ABSTRACT
In males, estrogens exert pleiotropic effects by acting on several tissue and organs, including the male reproductive system. The action of estrogens is manifest from prenatal life during which the exposure to estrogen excess might influence the development of some structures of the male reproductive tract. Male fertility is under the control of estrogens, especially in rodents. The loss of function of estrogen receptor alpha and/or of the aromatase enzyme leads to infertility in mice. In men, estrogens are able to exert their actions at several levels through the reproductive tract and on several different reproductive cells. However, the regulation of human male reproduction is complex, and the role of estrogens is less clear compared to mice. During fetal and perinatal life, estrogen acts on the central nervous system by modulating the development of some areas within the brain that are committed to controlling male sexual behavior in terms of setting gender identity, sexual orientation development and the evolution of normal adult male sexual behavior. This organizational, central effect of estrogens is of particular significance in other species (especially rodents and rams), but probably less important in men where psychosocial factors become more determining. Other relevant, non-reproductive physiological events, such as bone maturation and mineralization and glucose metabolism, depend on estrogen in men and an increasing body of evidence is disclosing new non-reproductive estrogen function. In this chapter we provide an update of estrogen’s role by reviewing the physiological actions of estrogen on male reproduction and the pathophysiology related to estrogen deficiency and estrogen excess. Phenotypes associated with estrogen deficiency and excess in rodents and in man have shed new light on the mechanisms involved in male reproduction, challenging the perception of the predominant importance of androgens in men. It is now clear that the imbalance between estrogen and androgen in men might affect male reproductive function even in presence of normal circulating androgens. Some uncertainties still remain, especially regarding the impact of abnormal serum estrogen levels on male health, particularly due to the fact that estrogen is not routinely measured in men in clinical practice. Advancements in methods to precisely measure estrogens in men, together with a reduction of their costs, should provide better evidence on this issue and inform clinical practice. In parallel, new basic, genetic, and clinical research is required to improve our knowledge on the role of estrogen in male reproductive function and men’s health in general.
INTRODUCTION
From an historical perspective, estrogens were identified about 85 years ago and estradiol was identified in 1940, reviewed in (1,2). The first evidence of estrogen production in the male was provided in 1934 by Zondek (3), who documented that male stallions excrete high levels of estrogens in the urine and hypothesized that estrogen production in the male occurs via the intratesticular conversion of androgens into estrogens (1,3,4).
In men, the conversion of androgens into estrogens was first demonstrated a few years later, when an increase in urinary estrogens after the administration of exogenous testosterone was recorded in normal men (5) (Figure 1).

Figure 1. Milestones in the advancement of research in the area of estrogens in men.
[E2: 17β-estradiol; ER: estrogen receptor; ERKO: Estrogen Receptor Knock out; ARKO: Aromatase Knock out]
A more detailed demonstration of estrogen production in the human male was provided several years later in 1979 by MacDonald et al. who showed that the aromatization of androgens to estrogens can occur also peripherally in several tissues other than in the testes (2,6).
Prior to the demonstration of estrogen production in males, the effects of estrogen excess on the development of male reproductive organs had been evident since the 1930s (7). Thus, the concept that male tissues are responsive to estrogens was not new, but it was thought that only a great amount of estrogens was able to induce such changes in males (2).
Notwithstanding the large amount of data accumulated in the last eighty years, research in the field of estrogen excess and its role on male reproductive system is still ongoing (8) (Figure 1).
The pioneering studies of Zondek and MacDonald opened the way for an appreciation of the physiological roles of estrogens in the male beyond their effects during embryogenesis (2). Several studies, year after year, provided further data on estrogen’s role in men (9-12), since the first pilot studies on estrogens and male reproductive function (13,14).
The progressive development of immunohistochemical studies and the subsequent progress in the field of molecular biology highlighted the actions of estrogens in the male [for further details see (4)] and opened the way for the creation of estrogen null mice, a useful animal model to study the physiological role of estrogens in vivo (15) (Figure 1).
The detailed characterization of estrogen receptors’ structure and function (15,16) together with the discovery and the characterization of genes involved in estrogens synthesis (17) disclosed the biomolecular mechanisms and related pathways involved in estrogen function and dysfunction. It is now clear that estrogen effects in the male are not confined to reproductive organs but are pleiotropic (18).
In addition, the development of male transgenic mice lacking functional estrogen receptors or the aromatase enzyme (responsible for estrogen biosynthesis) further contributed to advancements in this field (15,16). Finally, the discovery of mutations in both the human estrogen receptor alpha (19) and aromatase (20,21) genes contributed to an understanding of estrogen’s role in human male physiology and pathophysiology (11,12,22-25) (Figure 1).
Nowadays, notwithstanding this long history of studies, reviewed in (26-28), the role of estrogens in the physiology of the male reproductive tract is still not fully understood. The presence of estrogens in the human testis is well documented (29,30), and there is clear evidence that estrogens exert a wide range of biological effects in both men and women (10,12,18,24,25,31).
PHYSIOLOGY
Estrogen Biosynthesis in Males
The term estrogen refers to any substance, natural or synthetic, able to interact with the estrogen receptor (ER) (32,33). 17β-estradiol (estradiol) is the prevalent endogenous estrogen form in mammals, although many of its metabolites could be detected with several degrees of estrogenic activity (34). In humans, the three major endogenous estrogens are estrone (E1), estradiol (E2), and estriol (E3) (33) (Figure 2). In males, estrogens mainly derive from circulating androgens. The key step in estrogen biosynthesis is the aromatization of the C19 androgens, testosterone and androstenedione, to form estradiol and estrone, respectively (32). This step is under the control of the aromatase enzyme (32,35) (Figure 2).
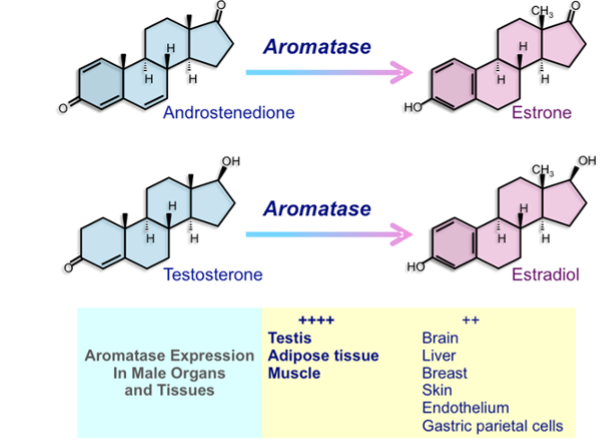
Figure 2. Biochemical pathway of testosterone conversion into estrogen.
However, a wide number of other endogenous products belongs to the category of estrogenic compounds, such as 27-hydroxycholesterol, dehydroepiandrosterone (DHEA), 7-oxo-DHEA, 7α-hydroxy-DHEA, 16α-hydroxy-DHEA, 7β-hydroxyepiandrosterone, Δ4-androstenedione, Δ5-androstenediol, 3α-androstanediol (3α-Adiol), 3β-androstanediol (3β-Adiol), 2-hydroxyestradiol, 2-hydroxyestrone, 4-hydroxyestradiol, and 4-hydroxyestrone and 16α-hydroxyestrone(33). In particular, dihydrotestosterone (DHT), an androgenic metabolite of testosterone that is synthesized by the enzyme 5 alpha reductase, can be metabolized into 3 β-Adiol, an intermediate metabolite with estrogenic activity (32,36). All these molecules differ in terms of ER affinity (33). Various exogenous substances also show estrogenic activity, such as bisphenol A, metalloestrogens, phytoestrogens (e.g., coumestrol, daidzein, genistein, miroestrol) and mycoestrogens (e.g., zeranol) (37). These exogenous estrogens can influence human physiology via environmental exposure or ingestion, however the real impact in vivo as well as critical thresholds and cumulative amount of exposure remain to be fully elucidated (38).
The aromatase enzyme is a P450 mono-oxygenase enzyme complex (17) present in the smooth endoplasmic reticulum, which acts through three consecutive hydroxylation reactions, with the final reaction being the aromatization of the A ring of androgens (17,34) (Figure 2). This enzymatic complex is composed of a ubiquitous and non-specific NADPH-cytochrome P450 reductase, together with the regulated form of cytochrome P450 aromatase (17,29). The latter is highly specific for androgens (39,40). The conversion of androgens into estrogens takes place mainly in the testes, adipose tissue and muscle tissue, even though other male tissues are also involved to a lesser extent (17,34,35) (Figure 2).
The P450 aromatase enzyme is encoded by the CYP19A1 gene: a gene of 123 kb of length, which consists of at least 16 exons and is located on the long arm of chromosome 15 in the q21.2 region in humans (9,17,34) (Figure 3). This gene belongs to the cytochrome P450 superfamily, similar to other enzymes involved in steroidogenesis (32).
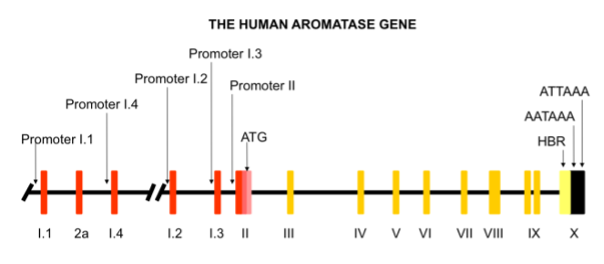
Figure 3. Schematic representation of the human aromatase (CYP19) gene.
[Red bars: first exons associated with upstream alternative, tissue-specific promoters; yellow bars: coding exons; black bar: heme-binding region].
Circulating estrogens are mainly reversibly bound to sex hormone binding globulin (SHBG), a β-globulin, and, to a lesser degree, to albumin (41). The amount of circulating free estradiol depends on several factors, of which the concentrations of albumin and SHBG are the most important (41). Serum free estradiol may be calculated by a complicated formula using total estradiol, SHBG, and albumin levels or may be measured by means of equilibrium dialysis or centrifugal ultrafiltration methodology; both, however, are too time consuming and expensive to be employed in routine clinical practice (41). When calculating free estradiol, the reliability of the value of total serum estradiol should be considered, since assays commonly used for estradiol in clinical laboratories have poor accuracy when measuring the low serum estrogen characteristic in males (42-44).
Estrogen Actions in Males
Estrogen action is mediated by interaction with specific nuclear estrogen receptors (ERs), which are ligand-inducible transcription factors regulating the expression of target genes after hormone binding (10,34,45). Two subtypes of ERs have been described: estrogen receptor α (ERα) and the more recently discovered estrogen receptor β (ERβ) (34,45). These two ER subtypes show different ligand specificity and transcriptional activity, and mediate the classical, direct, ligand-dependent pathway involving estrogen response elements in the promoters of targets genes and protein-protein interactions with several transcription factors (45). These two different ERs have different transcriptional activity (46). In particular, ERβ shows a weaker transcriptional activity compared to ERα (45). This difference is due to the presence of different ERβ isoforms, which can modulate estrogen signaling using different pathways and lead to different impacts on the regulation of target genes (45,46). In addition, it should be remarked that the co-expression of both ERα and ERβ in the same cell determines a complex cross-talk finally resulting in the antagonistic effect exerted by ERβ on ERα-dependent transcription (45,46). Thus, the presence/absence of ER subtypes together with their cross-talk determines a cell’s ability to respond to different ligands as well as the regulation of transcription of different target genes (45).
ERα in humans is encoded by the ESR1 gene located on the long arm of chromosome 6, while the ESR2 gene encodes ERβ and is located on band q22-24 of human chromosome 14 (45,46). The two ER proteins have a high degree of homology at the amino acid level (45) (Figure 4).
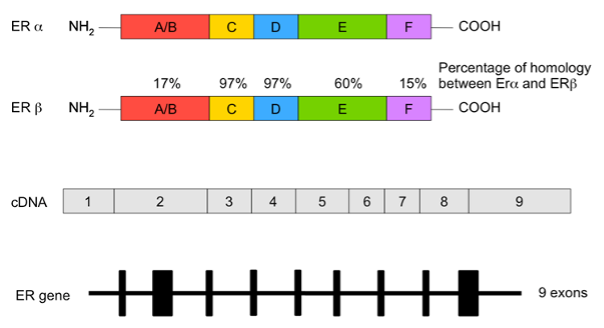
Figure 4. Estrogen receptor gene structure showing the 9 exons (lower panel), cDNA domains (indicating exons), and protein structures of both ERα and ERβ (upper panels: colored boxes denote the different functional domains of the protein).
ERs are nuclear receptors in which structurally and functionally distinct domains are recognized. Estrogens bind the COOH-terminal multifunctional ligand-binding domain (LBD), whereas the DNA-binding domain recognizes and binds DNA (45,46). The NH2-terminal domain, the most variable domain, is involved in the transcriptional activation (45). This domain recruits a range of coregulatory protein complexes to the DNA-bound receptor (45). The two ER forms share a high degree of sequence homology except in their NH2-terminal domains. This specificity accounts for different transcriptional effects on different target genes (45,46). The ER genomic pathway begins with the binding of estrogen to ER (45). This interaction induces conformational changes in the ER, allowing receptor dimerization and subsequent nuclear translocation prior to binding to estrogen response elements or to other regulatory sites within target genes (45,46). Thereafter, the availability of several coregulatory proteins influences the transcriptional response to estrogen (45,46).
While it is clear that estrogens regulate transcription via nuclear interaction with their receptors, a non-genomic action of estrogens has been also demonstrated, suggesting a different molecular mechanism involved in some estrogen actions (34,45-48). In vitro studies show a very short latency time between the administration of estrogens and the appearance of its biological effects. These actions seem to be mediated by a cell-surface G protein-coupled receptor, known as GPR30, that does not act through a transcriptional mechanism (34,47,48). Rapid effects of estrogens result from the actions of specific receptors localized most often to the plasma membrane; in particular it seems that a monomeric portion of the ERα is translocated from the nucleus to the plasma membrane (47,48).
Recently, immunohistochemical analysis of murine tissues reported the presence of GPR30 in the male reproductive tract, including testes, epididymis, vas deferens, seminal vesicles and prostate (49). Furthermore, a rapid response to estradiol suggests that non-genomic estrogen actions are involved also in human spermatozoa (50,51). The different intracellular pathways of estrogen action are summarized in Table 1.
|
Table 1. Characteristics of Estrogen Actions and Related Biomolecular Pathways |
||||
|
Estrogen Actions |
Receptors |
Mechanism/Pathway |
Final effect |
Features |
|
Genomic (Nuclear actions) |
ERα |
Transcriptional: nuclear interaction with estrogen-responsive elements |
Modulation of estrogen target gene expression |
Slow effects (minutes or hours) |
|
ERβ |
Transcriptional: nuclear interaction with estrogen-responsive elements |
Modulation of estrogen target gene expression |
Slow effects (minutes or hours) |
|
|
Non-genomic (cell membranes actions) |
Estrogen receptors on cells membrane (GPR30) |
Cells membrane changes |
Changes in ionic transport through cell surface |
Rapid effects (seconds) |
[ERα: estrogen receptor alpha; ERβ: estrogen receptor beta].
Aromatase enzyme and ERs are widely expressed in the male reproductive tract both in animals and humans (52,53), implying that estrogen biosynthesis occurs at this site and that both locally produced and circulating estrogens may interact with ERs in an intracrine/paracrine and/or endocrine fashion (34). Today, it is clear that not only testicular somatic cells, but also germ cells constitute a source of estrogens in human (29,54). Thus, the concept of a key role for estrogen in the male reproductive tract is strongly supported by the ability of the male reproductive structures to produce and respond to estrogens (26,52). In men, the aromatase enzyme and ERs are expressed in several tissues including those involved in male reproduction. The distribution and expression of aromatase and ERs described below concerns the male reproductive organs.
Aromatase and ERs in the Male Reproductive Tract
The distribution of ERs and aromatase in both the developing and adult male reproductive tract of rodents and humans is summarized below.
DISTRIBUTION OF ERs AND AROMATASE IN FETAL RODENTS
Aromatase and ERs are found at a very early stage of development in the rodent testis, thus suggesting a role for estrogens in influencing testicular development (4,26,55-57).
Leydig cells in fetal rodent testis express ERα before the androgen receptor. Moreover, ERα is abundant in the developing efferent ductules, which are the first male reproductive structures to express ERs during fetal development (58-60). Furthermore, the epididymis also expresses ERα in the fetal rodent. By contrast, it is unclear whether ERα is present within the seminiferous tubules of the fetal testis since conflicting results have been reported in literature (26,29,57).
ERβ is found early in fetal testis, particularly in gonocytes, Sertoli and Leydig cells, with the gonocytes showing the highest expression between 10-16 days post coitum (61). This suggests a role for estrogens in their maturation. In addition, ERβ is expressed by rat Wolffian ducts, the structures from which the efferent ductules and epididymis arise (26,57). ERα is widely expressed in efferent ductules from fetal life to adulthood, implying a crucial role in male reproduction that has been well documented in adult rodents (27,52,60). On the other hand, ERβ is mainly expressed during fetal life, suggesting a major role in the development of male reproductive structures until birth (26).
A recent study suggests that estradiol is also able to increase the production of stem cell factors by fetal human Sertoli cells, finally resulting in the proliferation and growth of spermatogonial stem cells (62). With this in view, estrogen deficiency during fetal life may conditioning the total amount of spermatogonia available in the future for the spermatogenetic maturation.
Aromatase is expressed in both Leydig and Sertoli cells in the fetal rodent testis, but not in gonocytes and immature structures of the seminal tract. ER and aromatase distribution in the fetal testes as summarized in Table 2. The presence of both aromatase and ERs in the developing fetal testis implies a possible involvement of estrogens in the process of differentiation and maturation of developing rodent testis just starting from an early stage of embryogenesis, with ERβ possibly playing a greater role than ERα (53,55,56).
Both ERα and ERβ are expressed in the fetal penile tissue and estrogens seem to be important for penile growth as well as for the normal differentiation of the terminal part of the urethra (63,64). In particular estrogen takes part together with androgens in the final fusion of the penile urethra (64) and estrogen deficiency due to both ERs disruption and aromatase deficiency may cause hypospadias in rodents (63-65).
|
Table 2. ERs and Aromatase Distribution in the Rodent Fetal Testis and Efferent Ducts |
|||
|
|
ERα |
ERβ |
Aromatase |
|
Leydig cells |
++ |
++ |
+ |
|
Sertoli cells |
- |
++ |
++ |
|
Gonocytes |
- |
+++ |
- |
|
Efferent Ducts |
+ |
+ |
- |
|
Penile tissue |
++ |
++ |
? |
The proposed distribution is based on information from various studies including immunohistochemical and mRNA expression studies.
[ERα: estrogen receptor alpha; ERβ: estrogen receptor beta].
DISTRIBUTION OF ERs AND AROMATASE IN ADULT RODENT REPRODUCTIVE TRACT
ERα is expressed (both in terms of mRNA and protein) in the Leydig cells of both adult rats and mice (66) but not in Sertoli cells, and is mainly expressed in the proximal (rete testis, efferent ductules, proximal epididymis), rather than in the distal (corpus and cauda of the epididymis, vas deferens) reproductive ducts (26). However, in neonatal and prepubertal rats, estradiol increases the expression of proteins involved in the proliferation and differentiation of Sertoli cells and of proteins involved in the adhesion of germ cells to Sertoli cells (67). Furthermore, ERα has been immunolocalized in ciliated and non-ciliated cell nuclei of the epididymal epithelium (59,68). This peculiar distribution explains several important estrogen actions in the proximal ducts, especially within the efferent ductules that are small and convoluted tubules connecting the rete testis (an anastomosing network of intricate and tenuous tubules located in the hilum of the testis) to the epididymis (60). In the efferent ductules, estrogens promote fluid reabsorption (52,60,69). Finally, the full-length form of ERα has been detected in purified rat germ cells, using a specific antibody directed against the C-terminal region of the protein (70) (Table 3).
ERβ is expressed (both in terms of mRNA and protein) in Leydig and Sertoli cells in adult rodents (26,57,60) and in monkey germ cells (71); furthermore, it is expressed also in epithelial and peritubular cells of efferent ducts (59,68). For many years the presence of ERβ in rodent germ cells has been the subject of some debate due to discrepancies in the results of different immunohistochemical studies (72). Immunolocalization of ERβ in differentiated germ cells of adult rodents has been revealed in various studies (61,73). Conversely, no ERβ immunoreactivity was found in rodent germ cells in other studies (74), while mRNA expression seems to decline from fetal life to adulthood in the rat (72). Nevertheless, ERβ seem to be involved in the regulation of gonocyte multiplication, which is under the influence of growth factors and estradiol (16), suggesting a functional role for ERβ at least in immature male germ cells. In addition, several studies have recently identified several pathways involving the ERβ in germ cells confirming both its presence and activity of these cells (75,76).
|
Table 3. ERs and Aromatase Distribution in the Adult Rodent Testis and Efferent Ducts |
||||
|
|
ERα |
ERβ |
GPR30 |
Aromatase |
|
Leydig cells |
+ |
+ / - |
+ |
+++ |
|
Sertoli cells |
- |
+ |
+ |
+ |
|
Germ cells Spermatogonia Pachytene Spermatocytes Round Spermatids Spermatozoa |
+ + + + + |
++ + + + + |
+ + + + ? |
++++ + + ++ + |
|
Efferent ductules |
++++ |
+ |
? |
+ |
The proposed distribution is based on information from various studies including immunohistochemical and mRNA expression studies.
[ER α: estrogen receptor alpha; ERβ: estrogen receptor beta; GPR30: G protein-coupled receptor].
GPR30 is widely expressed (both in terms of mRNA and protein) in rodent testis (77). In particular, this receptor is expressed in rat Leydig cells (78) and Sertoli cells (79,80), in the spermatogonia GC-1 cell line (81), in rat pachytene spermatocytes (70), and in round spermatids (82).
Rodent Leydig cells show higher aromatase expression than Sertoli cells (83). Aromatase is also expressed at high levels in germ cells throughout all stages of maturation, with its expression increasing as germ cells mature into spermatids. Aromatase mRNA expression and enzyme activity are present in both rat and mouse germ cells from the pachytene spermatocyte stage, and during their subsequent maturation into round spermatids (57,60,83) (Table 3). Carreau et al. demonstrated that aromatase activity in germ cells was more than 50% of that of the whole testis (29). This intensive activity suggests that germ cells may be a major source of estrogen in adult rodents (57,60,83) (Table 3). Specifically, when fully developed spermatids are released from the epithelium, aromatase is present in the residual body (the remains of the spermatid cytoplasm that is removed during spermiation) and is subsequently phagocytosed by the Sertoli cell. Aromatase activity also remains detectable in the cytoplasmic droplet attached to the flagellum when sperm passes through the epididymis, suggesting that mature spermatozoa are able to synthesize their own estrogen as they pass through the efferent ducts (29,84). The ability to synthesize estrogen gradually decreases as the droplet slowly moves to the end of the tail during epididymal transit until it is finally lost. The demonstration of aromatase in sperm is important as it suggests that the sperm itself could control the levels of estrogen present in the luminal fluid, and might directly modulate some functions such as the reabsorption of fluid from the efferent ductules (60).
DISTRIBUTION OF ERs AND AROMATASE IN THE HUMAN MALE REPRODUCTIVE SYSTEM
ERs are present in human testis and reproductive tract (29,60,85,86). In the male fetus both ERβ and aromatase are expressed in Sertoli, Leydig and germ cells from 13 to 24 weeks, whereas ERα expression is absent (86,87). Furthermore, ERβ immunoreactivity in the epididymis suggests a putative role for locally produced estrogens, the actions of which are likely mediated by ERβ in this site. This supports the importance of estrogens for the prenatal development and function of male reproductive structures, which is well documented in literature (87). In particular, estrogens play an important role in the development of the rete testis, efferent ductules, epididymis, and vas deferens (88).
Aromatase and ERβ, but not ERα, continue to be expressed (both in terms of mRNA and protein) during the prepubertal period in men, but their function during infancy remains unclear, especially if the very low levels of both circulating and locally produced sex steroids in this period of life is taken into account (89).
In adult men, ERα is expressed only in Leydig cells, while ERβ has been documented in both Leydig and Sertoli cells and in the efferent ducts (74) (Table 4). The presence of ERs in the human epididymis is still a matter of debate (27), even though ERα has been detected in the nuclei of epithelial cells of the caput of the epididymis (90), and recent data confirms its presence in the epididymis (86). Both ERs (ERα and β) have been identified in isolated immature germ cells (29). Furthermore, they were localized in mature spermatozoa (91) and in ejaculated spermatozoa (92). Luconi et al. first described an estrogen receptor-related protein in the sperm membrane (50,51). This protein is able to bind steroid hormones and may act through a calcium-calmodulin dependent pathway, accounting for a well-documented rapid non-genomic action (50,51). Subsequently, the expression (both in terms of mRNA and protein) of both ERs in human ejaculated spermatozoa (92,93) reinforced the concept that estrogens are able to modulate the spermatogenic process from its onset within the testes through to the final process of sperm maturation after ejaculation (4,29,92,93). The ERα and ERβ localize to different regions in human sperm, with ERα present in the compact zone in the equatorial segment of the upper post-acrosomal region of the sperm head, and ERβ in the mid-piece, at the site of the mitochondria (57). This confirms that each type of receptor probably has a distinct role in sperm physiology and in the process of fertilization (75,94).
|
Table 4. ERs and Aromatase Distribution in the Human Testis and Efferent Ductules |
||||
|
|
ERα |
ERβ |
GPR30 |
Aromatase |
|
Leydig cells |
+ |
+ / - |
+ |
+ |
|
Sertoli cells |
- |
+ |
+ |
+ |
|
Germ cells Spermatogonia Pachytene Spermatocytes Round Spermatids Spermatozoa |
- + + + |
+ + + ++ |
+ - - - |
ND + + + |
|
Efferent ductules |
+ |
+ |
? |
+ |
The proposed distribution is based on information from various studies including immunohistochemical and mRNA expression studies.
