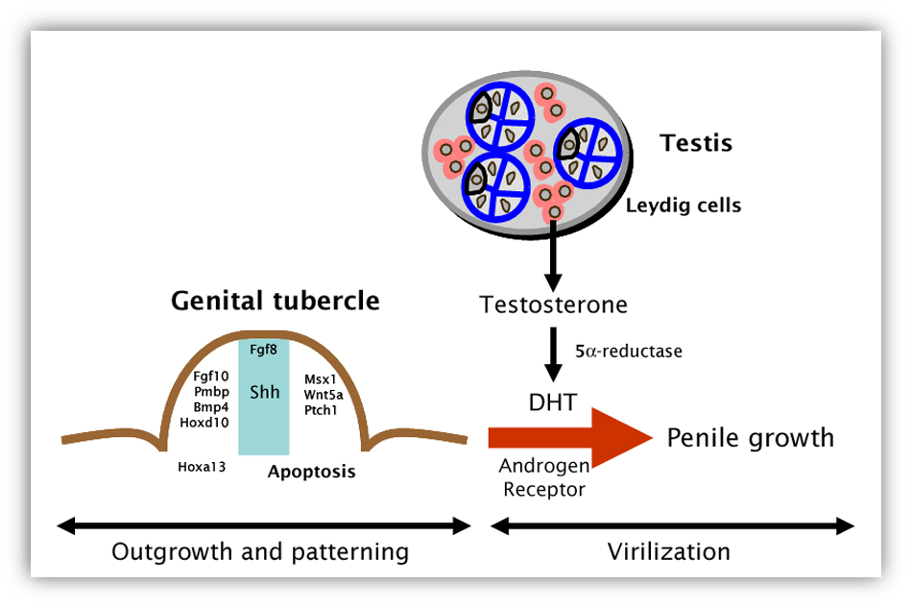
FIGURE 17. Growth factors regulating the outgrowth and ambisexual differentiation of the external genitalia. Role of sonic hedgehog (Shh) in the outgrowth and ambisexual differentiation of the genital tubercle (see table 5 for references). Most factors, with the exception of Hoxa13, are regulated by sonic hedgehog (Shh), expressed in the urethral epithelium (light green), and are identical to those regulating limb morphogenesis. Apoptosis is also affected by Shh. Data obtained from ref. (325): Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Developmental Biology, 270:1-18 (2004). http://www.sciencedirect.com/science/article/pii/S001216060400137X.
|
TABLE 5. Growth Factors in Urogenital Development
|
|
Growth factors
|
Role in urogenital development
|
References
|
|
BMP4
|
Restricts prostate ductal budding
|
(378)
|
|
BMP7
|
Closure of the distal urethra
|
(367)
|
|
FGF8
|
Initiation of genital swellings;
|
(379)
|
|
Ephrins
|
Urethral closure and scrotal fusion
|
(373)
|
|
FGF10
|
Development of the glans penis and clitoridis, and prostate
|
(364, 369, 379)
|
|
FGFR2-IIIB
|
Null mice exhibit severe hypospadias
|
(377)
|
|
HOXA10
|
Atrophic seminal vesicles in null mice
|
(380)
|
|
HOXA13
|
In mice, semi-dominant mutations lead to limb defects, vaginal hypoplasia and deficiency of the os penis (Hypodactyly syndrome)
In humans, an autosomal dominant mutation produces limb and uterine abnormalities and urinary tract malformations (Hand-Foot-Genital syndrome)
|
(354, 381)
|
|
HOXD13
|
Hoxd-13 null mice display decreased ductal branching in the prostate and seminal vesicle and agenesis of bulbourethral gland
|
(382)
|
|
HOXA13/HOXD13 null mutants
|
No genital tubercle, no partition of the cloaca in double mutants
|
(372)
|
|
LTAP
|
Vaginal opening
|
(362)
|
|
MSX2
|
Disruption of vaginal epithelium and lack of caudal Wolffian regression
|
(383)
|
|
PAX8
|
Vaginal opening
|
(357)
|
|
SHH/GLI2
|
Outgrowth and patterning of external genitalia and urogenital sinus
Development of prostatic ducts
Inhibition of apoptosis in penile smooth muscle
Masculinization of external genitalia
|
(369, 371, 384, 385)
|
|
SOX9
|
Lack of ventral prostate development
|
(363)
|
|
SFRP1 and 2
|
Testicular descent
|
(365)
|
|
VANGL2 (looptail mouse)
|
Imperforate vagina
|
(362, 386)
|
|
WNT/β-catenin
|
Masculinization of external genitalia
|
(387)
|
HORMONAL CONTROL OF MALE SEX DIFFERENTIATION
The classical experiments of Jost (58, 59) (Fig. 2) have taught us that the reproductive tract, whatever its genetic sex, will develop along female lines provided it is not exposed to testicular hormones, the main forces driving male sex differentiation (Fig. 18).
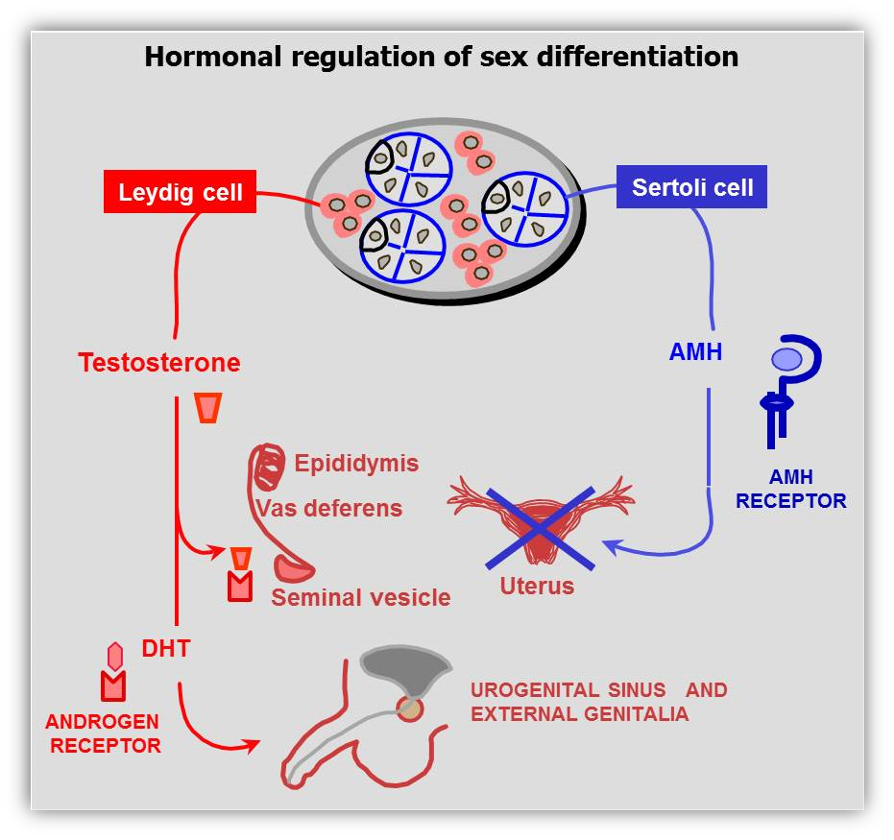
FIGURE 18. Hormonal control of male sex differentiation.
Anti-Müllerian Hormone (AMH)
Anti-Müllerian hormone (AMH), a member of the TGFβ family, triggers Müllerian regression, the first step of male sex somatic differentiation. AMH is expressed at high levels by Sertoli cells from the time of testicular differentiation (Fig. 19) until puberty and at lower levels thereafter (for reviews, see refs. (324, 388)). In the female, AMH begins to be produced in the second half of fetal life by granulosa cells of growing follicles (265, 266).
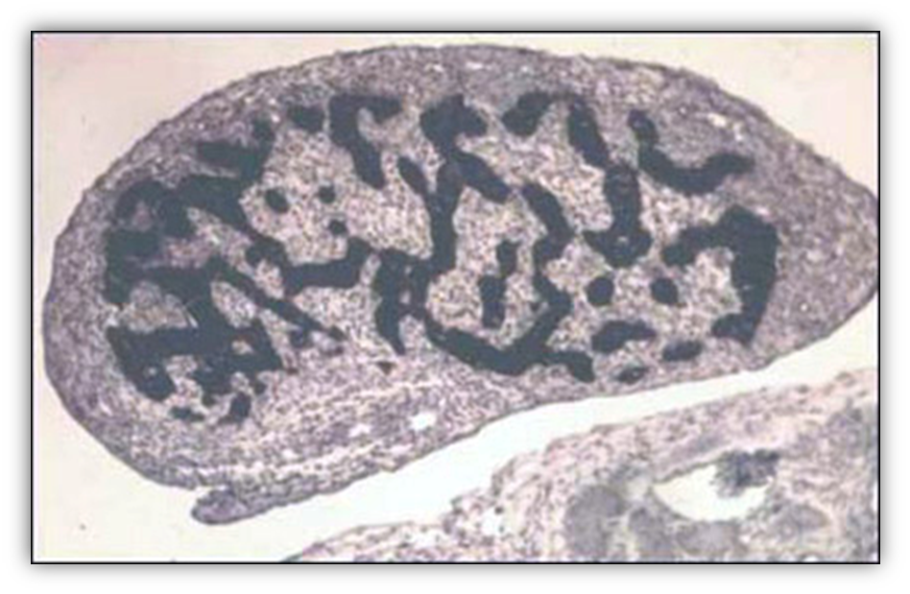
FIGURE 19. AMH protein expression by seminiferous tubules of an 11-week-old male human fetus, using an AMH-specific polyclonal antibody. Note the strong staining of seminiferous tubules.
Low expression of AMH and/or its type II receptor AMHR2 has also been identified in spermatocytes of maturing rat testis (389), the endometrium (390), the brain (391), hypothalamus (392), motor neurons (393) and female pituitary (394).
TGFβ family ligands are translated as dimeric precursor proteins comprising two polypeptide chains, each containing a large N-terminal pro-region and a much smaller C-terminal mature domain. Processing involves cleavage at sites between the two domains and dissociation of the pro-region domain. The AMH molecule is initially synthesized as a biologically inactive precursor. The precursor is cleaved by proteolytic enzymes into C and N terminal fragments which remain associated by non-covalent bonds (395, 396). Whether cleavage occurs at the time of secretion or within the target tissue is not clear at the present time. This step is required for binding of AMH to its primary receptor, at which time the AMH complex dissociates, releasing the mature ligand, the C-terminal homodimer and the N-terminal proregion (396). The homology of AMH to other members of the transforming growth factor-β (TGF-β) family is restricted to the C-terminus, for which a molecular model has been built, by analogy with crystallized members of the family (397) (Fig. 20). Cleavage and presumably bioactivity are enhanced if the endogenous cleavage site RAQR is replaced by a furin/kex2 RARR consensus site (398).
FIGURE 20. Molecular model of C-terminal AMH. A three-dimensional model of the C-terminal dimer was generated by comparative modeling using human BMP9 (399) as a template. The wrist epitope, the putative binding site for the type I receptor, is composed of the prehelix loop and alpha-helix of one monomer together with the concave side of the fingers of the second monomer (400). A mutation in the prehelix loop of AMH, Q496H, causes persistent Müllerian duct syndrome (397). Residues in the knuckle epitope of AMH, the putative binding site for AMHR2, are similar to those present in BMP7 and activin at the interface with ACTR2B (401, 402). Disulfide bonds (yellow) and Q496 residues (blue) are shown as sticks; residues in the knuckle epitopes are shown as spheres. Reprinted from ref. (397): Belville C, Van Vlijmen H, Ehrenfels C, Pepinsky RB, Rezaie AR, Picard J, Josso N, di Clemente N, Cate RL. Mutations of the anti-Müllerian hormone gene in patients with persistent Müllerian duct syndrome: biosynthesis, secretion and processing of the abnormal proteins and analysis using a three-dimensional model. Molecular Endocrinology 18:708-721 (2004). Copyright 2004 The Endocrine Society with permission. http://mend.endojournals.org/content/18/3/708.abstract?sid=22a37d21-69b5-499e-9996-8b1d4df81215
The human 2.8-kb gene has been cloned (403) and mapped to chromosome 19p13.3 (404). It consists of five exons, the last one coding for the C-terminal fragment. The AMH gene has been cloned in many other mammals (405-409), in the marsupial tammar wallaby (410), in the chick (411, 412) and American alligator (413), all of which carry Müllerian ducts which regress in the male. The gene is also present in the caudate amphibian, Pleurodeles waltl,whose Müllerian ducts persist in males (414). Even more surprisingly, AMH orthologs (415, 416) and the AMH type II receptor (417) have been cloned from the gonads of modern teleost fish, which do not possess Müllerian ducts at all. In fish, AMH appears to be involved essentially in germ cell proliferation and gonadal development (reviewed in ref. (418)), which suggests that AMH was initially a regulator of gonadal differentiation which acquired its anti-Müllerian activity during the course of evolution without completely relinquishing its former role. Indeed, in higher vertebrates, AMH inhibits Leydig cell differentiation (419) and follicle maturation (420).
The ontogeny of AMH expression differs widely between males and females. In the human fetal testis, AMH mRNA and protein can be detected from the 8th week, when Sertoli cells begin to form cord-like structures, the future seminiferous tubules (189) (Fig. 19). In the ovary, AMH production is detectable at 24 week gestation in granulosa cells of preantral follicles (265). The timing of the expression of AMH is crucial. In the male, high amounts of AMH must be expressed before Müllerian ducts lose their responsiveness, i.e. before the end of the 8th week in the human fetus. In the female, to avoid destroying the reproductive tract, it must be expressed after the window of sensitivity of the Müllerian ducts to its action has closed. Thus, in both sexes, the initiation of AMH transcription is under tight transcriptional control.
In the mammalian testis, but not in reptiles (413) or birds (421), SOX9 (97, 112, 113, 422, 423) -and to a lesser extent SOX8 (114)- triggers AMH expression in Sertoli cells by binding to a specific response element on the AMH promoter. Transcription factors SF1 (112, 113, 422, 424-431), GATA4 (113, 427, 432-438), WT1 (425, 439) increase, whereas DAX1 (425) and β-catenin (439) reduce, SOX9-activated AMH transcription either by binding to specific response elements or by protein-protein interaction (440, 441). In vivo, genes can affect AMH levels indirectly through their impact on testicular determination instead of acting on gene transcription.
Although initially gonadotropin-independent, AMH production falls under FSH control later in fetal life and after birth (113, 197, 442-444). FSH regulates AMH transcription through the FSH receptor-Gsα protein-adenylate cyclase-cyclic AMP pathway, resulting in a stimulation of protein kinase A (PKA) activity. PKA mediates phosphorylation of the transcriptional regulators SOX9, SF1 and AP2, as well as of IκB which releases NFκB. In the nucleus these factors activate AMH transcription by binding to their specific response elements on the AMH promoter (Fig. 21). LH and hCG do not have a direct effect on Sertoli cell AMH expression, but affect testicular AMH production through androgen action, as explained below.
FIGURE 21. Regulation of testicular AMH production. Left: the onset of AMH expression is gonadotropin-independent and depends on SOX9 binding to the proximal AMH promoter. Subsequently, SF1, GATA4 and WT1 enhance AMH expression by binding to specific promoter sequences or by interacting with transactivating factors. DAX1 impairs GATA4 and SF1 binding to the AMH promoters, resulting in lower AMH expression levels. Right: Later in fetal and postnatal life, FSH regulates AMH production through the FSH receptor-Gsα protein-adenylate cyclase (AC)-cyclic AMP (cAMP) pathway, resulting in a stimulation of protein kinase A (PKA) activity. PKA mediates phosphorylation of the transcriptional regulators SOX9, SF1 and AP2, as well as of IκB which releases NFκB. In the nucleus these factors bind to their specific response elements in proximal (SOX9, SF1) or distal (AP2 and NFκB) regions of the AMH promoter.
Right figure reprinted from ref. (113): Lasala C, Schteingart HF, Arouche N, Bedecarrás P, Grinspon R, Picard JY, Josso N, di Clemente N, Rey RA. SOX9 and SF1 are involved in cyclic AMP-mediated upregulation of anti-Müllerian gene expression in the testicular prepubertal Sertoli cells SMAT1. American Journal of Physiology – Endocrinology and Metabolism 2011; 301: E539-E547, Copyright 2011 the American Physiological Society. http://ajpendo.physiology.org/content/301/3/E539.abstract?sid=3829d833-dfdf-4310-bd6f-e7481c62be06
At puberty, FSH stimulation is antagonized by androgens resulting in a steep fall in AMH secretion by Sertoli cells (445). Androgen action requires the presence of the androgen receptor in Sertoli cells. This occurs relatively late after birth (Fig. 22) (204, 205, 446) allowing both AMH and testosterone to reach high levels in fetuses and neonates. In androgen-insensitive patients, affected by mutations of the androgen receptor, AMH levels are abnormally elevated during the perinatal and pubertal stages (447, 448), due to unopposed stimulation by FSH. Androgens act directly on pubertal Sertoli cells to inhibit AMH promoter activity in the presence of the androgen receptor (429), even though the AMH promoter does not carry consensus androgen response elements (449). For androgens to repress AMHexpression, the existence of intact sites for binding of the transactivating factor SF1 on the AMH promoter is crucial, suggesting that the inhibition of AMH promoter activity by androgens could be due to protein–protein interactions between the ligand-bound androgen receptor and SF1 or by blockage of SF1 binding to its sites (429).
Gonadotropins and steroid also regulate AMH in the ovary. FSH stimulates AMH transcription in cultured granulosa cells (450) while estrogens has differential effects according to which estrogen receptor is involved (451), while LH has no effect in normal cells (452).
FIGURE 22. Ontogeny of testicular AMH production. In the mammalian fetal testis, AMH expression is triggered by the increase of SOX9 levels. It is not prevented by the rise of intratesticular levels of testosterone because fetal Sertoli cells do not express the androgen receptor (AR). After birth the number of Sertoli cells expressing the AR progressively increases. At puberty, when testosterone increases again, AR is present and AMH production is inhibited.
AMH is measurable in human serum by ELISA. Initially, the procedure was used by pediatric endocrinologists to measure testicular AMH in boys, hence the first commercially available kits were suited to the high level of AMH concentration of prepubertal males (448). Following the discovery that AMH serum concentration in women mirrors ovarian reserve (453, 454), AMH assay has become a standard procedure in assisted reproduction centers and more sensitive methods, adapted to the low concentration of AMH in female serum, were developed (455)(456)(457). In parallel, automated assays, e.g. the electrochemiluminescence Roche Elecsys assay (458) and the Beckman Coulter Access AMH assay (459), are progressively gaining ground, due to increased reproducibility and accelerated turnaround time, only 18 minutes for the Roche Elecsys assay. There is reasonable correlation between the different manual kits after manipulation of standard curves by manufacturers but not between manual assays and automated ones, which yield 20-30% lower values (458, 460). It follows that AMH values obtained with different methods are not interchangeable (461). Since clinicians are not usually aware of the problem, serious interpretation errors may arise during patient follow-up. An international standard of human recombinant AMH needs to be developed, particularly since the Immunotech assay upon which many normative values have been based (462-464) has been pulled off the market.
The uncleaved AMH precursor and non-covalent cleaved AMH are both detectable by commercial ELISA kits, but attempts to discriminate between the various AMH forms have not proven clinically rewarding (465-468).
AMH is an exceptionally stable biomarker, variations during the menstrual cycle (469, 470) and diurnal variations in men (471) are minimal. Measurement of AMH in serum has diagnostic applications in disorders of sex development (324, 472) and as a marker of prepubertal testicular function in boys (473-477). In women, AMH levels are a reliable marker of follicular reserve (453, 454) and may be used with relative accuracy to predict the onset of menopause (478) or to follow the evolution of granulosa cell tumors (479, 480). Some AMH mutations with reduced in vitrobioactivity are associated with premature ovarian insufficiency (481). In contrast, the clinical usefulness of AMH in seminal fluid in men with non-obstructive azoospermia is debatable (482). Further discussion of the diagnostic and potentially therapeutic value of AMH in the adult ovary and testis is beyond the scope of this review.
AMH Transduction: Type I and II AMH Receptors
Like other members of the TGFβ family, AMH signals through two distinct membrane-bound receptors, both serine/threonine kinases. Unlike other members of the TGFβ family truncated forms of the AMH primary receptor AMHR2 are not secreted, unless the signal sequence is replaced by the TGFβ one, suggesting that the AMHR2 signal sequence is defective (483). A three-dimensional model of extra- and intracellular domains built by analogy with crystallized receptors of the TGFβ family (Fig. 23) has served to analyze structure/activity relationship of the receptor molecule (483, 484).
The AMHR2 gene, located on chromosome 12q13.13, spans 8 kb pairs and is divided into 11 exons. Exons 1-3 code for the signal sequence and extracellular domain, exon 4 for most of the transmembrane domain, and exons 5-11 for the intracellular serine/threonine kinase domains (485). AMHR2 is expressed in the mesenchymal cells which surround the Müllerian duct, and also in Sertoli, granulosa (486, 487), Leydig (419) and germ cells (389), endometrium (390), neurons (391, 393) and hypothalamus (392). Expression of the receptor in the peri-Müllerian mesenchyme requires the presence of the signaling molecule WNT7A (359). The activity of AMHR2 is enhanced by WT1 (488) and by SP600125, an inhibitor of the c-Jun N-terminal kinase (489).
