ABSTRACT
Gastrinomas are neuroendocrine neoplasms (NENs), that occur primarily in the duodenum and pancreas, which ectopically secrete gastrin, resulting in the Zollinger-Ellison syndrome (ZES), which is due to marked hypersecretion of gastric acid causing severe gastro-esophageal peptic disease. ZES patients have two management problems that must be dealt with: control of the acid hypersecretion and control of the gastrinoma, which is malignant in 60-90% of cases. Most gastrinomas are sporadic, but 20-25% of patients have it as part of the Multiple Endocrine Neoplasia-type 1 syndrome (MEN1), an autosomal dominant disorder characterized by endocrine tumors/hyperplasia of multiple endocrine organs (parathyroid> pancreatic islets>pituitary>adrenal). It is important to identify those with ZES/MEN1 as their management differs from those with sporadic disease. Acid hypersecretion is now controlled medically both acutely and long term, with proton pump inhibitors (PPI) the drugs of choice. In patients with sporadic ZES, after detailed imaging with cross-sectional imaging and somatostatin receptor imaging (SRI), resection of the gastrinomas should be considered whenever possible, with cures reported in 20-45% of patients. The role of surgical resection of the gastrinomas in MEN1/ZES is controversial and it is generally recommended it be reserved for patients with tumors>1.5/2 cm because of the multiplicity of small gastrinomas resulting in very low cure rates. The diagnosis of ZES requires demonstrating fasting hypergastrinemia in the presence of inappropriate acid secretion (pH<2), however, because of the widespread use of PPIs and the lack of gastric acid testing, the diagnosis of ZES is becoming more difficult and referral to a specialty group is frequently required. Patients with advanced metastatic disease are treated as other patients with advanced NENs including with somatostatin analogues, chemotherapy, everolimus, sunitinib, liver directed therapies, and peptide radio-receptor therapy (PRRT) with radiolabeled somatostatin analogues.
GENERAL/DEFINITIONS
ZES was first described in 1955 by two surgeons, RM Zollinger and EH Ellison, in two patients with intractable peptic ulcer disease (1). Although previous cases had been described (2,3), including one well described case by Roar Strom in 1952 (3-5), in Zollinger/Ellison’s two patients the authors were the first to propose the important association between the gastric hypersecretion and the presence of a pancreatic neuroendocrine neoplasm (PNEN) (1-3,6). Presently, the term gastrinoma and ZES are often used synonymous, however, in the past the term gastrinoma was also used to refer to a neoplasm synthesizing gastrin and ZES to the clinical manifestations (7). Numerous NENs and non-NENs can synthesize gastrin precursors which are not processed to the biologically active gastrin-17 or gastrin-34 as in ZES, and thus are generally not called gastrinomas by clinicians or in most current classification systems of pNEN (7-9). In addition to being well-described in humans, Zollinger-Ellison syndrome due to a gastrinoma have also been reported in dogs (10-17), cats (10,18-22), and a Mexican gray wolf (23).
Like most other functional pNEN syndromes (F-pNEN) (insulinomas, glucagonomas, VIPomas, etc.), in ZES the functional syndrome due to the ectopic hormone secretion requires immediate treatment because it was the most frequent cause of morbidity/death prior to effective treatments (24-38). In addition, treatment must be directed at the gastrinomas itself, because similar to all other pNEN, except insulinomas, the majority (60-90%) are malignant (9,26,27,39-42). Whereas effective surgical resection would cure both problems, in <50% of ZES patients is curative resection possible because of advanced disease or the patients have MEN1/ZES, which can only be cured with Whipple resections, which are not generally recommended (discussed below) (6,9,43-49). Therefore, treatment of patients with ZES requires management of two different treatment problems: the acid hypersecretion and the malignant nature of the gastrinoma.
This chapter will review important aspects of the management of patients with ZES and important treatment issues at present, including the most recent studies up to 2023. It will concentrate on the most current important aspects and not cover comprehensively all areas of ZES or numerous areas in depth. For more in depth considerations the reader is referred to recent papers/reviews which cover ZES generally (6,33,38,40,46,49-54); its diagnosis (29,51,55-61), clinical features (24,25,41,62-69); acid hypersecretion (24,50,70-72); gastrin provocative testing and the diagnosis of hypergastrinemia (36,50,51,55,57,73-86); MEN1/ZES (30,44,47,57,64,85-96,96-102); medical treatment of acid hypersecretion (50,51,69,72,78,80,103-108);clinical course and prognosis (41,65,87,93,109-117); surgical treatment of the gastrinoma (6,44,50-52,80,92,95,96,99,100,102,103,118-128); imaging and tumor localization (37,50,90,112,124,125,129-141); treatment of advanced disease in ZES and other NENs (42,48,50,51,58,135,142-156); diagnosis and treatment of all/functional pNEN (24-26,34-36,36,48,48-50,54,58,148,156-165) and pathology, pathogenesis and classification of gastrinomas/NENs (9,50,58,86,96,117,158,166-174).
Before considering the diagnosis and management of ZES in more detail it is important to realize that there are a number of misconceptions about ZES, often because of comparison with other pNEN and these need to be kept in mind. They are listed in the Table 1 below and briefly discussed in the following sections.
| Table 1. Widely Held Misconceptions About ZES |
|
1) Gastrinomas, similar to a number of other pNEN (insulinomas, gastrinomas, PPomas), primarily occur in the pancreas. FACT: In recent studies, 60-100% of gastrinomas in both sporadic ZES and MEN1/ZES occur in the duodenum, with only 0-15% in the pancreas (6,43,50,95,102,127,175-179) (Table 2).
|
|
2) MEN1 is uncommon in ZES, similar to other pNEN such as insulinomas (3-5%), glucagonomas (<5%), PPomas/nonfunctional pNEN (<3%). FACT: MEN1 is found in the highest frequency of all pNEN syndromes in ZES patients occurring in 20-25% and is important to diagnose because of its different treatment aspects (30,50,64,72,87,89,95,102).
|
|
3) With the increased awareness of ZES and widespread availability of gastrin assays and sensitive imaging modalities, similar to some other pNEN, gastrinomas are being diagnosed earlier. FACT: The time of onset of symptoms to diagnosis of ZES remains 4-7 years (24,26,48,60,62,89,134) and a number of factors are contributing to make the diagnosis even more difficult (See point #4 below).
|
|
4) As recommended in all guidelines (9,72,80,152,157,180-182), similar to other functional pNEN syndromes (F-pNENs), ZES is currently diagnosed by demonstrating excess hormone production (fasting hypergastrinemia) in the presence of an unphysiological effect of the hormone hypersecretion (i.e., inappropriate acid hypersecretion (elevated basal acid output>15 mEq/hr., pH<2)) (9,50,51,55,56,59,70,72,73,79,181,183,184). FACT: In contrast to, for example, insulinomas, which are uniformly diagnosed by demonstrating fasting hyperinsulinemia with accompanying hypoglycemia (frequently during a fasting study) (29,50,185-188), in a recent review of the last 20 cases of ZES reported in the literature in 2018 (55), 95% of the diagnoses were reported without performing a gastric analysis or gastric pH assessment (55) and thus not using classical established criteria. This approach has complicated the diagnosis of ZES and the factors leading to this confusion will be discussed below in detail in the ZES diagnosis section.
|
|
5) In MEN1 patients, similar to other MEN1 patients with F-pNEN such as insulinomas and glucagonomas, most gastrinomas can be cured by nonaggressive surgical resections in MEN1/ZES patients. FACT: In contrast to other F-pNEN (29,157,189), the 5-year surgical cure rate of MEN1/ZES is <5% (6,30,43,44,88,190) without aggressive surgical resections such as Whipple resection, which are not recommended (6,9,88,92,93,118,123,157,180,182). However, without these resections, most patients with small tumors and adequate acid secretory control have an excellent prognosis, which has led to controversy in their treatment, and will be discussed in the surgical section later (30,43,47,92,93,95,102,118,157,180,182,191).
|
The misconceptions listed in Table 1 above as well as the factors specific to ZES that led to these misconceptions have led to controversies that are complicating numerous aspects of the management of ZES patients. These extend particularly to the current diagnosis of ZES, the management of both gastrinomas and nonfunctional pNENs in MEN1/ZES patients, and various aspects of the surgical management of these patients. Each of these will be discussed in more detail in the specific later sections in this chapter.
EPIDEMIOLOGY: ZES
PNEN account in different series for 1-10% of all pancreatic tumors with a prevalence of 1/100,000 and annual incidence of 1-4/million, which is increasing in frequency (192-194). In older series, insulinomas, gastrinomas, and NF-pNEN were reported with similar frequencies, however, in recent series of pNEN patients NF-pNEN make up 60-80% of all cases (24,48). Currently, for F-pNENs, insulinomas and gastrinomas are the most frequent, with incidences of 0.5-3/million in different series (26,50). Generally, insulinomas/gastrinomas are 8-10-fold more frequent than VIPomas, 17-fold more than glucagonomas, and >20 fold more the other F-pNENs (GRFomas, pancreatic ACTHomas, etc.) (26,50). Gastrinomas are the most frequent malignant F-pNEN, because 60-90% are malignant, like the other less common F-PNEN, in contrast to insulinomas, which are malignant in only 5-10% in most series (26,50,158,188).
Gastrinoma, as well as other pNENs, can occur both sporadically or as part of an inherited syndrome (30,158,195-197). Gastrinomas occur more frequently with an associated inherited pNEN syndrome than other F-pNEN, particularly in the case of MEN1, where 20-25% of all ZES patients have MEN1/ZES, compared to <3-5% of other F-pNEN syndromes (30,50,65,86,87,89). ZES is also rarely reported in other inherited syndromes associated with pNEN including the autosomal dominant syndromes, von Hippel –Lindau Disease (30,196,198,199), tuberous sclerosis (30,200), and neurofibromatosis type 1 and type 2 (30,199,201-204).
PATHOPHYSIOLOGY: CLINICAL FEATURES
In the majority of patients with ZES (>90%), the presenting symptoms are due to the marked gastric acid hypersecretion (24,28,62,64,70,205,206). Generally, only in patients with advanced disease late in the disease course are the prominent symptoms due to the tumor per se (abdominal pain, weight loss, anorexia, etc.) (24,28,40,62,205,206). The acid dependency of the above symptoms is shown by numerous studies reporting in a typical ZES patient, all of the presenting symptoms (including the PUD, pain, diarrhea, GERD symptoms, weight loss) disappear if the gastric acid hypersecretion is adequately controlled by any means (surgical, medical, acid aspiration) (7,27,28,40,103,106,207).
The ectopic release of gastrin by the gastrinoma is the direct cause of the gastric hypersecretion (49,170,208). In a typical ZES patient the fasting hypergastrinemia results in a markedly increased basal acid output (BAO) of approximately 4-fold (42-mEq/hr.) (70) and in some patients the BAO is increased more than >10-fold (27,28,70,206,209-212). Chronic hypergastrinemia also has trophic effects on the gastric mucosa, stimulating an increase in number of parietal cells and gastric enterochromaffin-like cells (ECL cells) (7,76,213-217) with the result the parietal cell mass is increased up to 4-6-times normal (27,76,218,219). This contributes to both the elevated BAO and increased maximal capacity to secrete acid, as shown by ZES patients having increased maximal acid outputs (MAOs) (27,70,76,212,219-221). Diarrhea which is seen in >70% of ZES patients (Table 3) in recent prospective studies is due to the effects of the gastric acid hypersecretion by causing structural damage to the small intestine, it interferes with fat transport; inactivates pancreatic lipase; can precipitate bile acids; and if prolonged, leads to steatorrhea (27,158,222).
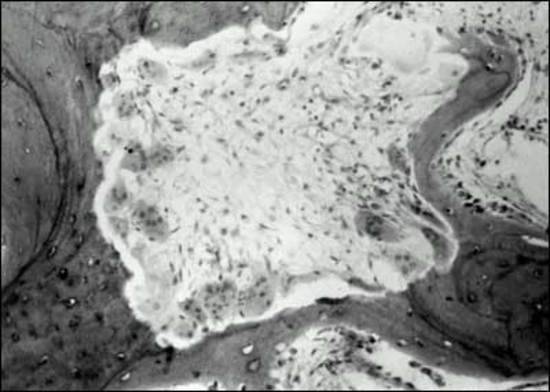
Long-standing hypergastrinemia stimulates proliferation of the gastric enterochromaffin-like cells (ECL cells), which show such a response in ZES-patients (223). Gastric ECL cells are increased a mean of twofold in ZES (76,212,223-225). ZES patients can develop advanced ECL-proliferative responses, similar to the findings in animal studies of chronic hypergastrinemia induced by various methods, and which, in some cases, results in neoplastic changes (7,76,213,217,226,227). It has been proposed that with chronic hypergastrinemia, the ECL cells undergo a progressive hyperplasia-neoplasia sequence of events beginning with simple hyperplasia, followed by linear hyperplasia, micronodular hyperplasia, adenomatoid hyperplasia, dysplasia (pre-carcinoid) and finally the development of carcinoids (7,76,217,226,228). In the prospective NIH studies greater than 98% of ZES patients demonstrated ECL hyperplasia (217,227), with 50% having advanced changes with sporadic ZES (7% dysplasia) (217) and 53% with MEN1/ZES (2%-dysplasia) (227). In ZES, there is a close correlation between the degree of ECL hyperplasia and the fasting serum gastrin level (76,217,227). Even though advanced ECL proliferative changes are seen in both sporadic and MEN1/ZES-patients, they have a marked difference in the rate of occurrence of gastric carcinoids. Gastric carcinoids occur in 0-33% of MEN1/ZES-patients (76,227), and in the one perspective NIH study were found in 23% (87,224,227,229-231). However, gastric carcinoids rarely occur (<1%) in sporadic-ZES patients (212,217,232-234), and it has been estimated they occur at least with 70-fold greater frequency in MEN1/ZES-patients (227). An important finding of the prospective NIH studies of ZES patients is there was no threshold effect of fasting gastrin on ECL growth, as had been previously proposed, with any increase in FSG being associated with increased ECL proliferation (76,217,227).
PATHOLOGY AND TUMOR CLASSFICATION
In the past, gastrinomas were frequently reported as nonbeta islet cell tumors (1), because they were originally thought to originate in the pancreas from the islets and to generally be pancreatic in location, similar to insulinomas (1,51,185,205,235,236). They were reported to occur in the pancreas with a distribution of pancreatic head: body: tail of 4:1:4 (27,40,63,205,237,238). Later studies described a small percentage of duodenal gastrinomas (239,240). Currently, duodenal gastrinomas are found 2-10 times more frequently than pancreatic (Table 2) (43,64,95,102,175-177,241-245). Therefore, prior to the mid-1980s, 80-95% of gastrinomas were reported in the pancreas, whereas now 45-100% are duodenal, and 0-45% pancreatic (40,175-177,236,241-244). Even as late as 1998, in Soga’s review of 359 cases of ZES, only 11% of the patients had a duodenal gastrinoma (7,27,43,63,235).This likely occurred because of the analogies to insulinomas which are almost always in the pancreas, as well as the fact that duodenal tumors were being missed on preoperative localization studies or with a standard laparotomy because of their small size (Table 2) (27,43,175-177,241) and in many series no gastrinoma was found in a significant percentage of patients (7,27,28,40,63,235). Furthermore, a number of the early cases were patients with MEN1/ZES, and intra-pancreatic tumors were found (which were generally NF-pNEN) and these were attributed to be the source of the gastrin, with the true source being in a duodenal gastrinoma, which was not explored for or detected. Recent studies show that when careful attention is paid to the duodenum at surgery (duodenotomy, intraduodenal palpation, transillumination on occasion), more duodenal tumors were found (6,45,95,102,175,176,241-244,246-248). Primary gastrinomas are rarely found in other intra-abdominal sites: (particularly the ovary and liver/bile duct, as well as very uncommonly in the pylorus, spleen, mesentery, stomach, kidney) and in a few cases(<5 total) (<0.5%) in extra-abdominal locations, including the cardiac intraventricular spectrum and due to nonsmall cell lung cancer (Table 2) (40,45,109,110,121,126,176,236,249-265). A number of studies provide strong evidence that gastrinomas can arise in lymph nodes as the primary site, however, this is not universally accepted and some have proposed that they represent metastases from occult primaries (27,40,43,258,259,266-274). The possibility that a lymph node primary tumor may occur is supported by studies demonstrating long-term cure after resection of only a lymph node gastrinoma (40,258,259,267). Furthermore, in 3-25% of patients without pNEN, chromogranin-positive rests occur in abdominal lymph-nodes (266,275). In the NIH prospective series, 11% of patients are classified as having primary lymph node gastrinomas (Table 2).
At surgery, it has been recently emphasized that 60-90% of gastrinomas occur within the “gastrinoma-triangle”, which is an area formed by the junction of the cystic/common bile ducts posteriorly, the junction of the second/third parts of the duodenum inferiorly, and the junction of the pancreatic neck/body medially (40,176,177,244,276). This occurs primarily because of the high frequency of duodenal gastrinomas which are now found that fall into this area. Duodenal gastrinomas do not occur in equal proportion in all parts of the duodenum, but instead demonstrate a decreasing occurrence distally, with almost 90% of duodenal gastrinomas occurring in the 1st/2nd part of the duodenum (Table 2) (175,277,278).
In early studies, 60-90% of gastrinomas were associated with metastases (primarily lymph-node/liver) and therefore they should all be considered potentially malignant (9,39,205,236,257,279). The presence of metastases or gross invasion of normal tissue remains the only generally accepted criterion for the diagnosis of malignancy (27,40,280). Gastrinomas metastasize initially primarily to regional lymph nodes and the liver (27,109,236). Duodenal gastrinomas are characteristically small in size (Table 2), frequently <1 cm in diameter; however, they are associated with lymph node metastases in 47% of the cases in the NIH prospective studies (20-80%-literature), which is a similar percentage seen with the larger pancreatic gastrinomas (mean size 3.8 cm) (Table 2). From this data it has been proposed that gastrinomas in these two sites are equally malignant (109,110,175,281). However, from the NIH prospective studies it is also proposed that duodenal and pancreatic gastrinomas are not equally aggressive, because liver metastases occur in 52% of the NIH patients with a pancreatic gastrinoma (15-45%-literature) (Table 2), whereas liver metastases occur in only 5% of duodenal gastrinomas (10%-literature) (Table 2) (109,110,281). This is a similar rate to a recent collective series of 24 ZES cases with a duodenal gastrinoma in which 4 of the patients (16%) had liver metastases but 75% had lymph node metastases (282).Similarly in a recent review of 52 ZES patients(33-sporadic/19-MEN1/ZES) the rate of liver metastases was significantly lower in those with MEN1/ZES (21% vs 51%, p=0.031) (64). At presence the basis for this difference in aggressive behavior of pancreatic and duodenal gastrinomas is unclear. A genomic analysis (172) identified a number of molecular similarities and differences between duodenal gastrinomas and pNENs. In a comparison of RNA-seq data, duodenal gastrinomas and pancreatic pNENs shared 1233 common co-expressed transcripts, however duodenal gastrinomas expressed 909 distinct transcripts not seen in either normal duodenum or pancreatic pNENs and pancreatic pNENs had 588 unique transcripts not shared in normal pancreas or duodenal gastrinomas (172).The duodenal gastrinomas strongly expressed two inflammatory mediators (IL-17 and TGF-alpha), enrichment of mesenchymal, cytoskeletal, neuroactive-ligand receptor interaction, and calcium signaling pathway genes (64). In both duodenal and pancreatic neuroendocrine tumors alterations in expression of genes were found that were involved in cellular signaling cascades as well as in associated immune cells, and presence of proinflammatory cytokines, however it is unclear how these are related to the differences in biologic behavior of these two groups of NENs.
Duodenal gastrinomas in sporadic cases (75-80%) differ from those in MEN1/ZES patients in that they are usually solitary tumors, whereas in MEN1/ZES they are multicentric, smaller and multiple (64,178,283,284).
Duodenal gastrinomas account for 44-66% of all duodenal NENs (285,286), however only 58 % are associated with the development of ZES (286). In a recent study (286) the characteristics of sporadic duodenal gastrinomas associated with ZES (n=24) or not associated with ZES (n=17) were compared. The duodenal gastrinomas associated with ZES had a higher mean Ki-67(1.74 vs 0.85, p=0.012), more frequently had associated lymph node metastases (75 vs 6%, p=0.012), more frequently were associated with liver metastases and presented more frequently with TNM stage ≥III (75 vs 6%, p<0.0010). In a recent collective study of 108 sporadic ZES patients (127) in which 68 had duodenal gastrinomas and 19 pancreatic tumors, the overall 5-yr survival was 94% and not affected by gastrinoma location. However, pancreatic location was associated with higher recurrence rate (p=0.0001) (127).
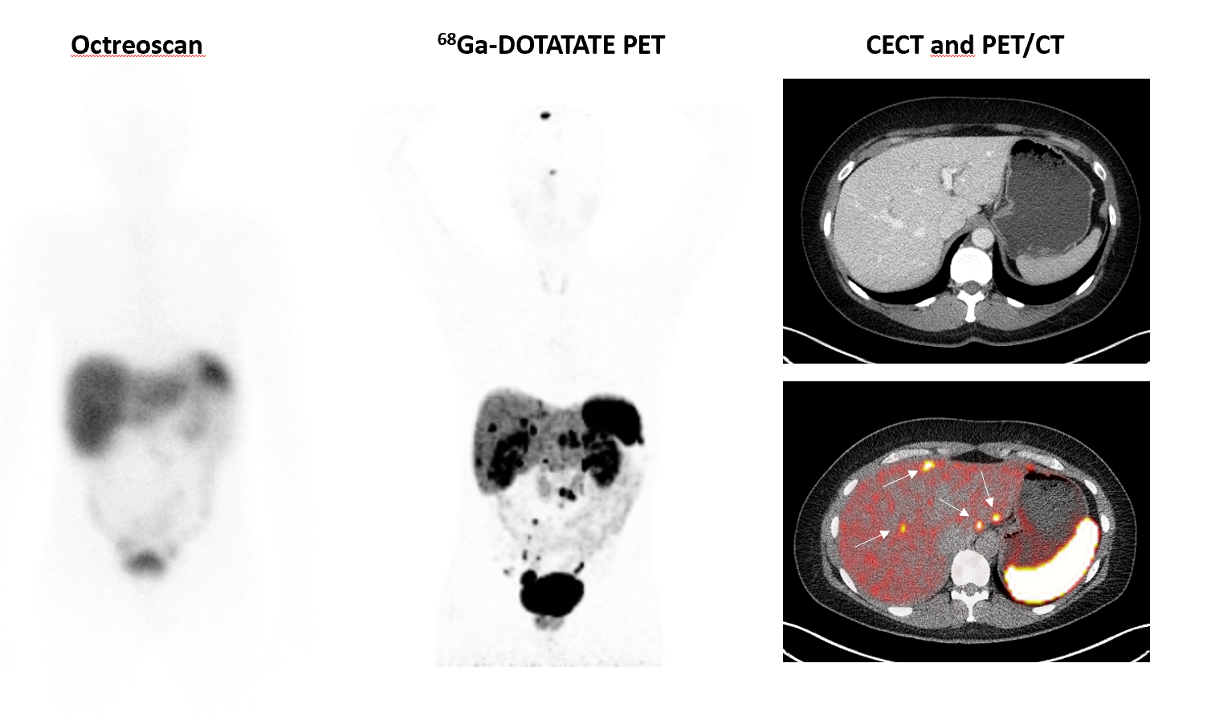
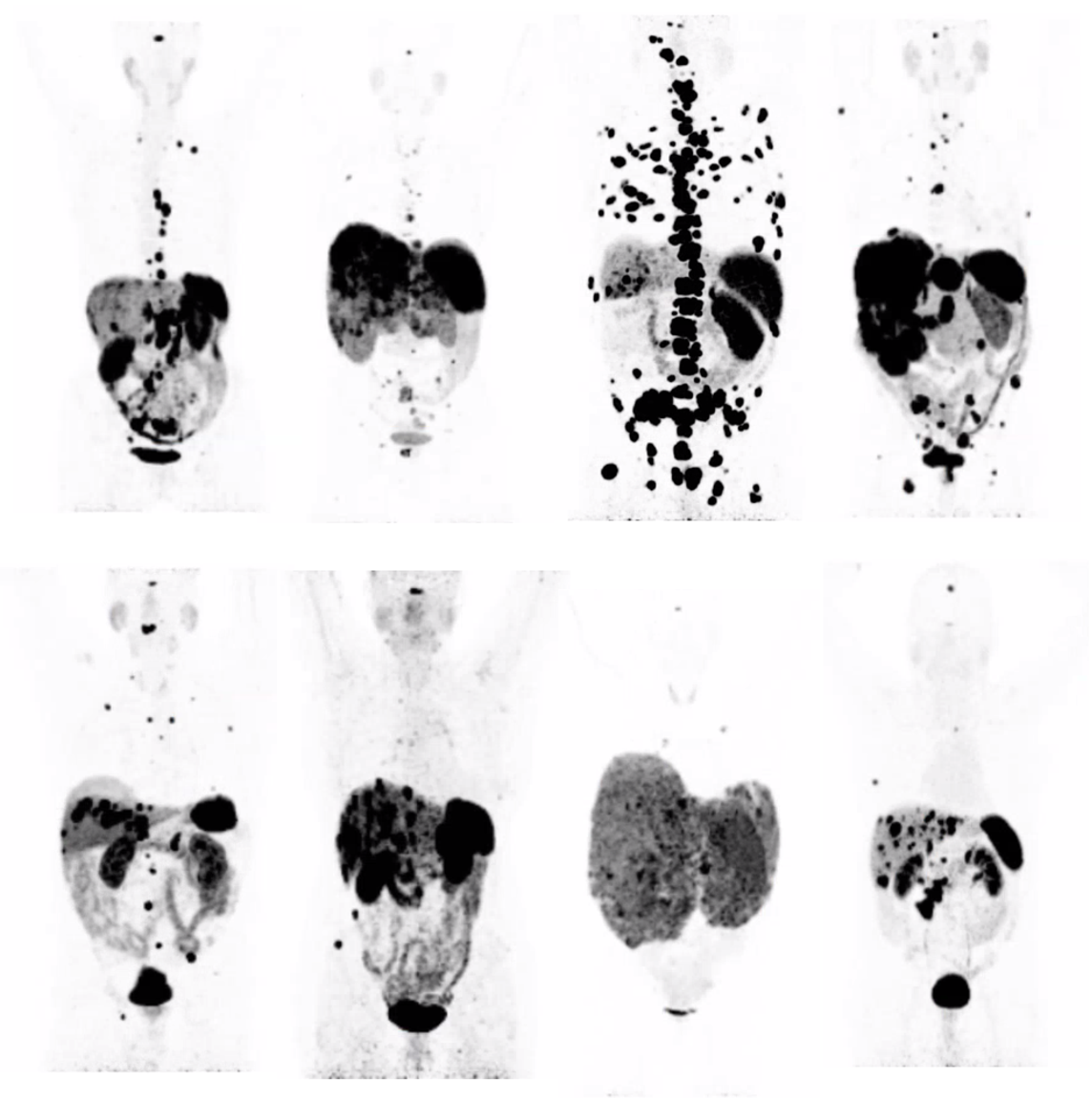
In the past literature, approximately one-third of ZES patients presented with metastatic liver disease, approximately one-third with no tumor found and one-third with localized disease (Table 2) (27,40). Some recent studies suggest an increasing proportion are being seen with earlier disease stages, without advanced disease (40) (Table 2). For example, in the last 221 patients seen at the NIH, the majority (65%) at presentation had localized disease, and in the remaining 35% of the patients, they were divided between those with hepatic metastases and those with no primary tumor found (40,109,110) (Table 2). This distribution of gastrinoma extent differs from that reported in various surgical series, because not all ZES patients are included in these series with exclusion of all non-operated patients including those with patients with diffuse liver metastases, most with MEN1/ZES and those with contra-indications to surgery (9,43,181,287,288). In the last 155 patients undergoing surgical exploration at NIH, 85% had limited disease and the remaining 15% either had limited hepatic metastases (8%) or no tumor was found (7%) (40). In older studies, up to 50% of patients had no tumor found (Table 2), whereas at present, gastrinomas are more frequently found, as evidenced by the recent NIH data in which in the last 81 patients explored for possible cure at NIH, a gastrinoma was found in all (43). As pointed out above this difference is almost entirely due to the careful exploration of the duodenal area with a Kocher maneuver, duodenotomy, intraluminal palpation, and transillumination, (43,63,175,176,244,247,289). It is likely the detection rate of primary gastrinomas will increase even further with the recent development and widespread use of somatostatin receptor imaging (SRI), which has superior sensitivity to conventional cross-sectional imaging (129,133,134,136,137,139,153). SRI was initially performed with 111Indium (diethylenediamine penta-acetic-D-phenylalanine-1) octreotide with single photon emission CT (SPECT) detection, but has now been replaced by 68Gallium DOTA (9,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid) labeled somatostatin analogues (generally 68Ga-DOTATOC PET/CT) with positron-emission tomography detection because of its even greater sensitivity (129,134-137,139,153,290-293).
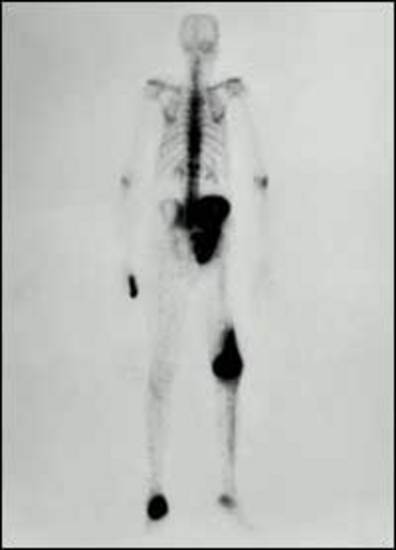
Distant, extrahepatic, metastases can occur with advanced gastrinomas (112,294-299). Metastases to bone are reported in 31% of ZES patients with advanced disease which occur primarily in the axial skeleton initially, however, they are uncommonly seen in ZES patients that do not have liver metastases (112,294,297,300). Their identification is important, because their detection frequently alters management (109,112,294,296,298,299).
Histologically, gastrinomas show the typical features of NENs, with cubical cells generally with few mitoses and having a granular, eosinophilic cytoplasm (236,280). They can demonstrate trabecular, gyriform or glandular morphology; however, no specific pattern is predictive of biologic behavior (27,235,280). Duodenal gastrinomas occur in the submucosa, frequently infiltrate the mucosa and in the case of tumors >1 cm, the muscular layer (236). Duodenal gastrinomas usually have proliferative rates <10%, whereas pancreatic gastrinomas frequently have higher proliferative rates (236,286). Both duodenal and pancreatic gastrinomas may demonstrate blood vessel invasion (236,280). Gastrinomas are usually identified as a NEN by their histological appearance and positivity with immunohistochemistry for the NEN markers (chromogranin A, synaptophysin) (27,236,280,301). Gastrin immunoreactivity (Gastrin-IR) can be detected in most gastrinomas (27,236,302,303) and approximately one-half produce multiple hormones (27,236,302,303).
Recently, it has been proposed that gastrinomas, as well as all pNEN/GI-NENs (carcinoid tumors), should have a common classification as NENs (166,168,304-306). Several classification systems (International Union for Cancer Control/American Joint Cancer Committee (UICC/AJCC), World Health Organization (WHO), European Neuroendocrine tumor Society (ENENs)) for both staging and grading NENs have been proposed recently, validated for pNEN, GI-NENs (carcinoids) and NENs (carcinoids) in other locations and recently updated (158,166,168,306-308). The use of these classification systems is essential to the management of NEN patients because they not only have overall prognostic significance, they also have predictive value for different treatment approaches and thus can dictate the treatment approach in some cases (115,115,116,116,158,166,167,306,308). These classification systems use primarily tumor size, extent, differentiation of the tumor and invasion for determination of stage (306). The grade of the tumor is determined by evaluating proliferative indices (Ki-67 and mitotic index (MI)) and the degree of differentiation of the tumor (well vs poor). NENs are divided into three grades based on the proliferative indices with Grade 1(G1) or low grade, having a Ki67<3%(MI <2 mitoses/10-HPF; Grade 2(G2) or intermediate grade having a Ki67>3-20%(MI-2-20/10 HPF), and high grade or Grade 3(G3) having a Ki67>20%(MI>20 mitosis/10 HPF) (115,116,158,166,167,306-309). Recently (WHO2017, 2019) Grade 3 was divided into two different groups depending on tumor differentiation with G3NEN having well differentiated tumor cells, and G3NEC (neuroendocrine cancer) having poorly differentiated tumor cells (115,116,158,166,167,306,308,310). Recent studies show G3NENs and G3NECs not only vary markedly in survival, but they also vary in their molecular pathogenesis and their treatment approaches (115,116,158,166,167,306,310,311). Proper classification of gastrinomas is essential, because recent studies demonstrate it has prognostic value and may affect the type of treatment recommended (115,116,304-306,312). Most gastrinomas are well-differentiated, pNEN Grade 1 or grade 2 (38,64,236,286). In one recent retrospective cohort study (64) (n=52), the grades of gastrinomas in patients with MEN1/ZES differed from those with sporadic ZES in having lower grade (G1: 83 % vs 39%; G2 (11% vs 54%) G3: (5.6% vs 6.1%), as well as being smaller in size (1.7 cm vs 3.1 cm). A review of 171 gastrinomas in various papers published up to 10/2020 in which tumor grade was reported, shows that 74% of the gastrinomas were grade 1, 22% were Grade 2 and only 4% were Grade 3(313). There is limited data on the correlation of tumor grade in ZES patients with survival. In one study (65)on univariate analysis in MEN1/ZES and sporadic ZES patients(n=37) the presence of grade 3 gastrinomas correlated with decreased survival (p=0.008), however not on multivariate analysis. In a recent review (127) of 108 patients with sporadic ZES, no predictive factors for survival, including tumor grade, were identified, however, for recurrence post- surgical resection, only tumor size (p=0.005) and tumor grade (p=0.01) were independent predictors of tumor recurrence. Two recent analyses (115,116) of prognostic factors in patients with any pNEN demonstrate that grade of the tumor was the most frequent significant prognostic factor cited in the studies analyzed both for overall survival and for disease free survival post-surgery (116) and in treatment of advanced resistant disease (115). These data would strongly suggest that the tumor grade of the gastrinoma in ZES patients will likely be a very important prognostic factor for assessing various aspects of long-term tumor behavior (survival/recurrence/aggressive growth).
|
Table 2. Characteristics of Gastrinomas (NIH Prospective Studies and Literature)
|
|
Characteristic
|
NIH Data (n=221)
Mean (range) Percent
|
Literature
Mean (range) Percent
|
|
Primary Location
Pancreas
Duodenum
Lymph node
Other (1)
Unknown
|
24
49
11
9
16
|
42 (0 – 70%)
15 (0 – 100%)
<1%
2 (0 – 18%)
30 (7 – 48%)
|
|
Duodenal Location
D-1
D-2
D-3
D-4
|
57
32
6
3
|
ND
ND
ND
ND
|
|
Percent Extent of Disease
No tumor found
Localized disease
Metastatic disease to liver
|
13
70
17
|
30 (7 – 50%)
36 (23 – 52%)
34 (13 – 54%)
|
|
Extent Metastases
Primary only
Primary + lymph nodes
Primary + liver metastases
Liver metastases only
Lymph node metastases only
|
36
29
23
3
16
|
32 (23 – 50%)
23 (8 – 61%)
32 (15 – 40%)
10 (4 – 15%)
11 (4 – 24)
|
|
Gastrinoma Size (cm)
Mean (largest)
Duodenal
Pancreatic
|
2 ± 0.2(0.1-4.8)
0.9 ±0.1(0.1-5)
4 ± 0.3 (0.5-7)
|
(1-6)
(0.2-5.5)
(0.5-10)
|
|
Metastases: Duo vs Pancreatic
Lymph node Metastases (%)
Duodenal
Pancreas
Liver Metastases (%)
Duodenal
Pancreas
|
47
48
5
52
|
(20-80%)
(up to 48% of patients had no primary 0-60%)
10
(15-45%)
|
Abbreviations: Duo-duodenal; D1-4-duodenal regions, 1,2,3,4;
Data are from (2,109,110,175,177,178,243,244,246,281,314-317).
(1) Other tumor locations include additional intra-abdominal sites (liver, bile duct, spleen pylorus, mesentery, ovary, lymph nodes) and very rarely extra-abdominal sites (heart, nonsmall cell lung cancer.
Tumors in a given patient in multiple locations can be monoclonal or polyclonal. In MEN1, multiple gastrinomas were reported to arise by independent clonal events in one study (318). A more recent study (114) which include 137 microscopic and macroscopic duodeno-pancreatic NENs and 36 matched metastases in 10 patients with MEN1 assessed tumoral ARX, PDX1, Ki67, gastrin expression and alternative lengthening of telomere. Most metastases (91%) originated from a single NET of origin, however, a few patients had likely multiple, metastatic primary NETS. In 6 patients with hypergastrinemia with MEN1, periduodeno-pancreatic lymph node metastases expressed gastrin and clustered with minute duodenal gastrinomas, not with larger pNEN. The pNEN frequently clustered with high grade or alternative lengthening of telomere positive primary tumors. It was concluded that in MEN-1 patients with ZES and pNEN a duodenal origin of the periduodeno-pancreatic lymph node metastases is likely even if preoperative localization studies do not reveal a duodenal tumor (114). Clonality (319) was analyzed in 20 sporadic gastrinomas from eight patients in whom the tumor was present in at least two separate sites. A combination of methods was used to assess clonality, including MEN1 gene mutation analysis, loss of heterozygosity analysis of the MEN1 locus, and analysis of X-chromosome inactivation at the human androgen receptor locus (human androgen receptor analysis). In three patients, a somatic MEN1 gene mutation was detected in the tumor. Identical mutations were found in other tumors at different sites within the same patients. Human androgen receptor analysis in three informative patients and loss of heterozygosity analysis in five patients revealed identical clonal patterns in the tumors from multiple sites in each patient. This study (319) concluded that sporadic gastrinomas at multiple sites are monoclonal and that MEN1 gene alterations in gastrinomas occur before the development of tumor metastases.
TUMOR BIOLOGY
Similar to other NENs, gastrinomas frequently synthesize (pancreatic polypeptide, insulin, glucagon, somatostatin) and also secrete multiple, gastrointestinal peptides as well as chromogranins, alpha-subunits of the glycoprotein hormones, and neuron-specific enolase (27,280,303,320-322). In one study (303) plasma levels of hormones other than gastrin are elevated in 62% of ZES-patients, with one additional hormone elevated in 44% and two in 18%. Motilin is the most common plasma hormone also elevated (30%), followed by human pancreatic polypeptide (27%), neurotensin (20%) and gastrin-releasing peptide (10%) (303). The occurrence of a second F-pNEN syndrome does occur in ZES patients (27,303,323,324) with cases of concomitant ZES and insulinoma (87,303,324-331),GRFomas (326,332,333), ectopic Cushing’s syndrome (66-68,113,325,334-345), glucagonomas (87,324,328,342,343,346-348), VIPoma (324,325), somatostatinomas (339,349), carcinoid syndrome (87,325,327,343,350) and PTHrPomas (351) all described. Even though secondary F-pNEN syndromes have been described in ZES, in general they are relatively infrequent, except for the development of Cushing’s syndrome in patients with advanced metastatic gastrinomas (66,68,109,113,325,336). In a prospective study from NIH of 45 ZES patients with a mean follow-up of 146 mos. from ZES, only one patient (2%) developed a second F-pNEN syndrome onset for a rate of 0.16%/yr (1% of patients every 6 yrs. of follow-up). This rate was considerably less than that reported in another study (352) of 353 patients with all pNEN(169=gastrinomas) in which 6.8% of all patients developed a secondary pNEN syndrome over a 19-mo. mean follow-up(rate=4.3%/yr.). Ectopic Cushing's syndrome has been more frequently reported in patients with ZES (27,113,334,336,345,353) as well as other pancreatic endocrine tumors (353-356). In a prospective study from the NIH (109) ectopic Cushing's syndrome developed in 4% of all patients with ZES studied (9/212), 17% (9/54) with liver metastases, 21% (7/33) dying of ZES-related causes and 25% (5/20) with bone metastases. It was an independent predictor of poor survival (p <0.005) with patients having a 10-year survival of 0%. Ectopic Cushing's syndrome only developed in patients with metastatic liver disease. Similar to bone metastases, development of ectopic Cushing's syndrome was a strong predictor of poor prognosis with patients only surviving a mean of 1.7+0.4 years after its onset (109).
The gastrin-gene covers a 4 kilobase area and consists of 3 exons and 2 introns, with the coding region translating into a 101-amino acid peptide, pre-progastrin (7,7,8,170,357,358). In normal antral G-cells, pre-progastrin undergoes a number of post-translational processing steps including dibasic cleavages, removal of the glycine extended COOH-terminal amino acids and sulfation, leading to the formation of progastrin, then COOH-terminal glycine-extended forms and finally the biologically active forms consisting of 2 COOH-amidated gastrins, gastrin-17 (G-17) and gastrin-34 (G-34), existing in sulfated and non-sulfated forms (7,8,170,357,358). Normally, >90% of antral gastrin is G-17, while in the duodenum only 40-50% is G-17(7,8,357,358). In the circulation, normal G34 is the predominant form (>60%) and sulfated/non-sulfated forms occur equally (7,8,357,358). In contrast, in patients with gastrinomas the relative concentrations of G-17 are higher (74-80%), and increased concentrations of partially processed forms are found (progastrin, NH2- and COOH- terminal fragments, COOH-glycine extended fragments, incompletely amidated fragments) (7,8,27,357-360). Alterations in post-translational processing have been correlated with the presence of metastatic disease (7,8,27,359,360); however, no prospective studies have established their usefulness in an individual case (7) and they are currently rarely measured.
Chromogranin A (CgA) is a 48-kilodalton protein stored in secretory granules of neuroendocrine cells and is widely used as an immunocytochemical marker to identify tumors as NENs (27,236,280,301,322,361-364). CgA is released simultaneously with the release of polypeptides and thus can be used as a general plasma tumor marker for NENs (322,361-363,365-368). Plasma CgA levels are elevated in 80-100% of ZES patients, as is the case in patients with other pNEN/GI-NENs (carcinoids) (322,365-370). Changes in plasma CgA levels are reported to be useful for assessing changes in tumor mass in some studies; however, in other studies, including in patients with gastrinomas, it has been found to be a relatively insensitive marker for tumor progression and/or NEN identification (115,116,364-369,371-375). One major problem with using plasma CgA as a tumor marker in ZES patients is that the chronic hypergastrinemia causes gastric ECL cell proliferation which increases plasma CgA (24,362-364,368,376). Thus, in ZES, elevated plasma CgA can come from the gastrinoma or from hyperplastic ECL cells (24,377-379). Unfortunately, plasma CgA is also increased by inflammatory disorders, other endocrine diseases, the use of proton pump inhibitors, gastrointestinal disorders, cardiovascular disorders and altered renal function, and therefore minimally or moderately elevated plasma CgA levels in the range frequently seen with small gastrinomas/pNEN overlap with values found in these other disorders (361-364,368).
In patients with gastrinoma, a number of agents stimulate the release of gastrin including secretin (61,74,75,84,380-384), glucagon (385-387), bombesin/GRP (380,388), muscarinic cholinergic agonists (380), beta-adrenergic agonists(389), calcium (74,75,380,383,384,390) and a standard meal (74,384,391,392); in addition, native and synthetic somatostatin analogues (octreotide, lanreotide) can decrease serum gastrin (7,103,393-396). Studies demonstrate that gastrinomas possess secretin receptors, somatostatin receptors, bombesin/GRP receptors, and calcium-sensing receptors (380,388,397-401). These findings have been used clinically for ZES diagnosis with the development of secretin, calcium, glucagon and standard meal provocative tests and the use of somatostatin analogues to control acid hypersecretion (7,56,74,75,103,391,394). The clinical aspects of gastrin provocative testing will be discussed in a later section on ZES diagnosis. Currently, somatostatin analogues are uncommonly used to control acid hypersecretion in ZES patient, because they must be given parenterally, whereas effective long-acting, oral antisecretory agents such as PPIs are available and are the drugs of choice (29,103,142,151,181,182,396). Somatostatin analogues are used for their anti-growth effects or to control ectopic secretion of other hormones in gastrinoma patients, as in other F-pNEN (25,58,142,148,152,155,402,403), and this will be discussed in later sections. Furthermore, the presence of somatostatin receptors on gastrinomas, as well as on other pNEN/NENs, is used for tumor localization, as well as to deliver cytotoxic radiotherapy to patients with advanced tumors (51,129,133,134,136,137,139,142,185,404), both of which will be discussed later in the treatment sections.
The exact mechanisms by which secretin, calcium, glucagon, or a meal stimulate an increase, and somatostatin analogues a decrease, in serum gastrin in ZES patients is not completely clear (27,74,397). The most likely explanation is a direct effect on gastrin release from the gastrinoma through activation of specific receptors which are known to be present on these cells, although others have proposed (in the case of the secretin-test) that it is an exaggerated physiological response (397,405,406). The evidence for a direct effect is that presence of receptors for these agents which have been shown on gastrinomas. Furthermore, in dispersed/cultured gastrinoma cells, calcium and secretin stimulate gastrin release, and secretin activates adenylate-cyclase in these cells which stimulates gastrin release (380,393,397,399,407-409). whereas somatostatin causes inhibition (393,409). Also, a direct relationship has been shown between the magnitude of expression of secretin receptors on gastrinomas and the magnitude of the secretin-stimulated response in ZES patients (397).