[ERα: estrogen receptor alpha; ERβ: estrogen receptor beta; GPR30: G protein-coupled receptor].
Of particular interest is the demonstration of differential expression in the human testis of wild type ERβ (ERβ1) and of a human variant form of ERβ, the latter arising from alternate splicing (known as ERβcx, or ERβ2), (95,96). ERβ2 expression seems to be associated with prevalent, negative inhibition of ER action by inhibiting ERα–induced transactivation (97); it is highest in spermatogonia and Sertoli cells in adult men, suggesting that these cells may be "protected" from estrogen action (95,96). Wild type ERβ1 was mostly present in pachytene spermatocytes and round spermatids, which have been proposed to be more estrogen sensitive (26), yet ERβ1 was low in less mature germ cells (95). In addition, the discovery of several splice variants of ERβ (including ERβ4) in human testicular cells suggests a specific and more complex estrogen action on spermatogenesis (96).
Besides, the cellular distribution of non-genomic GPR30 estrogen receptor in human testicular biopsies was examined (98). Immunohistochemical analysis of testicular sections identified the GPR30 receptor in the cytoplasm of Leydig cells, Sertoli cells and spermatogonia (98). This pattern of localization was further demonstrated by the analysis of GPR30 expression (both in terms of mRNA and protein) in isolated germ cells and in Sertoli cell culture (98). This peculiar distribution suggests that GPR30 may be involved in germ cell differentiation (98). Furthermore, the presence of GPR30 in human spermatozoa has been confirmed at both the mRNA and protein level, with this receptor being localized in the sperm mid-piece (99). The co-expression of the two classic ERs and of the GPR30 receptor in the same area within the spermatozoa (mid-piece and acrosome region) suggests a complex cross-talk among all these receptors able to influence physiological processes and pathological implications, such as tumorigenesis (100).
Aromatase expression in the human testis is present in both somatic and germ cells (53,88). Specifically, it is expressed in Leydig and Sertoli cells (101,102), in immature germ cells, from pachytene spermatocytes through elongated spermatids (57,101), and ejaculated sperm cells (103). Locally produced estrogens in sperm are proposed to exert a protective action on sperm DNA by preventing sperm DNA damage (104), thus accounting for estrogen’s potential role as a survival factor during sperm transit through the seminal vesicles (105). Unlike rodents, aromatase expression in human gametes persists during the transit through the genital tracts, since P450 aromatase has been demonstrated in human ejaculated spermatozoa at three different functional levels: mRNA expression, protein production and activity (92). Therefore, as in rodents, human sperm are considered a potential site of estrogen biosynthesis (4,92,101,102,104). The presence of functional aromatase in human spermatozoa allows the conversion of androgens into estrogens as they transit the reproductive tract, providing free estrogens in the seminal fluid able to act on the cells of the reproductive ducts. Thus, human spermatozoa should be considered a mobile endocrine unit (53,54,88,106).
In summary, the testes are able to synthesize and respond to estrogens throughout their development (53,88). The localization of ERα, ERβ and aromatase suggests that estrogen action is likely to be important for testicular and efferent ductule function. Differences among various polymorphisms of ER genes may account for different responses to estrogens in term of sperm count and sperm quality (107,108). The role of estrogens in the male reproductive system is clearer in rodents (see below), and the mapping of ERs and aromatase distribution in the human male reproductive system has led to the suggestion that estrogen plays a role in human male reproduction (4,53,55). As a consequence, a new field of research has evolved, aimed at improving our knowledge on estrogen action on male reproduction, and the molecular mechanisms involved in both animal models and in men.
ROLE OF ESTROGENS IN MALE REPRODUCTION
Estrogens in Animal Male Reproduction: Effects of Estrogen Deficiency
Estrogen-deficient knockout mice are useful models to investigate estrogen action in rodents (16,26). At present, four different lines of estrogen receptor-deficient knockout mice have been generated: 1) ERα knockout (α-ERKO) mice with disrupted ERα gene (109-111); 2) ERβ knockout (β-ERKO) mice, with an inactivated Erβ (112), 3) double ERα and ERβ knockout (αβ-ERKO) mice with non-functioning ERα and ERβ (16), and 4) GPR30 knockout mice (113-115). The αERKO, βERKO and αβERKO mice provide very helpful information on the loss of ER function, leading to estrogen resistance. The knockout of the aromatase gene in aromatase knockout (ArKO) mice is an experimental model useful for investigating the congenital lack of both circulating and locally produced estradiol (16,26,116,117). Estrogen-resistant mice (αERKO, βERKO, and αβERKO) have high levels of circulating estrogens with the non-genomic pathway still likely functional. Aromatase-deficient mice have no circulating estradiol however estrogen receptors could be activated by other estrogenic compounds produced outside the aromatase pathway (e.g. 3β-Adiol) or introduced by diet (e.g. phytoestrogens) (26). Furthermore, in 2009, Sinkevicius et al. created transgenic mice with a G525L point mutation in the ligand-binding domain of ERα (ENERKI mice) (118). This allows differentiation of ligand-dependent vs ligand-independent ER actions since these two different pathways could lead to different actions in vivo. The study of fertility of the ENERKI mouse shows that the efferent ductule fluid reabsorption is regulated by ligand-independent actions of ERα, whereas germ cell production and/or viability requires ligand-dependent ERα actions (118). Recently, Yao et al. mapped the Era-binding sites in the efferent ductules of male mice and they found 12105 peaks, of which about 50% were shared by the androgen receptor (119).
Recently, the creation of the knockout mice lacking GPR30 estrogen receptor (113,115) allowed an investigation of the reproductive phenotype of mice lacking a functional GPR30, with the results suggesting a minor role of this receptor in male fertility. GPR30 knockout mice did not show abnormalities of endocrine organs, alterations of spermatogenesis and mating behavior, or decreased fertility (114,120). A detailed study of spermatogenesis in this mouse model is, however, still lacking.
Studies on transgenic mice lacking ERs or the aromatase enzyme demonstrate that the lack of estrogen action is compatible with life (22,121). Congenital estrogen deficiency in mice leads to an impairment of male reproductive function ranging from normal fertility with a fully male phenotype in βERKO mice, to complete infertility in both αERKO and αβERKO mice. An intermediate pattern exists for the ArKO mice in which spermatogenesis is normal in young mice, but progressively worsens during aging (16,26,60,69,109-112,116,122). Reproductive characteristics of male mouse models are summarized in Table 5.
|
Table 5. Reproductive Phenotype of Male Mouse Models of Estrogen Deficiency |
|||
|
αERKO |
βERKO |
αβERKO |
ArKO |
|
Infertility |
Fully fertile |
Similar to αERKO mice |
Normal fertility in young mice, infertility with advancing age |
|
Normal FSH Elevated estradiol |
-- |
-- |
Normal FSH |
|
Germ cell loss and dilated seminiferous tubules |
Normal testicular histology |
Testicular histology similar to αERKO mice |
Histology of the testis is disrupted with advancing age |
|
Impairment of sexual behavior |
Normal sexual behavior |
Complete suppression of sexual behavior |
Impairment of sexual behavior |
The G protein-coupled receptor (GPR30) knockout mice have normal reproductive phenotype.
[ERKO: estrogen receptor knockout mice; α: estrogen receptor alpha; β: estrogen receptor beta; ArKO: Aromatase knockout mice].
Male αERKO mice are infertile as the seminiferous epithelium is atrophic and degenerated, and seminiferous tubules and rete testis are dilated (60,69,111), even though the development of male reproductive tract is largely unaffected (16,109,111,123). The disruption of spermatogenesis is progressive as the testicular histology is normal at postnatal day 10, but starts to degenerate at twenty-thirty days of age (69,111). From 40 to 60 days, tubules are markedly dilated with a corresponding significant increase in testicular volume, while the seminiferous epithelium becomes atrophic (16,69,111). A severe impairment in tubule fluid absorption at efferent ducts level is the cause of infertility in αERKO male mice, and this defect is partially mimicked also by the administration of an anti-estrogen drug in wild-type mice (59,60,69). In the male genital tract, the highest concentration of ERα is in the efferent ducts (69) and the estrogen-dependent fluid reabsorption at this site probably results from estrogen interaction with the ERα that seems to regulate the expression of the Na(+)/H(+) exchanger-3 (NHE3) (59,69). This mechanism appears to be the consequence of the ligand-independent ERα activation (118). In fact, the disruption of ERα, or the use of anti-estrogens, results in a decreased expression of NHE3 mRNA, as well as in a decrease of other proteins involved in water reabsorption, such as aquaporin I (124,125). The lack of fluid reabsorption in the efferent ductules of αERKO male mice and the consequent dilatation induces a retroactive progressive swelling of the seminiferous tubules (27,52,60,69,111,126). The seminiferous tubule damage results from the increased fluid pressure and severely impaired spermatogenesis coupled with testicular atrophy as seen at the age of 150 days of age (16,52,60,69). When germ cells from αERKO mice are transplanted in wild type mice, they show normal development (127). Recently, it has been demonstrated that some genes playing important role in the efferent ductules are regulated by Er both independently by estrogens or in combination with androgens where estrogen responsive elements colocalize with androgen responsive elements (119).
The αERKO mouse is also characterized by a reduced number, motility, and fertilizing capacity of the sperm levels (Table 5). In addition, αERKO male mice show increased serum luteinizing hormone (LH) and testosterone as well as Leydig cells hyperplasia, together with normal serum follicle-stimulating hormone (FSH) levels (Table 5) (16).
The production of both ArKO (122) and βERKO (112) mice added further insights in this field, supporting the idea that estrogen actions on the male reproductive tract are more complex than previously suggested on the basis of the studies performed on αERKO mice (16). In fact, unlike αERKO mice, male ArKO mice are initially fully fertile (122), but fertility decreases with advancing age (Table 5) (26,116,117). Furthermore, βERKO mice are fully fertile and apparently reproductively normal in adulthood (Table 5) (112).
The mechanism involved in the development of infertility in ArKO male mice therefore differs from that of the αERKO mice (26). Transgenic mice models suggest that ligand-independent ERα signaling is essential for concentrating epididymal sperm via regulation of efferent ductule fluid reabsorption, while ligand-dependent ERαis involved in germ cell production and/or viability (118). Thus, the lack of estrogen action at the level of the seminiferous epithelium rather than a problem due to impaired fluid reabsorption probably explains infertility in ArKO male mice (26,50). Accordingly, estradiol seems to be necessary for round spermatid survival and estrogen deficiency seems to promote apoptosis before differentiation into elongated spermatid (26,92,105).
Studies of αβERKO mice showed a male phenotype very close to that of αERKO mice characterized by infertility and dilated seminiferous tubules (16,26). On the contrary, βERKO male mice were fully fertile (112). These findings lead to the hypothesis that estrogen activity in the male reproductive tract depends on both the type of estrogen receptor involved, and the site of action through the male reproductive tract. Interestingly, results from mice lacking functional ERs or aromatase point to an important role for estrogen in the maintenance of mating behavior in male mice. For this reason, infertility in αERKO, αβERKO and ArKO mice is at least in part due to the reduction of various components of mating behavior from an early age (Table 5) (16,26).
The function of the hypothalamo-pituitary-testicular axis is impaired in both αERKO and ArKO male mice, leading to elevated serum LH levels in the presence of normal values of FSH, while, as expected, testosterone is augmented and estrogens are higher than normal or undetectable in αERKO and ArKO mice, respectively (26). Thus, negative effects on male reproduction are the direct result of estrogen deprivation in the reproductive structures or of indirect changes in the regulation of sex steroid secretion.
Taken together, all these studies support the concept that a functional ERα, but not ERβ and GPR30, is needed for the development and maintenance of a normal fertility in male mice (15,16,52,55,59,60,69,111,112). Anyhow, it should be remarked that estrogens are also able to self-regulate all these estrogen-related pathways in the male reproductive tract since estrogen receptor expression is regulated by estradiol in rats. In particular ERα positively regulates the expression of both ERα and ERβ while androgen receptor and ERβ negatively regulates ERs expression (128).
Estrogens in Human Male Reproduction: Effects of Estrogen Deficiency
The demonstration of wide expression of the aromatase enzyme, ERα and ERβ throughout the male reproductive system and within human sperm underlines the role of estrogens in human male reproductive function (4,29,55,106,129). Accordingly, estrogens seem to modulate sperm maturation (50,129), since spermatozoa express ERα and ERβ, and are responsive to estrogens throughout their journey from the testes to the urethra.
The characterization of human diseases leading to estrogen deficiency have increased our knowledge about the role of estrogen in male reproductive function as well as on other important physiological functions ranging from longitudinal growth, bone mass acquisition, and metabolic alterations (24,130-132).
Data from human subjects with congenital estrogen deficiency have provided conflicting and confusing results. Fertility has been investigated in only one man with estrogen resistance who exhibited a mutation in ERα, rendering him unable to respond to estrogen, thus he could be considered as a human equivalent of the αERKO mouse. However, this man had normal testicular volumes and normal sperm count but with slightly reduced motility (19) (Table 6). At present only one other patient has been diagnosed with estrogen resistance, but a semen analysis was not available for this 18 years-old boy, a possible impairment of fertility being hypothesized on the basis of low inhibin serum levels, reduced testis volume, and cryptorchidism (133). The human reproductive phenotype seems different from that observed in αERKO mice (16,26,52,69,111) since there was no clinical evidence of obstruction of the efferent ductules in the man with estrogen resistance, different to that observed in the rodent model (19). However, no data on the histology of the testis and efferent ductules is available from these two men with estrogen-resistance (19,133,134).
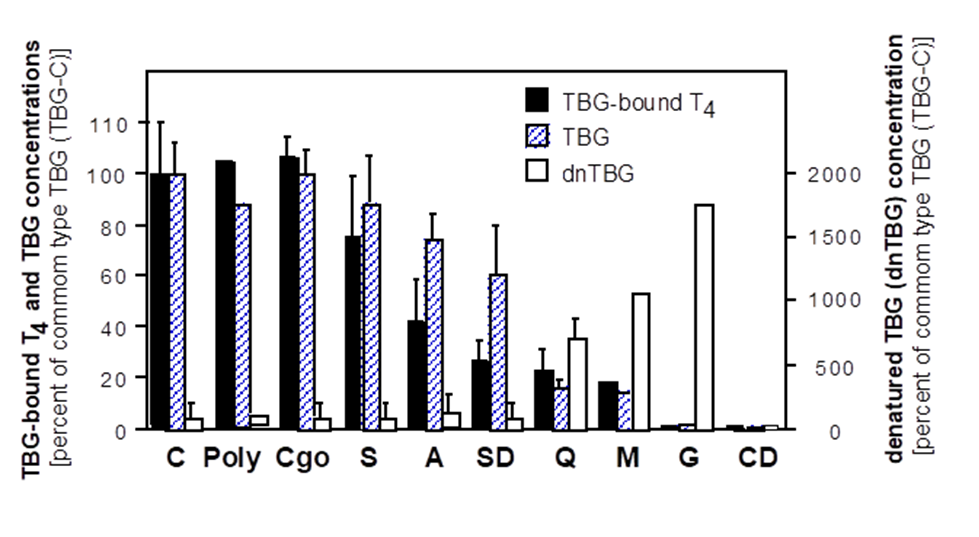
The other human model of estrogen deficiency is congenital aromatase deficiency (135). At present, sixteen men with aromatase deficiency have been described (Table 6 and Table 7) (20,136-149). For most of them the genetic diagnosis (143,144) and/or the clinical description (21,150-152), as well as the following clinical studies (153-160)were performed by our research group. These patients showed a variable degree of impaired spermatogenesis (4,11,143,144,161). The hormonal pattern of the patients affected by aromatase deficiency is summarized in Table 6(4,55,135,162). Testicular size in aromatase-deficient men is normal except for three cases having a smaller testes volume (Table 6), while data on testes volume are lacking in some reports (148). Among the eight patients with semen analysis available, six had normal sperm count (139,140,143,146,148,152) and the remaining two had oligospermia (21,22,138) from moderate (138) to severe (21) (Table 6). Anyhow, moderate to severe asthenospermia without teratospermia was also reported independently from the sperm count (21,22,138-140,152) (Table 6). Sperm count was unavailable in the other eight men with aromatase deficiency due to lack of data, diagnosis made at birth, and cases described in prepubertal age (20,136,137,141,143-145,147,149). Moreover, a variable degree of germ cell arrest, ranging from complete depletion of germ cells to arrest at the stage of primary spermatocytes, was described in three aromatase-deficient men who underwent biopsy of the testes (21,22,150,151) (Table 6).
Furthermore, a history of cryptorchidism was present in four patients (22,2%) being bilateral in two cases (145,150)and unilateral in the remaining two (139,140,152). These data suggest a possible role of estrogen in testis descent, although this was not seen in the transgenic mouse models. The small number of cases of cryptorchidism among men with aromatase deficiency does not allow any conclusion concerning a possible relationship between estrogen deficiency and the occurrence of abnormalities in testis development and descent. Besides, hypospadias has been reported in one case (145) and preliminary data speak in favor of estrogen role on penile tissue development during fetal growth (63-65), but this aspect needs to be confirmed by more robust studies.
In addition, a clinical condition of aromatase deficiency may be also caused by mutation in the cytochrome P450 oxidoreductase (POR) (163), as previously reviewed (162).
It should be remarked, however, that a clear cause-effect relationship between infertility and aromatase deficiency was not demonstrated in all these patients (4,135). For this reason, the different degree of fertility impairment found in men with congenital estrogen deficiency does not allow us to establish with certainty whether sperm abnormalities are a consequence of the lack of estrogen action or are an epiphenomenon. Again, this spermatogenetic pattern is different from that observed in ArKO mice (16,26,52,116,117,122).
|
Table 6. Reproductive Phenotypes of Men with Congenital Estrogen Deficiency |
||
|
|
Estrogen resistance |
Aromatase deficiency |
|
Total subjects |
2 |
16 |
|
Subjects diagnosed during adulthood |
1 |
11 |
|
Age at diagnosis (mean+DS; min-max) |
23 years; 18-28 |
25.8 + 8.6 years; 1-44 |
|
REPRODUCTIVE HORMONES |
|
|
|
LH |
High |
Normal to high |
|
FSH |
High |
High |
|
Testosterone |
Normal |
Normal to high |
|
Estradiol |
High |
Undetectable |
|
EXTERNAL GENITALIA |
|
|
|
Size testis |
Normal |
Small to normal |
|
Cryptorchidism |
Absent |
4 cases |
|
Hypospadias |
- |
1 case |
|
SEMEN ANALYSIS |
|
|
|
Sperm count |
Normozoospermia |
Oligo to normozoospermia |
|
Viability |
Asthenozoospermia |
Asthenozoospermia |
|
Testis biopsy |
Not performed |
Depletion or germ cell arrest at primary spematocyte level |
[LH: luteinizing hormone; FSH: follicle-stimulating hormone]
The frequency of sperm abnormalities in these patients together with the results from rodent studies suggests a possible role for estrogen in human spermatogenesis, however this requires further elucidation (4,12,24,30). Our knowledge on estrogen’s role in human male reproduction in vivo remains far from complete. The data available in the literature suggests that the action exerted by estrogens on male reproductive organs is more complex than that seen in mice and that estrogen alone does not directly control spermatogenesis to the same extent than in rodents, but are involved in a more complex and evolved network (26,118,164)
In addition to human models of congenital estrogen deficiency, other experimental settings have provided information on the role of estrogens on human male fertility.
Studies on the association between ER polymorphisms and infertility in men showed that two polymorphisms of ERα (XbaI and PvuII) are associated with azoospermia, severe oligospermia and impaired sperm motility (165-168) and the multiallele (TA)n polymorphism with male infertility (108). However, data available in literature provides conflicting results since Pvull resulted strongly associated with infertility (166), but also a strong protective factor of male fertility (169), depending on the research setting. The RsaI polymorphism of the ERβ has been associated with male infertility in one study (107), but not confirmed in another study (167). The ERβ (RsaI) polymorphism AluI was also associated with sperm motility, while no association with motility was found for the RsaI polymorphism (167). Thus, the association of polymorphisms of estrogen-related genes with both sperm concentration and motility, but not with sperm morphology, further supports a putative role of estrogen in controlling sperm production and quality (170).
Furthermore, the investigation of ERs in the nuclear matrix of human spermatozoa showed a reduction of ER levels in the nucleus of idiopathic infertile men compared to normospermic fertile men (171).
Interventional research studies show that the administration of aromatase inhibitors to infertile men with documented impaired testosterone-to-estradiol ratio may result in an improvement of their fertility rate, but further evidence is needed to verify their efficacy and safety (see paragraph below ‘Anti-estrogen treatment in men’ for further details) (172,173). These results suggest that such modulation of estrogen metabolism will influence sex hormone balance and the HPT axis while dissecting out direct effects of estrogen on spermatogenesis in vivo is extremely challenging.
It seems that exposure to increasing estradiol concentrations might influence glucose metabolism in spermatozoa and that the increase of aromatase activity and estradiol enhances glucose metabolism in capacitated, but not in non-capacitated sperm (93). Recently, intratesticular T and E2 were strongly correlated to aromatase expression in Leydig cells in infertile men; intratesticular T was higher and E2 lower in men with obstructive azoospermia compared to those with nonobstructive azoospermia, suggesting that an imbalance in aromatase expression and thus in the intratesticular T to E2 ratio might play a role in the pathogenesis of male infertility (174). In addition, serum estradiol was directly correlated with motility, sperm count and sperm morphology in male partners of infertile couples enrolled prospectively and low estradiol entered among risk factors for decreased semen quality in multivariate analysis (175).
It seems probable that most of estrogen actions operating in mice, such as regulation of sperm motility, sperm capacitation, acrosome reaction, and sperm metabolism also occur in men, but the contribution of estrogens to these processes is quantitatively less important in humans. It seems likely that most of these processes in humans are also regulated by other factors in a complex crosstalk system involving also estrogens. This could also explain why high amounts of estrogens or the exposure to an excess of environmental estrogens (or to xenoestrogens with high estrogenic potency) could negatively impact on male fertility. For these reasons, it is apparently difficult to reconcile existing data about effects of both estrogen deficiency and excess on male reproductive function (13,31,176-178).
Regulation of Gonadotropin Feedback
The regulation of gonadotropin feedback is an important and well-documented action of estrogen in males. While testosterone has been classically considered the key hormone for the control of gonadotropin feedback in the male, a role for estrogens was recently clarified in studies performed in normal and GnRH-deficient men. We now know that ERs are expressed both in the hypothalamus and the pituitary. In particular, GnRH neurons express ERβ but not ERα (179,180), thus the inhibitory effects of estrogens on these cells is mediated through other neuromediators (e.g. kisspeptin, neurokinin B) released by other neurons expressing also the ERα (181). ERs, especially ERα are expressed in gonadotropes cells (182).
The response of the hypothalamic-pituitary-gonadal axis to androgens is confirmed by the administration of dihydrotestosterone (DHT), which is able to partially decrease LH and FSH with a concomitant reduction in serum testosterone and estradiol (183). However, the discovery of men with congenital estrogen deficiency has provided further evidence for a relationship between estrogens and gonadotropin secretion also in men (22). In fact, serum gonadotropins are high in all adult patients with aromatase deficiency, notwithstanding normal to increased serum testosterone levels (135), thus implying that estrogens are also important for the regulation of circulating gonadotropins levels in men.
The effects of estrogens on gonadotropin secretion have been investigated in GnRH-deficient men whose gonadotropin secretion was normalized by pulsatile GnRH administration. Moreover, in order to determine the precise role of sex steroids on the hypothalamo-pituitary-testicular axis, several studies characterized by the administration of testosterone, testosterone plus testolactone (an aromatase inhibitor), or estradiol have been performed (184,185). Testosterone alone induced a significant decrease in mean basal LH and FSH levels as well as of LH pulse amplitude, demonstrating a direct suppressive effect on the pituitary of testosterone and its metabolites. In general, mean LH levels and LH pulse frequency are suppressed to a greater extent in normal control subjects under testosterone administration, suggesting the involvement of a hypothalamic site of action of testosterone (or its metabolites) in suppressing GnRH secretion. In order to discriminate the impact of testosterone from its aromatized products, both groups of subjects were treated with testosterone plus testolactone. The addition of this aromatase inhibitor completely inhibited the testosterone effect on gonadotropin secretion both in normal and GnRH-deficient men, thus leading to a significant increase in mean LH levels in both groups. The latter was greater in normal men who received testolactone alone than in normal men who received testosterone plus testolactone, thus confirming a direct effect of androgens on gonadotropin secretion in normal men. On the basis of the results of these studies, it is clear that the aromatization of testosterone to estradiol is, at least in part, required for normal gonadotropin feedback at the pituitary level (185). In fact, when the same experimental model was applied using estradiol administration instead of testolactone, mean LH and FSH levels as well as LH pulse amplitude decreased significantly during the treatment (184). These studies have demonstrated an important direct inhibitory effect of estradiol on gonadotropin secretion in both GnRH-deficient and normal men (184,185) and support the concept that, at least in part, the inhibitory effect on gonadotropin secretion is mediated by the conversion of testosterone to estradiol (4,186). Accordingly, the administration of the aromatase inhibitor letrozole to healthy adult males is able to suppress aromatase activity and serum estradiol levels leading to an increase of gonadotropins (187). Only the restoration of normal circulating estrogens, by means of transdermal estrogen administration, normalized gonadotropin secretion in this setting (187). In contrast, it seems that the 5α-reduction of testosterone to DHT does not play a very important role in pituitary secretion of gonadotropins (188); DHT, in fact, slightly decreases LH and FSH only after long-term administration (183).
