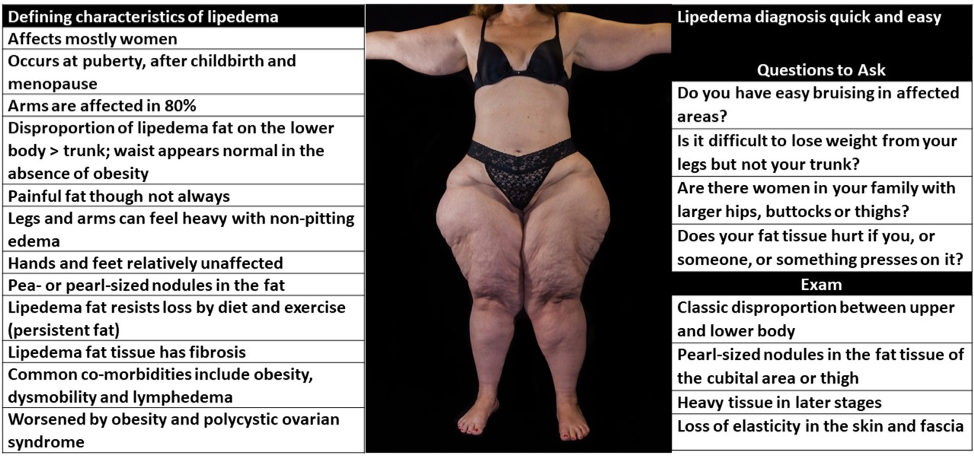
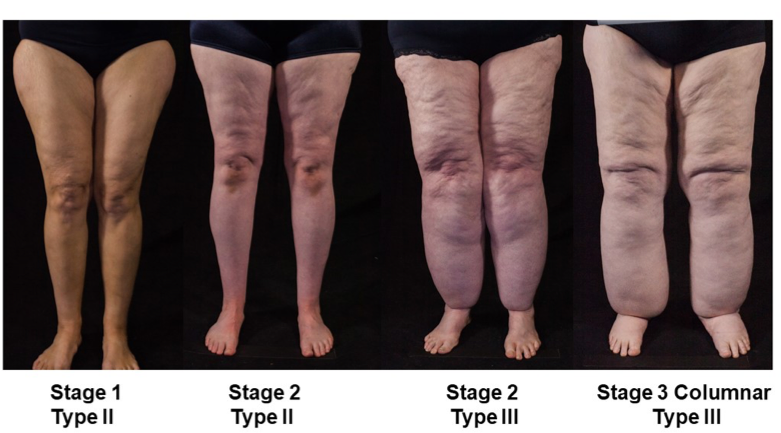
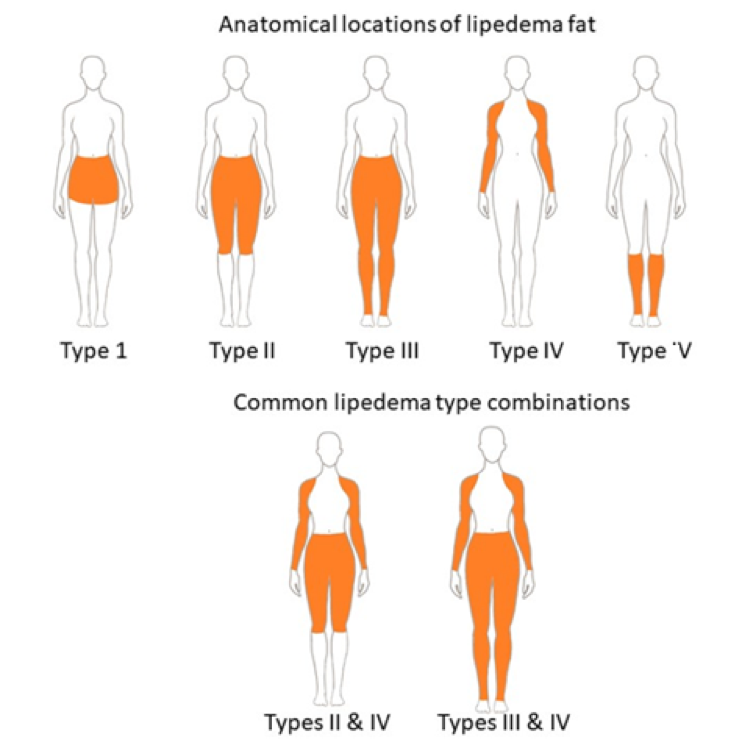
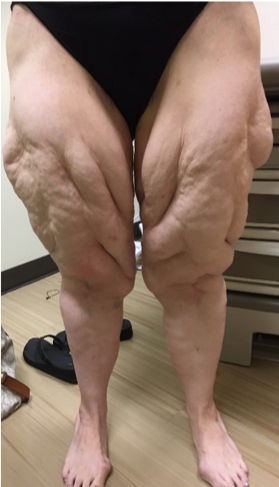
ABSTRACT
Type 1 diabetes (T1D) is an autoimmune disease characterized by progressive pancreatic beta-cell loss resulting in insulin deficiency and hyperglycemia. Exogenous insulin therapy is essential to prevent fatal complications from hyperglycemia. The Diabetes Control and Complications Trial and its long-term follow up, the Epidemiology of Diabetes and its Complications study, demonstrated that stringent glycemic control with intensive insulin therapy can prevent or postpone progression of microvascular disease and reduce risk for macrovascular disease and all-cause mortality. In addition, data obtained from the T1D Exchange, a registry of T1D patients founded in 2010, has become an invaluable resource for scientists worldwide, facilitating collaboration and accelerating understanding of prevailing diabetes practices. Insulin therapy using rapid- and long-acting insulin analogs is the mainstay of management of T1D. Insulin delivery is achieved subcutaneously using multiple daily injections or subcutaneous insulin infusion using insulin pumps. Effective management also involves use of self-monitoring of blood glucose using improved blood glucose meters, continuous glucose monitoring (CGM) devices, and newer insulin pumps with integrated sensor-augmented systems. Addressing psychosocial aspects of T1D plays a crucial role in effective disease management. Strategies to manage T1D are rapidly evolving. In addition to newer insulins, adjunctive non-insulin therapies such as use of incretin agents and SGLT-2 and combination SGLT-1/2 inhibitors are being actively pursued. CGM technology combined with glucose prediction algorithms has allowed for the development of artificial pancreas delivery systems. Cellular replacement options include pancreas and islet cell transplantation which can restore euglycemia but are limited by donor availability and the need for chronic immunosuppression. Newer strategies under development include islet cell encapsulation techniques, which might obviate the need for immunosuppression. Smart-insulin delivery systems, capable of releasing insulin depending on ambient glucose, are also being evaluated.
HISTORY OF TYPE 1 DIABETES TREATMENTS
Insulin Therapy
The discovery of insulin in 1921-22 was one of the greatest medical breakthroughs in history (1) (Figure 1). Initial work at the University of Toronto allowed for pancreatic extracts to be used to decrease blood glucoses in diabetic dogs. Developments by the pharmaceutical industry allowed for the large-scale commercial insulin production in 1923 (2). Individuals, mostly children with type 1 diabetes (T1D), whose life expectancies were measured in months were now able to prevent fatal ketoacidosis by taking injections of crude “soluble” (later known as regular) insulin. However, problems were soon noted. Hypoglycemia, occasionally life-threatening, was encountered as diabetes monitoring by urine testing for glycosuria was crude at best during those first decades after the discovery of insulin. The insulin itself was often impure and varied in potency from lot to lot. Allergic reactions were common and occasionally anaphylaxis would occur. Even more concerning was the appreciation that these patients often succumbed to chronic vascular complications which either dramatically reduced quality of life or resulted in a fatal cardiovascular event.
Tools to manage individuals with T1D improved over the decades since the discovery of insulin. Initial insulins were manufactured from bovine or porcine pancreata and production techniques became more efficient. Insulins with longer duration of action were first introduced in the 1930s, and over time purity and consistency of potency of these insulins improved (3). Nevertheless, “standard” animal insulins prior to 1980 contained 300-10000 parts per million of impurities, and elicited local and systemic effects when injected. Present day insulins sold in the United States today all contain less than 1 part per million of impurities.
Major improvements in insulin were developed in the late 1970s and early 1980s. First, not only was “purified” insulin introduced, but in 1982 the first human insulin was marketed both by Eli Lilly (recombinant DNA technology) and Novo (semi-synthetic methodology). These insulins were available as short-acting (regular) and longer-acting (Neutral protamine Hagedorn (NPH), lente, and ultralente) preparations. The other major advance with insulin therapy was with the delivery by the first continuous subcutaneous insulin infusion (CSII) pumps. While pumps were initially touted as providing less variable insulin absorption, the use of CSII had a greater impact: both patients and clinicians used this tool to teach themselves how to best use “basal bolus” insulin therapy, a strategy that would become a standard of care after the beginning of the next century with the development of insulin analogs.
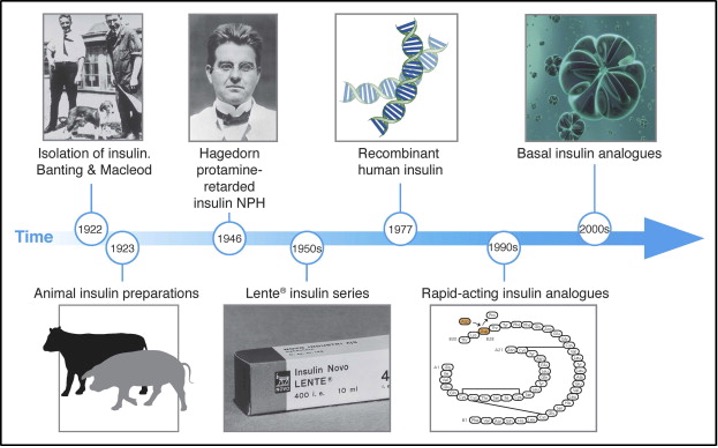
Figure 1. Time line of the evolution of insulin therapy. Figure source ref 3.
Monitoring Tools
At the same time as the development of human insulin and insulin pumps, improvements in glucose monitoring were introduced. Although there was initial skepticism if home blood glucose monitoring would be accepted by patients with diabetes, history has confirmed that this technology has revolutionized diabetes management and has allowed patients to titrate blood glucose to normal or near-normal levels. While self-monitoring of blood glucose (SMBG) allowed immediate evaluation of diabetes management, the introduction of hemoglobin A1c (HbA1c, or glycated hemoglobin, A1C) around the same time was used as a marker of objective longer-term (about 90 days) glucose control. When hemoglobin is exposed to glucose in the bloodstream, the glucose slowly becomes nonenzymatically bound to the hemoglobin in a concentration-dependent manner. The percentage of hemoglobin molecules that are glycated (have glucose bound to it) indicates what the average blood glucose concentration has been over the life of the red blood cell. Perhaps as importantly, A1C made it possible for researchers to study the effects of long-term glucose control and the development of vascular complications. New students of diabetes may now find it difficult to appreciate that one of the greatest medical controversies between the discovery of insulin and the early 1990s was the relationship between glucose control and diabetes complications. Improved insulins, pumps, SMBG, and A1C finally allowed this question to be properly studied.
THE DIABETES CONTROL AND COMPLICATIONS TRIAL
In 1993, all controversy regarding the impact of glucose control and vascular complications was dramatically answered with the publication of the Diabetes Control and Complications Trial (DCCT) (4). The trial showed definitively that stringent blood glucose control (for an average of 6.5 years) could slow or postpone the progression of retinal, renal, and neurological complications in individuals with T1D (Figure 2). In patients treated with “intensive therapy”—that is, therapy aimed at maintaining blood glucose levels as close to normal as possible—the risk of developing diabetic retinopathy was reduced by 76%, diabetic neuropathy by 60%, and diabetic nephropathy by 54%, compared with conventionally treated patients. Other benefits of intensive diabetes management include improved lipid profiles, reduced risk factors for macrovascular disease, and better maternal and fetal health.
Since the DCCT was completed in 1993, the research subjects have been followed in an observational study calledEpidemiology of Diabetes and its Complications (EDIC) (5). It was soon observed that the impact of this improved diabetes therapy for an average of 6.5 years (maintaining a A1C of approximately 7% with multiple injections or CSII compared to once or twice daily insulin and a A1C of approximately 9%) had long-lasting effects. Termed “metabolic memory”, there continued to be improvements in microvascular complications four years after the DCCT ended (Figure 3) (6-8). Despite the fact that A1C levels remained about 8% for both groups after the DCCT, the risk reduction for nonfatal myocardial infarction, stroke, or death were reduced by 57% eleven years after the conclusion of the formal study. The conclusions of this are profound since this was the first study to report a reduction of macrovascular disease with glucose control. Furthermore, these data confirmed the need to control blood glucose as meticulously as possible early in the course of the disease (9).
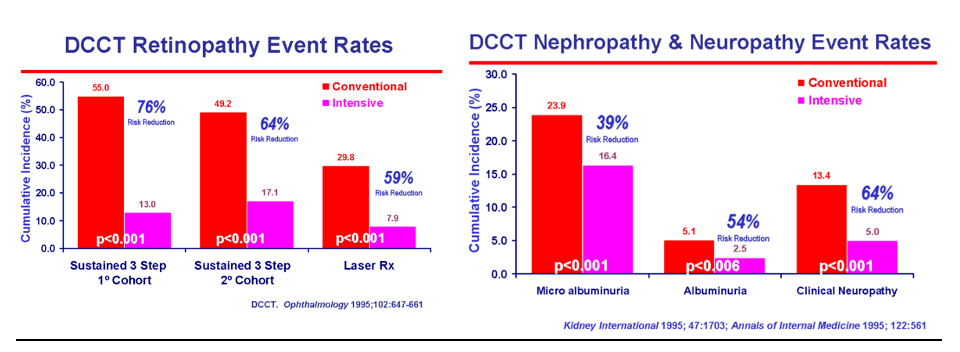
Figure 2. Relationship between microvascular complications and A1C in T1D
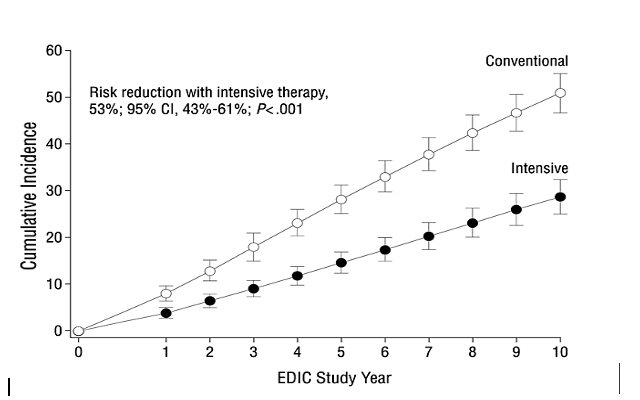
Figure 3. Cumulative incidence of further 3-step progression of retinopathy from DCCT closeout to EDIC study year 10 (adjusted for retinopathy level at DCCT end, cohort, entry A1C, baseline diabetes duration). From reference (10).