The exact pathogenesis or cell-of-origin of pancreatic or duodenal gastrinomas remains unclear. As mentioned above, gastrinomas and other pNEN were frequently called islet cell tumors, however it is still controversial that those arising in the pancreas actually originate from pancreatic islets (410,411). Numerous older studies have reported that gastrin is found only in the fetal/developing pancreas in islet cells so if pancreatic gastrinomas arose from islets, the possible cell of origin was unclear (8,27,412-414). Passaro and colleagues proposed two different subpopulations of gastrinomas existed (414-416). One group occurred in the gastrinoma triangle (duodenum, pancreatic head, peri-duodenal lymph nodes), which were to the right of the superior mesenteric artery, which originated form the ventral pancreatic bud and were relatively more benign with frequent positive lymph nodes, low rate of liver metastases and high cure rate (414-417). In contrast, the second group occurred outside the gastrinoma triangle, were entirely within the pancreas, were to the left of the superior mesenteric artery, arose from the dorsal pancreatic bud, and were more aggressive with lower cure rates and liver frequency of liver metastases (414-417). Numerous studies support the conclusion that duodenal and pancreatic gastrinomas differ in biologic behavior (109,110,127,170,172,281,415,418-420). Furthermore, in numerous studies gastrin-producing G cells were found in the adult duodenum, but not in the adult pancreas; therefore, supporting the proposal that different cells-of-origin were likely for duodenal and pancreatic gastrinomas (27,40,412,413,418,419). This proposal is further supported by a study (420) which demonstrates that all 15 duodenal gastrinomas show sonic hedgehog expression with none showing expression of pancreatic-duodenal homeobox 1, whereas the reverse pattern was seen in 11 pancreatic gastrinomas. It has been suggested that gastrinomas in the gastrinoma triangle area originate from stem cells in the ventral pancreatic bud, and that these cells become dispersed in lymphoid and duodenal tissue and give rise to the gastrinomas in this area (414) Others have proposed that gastrinomas originate from multi-potential, endocrine- programmed stem cells that undergo inappropriate and incomplete differentiation toward the G-cell in the islets/pancreas (27,413,418). Although some recent studies propose that cancer stem cells, which have been described in a number of solid tumors, could also be important in the pathogenesis of pNEN or GI-NENs, at present they have not been convincingly identified and isolated in GEP-NEN pathologic samples (421). A recent detailed lineage tracing study of gastrin expressing cells in pancreas provides some of the strongest evidence that pancreatic gastrinomas in sporadic ZES cases may originate from the islets (412). In this study (412) during fetal stages up to postnatal day 7 gastrin expressing cells were abundant, whereas a small population of gastrin expressing cells existed in adult islets which co-expressed glucagon or insulin and the pancreatic gastrin positive cells were found to originate from PTF1a+ and neurogenin 3 expressing progenitors that were a subpopulation of alpha and beta cells. Furthermore, disruption of the MEN1 gene in the progenitor cells, resulted in the development of pancreatic gastrin-expressing tumors, but no animals developed ZES (412). Recent studies provide evidence that gastrinomas in MEN1/ZES may have different pathogenesis than sporadic gastrinomas and also the development of the duodenal gastrinomas and pancreatic tumors differ in these patients. In MEN1/ZES patients, it has been proposed that the duodenal gastrinomas arise from the G cells by a process of hyperplasia similar to proposed for the response of ECL cells to gastrin in the stomach (422,423). In MEN1/ZES patients, it is proposed that the pivotal event in the development of the multifocal gastrin neoplasms is the allelic deletion of the second MEN1 allele (422,424). However, this sequence was not seen in sporadic duodenal gastrinomas (422,424). Previous studies (424) have reported that in MEN1 gastrinomas only 46% of the tumors exhibited LOH at the MEN1 locus with the remaining 55% not exhibiting allelic loss of the MEN1 gene locus, despite having precursor lesions such as hyperplastic G cells in the crypt base or in Brunner’s glands, suggesting that mechanisms besides loss of the wild type MEN1 allele may be involved in the transition from G-cell hyperplasia to duodenal gastrinoma (425). Recent studies (170,173,174,426,427) using mice with targeted MEN1 deletion bred onto a somatostatin null background and treated with omeprazole to induce hypergastrinemia developed gastric carcinoids as well as hyperplastic gastrin-expressing cells in the lamina propria of the proximal duodenum expressing markers for enteric glial cells such as glial fibrillary acidic protein. Because in these experiments, the MEN1gene had been deleted from the epithelial cells, this suggested a possible non-cell autonomous mechanism was involved. This conclusion was supported by a study (427) reporting duodenal gastrinomas as well as their metastatic lymph nodes showed immunohistochemical staining for enteric glial cell markers, whereas it was not seen in pancreatic gastrinomas (170,174,427). From these findings the authors (170,174) proposed that duodenal gastrinomas in these patients may arise from a neural crest-derived cell and /or an endodermally derived epithelial cell.
For pancreatic pNEN in MEN1 patients, two studies have come to different conclusions, with one concluding that PETs arise from duct cells (411) and the other concluding that they arise from islet cells (422,428).
Important insights into the natural history and prognosis of the gastrinoma per se have been provide by a number of long-term studies of patients with or without MEN1 (64,87,89,93,109-111,258,314,315,429-434). In ZES patients without MEN1(sporadic ZES), 25% of their gastrinomas show aggressive growth behavior (109,110). Aggressive growth is associated with a decreased ten-year survival (30%) compared to the excellent survival in those with nonaggressive disease (10 yr.-survival=96%) (110). A similar aggressive growth pattern has been described in patients with MEN1/ZES; however, the percentages are different, with only 14% demonstrating aggressive growth (111). In the sporadic ZES patients, those with aggressive growth are characterized by more frequently having liver metastases, a pancreatic primary, a large primary (>3 cm), a short disease history, higher gastrin levels, female gender, and sporadic ZES (109,110). In general, patients with MEN1/ZES have a better prognosis than patients with sporadic ZES (110). Finally, long-term studies demonstrate that even in patients with liver metastases, their rate of tumor growth may markedly vary with 42% demonstrating rapid growth, 26% having no tumor growth and 32% demonstrating a slow growth over a three-year period (429). Deaths only occurred in the subgroup with rapid tumor growth (62% died during follow-up) (429). This result has important implications for treatment in gastrinomas as well as other NENs with a number of studies demonstrating the rate of tumor growth prior to treatment is an important prognostic predictor of patient’s survival, outcome and even response to different therapies (402,429,435-440).
MOLECULAR PATHOGENESIS
The molecular pathogenesis of gastrinomas, similar to other pNEN /NENs, differs from more common adenocarcinomas, but has remained largely unknown until recently (29,170,172,174,427,441-443,443-448). In contrast to many adenocarcinomas, mutations of common tumor suppressor genes (p53, retinoblastoma, etc.) and oncogenes (Ras, myc, jun, Src, etc.), are infrequent in gastrinomas and other pNEN (29,158,308,310,311,441,443,443,446,448-453). This is not the case with G3NECs, which are uncommon in gastrinomas (<5%), which have a higher mutation rate for p53, Rb and p16(158,310). Whereas mutations of common oncogenes or tumor suppressor genes are uncommon in pNEN, recent studies provide evidence that both the p53 pathway and the retinoblastoma (RB) pathway are frequently altered in pNEN (454-457). The Rb pathway is inactivated in most pNEN (including gastrinomas) (455) by amplification of genes encoding the cyclin-dependent kinases Cdk4/Cdk6. A second study (454) found a low rate of p53 mutations in pNEN (<3%); however, the p53 pathway was altered in 70% of pNEN through aberrant activation of its negative regulators- MDM2 (22%), MDDM4 (320%), and WIPI (15%). A third study found the p53 target gene PHLDA3 is frequently inactivated in pNEN and this correlates with tumor progression and poor prognosis (456,457)
As discussed above, gastrinomas, as well as other pNEN not only occur sporadically (75%-gastrinomas), but can also occur as part of various inherited syndromes (30,114,158,195,197,458,458-462), including MEN1, tuberous sclerosis, neurofibromatosis, von Recklinghausen’s disease and von Hippel-Lindau disease (VHL), and investigations of the altered genes in these diseases have provided insights into the molecular pathogenesis of pNEN (30,195,196,442,449). Approximately 20-25% of patients (Table 3) (27,30,87,89,463) with ZES have Multiple Endocrine Neoplasia type 1 syndrome (Wermer’s syndrome) (MEN1/ZES). MEN1 is an autosomal dominant disorder due to mutations in the MEN1 gene on the long arm of chromosome 11 (11q13). The MEN1 gene has 10-exons encoding for a 610 amino acid protein, MENIN (30,87,97,317,448,464). A recent sequencing study (446) showed in sporadic pNEN, MENIN is also important with 44% having an inactivating mutations of the Multiple Endocrine Neoplasia-type 1(MEN1) gene. Mutations in the MEN1 gene occur in one-third of sporadic gastrinomas (30,441,449,450,465). Furthermore, 5-95% of patients with sporadic pNEN have loss of heterozygosity (LOH) at the MEN1 locus(11q13) including in 44% of sporadic gastrinomas (30,318,463). These results strongly suggest alterations in MENIN are important in the pathogenesis of sporadic gastrinomas and in the inherited syndrome, MEN1. The exact molecular alteration that occurs with MENIN mutations that results in pNEN, including gastrinomas, is not clear. However, it is known that MENIN is a nuclear protein that interacts with a large number of proteins (30,98,463,464,466,467). MENIN interacts with SMAD3; RPA2(a DNA-processing-factor); the AP1-transcription factor, JunD; nuclear factor-B(NF-B), Pem, FANCD2 (a DNA-repair-factor), nucleoside diphosphate kinase, NM23 cytoskeletal-associated proteins and various histone-modifying enzymes (30,463,464,466,467). A recent large WGS study (443) of pNENs found an MEN1 mutation in 41% of the pNENs and altered copy number in 70% and concluded that MEN1 played a central core pathway role in pNENs molecular pathogenesis interacting with each of the key cascades found to be altered in these tumors. This included MEN1(443) interacting with altered key genes involved in DNA damage repair (MLH1-4, MSH5, etc.), chromatin modification (SETD2, MLL3, etc.), altered telomere length (DAXX, ATRX, etc.), mTOR signaling (PTEN, TSC1-2,etc), homologous recombination and double break repair(CHEK2, BRAC1,TP53, etc.) and cell cycle regulation(CDK2C, JNK, etc.).
In recent sequence studies(446) of pNENs was carried out, and it was found that in addition to alterations in the MEN1 gene in 21-100%% (443,446,448), mutations were found in frequently in genes encoding for two subunits of a transcription/chromatin remodeling complex consisting of DAXX (death-domain associated-protein) (25-40%) and ATRX (alpha-thalassemia/mental retardation syndrome X-linked) (18-35%), followed by mutations in mTor pathway genes (15-54%) (443,446,448). MEN1/DAXX/ATRX are important in the epigenetic landscape including DNA methylation, histone modifications, posttranscriptional regulation, and are thought to play important roles in the pathogenesis of pNEN (308,443,446,448,452). Recent studies provide evidence that pNEN are heterogeneous (308,447,452,468,469). The presence of the MEN1/DAXX/ATRX mutant phenotype, which is present in 60% of pNEN, has been reported to correlate with a worse prognosis (448,452,470-474). The MEN1/DAXX/ATRX mutant profile of pNEN is associated with an islet alpha-cell lineage pattern (high ARX, low PDX1, high HNF1A expression) and has a much worse recurrence free survival (470). Numerous recent studies in pNENs (114,444,445,475,476) including gastrinomas support the importance of the cell lineage (alpha cell, beta cell, intermediate pattern), as well as alterations in DAXX, ATRX, alternative lengthening of telomeres and MEN1 mutations as determinants and prognostic factors for identify patients with pNENs showing aggressive growth and cohorts associated with decreased survival.
The VHL locus occurs at 3p25, and chromosome 3 alterations are reported in 21-50% of sporadic pNEN (449,477). However, these chromosome 3 alterations are rarely associated with a mutation at the VHL locus, suggesting that it is not involved in pNEN development; however, a locus telomeric to the VHL locus may be involved. Recent studies provide evidence for the importance in pNEN/gastrinomas of alterations in the DPC4/SMAD gene (20% in pNEN), the p16/MTS1 tumor suppressor gene (50-90%), mTor/Akt/PI3K pathway, amplification of the HER-2/neu proto-oncogene, as well as increased expression of a number of growth factors and/or their receptors (platelet-derived growth factor, hepatocyte growth-factor, epidermal growth factor, insulin-like growth-factor 1) (441,442,449,450,478,479). Numerous recent studies provide evidence that the mTor/Akt/PI3K pathway is particularly important for mediating the growth of pNEN (478,479). This evidence includes the success of the mTOR inhibitor, everolimus, in extending disease-free survival in patients with advanced pNEN (480), but also studies showing the mTor/Akt/PI3K/ signaling cascade plays a central role in pNEN cell growth and proliferation (442,478,479,481). Additional evidence for the importance of the mTor/Akt/PI3K pathway comes from a study showing mutations in mTor pathway genes (15%) in sporadic pNEN (443,446) as well as from a study (482) reporting the effects of a single nucleotide polymorphism. Replacing arginine by glycine in codon 388 (R388)) of the fibroblast growth factor receptor 4 (FGF4) (482) diminishes the responsiveness to mTor inhibitors in pNEN, and its presence in pNEN is associated with advanced tumor stage and liver metastases.
Numerous chromosomal alterations have been identified in sporadic pNEN and accumulate with advancing stage and tumor progression (158,308,452). Comparative genomic hybridization (CGH) and genomic-wide allelotyping studies report that chromosomal gains/losses occur frequently in pNEN, including in gastrinomas, and that the distribution of these changes differs between GI-NENs (carcinoids) and pNEN, supporting the conclusion that they have a different pathogenesis (29,48,449-451). In pNEN, allelic losses occur most frequently at chromosomal locus 1p (25-75%), 1q (20-90%), 3p (40-95%), 11p (30-50%), 11q (30-70%) and 22q (40-95%) (441,449,450,478). With pNEN, chromosomal gains occur most frequently at 17q (10-55%), 7q (15-70%), and 4 q (33%) (441,449,450,478). A number of these alterations are associated with malignant behavior including deletions at chromosome 1, 3p, 6, 11q, 17p and 22p, and gains on chromosome 4, 7, 14q, Xp (441,449,450,478). Deletions are more frequently seen in the primary tumor and gains in the metastases (452). The commonly mutated genes in pancreatic cancer such as KRAS, TP53, p16/cdk2A and SMAD4 and not commonly mutated in pNEN (446,469).
Results have been reported from a number of studies in which pNEN were studied using microarrays to perform gene expression profiling (449,450,478,483-485). Results from 8 studies in pNEN have been summarized (478) and they demonstrate a wide variation in the number of genes up-regulated (45-668) or down-regulated (25-323). These studies and others (483,484,486) describe a number of gene alterations that correlate with prognosis, survival, and relapse, but it is not clear presently which gene changes are of most important in the molecular pathogenesis of the pNEN.
CLINICAL FEATURES AND PRESENTATION: ZES
ZES most frequently occurs between the ages of 35-65 with a mean age of 41 yrs. (range-41-53) (7,27,62,235) but is reported in both children (487,488,488-491) and the elderly (27,62,63,235). There is a slight male predominance and in most series 20-35% of cases occur as part of the MEN1 syndrome (Table 3) (30,62,87,88). The main presenting symptoms are summarized in Table 3. Abdominal pain remains the most prominent symptom (>70%), and it is most frequently due to the presence of a duodenal ulcer, with a lesser subset presenting with pain due to gastro-esophageal reflux disease (GERD 20-44%%) (62). Whereas, in the older literature the ulcer was frequently described as occurring in abnormal locations outside the duodenum or as multiple ulcers, at present, most ZES patients present with a typical duodenal ulcer that is indistinguishable form that seen in idiopathic peptic ulcer disease (27,28,62). Similarly, the pain at presentation is similar to that seen in patients with idiopathic gastro-esophageal peptic disease (28,62). Diarrhea was uncommonly reported in older series, however in more recent series it is present in more than one-half the patients, and in 9-20% of patients it is the principal or a prominent presenting feature (Table 3) (51,55,60,62,87,490,492-495). The diarrhea differs from that seen with VIPomas in that it is characteristically not large volume (<1 L/day) and is more characterized by increased frequency and mild steatorrhea, if it is present (28,62,222). The presence of the diarrhea is an important clinical clue that when associated with peptic ulcer disease, should suggest the diagnosis of ZES (9,24,28,51,55,59,62,181), and this will be discussed in more detail in a later section on diagnosis of ZES.
|
Table 3. Clinical Features of Patients with ZES
|
|
Feature
|
NIH data (n= 261)
|
Literature data (range)
|
|
INITIAL SYMPTOM (percentage)
|
|
Abdominal pain
|
75
|
26–98
|
|
Diarrhea
|
73
|
17–89
|
|
Heartburn
|
44
|
0–56
|
|
Nausea
|
30
|
8–37
|
|
Vomiting
|
25
|
26–51
|
|
Bleeding
|
24
|
8–75
|
|
Pain and bleeding
|
19
|
19–44
|
|
Pain and diarrhea
|
55
|
28–56
|
|
FINDINGS AT PRESENTATION
|
|
Prominent gastric folds
|
94%
|
(10-30%)
|
|
OTHER CLINICAL FEATURES
|
|
Gender (percentage male)
|
56
|
44–70
|
|
Mean age onset (years)
|
41
|
41–53
|
|
MEN1 present (percentage)
|
22
|
10–48
|
|
PAST CLINICAL FEATURES
|
|
History-confirmed peptic ulcer (percentage)
|
71
|
71–93
|
|
History of Esophageal stricture (percentage)
|
4
|
4–6
|
|
History of Abdominal perforation (percentage)
|
5
|
5–18
|
Note. NIH data are from 261 patients with ZES prospectively studied (62). Literature data are from 11 series (50,64). Abbreviations: ZES-Zollinger-Ellison syndrome, MEN1-Multiple Endocrine Neoplasia type 1, ND-no data
In the past before effective nonsurgical methods to control acid hypersecretion was available, many ZES patients with ZES developed severe complications of the gastric acid hypersecretion (1,28,205,235). These included severe peptic ulcer disease (with perforation or penetration, with or without fistula formation), bleeding (22-45%), strictures leading to gastric outlet obstruction) (up to 20%) or GERD complications (esophageal ulcers, strictures, ulcers, bleeding, Barrett’s, rarely perforation) (up to 20) (1,28,62,205,235,496,497). At present, because of the widespread off label antisecretory drug use, it is uncommon to have patients present with symptoms due to complications from advanced peptic ulcer disease /GERD (62,498-501). In the NIH prospective study (62), only 4% of the 261 ZES patients had a perforation due to a peptic ulcer disease and 5% had esophageal strictures, although 10% had duodenal scarring due to chronic peptic ulcer disease (Table 3). At present, while a duodenal ulcer is usually present at diagnosis, it is not advanced, with 18-65% having no ulcer present (27,62,205), although up to 91% have a history of peptic ulcer disease (Table 3).
The diarrhea is a consequence of the acid hypersecretion and not due directly to the hypergastrinemia per se, as shown in numerous studies which report any method that controls the acid hypersecretion (nasogastric section, medications, surgery), without changing the level of hypergastrinemia, all lead to a decrease or cessation of the diarrhea (9,28,55,62,222,502).
In early studies of ZES patients, gastroesophageal reflux disease (GERD) symptoms (i.e., heartburn, pain) were either uncommon or not reported, so that 7 early series of ZES patients reported before 1986, the GERD symptoms were reported to occur in only at 0-2% of all patients (62). More recent GERD symptoms are increasingly reported in series of ZES patients, with 44% of 261 ZES patients having GERD symptoms at presentation in the prospective NIH series(62), and 49-61% in other series in the recent literature (Table 3) (62,498,503). Other gastrointestinal symptoms such as nausea (30%) and vomiting (25%) as well as weight loss (17%) are not infrequent in ZES patients at presentation (Table 3). The cause of the weight loss can be multifactorial, including from effect of the gastric acid hypersecretion on intestinal absorption causing malabsorption, decreased appetite, or from advanced metastatic disease resulting in anorexia, pain or other symptoms (62). In most patients early in their disease course or without widespread metastatic disease, the weight loss is due to maldigestion and malabsorption (28,62,222).
Approximately 20-25% of patients (Table 3) (27,30,87,89,190,463) with ZES have Multiple Endocrine Neoplasia type 1 syndrome (Wermer’s syndrome) (MEN1/ZES) and these patients have a number of important differences including clinical presentation and disease course from patients with ZES without MEN1(sporadic ZES) (27,30,64,87,89,190,463). These aspects will be discussed in the next section.
MEN1/ZES-GENERAL AND CLINCIAL FEATURES
MEN1/ZES-General
As discussed above, MEN1 is an autosomal dominant disorder resulting from mutations in the MEN1 gene located on the long arm of chromosome 11 (11q13) (30,87,317,464). The MEN1 gene has 10-exons with 9-exons encoding for a 610 amino acid protein, MENIN (30,87,317,464). The exact molecular alteration that occurs with MENIN mutations that results in pNEN, including gastrinomas, is not clear.
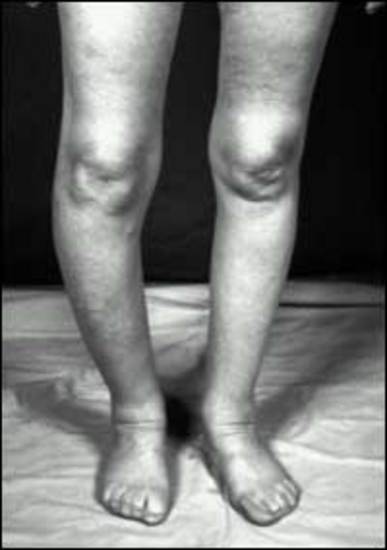
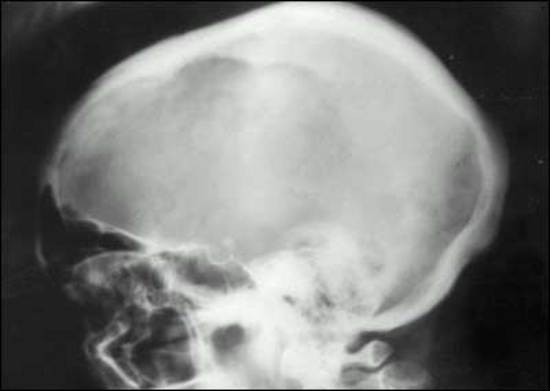
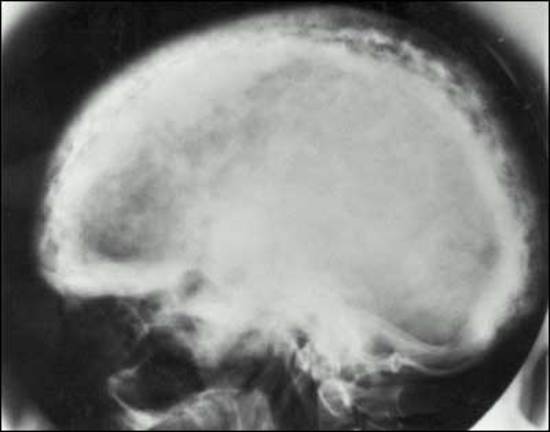
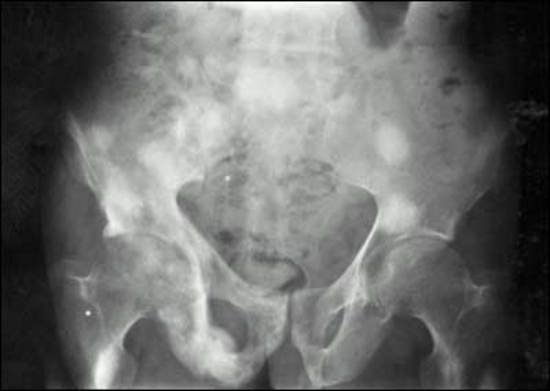
MEN1 causes NENs and hyperplasia in multiple endocrine organs (Table 4) that classically includes: hyperparathyroidism due to multi-gland parathyroid hyperplasia; pancreatic NENs (nonfunctional pNEN>gastrinoma> insulinoma>>other) (Table 4); and pituitary adenomas (prolactinomas>ACTH-secreting>growth hormone-secreting) (Table 4) (30,87,463,504,505). Each may be associated with a functional syndrome. The most frequent pNEN is a nonfunctional pNEN (NF-pNEN) with 80-100% developing microscopic NF-pNEN; however, NF-pNEN cause symptoms in only 0-13% (30,87). Gastrinomas are the most frequent functional pNEN (mean 54%, range 20-61%) (62,87,463,505,506) (Table 3). In addition, classically, adrenal tumors (rarely functional) and thyroid disease can occur in <50%, and these patients have an increased incidence of carcinoid (stomach, lung, thymus) (Table 4). Recently it has become recognized that these patients can develop a number of other tumors including smooth muscle tumors (leiomyomas, leiomyosarcomas), CNS tumors (meningiomas, schwannomas, ependymomas); and skin tumors (angiofibromas> collagenomas >lipomas >melanoma) (Table 4).
As will be discussed in the separate sections below the presence of MEN1 in ZES patients is important to recognize because it affects all aspects of the disease including: the pathogenesis, the pathologic findings; the clinical presentation; the treatment approaches; the prognosis and the role of surgery; and the need for genetic counseling (9,30,64,87-89,93,181,190,463,505,507-509).
|
Table 4. Clinical Features of Multiple Endocrine Neoplasia - Type I (MEN1)
|
|
|
Average Frequency (range)
% of all patients
|
|
Hyperparathyroidism
|
97 (78-100)
|
|
Pancreatic Endocrine Tumors
|
|
Pancreatic endocrine tumors (panNENs)
|
81-100
|
|
Nonfunctional or PPomas
|
80-100 (microscopic)
0-13 (symptomatic)
|
|
Gastrinomas
|
54 (20-60)
|
|
Insulinomas
|
18 (7-30)
|
|
Glucagonomas
|
3 (1-8)
|
|
VIPomas
|
1 (1-15)
|
|
Somatostatinomas
|
0-1
|
|
GRFoma
|
<1
|
|
Pituitary Tumors
|
54-65 (15-100)
|
|
Prolactin-secreting
|
15-45
|
|
Growth-hormone secreting
|
6-20
|
|
Cushing's syndrome
|
16
|
|
Adrenal Tumors
Cortical adenomas
Hyperplasia, carcinoma (uncommon)
|
27-36 (symptoms<2%)
|
|
Thyroid Tumors- adenomas
|
0-10 (0-30) (<1% symptomatic)
|
|
Carcinoid Tumors
|
|
Gastric (ECLoma)
|
7-35 (symptomatic<5%)
|
|
Lung
|
0-8
|
|
Thymic
|
0-8
|
|
Skin Tumors
|
40-100
|
|
Angiofibromas> collagenomas> café-au-lai> macules> lipomas
|
88%>72>38>34(symptomatic<1%)
|
|
Smooth muscle tumors- Leiomyomas, leiomyosarcomas
|
1-8% (symptomatic<1%)
|
|
CNS tumors- Meningiomas>ependymomas, schwanomas
|
0-8%>0-1% (symptomatic<1%)
|
Data from references (27,30,87,89,257,463,505).
MEN1/ZES-Clinical Features
For the 20-25% of patients (Tables 4 and 5) (27,30,87,89,463) with ZES with the Multiple Endocrine Neoplasia type 1 syndrome (MEN1/ZES), the presentation of mild hyperparathyroidism is best detected by an assessment of plasma ionized calcium levels, combined with assessment of plasma parathormone levels using a more sensitive assay such as intact PTH-IRMA assays (87,510). In general, the clinical manifestations of ZES are largely similar to those of patients with sporadic and MEN1/ZES, although patients with MEN1/ZES tend to have diarrhea less frequently as one of the presenting symptoms (26% vs 53%) (511). A carefully taken clinical, personal, and family history of endocrinopathies can be particularly important in suspecting MEN1/ZES, because up to 75% have a family history of MEN1 (Table 4) and 24-42% have a personal history compatible with renal colic (30,87,89). The presence of the MEN1 can affect the manifestations of ZES and aspects of its presentation, which will be discussed in a later section dealing with the diagnosis of ZES. In one study (64) the delay in diagnosis of ZES was greater in MEN1/ZES patients than in sporadic cases (7.4 ± 4.9 yrs. vs 3.9 ± 0.2 yrs, p=0.022).
|
Table 5. Features of Patients with MEN1/ ZES
|
|
Feature
|
NIH Data (n=106)
Mean (range)
|
Literature (range)
|
|
I. MEN1 Tumor/hyperplasia
|
|
Hyperparathyroidism
|
100 (94%)
|
88% (78-100%)
|
|
Pituitary disease
|
60%
|
31% (28-60%)
|
|
Adrenal abnormality
|
45%
|
13% (13-35%)
|
|
Other functional pNEN)
|
6%
|
15.7%
|
|
Smooth muscle tumor
|
7%
|
0.2%
|
|
Thyroid disease
|
6%
|
5% (3-25%)
|
|
CNS tumor (meningioma, etc.)
|
8%
|
<1%
|
|
Carcinoid
Gastric
Bronchial
Thymic
|
30%
20%
8%
6%
|
6%
4%
2%
2%
|
|
Skin tumor
Lipoma
Melanoma
Collagenoma
Angiofibroma
|
5%
2%
72%
88%
|
3%
<1%
<1%
<1%
|
|
II. Age/duration
|
|
Age (yrs.
Age at study
Age at onset ZES
Age onset MEN1
|
51.2 ± 1.2 (23.8 – 80)
29.8 ± 1.1 (10.2 – 61)
34.7 ± 1.0 (12.1 – 61)
|
43.5 ± 0.5 (43-51)
36.6 ± 0.6
34.1 ± 0.5
|
|
Duration (yrs.)
Of ZES
Of MEN1
|
16.6 ± 0.9 (1.4 – 43)
21.5 ± 1.1 (1.4 – 58)
|
ND
ND
|
|
III. Other MEN1 feature
|
|
Family History of MEN1
|
70%
|
76%
|
|
First MEN1 symptom
Asymptomatic (screening)
HPT
ZES
Pituitary
other
|
5%
38%
45%
8%
2%
|
1.3%
38%
41%
12%
8%
|
Abbreviations: MEN1 = multiple endocrine neoplasia type-1; ZES = Zollinger-Ellison syndrome; HPT = hyperparathyroidism; NIH = National Institutes of Health; ND=no data
NIH data are from (27,62,87,227,512-514)
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
Differential Diagnosis: When Should You Suspect ZES
Despite many articles on the diagnosis of ZES, the diagnosis is continuing to be delayed by 4-7 years from disease onset with no shortening occurring over the last few years (27,29,56,59,62,184,511), and in fact, numerous studies support the conclusion the diagnosis is becoming more difficult and may be delayed even further in the future (24,51,55,56,59,77,78,515-517). The diagnosis of ZES has historically been frequently missed and delayed, because ZES is an uncommon cause of PUD (1-3 new cases/million population/year), whereas idiopathic PUD is 1000-fold more frequent (2300 cases/ year /million) and their initial clinical manifestations can closely resemble each other (27,40,55,56,511,515). In the past when there was ineffective gastric antisecretory medications, ZES would often present with advanced, refractory peptic disease suggesting the diagnosis, however, at present, most patients present with a typical appearing duodenal ulcer, without complicated disease, as seen in patients with idiopathic PUD (28,40,511). This is occurring primarily because of the widespread available of potent gastric acid suppressant drugs (i.e., PPIs), which in conventional doses used to treat idiopathic GERD/PUD, also generally control the acid hypersecretion occurring in most ZES patients (70,105,518,519). The result of this change and others, are making the diagnosis more difficult primarily for two reasons: first, the widespread use of PPIs can both lead to a false negative diagnosis of ZES because the symptoms and acid secretion are well controlled on the PPI, as well as lead to a false-positive diagnosis of ZES because it can induce fasting hypergastrinemia (24,51,55,56,76-78,515,517). Secondly, there is an increasing unreliability of serum gastrin assays which are essential for the diagnosis of ZES (55,56,516,520-522). Each of these points will be discussed in detail later in this section.
A number of clinical/laboratory findings should suggest the diagnosis of ZES, and these are summarized in Table 6.
The presence of diarrhea with PUD is a particularly important clue to the possible presence of ZES, because in recent series when a history for diarrhea is careful sought it is present in >60% of ZES patients (Table 3,4,6). Conversely, in patients with idiopathic PUD/GERD, the occurrence of diarrhea is now uncommon, because the use of high doses of Mg containing antacids is now rare, which were a frequent cause of diarrhea in the past in patients with PUD/GERD (523,524).
|
Table 6. Findings That Should Suggest Possible Diagnosis of ZES
|
|
I. SYMPTOMS
|
|
A. Peptic ulcer disease or gastro-esophageal reflux disease (GERD) with:
|
|
diarrhea (>60%)
|
|
without H. pylori or use of NSAIDs (PUD) (10-50%)
|
|
with a long history of persistent or severe symptoms (i.e., >3 yrs.) (>50%)
|
|
with refractoriness to treatment
|
|
with a PUD complication (bleeding, perforation, penetration) (10-15%)
|
|
with a GERD complication (esophageal stricture, perforation, ulcer) (<5%)
|
|
with weight loss (15-20%)
|
|
with family history of PUD or GERD
|
|
with family history of endocrinopathy (esp. renal lithiasis, hyperparathyroidism)
|
|
B. Persistent diarrhea (50-80%) which is:
|
|
responsive to gastric acid antisecretory drug treatment (H2-R, PPIs)
|
|
secretory
|
|
associated with abdominal pain (50-70%)
|
|
associated with malabsorption that is unexplained
|
|
unexplained
|
|
with esophageal disease/symptoms (40-70%)
|
|
not responding to specific treatments of diarrheal diseases
|
|
with weight loss (15%)
|
|
with history of endocrinopathies or peptic ulcer disease (25%)
|
|
with family history of endocrinopathies (esp. renal lithiasis, hyperparathyroidism)
|
|
II. SIGNS
|
|
Multiple peptic ulcers in unusual locations (<10%)
|
|
Gastric outlet obstruction due to peptic ulcer disease (PUD) (3-10%)
|
|
Esophageal stricture due to peptic ulcer disease (3-5%)
|
|
PUD/GERD with findings of endocrinopathy or with MEN1-related tumors
|
|
Prominent gastric folds on UGI endoscopy/Imaging (94%)
|
|
III. LABORATORY/RADIOLOGY FINDINGS
|
|
PUD/GERD/unexplained diarrhea with:
Hypergastrinemia
Hypercalcemia
Positive somatostatin receptor imaging
Positive pancreatic mass
|
Numbers in parenthesis refer to percentage of ZES patients with these features. Table prepared from data in ref. (56,62,73,87,89,133,512,525).
Furthermore, in any patient with chronic diarrhea without an evident cause, especially if it is fasting in nature, ZES should be suspected (Tables 3,4,6) (60,62,63,87,183,222,490,492-494,498).
In idiopathic PUD, H. pylori infection (>80%) or the widespread use of NSAID/aspirin are a frequent contributing factor, whereas they are frequently not present in ZES patients with a duodenal ulcer (approximately 50%), thus the lack of their presence should raise the possibility of ZES (60,80,526-531). Although less common than in the past, any patient with severe PUD/GERD or with a PUD/GERD complication (stricture, obstruction, perforation, bleeding, penetration), ZES should be suspected (Tables 3,5,6). Because of the frequent occurrence of MEN1 in ZES patients (20-25%) (Table 5), any patient with PUD/GERD/unexplained diarrhea with a personal or family history of an endocrinopathy or a laboratory finding suggesting an endocrinopathy (especially hyperparathyroidism, renal stones, pituitary disease) should lead to suspicion of ZES (Tables 4,5,6). An unappreciated finding that was not emphasized in the past, but which recent studies show is present in up to 94% of ZES patients is the presence of prominent gastric folds on upper gastrointestinal endoscopy or imaging studies (Table 3) (62).
Establishing ZES Diagnosis
If ZES is suspected, a fasting serum gastrin level (FSG) is generally the initial study performed (9,29,55,56,59,182,184). FSG levels are elevated in almost all patients with ZES (>99%), except in some unusual circumstances, such as post-parathyroidectomy in MEN1/ZES or post-noncurative gastrinoma resection (73,74,190,498,508,509,511,532-534). Because of its high sensitivity, the assessment of FSG is an excellent screening test (40,56,498). However, an elevation of FSG alone has a low specificity for establishing the diagnosis of ZES, and no matter how high the FSG level, is not sufficient for a ZES diagnosis (29,40,51,55,56,59,184,498). Many physicians assume that a very high level of FSG (>10-100-fold elevated) is indicative of ZES; however, similar magnitudes of elevation in FSG levels can occur in patients with chronic atrophic gastritis/pernicious anemia, renal failure or those taking PPIs (55,56). For example, FSG levels 10-20-fold elevated are not uncommon in patients with chronic atrophic gastritis (367,535-537). Furthermore, in patients without ZES taking PPIs, although hypergastrinemia is frequent seen (see next paragraph) (80-100%), the FSG is usually increased<3-fold, although in some patients it is increased >10-fold (55,56,59,76,78,376,515,538-543).
Hypergastrinemia can either be physiological which develops as a physiological response to anything causing chronic hypo-/achlorhydria or it may be pathological or inappropriate which occurs in the presence of normal or even elevated gastric acid secretion, which would physiologically suppress gastrin release (Table 7). In humans the disorders causing physiological hypergastrinemia are due to CAG/pernicious anemia, use of PPIs, or H. pylori infections, which are much more frequent than ZES, and thus need to be excluded as a cause of the hypergastrinemia to establish a firm diagnosis of ZES.
|
Table 7. Causes of Chronic Hypergastrinemia
|
|
A. Associated with gastric acid hyposecretion/achlorhydria
|
|
Chronic atrophic gastritis (CAG)
|
|
Pernicious anemia
|
|
Treatment with potent gastric acid antisecretory agents (especially PPIs/uncommonly-H2-R)
|
|
H. pylori infections
|
|
Chronic renal failure
|
|
Post acid-reducing surgery/vagotomy
|
|
Inherited inactivating mutations in H+K+ATPase (1)
|
|
B. Associated with gastric acid hypersecretion
|
|
H. pylori infections
|
|
Antral G cell hyperfunction/hyperplasia
|
|
Gastric outlet obstruction
|
|
Chronic renal failure
|
|
Short bowel syndrome (rare)
|
|
Retained gastric antrum syndrome (rare)
|
|
ZES
|
1)-includes ATP4R mutations encoding for the alpha subunit of H+K+ATPase (544-547)
Therefore, historically, the next study generally recommended in a patient in whom fasting hypergastrinemia was detected and the possibility of ZES was being considered, was an assessment of gastric pH or fasting basal gastric secretory output (9,27,29,55,56,59,72,76,181,182,185,548). Gastric secretory rates are now rarely measured (55,70,72,105) and are available in only a few specialty centers and thus will be discussed briefly below for completeness. If the patient has fasting hypergastrinemia with a gastric pH≤2, ZES should be strongly suspected (55,59,70,73), as summarized in Table 8, because an NIH ZES study found that all ZES patients off of any antisecretory drug had a fasting gastric pH≤2 (70). As shown in Table 8 the diagnosis is established in the group with FSG increased>10-fold combined with gastric pH≤2. However, in the 60% of patients with FSG<10 fold elevated, the diagnosis is strongly suspected but not proven, because there are a number of other diseases (majority=rare) which can also cause these findings that are not ZES which are listed in Table 7 (51,55,56,59,73,74,76).
|
Table 8. Established and Recently Proposed (untested) Criteria for the Diagnosis of ZES
|
|
I. Established Criteria for diagnosis of ZES
Required all: Fasting serum hypergastrinemia (FSG) and gastric fluid pH≤2.
1. If FSG> 10 times elevated (over ULN) and gastric pH≤2, the diagnosis of ZES is established (exclude retained antrum almost always by history) (40% of ZES patients)
2. If FSG is <10 fold elevated and gastric pH≤2, need to perform additional testing to exclude other causes of FSG/ hyperchlorhydria) (60%)
a. Secretin test positive (≥120 pg/ml increase)
b. Elevated basal acid output (>15 mEq/Hr)
|
|
II. Possible new criteria for diagnosing ZES in patients with Fasting Hypergastrinemia in the absence of PPI therapy (gastric pH data not available) (Proposed; not evaluated or/and should not be routinely used)
A. Strongly supportive of ZES diagnosis
1. Active peptic ulcer disease (PUD) or a history compatible with recent PUD or improvement in diarrhea with PPIs combined with:
a. a positive somatostatin receptor scintigraphy imaging (SRI) with either 68Ga-DOTATATE PET/CT or 111In-DTPA-octreotide with SPECT/CT imaging.
b. a positive biopsy or cytology for a neuroendocrine tumor (NEN) (stronger support if a gastrinoma is found)
c. a positive secretin test.
d. known or strongly suspected MEN1 syndrome (i.e., a positive family history, hyperparathyroidism, or pituitary disease)
2. A patient with known MEN1 or strongly suspected MEN1 (i.e., a positive family history, hyperparathyroidism, or pituitary disease) with a positive gastrinoma by cytology/biopsy
B. Moderately supportive of ZES diagnosis (consider this a tentative diagnosis)
1. Positive somatostatin receptor scintigraphy imaging (SRI) with either 68Ga-DOTATATE PET/CT or 111In-DTPA-octreotide with SPECT/CT imaging (sporadic disease only) or positive cytology or biopsy for a NEN, ideally a gastrinoma, (sporadic disease or MEN1 syndrome present) with a biopsy-proven absence of atrophic gastritis and negative autoimmune markers. (1,2)
C. Weakly supportive of ZES diagnosis (insufficient alone for even a tentative diagnosis)
1. A patient with known MEN1 or strongly suspected MEN1 (i.e., a positive family history, hyperparathyroidism, or pituitary disease) with positive imaging or an SRI (1)
2. MEN1 syndrome absent but positive SRI or imaging for possible tumor (3).
|
|
III. Possible new criteria supporting the diagnosis of ZES in patients with Fasting Hypergastrinemia taking PPIs (4) (gastric pH data not available) (Proposed; not evaluated or and should not be routinely used)
A. Moderately supportive of ZES diagnosis (ZES is likely)
1. In a patient with or without MEN1 with active peptic ulcer disease (PUD) or a history compatible with recent PUD or improvement in diarrhea with PPIs combined with a positive biopsy or cytology for a neuroendocrine tumor (NEN) (stronger support if a gastrinoma is found.
2. In a patient without MEN1 with active peptic ulcer disease (PUD) or a history compatible with recent PUD or improvement in diarrhea with PPIs combined with
a positive somatostatin receptor scintigraphy imaging (SRI) with either 68Ga-DOTATATE PET/CT or 111In-DTPA-octreotide with SPECT/CT imaging. (5)
B. Weakly supportive of ZES diagnosis (consider this a tentative diagnosis)
1. In a patient without active PUD or history of diarrhea responding to PPIs without MEN1 with a biopsy-proven absence of atrophic gastritis and negative autoimmune markers with a positive SRI (6,7)
2. In a patient without active PUD or history of diarrhea responding to PPIs with known MEN1 or strongly suspected MEN1 (i.e., a positive family history, hyperparathyroidism, or pituitary disease) with a biopsy-proven absence of atrophic gastritis. (6) and negative autoimmune markers. (7)
C. Minimally supportive of ZES diagnosis (consider this a possible diagnosis only)
1. In a patient without active PUD or history of diarrhea responding to PPIs without MEN1 with a positive SRI
2. A patient with known MEN1 or strongly suspected MEN1 (i.e., a positive family history, hyperparathyroidism, or pituitary disease) without active PUD or history of diarrhea responding to PPIs with prominent gastric folds (8).
|
Part II and part III are from (55), Part I data from (55,73,74)
(1) Under such conditions a NEN is confirmed but since MEN1 patients develop multiple NENs in various locations NEN(s) identified on SRI may not be a gastrinoma(s) (30,89,90,549).
(2) Five biopsies (2-antrum, 2-corpus,1- incisura angularis) of the stomach are recommended to diagnose atrophic gastritis) (550,551).
(3) SRI can be positive in nonngastrinoma NENs, numerous other tumors and both physiological and pharmacologic processes, so alone is not specific for gastrinoma (134,401,552).
(4) The potential for a false-positive secretin test in patients with hypo-/achlorhydria limits the usefulness of the secretin test in patients taking PPIs unless the gastric pH≤2.
(5) Under these conditions a NEN is likely but since MEN1 patients develop multiple NENs in various locations NEN(s) a positive SRI or biopsy may not be a gastrinoma(s) (30,89,90,549)
(6) Five biopsies (2-antrum, 2-corpus,1- incisura angularis) of the stomach are recommended to diagnose atrophic gastritis) (550,551).
(7) Biopsy and autoimmune markers can both be negative in confirmed autoimmune gastropathy (550,551).