All these studies suggest possible estrogen action at the level of hypothalamus. In order to clarify the role of estrogen on the feedback regulation of gonadotropin secretion at hypothalamic level, Hayes et al. (189) conducted a study involving men affected by idiopathic hypogonadotropic hypogonadism (IHH), whose gonadotropin secretion was normalized by long-term pulsatile GnRH therapy, followed by treatment with the aromatase inhibitor anastrozole. They observed that the inhibition of estradiol synthesis led to an increase in mean gonadotropin levels that was greater in normal subjects than in IIH men, suggesting a hypothalamic involvement. The rise in mean LH concentrations in normal subjects due to anastrozole was due to increased LH pulse frequency and amplitude. The authors concluded that estrogen acts at the hypothalamic level by decreasing GnRH pulse frequency and pituitary responsiveness to GnRH (189). Subsequently, the same group (190) demonstrated that the administration of estradiol in normal subjects, whose endogenous testosterone and estradiol synthesis was inhibited through the use of ketoconazole, reduced mean LH levels by lowering LH pulse frequency, but not amplitude. These authors went on to report that the sex steroid component to FSH negative feedback was not androgenic but rather was mediated by estradiol effects on the frequency of GnRH stimulation (190,191).
For many years another important unresolved issue has been the relative role of circulating vs. locally produced estrogens in the control of gonadotropin secretion. Now we know that the effects of circulating estrogen are more relevant than that of locally produced, at both the hypothalamic and pituitary level (187) (Figure 5). Accordingly, the administration of both the aromatase inhibitor letrozole and estradiol at different dosages showed that serum testosterone and gonadotropins were inversely related to circulating estradiol, depending on the dose of exogenous estradiol (187). The serum estradiol required to obtain the same levels of gonadotropins were not different compared to that at baseline, suggesting that aromatase inhibition and the blockade of locally produced estrogens are less important than previously thought (187). In the same year, our group reached the same conclusions using a different approach. In men with aromatase deficiency, we demonstrated that circulating rather than locally produced estrogens are the main inhibitors of LH secretion (157). This implies that the role of locally produced estradiol on gonadotropin feedback at hypothalamic and pituitary levels is relatively modest in vivo (Figure 5). However, the role of locally produced estrogens has been poorly investigated since evaluating the effects of locally produced estrogens in vivo is challenging (186).
Data available in the literature demonstrate that (i) circulating estrogens are involved in gonadotropin suppression both at pituitary (187) and hypothalamic level (157,190), and (ii) estrogen effects on hypothalamus are independent from central aromatization, but requires adequate amounts of circulating estrogens in normal healthy men (187), in men with IHH (190,191), and in men with aromatase deficiency (157).
The effects of estrogen on gonadotropin secretion at the pituitary level operate from early- to mid-puberty (186,192,193) into old age in men (194). The administration of an aromatase inhibitor (anastrozole 1 mg daily for 10 weeks) to young men aged 15-22 years (192) resulted in a 50% decrease in serum estradiol concentrations, an increase in testosterone concentrations and an increase in both LH and FSH values during the study protocol. These hormonal parameters were restored after the discontinuation of anastrozole treatment (192). In addition, the administration of letrozole increased serum LH levels, LH pulse frequency and amplitude and the response of LH to GnRH administration in boys during early and mid-pubertal phases, confirming that estrogens act at the pituitary level during early phases of puberty (193), the role of estrogens in infancy and at the beginning of puberty remaining less known (186). The same mechanism continues to operate during adulthood and early senescence (195), as shown in fifteen eugonadal men, aged 65 years treated with 2 mg anastrozole for 9 weeks, in which serum FSH and LH levels increased significantly, in spite of an increase in serum testosterone levels (194). Similar results were replicated by using letrozole in older men (173). For these reasons, the use of aromatase inhibitors as blockers of the negative feedback on gonadotropin has been tested as a possible strategy useful for the treatment of late-onset male hypogonadism (196). The rationale was that increasing endogenous serum testosterone through the inhibition of the rate of conversion of testosterone into estradiol led to the consequent LH and FSH increase (196). After the first encouraging results (197-199), this kind of treatment seems to be not effective, especially on large-scale clinical trials and for long periods of time (195,196,200,201).
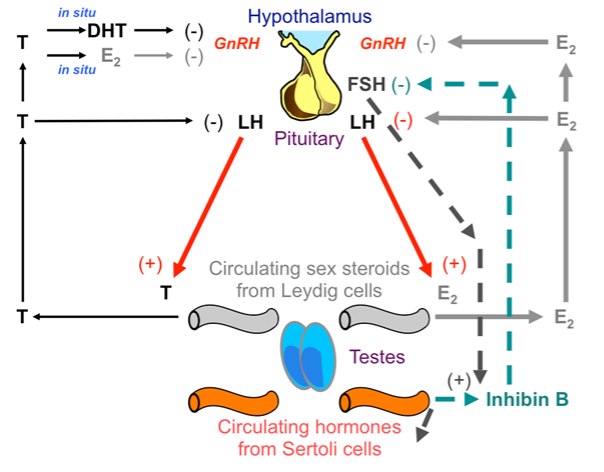
Figure 5. Sex steroid control of gonadotropin secretion after recent advances: estrogens, but not androgens, are the main regulator of gonadotropins and the action of circulating estradiol prevails with respect to that of locally produced estradiol.
[T: testosterone; DHT: dihydrotestosterone; E2: estradiol; GnRH: gonadotropin releasing hormone; LH: luteinizing hormone; FSH: follicle-stimulating hormone]
Previous data suggest that estradiol may modulate GnRH receptor number and function at hypothalamic-pituitary level (202), since ERs were detected in GnRH secreting neurons (203). Moreover, both genomic and non-genomic estrogen actions seems to be involved in the regulation of the gonadotropin feedback in males (203,204), although the precise mechanism remains unclear (205). Nevertheless, it is now well established that androgens need to be converted to estrogens in order to ensure the integrity of the gonadotropin feedback mechanism in men, testosterone itself having a lesser role than previously thought (Figure 5), and circulating estrogen, rather than locally produced estrogen, having a major role at the hypothalamic pituitary level (157,187,191).
In a complementary way, our knowledge on the role of estrogens in gonadotropin feedback has been enhanced through studies of men with congenital estrogen deficiency. The description of a man lacking a functional ERα (19)revealed a remarkable hormonal pattern consisting of normal serum testosterone, high estradiol and estrone levels, but increased serum FSH and LH concentrations; the serum testosterone remained in the normal range because of increased aromatization of testosterone to estradiol (Table 6). Other important information about estrogen’s role in the human male came from the discovery of naturally occurring mutations in the aromatase gene. To date, of the sixteen different cases of human male aromatase deficiency that have been described, all were discovered to be aromatase-deficient as adults, except one who was diagnosed as a child (137,141) and another one who was diagnosed at 15 months of age (145) (Table 6). Eight out of fourteen adult patients with aromatase deficiency had increased basal FSH concentrations (20,21,135,138-140,144,150-152,154), two had serum FSH in the upper normal range (143,144), and the remaining four had normal FSH (144,146-148). The subject diagnosed during childhood had normal FSH in infancy (137) and high to normal FSH levels at puberty (141). The unique patient diagnosed early at 15 months had normal serum T, LH, and FSH (145). LH was normal in all aromatase adult patients (138-140,144,146,147,150-152), except for one subject with elevated serum LH (20,136) and two subjects with high to normal LH levels (21,143,154)(Table 6). Serum testosterone concentrations were generally normal or high-to-normal except for the first case described with elevated serum levels (20,136), and two other aromatase-deficient men with testosterone slightly above the normal range (138,139). Conversely, another man with aromatase deficiency presented with low to normal serum testosterone levels (150,156). In all sixteen patients estradiol concentrations were undetectable (20,136-149). (Table 6). The detection of increased gonadotropin levels despite normal-to-increased serum testosterone levels, in these men, further highlights the key role for estrogen in regulating circulating gonadotropins in men (155,157), In normal men with pharmacologically induced sex steroid deprivation, estradiol but not testosterone, was able to restore normal FSH serum levels (191). Due to the concomitant impairment of the patient's spermatogenesis, complete normalization of serum FSH was not achieved in all aromatase-deficient men during estradiol treatment, even in the presence of physiological levels of circulating estradiol (135), only supraphysiological levels of estrogens were able to normalize FSH (21,135,154,155).
A detailed study of the effects of different doses of transdermal estradiol on pituitary function in two men with congenital aromatase deficiency demonstrated that estrogens might control not only basal secretion of gonadotropins but also their responsiveness to GnRH administration (138,155,157). In these studies, estrogen administration to three male patients with aromatase deficiency caused a decrease in both basal and GnRH-stimulated LH, FSH and α-subunit secretion with a dose-dependent response to GnRH administration (138,155,157). In 2006, Rochira et al. (157), demonstrated that estrogen’s effects on LH secretion are exerted both at pituitary and hypothalamic level, as shown by the decrease of basal and GnRH-stimulated secretion of LH and the LH pulse amplitude, and the reduction of the frequency of LH pulses respectively, during estrogen treatment to normalize estradiol serum levels in two aromatase-deficient men. In normal physiology, these data provide evidence that the negative feedback effects of circulating estrogens is more important than estrogen locally produced at the hypothalamic level (157). As previously explained, these data confirm data from healthy men (186).
Notwithstanding recent advances in the study of estrogen’s role in males, some difficulties remain when data from men with congenital estrogen deficiency are interpreted, particularly if phenotype heterogeneity is considered (161,186). No abnormalities were found in either gonadotropin secretion or in testis position and size in the patient with congenital aromatase deficiency diagnosed in childhood (137), unlike female newborns (206). For these reasons, the role of estrogens in the hypothalamo-pituitary-testicular axis should become relevant in a later stage of life than infancy in men. Furthermore, the smaller than expected increase in FSH levels (given the prevailing serum testosterone levels and impaired spermatogenesis) in two estrogen-deficient men (157), suggests a possible role of estrogens in priming and maturation of hypothalamus-pituitary-gonadal axis in men (155,156). Thus, the control of gonadotropin feedback exerted by sex steroids during early infancy and childhood remains a matter of debate in the human male (186).
In conclusion, estrogens are the main sex steroids involved in the control of gonadotropin secretion in men, testosterone having a minor but determinant role as demonstrated by evidence coming from complete androgen insensitivity (CAIS) syndrome in which serum LH is above normal as a consequence of androgen resistance notwithstanding elevated circulating estradiol (207).
Estrogens and Prostate
Androgens regulate prostate gland growth and differentiation, particularly during its development. Estrogens also act on prostate growth and differentiation through both ERα, and ERβ (208,209). In rodents, the prostate is sensitive to estrogen exposure during development (210).
Studies on animals have helped to better understand estrogen’s role in prostate growth. Studies in mice overexpressing aromatase (AROM+) demonstrated that prostate lobes are significantly reduced as a consequence of estrogen excess (211). On the other hand, aromatase-deficient mice presented a hyperplastic prostate gland probably due to the excess of circulating androgens (212) and consistent with hyperplasia of the epithelial, interstitial and luminal compartments (210). Furthermore, McPherson et al., using tissue recombination and an ERβ-specific agonist, demonstrated that ERβ activation results in an anti-proliferative response not influenced by systemic androgen levels, or activation of ERα (212). Moreover, studies on ArKO mice demonstrated that the administration of an ERβ-specific agonist reverted the existing hyperplastic epithelial pathology (212).
In terms of prostate carcinogenesis, it is generally assumed that androgenic hormones play a major role in tumor development, since the prostate gland is an androgen-dependent tissue, as is prostate cancer (213). However, considering the fact that testosterone can be converted to estradiol, and that ERs are present in the prostate epithelium (214), theoretically estrogen might also be involved in the induction of prostate cancer. Some polymorphisms (rs2470152, rs10459592, and rs4775936) of the CYP19A1 aromatase gene were associated with an increased risk of prostate cancer (215,216). Besides, patients with prostate cancer who are carriers of the rs4775936 polymorphism of the CYP19A1 aromatase gene show a significantly shorter time of cancer-specific survival compared to patients who do not carry this polymorphism (215). In line with this Bosland et al. found that combined treatment of rats with estradiol and testosterone lead to an increased incidence of prostate cancer from 35-40% with androgen alone to 90-100% (217). The estrogen pathways that may be involved at the molecular level in the process of prostate carcinogenesis are very complex (209). Several studies demonstrate that both ERα and β are involved in the transduction of estrogen signaling in prostate cancer such as cell proliferation pathways (209). Furthermore, ERβ seems mainly involved in pro-apoptotic pathways (e.g. FOXO3 and p-53), while ERα is involved in chronic inflammation, and the two ERs seem to act differently on oncogenes playing suppressive (ERβ) and oncogenic (ERα) roles (209). Proliferation of prostatic cells seems to be promoted by the activation of the ER while ER and GPER seems to exert an antiproliferative action (218,219). The different effects of each ER on the proliferation of prostate cells may accounts for the contrasting results (proliferative/antiproliferative) available in literature, depending on the prevailing activated pathway. However, estrogens also display a biphasic effects in vitro on prostate cells growth, which is enhanced by low estradiol and inhibited by high dose of estradiol (220). Probably, different pathways are activated in presence of estrogen excess, thus leading to a shift in the final effect on cell growth (218,220). At present studies on estrogen signaling in prostate cancer tissue are also providing promising results in term of the utilization of this signature as biomarker useful to tailor hormonal treatment (218).
Prostate was normal in aromatase-deficient men and did not change in volume during estrogen replacement therapy (Carani & Rochira; data not published data). Besides, the administration of selective inhibitors of aromatase are helpful for the evaluation of estrogen in vivo effects on prostate. Recently, the combined therapy with transdermal testosterone and the aromatase inhibitor anastrozole in older men with low or low-to-normal serum testosterone (< 350 ng/dL) prevented the increase of prostate volume, but not that of prostate-specific antigen seen in patients treated with testosterone alone (221). Similarly, high serum estradiol resulted directly related to prostate volume in 239 Chinese men with benign prostatic hyperplasia (222) even though these data are limited by the poor accuracy of estradiol measured by immunometric assay.
This is in line with the above-mentioned experimental results suggesting an active role of estrogens in prostate cell proliferation in prostate carcinogenesis. Traditionally, exogenous estrogens have been used for the treatment of prostate cancer since the 1940s thanks to their potent inhibitory effect on the HPT axis resulting in the suppression of circulating testosterone (223). However, diethylstilbestrol (DES) used in the past for prostate cancer was strongly associated with thromboembolic side effects(223). Recently, the use of exogenous estrogens for the therapy of prostate cancer is being reconsidered (224). Transdermal estradiol (patch) seems to be effective in inhibiting gonadotropins and in reducing serum testosterone in men with prostate cancer without increasing cardiovascular events (224).In the near future, if estrogen’s role in the prostate will be further elucidated, new treatment strategies will become available for benign prostate hypertrophy and cancer, especially in men with concomitant hypogonadism (225).
Estrogens and Male Sexual Behavior
Sex steroids act on several aspects of male sexual behavior (226). Sex steroids, mainly testosterone, modulate adult male sexual behavior in mammals (227). In men, sexual behavior is more complex than in other species since it results also from cognitive processes, cultural environment, and an individual system of beliefs (226,228). Thus, sexual behavior does not depend only on hormonal and genetic prerequisites in men (226,228). Traditionally, it was thought that only testosterone, the male hormone, is responsible for the control of male sexual behavior (229). In the last two decades, the possibility that estrogens may be involved in the control of male sexual behavior has received more attention, and an impact of estradiol on male sexuality has become evident (199).
Testosterone is mainly involved in the control of sexual desire and sexual drive and in the facilitation and maintenance of a normal sexual genital response (226). Erections, especially nocturnal erections, are also under the control of androgens (230,231). The role of estrogen on male sexual behavior has been poorly investigated and knowledge derives mainly from studies performed on animals or from rare models of human estrogen deficiency. The increasing interest on the treatment of transgender people (232) and on the cross actions of male and female hormones on both sexual behavior (233) and other physiological functions (234) probably have contributed to a better focus on this area of research. In recent years, however, several in vivo experimental settings have addressed this issue. As a result, nowadays all studies on steroid sex hormones action on male sexual behavior tend to investigate androgens and estrogens separately (199,235-238). Furthermore, steroid sex hormones may influence both gender-identity and sexual orientation (239,240), even though in humans this action is mitigated by the strong influence of psychosocial factors.
Estrogens and Gender Identity and Sexual Orientation
Testosterone aromatization to estradiol in the brain was traditionally considered the key step in the development of a male brain and in determining sexual dimorphism of the central nervous system in non-primate mammals (241-243). According to Dörner’s hypothesis (244), prenatal and perinatal brain exposure to estrogens may be responsible for the establishment of a male brain (240,245), an event occurring only in the male, but not female, brain. Accordingly, ovaries release a very small amount of estrogen, soon inactivated in rodents (4,245), while the testes produce a greater amount of androgen that is converted into estrogen. Thus, circulating estrogens are paradoxically greater in males than in females during fetal life (240,246) and this accounts for the sexual dimorphism of hypothalamic structures in rodents and other species like sheep (245-247).
The same mechanism seems to be also involved in the differentiation in hypothalamic structures between men and women (244,246,248). Prenatal hormonal exposure is classically considered to be involved in determining sexual orientation, on the basis of some differences in hypothalamic structures between heterosexual and homosexual men (243,248). This hypothesis is supported by the concept that brain sexual differentiation during fetal life occurs in parallel with the peak of testosterone secretion from the testis and the consequent increase in serum estradiol (240,241,243,245). Accordingly, the intrinsic pattern of mammalian brain development is female, and estrogen is required for the development of a male brain (240,243,244), thus emphasizing the role of locally produced estrogen (245). Permanent changes in the organization of different neural circuits, fundamental for sex-specific regulation of reproductive and sexual behavior, probably also occurs under the effects of estrogen (240,242,243,245,249). Considering all the above mentioned aspects, the lack of estrogen action on the developing brain in males should be considered strictly related to the direction of future development of sexual orientation, and of dimorphism of hypothalamic structures (240,241,243,245,248). Most of the data supporting this evidence, however, came from studies performed in rodents or other species, but not in humans (240,242,243,245).
The role of hypothalamic aromatase activity and expression in partner preference has been elegantly confirmed in rams (250). In this study, the choice of sexual partner was associated with both the volume of the ovine sexually dimorphic nucleus and different patterns of aromatase expression (250). This provides the first demonstration that differences in aromatase expression within the brain are related to partner choice and to the determination of adult sexual behavior (245,247,250). However, in humans, a clear cause-effect relationship between prenatal exposure to sex steroids (especially estrogens) and the differences in volume of some dimorphic brain areas (e.g. sexually dimorphic nucleus of the preoptic area and the intermediate nucleus) has not been demonstrated (240).
Aromatase-deficient men represent an interesting model to investigate the role of estradiol on human male sexual development and behavior from fetal life through adulthood (4,55,135). All men with aromatase deficiency who underwent a comprehensive evaluation of sexual behavior had male gender-identity and heterosexual orientation (4,20-22,135,138,143,144,150,152,153,156) (Table 7). The fact that congenital aromatase deficiency does not affect psychosexual orientation and gender-identity in humans suggests that estrogens do not mediate the organizational effects on male sexuality induced by early exposure to androgens. Differently from animals, psychological and social factors are the most relevant determinants of gender role behavior in men, with hormones probably having a minor role compared to other species (4,135,228,247). Accordingly, the prenatal exposure to DES, a potent estrogenic compound, is able to modify partner preference in animal studies, but not in humans (251).
In conclusion, aromatase plays a key role in controlling male reproductive behavior especially in animals (rodents and rams), by modulating organizational effects on the developing brain during fetal life (249,252); the latter are mediated by estrogen production within the brain and exposure to circulating estrogens. However, differences among species could explain the essential role of aromatization in rodents, rams, and monkeys (247,252,253) and its poor or minor effect in humans (4,135,153,156) and other primates, respectively (253). Thus the debate about nurture (254) versus nature (246) remains still open in humans.
Estrogens and Sexual Behavior
In adult men sexual behavior is partially dependent on testosterone, the main hormone involved in male sexuality (226,229,230). Accordingly, testosterone deficiency frequently causes loss of libido and erectile dysfunction (226,230,255). These are restored by testosterone replacement therapy, which is effective in increasing sexual interest and improving sexual function (226,227,255,256). Other hormones, however, are involved in the control of male sexual behavior, including estrogens (257,258).
In experimental animal models, the knockout of estrogen pathways or a pharmacologically induced estrogen deficiency results in severe impairment of sexual behavior (4,16,26). Accordingly, ArKO mice (259), αβERKO male mice (260) and αERKO mice (16,109) all exhibit a significant reduction in mounting frequency and prolonged latency to mount when compared with wild-type animals (16,26). On the contrary, βERKO mice did not show abnormalities of sexual behavior (16,112). These findings suggest that androgen receptor activation alone is not sufficient for fully normal sexual behavior in rodents and that a normal functioning ERα together with adequate levels of circulating or locally produced estrogen are required for mounting behavior in male mice (4,55).
Less is known about the role of estrogens in sexual behavior in men since the relative importance of testosterone and its metabolite estradiol on male sexual behavior is still not known. In the past five years, only a few studies have investigated the direct effect of estrogen on male sexual behavior (261,262), indirect evidence being available only from rare cases of men with congenital estrogen deficiency (4,55,130,135,143,144) (Table 7). A detailed sexual investigation of aromatase-deficient men documented an increase in all the parameters of sexual activity during estrogen treatment (153,156), with the best outcome in terms of sexual behavior obtained only when a concomitant normalization of both serum testosterone and estradiol was reached (156). These results support the concept that both sex steroids are required for normal sexual behavior in men. Outside the context of congenital lack of estrogens, it is difficult to reach conclusive information on the role of estrogen on male sexual behavior because of the inadequacy of studies and the conflicting results reported in the literature.
|
Table 7. Sexual Behavior in Men with Congenital Estrogen Deficiency |
||||
|
Subjects |
Authors |
Sexual function |
Gender identity |
Psychosexual orientation |
|
Estrogen Resistance (Age:28 years) |
Smith et al.1994(19) |
Libido: normal. Morning erections: normal. Nocturnal emissions: normal. Ejaculations: normal. |
Male |
Heterosexual |
|
Aromatase Deficiency (Age 24 years) |
Libido: modest. Morning erections: normal. Nocturnal emissions: normal. Ejaculations: normal. |
Male |
Heterosexual |
|
|
Aromatase Deficiency* (Age 38 years) |
Libido: normal. Morning erections: normal. Ejaculations: normal. |
Male |
Heterosexual |
|
|
Aromatase Deficiency* (Age 28 years) |
Morning erections: normal. Libido and sexual activity have not been investigated according to the religious thinking of the patient. |
Male |
Heterosexual |
|
|
Aromatase Deficiency (Age 27 years) |
Libido: normal. Morning erections: normal. Ejaculations: normal. |
Male |
Heterosexual |
|
|
Aromatase Deficiency (Age 25 years) |
Libido: normal. Morning erections: normal. Ejaculations: normal. |
Male |
Heterosexual |
|
|
Aromatase Deficiency (Age 27 years) |
Lanfranco et al. 2008(152) |
Libido: normal. Morning erections: normal. Ejaculations: mild praecox ejaculation. |
Male |
Heterosexual |
|
Aromatase Deficiency (Age 27 years) |
Baykan et al.2013(143) |
No sexual dysfunction reported before and during treatment |
Not reported |
Not reported |
|
Aromatase Deficiency 3 men (26-44 years) |
Pignatti et al.2013(144) |
No sexual dysfunction reported before and during treatment |
Not reported |
Not reported |
|
Aromatase Deficiency (Age 24 years) |
Chen et al.2015(146) |
Libido: normal. No sexual dysfunction reported
|
Not reported |
Not reported |
|
Aromatase Deficiency (Age 25 years) |
Miedlich et al.2016(147) |
Libido: normal. Morning erections: normal.
|
Not reported |
Not reported |
* Only these two patients underwent an extensive, well-designed study of sexual behavior in terms of psychosexual issues (gender identity and sexual orientation) and sexual function (desire and erectile function), while for other patients the information was obtained by patients’ interview and medical history. No data on sexual behavior are available for the other patient with estrogen resistance (133) and for the other aromatase deficient-men (139,140,145,148).
Recently, a very elegant study provided evidence-based information on the relative role of testosterone and estradiol on male sexual function in men (199). In this study, a considerable number of healthy men (n= 400) underwent gonadotropin suppression by the administration of a GnRH analogue (goserelin acetate), resulting in testosterone and estradiol suppression (199). In order to investigate the placebo effect and the effects of testosterone and estradiol, subjects were assigned to receive i) placebo, ii) testosterone treatment at different dosages, and iii) testosterone at different dosages plus the aromatase inhibitor anastrozole (199).
In the testosterone group, serum testosterone and estradiol varied from physiological levels to low levels according to the different doses of exogenous testosterone in each group and the estradiol to testosterone ratio remaining substantially unchanged in all groups (199). This pharmacologic scheme allowed testing the effects of lowering both serum testosterone and estradiol in a similar way on several physiological functions; the result was a decline of both sexual desire and erectile function in parallel with the decrease of both sex steroids (199). In the testosterone plus anastrozole group, the decline of serum testosterone paralleled that obtained in the testosterone group, while serum estradiol was quite suppressed and changed to a lesser degree in each testosterone dose group, thus fluctuating across very low values (199). In this group, both sexual desire and erectile function were severely affected in patients with low serum levels of estradiol despite normal serum testosterone in patients taking the higher doses of testosterone (199). Conversely, goserelin treatment resulted in the maximum reduction of both sexual desire and erectile function in the placebo group (199). These results confirm observations in aromatase-deficient men (135,153,156) and suggest that estrogen deficiency is largely responsible for the impairment in sexual function occurring when serum testosterone is suppressed in hypogonadal men (199). A possible role of estrogen on male sexual function is also provided by further studies showing that testosterone therapy is more effective on libido when the treatment produces serum estradiol levels greater than 5 ng/dL (235) and that this serum estradiol is directly related to sexual function in men (236). In particular, serum estradiol is associated with sexual activity and desire, but not with erectile function (263). In addition, exogenous estradiol improves sexual desire in men with low testosterone and prostate cancer (264).