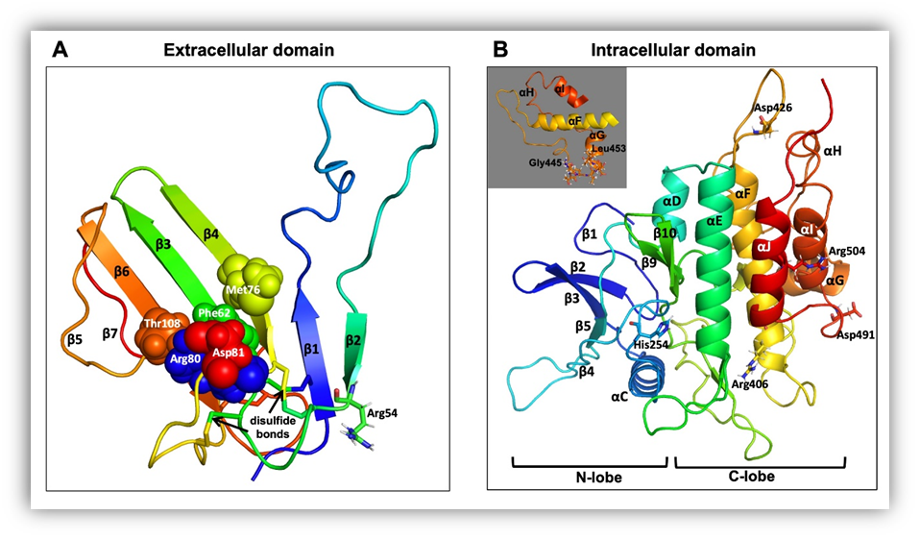
FIGURE 23. Molecular models of AMHR2 extracellular and intracellular domains. (A) The extracellular domain exhibits the general three-finger toxin fold of type II receptors and displays five disulfide bridges, four of which are conserved. Five amino acids (Phe62, Met76, Arg80, Asp81, and Thr108), implicated in binding AMH, are shown as spheres. (B) The intracellular domain exhibits the general fold of a two-domain kinase, with an N-lobe consisting mainly of a five-stranded β-sheet and a C-lobe, which is mainly α-helical. Some of the residues affected by PMDS mutations (Arg54, His254, Arg406, Asp426, Asp491, and Arg504) are shown as sticks. The inset shows residues affected by the p.((Gly445_Leu453del) mutation. Reprinted with permission from Elsevier, from ref. 364 (490): Josso N, Picard JY, Cate RL (2013). The Persistent Müllerian Duct Syndrome. In: New MI, Parsa A, Yuen TT, O'Malley BW, Hammer GD, eds. Genetic Steroid Disorders. New York, NY (USA): Elsevier
Binding of the receptor to its specific ligand requires proteolytic cleavage of the AMH precursor to yield the non-covalent complex AMH, but unlike other TGFβ family members, prior dissociation of this complex is not required. Dissociation is triggered by binding to AMHR2 (396) and is followed by the assembly of a tetrameric C-terminus/receptor complex with two molecules of type I receptor. Activated type I receptors then phosphorylate receptor-SMADS 1/5/8, which associate with SMAD4 and are then shuttled to the nucleus where they regulate transcription of target genes. (Fig. 24).
The AMH type II receptor is subject to processing (491). Increased expression of the receptor results in the removal of most of its extracellular domain and subsequent retention in the endoplasmic reticulum, resulting in a constitutive negative regulation.
The primary AMH receptor, AMHR2, is AMH-specific, a unique example of exclusive ligand-receptor pair within the TGFβ family (492). This specificity may be due to the presence of charged residues at the ligand binding interface (493). In contrast, the downstream elements of the AMH transduction pathway are shared with the bone morphogenetic protein family, namely ALK2 (or ACVR1, Activin a receptor, type I) ALK3 (or BMPR1A, Bone morphogenetic protein receptor, type IA) and all three BMP receptor SMADS, 1, 5 and 8 (494-496). Another BMP receptor, ALK6 (or BMPR1B, Bone morphogenetic protein receptor, type IB), is engaged by ligand-bound AMHR2 (494) but has an inhibitory effect on AMH activity (497). ALK3 is the more potent AMH type I receptor in the Müllerian duct (498), in the Leydig cell (499) and in the SMAT1 Sertoli cell line (497) but in its absence, ALK2 is capable of transducing the AMH signal (496, 497).
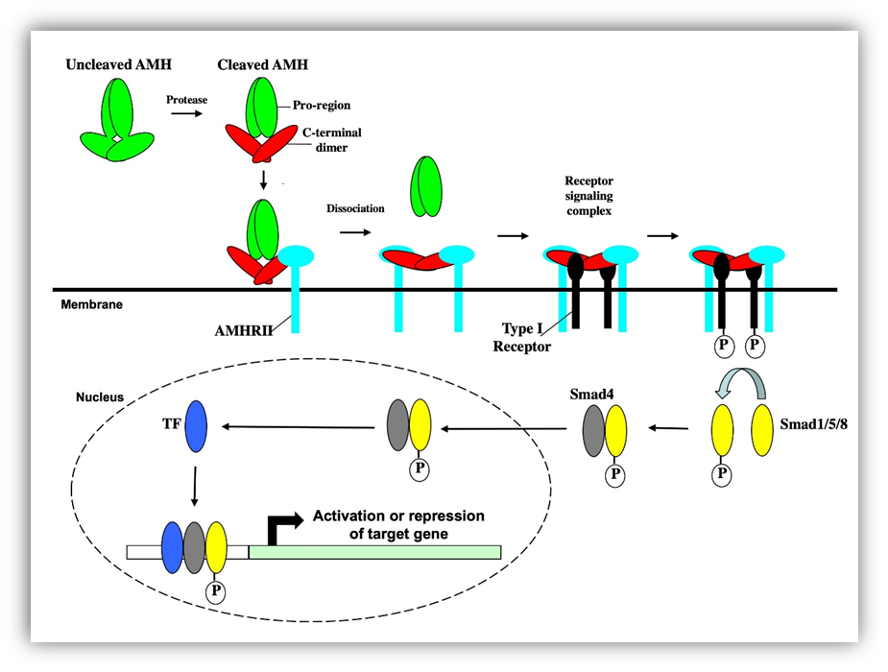
FIGURE 24. Model showing processing of AMH, assembly of the AMH receptor signaling complex, and intracellular signaling. Cleavage of the AMH precursor results in a conformational change in the C-terminal domain, which allows binding of the AMH non-covalent complex to AMRHII. After dissociation of the N terminal proregion, the type I receptor is recruited into the complex and phosphorylated by the type II receptor kinase. The activated type I receptor can then phosphorylate Smads 1/5/8, which associate with Smad 4, translocate to the nucleus and regulate AMH responsive genes. Courtesy of Dr. Richard Cate. Data obtained from ref. (396): di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, Picard J-Y, Whitty A, Josso N, Pepinsky RB, Cate RL. Processing of anti-Müllerian hormone regulates receptor activation by a mechanism distinct from TGF-β. Molecular Endocrinology 24:2193-2206 (2010). http://mend.endojournals.org/content/24/11/2193.abstract
The Persistent Müllerian Duct Syndrome
Mutations of human AMH or AMHR2 (324) and gene knockout in mice (500, 501) are associated with a rare form of disorder of sex development, the persistent Müllerian duct syndrome (PMDS). These XY individuals are externally normally virilized, Müllerian duct derivatives are discovered incidentally at surgery for either inguinal hernia or cryptorchidism (Fig. 25) or following discovery of the condition in a sibling. Older patients may seek medical attention because of an abdominal tumor, hematuria or hemospermia.
FIGURE 25. Operative findings in a patient with PMDS. The Fallopian tubes are tightly attached to the testes, preventing testicular descent. Note normal male external genitalia.
Reprinted from ref. (484): Abduljabbar M, Taheini K, Picard JY, Cate RL, Josso N. Mutations of the AMH type II receptor in two extended families with Persistent Mullerian Duct Syndrome: lack of phenotype/genotype correlation. Hormone Research in Paediatrics 77:291-297 (2012). Copyright 2012 S. Karger AG, Basel, with permission. http://www.karger.com/Article/FullText/338343.
In patients with PMDS, as in the normal female, the Müllerian ducts differentiate into Fallopian tubes, uterus, and upper vagina. They retain their close apposition to Wolffian duct derivatives, epididymis, and vas deferens while remaining tied to the pelvis by the broad ligament (Fig. 25). The clinical features of PMDS are similar in AMH and AMHR2 mutations and may vary within the same sibship. The mobility of the Müllerian structures determines testicular location. Bilateral cryptorchidism is observed most frequently: the uterus remains anchored to the pelvis, and mechanically prevents testicular descent. Alternatively, one or both testes may make it into the inguinal canal or the scrotum, dragging the uterus along. This may result either in unilateral cryptorchidism with a hernia containing the uterus on the opposite side, a condition known as “hernia uteri inguinalis”. The testis on the opposite side can be drawn into the same hemiscrotum by gentle traction or may already be present there; this condition typical of PMDS is named “transverse testicular ectopia”. It may be the only sign of an AMH or AMHR2 mutation in patients with normally regressed Müllerian derivatives (502). Approximately half the cases present with bilateral cryptorchidism, the rest with hernia uteri inguinalis or transverse testicular ectopia in similar proportions. The descended testis is only loosely anchored to the bottom of the processus vaginalis by a thin gubernaculum 4. It is exposed to an increased risk of torsion and subsequent degeneration (503). Associated abnormalities such as low birth weight with or without prematurity or complex metabolic syndromes are suggestive of idiopathic PMDS unrelated to defects in the AMH pathway. Intestinal malformations have been observed in four cases, consisting of either jejunal atresia or lymphangiectasis. Skeletal malformations suggestive of defects in the BMP pathway have not been reported.
Testicular tumors of every denomination, mostly seminomas, are a frequent mode of presentation of PMDS in older patients, particularly in settings where cryptorchidism has been neglected in childhood. Young patients may be affected by germ cell neoplasia in situ (502, 504). Early orchidopexy is not necessarily 100% effective to preserve against testicular degeneration (505, 506). Furthermore, the incidence in PMDS adults reaches 33% (502) compared to 18% for simple cryptorchidism (507), suggesting that misplacement of the testis may not be the only factor driving testicular cancer. Evolution depends upon the histological type of the tumor; choriocarcinomas and mixed germ cell tumors share a dim prognosis.
Uterine tumors occur less frequently, hematuria is usually the presenting symptom (508). They should not be confused with degeneration of the prostatic utricle often mistakenly called “Müllerian” cysts (509). Farikullah et al (510)reported 11 cases of Müllerian degeneration in males, but only 3 qualified as PMDS. Exceptionally, in an elderly PMDS patient, hematospermia may be due to endocrine imbalance with low testosterone and high estrogen secretion (511).
Infertility is the most common complication of PMDS. Pubertal development is normal, spermatogenesis is not unheard of (512), yet few patients actually father children and stringent evidence of paternity is lacking. In all cases at least one testis was in a scrotal position (reviewed in ref. (502). There are several causes of infertility: the excretory ducts may not be properly connected to the testis or the germinal epithelium may be damaged by longstanding cryptorchidism. Paradoxically, all fertile PMDS patients fathered children before their condition was diagnosed and surgically addressed. Surgery may compromise the testicular blood supply or the vasa deferentia, particularly if hysterectomy is undertaken without proper dissection of the male excretory ducts included in the uterine wall. The prognosis may improve with modern surgical and assisted reproduction techniques. In inbred populations where fertility is a crucial issue, as in the Middle East (513), genetic counseling is recommended and molecular screening should be carried out if a consanguineous union is contemplated.
Treatment should aim primarily towards the prevention of the two main complications of PMDS, cancer and infertility. Both goals are served by replacing the testes in the scrotum but excising the uterus to allow abdominal testes to descend into the scrotum carries significant risks. The primary testicular blood supply is through the internal spermatic and the deferential arteries. Often the spermatic vessels are too short and must be divided to allow orchidopexy. The viability of the testis then becomes wholly dependent upon the deferential artery, which is closely associated with the Müllerian structures and may be severely damaged by attempts to remove them (514). Most authors recommend partial hysterectomy, limited to the fundus and proximal Fallopian tubes, or the simple division of Müllerian structures in the midline. If the length of the gonadal vessels is the limiting factor; a Fowler–Stephens orchidopexy or microvascular autotransplantation (515) may produce good results. Intracytoplasmic sperm injection may be helpful in the case of ejaculatory duct defects. Orchidectomy is inevitable if the testes cannot be brought down.
The serum level of AMH in prepubertal patients depends on the molecular origin of the syndrome. Before puberty, the level of serum AMH allows easy discrimination between AMH and AMHR2 mutations. In nearly all patients with AMH mutations, AMH levels are extremely low or undetectable. AMH gene mutations with a normal AMH serum level are very unusual and should be regarded with suspicion. We have documented only one such case, a Gln 496 His mutation, which is thought to affect binding of AMH to its type 1 receptor ALK3 (397). Menabo et al (516) reported a case of PMDS attributed to AMH variants with a normal AMH level but they did not rule out an AMHR2 mutation. AMH levels are relatively low in normal infants shortly after birth, but then repeat determinations show a progressive rise with increasing age.
Serum AMH levels are within normal limits for age in AMHR2 mutations and in idiopathic PMDS, unrelated to defects in known components of the AMH pathway. Obviously, serum AMH is not detectable, whatever the genotype, in the case of anorchia (503) and may be abnormally low in cryptorchid patients. Testosterone and gonadotropin levels are normal for age. After pubertal maturation, serum AMH declines physiologically, and it may be difficult to discriminate between AMH and AMHR2.
Approximately 80% of PMDS cases are due to AMH or AMHR2 mutations, in about equal proportions. The first AMHmutation was reported in 1991 (517) in a Moroccan family. At the time of writing, early 2020, a total of 84 families affected by AMH mutations have been published in the world literature. All exons are affected (Fig 26). Exon 1, the site of most recurrent mutations (Table 6) is hit hardest, exon 5 is next but when the number of base pairs is taken into account, the relatively short exon 2 with its 13 mutations is the runner up. Although it is shorter, the 3’ end of exon 5 that codes for the bioactive C-terminal domain of AMH is targeted nearly twice as often as the 5’ end.
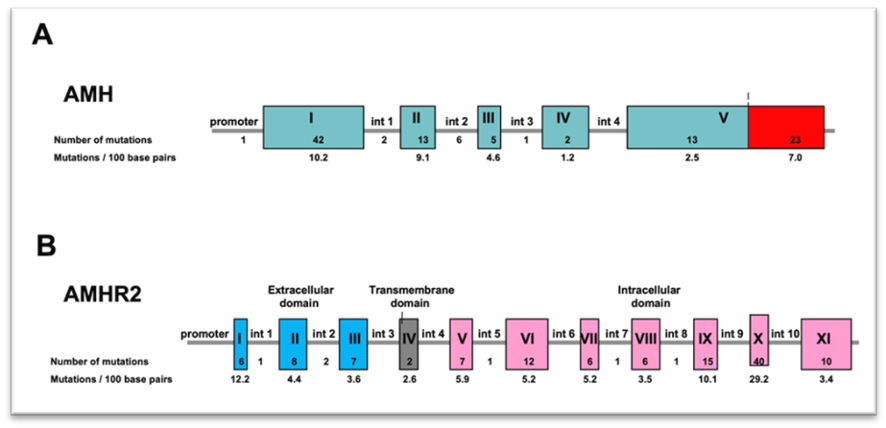
FIGURE 26. Mutations of the AMH and AMHR2 genes in the Persistent Müllerian Duct Syndrome (PMDS). Mutations of the AMH (top) and AMHR2 genes (bottom) in the Persistent Müllerian Duct Syndrome (PMDS). The 3' end of the AMH gene (picture in red) codes for the C-terminal domain, responsible for bioactivity, yet mutations are spread along the whole length of the gene. Similarly, mutations of the AMHR2 affect intracellular and extracellular domains alike.
Altogether, 67 different AMH alleles bearing all types of mutations have been described in PMDS. Missense and stop mutations are the most frequent, insertions are rare (see details in ref. (502). One deletion is of particular interest, because it disrupts the SF1 response element located at -228 in the AMH promoter. Inactivation of the -102 site does not prevent Müllerian regression in transgenic mice. The greater impact of the -228 deletion detected in the PMDS patient may be due to the vicinity of the -102 SF1 site to a GATA site, to which SF1 can indirectly bind through protein/protein interaction with GATA4. This hypothesis is supported by transactivation experiments showing that destruction of the GATA site adjacent to SF1-102 results in inactivation of the AMH promoter (430).
A few AMH mutations have been reproduced by site-directed mutagenesis, cloned into an expression vector and transfected into COS cells to allow study of the secretion of the mutant protein into the culture medium (397). These studies confirm that most single nucleotide variations of the AMH gene act by affecting the stability and secretion of the hormone, explaining why nearly all patients with AMH mutations, regardless of the site of the mutation, have a very low level of circulating AMH.
|
TABLE 6. Recurrent (n≥4) Mutations of AMH in PMDS
|
|
Exon
|
cDNA
|
Protein
|
Families (n)
|
|
1
|
c.35T>G
|
p.(Val12Gly)
|
4
|
|
1
|
c.283C>T
|
p.(Arg95*)
|
6
|
|
1
|
c.301G>A
|
p.(Gly101Arg)
|
4
|
|
1
|
c.343_344delCT
|
p.(Leu115Thrfs*58)
|
5
|
|
1
|
c.367C>T
|
p.(Arg123Trp)
|
7
|
|
2
|
c.500A>G
|
p.(Tyr167Cys)
|
5
|
Up to now, 90 families with various AMHR2 mutations have been published, the first in 1995 (485). Since AMHR2mutations lead to PMDS by blocking response to AMH, the level of circulating AMH is normal for age, in contrast to PMDS due to AMH mutations.
A total of 75 independent mutant alleles have been described, targeting all 11 exons and 5 introns; their location within the gene are shown in Fig.26B. Most are missense or stop mutations. Two cases of classical PMDS due to a microdeletion of the chromosomal region 12q13.13, the locus of the gene for AMHR2, have been reported. One case involved a homozygous microdeletion of five exons of the AMHR2 gene. In the second case, the whole AMHR2 gene was deleted from the maternally inherited chromosome. The patient’s paternal allele carried a stop mutation, which was initially thought to be homozygous by Sanger sequencing (518).
The most prevalent mutation, a 27-base deletion in exon 10 (c.1332_1358del) pictured on Fig. 23 results in the deletion of 9 amino acids from an alpha helix within the kinase domain and affects 37% of families with receptor mutations. The proportion reaches 62% of Northern European families, where it probably represents a founder effect. This mutation is easily detected by PCR, without the need for sequencing.
Not all PMDS cases have benefited from molecular study. In many countries, genetic studies are not readily available for PMDS, and cases have been published with only clinical data (519, 520). Owing to lack of molecular characterization, it is difficult to interpret the unusual sex-linked familial transmission of PMDS reported in two families (521, 522).
In approximately 20% of PMDS patients, careful sequencing of AMH and AMHR2 exons and adjacent portions of introns have failed to yield an explanation. Either a mutation has escaped detection or other genes are involved. The AMH and BMP families share type I receptors and cytoplasmic effectors, which could be implicated in PMDS. Initially, BMP receptors were considered unlikely candidates because an intact BMP pathway is required for survival beyond the embryonic stage. However, this might not hold for mild missense mutations (523). Alternatively, idiopathic PMDS could be caused by mutations in other genes involved in Müllerian duct development/regression such as β-catenin (320) or patterning genes not specifically involved in reproductive development. Studies with next generation sequencing are underway to resolve this issue. Women homozygous for AMH or AMHR2 mutations are normally fertile but it is too early to know whether, similar to “AMH-null” mice (286), they will experience premature ovarian failure due to follicular depletion
Androgens
Testosterone or dihydrotestosterone (DHT), binding to the same androgen receptor (AR), are the main factors involved in maintenance of the Wolffian duct and differentiation of male sex accessory organs and external genitalia.
Testosterone Biosynthesis
Beginning at 9 weeks, testosterone is produced from cholesterol by chorionic gonadotropin stimulation of fetal Leydig cells through the coordinated action of steroidogenic enzymes (Fig. 27 and Table 7), most of which are also expressed in the adrenal gland, explaining why many steroidogenic disorders are common to the testis and adrenal. Most steroidogenic enzymes are either hydroxysteroid dehydrogenases or cytochromes P450, residing either on the mitochondrial membrane (type I) or in the endoplasmic reticulum (type II) (524). The initial step in steroidogenesis, conversion of cholesterol into pregnenolone, is mediated by the P450 side-chain cleavage enzyme (P450scc), a type I cytochrome located at the inner mitochondrial membrane. However, the inner mitochondrial membrane contains relatively little cholesterol, so the rate-limiting step of steroidogenesis is the transfer of cholesterol from the outer to the inner mitochondrial membrane. This step is dependent on steroidogenic acute regulatory protein (StAR) regulated essentially by a trophic hormone stimulated cAMP/PKA pathway (525). The exact mechanism of StAR-mediated cholesterol transport into the mitochondria is not completely understood.
Pregnenolone is subsequently metabolized into 17α-hydroxypregnenolone and dehydroepiandrosterone (DHEA) by P450c17. This type II cytochrome bears two distinct activities: a 17α-hydroxylase activity responsible for the conversion of pregnenolone to 17α-hydroxypregnenolone and a 17-20 lyase activity, capable of converting 17α-hydroxypregnenolone to DHEA. P450c17 receives electrons from NADPH via the flavoprotein P450 oxidoreductase (POR) (526, 527). Cytochrome b5 is required for optimal 17,20 lyase activity (528, 529). P450c17 and its partner proteins also convert the Δ4 compound progesterone into 17α-hydroxyprogesterone and Δ4-androstenedione.