TYPE 1 DIABETES EXCHANGE
Compared with treatment methods used in the DCCT over 20 years ago, many new tools and technologies have now become available that enable patients and clinicians to attain target A1C levels more safely. Rapid- and long-acting insulin analogs, improved blood glucose meters, newer insulin pumps with integrated sensor-augmented systems and with automatic threshold suspend capabilities and continuous glucose monitoring (CGM) devices now play an integral part of T1D management. To evaluate how these advances in diabetes technology have impacted glycemic control in T1D, a broad-based, large-scale, multisite registry that includes patients at all ages across the life span in the U.S. was established in 2010 through a grant from the Leona M. and Harry B. Helmsley Charitable Trust. Called the T1D Exchange, this registry aims to provide an expansive data set to address important clinical and public health issues related to T1D. It comprises three complementary sections: i) a clinic network of adult and pediatric diabetes clinics; ii) a Web site called Glu, serving as an online community for patients; and iii) a biobank to store biological human samples for use by researchers. A statistical resource center provides statistical support to the Exchange as well as other T1D researchers. The data have provided information about various aspects of T1D, including metabolic control and management, in the United States and the opportunity to compare this data with registries from Europe and Australia (11). The clinic registry has provided valuable information regarding the state of T1D management and outcomes and allowed for addressing important clinical and public health issues. Registry data also have helped identify knowledge gaps leading to further advancements in clinical trials and epidemiologic research with over 47 publications as of March 2019 (12).
Currently there are over 35,000 patients enrolled in the registry, ranging in age from 1 - 93 years, with a duration of diabetes ranging from 1.5 to 83 years, 50% female, 82% were non-Hispanic white (13). Most recent data from the registry revealed that mean A1C in adults over age 30 ranged from 7.5-7.8%, which is lower than the value of 8% observed in the DCCT (14). However mean A1C levels increased in teens and emerging adults from 8.5% to 9.3%. Insulin pump use was observed in 63% of individuals. CGM use increased exponentially from 2010-12 to 2016-18 from 7% to 30%, with most participants using the Dexcom system (77%). CGM use increased significantly in the pediatric population. Many patients in the registry were able to achieve target A1C levels without an increase in the frequency of serious hypoglycemia as was observed in the DCCT. Use of adjunctive non-insulin glucose-lowering therapies was low overall and primarily included metformin, in 6% of adult participants over age 26 years.
CURRENT TECHNOLOGY IN TYPE 1 DIABETES
Glucose Meters
Current blood glucose monitoring systems (BGMS) are small electronic devices capable of analyzing glucose levels in capillary whole blood. To test blood glucose levels, patients are required to prick a finger using a lancing device to obtain a small drop of blood. The patient then places the drop of blood onto a glucose test strip, which has been previously inserted into the glucose meter. Typically, just a few seconds are required for the device to provide a blood glucose value.
BGMS use enzymatic reactions to provide estimates of blood glucose levels and the enzymes utilized include glucose oxidase, glucose dehydrogenase and hexokinase. The specific enzyme is usually packaged in a dehydrated form in a glucose test strip. Once blood is applied to the test strip, glucose in the patient’s blood sample rehydrates the enzyme activating a reaction. The product of this reaction can then be detected and measured by the glucose meter (15).
Notably, the advent of point-of-care BGMS has revolutionized diabetes care by allowing patients and practitioners to obtain real-time estimates of blood glucose values. These portable devices enabled patients to perform self-monitoring of blood glucose (SMBG), an integral component of effective diabetes self-management. The benefits of SMBG were confirmed during the DCCT which showed that intensive insulin therapy, requiring SMBG≥4 times/day with concomitant insulin dose titration, delayed the onset and slowed the progression of microvascular complications (4). Later, it was shown in the T1D Exchange that a higher frequency of testing (up to 10 times daily) is inversely associated with A1C levels in all age groups (16).
SMBG allows patients to guide management decisions (e.g., adjusting food intake, insulin therapy, and exercise) and determine whether glucose targets are being achieved. Further, it can help patients in monitoring and preventing asymptomatic hypoglycemia (17).
Patients with T1D should perform SMBG at a minimum of 4 times a day (before meals and at bedtime), as this will allow adjustments to prandial and basal insulin doses. In addition, SMBG should be considered prior to snacks, before and at completion of exercise, in the event of symptoms suggestive of hypoglycemia, and after treating hypoglycemia until blood glucose levels have normalized. Lastly, patients should test their blood glucose before performing critical tasks such as driving a motor vehicle or operating heavy machinery. Ultimately, frequency of SMBG will largely depend on patients’ individual needs (17).
An important point to make, however, is that patients should also be educated on avoiding “overuse” of SMBG. Testing too frequently may lead to administration of multiple correction doses within short periods of time, particularly if patients are anxious about their glucose levels not returning to target “fast enough”, leading to insulin “stacking” and resulting in iatrogenic hypoglycemia.
The technology of BGMS has evolved over the years and current devices are relatively easy to use and require minimal amounts of blood (Figure 4). Some instruments are able to capture events affecting glucose control (e.g., exercise, meals, insulin administration), provide customized reports, and calculate insulin bolus needs according to glycemia and intake of carbohydrate based on pre-established settings (i.e., insulin sensitivity factor and insulin-to-carbohydrate ratios). However, despite these unique advances in self-monitoring of blood glucose, independent analytic testing has shown that various BGMS do not fulfill the accuracy requirements set by the International Organization for Standardization (ISO) 151917 which requires for ≥95% of results to fall within ± 15 mg/dL of the reference result for samples with glucose concentrations <100 mg/dL and ±15% for samples with glucose concentrations ≥100 mg/dL (18). In addition, the FDA has stated that the ISO 15197 criteria are not sufficient to adequately protect lay-users of SMBGs because, for example, the standard does not adequately address the performance of over-the-counter blood glucose testing systems in the hypoglycemic range or across test strip lots. In view of this, the FDA has developed the “Self-Monitoring Blood Glucose Test Systems for Over-the-Counter use” guidance document which is intended to guide manufacturers in conducting appropriate performance studies and preparing 510(k) submissions for these device types (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/self-monitoring-blood-glucose-test-systems-over-counter-use). Thus, there is a pressing need for high quality standards to ensure improved accuracy and precision from BGMS.
SMBG has important drawbacks since blood is only sampled intermittently and therefore only glimpses of blood glucose concentrations are provided. SMBG does not offer information on glucose fluctuations even if performed frequently. Thus, there is potential for missing episodes of hyperglycemia and hypoglycemia.

Figure 4. Examples of a few blood glucose monitoring systems.
Glucose Downloads
The vast majority of currently available BGMS allow the generation of downloadable reports. These reports are a unique component of the patients’ evaluation allowing the identification of areas that require special attention in diabetes management. However, technical difficulties often compromise the usefulness of these data. For instance, it is not unusual for the date and/or time of the glucose meters to be inaccurate. Simple errors such as these have a huge impact on patient management as the data downloaded becomes largely uninterpretable. In addition, as each glucose meter usually has its own proprietary software, if a clinic does not have the specific software installed on their local computers, then the data may not be downloaded. The clinician is left with trying to review the data directly from the device, which is time consuming and does not offer the detailed overview from a customized printable report. There are platforms that are currently available which allow downloading various glucose meters, insulin pumps and CGM data and provide standardized reports (e.g., Clinipro®, Diasend®, Carelink®, Glooko®). However, there needs to be a unified effort by BGMS, insulin pump, and CGM companies in order to generate a universal download protocol as this would simplify data analysis and interpretation by practitioners (19).
Continuous Glucose Monitoring
Perhaps the most innovative technology for the treatment of T1D is the introduction of CGM (Figure 5). CGM technology allows for the measurement of glucose concentrations in the interstitial fluid (ISF) which correlates with plasma glucose values. However, when interpreting CGM values it is important to understand that ISF glucose consistently lags plasma glucose. A study in healthy adults analyzing glucose tracers following an overnight fast showed that it takes 5-6 minutes for glucose to be transported from the vascular to the interstitial space (physiological delay) (20). This is particularly relevant when glucose levels are trending up or down quickly as CGM data will not be as reliable in such scenarios and thus patients should confirm the direction of their glucose concentration by SMBG.
The components of CGM consist of a sensor that is inserted subcutaneously, a small electronic device that serves as the platform for the sensor, a transmitter, and a receiver device, which can be a standalone device or a smartphone (Figure 5). CGM Sensors can measure glucose levels up to every minute allowing for a glucose tracing to be generated and displayed in real-time (RT-CGM) on a receiver device, greatly improving the understanding of patients’ glucose profiles. Further, with the exception of the GuardianTM Connect system (Medtronic) which is pending approval, currently available CGM devices have obtained FDA approval for non-adjunctive use which means that patients can rely on their CGM values in order to guide management decisions (21).
Patients can customize alarms to activate for hypoglycemia or hyperglycemia. Understanding the trend allows patients to decide whether an increase or decrease in mealtime insulin dose is necessary. CGM thus also allows patients to intercept hypoglycemia (or hyperglycemia) prior to it occurring. Patients can also “flag” events thereby improving interpretation of glucose control associated with meals, insulin administration, and exercise. Also, most CGM devices allow users to share their RT-CGM data with others (e.g., family members or friends) which can then be monitored on a smartphone or other internet-enabled devices. This is of particular interest in the pediatric population as it allows parents to remotely monitor their child’s glucose profile when away from home or while exercising (e.g., participating in sports). Features of currently available CGM devices are listed in Figure 6.
Based on how the CGM data is delivered to the user, current CGM devices fall under 2 categories: Flash glucose monitoring (or intermittently scanned glucose monitoring) and Real-time glucose monitoring.
FLASH GLUCOSE MONITORING
Flash glucose monitoring requires the user to hold a reader device (which can be a smart phone) close to the subcutaneously inserted sensor (the patient “scans” the sensor with the reader) to have the real-time interstitial glucose value displayed. During a scan, the reader displays the real time glucose value, glucose alerts, a historic glucose trend of values recorded and a trend arrow indicating the glucose direction (22). There are currently 2 approved Flash CGM devices for patient use, the FreeStyle Libre 14 day and the FreeStyle Libre 2 (Figure 5). The Libre 14 days allows for real-time data sharing but is limited by the lack of alarms in case glucose values are dangerously high or low. Nonetheless, this device may be appealing to those patients who want to minimize capillary blood glucose measurements and complain of CGM sensor alarm fatigue (23, 24). On the other hand, the Libre 2 has optional real-time glucose alarms but currently it requires a dedicated stand-alone receiver (data cannot be sent to a smartphone) and it does not have the capability of real-time data sharing.
REAL-TIME GLUCOSE MONITORING
Real-time glucose monitoring allows for data to be continuously sent to a receiver device and apart from viewing the display to check glucose levels and the direction of glucose profile, no additional action is required by the patient. Further, real-time CGM systems provide real-time alerts which can be customized to prevent or treat hyper or hypoglycemia. In addition, all currently approved real-time CGMs allow for data sharing.
Another advantage of CGM is the amount of data that can be generated and downloaded in customizable reports (Figure 7). Health care professionals are not only able to download daily glucose profiles in a graphic display but can also obtain several statistics including means, medians, standard deviations, interquartile ranges, and minimum and maximum values. This provides a better assessment of glycemic variability (Figure 8). Most importantly, time in glucose ranges can be identified and evaluated. This is particularly helpful in patients who have hypoglycemia unawareness and allows for adjusting the treatment plan by both the patient and practitioners to eliminate occurrence of hypoglycemia.
KEY CGM METRICS
Key CGM metrics include: Time in target range (TIR) defined as the percentage of readings and time per day within the recommended target glucose range of 70-180 mg/dL; time below target glucose range (TBR); and time above target glucose range (TAR) (see Figures 7 and 9 for examples). Current recommendations are to achieve TIR >70% (>16 h, 48 min), TBR <4% (<1 h) and TAR <25% (<6 h). However, recommendations are different for older adults/high-risk populations and during pregnancy (25). In addition to time in glucose ranges, CGM data has also allowed to generate a formula to estimate the laboratory A1C based on CGM mean glucose levels. This estimated A1C has been named “Glucose Management Indicator” and offers the advantage of being unaltered by limitations inherent to the laboratory A1C measurement (e.g., anemia, iron deficiency, glycation abnormalities, drug interference). The enormous amount of data generated by CGMs can be overwhelming and difficult to follow and interpret and the need for a standardized report is critical for data interpretation and medical decision making. The Ambulatory Glucose Profile is a standardized report which incorporates all the core CGM metrics and recommended targets along with a 14-day composite glucose profile and is the recommended report by the International Consensus on Time in Range (Figure 9) (25, 26).

Figure 5. Examples of real-time continuous glucose monitoring systems.
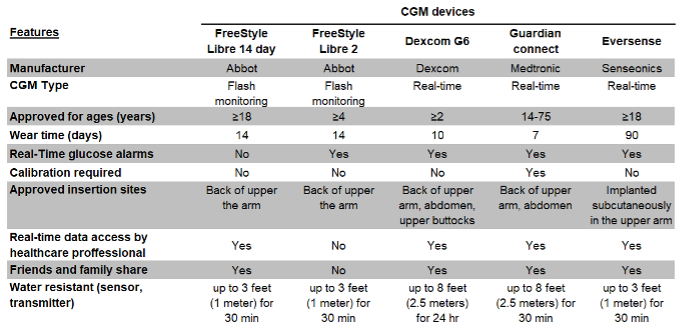
Figure 6. Features of currently approved CGM devices in the United States.
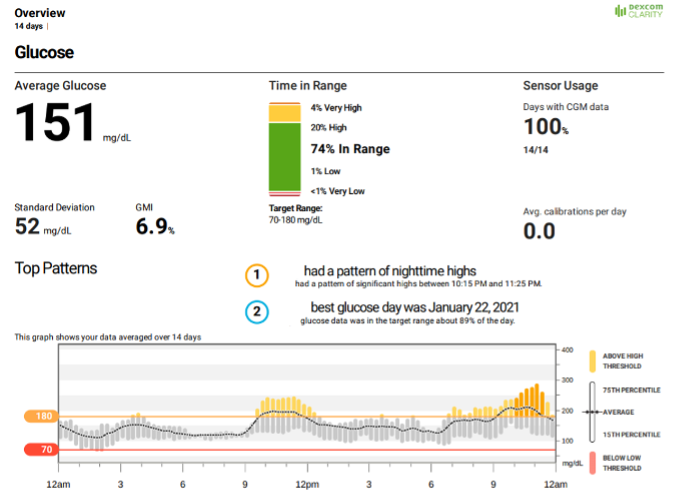
Figure 7. A 14-day DEXCOM CGM overview report showing sensor glucose data over a 24-hour period including mean (dotted line), standard deviation, glucose management indicator, interquartile range (grey bars), upper and lower glucose thresholds (orange and red lines, set by the user), percent time in range, sensor usage, top patterns, and average daily calibrations.
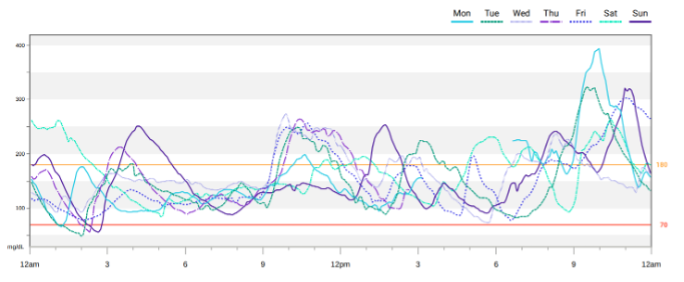
Figure 8. A 7-day DEXCOM CGM overlay report showing daily profiles allowing for the identification of trends and patterns.