Notwithstanding these studies, the role of estrogen in male sexual behavior remains controversial (257) since several studies reached opposite conclusions. In particular, Sartorius et al. found that DHT was effective in maintaining male sexual function in healthy, older men, despite its suppressive effect on both testosterone and estradiol, suggesting that male sexual function can be ensured without aromatization (237). Furthermore, other cross-sectional studies failed to demonstrate a clear association between serum estradiol and male sexual function (238,265).
To add further complexity, estrogen action on erectile function seems to be biphasic, since estrogen deficiency may affect the ability to achieve an erection, yet estrogen excess and an increased estradiol to testosterone ratio is associated with an impaired erectile function. A Chinese group reported that serum estradiol is higher in a large sample of adult men with erectile dysfunction compared to men with normal erectile function, while it was not different among men with and without premature ejaculation (266,267). The same group has proposed that high serum estradiol and reduced estradiol to testosterone ratio may be independent risk factor for organic erectile dysfunction (268). Similar results have been obtained by other authors in hypogonadal men where high serum estradiol was associated to more severe erectile dysfunction (269). Also in an experimental rabbit model of the metabolic syndrome (a model used to investigate erectile function), erectile dysfunction is associated with high serum estradiol rather than low testosterone in line with the above mentioned clinical data (270). Conversely, other authors did not find any correlation between testosterone to estradiol ratio and erectile dysfunction (271). All these data, however, needs to be confirmed by further studies since the strength of evidence is weak due to many flaws such as the lack of data on the cause of sexual dysfunction, the poor accuracy of estradiol assay, and the retrospective collection of data.
A possible explanation for these results is that a serum estradiol in the normal male range is required for a fully normal male sexual function in addition to testosterone, while both estrogen deficiency and estrogen excess have a negative impact on male sexual activity (156,236,238).
Finally, estrogen receptors and the aromatase enzyme have been identified in the penile tissue of a large number of species, including humans (272-274) suggesting direct estrogenic activity within the penis. At present, knowledge on estrogen action within the penis derives from the observation that: i) male offspring exposure to estrogen-like endocrine disruptors in utero induces micropenis and hypospadias (176), and that ii) penile development and function is estrogen-dependent in animals (275).
OTHER NON-REPRODUCTIVE PHYSIOLOGICAL ESTROGEN ACTIONS IN MEN
Estrogens, Metabolism, and Cardiovascular Diseases in Men
The role of estrogen on glucose and insulin metabolism in men is difficult to establish since it is challenging to differentiate androgen from estrogen actions in vivo. In estrogen-deficient men, both insulin resistance and fasting glucose are increased and improve during estrogen treatment (150,158,161), confirming data from mice models (16). Thus, severe estrogen to testosterone ratio imbalance (increased androgens and decreased estrogens) seems to favor the development of insulin resistance in men (150,151,158), and not only in estrogen-deficient men (276). In healthy men, the administration of an aromatase inhibitor in a double blind, randomized, controlled, crossover study led to a decrease of insulin sensitivity (277). Several other clinical studies confirmed that aromatase inhibition worsens insulin sensitivity in older men (278), and in obese men, independently from the increase in serum testosterone (279). The same results come from studies comparing exogenous estradiol to GnRH analogues for the treatment of prostate cancer showing that fasting both fasting glucose and cholesterol decreased in men treated with estradiol (224).
In accordance with this findings, relative estrogen deficiency is found in men with type 2 diabetes mellitus and low serum testosterone who display also low serum estradiol (280).
Furthermore, congenital estrogen deficiency is associated with an altered lipid profile (22,161), mainly characterized by higher total cholesterol and triglycerides serum levels, higher low-density lipoprotein (LDL) cholesterol and very low high-density lipoprotein (HDL) cholesterol (11,135). In these patients, estradiol treatment induces a moderate increase of HDL-cholesterol together with a small reduction of triglycerides, total cholesterol, and LDL cholesterol (21,135,138,150), resembling the effects of estrogen on lipid metabolism exerted in females (10). The administration of aromatase inhibitors leads to no change in serum lipids after 12 months of therapy (278). Outside the context of rare congenital diseases, the effects of estrogens and antiestrogens on serum lipids remains not well established due to conflicting results, and additional studies are needed (24).
In community-based men aged 20-70 years, high serum E2 is associated with reduced carotid plaque and serum E2 is directly related to carotid intima media thickness (281). The T to E2 ratio was associated with increased atheromatous plaque inflammation and increased risk of subsequent major adverse cardiovascular events (MACE) in men with documented atherosclerotic disease (282). In an aromatase-deficient man the normalization of serum E2 induced by estradiol replacement was able to reduce carotid atherosclerotic plaque volume on ultrasonography (150). While serum E2 may exert protective effects against atherosclerosis, the serum T to E2 ratio maybe a marker of low serum T and increased adiposity, especially in overweight and obese men and may be associated with increased cardiovascular (CV) risk (282,283). However, these clinical studies suffer from serum E2 measurement with commercially available immunoassay that are unreliable for E2 values in the normal male serum range (41,42,131). An association between coronary artery calcification (a surrogate radiological markers of coronary atherosclerosis) and both low serum testosterone and low serum estradiol measured by liquid chromatography/tandem mass spectrometry (LC-MS/MS) has been found in men participating to the Framingham Heart Study (284).
In hypogonadal men, estrogen deficiency, but not testosterone deficiency is responsible for vasomotor symptoms (i.e., hot flushes). In men treated with an LHRH analogue and replaced with placebo, different dosages of T alone or T plus aromatase inhibitors, only estrogen deficiency resulted in the occurrence of vasomotor symptoms (285). The main role of estrogen deficiency in the occurrence of hot flushes has been also confirmed in men with prostate cancer treated with exogenous estradiol or LHRH analogue, being hot flushes significantly more prevalent in men with estrogen deficiency due to LHRH therapy. Taken together these results reinforce the concept that in men with hypogonadism several clinical manifestations are due to relative estrogen deficiency rather than to testosterone deficiency per se (285).
Estrogens and the Male Bone
There is increasing evidence suggesting that circulating estrogens plays a key role in bone health in men, as in women (286). The relative contribution of androgen versus estrogens in the regulation of the male skeleton, however, is complex and relatively unclear (287). Some estrogen actions on male bone, such as bone maturation and the acceleration of growth arrest, are now well defined (286,288). The important role of estrogen in bone metabolism in men has been characterized in the last 15 years by means of the description of rare case reports of estrogen-deficient men (135,152) and by several epidemiological studies (289,290). All patients with congenital estrogen deficiency due to estrogen resistance (19,133,134) or aromatase deficiency (20,136-149). have unfused epiphyses in adulthood and fail to reach their closure and complete bone maturation (11,18,25,130,286). Estrogen replacement therapy led to skeletal maturation and improvement of bone mineral density in all aromatase-deficient men described so far (11,135,144) in a dose-dependent way (147,154) while testosterone treatment did not (21,22,159). During puberty and late adolescence, epiphyseal closure, growth arrest, the achievement of peak bone mass, and final bone maturation are mainly under the control of estrogens and all these processes do not progress in the case of severe estrogen deficiency leading to tall stature and osteoporosis (21,22,135,286). The eunuchoid body proportions of the skeleton typical of hypogonadal men are the effect of estrogen deficiency during late adolescence and of the disproportional growth between long bones and the appendicular skeleton (11,286). During adulthood, both normal circulating estradiol and testosterone are required for maintaining bone mineral density in aromatase-deficient men (136,159,160) as well as in the general male population (132,286,287,289-292). Estrogen action on bone seems to be possible only when circulating estradiol is above a threshold between approximately 15 and 25 pg/mL (55-92 pmol/L) (286,289). This mechanism has been suggested both for growth arrest and bone maturation (152) and for bone mineral density (BMD) (132,289,292) and suggest that circulating estrogens above this threshold are required for optimal skeletal maturation and mineralization in men (130,286). Relative estrogen deficiency, rather than testosterone deficiency, is responsible for bone loss in hypogonadal men as clearly demonstrated when using different doses of exogenous testosterone alone or in combination with a potent aromatase inhibitor in men treated with GnRH analogues (293). In this study, BMD decreased and indices of bone resorption increased only in the group of men treated with both testosterone and anastrozole independently from the dose of exogenous testosterone administered to men with pharmacologically-induced hypogonadism (293). Furthermore, this study confirms that serum estradiol below 10 pg/mL is particularly harmful for bone health (293).
Similarly, in men with prostate cancer, estrogen deficiency induced by Androgen Deprivation Therapy (ADT) is the main factor involved in bone loss, increase of bone fragility, and the occurrence of osteoporotic fractures since serum estradiol falls below 5 pg/mL in men receiving ADT (294-296) similar to what happens in women taking antiestrogens for breast cancer (132,292,297). Accordingly, new strategies for the treatment of prostate cancer by using blockers of the androgen receptor and exogenous estradiol are being investigated in order to mitigate the risk of bone fractures and other side effects of ADT (224,295,296). In particular, transdermal estradiol resulted effective in preventing bone loss compared to LHRH analogue in men with prostate cancer (298).
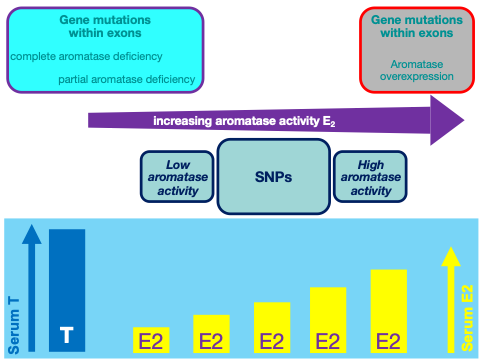
Figure 6. Genetically determined factors influencing the amount of serum estrogens in men starting from a determined amount of circulating serum T.
[SNPs: single-nucleotide polymorphisms; T: testosterone; DHT: dihydrotestosterone; E2: estradiol; GnRH: gonadotropin releasing hormone; LH: luteinizing hormone; FSH: follicle-stimulating hormone]
Except for rare cases of congenital estrogen deficiency (162), clinical experimental models of estrogen deficiency (199,285,293), and ADT (295,296), the most common condition in men is relative estrogen deficiency induced by hypogonadism and low serum levels of T, the precursor for estrogen production (Figure 1) (131,290,299,300). Relative estrogen deficiency may occur in hypogonadal men (299,300) especially in those with a less functioning aromatase enzyme (290,301), whose function is mainly under genetic determination (Figure 6) (131,132,292). The association between some polymorphisms of both ERα (302,303) and aromatase (304,305) genes and low bone mineral density (BMD) have clearly pointed out that the estrogen pathway is crucial for bone health in men confirming epidemiological and clinical data. These data have been recently confirmed by studies based on genome-wide association studies. These studies provided evidence on the role of aromatase enzyme in relative estrogen deficiency occurring in men with hypogonadism and its impact on BMD (Figure 6). Accordingly, a genome-wide association study demonstrated how some genetic variants of the aromatase enzyme are correlated with circulating serum E2 and BMD and that genetically determined values of circulating estradiol are linked to BMD by estimating that every 1 pg/mL of serum E2 corresponds to a genetically determined increase of BMD of 0.048 standard deviation (306). These results have been recently replicated by another GWAS study (307). Similarly, serum estradiol but not testosterone was identified as a causal factor in bone osteoporotic fractures in 175,583 men studied using a Mendelian randomization approach (308). Thus, the lower the serum estradiol the greater the extent of bone loss in hypogonadal men, the decrease of serum T exerting a direct, minor role (131,132,157,292,293). As a matter of fact, in male to female transexuals, high serum E2 induced by estrogen therapy maintains and increases bone mass despite low serum T (309,310). The degree or relative estrogen deficiency in hypogonadal men depends on several factors, including aromatase functioning (Figure6).
Estrogens, Body Composition, and Obesity in Men
The role of sex steroids action on body composition and adipose tissue metabolism in humans is long been recognized (311). Testosterone has been largely considered the main sex steroid involved in fat pathophysiology in men (311,312). However, it has become clear that estrogen also plays an important role in adipose tissue physiology in men since the first description of human cases of congenital estrogen deficiency (11,135,151) and the generation of knock-out mice models of estrogen deficiency (16,117,313,314) showing a phenotype of increased adiposity in spite of normal to increased serum testosterone.
Accordingly, both aromatase enzyme and ERs are expressed in the adipose tissue and in the skeletal muscle (12,18,24,25).
BODY COMPOSITION
Testosterone increases muscle mass and reduces fat mass in vivo (312). However, it is now clear that much these effects are due to its conversion into estradiol.
In the context of an experimental in vivo setting, healthy younger men treated with GnRH analogues and then substituted with different doses of exogenous testosterone alone together with placebo or in combination with an aromatase inhibitor, showed an increase in both subcutaneous and visceral fat mass mainly in presence of estrogen deficiency induced by the aromatase inhibitor (199). Similar results demonstrate that fat mass increases only in the group of healthy men with relative estrogen deficiency (below 15 pg/mL) induced pharmacologically by the short-term administration of GnRH analogue and aromatase inhibitors, but not in men with estradiol in the normal range (315). In accordance obese men treated with supraphysiological doses of transdermal testosterone or dutasteride showed an increase in fat mass only in the group taking an aromatase inhibitor compared to placebo (279).
Similarly, men treated with androgen deprivation therapy for prostate cancer increase their fat mass and weight and loose muscle mass (316-318). However, a double-blind randomized study tested the effects of 6 months of transdermal estradiol therapy in men on androgen deprivation therapy, and this study showed no beneficial effect of estradiol to prevent the increase of fat mass (319). This study was prematurely stopped due to Covid 19 pandemic, and the premature discontinuation might have resulted in insufficient power to detect a benefit (319). Estradiol therapy also seems to not influence fat-free mass and muscle size (199).
Congenital human models of sex steroids deficiency also support the importance of estrogen deficiency in the body fat mass increase since men with congenital estrogen deficiency show increased adiposity (11,135,150,151) and patients with complete androgen insensitivity syndrome do not accumulate fat mass even in absence of testosterone action thanks to a normal production of estrogens (320). The mechanisms through which estrogens modulate fat mass in men are not fully understood and several, different actions are involved and interlinked in a complex network.
ESTROGENS AND THE GH/IGF-1 AXIS
Estrogens modulate the GH/IGF-1 axis by enhancing both the GH and IGF-1 secretion in men (321). Thus, estrogen deficiency indirectly leads to body composition changes related to the inhibition of the GH-IGF-1 axis (321).
ESTROGEN ACTION ON ADIPOSE TISSUE
It is well known that the expression of the ER within adipose tissue is decreased in obese women (322) and that the deletion of the ERS1 gene encoding for the ER in adipocytes increases the adipocytes volume and total fat mass in both male and female rodents (323), but the underlying mechanisms needs to be fully ascertained.
Recently, the study performed in approximately 700 women and 800 men demonstrates that the expression of the estrogen receptor ESR1 within adipose tissue is inversely associated with abdominal fat mass and insulin insensitivity (324). Therefore, men with relative estrogen deficiency due to lower ESR1 expression in adipose tissue or low circulating estradiol, or both, tend to have higher fat mass and insulin resistance, thus pointing out on a main role of estrogens on weight and body composition in men. The reduction of ESR1 expression was associated with mitochondrial dysfunction of adipocytes, and these effects seem to be mediated by the reduction of expression of Polg1, a subunit of the polymerase enzyme involved in mitochondrial DNA replication and transcription (324). Reduced ESR1 expression in both white and brown adipocytes of ERKO mice (with deleted ESR1 gene) leads to increased fat in the former and reduced energy burning in the latter (324). Accordingly, in healthy adults of both sexes, serum E2 regulates brown adipose tissue thermogenesis, the latter being increased in women compared to men and associated with serum E2 (325,326).
ESTROGENS AND FEEDING BEHAVIOR
Sex hormones are able to influence adiposity in both men and women not only through peripheral mechanisms but also through a direct action on the brain (326,327) where they may modulate both energy balance and feeding behavior (328,329). Several data suggest that estrogens modulate energy balance at the hypothalamic level where they exert an anorexigenic action (326).
In addition to the energy expenditure, increasing evidence suggests that estrogens modulate appetitive behavior and food intake through their action on hedonic pathways operating at central level. Estrogens seem to lessen appetite and to reduce food intake. Recent studies have focused on the role of locally produced estrogens within the amygdala where its amount seems to be crucial for the regulation of feeding behavior. The amygdala is involved in the control of food intake both in humans and animals. In particular, functional imaging studies have demonstrated the activation of amygdala when images/videos of food or eating behavior are administered to volunteers and that functional dysregulation of this kind of activation is present in obese men and women, especially in hunger conditions (330). By measuring aromatase availability in the amygdala using positron emission tomography (PET) with the aromatase inhibitor [11C]vorozole in normal- weight, overweight, or obese men, Biegon et al. demonstrated that aromatase availability in the amygdala, but not circulating sex steroids, was inversely correlated to BMI (331). In particular, the aromatase inhibitor [11C]vorozole (a surrogate marker of aromatase expression/activity in vivo and of locally produced estrogens) was less available within the amygdala of obese men compared to healthy and normal-weight men (331). This suggests that locally produced estrogens within the amygdala can suppress eating behavior thus contributing to the modulation of weight gain since they facilitate the individual control of impulsive eating through cognitive/hedonic central effects within the brain (331).
Several actions of estrogens on food intake, energy homeostasis and body fat mass have been well characterized mainly in women (326) and till now the current knowledge was that estrogens are less important in men than in women in the control of these physiological functions, but recent evidence is changing this paradigm. In fact, all of the above findings point to an important role of estrogens on body composition, energy expenditure and control of feeding behavior in men and suggest that estrogen may represent a possible target to prevent and/or reverse weight gain in men (332)
Other Non-Reproductive Estrogen Functions in Men
During the last five years an increasing body of evidence suggest that estrogen may play a role on several other non-reproductive function in men. Among them the most investigated are cognitive function (333) and aging (334,335). However, at present more evidence is needed to confirm the putative role of estrogens on these functions in men.
EFFECTS OF ESTROGEN EXCESS
Effects of Exposure to Excess Estrogens in Animals
In order to evaluate the effect of estrogen excess on the reproductive tract, several studies have been performed in various animal species treated with diethylstilbestrol (DES), a synthetic, potent estrogenic compound (336). The period between 13 to 24 weeks of human fetal life corresponds with the highest susceptibility of male reproductive organs to endocrine disruptors (4,53,55,336). Many studies in rodents suggest that the inappropriate exposure to estrogen in utero and/or during the neonatal period impairs the hypothalamic-pituitary-gonadal axis, testicular descent, efferent ductule function and testicular function (26,31,176,178). The latter effect is a direct consequence of the exposure to estrogen excess, of the indirect effect of perturbations in circulating hormones, and of the ability of the efferent ductules to reabsorb fluid. It seems that ERβ may mediate the process through which excess estrogens produce negative effects on male reproduction (26,31,57). The effects of estrogen excess during the neonatal period can induce irreversible alterations of the testis that become manifest in adulthood, consisting of permanent changes in both testis function and spermatogenesis (26,31).
AROMATASE OVER EXPRESSION IN RODENTS
The transgenic model of mice overexpressing the aromatase enzyme (AROM+) exhibits highly elevated serum estradiol concentrations together with a decrease of serum testosterone levels due to gonadotropin suppression (211,337). The phenotypic abnormalities of AROM+ males are like those developed by mice that are perinatally exposed to estrogens. The most frequent abnormalities include: undescended testes, testicular interstitial cell hyperplasia, hypoandrogenism, and growth inhibition of accessory sex glands (211). The impairment of spermatogenesis observed in AROM+ may be due to multiple factors, including cryptorchidism, abnormal Leydig cell function, testosterone deficiency or hyperestrogenemia (211). Thus, estrogens are thought to inhibit Leydig cell development, growth and function, resulting in the final suppression of androgen production (26). Furthermore, the observation of numerous degenerating germ cells and the absence of spermatids within the seminiferous tubules of AROM+ mice suggest that germ cell development arrests at the pachytene spermatocyte stage (26). However, a possible role of cryptorchidism per se on germ cell arrest cannot be excluded since cryptorchidism is known to induce germ cell arrest in rodents (338). Interestingly, the spermatogenic arrest occurred at a stage where P450arom expression is generally high. The spermatogenic arrest found in the AROM+ mice could be explained, at least in part, by the suppression of FSH action (211,337). In fact, the reduced serum FSH levels associated with normal LH levels provide further evidence of the inhibiting actions of estrogens on FSH secretion in in AROM+ males (211,337).
Effects of Exposure to Excess Estrogens in Men
The observation that the clinical use of DES by pregnant women to prevent miscarriage is associated with a dramatic increase in the incidence of genital malformations in their sons represents the first evidence in humans on the potential for estrogen excess to provoke urogenital malformations (339). The most frequent structural and functional abnormalities include epididymal cysts, meatal stenosis, hypospadias, cryptorchidism and microphallus (339-341). The frequency of abnormalities is dependent on the timing of estrogen exposure; in fact, men who were exposed to DES before the 11th week of gestation (i.e. the time of Műllerian ducts formation) had a two-fold higher rate of abnormalities than those who were exposed later (339,341). These data support the hypothesis that the asynchrony between formation and regression of embryonic reproductive structures is probably strongly influenced by estrogen exposure.
Various reports demonstrated that semen quality of men exposed to DES in utero is significantly worse than in unexposed controls (342), even though sperm concentrations of most of these patients was average, with normal fertility (14). The implications for human spermatogenesis in terms of exposure to environmental estrogens remain less clear. The risk of testicular cancer among men exposed to DES in utero has been a controversial issue and several meta-analyses showed a doubling of testicular cancer risk, together with increased incidences of cryptorchidism, hypospadias, and impaired spermatogenesis (343). However, more direct evidence will be necessary in order to fully understand this issue and particularly to identify the exact estrogenic mechanism of action (343). It is clear that exogenous estrogens could interfere with the development of genital structures if administered during early organogenesis (341). The main effect is an impairment of gonadotropin secretion and the imbalance of estrogen to androgen ratio, which may account for impaired androgen receptor stimulation or inhibition according to the dose, the cell type and the timing of exposure (339,341). Furthermore, it seems that an excess of environmental estrogens could be a possible cause of impaired fertility in humans (176,177,341) since environmental estrogens are associated with an increased risk of subfertility in several studies (344). Although controversial, a proposed progressive decline in sperm count has been reported in some Western countries during the past 50 years, and has been suggested to involve negative effects of environmental contaminants, especially xenoestrogens, on male reproductive function(13,176,339,344).
In adult men the effects of estrogen excess are limited to rare causes of congenital aromatase overexpression and other rare conditions such as male to female transexuals taking exogenous estrogens.
AROMATASE OVER EXPRESSION IN HUMANS
Aromatase over-expression causes an increased conversion of androgens to estrogens with a consequent excess of estrogen. Excess estrogen in boys causes gynecomastia, a premature growth spurt, early fusion of epiphyses, and decreased adult height (162,345). Increased extraglandular aromatization was firstly reported in an adopted boy with prepubertal gynecomastia in 1977 (346). Four families were then described, in which several members had estrogen excess (manifested as gynecomastia in boys and men and premature thelarche in girls) due to increased extraglandular aromatization (347-349), and one case with a gain-of-function mutation of the aromatase gene (345). The latter seemed to be an autosomal dominant inherited disease (345,348). In adult men, elevated serum estradiol levels induce mild hypogonadotropic hypogonadism due to enhanced negative feedback on pituitary gonadotropins exerted by estrogens (162,345,348). This inhibitory effect of estrogen on reproductive function appears to be milder in males with aromatase excess syndrome than in patients receiving exogenous estrogens or having estrogen-secreting tumors, probably because serum estradiol and/or estrone levels are lower in the former (348). External genitalia in adult men with aromatase excess syndrome are characterized by normal penile and testicular size (162,345,348). This clinical reproductive phenotype has been observed also in other patients with aromatase excess syndrome due to gain-of-function mutations of the aromatase enzyme (162,350-352). Even though spermatogenesis and sexual behavior were not specifically studied, the adult men described were fertile and reported normal libido (345,348) and sperm count was normal in other studies (350). In these patients, treatment with an aromatase inhibitor reduces estrogen levels and normalized testosterone, LH and FSH serum levels (345,353), confirming a crucial role of estrogen in the suppression of both gonadotropins in men.
ESTROGEN EXCESS IN ADULT MEN
Klinefelter’s Syndrome has been classically considered a feminizing syndrome on the basis of signs (gynecomastia) and the observation of circulating estradiol higher than normal (354,355). In the literature, however, the data concerning hyperestrogenism in Klinefelter patients are not solid since they come from single case reports or studies using old assays for the measurement of serum estradiol. Data from mouse models of Klinefelter’s are not conclusive about the real increase of circulating estrogens and aromatase expression and activity (356). Infertility in these patients is mainly due to the genetic abnormalities rather than to the hormonal status (357). However, preliminary results from a recent meta-analysis does not confirm that is higher serum estradiol in Klinefelter’s patients compared with non-Klinefelter’s men, but show a condition of relative hyperestrogenism consisting with a slightly elevated estradiol to testosterone ratio in Klinefelter’s (358).
