Archives
Adrenal Incidentalomas
ABSTRACT
Wider application and technical improvement of abdominal imaging procedures in recent years, has led to the discovery of unsuspected adrenal tumors in an increasing frequency. These serendipitously detected lesions, also called adrenal incidentalomas, have become a common clinical problem and need to be investigated for evidence of hormonal hypersecretion and/or malignancy. In this chapter, information on the prevalence, etiology, radiological features, and appropriate biochemical evaluation are discussed in order to delineate the nature and hormonal status of adrenal incidentalomas. Despite the flurry of data accumulated, controversies are still present regarding the accuracy of diagnostic tests and cut-offs utilized to establish hormonal hypersecretion, potential long-term sequelae, indications for surgical treatment as well as duration and intensity of conservative management and follow-up. Recently, clinical guidelines proposing a diagnostic and therapeutic algorithm have been published to aid in clinical practice, however several areas are still debatable and require further research.
INTRODUCTION
Abdominal computed tomography (CT), since its introduction in the late 1970’s, has proven to be an excellent tool for identifying pathology in patients with suspected adrenal disease. It was also predicted that the ability of CT to image both adrenal glands could lead to the occasional discovery of asymptomatic adrenal disease (1). Nowadays, further technological advances and broader availability of CT and other imaging modalities such as Ultrasonography (US), Magnetic Resonance Imaging (MRI), and Positron Emission Tomography (PET) have made the detection of unexpected lesions in adrenal and other endocrine glands a common finding (2). Although earlier detection of adrenal disease may be important in certain cases, it is now recognized that diagnostic evaluation and follow-up of clinically inapparent adrenal masses, or so-called “adrenal incidentalomas” may put a significant burden on patient’s anxiety and health and produce increasing financial consequences for the health system (3). It is therefore important to develop cost-effective strategies to diagnose and manage patients with adrenal incidentalomas.
DEFINITION
According to the NIH State-of-the-Science Statement (4), adrenal incidentalomas (AIs) are defined as clinically inapparent adrenal masses discovered inadvertently in the course of diagnostic testing or treatment for conditions not related to the adrenals. Although an arbitrary cut-off of 1 cm or more has been employed to define an adrenal lesion as AI (5,6), this cut-off might be challenged following the higher resolution that modern imaging modalities offer, mainly MRI and CT. Nonetheless, in all published guidelines this cut-off is accepted as the minimum size above which additional diagnostic work-up should be performed, unless clinical signs and symptoms suggestive of adrenal hormone excess are present. Patients harboring an AI, by definition, should not have any history, signs or symptoms of adrenal disease prior to the imaging procedure that led to its discovery. This strict definition excludes cases in which symptomatic adrenal-dependent syndromes are “missed” during history taking or physical examination (6), but is also subject to some controversy regarding the ‘‘a priori’’ suspicion (7). In this context, an adrenal tumor detected in a patient undergoing abdominal imaging for staging and work-up of extra-adrenal malignancy should not be considered an AI, since adrenal metastases are a common finding in this setting, with a prevalence ranging from 3 to 40% in autopsy and from 6 to 20% in radiological series (8).
EPIDEMIOLOGY
The precise prevalence and incidence of AI is difficult to define since data from population-based studies are lacking. Most data are derived from autopsy or radiological studies that are relatively difficult to interpret due to their retrospective nature, insufficient clinical information provided, referral bias, and different patient selection criteria.
In autopsy studies the prevalence of AIs varies, depending on patient’s age and the size of the tumors. The mean prevalence in a total of 71,206 cases was found to be 2.3%, ranging from 1 to 8.7% (9–21), without any significant gender difference. The prevalence of AIs increases with age, being 0.2% in young subjects compared to 6.9% in subjects older than 70 years of age (22), and is higher in white, obese, diabetic, and hypertensive patients (8). The variability of the reported prevalence in different series also reflects the difficulty in distinguishing small nodules from adenomas, as in some post-mortem series, small nodules (<1 cm) were detected in more than half of the patients examined (13).
In radiological studies, the prevalence of AIs differs depending on the imaging modality used. Transabdominal US during a routine health examination identified AIs in 0.1% of those screened (23), while studies using CT reported a mean prevalence of 0.64% ranging from 0.35 to 1.9% in a total of 82,483 scans published in the literature between 1982 and 1994 (11,24–28). However, two recent studies utilizing high-resolution CT scanning technology, have reported a prevalence of 4.4% and 5% which are similar to that observed in autopsy studies (29,30). This increase in detection frequency paralleled by the technological advances in imaging modalities, can explain why AIs are considered a “disease of modern technology”. Age has also been found to affect AI detection rates, as these lesions are found in 0.2% of individuals younger than 30 years, in 3% in the age of 50 years and up to 10% in individuals above 70 years of age (22,29,31). The prevalence of AIs is very low in childhood and adolescence accounting for 0.3-0.4% of all tumors (32). Adrenal incidentalomas appear to be slightly more frequent in women in radiological series, in discordance with autopsy studies, probably because women undergo abdominal imaging more frequently than men (31). Adrenal masses are located bilaterally in 10-15% of cases (33), while distribution between the two adrenals appears to be similar in post-mortem and CT studies (8,31).
DIFFERENTIAL DIAGNOSIS
Adrenal Incidentalomas are not a single pathological entity, but rather comprise a spectrum of different pathologies that share the same path of discovery and include both benign and malignant lesions arising from the adrenal cortex, the medulla or being of extra-adrenal origin (Table 1).
In general, the vast majority (80-90%) of AIs are benign adrenal adenomas, as shown by accumulated follow-up data from their natural history, even in the absence of pathological confirmation, since adrenal adenomas are rarely excised (5). However, a number of these lesions may still be malignant and/or related to autonomous hormonal secretion that is not clinically detected due to subtle secretory pattern or periodical secretion. Therefore, the question a physician faces when dealing with an AI is to exclude pathology other than an adrenal adenoma, particularly an adrenocortical carcinoma (ACC), and evaluate its secretory potential.
|
Table 1: The Spectrum of Lesions Presenting as AIs (modified from (34)) |
|
Adrenal Cortex lesions |
|
· Adenoma (non-functioning) |
|
· Adenoma (functioning)- Cortisol-secreting, Aldosterone-secreting |
|
· Nodular hyperplasia (primary bilateral macronodular adrenal hyperplasia)* |
|
· Adrenocortical Carcinoma (secreting or non-secreting) |
|
Adrenal Medulla lesions |
|
· Pheochromocytoma (benign or malignant)* |
|
· Ganglioneuroma |
|
· Neuroblastoma, ganglioneuroblastoma |
|
Other adrenal lesions |
|
· Myelolipoma, lipoma |
|
· Hemangioma, angiosarcoma |
|
· Cyst |
|
· Hamartoma, teratoma |
|
Metastases (lung, breast, kidney, melanoma, lymphoma)* |
|
Infiltration* |
|
· Amyloidosis |
|
· Sarcoidosis |
|
· Lymphoma |
|
Infections* |
|
· Abscess · |
|
· Fungal/parasitic (histoplasmosis, coccidiomycosis, tuberculosis) |
|
· Cytomegalovirus |
|
Adrenal hemorrhage or hematomas* |
|
Adrenal pseudotumors |
|
Congenital Adrenal Hyperplasia (CAH)* |
* can present with bilateral adrenal lesions
Autonomous cortisol secretion (ACS) is the most frequent endocrine dysfunction detected in patients with AIs, with a prevalence ranging from 5 to 30%, depending on the study design, work-up protocols and mainly diagnostic criteria used (5). This condition exclusively identified in the setting of AIs, also termed subclinical Cushing’s syndrome or subclinical hypercortisolism, is characterized by the absence of the typical clinical phenotype of hypercortisolism and by the presence of subtle alterations of the hypothalamic-pituitary-adrenal (HPA) axis.
Pheochromocytomas (PCCs), albeit rare in the general population, are discovered in approximately 5% of patients with AIs (35), while more than 30% of PCCs are diagnosed as AIs (36). Clinical manifestations are highly variable and the classic clinical triad (headache, palpitations and diaphoresis) is not present in the majority of patients. In addition, several patients harbor ‘‘silent pheochromocytomas’’, being totally asymptomatic or having intermittent and subtle symptoms. In a large multicentric study, approximately half of the patients with PCCs presenting as AIs were normotensive, whereas the remaining had mild to moderate hypertension (31).
Primary aldosteronism (PA) has a median prevalence of 2% (range 1.1-10%) among patients with AIs (37). After excluding cases with severe hypertension and hypokalaemia a retrospective study found that 16 out of 1004 subjects with AIs (1.5%) had PA (31). This figure is relatively low when compared to the prevalence of PA in unselected hypertensive populations which ranges from 4.6 to 16.6% (38) and may be related to the different investigational protocols and cut-offs indicative of autonomous aldosterone secretion used. The absence of hypokalaemia does not exclude this condition but absence of hypertension makes PA unlikely, although normotensive patients with PA have occasionally been reported (39). A recent study using a new diagnostic approach, considering the stimulatory effect that adrenocorticotropin (ACTH) could exert on aldosterone secretion, revealed a 12% prevalence of PA in normotensive and normokalaemic patients with AIs (40).
Combining studies that used a broad definition of incidentaloma without clearly stated inclusion criteria and those that reported descriptions of individual cases, Mansmann et al found 41% of AIs to be adenomas, 19% metastases, 10% ACCs, 9% myelolipomas, and 8% PCCs, with other benign lesions, such as adrenal cysts, ganglioneuromas, hematomas and infectious or infiltrative lesions representing rare pathologies (41). However, the relative prevalence of any pathology depends on the inclusion criteria used and is highly influenced by referral bias. Surgical series tend to overestimate the prevalence of malignant and secretory tumors, because the suspicion of a carcinoma and a functioning or large tumor are considered as indications for surgery. The reported prevalence of ACCs in these studies is also misleading, as it is derived from highly selected patient populations and does not reflect the prevalence seen in population-based studies. Presence of primary adrenal malignancy is more related to mass size, as ACCs represent 2% of all tumors ≤4 cm in diameter, 6% of tumors with size 4.1-6 cm and 25% of the tumors >6 cm (4). On the other hand, benign adenomas comprise 65% of masses ≤4 cm, and 18% of masses >6 cm (41). Similarly, metastatic lesions are much more common when patients with known extra-adrenal cancer are included. Among patients with extra-adrenal malignancies (most commonly carcinomas of the lung, breast, kidney, and melanoma), up to 70% of AIs are metastases. In contrast, the probability of a serendipitously discovered adrenal lesion in a patient without a history of cancer to be metastatic is as low as 0.4% (27). Studies applying more strict inclusion criteria may identify a greater number of small masses and biochemically silent tumors. In a comprehensive review, Cawood et al. (3) concluded that the prevalence of malignant and functioning lesions among AIs is likely to be overestimated if strict inclusion and exclusion criteria for the study populations are not used. By analyzing 9 studies that more accurately simulated the clinical scenario of a patient referred for assessment of an AI, they reported a mean prevalence of 88.1% (range 86.4-93%) for non-functioning benign adrenal adenomas (NFAIs), 6% (range 4-8.3%) for ACS, 1.2% for aldosterinomas, 1.4% (range 0.8-3%) for ACCs, 0.2% (range 0-1.4%) for metastases and 3% (range 1.8-4.3%) for PCCs. These low rates for clinically significant tumors compared to those noted from previous studies, that did not use such a narrow definition of AI (6,8,41), highlighted the limitations of epidemiological data due to inherent bias and raised significant questions concerning the appropriate diagnostic and follow-up protocols.
In the case of bilateral AIs, a broader spectrum of diagnoses needs to be considered, particularly in a relevant clinical setting, including metastatic or infiltrative diseases of the adrenals, hemorrhage, congenital adrenal hyperplasia (CAH), bilateral PCCs, bilateral cortical adenomas, and primary bilateral macronodular adrenal hyperplasia (PBMAH) (42). Occasionally, adrenal tumors of different nature may simultaneously be present in the same patient or in the same adrenal gland (43–46). Adrenal pseudotumor is a term used to describe radiological images of masses that seem to be of adrenal origin, but arise from adjacent structures, such as the kidney, spleen, pancreas, vessels and lymph nodes or are results of technical artifacts.
PATHOGENESIS
The pathogenesis of AIs is largely unknown. Early observations in autopsy studies which revealed that AIs are more frequent in older patients, led to the notion that these tumors are a manifestation of the ageing adrenal and could represent focal hyperplasia in response to ischemic injury, a concept that was supported by histopathological findings of capsular arteriopathy (47). Clonal analysis of adrenal tumors later revealed that the vast majority are of monoclonal origin and only a few arise from polyclonal focal nodular hyperplasia under the putative effect of local or extra-adrenal growth factors (48,49). In this sense, it has been postulated that hyperinsulinemia associated with the insulin resistance in individuals with the metabolic syndrome, which frequently coexists in patients harboring AIs, could contribute to the development of these tumors, through the mitogenic action of insulin on the adrenal cortex (50,51). However, the opposite causal relationship, that subtle autonomous cortisol production from AIs results in insulin resistance, has also been proposed (52). Another interesting hypothesis involves alterations in the glucocorticoid feedback sensitivity of the HPA axis. In a recent study, unexpected ACTH and cortisol responses to the combined dexamethasone-CRH (corticotropin-releasing hormone) test were found, in about half of the patients with bilateral AIs, when compared to control and unilateral adenoma cases (53). Such a dysregulated ACTH secretion during lifetime may lead to subtle but chronic trophic stimulation of the adrenals by repeatedly inappropriately higher ACTH levels, particularly in response to stress, favoring nodular adrenal hyperplasia.
Although several genetic syndromes are known to be associated with adrenal tumors, germline or somatic genetic alterations are identified only in subgroups of sporadic tumors that are mainly functioning (54–56). Elucidation of specific signaling pathways involved in these familial syndromes has led to the identification of several mutations in genes not previously described in ACCs, cortisol- and aldosterone-secreting adenomas as well as PCCs, creating new insights in adrenal tumorigenesis (Figure 1). However, the genetics of benign non-functional AIs that account for the majority of AIs are poorly understood.
Figure 1. Genes Involved in the Development of Adrenocortical Tumors
MEN: Multiple Endocrine Neoplasia; CTNNB1: catenin beta-1 gene; CYP21A2: 21-hydroxylase gene; CAH: Congenital Adrenal Hyperplasia; APC: Adenomatous polyposis coli; FAP: Familial adenomatous polyposis; KCNJS: gene encoding potassium channel, inwardly rectifying subfamily J, member 5; ATP1A1: gene encoding sodium/potassium-transporting ATPase subunit alpha 1; ATP2B3: plasma membrane calcium-transporting ATPase 3; CACNA1D: gene encoding calcium channel, voltage-dependent, L type, alpha 1D subunit; ARMCS: Armadillo repeat containing 5; ZNRF3: gene encoding Zinc and Ring Finger3; IGF-2: Insulin-like growth factor 2; TP53: tumor protein p53; CDKN2A: cyclin-dependent kinase inhibitor 2A; RB1: retinoblastoma protein; DAXX: death-associated protein 6; GNAS: gene encoding G-protein alpha subunit: PDE8B: phosphodiesterase 8B; PRKACA: gene encoding catalytic subunit alpha of protein kinase A; SDH-A-B-C-D: gene encoding succinate dehydrogenase complex subunit A, B, C, and D; SDHAF2: succinate dehydrogenase complex assembly factor 2; VHL: von-Hippel-Landau; RET: rearranged during transfection proto-oncogene; MAX: myc-associated factor X; TMEM127: gene encoding transmembrane protein 127.
DIAGNOSTIC APPROACH
Although the prevalence of potentially life-threatening disorders associated with AIs is relatively low, the question of whether a lesion is malignant (mainly an ACC) or functioning needs to be addressed in patients with an incidentally discovered adrenal mass. A careful clinical examination and a detailed medical history, evaluation of the imaging characteristics of the adrenal tumor(s), and biochemical evaluation to exclude hormonal excess can help clinicians identify the few cases that pose a significant risk to the patient’s health.
Clinical Evaluation
Per definition, patients with AIs should have no signs or symptoms implying adrenal dysfunction before the radiological detection of the adrenal tumor(s). In everyday clinical practice though, physicians who are not familiar with endocrine diseases may overlook mild signs of hormone excess and pursue evaluation of adrenal function following the incidental discovery of an adrenal mass. In this setting, such cases should not be designated as AIs and highlight the need for detailed and careful clinical history and examination (57).
Imaging Evaluation
Current morphological imaging modalities mainly CT and MRI have proven to be reliable means to predict with high diagnostic accuracy the nature of the lesion and its malignant potential. The size of the lesion has been considered as indicative of malignancy as most ACCs are large or significantly larger than adenomas at the time of diagnosis (31). In a meta-analysis, ACCs represented 2% of all tumors ≤4 cm in diameter, but the risk of malignancy increased significantly with tumor size greater than 4 cm, being 6% in tumors with size 4.1-6 cm and 25% in tumors >6 cm (58). However, size alone has low specificity in distinguishing benign from malignant lesions, since ACCs can also be relatively small during early stages of development and exhibit subsequent progressive growth (5). Other than size, findings suggestive of malignancy include irregular shape and borders, tumor heterogeneity with central necrosis or hemorrhage and invasion into surrounding structures. Benign adenomas are usually small (<4 cm), homogenous, with well-defined margins. Slow growth rate or stable size of an adrenal mass have also been proposed as indicators of benign nature (4). However, studies on the natural history of AIs suggest that up to 25% of benign adenomas can display an increase in size by almost 1 cm, while adrenal metastases with no change in CT appearance over a period of 36 months have been described, not allowing for the introduction of a safe cut-off of absolute growth or growth rate to distinguish benign from malignant lesions (59).
Certain imaging properties of AIs, depending on the modality used, can be helpful for the differential diagnosis between benign and malignant adrenal lesions and are summarized in Table 2.
COMPUTED TOMOGRAPHY (CT)
CT has a high spatial and contrast resolution, which allows assessment of tissue density by measuring X-ray absorption compared to water (attenuation, expressed in Hounsfield Units - HU) (Figure 2). Water and air are conventionally allocated a HU value of 0 and -1000 respectively, while fat is usually characterized by a HU value between -40 and -100. Because there is an inverse linear relation between the fat content of a lesion and attenuation, lipid-rich adenomas express lower HU in unenhanced (without contrast medium) CT images compared to malignant lesions, which are usually lipid-poor (60). A value of 10 HU in unenhanced CT images is the most widely used and accepted attenuation value threshold for the diagnosis of a lipid-rich, benign adrenal adenoma (61,62). In several studies a density of ≤10 HU was found to be superior to size in differentiating benign from malignant masses, displaying a sensitivity of 96-100% and a specificity of 50-100% (63). In this context, the risk of malignancy in a homogeneous 5 cm adrenal mass with a CT attenuation value of 10 HU is close to 0% (42). On the other hand, up to 30-40% of benign adenomas are considered lipid-poor and have an attenuation value of >10 HU on non-contrast CT, which is considered indeterminate since it overlaps with those found in malignant lesions and PCCs. Hence, unenhanced CT attenuation is a useful screening tool to identify a lesion as benign and exclude malignancy but is less reliable in diagnosing a malignant mass with certainty. When considering patients with a history of extra-adrenal malignancy though, several studies evaluating the >10 HU cut-off as indicative of malignancy showed high sensitivity (93%) for the detection of malignancy but variable specificity, meaning that 7% of adrenal metastases were found to have a tumor density of ≤10 HU (62). Attenuation values in non-contrast CT can also reliably identify typical myelolipomas that have a density lower than minus 40 HU (42).
For these indeterminate adrenal lesions (>10HU) intravenous contrast administration reveals their hemodynamic and perfusion properties that can be utilized to distinguish benign from malignant lesions (Figure 2). The attenuation on delayed images (10-15 min post contrast administration) decreases more quickly in adenomas because they exhibit rapid uptake and clearance compared to malignant lesions that usually enhance rapidly but demonstrate a slower washout of contrast medium (64). There are two methods of estimating contrast medium washout: absolute percentage washout (APW) and relative percentage washout (RPW) and can be calculated from values of pre-contrast (PA), enhanced (EA, 60-70 seconds after contrast medium administration) and delayed (DA, 10-15 mins after contrast medium administration) attenuation values according to the formulas below:
APW=100 x (EA-DA) / (EA-PA)
RPW=100 x (EA-DA) / EA
Lipid-poor adenomas demonstrate rapid washout with APW >60% (sensitivity of 86-100%, specificity 83-92%) and a RPW >40% (sensitivity of 82-97%, specificity 92-100%) (65). The use of APW and RPW criteria can effectively discriminate benign from malignant adrenal masses. Metastases usually demonstrate slower washout on delayed images (APW<60%, RPW<40%) than adenomas and ACCs typically have an RPW of <40%. Furthermore, contrast-enhanced washout CT studies may not suffice for characterization of lesions such as PCCs, cysts, and myelolipomas; in these cases, further biochemical, anatomical and/or functional imaging may be required. Findings consistent, but not diagnostic, of PCC on CT include high attenuation values, prominent vascularity, and delayed washout of contrast medium (66).
Figure 2: CT images of adrenal pathologies presenting as adrenal incidentalomas. a,b,c: A patient with a benign (lipid-rich) adrenal adenoma with unenhanced attenuation value - 3 HU (a), early attenuation (60 seconds after i.v. contrast medium administration) 35 HU (b) and delayed attenuation (10 min post-contrast administration) 18 HU. ARW = 45% and RPW=49%. Absolute washout (APW) less than 60% is indeterminate. However, the low pre-contrast attenuation is suggestive of an adenoma. Relative washout (RPW) of 40% or higher is consistent with an adenoma; d,e,f: Biochemically and histologically proven pheochromocytoma with unenhanced attenuation of 49 HU (d), early attenuation 90 HU (e) and delayed attenuation 64 HU. ARW = 63% and RPW=29%. Absolute washout >60% is suggestive of an adenoma, however relative washout less than 40% and unenhanced attenuation >10 HU are indeterminate; g,h: A patient with a primary adrenocortical carcinoma characterized by heterogeneity an unenhanced attenuation value >10 HU (g) and inhomogeneous contrast medium uptake due to central areas of necrosis; i: Typical myelolipoma.
It is important to note that the aforementioned figures of sensitivity and specificity were produced in studies with limitations and high risk of bias because of lack of definitive pathological diagnosis, different timing in acquiring post-contrast images and the use of broad inclusion criteria, including not only AIs but also clinically overt adrenal masses. In agreement with this statement, a recent study (67), showed that only a minority (21%) of cortisol-secreting adenomas has the typical unenhanced attenuation value of <10 HU, because cortisol secretion is associated with decreased intra-cytoplasmic lipid droplets containing cholesterol esters which are necessary for cortisol synthesis. Nevertheless, among the adenomas with high pre-contrast density (>10 HU), washout analysis after contrast administration was consistent with the benign nature of the tumor in 60% of the cases.
Another crucial key point in clinical practice is that most abdominal and chest CT scans leading to the unexpected discovery of an adrenal mass are obtained with the use of intravenous contrast that may not fulfill current technical recommendations for an optimal CT study of the adrenal glands, such as analysis on contiguous 3-5 mm-thick CT slices, preferentially on multiple sections using multidetector (MDCT) row protocols (68). In such cases, it may be worthwhile to obtain a new CT scan, specifically aimed for the study of the adrenal glands, including washout protocols in order to avoid the radiation exposure of a subsequent third CT scan in case of indeterminate unenhanced attenuation values.
Finally, the importance of thorough and standardized reporting by radiologists (including common terminology, nodule size and HU) needs to be highlighted, in order to improve the percentage of patients with AIs that receive appropriate diagnostic testing and follow-up. This is a recently raised issue based on evidence that suggests that most of AIs are not adequately investigated according to international guidelines due to inconsistent use of terms and lack of specific details and recommendations in radiology reports (69,70).
MAGNETIC RESONANCE IMAGING (MRI)
Adrenal imaging with MRI can also aid in the differential diagnosis between benign and malignant adrenal pathology (Figure 3). Benign adrenal adenomas appear hypotense or isotense compared to the liver on T1-weighted images and have low signal intensity on T2-weighted images. The majority of PCCs show high signal intensity on T2-weighted imaging (“light bulb sign”) which is a non-specific finding; however, a wide range of imaging features of PCCs mimicking both benign and malignant adrenal lesions have also been described (66). Primary ACCs are characterized by intermediate to high signal intensity on T1- and T2-weighted images and heterogeneity (mainly on T2- sequence due to hemorrhage and/or necrosis) as well as avid enhancement with delayed washout. However, these features are not specific and display significant overlap between benign and malignant lesions. The MRI technique of chemical-shift imaging (CSI) exploits the different resonance frequencies of protons in water and triglyceride molecules oscillating in- or out-of-phase to each other under the effect of specific magnetic field sequences, to identify high lipid content in adrenal lesions (71). Adrenal adenomas with a high content of intracellular lipid usually lose signal intensity on out-of-phase images compared to in-phase images, whereas lipid-poor adrenal adenomas, malignant lesions and PCCs remain unchanged. Signal intensity loss can be assessed qualitatively by simple visual comparison or by quantitative analysis using the adrenal-to-spleen signal ratio and can identify adenomas with a sensitivity of 84-100% and a specificity of 92-100% (72). It must be remembered however, that ACC and clear renal cell cancer metastases may sometimes also show signal loss (73).
Overall, MRI is probably as effective as CT in distinguishing benign from malignant lesions. Although quality of data is poor and there are no randomized studies comparing the two conventional imaging modalities, a few studies have concluded that for lipid-rich adenomas, there is no apparent difference, but MRI with CSI might be superior when evaluating lipid-poor adenomas with an attenuation value up to 30 HU (74). Hence, CT is considered to be the primary radiological procedure for evaluating AIs because it is more easily available and cost-effective, whereas MRI should be employed when a CT is less desirable (as in pregnant women and in children), for lipid-poor adenomas with relatively high attenuation values, and for other suspected lesions such as PCCs (75). When MRI is the examination that revealed the AI, additional imaging with CT (unenhanced and/or PW studies) could be performed if the imaging phenotype is equivocal and following discussion of the individual case in a multidisciplinary team of experts.
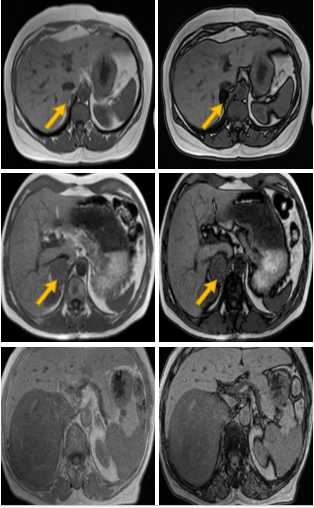
Figure 3: MRI images of different adrenal lesions presenting as incidentalomas, using the chemical shift imaging (CSI) technique. The loss of signal in out of phase images is typical in benign lipid-rich adenomas (a, b) in contrast with pheochromocytomas (c, d) and adrenocortical carcinomas (e, f) which do not display any signal loss.
SCINTIGRAPHY
In recent years, positron emission tomography (PET) using 18-fluoro-deoxyglucose (18F-FDG) has emerged as an effective tool in identifying malignant adrenal lesions. By utilizing the increased glucose uptake properties of cancer cells, 18F-FDG-PET combined with a CT scan (18F-FDG-PET/CT) achieves a sensitivity and specificity in identifying malignancy of 93-100% and 80-100% respectively (76,77). Both quantitative analysis of FDG uptake using maximum standardized uptake values (SUVmax) and qualitative assessment using a mass/liver SUV ratio have been used as a criterion, with the latter displaying better performance (78). A SUV ratio <1.45–1.6 between the adrenal and the liver is highly predictive of a benign lesion (79). Caveats in utilizing 18F-FDG-PET/CT include cost and availability, risk of false negative results in case of necrotic or hemorrhagic malignant lesions, size <1cm, extra-adrenal malignancies with low uptake (such as metastases from renal cell cancer or low-grade lymphoma) and false positive results in cases of sarcoidosis, tuberculosis, other inflammatory or infiltrative lesions and some adrenal adenomas and PCCs that show moderate FDG uptake (80). Because of its excellent negative predictive value, 18F-FDG-PET may help in avoiding unnecessary surgery in patients with non-secreting tumors with equivocal features in CT demonstrating low FDG uptake. Moreover, 18F-FDG-PET/CT may favor surgical removal of tumors with elevated uptake and no biochemical evidence of a PCC (76). Newer tracers such as 18F-fluorodihydroxyphenylalanine (F-DOPA) and 18F-fluorodopamine (FDA) for detection of PCC on PET have also been developed but their availability is limited (81).
Conventional adrenal scintigraphy using radiolabeled cholesterol molecules such as 131I-6-b-iodomethyl-norcholesterol (NP-59) and 75Se-selenomethyl-19-norcholesterol has been used in the past to discriminate benign from malignant lesions. These tracers enter adrenal hormone synthetic pathways and act as precursor-like compounds, providing information regarding the function of target tissue. Typically, benign hypersecreting tumors, and non-secreting adenomas, show tracer uptake, whereas primary and secondary adrenal malignancies, space-occupying or infiltrative etiologies of AIs appear as ‘cold’ masses, providing an overall sensitivity of 71-100% and a specificity of 50-100% (82). However, some benign adrenal tumors such as myelolipomas and some functioning ACCs, may also be visualized with these modalities. Several additional limitations of adrenal scintigraphy such as insufficient spatial resolution, lack of widespread expertise, limited availability of the tracer, being a time-consuming procedure (which requires serial scanning over 5-7 days), and high radiation doses received by the patient, have limited its value in routine clinical practice, especially when conventional imaging can provide more reliable information. Recently, 123I-iodometomidate has been introduced as a tracer because it binds specifically to adrenocortical enzymes, but its application is hampered by its limited availability and heterogeneous uptake by ACCs (83). Scintigraphy with 123I-meta-iodo-benzyl-guanidine (MIBG) is the preferred method for identifying PCCs when clinical, biochemical, and imaging features are not conclusive, or when multiple or malignant lesions need to be excluded (35).
|
Table 2: Imaging Findings Differentiating Common Adrenal Pathologies in AIs |
||||
|
FINDING |
Benign adenoma |
ACC |
Pheochromocytoma |
Metastases |
|
Size |
Usually <4cm |
Usually >4cm |
Variable |
Variable |
|
Growth rate |
Stable or <0.8cm/year |
Significant growth (>1cm/year) |
Slow growth |
Significant growth (>1cm/year) |
|
Shape & margins |
Round or oval with well-defined margins |
Irregular shape and margins. Invasion to surrounding tissues |
Variable |
Variable |
|
Composition |
Homogenous |
Heterogeneous (hemorrhage, necrosis) |
Heterogeneous (necrosis) |
Heterogeneous (hemorrhage, necrosis) |
|
CT Unenhanced attenuation |
≤10 HU (or >10 HU for lipid-poor adenomas) |
>10 HU |
>10 HU |
>10 HU |
|
CT Percent Washout (PW) |
APW >60% RPW>40% |
APW<60%, RPW<40% |
APW<60% RPW<40% |
APW<60%, RPW<40% |
|
MRI - CSI (out-of phase) |
Signal loss (except in lipid-poor adenomas) |
No change in signal intensity |
No change in signal intensity |
No change in signal intensity |
|
FDG uptake (PET) |
Low (some can have low to moderate uptake) |
High |
Low (malignant pheochromocytomas show high uptake) |
High |
|
NP-59 uptake |
Present |
Absent (except in some secreting tumors) |
Absent |
Absent |
ACC: Adrenocortical carcinoma; HU: Hounsfield Units; APW: Absolute PW; RPW: Relative PW; CSI: Chemical-shift Imaging; FDG: fluoro-deoxyglucose; NP-59: 131I-6-b-iodomethyl-norcholesterol
Hormonal Evaluation
Patients with AIs should be screened at presentation for evidence of excess catecholamine or cortisol secretion and, if hypertensive and/or hypokalemic, for aldosterone excess. As already discussed, the definition of AI per se implies the absence of clinical symptoms/signs related to these entities, however subtle hormonal hypersecretion not leading to the full clinical phenotype of a related syndrome may be present in patients with an AI (6).
SCREENING FOR CORTISOL EXCESS
According to the Endocrine Society’s Clinical Practice Guidelines for the diagnosis of Cushing’s syndrome and the AACE/AAES Medical Guidelines for the management of AIs, all patients with an incidentally discovered adrenal mass should be tested for the presence of hypercortisolism (57,84). Signs and symptoms of overt Cushing’s syndrome if present in a thorough clinical evaluation should prompt the physician to proceed with the recommended diagnostic approach described in the relevant Endocrine Society’s Clinical Guidelines (84). In this case, as discussed earlier, the validity of the term “incidentaloma” is debated.
In the absence of overt disease, biochemical investigation frequently reveals subtle cortisol hypersecretion and abnormalities of the HPA axis, a state previously termed as subclinical Cushing’s syndrome (6). Based on the most recent clinical practice guidelines by the European Society of Endocrinology (ESE) and European Network for the Study of Adrenal Tumors (ENSAT) the term “autonomous cortisol secretion” (ACS) is preferred and will also be used throughout this chapter. Although ACS is poorly defined, and its natural history is largely unknown (3), the prevalence of hypertension, diabetes, obesity, other features of the metabolic syndrome, and osteoporosis has been found to be increased in such patients (5,85). Because standard biochemical tests used to screen for Cushing’s syndrome were not designed to reveal the subtle changes encountered in ACS, and since a definitive clinical phenotype to ascertain the presence of this condition is missing, a combination of various parameters used to assess the integrity of the HPA axis have been employed. Alterations of the HPA axis suggestive of ACS in AIs include altered dexamethasone suppression (DST) and response to CRH, increased mean serum cortisol and urinary free cortisol (UFC) levels and reduced ACTH levels (31), although the latter has recently been questioned (86). Reduced dehydroepiandrosterone sulfate (DHEA-S) is considered a less reliable marker since it normally decreases with age and creates diagnostic problems in the elder AI patients, whereas the incorporation of midnight salivary cortisol as a means to diagnose ACS has produced inconsistent results (87,88). Recently, a study utilizing gas chromatography-tandem mass spectrometry (GC-MS/MS) to measure serum levels of several steroids in patients with ACS, non-functioning AIs and controls showed that decreased levels of adrenal androgens, their metabolites, and pregnenolone metabolites displayed sensitivity and specificity that were comparable to that of routine methods in selecting patients with ACS (89). Currently though, the 1 mg overnight DST, remains the most reliable and easily reproducible method and is the recommended test to detect cortisol secretion abnormalities based on pathophysiological reasoning, simplicity and the fact that it was incorporated in the diagnostic algorithms of most studies. (5,90).
Different cortisol cut-off values following the 1 mg DST have been advocated from different authors and were adopted by several authorities, ranging from 50 to 138 nmol/l (1.8 to 5 μg/dl) (57,91). Higher thresholds increase the specificity of the test but lower its sensitivity (92). It is also important to consider drugs or conditions that interfere with this test by altering dexamethasone absorption, metabolism by CYP3A4, or falsely elevate cortisol levels through increased cortisol-binding globulin (CBG) levels (93). The post 1 mg DST cortisol cutoff of >5 μg/dl (138 nmol/l) approach was substantiated by studies showing that all patients with such a cortisol value had uptake only on the side of the adenoma on adrenal scintigraphy (94). On the other hand, studies that used post-surgical hypoadrenalism as indicative of autonomous cortisol secretion suggested that lower cortisol cut-offs may be needed to identify these cases (95–97). Although two or more abnormal tests are usually required to establish the diagnosis of ACS (57), the 1 mg DST should be the initial screening test based on pathophysiological reasoning and the fact that it represents the most common HPA axis abnormality described in the majority of studies (42). The formal low dose dexamethasone suppression test (LDDST) can be used to confirm and quantify the degree of autonomous cortisol secretion or to exclude a false positive test (98,99). A negative DST using a cortisol cut-off value of 1.8 μg/dl (50 nmol/l) virtually excludes ACS. Furthermore, a number of studies have found that patients with post DST cortisol values >1.8 μg/dl (50 nmol/l) have increased morbidity or mortality (100,101). A value of >5 μg/dl (138 nmol/l) on the other hand, is highly suggestive of the presence of ACS (5). In the case of a positive test with intermediate cortisol values (1.8-5 μg/dl) the term “possible ACS” is proposed by recent guidelines (90) and consideration of further parameters, such as the presence of other abnormalities of the HPA axis and/or comorbidities, employment of a higher cortisol cut-off level, and re-testing after 3-6 months, have been suggested (102). In our opinion, the post-LDDST cortisol value should be considered in patients with such intermediate cortisol values following the 1 mg DST because, in addition to its high specificity, it correlates well with other indices of cortisol excess and the size of the adenoma, thus providing a quantitative measure of the degree of cortisol production from the adenoma and a more robust means for further follow-up (98,103).
SCREENING FOR PHEOCHROMOCYTOMA
Because catecholamine secretion can be intermittent, and there are cases of “silent” PCCs, screening should be performed even in normotensive patients with AIs in order to prevent the morbidity and mortality that may accompany this tumor (104). The initial recommended biochemical screening test is measurement of plasma free (from blood drawn in the supine position) or urinary fractionated metanephrines using liquid chromatography with mass spectrometric or electrochemical detection methods (35). This approach has a sensitivity and specificity of 99% and 97% respectively and has proven to be superior to measurement of plasma or urine catecholamines and vanillylmandelic acid (VMA) (105). Some studies have suggested higher specificity of the plasma than the urine test albeit without head-to-head comparisons using mass spectrometric-based methods. Thus, until data directly comparing plasma and urinary measurements are produced, urinary free fractionated metanephrines can be used as an alternative, if plasma free metanephrines measurement is not available (106). Sane et al suggested that routine biochemical screening for PCC in small (<2cm) homogenous AIs characterized by attenuation values <10 HU may not be necessary, since none of the 115 patients in his cohort with lipid-rich tumors (<10 HU) had constantly elevated 24-hour urinary metanephrines or normetanephrines, whereas all 10 histologically proven PCCs were larger than 2cm and were characterized by >10 HU in unenhanced CT scans (107). This was also confirmed from a recent multicenter retrospective study including 376 PCCs with sufficient data from CT imaging. Based on the lack of PCCs with an unenhanced attenuation of <10 HU and the low proportion (0.5%, 2/376) of PCCs with an attenuation of 10 HU, it was suggested that abstaining from biochemical testing for PCC in AIs with an unenhanced attenuation of ≤10 HU is reasonable, whereas contrast washout measurements were unreliable for ruling out PCC (108).
A recent study (109) comparing the clinical, hormonal, histological and molecular features of normotensive incidentally discovered PCCs (previously referred as “silent”) with tumors causing overt symptoms, revealed lower diagnostic sensitivity (75%) for plasma and urinary metanephrines irrespective of tumor size, while genetic and histological studies showed decreased expression of genes and proteins associated with catecholamine production and increased cellularity and mitotic activity in “silent” tumors. It was implied that asymptomatic incidentally discovered PCCs do not represent an early stage of development of PCCs but rather correspond to a distinct entity characterized by cellular defects in chromaffin machinery resulting in lower efficiency to produce or release catecholamines. It is, therefore, crucial to consider that normotensive patients with an AI and normal values of metanephrines, may indeed harbor a PCC. In such instance, the CT and MRI scan features of the tumor if suspicious for PCC, should alert the clinician to perform complementary investigations, such as plasma chromogranin A measurement, MIBG scintigraphy, 18F-FDG-PET/CT, or other alternative functional imaging (F-DOPA/PET or FDA/PET) to rule out this possibility.
SCREENING FOR ALDOSTERONE EXCESS
According to published guidelines from the Endocrine Society, all patients with an AI and hypertension, irrespective of serum potassium levels, should be tested for PA using the plasma aldosterone/renin ratio (ARR) as a screening test (37). However, the knowledge that PA can be diagnosed in normotensive patients with hypokalemia necessitates testing of all patients with hypertension or hypokalemia (39). Although there is no current consensus regarding the most diagnostic ARR cut-off, values >20-40 (plasma aldosterone expressed as ng/dl and plasma renin activity [PRA] as ng/ml/h) obtained in the morning from a seated patient are highly suggestive. However, the plasma aldosterone level also needs to be considered because extremely low PRA, even in the presence of normal aldosterone levels, will result in a high ARR; an aldosterone level less than 9 ng/dl makes the diagnosis of PA unlikely, whereas a level in excess of 15 ng/dl is suggestive (42). Attention should also be given to certain technical aspects required for the correct interpretation of the ARR such as unrestricted dietary salt intake, corrected potassium levels, and washout of interfering antihypertensive medication. Patients may be treated with a non-dihydropyridine calcium channel blocker (verapamil slow release) as a single agent or in combination with α-adrenergic blockers (such as doxazosin) and hydralazine for blood pressure control during the washout period, if needed. When suspected based on the ARR, PA should be verified with one of the commonly used confirmatory tests (oral sodium loading, saline infusion, fludrocortisone suppression, and captopril challenge). Admittedly, the extent that patients with AI should be investigated to exclude PA is still not known. Although PA has been reported with a low prevalence between patients with AIs (1-10%), substantially higher rates (24%) have recently been described using a recumbent post-low dose dexamethasone suppression (LDDST)-saline infusion test (PD-SIT) (40). Further studies evaluating the optimal biochemical diagnostic approach of PA in patients with AIs are required by comparing established versus evolving investigational protocols.
SCREENING FOR ANDROGEN/ESTROGEN EXCESS
Measurement of sex hormones is not recommended in patients with an AI on a routine basis (57). Elevated levels of serum DHEA-S, androstenedione, 17-OH progesterone as well as testosterone in women and estradiol in men and postmenopausal women can be found in more than half of patients with ACCs (110). Although cases of androgen or estrogen excess have been rarely described in patients with benign adrenocortical adenomas (111–114), they are usually accompanied by symptoms or signs of virilization in women (acne, hirsutism) or feminization in men (gynecomastia), and therefore such lesions cannot be considered as true AIs. Thus, the usefulness of measuring sex hormones and steroid precursors is limited in cases of adrenal lesions with indeterminate or suspicious for malignancy imaging characteristics, where elevated levels can point towards the adrenocortical origin of the tumor and suggest the presence of an ACC rather than a metastatic lesion. Additionally, increased basal or after consytropin stimulation levels of 17-OH progesterone can also indicate CAH in patients with bilateral AIs (6).
SCREENING FOR HYPOADRENALISM
Bilateral AIs caused by metastases of extra-adrenal malignancies or infiltrative diseases can rarely cause adrenal insufficiency (115). Therefore, in all patients with bilateral adrenal masses, adrenal insufficiency should be considered and evaluated clinically and if likely, diagnosis should be established using the standard 250μg consytropin stimulation test according to the Endocrine Society’s recently published clinical guidelines (116).
Fine-Needle Aspiration Biopsy (FNAB)
The use of percutaneous fine-needle aspiration biopsy (FNAB) as a mean to clarify the nature of an AI has now been surpassed by the non-invasive radiological methods because they have better diagnostic accuracy and are devoid of potential side effects (117,118). It should be noted that FNAB is not considered an accurate method of differentiating benign from malignant primary adrenal tumors but can be helpful in the diagnosis of metastases from extra-adrenal malignancies with a sensitivity of 73-100% and a specificity of 86-100% using variable population inclusion criteria, reference standards, and biopsy techniques (119–121). Therefore, in patients with suspicion of a rare tumor or a history of an underlying extra-adrenal malignancy and/or inconclusive imaging features of an AI (non-contrast CT attenuation value >10 HU and an RPW<40%), FNAB could be performed, but only if management would be altered by the histologic findings. FNAB has significant procedural risk with complications such as pneumothorax, bleeding, infection, pancreatitis, and dissemination of tumor cells along the needle track reported at a rate up to 14% by some, but not all available studies (117). To avoid the risk of a potentially lethal hypertensive crisis, PCC should always be excluded biochemically before FNA of an adrenal mass is attempted (122).
NATURAL HISTORY OF AIs
Since AIs do not represent a single clinical entity, their natural history varies depending on the underlying etiology. Primary malignant adrenal tumors typically display rapid growth (>2 cm/year) and a poor outcome with an overall 5-year survival of 47%. It is not known whether prognosis of patients with incidentally discovered ACC is different from symptomatic cases, however detection of the tumor at an early stage provides the possibility of definitive surgical cure (123). Patients with adrenal metastases have a clinical course depending on stage, grade, and site of the primary tumor (4). PCCs grow slowly and are mostly benign, but if untreated are potentially lethal displaying high cardiovascular mortality and morbidity, whereas 10-17% of the cases can be malignant (35). This is further emphasized by the fact that PCCs detected in autopsy series had not been suspected in 75% of the patients while they were alive, although they contributed to their death in approximately 55% of cases (124).
In benign adrenal tumors, which constitute the majority of AIs, the major concerns about their natural history revolve around their progressive growth, the possibility of malignant transformation, and the risk of evolution towards overt hypersecretion. Several cohort studies, despite their limitations, have shown that the majority of benign tumors remain stable in size; only 5-20% show a >1 cm increase in size, mostly within the first three years after prolonged follow-up (125,126), whereas occasional shrinkage, or even complete disappearance, of an adrenal mass have also been reported in about 4% of cases (8,127). Although there is not as yet a specific growth rate cut-off indicative of a benign nature, ACCs initially presenting as AIs, are invariably characterized by a rapid growth within months (at least > 0.8cm/year). The risk of an AI initially considered to be benign to become malignant has been estimated at <1/1000 (3,8) by Cawood et al, who found only two reports of a malignancy detected during the follow-up of AIs presenting as benign at diagnosis; the first was a renal carcinoma metastasis in a patient with a known history of renal carcinoma and the other was a non-Hodgkin’s lymphoma that showed a mass enlargement after 6 months (3). Two case reports of patients with a well-documented history of adrenal incidentalomas with totally benign imaging features on CT, who were diagnosed on follow-up (8 and 14 years later) with a malignant tumor in the same adrenal gland have recently been described (128,129). It is not known whether these cases can be explained by the independent occurrence of two events in a single adrenal (initially a typical benign adenoma and consequently the occurrence of an ACC) or whether a malignant transformation of a benign adenoma to carcinoma was the underlying course of events. Although there are some evidence to suggest the adenoma-carcinoma sequence is possible in the adrenal cortex (130,131), the high prevalence of adenomas contrasting with the extremely low prevalence of ACCs suggest that this process is probably exceptionally rare. These findings highlight the low risk of malignant transformation of AIs and the adequacy of current imaging to ascertain the diagnosis at presentation deterring the need for long-term imaging follow-up.
The appearance of hormonal hypersecretion over time in initially non-functioning AIs varies in different series. New-onset catecholamine or aldosterone overproduction is extremely rare (<0.3%), whereas development of overt hypercortisolism during follow-up is found in <1% (8). The most common disorder observed during follow-up is the occurrence of autonomous cortisol secretion eventually leading to ACS, reported with a frequency of up to 10% (127). This risk is higher for lesions >3 cm in size and during the first 2 years of follow-up but seems to plateau after 3-4 years, even if it does not subside completely (132). On the other hand, subtle hormonal alterations discovered at initial screening may also improve over time, indicating possible cyclical cortisol secretion from AIs and/or highlighting the inherent difficulty in biochemical confirmation of this condition (126).
Another issue of debate regarding the natural history of AIs that has attracted research, producing frequently conflicting data, is the sequelae of ACS on cardiovascular risk and subsequent mortality and morbidity. Several cross-sectional and cohort studies have reported a clustering of unfavorable cardiovascular risk factors in patients with AIs similar to those found in patients with overt Cushing’s syndrome (133,134). It is biologically plausible to anticipate that the presence of even mild to minimal cortisol excess may lead to some extent to the classic long-term consequences of overt hypercortisolism, such as hypertension, obesity, impaired glucose tolerance or frank diabetes, dyslipidemia and osteoporosis (figure 4). Because these metabolic derangements are common in the general and particularly the elderly population, in whom AIs are more frequently found, it is difficult to extrapolate whether there is a causal relationship between them. Whether these metabolic abnormalities in patients with AIs result in increased cardiovascular mortality and morbidity has not as yet been fully clarified. Although, some recent retrospective studies (100,101,135) have shown higher rates of cardiovascular events and mortality in patients with higher cortisol levels after the 1 mg DST, data from patients who underwent adrenalectomy are contradictory, regarding the outcome on metabolic and cardiovascular profile, whereas there are relatively few data on the risk of major cardiovascular events or mortality (97,136–138). Similarly, evidence on the detrimental effects of ACS on bone metabolism, such as lower bone density and high prevalence of vertebral fractures (43-72%) in postmenopausal women and eugonadal male patients with AIs (85,139–142) are conflicting with studies not showing reversal of these effects following surgical treatment (136,143). Additionally most of the detected vertebral fractures were minor and of uncertain clinical impact (85).
Moreover, there is growing evidence that even non-functioning AIs may be associated with similar metabolic disturbances and manifestations of the metabolic syndrome that are considered cardiovascular risk factors (144–146). Compared with controls, patients with non-functioning AIs exhibit subtle indices of atherosclerosis such as increased carotid intima-media thickness (IMT)(147), impaired flow-mediated vasodilatation (FMD) (148) and left ventricular hypertrophy (149). A recent study excluding patients with traditional risk factors (diabetes, hypertension or dyslipidemia) reported similar findings in patients harboring non-functioning AIs, with increased insulin resistance and endothelial dysfunction that correlated with subtle but not autonomous cortisol excess (150). Furthermore, an observational study suggested that patients with non-functioning AIs had a significantly higher risk of developing diabetes compared with control subjects without adrenal tumours prompting a re-assessment of whether the classification of benign adrenal tumors as “non-functional” adequately reflects the continuum of hormone secretion and metabolic risk they may harbor (151).
A recent meta-analysis (152) of 32 studies including patients with non-functioning AIs and adrenal tumors associated with ACS provided important insights on the natural history of such tumors that help in solving controversy and informing practice. First and foremost, it was observed that only a small proportion of patients with non-functioning AI or ACS had tumor growth or changes in hormone production during follow-up. Only 2.5% of adrenal incidentalomas grew by 10 mm or more over a mean follow-up of 41.5 months, whereas the mean difference in adenoma size between follow-up and baseline in all patients was negligible at 2.0 mm. Larger adenomas at diagnosis (≥25 mm) were even less likely than smaller tumors to grow during follow-up, which, according to the authors, suggests attainment of maximum growth potential. More importantly malignant transformation was never observed at the end of follow-up. Similarly, in patients with non-functioning AIs or ACS at diagnosis, the risk of developing clinically overt hormonal hypersecretion syndromes (Cushing’s, PA or catecholamine excess) was negligible (<0,1%), suggesting that these rare cases are probably attributed to the development of subsequent adrenal tumors and that ACS does not represent a preliminary stage of overt Cushing’s. Inapparent cortisol autonomy ensued only in 4,3% of patients with initially nonfunctioning tumours. The third and most novel finding of this thorough meta-analysis pertained to comorbidities, cardiovascular risk and mortality. It was confirmed, like in other similar studies, that patients with ACS had a high prevalence of cardiovascular risk factors (such as hypertension, obesity, dyslipidemia, and type 2 diabetes) and were more likely than those with non-functioning AIs to develop or show worsening of these factors during follow-up. However, the prevalence of such factors in patients with non-functioning AIs was also significant and higher than expected for Western populations. This finding could be explained by subtle degree of glucocorticoid excess not detected by current diagnostic criteria or perhaps by cyclical cortisol secretion or even by excess cortisol secretion in response to stress situations. It could also represent ascertainment bias since patients with diseases are more likely to have imaging tests that may detect an AI or could be a result of the previously theorized reverse causality concept that diabetes or the metabolic syndrome promote adrenal tumor development (153). Interestingly, reported all-cause and cardiovascular mortality in patients with non-functioning AI during follow-up were similar to those in patients with ACS, warranting close clinical follow-up and treatment for both groups of patients.
MANAGEMENT
Management of AIs is currently a debatable work in progress. Although the majority of AIs comprising of benign adenomas without evidence of hormone excess should not pose any compelling challenges, the few cases with equivocal imaging features, subtle hormone hypersecretion, or unusual evolution (i.e. significant tumor growth) should be ideally discussed in a multidisciplinary expert team meeting (90).
All published guidelines and expert reviews agree that patients with unilateral adrenal masses causing unambiguous hormonal overactivity, and those with suspected malignancy (mainly ACC), are candidates for surgical interventions (5,6,35,37,57,90,91,154,155). There is also broad consensus that the majority of AIs with clearly benign imaging phenotype in unenhanced CT and no relevant clinical activity do not require surgery. However, some authors have also advocated considering size as an indication for surgery. The 2002 NIH state-of-the-science report recommended surgical excision of all AIs greater than 6 cm and to use clinical judgment, based on the results of the initial or follow-up evaluations, when assessing masses between 4 and 6 cm for surgery (4). Considering the high prevalence of ACC in tumors >4cm, some have proposed lowering the size cut-off to 4 cm (156). Despite the paucity of data regarding the natural history of such large tumors, an attenuation value of ≤10 HU in unenhanced CT combined with washout properties consistent with a benign tumor and absence of significant growth over time, can be reassuring. Non-functioning lesions <4cm with indeterminate imaging features on unenhanced CT should be investigated further with contrast-washout studies, MRI-CSI, or 18F-FDG-PET/CT. If uncertainty remains, immediate surgery or repeat imaging after 3-6 months could be offered. It would also be prudent to exclude the possibility of a “silent” PCC in patients with an indeterminate lesion, before proceeding to surgery because hemodynamic instability during surgical excision may ensue.
The management of patients harboring AIs who have ACS is debatable and the beneficial effect of adrenalectomy has not been proven adequately in the literature. Some, but not all, predominantly retrospective studies have shown a beneficial effect in hypertension and diabetes mellitus in patients with AIs who underwent an adrenalectomy, compared to those who did not undergo such a procedure (97,136,138). In one prospective study with an 8-year follow-up, operated patients with ACS had an improvement in features of the metabolic syndrome, but not of osteoporosis, compared to those who were conservatively managed; however, no control group was included in the study (136). In a recent retrospective study, an improvement of blood pressure and blood glucose was noted in adrenalectomized patients with ACS, whereas these indices worsened in non-operated patients; even so, some patients apparently with non-functioning AI also showed an improvement in some of these parameters (97). Until results from randomized prospective trials, reporting outcome on metabolic and bone comorbidities as well as overall mortality and major cardiovascular events, become available, adrenalectomy should be considered on an individual basis. Since improvement of comorbidities and clinically relevant endpoints with adrenalectomy is not yet definitively proven, several other factors that are also linked to surgical outcome, such as patient’s age, duration and evolution of comorbidities and their degree of control, and presence and extent of end organ damage, should also be considered. Young patients with ACS and those with new onset and/or rapidly worsening comorbidities resistant to medical treatment (6,157) could thus be candidates for surgical intervention. A proposed algorithm for diagnostic approach and management of AIs is presented in Figure 4.
Before proceeding to surgical therapy, appropriate medical therapy must be given to all functioning lesions, aiming at symptom control. Apart from patients with Cushing’s syndrome, post-surgical adrenal insufficiency may ensue in ACS patients (158,159). Because the need for glucocorticoid coverage cannot be predicted before surgery, patients should be covered by steroids post-operatively until the HPA-axis can be formally assessed (95). Low morning cortisol levels the day after surgery, and before glucocorticoid replacement, provide evidence for post-surgical hypoadrenalism (97). All patients diagnosed with PCC, including normotensive patients with “silent” tumors should receive preoperative α-adrenergic blockade for 7 to 14 days to prevent perioperative cardiovascular complications. Treatment should also include a high-sodium diet and fluid intake to reverse catecholamine-induced blood volume contraction preoperatively and prevent severe hypotension after tumor removal (35). Finally, patients diagnosed with PA and bilateral tumors or a unilateral AI (if older than 40 years of age) who seek a potential surgical cure, should be considered for adrenal venous sampling (AVS) before proceeding to surgery, to confirm lateralization of the source of the excessive aldosterone secretion.
According to AACE/AAES Medical Guidelines for the management of adrenal incidentalomas, patients with AIs not elected for surgery after the initial diagnostic work-up, should undergo re-imaging 3-6 months after the initial diagnosis and then annually for the next 1-2 years, while annual biochemical testing is advised for up to 4-5 years following the diagnosis (57). As the natural history of AIs remains largely unknown, a widely accepted follow-up imaging and hormonal protocol has not been formulated yet. It has recently been suggested by some authors that given the low probability of the transformation of a benign and non-functioning adrenal mass to a malignant or functioning one, the routine application of the current strategies in all patients with AIs is likely to result in a number of unnecessary biochemical and radiological investigations (3,160,161). Such an approach is costly, and it does not take into account harmful consequences of diagnostic evaluation such as patients’ anxiety associated with repeated clinical visits and a high rate of false positive results leading to further testing or unnecessary adrenalectomy. Moreover, exposure to ionizing radiation from repeated CT scans increases the future cancer risk to the level that is similar to the risk of the adrenal lesion becoming malignant (3,162).