(8) Prominent gastric folds are present in 94% of ZES patients when initially seen, however they are not specific for ZES (62)
Abbreviations: ULN-upper limit of normal; FSG-fasting serum gastrin level; CAG-chronic atrophic gastritis, PPI-proton pump inhibitor; PUD-peptic ulcer disease; SRI-somatostatin imaging
The increased widespread use of PPIs has made the diagnosis of ZES more difficult (51,55,56,59,78,185). PPIs are potent gastric acid suppressants and because of their long durations of action (up to one week) (40,55,553-556) they induce hypergastrinemia in 80-100% of normal (55,56,59,78,184,376,515,538-543). The hypergastrinemia with PPIs develops rapidly (within 5 days); is a common finding among patients even without gastroesophageal disease since these agents are widely prescribed; are now available as over-the-counter medications; and are one of the most over-prescribed medications (185). The degree of hypergastrinemia is variable among PPI users, however in >20% of those taking PPIs in some studies the FSG increased >4-fold, and FSG levels>5-fold are not infrequent, with FSG levels even exceeding >10-fold increased have been reported (55,56,59,78,376,515,538-543). Furthermore, in contrast to H2R antagonists (cimetidine, ranitidine, nizatidine, famotidine), PPIs control symptoms in most ZES patients at conventional doses used in the treatment of idiopathic PUD/GERD (103,518,519,557-559), whereas with H2R antagonists, higher doses and/or more frequent dosing are usually needed than used to treat the typical patient with idiopathic GERD/PUD (40,72,103-106,559-562). In the past, ZES patients treated with conventional doses of H2R antagonists continued to have symptoms suggesting the diagnosis, whereas this is not the case with PPIs (56,56,59,515). Therefore, PPIs both mask and delay the diagnosis of ZES because of their effective symptom control at conventional doses and they also complicate the diagnosis of ZES by their ability to cause a false suspicion for ZES by inducing hypergastrinemia in normal subjects (56,515). However, the characteristic of PPIs which has most complicated the ability to diagnose ZES is their long duration of action which makes it difficult to take patients off the PPI, especially if ZES is present and can lead to complications (25,51,55,57,59,77,78,563) as will be discussed below.
If the gastric fluid is sampled in a patient with an elevated FSG while the patient is being treated with a PPI, and the gastric pH is >2 it is not possible with this information alone, to determine whether the hypergastrinemia is physiological or pathological. To resolve this problem, both historically and in the more recent American NANETs and European ENETs guidelines, as well as recommendation by experts for the diagnosis of ZES, it was recommended to stop the PPI for up to one week and then determining gastric pH and FSG (7,9,27,55,78,79,103,181,182,235,517,564). This approach should be performed with caution (25,55-57,59,77,520). In each of the above guidelines, it is pointed that this must be performed only after taking a careful history of the prior effects of stopping the PPIs, that high-dose H2R antagonists be substituted for the PPI (equivalent to ranitidine-300-600-every 4-6 hours), and this only be performed after it is established that acute PUD/GERD lesions are healed and the patient can be carefully followed during this time (55,56,59,77,520). After 5-7 days, the H2R can be stopped, antacids used and on the following day the repeat testing performed. A recent study (77) reported two patients with ZES who developed severe PUD/GERD complications when PPIs were suddenly stopped and recommended the diagnosis of ZES should be established by not stopping the PPI. A number of subsequently papers (55,56,59) have pointed out that it may be possible in some patients to decrease the dose/frequency of PPI to obtain gastric pH≤2 or use other findings (presence of gastrinoma) to establish the diagnosis; however, in most cases this will not be possible. The only established criteria, which usually require discontinuation of PPIs, are listed in Table 8 (Part I). Because of the potential risk in a patient who does have ZES, it has been recommended that in a patient suspected of having ZES on PPIs, that the trial off PPIs in such a patient is best performed at experienced centers (9,55,56,181,565).
In the past, gastric acid secretory studies were performed in most centers and the results used for ZES diagnosis. A study of gastric acid secretory results in 234 NIH ZES patients and 984 ZES patients from the literature reported study found that most ZES patients without previous gastric acid-reducing surgery have elevated basal and maximal acid outputs (BAO, MAO) with a mean BAO=42mEq/hr (normal<10 mEq/hr) and mean MAO=62.7 mEq/hr. (normal 48 mEq/hr. (men)/ 30 mEq/hr. (women) (70). In this study various levels of BAO, MAO, BAO/MAO ratios as well as basal gastric fluid volume and basal/maximal acid concentration or pH were proposed to identify ZES patients (70). A number of these secretory criteria had high sensitivity for identifying ZES patients with the commonly used BAO criteria of ≥15 mEq/hr. (no previous gastric surgery) or ≥5 mEq/hr (with previous gastric surgery) having a sensitivity of 87-90% and 81-100%, respectively (70). However, gastric acid secretion studies are now performed by very few centers, and thus not generally available, so these secretory criteria are no longer used. However, the above NIH study (70) demonstrated that >99% of ZES had a fasting gastric pH ≤2 off antisecretory drugs; therefore, this is a useful criterion that can be applied widely today. A recent study (566) described the validity of measuring gastric pH at the time of gastrointestinal endoscopy in ZES patients, so this criterion can be generally applied (70).
In a patient suspected of having ZES, who is found to have a FSG level >10-fold elevated and a gastric pH≤2 (which in 40% of ZES patients), the diagnosis is established without further testing (Table 8 (part I)), if the possibility of a retained antrum syndrome, which can mimic ZES (Table 7), has been ruled out by previous history/records (27,552,567). Unfortunately, most ZES patients (60%) present with a FSG<10-fold elevated (27,73,75,212) and are found to have a gastric-pH ≤2, which overlaps with a number of other disorders that can cause hyperchlorhydria with hypergastrinemia (Table 7, 8 (part b)) (7,28,81,105,134,183,184,527). The most frequent of this group are patients with H. pylori infection, which is most frequently thought to be associated with acid hyposecretion, but which can also result in hyperchlorhydria with hypergastrinemia (485,527,568,569), and may thus be particularly confusing. To exclude these other disorders (Table 7,8 (part b)) it is now recommended that a BAO and a secretin provocative test be performed (Table 8 (part b)). In the past, a number of gastrin provocative tests were reported to help identity the patients with ZES, which included tests using secretin (27,28,74,75,381,384,570), calcium (28,40,74,75,384,390,390,570) or a standard meal (27,28,74,384,391). The secretin/calcium tests were based on the finding that these agents stimulated an increase in serum gastrin in ZES patients compared to normal subjects (381,390), while with the standard meal test, ZES patients generally show <100%-increase in serum gastrin (74,384,391), whereas patients with antral G-cell hyperfunction/hyperplasia have an augmented and much larger response (74,384,391,571). At present, only the secretin test is widely used because of its convenience, sensitivity, specificity, and lack of side effects (33,61,74,83). A NIH study of 293 ZES patients (NIH) and 537 ZES cases(literature) (74) demonstrated that a value of 120 pg/mL increase with secretin had a sensitivity of 94% and specificity of 100% for ZES (74), and was more sensitive than previously proposed criteria of increases of 200 pg/ml, 50% over basal or 110 pg./ml (75,382,384,570), and therefore is the criterion recommended today (29,84,181,182). In some countries, secretin is not available, and a glucagon stimulation test has been proposed as an alternative (572); however, there is much less experience with the glucagon stimulation test. Unfortunately, the secretin tests results can be affected by PPI-inducted hypo/achlorhydria or by the presence of hypo/achlorhydria for other reasons; therefore, it cannot be reliably performed while the patient is taking PPIs or is hypo- or achlorhydric (573,574).
The availability of a reliable serum/plasma gastrin-assay is essential in all phases of the diagnostic evaluation of a patient with possible ZES. Unfortunately, a recent study (516) examined the accuracy of 12 widely used commercial assays for FSG assessment used by laboratories in both the US and Europe demonstrated and reported that only 5 assays reliably measured gastrin concentrations, with the others either overestimating or under-estimating the true value. Hence, 7 assays produced FSG values that could lead to false diagnoses or missed diagnoses (56,516,521). The inaccuracy occurred because inadequately characterized antibodies were used that either recognized precursor/inactive fragments or did not interact with all biologically active forms. The lack of a reliable FSG assay invalidates both the assessment of the FSG levels and the results of the secretin test. This is a potential major problem, and the best approach is to check to see if the laboratory performing the FSG assay for your patients uses one of the 5 reliable gastrin-assays listed in this paper or to obtain advice from a center that routinely performs FSG studies in your area for the assay they recommend. A recent study (82) reports a rapid method to measure serum G17 an G34 using liquid chromatography-tandem mass spectroscopy which might prove useful to circumvent the above problems using RIA’s.
A recent study (55) pointed out in regular practice the criteria that most physicians are using to make the diagnosis of ZES are not those outlined above. This report (55) for the first time proposing new criteria which would support the diagnosis of ZES that did not involve the assessment of gastric pH, because it was found that in the last 20 cases of ZES reported in the literature, in only 5% (1/20) was an assessment of gastric acidity used in establishing the proposed ZES diagnosis of the cases reported in these studies. This has occurred primarily because of the difficulty physicians are having in assessing the gastric fluid acidity in these patients. The failure to measure gastric acidity in newly selected patients is due to a number of different contributing factors. First, ln almost all community as well as many university hospitals, the assessment of gastric acid acidity is complicated by the general lack of its availability and the widespread use of PPIs. Secondly the vast majority of newly diagnosed ZES patients when the diagnosis is first suspected, the patients are almost all being treated with PPIs. As discussed above, these drugs have a long duration of action (up to 1 week), making it is difficult to assess the unsuppressed gastric acidity which can only be done by stopping the PPI for up to one week (40,55,70,74,75,254,553,555,556) which makes it difficult to take patients off the PPI. Third, because in a patient who has ZES, this approach is not without potential risk (51,55-57,59,77) and must be performed under control conditions, often using high doses of histamine H2 receptor antagonists, hence it is uncommonly performed. Although many current reports use the presence of an elevated FSG in combination with a positive SRI study to make the diagnosis of ZES (55), unfortunately, this is not specific for ZES, as patients can be achlorhydric/hypochlorhydria and have a non-gastrinoma neuroendocrine tumor that will be positive on SRI, and thus not have ZES. Furthermore, some propose the use of provocative test on PPIs to circumvent the need to stop the PPI to assess gastric pH (55), however a number of studies (573,574), but not all (61)conclude this is not a reliable alternative as the secretin test results are not reliable in a patient taking PPIs which frequently cause achlorhydria/marked hypochlorhydria which causes unreliable results (573,574). In a recent study (61) this conclusion has been challenged, because in 28 patients taking PPIs, no false positive or false negative secretin tests occurred and the sensitivity, specificity and positive predictive values for the secretin test were the same in patients taking or not taking PPIs.
It is important to remember that the new criteria (55) have been proposed to support the diagnosis of ZES, have not been widely evaluated and are not as strong as the classical criteria requiring increased FSG and gastric pH<2. These criteria were only proposed because 95% of physicians are not using the established criteria for the diagnosis of ZES (Table 8 (Part I)), and it is not apparent this practice will be reversed in the future. At present, it is best to refer these patients to a center that has expertise in the diagnosis of ZES to firmly establish the diagnosis by the established criteria. This is importance because it will dictate the course of management acutely and long term in the patient if ZES is present or not present (9,50,55).
TUMOR LOCALIZATION: ASSESSMENT OF PRIMARY LOCATION AND DISEASE
An assessment of both the primary tumor location and the tumor extent by various tumor localization modalities is needed at all steps in the management of ZES patients, similar to patients with other malignant NENS (9,27,29,33,53,135,140,140,142,181,575-582). It is initial needed in ZES patients to determine whether surgery should be considered and if so, to determine the extent of surgery; to determine the location, extent and in some cases the rate of growth of metastatic disease prior to any anti-tumor treatment; to assess in MEN1 patients the possible presence of extra-duodenal-pancreatic NENs, such as carcinoid tumors (especially of the lung/thymus); to assess post-resection status; and to assess changes in tumor load with antitumor therapies or extent of recurrence, with time (9,27,29,33,53,129,135,140,140,142,142,181,291,575-578,578,579,579-582). Generally, more than one imaging modalities is used in different patients with the most frequent cross-sectional imaging study being a being a triphasic CT scan with intravenous contrast. In the case of SRI, in the past primarily somatostatin receptor scintigraphy (SRS) using 111Indium-labeled somatostatin analogues with SPECT imaging was used (141,582). But now in most centers it is replaced by the use of SRI with 68Gallium-labeled somatostatin analogues with positron emission tomographic imaging (PET-scanning) (45,129,134-137,139,141,157,291,293,578,582).
A wide range of different imaging modalities have been used in the evaluation of ZES patients (Table 9) (9,130,134,135,141,291,293,576,582,583). These include cross-sectional imaging (CT scanning, magnetic-resonance imaging (MRI), transabdominal ultrasound); selective angiography; somatostatin receptor scintigraphy (SRS) using 111Indium-labeled somatostatin analogues with SPECT imaging or 68Gallium-labeled somatostatin analogues with positron emission tomographic imaging (PET-scanning); endoscopic ultrasound (EUS); and the assessment of serum gastrin gradients either determined in the portal venous drainage through transhepatic venous sampling or in hepatic veins after selective, intra-arterial secretin injections (9,27,29,37,130-132,135,138,142,153,181,291,293,323,577,583-592) vary in sensitivities for detection of the primary tumor, as well as metastatic tumor (Table 9).
|
Table 9. Tumor Localization Results in Patients with ZES
|
|
|
NIH studies
Mean(range)
|
Literature
Mean(range)
|
Literature
Mean(range)
|
|
Extra-hepatic lesions
|
|
Ultrasound
|
13 (9-16)
|
24 (0-28)
|
92 (92-93)
|
|
MRI
|
40 (30-57)
|
22 (20-25)
|
100 (99-100)
|
|
CT scan
|
38 (31-51)
|
38 (0-59)
|
90 (83-100)
|
|
Angiography
|
43 (28-57)
|
68 (35-68)
|
89 (84-94)
|
|
SRS
|
69 (58-78)
|
72 (57-77)
|
86 (86-100)
|
|
PVS
|
71
|
68 (60-94)
|
ND
|
|
Intra-arterial Secretin test
|
86
|
89 (40-100)
|
ND
|
|
EUS
|
ND
|
70 (28-86)
|
85 (80-93)
|
|
IOUS
|
83
|
83 (75-100)
|
|
|
Liver Metastases
|
|
Ultrasound
|
46
|
40 (15-77)
|
100 (99-100)
|
|
MRI
|
71
|
63 (60-75)
|
92 (88-100)
|
|
CT scan
|
42
|
48 (37-56)
|
99 (99-100)
|
|
Angiography
|
65
|
62 (33-86)
|
98 96-100
|
|
SRS
|
92
|
97 (92-100)
|
95 (90-100)
|
|
Intra-art. Secretin test
|
40
|
ND
|
ND
|
Data are from (9,27,40,119,129,133,142,295,525,591,593).
ND-no data.
At present, most patients when initially evaluated have performed a cross-sectional imaging study (CT, MRI. Ultrasound) and an SRI study to determine whether surgical resection should be considered (6,9,44,88,181,182,584). Gastrinomas, similar to other pNEN, are hypervascular and are thus their detection on imaging studies can be enhanced by the of administration of contrast; hence, in most patients, either a triphasic CT with intravenous contrast or an MRI with intravenous contrast (gadolinium (129,291,576,577,583). With the cross-sectional imaging modalities, the detection of lesions is influenced by their (40,141,525,577,591). In patients with gastrinoma lesions <1 cm in diameter, only <10-20% are detected, with 1-3 cm in diameter it increases to 15-40%, and with tumor lesions >3 cm, >80-90% are detected (40,525,577,591). Therefore, cross-sectional imaging studies will miss most primary duodenal gastrinomas, which are characteristically <1 cm in diameter; however, they detect most pancreatic primaries which are frequently > 3 cm in diameter (43,45,109,110,175,176,577). As summarized in Table 9, the sensitivity of cross-sectional imaging for detection of primary gastrinomas varies markedly among different series, with generally excellent specificity. In general, they detect <50% of the primaries, with lower yields in series with a high percentage of duodenal gastrinomas. For detection of a patient with liver metastases, cross-sectional CT/ultrasound identify approximately one-half the patients, whereas MRI detects nearly three-quarters (Table 9).
Selective angiography was widely used in the past, but is infrequently used now, however it is a sensitive method to image gastrinomas, (27,43,241,525,591,593). In most studies angiography was more sensitive than cross-sectional imaging studies for localizing primary gastrinomas, but it still did not localized approximately half of all primary gastrinomas, particularly missing small duodenal gastrinomas (40,43,175,241,525,591) (Table 9). However, angiography is increasingly not used, because it is an invasive procedure, but more importantly because of the increasing sensitivity of both cross-sectional imaging, and the increased availability of SRI which has a significantly higher sensitivity of SRS (Table 9). In the past frequently at the time of angiography, selective hormonal sampling was also used, and still used today in some centers for patients with ZES who have negative cross-sectional imaging and negative SRI studies (138,323,585,588,589,593-597) Two different methods for gastrin hormonal sampling have been used, with the first being the transhepatic catheterization of portal venous tributaries draining the pancreas (portal venous sampling (PVS)) (323,593,595) and the second, which is more frequently used, is the assessment of hepatic venous gastrin concentrations performed after secretin injection into selective arteries to various pancreatic/duodenal regions (585,588,589,593,594,596,597). This method is not dependent on tumor size and involves functional localization which can be very sensitive (Table 9), however, it is now rarely used being replaced by cross sectional imaging and SRI (588,589).
Greater than 90% of NENs/NTs including gastrinomas are well differentiated tumors which overexpress or ectopically express one of the subtypes of somatostatin receptors (sst1-5) in >90% of cases (primarily sst2) with the result that somatostatin receptor imaging (SRI) with various radiolabeled various somatostatin analogues is now widely used (90,134-137,139,141,153,291,292,582,598,599). This method is particularly sensitive method to identify both the primary and metastatic gastrinoma location (181,401,525,576,600-602). Of the five classes of somatostatin receptors (sst1-5), all can be detected in various gastrinomas; however, sst2(80-100%) and sst5 (30-60%) are the most often overexpressed (603). Whereas native somatostatin (som14) interacts with all 5 receptor subtypes with high affinity; it is rapidly degraded in the circulation, hence is not useful therapeutically or for radio-imaging studies (601,603). Two synthetic analogues of somatostatin, octreotide and lanreotide, which have high affinity only for sst2 and sst5, are metabolically stable, and are now widely used for both SRI, for their anti-tumor effects both alone or coupled to radiolabels that are cytotoxic to the tumor and will be discussed in the treatment section later (see PRRT) (90,129,292,601,603-609). Specifically in gastrinomas, in the NIH ZES prospective studies (Table 9), SRS using 111In-labeled somatostatin analogues (Octreoscan) and single photon emission computed tomographic scanning (SPECT) imaging detected primaries in 69% of patients and in one prospective study of 80 consecutive ZES patients (133), SRS was more sensitive than any single cross-sectional imaging study or angiography, and was equal in sensitivity to the combination of all three cross-sectional imaging studies (US, CT, MRI) and angiography together (58% vs 48%)(133). The sensitivity of SRS, similar to cross-sectional imaging, is influence by the size of the gastrinoma, with SRS using 111In-labeled somatostatin analogues (Octreoscan) and SPECT imaging visualizing only 20% of gastrinomas <0.5 cm in diameter, 30-40% <1 cm in diameter (610). Because the mean size of duodenal gastrinomas is <1 cm, SRS detects only 32% of duodenal gastrinomas (175,610). The use of 68Ga-labelled somatostatin analogues with positron emission tomography (PET-scanning) has greater resolution with increased sensitivity (129,134,141,290-292,582) and thus is an important recent advance. In the US and in many countries, the most commonly used ligand for SRI is now 68Gallium DOTA (9,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid) labeled somatostatin analogue (generally 68Ga-DOTATOC PET/CT) with positron-emission tomography detection (129,134,141,290,582). This SRI method has generally replaced the use of 111Indium (diethylenediamine penta-acetic-D-phenylalanine-1) octreotide with single photon emission CT (SPECT) detection, because of it greater sensitivity (129,134,290-292). SRI at present is the most sensitive method for assessing whole body localization of advanced NENs (129,134,290,292).
SRI is of particularly valuable for detecting distant metastases both to the liver and more distant, especially to bone, with a detection rate of 97% for identifying a patient with metastatic disease in the liver (Table 9). Studies demonstrate that bone metastases are relatively common in patients with advanced NENs including gastrinomas, in which they occur in up to 31% of ZES patients with liver metastases (58,112,294,611,612). The detection of bone metastases in ZES patient has been shown to have a high clinical importance, because they may not only require specific treatment, they also have important prognostic significance) (109,294,299,612,613). In one prospective study from NIH (112), SRS had greater sensitivity than bone scans for detecting bone metastases, and for imaging metastases in the spine was equal in sensitivity to MRI (112). Because 15-25% of the initial metastases occur outside the axial skeleton, SRI is recommended as the initial study over MRI to detect bone metastases (112).
Whereas endoscopic ultrasound (EUS) has proven to be one of the most sensitive modalities for detecting insulinomas/NF-pNEN and is reported to be sensitive for localizing gastrinomas in some studies, its use is controversial in gastrinomas (40,119,245,614-616). EUS detected a mean of 70% of gastrinomas in different studies (Table 9) and has the advantage of allowing histological verification of the presence of a NEN as well as obtaining samples for determining the grade of the NEN which is particularly important for prognosis (617,618). However, EUS’s result is operator-dependent and false positives can occur (119,133,245,615). An important issue in patients with ZES is EUS’s sensitivity for detecting gastrinomas in different locations such as the duodenum, which is the source of controversy in is use in ZES as opposed to its general use in patients with entirely intra-pancreatic NENs (insulinoma, NF-pNEN, etc.). In one review of EUS in ZES patients, EUS detected a pancreatic gastrinoma in 83%, whereas it detected a duodenal gastrinoma in only 43 % (119). This is a major problem for EUS in patients with ZES because in recent studies 3-10 times more gastrinomas are found duodenal than pancreatic (9,181,182). Because of this difference, many experts do not recommend EUS as a routine preoperative imaging study in patients with ZES, especially in the 75-85% of patients with sporadic ZES (119). As will be discussed further in a later section, serial EUS studies may be used in patients with MEN1/ZES to evaluate the possible growth of the pNETs in patients who do not undergo routine exploration (30,44,88,90,619,620). At present it is recommended that a cross-sectional imaging study and SRS with SPECT imaging be performed in all ZES patients to evaluate tumor location/extent (9,42,50,181,182). If negative, but where the diagnosis of ZES has otherwise been confirmed, MEN1/ZES is not present and surgery is being considered, there is not complete agreement on which if any localization procedure should be performed prior to surgery (45,119,254). This issue will be discussed further under the section on surgical management.
Recently, there has been increased interest in pNENs, including gastrinomas, as well as NENs in other locations of the use of 18F-Fluordeoxyglucose (18F-FDG) PET imaging particularly as a prognostic marker (42,90,115,116,129,134,291,621-623). 18F-FDG PET/CT assesses tumor metabolic activity by determining the glucose uptake and therefore measures a different tumor parameter than SRI which is assessing somatostatin receptor expression. Although 18F-FDG PET/CT is widely used in oncology, until recently, it was generally not thought helpful in patients with pNENs/NETs (134,624). However, numerous recent studies report high uptake by a proportion of NETs (291,625-627). In a number of studies, the high uptake/SUV of 18F-FDG PET/CT was reported to be associated with higher Ki67 values and was a predictor of overall survival as well as PFS (42,115,116,134,291,621-623). Lately there have been an increasing number of papers advocating either the use of FDG either alone or combined in dual imaging with 68Ga-DOTA-SSA PET/CT (42,622,623,625-631). Similar to its increasing use in other pNENs/NENs the use of 18F-FDG PET/CT in patients with gastrinomas may help in identifying those with aggressive disease, particular as a postoperative tool to stratify patients that may benefit by more aggressive postoperative treatments.
TREATMENT (ACID SECRETION/LOCALIZED DISEASE)
Like patients with other F-NEN syndrome, patients with ZES have two different aspects that require treatment and often can’t be controlled by a single treatment strategy (25,48,54,158,632-634): control of the hormone excess state and treatment directed at the NEN per se, because, except for insulinomas, but similar for gastrinomas and the other F-NEN, these NENs are malignant in 50-100% of cases and require treatment. Specifically, in the case of ZES treatment needs to be directed at two different problems: the control of the marked acid hypersecretion and the gastrinoma itself. Whereas a curative resection would solve both problems; unfortunately, it is possible in <30% of patients. Furthermore, in patients with MEN1/ZES which comprise 20-25%, treatment must be also directed at the other endocrinopathies these patients frequently develop, as well as genetic family counseling (30,65,96,99,100,203,463,505). The first section will discuss management of the acid hypersecretion, followed by the surgical management of the gastrinoma in patients without advanced metastatic disease. In the last section of treatment, the management of patients with advanced/metastatic disease will be discussed.
Management of Gastric Hypersecretion
GENERAL MANAGEMENT OF GASTRIC HYPERSECRETION
Numerous studies, especially older studies prior to adequate drug therapy to control the gastric acid hypersecretion in ZES patients, demonstrate that both the acute and long-term control of the acid hypersecretion is essential for long-term survival (9,46,72,89,103,181,184,205,313,635-637). Prior to the availability of effective acid antisecretory drugs, most ZES patients who did not have a total gastrectomy, eventually developed complications of the gastric acid hypersecretion, and the majority died from these complications rather than from tumor progression (1,27,28,46,72,89,103,184,205,235,313,635,638). This occurred largely because of the direct effect of the marked acid hypersecretion, with the mean basal-acid output (BAO) in ZES patients typically 4-times normal but reaching as high as 12-times the upper limit of normal in some patients (40,70). In a given patient it is not possible to predict when these elevated acid levels will overcome the defense mechanism (increased bicarbonate secretion, increased duodenal secretion, etc.), thus in all patients it is essential to acutely control the acid hypersecretion as soon as ZES is suspected and as the initial step in management (1,9,28,51,56,72,181,182,313,558,639).
SURGICAL TREATMENT OF GASTRIC HYPERSECRETION
While surgical management of the gastric acid hypersecretion in ZES patients is now rarely used, in the past (prior to the 1970’ s), the only effective means of adequately controlling gastric acid hypersecretion in these patients was by total gastrectomy (1,28,205,207,237,620,638,640-642). Lesser operations were almost invariably inadequate to prevent recurrence long-term (1,28,237,403,620,638,641). Because in ZES patients prior to any other means of controlling the acid hypersecretion, the total gastrectomy was often performed as an emergency procedure and was associated with considerable morbidity/mortality (1,40,205,638). However, later with the availability of histamine H2receptor antagonists, starting in the 1970’s, allowing preoperatively control of the acid hypersecretion medically in most patients, the total gastrectomy could then be performed electively and was relatively safe, with an overall mortality of 5.8% in 248 cases since 1980, and 2.4% for elective cases (207). However, the long-term morbidity remained unclear, and in some studies up to 50% of patients have moderate or severe side-effects, including weight loss, pain, stenosis of the anastomoses, vomiting and early satiety (27,40,643). At present, because of the effectiveness of medical therapy especially the PPIs, total gastrectomy is rarely performed and reserved for patients (<0.2%) (9,27,51,71,103,108,644,645) who cannot or will not regularly take oral antisecretory drugs.
Both vagotomy, as well as medical treatment with anticholinergic agents, can reduce the levels of gastric acid hypersecretion in ZES patients and also, they can potentiate the effectiveness of histamine H2R antagonists when added (27,40,646-648). After the availability of histamine H2R antagonists (1970+), but prior to the availability of PPIs(mid-1980s), most ZES patients were not cured at surgery, and because many continued to require frequent histamine H2R antagonists, it was proposed that parietal cell vagotomy, be performed at the time of surgery in ZES patients (211). In ZES patients that underwent selective vagotomy, (211,648), the BAO decreased by a mean of 50%, the histamine H2R antagonist dosage could be reduced by 40%, and in 36% of patients all antisecretory drugs could be stopped postoperatively. Today, with the development and availability of PPIs, which are highly effective in ZES patients, a form of vagotomy is rarely necessary or used.
In patients with MEN1/ZES with hyperparathyroidism, an effective parathyroidectomy can markedly reduce fasting gastrin levels (FSG), the BAO and can increase the sensitivity to gastric antisecretory drugs (190,508,509,533), with a mean decrease in BAO of 56% and the FSG of 55% (40,190,508,509). Moreover, in some patients, the FSG levels can decrease to the normal range, as well as a positive secretin-test can become negative (40,190,508,509). MEN1 patients, with or without ZES, have parathyroid hyperplasia which involves all four parathyroid glands, if recurrent hyperparathyroidism is to be avoided post-parathyroidectomy, it is recommend that either a 3.5 parathyroid gland resection or a 4-gland resection, with a parathyroid implant, should be performed in these patients (463,508,509,649-653).
Long-term, curative gastrinoma resection is possible in < 40% of patients with sporadic ZES undergoing surgery with the recommended surgical approaches in most guidelines with no-aggressive resections (non-Whipple resection) (6,43,175,254); and even when curative, it does not completely correct the gastric acid hypersecretion in some patients (654-656). In the NIH prospective studies of acid hypersecretion post-curative resection, the MAO decreased 50%, BAO decreased 75% within 6-12 mos. and then remained unchanged for up to 4 years, and the histamine H2R antagonists’ dose could be reduced by >60% (654-656). However, even though the BAO decreased by 75% after curative resection for up to 4 years, 60% of the patients remained acid hypersecretors (654,655). This group included 34% who were mild hypersecretors (BAO-15-24.9 mEq/hr.) and 28% who had marked to extreme hypersecretion (≥25 mEq/hr. (range-25-69 mEq/hr.)) (655). The mechanism of this continued hypersecretion post-curative resection is unclear (655). Practically, it means that all ZES patients should continue to be followed carefully post-curative resection and many will continue to need low doses of antisecretory drugs (655).
MEDICAL TREATMENT OF GASTRIC HYPERSECRETION
In all recent guidelines, medical treatment with oral gastric acid antisecretory drugs is the recommended method to control the gastric acid hypersecretion seen in ZES patients, both acutely and long-term (9,29,103,157,164,180,181). PPIs (omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole) are the recommended drug of choice because of their long durations of action and potency (7,9,25,29,40,72,103,157,164,180-182,657,658). Most ZES patients without complicated disease (MEN1/ZES, moderate-severe GERD, post-Billroth II surgery) require only once a day dosing and many are controlled on PPI doses equivalent to those used in idiopathic PUD disease (i.e., equivalent to 20 mg/day omeprazole) (103,184,518,519,557). In patients with complicated disease (MEN1/ZES (especially with active hyperparathyroidism), moderate-severe GERD, post-Billroth II surgery) higher doses/frequency are usually needed (72,184,518,519,557,659). For patients requiring higher doses, in general, increasing the dose frequency is more effective than increasing the dosage once-per-day (72,518,519). Most long-term studies were performed with omeprazole or lansoprazole as the PPI, however; other PPIs (pantoprazole, rabeprazole, esomeprazole) are effective in ZES, and it is not apparent anyone has an advantage over the others (103,184,660-662). There is no complete agreement on the starting dose of PPI to be recommended. This becomes an important point in patients with ZES because many of the PPI formulations are acid-labile and thus starting a patient on a low PPI-dose could delay its action, and in acutely ill ZES-patients with PUD this could result in complications (663). One study attempted to address this question (663) by starting patients with ZES on a low dose of omeprazole (20 mg/day) and found that in 32% acid secretion was not controlled and higher omeprazole doses were needed. This study proposed that ZES patients with uncomplicated ZES (no MEN1/ZES, moderate-severe-GERD, post-Billroth II surgery) be started on higher PPI doses (equivalent to omeprazole 60 mg/day) and then doses reduced during follow-up. Both the US NANETs guidelines (182) and the European ENETs guidelines (181) recommend that ZES patients with uncomplicated disease (no-MEN1/ZES, moderate-severe GERD, post-Billroth II surgery) be started on the equivalent of 60 mg/day of omeprazole and that patients with complicated disease be started on PPI doses equivalent to omeprazole 40-60 mg BID and then, with time, dose reduction be attempted. It is ideal to titrate the PPI dose to control the acid output (<10 mEq/hr for no gastric surgery, <5 mEq/hr for previous gastric surgery) (27,40,72,103,106,184,503,660), but few physicians now have access to units measuring gastric acid output. Symptom control (particularly diarrhea, pain, heartburn) can be used to guide management, and if mucosal disease is present, repeat UGI endoscopy should be performed after 6-8 weeks. Because of their potency, dose titration is less important with PPIs; however, it is essential with histamine H2R antagonists (see comments below in this section).
Only a few studies have reported the long-term results of continuous treatment with PPIs in ZES patients for 9-15 years (72,76,518,557,661). Tachyphylaxis does not develop with long-term PPI treatment in ZES patients, and on average <20% of patients require a PPI-dose increase/year (rate-0.13/patient), whereas with long-term histamine H2R antagonist treatment, an average of at least one dose increase/year was required (27,72,103,104,518,558-561). Long-term PPI use has proven safe; with fewer than 0.1% of patients stopping treatment because of a side-effect (103). A potential concern of long-term PPI-treatment is the drug-induced hypo-/achlorhydria, which may lead to effects on nutrient absorption (vitamin B12, iron, calcium) as well as enhanced hypergastrinemia resulting in an increased risk of gastric carcinoid tumors (76,502,664-669). Low vitamin B12 levels are frequent in ZES patients (666,667,670,671), are more frequent in ZES patients treated with PPIs, and correlate with the PPI-induced hypo/achlorhydria (670). While the PPI induced decrease in serum VB12 levels in ZES patients in the above study (670) was established in a prospective study of these patients, the question of whether PPIs systematically decrease VB12 levels in the nonZES, general population and thus should be monitored for, remains controversial (76,667,672).
In another study of ZES patients (673), deficiencies in body iron stores were not found with long-term PPI treatment. Recently, epidemiological, and various correlative studies report in the general population that long-term PPI use may result in an increased incidence of bone fractures, particularly in the spine/ hip, but there are no specific studies in ZES patients (76,666,667,674). In addition, in similar correlative studies in the general population a number of other possible side effects of long-term PPI treatment have been proposed: these are controversial and except for malabsorption of vitamin B12 have not been reported with increased occurrence in ZES patients (76,667). The proposed PPI-side-effects include an increased occurrence of such diverse problems as: dementia, chronic renal disease, hypomagnesemia, malabsorption of various nutrients (vitamin B12, iron, etc.), well as increased growth of various other cancers including gastric, pancreatic, and colorectal tumors (76,502,667,668,675,676). Hypomagnesemia has been rarely reported (3 case reports) in ZES patients (76,645,672,677) and in the prospective NIH studies involving 250 ZES patients, only a single patient developed hypomagnesemia despite chronic, continuous PPI with many patients taking higher PPI doses and with a mean treatment time >10 years, for rate of 0.4% over the treatment period (76). On the basis of these studies, it has been proposed (76,181,673) that only the serum vitamin B12-levels should be periodically assessed once a year in ZES patients with long-term PPI treatment, especially the group of patients who might have low vitamin B12 level initially or a poorer nutritional status (elderly patients with a long history of malabsorption).
Recently, there have been an increasing number of reports of medical failure in ZES patients of the long-term use of H2R (27,558,562,586,678) for maintenance acid control, and also even problems controlling acid with PPI therapy long-term (71,71,95,102,108,136,285,403,494,644,645,672,679-686). This is occurring in large part due to the lack of data from any extended long-term/ lifetime treatment studies (i.e., >10 yrs.-lifetime) of antisecretory acid control in ZES patients. This is in contrast to a number of studies of acute acid control and short-term (<5-6 yrs.) control with small number of ZES patients (225,518,519,554,561,661,662,677,687-693). This lack of information about the long-term efficacy of acid antisecretory drug’s in ZES is a particular problem because of the unique acid secretory condition in ZES. In ZES there is a constant hypersecretory drive due to constant ectopic secretion of gastrinoma from the gastrinoma, resulting in a constant acid hypersecretory state, which results in a constant requirement to inhibit the acid secretion, which because it is unique to ZES, its treatment can only be addressed by long-term/lifetime study data in these patients. In contrast to ZES, there are numerous long-term PPI studies in nonZES patients, particularly in patients with advanced idiopathic GERD, and these can provide evidence for safety issues that might occur with lifetime PPI treatment (76,667,694-696), which is applicable to chronic treatment of ZES patients, however, this is not the case with long-term/lifetime efficacy data in ZES. Another important variable contributing to the need to have data on long-term/lifelong antisecretory efficacy in ZES patients, occurs because of the marked variation of the dose requirement between individual ZES patients as well as in each patient, which has been well-examined in short-term ZES acid secretory studies (225,518,519,554,561,661,662,677,687-689,697). This issue was recently addressed (72)in an analysis of the results of acid antisecretory treatment in ZES patients, which examined in detailed the efficacy/pharmacology of long-term/lifetime medical treatment of acid hypersecretion in a large cohort of ZES patients. This study included results from all 303 patients with established ZES who were prospectively followed and had acid antisecretory treatment with either H2Rs or PPIs who had antisecretory doses individually titrated by the results of regular gastric acid testing. It includes both patients treated for short-term periods (<5 years), as well as patients treated long-term (>5 yrs.), and with lifetime treatment (30%), followed for up to 48 yrs. (mean-14 yrs.). Long-term/lifelong acid antisecretory treatment with H2Rs/PPIs could be successfully carried out in all patients with both uncomplicated and complicated ZES (i.e., with MEN1/ZES, previous Billroth 2, severe GERD). Successful treatment in this study was only possible because the drug doses were individually set by assessing acid secretory control by measuring the acid secretory rate and adjusting the various drug doses to establish proven criteria, with regular reassessments and readjustments. Frequent dose changes both up and down were needed; as well as regulation of dose-frequency and a primary reliance on the use of PPs. In this study (72) prognostic factors predicting patients who required PPI dose-changes were identified which need to be studied prospectively to develop a useful predictive algorithm which could be clinically useful for tailored long-term/lifetime therapy in these patients. These results clearly establish that long-term/lifelong medical control of the acid hypersecretion is possible in all ZES patients who can take acid antisecretory drugs, but requires it be performed in centers with the capability of titrating the drug dose over time by assessing the acid secretory rate, thus it is best if these patients are referred to centers with this capability.
Chronic hypergastrinemia in animals and man stimulates gastric enterochromaffin-like (ECL) cell proliferation and in animal models, gastric carcinoid tumors (ECLomas) can develop, some of which are malignant (76,217,224,227,502,665,666,698-701). In patients with ZES, ECL cell proliferative changes develop in >90% (76,217,227). However, patients with sporadic ZES (no MEN1) (75-80%), rarely develop gastric carcinoids (76,103,217,502), whereas MEN1/ZES patients have >70 greater risk of developing a gastric carcinoid (227). In one prospective study (227), 23% of MEN1/ZES patients had gastric carcinoids and other studies have indicated that these can be malignant in 10-30% of patients (76,227,502,666,702,703). In a similar prospective study of 106 patients (217) with sporadic ZES, none of the patients had a gastric carcinoid tumor, although 99% had ECL cell hyperplasia, and 50% had advanced ECL cell proliferative changes, including 7% with dysplasia. Even though there are a few case reports of gastric carcinoids found in sporadic ZES patients (76,212,228,229,232,233,704-709), the prospective NIH study discussed above(217), demonstrates that this is very uncommon, and differs markedly from the chronic atrophic gastritis patients in which 0.4-7% have gastric carcinoids on a routine endoscopy, and 5-35% in some series with long-term follow-up (76,710,711). There is no evidence the long-term use of PPIs accelerates gastric carcinoids development either in patients with sporadic ZES or with MEN1/ZES (76,103,502). However, because of the association of hypergastrinemia with gastric carcinoids, all patients with ZES should undergo an initial upper gastrointestinal endoscopy; those with MEN1/ZES should have a repeat UGI endoscopy yearly, while in those with sporadic ZES, if there are no upper GI symptoms, follow-up UGI endoscopy can be less frequent.
During the subsequent clinical course of many ZES patients after diagnosis, for their frequently occurs brief periods where they cannot take the oral antisecretory drugs (e.g., after surgery, chemotherapy, etc.) and during this period a parenterally administered gastric antisecretory drug may be necessary. Parental histamine H2R antagonists can be used, however, continuous infusions of high doses are required (27,103,105,586,712,713). In contrast, with parenteral PPIs (omeprazole, esomeprazole, lansoprazole, pantoprazole, etc.), because of their long durations of action, intermittent parenteral administration (every 6-12 hours) can be used (103,555,714-716).
At present histamine H2R antagonists are much less frequently used than in the past (76). Although histamine H2R antagonists can be effective if properly administered, they usually have to be taken every 4-6 hours, and the oral dose needs to be titrated so that acid hypersecretion one hour prior to the next dose is decreased to <10 mEq/hr (no previous-gastric-surgery, <5 mEq/hr.-previous gastric-acid surgery) (28,103-105,558,560,562,717). In most patients at this level of control, symptoms will be controlled, and mucosal lesions heal (27,103,105,106,586). For patients with complicated ZES (MEN1/ZES, moderate-severe GERD, previous Billroth II surgery), acid hypersecretion may have to be reduced to <1 mEq/hr in order to achieve complete healing (27,105,503,519,659). Using dose-titration, the average daily doses needed of oral histamine H2R antagonists in the prospective NIH studies were 4.9, 2.2 and 0.33 g/day for cimetidine, ranitidine, and famotidine, respectively (40,103). Despite these high doses, the drugs were generally free of dose related side effects, except for anti-androgen effects with cimetidine (gynecomastia, impotence) and were effective long-term, although approximately one dose-increase/ year was needed (27,40,103,104,558,561,718). Because of this need to titrate the histamine H2R antagonist dose for each patient, the need for frequent, high dosing and the need to adjust of dosage with time, PPIs (omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole) have now largely replaced the use of histamine H2R antagonists, and are currently the recommended drugs of choice, because of their long durations of action and potency (7,9,29,40,76,103,157,180-182,657,719). Most ZES patients without complicated disease (MEN1/ZES, moderate-severe GERD, post-Billroth II surgery) require only once a day dosing and many are controlled on PPI doses equivalent to those used in idiopathic PUD disease (i.e., equivalent to 20 mg/day omeprazole) (103,184,518,519,557). In patients with complicated disease (MEN1/ZES (especially with active hyperparathyroidism), moderate-severe GERD, post-Billroth II surgery) higher doses/frequency are usually needed (184,518,519,557).
Currently, somatostatin analogues are uncommonly used to control acid hypersecretion in ZES patient, because they must be given parenterally, whereas effective inexpensive long-acting, oral antisecretory agents such as PPIs are available and are the drugs of choice (29,103,142,151,181,182,396).
At presents most authorities, as well as all guidelines, agree that surgical resection for attempted cure should be performed in ZES patients whenever possible without undue risk, similar to the treatment of other potentially resectable pNENs (9,43,46,95,99,102,119,120,122,157,180,182,267,461,720-728). This approach is, in contrast to that in the recent past, wherein the role of routine surgery for cure was controversial, with some recommending that surgery not routinely be performed, because gastrinomas were frequently not found at surgery and cure was uncommon (729-731). In addition, many patients had negative preoperative imaging, and most patients with non-imaged or small gastrinomas had a good prognosis without surgery (729,731). The situation has changed because of results from a number of more systematic studies. In a NIH prospective surgical study (43) of sporadic ZES patients (n=123), the immediate postoperative cure rate was 51% and after 10-years was 34%. A number of other NIH surgical studies (45,175,176,241,254,258,732,733), as well as studies from other institutions (46,95,99,102,122,177,724) have provided additional support for routine surgery. This approach is further supported by two NIH studies on survival/disease course post-surgical resection of the primary gastrinoma, with the one study (732) demonstrating that patients who underwent routine exploration had a lower incidence of developing liver metastases post-resection (3% vs 23%, p<0.003). A subsequent NIH study (733) with more patients (n=160) and a longer follow-up (mean 12 yrs. postresection) demonstrated that patients undergoing surgery had a better overall survival (15 yrs., 98% vs 74%, p=0.0002); the survival advantage was disease-related (p=0.0012), due to less tumor progression, and fewer patients developed liver metastases (5% vs 29%, p=0.0002). This is a particularly important finding, because two NIH studies (109,110) in patients with gastrinomas, as well as a number of other studies both in patients with gastrinomas and other pNEN (115,116,235,276,433,639,734), have demonstrated that the development/presence of liver metastases is one of the most important prognostic markers of long-term survival in these patients. Neither of above NIH studies were randomized, but in each case the comparative groups were well matched (109,110,242,733).
In the past, imaging studies were not infrequently all negative on preoperative studies, and because these ZES patients had an excellent prognosis without surgery, and because surgery was often negative in these patients, this led a number of investigators to recommend against surgical exploration in this group (45,515,729-731,735-737). A subsequent NIH study (45) provided important information to challenge this approach by reporting the value of surgery in patients with preoperative negative imaging in an expert treatment center. In this study (45), in 58 ZES patients with negative preoperative imaging (40%=negative SRS), at surgical exploration, a gastrinoma was found in almost every patient (98%), and nearly 50% were cured. The postoperative cure rate was not different from ZES patients with positive preoperative imaging studies treated in a similar manner (45). This study demonstrated that if the diagnosis of ZES is appropriately established, that an experienced surgeon can find gastrinoma in almost every patient, even if imaging studies and negative, and almost one-half will be cured (45). This improvement in the surgical success of finding and curing gastrinomas in sporadic ZES patients has occurred because of a number of factors: particularly important is the appreciation that the majority of gastrinomas are not in the pancreas, as previously thought, and are, in fact, small duodenal tumors (often <1 cm) (6,109,110,175,177,236,286,738), which are frequently missed on even the most sensitive pre-operative imaging studies, including SRI (293); which will be missed at standard surgical operations if special duodenal gastrinoma localization procedures are not used, such as duodenotomy with or without duodenal-transillumination (175,176,244,247); the use of improved imaging including SRI (549); at surgical exploration the routine resection of pancreatic head area lymph nodes because of the possibility of lymph node primaries (258,259,268,269,269-272,739); and an understanding that patients with sporadic ZES have a different surgical outcome than those with MEN1/ZES (9,30,43,179,723).