Most male to female transexuals who undergo exogenous estrogen therapy continue to have sperm production and spermatogenesis progresses even after a long period of therapy with estrogens. Histological analysis of the testes removed as part of gender affirmation procedures in 72 male to female transexuals on long -term estrogen therapy (>1 year) shows the presence of germ cells and spermatids in about 80% and 40% of cases, respectively; these percentages being inversely related to testes volume (359). In particular, a reduced diameter of seminiferous tubules, Sertoli and Leydig cells abnormalities consisting with glycoproteins accumulation, germ cells and Leydig cells hypoplasia, and down regulation of Era expression in the seminiferous tubules have all been found in testes of male to female transexuals under long-term estrogen therapy (360,361). All these changes may be associated to impaired spermatogenesis ranging from the absence of spermatozoa production (362) to various degrees of reduction in number of spermatozoa and spermatozoa precursors (spermatids) in the seminiferous tubules (360,361,363). Serum T suppression below 50 ng/dL results almost constantly in a complete suppression of spermatogenesis (362), thus incomplete suppression of spermatogenesis may be considered a marker of inadequate hormonal treatment due to underdosage or lack of patients’ adherence to therapy (360,363).
CLINICAL IMPLICATIONS OF ESTROGENS IN MALES
Diagnostic Aspects: Significance of Serum Estradiol in Men
Approximately 50 μg of estradiol are produced daily: about 5-10μg in the testis (10 to 20%) and the remaining 40-45 μg (80 to 90%) in peripheral tissues (adipose tissue, muscle, breast, brain liver and bone) in which the aromatase enzyme is expressed (4,130,131). In adult men, the normal range of serum estradiol is around 14-43 pg/mL (51-157 pmol/L), accordingly to different studies (300,364). Based on chromatography techniques, such as liquid chromatography-tandem mass spectrometry, progress has been made in the measurement of serum estrogens within the low and low-normal range of men thereby overcoming the unreliability of immunometric commercially available assays (44). In clinical practice, the measurement of serum E2 in men is mandatory when a congenital condition of estrogen deficiency is suspected (135,162). In particular, the clinical work-up for the evaluation of male infertility may involve the serum estradiol assay when clinical aspects suggestive for aromatase deficiency, coupled with normal to high testosterone and gonadotropins levels and/or history of cryptorchidism are present (Table 6) (365). Outside this clinical context, the measurement of serum E2 could be helpful to identify a condition of relative estrogen deficiency in men with hypogonadism and osteoporosis and hot flushes. However, the accuracy of most of the major commercially available kits for the detection of serum estradiol remains poor, especially for low serum levels of estradiol typical of the male range (1,41,42,366,367) leaving the measurement of serum E2 in men substantially not indicated in the clinic (132,292,297,358). Therefore, the assay of serum estradiol is suggested only if the method used in clinical laboratories has a very high sensitivity and specificity (e.g. 3rd generation RIA or some immunometric assays with an acceptable accuracy) (290,301,368-370). At present, the gold standard test for E2 measurement remains the gas chromatography/tandem mass spectrometry (41-44,366,367) and its progressive introduction in laboratories for clinical routine evaluations of sex steroids in recent years (1,42-44,371,372) allows precise and accurate sex steroids measurement in a clinical setting (371,372) allowing ruling in/ruling out relative estrogen deficiency in men (131,132,292) and keeping serum E2 in the normal range in hypogonadal men treated with testosterone (373,374). Recently, the results from the Testosterone Trials have pointed out the importance of serum estradiol for the outcomes of testosterone replacement therapy (375). Accordingly, changes in serum estradiol best predicted not only BMD increase (an expected result) but also other classical outcomes of testosterone therapy in hypogonadal men such as sexual desire, hemoglobin, and HDL cholesterol suggesting that serum estradiol assayed by LC-MS/MS may be a good clinical marker of adequate testosterone substitution (375).
Estrogens and Male Infertility: Clinical and Therapeutic Implications
Estrogens are involved in male fertility and could represent a potential factor involved in the pathogenesis of infertility as well as a possible pathway to explore new therapies for human male infertility.
ESTROGEN TREATMENT
At present there is no indication to prescribe estrogen compounds to men, except for the treatment of rare diseases such as congenital estrogen deficiency (130,135,365) or in the management of transgender patients. The increasing evidence of the existence of several testosterone actions that are mainly mediated by estrogens theoretically support the concept that tailoring estrogens in the treatment of hypogonadal men may improve the outcome in terms of benefits for patients (197,198). However, at present, there is no evidence on the effectiveness and safety of such therapeutic strategy. In the future, advances in the field of routine clinical measurement of very low amounts of circulating estrogens (1,42-44,371) will open new frontiers for testing the effect of estrogen compound or of SERMs alone or combined to androgens in men with documented mild estrogen deficiency.
ESTROGEN TREATMENT OF AROMATASE DEFICIENT MEN
The clinical features common to all aromatase-deficient men are: tall stature, delayed bone maturation, osteopenia/osteoporosis, eunuchoid skeleton, bone pain, and progressive genu valgum (11,131,135,286). Estrogen replacement treatment, at the daily dose of 0.22 to 0.35 μg/kg of transdermal estradiol in adult men, should be started as soon as the diagnosis of estrogen deficiency has been reached (131,135,365). When the diagnosis is available at birth, or is achieved during infancy, low dosages of exogenous estradiol should be administered at the beginning of puberty (0.8 to 0.12 μg/kg daily) (135,141). The main target of estrogen replacement therapy in these patients is the skeleton in order to promote epiphyseal closure, bone maturation and mineralization and the completion of these physiological processes on time. Accordingly, high doses of estrogen in adult men with aromatase deficiency might be used to lead a rapid completion of skeletal maturation within 6-9 months in adults with epiphyseal cartilages still open, through rapid bone elongation and an increase in height followed by quick epiphyseal closure and growth arrest (131,135,147,154,365). Once epiphyseal closure has been achieved, estrogen replacement treatment should be continued lifelong. The main goal is to prevent bone loss and to reduce the risk of cardiovascular disease. In this case, the dose of estradiol should be reduced to ensure serum estradiol within the normal range for adult men (131,135,147,154,365). Moreover, estrogen treatment in aromatase deficient men is effective in normalizing or improving other aspects such as gonadotropin secretion, bone mineral density, glucose metabolism, insulin sensitivity, liver function, and circulating lipids (131,143,146-148,150,151,157-159). When estrogen treatment is started at puberty, the effects of estrogen treatment on spermatogenesis are unknown, but the administration of estrogens in a more physiological way could theoretically be associated with normal spermatogenesis in adulthood. Conversely in adult patients, impaired spermatogenesis is irreversible even when estradiol treatment is administered (135). Other aspects related to estrogen deficiency cannot be modified by estrogen treatment when the treatment is started during adulthood (e.g. eunuchoid body proportions, genu valgum, failure in reaching the bone peak mass, normal body weight restoration) (135,151).
Finally, the real impact of estrogen treatment on sexual behavior in adult aromatase-deficient men remains to be determined (135).
ANTI-ESTROGEN TREATMENT IN MEN
As estrogens act on gonadotropic feedback inhibition (157,187,190), they could be a good target in the clinical management of male infertility. The rationale is to employ anti-estrogen drugs in order to modulate gonadotropin feedback by blocking the inhibitory effect exerted by estrogen on gonadotropins and to increase both LH and FSH. This will result in increased testosterone and FSH with potential benefits on spermatogenesis (376,377). However, the real effectiveness of this approach in treating male infertility remains to be established, since conflicting results are available (4,129,172,173,200,377-381) and this kind of treatment remains empirical and ‘off label‘ (200,376,378). Thus, the real efficacy of anti-estrogens is far from being elucidated and whether the increase of sperm density induced by anti-estrogens is actually related to a real improvement of both sperm fertility and pregnancy rates is a matter of debate (4,129,200,377) (Table 8).
Since the 1960s, anti-estrogen agents have been used as an empirical treatment of male infertility (378,382) based on their modulation of the hypothalamic-pituitary testicular axis. The main classes of drug that have been tested are aromatase inhibitors. They are the most potent blockers of the estrogen-mediated negative feedback on gonadotropins and excites LH and FSH secretion aiming to stimulate spermatogenesis (383). However, no clear evidence of direct effects of anti-estrogens on spermatogenesis exists (200,376,383), but LH and FSH serum levels generally increase during aromatase inhibitor administration in infertile men (384).
Clomiphene at a dosage of 25-50 mg daily for 3-12 months, or tamoxifen at dosage of 20-30 mg daily for 3-6 months, represent the most frequently used anti-estrogen agents for the treatment of male infertility (385) (Table 8); on the contrary the new generation of selective estrogen receptor modulators does not result in significant changes in male fertility (386) (Table 8).
|
Table 8. Dosages and Time Duration of Oral Anti-Estrogen and Aromatase Inhibitors Used in Male Infertility and Their Different Effects on Semen Analysis |
|||
|
Treatment |
Dose (mg/daily) |
Duration (months) |
Effects on semen analysis |
|
Anti-estrogens |
|
|
|
|
Clomiphene |
25-50 |
3-12 |
Semen volume: No effect or ↑ Total sperm number: No effect or ↑ Sperm concentration: No effect or ↑ Sperm motility: No effect or ↑ Sperm morphology: No effect or ↑ |
|
Tamoxifen |
20-30 |
3-6 |
Semen volume: No effect Total sperm number: No effect Sperm concentration: No effect or ↑ Sperm motility: No effect Sperm morphology: No effect |
|
Tamoxifen and Testosterone undecanoate |
20 120 orally |
6 |
Semen volume: No effect Total sperm number: ↑ Sperm concentration: No effect Sperm motility: ↑ Sperm morphology: ↑ |
|
Aromatase inhibitors |
|
|
|
|
Testolactone |
2000 |
8 |
No effect |
|
Testolactone or Anastrozole |
100-200 |
6 |
Semen volume: ↑ Total sperm number: ↑ Sperm concentration: ↑ Sperm motility: ↑ Sperm morphology: ↑ |
|
Letrozole |
2,5 |
6 |
Semen volume: No effect Total sperm number: ↑ Sperm concentration: ↑ Sperm motility: ↑ Sperm morphology: No effect |
The use of these drugs is still off-label.
Clomiphene (25-50 mg/day) has been recently studied in a cohort of 86 men with hypogonadism for six months (387). This treatment represented an effective and apparently safe alternative to testosterone supplementation in hypogonadal men wishing to preserve their fertility (387). Furthermore, Ghanem et al. have recently found that combined treatment with clomiphene (25 mg/day) and an antioxidant drug (vitamin E) increased the pregnancy rate and improved sperm count and progressive motility in men with idiopathic oligoasthenozoospermia (388). More or less these data have been confirmed by several studies, most of them being retrospective or observational (389-391), with few RTCs studies available (392,393). Notwithstanding the improvement of sperm parameters in a variable percentage of men with infertility (393), a recent systematic review points out a possible impairment of sperm parameters (a decrease in semen count, concentration, motility, morphology and total motile sperm count) in up to 20% of patients treated with clomiphene citrate, this impairment of sperm remaining irreversible in 17% among men who had a decline in semen parameters after therapy discontinuation (394). In men with secondary hypogonadism treated with testosterone, enclomiphene (the transisomer of clomiphene) was able to prevent gonadotropin suppression and the related oligospermia compared to placebo (395) and these preliminary data have been confirmed by other studies (396)(Table 8). Clomiphene may be used off-label, but enclomiphene has not been approved by regulatory agencies and its use is limited to experimental trials (396).
Tamoxifen (20 mg/day) has been also used in combination with oral testosterone undecanoate (120 mg/day) in men affected by idiopathic oligozoospermia. This combined treatment was effective in improving not only the sperm parameters (total sperm number, sperm morphology and motility), but also the pregnancy rate (397). In 2012, Moein et al. studied thirty-two azoospermic infertile men with proven non-obstructive azoospermia, administrating Tamoxifen for 3 months (398). Tamoxifen treatment led to the recovery of spermatozoa in the ejaculates of six patients (398). These studies showed that treatment of patients with non-obstructive azoospermia with anti-estrogenic drugs like tamoxifen can improve the results of sperm recovery in testis samples and also increase the chance of pregnancy by microinjection. Also other non-controlled trials suggest improvements in sperm quality or sperm concentration(399,400), however, no well-performed clinical trial has confirmed these results (376), except for one RCT comparing tamoxifen alone and tamoxifen plus folate with placebo confirming that tamoxifen increased sperm concentration in men with sperm abnormalities (399) (Table 8). A recent meta-analysis including a very small number of studies supports the empirical use of the estrogen antagonists clomiphene and tamoxifen at the dose of 50 mg and 20 to 30 mg daily based on the finding of the detection of a doubling rate of pregnancy outcome among men with idiopathic infertility (401). The uncertain role of these therapies on male fertility may be related to the fact that idiopathic oligozoospermia constitutes a group of heterogeneous disorders of which only a subgroup might respond to anti-estrogen therapy. However, studies have failed to identify the characteristics of this subgroup and thus physicians cannot distinguish potential responders and non-responders (376).
Few data are available on the effect of aromatase inhibitors in male infertility (Table 8). An old study failed to demonstrate the efficacy of testolactone in the treatment of idiopathic oligozoospermic infertility (384). However, when aromatase inhibitors (testolactone or anastrozole) were administered in a selected group of infertile men with abnormal baseline testosterone-to-estradiol ratio, an improvement of fertility rate was generally obtained (172). In particular, letrozole treatment improved semen parameters and estradiol to testosterone imbalance in patients with low testosterone and increased estradiol to testosterone ratio (381). In 2011, Saylam et al. treated 27 infertile, hypogonadotropic men with 2.5 mg daily of letrozole for six months, finding an improvement of both testosterone serum levels and semen parameters after treatment (173). Thus, it seems that letrozole may facilitate some improvement in infertile men with azoospermia by improving the number of sperm in the ejaculate (173). Accordingly, a further study on the effects of letrozole on sperm parameters showed that letrozole but not placebo was effective in increasing sperm count and improving sperm motility after 6 months of treatment in a small group of 46 patients (22 on letrozole; 24 on placebo) who were azoospermic or cryptozoospermic at baseline (402) (Table 8). These results have been also replicated by not controlled studies (403-407). However, positive effects of aromatase inhibitors on sperm concentration and quality comes mainly from case series, retrospective, and cross-sectional studies, thus leaving the strength of evidence concerning the aromatase inhibitors efficacy on male fertility of low grade (200,201,377,408).
In men with hypogonadism, antiestrogens have the advantage to be effective in increasing serum T without suppressing gonadotropins if compared with testosterone replacement therapy, thus preserving spermatogenesis (200,201,377).
Data concerning the safety of anti-estrogens for treatment of male infertility are scant, especially as far as long-term treatment is concerned (195,196,376,377,409-411). Safety data regarding the use of clomiphene and tamoxifen for male infertility is limited, but information available supports their safety (410,411), the latter might be also derived indirectly from small groups of men with breast cancer (412). Conversely, more data are available on aromatase inhibitors (195,196,377). Six months of therapy with letrozole seems to not affect psychometric tests, glucose tolerance, serum circulating lipids, markers of bone turnover, and body composition, including BMD, in obese, hypogonadal men(413). In this study, however, moderate aromatase inhibition resulted in serum estradiol still within the normal male range and all the outcomes were obtained after a short period of treatment (413). In the literature, opposite results are available and suggest possible undesired effects of aromatase inhibitors, especially on metabolism and bone. Evidence exists that high-dose aromatase inhibition might lead to several side effects, especially when patients are treated for more than 12 months with an aromatase inhibitor (195,196). Both very short-term and short-term treatment with aromatase inhibitors had deleterious metabolic effects: one study demonstrated a prompt worsening of both insulin sensitivity and lipid profile in young and older men after 28 days of treatment with letrozole (414), while anastrozole reduced insulin sensitivity in healthy men after 6 weeks of treatment (338). In the case of longer treatments (with outcomes obtained after 1 year) vertebral deformities (415) and decreased BMD (173,197,416) were found in young and older men, respectively. In addition, treatment with aromatase inhibitors lowered HDL-cholesterol in peripubertal boys (417) and in both adult and older men (195,414) while data on total and LDL-cholesterol are conflicting (277,338,414). Data available from a very small subset of male patients operated on for male breast cancer and treated with anti-estrogens (most of them with tamoxifen) provides data on long-term effects and major adverse events (412). The authors concluded that side effects and major adverse events did not differ between men and women taking anti-estrogens and that cerebrovascular or coronary events, thromboembolic events (deep venous thrombosis)(418,419), depression, muscle cramps, and hot flashes might occur also in men during anti-estrogens treatment, hot flashes being the most frequent (411,412). These data, however, should be regarded with caution due to the small sample size, the lack of a control group, and the difficulties in proving a cause-effect relationship between major adverse events and the use of anti-estrogens in men. Besides, most of the studies on antiestrogens in men are based on short-term therapy, thus safety data for long-term therapy are not available.
As a result, it should be remarked that none of the drugs belonging to the category of anti-estrogens (i.e. clomiphene, tamoxifen, aromatase inhibitors) is approved for the indication of the treatment of male infertility by regulatory drug agencies (e.g. FDA, EMEA, TPD and TGA Regulations) nor is recommended by guidelines provided by Scientific Societies (e.g. NICE, ASA, EAA, SIAMS etc.) for use in idiopathic infertility (420,421). At present, all the data available on anti-estrogens in male infertility comes from the use of these drugs for research purposes outside the context of clinical practice. In addition, none of the studies are of adequate design, strength, and power. For all these reasons, their clinical use remains anecdotal and is off label (195,196,200,201).
In conclusion anti-estrogens, alone or in combination with testosterone, may represent a potential therapy for idiopathic oligozoospermia, however this remains an empirical off-label treatment (376,401). The data set does not yet provide sufficient evidence for these applications, but there is suggestive evidence that encourages further study (401). Further well-designed studies on adequate sample size (and homogeneous groups of men with infertility) are needed to detect their true efficacy in improving the pregnancy rate, or to identify the features of the responders.
FUTURE DIRECTIONS
Notwithstanding consistent advancements in the comprehension of estrogen role in men the pathophysiology of estrogens in males remains not fully explored and further studies are advocated. Preliminary data suggest that the decline in serum estradiol is associated with all-cause mortality in community-dwelling older men, but further investigations are needed to prove this relationship (422). In addition, the knowledge of the role of estrogen-related genetic determinants in male pathophysiology is still in its infancy. In recent years some research based on genome-wide association studies (GWAS) have provided new insights to this field (76,306-308,423). GWAS are useful in examining unexplored areas concerning physiological and pathological actions and related genetic determinants (Figure 6). Concerning estrogens in men a recent GWAS allowed disclosing more details on the association between low serum estradiol and increased adiposity and showed that higher estradiol levels were associated with lower adiposity (423). Thus, GWAS are promising for disclosing new important aspects related to estrogen pathophysiology in men and for improving the knowledge of genetic determinants of estrogens in health and disease. Advancement in the comprehension of individual genetically determined estrogen actions (Figure 6) together with the diffusion of LC-MS/MS in clinical laboratories allowing precise measurement of estrogens in men will pave the way to better tailor the management and therapy of both estrogen deficiency and excess in the human male.
CONCLUSIONS
Sex steroids account for sexual dimorphism because they are responsible for the establishment of primary and secondary sexual characteristics, which are under the control of androgens and estrogens in male and female, respectively. Advances in the understanding of the role of estrogens in animal and human models suggest a role for this sex steroid in the reproductive function of both sexes. The fact that both estrogen excess and estrogen deficiency influence male sexual development, testis function, the hypothalamic-pituitary-testis axis, spermatogenesis and ultimately male fertility, highlight the biological importance of estrogen action in males. Thus, estrogens, not only androgens, are responsible for some crucial physiological functions in men like fertility, reproduction, and bone health. In particular, the balance of serum estradiol to testosterone ratio is likely crucial for maintaining all these functions, thus suggesting that the homeostatic equilibrium between estrogens and androgens is important for the correct functioning of several physiological systems in men (11,22,34,121,158,172,381). From an evolutionary perspective, this relevance of estrogen actions in males provides an example of the parsimony operating in biological events that are crucial for the evolution of the human species such as growth and reproduction (Figure 7).
This chapter has addressed the reproductive effects of estrogens in males but there are emerging roles for estrogens in non-reproductive tissues. In particular, even though testosterone has traditionally been considered the sex hormone involved in bone maturation and growth arrest in men the key role of estrogens on growth has recently been revealed.
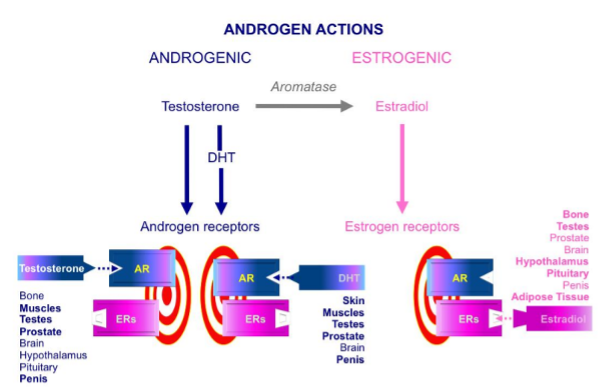
Figure 7. Direct and indirect (estrogen mediated) testosterone actions.
[DHT: dihydrotestosterone, AR: androgen receptor; ERs: estrogen receptors]
A major area of uncertainty is the possible role of estrogen in boys before puberty. It is known that low levels of circulating estradiol are detected in infancy when using ultrasensitive assays, but their significance is not known (130).
Several lines of evidence support the view that estrogens are required for, and in part mediate, androgen actions on several tissues and organs in men (Figure 7). The progress made in the last thirty years in this field have clarified the importance of estrogen in men but leaves some issues still unsolved. In particular, estrogen actions on bone and on gonadotropin secretion are now well characterized and part of the estrogen action on spermatogenesis is known, but further evidence is needed to clarify several aspects still under debate.
ACKNOWLEDGMENTS
We are indebted to Kenneth Korach S. (National Institutes of Health, Research Triangle Park, NC, United States), Evan Simpson (Hudson Institute of Medical Research, Clayton, Australia), Laura Maffei (Buenos Aires, Argentina) for their collaboration with our group in this research field.
A special thanks to Marco Faustini-Fustini (Ospedale Bellaria, Bologna, Italy), Antonio Balestrieri (Ospedale Bufalini, Cesena, Italy), Antonio R.M. Granata (University of Modena and Reggio Emilia), Elisa Pignatti (University of Modena and Reggio Emilia), Fabio Lanfranco (University of Turin, Italy), and Paolo Beck-Peccoz (Univesity of Milan, Italy) for fruitful discussion and collaboration in the research area of estrogen role in the human male.
We are grateful to Bruno Madeo, MD, PhD, Chiara Diazzi, MD, PhD Lucia Zirilli, MD, PhD Daniele Santi MD, PhD (University of Modena and Reggio Emilia) for their contribution in revising some parts of the previous version of this chapter (November 24, 2016).
REFERENCES
- Simpson E, Santen RJ. Celebrating 75 years of oestradiol. Journal of molecular endocrinology. 2015;55(3):T1-20.
- Hess RA, Cooke PS. Estrogen in the male: a historical perspective. Biology of reproduction. 2018;99(1):27-44.
- Zondek B. Mass excretion of oestrogenic hormone in the urine of the stallion. Nature. 1934;133(3354):209-210.
- Rochira V, Granata AR, Madeo B, Zirilli L, Rossi G, Carani C. Estrogens in males: what have we learned in the last 10 years? Asian journal of andrology. 2005;7(1):3-20.
- Steinach E, Kun H. Transformation of male sex hormones into a substance with the action of a female hormone. The Lancet. 1937;230(5954):845.
- MacDonald PC, Madden JD, Brenner PF, Wilson JD, Siiteri PK. Origin of estrogen in normal men and in women with testicular feminization. The Journal of clinical endocrinology and metabolism. 1979;49(6):905-916.
- Wolff E, Ginglinger A. Sur la transformation des poulets mâles en intersexués par injection d'hormone femelle (folliculine) aux embryons: avec 16 figures dans le texte. Ed. de la Librairie" Union.
- Johnson KJ, Heger NE, Boekelheide K. Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicological sciences : an official journal of the Society of Toxicology.2012;129(2):235-248.
- Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J, Clyne CD. Estrogen--the good, the bad, and the unexpected. Endocrine reviews. 2005;26(3):322-330.
- Deroo BJ, Korach KS. Estrogen receptors and human disease. The Journal of clinical investigation.2006;116(3):561-570.
- Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. The Journal of steroid biochemistry and molecular biology. 2008;109(3-5):212-218.
- Blakemore J, Naftolin F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology (Bethesda, Md). 2016;31(4):258-269.
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet (London, England). 1993;341(8857):1392-1395.
- Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL. Fertility in men exposed prenatally to diethylstilbestrol. The New England journal of medicine. 1995;332(21):1411-1416.
- Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reproductive biology. 2014;14(1):3-8.
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine reviews. 1999;20(3):358-417.
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine reviews. 1994;15(3):342-355.
- Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in Male Physiology. Physiological reviews.2017;97(3):995-1043.
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. The New England journal of medicine. 1994;331(16):1056-1061.
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. The Journal of clinical endocrinology and metabolism. 1995;80(12):3689-3698.
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. The New England journal of medicine.1997;337(2):91-95.
- Faustini-Fustini M, Rochira V, Carani C. Oestrogen deficiency in men: where are we today? European journal of endocrinology / European Federation of Endocrine Societies. 1999;140(2):111-129.
- Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiological reviews. 2017;97(1):135-187.