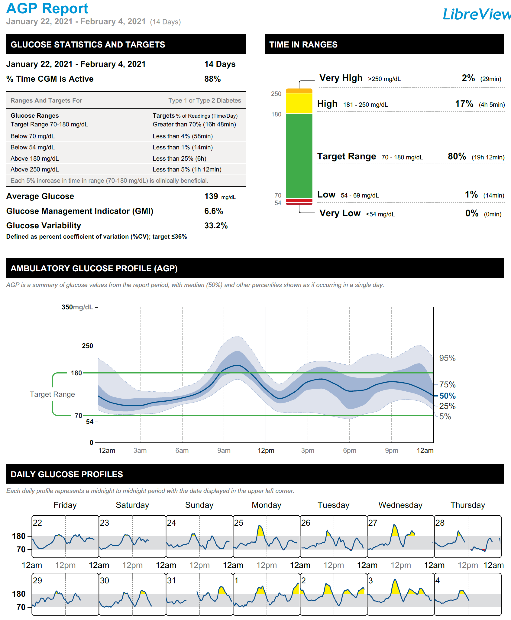
Figure 9. Ambulatory Glucose Profile (AGP) sample.
INSURANCE COVERAGE AND BILLING OF CGM DEVICES
Insurance coverage in the United States for devices is highly variable and challenging to navigate, and maybe unaffordable for some patients due to high copays or coverage issues. (These coverage requirements vary depending upon geographic area; practitioners are urged to follow guidelines in their country of practice). Understanding requirements for prescribing any CGM device is necessary and appropriate documentation is necessary. For individuals on Medicare to receive approval for a CGM device, documentation must include the following (as of 2021):
- The patient has diabetes mellitus and requires a therapeutic CGM.
- The patient is performing SMBG at least 4 times daily (Medicare only provides 3 test strips daily).
- The patient is treated with insulin and is injecting insulin at least 3 times daily or is on an insulin pump.
- The patient’s insulin treatment regimen requires frequent dose adjustment based on SMBG/CGM results.
- The patient had an in-person visit within 6 months prior to ordering the CGM with the treating practitioner to evaluate their diabetes and determine that criteria 1 to 4 are met. Subsequently, the patient must have an in-person visit every 6 months following the initial prescription to assess adherence to CGM and diabetes treatment plan.
There are billing codes for analyzing data from CGM devices. The patient visit should include certain key elements that need to be clearly documented in the chart as follows:
- A brief statement or narrative that the glucose sensor data were evaluated
- What patterns were noted
- Action steps and plan based on data interpretation provided to the patient
- Electronic or print of data report should be attached to the patient chart
CGM Integrated Insulin Pumps
As seen in Figure 10, some sensors are already integrated with insulin pumps (“sensor-augmented pumps”) so that the pump and receiver are in the same device. In addition, development of an integrated sensor and infusion set is currently being pursued, as this will simplify the incorporation of sensor technology into insulin pumps. Eventually, it is expected that all insulin pumps will be integrated with sensors. Yet, it should be appreciated that CGM is an equally important tool for MDI patients, and probably a more important diabetes management tool than using an insulin pump (21). Even after short periods of time, many patients can learn how to best use this technology to improve both mean glucose and glycemic variability. In a meta-analysis, comparing SMBG with RT-CGM, the latter achieved a lower A1C (between-group difference of change, -0.26%, (95% CI, -0.33% to -0.19%)) without increasing hypoglycemia (27). In the Juvenile Diabetes Research Foundation’s CGM trial, those individuals starting with baseline A1C levels under 7% overall had less hypoglycemia with CGM (28). A recent analysis of the T1D registry data suggests that CGM users, irrespective of insulin delivery method – i.e. multiple daily injections vs. pump therapy – had lower A1C levels than non-CGM users even after adjustment for confounding factors (29).
The American Association of Clinical Endocrinologists and American College of Endocrinology recommend the use of CGM for patients with T1D particularly for those with a history of severe hypoglycemia, hypoglycemia unawareness, and to assist in correction of hyperglycemia in patients not at goal. It may also be considered in pregnancy as it can help fine-tune insulin dosing, monitor for overnight hypoglycemia or hyperglycemia, and assess occurrence of postprandial hyperglycemia (30). The Endocrine Society guidelines on CSII Therapy and Continuous Glucose Monitoring in Adults recommend the use of RT-CGM for adult patients with T1D who either have A1C levels above target or well-controlled T1D and are willing and able to use these devices on a nearly daily basis (31).
Figure 10. Examples of modern-day insulin pumps.
OVERVIEW OF THERAPY FOR TYPE 1 DIABETES
Glycemic Targets
A1C is a measure of average glycemia over ~3 months and is a strong predictor of complications of diabetes (32). Current glycemic targets for adults from the American Diabetes Association (ADA) include a target A1C of <7%. However, it should be noted that this recommendation is a general target and the goal for the individual patient is as close to normal as possible (A1C of < 6%) without significant hypoglycemia. In addition, patients with T1D and hypoglycemia unawareness, long duration (> 25-30 years) of disease, limited life expectancies, very young children, or those with co-morbid conditions will require higher A1C targets. Individualized A1C targets need to be reviewed with each patient (17).
Thus, A1C testing should be performed routinely in all patients with diabetes as part of ongoing care. Frequency of A1C testing is determined based on the clinical situation, the treatment regimen used, and the clinician’s judgment. A1C measurements every 3 months help in the assessment of whether a patient’s glycemic targets have been reached. Although convenient, there are drawbacks to A1C measurements, as glycation rates may vary with patients’ race/ethnicity. Similarly, in patients with hemoglobinopathies, hemolytic anemia or other conditions that shorten the red blood cell life span, the A1C may not accurately reflect glycemic control or correlate with SMBG testing results. In such conditions, fructosamine may be considered as a substitute measure of long-term (average over 4 weeks) glycemic control. Clinicians should routinely compare downloaded SMBG or CGM averages with A1C as there are many reasons A1C may be altered due to a non-glycemic etiology and thus fructosamine or the downloaded glucose data itself would be a better metric to follow (33).
Non-Glycemic Treatment Targets
It should also be pointed out that in addition to glycemic targets, specific non-glycemic targets have also been recommended (34). Non-glycemic targets should also be tailored according to the individual with less stringent treatment goals for individuals with multiple coexisting illnesses and/or poor health and limited life expectancy. Recent real-world data from the T1D Exchange revealed that the incidence of cardiovascular disease (CVD) over 4.6 years was ~3.7% (35). Age, longer duration of diabetes, glycemic control, obesity, hypertension, dyslipidemia, and diabetic nephropathy were all associated with increased risk for CVD.
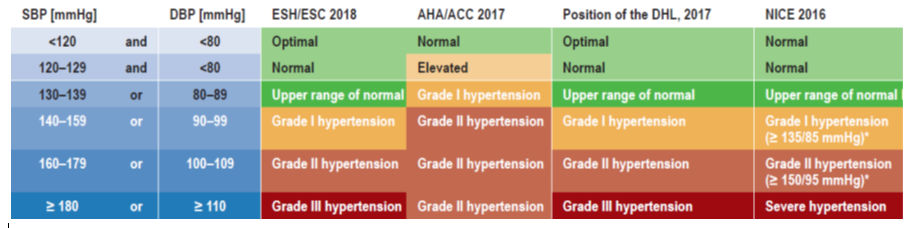
BLOOD PRESSURE
Good quality data to guide blood pressure management in T1D is lacking and most data are extrapolated from type 2 diabetes (T2D) clinical trials. The ADA recommends treatment to a goal of <140/90 mmHg for individuals with diabetes and hypertension at lower risk for CVD. Lower targets of <130/80 mmHg, should be considered for individuals who have higher cardiovascular risk or pre-existing ASCVD. Antihypertensive therapy should be initiated using a drug class that has demonstrated cardiovascular benefit such as angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), thiazide-like diuretics, or dihydropyridine calcium channel blockers. ACE inhibitors or ARBs are the preferred first line treatment for individuals with albuminuria.
LIPIDS
Very limited data exists for lipid management in patients with T1D of any age. Limited evidence suggests that primary prevention with lipid-lowering medications decreases the incidence of CVD (36). The ADA has adopted the approach of the 2018 American College of Cardiology/ American Heart Association multi-society cholesterol guidelines and recommends similar statin approaches for individuals with T1D (34). All patients with T1D and CVD should be treated with high intensity statins. Addition of non-statin therapies such as ezetimibe and PCSK9 inhibitors should be considered based on overall risk and achieved LDL-C thresholds. Patients with T1D over the age of 40 should be offered statin therapy. In individuals younger than age 40 with T1D and additional risk factors (such as albuminuria, HTN, strong family history, long duration of diabetes >20 years), moderate intensity statin therapy should be considered after clinical discussion. Recently, a prediction model for CVD events in T1D to help decision making for primary prevention that has been developed and shows promise but needs further validation (37). There is new evidence of the contribution of cardiac autoimmunity to CVD in T1D in the DCCT/EDIC cohort that warrants further investigation (38).
INSULIN THERAPY
Insulin therapy is the cornerstone of management of T1D as beta cell dysfunction or destruction progressively leads to absolute insulin deficiency. Physiologic insulin replacement that aims to mimic normal pancreatic insulin secretion is the preferred method of treatment of T1D patients. Basal insulin is the background insulin required to suppress hepatic glucose production overnight and between meals. Prandial (bolus or meal-time) insulin replacement, provides enough insulin to dispose of glucose after eating. Such a therapeutic insulin regimen providing both basal and bolus insulin allows flexibility of dosing. Older twice-daily combination of regular and NPH regimens generally should not be used in T1D as they are less effective since the time-action profile of these two standard insulins do not readily allow for the clear separation of basal and prandial insulin action. However, it may be necessary to use such regimens in patients who cannot otherwise afford insulin. It also should be pointed out that for newly diagnosed patients with T1D, transient use of once- or twice-daily basal injections is sometimes adequate.
Principles of Management of T1DM
Management of T1D involves a multidisciplinary framework that includes the following:
- Physiologic insulin replacement using basal-bolus therapy, either as MDI or CSII
- Blood glucose monitoring with SMBG and/or CGM with development of individualized A1c goals
- Patient education
- A supportive team of providers including endocrinologists, nurses, certified diabetes care and education specialists (CDCES)s, pharmacists, psychologists, dietitians, social workers, other specialists such as cardiologists, nephrologists, psychiatrists as well as family members, social support groups etc.
Types of Insulin
Selecting the appropriate insulin depends largely on the desired time course of insulin action. Table 1 shows the pharmacokinetic characteristics—time to onset of action, time of peak action, effective duration of action, and maximum duration of action—of currently available insulins; however, these can vary considerably among individuals.
Insulin products are categorized according to their action profiles:
- Rapid-acting: e.g., insulin lispro, insulin aspart, and insulin glulisine (genetically engineered insulin analogs)
- Short-acting: regular (soluble) insulin
- Intermediate-acting: NPH (isophane)
- Long-acting, e.g., insulin glargine, insulin detemir, and insulin degludec (genetically engineered insulin analogs)
- Pre-mixed insulin
- Inhaled insulin
Insulin analogs are insulin molecules modified by genetic engineering and recombinant DNA technology. The amino acid structure of insulin is altered to change the properties of insulin – i.e., time to onset, peak, and duration of action, compared to human regular insulin. However, the biological properties and stability of the insulin molecule are intact. A general principle to bear in mind is the longer the time to peak, the broader the peak and the longer the duration of action. Additionally, the breadth of the peak and the duration of action will be extended with increasing dose. Figure 11 should therefore be considered a conceptual representation of insulin action curves.
Mealtime (Prandial) Insulins
RAPID-ACTING INSULIN
These are insulin analogs with a rapid onset in 15-30 minutes, peak in 30-90 minutes, and an effective duration of 4 to 5 hours when injected subcutaneously. They have a shorter time action profile compared to human (regular) insulin because they do not self-aggregate in solution. All rapid-acting insulin analogs have a 1 - 2 amino acid difference from the primary structure of human insulin. Insulin lispro differs from human insulin by an amino acid exchange of lysine and proline at positions B28 and B29 (39). The substitution of aspartic acid for proline at position B28 characterizes insulin aspart (40). Insulin glulisine differs from human insulin in that the B3 asparagine is replaced by lysine, and B29 lysine is replaced by glutamic acid (41). These modifications in the primary structure of human insulin increase the rapidity of breakdown of insulin hexamers in the analogs and thus result in more rapid absorption. When administered before meals, rapid-acting insulins used as part of multiple daily injections (Figure 11) or with CSII, resemble physiologic insulin increases stimulated by food. Doses can be adjusted proportionate to food consumed; in patients with gastroparesis or poor appetite, insulin can be injected halfway through or after the meal. A follow-on biologic to insulin lispro (biosimilar lispro) is now available as Admelog.
Ultra-rapid acting insulin aspart (Fiasp) available since 2018 is insulin aspart with added niacinamide. This results in quicker absorption with faster onset of action after injection and therefore can be injected right before the start of a meal (or within 20 minutes after the start of a meal). This allows for some flexibility of dosing. Safety and efficacy data in adults and children is similar to insulin aspart (42). Fiasp has recently also been approved for use in insulin pumps. Data in pregnant women is lacking. Recently, ultra-rapid acting lispro (lispro-aabc) has become available in several countries including the United States. This insulin has been shown to appear in the bloodstream within 1 minute of injection (43). Ultra-rapid acting lispro was found to be non-inferior to rapid-acting lispro and superior for postprandial blood glucose control in T1D and T2D (44, 45).
INHALED INSULIN
Currently, one form of inhaled insulin is available in the market. Afrezza was approved by the FDA in 2014. This is a drug-device combination that contains powdered human insulin in single use dose cartridges delivered via a small inhaler. When inhaled, it dissolves immediately on contact with the alveolar surface of the lung and is rapidly absorbed into the systemic circulation, reaching a peak within 15 minutes. Thus, Afrezza acts similar to rapid-acting insulin analogs but with a much faster peak of action, and shorter duration of action. Prior to initiation of its use, patients should be screened for underlying lung disease with spirometry. Follow-up spirometry is recommended after 6 months’ use, and annually thereafter. The main advantages of inhaled insulin are avoidance of injections, faster onset of action, less weight gain, and less hypoglycemia (46). Dosing is not flexible as cartridges are available in fixed doses (4, 8 and 12 units). Afrezza is contraindicated in patients with chronic lung disease such as asthma or chronic obstructive pulmonary disease (COPD).
SHORT-ACTING INSULIN
Regular insulin is structurally similar to endogenous human insulin. It consists of dissolved zinc-insulin crystals which self-aggregate in the subcutaneous tissue and results in a delayed onset of action of 30 to 60 minutes, a peak of 2 to 3 hours, and an effective duration of 6 to 8 hours. Proper use requires injection at least 20 to 30 minutes prior to meals to match insulin availability and carbohydrate absorption. Use of regular insulin is associated with greater hypoglycemia risk (47). Regular insulin acts almost instantly when injected intravenously.
Basal Insulins
INTERMEDIATE-ACTING INSULIN
Neutral protamine Hagedorn (NPH) insulin, developed in the 1950s, is a combination of recombinant human insulin with protamine which results in crystal formation. When injected subcutaneously, precipitated crystals of NPH insulin are released slowly resulting in a longer duration of action compared to regular insulin. Action of NPH varies quite widely within the same patient as well as between patients. Its onset of action occurs 2 to 4 hours from the time of injection, with a peak effect lasting 6 to 10 hours, and an effective duration of 10 to 16 hours. Due to this peak effect, NPH insulin acts as a basal and a prandial insulin, necessitating that patients eat a meal at the time the insulin is peaking. NPH typically requires twice a day dosing (48).