The standard operation includes besides a careful inspection of the duodenum, pancreas and general abdominal inspection, a Kocher maneuver to explore the pancreatic/head; a duodenotomy with or without duodenal transillumination; routine resection of pancreatic/duodenal lymph nodes; careful inspection of biliary tract and liver, and an intra-operative ultrasound(IOUS) examination of the pancreas (43,95,120,175,176,247,249,258,288,738,740-743). This detailed examination is based on the fact that the relative order of occurrence of gastrinomas is duodenum>>pancreas>lymph node primary>primary liver/biliary tract> other (ovary, mesentery, gastric, etc.) (27,40,50,744). The most important procedure is a careful inspection of the duodenum. This requires the performance of a duodenotomy which is characteristically a 3-cm longitudinal duodenotomy centered on the anterolateral surface of the descending part (second portion) of the duodenum accompanied in the NIH protocol with transillumination of the duodenum (175,176,244,247). A duodenotomy is required to carefully inspect the duodenum because intraoperative ultrasound (IOUS) has been found to be relatively insensitive for duodenal wall tumors in patients with ZES (740). The use of a duodenotomy was proposed by Norman Thompson, University of Michigan in 1989 (246) at a time when most physicians though gastrinomas were primarily intrapancreatic, similar to insulinomas and its benefits and risks for detecting occult duodenal gastrinomas was debated (175,175,246,247,721). Its routine use was firmly established by a prospective study at NIH involving 35 patients with ZES in which all patients first had the standard exploration for a duodenal tumor involving careful palpation without a duodenotomy/or intraoperative transillumination of duodenum, followed IOUS, then duodenal transillumination and finally a duodenotomy (244). Standard palpation identified only 61% of all duodenal tumors found by any method, IOUS found only 26% and no new lesions; transillumination identified 64% of all duodenal tumors and 6 of these were new tumors, whereas duodenotomy identified all 31 duodenal tumors of which 5 were not identified by any other method.
This result was corroborated by another NIH study (176) which compared the surgical results from 36 patients (Group 1) who underwent the standard laparotomy (1980-1986) without duodenotomy (prior to its routine use) to a group receiving the same operation but with transillumination and a duodenotomy (19987-1990-37 patients) (Group 2). Gastrinomas were found in significantly more patients in Group 2(92% vs 64%, p<0.01); this increase was due to more duodenal gastrinomas detected in Group 2 (43% vs 11%, p<0.01) which resulted in an increased disease-free rate in group 2 (176). Most importantly, a NIH 2004 study (175) examined the long-term effects of adding a duodenotomy on the long-term cure rate. This study (176) compared results in 143 ZES patients, of which all had the standard exploration protocol, but 79 had a duodenotomy and the others had not had one. Gastrinomas were found in a higher percentage of patients who had underwent a duodenotomy (98 vs 76%, p<0.000011); as were duodenal gastrinomas (62 vs 18%, p<0.00001), whereas the detection rate of pancreatic tumors was similar; the duodenotomy group had a postoperative cure rate that was higher (62 vs 44%, p=0.010) as well as the long-term cure rate (52 vs 26%, p=0.0012). These results have established the need for all patients to have duodenotomy at exploration (9,157,181,182,745).
The routine resection of peri-duodena/peripancreatic lymph nodes is recommend for two reasons. First, as discussed earlier, a number of different groups have reported lymph node primary gastrinomas (27,40,43,258,259,266-274)which in the NIH series (258) was the third largest primary tumor group after pancreaticoduodenal tumors, comprising 10 % and their resection resulted in a disease-free state. Secondly, lymphadenopathy is reported to increase the disease-free rate postresection in ZES patients, as well as to increase overall survival in patients with sporadic ZES (746).
In contrast to the situation with sporadic ZES (no MEN1), the surgical management of MEN1/ZES patients remains controversial (9,30,87,92,93,95,95,96,99,120,122,123,128,157,181,182,745,747). This has occurred because almost all studies demonstrate that these patients are rarely cured by the standard ZES operation involving local tumor resection/enucleation even with a duodenotomy, and that cure only occurs if a Whipple resection is performed, which is not routinely recommended (9,43,44,88,89,92,122,181,182,747,748). Even though pancreaticoduodenectomy (Whipple resection) will cure the ZES in MEN1 patients (30,92,119,747,748), it is not routinely or generally recommended by most groups or in most guidelines in patients with MEN1/ZES, primarily because of the long-term potential complications (119,748,749). Also, in patients with NF-pNEN, because of the multiplicity of small adenomas, a total pancreatectomy would be required, which because of its morbidity, is not recommended (30,750). This low cure rate with nonaggressive resections occurs because MEN1/ZES patients almost invariably have multiple, duodenal gastrinomas which are microscopic to small in size (many <0.5 cm) and thus difficult to find at surgery, as well as >50% have metastatic lymph nodes at surgery (43,95,119,178,179,191,284). On preoperative imaging studies in MEN1/ZES patients, duodenal-pancreatic NENs are frequently visualized, however, the peripancreatic tumors are often not the primary but an adjacent positive metastatic lymph node, whereas the pancreatic NENs frequently visualized are usually not gastrinomas (0-<15%) (mostly-nonfunctional-pNEN) (88,191,284). Numerous studies report that if the preoperative imaging studies identify a tumor <1.5-2 cm in diameter, that these patients have an excellent long-term prognosis; in fact, survival is not different from MEN1 patients without a pNEN seen in some studies (9,27,191,751).
A number of other points complicate the decision for surgery and the management of the pancreatic-duodenal lesions in MEN1 patients and have particularly importance in recommending a more conserve approach than aggressive surgical resection in MEN1/ZES patients. First, pNEN present approximately 10-years earlier in MEN1 than sporadic cases (30,87), even occasionally occurring in patients < 20 years old (101,752). This has led to added controversy on whether such young patients should undergo surgery or have continued surveillance. Second, MEN1 patients have an increased incidence of glucose intolerance and diabetes (753,754). This could become an important consideration, particularly in younger patients if they underwent extensive pancreatic resections such as Whipple resection, because the occurrence of glucose intolerance/diabetes after such procedures is reported in different series as, 10% (749), 34 % (755),40% (102) and 86%(756) if MEN1 patients underwent a major pancreatic resection and in 8-27 % of any patients undergoing pancreaticoduodenectomy (102,757,758). Furthermore, pancreatic insufficiency develops in 41-50% after major resections or un 40% after Whipple resections (102) which can complicate the post-surgical clinical management. Third, is the potential importance of continued radiation exposure in MEN1 patients who require life-long monitoring (90). This could become an important issue if these patients are followed, and continued imaging surveillance is required. Some recommend endoscopic ultrasound (EUS) for this purpose (90), which is the most sensitive modality, however, it is an invasive procedure which is done under general anesthesia in many centers, and therefore other imaging modalities that allow serial assessment of changes in pNEN size, would be of value, such as repeated cross-sectional imaging studies (MRI, CT scanning), however they are less sensitive. These cross-sectional imaging modalities (CT, MRI) very frequently miss small pNEN<1.5-2 cm in diameter, a group that numerous studies shows do not have an increased mortality from pNEN (181,505,751,759,760). For the above reasons, there has been increased interest in MEN1 patients, especially younger patients, in imaging studies not involving radiation such as MRI, but because MRI does not detect a significant number of small pNEN in MEN1 patients, there also is increased interest in more sensitive imaging studies such as 68Ga-DOTATOC positron emission tomographic/CT imaging (68Ga-DOTATOC-PET/CT) which involve radiation. This interest has especially increased with recent studies reporting for the first time prospective (549,761,762) and non-prospective studies (763,764) demonstrating enhanced sensitivity/specificity for localizing NETs, including pNEN, in MEN1 patients, using 68Ga-DOTATOC-PET/CT. Lifetime exposure to radiation may be a particular issue in MEN1 patients because basic science studies demonstrate that menin, the protein altered in patients with MEN1, is involved in DNA repair, cell cycle control and transcriptional regulation, and when there is a loss of menin activity, as occurs in MEN1 patients, cells become more sensitive to the effects of ionizing radiation as well as other cell damaging injuries (765-767). As a result, a number of studies have raised concerns about the use of imaging studies involving radiation in younger patients (without MEN1) (768-770), and whether younger MEN1 patients are at increased risk is unclear. These points raise controversies about when and how frequent these serial imaging studies should be used.
For these reasons, most current guidelines, and expert opinions (9,44,88,157,180-182) for the treatment of pNEN in MEN1 patients recommend that MEN1/ZES patients with preoperative imaging studies demonstrating pNETs <1.5-2 cm in diameter not undergo routine surgical exploration. These guidelines also recommend that when surgical exploration is performed that Whipple resections not be routinely performed.
There are however, increasing concerns raised by the number of recent studies with this general conservative approach. Important points being raised is that these patients have a markedly shortened life- expectancy (i.e., 55 yrs. in the large prospective NIH review (89) with the major cause of death being malignant NENs, although presumed pNEN in origin it is not proven at this point (89). A second major point reviewed above is the enhanced ability to localize the primary NENs and their extent preoperative with the availability of SLI, allowing an enhanced ability to plan the operation and extend of surgery needed and likely enhancing the probability of cure. A third major point is that there have been a number of series (95,99,102) from different institutions reporting high cure rates and excellent long term survival in these patients after Whipple resections (95,99,102,119,178,191,683,747,748,771-801) . Furthermore, in a number of these studies the rate of post operative diabetes is less than previous reported in some studies and even not higher than seen with the recommended more conservative resections (273).
Increasingly, both patients with sporadic ZES, as well as those with MEN1/ZES, who had undergone an initial surgical resection, are being reoperated with time for either a recurrence after being initial rendered disease-free or due to increasing tumor growth with persistent disease (92,127,254,747,802-805). In a recent prospective NIH study of 52 ZES patients (254) with recurrence who underwent reoperation, the reoperation occurred a mean of 6 years after the initial surgery. After the reoperation, 35% were disease-free immediately postoperative and on the last follow-up after the repeat surgery (mean-8 years), 25% remained disease free, which are lower percentages than seen with the initial operation in NIH studies (43,45,175,254,805). In this study (254), the 20-year survival was 84% and the presence or absence of MEN1/ZES did not affect survival, but the length of the disease-free interval postresection and presence of liver metastases did. A recent study (127) reported recurrence in 108 sporadic ZES patients who had underwent an initial elective surgery between 2000-2020 in 15 different European hospitals. In these patients (127) 68 had duodenal gastrinomas, 19 (18%) had pancreatic gastrinomas, and 21 (19%) had a primary lymph node gastrinoma in the original surgery. During the initial surgery 74% of the patients with duodenal gastrinomas had a pancreaticoduodenectomy (Whipple Resection). For all gastrinoma patients (127) their mean OS was 173 mos., 5-yr survival 94%, and no predictive factors were found. The median DF-survival was 93 mos., and the 5 yr DF survival rate was 63%. For recurrence, significant prognostic factors were tumor size> 2 cm, (P=0.00001), tumor grade (p=0.00001) and pancreatic gastrinoma location (p=0.0001), however on multivariate analysis only tumor size >2 cm (p=0.005) and grade (p-0.013) were significant. Specifically, not a significant prognostic factor was age, sex, preoperative gastrin level, lymphadenopathy <10 nodes or metastatic lymph nodes in resected nodes. Also, for duodenal gastrinoma the recurrence rate was similar in patients with a Whipple operation to that in patients with excisions of duodenal tumors and lymphadenectomy (127).
A recent study also reported the results of duodenopancreatic reoperations in patients with MEN1(12 patients), of whom 5 patients (42%) had MEN1/ZES (92,747). In this study (92,747) the mean time to reoperation was 5.5 yrs., and with a long-term mean follow-up of 18 years, 83% (10/12) remained alive. The authors (92,747) concluded reoperations in this group of patients are not uncommon, there is no increased perioperative morbidity with reoperation in a specialty center, the patients can have prolonged survival after reoperation and that organ-sparing resections are preferred in these patients.
General Points
With the increased ability to medically control the gastric acid hypersecretory state in ZES patients, the natural history/growth of the gastrinoma is becoming the major determinant of long-term survival in ZES patients (46,89,109,110,314,433). Natural history studies show that gastrinomas are malignant in 60-90% of patients, and at present, approximately one-third of ZES patients present with metastatic disease to the liver, and because most patients are not cured surgically, an increasing proportion develop advanced metastatic disease over time (40,46,89,109,110,254,314,433,639). Overall, in NIH prospective studies, 25% of patients with sporadic ZES (109,110) and 15% of MEN1/ZES patients (111) have tumors showing an aggressive growth pattern, and in 40% of patients with hepatic metastases, aggressive growth occurs (429). As a result, currently one-half of ZES patients have tumor-related deaths (109).
A number of clinical, laboratory, pathological and other tumoral features in ZES patients are associated with a poor prognosis and are summarized in Table 10. A number of studies report one of the most important prognostic factors is the presence of any liver metastases (initially or their development) (Table 10). For example, in the NIH studies, the 10-year survival of ZES patients with no liver metastases initially is 96%, with liver metastases limited to one hepatic lobe is 78%, and with diffuse liver metastases is 16% (109,110). If liver metastases develop for the first time during the follow-up period after an initial evaluation wherein no liver metastases were present, the ten-year survival is decreased to 85% (110). However, in different studies at different times in the NIH cohort of ZES patients, the presence of lymph node metastases alone was, at best, only a weak predictor of poor prognosis, and in fact, was not predictive in a number of early studies (Table 10) (109,110,314,806). In a detailed analysis (806) of 216 pNEN patients at NIH in which >90% were ZES, with a prolonged follow-up (mean 11 years), overall survival decreased not only in patients with any lymph node positive, but also the extent of decrease in survival correlated with the number of positive lymph nodes. This result is consistent with some general studies in patients with various pNEN (807-809), but differs from others, which found no effect of lymph node metastases on survival in patients with various pNEN (130,314,806,810-813).
Numerous characteristics of the gastrinoma itself correlate with decreased survival including (Table 10): pancreatic location over duodenal location; increasing primary size; rate of growth overtime; in addition to the presence of liver or lymph node metastases the development of bone metastases has a poor prognosis. The development of ectopic, Cushing’s syndrome or bone-metastases has a particularly poor prognosis with survival averaging only one year (109,113,294,611). The fact that duodenal and pancreatic gastrinomas are equally malignant (40-70%=lymph node metastases), but not equally aggressive, with liver metastases present in 25-40% of pancreatic gastrinomas, but in only 2% of duodenal gastrinomas; results in pancreatic gastrinomas having a worse prognosis (Table 10) (27,109,110,314,433). Other features of gastrinomas associated with a poor prognosis including advanced ENET/WHO classification, higher ENET/WHO grade, poor differentiation, other histological features, and rapid growth (Table 10) (65,142,301,814).
As mentioned earlier in the pathology section of this paper, both the recently developed TNM tumor classification systems (ENETs, UICC/AJCC/WHO) and the tumor grading systems have been shown to be the most important single factors in numerous multivariate analyses for predicting overall survival or disease-free survival in all NENs ( pNEN, GI-NENs (Carcinoids) (Table 10) (115,116,142,815-817). Most (>90%) of gastrinomas are well differentiated NENs (Grade G1 or G2), and at present there is only one study just including only gastrinomas showing the importance prognostic effect of grade on survival of ZES patients (65). However, because of the almost universal importance of these classification/grading systems in studies involving all pNEN, it is almost certain this will be true of gastrinomas also.
|
Table 10. Prognostic Factors in Patients with Gastrinomas (overall survival or associated with increased development liver metastases)
|
|
Prognostic factor for decreased survival
|
Reference(s)
|
|
I. GASTRINOMAS ONLY
|
|
I.A. Acid Control
|
|
Uncontrolled acid hypersecretion
|
(1,27,205,433,638)
|
|
I.B. Demographic Features
|
|
Female gender (p=0.024)
|
(109,110)
|
|
Diagnosis before 1980 (p=0.010)
|
(315)
|
|
Older age at diagnosis (p=0.001)
|
(65,315)
|
|
I.C. Disease Clinical/Lab Features
|
|
MEN1 absent (sporadic ZES) (p<0.03) (Fig.3.D)
|
(64,65,110,207,734,818)
|
|
Short disease history prior diagnosis (<3 yrs.) (p<0.001)
|
(109,110,819)
|
|
High gastrin (p=0.022)
|
(109,110,191,507,819)
|
|
I.D. Disease Course
|
|
Recurrence postop with short disease-free interval
|
(254)
|
|
Develop ectopic Cushing’s syndrome (p=0.0049)
|
(109,113)
|
|
Primary gastrinoma location
|
|
|
Pancreatic >duodenal (p<0.004)
|
(109,110,281,315,734,819)
|
|
I.E. Tumor size, Location, Extent, Growth Rate
|
|
Large primary tumor size (>2-3 cm)
|
(109,110,315,433,507,819)
|
|
Gastrinoma located to the Left of the SMA> right of SMA (gastrinoma triangle)
|
(415)
|
|
Presence of Lymph node metastases (p<0.004)
|
(109,734,806)
|
|
Extent/presence of liver metastases (p<0.0001)
|
(27,64,109,110,254,314,314,433,507,734)
|
|
Diffuse>localized (p<0.0001)
|
|
|
Diffuse>both lobes>single lobe>none
|
|
|
Rate of growth of liver metastases or tumor
|
|
|
Rapid> slow, none
|
(111,402,429)
|
|
Time liver metastases diagnosed
|
|
|
Present initially>develop on follow-up (p=0.02)
|
(109)
|
|
Develop bone or extrahepatic metastases (p<0.0001)
|
(65,109,112,433,611)
|
|
I.F. Specific Tumor Features
|
|
Flow cytometric results
|
|
|
High S phase, low % nontetraploid aneuploid, multiple stem line aneuploid frequent
|
(820)
|
|
Molecular changes
|
(821)
|
|
(Chromosome 1qLOH) (p=0.019))
|
(822)
|
|
(Chromosome XLOH) (p=0.042))
|
|
|
Tumor grade
|
(65)
|
|
II. ADDITIONAL FEATURES SHARED WITH OTHER pNEN
|
|
II.A. Classification
|
|
Advanced TNM classification (ENETs, UICC/AJCC/WHO)
|
(41,115,116,142,816,817,823)
|
|
IIB. Histological Features
|
|
Poorly differentiated
|
(41,115,116,142,824,825)
|
|
High Ki67>low Ki67 proliferative index
|
(115,116,142,815-817)
|
|
Cytokeratin 19-IR positivity
|
(115,116,826-828)
|
|
Vascular, neural invasion
|
(115,116,639,829)
|
|
Decreased expression of autophagic genes
|
(117)
|
|
Alternative lengthening of telomeres, ATRX/DAXX loss
|
(444)
|
|
Alpha cell origin over Beta cell origin
|
(444)
|
|
IIC. Other Features
|
|
Age
|
(41,825)
|
|
Gender
|
(825)
|
|
Poor symptom control post-resection
|
(32)
|
|
No surgical resection
|
(41,825)
|
|
NF-pNEN rather than F-pNEN
|
(825)
|
|
Tumor size
|
(825)
|
Abbreviations: SMA, superior mesenteric artery; LOH, loss of heterozygosity; IR, immunoreactivity; ENETs, European Neuroendocrine Tumor network; postop-postoperative
A wide range of different anti-tumor treatments is used in patients with advanced pNEN, which are similar to that used in all advanced NENs. These include: surgical resection including cytoreductive (debunking) surgery; liver-directed therapies including radio-frequency ablation (RFA)/other local ablative therapies; trans-arterial embolization (TAE) or chemo-embolization (TACE), radio-embolization or selective internal radiation therapy (SIRT); chemotherapy; biotherapy with somatostatin analogues or interferon-Alfa; molecular targeted therapy with mTOR (everolimus) or tyrosine kinase inhibitors; peptide radio-receptor therapy (PRRT), liver transplantation and immunotherapy (38,50,142,158,604,830-839). There are only a few small, specific studies including only patients with metastatic gastrinomas as they are usually included in series with other metastatic pNEN, and in some cases even with GI-NENs (Carcinoids). Thus, below the results will primarily be from series containing pNEN with some gastrinomas.
One of the main problems with all forms of anti-tumor treatment in patients with advanced pNENs and other NENs, is the development of resistant to the therapies with time. This occurs to varying degrees at different times with all of the current therapies except complete surgical removal without recurrence (840,841). Numerous experimental approaches have been tried after failure of the different primary therapies, with the most frequent approach used is to switch to another primary established approach (840,841). The development of resistant with treatment and approaches recommended to deal with it will be briefly covered in each of the following sections dealing with the various established specific primary anti-tumor therapies.
Cytoreductive Surgery
In patients with gastrinomas with advanced metastatic disease, similar to all malignant NENs, it is recommended that the possibility of surgical removal of all resectable tumor (cytoreductive surgery, debunking surgery) should be considered by many authorities although there are no controlled studies to support its value (9,41,128,142,143,157,171,181,191,258,288,831,842-862,862-864). Surgery is generally recommended if ≥90% of all imageable disease can be removed, although others, have recommended lower numbers. There are only a few reports containing primarily gastrinomas treated with this approach (122,258,288,403,842-844,865), with most studies reporting results from different malignant pNEN and in some cases combined with GI-NENs (carcinoids). In various studies using this approach five-year survivals of 75-80% are reported and increased survival over patients not undergoing such surgery (128,142,143,157,831,849-859,861,862,862,864,866). This approach is primarily used in patients with well differentiated NENs, which is the case in >90% of gastrinomas (G1, G2,G3NET). Unfortunately, this approach is possible in the minority of even patients with advanced well differentiated gastrinomas or other NENs (<15-20%), and because of lack of control studies establishing its efficacy, it is not uniformly used. In only a small minority of G3NEC patients, is such an approach considered and even then, it is controversial (857,867).
At the time of any abdominal surgery, it is generally recommended that prophylactic cholecystectomy be performed because of the widespread use of somatostatin analogues for their anti-tumor activity and the ability of long-term treatment with them to cause biliary stasis and gallstones (143,180,849,868). Lastly, recent non-controlled studies, report that removal of the primary tumor increases the survival rate with PRRT and suggest it routinely be performed, although this approach is not widely used at present (869).
Liver Directed Therapies
GENERAL
These approaches include the use of local ablative techniques (radiofrequency ablation (RFA), ethanol injections, cryotherapy), which are frequently used in combination with other anti-tumor treatments, as well as various more general hepatic cytotoxic approaches using trans-arterial embolization (TAE)/chemo-embolization (TACE) or radioembolization (42,142,143,831,834,851,859,870-872,872-877). The embolization approaches in gastrinoma patients are generally reserved for patients who have metastatic unresectable hepatic metastases either limited to the liver or with liver-predominant disease, particularly if locally symptomatic, whereas in patients with other F-NENs, they are also frequently used for patients in whom the symptoms due to F-NEN excess-state not controlled by other modalities (25,142,143,831,834,851,870-872,876,878,879).
RADIOFREQUENCY AND OTHER ABLATIVE THERAPIES
Of the all of the liver-directed therapies, RFA is the most widely used, which converts RF waves to heat resulting in cellular destruction (142,831,880-883). RFA and other ablative techniques (cryotherapy, ethanol injections) are administered either at the time of surgery (+/- laparoscopic) to ablate isolated metastases or by radiological techniques for guidance (25,142,844,847,881,882,884-887).In different studies, relative contra-indications to its use are the presence of large lesions (>3.5-5 cm), a large number of lesions (>5-15), and he presence of metastases near vital structures (831,848,880,880,881,883,885,887-889). RFA has response rates of 80-95% which last up to 3 years, has the lowest complication rate of all liver-directed therapies (<15%), and can be used alone for a palliative procedure or to supplement a surgical resection by removing additional isolated liver metastases (25,831,880,885,888).
Embolization and Chemoembolization
Embolization of advanced metastases in the liver in patients with metastatic gastrinomas or other NENs, can be used because the blood supply to the tumor is primarily arterial, whereas in normal liver only 20-25% is arterial, with the majority coming from the portal vein (42,142,831,870,872-876,880,882). Therefore, interrupting the tumoral area arterial supply preferentially affects metastases (142,870,880,882). At present, this procedure is usually performed radiological, rather than at surgery and can be done alone (trans-arterial embolization or TAE) or accompanied by administration of chemotherapeutic agents (trans-arterial chemoembolization or TACE) such as doxorubicin, cisplatin, 5-fluorouracil, mitomycin C or streptozotocin (42,142,837,870,872,872,873,875,876,880,882,888,890). TAE or TACE is performed using gel foam powder or polyvinyl alcohol particles. In various studies a response is seen in 55-100% of symptomatic patients, 25-85% have an objective tumor response, and responses last from 6-45 mos. (142,831,834,870,872,880,891). Five-year survival rates for TAE/TACE are 20-35% and the progression free survival is 1.5 years (142,870,892). Contra-indications are the presence of portal venous occlusion, liver failure, extensive liver involvement (>50-75%), poor performance score, and previous biliary surgical reconstruction (142,870,880,888,890-893). Both TAE and TACE are associated with side-effects including a mortality rate of <6%, complications in 10-80%, particularly post embolization syndrome (pain, fever, nausea/vomiting), and occasionally gallbladder necrosis, hepatic failure, abscess formation and liver/renal failure (831,834,870,880,882,888,890,891,894). TAE/TACE are generally considered for palliative therapy in patients with non-resectable liver metastases with hepatic predominant disease (142,143,180,834,872,891). There are no prospective studies that have established the value of TAE/TACE; however, both the NANETS and ENETs guidelines recommend TAE/TACE be considered for palliative treatment in an experienced center if the patient has hepatic-only or hepatic-predominant disease that is not surgically resectable (142,143,180,182,834,848,891).
Radioembolization or Selective Internal Radiation Therapy (SIRT)
Radio-embolization or selective internal radiation therapy (SIRT) utilizes 90Yttrium-labeled microspheres (Sir-spheres-20-60 um diameter, load-50Bq/sphere or Theraspheres-glass sphere, 20-30um diameter, 2500 Bq/sphere), which are administered by selective intra-arterial injection after a pretreatment angiogram to allow correct catheter localization (9,142,181,182,831,848,871,872,876,876-878,895-905). Prior to their administration, the position of the catheter tip needs to be properly established so that microsphere administration does not enter the cystic or duodenal arteries, which can result in cholecystitis or ulceration, and the amount of lung shunting must be determined to avoid radiation pneumonitis (142,831,871,871,872). Contraindications include: the presence of excessive shunting to the lung/GI tract; inadequate liver reserve; and the inability to isolate the liver arterial tree from the gastric/small intestinal branches (142,831,871,872,905,906). The mean objective response rate from 12 studies in patients with advanced NENs was 55% (range-12-90%) with stable disease seen in 32% (range-10-60% (142,831,871,905,907) and the disease control rate was 91% in a recent multicenter international study (908). The mean survival is 30-months and 50% of patients have symptomatic improvement in quality-of-life indices (142,871,897-899). Side-effects include post-embolization (fever, nausea, vomiting, abdominal pain) (25-45%), \and rarely ulceration or cholecystitis (<1%) if the catheter is not properly positioned (142,831,871,905,907,909).
At present the exact embolization procedure that is preferable in which clinical situation is not clear because of lack of prospective comparative studies. There have been no randomized control trials comparing radioembolization to the other liver-directed therapies, so at present at is unclear which should be preferred.
CHEMOTHERAPY
Chemotherapy has a poor response rate (<15%) in well-differentiated NENs outside the pancreas (lung/GI-NETs, carcinoids) and thus is uncommonly used for these tumors, it has higher response rates in different series of malignant, well-differentiated pNEN, varying from 25% to 70 % (9,147,181,182,832,848,893,904,910-920). Until recently the generally used chemotherapeutic regimen was streptozotocin (STZ) based for advanced pNEN (most frequently combined with doxorubicin, 5-FU or cyclophosphamide) (9,142,147,181,182,832,878,893,910,916-919,921). STZ is a glycosamine-nitrourea derivative which was found to have cytotoxic effect on pancreatic islets (28), and since 1968 has been used for the treatment of patients with metastatic pNEN/gastrinomas (9,142,181,182,878,893,916-918,921). However, recent studies report temozolomide and capecitabine may have at least similar if not better activity than STX based regimens (827,830,911-913,918-920,922-927). Recently, in a randomized, prospective trial (ECOG-ACRIN E2211) (928) the Eastern Cooperative Oncology group compared the combination of temozolomide (TMZ) and capecitabine (CAP)(CAPTEM) to TMZ alone in 144 patients with advanced G1/G2 pNENs. At the interim analysis the mean PFS was 14.4 mos. in the TMZ alone group and 22.7 MS FR CAPTEM (P=0.022). At the final analysis the mean S was 53,6 mos. for TMZ and 58.7. mos. with CAPTEM. MGMT deficiency was associated with the response in this study. The authors concluded that the CAPTEM combination was superior to TMZ alone and that MGMT deficiency correlated with a response (928). In a recent systematic review of CAPTEM treatment of patients with advanced NENs, involving 42 articles with 1818 patients the overall disease control rate was 77% (range 44-100%), the median PFS ranged from 4 to 38 mos., and the media OS ranged from 8 to 103 mos. In this review (920) the safety analysis showed an occurrence of G3-G4 toxicities in 16% of the patients treated. The most common toxicities were hematological (27%), gastrointestinal (8%) and cutaneous (3%). This systematic analysis (920) concluded CAPTEM was an effective and relatively safe treatment for patients with well-moderately differentiated NENs of pancreatic, GI, lung and unknown origin.
STZ-based regimens have considerable morbidity with 70-100% developing some side-effect including nausea/vomiting (70-100%), abnormalities in hepatic function, leukopenia, and thrombocytopenia in 6%, and 15-40% developing some degree of renal toxicity including proteinuria (40-60%) and decreased creatinine clearance (893,913,916,917). The combination of STZ/doxorubicin (±5-FU) has an objective response rate of 20-45%, but complete responses are rare, and the median-response duration is 5-20 months (9,142,181,182,830,878,893,914,916-919,921). In patients with advanced gastrinomas only, the response rate varied from 5 to 40% (147,929).
Poorly differentiated pNETs comprise only 2-6.5% of all pNETs; however, it is important that they be identified because they have an aggressive course and poor prognoses and are generally treated differently than well-differentiated pNEN (54,142,848,912,913,918,930-935). The recommended chemotherapeutic drug combinations for treatment of well differentiated pNEN differs from that for treatment of poorly differentiated NENs (Grade 3) in any location (142,143,180,912,913,932,935-937). In contrast to the combinations listed above to treat advanced well differentiated pNEN, in patients with poorly differentiated NENs, a cisplatin-based drug combination with etoposide is generally the initial treatment (142,143,180,848,912,913,918,919,931,932,936,938,939). This combination results in an objective response in 30-80% of patients with mean duration of <12 months (142,143,180,912,932,936). The median survival is 4-16 mos., and the 5-year survival is 11% (range 0-31%) (142,143,180,912,913,918,931,932,936,938). This chemotherapeutic regiment can be associated with significant toxicity including GI toxicity (nausea/vomiting), myeloid-suppression and renal toxicity (142,143,180,912,913,931,932,936,938,939). In a recent multicenter study promising results were reported with the use of temozolomide/capecitabine (92%/8%=TMZ alone) (CAPTEM) in patients with Gr3 GP-NENs (933). In this study (933) the results of treatment with CAPTEM were reported from 130 patients (67% pNENs) and a radiological response was seen in 36%, the median TTF was 3.6 mos., OS was 9.2 mos., with the TTF being longer in pNENs than patients with GI-NENs Gr 3 tumors (5.8 vs 1.8 m0s, p=0.04). The role of surgery in patients with high grade NENs is controversial (940), although it is reported to be associated with higher survival in those with Gr3 WDs in some studies (940).
Recently, some studies (926,928,941-948) report that the effectiveness of alkylating agents in NENs correlated with the expression of, but not all (489,945,949-951) the DNA repair enzyme, O6-methylguanine DNA methyl transferase (MGMT) in these tumors. MGMT, in its role as a DNA repair enzyme, specifically removes the methyl/alkyl group form the O6 position of guanine, whereas alkylating agents induce methylation at this site which leads to DNA mismatch occurring and results in cell death/apoptosis. Some studies show that pNEN have a higher response rate to alkylating agents due to their low level of MGMT (926) compared to GI-NENs (carcinoids) having higher MGMT levels and lower response rate. Perspective studies are needed before recommending the routine determination of NEN tumoral MGMTs to help predict, for a given patient the subsequent response to an alkylating agent, and therefore its routine use is not generally recommended at this time (928,945-948).
BIOTHERAPY
Somatostatin Analogues
Similar to NENs in general, most well-differentiated G1, G2 pNEN, as well as a proportion of G3 pNEN, overexpress one of the 5 subtypes of somatostatin receptors (sst1-5) (most frequently sst2) (142,851,952-955). Numerous studies, including both non- controlled and randomized controlled studies (PROMID, CLARINET studies) on NENs and pNEN(including gastrinomas) (142,954,956,957), demonstrate that somatostatin agonist analogs (octreotide, lanreotide) are not only are effective for controlling the hormone-excess state in F-NENs(discussed in a previous section), but also have anti-tumor growth effects in NENs (25,142,155,402,603,609,848,851,952-955,958-965). The exact molecular basis for this antiproliferative-effect is not entirely clear, but somatostatin analogues inhibit the release of growth factors from NETs, have antiproliferative effects on neighboring cells (stromal, immune, vascular, etc.) and activate intracellular cascades that have antiproliferative effects (phosphatases, inhibition of adenylate -cyclase, etc.) (603,954,959,962-964). In these studies the anti-tumor effect of the somatostatin analogues is almost entirely a tumoristatic effect (only 10-15% show decreased tumor-size), resulting in disease stabilization with prolongation of progressive free survival, and because of study design and the multiple treatments the patients received, an effect on overall survival has not been established (155,402,603,609,848,956,958,961-964,966). However, these agents are very well tolerated, and are generally the first line treatment of patients with advanced well-differentiated gastrinomas and other pNEN as well as with lung/GI-NENs(carcinoids) (25,142,143,150,164,180,833,851,952-956). In a recent study of the use somatostatin analogs (SSAs) on tumor progression in 12 ZES patients (WD, G1, G2) (155), 67% had a sustained response to SSAs and 33% showed early progression. There was a significant difference in PFS between the early and late progression groups (84 vs 2 mos., p=0.004) (155). However, there was no difference OS or PFS between these 12 SSA treated ZES patients and 21 other ZES patients not treated with SSA analogues (155).
With time the tumor may become refractory to the antigrowth effect of the somatostatin analogue, and its efficacy may be restored by either increasing the dosage or shortening the time interval between doses (965). Side effects that result in somatostatin analogue therapy discontinuation are rare with any side-effect occurring in 50% of patients (including pain at injection site, GI symptoms), which may improve with continued treatment (25,29,142,848,954,955,958,961,964,967). Long-term more serious side-effects include the development of biliary sludge/gallstones which cause symptomatic disease in <1%, developing glucose intolerance/diabetes or developing steatorrhea which is usually mild (25,29,142,142,848,868,954,955,958,961,964,967).
Interferon
Interferon-alpha, similar to somatostatin analogues, is able to control symptoms of the hormone hypersecretory state and has anti-proliferative effects in pNEN/NENs which result in primarily disease stabilization, rather than a decrease in tumor size (<15%) (142,848,961,968-973). In the only study of interferon limited to gastrinoma patients (13 patients, advanced metastatic progressive disease) (972), 46% of patients showed disease stabilization, and in 23% it lasted almost 2 years. The antiproliferative effect on pNENs/NETs of interferon is partially mediated by blocking cell-cycle progression in G1, inhibiting DNA synthesis, stimulating an increase in Bcl-2, inhibiting protein synthesis, inhibiting angiogenesis, and induction of apoptosis (961,968,970,971). Side effects develop in the majority of patients (>70%) with the most frequent being flu-like symptoms (40-80%), weight loss/anorexia (60%), and fatigue (51%), which frequently decrease in severity with continued treatment or with decreased dose (848,961,970-972). More serious side effects include hepatotoxicity (31%), hyperlipemia (31%), and bone marrow toxicity; autoimmune disorders particularly thyroid disease and rarely CNS side effects such as depression or mental disorders (142,961,968,970-972). While interferon-alpha was frequently used in the past either alone or with somatostatin, at present it is uncommonly used because of the availability of other agents with fewer side effects.
MOLECULAR TARGETED THERAPIES
mTor-Inhibitors (Everolimus)
Both extensive in vitro and in vivo studies demonstrate that activation of the mTOR cascade, plays and important role in the proliferation, growth, and apoptosis of pNEN, as well as NENs in other locations (142,150,479,882,915,957,964,974-979). mTor is a serine-threonine kinase critically involved in a variety of cellular functions including apoptosis, cell-growth, and proliferation (150,957,974,977,978,980). A number of different mTOR antagonists have been developed and shown to have anti-proliferative effects in NENs in various in vitro and in vivo studies, however, only one, everolimus, has been approved by the FDA for patients with advanced NENs (pNEN (including gastrinomas) and GI-NENs (carcinoids) (142,150,480,974-976,978-981). The approval of everolimus for use in both advanced pNEN and GI-NENs(carcinoids) was based on the positive results of two randomized, double-blind, prospective, placebo-controlled studies, RADIANT-3 (pNEN) (480) and RADIANT-4 (lung/GI-NENs) (982), which each demonstrated almost a 3-fold increase in PFS (p<0.001). There are no specific studies on the effects of everolimus on gastrinomas only, and the only data comes from general trials of all pNEN.
Everolimus treatment was associated with a 2-fold increase in adverse events, the majority being grade 1 or 2, with grade 3 or 4 side effects occurring in 3-7% (primarily hematological, stomatitis, or hyperglycemia) which could be managed by dose-reduction or drug interruption (480). At present, it is not established whether everolimus’ ability to increase progression-free survival will result in an increase in overall survival (142,981).
Long-term treatment with everolimus is associated with primary and acquired resistance, which has frequently limited its long-term benefit for patients with advanced NEN (150,983-985). Numerous mechanisms for this have been described but none has been sufficiently successful to lead to its widespread use or its ability to function as a biomarker for the occurrence of resistance (983-985).
Numerous studies have provided information on the importance of angiogenesis in the pathogenesis of pNENs as well as other NENs (986). A recent randomized Phase II study compared the effectiveness of everolimus alone versus everolimus plus the anti-VEGF agent, bevacizumab in 150 patients with advanced pNENs (987). The combination resulted in improved PFS compared to everolimus alone (16.7 mos. vs 14 mos., complete tumor response in 31% with the combination compared to 12% with everolimus alone (p=0.0053), with similar median overall responses. Toxicities were more frequently seen in the combination group (987). The authors concluded that these results support the need for continued evaluation of VEGF pathway inhibitors for the treatment of advanced pNENs (987).
Tyrosine Kinase Receptor Inhibitors (Sunitinib and Surufatinib)
PNEN including gastrinomas, similar to other NENs, as well as other neoplasms and normal tissues, frequently possess multiple tyrosine kinase (TK) receptors, which are important in mediating growth, angiogenesis, differentiation, and apoptosis (26,142,848,895,957,964,988-995). TK receptors comprise >20 families of transmembrane receptors that mediate the actions of a number of different growth factors that include the receptors for insulin-like growth factor (IGF1R), epidermal growth factor family (EGFRs); hepatocyte growth factor (c-Met); platelet-derived growth factor family (PDGFRs); vascular endothelial growth factor family (VEGFRs); stem cell factor (c-Kit) and a number of others (142,957,992,994). A number to tyrosine kinase receptor antagonists (sunitinib, axitinib, cabozantinib, famitinib, nintedanib, pazopanib, sorafinib, sulfatinib, surufatinib) (979,994,996-998,998-1001) have been shown to have anti-growth/anti-angiogenic effects on pNEN/NENs in both in vitro and in vivo animal studies, however, at present the only two are approved for use in various countries which are sunitinib (US, Europe, other countries) which is an inhibitor of a number of tyrosine kinase receptors (PDGFR, VEGFR1/2,c-KIT, FLT-3) (142,914,989,991,994,996,1002-1004) and surufatinib (approved in China) which is an inhibitor of VEGFR, FGFR1, and colony stimulating factor 1 (CSF1R) (998,998-1001). In a Phase 3 double blind, randomized trial (1002) in 171 patients with progressive well-differentiated nonresectable pancreatic NENs (including 19 patients with ZES), sunitinib resulted in a doubling of PFS (11.4 vs. 4.5 mos., p<0.001), which lead to its approval for advanced pNEN. There are no specific studies on the effects of sunitinib on gastrinomas only, and the only data comes from general trials of all pNEN.
Sunitinib treatment is associated with frequent grade 1/2 side effects and some grade 3/4 side effects particularly neutropenia (12%) and hypertension (10%) (994,1002,1005). A quality-of -life analysis (1002) showed sunitinib did not have a significant effect, with most side-effects able to be managed by dose-reduction and/or temporary cessation of treatment.
Surufatinib was evaluated in two multicenter studies, one a randomized, double-blind, placebo controlled Phase III involving 172 patients with advanced pNENs in 21 different Chinese hospitals (999) and a second study (SANET-ep) involving 198 patients with advanced extra-pancreatic NENs 1in 24 hospitals in China (998), In the first study in patients with pNENs (999) the PFS was 19.3 mos. with surufatinib vs 3.7 with placebo (p=0.00110) with the most common Gr ¾ side-effects being hypertension (38% vs 7%), proteinuria (10% vs 1%) and hypertriglyceridemia (7% vs 0%). In the second study on patients with advanced extra-pancreatic NENs (998) the PFS was 9.2 mos. with surufatinib vs 3.8 with placebo (p<0.0001) with the most common Gr ¾ side-effects being hypertension (47% vs 13%) and proteinuria (19% vs 0%). Currently, surufatinib is not approved by either the FDA or European regulatory agencies.
Long-term treatment with sunitinib is associated with primary and acquired resistance, which has frequently limited their long-term benefit for patients with advanced NEN (840,841,983,984,994,995). Numerous mechanisms for this have been described but none has been sufficiently successful to lead to its widespread use or its ability to function as a biomarker for the occurrence of resistance (840,841,983,984,995).
Peptide Radioreceptor Therapy (PRRT) Using Radiolabeled Somatostatin Analogues
PRRT utilizes the fact that almost all well-differentiated NENs, as well as a proportion of G3NECs, overexpress somatostatin receptors (sst1-5) particularly sst2, which can bind radiolabeled somatostatin analogues resulting in the targeted delivery of cytotoxic radiation to the tumor cells (142,154,603-605,607,608,836,952,953,1006-1018). Two different isotopes have been used in most studies: 90Yttrium(90Y)- or 177Lutetium(177Lu)- labeled somatostatin analogues (142,154,836,1008-1016,1018). 177Lu emits beta-particles and gamma rays, has a maximum tissue penetration of 2 mm, and a half-life of 6.7 days, whereas 90Y strongly emits beta-particles, has a maximal tissue penetration of 12 mm, and a half-life of 2.7 days (1011-1015). A number of different synthetic somatostatin analogues have been used, with the most frequent being octreotide or octreotate coupled to the radiolabel by different chelators, including diethylene triamine penta-acetic acid (DTPA) and 1,4,7,10-tetraazaacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (142,1010,1011,1013,1014).
At present the only approved formulation for pNEN is 177Lu-DOTATATE (604,605,607,608,1006-1010). The approval of this therapy is based on results of a double-blinded, control phase 3 trial (NETTER-1) (1019) in patients with advanced unresectable, midgut carcinoids which showed a marked prolongation of PFS (from 8.4 mos. to >40 mos., p<0.0001), with an increased overall survival from 3 to 18%, combined with the results of treatment of 510 patients with advanced pNEN and other NENs, in Rotterdam which showed complete response in 2%, partial response in 28%, and tumor stabilization in 35% (404,1015). Of 440 patients (10 studies) with various malignant pNEN/NETs, including gastrinomas, treated with 90Y-labeled somatostatin analogues, complete tumor remission was rare (0-6%), partial remission occurred in 7-37%, and tumor stabilization in 40-86% (29,142,1011,1013,1014). In a recent meta-analysis of 22 studies (1758 patients) with advanced NENs treated with PRRT the pooled disease response rate (complete/partial tumor response) was 33% with RECIST criteria, and the pooled disease control rate (compete/partial response or stable disease) was 79% (1020). In a second recent meta-analysis of PRRT results (1018) involving 15 studies selected from 715 references in patients with advanced NENs, the pooled response rate was 27.6% by RECIST criteria, and 20.6 % by SWOG criteria, with respective DCR rates of 79.1% and 78.3% by the two criteria demonstrating excellent agreement with these two different criteria of response.
In two different studies there was no significant difference in the PFS overall survival rates between NEN patients treated with PRRT with advanced pNEN compared to NENs in other sites (869,1010).
Gastrinomas are one of the malignant pNEN/NETs that were most responsive to PRRT (42%-3mos); however, they also had one of the highest recurrence rates leading to a poorer prognosis (142,404,1011,1013-1015,1021). In one detailed study of 11 patients with metastatic ZES (1022) treated with either 90Y-and/or 177Lu-labeled somatostatin analogues, the mean serum gastrin decreased by 81%, compete response occurred in 9%, partial tumor response in 45%, tumor stabilization in 45%, and in 64% the antitumor effect persisted for a median period of 14 months. In a second study (1023) involving 30 gastrinoma patients treated with 90Y- labeled somatostatin analogues the tumoral partial response rate was 33% with a mean overall survival time of 40 mos.
The treatment was well tolerated when given with renal protective amino acid infusions, with no Grade 3/4 nephrotoxicity. In various studies the most serious side effects are hematological (15%-transient, 0.8% developing a myeloproliferative disorder), liver toxicity (0.6%) and renal toxicity, with the latter occurring primarily in patients receiving 90Y-labeled somatostatin analogues (154,404,604,605,607,608,1006,1007,1010,1015,1019).
Recent studies show that in patients with refractory hormonal symptoms due to a F-NEN, PRRT may be of great help in control the hormone excess state’s symptoms independent of its effect on tumor proliferation (25,148,350,860,1010,1024). Although this is almost never an issue in patients with gastrinoma because of the effectiveness of PPIs in controlling the symptoms of the gastric acid hypersecretion, numerous recent studies suggest that PRRT may be particularly helpful in patients with refractory advanced F-NEN syndromes in controlling the hormone excess state, particularly in patients with carcinoid syndrome, VIPomas and insulinomas (25,148,350,860,1010,1024).