- Russell N, Grossmann M. MECHANISMS IN ENDOCRINOLOGY: Estradiol as a male hormone. European journal of endocrinology / European Federation of Endocrine Societies. 2019;181(1):R23-r43.
- Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. The Journal of clinical investigation. 2019;129(5):1818-1826.
- O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocrine reviews.2001;22(3):289-318.
- Hess RA. Estrogen in the adult male reproductive tract: a review. Reproductive biology and endocrinology : RB&E. 2003;1:52.
- Carreau S, Silandre D, Bourguiba S, Hamden K, Said L, Lambard S, Galeraud-Denis I, Delalande C. Estrogens and male reproduction: a new concept. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al]. 2007;40(6):761-768.
- Carreau S, Bouraima-Lelong H, Delalande C. Role of estrogens in spermatogenesis. Frontiers in bioscience (Elite edition). 2012;4:1-11.
- Schulster M, Bernie AM, Ramasamy R. The role of estradiol in male reproductive function. Asian journal of andrology. 2016;18(3):435-440.
- Sharpe RM. The roles of oestrogen in the male. Trends in endocrinology and metabolism: TEM. 1998;9(9):371-377.
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews. 2011;32(1):81-151.
- Baker ME. What are the physiological estrogens? Steroids. 2013;78(3):337-340.
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. The New England journal of medicine. 2002;346(5):340-352.
- Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77(1-2):27-35.
- Ghayee HK, Auchus RJ. Clinical implications of androgen synthesis via 5alpha-reduced precursors. Endocrine development. 2008;13:55-66.
- Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, Hass BS, Xie Q, Dial SL, Moland CL, Sheehan DM. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chemical research in toxicology. 2001;14(3):280-294.
- Amir S, Shah STA, Mamoulakis C, Docea AO, Kalantzi OI, Zachariou A, Calina D, Carvalho F, Sofikitis N, Makrigiannakis A, Tsatsakis A. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int J Environ Res Public Health. 2021;18(4).
- Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457(7226):219-223.
- Lo J, Di Nardo G, Griswold J, Egbuta C, Jiang W, Gilardi G, Ghosh D. Structural basis for the functional roles of critical residues in human cytochrome p450 aromatase. Biochemistry. 2013;52(34):5821-5829.
- Rosner W. Free estradiol and sex hormone-binding globulin. Steroids. 2015;99(Pt A):113-116.
- Demers LM, Hankinson SE, Haymond S, Key T, Rosner W, Santen RJ, Stanczyk FZ, Vesper HW, Ziegler RG. Measuring Estrogen Exposure and Metabolism: Workshop Recommendations on Clinical Issues. The Journal of clinical endocrinology and metabolism. 2015;100(6):2165-2170.
- Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72(8):666-671.
- Santen RJ, Demers LM, Ziegler RG. Workshop on measuring estrogen exposure and metabolism: Summary of the presentations. Steroids. 2015;99(Pt A):1-7.
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiological reviews.2007;87(3):905-931.
- Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135-170.
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocrine reviews. 2007;28(7):726-741.
- Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology.2011;152(12):4489-4495.
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annual review of physiology. 2008;70:165-190.
- Luconi M, Muratori M, Forti G, Baldi E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. The Journal of clinical endocrinology and metabolism. 1999;84(5):1670-1678.
- Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G. Nongenomic activation of spermatozoa by steroid hormones: facts and fictions. Molecular and cellular endocrinology. 2009;308(1-2):39-46.
- Hess RA. Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segment of the epididymis. Spermatogenesis. 2014;4(2):e979103.
- Li X, Li H, Jia L, Li X, Rahman N. Oestrogen action and male fertility: experimental and clinical findings. Cellular and molecular life sciences : CMLS. 2015;72(20):3915-3930.
- Carreau S, Bouraima-Lelong H, Delalande C. Estrogens in male germ cells. Spermatogenesis. 2011;1(2):90-94.
- Rochira V, Balestrieri A, Madeo B, Baraldi E, Faustini-Fustini M, Granata AR, Carani C. Congenital estrogen deficiency: in search of the estrogen role in human male reproduction. Molecular and cellular endocrinology.2001;178(1-2):107-115.
- Luconi M, Forti G, Baldi E. Genomic and nongenomic effects of estrogens: molecular mechanisms of action and clinical implications for male reproduction. The Journal of steroid biochemistry and molecular biology.2002;80(4-5):369-381.
- Carreau S, de Vienne C, Galeraud-Denis I. Aromatase and estrogens in man reproduction: a review and latest advances. Advances in medical sciences. 2008;53(2):139-144.
- Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991;128(6):2874-2879.
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. Journal of andrology.1997;18(6):602-611.
- Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS. Estrogen and its receptors in efferent ductules and epididymis. Journal of andrology. 2011;32(6):600-613.
- van Pelt AM, de Rooij DG, van der Burg B, van der Saag PT, Gustafsson JA, Kuiper GG. Ontogeny of estrogen receptor-beta expression in rat testis. Endocrinology. 1999;140(1):478-483.
- Tao K, Sun Y, Chao Y, Xing L, Leng L, Zhou D, Zhu W, Fan L. β-estradiol promotes the growth of primary human fetal spermatogonial stem cells via the induction of stem cell factor in Sertoli cells. J Assist Reprod Genet. 2021.
- Cripps SM, Mattiske DM, Black JR, Risbridger GP, Govers LC, Phillips TR, Pask AJ. A loss of estrogen signaling in the aromatase deficient mouse penis results in mild hypospadias. Differentiation. 2019;109:42-52.
- Baskin L, Cao M, Sinclair A, Li Y, Overland M, Isaacson D, Cunha GR. Androgen and estrogen receptor expression in the developing human penis and clitoris. Differentiation. 2020;111:41-59.
- Govers LC, Phillips TR, Mattiske DM, Rashoo N, Black JR, Sinclair A, Baskin LS, Risbridger GP, Pask AJ. A critical role for estrogen signaling in penis development. Faseb j. 2019;33(9):10383-10392.
- Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. The Journal of endocrinology. 1997;153(3):485-495.
- Macheroni C, Lucas TFG, Porto CS. The role of estrogen receptors in rat Sertoli cells at different stages of development. Heliyon. 2020;6(11):e05363.
- Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, Franca LR, Hess RA. Differential hormonal regulation of estrogen receptors ERalpha and ERbeta and androgen receptor expression in rat efferent ductules. Reproduction (Cambridge, England). 2004;128(1):73-86.
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390(6659):509-512.
- Chimento A, Sirianni R, Delalande C, Silandre D, Bois C, Ando S, Maggiolini M, Carreau S, Pezzi V. 17 beta-estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ER alpha. Molecular and cellular endocrinology. 2010;320(1-2):136-144.
- Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63(10):498-504.
- Mowa CN, Iwanaga T. Expression of estrogen receptor-alpha and -beta mRNAs in the male reproductive system of the rat as revealed by in situ hybridization. Journal of molecular endocrinology. 2001;26(3):165-174.
- Saunders PT, Fisher JS, Sharpe RM, Millar MR. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. The Journal of endocrinology. 1998;156(3):R13-17.
- Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. The Journal of clinical endocrinology and metabolism. 2000;85(12):4835-4840.
- Dumasia K, Kumar A, Deshpande S, Balasinor NH. Estrogen signaling, through estrogen receptor β, regulates DNA methylation and its machinery in male germ line in adult rats. Epigenetics. 2017;12(6):476-483.
- Raut S, Kumar AV, Khambata K, Deshpande S, Balasinor NH. Genome-wide identification of estrogen receptor binding sites reveals novel estrogen-responsive pathways in adult male germ cells. Biochem J.2020;477(12):2115-2131.
- Chimento A, Sirianni R, Casaburi I, Pezzi V. GPER Signaling in Spermatogenesis and Testicular Tumors. Frontiers in endocrinology. 2014;5:30.
- Vaucher L, Funaro MG, Mehta A, Mielnik A, Bolyakov A, Prossnitz ER, Schlegel PN, Paduch DA. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PloS one. 2014;9(4):e92425.
- Lucas TF, Pimenta MT, Pisolato R, Lazari MF, Porto CS. 17beta-estradiol signaling and regulation of Sertoli cell function. Spermatogenesis. 2011;1(4):318-324.
- Lucas TF, Royer C, Siu ER, Lazari MF, Porto CS. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biology of reproduction. 2010;83(2):307-317.
- Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Ando S, Maggiolini M, Pezzi V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149(10):5043-5051.
- Chimento A, Sirianni R, Zolea F, Bois C, Delalande C, Ando S, Maggiolini M, Aquila S, Carreau S, Pezzi V. Gper and ESRs are expressed in rat round spermatids and mediate oestrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. International journal of andrology. 2011;34(5 Pt 1):420-429.
- Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biology of reproduction. 1998;58(4):919-926.
- Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Millette CF, Osawa Y, Shizuta Y, Toda K, Bahr JM. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132(3):1396-1401.
- Cavaco JE, Laurentino SS, Barros A, Sousa M, Socorro S. Estrogen receptors alpha and beta in human testis: both isoforms are expressed. Systems biology in reproductive medicine. 2009;55(4):137-144.
- Cunha GR, Li Y, Mei C, Derpinghaus A, Baskin LS. Ontogeny of estrogen receptors in human male and female fetal reproductive tracts. Differentiation. 2021;118:107-131.
- Boukari K, Ciampi ML, Guiochon-Mantel A, Young J, Lombes M, Meduri G. Human fetal testis: source of estrogen and target of estrogen action. Human reproduction (Oxford, England). 2007;22(7):1885-1892.
- Hess RA, Sharpe RM, Hinton BT. Estrogens and development of the rete testis, efferent ductules, epididymis and vas deferens. Differentiation. 2021;118:41-71.
- Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, Rivarola MA, Belgorosky A. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatric research. 2006;60(6):740-744.
- Kolasa A, Wiszniewska B, Marchlewicz M, Wenda-Rozewicka L. Localisation of oestrogen receptors (ERalpha and ERbeta) in the human and rat epididymides. Folia morphologica. 2003;62(4):467-469.
- Lambard S, Galeraud-Denis I, Saunders PT, Carreau S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. Journal of molecular endocrinology. 2004;32(1):279-289.
- Aquila S, Sisci D, Gentile M, Middea E, Catalano S, Carpino A, Rago V, Ando S. Estrogen receptor (ER)alpha and ER beta are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. The Journal of clinical endocrinology and metabolism.2004;89(3):1443-1451.
- Aquila S, De Amicis F. Steroid receptors and their ligands: effects on male gamete functions. Experimental cell research. 2014;328(2):303-313.
- Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Human reproduction (Oxford, England). 2005;20(12):3481-3487.
- Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. The Journal of clinical endocrinology and metabolism. 2002;87(6):2706-2715.
- Aschim EL, Saether T, Wiger R, Grotmol T, Haugen TB. Differential distribution of splice variants of estrogen receptor beta in human testicular cells suggests specific functions in spermatogenesis. The Journal of steroid biochemistry and molecular biology. 2004;92(1-2):97-106.
- Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic acids research. 1998;26(15):3505-3512.
- Oliveira PF, Alves MG, Martins AD, Correia S, Bernardino RL, Silva J, Barros A, Sousa M, Cavaco JE, Socorro S. Expression pattern of G protein-coupled receptor 30 in human seminiferous tubular cells. General and comparative endocrinology. 2014;201:16-20.
- Rago V, Giordano F, Brunelli E, Zito D, Aquila S, Carpino A. Identification of G protein-coupled estrogen receptor in human and pig spermatozoa. Journal of anatomy. 2014;224(6):732-736.
- Chimento A, De Luca A, Nocito MC, Avena P, La Padula D, Zavaglia L, Pezzi V. Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis. Cells. 2020;9(9).
- Carreau S. Germ cells: a new source of estrogens in the male gonad. Molecular and cellular endocrinology.2001;178(1-2):65-72.
- Carreau S, Bourguiba S, Lambard S, Galeraud-Denis I, Genissel C, Bilinska B, Benahmed M, Levallet J. Aromatase expression in male germ cells. The Journal of steroid biochemistry and molecular biology.2001;79(1-5):203-208.
- Lambard S, Galeraud-Denis I, Bouraima H, Bourguiba S, Chocat A, Carreau S. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Molecular human reproduction. 2003;9(3):117-124.
- Meeker JD, Singh NP, Hauser R. Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. Journal of andrology. 2008;29(4):379-388.
- Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. The Journal of clinical endocrinology and metabolism. 2000;85(5):2057-2067.
- Carreau S, Bouraima-Lelong H, Delalande C. Estrogens: new players in spermatogenesis. Reproductive biology. 2011;11(3):174-193.
- Aschim EL, Giwercman A, Stahl O, Eberhard J, Cwikiel M, Nordenskjold A, Haugen TB, Grotmol T, Giwercman YL. The RsaI polymorphism in the estrogen receptor-beta gene is associated with male infertility. The Journal of clinical endocrinology and metabolism. 2005;90(9):5343-5348.
- Guarducci E, Nuti F, Becherini L, Rotondi M, Balercia G, Forti G, Krausz C. Estrogen receptor alpha promoter polymorphism: stronger estrogen action is coupled with lower sperm count. Human reproduction (Oxford, England). 2006;21(4):994-1001.
- Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science (New York, NY).1994;266(5190):1524-1527.
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(23):11162-11166.
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology.1996;137(11):4796-4805.
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15677-15682.
- Sharma G, Prossnitz ER. GPER/GPR30 Knockout Mice: Effects of GPER on Metabolism. Methods in molecular biology (Clifton, NJ). 2016;1366:489-502.
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biology of reproduction. 2009;80(1):34-41.
- Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154(11):4136-4145.
- Robertson KM, O'Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):7986-7991.
- Murata Y, Robertson KM, Jones ME, Simpson ER. Effect of estrogen deficiency in the male: the ArKO mouse model. Molecular and cellular endocrinology. 2002;193(1-2):7-12.
- Sinkevicius KW, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL. Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology.2009;150(6):2898-2905.
- Yao G, Hu S, Yu L, Ru Y, Chen CD, Liu Q, Zhang Y. Genome-Wide Mapping of In Vivo ERα-Binding Sites in Male Mouse Efferent Ductules. Endocrinology. 2017;158(11):3724-3737.
- Chimento A, Sirianni R, Casaburi I, Pezzi V. Role of estrogen receptors and g protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis. Frontiers in endocrinology.2014;5:1.
- Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. The Journal of clinical endocrinology and metabolism. 1999;84(12):4677-4694.
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):6965-6970.
- Donaldson KM, Tong SY, Washburn T, Lubahn DB, Eddy EM, Hutson JM, Korach KS. Morphometric study of the gubernaculum in male estrogen receptor mutant mice. Journal of andrology. 1996;17(2):91-95.
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proceedings of the National Academy of Sciences of the United States of America.2001;98(24):14132-14137.
- Lee KH, Finnigan-Bunick C, Bahr J, Bunick D. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta. Biology of reproduction. 2001;65(5):1534-1541.
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biology of reproduction. 2000;63(6):1873-1880.
- Mahato D, Goulding EH, Korach KS, Eddy EM. Spermatogenic cells do not require estrogen receptor-alpha for development or function. Endocrinology. 2000;141(3):1273-1276.
- Kumar A, Dumasia K, Deshpande S, Raut S, Balasinor NH. Delineating the regulation of estrogen and androgen receptor expression by sex steroids during rat spermatogenesis. The Journal of steroid biochemistry and molecular biology. 2018;182:127-136.
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2010;365(1546):1517-1535.
- Rochira VS, D; Carani, C. Pathophysiology of estrogen action in men. In: Behre ENHM, ed. Testosterone Action, Deficiency, Substitution. Fourth Edition ed. Cambridge, UK: Cambridge University Press; 2012.
- Rochira V, Carani C. Estrogen Deficiency in Men. In: Simoni M, Huhtaniemi IT, eds. Endocrinology of the Testis and Male Reproduction. Cham: Springer International Publishing; 2017:797-828.
- Rochira V, Madeo B. Estrogens and Male Osteoporosis. In: Ferlin A, Migliaccio S, eds. Male Osteoporosis: Gender Differences in Pathophysiology, Clinical Aspects, Diagnosis and Treatment. Cham: Springer International Publishing; 2020:67-84.
- Bernard V, Kherra S, Francou B, Fagart J, Viengchareun S, Guéchot J, Ladjouze A, Guiochon-Mantel A, Korach KS, Binart N, Lombès M, Christin-Maitre S. Familial Multiplicity of Estrogen Insensitivity Associated With a Loss-of-Function ESR1 Mutation. The Journal of clinical endocrinology and metabolism. 2017;102(1):93-99.
- Smith EP, Specker B, Bachrach BE, Kimbro KS, Li XJ, Young MF, Fedarko NS, Abuzzahab MJ, Frank GR, Cohen RM, Lubahn DB, Korach KS. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. The Journal of clinical endocrinology and metabolism. 2008;93(8):3088-3096.
- Rochira V, Carani C. Aromatase deficiency in men: a clinical perspective. Nature reviews Endocrinology.2009;5(10):559-568.
- Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. The New England journal of medicine. 1998;339(9):599-603.
- Deladoey J, Fluck C, Bex M, Yoshimura N, Harada N, Mullis PE. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. The Journal of clinical endocrinology and metabolism. 1999;84(11):4050-4054.
- Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. The Journal of clinical endocrinology and metabolism. 2002;87(12):5476-5484.
- Pura MM, H; Carreau, S; Kottler, ML Clinical findings in an adult man with a novel mutation in the aromatase gene. Paper presented at: Abstract Book of the 85th Annual Meeting of the Endocrine Society2003; Philadelphia, PA, USA
- Mittre Herve MH, Kottler ML, Pura M. Human gene mutations. Gene symbol: CYP19. Disease: Aromatase deficiency. Human genetics. 2004;114(2):224.
- Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. The Journal of clinical endocrinology and metabolism. 2004;89(12):6025-6029.
- Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K. Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2005;37(3):178-183.
- Baykan EK, Erdogan M, Ozen S, Darcan S, Saygili LF. Aromatase deficiency, a rare syndrome: case report. Journal of clinical research in pediatric endocrinology. 2013;5(2):129-132.
- Pignatti EU, Kursad; Kartal, Ermine; Ajlouni, Kamel; Khawaja, Nahla;Carani, Cesare; Marino, Marco; Simoni, Manuela; Vighi, Eleonora; Rochira, Vincenzo. Complete aromatase deficiency in four adult men: detection of a novel mutation and two known mutations in the CYP19A1 gene. Endocrine Abstracts. 2013;32(32):P640.
- Bouchoucha N, Samara-Boustani D, Pandey AV, Bony-Trifunovic H, Hofer G, Aigrain Y, Polak M, Flück CE. Characterization of a novel CYP19A1 (aromatase) R192H mutation causing virilization of a 46,XX newborn, undervirilization of the 46,XY brother, but no virilization of the mother during pregnancies. Molecular and cellular endocrinology. 2014;390(1-2):8-17.
- Chen Z, Wang O, Nie M, Elison K, Zhou D, Li M, Jiang Y, Xia W, Meng X, Chen S, Xing X. Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Molecular and cellular endocrinology. 2015;399:32-42.
- Miedlich SU, Karamooz N, Hammes SR. Aromatase deficiency in a male patient - Case report and review of the literature. Bone. 2016;93:181-186.
- Abur U, Atmaca A, Scott H, Gagliardi L, Altundağ E, Akar OS, Bayrak IK, Oğur G. A Male Case of Aromatase Deficiency with a Novel CYP19A1 Mutation. Journal of clinical research in pediatric endocrinology. 2017;9:2-2.
- Kim SY, Colaiacovo S, Dave S, Coughlin K, Langdon K, Stein R, Saleh M. Aromatase deficiency in an Ontario Old Order Mennonite family. J Pediatr Endocrinol Metab. 2021;34(12):1615-1618.
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. The Journal of clinical endocrinology and metabolism. 2004;89(1):61-70.
- Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clinical endocrinology. 2007;67(2):218-224.
- Lanfranco F, Zirilli L, Baldi M, Pignatti E, Corneli G, Ghigo E, Aimaretti G, Carani C, Rochira V. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628-635.
- Carani C, Rochira V, Faustini-Fustini M, Balestrieri A, Granata AR. Role of oestrogen in male sexual behaviour: insights from the natural model of aromatase deficiency. Clinical endocrinology. 1999;51(4):517-524.
- Rochira V, Faustini-Fustini M, Balestrieri A, Carani C. Estrogen replacement therapy in a man with congenital aromatase deficiency: effects of different doses of transdermal estradiol on bone mineral density and hormonal parameters. The Journal of clinical endocrinology and metabolism. 2000;85(5):1841-1845.
- Rochira V, Balestrieri A, Faustini-Fustini M, Borgato S, Beck-Peccoz P, Carani C. Pituitary function in a man with congenital aromatase deficiency: effect of different doses of transdermal E2 on basal and stimulated pituitary hormones. The Journal of clinical endocrinology and metabolism. 2002;87(6):2857-2862.
- Carani C, Granata AR, Rochira V, Caffagni G, Aranda C, Antunez P, Maffei LE. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroendocrinology.2005;30(5):413-417.
- Rochira V, Zirilli L, Genazzani AD, Balestrieri A, Aranda C, Fabre B, Antunez P, Diazzi C, Carani C, Maffei L. Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155(4):513-522.
- Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabetic medicine : a journal of the British Diabetic Association.2007;24(12):1491-1495.
- Rochira V, Zirilli L, Madeo B, Aranda C, Caffagni G, Fabre B, Montangero VE, Roldan EJ, Maffei L, Carani C. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone.2007;40(6):1662-1668.
- Zirilli L, Maffei L, Meunier PJ, Chavassieux P, Carani C, Rochira V. The effects of long-term raloxifene and estradiol treatments on bone in a patient with congenital aromatase deficiency. Bone. 2009;45(5):827-832.
- Rochira V, Balestrieri A, Madeo B, Spaggiari A, Carani C. Congenital estrogen deficiency in men: a new syndrome with different phenotypes; clinical and therapeutic implications in men. Molecular and cellular endocrinology. 2002;193(1-2):19-28.
- Fukami M, Ogata T. Congenital disorders of estrogen biosynthesis and action. Best practice & research Clinical endocrinology & metabolism. 2021:101580.
- Parween S, Fernández-Cancio M, Benito-Sanz S, Camats N, Rojas Velazquez MN, López-Siguero JP, Udhane SS, Kagawa N, Flück CE, Audí L, Pandey AV. Molecular Basis of CYP19A1 Deficiency in a 46,XX Patient With R550W Mutation in POR: Expanding the PORD Phenotype. The Journal of clinical endocrinology and metabolism. 2020;105(4).
- Cooke PS, Walker WH. Nonclassical androgen and estrogen signaling is essential for normal spermatogenesis. Semin Cell Dev Biol. 2021.
- Suzuki Y, Sasagawa I, Itoh K, Ashida J, Muroya K, Ogata T. Estrogen receptor alpha gene polymorphism is associated with idiopathic azoospermia. Fertility and sterility. 2002;78(6):1341-1343.
- Kukuvitis A, Georgiou I, Bouba I, Tsirka A, Giannouli CH, Yapijakis C, Tarlatzis B, Bontis J, Lolis D, Sofikitis N, Papadimas J. Association of oestrogen receptor alpha polymorphisms and androgen receptor CAG trinucleotide repeats with male infertility: a study in 109 Greek infertile men. International journal of andrology.2002;25(3):149-152.
- Lazaros LA, Xita NV, Kaponis AI, Zikopoulos KA, Plachouras NI, Georgiou IA. Estrogen receptor alpha and beta polymorphisms are associated with semen quality. Journal of andrology. 2010;31(3):291-298.
- Lazaros L, Xita N, Kaponis A, Hatzi E, Plachouras N, Sofikitis N, Zikopoulos K, Georgiou I. The association of aromatase (CYP19) gene variants with sperm concentration and motility. Asian journal of andrology.2011;13(2):292-297.
- Mobasseri N, Nikzad H, Karimian M. Protective effect of oestrogen receptor α-PvuII transition against idiopathic male infertility: a case-control study and meta-analysis. Reprod Biomed Online. 2019;38(4):588-598.
- Lee IW, Kuo PH, Su MT, Kuan LC, Hsu CC, Kuo PL. Quantitative trait analysis suggests polymorphisms of estrogen-related genes regulate human sperm concentrations and motility. Human reproduction (Oxford, England). 2011;26(6):1585-1596.
- Gonzalez-Unzaga M, Tellez J, Calzada L. Clinical significance of nuclear matrix-estradiol receptor complex in human sperm. Archives of andrology. 2003;49(1):77-81.
- Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. The Journal of urology. 2002;167(2 Pt 1):624-629.
- Saylam B, Efesoy O, Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertility and sterility. 2011;95(2):809-811.
- Shiraishi K, Oka S, Matsuyama H. Testicular Testosterone and Estradiol Concentrations and Aromatase Expression in Men with Nonobstructive Azoospermia. The Journal of clinical endocrinology and metabolism.2021;106(4):e1803-e1815.
- Hagiuda J, Ishikawa H, Marumo K. Serum oestradiol levels in male partners of infertile couples. Andrologia.2015;47(6):669-673.
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr., Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environmental health perspectives. 1996;104 Suppl 4:741-803.
- Pflieger-Bruss S, Schuppe HC, Schill WB. The male reproductive system and its susceptibility to endocrine disrupting chemicals. Andrologia. 2004;36(6):337-345.
- Sharpe RM. Do males rely on female hormones? Nature. 1997;390(6659):447-448.
- Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. The Journal of clinical endocrinology and metabolism.2007;92(7):2827-2830.
- Wolfe A, Wu S. Estrogen receptor-β in the gonadotropin-releasing hormone neuron. Seminars in reproductive medicine. 2012;30(1):23-31.
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800-2812.