LONG-ACTING INSULIN ANALOGS
Long acting insulin analogs were created by modifying the amino acid sequence on the beta chain of insulin (49). They exhibit much improved pharmacokinetics and pharmocodynamics without a peak effect and maintain a longer duration of action. Improved absorption rates result in significantly decreased inter-individual and intra-individual variability with improvement in glycemic control and reduced hypoglycemia risk.
Insulin glargine is a modified human insulin produced by the substitution of glycine for asparagine at position A21 of the insulin molecule and by the addition of two arginine molecules at position B30 (48). These changes result in an insulin molecule that is less soluble at the injection site forming a precipitate in the subcutaneous tissue to form a depot from which insulin is slowly released after injection and is slowly released into the circulation. It has no pronounced peak and a longer duration of action of about 20 to 24 hours in most patients, allowing for once daily dosing. In clinical practice, many patients with T1DM may benefit from twice-daily injections. Insulin glargine is solubilized in acidic pH and should not be mixed with rapid-acting insulins as the kinetics of both insulins will be altered. Insulin glargine shows a greater reduction in A1C and decreased hypoglycemia in patients with T1DM compared to NPH insulin (50).
Insulin detemir is a soluble basal insulin analog. It is covalently acylated with fatty acids on the lysine at position B29, which allows for reversible binding to albumin (51). This delays its absorption from subcutaneous tissue and prolongs its time in the circulation. Although the mean duration of action of insulin detemir has been shown to be 24h, one study showed shorter duration of action (about 17h), which suggests that most patients with T1D may require twice-daily dosing of insulin detemir (52).
ULTRALONG-ACTING INSULIN ANALOGS
Insulin degludec is an ultra-long acting basal insulin available in the US since 2015 that has the same amino acid sequence as human insulin, apart from the deletion of the threonine amino acid residue at B30 and the addition of a fatty acid to the lysine at B29 (53). The fatty acid moiety causes self-aggregation of insulin molecules into soluble multihexamers. Slow dissociation of zinc from the insulin allows for gradual and stable absorption of insulin monomers resulting in a long half-life and a prolonged duration of action of 42 hours at steady state. In patients with T1D, similar A1C reduction with lower rates of nocturnal hypoglycemia have been reported with insulin degludec compared with insulin glargine (54, 55). The extended duration of insulin degludec allows for more flexibility of day-to-day dose timing without compromising glycemic control or safety (56).
U-300 glargine (Gla-300) is a formulation of insulin glargine that delivers the same number of insulin units as insulin glargine 100 units/mL (Gla-100), but in a third of the volume. The compact depot renders a smaller surface area of insulin glargine for a given dose, leading to a slower release of insulin glargine over time. This translates into a more constant PK/PD profile, with a prolonged duration of action (up to 30 hours) with Gla-300 compared with Gla-100 in patients with T1DM (57). Gla-300 has been shown to provide similar glucose control compared to Gla-100 with less weight gain and hypoglycemia (58).
Pre-Mixed Insulins
Premixed insulins are mixtures of prandial and intermediate acting insulins (the same prandial insulin attached to protamine so that it becomes intermediate acting). Insulin mixtures are available as human insulin mixtures (NPH and regular mixture) as well as analog mixtures. In the US, insulin lispro protamine mixtures are available in two forms: 75% insulin lispro protamine suspension and 25% insulin lispro injection (75/25) and 50% insulin lispro protamine suspension and 50% insulin lispro injection (50/50). Available preparations of insulin aspart protamine mixtures include 50/50 and 70/30 suspensions. A variety of other ratios are available in Europe. There is only one mixture of analog-analog without protamine (aspart 30% +degludec 70%, Ryzodeg). These insulin mixtures are typically administered before breakfast and dinner. This alleged twice daily dosing is the primary advantage of these insulins. In general, use of premixed insulins restricts adjustment of doses and meal timing. Therefore, premixed insulins are not recommended for adult patients with T1D, where intensive regimens with ability to make adjustments in the premeal short-acting insulin bolus are better suited for glycemic control. Premixed insulin in T1D could have benefit for some patients who do not adhere to an intensive insulin regimen, and with consistent food intake and timing of meals.
Concentrated Insulins
U-500 INSULIN
U-500 insulin is highly concentrated regular insulin, administered 2-3 times a day without basal insulin. Due to its concentration, the action is prolonged and variable. In T1D, use is primarily limited to individuals with significant insulin resistance (requiring >200 units of insulin a day). Caution should be used while prescribing this insulin as confusion may occur among clinicians, pharmacists, nurses, and patients who are unfamiliar with its use. U-500 insulin is also available in a pen delivery system allowing patients to administer insulin by 5 units increments up to a maximum of 300 units at a time. Units to be delivered are clearly readable through the pen “dose window” which should minimize or eliminate confusion when administering this highly concentrated insulin formulation.
CONCENTRATED INSULIN ANALOGS
U-200 formulations of insulin lispro and insulin degludec are also available and allow for delivery of lower volumes and therefore better absorption. U-300 glargine is available in pen form and holds up to 900 units of insulin with dosing capability up to 160 units per dose.
CONVERSION FROM U-100 TO CONCENTRATED INSULIN
Switching from U-100 insulin to concentrated insulin may occasionally be necessary in the setting of severe insulin resistance and use of large amount of U-100 insulin. U-200 lispro is bioequivalent to U-100 lispro, and U-200 degludec is bioequivalent to U-100 degludec. This means that the dose can be converted 1:1 on a unit basis when switching from U-100 to U-200 formulation. The insulin is delivered at 50% less volume. U-300 glargine, on the other hand is not bioequivalent to U-100 glargine. Individuals with T1D often require 15-20% higher dose of U-300 glargine. Similarly, a dose reduction of 20% is essential when switching from U-300 glargine back to U-100 glargine to avoid hypoglycemia. When initiating U-500R, dosing should be determined based on current and targeted glycemic goals to optimize efficacy and safety. U-500R provides mealtime coverage and its extended duration of action provides basal coverage also.
Biosimilar Insulins/Follow-on Biologics
According to the FDA, a “biosimilar” is a biological product that is highly similar to a US-licensed reference biological product not withstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product. As of 2020, there are 4 follow-on biologics approved. These include Basaglar (US, Europe - insulin glargine), Basalin (China- insulin glargine), Semglee (EU, Australia, insulin glargine) and
|
Table 1. Currently Available Insulin Preparations |
||||
|
Insulin Preparation |
Onset of action (h) |
Peak Action (h) |
Effective duration of action (h) |
Maximum duration(h) |
|
Rapid-acting analogs |
|
|
|
|
|
Insulin lispro (Humalog, Admelog) |
¼ - ½ |
½-1 ½ |
3-4 |
4-6 |
|
Insulin aspart (NovoLog) |
¼ - ½ |
½ -1 ¼ |
3-4 |
4-6 |
|
Insulin glulisine (Apidra) |
¼ - ½ |
½ -1 ¼ |
3-4 |
4-6 |
|
Insulin aspart (Fiasp) |
¼ -1/3 |
1.5-2.5 |
3-4 |
5-7 |
|
Insulin lispro-aabc (Lyumjev) |
1/8 |
2 |
|
4-6 |
|
Inhaled insulin (Afrezza) |
seconds |
12-17 min |
2-3 |
2-3 |
|
Short-acting |
|
|
|
|
|
Regular (soluble) |
½ - 1 |
2-3 |
3-6 |
6-8 |
|
Intermediate-acting |
|
|
|
|
|
NPH (isophane) |
2-4 |
6-10 |
10-16 |
14-16 |
|
Long-acting analog |
|
|
|
|
|
Insulin glargine (Lantus, Basaglar) |
0.5-1.5 |
8-16 |
18-20 |
20-24 |
|
Insulin glargine U-300 (Toujeo) |
0.5-1.5 |
none |
24 |
30 |
|
Insulin detemir (Levemir) |
0.5-1.5 |
6-8 |
14 |
~20 |
|
Insulin degludec (Tresiba) |
0.5-1.5 |
none |
24 |
40 |
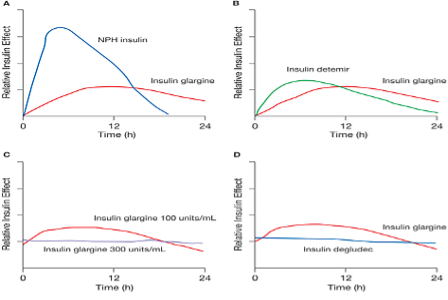
Figure 11. Available basal insulins and duration of action. Figure source Ref (59).
Factors Influencing Insulin Absorption
Insulin absorption variability is one of the greatest obstacles to replicating physiologic insulin secretion. Among the many factors that affect insulin absorption and availability (Table 2) are injection site, the timing, type or dose of insulin used, and physical activity. Day-to-day intra-individual variation in insulin absorption is approximately 25%, and the variation between patients may be as high as 50%. This occurs more commonly with larger doses of human insulin which form a depot and can unpredictably prolong duration of action; however, this is less of an issue with rapid-acting insulin analogs. In general, any strategy that increases the consistency of delivery should decrease glucose fluctuations; and insulin regimens that emphasize rapid-acting insulin are more reproducible in their effects on blood glucose levels. Insulin pumps using a rapid-acting insulin analog can significantly reduce glucose variability. Like multiple-injection regimens, use of an insulin pump requires frequent blood glucose monitoring. In addition, pump users need a back-up method of insulin administration, and attention to mechanical and injection site issues.
Reducing Variability of Insulin Absorption
INJECTION SITES
Subcutaneous insulin is absorbed most rapidly when injected into the abdomen, followed by the arms, buttocks and thighs. These differences are likely due to variations in regional blood flow. A single region should be utilized for injections without rotation between regions, as this may result in day-to-day variation of insulin absorption. However, while using a region, site rotation (i.e. – rotating injections systematically within the abdomen) is important to avoid development of lipohypertrophy or atrophy due to repeated injections at the same site. Injection into lipohypertrophic areas results in erratic, slower absorption of insulin. Exercise increases the rate of absorption from injection sites, likely by increasing blood flow to the skin; local effects may also be involved.
TIMING OF PRE-MEAL INJECTIONS
Gauging the appropriate interval between preprandial injections and eating, known as the “lag time,” is essential for coordinating insulin availability with glycemic excursions following meals. The timing of the injections should also be adapted to the level of premeal glycemia. Insulin lispro, insulin aspart, and insulin glulisine have rapid onset of action and, ideally, should be given approximately 10-20 minutes before mealtime when blood glucose is in the target range, keeping in mind that if the meal is delayed, hypoglycemia may ensue. When blood glucose levels are above a patient’s target range, the lag time should be increased to permit the insulin to begin to have an effect sooner. In this case, rapid-acting acting insulin analogs can be given 20-30 minutes before the meal, depending upon the degree of hyperglycemia. If premeal blood glucose levels are below target range, administration of rapid-acting insulin should be postponed until after some carbohydrates have been consumed. Use of frequent home glucose monitoring or CGM can assist in determining appropriate lag times. It is important to emphasize the effect of administering prandial insulin up to 20 minutes before a meal. Pre-bolusing has been shown to reduce post-prandial glucose spike by up to 50 mg/dL.
OTHER FACTORS
Exercise, as discussed earlier, results in increased blood flow to muscle groups and can increase rate of insulin absorption. Heat can also increase the rate at which insulin is absorbed from the skin. For example, being out in the sun or injection before going into a hot tub may lead to hypoglycemia. Intra-muscular injections result in a more rapid onset of action compared to subcutaneous tissue. This route can be utilized under certain situations such as ketoacidosis, insulin pump failure or in the event of profound hyperglycemia.
|
Table 2. Factors Affecting the Bioavailability and Absorption Rate of Subcutaneously Injected Insulin |
|
|
Factor |
Effects |
|
Site of injection |
Abdominal injection (particularly if above the umbilicus) results in the quickest absorption; arm injection results in quicker absorption than thigh or hip injection. |
|
Depth of injection |
Intramuscular injections are absorbed more rapidly than subcutaneous injections. |
|
Insulin concentration |
U-40 insulin (40 units per mL) is absorbed more rapidly than U-100 insulin (100 units per mL). U-40 insulin is an old insulin formulation not available in the United States for patient use. Currently, it is used for treating canine and feline diabetes mellitus |
|
Insulin dose |
Higher doses have prolonged duration of action compared with lower doses. |
|
Insulin mixing |
Regular insulin maintains its potency and time-action profile when it is mixed with NPH insulin |
|
Exercise |
Exercising a muscle group before injecting insulin into that area Increases the rate of insulin absorption. |
|
Heat application or Massage |
Local application of heat or massage after an insulin injection increases the rate of insulin absorption. |
Role of Insulin Analogs in Management of T1D
Most of the problems of insulin replacement in T1D arise from the fact that subcutaneous injection or pump infusion remains a relatively poor route of administration. From the subcutaneous site of injection, insulin is absorbed into the systemic, not portal circulation. More importantly, subcutaneous injection leads to variable absorption from one injection to another, due largely to the non-physiologic pharmacokinetics of standard insulins. Insulin analogs were developed to overcome this problem.
Currently there are three rapid-acting insulin analogs: insulin lispro, insulin aspart, and insulin glulisine, all of which have a rapid onset of action and peak, thereby improving 1- to 2-hour postprandial blood glucose control compared with regular insulin. These rapid-acting analogs must be used in conjunction with a basal insulin to improve overall glycemic control (Figures 11 and 12). Importantly, the rapid-acting analogs have consistently outperformed regular insulin in terms of post-absorptive hypoglycemia. This finding should not be surprising since the duration of regular insulin is much longer than the gut absorption of a typical mixed meal.

Figure 12. Idealized insulin curves for prandial insulin with a rapid-acting analog (RAA) with basal insulin glargine or insulin detemir. Each insulin preparation is responsible for either the prandial or basal component. Many patients find the basal insulins do not last the entire 24 hours and they give the basal insulin twice daily. B=breakfast; L=lunch; S=supper; HS=bedtime
Clinical trials have demonstrated lower fasting glucose levels and less nocturnal hypoglycemia with insulin glargine than with NPH insulin, advantages that are especially relevant in patients aiming for meticulous control (A1C <7%) or those with hypoglycemia unawareness. Trials with T1D have shown similar results with insulin detemir which compared with NPH insulin was equally effective in maintaining glycemic control, although detemir was administered at a higher molar dose. The newest basal insulin preparations, insulin degludec and U-300 insulin glargine are claimed to show less nocturnal hypoglycemia than insulin glargine or insulin detemir. In general, hypoglycemia is reduced with any of these basal analog insulins compared to NPH insulin. Since hypoglycemia is clearly one of the treatment-limiting aspects of T1D therapy, the use of these analogs has gained wide-spread acceptance.
Multiple Daily Injection (MDI) Insulin Therapy
A simpler conceptual approach preferred by most patients with T1D is using a prandial insulin analog for each meal (i.e., insulin lispro, insulin aspart, or insulin glulisine) and a separate basal insulin analog (i.e., insulin glargine, insulin detemir, or insulin degludec). Although these true basal-prandial regimens require more shots than conventional twice-daily regimens, they are considerably more flexible, allowing greater freedom to skip meals or change mealtimes. Moreover, use of the long-acting basal and rapid-acting insulin analogs, allows strategies to achieve individual, defined blood glucose targets more easily. Such modifications might include changing the timing of insulin injections in relation to meals, changing the portions or content of food to be consumed, or adjusting insulin doses or supplements for premeal hyperglycemia.