With 177Lu-(DOTA0, Tyr3) octreotate, a number of prognostic factors were identified which predicted a poor outcome after PRRT, which included; presence of progressive metastatic disease prior to treatment; Karnofsky performance score of ≤70; no tumor-response to PRRT; weight loss at the time of treatment; presence of bone metastases; extensive liver involvement; poor uptake of the radiolabeled analogue by the tumor; the presence of malignant gastrinoma, VIPoma, or insulinoma and the presence of positive lesions on 18F-FDG Pet scans(142,404,612,1011,1013-1015,1021,1025,1026).
Recent studies support the conclusion that retreatment of NEN patients with PRRT who develop progressive disease after an initial PRRT treatment, is a feasible option with good efficacy and acceptable toxicity (1027,1028). This conclusion was supported by two recent systematic reviews and met analyses (1027,1029). In the report by (1027) 9 articles with 426 patients were analyzed and the ORR after retreatment was 17.1%, DCR was 76.9%, PFS was 14.1 mos. and OS was 26.9 mos. Pooled proportions of hematologic and renal toxicities were 11% and 0.7%. In a subgroup allowing direct comparison to initial PRRT treatment data, the salvage PRRT had a lower therapeutic efficacy (ORR, DCR, p<0.001) and shorter PFS (P=0.03), despite similar hematologic and renal toxicity. In a second analysis (1029) 13 studies were identified containing retreatment data (177Lu-PRRT, or 90Y-PRRT) from 567 original studies. With 177Lu-PRRT retreatment in 7 studies PFS was 12.5 mos., mean OS was 26.8 mos., and DCT was 71%. Grade ¾ toxicity occurred in 5% of 177Lu-PRRT retreated patients, with no Gr ¾ renal toxicity and no myelodysplastic and acute myeloid leukemia incidence.
Liver Transplantation
In contrast to many metastatic tumors, liver transplantation is both recommended for pNEN/NENs (including patients with advanced gastrinomas) and is selectively used in a small number of patients with metastatic advance metastatic pNEN/NENs, although it use remains controversial (142,143,1030-1038). In one recent systematic analysis of reported NEN series the 1-,3- and 5-yr survival rates were 89%, 69% and 63% with a recurrence rate after transplantation of 31-57% (1032). In another recent (2020) review of 206 patients with metastatic pNEN/NENs who underwent liver transplantation the overall survival rates at 1,3,5 and 10 years were 89%,75%, 65% and 46% respectively (1039). In this study (1039) the recurrence rate was 34% with a mean time to recurrence of 28 mos. Important prognostic factors in the systematic analysis (1032) were >50% liver involvement by tumor, high Ki67, or the presence of a pancreatic primary over a GI-NEN. In other studies important other factors predict a poorer prognosis include: older age of patient(>50), older age of donor, cold ischemic time MELD score, tumor recurrence, presence of a symptomatic tumor, a primary tumor in the pancreas as opposed to an extra-pancreatic NEN (carcinoid), poor tumor differentiation, transplantation associated with a simultaneous and extensive digestive tract resection, presence of hepatomegaly, presence of extra-hepatic metastases and a patient with a primary tumor not resected (1034-1036,1039). Liver transplantation is generally reserved for patients with life-threatening hormonal disturbances refractory to other treatments (which is very rare in ZES) or to selected patients with pNEN/NENs with diffuse liver involvement refractory to all other treatments (26,142,1033-1040).
If liver transplantation is considered, important selection criteria include the presence of a well-differentiated NEN/pNEN; age <45-50; a Ki-67 index <10%; <50% liver involvement; the absence of extra-hepatic metastases as determined using the most sensitive methodology (68Ga-labeled somatostatin-analogues and PET imaging) ; the absence of extra-hepatic disease(resected primary tumor); the absence of other resections at the time of liver-transplantation; and some groups consider various histological features such as E-cadherin-tumor staining characteristics (142,848,930,1032,1034-1039,1041,1042).
Immunotherapy
Since the recent widespread effectiveness of immune check point inhibitors in a number of tumors (melanomas, etc.), including in patients with poorly differentiated lung NENs with small cell neuroendocrine tumors, there have been several studies of the efficacy and safety in other tumors such as patients with advanced NENs including pNEN such as advanced gastrinomas (995,1043-1049).
A number of these studies suggest single agent (Anti-PDL1) immunotherapy may be a useful approach in only small subset of patients with advanced disease (12% in Keynote 02 study (1047) , 0-5% in other studies not selecting for PD-L1 activity (1049-1051) and this subset is primarily patients with high-grade poorly differentiated NENs (995,1043-1046). In various GEP-NENs the presence of high tumor-infiltrating lymphocyte (TILs) numbers and high PD-1 expression show a significant correlation with decreased survival and higher grading of the tumor supporting the finding that immunotherapy might be most promising in GEP-NENs with high TILs (1044). In other tumors particularly melanomas, renal cell carcinoma and non-small cell lung tumors, a combination of immunotherapies using anti-PD-L1 and CTLA-4 blockade resulted in increased efficacy, and a recent study in patients with various advanced NENs reported an objective response rate of 24 % with increased activity in patients with both atypical lung NENs and with high-grade pNEN (1046). Other studies report ORR of 14.7-25% almost entirely in patients with high grade NENs (995). Ongoing and future studies are in progress to exactly define the best combinations as well as define which specific NEN types will best respond (995,1043-1045).
Neoadjuvant Therapy
Because the large majority (>80%) of patients with advanced gastrinomas or other advanced NENs present with surgically unresectable disease, primarily with widespread liver metastases or unresectable locally invasive tumors, the possible benefit of surgical resection is not an option. Therefore, with the increasingly effectiveness of various anti-tumor modalities, there is increasing interest in a possible role of neoadjuvant treatments which could allow tumor downsizing to the point surgical resection can become an option (1052,1053). In one recent systematic review and meta-analysis of 9 studies from the literature (1052), involving 468 patients with advanced pNENs who had adjuvant therapies, there were no complete responders, 43.6% had a partial response; 51.3% had stable disease; and 4.3% had progressive disease. The estimated resection rate was 68.2%, and the RO resection rate was 60.2% (1052). There was no difference in the resection rate between different chemotherapy regimens (41% vs 34%), as well as the RO resection rate (62% to 68%) and the ORR was similar with CAPTEM and FAS (42% and 34%). PRRT showed a higher numerical ORR than chemotherapy although the difference did not reach statistical significance (49% vs 37%, p=0.154). The authors concluded these results were promising for some patients, however the best neoadjuvant regime to use remains unclear (1052).
REFERENCES
- Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg 1955;142:709-728
- Stabile BE. Gastrinoma before Zollinger and Ellison. Am J Surg 1997;174:232-236
- Wilson SD, Ellison EC. Invited Commentary. Ann Surg 2019;270:e22
- Soreide JA, Lea D. The Gastrinoma Saga Before Zollinger and Ellison: The Strom Case Revisited. Ann Surg 2019;270:e19-e21
- Soreide JA, Hem E. Roar Strom - the Norwegian surgeon who was three years ahead of Zollinger and Ellison. Tidsskr Nor Laegeforen 2019;139:
- Norton JA, Foster DS, Ito T , et al. Gastrinomas: Medical and SurgicalTreatment. Endocrinol Metab Clin North Am 2018;47:577-601
- Jensen RT. Gastrointestinal endocrine tumors.Gastrinoma. Bailliere's Clin Gastroenterol 1996;10:555-766
- Rehfeld JF, van Solinge WW. The tumor biology of gastrin and cholecystokinin. Adv Cancer Res 1994;63:295-347
- Jensen RT, Niederle B, Mitry E , et al. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173-182
- Shaw DH. Gastrinoma (Zollinger-Ellison Syndrome) in the Dog and cat. Can Vet J 1988;29:448-452
- Hoenerhoff M, Kiupel M. Concurrent gastrinoma and somatostatinoma in a 10-year-old Portuguese water dog. J Comp Pathol 2004;130:313-318
- Brooks D, Watson GL. Omeprazole in a dog with gastrinoma. J Vet Intern Med 1997;11:379-381
- Green RA, Gartrell CL. Gastrinoma: a retrospective study of four cases (1985-1995). J Am Anim Hosp Assoc 1997;33:524-527
- Hayden DW, Henson MS. Gastrin-secreting pancreatic endocrine tumor in a dog (putative Zollinger-Ellison syndrome). J Vet Diagn Invest 1997;9:100-103
- Straus E, Johnson GF, Yalow RS. Canine Zollinger-Ellison syndrome. Gastroenterology 1977;72:380-381
- Fukushima U, Sato M, Okano S , et al. A case of gastrinoma in a Shih-Tzu dog. J Vet Med Sci 2004;66:311-313
- Hughes SM. Canine gastrinoma: a case study and literature review of therapeutic options. N Z Vet J 2006;54:242-247
- Patnaik AK, Lieberman PH, Erlandson RA , et al. Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol 2005;42:331-337
- van den Ingh ThS, Lamers CB, Lindeman J. Zollinger-Ellison syndrome in a cat. Vet Q 1988;10:151-155
- Middleton DJ, Watson AD, Vasak E , et al. Duodenal ulceration associated with gastrin-secreting pancreatic tumor in a cat. J Am Vet Med Assoc 1983;183:461-462
- Lane M, Larson J, Hecht S , et al. Medical management of gastrinoma in a cat. JFMS Open Rep 2016;2:2055116916646389
- Myers-Nodes J, Mazepa ASW. Combined surgical and medical management of a cat with gastrinoma. J Small Anim Pract 2022;63:632-634
- Struthers JD, Robl N, Wong VM , et al. Gastrinoma and Zollinger-Ellison syndrome in canids: a literature review and a case in a Mexican gray wolf. J Vet Diagn Invest 2018;30:584-588
- Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: Advances. Best Pract Res Clin Gastroenterol 2012;26:737-753
- Ito T, Lee L, Jensen RT. Treatment of symptomatic neuroendocrine tumor syndromes: recent advances and controversies. Expert Opin Pharmacother 2016;17:2191-2205
- Jensen RT, Norton JA, Oberg K. Neuroendocrine Tumors. In: Feldman M, Friedman LS, Brandt LJ eds. Sleisenger and Fordtran's Gastrointestinal and Liver Diseases. Philadelphia: Elsevier Saunders; 2016:501-541.
- Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD et al. eds. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press Publishing Co.; 1993:931-978.
- Jensen RT, Gardner JD, Raufman JP , et al. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med 1983;98:59-75
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors:; Pancreatic endocrine tumors. Gastroenterology 2008;135:1469-1492
- Jensen RT, Berna MJ, Bingham MD , et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management and controversies. Cancer 2008;113(7 suppl):1807-1843
- Norton JA, Krampitz G, Zemek A , et al. Better Survival But Changing Causes of Death in Patients With Multiple Endocrine Neoplasia Type 1. Ann Surg 2015;261:e147-e148
- Zaidi MY, Lopez-Aguiar AG, Poultsides GA , et al. The impact of failure to achieve symptom control after resection of functional neuroendocrine tumors: An 8-institution study from the US Neuroendocrine Tumor Study Group. J Surg Oncol 2019;119:5-11
- Rossi RE, Elvevi A, Citterio D , et al. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J Gastroenterol 2021;27:5890-5907
- Bevere M, Gkountakos A, Martelli FM , et al. An Insight on Functioning Pancreatic Neuroendocrine Neoplasms. Biomedicines 2023;11:303
- Magi L, Marasco M, Rinzivillo M , et al. Management of Functional Pancreatic Neuroendocrine Neoplasms. Curr Treat Options Oncol 2023;24:725-741
- Hofland J, de Herder WW. Effective strategies for adequate control of hormonal secretion in functioning neuroendocrine neoplasms. Best Pract.Res Clin Endocrinol Metab , 101787. 2023.
- Knapp TG, Duan S, Merchant JL , et al. Quantitative characterization of duodenal gastrinoma autofluorescence using multiphoton microscopy. Lasers Surg Med 2023;55:208-225
- Rydzewska G, Strzelczyk J, Bednarczuk T , et al. Gastroduodenal neuroendocrine neoplasms including gastrinoma - update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol 2022;73:455-490
- Creutzfeldt W, Arnold R, Creutzfeldt C , et al. Pathomorphologic, biochemical and diagnostic aspects of gastrinomas (Zollinger-Ellison syndrome). Hum Pathol 1975;6:47-76
- Jensen RT. Zollinger-Ellison syndrome. In: Doherty GM, Skogseid B eds. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lippincott Williams & Wilkins; 2001:291-344.
- Kaur A, Wang S, Herbert J , et al. Analysis of Clinical Characteristics and Survival in Patients With Functional Neuroendocrine Tumors of Gastrointestinal Origin. Pancreas 2022;51:1171-1178
- Clift AK, Thomas R, Frilling A. Developments in interventional management of hepatic metastases from neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 2023;101798
- Norton JA, Fraker DL, Alexander HR , et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med 1999;341:635-644
- Jensen RT, Norton JA. Treatment of Pancreatic Neuroendocrine Tumors in Multiple Endocrine Neoplasia Type 1: Some Clarity But Continued Controversy. Pancreas 2017;46:589-594
- Norton JA, Fraker DL, Alexander HR , et al. Value of surgery in patients with negative imaging and sporadic zollinger-ellison syndrome. Ann Surg 2012;256:509-517
- Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg 2009;46:13-106
- Nieman LK. Cushing's syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol 2015;173:M33-M38
- Wolin EM, Jensen RT. Neuroendocrine Tumors. In: Goldman L, Schaffer AI, Crow MK et al. eds. GOLDMAN-CECIL MEDICINE. Philadelphia: Elsevier; 2020:1520-1528.
- Metz DC, Jensen RT. Zollinger-Ellison syndrome. In: Podoloff DA, Camilleri M, Fitz JGKAN et al. eds. Yamada"s Textbook of Gastroenterogy. Chichester,West Sussex,UK: Wiley-Blackwell; 2020:In press.
- Jensen RT. Zollinger-Ellison syndrome. In: Podolsky DK, Camilleri M, Fitz JGKAN et al. eds. Yamada's Textbook of Gastroenterology. West Sussex. UK: John Wiley and Sons,Ltd.; 2016:1078-1102.
- Ito T, Igarashi H, Jensen RT. Zollinger-Ellison syndrome: Recent advances and controversies. Current Opinion in Gastroenterology 2013;29:650-661
- Krampitz GW, Norton JA. Current management of the Zollinger-Ellison syndrome. Adv Surg 2013;47:59-79
- Chatzipanagiotou O, Schizas D, Vailas M , et al. All you need to know about gastrinoma today | Gastrinoma and Zollinger-Ellison syndrome: A thorough update. J Neuroendocrinol 2023;35:e13267
- Chang A, Sherman SK, Howe JR , et al. Progress in the Management of Pancreatic Neuroendocrine Tumors. Annu Rev Med 2022;73:213-229
- Metz DC, Cadiot G, Poitras P , et al. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int J Endocr Oncol 2017;4:167-185
- Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: Increasingly difficult. World J Gastroenterol 2012;18:5495-5503
- Singh Ospina N, Donegan D, Rodriguez-Gutierrez R , et al. Assessing for Multiple Endocrine Neoplasia Type 1 in Patients Evaluated for Zollinger-Ellison Syndrome-Clues to a Safer Diagnostic Process. Am J Med 2017;130:603-605
- Hofland J, Kaltsas G, de Herder WW. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr Rev 2019;41:371-403
- Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol 2012;10:126-130
- Klimko A, Plotogea O, Constantinescu A , et al. Delayed Management of Zollinger-Ellison Syndrome in a Noncompliant Patient. Cureus 2020;12:e8471
- Bhattacharya S, Blau JE, Cochran C , et al. Validity of Secretin Stimulation Testing on Proton Pump Inhibitor Therapy for Diagnosis of Zollinger-Ellison Syndrome. Am J Gastroenterol 2021;116:2216-2221
- Roy PK, Venzon DJ, Shojamanesh H , et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379-411
- Soga J, Yakuwa Y. The gastrinoma/Zollinger-Ellison syndrome: statistical evaluation of a Japanese series of 359 cases. J Hep Bil Pancr Surg 1998;5:77-85
- Guo Y, Chen LH, Liu M , et al. (Comparison of clinical characteristics between sporadic gastrinoma and multiple endocrine neoplasia type 1-related gastrinoma). Zhonghua Wei Chang Wai Ke Za Zhi 2021;24:875-882
- Massironi S, Rossi RE, Laffusa A , et al. Sporadic and MEN1-related gastrinoma and Zollinger-Ellison syndrome: differences in clinical characteristics and survival outcomes. J Endocrinol Invest 2023;46:957-965
- Zheng M, Chen L, Nie X , et al. Pancreatic neuroendocrine tumor with ectopic adrenocorticotropic hormone syndrome: a case report and 5-year follow-up. Endocr J 2022;69:243-251
- Nakaoka K, Hashimoto S, Kawabe N , et al. A rare case of pancreatic neuroendocrine neoplasm causing Cushing's syndrome. Clin J Gastroenterol 2022;15:256-262
- Wydra A, Cylke-Falkowska K, Czajka-Oraniec I , et al. Severe ectopic Cushing syndrome in a transgender man with a metastatic gastrinoma and an adrenal tumor-A case report and review of the literature. Front Endocrinol (Lausanne) 2023;14:1135016
- Bahrin MH, Hagag R, Ali A , et al. Benign Oesophageal Stricture and Chronic Diarrhoea As Atypical Presenting Symptoms of an Advanced Metastatic Pancreatic Gastrinoma: A Case Report and Review of Literature. Cureus 2021;13:e16593
- Roy PK, Venzon DJ, Feigenbaum KM , et al. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis - A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore) 2001;80:189-222
- Perrier M, Delemer B, Deguelte S , et al. Total gastrectomy for severe proton pump inhibitor-induced hypomagnesemia in a MEN1/Zollinger Ellison syndrome patient. Pancreatology 2021;21:236-239
- Ito T, Ramos-Alvarez I, Jensen RT. Successful lifetime/lifelong medical treatment of acid in Zollinger-Ellison syndrome (ZES): Myth or fact? Insights from an analysis of results of NIH-long-term prospective studies of ZES. Cancers (Basel) 2023;15:1377
- Berna MJ, Hoffmann KM, Serrano J , et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295-330
- Berna MJ, Hoffmann KM, Long SH , et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85:331-364
- Frucht H, Howard JM, Slaff JI , et al. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome: A prospective study. Ann Intern Med 1989;111:713-722
- Lee L, Ramos-Alvarez I, Ito T , et al. Insights into Effects/Risks of Chronic Hypergastrinemia and Lifelong PPI Treatment in Man Based on Studies of Patients with Zollinger-Ellison Syndrome. Int J Mol Sci 2019;20:E5128
- Poitras P, Gingras MH, Rehfeld JF. The Zollinger-Ellison syndrome: dangers and consequences of interrupting antisecretory treatment. Clin Gastroenterol Hepatol 2012;10:199-202
- Banasch M, Schmitz F. Diagnosis and treatment of gastrinoma in the era of proton pump inhibitors. Wien Klin Wochenschr 2007;119:573-578
- Mendelson AH, Donowitz M. Catching the Zebra: Clinical Pearls and Pitfalls for the Successful Diagnosis of Zollinger-Ellison Syndrome. Dig Dis Sci 2017;62:2258-2265
- De Angelis C, Cortegoso VP, Venezia L , et al. Diagnosis and management of Zollinger-Ellison syndrome in 2018. Minerva Endocrinol 2018;43:212-220
- Murakami T, Usui T, Nakamoto Y , et al. Challenging Differential Diagnosis of Hypergastremia and Hyperglucagonemia with Chronic Renal Failure: Report of a Case with Multiple Endocrine Neoplasia Type 1. Intern Med 2017;56:1375-1381
- Yu S, Wang D, Ma X , et al. Analytical and Clinical Performance of a Liquid Chromatography-Tandem Mass Spectrometry Method for Measuring Gastrin Subtypes G34 and G17 in Serum. Clin Chem 2021;67:1220-1229
- Veysey-Smith R, Moore AR, Murugesan SV , et al. Effects of Proton Pump Inhibitor Therapy, H. pylori Infection and Gastric Preneoplastic Pathology on Fasting Serum Gastrin Concentrations. Front Endocrinol (Lausanne) 2021;12:741887
- Giusti F, Cioppi F, Fossi C , et al. Secretin Stimulation Test and Early Diagnosis of Gastrinoma in MEN1 Syndrome: Survey on the MEN1 Florentine Database. J Clin Endocrinol Metab 2022;107:e2110-e2123
- Endall R, Thompson M, Parameswaran V , et al. The Relationship of Gastrinoma in MEN 1 to Helicobacter pylori infection. J Clin Endocrinol Metab 2020;105:e 676-e682
- Hackeng WM, Dreijerink KMA, Offerhaus GJA , et al. A Parathyroid-Gut Axis: Hypercalcemia and the Pathogenesis of Gastrinoma in Multiple Endocrine Neoplasia 1. Mol Cancer Res 2021;19:946-949
- Gibril F, Schumann M, Pace A , et al. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine (Baltimore) 2004;83:43-83
- Norton JA, Krampitz G, Jensen RT. Multiple Endocrine Neoplasia: Genetics and Clinical Management. Surg Oncol Clin N Am 2015;24:795-832
- Ito T, Igarashi H, Uehara H , et al. Causes of Death and Prognostic Factors in Multiple Endocrine Neoplasia Type 1: A Prospective Study: Comparison of 106 MEN1/Zollinger-Ellison Syndrome Patients With 1613 Literature MEN1 Patients With or Without Pancreatic Endocrine Tumors. Medicine (Baltimore) 2013;92:135-181
- Ito T, Jensen RT. Imaging in multiple endocrine neoplasia type 1: recent studies show enhanced sensitivities but increased controversies. Int J Endocr Oncol 2016;3:53-66
- Mandl A, Welch JM, Kapoor G , et al. Two distinct classes of thymic tumors in patients with MEN1 show LOH at the MEN1 locus. Endocr Relat Cancer 2021;28:L15-L19
- Albers MB, Manoharan J, Bollmann C , et al. Results of Duodenopancreatic Reoperations in Multiple Endocrine Neoplasia Type 1. World J Surg 2019;43:552-558
- Vinault S, Mariet AS, Le BM , et al. Metastatic Potential and Survival of Duodenal and Pancreatic Tumors in Multiple Endocrine Neoplasia Type 1: A GTE and AFCE Cohort Study (Groupe d'etude des Tumeurs Endocrines and Association Francophone de Chirurgie Endocrinienne). Ann Surg 2020;272:1094-1101
- Febrero B, Segura P, Ruiz-Manzanera JJ , et al. Uncommon tumors in multiple endocrine neoplasia (MEN) type 1: Do they have a relationship with the prognosis of these patients? J Endocrinol Invest 2021;44:1327-1330
- Imamura M, Komoto I, Taki Y. How to treat gastrinomas in patients with multiple endocrine neoplasia type1: surgery or long-term proton pump inhibitors? Surgery Today . 2023. (In Press)
- Geslot A, Vialon M, Caron P , et al. New therapies for patients with multiple endocrine neoplasia type 1. Ann Endocrinol (Paris) 2021;82:112-120
- Mele C, Mencarelli M, Caputo M , et al. Phenotypes Associated With MEN1 Syndrome: A Focus on Genotype-Phenotype Correlations. Front Endocrinol (Lausanne) 2020;11:591501
- Naruoka A, Ohnami S, Nagashima T , et al. Genomic profiling of multiple tissues in two patients with multiple endocrine neoplasia type 1. Biomed Res 2021;42:89-94
- Santucci N, Gaujoux S, Binquet C , et al. Pancreatoduodenectomy for Neuroendocrine Tumors in Patients with Multiple Endocrine Neoplasia Type 1: An AFCE (Association Francophone de Chirurgie Endocrinienne) and GTE (Groupe d'étude des Tumeurs Endocrines) Study. World J Surg 2021;45:1794-1802
- Gong S, Li Z, Liu XB , et al. Gastrinoma in multiple endocrine neoplasia type 1 after total pancreatectomy: A case report. Medicine (Baltimore) 2019;98:e18275
- Shariq OA, Lines KE, English KA , et al. Multiple endocrine neoplasia type 1 in children and adolescents: Clinical features and treatment outcomes. Surgery 2022;171:77-87
- Kong W, Albers MB, Manoharan J , et al. Pancreaticoduodenectomy Is the Best Surgical Procedure for Zollinger-Ellison Syndrome Associated with Multiple Endocrine Neoplasia Type 1. Cancers (Basel) 2022;14:1928
- Ito T, Igarashi H, Uehara H , et al. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacotherapy 2013;14:307-321
- Collen MJ, Howard JM, McArthur KE , et al. Comparison of ranitidine and cimetidine in the treatment of gastric hypersecretion. Ann Intern Med 1984;100:52-58
- Metz DC, Pisegna JR, Fishbeyn VA , et al. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J Surg 1993;17:468-480
- Raufman JP, Collins SM, Pandol SJ , et al. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology 1983;84:108-113
- Kusano K, Uno K, Koike T , et al. A case of refractory esophageal stricture due to occult gastrinoma of the duodenum. Clin J Gastroenterol 2022;15:859-863
- Martino BR, Manibusan P. Zollinger Ellison Syndrome Refractory to Medical Therapy in the Setting of Multiple Endocrine Neoplasia Type I. Cureus 2022;14:e26468
- Yu F, Venzon DJ, Serrano J , et al. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J Clin Oncol 1999;17:615-630
- Weber HC, Venzon DJ, Lin JT , et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology 1995;108:1637-1649
- Gibril F, Venzon DJ, Ojeaburu JV , et al. Prospective study of the natural history of gastrinoma in patients with MEN1: Definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab 2001;86:5282-5293
- Gibril F, Doppman JL, Reynolds JC , et al. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J Clin Oncol 1998;16:1040-1053
- Maton PN, Gardner JD, Jensen RT. Cushing's syndrome in patients with Zollinger-Ellison syndrome. N Engl J Med 1986;315:1-5
- Hackeng WM, van Beek DJ, Kok ASM , et al. Metastatic Patterns of Duodenopancreatic Neuroendocrine Tumors in Patients With Multiple Endocrine Neoplasia Type 1. Am J Surg Pathol 2022;46:159-168
- Lee L, Ramos-Alvarez I, Jensen RT. Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies. Cancers (Basel) 2022;14:1250
- Lee L, Ito T, Jensen RT. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: recent advances and controversies. Expert Rev Anticancer Ther 2019;19:1029-1050
- Matrood S, Melms LE, Bartsch DK , et al. The Expression of Autophagy-Associated Genes Represents a Valid Footprint for Aggressive Pancreatic Neuroendocrine Neoplasms. Int J Mol Sci 2023;24:3636
- Bartsch DK, Albers MB. Controversies in surgery for multiple endocrine neoplasia type-1- associated Zollinger-Ellison syndrome. Int J Endo Oncol 2015;2:263-271
- Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg 2004;240:757-773
- Souche R, Hobeika C, Hain E , et al. Surgical Management of Neuroendocrine Tumours of the Pancreas. J Clin Med 2020;9:2993
- Sampathirao N, Basu S. Rare Occurrence Of Hypergastrinemia due to Thoracic Neuroendocrine Tumor: Detection and Characterization by 68Ga-DOTATATE PET-CT. J Nucl.Med.Technol. 2016.
- Shao QQ, Zhao BB, Dong LB , et al. Surgical management of Zollinger-Ellison syndrome: Classical considerations and current controversies. World J Gastroenterol 2019;25:4673-4681
- Sauvanet A. Gastroenteropancreatic neuroendocrine tumors: Role of surgery. Ann Endocrinol (Paris) 2019;80:175-181
- Cockburn KC, Toumi Z, Mackie A , et al. Radioguided Surgery for Gastroenteropancreatic Neuroendocrine Tumours: a Systematic Literature Review. J Gastrointest Surg 2021;25:3244-3257
- Okasha HH, Aboubakr A, Elenin SA , et al. Unexpected adverse events of endoscopic polypectomy of a juxtapapillay duodenal gastrinoma. Gastrointest Endosc 2022;95:589-590
- Okamoto T, Yoshimoto T, Ohike N , et al. Spontaneous regression of gastric gastrinoma after resection of metastases to the lesser omentum: A case report and review of literature. World J Gastroenterol 2021;27:129-142
- Robin L, Sauvanet A, Walter T , et al. Recurrence after surgical resection of nonmetastatic sporadic gastrinoma: Which prognostic factors and surgical procedure? Surgery 2023;173:1144-1152
- Titan AL, Norton JA, Fisher AT , et al. Evaluation of Outcomes Following Surgery for Locally Advanced Pancreatic Neuroendocrine Tumors. JAMA Netw Open 2020;3:e2024318
- Lee L, Ito T, Jensen RT. Imaging of pancreatic neuroendocrine tumors: recent advances, current status and controversies. Expert Rev Anticancer Ther 2018;18:837-860
- Krudy AG, Doppman JL, Jensen RT , et al. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. Am J Roentgenol 1984;143:585-589
- Maton PN, Miller DL, Doppman JL , et al. Role of selective angiography in the management of Zollinger- Ellison syndrome. Gastroenterology 1987;92:913-918
- Frucht H, Doppman JL, Norton JA , et al. Gastrinomas: Comparison of MR Imaging with CT, angiography and US. Radiology 1989;171:713-717
- Gibril F, Reynolds JC, Doppman JL , et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: a prospective study. Ann Intern Med 1996;125:26-34
- Ito T, Jensen RT. Molecular imaging in neuroendocrine tumors: recent advances, controversies, unresolved issues, and roles in management. Curr Opin Endocrinol Diabetes Obes 2017;24:15-24
- Desai H, Borges-Neto S, Wong TZ. Molecular Imaging and Therapy for Neuroendocrine Tumors. Curr Treat Options Oncol 2019;20:78
- Lui J, Terra SBSP, de la FJ. Metastatic Gastrinoma Localized on Gallium-68 DOTATATE Imaging. Mayo Clin Proc 2021;96:285-286
- Stolniceanu CR, Grierosu IC, Matovic M , et al. Somatostatin receptor molecular imaging in a misdiagnosed gastrinoma case. World J Nucl Med 2020;19:417-420
- Higashino N, Sonomura T, Ito D , et al. Usefulness of computed tomography during arteriography in a selective arterial calcium agent injection test for pancreatic gastrinoma: a case description. Quant Imaging Med Surg 2021;11:3898-3903
- Jayasekera M, Sartin S, Bhargava P. Ga-68 DOTATATE PET/CT in a patient with Zollinger-Ellison syndrome. Radiol Case Rep 2023;18:1046-1048
- Pellegrino F, Granata V, Fusco R , et al. Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists. Tomography 2023;9:217-246
- Duan H, Iagaru A. Neuroendocrine Tumor Diagnosis: PET/MR Imaging. PET Clin 2023;18:259-266
- Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol 2012;47:941-960
- Pavel M, O'Toole D, Costa F , et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016;103:172-185
- Akirov A, Larouche V, Alshehri S , et al. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers (Basel) 2019;11:828
- Dimitriadis GK, Weickert MO, Randeva HS , et al. Medical management of secretory syndromes related to gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2016;23:R423-R436
- Gut P, Waligorska-Stachura J, Czarnywojtek A , et al. Management of the hormonal syndrome of neuroendocrine tumors. Arch Med Sci 2017;13:515-524
- von Schrenck T, Howard JM, Doppman JL , et al. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology 1988;94:1326-1334
- Zandee WT, Brabander T, Blazevic A , et al. Symptomatic and Radiological Response to 177Lu-DOTATATE for the Treatment of Functioning Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab 2019;104:1336-1344
- Halfdanarson TR, Strosberg JR, Tang L , et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:863-881
- Lee L, Ito T, Jensen RT. Everolimus in the treatment of neuroendocrine tumors: efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin Pharmacother 2018;19:909-928
- Guarnotta V, Martini C, Davi MV , et al. The Zollinger-Ellison syndrome: is there a role for somatostatin analogues in the treatment of the gastrinoma? Endocrine 2018;60:15-27
- Shah MH, Goldner WS, Halfdanarson TR , et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693-702
- Patil VA, Goroshi MR, Shah H , et al. Comparison of (68)Ga-DOTA-NaI(3)-Octreotide/tyr(3)-octreotate positron emission tomography/computed tomography and contrast-enhanced computed tomography in localization of tumors in multiple endocrine neoplasia 1 syndrome. World J Nucl Med 2020;19:99-105
- Hofland J, Brabander T, Verburg FA , et al. Peptide Receptor Radionuclide Therapy. J Clin Endocrinol Metab 2022;107:3199-3208
- Massironi S, Cavalcoli F, Elvevi A , et al. Somatostatin analogs in patients with Zollinger Ellison syndrome (ZES): an observational study. Endocrine 2022;75:942-948
- Ito T, Masui T, Komoto I , et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol 2021;56:1033-1044
- Falconi M, Eriksson B, Kaltsas G , et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103:153-171
- Jensen RT. Neuroendocrine Tumors of the Gastrointestintal Tract (GI) and Pancreas. In: Jamieson LL, Fauci AS, Kasper DL et al. eds. Harrison's Principles of Internal Medicine-ED.20. New York, New York: McGraw Hill Education Medical Publishing Division; 2018:596-615.
- Del Olmo-Garcia MI, Muros MA, Lopez-de-la-Torre M , et al. Prevention and Management of Hormonal Crisis during Theragnosis with LU-DOTA-TATE in Neuroendocrine Tumors. A Systematic Review and Approach Proposal. J Clin Med 2020;9:2203
- Wolin EM, Benson III AB. Systemic Treatment Options for Carcinoid Syndrome: A Systematic Review. Oncology 2019;96:273-289
- Pavel M, Oberg K, Falconi M , et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844-860
- Tsoli M, Alexandraki K, Xanthopoulos C , et al. Medical Treatment of Gastrointestinal Neuroendocrine Neoplasms. Horm Metab Res 2020;52:614-620
- Murakami M, Fujimori N, Matsumoto K , et al. A clinical analysis on functioning pancreatic neuroendocrine tumors (focusing on VIPomas): a single-center experience. Endocr J 2022;69:1201-1209
- Ito T, Jensen RT. Perspectives on the Current Pharmacotherapeutic Strategies for Management of Functional Neuroendocrine Tumor Syndromes. Expert Opin Pharmacother 2021;22:685-693
- Marchese U, Gaillard M, Pellat A , et al. Multimodal Management of Grade 1 and 2 Pancreatic Neuroendocrine Tumors. Cancers (Basel) 2022;14:433
- Klimstra DS. Pathologic Classification of Neuroendocrine Neoplasms. Hematol Oncol Clin North Am 2016;30:1-19
- Inzani F, Petrone G, Rindi G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am 2018;47:463-470
- Klimstra DS, La Rosa S, Rindi G. Classification of Neuroendocrine Neopasms of the digestive system. In: WHO Classsification of Tumors Editorial Board ed. WHO classification of tumours:digestive system tumours. Lyon, France: IARC Press; 2019:16-19.
- Kasajima A, Kloppel G. Neuroendocrine neoplasms of lung, pancreas and gut: a morphology-based comparison. Endocr Relat Cancer 2020;27:R417-R432
- Duan S, Rico K, Merchant JL. Gastrin: From Physiology to Gastrointestinal Malignancies. Function (Oxf) 2022;3:zqab062
- Maekawa A, Kudo A, Kishino M , et al. Hormonal tumor mapping for liver metastases of gastroenteropancreatic neuroendocrine neoplasms: a novel therapeutic strategy. J Cancer Res Clin Oncol 2022;148:697-706
- Rico K, Duan S, Pandey RL , et al. Genome analysis identifies differences in the transcriptional targets of duodenal versus pancreatic neuroendocrine tumours. BMJ Open Gastroenterol 2021;8:e000765
- Duan S, Sawyer TW, Sontz RA , et al. GFAP-directed Inactivation of Men1 Exploits Glial Cell Plasticity in Favor of Neuroendocrine Reprogramming. Cell Mol Gastroenterol Hepatol 2022;14:1025-1051
- Elvis-Offiah UB, Duan S, Merchant JL. MENIN-mediated regulation of gastrin gene expression and its role in gastrinoma development. FASEB J 2023;37:e22913
- Norton JA, Alexander HR, Fraker DL , et al. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann Surg 2004;239:617-626
- Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: Results of a 10-year prospective study. Ann Surg 1992;215:8-18
- Howard TJ, Zinner MJ, Stabile BE , et al. Gastrinoma excision for cure. A prospective analysis. Ann Surg 1990;211:9-14
- Pipeleers-Marichal M, Somers G, Willems G , et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med 1990;322:723-727
- Anlauf M, Garbrecht N, Henopp T , et al. Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features. World J Gastroenterol 2006;12:5440-5446
- Kunz PL, Reidy-Lagunes D, Anthony LB , et al. Consensus Guidelines for the Management and Treatment of Neuroendocrine Tumors. Pancreas 2013;42:557-577
- Jensen RT, Cadiot G, Brandi ML , et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms: Functional Pancreatic Endocrine Tumor Syndromes. Neuroendocrinology 2012;95:98-119
- Kulke MH, Anthony LB, Bushnell DL , et al. NANETS Treatment Guidelines: Well-Differentiated Neuroendocrine Tumors of the Stomach and Pancreas. Pancreas 2010;39:735-752
- Phan J, Benhammou JN, Pisegna JR. Gastric Hypersecretory States: Investigation and Management. Curr Treat Options Gastroenterol 2015;13:386-397
- Osefo N, Ito T, Jensen RT. Gastric Acid hypersecretory States: recent insights and advances. Curr Gastroenterol Rep 2009;11:433-441
- Ito T, Jensen RT. Neuroendocrine Neoplasms and Functional Syndromes. In: Weber HC ed. Gastrointestinal Endocrinology. New York: Springer; 2023(In Press).
- Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol 2005;19:783-798
- Bansal N, Weinstock RS. Non-Diabetic Hypoglycemia. Endotext(Internet) 2000;
- Maggio I, Mollica V, Brighi N , et al. The functioning side of the pancreas: a review on insulinomas. J Endocrinol Invest 2020;43:139-148
- O'Toole D, Kianmanesh R, Caplin M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology 2016;103:113-195
- Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med 1998;243:477-488
- Norton JA, Alexander HR, Fraker DL , et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg 2001;234:495-506
- Dasari A, Shen C, Halperin D , et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-1342
- Yao JC, Eisner MP, Leary C , et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007;14:3492-3500
- Yao JC, Hassan M, Phan A , et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-3072
- Geurts JL. Inherited syndromes involving pancreatic neuroendocrine tumors. J Gastrointest Oncol 2020;11:559-566
- Glasker S, Vergauwen E, Koch CA , et al. Von Hippel-Lindau Disease: Current Challenges and Future Prospects. Onco Targets Ther 2020;13:5669-5690
- Carrera S, Sancho A, Azkona E , et al. Hereditary pancreatic cancer: related syndromes and clinical perspective. Hered Cancer Clin Pract 2017;15:9
- Sala Hernandez A, Montalva Oron EM, Pareja Ibars E , et al. Management of pancreatic gastrinoma associated with Von Hippel-Lindau disease: a case report. Rev Esp Enferm Dig 2016;109:
- Alshikho MJ, Noureldine SI, Talas JM , et al. Zollinger-Ellison Syndrome Associated with von Recklinghausen Disease: Case Report and Literature Review. Am J Case Rep 2016;17:398-405
- Schwarzkopf G, Pfisterer J. Metastasizing gastrinoma and tuberous sclerosis complex. Zentralbl Pathol 1994;139:477-481
- Chagnon JP, Barge J, Henin D , et al. (Recklinghausen's disease with digestive localizations associated with gastric acid hypersecretion suggesting Zollinger-Ellison syndrome). Gastroenterol Clin Biol 1985;9(1):65-69
- Massironi S, Zilli A, Rossi RE , et al. Gastrinoma and neurofibromatosis type 2: the first case report and review of the literature. BMC Gastroenterol 2014;14:110
- Arif AA, Kim PTW, Melck A , et al. Pancreatic Gastrinoma, Gastrointestinal Stromal Tumor (GIST), Pheochromocytoma, and Hürthle Cell Neoplasm in a Patient with Neurofibromatosis Type 1: A Case Report and Literature Review. Am J Case Rep 2021;22:e927761
- Gauci J, Azzopardi N, Babic D , et al. Neurofibromatosis Type 1: A Rare Predisposition for Gastrinomas and Other Neuroendocrine Tumors. Pancreas 2022;51:559-562
- Ellison EH, Wilson SD. The Zollinger-Ellison syndrome: Re-appraisal and evaluation of 260 registered cases. Ann Surg 1964;160:512-530
- Stage JG, Stadil F. The clinical diagnosis of the Zollinger-Ellison syndrome. Scand J Gastroenterol Suppl 1979;53:79-91
- Thompson JC, Lewis BG, Wiener I , et al. The role of surgery in the Zollinger-Ellison syndrome. Ann Surg 1983;197(5):594-607
- Schubert ML. Hormonal regulation of gastric acid secretion. Curr Gastroenterol Rep 2008;10:523-527
- Aoyagi T, Summerskill WH. Gastric secretion with ulcerogenic islet cell tumor. Importance of basal acid output. Arch Intern Med 1966;117:667-672
- Way L, Goldman L, Dunphy JE. Zollinger-Ellison syndrome. An analysis of twenty-five cases. Am J Surg 1968;116(2):293-304
- Richardson CT, Peters MN, Feldman M , et al. Treatment of Zollinger-Ellison syndrome with exploratory laparotomy, proximal gastric vagotomy, and H2-receptor antagonists. A prospective study. Gastroenterology 1985;89:357-367
- Jensen RT. Gastrinoma as a model for prolonged hypergastrinemia in man. In: Walsh JH ed. Gastrin. New York, NY: Raven Press Publishing Co.; 1993:373-393.
- Willems G. Trophic action of gastrin on specific target cells in the gut. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:30-44.
- Hakanson R, Oscarson J, Sundler F. Gastrin and the trophic control of gastric mucosa. Scand J Gastroenterol 1986;Suppl 118:18-30
- Hakanson R, Sundler FH. Regulation of gastric endocrine cell proliferation. In: Walsh JH ed. Gastrin. New York: Raven Press, Ltd.; 1993:307-318.
- Hakanson R, Ekelund M, Sundler F. Activation and proliferation of gastric endocrine cells. In: Falkmer S, Hakanson R, Sundler F eds. Evolution and tumor pathology of the neuroendocrine system. Amsterdam: Elsevier; 1984:371-398.
- Peghini PL, Annibale B, Azzoni C , et al. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology 2002;123:68-85
- Neuburger P, Lewin M, Recherche Cd , et al. Parietal and chief cell population in four cases of the Zollinger-Ellison syndrome. Gastroenterology 1972;63:937-942
- Polacek MA, Ellison EH. Parietal cell mass and gastric acid secretion in the Zollinger-Ellison syndrome. Surgery 1966;60:606-614
- Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology 1973;65:140-165
- Plockinger U, Rindi G, Arnold R , et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology 2004;80:394-424
- Jensen RT. Overview of chronic diarrhea caused by functional neuroendocrine neoplasms. Semin Gastrointest Dis 1999;10:156-172
- D'Adda T, Corleto V, Pilato FP , et al. Quantitative ultrastructure of endocrine cells of oxyntic mucosa in Zollinger-Ellison syndrome. Correspondence with light microscopic findings. Gastroenterology 1990;99:17-26
- Maton PN, Lack EE, Collen MJ , et al. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology 1990;99:943-950
- Lehy T, Mignon M, Cadiot G , et al. Gastric endocrine cell behavior in Zollinger-Ellison patients upon long-term potent antisecretory treatment. Gastroenterology 1989;96:1029-1040
- Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion 1988;39:61-79
- Berna MJ, Annibale B, Marignani M , et al. A prospective study of gastric carcinoids and enterochromaffin-like cells changes in Multple Endocrine Neoplaisa Type 1 and Zollinger-Ellison syndrome: Identification of risk factors. J Clin Endocrinol Metab 2008;93:1582-1591
- Solcia E, Bordi C, Creutzfeldt W , et al. Histopathological classification of nonantral gastric endocrine growths in man. Digestion 1988;41:185-200
- Rindi G, Luinetti O, Cornaggia M , et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993;104:994-1006
- Cadiot G, Lehy T, Ruszniewski P , et al. Gastric endocrine cell evolution in patients with Zollinger-Ellison syndrome. Influence of gastrinoma growth and long-term omeprazole treatment. Dig Dis Sci 1993;38:1307-1317
- Lehy T, Cadiot G, Mignon M , et al. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut 1992;33:1275-1279
- Feurle GE. Argyrophil cell hyperplasia and a carcinoid tumour in the stomach of a patient with sporadic Zollinger-Ellison syndrome. Gut 1994;35:275-277
- Cadiot G, Vissuzaine C, Potet F , et al. Fundic argyrophil carcinoid tumor in a patient with sporadic-type Zollinger-Ellison syndrome. Dig Dis Sci 1995;40:1275-1278
- Rais R, Trikalinos NA, Liu J , et al. Enterochromaffin-like Cell Hyperplasia-Associated Gastric Neuroendocrine Tumors May Arise in the Setting of Proton Pump Inhibitor Use. Arch Pathol Lab Med 2022;146:366-371
- Jensen RT, Doppman JL, Gardner JD. Gastrinoma. In: Go VLW, Brooks FA, DiMagno EP et al. eds. The Exocrine Pancreas: Biology, Pathobiology and Disease. New York: Raven Press; 1986:727-744.