- Shupnik MA. Oestrogen receptors, receptor variants and oestrogen actions in the hypothalamic-pituitary axis. Journal of neuroendocrinology. 2002;14(2):85-94.
- Idan A, Griffiths KA, Harwood DT, Seibel MJ, Turner L, Conway AJ, Handelsman DJ. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: a randomized, placebo-controlled trial. Annals of internal medicine. 2010;153(10):621-632.
- Finkelstein JS, O'Dea LS, Whitcomb RW, Crowley WF, Jr. Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin-releasing hormone-deficient men. The Journal of clinical endocrinology and metabolism. 1991;73(3):621-628.
- Finkelstein JS, Whitcomb RW, O'Dea LS, Longcope C, Schoenfeld DA, Crowley WF, Jr. Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. The Journal of clinical endocrinology and metabolism. 1991;73(3):609-620.
- Guercio G, Saraco N, Costanzo M, Marino R, Ramirez P, Berensztein E, Rivarola MA, Belgorosky A. Estrogens in Human Male Gonadotropin Secretion and Testicular Physiology From Infancy to Late Puberty. Frontiers in endocrinology. 2020;11:72.
- Raven G, de Jong FH, Kaufman JM, de Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. The Journal of clinical endocrinology and metabolism. 2006;91(9):3324-3328.
- Bagatell CJ, Dahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. Journal of andrology. 1994;15(1):15-21.
- Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF, Jr. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. The Journal of clinical endocrinology and metabolism.2000;85(9):3027-3035.
- Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF, Jr., Hayes FJ. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. The Journal of clinical endocrinology and metabolism. 2008;93(3):784-791.
- Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF, Jr., Hayes FJ. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. The Journal of clinical endocrinology and metabolism. 2008;93(7):2686-2692.
- Mauras N, O'Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. The Journal of clinical endocrinology and metabolism. 2000;85(7):2370-2377.
- Wickman S, Dunkel L. Inhibition of P450 aromatase enhances gonadotropin secretion in early and midpubertal boys: evidence for a pituitary site of action of endogenous E. The Journal of clinical endocrinology and metabolism. 2001;86(10):4887-4894.
- Taxel P, Kennedy DG, Fall PM, Willard AK, Clive JM, Raisz LG. The effect of aromatase inhibition on sex steroids, gonadotropins, and markers of bone turnover in older men. The Journal of clinical endocrinology and metabolism. 2001;86(6):2869-2874.
- de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reproductive biology and endocrinology : RB&E. 2011;9:93.
- de Ronde W. Therapeutic uses of aromatase inhibitors in men. Current opinion in endocrinology, diabetes, and obesity. 2007;14(3):235-240.
- Burnett-Bowie SA, Roupenian KC, Dere ME, Lee H, Leder BZ. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clinical endocrinology. 2009;70(1):116-123.
- Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. The Journal of clinical endocrinology and metabolism.2004;89(3):1174-1180.
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. The New England journal of medicine. 2013;369(11):1011-1022.
- Awouters M, Vanderschueren D, Antonio L. Aromatase inhibitors and selective estrogen receptor modulators: Unconventional therapies for functional hypogonadism? Andrology. 2020;8(6):1590-1597.
- Ide V, Vanderschueren D, Antonio L. Treatment of Men with Central Hypogonadism: Alternatives for Testosterone Replacement Therapy. Int J Mol Sci. 2020;22(1).
- McArdle CA, Schomerus E, Groner I, Poch A. Estradiol regulates gonadotropin-releasing hormone receptor number, growth and inositol phosphate production in alpha T3-1 cells. Molecular and cellular endocrinology.1992;87(1-3):95-103.
- Hu L, Gustofson RL, Feng H, Leung PK, Mores N, Krsmanovic LZ, Catt KJ. Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Molecular endocrinology (Baltimore, Md). 2008;22(10):2250-2259.
- Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(38):10153-10164.
- Vanderschueren D, Bouillon R. Estrogen deficiency in men is a challenge for both the hypothalamus and pituitary. The Journal of clinical endocrinology and metabolism. 2000;85(9):3024-3026.
- Marino R, Perez Garrido N, Costanzo M, Guercio G, Juanes M, Rocco C, Ramirez P, Warman DM, Ciaccio M, Pena G, Feyling JG, Miras M, Rivarola MA, Belgorosky A, Saraco N. Five new cases of 46,XX aromatase deficiency: clinical follow-up from birth to puberty, a novel mutation, and a founder effect. The Journal of clinical endocrinology and metabolism. 2015;100(2):E301-307.
- Mongan NP, Tadokoro-Cuccaro R, Bunch T, Hughes IA. Androgen insensitivity syndrome. Best practice & research Clinical endocrinology & metabolism. 2015;29(4):569-580.
- Harkonen PL, Makela SI. Role of estrogens in development of prostate cancer. The Journal of steroid biochemistry and molecular biology. 2004;92(4):297-305.
- Kowalska K, Piastowska-Ciesielska AW. Oestrogens and oestrogen receptors in prostate cancer. SpringerPlus.2016;5:522.
- McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142(6):2458-2467.
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142(6):2435-2442.
- McPherson SJ, Ellem SJ, Patchev V, Fritzemeier KH, Risbridger GP. The role of Eralpha and ERbeta in the prostate: insights from genetic models and isoform-selective ligands. Ernst Schering Foundation symposium proceedings. 2006(1):131-147.
- Bosland MC. The role of steroid hormones in prostate carcinogenesis. Journal of the National Cancer Institute Monographs. 2000(27):39-66.
- Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. The Journal of clinical endocrinology and metabolism. 2004;89(5):2434-2441.
- Kanda S, Tsuchiya N, Narita S, Inoue T, Huang M, Chiba S, Akihama S, Saito M, Numakura K, Tsuruta H, Satoh S, Saito S, Ohyama C, Arai Y, Ogawa O, Habuchi T. Effects of functional genetic polymorphisms in the CYP19A1 gene on prostate cancer risk and survival. Int J Cancer. 2015;136(1):74-82.
- Beuten J, Gelfond JA, Franke JL, Weldon KS, Crandall AC, Johnson-Pais TL, Thompson IM, Leach RJ. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1869-1880.
- Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17 beta or diethylstilbestrol. Carcinogenesis. 1995;16(6):1311-1317.
- Qu LG, Wardan H, Davis ID, Pezaro C, Sluka P. Effects of estrogen receptor signaling on prostate cancer carcinogenesis. Transl Res. 2020;222:56-66.
- Lau KM, To KF. Importance of Estrogenic Signaling and Its Mediated Receptors in Prostate Cancer. Int J Mol Sci. 2016;17(9).
- Nakajima Y, Osakabe A, Waku T, Suzuki T, Akaogi K, Fujimura T, Homma Y, Inoue S, Yanagisawa J. Estrogen Exhibits a Biphasic Effect on Prostate Tumor Growth through the Estrogen Receptor β-KLF5 Pathway. Mol Cell Biol. 2016;36(1):144-156.
- Dias JP, Melvin D, Shardell M, Ferrucci L, Chia CW, Gharib M, Egan JM, Basaria S. Effects of Transdermal Testosterone Gel or an Aromatase Inhibitor on Prostate Volume in Older Men. The Journal of clinical endocrinology and metabolism. 2016;101(4):1865-1871.
- Xu D, Wu Y, Shen H, Qian S, Qi J. High serum concentration of estradiol may be a risk factor of prostate enlargement in aging male in China. Aging Male. 2020;23(1):1-6.
- Reis LO, Zani EL, García-Perdomo HA. Estrogen therapy in patients with prostate cancer: a contemporary systematic review. Int Urol Nephrol. 2018;50(6):993-1003.
- Langley RE, Gilbert DC, Duong T, Clarke NW, Nankivell M, Rosen SD, Mangar S, Macnair A, Sundaram SK, Laniado ME, Dixit S, Madaan S, Manetta C, Pope A, Scrase CD, McKay S, Muazzam IA, Collins GN, Worlding J, Williams ST, Paez E, Robinson A, McFarlane J, Deighan JV, Marshall J, Forcat S, Weiss M, Kockelbergh R, Alhasso A, Kynaston H, Parmar M. Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised Prostate Adenocarcinoma Transcutaneous Hormone (PATCH) trial programme. Lancet (London, England). 2021;397(10274):581-591.
- Brodie AM, Son C, King DA, Meyer KM, Inkster SE. Lack of evidence for aromatase in human prostatic tissues: effects of 4-hydroxyandrostenedione and other inhibitors on androgen metabolism. Cancer research.1989;49(23):6551-6555.
- Rochira V, Zirilli L, Madeo B, Balestrieri A, Granata AR, Carani C. Sex steroids and sexual desire mechanism. Journal of endocrinological investigation. 2003;26(3 Suppl):29-36.
- Robbins A. Androgens and male sexual behavior from mice to men. Trends in endocrinology and metabolism: TEM. 1996;7(9):345-350.
- van Anders SM. Beyond masculinity: testosterone, gender/sex, and human social behavior in a comparative context. Frontiers in neuroendocrinology. 2013;34(3):198-210.
- Funk CH, B; Lejwa, A. The male hormone. American Journal of Physiology. 1930;92:440-449.
- Granata AR, Rochira V, Lerchl A, Marrama P, Carani C. Relationship between sleep-related erections and testosterone levels in men. Journal of andrology. 1997;18(5):522-527.
- Rochira V, Balestrieri A, Madeo B, Granata AR, Carani C. Sildenafil improves sleep-related erections in hypogonadal men: evidence from a randomized, placebo-controlled, crossover study of a synergic role for both testosterone and sildenafil on penile erections. Journal of andrology. 2006;27(2):165-175.
- Gooren LJ. Clinical practice. Care of transsexual persons. The New England journal of medicine.2011;364(13):1251-1257.
- Van Goozen SH, Cohen-Kettenis PT, Gooren LJ, Frijda NH, Van de Poll NE. Gender differences in behaviour: activating effects of cross-sex hormones. Psychoneuroendocrinology. 1995;20(4):343-363.
- Gooren LJ, Giltay EJ. Men and women, so different, so similar: observations from cross-sex hormone treatment of transsexual subjects. Andrologia. 2014;46(5):570-575.
- Ramasamy R, Scovell JM, Kovac JR, Lipshultz LI. Elevated serum estradiol is associated with higher libido in men on testosterone supplementation therapy. European urology. 2014;65(6):1224-1225.
- Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Ellenberg SS, Matsumoto AM, Bhasin S, Molitch ME, Farrar JT, Cella D, Barrett-Connor E, Cauley JA, Cifelli D, Crandall JP, Ensrud KE, Fluharty L, Gill TM, Lewis CE, Pahor M, Resnick SM, Storer TW, Swerdloff RS, Anton S, Basaria S, Diem S, Tabatabaie V, Hou X, Snyder PJ. Association of sex hormones with sexual function, vitality, and physical function of symptomatic older men with low testosterone levels at baseline in the testosterone trials. The Journal of clinical endocrinology and metabolism. 2015;100(3):1146-1155.
- Sartorius GA, Ly LP, Handelsman DJ. Male sexual function can be maintained without aromatization: randomized placebo-controlled trial of dihydrotestosterone (DHT) in healthy, older men for 24 months. The journal of sexual medicine. 2014;11(10):2562-2570.
- O'Connor DB, Lee DM, Corona G, Forti G, Tajar A, O'Neill TW, Pendleton N, Bartfai G, Boonen S, Casanueva FF, Finn JD, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Labrie F, Lean ME, Punab M, Silman AJ, Vanderschueren D, Wu FC. The relationships between sex hormones and sexual function in middle-aged and older European men. The Journal of clinical endocrinology and metabolism. 2011;96(10):E1577-1587.
- Meyer-Bahlburg HF. Sex steroids and variants of gender identity. Endocrinology and metabolism clinics of North America. 2013;42(3):435-452.
- Bao AM, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Frontiers in neuroendocrinology. 2011;32(2):214-226.
- Gorski R. Sexual differentiation of the endocrine brain and its control. In: Motta M, ed. Brain Endocrinology. 2nd ed. New York: Raven Press; 1991:71-104.
- Pilgrim C, Reisert I. Differences between male and female brains--developmental mechanisms and implications. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme.1992;24(8):353-359.
- Garcia-Falgueras A, Swaab DF. Sexual hormones and the brain: an essential alliance for sexual identity and sexual orientation. Endocrine development. 2010;17:22-35.
- Dorner G. Neuroendocrine response to estrogen and brain differentiation in heterosexuals, homosexuals, and transsexuals. Archives of sexual behavior. 1988;17(1):57-75.
- Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Seminars in reproductive medicine. 2009;27(3):207-217.
- Swaab DF, Wolff SEC, Bao AM. Sexual differentiation of the human hypothalamus: Relationship to gender identity and sexual orientation. Handb Clin Neurol. 2021;181:427-443.
- Roselli CE, Estill CT, Stadelman HL, Meaker M, Stormshak F. Separate critical periods exist for testosterone-induced differentiation of the brain and genitals in sheep. Endocrinology. 2011;152(6):2409-2415.
- LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science (New York, NY). 1991;253(5023):1034-1037.
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139(1):61-72.
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology.2004;145(2):478-483.
- Troisi R, Palmer JR, Hatch EE, Strohsnitter WC, Huo D, Hyer M, Fredriksen-Goldsen KI, Hoover R, Titus L. Gender Identity and Sexual Orientation Identity in Women and Men Prenatally Exposed to Diethylstilbestrol. Archives of sexual behavior. 2020;49(2):447-454.
- Wright CL, Schwarz JS, Dean SL, McCarthy MM. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends in endocrinology and metabolism: TEM. 2010;21(9):553-561.
- Wallen K, Hassett JM. Sexual differentiation of behaviour in monkeys: role of prenatal hormones. Journal of neuroendocrinology. 2009;21(4):421-426.
- Jannini EA, Blanchard R, Camperio-Ciani A, Bancroft J. Male homosexuality: nature or culture? The journal of sexual medicine. 2010;7(10):3245-3253.
- Carani CR, V; Granata, ARM. Sexuality and erectile dysfunction. In: Wass JS, SM ed. Oxford Textbook of Endocrinology and Diabetes. 2nd ed. Oxford, UK: Oxford University Press; 2011:1449-1458.
- Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, Rochira V, Sforza A, Lenzi A, Mannucci E, Maggi M. Testosterone supplementation and sexual function: a meta-analysis study. The journal of sexual medicine. 2014;11(6):1577-1592.
- Corona G, Isidori AM, Aversa A, Burnett AL, Maggi M. Endocrinologic Control of Men's Sexual Desire and Arousal/Erection. The journal of sexual medicine. 2016;13(3):317-337.
- Buvat J, Maggi M, Gooren L, Guay AT, Kaufman J, Morgentaler A, Schulman C, Tan HM, Torres LO, Yassin A, Zitzmann M. Endocrine aspects of male sexual dysfunctions. The journal of sexual medicine. 2010;7(4 Pt 2):1627-1656.
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochemical and biophysical research communications.1998;252(2):445-449.
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO). Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14737-14741.
- Luisi M, Franchi F. Double-blind group comparative study of testosterone undecanoate and mesterolone in hypogonadal male patients. Journal of endocrinological investigation. 1980;3(3):305-308.
- Davidson JM, Camargo C, Smith ER, Kwan M. Maintenance of sexual function in a castrated man treated with ovarian steroids. Archives of sexual behavior. 1983;12(3):263-274.
- Cunningham GR, Stephens-Shields AJ, Rosen RC, Wang C, Bhasin S, Matsumoto AM, Parsons JK, Gill TM, Molitch ME, Farrar JT, Cella D, Barrett-Connor E, Cauley JA, Cifelli D, Crandall JP, Ensrud KE, Gallagher L, Zeldow B, Lewis CE, Pahor M, Swerdloff RS, Hou X, Anton S, Basaria S, Diem SJ, Tabatabaie V, Ellenberg SS, Snyder PJ. Testosterone Treatment and Sexual Function in Older Men With Low Testosterone Levels. The Journal of clinical endocrinology and metabolism. 2016;101(8):3096-3104.
- Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. The Journal of urology. 2011;185(1):17-23.
- Hsu B, Cumming RG, Blyth FM, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ. The longitudinal relationship of sexual function and androgen status in older men: the Concord Health and Ageing in Men Project. The Journal of clinical endocrinology and metabolism. 2015;100(4):1350-1358.
- Wu F, Chen T, Mao S, Jiang H, Ding Q, Xu G. Levels of estradiol and testosterone are altered in Chinese men with sexual dysfunction. Andrology. 2016;4(5):932-938.
- Chen T, Wu F, Wang X, Ma G, Xuan X, Tang R, Ding S, Lu J. Different levels of estradiol are correlated with sexual dysfunction in adult men. Sci Rep. 2020;10(1):12660.
- Chen HR, Tian RH, Li P, Chen HX, Xia SJ, Li Z. Estradiol is an independent risk factor for organic erectile dysfunction in eugonadal young men. Asian journal of andrology. 2020;22(6):636-641.
- El-Sakka AI. Impact of the association between elevated oestradiol and low testosterone levels on erectile dysfunction severity. Asian journal of andrology. 2013;15(4):492-496.
- Vignozzi L, Filippi S, Comeglio P, Cellai I, Morelli A, Marchetta M, Maggi M. Estrogen mediates metabolic syndrome-induced erectile dysfunction: a study in the rabbit. The journal of sexual medicine. 2014;11(12):2890-2902.
- Castelló-Porcar AM, Martínez-Jabaloyas JM. Testosterone/estradiol ratio, is it useful in the diagnosis of erectile dysfunction and low sexual desire? Aging Male. 2016;19(4):254-258.
- Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, Mancina R, Muratori M, Forti G, Serio M, Luconi M. Expression of functional estrogen receptors in human fetal male external genitalia. The Journal of clinical endocrinology and metabolism. 2003;88(4):1815-1824.
- Dietrich W, Haitel A, Huber JC, Reiter WJ. Expression of estrogen receptors in human corpus cavernosum and male urethra. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society.2004;52(3):355-360.
- Goyal HO, Braden TD, Williams CS, Williams JW. Role of estrogen in induction of penile dysmorphogenesis: a review. Reproduction (Cambridge, England). 2007;134(2):199-208.
- Mowa CN, Jesmin S, Miyauchi T. The penis: a new target and source of estrogen in male reproduction. Histology and histopathology. 2006;21(1):53-67.
- Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends in endocrinology and metabolism: TEM. 2011;22(1):24-33.
- Gibb FW, Homer NZ, Faqehi AM, Upreti R, Livingstone DE, McInnes KJ, Andrew R, Walker BR. Aromatase Inhibition Reduces Insulin Sensitivity in Healthy Men. The Journal of clinical endocrinology and metabolism.2016;101(5):2040-2046.
- Dias JP, Shardell MD, Carlson OD, Melvin D, Caturegli G, Ferrucci L, Chia CW, Egan JM, Basaria S. Testosterone vs. aromatase inhibitor in older men with low testosterone: effects on cardiometabolic parameters. Andrology. 2017;5(1):31-40.
- Juang PS, Peng S, Allehmazedeh K, Shah A, Coviello AD, Herbst KL. Testosterone with dutasteride, but not anastrazole, improves insulin sensitivity in young obese men: a randomized controlled trial. The journal of sexual medicine. 2014;11(2):563-573.
- Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care. 2011;34(8):1854-1859.
- Chan YX, Knuiman MW, Hung J, Divitini ML, Handelsman DJ, Beilby JP, McQuillan B, Yeap BB. Testosterone, dihydrotestosterone and estradiol are differentially associated with carotid intima-media thickness and the presence of carotid plaque in men with and without coronary artery disease. Endocr J. 2015;62(9):777-786.
- van Koeverden ID, de Bakker M, Haitjema S, van der Laan SW, de Vries JPM, Hoefer IE, de Borst GJ, Pasterkamp G, den Ruijter HM. Testosterone to oestradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res. 2019;115(2):453-462.
- Figtree GA, Ngo DTM, Bubb KJ. Testosterone to estradiol ratio and plaque inflammation: mechanistic insights and biomarker potential? Cardiovasc Res. 2019;115(2):255-257.
- Travison TG, O'Donnell CJ, Bhasin S, Massaro JM, Hoffmann U, Vasan RS, D'Agostino RB, Sr., Basaria S. Circulating Sex Steroids and Vascular Calcification in Community-Dwelling Men: The Framingham Heart Study. The Journal of clinical endocrinology and metabolism. 2016;101(5):2160-2167.
- Taylor AP, Lee H, Webb ML, Joffe H, Finkelstein JS. Effects of Testosterone and Estradiol Deficiency on Vasomotor Symptoms in Hypogonadal Men. The Journal of clinical endocrinology and metabolism.2016;101(9):3479-3486.
- Rochira V, Kara E, Carani C. The endocrine role of estrogens on human male skeleton. International journal of endocrinology. 2015;2015:165215.
- Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Borjesson AE, Ohlsson C. Sex steroid actions in male bone. Endocrine reviews. 2014;35(6):906-960.
- Khosla S. Estrogen and bone: insights from estrogen-resistant, aromatase-deficient, and normal men. Bone.2008;43(3):414-417.
- Khosla S. Update on estrogens and the skeleton. The Journal of clinical endocrinology and metabolism.2010;95(8):3569-3577.
- Rochira V, Balestrieri A, Madeo B, Zirilli L, Granata AR, Carani C. Osteoporosis and male age-related hypogonadism: role of sex steroids on bone (patho)physiology. European journal of endocrinology / European Federation of Endocrine Societies. 2006;154(2):175-185.
- Rochira V, Antonio L, Vanderschueren D. EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology. 2018;6(2):272-285.
- Rochira V. Late-onset Hypogonadism: Bone health. Andrology. 2020;8(6):1539-1550.
- Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, Hirsch SC, Linker A, Perros N, Servais AB, Taylor AP, Webb ML, Youngner JM, Yu EW. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. The Journal of clinical investigation. 2016;126(3):1114-1125.
- Decaroli MC, Rochira V. Aging and sex hormones in males. Virulence. 2017;8(5):545-570.
- Russell N, Hoermann R, Cheung AS, Ching M, Zajac JD, Handelsman DJ, Grossmann M. Short-term effects of transdermal estradiol in men undergoing androgen deprivation therapy for prostate cancer: a randomized placebo-controlled trial. European journal of endocrinology / European Federation of Endocrine Societies.2018;178(5):565-576.
- Khalil R, Antonio L, Laurent MR, David K, Kim NR, Evenepoel P, Eisenhauer A, Heuser A, Cavalier E, Khosla S, Claessens F, Vanderschueren D, Decallonne B. Early effects of androgen deprivation on bone and mineral homeostasis in adult men: a prospective cohort study. European journal of endocrinology / European Federation of Endocrine Societies. 2020;183(2):181-189.
- Decaroli MC, De Vincentis S, Rochira V. Aging and sex hormones in males. Vitam Horm. 2021;115:333-366.
- Langley RE, Kynaston HG, Alhasso AA, Duong T, Paez EM, Jovic G, Scrase CD, Robertson A, Cafferty F, Welland A, Carpenter R, Honeyfield L, Abel RL, Stone M, Parmar MK, Abel PD. A Randomised Comparison Evaluating Changes in Bone Mineral Density in Advanced Prostate Cancer: Luteinising Hormone-releasing Hormone Agonists Versus Transdermal Oestradiol. European urology. 2016;69(6):1016-1025.
- Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. European journal of endocrinology / European Federation of Endocrine Societies.2015;173(6):809-817.
- Frederiksen H, Johannsen TH, Andersen SE, Albrethsen J, Landersoe SK, Petersen JH, Andersen AN, Vestergaard ET, Schorring ME, Linneberg A, Main KM, Andersson AM, Juul A. Sex-specific Estrogen Levels and Reference Intervals from Infancy to Late Adulthood Determined by LC-MS/MS. The Journal of clinical endocrinology and metabolism. 2020;105(3):754-768.
- Vottero A, Rochira V, Capelletti M, Viani I, Zirilli L, Neri TM, Carani C, Bernasconi S, Ghizzoni L. Aromatase is differentially expressed in peripheral blood leukocytes from children, and adult female and male subjects. European journal of endocrinology / European Federation of Endocrine Societies. 2006;154(3):425-431.
- Ongphiphadhanakul B, Rajatanavin R, Chanprasertyothin S, Piaseu N, Chailurkit L. Serum oestradiol and oestrogen-receptor gene polymorphism are associated with bone mineral density independently of serum testosterone in normal males. Clinical endocrinology. 1998;49(6):803-809.
- Khosla S, Riggs BL, Atkinson EJ, Oberg AL, Mavilia C, Del Monte F, Melton LJ, 3rd, Brandi ML. Relationship of estrogen receptor genotypes to bone mineral density and to rates of bone loss in men. The Journal of clinical endocrinology and metabolism. 2004;89(4):1808-1816.
- Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S, Lucani B, Gennari C, Brandi ML. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. The Journal of clinical endocrinology and metabolism.2004;89(6):2803-2810.
- Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. The Journal of clinical endocrinology and metabolism. 2003;88(7):3075-3081.
- Eriksson AL, Perry JRB, Coviello AD, Delgado GE, Ferrucci L, Hoffman AR, Huhtaniemi IT, Ikram MA, Karlsson MK, Kleber ME, Laughlin GA, Liu Y, Lorentzon M, Lunetta KL, Mellstrom D, Murabito JM, Murray A, Nethander M, Nielson CM, Prokopenko I, Pye SR, Raffel LJ, Rivadeneira F, Srikanth P, Stolk L, Teumer A, Travison TG, Uitterlinden AG, Vaidya D, Vanderschueren D, Zmuda JM, Marz W, Orwoll ES, Ouyang P, Vandenput L, Wu FCW, de Jong FH, Bhasin S, Kiel DP, Ohlsson C. Genetic Determinants of Circulating Estrogen Levels and Evidence of a Causal Effect of Estradiol on Bone Density in Men. The Journal of clinical endocrinology and metabolism. 2018;103(3):991-1004.