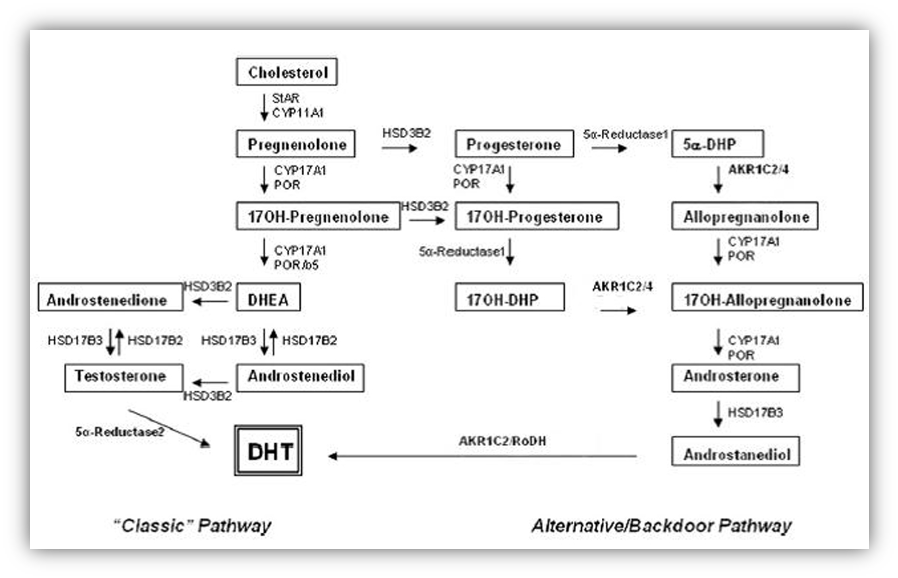
FIGURE 27. Steroidogenesis. Steroidogenesis: the “classic” and “backdoor” pathways for dihydrotestosterone (DHT) synthesis. See Table 7 for enzyme nomenclature. DHEA: dehydroepiandrosterone, DHP: dihydroprogesterone. Reprinted from ref. (530): Fluck CE, Meyer-Boni M, Pandey AV, Kempna P, Miller WL, Schoenle EJ, Biason-Lauber A. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. American Journal of Human Genetics 89:201-218 (2011). Copyright 2011, with permission from Elsevier. http://www.cell.com/AJHG/abstract/S0002-9297(11)00262-X (top figure), and ref. (531): Wilson JD, Shaw G, Leihy ML, Renfree MB. The marsupial model for male phenotypic development. Trends in Endocrinology and Metabolism, 13:78-83 (2002), Copyright 2002, with permission from Elsevier. http://www.sciencedirect.com/science/article/pii/S1043276001005252 (bottom figure).
Two additional enzymes, 3β- and 17β-hydroxysteroid dehydrogenases are required for the synthesis of testosterone. Two isoforms of 3ß-hydroxysteroid dehydrogenases have been identified: 3ß-HSD type 1, expressed mainly in the placenta, mammary gland and skin, and 3ß-HSD type 2, expressed in the gonads and adrenal glands. Only mutations in the type 2 gene result in congenital adrenal hyperplasia and/or DSD (532, 533).
The final testicular enzyme in testosterone biosynthesis is 17ß-hydroxysteroid dehydrogenase (17β-HSD), formerly known as 17-ketosteroid reductase, which reduces 17-ketosteroids to 17β-hydroxysteroids, i.e. Δ4-androstenedione to testosterone and the Δ5 steroid DHEA to androstenediol. Three isoforms of 17ß-HSD have been identified. The type 3 isoform, HSD17B3, is expressed in the testis and is the only one involved in fetal male sexual differentiation (534). XY patients with impaired HSD17B3 usually develop with female or ambiguous external genitalia; however, Wolffian ducts derivatives are present in most, probably due to accumulation of the weak androgens Δ4-androstenedione and Δ5-DHEA. The type 2, HSD17B2, is expressed in the liver and has the capacity for testosterone synthesis. This could explain the virilization observed at puberty in XY patients with HSD17B3 deficiency (534, 535).
|
TABLE 7. Proteins Involved in Androgen Production
|
|
Protein
|
Main Role
|
Gene
|
Chromosome
|
|
Steroidogenic acute Regulatory Protein (StAR)
|
Cholesterol trafficking
|
STAR
|
8p11.2
|
|
P450scc (P450 side chain cleavage enzyme)
Cytochrome P450, family 11, subfamily A, polypeptide 1
|
Cholesterol side-chain cleavage
|
CYP11A1
|
15q24.1
|
|
P450c17 (17α-hydroxylase/17,20-lyase)
Cytochrome P450, family 17, subfamily A, polypeptide 1
|
Metabolizes pregnenolone
|
CYP17A1
|
10q24.32
|
|
P450 oxidoreductase (POR)
|
Electron donor to P450c17
|
POR
|
7q11.23
|
|
Cytochrome b5, type A
|
Regulation of 17,20-lyase activity
|
CYB5A
|
18q22.3
|
|
3β-hydroxysteroid dehydrogenenase 2 (3β-HSD 2)
|
Conversion of Δ5 to Δ4 steroids
|
HSD3B2
|
1p12
|
|
17β-hydroxysteroid dehydrogenenase 3 (17β-HSD 3)
|
Reduction of 17 keto to 17β-hydroxysteroids
|
HSD17B3
|
9q22.32
|
|
5α-reductase type 2
|
Reduction of T to DHT
|
SRD5A2
|
2p23.1
|
|
5α-reductase type 1
|
Reduction of progesterone and 17OH-progesterone
|
SRD5A1
|
5p15.31
|
|
Aldo-keto reductase family 1, member C2
3α-hydroxysteroid dehydrogenase, type III (3α-HSD)
|
Oxidoreduction of 3α-androstanediol/ DHT *
|
AKR1C2
|
10p15.1
|
|
Aldo-keto reductase family 1, member C4
3α-hydroxysteroid dehydrogenase, type I
|
id but less efficient
|
AKR1C4
|
10p15.1
|
|
17β-hydroxysteroid dehydrogenase 6 (17β-HSD 6)
Retinol dehydrogenase 1
3α-hydroxysteroid epimerase
|
Oxidizes 3α-androstanediol to DHT
|
HSD17B6
RODH
|
12q13.3
|
|
P450aro (aromatase)
Cytochrome P450, family 19, subfamily A, polypeptide 1
|
Aromatizes androgens to estrogens
|
CYP19A1
|
15.q21.2
|
|
Steroidogenic factor 1 (SF1)
Nuclear receptor subfamily 5, group A, member 1
Adrenal-4 binding protein (AD4BP)
Fushi tarazu factor 1 (FTZF1)
|
Regulates several steroidogenic enzymes
|
NR5A1
|
9q33.3
|
* The direction of the reaction depends on cofactor availability (530). The four last enzymes act exclusively in the alternate pathway of DHT synthesis.
DHT Production: Classic and Alternative (Backdoor) Pathways
Testosterone itself is not a very active androgen; its metabolite DHT is the main virilizing agent during male reproductive development. The conversion of testosterone to DHT amplifies the androgenic signal through several mechanisms. DHT cannot be aromatized to estrogen, and thus its effects are purely androgenic. Testosterone and DHT bind to the same androgen receptor but DHT does so with greater affinity which results in a stabilization of the hormone-receptor complex for a longer period of time (536).
In the classic pathway of DHT production (Fig. 27, top), testosterone is converted to DHT inside the target cell by the enzyme 5α-reductase type 2 coded by the SRD5A2 gene expressed in fetal genital skin, in male accessory sex glands and in the prostate (537). In tissues equipped with 5α-reductase at the time of sex differentiation, such as the urogenital sinus and external genitalia, DHT is the active androgen (538). During embryogenesis, 5α-steroid reductase type-2 encoded by the SRD5A2 gene plays a central role in the differentiation of the male phenotype. Patients with 5α-reductase deficiency virilize very poorly at these levels (539, 540). Another functional isoenzymes of 5α-reductase, with a different pH optimum, has been characterized (537): 5α-reductase type 1, transiently active in newborn skin and scalp and permanently expressed in liver after birth and in skin from the time of puberty, is not expressed in the fetus. Tissue distribution and ontogeny of both isoforms as well as mutation studies in humans with 46,XY DSD clearly indicate that type 2 plays the major role in sexual differentiation but the emergence of type 1 probably accounts for the pubertal virilization of the type 2-deficient patients.
Testosterone, however, is not an obligatory precursor of DHT (Fig. 27, bottom). Observations in a marsupial, the tammar wallaby (531), have shown that the testis itself produces biologically significant amounts of DHT through an alternate or “backdoor” pathway without using testosterone, DHEA or androstenediol as intermediates. Additional enzymes not part of the classic pathway can mediate the direct oxidation of 5α-androstanediol to DHT (524, 530). This "backdoor" pathway contributes to virilization in the human fetus as demonstrated by the genetic studies of Flück and her colleagues (530) in two families with 46,XY DSD. After they failed to demonstrate mutations in known steroidogenic enzymes, they explored genes acting in the alternate pathway of androgen synthesis. This led to discovery of mutations in the genes AKR1C2 and AKR1C4, alias 3α-hydroxysteroid dehydrogenase (or reductase) type I and type III. In the alternate pathway these enzymes catalyze the reduction of dihydroprogesterone and 17OH-dihydroprogesterone to allopregnanolone and 17OH-allopregnanolone, the precursor of androsterone and androstanediol. Their role in the oxidation of the latter to testosterone is hypothetical because they have very high affinity for NADP(H), which favors reductive reactions and low affinity for NAD(H) which favors the opposite, thus they are expected to function primarily as a reductase (541). AKR1C2 is expressed in the fetal, but not the adult testis, AKR1C4 is expressed at low levels in both tissues. The deleterious effect of AKR1C2/4 mutations proves that testicular DHT synthesis through the alternate pathway is required for normal fetal sex differentiation.
Gonadotropin Control of Testosterone Production
Testosterone production by the human fetal testis is detectable at 9 weeks, peaks between 14 and 17 weeks and then falls sharply, so that in late pregnancy the serum concentrations of testosterone overlap in males and females. Gonadotropin stimulation is not required for the initiation of steroid synthesis (220) but is necessary to maintain Leydig cell function subsequently. Testicular and serum levels of testosterone are closely correlated with human chorionic gonadotropin (hCG) concentration; the peak of fetal testicular steroidogenic activity coincides with the acme of concentrations of hCG in the circulation. In adult Leydig cells, the capacity to respond to sustained gonadotropic stimulation by increased androgen production is curtailed by the development of a refractory state, due to receptor down-regulation (542). Fetal Leydig cells apparently escape desensitization, allowing them to maintain a high testosterone output during the several weeks necessary to male differentiation of the genital tract. The fetal pituitary takes over when chorionic gonadotropin declines in the 3rd trimester (reviewed in ref. (543) (Fig. 28). Impaired LH secretion in 46,XY fetuses does not result in DSD because the most important steps of sexual differentiation, with the exception of penile growth, occur at the time Leydig cells are controlled by hCG.
In contrast, mutations in the LH/CG receptor of Leydig cells result in severe virilization defects (544). LH and hCG signal through a common seven-transmembrane domain receptor coupled to G proteins present on testicular Leydig cells. The human gene located on chromosome 2p21, contains 11 exons. The first ten encode a long N-terminal extracellular domain responsible for hormone binding, while the 11th exon encodes the whole transmembrane domain, involved in the cAMP/PKA signal transduction pathway. A functioning LH/CG receptor is absolutely necessary to achieve a normal development of the fetal Leydig cell population and androgen production. Loss of function mutations lead to 46,XY DSD (reviewed in ref. (545), with the exception of the deletion of exon 10, which was identified in a patient with normal male phenotype but lack of pubertal development (546, 547). This suggests that exon 10 is required for signal transduction of pituitary LH but not hCG.
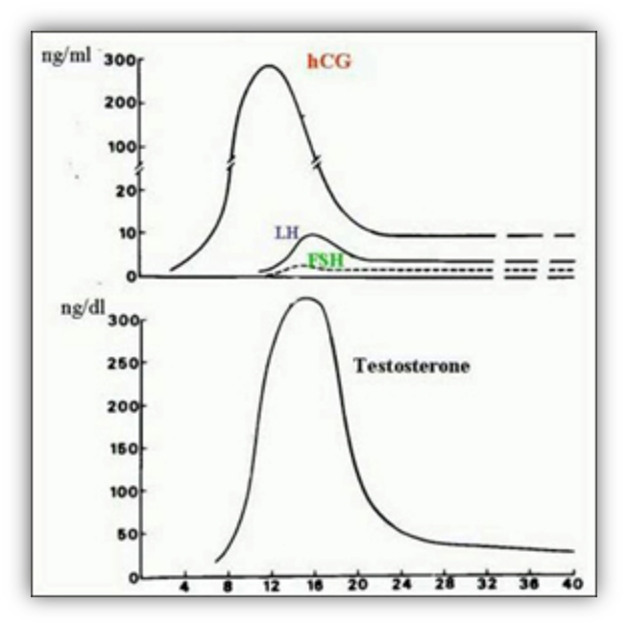
FIGURE 28. Control of testosterone production in the human fetus. Note the low testosterone concentration during the last trimester, at the time that hCG production by the placenta has abated. Data obtained from ref. (548): Winter JSD, Faiman C, Reyes F (1981). Sexual endocrinology of fetal and perinatal life. In: Austin CR, ed. Mechanisms of Sex Differentiation in Animals and Man. London: Academic Press; p.205-253.
The Androgen Receptor
Testosterone and DHT exert their action on androgen-dependent tissues by binding to the androgen receptor, a member of the steroid receptor family (Fig. 29). Mutations of this receptor lead to the androgen insensitivity syndrome, a relatively common disorder of sex development typically characterized by a female external genital appearance in XY patients despite a normal or excessive production of testicular hormones (see ref. (549) for review). The androgen receptor is encoded by a single-copy gene located on the long arm of the X chromosome, locus Xq12 (550). It spans 75-90 kb and its open reading frame of 2.75 kb comprises 8 exons. Exon 1 is the longest and codes for the amino-terminal transactivation domain. A highly polymorphic CAG triplet containing 14-35 repeats towards the 5’-end of exon 1, is useful as a genetic marker for inheritance of X chromosomes. Interestingly, expansion of the trinucleotide repeat which encodes this long tract of glutamine residues segregates with X-linked spinal and bulbar atrophy a degenerative neuropathy characterized by the accumulation of the mutated receptor in the nucleus and cytoplasm of motor neurons (reviewed in ref. (551). Exons 2 and 3 code for sequences containing two zinc fingers implicated in DNA binding. Most mutations occur in exons 4 to 8, which encode the steroid hormone binding domain. The 5’-portion of exon 4 codes for the hinge region between the DNA- and steroid-binding domains, and plays a regulatory role (552). A complete database of androgen receptor mutations is available from McGill University in Montreal (553).
In contrast to receptors for other steroid sex hormones, which reside in the nucleus even in the absence of ligand binding, the androgen receptor resides mainly in the cytoplasm, associated with heat-shock and other chaperone proteins, in the absence of hormone and translocates into the nucleus in the presence of ligand (554). Nuclear localization is controlled by a nuclear localization signal spanning the second zinc finger and the hinge region competing with an androgen-regulated nuclear export signal in the ligand binding domain (555). The androgen/AR complex can also signal through non-DNA binding-dependent pathways. However, the physiological relevance of these actions remaining largely unknown (554).
The androgen receptor binds to specific DNA motifs, the androgen response elements (ARE), present in the promoter regions of androgen-activated genes. The consensus or classic ARE consists of two palindromic half sites spaced by three base pairs (AGAACAnnnTGTTCT).while the so-called "selective" AREs, such as the one in intron 1 of the SRD5A2 gene (556) resemble direct repeats of the same hexamer (557). After binding to AREs on the promoters of androgen-responsive genes, the androgen receptor regulates their transcriptional activity. It is aided in this task by co-regulators, partner proteins that facilitate assembly of the preinitiation complex through chromatin remodeling. These include the p160 family of coactivators, which interact selectively with the agonist-bound form of AR (558-560). Attempts at blocking the androgen receptor by preventing its interaction with co-activators are part of the therapeutic strategy in prostate cancer (554).
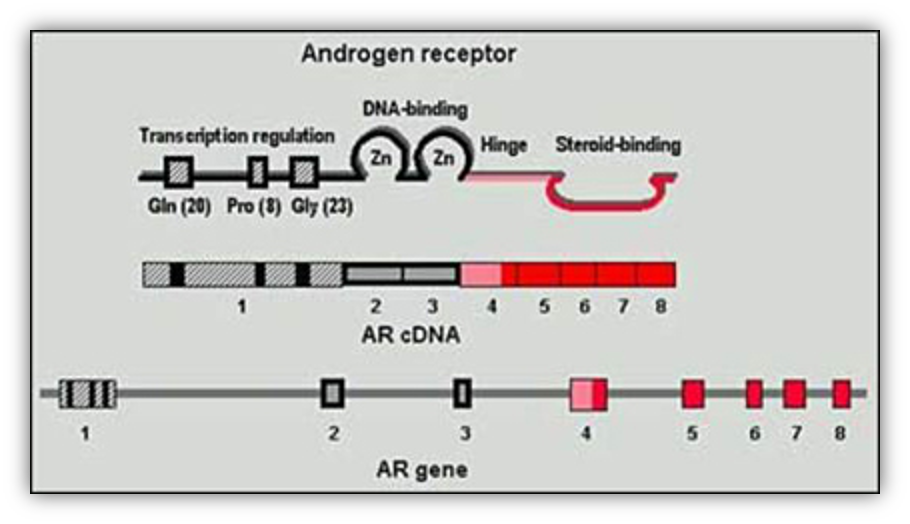
FIGURE 29. Androgen receptor protein, cDNA and gene.
The Case of the Wolffian Ducts: The Role of Local Testosterone
In fetal Wolffian ducts, 5α-reductase is expressed only after the ambisexual, critical, stage of male sex differentiation, thus testosterone itself, not DHT, saves them from degeneration (537, 538). Because of its close proximity to the testis, the Wolffian duct is exposed to a very high local concentration of testosterone, a source of androgen not available to organs receiving testosterone only via the peripheral circulation (Fig. 30) (297). Patients with androgen insensitivity whose androgen receptor retains very low but significant residual activity have a female phenotype but retain an epididymis or vas deferens (561). Wolffian duct differentiation is programmed during a critical time window, between 15.5 and 17.5 dpc in the rat fetus. Because the androgen receptor is expressed in the Wolffian duct stroma but not in the epithelium during this time, Wolffian duct differentiation is likely to be dependent on androgen-mediated signaling from the stroma to the epithelium (562).
Two phase can be described in the development of the Wolffian ducts (297). In the first phase, testosterone induces the stabilization of the ducts (in rodents, this occurs between embryonic days 13 and 16). Afterwards the Wolffian ducts undergo elongation and convolution of the cranial end, where the epididymis and vas deferens differentiate, and the seminal vesicles form at the caudal end.
Control of Testicular Descent
Androgens are required to mediate the disappearance of the cranial suspensory ligament (563, 564) and later for the inguinoscrotal phase of testicular descent. The mechanism of androgenic action on the gubernaculum is controversial. Androgens could act through the genitofemoral nerve and the neuropeptide calcitonin gene-related peptide (565, 566). Thus, any condition associated with decrease of fetal testicular production or action may impair testicular descent.
The first, transabdominal, phase of testicular descent is controlled by Insulin-like factor 3 (INSL3), a member of the insulin/relaxin hormone superfamily secreted by Leydig cells, signaling through its G protein-coupled receptor LGR8, now known as relaxin family peptide receptor 2 (RXFP2) (217, 567). INSL3 acts by inducing male development of the gubernaculum testis. Mutations of INSL3 have been detected in cryptorchid patients (568), similarly deletion of Rxfp2 targeted to mesenchymal gubernacular cells leads to high cryptorchidism in mice (569). Prenatal DES treatment, which is associated with cryptorchidism, impairs Insl3 expression in mouse testis and interferes with gubernacular development (570).
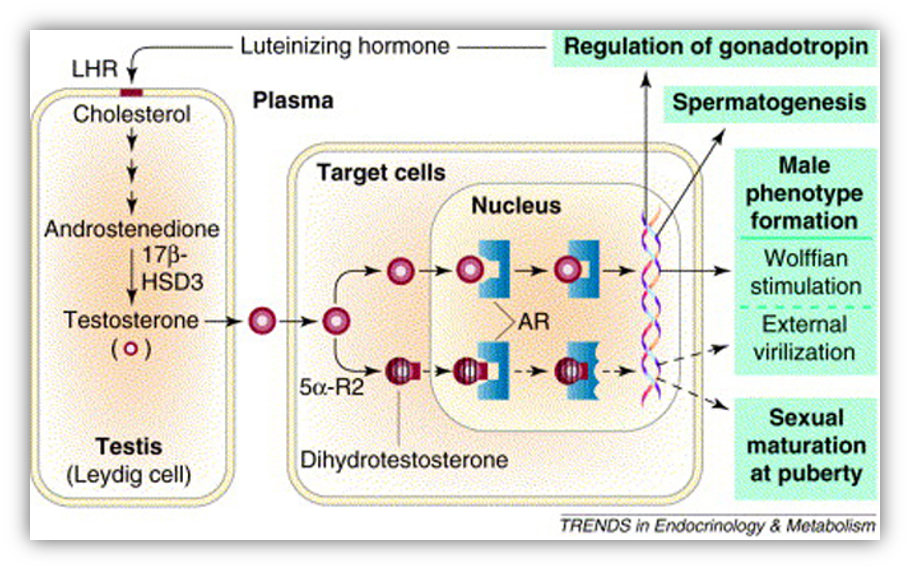
FIGURE 30. Respective roles of testosterone(T) and dihydrotestosterone (DHT) in sex differentiation. Normal androgen physiology in mammals. Testosterone and dihydrotestosterone are assumed to work by binding to the same receptor protein and forming hormone–receptor complexes of different allosteric configurations. Abbreviations: AR, androgen receptor; 17β-HSD3, 17β-hydroxysteroid dehydrogenase type 3; LHR, luteinizing hormone receptor; 5α-R2, steroid 5α-reductase type 2. Reprinted from ref. (531): Wilson JD, Shaw G, Leihy ML, Renfree MB. The marsupial model for male phenotypic development. Trends in Endocrinology and Metabolism, 13:78-83 (2002), Copyright 2002, with permission from Elsevier. http://www.sciencedirect.com/science/article/pii/S1043276001005252.