Based on available data, it is safe to conclude that lesions <2 cm in size, and with an attenuation value <10 HU, have the lowest possibility of growth and thus long-term imaging follow-up is probably unnecessary. For larger tumors despite the high sensitivity and adequate specificity of unenhanced CT for identifying adenomas, the lack of prospective studies precludes suggesting stringent recommendations regarding optimal radiological follow-up (5). It is our practice for large lesions, particularly those >4 cm with attenuation values <10 HU, to repeat a CT scan after 6-12 months and if there is no increase in size and the imaging features remain unaltered, to defer further radiological follow-up. It is thought that a one-time follow-up scan in 6-12 months may be reassuring to the physician and the patient (42). An increase of >20% of the largest tumor diameter together with an at least 5 mm increase in this diameter (90), or an absolute increase by >8 mm over 12 months (59), probably warrant further follow-up and re-evaluation of radiological features.
The appropriate hormonal follow-up of patients not elected for surgery is also not established. Patients without any biochemical abnormalities at presentation could be spared the burden of repeated testing, since the risk of developing clinically overt hormonal excess is extremely low. Clinical follow-up with assessment of cardiovascular risk factors that have been associated with the presence of AIs may be adequate to detect the reported ~10% of the cases of new-onset ACS (5). Patients with worsening of their metabolic parameters should be retested with the 1mg DST and be advised to apply lifestyle changes and effective medical treatment to reduce cardiovascular risk. If biochemical abnormalities suggesting ACS are present during the initial screening, annual clinical follow-up including evaluation of potentially cortisol excess-related comorbidities, as well as periodic testing of the HPA axis, is advisable. Patients with ACS who do not reach the treatment goals despite an adequate medical therapy could be offered surgery. Duration of follow-up is also under debate, however based on available data, annual hormonal evaluation may be suggested for up to five years, and especially for lesions >3 cm (57).
CONCLUSION
AIs are increasingly being recognized, particularly in the aging population. Adrenal CT and MRI can reliably distinguish benign lesions, while 18F-FDG-PET/CT scan can be helpful in identifying tumors with malignant potential. ACS is the most common hyperfunctional state that is best substantiated using the 1 mg DST; urinary/plasma metanephrines and aldosterone/renin ratio are used to screen for PCCs and hyperaldosteronism. Adrenal lesions with suspicious radiological findings, PCCs, and tumors causing overt clinical syndromes, as well as those with considerable growth during follow-up, should be treated with surgical resection. Although there is no consensus, the interval for diagnostic follow-up testing relies on the radiological and hormonal features of the tumors at presentation. The benefit of surgical resection in patients with substantial comorbidities and associated subclinical adrenal hyperfunction, mainly in the form of ACS, is still under investigation.
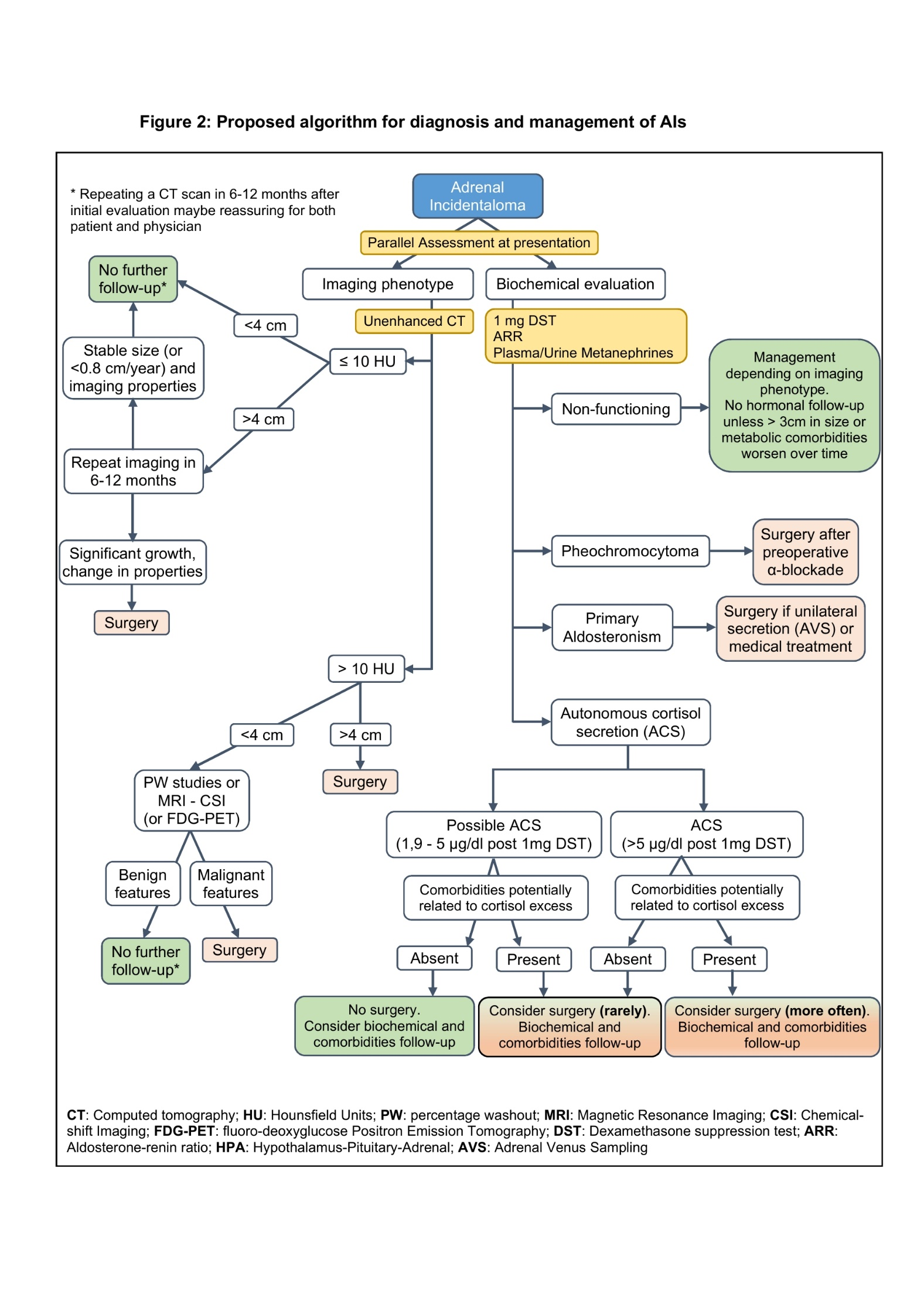
Figure 4. Proposed Algorithm for Diagnosis and Management of AIs
REFERENCES
- Korobkin M, White E, Kressel H, Moss A, Montagne J. Computed tomography in the diagnosis of adrenal disease. Am. J. Roentgenol. 1979;132(2):231–238.
- Vassiliadi D a, Tsagarakis S. Endocrine incidentalomas--challenges imposed by incidentally discovered lesions. Nat. Rev. Endocrinol. 2011;7(11):668–80.
- Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur. J. Endocrinol. 2009;161(4):513–527.
- Grumbach MM, Biller BMK, Braunstein GD, Campbell KK, Aidan Carney J, Godley PA, Harris EL, Lee JKT, Oertel YC, Posner MC, Schlechte JA, Wieand S, Marciel K, Carney JA, Godley PA, Harris EL, Lee JKT, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically inapparent adrenal mass (“incidentaloma”). In: Annals of Internal Medicine.Vol 138.; 2003:424–429.
- Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, Reimondo G, Pia A, Toscano V, Zini M, Borretta G, Papini E, Garofalo P, Allolio B, Dupas B, Mantero F, Tabarin A. AME position statement on adrenal incidentaloma. Eur. J. Endocrinol. 2011;164(6):851–870.
- Young WF. The Incidentally Discovered Adrenal Mass. N. Engl. J. Med. 2007;356(6):601–610.
- Nawar R. Adrenal incidentalomas -- a continuing management dilemma. Endocr. Relat. Cancer 2005;12(3):585–598.
- Barzon L, Sonino N, Fallo F, Palù G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur. J. Endocrinol. 2003;149(4):273–285.
- Rineheart JF WO and CW. Adenomatous hyperplasia of the adrenal cortex associated with essential hypertension. Arch. Pathol. 1941;(34):1031–1034.
- RUSSI S, BLUMENTHAL HT, GRAY SH. Small adenomas of the adrenal cortex in hypertension and diabetes. Arch. Intern. Med. (Chic). 1945;76:284–91.
- Abecassis M, McLoughlin MJ, Langer B, Kudlow JE. Serendipitous adrenal masses: Prevalence, significance, and management. Am. J. Surg. 1985;149(6):783–788.
- Meagher AP, Hugh TB, Casey JH, Chisholm DJ, Farrell JC, Yeates M. Primary adrenal tumours--a ten-year experience. Aust. N. Z. J. Surg. 1988;58(6):457–62.
- Reinhard C, Saeger W, Schubert B. Adrenocortical nodules in post-mortem series. Development, functional significance, and differentiation from adenomas. Gen. Diagn. Pathol. 1996;141(3–4):203–8.
- COMMONS RR, CALLAWAY CP. Adenomas of the adrenal cortex. Arch. Intern. Med. (Chic). 1948;81(1):37–41.
- Schroeder H. Clinical types - the endocrine hypertensive syndrome. In: Schroeder H, ed. Hypertensive Diseases: Causes and Control. Philadelphia: Lea & Febiger; 1953:295–333.
- Dévényi I. Possibility of normokalaemic primary aldosteronism as reflected in the frequency of adrenal cortical adenomas. J. Clin. Pathol. 1967;20(1):49 LP – 51.
- Kokko JP, Brown T, Berman M. ADRENAL ADENOMA AND HYPERTENSION. Lancet 1967;289(7488):468–470.
- Hedeland H, Östberg G, Hökfelt B. ON THE PREVALENCE OF ADRENOCORTICAL ADENOMAS IN AN AUTOPSY MATERIAL IN RELATION TO HYPERTENSION AND DIABETES. Acta Med. Scand. 1968;184(1‐6):211–214.
- Yamada EY, Fukunaga FH. Adrenal adenoma and hypertension. A study in the Japanese in Hawaii. Jpn. Heart J. 1969;10(1):11–9.
- Granger P, Genest J. Autopsy study of adrenals in unselected normotensive and hypertensive patients. Can. Med. Assoc. J. 1970;103(1):34–6.
- Russell RP, Masi AT, Richter ED. Adrenal cortical adenomas and hypertension. A clinical pathologic analysis of 690 cases with matched controls and a review of the literature. Medicine (Baltimore). 1972;51(3):211–25.
- Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally Discovered Adrenal Masses*. Endocr. Rev. 1995;16(4):460–484.
- Masumori N, Adachi H, Noda Y, Tsukamoto T. Detection of adrenal and retroperitoneal masses in a general health examination system. Urology 1998;52(4):572–576.
- Glazer HS, Weyman PJ, Sagel SS, Levitt RG, McClennan BL. Nonfunctioning adrenal masses: incidental discovery on computed tomography. AJR. Am. J. Roentgenol. 1982;139(1):81–5.
- Prinz RA, Brooks MH, Churchill R, Graner JL, Lawrence AM, Paloyan E, Sparagana M. Incidental asymptomatic adrenal masses detected by computed tomographic scanning. Is operation required? JAMA 1982;248(6):701–4.
- Belldegrun A, Hussain S, Seltzer SE, Loughlin KR, Gittes RF, Richie JP. Incidentally discovered mass of the adrenal gland. Surg. Gynecol. Obstet. 1986;163(3):203–8.
- Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery 1991;110(6):1014–21.
- Caplan RH, Strutt PJ, Wickus GG. Subclinical Hormone Secretion by Incidentally Discovered Adrenal Masses. Arch. Surg. 1994;129(3):291.
- Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti G V., Angeli A, Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Invest. 2006;29(4):298–302.
- Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: Prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. Am. J. Roentgenol. 2008;190(5):1163–1168.
- Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, Giovagnetti M, Opocher G, Angeli A. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 2000;85(2):637–644.
- Mayer S., Oligny L., Deal C, Yazbeck S, Gagné N, Blanchard H. Childhood adrenocortical tumors: Case series and reevaluation of prognosis—A 24-year experience. J. Pediatr. Surg. 1997;32(6):911–915.
- Angeli A, Osella G, Alì A, Terzolo M. Adrenal incidentaloma: an overview of clinical and epidemiological data from the National Italian Study Group. Horm. Res. 1997;47(4–6):279–83.
- Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26(1):69–82.
- Lenders JWM, Duh Q-Y, Eisenhofer G, Gimenez-Roqueplo A-P, Grebe SKG, Murad MH, Naruse M, Pacak K, Young WF. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014;99(6):1915–1942.
- Kopetschke R, Slisko M, Kilisli A, Tuschy U, Wallaschofski H, Fassnacht M, Ventz M, Beuschlein F, Reincke M, Reisch N, Quinkler M. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur. J. Endocrinol. 2009;161(2):355–61.
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016;101(5):1889–1916.
- Piaditis G, Markou A, Papanastasiou L, Androulakis II, Kaltsas G. Progress in aldosteronism: A review of the prevalence of primary aldosteronism in pre-hypertension and hypertension. Eur. J. Endocrinol. 2015;172(5):R191–R203.
- Médeau V, Moreau F, Trinquart L, Clemessy M, Wémeau J-L, Vantyghem MC, Plouin P-F, Reznik Y. Clinical and biochemical characteristics of normotensive patients with primary aldosteronism: a comparison with hypertensive cases. Clin. Endocrinol. (Oxf). 2008;69(1):20–28.
- Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, Dimitriou K, Ragkou D, Markou A, Vamvakidis K, Zografos G, Chrousos G. High prevalence of autonomous cortisol and aldosterone secretion from adrenal adenomas. Clin. Endocrinol. (Oxf). 2009;71(6):772–778.
- Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The Clinically Inapparent Adrenal Mass: Update in Diagnosis and Management. Endocr. Rev. 2004;25(2):309–340.
- Zeiger MA, Siegelman SS, Hamrahian AH. Medical and surgical evaluation and treatment of adrenal incidentalomas. J. Clin. Endocrinol. Metab. 2011;96(7):2004–2015.
- Fallo F, Barzon L, Boscaro M, Sonino N. Coexistence of aldosteronoma and contralateral nonfunctioning adrenal adenoma in primary aldosteronism. Am. J. Hypertens. 1997;10(4 Pt 1):476–8.
- Satoh F, Murakami O, Takahashi K, Ueno J, Nishikawa T, Abe K, Mouri T, Sasano H. Double adenomas with different pathological and hormonal features in the left adrenal gland of a patient with Cushing’s syndrome. Clin. Endocrinol. (Oxf). 1997;46(2):227–34.
- Morimoto S, Sasaki S, Moriguchi J, Miki S, Kawa T, Nakamura K, Fujita H, Itoh H, Nakata T, Takeda K, Nakagawa M. Unique association of pheochromocytoma with contralateral nonfunctioning adrenal cortical adenoma. Am. J. Hypertens. 1998;11(1 Pt 1):117–21.
- Chortis V, May CJH, Skordilis K, Ayuk J, Arlt W, Crowley RK. Double trouble: two cases of dual adrenal pathologies in one adrenal mass. Endocrinol. diabetes Metab. case reports 2019;2019. doi:10.1530/EDM-18-0151.
- Dobbie JW. Adrenocortical nodular hyperplasia: The ageing adrenal. J. Pathol. 1969;99(1):1–18.
- Beuschlein F, Reincke M, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S, Chrousos GP, Allolio B. Clonal composition of human adrenocortical neoplasms. Cancer Res. 1994;54(18):4927–32.
- Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuls Y, Luton J-P, Girard F, Bouc Y. Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clin. Endocrinol. (Oxf). 1994;40(4):465–477.
- Pillion DJ, Arnold P, Yang M, Stockard CR, Grizzle WE. Receptors for insulin and insulin-like growth factor-I in the human adrenal gland. Biochem. Biophys. Res. Commun. 1989;165(1):204–11.
- Reincke M, Fassnacht M, Väth S, Mora P, Allolio B. Adrenal incidentalomas: a manifestation of the metabolic syndrome? Endocr. Res. 1996;22(4):757–61.
- Angeli A, Terzolo M. Adrenal incidentaloma--a modern disease with old complications. J. Clin. Endocrinol. Metab. 2002;87(11):4869–71.
- Vassiliadi DA, Tzanela M, Tsatlidis V, Margelou E, Tampourlou M, Mazarakis N, Piaditis G, Tsagarakis S. Abnormal responsiveness to dexamethasone-suppressed CRH test in patients with bilateral adrenal incidentalomas. J. Clin. Endocrinol. Metab. 2015;100(9):3478–3485.
- Bertagna X. Genetics of adrenal diseases in 2014: Genetics improves understanding of adrenocortical tumours. Nat. Rev. Endocrinol. 2014;11(2):77–78.
- Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol. Cell. Endocrinol. 2014;386(1–2):67–84.
- Bonnet-Serrano F, Bertherat J. Genetics of tumors of the adrenal cortex. Endocr. Relat. Cancer 2018;25(3):R131–R152.
- Zeiger M, Thompson G, Duh Q-Y, Hamrahian A, Angelos P, Elaraj D, Fishman E, Kharlip J. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas. Endocr. Pract. 2009;15(Supplement 1):1–20.
- Lau J, Balk E, Rothberg M, Ioannidis JPA, DeVine D, Chew P, Kupelnick B, Miller K. Management of clinically inapparent adrenal mass. Evid. Rep. Technol. Assess. (Summ). 2002;(56):1–5.
- Pantalone K, Gopan T, Remer E, Faiman C, Ioachimescu A, Levin H, Siperstein A, Berber E, Shepardson L, Bravo E, Hamrahian A. Change in Adrenal Mass Size as a Predictor of a Malignant Tumor. Endocr. Pract. 2010;16(4):577–587.
- Kaltsas G, Chrisoulidou A, Piaditis G, Kassi E, Chrousos G. Current status and controversies in adrenal incidentalomas. Trends Endocrinol. Metab. 2012;23(12):602–609.
- Blake MA, Holalkere N-S, Boland GW. Imaging Techniques for Adrenal Lesion Characterization. Radiol. Clin. North Am. 2008;46(1):65–78.
- Dinnes J, Bancos I, di Ruffano LF, Chortis V, Davenport C, Bayliss S, Sahdev A, Guest P, Fassnacht M, Deeks JJ, Arlt W. MANAGEMENT OF ENDOCRINE DISEASE: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur. J. Endocrinol. 2016;175(2):R51–R64.
- Hamrahian AH, Ioachimescu AG, Remer EM, Motta-Ramirez G, Bogabathina H, Levin HS, Reddy S, Gill IS, Siperstein A, Bravo EL. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J. Clin. Endocrinol. Metab. 2005;90(2):871–7.
- Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. Am. J. Roentgenol. 1998;170(3):747–752.
- Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, Raghupathi KI. Adrenal Masses: Characterization with Combined Unenhanced and Delayed Enhanced CT. Radiology 2002;222(3):629–633.
- Motta-Ramirez GA, Remer EM, Herts BR, Gill IS, Hamrahian AH. Comparison of CT Findings in Symptomatic and Incidentally Discovered Pheochromocytomas. Am. J. Roentgenol. 2005;185(3):684–688.
- Chambre C, McMurray E, Baudry C, Lataud M, Guignat L, Gaujoux S, Lahlou N, Guibourdenche J, Tissier F, Sibony M, Dousset B, Bertagna X, Bertherat J, Legmann P, Groussin L. The 10 Hounsfield units unenhanced computed tomography attenuation threshold does not apply to cortisol secreting adrenocortical adenomas.; 2015:325–332.
- Blake MA, Kalra MK, Sweeney AT, Lucey BC, Maher MM, Sahani D V, Halpern EF, Mueller PR, Hahn PF, Boland GW. Distinguishing benign from malignant adrenal masses: multi-detector row CT protocol with 10-minute delay. Radiology 2006;238(2):578–85.
- Wickramarachchi BN, Meyer-Rochow GY, McAnulty K, Conaglen J V., Elston MS. Adherence to adrenal incidentaloma guidelines is influenced by radiology report recommendations. ANZ J. Surg. 2016;86(6):483–486.
- de Haan RR, Schreuder MJ, Pons E, Visser JJ. Adrenal Incidentaloma and Adherence to International Guidelines for Workup Based on a Retrospective Review of the Type of Language Used in the Radiology Report. J. Am. Coll. Radiol. 2019;16(1):50–55.
- Korobkin M, Francis IR, Kloos RT, Dunnick NR. The incidental adrenal mass. Radiol. Clin. North Am. 1996;34(5):1037–54.
- Boland GWL. Adrenal Imaging: Why, When, What, and How? Part 3. The Algorithmic Approach to Definitive Characterization of the Adrenal Incidentaloma. Am. J. Roentgenol. 2011;196(2):W109–W111.
- McDermott S, O’Connor OJ, Cronin CG, Blake MA. Radiological evaluation of adrenal incidentalomas – Current methods and future prospects. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26(1):21–33.
- Israel GM, Korobkin M, Wang C, Hecht EN, Krinsky GA. Comparison of Unenhanced CT and Chemical Shift MRI in Evaluating Lipid-Rich Adrenal Adenomas. Am. J. Roentgenol. 2004;183(1):215–219.
- Elsayes KM, Menias CO, Siegel CL, Narra VR, Kanaan Y, Hussain HK. Magnetic Resonance Characterization of Pheochromocytomas in the Abdomen and Pelvis. J. Comput. Assist. Tomogr. 2010;34(4):548–553.
- Nunes ML, Rault A, Teynie J, Valli N, Guyot M, Gaye D, Belleannee G, Tabarin A. 18F-FDG PET for the Identification of Adrenocortical Carcinomas among Indeterminate Adrenal Tumors at Computed Tomography Scanning. World J. Surg. 2010;34(7):1506–1510.
- Tessonnier L, Sebag F, Palazzo FF, Colavolpe C, De Micco C, Mancini J, Conte-Devolx B, Henry JF, Mundler O, Taïeb D. Does 18F-FDG PET/CT add diagnostic accuracy in incidentally identified non-secreting adrenal tumours?; 2008:2018–2025.
- Boland GWL, Blake MA, Holalkere NS, Hahn PF. PET/CT for the characterization of adrenal masses in patients with cancer: Qualitative versus quantitative accuracy in 150 consecutive patients. Am. J. Roentgenol. 2009;192(4):956–962.
- Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G, Libé R, Bienvenu M, Alberini J-L, Salenave S, Bouchard P, Bertherat J, Dousset B, Legmann P, Richard B, Foehrenbach H, Bertagna X, Tenenbaum F. 18 F-Fluorodeoxyglucose Positron Emission Tomography for the Diagnosis of Adrenocortical Tumors: A Prospective Study in 77 Operated Patients. J. Clin. Endocrinol. Metab. 2009;94(5):1713–1722.
- Sharma P, Singh H, Dhull VVS, Suman KC S, Kumar A, Bal C, Kumar R. Adrenal Masses of Varied Etiology: Anatomical and Molecular Imaging Features on PET-CT. Clin. Nucl. … 2014;00(00):1–10.
- Havekes B, King K, Lai EW, Romijn JA, Corssmit EPM, Pacak K. New imaging approaches to phaeochromocytomas and paragangliomas. Clin. Endocrinol. (Oxf). 2010;72(2):137–45.
- Gross MD, Shapiro B, Francis IR, Glazer GM, Bree RL, Arcomano MA, Schteingart DE, McLeod MK, Sanfield JA, Thompson NW. Scintigraphic evaluation of clinically silent adrenal masses. J. Nucl. Med. 1994;35(7):1145–52.
- Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H, Beuschlein F, Zink M, Lang K, Allolio B, Schirbel A. [123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J. Clin. Endocrinol. Metab. 2008;93(6):2358–65.
- Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM, Edwards H. The diagnosis of Cushing’s syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008;93(5):1526–1540.
- Morelli V, Eller-Vainicher C, Salcuni AS, Coletti F, Iorio L, Muscogiuri G, Della Casa S, Arosio M, Ambrosi B, Beck-Peccoz P, Chiodini I. Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: A multicenter longitudinal study. John Wiley & Sons, Ltd; 2011:1816–1821.
- Olsen H, Kjellbom A, Löndahl M, Lindgren O. Suppressed ACTH Is Frequently Unrelated to Autonomous Cortisol Secretion in Patients With Adrenal Incidentalomas. J. Clin. Endocrinol. Metab. 2019;104(2):506–512.
- Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM, Maffini MA, Scillitani A, Ambrosi B, Beck-Peccoz P, Chiodini I. The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur. J. Endocrinol. 2009;160(1):87–92.
- Bencsik Z, Szabolcs I, Kovács Z, Ferencz A, Vörös A, Kaszás I, Bor K, Gönczi J, Góth M, Kovács L, Dohán O, Szilágyi G. Low dehydroepiandrosterone sulfate (DHEA-S) level is not a good predictor of hormonal activity in nonselected patients with incidentally detected adrenal tumors. J. Clin. Endocrinol. Metab. 1996;81(5):1726–9.
- Hána V, Ježková J, Kosák M, Kršek M, Hána V, Hill M. Novel GC-MS/MS Technique Reveals a Complex Steroid Fingerprint of Subclinical Hypercortisolism in Adrenal Incidentalomas. J. Clin. Endocrinol. Metab. 2019;104(8):3545–3556.
- Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016;175(2):G1–G34.
- Tabarin A, Bardet S, Bertherat J, Dupas B, Chabre O, Hamoir E, Laurent F, Tenenbaum F, Cazalda M, Lefebvre H, Valli N, Rohmer V. Exploration and management of adrenal incidentalomas. Ann. Endocrinol. (Paris). 2008;69(6):487–500.
- Morelli V, Masserini B, Salcuni AS, Eller-Vainicher C, Savoca C, Viti R, Coletti F, Guglielmi G, Battista C, Iorio L, Beck-Peccoz P, Ambrosi B, Arosio M, Scillitani A, Chiodini I. Subclinical Hypercortisolism: correlation between biochemical diagnostic criteria and clinical aspects. Clin. Endocrinol. (Oxf). 2010;73(2):161–6.
- Lopez A-G, Fraissinet F, Lefebvre H, Brunel V, Ziegler F. Pharmacological and analytical interference in hormone assays for diagnosis of adrenal incidentaloma. Ann. Endocrinol. (Paris). 2019;80(4):250–258.
- Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. J. Clin. Endocrinol. Metab. 1999;84(2):520–6.
- Eller-Vainicher C, Morelli V, Salcuni AS, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Carnevale V, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A, Chiodini I. Post-surgical hypocortisolism after removal of an adrenal incidentaloma: is it predictable by an accurate endocrinological work-up before surgery? Eur. J. Endocrinol. 2010;162(1):91–9.
- Eller-Vainicher C, Morelli V, Salcuni AS, Battista C, Torlontano M, Coletti F, Iorio L, Cairoli E, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A, Chiodini I. Accuracy of several parameters of hypothalamic-pituitary-adrenal axis activity in predicting before surgery the metabolic effects of the removal of an adrenal incidentaloma. Eur. J. Endocrinol. 2010;163(6):925–35.
- Chiodini I, Morelli V, Salcuni AS, Eller-Vainicher C, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Pellegrini F, Copetti M, Beck-Peccoz P, Arosio M, Ambrosi B, Trischitta V, Scillitani A. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J. Clin. Endocrinol. Metab. 2010;95(6):2736–2745.
- Tsagarakis S, Vassiliadi D, Thalassinos N. Endogenous subclinical hypercortisolism: Diagnostic uncertainties and clinical implications. J. Endocrinol. Invest. 2006;29(5):471–82.
- Theodoraki A, Khoo B, Hamda A, Schwappach A, Perera S, Vanderpump MP, Bouloux P. Outcomes in 125 Individuals with Adrenal Incidentalomas from a Single Centre. A Retrospective Assessment of the 1 mg Overnight and Low Dose Dexamethasone Suppression Tests. Horm. Metab. Res. 2011;43(13):962–969.
- Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: A 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405.
- Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J. Clin. Endocrinol. Metab. 2014;99(12):4462–4470.
- Stewart PM. Is subclinical Cushing’s syndrome an entity or a statistical fallout from diagnostic testing? Consensus surrounding the diagnosis is required before optimal treatment can be defined. J. Clin. Endocrinol. Metab. 2010;95(6):2618–20.
- Tsagarakis S, Roboti C, Kokkoris P, Vasiliou V, Alevizaki C, Thalassinos N. Elevated post-dexamethasone suppression cortisol concentrations correlate with hormonal alterations of the hypothalamo-pituitary adrenal axis in patients with adrenal incidentalomas. Clin. Endocrinol. (Oxf). 1998;49(2):165–71.
- Grozinsky-Glasberg S, Szalat A, Benbassat CA, Gorshtein A, Weinstein R, Hirsch D, Shraga-Slutzky I, Tsvetov G, Gross DJ, Shimon I. Clinically silent chromaffin-cell tumors: Tumor characteristics and long-term prognosis in patients with incidentally discovered pheochromocytomas. J. Endocrinol. Invest. 2010;33(10):739–44.
- Lenders JWM, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 2002;287(11):1427–34.
- Boyle JG, Davidson DF, Perry CG, Connell JMC. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. J. Clin. Endocrinol. Metab. 2007;92(12):4602–4608.
- Sane T, Schalin-Jäntti C, Raade M. Is biochemical screening for pheochromocytoma in adrenal incidentalomas expressing low unenhanced attenuation on computed tomography necessary? J. Clin. Endocrinol. Metab. 2012;97(6):2077–83.
- Canu L, Van Hemert JAW, Kerstens MN, Hartman RP, Khanna A, Kraljevic I, Kastelan D, Badiu C, Ambroziak U, Tabarin A, Haissaguerre M, Buitenwerf E, Visser A, Mannelli M, Arlt W, Chortis V, Bourdeau I, Gagnon N, Buchy M, Borson-Chazot F, Deutschbein T, Fassnacht M, Hubalewska-Dydejczyk A, Motyka M, Rzepka E, Casey RT, Challis BG, Quinkler M, Vroonen L, Spyroglou A, Beuschlein F, Lamas C, Young WF, Bancos I, Timmers HJLM. CT Characteristics of Pheochromocytoma: Relevance for the Evaluation of Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2019;104(2):312–318.
- Haissaguerre M, Courel M, Caron P, Denost S, Dubessy C, Gosse P, Appavoupoulle V, Belleannée G, Jullié ML, Montero-Hadjadje M, Yon L, Corcuff JB, Fagour C, Mazerolles C, Wagner T, Nunes ML, Anouar Y, Tabarin A. Normotensive incidentally discovered pheochromocytomas display specific biochemical, cellular, and molecular characteristics. J. Clin. Endocrinol. Metab. 2013;98(11):4346–4354.
- Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr. Rev. 2014;35(2):282–326.
- Phornphutkul C, Okubo T, Wu K, Harel Z, Tracy TF, Pinar H, Chen S, Gruppuso PA, Goodwin G. Aromatase p450 expression in a feminizing adrenal adenoma presenting as isosexual precocious puberty. J. Clin. Endocrinol. Metab. 2001;86(2):649–52.
- Goto T, Murakami O, Sato F, Haraguchi M, Yokoyama K, Sasano H. Oestrogen producing adrenocortical adenoma: clinical, biochemical and immunohistochemical studies. Clin. Endocrinol. (Oxf). 1996;45(5):643–8.
- Fukushima A, Okada Y, Tanikawa T, Kawahara C, Misawa H, Kanda K, Morita E, Sasano H, Tanaka Y. Virilizing adrenocortical adenoma with Cushing’s syndrome, thyroid papillary carcinoma and hypergastrinemia in a middle-aged woman. Endocr. J. 2003;50(2):179–87.
- Rodríguez-Gutiérrez R, Bautista-Medina MA, Teniente-Sanchez AE, Zapata-Rivera MA, Montes-Villarreal J. Pure androgen-secreting adrenal adenoma associated with resistant hypertension. Case Rep. Endocrinol. 2013;2013:356086.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet (London, England) 2014;383(9935):2152–67.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016;101(2):364–389.
- Quayle FJ, Spitler JA, Pierce RA, Lairmore TC, Moley JF, Brunt LM. Needle biopsy of incidentally discovered adrenal masses is rarely informative and potentially hazardous. Surgery 2007;142(4):497–502; discussion 502-4.
- Bancos I, Tamhane S, Shah M, Delivanis DA, Alahdab F, Arlt W, Fassnacht M, Murad MH. Diagnosis of endocrine disease: The diagnostic performance of adrenal biopsy: A systematic review and meta-analysis. Eur. J. Endocrinol. 2016;175(2):R65–R80.
- Lumachi F, Borsato S, Tregnaghi A, Marino F, Fassina A, Zucchetta P, Marzola MC, Cecchin D, Bui F, Iacobone M, Favia G. High risk of malignancy in patients with incidentally discovered adrenal masses: Accuracy of adrenal imaging and image-guided fine-needle aspiration cytology. Tumori 2007;93(3):269–274.
- Harisinghani MG, Maher MM, Hahn PF, Gervais DA, Jhaveri K, Varghese J, Mueller PR. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin. Radiol. 2002;57(10):898–901.
- Welch TJ, Sheedy PF, Stephens DH, Johnson CM, Swensen SJ. Percutaneous adrenal biopsy: review of a 10-year experience. Radiology 1994;193(2):341–4.
- Vanderveen KA, Thompson SM, Callstrom MR, Young WF, Grant CS, Farley DR, Richards ML, Thompson GB. Biopsy of pheochromocytomas and paragangliomas: potential for disaster. Surgery 2009;146(6):1158–66.
- Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23(2):273–289.
- Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin. Proc. 1981;56(6):354–60.
- Bülow B, Jansson S, Juhlin C, Steen L, Thorén M, Wahrenberg H, Valdemarsson S, Wängberg B, Ahrén B, __. Adrenal incidentaloma - follow-up results from a Swedish prospective study. Eur. J. Endocrinol. 2006;154(3):419–23.
- Bernini GP, Moretti A, Oriandini C, Bardini M, Taurino C, Salvetti A. Long-term morphological and hormonal follow-up in a single unit on 115 patients with adrenal incidentalomas. Br. J. Cancer 2005;92(6):1104–9.
- Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, Angeli A. Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol. Metab. Clin. North Am. 2005;34(2):423–39, x.
- Belmihoub I, Silvera S, Sibony M, Dousset B, Legmann P, Bertagna X, Bertherat J, Assié G. From benign adrenal incidentaloma to adrenocortical carcinoma: An exceptional random event. Eur. J. Endocrinol. 2017;176(6):K15–K19.
- Rebielak ME, Wolf MR, Jordan R, Oxenberg JC. Adrenocortical carcinoma arising from an adrenal adenoma in a young adult female. J. Surg. Case Reports 2019;2019(7):rjz200.
- Ronchi CL, Sbiera S, Leich E, Henzel K, Rosenwald A, Allolio B, Fassnacht M. Single Nucleotide Polymorphism Array Profiling of Adrenocortical Tumors - Evidence for an Adenoma Carcinoma Sequence? Veitia RA, ed. PLoS One 2013;8(9):e73959.
- Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, René-Corail F, Elarouci N, Sbiera S, Kroiss M, Allolio B, Waldmann J, Quinkler M, Mannelli M, Mantero F, Papathomas T, De Krijger R, Tabarin A, Kerlan V, Baudin E, Tissier F, Dousset B, Groussin L, Amar L, Clauser E, Bertagna X, Ragazzon B, Beuschlein F, Libé R, de Reyniès A, Bertherat J. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014;46(6):607–612.
- Libè R, Dall’Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur. J. Endocrinol. 2002;147(4):489–94.
- Androulakis II, Kaltsas G, Piaditis G, Grossman AB. The clinical significance of adrenal incidentalomas. Eur. J. Clin. Invest. 2011;41(5):552–560.
- Terzolo M, Pia A, Alì A, Osella G, Reimondo G, Bovio S, Daffara F, Procopio M, Paccotti P, Borretta G, Angeli A. Adrenal incidentaloma: a new cause of the metabolic syndrome? J. Clin. Endocrinol. Metab. 2002;87(3):998–1003.
- Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: An Italian multicenter study. J. Clin. Endocrinol. Metab. 2014;99(3):827–834.
- Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann. Surg. 2009;249(3):388–91.
- Sereg M, Szappanos Á, Tőke J, Karlinger K, Feldman K, Kaszper É, Varga I, Gláz E, Rácz K, Tóth M. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: a long-term follow-up study. Eur. J. Endocrinol. 2009;160(4):647–655.
- TSUIKI M, TANABE A, TAKAGI S, NARUSE M, TAKANO K. Cardiovascular Risks and Their Long-Term Clinical Outcome in Patients with Subclinical Cushing’s Syndrome. Endocr. J. 2008;55(4):737–745.
- Chiodini I, Viti R, Coletti F, Guglielmi G, Battista C, Ermetici F, Morelli V, Salcuni A, Carnevale V, Urbano F, Muscarella S, Ambrosi B, Arosio M, Beck-Peccoz P, Scillitani A. Eugonadal male patients with adrenal incidentalomas and subclinical hypercortisolism have increased rate of vertebral fractures. Clin. Endocrinol. (Oxf). 2009;70(2):208–213.
- Tauchmanovà L, Pivonello R, De Martino MC, Rusciano A, De Leo M, Ruosi C, Mainolfi C, Lombardi G, Salvatore M, Colao A. Effects of sex steroids on bone in women with subclinical or overt endogenous hypercortisolism. Eur. J. Endocrinol. 2007;157(3):359–366.
- Chiodini I, Mascia ML, Muscarella S, Battista C, Minisola S, Arosio M, Santini SA, Guglielmi G, Carnevale V, Scillitani A. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann. Intern. Med. 2007;147(8):541–8.
- Chiodini I, Vainicher CE, Morelli V, Palmieri S, Cairoli E, Salcuni AS, Copetti M, Scillitani A. MECHANISMS IN ENDOCRINOLOGY: Endogenous subclinical hypercortisolism and bone: a clinical review. Eur. J. Endocrinol. 2016;175(6):R265–R282.
- Guerrieri M, Campagnacci R, Patrizi A, Romiti C, Arnaldi G, Boscaro M. Primary adrenal hypercortisolism: minimally invasive surgical treatment or medical therapy? A retrospective study with long-term follow-up evaluation. Surg. Endosc. 2010;24(10):2542–2546.
- Peppa M, Boutati E, Koliaki C, Papaefstathiou N, Garoflos E, Economopoulos T, Hadjidakis D, Raptis SA. Insulin resistance and metabolic syndrome in patients with nonfunctioning adrenal incidentalomas: a cause-effect relationship? Metabolism. 2010;59(10):1435–41.
- Garrapa GGM, Pantanetti P, Arnaldi G, Mantero F, Faloia E. Body Composition and Metabolic Features in Women with Adrenal Incidentaloma or Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 2001;86(11):5301–5306.
- Fernández-Real JM, Engel WR, Simó R, Salinas I, Webb SM. Study of glucose tolerance in consecutive patients harbouring incidental adrenal tumours. Study Group of Incidental Adrenal Adenoma. Clin. Endocrinol. (Oxf). 1998;49(1):53–61.
- Yener S, Genc S, Akinci B, Secil M, Demir T, Comlekci A, Ertilav S, Yesil S. Carotid intima media thickness is increased and associated with morning cortisol in subjects with non-functioning adrenal incidentaloma. Endocrine 2009;35(3):365–70.
- Yener S, Baris M, Secil M, Akinci B, Comlekci A, Yesil S. Is there an association between non-functioning adrenal adenoma and endothelial dysfunction? J. Endocrinol. Invest. 2011;34(4):265–70.
- Ermetici F, Dall’Asta C, Malavazos AE, Coman C, Morricone L, Montericcio V, Ambrosi B. Echocardiographic alterations in patients with non-functioning adrenal incidentaloma. J. Endocrinol. Invest. 2008;31(6):573–7.
- Androulakis II, Kaltsas GA, Kollias GE, Markou AC, Gouli AK, Thomas DA, Alexandraki KI, Papamichael CM, Hadjidakis DJ, Piaditis GP. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J. Clin. Endocrinol. Metab. 2014;99(8):2754–2762.
- Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” Adrenal Tumors and the Risk for Incident Diabetes and Cardiovascular Outcomes. Ann. Intern. Med. 2016;165(8):533.
- Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L, Murad MH, O’Reilly MW, Arlt W, Bancos I. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess. Ann. Intern. Med. 2019;171(2):107.
- Terzolo M, Reimondo G. Insights on the Natural History of Adrenal Incidentalomas. Ann. Intern. Med. 2019;171(2):135.
- Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015;100(8):2807–2831.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. J. Clin. Endocrinol. Metab. 2010;95(9):4106–4113.
- Mantero F, Arnaldi G. Management approaches to adrenal incidentalomas. A view from Ancona, Italy. Endocrinol. Metab. Clin. North Am. 2000;29(1):107–25, ix.
- Terzolo M, Bovio S, Pia A, Reimondo G, Angeli A. Management of adrenal incidentaloma. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23(2):233–243.
- Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal Function After Adrenalectomy for Subclinical Hypercortisolism and Cushing’s Syndrome: A Systematic Review of the Literature. J. Clin. Endocrinol. Metab. 2014;99(8):2637–2645.
- Khawandanah D, ElAsmar N, Arafah BM. Alterations in hypothalamic-pituitary-adrenal function immediately after resection of adrenal adenomas in patients with Cushing’s syndrome and others with incidentalomas and subclinical hypercortisolism. Endocrine 2019;63(1):140–148.
- Kastelan D, Kraljevic I, Dusek T, Knezevic N, Solak M, Gardijan B, Kralik M, Poljicanin T, Skoric-Polovina T, Kastelan Z. The clinical course of patients with adrenal incidentaloma: Is it time to reconsider the current recommendations? Eur. J. Endocrinol. 2015;173(2):275–282.
- Yeomans H, Calissendorff J, Volpe C, Falhammar H, Mannheimer B. Limited value of long-term biochemical follow-up in patients with adrenal incidentalomas-a retrospective cohort study. BMC Endocr. Disord. 2015;15(1):6.
- Muth A, Taft C, Hammarstedt L, Björneld L, Hellström M, Wängberg B. Patient-reported impacts of a conservative management programme for the clinically inapparent adrenal mass.; 2013:228–236.
Normal Physiology of Growth Hormone in Adults
ABSTRACT
Growth hormone (GH) is an ancestral hormone secreted episodically from somatotroph cells in the anterior pituitary. Since the recognition of its multiple and complex effects in the early 1960s, the physiology and regulation of GH has become a major area of research interest in the field of endocrinology. In adulthood, its main role is to regulate the metabolism. Pituitary synthesis and secretion of GH is stimulated by episodic hypothalamic secretion of GH releasing factor and inhibited by somatostatin. Insulin-like Growth Factor I (IGF-I) inhibits GH secretion by a negative loop at both hypothalamic and pituitary levels. In addition, age, gender, pubertal status, food, exercise, fasting, sleep and body composition play important regulatory roles. GH acts both directly through its own receptors and indirectly through the induced production of IGF-I. Their effects may be synergistic (stimulate growth) or antagonistic, as for the effect on glucose metabolism: GH stimulates lipolysis and promotes insulin resistance, whereas IGF-I acts as an insulin agonist. The bioactivity of IGF-I is tightly controlled by several IGF-I binding proteins. The mechanisms underlying the insulin antagonist effect of GH in humans are causally linked to lipolysis and the ensuing elevated levels of circulating free fatty acids. The nitrogen retaining properties of GH predominantly involve stimulation of protein synthesis, which could be either direct or mediated through IGF-I, insulin or lipid intermediates. In the present chapter, the normal physiology of GH secretion and the effects of GH on intermediary metabolism throughout adulthood, focusing on human studies, are presented.
INTRODUCTION
Harvey Cushing proposed in 1912 in his monograph "The Pituitary Gland" the existence of a "hormone of growth", and was thereby among the first to indicate that the primary action of growth hormone (GH) was to control and promote skeletal growth. In clinical medicine GH (also called (somatotrophin) was previously known for its role on promoting growth of hypopituitary children, and for its adverse effects in connection with hypersecretion as observed in acromegaly. The multiple and complex actions of human GH were, however, acknowledged shortly after the advent of a pituitary-derived preparation of the hormone in the late fifties - as reviewed by Raben in 1962 (1).
GROWTH HORMONE
GH is a single chain protein with 191 amino-acids and two disulfide bonds. The human GH gene is located on chromosome 17q22 as part of a locus that comprises five genes. In addition to two GH related genes (GH1 that codes for the main adult growth hormone, produced in the somatotrophic cells found in the anterior pituitary gland and, to a minor extent, in lymphocytes, and GH2 that codes for placental GH), there are three genes coding for chorionic somatomammotropin (CSH1, CSH2 and CSHL) (also known as placental lactogen) genes (2,3). The GH1 gene encodes two distinct GH isoforms (22 kDa and 20 kDa). The principal and most abundant GH form in the pituitary and blood is the monomeric 22K-GH isoform, representing also the recombinant GH available for therapeutic use (and subsequently for doping purposes) (3). Administration of recombinant 22K-GH exogenously leads to a decrease in the 20K-GH isoform, and thus testing both isoforms is used to detect GH doping in sports (4).
As already mentioned, GH is secreted by the somatotroph cells located primarily in the lateral wings of the anterior pituitary. A recent single cell RNA sequencing study performed in mice showed that GH-expressing cells, representing the somatotrophs, are the most abundant cell population in the adult pituitary gland (5). The differentiation of somatotroph cell is governed by the pituitary transcription factor 1 (Pit-1). Data in mice suggest that the pituitary holds regenerative competence, the GH-producing cells being regenerated form the pituitary’s stem cells in young animals after a period of 5 months (6).
Physiological Regulation of GH Secretion
The morphological characteristics and number of somatotrophs are remarkably constant throughout life, while their secretion pattern changes. GH secretion occurs in a pulsatile fashion, and in a circadian rhythm with a maximal release in the second half of the night. So, sleep is an important physiological factor that increases the GH release. Interestingly, the maximum GH levels occur within minutes of the onset of slow wave sleep and there is marked sexual dimorphism of the nocturnal GH increase in humans, constituting only a fraction of the total daily GH release in women, but the bulk of GH output in men (7).
GH secretion is also gender-, pubertal status- and age- dependent (Figure 1 and Figure 4) (8). Integrated 24h GH concentration is significantly greater in women than in men and greater in the young than in older adults. The serum concentration of free estradiol, but not free testosterone, correlates with GH, and when correcting for the effects of estradiol, neither gender nor age influence GH concentration. This suggests that estrogens play a crucial role in modulating GH secretion (8). During puberty, a 3-fold increase in pulsatile GH secretion occurs that peaks around the age of 15 years in girls and 1 year later in boys (9).
Figure 1 The secretory pattern of GH in young and old female and male. In young individuals the GH pulses are larger and more frequent and that female secrete more GH than men (modified from (8)).
Pituitary synthesis and secretion of GH is stimulated by episodic hypothalamic hormones. Growth hormone releasing hormone (GHRH) stimulates while somatostatin (SST) inhibits GH production and release. GH stimulates IGF-I production which in turn inhibits GH secretion at both hypothalamic and pituitary levels. The gastric peptide ghrelin is also a potent GH secretagogue, which acts to amplify hypothalamic GHRH secretion and synergize with its pituitary GH-stimulating effects (Figure 2) (10). Interestingly, recently germline or somatic duplication of GPR101 has been shown to constitutively activate the cAMP pathway in the absence of a ligand, leading to GH release. Although the precise physiology of GPR101 is unclear, it is worth mentioning it since it clearly has an effect on GH pathophysiology (11).
In addition, a multitude of other factors may impact the GH axis, most probably due to interaction with GRHR, somatostatin, and ghrelin. Estrogens stimulate the secretion of GH, but inhibit the action of GH on the liver by suppressing GH receptor (GHR) signaling. In contrast, androgens enhance the peripheral actions of GH (12). Exogenous estrogens potentiate pituitary GH responses to submaximal effective pulses of exogenous GHRH (13) and mute inhibition by exogenous SST (14). Also, exogenous estrogen potentiates ghrelin’s action (15).
GH release correlates inversely with intraabdominal visceral adiposity via mechanisms that may depend on increased free fatty acids (FFA) flux, elevated insulin, or free IGF-I.
Figure 2. Factors that stimulate and suppress GH secretion under physiological conditions.
GROWTH HORMONE RELEASING HORMONE
GHRH is a 44 amino-acid polypeptide produced in the arcuate nucleus of the hypothalamus. These neuronal terminals secrete GHRH to reach the anterior pituitary somatotrophs via the portal venous system, which leads to GH transcription and secretion. Moreover, animal studies have demonstrated that GHRH plays a vital role in the proliferation of somatotrophs in the anterior pituitary, whereas the absence of GHRH leads to anterior pituitary hypoplasia (16). In addition, GHRH up-regulates GH gene expression and stimulates GH release (17). The secretion of GHRH is stimulated by several factors including depolarization, α2-adrenergic stimulation, hypophysectomy, thyroidectomy and hypoglycemia, and it is inhibited by SST, IGF-I, and activation of GABAergic neurons.
GHRH acts on the somatotrophs via a seven trans-membrane G protein-coupled stimulatory cell-surface receptor. This receptor has been extensively studied over the last decade leading to the identification of several important mutations. Point mutations in the GHRH receptors, as illustrated by studies done on the lit/lit dwarf mice, showed a profound impact on subsequent somatotroph proliferation leading to anterior pituitary hypoplasia (18). Unlike the mutations in the Pit-1 and PROP-1 genes, which lead to multiple pituitary hormone deficiencies and anterior pituitary hypoplasia, mutations in the GHRH receptor lead to profound GH deficiency with anterior pituitary hypoplasia. Subsequent to the first GHRH receptor mutation described in 1996 (19), an array of familial GHRH receptor mutations have been recognized over the last decade. These mutations account for almost 10% of familial isolated GH deficiencies. An affected individual will present with short stature and a hypoplastic anterior pituitary. However, they lack certain typical features of GH deficiency such as midfacial hypoplasia, microphallus, and neonatal hypoglycemia (20).
SOMATOSTATIN (SST)
SST is a cyclic peptide, encoded by a single gene in humans, which mostly exerts inhibitory effects on endocrine and exocrine secretions. Many cells in the body, including specialized cells in the anterior paraventricular nucleus and arcuate nucleus, produce SST. These neurons secrete SST into the adenohypophyseal portal venous system, via the median eminence, to exert effects on the anterior pituitary. SST has a short half-life of approximately 2 minutes as it is rapidly inactivated by tissue peptidase in humans.
SST acts via a seven trans-membrane, G protein coupled receptor and, thus far, five subtypes of the receptor have been identified in humans (SSTR1-5). Although all five receptor subtypes are expressed in the human fetal pituitary, the adult pituitary only expresses 4 subtypes (SSTR1, SSTR2, SSTR3, SSTR5). Of these four subtypes, somatotrophs exhibit more sensitivity to SSTR2 and SSTR5 ligands in inhibiting the secretion of GH in a synergistic manner (21). Somatostatin inhibits GH release but not GH synthesis.
GHRELIN
Ghrelin is a 28 amino-acid peptide that is the natural ligand for the GH secretagogue receptor. In fact, ghrelin and GHRH have a synergistic effect in increasing circulating GH levels (7). Ghrelin is primarily secreted by the stomach and may be involved in the GH response to fasting and food intake.
Clinical Implications
GH levels – influence of body composition, physical fitness and age
With the introduction of dependable radioimmunological assays, it was recognized that circulating GH is blunted in obese subjects, and that normal aging is accompanied by a gradual decline in GH levels (22,23). It has been hypothesized that many of the senescent changes in body composition and organ function are related to or caused by decreased GH (24), also known as "the somatopause".
Studies carried out in the late 90s have uniformly documented that adults with severe GH deficiency are characterized by increased fat mass and reduced lean body mass (LBM) (25). It is also known that normal GH levels can be restored in obese subjects following massive weight loss (26), and that GH substitution in GH-deficient adults normalizes body composition. What remains unknown is the cause-effect relationship between decreased GH levels and senescent changes in body composition. Is the propensity for gaining fat and losing lean mass initiated or preceded by a primary age-dependent decline in GH secretion and action? Alternatively, accumulation of fat mass secondary to non-GH dependent factors (e.g. life style, dietary habits) results in a feedback inhibition of GH secretion. Moreover, little is known about possible age-associated changes in GH pharmacokinetics and bioactivity.
Cross-sectional studies performed to assess the association between body composition and stimulated GH release in healthy subjects show that adult people (mean age 50 yr) have a lower peak GH response to secretagogues (clonidine and arginine), while females had a higher response to arginine when compared to males. Multiple regression analysis, however, reveal that intra-abdominal fat mass is the most important and negative predictor of peak GH levels, as previously mentioned (27). In the same population, 24-h spontaneous GH levels also predominantly correlated inversely with intra-abdominal fat mass (Figure 3) (28).
Figure 3. Correlation between intra-abdominal fat mass and 24-hour GH secretion.
A detailed analysis of GH secretion in relation to body composition in elderly subjects has, to our knowledge, not been performed. Instead, serum IGF-I has been used as a surrogate or proxy for GH status in several studies of elderly men (29-31). These studies comprise large populations of ambulatory, community-dwelling males aged between 50-90 yr. As expected, the serum IGF-I declined with age (Figure 4), but IGF-I failed to show any significant association with body composition or physical performance.
Figure 4. Changes in serum IGF-I with age; modified from (32).
GH action: Influence of age, sex and body composition
Considering the great interest in the actions of GH in adults, surprisingly few studies have addressed possible age-associated differences in the responsiveness or sensitivity to GH. In normal adults the senescent decline in GH levels is paralleled by a decline in serum IGF-I, suggesting a down-regulation of the GH-IGF-I axis. Administration of GH to elderly healthy adults has generally been associated with predictable, albeit modest, effects on body composition and side effects in terms of fluid retention and modest insulin resistance (33). Whether this reflects an unfavorable balance between effects and side effects in older people or the employment of excessive doses of GH is uncertain, but it is evident that older subjects are not resistant to GH. Short-term dose-response studies clearly demonstrate that older patients require a lower GH dose to maintain a given serum IGF-I level (34,35), and it has been observed that serum IGF-I increases in individual patients on long-term therapy if the GH dosage remains constant. Moreover, patients with GH deficiency older than 60 years are highly responsive to even a small dose of GH (36). Interestingly, there is a gender difference response to GH treatment with men being more responsive in terms of IGF-I generation and fat loss during therapy, most probably due to lower estrogen levels that negatively impact the GH effect on IGF-I generation in the liver (37).
The pharmacokinetics and short-term metabolic effects of a near physiological intravenous GH bolus (200μg) were compared in a group of young (30 year) and older (50 year) healthy adults (38). The area under the GH curve was significantly lower in older subjects, whereas the elimination half-life was similar in the two groups, suggesting both an increased metabolic clearance rate and apparent distribution volume of GH in older subjects. Both parameters showed a strong positive correlation with fat mass, although multiple regression analysis revealed age to be an independent positive predictor. The short-term lipolytic response to the GH bolus was higher in young as compared to older subjects. Interestingly, the same study showed that the GH binding proteins correlated strongly and positively with abdominal fat mass (39).
A prospective long-term study of normal adults with serial concomitant estimations of GH status and adiposity would provide useful information about the cause-effect relationship between GH status and body composition as a function of age. In the meantime, the following hypothesis is proposed (Figure 5): 1. Changes in life-style and genetic predispositions promote accumulation of body fat with aging; 2. The increased fat mass, leads to increased FFA availability, and induces insulin resistance and hyperinsulinemia; 3. High insulin levels suppress IGF binding protein (IGFBP)-1 resulting in a relative increase in free IGF-I levels; 4. Systemic elevations of FFA, insulin and free IGF-I suppress pituitary GH release, which further increases fat mass; 5. Endogenous GH is cleared more rapidly in subjects with a high amount of fat tissue.
At present it is not justified to treat the age-associated deterioration in body composition and physical performance with GH especially due to concern that the ensuing elevation of IGF-I levels may increase the risk for the development of neoplastic disease (For an extensive discussion of GH in the elderly see the chapter on this topic in the Endocrinology of Aging section of Endotext).