- Schmitz D, Ek WE, Berggren E, Höglund J, Karlsson T, Johansson Å. Genome-wide Association Study of Estradiol Levels and the Causal Effect of Estradiol on Bone Mineral Density. The Journal of clinical endocrinology and metabolism. 2021;106(11):e4471-e4486.
- Nethander M, Vandenput L, Eriksson AL, Windahl S, Funck-Brentano T, Ohlsson C. Evidence of a Causal Effect of Estradiol on Fracture Risk in Men. The Journal of clinical endocrinology and metabolism. 2019;104(2):433-442.
- Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Kaufman JM, T'Sjoen G. Preservation of volumetric bone density and geometry in trans women during cross-sex hormonal therapy: a prospective observational study. Osteoporos Int. 2015;26(1):35-47.
- Laurent MR, Gielen E, Vanderschueren D. Estrogens, the be-all and end-all of male hypogonadal bone loss? Osteoporos Int. 2015;26(1):29-33.
- Björntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord.1996;20(4):291-302.
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical endocrinology. 2005;63(3):280-293.
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(23):12729-12734.
- Misso ML, Murata Y, Boon WC, Jones ME, Britt KL, Simpson ER. Cellular and molecular characterization of the adipose phenotype of the aromatase-deficient mouse. Endocrinology. 2003;144(4):1474-1480.
- Chao J, Rubinow KB, Kratz M, Amory JK, Matsumoto AM, Page ST. Short-Term Estrogen Withdrawal Increases Adiposity in Healthy Men. The Journal of clinical endocrinology and metabolism. 2016;101(10):3724-3731.
- Smith MR. Changes in body composition during hormonal therapy for prostate cancer. Clin Prostate Cancer.2003;2(1):18-21.
- Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63(4):742-745.
- Haseen F, Murray LJ, Cardwell CR, O'Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv.2010;4(2):128-139.
- Russell N, Hoermann R, Cheung AS, Zajac JD, Handelsman DJ, Grossmann M. Effects of estradiol on fat in men undergoing androgen deprivation therapy: a randomized trial. European journal of endocrinology / European Federation of Endocrine Societies. 2021;186(1):9-23.
- Gava G, Mancini I, Orsili I, Bertelloni S, Alvisi S, Seracchioli R, Meriggiola MC. Bone mineral density, body composition and metabolic profiles in adult women with complete androgen insensitivity syndrome and removed gonads using oral or transdermal estrogens. European journal of endocrinology / European Federation of Endocrine Societies. 2019;181(6):711-718.
- Birzniece V, Ho KKY. Sex steroids and the GH axis: Implications for the management of hypopituitarism. Best practice & research Clinical endocrinology & metabolism. 2017;31(1):59-69.
- Nilsson M, Dahlman I, Rydén M, Nordström EA, Gustafsson JA, Arner P, Dahlman-Wright K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond). 2007;31(6):900-907.
- Drew BG, Hamidi H, Zhou Z, Villanueva CJ, Krum SA, Calkin AC, Parks BW, Ribas V, Kalajian NY, Phun J, Daraei P, Christofk HR, Hewitt SC, Korach KS, Tontonoz P, Lusis AJ, Slamon DJ, Hurvitz SA, Hevener AL. Estrogen receptor (ER)α-regulated lipocalin 2 expression in adipose tissue links obesity with breast cancer progression. J Biol Chem. 2015;290(9):5566-5581.
- Zhou Z, Moore TM, Drew BG, Ribas V, Wanagat J, Civelek M, Segawa M, Wolf DM, Norheim F, Seldin MM, Strumwasser AR, Whitney KA, Lester E, Reddish BR, Vergnes L, Reue K, Rajbhandari P, Tontonoz P, Lee J, Mahata SK, Hewitt SC, Shirihai O, Gastonbury C, Small KS, Laakso M, Jensen J, Lee S, Drevon CA, Korach KS, Lusis AJ, Hevener AL. Estrogen receptor α controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci Transl Med. 2020;12(555).
- Fuller-Jackson JP, Dordevic AL, Clarke IJ, Henry BA. Effect of sex and sex steroids on brown adipose tissue heat production in humans. European journal of endocrinology / European Federation of Endocrine Societies.2020;183(3):343-355.
- López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends in endocrinology and metabolism: TEM. 2015;26(8):411-421.
- Davis KE, M DN, Sun K, W MS, J DB, J AZ, Zeve D, L DH, D WC, L MG, Xu Y, Z VW, S AK, Clegg DJ. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227-242.
- Sample CH, Davidson TL. Considering sex differences in the cognitive controls of feeding. Physiol Behav.2018;187:97-107.
- Rivera HM, Stincic TL. Estradiol and the control of feeding behavior. Steroids. 2018;133:44-52.
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493-500.
- Biegon A, Alia-Klein N, Alexoff DL, Fowler JS, Kim SW, Logan J, Pareto D, Preston-Campbell R, Wang GJ, Hildebrandt T. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. Proc Natl Acad Sci U S A. 2020;117(37):22962-22966.
- Hampton T. How Targeting Fat Cells' Estrogen Receptors Could Fight Obesity. Jama. 2020;324(21):2146.
- Taxier LR, Gross KS, Frick KM. Oestradiol as a neuromodulator of learning and memory. Nat Rev Neurosci.2020;21(10):535-550.
- Yeap BB, Hui J, Knuiman MW, Handelsman DJ, Flicker L, Divitini ML, Arscott GM, McLennan SV, Twigg SM, Almeida OP, Hankey GJ, Golledge J, Norman PE, Beilby JP. Cross-sectional associations of sex hormones with leucocyte telomere length, a marker of biological age, in a community-based cohort of older men. Clinical endocrinology. 2019;90(4):562-569.
- Yeap BB, Hui J, Knuiman MW, C SAP, Ken KYH, Flicker L, Divitini ML, Arscott GM, Twigg SM, Almeida OP, Hankey GJ, Golledge J, Beilby JP. Associations of plasma IGF1, IGFBP3 and estradiol with leucocyte telomere length, a marker of biological age, in men. European journal of endocrinology / European Federation of Endocrine Societies. 2020;182(1):23-33.
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, Hoover RN. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). International journal of andrology. 2010;33(2):377-384.
- Streng T, Li X, Lehtoranta M, Makela S, Poutanen M, Talo A, Tekmal RR, Santti R. Infravesical obstruction in aromatase over expressing transgenic male mice with increased ratio of serum estrogen-to-androgen concentration. The Journal of urology. 2002;168(1):298-302.
- Kumar V, Misro MM, Datta K. Simultaneous accumulation of hyaluronan binding protein 1 (HABP1/p32/gC1qR) and apoptotic induction of germ cells in cryptorchid testis. Journal of andrology. 2012;33(1):114-121.
- Toppari J, Skakkebaek NE. Sexual differentiation and environmental endocrine disrupters. Bailliere's clinical endocrinology and metabolism. 1998;12(1):143-156.
- Virtanen HE, Adamsson A. Cryptorchidism and endocrine disrupting chemicals. Molecular and cellular endocrinology. 2012;355(2):208-220.
- Toppari J, Rodprasert W, Koskenniemi JJ. Exposure Variation and Endocrine Disruption of the Male Reproductive System. Hormone research in paediatrics. 2016;85(6).
- Norgil Damgaard I, Main KM, Toppari J, Skakkebaek NE. Impact of exposure to endocrine disrupters in utero and in childhood on adult reproduction. Best practice & research Clinical endocrinology & metabolism.2002;16(2):289-309.
- Martin OV, Shialis T, Lester JN, Scrimshaw MD, Boobis AR, Voulvoulis N. Testicular dysgenesis syndrome and the estrogen hypothesis: a quantitative meta-analysis. Environmental health perspectives. 2008;116(2):149-157.
- Den Hond E, Tournaye H, De Sutter P, Ombelet W, Baeyens W, Covaci A, Cox B, Nawrot TS, Van Larebeke N, D'Hooghe T. Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients. Environment international. 2015;84:154-160.
- Shozu M, Sebastian S, Takayama K, Hsu WT, Schultz RA, Neely K, Bryant M, Bulun SE. Estrogen excess associated with novel gain-of-function mutations affecting the aromatase gene. The New England journal of medicine. 2003;348(19):1855-1865.
- Hemsell DL, Edman CD, Marks JF, Siiteri PK, MacDonald PC. Massive extranglandular aromatization of plasma androstenedione resulting in feminization of a prepubertal boy. The Journal of clinical investigation.1977;60(2):455-464.
- Berkovitz GD, Guerami A, Brown TR, MacDonald PC, Migeon CJ. Familial gynecomastia with increased extraglandular aromatization of plasma carbon19-steroids. J Clin Invest. 1985;75(6):1763-1769.
- Stratakis CA, Vottero A, Brodie A, Kirschner LS, DeAtkine D, Lu Q, Yue W, Mitsiades CS, Flor AW, Chrousos GP. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. The Journal of clinical endocrinology and metabolism. 1998;83(4):1348-1357.
- Leiberman E, Zachmann M. Familial adrenal feminization probably due to increased steroid aromatization. Hormone research. 1992;37(3):96-102.
- Fukami M, Shozu M, Soneda S, Kato F, Inagaki A, Takagi H, Hanaki K, Kanzaki S, Ohyama K, Sano T, Nishigaki T, Yokoya S, Binder G, Horikawa R, Ogata T. Aromatase excess syndrome: identification of cryptic duplications and deletions leading to gain of function of CYP19A1 and assessment of phenotypic determinants. The Journal of clinical endocrinology and metabolism. 2011;96(6):E1035-1043.
- Shihara D, Miyado M, Nakabayashi K, Shozu M, Ogata T, Nagasaki K, Fukami M. Aromatase excess syndrome in a family with upstream deletion of CYP19A1. Clinical endocrinology. 2014;81(2):314-316.
- Tan X, Wu X, Chen J, Wu Y, Li S, Chen X, Zhang X. Aromatase excess syndrome in a Chinese boy due to a novel duplication at 15q21.2. J Pediatr Endocrinol Metab. 2019;32(1):85-88.
- Binder G, Nakamura A, Schweizer R, Ogata T, Fukami M, Nagasaki K. Long-term Effect of Aromatase Inhibition in Aromatase Excess Syndrome. The Journal of clinical endocrinology and metabolism. 2021;106(5):1491-1500.
- Klinefelter HF RE, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A-Leydigism, and increased excretion of follicle stimulating hormone. Journal of Clinical Endocrinology.1942;2(11):615-627.
- Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet (London, England).2004;364(9430):273-283.
- Wistuba J. Animal models for Klinefelter's syndrome and their relevance for the clinic. Molecular human reproduction. 2010;16(6):375-385.
- Bonomi MR, V; Pasquali, D; Balercia, G; Jannini, EA; Ferlin, A. Klinefelter Syndrome (KS): genetics, clinical phenotype and hypogonadism. Journal of Endocrinological Investigation. 2016.
- Santi D, De Vincentis S, Scaltriti S, Rochira V. Relative hyperestrogenism in Klinefelter Syndrome: results from a meta-analysis. Endocrine. 2019;64(2):209-219.
- Jiang DD, Swenson E, Mason M, Turner KR, Dugi DD, Hedges JC, Hecht SL. Effects of Estrogen on Spermatogenesis in Transgender Women. Urology. 2019;132:117-122.
- Leavy M, Trottmann M, Liedl B, Reese S, Stief C, Freitag B, Baugh J, Spagnoli G, Kölle S. Effects of Elevated β-Estradiol Levels on the Functional Morphology of the Testis - New Insights. Sci Rep. 2017;7:39931.
- Matoso A, Khandakar B, Yuan S, Wu T, Wang LJ, Lombardo KA, Mangray S, Mannan A, Yakirevich E. Spectrum of findings in orchiectomy specimens of persons undergoing gender confirmation surgery. Hum Pathol. 2018;76:91-99.
- Vereecke G, Defreyne J, Van Saen D, Collet S, Van Dorpe J, T'Sjoen G, Goossens E. Characterisation of testicular function and spermatogenesis in transgender women. Human reproduction (Oxford, England).2021;36(1):5-15.
- Schneider F, Kliesch S, Schlatt S, Neuhaus N. Andrology of male-to-female transsexuals: influence of cross-sex hormone therapy on testicular function. Andrology. 2017;5(5):873-880.
- Mezzullo M, Pelusi C, Fazzini A, Repaci A, Di Dalmazi G, Gambineri A, Pagotto U, Fanelli F. Female and male serum reference intervals for challenging sex and precursor steroids by liquid chromatography - tandem mass spectrometry. The Journal of steroid biochemistry and molecular biology. 2020;197:105538.
- Balestrieri A, Faustini-Fustini M, Rochira V, Carani C. Clinical implications and management of oestrogen deficiency in the male. Clinical endocrinology. 2001;54(4):431-432.
- Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. The Journal of clinical endocrinology and metabolism.2013;98(4):1376-1387.
- Handelsman DJ, Newman JD, Jimenez M, McLachlan R, Sartorius G, Jones GR. Performance of direct estradiol immunoassays with human male serum samples. Clinical chemistry. 2014;60(3):510-517.
- Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB, Jr. Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. The Journal of clinical investigation. 1994;94(6):2475-2480.
- Huhtaniemi IT, Tajar A, Lee DM, O'Neill TW, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Forti G, Wu FC. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. European journal of endocrinology / European Federation of Endocrine Societies. 2012;166(6):983-991.
- Ketha H, Girtman A, Singh RJ. Estradiol assays--The path ahead. Steroids. 2015;99(Pt A):39-44.
- Simoni MF, F; Roli, L; Pagotto, U. Methodology for measuring testosterone, dihydrotestosterone and sex hormone-binding globulin in a clinical setting. In: Behre ENHM, ed. Testosterone Action, Deficiency, Substitution. Fourth Edition ed. Cambridge, UK: Cambridge University Press; 2012:60-86.
- Pagotto U, Fanelli F, Granata AR. Hormonal Laboratory Examination. In: Simoni M, Huhtaniemi IT, eds. Endocrinology of the Testis and Male Reproduction. Cham: Springer International Publishing; 2017:495-516.
- Aguirre LE, Colleluori G, Fowler KE, Jan IZ, Villareal K, Qualls C, Robbins D, Villareal DT, Armamento-Villareal R. High aromatase activity in hypogonadal men is associated with higher spine bone mineral density, increased truncal fat and reduced lean mass. European journal of endocrinology / European Federation of Endocrine Societies. 2015;173(2):167-174.
- Aguirre LE, Colleluori G, Robbins D, Dorin R, Shah VO, Chen R, Jan IZ, Qualls C, Villareal DT, Armamento-Villareal R. Bone and body composition response to testosterone therapy vary according to polymorphisms in the CYP19A1 gene. Endocrine. 2019;65(3):692-706.
- Stephens-Shields AJ, Snyder PJ, Ellenberg SS, Taylor L, Bhasin S. Relation of Testosterone, Dihydrotestosterone, and Estradiol With Changes in Outcomes Measures in the Testosterone Trials. The Journal of clinical endocrinology and metabolism. 2022;107(5):1257-1269.
- Garg H, Kumar R. Empirical Drug Therapy for Idiopathic Male Infertility: What is the New Evidence? Urology.2015;86(6):1065-1075.
- Yang C, Li P, Li Z. Clinical application of aromatase inhibitors to treat male infertility. Hum Reprod Update.2021;28(1):30-50.
- Kamischke A, Nieschlag E. Analysis of medical treatment of male infertility. Hum Reprod. 1999;14 Suppl 1:1-23.
- Vandekerckhove P, Lilford R, Vail A, Hughes E. Clomiphene or tamoxifen for idiopathic oligo/asthenospermia. The Cochrane database of systematic reviews. 2000(2):CD000151.
- Vandekerckhove P, Lilford R, Vail A, Hughes E. WITHDRAWN: Clomiphene or tamoxifen for idiopathic oligo/asthenospermia. The Cochrane database of systematic reviews. 2007(4):CD000151.
- Gregoriou O, Bakas P, Grigoriadis C, Creatsa M, Hassiakos D, Creatsas G. Changes in hormonal profile and seminal parameters with use of aromatase inhibitors in management of infertile men with low testosterone to estradiol ratios. Fertility and sterility. 2012;98(1):48-51.
- Mellinger RC, Thompson RJ. The effect of clomiphene citrate in male infertility. Fertility and sterility.1966;17(1):94-103.
- Kotoulas IG, Cardamakis E, Michopoulos J, Mitropoulos D, Dounis A. Tamoxifen treatment in male infertility. I. Effect on spermatozoa. Fertility and sterility. 1994;61(5):911-914.
- Clark RV, Sherins RJ. Treatment of men with idiopathic oligozoospermic infertility using the aromatase inhibitor, testolactone. Results of a double-blinded, randomized, placebo-controlled trial with crossover. Journal of andrology. 1989;10(3):240-247.
- Unal D, Yeni E, Verit A, Karatas OF. Clomiphene citrate versus varicocelectomy in treatment of subclinical varicocele: a prospective randomized study. International journal of urology : official journal of the Japanese Urological Association. 2001;8(5):227-230.
- Cappon GD, Horimoto M, Hurtt ME. Reproductive toxicity assessment of lasofoxifene, a selective estrogen receptor modulator (SERM), in male rats. Birth defects research Part B, Developmental and reproductive toxicology. 2004;71(3):142-149.
- Katz DJ, Nabulsi O, Tal R, Mulhall JP. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU international. 2012;110(4):573-578.
- Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertility and sterility. 2010;93(7):2232-2235.
- Surbone A, Vaucher L, Primi MP, Leyvraz C, Pitteloud N, Ballabeni P, Mathevet P, Vulliemoz N. Clomiphene citrate effect on testosterone level and semen parameters in 18 infertile men with low testosterone level and normal/low gonadotropines level. Eur J Obstet Gynecol Reprod Biol. 2019;238:104-109.
- Sharma D, Zillioux J, Khourdaji I, Reines K, Wheeler K, Costabile R, Kavoussi P, Smith R. Improvements in semen parameters in men treated with clomiphene citrate-A retrospective analysis. Andrologia.2019;51(5):e13257.
- Patel DP, Brant WO, Myers JB, Presson AP, Johnstone EB, Dorais JA, Aston KI, Carrell DT, Hotaling JM. The safety and efficacy of clomiphene citrate in hypoandrogenic and subfertile men. Int J Impot Res. 2015;27(6):221-224.
- Helo S, Ellen J, Mechlin C, Feustel P, Grossman M, Ditkoff E, McCullough A. A Randomized Prospective Double-Blind Comparison Trial of Clomiphene Citrate and Anastrozole in Raising Testosterone in Hypogonadal Infertile Men. The journal of sexual medicine. 2015;12(8):1761-1769.
- Cannarella R, Condorelli RA, Mongioì LM, Barbagallo F, Calogero AE, La Vignera S. Effects of the selective estrogen receptor modulators for the treatment of male infertility: a systematic review and meta-analysis. Expert Opin Pharmacother. 2019;20(12):1517-1525.
- Gundewar T, Kuchakulla M, Ramasamy R. A paradoxical decline in semen parameters in men treated with clomiphene citrate: A systematic review. Andrologia. 2021;53(1):e13848.
- Wiehle RD, Fontenot GK, Wike J, Hsu K, Nydell J, Lipshultz L. Enclomiphene citrate stimulates testosterone production while preventing oligospermia: a randomized phase II clinical trial comparing topical testosterone. Fertility and sterility. 2014;102(3):720-727.
- Earl JA, Kim ED. Enclomiphene citrate: A treatment that maintains fertility in men with secondary hypogonadism. Expert Rev Endocrinol Metab. 2019;14(3):157-165.
- Adamopoulos DA, Pappa A, Billa E, Nicopoulou S, Koukkou E, Michopoulos J. Effectiveness of combined tamoxifen citrate and testosterone undecanoate treatment in men with idiopathic oligozoospermia. Fertility and sterility. 2003;80(4):914-920.
- Moein MR, Tabibnejad N, Ghasemzadeh J. Beneficial effect of tamoxifen on sperm recovery in infertile men with nonobstructive azoospermia. Andrologia. 2012;44 Suppl 1:194-198.
- Boonyarangkul A, Vinayanuvattikhun N, Chiamchanya C, Visutakul P. Comparative Study of the Effects of Tamoxifen Citrate and Folate on Semen Quality of the Infertile Male with Semen Abnormality. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2015;98(11):1057-1063.
- Tsourdi E, Kourtis A, Farmakiotis D, Katsikis I, Salmas M, Panidis D. The effect of selective estrogen receptor modulator administration on the hypothalamic-pituitary-testicular axis in men with idiopathic oligozoospermia. Fertility and sterility. 2009;91(4 Suppl):1427-1430.
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology.2013;1(5):749-757.
- Cavallini G, Biagiotti G, Bolzon E. Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian journal of andrology.2013;15(6):806-811.
- Shoshany O, Abhyankar N, Mufarreh N, Daniel G, Niederberger C. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertility and sterility. 2017;107(3):589-594.
- Shuling L, Sie Kuei ML, Saffari SE, Jiayun Z, Yeun TT, Leng JPW, Viardot-Foucault V, Nadarajah S, Chan JKY, Hao TH. Do men with normal testosterone-oestradiol ratios benefit from letrozole for the treatment of male infertility? Reprod Biomed Online. 2019;38(1):39-45.
- Peivandi S, Jafarpour H, Abbaspour M, Ebadi A. Effect of letrozole on spermogram parameters and hormonal profile in infertile men: A clinical trial study. Endocr Regul. 2019;53(4):231-236.
- Shah T, Nyirenda T, Shin D. Efficacy of anastrozole in the treatment of hypogonadal, subfertile men with body mass index ≥25 kg/m(2). Transl Androl Urol. 2021;10(3):1222-1228.
- Kooshesh L, Bahmanpour S, Zeighami S, Nasr-Esfahani MH. Effect of Letrozole on sperm parameters, chromatin status and ROS level in idiopathic Oligo/Astheno/Teratozoospermia. Reproductive biology and endocrinology : RB&E. 2020;18(1):47.
- Ribeiro MA, Gameiro LF, Scarano WR, Briton-Jones C, Kapoor A, Rosa MB, El Dib R. Aromatase inhibitors in the treatment of oligozoospermic or azoospermic men: a systematic review of randomized controlled trials. JBRA Assist Reprod. 2016;20(2):82-88.
- Kumar R, Gautam G, Gupta NP. Drug therapy for idiopathic male infertility: rationale versus evidence. J Urol.2006;176(4 Pt 1):1307-1312.
- Chandrapal JC, Nielson S, Patel DP, Zhang C, Presson AP, Brant WO, Myers JB, Hotaling JM. Characterising the safety of clomiphene citrate in male patients through prostate-specific antigen, haematocrit, and testosterone levels. BJU international. 2016;118(6):994-1000.
- Wibowo E, Pollock PA, Hollis N, Wassersug RJ. Tamoxifen in men: a review of adverse events. Andrology.2016;4(5):776-788.
- Bradley KL, Tyldesley S, Speers CH, Woods R, Villa D. Contemporary systemic therapy for male breast cancer. Clinical breast cancer. 2014;14(1):31-39.
- Loves S, de Jong J, van Sorge A, Telting D, Tack CJ, Hermus A, Westerterp K, de Boer H. Somatic and psychological effects of low-dose aromatase inhibition in men with obesity-related hypogonadotropic hypotestosteronemia. European journal of endocrinology / European Federation of Endocrine Societies.2013;169(5):705-714.
- Lapauw B, T'Sjoen G, Mahmoud A, Kaufman JM, Ruige JB. Short-term aromatase inhibition: effects on glucose metabolism and serum leptin levels in young and elderly men. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160(3):397-402.
- Hero M, Toiviainen-Salo S, Wickman S, Makitie O, Dunkel L. Vertebral morphology in aromatase inhibitor-treated males with idiopathic short stature or constitutional delay of puberty. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(7):1536-1543.
- Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. The Journal of clinical endocrinology and metabolism.2009;94(12):4785-4792.
- Hero M, Ankarberg-Lindgren C, Taskinen MR, Dunkel L. Blockade of oestrogen biosynthesis in peripubertal boys: effects on lipid metabolism, insulin sensitivity, and body composition. European journal of endocrinology / European Federation of Endocrine Societies. 2006;155(3):453-460.
- Allasia S, Motta G, Mirabelli M, Tagliabue MP, Lanfranco F. A case of deep vein thrombosis in a young male treated with tamoxifen for idiopathic infertility. Asian journal of andrology. 2017;19(5):615-616.
- Sun D, Jin B, Cai B, Deng W. Deep vein thrombosis in a nonobstructive azoospermia male taking tamoxifen: a rare case report. Transl Androl Urol. 2020;9(4):1769-1772.
- Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, Krausz C, Giwercman A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology. 2018;6(4):513-524.
- Ferlin A, Calogero AE, Krausz C, Lombardo F, Paoli D, Rago R, Scarica C, Simoni M, Foresta C, Rochira V, Sbardella E, Francavilla S, Corona G. Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS) : Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR). Journal of endocrinological investigation. 2022;45(5):1085-1113.
- Hsu B, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Hirani V, Waite LM, Seibel MJ, Handelsman DJ. Temporal Changes in Androgens and Estrogens Are Associated With All-Cause and Cause-Specific Mortality in Older Men. The Journal of clinical endocrinology and metabolism. 2016;101(5):2201-2210.
- Loh NY, Humphreys E, Karpe F, Tomlinson JW, Noordam R, Christodoulides C. Sex hormones, adiposity, and metabolic traits in men and women: a Mendelian randomisation study. European journal of endocrinology / European Federation of Endocrine Societies. 2022;186(3):407-416.