- Kloppel G, Anlauf M. Gastrinoma - morphological aspects. Wien Klin Wochenschr 2007;119:579-584
- Zollinger RM, Ellison EC, Fabri PJ , et al. Primary peptic ulcerations of the jejunum associated with islet cell tumors. Twenty-five-year appraisal. Ann Surg 1980;192:422-430
- Deveney CW, Deveney KS, Stark D , et al. Resection of gastrinomas. Ann Surg 1983;198:546-553
- Oberhelman HA, Jr., Nelsen TS, Johnson AN , et al. Ulcerogenic tumors of the duodenum. Ann Surg 1961;153:214-227
- Hofmann JW, Fox PS, Wilson SD. Duodenal wall tumors and the Zollinger-Ellison syndrome. Surgical management. Arch Surg 1973;107:334-339
- Norton JA, Doppman JL, Collen MJ , et al. Prospective study of gastrinoma localization and resection in patients with Zollinger-Ellison syndrome. Ann Surg 1986;204:468-479
- Raufman JP, Kasbekar DK, Jensen RT , et al. Potentiation of pepsinogen secretion from dispersed glands from rat stomach. Am J Physiol 1983;245:G525-G530
- Thompson NW. Current concepts in the surgical management of multiple endocrine neoplasia type 1 pancreatic-duodenal disease. Results in the treatment of 40 patients with Zollinger-Ellison syndrome, hypoglycaemia or both. J Intern Med 1998;243:495-500
- Sugg SL, Norton JA, Fraker DL , et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg 1993;218:138-144
- Cadiot G, Lebtahi R, Sarda L , et al. Preoperative detection of duodenal gastrinomas and peripancreatic lymph nodes by somatostatin receptor scintigraphy. Gastroenterology 1996;111:845-854
- Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum. A cause of failed operations for the Zollinger-Ellison syndrome. Ann Surg 1989;209:396-404
- Frucht H, Norton JA, London JF , et al. Detection of duodenal gastrinomas by operative endoscopic transillumination: a prospective study. Gastroenterology 1990;99:1622-1627
- Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg 1993;17:455-462
- Norton JA, Foster DS, Blumgart LH , et al. Incidence and Prognosis of Primary Gastrinomas in the Hepatobiliary Tract. JAMA Surg 2018;153:e175083
- Norton JA, Harris EJ, Chen Y , et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg 2011;146:724-732
- Moraes AB, Treistman N, Studart MC , et al. Gastrinoma of Cystic Duct: A Rare Association With Multiple Endocrine Neoplasia Type 1. J Clin Med Res 2018;10:843-847
- Pipek LZ, Jardim YJ, de Mesquita GHA , et al. Large primary hepatic gastrinoma in young patient treated with trisegmentectomy: A case report and review of the literature. World J Hepatol 2018;10:517-522
- Wu PC, Alexander HR, Bartlett DL , et al. A prospective analysis of the frequency, location, and curability of ectopic (non-pancreaticoduodenal, non-nodal) gastrinoma. Surgery 1997;122:1176-1182
- Norton JA, Krampitz GW, Poultsides GA , et al. Prospective Evaluation of Results of Reoperation in Zollinger-Ellison Syndrome. Ann Surg 2018;267:782-788
- Gibril F, Curtis LT, Termanini B , et al. Primary cardiac gastrinoma causing Zollinger-Ellison syndrome. Gastroenterology 1997;112:567-574
- Abou-Saif A, Lei J, McDonald TJ , et al. A new cause of Zollinger-Ellison syndrome: non-small cell lung cancer. Gastroenterology 2001;120:1271-1278
- Gibril F, Jensen RT. Advances in evaluation and management of gastrinoma in patients with Zollinger-Ellison syndrome. Curr Gastroenterol Rep 2005;7:114-121
- Norton JA, Alexander HA, Fraker DL , et al. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: A prospective study. Ann Surg 2003;237:650-659
- Arnold WS, Fraker DL, Alexander HR , et al. Apparent lymph node primary gastrinoma. Surgery 1994;116:1123-1130
- Ito T, Jensen RT. Primary hepatic gastrinoma: an unusual case of zollinger-ellison syndrome. Gastroenterol Hepatol (N Y ) 2010;6:57-59
- Tonelli F, Giudici F, Nesi G , et al. Biliary tree gastrinomas in multiple endocrine neoplasia type 1 syndrome. World J Gastroenterol 2013;19:8312-8320
- Luiso D, Zuccarino F, Tizon-Marcos H , et al. Primary intracardiac gastrinoma causing Zollinger-Ellison syndrome. Eur Heart J 2019;41:3376
- Rafieian S, Vahedi M, Jahanbin B , et al. Primary thoracic gastrinoma causing Zollinger-Ellison syndrome. Indian J Thorac Cardiovasc Surg 2021;37:706-709
- Gonzalez-Lopez R, Ramlrez-Castaneda J, Ortega-Jimenez JA , et al. Primary hepatic gastrinoma, extremely rare case presentation and its surgical resolution, in a third level hospital in Mexico. Cir Cir 2022;90:109-113
- Rosiek V. Sequential therapies in gastric gastrinoma. Endokrynol Pol 2022;73:171-172
- Perrier ND, Batts KP, Thompson GB , et al. An immunohistochemical survey for neuroendocrine cells in regional pancreatic lymph nodes: a plausible explanation for primary nodal gastrinomas? Surgery 1995;118:957-965
- Atema JJ, Amri R, Busch OR , et al. Surgical treatment of gastrinomas: a single-centre experience. HPB (Oxford) 2012;14:833-838
- Anlauf M, Enosawa T, Henopp T , et al. Primary lymph node gastrinoma or occult duodenal microgastrinoma with lymph node metastases in a MEN1 patient: the need for a systematic search for the primary tumor. Am J Surg Pathol 2008;32:1101-1105
- Chen Y, Deshpande V, Ferrone C , et al. Primary lymph node gastrinoma: A single institution experience. Surgery 2017;162:1088-1094
- Singh D, Lal SB, Sood A , et al. Management of Primary Lymph Nodal Gastrinoma With Liver Metastases Resulting in Zollinger-Ellison Syndrome. Clin Nucl Med 2019;44:e36-e39
- Abu Ghanimeh M, Abuamr K, Sadeddin E , et al. Severe chronic diarrhoea secondary to primary lymph node gastrinoma. BMJ Case Rep 2017;bcr2016216855
- Cavalcanti E, Stasi E, Coletta S , et al. Primary lymph node gastrinoma: a case report and review of the literature. World J Surg Oncol 2020;18:80
- Marcos Enriquez JC, Coayla CF, Caceres R.M. , et al. (Primary lymph node gastrinoma). Rev Gastroenterol Peru 2022;42:122-125
- Zhang G, Jiang Q, Zhang S , et al. The sun beside the pancreatic neck: The lymph node gastrinoma (with video). Endosc Ultrasound 2023;12:152-154
- Herrmann ME, Ciesla MC, Chejfec G , et al. Primary Nodal gastrinomas - an immunohistochemical study in support of a theory. Arch Pathol Lab Med 2000;124:832-835
- Stabile BE, Morrow DJ, Passaro E, Jr. The gastrinoma triangle: operative implications. Am J Surg 1984;147:25-31
- Thom AK, Norton JA, Axiotis CA , et al. Location, incidence and malignant potential of duodenal gastrinomas. Surgery 1991;110:1086-1093
- Delcore R, Jr., Cheung LY, Friesen SR. Characteristics of duodenal wall gastrinomas. Am J Surg 1990;160:621-623
- Metz DC, Jensen RT, Bale AE et al. Multiple endocrine neoplasia type 1: clinical features and management. In: Bilezekian JP, Levine MA, Marcus R eds. The Parathyroids. New York: Raven Press Publishing Co.; 1994:591-646.
- Kloppel G, Schroder S, Heitz PU. Histopathology and immunopathology of pancreatic endocrine tumors. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:99-120.
- Donow C, Pipeleers-Marichal M, Schroder S , et al. Surgical pathology of gastrinoma: site, size, multicentricity, association with multiple endocrine neoplasia type 1, and malignancy. Cancer 1991;68:1329-1334
- Creutzfeldt W, Arnold R. Somatostatin and the stomach: exocrine and endocrine aspects. Metabolism 1978;27:1309-1315
- Pipeleers-Marichal M, Donow C, Heitz PU , et al. Pathologic aspects of gastrinomas in patients with Zollinger-Ellison syndrome with and without multiple endocrine neoplasia type I. World J Surg 1993;17:481-488
- MacFarlane MP, Fraker DL, Alexander HR , et al. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-Type 1. Surgery 1995;118:973-980
- Hoffmann KM, Gibril F, Entsuah LK , et al. Patients with multiple endocrine neoplasia type 1 with gastrinomas have an increased risk of severe esophageal disease including stricture and the premalignant condition, Barrett's esophagus. J Clin Endocrinol Metab 2006;91:204-212
- Rosentraeger MJ, Garbrecht N, Anlauf M , et al. Syndromic versus non-syndromic sporadic gastrin-producing neuroendocrine tumors of the duodenum: comparison of pathological features and biological behavior. Virchows Arch 2016;468:277-287
- Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1). Surg Oncol 2003;12:145-151
- Norton JA, Doherty GD, Fraker DL , et al. Surgical treatment of localized gastrinoma within the liver: A prospective study. Surgery 1998;124:1145-1152
- Zogakis TG, Gibril F, Libutti SK , et al. Management and outcome of patients with sporadic gastrinomas arising in the duodenum. Ann Surg 2003;238:42-48
- Oberg K, Sundin A. Imaging of Neuroendocrine Tumors. Front Horm Res 2016;45:142-151
- Sundin A, Arnold R, Baudin E , et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017;105:212-244
- Sundin A. Novel Functional Imaging of Neuroendocrine Tumors. Endocrinol Metab Clin North Am 2018;47:505-523
- Gabriel S, Garrigue P, Dahan L , et al. Prospective evaluation of (68) Ga-DOTATATE PET/CT in limited disease neuroendocrine tumours and/or elevated serum neuroendocrine biomarkers. Clin Endocrinol (Oxf) 2018;89:155-163
- Lebtahi R, Cadiot G, Delahaye N , et al. Detection of bone metastases in patients with endocrine gastroenteropancreatic tumors: bone scintigraphy compared with somatostatin receptor scintigraphy. J Nucl Med 1999;40:1602-1608
- Jensen RT. Endocrine Neoplasms of the Pancreas. In: Yamada T, Alpers DH, Kalloo AN et al. eds. Textbook of Gastroenterology. Oxford, England: Wiley-Blackwell; 2009:1875-1920.
- Kos-Kudla B, O'Toole D, Falconi M , et al. ENETS consensus guidelines for the management of bone and lung metastases from neuroendocrine tumors. Neuroendocrinology 2010;91:341-350
- Leboulleux S, Dromain C, Vataire AL , et al. Prediction and diagnosis of bone metastases in well-differentiated gastro-entero-pancreatic endocrine cancer: a prospective comparison of whole body magnetic resonance imaging and somatostatin receptor scintigraphy. J Clin Endocrinol Metab 2008;93:3021-3028
- Van Loon K, Zhang L, Keiser J , et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr Connect 2015;4:9-17
- Altieri B, Di Dato C, Martini C , et al. Bone Metastases in Neuroendocrine Neoplasms: From Pathogenesis to Clinical Management. Cancers (Basel) 2019;11:1332
- Cortes M, Mora J, Benitez A , et al. (Bone metastasis from gastrinoma without hepatic involvement. Utility of the scintigraphy with 111 In-pentetreotide). Rev Esp Med Nucl 2001;20:23-26
- Klimstra DS, Modlin IR, Coppola D , et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-712
- Heitz PU, Kasper M, Polak JM , et al. Pancreatic endocrine tumors. Immunohistochemial analysis of 125 tumors. Hum Pathol 1982;13:263-271
- Chiang HC, O'Dorisio TM, Huang SC , et al. Multiple hormone elevations in patients with Zollinger-Ellison syndrome: Prospective study of clinical significance and of the development of a second symptomatic pancreatic endocrine tumor syndrome. Gastroenterology 1990;99:1565-1575
- Klimstra DS. Pathology reporting of neuroendocrine tumors: essential elements for accurate diagnosis, classification, and staging. Semin Oncol 2013;40:23-36
- Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2011;18 Suppl 1:S1-S16
- Rindi G, Mete O, Uccella S , et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol 2022;33:115-154
- Kloppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med 2017;33:324-330
- Tang LH. Pancreatic Neuroendocrine Neoplasms: Landscape and Horizon. Arch Pathol Lab Med 2020;144:816-828
- Anonymous. WHO classification of tumours of endocrine organs. Vol. 10, 4th Edition. Lyon, France: Agency for Research on Cancer; 2017.
- Sorbye H, Baudin E, Perren A. The Problem of High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumors, Neuroendocrine Carcinomas, and Beyond. Endocrinol Metab Clin North Am 2018;47:683-698
- Milione M, Maisonneuve P, Spada F , et al. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology 2017;104:85-93
- Rindi G, Falconi M, Klersy C , et al. TNM Staging of Neoplasms of the Endocrine Pancreas: Results From a Large International Cohort Study. J Natl Cancer Inst 2012;104:764-777
- Jensen RT, Ito T. Gastrinoma. Endotext(Internet){www endotext org) 2020;
- Stabile BE, Passaro E, Jr. Benign and malignant gastrinoma. Am J Surg 1985;49:144-150
- Cadiot G, Vuagnat A, Doukhan I , et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Gastroenterology 1999;116:286-293
- Ruszniewski P, Podevin P, Cadiot G , et al. Clinical, anatomical, and evolutive features of patients with the Zollinger-Ellison syndrome combined with type I multiple endocrine neoplasia. Pancreas 1993;8:295-304
- Chandrasekharappa SC, Guru SC, Manickam P , et al. Positional cloning of the gene for multiple endocrine neoplasia-Type 1. Science 1997;276:404-407
- Debelenko LV, Zhuang ZP, Emmert-Buck MR , et al. Allelic deletions on chromosome 11q13 in Multiple Endocrine Neoplasia Type-I-associated sporadic gastrinomas and pancreatic endocrine tumors. Cancer Res 1997;57:2238-2243
- Goebel SU, Vortmeyer O, Zhang ZP , et al. Identical clonality of sporadic gastrinomas at multiple sites. Cancer Res 2000;60:60-63
- Gurevich L, Kazantseva I, Isakov VA , et al. The analysis of immunophenotype of gastrin-producing tumors of the pancreas and gastrointestinal tract. Cancer 2003;98:1967-1976
- Kimura H, Ohtsuka T, Fujimoto T , et al. Different Hormonal Expression Patterns Between Primary Pancreatic Neuroendocrine Tumors and Metastatic Sites. Pancreas 2016;45:947-952
- Hong L, Wang Y, Zhang T , et al. Chromogranin A: A Valuable Serum Diagnostic Marker for Non-Insulinoma Neuroendocrine Tumors of the Pancreas in a Chinese Population. Med Sci Monit 2020;26:e926635
- Cherner JA, Doppman JL, Norton JA , et al. Selective venous sampling for gastrin to localize gastrinomas. A prospective study. Ann Intern Med 1986;105:841-847
- Wynick D, Williams SJ, Bloom SR. Symptomatic secondary hormone syndromes in patients with established malignant pancreatic endocrine tumors. N Engl J Med 1988;319:605-607
- Lase I, Strele I, Gronberg M , et al. Multiple hormone secretion may indicate worse prognosis in patients with ectopic Cushing's syndrome. Hormones (Athens ) 2020;19:351-360
- Price DE, Absalom SR, Davidson K , et al. A case of multiple endocrine neoplasia: hyperparathyroidism, insulinoma, GRF-oma, hypercalcitoninaemia and intractable peptic ulceration. Clin Endocrinol (Oxf) 1992;37:187-188
- Liaw CC, Lin JT, Chen TJ. Multiple-hormone-producing islet cell carcinoma: report of a case. Taiwan Yi Xue Hui Za Zhi 1989;88:722-725
- Murray-Lyon IM, Eddleston AL, Williams R , et al. Treatment of multiple-hormone-producing malignant islet-cell tumor with streptozotocin. Lancet 1968;2:895-898
- Lodish MB, Powell AC, Abu-Asab M , et al. Insulinoma and gastrinoma syndromes from a single intrapancreatic neuroendocrine tumor. J Clin Endocrinol Metab 2008;93:1123-1128
- Rustagi T, Siegel RD. Zollinger-ellison syndrome with subsequent association of insulinoma. JOP 2010;11:486-488
- Mizuno N, Naruse S, Kitagawa M , et al. Insulinoma with subsequent association of Zollinger-Ellison syndrome. Intern Med 2001;40:386-390
- Wilson DM, Ceda GP, Bostwick DG , et al. Acromegaly and Zollinger-Ellison syndrome secondary to an islet cell tumor: characterization and quantification of plasma and tumor human growth hormone-releasing factor. J Clin Endocrinol Metab 1984;59:1002-1005
- Ching JL, Christofides ND, Kraenzlin ME , et al. Growth hormone secretion dynamics in a patient with ectopic growth hormone-releasing factor production. Am J Med 1985;79:135-138
- Kubicka E, Zawadzka K, Syrycka J , et al. A case of gastrinoma associated with ectopic Cushing syndrome. Pol Arch Intern Med 2020;130:328-329
- Amikura K, Alexander HR, Norton JA , et al. The role of surgery in the management of ACTH-producing islet cell tumors of the pancreas. Surgery 1995;118:1125-1130
- Doppman JL, Nieman LK, Cutler GB, Jr. , et al. Adrenocorticotropic hormone-secreting islet cell tumors: Are they always malignant? Radiology 1994;190:59-64
- Ilias I, Torpy DJ, Pacak K , et al. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab 2005;90:4955-4962
- Raddatz D, Horstmann O, Basenau D , et al. Cushing's syndrome due to ectopic adrenocorticotropic hormone production by a non-metastatic gastrinoma after lonterm conservative treatment of Zollinger-Ellison syndrome. Ital J Gastroenterol Hepatol 1998;30:636-640
- Asa SL, Kovacs K, Killinger DW , et al. Pancreatic islet cell carcinoma producing gastrin, ACTH, alpha- endorphin, somatostatin and calcitonin. Am J Gastroenterol 1980;74(1):30-35
- Law DH, Liddle GW, Scott HW, Jr. , et al. Ectopic production of multiple hormones (ACTH, MSH and gastrin) by a single malignant tumor. N Engl J Med 1965;273:292-296
- Lyons DF, Eisen BR, Clark MR , et al. Concurrent Cushing's and Zollinger-Ellison syndromes in a patient with islet cell carcinoma: case report and review of the literature. Am J Med 1984;76:729-733
- Belchetz PE, Brown CL, Makin HL , et al. ACTH, glucagon and gastrin production by a pancreatic islet cell carcinoma and its treatment. Clin Endocrinol 1973;2:307-316
- O'Neal LW, Kipnis DM, Luse SA , et al. Secretion of various endocrine substances by ACTH-secreting tumors--gastrin, melanotropin, norepinephrine, serotonin, parathormone, vasopressin, glucagon. Cancer 1968;21:1219-1232
- Park SY, Rhee Y, Youn JC , et al. Ectopic Cushing's Syndrome Due to Concurrent Corticotropin-Releasing Hormone (CRH) and Adrenocorticotropic Hormone (ACTH) Secreted by Malignant Gastrinoma. Exp Clin Endocrinol Diabetes 2007;115:13-16
- Ahmad NA, Furth EE, Schwartz SS , et al. Sporadic Zollinger-Ellison syndrome with ectopic production of corticotropin: surgical management. Endocr Pract 1999;5:261-265
- Kido-Nakahara M, Nakahara T, Miki M , et al. Necrolytic migratory erythema associated with alteration from predominantly gastrin-secreting to predominantly glucagon-secreting pancreatic neuroendocrine tumor. Eur J Dermatol 2014;24:702-703
- Balian A, Fromont C, Naveau S , et al. A particularly aggressive combined glucagonoma and gastrinoma syndrome. Eur J Gastroenterol Hepatol 1999;11:1417-1419
- Lokich J, Bothe A, O'Hara C , et al. Metastatic islet cell tumor with ACTH, gastrin, and glucagon secretion. Clinical and pathologic studies with multiple therapies. Cancer 1987;59:2053-2058
- Soga J, Yakuwa Y. Somatostatinoma/inhibitory syndrome: a statistical evaluation of 173 reported cases as compared to other pancreatic endocrinomas. J Exp Clin Cancer Res 1999;18:13-22
- Ito T, Lee L, Jensen RT. Carcinoid-syndrome: recent advances, current status and controversies. Curr Opin Endocrinol Diabetes Obes 2018;25:22-35
- Morita Y, Suzuki S, Sakaguchi T , et al. Pancreatic neuroendocrine cell tumor secreting parathyroid hormone-related protein and gastrin: Report of a case. Surg Today 2010;40:1192-1196
- Cho KJ, Vinik AI, Thompson NW , et al. Localization of the source of hyperinsulinism: percutaneous transhepatic portal and pancreatic vein catheterization with hormone assay. AJR Am J Roentgenol 1982;139:237-245
- Fraker DL, Jensen RT. Pancreatic endocrine tumors. In: DeVita VT, Hellman S, Rosenberg SA eds. Cancer: Principles and Practice of Oncology. Philadelphia, PA: Lippincott-Raven Publishers; 1997:1678-1704.
- Jensen RT, Norton JA. Endocrine neoplasms of the pancreas. In: Yamada T, Alpers DH, Owyang C et al. eds. Textbook of Gastroenterology. Philadelphia: J.B. Lippincott Company; 1998:2193-2228.
- Jensen RT, Norton JA. Endocrine tumors of the pancreas. In: Feldman M, Scharschmidt BF, Sleisenger MH eds. Gastrointestinal and Liver Disease. Philadelphia: W.B. Saunders; 1998:871-894.
- Jensen RT. Pancreatic endocrine tumors: recent advances. Ann Oncol 1999;10:170-176
- Rehfeld JF, Friis-Hansen L, Goetze JP , et al. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem 2007;7:1154-1165
- Walsh JH. Gastrin. In: Walsh JH, Dockray GJ eds. Gut Peptides. New York: Raven Press; 1994:75-121.
- Bardram L. Progastrin in pancreas and the Zollinger-Ellison syndrome. Scand J Gastroenterol 1990;25:1185-1195
- Bardram L. Progastrin in serum from Zollinger-Ellison patients. An indicator of malignancy? Gastroenterology 1990;98:1420-1426
- d'Herbomez M, Do Cao C, Vezzosi D , et al. Chromogranin A assay in clinical practice. Ann Endocrinol (Paris) 2010;71:274-280
- Lawrence B, Gustafsson BI, Kidd M , et al. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:111-34, viii
- Baekdal J, Krogh J, Klose M , et al. Limited Diagnostic Utility of Chromogranin A Measurements in Workup of Neuroendocrine Tumors. Diagnostics (Basel) 2020;10:881
- Jacoba IM, Weber HC. Biomarkers in gastroenteropancreatic neuroendocrine neoplasms. Curr Opin Endocrinol Diabetes Obes 2023;30:175-180
- Nobels FR, Kwekkeboom DJ, Bouillon R , et al. Chromogranin A: its clinical value as marker of neuroendocrine tumours. Eur J Clin Invest 1998;28:431-440
- Goebel SU, Serrano J, Yu F , et al. Prospective study of the value of serum chromogranin A or serum gastrin levels in assessment of the presence, extent, or growth of gastrinomas. Cancer 1999;85:1470-1483
- Nobels FR, Kwekkeboom DJ, Coopmans W , et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab 1997;82:2622-2628
- Ito T, Igarashi H, Jensen RT. Serum pancreastatin: the long sought universal, sensitive, specific tumor marker for neuroendocrine tumors? Pancreas 2012;41:505-507
- Abou-Saif A, Gibril F, Ojeaburu JV , et al. Prospective study of the ability of serial measurements of serum chromogranin A and gastrin to detect changes in tumor burden in patients with gastrinomas. Cancer 2003;98:249-261
- Rehfeld JF, Bardram L, Hilsted L , et al. An evaluation of chromogranin A versus gastrin and progastrin in gastrinoma diagnosis and control. Biomark Med 2014;8:571-580
- Massironi S, Rossi RE, Casazza G , et al. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: a large series from a single institution. Neuroendocrinology 2014;100:240-249
- Rehfeld JF. Chromogranin A in gastrinomas: Promises and pitfalls. Clin Chim Acta 2015;446:15-20
- Malczewska A, Oberg K, Kos-Kudla B. NETest is superior to chromogranin A in neuroendocrine neoplasia: a prospective ENETS CoE analysis. Endocr Connect 2021;10:110-123
- Malczewska A, Witkowska M, Wójcik-Giertuga M , et al. Prospective Evaluation of the NETest as a Liquid Biopsy for Gastroenteropancreatic and Bronchopulmonary Neuroendocrine Tumors: An ENETS Center of Excellence Experience. Neuroendocrinology 2021;111:304-319
- Modlin IM, Kidd M, Falconi M , et al. A multigenomic liquid biopsy biomarker for neuroendocrine tumor disease outperforms CgA and has surgical and clinical utility. Ann Oncol 2021;32:1425-1433
- Raines D, Chester M, Diebold AE , et al. A prospective evaluation of the effect of chronic proton pump inhibitor use on plasma biomarker levels in humans. Pancreas 2012;41:508-511
- Syversen U, Mignon M, Bonfils S , et al. Chromogranin A and pancreastatin-like immunoreactivity in serum of gastrinoma patients. Acta Oncol 1993;32:161-165
- Stabile BE, Howard TJ, Passaro E, Jr. , et al. Source of plasma chromogranin A elevation in gastrinoma patients. Arch Surg 1990;125:451-453
- Hirschowitz BI, Worthington J, Mohnen J , et al. Chromogranin A in patients with acid hypersecretion and/or hypergastrinaemia. Aliment Pharmacol Ther 2007;26:869-878
- Elouaer-Blanc L, Sobhani I, Ruszniewski P , et al. Gastrin secretion by gastrinoma cells in long-term culture. Am J Physiol 1988;255:G596-G602
- Isenberg JI, Walsh JH, Passaro E, Jr. , et al. Unusual effect of secretin on serum gastrin, serum calcium and gastric acid secretion in a patient with suspected Zollinger- Ellison syndrome. Gastroenterology 1972;62:626-631
- McGuigan JE, Wolfe MM. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology 1980;79:1324-1331
- Deveney CW, Deveney KS, Jaffe BM , et al. Use of calcium and secretin in the diagnosis of gastrinoma (Zollinger-Ellison syndrome). Ann Intern Med 1977;87:680-686
- Lamers CB, Van Tongeren JHM. Comparative study of the value of calcium, secretin, and meal stimulated increase in serum gastrin in the diagnosis of the Zollinger-Ellison syndrome. Gut 1977;18:128-134
- Korman MG, Soveny C, Hansky J. Effect of glucagon on serum gastrin. II. Studies in pernicious anaemia and the Zollinger-Ellison syndrome. Gut 1973;14:459-461
- Shibata C, Funayama Y, Fukushima K , et al. Role of selective arterial secretin injection test in treatment of gastrinoma. Hepatogastroenterology 2006;53:960-963
- Shibata C, Funayama Y, Fukushima K , et al. The Glucagon Provocative Test for the Diagnosis and Treatment of Zollinger-Ellison Syndrome. J Gastrointest Surg 2008;12:344-349
- Kawai K, Mukai H, Yuzawa K , et al. Effects of neuromedin B and GRP-10 on gastrin and insulin release from cultured tumor cells of a malignant gastrinoma. Endocrinol Jpn 1990;37:857-865
- Sakamoto T, Miyata M, Hamaji M , et al. Beta-adrenergic regulation of gastrin release from gastrinoma cells. Surgery 1990;107:282-288
- Passaro E, Jr., Basso N, Walsh JH. Calcium challenge in the Zollinger-Ellison syndrome. Surgery 1972;72:60-67
- Frucht H, Howard JM, Stark HA , et al. Prospective study of the standard meal provocative test in Zollinger-Ellison syndrome. Am J Med 1989;87:528-536
- Malagelada JR. Pathophysiological responses to meals in the Zollinger-Ellison syndrome: 1. Paradoxical postprandial inhibition of gastric secretion. Gut 1978;19:284-289
- Ellison EC, Gower WR, Elkhammas E , et al. Characterization of the in vivo and in vitro inhibition of gastrin secretion from gastrinoma by a somatostatin analogue (SMS 201-995). Am J Med 1986;81:56-64
- Vinik AI, Tsai S, Moattari AR , et al. Somatostatin analogue (SMS 201-995) in patients with gastrinomas. Surgery 1988;104:834-842
- Ruszniewski P, Ramdani A, Cadiot G , et al. Long-term treatment with octreotide in patients with the Zollinger-Ellison syndrome. Eur J Clin Invest 1993;23:296-301
- Auernhammer CJ, Goke B. Medical treatment of gastrinomas. Wien Klin Wochenschr 2007;119:609-615
- Long SH, Berna MJ, Thill M , et al. Secretin-receptor and secretin-receptor-variant expression in gastrinomas: correlation with clinical and tumoral features and secretin and calcium provocative test results. J Clin Endocrinol Metab 2007;92:4394-4402
- Goebel SU, Peghini PL, Goldsmith PK , et al. Expression of the calcium-sensing receptor in gastrinomas. J Clin Endocrinol Metab 2000;85:4131-4137
- Itami A, Kato M, Komoto I , et al. Human gastrinoma cells express calcium-sensing receptor. Life Sci 2001;70:119-129
- Reubi JC, Kvols LK, Waser B , et al. Detection of somatostatin receptors in surgical and percutaneous needle biopsy samples of carcinoids and islet cell carcinomas. Cancer Res 1990;50:5969-5977
- Krenning EP, Kwekkeboom DJ, Bakker WH , et al. Somatostatin receptor scintigraphy with (111In-DTPA-D-Phe1)- and (123I-Tyr3)-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716-731
- Shojamanesh H, Gibril F, Louie A , et al. Prospective study of the anti-tumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinomas. Cancer 2002;94:331-343
- Daniels LM, Khalili M, Morano WF , et al. Case report: optimal tumor cytoreduction and octreotide with durable disease control in a patient with MEN-1 and Zollinger-Ellison syndrome-over a decade of follow-up. World J Surg Oncol 2019;17:213
- Kwekkeboom DJ, de Herder WW, Kam BL , et al. Treatment with the radiolabeled somatostatin analog (177 Lu-DOTA 0,Tyr3)octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124-2130
- Brady CE, III. Secretin provocation test in the diagnosis of Zollinger-Ellison syndrome. Am J Gastroenterol 1991;86:129-134
- Brady CE, Utts SJ, Dev J. Secretin provocation in normal and duodenal ulcer subjects. Is the gastrin rise in Zollinger-Ellison syndrome paradoxic or exaggeration? Dig Dis Sci 1987;32:232-238
- Gower WR, Jr., Ellison EC, Knierim TH , et al. Gastrinoma in vitro: morphological and physiological studies of primary cell cultures. Gastroenterology 1990;98:936-954
- Imamura M, Adachi H, Takahashi K , et al. Gastrin release from gastrinoma cells stimulated with secretin. Dig Dis Sci 1982;27:1130-1136
- Chiba T, Yamatani T, Yamaguchi A , et al. Mechanism for increase of gastrin release by secretin in Zollinger-Ellison syndrome. Gastroenterology 1989;96:1439-1444
- Sawicki MP, Wan YJ, Johnson CL , et al. Loss of heterozygosity on chromosome 11 in sporadic gastrinomas. Hum Genet 1992;89:445-449
- Vortmeyer AO, Huang S, Lubensky I , et al. Non-islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab 2004;89:1934-1938
- Bonnavion R, Teinturier R, Jaafar R , et al. Islet Cells Serve as Cells of Origin of Pancreatic Gastrin-Positive Endocrine Tumors. Mol Cell Biol 2015;35:3274-3283
- Larsson LI, Rehfeld JF, Goltermann N. Gastrin in the human fetus: Distribution and molecular forms of gastrin in the antro-pyloric gland area, duodenum and pancreas. Scand J Gastroenterol 1977;12:869-872
- Passaro E, Jr., Howard TJ, Sawicki MP , et al. The origin of sporadic gastrinomas within the gastrinoma triangle: a theory. Arch Surg 1998;133:13-16
- Howard TJ, Sawicki MP, Stabile BE , et al. Biologic behavior of sporadic gastrinoma located to the right and left of the superior mesenteric artery. Am J Surg 1993;165:101-105
- Howard TJ, Stabile BE, Zinner MJ , et al. Anatomic distribution of pancreatic endocrine tumors. Am J Surg 1990;159:258-264
- Sawicki MP, Howard TJ, Dalton M , et al. The dichotomous distribution of gastrinomas. Arch Surg 1990;125:1584-1587
- Solcia E, Capella C, Buffa R , et al. Endocrine cells of the gastrointestinal tract and related tumors. Pathobiol Annu 1979;9:163-204
- Solcia E, Capella C, Buffa R , et al. Pathology of the Zollinger-Ellison syndrome. Prog Surg Pathol 1980;I:119-133
- Fendrich V, Ramerth R, Waldmann J , et al. Sonic hedgehog and pancreatic-duodenal homeobox 1 expression distinguish between duodenal and pancreatic gastrinomas. Endocr Relat Cancer 2009;16:613-622
- Grande E, Capdevila J, Barriuso J , et al. Gastroenteropancreatic neuroendocrine tumor cancer stem cells: do they exist? Cancer Metastasis Rev 2012;31:47-53
- Kloppel G, Anlauf M, Perren A. Endocrine precursor lesions of gastroenteropancreatic neuroendocrine tumors. Endocr Pathol 2007;18:150-155
- Anlauf M, Perren A, Meyer CL , et al. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 2005;128:1187-1198
- Anlauf M, Perren A, Henopp T , et al. Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions. Gut 2007;56:637-644
- Anlauf M, Perren A, Kloppel G. Endocrine precursor lesions and microadenomas of the duodenum and pancreas with and without MEN1: criteria, molecular concepts and clinical significance. Pathobiology 2007;74:279-284
- Sundaresan S, Kang AJ, Hayes MM , et al. Deletion of Men1 and somatostatin induces hypergastrinemia and gastric carcinoids. Gut 2017;66:1012-1021
- Sundaresan S, Meininger CA, Kang AJ , et al. Gastrin Induces Nuclear Export and Proteasome Degradation of Menin in Enteric Glial Cells. Gastroenterology 2017;153:1555-1567
- Perren A, Anlauf M, Henopp T , et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab 2007;92:1118-1128
- Sutliff VE, Doppman JL, Gibril F , et al. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J Clin Oncol 1997;15:2420-2431
- Goudet P, Peschaud F, Mignon M , et al. Les gastrinomes dans les neoplasies endocriniennes multiples de type 1. Une etude de cohorte de 127 cas du groupe des tumeurs endocrines (GTE). Ann Chir 2004;129:149-155
- Goudet P, Murat A, Binquet C , et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg 2010;34:249-255
- Giudici F, Cavalli T, Giusti F , et al. Natural History of MEN1 GEP-NET: Single-Center Experience After a Long Follow-Up. World J Surg 2017;41:2312-2323
- Mignon M, Cadiot G. Natural history of gastrinoma: lessons from the past. Ital J Gastroenterol Hepatol 1999;31:S98-S103
- Thompson JC, Reeder DD, Villar HV , et al. Natural history and experience with diagnosis and treatment of the Zollinger-Ellison syndrome. Surg Gynecol Obstet 1975;140:721-739
- Madeira I, Terris B, Voss M , et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut 1998;43:422-427
- Dromain C, Pavel ME, Ruszniewski P , et al. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer 2019;19:66
- Dromain C, Sundin A, Najran P , et al. Tumour Growth Rate to predict the outcome of patients with Neuroendocrine Tumours: Performance and sources of variability. Neuroendocrinology 2021;111:831-839
- Palazzo M, Lombard-Bohas C, Cadiot G , et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol 2013;25:232-238
- Lamarca A, Ronot M, Moalla S , et al. Tumor Growth Rate as a Validated Early Radiological Biomarker Able to Reflect Treatment-Induced Changes in Neuroendocrine Tumors: The GREPONET-2 Study. Clin Cancer Res 2019;25:6692-6699
- Lamarca A, Crona J, Ronot M , et al. Value of Tumor Growth Rate (TGR) as an Early Biomarker Predictor of Patients' Outcome in Neuroendocrine Tumors (NET)-The GREPONET Study. Oncologist 2019;24:e1082-e1090
- Oberg K. The genetics of neuroendocrine tumors. Semin Oncol 2013;40:37-44
- Zhang J, Francois R, Iyer R , et al. Current understanding of the molecular biology of pancreatic neuroendocrine tumors. J Natl Cancer Inst 2013;105:1005-1017
- Scarpa A. The landscape of molecular alterations in pancreatic and small intestinal neuroendocrine tumours. Ann Endocrinol (Paris) 2019;80:153-158
- Hackeng WM, Assi HA, Westerbeke FHM , et al. Prognostic and Predictive Biomarkers for Pancreatic Neuroendocrine Tumors. Surg Pathol Clin 2022;15:541-554
- Di Domenico A, Pipinikas CP, Maire RS , et al. Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Commun Biol 2020;3:740
- Jiao Y, Shi C, Edil BH , et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-1203
- Cejas P, Drier Y, Dreijerink KMA , et al. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat Med 2019;25:1260-1265
- Mohindroo C, McAllister F, De Jesus-Acosta A. Genetics of Pancreatic Neuroendocrine Tumors. Hematol Oncol Clin North Am 2022;36:1033-1051
- Duerr EM, Chung DC. Molecular genetics of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab 2007;21:1-14
- Corleto VD, Delle Fave G, Jensen RT. Molecular insights into gastrointestinal neuroendocrine tumors: importance and recent advances. Dig Liver Dis 2002;34:668-680
- Di Domenico A, Wiedmer T, Marinoni I , et al. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr Relat Cancer 2017;24:R315-R334
- Pipinikas CP, Berner AM, Sposito T , et al. The evolving (epi)genetic landscape of pancreatic neuroendocrine tumours. Endocr Relat Cancer 2019;26:R519-R544
- Wang H, Sun L, Bao H , et al. Genomic dissection of gastrointestinal and lung neuroendocrine neoplasm. Chin J Cancer Res 2019;31:918-929
- Hu W, Feng Z, Modica I , et al. Gene Amplifications in Well-Differentiated Pancreatic Neuroendocrine Tumors Inactivate the p53 Pathway. Genes Cancer 2010;1:360-368
- Tang LH, Contractor T, Clausen R , et al. Attenuation of the retinoblastoma pathway in pancreatic neuroendocrine tumors due to increased cdk4/cdk6. Clin Cancer Res 2012;18:4612-4620
- Chen Y, Ohki R. p53-PHLDA3-Akt Network: The Key Regulators of Neuroendocrine Tumorigenesis. Int J Mol Sci 2020;21:
- Ohki R, Saito K, Chen Y , et al. PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A 2014;111:E2404-E2413
- Mowrey K, Northrup H, Rougeau P , et al. Frequency, Progression, and Current Management: Report of 16 New Cases of Nonfunctional Pancreatic Neuroendocrine Tumors in Tuberous Sclerosis Complex and Comparison With Previous Reports. Front Neurol 2021;12:627672
- Ahmad S, Naber MR, Giles RH , et al. Diagnostic and management strategies for pNETs in Von Hippel-Lindau: a systematic review. Endocr Relat Cancer 2021;28:151-160
- Evans LM, Geenen KR, O'Shea A , et al. Tuberous sclerosis complex-associated nonfunctional pancreatic neuroendocrine tumors: Management and surgical outcomes. Am J Med Genet A 2022;188:2666-2671
- Halperin R, Tirosh A. Non-Interventional Management of Advanced Pancreatic Neuroendocrine Neoplasms in Patients with von Hippel-Lindau Disease. Cancers (Basel) 2023;15:1739
- Mestre-Alagarda C, Srirajaskanthan R, Zen Y , et al. Genetic and epigenetic prognosticators of neuroendocrine tumours of the GI tract, liver, biliary tract and pancreas: A systematic review and meta-analysis. Histopathology 2023 (In Press)
- Thakker RV. Multiple endocrine neoplasia type 1. Endocrinol Metab Clin North Am 2000;29:541-567
- Thakker RV. Multiple endocrine neoplasia type 1 (MEN1). Best Pract Res Clin Endocrinol Metab 2010;24:355-370
- Goebel SU, Heppner C, Burns AD , et al. Geneotype/phenotype correlations of MEN1 gene mutations in sporadic gastrinoma. J Clin Endocrinol Metab 2000;85:116-123
- Falchetti A, Marini F, Luzi E , et al. Multiple endocrine neoplasms. Best Pract Res Clin Rheumatol 2008;22:149-163
- Duan S, Sheriff S, Elvis-Offiah UB , et al. Clinically Defined Mutations in MEN1 Alter Its Tumor-suppressive Function Through Increased Menin Turnover. Cancer Res Commun 2023;3:1318-1334
- Scarpa A, Chang DK, Nones K , et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017;543:65-71
- Yachida S, Vakiani E, White CM , et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012;36:173-184
- Chan CS, Laddha SV, Lewis PW , et al. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 2018;9:4158
- Marinoni I, Kurrer AS, Vassella E , et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014;146:453-460
- Wang F, Xu X, Ye Z , et al. Prognostic Significance of Altered ATRX/DAXX Gene in Pancreatic Neuroendocrine Tumors: A Meta-Analysis. Front Endocrinol (Lausanne) 2021;12:691557
- Heaphy CM, Singhi AD. The diagnostic and prognostic utility of incorporating DAXX, ATRX, and alternative lengthening of telomeres to the evaluation of pancreatic neuroendocrine tumors. Hum Pathol 2022;129:11-20
- Hong X, Qiao S, Li F , et al. Whole-genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: leading to a new classification system. Gut 2020;69:877-887
- Lund KA, Lazar CS, Chen WS , et al. Phosphorylation of the epidermal growth factor receptor at threonine 654 inhibits ligand-induced internalization and down- regulation. J Biol Chem 1990;265:20517-20523
- Simon T, Riemer P, Jarosch A , et al. DNA methylation reveals distinct cells of origin for pancreatic neuroendocrine carcinomas and pancreatic neuroendocrine tumors. Genome Med 2022;14:24
- Amato E, Barbi S, Malpeli G , et al. Chromosome 3p alterations in pancreatic endocrine neoplasia. Virchows Arch 2011;458:39-45
- Capurso G, Festa S, Valente R , et al. Molecular pathology and genetics of pancreatic endocrine tumours. J Mol Endocrinol 2012;49:R37-R50
- Wolin EM. PI3K/Akt/mTOR Pathway Inhibitors in the Therapy of Pancreatic Neuroendocrine Tumors. Cancer Lett 2013;335:1-8
- Yao JC, Shah MH, Ito T , et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N Engl J Med 2011;364:514-523
- Missiaglia E, Dalai I, Barbi S , et al. Pancreatic Endocrine Tumors: Expression Profiling Evidences a Role for AKT-mTOR Pathway. J Clin Oncol 2010;28:245-255
- Serra S, Zheng L, Hassan M , et al. The FGFR4-G388R single-nucleotide polymorphism alters pancreatic neuroendocrine tumor progression and response to mTOR inhibition therapy. Cancer Res 2012;72:5683-5691
- Zhou H, Chen Q, Tan W , et al. Integrated clinicopathological features and gene microarray analysis of pancreatic neuroendocrine tumors. Gene 2017;625:72-77
- Wang DD, Liu ZW, Han MM , et al. Microarray based analysis of gene expression patterns in pancreatic neuroendocrine tumors. Eur Rev Med Pharmacol Sci 2015;19:3367-3374
- Waldum HL, Hauso O, Fossmark R. The regulation of gastric acid secretion - clinical perspectives. Acta Physiol (Oxf) 2014;210:239-256
- Hua J, Shi S, Xu J , et al. Expression Patterns and Prognostic Value of DNA Damage Repair Proteins in Resected Pancreatic Neuroendocrine Neoplasms. Ann Surg 2022;275:443-452
- Wilson SD. The role of surgery in children with the Zollinger-Ellison syndrome. Surgery 1982;92(4):682-692
- Nazir Z. Long-term follow-up of a child with primary lymph node gastrinoma and Zollinger-Ellison syndrome. J Pediatr Surg 2011;46:969-972
- Cives M, Ghayouri M, Morse B , et al. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:759-767
- Chang FY, Liao KY, Wu L , et al. An uncommon cause of abdominal pain and diarrhea-gastrinoma in an adolescent. Eur J Pediatr 2010;169:355-357
- Citak EC, Taskinlar H, Arpaci RB , et al. Primary lymph node gastrinoma: a rare cause of abdominal pain in childhood. J Pediatr Hematol Oncol 2013;35:394-398
- Goyal R, Debi U, Dey P , et al. Zollinger-Ellison syndrome: an unusual case of chronic diarrhoea in a child. Malays J Pathol 2016;38:321-325
- Anderson B, Sweetser S. Gastrointestinal: Zollinger-Ellison Syndrome: A rare cause of chronic diarrhea and abdominal pain. J Gastroenterol Hepatol 2017;32:1281
- Aamar A, Madhani K, Virk H , et al. Zollinger-Ellison Syndrome: A Rare Case of Chronic Diarrhea. Gastroenterology Res 2016;9:103-104
- Eads JR, Reidy-Lagunes D, Soares HP , et al. Differential Diagnosis of Diarrhea in Patients With Neuroendocrine Tumors. Pancreas 2020;49:1123-1130
- Bondeson AG, Bondeson L, Thompson NW. Stricture and perforation of the esophagus: overlooked threats in the Zollinger-Ellison syndrome. World J Surg 1990;14:361-363
- Siewert R, Jennewein HM, Arnold R , et al. The lower oesophageal sphincter in the Zollinger-Ellison syndrome. Ger Med 1973;3:101-102
- Mignon M, Jais P, Cadiot G et al. Clinical features and advances in biological diagnostic criteria for Zollinger-Ellison syndrome. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:223-239.