Figure 5. Hypothetical model for the association between low GH levels and increased visceral fat in adults.
Life-long GH deficiency
A real-life model for GH effects in human physiology is represented by patients with life-long severe reduction in GH signaling due to GHRH or GHRH receptor mutations, combined deficiency of GH, prolactin, and TSH, or global deletion of GHR. They show short stature, doll facies, high-pitched voices, and central obesity, and are fertile (40). Despite central obesity and increased liver fat, they are insulin sensitive, partially protected from cancer and present a major reduction in pro-aging signaling and perhaps increased longevity (41). The decrease of cancer risk in life-long GH deficiency together with reports on the permissive role of GH for neoplastic colon growth (42), pre-neoplastic mammary lesions (43), and progression of prostate cancer (44) demands, at least, a careful tailoring of GH substitution dosage in the GH deficient patients.
GH and the immune system
Although the majority of data on the relation between GH and the immune system are from animal studies, it seems that GH may possess immunomodulatory actions. Immune cells, including several lymphocyte subpopulations, express receptors for GH, and respond to its stimulation (45). GH stimulates in vitro T and B-cell proliferation and immunoglobulin synthesis, enhances human myeloid progenitor cell maturation, and modulates in vivo Th1/Th2 (8) and humoral immune responses (46). It has been shown that GH can induce de novo T cell production and enhance CD4 recovery in HIV+ patients. Another study with possible clinical relevance showed that sustained GH expression reduced prodromal disease symptoms and eliminated progression to overt diabetes in mouse model of type 1 diabetes, a T-cell–mediated autoimmune disease. GH altered the cytokine environment, triggered anti-inflammatory macrophage (M2) polarization, maintained activity of the suppressor T-cell population, and limited Th17 cell plasticity (46). JAK/STAT signaling, the principal mediator of GHR activation, is well-known to be involved in the modulation of the immune system, so is tempting to assume that GH may have a role too, but clear data in humans are needed.
Growth Hormone Signaling in Humans
Growth hormone RECEPTOR (GHR) activation
GHR signaling is a separate and prolific research field by itself (47), so this section will focus on recent data obtained in human models.
GHRs have been identified in many tissues including fat, lymphocytes, liver, muscle, heart, kidney, brain and pancreas (48,49). Activation of receptor-associated Janus kinase (JAK)-2 is the critical step in initiating GH signaling. One GH molecule binds to two GHR molecules that exist as preformed homodimers. Following GH binding, the intracellular domains of the GHR dimer undergo rotation, which brings together the two intracellular domains each of them binding one JAK2 molecule. This, in turn, induces cross-phosphorylation of tyrosine residues in the kinase domain of each JAK2 molecule followed by tyrosine phosphorylation of the GHR (48,50). Phosphorylated residues on GHR and JAK2 form docking sites for different signaling molecules including signal transducers and activators of transcription (STAT) 1, 3, 5a and 5b. STATs bound to the activated GHR-JAK2 complex are subsequently phosphorylated on a single tyrosine by JAK2 allowing dimerization and translocation to the nucleus, where they bind to DNA and activate gene transcription. A STAT5b binding site has been characterized in the IGF-I gene promoter region (51). Attenuation of JAK2-associated GH signaling is mediated by a family of cytokine-inducible suppressors of cytokine signaling (SOCS) (52). SOCS proteins bind to phosphotyrosine residues on the GHR or JAK2 and suppress GH signaling by inhibiting JAK2 activity and competing with STATs. For example, it has been reported that the inhibitory effect of estrogen on hepatic IGF-I production seems to be mediated via up regulation of SOCS-2 (53).
Data on GHR signaling derive mainly from rodent models and experimental cell lines, although GH-induced activation of the JAK2/STAT5b and the mitogen activated protein kinase (MAPK) pathways have been recorded in cultured human fibroblasts from healthy human subjects (54). STAT5b in human subjects is critical for GH-induced IGF-I expression and growth promotion as demonstrated by the identification of mutations in the STAT5b gene of patients presenting with severe GH insensitivity in the presence of a normal GHR (55). Activation of GHR signaling in vivo has been reported in healthy young male subjects exposed to an intravenous GH bolus vs. saline (56). Significant tyrosine phosphorylation of STAT5b was recorded after GH exposure at 30-60 minutes in muscle and fat biopsies, but there was no evidence of GH-induced activation of PI 3-kinase, Akt/PKB, or MAPK (56).
GH and insulin signaling
GH impairs the insulin mechanism but the exact mechanisms in humans are still a matter of debate. There is no evidence of a negative effect of GH on insulin binding to the receptor (57,58), which obviously implies post-receptor metabolic effects.
There is animal and in vitro evidence to suggest that insulin and GH share post-receptor signaling pathways (59). Convergence has been reported at the levels of STAT5 and SOCS3 (60) as well as on the major insulin signaling pathway: insulin receptor substrates (IRS) 1 and 2, PI 3-kinase (PI3K), Akt, and extracellular regulated kinases (ERK) 1 and 2 (61-63). Studies in rodent models suggest that the insulin-antagonistic effects of GH in adipose involve suppression of insulin-stimulated PI3-kinase activity (59,64). In 2001 it was demonstrated that GH induces cellular insulin resistance by uncoupling PI3K and its downstream signals in 3T3-L1 adipocytes (65)]. A follow up study has shown that GH increased p85α expression and decreased PI3K activity in adipose tissue of mice, supporting the previous report of a direct inhibitory effect of GH on PI3K activity (64). However, a study performed in healthy human skeletal muscle showed, as expected, that the infusion of GH induced a sustained increase in FFA levels and subsequently insulin resistance as assessed by the euglycemic clamp technique, but was not associated with any change in the insulin-stimulated increase in either IRS-1/PI3K or PKB/Akt activity (66). It was subsequently showed that insulin had no impact on GH-induced STAT5b activation or SOCS3 mRNA expression (67).
Because GH and insulin share some common intracellular substrates, a hypothesis arose claiming that competition for intracellular substrates explains the negative effect of GH on insulin signaling (59). Furthermore, studies have shown that SOCS proteins negatively regulate the insulin signaling pathway (68). Therefore, another possible mechanism by which GH alters the action of insulin is by increasing the expression of SOCS genes.
INSULIN-LIKE GROWTH FACTOR-I
Physiology of IGF-I
GH acts both directly through its own receptor and indirectly through the induced production of IGF-I. GH stimulates synthesis of IGF-I in the liver and many other target tissues (Figure 6); about 75% of circulating IGF-I is liver-derived. IGF-I is a 70 amino-acid peptide, found in the circulation, 99% bound to transport proteins (IGFBP) in the circulation.
Following the initial discovery of IGF-I, it was thought that GH governs somatic growth only by IGF-I produced by the liver (69). However, in the 1980s this hypothesis was challenged by the identification of IGF-I production in numerous tissues. IGF-I is known as a global and tissue-specific growth factor as well as an endocrine factor. In some tissues IGF-I acts as a potent inhibitor of cellular apoptosis.
Figure 6. GH is produced in the pituitary gland. In the periphery, GH acts directly and indirectly through stimulation of IGF-I production. In the circulation, the liver is the most important source of IGF-I (75%) but other tissues (e.g. brain, adipose tissue, kidney, bone, and muscles) may contribute. Under GH stimulation the muscle, adipose tissue, and bone have been shown to secrete IGF-I that has a paracrine/autocrine effect.
Interestingly, insulin and IGF-I share many structural and functional similarities, implying that they originated from the same ancestral molecule. Both molecules could have been part of the cycle of food intake and consequent tissue growth. The IGF-I gene is a member of the insulin gene family and the IGF-I receptor is structurally similar to the insulin receptor in its tetrameric structure, with 2 alpha and 2 beta subunits (70). The alpha subunit binds IGF-I, IGF-II, and insulin; however, the subunit has a higher affinity towards IGF-I compared to IGF-II and insulin. Although insulin and IGF-I share many similarities, during evolution the functionality of the two molecules has become more divergent, where insulin plays a more metabolic role and IGF-I is more involved in cell growth.
The IGF-I receptor is expressed in many tissues in the body. However, the receptor number on each cell is strictly regulated by several systemic and tissue factors including circulating GH, iodothyronines, platelet-derived growth factor, and fibroblast growth factor. Following the binding of the IGF-I molecule, the receptor undergoes a conformational change which activates tyrosine kinase, leading to auto-phosphorylation of tyrosine. The activated receptor phosphorylates IRS-2, which in-turn activates the RAS activating protein SOS. This complex activates the MAPK pathway leading to the stimulation of cell growth (71,72).
The IGFBP family comprises six binding proteins (IGFBP 1-6) with a high affinity towards IGF-I and II. Apart from regulating the free plasma IGF fraction, IGFBPs also play an important role in the transport of IGF into different tissues and extravascular space. IGFBP-3 and IGFBP-2 are the most abundant forms seen in plasma and are saturated with IGF-I due to their high affinity: 75% of IGF-I is bound to IGFBP-3. Interestingly, similar to IGF-I, IGFBP-3 production is also regulated by GH. In the plasma, IGFBP-3 is bound to a protein called acid labile subunit (ALS), which stabilizes the “IGFBP3-IGF-I” complex, prolonging its half-life to approximately 16 hours (73). IGFBP-1, on the other hand, is present in lower concentration in plasma than IGFBP-2 and 3. However, due to lower affinity for IGF-I, IGFBP-1 is usually in an unsaturated state and changing plasma concentrations of IGFBP-1 become important in determining the unbound fraction of IGF-I. A recently new discovered player in the regulation of IGF-I bioavailability is the pregnancy-associated plasma protein-A2 (PAPP-A2) that cleaves IGFBP3 and 5 and releases IGF-I. Homozygous mutations in PAPP-A2 result in growth failure with elevated total but low free IGF-I (74). Low IGF-I bioavailability impairs growth and glucose metabolism in a mouse model of human PAPP-A2 deficiency and treatment with recombinant human IGF-I in PAPP-A2 deficient patients improves growth and bone mass and ameliorates glucose metabolism (74,75).
Effects of IGF-I
Studies on hypophysectomized animals overexpressing IGF-I demonstrate the independent anabolic effects of IGF-I (76). IGF-I plays a key role in growth, where it acts not only as a determinant of postnatal growth, but also as an intra-uterine growth promoter. Total inactivation of the IGF-I gene in mice produce a perinatal mortality of 80% with the surviving animal showing significant growth retardation compared to controls (77). Human IGF-I deficiency can be either due to GH deficiency, GHR inactivation, or IGF-I gene mutation. Interestingly, infants with congenital GH deficiency and GHR mutations present with only minor growth retardation, whereas the rare patient with IGF-I deficiency, secondary to a homozygous partial deletion of the IGF-I gene, presents with severe pre- and postnatal growth failure, mental retardation, sensorineural deafness and microcephaly (78-80). The differences in the clinical presentation are most likely due to the fact that some degree of IGF-I production is present in patients with GH deficiency, and GHR and GHRH defects. The important growth promoting role of IGF-I is further demonstrated by studies on transgenic mice. Only 6-8% postnatal growth retardation is presented in mice with liver-selective deletion of IGF-I gene showing low serum IGF-I concentrations, whereas animals with total IGF-I deletion or those with only peripherally produced IGF-I deletion showed marked growth retardation (81).
Both elevated and reduced levels of serum IGF-I are associated with excess mortality in human adults (82). In addition, it is well recognized in many species including worms, flies, rodents and primates that a reciprocal relationship exists between longevity and activation of the insulin/IGF axis (82). In this regard, it is noteworthy that calorie restriction is associated with increased longevity and reduced insulin/IGF activity in many species (83), albeit GH levels being increased by calorie restriction and fasting (84).
In the context of GH and IGF-I physiology it can be concluded that 1) during childhood and adolescence the combined actions of GH and IGF-I in the presence of sufficient nutrition promote longitudinal growth and somatic maturation, 2) continued excess IGF-I activity in adulthood increases the risk for cardiovascular and neoplastic diseases and hence reduces longevity, and 3) calorie restriction, which suppresses IGF-I activity and stimulates GH secretion, may promote longevity also in human adults (84).
METABOLIC EFFECTS OF GROWTH HORMONE
The nutritional status dictates the effects of GH. In the state of ‘feast’ and sufficient nutrient intake where insulin is increased in the liver and IGF-I production is stimulated, GH promotes protein anabolism. Whereas, in a state with decreased nutrient intake and during the sleep and exercise, the direct effects of GH are more predominant and this is mainly characterized by stimulation of lipolysis.
Glucose Homeostasis and Lipid Metabolism
The involvement of the pituitary gland in the regulation of substrate metabolism was originally detailed in the classic dog studies by Houssay (85). Fasting hypoglycemia and pronounced sensitivity to insulin were distinct features of hypophysectomized animals. These symptoms were readily corrected by administration of anterior pituitary extracts. It was also noted that pancreatic diabetes was alleviated by hypophysectomy. Finally, excess of anterior pituitary lobe extracts aggravated or induced diabetes in hypophysectomized dogs. Furthermore, glycemic control deteriorated following exposure to a single supraphysiological dose of human GH in hypophysectomized adults with type 1 diabetes mellitus (86). Somewhat surprisingly, only modest effects of GH on glucose metabolism were recorded in the first metabolic balance studies involving adult hypopituitary patients (87,88).
More recent studies on glucose homeostasis in GH deficient adults have generated results which at first glance may appear contradictory. Insulin resistance may be more prevalent in untreated GH deficient adults, whereas the impact of GH replacement on this feature seems to depend on the duration and the dose (89).
Below, some of the metabolic effects of GH in human subjects, with special reference to the interaction between glucose and lipid metabolism, will be reviewed.
Studies in Normal Adults
More than fifty years ago, it was shown that infusion of high-dose GH into the brachial artery of healthy adults reduced forearm glucose uptake in both muscle and adipose tissue, which was paralleled by increased uptake and oxidation of FFA (90). This pattern was opposite to that of insulin, and GH in the same model abrogated the metabolic actions of insulin.
Administration of a GH bolus in the post-absorptive state stimulates lipolysis following a lag time of 2-3 hours (91). Plasma levels of glucose and insulin, on the other hand, change very little. This is associated with small reductions in muscular glucose uptake and oxidation, which could reflect substrate competition between glucose and fatty acids (i.e. the glucose/fatty acid cycle) (Figure 7). In line with this, sustained exposure to high GH levels induces both hepatic and peripheral (muscular) resistance to the actions of insulin on glucose metabolism together with increased (or inadequately suppressed) lipid oxidation. Apart from enhanced glucose/fatty acid cycling, it has been shown that GH-induced insulin resistance is accompanied by reduced muscle glycogen synthase activity (57) and diminished glucose dependent glucose disposal (92). However, insulin binding and insulin receptor kinase activity from muscle biopsies is not affected by GH (57).
Lessons from Acromegaly
Active acromegaly clearly unmasks the diabetogenic properties of GH. In the basal state plasma glucose is elevated despite compensatory hyperinsulinemia. In the basal and insulin-stimulated state (euglycemic glucose clamp) hepatic and peripheral insulin resistance is associated with enhanced lipid oxidation and energy expenditure (93). There is evidence to suggest that this hyper-metabolic state ultimately leads to beta cell exhaustion and overt diabetes mellitus (94), but it is also shown that the abnormalities are completely reversed after successful surgery (93). Conversely, it has been shown that administration of GH in supraphysiological doses for only two weeks induces comparable acromegaloid - and reversible - abnormalities in substrate metabolism and insulin sensitivity (95).
Interaction of Glucose and Lipid Metabolism
The effect of FFA on the partitioning of intracellular glucose fluxes was originally described by Randle et al. (96). According to this hypothesis (the glucose/fatty acid cycle), oxidation of FFA initiates an upstream, chain-reaction-like, inhibition of glycolytic enzymes, which ultimately inhibits glucose uptake (Figure 7).
Figure 7. The glucose fatty-acid cycle. A. Randle proposed in 1963 that increased FFA compete with and displace glucose utilization leading to a decreased glucose uptake. The hypothesis stated that an increase in fatty acid oxidation in muscle and fat results in higher acetyl CoA in mitochondria leading to inactivation of two rate-limiting enzymes of glycolysis (i.e., phosphofructokinase (PFK) and pyruvate dehydrogenase (PDH) complex). A subsequent increase in intracellular glucose-6-phosphate (glucose 6-P) results in high intracellular glucose concentrations and decreased glucose uptake by muscle and fat. B. However, in contrast to the proposed hypothesis by Randle, studies using MR spectroscopy have shown reductions in intramyocellular glucose 6-P and glucose concentrations and have led to an alternative hypothesis. The new hypothesis proposes that a transient increase of intracellular diacylglycerol (DAG) activates the theta isoform of protein kinase C (PKCθ) that causes increased serine phosphorylation of IRS-1/2 and consecutively decrease PI3K activation and glucose-transport activity leading to decrease intracellular glucose concentrations.
The Randle hypothesis remains an appealing model to explain the insulin-antagonistic effects of GH when considering its pronounced lipolytic effects. To support this, experiments have shown that co-administration of anti-lipolytic agents and GH reverses GH-induced insulin resistance (97). Moreover it has been shown that GH-induced insulin resistance is associated with suppressed pyruvate dehydrogenase activity in skeletal muscle (98). However, according to the Randle hypothesis, the fatty acid-induced insulin resistance will result in elevated intracellular levels of both glucose and glucose-6-phosphate (Figure 7), whereas the muscle biopsies from GH deficient adults after GH treatment have revealed increased glucose but low-normal glucose-6-phosphate levels (99).
Implications for GH Replacement
Regardless of the exact mechanisms, the insulin antagonistic effects may cause concern when replacing adult GH deficient patients with GH, since some of these patients are insulin resistant in the untreated state. There is evidence to suggest that the direct metabolic effects on GH may be balanced by long-term beneficial effects on body composition and physical fitness, but some studies report impaired insulin sensitivity in spite of favorable changes in body composition. There is little doubt that these effects of GH are dose-dependent and may be minimized or avoided if an appropriately low replacement dose is used. Still, the pharmacokinetics of subcutaneous (s.c.) GH administration is unable to mimic the endogenous GH pattern with suppressed levels after meals and elevations only during post absorptive periods, such as during the night. This may be considered the natural domain of GH action, which coincides with minimal beta-cell challenge. This reciprocal association between insulin and GH and its potential implications for normal substrate metabolism was initially described by Rabinowitz & Zierler (100). The problem arises when GH levels are elevated during repeated prandial periods. The classic example is active acromegaly, but prolonged high dose s.c. GH administration may cause similar effects. Administration of GH in the evening probably remains the best compromise between effects and side effects (101), but it is far from physiological.
We know and understand that hypoglycemia is a serious and challenging side effect of insulin therapy as a consequence of inappropriately high insulin levels (during fasting). As a corollary, we must realize that hyperglycemia may result from GH therapy. It is therefore important to carefully monitor glucose metabolism and to use the lowest effective dose when replacing adults with GH.
Effects of GH on Muscle Mass and Function
The anabolic nature of GH is clearly evident in patients with acromegaly and vice versa in patients with GH deficiency. A large number of in vitro and animal studies throughout several decades have documented stimulating effects of GH on skeletal muscle growth. The methods employed to document in vivo effects of GH on muscle mass in humans have been exhaustive, including whole body retention of nitrogen and potassium, total and regional muscle protein metabolism using labeled amino-acids, estimation of LBM by total body potassium or dual x-ray absorptiometry, and direct calculation of muscle area or volume by computerized tomography and magnetic resonance imaging.
Effects of GH on Skeletal Muscle Metabolism in Vitro and in Vivo
The clinical picture of acromegaly and gigantism includes increased LBM of which skeletal muscle mass accounts for approximately 50%. Moreover, retention of nitrogen was one of the earliest observed and most reproducible effects of GH administration in humans (1). Thoroughly conducted studies with GH administration in GH deficient children, using a variety of classic anthropometric techniques, strongly suggested that skeletal muscle mass increased significantly during treatment (102,103). Indirect evidence of an increase in muscle cell number following GH treatment was also presented (103).
These early clinical studies were paralleled by experimental studies in rodent models. GH administration in hypophysectomized rats increased not only muscle mass, but also muscle cell number (i.e. muscle DNA content) (103). Interestingly, the same series of experiments revealed that work-induced muscle hypertrophy could occur in the absence of GH. The ability of GH to stimulate RNA synthesis and amino-acid incorporation into protein of isolated rat diaphragm suggested direct mechanisms of actions, whereas direct effects of GH on protein synthesis could not be induced in liver cell cultures (104). Another important observation of that period was that GH directly increases the synthesis of both sarcoplasmic and myofibrillar protein without affecting proteolysis in a rat model (105).
In a human study, the in vivo effects of systemic and local GH and IGF-I administration on total and regional protein metabolism revealed that GH administration for 7 days in normal adults increased whole body protein synthesis without affecting proteolysis (106), and comparable results have been obtained in other human studies (107-110).
Based on these studies it seems that the nitrogen-retaining properties of GH predominantly involve stimulation of protein synthesis without affecting (lowering) proteolysis. Theoretically, the protein anabolic effects of GH could be either direct or mediated through IGF-I, insulin, or lipid intermediates. GHR are present in skeletal muscle (49), which allows for direct GH effects; alternatively, GH may stimulate local muscle IGF-I release, which subsequently acts in an autocrine/paracrine manner. The effects of systemic IGF-I administration on whole body protein metabolism seem to depend on ambient amino-acid levels in the sense that IGF-I administered alone suppresses proteolysis (111) whereas IGF-I in combination with an amino-acid infusion increase protein synthesis (112). It is therefore likely that the muscle anabolic effects of GH, at least to some extent, are mediated by IGF-I. By contrast, it is repeatedly shown that insulin predominantly acts through suppression of proteolysis and this effect(s) appears to be blunted by co-administration of GH (113). The degree to which mobilization of lipids contributes to the muscle anabolic actions of GH has so far not been specifically investigated.
An interesting discovery has been that infusion of GH and IGF-I into the brachial artery increases forearm blood flow several fold (110,114). This effect appears to be mediated through stimulation of endothelial nitric oxide release leading to local vasodilatation (115,116). Thus, it appears that an IGF-I mediated increase in muscle nitric oxide release accounts for some of the effects of GH on skeletal muscle protein synthesis. This increase in muscle blood flow may also contribute to the GH-induced increase in resting energy expenditure, since skeletal muscle metabolism is a major determinant of resting energy expenditure (23). Moreover, it is plausible that the reduction in total peripheral resistance seen after GH administration in adult growth hormone deficiency is mediated by nitric oxide (116).
Effects of GH Administration on Muscle Mass and Function in Adults without GH-Deficiency
As previously mentioned, the ability of acute and more prolonged GH administration to retain nitrogen in healthy adults has been known for decades, and more recent studies have documented a stimulatory effect on whole body and forearm protein synthesis.
Rudman et al. were the first to suggest that the senescent changes in body composition were causally linked to the concomitant decline in circulating GH and IGF-I levels (23). This concept has been recently reviewed (117), and a number of studies with GH and other anabolic agents for treating the sarcopenia of ageing are currently in progress.
Placebo-controlled GH administration in young healthy adults undergoing a resistance exercise program for 12 weeks showed a GH induced increase in LBM, whole body protein balance, and whole body protein synthesis, whereas quadriceps muscle protein synthesis rate and muscle strength increased to the same degree in both groups during training (118). In a similar study in older men, GH also increased LBM and whole body protein synthesis, without significantly amplifying the effects of exercise on muscle protein synthesis or muscle strength (119). An increase in LBM but unaltered muscle strength following 10 weeks of GH administration plus resistance exercise training was also recorded (120). A more recent study in older men observed a significant increase (4.4 %) in LBM with GH, but no significant effects on muscle strength (121). Finally, a meta-analysis of studies administering GH to healthy adult subjects showed that it increases LBM and reduces fat mass without improving muscle strength or aerobic exercise capacity (122).
Numerous studies have evaluated the effects of GH administration in chronic and acute catabolic illness. A comprehensive survey of the prolific literature within this field is beyond the scope of this review, but it is noteworthy that HIV-associated body wasting is a licensed indication for GH treatment in the USA. In these patients, GH treatment for 12 weeks has been associated with significant increments in LBM and physical fitness (123,124).
CONCLUSIONS
The GH/IGF-I axis is specifically regulated and is involved in a multitude of processes during all the aspects of life from intrauterine growth, to childhood and puberty, adulthood and lastly elderly stages of life. GH acts directly or via its principal metabolite, IGF-I, and has a wide range of physiological roles being a metabolic active hormone in adulthood. The nutritional status of an organism dictates the effects of GH, either an impairment of insulin action (fasting state) or promoting protein anabolism (fed state). As our knowledge of GH normal physiology increases, our ability to understand and specifically target the GH/IGF-I pathway for a diverse range of therapeutic purposes should also increase. Normal aging is associated with a gradual decline in serum IGF-I levels that run in parallel with reductions in muscle mass and function and other senescent changes in organ function. The cause-effect relationship is uncertain, but GH administration to elderly people without pituitary disease has not proven beneficial and sustained supra-physiological IGF levels and actions are likely to be harmful. On the other hand, a stimulation of endogenous GH secretion induced by exercise and calorie restriction may contribute to healthy aging.
REFERENCES
- Raben MS. Growth hormone. 1. Physiologic aspects. N Engl J Med. 1962;266:31-35.
- Petronella N, Drouin G. Gene conversions in the growth hormone gene family of primates: stronger homogenizing effects in the Hominidae lineage. Genomics. 2011;98(3):173-181.
- Baumann GP. Growth hormone doping in sports: a critical review of use and detection strategies. Endocr Rev. 2012;33(2):155-186.
- Holt RIG, Ho KKY. The Use and Abuse of Growth Hormone in Sports. Endocr Rev. 2019;40(4):1163-1185.
- Cheung LYM, George AS, McGee SR, Daly AZ, Brinkmeier ML, Ellsworth BS, Camper SA. Single-Cell RNA Sequencing Reveals Novel Markers of Male Pituitary Stem Cells and Hormone-Producing Cell Types. Endocrinology. 2018;159(12):3910-3924.
- Willems C, Fu Q, Roose H, Mertens F, Cox B, Chen J, Vankelecom H. Regeneration in the pituitary after cell-ablation injury: time-related aspects and molecular analysis. Endocrinology. 2015:en20151741.
- Ribeiro-Oliveira A, Barkan AL. Growth Hormone Pulsatility and its Impact on Growth and Metabolism in Humans. in K Ho (ed), Growth Hormone Related Diseases and Therapy: A Molecular and Physiological Perspective for the Clinician, Contemporary Endocrinology. 2011:33-56.
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64(1):51-58.
- Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25(5):693-721.
- Kargi AY, Merriam GR. Diagnosis and treatment of growth hormone deficiency in adults. Nat Rev Endocrinol. 2013;9(6):335-345.
- Iacovazzo D, Hernandez-Ramirez LC, Korbonits M. Sporadic pituitary adenomas: the role of germline mutations and recommendations for genetic screening. Expert Rev Endocrinol Metab. 2017;12(2):143-153.
- Birzniece V, Ho KKY. Sex steroids and the GH axis: Implications for the management of hypopituitarism. Best Pract Res Clin Endocrinol Metab. 2017;31(1):59-69.
- Veldhuis JD, Evans WS, Bowers CY, Anderson S. Interactive regulation of postmenopausal growth hormone insulin-like growth factor axis by estrogen and growth hormone-releasing peptide-2. Endocrine. 2001;14(1):45-62.
- Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD. Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin's dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab. 2001;86(7):3143-3149.
- Anderson SM, Shah N, Evans WS, Patrie JT, Bowers CY, Veldhuis JD. Short-term estradiol supplementation augments growth hormone (GH) secretory responsiveness to dose-varying GH-releasing peptide infusions in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86(2):551-560.
- Murray PG, Higham CE, Clayton PE. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-GH axis: the past 60 years. J Endocrinol. 2015;226(2):T123-140.
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci U S A. 2005;102(46):16880-16885.
- Le Tissier PR, Carmignac DF, Lilley S, Sesay AK, Phelps CJ, Houston P, Mathers K, Magoulas C, Ogden D, Robinson IC. Hypothalamic growth hormone-releasing hormone (GHRH) deficiency: targeted ablation of GHRH neurons in mice using a viral ion channel transgene. Mol Endocrinol. 2005;19(5):1251-1262.
- Wajnrajch MP, Gertner JM, Harbison MD, Chua SC, Jr., Leibel RL. Nonsense mutation in the human growth hormone-releasing hormone receptor causes growth failure analogous to the little (lit) mouse. Nat Genet. 1996;12(1):88-90.
- Alatzoglou KS, Dattani MT. Genetic causes and treatment of isolated growth hormone deficiency-an update. Nat Rev Endocrinol. 2010;6(10):562-576.
- Ren SG, Taylor J, Dong J, Yu R, Culler MD, Melmed S. Functional association of somatostatin receptor subtypes 2 and 5 in inhibiting human growth hormone secretion. J Clin Endocrinol Metab. 2003;88(9):4239-4245.
- Copinschi G, Wegienka LC, Hane S, Forsham PH. Effect of arginine on serum levels of insulin and growth hormone in obese subjects. Metabolism. 1967;16(6):485-491.
- Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67(5):1361-1369.
- Rudman D. Growth hormone, body composition, and aging. J Am Geriatr Soc. 1985;33(11):800-807.
- Jorgensen JO, Vahl N, Hansen TB, Thuesen L, Hagen C, Christiansen JS. Growth hormone versus placebo treatment for one year in growth hormone deficient adults: increase in exercise capacity and normalization of body composition. Clin Endocrinol (Oxf). 1996;45(6):681-688.
- Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA. Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med. 1984;311(22):1403-1407.
- Vahl N, Jorgensen JO, Jurik AG, Christiansen JS. Abdominal adiposity and physical fitness are major determinants of the age associated decline in stimulated GH secretion in healthy adults. J Clin Endocrinol Metab. 1996;81(6):2209-2215.
- Vahl N, Jorgensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol. 1997;272(6 Pt 1):E1108-1116.
- Papadakis MA, Grady D, Tierney MJ, Black D, Wells L, Grunfeld C. Insulin-like growth factor 1 and functional status in healthy older men. J Am Geriatr Soc. 1995;43(12):1350-1355.
- Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145(11):970-976.
- Kiel DP, Puhl J, Rosen CJ, Berg K, Murphy JB, MacLean DB. Lack of an association between insulin-like growth factor-I and body composition, muscle strength, physical performance or self-reported mobility among older persons with functional limitations. J Am Geriatr Soc. 1998;46(7):822-828.
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78(3):744-752.
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1-6.
- Jorgensen JO, Flyvbjerg A, Lauritzen T, Alberti KG, Orskov H, Christiansen JS. Dose-response studies with biosynthetic human growth hormone (GH) in GH-deficient patients. J Clin Endocrinol Metab. 1988;67(1):36-40.
- Moller J, Jorgensen JO, Lauersen T, Frystyk J, Naeraa RW, Orskov H, Christiansen JS. Growth hormone dose regimens in adult GH deficiency: effects on biochemical growth markers and metabolic parameters. Clin Endocrinol (Oxf). 1993;39(4):403-408.
- Toogood AA, Shalet SM. Growth hormone replacement therapy in the elderly with hypothalamic-pituitary disease: a dose-finding study. J Clin Endocrinol Metab. 1999;84(1):131-136.
- Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA. Growth hormone (GH)-deficient men are more responsive to GH replacement therapy than women. J Clin Endocrinol Metab. 1997;82(2):550-555.
- Vahl N, Moller N, Lauritzen T, Christiansen JS, Jorgensen JO. Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: relation to age, sex, and body composition. J Clin Endocrinol Metab. 1997;82(11):3612-3618.
- Fisker S, Vahl N, Jorgensen JO, Christiansen JS, Orskov H. Abdominal fat determines growth hormone-binding protein levels in healthy nonobese adults. J Clin Endocrinol Metab. 1997;82(1):123-128.
- Aguiar-Oliveira MH, Bartke A. Growth Hormone Deficiency: Health and Longevity. Endocr Rev. 2019;40(2):575-601.
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13.
- Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, Barrett R, Workman M, Wawrowsky K, Ljubimov VA, Uhart M, Melmed S. Growth hormone is permissive for neoplastic colon growth. Proc Natl Acad Sci U S A. 2016;113(23):E3250-3259.
- Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30(1):51-74.
- Slater MD, Murphy CR. Co-expression of interleukin-6 and human growth hormone in apparently normal prostate biopsies that ultimately progress to prostate cancer using low pH, high temperature antigen retrieval. J Mol Histol. 2006;37(1-2):37-41.
- Bodart G, Farhat K, Charlet-Renard C, Salvatori R, Geenen V, Martens H. The Somatotrope Growth Hormone-Releasing Hormone/Growth Hormone/Insulin-Like Growth Factor-1 Axis in Immunoregulation and Immunosenescence. Front Horm Res. 2017;48:147-159.
- Villares R, Kakabadse D, Juarranz Y, Gomariz RP, Martinez AC, Mellado M. Growth hormone prevents the development of autoimmune diabetes. Proc Natl Acad Sci U S A. 2013.
- Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2006;7(4):225-235.
- Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front Endocrinol (Lausanne). 2018;9:35.
- Kelly PA, Djiane J, Postel-Vinay MC, Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991;12(3):235-251.
- Brooks AJ, Dai W, O'Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344(6185):1249783.
- Woelfle J, Chia DJ, Rotwein P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem. 2003;278(51):51261-51266.
- Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279(2):821-824.
- Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CK, Low TH, Leong GM, Ross RJ, Ho KK. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci U S A. 2003;100(3):1016-1021.
- Silva CM, Kloth MT, Whatmore AJ, Freeth JS, Anderson N, Laughlin KK, Huynh T, Woodall AJ, Clayton PE. GH and epidermal growth factor signaling in normal and Laron syndrome fibroblasts. Endocrinology. 2002;143(7):2610-2617.
- Hwa V, Little B, Adiyaman P, Kofoed EM, Pratt KL, Ocal G, Berberoglu M, Rosenfeld RG. Severe growth hormone insensitivity resulting from total absence of signal transducer and activator of transcription 5b. J Clin Endocrinol Metab. 2005;90(7):4260-4266.
- Jorgensen JO, Jessen N, Pedersen SB, Vestergaard E, Gormsen L, Lund SA, Billestrup N. GH receptor signaling in skeletal muscle and adipose tissue in human subjects following exposure to an intravenous GH bolus. Am J Physiol Endocrinol Metab. 2006;291(5):E899-905.
- Bak JF, Moller N, Schmitz O. Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am J Physiol. 1991;260(5 Pt 1):E736-742.
- Rosenfeld RG, Wilson DM, Dollar LA, Bennett A, Hintz RL. Both human pituitary growth hormone and recombinant DNA-derived human growth hormone cause insulin resistance at a postreceptor site. J Clin Endocrinol Metab. 1982;54(5):1033-1038.
- Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res. 2005;15(5):324-336.
- Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275(21):15985-15991.
- Ridderstrale M, Degerman E, Tornqvist H. Growth hormone stimulates the tyrosine phosphorylation of the insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase in primary adipocytes. J Biol Chem. 1995;270(8):3471-3474.
- Thirone AC, Carvalho CR, Saad MJ. Growth hormone stimulates the tyrosine kinase activity of JAK2 and induces tyrosine phosphorylation of insulin receptor substrates and Shc in rat tissues. Endocrinology. 1999;140(1):55-62.
- Olarescu NC, Bollerslev J. The Impact of Adipose Tissue on Insulin Resistance in Acromegaly. Trends Endocrinol Metab. 2016;27(4):226-237.
- del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, Kopchick JJ, Friedman JE, Draznin B, Thorner MO. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56(6):1638-1646.
- Takano A, Haruta T, Iwata M, Usui I, Uno T, Kawahara J, Ueno E, Sasaoka T, Kobayashi M. Growth hormone induces cellular insulin resistance by uncoupling phosphatidylinositol 3-kinase and its downstream signals in 3T3-L1 adipocytes. Diabetes. 2001;50(8):1891-1900.
- Jessen N, Djurhuus CB, Jorgensen JO, Jensen LS, Moller N, Lund S, Schmitz O. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab. 2005;288(1):E194-199.
- Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Moller N, Lund S, Jorgensen JO. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab. 2008;93(7):2842-2850.
- Feng X, Tang H, Leng J, Jiang Q. Suppressors of cytokine signaling (SOCS) and type 2 diabetes. Mol Biol Rep. 2014;41(4):2265-2274.
- Salmon WD, Jr., Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49(6):825-836.
- Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Arch Physiol Biochem. 2008;114(1):17-22.
- Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16(4-5):421-439.
- Kim JJ, Accili D. Signalling through IGF-I and insulin receptors: where is the specificity? Growth Horm IGF Res. 2002;12(2):84-90.
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23(6):824-854.
- Cabrera-Salcedo C, Mizuno T, Tyzinski L, Andrew M, Vinks AA, Frystyk J, Wasserman H, Gordon CM, Hwa V, Backeljauw P, Dauber A. Pharmacokinetics of IGF-1 in PAPP-A2-Deficient Patients, Growth Response, and Effects on Glucose and Bone Density. J Clin Endocrinol Metab. 2017;102(12):4568-4577.
- Fujimoto M, Andrew M, Liao L, Zhang D, Yildirim G, Sluss P, Kalra B, Kumar A, Yakar S, Hwa V, Dauber A. Low IGF-I Bioavailability Impairs Growth and Glucose Metabolism in a Mouse Model of Human PAPPA2 p.Ala1033Val Mutation. Endocrinology. 2019;160(6):1363-1376.
- Behringer RR, Lewin TM, Quaife CJ, Palmiter RD, Brinster RL, D'Ercole AJ. Expression of insulin-like growth factor I stimulates normal somatic growth in growth hormone-deficient transgenic mice. Endocrinology. 1990;127(3):1033-1040.
- Powell-Braxton L, Hollingshead P, Giltinan D, Pitts-Meek S, Stewart T. Inactivation of the IGF-I gene in mice results in perinatal lethality. Ann N Y Acad Sci. 1993;692:300-301.
- Gluckman PD, Gunn AJ, Wray A, Cutfield WS, Chatelain PG, Guilbaud O, Ambler GR, Wilton P, Albertsson-Wikland K. Congenital idiopathic growth hormone deficiency associated with prenatal and early postnatal growth failure. The International Board of the Kabi Pharmacia International Growth Study. J Pediatr. 1992;121(6):920-923.
- Savage MO, Blum WF, Ranke MB, Postel-Vinay MC, Cotterill AM, Hall K, Chatelain PG, Preece MA, Rosenfeld RG. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). J Clin Endocrinol Metab. 1993;77(6):1465-1471.
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335(18):1363-1367.
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229(1):141-162.
- Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93(2):571-598.
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321-326.
- Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177.
- BA H. The hypophysis and metabolism. N Engl J Med. 1936;214:961-985.
- Luft R, Ikkos D, Gemzell CA, Olivecrona H. Effect of human growth hormone in hypophysectomised diabetic subjects. Lancet. 1958;1(7023):721-722.
- Raben MS, Hollenberg CH. Effect of growth hormone on plasma fatty acids. J Clin Invest. 1959;38(3):484-488.
- Henneman DH, Henneman PH. Effects of human growth hormone on levels of blood urinary carbohydrate and fat metabolites in man. J Clin Invest. 1960;39:1239-1245.
- Hew FL, Koschmann M, Christopher M, Rantzau C, Vaag A, Ward G, Beck-Nielsen H, Alford F. Insulin resistance in growth hormone-deficient adults: defects in glucose utilization and glycogen synthase activity. J Clin Endocrinol Metab. 1996;81(2):555-564.
- Rabinowitz D, Klassen GA, Zierler KL. Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest. 1965;44:51-61.
- Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG, Orskov H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol. 1990;258(1 Pt 1):E86-91.
- Orskov L, Schmitz O, Jorgensen JO, Arnfred J, Abildgaard N, Christiansen JS, Alberti KG, Orskov H. Influence of growth hormone on glucose-induced glucose uptake in normal men as assessed by the hyperglycemic clamp technique. J Clin Endocrinol Metab. 1989;68(2):276-282.
- Moller N, Schmitz O, Joorgensen JO, Astrup J, Bak JF, Christensen SE, Alberti KG, Weeke J. Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab. 1992;74(5):1012-1019.
- Sonksen PH, Greenwood FC, Ellis JP, Lowy C, Rutherford A, Nabarro JD. Changes of carbohydrate tolerance in acromegaly with progress of the disease and in response to treatment. J Clin Endocrinol Metab. 1967;27(10):1418-1430.
- Moller N, Moller J, Jorgensen JO, Ovesen P, Schmitz O, Alberti KG, Christiansen JS. Impact of 2 weeks high dose growth hormone treatment on basal and insulin stimulated substrate metabolism in humans. Clin Endocrinol (Oxf). 1993;39(5):577-581.
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785-789.
- Nielsen S, Moller N, Christiansen JS, Jorgensen JO. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes. 2001;50(10):2301-2308.
- Nellemann B, Vendelbo MH, Nielsen TS, Bak AM, Hogild M, Pedersen SB, Bienso RS, Pilegaard H, Moller N, Jessen N, Jorgensen JO. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol (Oxf). 2014;210(2):392-402.
- Christopher M, Hew FL, Oakley M, Rantzau C, Alford F. Defects of insulin action and skeletal muscle glucose metabolism in growth hormone-deficient adults persist after 24 months of recombinant human growth hormone therapy. J Clin Endocrinol Metab. 1998;83(5):1668-1681.
- Rabinowitz D, Zierler KL. A METABOLIC REGULATING DEVICE BASED ON THE ACTIONS OF HUMAN GROWTH HORMONE AND OF INSULIN, SINGLY AND TOGETHER, ON THE HUMAN FOREARM. Nature. 1963;199:913-915.
- Jorgensen JO. Human growth hormone replacement therapy: pharmacological and clinical aspects. Endocr Rev. 1991;12(3):189-207.
- Tanner JM, Hughes PC, Whitehouse RH. Comparative rapidity of response of height, limb muscle and limb fat to treatment with human growth hormone in patients with and without growth hormone deficiency. Acta Endocrinol (Copenh). 1977;84(4):681-696.
- DB C. Effect of growth hormone on cell and somatic growth. . In: Handbook of physiology (Eds Knobil and Sawyer)Washington DC. 1974:159-186.
- Korner A. Growth hormone control of biosynthesis of protein and ribonucleic acid. Recent Prog Horm Res. 1965;21:205-240.
- Goldberg AL. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969;244(12):3217-3222.
- Horber FF, Haymond MW. Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J Clin Invest. 1990;86(1):265-272.
- Russell-Jones DL, Weissberger AJ, Bowes SB, Kelly JM, Thomason M, Umpleby AM, Jones RH, Sonksen PH. The effects of growth hormone on protein metabolism in adult growth hormone deficient patients. Clin Endocrinol (Oxf). 1993;38(4):427-431.
- Fryburg DA, Barrett EJ. Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism. 1993;42(9):1223-1227.
- Copeland KC, Nair KS. Acute growth hormone effects on amino acid and lipid metabolism. J Clin Endocrinol Metab. 1994;78(5):1040-1047.
- Fryburg DA, Gelfand RA, Barrett EJ. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol. 1991;260(3 Pt 1):E499-504.
- Turkalj I, Keller U, Ninnis R, Vosmeer S, Stauffacher W. Effect of increasing doses of recombinant human insulin-like growth factor-I on glucose, lipid, and leucine metabolism in man. J Clin Endocrinol Metab. 1992;75(5):1186-1191.
- Russell-Jones DL, Umpleby AM, Hennessy TR, Bowes SB, Shojaee-Moradie F, Hopkins KD, Jackson NC, Kelly JM, Jones RH, Sonksen PH. Use of a leucine clamp to demonstrate that IGF-I actively stimulates protein synthesis in normal humans. Am J Physiol. 1994;267(4 Pt 1):E591-598.
- Fryburg DA, Louard RJ, Gerow KE, Gelfand RA, Barrett EJ. Growth hormone stimulates skeletal muscle protein synthesis and antagonizes insulin's antiproteolytic action in humans. Diabetes. 1992;41(4):424-429.
- Copeland KC, Nair KS. Recombinant human insulin-like growth factor-I increases forearm blood flow. J Clin Endocrinol Metab. 1994;79(1):230-232.
- Fryburg DA. NG-monomethyl-L-arginine inhibits the blood flow but not the insulin-like response of forearm muscle to IGF- I: possible role of nitric oxide in muscle protein synthesis. J Clin Invest. 1996;97(5):1319-1328.
- Boger RH, Skamira C, Bode-Boger SM, Brabant G, von zur Muhlen A, Frolich JC. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study. J Clin Invest. 1996;98(12):2706-2713.
- Bartke A, Darcy J. GH and ageing: Pitfalls and new insights. Best Pract Res Clin Endocrinol Metab. 2017;31(1):113-125.
- Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol. 1992;262(3 Pt 1):E261-267.
- Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol. 1995;268(2 Pt 1):E268-276.
- Taaffe DR, Pruitt L, Reim J, Hintz RL, Butterfield G, Hoffman AR, Marcus R. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79(5):1361-1366.
- Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):708-716.
- Hermansen K, Bengtsen M, Kjaer M, Vestergaard P, Jorgensen JOL. Impact of GH administration on athletic performance in healthy young adults: A systematic review and meta-analysis of placebo-controlled trials. Growth Horm IGF Res. 2017;34:38-44.
- Waters D, Danska J, Hardy K, Koster F, Qualls C, Nickell D, Nightingale S, Gesundheit N, Watson D, Schade D. Recombinant human growth hormone, insulin-like growth factor 1, and combination therapy in AIDS-associated wasting. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125(11):865-872.
- Schambelan M, Mulligan K, Grunfeld C, Daar ES, LaMarca A, Kotler DP, Wang J, Bozzette SA, Breitmeyer JB. Recombinant human growth hormone in patients with HIV-associated wasting. A randomized, placebo-controlled trial. Serostim Study Group. Ann Intern Med. 1996;125(11):873-882.
Social and Environmental Factors Influencing Obesity
ABSTRACT
The evidence for social and environmental factors that contribute to obesity are often underappreciated. Obesity prevalence is significantly associated with sex, racial ethnic identity, and socioeconomic status, which creates complex relationships between each of these characteristics. Food availability remains an important factor associated with obesity that relates to differences in prevalence seen across geographical areas and higher rates of obesity within low socioeconomic status individuals. Proliferation of high calorie, energy dense food options that are or perceived as more affordable combined with reductions in occupational and transportation related physical activity can contribute to a sustained positive energy balance. Additionally, environments experiencing deprivation, disorder, or high crime have been shown to be associated with higher odds of obesity, which may appear more frequently in low social status individuals. Both objective and subjective measures of social status and inequality are associated with increased energy intake and decreased energy expenditure, which could place individuals of low social status at greater risk for obesity development. Given the complexity of this multifactorial disease, effective obesity care requires knowledge of these complex relationships and an integration between the health systems and surrounding community. Resources for practicing clinicians regarding methods of screening for social and environmental factors in clinical care are provided in addition to information on a program that has been widely dispersed and made accessible to those who may be the most at risk.
INTRODUCTION
Many medical providers appreciate the significant social and environmental determinants of obesity but are unsure how to address them. Others consider these factors outside of their control and scope of practice, and are thus hesitant to even broach the topic with their patients. Finally, many medical providers still attribute obesity to causes within a person’s control, such as dietary choices, amount of exercise, or willpower, (1, 2) which perpetuates a stigma that accompanies this disease. Specifically, the prevailing stigma is that those who suffer from obesity represent a population who lack the willingness to change their poor lifestyle habits or harbor a character flaw that, at its extreme, infers immoral behaviors (e.g., gluttony). In reality, obesity is a multifactorial disease (3) that is caused by a combination of biological, genetic, social, environmental, and behavioral determinants. In order to address this gap in the understanding of the social and environmental determinants of obesity and improve the care of patients with obesity, this chapter will review the evidence for the social and environmental determinants of obesity development. The specific areas to be covered include social identity, social status, societal trends, and influences of the built, industrial, and social environments, all factors that are closely associated with the prevalence or incidence of obesity or that impact efforts to prevent and treat this disease. Resources for the busy clinician that will support implemental changes in one’s practice to improve the care and management of patients with obesity, as well as evidenced-based opportunities for advocacy in the community, will be included in the final section.
This chapter is divided into three primary sections based on the progression of thought and evidence surrounding the social and environmental determinants of obesity: individual characteristics, environmental characteristics, and social hierarchy influences. Individual characteristics are those that are attributed to the individual with obesity such as their sex, age, race, ethnicity, and socioeconomic status (SES). Environmental characteristics surround the individual, including the physical spaces where people live, work, and play, as well as sociocultural norms. The social hierarchy refers to social status or social rank of individuals within larger society or a local community.
INDIVIDUAL CHARACTERISTICS
The prevalence of obesity varies according to key individual characteristics such as age, sex, race and ethnicity, and SES. The prevalence of obesity increases cross-sectionally across the lifespan: from 13.9%, in early childhood (2-5 years old) to 18.4% in childhood (6-11 years old), 20.6% in adolescence (12-19 years old), 35.7%, in young adulthood (20-39 years old), 42.8% in adulthood (40-59 years old), and 41.0% in older adulthood (≥60 years old) (4). As of 2016, the prevalence of adult obesity in women in the United States was 41.1% and in men was 37.9% (4). In the decade between 2007-2008 and 2015-2016, obesity significantly increased only in women (4), suggesting a sex-specific vulnerability to expression of this disease. Additionally, when race and ethnicity are considered, significant interactions between race and sex emerge. Non-Hispanic black, non-Hispanic Asian, and Hispanic women all have significantly higher prevalence of obesity than men with the same racial ethnic identity (5). In men and women, non-Hispanic Asians have significantly lower prevalence of obesity compared to all other major races and ethnicities in the United States (Note: not adjusted for ethnic specific cut points for Asians), and Non-Hispanic blacks and Hispanics have significantly higher prevalence of obesity compared to Non-Hispanic whites (5). It is not fully clear why differences in obesity prevalence by race and ethnicity are present, but some evidence points to differences in genetic backgrounds that affect body composition and fat distribution (6, 7), and to differences in cultural body image standards (8). Additionally, in the United States, race and ethnicity are confounded with SES, which is one of the most potent indicators of overall health in the United States (9).
A significantly greater proportion of underrepresented racial ethnic minorities are considered low SES compared to non-Hispanic Asians and non-Hispanic whites in the United States. Socioeconomic status is a composite measure that can be represented by measures of income, educational attainment, or occupational status. In the 2017 Census, 21.2% of non-Hispanic blacks and 18.3% of Hispanics lived below the poverty level compared to 8.7% of non-Hispanic whites and 10% of non-Hispanic Asians (10). Non-Hispanic Asians (53.9%) and non-Hispanic whites (36.2%) are more likely to earn a bachelor’s degree than non-Hispanic blacks (22.5%) and Hispanics (15.5%) (11). In terms of health, low SES in childhood is associated with adult development of cardiovascular risk factors and a 20% increase in the odds of having central obesity (as defined by a waist circumference >102 cm for men or > 88 cm for women) (12). In adult women, obesity prevalence increases with decreasing income and educational attainment; however, in non-Hispanic black women, obesity prevalence differs by education gradients but not by income gradients (13). Conversely, non-Hispanic black men have a higher prevalence of obesity in the highest income group, but all the men’s racial ethnic groups showed similar relationships between obesity rates and education gradients as women (13). Higher SES is also associated with healthy lifestyle behaviors that are often the first line of prevention or treatment for obesity. On the other hand, low SES is associated with less leisure time physical activity (14) and consumption of energy-dense diets that are nutrient poor (15); however, SES is not the only factor that influences these behaviors. Further exploration of how SES affects resources and the ability to practice healthy behaviors is expounded upon in the next section.
ENVIRONMENTAL CHARACTERISTICS
Geography
Obesity prevalence differs by geographical region in the United States with the South and the Midwest having the highest level of obesity among adults (16). The Midwest and South also have high rates of diabetes and metabolic syndrome, which frequently accompany obesity (16). Approximately 55% of global increases in BMI can be attributed to rising BMI in rural areas, and this may be as high as 80% in low- and middle-income countries (17). Rural areas are associated with 1.36 higher odds of obesity compared to urban areas; however, mediation analysis shows that individual educational attainment, neighborhood median household income, and neighborhood-built environment features reduce these odds by 94% and render the relationship statistically insignificant (18). Rural areas tend to have farther distances between residences and supermarkets, clinical settings, and recreational opportunities, which may be impacting the ability to practice healthy behaviors that prevent obesity. This is one example of the “built environment”, which alludes to the infrastructure of a geographic area that influences proximity to and types of resources, transportation methods, and neighborhood quality.
Food Availability
The frequency and type of food vendors in a neighborhood determines the types of foods that residents can purchase. Historically, evidence has suggested that fast food restaurant density is associated with obesity prevalence. A state-level analysis of fast food restaurant density and the number of residents per restaurant accounted for 6% of the variance in state obesity prevalence (19). Individual-level factors can interact with built environmental factors (like fast food restaurant density) to increase the odds of obesity. For example, one study in older adults showed that residents who ate 1-2 times per week at a fast food restaurant (odds ratio [OR]: 1.878), did not meet current physical activity guidelines (OR: 1.792), had low self-efficacy for eating healthy food (OR: 1.212), or identified as non-Hispanic black (OR: 8.057) and lived in a high density fast food neighborhood were more likely to have obesity than older adults who lived in a low density fast food neighborhood (20). On the other hand, recent research suggests that fast food restaurant density is not associated with obesity prevalence and the food consumed in these establishments’ accounts for less than 20% of the total energy intake (21). This could reflect the widespread availability of fast food nationally, which weakens the ability to dissect links between its presence and increased consumption specific to obesity.
The term “food desert” is often used to describe areas with limited access to affordable and nutritious food (e.g. supermarkets) and these vary significantly according to neighborhood socioeconomic and racial/ethnic composition (22, 23). Food desert designation has been positively linked to obesity in the United States and simply switching from a non-food desert census tract to a food desert census tract can increase the odds of obesity by 30%, when all other relevant factors are held constant (24). Conversely, access to supermarkets does not automatically result in healthier eating behavior and weight status. A systematic review showed that five out of six studies looking at supermarket access did not find increased fruit and vegetable consumption with greater accessibility; however, four out of five studies looking at changes in weight status found lower BMI and prevalence of obesity in areas with high access to supermarkets compared to low access areas (25). A large natural experiment found that the opening of a new supermarket improved overall diet quality in the neighborhood, but did not affect fruit and vegetable intake or BMI (26). Interestingly, the only positive outcome directly associated with regular use of the new supermarket was higher perceived access to healthy food (26). Although these findings are mixed, it is important to acknowledge that changes in food choices at a neighborhood level might occur too slowly to be captured in these studies.
In addition to food availability and quality, the shift in food type, amount, and pricing is also relevant to the obesity epidemic. For example, available evidence strongly supports a greater risk of weight gain and type 2 diabetes with increased consumption of sugar-sweetened beverages (27). North America still has the highest per capita sales of calorie sugar-sweetened beverages, but is slowly starting to shift to low-calorie sugar sweetened beverages, though sports and energy drink consumption continue to increase (28). Portion sizes in the most popular fast-food, take-out, and family style restaurants exceed current USDA and FDA standard-recommended portion amounts as well as what had been historically served in past decades (29). Increased portion sizes have been robustly linked to increases in energy intake in both adults and children; however, evidence is limited that decreasing portion size results in decreased energy intake (30). In addition, fast foods, snack foods, and foods available through convenience stores are typically ultra-processed (high in processed grains and added sugars; low in fiber and unsaturated fats). A recent study found that keeping macronutrient content the same, meals that were ultra-processed resulted in greater food intake and weight gain over a two-week follow-up compared to consumption of non-processed foods (31). Contributing to increased intake of fast-foods and ultra-processed foods is the marketing techniques implemented by food industries across multiple mediums. Though adults have shown to be less susceptible to the effects of food advertising, experimental studies with children produce a moderate effect size for increased food consumption after food advertising exposure (32). Food advertising targeted at children is focused on brand building and emotive messages may not be discerned as such by this vulnerable population (33). Another common misconception confronting consumers is that healthy foods are more expensive, but research suggests this perception is based on misleading price metrics as well as changes in fruit and vegetable convenience and level of preparedness (34). Price per calorie metrics show fruits and vegetables to be more expensive than less healthy foods; however, price per average portion and price per edible 100 grams actually shows that fruits and vegetables are less expensive (34). In times of financial constraint, socioeconomically disadvantaged groups maximize energy value for money resulting in energy-dense, nutrient poor diets that contribute to obesity (35).