The basic treatment principles of insulin dosing include establishing a total daily dose, an insulin to carbohydrate ratio and an insulin sensitivity or correction factor.
ESTABLISHING A TOTAL DAILY DOSE (TDD) OF INSULIN
This is the first step in starting treatment in a patient with newly diagnosed diabetes. This dose can vary based on the individual and can range from 0.3- 1.5 units/kg/day. A good starting dose is ~0.5 units/kg/day. Once the TDD is determined, this number is divided by half to establish the basal and bolus requirements. As a general rule of thumb, half the insulin is used as basal insulin, while the other half is used as prandial or mealtime insulin.For example, in a person weighing 75 kg, a typical total daily insulin dose might be 75 kg X 0.7 units/kg = roughly 37 units/day. The basal insulin dose would be roughly 18 units and bolus insulin total would be 18 units (divided amongst meals, see below).
Long-acting insulin analogs U-100 glargine and detemir can be administered once or twice daily. Insulin degludec or U-300 insulin glargine can be administered once a day.
USING PRANDIAL INSULIN
Establishing an Insulin to Carbohydrate (Carb) Ratio
Patients with T1D derive the greatest therapeutic benefit when basal and prandial analogs are used together, because the physiologic pharmacokinetics and pharmacodynamics of these analogs make separating the basal and prandial components of insulin replacement easier. In general, administering the appropriate amount of pre-meal insulin requires that the patient know at least their current blood glucose level and the estimated amount of carbohydrates for a meal. Initially, the amount of prandial insulin can be determined by approximating the percentage of calories consumed at each meal. As patients become more educated, however, they may alter the prandial dose by estimating the carbohydrate component of each meal or snack. As patients become more sophisticated, they may note that the same carbohydrate quantity may have a different effect on their blood glucose level depending upon the specific type of meal consumed.
The carb ratio provides the dose of rapid acting insulin (lispro, aspart, glulisine) to cover the carbohydrate content of a meal. A typical starting point in patients with T1D is to give 1 unit of rapid acting insulin for every 15 grams of carbohydrates. This ratio is variable ranging from 1 unit for every 5g to 30 g of carbohydrate. To estimate the carb ratio, the “500 rule” can be used:
500/total daily dose (TDD) = grams of carbohydrate covered by 1 unit of insulin.
Example: A person who takes a total of 50 units of insulin per day (both basal and prandial combined) will need 1 unit of rapid acting prandial insulin for every 10g carbohydrate (500/50 = 10g of carbohydrate covered by 1 unit of insulin, using above formula).
Alternative way to calculate the carb ratio – Add all carbohydrates consumed in a day and divide this by the total units of prandial insulin taken that day, using an average over 3 days.
Prandial insulin may be reduced/skipped when:
- Extra carbohydrates are used to raise low blood sugars or cover increased physical activity
- Recent dose of correction insulin within past 1-2 h
- Nausea or vomiting preventing oral intake
Determining the Correction Dose or “Insulin Sensitivity Factor” (ISF)
In addition to covering the carbohydrate load of a meal, individuals will also need to correct hyperglycemia, called the “correction dose”. The method commonly used for this is the “1800 Rule”. This estimates the point drop in glucose for every unit of rapid-acting insulin administered:
1800/TDD = Point drop in glucose for 1 unit of rapid-acting insulin
This ISF (also called the correction factor) can be used for between-meal elevations in blood glucose. Thus, in general this correction dose can be utilized anytime provided the patient has not taken an injection of rapid acting insulin over the past 2-4 hours (insulin on board, Figure 12).
Target glucose: The ISF enables achieving appropriate individualized blood glucose targets.
For example: A person who takes a total of 60 units of insulin per day will require 1 unit of rapid acting insulin to drop the glucose by 30 points. If the patient’s glucose is 180 mg/dL and the glucose target has been set at 120 mg/dL, a correction dose of 2 units would be required to bring the glucose down to target:
- ISF = 1800/60 (TDD) = 30; 1 unit of rapid-acting insulin will decrease glucose by 30 points
- 180 mg/dL (actual glucose level) – 120 mg/dL (target glucose level) = 60; this is the excess glucose, that is, the value that is above target and that needs to be corrected
- 60/30 (ISF) = 2; dividing the excess glucose by the ISF will provide the amount of correction insulin units that are required to bring down the glucose to target, in this case it will be 2 units.
Putting it All Together - Combining the Carb Ratio and ISF
Combining the carbohydrate load and ISF will enable patients to appropriately target their pre-meal glucose.
For example: An individual with a carb ratio of 1:15 and ISF of 1 unit/50mg/dL, prior to a meal of 60g carbohydrates and a pre-meal blood glucose of 220mg/dL and target of 120mg/dL would take the following steps to administer the appropriate amount of prandial insulin as follows:
- To cover carbohydrate intake: 60g/15g per unit =4 units
- Correction dose: 220 mg/dL (actual glucose)– 120mg/dL (target glucose) = 100mg/dL. ISF is 100/50 = 2 units to correct.
- Total amount of prandial insulin: 4+2= 6 units
Insulin Titration and Pattern Adjustments
Reviewing blood glucoses and recognizing patterns is one of the most important aspects of diabetes management, allowing for timely and appropriate adjustments in insulin dose, food intake, and managing physical activity. Pattern management is aided by valuable tools such as SMBG with information obtained through download software (see above) or logbooks and CGM data. These tools can be used in order of priority, for assessment of hypoglycemia, hyperglycemia, glycemic variability, frequency of SMBG readings, etc.
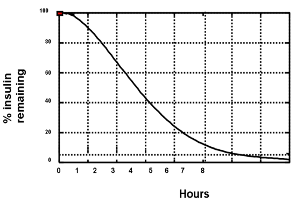
Figure 13. The appearance of insulin into the blood stream (pharmacokinetics) is different than the measurement of insulin action (pharmacodynamics). This figure is a representation of timing of insulin action for insulin aspart from euglycemic clamp data (0.2 U/kg into the abdomen). Using this graph assists patients to avoid “insulin stacking”. For example, 3 hours after administration of 10 units of insulin aspart, one can estimate that there is still 40% X 10 units, or 4 units of insulin remaining. By way of comparison, the pharmacodynamics of regular insulin is approximately twice that of insulin aspart or insulin lispro. Currently used insulin pumps keep track of this “insulin-on-board” to avoid insulin stacking. Adapted from reference (40).
INSULIN DELIVERY SYSTEMS
Significant improvement in pharmacokinetics and pharmacodynamics of insulin analogs and advances in technology has allowed for insulin delivery systems to resemble endogenous insulin secretion as closely as possible.
Insulin Pens
Insulin pens were first introduced in 1981 as injection devices. These pens contain a cartridge holding insulin which is injected into the subcutaneous tissue through a fine, replaceable needle. Insulin pens are convenient, portable and are widely used as a part of MDI therapy. Currently, insulin pens are available as disposable pens containing prefilled cartridges or reusable insulin pens with replaceable insulin cartridges. Several insulin pens allow the convenience of ½ unit dosing, a critical need for pediatric patients and those adults with high insulin sensitivity and low insulin requirements.
Insulin smart pens - The first insulin smart pen was approved for use in the United States (InPen, Companion Medical, California, USA) in 2017 (Figure 14). Smart pens can record timing and amount of each administered insulin dose, display the last dose and insulin onboard and also make dosing recommendations based on pre-specified information (Figure 15). This information is wirelessly transmitted via Bluetooth to a dedicated mobile application on a smartphone device. Other similar devices are in development including the NovoPen 6 and NovoPen Echo Plus reusable insulin pens equipped with near-field communication technology (Novo Nordisk, Denmark), recently approved in the European Union.

Figure 14. InPen Smart Insulin Pen
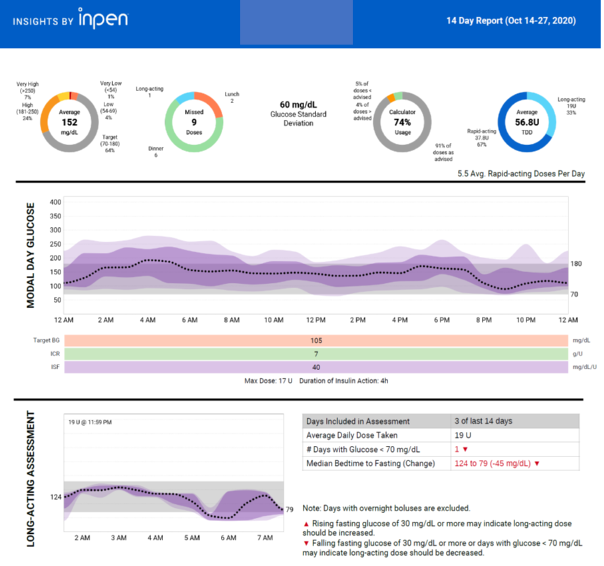
Figure 15. InPen Insight report. Report provides missed doses, bolus calculator dosage, long-acting insulin assessment and CGM data (if paired with the InPen app).
Continuous Subcutaneous Insulin Infusion Therapy (CSII)
While not a new tool, insulin pump therapy remains the gold standard of insulin delivery for T1D (Figure 9). CSII is the most precise way to mimic normal insulin secretion because basal insulin infusion rates can be programmed throughout a 24-hour period. Essentially, the CSII pump may be thought of as a computerized mechanical syringe automatically delivering insulin in physiologic fashion. Patients can accommodate metabolic changes related to eating, exercise, illness, or varying work and travel schedules by modifying insulin availability. Basal rates can be adjusted to match lower insulin demands at night (between approximately 11 PM and 4 AM) and higher requirements between 3 AM or 4 AM and 9 AM.
Various studies comparing glycemic control during CSII versus intensive insulin injection regimens have been published. A meta-analysis of 12 randomized controlled trials of CSII versus multiple injection regimens showed a weighted mean difference in blood glucose concentration of 16 mg/dL (95% CI 9-22) and a difference in A1C of 0.5% (0.2-0.7) favoring CSII (60). The slightly but significantly better control in patients on CSII was accomplished with a 14% average reduction in daily insulin dose.
A meta-analysis funded by the Agency for Healthcare Research and Quality showed that in adults with T1D A1C levels decreased more with CSII than multiple injections, but one study heavily influenced this finding (27). For both children and adults, there was no difference in severe hypoglycemia. The common misconception that CSII leads to more hypoglycemia is not valid.
Modern insulin pumps are much smaller and easier to use than the pumps of the past (Figure 10).
With the exception of insulin lispro-aabc (Lyumjev), all rapid-acting analogs are approved in the United States for use in insulin pumps. The basal rate of the insulin pump replaces the use of daily injections of basal insulin. The boluses given before each meal are essentially the same as normal insulin injections of rapid acting insulin. The pump allows programming of several different basal infusion rates at increments that can range from 0.025 up to 35.0 units/hour (usually ranging from 0.4 to 2.0 units/hour) to meet non-prandial insulin demands, though it is unlikely that the average patient will require more than 2 or 3 different rates (Figure 16). As with MDI, correction doses can be provided before or between meals. Figures 17 and 18 show data that is typically downloaded from a pump.
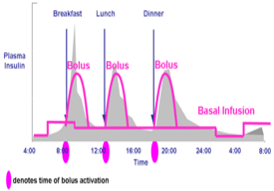
Figure 16. Idealized insulin curves for CSII with either insulin lispro, insulin aspart, or insulin glulisine. Note the basal insulin component can be altered based on changing basal insulin requirements. Typically, insulin rates need to be lowered between midnight and 0400 h (predawn phenomenon) and raised between 0400 h and 0800 h (dawn phenomenon). The basal rate the rest of the day is usually intermediate to the other two. Modern-day pumps can calculate prandial insulin dose by the patient entering the blood glucose concentration and the anticipated amount of carbohydrate to be consumed. The pump calculates how much previous prandial insulin is still active and provides the patient a final suggested dose which the patient may activate or override.
There are many fundamental differences between CSII and MDI. These include:
TITRATION OF BASAL RATES
From a practical point of view, the first and most important insulin dose to provide in a correct amount is the basal rate. If the basal dose is set incorrectly, neither the bolus doses nor the correction doses will be appropriate. A common mistake observed in CSII therapy is that the basal dose is set too high, making the administration of even small insulin correction doses result in hypoglycemia. The greatest advantage of CSII is it allows more flexibility and titration of the basal doses.
The basal dose can be titrated throughout the day to meet patients’ individual needs and this should be done in a systematic manner by performing “basal checks.” Prior to starting a basal rate assessment (basal check), the following conditions should be met for the day of the test: last meal and/or insulin bolus should have occurred at least 4 hours prior to starting the assessment; last meal should preferentially be low in fat and not have too much protein; avoid exercise and alcohol; do not perform the assessment if hypoglycemia has occurred earlier in the day or there is an inter-current illness. Of note, it is recommended to repeat the assessment on several occasions to identify a pattern prior to making adjustments to the basal rate.
Nighttime Basal Rate
It is usually best to start by addressing the overnight basal rate. An overnight basal assessment is performed on a night the patient has a bedtime glucose level within target. The patient is asked not to have anything to eat during the assessment. The patient then measures glucose levels at bedtime, midnight, 3AM and upon awakening to assess for changes in glucose profile (the use of a CGM obviously makes this exercise much easier). Glucose should also be checked in case of hypoglycemic symptoms. If hypoglycemia ensues or glucose level rises above target, the assessment is stopped and the patient treats the glucose level accordingly. Rises or falls of ≤ 30 mg/dl from bedtime to morning (upon awakening) are usually acceptable. By contrast, glucose changes > 30 mg/dl will require adjustments in basal rates usually consisting of 10-20% changes in insulin dose (as deemed clinically appropriate) starting 2 hours before the observed rise or fall in glucose levels. In general, a change in a basal dose takes two to four hours to result in a change in blood glucose.
Daytime Basal Rates
Daytime basal rates are checked by assessing the glucose profile across a skipped-meal time segment (i.e., pre-breakfast to pre-lunch, pre-lunch to pre-dinner, and pre-dinner to bedtime). To check the “pre-breakfast to pre-lunch” time segment, breakfast is skipped and glucose level is checked at 1-2 hour intervals for the duration of the time segment (prior to lunch). Glucose levels should also be checked in the event of hypoglycemic symptoms. The same recommendations regarding changes in glycemic levels requiring insulin dose adjustments described for the overnight basal assessment apply here.
TRACKING OF INSULIN-ON-BOARD
Another major difference between CSII and MDI is the pump can accurately track the insulin-on-board for safer use of correction doses (Figure 13). As noted above, doing this accurately can have a major impact in preventing insulin stacking.
INSULIN DOSE CALCULATOR
Insulin-to-carbohydrate ratios and insulin sensitivity factors with corresponding target glucose values can be set and modified as needed in insulin pumps. Patients are only required to enter their glucose level and/or anticipated carbohydrate amount to be consumed and the insulin pump will calculate the insulin dose and recommend a bolus dose. So, the complicated mathematics to best utilize MDI are done automatically with CSII.
MODIFICATIONS TO BOLUS DELIVERY
Pumps can be programmed for individual boluses to be administered over an extended period of time (“extended” or “square wave” bolus). This feature may be particularly helpful for very high-fat meals or those patients with delayed gastric emptying, seen with gastroparesis or in those receiving pramlintide (see below).