- Freund MR, Reissman P, Schwarz AD. Jejunal Perforations in Zollinger-Ellison Syndrome: Expect the Unexpected. Am Surg 2020;86:171-172
- Waxman I, Gardner JD, Jensen RT , et al. Peptic ulcer perforation as the presentation of Zollinger- Ellison syndrome. Dig Dis Sci 1991;16:19-24
- Pakzad N, Salonia J, Mathew J. A Young Man With Abdominal Pain, Shock, and Respiratory Distress. Chest 2018;154:e119-e121
- Jensen RT. Consequences of long-term proton pump blockade: Highlighting insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol 2006;98:4-19
- Miller LS, Vinayek R, Frucht H , et al. Reflux esophagitis in patients with Zollinger-Ellison syndrome. Gastroenterology 1990;98:341-346
- Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci 1991;36:933-942
- Thakker RV, Newey PJ, Walls GV , et al. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1). J Clin Endocrinol Metab 2012;97:2990-3011
- Langer P, Wild A, Celik I , et al. Prospective controlled trial of a standardized meal stimulation test in the detection of pancreaticoduodenal endocrine tumours in patients with multiple endocrine neoplasia type 1. Br J Surg 2001;88:1403-1407
- van Beek DJ, Nell S, Pieterman CRC , et al. Prognostic factors and survival in MEN1 patients with gastrinomas: Results from the DutchMEN study group (DMSG). J Surg Oncol 2019;120:966-975
- Norton JA, Cornelius MJ, Doppman JL , et al. Effect of parathyroidectomy in patients with hyperparathyroidism, Zollinger-Ellison syndrome and multiple endocrine neoplasia Type I: A prospective study. Surgery 1987;102:958-966
- Norton JA, Venzon DJ, Berna MJ , et al. Prospective study of surgery for primary hyperaparathyroidism (HPT) in Multiple Endocrine Neoplasia type 1 (MEN1), and Zollinger-Ellison syndrome (ZES): longterm outcome of a more virulent form of HPT. Ann Surgery 2008;247:501-510
- Benya RV, Metz DC, Venzon DJ , et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am J Med 1994;97:436-444
- Mignon M, Cadiot G. Diagnostic and therapeutic criteria in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. J Intern Med 1998;243:489-494
- Asgharian B, Turner ML, Gibril F , et al. Cutaneous tumors in patients with MEN1 and gastrinomas: Prospective study of frequency and development of criteria with high sensitivity and specificity. J Clin Endocrinol Metab 2004;89:5328-5336
- Asgharian B, Chen YJ, Patronas NJ , et al. Meningiomas may be a component tumor of MEN1. Clin Cancer Res 2004;10:869-880
- Gibril F, Chen Y-J, Schrump DS , et al. Prospective study of thymic carcinoids in patients with Multiple Endocrine Neoplasia Type 1. J Clin Endocrinol Metab 2003;88:1066-1081
- Corleto VD, Annibale B, Gibril F , et al. Does the widespread use of proton pump inhibitors mask, complicate and/or delay the diagnosis of Zollinger-Ellison syndrome? Aliment Pharmacol Ther 2001;15:1555-1561
- Rehfeld JF, Gingras MH, Bardram L , et al. The Zollinger-Ellison Syndrome and Mismeasurement of Gastrin. Gastroenterology 2011;140:1444-1453
- Murugesan SV, Varro A, Pritchard DM. Review article: Strategies to determine whether hypergastrinaemia is due to Zollinger Ellison syndrome rather than a more common benign cause. Aliment Pharmacol Ther 2009;29:1055-1068
- Metz DC, Strader DB, Orbuch M , et al. Use of omeprazole in Zollinger-Ellison: A prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther 1993;7:597-610
- Metz DC, Pisegna JR, Fishbeyn VA , et al. Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology 1992;103:1498-1508
- Poitras P, Gingras MH, Rehfeld JF. Secretin stimulation test for gastrin release in zollinger-ellison syndrome: to do or not to do? Pancreas 2013;42:903-904
- Rehfeld JF, Bardram L, Hilsted L , et al. Pitfalls in diagnostic gastrin measurements. Clin Chem 2012;58:831-836
- Rehfeld JF. The art of measuring gastrin in plasma: a dwindling diagnostic discipline? Scand J Clin Lab Invest 2008;68:353-361
- Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf 2000;22:53-72
- Green FW, Norton RA, Kaplan MM. Pharmacology and clinical use of antacids. Am J Hosp Pharm 1975;32:425-429
- Gibril F, Jensen RT. Diagnostic uses of radiolabelled somatostatin-receptor analogues in gastroenteropancreatic endocrine tumors. Dig Liver Dis 2004;36:S106-S120
- Saeed ZA, Evans DJ, Jr., Evans DG , et al. Helicobacter pylori and the Zollinger-Ellison syndrome. Dig Dis Sci 1991;36:15-18
- Metz DC, Weber HC, Orbuch M , et al. Helicobacter pylori infection: a reversible cause of hypergastrinemia and hyperchlorhydria which can mimic Zollinger-Ellison syndrome. Dig Dis Sci 1995;40:153-159
- Fich A, Talley NJ, Shorter RG , et al. Zollinger-Ellison syndrome. Relation to Helicobacter pylori-associated chronic gastritis and gastric acid secretion. Dig Dis Sci 1991;36:10-14
- Weber HC, Venzon DJ, Jensen RT , et al. Studies on the interrelation between Zollinger-Ellison syndrome, Helicobacter pylori and proton pump inhibitor therapy. Gastroenterology 1997;112:84-91
- Watanabe T, Matsushima Y, Nakase H , et al. Effects of Helicobacter pylori infection on Zollinger-Ellison syndrome. J Gastroenterol 2000;35:735-741
- McColl KE. Helicobacter pylori-negative nonsteroidal anti-inflammatory drug-negative ulcer. Gastroenterol Clin North Am 2009;38:353-361
- Fishbeyn VA, Norton JA, Benya RV , et al. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med 1993;119:199-206
- McCarthy DM, Peikin SR, Lopatin RN , et al. Hyperparathyroidism a reversible cause of cimetidine-resistant gastric hypersecretion. Br Med J 1979;1:1765-1766
- Winczura A, Saggi B, Savage-Lobeck D. Gastrinoma With Relatively Low Gastrin Levels: A Case Report. Cureus 2023;15:e41686
- Borch K, Renvall H, Liedberg G , et al. Relations between circulating gastrin and endocrine cell proliferation in the atrophic gastric fundic mucosa. Scand J Gastroenterol 1986;21:357-363
- Vannella L, Sbrozzi-Vanni A, Lahner E , et al. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment Pharmacol Ther 2011;33:1361-1369
- Delle Fave G, Marignani M, Moretti A , et al. Hypergastrinemia and enterochromaffin-like cell hyperplasia. Yale J Biol Med 1998;71:291-301
- Dhillo WS, Jayasena CN, Lewis CJ , et al. Plasma gastrin measurement cannot be used to diagnose a gastrinoma in patients on either proton pump inhibitors or histamine type-2 receptor antagonists. Ann Clin Biochem 2006;43:153-155
- Lamberts R, Creutzfeldt W, Stockmann F , et al. Long term omeprazole treatment in man: effects on gastric endocrine cell populations. Digestion 1988;39:126-135
- Pregun I, Herszenyi L, Juhasz M , et al. Effect of proton-pump inhibitor therapy on serum chromogranin a level. Digestion 2011;84:22-28
- Jansen JB, Klinkenberg-Knol EC, Meuwissen SG , et al. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology 1990;99:621-628
- Arnold R. Diagnosis and differential diagnosis of hypergastrinemia. Wien Klin Wochenschr 2007;119:564-569
- Lundell L, Vieth M, Gibson F , et al. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther 2015;42:649-663
- Calvete O, Reyes J, Zuniga S , et al. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum Mol Genet 2015;24:2914-2922
- Fossmark R, Calvete O, Mjones P , et al. ECL-cell carcinoids and carcinoma in patients homozygous for an inactivating mutation in the gastric H(+) K(+) ATPase alpha subunit. APMIS 2016;124:561-566
- Ihara Y, Umeno J, Hori Y. Type IV Gastric Carcinoids in the Stomach Caused by ATP4A Gene Mutations. Clin Gastroenterol Hepatol 2020;18:A22
- Calvete O, Varro A, Pritchard DM , et al. A knockin mouse model for human ATP4aR703C mutation identified in familial gastric neuroendocrine tumors recapitulates the premalignant condition of the human disease and suggests new therapeutic strategies. Dis Model Mech 2016;9:975-984
- Maltz C, Lightdale CJ. The Zollinger-Ellison syndrome: a new approach. Clin Bull 1979;9:165-167
- Sadowski SM, Millo C, Cottle-Delisle C , et al. Results of (68)Gallium-DOTATATE PET/CT Scanning in Patients with Multiple Endocrine Neoplasia Type 1. J Am Coll Surg 2015;221:509-517
- Minalyan A, Benhammou JN, Artashesyan A , et al. Autoimmune atrophic gastritis: current perspectives. Clin Exp Gastroenterol 2017;10:19-27
- Dixon MF, Genta RM, Yardley JHCP. Classification and grading of gastritis. The upgraded Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-1181
- Gibril F, Reynolds JC, Chen CC , et al. Specificity of somatostatin receptor scintigraphy: a prospective study and the effects of false positive localizations on management in patients with gastrinomas. J Nucl Med 1999;40:539-553
- Festen HP, Thijs JC, Lamers CB , et al. Effect of oral omeprazole on serum gastrin and serum pepsinogen I levels. Gastroenterology 1984;87:1030-1034
- McArthur KE, Collen MJ, Maton PN , et al. Omeprazole: effective, convenient therapy for Zollinger-Ellison syndrome. Gastroenterology 1985;88:939-944
- Vinayek R, Amantea MA, Maton PN , et al. Pharmacokinetics of oral and intravenous omeprazole in patients with the Zollinger-Ellison syndrome. Gastroenterology 1991;101:138-147
- Metz DC, Pisegna JR, Ringham GL , et al. Prospective study of efficacy and safety of lansoprazole in Zollinger-Ellison syndrome. Dig Dis Sci 1993;38:245-256
- Hirschowitz BI, Mohnen J, Shaw S. Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome. Aliment Pharmacol Ther 1996;10:507-522
- Jensen RT. Use of omeprazole and other proton pump inhibitors in the Zollinger-Ellison syndrome. In: Olbe L ed. Milestones in Drug Therapy. Basel, Switzerland: Birkhauser Verlag AG Publish. Co.; 1999:205-221.
- Vinayek R, Howard JM, Maton PN , et al. Famotidine in the therapy of gastric hypersecretory states. Am J Med 1986;81:49-59
- Jensen RT, Collen MJ, McArthur KE , et al. Comparison of the effectiveness of ranitidine and cimetidine in inhibiting acid secretion in patients with gastric acid hypersecretory states. Am J Med 1984;77(5B):90-105
- Howard JM, Chremos AN, Collen MJ , et al. Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology 1985;88:1026-1033
- Jensen RT. Basis for failure of cimetidine in patients with Zollinger- Ellison syndrome. Dig Dis Sci 1984;29:363-366
- Jensen RT. Endocrine tumors of the Gastrointestinal Tract and Pancreas. In: Longo DL, Fauci AS, Kasper DL et al. eds. Harrison's Principles of Internal Medicine. New York: McGraw-Hill companies; 2012:3056-3072.
- Arnold R. Endocrine tumours of the gastrointestinal tract. Introduction: definition, historical aspects, classification, staging, prognosis and therapeutic options. Best Pract Res Clin Gastroenterol 2005;19:491-505
- Kulke MH, Shah MH, Benson AB , et al. Neuroendocrine Tumors: version 3.2017: NCCN Clinical Practice Guidelines in Oncology. NCCN Clinical Practice Guidelines in Oncology 2017;1-116
- Oh DS, Wang HS, Ohning GV , et al. Validation of a new endoscopic technique to assess acid output in Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol 2006;4:1467-1473
- Gibril F, Lindeman RJ, Abou-Saif A , et al. Retained gastric antrum syndrome. A forgotten, treatable cause of refractory peptic ulcer disease. Dig Dis Sci 2001;46:610-617
- Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol 2012;28:587-593
- Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis 2011;29:459-464
- Deveney CW, Deveney KE, Jones S, Jaffe BM. Calcium and secretin tests in diagnosis of gastrinoma. Gastroenterology 70, 968. 1976.
- Annibale B, Rindi G, D'Ambra G , et al. Antral gastrin cell hyperfunction and helicobacter pylori infection. Aliment Pharmacol Ther 1996;10:607-615
- Shibata C, Kakyo M, Kinouchi M , et al. Criteria for the glucagon provocative test in the diagnosis of gastrinoma. Surg Today 2013;43:1281-1285
- Feldman M, Schiller LR, Walsh JH , et al. Positive intravenous secretin test in patients with achlorhydria-related hypergastrinemia. Gastroenterology 1987;93:59-62
- Shah P, Singh MH, Yang YX , et al. Hypochlorhydria and achlorhydria are associated with false-positive secretin stimulation testing for zollinger-ellison syndrome. Pancreas 2013;42:932-936
- Leung D, Schwartz L. Imaging of neuroendocrine tumors. Semin Oncol 2013;40:109-119
- Sundin A. Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 2012;26:803-818
- Klose KJ, Heverhagen JT. Localisation and staging of gastrin producing tumours using cross-sectional imaging modalities. Wien Klin Wochenschr 2007;119:588-592
- Singh A, Hines JJ, Friedman B. Multimodality Imaging of the Pancreatic Neuroendocrine Tumors. Semin Ultrasound CT MR 2019;40:469-482
- Fottner C, Ferrata M, Weber MM. Hormone secreting gastro-entero-pancreatic neuroendocrine neoplasias (GEP-NEN): When to consider, how to diagnose? Rev Endocr Metab Disord 2017;18:393-410
- Gibril F, Doppman JD, Jensen RT. Comparative analysis of tumor localization techniques for neuroendocrine tumors. Yale J Biol Med 1997;70:481-500
- Jensen RT. Carcinoid and pancreatic endocrine tumors: recent advances in molecular pathogenesis, localization, and treatment. Curr Opin Oncol 2000;12:368-377
- Szidonya L, Park EA, Kwak JJ , et al. Molecular and Anatomic Imaging of Neuroendocrine Tumors. Surg Oncol Clin N Am 2022;31:649-671
- Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US). Best Pract Res Clin Endocrinol Metab 2007;21:43-68
- Wank SA, Doppman JL, Miller DL , et al. Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology 1987;92:905-912
- Doppman JL, Miller DL, Chang R , et al. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology 1990;174:25-29
- Metz DC, Jensen RT. Advances in gastric antisecretory therapy in Zollinger-Ellison syndrome. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:240-257.
- Sundin A. Imaging of neuroendocrine tumors. Expert Opin Med Diagn 2012;6:473-483
- Hussain JS, Srinivasa RN, Hage A , et al. Bringing SASI back: Single session selective arterial secretin injection and transarterial embolization of intrahepatic pancreatic neuroendocrine metastasis in a MEN-1 patient. Radiol Case Rep 2018;13:333-335
- Hayashi R, Minami I, Sasahara Y , et al. Diagnostic accuracy of selective arterial calcium injection test for localization of gastrinoma. Endocr J 2020;67:305-315
- Manoharan J, Bollmann C, Kann PH , et al. Gender Differences in Multiple Endocrine Neoplasia Type 1: Implications for Screening? Visc Med 2020;36:3-9
- Orbuch M, Doppman JL, Strader DB et al. Imaging for pancreatic endocrine tumor localization: recent advances. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:268-281.
- Yang J, Kan Y, Ge BH , et al. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol 2014;55:389-398
- Strader DB, Doppman JL, Orbuch M et al. Functional localization of pancreatic endocrine tumors. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: Karger Publishing Co.; 1995:282-297.
- Thom AK, Norton JA, Doppman JL , et al. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery 1992;112(#6):1002-1008
- Miller DL, Doppman JL, Metz DC , et al. Zollinger-Ellison syndrome: technique, results and complications of portal venous sampling. Radiology 1992;182:235-241
- Gibril F, Doppman JL, Chang R , et al. Metastatic gastrinomas: localization with selective arterial injection of secretin. Radiology 1996;198:77-84
- Imamura M, Takahashi K. Use of selective arterial secretin injection test to guide surgery in patients with Zollinger-Ellison syndrome. World J Surg 1993;17:433-438
- Vannella L, Lahner E, Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: a critical reappraisal. World J Gastroenterol 2012;18:1279-1285
- Otto AI, Marschalko M, Zalatnai A , et al. Glucagon cell adenomatosis: a new entity associated with necrolytic migratory erythema and glucagonoma syndrome. J Am Acad Dermatol 2011;65:458-459
- el-Omar EM, Penman ID, Ardill JE , et al. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 1995;109:681-691
- Kwekkeboom DJ, Kam BL, Van Essen M , et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 2010;17:R53-R73
- Behe M, Gotthardt M, Behr TM. Imaging of gastrinomas by nuclear medicine methods. Wien Klin Wochenschr 2007;119:593-596
- Oberg KE, Reubi JC, Kwekkeboom DJ , et al. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010;139:742-53, 753
- Kong G, Hicks RJ. Peptide Receptor Radiotherapy: Current Approaches and Future Directions. Curr Treat Options Oncol 2019;20:77
- Kwekkeboom DJ, Krenning EP. Peptide Receptor Radionuclide Therapy in the Treatment of Neuroendocrine Tumors. Hematol Oncol Clin North Am 2016;30:179-191
- Murtha TD, Lupsa BC, Majumdar S , et al. A Systematic Review of Proinsulin-Secreting Pancreatic Neuroendocrine Tumors. J Gastrointest Surg 2017;21:1335-1341
- Bushnell DL, Bodeker KL. Overview and Current Status of Peptide Receptor Radionuclide Therapy. Surg Oncol Clin N Am 2020;29:317-326
- Feijtel D, de Jong M, Nonnekens J. Peptide receptor radionuclide therapy: Looking back, looking forward. Curr Top Med Chem 2020;20:2959-2969
- La Salvia A, Modica R, Rossi RE , et al. Targeting neuroendocrine tumors with octreotide and lanreotide: Key points for clinical practice from NET specialists. Cancer Treat Rev 2023;117:102560
- Alexander HR, Fraker DL, Norton JA , et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg 1998;228:228-238
- Barton JC, Hirschowitz BI, Maton PN , et al. Bone metastases in malignant gastrinoma. Gastroenterology 1986;91:1179-1185
- Mathew A, Fendler WP, Theysohn J , et al. Bone Metastases in Patients with Pancreatic NETs: Prevalence and Prognosis. Horm Metab Res 2023 (In Press)
- Cives M, Pelle E, Rinzivillo M , et al. BONE METASTASES IN NEUROENDOCRINE TUMORS: MOLECULAR PATHOGENESIS AND IMPLICATIONS IN CLINICAL PRACTICE. Neuroendocrinology 2020;111:207-216
- Ruszniewski P, Amouyal P, Amouyal G , et al. Localization of gastrinomas by endoscopic ultrasonography in patients with Zollinger-Ellison syndrome. Surgery 1995;117:629-635
- Thompson NW, Czako PF, Fritts LL , et al. Role of endoscopic ultrasonography in the localization of insulinomas and gastrinomas. Surgery 1994;116:1131-1138
- Rosch T, Lightdale CJ, Botet JF , et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med 1992;326:1721-1726
- Kamata K, Ashida R, Yasukawa S , et al. Histological diagnosis and grading of pancreatic neuroendocrine tumor by endoscopic ultrasound-guided fine needle biopsy using a 25-gauge needle with a core trap: A multicenter prospective trial. Pancreatology 2020;20:1428-1433
- Kann PH. Is endoscopic ultrasonography more sensitive than magnetic resonance imaging in detecting and localizing pancreatic neuroendocrine tumors? Rev Endocr Metab Disord 2018;19:133-137
- Thomas-Marques L, Murat A, Delemer B , et al. Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol 2006;101:266-273
- Kann PH, Balakina E, Ivan D , et al. Natural course of small, asymptomatic neuroendocrine pancreatic tumours in multiple endocrine neoplasia type 1: an endoscopic ultrasound imaging study. Endocr Relat Cancer 2006;13:1195-1202
- Binderup T, Knigge U, Loft A , et al. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;16:978-985
- Carideo L, Prosperi D, Panzuto F , et al. Role of Combined ((68)Ga)Ga-DOTA-SST Analogues and ((18)F)FDG PET/CT in the Management of GEP-NENs: A Systematic Review. J Clin Med 2019;8:1032
- Mohamed A, Asa SL, Lee Z , et al. The predictive impact of dual somatostatin receptor/fluorodeoxyglucose (FDG) positron emission tomography (PET) in metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs): review of literature and a single institution experience. J Gastrointest Oncol 2023;14:1087-1094
- Dromain C, Deandreis D, Scoazec JY , et al. Imaging of neuroendocrine tumors of the pancreas. Diagn Interv Imaging 2016;97:1241-1257
- Naswa N, Sharma P, Gupta SK , et al. Dual tracer functional imaging of gastroenteropancreatic neuroendocrine tumors using 68Ga-DOTA-NOC PET-CT and 18F-FDG PET-CT: competitive or complimentary? Clin Nucl Med 2014;39:e27-e34
- Rinzivillo M, Partelli S, Prosperi D , et al. Clinical Usefulness of (18)F-Fluorodeoxyglucose Positron Emission Tomography in the Diagnostic Algorithm of Advanced Entero-Pancreatic Neuroendocrine Neoplasms. Oncologist 2018;23:186-192
- Sharma P, Naswa N, Kc SS , et al. Comparison of the prognostic values of Ga-DOTANOC PET/CT and F-FDG PET/CT in patients with well-differentiated neuroendocrine tumor. Eur J Nucl Med Mol Imaging 2014;41:2194-2202
- Has Simsek D, Kuyumcu S, Turkmen C , et al. Can complementary 68Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? J Nucl Med 2014;55:1811-1817
- Hindie E. The NETPET Score: Combining FDG and Somatostatin Receptor Imaging for Optimal Management of Patients with Metastatic Well-Differentiated Neuroendocrine Tumors. Theranostics 2017;7:1159-1163
- Chan DL, Pavlakis N, Schembri GP , et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017;7:1149-1158
- Nilica B, Waitz D, Stevanovic V , et al. Direct comparison of (68)Ga-DOTA-TOC and (18)F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur J Nucl Med Mol Imaging 2016;43:1585-1592
- Ito T, Hijioka S, Masui T , et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol 2017;52:9-18
- Mignon M, Bonfils S. Diagnosis and treatment of Zollinger-Ellison syndrome. Bailliere's Clin Gastroenterol 1988;2:677-698
- Maton PN, Gardner JD, Jensen RT. Diagnosis and management of Zollinger-Ellison syndrome. Endocrinol Metab Clin North Am 1989;18:519-543
- Atalar K, Warren OJ, Jacyna M , et al. Laparoscopic resection for primary lymph node gastrinoma. Pancreas 2013;42:723-725
- Jensen RT. Gastrin-producing tumors. Cancer Treat Res 1997;89:293-334
- Jensen RT, Maton PN, Gardner JD. Current management of Zollinger-Ellison syndrome. Drugs 1986;32:188-196
- Fox PS, Hofmann JW, Wilson SD , et al. Surgical management of the Zollinger-Ellison syndrome. Surg Clin North Am 1974;54:395-407
- Jensen RT. Natural history of digestive endocrine tumors. In: Mignon M, Colombel JF eds. Recent advances in pathophysiology and management of inflammatory bowel diseases and digestive endocrine tumors. Paris, France: John Libbey Eurotext Publishing Co.; 1999:192-219.
- Quatrini M, Castoldi L, Rossi G , et al. A follow-up study of patients with Zollinger-Ellison syndrome in the period 1966-2002: effects of surgical and medical treatments on long-term survival. J Clin Gastroenterol 2005;39:376-380
- Zollinger RM, Ellison EC, O'Dorisio TM , et al. Thirty years' experience with gastrinoma. World J Surg 1984;8:427-435
- Jensen RT. Gastrinomas: advances in diagnosis and management. Neuroendocrinology 2004;80:23-27
- Malagelada JR, Edis AJ, Adson MA , et al. Medical and surgical options in the management of patients with gastrinoma. Gastroenterology 1983;84:1524-1532
- Franz RC, Penzhorn HO. Is total gastrectomy still a viable option in the management of patients with the Zollinger-Ellison syndrome? S Afr J Surg 2007;45:58-60
- Stroker E, Leone L, Vandeput Y , et al. Severe symptomatic hypomagnesaemia induced by the chronic use of proton pump inhibitors: a case report of a patient with Zollinger-Ellison syndrome. Acta Clin Belg 2014;69:62-65
- Richardson CT, Walsh JH. The value of a histamine H2-receptor antagonist in the management of patients with the Zollinger-Ellison syndrome. N Engl J Med 1976;294:133-135
- Richardson CT, Feldman M, McClelland RN , et al. Effect of vagotomy in Zollinger-Ellison syndrome. Gastroenterology 1979;77:682-686
- McArthur KE, Richardson CT, Barnett CC , et al. Laparotomy and proximal gastric vagotomy in Zollinger-Ellison syndrome: results of a 16-year prospective study. Am J Gastroenterol 1996;91:1104-1111
- Lamas C, Navarro E, Casteras A , et al. MEN1-associated primary hyperparathyroidism in the Spanish Registry: clinical characterictics and surgical outcomes. Endocr Connect 2019;8:1416-1424
- Goudet P, Cougard P, Vergès B , et al. Hyperparathyroidism in multiple endocrine neoplasia type I: surgical trends and results of a 256-patient series from Groupe d'Etude des Néoplasies Endocriniennes Multiples study group. World J Surg 2001;25:886-890
- Nilubol N, Weinstein LS, Simonds WF , et al. Limited Parathyroidectomy in Multiple Endocrine Neoplasia Type 1-Associated Primary Hyperparathyroidism: A Setup for Failure. Ann Surg Oncol 2016;23:416-423
- Kivlen MH, Bartlett DL, Libutti SK , et al. Reoperation for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery 2001;130:991-998
- Elaraj DM, Skarulis MC, Libutti SK , et al. Results of initial operation for hyperparathryoidism (HPT) in patients with Multiple Endocrine Neoplasia Type 1 (MEN1). Surgery 2003;134:858-864
- Pisegna JR, Norton JA, Slimak GG , et al. Effects of curative resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology 1992;102:767-778
- Ojeaburu JV, Ito T, Crafa P , et al. Mechanism of Acid hypersecretion post curative gastrinoma resection. Dig Dis Sci 2011;56:139-154
- Metz DC, Benya RV, Fishbeyn VA , et al. Prospective study of the need for long-term antisecretory therapy in patients with Zollinger-Ellison syndrome following successful curative gastrinoma resection. Aliment Pharmacol Ther 1993;7(#3):247-257
- Weinstein MC, Feinberg HV. Clinical decision analysis. Philadelphia, PA: W.B. Saunders; 1980.
- Maton PN, Vinayek R, Frucht H , et al. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology 1989;97:827-836
- Maton PN, Frucht H, Vinayek R , et al. Medical management of patients with Zollinger-Ellison syndrome who have had previous gastric surgery: A prospective study. Gastroenterology 1988;94:294-299
- Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep 2004;6:454-463
- Hirschowitz BI, Simmons J, Mohnen J. Clinical outcome using lansoprazole in acid hypersecretors with and without Zollinger-Ellison syndrome: a 13-year prospective study. Clin Gastroenterol Hepatol 2005;3:39-48
- Hirschowitz BI, Simmons J, Mohnen J. Long-term lansoprazole control of gastric acid and pepsin secretion in ZE and non-ZE hypersecretors: a prospective 10-year study. Aliment Pharmacol Ther 2001;15:1795-1806
- Termanini B, Gibril F, Stewart CA , et al. A prospective study of the effectiveness of low dose omeprazole as initial therapy in Zollinger-Ellison syndrome. Aliment Pharmacol Ther 1996;10:61-71
- Heidelbaugh JJ, Metz DC, Yang YX. Proton pump inhibitors: are they overutilised in clinical practice and do they pose significant risk? Int J Clin Pract 2012;66:582-591
- Frucht H, Maton PN, Jensen RT. Use of omeprazole in patients with the Zollinger-Ellison syndrome. Dig Dis Sci 1991;36:394-404
- Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology 2010;139:1115-1127
- Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin b(12), iron, and magnesium. Curr Gastroenterol Rep 2010;12:448-457
- Linder L, Tamboue C, Clements JN. Drug-Induced Vitamin B12 Deficiency: A Focus on Proton Pump Inhibitors and Histamine-2 Antagonists. J Pharm Pract 2017;30:639-642
- Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol Toxicol 2002;91:333-350
- Termanini B, Gibril F, Sutliff VE, III , et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med 1998;104:422-430
- Hirschowitz BI, Worthington J, Mohnen J. Vitamin B12 deficiency in hypersecretors during long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther 2008;27:1110-1121
- Eyal A, Sueissa A, Braun E , et al. From hypomagnesaemia to Zollinger-Ellison syndrome: an adverse effect of a proton pump inhibitor. BMJ Case Rep 2014;2014:bcr2014205165
- Stewart CA, Termanini B, Sutliff VE , et al. Assessment of the risk of iron malabsorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther 1998;12:83-98
- Yang YX, Lewis JD, Epstein S , et al. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296:2947-2953
- Nehra AK, Alexander JA, Loftus CG , et al. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc 2018;93:240-246
- Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf 2018;10:2042098618809927
- Metz DC, Sostek MB, Ruszniewski P , et al. Effects of esomeprazole on Acid output in patients with zollinger-ellison syndrome or idiopathic gastric Acid hypersecretion. Am J Gastroenterol 2007;102:2648-2654
- Deveney CW, Stein S, Way LW. Cimetidine in the treatment of Zollinger-Ellison syndrome. Am J Surg 1983;146:116-123
- Pratap T, Jalal MJA, Jacob D , et al. Radiological Features of Zollinger-Ellison Syndrome: A Report of Two Cases. Indian J Radiol Imaging 2022;32:395-402
- Venkat PG, Longstreth GF. Gastroesophageal Reflux Disease and the Zollinger-Ellison Syndrome. Am J Gastroenterol 2022;117:1012-1013
- Simmons LH, Guimaraes AR, Zukerberg LR. Case records of the Massachusetts General Hospital. Case 6-2013. A 54-year-old man with recurrent diarrhea. N Engl J Med 2013;368:757-765
- Bhattacharya S, Blau JE, Koh C. Acute oesophageal necrosis in multiple endocrine neoplasia type 1: an undescribed complication. BMJ Case Rep 2021;14:e238214
- Matsubayashi H, Kawata N, Kakushima N , et al. A case of type 1 multiple endocrine neoplasia with esophageal stricture successfully treated with endoscopic balloon dilation and local steroid injection combined with surgical resection of gastrinomas. BMC Gastroenterol 2017;17:37
- Gaztambide S, Vazquez JA. Short- and long-term effect of a long-acting somatostatin analogue, lanreotide (SR-L) on metastatic gastrinoma. J Endocrinol Invest 1999;22:144-146
- Wilson SD, Doffek KM, Krzywda EA , et al. Zollinger-Ellison syndrome associated with a history of alcohol abuse: Coincidence or consequence? Surgery 2011;150:1129-1135
- Thodiyil PA, El-Masry NS, Williamson RC. Supergastrinoma: simultaneous peptic ulceration of esophagus, stomach, and small intestine. Int Surg 2003;88:155-158
- Lloyd-Davies KA, Rutgersson K, Solvell L. Omeprazole in the treatment of Zollinger-Ellison syndrome: a 4-year international study. Aliment Pharmacol Ther 1988;2:13-32
- Morocutti A, Merrouche M, Bjaaland T , et al. An open-label study of rabeprazole in patients with Zollinger-Ellison syndrome or idiopathic gastric acid hypersecretion. Aliment Pharmacol Ther 2006;24:1439-1444
- Metz DC, Comer GM, Soffer E , et al. Three-year oral pantoprazole administration is effective for patients with Zollinger-Ellison syndrome and other hypersecretory conditions. Aliment Pharmacol Ther 2006;23:437-444
- Riff BP, Leiman DA, Bennett B , et al. Weight Gain in Zollinger-Ellison Syndrome After Acid Suppression. Pancreas 2016;45:193-197
- Paul G, Ramdani A, Mignon M , et al. (Comparative efficacy of lansoprazole and omeprazole on the intragastric pH measured over a period of 24 hours and on the basal). Gastroenterol Clin Biol 1994;18:695-701
- Mignon M, Hochlaf S, Forestier S , et al. (Dose-response effect of lansoprazole in patients with Zollinger-Ellison syndrome). Gastroenterol Clin Biol 1994;18:13-16
- Ramdani A, Mignon M, Samoyeau R. Effect of pantoprazole versus other proton pump inhibitors on 24-hour intragastric pH and basal acid output in Zollinger-Ellison syndrome. Gastroenterol Clin Biol 2002;26:355-359
- Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017;11:27-37
- Katz PO, Dunbar KB, Schnoll-Sussman FH , et al. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol 2022;117:27-56
- Chinzon D, Domingues G, Tosetto N , et al. SAFETY OF LONG-TERM PROTON PUMP INHIBITORS: FACTS AND MYTHS. Arq Gastroenterol 2022;59:219-225
- Richter JE, Pandol SJ, Castell DO , et al. Gastroesophageal reflux disease in the Zollinger-Ellison syndrome. Ann Intern Med 1981;95:37-43
- Waldum HL, Fossmark R, Bakke I , et al. Hypergastrinemia in animals and man: causes and consequences. Scand J Gastroenterol 2004;39:505-509
- Waldum HL, Sordal O, Fossmark R. Proton pump inhibitors (PPIs) may cause gastric cancer - clinical consequences. Scand J Gastroenterol 2018;53:639-642
- Wormsley KG. Is chronic long-term inhibition of gastric secretion really dangerous. Scand J Gastroenterol Suppl 1988;146:166-174
- Mignon M, Lehy T, Bonnefond A , et al. Development of gastric argyrophil carcinoid tumors in a case of Zollinger-Ellison syndrome with primary hyperparathyroidism during long term antisecretory treatment. Cancer 1987;59:1959-1962
- Norton JA, Melcher ML, Gibril F , et al. Gastric carcinoid tumors in multiple endocrine neoplasia-1 patients with Zollinger-Ellison syndrome can be symptomatic, demonstrate aggressive growth, and require surgery. Surgery 2004;136:1267-1274
- Scherubl H, Cadiot G, Jensen RT , et al. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy 2010;42:664-671
- Bordi C, D'Adda T, Azzoni C , et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 1995;19:S8-S19
- Borch K, Renvall H, Liedberg G. Gastric endocrine cell hyperplasia and carcinoid tumors in pernicious anemia. Gastroenterology 1985;88:638-648
- Hage E, Hendel L, Gustafsen J , et al. Histopathology of the gastric oxyntic mucosa in two different patient groups during long-term treatment with omeprazole. Eur J Gastroenterol Hepatol 2003;15:781-789
- Konturek SJ, Konturek PC, Bielanski W , et al. Case presentation of gastrinoma combined with gastric carcinoid with the longest survival record--Zollinger-Ellison syndrome: pathophysiology, diagnosis and therapy. Med Sci Monit 2002;8:CS43-CS59
- Lee HW, Chung JW, Kim YJ , et al. Synchronous Peripancreatic Lymph Node Gastrinoma and Gastric Neuroendocrine Tumor Type 2. Clin Endosc 2016;49:483-487
- Tariq H, Kamal MU, Vootla V , et al. A Rare Cause of Abdominal Pain and Mass in an 18-Year-Old Patient: A Diagnostic Dilemma. Gastroenterology Res 2018;11:75-78
- Campana D, Ravizza D, Ferolla P , et al. Risk factors of type 1 gastric neuroendocrine neoplasia in patients with chronic atrophic gastritis. A retrospective, multicentre study. Endocrine 2017;56:633-638
- Massironi S, Zilli A, Elvevi A , et al. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev 2019;18:215-222
- Vinayek R, Hahne WF, Euler AR , et al. Parenteral control of gastric hypersecretion in patients with Zollinger-Ellison syndrome. Dig Dis Sci 1993;38:1857-1865
- Saeed ZA, Norton JA, Frank WO , et al. Parenteral antisecretory drug therapy in patients with Zollinger- Ellison syndrome. Gastroenterology 1989;96:1393-1402
- Metz DC, Forsmark C, Lew EA , et al. Replacement of oral proton pump inhibitors with intravenous pantoprazole to effectively control gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Am J Gastroenterol 2001;96:3274-3280
- Vinayek R, Frucht H, London JF , et al. Intravenous omeprazole in patients with Zollinger-Ellison syndrome undergoing surgery. Gastroenterology 1990;99:10-16
- Pang SH, Graham DY. A clinical guide to using intravenous proton-pump inhibitors in reflux and peptic ulcers. Therap Adv Gastroenterol 2010;3:11-22
- Maton PN, Gardner JD, Jensen RT. Recent advances in the management of gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterol Clin North Am 1989;18:847-863
- Jensen RT, Collen MJ, Allende HD , et al. Cimetidine-induced impotence and breast changes in patients with gastric hypersecretory states. N Engl J Med 1983;308:883-887
- Jensen RT, Metz DC, Koviack PD , et al. Prospective study of the long-term efficacy and safety of lansoprazole in patients with Zollinger-Ellison syndrome. Aliment Pharmacol Ther 1993;7 Suppl 1:41-50
- Hajri A, Aprahamian M, Damge C. Effect of a new CCK-receptor antagonist, CR 1409, on pancreatic growth induced by caerulein, CCK-8, bombesin and gastrin-releasing peptide in the rat. Digestion 1989;43:66-72
- Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with the Zollinger-Ellison syndrome. World J Surg 1991;15:151-159
- Ellison EC, Sparks J, Verducci JS , et al. 50-year appraisal of gastrinoma: recommendations for staging and treatment. J Am Coll Surg 2006;202:897-905
- Lorenz K, Dralle H. Surgical treatment of sporadic gastrinoma. Wien Klin Wochenschr 2007;119:597-601
- Fendrich V, Langer P, Waldmann J , et al. Management of sporadic and multiple endocrine neoplasia type 1 gastrinomas. Br J Surg 2007;94:1331-1341
- Fendrich V, Bartsch DK. Surgical Therapy of Sporadic Pancreatic Neuroendocrine Neoplasias G1/G2. Visc Med 2017;33:344-350
- Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg 1994;31:1-156
- Norton JA, Jensen RT. Role of surgery in Zollinger-Ellison syndrome. J Am Coll Surg 2007;205:S34-S37
- Mignon M, Ruszniewski P, Haffar S , et al. Current approach to the management of tumoral process in patients with gastrinoma. World J Surg 1986;10:703-710
- McCarthy DM. The place of surgery in the Zollinger-Ellison syndrome. N Engl J Med 1980;302:1344-1347
- Hirschowitz BI. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med 1999;341:2096-2097
- Hirschowitz BI. Clinical course of nonsurgically treated Zollinger-Ellison syndrome. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:360-371.
- Fraker DL, Norton JA, Alexander HR , et al. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg 1994;220:320-330
- Norton JA, Fraker DL, Alexander HR , et al. Surgery increases survival in patients with gastrinoma. Ann Surg 2006;244:410-419
- Zollinger RM. Gastrinoma: factors influencing prognosis. Surgery 1985;97:49-54
- McNamara D, Lewis T, O'Moran C. Zollinger-Ellison syndrome with fasting hypoglycaemia. J Royal Soc Med 1998;91:92-93
- Wilcox CM, Seay T, Arcury JT , et al. Zollinger-Ellison syndrome: presentation, response to therapy, and outcome. Dig Liver Dis 2011;43:439-443
- Collins JSA, Buchanan KD, Kennedy TL , et al. Changing patterns in presentation and management of the Zollinger-Ellison syndrome in Northern Ireland, 1970-1988. Q J Med 1991;78:215-225
- Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol 2005;19:675-697
- Harper S, Carroll RW, Frilling A , et al. Primary lymph node gastrinoma: 2 cases and a review of the literature. J Gastrointest Surg 2015;19:651-655
- Norton JA. Intraoperative methods to stage and localize pancreatic and duodenal tumors. Ann Oncol 1999;10:182-184
- Norton JA, Cromack DT, Shawker TH , et al. Intraoperative ultrasonographic localization of islet cell tumors. A prospective comparison to palpation. Ann Surg 1988;207:160-168
- Norton JA. Surgery and prognosis of duodenal gastrinoma as a duodenal neuroendocrine tumor. Best Pract Res Clin Gastroenterol 2005;19:699-704
- Norton JA. Surgical treatment of islet cell tumors with special emphasis on operative ultrasound. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:309-332.
- Maton PN, Mackem SM, Norton JA , et al. Ovarian carcinoma as a cause of Zollinger-Ellison syndrome. Natural history, secretory products and response to provocative tests. Gastroenterology 1989;97:468-471
- Carty SE, Jensen RT, Norton JA. Prospective study of aggressive resection of metastatic pancreatic endocrine tumors. Surgery 1992;112:1024-1031
- Bartsch DK, Waldmann J, Fendrich V , et al. Impact of lymphadenectomy on survival after surgery for sporadic gastrinoma. Br J Surg 2012;99:1234-1240
- Albers MB, Manoharan J, Bartsch DK. Contemporary surgical management of the Zollinger-Ellison syndrome in multiple endocrine neoplasia type 1. Best Pract Res Clin Endocrinol Metab 2019;33:101318
- Lopez CL, Falconi M, Waldmann J , et al. Partial pancreaticoduodenectomy can provide cure for duodenal gastrinoma associated with multiple endocrine neoplasia type 1. Ann Surg 2013;257:308-314
- Lopez CL, Waldmann J, Fendrich V , et al. Long-term results of surgery for pancreatic neuroendocrine neoplasms in patients with MEN1. Langenbecks Arch Surg 2011;396:1187-1197
- Falconi M, Bartsch DK, Eriksson B , et al. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms of the Digestive System: Well-Differentiated Pancreatic Non-Functioning Tumors. Neuroendocrinology 2012;95:120-134
- Triponez F, Goudet P, Dosseh D , et al. Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg 2006;30:654-662
- Goudet P, Dalac A, Le BA , et al. MEN1 disease occurring before 21 years old. A 160-patient cohort study from the GTE (Groupe d'etude des Tumeurs Endocrines). J Clin Endocrinol Metab 2015;100:1568-1577
- van Wijk JP, Dreijerink KM, Pieterman CR , et al. Increased prevalence of impaired fasting glucose in MEN1 gene mutation carriers. Clin Endocrinol (Oxf) 2012;76:67-71
- McCallum RW, Parameswaran V, Burgess JR. Multiple endocrine neoplasia type 1 (MEN 1) is associated with an increased prevalence of diabetes mellitus and impaired fasting glucose. Clin Endocrinol (Oxf) 2006;65:163-168
- Kouvaraki MA, Shapiro SE, Cote GJ , et al. Management of pancreatic endocrine tumors in multiple endocrine neoplasia type 1. World J Surg 2006;30:643-653
- Sakurai A, Katai M, Yamashita K , et al. Long-term follow-up of patients with multiple endocrine neoplasia type 1. Endocr J 2007;54:295-302
- Timofte D, Livadariu R, Bintintan V , et al. Metabolic disorders in patients operated for pancreatic cancer. Rev Med Chir Soc Med Nat Iasi 2014;118:392-398
- van Beek DJ, Nell S, Vorselaars WMCM , et al. Complications After Major Surgery for Duodenopancreatic Neuroendocrine Tumors in Patients with MEN1: Results from a Nationwide Cohort. Ann Surg Oncol 2021;28:4387-4399
- Barbe C, Murat A, Dupas B , et al. Magnetic resonance imaging versus endoscopic ultrasonography for the detection of pancreatic tumours in multiple endocrine neoplasia type 1. Dig Liver Dis 2012;44:228-234
- Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol 2015;3:895-905
- Froeling V, Elgeti F, Maurer MH , et al. Impact of Ga-68 DOTATOC PET/CT on the diagnosis and treatment of patients with multiple endocrine neoplasia. Ann Nucl Med 2012;26:738-743
- Lastoria S, Marciello F, Faggiano A , et al. Role of Ga-DOTATATE PET/CT in patients with multiple endocrine neoplasia type 1 (MEN1). Endocrine 2016;52:488-494
- Sharma P, Mukherjee A, Karunanithi S , et al. Accuracy of 68Ga DOTANOC PET/CT Imaging in Patients With Multiple Endocrine Neoplasia Syndromes. Clin Nucl Med 2015;40:e351-e356
- Kumar Gupta S, Singla S, Damle NA , et al. Diagnosis of Men-I Syndrome on (68)Ga-DOTANOC PET-CT and Role of Peptide Receptor Radionuclide Therapy With (177)Lu-DOTATATE. Int J Endocrinol Metab 2012;10:629-633
- Kottemann MC, Bale AE. Characterization of DNA damage-dependent cell cycle checkpoints in a menin-deficient model. DNA Repair (Amst) 2009;8:944-952
- Busygina V, Kottemann MC, Scott KL , et al. Multiple endocrine neoplasia type 1 interacts with forkhead transcription factor CHES1 in DNA damage response. Cancer Res 2006;66:8397-8403
- Busygina V, Suphapeetiporn K, Marek LR , et al. Hypermutability in a Drosophila model for multiple endocrine neoplasia type 1. Hum Mol Genet 2004;13:2399-2408
- Miglioretti DL, Johnson E, Williams A , et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 2013;167:700-707
- Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol 2008;81:362-378
- Pearce MS, Salotti JA, Little MP , et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380:499-505
- Stadil F. Treatment of gastrinomas with pancreaticoduodenectomy. In: Mignon M, Jensen RT eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Series: Frontiers in Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995:333-341.