Transportation
Infrastructure can dictate means of transportation and neighborhood walkability, which is associated with weight status. High neighborhood walkability has been found to be associated with decreased prevalence of overweight and obesity (36), which can link back to structural differences discussed earlier between urban and rural areas (urban areas having higher walkability). Transport-related physical activity decreased by 17.8% between 1965 and 2009 in the United States, which could be due to growing ubiquity of car ownership and supportive infrastructure for automotive transport in the United States (37). Proximity to recreational facilities, recreational facility density, access to sidewalks and paths that remove pedestrians from traffic hazards, and access to parks, have all been reported to be facilitators of physical activity in qualitative and quantitative research (38, 39).
The quality of infrastructure in a neighborhood and the perceived aesthetics of homes, shops, and recreational facilities can impact the use of these facilities. A study in a high-income neighborhood and a low-income neighborhood showed that even though the number of recreational facilities was equitable in the neighborhoods, the residents of the low-income neighborhood perceived that they had less access to recreational facilities (40).
Additional neighborhood descriptors that are associated with obesity include neighborhood deprivation, disorder, and crime. Neighborhood deprivation, a composite score of socioeconomic position of individuals in a neighborhood that is used to assign a rank to that neighborhood, shows that high levels of deprivation are associated with a 20% increased odds of overweight (41). Neighborhood physical disorder refers to the presence of vandalism, abandoned lots or vehicles, garbage, and quality of building conditions. Women in an urban area with high neighborhood physical disorder have a 1.43 greater odds of obesity (42). Persons living in areas of high crime have a 28% reduced odds of achieving higher levels of physical activity and, conversely, perceived safety increases the odds of achieving higher levels of physical activity by 27% (43). Living in a neighborhood with high crime has been found to be associated with increased weekly snack consumption in women (42). The relevance of the neighborhood environment to obesity is further exemplified in the Moving to Opportunities Study (44). The Department of Housing and Urban Development randomly assigned just under 5000 families in Chicago, Baltimore, Boston, Los Angeles, and New York public housing to 3 possible conditions: receive a housing voucher to move to a low-poverty census track with moving counseling, receive a standard unrestricted housing voucher and no moving counseling, or receive nothing. Despite the fact that this study was not focused on weight or diabetes outcomes, participants that received the voucher to move to a low-poverty census track had 4.61 percentage points lower prevalence of BMI > 35, BMI > 40, and glycated hemoglobin ≥ 6.5% than participants who received nothing (44), showing that a mere change in environment from high- to low-poverty rates was enough to have a significant impact.
Work Environment and Advances in Communication Technology
As the built environment and food environment have changed in the United States, so has the work environment. From 1960 to 2010, jobs in the U.S. private industry shifted from 50% requiring at least moderate to vigorous physical activity to less than 20% requiring this level of activity intensity (45). National Health and Nutrition Examination Survey data has documented an association between decreases in work-related energy expenditure and weight gain over the same time period (45). These changes in occupation related physical activity could be due to improvements in labor-saving technology. Technology advances are not confined to the work environment and have spread into many facets of daily life, such as improvements in smart personal communication devices, internet media platforms, marketing techniques, and enhanced audio-visual media. Studies show that marketing for unhealthy foods is often targeted at more vulnerable populations such as Non-Hispanic blacks (46) and Hispanics (47). Additionally, the availability of information about healthy weight-loss behaviors on the internet is poor when searched for in Spanish (48). “Screen time” or the time spent using technology that utilizes a screen interface has been found to be associated with increased risk for obesity (49-51); however, many app companies and academic researchers are now using that same technology to help with obesity prevention and treatment (52-54).
SOCIAL HIERARCHY
Animal research consistently shows that animals of subordinate status experience adverse physiological and behavioral changes compared to their high status counterparts: higher levels of cortisol (primates) (55), elevated blood pressure (rats, rabbits, baboons, macaques) (56), elevated heart rate (primates) (56), accumulation of visceral fat (rats) (57), increased ad-libitum energy-dense food consumption (macaques, rats) (57, 58), cardiovascular disease (mice) (59), and shortened lifespan (mice) (59). This implies that social standing, regardless of species, has physiological implications and could be contributing to obesity development and poor health. The findings from animal models thus serve as the basis for parallel outcomes reported in humans of low social status.
Social status can be measured objectively or subjectively. Objective measures typically include socioeconomic status (SES) variables, such as income, education, or occupation, which were discussed as individual level factors at the beginning of this chapter. Social status can also be represented by manifestations of status differentials, including inequality between groups or measurable differences in the ability for someone to obtain basic life necessities, such as food security. High levels of absolute income/wealth may be related to health not only through better material conditions, but also through social position. However, in an analysis of two nationally representative British panel studies, ranked position of income/wealth, not absolute income/wealth, predicted adverse health outcomes such as obesity, presence of chronic disease, and poor ratings of physical functioning and pain (60). In a worldwide study of physical activity, countries with large activity inequality predicted obesity better than the total volume of physical activity within the country (61). Activity inequality is identified by calculating a Gini coefficient for population step count data from each country, 0 = complete equality, 1= complete inequality. Individuals in the top five countries for physical activity inequality (Saudi Arabia, USA, Egypt, Canada, Australia) were 196% more likely to have obesity than individuals from more equal societies that did not have large disparities in step counts across the population. Gender differences account for 43% of the inequality observed, however, this effect was mitigated in societies that rated higher in walkability (61). Inequality can also drive calorie consumption. Individuals who are experimentally induced to view themselves as poor in reference to others exhibited increased calorie intake (62). Additionally, individuals who believed they were poorer or wealthier than an interaction partner exhibited higher levels of anxiety in regards to that difference in status that, in turn, led to increased calorie consumption (62).
Food insecurity affects approximately 11.8 percent of families in the United States and has been linked to obesity and diabetes. Food insecurity occurs when “the intake of one or more members of a household is reduced and eating patterns are disrupted (sometimes resulting in hunger) because of insufficient money and other resources for food” (63). In women, food insecurity status predicts overweight/obese status differentially across racial ethnic groups. Non-Hispanic white women who are food insecure are 41% more likely to have overweight or obesity whereas Hispanic women who are food insecure are 29% more likely to have overweight and obesity (64). Among non-Hispanic black women and men, food insecurity did not predict overweight or obesity status (64). A population-based study in Canada revealed that persons in food insecure households had double the risk of developing type 2 diabetes compared to persons in food secure households, even after controlling for age, gender, income, race, physical activity, smoking status, alcohol consumption, diet quality, and BMI (65). Reduced food availability is theorized to initiate compensatory biological mechanisms that boost caloric intake, decrease resting metabolic rate, and increase storage of adipose tissue as a protective mechanism for survival (66). Research in youth has provided evidence for a moderating effect of food insecurity on the relationship between income and subjective social status (67). This means that low income is more strongly associated with low subjective social status when the household is also food insecure.
Subjective measures of social status (SSS) are typically measured by asking individuals to place themselves on 10-rung ladders based on where they perceive their rank within society and the community. Experimental evidence demonstrates a relationship between feelings of low social status and increased calorie intake. Cornil and Chandon showed that hometowns of National Football League teams consumed more calories after a team loss than hometowns of winning teams or of hometowns where teams didn’t play (68). Manipulations of social status in an experimental setting show that acute eating behavior post experimental manipulation consists of higher calorie food choices and higher total calorie intake in the low status group (69). Additionally, individuals randomized to a low social status condition, had increased levels of ghrelin, a hormone that stimulates appetite, as compared to the high social status condition, suggesting a physiological hunger response to low perceived social status (70). Studies of physical activity and SSS show that low SSS is associated with significantly lower levels of moderate to vigorous physical activity (71, 72), which could contribute to a lower overall energy expenditure. Closely related to SSS are other perceptive representations of status differentials, such as perceived discrimination, which is associated with increased weight and BMI in women (73) and increased abdominal adiposity in non-Hispanic whites (74).
Researchers have integrated individual and environmental factors into design and development of interventions to improve weight outcomes or weight-related behaviors (healthy eating, physical activity); however, not all of them are successful. For example, a study among low-income women with children in rural Mexico randomly assigned families to cash or in-kind transfers (food baskets) and found that women in the food basket and cash groups actually gained weight compared to women in the control group (75). This study and others that show weight gain occurring in spite of access to resources or poverty relief imply accounting for individual and environmental factors alone may not paint a complete picture of obesity development. Granted, it is important to consider that systemic environmental changes, such as placement of sidewalks or fruits and vegetables in a corner store, may not be adequately captured in a short time frame typical of academic studies. However, the small or nonexistent changes observed when resources are supplied warrants further investigation into deeper realms of social hierarchical constructs, as well as continued study of individual and environmental factors to improve treatment and prevention of obesity.
CLINICAL IMPLICATIONS AND CONCLUSIONS
Given the extent of the information on individual, environmental, and social hierarchy constraints on obesity development, it is important to understand how these can merge with clinical care. It is evident that there is no one simple solution and effective care requires knowledge of these complex relationships and an integration between the health system and the surrounding community. For example, based on the knowledge that the social determinants of health can influence diabetes and its comorbidities, the American Diabetes Association recommends in its clinical guidelines that providers “assess the social context… and apply that information to treatment decisions” (76). In conjunction with recognition of the impact of social and environmental determinants on multiple chronic diseases, some researchers propose that “community vital signs” be integrated into the electronic health record (EHR) (77) and some community health centers have begun pilot testing a social determinants questionnaire in their HER (78). Knowledge provided by these “vital signs” and social determinants could help providers make appropriate lifestyle-tailored recommendations for the patient.
Discussing context surrounding food in a patient’s life can provide insight into the realistic expectations for a patient’s diet. Food insecurity can be identified with a short two question screener (79) and implementation in clinics has shown that screening improves clinician awareness of food insecurity, helping to better understand the lengths to which it affects patient treatment (80). Positive responses from physicians after pilot testing that incorporates screening into clinical practice mitigates concerns that discussions about food security would be stigmatizing to the patient (80). Patients who identify as food insecure can be referred to local food banks or community programs that will connect patients with resources at a federal and community level.
Patients that are finding it difficult to follow lifestyle modification recommendations to lose weight to prevent diabetes development may benefit from the Diabetes Prevention Program. The Diabetes Prevention Program is a lifestyle program focused on weight loss through dietary change and increased physical activity. While the overall weight loss was modest (~4% after 4 years), participants lowered their chances of developing diabetes by 58% during long-term follow-up (81). This program has been adapted for implementation and dissemination purposes and now the CDC’s National Diabetes Prevention (National DPP) program is available at almost 2,000 sites across the United States including many YMCAs, with a mix of online and in-person options. This program is covered for eligible individuals by Medicare and many private insurers and cost for non-covered patients is variable and often income-based or free. Initial evaluation of the real-world evidence for implementation of the National DPP have been promising with 35% achieving 5% weight loss and 42% meeting the activity goal of 150 minutes per week (82). Locations with the best participant retention and attendance share the following qualities: referrals from healthcare providers or health systems, provision of non-monetary incentives for participation, and use of cultural adaptations to address participant needs (83). The National DPP provides an affordable, easy and local referral source so that the provider can be assured their patients are receiving evidence-based lifestyle management in an ongoing program.
RESOURCES
Figure 1 below shows the age-adjusted prevalence of obesity in adults by race and ethnicity, and sex from the Centers for Disease Control 2017 National Center for Health Statistics Data Brief (5).
Figure 1. Prevalence of Obesity by Race/Ethnicity and Sex
Questions to Incorporate into Your EHR About Food Insecurity
- “We worried whether (my/our) food would run out before (I/we) got money to buy more” Was that often true, sometimes true, or never true for (you/your household) in the last 12 months?
- “The food that (I/we) bought just didn't last and (I/we) didn't have money to get more” Was that often true, sometimes true, or never true for (you/your household) in the last 12 months?
Information on the Diabetes Prevention Program
https://nccd.cdc.gov/DDT_DPRP/Registry.aspx
Opportunities for Advocacy
The Obesity Action Coalition: https://www.obesityaction.org/
The Obesity Society: https://www.obesity.org/
STOP Obesity Alliance: http://stop.publichealth.gwu.edu/
Rudd Center for Food Policy and Obesity: http://www.uconnruddcenter.org/weight-bias-stigma
REFERENCES
- Sikorski C, Luppa M, Kaiser M, et al. The stigma of obesity in the general public and its implications for public health - A systematic review. BMC Public Health. 2011;11(1):661. doi:10.1186/1471-2458-11-661
- Tsai AG, Histon T, Kyle TK, Rubenstein N, Donahoo WT. Evidence of a gap in understanding obesity among physicians. Obes Sci Pract. 2018;4(1):46-51. doi:10.1002/osp4.146
- Allison (chair) DB, Downey (co-chair) M, Atkinson RL, et al. Obesity as a Disease: A White Paper on Evidence and Arguments Commissioned by the Council of The Obesity Society. Obesity. 2008;16(6):1161-1177. doi:10.1038/oby.2008.231
- Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in usyouth and adults by sex and age, 2007-2008 to 2015-2016. JAMA - J Am Med Assoc. 2018;319(16):1723-1725. doi:10.1001/jama.2018.3060
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth : United States, 2015–2016. (U.S.) NC for HS, ed. https://stacks.cdc.gov/view/cdc/49223.
- Fernández JR, Shiver MD. Using genetic admixture to study the biology of obesity traits and to map genes in admixed populations. Nutr Rev. 2004;62(7 Pt 2):S69-S74. doi:10.1301/nr.2004.jul.S69-S74
- Cardel M, Higgins PB, Willig AL, et al. African genetic admixture is associated with body composition and fat distribution in a cross-sectional study of children. Int J Obes. 2011;35(1):60-65. doi:10.1038/ijo.2010.203
- Kronenfeld LW, Reba-Harrelson L, Von Holle A, Reyes ML, Bulik CM. Ethnic and racial differences in body size perception and satisfaction. Body Image. 2010;7(2):131-136. doi:10.1016/j.bodyim.2009.11.002
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic Disparities in Health in the United States: What the Patterns Tell Us. Am J Public Health. 2010;100(S1):S186-S196. doi:10.2105/AJPH.2009.166082
- Fontenot K, Semega J, Kollar M. Income and and Poverty Poverty the United States : 2017. 2018;(September 2018).
- Ryan CL, Bauman K. Educational attainment in the United States: 2015 population characteristics. United States Census Bur. 2016;2010:20-578. doi:P20-578
- Kivimäki M, Davey Smith G, Juonala M, et al. Socioeconomic position in childhood and adult cardiovascular risk factors, vascular structure, and function: Cardiovascular risk in young Finns study. Heart. 2006. doi:10.1136/hrt.2005.067108
- Ogden CL, Fakhouri TH, Carroll MD, et al. Prevalence of Obesity Among Adults, by Household Income and Education — United States, 2011–2014. MMWR Morb Mortal Wkly Rep. 2017;66(50):1369. doi:10.15585/MMWR.MM6650A1
- O’Donoghue G, Kennedy A, Puggina A, et al. Socio-economic determinants of physical activity across the life course: A “DEterminants of DIet and Physical ACtivity” (DEDIPAC) umbrella literature review. Henchoz Y, ed. PLoS One. 2018;13(1):e0190737. doi:10.1371/journal.pone.0190737
- Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117. doi:10.1093/ajcn/87.5.1107
- Gurka MJ, Filipp SL, DeBoer MD. Geographical variation in the prevalence of obesity, metabolic syndrome, and diabetes among US adults. Nutr Diabetes. 2018;8(1):14. doi:10.1038/s41387-018-0024-2
- Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569(7755):260-264. doi:10.1038/s41586-019-1171-x
- Wen M, Fan JX, Kowaleski-Jones L, Wan N. Rural–Urban Disparities in Obesity Prevalence Among Working Age Adults in the United States: Exploring the Mechanisms. Am J Heal Promot. 2018;32(2):400-408. doi:10.1177/0890117116689488
- Maddock J. The relationship between obesity and the prevalence of fast food restaurants: State-level analysis. Am J Heal Promot. 2004;19(2):137-143. doi:10.4278/0890-1171-19.2.137
- Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D. Obesity and the built environment: does the density of neighborhood fast-food outlets matter? Am J Health Promot. 2009;23(3):203-209. doi:10.4278/ajhp.071214133
- Mazidi M, Speakman JR. Higher densities of fast-food and full-service restaurants are not associated with obesity prevalence. Am J Clin Nutr. 2017;106(2):603-613. doi:10.3945/ajcn.116.151407
- Moore L V., Diez Roux A V. Associations of Neighborhood Characteristics With the Location and Type of Food Stores. Am J Public Health. 2006;96(2):325-331. doi:10.2105/AJPH.2004.058040
- Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Neighborhood Racial Composition, Neighborhood Poverty, and the Spatial Accessibility of Supermarkets in Metropolitan Detroit. Am J Public Health. 2005;95(4):660-667. doi:10.2105/AJPH.2004.042150
- Chen D, Jaenicke EC, Volpe RJ. Food Environments and Obesity: Household Diet Expenditure Versus Food Deserts. Am J Public Health. 2016;106(5):881-888. doi:10.2105/AJPH.2016.303048
- Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev. 2011;12(5):e95-e106. doi:10.1111/j.1467-789X.2010.00769.x
- Dubowitz T, Ghosh-Dastidar M, Cohen DA, et al. Diet And Perceptions Change With Supermarket Introduction In A Food Desert, But Not Because Of Supermarket Use. Health Aff. 2015;34(11):1858-1868. doi:10.1377/hlthaff.2015.0667
- Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14(8):606-619. doi:10.1111/obr.12040
- Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4(2):174-186. doi:10.1016/S2213-8587(15)00419-2
- Young LR, Nestle M. The contribution of expanding portion sizes to the US obesity epidemic. Am J Public Health. 2002;92(2):246-249. doi:10.2105/AJPH.92.2.246
- Livingstone MBE, Pourshahidi LK. Portion Size and Obesity. Adv Nutr. 2014;5(6):829-834. doi:10.3945/an.114.007104
- Hall KD, Ayuketah A, Brychta R, et al. Clinical and Translational Report Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake Cell Metabolism Clinical and Translational Report Ultra-Processed Diets Cause Excess Ca. Cell Metab. 2019;30(1):1-11. doi:10.1016/j.cmet.2019.05.008
- Boyland EJ, Nolan S, Kelly B, et al. Advertising as a cue to consume: a systematic review and meta-analysis of the effects of acute exposure to unhealthy food and nonalcoholic beverage advertising on intake in children and adults. Am J Clin Nutr. 2016;103(2):519-533. doi:10.3945/ajcn.115.120022
- Story M, French S. Food Advertising and Marketing Directed at Children and Adolescents in the US. Int J Behav Nutr Phys Act. 2004;1(1):3. doi:10.1186/1479-5868-1-3
- Carlson A, Frazão E. Food costs, diet quality and energy balance in the United States. Physiol Behav. 2014;134(C):20-31. doi:10.1016/j.physbeh.2014.03.001
- Lee A, Mhurchu CN, Sacks G, et al. Monitoring the price and affordability of foods and diets globally. Obes Rev. 2014;14(November 2012):82-95. doi:10.1111/obr.12078
- Creatore MI, Glazier RH, Moineddin R, et al. Association of Neighborhood Walkability With Change in Overweight, Obesity, and Diabetes. JAMA. 2016;315(20):2211. doi:10.1001/jama.2016.5898
- Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13(8):659-680. doi:10.1111/j.1467-789X.2011.00982.x
- Salvo G, Lashewicz BM, Doyle-Baker PK, McCormack GR. Neighbourhood Built Environment Influences on Physical Activity among Adults: A Systematized Review of Qualitative Evidence. Int J Environ Res Public Health. 2018;15(5). doi:10.3390/ijerph15050897
- Smith M, Hosking J, Woodward A, et al. Systematic literature review of built environment effects on physical activity and active transport - an update and new findings on health equity. Int J Behav Nutr Phys Act. 2017;14(1):158. doi:10.1186/s12966-017-0613-9
- Giles-Corti B, Donovan RJ. Socioeconomic status differences in recreational physical activity levels and real and perceived access to a supportive physical environment. Prev Med (Baltim). 2002. doi:10.1006/pmed.2002.1115
- van Lenthe F, Mackenbach J. Neighbourhood deprivation and overweight: the GLOBE study. Int J Obes. 2002;26(2):234-240. doi:10.1038/sj.ijo.0801841
- Mayne SL, Jose A, Mo A, et al. Neighborhood disorder and obesity-related outcomes among women in Chicago. Int J Environ Res Public Health. 2018;15(7). doi:10.3390/ijerph15071395
- Rees-Punia E, Hathaway ED, Gay JL. Crime, perceived safety, and physical activity: A meta-analysis. Prev Med (Baltim). 2018;111(October 2017):307-313. doi:10.1016/j.ypmed.2017.11.017
- Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, Obesity, and Diabetes — A Randomized Social Experiment. N Engl J Med. 2011. doi:10.1056/NEJMsa1103216
- Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 Decades in U.S. Occupation-Related Physical Activity and Their Associations with Obesity. Lucia A, ed. PLoS One. 2011;6(5):e19657. doi:10.1371/journal.pone.0019657
- Grier SA, Kumanyika SK. The Context for Choice: Health Implications of Targeted Food and Beverage Marketing to African Americans. Am J Public Health. 2008;98(9):1616-1629. doi:10.2105/AJPH.2007.115626
- Adeigbe RT, Baldwin S, Gallion K, Grier S, Ramirez AG. Food and Beverage Marketing to Latinos. Heal Educ Behav. 2015;42(5):569-582. doi:10.1177/1090198114557122
- Cardel MI, Chavez S, Bian J, et al. Accuracy of weight loss information in Spanish search engine results on the internet. Obesity. 2016;24(11):2422-2434. doi:10.1002/oby.21646
- Robinson TN, Banda JA, Hale L, et al. Screen Media Exposure and Obesity in Children and Adolescents. Pediatrics. 2017;140(Suppl 2):S97-S101. doi:10.1542/peds.2016-1758K
- Banks E, Jorm L, Rogers K, Clements M, Bauman A. Screen-time, obesity, ageing and disability: findings from 91 266 participants in the 45 and Up Study. Public Health Nutr. 2011;14(1):34-43. doi:10.1017/S1368980010000674
- Mitchell JA, Rodriguez D, Schmitz KH, Audrain-McGovern J. Greater screen time is associated with adolescent obesity: A longitudinal study of the BMI distribution from Ages 14 to 18. Obesity. 2013;21(3):572-575. doi:10.1002/oby.20157
- Lee AM, Chavez S, Bian J, et al. Efficacy and effectiveness of mobile health technologies for facilitating physical activity in adolescents: Scoping review. JMIR mHealth uHealth. 2019;7(2). doi:10.2196/11847
- D.E. S, G. T-M, S.J. J, S. W. Mobile apps for pediatric obesity prevention and treatment, healthy eating, and physical activity promotion: Just fun and games? Transl Behav Med. 2013;3(3):320-325. http://link.springer.com/article/10.1007/s13142-013-0206-3. Accessed June 10, 2016.
- Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16(5):376-392. doi:10.1111/obr.12268
- Abbott DH, Keverne EB, Bercovitch FB, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43(1):67-82. http://www.ncbi.nlm.nih.gov/pubmed/12614636. Accessed April 16, 2019.
- Sapolsky RM. Social Status and Health in Humans and Other Animals. Annu Rev Anthropol. 2004;33(1):393-418. doi:10.1146/annurev.anthro.33.070203.144000
- Tamashiro KLK, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89(4):536-542. doi:10.1016/j.physbeh.2006.05.026
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiol Behav. 2008;94(4):586-594. doi:10.1016/j.physbeh.2008.03.019
- Razzoli M, Nyuyki-Dufe K, Gurney A, et al. Social stress shortens lifespan in mice. Aging Cell. 2018. doi:10.1111/acel.12778
- Daly M, Boyce C, Wood A. A social rank explanation of how money influences health. Heal Psychol. 2015. doi:10.1037/hea0000098
- Althoff T, Sosič R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017;547(7663):336-339. doi:10.1038/nature23018
- Bratanova B, Loughnan S, Klein O, Claassen A, Wood R. Poverty, inequality, and increased consumption of high calorie food: Experimental evidence for a causal link. Appetite. 2016;100:162-171. doi:10.1016/j.appet.2016.01.028
- Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household Food Security in the United States in 2016. 2017.
- Hernandez DC, Reesor LM, Murillo R. Food insecurity and adult overweight/obesity: Gender and race/ethnic disparities. Appetite. 2017;117:373-378. doi:10.1016/j.appet.2017.07.010
- Tait CA, L’Abbé MR, Smith PM, Rosella LC. The association between food insecurity and incident type 2 diabetes in Canada: A population-based cohort study. PLoS One. 2018. doi:10.1371/journal.pone.0195962
- Dhurandhar EJ. The food-insecurity obesity paradox: A resource scarcity hypothesis. Physiol Behav. 2016. doi:10.1016/j.physbeh.2016.04.025
- Cardel MI, Tong S, Pavela G, et al. Youth Subjective Social Status (SSS) is Associated with Parent SSS, Income, and Food Insecurity but not Weight Loss Among Low-Income Hispanic Youth. Obesity. 2018;26(12):1923-1930. doi:10.1002/oby.22314
- Cornil Y, Chandon P. From Fan to Fat? Vicarious Losing Increases Unhealthy Eating, but Self-Affirmation Is an Effective Remedy. Psychol Sci. 2013;24(10):1936-1946. doi:10.1177/0956797613481232
- Cardel MI, Johnson SL, Beck J, et al. The effects of experimentally manipulated social status on acute eating behavior: A randomized, crossover pilot study. Physiol Behav. 2015;162:93-101. doi:10.1016/j.physbeh.2016.04.024
- Cheon BK, Hong Y-Y. Mere experience of low subjective socioeconomic status stimulates appetite and food intake. Proc Natl Acad Sci. 2017. doi:10.1073/pnas.1607330114
- Frerichs L, Huang TTK, Chen DR. Associations of subjective social status with physical activity and body mass index across four asian countries. J Obes. 2014. doi:10.1155/2014/710602
- Rajala K, Kankaanpää A, Laine K, Itkonen H, Goodman E, Tammelin T. Associations of subjective social status with accelerometer-based physical activity and sedentary time among adolescents. J Sports Sci. June 2018:1-8. doi:10.1080/02640414.2018.1485227
- Bernardo C de O, Bastos JL, González-Chica DA, Peres MA, Paradies YC. Interpersonal discrimination and markers of adiposity in longitudinal studies: a systematic review. Obes Rev. 2017;18(9):1040-1049. doi:10.1111/obr.12564
- Hunte HER, Williams DR. The association between perceived discrimination and obesity in a population-based multiracial and multiethnic adult sample. Am J Public Health. 2009;99(7):1285-1292. doi:10.2105/AJPH.2007.128090
- Leroy JL, Gadsden P, Gonzalez de Cossio T, Gertler P. Cash and in-Kind Transfers Lead to Excess Weight Gain in a Population of Women with a High Prevalence of Overweight in Rural Mexico. J Nutr. 2013. doi:10.3945/jn.112.167627
- American Diabetes Association AD. 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S7-S12. doi:10.2337/dc19-S001
- Bazemore AW, Cottrell EK, Gold R, et al. “Community vital signs” : incorporating geocoded social determinants into electronic records to promote patient and population health. J Am Med Informatics Assoc. 2016;23(2):407-412. doi:10.1093/jamia/ocv088
- Gold R, Bunce A, Cowburn S, et al. Adoption of Social Determinants of Health EHR Tools by Community Health Centers. Ann Fam Med. 2018;16(5):399-407. doi:10.1370/afm.2275
- Gundersen C, Engelhard EE, Crumbaugh AS, Seligman HK. Brief assessment of food insecurity accurately identifies high-risk US adults. Public Health Nutr. 2017;20(8):1367-1371. doi:10.1017/S1368980017000180
- Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons Learned from Implementation of the Food Insecurity Screening and Referral Program at Kaiser Permanente Colorado. Perm J. 2018;22:18-093. doi:10.7812/TPP/18-093
- Diabetes Prevention Program (DPP) | NIDDK. National Institute of Diabetes and Digestive and Kidney Disease. https://www.niddk.nih.gov/about-niddk/research-areas/diabetes/diabetes-prevention-program-dpp. Published 2018. Accessed May 6, 2019.
- Ely EK, Gruss SM, Luman ET, et al. A National Effort to Prevent Type 2 Diabetes: Participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331-1341. doi:10.2337/dc16-2099
- Nhim K, Gruss SM, Porterfield DS, et al. Using a RE-AIM framework to identify promising practices in National Diabetes Prevention Program implementation. Implement Sci. 2019;14(1):81. doi:10.1186/s13012-019-0928-9
Growth Hormone in Aging
ABSTRACT
Growth hormone (GH) serves important roles in adult life, including maintenance of lean body mass and bone mass, promoting lipolysis, thereby limiting visceral adiposity, and regulating carbohydrate metabolism, cardiovascular system function, aerobic exercise capacity, and cognitive function. Younger adults with growth hormone deficiency (AGHD) exhibit abnormalities in body composition, physical and cognitive function, and quality of life which are reversed by GH replacement therapy. With advancing age GH production declines, paralleled by physical and functional alterations similar to those of AGHD; however, the degree to which the decrease in GH contributes to these age-related changes is unknown. Seemingly in opposition to the theory that the diminished GH secretion of older age is a net detriment are observations that animal models of congenital GH deficiency have remarkably increased life span and humans with congenital GH deficiency may have decreased rates of age-related diseases such as diabetes and cancer. Several short-term studies aiming to increase GH in older adults by a variety of interventions including exercise, administration of GH, or treatment with GH secretagogues have demonstrated consistent effects to improve body composition, yet inconsistent effects on physical and cognitive function. While side effects of GH administration in older adults include edema, arthralgias, and elevated blood glucose, data regarding the possible long-term effects on “hard end points” such as risk of fractures, cancer, cardiovascular disease, life expectancy, and mortality are lacking.
INTRODUCTION
The decline in growth hormone secretion observed with aging is associated with changes in body composition and physical and psychological function that are similar to those seen in younger adult patients with growth hormone deficiency. These changes include reductions in lean body mass and muscle strength and an increase in body fat, particularly in the visceral compartment. Memory and cognitive function gradually deteriorate with age. Deep (slow-wave) sleep also decreases markedly with age, together with a decrease in nighttime growth hormone secretion, and sleep disorders become a significant clinical problem in old age. Although these changes only show an association, and it is still unknown whether there is any causal link between them, they have led to speculation that replacing or stimulating growth hormone may reverse some of the detrimental features of the aging process (1).
AGE-RELATED CHANGES IN GROWTH HORMONE SECRETION
The trophic hormones which rise at puberty, including sex steroids and GH, have dramatic effects on body composition and strength. Their levels plateau in young adult life and then decline progressively, accompanied by a loss of muscle mass and aerobic capacity, and an increase in abdominal fat. These changes resemble some features of hypogonadism and adult GH deficiency (2).
After the third decade of life, there is a progressive decline of GH secretion by approximately 15% for every decade of adult life. Integrated measurements of daily GH secretion demonstrate that secretion peaks at puberty at about 150 µg/kg day, then decreases to approximately 25 µg/kg/day by age 55 (3). The reduction in GH secretion results from a marked reduction in GH pulse amplitude, with only very little change in pulse frequency (4). This process is characterized by lack of day-night GH rhythm resulting from loss of nocturnal sleep-related GH pulses (Figure 1) (4). Growth Hormone Binding Protein decreases from 60 years of age, theoretically increasing the amount of bioavailable growth hormone (5). This decrease is thought to parallel the decrease in growth hormone receptors with age. Though slow-wave sleep (SWS) decreases with age most studies administering GH or GHRH to seniors did not improve SWS. This finding suggests that the age-related decline in GH does not cause reduced SWS, although the reverse may be true.
FIGURE 1. Patterns of GH secretion in younger and older women and men. There is a marked age-related decline in GH secretion in both sexes and a loss of the nighttime enhancement of GH secretion seen during deep (slow-wave) sleep. This decrease is primarily due to a reduction in GH pulse amplitude, with little change in pulse frequency. L = large GH pulses, S = small GH pulses. From Ho et al. 1987 (4).
Circulating levels of IGF-I, the main mediator for the trophic effects of growth hormone, also decline with age (Figure 2). The majority of circulating IGF-I is produced in the liver under the control of GH. It appears that the age-related decline in IGF-I production is a direct result of decreases in GH and there is no evidence to suggest increased “GH resistance”. In fact, studies of GH replacement therapy in patients with pituitary disorders and dose-response studies demonstrate a reduction in GH dose necessary to maintain normal IGF-I concentrations in older subjects, although this is due at least in part to the higher susceptibility to side effects from GH and also that their target IGF-1 is lower (6-7).
FIGURE 2. Changes in serum IGF-I with increasing age. Modified from Juul A et al,1994 (8).
POTENTIAL MECHANISMS UNDERLYING THE DECLINE IN GH SECRETION WITH AGE
Three hypothalamic factors regulate GH secretion: somatostatin (SRIF), growth-hormone releasing hormone (GHRH) and ghrelin (9) (Figure 3). Somatostatin is a noncompetitive inhibitor of GH secretion as well as of other hormones. It modulates the GH response to GHRH. GHRH is the principal stimulator for GH synthesis and release. Ghrelin is the endogenous ligand to the growth hormone secretagogue receptor-1a (GHSR-1a). Ghrelin is secreted primarily by the stomach and has appetite-stimulating activity separate from its effect on GH secretion. Although recent preclinical data suggest that not all the effects of ghrelin are mediated through GHSR-1a (10), its orexigenic and GH secretagogue effects require the presence of the GHSR-1a (11).Acylated Ghrelin levels decrease with age (12) Growth hormone secretagogues (GHS) are synthetic molecules that stimulate the GHSR-1a exhibiting strong growth hormone-releasing activity.
A variety of stimuli and inhibitors, such as exercise, sleep, food intake, stress and body composition have effects on the hypothalamic factors that regulate GH production (13). All of these factors interact to generate the physiological patterns of pulsatile GH secretion.
FIGURE 3. Major components of the GH neuroregulatory system (3).
There are several mechanisms that could explain the age-related decrease in GH secretion. Possibilities include decreased secretion of GHRH or ghrelin, increase in inhibition by somatostatin, increased sensitivity of somatotrophs to negative feedback inhibition by IGF-I, decline in pituitary responsiveness to GHRH, and pituitary and/or hypothalamic responsiveness to ghrelin.
Whether the aging pituitary responds normally to GHRH and ghrelin is still a matter of debate. Although earlier studies suggest no age–related decline in GH responsiveness to GHRH (13), more recent reports suggest a gender-independent, age-related decline in the GH responsiveness to GHRH and ghrelin (14) (Figure 4).There is no age-related increase in GH sensitivity to IGF-1 (15); however, there may be relative deficiency in GHRH and ghrelin secretion, and an increase in SRIF secretion, in older individuals (16). The density of GHRS-1a receptors in the hypothalamus decreases with aging and this is thought to be responsible for the age-related decreased response to some GHS (17). The aging pituitary is also less responsive to exercise, sleep, and other physiologic stimuli. Based on these observations it is most likely that the age-related change in GH secretion is multifactorial in etiology and is caused by changes above the level of the pituitary.
FIGURE 4. Approximate peak GH relative change in response to I.V. bolus of Ghrelin and GHRH is diminished in older adults compared to young males. Adapted from Broglio F et al. (14).
DECREASE IN GH IN NORMAL AGING: SIMILARITIES WITH AND DIFFERENCES FROM ADULT GROWTH HORMONE DEFICIENCY
Although not a perfect parallel with aging, adult growth hormone deficiency (AGHD) is the best documented source of information on signs and symptoms of reduced GH secretion, effects of treatment, dosing strategies appropriate for adults, and side effects and safety of GH replacement.
Several aspects of normal aging resemble features of the AGHD syndrome, including decrease in muscle and bone mass, increased visceral fat, diminishing exercise and cardiac capacity, atherogenic alterations in lipid profile, thinning of skin, and many psychological and cognitive problems (18-19) (Table 1). Although these changes and the GH deficit of aging are milder than seen in AGHD, they remain clinically significant (20).
|
Table 1. Features of Adult Growth Hormone Deficiency (3) |
|
Increased fat mass especially abdominal fat |
|
Decreased lean body mass |
|
Decreased muscle strength |
|
Decreased cardiac capacity |
|
Decreased exercise performance |
|
Decreased bone mass |
|
Decreased RBC volume |
|
Atherogenic lipid profile |
|
Thin dry skin |
|
Impaired sweating |
|
Poor venous access |
|
Psychosocial problems- Ø low self-esteem Ø depression Ø anxiety Ø fatigue and listlessness Ø sleep disturbances Ø emotional lability and poor self-control Ø social isolation Ø poor marital and social-economic performance |
It is important to distinguish the normal decrease in GH secretion associated with aging from true AGHD. Although aging is a state of relative physiologic GH deficiency, it is not a disease in itself and is clearly a separate entity from AGHD. This is demonstrated by higher GH secretion and physiological responses seen in older adults when compared with AGHD patients of similar age (2, 20). Moreover, aging per-se is not an indication for AGHD diagnosis testing or administration (21, 22).
Biochemical tests for AGHD diagnosis are imperfect, and their accuracy is strongly affected by the pre-test probability of the condition. Therefore, the most important indicator of the likelihood of GHD is the clinical context (21). In the majority of cases this is due to tumors arising in the region of the sella turcica or the treatment for these tumors including surgery and radiation, but there are other etiologies. Traumatic brain injury is an increasingly recognized cause of AGHD and may occur without coexisting deficiencies in other anterior pituitary hormones (22). IGF-1 levels alone are generally not enough for AGHD diagnosis, hence the need for provocative testing with the insulin tolerance test, glucagon stimulation test, or when available the combined GHRH-arginine test (23).
The GHS and ghrelin mimetic, macimorelin, has recently been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for AGHD diagnosis., Macimorelin provides a simple, orally-available, well-tolerated, reproducible, and safe diagnostic test for AGHD with comparable accuracy to the insulin tolerance test in adults (24). Since studies of macimorelin excluded individuals over the age of 65 or with a BMI >40, the safety and efficacy of this test in these populations has not been established.
CLINICAL CONSEQUENCES OF AGE-RELATED DECREASE IN GH SECRETION
The age-related increase in body mass index, changes in body composition, and diminished functional capacity parallel the age-associated decline in growth hormone secretion (18, 19). The alterations in body composition that are most pronounced in normal aging include a reduction in bone density and in muscle mass and strength, an increase in body fat, and adverse changes in lipoprotein profiles (2, 16). This decline in GH production is initially clinically silent, but over time may contribute to sarcopenic obesity and frailty.
The decline in GH may also play a role in cognitive changes observed with aging. One of the many systems for classifying different cognitive domains is grouping them as either “crystallized” or “fluid” intelligence. Crystallized intelligence generally refers to vocabulary and long-term memory; whereas the fluid intelligence includes short term memory and active problem-solving and demonstrates a more marked age-related decline. Several studies have shown a correlation between plasma IGF-I concentrations and performance on tests of fluid intelligence (25), suggesting that GH may play a role in maintenance of fluid intelligence.
Mechanistic insights into the role of growth hormone and IGF-I in age-related alterations in cognitive function were assessed in several studies by Sonntag and colleagues demonstrating somatotrophic effects on rodent brain aging (26). These studies suggest that deficiencies in GH and IGF-I contribute to the functional decline in senescent rats whereas augmentation of GH or IGF-I improved cognitive function, increased glucose utilization throughout the brain, increased cortical vascularity, and ameliorated age-related decline in hippocampal neurogenesis. Although some small studies suggest a positive effect of GHRH or GH replacement on cognition (27), there is insufficient data to recommend GH deficiency testing or GH treatment solely for this purpose (22).
GROWTH HORMONE IN AGING: CAVEATS REGARDING LONGEVITY
Animal studies have called into question the hypothesis that interventions aiming to increase growth hormone secretion and IGF-I should be considered a net benefit (28). In numerous species from nematodes to rodents, caloric restriction, which lowers IGF-1, has been associated with an increased life span. Mice with growth hormone resistance and profoundly reduced IGF-I levels appear healthy and have increased life expectancy, along with reduced fasting glucose and insulin levels (29, 30). In mice with mutations in the gene necessary for the differentiation of pituitary cells to produce GH, life span increases by 42% and tumor development is delayed (30). These findings are accentuated when combined with caloric restriction, while treatment with GH actually reduces lifespan. Mice treated with a metalloproteinase that cleaves an IGF-binding protein to decrease the bioavailability of IGF-1 have a 38% longer lifespan and a lower incidence of tumors. In experiments conducted with a mouse strain prone to developing age-related cognitive decline and decreased life expectancy, treatment with a GHRH-receptor antagonist resulted in increased telomerase activity, improvements in some markers of oxidative stress, improved cognition, and increased mean life expectancy (31) On the other hand, mice treated with a growth hormone antagonist made by a single amino acid substitution in GH do not show increased lifespan.
The mechanism of increased longevity seen in these mice populations is complicated. Some mouse models with increased lifespan have insulin resistance, while those that develop overt diabetes have a shortened lifespan. Long living mice are either leaner than normal or have increased subcutaneous adipose tissue, both of which may have protective aging effects. Caloric restriction and decreased GH/IGF-1 signaling improves resistance to cellular stress, inhibits mTOR by rapamycin, leading to longevity and may be responsible for enhanced tumor resistance.
The parallel between these results and human senescence is not clear. This last assertion is underscored by the recent report that longevity is not increased, but rather reduced in women in a Brazilian population with a GHRH receptor mutation (32) and in a Swiss cohort of patients with isolated GH deficiency from a homozygous mutation spanning the GH1 gene (33). In an Ecuadorian-kindred with GH receptor deficiency and very low levels of IGF-I, however, rates of cancer and diabetes were markedly reduced compared to unaffected people in the same communities (34). A group of Croatian patients with dwarfism and deficiencies in GH, TSH, prolactin, FSH, and LH as a result of a homozygous PROP1 mutation do not die prematurely, do not develop diabetes mellitus, and have delayed appearance of grey hair (30). Ultimately, although size and lifespan appear to have an inverse relationship in some animal studies, no formal longevity studies have been performed in humans with dwarfism.
GROWTH HORMONE THERAPY IN NORMAL AGING
A large literature of over 2000 published papers has led to a general consensus that GH replacement can reverse many abnormalities in AGHD patients. Recent reviews of this literature report reduced fat mass, increased lean body mass, improved exercise capacity and cardiac function, improved bone mineral density, and enhanced quality of life by subjective and objective measures (35-37).
The similarities between aging and adult GH deficiency, while not exact, have led to interest in administering GH directly or stimulating GH secretion in aging individuals. However, the starting point and the target are not the same in the two conditions, and we cannot assume safety and efficacy will be the same.
To date, studies of interventions to increase GH effect in elderly subjects include administration of GH, IGF-I, GHRH, and ghrelin mimetic (GHS) either alone or in combination with each other, sex steroids, or exercise. The first studies of GH treatment in non-GHD older adults were performed not long after its effects in AGHD were demonstrated. In 1990 Rudman and colleagues reported that healthy men above the age of 60 who were treated with GH for 6 months responded with an 8.8% increase in lean body mass, a 14.4% decrease in adipose tissue mass, and a 1.6% increase bone mineral density (BMD) only at the vertebral spine (38). Although the change in BMD was quite small it was especially remarkable considering that most studies of AGHD have required one year or more of therapy to show an improvement in bone density. The changes in body composition persisted after one year of growth hormone treatment (39).
Although the Rudman study did not include any functional measures, given these results, it was postulated that GH treatment might also improve muscle strength and functional performance. Despite an absence of demonstrated functional efficacy, some clinics began to prescribe GH treatment to healthy older persons. Acknowledging this growing practice and the lack of information on the subject, the NIH National Institute on Aging issued a call for applications in 1991 to study trophic factors in aging. Several studies of GH, either alone or in combination with sex steroids, IGF-I, or exercise conditioning, and one study of GHRH were funded and have since been completed. These reports generally demonstrate that GH replacement in normal seniors can increase levels of IGF-I to the young adult normal range. However, lower doses of GH were used in subsequent studies to maintain IGF-I levels in the normal range for healthy young adults, since attempts to reproduce the doses of the initial study of Rudman and colleagues led to severe side effects. Following is a summary of the findings of these studies.
Effects of GH Treatment on Strength and Functional Performance
Though GH treatment of otherwise healthy seniors has been shown to have potentially beneficial effects on body composition, studies of physical functional effects have been generally disappointing and inconsistent.
In a relatively large study, comparing the effects of 6 months of growth hormone treatment with placebo in men aged 70 to 85, Papadakis and colleagues reported a 13% reduction in fat mass and a 4% increase in lean body mass in the treatment group, effects consistent with earlier studies; however, there was no effect of growth hormone on knee or handgrip strength or endurance (40). It is important to point out that this seemingly negative result is likely undercut and confounded by the excellent baseline functional status of the study subjects, who were close to the top of the range on many of the tests used, even before treatment. It is very likely that such a "ceiling effect" led to difficulties in demonstrating further improvements due to treatment with growth hormone.
In a separate study, Taaffe and co-workers showed that exercise training improved strength and exercise capacity, but growth hormone treatment did not further augment this effect (41). Several other similar studies have since been completed (42). These studies were conducted for 6–12 months, each at a single site; therefore, only short-term outcomes and side effects, not long-term risks, could be observed. Their results do not provide guidance on the effects of GH on long-term clinical outcomes or “hard” endpoints such as falls or fractures, maintenance of functional status, or effects on cardiovascular morbidity and mortality – outcomes that could establish more definitively the rationale for GH treatment in normal aging. Though few long-term risks have been observed, this is mainly indicative of an absence of information rather than a demonstration of safety. In 2004 a review of various interventions for sarcopenia and muscle weakness in the elderly concluded that GH therapy produces a high incidence of side-effects, does not increase strength, and that resistance training is the most effective intervention for increasing muscle mass and strength in the elderly (43).
In 2007 Liu and colleagues published a systematic review of the safety and efficacy of growth hormone in the healthy elderly (42). After a mean treatment duration of 27 weeks, GH treated individuals had decrease in fat mass of 2.1 kg and an equal increase in lean body mass of 2.1 kg, with no change in weight overall. Total cholesterol levels trended downward (0.29 mmol/L), though not significantly after adjustment for the change in body composition. Other outcomes, including bone density and other serum lipid levels, did not change. Despite higher doses of GH per kilogram of body weight, women treated with GH did not increase lean body mass and achieved only borderline significant decreases in fat mass, indicating a difference in response to GH therapy between genders. Persons treated with GH were significantly more likely to experience soft tissue edema, arthralgias, carpal tunnel syndrome, and gynecomastia and were somewhat more likely to experience the onset of diabetes mellitus and impaired fasting glucose (Figure 5).
Figure 5. Adverse events in participants treated with growth hormone versus those not treated. From Liu H et al. (42)
Effects of GH Treatment on Cognition, Sleep, and Mood
As noted above, rodent studies have shown that GH administration increases brain vascularity and improves performance on some cognitive tests, but systematic tests of cognitive effects of GH treatment in humans are lacking (44). A seemingly contradictory finding was reported in 2017 by Basu and colleagues who demonstrated that spatial learning and memory were improved in 12-month-old GH receptor antagonist transgenic mice when compared to their wild type controls, proposing that GH antagonism as well may have cognitive benefits in aging rodents (45). A trial of GH therapy in patients with Down syndrome showed an increase in head circumference but no effect on cognitive performance (46). Early reports suggested that GH increased deep sleep (SWS), but subsequent studies have failed to confirm this and indeed have found increased sleep fragmentation and reduced total deep sleep (27). While GH treatment of adult GH deficiency improves self-rated quality of life (QoL) scores using any of a number of questionnaires, there are no solid comparable data for normal aging.
Combination Interventions with GH and Sex Steroids
In a 6-month study of healthy men and women over the age of 65 using GH alone or in combination with estrogen/progestin in women and testosterone in men, GH administration increased lean body mass (LBM) in women with or without estrogen/progestin (47). In men, GH and testosterone increased LBM when given alone and had an additive effect in combination. In men, total fat mass decreased with either testosterone or GH alone, but the decrease was greatest with the combination, whereas in women GH decreased fat mass while sex steroids did not change fat mass. Body strength did not improve in women and slightly increased in men only in the GH + testosterone arm of the trial. There was no evidence that co-administration of sex steroids altered the frequency or severity of GH-related side effects.
A 2006 British study randomized healthy older men to 6 months treatment with GH, testosterone patch (Te), or combination of both GH and testosterone (GHTe) and compared results to placebo (48). Both GH treated groups experienced similar increases in lean body mass, while this parameter was unchanged by testosterone treatment alone. Fat mass decreased only in the GH/testosterone combination group. Similarly, mid-thigh muscle cross-sectional area and exercise capacity (VO2 max) was increased only in GHTe and not in the GH or Te groups. There was no difference among the groups in 5 of 6 muscle strength measures except for strength of knee flexion that was found to be increased in the GHTe group. Both GH treated groups reported improvement in a quality of life questionnaire. Overall GH treatment was well tolerated in this study, with most GH-related side effects resolving with dose adjustment.
A study published in 2009 randomized men over the age of 65 having IGF-I levels in the bottom tertile of the reference range, and who were treated with testosterone after a “Leydig cell clamp”, to three groups of daily doses of GH either 0, 5 mcg/kg or 10 mcg/kg (49). After 16 weeks the investigators were able to demonstrate significant synergistic effects of GH treatment, when added to testosterone replacement, on all parameters studied including decrease in total fat mass and truncal fat as well as increase in lean body mass and maximal voluntary muscle strength and aerobic endurance. A slight increase in systolic blood pressure was noted in the study, but did not appear to be related to GH therapy.
Growth Hormone and Exercise
Regular exercise has been shown to increase lean body mass, muscle strength, and aerobic capacity in older men (50). Vigorous exercise acutely stimulates growth hormone secretion, a physiologic response that has been utilized as a screening test for growth hormone deficiency in children.
The growth hormone response to exercise decreases with aging (51). This finding led to speculation that some of the effects of exercise might be mediated via effects on growth hormone and IGF-I. Although exercise stimulates an acute rise in growth hormone secretion, subsequent overnight growth hormone secretion is inhibited (52). In older adults, even intensive exercise does not elevate serum IGF-I level (53). Therefore, the effects of exercise on muscle mass and function seem to be separate from those of growth hormone.
Studies assessing the effects of adding GH to progressive resistance training regimens in older adults have found little to no additional benefit of GH therapy on measures of muscle strength or other measures of muscle composition, but did find that GH therapy led to greater reductions in fat mass than resistance training alone (41, 54, 55).
Adverse Effects of GH Treatment
Side effects observed during clinical trials of growth hormone treatment in normal aging must be taken into consideration in a different way from those in patients treated for adult growth hormone deficiency. The possibility that some of the hormonal changes observed with aging could represent adaptive responses must be considered. Whether increasing growth hormone above the age-appropriate normal range may have as many risks, both acute and delayed, as benefits is a worthwhile hypothesis to examine. The acute side effects of growth hormone are largely hormonal. The most worrisome long-term potential side effect, of special importance in the older population where baseline risk is elevated, is the risk of cancer. Though there is no definitive evidence that GH replacement in AGHD increases the risk of de novo or recurrent malignancy, but several case reports note development of cancer after treatment with GH and because it is a mitogen, the use of GH is contraindicated in active malignancy (35, 36). Nevertheless, GH replacement is not associated with tumor regrowth in AGHD patients with pituitary tumors (56).
Older persons are more sensitive to replacement with GH and more susceptible to the side effects of therapy. The acute side effects are due to the hormonal effects of over-replacement, which can be avoided or relieved with careful dose titration. Patients who are older, heavier, or female are more prone to develop complications (22). Common side effects of GH replacement include fluid retention, with peripheral edema, arthralgia, and carpal tunnel syndrome (Figure 5) Although glucose levels often increase with initiation of GH, these levels generally return toward normal with the improvement in body composition and reduced insulin resistance. However, some studies report persistent elevations in fasting glucose and insulin with chronic GH treatment. Other less frequently reported side effects include headache, tinnitus, and benign intracranial hypertension (22). Hypothyroidism is common in the elderly. GH can accelerate both the clearance of thyroxine and promote its conversion to triiodothyronine, and therefore may have variable effects in hypothyroid patients who are on thyroid hormone replacement.
GROWTH HORMONE RELEASING HORMONE (GHRH) AND GROWTH HORMONE SECRETAGOGUES (GHS)
GHRH and GHS stimulate the secretion of GH. Since most AGHD is caused by pituitary lesions, and these patients, unlike healthy seniors, are unresponsive to GHRH or GHS, there are few studies of treatment with these agents.
Theoretically, treatment with GHRH or GHS should lead to more physiologic GH replacement, leading to a pulsatile rather than prolonged elevation in GH and preserving the ability for negative feedback inhibition of GH by increasing IGF-I. GHRH and GHS effects are influenced by the same factors which modulate endogenous GHRH secretion, such as negative feedback by somatostatin. This normal negative feedback regulation would be expected to result in buffering against overdose. The side effects of GHRH treatment are similar in character to GH treatment but are milder and less frequent. Since the GHS are smaller molecules than GH, and generally resistant to digestive enzymes, they can be administered via the oral, transdermal or nasal routes.
Growth Hormone Releasing Hormone (GHRH)
There are several published trials of GHRH treatment in normal aging (57, 58). Once daily GHRH injections can stimulate increases in GH and IGF-I at least to the lower part of the young adult normal range (57). In a study of 6 months treatment with daily bedtime subcutaneous injections of GHRH(1–29)NH2, alone or in combination with formal exercise conditioning, IGF-I levels increased by 35% (56). Participants had an increase in lean body mass and decrease in body fat (mainly abdominal visceral fat). However, there was no improvement in strength or aerobic fitness with GHRH injections. This study confirmed the benefits of exercise but showed no effect upon IGF-I levels; thus, it appears that GH/GHRH and exercise work through different mechanisms. Subjects receiving GHRH also showed no change in scores on an integrated physical functional performance test of activities of daily living, but there was a significant decline in physical function in the placebo group. This finding, suggesting that GHRH can stabilize if not improve physical function, needs confirmation.
Sleep and cognition were also studied in this GHRH trial, with unexpected results. GHRH failed to improve and may even have impaired deep sleep, despite the rise in IGF-I and pulsatile GH. However, GHRH treatment was associated with improved scores in several domains of fluid (but not crystallized) intelligence – those measures previously found to be correlated with circulating IGF-I levels (25).
A 2006 study of the effects of 6-months daily treatment with sermorelin acetate, a GHRH analogue, on cognitive function of 89 elderly adults found significant improvement on several cognitive assessments, particularly those involving problem solving, psychomotor processing speed, and working memory, but no change on tests reflecting crystallized intelligence (27). Higher GH levels were associated with higher Wechsler Adult Intelligence Scale performance IQ scores, and greater increases in IGF-1 were associated with higher verbal fluency test scores, while gender, estrogen status, and initial cognitive function did not interact with the GHRH effect on cognition.
A 2013 pilot study of 30 elderly adults given a stabilized analogue of GHRH, tesamorelin, versus placebo, used magnetic resonance spectroscopy to examine the effects of inhibitory and excitatory neurotransmitters (60). After 20 weeks GABA levels were increased in all brain regions, N-acetylaspartylglutamate levels were increased in the dorsolateral frontal cortex, and myo-inositol (an osmolyte linked to Alzheimer disease) levels were decreased in the posterior cingulate, with similar results across adults with mild cognitive impairment (MCI) and those with normal cognitive function. Treatment related changes in serum IGF-1 were positively correlated with changes in GABA and negatively correlated with myo-inositol. There was a favorable treatment effect on cognition (p = .03), but no significant associations were observed between treatment-related changes in neurochemical and cognitive outcomes.
The follow-up study of 152 elderly patients on tesamorelin versus placebo included those with amnestic MCI (early stage Alzheimer’s disease) and analyzed executive function, episodic memory, mood, sleep, insulin sensitivity, glucose tolerance, body composition, and IGF-1 levels (61). GHRH had a favorable effect on cognition (P = .002) in both groups. Treatment related increases in IGF-I were associated with higher composite change scores in executive function (p = .03). Visual memory, mood, sleep, hemoglobin A1c, and 2-hour OGTT glucose and insulin responses were not affected in either population, though GHRH treatment was associated with increased fasting plasma insulin levels in adults with MCI. Treatment with GHRH reduced body fat by 7.4% (p < .001) and increased lean muscle mass by 3.7% (p < .001), across both populations. Ultimately though, the clinical significance of these results cannot be assessed as no data was collected regarding functional status.