HORMONAL CONTROL OF FEMALE DIFFERENTIATION
Estrogens, Diethylstilbestrol, Xenoestrogens
The conclusion that ovarian hormones are not necessary to female development of the female reproductive tract (58, 59) is supported by the female phenotypic development of 45,X or 46,XY subjects with bilateral gonadal aplasia and of aromatase knockout mice unable to synthesize estrogens. Yet, inappropriate estrogen exposure is clearly detrimental. The most tragic illustration of estrogen toxicity is the « DES story ». Diethylstilbestrol (DES), a synthetic estrogen, was widely administered to pregnant women in the early 1940s in the hope of preventing abortion. It was later discovered that female progeny exhibited severe abnormalities of the reproductive tract: vaginal clear-cell adenocarcinoma, vaginal adenosis and squamous metaplasia, transverse vaginal ridges and structural malformations of the cervix and uterus (571, 572).
Environmental chemicals that exert deleterious effects upon the endocrine axis are called endocrine disruptors. By binding to nuclear hormone receptors, they may affect sexual differentiation. Unregulated exposure to xenoestrogens such as bisphenol A is now incriminated in the occurrence of cryptorchidism and hypospadias (573-575). Phthalates also adversely affect male differentiation by increasing the expression of COUP-TF2, a transcription factor which represses steroidogenic enzymes (576). Evidence from animal studies show that environmental exposure to endocrine disrupting chemicals is at least partially responsible (reviewed in (577, 578). Phthalates may act as pseudo-estrogens (biphenol A, alias BPA) or as antiandrogens (diethylhexylphthalate, alias DEHP) (579); however caution is required for interpretation of animal studies because of species differences. In human testes, germ cells appear the most susceptible to damage by phthalates (580). Atrazine, a herbicide widely used in the United States, demasculinizes male gonads and reduces sperm count by interfering with phosphodiesterase enzymes and SF1 (581).
CONCLUSION
A bewildering number of hormones and growth factors is involved in sex determination and differentiation, making it one of the best studied developmental processes. The uncovering of an active genetic pathway towards ovarian development has overturned the dogma of a default pathway towards female gonadal differentiation. For the moment, testicular hormones retain their primacy in modeling the reproductive tract but who knows what surprises the future holds in store?
REFERENCES
1. Nef S, Stevant I, Greenfield A (2019) Characterizing the bipotential mammalian gonad. Curr Top Dev Biol 134:167-194
2. Yoshino T, Murai H, Saito D (2016) Hedgehog-BMP signalling establishes dorsoventral patterning in lateral plate mesoderm to trigger gonadogenesis in chicken embryos. Nat Commun 7:12561
3. O'Rahilly R (1983) The timing and sequence of events in the development of the human reproductive system during the embryonic period proper. Anat. Embryol. (Berl.) 166:247-261
4. Shawlot W, Behringer RR (1995) Requirement for Lim1 in head-organizer function. Nature 374:425-430
5. Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S (1997) Defects of urogenital development in mice lacking Emx2. Development 124:1653-1664
6. Kusaka M, Katoh-Fukui Y, Ogawa H, Miyabayashi K, Baba T, Shima Y, Sugiyama N, Sugimoto Y, Okuno Y, Kodama R, Iizuka-Kogo A, Senda T, Sasaoka T, Kitamura K, Aizawa S, Morohashi K (2010) Abnormal epithelial cell polarity and ectopic epidermal growth factor receptor (EGFR) expression induced in Emx2 KO embryonic gonads. Endocrinology 151:5893-5904
7. Torres M, Gómez-Pardo E, Dressler GR, Gruss P (1995) Pax-2 controls multiple steps of urogenital development. Development 121:4057-4065
8. Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H (2000) The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403:909-913
9. Wilhelm D, Englert C (2002) The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 16:1839-1851
10. Mazaud S, Oréal E, Guigon CJ, Carré-Eusèbe D, Magre S (2002) Lhx9 expression during gonadal morphogenesis as related to the state of cell differentiation. Gene Expression Patterns 2:373-377
11. Zhao L, Wang C, Lehman ML, He M, An J, Svingen T, Spiller CM, Ng ET, Nelson CC, Koopman P (2018) Transcriptomic analysis of mRNA expression and alternative splicing during mouse sex determination. Mol Cell Endocrinol 478:84-96
12. Pitetti JL, Calvel P, Romero Y, Conne B, Truong V, Papaioannou MD, Schaad O, Docquier M, Herrera PL, Wilhelm D, Nef S (2013) Insulin and IGF1 Receptors Are Essential for XX and XY Gonadal Differentiation and Adrenal Development in Mice. PLoS Genet 9:e1003160
13. Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE (2004) Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131:4095-4105
14. Hu YC, Okumura LM, Page DC (2013) Gata4 is required for formation of the genital ridge in mice. PLoS Genet 9:e1003629
15. Fujimoto Y, Tanaka SS, Yamaguchi YL, Kobayashi H, Kuroki S, Tachibana M, Shinomura M, Kanai Y, Morohashi K, Kawakami K, Nishinakamura R (2013) Homeoproteins Six1 and Six4 regulate male sex determination and mouse gonadal development. Dev. Cell 26:416-430
16. Lin YT, Barske L, DeFalco T, Capel B (2017) Numb regulates somatic cell lineage commitment during early gonadogenesis in mice. Development 144:1607-1618
17. Gregoire EP, Stevant I, Chassot AA, Martin L, Lachambre S, Mondin M, de Rooij DG, Nef S, Chaboissier MC (2018) NRG1 signalling regulates the establishment of Sertoli cell stock in the mouse testis. Mol Cell Endocrinol 478:17-31
18. Schmahl J, Capel B (2003) Cell proliferation is necessary for the determination of male fate in the gonad. Dev.Biol. 258:264-276
19. Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B (2004) Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development 131:3627-3636
20. Sekido R, Lovell-Badge R (2013) Genetic control of testis development. Sex Dev. 7:21-32
21. Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A (2001) Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106:319-329
22. Pritchard Jones K, Fleming S, Davidson D, Bickmore W, Porteous D, Gosden C, Bard J, Buckler A, Pelletier J, Housman D (1990) The candidate Wilms' tumour gene is involved in genitourinary development. Nature 346:194-197
23. Hanley NA, Ball SG, Clement-Jones M, Hagan DM, Strachan T, Lindsay S, Robson S, Ostrer H, Parker KL, Wilson DI (1999) Expression of steroidogenic factor 1 and Wilms' tumour 1 during early human gonadal development and sex determination. Mech. Dev. 87:175-180
24. Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R (1993) WT-1 is required for early kidney development. Cell 74:679-691
25. Pelletier J, Bruening W, Li FP, Haber DA, Glaser T, Housman DE (1991) WT1 mutations contribute to abnormal genital system development and hereditary Wilms' tumour. Nature 353:431-434
26. Royer-Pokora B, Beier M, Henzler M, Alam R, Schumacher V, Weirich A, Huff V (2004) Twenty-four new cases of WT1 germline mutations and review of the literature: Genotype/phenotype correlations for Wilms tumor development. Am. J. Med. Genet. A 127A:249-257
27. Köhler B, Biebermann H, Friedsam V, Gellermann J, Maier RF, Pohl M, Wieacker P, Hiort O, Grüters A, Krude H (2011) Analysis of the Wilms' tumor suppressor gene (WT1) in patients 46,XY disorders of sex development. J. Clin. Endocrinol. Metab. 96:E1131-1136
28. Schimmer BP, White PC (2010) Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol. Endocrinol. 24:1322-1337
29. El-Khairi R, Achermann JC (2012) Steroidogenic factor-1 and human disease. Semin. Reprod. Med. 30:374-381
30. Suntharalingham JP, Buonocore F, Duncan AJ, Achermann JC (2015) DAX-1 (NR0B1) and steroidogenic factor-1 (SF-1, NR5A1) in human disease. Best practice & research. Clinical endocrinology & metabolism 29:607-619
31. Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481-490
32. Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL (2004) Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol. Endocrinol. 18:1610-1619
33. Bashamboo A, Donohoue PA, Vilain E, Rojo S, Calvel P, Seneviratne SN, Buonocore F, Barseghyan H, Bingham N, Rosenfeld JA, Mulukutla SN, Jain M, Burrage L, Dhar S, Balasubramanyam A, Lee B, Members of UDN, Dumargne MC, Eozenou C, Suntharalingham JP, de Silva K, Lin L, Bignon-Topalovic J, Poulat F, Lagos CF, McElreavey K, Achermann JC (2016) A recurrent p.Arg92Trp variant in steroidogenic factor-1 (NR5A1) can act as a molecular switch in human sex development. Hum. Mol. Genet. 25:3446-3453
34. Domenice S, Machado AZ, Ferreira FM, Ferraz-de-Souza B, Lerario AM, Lin L, Nishi MY, Gomes NL, da Silva TE, Silva RB, Correa RV, Montenegro LR, Narciso A, Costa EM, Achermann JC, Mendonca BB (2016) Wide spectrum of NR5A1-related phenotypes in 46,XY and 46,XX individuals. Birth Defects Res C Embryo Today 108:309-320
35. Buaas FW, Val P, Swain A (2009) The transcription co-factor CITED2 functions during sex determination and early gonad development. Hum.Mol.Genet. 18:2989-3001
36. Combes AN, Spiller CM, Harley VR, Sinclair AH, Dunwoodie SL, Wilhelm D, Koopman P (2010) Gonadal defects in Cited2-mutant mice indicate a role for SF1 in both testis and ovary differentiation. Int J Dev Biol 54:683-689
37. Stevant I, Nef S (2019) Genetic Control of Gonadal Sex Determination and Development. Trends Genet 35:346-358
38. Liu C, Rodriguez K, Yao HH (2016) Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development 143:3700-3710
39. Edson MA, Nagaraja AK, Matzuk MM (2009) The mammalian ovary from genesis to revelation. Endocr. Rev. 30:624-712
40. Wylie C (1999) Germ cells. Cell 96:165-174
41. Johnson AD, Alberio R (2015) Primordial germ cells: the first cell lineage or the last cells standing? Development 142:2730-2739
42. Marlow F (2015) Primordial Germ Cell Specification and Migration. F1000Res 4
43. Spiller C, Bowles J (2019) Sexually dimorphic germ cell identity in mammals. Curr Top Dev Biol 134:253-288
44. Saitou M, Payer B, O'Carroll D, Ohinata Y, Surani MA (2005) Blimp1 and the emergence of the germ line during development in the mouse. Cell Cycle 4:1736-1740
45. Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13:424-436
46. Ying Y, Qi X, Zhao GQ (2001) Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc. Natl. Acad. Sci. USA 98:7858-7862
47. Ying Y, Zhao GQ (2001) Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev.Biol. 232:484-492
48. Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M (2009) A signaling principle for the specification of the germ cell lineage in mice. Cell 137:571-584
49. Surani MA (2015) Human Germline: A New Research Frontier. Stem Cell Reports 4:955-960
50. Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA (2015) SOX17 is a critical specifier of human primordial germ cell fate. Cell 160:253-268
51. Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H, Tam PP (2005) IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev. Cell 9:745-756
52. Bendel-Stenzel M, Anderson R, Heasman J, Wylie C (1998) The origin and migration of primordial germ cells in the mouse. Semin Cell Dev Biol 9:393-400
53. Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C, Lehmann R (2003) The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 130:4279-4286
54. Rucker EB, Dierisseau P, Wagner KU, Garrett L, Wynshaw Boris A, Flaws JA, Hennighausen L (2000) Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol. Endocrinol. 14:1038-1052
55. Hu YC, Nicholls PK, Soh YQ, Daniele JR, Junker JP, van Oudenaarden A, Page DC (2015) Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet 11:e1005019
56. Lin Y, Gill ME, Koubova J, Page DC (2008) Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 322:1685-1687
57. Nicholls PK, Schorle H, Naqvi S, Hu YC, Fan Y, Carmell MA, Dobrinski I, Watson AL, Carlson DF, Fahrenkrug SC, Page DC (2019) Mammalian germ cells are determined after PGC colonization of the nascent gonad. Proc Natl Acad Sci U S A 116:25677-25687
58. Jost A (1947) Recherches sur la différenciation sexuelle de l'embryon de lapin. III. Rôle des gonades foetales dans la différenciation sexuelle somatique. Arch. Anat. Microsc. Morphol. Exp. 36:271-315
59. Jost A (1953) Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog. Horm. Res. 8:379-418
60. Rotgers E, Jorgensen A, Yao HH (2018) At the Crossroads of Fate-Somatic Cell Lineage Specification in the Fetal Gonad. Endocr. Rev. 39:739-759
61. Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH (1959) A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome). Lancet 1:711-713
62. Jacobs PA, Strong JA (1959) A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183:302-303
63. Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240-244
64. Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes [see comments]. Nature 346:245-250
65. Lovell-Badge R, Robertson E (1990) XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109:635-646
66. Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351:117-121
67. Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M (1990) Genetic evidence equating SRY and the testis-determining factor. Nature 348:448-450
68. Jager RJ, Anvret M, Hall K, Scherer G (1990) A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature 348:452-454
69. Hiramatsu R, Matoba S, Kanai-Azuma M, Tsunekawa N, Katoh-Fukui Y, Kurohmaru M, Morohashi K, Wilhelm D, Koopman P, Kanai Y (2009) A critical time window of Sry action in gonadal sex determination in mice. Development 136:129-138
70. Polanco JC, Koopman P (2007) Sry and the hesitant beginnings of male development. Dev. Biol. 302:13-24
71. Capel B (2017) Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat Rev Genet 18:675-689
72. Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M (2009) Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139:1130-1142
73. Weil D, Wang I, Dietrich A, Poustka A, Weissenbach J, Petit C (1994) Highly homologous loci on the X and Y chromosomes are hot-spots for ectopic recombinations leading to XX maleness. Nat. Genet. 7:414-419
74. Hawkins JR (1993) Mutational analysis of SRY in XY females. Hum. Mutat. 2:347-350
75. Grinspon RP, Rey RA (2016) Disorders of sex development with testicular differentiation in SRY-negative 46,XX individuals: clinical and genetic aspects. Sex. Dev. 10:1-11
76. Thevenet L, Albrecht KH, Malki S, Berta P, Boizet-Bonhoure B, Poulat F (2005) NHERF2/SIP-1 Interacts with Mouse SRY via a Different Mechanism than Human SRY. J. Biol. Chem. 280:38625-38630
77. Oh HJ, Li Y, Lau YF (2005) Sry associates with the heterochromatin protein 1 complex by interacting with a KRAB domain protein. Biol Reprod 72:407-415
78. Hanley NA, Hagan DM, Clement-Jones M, Ball SG, Strachan T, Salas-Cortes L, McElreavey K, Lindsay S, Robson S, Bullen P, Ostrer H, Wilson DI (2000) SRY, SOX9, and DAX1 expression patterns during human sex determination and gonadal development. Mech. Dev. 91:403-407
79. Shimamura R, Fraizer GC, Trapman J, Lau Y, Saunders GF (1997) The Wilms' tumor gene WT1 can regulate genes involved in sex determination and differentiation: SRY, Mullerian-inhibiting substance, and the androgen receptor. Clin. Cancer Res. 3:2571-2580
80. Hossain A, Saunders GF (2001) The human sex-determining gene SRY is a direct target of WT1. J Biol Chem 276:16817-16823
81. Desclozeaux M, Poulat F, de Santa Barbara P, Soullier S, Jay P, Berta P, Boizet-Bonhoure B (1998) Characterization of two Sp1 binding sites of the human sex determining SRY promoter. Biochim. Biophys. Acta 1397:247-252
82. Assumpcao JG, Ferraz LF, Benedetti CE, iel-Guerra AT, Guerra-Junior G, Marques de Faria AP, Baptista MT, de Mello MP (2005) A naturally occurring deletion in the SRY promoter region affecting the Sp1 binding site is associated with sex reversal. J.Endocrinol.Invest. 28:651-656
83. Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH (2002) Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129:4627-4634
84. Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS (2008) A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol. Biol. 9:44
85. Gierl MS, Gruhn WH, von Seggern A, Maltry N, Niehrs C (2012) GADD45G functions in male sex determination by promoting p38 signaling and Sry expression. Dev. Cell 23:1032-1042
86. Warr N, Carre GA, Siggers P, Faleato JV, Brixey R, Pope M, Bogani D, Childers M, Wells S, Scudamore CL, Tedesco M, del Barco Barrantes I, Nebreda AR, Trainor PA, Greenfield A (2012) Gadd45gamma and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev. Cell 23:1020-1031
87. Bogani D, Siggers P, Brixey R, Warr N, Beddow S, Edwards J, Williams D, Wilhelm D, Koopman P, Flavell RA, Chi H, Ostrer H, Wells S, Cheeseman M, Greenfield A (2009) Loss of mitogen-activated protein kinase kinase kinase 4 (MAP3K4) reveals a requirement for MAPK signalling in mouse sex determination. PLoS Biol. 7:e1000196
88. Kuroki S, Matoba S, Akiyoshi M, Matsumura Y, Miyachi H, Mise N, Abe K, Ogura A, Wilhelm D, Koopman P, Nozaki M, Kanai Y, Shinkai Y, Tachibana M (2013) Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science 341:1106-1109
89. Ion A, Telvi L, Galacteros F, Valayer J, Fellous M, McElreavey K (1996) A novel mutation in the putative DNA helicase XH2 is responsible for male- to-female sex reversal associated with an atypical form of the ATR-X syndrome. Am.J.Hum.Genet. 58:1185-1191
90. Gibbons RJ, Picketts DJ, Villard L, Higgs DR (1995) Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome). Cell 80:837-845
91. Reardon W, Gibbons RJ, Winter RM, Baraitser M (1995) Male pseudohermaphroditism in sibs with the alpha-thalassemia/mental retardation (ATR-X) syndrome. Am.J.Med.Genet. 55:285-287
92. Tachibana M (2015) Epigenetic regulation of mammalian sex determination. J. Med. Invest. 62:19-23
93. Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanoviç M, Weissenbach J, Mansour S, Young ID, Goodfellow PN (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372:525-530
94. Jakob S, Lovell-Badge R (2011) Sex determination and the control of Sox9 expression in mammals. FEBS J. 278:1002-1009
95. Sekido R, Lovell-Badge R (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 453:930-934
96. Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122:2813-2822
97. Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat. Genet. 14:62-68
98. Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R (2004) SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 274:271-279
99. Sreenivasan R, Ludbrook L, Fisher B, Declosmenil F, Knower KC, Croft B, Bird AD, Ryan J, Bashamboo A, Sinclair AH, Koopman P, McElreavey K, Poulat F, Harley VR (2018) Mutant NR5A1/SF-1 in patients with disorders of sex development shows defective activation of the SOX9 TESCO enhancer. Hum. Mutat. 39:1861-1874
100. Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA (2000) A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat. Genet. 26:490-494
101. Vidal VP, Chaboissier MC, de Rooij DG, Schedl A (2001) Sox9 induces testis development in XX transgenic mice. Nat. Genet. 28:216-217
102. Chaboissier MC, Kobayashi A, Vidal VIP, Lutzkendorf S, van de Kant HJG, Wegner M, de Rooij DG, Behringer RR, Schedl A (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131:1891-1901
103. Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79:1111-1120
104. Huang B, Wang SB, Ning Y, Lamb AN, Bartley J (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 87:349-353
105. Bagheri-Fam S, Barrionuevo F, Dohrmann U, Gunther T, Schule R, Kemler R, Mallo M, Kanzler B, Scherer G (2006) Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev. Biol. 291:382-397
106. Georg I, Bagheri-Fam S, Knower KC, Wieacker P, Scherer G, Harley VR (2010) Mutations of the SRY-responsive enhancer of SOX9 are uncommon in XY gonadal dysgenesis. Sex. Dev. 4:321-325
107. Kim GJ, Sock E, Buchberger A, Just W, Denzer F, Hoepffner W, German J, Cole T, Mann J, Seguin JH, Zipf W, Costigan C, Schmiady H, Rostasy M, Kramer M, Kaltenbach S, Rosler B, Georg I, Troppmann E, Teichmann AC, Salfelder A, Widholz SA, Wieacker P, Hiort O, Camerino G, Radi O, Wegner M, Arnold HH, Scherer G (2015) Copy number variation of two separate regulatory regions upstream of SOX9 causes isolated 46,XY or 46,XX disorder of sex development. J Med Genet 52:240-247
108. Gonen N, Futtner CR, Wood S, Garcia-Moreno SA, Salamone IM, Samson SC, Sekido R, Poulat F, Maatouk DM, Lovell-Badge R (2018) Sex reversal following deletion of a single distal enhancer of Sox9. Science 360:1469-1473
109. White S, Ohnesorg T, Notini A, Roeszler K, Hewitt J, Daggag H, Smith C, Turbitt E, Gustin S, van den Bergen J, Miles D, Western P, Arboleda V, Schumacher V, Gordon L, Bell K, Bengtsson H, Speed T, Hutson J, Warne G, Harley V, Koopman P, Vilain E, Sinclair A (2011) Copy number variation in patients with disorders of sex development due to 46,XY gonadal dysgenesis. PLoS One 6:e17793
110. Cox JJ, Willatt L, Homfray T, Woods CG (2011) A SOX9 Duplication and Familial 46,XX Developmental Testicular Disorder. N. Engl. J. Med. 364:91-93
111. Vetro A, Ciccone R, Giorda R, Patricelli MG, Della ME, Forlino A, Zuffardi O (2011) XX males SRY negative: a confirmed cause of infertility. J Med Genet 48:710-712
112. Arango NA, Lovell-Badge R, Behringer RR (1999) Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99:409-419
113. Lasala C, Schteingart HF, Arouche N, Bedecarrás P, Grinspon RP, Picard JY, Josso N, di Clemente N, Rey RA (2011) SOX9 and SF1 are involved in cyclic AMP-mediated upregulation of anti-Mullerian gene expression in the testicular prepubertal Sertoli cell line SMAT1. Am J Physiol Endocrinol Metab 301:E539-547