TEMPORARY BASAL RATES
The other major advantage of CSII is that it allows the use of “temporary basal rates.” This is extremely helpful in situations where metabolic demands have “temporarily” changed such as during illness (requiring an increase in insulin dose) or during exercise (requiring a dose reduction). Again, due to the time action of the rapid-acting analogs, sufficient time must be incorporated when using a temporary basal rate.
DOWNLOAD CAPABILITY
Pump data can be downloaded, and the data obtained is extremely helpful in understanding patients’ glycemic responses to an established insulin regimen (Figure 17). Also, it can assist in evaluating patients’ behaviors pertaining to their glucose management. Downloads provide information regarding the total daily insulin use broken down into percentages corresponding to basal and bolus delivery. This allows determining if patients are consistently administering boluses or whether they are essentially “running on basal.” Some of the additional data that can be downloaded includes average glucose levels, frequency of glucose monitoring, days between site changes, amount of time patients are suspending the pump or using temporary basal rates, frequency of boluses (which allows to identify non-compliance or insulin stacking behaviors), and average daily carbohydrates consumed (Figure 18).
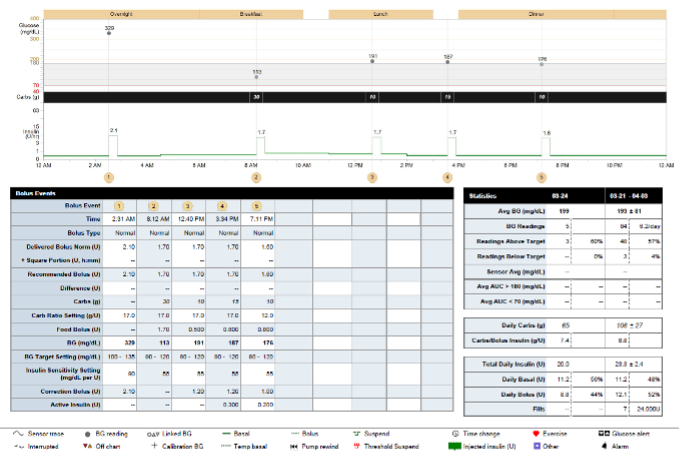
Figure 17. A patient’s insulin pump download showing comprehensive data for one day including basal rates, boluses and use of bolus calculator, glucose monitoring, carbohydrate intake, and percentage of glucose at target.
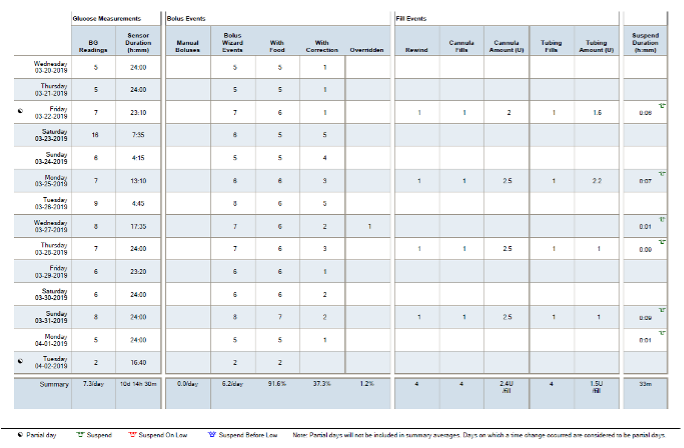
Figure 18. A patient’s pump download showing glucose measurements, bolus events, fill events (denoting frequency of site and set changes), as well as insulin pump suspension duration for a 14-day period).
However, despite the multiple benefits of CSII therapy there are also several risks. The first is an abrupt stoppage of insulin delivery either from an occlusion or dislodging of the catheter. For most patients who measure glucose levels at least 4 times daily the problem can be discovered and rectified quickly. However, for the occasional patient who tests infrequently or misses several glucose tests the discontinuation of the insulin infusion can result in ketoacidosis. Fortunately, this is rare. When glucose levels are found to be elevated for no apparent reason, it is appropriate to bolus the appropriate correction dose and if after 1 to 2 hours glucose levels are not improved, an injection of insulin is recommended, and the infusion site should be changed.
Another potential complication is infection, often an abscess, at the infusion site. This is also rare and can be minimized with meticulously cleaning the pump site prior to insertion. Although not as severe, inflammation from pump sites can be problematic. This can be improved by changing the infusion set every 24 to 72 hours and rotating pump sites. Similarly, some patients develop lipohypertrophy from infusing the insulin in the same area. This can result in extreme variability in insulin absorption. Again, frequent rotation of pump sites can alleviate this problem which is under-reported. Clinicians should therefore make pump site observation a part of every clinic visit.
CLASSIFICATION OF INSULIN PUMPS
Insulin pumps can be classified by the way insulin is delivered into:
Pumps with Tubing
These insulin pumps require an infusion set for insulin delivery. They house an insulin-filled cartridge connected to a tubing with a prespecified length, allowing patients to select the length that better accommodates to their needs. At the end of the tubing is a needle or soft Teflon cannula that can be inserted into the subcutaneous tissue at a 30- to 45- or 90-degree angle, depending on the type of infusion set used. The abdomen is the preferred infusion site because placement of the catheter there is convenient and comfortable and insulin absorption is most consistent in this region. However, the upper outer quadrant of the buttocks, upper thighs, and triceps fat pad of the arms may also be used.
Infusion sets allow removal of the insertion needle, leaving only the soft cannula in place subcutaneously. Patients who experience frequent soft cannula kinking or those with Teflon allergies can opt for infusion sets that use a small stainless-steel needle to infuse insulin instead of a Teflon cannula. Infusion sets have a quick-release mechanism, allowing them to be temporarily disconnected from the insertion site. This quick-release feature makes dressing, swimming, showering, and other activities more convenient.
Tubeless Pump
Patients may also choose the convenience of a tubeless or patch pump. This pump consists of disposable “pods” which are discarded every three days. The pods are essentially small self-contained insulin pumps with an internal insulin cartridge, an insertion needle and cannula, and the necessary hardware required for insulin administration. Insulin is infused directly from the pod through a catheter without the use of any tubing. Both basal and bolus insulin dosing is communicated to the pod through either audio frequency or Bluetooth technology via a separate “personal diabetes manager” device.
Artificial Pancreas Device Systems
Improvements in CGM sensor technology have allowed for the integration of CGM systems with insulin pumps and the development of artificial pancreas device systems (APDS), also known as closed-loop (CL) systems. An APDS consists of an insulin pump, a CGM device, and an insulin infusion algorithm designed for safety and glucose control optimization.
In 2009, the JDRF developed an artificial pancreas road map defining 6 stages of APDS technology based on the level of automation (61):
- First generation:
- Stage 1: Very-Low-Glucose Insulin Off Pump. Pump shuts off when user not responding to low-glucose alarm.
- Stage 2: Hypoglycemia Minimizer. Predictive hypoglycemia causes alarms, followed by reduction or cessation of insulin delivery before blood glucose gets low.
- Stage 3: Hypoglycemia/Hyperglycemia Minimizer. Same product as #2 but with added feature allowing insulin dosing above high threshold.
- Second Generation:
- Stage 4: Automated Basal/Hybrid Closed Loop. Closed loop at all times with mealtime manual assist bolus.
- Stage 5: Fully Automated Insulin Closed Loop. Manual mealtime bolus eliminated.
- Third Generation
- Stage 6: Fully Automated Multihormone Closed Loop.
First generation devices focused primarily on prevention of hypoglycemia. Second generation devices have introduced automation of basal insulin delivery with or without automatic correction boluses. Lastly, third generation devices are expected to fully close the loop while providing a multi-hormonal (e.g., insulin, glucagon, amylin) delivery approach.
It is important to note that the development of any of these specific stages is not dependent on the previous one being completed and can occur in tandem.
APDS can also be classified according to the type of control algorithm used to determine insulin delivery (62):
- Proportional Integral Derivative (PID). This algorithm responds to measured glucose levels where: “proportional” refers to the difference between the measured sensor glucose and the target glucose; “integral” refers to how long the sensor glucose has been away from the target; and “derivative” refers to how rapidly the sensor glucose is changing.
- Model Predictive Control (MPC). This algorithm allows prediction of glucose levels at a specific point in the future and based on this data, modulation of insulin delivery.
- Fuzzy logic. The calculation of insulin doses is similar to what a diabetes specialist would recommend based on CGM data.
- Bio-inspired. Uses a mathematical model of beta cell insulin production in response to changes in blood glucose.
A list of currently approved APDS and features is listed in Figure 19 (63-67)
Figure 19. Features and CGM outcomes from pivotal studies on currently available artificial pancreas device systems.
ADJUNCTIVE NON-INSULIN THERAPIES IN TYPE 1 DIABETES
Intensive insulin therapy for T1D is associated with increased risk of hypoglycemia. Additionally, glycemic variability and weight gain with resultant non-adherence to insulin are commonly encountered. Weight gain also contributes to increased cardiometabolic risk such as hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Insulin therapy also does not address glucagon excess and altered gastric emptying that is seen in patients with T1D. Hence adjunctive therapies could be of potential benefit in management of T1D.
Amylin Analog - Pramlintide
Amylin is a neuroendocrine hormone co-secreted with insulin by the pancreatic beta cells in a fixed ratio (68); T1D is a state of deficiency. Amylin reduces postprandial hyperglycemia by reducing mealtime glucagon secretion. It also delays gastric emptying, increases satiety and enables weight loss. Overall, amylin complements the action of insulin by targeting postprandial hyperglycemia.
Pramlintide is an injectable amylin analog approved for use in T1D as an adjunct to prandial insulin. Pramlintide has similar physiological effects as amylin, such as decreased food intake, and decreases mean A1C by 0.3-0.5% with modest weight loss (69). A recent crossover study of pramlintide infusion co-administered with human regular insulin via a pump over 24h improved glycemic variability and postprandial hyperglycemia in adults with T1D (70). Pramlintide is injected just prior to meals at an initial dose of 15 mcg and increased as tolerated to a final dose of 60 mcg. It should be administered only prior to major meals consisting of 250 calories or 30 grams of carbohydrate. Prandial insulin doses of insulin (in MDI or CSII therapy) should be reduced as food intake decreases and gastric emptying is delayed. For those receiving insulin via a pump, using an “extended bolus” (see above) works best to avoid postprandial hypoglycemia. For those using MDI, some patients administer their insulin just prior to eating (without a lag time) or after eating. Use of pramlintide is limited by nausea, often mild and self-limited. Severe insulin-induced hypoglycemia has also been noted with the use of pramlintide if insulin doses are not sufficiently reduced on initiation of pramlintide therapy. However widespread use of pramlintide as a therapeutic adjunct in T1D has been limited due to concerns of nausea, hypoglycemia and additional injection burden. Long-term use of pramlintide is unclear at this time.
Metformin
Metformin, a biguanide, is used as first-line therapy in patients with T2D. It decreases hepatic gluconeogenesis and improves insulin sensitivity (71). Metformin may have some benefit in reducing insulin doses and possibly improve metabolic control in obese/overweight individuals as observed in small studies in patients with T1D. An early meta-analysis of 5 studies suggested that addition of metformin resulted in a decrease in insulin requirement (6.6 units/day), and a decrease in weight with minimal change in A1C (72). A randomized placebo-controlled trial in 140 overweight adolescents with T1D evaluated the addition of metformin to insulin (73). There was no improvement in glycemic control after 6 months but use of metformin resulted in decreased insulin dose and improved measures of adiposity, despite increased gastrointestinal adverse events. A meta-analysis of 19 RCTs suggests short term improvement in A1C that is not sustained after 3 months and associated with higher incidence of GI side effects (74). Although metformin has been shown to decrease CVD morbidity in T2D, data in T1D is lacking. Recent evidence suggests that metformin decreases insulin resistance and improves vascular health in adolescents with T1D (75). The REMOVAL trial assessed benefit of metformin in T1D and cardiovascular risk and showed no evidence of sustained A1C reduction, and no benefit in carotid intima-media thickness (the study’s primary endpoint); however, reductions in body weight, LDL-C and total insulin requirements was observed (76). Therefore, based on current evidence, concomitant use of metformin in patients with T1D and is not recommended in current published guidelines.
Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors
SGLT2 is a protein expressed in the proximal convoluted tubule (PCT) of the kidney and is responsible for re-absorption of filtered glucose. Inhibition of SGLT2 prevents glucose reabsorption in the PCT and increases glucose excretion by the kidney. SGLT1 is the major intestinal glucose transporter. SGLT1 inhibition also increases postprandial release of the gastrointestinal hormones GLP-1 and polypeptide YY, probably by increasing delivery of glucose to the distal small intestine, thereby regulating glucose and appetite control. Notably, the action of these agents is insulin-independent, therefore this class of drugs has potential as adjunctive therapy for T1D. Additionally recent clinical trials have also demonstrated improvements in cardiovascular outcomes trials as well as reductions in renal outcomes in T2D; therefore, there is significant interest for use in T1D. Early small studies of SGLT2 inhibitors in T1D showed promising results with evidence of decreased total daily insulin dosage, improvement in fasting glucose and A1C, measures of glycemic variability, rates of hypoglycemia and body weight (77-79).
Common side effects associated with this class of drugs include genital and urinary infections. Euglycemic diabetic ketoacidosis has been recognized in patients with T1D due to glycosuria masking hyperglycemia but with a catabolic state (due to insulin deficiency and hyperglucagonemia) with ketonemia (80, 81).
A dual inhibitor of SGLT1 and 2 sotagliflozin is under development and shows promise in T1D patients (82). Currently in the US, SGLT2 inhibitors are approved for use in T2D only. SGLT2 and mixed SGLT1/2 inhibitors are approved for use in T1D by the European Medicines Agency.
All four available SGLT2 inhibitors have been studied in T1D. When added to insulin therapy, all SGLT2 inhibitors appear to decrease A1C levels, averaging 0.35-0,5% within 6 months of initiation; however, this effect does not appear to be sustained at 1 year in clinical trials and effects appear to wane with time (83). Insulin dosing should be adjusted with caution to avoid hypoglycemia. There is no data on efficacy comparing the different agents currently. It is estimated that these agents increase risk of diabetic ketoacidosis by 8-fold, and therefore are not approved for use in T1D in the US.
Incretin Therapies
Endogenous glucagon-like peptide-1 (GLP-1) is secreted from L cells (present in the small and large intestine) in response to food ingestion. GLP-1 enhances glucose-induced insulin secretion, inhibits glucagon secretion, delays gastric emptying, and induces satiety. GLP-1 secretion in T1D patients is similar to that seen in healthy individuals. In vitro studies suggest that incretin-based therapies can expand beta cell mass, stimulate beta cell proliferation and inhibit beta cell apoptosis, although this has not been demonstrated in humans. Thus, due to their putative effects on beta cell integrity and function, GLP-1 receptor agonists and oral dipeptidyl peptidase-4 (DPP-4) inhibitors are of interest in T1D. GLP-1 receptor agonists delay gastric emptying, suppress postprandial glucagon secretion, and increase satiety. Studies suggest that these agents may decrease insulin requirements and facilitate weight loss (84, 85). Early RCTs of liraglutide in T1D revealed weight loss and some A1C lowering benefit (85, 86). Recent data suggests benefit of liraglutide 1.8 mg in individuals with T1D and higher BMI in decreasing A1C, weight and no increased hypoglycemia risk (87). However, these effects may not be sustained, based on results from a weekly exenatide study (88). At this time, GLP-1 receptor agonists are not a recommended treatment option in T1D.