- Imamura M, Komoto I, Ota S , et al. Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. World J Gastroenterol 2011;17:1343-1353
- Imamura M, Takahashi K, Isobe Y , et al. Curative resection of multiple gastrinomas aided by selective arterial secretin injection and intraoperative secretin test. Ann Surg 1989;210:710-715
- Bartsch DK, Fendrich V, Langer P , et al. Outcome of duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg 2005;242:757-64, discussion
- Waddell WR, Coppinger WR, Loughry RW. Pancreaticoduodenectomy for Zollinger-Ellison syndrome. Ann Surg 1968;168(4):641-654
- Oberhelman HA, Jr. Excisional therapy for ulcerogenic tumors of the duodenum: long- term results. Arch Surg 1972;104:447-453
- Roche A, Raisonnier A, Gillon-Savouret MC. Pancreatic venous sampling and arteriography in localizing insulinomas and gastrinomas: procedure and results in 55 cases. Radiology 1982;145:621-627
- Cavina E, Pagliai EF, Goletti O , et al. Pylorus preserving pancreatoduodenectomy for gastrinoma. A case report. Ital J Surg Sci 1986;16:291-295
- Stamm B, Hedinger CE, Saremaslani P. Duodenal and ampullary carcinoid tumors. A report of 12 cases with pathological characteristics, polypeptide content and relation to MEN 1 syndrome and von Recklinghausen's disease (neurofibromatosis). Virchows Arch A Pathol Anat Histopathol 1986;408:475-489
- Vogel SB, Wolfe MM, McGuigan JE , et al. Localization and resection of gastrinomas in Zollinger-Ellison syndrome. Ann Surg 1987;205:550-556
- Lind T, Olbe L. Long term follow up of patients with Zollinger-Ellison syndrome (ZES). Acta Chir Scand 1989;155:383-388
- Rosato FE, Bonn F, Shapiro M , et al. Selective arterial stimulation of secretin in localization of gastrinomas. Surg Gynecol Obstet 1990;171:196-200
- Delcore R, Friesen SR. Role of pancreatoduodenectomy in the management of primary duodenal wall gastrinomas in patients with Zollinger-Ellison syndrome. Surgery 1992;112:1016-1022
- Farley DR, Van Heerden JA, Grant CS , et al. The Zollinger-Ellison syndrome. A collective surgical experience. Ann Surg 1992;215:561-569
- Udelsman R, Yeo CJ, Hruban RH , et al. Pancreaticoduodenectomy for selected pancreatic endocrine tumors. Surg Gynecol Obstet 1993;177:269-278
- Stadil F, Bradram L, Gustafsen J , et al. Surgical treatment of the Zollinger-Ellison syndrome. World J Surg 1993;17:463-467
- Schroder W, Holscher AH, Beckurts KTE , et al. Chirurgische therapie des gastrinoms mit assoziiertem Zollinger-Ellison-Syndrom. Z Gastroenterol 1996;34:465-472
- Phan GQ, Yeo CJ, Cameron JL , et al. Pancreaticoduodenectomy for selected periampullary neuroendocrine tumors: Fifty patients. Surgery 1997;122:989-997
- Jordan PH, Jr. A personal experience with pancreatic and duodenal neuroendocrine tumors. J Am Coll Surg 1999;189:470-482
- Partensky C, Berger F, Owono P , et al. Cephalic duodenopancreatectomy for endocrine tumor of the ampulla of Vater and of the minor papilla. Gastroenterol Clin Biol 1999;23:832-836
- Tonelli F, Fratini G, Falchetti A , et al. Surgery for gastroenteropancreatic tumours in multiple endocrine neoplasia type 1: review and personal experience. J Intern Med 2005;257:38-49
- Bartsch DK, Langer P, Wild A , et al. Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: Surgery or surveillance? Surgery 2000;128:958-966
- Kato M, Imamura M, Hosotani R , et al. Curative resection of microgastrinomas based on the intraoperative secretin test. World J Surg 2000;24:1425-1430
- Lairmore TC, Chen VY, DeBenedetti MK , et al. Duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg 2000;231:909-918
- Siech M, Mattfeldt T, Schlosser W , et al. Duodenum-preserving pancreatic head resection in patients with benign and borderline tumors of the pancreatic head. Langenbeck's Arch Surg 2000;385:229-233
- Thodiyil PA, El-Masry NS, Williamson RC. Achieving eugastrinaemia in Zollinger-Ellison syndrome: resection or enucleation? Dig Surg 2001;18:118-123
- Huang JJ, Yeo CJ, Sohn TA , et al. Quality of life and outcomes after pancreaticoduodenectomy. Ann Surg 2000;231:890-898
- Tartaglia A, Portela-Gomes GM, Oberg K , et al. Chromogranin A in gastric neuroendocrine tumours: an immunohistochemical and biochemical study with region-specific antibodies. Virchows Arch 2006;448:399-406
- Price TN, Thompson GB, Lewis JT , et al. Zollinger-Ellison syndrome due to primary gastrinoma of the extrahepatic biliary tree: three case reports and review of literature. Endocr Pract 2009;15:737-749
- Giovinazzo F, Butturini G, Monsellato D , et al. Lymph nodes metastasis and recurrences justify an aggressive treatment of gastrinoma. Updates Surg 2013;65:19-24
- Fernandez-Cruz L, Pelegrina A. Surgery for gastrinoma: Short and long-term results. Cir Esp 2015;93:390-395
- Maire F, Sauvanet A, Couvelard A , et al. Recurrence after surgical resection of gastrinoma: who, when, where and why? Eur J Gastroenterol Hepatol 2012;24:368-374
- Grobmyer SR, Vogel SB, McGuigan JE , et al. Reoperative surgery in sporadic Zollinger-Ellison Syndrome: longterm results. J Am Coll Surg 2010;208:718-722
- Nishio K, Nishio A, Nishikawa T , et al. Recurrent gastrinoma in the mesentery 19 years after primary resection. Dig Dis Sci 2007;52:3104-3108
- Jaskowiak NT, Fraker DL, Alexander HR , et al. Is reoperation for gastrinoma excision in Zollinger-Ellison syndrome (ZES) indicated? Surgery 1996;120:1057-1063
- Krampitz GW, Norton JA, Poultsides GA , et al. Lymph nodes and survival in duodenal and pancreatic neuroendocrine tumors. Arch Surg 2012;147:820-827
- Tomassetti P, Campana D, Piscitelli L , et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 2005;16:1806-1810
- Bettini R, Boninsegna L, Mantovani W , et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol 2008;19:903-908
- Boninsegna L, Panzuto F, Partelli S , et al. Malignant pancreatic neuroendocrine tumour: Lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer 2012;48:1608-1615
- Roland CL, Bian A, Mansour JC , et al. Survival impact of malignant pancreatic neuroendocrine and islet cell neoplasm phenotypes. J Surg Oncol 2011;
- Chu QD, Hill HC, Douglass HO, Jr. , et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol 2002;9:855-862
- Bilimoria KY, Talamonti MS, Tomlinson JS , et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 2008;247:490-500
- DiNorcia J, Lee MK, Reavey PL , et al. One hundred thirty resections for pancreatic neuroendocrine tumor: evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg 2010;14:1536-1546
- Capelli P, Fassan M, Scarpa A. Pathology - Grading and staging of GEP-NETs. Best Pract Res Clin Gastroenterol 2012;26:705-717
- Arnold R, Rinke A, Klose KJ , et al. Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol 2005;3:761-771
- Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis in patients with metastatic pancreatic endocrine carcinomas. Pancreas 2009;38:255-258
- Strosberg JR, Cheema A, Weber J , et al. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol 2011;29:3044-3049
- Zollinger RM. The Zollinger-Ellison syndrome. World J Surg 1981;5:773-775
- Berger AC, Gibril F, Venzon DJ , et al. Prognostic value of initial fasting serum gastrin level in patients with Zollinger-Ellison syndrome. J Clin Oncol 2001;19:3051-3057
- Metz DC, Kuchnio M, Fraker DL , et al. Flow cytometry and Zollinger-Ellison syndrome: relationship to clinical course. Gastroenterology 1993;105:799-813
- Chen Y-J, Zortmeyer A, Zhuang ZP , et al. Chromosome X loss of heterozygosity frequently occurs in gastrinomas and is correlated with aggressive tumor growth. Cancer 2004;100:1379-1387
- Chen YJ, Vortmeyer A, Zhuang Z , et al. Loss of heterozygosity of chromosome 1q in gastrinomas: occurrence and prognostic significance. Cancer Res 2003;63:817-823
- Pape UF, Jann H, Muller-Nordhorn J , et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008;113:256-265
- Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology 2007;132:899-904
- Yang Z, Shi G. Comparative outcomes of pancreatic neuroendocrine neoplasms: A population-based analysis of the SEER database. Eur J Surg Oncol 2022;48:2181-2187
- Schmitt AM, Anlauf M, Rousson V , et al. WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am J Surg Pathol 2007;31:1677-1682
- Maire F, Hammel P, Faivre S , et al. Temozolomide: a safe and effective treatment for malignant digestive endocrine tumors. Neuroendocrinology 2009;90:67-72
- La Rosa S, Rigoli E, Uccella S , et al. Prognostic and biological significance of cytokeratin 19 in pancreatic endocrine tumours. Histopathology 2007;50:597-606
- La Rosa S, Sessa F, Capella C , et al. Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Arch 1996;429:323-333
- Hauser H, Gerson DS, Reidy-Lagunes D , et al. Systemic Therapies for Metastatic Pancreatic Neuroendocrine Tumors. Curr Treat Options Oncol 2019;20:87
- Dermine S, Palmieri LJ, Lavole J , et al. Non-Pharmacological Therapeutic Options for Liver Metastases in Advanced Neuroendocrine Tumors. J Clin Med 2019;8:1907
- Scoville SD, Cloyd JM, Pawlik TM. New and emerging systemic therapy options for well-differentiated gastroenteropancreatic neuroendocrine tumors. Expert Opin Pharmacother 2020;21:183-191
- Mohamed A, Strosberg JR. Medical Management of Gastroenteropancreatic Neuroendocrine Tumors: Current Strategies and Future Advances. J Nucl Med 2019;60:721-727
- Kanabar R, Barriuso J, McNamara MG , et al. Liver embolisation for patients with neuroendocrine neoplasms: systematic review. Neuroendocrinology 2021;111:354-369
- Li YL, Cheng ZX, Yu FH , et al. Advances in medical treatment for pancreatic neuroendocrine neoplasms. World J Gastroenterol 2022;28:2163-2175
- Ngongoni R, Visser B. Surgery, Liver Directed Therapy and Peptide Receptor Radionuclide Therapy for Pancreatic Neuroendocrine Tumor Liver Metastases. Cancers (Basel) 2022;14:
- Chauhan A, De Rivero J, Ramirez RA , et al. Treatment Sequencing Strategies in Advanced Neuroendocrine Tumors: A Review. Cancers (Basel) 2022;14:5049
- Modica R, Liccardi A, Minotta R , et al. Therapeutic strategies for patients with neuroendocrine neoplasms: current perspectives. Expert Rev Endocrinol Metab 2022;17:389-403
- Cuthbertson DJ, Shankland R, Srirajaskanthan R. Diagnosis and management of neuroendocrine tumours. Clin Med (Lond) 2023;23:119-124
- Shi C, Morse MA. Mechanisms of Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers (Basel) 2022;14:6114
- McClellan K, Chen EY, Kardosh A , et al. Therapy Resistant Gastroenteropancreatic Neuroendocrine Tumors. Cancers (Basel) 2022;14:
- Norton JA, Sugarbaker PH, Doppman JL , et al. Aggressive resection of metastatic disease in selected patients with malignant gastrinoma. Ann Surg 1986;203:352-359
- Norton JA, Warren RS, Kelly MG , et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery 2003;134:1057-1065
- Norton JA, Kirlen MA, Li M , et al. Morbidity and mortality of aggressive resections in patients with advanced neuroendocrine tumors. Arch Surg 2003;138:859-866
- Que FG, Sarmiento JM, Nagorney DM. Hepatic surgery for metastatic gastrointestinal neuroendocrine tumors. Adv Exp Med Biol 2006;574:43-56
- Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin North Am 2003;12:231-242
- Wang SC, Fidelman N, Nakakura EK. Management of well-differentiated gastrointestinal neuroendocrine tumors metastatic to the liver. Semin Oncol 2013;40:69-74
- Pavel M, Baudin E, Couvelard A , et al. ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2012;95:157-176
- Howe JR, Cardona K, Fraker DL , et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017;46:715-731
- Tamburrino D, Spoletini G, Partelli S , et al. Surgical management of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab 2016;30:93-102
- Uri I, Grozinsky-Glasberg S. Current treatment strategies for patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Clin Diabetes Endocrinol 2018;4:16
- Chan MY, Ma KW, Chan A. Surgical management of neuroendocrine tumor-associated liver metastases: a review. Gland Surg 2018;7:28-35
- Ejaz A, Reames BN, Maithel S , et al. Cytoreductive debulking surgery among patients with neuroendocrine liver metastasis: a multi-institutional analysis. HPB (Oxford) 2018;20:277-284
- Lesurtel M, Nagorney DM, Mazzaferro V , et al. When should a liver resection be performed in patients with liver metastases from neuroendocrine tumours? A systematic review with practice recommendations. HPB (Oxford) 2015;17:17-22
- Addeo P, Bertin JB, Imperiale A , et al. Outcomes of Simultaneous Resection of Small Bowel Neuroendocrine Tumors with Synchronous Liver Metastases. World J Surg 2020;44:2377-2384
- Jeune F, Taibi A, Gaujoux S. Update on the Surgical Treatment of Pancreatic Neuroendocrine Tumors. Scand J Surg 2020;109:42-52
- Merola E, Rinke A, Partelli S , et al. Surgery with Radical Intent: Is There an Indication for G3 Neuroendocrine Neoplasms? Ann Surg Oncol 2020;27:1348-1355
- Pozzari M, Maisonneuve P, Spada F , et al. Systemic therapies in patients with advanced well-differentiated pancreatic neuroendocrine tumors (PanNETs): When cytoreduction is the aim. A critical review with meta-analysis. Cancer Treat Rev 2018;71:39-46
- Kacmaz E, Heidsma CM, Besselink MGH , et al. Treatment of Liver Metastases from Midgut Neuroendocrine Tumours: A Systematic Review and Meta-Analysis. J Clin Med 2019;8:403
- Veltroni A, Cosaro E, Spada F , et al. Clinico-pathological features, treatments and survival of malignant insulinomas: a multicenter study. Eur J Endocrinol 2020;182:439-446
- Sham JG, Ejaz A, Gage MM , et al. The Impact of Extent of Liver Resection Among Patients with Neuroendocrine Liver Metastasis: an International Multi-institutional Study. J Gastrointest Surg 2019;23:484-491
- Cloyd JM, Wiseman JT, Pawlik TM. Surgical management of pancreatic neuroendocrine liver metastases. J Gastrointest Oncol 2020;11:590-600
- Jensen RT, Bodei L, Capdevila J , et al. Unmet Needs in Functional and Nonfunctional Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2019;108:26-36
- Tran CG, Sherman SK, Chandrasekharan C , et al. Surgical Management of Neuroendocrine Tumor Liver Metastases. Surg Oncol Clin N Am 2021;30:39-55
- Cherner JA, Sawyers JL. Benefit of resection of metastatic gastrinoma in multiple endocrine neoplasia type I. Gastroenterology 1992;102:1049-1053
- Masui T, Nagai K, Anazawa T , et al. Risk factors for short recurrence-free survival after resection of pancreatic neuroendocrine tumor (PanNET) liver metastases: which patients should undergo resection? Scand J Gastroenterol 2020;55:479-484
- Merola E, Falconi M, Rinke A , et al. Radical intended surgery for highly selected stage IV neuroendocrine neoplasms G3. Am J Surg 2020;220:284-289
- Brighi N, Lamberti G, Maggio I , et al. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig Liver Dis 2019;51:689-694
- Bertani E, Fazio N, Radice D , et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1-G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann Surg Oncol 2016;23:981-989
- Nazario J, Gupta S. Transarterial liver-directed therapies of neuroendocrine hepatic metastases. Semin Oncol 2010;37:118-126
- Vyleta M, Coldwell D. Radioembolization in the treatment of neuroendocrine tumor metastases to the liver. Int J Hepatol 2011;2011(Article ID 785315):1-5
- Barat M, Cottereau AS, Kedra A , et al. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J Clin Med 2020;9:2302
- Kessler J, Singh G, Ituarte PHG , et al. A Comparison of Liver-Directed Therapy and Systemic Therapy for the Treatment of Liver Metastases in Patients with Gastrointestinal Neuroendocrine Tumors: Analysis of the California Cancer Registry. J Vasc Interv Radiol 2021;32:393-402
- Nanno Y, Toyama H, Ueshima E , et al. Transarterial chemoembolization for liver metastases of a pancreatic neuroendocrine neoplasm: a single-center experience. Surg Today 2023 (In Press)
- Cazzato RL, Hubele F, De Marini P , et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers (Basel) 2021;13:6368
- Egger ME, Armstrong E, Martin RC , et al. Transarterial Chemoembolization vs Radioembolization for Neuroendocrine Liver Metastases: A Multi-Institutional Analysis. J Am Coll Surg 2020;230:363-370
- Ramdhani K, Braat AJAT. The Evolving Role of Radioembolization in the Treatment of Neuroendocrine Liver Metastases. Cancers (Basel) 2022;14:3415
- The NCCN Clinical Practice Guidelines in Oncology for Neuroendocrine tumors version 1.2012. ( online. go to www.nccn.org.: 2012.
- Klein A, Clemens J, Cameron J. Periampullary neoplasms in von Recklinghausen's disease. Surgery 1989;106:815-819
- O'Toole D, Ruszniewski P. Chemoembolization and other ablative therapies for liver metastases of gastrointestinal endocrine tumours. Best Pract Res Clin Gastroenterol 2005;19:585-594
- Elias D, Goere D, Leroux G , et al. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. Eur J Surg Oncol 2009;35:1092-1097
- Harring TR, Nguyen NT, Goss JA , et al. Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol 2011;2011:1-11
- Figueiredo Ferreira M, Garces-Duran R, Eisendrath P , et al. EUS-guided radiofrequency ablation of pancreatic/peripancreatic tumors and oligometastatic disease: an observational prospective multicenter study. Endosc Int Open 2022;10:E1380-E1385
- Guillermet-Guibert J, Lahlou H, Pyronnet S , et al. Somatostatin receptors as tools for diagnosis and therapy: Molecular aspects. Best Pract Res Clin Gastroenterol 2005;19:535-551
- Karabulut K, Akyildiz HY, Lance C , et al. Multimodality treatment of neuroendocrine liver metastases. Surgery 2011;150:316-325
- Akyildiz HY, Mitchell J, Milas M , et al. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: Long-term follow-up. Surgery 2010;148:1288-1293
- Eriksson J, Stalberg P, Nilsson A , et al. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg 2008;32:930-938
- Vogl TJ, Naguib NN, Zangos S , et al. Liver metastases of neuroendocrine carcinomas: interventional treatment via transarterial embolization, chemoembolization and thermal ablation. Eur J Radiol 2009;72:517-528
- Mazzaglia PJ, Berber E, Milas M , et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery 2007;142:10-19
- Kvols LK, Turaga KK, Strosberg J , et al. Role of interventional radiology in the treatment of patients with neuroendocrine metastases in the liver. J Natl Compr Canc Netw 2009;7:765-772
- de Mestier L, Zappa M, Hentic O , et al. Liver transarterial embolizations in metastatic neuroendocrine tumors. Rev Endocr Metab Disord 2017;18:459-471
- Maire F, Lombard-Bohas C, O'Toole D , et al. Hepatic arterial embolization versus chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: a prospective randomized study. Neuroendocrinology 2012;96:294-300
- Toumpanakis C, Meyer T, Caplin ME. Cytotoxic treatment including embolization/chemoembolization for neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 2007;21:131-144
- Lewis MA, Jaramillo S, Roberts L , et al. Hepatic Artery Embolization for Neuroendocrine Tumors: Postprocedural Management and Complications. Oncologist 2012;17:725-731
- Memon K, Lewandowski RJ, Mulcahy MF , et al. Radioembolization for Neuroendocrine Liver Metastases: Safety, Imaging, and Long-term Outcomes. Int J Radiat Oncol Biol Phys 2012;83:887-894
- Kennedy A, Coldwell D, Sangro B , et al. Integrating Radioembolization into the Treatment Paradigm for Metastatic Neuroendocrine Tumors in the Liver. Am J Clin Oncol 2012;35:293-301
- Paprottka PM, Hoffmann RT, Haug A , et al. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc Intervent Radiol 2012;35:334-342
- Lacin S, Oz I, Ozkan E , et al. Intra-arterial treatment with 90yttrium microspheres in treatment-refractory and unresectable liver metastases of neuroendocrine tumors and the use of 111in-octreotide scintigraphy in the evaluation of treatment response. Cancer Biother Radiopharm 2011;26:631-637
- Shaheen M, Hassanain M, Aljiffry M , et al. Predictors of response to radio-embolization (TheraSphere(R)) treatment of neuroendocrine liver metastasis. HPB (Oxford) 2012;14:60-66
- King J, Quinn R, Glenn DM , et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008;113:921-929
- Whitney R, Valek V, Fages JF , et al. Transarterial chemoembolization and selective internal radiation for the treatment of patients with metastatic neuroendocrine tumors: a comparison of efficacy and cost. Oncologist 2011;16:594-601
- Kennedy AS, Dezarn WA, McNeillie P , et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-279
- Saxena A, Chua TC, Bester L , et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg 2010;251:910-916
- Ramage JK, Ahmed A, Ardill J , et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012;61:6-32
- Deleporte A, Flamen P, Hendlisz A. State of the art: radiolabeled microspheres treatment for liver malignancies. Expert Opin Pharmacother 2010;11:579-586
- Lee E, Leon Pachter H, Sarpel U. Hepatic arterial embolization for the treatment of metastatic neuroendocrine tumors. Int J Hepatol 2012;2012(Article ID 471203):1-8
- Barbier CE, Garske-Roman U, Sandstrom M , et al. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging 2016;43:1425-1431
- Braat AJAT, Kappadath SC, Ahmadzadehfar H , et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases: International Multicenter Study on Efficacy and Toxicity. Cardiovasc Intervent Radiol 2019;42:413-425
- Currie BM, Hoteit MA, Ben-Josef E , et al. Radioembolization-Induced Chronic Hepatotoxicity: A Single-Center Cohort Analysis. J Vasc Interv Radiol 2019;30:1915-1923
- Krug S, Gress TM, Michl P , et al. The Role of Cytotoxic Chemotherapy in Advanced Pancreatic Neuroendocrine Tumors. Digestion 2017;96:67-75
- de Mestier L, Walter T, Brixi H , et al. Comparison of Temozolomide-Capecitabine to 5-Fluorouracile-Dacarbazine in 247 Patients with Advanced Digestive Neuroendocrine Tumors Using Propensity Score Analyses. Neuroendocrinology 2019;108:343-353
- Cives M, Pelle' E, Quaresmini D , et al. The Role of Cytotoxic Chemotherapy in Well-Differentiated Gastroenteropancreatic and Lung Neuroendocrine Tumors. Curr Treat Options Oncol 2019;20:72
- Chan DL, Singh S. Current Chemotherapy Use in Neuroendocrine Tumors. Endocrinol Metab Clin North Am 2018;47:603-614
- Palmieri LJ, Dermine S, Barre A , et al. Medical Treatment of Advanced Pancreatic Neuroendocrine Neoplasms. J Clin Med 2020;9:1860
- Riccardi F, Rizzo M, Festino L , et al. Therapy innovation for the treatment of pancreatic neuroendocrine tumors. Expert Opin Ther Targets 2012;16 Suppl 2:S91-102
- Kouvaraki MA, Ajani JA, Hoff P , et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-4771
- Delaunoit T, Ducreux M, Boige V , et al. The doxorubicin-streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma; a judicious option? Eur J Cancer 2004;40:515-520
- Kulke MH. Systemic therapy for advanced pancreatic neuroendocrine tumors. Semin Oncol 2013;40:75-83
- Costa FP, Gumz B, Pasche B. Selecting patients for cytotoxic therapies in gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 2012;26:843-854
- Arrivi G, Verrico M, Roberto M , et al. Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs): A Systematic Review and Pooled Analysis. Cancer Manag Res 2022;14:3507-3523
- O'Toole D, Hentic O, Corcos O , et al. Chemotherapy for gastro-enteropancreatic endocrine tumours. Neuroendocrinology 2004;80 Suppl 1:79-84
- Lu Y, Zhao Z, Wang J , et al. Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: A meta-analysis. Medicine (Baltimore) 2018;97:e12784
- Thomas K, Voros BA, Meadows-Taylor M , et al. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers (Basel) 2020;12:206
- Strosberg JR, Fine RL, Choi J , et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-275
- Ekeblad S, Sundin A, Janson ET , et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986-2991
- Kulke MH, Hornick JL, Frauenhoffer C , et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res 2009;15:338-345
- Spada F, Maisonneuve P, Fumagalli C , et al. Temozolomide alone or in combination with capecitabine in patients with advanced neuroendocrine neoplasms: an Italian multicenter real-world analysis. Endocrine 2021;72:268-278
- Kunz PL, Graham NT, Catalano PJ , et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J Clin Oncol 2023;41:1359-1369
- Ruszniewski P, Hochlaf S, Rougier P , et al. (Intravenous chemotherapy with streptozotocin and 5 fluorouracil for hepatic metastases of Zollinger-Ellison syndrome. A prospective multicenter study in 21 patients). Gastroenterol Clin Biol 1991;15:393-398
- Steinmuller T, Kianmanesh R, Falconi M , et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2008;87:47-62
- Nilsson O, Van Cutsem E, Delle Fave G , et al. Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic). Neuroendocrinology 2006;84:212-215
- Smith J, Reidy-Lagunes D. The management of extrapulmonary poorly differentiated (high-grade) neuroendocrine carcinomas. Semin Oncol 2013;40:100-108
- Chan DL, Bergsland EK, Chan JA , et al. Temozolomide in grade III gastroenteropancreatic neuroendocrine neoplasms (G3 GEPNENs): A multicentre retrospective review. Oncologist 2021;26:950-955
- Garcia-Alvarez A, Cubero JH, Capdevila J. What Is the Status of Immunotherapy in Neuroendocrine Neoplasms? Curr Oncol Rep 2022;24:451-461
- Alheraki SZ, Almquist DR, Starr JS , et al. Treatment landscape of advanced high-grade neuroendocrine neoplasms. Clin Adv Hematol Oncol 2023;21:16-26
- Iwasa S, Morizane C, Okusaka T , et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol 2010;40:313-318
- Garcia-Carbonero R, Anton-Pascual B, Modrego A , et al. Advances in the Treatment of Gastroenteropancreatic Neuroendocrine Carcinomas: Are we Moving Forward? Endocr Rev 2023;44:724-736
- Strosberg JR, Coppola D, Klimstra DS , et al. The NANETS Consensus Guidelines for the Diagnosis and Management of Poorly Differentiated (High-Grade) Extrapulmonary Neuroendocrine Carcinomas. Pancreas 2010;39:799-800
- Olsen IH, Langer SW, Jepsen I , et al. First-line treatment of patients with disseminated poorly differentiated neuroendocrine carcinomas with carboplatin, etoposide, and vincristine: a single institution experience. Acta Oncol 2012;51:97-100
- Que QY, Zhang LC, Bao JQ , et al. Role of surgical treatments in high-grade or advanced gastroenteropancreatic neuroendocrine neoplasms. World J Gastrointest Surg 2022;14:397-408
- Walter T, van Brakel B, Vercherat C , et al. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: prognostic relevance and association with response to alkylating agents. Br J Cancer 2015;112:523-531
- Campana D, Walter T, Pusceddu S , et al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: an observational retrospective multicenter study. Endocrine 2018;60:490-498
- Schmitt AM, Pavel M, Rudolph T , et al. Prognostic and predictive roles of MGMT protein expression and promoter methylation in sporadic pancreatic neuroendocrine neoplasms. Neuroendocrinology 2014;100:35-44
- Cros J, Hentic O, Rebours V , et al. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:625-633
- Lemelin A, Barritault M, Hervieu V , et al. O6-methylguanine-DNA methyltransferase (MGMT) status in neuroendocrine tumors: a randomized phase II study (MGMT-NET). Dig Liver Dis 2019;51:595-599
- Yagi K, Ono H, Kudo A , et al. MGMT is frequently inactivated in pancreatic NET-G2 and is associated with the therapeutic activity of STZ-based regimens. Sci Rep 2023;13:7535
- Brighi N, Lamberti G, Andrini E , et al. Prospective Evaluation of MGMT-Promoter Methylation Status and Correlations with Outcomes to Temozolomide-Based Chemotherapy in Well-Differentiated Neuroendocrine Tumors. Curr Oncol 2023;30:1381-1394
- Trillo Aliaga P, Spada F, Peveri G , et al. Should temozolomide be used on the basis of O(6)-methylguanine DNA methyltransferase status in patients with advanced neuroendocrine tumors? A systematic review and meta-analysis. Cancer Treat Rev 2021;99:102261
- Wang W, Zhang Y, Peng Y , et al. A Ki-67 Index to Predict Treatment Response to the Capecitabine Temozolomide (CAPTEM) Regimen in Neuroendocrine Neoplasms: A Retrospective Multicenter Study. Neuroendocrinology 2021;111:752-763
- Girot P, Dumars C, Mosnier JF , et al. Short article: Evaluation of O6-methylguanine-DNA methyltransferase as a predicting factor of response to temozolomide-based chemotherapy in well-differentiated metastatic pancreatic neuroendocrine tumors. Eur J Gastroenterol Hepatol 2017;29:826-830
- Raj N, Klimstra DS, Horvat N , et al. O6-Methylguanine DNA Methyltransferase Status Does Not Predict Response or Resistance to Alkylating Agents in Well-Differentiated Pancreatic Neuroendocrine Tumors. Pancreas 2017;46:758-763
- Zhang J, Kulkarni HR, Singh A , et al. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J Nucl Med 2019;60:377-385
- Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer 2020;27:R67-R77
- Stueven AK, Kayser A, Wetz C , et al. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int J Mol Sci 2019;20:3049
- Narayanan S, Kunz PL. Role of Somatostatin Analogues in the Treatment of Neuroendocrine Tumors. Hematol Oncol Clin North Am 2016;30:163-177
- Caplin ME, Pavel M, Cwikla Jb , et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-233
- Capurso G, Fazio N, Festa S , et al. Molecular target therapy for gastroenteropancreatic endocrine tumours: biological rationale and clinical perspectives. Crit Rev Oncol Hematol 2009;72:110-124
- Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res 2010;29:19-31
- Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol 2010;16:2963-2970
- Sideris L, Dube P, Rinke A. Antitumor Effects of Somatostatin Analogs in Neuroendocrine Tumors. Oncologist 2012;17:747-755
- Plockinger U, Wiedenmann B. Biotherapy. Best Pract Res Clin Endocrinol Metab 2007;21:145-162
- Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol 2013;40:56-68
- Modlin IM, Pavel M, Kidd M , et al. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther 2010;31:169-188
- Panzuto F, Di Francesco V, Iannicelli E , et al. Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol 2006;17:461-466
- Sharp AJ, Hayes AR, Grossman A. High-dose Somatostatin Analogues for Progressive Neuroendocrine Tumours. Eur Endocrinol 2020;16:93-95
- Rinke A, Muller HH, Schade-Brittinger C , et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-4663
- Oberg K, Ferone D, Kaltsas G , et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: biotherapy. Neuroendocrinology 2009;90:209-213
- Shah T, Caplin M. Biotherapy for metastatic endocrine tumours. Best Pract Res Clin Gastroenterol 2005;19:617-636
- Amoroso V, Pavel M, Claps M , et al. IFN-a in advanced well-differentiated neuroendocrine tumors: the neglected drug? Future Oncol 2018;14:897-899
- Öberg K. Interferon in the management of neuroendocrine GEP-tumors. Digestion 2000;62:92-97
- Oberg K. Biotherapies for GEP-NETs. Best Pract Res Clin Gastroenterol 2012;26:833-841
- Pisegna JR, Slimak GG, Doppman JL , et al. An evaluation of human recombinant alpha interferon in patients with metastatic gastrinoma. Gastroenterology 1993;105:1179-1183
- Ozdirik B, Tacke F, Benz F , et al. A case report of an excellent response to interferon-a in a patient with functional metastasized neuroendocrine tumor refractory to other treatments. Medicine (Baltimore) 2020;99:e20820
- Cingarlini S, Bonomi M, Corbo V , et al. Profiling mTOR pathway in neuroendocrine tumors. Target Oncol 2012;7:183-188
- Lamberti G, Brighi N, Maggio I , et al. The Role of mTOR in Neuroendocrine Tumors: Future Cornerstone of a Winning Strategy? Int J Mol Sci 2018;19:747
- Gajate P, Alonso-Gordoa T, Martinez-Saez O , et al. Prognostic and predictive role of the PI3K-AKT-mTOR pathway in neuroendocrine neoplasms. Clin Transl Oncol 2018;20:561-569
- Yao JC. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab 2007;21:163-172
- Grozinsky-Glasberg S, Pavel M. Inhibition of mTOR in carcinoid tumors. Target Oncol 2012;7:189-195
- Vijayvergia N, Dasari A. Targeted Therapies in the Management of Well-Differentiated Digestive and Lung Neuroendocrine Neoplasms. Curr Treat Options Oncol 2020;21:96
- Fazio N, Cinieri S, Lorizzo K , et al. Biological targeted therapies in patients with advanced enteropancreatic neuroendocrine carcinomas. Cancer Treat Rev 2010;36 Suppl 3:S87-S94
- Jensen RT, Delle Fave G. Promising advances in the treatment of malignant pancreatic endocrine tumors. N Engl J Med 2011;364:564-565
- Yao JC, Fazio N, Singh S , et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016;387:968-977
- Beyens M, Vandamme T, Peeters M , et al. Resistance to targeted treatment of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 2019;26:R109-R130
- Pozas J, San RM, Alonso-Gordoa T , et al. Targeting Angiogenesis in Pancreatic Neuroendocrine Tumors: Resistance Mechanisms. Int J Mol Sci 2019;20:4949
- Fazio N. Neuroendocrine tumors resistant to mammalian target of rapamycin inhibitors: A difficult conversion from biology to the clinic. World J Clin Oncol 2015;6:194-197
- Lauricella E, Mandriani B, Cavallo F , et al. Angiogenesis in NENs, with a focus on gastroenteropancreatic NENs: from biology to current and future therapeutic implications. Front Oncol 2022;12:957068
- Kulke MH, Ou FS, Niedzwiecki D , et al. Everolimus with or without bevacizumab in advanced pNET: CALGB 80701 (Alliance). Endocr Relat Cancer 2022;29:335-344
- Fjallskog ML, Lejonklou MH, Oberg KE , et al. Expression of molecular targets for tyrosine kinase receptor antagonists in malignant endocrine pancreatic tumors. Clin Cancer Res 2003;9:1469-1473
- Capdevila J, Salazar R. Molecular targeted therapies in the treatment of gastroenteropancreatic neuroendocrine tumors. Target Oncol 2009;4:287-296
- Gilbert JA, Adhikari LJ, Lloyd RV , et al. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr Relat Cancer 2010;17:623-636
- Faivre S, Sablin MP, Dreyer C , et al. Novel anticancer agents in clinical trials for well-differentiated neuroendocrine tumors. Endocrinol Metab Clin North Am 2010;39:811-826
- Raymond E, Hobday T, Castellano D , et al. Therapy innovations: tyrosine kinase inhibitors for the treatment of pancreatic neuroendocrine tumors. Cancer Metastasis Rev 2011;30 Suppl 1:19-26
- Pavel ME, Wiedenmann B. Novel therapeutic agents for the treatment of gastroenteropancreatic neuroendocrine tumors. Horm Metab Res 2011;43:844-853
- Fazio N, Cella CA, Del Re M , et al. Pharmacodynamics, clinical findings and approval status of current and emerging tyrosine-kinase inhibitors for pancreatic neuroendocrine tumors. Expert Opin Drug Metab Toxicol 2019;15:993-1004
- Mosalem O, Sonbol MB, Halfdanarson TR , et al. Tyrosine Kinase Inhibitors and Immunotherapy Updates in Neuroendocrine Neoplasms. Best Pract Res Clin Endocrinol Metab 2023;101796
- Grillo F, Florio T, Ferrau F , et al. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr Relat Cancer 2018;25:R453-R466
- Toumpanakis C, Fazio N, Tiensuu JE , et al. Unmet Needs in Appendiceal Neuroendocrine Neoplasms. Neuroendocrinology 2019;108:37-44
- Xu J, Shen L, Zhou Z , et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:1500-1512
- Xu J, Shen L, Bai C , et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:1489-1499
- Ali MA, Shah SS, Tahir N , et al. Efficacy and toxicity of surufatinib in neuroendocrine tumors: A systematic review and meta-analysis. J Neuroendocrinol 2022;34:e13149
- Lu X, Yan S, Koral KA , et al. Surufatinib for the treatment of advanced extrapancreatic neuroendocrine tumors. Expert Rev Anticancer Ther 2021;21:917-926
- Raymond E, Dahan L, Raoul JL , et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N Engl J Med 2011;364:501-513
- Blumenthal GM, Cortazar P, Zhang JJ , et al. FDA approval summary: sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist 2012;17:1108-1113
- Raymond E, Kulke MH, Qin S , et al. Efficacy and Safety of Sunitinib in Patients with Well-Differentiated Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2018;107:237-245
- Valle JW, Borbath I, Rosbrook B , et al. Sunitinib in patients with pancreatic neuroendocrine tumors: update of safety data. Future Oncol 2019;15:1219-1230
- Cives M, Strosberg J. Radionuclide Therapy for Neuroendocrine Tumors. Curr Oncol Rep 2017;19:9
- Hicks RJ, Kwekkeboom DJ, Krenning E , et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017;105:295-309
- Ramage J, Naraev BG, Halfdanarson TR. Peptide receptor radionuclide therapy for patients with advanced pancreatic neuroendocrine tumors. Semin Oncol 2018;45:236-248
- Alsadik S, Yusuf S, Al-Nahhas A. Peptide Receptor Radionuclide Therapy for Pancreatic Neuroendocrine Tumours. Curr Radiopharm 2019;12:126-134
- Starr JS, Sonbol MB, Hobday TJ , et al. Peptide Receptor Radionuclide Therapy for the Treatment of Pancreatic Neuroendocrine Tumors: Recent Insights. Onco Targets Ther 2020;13:3545-3555
- van Vliet EI, Teunissen JJ, Kam BL , et al. Treatment of Gastroenteropancreatic Neuroendocrine Tumors with Peptide Receptor Radionuclide Therapy. Neuroendocrinology 2013;97:74-85
- Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:173-185
- Bergsma H, van Vliet EI, Teunissen JJ , et al. Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Pract Res Clin Gastroenterol 2012;26:867-881
- Van Essen M, Sundin A, Krenning EP , et al. Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol 2014;10:102-114
- Kwekkeboom DJ, Teunissen JJ, Bakker WH , et al. Radiolabeled somatostatin analog (177Lu-DOTA0,Tyr3)octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005;23:2754-2762
- Merola E, Grana CM. Peptide Receptor Radionuclide Therapy (PRRT): Innovations and Improvements. Cancers (Basel) 2023;15:
- Kipnis ST, Hung M, Kumar S , et al. Laboratory, Clinical, and Survival Outcomes Associated With Peptide Receptor Radionuclide Therapy in Patients With Gastroenteropancreatic Neuroendocrine Tumors. JAMA Netw Open 2021;4:e212274
- Zhang J, Song Q, Cai L , et al. The efficacy of (177)Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) in patients with metastatic neuroendocrine tumours: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2020;146:1533-1543
- Strosberg J, El-Haddad G, Wolin E , et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-135
- Wang LF, Lin L, Wang MJ , et al. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine (Baltimore) 2020;99:e19304
- Forrer F, Valkema R, Kwekkeboom DJ , et al. Neuroendocrine tumors.Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab 2007;21:111-129
- Grozinsky-Glasberg S, Barak D, Fraenkel M , et al. Peptide receptor radioligand therapy is an effective treatment for the long-term stabilization of malignant gastrinomas. Cancer 2011;117:1377-1385
- Dumont RA, Seiler D, Marincek N , et al. Survival after somatostatin based radiopeptide therapy with (90)Y-DOTATOC vs. (90)Y-DOTATOC plus (177)Lu-DOTATOC in metastasized gastrinoma. Am J Nucl Med Mol Imaging 2015;5:46-55
- Hofland J, Herrera-Martinez AD, Zandee WT , et al. Management of carcinoid syndrome: a systematic review and meta-analysis. Endocr Relat Cancer 2019;26:R145-R156
- Zhang J, Liu Q, Singh A , et al. Prognostic Value of (18)F-FDG PET/CT in a Large Cohort of 495 Patients with Advanced Metastatic Neuroendocrine Neoplasms (NEN) Treated with Peptide Receptor Radionuclide Therapy (PRRT). J Nucl Med 2020;61:1560-1569
- Binderup T, Knigge U, Johnbeck CB , et al. (18)F-FDG-PET is superior to WHO grading as prognostic tool in neuroendocrine neoplasms and useful in guiding peptide receptor radionuclide therapy: a prospective 10-year follow-up study of 166 patients. J Nucl Med 2020;
- Kim YI. Salvage peptide receptor radionuclide therapy in patients with progressive neuroendocrine tumors: a systematic review and meta-analysis. Nucl Med Commun 2021;42:451-458
- Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev 2021;93:102141
- Sachs G, Shin JM, Briving C , et al. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol 1995;35:277-305
- Gedaly R, Daily MF, Davenport D , et al. Liver Transplantation for the Treatment of Liver Metastases From Neuroendocrine Tumors: An Analysis of the UNOS Database. Arch Surg 2011;146:953-958
- Le Treut YP, Gregoire E, Klempnauer J , et al. Liver Transplantation for Neuroendocrine Tumors in Europe-Results and Trends in Patient Selection: A 213-Case European Liver Transplant Registry Study. Ann Surg 2013;257:807-815
- Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I , et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017;162:525-536
- Kim J, Zimmerman MA, Hong JC. Liver transplantation in the treatment of unresectable hepatic metastasis from neuroendocrine tumors. J Gastrointest Oncol 2020;11:601-608
- Fan ST, Le Treut YP, Mazzaferro V , et al. Liver transplantation for neuroendocrine tumour liver metastases. HPB (Oxford) 2015;17:23-28
- Lim C, Lahat E, Osseis M , et al. Liver Transplantation for Neuroendocrine Tumors: What Have We Learned? Semin Liver Dis 2018;38:351-356
- Spolverato G, Bagante F, Tsilimigras DI , et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors. Minerva Chir 2019;74:399-406
- Muller PC, Pfister M, Eshmuminov D , et al. Liver transplantation versus liver resection for treatment of neuroendocrine liver metastasis: Appraisal of the current evidence. Hepatobiliary Pancreat Dis Int 2023;
- Shah T, Manas DM, Ford SJ , et al. Where Are We Now with Liver Transplantation in Neuroendocrine Neoplasms? The Place of Liver Transplantation for Grades 1 and 2 Well-Differentiated Unresectable Liver Metastatic Neuroendocrine Tumours. Curr Oncol Rep 2023;25:135-144
- Valvi D, Mei X, Gupta M , et al. Younger Age Is Associated with Improved Survival in Patients Undergoing Liver Transplantation Alone for Metastatic Neuroendocrine Tumors. J Gastrointest Surg 2020;
- Vilchez V, Gedaly R. Liver transplantation for the treatment of neuroendocrine liver metastases. Best Pract Res Clin Endocrinol Metab 2016;30:141-147
- Gregoire E, Le Treut YP. Liver transplantation for primary or secondary endocrine tumors. Transpl Int 2010;23:704-711
- Le Treut YP, Gregoire E, Belghiti J , et al. Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: an 85-case French multicentric report. Am J Transplant 2008;8:1205-1213
- Maggio I, Manuzzi L, Lamberti G , et al. Landscape and Future Perspectives of Immunotherapy in Neuroendocrine Neoplasia. Cancers (Basel) 2020;12:832
- Bosch F, Bruwer K, Altendorf-Hofmann A , et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr Relat Cancer 2019;26:293-301
- Weber MM, Fottner C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol Res Treat 2018;41:306-312
- Klein O, Kee D, Markman B , et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin Cancer Res 2020;26:4454-4459
- Mehnert JM, Bergsland E, O'Neil BH , et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020;126:3021-3030
- Xu G, Wang Y, Zhang H , et al. Immunotherapy and potential predictive biomarkers in the treatment of neuroendocrine neoplasia. Future Oncol 2020;
- Al-Toubah T, Cives M, Strosberg J. Novel immunotherapy strategies for treatment of neuroendocrine neoplasms. Transl Gastroenterol Hepatol 2020;5:54
- Rindi G, Wiedenmann B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: towards precision medicine. Nat Rev Endocrinol 2020;16:590-607
- Young K, Lawlor RT, Ragulan C , et al. Immune landscape, evolution, hypoxia-mediated viral mimicry pathways and therapeutic potential in molecular subtypes of pancreatic neuroendocrine tumours. Gut 2020;
- Li Y, Fan Z, Zhang F , et al. Neoadjuvant therapy in pancreatic neuroendocrine neoplasms: A systematic review and meta-analysis. Front Oncol 2022;12:981575
- Lania A, Ferraù F, Rubino M , et al. Neoadjuvant Therapy for Neuroendocrine Neoplasms: Recent Progresses and Future Approaches. Front Endocrinol (Lausanne) 2021;12:651438