114. Schepers G, Wilson M, Wilhelm D, Koopman P (2003) SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J Biol Chem 278:28101-28108
115. Barrionuevo F, Georg I, Scherthan H, Lécureuil C, Guillou F, Wegner M, Scherer G (2009) Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev. Biol. 327:301-312
116. Polanco JC, Wilhelm D, Davidson TL, Knight D, Koopman P (2010) Sox10 gain-of-function causes XX sex reversal in mice: implications for human 22q-linked disorders of sex development. Hum. Mol. Genet. 19:506-516
117. Georg I, Barrionuevo F, Wiech T, Scherer G (2012) Sox9 and Sox8 Are Required for Basal Lamina Integrity of Testis Cords and for Suppression of FOXL2 During Embryonic Testis Development in Mice. Biol. Reprod. 87:(99): 91-11
118. Portnoi MF, Dumargne MC, Rojo S, Witchel SF, Duncan AJ, Eozenou C, Bignon-Topalovic J, Yatsenko SA, Rajkovic A, Reyes-Mugica M, Almstrup K, Fusee L, Srivastava Y, Chantot-Bastaraud S, Hyon C, Louis-Sylvestre C, Validire P, de Malleray Pichard C, Ravel C, Christin-Maitre S, Brauner R, Rossetti R, Persani L, Charreau EH, Dain L, Chiauzzi VA, Mazen I, Rouba H, Schluth-Bolard C, MacGowan S, McLean WHI, Patin E, Rajpert-De Meyts E, Jauch R, Achermann JC, Siffroi JP, McElreavey K, Bashamboo A (2018) Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Hum. Mol. Genet. 27:1228-1240
119. Sutton E, Hughes J, White S, Sekido R, Tan J, Arboleda V, Rogers N, Knower K, Rowley L, Eyre H, Rizzoti K, McAninch D, Goncalves J, Slee J, Turbitt E, Bruno D, Bengtsson H, Harley V, Vilain E, Sinclair A, Lovell-Badge R, Thomas P (2011) Identification of SOX3 as an XX male sex reversal gene in mice and humans. J. Clin. Invest. 121:328-341
120. McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M (1993) A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc. Natl. Acad. Sci. USA 90:3368-3372
121. Ottolenghi C, Omari S, García-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D (2005) Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 14:2053-2062
122. Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P (2005) Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev. Biol. 287:111-124
123. Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G (2006) Homozygous Inactivation of Sox9 Causes Complete XY Sex Reversal in Mice. Biol. Reprod. 74:195-201
124. Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Capel B (2007) Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc. Natl. Acad. Sci. USA 104:16558-16563
125. Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR (2008) Loss of Fgfr2 leads to partial XY sex reversal. Dev.Biol. 314:71-83
126. Carré GA, Greenfield A (2014) Characterising novel pathways in testis determination using mouse genetics. Sex. Dev. 8:199-207
127. Moniot B, Declosmenil F, Barrionuevo F, Scherer G, Aritake K, Malki S, Marzi L, Cohen-Solal A, Georg I, Klattig J, Englert C, Kim Y, Capel B, Eguchi N, Urade Y, Boizet-Bonhoure B, Poulat F (2009) The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 136:1813-1821
128. Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, Berta P, Poulat F, Boizet-Bonhoure B (2005) Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J 24:1798-1809
129. Freire AV, Ropelato MG, Rey RA (2020) Ovaries and Testes. In: Kovacs CS, Deal C eds. Maternal-Fetal and Neonatal Endocrinology. 1st ed. Boston, MA, USA: Academic Press-Elsevier; 625-641
130. Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier M-C, Poulat F, Behringer RR, Lovell-Badge R, Capel B (2006) Fgf9 and Wnt4 Act as Antagonistic Signals to Regulate Mammalian Sex Determination. PLoS Biol. 4:e187
131. Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM (2001) Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104:875-889
132. Loke J, Pearlman A, Radi O, Zuffardi O, Giussani U, Pallotta R, Camerino G, Ostrer H (2014) Mutations in MAP3K1 tilt the balance from SOX9/FGF9 to WNT/beta-catenin signaling. Hum. Mol. Genet. 23:1073-1083
133. Matson CK, Zarkower D (2012) Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet 13:163-174
134. Zhao L, Svingen T, Ng ET, Koopman P (2015) Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development 142:1083-1088
135. Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D (2011) DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476:101-104
136. Lindeman RE, Gearhart MD, Minkina A, Krentz AD, Bardwell VJ, Zarkower D (2015) Sexual cell-fate reprogramming in the ovary by DMRT1. Curr Biol 25:764-771
137. Ottolenghi C, McElreavey K (2000) Deletions of 9p and the quest for a conserved mechanism of sex determination. Mol. Genet. Metab. 71:397-404
138. Ottolenghi C, Veitia R, Quintana Murci L, Torchard D, Scapoli L, Souleyreau-Therville N, Beckmann J, Fellous M, McElreavey K (2000) The region on 9p associated with 46,XY sex reversal contains several transcripts expressed in the urogenital system and a novel doublesex-related domain. Genomics 64:170-178
139. Bardoni B, Zanaria E, Guioli S, Floridia G, Worley KC, Tonini G, Ferrante E, Chiumello G, McCabe ER, Fraccaro M (1994) A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat. Genet. 7:497-501
140. Ludbrook LM, Bernard P, Bagheri-Fam S, Ryan J, Sekido R, Wilhelm D, Lovell-Badge R, Harley VR (2012) Excess DAX1 leads to XY ovotesticular disorder of sex development (DSD) in mice by inhibiting steroidogenic factor-1 (SF1) activation of the testis enhancer of SRY-box-9 (Sox9). Endocrinology 153:1948-1958
141. Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R (1998) Dax1 antagonizes Sry action in mammalian sex determination. Nature 391:761-767
142. Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL (1998) Role of Ahch in gonadal development and gametogenesis. Nat. Genet. 20:353-357
143. Bouma GJ, Albrecht KH, Washburn LL, Recknagel AK, Churchill GA, Eicher EM (2005) Gonadal sex reversal in mutant Dax1 XY mice: a failure to upregulate Sox9 in pre-Sertoli cells. Development 132:3045-3054
144. Park SY, Lee EJ, Emge D, Jahn CL, Jameson JL (2008) A Phenotypic Spectrum of Sexual Development in Dax1 (Nr0b1)-Deficient Mice: Consequence of the C57BL/6J Strain on Sex Determination. Biol. Reprod. 79:1038-1045
145. Ludbrook LM, Harley VR (2004) Sex determination: a "window" of DAX1 activity. Trends Endocrinol.Metab. 15:116-121
146. Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, Higashinakagawa T (1998) Male-to-female sex reversal in M33 mutant mice. Nature 393:688-692
147. Katoh-Fukui Y, Miyabayashi K, Komatsu T, Owaki A, Baba T, Shima Y, Kidokoro T, Kanai Y, Schedl A, Wilhelm D, Koopman P, Okuno Y, Morohashi K (2012) Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology 153:913-924
148. Garcia-Moreno SA, Lin YT, Futtner CR, Salamone IM, Capel B, Maatouk DM (2019) CBX2 is required to stabilize the testis pathway by repressing Wnt signaling. PLoS Genet 15:e1007895
149. Biason-Lauber A, Konrad D, Meyer M, deBeaufort C, Schoenle EJ (2009) Ovaries and Female Phenotype in a Girl with 46,XY Karyotype and Mutations in the CBX2 Gene. Am. J. Hum. Genet. 84:658-663
150. Warr N, Bogani D, Siggers P, Brixey R, Tateossian H, Dopplapudi A, Wells S, Cheeseman M, Xia Y, Ostrer H, Greenfield A (2011) Minor abnormalities of testis development in mice lacking the gene encoding the MAPK signalling component, MAP3K1. PLoS One 6:e19572
151. Pearlman A, Loke J, Le CC, White S, Chin L, Friedman A, Warr N, Willan J, Brauer D, Farmer C, Brooks E, Oddoux C, Riley B, Shajahan S, Camerino G, Homfray T, Crosby AH, Couper J, David A, Greenfield A, Sinclair A, Ostrer H (2010) Mutations in MAP3K1 cause 46,XY disorders of sex development and implicate a common signal transduction pathway in human testis determination. Am. J. Hum. Genet. 87:898-904
152. Chamberlin A, Huether R, Machado AZ, Groden M, Liu HM, Upadhyay K, O V, Gomes NL, Lerario AM, Nishi MY, Costa EMF, Mendonca B, Domenice S, Velasco J, Loke J, Ostrer H (2019) Mutations in MAP3K1 that cause 46,XY disorders of sex development disrupt distinct structural domains in the protein. Hum. Mol. Genet. 28:1620-1628
153. Harris A, Siggers P, Corrochano S, Warr N, Sagar D, Grimes DT, Suzuki M, Burdine RD, Cong F, Koo BK, Clevers H, Stevant I, Nef S, Wells S, Brauner R, Ben Rhouma B, Belguith N, Eozenou C, Bignon-Topalovic J, Bashamboo A, McElreavey K, Greenfield A (2018) ZNRF3 functions in mammalian sex determination by inhibiting canonical WNT signaling. Proc Natl Acad Sci U S A 115:5474-5479
154. Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F (2012) ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485:195-200
155. Zebisch M, Xu Y, Krastev C, MacDonald BT, Chen M, Gilbert RJ, He X, Jones EY (2013) Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun 4:2787
156. Bashamboo A, Eozenou C, Jorgensen A, Bignon-Topalovic J, Siffroi JP, Hyon C, Tar A, Nagy P, Solyom J, Halasz Z, Paye-Jaouen A, Lambert S, Rodriguez-Buritica D, Bertalan R, Martinerie L, Rajpert-De Meyts E, Achermann JC, McElreavey K (2018) Loss of Function of the Nuclear Receptor NR2F2, Encoding COUP-TF2, Causes Testis Development and Cardiac Defects in 46,XX Children. Am. J. Hum. Genet. 102:487-493
157. Fukami M, Wada Y, Miyabayashi K, Nishino I, Hasegawa T, Nordenskjöld A, Camerino G, Kretz C, Buj-Bello A, Laporte J, Yamada G, Morohashi K, Ogata T (2006) CXorf6 is a causative gene for hypospadias. Nat.Genet. 38:1369-1371
158. Fukami M, Wada Y, Okada M, Kato F, Katsumata N, Baba T, Morohashi K, Laporte J, Kitagawa M, Ogata T (2008) Mastermind-like domain-containing 1 (MAMLD1 or CXorf6) transactivates the Hes3 promoter, augments testosterone production, and contains the SF1 target sequence. J Biol Chem. 283:5525-5532
159. Flück CE, Audí L, Fernández-Cancio M, Sauter KS, Martínez de LaPiscina I, Castaño L, Esteva I, Camats N (2019) Broad Phenotypes of Disorders/Differences of Sex Development in MAMLD1 Patients Through Oligogenic Disease. Front Genet 10:746
160. Miyado M, Yoshida K, Miyado K, Katsumi M, Saito K, Nakamura S, Ogata T, Fukami M (2017) Knockout of Murine Mamld1 Impairs Testicular Growth and Daily Sperm Production but Permits Normal Postnatal Androgen Production and Fertility. Int. J. Mol. Sci. 18
161. Brennan J, Karl J, Capel B (2002) Divergent Vascular Mechanisms Downstream of Sry Establish the Arterial System in the XY Gonad. Dev. Biol. 244:418-428
162. Coveney D, Cool J, Oliver T, Capel B (2008) Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc. Natl. Acad. Sci. U. S. A. 105:7212-7217
163. Byskov AG (1986) Differentiation of mammalian embryonic gonad. Physiol Rev. 66:71-117
164. Cool J, Carmona FD, Szucsik JC, Capel B (2008) Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2:128-133
165. Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B (1997) Male-specific cell migration into the developing gonad. Curr. Biol. 7:958-968
166. Tilmann C, Capel B (1999) Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development 126:2883-2890
167. Yao HHC, DiNapoli L, Capel B (2003) Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 130:5895-5902
168. Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, Koopman P (2009) Endothelial cell migration directs testis cord formation. Dev Biol 326:112-120
169. Cool J, DeFalco TJ, Capel B (2011) Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A 108:167-172
170. Ungewitter EK, Yao HH (2013) How to make a gonad: cellular mechanisms governing formation of the testes and ovaries. Sex Dev. 7:7-20
171. Grinspon RP, Rey RA (2019) Molecular Characterization of XX Maleness. Int. J. Mol. Sci. 20:6089
172. Karl J, Capel B (1998) Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev.Biol. 203:323-333
173. Albrecht KH, Eicher EM (2001) Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev.Biol. 240:92-107
174. Bullejos M, Koopman P (2001) Spatially dynamic expression of Sry in mouse genital ridges. Dev.Dyn. 221:201-205
175. Tilmann C, Capel B (2002) Cellular and molecular pathways regulating mammalian sex determination. Recent Prog.Horm.Res. 57:1-18
176. Palmer SJ, Burgoyne PS (1991) In situ analysis of fetal, prepuberal and adult XX----XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development 112:265-268
177. Colvin JS, White AC, Pratt SJ, Ornitz DM (2001) Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development 128:2095-2106
178. Grimmond S, Van Hateren N, Siggers P, Arkell R, Larder R, Soares MB, de Fatima B, Smith L, Tymowska-Lalanne Z, Wells C, Greenfield A (2000) Sexually dimorphic expression of protease nexin-1 and vanin-1 in the developing mouse gonad prior to overt differentiation suggests a role in mammalian sexual development. Hum. Mol. Genet. 9:1553-1560
179. Pierucci Alves F, Clark AM, Russell LD (2001) A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod 65:1392-1402
180. Clark AM, Garland KK, Russell LD (2000) Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63:1825-1838
181. Bitgood MJ, Shen L, McMahon AP (1996) Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 6:298-304
182. Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ (1998) Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc. Natl. Acad. Sci. USA 95:13630-13634
183. Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M (2000) A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 67:1302-1305
184. Canto P, Soderlund D, Reyes E, Mendez JP (2004) Mutations in the desert hedgehog (DHH) gene in patients with 46,XY complete pure gonadal dysgenesis. J. Clin. Endocrinol. Metab. 89:4480-4483
185. Callier P, Calvel P, Matevossian A, Makrythanasis P, Bernard P, Kurosaka H, Vannier A, Thauvin-Robinet C, Borel C, Mazaud-Guittot S, Rolland A, Desdoits-Lethimonier C, Guipponi M, Zimmermann C, Stevant I, Kuhne F, Conne B, Santoni F, Lambert S, Huet F, Mugneret F, Jaruzelska J, Faivre L, Wilhelm D, Jegou B, Trainor PA, Resh MD, Antonarakis SE, Nef S (2014) Loss of function mutation in the palmitoyl-transferase HHAT leads to syndromic 46,XY disorder of sex development by impeding Hedgehog protein palmitoylation and signaling. PLoS Genet 10:e1004340
186. Bendsen E, Byskov AG, Laursen SB, Larsen HP, Andersen CY, Westergaard LG (2003) Number of germ cells and somatic cells in human fetal testes during the first weeks after sex differentiation. Hum. Reprod. 18:13-18
187. Magre S, Jost A (1980) The initial phases of testicular organogenesis in the rat. Arch Anat Microsc Morphol Exp 69:297-318
188. Fröjdman K, Paranko J, Virtanen I, Pelliniemi LJ (1992) Intermediate filaments and epithelial differentiation of male rat embryonic gonad. Differentiation 50:113-123
189. Josso N, Lamarre I, Picard JY, Berta P, Davies N, Morichon N, Peschanski M, Jeny R (1993) Anti-Müllerian hormone in early human development. Early Hum. Dev. 33:91-99
190. Josso N, Rey RA, Picard JY (2013) Anti-müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Int. J. Endocrinol. 2013:674105
191. Werner R, Merz H, Birnbaum W, Marshall L, Schroder T, Reiz B, Kavran JM, Baumer T, Capetian P, Hiort O (2015) 46,XY Gonadal Dysgenesis due to a Homozygous Mutation in Desert Hedgehog (DHH) Identified by Exome Sequencing. J. Clin. Endocrinol. Metab. 100:E1022-1029
192. Yao HH, Whoriskey W, Capel B (2002) Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 16:1433-1440
193. Cupp AS, Uzumcu M, Skinner MK (2003) Chemotactic Role of Neurotropin 3 in the Embryonic Testis That Facilitates Male Sex Determination. Biol. Reprod. 68:2033-2037
194. Cupp AS, Tessarollo L, Skinner MK (2002) Testis Developmental Phenotypes in Neurotropin Receptor trkA and trkC Null Mutations: Role in Formation of Seminiferous Cords and Germ Cell Survival. Biol. Reprod. 66:1838-1845
195. Svingen T, Koopman P (2013) Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev 27:2409-2426
196. O'Shaughnessy PJ, Baker PJ, Monteiro A, Cassie S, Bhattacharya S, Fowler PA (2007) Developmental changes in human fetal testicular cell numbers and messenger ribonucleic acid levels during the second trimester. J. Clin. Endocrinol. Metab. 92:4792-4801
197. Al-Attar L, Noël K, Dutertre M, Belville C, Forest MG, Burgoyne PS, Josso N, Rey R (1997) Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J. Clin. Invest. 100:1335-1343
198. Kumar TR, Low MJ, Matzuk MM (1998) Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology 139:3289-3295
199. Kumar TR, Wang Y, Lu N, Matzuk MM (1997) Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 15:201-204
200. O'Shaughnessy PJ, Morris ID, Huhtaniemi I, Baker PJ, Abel MH (2009) Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: Data from mutant and genetically modified mice. Mol. Cell. Endocrinol. 306:2-8
201. Bougnères P, François M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, Roger D, Lahlou N (2008) Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J. Clin. Endocrinol. Metab. 93:2202-2205
202. Braslavsky D, Grinspon RP, Ballerini MG, Bedecarrás P, Loreti N, Bastida G, Ropelato MG, Keselman A, Campo S, Rey RA, Bergada I (2015) Hypogonadotropic Hypogonadism in Infants with Congenital Hypopituitarism: A Challenge to Diagnose at an Early Stage. Horm. Res. Paediatr. 84:289-297
203. Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, Rivarola MA, Belgorosky A (2006) Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr. Res. 60:740-744
204. Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, Gonzalez-Peramato P, Serrano A (2008) Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J. Clin. Endocrinol. Metab. 93:4408-4412
205. Boukari K, Meduri G, Brailly-Tabard S, Guibourdenche J, Ciampi ML, Massin N, Martinerie L, Picard JY, Rey R, Lombes M, Young J (2009) Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J. Clin. Endocrinol. Metab. 94:1818-1825
206. Rey RA (2014) Mini-puberty and true puberty: differences in testicular function. Ann. Endocrinol. (Paris) 75:58-63
207. Mayerhofer A, Lahr G, Seidl K, Eusterschulte B, Christoph A, Gratzl M (1996) The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J. Androl. 17:223-230
208. DeFalco T, Takahashi S, Capel B (2011) Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352:14-26
209. Hatano O, Takakusu A, Nomura M, Morohashi K (1996) Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1:663-671
210. Swain A (2006) Sex Determination: Time for Meiosis? The Gonad Decides. Curr. Biol. 16:R507-R509
211. Hu L, Monteiro A, Johnston H, King P, O'Shaughnessy PJ (2007) Expression of Cyp21a1 and Cyp11b1 in the fetal mouse testis. Reproduction 134:585-591
212. Martinez-Aguayo A, Rocha A, Rojas N, García C, Parra R, Lagos M, Valdivia L, Poggi H, Cattani A, Chilean Collaborative Testicular Adrenal Rest Tumor Study G (2007) Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 92:4583-4589
213. Kumar DL, DeFalco T (2018) A perivascular niche for multipotent progenitors in the fetal testis. Nat Commun 9:4519
214. Griswold SL, Behringer RR (2009) Fetal Leydig cell origin and development. Sex Dev. 3:1-15
215. Codesal J, Regadera J, Nistal M, Regadera-Sejas J, Paniagua R (1990) Involution of human fetal Leydig cells. An immunohistochemical, ultrastructural and quantitative study. J Anat. 172:103-14.:103-114
216. Nef S, Parada LF (1999) Cryptorchidism in mice mutant for InsI3. Nat. Genet. 22:295-299
217. Feng S, Ferlin A, Truong A, Bathgate R, Wade JD, Corbett S, Han S, Tannour-Louet M, Lamb DJ, Foresta C, Agoulnik AI (2009) INSL3/RXFP2 signaling in testicular descent. Ann.N.Y.Acad.Sci. 1160:197-204
218. Ivell R, Wade JD, Anand-Ivell R (2013) INSL3 as a biomarker of Leydig cell functionality. Biol Reprod 88:147
219. Brennan J, Tilmann C, Capel B (2003) Pdgfr-a mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 17:800-810
220. Word RA, George FW, Wilson JD, Carr BR (1989) Testosterone synthesis and adenylate cyclase activity in the early human fetal testis appear to be independent of human chorionic gonadotropin control. J. Clin. Endocrinol. Metab. 69:204-208
221. Kremer H, Kraaij R, Toledo SP, Post M, Fridman JB, Hayashida CY, van Reen M, Milgrom E, Ropers HH, Mariman E (1995) Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat. Genet. 9:160-164
222. Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E (2001) Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am. J. Hum. Genet. 68:1102-1109
223. Crawford PA, Dorn C, Sadovsky Y, Milbrandt J (1998) Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol 18:2949-2956
224. Suzuki T, Mizusaki H, Kawabe K, Kasahara M, Yoshioka H, Morohashi K (2002) Concerted regulation of gonad differentiation by transcription factors and growth factors. Novartis Found Symp 244:68-77
225. Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K (2002) Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat. Genet. 32:359-369
226. Merlet J, Racine C, Moreau E, Moreno SG, Habert R (2007) Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc. Natl. Acad. Sci. USA 104:3615-3620
227. McLaren A, Southee D (1997) Entry of mouse embryonic germ cells into meiosis. Dev.Biol. 187:107-113
228. Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P (2006) Retinoid Signaling Determines Germ Cell Fate in Mice. Science 312:596-600
229. Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 103:2474-2479
230. Bowles J, Feng CW, Spiller C, Davidson TL, Jackson A, Koopman P (2010) FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev.Cell 19:440-449
231. Feng CW, Bowles J, Koopman P (2014) Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol 382:488-497
232. Edelsztein NY, Kashimada K, Schteingart HF, Rey RA (2019) CYP26B1 declines postnatally in Sertoli cells independently of androgen action in the mouse testis. Mol. Reprod. Dev.