In a non-controlled 3-month trial of GHRH(1-44)amide in 10 postmenopausal women, increases in both GH and IGF-I levels as well as decreased visceral fat were demonstrated (57). This study also reported improvements from baseline in selected measures of functional performance including timed walking and stair climbing.
Thus, as is the case with GH, studies of treatment of healthy seniors with GHRH have arrived at a consensus on hormonal and body composition effects but inconsistent functional effects. There is a very encouraging but still unconfirmed positive effect on some domains of fluid intelligence.
Ghrelin Mimetics and Growth Hormone Secretagogues (GHS)
Ghrelin, a 28 amino-acid octanoylated peptide, is produced in the stomach and increases before meals and during overnight fasting. Ghrelin acts at both hypothalamic and pituitary levels via mechanisms distinct from GHRH. Ghrelin therefore has different effects from GHRH or GH; subjects often gain body weight, lean and fat mass via a number of GH dependent and independent mechanisms (62). The effects of ghrelin on GH secretion depend in part on the presence of GHRH. If GHRH secretion declines with aging, as is thought to be the case, ghrelin’s effects may be blunted. While the effects of these two GHS differ clinically, they have synergistic effects on GH release, and therefore supplementation of both substances may be more effective than either alone. Nevertheless, ghrelin is more potent than GHRH at eliciting GH secretion (14). Additionally, there are other substances which can enhance GH response to GHS by suppressing somatostatin secretion, including arginine and beta-adrenergic antagonists, which could potentially enhance treatment effects (59).
Several studies have shown short-term effects of GHS on GH secretion, but few studies of their chronic effects in normal aging have been reported. Bowers and colleagues showed that chronic repeated injections or subcutaneous infusions of GH-releasing peptide-2 (GHRP-2) could stimulate and maintain increases in episodic GH secretion and raise IGF-I levels (63).
Results of a one-year double-blind, randomized, placebo-controlled, modified-crossover clinical trial of the Merck orally active ghrelin mimetic MK-677 in healthy high functioning older adults were published in 2008 (64). Daily administration of MK-677 significantly increased growth hormone and IGF-I levels to those of healthy young adults without serious adverse effects. Mean fat-free mass decreased in the placebo group but increased in the MK-677 group. No significant differences were observed in abdominal visceral fat or total fat mass. Body weight increased 0.8 kg in the placebo group and 2.7 kg in the MK-677 group (P = 0.003). Fasting blood glucose level increased an average of 0.3 mmol/L (5 mg/dL) in the MK-677 group (P = 0.015), and insulin sensitivity decreased. The most frequent side effects were an increase in appetite that subsided in a few months and transient, mild lower-extremity edema and muscle pain. Low-density lipoprotein cholesterol levels decreased in the MK-677 group relative to baseline values (change, -0.14 mmol/L) (-5.4 mg/dL) P = 0.026); no differences between groups were observed in total or high-density lipoprotein cholesterol levels. Changes in bone mineral density consistent with increased bone remodeling occurred in MK-677 recipients. Increased fat-free mass did not result in changes in strength or function.
A multicenter trial of the Pfizer investigational oral GHS, capromorelin, in pre-frail older men and women recruited over 300 subjects and was initially planned as a two-year intervention (65). The study was stopped, however, after all subjects had been treated for 6 months and many for 12 months, due to failure to see an increase in percent lean body mass, which was a pre-set non-efficacy termination criterion. Absolute lean body mass did increase significantly, but due to the appetite-stimulating and lipogenic/anti-lipolytic effect of ghrelin mimetics – unforeseen in early 1999 when the study was designed and ghrelin was still unknown – subjects also gained weight (about 1.5 Kg) and this washed out the effect on percent lean body mass. However, even this truncated study is currently the largest clinical trial of chronic GHS treatment in aging. It showed the expected increases in IGF-I levels and (as noted) total lean body mass. There were also encouraging effects on physical functional performance. Of seven functional tests, one improved significantly after 6 months of treatment, and another after 12 months. Two other measures showed non-significant trends toward improvement, and the three remaining measures showed no effect. Effects on clinical endpoints such as falls could not be assessed with this relatively brief duration of treatment. Side effects were generally mild, including increases in fasting blood sugar within the normal range. Interestingly, there was a self-reported deterioration of sleep quality, though formal sleep testing was not performed. Cognition was not studied in this trial. The reasons for the difference in functional outcomes between the two trials are not clear, but it is speculated that this may reflect differences in the populations studied. The MK-677 study recruited a robustly healthy population of seniors in whom further improvement in physical function might be difficult to achieve, while the capromorelin trial was limited to participants already manifesting a decline in function.
Thus, as with GH and GHRH, reports of the hormonal and body composition effects of ghrelin mimetic GHS in normal aging are relatively consistent, but there is no consensus on functional effects among these very few studies, and of course none could assess long-term clinical outcomes or risks.
The novel GHS, anamorelin, is currently under clinical development for cancer anorexia and cachexia syndrome (CACS), a syndrome overrepresented in the elderly. In a phase II randomized, double-blind, placebo-controlled study, 3 days of treatment increased body weight and appetite in these patients when compared to placebo (66). Over 3 months of treatment, anamorelin increased body weight, LBM, hand grip strength, and quality of life (QOL). Anamorelin also increased IGF-1 and IGF binding protein (IGFBP)-3. It was well-tolerated, but it induced a small increase in glucose and insulin concentrations (67). Unfortunately, two large, international, randomized, double-blind, placebo-controlled phase III studies in patients with advanced non-small cell lung cancer and CACS (ROMANA 1 and 2) did not show improvements in handgrip strength with anamorelin, in spite of increased LBM, fat mass, body weight and appetite-related QoL compared to placebo (68). Although these studies were not restricted to the elderly, the mean age of the population was above 60 years of age in all studies.
CONCLUSIONS
While aging is not a disease, it results in alterations in body composition and functional decline with subsequent frailty and loss of independence. Interventions that slow this decline could potentially prolong the capacity for independent living and improve quality of life, but this has not yet been demonstrated. It is unknown whether the decrease in trophic hormones including sex steroids and growth hormone that occur with aging represents an adaptive or pathological process. Aging may represent a milder form of adult GHD, and since GH replacement in frank AGHD has met with success, it may be logical to reason that GH replacement or stimulation by GHRH or GHS might be beneficial in aging. However, older persons are more sensitive to GH, and thus more susceptible to the side effects of replacement. To date, definitive conclusions regarding functional effects of treatments in normal aging aimed at increasing GH levels to those of young healthy persons have been elusive. Until more studies are undertaken to determine the long-term effects of GH and GHS supplementation, conclusive statements about the merits of treatment cannot be made. Long term studies on hard clinical endpoints, such as falls and fracture rates, function measures, quality of life, and decreased morbidity and mortality from vascular disease need to be performed in order to establish the role, if any, for GH and GHS treatment in normal aging. In the meantime, GH use for anti-aging purposes is currently prohibited by US federal law (69, 70).
REFERENCES
- Sattler FR. Growth hormone in the aging male. Best Pract Res Clin Endocrinol Metab. 2013; 27 (4): 51-55.
- Anawalt BD, Merriam GR. Neuroendocrine aging in men: andropause and somatopause. Endocrinology and Metabolism Clinics of North America. 2001; 30:647–69.
- Merriam GR, Hersch EC. Growth hormone (GH)-releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clin Interv Aging. 2008; 3(1):121-9.
- Ho KY, Evans WS, Blizzard RM, Velduis JD, Merriam GR, Samojlik R, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. Effects of sex and age on the 24 hour profile of growth hormone secretion in man: Importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987; 64:51–8.
- Maheshwari H, Sharma L, Baumann G. Decline of plasma growth hormone binding protein in old age. J Clin Endocrinol Metab. 1996 Mar;81(3):995-7
- Jørgensen JOL, Flyvbjerg A, Lauritzen T, Alberti KGMM, Ørskov H, Christiansen JS. Dose-response studies with biosynthetic human growth hormone deficient patients. J Clin Endocrinol Metab. 1988; 67: 36-40.
- Møller J, Jørgensen JOL, Laursen T, Frystyk J, Naeraa R, Ørskov H, Christiansen JS. Growth hormone (GH) dose regimens in GH deficiency: effects on biochemical growth markers and metabolic parameters. Clin Endocrinol. 1993; 39: 403-408.
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, Müller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents,and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994; 78:744-752.
- Veldhuis JD, Bowers CY. Human GH pulsatility: an ensemble property regulated by age and gender.J Endocrinol Invest. 2003; Sep; 26:799-813.
- Chen JA, SpencerA, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM. Ghrelin prevents tomour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle. 2015; Jun(6)2: 132-43.
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004; Mar 30; 101(13) 4670-84.
- Di Francesco V, Fantin F, Residori L, Bissoli L, Micciolo R, Zivelonghi A, Zoico E, Omizzolo F, Bosello O, Zamboni M. Effect of age on the dynamics of acylated ghrelin in fasting conditions and in response to a meal.J Am Geriatr Soc. 2008 Jul;56(7):1369-70. doi: 10.1111/j.1532-5415.2008.01732.
- Pavlov EP, Harman SM, Merriam GR, Gelato MC, Blackman MR. Responses of growth hormone (GH) and somatomedin C to GH-releasing hormone in healthy aging men. J Clin Endocrinol Metab. 1986; 62:595.
- Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Guana C, van de Lely AJ, Deghenghi R, Bo, M, Arvat E, Ghigo E. The endocrine response to ghrelin as a response to gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003; Apr 88(4); 1537-42.
- Chapman IM, Hartman ML, Pezzoli SS, Harrell FE Jr, Hintz RL, Alberti KG, Thorner MO. Effect of aging on the sensitivity of growth hormone secretion to insulin-like growth factor-I negative feedback. J Clin Endocrinol Metab. 1997; 82:2996.
- Russell-Aulet M, Jaffe CA, Demott-Friberg R, Barkan AL. In vivo semiquantification of hypothalamic growth hormone-releasing hormone (GHRH) output in humans: Evidence for relative GHRH deficiency in aging. J Clin Endocrinol Metab. 1999; 84:3490.
- Ghigo E, Arvat E, Giordano R, Broglio F, Gianotti L, Maccario M, Bisi G, Graziani A, Papotti M, Muccioli G, Deghenghi R, Camanni F. Biologic activities of growth hormone secretagogues in humans. Endocrine. 2001; Feb 14(1):87-93.
- Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr. Rev. 1993; 14:20–39.
- Martin FC, Yeo A-L, Sönksen PH. Growth hormone secretion in the elderly: aging and the somatopause. Balliere’s Clin Endocrinol Metab. 1997; 11:223–50.
- Toogood AA, O’Neill PA, Shalet SM. Beyond the somatopause: growth hormone deficiency in adults over the age of 60 years. J Clin Endocrinol Metab. 1996; 81:460–65.
- h1>Merriam GR, Wyatt FG. Diagnosis and treatment of growth hormone deficiency in adults: current perspectives. Current Opinion in Endocrinology and Diabetes. 2006; 13:362–8.
- Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML. Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011; Jun; 96(6): 1587-1609.
- Kargi AY, Merriam GR. Testing for growth hormone deficiency in adults: doing without growth hormone-releasing hormone. Curr Opin Endocrinol Diabetes Obes. 2012; 19 (4): 300-5.
- Garcia JM, Biller BMK, Korbonits M, Popovic V, Luger A, Strasburger CJ, Chanson P, Medic-Stojanoska M, Schophol J, Zakrzewska A, Pekic S, Bolanowski M, Swerdloff R, Wang C, Blevins T, Marcelli M, Ammer N, Sachse R, Yuen KCJ. Macimorelin as a diagnostic test for adult GH deficiency. J Clin Endocrinol Metab. 2018; Aug 1;103(8):3083-3093.
- Aleman A, Verhaar HJ, DeHaan EH, De Vries WR, Samson MM, Drent ML, Van de Veen EA, Koppeschaar HP. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999; 84:471–5.
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Aging Res Rev.h1>2005; 4(2):195-212.
- Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging.h1>2006; 27:318-23.
- Bartke A. Growth hormone and aging: updated review. World J Mens Health. 2019; Jan 37(1):19-30.
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000; 141:2608
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013; 9: 366-376.
- Banks WA, MorleyJE, Farr SA, Price TO, Ercal N, Vidaurre I, Schally AV Effects of a growth hormone-releasing hormone antagonist on telomerase activity, oxidative stress, longevity, and aging in mice. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):22272-7.
- Aguiar-Oliveira MH, Oliveira FT, Pereira RM, Oliveira CR, Blackford A, Valenca EH, Santos EG, Gois-Junior MB, Meneguz-Moreno RA, Araugo VP, Oliveira-Neto LA, Almeida RP, Santos MA, Farias NT, Silveira DC, Cabral GW, Calazans FR, Seabra JD, Lopes TF, Rodrigues EO, Porto LA, Oliveira IP, Melo EV, Martari M, Salvatori R. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene.J Clin Endocrinol Metab. 2010; 95:714-21.
- Besson AS, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab. 2003; 88 (8): 3664-7.
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med.h1>2011; 3: 70 ra13.
- Reed, ML, Merriam GR, Kargi AY. Adult growth hormone deficiency - benefits, side effects, and risks of growth hormone replacement. Front Endocrinol. 2013; 4: 64.
- Kargi AY & Merriam GR. Diagnosis and treatment of growth hormone deficiency in adults. Nature Reviews Endocrinology. 2013; 9: 335-345.
- Melmed S. Pathogenesis and diagnosis of growth hormone deficiency in adults. N Engl J Med. 2019; Jun 27;380(26): 2551-2562.
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med 1990; 323:1-6
- Rudman D, Feller AG, Cohn L, Shetty KR, Rudman IW, Draper MW. Effects of human growth hormone on body composition in elderly men. Horm Res 1991 36(suppl):73
- Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996; 124:708.
- Taaffe DR, Jin IH, Vu TH, Hoffman AR, Marcus R. Lack of effect of recombinant human growth hormone on muscle morphology and GH-insulin-like growth factor expression in resistance-trained elderly men. J Clin Endocrinol Metab. 1996; 81:421
- Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Int Med. 2007; 146: 104-115.
- Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004; 33 (6): 548-555.
- Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2014; S0531-5565(14)00277-0 E-pub.
- Basu A, McFarlane HG, Kopchick JJ. Spatial learning and memory in male mice with altered growth hormone action. Hormone Behav 2017; 93: 18-30.
- Growth Hormone Treatment in Down's Syndrome, eds. S.Castells and K.E.Wisniewski, London, J.Wiley,1993).
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart JK, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002; 288:2282-92.
- Giannoulis MG, Sonsken PH, Umpleby M, Breen L, Pentecost C, Whyte M, McMillan CV, Bradley C, Martin FC. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006 Feb;91(2):477-84
- Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009; 94 (6): 1991-2001.
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC. Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995; 273:402.
- Kraemer WJ, Hakkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, Gotshalk LA, Gordon SE, Fleck SJ, Campbell WW, Putukian M, Evans WJ. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999; 87:982.
- Nindl BC, Hymer WC, Deaver DR, Kraemer WJ. Growth hormone pulsatility profile characteristics following acute heavy resistance exercise. J Appl Physiol. 2001; 91(1):163-72.
- Vitiello MV, Wilkinson CW, Merriam GR, Moe KE, Prinz PN, Ralph DD, Colasurdo EA, Schwartz RS. Successful 6-month endurance training does not alter insulin-like growth factor-I in healthy older men and women. J Gerontol Med Sci. 1997; 52A:149-154.
- Yarasheski KE, Zachwieja JJ, Campbell JA, et al. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. American Journal of Physiology. 1995;268(2 Pt 1):E268–E276.
- Hennessey JV, Chromiak JA, DellaVentura S, et al. Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. Journal of the American Geriatrics Society. 2001;49(7):852–858.
- Veldhuis JD, Patri JM, Frick K, Weltman JY, Weltman AL. Administration of recombinant human GHRH-1,44-amide for 3 months reduces abdominal visceral fat mass and increases physical performance measures in postmenopausal women. Eur J Endocrinol. 2005; 153:669–77.
- Merriam GR, Kletke M, Barsness S, Buchner D, Hirth V, Moe KE, Schwartz RS, Vitiello MV. Growth hormone-releasing hormone in normal aging: An Update. Today’s Therapeutic Trends. 2000; 18:335–54.
- Merriam GR, Buchner DM, Prinz PN, Schwartz RS, Vitiello MV. Potential applications of GH secretagogs in the evaluation and treatment of the age-related decline in growth hormone secretion. Endocrine. 1997; 7:1–3.
- Friedman SD, Baker LD, Borson S, Jensen JE, Barsness SM, Craft S, Merriam GR, Otto RK, Novotny EJ, Vitiello MV. Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA Neurol. 2013; 70 (7): 883-890.
- Baker LD, Barsness SM, Borson S, Merriam GR, Friedman SD, Craft S, Vitiello MV. Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: Results of a controlled trial. Arch Neurol. 2012; 69 (11): 1420-1429.
- Guillory B, Splenser A, Garcia J. The role of ghrelin in anorexia-cachexia syndromes. Vitam Horm. 2013; 92:61-106.
- Bowers CY, Granda R, Mohan S, Kuipers J, Baylink D, Veldhuis JD. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J Clin Endocrinol Metab. 2004; 89:2290–300.
- Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE Jr, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med.h1>2008;149 (9):601-11.
- White HK, Petrie CD, Landschulz W, MacLean D, Taylor A, Lyles K, Wei JY, Hoffman AR, Salvatori R, Ettinger MP, Morey MC, Blackman MR, Merriam GR. Capromorelin Study Group. Effects of an oral growth hormone secretagogue in older adults.J Clin Endocrinol Metab. 2009; 94(4):1198-206.
- Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: A multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21(1):129–13
- Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J. Anamorelin for patients with cancer cachexia: An integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16(1):108–116
- Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (romana 1 and romana 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519–531. Phase III clinical trial results of anamorelin in CACS.
- Perls TT, Reisman NR, Olshansky SJ. Provision or distribution of growth hormone for “antiaging” clinical and legal issues. 2005;294(16):2086-2090.
- Sonksen P. Idiopathic growth hormone deficiency in adults, Ben Johnson, and the somatopause. J Clin Endocrinol Metab. 2013 Jun;98(6):2270-3.
Endocrine Hypertension
CLINICAL RECOGNITION
Hypertension is defined differently by various societies with a blood pressure exceeding 139/89 mm Hg for adults aged 18 years or older generally considered being elevated, based on the mean of 2 or more properly measured seated BP readings on each of 2 or more office visits. Hypertension affects approximately 31% of Americans when using the above cutoff level. Blood pressure control is suboptimal and is achieved in less than 1 in 3. For children, hypertension is defined as an average systolic BP and/or diastolic BP that is greater than the 95th percentile for age, gender, and height on more than 3 occasions. Normal BP in children is defined as a SBP and DBP less than the 90th percentile for age, gender, and height. Figure 1 provides an overview of classification of BP for adults 18 years and older.
Figure 1. Classification of Hypertension. AHA, American Heart Association; ACC, American College of Cardiology; ESC, European Society of Cardiology; ESH, European Society of Hypertension; DHL, German Hypertension League; NICE, National Institute for Health and Care Excellence of the United Kingdom. DBP, diastolic blood pressure; SBP, systolic blood pressure. Modified from: Jordan J, Kurschat C, Reuter H. Arterial hypertension. Dtsch Arztebl Int. 2018 Aug 20;115(33-34):557-568
Less than 5% of hypertension is endocrine related, the vast majority being “essential”. Endocrine hypertension is suggested by finding physical or historical clues suggesting a specific endocrine disease or patient’s failure to respond to conventional therapy. The first step when evaluating a patient with suspected endocrine-related hypertension is to exclude other causes of secondary hypertension. A detailed medical history and review of systems should be obtained. The onset of hypertension and the response to previous anti-hypertensive treatment should be determined. A history of target organ damage (i.e. retinopathy, nephropathy, claudication, heart disease, abdominal or carotid artery disease) and the overall cardiovascular risk status should also be explored in detail. Moreover, a detailed family history may provide valuable insights into familial forms of endocrine hypertension.
A secondary cause of hypertension should be suspected with the following:
- Young age
- Resistant hypertension
- Need for more than 3 antihypertensives to control blood pressure
- Very high blood pressure >180/110 mm Hg
- Family history of kidney disease
- Hypokalemia
- Plethora with features of Cushing’s syndrome
- Spells with variable blood pressure spikes
- Features of growth hormone excess
- Features of hypothyroidism, i.e. swollen eyes, dry skin
- Signs and symptoms of hyperthyroidism, i.e. palpitations, weight loss
- Retinal angiomas (?von Hippel Lindau disease)
Table 1 provides a specific description of the clinical presentation of endocrine conditions related to hypertension.
|
Table 1. Clinical Findings in Patients with Endocrine Hypertension |
|
|
Condition |
Clinical presentation |
|
Primary hyperaldosteronism |
Diastolic hypertension, headache, muscle weakness, hypokalemia, metabolic alkalosis |
|
Cushing’s syndrome |
Fatigue, weight gain, round face, proximal myopathy, plethora, hirsutism, buffalo hump, central obesity |
|
Pheochromocytoma |
Headache, palpitation, sweating, pallor, paroxysmal BP |
|
Hyperthyroidism
|
Tremor, tachycardia, atrial fibrillation, weight loss, goiter, ophthalmopathy, pretibial myxedema |
|
Hypothyroidism
|
Fatigue, cold intolerance, weight gain, nonpitting edema, periorbital puffiness |
|
CAH: 11beta-hydroxylase deficiency |
Virilization, tall stature, hirsutism, advanced bone age, amenorrhea |
|
CAH: 17alpha-hydroxylase deficiency |
Pseudohermaphroditism (male), sexual infantilism (female), hypokalemia |
|
Liddle syndrome |
Severe hypertension, hypokalemia, and metabolic alkalosis |
|
Apparent mineralocorticoid excess |
Growth retardation/short stature, hypertension, hypokalemia, diabetes insipidus, |
|
Pseudohypoaldosteronism type 2 |
Short stature, hyperkalemic metabolic acidosis, normal aldosterone |
|
Glucocorticoid Resistance
|
Ambiguous genitalia, precocious puberty, hirsutism, oligo/anovulation |
|
Hyperparathyroidism |
Bones, stones, abdominal groans, and psychic moans |
|
Acromegaly
|
Headache, jaw enlargement, macroglossia, amenorrhea, impotence, diabetes mellitus, hypertension, heart failure |
|
Insulin Resistance
|
Hypertension, abdominal/visceral obesity, dyslipidemia, and insulin resistance |
It is also important to identify correctly patients with hypertensive emergencies (increased BP and acute target-organ damage) and provide the necessary urgent treatment. A focused exam must be undertaken quickly with the purpose of rapid identification of the acute target-organ damage. Hypertensive urgency is defined as a SBP > 180 mm Hg or DBP >120 mm Hg with minimal or no target-organ damage. The following tables shows the common hypertensive emergencies and the possible types of acute end-organ injury. Approx. 1% of Americans with hypertension will present with a hypertensive emergency.
|
Table 2. Common Causes of Hypertensive Emergencies |
|
Medication noncompliance Renovascular and renoparenchymal disease Pre-eclampsia/eclampsia Malignant hypertension Acute increase in sympathetic activity (Pheochromocytoma crisis) Autonomic dysfunction (Guillain-Barré syndrome, post-spinal cord injury) and Central nervous system disorders (head injury, cerebral infarction / hemorrhage) Drugs Sympathomimetics (cocaine, amphetamines incl. crystal meth, phencyclidine, etc) MAO inhibitors and the ingestion of tyramine-containing foods Withdrawal from clonidine and other central alpha2 adrenergic receptor agonists |
|
Table 3. Hypertensive Emergency Acute End-Organ Injury |
|
Cerebrovascular Subarachnoid or intracerebral hemorrhage Ischemic stroke Encephalopathy Renal damage Acute renal failure, scleroderma renal crisis, microangiopathic hemolytic anemia Cardiac Heart failure Acute coronary syndromes Acute aortic dissection Eye Hemorrhage Exudate Papilledema |
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
Idiopathic (primary or essential) hypertension accounts for approximately 95% of diagnosed cases. It is estimated that approximately 5% of hypertensive patients have identifiable conditions that result in blood pressure elevation (secondary hypertension). Endocrine hypertension accounts for approximately 3% of the secondary forms of hypertension and is a term assigned to states in which hormonal derangements result in clinically significant hypertension. The major causes of secondary hypertension are summarized in table 4.
|
Table 4. Classification of Hypertension |
|
Essential (95%) |
|
Secondary causes (5%) |
|
Endocrine Hypertension Adults Cushing’s Syndrome Primary aldosteronism Pheochromocytoma Hyperthyroidism Hypothyroidism Hyperparathyroidism Acromegaly Insulin Resistance Children CAH: 11beta-hydroxylase deficiency CAH: 17alpha-hydroxylase deficiency Apparent mineralocorticoid excess Liddle syndrome Pseudohypoaldosteronism type 2 Glucocorticoid Resistance Insulin resistance Constitutive activation of the MR (Geller syndrome) Non-Endocrine Hypertension Polycystic kidney disease Glomerular disease Renovascular · Atherosclerosis (older individuals) · Fibromuscular dysplasia (women) · Other: Scleroderma, vasculitis (PAN) Medications (Contraceptive drugs, NSAIDs, nasal decongestants with adrenergic effects, MAOIs, steroids, methamphetamine, cocaine) Obstructive sleep apnea Coarctation of aorta Pre-eclampsia, eclampsia Polycythemia vera |
PATHOPHYSIOLOGY
Cushing’s Syndrome
Hypercortisolemia is associated with hypertension in approximately 80% of adult cases and half of children. In Cushing’s syndrome there is increased hepatic production of angiotensinogen and cardiac output, reduced production of prostaglandins via inhibition of phospholipase A, increased insulin resistance, and oversaturation of 11beta-Hydroxysteroid Dehydrogenase activity with increased mineralocorticoid effect through stimulation of the mineralocorticoid receptor.
Primary Aldosteronism (PA)
PA can be a sporadic or familial condition. Most cases of PA are caused by bilateral adrenal hyperplasia and less commonly by an aldosterone-producing adrenal adenoma. Very rarely, PA can be caused by an adrenal carcinoma or unilateral adrenal cortex hyperplasia (also called primary adrenal hyperplasia). Familial aldosteronism is estimated to affect at least 2% of all patients with primary hyperaldosteronism and is classified as type 1, 2, 3, and 4. In familial hyperaldosteronism type 1, an autosomal dominantly inherited chimeric gene defect in CYP11B1/CYPB2 (coding for 11beta-hydroxylase/aldosterone synthase) causes ectopic expression of aldosterone synthase activity in the cortisol-producing zona fasciculata, making mineralocorticoid production regulated by corticotropin. The hybrid gene has been identified on chromosome 8. Familial hyperaldosteronism type 2 is not glucocorticoid-remediable. During the last years, other forms of familial aldosteronism were identified with 18-oxoF 10-1,000 higher (in type 3) than seen in familial hyperaldosteronism type 1 and/or type 2. Familial hyperaldosteronism type 3 is caused by germline mutations in the potassium channel subunit KCNJ5 and familial hyperaldosteronism type 4 is caused by germline mutations in the CACNA1H gene, which encodes the alpha subunit of an L-type voltage-gated calcium channel (Cav3.2).
Pheochromocytoma
These rare neuroendocrine tumors are composed of chromaffin tissue containing neurosecretory granules. Adrenal pheochromocytomas and most paragangliomas located in the abdomen produce and secrete catecholamines which can cause paroxysmal or sustained hypertension with hypertensive crisis.
Hyperthyroidism
Hyperthyroidism increases systolic blood pressure by increasing heart rate, decreasing systemic vascular resistance, and raising cardiac output. In thyrotoxicosis, patients usually are tachycardic and have high cardiac output with an increased stroke volume and elevated systolic blood pressure.
Hypothyroidism
Hypothyroid patients have impaired endothelial function, increased systemic vascular resistance, extracellular volume expansion, and an increased diastolic blood pressure. Hypothyroid patients have higher mean 24-h systolic BP and BP variability on 24-h ambulatory BP monitoring.
Congenital Adrenal Hyperplasia: 11beta-hydroxylase deficiency (5% of CAH)
11beta-hydroxylase is responsible for the conversion of deoxycorticosterone (DOC) to corticosterone (precursor of aldosterone) and 11-deoxycortisol to cortisol. In approximately 2/3 of individuals affected by a deficiency of this enzyme, monogenic low renin hypertension with low aldosterone levels ensues caused by accumulation of 11-deoxycortisol and DOC.
Congenital Adrenal Hyperplasia: 17alpha-hydroxylase deficiency
This enzyme deficiency is rare and leads to diminished production of cortisol and sex steroids. Chronic elevation of ACTH causes an increased production of DOC and corticosterone with subsequent hypertension, hypokalemia, low aldosterone concentrations with suppressed renin.
Apparent Mineralocorticoid Excess
Low-renin hypertension can present in various forms; one of them is apparent mineralocorticoid excess (AME), an autosomal recessive disorder caused by deficiency of the 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) enzyme. This enzyme converts cortisol to the inactive cortisone in renal tubular cells. The lack of this enzyme results in high levels of cortisol in renal tubule cells, which activates the mineralocorticoid receptor.
Liddle Syndrome
Liddle described patients with severe hypertension, hypokalemia, and metabolic alkalosis, who had low plasma aldosterone levels and plasma renin activity. “Gain of function” mutations in the genes coding for the beta- or gamma-subunit of the renal epithelial sodium channel, located at chromosome 16p13, lead to constitutive activation of renal sodium resorption and subsequent volume expansion.
Pseudohypoaldosteronism Type 2
This condition is transmitted in an autosomal dominant fashion, and can cause low renin hypertension. Hypertension in these patients may develop as a consequence of increased renal salt reabsorption, and hyperkalemia ensues as a result of reduced renal K excretion despite normal glomerular filtration and aldosterone secretion. Abnormalities such as activating mutations in the amiloride-sensitive sodium channel of the distal renal tubule are responsible for the clinical phenotype.
Glucocorticoid Resistance or Chrousos Syndrome
This autosomal recessive or dominant inherited disorder is rare and caused by inactivating mutations of the glucocorticoid receptor gene. Permanent elevation of ACTH can lead to stimulation of adrenal compounds with mineralocorticoid activity (corticosterone, DOC), and elevation of cortisol may lead to stimulation of the mineralocorticoid receptor, resulting in hypertension. In women, hirsutism and oligomenorrhea may develop through stimulation of androgens.
Constitutive Activation of the Mineralocorticoid Receptor (MC receptor)
The MC receptor can be mutated leading to the onset of hypertension before age 20. “Gain of function” mutations in the MC gene on chromosome 4q31 were identified. The inheritance pattern is autosomal-dominant.
DIAGNOSTIC TESTS NEEDED AND SUGGESTED
The presence of clinical signs and symptoms suggestive of endocrine hypertension (see table 1) should lead to a general screening for the most common forms of endocrine hypertension (Table 5).
|
Table 5. Screening Tests for Endocrine Causes of Hypertension |
|
|
Cushing’s Syndrome |
24-hour urinary cortisol, overnight dexamethasone suppression test, midnight salivary cortisol |
|
Primary Hyperaldosteronism |
Plasma aldosterone: renin ratio |
|
Pheochromocytoma |
Urinary or plasma metanephrines, urinary catecholamines |
|
Thyroid Dysfunction |
TSH, FT4, T3 |
In patients with a positive screening test, subsequent confirmation by various testing modalities is necessary (Table 6). These steps may involve supplementary laboratory tests and localization imaging tests (CT, MRI).
|
Table 6. Tests for Diagnosing the Most Prevalent Forms of Endocrine Hypertension |
|
Cushing’s Syndrome High-dose Dexamethason suppression test or CRH test If positive, then pituitary MRI and/or bilateral inferior petrosal sinus sampling If negative, then chest/abdomen MRI and/or 68Ga-DOTATATE PET/CT scan or Octreoscan ACTH-independent (90-95%) (ACTH <10 ng/L) Adrenal CT or MRI |
|
Hyperaldosteronism positive if aldosterone excretion > 12 to 14 µg/d while urine Na > 200 mEq/day or other suppression tests: fludrocortisone suppression and captopril challenge Adrenal CT or MRI Adrenal vein sampling |
|
Pheochromocytoma abd/pelvis if negative then chest/head and neck Functional imaging [123/131] Iodine-Metaiodobenzylguanidine scan specific PET ([18F] Fluorodopamine, [18F]Fluorodopa) scan non-specific PET ([18F] Fluorodeoxyglucose) Genetic testing |
If the above conditions have been ruled out but the suspicion of an endocrine cause of hypertension is still high, we should move to the next step and test for rare causes of hypertension. The diagnostic strategy is described in table 7.
|
Table 7. Testing for Rare Causes of Endocrine Hypertension |
|
CAH: 11beta-hydroxylase deficiency ↓renin, ↓↓ aldosterone, ↑urinary 100*THS/(THE+THF+5αTHF) and 100*THDOC/(THE+THF+5αTHF) ratios Genetic testing |
|
CAH: 17alpha-hydroxylase deficiency ↑DOC, ↓11-deoxycortisol, ↓↓ aldosterone ↓renin, ↓plasma 17-hydroxyprogesterone, Genetic testing |
|
Apparent mineralocorticoid excess ↓renin, ↓K, low aldosterone ↑ 24 h urinary free cortisol / cortisone Genetic testing |
|
Liddle Syndrome |
|
Pseudohypoaldosteronism type 2 ↓urinary THALDO Genetic testing (ENaC gene) |
|
Glucocorticoid Resistance Syndrome Genetic testing |
|
Constitutive Activation of the Mineralocorticoid Receptor ↑K, ↓aldosterone, ↓renin ↓urinary THALDO Genetic testing |
THE-tetrahydrocortisone; THF- tetrahydrocortisol; THA-tetrahydro 11-dehydro-corticosterone; THB-tetrahydrocorticosterone; DOC-deoxycorticosterone; THALDO-tetrahydro aldosterone
THERAPY
In the face of a hypertensive crisis, rapid action is important and the underlying disorder and the individual patient’s comorbidities determine the treatment approach. Aortic dissection will require rapid lowering of blood pressure, whereas blood pressure in an ischemic cerebrovascular event should be lowered modestly considering the cerebral perfusion and intracranial pressures. Among 1000 participants with intracerebral hemorrhage and a mean systolic blood pressure of 201 mm Hg at baseline lowering the SBP to 110 to 139 mm Hg did not result in a lower rate of death or disability than standard reduction to a target of 140 to 179 mm Hg (Qureshi AI et al. NEJM 2016). For acute hypertension following stroke, labetalol, nicardipine, and nitroprusside are commonly administered with labetalol being considered first line therapy. For cocaine intoxication, phentolamine and nitroprusside are recommended. For an adrenergic crisis due to pheochromocytoma, phentolamine, nitroprusside and urapidil are preferred. For the management of a hypertensive emergency in pregnant and postpartal women, intravenous labetalol next to magnesium sulfate, ketanserine, hydralazine, and nicardipine are considered first line medications. Immediate release oral nifedipine can also be given, especially when no intravenous access is available.
In general, in the first hour of treatment the mean arterial blood pressure should be reduced by 15% to 20% from baseline and then another 10%-15% over the following 2 to 6 h with a further gradual reduction over the next 24 h to reach normal blood pressure levels.
The most common used intravenous drugs and their dose and duration of action are listed in the table 8.
|
Table 8. Commonly Used Intravenous Drugs |
||
|
Agent |
Dose |
Onset/ duration of action |
|
Vasodilators |
|
|
|
Nitroprusside |
0.25-10 mcg/kg/min |
0.5-1 min/ 1-10 minutes |
|
Nitroglycerine |
5-200 mcg/kg/min |
1-2 min/ 3-5 minutes |
|
Nicardipine |
5-15 mg/h, increase every 15 min |
5-10 min/ 1-4h |
|
Fenoldopam |
Initial dose:0.1 mg/kg/min followed by 0.05 to 0.1 mcg/kg/min q 15-20min till normal BP |
10 min/ 30 minutes |
|
Hydralazine |
10-20 mg q 20-30min |
10-20 min/3-8h |
|
Beta-blockers |
|
|
|
Labetalol |
20-80 mg as bolus every 10-20 min. or 0.5-2 mg/kg/min |
5-10 min/2-6h |
|
Esmolol |
0.5-1 mg/kg bolus; 50-300 mcg/kg/min |
1-2 min / 10-30 min |
|
Alpha-blocker |
|
|
|
Phentolamine |
1-5 mg bolus q 5-15min; 0.5-1 mg/h infusion |
1-2 min/ 3-10 min |
|
Urapidil |
12.5-25 mg bolus; 5-40 mg/h infusion |
3-5 min / 4-6 h |
|
Antagonist of 5-HT2 (hydroxytryptamine) receptors |
||
|
Ketanserin |
5 mg bolus, repeat; 2-6 mg/h infusion |
1-2 min / 30-60 min |
Once the diagnosis of a specific cause of endocrine hypertension has been established, treatment oriented toward the endocrine diseases should be instituted (see specific chapters in Endotext that discuss the treatment of these disorders in depth).
|
Table 9. Treatment for Endocrine Causes of Hypertension |
|
Cushing’s Syndrome Adrenolytic Therapy Metyrapone 250-6000 mg/day in 3-4 doses daily (oral) Ketoconazole 200-1200 mg/day in up to 4 daily doses (oral) Mitotane up to 4-12 g/day (oral) Etomidate intravenously at 0.3 mg/kg/h based on the serum cortisol levels Somatostatin analogues Pasireotide 600-900 µg twice daily s.c. Dopamine agonists Cabergoline initially 0.5 mg/week, titrated to 4.5 mg/week (oral) Alkylating drugs Temozolomide (experimental, oral) Glucocorticoid receptor antagonists Mifepristone, CORT112716, 113083 (oral) |
|
Primary aldosteronism Mineralocorticoid receptor antagonist Eplerenone 50 - 300 mg / day (oral) Spironolactone 50-225 mg/day (oral) Glucocorticoids (GRA) Dexamethasone (low dose i.e. 0.5 mg) |
|
Pheochromocytoma a-adrenoceptor blocker± Β-blockers Phenoxybenzamine at 10-20 mg (titrated up based on SBP) twice daily for 2 weeks before surgery Propranolol or other beta-blocker for reflex tachycardia Hypertensive crisis Phentolamine i.v. bolus of 2.5 mg-5 mg at 1 mg/min Sodium nitroprusside as an alternative at 0.25-10 mcg/kg/min |
|
Hyperthyroidism Aggressive hydration of up to 3-4 L/d of crystalloid Antithyroid drugs Methimazole 20-30 mg q 6-12h, then 5-40 mg/d Propylthiouracil (second line) 200 mg q 4-6hr initially then 100-150 mg/day BID Dexamethasone (up to 2 mg q6h) β-blocker Propranolol 40 mg q6h titrated to SBP Iodide i.e. Lugol’s solution 1-2 drops |
|
Hypothyroidism Levothyroxine (1.6 mcg/kg/day)-lower dose for patients at risk for ischemic heart disease Myxedema coma Loading dose 5-10 mcg/kg T4 iv then 50-100 mcg iv qd and steroid replacement (i.e.hydrocortisone 5-10 mg/hr) until normalization of adrenal function |
GRA- Glucocorticoid-remediable aldosteronism
FOLLOW-UP
The long-term management of patients with the respective underlying endocrine disorder is discussed in depth in other sections of ENDOTEXT, for instance, the adrenal and pituitary sections.
REFERENCES
- Jordan J, Kurschat C, Reuter H. Arterial hypertension. Dtsch Arztebl Int. 2018 Aug 20;115(33-34):557-568
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016 May;101(5):1889-916
- Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014 Jun;99(6):1915-42
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM.The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008 May;93(5):1526-40
- Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A; Endocrine Society. Treatment of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015 Aug;100(8):2807-31
- Ferrari P, Bianchetti MG. Diagnostic investigations in inherited endocrine disorders of sodium regulations. In: Ranke MB, Mullis P-E (eds): Diagnostics of Endocrine Function in Children and Adolescents, ed 4. Basel, Karger, 2011, pp 210–234 (DOI:10.1159/000327410)
- Ong KL, Cheung BM, Man YB, et al: Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007, 49: (1): 69-75.
- Endocrine Hypertension (editors: Koch CA & Chrousos GP), Contemporary
Endocrinology Series, Springer, New York, 2013, ISBN: 978-1-60761-547-7 (Print), ISBN-10: 978-1-60761-548-4 (online)
Emergencies Related To Pheochromocytoma/ Paraganglioma Syndrome
INTRODUCTION
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are rare chromaffin cells tumors (PPGLs) characterized by the production, storage, metabolism, and secretion of catecholamines and their metabolites, metanephrines and methoxytyramine. These tumors can raise significant challenges in clinical recognition, diagnosis, and therapy and when undiagnosed can result in severe morbidity as well as mortality, especially due to cardiovascular system toxicity. Despite anatomical dissimilarity – PCC arising from adrenal medulla and PGLs from extra-adrenal sympathetic or parasympathetic paraganglia – these tumors display common embryonic origin, enzymatic milieu, and the ability to produce catecholamines and their metabolites. While once thought as mostly benign and biochemically active, these tumors can show a wide spectrum of cellular and biochemical dedifferentiation, including an aggressive metastatic course and biochemical silence.
The PPGL field has undergone a significant transformation in recent years. We now know that PPGLs represent the highest hereditary-driven endocrine condition with up to 40% of cases related to mutations in 15 well-established driver genes and a growing number of disease-modifying genes (now about 25). We now also appreciate that a significant proportion of what previously was thought to be almost exclusively benign disease is actually malignant and can display biochemical silence and a specific secretory profile with mainly elevated norepinephrine and/or dopamine.
Definition
What would define actual urgency or emergency in PPGL? Is it a biochemical phenotype closely associated with a particular catecholamine secretion or various biomarkers suggestive of dedifferentiation and thus a malignant course, disease symptomatology, or the rapidity of disease progression? Is it an overall basic patient’s health status that can rapidly deteriorate or the expected complications from surgery? Is it the level of comfort and experience of the managing endocrinologist or abilities of the operating surgeon or possible lack of these? Or maybe it is the ability of the patient to follow-up frequently enough for appropriate management or the affordability of tests and medications or lack of those (some due to their price) which would eventually associate with a grim outcome. At the end of the day, it is probably all of the above and even more including some psychological aspects and fear to develop metastatic disease. Any disease that can potentially deteriorate to severely morbid outcomes needs to be seen as urgent and emergent most of the time. Obviously, in the case of a severe hypertensive crisis in the operating room that developed shortly after a previously undiagnosed/misdiagnosed abdominal mass was manipulated, the diagnosis that will drive appropriate therapy will be acute catecholamine crisis and there is a universal awareness of such a situation. Same should be true in case of severe therapy-resistant hypertension which rapidly deteriorates with use of β-adrenoceptor blockers. Unfortunately, there are many other possible scenarios that could start relatively slow and rapidly deteriorate to true medical emergencies. An example is our recent case that shows all aspects of PPGL management, including emergencies. A young female in late pregnancy was admitted for increased BP, thought to be related to noncompliance with BP medications and possible development of pre-eclampsia. Because of resistance to therapy while hospitalized the patient had an assessment of plasma catecholamines, which came back markedly elevated. Her imaging studies showed a 12 cm abdominal PGL with the fetus’ head laying directly on it. The tumor was massively vascularized and engulfed major abdominal vessels. The team that discussed her management could fill a lecture hall and included obstetrics, gynecologic surgery, endocrine surgery, general surgery, vascular surgery, anesthesiology, neonatology ICU, medical ICU, endocrinology, nursing and many more. All of the above worked hard on the day of combined caesarian section delivery and open abdominal surgery, which was complicated, but resulted in full recovery for both mother and baby.
CLINICAL ASPECTS
Clinical course and outcomes of excessive catecholamine secretion by PPGLs closely correlate with multiple factors related to the biochemistry of catecholamine action, secretory profile, acuity, and severity of actual hypercatecholaminemia. This gets very complicated by the fact that clinical symptomatology of hypercatecholaminemia lacks specificity and often presents as much more prevalent conditions, like hypertension, anxiety, or cardiac arrhythmias. If there is a single most important factor to define the overall outcome of the disease, we personally would pick timely suspicion and initiation of appropriate workup. While hypertension – paroxysmal or sustained – usually represents the initial or most common symptom, the overall clinical symptomatology varies widely and is summarized in Table 1.
|
Table 1. Clinical Syndromes Related to PPGL |
|||
|
Organ |
Syndrome |
Mechanism |
Receptor action |
|
Heart |
§ Angina § Heart attack § Cardiomyopathies § Myocarditis § Arrythmias § Heart failure |
§ Coronary spasm § Positive inotropy § Positive chronotropy § Unmatched O2 demand § Hypoperfusion |
§ Coronary α1, β2 § Conducting system β1, β2 § Conducting β1, β2 |
|
Brain |
§ Stroke § Encephalopathy |
§ Vasoconstriction § Unmatched O2 demand § Hypoperfusion |
§ Cerebral arterioles α1 § Effect of systemic HTN |
|
Vascular |
§ Shock § Postural hypotension § Aortic dissection § Organ ischemia § Limb ischemia |
§ Arteriolar vasoconstriction § Arteriolar vasodilation § Vasodilation § Unmatched O2 demand § Hypoperfusion |
§ Vascular α1, α2, β2 |
|
Kidneys |
§ ARF § Hematuria |
§ Vasoconstriction § Vasodilation § Increased renin secretion § Unmatched O2 demand § Hypoperfusion |
§ Vascular α1, α2, β1, β2 |
|
Lungs |
§ Pulmonary edema § ARDS § Fibrosis § Pulmonary HTN |
§ Vasoconstriction § Vasodilation § Bronchodilation |
§ Vascular α1, α2, β2 |
|
GI |
§ Intestinal ischemia |
§ Vasoconstriction § Unmatched O2 demand § Hypoperfusion |
§ Visceral arterioles α1, β2 |
Physiology of Catecholamine Action
Catecholamine production takes place in both adrenals, as well as sympathetic paraganglia. The synthetic pathway is specific for the abovementioned organs, defined by the unique set of intracellular enzymes able to convert the amino acid tyrosine to end product epinephrine or norepinephrine, dependent on the site of the synthesis. The actual catecholamine is related to the site/type of the cell, as well as degree or differentiation or lack of such (Figure 1). The transport of tyrosine through the cell membrane is active process and carried out by a member of the amino acid transporter family – large neutral amino acid transporters of the L family, mostly LAT1. These transporters can be induced and show overexpression, especially in some cancers. Dedifferentiated/malignant PPGL can produce a phenomenon of massive or predominantly norepinephrine production/secretion profile driven by the both overexpressed LAT1 and the lack of phenylethanolamine N-methyltransferase (PNMT) – the enzyme that converts norepinephrine to epinephrine.
Figure 1. Catecholamine Synthesis
Although currently we manage both conditions as part of a single syndrome, the physiology of catecholamine production and secretion from both systems is relatively distinct. Adrenals – the mastermind of the fight and flight response – are designed to produce significant amounts of epinephrine/adrenaline, to be secreted in response to stress. The secretion pattern is episodic/paroxysmal and relatively short lived. Epinephrine, the end-product of the synthetic pathway, is stored in secretory granules and secreted on an as-needed basis. Norepinephrine in this case is a co-secretory catecholamine, somewhat different in affinity to adrenergic receptors – through which both substances are signaling (Figure 2 and 3). In cases of catecholamine-producing tumors, the pattern of secretion can vary between paroxysmal or sustained, while the ratio between two catecholamines relates to some degree to the level of differentiation of the adenomatous tissue. Sympathetic paraganglia, on the other hand, is mostly involved in production of norepinephrine as a major sympathetic neurotransmitter rather than as a systemic hormone. The product is mostly secreted into the synaptic space, which than spills over into the systemic circulation. In the physiologic state, a significant amount of norepinephrine re-uptake back into the pre-synapse for repeated use occurs. In the case of PGLs, the increased amount of product reaches the systemic circulation to produce symptoms and signs indistinguishable from adrenally secreted norepinephric clinical picture of adrenergic overactivity.
Figure 2. Adrenergic Receptors and Ligands
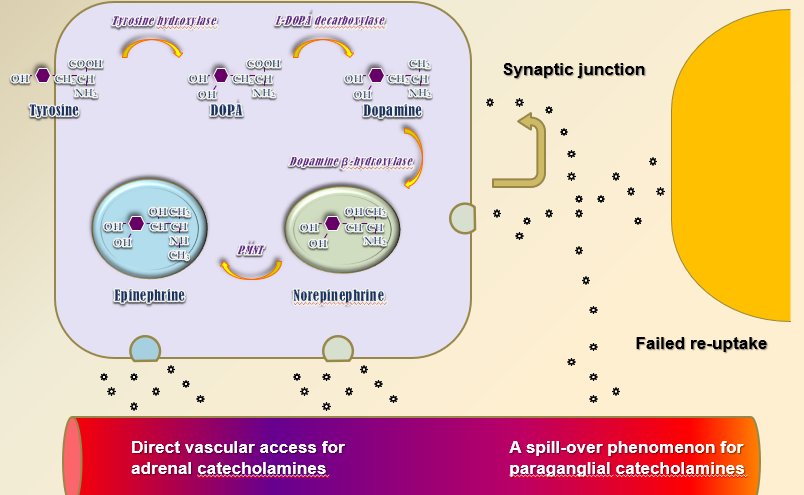
Figure 3. Catecholamine Secretion
Catecholamines, both epinephrine and norepinephrine act through activation of the G protein coupled adrenergic receptors (GPCRs), both α1 and 2 and β1 and 2 with minor difference in the fact that norepinephrine has lower affinity to β2-adrenoceptors and thus norepinephric hypercatecholaminemia lack a mild component of peripheral vasodilation and could have slightly different clinical appearance compared to purely epinephric hypercatecholaminemia Table 1 and Figure 2). As with other GPCRs, adrenoceptors can undergo desensitization, which could explain the different clinical presentations in relatively mild long standing disease compared to more rapidly developing hypercatecholaminemia. One also needs to remember that in massive biochemical hypercatecholaminemia, competitive α- and β-adrenoceptor blockers could be overwhelmed by the concentration of the ligand and safe preoperative adrenoceptor blockade can take longer to achieve and can be partial rather than complete.
Clinical Features
Hypercatecholaminemia-related endocrine emergencies define rare, but truly severe and potentially deadly end of the clinical spectrum of the PPGL syndrome. While it is called a great masquerader, this is misleading because it is not that the disease that masquerades, but rather because of the fact that clinical symptomology is completely non-specific and lacks any definitive symptom or signs that would point towards PPGL as a sole contender. It rather presents with symptoms and signs of much more prevalent conditions – like hypertension, benign cardiac arrhythmias, anxiety – and thus, progresses towards acute or chronic complications without being suspected. Needless to say, that unless the disease is severe or acute, it could be treated as a mainstay symptom-driven state – like hypertension – with at least some success. Clinical emergencies, related to the PPGL represent a completely different scenario – these are usually either unsuspected or only partially treated cases with severe short-term morbidity and significant mortality. In these cases, clinical suspicion is an absolute cornerstone of the management and the delay in diagnosis is adversely proportional to the overall outcome. Clinical scenarios with resultant PPGL-related emergencies usually include unrelated surgeries, where overall stress or tumor manipulation results in massive and acute hypercatecholaminemia with fully sensitized adrenergic receptors and lack of any adrenoceptor blockade, which precipitates acute and severe hypertensive crisis and potentially multiorgan failure. Less dramatic, but still a potentially severe condition includes treating progressive hypertension in the general or obstetric population with a medication that predisposes to unopposed α-adrenoceptor stimulation and thus precipitates severe peripheral vasoconstriction and either worsening of hypertension or heart failure. Obviously, patients with pre-existing heart or renal failure will be much more susceptible to severe outcomes. Because of the fact that some tumors express slow biochemical progression, we need to keep a high index of suspicion not only for patients with resistant HTN, familial HTN, or young age of onset, but for any patient who might have potential to have this disease.
ACUTE VS CHRONIC HYPERCATECHOLAMINEMIA
While an acute increase in catecholamine levels is directly responsible for precipitation of a hypertensive crisis through vascular vasoconstriction and positive inotropy, a long-lasting increase in catecholamine levels, especially of relatively mild degree, can be completely asymptomatic. This can probably be explained to some degree by several physiologic processes, including desensitization of the adrenergic receptors. Slowly progressive disease will mask, at least partially, clinical symptomatology, as well as allow sometime for the patient to try antihypertensive, antiarrhythmic, or antianxiety therapies as part of the therapy for aforementioned nonspecific conditions, as well as clinically desensitize the patient to mild hypertensive symptoms.
As mentioned above, clinical scenarios will mostly associate with unrelated surgeries, obstetric conditions like delivery and pre-eclampsia, as well as sudden or rapidly progressive deterioration of a previously stable person with significant conditions that would be sensitive to rapid increases in BP, pulse rate, or overall oxygen requirement. Currently, there is a well-accepted awareness, especially in the operating/delivery rooms, that sudden and rapid increases in systolic BP must be treated immediately by medications capable to act in the hypercatecholaminemic state. There is also a sufficient awareness of predominant β-adrenoceptor activity of labetalol, which could provide only partial α-blockage and be insufficient alone in full hypercatecholaminemic crisis. On the other hand, IV phentolamine is not a readily available operating room medication and this leaves nitroprusside as a medication mostly available in the operating room settings. Its use for prolonged and complicated surgery or delivery could possibly be associated with generation of methemoglobin and thiocyanite in the patient or the newborn. Because of the fact that majority of acute and severe hypercatecholaminemic states will have either mixed adrenergic/noradrenergic or noradrenergic biochemical phenotype, there is less expectation of β2 -adrenoceptor driven vasodilation and orthostasis.
SEVERE VS MILD HYPERCATECHOLAMINEMIA
It is accepted that patients with mild hypercatecholaminemia can be relatively asymptomatic or mildly symptomatic with some response to the usual antihypertensive therapy, thus disease can be present for a significant length of time undiagnosed. Severe hypercatecholaminemia, on the other hand, is markedly symptomatic and should be suspected right away. Clinical problems arise in cases when this happens during unrelated surgeries, as stated above, especially when an unrecognized abdominal mass, which in this case will be a PGL, is manipulated and releases massive amounts of pre-synthesized catecholamines. These cases are rare and close to impossible to predict, but in cases of severe intra-operative or intra-labor hypertension, should be immediately suspected and treated. Another scenario represents rapidly progressive disease in a younger patient – these are usually familial paragangliomas that can rapidly progress and metastasize. In this case, younger patients present with what suggest anxiety, especially in patients with an episodic secretory profile. Appropriate diagnosis can be significantly delayed when these patients enter the “outpatient workup mode” with infrequent appointments to assess the efficacy of anti-anxiety medications. This delay in diagnosis can associate with development of significant complications in patients with other pre-existing conditions. Obviously, acute concomitant illnesses will precipitate acute hypertensive crisis. Although over-suspicion could result in significant number of questionably necessary tests, it seems reasonable to test keeping in mind potentially morbid outcomes of severe untreated hypercatecholaminemia.
EPISODIC VS CONTINUOUS SECRETION
Both episodic and sustained secretion of catecholamines can produce hypertension as well as an acute crisis. One can argue that the episodic form is more symptomatic owing to the nature of an on and off symptomatology that can be easier to detect for both the patient and the physician. We are not aware of differential adrenoceptor desensitization of episodic hypercatecholaminemia when compared to a persistent secretory state. Both forms are capable of rapid secretion of massive amounts of catecholamines in case of stress or manipulation, so the actual presentation or management of acute hypertensive emergency will not differ.
LARGE VS SMALL MASS
Historically, the size of the mass was thought to be proportional to the biochemical activity of PCC, with the exception of larger tumors, which were thought to overgrow their vascular supply, become necrotic and decrease the ability to be significantly active biochemically. Current knowledge complicates this to a significant degree because of several added details. The degree of differentiation of PPGL can massively affect both the actual profile of the secreted catecholamines (the higher the differentiation, the more probable the synthetic catecholamine pathway leads to epinephrine), as well as the amount of secreted catecholamines, where lesser differentiation could associate with a significant decrease in the amount of the synthetic catecholamine.
One should also remember that larger intra-abdominal masses can also result in local tissue invasion, including large or multiple vessels, adjacent organs etc. In this case, knowing the anatomical relationship between the tumor and the adjacent tissues can help avoid a potentially prolonged and complicated surgery.