The DPP-4 enzyme degrades endogenous GLP-1 and removes it from the circulation. DPP-4 inhibitors lower blood glucose by preventing breakdown of endogenous GLP-1, thereby increasing concentration in the circulation. In patients with T2D, DPP-4 inhibitors potentiate glucose-dependent insulin secretion and inhibit glucagon release without effect on gastric emptying or bodyweight. Patients with T1D have inappropriately raised glucagon secretion and DPP-4 inhibitors added to insulin could potentially enhance insulin secretion in patients with residual endogenous insulin secretion and improve glycemic control. However, observed effects in patients with T1D are limited with modest improvements in A1C that are short-term and not sustained (89). Therefore, these agents cannot be recommended for use in T1D.
Bariatric Surgery
Bariatric and other metabolic surgeries are effective weight loss treatments in severe obesity. In T1D individuals with morbid obesity, bariatric surgery has been shown to result in significant weight loss, decrease in insulin requirements and an overall improvement in metabolic profile. However, DKA and hypoglycemia occur in the post-operative period. Longer term and larger studies are required to further evaluate the role of bariatric surgery in T1D (90).
OTHER ASPECTS OF MANAGEMENT
Psychosocial Aspects
Assessment and management of psychosocial issues are an important component of care in individuals with T1D throughout their life span (91). While the individual patient is the focus of care, family support should be encouraged when appropriate. Evaluation and discussion of psychosocial issues and screening for depression screening should be included as part of each clinic visit. Many patients experience “diabetes distress” related to the multitude of self-care responsibilities to optimize glycemic control. Diabetes distress is frequently associated with suboptimal glycemic control, low self-efficacy and reduced self-care. Depression, anxiety from fear of hypoglycemia, and eating disorders can develop and are associated with poor glycemic control. In young adults, comprehensive management of diabetes that addresses these psychosocial issues can improve glycemic control and reduce hospitalization due to diabetic ketoacidosis. Strategic interventions such as cognitive restructuring, goal setting and problem solving can help individuals particularly adolescents and young adults reduce diabetes distress (92). Thus, early identification and treatment including referral to a mental health specialist can help aid management of diabetes.
Management in Exercise
The benefits of exercise and physical activity in patients with type 1 diabetes have been well documented (93, 94). However, achieving adequate glycemic control during and after completion of exercise remains a rather challenging aspect of type 1 diabetes management. Glycemia at the initiation of exercise, sensor glucose trend (if using a CGM), timing from the previous meal, carbohydrate content in the meal preceding exercise, type and duration of exercise, are all but a few of the factors that need to be considered to ensure that glycemic control remains stable during and after cessation of exercise.
In 2017, an international consensus statement for exercise management in type 1 diabetes was published (95). This consensus is a unique resource which provides detailed glucose management strategies. Recommended adjustments to basal and prandial insulin, for both insulin pump and multiple daily insulin injection users, as well as carbohydrate intake requirements depending on the intensity and duration of activity are clearly presented. Quite importantly, the consensus also covers factors that would preclude exercise including the presence of elevated ketones, recent hypoglycemia, and diabetes-related complications which may be exacerbated in the context of vigorous exercise and/or competitive endurance events. We encourage the reader to refer to this publication for additional guidance.
For those patients on hybrid closed loop systems, a way to minimize the occurrence of exercise-induced hypoglycemia is the use of a higher glucose target for exercise. For the Medtronic 670G, the standard Auto-Mode target is 120 mg/dL which can be temporarily changed to 150 mg/dL. For the Tandem X2 with Control IQ, the standard target for regular activity is between 112.5 and 160 mg/dL and can be temporarily changed to 140-160 mg/dL.
A study in open loop insulin pump users found that a basal rate reduction starting 90 min before exercise was superior to pump suspension at exercise onset for reduction of hypoglycemia risk during exercise and did not compromise the post-exercise meal glycemic control (96).
Another strategy that may be more effective than basal rate reduction for prevention of exercise induced hypoglycemia is the use of a subcutaneously administered mini-dose of glucagon. A small study including 15 subjects with type 1 diabetes on insulin pump therapy who exercised in the fasting state in the morning for 45 min, found that a dose of 150 µg of subcutaneous glucagon, compared to a 50% basal insulin reduction or 40-g oral glucose tablets, resulted in no hypoglycemia (vs. basal insulin reduction) and no hyperglycemia (vs. oral glucose tablets) (97). However, larger and long-term studies are required before determining if a mini-dose of glucagon is safe and effective for prevention of exercise induced hypoglycemia in subjects with type 1 diabetes.
Management of Special Populations
OLDER ADULTS
Adults with T1D now span a very large age spectrum—from 18 to 100 years of age and beyond. These individuals are unique in that they usually have lived with a complex disease for many years (91). An understanding of each individual’s circumstances is vital and management often requires assessment of medical, functional, mental, and social domains. The ADA emphasizes that glycemic targets should be individualized with the goal of achieving the best possible control while minimizing the risk of severe hyperglycemia and hypoglycemia (98).
Glycemic goals in older adults vary. Most older adults with T1D have long-standing disease (unlike individuals with T2DM where diabetes can be long-standing or new onset). Additionally, there is a wide range of health in older individuals, with some patients enjoying good functional status and no comorbid conditions, while others are limited by multiple comorbidities as well as physical or cognitive impairments. Older T1D patients may develop diabetes related complications which pose a challenge in disease management. Insulin dosing errors, hypoglycemia unawareness, and inability to manage hypoglycemia when it occurs may result from physical and cognitive decline. Special attention should be focused on meal planning and physical activities in this population. Severe hyperglycemia can lead to dehydration and hyperglycemic crises (91). Issues related to self-care capacity, mobility, and autonomy should be promptly addressed.
Thus, treatment goals should be reassessed and individualized based on patient factors. Older patients with long life expectancy and little comorbidity should have treatment targets similar to those of middle-aged or younger adults. In patients with multiple comorbid conditions, treatment targets may be relaxed, while avoiding symptomatic hyperglycemia or the risk of diabetic ketoacidosis (91). Therefore, it is important to assess the clinical needs of the patient, setting specific goals and expectations that may differ quite significantly between a healthy 24-year-old and a frail 82-year-old with retinopathy and cardiovascular disease.
There are few long-term studies in older adults demonstrating the benefits of intensive glycemic, blood pressure, and lipid control (98). As with younger adults, glycemic control should be assessed based on frequent SMBG levels (and CGM data, if available) as well as A1C to help direct changes in therapy. More stringent A1C goals (~6.5-7%) can be recommended in select older adults if this can be achieved without hypoglycemia or other adverse effects. This is appropriate for older individuals with anticipated long-life expectancy, hypoglycemia awareness and no CVD. Less stringent A1C goals (for example A1C<8.5%) may be appropriate for patients with a history of severe hypoglycemia, hypoglycemia unawareness, limited life expectancy, advanced microvascular/macrovascular complications, or extensive comorbid conditions (91, 99).
INPATIENT MANAGEMENT AND OUTPATIENT PROCEDURES
The challenges involved in management of individuals with T1D in the hospital and in preparation for scheduled outpatient procedures include difficulties associated with fasting, maintaining a consistent source of carbohydrate, and facilitating inpatient blood glucose management while modifying scheduled insulin therapy. Individuals with T1D may have difficulty fasting for long periods of time (more than 10 h) prior to a procedure. Patients with T1D should be prepared with a treatment plan for insulin dose adjustments and oral glucose intake prior to any procedure that requires alterations in dietary intake and/or fasting.
In general, goals for blood glucose levels in individuals with T1D are the same as for people with T2D or hospital-related hyperglycemia (100) . It is imperative that the entire health care team, including anesthesiologists and surgeons as well as other specialists who perform procedures, understands T1D and how it factors into the comprehensive delivery of care. First, the diagnosis of T1D should be clearly identified in the patient’s record. Second, the awareness that people with T1D will be at high risk for hypoglycemia during prolonged fasting and are at risk for ketosis if insulin is inappropriately withheld. Under anesthesia, individuals with T1D must be carefully monitored for hypoglycemia and hyperglycemia. Third, a plan for preventing and treating hypoglycemia should be established for each patient.
SMBG should be ordered to fit the patient’s usual insulin regimen with modifications as needed based on clinical status. Self-management in the hospital may be appropriate for some individuals with T1D including those who successfully manage their disease at home, have cognitive skills to perform necessary tasks such as administer insulin and perform SMBG, count carbohydrates and have a good understanding of their condition (100). For some individuals, once the most acute phase of an illness has resolved or improved, patients may be able to self-administer their prior multiple-dose or CSII insulin regimen under the guidance of hospital personnel who are knowledgeable in glycemic management. Individuals managed with insulin pumps and/or multiple-dose regimens with carbohydrate counting and correction dosing may be allowed to manage their own diabetes if this is what they desire, once they are capable of doing so.
The need for uninterrupted basal insulin to prevent hyperglycemia and ketoacidosis is important to recognize. Insulin dosing adjustments should also be made in the perioperative period and inpatient setting with consideration of oral intake and blood glucose trends.
The use of CGM in the inpatient setting is an area of ongoing research. Currently, the Endocrine Society recommends against the use of real-time CGM (RT-CGM) alone in the intensive care unit or operating room settings due to limited available data on accuracy (101). A study in T2D patients on basal bolus insulin therapy admitted to the general ward evaluated the use of retrospective CGM versus point of care capillary glucose testing for inpatient glycemic control (102). Although average daily glucose levels were comparable between CGM and capillary blood glucose testing, CGM detected a higher number of hypoglycemic episodes (55 vs 12, P < 0.01) suggesting that CGM may be beneficial for identification of hypoglycemia in the general ward particularly in patients with hypoglycemia unawareness. We feel it is reasonable to allow T1D patients who already benefit from use of RT-CGM to continue the use of this technology in the non-ICU inpatient setting under the supervision of the care team. Large prospective randomized trials will be required to establish benefit or lack thereof of RT-CGM use on inpatient glycemic control.
BETA-CELL REPLACEMENT STRATEGIES
Pancreas Transplantation
Pancreas transplantation is a currently available therapeutic option for patients with diabetes who meet specific clinical criteria. Patients with end-stage renal disease are eligible to undergo simultaneous pancreas kidney (SPK) transplantation. Also, pancreas transplantation may be offered as a separate procedure after a patient has already received a kidney transplant (pancreas after kidney (PAK)). In addition, solitary pancreas transplantation may also be offered to those individuals presenting with severe metabolic complications attributed to poor glycemic control (pancreas transplant alone (PTA)). Pancreas transplantation procedures have been performed since the 1960’s. A 2011 update on Pancreas Transplantation from the International Pancreas Transplant Registry reported improvements in patient survival and graft function over a course of 24 years of pancreas transplantation (103). These improved outcomes were related to changes in surgical technique and immunosuppressive regimens as well as tighter donor selection criteria. At 5-years post-transplantation, pancreas graft survival is now reported at ~70% for SPK and at ~ 50% for PAK and PTA. Further, patient survival at 10 years exceeds 70% with the highest survival rate observed in PTA recipients (82%).
Islet Transplantation
Islet transplantation provides a less invasive surgical alternative for beta-cell replacement in patients with labile diabetes and has the potential to restore normoglycemia, eliminate severe hypoglycemia and restore hypoglycemia awareness. However, this procedure is still considered experimental in the United States. Marked improvements have also been noted in the field of islet transplantation over the past decade which have led to insulin independence rates at 5 years being comparable to pancreas transplantation outcomes (104). A pivotal study of islet transplantation in patients with T1D showed that at 1-year post transplant, 87% of study participants achieved the primary endpoint of a A1C <7.0% and freedom from severe hypoglycemia (from day 28 to 365) (105). Further details about islet transplantation can be found in the Endotext chapter on this topic.
FUTURE DIRECTIONS IN MANAGEMENT OF TYPE 1 DIABETES
Artificial Pancreas Device Systems - Closed Loop Systems
In addition to insulin-only CL-systems, bi-hormonal closed loop systems are also being actively explored. Additional manufacturers utilizing insulin-only CL-systems are expected to launch their devices in the near future. The introduction of faster-acting insulins (biochaperone lispro and faster-acting insulin aspart (FIAsp)) could potentially make these strategies more effective. As this technology advances, we are getting closer to the goal of a fully automated device which will be able to predict with high accuracy changes in glucose profiles and respond accordingly with stringent modulation of infusion of hormones (e.g., insulin, glucagon, amylin) to maintain glycemia within normal ranges.
Implantation of Encapsulated Islets
Some of the limitations of islet transplantation currently include the limited availability of donors and the need for long term immunosuppression to prevent rejection of the transplanted graft. Protecting the islets from the immunologic environment may allow both the use of non-human islets for transplantation and minimize or eliminate the need for systemic immunosuppression. Thus, the encapsulation of islets to attain these goals has been sought for several years but unfortunately this technology is still not at the stage to make it to the clinical arena. Although initial attempts at encapsulation of islets resulted in damage of the capsule by local tissue responses, newer techniques allowing for conformal coating of human islets have shown promising results in pre-clinical models and are currently being explored (106).
Islet Xenotransplantation
An alternative to human pancreas and islet transplantation which is currently being explored is the use of pig islets. Pig islets have major physiologic similarities to human islets. Notably, pig insulin differs from human insulin by only one amino acid. Donor pigs may be genetically engineered to be protected from the human immune system thus reducing the need for potent immunosuppression. Studies in non-human primates using encapsulated pig islets have resulted in graft survival for more than 6 months (107). Research in this field in actively ongoing.
Stem Cell Based Therapies
Stem cell research has allowed the generation of insulin-producing pancreatic β-cells from human pluripotent stem cells (108). Further, scientists can now also generate alpha and delta cells from stem cells therefore more closely mimicking a fully functional human islet. This technology has the potential to generate vast amounts of glucose-responsive β-cells and allow for the development of customizable islets containing predetermined amounts of specific cell lines. Results in preclinical models are encouraging and a clinical trial is expected in 2021.
Glucose Responsive Insulins (Smart Insulins)
Another area of ongoing research is the development of “smart” drug delivery systems able to respond to environmental or external triggers greatly improving therapeutic performance. Conceptually, “smart” insulins should be able to respond to changes in ambient glucose which would dictate activation or cessation of insulin delivery. Several efforts have been made to generate glucose-responsive insulin delivery systems and some have shown promising results in pre-clinical studies including the utilization of enzymatic triggers, glucose-binding proteins, and synthetic molecules able to bind to glucose. However, current limitations include the potential for immunogenicity and poor glucose selectivity (109). Continued progress in this field in the coming years to reduce the burden of diabetes is anticipated.
CONCLUSIONS
No disease has had such an evolution of therapy in the past 100 years as T1D. From certain death to the discovery of insulin, from impure animal insulin preparations to purified human insulins, from once daily long-acting insulin to CSII, from urine glucose testing to real-time continuous glucose sensors and closed loop insulin pumps, treatments continue to emerge that improve the lives of people with T1D. Our current challenges remain teaching the providers how to best use these new tools, directing our medical systems to allow us to best utilize these therapies, and perhaps most importantly, transferring diabetes technologies to the patients who can best apply them. Although the future is exciting, we need to continually master the use of our current tools before we can successfully move forward. Hopefully, soon the successful management of T1D will become a reality for all with this disease.