233. Suzuki A, Saga Y (2008) Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 22:430-435
234. Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT (2011) Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS One 6:e20249
235. Frydman N, Poulain M, Arkoun B, Duquenne C, Tourpin S, Messiaen S, Habert R, Rouiller-Fabre V, Benachi A, Livera G (2017) Human foetal ovary shares meiotic preventing factors with the developing testis. Hum. Reprod. 32:631-642
236. McLaren A (2000) Germ and somatic cell lineages in the developing gonad. Mol. Cell. Endocrinol. 163:3-9
237. Tevosian SG (2013) Genetic control of ovarian development. Sex Dev. 7:33-45
238. Lin YT, Capel B (2015) Cell fate commitment during mammalian sex determination. Curr Opin Genet Dev 32:144-152
239. Biason-Lauber A, Chaboissier MC (2015) Ovarian development and disease: The known and the unexpected. Semin Cell Dev Biol 45:59-67
240. Biason-Lauber A (2012) WNT4, RSPO1, and FOXL2 in sex development. Semin. Reprod. Med. 30:387-395
241. Manuylov NL, Smagulova FO, Leach L, Tevosian SG (2008) Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development 135:3731-3743
242. Heikkila M, Prunskaite R, Naillat F, Itaranta P, Vuoristo J, Leppaluoto J, Peltoketo H, Vainio S (2005) The partial female to male sex reversal in Wnt-4-deficient females involves induced expression of testosterone biosynthetic genes and testosterone production, and depends on androgen action. Endocrinology 146:4016-4023
243. Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E (2008) SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am. J. Hum. Genet. 82:39-47
244. Koizumi M, Oyama K, Yamakami Y, Kida T, Satoh R, Kato S, Hidema S, Oe T, Goto T, Clevers H, Nawa A, Nishimori K (2015) Lgr4 controls specialization of female gonads in mice. Biol Reprod 93:90
245. Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G (2006) R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 38:1304-1309
246. Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC (2008) Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum.Mol Genet. 17:1264-1277
247. Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M (2008) R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum.Mol Genet. 17:1278-1291
248. Nicol B, Yao HH (2014) Building an ovary: insights into establishment of somatic cell lineages in the mouse. Sex. Dev. 8:243-251
249. Niehrs C (2012) The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13:767-779
250. Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC (2008) Genetics of ovarian differentiation: Rspo1, a major player. Sex. Dev. 2:219-227
251. Duffin K, Bayne RA, Childs AJ, Collins C, Anderson RA (2009) The forkhead transcription factor FOXL2 is expressed in somatic cells of the human ovary prior to follicle formation. Mol.Hum.Reprod.
252. Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27:159-166
253. Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M (2004) The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933-942
254. Takasawa K, Kashimada K, Pelosi E, Takagi M, Morio T, Asahara H, Schlessinger D, Mizutani S, Koopman P (2014) FOXL2 transcriptionally represses Sf1 expression by antagonizing WT1 during ovarian development in mice. FASEB J. 28:2020-2028
255. Yao HH, Matzuk MM, Jorgez CJ, Page DC, Swain A, Capel B (2004) Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev.Dyn. 230:210-215
256. Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A (2003) Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130:3663-3670
257. Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P (2009) Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech.Dev. 126:324-336
258. Pailhoux E, Vigier B, Chaffaux S, Servel N, Taourit S, Furet JP, Fellous M, Grosclaude F, Cribiu EP, Cotinot C, Vaiman D (2001) A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 29:453-458
259. De Baere E, Dixon MJ, Small KW, Jabs EW, Leroy BP, Devriendt K, Gillerot Y, Mortier G, Meire F, Van Maldergem L, Courtens W, Hjalgrim H, Huang S, Liebaers I, Van Regemorter N, Touraine P, Praphanphoj V, Verloes A, Udar N, Yellore V, Chalukya M, Yelchits S, De Paepe A, Kuttenn F, Fellous M, Veitia R, Messiaen L (2001) Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype--phenotype correlation. Hum. Mol. Genet. 10:1591-1600
260. George FW, Wilson JD (1978) Conversion of androgen to estrogen by the human fetal ovary. J. Clin. Endocrinol. Metab. 47:550-555
261. Bowles J, Feng CW, Miles K, Ineson J, Spiller C, Koopman P (2016) ALDH1A1 provides a source of meiosis-inducing retinoic acid in mouse fetal ovaries. Nat Commun 7:10845
262. Pryse-Davies J, Dewhurst CJ (1971) The development of the ovary and uterus in the foetus, newborn and infant : a morphological and enzyme histochemical study. J. Pathol. 103:5-25
263. Reynaud K, Cortvrindt R, Verlinde F, De Schepper J, Bourgain C, Smitz J (2004) Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil Steril 81:1112-1119
264. Baker TG (1963) A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Med London 158:417-433
265. Kuiri-Hänninen T, Kallio S, Seuri R, Tyrvainen E, Liakka A, Tapanainen J, Sankilampi U, Dunkel L (2011) Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J. Clin. Endocrinol. Metab. 96:3432-3439
266. Rajpert-De Meyts E, Jørgensen N, Græm N, Müller J, Cate RL, Skakkebæk NE (1999) Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J. Clin. Endocrinol. Metab. 84:3836-3844
267. Rey R, Sabourin JC, Venara M, Long WQ, Jaubert F, Zeller WP, Duvillard P, Chemes H, Bidart JM (2000) Anti-Mullerian hormone is a specific marker of sertoli- and granulosa-cell origin in gonadal tumors. Hum. Pathol. 31:1202-1208
268. Weenen C, Laven JSE, vonBergh ARM, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BCJM, Themmen APN (2004) Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol.Hum.Reprod. 10:77-83
269. Merchant-Larios H (1975) Rat gonadal and ovarian organogenesis with and without germ cells. An ultrastructural study. Dev.Biol. 44:1-21
270. McLaren A (1984) Meiosis and differentiation of mouse germ cells. Symp.Soc.Exp.Biol. 38:7-23
271. Singh RP, Carr DH (1966) The anatomy and histology of XO human embryos and fetuses. Anat.Rec. 155:369-383
272. Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL (1990) Abnormal sexual development in transgenic mice chronically expressing müllerian inhibiting substance. Nature 345:167-170
273. Liu CF, Liu C, Yao HH (2010) Building pathways for ovary organogenesis in the mouse embryo. Curr.Top.Dev.Biol. 90:263-290
274. Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B (2012) Temporal Differences in Granulosa Cell Specification in the Ovary Reflect Distinct Follicle Fates in Mice. Biol. Reprod. 86:37
275. Orisaka M, Tajima K, Mizutani T, Miyamoto K, Tsang BK, Fukuda S, Yoshida Y, Kotsuji F (2006) Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol.Reprod. 75:734-740
276. Yokobayashi S, Liang CY, Köhler H, Nestorov P, Liu Z, Vidal M, van Lohuizen M, Roloff TC, Peters AH (2013) PRC1 coordinates timing of sexual differentiation of female primordial germ cells. Nature 495:236-240
277. Kumar S, Cunningham TJ, Duester G (2013) Resolving molecular events in the regulation of meiosis in male and female germ cells. Sci Signal 6:pe25
278. Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao X, Duester G (2011) Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun 2:151
279. Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R (1999) Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat. Genet. 21:123-127
280. Menke DB, Koubova J, Page DC (2003) Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev.Biol. 262:303-312
281. Pepling ME (2006) From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 44:622-632
282. Matzuk MM, Burns KH (2012) Genetics of Mammalian Reproduction: Modeling the End of the Germline. Annu. Rev. Physiol. 74:503-528
283. Fowler PA, Flannigan S, Mathers A, Gillanders K, Lea RG, Wood MJ, Maheshwari A, Bhattacharya S, Collie-Duguid ES, Baker PJ, Monteiro A, O'Shaughnessy PJ (2009) Gene expression analysis of human fetal ovarian primordial follicle formation. J. Clin. Endocrinol. Metab. 94:1427-1435
284. Kerr B, García-Rudaz C, Dorfman M, Paredes A, Ojeda SR (2009) NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction 138:131-140
285. Soyal SM, Amleh A, Dean J (2000) FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development 127:4645-4654
286. Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP (1999) Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 140:5789-5796
287. Visser JA, de Jong FH, Laven JSE, Themmen APN (2006) Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 131:1-9
288. Laitinen M, Vuojolainen K, Jaatinen R, Ketola I, Aaltonen J, Lehtonen E, Heikinheimo M, Ritvos O (1998) A novel growth differentiation factor-9 (GDF-9) related factor is co-expressed with GDF-9 in mouse oocytes during folliculogenesis. Mech.Dev. 78:135-140
289. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531-535
290. Di Pasquale E, Beck-Peccoz P, Persani L (2004) Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am. J. Hum. Genet. 75:106-111
291. Belli M, Shimasaki S (2018) Molecular Aspects and Clinical Relevance of GDF9 and BMP15 in Ovarian Function. Vitam Horm 107:317-348
292. de Mello Santos T, Hinton BT (2019) We, the developing rete testis, efferent ducts, and Wolffian duct, all hereby agree that we need to connect. Andrology 7:581-587
293. Murashima A, Xu B, Hinton BT (2015) Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl 17:749-755
294. Gonçalves A, Zeller R (2011) Genetic analysis reveals an unexpected role of BMP7 in initiation of ureteric bud outgrowth in mouse embryos. PLoS.One. 6:e19370
295. Hoshi M, Batourina E, Mendelsohn C, Jain S (2012) Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development 139:2405-2415
296. Kuschert S, Rowitch DH, Haenig B, McMahon AP, Kispert A (2001) Characterization of Pax-2 regulatory sequences that direct transgene expression in the Wolffian duct and its derivatives. Dev Biol 229:128-140
297. Shaw G, Renfree MB (2014) Wolffian duct development. Sex. Dev. 8:273-280
298. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev.Cell 9:283-292
299. Caterino S, Lorenzon L, Cavallini M, Cavaniglia D, Ferro F (2014) Epididymal-testicular fusion anomalies in cryptorchidism are associated with proximal location of the undescended testis and with a widely patent processus vaginalis. J Anat 225:473-478
300. Hoshi M, Reginensi A, Joens MS, Fitzpatrick JAJ, McNeill H, Jain S (2018) Reciprocal Spatiotemporally Controlled Apoptosis Regulates Wolffian Duct Cloaca Fusion. J. Am. Soc. Nephrol. 29:775-783
301. Price D, Zaaijer JJP, Ortiz E, Brinkmann AO (1975) Current view on embryonic sex differentiation in reptiles, birds and mammals. Am.Zool. 15, suppl 1:173-195
302. Orvis GD, Behringer RR (2007) Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 306:493-504
303. Mullen RD, Behringer RR (2014) Molecular genetics of Mullerian duct formation, regression and differentiation. Sex. Dev. 8:281-296
304. Kobayashi A, Shawlot W, Kania A, Behringer RR (2004) Requirement of Lim1 for female reproductive tract development. Development 131:539-549
305. Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, Teixeira J, Donahoe PK (2006) Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development 133:2359-2369
306. Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR (2008) A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol. Reprod. Dev. 75:1154-1162
307. Davis RJ, Harding M, Moayedi Y, Mardon G (2008) Mouse Dach1 and Dach2 are redundantly required for Mullerian duct development. Genesis 46:205-213
308. Huang CC, Orvis GD, Kwan KM, Behringer RR (2014) Lhx1 is required in Mullerian duct epithelium for uterine development. Dev Biol 389:124-136
309. Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405-409
310. Fujino A, Arango N, Zhan Y, Manganaro T, Li X, MacLaughlin D, Donahoe P (2009) Cell migration and activated PI3K/AKT-directed elongation in the developing rat Mullerian duct. Dev. Biol. 325:351-362
311. Guioli S, Sekido R, Lovell-Badge R (2007) The origin of the Müllerian duct in chick and mouse. Dev. Biol. 302:389-398
312. Zhao F, Zhou J, Li R, Dudley EA, Ye X (2016) Novel function of LHFPL2 in female and male distal reproductive tract development. Sci. Rep. 6:23037
313. Cunha GR, Robboy SJ, Kurita T, Isaacson D, Shen J, Cao M, Baskin LS (2018) Development of the human female reproductive tract. Differentiation 103:46-65
314. Moses MM, Behringer RR (2019) A gene regulatory network for Mullerian duct regression. Environ Epigenet 5:dvz017
315. Picon R (1969) Action du testicule foetal sur le développement in vitro des canaux de Müller chez le rat. Arch.Anat.Microsc.Morph.Exp. 58:1-19
316. Roberts LM, Hirokawa Y, Nachtigal MW, Ingraham HA (1999) Paracrine-mediated apoptosis in reproductive tract development. Dev.Biol. 208:110-122
317. Allard S, Adin P, Gouédard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F (2000) Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development 127:3349-3360
318. Paranko J, Foidart JM, Pelliniemi LJ (1986) Developmental changes in interstitial collagens of fetal rat genital ducts. Dev. Biol. 113:364-372
319. Trelstad RL, Hayashi A, Hayashi K, Donahoe PK (1982) The epithelial-mesenchymal interface of the male rate Mullerian duct: loss of basement membrane integrity and ductal regression. Dev.Biol. 92:27-40
320. Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, Behringer RR (2011) b-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development 138:1967-1975
321. Mullen RD, Wang Y, Liu B, Moore EL, Behringer RR (2018) Osterix functions downstream of anti-Mullerian hormone signaling to regulate Mullerian duct regression. Proc Natl Acad Sci U S A 115:8382-8387
322. Park JH, Tanaka Y, Arango NA, Zhang L, Benedict LA, Roh MI, Donahoe PK, Teixeira JM (2014) Induction of WNT inhibitory factor 1 expression by Mullerian inhibiting substance/antiMullerian hormone in the Mullerian duct mesenchyme is linked to Mullerian duct regression. Dev Biol 386:227-236
323. Hannema SE, Hughes IA (2007) Regulation of Wolffian duct development. Horm. Res. 67:142-151
324. Josso N, Rey R, Picard JY (2012) Testicular anti-Mullerian hormone: clinical applications in DSD. Semin. Reprod. Med. 30:364-373
325. Klonisch T, Fowler PA, Hombach-Klonisch S (2004) Molecular and genetic regulation of testis descent and external genitalia development. Dev. Biol. 270:1-18
326. Zhao F, Franco HL, Rodriguez KF, Brown PR, Tsai MJ, Tsai SY, Yao HH (2017) Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science 357:717-720
327. Jirásek JE (1977) Morphogenesis of the genital system in the human. Birth Defects Orig. Artic. Ser. 13:13-39
328. Bouty A, Ayers KL, Pask A, Heloury Y, Sinclair AH (2015) The Genetic and Environmental Factors Underlying Hypospadias. Sex. Dev. 9:239-259
329. Penington EC, Hutson JM (2002) The urethral plate : does it grow into the genital tubercle or within it ? Br.J.Urol. 89:733-739
330. Blaschko SD, Cunha GR, Baskin LS (2012) Molecular mechanisms of external genitalia development. Differentiation 84:261-268
331. Glenister TW (1962) The development of the utricle and of the so-called "middle" or "median" lobe of the human prostate. J. Anat. 96:443-455
332. Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR (2001) Urethral seam formation and hypospadias. Cell Tissue Res. 305:379-387
333. Cunha GR, Sinclair A, Risbridger G, Hutson J, Baskin LS (2015) Current understanding of hypospadias: relevance of animal models. Nat Rev Urol 12:271-280
334. Rey RA, Codner E, Iñíguez G, Bedecarrás P, Trigo R, Okuma C, Gottlieb S, Bergadá I, Campo SM, Cassorla FG (2005) Low risk of impaired testicular Sertoli and Leydig cell functions in boys with isolated hypospadias. J. Clin. Endocrinol. Metab. 90:6035-6040
335. Zalel Y, Pinhas-Hamiel O, Lipitz S, Mashiach S, Achiron R (2001) The development of the fetal penis--an in utero sonographic evaluation. Ultrasound Obstet. Gynecol. 17:129-131
336. Scott HM, Mason JI, Sharpe RM (2009) Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr. Rev. 30:883-925
337. Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, Baskin LS (2002) Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res 307:145-153
338. Laron Z, Sarel R (1970) Penis and testicular size in patients with growth hormone insufficency. Acta Endocrinol. (Copenh.) 63:625-633
339. Lee PA, Mazur T, Houk CP, Blizzard RM (2018) Growth Hormone Deficiency Causing Micropenis: Lessons Learned From a Well-Adjusted Adult. Pediatrics 142
340. Oh JK, Im YJ, Park K, Paick JS (2018) Effects of combined growth hormone and testosterone treatments in a rat model of micropenis. Endocr Connect
341. Fritsch H, Hoermann R, Bitsche M, Pechriggl E, Reich O (2013) Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. J Anat.