We are also historically aware of the fact that the actual size of the adrenal tumor correlates with possible metastatic/malignant state/course. In PPGL this postulate is also relative, making genetic milieu more important factor for the prediction of malignancy (like SDHB mutation or younger age), then the actual size of the initial adrenal mass. It worth mentioning that multiple masses and PGLs per se will have a higher predisposition to malignancy as compared to a single adrenal pheochromocytoma.
In any case, finding a small PPGL and assuming that there would be no significant hypercatecholaminemia during stress or surgery is as wrong as finding a large mass and assuming that it had outgrown the vascular supply and thus is necrotic and incapable of acute delivery of massive hypercatecholaminemia.
PHEOCHROMOCYTOMA VS PARAGANGLIOMA
The division of PPGL tumors into PCC and PGL is mostly anatomical rather than functional. The only major difference is that PCCs express significantly higher content of PNMT and thus higher probability of predominantly the adrenergic or mixed biochemical phenotype, as compared to predominantly noradrenergic phenotype of PGLs. With that said, the actual profile will strongly depend on the degree of tumor differentiation, as well as possibility of mixed PPGL cases.
Another possible cause of differences in the acute conditions associated with different PPGL tumors is the fact that adrenal incidentalomas are readily diagnosed on unrelated imaging studies, especially in recent years when both chest and abdominal CT scans, which both image adrenal glands, are done for progressively increasing number of conditions. PGL are frequently missed, especially in cases where clinical symptomatology is less severe or the patient is young and is otherwise seen as “healthy”. Acute and severe hypercatecholaminemic crisis can occur when a previously unknown abdominal or chest mass is seen during unrelated surgery or invasive procedure and is manipulated, causing release of massive amount of pre-synthesized catecholamines. In these cases, surgical awareness of uncommon locations and anesthesiology readiness for appropriate therapy of potentially life-threatening crisis is the true cornerstone of the management of this endocrine emergency.
SINGLE TUMOR VS METASTATIC DISEASE
The main difference in the approach to the possibility of metastatic disease is based on the expectation that rapid postoperative withdrawal of adrenoceptor blockade will associate with rebound hypertensive crisis. In addition to this, possibility of distant metastatic disease with significant morbidity associated with involvement of affected organs needs to be kept in mind.
SPONTANEOUS VS FAMILIAL/SYNDROMAL CASE
Recent years have tremendously changed many aspects of our understanding of the biology and management of the PPGL particularly the progress in understanding the genetics of the disease. While possibility of a genetically driven condition should be increased in younger patients or ones with a positive family history, finding a predominantly noradrenergic biochemical phenotype, multiple masses on imaging studies, or additional clinical findings – thyroid nodules happen to be medullary thyroid cancer (MTC), renal tumors etc. – should strongly suggest a genetic condition. The opposite is even more important – like sending patient with thyroid nodule of unclear pathology to surgery and ending up with it being MTC and patient having a hypertensive crisis during the surgery. Possible syndromal association with SDHB mutation should prompt assessment of multiple tumors, as well as early recurrence and metastatic disease to prevent early post-operative discontinuation of medical therapy and rebound hypertension or discontinuation of long term follow up. In addition, the head and neck PGLs, rarely seen by endocrinologists in the past, are associated with a SDHD gene mutation and can metastasize and locally invade, while being secretory silent. Establishment of a genetic disorder requires institution of testing and biochemical screening of relatives.
DOPAMINE VS NOREPINEPHRINE VS EPINEPHRINE SECRETING TUMORS
Based on the differences in the affinity of epinephrine and norepinephrine to adrenoceptors, with norepinephrine having lesser action on the β2-adrenoceptor, one can expect a pure “vasoconstrictive” clinical presentation in cases with pure norepinephric secretory profile, while with epinephrine and dopamine-secreting tumors, orthostatic or episodic hypotension will be much more frequent.
PPGL IN PREGNANCY
PPGL during pregnancy is a rare clinical entity. In the case of pregnancy, there are 2 patients at the same time, both the mother and the fetus. Both can be severely affected by the disease, although in a somewhat different manner. PPGL is difficult to suspect during pregnancy because of pre-eclampsia-driven management attitude. Diagnosis can be significantly delayed causing fetal morbidity and affecting both the pregnancy and delivery. Several physiologic phenomena drive the unique behavior of PPGL in pregnancy. These include high placental expression of catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO) and lack of autoregulation in uteroplacental circulation. While both enzymes are responsible for production of inactive catecholamine metabolites, they provide some kind of “fetal barrier”, shielding the fetus from exposure to increased catecholamine levels. Lack of uteroplacental vascular autoregulation, on the other hand, directly affects placental blood flow and fetal blood supply in the hypertensive vasoconstricted mother and can associate with rapid development of uteroplacental insufficiency. As far as management – MRI will be the preferred imaging modality, medical therapy will be started with same medications as in non-pregnant patients, and the management of acute severe hypercatecholaminemia will be similar to non-pregnant cases, with exception for the need to avoid methyldopa and more prevalent use of intravenous magnesium sulfate, which will be effective in both PPGL and pre-eclampsia. Surgery still remains the treatment of choice and there is continuous debate about the sequence of delivery versus surgery.
PPGL IN PEDIATRIC POPULATION
Hypertension in the pediatric population is mostly secondary and is mostly related to renal disease with endocrine causes happening much less frequently. With this said, the possibility of both genetically-driven as well as a malignant course is much higher and needs to be assessed in every pediatric case. Overall management is similar to adult PPGL. On the other hand, the patient will need an extended follow up to assure that any possibility of recurrence is monitored.
CO-SECRETORY SUBSTANCES
The unique enzymatic machinery of PPGL cells provides a series of steps that transforms an amino acid to an amine. In this case, the amino acid is tyramine and the end product are catecholamines. One should appreciate that PPGLs can co-secrete multiple active substances, most clinically relevant of which will probably be ACTH/CRH, which can cause frank and at times severe Cushing syndrome. This needs to be kept in mind, especially when the patient presents with suspicious symptoms or biochemical findings. PPGL as part of MEN2 will associate with overproduction of calcitonin and disseminated metastatic disease, which needs to be diagnosed, hopefully prior to PPGL surgery.
SYMPTOMATIC VS SILENT
While we had discussed in length symptomatic PPGL, parasympathetic PGL could associate with silent tumors, which could be associated with SDHB/D mutation and might have a malignant/metastatic course with local involvement of carotid sinus, as well as major neck vessels, in times associated with different – “silent/local” urgencies/emergencies.
TREATMENT-ASSOCIATED SEVERE HYPERCATECHOLAMINEMIA
Inoperable or recurrent metastatic disease can be treated through multiple modalities, which usually cause different degrees of tumor destruction. These include older therapies (radiofrequency ablation, cryotherapy, external beam radiation, transarterial chemoembolization, ethanol injection), as well as newly rediscovered 131I-MIBG and somatostatin receptor-driven peptide receptor radionuclide therapy (PRRT) with 90Y-DOTATOC/DOTATATE and 177Lu-DOTATATE. This tumor destruction is associated with the potential of massive release of the pre-synthesized catecholamines and could generate severe hypercatecholaminemia for a prolonged period of time. In preparation for therapy, patients need to undergo a protocol, identical to surgical preparation and their biochemical response needs to be followed for weeks after therapy. Overtly secreting or very large tumors should probably generate post-procedural admission for closer monitoring to make sure that the patient will not develop a hypertensive emergency. As we had discussed above, pre-treatment with a competitive α-adrenoceptor antagonist must be used in almost all patients but may provide insufficient α-adrenoceptor blockade with massive hypercatecholaminemia. On the other hand, use of phenoxybenzamine in a full dose, can potentially result in prolonged hypotension but is less problematic than a severe hypertensive crisis and its consequences.
CLINICAL SYNDROMES ASSOCIATED WITH PPGL
Multisystem Failure
This is by far most feared complication because of the high morbidity and mortality associated with a rapid and at times unexpected and unpredicted development, resembling an avalanche starting small but rapidly leaping into a clinical disaster. While it could be preceded by a hypertensive crisis, patients who are sicker and fragile at baseline can develop it with little or no warning symptoms. The blood pressure pattern can show either hypertension or hypotension in case of progressive shock and cardiac failure. It can associate with fever, encephalopathy, as well as renal failure, pulmonary edema and even disseminated intravascular coagulation. Clinical outcomes mostly depend on delays in diagnosis and initiation of appropriate therapy.
Cardiovascular Emergencies
HYPERTENSIVE CRISIS
While hypertension in patients with PPGL can be both paroxysmal and sustained, a severe hypertensive crisis is usually precipitated by stress, postural changes, food containing large amounts of catecholamine precursors, as well as local manipulation of an unsuspected tumor. Medications can also induce hypertensive crisis through direct stimulation of release of stored catecholamines – which could be of a massive quantity. These medications include ACTH, tricyclic antidepressants, phenothiazine, nasal decongestants containing sympathomimetic or histamine, and metoclopramide. Treatment needs to be initiated immediately, intravenously and one needs to remember that α-adrenoceptor blockade is the drug of choice, as well as the fact that β-adrenoceptor medication can both cause and precipitate acute deterioration of the hypertensive crisis.
HYPOTENSIVE SHOCK
Hypotension in PPGL is usually perceived as exclusively related to dopamine or epinephrine-secreting tumors. While this is true, hypovolemia and acute heart failure due to an acute coronary event, myocarditis, or pulmonary edema can produce profound hypotension and shock in norepinephrine-secreting tumors too.
CARDIAC ARRHYTHMIAS
Tachyarrhythmias are frequently associated with PPGL and are related to β-adrenergic stimulation-driven positive inotropy. These are mostly supraventricular including atrial fibrillation and flutter, as well as wide complex ventricular tachycardia. One needs to remember that in case of myocarditis, cardiomyopathy, or an acute coronary event, myocardial susceptibility to any type of rhythm disturbances is significantly increased and can manifest with bradyarrhythmia’s.
MYOCARDITIS AND CARDIOMYOPATHY
Development of myocarditis and cardiomyopathy in PPGL is well known and described, but still remains poorly understood as far as the actual mechanistic process. It could relate to direct myocardial toxicity of significant and prolonged hypercatecholaminemia, as well as prolonged hypertension or a coronary event. It could be of any type – either hypertrophic or dilated – as well as asymmetric (tako-tsubo type). It could improve to some degree after successful treatment.
ACUTE MYOCARDIAL OR PERIPHERAL ISCHEMIA
Both could be caused by prolonged hypertension, resulting in intimal hypertrophy, as well as local spasm in the naïve or already sclerotic vessel. It can also result from increased and uncompensated oxygen demand.
Pulmonary Emergencies
Pulmonary edema can be both cardiac and non-cardiac of origin. The first is discussed above, while the last is mostly related to increased capillary pressure together with vasoconstriction related stasis and an increase in vascular permeability.
Gastrointestinal Emergencies
Clinically, acute GI emergencies are usually associated with abdominal pain and vomiting. These could be related to mesenteric ischemia, which consequently can result in bowel perforation, ileus, and GI bleeding. Ileus in PPGL can be both paralytic and pseudo-obstructive and can also associate with megacolon. Although rarely thought to be related to PPGL, these disorders need to be diagnosed early and treated to prevent rapid deterioration and the need for urgent surgery. Severe hypertension can also associate with an aneurysm of the aorta that can undergo dissection with a hypertensive spike.
Renal Emergencies
Acute vasoconstriction of the renal arteries can result in acute renal failure, while prolonged hypertension can cause progressive deterioration of renal function over a relatively short period of time, especially in patients with underlying hypertension or susceptibility to significant vascular changes.
Neurologic Emergencies
Strokes are known to occur with both paroxysmal and sustained hypertension, but other neurologic emergencies could include hypertensive encephalopathy, subarachnoid hemorrhage, and seizures. Neurologic deficiencies related to brain or spinal metastases, as well as local neurologic deficits caused by paragangliomas are also seen.
MANAGEMENT
The diagnostic approach and treatment of PPGL are discussed in detail in the PPGL section of Endotext and shown in Table 2, but what we will discuss here is the approach to PPGL-related medical emergencies.
|
Table 2. Treatment of PPGL |
|||
|
Stage |
Goal |
Primary |
Alternative |
|
Initial oral |
Normalization of BP Minimal organ effect |
In the following order: α-blocker β blocker Metyrosine |
Calcium channel blocker Labetalol |
|
Pre-operative |
Normal BP Normovolemia Optimized cardiac performance |
As Initial Fluids to normovolemia |
As Initial |
|
Intra-operative |
Prevention of the following: Severe hypercatecholaminemia Severe hypertension Severe hypotension |
Phentolamine IV Nitroprusside IV Aggressive fluid replacement |
Labetalol IV |
|
Post-operative |
Prevention of hypotension Prevention of hypoglycemia |
Aggressive fluid replacement Glucose supplementation |
|
|
Inoperable disease |
Maintenance of normal BP Treatment of metastatic disease |
Chemotherapy Radiotherapy Debulking |
Experimental Therapy |
As with non-urgent PPGL, the main part of successful management and prevention of its deterioration into a medical emergency is timely suspicion and diagnosis. Obviously, this cannot happen in each and every case and we will continue to see acute severe hypertensive crises and poor outcomes that could not be prevented. But otherwise, the overall suspicion should be relatively high, even if it will generate some unnecessary workups, while preventing avoidable death. We also feel that the common flamboyancy of PPGL being a great and friendly masquerader should probably be revised to some degree. It should include a quite real possibility of metamorphosis of this apparent benignity into behavioral tendencies of a Grim Reaper; just to make sure that the reality of severely morbid outcomes is known and respected.
As was discussed above, in case of acute intra-operative hypertensive crisis with or without identifiable mass, therapy should be initiated assuming PPGL-related severe hypercatecholaminemia. Medications are to be administered IV and should include phentolamine or nitroprusside. Nitroprusside is more readily available in ORs compared to phentolamine, which needs to be prepared by the pharmacy. Nitroprusside can cause adverse effects when administered over an extended period during complicated surgery (discussed above). If existence of a PPGL is established prior to surgery, it would definitely be advisable to procure phentolamine to be available in OR/ICU. Phentolamine, an α-adrenoceptor antagonist, is given as an i.v. bolus of 2.5 mg to 5 mg at 1 mg/min, which can be repeated every 5 min for adequate control of hypertension. Alternatively, it can be given as a continuous infusion (100 mg of phentolamine in 500 mL of 5% dextrose in water, not available in USA) with an infusion rate adjusted to the patient’s blood pressure during continuous blood pressure monitoring. Sodium nitroprusside can be administered at 0.5 to 10.0 mcg/kg per minute (stop if no results are seen after 10 minutes). Magnesium sulfate acts as vasodilator and antiarrhythmic and is administered as a 1-2 gm bolus and then continuously at 1 to 3 gm/h. Esmolol, a short acting β1-adrenoceptor antagonist can improve uncontrolled tachycardia. Continuous infusion of Nicardipine, that is usually a very good initial choice, can prevent catecholamine-induced coronary vasospasm, hypertension, and tachycardia and it is given intravenously at 1 to 2.5 mg for 2 min, then at 5 to 15 mg/h. If the patient was not on a α-adrenoceptor blocker prior to the surgery, use of Labetalol could precipitate deterioration in blood pressure because of 1:7 α:β-specific blocking effect. If the patient is on a short-acting competitive α-adrenoceptor blocker, using i.v. Labetalol bolus could be beneficial for better control of blood pressure.
The same approach should be carried out in cases of a severe hypertensive crisis if it happens acutely in a patient with known and insufficiently treated PPGL (recurrence, lack of compliance), acute deterioration of hypertension, or resistant to initial therapy including pre-eclampsia. There will be little time to sufficiently and efficiently pre-load patient with oral therapy, which should be carried out after resolution of the crisis if surgery was not performed.
If, on the other hand, there is time for oral therapy in less urgent situations or while awaiting upcoming surgery, α-adrenoceptor blockade should be initiated as soon as possible and the patient should be clinically evaluated on a frequent basis to adjust therapy as tolerated. The choice of medication should be dictated by several factors. In cases of severe hypercatecholaminemia or relatively recent onset, where one should expect less time available for significant desensitization of adrenoceptors, competitive α-adrenoceptor blockers could provide lesser control of symptoms just by the virtue of pharmacokinetics against massive concentration of catecholamines. In such cases, use of a non-competitive agonist – phenoxybenzamine - will make more sense. Otherwise, competitive adrenoceptor blockers seem to be efficient and safe and could result in shorter hospitalization due to shorter action and lesser postoperative hypotension. Also, doxazosin (as well as others (prazosin and terazosin, which seem to be used less frequently) seems to be both safe and efficient in PPGL management. Physician’s preferences and experience play a major role in the selection of prescribed medication. In addition to this, the cost and availability affects the choice of medication. Endocrinologist need to be comfortable with multiple different classes of medications used for therapy. Calcium channel blockers (nicardipine/amlodipine) proved to be also safe and efficient, but, again, we would suggest avoiding them alone in cases of severe hypercatecholaminemia, especially with concomitant congestive heart failure. A competitive inhibitor of tyrosine hydroxylase - α-methyl-para-tyrosine (metyrosine, Demser) can be used to both compete with tyrosine (the substrate for catecholamine synthesis), as well as a direct inhibitor of tyrosine hydroxylase.
Hypotension is managed by fluid administration and/or vasopressors including phenylephrine. Hypoglycemia can occur after removal of PPGL-related catecholamine excess as a result of rebound release of insulin secretion and is treated with intravenous glucose.
GUIDELINES
Lenders JW, Duh QY, Eisenhofer G et al.; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942.
REFERENCES
Pacak K, Tella SH. Pheochromocytoma and Paraganglioma. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-2018 Jan 4.
Crona J, Taieb D, Pacak K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocrine Reviews. 2017;38(6):489-515.
Fishbein L, Leshchiner I, Walter V, et al. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181–193
Prete A, Paragliola RA, Salvatori R, Salvatore MC. Management of catecholamine-secreting tumors in pregnancy: a review. Endocrine Practice. 2015;22(3):357-70.
Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108–119.
Tischler AS, Pacak K, Eisenhofer G.The Adrenal Medulla and Extra-adrenal Paraganglia: Then and Now. Endocr Pathol. 2013 Dec 24
Pacak K Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007:92(11):4069-79.
Congenital Hypothyroidism
INTRODUCTION
Thyroid hormones are essential for normal development and growth of many target tissues, including the brain and the skeleton. Thyroid hormone (TH) action on critical genes for neurodevelopment is limited to a specific time window, and even a short period of deficiency of TH can cause irreversible brain damage. During the first trimester of pregnancy fetal brain development is totally dependent on maternal thyroid function. Congenital hypothyroidism (CH) is one of the most preventable causes of mental retardation, but early diagnosis is needed in order to prevent irreversible damage. Today more than 70% of the babies worldwide are born in areas without an organized screening program. Screening for CH has enabled the virtual eradication of the devastating effects of mental retardation due to sporadic CH in most developed countries of the world. The survival of increasingly small and premature fetuses has resulted in a growing number of neonates with abnormalities in thyroid function and a continuing controversy as to which of these infants require therapy.
Non endemic CH is one of the commonest treatable causes of mental retardation. The importance of early treatment in diminishing the neuro-psychological abnormalities of CH was demonstrated convincingly in the 1970’s. The development of a sensitive and specific radioimmunoassay for the measurement of T4 in dried whole blood and later tests for T4 and TSH using 1/8″ discs provided the technical means to screen all newborns for CH prior to the development of clinical manifestations. Thus, CH includes all the characteristics of a disease for which screening is justified: 1) it is common (4-5 times more common than phenylketonuria for which screening programs were initially developed); 2) to prevent mental retardation, the diagnosis must be made early, preferably within the first few days of life; 3) at that age, clinical recognition is difficult if not impossible; 4) sensitive, specific screening tests are available; 5) simple, cheap effective treatment is available; and 6) the cost-benefit ratio is highly favorable. Newborn screening programs have been introduced throughout the industrialized nations and are under development in many other parts of the world. Although there continues to be some disagreement as to whether minor neuro-intellectual sequelae remain in the most severely affected infants, accumulating evidence suggests that a normal outcome is possible even in the latter group of babies as long as treatment is started sufficiently early and is adequate.
National screening programs are well organized in many developed countries. However, it must be emphasized that approximately 71% of babies worldwide are not born in an area with an established national screening program for CH. The economic burden of disability owing to CH is still a significant public health challenge.
CLINICAL RECOGNITION
Clinical findings of CH are usually difficult to appreciate in the newborn period except in the unusual situation of combined maternal-fetal hypothyroidism. Many of the classic features (large tongue, hoarse cry, facial puffiness, umbilical hernia, hypotonia, mottling, cold hands and feet and lethargy), when present, are subtle and develop only with the passage of time. In addition to the aforementioned findings, nonspecific signs that suggest the diagnosis of neonatal hypothyroidism include: prolonged, unconjugated hyperbilirubinemia, gestation longer than 42 weeks, feeding difficulties, delayed passage of stools, hypothermia, or respiratory distress in an infant weighing over 2.5 kg. A large anterior fontanelle and/or a posterior fontanelle > 0.5 cm is frequently present in affected infants but may not be appreciated. In general, the extent of the clinical findings depends on the cause, severity, and duration of hypothyroidism. Babies in whom severe feto-maternal hypothyroidism was present in utero tend to be the most symptomatic at birth. Similarly, babies with athyreosis or a complete block in thyroid hormonogenesis tend to have more signs and symptoms at birth than infants with an ectopic thyroid, the most common cause of CH. Unlike acquired hypothyroidism, babies with CH are of normal size. However, if diagnosis is delayed, subsequent linear growth is impaired. The finding of palpable thyroid tissue suggests that the hypothyroidism is due to an abnormality in thyroid hormonogenesis or in thyroid hormone action.
Bone maturation reflects the duration and the severity of hypothyroidism. Signs of delayed epiphyseal maturation on knee x-rays, persistence of the posterior fontanelle, a large anterior fontanelle, and a wide sagittal suture all reflect delayed bone maturation. The absence of one or both knee epiphyses has been shown to be related to T4 concentration at diagnosis and to IQ outcome, and is thus a reliable index of intrauterine hypothyroidism.
PATHOPHYSIOLOGY
For a detailed discussion of the cause of CH and hypothyroidism in infants see the chapter in Endotext entitled Disorders of the Thyroid Gland in Infancy, Childhood and Adolescence by Segni in the Thyroid section.
Permanent Primary Congenital Hypothyroidism
Permanent primary CH can be the consequence of a disorder in thyroid development and/or migration (thyroid dysgenesis), or due to defects at every step-in thyroid hormone synthesis (thyroid dyshormonogenesis). Although CH is in the great majority of cases a sporadic disease, the recent guidelines for CH recommend genetic counseling in targeted cases. Positive family history for CH, association with cardiac or kidney malformation, midline malformations, deafness, neurological sigs (i.e., choreoathetosis, hypotonia), any sign of Albright hereditary osteodystrophy, lung disorders, suggest genetic counseling, in order to assess the risk of recurrence and to provide further information about a possible genetic etiology of CH. Genetic causes of CH are described in table 1.
|
TABLE 1. GENETIC CAUSES OF CONGENITAL HYPOTHYROIDISM |
||
|
Gene locus |
Inheritance |
|
|
PRIMARY HYPOTHYROIDISM |
|
|
|
Monogenic forms of thyroid dysgenesis |
|
|
|
Thyroid stimulating hormone receptor (TSHR) |
|
AR |
|
NK2 1 (NK2-1, TTF1) brain-lung thyroid syndrome |
14q13 |
AD |
|
Paired box gene 8 (PAX8) |
2q11.2 |
AD |
|
Forkhead boxE1 (FOXE1, TTF2) (Bambforth-Lazarus syndrome) |
9q22 |
AR |
|
NK2 homeobox 5 (NKX2-5) |
|
|
|
New candidate genes |
|
|
|
Nertrin 1 (NTN-1) |
|
|
|
JAG1 |
20p.12.2 |
|
|
Glis3 |
9p24.2 |
AR |
|
Inborn errors of thyroid hormonogenesis |
|
|
|
Sodium/Iodide symporter (SLC5A5, NIS) |
19p13.2 |
AR |
|
Thyroid peroxidase (TPO) |
2p25 |
AR |
|
Pendred syndrome (SLC26A4, PDS) |
7q31 |
AR |
|
Thyroglobulin (TG) |
8q24 |
AR |
|
Iodothyrosine deiodinase (IYD, DEHAL1) |
6q24-25 |
AR |
|
Dual oxidase 2 (DUOX2) |
15q15.3 |
AR/AD |
|
Dual oxidase maturation factor 2 (DUOXA2) |
|
AR/AD |
|
CENTRAL HYPOTHYROIDISM |
|
|
|
Isolated TSH deficiency |
|
|
|
TRHR |
14q31 |
AR |
|
TSHB |
1p13 |
AR |
|
Isolated TSH deficiency or combined pituitary hormone deficiency |
|
|
|
Immunoglobulin superfamily member1 (IGSF1) gene defects |
Xq26.1 |
X-Linked |
|
Combined pituitary hormone deficiency |
|
|
|
POU1F1 |
3p11 |
AR, AD |
|
PROP1 |
5q |
AR |
|
HESX1 |
3p21.2-21.2 |
AR/AD |
|
LHX3 |
9q.34 |
AR |
|
LHX4 |
1q25 |
AD |
|
SOX3 |
|
X-linked |
|
OTX2 |
|
AD |
Thyroid Dysgenesis
The majority (85 to 90%) of cases of permanent CH in North America, Western Europe, and Japan are due to an abnormality of thyroid gland development (thyroid dysgenesis). Thyroid dysgenesis may result in the complete absence of thyroid tissue (agenesis, 20-30%) owing to a defect in survival of the thyroid follicular cells precursors) or it may be partial (hypoplasia); the latter often is accompanied by a failure to descend into the neck (ectopy) mostly located in a sublingual position as a result of a premature arrest of its migratory process. Lowering of cut off TSH values for newborn screening increases the percentage of CH with thyroid in situ. Females are affected twice as often as males. In the United States, thyroid dysgenesis, is less frequent among African Americans and more common among Hispanics and Asians. Babies with CH have an increased incidence of cardiac anomalies, particularly atrial and ventricular septal defects. An increased prevalence of renal and urinary tract anomalies has also been reported. Most cases of thyroid dysgenesis are sporadic. Familial cases represent approximately 2% of cases.
Genetic causes of congenital hypothyroidism are described in table 1. Thyroid transcription factors (TTF) such as NKX2-1 (or formerly TTF1/TITF1), FOXE1 (Forkhread Box E1, formerly TTF2/TITF2), PAX8 (Paired box gene 8), and NKX2-5, are expressed during early phases of thyroid organogenesis (budding and migration), and thyroid stimulating hormone receptor gene (TSHR) is expressed during the later phases of thyroid development. All these genes are involved in normal thyroid development and in thyroid dysgenesis, however, abnormalities in these genes have been found in only a small proportion of affected patients, usually in association with other developmental abnormalities. Alternately, epigenetic modifications, early somatic mutations, or stochastic developmental events may play a role. Five monogenic forms due to mutations in TSHR, NXK2-1, PAX8, FOXE-1. NXK2-5 have been reported. Monogenic forms represent less than 10% in thyroid dysgenesis. Inactivating TSHR mutations are the most frequent cause of monogenic thyroid dysgenesis and non-syndromic CH, with prevalence in CH cohorts around 4 %.
Inborn Errors of Thyroid Hormonogenesis
Inborn errors of thyroid hormonogenesis (thyroid dyshormonogenesis) are responsible for most of the remaining cases (15%) of neonatal thyroidal hypothyroidism. Unlike thyroid dysgenesis, most are sporadic condition. These inborn errors of thyroid hormonogenesis are commonly associated with an autosomal recessive form of inheritance, consistent with a single gene abnormality. DUOX2 mutations can be transmitted in autosomal dominant way. Thyroid dyshormonogenesis is caused by genetic defects in proteins involved in all steps of thyroid hormone synthesis and often associated with goiter formation. Goiter can be present in utero or at birth. .A number of different defects have been characterized based on radioiodine uptake and perchlorate test and include: 1) Iodide transport defect that shows failure to concentrate iodide, with low or absent radioiodine uptake; 2) Iodide organification defects due to thyroid peroxidase mutations (TPO), Dual Oxidase 2 (DUOX2), Dual Oxidase Maturation Factor 2 mutations (DUOX2A), SLC26A4, and pendrin defects that have normal radioiodine uptake and altered perchlorate discharge test; and 3) Forms with normal radioiodine uptake and a normal perchlorate test due to thyroglobulin TG mutations, iodide recycling defects, and iodothyrosine deiodinase mutations.
Pendred Syndrome
Pendred syndrome is defined by the association of familial profound deafness with multinodular goiter. It is caused by biallelic mutation in the pendrin gene. Pendred syndrome is the only form of thyroid dyshormonogenesis associated with a malformation. The inner ear presents a characteristic malformation of the cochlea. Congenital hypothyroidism is present in only 30% of cases, goiter occurs often in childhood. Perchlorate test shows a partial organification defect. Pendred syndrome is the most frequent etiology of familial deafness.
Central Congenital Hypothyroidism (CCH)
CCH is caused by an insufficient thyroid hormone biosynthesis due to a defective stimulation by TSH, in the presence of an otherwise normal thyroid. This condition includes all causes of CH due to a pituitary or hypothalamic pathology (secondary or tertiary hypothyroidism). CCH was previously considered a very rare disease with a prevalence initially estimated to be 1:100,000 in newborns. In more recent data, CCH had an incidence that could reach 1:16,000, as shown from results from screening for CH in the Netherlands.
CCH is sometime not identified at birth, because the limiting step is “how low is a low T4”, low enough to be considered an effective cutoff value and allow the determination of TSH and TBG. Many cases are diagnosed in infancy or childhood, if not later in adulthood. The majority of screening programs are based on TSH determination and a high index of suspicion is needed to identify CCH in the preclinical phase. Delayed diagnosis may result in neurodevelopment delay. More than 50% of children with CCH have moderate or severe hypothyroidism, so, if not treated, the risk of neurodevelopmental delay should not be underestimated.
In the majority of cases identified early, TSH deficiency is a part of combined pituitary hormone deficiency. A timely correction of ACTH and cortisol deficiency, and/or GH deficiency may avoid life threatening emergencies. CCH can be transient (mostly due to drugs or maternal hyperthyroidism), or permanent. Genetic causes are listed in Table 1.
Defects of Thyroid Hormone Transport in Serum
For complete coverage of this and related areas visit the chapter entitled “Defects of thyroid
hormone transport in serum” in the thyroid section of Endotext by Samuel Refetoff. Inherited abnormalities of the iodothyronine-binding serum proteins include TBG deficiency (partial or complete), TBG excess, transrethyretin (TTR) (prealbumin) variants, and familial
dysalbuminemic hyperthyroxinemia (FDH). In these conditions the concentration of free hormones is unaltered, but the abnormal total thyroxine concentrations can be misleading during neonatal screening and in the evaluation of thyroid function.
Impaired Sensitivity to Thyroid Hormone
For complete coverage of this and related areas visit the chapter entitled: “Impaired sensitivity to thyroid hormone: defects of transport, metabolism and action” in the thyroid section of Endotext by Alexandra M. Dumitrescu and Samuel Refetoff. Impaired sensitivity to thyroid hormone includes defects in thyroid hormone action, transport, and metabolism. They are classified as a) thyroid hormone cell membrane transport defects, b) thyroid hormone metabolism defect, and c) thyroid hormone action defect that include resistance to thyroid hormone.
Causes of Transient Neonatal Hypothyroidism
Transient neonatal hypothyroidism should be distinguished from a ‘false positive’ result in which the screening result is abnormal but the confirmatory serum sample is normal. Causes of transient abnormalities of thyroid function in the newborn period are listed in Table 2. While iodine deficiency, iodine excess, drugs and maternal TSH receptor blocking antibodies are the most common causes of transient hypothyroidism, in some cases the cause is unknown.
|
TABLE 2. CAUSES OF TRANSIENT HYPOTHYROIDISM IN THE NEWBORN |
|
PRIMARY HYPOTHYROIDISM Prenatal or postnatal iodine deficiency or excess Maternal antithyroid medication Maternal TSH receptor blocking antibodies Mild gene mutations (i.e. DUOX2, TSH-R) Maternal hypothyroidism Prematurity, VLBW Drugs, (i.e. Dopamine, steroids) Hypothyroxinemia (low T4, normal TSH) |
|
CENTRAL HYPOTHYROIDISM Prenatal exposure to maternal hyperthyroidism Prematurity (particularly <27 weeks gestation) Drugs |
Iodine Deficiency or Excess
In addition to iodine deficiency, both the fetus and newborn infant are sensitive to the thyroid- suppressive effects of excess iodine, whether administered to the mother during pregnancy, lactation, or directly to the baby. This occurs because recovery from the thyroid-suppressive effect of iodine does not mature before 36 weeks gestation. Reported sources of iodine include drugs (e.g., potassium iodide, amiodarone), radiocontrast agents, and antiseptic solutions (e.g., povidone-iodine) used for skin cleansing or vaginal douches. In contrast to Europe, iodine-induced transient hypothyroidism has not been documented frequently in North America.
Maternal Antithyroid Medication
Transient neonatal hypothyroidism may develop in babies whose mothers are being treated with antithyroid medication (either propylthiouracil, PTU or methimazole) for the treatment of Graves’ disease. Even maternal PTU doses of 200 mg or less have been associated with an effect on neonatal thyroid function, illustrating the increased fetal sensitivity to these drugs. Babies with PTU- or methimazole-induced hypothyroidism characteristically develop an enlarged thyroid gland and if the dose is sufficiently large, respiratory embarrassment may occur. Both the hypothyroidism and goiter resolve spontaneously with clearance of the drug from the baby’s circulation. Usually replacement therapy is not required.
Maternal TSH Receptor Antibodies
Maternal TSH receptor blocking antibodies, a population of antibodies closely related to the TSH receptor stimulating antibodies in Graves’ disease, may be transmitted to the fetus in sufficient titer to cause transient neonatal hypothyroidism. The incidence of this disorder has been estimated to be 1 in 180,000. TSH receptor blocking antibodies most often are found in mothers who have been treated previously for Graves’ disease or who have the non-goitrous form of chronic lymphocytic thyroiditis (primary myxedema). Occasionally these mothers are not aware that they are hypothyroid and the diagnosis is made in them only after CH has been recognized in their infants. Unlike TSH receptor stimulating antibodies that mimic the action of TSH, TSH receptor blocking antibodies inhibit both the binding and action of TSH. Because TSH-induced growth is blocked, these babies do not have a goiter. Similarly, inhibition of TSH-induced radioactive iodine uptake may result in a misdiagnosis of thyroid agenesis. Usually the hypothyroidism resolves in 3 or 4 months. Babies with TSH receptor blocking-antibody induced hypothyroidism are difficult to distinguish at birth from the more common thyroid dysgenesis but they differ from the latter in a number of important ways (Table 3). They do not require lifelong therapy, and there is a high recurrence rate in subsequent offspring due to the tendency of these antibodies to persist for many years in the maternal circulation. Unlike babies with thyroid dysgenesis in whom a normal cognitive outcome is found if postnatal therapy is early and adequate, babies with maternal blocking-antibody induced hypothyroidism may have a permanent deficit in intellectual development if feto-maternal hypothyroidism was present in utero.
|
TABLE 3. CLINICAL FEATURES OF THYROID DYSGENESIS VERSUS TSH RECEPTOR BLOCKING ANTIBODY INDUCED CONGENITAL HYPOTHYROIDISM |
||
|
|
Dysgenesis |
Blocking Ab |
|
Severity of CH |
+ to ++++ |
+ to ++++ |
|
Palpable thyroid |
No |
No |
|
123I uptake |
None to low |
None to normal |
|
Clinical Course |
Permanent |
Transient |
|
Familial risk |
No |
Yes |
|
TPO Abs |
Variable |
Variable |
|
TSH Receptor Abs |
Absent |
Potent |
Transient Central Hypothyroidism Due to Maternal Hyperthyroidism
Occasionally, babies born to mothers who were hyperthyroid during pregnancy develop transient hypothalamic-pituitary suppression. This hypothyroxinemia is usually self-limited, but in some cases, it may last for years and require replacement therapy. In general, the titer of TSH receptor stimulating antibodies in this population of infants is lower than in those who develop transient neonatal hyperthyroidism.
Prematurity
Hypothyroxinemia in the presence of a ‘normal’ TSH occurs most commonly in premature infants in whom it is found in 50% of babies of less than 30 weeks gestation. Often the free T4 when measured by equilibrium dialysis is less affected than the total T4. The reasons for the hypothyroxinemia of prematurity are complex. As well as hypothalamic-pituitary immaturity, premature infants frequently have TBG deficiency due to both immature liver function and undernutrition, and they may have “sick euthyroid syndrome”. They may also be treated with drugs that suppress the hypothalamic-pituitary-thyroid axis. Hypothyroxinemia of prematurity may be associated with adverse neurodevelopmental outcomes. L-T4 treatment overall has no proven benefit and can be harmful. Long term outcome evaluation in young adults did not find an association between transient hypothyroxinemia of prematurity and neurodevelopmental outcome. Whether or not premature infants with hypothyroxinemia should be treated remains controversial at the present time. Although several retrospective, cohort studies have documented a relationship between severe hypothyroxinemia and both developmental delay and disabling cerebral palsy in preterm infants <32 weeks gestation a causal relationship could not be determined since the serum T4 in premature infants, as in adults, has been shown to reflect the severity of illness and risk of death.
Drugs
Drugs that suppress the hypothalamic-pituitary axis include known agents such as steroids and dopamine, but also aminophylline, caffeine and diamorphine, which are commonly used in sick premature infants.
Other Causes of Hypothyroidism in Infancy
Chronic lymphocytic thyroiditis
Chronic lymphocytic thyroiditis (CLT) is a rare disease in infancy, but if not recognized and treated, can cause severe hypothyroidism with permanent brain damage. CLT can be associated with other autoimmune disease such as type 1 diabetes or as a manifestation of the IPEX syndrome. Clinical manifestations and biochemical hypothyroidism (TSH ranged from >42 to 928 mU/L) were severe and very high levels of antibodies were detectable.
IPEX related disorders
Lymphocytic thyroiditis has also been described in newborns with severe defects in tolerance and autoimmunity with immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, a polyglandular disorder characterized by early-onset diabetes and colitis. IPEX disorders are an expanding spectrum of disease with mutations in FOXP3, CD25 deficiency, STAT5 deficiency, and others.
Hepatic hemangiomas: consumptive hypothyroidism
Hepatic hemangioendothelioma is a rare tumor typically presenting in infancy. Hypothyroidism is caused by a production of type 3 deiodinase by the vascular tumor. D3 deiodinase increases inactivation of T4 and T3 to reverse T3 and T2 and a large amount of LT4 (up to 94/ µg/kg/day) is needed to compensate for this inactivation. Frequent monitoring is required, adapting the LT4 treatment to the growing proliferative phase of the tumor. Today hemangioendotheliomas in infancy may successfully being treated with steroids and propranolol and may undergo spontaneous regression. Some babies underwent liver transplantation.
SCREENING STRATEGIES
The aim of neonatal screening is the earliest identification of any form of CH, but particularly those patients with severe hypothyroidism in whom disability is greatest if not treated. The identification of Central CH by screening programs is under debate. Two screening strategies for the detection of CH have evolved. In the primary T4/backup TSH method, still favored in much of North America and the Netherlands, T4 is measured initially while TSH is checked on the same blood spot in those specimens in which the T4 concentration is low. In the primary TSH approach, favored in most parts of Europe and Japan, blood TSH is measured initially.
A primary T4/backup TSH program will detect overt primary hypothyroidism, secondary or tertiary hypothyroidism, babies with a low serum T4 level but delayed rise in the TSH concentration, TBG deficiency and hypothyroxinemia; this approach may, however, miss subclinical hypothyroidism. A primary TSH strategy, on the other hand, will detect both overt and subclinical hypothyroidism, but will miss secondary or tertiary hypothyroidism, a delayed TSH rise, TBG deficiency and hypothyroxinemia. There are fewer false positives with a primary TSH strategy. Both programs will miss the rare infant whose T4 level on initial screening is normal but who later develops low T4 and elevated TSH concentrations. This pattern has been termed “atypical” CH or “delayed TSH” and is observed most commonly in premature babies with transient hypothyroidism or infants with less severe forms of permanent disease.
According to the European Society for Pediatric Endocrinology (ESPE) guidelines, the most sensitive test for detecting primary CH is the determination of TSH concentration that detects primary CH more effectively than primary T4 screening Primary T4 screening with confirmatory TSH testing can detect some cases of central CH, but some cases of mild CH can be missed, depending on the cutoff T4 value used.
Measurement of T4 and/or TSH is performed on an eluate of dried whole blood (DBS) collected on filter paper by skin puncture on day 1-4 of life. Primary CH screening has been shown to be effective for the testing of cord blood or the blood collected on filter paper after the age of 24 hours. Blood is applied directly to the filter paper and after drying the card is sent to the laboratory. The best time to collect blood for TSH screening is 48 to 72 hours of age. The practice of early discharge from the hospital of otherwise healthy full-term infants has resulted in a greater proportion of babies being tested before this time. For example, it has been estimated that in North America 25% or more of newborns are now discharged within 24 hours of delivery and 40% in the second 24 hours of life. Because of the neonatal TSH surge and the dynamic changes in serum T4 and T3 concentrations that occur within the first few days of life, early discharge increases the number of false positive results. It is important that in the screening laboratory the results of TSH are interpreted in relation to time of sampling.
Physicians caring for infants need to appreciate that there is always the possibility for human error in failing to identify affected infants, whichever screening program is utilized. This can occur due to poor communication, lack of receipt of requested specimens, or the failure to test an infant who is transferred between hospitals during the neonatal period. Therefore, if the diagnosis of hypothyroidism is suspected clinically, the infant should always be tested. Adult normative values, provided by many general hospital laboratories, differ from those in the newborn period and should never be employed.
Special categories of neonates with CH can be missed at screening performed at the usual time, particularly preterm babies and neonates with serious illnesses and multiple births. Drugs used in neonatal intensive care (i.e., dopamine, glucocorticoids that suppresses TSH), immaturity of hypothalamic-pituitary thyroid axis, decreased hepatic production of thyroid binding globulin, reduced transfer of maternal T4, reduced intake of iodine or excess iodine exposure, fetal blood mixing in multiple births can affect the first sample, and in many centers a second specimen is required to rule out CH. Preterm babies have a higher incidence of a unique form of hypothyroidism, characterized by a delayed elevation of TSH. These babies can later develop low T4 and elevated TSH concentrations. This pattern has been termed “atypical” CH or “delayed TSH”. Preterm babies with a birth weight of less than 1500 gr. have an incidence of CH of 1:300. Survival of even extremely premature babies (<28 weeks of gestation) is around 90% in developed countries, and the incidence of prematurity is around 11.5 % in US and 11.8 % worldwide. So, an increasing subpopulation of preterm babies and high-risk newborns deserves a special screening and follow up for CH.
In these categories a second specimen 2-6 weeks from the first (ESPE guidelines suggested at about 15 days, or after 15 days from the first) may be indicated in a) preterm neonates with a gestational age of less than 37 weeks, b) Low Birth Weight and Very Low Birth Weight neonates, c) ill and preterm neonates admitted to neonatal intensive care unit, d) if specimen collection was within the first 24 hours of life, and e) multiple births, particularly in the case of same sex twins. The interpretation of the screening results should consider the results of a multiple sampling strategy, the age of sampling, and the maturity (GA/birth weight) of the neonate. A second screen (using a lower TSH cutoff) is able to detect the delayed elevation of TSH that occurs in these babies.
CH is defined on the basis of serum FT4 levels as severe when FT4 is <5 pmol/l, moderate when FT4 is 5 to 10 pmol/l, and mild when FT4 is 10 to 15 pmol/l, respectively. Determination of serum thyroglobulin (Tg) is useful, if below the detection threshold, to suggest athyreosis or a complete thyroglobulin synthesis defect. Measurement of Tg is most helpful when a defect in Tg synthesis or secretion is being considered. In the latter condition the serum Tg concentration is low or undetectable despite the presence of a normal or enlarged, eutopic thyroid gland. Serum Tg concentration also reflects the amount of thyroid tissue present and the degree of stimulation. For example, Tg is undetectable in most patients with thyroid agenesis, intermediate in babies with an ectopic thyroid gland, and may be elevated in patients with abnormalities of thyroid hormonogenesis not involving Tg synthesis and secretion. Considerable overlap exists, and so, the Tg value needs to be considered in association with the findings on imaging. In patients with inactivating mutations of the TSH receptor discordance between findings on thyroid imaging and the serum Tg concentration has been described in some but not all studies.
IMAGING TECHNIQUES IN CH
Imaging studies are helpful to determine the specific etiology of CH. Both scintigraphy and ultrasound (US) should be considered in neonates with high TSH concentrations. Ideally, the association of US and scintigraphy gives the best information in a child with primary hypothyroidism. Scintigraphy shows the presence/absence (athyreosis), position (ectopic gland, in any point from the foramen caecum at the base of the tongue to the anterior mediastinum) and rough anatomic structure of the thyroid gland. US, is a useful tool in defining size and morphology of a eutopic thyroid gland, however, US alone is less effective in detecting ectopic glands. Color Doppler US improves the effectiveness of US. It is important to remember that an attempt to obtain imaging in a newborn should never delay the initiation of treatment. Scintigraphy should be carried out within 7 days of starting LT4 treatment. Scintigraphy may be carried out with either 10-20 MBq of technetium 99m (99mTc) or 1-2 MBq of iodine123 (I123). Tc is more widely available, less expensive, and quicker to use than I123. Scintigraphy with I123, if available, is usually preferred because of the greater sensitivity and because, I123, unlike of technetium99, is organified. Therefore, imaging with this isotope allows quantitative uptake measurements and tests for both iodine transport defects and abnormalities in thyroid oxidation. An enrichment of the tracer within the salivary gland can lead to misinterpretation, especially on lateral views, but this can be avoided by allowing the infant to feed before scintigraphy, thus empting the salivary glands and keeping the child calm under the camera. The perchlorate discharge test is considered indicative of an organification defect when a discharge of > 10% of the administered I123 dose occurs in a thyroid in normal position (when perchlorate is given at 2 hours).
Excess iodine intake through exposure, maternal TSH receptor blocking antibodies, inactivating mutation in the TSH receptor and in the sodium/iodide symporter (NIS), and TSH suppression from LT4 treatment can interfere with the I123 uptake, showing no uptake in the presence of a thyroid in situ (apparent athyreosis).
Thyroid ultrasonography is performed with a high frequency linear array transducer (10-15 MHz) and allows a resolution of 0.7 to 1mm. Thyroid tissue is more echogenic than muscle and less echogenic than fat. In the case of absence of the thyroid, fat tissue can be misdiagnosed as dysplastic thyroid gland in situ. Distinguishing between thyroid hypoplasia and dysplastic non-thyroidal tissue in a newborn requires an experience and reevaluation at a later age can result in a different diagnosis.
Combining scintigraphy and thyroid ultrasound improves diagnostic accuracy and helps to address further investigations, including molecular genetic studies. Infants found to have a normal sized gland in situ in the absence of a clear diagnosis should undergo further reassessment of the thyroid axis and imaging at a later age.
THERAPY
Timing of normalization of thyroid hormones is critical for brain development and therefore replacement therapy with L-thyroxine (L-T4) should be begun as soon as the diagnosis of CH is confirmed. Treatment should be started immediately if DBS TSH concentration is >40 mUI/l because this value strongly suggests decompensated hypothyroidism. If TSH is < 40 mUI/l the clinician may postpone treatment, pending the serum results, for 1-2 days. ESPE guidelines suggest treatment should be started if venous TSH concentration is persistently >20 mUI/l, even if serum FT4 is normal. Severe hypothyroidism is defined by T4 <5 mcg/dL (64 nmol/L) and/or TSH >40 mU. According to ESPE guidelines, CH is defined on the basis of serum FT4 levels as severe when FT4 is <5 pmol/l, moderate when FT4 is 5 to 10 pmol/l, and mild when FT4 is 10 to 15 pmol/l. As noted above, treatment need not be delayed in anticipation of performing thyroid imaging studies as long as the latter are done within 5-7 days of initiating treatment (before suppression of the serum TSH). Parents should be counseled regarding the causes of CH, the importance of compliance, and the excellent prognosis in most babies if therapy is initiated sufficiently early and is adequate. Educational materials should be provided. An initial dosage of 10-15 mcg/kg/day of L-T4 is generally recommended to normalize the T4 as soon as possible. The highest dose is indicated in infants with severe disease, and the lower dose in those with a mild to moderate CH. L-T4 tablets can be crushed and given via a small spoon, with suspension, if necessary in a few milliliters of water or breast milk or formula or juice, but care should be taken that all of the medicine has been swallowed. Thyroid hormone should not be given with substances that interfere with its absorption, such as iron, calcium, soy, or fiber. Drugs such as antacids (aluminum hydroxide) or infantile colic drops (simethicone) can interfere with L-thyroxine absorption. Many babies will swallow the pills whole or will chew the tablets with their gums even before they have teeth. Reliable liquid preparations are not available commercially in the US, although they have been used successfully in Europe. A brand name rather a generic formulation of L-T4 is recommended because they are not bioequivalent.
It is still a matter of debate if treatment can be beneficial in otherwise healthy babies with venous TSH concentration between 6-20 mUI/l and FT4 concentration within the normal limits for age. In these cases, diagnostic imaging is recommended to try to establish a definitive diagnosis. If TSH concentration remains high for more than 3 or 4 weeks, it is possible (in discussion with the family) to either start LT4 supplementation immediately and then retest off treatment at a later stage or retest two weeks later without treatment. Given the irreversibility of possible harm, treating during early childhood and revaluating thyroid function after myelination of the central nervous system is completed (by 36 to 40 months of age) can be a prudent approach. LT4 treatment must be started immediately if FT4 or TT4 levels are low, given the known adverse effect of untreated decompensated CH on neurodevelopment and somatic growth.
The aims of therapy are to normalize the T4 as soon as possible, to avoid hyperthyroidism where possible, and to promote normal growth and development. When an initial dosage of 10-15 mcg/kg is used, the T4 will normalize in most infants within 1 week and the TSH will normalize within 1-month. Subsequent adjustments in the dosage of medication are made according to the results of thyroid function tests and the clinical picture. Often small increments or decrements of L-thyroxine (12.5 mcg) are needed. This can be accomplished by 1/2 tablet changes, by giving an alternating dosage on subsequent days, or by giving an extra tablet once a week.
As stated in ESPE guidelines: “L-T4 alone is recommended as the medication of choice and should be started as soon as possible, no later than two weeks of life or immediately after confirmatory test results in infants identified in a second routine screening test. L-T4 should be given orally. If intravenous administration is necessary, the dose should be no more than 80% of the oral dose”. Serum or plasma FT4 (or TT4) and TSH concentration should be determined at least 4 hours after the last L-T4 administration. TSH should be maintained in the age-specific reference range and FT4 in the upper half of the age- specific reference range. “The first follow up examination is indicated after 1-2 weeks after the start of LT4 treatment and then every 2 weeks until TSH levels are completely normalized and then every 1- 3 months until 12 months of age. Between the age of one and three years, children should undergo frequent clinical and laboratory evaluations (every 2 to 4 months).” Thereafter, evaluations should be carried out every 3 to 12 months until growth is completed. “More frequent evaluations should be carried out if compliance is questioned or abnormal values are obtained. Any reduction of L-T4 dose should not be based on a single increase of FT4 concentration during treatment. “Measurements should be performed after 4-6 weeks any change in the dosage or in the L-T4 formulation”.
RE-EVALUATION AND TRIAL OFF THERAPY
In hypothyroid babies in whom an organic basis was not established at birth and in whom transient disease is suspected, a trial off replacement therapy can be initiated after the age of 3 years when most thyroxine-dependent brain maturation has occurred, as shown by MRI studies. Re-evaluation is recommended if the treatment was started in a sick child (i.e. preterm), if thyroid antibodies were detectable, if no diagnostic assessment was completed, and in children who have required no increase in L-T4 dosage since infancy. Re-evaluation is recommended also in the case of a eutopic gland with or without goiter, if no enzyme defects have been detected, or if any other cause of transient hypothyroidism is suspected.
Re-evaluation is not necessary if venous TSH concentration has risen during the first year of life, due to either LT4 underdosage or poor compliance. To perform a precise diagnosis, LT4 treatment is suspended for 4-6 weeks, and biochemical testing and thyroid imaging are carried out. To establish the presence of primary hypothyroidism, without defining the cause, L-T4 dose may be decreased by 20-30% for 2 to 3 weeks. If TSH serum levels rise to > 10 mU/L during this period, the hypothyroidism can be confirmed.
PROGNOSIS
Although all agree that the mental retardation associated with untreated CH has been largely eradicated by newborn screening, controversy persists as to whether subtle cognitive and behavioral deficits remain, particularly in the most severely affected infants. Both the initial treatment dose and early onset of treatment (before 2 weeks) are important. Time to normalization of circulating thyroid hormone levels, the initial free T4 concentration, maternal IQ, socioeconomic status, and ethnic status have also been related to outcome. The long-term problems for these babies appear to be in the areas of memory, language, fine motor, attention, and visual spatial. Inattentiveness can occur both in patients who are overtreated and those in whom treatment was initiated late or was inadequate. In addition to adequate dosage, assurance of compliance and careful long-term monitoring are essential for an optimal developmental outcome. More details about long term follow up are reported in ESPE guidelines. Progressive hearing loss in CH should be recognized and corrected, because they strongly influenced the outcome.
ACKNOWLEDGEMENTS
This chapter is, in part, based on the previous version written by Prof. Rosalind Brown.
GUIDELINES
Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017 Mar;27(3):315-389.
Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, Polak M, Butler G on behalf of ESPE-PES-SLEP-JSPE-APEG-APPE-ISPAE, and the Congenital Hypothyroidism Consensus Conference Group. European Society for Pediatric Endocrinology Consensus Guidelines on Screening, Diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014; 99:363-384.
REFERENCES
Segni M. Disorders of the Thyroid Gland in Infancy, Childhood and Adolescence. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000- 2017 Mar 18
Dumitrescu AM, Refetoff S. Impaired Sensitivity to Thyroid Hormone: Defects of Transport, Metabolism and Action. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000- 2015 Aug 20
Refetoff S. Abnormal Thyroid Hormone Transport. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000- 2015 Jul 15
Bernal J. Thyroid Hormones in Brain Development and Function. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000- 2015 Sep 2.
Kaluarachchi DC, Allen DB, Eickhoff JC, Dawe SJ, Baker MW. Thyroid-Stimulating Hormone Reference Ranges for Preterm Infants. Pediatrics. 2019 Aug;144(2).
Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Practice & Research Clinical Endocrinology & Metabolism. 2014; 28:175-187.
Bauer AJ, Wassner AJ. Thyroid hormone therapy in congenital hypothyroidism and pediatric hypothyroidism. Endocrine. 2019 Jul 26. doi: 10.1007/s12020-019-02024-6. [Epub ahead of print]
Evaluation and Treatment of Gender-Dysphoric/Gender Incongruent Adults
ABSTRACT
Gender dysphoria refers to the suffering due to an incongruence between one’s sex assigned at birth and one’s self-perceived gender. Primary care physicians often play an important role in diagnosis and initiation of treatment of gender dysphoria. However, gender dysphoria is preferentially diagnosed by a specialized psychologist or psychiatrist. This does not imply that gender dysphoria in itself is a mental disorder, but co-morbidity needing attention, is frequently present. The prevalence of transgender people who receive medical treatment has steeply increased in the last decades. The current prevalence is estimated at 1:2,800 for transwomen (male sex assigned at birth, female gender identity) and 1:5,200 for transmen (female sex assigned at birth, male gender identity). Treatment of transgender people often includes gender-affirming hormonal therapy and/or surgery, and is optimally provided by a multidisciplinary team consisting of psychologists, endocrinologists, plastic surgeons, gynecologists, urologists, otorhinolaryngologists, and/or dermatologists. Medical treatment usually improves the quality of life of transgender people, but might also have side-effects such as increased risk for cardiovascular disease or hormone-sensitive tumors. There is still little known about the optimal therapy (for specific transgender subpopulations) and its long-term side-effects. Nowadays, guidelines are mainly based on clinical experience instead of evidence. However, transgender medicine is a growing field and the increasing number of good quality studies are helping to improve care of transgender people. In this contribution we mainly focus on what is known about the side-effects of hormonal therapy. In addition, we provide information about surgical and fertility preservation options for transgender people. We conclude this contribution with remarks about special conditions such as older age and unsupervised hormone use.