REFERENCES
- Rosenfeld, L., Insulin: discovery and controversy. Clin Chem, 2002. 48(12): p. 2270-88.
- Hirsch, I.B., et al., The Evolution of Insulin and How it Informs Therapy and Treatment Choices. Endocr Rev, 2020. 41(5).
- Lauritzen, T., O.K. Faber, and C. Binder, Variation in 125I-insulin absorption and blood glucose concentration. Diabetologia, 1979. 17(5): p. 291-5.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med, 1993. 329(14): p. 977-86.
- Nathan, D.M., et al., Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med, 2005. 353(25): p. 2643-53.
- Martin, C.L., et al., Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care, 2006. 29(2): p. 340-4.
- Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med, 2000. 342(6): p. 381-9.
- Writing Team for the Diabetes, C., I. Complications Trial/Epidemiology of Diabetes, and G. Complications Research, Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA, 2003. 290(16): p. 2159-67.
- Genuth, S., Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes. Endocr Pract, 2006. 12 Suppl 1: p. 34-41.
- White, N.H., et al., Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol, 2008. 126(12): p. 1707-15.
- Beck, R.W., et al., The T1D Exchange clinic registry. J Clin Endocrinol Metab, 2012. 97(12): p. 4383-9.
- Beck, R.W., K.M. Miller, and N.C. Foster, The T1D Exchange Clinic Network and Registry: 10 Years of Enlightenment on the State of Type 1 Diabetes in the United States. Diabetes Technol Ther, 2019. 21(6): p. 310-312.
- Foster, N.C., et al., State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther, 2019. 21(2): p. 66-72.
- Miller, K.M., et al., Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care, 2015. 38(6): p. 971-8.
- Tonyushkina, K. and J.H. Nichols, Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol, 2009. 3(4): p. 971-80.
- Miller, K.M., et al., Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care, 2013. 36(7): p. 2009-14.
- American Diabetes, A., 6. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care, 2021. 44(Suppl 1): p. S73-S84.
- Freckmann, G., et al., System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol, 2012. 6(5): p. 1060-75.
- Bergenstal, R.M., et al., Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther, 2013. 15(3): p. 198-211.
- Basu, A., et al., Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes, 2013. 62(12): p. 4083-7.
- Castle, J.R. and P.G. Jacobs, Nonadjunctive Use of Continuous Glucose Monitoring for Diabetes Treatment Decisions. J Diabetes Sci Technol, 2016. 10(5): p. 1169-73.
- Bailey, T., et al., The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther, 2015. 17(11): p. 787-94.
- New, J.P., et al., Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med, 2015. 32(5): p. 609-17.
- Shivers, J.P., et al., "Turn it off!": diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol, 2013. 7(3): p. 789-94.
- Battelino, T., et al., Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care, 2019. 42(8): p. 1593-1603.
- Bergenstal, R.M., et al., Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol, 2013. 7(2): p. 562-78.
- Yeh, H.C., et al., Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med, 2012. 157(5): p. 336-47.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study, G., et al., The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care, 2009. 32(8): p. 1378-83.
- Foster, N.C., et al., Continuous Glucose Monitoring in Patients With Type 1 Diabetes Using Insulin Injections. Diabetes Care, 2016. 39(6): p. e81-2.
- Bailey, T.S., et al., American Association of Clinical Endocrinologists and American College of Endocrinology 2016 Outpatient Glucose Monitoring Consensus Statement. Endocr Pract, 2016. 22(2): p. 231-61.
- Peters, A.L., et al., Diabetes Technology-Continuous Subcutaneous Insulin Infusion Therapy and Continuous Glucose Monitoring in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 2016: p. jc20162534.
- Sacks, D.B., et al., Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care, 2011. 34(6): p. 1419-23.
- Rubinow, K.B. and I.B. Hirsch, Reexamining metrics for glucose control. JAMA, 2011. 305(11): p. 1132-3.
- American Diabetes, A., 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care, 2021. 44(Suppl 1): p. S125-S150.
- Shah, V.N., et al., Risk Factors for Cardiovascular Disease (CVD) in Adults with Type 1 Diabetes: Findings from Prospective Real-life T1D Exchange Registry. J Clin Endocrinol Metab, 2020. 105(5).
- Schofield, J., J. Ho, and H. Soran, Cardiovascular Risk in Type 1 Diabetes Mellitus. Diabetes Ther, 2019. 10(3): p. 773-789.
- Vistisen, D., et al., Prediction of First Cardiovascular Disease Event in Type 1 Diabetes Mellitus: The Steno Type 1 Risk Engine. Circulation, 2016. 133(11): p. 1058-66.
- Sousa, G.R., et al., Glycemic Control, Cardiac Autoimmunity, and Long-Term Risk of Cardiovascular Disease in Type 1 Diabetes Mellitus. Circulation, 2019. 139(6): p. 730-743.
- Howey, D.C., et al., (Lys(B28), Pro(B29))-human insulin. A rapidly absorbed analogue of human insulin. Diabetes, 1994. 43(3): p. 396-402.
- Mudaliar, S.R., et al., Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care, 1999. 22(9): p. 1501-6.
- Garg, S.K., S.L. Ellis, and H. Ulrich, Insulin glulisine: a new rapid-acting insulin analogue for the treatment of diabetes. Expert Opin Pharmacother, 2005. 6(4): p. 643-51.
- Davis, A., J. Kuriakose, and J.N. Clements, Faster Insulin Aspart: A New Bolus Option for Diabetes Mellitus. Clin Pharmacokinet, 2019. 58(4): p. 421-430.
- Linnebjerg, H., et al., Pharmacokinetics and Glucodynamics of Ultra Rapid Lispro (URLi) versus Humalog((R)) (Lispro) in Younger Adults and Elderly Patients with Type 1 Diabetes Mellitus: A Randomised Controlled Trial. Clin Pharmacokinet, 2020. 59(12): p. 1589-1599.
- Blevins, T., et al., Randomized Double-Blind Clinical Trial Comparing Ultra Rapid Lispro With Lispro in a Basal-Bolus Regimen in Patients With Type 2 Diabetes: PRONTO-T2D. Diabetes Care, 2020. 43(12): p. 2991-2998.
- Klaff, L., et al., Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: Results from the 26-week PRONTO-T1D study. Diabetes Obes Metab, 2020. 22(10): p. 1799-1807.
- Bode, B.W., et al., Inhaled Technosphere Insulin Compared With Injected Prandial Insulin in Type 1 Diabetes: A Randomized 24-Week Trial. Diabetes Care, 2015. 38(12): p. 2266-73.
- Borgono, C.A. and B. Zinman, Insulins: past, present, and future. Endocrinol Metab Clin North Am, 2012. 41(1): p. 1-24.
- Hirsch, I.B., Insulin analogues. N Engl J Med, 2005. 352(2): p. 174-83.
- Switzer, S.M., et al., Intensive insulin therapy in patients with type 1 diabetes mellitus. Endocrinol Metab Clin North Am, 2012. 41(1): p. 89-104.
- Ratner, R.E., et al., Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care, 2000. 23(5): p. 639-43.
- Havelund, S., et al., The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res, 2004. 21(8): p. 1498-504.
- Heller, S., C. Koenen, and B. Bode, Comparison of insulin detemir and insulin glargine in a basal-bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: a 52-week, multinational, randomized, open-label, parallel-group, treat-to-target noninferiority trial. Clin Ther, 2009. 31(10): p. 2086-97.
- Keating, G.M., Insulin degludec and insulin degludec/insulin aspart: a review of their use in the management of diabetes mellitus. Drugs, 2013. 73(6): p. 575-93.
- Mathieu, C., et al., Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab, 2013. 98(3): p. 1154-62.
- Russell-Jones, D., et al., Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: A meta-analysis of seven clinical trials. Nutr Metab Cardiovasc Dis, 2015. 25(10): p. 898-905.
- Meneghini, L.F., Insulin therapy for type 2 diabetes. Endocrine, 2013. 43(3): p. 529-34.
- Dailey, G. and F. Lavernia, A review of the safety and efficacy data for insulin glargine 300 units/ml, a new formulation of insulin glargine. Diabetes Obes Metab, 2015. 17(12): p. 1107-14.
- Home, P.D., et al., New Insulin Glargine 300 Units/mL Versus Glargine 100 Units/mL in People With Type 1 Diabetes: A Randomized, Phase 3a, Open-Label Clinical Trial (EDITION 4). Diabetes Care, 2015. 38(12): p. 2217-25.
- Pettus, J., et al., The past, present, and future of basal insulins. Diabetes Metab Res Rev, 2016. 32(6): p. 478-96.
- Hirsch, I.B., et al., Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care, 2005. 28(3): p. 533-8.
- Kowalski, A.J., Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther, 2009. 11 Suppl 1: p. S113-9.
- Trevitt, S., S. Simpson, and A. Wood, Artificial Pancreas Device Systems for the Closed-Loop Control of Type 1 Diabetes: What Systems Are in Development? J Diabetes Sci Technol, 2016. 10(3): p. 714-23.
- Bergenstal, R.M., et al., Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA, 2016. 316(13): p. 1407-1408.
- Breton, M.D., et al., A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. N Engl J Med, 2020. 383(9): p. 836-845.
- Brown, S.A., et al., Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med, 2019. 381(18): p. 1707-1717.
- Forlenza, G.P., et al., Safety Evaluation of the MiniMed 670G System in Children 7-13 Years of Age with Type 1 Diabetes. Diabetes Technol Ther, 2019. 21(1): p. 11-19.
- Garg, S.K., et al., Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther, 2017. 19(3): p. 155-163.
- Lebovitz, H.E., Adjunct therapy for type 1 diabetes mellitus. Nat Rev Endocrinol, 2010. 6(6): p. 326-34.
- Edelman, S., et al., A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care, 2006. 29(10): p. 2189-95.
- Riddle, M.C., et al., Control of Postprandial Hyperglycemia in Type 1 Diabetes by 24-Hour Fixed-Dose Coadministration of Pramlintide and Regular Human Insulin: A Randomized, Two-Way Crossover Study. Diabetes Care, 2018. 41(11): p. 2346-2352.
- Schimmack, G., R.A. Defronzo, and N. Musi, AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes Metab, 2006. 8(6): p. 591-602.
- Vella, S., et al., The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia, 2010. 53(5): p. 809-20.
- Libman, I.M., et al., Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA, 2015. 314(21): p. 2241-50.
- Liu, Y.S., et al., Vascular and metabolic effects of metformin added to insulin therapy in patients with type 1 diabetes: A systematic review and meta-analysis. Diabetes Metab Res Rev, 2020. 36(6): p. e3334.
- Bjornstad, P., et al., Metformin Improves Insulin Sensitivity and Vascular Health in Youth With Type 1 Diabetes Mellitus. Circulation, 2018. 138(25): p. 2895-2907.
- Petrie, J.R., et al., Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol, 2017. 5(8): p. 597-609.
- Henry, R.R., et al., Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care, 2015. 38(12): p. 2258-65.
- Perkins, B.A., et al., Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care, 2014. 37(5): p. 1480-3.
- Pieber, T.R., et al., Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab, 2015. 17(10): p. 928-35.
- Bode, B.W. and S.K. Garg, The Emerging Role of Adjunctive Noninsulin Antihyperglycemic Therapy in the Management of Type 1 Diabetes. Endocr Pract, 2016. 22(2): p. 220-30.
- Janssens, B., S. Caerels, and C. Mathieu, SGLT inhibitors in type 1 diabetes: weighing efficacy and side effects. Ther Adv Endocrinol Metab, 2020. 11: p. 2042018820938545.
- Sands, A.T., et al., Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care, 2015. 38(7): p. 1181-8.
- Taylor, S.I., et al., SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol, 2019. 7(12): p. 949-958.
- Dejgaard, T.F., et al., Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol, 2016. 4(3): p. 221-32.
- Ahren, B., et al., Efficacy and Safety of Liraglutide Added to Capped Insulin Treatment in Subjects With Type 1 Diabetes: The ADJUNCT TWO Randomized Trial. Diabetes Care, 2016. 39(10): p. 1693-701.
- Mathieu, C., et al., Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care, 2016. 39(10): p. 1702-10.
- Dejgaard, T.F., et al., Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: The Lira Pump trial-a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab, 2020. 22(4): p. 492-500.
- Herold, K.C., et al., Exenatide extended release in patients with type 1 diabetes with and without residual insulin production. Diabetes Obes Metab, 2020. 22(11): p. 2045-2054.
- Ellis, S.L., et al., Effect of sitagliptin on glucose control in adult patients with Type 1 diabetes: a pilot, double-blind, randomized, crossover trial. Diabet Med, 2011. 28(10): p. 1176-81.
- Kirwan, J.P., et al., Bariatric Surgery in Obese Patients With Type 1 Diabetes. Diabetes Care, 2016. 39(6): p. 941-8.
- Chiang, J.L., et al., Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care, 2014. 37(7): p. 2034-54.
- Hagger, V., et al., Diabetes Distress Among Adolescents with Type 1 Diabetes: a Systematic Review. Curr Diab Rep, 2016. 16(1): p. 9.
- Chimen, M., et al., What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia, 2012. 55(3): p. 542-51.
- Sluik, D., et al., Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch Intern Med, 2012. 172(17): p. 1285-95.
- Riddell, M.C., et al., Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol, 2017. 5(5): p. 377-390.
- Zaharieva, D.P., et al., Improved Open-Loop Glucose Control With Basal Insulin Reduction 90 Minutes Before Aerobic Exercise in Patients With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Care, 2019. 42(5): p. 824-831.
- Rickels, M.R., et al., Mini-Dose Glucagon as a Novel Approach to Prevent Exercise-Induced Hypoglycemia in Type 1 Diabetes. Diabetes Care, 2018. 41(9): p. 1909-1916.
- American Diabetes, A., 12. Older Adults: Standards of Medical Care in Diabetes-2021. Diabetes Care, 2021. 44(Suppl 1): p. S168-S179.
- Kirkman, M.S., et al., Diabetes in older adults. Diabetes Care, 2012. 35(12): p. 2650-64.
- American Diabetes, A., 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2021. Diabetes Care, 2021. 44(Suppl 1): p. S211-S220.
- Gomez, A.M. and G.E. Umpierrez, Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol, 2014. 8(5): p. 930-6.
- Gomez, A.M., et al., Continuous Glucose Monitoring Versus Capillary Point-of-Care Testing for Inpatient Glycemic Control in Type 2 Diabetes Patients Hospitalized in the General Ward and Treated With a Basal Bolus Insulin Regimen. J Diabetes Sci Technol, 2016. 10(2): p. 325-9.
- Gruessner, A.C., 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud, 2011. 8(1): p. 6-16.
- Bellin, M.D., et al., Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant, 2012. 12(6): p. 1576-83.
- Hering, B.J., et al., Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care, 2016. 39(7): p. 1230-40.
- Tomei, A.A., et al., Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci U S A, 2014. 111(29): p. 10514-9.
- Park, C.G., R. Bottino, and W.J. Hawthorne, Current status of islet xenotransplantation. Int J Surg, 2015. 23(Pt B): p. 261-6.
- Pagliuca, F.W., et al., Generation of functional human pancreatic beta cells in vitro. Cell, 2014. 159(2): p. 428-39.
- Webber, M.J. and D.G. Anderson, Smart approaches to glucose-responsive drug delivery. J Drug Target, 2015. 23(7-8): p. 651-5.