342. Drews U (2007) Helper function of the Wolffian ducts and role of androgens in the development of the vagina. Sex. Dev. 1:100-110
343. Kobayashi A, Behringer RR (2003) Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 4:969-980
344. Philibert P, Biason-Lauber A, Gueorguieva I, Stuckens C, Pienkowski C, Lebon-Labich B, Paris F, Sultan C (2011) Molecular analysis of WNT4 gene in four adolescent girls with mullerian duct abnormality and hyperandrogenism (atypical Mayer-Rokitansky-Kuster-Hauser syndrome). Fertil Steril 95:2683-2686
345. Philibert P, Biason-Lauber A, Rouzier R, Pienkowski C, Paris F, Konrad D, Schoenle E, Sultan C (2008) Identification and Functional Analysis of a New WNT4 Gene Mutation among 28 Adolescent Girls with Primary Amenorrhea and Mullerian Duct Abnormalities: A French Collaborative Study. J. Clin. Endocrinol. Metab. 93:895-900
346. Biason-Lauber A, De Filippo G, Konrad D, Scarano G, Nazzaro A, Schoenle EJ (2007) WNT4 deficiency--a clinical phenotype distinct from the classic Mayer-Rokitansky-Kuster-Hauser syndrome: a case report. Hum. Reprod. 22:224-229
347. Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ (2004) A WNT4 Mutation Associated with Mullerian-Duct Regression and Virilization in a 46,XX Woman. N. Engl. J. Med. 351:792-798
348. Ravel C, Lorenco D, Dessolle L, Mandelbaum J, McElreavey K, Darai E, Siffroi JP (2009) Mutational analysis of the WNT gene family in women with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil Steril 91:1604-1607
349. Parr BA, McMahon AP (1998) Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707-710
350. Chen H, Ruan YC, Xu WM, Chen J, Chan HC (2012) Regulation of male fertility by CFTR and implications in male infertility. Hum. Reprod. Update 18:703-713
351. Deutscher E, Hung-Chang Yao H (2007) Essential roles of mesenchyme-derived beta-catenin in mouse Mullerian duct morphogenesis. Dev Biol 307:227-236
352. Liu CF, Bingham N, Parker K, Yao HHC (2009) Sex-specific roles of beta-catenin in mouse gonadal development. Hum. Mol. Genet. 18:405-417
353. Hong XM, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK (2008) Dicer1 Is essential for female fertility and normal development of the female reproductive system. Endocrinology 149:6207-6212
354. Podlasek CA, Clemens JQ, Bushman W (1999) Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J. Urol. 161:1655-1661
355. Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A (1996) Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 10:903-918
356. Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M (2002) Nephric lineage specification by Pax2 and Pax8. Genes Dev 16:2958-2970
357. Mittag J, Winterhager E, Bauer K, Grummer R (2007) Congenital hypothyroid female Pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology 148:719-725
358. Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M (1994) Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120:2749-2771
359. Miller C, Sassoon DA (1998) Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125:3201-3211
360. Vandenberg AL, Sassoon DA (2009) Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development 136:1559-1570
361. Miller C, Degenhardt K, Sassoon DA (1998) Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis [letter]. Nat. Genet. 20:228-230
362. Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P (2001) Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat.Genet. 28:251-255
363. Thomsen MK, Butler CM, Shen MM, Swain A (2008) Sox9 is required for prostate development. Dev. Biol. 316:302-311
364. Donjacour AA, Thomson A, Cunha G (2003) FGF-10 plays an essential role in the growth of the fetal prostate. Dev. Biol. 261:39-54
365. Warr N, Siggers P, Bogani D, Brixey R, Pastorelli L, Yates L, Dean CH, Wells S, Satoh W, Shimono A, Greenfield A (2009) Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol 326:273-284
366. Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB, III, Yamada G (2003) Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia: development by concerted actions of BMP signaling. Development 130:6209-6220
367. Morgan EA, Nguyen SB, Scott V, Stadler HS (2003) Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development 130:3095-3109
368. Lin C, Yin Y, Long F, Ma L (2008) Tissue-specific requirements of beta-catenin in external genitalia development. Development 135:2815-2825
369. Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ (2002) Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev.Biol. 247:26-46
370. Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT (2007) Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod 76:19-28
371. Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, Motoyama J, Iguchi T, Nakagata N, Hui CC, Yamada G (2011) The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 152:2894-2903
372. Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dollé P (1997) Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development 124:4781-4791
373. Yucel S, Dravis C, García N, Henkemeyer M, Baker LA (2007) Hypospadias and anorectal malformations mediated by Eph/ephrin signaling. J. Pediatr. Urol. 3:354-363
374. Shen J, Liu B, Sinclair A, Cunha G, Baskin LS, Choudhry S (2015) Expression Analysis of DGKK during External Genitalia Formation. J. Urol. 194:1728-1736
375. van der Zanden LF, van Rooij IA, Feitz WF, Knight J, Donders AR, Renkema KY, Bongers EM, Vermeulen SH, Kiemeney LA, Veltman JA, Arias-Vasquez A, Zhang X, Markljung E, Qiao L, Baskin LS, Nordenskjold A, Roeleveld N, Franke B, Knoers NV (2011) Common variants in DGKK are strongly associated with risk of hypospadias. Nat. Genet. 43:48-50
376. Carmichael SL, Mohammed N, Ma C, Iovannisci D, Choudhry S, Baskin LS, Witte JS, Shaw GM, Lammer EJ (2013) Diacylglycerol kinase K variants impact hypospadias in a California study population. J. Urol. 189:305-311
377. Petiot A, Perriton CL, Dickson C, Cohn MJ (2005) Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development 132:2441-2450
378. Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W (2001) Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev.Biol. 232:301-314
379. Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, Yamada G (2000) Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127:2471-2479
380. Podlasek CA, Seo RM, Clemens JQ, Ma L, Maas RL, Bushman W (1999) Hoxa-10 deficient male mice exhibit abnormal development of the accessory sex organs. Dev.Dyn. 214:1-12
381. Mortlock DP, Innis JW (1996) Mutation of HOXA13 in hand-foot-genital syndrome. Nat.Genet. 15:179-180
382. Podlasek CA, Duboule D, Bushman W (1997) Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev.Dyn. 208:454-465
383. Yin Y, Lin CX, Ma L (2006) Msx2 promotes vaginal epithelial differentiation and Wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol. Endocrinol. 20:1535-1546
384. Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, McVary KT (2003) Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol.Reprod 68:423-438
385. Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, AbateShen C, Beachy PA, Shen MM (2004) Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Develop.Biol 267:387-398
386. Strong LC, Hollander WF (1949) Hereditary loop-tail in the house mouse accompanied by imperforate vaginal and craniorachischisis when homozygous. J. Hered. 40:329-334
387. Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G (2009) Genetic interactions of the androgen and Wnt/ß-catenin pathways for the masculinization of external genitalia. Mol. Endocrinol. 23:871-880
388. Edelsztein NY, Grinspon RP, Schteingart HF, Rey RA (2016) Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int. J. Pediatr. Endocrinol. 2016:20
389. Ohyama K, Ohta M, Hosaka YZ, Tanabe Y, Ohyama T, Yamano Y (2015) Expression of anti-Mullerian hormone and its type II receptor in germ cells of maturing rat testis. Endocr J 62:997-1006
390. Wang J, Dicken C, Lustbader JW, Tortoriello DV (2009) Evidence for a Mullerian-inhibiting substance autocrine/paracrine system in adult human endometrium. Fertil.Steril. 91:1195-1203
391. Lebeurrier N, Launay S, Macrez R, Maubert E, Legros H, Leclerc A, Jamin SP, Picard JY, Marret S, Laudenbach V, Berger P, Sonderegger P, Ali C, di Clemente N, Vivien D (2008) Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J Cell Sci 121:3357-3365
392. Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D, Pigny P, Prescott M, Campbell R, Herbison AE, Prevot V, Giacobini P (2016) Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nature Communications 7:10055
393. Wang PY, Protheroe A, Clarkson AN, Imhoff F, Koishi K, McLennan IS (2009) Mullerian inhibiting substance contributes to sex-linked biases in the brain and behavior. Proc. Natl. Acad. Sci. U. S. A. 106:7203-7208
394. Garrel G, Racine C, L'Hote D, Denoyelle C, Guigon CJ, di Clemente N, Cohen-Tannoudji J (2016) Anti-Mullerian hormone: a new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci. Rep. 6:23790
395. Pepinsky RB, Sinclair LK, Chow EP, Mattaliano RJ, Manganaro TF, Donahoe PK, Cate RL (1988) Proteolytic processing of mullerian inhibiting substance produces a transforming growth factor-beta-like fragment. J. Biol. Chem. 263:18961-18964
396. di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, Picard J-Y, Whitty A, Josso N, Pepinsky RB, Cate RL (2010) Processing of Anti-Mullerian Hormone Regulates Receptor Activation by a Mechanism Distinct from TGF-b. Mol. Endocrinol. 24:2193-2206
397. Belville C, Van Vlijmen H, Ehrenfels C, Pepinsky B, Rezaie AR, Picard JY, Josso N, di Clemente N, Cate RL (2004) Mutations of the anti-mullerian hormone gene in patients with persistent mullerian duct syndrome: biosynthesis, secretion, and processing of the abnormal proteins and analysis using a three-dimensional model. Mol. Endocrinol. 18:708-721
398. Pépin D, Hoang M, Nicolaou F, Hendren K, Benedict LA, Al-Moujahed A, Sosulski A, Marmalidou A, Vavvas D, Donahoe PK (2013) An albumin leader sequence coupled with a cleavage site modification enhances the yield of recombinant C-terminal Mullerian Inhibiting Substance. Technology 1:63-71
399. Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, Singh M, Tsareva T, Parice Y, Mahoney A, Roschke V, Sanyal I, Choe S (2005) Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J. Biol. Chem. 280:25111-25118
400. Sebald W, Nickel J, Zhang JL, Mueller TD (2004) Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol. Chem. 385:697-710
401. Greenwald J, Groppe J, Gray P, Wiater E, Kwiatkowski W, Vale W, Choe S (2003) The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol. Cell 11:605-617
402. Thompson TB, Woodruff TK, Jardetzky TS (2003) Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions. EMBO J 22:1555-1566
403. Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP (1986) Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell 45:685-698
404. Cohen-Haguenauer O, Picard JY, Mattei MG, Serero S, Nguyen VC, de Tand MF, Guerrier D, Hors Cayla MC, Josso N, Frézal J (1987) Mapping of the gene for anti-Müllerian hormone to the short arm of human chromosome 19. Cytogenet. Cell Genet. 44:2-6
405. Picard JY, Benarous R, Guerrier D, Josso N, Kahn A (1986) Cloning and expression of cDNA for anti-Müllerian hormone. Proc. Natl. Acad. Sci. U. S. A. 83:5464-5468
406. Münsterberg A, Lovell-Badge R (1991) Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development 113:613-624
407. King TR, Lee BK, Behringer RR, Eicher EM (1991) Mapping anti-müllerian hormone (Amh) and related sequences in the mouse: identification of a new region of homology between MMU10 and HSA19p. Genomics 11:273-283
408. Haqq C, Lee MM, Tizard R, Wysk M, DeMarinis J, Donahoe PK, Cate RL (1992) Isolation of the rat gene for Mullerian inhibiting substance. Genomics 12:665-669
409. Lahbib Mansais Y, Barbosa A, Yerle M, Parma P, Milan D, Pailhoux E, Gellin J, Cotinot C (1997) Mapping in pig of genes involved in sexual differentiation: AMH, WT1, FTZF1, SOX2, SOX9, AHC, and placental and embryonic CYP19. Cytogenet Cell Genet 76:109-114
410. Pask AJ, Whitworth DJ, Mao CA, Wei KJ, Sankovic N, Marshall Graves JA, Shaw G, Renfree MB, Behringer RR (2004) Marsupial Anti-Mullerian Hormone Gene Structure, Regulatory Elements, and Expression. Biol. Reprod. 70:160-167
411. Carré-Eusèbe D, di Clemente N, Rey R, Pieau C, Vigier B, Josso N, Picard JY (1996) Cloning and expression of the chick anti-Müllerian hormone gene. J Biol Chem 271:4798-4804
412. Neeper M, Lowe R, Galuska S, Hofmann KJ, Smith RG, Elbrecht A (1996) Molecular cloning of an avian anti-Müllerian hormone homologue. Gene 176:203-209
413. Western PS, Harry JL, Marshall Graves JA, Sinclair AH (1999) Temperature-dependent sex determination in the American alligator: AMH precedes SOX9 expression. Dev.Dyn. 216:411-419
414. Al-Asaad I, Chardard D, di Clemente N, Picard JY, Dumond H, Chesnel A, Flament S (2013) Mullerian inhibiting substance in the caudate amphibian Pleurodeles waltl. Endocrinology 154:3931-3936
415. Halm S, Rocha A, Miura T, Prat F, Zanuy S (2007) Anti-Mullerian hormone (AMH/AMH) in the European sea bass: Its gene structure, regulatory elements, and the expression of alternatively-spliced isoforms. Gene 388:148-158
416. Pala I, Kluver N, Thorsteinsdottir S, Schartl M, Coelho MM (2008) Expression pattern of anti-Mullerian hormone (amh) in the hybrid fish complex of Squalius alburnoides. Gene 410:249-258
417. Kluver N, Pfennig F, Pala I, Storch K, Schlieder M, Froschauer A, Gutzeit HO, Schartl M (2007) Differential expression of anti-Mullerian hormone (amh) and anti-Mullerian hormone receptor type II (amhrII) in the teleost medaka. Dev.Dyn. 236:271-281
418. Pfennig F, Standke A, Gutzeit HO (2015) The role of Amh signaling in teleost fish--Multiple functions not restricted to the gonads. Gen Comp Endocrinol 223:87-107
419. Racine C, Rey R, Forest MG, Louis F, Ferré A, Huhtaniemi I, Josso N, di Clemente N (1998) Receptors for anti-müllerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 95:594-599
420. Visser JA, Themmen AP (2005) Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol 234:81-86
421. Oreal E, Mazaud S, Picard JY, Magre S, Carre-Eusebe D (2002) Different patterns of anti-Mullerian hormone expression, as related to DMRT1, SF-1, WT1, GATA-4, Wnt-4, and Lhx9 expression, in the chick differentiating gonads. Dev Dyn 225:221-232
422. de Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol 18:6653-6665
423. Katoh-Fukui Y, Igarashi M, Nagasaki K, Horikawa R, Nagai T, Tsuchiya T, Suzuki E, Miyado M, Hata K, Nakabayashi K, Hayashi K, Matsubara Y, Baba T, Morohashi K, Igarashi A, Ogata T, Takada S, Fukami M (2015) Testicular dysgenesis/regression without campomelic dysplasia in patients carrying missense mutations and upstream deletion of SOX9. Mol Genet Genomic Med 3:550-557
424. Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA (1994) Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell 77:651-661
425. Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA (1998) Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445-454
426. Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK (2000) Endogenous expression of Mullerian inhibiting substance in early postnatal rat sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc. Natl. Acad. Sci. USA 97:1624-1629
427. Tremblay JJ, Viger RS (2001) Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol Reprod 64:1191-1199
428. Tantawy S, Mazen I, Soliman H, Anwar G, Atef A, El-Gammal M, El-Kotoury A, Mekkawy M, Torky A, Rudolf A, Schrumpf P, Gruters A, Krude H, Dumargne MC, Astudillo R, Bashamboo A, Biebermann H, Kohler B (2014) Analysis of the gene coding for steroidogenic factor 1 (SF1, NR5A1) in a cohort of 50 Egyptian patients with 46,XY disorders of sex development. Eur J Endocrinol 170:759-767
429. Edelsztein NY, Racine C, di Clemente N, Schteingart HF, Rey RA (2018) Androgens downregulate anti-Mullerian hormone promoter activity in the Sertoli cell through the androgen receptor and intact SF1 sites. Biol Reprod 99:1303-1312
430. Schteingart HF, Picard JY, Valeri C, Marshall I, Treton D, di Clemente N, Rey RA, Josso N (2019) A mutation inactivating the distal SF1 binding site on the human anti-Mullerian hormone promoter causes persistent Mullerian duct syndrome. Hum. Mol. Genet. 28:3211-3218
431. Pan S, Guo S, Liu L, Yang X, Liang H (2020) Functional study of a novel c.630delG (p.Y211Tfs*85) mutation in NR5A1 gene in a Chinese boy with 46,XY disorders of sex development. J. Assist. Reprod. Genet. 37:477-486
432. Viger RS, Mertineit C, Trasler JM, Nemer M (1998) Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development 125:2665-2675
433. Tremblay JJ, Viger RS (1999) Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol. Endocrinol. 13:1388-1401
434. Tremblay JJ, Robert NM, Viger RS (2001) Modulation of endogenous GATA-4 activity reveals its dual contribution to Müllerian inhibiting substance gene transcription in Sertoli cells. Mol. Endocrinol. 15:1636-1650
435. Robert NM, Tremblay JJ, Viger RS (2002) Friend of GATA (FOG)-1 and FOG-2 differentially repress the GATA-dependent activity of multiple gonadal promoters. Endocrinology 143:3963-3973
436. Lourenço D, Brauner R, Rybczynska M, Nihoul-Fekete C, McElreavey K, Bashamboo A (2011) Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc Natl Acad Sci U S A 108:1597-1602
437. Bouchard MF, Bergeron F, Grenier Delaney J, Harvey LM, Viger RS (2019) In Vivo Ablation of the Conserved GATA-Binding Motif in the Amh Promoter Impairs Amh Expression in the Male Mouse. Endocrinology 160:817-826
438. van den Bergen JA, Robevska G, Eggers S, Riedl S, Grover SR, Bergman PB, Kimber C, Jiwane A, Khan S, Krausz C, Raza J, Atta I, Davis SR, Ono M, Harley V, Faradz SMH, Sinclair AH, Ayers KL (2020) Analysis of variants in GATA4 and FOG2/ZFPM2 demonstrates benign contribution to 46,XY disorders of sex development. Mol Genet Genomic Med 8:e1095
439. Hossain A, Saunders GF (2003) Role of Wilms Tumor 1 (WT1) in the Transcriptional Regulation of the Mullerian-Inhibiting Substance Promoter. Biol. Reprod. 69:1808-1814
440. Hossain A, Saunders GF (2003) Synergistic Cooperation between the beta-Catenin Signaling Pathway and Steroidogenic Factor 1 in the Activation of the Mullerian Inhibiting Substance Type II Receptor. J. Biol. Chem. 278:26511-26516
441. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M (2008) Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol.Endocrinol 22:781-798
442. Lukas-Croisier C, Lasala C, Nicaud J, Bedecarrás P, Kumar TR, Dutertre M, Matzuk MM, Picard JY, Josso N, Rey R (2003) Follicle-stimulating hormone increases testicular Anti-Müllerian hormone (AMH) production through sertoli cell proliferation and a nonclassical cyclic adenosine 5'-monophosphate-mediated activation of the AMH gene. Mol. Endocrinol. 17:550-561
443. Lasala C, Carré-Eusèbe D, Picard JY, Rey R (2004) Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 23:572-585
444. Rey RA, Venara M, Coutant R, Trabut JB, Rouleau S, Lahlou N, Sultan C, Limal JM, Picard JY, Lumbroso S (2006) Unexpected mosaicism of R201H-GNAS1 mutant-bearing cells in the testes underlie macro-orchidism without sexual precocity in McCune-Albright syndrome. Hum. Mol. Genet. 15:3538-3543
445. Rey R, Lordereau-Richard I, Carel JC, Barbet P, Cate RL, Roger M, Chaussain JL, Josso N (1993) Anti-müllerian hormone and testosterone serum levels are inversely related during normal and precocious pubertal development. J. Clin. Endocrinol. Metab. 77:1220-1226
446. Rey RA, Musse M, Venara M, Chemes HE (2009) Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc. Res. Tech. 72:787-795
447. Rey R, Mebarki F, Forest MG, Mowszowicz I, Cate RL, Morel Y, Chaussain JL, Josso N (1994) Anti-müllerian hormone in children with androgen insensitivity. J. Clin. Endocrinol. Metab. 79:960-964
448. Rey RA, Belville C, Nihoul-Fékété C, Michel-Calemard L, Forest MG, Lahlou N, Jaubert F, Mowszowicz I, David M, Saka N, Bouvattier C, Bertrand AM, Lecointre C, Soskin S, Cabrol S, Crosnier H, Léger J, Lortat-Jacob S, Nicolino M, Rabl W, Toledo SP, Bas F, Gompel A, Czernichow P, Josso N (1999) Evaluation of gonadal function in 107 intersex patients by means of serum antimüllerian hormone measurement. J. Clin. Endocrinol. Metab. 84:627-631
449. Rey R, Josso N (1996) Regulation of testicular anti-Müllerian hormone secretion. Eur.J.Endocrinol. 135:144-152
450. Taieb J, Grynberg M, Pierre A, Arouche N, Massart P, Belville C, Hesters L, Frydman R, Catteau-Jonard S, Fanchin R, Picard JY, Josso N, Rey RA, di Clemente N (2011) FSH and its second messenger cAMP stimulate the transcription of human anti-Mullerian hormone in cultured granulosa cells. Mol. Endocrinol. 25:645-655
451. Grynberg M, Pierre A, Rey R, Leclerc A, Arouche N, Hesters L, Catteau-Jonard S, Frydman R, Picard JY, Fanchin R, Veitia R, di Clemente N, Taieb J (2012) Differential regulation of ovarian anti-mullerian hormone (AMH) by estradiol through alpha- and beta-estrogen receptors. J. Clin. Endocrinol. Metab. 97:E1649-1657
452. Pierre A, Peigne M, Grynberg M, Arouche N, Taieb J, Hesters L, Gonzales J, Picard JY, Dewailly D, Fanchin R, Catteau-Jonard S, di Clemente N (2013) Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum. Reprod. 28:762-769
453. van Rooij IAJ, Broekmans FJM, te Velde ER, Fauser BCJM, Bancsi LFJM, Jong FH, Themmen APN (2002) Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum. Reprod. 17:3065-3071
454. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER (2005) Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83:979-987
455. Li HW, Ng EH, Wong BP, Anderson RA, Ho PC, Yeung WS (2012) Correlation between three assay systems for anti-Mullerian hormone (AMH) determination. J.Assist.Reprod.Genet. 29:1443-1446
456. Han X, McShane M, Sahertian R, White C, Ledger W (2014) Pre-mixing serum samples with assay buffer is a prerequisite for reproducible anti-Mullerian hormone measurement using the Beckman Coulter Gen II assay. Hum. Reprod. 29:1042-1048
457. Roberts SC, Seav SM, McDade TW, Dominick SA, Gorman JR, Whitcomb BW, Su HI (2016) Self-collected dried blood spots as a tool for measuring ovarian reserve in young female cancer survivors. Hum. Reprod.
458. Gassner D, Jung R (2014) First fully automated immunoassay for anti-Mullerian hormone. Clin. Chem. Lab. Med. 52:1143-1152
459. Pearson K, Long M, Prasad J, Wu YY, Bonifacio M (2016) Assessment of the Access AMH assay as an automated, high-performance replacement for the AMH Generation II manual ELISA. Reprod Biol Endocrinol 14:8
460. Nelson SM, Pastuszek E, Kloss G, Malinowska I, Liss J, Lukaszuk A, Plociennik L, Lukaszuk K (2015) Two new automated, compared with two enzyme-linked immunosorbent, antimullerian hormone assays. Fertil Steril 104:1016-1021 e1016
461. Su HI, Sammel MD, Homer MV, Bui K, Haunschild C, Stanczyk FZ (2014) Comparability of antimullerian hormone levels among commercially available immunoassays. Fertil Steril 101:1766-1772 e1761
462. Aksglæde L, Sorensen K, Boas M, Mouritsen A, Hagen CP, Jensen RB, Petersen JH, Linneberg A, Andersson AM, Main KM, Skakkebæk NE, Juul A (2010) Changes in anti-Mullerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J. Clin. Endocrinol. Metab. 95:5357-5364
463. Grinspon RP, Bedecarrás P, Ballerini MG, Iñíguez G, Rocha A, Mantovani Rodrigues Resende EA, Brito VN, Milani C, Figueroa Gacitua V, Chiesa A, Keselman A, Gottlieb S, Borges MF, Ropelato MG, Picard JY, Codner E, Rey RA (2011) Early onset of primary hypogonadism revealed by serum anti-Müllerian hormone determination during infancy and childhood in trisomy 21. Int. J. Androl. 34:e487-e498
464. Hagen CP, Aksglæde L, Sorensen K, Main KM, Boas M, Cleemann L, Holm K, Gravholt CH, Andersson AM, Pedersen AT, Petersen JH, Linneberg A, Kjaergaard S, Juul A (2010) Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J. Clin. Endocrinol. Metab. 95:5003-5010
465. Mamsen LS, Munthe-Fog L, Petersen TS, Jeppesen JV, Mollgard K, Grondahl ML, Larsen A, Ernst E, Oxvig C, Kumar A, Kalra B, Andersen CY (2016) Reply: Methodological considerations in measuring different AMH splice forms using ELISA: validity of proAMH ELISA. Mol. Hum. Reprod. 22:374-375
466. Mamsen LS, Petersen TS, Jeppesen JV, Mollgard K, Grondahl ML, Larsen A, Ernst E, Oxvig C, Kumar A, Kalra B, Andersen CY (2015) Proteolytic processing of anti-Mullerian hormone differs between human fetal testes and adult ovaries. Mol. Hum. Reprod. 21:571-582
467. Pankhurst MW, McLennan IS (2016) A specific immunoassay for proAMH, the uncleaved proprotein precursor of anti-Mullerian hormone. Mol Cell Endocrinol 419:165-171
468. Pierre A, Racine C, Rey RA, Fanchin R, Taieb J, Cohen-Tannoudji J, Carmillo P, Pepinsky RB, Cate RL, di Clemente N (2016) Most Cleaved Anti-Mullerian Hormone Binds Its Receptor in Human Follicular Fluid but Little Is Competent in Serum. J. Clin. Endocrinol. Metab. 101:4618-4627
469. van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, Broekmans FJ (2010) Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum. Reprod. 25:221-227
470. La Marca A, Grisendi V, Griesinger G (2013) How Much Does AMH Really Vary in Normal Women? Int. J. Endocrinol. 2013:959487
471. Chong YH, Pankhurst MW, McLennan IS (2015) The Daily Profiles of Circulating AMH and INSL3 in Men are Distinct from the Other Testicular Hormones, Inhibin B and Testosterone. PLoS One 10:e0133637
472. Rey RA, Grinspon RP (2011) Normal male sexual differentiation and aetiology of disorders of sex development. Best practice & research. Clinical endocrinology & metabolism 25:221-238
473. Grinspon RP, Rey RA (2010) Anti-mullerian hormone and Sertoli cell function in paediatric male hypogonadism. Horm. Res. Paediatr. 73:81-92
474. Rey RA, Grinspon RP, Gottlieb S, Pasqualini T, Knoblovits P, Aszpis S, Pacenza N, Stewart Usher J, Bergadá I, Campo SM (2013) Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology 1:3-16
475. Rohayem J, Nieschlag E, Kliesch S, Zitzmann M (2015) Inhibin B, AMH, but not INSL3, IGF1 or DHEAS support differentiation between constitutional delay of growth and puberty and hypogonadotropic hypogonadism. Andrology 3:882-887
476. Grinspon RP, Arozarena M, Prada S, Bargman G, Sanzone M, Morales Bazurto M, Gutiérrez M, Bedecarrás P, Kannemann A, Elena GO, Gottlieb S, Berenstein AJ, Ropelato MG, Bergadá I, Aversa LA, Rey RA (2019) Safety of standardised treatments for haematologic malignancies as regards to testicular endocrine function in children and teenagers. Hum. Reprod. 34:2480-2494
477. Grinspon RP, Gottlieb S, Bedecarras P, Rey RA (2018) Anti-Müllerian Hormone and Testicular Function in Prepubertal Boys With Cryptorchidism. Front. Endocrinol. (Lausanne) 9:182; 181-114
478. Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F (2013) Modeling Age at Menopause Using Serum Concentration of Anti-Mullerian Hormone. J. Clin. Endocrinol. Metab.