INTRODUCTION
Gender identity is the personal sense of one's own gender. A significant incongruence between one’s physical phenotype and one’s gender identity is defined as gender dysphoria. While care for transgender people has long been based on a binary understanding of gender (male versus female), the existence of non-binary or gender queer genders is getting increasing attention. Non-binary or gender queer peopleidentify with a gender that is neither exclusively male nor female, but is composed of both, oscillates between genders, is situated beyond the binary, or rejects the binary (1). Gender dysphoria is usually diagnosed by a specialized psychologist, which does not imply that it is a mental disorder per se. People who have a male sex assigned at birth but have developed a female gender identity are called transwomen and people who have a female sex assigned at birth but experience a male gender identity are called transmen.The prevalence of transgender people who seek medical treatment has dramatically increased in the last years, and a recent Dutch study estimated a prevalence of 1:2,800 for transwomen and 1:5,200 for transmen (2). While the etiology is complex and probably multifactorial, the most widely believed hypothesis is that transgender people have experienced a sex-atypical differentiation of the brain during fetal development (3,4).
To many physicians, transgender medicine is a novel area of medicine. Most physicians need intensive interaction with a transgender individual to empathize the suffering, and to arrive at the insight that hormonal and surgical treatment might alleviate gender dysphoria-related distress. It is usually assumed that medical interventions can only be justified when objective, identifiable pathological processes account for human suffering. The role that biological factors play in the development of gender identity is still not solidly established but an increasingly number of reports provide evidence that androgens play a role in the development of gender identity. The association is not absolute, and the information is not robust enough to draw solid conclusions. Nevertheless, it has been demonstrated that gender-affirming hormonal therapy and/or surgery, which is only recommended in people with well-documented gender dysphoria (5), contributes to an improved well-being (6). Medical treatment is preferably provided by a multidisciplinary team consisting of psychologists, endocrinologists, plastic surgeons, gynecologists, urologists, otorhinolaryngologists, and/or dermatologists.
When providing transgender care, physicians are confronted with a lack of systematically and accurately collected data. Transgender people are not a well-defined group, and show considerable heterogeneity, for instance in age of onset, in the degree of suffering, and in the wish for treatment. The attention that transgender people receive in medicine is certainly improving, however transgender people still experience health disparities. There is a dearth of research and evidence-based guidelines for treatment, and the specific health needs for transgender people are understudied. It is problematic to include sufficient numbers of participants to perform statistically robust studies with regard to the best transgender hormonal therapy, and the long-term side-effects of treatment. Nowadays, physicians with extensive clinical experience have drafted guidelines based primarily on empiric observation. Fortunately, the number of good quality studies is currently increasing.
HORMONAL THERAPY
In transwomen, hormonal therapy usually consists of estrogens ± antiandrogens (e.g. cyproterone acetate, spironolactone, or GnRH analogues) (9,10). Estrogen in the form of estradiol is recommended to minimalize the risk of venous thrombosis and cardiovascular disease. Since progestogens, other than progestogenic antiandrogens, have not been proven to have an additional effect on feminization in transwomen, they are usually not recommended (5,9,10), although they might have beneficial effects in cisgender women (11). In transmen, hormonal therapy usually consists of testosterone only (10). See Table 1 for an overview of the hormonal options for transwomen, and Table 2 for the hormonal options for transmen. For details on the hormonal regimens for transwomen and transmen the Endocrine Society Clinical Practice Guideline is an excellent source of information (10).
|
Table 1. Hormonal Regimens Regularly Used in Transwomen |
|
|
Estrogens |
Recommended Dose |
|
Oral estradiol |
2-6 mg daily |
|
Intramuscular estradiol valerate/cypionate |
10-20 mg/1-2 weeks |
|
Estradiol patch* |
50-100 mcg/24 hours |
|
Estradiol gel* |
0.75 mg-2.25 mcg daily |
|
Antiandrogens** |
|
|
Cyproterone acetate |
10-50 mg daily |
|
Spironolactone |
50-200 mg daily |
|
GnRH analogue |
Varies per preparation |
* Transdermal preparations are recommended in transwomen
aged ≥40 years or in those with an increased cardiovascular risk
** Antiandrogens are discontinued after orchiectomy
|
Table 2. Hormonal Regimens Regularly Used in Transmen |
|
|
Testosterone |
Recommended dose |
|
Intramuscular enanthate or cypionate* Intramuscular mixed esters (Sustanon®) Intramuscular undecanoate |
100–200 mg/2 weeks 250 mg/2-3 weeks |
|
Testosterone gel |
25-100 mg/day |
|
Testosterone patch |
2 or 4 mg/day |
Preparation availability will differ between countries
* Due to associated serum testosterone peaks, these injections may not the best option for transmen who develop polycythemia (12)
** The above are the conventional dosages for cismen. To virilize transmen 50-75% of these dosages are usually sufficient
Effects of Hormonal Therapy
TRANSWOMEN
In transwomen, hormonaltherapy induces feminization such as breast growth, skin softness, fat redistribution, and a decrease of body hair (9,10). Figure 1 shows the expected effects of hormonal therapy in transwomen, and the period over which achievement of maximum effects could be expected (9,10,13). Most of the hormone-induced effects start within the first months of treatment. It is important to realize that transwomen might not achieve the desired breast size; one year of hormonal therapy in transwomen usually results in less than an AAA cup size (13). As a result, many transwomen choose for breast augmentation surgery (14). It is also important to note that for complete permanent removal of (facial) hair, additional laser skin treatments are required. Furthermore, hormonal therapy does not induce voice changes. Therefore, transwomen who desire raising of their voice pitch may benefit from referral to a speech therapist (10).
Figure 1. Hormone Effects and the Term of Maximum Expected Effects in Transwomen
TRANSMEN
In transmen, hormonal therapy induces masculinization such as an increase in facial and body hair, an increase in muscle mass and strength, a masculinized voice, and a cessation of the menstruation. Figure 2 shows the expected effects of hormonal therapy in transmen, and the term that maximum effects could be expected (10). Most of the hormone-induced effects start within the first months of treatment. In most cases the menses cease during testosterone therapy. However, some transmen who experience persistent vaginal bleeding need additional therapy such as the progestin lynesterol or GnRH analogues, which suppress gonadotropin secretion.
Figure 2. Hormone Effects and Term of Maximum Expected Effects in Transmen
Hormonal Effects on Bone Health
Before puberty, the size and volume of the skeleton are similar in the two sexes. But upon the rise of androgens during puberty, a higher peak bone mass is attained in boys than in girls. Bone mass accrual, bone growth and maintenance of skeletal integrity in adulthood are critically determined by sex hormone production. In both sexes, hypogonadism leads to loss of bone. In men a significant role of estrogens in bone metabolism has been demonstrated. It is of note that testosterone is partially aromatized to estradiol, and it is well established that estradiol also plays a pivotal role in the bone health of men (15,16). These mechanisms underscore that hormonal therapy in transgender people will affect bone metabolism.
TRANSWOMEN
Unexpectedly, transwomenhave a lower bone mineral density than controls before starting with hormonal therapy. This might be explained by a less active lifestyle and/or a lower vitamin D status (17,18). As remarked above, in both sexes’ estrogens are important in the maintenance of bone health in adulthood. Initial studies examining the effect of long‐term hormonal therapy on bone mineral density showed contradictory results. However, most of these studies were small cross-sectional studies in which a baseline difference in bone mineral density was not taken into account (19–21). The most recent cohort study in transwomen showed that hormonal therapy does not have negative effects on bone mineral density, and that the lower bone mineral density in transwomen found in previous studies is solely based on a baseline lower bone mineral density (18). Therefore, it would be worthwhile to give lifestyle advice regarding physical exercise, adequate vitamin D status, and calcium intake to transwomen.
In postmenopausal women, bone mineral density depends on estrogens derived from aromatization of ovarian androgen production (22). Many transwomen have undergone orchiectomy in the process of their transition. Their status could probably be compared with a surgically-induced menopause in a ciswoman. Women with a surgically-induced menopause experience rapid bone loss during the first five years after oophorectomy. Based on this information, it has become clear that complete discontinuation of hormonal therapy in transwomen above the age of 50 leads to a profound loss of bone strength. Therefore, it is advisable to not discontinue estrogen therapy in older transwomen (23).
TRANSMEN
In contrast to transwomen, transmen show no lower bone mineral density before starting hormonal therapy (24,25). Furthermore, no negative effects of testosterone therapy on bone mineral density have been found (18,20,24).
Hormonal Effects on Cardiovascular Health
Cardiovascular disease is a prominent cause of morbidity and mortality in both women and men. Sex is known to affect one’s risk for cardiovascular disease. Men have a higher (age-adjusted) risk of strokes and acute coronary events than women (26–28). Strokes are 33% more incident in men than in women (28), and acute coronary events 172% (29). In addition, during reproductive age, women have a 100% higher risk of venous thromboembolic events than men (30,31). These data suggest that sex hormones play a role in the occurrence of cardiovascular events. Based on this information, it is surprising that recent studies found that estrogen therapy (without progestogen) increases the risk for developing strokes in menopausal women (32). There is also evidence that suggests a relationship between testosterone therapy and an increased risk of cardiovascular events (33–35). If exogenous sex hormones indeed have impact on the cardiovascular system, this might have consequences for transgender people receiving hormonal therapy. At the Amsterdam University Medical Center, the Netherlands, we have analyzed the development of cardiovascular disease in the population of the Gender Clinic. The Gender Clinic started in 1972 and there is now a large cohort of transgender people being followed-up including a growing number of older transgender people. The findings are summarized below.
TRANSWOMEN
Upon analysis of our transgender population in 1989 and in 1997 (36,37), cardiovascular disease, other than venous thromboembolism, was not increased in transwomen compared to cismen. However, the most recent evidence from 2011 and 2018 shows that transwomen receiving hormonal therapy have an increased risk for strokes and venous thromboembolism (but not acute coronary events) compared to cismen (38,39). The current estimated incidence rate for strokes in transwomen on hormonal therapy is 127 per 100,000 person-years, which is 80% higher than in cismen. The current estimated incidence rate for venous thromboembolic events is 320 per 100,000 person-years, which is 355% higher than in cismen (38). While the increased cardiovascular risk in transwomen was initially attributed to the usage of ethinylestradiol, recent studies found that transwomen who use other types of estrogens also have an increased risk for strokes and venous thromboembolism (38–40). The hypercoagulable effect of hormonal therapy (41)may be one of the mediators of the increased cardiovascular risk in transwomen.
Possibly, venous and arterial cardiovascular side-effects become more prominent past the age of 40-50 years, and in people with cardiovascular risk factors. While strong evidence is currently lacking, transdermal estradiol might be preferred over oral estrogens in these transwomen (9,42,43). In addition, one should be aware that progestogenic antiandrogens (e.g. cyproterone acetate) may further increase one’s risk for venous thromboembolism (44), and should therefore be continued no longer than necessary. Modifiable cardiovascular risk factors such as lipid concentrations, glucose concentrations, and blood pressure should be regularly monitored and treated in accordance with guidelines for ciswomen.
TRANSMEN
As in transwomen, first analyses from our center did not show an increased risk of cardiovascular disease in transmen using testosterone (36,37,45). However, the most recent analysis shows an increased risk for acute coronary events in transmen receiving testosterone, with a current estimated incidence rate of 100 per 100,000 person-years, which is 269% higher than the rate in ciswomen (38). The increased risk of acute coronary events in transmen receiving testosterone may be (partly) explained by the testosterone-induced combination of increases in hematocrit, thromboxane, triglycerides, and low-density lipoprotein cholesterol, and a decrease in plasma high-density lipoprotein cholesterol concentrations (35,46,47). Although, the design of the study made it impossible to draw any conclusions about a causal relationship we recommend to regularly monitor cardiovascular risk factors in transmen on testosterone therapy.
Hormonal Effects on Tumor Risk
Malignant neoplasms are the second leading cause of death worldwide (48). The risk for certain types of tumors differs between men and women. While this is obvious for neoplasms that develop in sex-specific organs such as the ovaries or the prostate, it is also the case for other types of tumors such as those of the meninges (49)and thyroid gland (50). Some of these differences are attributed to the exposure of sex hormones. Combined hormonal therapy in postmenopausal women has been found to increase the risk of breast cancer and death from lung cancer (51). In women with polycystic ovary syndrome a higher risk for endometrial cancer has been described, which is probably explained by the prolonged endometrial exposure to unopposed estrogen that results from anovulation (52). Testosterone therapy in hypogonadal men is not clearly associated with an increased cancer risk, but breast cancer risk in prostate cancer patients who receive estrogen therapy seems 3.91 times higher than in prostate cancer patients not receiving estrogen therapy (53). If exogenous sex hormones indeed are able to induce cell proliferation, this might have consequences for transgender people receiving hormonal therapy. It is good to keep in mind that transwomen and transmen remain susceptible to cancers of reproductive organs that are no longer in alignment with their gender. For example, postsurgical transwomen, and attending physicians, might not recognize their persisting risk of prostate cancer (the prostate is not removed during vaginoplasty). In addition, transmen who have not undergone removal of the uterus still have risk for cervical cancer. It is also important to realize that transgender people may opt out of cancer screening and examinations because of emotional or physical distress associated with the discordance between their experienced gender and their birth assigned sex.
To date, large-scale studies investigating neoplasms in transgender people are scarce and the literature mainly consists of case reports. One of the first reviews was presented in 2008 (54), and an extensive, high quality review appeared in 2017 (55). A cautious comparison of the two reports helps us to provide insight into the neoplasm-related morbidity and mortality in transgender people.
TRANSWOMEN
Breast Cancer
Estrogen in combination with antiandrogen therapy in transwomen stimulate the development of breast lobules, ducts, and acini which are histologically identical to those of ciswomen (56). While for a long time it was believed that the risk of breast cancer in transwomen receiving hormonal therapy was not higher than those of men (57,58), most recent evidence show that transwomen receiving hormonal therapy do have a 46-fold higher risk for breast cancer compared to men (59). As became clear in the Women’s Health Initiative study, addition of progestin to estrogen leads to an increase of the risk of breast cancer in women (60). Although evidence regarding breast cancer and the usage of the progestogenic cyproterone acetate is lacking, the above described data suggest that cyproterone acetate should be continued no longer than necessary. In addition, based on the most recent study that shows a much higher risk of breast cancer in transwomen compared to men, it is reasonable to recommend transwomen on hormonal therapy to participate in population-based breast cancer screening programs (9).
Prostate Cancer
While in the past, estrogens have been used to treat prostate cancer, estrogen and its related compounds have also been suggested as potential causative agents (61). Current literature, suggests that prostate cancer is very rare among transwomen. The few cases that have been reported in transwomen were in those who had not been screened for prostate cancer before starting hormonal therapy. Consequently, it remained unclear whether the prostate cancer was already present before hormonal therapy had been initiated (62). While prostate cancer has been rarely reported, underdiagnosis is possible due to lack of close prostate monitoring. Based on available evidence it does not seem necessary to screen transwomen in a different way to cismen, for which population-based screening is not recommended. But similarly, a transwoman with a first-degree male relative with prostate cancer should be made aware of her increased risk and prostate cancer [PSA] testing should be discussed to allow informed decision making. However, when interpreting PSA values in this context, it has to be kept in mind that suppression of testosterone by antiandrogens or due to gonadectomy lowers PSA values. A cross-sectional study of Wierckx et al. (63)found median PSA levels of 0.003 ng/mL with an interquartile range of 0.03 to 0.09 in a group of 50 postoperative transwomen using hormonal therapy on an average of 10 years. Therefore a serum level of PSA >1.0 ng/mL may already be a reason for suspicion in transwomen (64).
Prolactinoma
Serum prolactin concentrations usually rise slightly in response to estrogen administration and more so by cyproterone acetate (65,66). Based on case reports, it was initially believed that prolactin concentrations in transwomen had to be regularly monitored because of their increased risk for prolactinomas. Surprisingly, a very recent cohort study suggests that the occurrence of prolactinomas in transwomen using hormonal therapy is not higher than that in ciswomen, and that regular prolactin checks are not necessary (67). However, cyproterone acetate should be continued no longer than necessary.
Meningioma
Several meningiomas have been reported in transwomen. The current estimated incidence rate of this type of tumor is 33 per 100,000 person-years. This incidence rate is 4 times higher than the incidence rate in ciswomen and 12 times higher than the incidence rate in cismen (67,68). It has been suggested that the occurrence of meningiomas in transwomen is mainly related to cyproterone acetate usage as progesterone receptors are abundantly expressed in human meningiomas (67). Since the occurrence of meningiomas is still rare in transwomen, regular screening for this type of tumor seems not necessary. It is recommended to continue cyproterone acetate no longer than necessary.
Other Types of Cancer
As sexually transmitted infections may be more prevalent in transwomen, tumors related to sexually transmitted infections, such as Kaposi sarcoma or anal cancer, may also occur more often. Indeed, disproportionately high incidences of these types of tumors have been found in the transgender population (55,69). Some case reports have been published on cancer in surgically constructed organs like the neo-vagina in transwomen (70,71). While the incidence of these types of tumors seem to be very low it is important to be aware of this possibility.
TRANSMEN
Breast Cancer
Cases of breast cancer have been reported in transmen before mastectomy (59,72,73). It is important to know that because of cosmetic reasons not all glandular tissue is removed during a mastectomy in transmen. Indeed, several cases of breast cancer have been reported in transmen who already had received mastectomy (59,73–75). The incidence of breast cancer in transmen who have received mastectomy seems higher than in cismen, but much lower than in ciswomen (59). While physicians and transmen have to be aware of their risk of breast carcinoma after mastectomy, it seems unnecessary for transmen to participate in the screening programs for women. However, for transmen with a genetic predisposition for breast cancer, more radical forms of mastectomy could be considered.
Endometrial Cancer
Not all transmen choose to remove their uterus. Menstruation usually ceases in transmen receiving testosterone therapy. Testosterone can be converted into estradiol, which may induce proliferation of the endometrium. These mechanisms may induce a higher risk of endometrial cancer in transmen. Women with polycystic ovary syndrome who do not menstruate and suffer from hyperestrogenism, have a thicker endometrium and a higher risk of endometrial cancer (76). It is also possible that the risk for endometrial cancer in transmen using testosterone is lower due to complete atrophy of the endometrium (55). There is currently only 1 case of endometrial cancer reported in a transman using testosterone (77). But it is important to know that, until recently, many countries required removal of female sex organs before transmen could change their sex on the birth certificate. Therefore, long-term follow-up data about testosterone receiving transmen with a uterus are lacking. This makes it impossible to draw hard conclusions. Nevertheless, in transmen with non-cyclic vaginal blood loss, we recommend to perform a vaginal ultrasound.
Cervical Cancer
Transmen in whom the uterus has not been removed have a risk of cervical carcinoma. Human papilloma virus is the most important risk factor for developing cervix carcinoma. Studies in ciswomen show that testosterone may also be a risk factor (78). To date, only 2 cases of cervical carcinoma in transmen have been described (77,79). Again, it is important to keep in mind that, until recently, many countries required removal of female sex organs before a transman could change the sex on the birth certificate, which makes the data available limited. As there is no evidence for a decreased risk of cervical carcinoma in transmen, it seems reasonable for transmen with a uterus to participate in screening/HPV vaccination programs for ciswomen. It is important to inform transmen about the need for this screening as they probably do not receive invitations from screening organizations.
Ovarian Cancer
Endometrial epidermal growth factor receptor, which is stimulated by testosterone, is commonly found in ovarian cancer cells, and its expression has been associated with poor prognosis (80). However, whether testosterone therapy increases the risk for ovarian cancer in transmen has not been elucidated yet. To date, 3 cases of ovarian cancer have been reported in transmen using testosterone (81,82). Future studies need to provide more evidence about the risk of gynecological cancers in transmen. Until then, screening for ovarian cancer seems unnecessary.
EVALUATION OF TRANSGENDER PEOPLE RECEIVING HORMONAL THERAPY
Since hormonal therapy is associated with several side-effects it is recommended that medical conditions which can be exacerbated by hormonal therapy are addressed before the start of therapy. During hormonal therapy it is advisable to regularly measure hormone concentrations and maintain them in the normal physiological range. For transwomen estradiol levels between 100 to 200 pg/mL (367 pmol/L to 734 pmol/L) and testosterone levels of <50 ng/dL (<2 nmol/L) are recommended. For transmen, the testosterone level is dependent on the specific assay, but is typically 320 to 1000 ng/dL (11 nmol/L to 35 nmol/L) (10). However, the peak testosterone level after a short acting testosterone injection is often (much) higher than 1200 ng/dL (42 nmol/L). It is also recommended to regularly measure glucose concentrations, lipid panel, and blood pressure during hormonal therapy in both transwomen and transmen, hematocrit in transmen, and electrolytes in transwomen receiving spironolactone (in the first year at baseline and 3 and 12 months, hereafter every 6 months to 2 years).
SURGERY
Many transgender people choose to have surgery in addition to hormonal therapy. There are several surgical options. While some types of surgery affect fertility, such as vaginoplasty in transwomen or oophorectomy/hysterectomy in transmen, others do not, such as breast surgery in both transwomen and transmen. For surgery which affects fertility, most guidelines recommend the usage of gender-affirming hormones for at least 12 months, which is based on expert consensus that 12 continuous months of living in the experienced gender role is needed for transgender individuals to experience and socially adjust in the desired gender role (5,10).
Surgical Options in Transwomen
ORCHIECTOMY
Orchiectomy can be performed independently or as part of a vaginoplasty. Orchiectomy is a relatively low-risk procedure (83). After orchiectomy the antiandrogens are no longer necessary and can discontinued.
VAGINOPLASTY
During a vaginoplasty an orchiectomy (if not previously performed) is performed, in combination with an amputation of the penis, the creation of a neovaginal cavity with lining, the reconstruction of a urethral meatus, and the creation of labia and clitoris. The most frequent procedure is penile inversion vaginoplasty during which the penile skin is used a pedicled flap for the vaginal lining. Since the amount of penile skin is limited, the penile skin flap is often combined with a scrotal skin flap. To prevent hair in the posterior lining of the vagina, hair removal therapy is desirable before penile inversion vaginoplasty. An alternative for penile inversion vaginoplasty is intestinal vaginoplasty, which is a good option in cases in which insufficient skin is available (for example in transwomen who have received puberty blockers during adolescence (83)).
BREAST AUGMENTATION
Many transwomen choose for breast augmentation since they are not satisfied with their hormone-induced breast growth. The breast augmentation procedure does not differ from an breast augmentation in ciswomen (83).
FACIAL FEMINIZATION SURGERY
Facial feminization surgery includes a wide range of craniomaxillofacial surgical procedures which are designed to create more feminine facial features. Overall, facial feminization surgery seems a highly efficacious and beneficial procedure for transwomen (84).
VOCAL CORD SURGERY
Surgically shortening of the vibrating vocal cords or increasing the vocal cord tension can raise the voice pitch in cases that voice therapy does not achieve the desired effect (83).
CHONDROLARYNGOPLASTY
During chondrolaryngoplasty (tracheal shaving) the prominence of the thyroid cartilage is reduced. Chondroplasty can be performed alone or in combination with vocal cord surgery. Reduction of the Adam’s apple can have positive effects on the psychological well-being of transwomen (85).
Surgical Options in Transmen
SUBCUTANEOUS MASTECTOMY
Most transmen choose mastectomy. A mastectomy in transmen which is performed for aesthetic reasons differs from a mastectomy in ciswomen which is performed because of breast cancer. During the mastectomy in transmen not all glandular tissue is removed. In addition, (more) attention has to be paid to reduction and adequate positioning of the nipple areola complex, destruction of the inframammary fold, and minimization of scars (83).
HYSTERECTOMY
Many transmen desire uterus extirpation with or without a salpingo-oophorectomy for gender affirmation, pelvic pain, persistent vaginal blood loss, or cancer-risk reduction. It is preferred to use a vaginal approach instead of a transabdominal approach although this could be technically challenging as many transmen have not experienced penetrative intercourse and are on testosterone therapy (86). Transmen who also want a colpectomy can also choose to have a robot-assisted laparoscopic hysterectomy (with salpingo-oophorectomy) in combination with a robot-assisted laparoscopic colpectomy (87).
COLPECTOMY
Transmen may choose for a colpectomy (removal of the vaginal epithelium) for several reasons, such as unwanted vaginal discharge in general or as result of sexual arousal, or the wish for phalloplasty with urethral extension. There are two options for colpectomy, the vaginal approach and the robot-assisted laparoscopic colpectomy in combination with hysterectomy and salpingo-oophorectomy. The robot-assisted procedure seems to be safer than the vaginal approach (87).
PHALLOPLASTY
Phalloplasty is the surgical creation of a full-size penis. It is a difficult surgical procedure with high rates of complications such as urethral stenosis. An ideal phallus has sufficient length for vaginal penetration, has sensibility, and, if desired, enables the transman to urinate in standing position. Multiple flaps have been used to create the phallus, but for penile reconstruction the free radial forearm flap remains the gold standard (83,88).
METADOIDIOPLASTY
During a metoidioplasty a microphallus is created by using the testosterone-induced hypertrophied clitoris. While a metadoidioplasty gives a lower risk for complications than a phalloplasty, it cannot be guaranteed that voiding in the standing position is possible. In addition, vaginal penetration will not be possible (83,88).
FERTILITY PRESERVATION
It is estimated that about 47% of transgender individuals would like to have a child to whom they are genetically related (89). Gender affirmation therapy, both hormonal and surgical, is an indication for fertility preservation since hormonal therapy adversely affects fertility, and surgery may include gonadal removal. While the adverse effects of hormonal therapy may be reversible when the therapy is ceased, it is important to discuss fertility preservation options with a transgender individual before the start of hormonal therapy (90). In transwomen the percentage that would have frozen sperm if this option was offered, varied from 13% in asexual or heterosexual (being attracted to men) transwomen to 56% in homosexual (being attracted to women) and bisexual transwomen (91). It is estimated that about 37% (92)of the transmen wish to have their gametes preserved before any gender affirming therapy. For transgender adolescents it is important to involve parents in the fertility preservation counseling as they play an important role in exploring options for their children and usually have to give their consent to interventions. A recent study found that parents overall did not emphasize the importance of their child having children to whom they are genetically related but they agreed that that fertility preservation counseling is relevant (93).
Transwomen
Semen cryopreservation using specimens obtained from masturbation or penile vibratory stimulation is technically the most easy, reliable and inexpensive method for fertility preservation in transwomen. However, for some transwomen this option may not be possible because of the psychologically distress induced by this procedure, or the difficulties in erection and ejaculation. Alternatives are electro-stimulation or surgical sperm retrieval, or in case of azoospermia, testicular sperm extraction (90). The obtained sperm can be used to fertilize the partner of the transwoman if this partner is female. In case of a male partner a gestational surrogate is needed for fertilization.
Transmen
For transmen fertility options are embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation. As long as the ovaries and uterus are in situ, it is also possible for a transgender man to become pregnant spontaneously. Since testosterone therapy may be dangerous for fetal development it is important that testosterone therapy be discontinued before the transman becomes pregnant. In contrast to the other options, ovarian tissue cryopreservation is more experimental and not widely available. For embryo creation or to fertilize a preserved oocyte, sperm from an intimate partner or an anonymous donor, is needed. When a transgender man does not want to carry the child, a gestational surrogate is also needed. However, surrogacy for transgender individuals is still not widely available due to ethical and legal issues. Furthermore it is important to note that all fertility options in transmen are sooner or later accompanied with controlled ovarian hyperstimulation, which could be very distressing for a transman (90).
SPECIAL TOPICS
Hormonal Therapy in Older Transgender People
Some transgender people start the transition to their experienced gender at an older age (often after a long time of struggling), even past the age of 50 or 60 years (23,94). The majority of these people are currently transwomen (94), but the number of transgender individuals, and especially transmen is currently rapidly growing, possibly due to a greater tolerance and acceptability in society (2). There is no evidence that the manifestations of biological effects of sex hormones will be less in the elderly than in younger people (13,95,96). Age itself should not be regarded as a contraindication to start with hormonal therapy, but the risks of side-effects may be higher at an older age (97).
Unsupervised Use of Hormonal Therapy
Ideally, the indication for hormonal therapy is the result of psychological assessment that concludes that medical treatment will bring relief to an individual suffering from gender dysphoria (98). However, it is not uncommon that transgender people self-medicate. The use of health care facilities specialized in gender care may be unaffordable, difficult to assess due to long waiting lists, or people are unwilling to undergo psychodiagnostic assessment of their gender problems. Hormones are relatively easy to obtain, and peer groups and the internet provide (sometimes misguided) information on their use (99–101). Frequently, contraceptive pills containing ethinylestradiol with its associated risks (45,102)are used in high doses. Our knowledge about self-medication in the transgender population is very limited, but a study has indicated increased side-effects with illicit use of hormones (101). Another study found 23% of applicants for treatment had used sex hormones already. Remarkably, 32% were transwomen and 6% transmen. The individuals who had used self-prescribed hormones had much less knowledge about appropriate use and potential side-effects (103). Physicians should be aware of illicit hormone use and intervene when necessary.
CONCLUSIONS
Transgender care is a challenging, multidisciplinary, and developing field in medicine. The transgender population is rapidly growing and the existence of non-binary or gender queer genders gets increasingly more attention.Before the start of any type of therapy, the physician needs to discuss the pros and cons of the several treatment options so that the transgender individual can make a well-considered decision. Due to the increase of high-quality studies, hormonal and surgical therapy will probably be further optimized in the (near) future. Therefore, it is important for physicians who provide transgender care to stay up-to-date with the latest literature. In addition, as the waiting lists may be growing due to the rapidly the increasing number of new applications to gender clinics, physicians should be aware of a growing number of transgender individuals who use self-prescribed hormones.
REFERENCES
- Koehler A, Eyssel J, Nieder TO.Genders and Individual Treatment Progress in (Non-)Binary Trans Individuals. J. Sex. Med.2018;15(1):102–113.
- Wiepjes CM, Nota NM, Blok CJM De, Klaver M, Vries ALC De, Wensing-kruger SA, Jongh RT De, Bouman M, Steensma TD, Cohen-kettenis P, Gooren LJG, Kreukels BPC, den Heijer M.The Amsterdam Cohort of Gender Dysphoria Study ( 1972 e 2015 ): Trends in Prevalence , Treatment , and Regrets. J. Sex. Med.2018;15:582–590.
- Polderman TJC, Kreukels BPC, Irwig MS, Beach L, Chan YM.The Biological Contributions to Gender Identity and Gender Diversity : Bringing Data to the Table. Behav. Genet.2018;48(2):95–108.
- Swaab DF.Sexual differentiation of the brain and behavior. Best Pract. Res. Clin. Endocrinol. Metab.2007;21(3):431–444.
- Coleman E, Bockting W, Botzer M, Cohen-Kettenis PT, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ, Monstrey S, Adler RK, Brown GR, Zucker K.Standards of Care, for the Health of Transsexual, Transgender, and Gender Nonconforming People. Int. J. Tansgenderism2012;13:165–232.
- Van De Grift TC, Elaut E, Cerwenka SC, Cohen-Kettenis PT, De Cuypere G, Richter-Appelt H, Kreukels BPC.Effects of Medical Interventions on Gender Dysphoria and Body Image: A Follow-Up Study.Psychosom. Med.2017;79:815–823.
- Bradford J, Reisner SL, Honnold JA, Xavier J.Experiences of Transgender-Related Discrimination and Implications for Health : Results From the Virginia Transgender Health Initiative Study. Res. Pract.2013;103(10):1820–1829.
- Budge SL, Adelson JL, Howard KAS.Anxiety and Depression in Transgender Individuals: The Roles of Transition Status , Loss , Social Support, and Coping. J. Consult. Clin. Psychol.2013;81(3):545–557.
- Tangpricha V, den Heijer M.Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol.2017;5:291–300.
- Hembree WC, Cohen-kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen GG.Endocrine Treatment of Gender-Dysphoric/ Gender-Incongruent Persons: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab.2017;102(11):1–35.
- Prior JC.Progesterone is important for transgender women’s therapy-applying evidence for the benefits of progesterone in ciswomen. J. Clin. Endocrinol. Metab.2019;104:1181–1186.
- Ohlander SJ, Varghese B, Pastuszak AW.Erythrocytosis Following Testosterone Therapy. Sex. Med. Rev.2018;6:77–85.
- De Blok CJM, Klaver M, Wiepjes CM, Nota NM, Heijboer AC, Fisher AD, Schreiner T, T’Sjoen G, Den Heijer M.Breast development in transwomen after 1 year of cross-sex hormone therapy: Results of a prospective multicenter study. J. Clin. Endocrinol. Metab.2018;103:532–538.
- Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barrett J.Predictive Markers for Mammoplasty and a Comparison of Side Effect Profiles in Transwomen Taking Various Hormonal Regimens. J Clin Endocrinol Metab2012;97:4422–4428.
- Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D.Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol.2010;207:127–34.
- Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ, Murad MH.Effect of sex steroids on the bone health of transgender individuals: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab.2017;102:3904–3913.
- Van Caenegem E, Taes Y, Wierckx K, Vandewalle S, Toye K, Kaufman JM, Schreiner T, Haraldsen I, T’Sjoen G.Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone2013;54:92–97.
- Wiepjes CM, de Jongh RT, de Blok CJ, Vlot MC, Lips P, Twisk JW, den Heijer M.Bone Safety During the First Ten Years of Gender-Affirming Hormonal Treatment in Transwomen and Transmen. J. Bone Miner. Res.2018:doi: 10.1002/jbmr.3612. [Epub ahead of print].
- Lapauw B, Taes Y, Simoens S, Van Caenegem E, Weyers S, Goemaere S, Toye K, Kaufman JM, T’Sjoen GG.Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone2008;43:1016–1021.
- Ruetsche AG, Kneubuehl R, Birkhaeuser MH, Lippuner K.Cortical and trabecular bone mineral density in transsexuals after long-term cross-sex hormonal treatment: A cross-sectional study. Osteoporos. Int.2005;16:791–798.
- Sosa M, Jódar E, Arbelo E, Domínguez C, Saavedra P, Torres A, Salido E, de Tejada MJG, Hernández D.Bone Mass, Bone Turnover, Vitamin D, and Estrogen Receptor Gene Polymorphisms in Male to Female Transsexuals. J. Clin. Densitom.2003;6:297–304.
- Kuchuk NO, Van Schoor NM, Pluijm SMF, Smit JH, De Ronde W, Lips P.The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin. Endocrinol. (Oxf).2007;67:295–303.
- Fabbre VD.Gender Transitions in Later Life: The Significance of Time in Queer Aging. J. Gerontol. Soc. Work2014;57:161–175.
- Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van De Peer F, Toye K, Kaufman JM, T’Sjoen G.Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J. Clin. Endocrinol. Metab.2012;97:2503–2511.
- Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S.Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm. Behav.2007;52:334–343.
- Petrea RE, Beiser AS, Seshadri S, Kelly-hayes M, Kase CS, Wolf PA.Gender Differences in Stroke Incidence and Poststroke Disability in the Framingham Heart Study. Stroke2009;40:1032–1037.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler IER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB.Heart Disease and Stroke Statistics - 2014 Update: A report from the American Heart Association. Circulation2014;129:e28–e292.
- Appelros P, Stegmayr B, Terént A.Sex Differences in Stroke Epidemiology. Stroke2009;40:1082–1090.
- Albrektsen G, Heuch I, Løchen ML, Thelle DS, Wilsgaard T, Njølstad I, Bønaa KH.Lifelong gender gap in risk of incident myocardial infarction: The Tromsø study. JAMA Intern. Med.2016;176:1673–1679.
- Bleker SM, Coppens M, Middeldorp S.Blood Reviews Sex , thrombosis and inherited thrombophilia. Blood Rev.2014;28(3):123–133.
- Roach REJ, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S.Sex Difference in Risk of Second but Not of First Venous Thrombosis. Circulation2014;129:51–56.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J.Long-termhormone therapy for perimenopausal and postmenopausalwomen. Cochrane Database Syst Rev.2017;1:CD004143. doi:10.1002/14651858.CD004143.pub5.www.cochranelibrary.com.
- Onasanya O, Iyer G, Lucas E, Lin D, Singh S, Alexander GC.Association between exogenous testosterone and cardiovascular events: an overview of systematic reviews. LANCET Diabetes Endocrinol.2016;8587(16):943–956.
- Martinez C, Suissa S, Rietbrock S, Katholing A, Freedman B, Cohen AT, Handelsman DJ.Testosterone treatment and risk of venous thromboembolism : population based case-control study. BMJ2016;355(June):i.5968.
- Xu L, Freeman G, Cowling BJ, Schooling CM.Testosterone therapy and cardiovascular events among men : a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med.2013;11:1–12.
- Asscheman H, Gooren LJ, Eklund PL.Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism.1989;38:869–873.
- van Kesteren PJM, Asscheman H, Megens JAJ, Gooren LJG.Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin. Endocrinol. (Oxf).1997;47:337–342.
- Nota NM, Wiepjes CM, de Blok CJ, Gooren LJ, Kreukels BP, den Heijer M.The occurrence of acute cardiovascular events in transgender individuals receiving hormone therapy: results from a large cohort study. Circulation2019:doi: 10.1161/CIRCULATIONAHA.118.038584.
- Getahun D, Nash R, Flanders WD, Baird TC, Becerra-culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M.Cross-sex Hormones and Acute Cardiovascular Events in Transgender Persons. Ann. Intern. Med.2018;169:205–213.
- Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T’Sjoen G.Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case – control study. Eur. J. Endocrinol.2013;169:471–478.
- Selier N, Cannegieter S, Venemans-Jellema A, den Heijer M.Moderate cross-sex hormone-induced prothrombotic changes of hemostatic variables in transgender subjects. In: Endocrine Abstracts (2016) 41 EP207 | DOI: 10.1530/endoabs.41.EP207.
- Canonico M, Plu-Bureau G, Lowe GDO, Scarabin PY.Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: Systematic review and meta-analysis. BMJ2008;336:1227–1231.
- Renoux C, Dell’Aniello S, Garbe E, Suissa S.Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ2010;341:83.
- Dragoman M V., Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME.A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int. J. Gynecol. Obstet.2018;141:287–294.
- Asscheman H, Giltay EJ, Megens JAJ, Ronde WP De, Trotsenburg MAA Van.A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur. J. Endocrinol.2011;164:635–642.
- Van Velzen DM, Paldino A, Klaver M, Nota NM, Defreyne J, Kees Hovingh G, Thijs A, Simsek S, Sjoen GT, Den Heijer M.Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J. Clin. Endocrinol. Metab.2019;104:1937–1947.
- Maraka S, Singh Ospina N, Rodriguez-gutierrez R, Davidge-pitts CJ, Nippoldt TB, Prokop LJ, Murad MH.Sex Steroids and Cardiovascular Outcomes in Transgender Individuals: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab.2017;102:3914–3923.
- Collaborators. G 2015 M and C of D.Global , regional , and national life expectancy , all-cause mortality , and cause-specifi c mortality for 249 causes of death , 1980 – 2015 : a systematic analysis for the Global Burden of Disease Study 2015. Lancet2016;388:1459–1544.
- Blankenstein MA, Verheijen M, Jacobs JM, Donker TH, Duijnhoven MWF Van, Thijssen JHH.Occurrence , regulation , and significance of progesterone receptors in human meningioma. Steroids2000;65:795–800.
- Rahbari R, Zhang L, Kebebew E.Thyroid cancer gender disparity. Futur. Oncol.2010;6:1771–1779.
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J.Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev.2017:DOI: 10.1002/14651858.CD004143.pub5.
- Dumesic DA, Lobo RA.Cancer risk and PCOS. Steroids2013;78:782–785.
- Karlsson CT, Malmer B, Wiklund F, Grönberg H.Breast Cancer as a Second Primary in Patients With Prostate Cancer — Estrogen Treatment or Association With Family History of Cancer ? J. Urol.2006;176(August):538–543.
- Mueller A, Gooren L.Hormone-related tumors in transsexuals receiving treatment with cross-sex hormones. Eur. J. Endocrinol.2008;159(3):197–202.
- Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M.Cancer in transgender people: Evidence and methodological considerations. Epidemiol. Rev.2017;39:93–107.
- Maglione K, Margolies L, Jaffer S, Szabo J, Schmidt H, Weltz C, Sonnenblick E.Breast cancer in male-to-female transsexuals: use of breast imaging for detection. Am. J. Roentgenol.2014;47:W735–W740.
- Gooren LJ, van Trotsenburg MAA, Giltay EJ, van Diest PJ.Breast Cancer Development in Transsexual Subjects Receiving Cross-Sex Hormone Treatment. J. Sex. Med.2013;10:3129–3134.
- Brown GR, Jones KT.Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res. Treat.2015;149:191–198.
- de Blok CJ, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KM, Barbé E, Konings IR, den Heijer M.Breast cancer risk in transgender people receiving hormone treatment: nationwide cohort study in the Netherlands. BMJ2019:BMJ 2019;365:l1652.
- Chlebowski RT, Aragaki AK, Anderson GL.Menopausal hormone therapy influence on breast cancer outcomes in the women’s health initiative. JNCCN J. Natl. Compr. Cancer Netw.2015;13:917–924.
- Nelles JL, Hu WY, Prins GS.Estrogen action and prostate cancer. Expert Rev. Endocrinol. Metab.2011;6:437–451.
- Deebel NA, Morin JP, Autorino R, Vince R, Grob B, Hampton LJ.Prostate Cancer in Transgender Women: Incidence, Etiopathogenesis, and Management Challenges. Urology2017;110:166–171.
- Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T’Sjoen G.Long-term evaluation of cross-sex hormone treatment in transsexual persons. J. Sex. Med.2012;9(10):2641–51.
- Gooren L, Morgentaler A.Prostate cancer incidence in orchidectomised male-to-female transsexual persons treated with oestrogens. Andrologia2014;46:1156–1160.
- Defreyne J, Nota N, Pereira C, Schreiner T, Fisher AD, den Heijer M TG.Transient Elevated Serum Prolactin in Trans Women Is Caused by Cyproterone Acetate Treatment. LGBT Heal.2017;4(5):328–336.
- Nota N, Dekker MJHJ, Wiepjes MKCM, Van Trotsenburg M, Heijboer MC, Den Heijer M.Prolactin levels during short- and long- term cross- sex hormone treatment : an observational study in transgender persons. Andrologia2017;49:Epub 2016 Aug 25.
- Nota NM, Wiepjes CM, De Blok CJM, Gooren LJG, Peerdeman SM, Kreukels BPC, Den Heijer M.The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment. Brain2018;141:2047–2054.
- Ter Wengel P V, Martin E, Gooren L, den Heijer M, Peerdeman SM.Meningiomas in three male-to-female transgender subjects using oestrogens / progestogens and review of the literature. Andrologia2016;48(48):1130–37.
- Hutchison LM, Boscoe FP, Feingold BJ.Cancers disproportionately affecting the New York state transgender population, 1979-2016. Am. J. Public Health2018;108:1260–1262.
- Bollo J, Balla A, Rodriguez Luppi C, Martinez C, Quaresima S, Targarona EM.HPV-related squamous cell carcinoma in a neovagina after male-to-female gender confirmation surgery. Int. J. STD AIDS2018;29:306–308.
- Fernandes HM, Manolitsas TP, Jobling TW.Carcinoma of the neovagina after male-to-female reassignment. J. Low. Genit. Tract Dis.2014;18:E43-5.
- Shao T, Grossbard ML, Klein P.Breast cancer in female-to-male transsexuals: Two cases with a review of physiology and management. Clin. Breast Cancer2011;11:417–419.
- Stone JP, Hartley RL, Temple-Oberle C.Breast cancer in transgender patients: A systematic review. Part 2: Female to Male. Eur. J. Surg. Oncol.2018;44:1463–1468.
- Nikolic D V., Djordjevic ML, Granic M, Nikolic AT, Stanimirovic V V., Zdravkovic D, Jelic S.Importance of revealing a rare case of breast cancer in a female to male transsexual after bilateral mastectomy. World J. Surg. Oncol.2012;10:280.
- Burcombe RJ, Makris A, Pittam M, Finer N.Breast cancer after bilateral subcutaneous mastectomy in a female-to-male trans-sexual. Breast2003;12:290–293.
- Daniilidis A, Dinas K.Long term health consequences of polycystic ovarian syndrome : a review analysis. Hippokratia2009;13:90–92.
- Urban RR, Teng NNH, Kapp DS.Gynecologic malignancies in female-to-male transgender patients: The need of original gender surveillance. Am. J. Obstet. Gynecol.2011;204:e9–e12.
- Rinaldi S, Plummer M, Biessy C, Castellsagué X, Overvad K, Kjær SK, Tjønneland A, Clavel-Chapelon F, Chabbert-Buffet N, Mesrine S, Lukanova A, Kaaks R, Weikert C, Boeing H, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Agnoli C, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita B, Van Kranen HJ, Peeters PHM, Bakken K, Lund E, Gram IT, Rodríguez L, Xavier Bosch F, Sańchez MJ, Dorronsoro M, Navarro C, Gurrea AB, Kjellberg L, Dillner J, Manjer J, Butt S, Khaw KT, Wareham N, Allen NE, Travis R, Romieu I, Ferrari P, Riboli E, Franceschi S.Endogenous sex steroids and risk of cervical carcinoma: Results from the EPIC study. Cancer Epidemiol. Biomarkers Prev.2011;20:2532–2540.
- Driák D, Samudovský M.Could a man be affected with carcinoma of cervix?--The first case of cervical carcinoma in trans-sexual person (FtM)--case report. Acta medica (Hradec Kral.2005;48:53–5.
- Bartlett JMS, Langdon SP, Simpson BJB, Stewart M, Katsaros D, Sismondi P, Love S, Scott WN, Williams ARW, Lessells AM, Macleod KG, Smyth JF, Miller WR.The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br. J. Cancer1996;73:301–306.
- Hage JJ, Dekker JJML, Karim RB, Verheijen RHM, Bloemena E.Ovarian cancer in female-to-male transsexuals: Report of two cases. Gynecol. Oncol.2000;76:413–415.
- Dizon DS, Tejada-Berges T, Koelliker S, Steinhoff M, Granai CO.Ovarian cancer associated with testosterone supplementation in a female-to-male transsexual patient. Gynecol. Obstet. Invest.2006;62:226–228.
- Colebunders B, Brondeel S, D’Arpa S, Hoebeke P, Monstrey S.An Update on the Surgical Treatment for Transgender Patients. Sex. Med. Rev.2017;5:103–109.
- Morrison SD, Vyas KS, Motakef S, Gast KM, Chung MT, Rashidi V, Satterwhite T, Kuzon W, Cederna PS.Facial Feminization: Systematic Review of the Literature. Plast. Reconstr. Surg.2016;137:1759–1770.
- Cohen MB, Insalaco LF, Tonn CR, Spiegel JH.Patient Satisfaction after Aesthetic Chondrolaryngoplasty. Plast. Reconstr. Surg. - Glob. Open2018;6:e1877.
- Louie M, Moulder JK.Hysterectomy for the Transgender Man. Curr. Obstet. Gynecol. Rep.2017;6:126–132.
- Groenman F, Nikkels C, Huirne J, van Trotsenburg M, Trum H.Robot-assisted laparoscopic colpectomy in female-to-male transgender patients; technique and outcomes of a prospective cohort study. Surg. Endosc.2017;31:3363–3369.
- Djordjevic ML.Novel surgical techniques in female to male gender confirming surgery. Transl. Androl. Urol.2018;7:628–638.
- Tornello SL, Bos H.Parenting Intentions Among Transgender Individuals. LGBT Heal.2017;4:115–120.
- Mattawanon N, Spencer JB, Schirmer DA, Tangpricha V.Fertility preservation options in transgender people: A review. Rev. Endocr. Metab. Disord.2018;19:231–242.
- De Sutter P, Kira K, Verschoor A, Hotimsky A.The desire to have children and the preservation of fertility in transsexual women: A survey. Int. J. Transgenderism2002;6:1–12.
- Wierckx K, Van Caenegem E, Pennings G, Elaut E, Dedecker D, Van De Peer F, Weyers S, De Sutter P, T’Sjoen G.Reproductive wish in transsexual men. Hum. Reprod.2012;27:483–487.
- Walton-Betancourth S, Monti E, Adu-Gyamfi K, Roberts A, Clarkson K, Ward S, Butler G.Fertility preservation for transgender adolescents: The parent’s view. Endocr. Abstr. 58 P030 | DOI 10.1530/endoabs.58.P030. doi:10.1530/endoabs.58.p030.
- Bouman WP, Claes L, Marshall E, Pinner GT, Longworth J, Maddox V, Witcomb G, Jimenez-Murcia S, Fernandez-Aranda F, Arcelus J.Sociodemographic Variables, Clinical Features, and the Role of Preassessment Cross-Sex Hormones in Older Trans People. J. Sex. Med.2016;13:711–719.
- Bhattacharya RK, Khera M, Blick G, Kushner H, Miner MM.Testosterone replacement therapy among elderly males: The Testim Registry in the US (TRiUS). Clin. Interv. Aging2012;7:321–330.
- Klaver M, De Blok CJM, Wiepjes CM, Nota NM, Dekker MJHJ, De Mutsert R, Schreiner T, Fisher AD, T’Sjoen G, Den Heijer M.Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: Results from a multicenter prospective study.Eur. J. Endocrinol.2018;178:163–171.
- Streed CG, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M.Cardiovascular disease among transgender adults receiving hormone therapy: A narrative review. Ann. Intern. Med.2017;167:256–267.
- Cohen-Kettenis PT, Pfäfflin F.The DSM diagnostic criteria for gender identity disorder in adolescents and adults. Arch. Sex. Behav.2010;39:499–513.
- Rotondi NK, Bauer GR, Scanlon K, Kaay M, Travers R, Travers A.Nonprescribed hormone use and self-performed surgeries: “do-it-yourself” transitions in transgender communities in Ontario, Canada. Am. J. Public Health2013;103:1830–1836.
- Gooren LJ, Sungkaew T, Giltay EJ.Exploration of functional health, mental well-being and cross-sex hormone use in a sample of Thai male-to-female transgendered persons (kathoeys). Asian J. Androl.2013;15:280–285.
- Becerra-Fernández A, de Luis Roman DA, Piedrola Maroto G.Morbidity in transsexual patients with cross-gender hormone self-treatment. Med. Clin. (Barc).1999;113:484–487.
- Toorians AWFT, Thomassen MCLGD, Zweegman S, Magdeleyns EJP, Tans G, Gooren LJG, Rosing J.Venous Thrombosis and Changes of Hemostatic Variables during Cross-Sex Hormone Treatment in Transsexual People. J. Clin. Endocrinol. Metab.2003;88:5723–5729.
- Mepham N, Bouman WP, Arcelus J.People with Gender Dysphoria Who Self-Prescribe Cross-Sex Hormones: Prevalence, Sources, and Side Effects Knowledge. J Sex Med2014;11:2995–3001.
Lipid and Lipoprotein Metabolism in Liver Disease
ABSTRACT
The liver plays a central role in lipid metabolism, serving as the center for lipoprotein uptake, formation, and export to the circulation. Alterations in hepatic lipid metabolism can contribute to the development of chronic liver disease, such as nonalcoholic fatty liver disease (NAFLD) and add to the progression of other chronic liver disease, as occurs in hepatitis C. Moreover, chronic liver disease can impact hepatic lipid metabolism leading to alterations in circulating lipid levels contributing to dyslipidemia. This chapter discusses the interplay between lipid metabolism and chronic liver diseases focusing on NAFLD, alcoholic liver disease, hepatitis C, hepatitis B, cholestatic liver disease, and cirrhosis.
NONALCOHOLIC FATTY LIVER DISEASE
Case Presentation
A 60-year-old woman with a past medical history significant for hypertension, dyslipidemia and diabetes mellitus presents for management of newly diagnosed nonalcoholic steatohepatitis (NASH). She has a strong family history of coronary artery disease and a personal history of dyslipidemia characterized by a serum triglyceride level of 220 mg/dl, low-density lipoprotein (LDL) cholesterol of 180 mg/dl, high-density lipoprotein (HDL) cholesterol of 50 mg/dl and total cholesterol of 274 mg/dl. Based on these values, her primary physician has recommended she start a lipid lowering medication. However, with her history of liver disease she is uncertain whether she can safely take lipid-lowering medications.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States, affecting up to a third of adults (1,2). NASH is the progressive form of NAFLD and can lead to cirrhosis, hepatocellular carcinoma, and the need for liver transplantation. In addition to significant morbidity and mortality from end-stage liver disease, NAFLD confers an increased risk of cardiovascular disease (CVD) (3). CVD is the leading cause of mortality among individuals with NAFLD (4). The dyslipidemia of NAFLD may be one of several important and modifiable CVD risk factors.
Changes in Lipoprotein Metabolism and Clinical Manifestations
DEVELOPMENT OF STEATOSIS
NAFLD is characterized in part by steatosis, excess lipid deposition as lipid droplets within hepatocytes. These lipid droplets consist largely of triglycerides and are the result of an imbalance of hepatic lipid handling. Steatosis can occur when one or more of the following conditions is present; 1) excess delivery of free fatty acids (FFA) to the liver from adipose tissue, 2) increased de novo lipogenesis (DNL) within the liver, 3) decreased oxidation of fatty acids within hepatocytes and 4) impaired export of triglycerides from the liver in the form of very-low density lipoproteins (VLDL).
Excess FFA Delivery to the Liver
When excess adiposity and insulin resistance are present, FFA release from adipocytes is increased (5). Upon release FFA are then delivered via the circulation to the liver and may overwhelm the liver’s capacity to oxidize or export lipids, contributing to the development of steatosis. The fatty acid translocase FAT/CD36 mediates uptake of FFA into the liver and is upregulated in human and experimental NAFLD, which may contribute to steatosis (6,7,8).
Increased DNL
Hyperinsulinemia, often seen in the setting of obesity and the metabolic syndrome, can also contribute to DNL as the result of increased transcriptional activities of sterol regulatory element binding protein (SREBP) 1c- and peroxisome proliferator-activated receptor (PPAR)-γ (5,9,10). Increased circulating glucose levels also mediate lipogenesis via cholesterol regulatory element binding protein (ChREBP) activation (11). The increased synthesis of lipids within the liver can lead to accumulation within hepatocytes and can promote the development of steatosis.
Insufficient Export of Hepatic Triglycerides
Export of triglycerides from the liver requires the formation of VLDL and when VLDL formation is impaired steatosis can develop. VLDL are formed when triglycerides are complexed to apolipoprotein B100 (apoB100) via the action of microsomal triglyceride transfer protein (MTP). Steatosis can develop when any of the components of VLDL formation are missing or impaired. Genetic or pharmacologic alteration of MTP or the truncation or absence of ApoB100 can lead to steatosis (12-16). In addition, ApoB100 levels can be decreased by FFA accumulation. FFA accumulation within the liver can lead to chronic stress of the hepatocyte endoplasmic reticulum (ER). Increased ER stress results in increased ApoB100 degradation, decreasing the ability of the liver to export triglycerides and potentially worsen steatosis.
Complete VLDL assembly and secretion relies on several additional steps. Following the formation of nascent VLDL particles, further lipidation is needed to create mature VLDL particles. The process of this lipidation is not well understood but may rely on fusion with lipid droplets (17). Interruption of this process of lipid mobilization from lipids droplets to VLDL may also contribute to the development of steatosis (18). Recent genetic studies have shown a strong link between a polymorphism in the gene patatin-like phospholipase domain-containing 3 (PNPLA3) and NAFLD. This coding region polymorphism (I148M) reduces hepatic VLDL secretion, possibly by interfering with triglyceride mobilization and results in hepatic steatosis (19-21). However, conflicting data indicates there may be a compensatory increase in VLDL export in some NAFLD patients, although this increase is insufficient to counterbalance the elevated hepatic triglyceride content (22). The transmembrane 6 superfamily 2 (TM6SF2) E167K variant results in decreased hepatic VLDL secretion and is associated with NAFLD, fibrosis and cirrhosis in the setting of decreased LDL and triglyceride levels. This variant is associated with progressive liver disease but a decreased risk of cardiovascular disease (23,24). Familial hypobetalipoproteinemia (FHBL) is a condition characterized by diminished levels of functional ApoB100, resulting in impaired VLDL export and the development of hepatic steatosis. Magnetic resonance spectroscopy studies have shown liver fat content in individuals with FHBL to be five times greater than in controls (25,26). Progress to steatohepatitis, cirrhosis and hepatocellular carcinoma (HCC) has been noted in this population (27,28,29,30).