479. Gustafson ML, Lee MM, Scully RE, Moncure AC, Hirakawa T, Goodman A, Muntz HG, Donahoe PK, MacLaughlin DT, Fuller AF (1992) Müllerian inhibiting substance as a marker for ovarian sex-cord tumor. N. Engl. J. Med. 326:466-471
480. Long WQ, Ranchin V, Pautier P, Belville C, Denizot P, Cailla H, Lhommé C, Picard JY, Bidart JM, Rey R (2000) Detection of minimal levels of serum anti-Müllerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. J. Clin. Endocrinol. Metab. 85:540-544
481. Alvaro Mercadal B, Imbert R, Demeestere I, Gervy C, De Leener A, Englert Y, Costagliola S, Delbaere A (2015) AMH mutations with reduced in vitro bioactivity are related to premature ovarian insufficiency. Hum. Reprod. 30:1196-1202
482. La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A (2010) Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 16:113-130
483. Belville C, Maréchal JD, Pennetier S, Carmillo P, Masgrau L, Messika-Zeitoun L, Galey J, Machado G, Treton D, Gonzales J, Picard JY, Josso N, Cate RL, di Clemente N (2009) Natural mutations of the anti-Mullerian hormone type II receptor found in persistent Mullerian duct syndrome affect ligand binding, signal transduction and cellular transport. Hum. Mol. Genet. 18:3002-3013
484. Abduljabbar M, Taheini K, Picard JY, Cate RL, Josso N (2012) Mutations of the AMH type II receptor in two extended families with Persistent Mullerian Duct Syndrome: lack of phenotype/genotype correlation. Horm.Res.Paediatr. 77:291-297
485. Imbeaud S, Faure E, Lamarre I, Mattei MG, di CN, Tizard R, Carre-Eusebe D, Belville C, Tragethon L, Tonkin C, Nelson J, McAuliffe M, Bidart JM, Lababidi A, Josso N, Cate RL, Picard JY (1995) Insensitivity to anti-mullerian hormone due to a mutation in the human anti-mullerian hormone receptor. Nat. Genet. 11:382-388
486. di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard JY, Vigier B, Josso N, Cate R (1994) Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol. Endocrinol. 8:1006-1020
487. Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels B, Wilming LG, Meijers JH (1994) A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the müllerian duct. Development 120:189-197
488. Klattig J, Sierig R, Kruspe D, Besenbeck B, Englert C (2007) Wilms' tumor protein Wt1 is an activator of the anti-Mullerian hormone receptor gene Amhr2. Mol Cell Biol 27:4355-4364
489. Renlund N, Pieretti-Vanmarcke R, O'Neill FH, Zhang LH, Donahoe PK, Teixeira J (2008) c-Jun N-terminal kinase inhibitor II (SP600125) activates mullerian inhibiting substance type II receptor-mediated signal transduction. Endocrinology 149:108-115
490. Josso N, Picard JY, Cate RL (2013) The Persistent Müllerian Duct Syndrome. In: New MI, Parsa A, Yuen TT, O'Malley BW, Hammer GD eds. Genetic Steroid Disorders. New York, NY (USA): Elsevier; 265-278
491. Hirschhorn T, di Clemente N, Amsalem AR, Pepinsky RB, Picard JY, Smorodinsky NI, Cate RL, Ehrlich M (2015) Constitutive negative regulation in the processing of the anti-Mullerian hormone receptor II. J Cell Sci 128:1352-1364
492. Mishina Y, Whitworth DJ, Racine C, Behringer RR (1999) High specificity of Müllerian-inhibiting substance signaling in vivo. Endocrinology 140:2084-2088
493. Hart KN, Pepin D, Czepnik M, Donahoe PK, Thompson TB (2020) Mutational analysis of the putative Anti-Mullerian Hormone (AMH) binding interface on its type II receptor, AMHR2. Endocrinology
494. Gouédard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massagué J, di Clemente N (2000) Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Müllerian hormone and its type II receptor. J Biol Chem 275:27973-27978
495. Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK (2001) Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol. Endocrinol. 15:946-959
496. Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, Roberts AB, Umans L, Huylebroeck D, Zwijsen A, Wang D, Martin JF, Behringer RR (2008) Functional Redundancy of TGF-beta Family Type I Receptors and Receptor-Smads in Mediating Anti-Mullerian Hormone-Induced Mullerian Duct Regression in the Mouse. Biol. Reprod. 78:994-1001
497. Belville C, Jamin SP, Picard JY, Josso N, di Clemente N (2005) Role of type I receptors for anti-Mullerian hormone in the SMAT-1 Sertoli cell line. Oncogene 24:4984-4992
498. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR (2002) Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat. Genet. 32:408-410
499. Wu X, Zhang N, Lee MM (2012) Mullerian inhibiting substance recruits ALK3 to regulate Leydig cell differentiation. Endocrinology 153:4929-4937
500. Behringer RR, Finegold MJ, Cate RL (1994) Müllerian-inhibiting substance function during mammalian sexual development. Cell 79:415-425
501. Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR (1996) Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 10:2577-2587
502. Picard JY, Cate RL, Racine C, Josso N (2017) The Persistent Mullerian Duct Syndrome: An Update Based Upon a Personal Experience of 157 Cases. Sex. Dev. 11:109-125
503. Imbeaud S, Rey R, Berta P, Chaussain JL, Wit JM, Lustig RH, De Vroede MA, Picard JY, Josso N (1995) Testicular degeneration in three patients with the persistent müllerian duct syndrome. Eur. J. Pediatr. 154:187-190
504. Williams JC, Merguerian PA, Schned AR, Amdur RJ (1994) Bilateral testicular carcinoma in situ in persistent mullerian duct syndrome: a case report and literature review. Urology 44:595-598
505. Melman A, Leiter E, Pérez JM, Driscoll D, Palmer C (1981) The influence of neonatal orchiopexy upon the testis in persistent Mullerian duct syndrome. J. Urol. 125:856-858
506. Manassero F, Cuttano MG, Morelli G, Salinitri G, Spurio M, Selli C (2004) Mixed germ cell tumor after bilateral orchidopexy in persistent Mullerian duct syndrome with transverse testicular ectopia. Urol. Int. 73:81-83
507. Bucci S, Liguori G, Buttazzi L, Bussani R, Trombetta C (2002) Bilateral testicular carcinoma in patient with the persistent mullerian duct syndrome. J.Urol. 167:1790
508. Thiel DD, Erhard MJ (2005) Uterine adenosarcoma in a boy with persistent mullerian duct syndrome: first reported case. J.Pediatr.Surg. 40:e29-e31
509. Kato H, Komiyama I, Maejima T, Nishizawa O (2002) Histopathological study of the Müllerian duct remnant: Clarification of disease categories and terminology. J. Urol. 167:133-136
510. Farikullah J, Ehtisham S, Nappo S, Patel L, Hennayake S (2012) Persistent Mullerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Mullerian remnants. BJU Int. 110:E1084-1089
511. Gricourt S, Treton D, Renard-Pennat R, Samuel Lajeunesse J, Bitker MO, Bichet JC, Picard JY, Touraine P (2011) Novel anti-mullerian hormone mutation revealed by haematospermia in a 60-year-old patient. Clin. Endocrinol. (Oxf.) 74:404-405
512. Martin EL, Bennett AH, Cromie WJ (1992) Persistent Mullerian Duct Syndrome with transverse testicular ectopia and spermatogenesis. J. Urol. 147:1615-1617
513. Josso N, Audi L, Shaw G (2011) Regional variations in the management of testicular or ovotesticular disorders of sex development. Sex. Dev. 5:225-234
514. Vandersteen DR, Chaumeton AK, Ireland K, Tank ES (1997) Surgical management of persistent müllerian duct syndrome. Urology 49:941-945
515. Harrison CB, Kaplan GW, Scherz HC, Packer MG, Jones J (1990) Microvascular autotransplantation of the intra-abdominal testis. J. Urol. 144:506-507; discussion 512-503
516. Menabo S, Balsamo A, Nicoletti A, Gennari M, Pirazzoli P, Cicognani A, Baldazzi L (2008) Three Novel AMH Gene Mutations in a Patient with Persistent Mullerian Duct Syndrome and Normal AMH Serum Dosage. Horm. Res. 70:124-128
517. Knebelmann B, Boussin L, Guerrier D, Legeai L, Kahn A, Josso N, Picard JY (1991) Anti-Müllerian hormone Bruxelles: a nonsense mutation associated with the persistent Müllerian duct syndrome. Proc. Natl. Acad. Sci. USA 88:3767-3771
518. Tosca L, Giltay JC, Bouvattier C, Klijn AJ, Bouligand J, Lambert AS, Lecerf L, Josso N, Tachdjian G, Picard JY (2020) Persistent Mullerian duct syndrome due to anti-Mullerian hormone receptor 2 microdeletions: a diagnostic challenge. Hum. Reprod. 35:999-1003
519. Sigdel PR, Dhital P, Kulung Rai BD, Poudyal S, Luitel B, Sharma UK (2019) Persistent Mullerian duct syndrome with transverse testicular ectopia: A case report. Urol Case Rep 25:100888
520. Samet A, Mseddi MA, Bahloul R, Rebai N, Bahloul A, Slimene MH (2019) Cancer on cryptorchid testis revealing a Persistent Mullerian Duct Syndrome: A rare case. Urol Case Rep 26:100977
521. Sloan WR, Walsh PC (1976) Familial persistent Mullerian duct syndrome. J.Urol. 115:459-461
522. Naguib KK, Teebi AS, Farag TI, al-Awadi SA, el-Khalifa MY, el-Sayed M (1989) Familial uterine hernia syndrome: report of an Arab family with four affected males. Am.J.Med.Genet. 33:180-181
523. Renault L, Patino LC, Magnin F, Delemer B, Young J, Laissue P, Binart N, Beau I (2020) BMPR1A and BMPR1B Missense Mutations Cause Primary Ovarian Insufficiency. J. Clin. Endocrinol. Metab. 105
524. Auchus RJ, Miller WL (2012) Defects in androgen biosynthesis causing 46,XY disorders of sexual development. Semin. Reprod. Med. 30:417-426
525. Manna PR, Slominski AT, King SR, Stetson CL, Stocco DM (2014) Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: involvement of cAMP/PKA signaling. Endocrinology 155:576-591
526. Miller WL (2012) The syndrome of 17,20 lyase deficiency. J. Clin. Endocrinol. Metab. 97:59-67
527. Auchus RJ (2017) Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol 165:71-78
528. Pandey AV, Miller WL (2005) Regulation of 17,20 Lyase Activity by Cytochrome b5 and by Serine Phosphorylation of P450c17. J. Biol. Chem. 280:13265-13271
529. Simonov AN, Holien JK, Yeung JC, Nguyen AD, Corbin CJ, Zheng J, Kuznetsov VL, Auchus RJ, Conley AJ, Bond AM, Parker MW, Rodgers RJ, Martin LL (2015) Mechanistic Scrutiny Identifies a Kinetic Role for Cytochrome b5 Regulation of Human Cytochrome P450c17 (CYP17A1, P450 17A1). PLoS One 10:e0141252
530. Flück CE, Meyer-Boni M, Pandey AV, Kempna P, Miller WL, Schoenle EJ, Biason-Lauber A (2011) Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am. J. Hum. Genet. 89:201-218
531. Wilson JD, Shaw G, Leihy ML, Renfree MB (2002) The marsupial model for male phenotypic development. Trends Endocrinol. Metab. 13:78-83
532. Mebarki F, Sanchez R, Rhéaume E, Laflamme N, Simard J, Forest MG, Bey-Omar F, David M, Labrie F, Morel Y (1995) Nonsalt-losing male pseudohermaphroditism due to the novel homozygous N100S mutation in the type II 3 beta-hydroxysteroid dehydrogenase gene. J. Clin. Endocrinol. Metab. 80:2127-2134
533. Burckhardt MA, Udhane SS, Marti N, Schnyder I, Tapia C, Nielsen JE, Mullis PE, Rajpert-De Meyts E, Fluck CE (2015) Human 3beta-hydroxysteroid dehydrogenase deficiency seems to affect fertility but may not harbor a tumor risk: lesson from an experiment of nature. Eur J Endocrinol 173:K1-K12
534. Andersson S, Russell DW, Wilson JD (1996) 17b-hydroxysteroid dehydrogenase 3 deficiency. Trends Endocrinol. Metab. 7:121-126
535. George MM, New MI, Ten S, Sultan C, Bhangoo A (2010) The clinical and molecular heterogeneity of 17betaHSD-3 enzyme deficiency. Horm. Res. Paediatr. 74:229-240
536. Imperato-McGinley J, Zhu YS (2002) Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency. Mol. Cell. Endocrinol. 198:51-59
537. Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW (1993) Tissue distribution and ontogeny of steroid 5alpha-reductase isoenzyme expression. J. Clin. Invest. 92:903-910
538. Wilson JD, Lasnitzki I (1971) Dihydrotestosterone formation in fetal tissues of the rabbit and rat. Endocrinology 89:659-668
539. Maimoun L, Philibert P, Cammas B, Audran F, Bouchard P, Fénichel P, Cartigny M, Pienkowski C, Polak M, Skordis N, Mazen I, Ocal G, Berberoglu M, Reynaud R, Baumann C, Cabrol S, Simon D, Kayemba-Kay's K, De Kerdanet M, Kurtz F, Leheup B, Heinrichs C, Tenoutasse S, Van Vliet G, Grüters A, Eunice M, Ammini AC, Hafez M, Hochberg Z, Einaudi S, Al Mawlawi H, Nuñez CJ, Servant N, Lumbroso S, Paris F, Sultan C (2011) Phenotypical, biological, and molecular heterogeneity of 5alpha-reductase deficiency: an extensive international experience of 55 patients. J. Clin. Endocrinol. Metab. 96:296-307
540. Chavez B, Ramos L, Gomez R, Vilchis F (2014) 46,XY disorder of sexual development resulting from a novel monoallelic mutation (p.Ser31Phe) in the steroid 5alpha-reductase type-2 (SRD5A2) gene. Mol Genet Genomic Med 2:292-296
541. Rizner TL, Penning TM (2014) Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids 79:49-63
542. Cigorraga SB, Dufau ML, Catt KJ (1978) Regulation of luteinizing hormone receptors and steroidogenesis in gonadotropin-desensitized Leydig cells. J Biol Chem 253:4297-4304
543. Rabinovici J, Jaffe RB (1990) Development and regulation of growth and differentiated function in human and subhuman primate fetal gonads. Endocr. Rev. 11:532-557
544. Themmen APN, Huhtaniemi IT (2000) Mutations of Gonadotropins and Gonadotropin Receptors: Elucidating the Physiology and Pathophysiology of Pituitary-Gonadal Function. Endocr. Rev. 21:551-583
545. Latronico AC, Arnhold IJ (2012) Inactivating mutations of the human luteinizing hormone receptor in both sexes. Semin. Reprod. Med. 30:382-386
546. Gromoll J, Eiholzer U, Nieschlag E, Simoni M (2000) Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J. Clin. Endocrinol. Metab. 85:2281-2286
547. Bruysters M, Christin-Maitre S, Verhoef-Post M, Sultan C, Auger J, Faugeron I, Larue L, Lumbroso S, Themmen AP, Bouchard P (2008) A new LH receptor splice mutation responsible for male hypogonadism with subnormal sperm production in the propositus, and infertility with regular cycles in an affected sister. Hum.Reprod 23:1917-1923
548. Winter JSD, Faiman C, Reyes F (1981) Sexual endocrinology of fetal and perinatal life. In: Austin CR ed. Mechanisms of Sex Differentiation in Animals and Man. London: Academic Press; 205-253
549. Hughes IA, Werner R, Bunch T, Hiort O (2012) Androgen insensitivity syndrome. Semin. Reprod. Med. 30:432-442
550. Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM (1988) Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327-330
551. Finsterer J (2009) Bulbar and spinal muscular atrophy (Kennedy's disease): a review. Eur.J.Neurol. 16:556-561
552. Deeb A, Jaaskelainen J, Dattani M, Whitaker HC, Costigan C, Hughes IA (2008) A novel mutation in the human androgen receptor suggests a regulatory role for the hinge region in amino-terminal and carboxy-terminal interactions. J. Clin. Endocrinol. Metab. 93:3691-3696
553. Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M (2012) The androgen receptor gene mutations database: 2012 update. Hum. Mutat. 33:887-894
554. Davey RA, Grossmann M (2016) Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 37:3-15
555. Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z (2003) Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J.Biol.Chem. 278:41998-42005
556. Kerkhofs S, Dubois V, De GK, Helsen C, Clinckemalie L, Spans L, Schuit F, Boonen S, Vanderschueren D, Saunders PT, Verhoeven G, Claessens F (2012) A role for selective androgen response elements in the development of the epididymis and the androgen control of the 5alpha reductase II gene. FASEB J. 26:4360-4372
557. Denayer S, Helsen C, Thorrez L, Haelens A, Claessens F (2010) The rules of DNA recognition by the androgen receptor. Mol.Endocrinol. 24:898-913
558. Black BE, Paschal BM (2004) Intranuclear organization and function of the androgen receptor. Trends Endocrinol.Metab. 15:411-417
559. van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G (2012) Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol.Cell Endocrinol. 352:57-69
560. Edelsztein NY, Rey RA (2019) Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis. Cells 8:1-17
561. Hannema SE, Scott IS, Hodapp J, Martin H, Coleman N, Schwabe JW, Hughes IA (2004) Residual Activity of Mutant Androgen Receptors Explains Wolffian Duct Development in the Complete Androgen Insensitivity Syndrome. J. Clin. Endocrinol. Metab. 89:5815-5822
562. Welsh M, Saunders PT, Sharpe RM (2007) The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology 148:3185-3195
563. Clarnette TD, Sugita Y, Hutson JM (1997) Genital anomalies in human and animal models reveal the mechanisms and hormones governing testicular descent. Br. J. Urol. 79:99-112
564. van der Schoot P, Elger W (1992) Androgen induced prevention of the outgrowth of cranial gonadal suspensory ligaments in fetal rats. J. Androl. 13:534-542
565. Su S, Farmer PJ, Li R, Sourial M, Buraundi S, Bodemer D, Southwell BR, Hutson JM (2012) Regression of the mammary branch of the genitofemoral nerve may be necessary for testicular descent in rats. J.Urol. 188:1443-1448
566. Mamoulakis C, Antypas S, Sofras F, Takenaka A, Sofikitis N (2015) Testicular Descent. Hormones (Athens) 14:515-530
567. Ivell R, Anand-Ivell R (2011) Biological role and clinical significance of insulin-like peptide 3. Curr.Opin.Endocrinol Diabetes Obes. 18:210-216
568. Tomboc M, Lee PA, Mitwally MF, Schneck FX, Bellinger M, Witchel SF (2000) Insulin-like 3/relaxin-like factor gene mutations are associated with cryptorchidism. J. Clin. Endocrinol. Metab. 85:4013-4018
569. Huang Z, Rivas B, Agoulnik AI (2012) Insulin-Like 3 Signaling Is Important for Testicular Descent but Dispensable for Spermatogenesis and Germ Cell Survival in Adult Mice. Biol. Reprod. 87:143
570. Emmen JMA, McLuskey A, Adham IM, Engel W, VerhoefPost M, Themmen APN, Grootegoed JA, Brinkmann AO (2000) Involvement of insulin-like factor 3 (Insl3) in diethylstilbestrol-induced cryptorchidism. Endocrinology 141:846-849
571. Sassoon D (1999) Wnt genes and endocrine disruption of the female reproductive tract: a genetic approach. Mol. Cell. Endocrinol. 158:1-5
572. Newbold RR, Padilla-Banks E, Jefferson WN (2006) Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147:S11-S17
573. Sharpe RM (2003) The 'oestrogen hypothesis' - where do we stand now? Int. J. Androl. 26:2-15
574. Gaspari L, Paris F, Jandel C, Kalfa N, Orsini M, Daures JP, Sultan C (2011) Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case-control study. Hum Reprod.
575. N'tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud'homme SM, Pozzi-Gaudin S, Frydman R, Benachi A, Livera G, Rouiller-Fabre V, Habert R (2012) Differential effects of bisphenol a and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS.One. 7:e51579
576. van den Driesche S, Walker M, McKinnell C, Scott HM, Eddie SL, Mitchell RT, Seckl JR, Drake AJ, Smith LB, Anderson RA, Sharpe RM (2012) Proposed role for COUP-TFII in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents. PLoS.One. 7:e37064
577. Skakkebæk NE (2016) A Brief Review of the Link between Environment and Male Reproductive Health: Lessons from Studies of Testicular Germ Cell Cancer. Horm. Res. Paediatr.
578. Skakkebæk NE, Rajpert-De Meyts E, Main KM (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: Opinion. Hum. Reprod. 16:972-978
579. Abdel-Maksoud FM, Leasor KR, Butzen K, Braden TD, Akingbemi BT (2015) Prenatal Exposures of Male Rats to the Environmental Chemicals Bisphenol A and Di(2-Ethylhexyl) Phthalate Impact the Sexual Differentiation Process. Endocrinology 156:4672-4683
580. van den Driesche S, McKinnell C, Calarrao A, Kennedy L, Hutchison GR, Hrabalkova L, Jobling MS, Macpherson S, Anderson RA, Sharpe RM, Mitchell RT (2015) Comparative effects of di(n-butyl) phthalate exposure on fetal germ cell development in the rat and in human fetal testis xenografts. Environ Health Perspect 123:223-230
581. Mizoguchi BA, Valenzuela N (2016) Ecotoxicological Perspectives of Sex Determination. Sex. Dev. 10:45-57