Hepatic Accumulation of Free Cholesterol
The degree of hepatic free cholesterol accumulation in NAFLD correlates with presence and severity of cytologic ballooning (31). Decreased expression of ATP-binding cassette (ABC) A1 and ABCG8 cholesterol efflux proteins, may disrupt transfer of cholesterol from hepatocytes, driving up hepatocyte cholesterol (32,33). There is conflicting evidence regarding changes to hepatic uptake of LDL in individuals with NAFLD, with some studies indicating upregulation of LDL receptors resulting in cholesterol overloading (34).
CHANGES IN LIPID METABOLISM
Dyslipidemia is frequent in adults with radiographic and biopsy-proven NAFLD and is characterized by hypertriglyceridemia, increased LDL particle concentrations, decreased LDL particle size, and decreased HDL levels (35). High ratios of total cholesterol or triglyceride to HDL-cholesterol are associated with NAFLD (36). In addition, non-HDL-cholesterol (non-HDL-C), a composite measure of apolipoprotein-B containing lipoproteins and an important marker of CVD risk, is elevated in individuals with NASH (19). NASH is also characterized by alterations in lipoprotein subfractions. Lipoprotein subfraction assays measure lipoprotein particle size, density and composition. NASH is characterized by large VLDL particle size and decreased LDL and HDL particle size (35). However, there is conflicting data on the association between NASH and VLD particle size (17,18). Furthermore, increased levels of LDL-III and IV particles, atherogenic forms of LDL, and reduced HDL2b levels, a cardioprotective lipoprotein, are observed in NASH (36,37). Fortunately, resolution of NASH is associated with increases in HDL, decreases in triglycerides, and increases in mean LDL particle diameter and the frequency of LDL phenotype A (39).
Insulin Resistance
Insulin resistance is a fundamental aspect of NAFLD and can result in many of the alterations in lipid metabolism and circulating lipid levels seen in NAFLD.
INSULIN RESISTANCE INCREASES CIRCULATING LDL, VLDL AND TRIGLYCERIDES LEVELS
Insulin resistance can increase circulating VLDL and triglyceride levels via several mechanisms. Insulin resistance leads to a loss of suppression of MTP transcription, which increases the efficiency of VLDL assembly (40,41). Insulin resistance also impacts VLDL levels by decreasing lipoprotein lipase (LPL) levels. LPL is an enzyme found on the endothelial cells within muscle and adipose tissue. LPL hydrolyzes triglycerides from circulating VLDL and facilitates triglyceride delivery to muscle and adipose tissues. In the setting of insulin resistance, LPL is downregulated decreasing the clearance of VLDL from the circulation and increasing circulating VLDL levels (42).
Insulin resistance can also act via ApoCIII levels to increase circulating VLDL and triglyceride levels. ApoCIII, a lipoprotein found on VLDL, inhibits LPL and can decrease VLDL clearance from the circulation (43). In the setting of insulin resistance, ApoCIII levels are increased, leading to decreased VLDL/triglyceride clearance and resulting in hypertriglyceridemia and increased VLDL levels. ApoCIII also appears to modulate plasma triglyceride levels via LPL-independent mechanisms. In patients with LPL deficiency due to familial chylomicronemia syndrome, administration of an ApoCIII mRNA inhibitor for 13 weeks reduced plasma triglycerides by 56-86% (44).
Insulin resistance also impacts LDL metabolism via upregulation of hepatic lipase and increased LDL receptor degradation. Hepatic lipase is an enzyme that remove triglycerides from intermediate-density lipoproteins (IDL) leading to the development of smaller, denser low-density lipoproteins. In NAFLD and insulin resistance, hepatic lipase levels are upregulated leading to increased levels of small, dense LDL (sdLDL) (45). Insulin can also increase circulating LDL levels via its effects on the LDL receptor. Insulin upregulates proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein that can bind and degrade the LDL receptor (46). Upregulation of PCSK9 leads to decreased LDL receptor availability on hepatocytes and increased circulating LDL levels.
INSULIN RESISTANCE DECREASES CIRCULATING HDL LEVELS
Insulin resistance decreases circulating HDL levels by interfering with HDL particle assembly. HDL is formed within plasma at the surface of the hepatocyte and requires the interaction of ApoA-1 and ABCA1 (47). Nascent HDL particles are formed when ApoA-1, secreted by the liver or released from other lipoproteins, is lipidated by ABCA1 with phospholipids and free cholesterol. Insulin resistance hampers HDL formation by promoting the phosphorylation and degradation of ABCA1 and by reducing ABCA1 activity (48). In addition to hampering HDL production, insulin resistance may interfere with reverse cholesterol transport. Insulin resistance can result in the formation of particularly triglyceride-rich HDL particles via the action of cholesterol ester transfer protein (CETP) (49). Triglyceride-rich HDL are taken up more rapidly by the liver and may result in lower circulating HDL levels.
Management
Diet and exercise are the foundations of the management of both NAFLD and the dyslipidemia of NAFLD. Small studies have indicated that both a low carbohydrate diet as well as the Mediterranean diet may improve serum lipid levels and NAFLD (50-52). Further, adherence to a Mediterranean diet reduces the development of CVD (53). As CVD is a cause of considerable morbidity and mortality in NAFLD patients, adherence to a Mediterranean diet may have multiple benefits.
Routine aerobic exercise, defined as 30 minutes of moderate exercise most days of the week, can result in significant improvements in lipid levels and may improve hepatic lipid content (54,55). Individuals with NAFLD should be advised to participate in regular, aerobic exercise.
LIPID LOWERING MEDICATIONS
HMG-CoA Reductase Inhibitors
When diet and exercise are insufficient in individuals with NAFLD, HMG-CoA reductase inhibitors or “statins” are recommended. Statins play an important role in both the primary and secondary prevention of CVD and should be used in patients with NAFLD and dyslipidemia. Compared to placebo, statins have been shown, in a post-hoc analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study, to significantly reduce cardiovascular events in individuals with NAFLD (56). Statins have also been shown to exert a protective effect on liver histology in patients with NAFLD/NASH, with dose-dependent reduction in steatosis, steatohepatitis and fibrosis stages F2-F4, although protection against steatohepatitis in the presence of the I148M PNPLA3 risk variant did not reach statistical significance (57).
It is important to note that while there remains a concern among physicians about statin hepatotoxicity, the incidence of statin-induced hepatotoxicity in the general population is extremely low and is not increased in individuals with NAFLD or NASH (58-60). Apprehension among physicians may partly account for the current under prescribing of statins in patients with NAFLD (61,62).
Omega-3 Fatty Acids
Omega-3 fatty acids can be used in patients with NAFLD for the treatment of isolated hypertriglyceridemia or when statins alone are insufficient to control triglyceride levels. Omega-3 fatty acids act to reduce hepatic VLDL secretion and lower serum triglyceride levels. Doses of up to 4 grams daily can decrease triglycerides by 25-35% (63). Omega-3 fatty acids may reduce radiographic steatosis and several randomized controlled trials (RCTs) of omega-3 fatty acids are ongoing to determine their impact on NASH histology (64-66).
Cholesterol Absorption Inhibitors
A further class of drugs which may hold promise are the cholesterol absorption inhibitors, of which ezetimibe has been most extensively studied. A recently conducted RCT involving 32 NAFLD patients found that ezetimibe use led to significant improvement in fibrosis stage and ballooning score (67). Of note, Loomba et al. reported no significant impact of ezetimibe on liver fat content, as assessed by magnetic resonance imaging proton density-fat fraction and liver biopsy (68). The influence of ezetimibe on the various stages of NAFLD pathogenesis remains to be fully characterized. Further large-scale RCTs are warranted to explore ezetimibe’s potential as a component of NAFLD/NASH therapy alongside statins.
LIPID TREATMENT GOALS
We recommend that patients with NAFLD adhere to the Cholesterol Clinical Practice Guidelines from the American Heart Association and American College of Cardiology released in 2018. The guidelines recommend that all adults with any form of CVD or an LDL ≥ 190 mg/dL should be treated with high intensity statins for a goal 50% reduction in LDL. Patients aged 45-70 years with diabetes with LDL < 189 mg/dL or patients with > 7.5% global 10-year CVD-risk should receive moderate intensity statins for a goal 30-50% reduction in LDL. A specific target LDL is no longer formally recommended.
Return to Case
For our patient with NAFLD it would be both safe and important for her to take lipid-lowering medication to manage her dyslipidemia and reduce her risk of a CVD development. She would benefit from administration of a statin of either moderate or high intensity, based on the outcome of risk assessment.
|
Table 1. Key Points- Non-Alcoholic Fatty Liver Disease |
|
NAFLD is associated with insulin resistance which results in atherogenic dyslipidemia characterized by increased small dense LDL and triglyceride levels and decreased HDL levels. |
|
The dyslipidemia of NAFLD may contribute to the increased risk of CVD observed in individuals with NAFLD |
|
Patients with NAFLD and NASH should be treated for their dyslipidemia to reduce their CVD risk. |
|
Individuals with NAFLD can be treated with statins without increased risk of hepatotoxicity. |
ALCOHOLIC LIVER DISEASE
Case Presentation
An obese 48-year-old man with a past medical history significant for coronary heart disease, hypertension, and diabetes mellitus presents for management of newly diagnosed hepatic steatosis. He has a family history of coronary artery disease. He admits to consuming 3 glasses of wine per night during the week and an additional two per evening on weekends. His fasting plasma triglyceride concentration is 350 mg/dl, his LDL cholesterol is 130 mg/dl, and HDL cholesterol is 55 mg/dl. The alanine aminotransferase level (ALT) is modestly elevated at 55 IU/ml. He would like to know whether he has NAFLD and whether you recommend continuing his current alcohol intake to protect against CVD, especially since he was told that his good cholesterol was elevated.
Introduction
Alcoholic liver disease (ALD) accounts for nearly half of cirrhosis-related mortality in the United States (69). A hallmark feature of ALD is hepatic steatosis, which develops in more than 90% of heavy drinkers. However, less than one third of these individuals develop complications that include alcoholic hepatitis, cirrhosis and HCC (69). Risk factors for disease progression include female sex, obesity, drinking patterns, dietary factors, non–sex-linked genetic factors, and cigarette smoking (70,71). Alcohol also synergizes with other etiologies of chronic liver disease, including NAFLD and viral hepatitis to accelerate progression (69). Hypertriglyceridemia is the primary dyslipidemia associated with alcohol ingestion (72), and a J-shaped association exists between alcohol intake and CVD (73), which may reflect a parallel effect of plasma triglycerides (72). Although its contribution to metabolic syndrome is unclear, alcohol intake appears to interact with obesity to further increase plasma triglyceride concentrations (72).
Changes in Lipoprotein Metabolism and Clinical Manifestations
DEVELOPMENT OF STEATOSIS
As with NALFD, the development of steatosis in response to alcohol is multifactorial. Alcohol impairs the β-oxidation of fatty acids by mitochondria, promotes de novolipogenesis in the liver, and increases fatty acid uptake. As is the case in NALFD, VLDL secretion is also increased due to alcohol.
Excess FFA Delivery to the Liver
As is the case for NAFLD, fatty acids from extrahepatic sources appear to contribute to hepatic steatosis. In addition to increasing mobilization of fatty acids from adipose tissue (74), alcohol intake augments the supply of lipids to the liver from the small intestine in the form of chylomicron remnants (75).
Increased DNL
Increased DNL contributes to alcohol-related steatosis by direct and indirect mechanisms (69). The alcohol metabolite acetaldehyde increases transcription of SREBP1c, which upregulates transcription of lipogenic genes. Alcohol-induced endoplasmic reticulum stress and inflammation leads to increased processing of the SREBP1c protein within hepatocytes. Alcohol also inhibits proteins that suppress lipogenesis. The protein deacetylase Sirtuin 1 (SIRT1), plays a central role (76). Suppression of SIRT1 by alcohol leads to hyperacetylation of a group of molecules, including those that promote lipogenesis. Inhibition of adenosine monophosphate kinase (AMPK) contributes, because AMPK-mediated phosphorylation of SREBP1c reduces transcriptional activity. AMPK also phosphorylates and inhibits acetyl-CoA carboxylate (ACC), the rate-limiting step in lipogenesis.
Impaired Oxidation and Degradation of Fatty Acids
Alcohol decreases mitochondrial fatty acid oxidation principally by decreasing activity of the transcription factor peroxisome proliferator activated receptor (PPAR) α. This occurs in response to increased NADH/NAD+ ratios and decreased AMPK activity, among other factors (69). PPARα promotes the transcription of genes that mediate fatty acid oxidation. Alcohol intake may also inhibit autophagy (69), which plays an important role removing lipids from the liver (77).
Insufficient Export of Hepatic Triglycerides
Alcohol increases VLDL secretion (72,78), apparently by increasing the transcription of MTP (74). The increased in export of hepatic triglycerides is insufficient to offset the accumulation due to increases in fatty acid uptake and synthesis in the setting of decreased oxidation.
HYPERTRIGLYCERIDEMIA
Increased VLDL secretion contributes to hypertriglyceridemia that is observed in the setting of alcohol consumption. This is exacerbated by decreased expression of LPL (79), which promotes clearance of VLDL triglycerides into muscle and fat tissue. There is also an interaction between alcohol consumption and genetic polymorphisms in apoCIII, which circulates in the plasma and functions to inhibit lipoprotein lipase activity (80).
CIRCULATING HDL LEVELS
Alcohol increases HDL lipids and apolipoproteins in patterns that depend upon amount of consumption: Moderate consumption tends to increase plasma concentrations of smaller HDL particles, whereas heavier consumption favors larger HDL particles (81). Alcohol interacts with HDL metabolism in multiple steps, which can ultimately lead to increased reverse cholesterol transport, the process by which cellular cholesterol is transported to the liver for elimination into bile (81,82). Heavier alcohol consumption impairs CETP activity, so the typical inverse relationship observed under circumstances associate with NAFLD is not necessarily observed in the setting of alcohol use and HDL may be increased as well (72,83). Moderate alcohol consumption also appears to enhance the anti-inflammatory and anti-oxidant properties of HDL particles (81).
CIRCULATING LDL LEVELS
The effects of alcohol on plasma LDL cholesterol concentrations is less consistent than observed for HDL, with different patterns observed in different populations, which may be attributable to genetic polymorphisms with these populations (81).
Management
Although considerable anecdotal evidence exists to support a CVD benefit of moderate alcohol consumption, insufficient data are available to translate this concept into a clinical recommendation. In the setting of alcohol-related hepatic steatosis, cessation of drinking, along with therapeutic lifestyle modifications, are the mainstays of therapy.
Return to Case
The diagnosis of NAFLD is based on the absence of significant alcohol consumption. For a man, the upper limit of alcohol intake is 2 drinks per day. This means that this patient cannot be categorized simply as NAFLD, although the coexistence of alcoholic liver disease and NAFLD is likely in this patient. He is at high risk for CVD, so should be managed accordingly, including lipid lowering therapy with statins. His alcohol consumption should be reduced to less than 2 drinks per day, which may help reduce his fasting triglyceride concentrations. He should not be falsely reassured by his elevated HDL cholesterol concentration.
|
Table 2. Key Points- Alcoholic Liver Disease |
|
The consumption of alcohol is a common cause of excess fat accumulation in the liver. |
|
There are multiple mechanisms by which alcohol promotes hepatic steatosis. |
|
Alcohol can increase plasma HDL cholesterol concentrations and fasting triglyceride concentrations. |
|
Although modest alcohol consumption is associated with reduced CVD risk, this cannot be recommended due to other potential adverse effects, including alcoholic liver disease. |
VIRAL HEPATITIS-- C
Case Presentation
A 65-year-old woman with a past medical history of CVD and untreated genotype 1 chronic hepatitis C presents for management of CVD. Her lipid levels are notable for an LDL of 99. She has read that since her LDL is below the recommended level for patients with CVD she would not benefit from lipid lowering therapy. What would you advise her?
Introduction
Hepatitis C virus (HCV) is a positive-strand RNA virus of the family Flaviviridaethat can lead to chronic infection as well as the development of cirrhosis, HCC, and the need for liver transplantation. Chronic HCV (CHC) infection impacts between 130 and 170 million individuals worldwide (84).
Changes in Lipoprotein Metabolism
HCV replication is intricately linked with host cell lipids and impacts host lipid metabolism. Circulating HCV virions complex with host lipoproteins and form lipoviroparticles (85). This lipid composition is a prerequisite for maintenance of viral particle morphology and HCV infectivity (86,87,88,89). For example, lipids on the virion surface shield viral envelope epitopes, protecting them from antibody engagement (90). Lipoviroparticles can enter hepatocytes via multiple receptors including the hepatocyte LDL receptor (which may also facilitate the replication step of the HCV cycle (91)) and utilizes cell surface molecules including Niemann-Pick C1-like 1 (NPC1L1), a receptor for cholesterol resorption, and scavenger receptor class B member 1 (SRB1), which acts to promote cholesterol uptake from lipoproteins, and interacts with HCV envelope glycoprotein E2 to promote HCV entry (92,93,94). LDL receptor and SRB1 appear to have a redundant role in HCV entry (95). Several apolipoproteins influence HCV uptake: apoC1 interacts with HCV glycoproteins to promote infection, and apoE mediates initial attachment between virus and hepatocyte. Hepatocyte VLDL receptor mediates an additional HCV entry mechanism, involving E2 and apoE, with increased VLDL receptor expression conferring greater susceptibility to infection (96). Formation of the HCV core protein involves interaction with host cytosolic lipid droplets and interaction with diacylglycerol O-acetyltransferase 1, a host enzyme involved in triglyceride synthesis. HCV replication also interacts with host cholesterol synthesis within hepatocytes. The host protein FBL2 undergoes geranylgeranylation, an intermediate of the cholesterol synthesis pathway (97). When this pathway is interrupted, the HCV replication complex is extinguished (98). Finally, HCV secretion from hepatocytes involves complexing with apoE-containing host lipoproteins in the form of VLDL or HDL (99).
Clinical Manifestations
Like NAFLD, HCV infection is associated with the development of hepatic steatosis. However, unlike NAFLD, HCV is also associated with hypolipidemia. CHC infection is associated with significantly lower host LDL and total cholesterol levels than in uninfected controls (100). Treatment is associated with increases in both LDL and cholesterol levels in patients with HCV who achieve a cure, defined as a sustained virologic response (SVR). Changes in host serum lipids are also seen in patients with acute HCV. Acute HCV infection is associated with a decrease in total cholesterol, LDL and non-HDL-cholesterol from pre-infection levels. In addition, total cholesterol, LDL, triglycerides and non-HDL-C progressively decline over a 10-year period following HCV seroconversion, after adjusting for BMI and FIB-4 score (101). In patients who achieved viral clearance, either spontaneous or treatment-induced, total cholesterol, LDL and non-HDL-C increased significantly from infection levels. In an important proportion of patients with both acute and chronic infection, post-viral clearance lipid levels exceed pre-infection levels (102).
While HCV infection is associated with a decrease in LDL and non-HDL-C, important CVD risk factors, HCV infection is associated with an increased overall risk of CVD (103,104). When non-HCV infected individuals with similar lipid levels are compared to those with CHC, HCV infection independently confers an increased risk of acute myocardial infarction (AMI), with a more pronounced increase seen in younger individuals (105). Further, lipid-lowering therapy among individuals with CHC was associated with a greater reduction in AMI risk than uninfected persons with similar lipid levels. Therefore, lipid levels may not accurately reflect CVD risk in patients with CHC.
Management
Lipid treatment goals for individuals with CHC are not well established. We recommend that patients with CHC adhere to the Cholesterol Clinical Practice Guidelines from the American Heart Association and American College of Cardiology released in 2018 (106). Retrospectively-collected data links statin use to improved liver-related outcomes, with higher likelihood of achieving SVR, and lower rates of fibrosis progression, cirrhosis development, HCC incidence, and mortality amongst patients with CHC (107,108,109,110). Simon et al. identified that atorvastatin and fluvastatin have the most significant anti-fibrotic benefit, compared with simvastatin, pravastatin, lovastatin or no statin use (111). It is important to note that for individuals who have achieved an SVR after HCV treatment, lipid levels often increased to or above pre-infection levels. Induction of SVR using DAA therapy led to pro-atherogenic lipid changes (increased total cholesterol, LDL, LDL/HDL ratio, and non-HDL-C), irrespective of DAA regimen or fibrotic stage, with a parallel reduction in insulin resistance. The balance of these effects with respect to CVD risk remains to be determined (112). Hashimoto et al. found greater increases in serum LDL-cholesterol (LDL-C) levels in patients undergoing therapy with ledipasvir/sofosbuvir compared to daclatasvir/asunaprevir. Decline in HCV core protein was also independently associated with rises in LDL-C (113). Thus, practitioners should be mindful to monitor post-treatment lipid levels and treat appropriately.
Return to Case
For our patient with CHC and CVD it would be important for her to take a lipid-lowering medication to reduce her risk of a second CVD event. Based on the guidelines, she would benefit from high intensity statin therapy, with a goal of decreasing LDL cholesterol by >50%.
|
Table 3. Key Points- Hepatitis C |
|
The hepatitis C virus interacts with host lipids for hepatocyte entry, viral replication and secretion. |
|
HCV infection decreases host serum LDL and total cholesterol levels. |
|
HCV infection is still associated with an increased risk of AMI and treatment with statins reduces this risk. |
|
Treatment of HCV results in increase in serum lipid levels to at least pre-infection levels. |
VIRAL HEPATITIS –HEPATITIS B
Introduction
Approximately 240 million individuals are chronically infected with the hepatitis B virus (HBV) (114). Like HCV, chronic HBV infection can lead to cirrhosis and hepatocellular carcinoma.
Lipoprotein Metabolism in Hepatitis B
HBV interacts with host lipid metabolism in several important ways including during viral cell entry and formation of a vital viral protein, the HBV surface antigen. HBV uses the Na+-taurocholate cotransporting polypeptide (NTCP), a peptide that normally allows for hepatocyte uptake of host bile acids, to gain access to hepatocytes (115). HBV binding to NTCP impairs the ability of NTCP to promote hepatocyte uptake of bile acids. This results in an increase in conversion of cholesterol to bile acids.
The formation of the HBV surface antigen within hepatocytes relies in part on host cell cholesterol (116). The surface antigen particle is synthesized in the membrane of the hepatocyte endoplasmic reticulum (ER) and is associated with the host ER lipid bilayer. Association with the lipid bilayer helps make the particle resistant to degradation by cellular proteases. The surface antigen is then transported to the ER lumen and exported from the hepatocyte as a lipoprotein particle. Approximately 25% of the surface antigen complex is composed of host lipids including phosphatidylcholine, triglycerides, cholesterol and cholesterol esters (116).
HBV infection may also alter lipogenic gene expression. Two studies have demonstrated increased in lipogenic gene expression in HBV-infected transgenic mice compared to uninfected mice. HBV-infected transgenic mice have increased gene expression of SREBP2, 3-hydroxy-3-methylglutaryl-coenzyme A reductase, LDL receptor, fatty acid synthase, and ATP citrate lyase, all of which play a role in either cholesterol metabolism or fatty acid synthesis (117,118). Oehler et al also found that in HBV infected humanized mice, gene expression of human apolipoprotein A1, a lipoprotein found in HDL which plays a role in reverse cholesterol transport and PPAR-gamma which regulates adipocyte differentiation and fatty acid storage, was significantly enhanced.
HBV-infected transgenic mice also demonstrate elevated levels of 7α-hydroxylase (hCYP7A1), which promotes bile acid formation from cholesterol. In liver biopsy samples from patients with chronic HBV infection, hCYP7A1 was significantly induced when compared to uninfected controls. These findings suggest that HBV replication may impact cholesterol metabolism.
Clinical Manifestations
Data on the impact of HBV infection on circulating lipid levels in humans is limited. HBV infection may be associated with lower triglyceride levels than in uninfected patients (119), however its influence on HDL remains ambiguous. Hsu et al performed a case control study comparing 322 individuals with chronic HBV infection to 870 age-matched, uninfected controls. Individuals with HBV infection were found to have significantly lower triglyceride and HDL levels when compared to controls. In a second retrospective cohort of 122 individuals with chronic HBV, HBV DNA levels was inversely proportional to serum triglyceride levels but no relationship was seen with HDL levels (119). Amongst a cohort of non-diabetic patients, HBsAg-seropositivity was inversely correlated with hypertriglyceridemia and low serum HDL cholesterol. Hence, chronic HBV infection may favorably impact lipid profiles, which could partly account for the inverse relationship between HBsAg-seropositivity and metabolic syndrome seen in this cohort (120). Similarly, Joo et al. demonstrated that in patients who were initially free of dyslipidemia, HBsAg-positivity was associated with lower risk of developing dyslipidemia during an average follow up of 4.46 years (121).
Circulating lipid levels may be predictive of clinical outcomes in HBV-infected patients. Chen et al. found that average plasma apolipoprotein A-V level was decreased amongst 209 non-survivors of HBV-acute on chronic liver failure versus 121 survivors (122).
Like HCV, chronic HBV infection is frequently associated with hepatic steatosis. Between 25% and 51% of patients with HBV are found to have steatosis on imaging or biopsy (123). However, while concurrent steatosis is common in HBV infections, steatohepatitis is not frequently described. Further, the pathogenesis of steatosis in HBV is not well understood and may be related to co-existing metabolic factors such as body mass index (BMI) and insulin resistance rather than the viral infection itself (124).
Management
As data on the impact of HBV infection on circulating lipids is limited there are no formal guidelines for dyslipidemia management in this population. Clinicians should be mindful of a possible decrease in HDL in this population and follow standard guidelines from the American Heart Association and American College of Cardiology on lipid management. Recent studies have shown reduced risk of cirrhosis development (125), decompensation (125, 126, 127, 128), mortality (126, 127) and portal hypertension (126) amongst statin users compared to non-users with chronic HBV- and HCV-related hepatitis. Furthermore, statin use was associated with a 32% reduced HCC risk. Concomitant use of statin and nucleos(t)ide analogue led to an additive chemopreventive effect (129). Large-scale RCTs to comprehensively evaluate statins as a means of protection against disease progression in patients with viral hepatitis are warranted.
|
Table 4. Key Points- Hepatitis B |
|
HBV infection may interact with host lipids and enhance lipogenic gene expression |
|
The clinical manifestations of HBV on host lipids are not well studied but HBV infection may decrease serum triglyceride and HDL levels. |
|
Management of patients with HBV and dyslipidemia should be guided by standard recommendations for the treatment of dyslipidemia. |
CHOLESTATIC DISEASES
Case Presentation
A 58-year-old woman is referred with primary biliary cirrhosis (PBC) for the management of an elevated plasma total cholesterol of 450. She reports symptoms only consistent with mild and intermittent pruritis. She is currently taking ursodeoxycholic acid. Her physical is notable for xanthelasma under the eyes.
Introduction
Bile is the route for cholesterol elimination from the body. Plasma cholesterol is taken up by the liver in the form of apolipoprotein B-containing lipoproteins (i.e. remnant lipoproteins and LDL) by receptor-mediated endocytosis or by selective uptake of HDL cholesterol (130). Cholesterol is eliminated by conversion to bile salts and by biliary secretion. Biliary obstruction, notable when due to cholestatic diseases, can interfere with cholesterol elimination leading to hypercholesterolemia. This occurs most commonly in the setting of primary biliary cirrhosis (PBC), which is an autoimmune-mediated destruction of intrahepatic bile ducts. It can also occur in primary sclerosing cholangitis (PSC), in which there is inflammatory stricturing of larger bile ducts by poorly understood mechanisms.
Lipoprotein Metabolism in Cholestasis
Hypercholesterolemia associated with cholestasis is largely attributable to the formation of lipoprotein X, an atypical lipoprotein particle. Lipoprotein X comprises principally unesterified cholesterol and phospholipids (131), resembling the cholesterol-phospholipid vesicles that are secreted by the liver into bile (132). The principal proteins associated with lipoprotein-X are apoC and albumin contained within the core (133,134). The lipids of the particle comprise a sphere, with an aqueous core. Lipoprotein-X is devoid of apoB. It appears to be formed due to the secretion of biliary-type particles into plasma in the setting of obstruction to bile flow (135), although defects in plasma cholesterol esterification may also contribute (131). Lipoprotein X has similar characteristics as LDL including density, so that its presence in plasma requires electrophoretic separation (136).
Plasma total cholesterol concentrations are increased in PBC in proportion to disease severity, with elevations that can be striking and exceed 1,000 mg/dl, and can be a rare cause of pseudohyponatremia (133). Where these elevations are primarily attributable to lipoprotein-X, apolipoprotein B concentrations may also be elevated due abnormal lipoprotein metabolism associated with liver disease (131,133). Serum metabolomics analysis of patients with PBC revealed elevated levels of VLDL and LDL compared to controls (137). HDL cholesterol concentrations are elevated in the early stages of PBC and tend to decline as the disease progresses (138), apparently because of increased circulating hepatic lipase activity that promotes HDL catabolism (131). Patients with more advanced PBC exhibit increased plasma triglycerides (131), presumably attributable to decreased hepatic lipase activity (138).
Plasma lipids in PSC have been less well characterized than in PBC. In a small series (139), the hypercholesterolemia was more modest than generally observed in PBC, but did increase in concert with disease severity. HDL cholesterol levels tended to be high, and triglyceride elevations were uncommon.
Clinical Manifestations
An important consideration has been whether the lipid abnormalities associated with cholestatic diseases confer increased CVD risk. This has been studied more extensively in PSC in the form of prospective trials (138,140). Although each had limitations, collectively there was no suggestion of increased atherosclerotic events, which is in keeping with the relative absence of elevations in atherogenic particles There is also evidence in vitroto suggest that lipoprotein-X may be atheroprotective by reducing oxidation of LDL (136). In patients with PBC, the presence of xanthelasma does not appear to connote an increased CVD burden (138).
As with PBC, the cholesterol elevations associated with PSC do not tend to confer CVD risk. None was observed in the small series cited previously, but it was acknowledged that patients were young enough that excess CVD complications would not have been expected (139). Lipid levels ultimately fell in patients who had progressed to cirrhosis and hepatic failure.
Management
Due to the overall lack of clinical evidence, the management of hypercholesterolemia associated with cholestasis lacks formal recommendations. In PBC, ursodeoxycholic acid (UDCA) slows the progression of disease and prolongs survival (141).Chronic UDCA administration also reduces plasma LDL concentrations. In PBC patients, statin therapy is generally safe and is effective at lowering LDL cholesterol in PBC patients (58,142-144). At present, UDCA is generally not recommended in the management of PSC (145), and data are lacking regarding lipid-lowering therapies in these patients. Of note, some patients with obstructive jaundice are treated with bile acid binders to reduce pruritus and not primarily to reduce plasma cholesterol concentrations.
Return to Case
For our patient with PBC, the presence of lipoprotein-X may be confirmed by lipoprotein electrophoresis. The possible contribution of atherogenic particles may be estimated by the measurement of the plasma apoB concentration. The institution of statin therapy should be based on standard estimates of CVD risk.
|
Table 5. Key Points- Cholestatic Disease |
|
Plasma total cholesterol concentrations are commonly elevated in the setting of cholestasis. |
|
Lipoprotein-X is an abnormal lipoprotein that circulates in patients with cholestasis and is primarily responsible for the elevations in plasma total cholesterol concentrations. |
|
Elevations in plasma cholesterol concentrations due to cholestasis do not appear to confer excess CVD risk. |
|
Patients with cholestatic disorders may be candidates for lipid lowering therapy if they are otherwise at risk for CVD. |
CIRRHOSIS
Introduction
Cirrhosis is the common advanced histologic endpoint for chronic liver diseases in which the formation of fibrotic nodules in the liver often obscured the etiology of the responsible disease process. The clinical correlates range widely from well-compensated liver function with no apparent clinical manifestations to advanced decompensated liver disease with portal hypertension, with complications that include hepatic encephalopathy, esophageal varices, and ascites. Moreover, the development of cirrhosis confers increased risk of HCC.
Changes in Lipoprotein Metabolism and Clinical Manifestations
The changes in lipoprotein metabolism associated with cirrhosis generally reflect the degree of impairment of hepatic function. In one study (146), plasma concentrations of total cholesterol, HDL cholesterol, LDL cholesterol, and VLDL cholesterol varied with increases in prothrombin time and decreases in albumin, which reflect hepatic synthetic function.These findings are in general agreement with other studies (147). Lipoprotein compositions are also altered in the setting of cirrhosis, with LDL particles enriched with triglycerides and deficient in cholesteryl esters, and HDL particles enriched with triglycerides, free cholesterol and phospholipids (147). These changes are secondary to characteristic abnormalities in plasma enzymes that remodel lipoproteins, including lecithin-cholesterol acyl transferase (LCAT), hepatic lipase, and phospholipid transfer protein (PLTP) (147). HDL-C and enzymes involved in HDL maturation and metabolism are decreased in patients with cirrhosis. There is a shift in the composition of HDL in those with cirrhosis towards the larger HDL2 subclass, with a reduction in small HDL3 particles. The latter is associated with diminished cholesterol efflux capacity which in turn independently predicts 1-year mortality (148).
Hepatocellular carcinoma can occur in the setting of cirrhosis and may be associated with alterations in plasma lipids (149-151). In instances of hypercholesterolemia, the increase may be driven by elevated rates of cholesterol synthesis and cellular levels of 3-hydroxy-3-methylglutarylcoenzyme A. It is unclear whether this hypercholesterolemia confers increased CVD risk (152,153).
CVD risk is dependent upon the etiology of cirrhosis, at least in part due to the association of type 2 diabetes. Cirrhosis due to NASH, HCV, and alcoholic liver disease increases the risk of type 2 diabetes, which is not observed in cholestatic liver diseases and presumably contributes to CVD risk (147,154). Statin therapy may be safely administered in patients with compensated cirrhosis and increased CVD risk (58). In patients with non-cholestatic cirrhosis, low HDL cholesterol serves as a liver function test that is an indicator of poor prognosis, increasing the risk of cirrhotic death (155).
REFERENCES
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140:124-131
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40:1387-1395
- Corey KE, Chalasani N. Management of Dyslipidemia as a Cardiovascular Risk Factor in Individuals With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2014;12:1077-84
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363:1341-1350
- Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 2013; 48:434-441
- Arguello G, Balboa E, Arrese M, Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta 2015;1852:1765-78.
- Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011;60:1394-402.
- He J,Lee JH,Febbraio M,Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med (Maywood).2011 236:1116-21.
- Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011; 22:353-363
- Sanders FW,Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc.2016;91:452-68.
- Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 2006; 4:107-110
- Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 1992; 258:999-1001
- Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr 2000; 20:663-697
- Goh VJ, Silver DL. The lipid droplet as a potential therapeutic target in NAFLD. Semin Liver Dis 2013; 33:312-320
- Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res 2004; 45:941-947
- McGowan MP, Tardif JC, Ceska R, Burgess LJ, Soran H, Gouni-Berthold I, Wagener G, Chasan-Taber S. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One 2012; 7:e49006
- Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 2002; 277:17377-17380
- Chamberlain JM, O'Dell C, Sparks CE, Sparks JD. Insulin suppression of apolipoprotein B in McArdle RH7777 cells involves increased sortilin 1 interaction and lysosomal targeting. Biochem Biophys Res Commun 2013; 430:66-71
- Chamoun Z, Vacca F, Parton RG, Gruenberg J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol Cell 2013; 105:219-233
- Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, Cohen JC, Hobbs HH. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest 2012; 122:4130-4144
- Pirazzi C, Adiels M, Burza MA, Mancina RM, Levin M, Stahlman M, Taskinen MR, Orho-Melander M, Perman J, Pujia A, Andersson L, Maglio C, Montalcini T, Wiklund O, Boren J, Romeo S. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol 2012; 57:1276-1282
- Fabbrini E,Mohammed BS,Magkos F,Korenblat KM,Patterson BW,Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology2008;134:424-31
- KozlitinaJ, SmagrisE, Stender S,NordestgaardBG, ZhouHH,Tybjærg-HansenA, VogtTF, HobbsHH, Cohen Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics 2014;46:352-6
- Dongiovanni P, Petta S, Maglio Cet al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 2015;61:506-14
- Schonfeld G. Familial hypobetalipoproteinemia: a review. J Lipid Res 2003; 44:878-83
- Schonfeld G, Patterson BW, Yablonskiy DA, et al. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res 2003;44:470–478
- Welty FK. Hypobetalipoproteinemia and abetalipoproteinemia. Curr Opin Lipidol 2014; 25:161-8
- Tarugi P, Lonardo A. Heterozygous familial hypobetalipoproteinemia associated with fatty liver. Am J Gastroenterol 1997; 92:1400–1402
- Bonnefont-Rousselot D, Condat B, Sassolas A, et al. Cryptogenic cirrhosis in a patient with familial hypocholesterolemia due to a new truncated form of apolipoprotein B. Eur J Gastroenterol Hepatol 2009; 21:104–108
- Lonardo A, Tarugi P, Ballarini G, Bagni A. Familial heterozygous hypobetalipoproteinemia, extrahepatic primary malignancy, and hepatocellular carcinoma. Dig Dis Sc 1998; 43:2489–2492
- Caballero F,Fernández A,De Lacy AM,Fernández-Checa JC,Caballería J,García-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol2009;50:789-96.
- Min HK,Kapoor A,Fuchs Met al. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab.2012;15:665-74
- Yang Y,Jiang Y,Wang Y,An W. Suppression of ABCA1 by unsaturated fatty acids leads to lipid accumulation in HepG2 cells. Biochimie2010;92:958-63
- Van Rooyen DM,Larter CZ,Haigh WG. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology2011;141:1393-403
- DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2013; 227:429-436
- Wu KT,Kuo PL,Su SB. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol2016;10:420-5
- Sonmez A,Nikolic D,Dogru T. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol2015;9:576-82
- Corey KE, Misdraji J, Gelrud L, Zheng H, Chung RT, Krauss RM. Nonalcoholic steatohepatitis is associated with an atherogenic lipoprotein subfraction profile. Lipids Health Dis 2014; 13:100
- Corey KE, Vuppalanchi R, Wilson LA, Cummings OW, Chalasani N. NASH Resolution is Associated with Improvements in HDL and Triglyceride Levels But Not Improvement in LDL or Non-HDL-C Levels. Aliment Pharmacol Ther 2015;41:301–9
- Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol 2012; 23:206-212
- Sparks CE, Sparks JD. Hepatic steatosis and VLDL hypersecretion. Curr Opin Lipidol 2012; 23:395-397
- Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 2009; 297:E271-288
- Shachter NS. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr Opin Lipidol 2001; 12:297-304
- Gaudet D, Brisson D, Tremblay K et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014; 371:2200-6
- Miksztowicz V, Lucero D, Zago V, Cacciagiu L, Lopez G, Gonzalez Ballerga E, Sorda J, Fassio E, Schreier L, Berg G. Hepatic lipase activity is increased in non-alcoholic fatty liver disease beyond insulin resistance. Diabetes Metab Res Rev 2012; 28:535-541
- Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res 2012; 53:2515-2524
- Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 2006; 84:276-294
- Nonomura K, Arai Y, Mitani H, Abe-Dohmae S, Yokoyama S. Insulin down-regulates specific activity of ATP-binding cassette transporter A1 for high density lipoprotein biogenesis through its specific phosphorylation. Atherosclerosis 2011; 216:334-341
- Lucero D, Zago V, Lopez GI, Graffigna M, Lopez GH, Fainboim H, Miksztowicz V, Gomez Rosso L, Belli S, Levalle O, Berg G, Brites F, Wikinski R, Schreier L. Does non-alcoholic fatty liver impair alterations of plasma lipoproteins and associated factors in metabolic syndrome? Clin Chim Acta 2011; 412:587-592
- Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O'Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 2013; 59:138-143
- Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009; 136:1552-1560
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ, Dietary Intervention Randomized Controlled Trial G. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008; 359:229-241
- Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA, Investigators PS. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368:1279-1290
- Wood PD, Haskell WL, Blair SN, Williams PT, Krauss RM, Lindgren FT, Albers JJ, Ho PH, Farquhar JW. Increased exercise level and plasma lipoprotein concentrations: a one-year, randomized, controlled study in sedentary, middle-aged men. Metabolism 1983; 32:31-39
- Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009; 50:1105-1112
- Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP, Group GSC. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 2010; 376:1916-1922
- Dongiovanni P, Petta S, Mannisto V et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol 2015;63:705-12
- Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol 2006; 97:77C-81C
- Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004; 126:1287-1292
- Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007; 46:1453-1463
- Dongiovanni P,Petta S,Mannisto V. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol2015;63:705-12
- Del Ben M, Baratta F, Polimeni L. Under-prescription of statins in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis2017;27:161-7
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006; 84:5-17
- Cussons AJ, Watts GF, Mori TA, Stuckey BG. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab 2009; 94:3842-3848
- Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Alagona C, Papa G, Rabuazzo AM, Purrello F. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis 2008; 40:194-199
- Zhu FS, Liu S, Chen XM, Huang ZG, Zhang DW. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol 2008; 14:6395-6400
- Takeshita Y, Takamura T, Honda M et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia 2014;57:878-90
- Loomba R, Sirlin CB, Ang B et al. Ezetimibe for the Treatment of Nonalcoholic Steatohepatitis: Assessment by Novel Magnetic Resonance Imaging and Magnetic Resonance Elastography in a Randomized Trial (MOZART Trial). Hepatology 2015;61:1239-50
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011; 141:1572-1585
- O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology 2010; 51:307-328
- Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis 2007; 27:44-54
- Klop B, do Rego AT, Cabezas MC. Alcohol and plasma triglycerides. Curr Opin Lipidol 2013; 24:321-326
- Foerster M, Marques-Vidal P, Gmel G, Daeppen JB, Cornuz J, Hayoz D, Pecoud A, Mooser V, Waeber G, Vollenweider P, Paccaud F, Rodondi N. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol 2009; 103:361-368
- Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol 2012; 180:998-1007
- Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res 1979; 20:289-315
- You M, Jogasuria A, Taylor C, Wu J. SIRT1 signaling and alcoholic fatty liver disease. Hepatobiliary Surg Nutr 2015; 4:88-100
- Czaja MJ, Ding WX, Donohue TM, Jr., Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin XM. Functions of autophagy in normal and diseased liver. Autophagy 2013; 9:1131-1158
- Mudrakova E, Poledne R, Kovar J. Postprandial triglyceridemia after single dose of alcohol in healthy young men. Nutr Metab Cardiovasc Dis 2013; 23:183-188
- Schneider J, Liesenfeld A, Mordasini R, Schubotz R, Zofel P, Kubel F, Vandre-Plozzitzka C, Kaffarnik H. Lipoprotein fractions, lipoprotein lipase and hepatic triglyceride lipase during short-term and long-term uptake of ethanol in healthy subjects. Atherosclerosis 1985; 57:281-291
- Ruixing Y, Yiyang L, Meng L, Kela L, Xingjiang L, Lin Z, Wanying L, Jinzhen W, Dezhai Y, Weixiong L. Interactions of the apolipoprotein C-III 3238C>G polymorphism and alcohol consumption on serum triglyceride levels. Lipids Health Dis 2010; 9:86
- Brinton EA. Effects of ethanol intake on lipoproteins. Curr Atheroscler Rep 2012; 14:108-114
- Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci 2002; 39:225-283
- Kuusisto SM, Peltola T, Laitinen M, Kumpula LS, Makinen VP, Salonurmi T, Hedberg P, Jauhiainen M, Savolainen MJ, Hannuksela ML, Ala-Korpela M. The interplay between lipoprotein phenotypes, adiponectin, and alcohol consumption. Ann Med 2012; 44:513-522
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29 Suppl 1:74-81
- Andre P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Brechot C, Paranhos-Baccala G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol 2002; 76:6919-6928
- Lavie M, Dubuisson J. Interplay between hepatitis C virus and lipid metabolism during virus entry and assembly. Biochimie 2017;141:62-9
- Aizaki H, Morikawa K, Fukasawa M et al. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol 2008;82:5715-24
- Shimizu Y, Hishiki T, Sugiyama K et al. Lipoprotein lipase and hepatic triglyceride lipase reduce the infectivity of hepatitis C virus (HCV) through their catalytic activities on HCV-associated lipoproteins. Virology 2010;407:152-9
- Yamamoto M, Aizaki H, Fukasawa M et al. Structural requirements of virion-associated cholesterol for infectivity, buoyant density and apolipoprotein association of hepatitis C virus. J Gen Virol 2011;92:2082-7
- Fauvelle C, Felmlee DJ, Crouchet E et al. Apolipoprotein E mediates evasion from hepatitis C virus neutralizing antibodies. Gastroenterology 2016;150:206-17
- Albecka A, Belouzard S, de Beeck AO et al. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology 2012;55:998-1007
- Acuna-Alonzo V, Flores-Dorantes T, Kruit JK, Villarreal-Molina T, Arellano-Campos O, Hunemeier T, Moreno-Estrada A, Ortiz-Lopez MG, Villamil-Ramirez H, Leon-Mimila P, Villalobos-Comparan M, Jacobo-Albavera L, Ramirez-Jimenez S, Sikora M, Zhang LH, Pape TD, Granados-Silvestre MD, Montufar-Robles I, Tito-Alvarez AM, Zurita-Salinas C, Bustos-Arriaga J, Cedillo-Barron L, Gomez-Trejo C, Barquera-Lozano R, Vieira-Filho JP, Granados J, Romero-Hidalgo S, Huertas-Vazquez A, Gonzalez-Martin A, Gorostiza A, Bonatto SL, Rodriguez-Cruz M, Wang L, Tusie-Luna T, Aguilar-Salinas CA, Lisker R, Moises RS, Menjivar M, Salzano FM, Knowler WC, Bortolini MC, Hayden MR, Baier LJ, Canizales-Quinteros S. A functional ABCA1 gene variant is associated with low HDL-cholesterol levels and shows evidence of positive selection in Native Americans. Hum Mol Genet
- Wunschmann S, Muller HM, Stipp CS, Hemler ME, Stapleton JT. In vitro interaction between hepatitis C virus (HCV) envelope glycoprotein E2 and serum lipoproteins (LPs) results in enhanced cellular binding of both HCV E2 and LPs. J Infect Dis 2006; 194:1058-1067
- Scarselli E, Ansuini H, Cerino R et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J 2002;21:5017-25
- Yamamoto S, Fukuhara T, Ono C et al. Lipoprotein receptors redundantly participate in entry of hepatitis C virus. PLoS Pathog 2016;12:1005610
- Ujinoa S, Nishitsujia H, Hishikib T, Sugiyamac K, Takakud H, and Shimotohno K. Hepatitis C virus utilizes VLDLR as a novel entry pathway. Proc Natl Acad Sci USA 2016;113:188-93
- Wang C, Gale M, Jr., Keller BC, Huang H, Brown MS, Goldstein JL, Ye J. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol Cell 2005; 18:425-434
- Guthery SL, Pohl JF, Bucuvalas JC, Alonso MH, Ryckman FC, Balistreri WF, Heubi JE. Bone mineral density in long-term survivors following pediatric liver transplantation. Liver Transpl 2003; 9:365-370
- Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol 2008; 82:2120-2129
- Corey KE, Kane E, Munroe C, Barlow LL, Zheng H, Chung RT. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology 2009; 50:1030-1037
- Butt AA, Yan P, Simon TG, Chung RT, Abou-Samra AB; ERCHIVES study team. Changes in circulating lipids level over time after acquiring HCV infection: results from ERCHIVES. BMC Infect Dis 2015;15:510
- Corey KE, Mendez-Navarro J, Barlow LL, Patwardhan V, Zheng H, Kim AY, Lauer GM, Chung RT. Acute hepatitis C infection lowers serum lipid levels. J Viral Hepat 2011; 18:e366-371
- Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009; 49:225-232
- Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging 2012; 32:421-430
- Butt AA, Yan P, Chew KW. Risk of Acute Myocardial Infarction Among Hepatitis C Virus (HCV)-Positive and HCV-Negative Men at Various Lipid Levels: Results From ERCHIVES. Clin Infect Dis. 2017;65:557-65
- Grundy SM et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:1082-1143.
- Butt AA, Yan P, Bonilla H. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: Results from ERCHIVES. Hepatology 2015;62:365-74
- Yang YH, Tsan YT, Chen MJ, Shih WT, Tsai YH, Chen PC. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol 2015;63:1111-7
- Zheng YX, Zhou PC, Zhou RR, Fan XG. The benefit of statins in chronic hepatitis C patients: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017;29:759-66
- Simon TG, KingLY, ZhengH, Chung Statin Use is Associated with a Reduced Risk of Fibrosis Progression in Chronic Hepatitis C. J Hepatol 2015;62:18–23.
- SimonTG, BonillaH, YanP, ChungRT, MD, Butt Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and HCC, among patients with HCV Results from ERCHIVES. Hepatology 2016; 64:47–57.
- Gitto S, Cicero AFG, Loggi E. Worsening of Serum Lipid Profile after Direct Acting Antiviral Treatment. Ann Hepatol 2018;17:64-75
- Satoru Hashimoto, Hiroshi Yatsuhashi, Seigo Abiru et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS One2016;28:11
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012; 30:2212-2219
- Yan H, Peng B, Liu Y, Xu G, He W, Ren B, Jing Z, Sui J, Li W. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol 2014; 88:3273-3284
- Lin YL, Shiao MS, Mettling C, Chou CK. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion. Virology 2003; 314:253-260
- Oehler N, Volz T, Bhadra OD, Kah J, Allweiss L, Giersch K, Bierwolf J, Riecken K, Pollok JM, Lohse AW, Fehse B, Petersen J, Urban S, Lutgehetmann M, Heeren J, Dandri M. Binding of hepatitis B virus to its cellular receptor alters the expression profile of genes of bile acid metabolism. Hepatology 2014; 60:1483-1493
- Hajjou M, Norel R, Carver R, Marion P, Cullen J, Rogler LE, Rogler CE. cDNA microarray analysis of HBV transgenic mouse liver identifies genes in lipid biosynthetic and growth control pathways affected by HBV. J Med Virol 2005; 77:57-65
- Hsu CS, Liu CH, Wang CC, Tseng TC, Liu CJ, Chen CL, Chen PJ, Chen DS, Kao JH. Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat 2012; 19:e48-57
- Huang CY,Lu CW,Liu YL,Chiang CH,Lee LT,Huang KC. Relationship between chronic hepatitis B and metabolic syndrome: A structural equation modeling approach. Obesity (Silver Spring)2016;24:483-9
- Joo EJ,Chang Y,Yeom JS,Cho YK,Ryu S. Chronic hepatitis B virus infection and risk of dyslipidaemia: A cohort study. J Viral Hepat2019;26:162-9
- Chen EQ,Wang ML,Zhang DM. Plasma Apolipoprotein A-V Predicts Long-term Survival in Chronic Hepatitis B Patients with Acute-on-Chronic Liver Failure. Sci Rep2017;7:45576
- Lefkowitch JH, Schiff ER, Davis GL, Perrillo RP, Lindsay K, Bodenheimer HC, Jr., Balart LA, Ortego TJ, Payne J, Dienstag JL, et al. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The Hepatitis Interventional Therapy Group. Gastroenterology 1993; 104:595-603
- Gordon A, McLean CA, Pedersen JS, Bailey MJ, Roberts SK. Hepatic steatosis in chronic hepatitis B and C: predictors, distribution and effect on fibrosis. J Hepatol 2005; 43:38-44
- Huang YW,Lee CL,Yang SS. Statins Reduce the Risk of Cirrhosis and Its Decompensation in Chronic Hepatitis B Patients: A Nationwide Cohort Study. Am J Gastroenterol2016;111:976-85
- Kim RG,Loomba R,Prokop LJ,Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol2017;15:1521-30
- Wong JC,Chan HL,Tse YK,Yip TC,Wong VW,Wong GL. Statins reduce the risk of liver decompensation and death in chronic viral hepatitis: a propensity score weighted landmark analysis. Aliment Pharmacol Ther2017;46:1001-10
- Chang FM,Wang YP,Lang HC,Tsai CF,Hou MC,Lee FY,Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. 2017;66:896-907
- Hsiang JC,Wong GL,Tse YK,Wong VW,Yip TC,Chan HL. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J Hepatol2015;63:1190-7
- Cohen DE, Fisher EA. Lipoprotein metabolism, dyslipidemia, and and nonalcoholic fatty liver disease. Semin Liver Dis 2013; 33:380-388
- Jahn CE, Schaefer EJ, Taam LA, Hoofnagle JH, Lindgren FT, Albers JJ, Jones EA, Brewer HB, Jr. Lipoprotein abnormalities in primary biliary cirrhosis. Association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology 1985; 89:1266-1278
- Cohen DE. Hepatocellular transport and secretion of biliary lipids. Curr Opin Lipidol 1999; 10:295-302
- Hussain I, Ahmad Z, Garg A. Extreme hypercholesterolemia presenting with pseudohyponatremia - a case report and review of the literature. J Clin Lipidol 2015; 9:260-264
- Narayanan S. Biochemistry and clinical relevance of lipoprotein X. Ann Clin Lab Sci 1984; 14:371-374
- Elferink RP, Ottenhoff R, van Marle J, Frijters CM, Smith AJ, Groen AK. Class III P-glycoproteins mediate the formation of lipoprotein X in the mouse. J Clin Invest 1998; 102:1749-1757
- Chang PY, Lu SC, Su TC, Chou SF, Huang WH, Morrisett JD, Chen CH, Liau CS, Lee YT Lipoprotein-X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res 2004; 45:2116-2122
- Hao J,Yang T,Zhou Y. Serum Metabolomics Analysis Reveals a Distinct Metabolic Profile of Patients with Primary Biliary Cholangitis. Sci Rep2017;7:784
- Sorokin A, Brown JL, Thompson PD. Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis 2007; 194:293-299
- Jorgensen RA, Lindor KD, Sartin JS, LaRusso NF, Wiesner RH. Serum lipid and fat-soluble vitamin levels in primary sclerosing cholangitis. J Clin Gastroenterol 1995; 20:215-219
- Doycheva I, Chen C, Pan JJ, Levy C. Asymptomatic primary biliary cirrhosis is not associated with increased frequency of cardiovascular disease. World J Hepatol 2011; 3:93-98
- Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology 2009; 50:291-308
- Cash WJ, O'Neill S, O'Donnell ME, McCance DR, Young IS, McEneny J, McDougall NI, Callender ME. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int 2013; 33:1166-1174
- Ritzel U, Leonhardt U, Nather M, Schafer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol 2002; 36:454-458
- Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Sourij H, Stauber RE, Winkler K, Marz W, Wascher TC, Trauner M. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis 2010; 209:178-183
- Lindor KD, Kowdley KV, Harrison ME. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol 2015; 110:646-659
- Cicognani C, Malavolti M, Morselli-Labate AM, Zamboni L, Sama C, Barbara L. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med 1997; 157:792-796
- Loria P, Marchesini G, Nascimbeni F, Ballestri S, Maurantonio M, Carubbi F, Ratziu V, Lonardo A. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis 2014; 232:99-109
- Trieb M,Horvath A,Birner-Gruenberger R. Liver disease alters high-density lipoprotein composition, metabolism and function.Biochim Biophys Acta2016;1861:630-8
- Hwang SJ, Lee SD, Chang CF, Wu JC, Tsay SH, Lui WY, Chiang JH, Lo KJ. Hypercholesterolaemia in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 1992; 7:491-496
- Sohda T, Iwata K, Hirano G, Sakurai K, Yokoyama K, Morihara D, Takeyama Y, Irie M, Shakado S, Sakisaka S. 3-Hydroxyl-3-methylglutaryl-coenzyme A reductase is up regulated in hepatocellular carcinoma associated with paraneoplastic hypercholesterolemia. Med Mol Morphol 2013; 46:239-242
- Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis 2006; 5:4
- Erickson SK, Cooper AD, Barnard GF, Havel CM, Watson JA, Feingold KR, Moser AH, Hughes-Fulford M, Siperstein MD. Regulation of cholesterol metabolism in a slow-growing hepatoma in vivo. Biochim Biophys Acta 1988; 960:131-138
- Feingold KR, Wiley MH, Moser AH, Siperstein MD. Altered activation state of hydroxymethylglutaryl-coenzyme A reductase in liver tumors. Arch Biochem Biophys 1983; 226:231-241
- Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol 2000; 32:209-217
- Habib A, Mihas AA, Abou-Assi SG, Williams LM, Gavis E, Pandak WM, Heuman DM. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin Gastroenterol Hepatol 2005; 3:286-291
